
Consolidated Financial Statements Years Ended December 31, 2023 and 2022

DECOY THERAPEUTICS, INC. Contents Page Reports of Independent Registered Public Accounting Firm F-1 Consolidated Financial Statements Consolidated Balance Sheets as of December 31, 2023 and December 31, 2022 F-2 Consolidated Statements of Operations for the years ended December 31, 2023 and 2022 F-3 Consolidated Statements of Shareholders’ Equity for the years ended December 31, 2023 and 2022 F-4 Consolidated Statements of Cash Flows for the years ended December 31, 2023 and 2022 F-5 Notes to consolidated financial statements F-6 – F-25

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM To the Board of Directors and Stockholders of Decoy Therapeutics, Inc. Opinion on the Financial Statements We have audited the accompanying consolidated balance sheets of Decoy Therapeutics, Inc. (“the Company”) as of December 31, 2023 and 2022, and the related consolidated statements of operations, shareholders’ equity, and cash flows for the years then ended, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America. Going Concern The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has generated an accumulated deficit of $15.1 million since its inception. Until the Company is successful in gaining regulatory approvals, it is unable to sell the Company’s product in any market. These factors, among others, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. Basis for Opinion These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB. We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion. Critical Audit Matters The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate. Grant Income

As stated in Note 3 in financial statements, the Company has received grants from two funding sources. The grant amounts are considered significant to the overall financial statements and requires auditor subjectivity and judgment to assess the appropriate guidance to which these transactions apply, as well as significant auditor effort to test the appropriateness of deferred grant income released to income during the periods. How the Critical Audit Matter Was Addressed in the Audit Our principal audit procedures to evaluate management’s analysis of revenue recognition on grants consisted of the following, among others: 1. Obtained and analyzed grant agreements and other supporting documents to identify potential provisions that would significantly impact the manner in which the related income could be recognized. 2. Performed GAAP analysis to determine grant related income was being recognized pursuant to the appropriate guidance. 3. Tested selections of expenses asserted to be related to specific grants to determine that deferred grant funds released to income were appropriately recorded. Fruci & Associates II, PLLC – PCAOB ID #05525 We have served as the Company’s auditor since 2024. Spokane, Washington November 26, 2024

DECOY THERAPEUTICS, INC. F-2 Consolidated Balance Sheets December 31, December 31, 2023 2022 ASSETS Current assets: Cash and cash equivalents 4,156,433$ 1,624,242$ Prepaid expenses and other current assets 194,664 65,864 Total current assets 4,351,097 1,690,106$ Fixed assets, net of depreciation 105,450 140,758 Other assets - long term 41,000 40,280 Total assets 4,497,547$ 1,871,144$ LIABILITIES AND SHAREHOLDERS' EQUITY Current liabilities: Accounts payable 400,495$ 434,462$ Accrued expenses 185,024 59,501 Accrued interest and financing expense 1,541,863 408,257 Deferred income - grants 4,077,453 143,654 Promissory note 1,425,939 - Convertible note - seed tranche A 4,122,000 1,381,000 Convertible note - seed 1,073,000 749,000 Convertible note - senior 6,523,556 4,038,000 Total current liabilities 19,349,330 7,213,874 Warrants 131,000 382,000 Total liabilities 19,480,330$ 7,595,874$ Commitments and contingencies - - Shareholders' equity: - - 1,288 1,288 Additional paid in capital 74,512 4,026 Accumulated deficit (15,058,583) (5,730,044) Total shareholders' equity (deficit) (14,982,783)$ (5,724,730)$ Total liabilities and shareholders' equity 4,497,547$ 1,871,144$ Common stock; par value $.001 per share; 6,000,000 shares authorized (includes 1,000,000 non-voting shares) at December 31, 2023 and 2022; 1,287,930 shares issued and outstanding at December 31, 2023 and 2022. Preferred stock; par value $0.001 par value – 2,000,000 shares authorized -0- shares issued and outstanding at December 31, 2023 and 2022. The accompanying footnotes are an integral part of these consolidated financial statements

DECOY THERAPEUTICS, INC. F-3 Consolidated Statements of Operations 2023 2022 Operating expenses General and administrative 1,065,022$ 1,024,835$ Research and development 2,384,897 2,265,601 Total operating expenses 3,449,919$ 3,290,436$ Other (income) and expenses Grant income (666,201)$ (738,990)$ Fair value adjustment to convertible notes payable 5,643,000 703,000 Warrant liability (income) expense (251,000) 108,000 Financing expense 52,556 22,500 Unrealized loss (gain) - (315) Interest and financing expense 1,100,265 331,011 Total other (income) expense 5,878,620 425,206 Net loss (9,328,539)$ (3,715,643)$ Net loss attributable to shareholders - per share Basic (7.24)$ (2.56)$ Fully-diluted (7.24)$ (2.56)$ Weighted average number of common shares Basic 1,287,930 1,449,292 Fully-diluted 1,287,930 1,449,292 Years Ended December 31, The accompanying footnotes are an integral part of these consolidated financial statements

DECOY THERAPEUTICS, INC. F-4 Consolidated Statements of Shareholders’ Equity Years Ended December 31, 2023 and 2022 Shares Amount Shares Amount Additional Paid in Accumulated Deficit Total Balance at December 31, 2021 - -$ 1,500,000 1,500$ 3,659$ (2,014,402)$ (2,009,243)$ Sale of common stock - - 3,930 4 47 - 51 Purchase of common stock - - (216,000) (216) - - (216) Stock based compensation - - - - 320 - 320 Net loss - - - - - (3,715,643) (3,715,643) Balance at December 31, 2022 - -$ 1,287,930 1,288$ 4,026$ (5,730,045)$ (5,724,731)$ Stock based compensation - - - - 70,486 - 70,486 Net loss - - - - - (9,328,539) (9,328,539) Balance at December 31, 2023 - -$ 1,287,930 1,288$ 74,512$ (15,058,584)$ (14,982,784)$ Common Shares Preferred Shares The accompanying footnotes are an integral part of these consolidated financial statements

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-5 Consolidated Statements of Cash Flows Years Ended December 31, 2023 2022 Cash flow s used in operating activities: Net loss (9,328,539)$ (3,715,643)$ Depreciation and amorization 96,533 39,840 Fair value adjustment to convertible notes payable 5,643,000 703,000 Change in fair value of w arrant liability (251,000) 108,000 Stock based compensation 70,486 320 Non-cash interest expense related to notes 1,133,605 366,011 Changes in assets and liabilities: Increase in prepaid expenses & other assets (129,520) (98,045) Increase in accounts payable and accrued expenses 91,556 203,699 Increase (decrease) in deferred revenue - grants 3,933,799 (314,746) Net cash used in operating activities 1,259,921$ (2,707,565)$ Cash f low s provided by (used in) investing activities: Purchase of property, plant and equiptment (8,669)$ (158,098)$ Net cash provided by (used in) investing activities (8,669)$ (158,098)$ Cash f low s provided by f inancing activities: Proceeds from notes, (net) 1,280,939$ 2,250,000$ Payment of notes - (250,000) Net (purchases) and sales of common stock - (165) Net cash provided by f inancing activities 1,280,939$ 1,999,835$ Net change in cash and cash equivalents: 2,532,191 (865,828) Cash and cash equivalents - beginning of year 1,624,242 2,490,070 Cash and cash equivalents - end of year 4,156,433$ 1,624,242$ Supplemental cash f low disclousres: Income taxes paid 726$ 5,020$ Interest paid -$ 25,000$ The accompanying footnotes are an integral part of these consolidated financial statements

