 |
| |
| (formerly Stem Cell Therapeutics Corp.) |
| |
| |
| |
| ANNUAL INFORMATION FORM |
| |
| FOR THE YEAR ENDED DECEMBER 31, 2014 |
| |
| |
| |
| 96 Skyway Avenue |
| Toronto, Ontario M9W 4Y9 |
| www.trilliumtherapeutics.com |
| |
| |
| Unless otherwise indicated, all information in the Annual Information Form |
| is presented as at and for the year ended December 31, 2014 |
| |
| |
| March 23, 2015 |
TABLE OF CONTENTS
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This Annual Information Form, or AIF, contains forward-looking statements within the meaning of applicable securities laws. All statements contained herein that are not clearly historical in nature are forward-looking, and the words “anticipate”, “believe”, “expect”, “estimate”, “may”, “will”, “could”, “leading”, “intend”, “contemplate”, “shall” and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements in this AIF include, but are not limited to, statements with respect to:
| • | our expected future loss and accumulated deficit levels; |
| • | our projected financial position and estimated cash burn rate; |
| • | our expectations about the timing of achieving milestones and the cost of our development programs; |
| • | our observations and expectations regarding the binding profile of SIRPαFc with red blood cells compared to anti-CD47 monoclonal antibodies and proprietary CD47-blocking agents and the potential benefits to patients; |
| • | our requirements for, and the ability to obtain, future funding on favorable terms or at all; |
| • | our projections for the SIRPαFc development plan and progress of each of our products and technologies, particularly with respect to the timely and successful completion of studies and trials and availability of results from such studies and trials; |
| • | our expectations about our products’ safety and efficacy; |
| • | our expectations regarding our ability to arrange for the manufacturing of our products and technologies; |
| • | our expectations regarding the progress, and the successful and timely completion, of the various stages of the regulatory approval process; |
| • | our ability to secure strategic partnerships with larger pharmaceutical and biotechnology companies; |
| • | our strategy to acquire and develop new products and technologies and to enhance the capabilities of existing products and technologies; |
| • | our plans to market, sell and distribute our products and technologies; |
| • | our expectations regarding the acceptance of our products and technologies by the market; |
| • | our ability to retain and access appropriate staff, management, and expert advisers; |
| • | our expectations with respect to existing and future corporate alliances and licensing transactions with third parties, and the receipt and timing of any payments to be made by us or to us in respect of such arrangements; and |
| • | our strategy with respect to the protection of our intellectual property. |
All forward-looking statements reflect our beliefs and assumptions based on information available at the time the assumption was made. These forward-looking statements are not based on historical facts but rather on management’s expectations regarding future activities, results of operations, performance, future capital and other expenditures (including the amount, nature and sources of funding thereof), competitive advantages, business prospects and opportunities. By its nature, forward-looking information involves numerous assumptions, inherent risks and uncertainties, both general and specific, known and unknown, that contribute to the possibility that the predictions, forecasts, projections or other forward-looking statements will not occur. Factors which could cause future outcomes to differ materially from those set forth in the forward-looking statements include, but are not limited to:
| • | the effect of continuing operating losses on our ability to obtain, on satisfactory terms, or at all, the capital required to maintain us as a going concern; |
| • | the ability to obtain sufficient and suitable financing to support operations, preclinical development, clinical trials, and commercialization of products; |
| • | the risks associated with the development of novel compounds at early stages of development in our intellectual property portfolio; |
| • | the risks of reliance on third-parties for the planning, conduct and monitoring of clinical trials and for the manufacture of drug product; |
| • | the risks associated with the development of our product candidates including the demonstration of efficacy and safety; |
| • | the risks related to clinical trials including potential delays, cost overruns and the failure to demonstrate efficacy and safety; |
3
| • | the risks of delays and inability to complete clinical trials due to difficulties enrolling patients; |
| • | risks associated with our inability to successfully develop companion diagnostics for our development candidates; |
| • | delays or negative outcomes from the regulatory approval process; |
| • | our ability to successfully compete in our targeted markets; |
| • | our ability to attract and retain key personnel, collaborators and advisors; |
| • | risks relating to the increase in operating costs from expanding existing programs, acquisition of additional development programs and increased staff; |
| • | risk of negative results of clinical trials or adverse safety events by us or others related to our product candidates; |
| • | the potential for product liability claims; |
| • | our ability to achieve our forecasted milestones and timelines on schedule; |
| • | financial risks related to the fluctuation of foreign currency rates and expenses denominated in foreign currencies; |
| • | our ability to adequately protect proprietary information and technology from competitors; |
| • | risks related to changes in patent laws and their interpretations; |
| • | our ability to source and maintain licenses from third-party owners; |
| • | the risk of patent-related litigation and the ability to protect trade secrets; and |
| • | the risk of reduced liquidity and market value decline resulting from a potential share consolidation, |
all as further and more fully described under the section of this AIF entitled “Risk Factors”.
Although the forward-looking statements contained in this AIF are based upon what our management believes to be reasonable assumptions, we cannot assure readers that actual results will be consistent with these forward-looking statements.
Any forward-looking statements represent our estimates only as of the date of this AIF and should not be relied upon as representing our estimates as of any subsequent date. We undertake no obligation to update any forward-looking statement or statements to reflect events or circumstances after the date on which such statement is made or to reflect the occurrence of unanticipated events, except as may be required by securities legislation.
All references in this AIF to “the Company”, “Trillium”, “we”, “us”, or “our” refer to Trillium Therapeutics Inc. and the subsidiaries through which it conducts its business, unless otherwise indicated.
All amounts are in Canadian dollars, unless otherwise indicated.
4
CORPORATE INFORMATION
We were incorporated under the Business Corporations Act (Alberta) on March 31, 2004 as Neurogenesis Biotech Corp. On October 19, 2004, we amended our articles of incorporation to change our name from Neurogenesis Biotech Corp. to Stem Cell Therapeutics Corp., or SCT. On November 7, 2013 SCT was continued under the Business Corporations Act (Ontario), or OBCA. On June 1, 2014 we filed articles of amalgamation to amalgamate SCT with our wholly-owned subsidiary, Trillium Therapeutics Inc., or TrilliumPrivateco, and renamed the combined company Trillium Therapeutics Inc. We are a company domiciled in Ontario, Canada.
We had one wholly-owned subsidiary, Stem Cell Therapeutics Inc., which was incorporated under the Business Corporations Act (Alberta) on December 22, 1999 and was continued under the OBCA on November 12, 2003 and then extra-provincially registered in Alberta on January 11, 2005. This inactive subsidiary was dissolved on September 17, 2014.
Our head and registered offices are located at 96 Skyway Avenue, Toronto, Ontario, Canada M9W 4Y9. Our website address is www.trilliumtherapeutics.com.
BUSINESS
Overview
We are an immuno-oncology company developing innovative therapies for the treatment of cancer. Our lead program, SIRPαFc, is a novel, antibody-like protein that harnesses the innate immune system by blocking the activity of CD47, a molecule whose expression is increased on cancer cells to evade the host immune system. Expressed at high levels on the cell surface of a variety of liquid and solid tumors, CD47 functions as a signal that inhibits the destruction of tumor cells by macrophages via phagocytosis. By blocking the activity of CD47, we believe SIRPαFc has the ability to promote the macrophage-mediated killing of tumor cells in a broad variety of cancers both as a monotherapy and in combination with other immune therapies. We expect to file an investigational new drug, or IND, application in the third quarter of 2015, and shortly thereafter commence phase I studies in acute myeloid leukemia, or AML, and myelodysplastic syndrome, or MDS. In the first phase I study we are also considering dosing patients with solid tumors or lymphomas who have normal bone marrow function in order to more clearly assess the potential hematological toxicity of SIRPαFc. We also plan to continue to conduct preclinical studies in other liquid and solid tumors to identify future clinical indications.
The immune system is the body’s mechanism to identify and eliminate pathogens, and can be divided into the innate immune system and the adaptive immune system. The innate immune system is the body’s first line of defense and consists of proteins and cells, such as macrophages, that identify and provide an immediate response to pathogens. The adaptive immune system is activated by, and adapts to, pathogens, creating a targeted and durable response. Cancer cells often have the ability to reduce the immune system’s ability to recognize and destroy them. We believe SIRPαFc plays a critical role in enhancing the innate immune system and importantly, because of its ability to target macrophages, also has an important downstream effect on the adaptive immune system.
Immuno-oncology is altering cancer treatment
While the concept of using the immune system to treat cancer can trace its roots back to late nineteenth century, it was only recently that the field of immuno-oncology received validation with the approval of antibodies that block CTLA-4 (ipilimumab) and PD-1 (pembrolizumab and nivolumab). These drugs have established the clinical principle that anti-tumor responses can be induced by interfering with the negative signals that normally shut down immune responses. The field of immuno-oncology is now regarded as causing a “paradigm shift” in cancer treatment and has been predicted by equity research analysts to generate US$20 to US$44 billion in revenue by the mid-2020s.
5
Our Strategy
Our goal is to become a leading innovator in the field of immuno-oncology by targeting immune-regulatory pathways that tumor cells exploit to evade the host immune system. We believe that SIRPαFc has the potential to improve survival and quality of life for cancer patients.
| • | Rapidly advance the clinical development of SIRPαFc. Following the completion of ongoing toxicology studies and cGMP production of our clinical lot, we plan to file an IND in the third quarter of 2015 for a first-in-human trial of SIRPαFc in AML and MDS patients, and possibly solid tumor or lymphoma patients with normal bone marrow function. |
| • | Expand our SIRPαFc clinical program to include additional cancer indications. Because CD47 is highly expressed by multiple liquid and solid tumors, and high expression is correlated with worse clinical outcomes, we believe SIRPαFc has potential to be effective in a wide variety of tumors. We plan to carry out the preclinical work necessary to select additional, high potential cancer indications for clinical development. |
| • | Maximize value of SIRPαFc through scientific collaborations. SIRPαFc has broad potential applicability in various cancer indications, and we believe it is well suited for use as both a monotherapy and in combination with other agents. We plan to selectively and opportunistically pursue scientific collaborations with academic researchers as well as other companies, in order to realize the full value proposition of SIRPαFc. |
| • | To become a leading integrated immuno-oncology company. Developing cancer immunotherapies requires significant experience and development expertise. Our experienced management team, board of directors, scientific advisory board and in-house capabilities enable us to execute our product development plan. We intend to continue to invest in the infrastructure and personnel to support our continued growth that will enable us to become a leading immuno-oncology company. |
SIRPαFc Key Attributes
| • | Potential efficacy in a broad range of cancers. SIRPαFc blocks the tumor’s ability to transmit a “do not eat” signal allowing macrophages to destroy tumor cells; a mechanism that we believe has broad applicability. |
| • | Potential for use as monotherapy and in combination with other therapies. We intend to develop SIRPαFc as a monotherapy as well as potentially for use in combination with other cancer immuno- therapies. |
| • | Differentiated pharmacokinetic and safety profile. We believe SIRPαFc’s low level of binding to red blood cells lowers the risk of anemia and lowers the loss of drug from circulation. This pharmacokinetic profile potentially allows for lower dosing and a positive safety profile. |
| • | May enhance both innate and adaptive immune response. SIRPαFc may enhance stimulation of tumor attacking T-cells, since macrophages, in addition to their role in phagocytosis, can also prime T-cells through antigen-presentation. |
Blocking the CD47 “do not eat” signal using the SIRPαFc decoy receptor
Macrophages are a type of white blood cell that can ingest and destroy (phagocytose) other cells. They are part of the innate immune system that helps to protect the body from infection. More recently, a role for macrophages in the control of tumors has been described.
Macrophage activity is controlled by both positive “eat” and negative “do not eat” signals. Tumor cells may express “eat” signals (e.g., calreticulin) that make themselves visible to macrophages. To counterbalance this increased visibility the tumor cells often express high levels of CD47, which transmits a “do not eat” signal by binding the signal regulatory protein alpha, or SIRPα, on the surface of macrophages. We believe that the higher expression of CD47 on the tumor cell helps it evade destruction by the macrophage by overwhelming any activating “eat” signals.
SIRPαFc is an antibody-like protein that consists of the CD47-binding domain of human SIRPα linked to the Fc region of a human immunoglobulin. It is designed to act as a soluble decoy receptor, preventing CD47 from delivering its inhibitory signal. Neutralization of the inhibitory CD47 signal enables the activation of macrophage anti-tumor effects by the pro-phagocytic “eat” signals. The Fc region of SIRPαFc may, depending on its identity, also assist in the activation of macrophages by engaging Fc receptors.
6
Figure 1, below, illustrates how SIRPαFc blocks the CD47 “do not eat” signal.
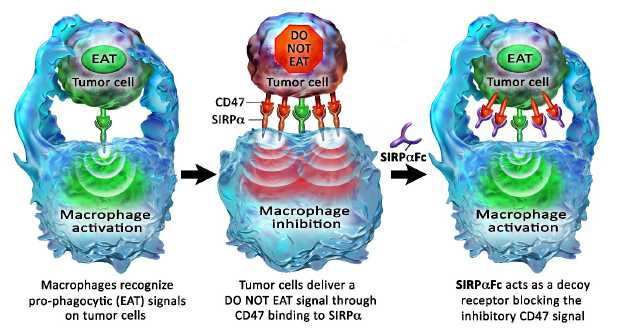
In addition to their direct anti-tumor activity, macrophages can also function as antigen-presenting cells and stimulate antigen-specific T cells. Recent data in mice have demonstrated that CD47 antibody blockade increases antigen presentation by macrophage and stimulates the development of anti-tumor cytotoxic T cell responses. We hypothesize that increasing macrophage phagocytosis by SIRPαFc treatment may also enhance tumor-specific T cell responses. Thus, SIRPαFc may enhance both the innate (macrophage) and adaptive (T cell) components of an anti-tumor immune response.
SIRPαFc has broad clinical potential
We believe that SIRPαFc has broad clinical potential in both hematological and solid tumors. High expression of the CD47 “do not eat” signal on tumor cells has been observed in acute myeloid leukemia, or AML, myelodysplastic syndrome, or MDS, chronic myeloid leukemia, or CML, acute lymphoblastic leukemia, or ALL, diffuse large B cell lymphoma, chronic lymphocytic leukemia, follicular lymphoma, mantle cell lymphoma, marginal zone lymphoma, multiple myeloma and in the following solid tumors: bladder, brain, breast, colon, leiomyosarcoma, liver, melanoma, ovarian and prostate. In a number of these cancers high CD47 expression was shown to have negative clinical consequences, correlating with more aggressive disease and poor survival. In normal karyotype AML patients, for example, high CD47 expression was correlated with worse event-free survival (6.8 vs. 17.1 months) and worse overall survival (9.1 vs. 22.1 months) compared to low CD47 expression. These data are consistent with CD47 providing a survival advantage to tumor cells. Furthermore, numerous studies have shown that antibody blockade of CD47 has demonstrated activity in mice engrafted with human tumors.
