Exhibit 99.1

Breakthrough Technology That Could Transform Nerve - Targeted Treatments

Disclaimer All statements contained herein other than statements of historical fact, including statements regarding our future results of operations and financial position, our business strategy and plans, and our objectives for future operations, are forward - looking statements . The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward looking statements . We have based these forward - looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short - term and long - term business operations and objectives, and financial needs . These statements are only predictions and involve known and unknown risks, uncertainties, and other factors, including those discussed under "Risk Factors" in our Form 1 - A filed January 19 , 2024 with the Securities and Exchange Commission ("SEC") and updated from time to time in our Form 10 - Q filings and in our other public filings with the SEC . Any forward - looking statements contained in this release speak only as of its date . Moreover, we operate in a very competitive and rapidly changing environment . New risks emerge from time to time . It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward - looking statements we may make . In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward - looking statements . More detailed information about Autonomix is set forth in our filings with the Securities and Exchange Commission . Investors and security holders are urged to read these documents free of charge on the SEC’s web site at http : //www . sec . gov . The data contained herein is derived from various internal and external sources . No representation is made as to the reasonableness of the assumptions made within or the accuracy or completeness of any projections or modeling or any other information contained herein . Any data on past performance or modeling contained herein is not an indication as to future performance . Autonomix assumes no obligation to update the information in this presentation . 2

Unleashing the Power of Nerve - Targeted Treatments 3 3,000 times greater sensitivity than currently available technologies Lead program in pancreatic cancer - related pain represents compelling multi - billion - dollar opportunity Human POC clinical trial ongoing with enrollment is expected before the end of 2024 100+ patents issued and pending Advancing first - in - class catheter - based sensing technology that has ability to sense neural signals that indicate pain or disease and destroy those nerves at the source Targeting total combined $100 Billion market opportunity

The Nervous System is Responsible for Key Bodily Functions 4

The Problem The ability to target and treat nerves is severely limited because physicians lack the necessary tools to sense, target and influence neuronal signals safely and reliably. Essentially, making otherwise potentially effective treatments very risky. Creating an approach that’s been called “treat and pray.” “Treat and Pray” No More Clearer visibility and a better understanding of the nervous system can open doors to new treatments and diagnostic techniques that are currently unavailable . 5
POTENTIAL APPLICATIONS This could change everything. When it comes to the fields of neurophysiology and electrophysiology, we believe we can enable procedures like Medtronic’s renal denervation to advance from “treat and pray” to “sense, treat and verify,” which we believe may translate into faster, safer and more effective treatments. 6

It’s almost universally accepted that the current approach to pain management is broken. In 2020, pharmacies filled 142 million opioid prescriptions. Enough for 43% of Americans to receive one. These highly addictive opioids are prescribed for all sorts of pain - From cancer pain to hip replacements, and even wisdom teeth removal. Shockingly 50,000 people die each year from opioid painkiller overdoses. National Institute on Drug Abuse, 2019 Limitations of existing nerve sensing technologies result in many current non - drug based procedures being done "blind," increasing the risk of unintended adverse events. 7 POTENTIAL APPLICATIONS Pain Management

Compelling Opportunity PROBLEM OPPORTUNITY Durable Pain Relief Pancreatic tumors often cause severe pain that is very difficult to manage. We are seeking to create a minimally invasive procedure to deliver a durable and meaningful reduction in the pain of the patient. PROBLEM OPPORTUNITY Targeted Treatments Injections of ethanol are difficult to control and can destroy nerve tissue indiscriminately. Our device is being developed to travel within the vascular system and detect the individual nerves causing pain. Debilitating & Painful Lack of Precision Our first target will be the treatment of pain associated with pancreatic cancer 8 1 . Precedence Research (https://www.precedenceresearch.com/ pancreatic - cancer - market) $2.2 Billion Market 1 Significant Unmet Need Speed to Market Cost of Development

Ongoing Proof of Concept Clinical Trial in Pain Associated with Pancreatic Cancer 9 Trial to Assess the Use of Transvascular RF Ablation for the Treatment of Pancreatic C ancer P ain Study Design Timelines Number of Subjects: 20 Clinical Sites 1 Objective Ablate relevant somatic nerves and mitigate pain Q1 2024 Q1 2024 Before Year End 2024 x Secures Principal Investigators Commence Enrollment and Treatments Complete Enrollment 1H 2025 Topline data from POC Trial

Proof - of - Concept in Lead Indication Opens Up a >$100 Billion Total Market Opportunity 10 1. https:// www.mordorintelligence.com /industry - reports/pain - management - market 2. https:// www.polarismarketresearch.com /industry - analysis/global - hypertension - drug - market 3. https:// www.precedenceresearch.com /chronic - obstructive - pulmonary - disease - treatment - market