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-6 NOTE 1 – ORGANIZATION, BUSINESS AND BASIS OF PRESENTATION Decoy Therapeutics, Inc. (the “Company”) is a development stage biopharmaceutical company with a mission to revolutionize the design, development, and commercialization of peptide-conjugate therapeutics. The Company believes that its evolving, proprietary Immediate Peptide/PPMO/PNA Alpha-helical Conjugate Technology (IMP3ACT) platform represents a fundamental revolution in peptide-conjugate drug discovery by substantially accelerating the time to design and validate new lead quality drug candidates from years to months or even weeks. The Company’s IMP3ACT platform tames the complexity of the peptide-conjugate modality by using machine learning (ML) and artificial intelligence (AI), coupled with world-leading high-speed synthesis of peptide-conjugates and a strong understanding of target biology, to rapidly interrogate and reengineer naturally existing peptides that bind to disease mediating targets. The Company employs a multi-parameter approach to design and optimization, simultaneously focusing on a broad set of characteristics that will be important through the development and commercialization of the drug, such as chemical affinity, agonist/antagonist activity, enzymatic resistance for enhanced pharmacokinetics, formulation, and manufacturing. The Company believes its approach will significantly decrease timelines, risk, and expense downstream in the therapeutic development process, and can still be executed quickly by the IMP3ACT platform during the design and lead optimization phase. The Company plans to deploy the IMP3ACT platform in two major target areas: (a) antiviral fusion inhibitors and (b) G-Protein Coupled Receptors (GPCRs). In both target areas there is strong evidence that single peptide-conjugates can be designed to affect multiple disease states, creating the potential for multi-indication therapeutics with broad activity from a single drug. The Company believes both target areas also offer substantial commercial opportunities to address significant unmet medical needs. The Company was incorporated in Delaware on April 17, 2020 and has a principal place of business in Cambridge, Massachusetts. The Company has a wholly-owned Canadian subsidiary, Decoy Drug Discovery Canada, which was incorporated on July 8, 2021. The Company’s Canadian subsidiary’s primary activities have been related to sponsored research activities at the University of Toronto and The University of Waterloo. The Company is devoting substantially all of its efforts to product research and development, initial market development, and raising capital. The Company has not generated any product revenue related to its primary business purpose to date and is subject to a number of risks similar to those of other early stage life science companies, including dependence on key individuals, competition from other companies, the need for development of commercially viable products, and the need to obtain adequate additional financing to fund the development of its product candidates. The Company is also subject to a number of risks similar to other companies in the industry, including rapid technological change, regulatory approval of products, uncertainty of market acceptance of products, competition from substitute products and larger companies, the need to obtain additional financing, compliance with government regulations, protection of proprietary technology, dependence on third parties, product liability, and dependence on key individuals. Going Concern Evaluation: As of December 31, 2023, the Company’s primary source of liquidity is its cash and cash equivalent balances. Until the Company is successful in gaining regulatory approvals, it is unable to sell the Company’s product in any market. Without revenues, the Company is reliant on funding obtained from investment in the Company to maintain business operations until the Company can generate positive cash flows from operations. The Company cannot predict the extent of future operating losses and accumulated deficit, and it may never generate sufficient revenues to achieve or sustain profitability.

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-7 The Company has generated an accumulated deficit of $15.1 million since its inception and will require substantial additional capital to fund its research and development and ongoing operating expenses. It is subject to risks common to companies in the biotechnology industry, including, but not limited to, development by the Company or its competitors of technological innovations, risks of failure of clinical studies, dependence on key personnel, protection of proprietary technology and compliance with government regulations. If access to capital is not achieved in the near term, it will materially harm the Company’s business, financial condition and results of operations to the extent that the Company may be required to cease operations altogether, file for bankruptcy, or undertake any combination of the foregoing. These factors raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that these consolidated financial statements are issued. NOTE 2 - LIQUIDITY RISKS AND OTHER UNCERTAINTIES The Company has incurred net losses every year since inception and has an accumulated deficit of approximately $15.1 million at December 31, 2023. The Company has historically funded its operations through debt and equity financings. At December 31, 2023, the Company had cash balances totaling $4.2 million. The Company will need to arrange additional financing in order to continue to pursue its current business objectives as planned and to continue to fund its operations. The Company is looking to raise additional funds through any combination of additional equity and debt financings or from other sources, however, the Company has no guaranteed source of capital that will sustain operations for a period of one year from the date these financial statements are available to be issued. There can be no assurance that any such potential financing opportunities will be available on acceptable terms, if at all. Other risks and uncertainties: The Company is subject to risks common to development stage biopharmaceutical companies including, but not limited to, new technological innovations, dependence on key personnel, protection of proprietary technology, compliance with government regulations, product liability, pre-clinical and clinical trial outcome risks, regulatory approval risks, uncertainty of market acceptance and additional financing requirements. The Company’s products require approval or clearance from the FDA prior to commencing commercial sales in the United States. There can be no assurance that the Company’s products will receive all of the required approvals or clearances. Approvals or clearances are also required in foreign jurisdictions in which the Company may license or sell its products. There can be no assurance that the Company’s products, if approved, will be accepted in the marketplace, nor can there be any assurance that any future products can be developed or manufactured at an acceptable cost and with appropriate performance characteristics, or that such products will be successfully marketed. NOTE 3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES Basis of Presentation: The accompanying consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States (“U.S. GAAP”), which have been consistently applied, reflecting the operations of Decoy Therapeutics Inc. since inception. All intercompany accounts and transactions have been eliminated in consolidation.

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-8 Principles of Consolidation: The accompanying consolidated financial statements include the accounts of Decoy Therapeutics, Inc. and its wholly owned subsidiary. All intercompany transactions and balances are eliminated in consolidation. The functional currency of Decoy Drug Discovery Canada, Inc., a wholly-owned subsidiary of the Company, is the U.S. dollar. Consolidated balance sheet accounts of the Company’s subsidiary are remeasured into U.S. dollars using the exchange rate in effect at the consolidated balance sheet date while expenses are remeasured using the average exchange rate in effect during the period. Gains and losses arising from remeasurement of the wholly owned subsidiary’s financial statements are included in the determination of net loss. Use of estimates: The preparation of financial statements in conformity with U.S. GAAP requires the Company’s management to make estimates and judgments that may affect the reported amounts of assets, liabilities, revenues and expenses, and the related disclosure of contingent assets and liabilities. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. Changes in estimates are reflected in reported results in the period in which they become known. Cash and cash equivalents: The Company considers all highly liquid investments and short-term debt instruments with original maturities of three months or less to be cash equivalents. From time to time during the periods presented, the Company has had bank account balances in excess of federally insured limits where substantially all cash is held in the United States. The Company has not experienced losses in such accounts. The Company believes that it is not subject to unusual credit risk beyond the normal credit risk associated with commercial banking relationships. Fair value of financial instruments: The Company considers its cash and cash equivalents, accounts payable, accrued expenses to meet the definition of financial instruments. The carrying amounts of these financial instruments approximated their fair values due to the short maturities. The Company measures fair value as required by ASC Topic 820, Fair Value Measurements and Disclosures (“ASC Topic 820”). ASC Topic 820 defines fair value, establishes a framework and gives guidance regarding the methods used for measuring fair value, and expands disclosures about fair value measurements. ASC Topic 820 clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants (see Note 5). Property and equipment: Property and equipment are recorded at cost and are depreciated when placed in service using the straight-line method based on their estimated useful lives as follows: Estimated Useful Life Laboratory equipment 5 years Computer equipment and software 3 years Office furniture and equipment 5 years

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-9 For the years ended December 31, 2023 and 2022, the Company's had property and equipment depreciation expense of $43,977 and $17,340, respectfully. Impairment of long-lived assets: The Company reviews long-lived assets when events or changes in circumstances indicate the carrying value of the assets may not be recoverable. Recoverability is measured by comparison of the book values of the assets to future net undiscounted cash flows that the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the book value of the assets exceed their fair value, which is measured based on the projected discounted future net cash flows arising from the assets. No impairment losses have been recorded during the years ended December 31, 2023 and 2022. Warrants: The Company classifies as equity any contracts that (i) require physical settlement or net-share settlement or (ii) provide the Company with a choice of net-cash settlement or settlement in its own shares (physical settlement or net- share settlement), provided that such contracts are indexed to the Company’s own stock. The Company classifies as assets or liabilities any contracts that (a) require net-cash settlement (including a requirement to net cash settle the contract if an event occurs and if that event is outside the Company’s control) or (b) give the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement). The Company assesses classification of its warrants and other free-standing derivatives at each reporting date to determine whether a change in classification between assets, liabilities and equity is required. The Company evaluated its issued and outstanding warrants to assess their proper classification using the applicable criteria enumerated under U.S. GAAP and determined that such warrants meet the criteria for liability classification in the accompanying consolidated balance sheets as of December 31, 2023 and December 31, 2022, respectively. Grant income: The Company has received grants from two funding sources, including a private not-for-profit organization and a federal agency. Grant income consists of income earned from grants to conduct development research. Funds received in advance of services being performed are recorded as deferred income. Income under the not-for-profit and federal agency grants is recognized as labor and material costs are incurred. Labor costs are recognized based on actual salary costs incurred related to the projects, and material costs are recognized based on actual expenditures. As of December 31, 2023 and December 31, 2022, the Company has recognized a total of $0.67 million and $0.74 million of income related to these grants, and has received a total of $1.1 million and $424,000 in cash receipts. Research and development: Research and development costs are expensed as incurred. Research and development expenses include personnel costs associated with research and development activities, including third-party contractors to perform research, conduct clinical trials and manufacture drug supplies and materials. The Company accrues costs incurred by external service providers, based on its estimates of service performed and costs incurred. These estimates include the level of services performed by third parties, administrative costs incurred by third parties, and other indicators of the services completed. Based on the timing of amounts invoiced by service providers, the Company may also record payments made to those providers as prepaid expenses that will be recognized as expenses in future periods as the related services are rendered.

