
Company Overview Q3 2019

Safe Harbor Statement This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statement in this presentation that is not a historical fact is a forward-looking statement. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially and reported results should not be considered as an indication of future performance. Examples of such statements include, but are not limited to: statements relating to the commercial or market opportunity and expansion; the adequacy of Salarius’ capital to support its future operations and its ability to successfully initiate and complete clinical trials and regulatory submissions; expected dose escalation and dose expansion; number of additional clinical sites; expected cohort readouts; expected therapeutic options for SP-2577 and related effects; timing of development and future milestones; the nature, strategy and focus of Salarius; future economic conditions or performance; and the development, expected timeline and commercial potential of any product candidates of Salarius. Salarius may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in the forward-looking statements and you should not place undue reliance on these forward-looking statements. Such statements are based on management’s current expectations and involve risks and uncertainties. Actual results and performance could differ materially from those projected in the forward-looking statements as a result of many factors, including, without limitation: risks and uncertainties associated with the availability of sufficient resources of Salarius to meet its business objectives and operational requirements; the ability to project future cash utilization and reserves needed for contingent future liabilities and business operations; the fact that the results of earlier studies and trials may not be predictive of future clinical trial results; the ability to protect Salarius’ intellectual property rights; risks related to the drug development and the regulatory approval process; and the impact of competitive products and technological changes. Salarius disclaims any intent or obligation to update these forward-looking statements to reflect events or circumstances that exist after