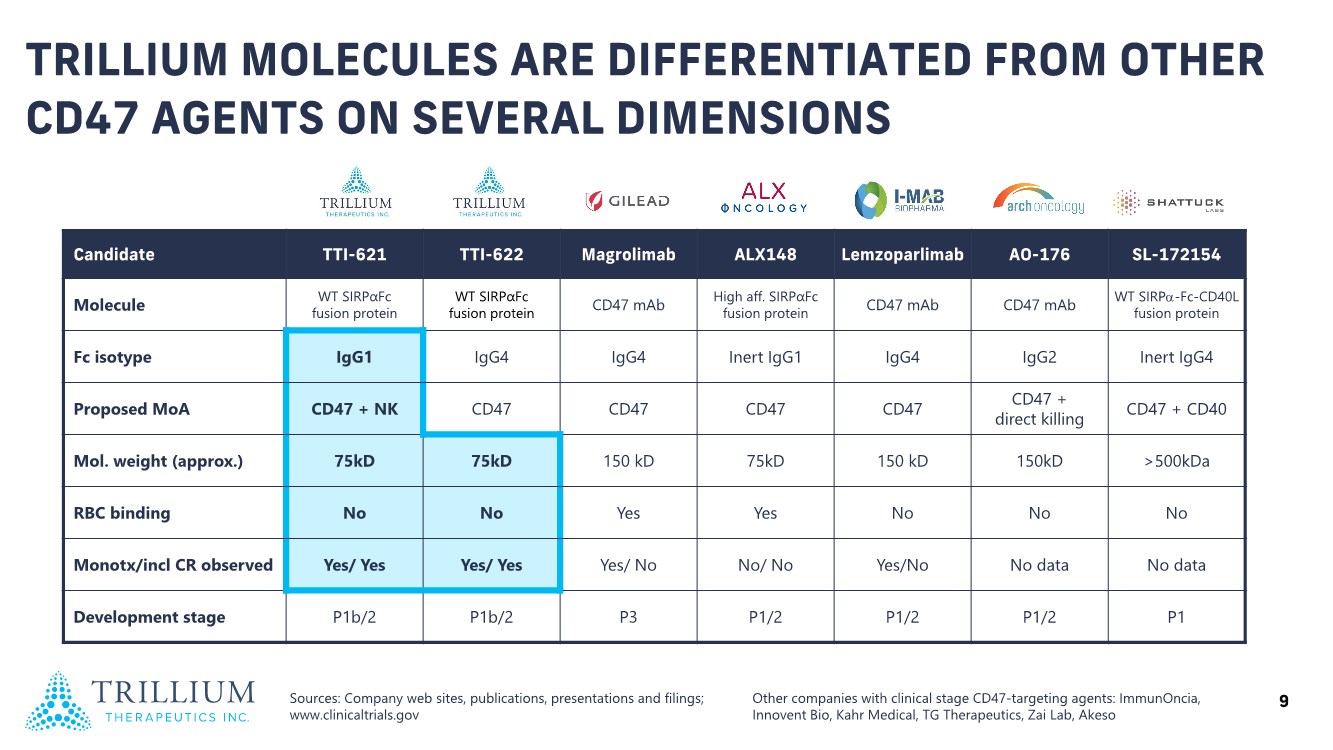
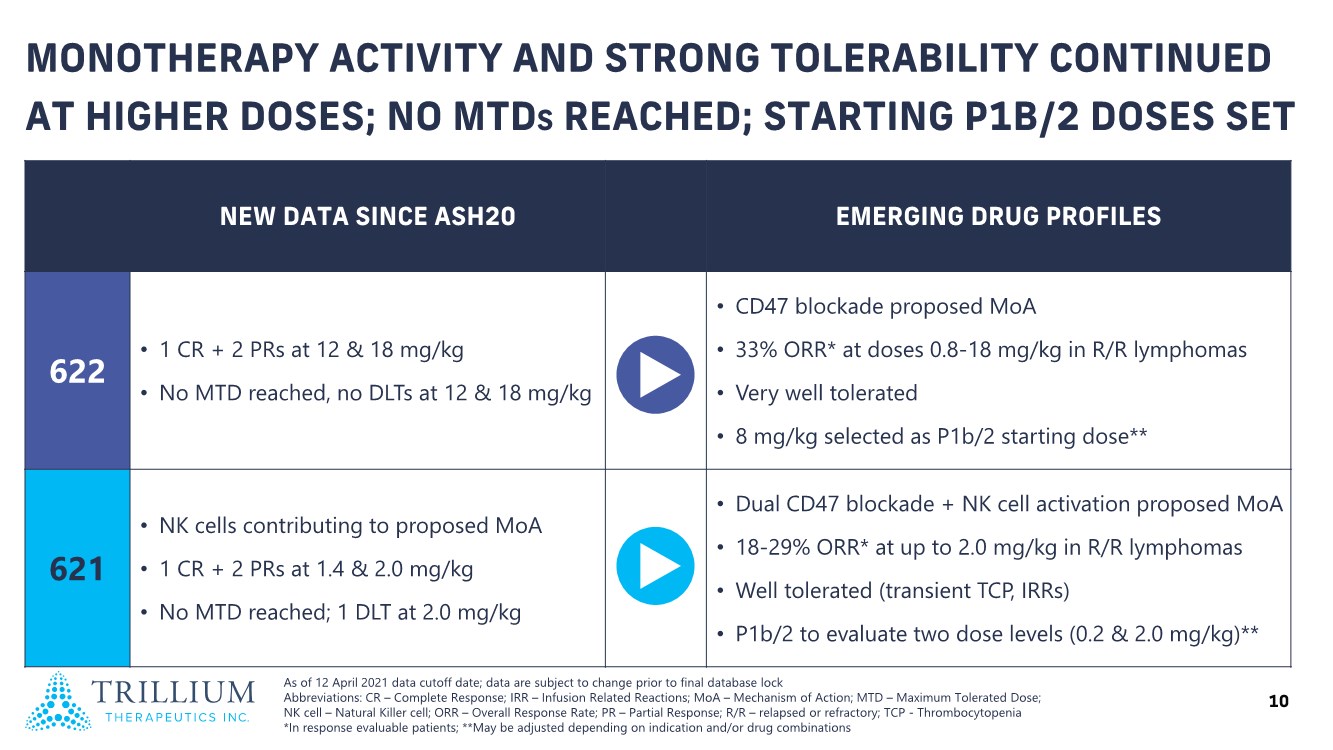
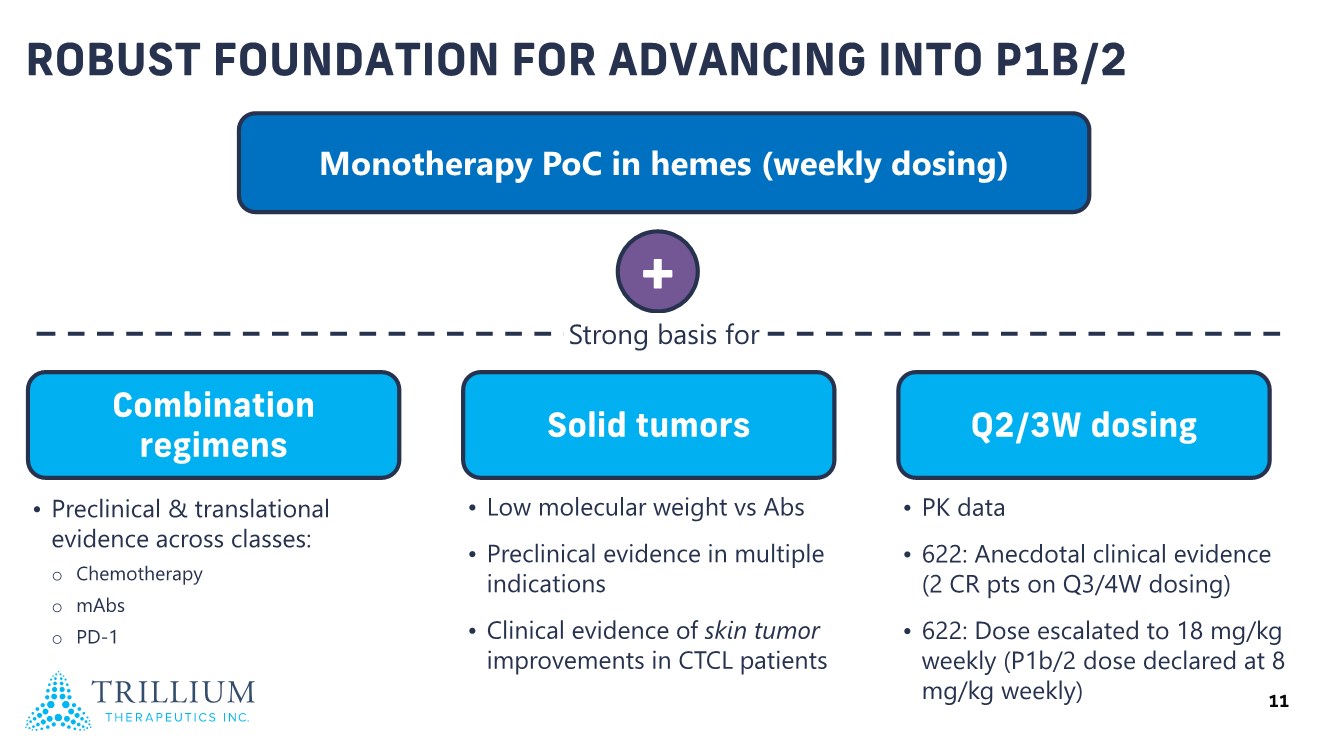
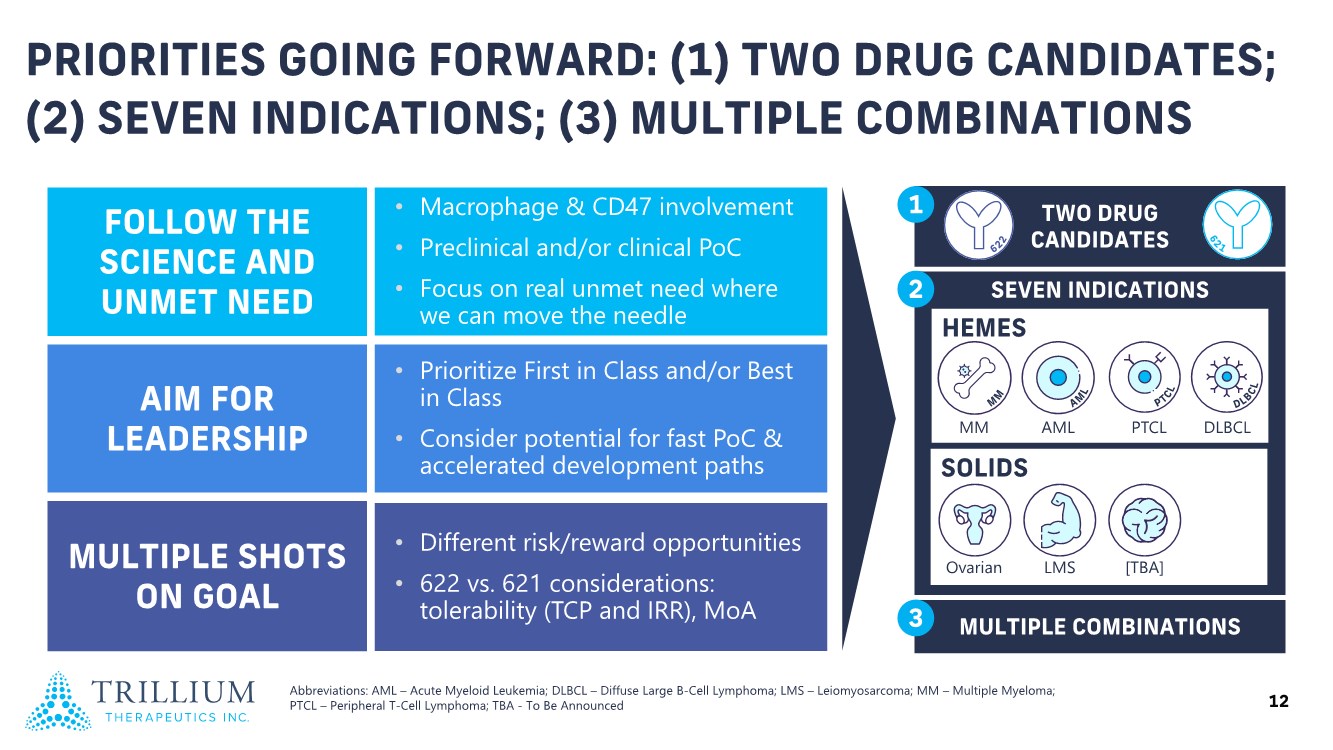
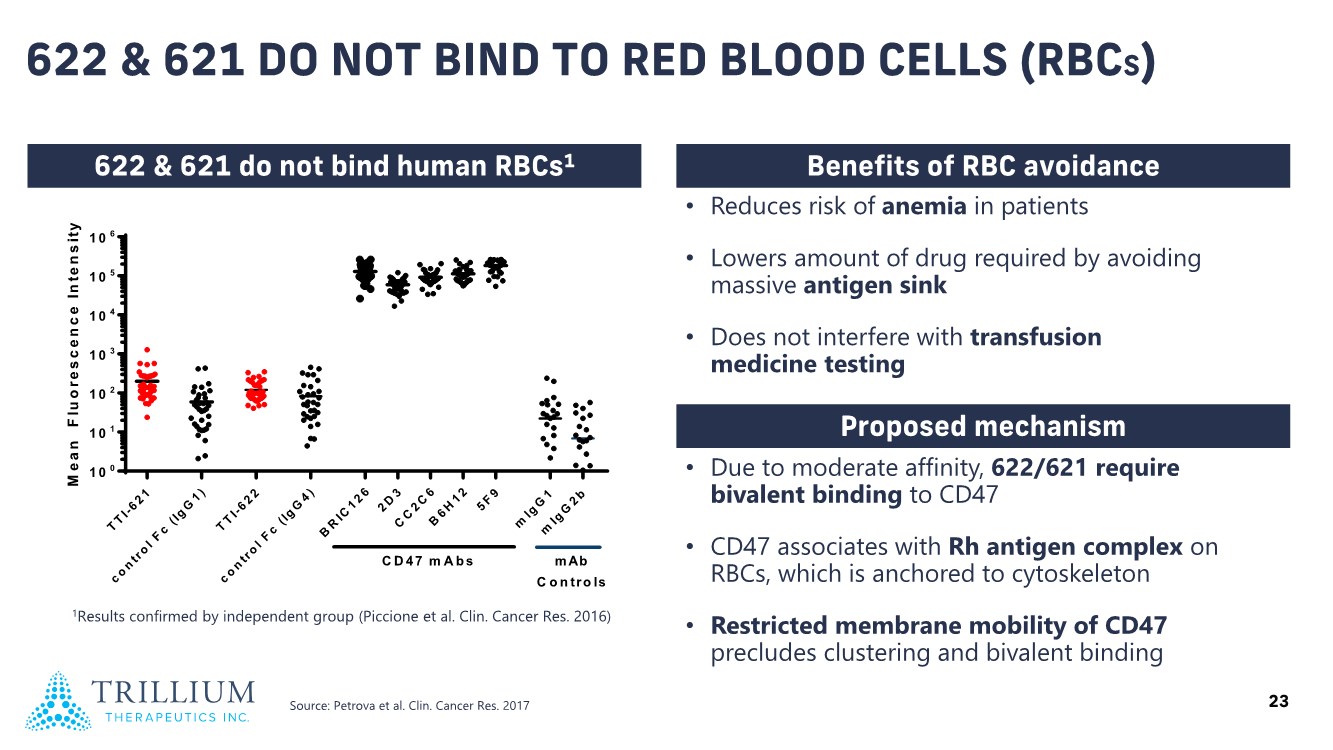
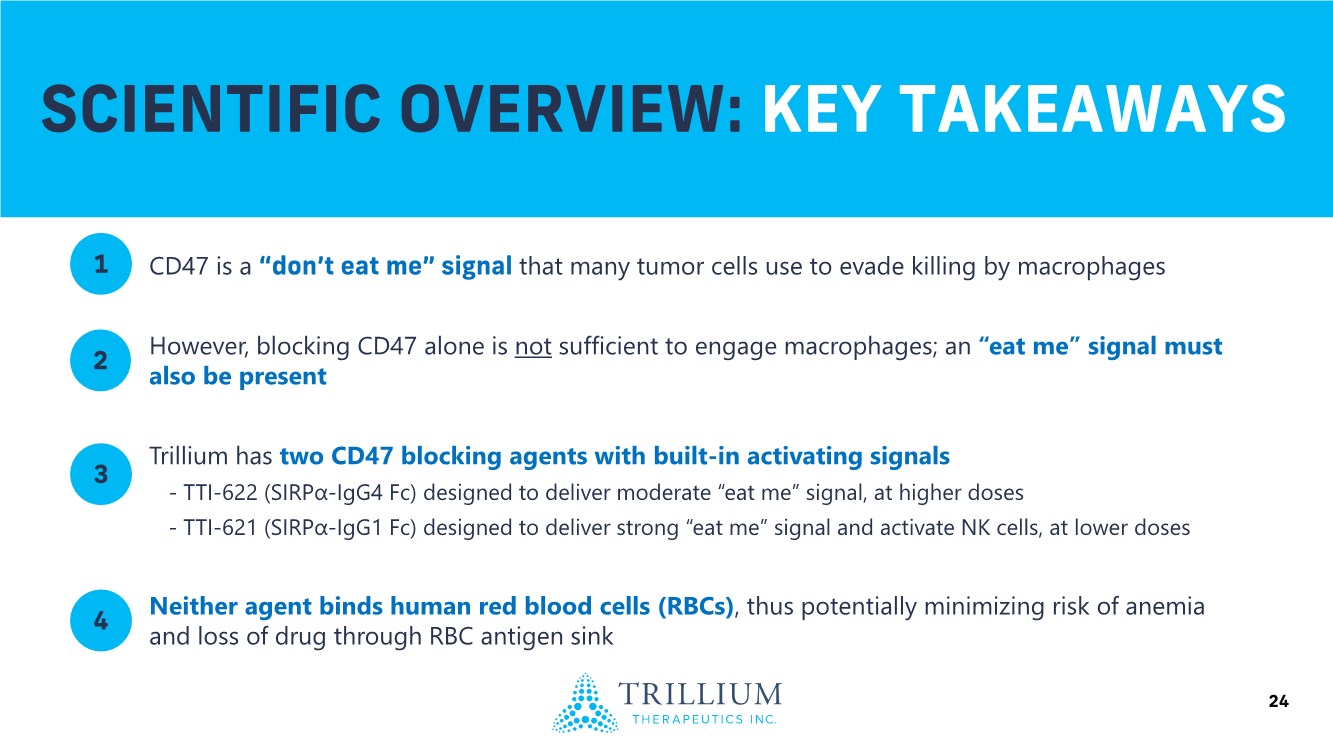
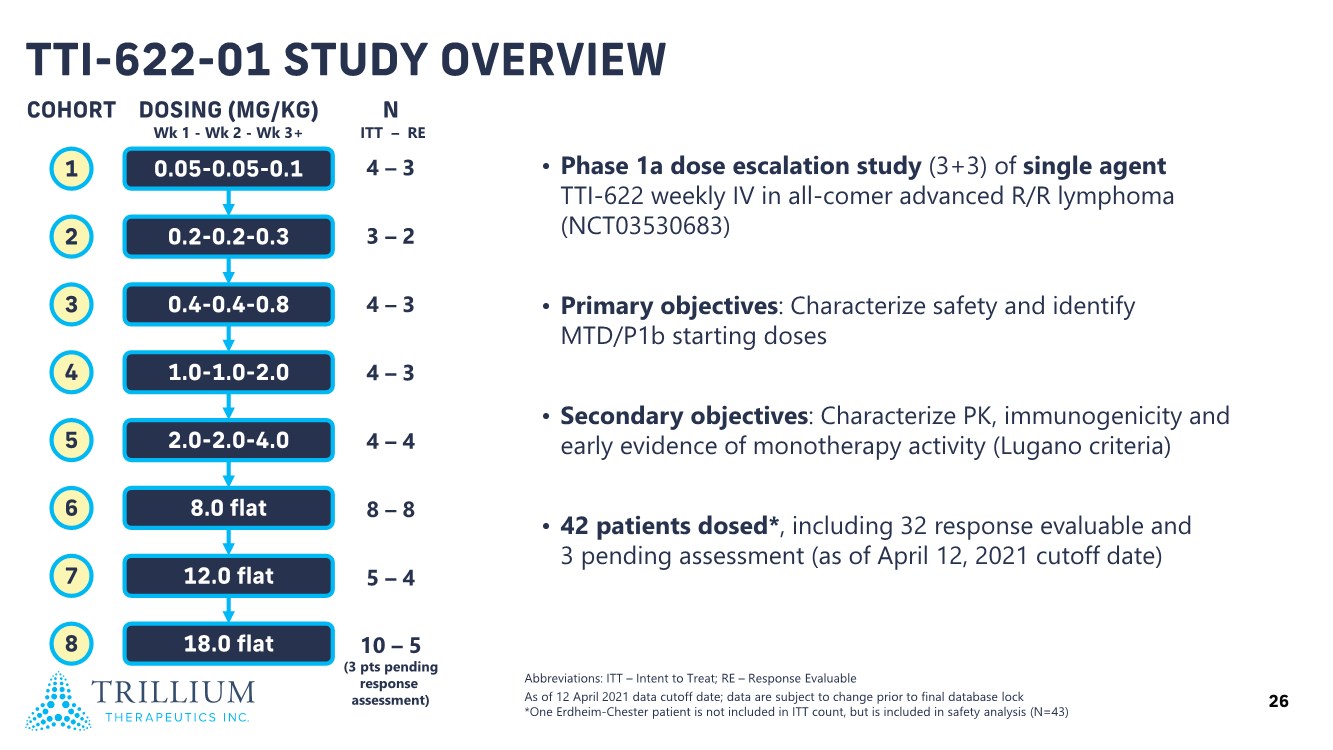
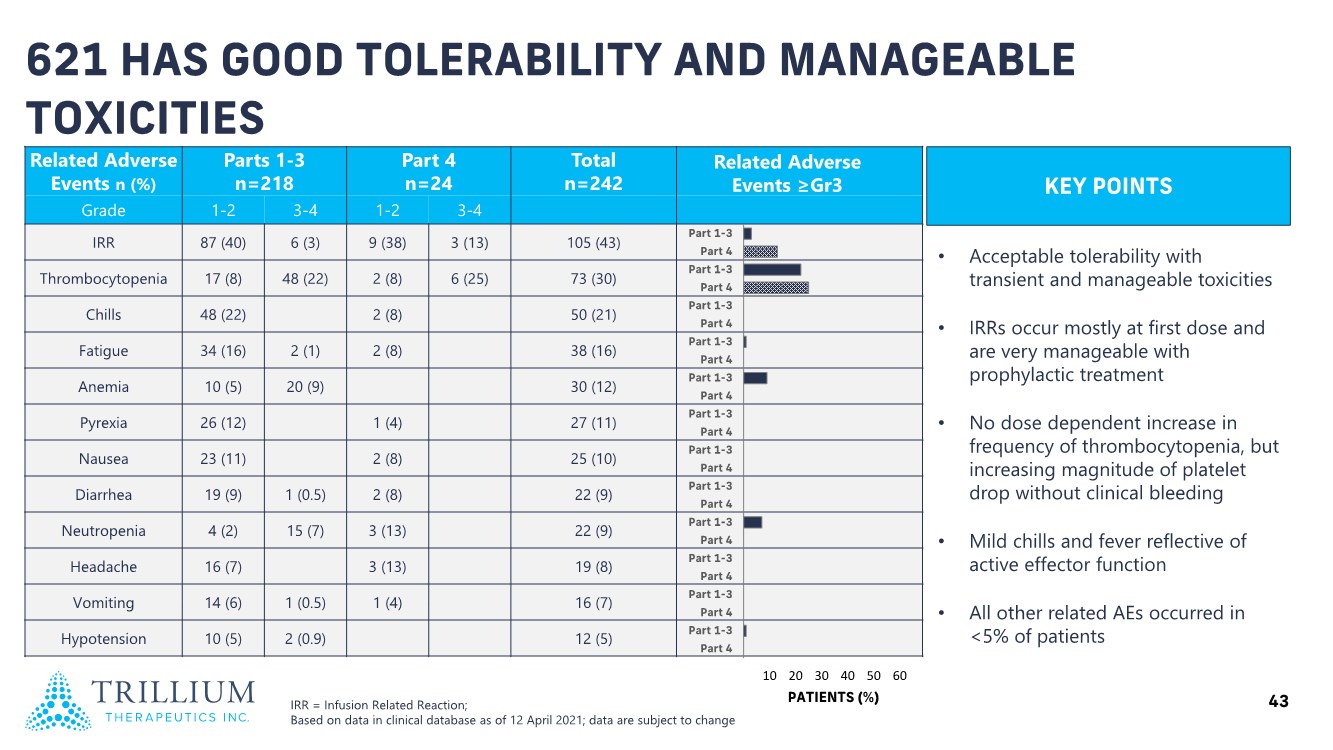
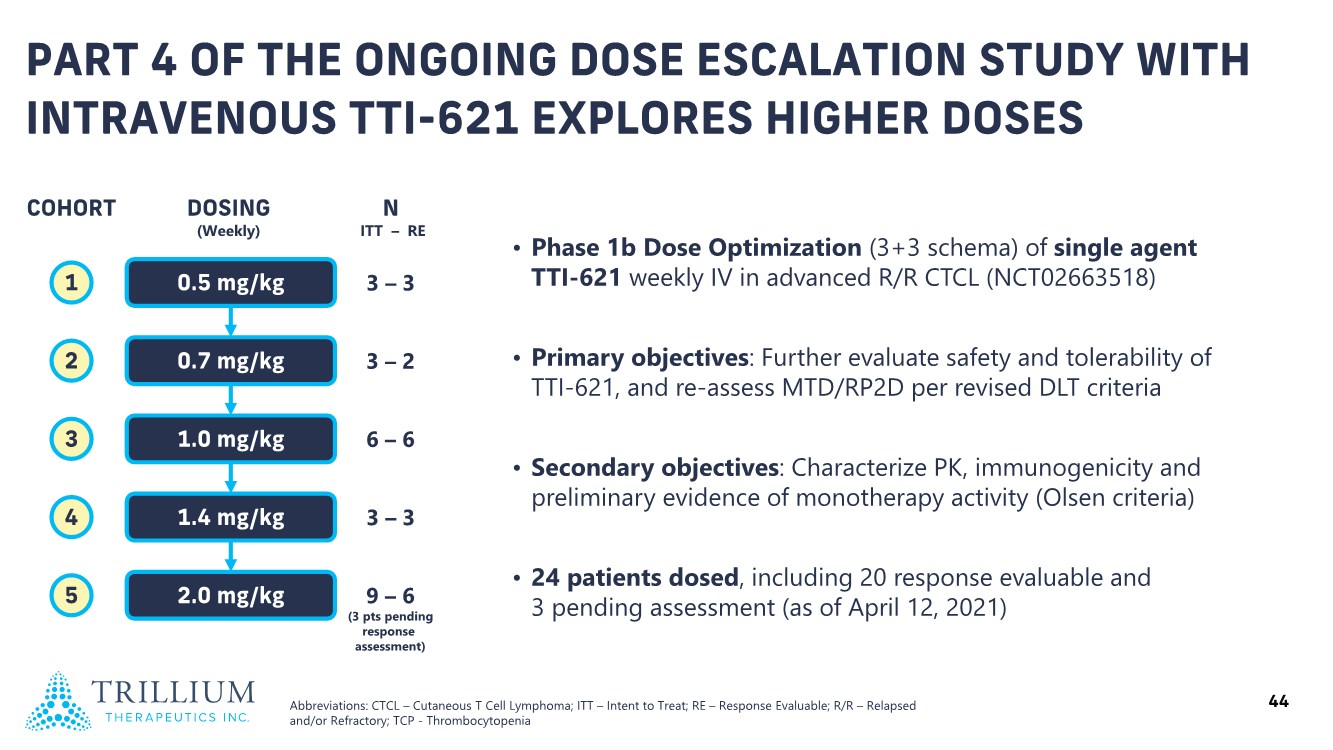
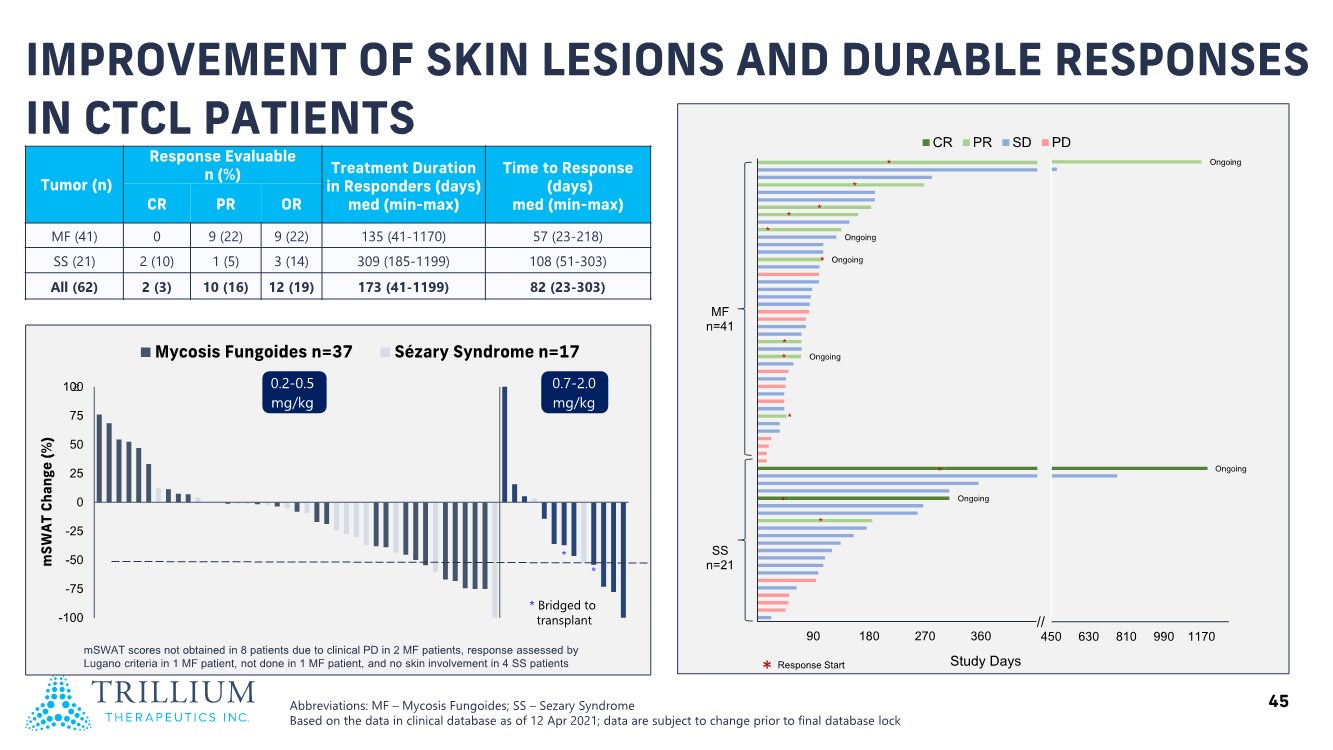
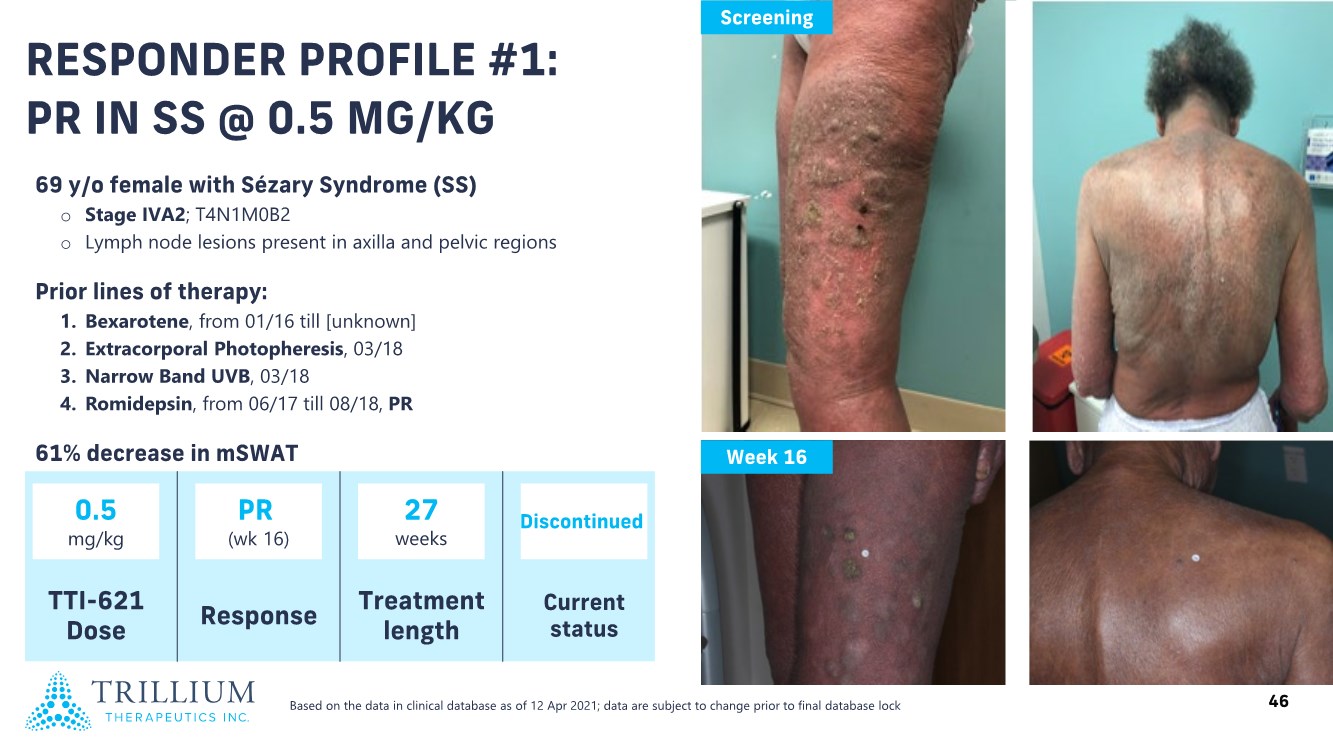
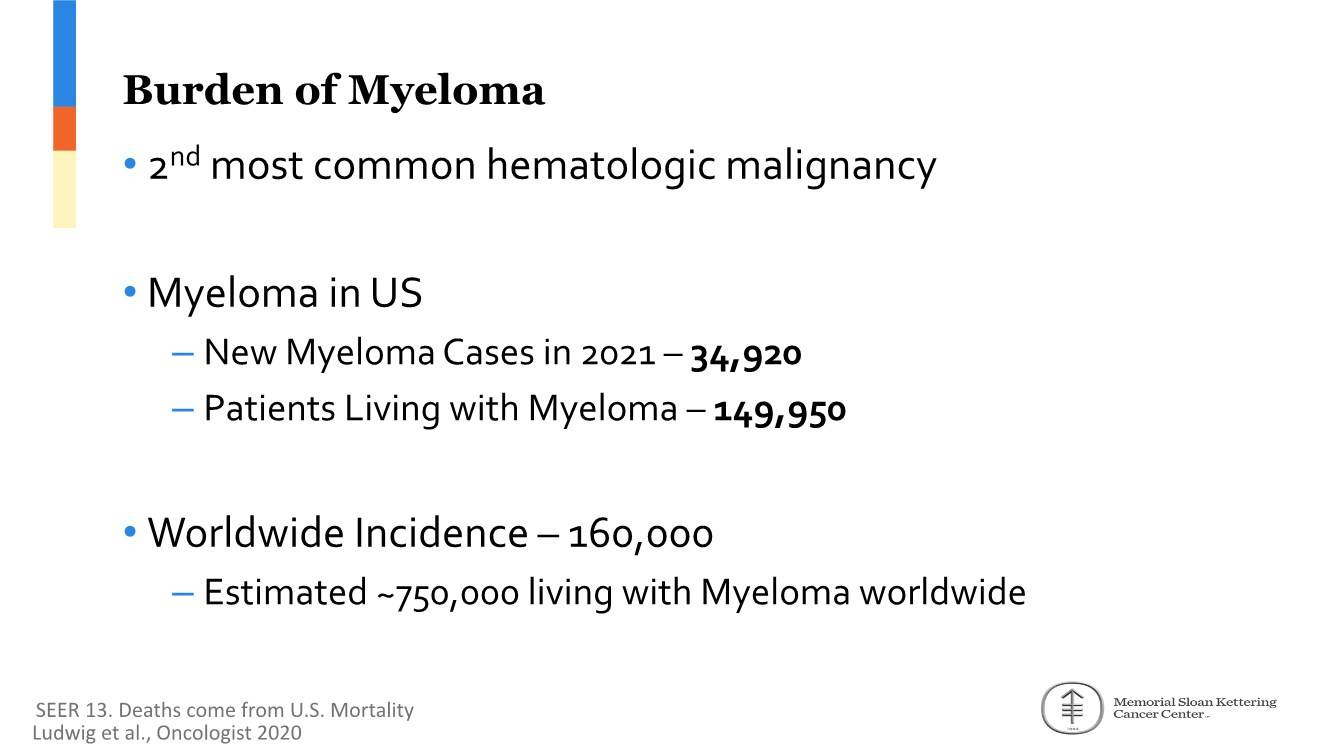
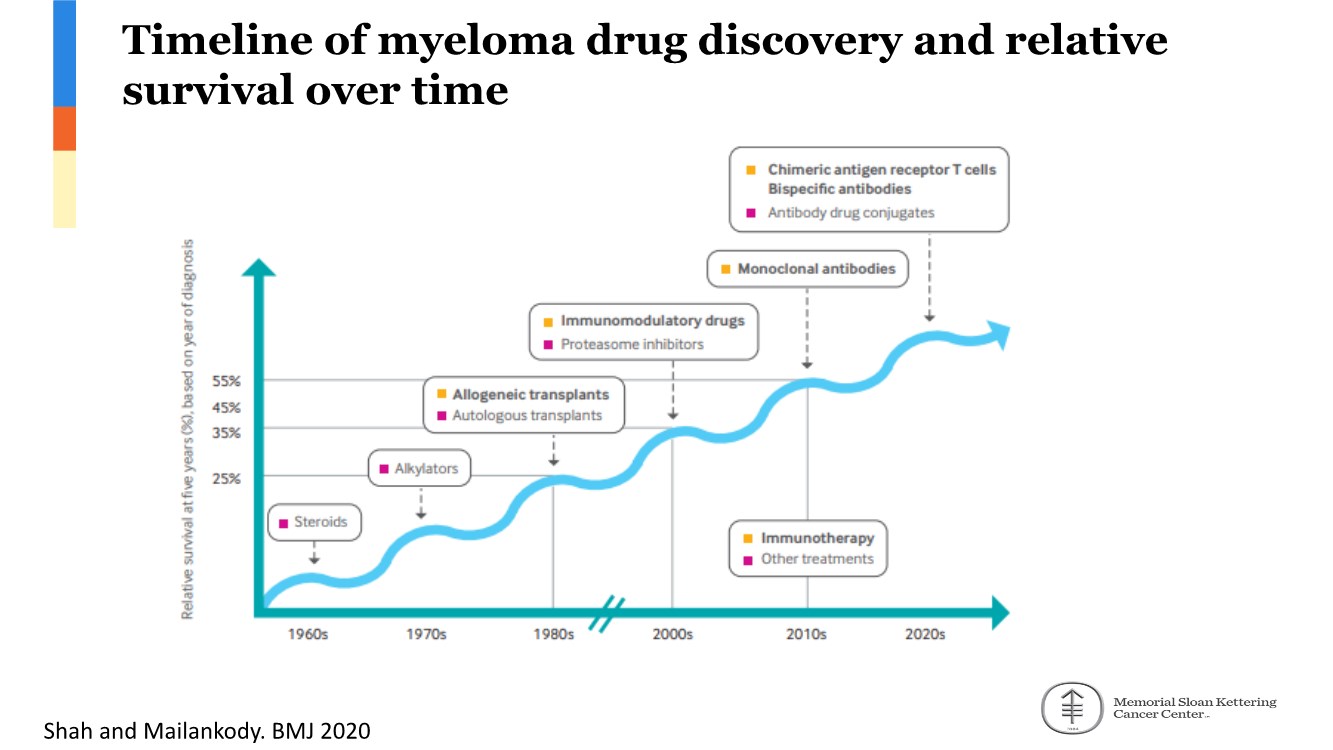
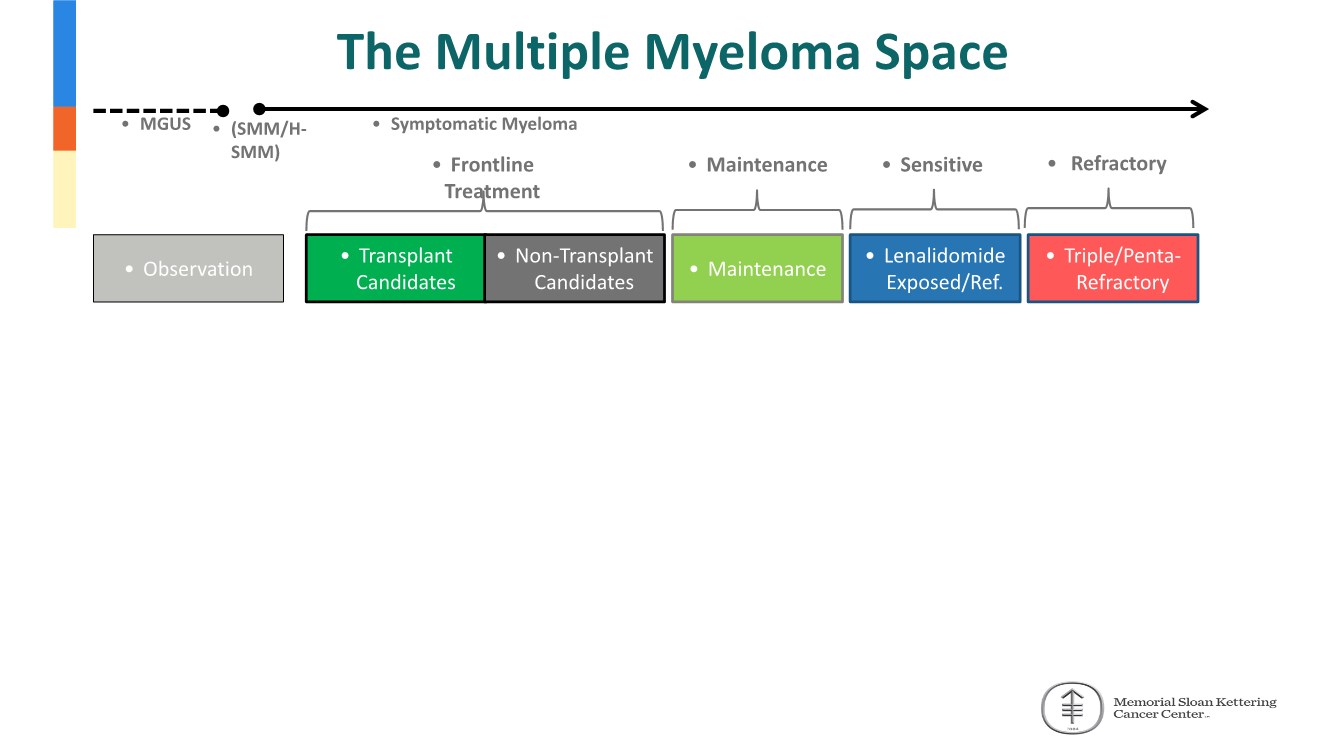
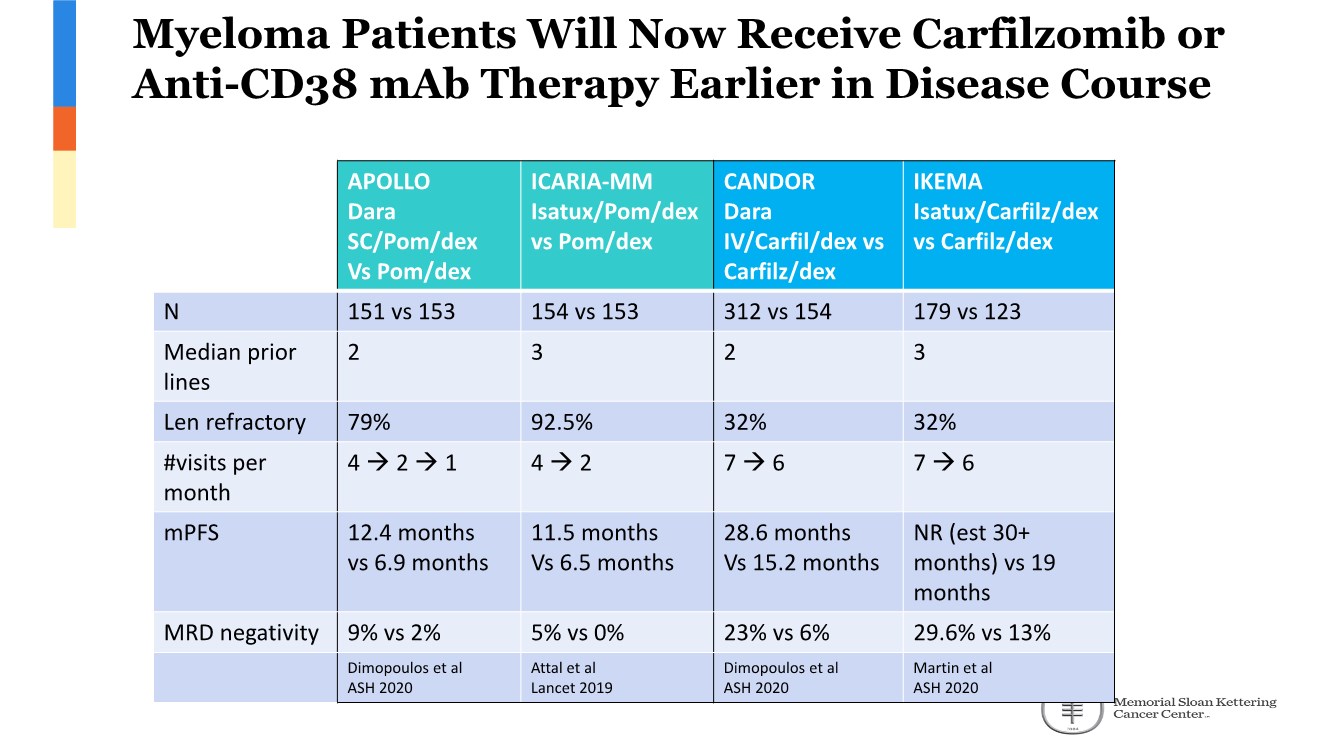
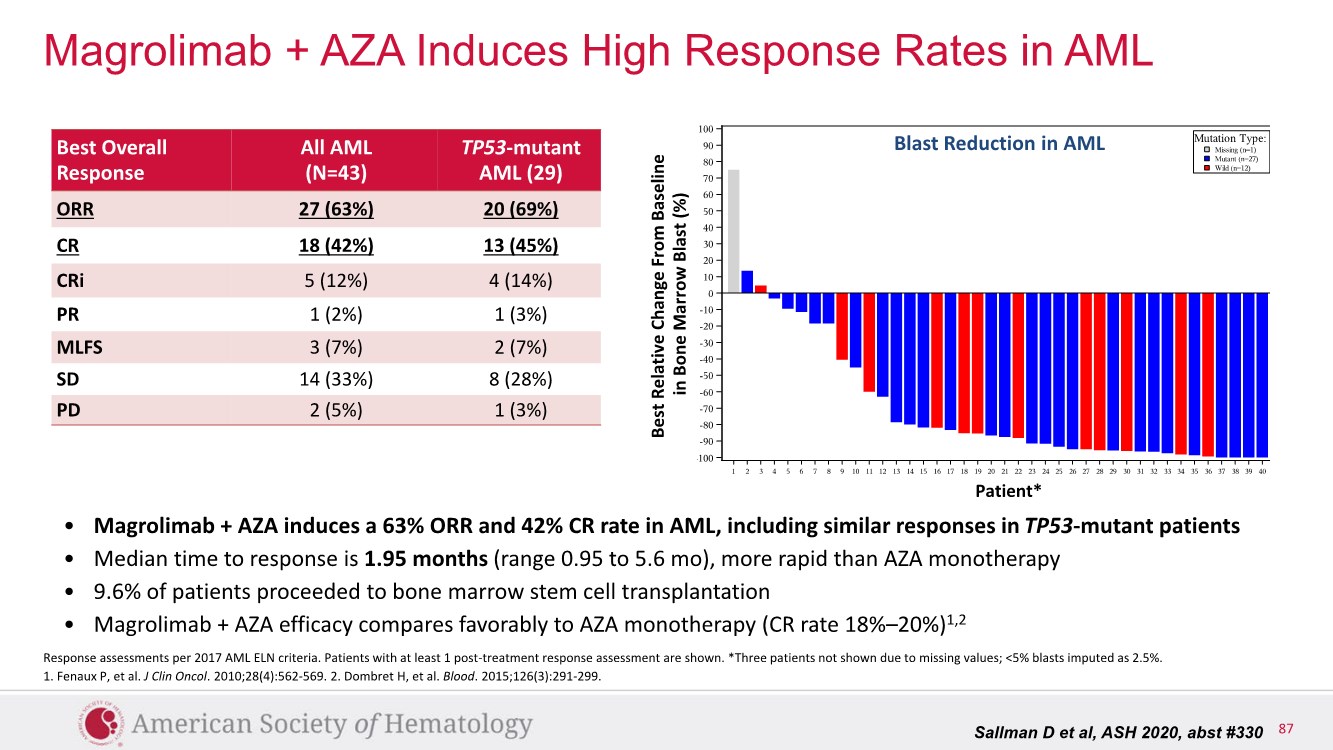
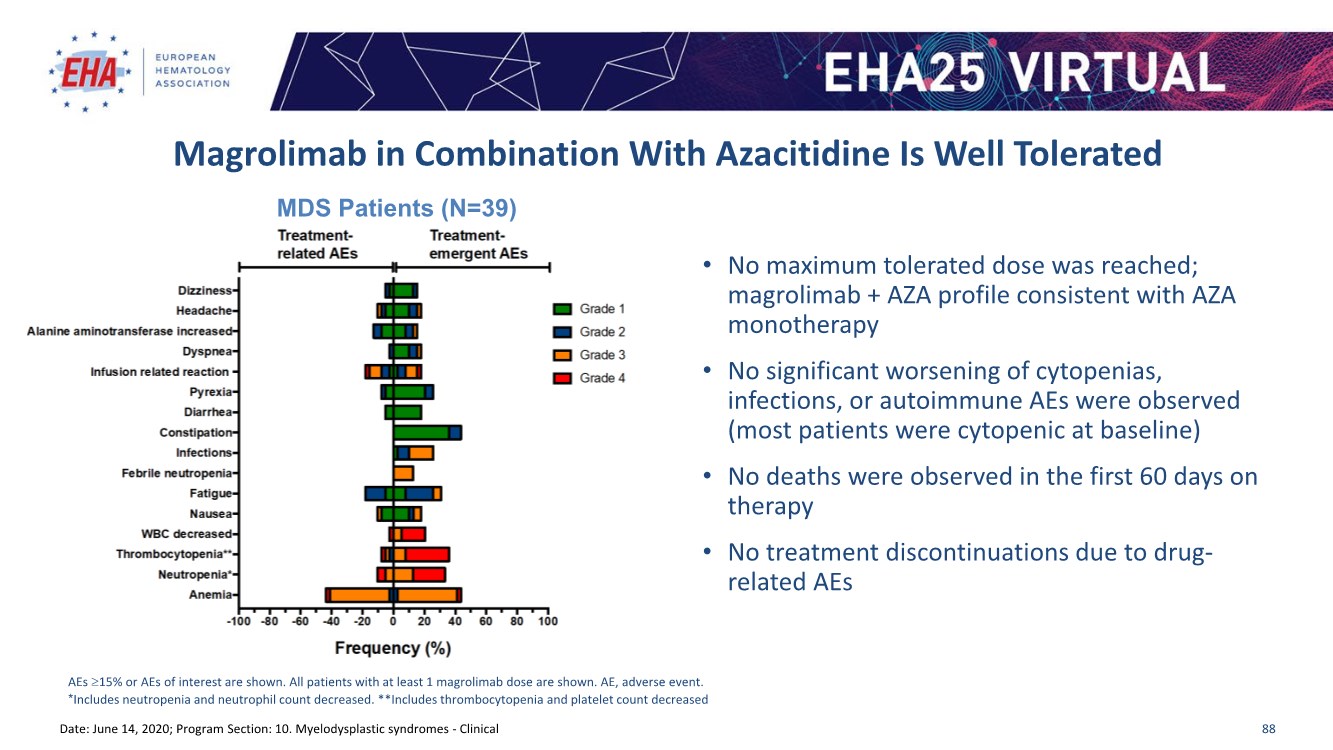