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-10 Key Relationships & Licenses: In June 2020, the Company entered into a one-year, non-exclusive licensing agreement with the Massachusetts Institute of Technology (“MIT”) related to developing potential treatments for Covid-19 using a variety available resources, services and technologies from MIT. Additionally, in July 2020, the Company entered into a Sponsored Research Agreement and option agreement with Columbia University to evaluate a molecule to block the transmission of Covid-19, Neither collaboration remains active. The Company has attracted non-dilutive investments from the European Union’s IMI-CARE Consortium, The Bill & Melinda Gates Foundation (“BMGF”), The U.S. Government’s Biological Research and Development Authority (“BARDA”) and Johnson & Johnson through the U.S. Government’s Blue Knight Blue Knight Program. Income taxes: The Company accounts for its income taxes using the asset and liability method. Accordingly, deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and tax credit carry-forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company maintains a full valuation allowance on its existing deferred tax assets. The Company also accounts for uncertain tax positions using the more-likely-than-not threshold for financial statement recognition and measurement of a tax position taken in the Company’s income tax returns. As of December 31, 2023 and 2022, the Company had no uncertain tax positions which affected its financial position and its results of operations or its cash flows. The Company will continue to evaluate for uncertain tax positions in the future. If at any time the Company should record interest and penalties in connection with income taxes, the interest and the penalties will be expensed within the interest and general and administrative expenses, respectively. Stock based compensation: The Company recognizes compensation costs resulting from the issuance of stock-based awards to employees, non- employees and directors as an expense in the consolidated statements of operations over the requisite service period based on a measurement of fair value for each stock-based award. The fair value of each option grant is estimated as of the date of grant using the Black-Scholes option-pricing model, net of actual forfeitures. The fair value is amortized as compensation cost on a straight-line basis over the requisite service period of the awards, which is generally the vesting period. The Company utilizes the simplified method to estimate the expected term. The risk-free interest rate was determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. The expected dividend yield was assumed to be zero as the Company has not paid and dividends since its inception and does not anticipate paying dividends in the foreseeable future. Earnings (loss) per share: The Company reports loss per share in accordance with ASC 260-10, Earnings Per Share, which provides for calculation of “basic” and “diluted” earnings per share. Basic earnings per share includes no dilution and is computed by dividing net income or loss available to shareholders by the weighted average shares outstanding for the period. Diluted earnings per share reflect the potential dilution of securities that could share in the earnings of an entity. The

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-11 calculation of diluted net earnings (loss) per share gives effect to ordinary shares equivalents; however, potential shares are excluded if their effect is anti-dilutive. For the year ended December 31, 2023, the number of shares excluded from the diluted net earnings (loss) per share included outstanding warrants to purchase 232,092 shares, 886,439 shares from the conversion of outstanding convertible notes and outstanding stock options to purchase 469,350. For the year ended December 31, 2022, the number of shares excluded from the diluted net earnings (loss) per share included outstanding warrants to purchase 232,092 shares, 783,776 in shares from the conversion of outstanding convertible notes and outstanding stock options to purchase 237,850. The inclusion of these warrants, shares from convertible notes and stock options for both 2023 and 2022 in the denominator would be anti-dilutive. NOTE 4 – NOTES Unsecured Promissory Note In October 20, 2021, the Company issued an unsecured promissory note (the “2021 Promissory Note”) in exchange for cash proceeds of $250,000. The term of the 2021 Promissory Note is nine months unless earlier settled upon an automatic payment condition, as further described below. The specific terms and conditions are as follows: 1. Interest: Interest of $25,000 shall accrue on the outstanding principal amount for the first 90 days of the loan and thereafter interest shall accrue on $275,000 at a rate per annum equal to 5% compounded each month thereafter until paid. 2. Automatic Payment: If at any time during the term of the 2021 Promissory Note the Company had issued and sold $650,000 or more in additional capital exclusive of the 2021 Promissory Note and via the sale of the Company’s common stock, $0.001 par value per share (the “Common Stock”) or any securities convertible into Common Stock (such event being an “Automatic Payment Date”), then the Company would be required to pay the holder the entire outstanding principal amount within fifteen days after the closing of the relevant transactions. If the Automatic Payment Date occurred before 90 days from the date of the 2021 Promissory Note, then interest of $25,000 would immediately accrue on the outstanding principal amount on the Automatic Payment Date. No such Automatic Payment Date occurred during the term of the 2021 Promissory Note. 3. Issuance of Company Equity Securities: Within 90 days from the date of the Promissory Note, the Company issued 3,930 shares of Common Stock to the holder at a price of $0.013 (representing current fair market value based on the Company’s most recent 409A valuation at that time) as additional consideration for extending the loan evidenced by the 2021 Promissory Note. On January 5, 2022, the Company repaid the 2021 Promissory Note in full, including the $25,000 interest specified above. The 2021 Promissory Note was considered settled at that time. Accounting Guidance The 2021 Promissory Note is considered an obligation (or liability) of the Company as prescribed by Accounting Standards Codification (“ASC”) 470-10. The Company has elected the fair value option under ASC 825 and ASC 825-10-15-4(a) for the 2021 Promissory Note and will measure the 2021 Promissory Note, as a whole, at fair value, with changes in fair value reported in earnings. As neither ASC 815 nor ASC 825 prescribes the location in which the Company should report fair value changes in the income statement, the Company will elect a policy to present all changes in fair value of the 2021 Promissory Note as a component of interest expense.