In vitro studies with blood cancer lines derived from ALL, B lymphoma, AML, CML and multiple myeloma patients demonstrated that SIRPαFc frequently triggered significant macrophage-mediated tumor cell phagocytosis compared to control treatment.
Figure 2, below, shows how SIRPαFc triggers macrophage-mediated phagocytosis of many different human blood cancer cell lines.
7

***p<0.0001; **p<0.01; *p<0.05: NS: Thep value is a probability value, with values <0.05 considered statistically significant versus control treatment. NS indicatesp>0.05, which is considered not statistically significant versus control treatment.
In August 2014, we entered into a collaboration with academic investigators to explore the therapeutic potential of SIRPαFc in a variety of solid tumor models. The research is being conducted in the laboratories of Drs. James Koropatnick and Ting-Yim Lee, at the Lawson Health Research Institute and the Robarts Research Institute, University of Western Ontario. Our funding is matched 1:1 by a grant from the Ontario Research Fund – Research Excellence providing the collaboration with a research budget approximating $600,000. We are also working with collaborators at from University Health Network, or UHN, and Hospital for Sick Children, or HSC, and plan to further expand our collaboration network in 2015.
SIRPαFc for the Treatment of AML and MDS
We currently expect our first clinical evaluation of a potential indication for SIRPαFc will be for the treatment of AML and MDS patients. We may also add solid tumor or lymphoma patients with normal bone marrow function. AML is the most common type of acute leukemia in adults, with approximately 13,000 new cases diagnosed each year in the United States. The majority of AML patients receive induction chemotherapy. In patients under 60 years of age, remission rates of up to 75% can be achieved, and patients with good-risk or standard-risk chromosomal changes will typically receive post-remission therapy with high dose cytarabine. However, relapses are common, and the majority of patients will die from their disease. AML in patients over 60 years of age is very difficult to treat, with five-year survival rates reported of less than 10%. There is ample evidence that AML is sustained by leukemic stem cells, or LSCs, and the resistance of these LSCs to conventional chemotherapy is thought to be responsible for disease relapse. CD47 is overexpressed by both bulk differentiated AML cancer cells as well as LSCs.
MDS is a heterogeneous group of closely related blood disorders characterized by one or more peripheral blood cytopenias secondary to bone marrow dysfunction. It is considered a premalignant condition and often progresses to AML when additional genetic abnormalities are acquired. There are approximately 13,000 new cases of MDS diagnosed in the United States each year. The median survival times for MDS patients range from 5.7 years (low risk) to 5 months (high risk).
8
As described above, AML tumor cells have been shown to have high levels of CD47, and CD47 expression has been shown to correlate with worse clinical outcome. Antibody blockade of CD47 has been shown to promote phagocytosis of AML cells in vitro and promote anti-leukemic activity in mouse models. CD47 expression has also been shown to be elevated in bone marrow progenitor cells from high risk MDS patients compared to low risk.
In cell-based experiments, SIRPαFc has been shown to promote the phagocytosis of patient-derived AML tumor samples. In the absence of SIRPαFc treatment, human macrophages are poorly phagocytic. However, treatment of tumor cells with SIRPαFc at doses equal or greater to 10 nanomolar (nM) results in a dramatic increase in tumor cell phagocytosis. This effect is seen visually using confocal microscopy and quantified by expressing a phagocytosis index (number of engulfed tumor cells per 100 macrophages).
Figure 3, below, illustrates how SIRPαFc enables macrophages to kill human AML tumor cells.
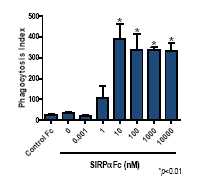
*p<0.01: Thep value is a probability value, with values <0.05 considered statistically significant versus control treatment.
Since AML is a heterogeneous disease, we assessed the impact of SIRPαFc on a panel of 10 AML tumor samples isolated from a diverse patient population at various disease stages and different genetic abnormalities. SIRPαFc was shown to be active against 9 of these 10 samples, indicating that the pro-phagocytic effect of SIRPαFc is not restricted to a subset of AML tumors.
Figure 4, below, illustrates how SIRPαFc is active against a diverse panel of AML samples.

9
***p<0.0001; **p<0.01; *p<0.05; NS: The p value is a probability value, with values <0.05 considered statistically significant versus control treatment. NS indicatesp>0.05, which is considered not statistically significant versus control treatment.
The below table sets forth the AML patient characteristics supporting the information in Figure 4, above.
| Patient | | Age | | Sex | | FAB Subtype(1) | | Blast (%)(2) |
| 80559 | | 68 | | M | | M5a | | 20 |
| 80567 | | 69 | | F | | M5a | | 90 |
| 90174 | | 41 | | M | | M4 | | N/A |
| 90543 | | 33 | | M | | M2 | | 82 |
| 90596 | | 69 | | M | | M0 | | 97 |
| 90650 | | 67 | | M | | M1 | | 90 |
| 90765 | | 94 | | F | | M2 | | 90 |
| 100116 | | 66 | | F | | M4 | | N/A |
| 100622 | | 65 | | F | | M4Eo | | 40 |
| 100857 | | 73 | | M | | M2 | | 10 |
Notes:
| (1) | The French-American-British, or FAB, classification system divides AML into eight subtypes, M0 through to M7, based on the type of cell from which the leukemia developed and its degree of maturity. |
| | |
| (2) | Blast (%) refers to the percentage of leukemic myeloblasts, or blood cells affected by disease, compared to all blood cells in the sample. |
The ability of macrophages to kill normal cells in the presence of SIRPαFc was also assessed by confocal microscopy. SIRPαFc enabled macrophages to phagocytose AML tumor cells but spared normal peripheral blood-derived mononuclear cells. This indicates that SIRPαFc-mediated phagocytosis is tumor cell-specific, and strongly suggests that a therapeutic window exists in which SIRPαFc can promote the killing of tumor cells while sparing normal hematopoietic targets. These results are consistent with similar published data using CD47 antibodies. The strong selectivity for tumor cells may be due to the expression of pro-phagocytic “eat” signals such as calreticulin on malignant cells but not on normal cells.
Figure 5, below, illustrates how SIRPαFc induces tumor cell-specific phagocytosis.
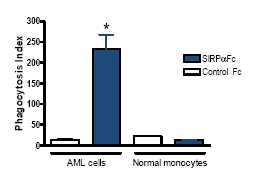
*Thep value is a probability value, with values <0.05 considered statistically significant versus control treatment.
10
The activity of SIRPαFc was assessed by engrafting AML patient tumor cells into nonobese diabetic NOD.SCID, or NS, mice. In this model, tumor cells were injected directly into the bone marrow of an NS mouse and three weeks later therapy was initiated with SIRPαFc or a control Fc protein. After treating for 4 weeks, the mice were sacrificed and leukemia in the bone marrow (harvested from the injected and non-injected femurs) and spleen was measured by flow cytometry. Treatment with SIRPαFc in this model resulted in the reduction of leukemia to non-detectable levels in all three tissues in most mice. These results are further validated by independent research demonstrating activity of CD47 blockade using monoclonal antibodies.
Figure 6, below, illustrates how SIRPαFc has potent anti-leukemic activityin vivo.The information presented in Figure 6 is based on the treatment of mice with a human SIRPαFc at a dose of 8 mg/kg intraperitoneal, or IP, three times per week for four weeks, starting 21 days after engraftment.

Thep value is a probability value, with values <0.05 considered statistically significant versus control treatment.
The human SIRPαFc used in the above model does not bind mouse CD47 and thus does not model a potential “antigen sink” effect, whereby drug is rapidly removed from circulation by binding target on non-tumor (host) cells. To overcome this limitation, tumor studies were conducted using a mouse surrogate drug that contains the SIRPα sequence from the NS mouse strain, which has the unusual property of binding both mouse and human CD47 and thus can be used in studies in which human tumor cells are transplanted into mice. The mouse surrogate drug was shown to mediate strong anti-leukemic activity in AML tumor studies, even at relatively low doses (<1 mg/kg). These data demonstrate that the presence of CD47 target on normal cells does not interfere with drug activity.
Figure 7, below, illustrates how SIRPαFc has strong anti-leukemic activity even at low doses. The information presented in Figure 7 is based on the treatment of mice with a mouse SIRPαFc at doses of 0.2, 1 or 5 mg/kg IP for three times per week for four weeks, starting 21 days after engraftment.
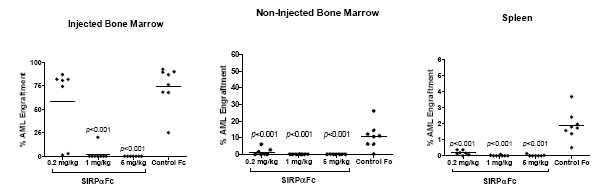
Thep value is a probability value, with values <0.05 considered statistically significant versus control treatment.
Additional AML tumor studies have been conducted, examining various therapeutic dosing regimens, as well as the effect of different Fc regions. The data continue to demonstrate significant anti-tumor activity at doses below 1 mg/kg. The results have also shown that the identity of the Fc region directly impactsin vivo potency.
11
Several preliminary non-good laboratory practice safety studies have been conducted in cynomolgus monkeys. The results have provided supportive safety and pharmacokinetic data and provided valuable guidance for the design of our formal IND-enabling toxicology program which commenced in February 2015.
Clinical development plan
Figure 8, below, illustrates the SIRPαFc preclinical development plan. We have completed production of the SIRPαFc toxicology lots and commenced formal IND-enabling toxicology studies. We expect to complete current Good Manufacturing Practice, or cGMP, production of our clinical lot in the second quarter of 2015 and file an IND in the third quarter of 2015 for a first-in-human trial of SIRPαFc in AML and MDS patients. The first clinical study may also involve patients with solid tumors or lymphoma who have normal bone marrow function.
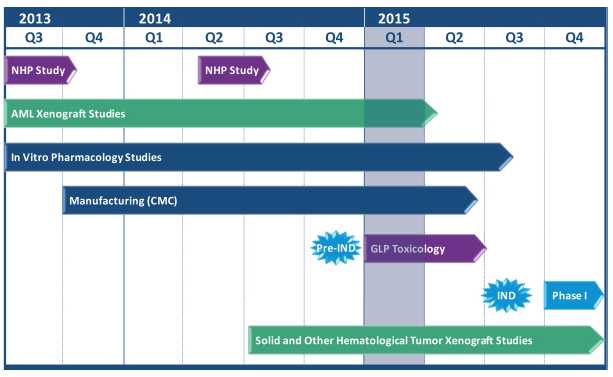
Combination Therapy
SIRPαFc is currently being developed as a monotherapy. However, we believe that SIRPαFc enhancement of macrophage activity and possibly T cell responses could be synergistic with other immune-mediated therapies. Studies conducted by third parties provide evidence that SIRPαFc may be useful in combination with approved anti-cancer antibodies (e.g., Rituxan®, Herceptin®, Campath®). Since many cancer antibodies work at least in part by activating cells of the innate immune system, it may be possible to enhance the potency of these agents by blocking the negative “do not eat” CD47 signal that tumor cells deliver to macrophages. We hypothesize that SIRPαFc may act synergistically with other immunological agents, including T cell checkpoint inhibitors, cancer vaccines, oncolytic viruses or chimeric antigen receptor, or CAR, T cells. We therefore plan to explore SIRPαFc in combination studies using preclinical tumor models.
Competition - CD47/ AML
SIRPαFc competes directly with CD47 blocking antibodies from Stanford University and Celgene Corporation, both of which have entered early clinical development. Novimmune SA also has a bispecific (anti-CD47/anti-CD19) antibody program, although it is still in the discovery stage.
12
We believe that our approach of using SIRPα, the natural binding partner for CD47, may have important advantages over treatment with CD47-specific antibodies. In April 2014, we presented data at the 105th American Association for Cancer Research annual meeting demonstrating that our SIRPαFc fusion proteins bind very poorly to human red blood cells, or RBCs, compared to both commercial anti-CD47 monoclonal antibodies and proprietary CD47-blocking agents. In Figure 9, below, on the left, we demonstrate the poor binding of SIRPαFc to RBCs compared to four anti-CD47 antibodies. This profound difference in binding was not seen with other cell types, including an AML cell line (AML-2, right side), suggesting it is an RBC-specific phenomenon. Our observation is consistent with a prior report demonstrating a notable difference in human RBC binding between CD47 antibody and recombinant SIRPα. Furthermore, the lack of significant binding of SIRPαFc to RBCs is unique to humans, since SIRPαFc bound strongly to mouse and monkey RBCs. While the mechanism behind this observation is still under investigation, our preliminary data suggest that it may relate to unique structural features of CD47 in the human RBC membrane.
Figure 9, below, illustrates how SIRPαFc binds very poorly to human RBCs compared to CD47 antibodies. The information presented in Figure 9 is based on the binding of commercially available anti-CD47 monoclonal antibodies and SIRPαFc to human RBCs (n=14 donors) and AML-2 cells.

The very low RBC binding profile of our SIRPαFc proteins may provide two important advantages over other CD47 blocking agents. First, there may be a lower risk of RBC toxicity (anemia) with SIRPαFc. Anemia is a condition in which the blood is deficient in red blood cells, in hemoglobin, or in total volume. This potential advantage would translate into improved patient care, particularly in diseases such as AML where patients are predisposed to developing anemia. Second, there may be much lower loss of SIRPαFc from circulation in treated patients compared to CD47 antibodies, which bind strongly to circulating RBCs. The ability of RBCs to absorb and remove circulating CD47-specific antibodies, a so called “antigen-sink effect”, may necessitate high doses of drug in order to effectively target the tumor cells, which could lead to potentially higher off-target toxicity. Therefore, SIRPαFc may have a better safety profile and drug kinetics than CD47-specific antibodies based on these preclinical analyses.
While there is significant competitive clinical activity in AML, there are very few agents in development that are pursuing the same unique mechanism of action (enhancement of macrophage phagocytic activity) as SIRPαFc. To our knowledge, there are a few parties pursuing CD47-blocking antibodies, but no one else developing a SIRPαFc protein. In addition, there are few agents in development targeting AML LSCs. Similar to our targeting of CD47, we believe other agents in development targeting AML LSCs are largely focused on cell surface proteins such as CD123 and CD44.
Plan of Operations
Over the next 12 months, our primary focus is the advancement of our SIRPαFc development program with the intent to initiate the first phase I clinical trial of SIRPαFc in AML and MDS patients. This study may also involve patients with solid tumors or lymphomas who have normal bone marrow function. The major tasks to be performed to accomplish this include completion of manufacturing of cGMP material suitable for clinical testing, completion of IND-enabling toxicology and pharmacology studies, submission of our IND application for FDA approval to start the trial in the third quarter of 2015 and the initiation of the clinical trial in the fourth quarter of 2015. We will also continue preclinical studies in other blood cancers and solid tumors to identify potential future clinical indications.