the date on which they were made. You should review additional disclosures we make in our filings with the Securities and Exchange Commission, including our Quarterly Reports on Form 10-Q and our Annual Report on Form 10-K. You may access these documents for no charge at http://www.sec.gov. A registration statement on Form S-3 of Flex Pharma, Inc. has been filed with the Securities and Exchange Commission and declared effective. The offering of these securities will be made only by means of a written prospectus supplement and base prospectus forming part of the effective registration statement relating to the shares. Copies of the prospectus for this offering may be obtained, when available, by contacting Oppenheimer & Co. Inc., 85 Broad Street, 26th Floor, New York, NY 10004, Attn: Syndicate Prospectus Department, by calling (212) 667-8563, or by email to EquityProspectus@opco.com. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction. © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL 2

Investor Highlights: Salarius Pharmaceuticals is an Epigenetic Focused Clinical-stage Oncology Biotech Company 1 Salarius has a differentiated LSD1 inhibitor with expected human data in 2020 • Multi-company interest and clinical data validates LSD1 as a therapeutic target 2 Development strategy focused on Speed to Market and Market Expansion • Speed to Market: Ewing sarcoma trial Rare Pediatric Disease and Orphan Status Designation • Market Expansion: Advanced Solid Tumor trial Hormonal cancers, sarcomas ($1B+ markets) 3 Seasoned management team leading Salarius • Experienced in product, clinical and early stage development 4 Lead clinical program funded by extensive non-dilutive capital • $18.7M CPRIT award and support from the National Pediatric Cancer Foundation 5 Opportune time to capitalize on growth potential • Potential to expand into other indications of high value (including immunotherapy) • Relatively short timeline to pivotal inflection points 3 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL