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-12 The embedded forward found in the 2021 Promissory Note requiring the Company to issue 3,930 shares of its Common Stock does not represent an embedded derivative. Since the contract itself does not permit/require net settlement, the contract cannot be traded on active markets, and the shares underlying the forward are not readily convertible to cash, a separate instrument with the same terms as the embedded forward would not meet the definition of a derivative. This means that the embedded forward does not meet the condition in ASC 815-15-25-1(c) and does not need to be bifurcated from the 2021 Promissory Note. Upon issuance of the shares underlying the forward, the Company will record the cash proceeds received, Common Stock (at par), and additional paid in capital. SEED Tranche A Convertible Promissory Note On November 4, 2020, the Company entered into a Convertible Promissory Note (“Tranche A Note”) in exchange for $250,000 cash proceeds. The Tranche A Note bears interest at 5% per annum computed on a 356-day year. The stated maturity date of the Tranche A Note is December 31, 2021, though this was subsequently amended on February 28, 2022 to extend the maturity date through April 12, 2023, and again amended on November 13, 2023 to extend the maturity date through June 30, 2024. The Tranche A Note contains a variety of variable share settlement provisions, as indicated below: Elective Conversion. In the event that the Company issues and sells shares of its equity securities to investors (a “Subsequent Financing"), then the holder of the Tranche A Note shall have the rights and option to convert the outstanding principal amount of the Tranche A Note and any unpaid accrued interest in whole into the equity securities sold in the Subsequent Financing at a conversion price equal to the lowest of the following: (i) the cash price paid per share for equity securities by the investors in the Subsequent Financing; (ii) $0.8333334 per share (equitably adjusted to account for stock splits, stock dividends and similar events with respect to the Common Stock between the date of the Tranche A Note and the date of such conversion); and (iii) the lowest exercise or conversion price per share of Common Stock underlying any stock option, stock appreciation right, or other stock-based equity award under the Company's stock-based awards (the "Stock Plan"). The issuance of equity securities pursuant to the conversion of this Tranche A Note shall otherwise be upon and subject to the same terms and conditions applicable to equity securities sold in the Subsequent Financing. Automatic Conversion upon a Qualified Financing. In the event that the Company issues and sells shares of its equity securities to investors while the Tranche A Note remains outstanding in an equity financing with total proceeds to the Company of not less than $5,000,000, excluding the conversion of the Tranche A Note or other convertible securities issued for capital raising purposes (a “Qualified Financing”), then the outstanding principal amount of the Tranche A Note and any unpaid accrued interest shall automatically convert in whole without any further action by the holder into equity securities sold in the Qualified Financing at a conversion price equal to the lowest of the following: (i) the cash price paid per share for equity securities by the investors in the Qualified Financing; (ii) $0.8333334 per share (equitably adjusted to account for stock splits, stock dividends and similar events with respect to the Company’s Common Stock between the date of the Tranche A Note and the date of such conversion); and (iii) the lowest exercise or conversion price per share of Common Stock underlying any stock option, stock appreciation right, or other stock- based equity award under the Company's Stock Plan, in each case granted to any of the current four stockholders of the Company between the date of the Tranche A Note and the date of such conversion (as equitably adjusted as provided in clause (ii) above). The issuance of equity securities pursuant to the conversion of this Tranche A Note shall otherwise be upon and subject to the same terms and conditions applicable to equity securities sold in the Qualified Financing. Change of Control. If the Company consummates a change of control (as further defined below) while the Tranche A Note remains outstanding, the Company shall repay the holder in cash in an amount equal to the outstanding principal amount of this Tranche A Note plus any unpaid accrued interest on the original principal; provided, however, that upon the written election of the holder made not less than five days prior to such change of control, the Company shall

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-13 convert the outstanding principal balance of this Tranche A Note and any unpaid accrued interest into shares of Common Stock at a conversion price equal to the lower of the following: (i) $0.8333334 per share (equitably adjusted to account for stock splits, stock dividends and similar events with respect to the Common Stock between the date hereof and the date of such conversion); and (ii) the lowest exercise or conversion price per share of Common Stock underlying any stock option, stock appreciation right, or other stock-based equity award under the Company's Stock Plan, in each case granted to any of the current four stockholders of the Company between the date hereof and the date of such conversion (as equitably adjusted as provided in clause (i) above). For purposes of the Tranche A Note, a change of control means (i) a consolidation or merger of the Company with or into any other corporation or other entity or person, or any other corporate reorganization, other than any such consolidation, merger or reorganization in which the shares of capital stock of the Company immediately prior to such consolidation, merger or reorganization continue to represent a majority of the voting power of the surviving entity immediately after such consolidation, merger or reorganization; (ii) any transaction or series of related transactions to which the Company is a party in which in excess of 50% of the Company's voting power is transferred; or (iii) the sale or transfer of all or substantially all of the Company's assets, or the exclusive license of all or substantially all of the Company's material intellectual property; provided that a change of control shall not include any transaction or series of transactions principally for bona fide equity financing purposes in which cash is received by the Company or any successor, indebtedness of the Company is cancelled or converted or a combination thereof. Neither party has the ability to redeem the Tranche A Note prior to the stated maturity date and there are no other provisions requiring accounting analysis. The Tranche A Note is considered an obligation (or liability) of the Company as prescribed by ASC 470-10 and/or 480-10-25-14(a). ). The Company has elected the fair value option under ASC 825-10-15-4(a) and paragraphs 4-5 of ASC 815-15-25 for each Tranche A Note and will instead measure each Tranche A Note, as a whole, at fair value, with changes in fair value reported in earnings. The Company will present all changes in fair value of the Tranche A Note as a component of interest expense. For the years ended December 31, 2023 and 2022, the Company recorded approximately $13,000 and $13,000 respectively, of accrued interest related to the Tranche A Note. For the years ended December 31, 2023 and 2022, the Company recorded income (expense) approximately of ($2,741,000) and ($278,000), respectively, as a change in the fair value of debt in the statement of operations. SEED Convertible Promissory Notes On March 25, 2021, April 12, 2021, and April 5, 2022, the Company entered into three separate Convertible Promissory Notes (the “Seed Notes”) in exchange for $650,000 total cash proceeds. The Seed Notes bear interest at 8% per annum computed on a 365-day year. The stated maturity date of each Seed Note is two years (24 months) after the Issuance Date. The maturity date of the April 5, 2022 Seed Note has been extended to December 31, 2024. The maturity dates of the March 25, 2021 and April 12, 2021 Seed Notes have been extended to June 30, 2024. At the time of issuance of these financials, the Company is in process of further extending the maturity dates for the March 25, 2021 and April 12, 2021 Seed Notes. The Seed Notes are considered an obligation (or liability) of the Company as prescribed by ASC 470-10 and/or 480-10-25-14(a). The Company has elected the fair value option under ASC 825-10-15-4(a) and paragraphs 4-5 of ASC 815-15-25 for each Seed Note and will instead measure each Seed Note, as a whole, at fair value, with changes in fair value reported in earnings. The Seed Notes are convertible at the option of the Holder subject to the following conditions which are identical across the three Seed Notes: Conversion upon a Qualified Financing. In the event that the Company issues and sells shares of its equity securities to investors while the Seed Notes remain outstanding in an equity financing with total proceeds to the Company of not less than $5,000,000 (excluding the conversion of the Seed Notes or other convertible securities issued for capital raising purposes (a “Seed Note Qualified Financing”), then the outstanding principal amount of the Seed Notes and any unpaid accrued interest shall automatically convert in whole without any further action by the holders into equity securities sold in the Seed Note Qualified Financing at a conversion price equal to the lesser of (i) the cash price paid

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-14 per share for equity securities by the investors in the Seed Notes Qualified Financing multiplied by 0.80, and (ii) the quotient resulting from dividing $20,000,000 by the number of outstanding shares of Common Stock of the Company immediately prior to the Seed Note Qualified Financing (assuming conversion of all securities convertible into Common Stock and exercise of all outstanding options and warrants, but excluding the shares of equity securities of the Company issuable upon the conversion of Seed Notes or other convertible securities issued for capital raising purposes. The issuance of equity securities pursuant to the conversion of the Seed Notes shall be upon and subject to the same terms and conditions applicable to equity securities sold in the Seed Note Qualified Financing. If the conversion price of the Seed Notes is less than the price per share at which equity securities are issued in the Seed Note Qualified Financing, the Company may, solely at its option, elect to convert the Seed Notes into shares of a newly created series of preferred stock having the identical rights, privileges, preferences and restrictions as the equity securities issued in the Seed Note Qualified Financing, and otherwise on the same terms and conditions, other than with respect to: (i) the per share liquidation preference and the conversion price for purposes of price-based anti- dilution protection, which will equal the conversion price; and (ii) the per share dividend, which will be the same percentage of the conversion price as applied to determine the per share dividends of the investors in the Seed Note Qualified Financing relative to the purchase price paid by the investors. Optional Conversion at non-Qualified Financing. In the event the Company consummates, while this Seed Notes remain outstanding, an equity financing pursuant to which the Company sells shares of preferred stock in a transaction that does not constitute a Seed Note Qualified Financing, then the Seed Note holders shall have the option to treat such equity financing as a Seed Note Qualified Financing on the same terms set forth herein. Maturity Date Conversion. In the event that the Seed Notes remain outstanding on the maturity sate, then the outstanding principal balance of the Seed Notes and any unpaid accrued interest shall automatically without any further action by the holders convert as of the maturity date into shares of Common Stock at a conversion price equal to the quotient resulting from dividing $20,000,000 by the number of outstanding shares of Common Stock as of the maturity date assuming conversion of all securities convertible into Common Stock and exercise of all outstanding options and warrants, but excluding the shares of equity securities of the Company issuable upon the conversion of the Seed Notes or other convertible securities issued for capital raising purposes. Change of Control. If the Company consummates a change of control (as further defined below) while the Seed Notes remain outstanding, the Company shall repay the holders in cash in an amount equal to the outstanding principal amount of the Seed Notes plus any unpaid accrued interest on the original principal; provided, however, that upon the written election of the holders made not less than five days prior to the change of control, the Company shall convert the outstanding principal balance of the Seed Notes and any unpaid accrued interest into shares of Common Stock at a conversion price equal to the quotient resulting from dividing $20,000,000 by the number of outstanding shares of Common Stock of the Company immediately prior to the change of control. For purposes of the Seed Notes, a change of control means (i) a consolidation or merger of the Company with or into any other corporation or other entity or person, or any other corporate reorganization, other than any such consolidation, merger or reorganization in which the shares of capital stock of the Company immediately prior to such consolidation, merger or reorganization continue to represent a majority of the voting power of the surviving entity immediately after such consolidation, merger or reorganization; (ii) any transaction or series of related transactions to which the Company is a party in which in excess of 50% of the Company’s voting power is transferred; or (iii) the sale or transfer of all or substantially all of the Company’s assets, or the exclusive license of all or substantially all of the Company’s material intellectual property. The Seed Notes contain as side letter that contains participation and put rights. Aside from the conditions noted in the side letter, neither party has the ability to redeem the loan prior to the stated maturity date and there are no other provisions requiring accounting analysis. For the year ended December 31, 2023 and 2022 the Company recorded approximately $52,000 and $46,000 of accrued interest. For the year ended December 31, 2023 and 2022 the Company recorded income (expense) related