13
Agreements with Catalent Pharma Solutions
In connection with our development of SIRPαFc, we entered into two agreements on August 12, 2014 with Catalent pursuant to which we acquired the right to use two of Catalent’s proprietary GPEx® expression cell lines for the manufacture of two SIRPαFc proteins: TTI-621 and TTI-622. One agreement relates to the manufacture of TTI-621 and the other agreement relates to the manufacture of TTI-622. In consideration for the purchase of the expression cell lines, each agreement provides that we will pay Catalent up to US$875,000 upon reaching certain pre-marketing approval milestones and up to an additional US$28.8 million for reaching certain sales milestones. We will also pay Catalent an annual product maintenance fee until the first product derived from the expression cell lines receives a regulatory approval other than a pricing or reimbursement approval.
Under the agreements, we may use the two expression cell lines to secure such regulatory approvals and to develop, test, market and otherwise commercially exploit products originating from the cell lines. We may transfer the expression cell lines to a third party contract manufacturer who may utilize the cell lines in a similar fashion. We, or a third-party, cannot use or modify the cell lines, or any portions of the cell lines, to create a new cell line.
We plan to further develop the expression cell lines for use in our pre-IND toxicology and pharmacology studies, as well as to supply our phase I clinical trial. We will be required to indemnify Catalent for any costs Catalent incurs related to regulatory filings and related claims or proceedings, for the conduct of any clinical trials and for any manufacture, packaging, sale, promotion, distribution, use of or exposure to the expression cell lines or products. As a result of this risk, we are obligated to maintain several designated insurance policies throughout the term of the agreements.
We may terminate the agreements upon 90 days’ written notice to Catalent, upon their bankruptcy or upon their material breach and failure to cure within 30 days. Similarly, Catalent may terminate the agreements upon our bankruptcy or upon our material breach and failure to cure within 30 days. If our material breach is for nonpayment, however, we will only have 10 days to cure before Catalent may terminate the agreement.
Product for Development with Partners – CD200 Monoclonal Antibodies
Our CD200 monoclonal antibody program also targets a key pathway that tumor cells use to evade attack from the immune system. There is evidence that CD200 is highly expressed by many different types of blood cell and solid tumors, and in numerous cases this high expression correlates with disease progression and poor clinical outcome. This is consistent with tumor cells using CD200 as a means of evading immune-mediated destruction. Tumor-expressed CD200 can modulate anti-tumor responses in vitro and in vivo, and antibodies that block CD200 have been shown to promote anti-tumor immunity in animal models of cancer.
Our CD200 monoclonal antibody is a fully human monoclonal antibody that blocks the activity of CD200. We have conducted in vitro and in vivo preclinical oncology-focused studies. Our antibodies bind strongly to human CD200, potently neutralizing CD200 function in vitro, and have shown anti-tumor activity in a transplanted human tumor cell model. We are seeking a development partner to advance this program into formal preclinical IND-enabling studies.
Intellectual Property
We own or control patent rights covering our key products and their therapeutic end uses. The patents and patent applications are either granted or pending in major pharmaceutical markets. In all, the patent estate includes five key patent families, including five key issued patents and two key pending patent applications in the U.S. and various other patent applications pending in Europe, Canada, Australia, China, India, and Japan. These include four granted patents in the U.S. relating to the immune modulating use of CD200 monoclonal antibodies, and a granted patent in the U.S. for an assay useful to detect CD200 in cancer patients, as well as a filing for novel human antibodies to CD200.
14
In connection specifically with patent applications relating to SIRPαFc, we control two patent families that comprise eight individual filings. One family has claims that embrace species of SIRPαFc found to have certain therapeutic properties and their use for the treatment of cancer. These patent rights are owned outright by Trillium and national patent filings are planned. Patents emerging from this family will expire in 2033. A second SIRPα patent family was in-licensed on an exclusive basis from co-owners UHN and HSC. This family has been filed in major markets, including the U.S., Europe, Japan, Canada, Australia, China and India. The claims cover the use of various forms of SIRPα to treat CD47-positive cancers. Patents issuing from this family begin to expire in the year 2030.
In connection specifically with CD200 monoclonal antibody patents, we have four granted U.S. patents that cover the use of CD200 antibodies for the treatment of autoimmune and related disorders that will begin to expire in 2018. We more recently made a patent filing that covers particular species of human antibodies to human CD200. A patent family based on this filing would expire in 2035.
We intend to protect additional intellectual property developed by us through the filing of patent applications within appropriate jurisdictions throughout the world.
Regulatory Process
Government authorities in the United States, including federal, state, and local authorities, and in other countries, extensively regulate, among other things, the manufacturing, research and clinical development, marketing, labeling and packaging, storage, distribution, post-approval monitoring and reporting, advertising and promotion, and export and import of biological products, such as those we are developing. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources.
Securing final regulatory approval for the manufacture and sale of biological products in the U.S., Europe, Canada and other commercial territories, is a long and costly process that is controlled by that particular territory’s regulatory agency. The regulatory agency in the U.S. is the FDA, in Canada it is HC, and in Europe it is the European Medicines Agency, or EMA. Other regulatory agencies have similar regulatory approval processes, but each regulatory agency has its own approval processes. Approval in the U.S., Canada or Europe does not assure approval by other regulatory agencies, although often test results from one country may be used in applications for regulatory approval in another country.
None of our products have been completely developed or tested and, therefore, we are not yet in a position to seek final regulatory approval to market any of our products.
U.S. Government Regulation
In the U.S., the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and its implementing regulations, and biologics under the FDCA and the Public Health Service Act, or PHSA, and its implementing regulations. FDA approval is required before any new unapproved drug or biologic or dosage form, including a new use of a previously approved drug, can be marketed in the United States. Drugs and biologics are also subject to other federal, state, and local statutes and regulations. If we fail to comply with applicable FDA or other requirements at any time during the product development process, clinical testing, the approval process or after approval, we may become subject to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, civil monetary penalties or criminal prosecution. Any FDA enforcement action could have a material adverse effect on us.
15
The process required by the FDA before product candidates may be marketed in the United States generally involves the following:
| • | completion of extensive preclinical laboratory tests and preclinical animal studies, all performed in accordance with the Good Laboratory Practices regulations; |
| • | submission to the FDA of an investigational new drug application, or IND, which must become effective before human clinical trials may begin and must be updated annually; |
| • | approval by an independent institutional review board, or IRB, or ethics committee representing each clinical site before each clinical trial may be initiated; |
| • | performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the product candidate for each proposed indication; |
| • | preparation of and submission to the FDA of a new drug application, or NDA, or biologics license application, or BLA, after completion of all pivotal clinical trials; |
| • | potential review of the product application by an FDA advisory committee, where appropriate and if applicable; |
| • | a determination by the FDA within 60 days of its receipt of an NDA or BLA to file the application for review; |
| • | satisfactory completion of an FDA pre-approval inspection of the manufacturing facilities where the proposed product is produced to assess compliance with current Good Manufacturing Practices, or cGMP; |
| • | a potential FDA audit of the preclinical research and clinical trial sites that generated the data in support of the NDA or BLA; and |
| • | FDA review and approval of an NDA or BLA prior to any commercial marketing or sale of the product in the U.S. |
The preclinical research and clinical testing and approval process require substantial time, effort, and financial resources, and we cannot be certain that any approvals for our product candidates will be granted on a timely basis, if at all.
An IND is a request for authorization from the FDA to administer an investigational new drug product to humans in clinical trials. The central focus of an IND submission is on the general investigational plan and the protocol(s) for human clinical trials. The IND also includes results of animal studies assessing the toxicology, pharmacokinetics, pharmacology, and pharmacodynamic characteristics of the product; chemistry, manufacturing, and controls information; and any available human data or literature to support the use of the investigational new drug. An IND must become effective before human clinical trials may begin. An IND will automatically become effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to the proposed clinical trials. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before clinical trials can begin. Accordingly, submission of an IND may or may not result in the FDA allowing clinical trials to commence.
Clinical Trials
Clinical trials involve the administration of the investigational new drug to human subjects under the supervision of qualified investigators in accordance with Good Clinical Practices, or GCPs, which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety, and the efficacy criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. Additionally, approval must also be obtained from each clinical trial site’s IRB or ethics committee, before the trials may be initiated, and the IRB or ethics committee must monitor the trial until completed. There are also requirements governing the reporting of ongoing clinical trials and clinical trial results to public registries.
The clinical investigation of a drug is generally divided into three or four phases. Although the phases are usually conducted sequentially, they may overlap or be combined.
16
| • | Phase I. The drug is initially introduced into healthy human subjects or patients with the target disease or condition. These studies are designed to evaluate the safety, dosage tolerance, metabolism and pharmacologic actions of the investigational new drug in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on effectiveness. |
| • | Phase II. The drug is administered to a limited patient population to evaluate dosage tolerance and optimal dosage, identify possible adverse side effects and safety risks, and preliminarily evaluate efficacy. |
| • | Phase III. The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites to generate enough data to statistically evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the investigational new drug product, and to provide an adequate basis for physician labeling. |
| • | Phase IV. In some cases, the FDA may condition approval of an NDA or BLA for a product candidate on the sponsor’s agreement to conduct additional clinical trials after approval. In other cases, a sponsor may voluntarily conduct additional clinical trials after approval to gain more information about the drug. Such post-approval studies are typically referred to as phase IV clinical trials. |
Clinical trial sponsors must also report to the FDA, within certain timeframes, serious and unexpected adverse reactions, any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator’s brochure, or any findings from other studies or animal testing that suggest a significant risk in humans exposed to the product candidate. The FDA, the IRB or ethics committee, or the clinical trial sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group provides authorization for whether or not a trial may move forward at designated check points based on access to certain data from the trial.
The clinical trial process can take years to complete, and there can be no assurance that the data collected will support FDA approval or licensure of the product. Results from one trial are not necessarily predictive of results from later trials. We may also suspend or terminate a clinical trial based on evolving business objectives and/or competitive climate.
Submission of an NDA or BLA to the FDA
Assuming successful completion of all required preclinical studies and clinical testing in accordance with all applicable regulatory requirements, detailed investigational new drug product information is submitted to the FDA in the form of an NDA or BLA requesting approval to market the product for one or more indications. Under federal law, the submission of most NDAs and BLAs is subject to an application user fee. For fiscal year 2015, the application user fee exceeds $2.3 million, and the sponsor of an approved NDA or BLA is also subject to annual product and establishment user fees, set at $110,370 per product and $569,200 per establishment. These fees are typically increased annually. Applications for orphan drug products are exempted from the NDA and BLA application user fee, unless the application includes an indication for other than a rare disease or condition, and may be exempted from product and establishment user fees under certain conditions.
An NDA or BLA must include all relevant data available from pertinent preclinical studies and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls, and proposed labeling, among other things. Data comes from company-sponsored clinical trials intended to test the safety and effectiveness of a use of a product, and may also come from a number of alternative sources, including trials initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and effectiveness of the investigational new drug product to the satisfaction of the FDA.
Once an NDA or BLA has been submitted, the FDA’s goal is to review the application within ten months after it accepts the application for filing, or, if the application relates to an unmet medical need in a serious or life-threatening indication, six months after the FDA accepts the application for filing. The review process is often significantly extended by the FDA’s requests for additional information or clarification.
17
Before approving an NDA or BLA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA or BLA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP.
The FDA is required to refer an NDA or BLA for a novel drug (in which no active ingredient has been approved in any other application) to an advisory committee or explain why such referral was not made. Typically, an advisory committee is a panel of independent experts, including clinicians and other scientific experts, that reviews, evaluates and provides a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
The FDA’s Decision on an NDA or BLA
After the FDA evaluates the NDA or BLA and conducts inspections of manufacturing facilities where the product will be produced, the FDA will issue either an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete and the application is not ready for approval. In order to satisfy deficiencies identified in a Complete Response Letter, additional clinical data and/or an additional phase III clinical trial(s), and/or other significant, expensive and time-consuming requirements related to clinical trials, preclinical studies or manufacturing may be required for the product candidate. Even if such additional information is submitted, the FDA may ultimately decide that the NDA or BLA does not satisfy the criteria for approval. The FDA could also approve the NDA or BLA with a risk evaluation and mitigation strategy, plan to mitigate risks, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling, development of adequate controls and specifications, or a commitment to conduct one or more post-market studies or clinical trials. Such post-market testing may include phase IV clinical trials and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization. New government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
Patent Term Restoration
Depending upon the timing, duration, and specifics of the FDA approval of the use of our product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of an NDA or BLA, plus the time between the submission date and the approval of that application. Only one patent applicable to an approved product is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent and within 60 days of the product’s approval. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we may apply for restoration of patent term for one of our currently owned or licensed patents to add patent life beyond its current expiration date, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant NDA or BLA.
Companion Diagnostics
In its August 6, 2014, guidance document entitled “In Vitro Companion Diagnostic Devices,” the FDA defines an IVD companion diagnostic device to be an in vitro diagnostic device that provides information that is essential for the safe and effective use of a corresponding therapeutic product. Use of an IVD companion diagnostic device is considered essential when its use is required in the labeling of a therapeutic product, for example, to select appropriate patients for a product or those who should not use the product, or to monitor patients to achieve safety or effectiveness. In most circumstances, the IVD companion diagnostic device should be approved or cleared by FDA under the device authorities of the FDCA contemporaneously with the therapeutic product’s approval under section 505 of the FDCA for a drug or section 351 of the PHSA for a biological product. FDA expects the therapeutic product sponsor to address the need for an approved or cleared IVD companion diagnostic device in its therapeutic product development plan. The therapeutic product sponsor may develop its own IVD companion diagnostic device, partner with a diagnostic device sponsor to develop an IVD companion diagnostic device, or explore modifying an existing IVD diagnostic device to develop a new intended use. The FDA explains if a diagnostic device and a therapeutic device are studied together to support their respective approvals, both products can be studied in the same investigational study that meets both the requirements of the Investigational Device Exemption, or IDE, regulations and the IND regulations. Depending on the study plan and participants, a sponsor may seek to submit an IND alone, or both an IND and IDE.
18
Raw Materials, Manufacturing, and Supply
We have limited experience in manufacturing products for clinical or commercial purposes. We produce small quantities of SIRPαFc and CD200 monoclonal antibody in our laboratories for internal use. We believe that sources of raw materials pertinent to our laboratory operations and for manufacturing of our SIRPαFc product by our CMO are generally available.
We have established a contract manufacturing relationship for the supply of SIRPαFc that we believe will provide sufficient material for early clinical trials. In addition, we are establishing the basis for long-term commercial production capabilities. However, there can be no assurance that our contract manufacturer will be successful at scaling up and producing our product with the required quality and in the quantities and timelines that we will need for clinical and/or commercial purposes.
We expect to similarly rely on contract manufacturing relationships for any products that we may further develop, or in-license or acquire in the future. However, there can be no assurance that we will be able to successfully contract with such manufacturers on terms acceptable to us, or at all.
Contract manufacturers are subject to ongoing periodic and unannounced inspections by the FDA, the U.S. Drug Enforcement Administration and corresponding state agencies to ensure strict compliance with cGMP and other state and federal regulations. We do not have control over third-party manufacturers’ compliance with these regulations and standards, other than through contractual obligations and periodic auditing. If they are deemed out of compliance with such regulations, approvals could be delayed, product recalls could result, inventory could be destroyed, production could be stopped and supplies could be delayed or otherwise disrupted.