Seasoned Management Team Margaret Dugan, MD Steve Horrigan, PhD Senior Medical Advisor Chief Scientific Officer Scott Jordan Daniela Y Santiesteban Chief Financial Officer PhD Business Development Manager Board of Directors David Arthur, Jonathan Northrup, Tess Burleson, Paul Lammers, Bruce McCreedy, William McVicar, Arnold Hanish, MBA MBA CPA MD MSc PhD PhD CPA Salarius Stingray Translational Triumvira Precision Medicine Flex Pharma Omeros Corporation Pharmaceuticals Therapeutics Genomics 4 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL
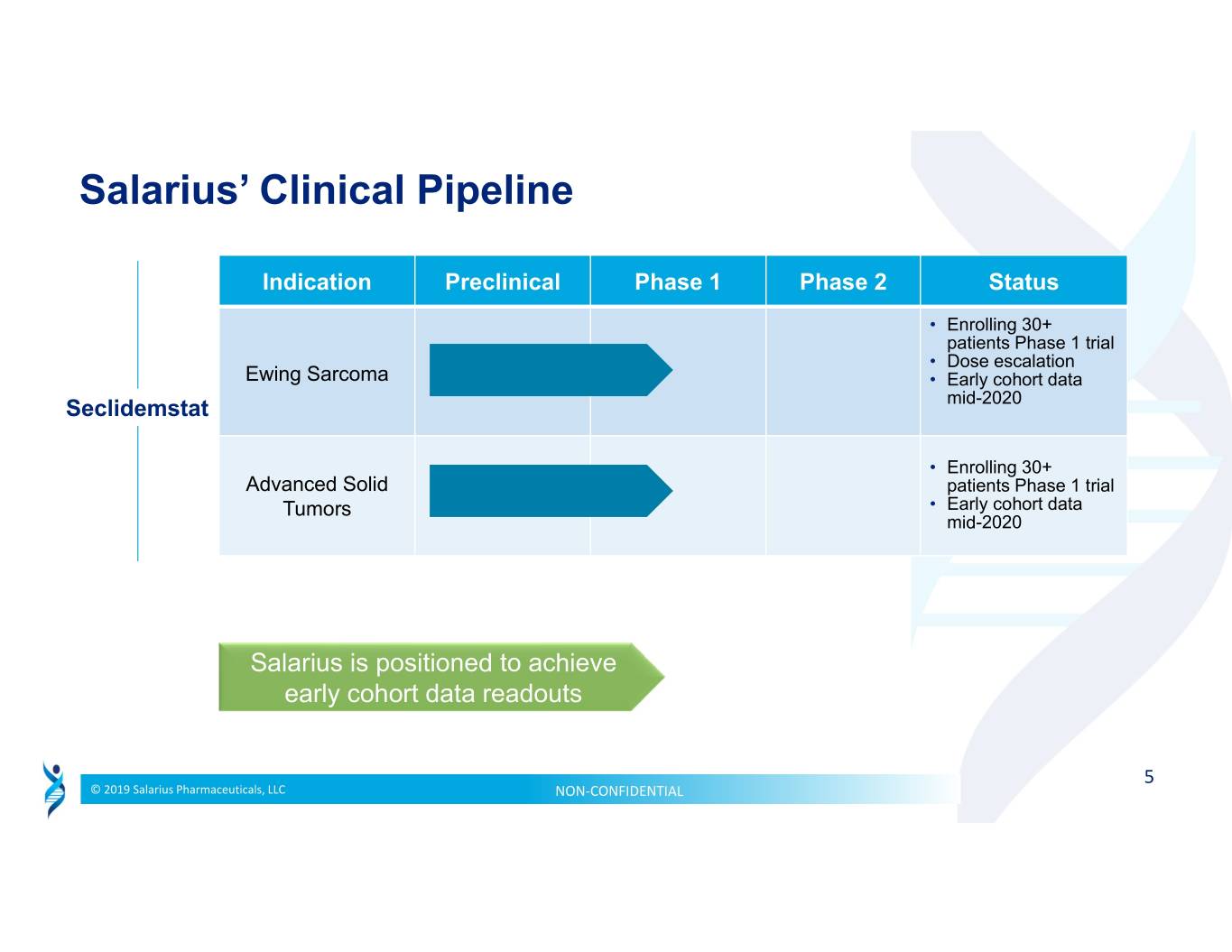
Salarius’ Clinical Pipeline Indication Preclinical Phase 1 Phase 2 Status • Enrolling 30+ patients Phase 1 trial • Dose escalation Ewing Sarcoma • Early cohort data Seclidemstat mid-2020 • Enrolling 30+ Advanced Solid patients Phase 1 trial Tumors • Early cohort data mid-2020 Salarius is positioned to achieve early cohort data readouts 5 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL

Salarius is Poised to Add to the Growing Epigenetic Wave The epigenetic space has been increasing in activity since 2018 Clinical Preclinical Drug registration Phase 1: LSD1; Ewing’s and Solid Tumors & Phase 1: EZH2 and BET inhibitors; solid/heme ~$1B deal ($40M upfront) to Submitted an NDA for advance a preclinical asset (lead Phase 2: LSD1; AML and Epithelioid Sarcoma optimization) SCLC (1H2019) and has plans to submit another for Follicular Lymphoma (2H2019) Phase 2b: Raised $40M to advance LSD1 program 6 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL
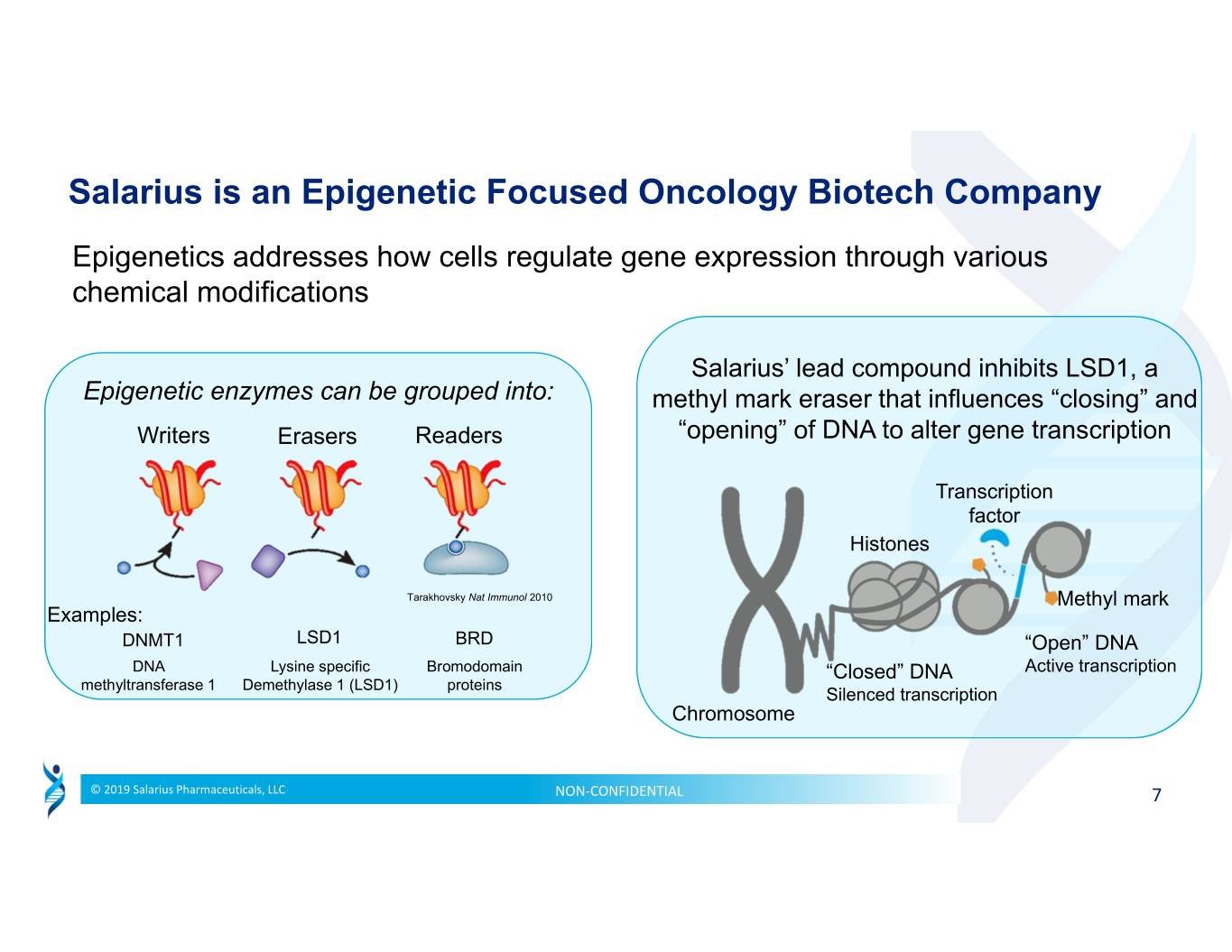
Salarius is an Epigenetic Focused Oncology Biotech Company Epigenetics addresses how cells regulate gene expression through various chemical modifications Salarius’ lead compound inhibits LSD1, a Epigenetic enzymes can be grouped into: methyl mark eraser that influences “closing” and WritersErasers Readers “opening” of DNA to alter gene transcription Transcription factor Histones Tarakhovsky Nat Immunol 2010 Methyl mark Examples: DNMT1 LSD1 BRD “Open” DNA DNA Lysine specific Bromodomain “Closed” DNA Active transcription methyltransferase 1 Demethylase 1 (LSD1) proteins Silenced transcription Chromosome © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL 7