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-15 to the Seed Notes of approximately of ($324,000) and ($98,000), respectively as a change in the fair value of debt in the Statement of Operations. Senior Secured Convertible Promissory Notes On December 22, 2021, and December 23, 2021, the Company entered into two separate Senior Secured Convertible Promissory Notes (the “Senior Notes”) in exchange for up to a combined $4M total cash proceeds. The stated maturity date for each Senior Note is March 22, 2023, and March 23, 2023, respectively. Per an amendment dated March 22, 2023, the Company elected to extend the maturity date for an additional six months for both Senior Notes. The Company subsequently extended the maturity date for both Senior Notes to June 30, 2024. At the time of issuance of these financials, the Company is working on an updated extension to these Senior Notes. The Senior Notes are considered an obligation (or liability) of the Company as prescribed by ASC 470-10 and/or 480-10-25-14(a). The Company has elected the fair value option under ASC 825-10-15-4(a) and paragraphs 4-5 of ASC 815-15-25 for each Senior Note and will instead measure each Senior Note, as a whole, at fair value, with changes in fair value reported in earnings. The Senior Notes contain an option to extend the maturity date by an additional six months for an extension premium of 110% which is exercisable by the issuer. The Senior Notes bear interest at 12% per annum computed on a 360-day year and contain the following conversion and redemption features: At any time after the issuance sate of the Senior Notes, the Senior Notes shall be convertible into validly issued, fully paid and non-assessable shares of Common Stock. The number of shares of Common Stock issuable upon conversion of any conversion amount, including all accrued and unpaid interest with respect to such portion of the principal amount, divided by the conversion amount. The conversion amount attributable to the first disbursement conversion price (initially $10.47). If a subsequent disbursement is made in a six month period following the issuance date, initially 110% of the first disbursement conversion price, or if a subsequent disbursement is made after the six month period following the issuance date but prior to the date that is one year from the issuance date, initially 125% of the first disbursement conversion price. From and after a date upon which the Company becomes a publicly traded entity (as defined in the Senior Note), the Company shall not implement the conversion of any portion of the Senior Notes, and the holders shall not have the right to convert any portion of the Senior Notes. The Company issued the Senior Notes together with detachable warrants (the “Warrants”) to purchase shares of the Company’s Common Stock pursuant to a warrant purchase agreement. The Warrants were issued after each scheduled disbursement. The Company believes that the Warrants issued in connection with the Senior Notes are liability- classified under ASC 480-10-25-8, because the Company could be required to repurchase the Warrants under the terms thereof for reasons outside the control of the Company, including in the event of default (as defined in the Warrants). Even if the Warrants were not liability-classified under ASC 480, they would be classified as liabilities under ASC 815 because the Warrants meet the definition of a derivative under ASC 815-10-15-8. Because the Warrants are liability-classified, they will be initially and subsequently measured at fair value until settlement or expiry, with changes in fair value reported in the Statement of Income. The Company will also be measuring the related Senior Notes issued in conjunction with the Warrants at fair value. To the extent that the proceeds received from investors are less than the combined fair values of the Senior Notes and Warrants, the difference will be reported as an immediately loss in the statement of operations. For the years ended December 31, 2023 and 2022 the Company recorded income (expense) approximately of $251,000 and ($108,000) as a change in the fair value of warrant in the Statement of Operations. Finally, in connection with the issuance of the Senior Notes, the Company also entered into a Registration Rights Agreement (the “RRA”) that outlines the actions the Company will take to register the securities underlying the Senior Notes and Warrants with the U.S, Securities and Exchange Commission. If the Company does not comply with the registration requirements under the RRA, the holders are entitled to receive payments if the Company is unable to

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-16 comply with the promises in the RRA. The Company shall pay to each holder an amount in cash, as partial liquidated damages and not as a penalty, equal to 1% of the purchase price paid by such holder pursuant to the purchase agreement for the Senior Notes. The Company analyzed ASC 825-20-25-1 for the accounting treatment for registration agreements related to financing arrangements. The Company determined the existence of the registration payment arrangement does not affect the accounting for the Senior Notes and the registration payment arrangement should not be recognized at this time under ASC Subtopic 450-20. ASC 450-20-25-1, requires contingent obligations to be recorded when a loss is probable of occurrence and reasonably estimable. As of December 31, 2023, it is not probable that the Company will be subject to penalties related to the RRA. The Company will reassess this conclusion each reporting period. For the year ended December 31, 2023 and 2022 the Company recorded approximately $480,000 and $307,000 of accrued interest related to the SSCPN. For the year ended December 31, 2023 and 2022 the Company recorded income (expense) approximately of $2,578,000 and $327,000 as a change in the fair value of debt in the Statement of Operations. At origination the Company incurred $45,000 of dept issuance cost related to these SSCPN, during the years ended December 31, 2023 and 2022 the company amortized $22,000 and $22,500 of these costs. Bridge Notes During the year ended December 31, 2023, the Company entered into a series of Promissory Notes (“Bridge Notes”) in exchange for notional proceeds totaling $1,448,899. The terms and conditions of each Bridge Note are identical except for the proceeds invested by each investor and the maturity date of each Bridge Note. The stated maturity date for these Bridge Notes is twelve months from the date of issuance. At the time of issuance of these financials, the Company is working on an updated extensions to these Bridge Notes. Each of the Bridge Notes was issued at an original issue discount with principal and accrued interest due and payable on the earlier of one year from issuance date, or the date of the closing of an initial public offering of the Company (“IPO”). The Company also has the option to repay the loan before the stated maturity date without penalty. The securities purchase agreement pursuant to which the Bridge Notes were sold requires that, in addition to repayment of principal and interest, shares of the Company’s Common Stock be issued to the holder according to the following conditions: 100% of the principal value of the note divided by (A) the Company’s IPO price or (B) if the Company fails to complete an IPO before maturity, the number of shares calculated using a $40 million pre-money valuation for the Company and the number of the Company’s shares outstanding at maturity. Interest shall accrue to the holders on the aggregate then outstanding principal amount of the Bridge Notes at the rate of 10% per annum, calculated on the basis of a 360-day year and shall accrue daily commencing on the original issue date of the Bridge Notes until payment in full of the outstanding principal, together with all accrued and unpaid interest, liquidated damages and other amounts which may become due hereunder, has been made. The Bridge Notes are obligations of the Company that could be settled in cash (traditional debt under ASC 470) and a variable number of shares as per ASC 480-10-25-14(a). Under either ASC Topic, pursuant to U.S. GAAP the Bridge Notes would be presented the balance sheet as a liability at amortized cost. The Bridge Notes contain a number of embedded features that should be evaluated for bifurcation. The Company will elect the fair value option to account for each of the Bridge Notes, as permitted by ASC 825-10-15 and ASC 815-15- 25. Based on the Company’s analysis, all three conditions under ASC 815-15-25-1 are met and the embedded share settlement feature would ordinarily require bifurcation as an embedded derivative. Based on the conditions in ASC 825-10-15 and ASC 815-15-25, the Company elected to apply the fair value option for each of the Bridge Notes and will not be required to bifurcate any embedded features. Neither ASC 825 nor ASC 815 prescribes the location in