If we need to change manufacturers after commercialization, the FDA and corresponding foreign regulatory agencies must approve these new manufacturers in advance, which will involve testing and additional inspections to ensure compliance with FDA regulations and standards and may require significant lead times and delay, and disruption of supply. Furthermore, switching manufacturers may be difficult because the number of potential manufacturers is limited. It may be difficult or impossible for us to find a replacement manufacturer quickly or on terms acceptable to us, or at all.
Property, Plant and Equipment
We operate from approximately 10,000 square feet of leased laboratory and office space at 96 Skyway Avenue, Toronto, Ontario, Canada, M9W 4Y9. We perform research and development in our facility and use qualified vendors and collaborators to conduct research and development and manufacturing on our behalf. We incur capital expenditures mainly for laboratory equipment, office equipment, computer equipment and leaseholds in the operation of our business. As at December 31, 2014 the net carrying value of our property and equipment was $235,402.
19
Employees
As at March 23, 2015, we had eighteen full-time employees including four senior management, twelve research and development staff and two finance and administrative staff. All employees are located at our head office and lab facilities in Toronto, Ontario, Canada.
We also use consultants and outside contractors to carry on many of our activities, including preclinical testing and validation, formulation, assay development, manufacturing, clinical and regulatory affairs, toxicology and clinical trials.
Legal Proceedings
To our knowledge, there have not been any legal or arbitration proceedings, including those relating to bankruptcy, receivership or similar proceedings, those involving any third party, and governmental proceedings pending or known to be contemplated, which may have, or have had in the recent past, significant effect our financial position or profitability.
Also, to our knowledge, there have been no material proceedings in which any director, any member of senior management, or any of our affiliates is either a party adverse to us or any of our subsidiaries or has a material interest adverse to us or any of our subsidiaries.
GENERAL DEVELOPMENT OF THE BUSINESS – 3 YEAR SUMMARY
Acquisition of Trillium Privateco
During 2013, we refocused our product pipeline through the strategic acquisition of Trillium Privateco, a private biopharmaceutical company specializing in immune regulation and the development of cancer therapeutics, which included the SIRPαFc and CD200 preclinical cancer programs. On April 9, 2013, we completed a merger with Trillium Privateco, to access its SIRPαFc immuno-oncology program. Prior to this merger, we were focused on regenerative stem cell technologies. Following the merger, in July 2013, we ceased all activities associated with our regenerative neurology programs and abandoned all related intellectual property filings.
As consideration for the merger, we paid $1,200,000 in cash and issued 92,639 common shares and 110,000 units. Each unit consisted of one common share and 30 common share purchase warrants, with each 30 common share purchase warrants allowing their holder to acquire one additional common share at an exercise price of $12.00 until March 15, 2018. Cash used in the investment of $647,996 was determined by subtracting cash acquired of $552,004 from cash consideration of $1,200,000. Post-closing, Trillium Privateco shareholders held approximately 16% of the issued and outstanding common shares, and Trillium Privateco became our wholly-owned subsidiary.
Trillium Privateco’s SIRPαFc program originated from leading researchers in the field, including Drs. John Dick and Jean Wang of the UHN and Dr. Jayne Danska of HSC. Exclusive rights to SIRPαFc have been licensed from UHN and HSC pursuant to a license agreement amended and restated as of June 1, 2012. The licensed intellectual property relates to methods and compounds used in the modulation of SIRPα-CD47 interaction for therapeutic cancer applications. The license agreement requires us to use commercially reasonable efforts to commercialize the licensed technology. The license agreement will terminate on a country-by-country basis, in countries where a valid claim exists, when the last valid claim expires in such country, or if no valid claim exists, when the last valid claim expires in the U.S.
The license includes commitments to pay an annual maintenance fee of $25,000, as well as payments on patent issuances, development milestone payments ranging from $100,000 to $300,000 on the initiation of phase I, II and III clinical trials, and payments on the achievement of certain regulatory milestones as well as low single digit royalties on commercial sales. The payments due on submission of the first biologics license application, or BLA, and receipt of a first regulatory approval in the U.S. are $1,000,000 for each milestone. The aggregate milestones payable on their first achievement under the agreement in the major markets of the U.S., Europe and Asia combined are $5,660,000. We are also required to pay 20% of any sublicensing revenues to the licensors on the first $50 million of sublicensing revenues received and 15% thereafter.
20
Share capital issued – for the year ended December 31, 2014
On November 14, 2014, we consolidated our outstanding common shares issuing one post-consolidated share for each 30 pre-consolidated shares. All references in this AIF to the number of common shares, stock options and deferred share units, or DSUs, refer to the post-consolidated amounts.
In the year ended December 31, 2014, 2,596,251 warrants were exercised for 86,540 common shares and for proceeds of $946,813 and 2,614 stock options were exercised for proceeds of $19,600. Also, 909,091 warrants issued in March 2011 expired unexercised.
During the year ended December 31, 2014, 8,390,476 Series I First Preferred Shares were converted into 279,682 common shares.
Share capital issued – for the year ended December 31, 2013
We consolidated our outstanding common shares issuing one post-consolidated share for each 10 pre-consolidated shares and the common shares began trading on a post-consolidated basis on February 6, 2013.
In March 2013, we completed an offering pursuant to a base shelf prospectus and prospectus supplement and in the U.S. pursuant to a private placement memorandum for a total of 424,500 units at a price of $7.50 per unit, for aggregate gross proceeds of $3,185,080 ($2,615,240 net of issuance costs). Each unit consisted of one common share and 30 common share purchase warrants. Each 30 warrants entitle the holder to purchase one common share at a price of $12.00 per share at any time prior to expiry on March 15, 2018 (12,315,000 warrants) and March 27, 2018 (420,000 warrants). In connection with the financing, we issued 814,051 compensation warrants having an aggregate fair value of $48,843 estimated using the Black-Scholes option pricing model. Each 30 compensation warrants entitle the holder to acquire one common share at an exercise price of $7.50 per share prior to expiry on March 16, 2015.
The allocation of the $7.50 common share unit issue price to the common shares and unit warrants was based on the relative fair values of the common shares and the warrants. The fair value of the warrants was determined using the Black-Scholes option pricing model. The common shares were allocated a price of $6.00 per share and the warrants were allocated a price of $1.50 per 30 warrants. The costs of the issue were allocated on a pro rata basis to the common shares and warrants. Accordingly, $2,092,192 was allocated to common shares and $523,048 to warrants, net of issuance costs. Assumptions used to determine the value of the unit warrants were: dividend yield of 0%; risk-free interest rate of 1.1%; expected volatility of 64%; and average expected life of 3 years. Assumptions used to determine the value of the compensation warrants were: dividend yield of 0%; risk-free interest rate of 1.0%; expected volatility of 80%; and average expected life of 2 years.
On April 9, 2013, we issued 202,639 common shares and 3,300,000 common share purchase warrants for partial consideration for the acquisition of Trillium Privateco.
On April 16, 2013, we issued 167,619 common shares and 1,600,000 common share purchase warrants as consideration for the acquisition of certain rights related to tigecycline from UHN.
On December 13, 2013, we completed a private placement of 2,641,590 common share units (each common share unit consisting of one common share and 22.5 common share purchase warrants) at a price of $6.30 per unit and 77,895,165 preferred share units (each preferred share unit consisting of one Series I First Preferred Share and three-quarters of a common share purchase warrant) at a price of $0.21 per unit for gross proceeds of $32,866,025 ($30,713,841 net of issuance costs). Each 30 warrants entitle the holder to purchase one common share at a price of $8.40 at any time prior to expiry on December 13, 2018. In connection with the financing, we issued 5,014,839 compensation warrants having an aggregate fair value of $651,929 estimated using the Black-Scholes option pricing model. Each 30 compensation warrants entitle the holder to acquire one common share at an exercise price of $6.30 per share prior to expiry on December 13, 2015.
21
The allocation of the $6.30 unit issue price to the common shares, 30 Series I First Preferred Shares and 22.5 unit warrants was based on the relative fair values of the common shares, Series I First Preferred Shares and the warrants. The fair value of the warrants was determined using the Black-Scholes option pricing model. The common shares were allocated a price of $4.80 per share, the Series I First Preferred Shares were allocated a price of $4.80 per 30 shares, and the warrants were allocated a price of $1.50 per each 22.5 warrants. The costs of the issue were allocated on a pro rata basis. Accordingly, $11,488,602 was allocated to common shares, $11,292,525 to the Series I First Preferred Shares and $7,932,714 to warrants, net of issuance costs. Assumptions used to determine the value of the unit warrants were: dividend yield of 0%; risk-free interest rate of 1.2%; expected volatility of 70%; and average expected life of 3 years. Assumptions used to determine the value of the compensation warrants were: dividend yield of 0%; risk-free interest rate of 1.1%; expected volatility of 66%; and average expected life of 2 years.
All securities issued under the offering (including the compensation warrants) are subject to statutory resale restrictions.
In the December 2013 private placement, subscribers who purchased preferred share units and certain subscribers who purchased common share units were subject to restrictions on the conversion and exercise of securities of ours convertible into common shares. Such subscribers cannot convert or exercise securities of ours convertible or exercisable into common shares if, after giving effect to the exercise of conversion, the subscriber and its joint actors would have beneficial ownership or direction or control over common shares in excess of 4.99% of the then outstanding common shares. This limit may be increased at the option of the subscriber on 61 days prior written notice: (i) up to 9.99%, (ii) up to 19.99%, subject to stock exchange clearance of a personal information form submitted by the subscriber, and (iii) above 19.99%, subject to stock exchange approval and shareholder approval.
Subject to receipt of any required regulatory approvals, subscribers who purchased a minimum of 10% of the securities sold under the offering have been given rights to purchase securities of ours in future financings to enable each such subscriber to maintain its percentage holding in us for so long as the subscriber holds at least 10% of the outstanding common shares on a fully-diluted basis.
Share capital issued – for the year ended December 31, 2012
There were no common or preferred shares issued in the year ended December 31, 2012.
Tigecycline License
On April 16, 2013, we signed an agreement with UHN to gain rights to intellectual property related to the use of tigecycline for the treatment of leukemia which included a UHN sponsored, open label phase I multicenter dose-escalation tigecycline trial in patients with relapsed or refractory AML. The initial consideration for the UHN license of $1,085,714 was satisfied by the issuance of 167,619 common shares and 1,600,000 common share purchase warrants, with each 30 warrants allowing their holder to acquire one additional common share at an exercise price of $12.00 until March 15, 2018. Additional consideration under the UHN license included an annual license maintenance fee and future development milestones. The fair value of the common shares issued was based on the closing price of our common shares of $6.00 on April 16, 2013 and $7.50 per unit based on the fair value of the common share and common share purchase warrant components as of the date of acquisition.
Since acquiring these rights, we monitored the results of the phase I trial and assessed alternate development strategies for the technology. In August 2014, we completed an evaluation of this program with respect to its scientific merit, commercial potential, strength of intellectual property, as well as its overall fit with our current focus and expertise. We concluded that we should focus our business plan on expanding the SIRPαFc program, rather than continue development of tigecycline, and returned the licensed rights to UHN and recorded an impairment loss of $429,763 in the second quarter of 2014.
Capital Markets
We were listed on the TSX Venture Exchange, or TSXV, until April 22, 2014 when we migrated to the Toronto Stock Exchange, or TSX. We traded under the symbol “SSS” until June 6, 2014 when the symbol was changed to “TR”. We were listed on the OTCQX International under the symbol “SCTPF” from May 20, 2013 until we began trading on the NASDAQ Capital Market under the symbol “TRIL” on December 19, 2014.
22
Capital Expenditures
Prior to the April 2013 merger with Trillium Privateco, we incurred minimal capital expenditures. Capital expenditures are required mainly for laboratory equipment, office equipment, computers and leasehold improvements. Capital expenditures in the years ended December 31, 2014 and 2013 are set out in the following table.
| | | Year ended December 31, | |
| | | 2014 | | | 2013 | |
| Capital expenditures | $ | 173,603 $ | | | 34,017 | |
Trend Information
Historical patterns of expenditures cannot be taken as an indication of future expenditures. The amount and timing of expenditures and therefore liquidity and capital resources vary substantially from period to period depending on the number of research and development programs being undertaken at any one time, the stage of the development programs, the timing of significant expenditures for manufacturing, toxicology and pharmacology studies and clinical trials, and the availability of funding from investors and prospective commercial partners.
Expenses for the three months ended March 31, 2013 were lower due to low development activity levels while we sought new products for development. Subsequent to the April 9, 2013 Trillium Privateco merger, expenses increased significantly as we acquired twelve personnel and an approximately 10,000 square foot leased laboratory and office facility, and thereafter licensed additional technology assets from UHN. In December 2013, we completed a private placement raising gross proceeds of $33 million to advance our SIRPαFc program through phase I clinical trials. In 2014, we advanced the manufacturing of our products. Management expects 2015 expenditures will increase from 2014 levels as we expect to advance our SIRPαFc program through IND-enabling studies, complete manufacturing for and initialize the phase I clinical trial, hire additional personnel, and carry out an expanded preclinical program that includes additional cancer indications and combination studies.
RISK FACTORS
An investment in our common shares involves a high degree of risk and should be considered speculative. An investment in our common shares should only be undertaken by those persons who can afford the total loss of their investment. You should carefully consider the risks and uncertainties described below, as well as other information contained in this AIF. The risks and uncertainties below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we believe to be immaterial may also adversely affect our business. If any of the following risks occur, our business, financial condition and results of operations could be seriously harmed and you could lose all or part of your investment. Further, if we fail to meet the expectations of the public market in any given period, the market price of our common shares could decline. We operate in a highly competitive environment that involves significant risks and uncertainties, some of which are outside of our control.
Risks Related to our Business and our Industry
We expect to incur future losses and we may never become profitable.
We have incurred losses of $12.9 million, $4.3 million and $1.1 million during 2014, 2013 and 2012, respectively, and expect to incur an operating loss in 2015. We have an accumulated deficit since inception through December 31, 2014 of $50.6 million. We believe that operating losses will continue in and beyond 2015 because we are planning to incur significant costs associated with the preclinical and clinical development of SIRPαFc. Our net losses have had and will continue to have an adverse effect on, among other things, our shareholders’ equity, total assets and working capital. We expect that losses will fluctuate from quarter to quarter and year to year, and that such fluctuations may be substantial. We cannot predict when we will become profitable, if at all.
23
We currently have no product revenue and will not be able to maintain our operations and research and development without additional funding.
To date, we have generated no product revenue and cannot predict when and if we will generate product revenue. Our ability to generate product revenue and ultimately become profitable depends upon our ability, alone or with partners, to successfully develop our product candidates, obtain regulatory approval and commercialize products, including any of our current product candidates, or other product candidates that we may develop, in-license or acquire in the future. We do not anticipate generating revenue from the sale of products for the foreseeable future. We expect our research and development expenses to increase substantially in connection with our ongoing activities, particularly as we advance our product candidates into clinical trials.