LSD1 Is An Attractive Target For Cancer Therapy • Lysine Specific Demethylase 1 (LSD1) is an epigenetic “eraser” that is a target of interest for solid tumors and hematological cancers . LSD1 overexpression is often correlated with poor prognosis via regulation of pathways involved in: • Cell differentiation • Cell motility • Stem-like phenotype • Cell cycle LSD1 affects gene expression via . LSD1 associates with over 60 gene regulatory enzymatic and scaffolding properties proteins1 Lead compound, Seclidemstat (SP-2577), comprehensively inhibits LSD1 8 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL 1Majello,B. Cancers 2019.

LSD1 is a target of interest given Recent works demonstrate LSD1’s its role in cancer progression demethylation independent activity 2019 2019 2018 2019 9 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL

Competitive Landscape and Differentiation
Seclidemstat is a Differentiated Inhibitor Addressing Areas of High Unmet Need Supported by Strong IP • Seclidemstat (SP-2577) is a small molecule oral therapeutic differentiated by: (1) Mechanism – reversible vs. irreversible (2) Binding location – comprehensive inhibition of enzymatic and scaffolding properties • Strategically positioned in indications of high unmet need w/ strong mechanistic rationale: o Ewing Sarcoma – Aggressive childhood bone cancer, no approved targeted treatments o Other Sarcomas – Share a similar biology to Ewing sarcoma o Late Stage Prostate/Breast/Ovarian and other cancers are upside • Composition of matter patents allowed globally – US patent expires in 2032 exclusive of possible extensions 11 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL
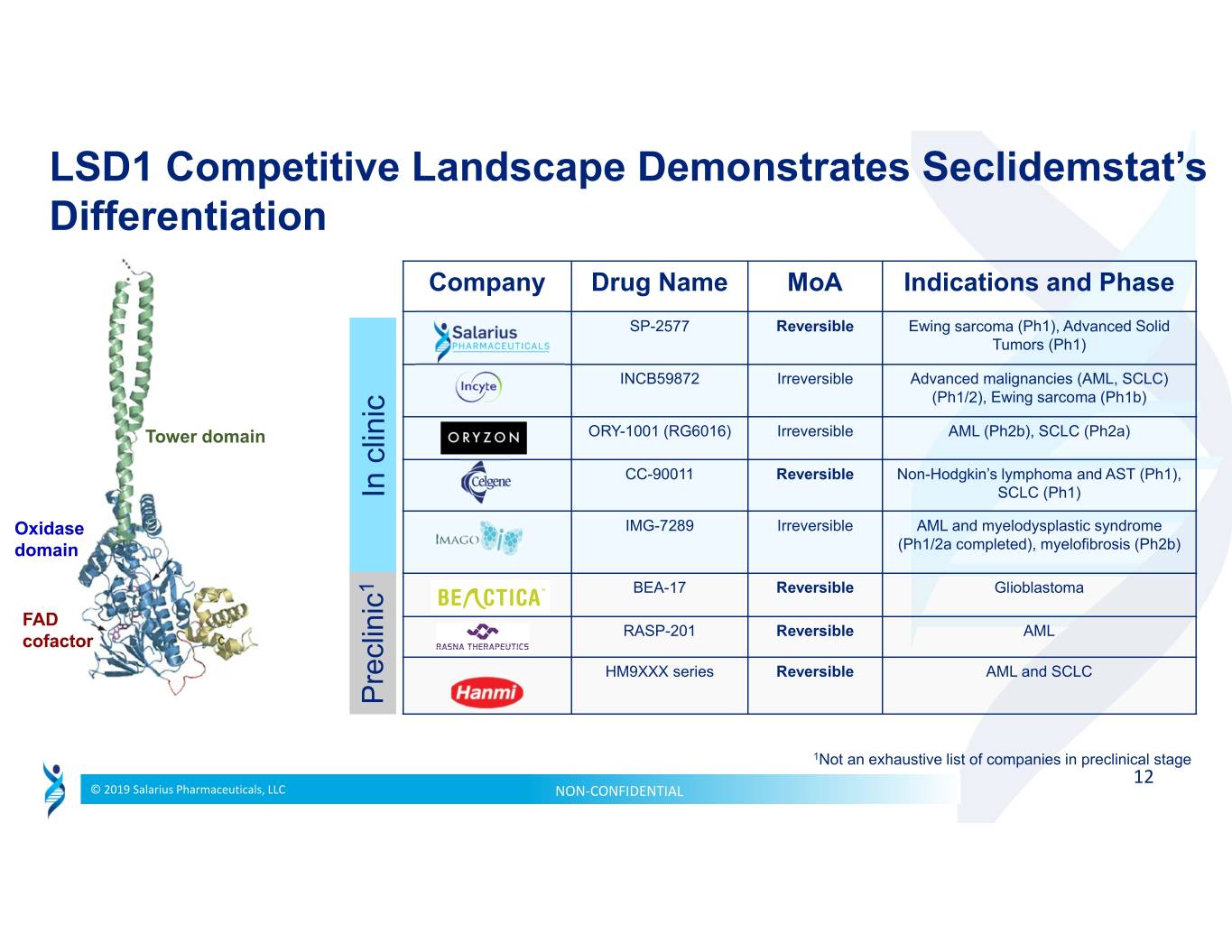
LSD1 Competitive Landscape Demonstrates Seclidemstat’s Differentiation Company Drug Name MoA Indications and Phase SP-2577 Reversible Ewing sarcoma (Ph1), Advanced Solid Tumors (Ph1) INCB59872 Irreversible Advanced malignancies (AML, SCLC) (Ph1/2), Ewing sarcoma (Ph1b) Tower domain ORY-1001 (RG6016) Irreversible AML (Ph2b), SCLC (Ph2a) CC-90011 Reversible Non-Hodgkin’s lymphoma and AST (Ph1), In clinic SCLC (Ph1) Oxidase IMG-7289 Irreversible AML and myelodysplastic syndrome domain (Ph1/2a completed), myelofibrosis (Ph2b) 1 BEA-17 Reversible Glioblastoma FAD RASP-201 Reversible AML cofactor HM9XXX series Reversible AML and SCLC Preclinic 1Not an exhaustive list of companies in preclinical stage 12 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL