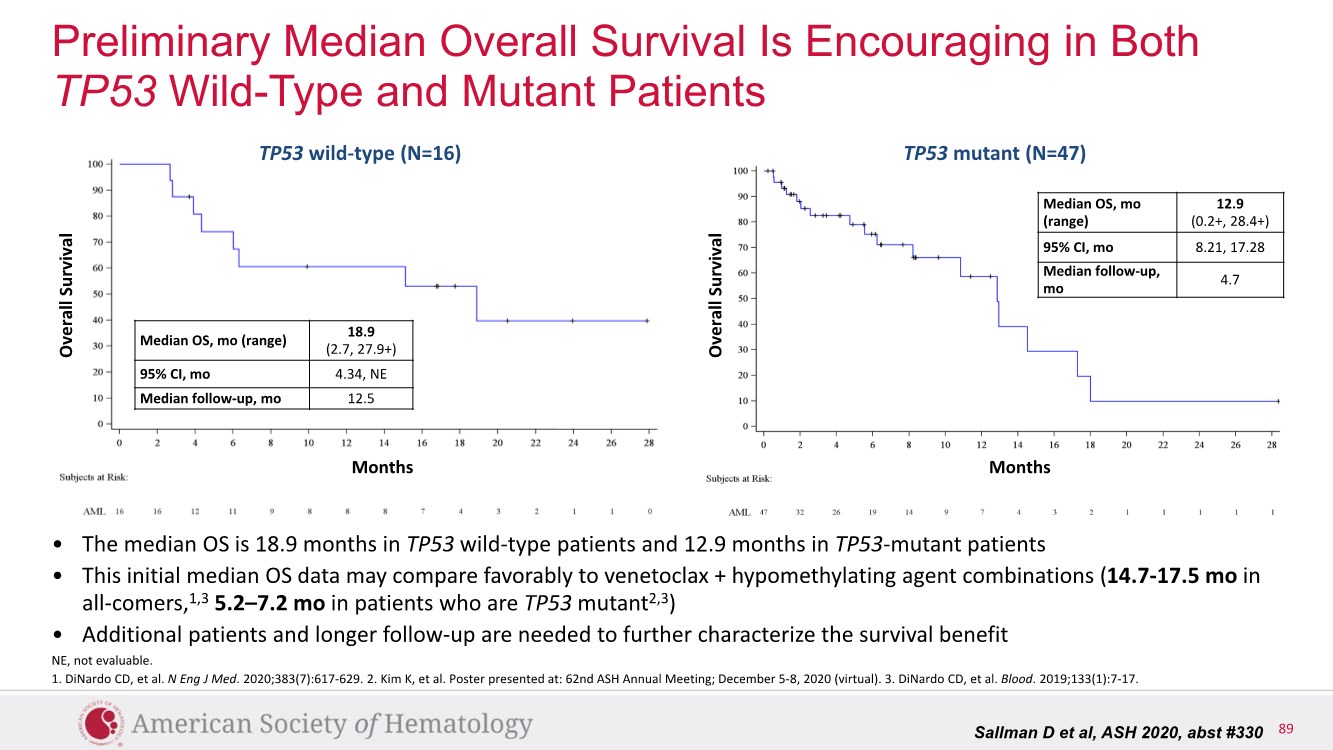
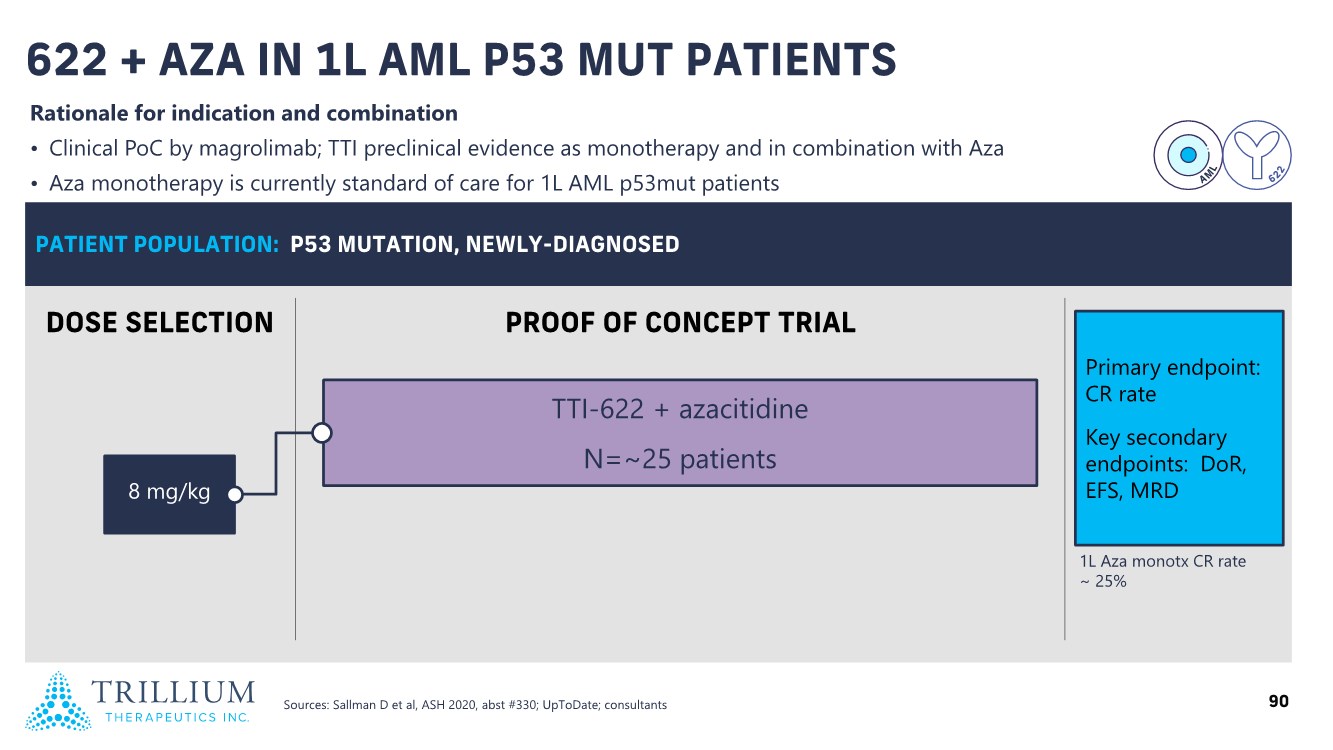
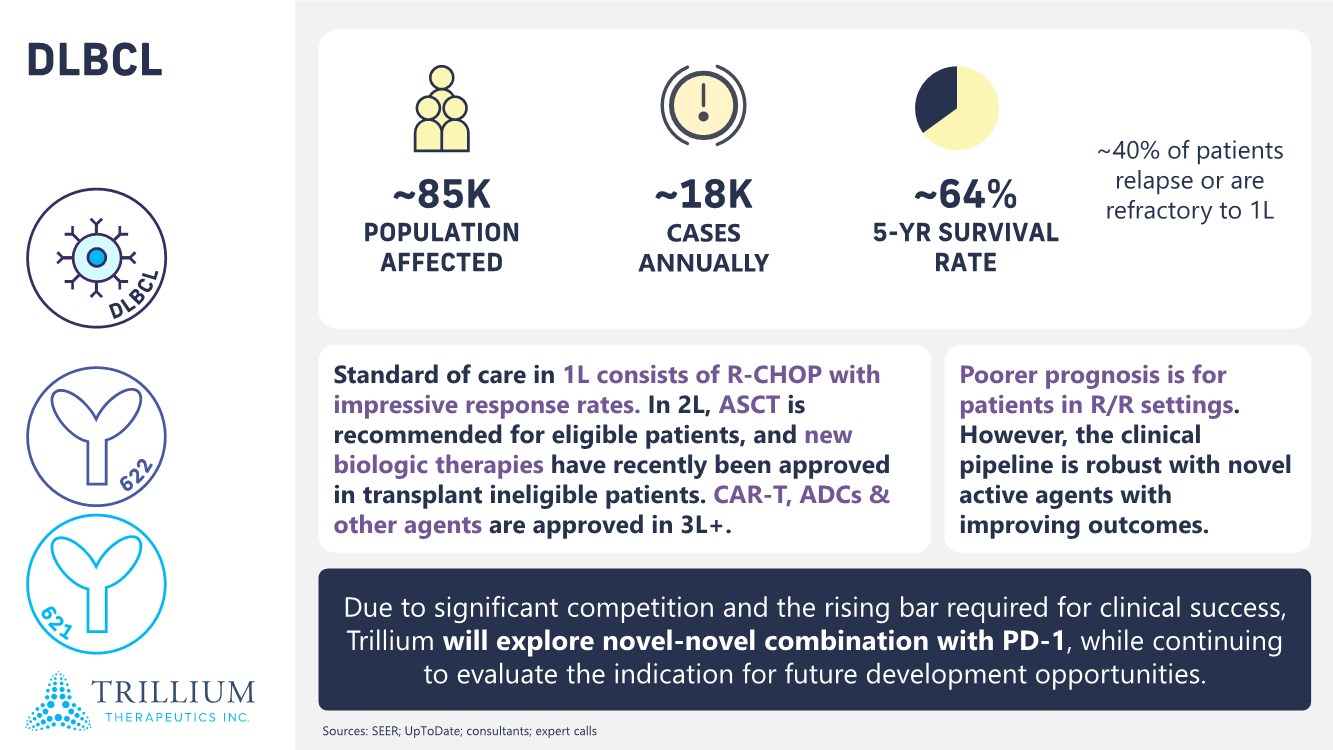
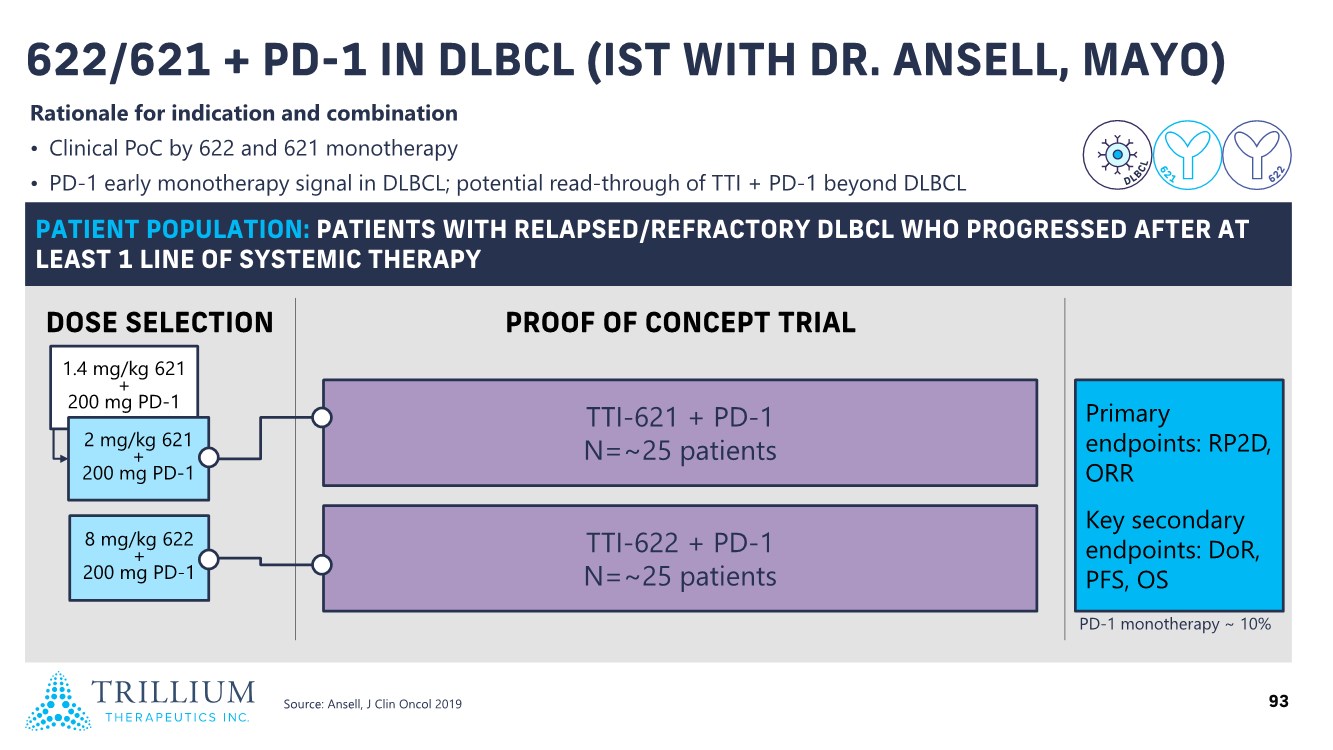
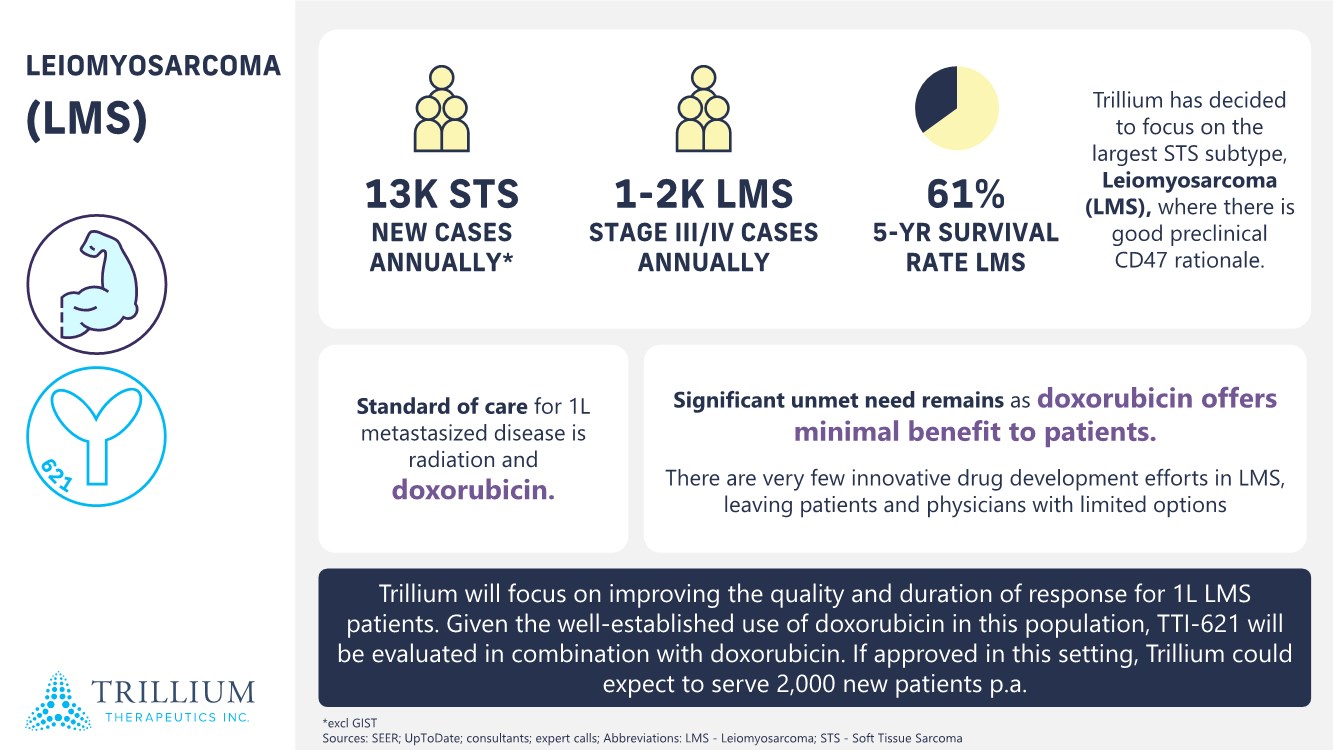
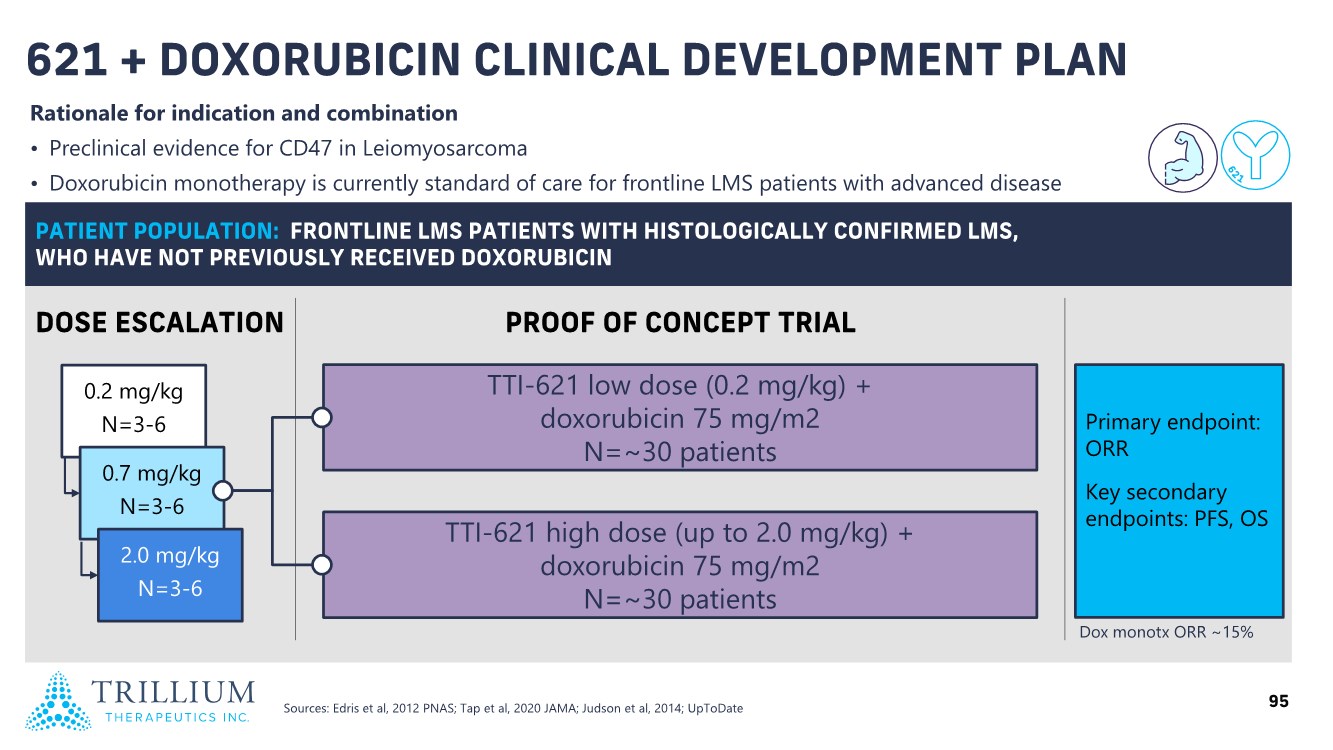
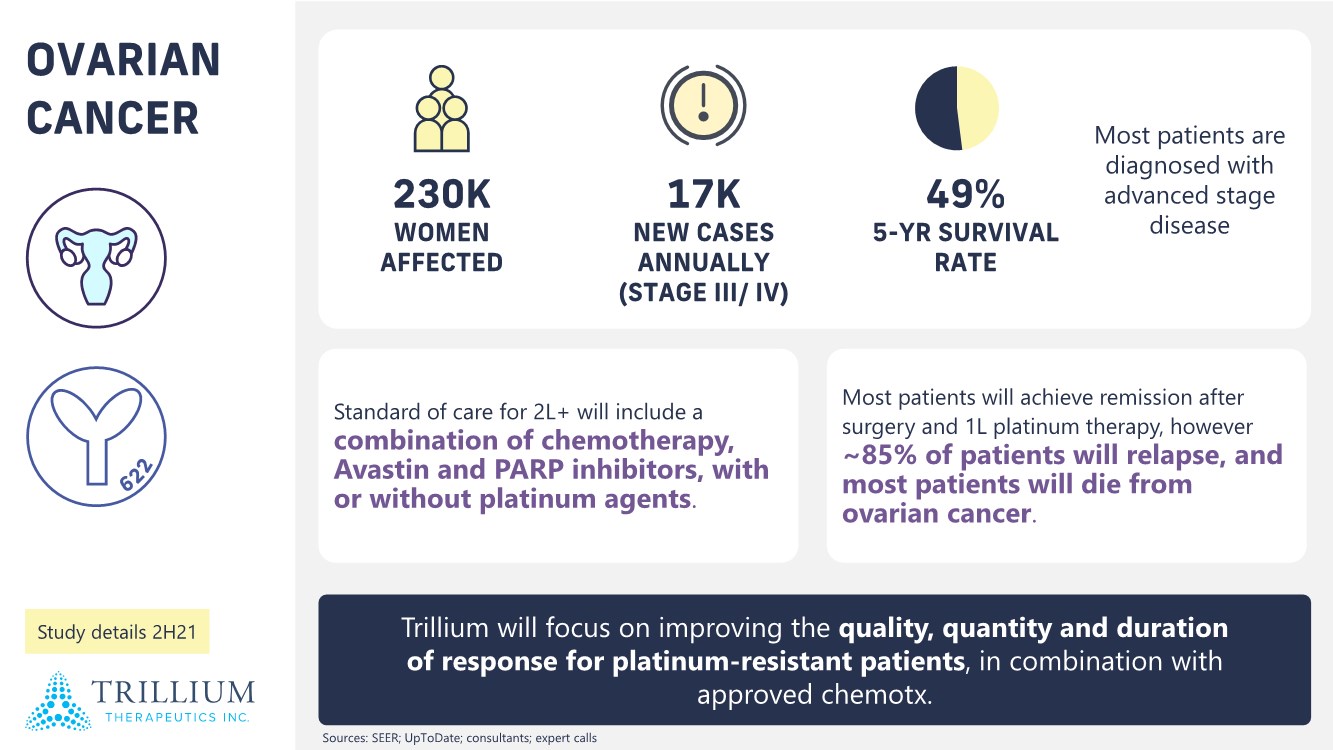
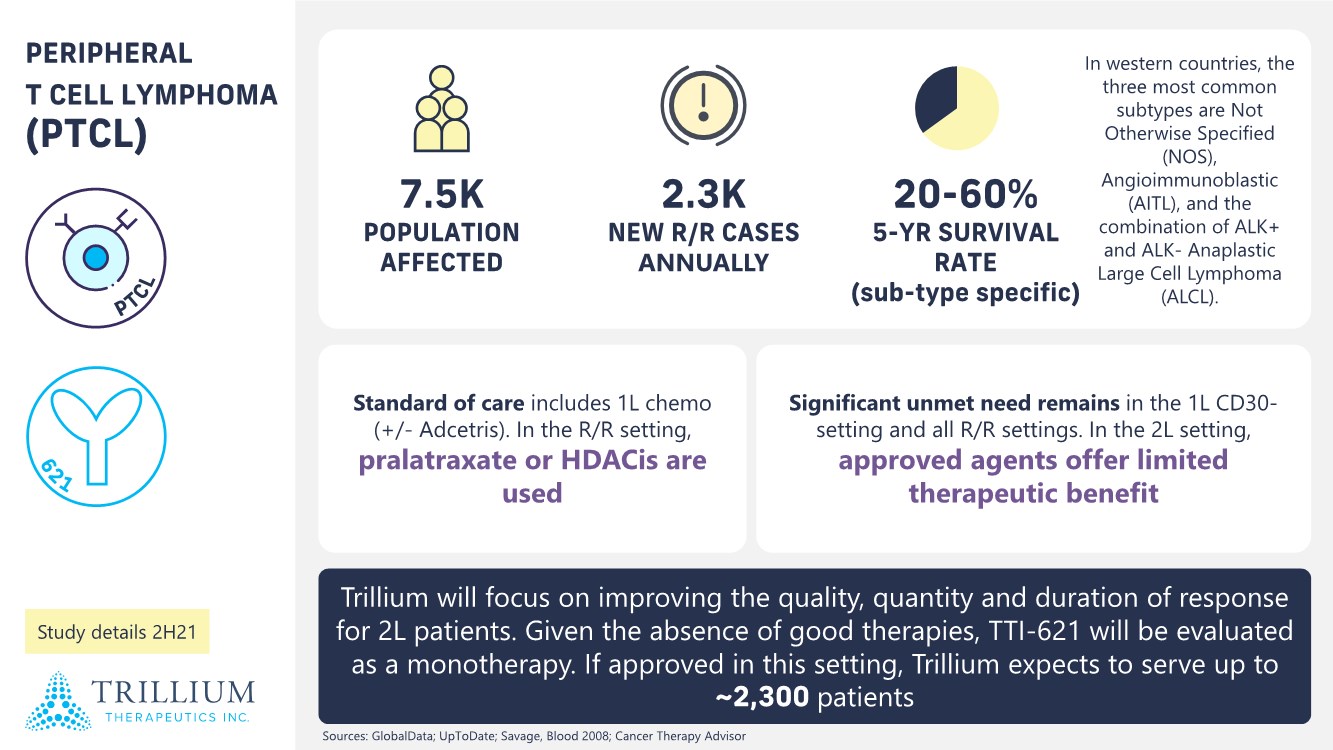
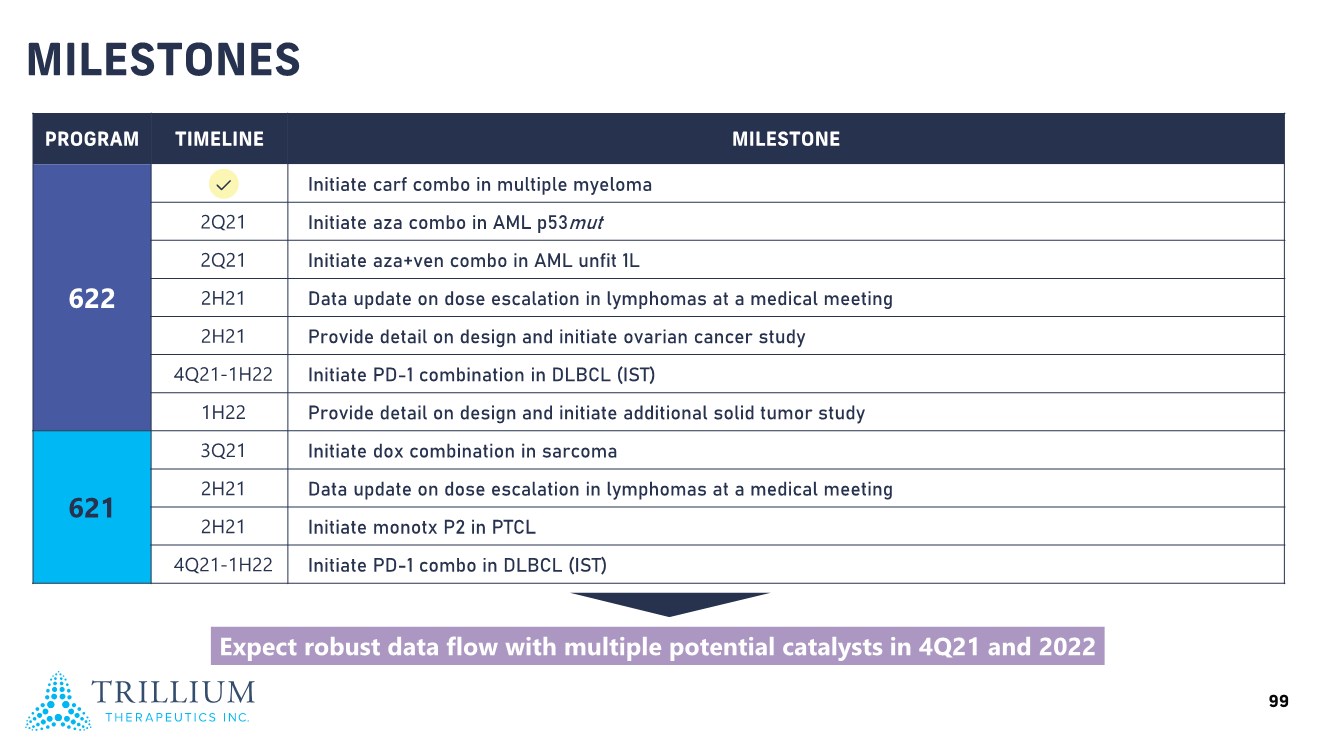
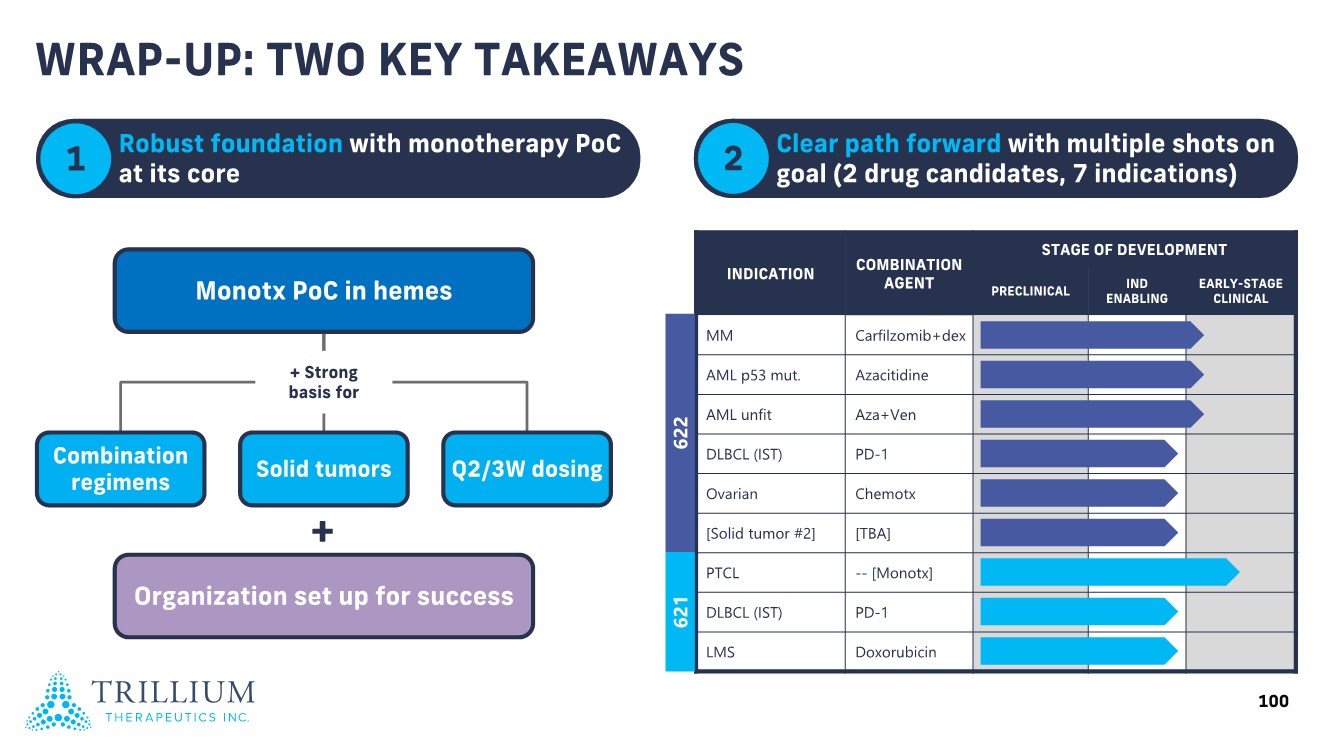
| 2 FORWARD-LOOKING STATEMENTS SAFE HARBOR This presentation contains forward-looking statements within the meaning of applicable securities laws including the Private Securities Litigation Reform Act of 1995, as amended. All statements contained herein