Our prospects depend on the success of our product candidates which are at early stages of development, and we may not generate revenue for several years, if at all, from these products.
Given the early stage of our product development, we can make no assurance that our research and development programs will result in regulatory approval or commercially viable products. To achieve profitable operations, we, alone or with others, must successfully develop, gain regulatory approval and market our future products. We currently have no products that have been approved by the U.S. Food and Drug Administration, or FDA, Health Canada, or HC, or any similar regulatory authority. To obtain regulatory approvals for our product candidates being developed and to achieve commercial success, clinical trials must demonstrate that the product candidates are safe for human use and that they demonstrate efficacy. While SIRPαFc has begun preclinical studies to support an IND application, we have not yet completed cGMP manufacturing, formal toxicology studies, a phase I clinical trial or subsequent required clinical trials. Our CD200 program is ready to enter formal IND-enabling preclinical studies, but we do not plan to initiate these studies until we have a development partner to share in the cost and responsibility of the program.
Many product candidates never reach the stage of clinical testing and even those that do have only a small chance of successfully completing clinical development and gaining regulatory approval. Product candidates may fail for a number of reasons, including, but not limited to, being unsafe for human use or due to the failure to provide therapeutic benefits equal to or better than the standard of treatment at the time of testing. Unsatisfactory results obtained from a particular study relating to a research and development program may cause us or our collaborators to abandon commitments to that program. Positive results of early preclinical research may not be indicative of the results that will be obtained in later stages of preclinical or clinical research. Similarly, positive results from early-stage clinical trials may not be indicative of favorable outcomes in later-stage clinical trials. We can make no assurance that any future studies, if undertaken, will yield favorable results. We can make no assurances that toxicology studies in non-human primates will yield results that will allow us to proceed with clinical trials in humans.
The early stage of our product development makes it particularly uncertain whether any of our product development efforts will prove to be successful and meet applicable regulatory requirements, and whether any of our product candidates will receive the requisite regulatory approvals, be capable of being manufactured at a reasonable cost or be successfully marketed. If successful in developing our current and future product candidates into approved products, we will still experience many potential obstacles such as the need to develop or obtain manufacturing, marketing and distribution capabilities. If we are unable to successfully commercialize any of our products, our financial condition and results of operations may be materially and adversely affected.
24
We rely and will continue to rely on third parties to plan, conduct and monitor our preclinical studies and clinical trials, and their failure to perform as required could cause substantial harm to our business.
We rely and will continue to rely on third parties to conduct a significant portion of our preclinical and clinical development activities. Preclinical activities include in vivo studies providing access to specific disease models, pharmacology and toxicology studies, and assay development. Clinical development activities include trial design, regulatory submissions, clinical patient recruitment, clinical trial monitoring, clinical data management and analysis, safety monitoring and project management. If there is any dispute or disruption in our relationship with third parties, or if they are unable to provide quality services in a timely manner and at a feasible cost, our active development programs will face delays. Further, if any of these third parties fails to perform as we expect or if their work fails to meet regulatory requirements, our testing could be delayed, cancelled or rendered ineffective.
We rely on contract manufacturers over whom we have limited control. If we are subject to quality, cost or delivery issues with the preclinical and clinical grade materials supplied by contract manufacturers, our business operations could suffer significant harm.
We have limited manufacturing experience and rely on contract manufacturing organizations, or CMOs, to manufacture our product candidates for larger preclinical studies and clinical trials. We produce small quantities of our product candidates at bench scale in our laboratory facilities for use in smaller preclinical studies. We rely on CMOs for manufacturing, filling, packaging, storing and shipping of drug product in compliance with cGMP regulations applicable to our products. The FDA ensures the quality of drug products by carefully monitoring drug manufacturers’ compliance with cGMP regulations. The cGMP regulations for drugs contain minimum requirements for the methods, facilities and controls used in manufacturing, processing and packing of a drug product.
We have contracted with Catalent Pharma Solutions, LLC, or Catalent, for the manufacture of the SIRPαFc protein needed for our pre-IND toxicology and pharmacology studies and to supply drug product for our phase I clinical trial. The manufacture of recombinant proteins uses well established processes including a protein expression system. Catalent is producing SIRPαFc using their proprietary GPEx® expression system. We believe that Catalent has the capacity, the systems and the experience to supply SIRPαFc for our planned phase I clinical trial and we may consider using Catalent for manufacturing for later clinical trials. However, since the Catalent manufacturing facility where SIRPαFc is being produced was only recently established, it has not been inspected by the FDA. Any manufacturing failures or delays or compliance issues could cause delays in the completion of our preclinical studies of SIRPαFc, and delays in the submission or approval of our IND and the initiation of our phase I clinical trial.
There can be no assurances that CMOs will be able to meet our timetable and requirements. We have not contracted with alternate suppliers in the event Catalent is unable to scale up production, or if Catalent otherwise experiences any other significant problems. If we are unable to arrange for alternative third-party manufacturing sources on commercially reasonable terms or in a timely manner, we may be delayed in the development of our product candidates. Further, contract manufacturers must operate in compliance with cGMP and failure to do so could result in, among other things, the disruption of product supplies. Our dependence upon third parties for the manufacture of our products may adversely affect our profit margins and our ability to develop and deliver products on a timely and competitive basis.
If clinical trials of our product candidates fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or do not otherwise produce positive results, we would incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates.
Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must conduct preclinical studies in animals and extensive clinical trials in humans to demonstrate the safety and efficacy of the product candidates. Clinical testing is expensive and difficult to design and implement, can take many years to complete and has uncertain outcomes. The outcome of preclinical studies and early clinical trials may not predict the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in advanced clinical trials due to lack of efficacy or unacceptable safety profiles, notwithstanding promising results in earlier trials. We do not know whether the clinical trials we may conduct will demonstrate adequate efficacy and safety to result in regulatory approval to market any of our product candidates in any jurisdiction. A product candidate may fail for safety or efficacy reasons at any stage of the testing process. A major risk we face is the possibility that none of our product candidates under development will successfully gain market approval from the FDA or other regulatory authorities, resulting in us being unable to derive any commercial revenue from them after investing significant amounts of capital in multiple stages of preclinical and clinical testing.
25
If we experience delays in clinical testing, we will be delayed in commercializing our product candidates, and our business may be substantially harmed.
We cannot predict whether any clinical trials will begin as planned, will need to be restructured or will be completed on schedule, or at all. Our product development costs will increase if we experience delays in clinical testing. Significant clinical trial delays could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before us, which would impair our ability to successfully commercialize our product candidates and may harm our financial condition, results of operations and prospects. The commencement and completion of clinical trials for our products, including our planned SIRPαFc phase I clinical trial, may be delayed for a number of reasons, including delays related, but not limited, to:
| • | failure by regulatory authorities to grant permission to proceed or placing the clinical trial on hold; |
| • | patients failing to enroll or remain in our trials at the rate we expect; |
| • | suspension or termination of clinical trials by regulators for many reasons, including concerns about patient safety or failure of our contract manufacturers to comply with cGMP requirements; |
| • | any changes to our manufacturing process that may be necessary or desired; |
| • | delays or failure to obtain clinical supply from contract manufacturers of our products necessary to conduct clinical trials; |
| • | product candidates demonstrating a lack of safety or efficacy during clinical trials; |
| • | patients choosing an alternative treatment for the indications for which we are developing any of our product candidates or participating in competing clinical trials; |
| • | patients failing to complete clinical trials due to dissatisfaction with the treatment, side effects or other reasons; |
| • | reports of clinical testing on similar technologies and products raising safety and/or efficacy concerns; |
| • | competing clinical trials and scheduling conflicts with participating clinicians; |
| • | clinical investigators not performing our clinical trials on their anticipated schedule, dropping out of a trial, or employing methods not consistent with the clinical trial protocol, regulatory requirements or other third parties not performing data collection and analysis in a timely or accurate manner; |
| • | failure of our contract research organizations, or CROs, to satisfy their contractual duties or meet expected deadlines; |
| • | inspections of clinical trial sites by regulatory authorities or Institutional Review Boards, or IRBs, or ethics committees finding regulatory violations that require us to undertake corrective action, resulting in suspension or termination of one or more sites or the imposition of a clinical hold on the entire study; |
| • | one or more IRBs or ethics committees rejecting, suspending or terminating the study at an investigational site, precluding enrollment of additional subjects, or withdrawing its approval of the trial; or |
| • | failure to reach agreement on acceptable terms with prospective clinical trial sites. |
Our product development costs will increase if we experience delays in testing or approval or if we need to perform more or larger clinical trials than planned. Additionally, changes in regulatory requirements and policies may occur, and we may need to amend study protocols to reflect these changes. Amendments may require us to resubmit our study protocols to regulatory authorities or IRBs or ethics committees for re-examination, which may impact the cost, timing or successful completion of that trial. Delays or increased product development costs may have a material adverse effect on our business, financial condition and prospects.
If we have difficulty enrolling patients in clinical trials, the completion of the trials may be delayed or cancelled.
As our product candidates advance from preclinical testing to clinical testing, and then through progressively larger and more complex clinical trials, we will need to enroll an increasing number of patients that meet our eligibility criteria. There is significant competition for recruiting cancer patients in clinical trials, and we may be unable to enroll the patients we need to complete clinical trials on a timely basis or at all. The factors that affect our ability to enroll patients are largely uncontrollable and include, but are not limited to, the following:
26
| • | size and nature of the patient population; |
| • | eligibility and exclusion criteria for the trial; |
| • | design of the study protocol; |
| • | competition with other companies for clinical sites or patients; |
| • | the perceived risks and benefits of the product candidate under study; |
| • | the patient referral practices of physicians; and |
| • | the number, availability, location and accessibility of clinical trial sites. |
If we are unable to successfully develop companion diagnostics for our therapeutic product candidates, or experience significant delays in doing so, we may not achieve marketing approval or realize the full commercial potential of our therapeutic product candidates.
We plan to develop companion diagnostics for our therapeutic product candidates. We expect that, at least in some cases, regulatory authorities may require the development and regulatory approval of a companion diagnostic as a condition to approving our therapeutic product candidates. We have limited experience and capabilities in developing or commercializing diagnostics and plan to rely in large part on third parties to perform these functions. We do not currently have any agreement in place with any third party to develop or commercialize companion diagnostics for any of our therapeutic product candidates.
Companion diagnostics are subject to regulation by the FDA, HC and comparable foreign regulatory authorities as medical devices and may require separate regulatory approval or clearance prior to commercialization. If we, or any third parties that we engage to assist us, are unable to successfully develop companion diagnostics for our therapeutic product candidates, or experience delays in doing so, our business may be substantially harmed.
Regulatory approval processes are lengthy, expensive and inherently unpredictable. Our inability to obtain regulatory approval for our product candidates would substantially harm our business.
Our development and commercialization activities and product candidates are significantly regulated by a number of governmental entities, including the FDA, HC and by comparable authorities in other countries. Regulatory approvals are required prior to each clinical trial and we may fail to obtain the necessary approvals to commence or continue clinical testing. We must comply with regulations concerning the manufacture, testing, safety, effectiveness, labeling, documentation, advertising and sale of products and product candidates and ultimately must obtain regulatory approval before we can commercialize the product candidate. The time required to obtain approval by such regulatory authorities is unpredictable but typically takes many years following the commencement of preclinical studies and clinical trials. Any analysis of data from clinical activities we perform is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. Even if we believe results from our clinical trials are favorable to support the marketing of our product candidates, the FDA or other regulatory authorities may disagree. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate and it is possible that none of our existing product candidates or any future product candidates will ever obtain regulatory approval.
We could fail to receive regulatory approval for our product candidates for many reasons, including, but not limited to:
| • | disagreement with the design or implementation of our clinical trials; |
| • | failure to demonstrate that a product candidate is safe and effective for its proposed indication; |
| • | failure of clinical trials to meet the level of statistical significance required for approval; |
| • | failure to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| • | disagreement with our interpretation of data from preclinical studies or clinical trials; |
| • | the insufficiency of data collected from clinical trials of our product candidates to support the submission and filing of a biologics license application, or BLA, or other submission or to obtain regulatory approval; |
| • | deficiencies in the manufacturing processes or the failure of facilities of CMOs with whom we contract for clinical and commercial supplies to pass a pre-approval inspection; or |
27
| • | changes in the approval policies or regulations that render our preclinical and clinical data insufficient for approval. |
A regulatory authority may require more information, including additional preclinical or clinical data to support approval, which may delay or prevent approval and our commercialization plans, or we may decide to abandon the development program. If we were to obtain approval, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. Moreover, depending on any safety issues associated with our product candidates that garner approval, the FDA may impose a risk evaluation and mitigation strategy, thereby imposing certain restrictions on the sale and marketability of such products.
We will require additional capital to finance our operations, which may not be available to us on acceptable terms, or at all. As a result, we may not complete the development and commercialization of our product candidates or develop new product candidates.
As a research and development company, our operations have consumed substantial amounts of cash since inception. We expect to spend substantial funds to continue the research, development and testing of our product candidates that are in the preclinical testing stages of development, and to prepare to commercialize products subject to FDA approval in the U.S. and similar approvals in other jurisdictions. We will also require significant additional funds if we expand the scope of our current clinical plans for the SIRPαFc program or if we were to acquire any new assets and advance those towards clinical testing. Therefore, for the foreseeable future, we will have to fund all of our operations and development expenditures from cash on hand, equity or debt financings, through collaborations with other biotechnology or pharmaceutical companies or through financings from other sources. We expect that our existing cash at December 31, 2014 of $26,165,056 will enable us to fund our current operating plan requirements into the fourth quarter of 2016. Additional financing will be required to meet our long term liquidity needs. If we do not succeed in raising additional funds on acceptable terms, we might not be able to complete planned preclinical studies and clinical trials or pursue and obtain approval of any product candidates from the FDA and other regulatory authorities. It is possible that future financing will not be available or, if available, may not be on favorable terms. The availability of financing will be affected by the achievement of our corporate goals, the results of scientific and clinical research, the ability to obtain regulatory approvals, the state of the capital markets generally and with particular reference to drug development companies, the status of strategic alliance agreements and other relevant commercial considerations. If adequate funding is not available, we may be required to delay, reduce or eliminate one or more of our product development programs, or obtain funds through corporate partners or others who may require us to relinquish significant rights to product candidates or obtain funds on less favorable terms than we would otherwise accept. To the extent that external sources of capital become limited or unavailable or available on onerous terms, our intangible assets and our ability to continue our clinical development plans may become impaired, and our assets, liabilities, business, financial condition and results of operations may be materially or adversely affected.
We face competition from other biotechnology and pharmaceutical companies and our financial condition and operations will suffer if we fail to effectively compete.