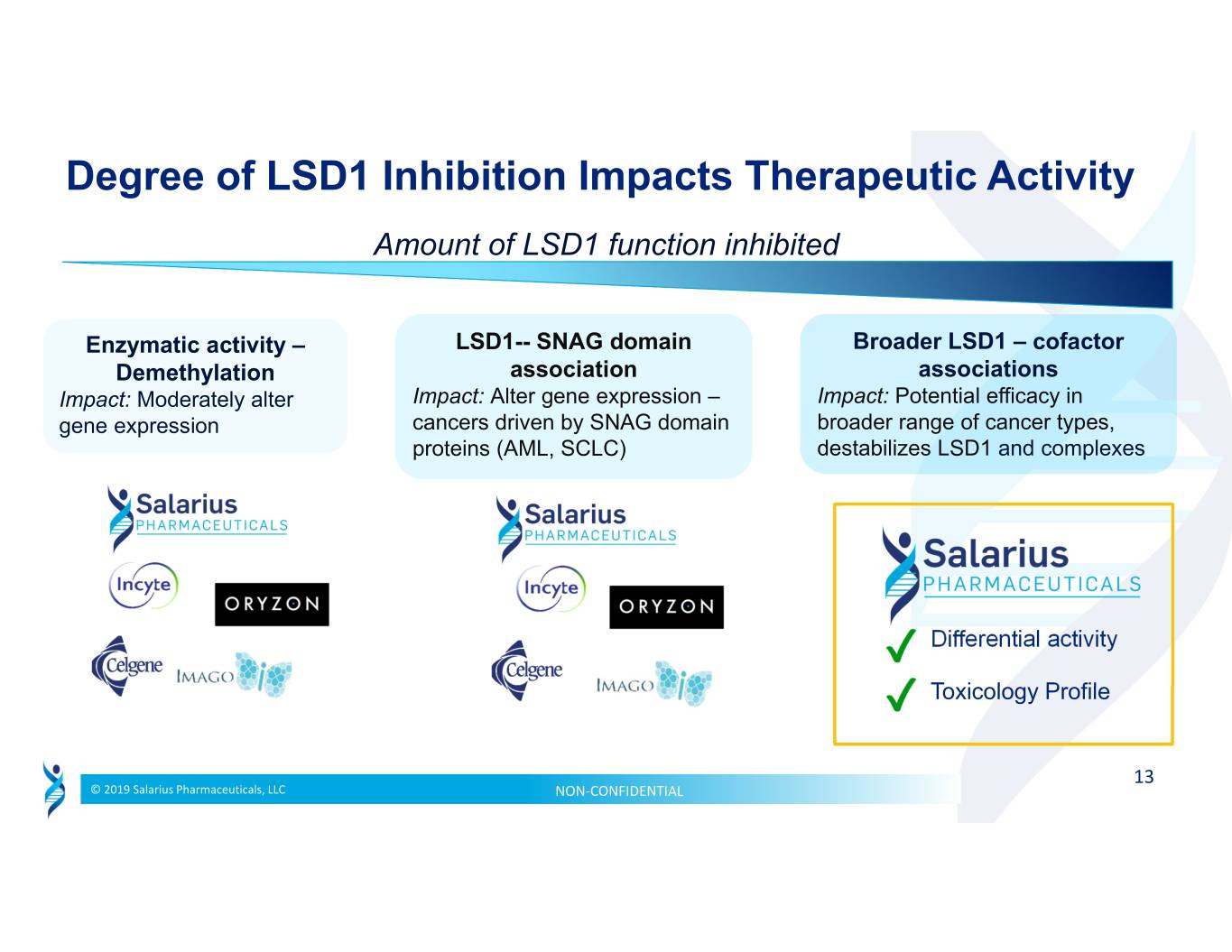
Degree of LSD1 Inhibition Impacts Therapeutic Activity Amount of LSD1 function inhibited Enzymatic activity – LSD1-- SNAG domain Broader LSD1 – cofactor Demethylation association associations Impact: Moderately alter Impact: Alter gene expression – Impact: Potential efficacy in gene expression cancers driven by SNAG domain broader range of cancer types, proteins (AML, SCLC) destabilizes LSD1 and complexes Differential activity Toxicology Profile 13 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL

Speed to Market: Seclidemstat in Ewing sarcoma
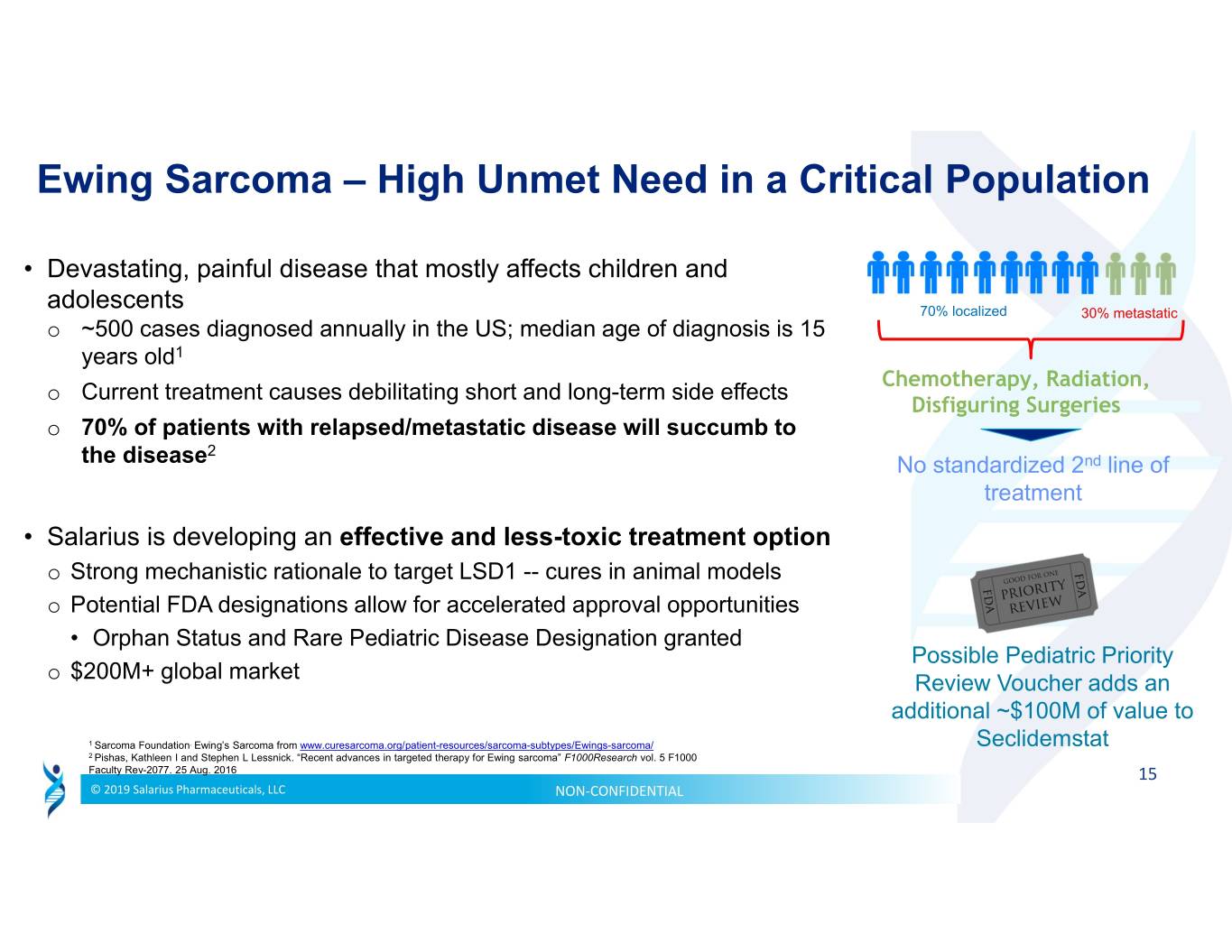
Ewing Sarcoma – High Unmet Need in a Critical Population • Devastating, painful disease that mostly affects children and adolescents 70% localized 30% metastatic o ~500 cases diagnosed annually in the US; median age of diagnosis is 15 years old1 Chemotherapy, Radiation, Current treatment causes debilitating short and long-term side effects o Disfiguring Surgeries o 70% of patients with relapsed/metastatic disease will succumb to 2 the disease No standardized 2nd line of treatment • Salarius is developing an effective and less-toxic treatment option o Strong mechanistic rationale to target LSD1 -- cures in animal models o Potential FDA designations allow for accelerated approval opportunities • Orphan Status and Rare Pediatric Disease Designation granted Possible Pediatric Priority $200M+ global market o Review Voucher adds an additional ~$100M of value to 1 Sarcoma Foundation. Ewing’s Sarcoma from www.curesarcoma.org/patient-resources/sarcoma-subtypes/Ewings-sarcoma/ Seclidemstat 2 Pishas, Kathleen I and Stephen L Lessnick. “Recent advances in targeted therapy for Ewing sarcoma” F1000Research vol. 5 F1000 Faculty Rev-2077. 25 Aug. 2016 15 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL
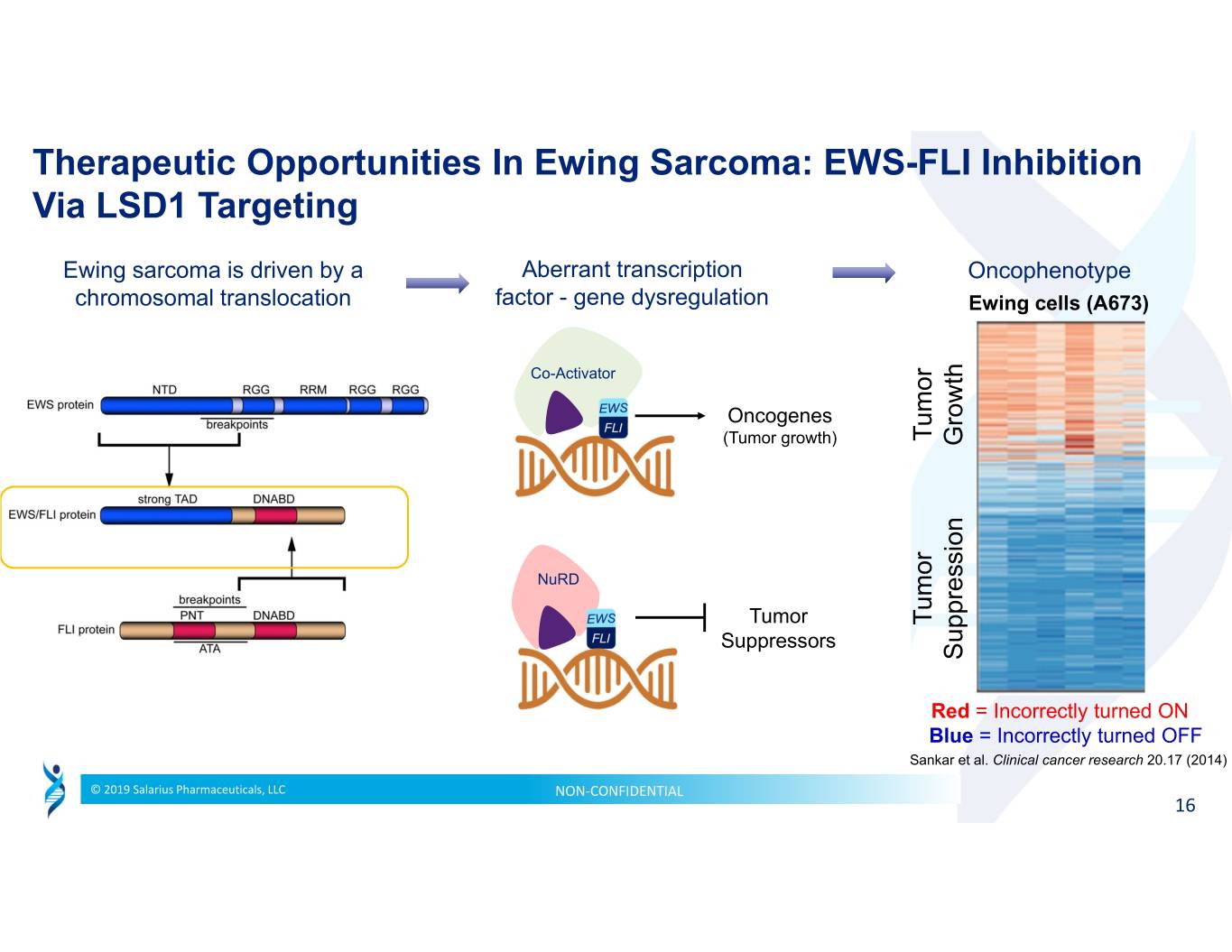