that are not clearly historical in nature are forward-looking, and the words “anticipate”, “believe”, “expect”, “estimate”, “may”, “will”, “could”, “leading”, “intend”, “contemplate”, “shall”, “propose”, “plan” and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements in this presentation include statements about, without limitation, our clinical plans and objectives for our TTI-621 and TTI-622 programs including additional clinical trials and the expected timing thereof, the additional indications, patient populations and combination therapeutics for which we intend to study our product candidates, the timing and ability to achieve certain milestones relating to our programs, our expectation regarding accelerated regulatory pathways, and our expectations regarding our product candidates, including with respect to monotherapy activity, method of action, safety profile, solid tumor advantage and first-in-class or best-in-class status. These forward-looking statements involve risks and uncertainties that may cause actual results, events or developments to be materially different from any future results, events or developments expressed or implied by such forward-looking statements. Such factors include, but are not limited to, that preliminary or interim data from a clinical trial may not be indicative of final trial results, that clinical trial results may not be favorable; that early clinical trial results may not be indicative of later-stage clinical trial results; uncertainties inherent in the product development process (including with respect to the timing of results and whether such results will be predictive of future results); that we may not be able to obtain accelerated regulatory approval for our product candidates or at all; our ability to obtain financing to advance product candidates in our development portfolio; changing market conditions; the successful and timely completion of pre-clinical and clinical studies; the severity, duration and spread of the COVID-19 outbreak, as well as the