The biotechnology and pharmaceutical industries are intensely competitive and subject to rapid and significant technological change. Our competitors include large, well-established pharmaceutical companies, biotechnology companies, and academic and research institutions developing cancer therapeutics for the same indications we are targeting and competitors with existing marketed therapies. Many other companies are developing or commercializing therapies to treat the same diseases or indications for which our product candidates may be useful. Although there are no approved therapies that specifically target the CD47 pathway, some competitors use therapeutic approaches that may compete directly with our product candidates. For example, SIRPαFc is in direct competition with CD47 blocking antibodies from Stanford University, Celgene Corporation and Novimmune SA.
Many of our competitors have substantially greater financial, technical and human resources than we do and have significantly greater experience than us in conducting preclinical testing and human clinical trials of product candidates, scaling up manufacturing operations and obtaining regulatory approvals of products. Accordingly, our competitors may succeed in obtaining regulatory approval for products more rapidly than we do. Our ability to compete successfully will largely depend on:
28
| • | the efficacy and safety profile of our product candidates relative to marketed products and other product candidates in development; |
| • | our ability to develop and maintain a competitive position in the product categories and technologies on which we focus; |
| • | the time it takes for our product candidates to complete clinical development and receive marketing approval; |
| • | our ability to obtain required regulatory approvals; |
| • | our ability to commercialize any of our product candidates that receive regulatory approval; |
| • | our ability to establish, maintain and protect intellectual property rights related to our product candidates; and |
| • | acceptance of any of our product candidates that receive regulatory approval by physicians and other healthcare providers and payers. |
If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition and operations will substantially suffer.
We heavily rely on the capabilities and experience of our key executives and scientists and the loss of any of them could affect our ability to develop our products.
The loss of Dr. Niclas Stiernholm, our President and Chief Executive Officer, or other key members of our staff, including Dr. Robert Uger, our Chief Scientific Officer, James Parsons, our Chief Financial Officer, or Dr. Penka Petrova, our Vice President, Drug Development, could harm us. We have employment agreements with Drs. Stiernholm, Uger and Petrova and Mr. Parsons, although such employment agreements do not guarantee their retention. We also depend on our scientific and clinical collaborators and advisors, all of whom have outside commitments that may limit their availability to us. In addition, we believe that our future success will depend in large part upon our ability to attract and retain highly skilled scientific, managerial, medical, clinical and regulatory personnel, particularly as we expand our activities and seek regulatory approvals for clinical trials. We routinely enter into consulting agreements with our scientific and clinical collaborators and advisors, key opinion leaders and academic partners in the ordinary course of our business. We also enter into contractual agreements with physicians and institutions who will recruit patients into our clinical trials on our behalf in the ordinary course of our business. Notwithstanding these arrangements, we face significant competition for these types of personnel from other companies, research and academic institutions, government entities and other organizations. We cannot predict our success in hiring or retaining the personnel we require for continued growth. The loss of the services of any of our executive officers or other key personnel could potentially harm our business, operating results or financial condition.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include failures to comply with FDA regulations, provide accurate information to the FDA, comply with manufacturing standards we have established, comply with federal and state health-care fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a substantial impact on our business and results of operations, including the imposition of substantial fines or other sanctions.
29
We may expand our business through the acquisition of companies or businesses or by entering into collaborations or by in-licensing product candidates, each of which could disrupt our business and harm our financial condition.
We have in the past and may in the future seek to expand our pipeline and capabilities by acquiring one or more companies or businesses, entering into collaborations or in-licensing one or more product candidates. For example, in April 2013, we acquired Trillium Privateco to acquire novel cancer therapeutics, an experienced scientific management team and internal development capabilities. We licensed intellectual property relating to methods and compounds for the modulation of the SIRPα-CD47 interaction for therapeutic cancer applications from University Health Network, or UHN, and Hospital for Sick Children, or HSC. In April 2013, we also in-licensed tigecycline which was subsequently returned to the UHN in 2014. Acquisitions, collaborations and in-licenses involve numerous risks, including, but not limited to:
| • | substantial cash expenditures; |
| • | technology development risks; |
| • | potentially dilutive issuances of equity securities; |
| • | incurrence of debt and contingent liabilities, some of which may be difficult or impossible to identify at the time of acquisition; |
| • | difficulties in assimilating the operations of the acquired companies; |
| • | potential disputes regarding contingent consideration; |
| • | diverting our management’s attention away from other business concerns; |
| • | entering markets in which we have limited or no direct experience; and |
| • | potential loss of our key employees or key employees of the acquired companies or businesses. |
We have experience in making acquisitions, entering collaborations and in-licensing product candidates, however, we cannot provide assurance that any acquisition, collaboration or in-license will result in short-term or long-term benefits to us. We may incorrectly judge the value or worth of an acquired company or business or in-licensed product candidate. In addition, our future success would depend in part on our ability to manage the rapid growth associated with some of these acquisitions, collaborations and in-licenses. We cannot assure you that we would be able to successfully combine our business with that of acquired businesses, manage a collaboration or integrate in-licensed product candidates. Furthermore, the development or expansion of our business may require a substantial capital investment by us.
Negative results from clinical trials or studies of others and adverse safety events involving the targets of our products may have an adverse impact on our future commercialization efforts.
From time to time, studies or clinical trials on various aspects of biopharmaceutical products are conducted by academic researchers, competitors or others. The results of these studies or trials, when published, may have a significant effect on the market for the biopharmaceutical product that is the subject of the study. The publication of negative results of studies or clinical trials or adverse safety events related to our product candidates, or the therapeutic areas in which our product candidates compete, could adversely affect our share price and our ability to finance future development of our product candidates, and our business and financial results could be materially and adversely affected.
We face the risk of product liability claims, which could exceed our insurance coverage and produce recalls, each of which could deplete our cash resources.
We are exposed to the risk of product liability claims alleging that use of our product candidates caused an injury or harm. These claims can arise at any point in the development, testing, manufacture, marketing or sale of our product candidates and may be made directly by patients involved in clinical trials of our product candidates, by consumers or healthcare providers or by individuals, organizations or companies selling our products. Product liability claims can be expensive to defend, even if the product or product candidate did not actually cause the alleged injury or harm.
30
Insurance covering product liability claims becomes increasingly expensive as a product candidate moves through the development pipeline to commercialization. We currently maintain clinical trial liability insurance coverage of $10 million. However, there can be no assurance that such insurance coverage is or will continue to be adequate or available to us at a cost acceptable to us or at all. We may choose or find it necessary under our collaborative agreements to increase our insurance coverage in the future. We may not be able to secure greater or broader product liability insurance coverage on acceptable terms or at reasonable costs when needed. Any liability for damages resulting from a product liability claim could exceed the amount of our coverage, require us to pay a substantial monetary award from our own cash resources and have a material adverse effect on our business, financial condition and results of operations. Moreover, a product recall, if required, could generate substantial negative publicity about our products and business, inhibit or prevent commercialization of other products and product candidates or negatively impact existing or future collaborations.
In addition, some of our licensing and other agreements with third parties require or might require us to maintain product liability insurance. If we cannot maintain acceptable amounts of coverage on commercially reasonable terms in accordance with the terms set forth in these agreements, the corresponding agreements would be subject to termination, which could have a material adverse impact on our operations.
We may not achieve our publicly announced milestones according to schedule, or at all.
From time to time, we may announce the timing of certain events we expect to occur, such as the anticipated timing of results from our clinical trials. These statements are forward-looking and are based on the best estimates of management at the time relating to the occurrence of such events. However, the actual timing of such events may differ from what has been publicly disclosed. The timing of events such as initiation or completion of a clinical trial, filing of an application to obtain regulatory approval, or announcement of additional clinical trials for a product candidate may ultimately vary from what is publicly disclosed. For example, we cannot provide assurances that the SIRPαFc phase I clinical trial will be initiated on schedule, if at all, or that we will make regulatory submissions or receive regulatory approvals as planned. These variations in timing may occur as a result of different events, including the nature of the results obtained during a clinical trial or during a research phase, problems with a CMO or a CRO or any other event having the effect of delaying the publicly announced timeline. We undertake no obligation to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as otherwise required by law. Any variation in the timing of previously announced milestones could have a material adverse effect on our business plan, financial condition or operating results and the trading price of common shares.
We are exposed to the financial risk related to the fluctuation of foreign exchange rates and the degrees of volatility of those rates.
We may be adversely affected by foreign currency fluctuations. To date, we have been primarily funded through issuances of equity, proceeds from the exercise of warrants and stock options and from interest income on funds available for investment, which are all denominated in Canadian dollars. A growing portion of our expenditures are in U.S. dollars, however, and we are, therefore, subject to foreign currency fluctuations which may, from time to time, impact our financial position and results of operations.
Risks Related to Intellectual Property
If we are unable to adequately protect and enforce our intellectual property, our competitors may take advantage of our development efforts or acquired technology and compromise our prospects of marketing and selling our key products.
We own or hold licenses to a number of U.S.-issued patents and U.S. and Canadian pending patent applications, as well as foreign patents and foreign counterparts. In the U.S., we have our own rights to five key issued patents and two pending key patent applications. In addition, we have numerous patent applications pending in Europe, Australia, China, India, and Japan. Our success will depend in part upon our ability to protect our intellectual property and proprietary technologies and upon the nature of the intellectual property protection we receive. For example, some of our patent portfolio covers primarily methods of use but not compositions of matter. The ability to compete effectively and to achieve partnerships will depend on our ability to develop and maintain proprietary aspects of our technology and to operate without infringing on the proprietary rights of others. The presence of such proprietary rights of others could severely limit our ability to develop and commercialize our products, to conduct our existing research and/or require financial resources to defend litigation, which may be in excess of our ability to raise such funds. There is no assurance that our pending patent applications or those that we intend to acquire will be approved in a form that will be sufficient to protect our proprietary technology and gain or keep any competitive advantage that we may have.
31
The patent positions of pharmaceutical companies can be highly uncertain and involve complex legal, scientific and factual questions for which important legal principles remain unresolved. Patents issued to us or our respective licensors may be challenged, invalidated or circumvented. To the extent our intellectual property, including licensed intellectual property, offers inadequate protection, or is found to be invalid or unenforceable, we are exposed to a greater risk of direct competition. If our intellectual property does not provide adequate protection against our competitors’ products, our competitive position could be adversely affected, as could our business, financial condition and results of operations. Both the patent application process and the process of managing patent disputes can be time consuming and expensive, and the laws of some foreign countries may not protect our intellectual property rights to the same extent as do the laws of Canada and the United States.
We will be able to protect our intellectual property from unauthorized use by third parties only to the extent that our proprietary technologies, key products and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets and provided we have the funds to enforce our rights, if necessary.
If we lose our licenses from third-party owners we may be unable to continue a substantial part of our business.
We are party to a number of licenses that give us rights to intellectual property that is necessary or useful for a substantial part of our business. Pursuant to our exclusive license agreement with UHN and HSC under which we license certain patent rights for our key products and their uses, we are required to use commercially reasonable efforts to commercialize products based on the licensed rights and pay certain royalties and sublicensing revenue to UHN and HSC. These licenses require that we pay development milestone payments, regulatory milestone payments, royalties on net sales and sublicensing revenues as well as annual maintenance fees.
We have also entered into agreements allowing us to manufacture SIRPαFc using Catalent’s proprietary GPEx® expression system. The consideration includes payments at the time we successfully reach a series of development and sales milestones. We may also enter into licenses in the future to access additional third-party intellectual property.
If we fail to pay annual maintenance fees, development and sales milestones, or it is determined that we did not use commercially reasonable efforts to commercialize licensed products, we could lose our licenses which could have a material adverse effect on our business and financial condition.
We may require additional third-party licenses to effectively develop and manufacture our key products and are currently unable to predict the availability or cost of such licenses.
A substantial number of patents have already been issued to other biotechnology and pharmaceutical companies. To the extent that valid third-party patent rights cover our products or services, we or our strategic collaborators would be required to seek licenses from the holders of these patents in order to manufacture, use or sell these products and services, and payments under them would reduce our profits from these products and services. We are currently unable to predict the extent to which we may wish or be required to acquire rights under such patents and the availability and cost of acquiring such rights, or whether a license to such patents will be available on acceptable terms or at all. There may be patents in the U.S. or in foreign countries or patents issued in the future that are unavailable to license on acceptable terms. Our inability to obtain such licenses may hinder or eliminate our ability to manufacture and market our products.
32
Changes in patent law and its interpretation could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other biotechnology and pharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves technological and legal complexity, and obtaining and enforcing biopharmaceutical patents is costly, time-consuming and inherently uncertain. The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our and our licensors’ or collaborators’ ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. Patent and Trademark Office, or USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our and our licensors’ or collaborators’ ability to obtain new patents or to enforce existing patents and patents we and our licensors or collaborators may obtain in the future.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our and our licensors’ or collaborators’ patent applications and the enforcement or defense of our or our licensors’ or collaborators’ issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The USPTO recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our or our licensors’ or collaborators’ patent applications and the enforcement or defense of our or our licensors’ or collaborators’ issued patents, all of which could have a material adverse effect on our business and financial condition.
Litigation regarding patents, patent applications and other proprietary rights may be expensive, time consuming and cause delays in the development and manufacturing of our key products.
Our success will depend in part on our ability to operate without infringing the proprietary rights of third parties. The pharmaceutical industry is characterized by extensive patent litigation. Other parties may have, or obtain in the future, patents and allege that the use of our technologies infringes these patent claims or that we are employing their proprietary technology without authorization.
In addition, third parties may challenge or infringe upon our existing or future patents. Proceedings involving our patents or patent applications or those of others could result in adverse decisions regarding:
| • | the patentability of our inventions relating to our key products; and/or |
| • | the enforceability, validity or scope of protection offered by our patents relating to our key products. |
If we are unable to avoid infringing the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court. Regardless of the outcome, patent litigation is costly and time consuming. In some cases, we may not have sufficient resources to bring these actions to a successful conclusion. In addition, if we do not obtain a license, develop or obtain non-infringing technology, fail to defend an infringement action successfully or have infringed patents declared invalid, we may:
| • | incur substantial monetary damages; |
| • | encounter significant delays in bringing our key products to market; and/or |
33
| • | be precluded from participating in the manufacture, use or sale of our key products or methods of treatment requiring licenses. |
Even if we are successful in these proceedings, we may incur substantial costs and divert management time and attention in pursuing these proceedings, which could have a material adverse effect on us.
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them.
Because we rely on third parties to develop our products, we must share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements typically restrict the ability of our collaborators, advisors, employees and consultants to publish data potentially relating to our trade secrets. Our academic collaborators typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. In other cases, publication rights are controlled exclusively by us, although in some cases we may share these rights with other parties. We also conduct joint research and development programs which may require us to share trade secrets under the terms of research and development collaboration or similar agreements. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication. A competitor’s discovery of our trade secrets would impair our competitive position and could have a material adverse effect on our business and financial condition.
Risks Related to Our Common Shares
Our common share price has been volatile in recent years, and may continue to be volatile.