Therapeutic Opportunities In Ewing Sarcoma: EWS-FLI Inhibition Via LSD1 Targeting Ewing sarcoma is driven by a Aberrant transcription Oncophenotype chromosomal translocation factor - gene dysregulation Ewing cells (A673) Co-Activator Oncogenes Tumor (Tumor growth) Growth NuRD Tumor Tumor Suppressors Suppression Red = Incorrectly turned ON Blue = Incorrectly turned OFF Sankar et al. Clinical cancer research 20.17 (2014) © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL 16
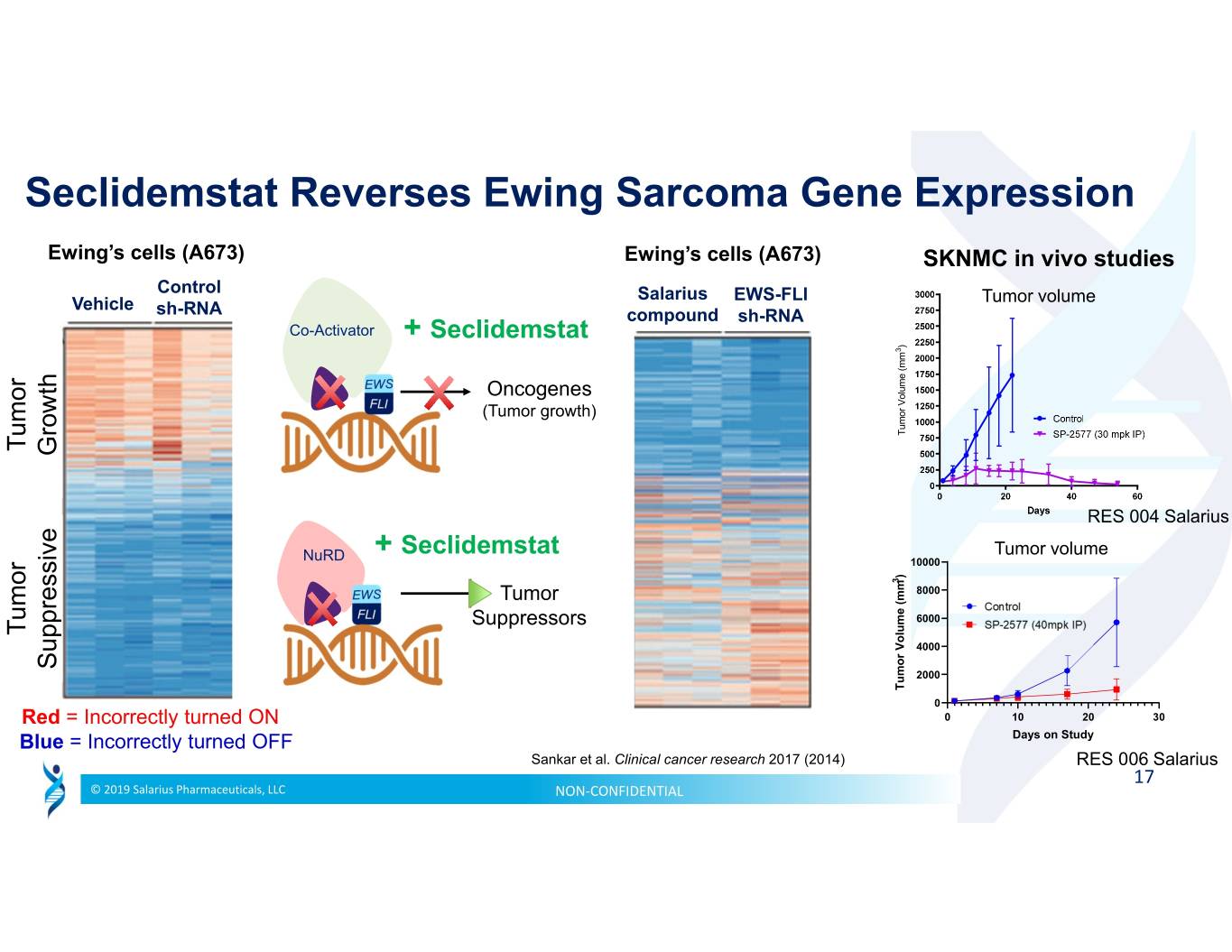
Seclidemstat Reverses Ewing Sarcoma Gene Expression Ewing’s cells (A673) Ewing’s cells (A673) SKNMC in vivo studies Control Salarius EWS-FLI Vehicle Tumor volume sh-RNA compound sh-RNA Co-Activator + Seclidemstat ) 3 Oncogenes (Tumor growth) Tumor Volume (mm Tumor Growth RES 004 Salarius + Seclidemstat Tumor volume NuRD 10000 ) 3 Tumor 8000 Suppressors 6000 Tumor 4000 Suppressive 2000 Tumor (mm Volume Tumor 0 Red = Incorrectly turned ON 0 102030 Blue = Incorrectly turned OFF Days on Study Sankar et al. Clinical cancer research 2017 (2014) RES 006 Salarius 17 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL
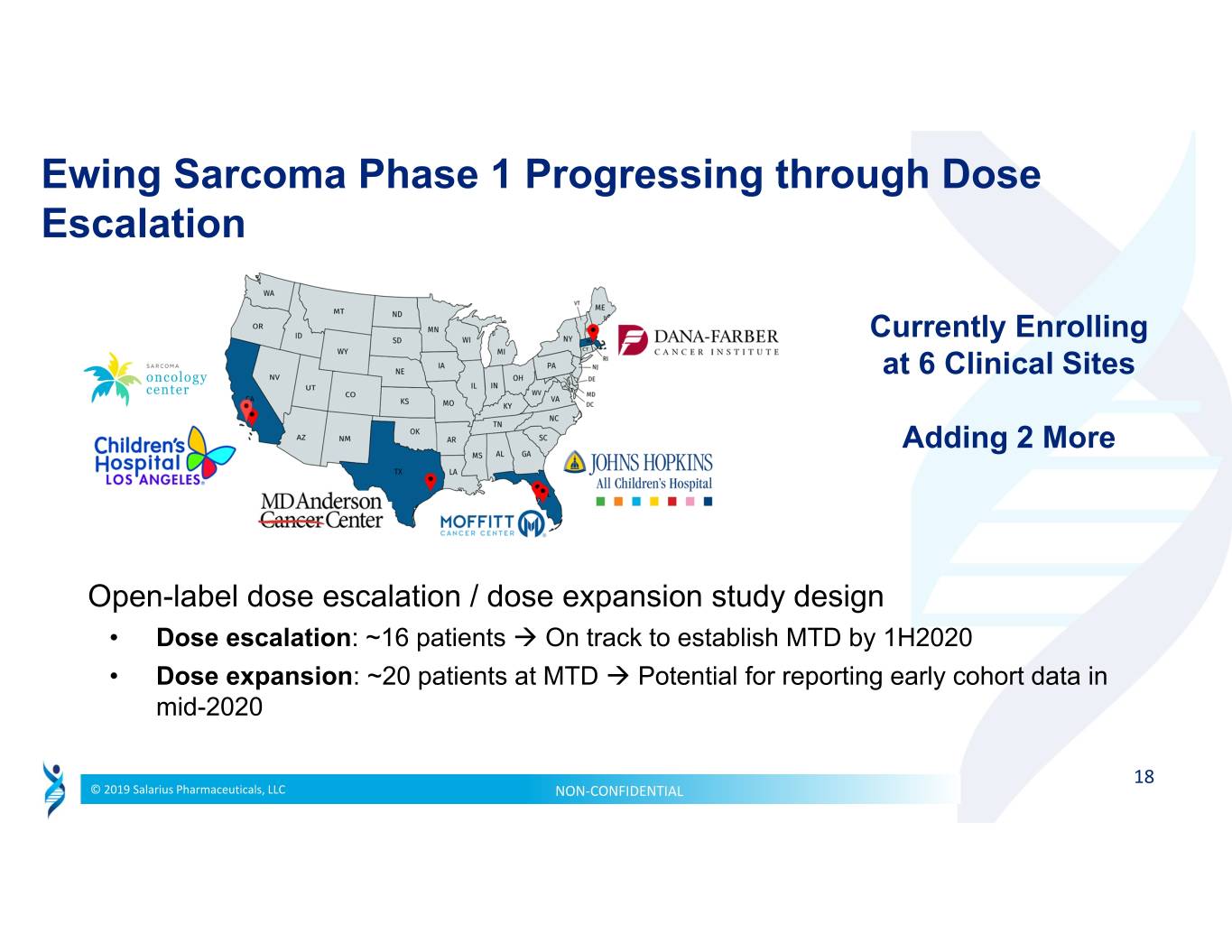
Ewing Sarcoma Phase 1 Progressing through Dose Escalation Currently Enrolling at 6 Clinical Sites Adding 2 More Open-label dose escalation / dose expansion study design • Dose escalation: ~16 patients ��� On track to establish MTD by 1H2020 • Dose expansion: ~20 patients at MTD Potential for reporting early cohort data in mid-2020 18 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL
Market Expansion: Seclidemstat in Advanced Solid Tumors
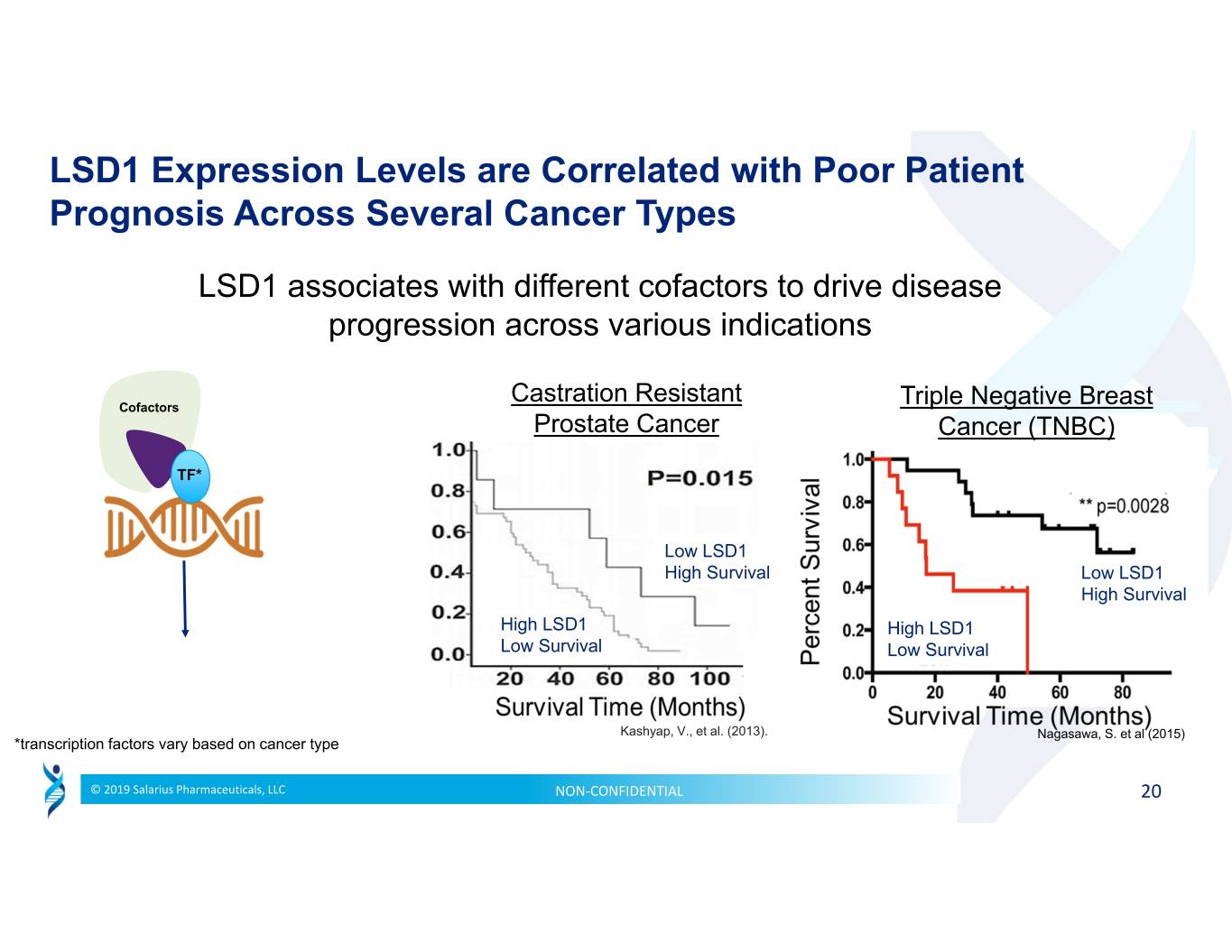
LSD1 Expression Levels are Correlated with Poor Patient Prognosis Across Several Cancer Types LSD1 associates with different cofactors to drive disease progression across various indications Castration Resistant Cofactors Triple Negative Breast Prostate Cancer Cancer (TNBC) TF* Low LSD1 High Survival Low LSD1 High Survival High LSD1 High LSD1 Low Survival Low Survival Kashyap, V., et al. (2013). Nagasawa, S. et al (2015) *transcription factors vary based on cancer type © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL 20
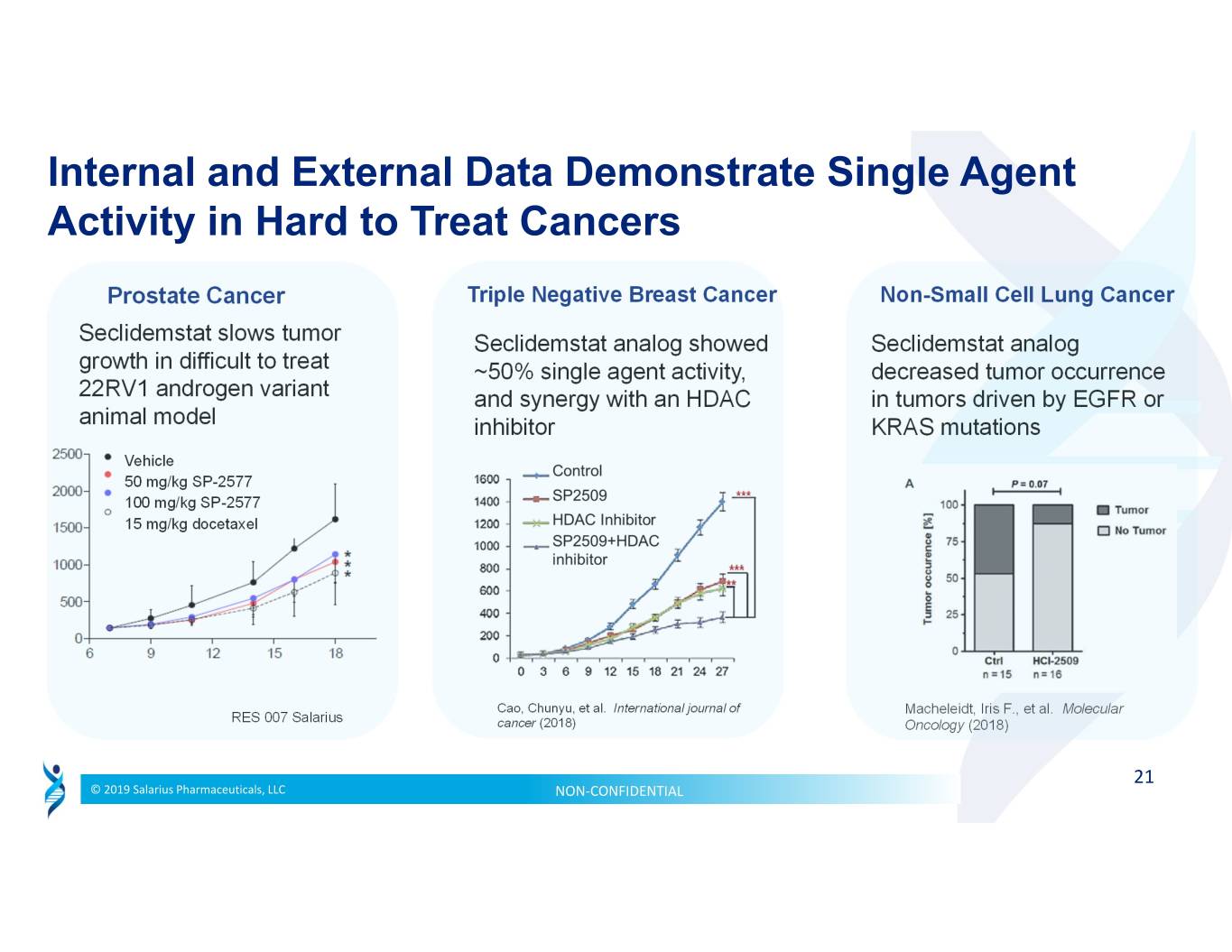