direct and indirect impacts that the pandemic may have on our operations; the establishment of corporate alliances; the impact of competitive products and pricing; new product development risks; uncertainties related to the regulatory approval process or the ability to obtain drug product in sufficient quantity or at standards acceptable to health regulatory authorities to complete clinical trials or to meet commercial demand; and other risks detailed from time to time in Trillium's ongoing quarterly and annual reporting. A discussion of risks and uncertainties facing us appears in our annual report on Form 10-K for the year ended December 31, 2020 filed with the U.S. Securities Exchange Commission and available at www.sec.gov and www.sedar.com, each as updated by Trillium's continuous disclosure filings, which are available at www.sedar.com and at www.sec.gov. Forward-looking statements are not guarantees of future performance and accordingly undue reliance should not be put on such statements due to the inherent uncertainty therein. Any forward-looking statements speaks only as of the date on which it is made and, except as may be required by applicable securities laws, the Company disclaims any intent or obligation, whether as a result of new information, future events or results or otherwise. All forward-looking statements herein are qualified in their entirety by this cautionary statement. Note Regarding Principal Investigators: Certain portions of this presentation are provided by third-party principal investigators for the ongoing clinical trials of our product candidates. The statements and opinions contained in this presentation and made by the presenting principal investigators are solely those of the principal investigators. Note Regarding Trademarks: Any trademarks referred to in this presentation are the property of their respective owners. Appearances of such other trademarks herein should not be construed as any indicator that their respective owners will not assert their rights thereto. |