The market prices for securities of biopharmaceutical companies, including ours, have historically been volatile. In the year ended December 31, 2013, our common shares traded on the TSX Venture Exchange, or TSXV, at a high of $18.00 and a low of $4.20 per share. In the year ended December 31, 2014, on the TSXV/TSX our common shares traded at a high of $22.20 and a low of $6.30 per share. A number of factors could influence the volatility in the trading price of our common shares, including changes in the economy or in the financial markets, industry related developments, the results of product development and commercialization, changes in government regulations, and developments concerning proprietary rights, litigation and cash flow. Our quarterly losses may vary because of the timing of costs for manufacturing and initiating and completing preclinical and clinical trials. Also, the reporting of adverse safety events involving our products and public rumors about such events could cause our share price to decline or experience periods of volatility. Each of these factors could lead to increased volatility in the market price of our common shares. In addition, changes in the market prices of the securities of our competitors may also lead to fluctuations in the trading price of our common shares.
We have never paid dividends and do not expect to do so in the foreseeable future.
We have not declared or paid any cash dividends on our common or preferred shares to date. The payment of dividends in the future will be dependent on our earnings and financial condition in addition to such other factors as our board of directors considers appropriate. Unless and until we pay dividends, shareholders may not receive a return on their shares. There is no present intention by our board of directors to pay dividends on our shares.
34
Future sales or issuances of equity securities and the conversion of outstanding securities to common shares could decrease the value of the common shares, dilute investors’ voting power and reduce our earnings per share.
We may sell additional equity securities in future offerings, including through the sale of securities convertible into equity securities, to finance operations, acquisitions or projects, and issue additional common shares if outstanding warrants or stock options are exercised, or preferred shares are converted to common shares, which may result in dilution. See the information in the section of this AIF entitled “Description of Share Capital” for details of our outstanding securities convertible into common shares. On March 11, 2015, we filed a Form F-1 registration statement with the U.S. Securities and Exchange Commission with respect to a proposed public offering of up to US$50 million of our common shares. We filed a base shelf prospectus with securities commissions in Canada on May 26, 2014 that provides that we may sell under the prospectus from time to time over the following 25 months up to $50 million, in one or more offerings, of common shares, preferred shares, warrants to purchase common shares, or units comprising a combination of common shares, preferred shares and/or warrants. Subject to receipt of any required regulatory approvals, subscribers of the December 2013 private placement who purchased a minimum of 10% of the securities sold under the offering received rights to purchase our securities in future financings to enable each such shareholder to maintain their percentage holding in our common shares for so long as the subscriber holds at least 10% of the outstanding common shares on a fully-diluted basis. Shareholders who do not have this future financing participation right may be disadvantaged in participating in such financings.
Our board of directors has the authority to authorize certain offers and sales of additional securities without the vote of, or prior notice to, shareholders. Based on the need for additional capital to fund expected expenditures and growth, it is likely that we will issue additional securities to provide such capital. Such additional issuances may involve the issuance of a significant number of common shares at prices less than the current market price for our common shares.
Sales of substantial amounts of our securities, or the availability of such securities for sale, as well as the issuance of substantial amounts of our common shares upon conversion of outstanding convertible equity securities, could adversely affect the prevailing market prices for our securities and dilute investors’ earnings per share. A decline in the market prices of our securities could impair our ability to raise additional capital through the sale of securities should we desire to do so.
We are likely a “passive foreign investment company”, which may have adverse U.S. federal income tax consequences for U.S. shareholders.
U.S. investors should be aware that we believe we were classified as a passive foreign investment company, or PFIC, during the tax year ended December 31, 2014, and based on current business plans and financial expectations, we expect that we will be a PFIC for the current tax year and may be a PFIC in future tax years. If we are a PFIC for any year during a U.S. shareholder’s holding period of our common shares, then such U.S. shareholder generally will be required to treat any gain realized upon a disposition of our common shares, or any so-called “excess distribution” received on our common shares, as ordinary income, and to pay an interest charge on a portion of such gain or distributions, unless the shareholder makes a timely and effective “qualified electing fund” election, or QEFElection, or a “mark-to-market” election with respect to our shares. A U.S. shareholder who makes a QEF Election generally must report on a current basis its share of our net capital gain and ordinary earnings for any year in which we are a PFIC, whether or not we distribute any amounts to our shareholders. A U.S. shareholder who makes the mark-to-market election generally must include as ordinary income each year the excess of the fair market value of the common shares over the shareholder’s adjusted tax basis therein. Each U.S. shareholder should consult its own tax advisors regarding the PFIC rules and the U.S. federal income tax consequences of the acquisition, ownership and disposition of our common shares.
It may be difficult for non-Canadian investors to obtain and enforce judgments against us because of our Canadian incorporation and presence.
We are a corporation existing under the laws of the Province of Ontario, Canada. Several of our directors and officers, and several of the experts are residents of Canada, and all or a substantial portion of their assets, and a substantial portion of our assets, are located outside the United States. Consequently, although we have appointed an agent for service of process in the United States, it may be difficult for holders of our securities who reside in the United States to effect service within the United States upon those directors and officers, and the experts who are not residents of the United States. It may also be difficult for holders of our securities who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers and experts under the United States federal securities laws. Investors should not assume that Canadian courts (i) would enforce judgments of United States courts obtained in actions against us or such directors, officers or experts predicated upon the civil liability provisions of the United States federal securities laws or the securities or “blue sky” laws of any state or jurisdiction of the United States or (ii) would enforce, in original actions, liabilities against us or such directors, officers or experts predicated upon the United States federal securities laws or any securities or “blue sky” laws of any state or jurisdiction of the United States. In addition, the protections afforded by Canadian securities laws may not be available to investors in the United States.
35
If there are substantial sales of our common shares, the market price of our common shares could decline.
Sales of substantial numbers of our common shares could cause a decline in the market price of our common shares. Any sales by existing shareholders or holders who exercise their warrants or stock options may have an adverse effect on our ability to raise capital and may adversely affect the market price of our common shares.
We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common shares less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier. We cannot predict if investors will find our common shares less attractive because we may rely on these exemptions. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and our share price may be more volatile.
Any failure to maintain an effective system of internal controls may result in material misstatements of our consolidated financial statements or cause us to fail to meet our reporting obligations or fail to prevent fraud; and in that case, our shareholders could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our common shares.
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud. If we fail to maintain an effective system of internal controls, we might not be able to report our financial results accurately or prevent fraud; and in that case, our shareholders could lose confidence in our financial reporting, which would harm our business and could negatively impact the price of our common shares. While we believe that we have sufficient personnel and review procedures to allow us maintain an effective system of internal controls, we cannot assure you that we will not experience potential material weaknesses in our internal control. Even if we conclude that our internal control over financial reporting provides reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with International Financial Reporting Standards, as issued by the International Accounting Standards Board, because of its inherent limitations, internal control over financial reporting may not prevent or detect fraud or misstatements. Failure to implement required new or improved controls, or difficulties encountered in their implementation, could harm our results of operations or cause us to fail to meet our future reporting obligations.
If we fail to timely achieve and maintain the adequacy of our internal control over financial reporting, we may not be able to produce reliable financial reports or help prevent fraud. Our failure to achieve and maintain effective internal control over financial reporting could prevent us from complying with our reporting obligations on a timely basis, which could result in the loss of investor confidence in the reliability of our consolidated financial statements, harm our business and negatively impact the trading price of our common shares.
36
As a foreign private issuer, we are not subject to certain United States securities law disclosure requirements that apply to a domestic United States issuer, which may limit the information which would be publicly available to our shareholders.
As a foreign private issuer, we are not required to comply with all the periodic disclosure requirements of the Exchange Act, and therefore, there may be less publicly available information about us than if we were a United States domestic issuer. For example, we are not subject to the proxy rules in the United States and disclosure with respect to our annual meetings will be governed by Canadian requirements.
Our charter documents and certain Canadian legislation could delay or deter a change of control, limit attempts by our shareholders to replace or remove our current management and limit the market price of our common shares.
Our authorized preferred shares are available for issuance from time to time at the discretion of our board of directors, without shareholder approval. Our articles grant our board of directors the authority to determine the special rights and restrictions granted to or imposed on any unissued series of preferred shares, and those rights may be superior to those of our common shares. Further, the Investment Canada Act subjects any acquisition of control of a company by a non-Canadian to government review if the value of the assets as calculated pursuant to the legislation exceeds a threshold amount or in other circumstances determined at the discretion of the Canadian government. A reviewable acquisition may not proceed unless the relevant minister is satisfied that the investment is likely to be of net benefit to Canada and the Canadian government is satisfied that no other important concerns arise from the acquisition of control. Any of the foregoing could prevent or delay a change of control and may deprive or limit strategic opportunities to our shareholders to sell their shares.
DESCRIPTION OF SHARE CAPITAL
Overview
Our authorized share capital consists of an unlimited number of common shares, Class B Shares and Series I First Preferred Shares, in each case without nominal or par value.
The holders of common shares are entitled to receive notice of and to attend all annual and special meetings of our shareholders and to one vote per share held at each such meeting, and they are entitled to receive dividends as determined and declared by our board of directors.
Subject to the rights of the holders of any other class of our shares entitled to receive dividends in priority to or concurrently with the holders of the common shares, our board of directors may in its sole discretion declare dividends on the common shares to the exclusion of any other class of shares of the Company.
In the event of our liquidation, dissolution or winding up or other distribution of our assets among our shareholders for the purpose of winding up our affairs, the holders of the common shares shall, subject to the rights of the holders of any other class of shares entitled to receive our assets upon such a distribution in priority to or concurrently with the holders of the common shares, be entitled to participate in the distribution. Such distribution shall be made in equal amounts per share on all the common shares at the time outstanding without preference or distinction.
The holders of the Class B Shares are entitled to receive notice of and to attend any meeting of our shareholders but shall not be entitled to vote any of their Class B Shares at any such meeting. Each issued and fully paid Class B Share may at any time be converted, at the option of the holder, into one common share. In the event of our liquidation, dissolution or winding up or other distribution of our assets among our shareholders for the purpose of winding up our affairs, the holders of the Class B Shares shall be entitled to participate rateably with the common shares in any distribution of the assets of the Company.
The First Preferred Shares may at any time and from time to time be issued in one or more series and our board of directors may before the issue thereof fix the number of shares in, and determine the designation, rights, privileges, restrictions and conditions attaching to the shares of, each series of First Preferred Shares.
37
The First Preferred Shares shall be entitled to priority over the common shares and Class B Shares and all other shares ranking junior to the First Preferred Shares with respect to the payment of dividends and the distribution of our assets in the event of our liquidation, dissolution or winding up or other distribution of our assets among our shareholders for the purpose of winding up our affairs.
The First Preferred Shares of each series rank on a parity with the First Preferred Shares of every other series with respect to priority in the payment of dividends and in the distribution of our assets in the event of our liquidation, dissolution or winding up or other distribution of our assets among our shareholders for the purpose of winding up our affairs.
During 2013, we created a new series of shares, our Series I Preferred Shares. The holders of Series I Preferred Shares are not entitled to vote at any meeting of our shareholders (except in limited circumstances provided for in the OBCA, and they are entitled to receive dividends as determined and declared at the discretion of our board of directors equally on a one-for-one basis with the holders of shares of the other series of First Preferred Shares. Each issued and fully paid Series I Preferred Share may at any time be converted, at the option of the holder, into one-thirtieth (1/30th) of a common share, subject to adjustment.
As at December 31, 2014, 4,427,244 common shares were outstanding, 69,504,689 Series I Preferred Shares were outstanding and convertible into 2,316,823 common shares, and no Class B Shares were outstanding. As at December 31, 2014, 138,724,781 common share purchase warrants were outstanding and convertible into 4,624,159 common shares at a weighted average exercise price of $8.73 per common share.
As at December 31, 2014, there were 590,141 stock options outstanding to purchase common shares. The terms and conditions of such stock options are contained in the 2014 Stock Option Plan.
As at December 31, 2014, we have issued 28,777 deferred share units. The terms and conditions of such deferred share units are contained in the 2014 DSU Plan.
Warrants
Our board of directors authorized or ratified the issuances of the warrants set forth in the table below and the issuance of one common share upon the due exercise of each 30 warrants in accordance with its terms and the receipt by us of the designated exercise price payable in respect of the share prior to the time of expiry on the designated expiry date.
As at December 31, 2014, we had the following outstanding warrants (on a post-consolidation basis):
| Number of Warrants | | | | |
| Outstanding | | Exercise Price | | Expiry Date |
| 202,800 | | $7.50 | | March 16, 2015 |
| 5,014,839 | | $6.30 | | December 13, 2015 |
| 15,230,000 | | $12.00 | | March 15, 2018 |
| 420,000 | | $12.00 | | March 27, 2018 |
| 117,857,142 | | $8.40 | | December 13, 2018 |
| Total: 138,724,781 | | | | |
Stock Options
Our board of directors authorized or ratified the issuances of the options set forth in the table below and the issuance of one common share upon the due exercise of each option in accordance with its terms and the receipt by us of the designated exercise price payable in respect of the share prior to the time of expiry on the designated expiry date.
38
As at December 31, 2014, we had the following outstanding stock options (on a post-consolidation basis):
| Number of Stock | | | | | | |
| Options Outstanding | | Number Exercisable | | Exercise Price | | Expiry Date |
| 3,333 | | 3,333 | | $7.50 | | March 6, 2015 |
| 1,667 | | 1,667 | | $30.00 | | March 6, 2015 |
| 3,333 | | 3,333 | | $7.50 | | March 7, 2015 |
| 1,333 | | 1,333 | | $30.00 | | March 7, 2015 |
| 1,667 | | 1,667 | | $30.00 | | June 28, 2016 |
| 3,334 | | 3,334 | | $30.00 | | July 18, 2016 |
| 417 | | 417 | | $30.00 | | August 25, 2016 |
| 333 | | 333 | | $30.00 | | June 1, 2017 |
| 73,675 | | 36,842 | | $7.50 | | April 8, 2023 |
| 1,166 | | 584 | | $7.50 | | May 23, 2023 |
| 6,666 | | 2,222 | | $15.30 | | January 29, 2024 |
| 13,332 | | 4,444 | | $18.90 | | March 6, 2024 |
| 264,127 | | 88,043 | | $10.35 | | April 17, 2024 |
| 215,758 | | 71,918 | | $8.34 | | May 27, 2024 |
| Total: 590,141 | | Total: 219,470 | | | | |
Deferred Share Units
As at December 31, 2014, we had 28,777 (post-consolidated) issued and outstanding DSUs which are convertible into common shares.
Prior Sales
The following table summarizes details of each class of securities that is outstanding but not listed or quoted on a marketplace issued by the Company during the year ended December 31, 2014. On November 14, 2014, the Company consolidated its outstanding common shares issuing one post-consolidated share for each 30 pre-consolidated shares. The price and number of securities in the table below have been adjusted to reflect the post-consolidation values.