Internal and External Data Demonstrate Single Agent Activity in Hard to Treat Cancers Prostate Cancer Triple Negative Breast Cancer Non-Small Cell Lung Cancer Seclidemstat slows tumor Seclidemstat analog showed Seclidemstat analog growth in difficult to treat ~50% single agent activity, decreased tumor occurrence 22RV1 androgen variant and synergy with an HDAC in tumors driven by EGFR or animal model inhibitor KRAS mutations Vehicle 50 mg/kg SP-2577 100 mg/kg SP-2577 15 mg/kg docetaxel Cao, Chunyu, et al. International journal of Macheleidt, Iris F., et al. Molecular RES 007 Salarius cancer (2018) Oncology (2018) 21 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL
AST Clinical Trial Overview Open-label dose escalation / dose expansion study design • Enrolling advanced malignancies and enriching for indications Seclidemstat has shown efficacy – Prostate, breast, patients with specific genetic backgrounds + others • Potential for early signs of therapeutic activity with established biomarker • Early cohort readouts in mid-2020 22 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL

Future Opportunities
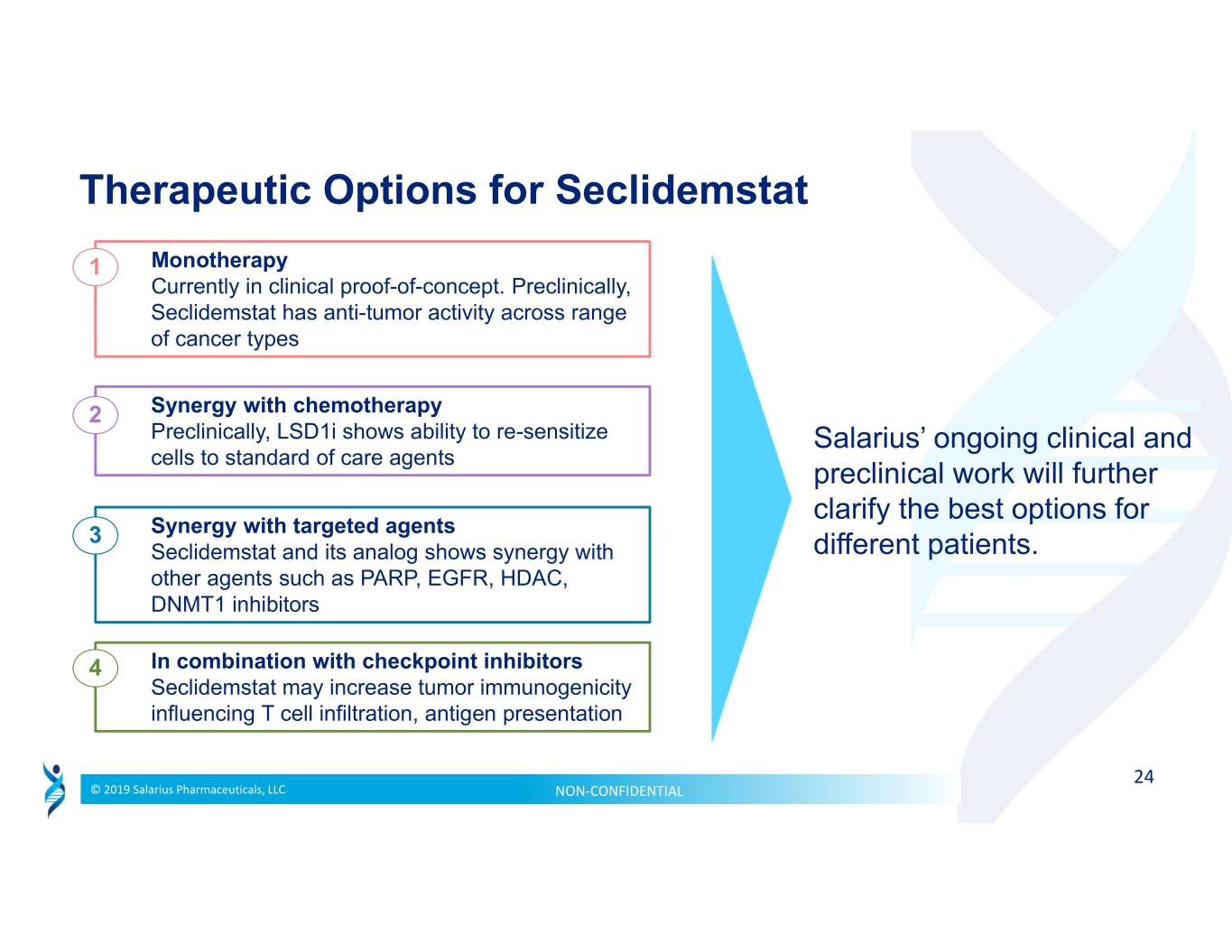
Therapeutic Options for Seclidemstat 1 Monotherapy Currently in clinical proof-of-concept. Preclinically, Seclidemstat has anti-tumor activity across range of cancer types 2 Synergy with chemotherapy Preclinically, LSD1i shows ability to re-sensitize Salarius’ ongoing clinical and cells to standard of care agents preclinical work will further clarify the best options for 3 Synergy with targeted agents Seclidemstat and its analog shows synergy with different patients. other agents such as PARP, EGFR, HDAC, DNMT1 inhibitors 4 In combination with checkpoint inhibitors Seclidemstat may increase tumor immunogenicity influencing T cell infiltration, antigen presentation 24 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL
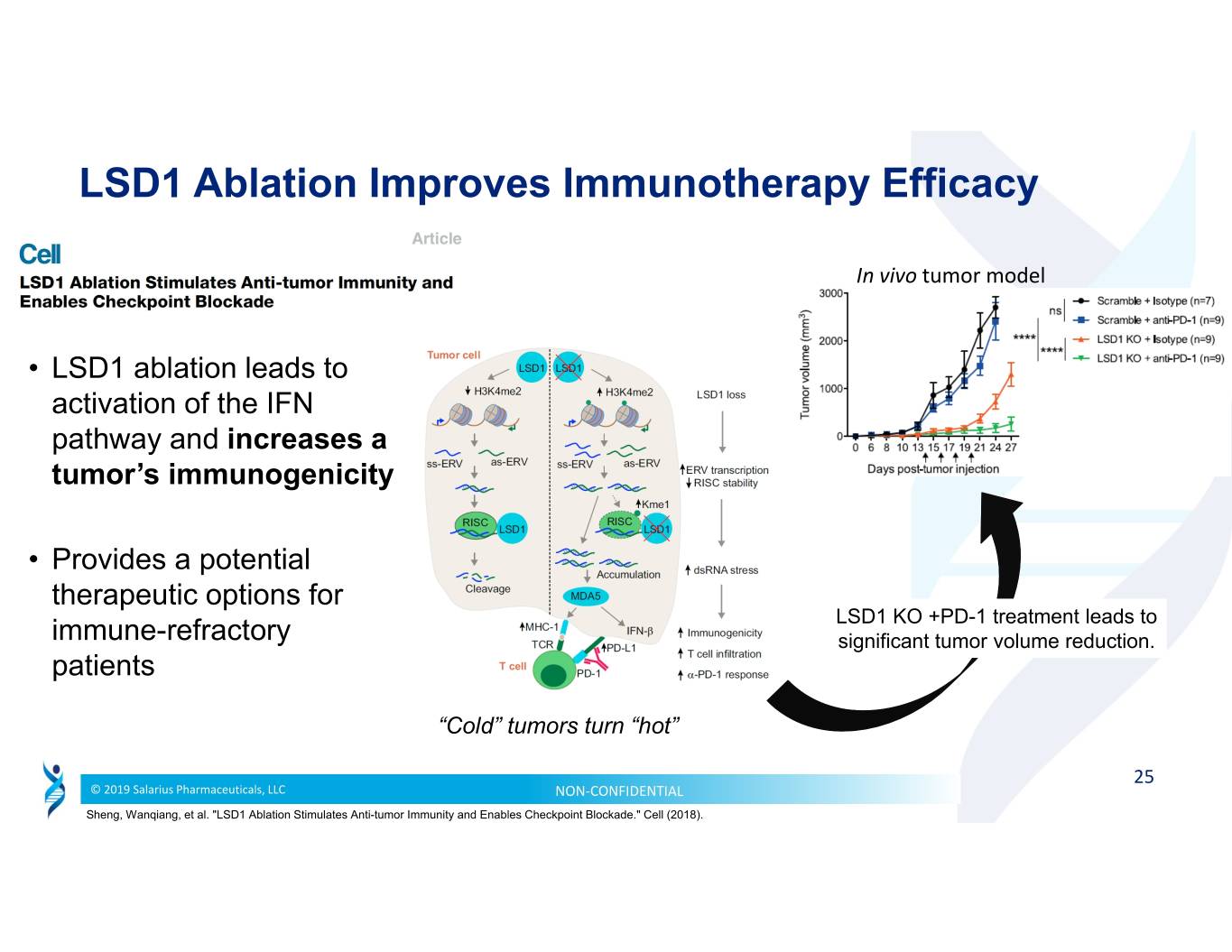
LSD1 Ablation Improves Immunotherapy Efficacy In vivo tumor model • LSD1 ablation leads to activation of the IFN pathway and increases a tumor’s immunogenicity • Provides a potential therapeutic options for LSD1 KO +PD-1 treatment leads to immune-refractory significant tumor volume reduction. patients “Cold” tumors turn “hot” 25 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL Sheng, Wanqiang, et al. "LSD1 Ablation Stimulates Anti-tumor Immunity and Enables Checkpoint Blockade." Cell (2018).