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-17 which the Company should report fair value changes in the income statement, the Company will elect a policy to present all changes in fair value of the Bridge Notes as a component of interest expense in the Statement of Income. For the year ended December 31, 2023 the Company recorded approximately $21,000 of accrued interest related to these Bridge Notes. At origination during the year ended December 31, 2023 the Company incurred $145,000 of dept issuance cost related to these Bridge Notes, during the year ended December 31, 2023 the company amortized $30,556 of these costs. Demand Notes On June 13, 2023, the Company issued three separate notes (“Demand Notes”) in exchange for gross cash proceeds totaling $150,000, prior to the payment of offering costs. The terms and conditions of each Demand Note are identical. Each Demand Note was issued at a discount and must be repaid upon the earlier of the maturity date or within 5 days of the demand by the holder. The specific terms of the Demand Notes are as follows: In exchange for receipt of Demand Notes, the Company promises to pay the holders, the principal sum of $166,665 together with interest thereon from the date hereof, at 10% per annum, with interest and principal being immediately payable on the earlier of (i) the maturity date and (ii) five days from the date that the Holder demands repayment. The maturity date shall be 90 days from the issuance date of each Demand Note. At maturity on August 12, 2023 two of these three Demand Notes were repaid in full, the one remaining note is outstanding to an employee/founder of the Company – See related party (Note 9) The Demand Notes are obligations of the Company that will be settled in cash and therefore represent traditional debt. Traditional debt is accounted for under ASC 470 and requires presentation on the balance sheet as a liability at amortized cost. The Demand Notes contain an embedded written put right. Specifically, the investors of each Demand Note have the right to demand repayment with five days’ notice. The Demand Notes were evaluated to determine whether the embedded features should be bifurcated, or detached from the note and accounted for separately if it meets the criteria in ASC 815-15-25-1. As neither ASC 825 nor ASC 815 prescribes the location in which the Company should report fair value changes in the income statement, the Company elected a policy to present all changes in fair value of the Demand Notes as a component of interest expense in the Statement of Operations. For the years ended December 31, 2023 the Company recorded approximately $3,000 of accrued interest related to these Demand Notes. Below is a schedule of note balances as of the years ended December 31, 2023 and 2022: Senior Secured Promissory Seed Convertible Notes Tranche A Seed Note Beginning Balance December 31, 2021 -$ 400,000$ 250,000$ 2,000,000$ Change in principal balance - 250,000 - 2,000,000 Beginning Balance December 31, 2022 -$ 650,000$ 250,000$ 4,000,000$ Change in principal balance 1,544,444 - - - Ending Balance December 31, 2023 1,544,444$ 650,000$ 250,000$ 4,000,000$

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-18 NOTE 5 - FAIR VALUE OF FINANCIAL INSTRUMENTS The Company applies fair value accounting for all assets and liabilities that are recognized or disclosed at fair value in the financial statements on a recurring basis. Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities the Company considers the principal or most advantageous market in which it would transact and the market-based risk measurements or assumptions that market participants would use in pricing the asset or liability, such as risks inherent in valuation techniques, transfer restrictions and credit risk. For certain instruments, including cash and cash equivalents, accounts payable, and accrued expenses, it was estimated that the carrying amount approximated fair value because of the short maturities of these instruments. Fair value is estimated using various valuation models, which utilize certain inputs and assumptions that market participants would use in pricing the asset or liability. The inputs and assumptions used in valuation models are classified in the fair value hierarchy as follows: Level 1: Quoted prices (unadjusted) in active markets that are accessible at the measurement date for assets or liabilities. The fair value hierarchy gives the highest priority to Level 1 inputs. Level 2: Quoted market prices for similar instruments in an active market; quoted prices for identical or similar assets and liabilities in markets that are not active; and model-derived valuations inputs of which are observable and can be corroborated by market data. Level 3: Unobservable inputs and assumptions that are supported by little or no market activity and that are significant to the fair value of the asset and liability. The fair value hierarchy gives the lowest priority to Level 3 inputs. In determining the appropriate hierarchy levels, the Company analyzes the assets and liabilities that are subject to fair value disclosure. Financial assets and liabilities are classified in their entirety based on the lowest level of input that is significant to their fair value measurement.
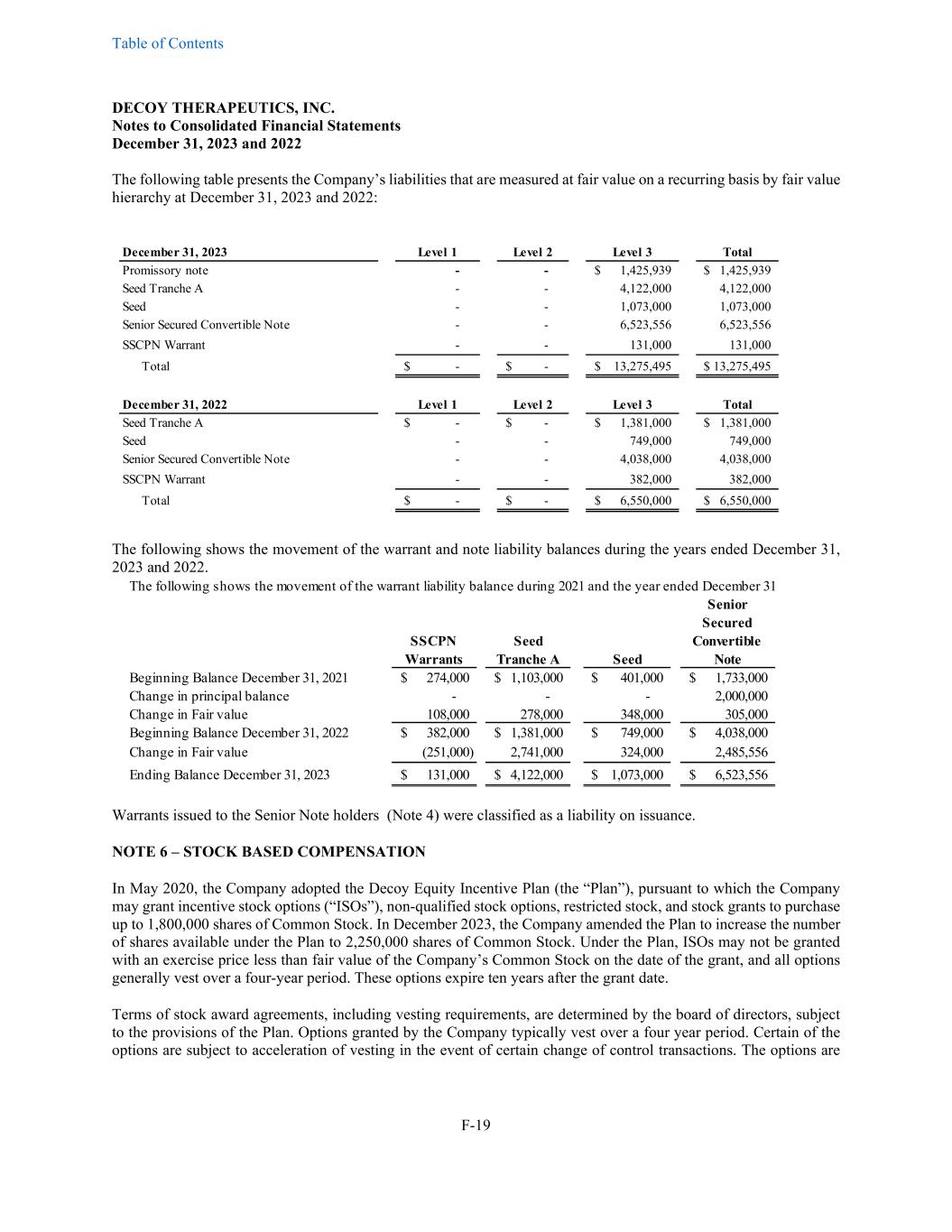
Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-19 The following table presents the Company’s liabilities that are measured at fair value on a recurring basis by fair value hierarchy at December 31, 2023 and 2022: December 31, 2023 Level 1 Level 2 Level 3 Total Promissory note - - 1,425,939$ 1,425,939$ Seed Tranche A - - 4,122,000 4,122,000 Seed - - 1,073,000 1,073,000 Senior Secured Convertible Note - - 6,523,556 6,523,556 SSCPN Warrant - - 131,000 131,000 Total -$ -$ 13,275,495$ 13,275,495$ December 31, 2022 Level 1 Level 2 Level 3 Total Seed Tranche A -$ -$ 1,381,000$ 1,381,000$ Seed - - 749,000 749,000 Senior Secured Convertible Note - - 4,038,000 4,038,000 SSCPN Warrant - - 382,000 382,000 Total -$ -$ 6,550,000$ 6,550,000$ The following shows the movement of the warrant and note liability balances during the years ended December 31, 2023 and 2022. The following shows the movement of the warrant liability balance during 2021 and the year ended December 31 Senior Secured SSCPN Seed Convertible Warrants Tranche A Seed Note Beginning Balance December 31, 2021 274,000$ 1,103,000$ 401,000$ 1,733,000$ Change in principal balance - - - 2,000,000 Change in Fair value 108,000 278,000 348,000 305,000 Beginning Balance December 31, 2022 382,000$ 1,381,000$ 749,000$ 4,038,000$ Change in Fair value (251,000) 2,741,000 324,000 2,485,556 Ending Balance December 31, 2023 131,000$ 4,122,000$ 1,073,000$ 6,523,556$ Warrants issued to the Senior Note holders (Note 4) were classified as a liability on issuance. NOTE 6 – STOCK BASED COMPENSATION In May 2020, the Company adopted the Decoy Equity Incentive Plan (the “Plan”), pursuant to which the Company may grant incentive stock options (“ISOs”), non-qualified stock options, restricted stock, and stock grants to purchase up to 1,800,000 shares of Common Stock. In December 2023, the Company amended the Plan to increase the number of shares available under the Plan to 2,250,000 shares of Common Stock. Under the Plan, ISOs may not be granted with an exercise price less than fair value of the Company’s Common Stock on the date of the grant, and all options generally vest over a four-year period. These options expire ten years after the grant date. Terms of stock award agreements, including vesting requirements, are determined by the board of directors, subject to the provisions of the Plan. Options granted by the Company typically vest over a four year period. Certain of the options are subject to acceleration of vesting in the event of certain change of control transactions. The options are