Date of Issuance
| Price per Security or
Exercise Price as Applicable
| Number of and
Description of Securities
|
|
|
January 29, 2014
| $15.30
| 6,666
Options(1) |
|
March 6, 2014
| $18.90
| 13,332
Options(1) |
|
April 17, 2014
| $10.35
| 264,127
Options(1) |
|
May 27, 2014
| $8.34
| 215,758
Options(1) |
|
May 27, 2014
| $8.34
| 28,777
Deferred Share Units(2) |
|
Note:
39
| (1) | Issued under the 2014 Stock Option Plan. |
| (2) | Issued under the 2014 DSU Plan. |
MARKET FOR SECURITIES
We were listed on the TSXV until April 22, 2014 when we delisted from the TSXV and began trading on the TSX. We traded under the symbol “SSS” until June 6, 2014 when the symbol was changed to “TR”. We were listed on the OTCQX International under the symbol “SCTPF” from May 20, 2013 until we delisted and began trading on the NASDAQ Capital Market under the symbol “TRIL” on December 19, 2014. The following table shows the price ranges and volumes traded on the TSXV/TSX and OTCQX/NASDAQ for the periods noted:
Month | TSXV/TSX | OTCQX/NASDAQ |
| High ($) | Low ($) | Volume (#) | High (US$) | Low (US$) | Volume (#) |
| December 2014 | $ 10.500 | $ 7.020 | 209,040 | $ 9.100 | $ 6.017 | 893,391 |
| November 2014 | $ 10.350 | $ 8.250 | 183,076 | $ 9.090 | $ 7.350 | 177,862 |
| October 2014 | $ 8.400 | $ 6.300 | 59,873 | $ 7.650 | $ 5.637 | 68,959 |
| September 2014 | $ 9.150 | $ 7.200 | 66,472 | $ 8.550 | $ 6.300 | 40,676 |
| August 2014 | $ 9.150 | $ 8.250 | 51,381 | $ 8.409 | $ 7.500 | 45,757 |
| July 2014 | $ 10.350 | $ 8.400 | 60,773 | $ 9.960 | $ 7.608 | 32,116 |
| June 2014 | $ 12.300 | $ 8.100 | 103,066 | $ 11.490 | $ 7.278 | 32,151 |
| May 2014 | $ 12.150 | $ 7.950 | 83,797 | $ 11.310 | $ 7.140 | 34,901 |
| April 2014 | $ 15.600 | $ 9.750 | 136,717 | $ 14.235 | $ 8.700 | 50,706 |
| March 2014 | $ 22.200 | $ 12.600 | 182,545 | $ 19.596 | $ 11.064 | 88,116 |
| February 2014 | $ 18.000 | $ 14.700 | 65,422 | $ 16.470 | $ 13.200 | 39,449 |
| January 2014 | $ 19.500 | $ 11.400 | 243,433 | $ 17.922 | $ 10.605 | 179,312 |
Note:
| (1) | Common share market prices are restated to reflect the 30 for 1 share consolidation completed in November 2014. |
BOARD OF DIRECTORS AND MANAGEMENT
The following table and summary of business experience set forth the name, office held, and functions and areas of experience in the Company, principal business activities and other principal directorships of each of our directors and senior management:
| Name | | |
| Present Office Held | | |
| Province/State and | | |
| Country of Residence | | |
| Position Held Since | | Principal Business Activities, Other Principal Directorships and Function |
Luke Beshar
Director(1)
New Jersey, USA
March 10, 2014 | | Mr. Beshar was the Executive/Senior Vice President and Chief Financial Officer of NPS Pharmaceuticals, Inc., a global biopharmaceutical company from November 2007 to February 2015. As an independent director, Mr. Beshar supervises our management and helps to ensure compliance with our corporate governance policies and standards. |
40
Henry Friesen
Director(1)(2)
Manitoba, Canada
June 28, 2011 | | Dr. Friesen is a Distinguished University Professor Emeritus at University of Manitoba since October 2000. As an independent director, Dr. Friesen supervises our management and helps to ensure compliance with our corporate governance policies and standards. |
Robert Kirkman
Director(1)(3)
Washington, USA
December 17, 2013 | | Dr. Kirkman is President and Chief Executive Officer and director of Oncothyreon Inc., an oncology-focused biotechnology company since September 2006. As an independent director, Dr. Kirkman supervises our management and helps to ensure compliance with our corporate governance policies and standards. |
Michael Moore
Director(2)(3)
Surrey, UK
April 9, 2013 | | Dr. Moore is the Executive Chair ofMISSIONTherapeutics Ltd. since 2012 and a Board Chair or Director of several private biopharmaceutical companies in the UK. From 2004 to 2013, Dr. Moore was the Chair of Trillium Privateco, and from 2003 to 2008, Dr. Moore was the Chief Executive Officer and Director of PIramed Ltd, a UK- based oncology company sold to Roche in 2008. As an independent director, Dr. Moore supervises our management and helps to ensure compliance with our corporate governance policies and standards. |
Thomas Reynolds
Director(2)(3)
Washington, USA
March 10, 2014 | | Dr. Reynolds is an independent biotechnology consultant since February 2013, and was Chief Medical Officer of Seattle Genetics, Inc., a biotechnology company focused on antibody-based therapies for the treatment of cancer from March 2007 to January 2013. Dr. Reynolds also sits on the board of MEI Pharma, Inc. As an independent director, Dr. Reynolds supervises our management and helps to ensure compliance with our corporate governance policies and standards. |
Calvin Stiller
Director, Chair of theBoard
Ontario, Canada
July 18, 2011 | | Dr. Stiller is the Chair of Ontario Institute for Cancer Research and Professor Emeritus at Western University. Dr. Stiller also sits on the board of Revera Corporation and Magor Corporation. As an independent director, Dr. Stiller supervises our management and helps to ensure compliance with our corporate governance policies and standards. |
Niclas Stiernholm
President and ChiefExecutive Officer, Director
Ontario, Canada
Director since July 18, 2011; President and CEO since
April 9, 2013 | | Dr. Stiernholm is the President and Chief Executive Officer of Trillium since April 9, 2013 and was the President and Chief Executive Officer of Trillium Privateco since 2002. He joined Trillium from YM BioSciences where he was Executive Vice President and Chief Scientific Officer. As President and Chief Executive Officer, Dr. Stiernholm is responsible for overseeing our strategic direction, executing business development plans and ensuring that our scientific programs remain funded and advance on schedule. As a director, Dr. Stiernholm participates in management oversight and helps to ensure compliance with our corporate governance policies and standards. |
Robert Uger
Chief Scientific Officer
Ontario, Canada
April 9, 2013 | | Dr. Uger is the Chief Scientific Officer of Trillium since April 9, 2013 and was the Vice President, Research of Trillium Privateco since 2003. He joined Trillium from Aventis Pasteur where he was a Senior Research Scientist involved in cancer vaccine research. As Chief Scientific Officer, Dr. Uger is responsible for developing and implementing our scientific direction, and oversees both internal product development and external research and development programs. |
41
James Parsons
Chief Financial Officer
Ontario, Canada
August 25, 2011 | | Mr. Parsons is the Chief Financial Officer of Trillium since August 25, 2011 and was also the Director, Finance of Trillium Privateco. He was previously the Vice President, Finance of DiaMedica Inc. from October 2010 to May 2014, and Chief Financial Officer of Amorfix Life Sciences Ltd. from 2006 to 2010. Mr. Parsons sits on the board of Sernova Corp. As Chief Financial Officer, Mr. Parsons is responsible for financial and risk management, investor relations, corporate governance and administration. |
Penka Petrova
Vice-President, DrugDevelopment
Ontario, Canada
April 9, 2013 | | Dr. Petrova is the Vice President, Drug Development of Trillium since April 9, 2013 and was the Director, Drug Development of Trillium Privateco from June 2011 to March 2013. Dr. Petrova joined Trillium Privateco from Prescient Neuropharma in 2003. As Vice President, Drug Development, Dr. Petrova is responsible for managing our formal drug development efforts, including all outsourced activities to contract manufacturers and contract research organizations. |
Notes:
| (1) | Member of our audit committee. |
| (2) | Member of our corporate governance and nominating committee. |
| (3) | Member of our compensation committee. |
Directors are elected annually and hold office until a successor is elected at a subsequent annual meeting of the Company, unless a director’s office is earlier vacated in accordance with the by-laws of the Company. The business address for our directors and senior management is Trillium Therapeutics Inc., 96 Skyway Avenue, Toronto, Ontario, Canada, M9W 4Y9.
As at December 31, 2014, the directors and senior officers of the Company, as a group, beneficially owned, directly or indirectly, 31,000 common shares of the Company constituting approximately 0.7% of the issued and outstanding common shares.
CONFLICTS OF INTEREST
Certain of our officers and directors are also officers and/or directors of other companies engaged in the biotechnology industry and research business generally. As a result, situations arise where the interest of such directors and officers conflict with their interests as directors and officers of other companies. The resolution of such conflicts is governed by applicable corporate laws, which require that directors act honestly, in good faith and with a view to the best interests of the Company. In addition, the OBCA, our governing statute, requires our officers and directors to disclose any personal interest which they may have in any material contract or transaction which is proposed to be entered into with the Company and, in the case of directors, to abstain from voting as a director for the approval of any such contract or transaction, unless otherwise permitted under the OBCA.
LEGAL PROCEEDINGS
We are not a party to, and none of our assets are subject to, any material legal proceedings, nor to our knowledge are any such proceedings contemplated.
INTEREST OF MANAGEMENT AND OTHERS IN MATERIAL TRANSACTIONS
There are no material interests, direct or indirect, of directors, senior officers, any shareholders who beneficially own, directly or indirectly, more than 10% of our outstanding common shares, or any known associates or affiliates of such persons, in any transaction within the last three years or in any proposed transaction which has materially affected or would materially affect the Company.
42
For the years ended December 31, 2014 and 2013, $7,916 and $138,750, respectively, was paid to our former Executive Chair, a director, for consulting fees. These transactions were in the normal course of operations and were measured at the exchange amount, which is the amount of consideration established and agreed to by the parties.
INTEREST OF EXPERTS
Our auditors are Ernst & Young LLP, Chartered Professional Accountants, Licensed Public Accountants, Toronto, Ontario, Canada. Our consolidated financial statements as at December 31, 2014 and 2013 have been audited by Ernst & Young LLP, Independent Registered Public Accounting Firm, as indicated in their report dated March 23, 2015. Ernst & Young LLP has been the Corporation's auditors since inception on March 31, 2004.
Ernst & Young LLP has advised that they are independent with respect to the Corporation within the meaning of the Rules of Professional Conduct of the Chartered Professional Accountants of Ontario (registered name of the Institute of Chartered Accountants of Ontario) and the rules and standards of the Public Company Accounting Oversight Board (PCAOB) (United States) and the securities laws and regulations administered by the United States Securities and Exchange Commission.
TRANSFER AGENT
Our registrar and transfer agent is Computershare Trust Company of Canada, located at 100 University Avenue, Toronto, Ontario, M5J 2Y1.
MATERIAL CONTRACTS
There are no other contracts, other than those disclosed in this AIF and those entered into in the ordinary course of our business, that are material to us and which were entered into in the last completed fiscal year or which were entered into before the most recently completed fiscal year but are still in effect as of the date of this AIF:
| 1. | License Agreement between Trillium Privateco, UHN and HSC dated February 1, 2010 pursuant to which we licensed intellectual property relating to methods and compounds for the modulation of the SIRPα-CD47 interaction for therapeutic cancer applications. The license agreement requires us to use commercially reasonable efforts to commercialize the licensed technology. The license agreement will terminate on a country-by-country basis, in countries where a valid claim exists, when the last valid claim expires in such country, or if no valid claim exists, when the last valid claim expires in the U.S. We paid an up-front license fee of $150,000 and committed to pay an annual maintenance fee of $25,000, as well as payments on patent issuances, development milestone payments ranging from $100,000 to $300,000 on the initiation of phase I, II and III clinical trials respectively, and payments upon the achievement of certain regulatory milestones as well as royalties of either 3% or 1% of net revenues on commercial sales. The regulatory milestone payments amount to $1 million on each of the submission of a first BLA in the U.S. and receipt of first regulatory approval in the U.S. and proportionate payments in other territories worldwide. The aggregate milestones payable on their first achievement under the agreement in the major markets of the U.S., Europe and Asia combined are $5,660,000. Under the license agreement, Trillium is required to pay 20% of any sublicensing revenues to the licensors on the first $50 million of sublicensing revenues, and pay 15% of any sublicensing revenues to the licensors after the first $50 million of sublicensing revenue received. |
| | |
| 2. | GPEx®-Derived Cell Line Sale Agreement between Trillium Therapeutics Inc. and Catalent Pharma Solutions, LLC dated August 12, 2014 pursuant to which we acquired the right to use the GPEx® expression system for the manufacture of TTI-621 (SIRPαFc). Consideration for the license includes potential pre-marketing approval milestones of up to US$875,000 and aggregate sales milestone payments of up to US$28.8 million. |
| | |
| 3. | GPEx®-Derived Cell Line Sale Agreement between Trillium Therapeutics Inc. and Catalent Pharma Solutions, LLC dated August 12, 2014 pursuant to which we acquired the right to use the GPEx® expression system for the manufacture of TTI-622 (SIRPαFc). Consideration for the license includes potential pre-marketing approval milestones of up to US$875,000 and aggregate sales milestone payments of up to US $28.8 million. |
43
| 4. | Shareholder rights plan between Trillium and Computershare Investor Services Inc. dated September 16, 2013 and amended June 3, 2014. This plan was approved by our shareholders on May 27, 2014. See the discussion in the section of this prospectus entitled “Description of Share Capital – Shareholder Rights Plan”. |
| | |
| 5. | 2014 Stock Option Plan that was approved by our shareholders on May 27, 2014. See the discussion in the section of this prospectus entitled “Board of Directors and Management – Stock Option Plan”. |
| | |
| 6. | 2014 DSU Plan that was approved by our shareholders on May 27, 2014. See the discussion in the section of this prospectus entitled “Board of Directors and Management – Deferred Share Unit Plan”. |
| | |
| 7. | Warrant Indenture between the Company and Computershare Trust Company of Canada, or Computershare, dated March 15, 2013. This indenture provides that Computershare will act as the trust agent for the administration of the issued warrants. |
| | |
| 8. | Warrant Indenture between the Company and Computershare dated April 8, 2013. This indenture provides that Computershare will act as the trust agent for the administration of the issued warrants. |
| | |
| 9. | Warrant Indenture between the Company and Computershare dated December 13, 2013. This indenture provides that Computershare will act as the trust agent for the administration of the issued warrants. |
ADDITIONAL INFORMATION
Additional information about us may be found on SEDAR at www.sedar.com. Additional information including directors’ and officers’ remuneration and indebtedness, principal holders of our securities, options to purchase securities and securities authorized for issuance under equity compensation plans, is contained in our Management Information Circular dated April 22, 2014 provided for the last annual meeting of our shareholders held on May 27, 2014. Additional information may also be found in our audited financial statements and related MD&A for the year ended December 31, 2014. Each of these documents is available on SEDAR at www.sedar.com and is incorporated herein by reference.
44