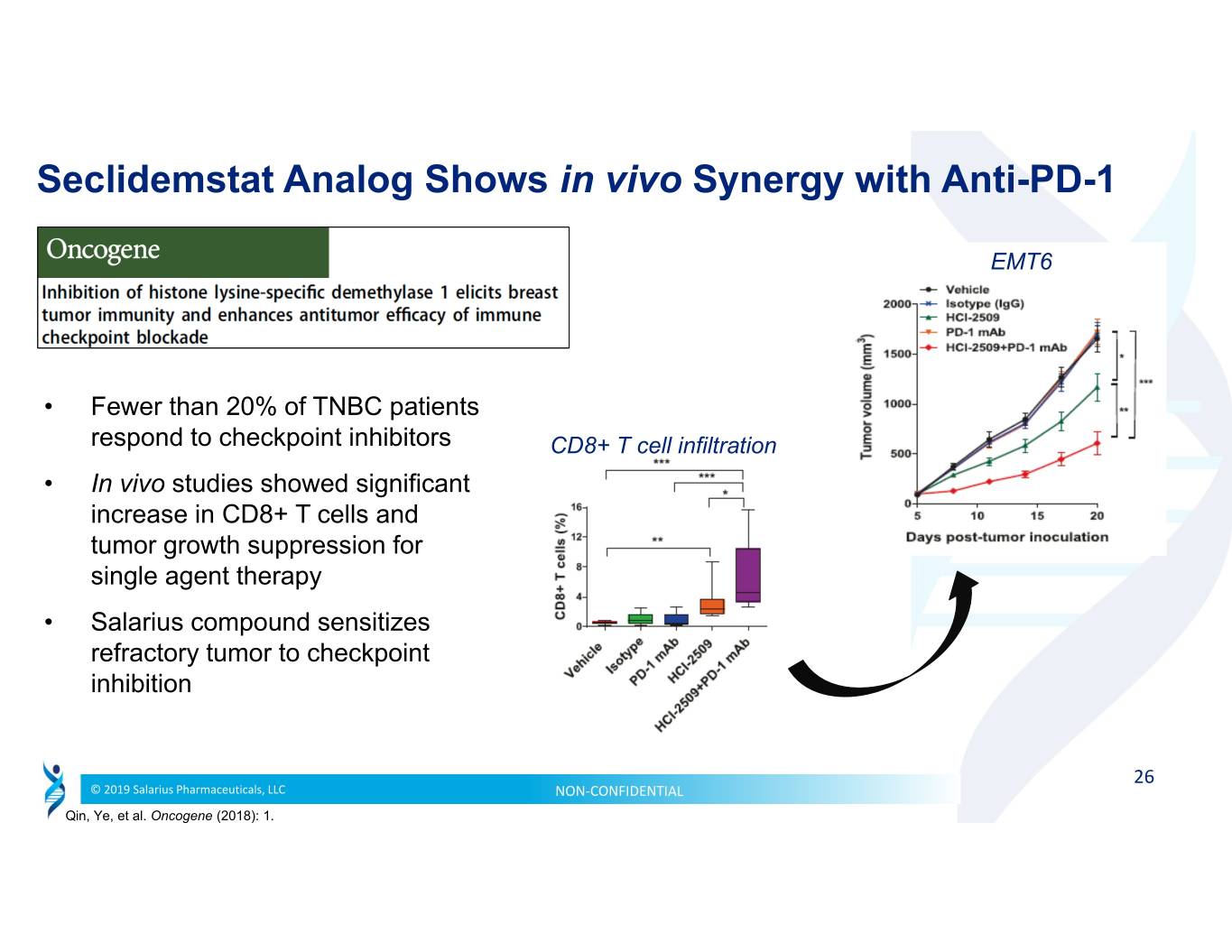
Seclidemstat Analog Shows in vivo Synergy with Anti-PD-1 EMT6 • Fewer than 20% of TNBC patients respond to checkpoint inhibitors CD8+ T cell infiltration • In vivo studies showed significant increase in CD8+ T cells and tumor growth suppression for single agent therapy • Salarius compound sensitizes refractory tumor to checkpoint inhibition 26 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL Qin, Ye, et al. Oncogene (2018): 1.
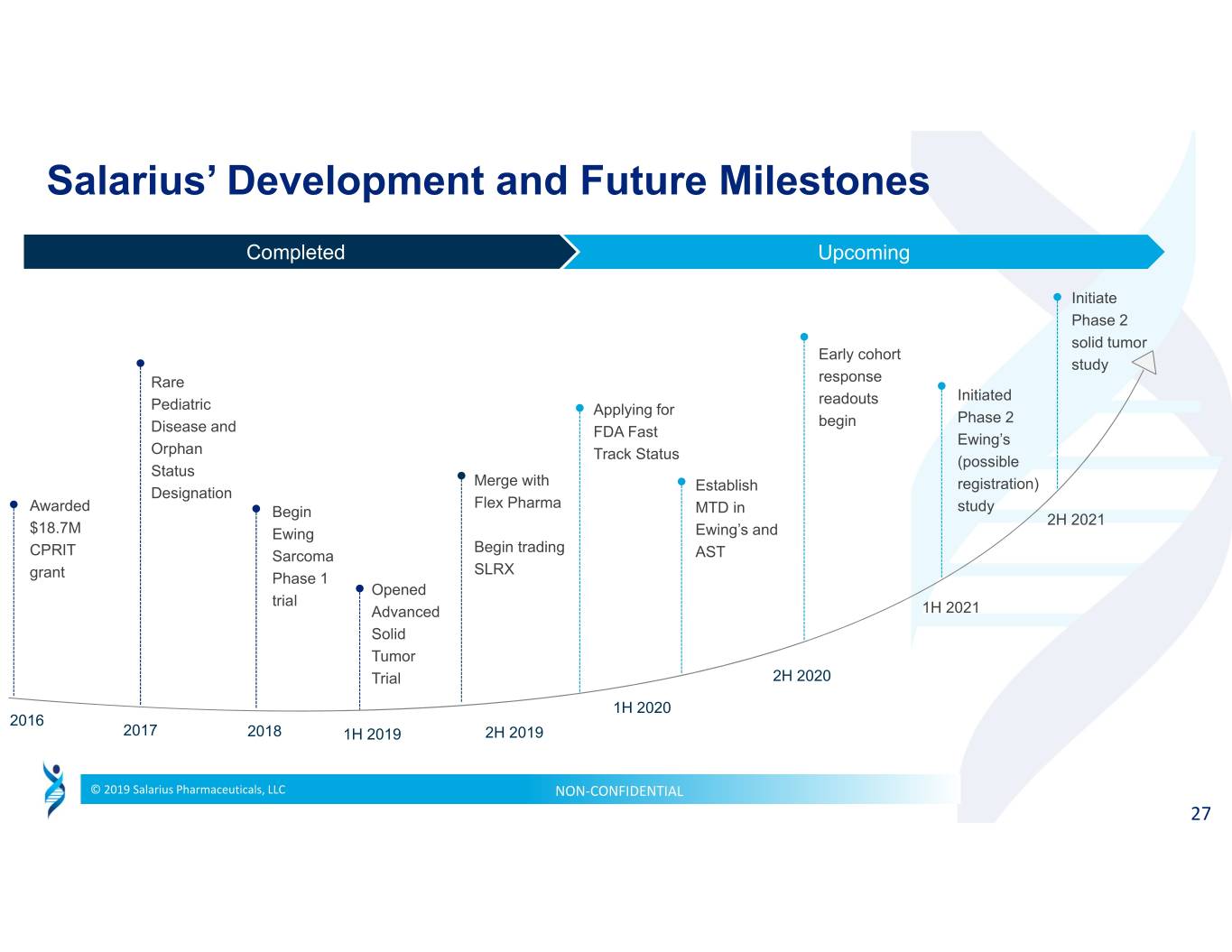
Salarius’ Development and Future Milestones Completed Upcoming Initiate Phase 2 solid tumor Early cohort study Rare response Initiated Pediatric readouts Applying for Phase 2 Disease and begin FDA Fast Ewing’s Orphan Track Status (possible Status Merge with Establish registration) Designation Flex Pharma Awarded Begin MTD in study 2H 2021 $18.7M Ewing Ewing’s and Begin trading CPRIT Sarcoma AST SLRX grant Phase 1 Opened trial Advanced 1H 2021 Solid Tumor Trial 2H 2020 1H 2020 2016 2017 2018 1H 2019 2H 2019 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL 27

Investor Highlights: Salarius Pharmaceuticals is an Epigenetic Focused Clinical-stage Oncology Biotech Company 1 Salarius has a differentiated LSD1 inhibitor with expected human data in 2020 • Multi-company interest and clinical data validates LSD1 as a therapeutic target 2 Development strategy focused on Speed to Market and Market Expansion • Speed to Market: Ewing sarcoma trial Rare Pediatric Disease and Orphan Status Designation • Market Expansion: Advanced Solid Tumor trial Hormonal cancers, sarcomas ($1B+ markets) 3 Seasoned management team leading Salarius • Experienced in product, clinical and early stage development 4 Lead clinical program funded by extensive non-dilutive capital • $18.7M CPRIT award and support from the National Pediatric Cancer Foundation 5 Opportune time to capitalize on growth potential • Potential to expand into other indications of high value (including immunotherapy) • Relatively short timeline to pivotal inflection points 28 © 2019 Salarius Pharmaceuticals, LLC NON‐CONFIDENTIAL

Thank you!




