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-20 exercisable from the date of grant for a period of ten years. For options granted to date, the exercise price equaled the estimated fair value of the Common Stock as determined by the board of directors on the date of grant. The following table summarizes stock-based activities under the Amended Plan: Options Weighted Average Exercise Price Weighted Average Contractual Terms Outstanding at December 31, 2021 64,500 0.01$ 9.16 Granted 174,600 0.72 Forfeited/Cancelled (1,250) 0.01 Outstanding at December 31, 2022 237,850 0.53$ 9.08 Granted 231,500 3.56 Forfeited/Cancelled - - Outstanding at December 31, 2023 469,350 2.03$ 8.96 Exercisable options at December 31, 2023 209,094 0.50$ 8.05 The intrinsic value of outstanding options at December 31, 2023 was approximately $704,000. Stock options granted during the year ended December 31, 2023, were valued using the Black-Scholes option-pricing model with the following weighted average assumptions: December 31, 2023 December 31, 2022 Expected volatility 97.9% 103.1% Risk-free interest rate 3.9% 3.0% Expected dividend yield 0.0% 0.0% Expected life of options in years 5.5 5.5 Exercise Price 3.56$ 0.72$ Fair value of common stock 2.61$ 0.01$ Estimate fair value of option -$ -$ Stock based compensation expense was $70,486 ($45,767 included in research and development expense and $24,719 included in general and administrative expenses) in the year ended December 31, 2023. Stock based compensation expense was $320 ($203 included in research and development expense and $117 included in general and administrative expenses) in the year ended December 31, 2022. At December 31, 2023, the total unrecognized compensation expense related to non-vested options was approximately $534,000 and is expected to be recognized over the remaining weighted average service period of approximately 1.9 years.

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-21 NOTE 7 – PREPAID EXPENSES AND OTHER CURRENT ASSETS Prepaid expenses and other current assets are as follows: December 31, December 31, 2023 2022 Prepaid R&D costs $ 95,360 $ 17,001 Prepaid rent 16,023 15,436 Prepaid software subscriptions 49,828 22,267 Prepaid professional fees 20,000 - Prepaid other 13,453 11,160 Total $ 194,664 $ 65,864 NOTE 8 – ACCRUED EXPENSES Accrued expenses are as follows: December 31, December 31, 2023 2022 Payroll $ 72,039 $ 50,143 Professional fees 103,071 - Other expenses 9,914 9,358 Total $ 185,024 $ 59,501 NOTE 9 – RELATED PARTY TRANSACTIONS Due to Officers/Founders: As of December 31, 2023, one officer/founder of the Company had an outstanding Demand Note (See Note 4) in the principal amount of $55,555, plus accrued interest of $2,968. This note accrues interest at 10% and has a maturity date of December 28, 2024. NOTE 10 – LICENSE AGREEMENTS AND GRANTS The Company has received significant grants are from The Bill and Melinda Gates Foundation, Johnson & Johnson through the U.S. government’s Blue Knight Program, the European Union’s IMI.CARE.EU Consortium, the Canadian government’s National Research Council, and GOOGLE’s AI Startup Program. Key Relationships, Licenses and Grants The Company received a foundation grant from the Bill and Melinda Foundation for the development of a nasally inhaled, low cost, peptide conjugate pan-Coronavirus antiviral inhibitor. The initial award in September 6, 2021 provided up to a total of approximately $904,000 and expired on February 28, 2023. The Company initially recorded the proceeds in Deferred income. As work was commenced under the grant the company recognizes income from deferred income.

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-22 In 2023 the Company entered into a supplemental grant with the Bill and Melinda Foundation for an additional $4,084,500 for continued work on the nasally inhaled, low cost, peptide conjugate pan-Coronavirus antiviral inhibitor reference above. The Company received payment of $3,500,000 in September 28, 2023, the remaining $584,500 will be received after the completion of certain milestones . The Company recognized income of approximately $235,000 and $739,000 in the years ended December 31, 2023 and 2022, respectively. The Company had approximately $3,408,000 and $144,000 in deferred income balances related to this grant for the years ended December 31, 2023 and 2022, respectably. Johnson and Johnson Quickfire Grants The Company received a grant from the Johnson and Johnson through the U.S. government’s Blue Knight Program (Quickfire Grant) for experiments relating to the pharmacokinetics and tolerability of the aforementioned pan- Coronavirus inhibitor in the Human Airway Epithelium (HAE) model. The initial award to the Company in January 31, 2023 provided for $100,000. The Company initially recorded the proceeds in deferred income. As work was commenced under the grant the company recognizes income from deferred income. In September 22, 2023 the Company received the first $500,000 of an additional Quickfire grant for $1,000,000 for work to investigate the potential for broader therapeutic use of the aforementioned pan-Coronavirus inhibitor. The Company initially recorded the proceeds in deferred revenue. As work was commenced under the grant the company recognizes income from deferred income. In December 1, 2023 the Company received an the second $500,000 of the Quickfire grant mentioned above. The Company initially recorded the proceeds in deferred income. As work was commenced under the grant the company recognizes income from deferred income. The Company recognized income of approximately $431,000 and $0 in the years ended December 31, 2023 and 2022, respectively. The Company had approximately $669,000 and $0 in deferred income balances related to these grants for the years ended December 31, 2023 and 2022, respectably. NOTE 11 – SHAREHOLDERS’ EQUITY (DEFICIT) As of December 31, 2023, the total authorized capital stock of the Company was 8,000,000 shares, which consisted of 5,000,000 shares of Common Stock, $0.001 par value per share; 1,000,000 shares of Nonvoting Common Stock $0.001 par value per share; 2,000,000 shares of Preferred Stock, $0.001 par value per share. Common stock At December 31, 2023 and 2022, the Company has authorized 5,000,000 shares of Common Stock, par value $0.001 per share, of which, 1,287,930 were issued. General The voting, dividend and liquidation rights of the holders of shares of Common Stock are subject to and qualified by the rights, powers and preferences of the holders of shares of preferred stock. The Common Stock has the following characteristics:

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-23 Voting The holders of shares of Common Stock are entitled to one vote for each share of Common Stock held at all meetings of stockholders and written actions in lieu of meetings. Dividends The holders of shares of Common Stock are entitled to receive dividends, if and when declared by the board of directors. Cash dividends may not be declared or paid to holders of shares of Common Stock until paid on each series of outstanding preferred stock in accordance with their respective terms. As of December 31, 2023, no dividends have been declared or paid since the Company’s inception. Liquidation After payment to the holders of shares of preferred stock of their liquidation preferences, the holders of the Common Stock are entitled to share ratably in the Company’s assets available for distribution to stockholders, in the event of any voluntary or involuntary liquidation, dissolution or winding up of the Company or upon the occurrence of a deemed liquidation event. Preferred stock At December 31, 2023 and 2022, the Company authorized 2,000,000 shares of $0.001 per share par value preferred stock, of which none have been issued. Nonvoting Common stock At December 31, 2023 and 2022, the Company authorized 1,000,000 shares of $0.001 per share par value Nonvoting Common Stock, of which, none have been issued. General The voting, dividend and liquidation rights of the holders of shares of Nonvoting Common Stock are subject to and qualified by the rights, powers and preferences of the holders of shares of preferred stock. The Nonvoting Common Stock has the following characteristics: Voting The holders of shares of Nonvoting Common Stock are not entitled to vote. Only in special and limited case where mandated by Delaware law, Nonvoting shareholders shall be entitled to one half ( ½) vote for each share of Nonvoting Common Stock. Dividends The holders of shares of Nonvoting Common Stock are entitled to receive dividends on a one for one basis, if and when declared by the board of directors on Common Stock. Cash dividends may not be declared or paid to holders of shares of Nonvoting Common Stock until paid on each series of outstanding preferred stock in accordance with their respective terms. For the years ended December 31, 2023 and 2022, no dividends have been declared or paid since the Company’s inception. Liquidation

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-24 After payment to the holders of shares of preferred stock of their liquidation preferences, the holders of the Nonvoting Common Stock are entitled to share ratably in the Company’s assets available for distribution to stockholders, in the event of any voluntary or involuntary liquidation, dissolution or winding up of the Company or upon the occurrence of a deemed liquidation event. Warrants As noted in Note 4, Senior Secured Convertible Promissory Notes, the Company issued an additional 88,826 warrant shares during the year ended December 31, 2022. As of December 31, 2023 these warrants have not been exercised and remain outstanding. Warrants Weighted Average Exercise Price Weighted Average Contractual Terms Outstanding at December 31, 2021 143,266 10.47$ 5.50 Granted 88,826 10.47 Forfeited/Cancelled - - Outstanding at December 31, 2022 232,092 10.47$ 4.50 Granted - - Forfeited/Cancelled - - Outstanding at December 31, 2023 232,092 10.47$ 3.50 As of December 31, 2023, outstanding warrants expire in June 19, 2027, and have a fair value of $382,000. NOTE 12 – INCOME TAXES Significant components of the Company’s deferred tax assets and liabilities at December 31, 2023 and December 31, 2022 are as follows: (table in thousands) 2023 2022 Net operating losses 1,116,404$ 729,814$ Accrued Expenses and Other 31,582 13,699 R&D Credit Carryforward 20,004 10,001 R&D Capitalization 710,455 370,551 Nondeductible Interest Expense 433,989 111,199 Other (11,897) (38,694) Total gross deferred tax assets/(liabilities) 2,300,537$ 1,196,570$ Less valuation allowance (2,300,537) (1,196,570) Net deferred tax assets/(liabilities) -$ -$ The income tax benefit for the years ended December 31, 2023 and December 31, 2022 differed from the amounts computed by applying the U.S. federal income tax rate of 21% to loss before tax benefit as a result of nondeductible expenses, tax credits generated, utilization of net operating loss carryforwards, and increases in the Company’s valuation allowance.

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-25 (table in thousands) 2023 2022 Federal Statutory Rate (1,958,993)$ (780,285)$ State Income Tax, Net of Federal Benefit (992) (992) Permanent Differences 1,150,417 197,715 Stock Based Compensation 14,802 67 Research and Development (571,473) (350,803) Change in Valuation Allowance 1,366,239 934,298 Effective Tax -$ -$ A valuation allowance is required to reduce the deferred tax assets reported if, based on the weight of the evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized. After consideration of the available evidence, both positive and negative, the Company determined that valuation allowances of $2,300,537 and $1,196,570 at December 31, 2023 and December 31, 2022 were necessary to reduce the deferred tax assets to the amount that will more likely than not be realized. At December 31, 2023 and 2022, the Company had gross U.S. Federal income tax net operating loss ("NOL") carryforward of approximately $2,067,595 and $883,348, respectively that may be used to offset future taxable income. The NOL was generated after 2017 and can be carried forward indefinitely under the Tax Cuts and Jobs Act. The company also had gross $2,052,767 of state net operating losses that will begin to expire in 2038. At December 31, 2023, the Company had approximately $12,662 of federal Research and Development (R&D) tax credit carry- forwards. If not utilized, the federal R&D credits will begin to expire in 2042. The Internal Revenue Code (the "IRC") contains limitations on the use of net operating loss carryforwards after the occurrence of a substantial ownership change as defined by IRC Section 382. The Company has not performed a detailed analysis, however utilization of such net operating loss carryforwards will likely be significantly limited due to the shares issued in the Primary Financing and the Merger. The income tax benefit for the years ended December 31, 2023 and 2022 differed from the amounts computed by applying the US federal income tax rate of 21% primarily because of the increase in the valuation allowance and the tax impact of other permanent items, which resulted in an effective tax rate of zero for both years. The Tax Cuts and Jobs Act of 2017 (TCJA) has modified the IRC 174 expenses related to research and development for the tax years beginning after December 31, 2021. Under the TCJA, the Company must now capitalize the expenditures related to research and development activities and amortize over five years for U.S. activities and 15 years for non-U.S. activities using a mid-year convention. Therefore, the capitalization of research and development costs in accordance with IRC 174 resulted in a gross deferred tax asset of $3,206,283. NOTE 13 - CONTINGENCIES From time to time, the Company may become involved in various legal matters arising in the ordinary course of business. Management is unaware of any matters requiring accrual for related losses in the financial statements. NOTE 14 - SUBSEQUENT EVENTS Management has evaluated subsequent events through November 26, 2024, which is the date the financial statements were available to be issued. Other than disclosed below, there were no subsequent events that require adjustment or disclosure in the consolidated financial statements.

Table of Contents DECOY THERAPEUTICS, INC. Notes to Consolidated Financial Statements December 31, 2023 and 2022 F-26 Between May 2024 and September 2024, the Company issued $655,555 of notional principal of promissory notes with an original issue discount of 10%, an annual interest rate of 10%, and a maturity date of 180-days from issuance to a series of investors. In connection with the issuance of these notes, the Company issued to the holders of the notes an aggregate of 89,000 warrants to purchase shares of the Company’s Common Stock at a price of $16.00 per share, with a term of 5 years from the date of issue. Additionally, as compensation for investment banking services the company issued 45,542 warrants to purchase shares of the Company’s Common Stock to Sutter Securities and related parties at a range of prices with a weighted average purchase price of $11.61. On October 30, 2024, the Company issued convertible notes with principal amount of $250,000, with a Company option to call an additional $250,000 under equivalent terms. These notes will convert into shares of the Company’s Common Stock at a price of $5.23 per share in a manner substantially similar to the conversion rights under the Senior Secured Convertible Notes described above, have an annual interest rate of 10%, and a maturity date 180 days from the date of issuance. During the second half of 2024, founders of the Company loaned the Company approximately $100,000 through non- interest bearing, open-ended maturity notes.




