Think of our device candidate as a GPS for the nervous system. Like the MRI, our technology may provide a significant leap forward in diagnostic abilities. Detects and differentiates signals from the nervous system throughout the body with extremely high sensitivity. Our catheter - based platform is being designed to be small enough to reach any organ, with a low - cost disposable. Our Breakthrough Solution 11

Patented Catheter Technology Custom Antenna Array Our state - of - the - art custom antennae array is being designed to detect, map, display and modulate the problem accurately and safely. Guidewire & Catheter Bodies Small enough to reach any organ. Digital rendering, not final device. Proprietary Chipset Equipped with embedded microelectronics, our patented chipset enables robust nerve signal processing at the source. 12

13 100 μ V 1 - 2 μ V Cardiac Signal Strength Neural Signal Strength
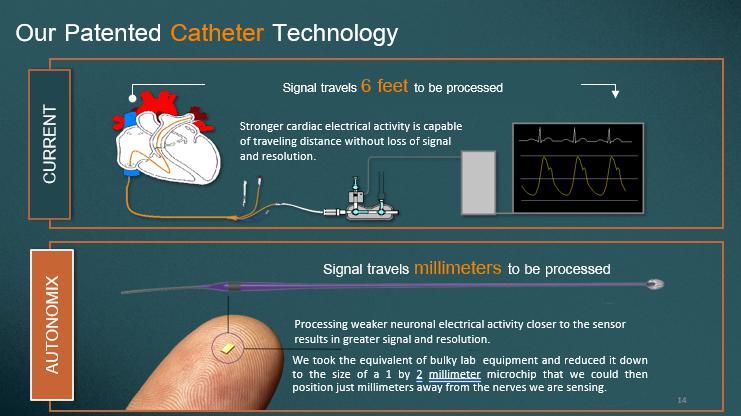
Our Patented Catheter Technology We took the equivalent of bulky lab equipment and reduced it down to the size of a 1 by 2 millimeter microchip that we could then position just millimeters away from the nerves we are sensing . 14 Signal travels millimeters to be processed AUTONOMIX Stronger cardiac electrical activity is capable of traveling distance without loss of signal and resolution. CURRENT Signal t ravels 6 feet to be p rocessed Processing weaker neuronal electrical activity closer to the sensor results in greater signal and resolution.

Replaces This (Typical Catheter Lab) Our Patented Catheter Technology Such a small form factor microchip, similar to the one used in today's Apple Watch, wasn't commercially available until recently . We not only own the exclusive patent rights to this intravascular approach, but we’ve proven it works . 15

PROPRIETARY CATHETER IN ARTERY TARGET NERVES (SHOWN IN YELLOW) By placing our proprietary chip on the tip of the catheter, we expect to be able to sense, identify, and treat nerves with 3000x more sensitivity than alternative methods. 16

We are developing a disruptive technology at the center of the same industry as Elon Musk’s Neuralink and where players like Medtronic and Boston Scientific have been actively engaged in acquisitions. $ 100 b EP Market Opportunity 50 + Years of Combined Experience 100 + Over 100 Patents Issued or Pending with 15 Distinct, Wholly - Owned Patent Families 17

Potential Exit Opportunity Early Exit Opportunities All Potential Partners Multiple Large Players Robust M&A Environment Target Transaction Focus Stage $925 Million $575 Million $761 Million Ser A $425 Million $205 Million Ser C Undisclosed (1/22) (6/22) (1/22) (10/22) (1/22) (7/18) A - Fib Ablation A - Fib Ablation Nerve Stim Cuff Neural Implants Neural Implants U - Sound Ablation Clinical Pivotal / CE Clinical Animal Only Preclinical Pivotal / CE 18

Financial Snapshot Nasdaq: AMIX 19 $11.2 Million Initial Public Offering Closed January 26, 2024 $ 84 M 112 K 18.6 M Market Cap 1 Average Volume 1 Shares Outstanding 2 1: As of February 26, 2024 with a closing price of $4.53 2: As of January 26, 2024

Timeline 2024 Sensing Technology Development Sensing Safety in Animals Animal Safety Testing Human Clinical Ablation Device Development POC - First in Human Clinical Trial - Pancreatic Cancer Pain Combined Sensing / Ablation Clinical Trial 2025 Ablation System and Catheter Ready for Human Pivotal Combined Sensing / Ablation Clinical Trial SENSING DEVELOPMENT ABLATION DEVELOPMENT CLINICAL TRIALS REGULATORY 2026 De Novo Submission Transfer t o Manufacturing FDA Clearance 2027 Industry exit window 20 *Planned development timeline. These timeframes are not guarantees and this timeline is subject to change.

Proven Team with Multiple Successful Exits EXECUTIVE CHAIRMAN CHIEF EXECUTIVE OFFICER CO - FOUNDER, CMO CO - FOUNDER, CTO CHIEF FINANCIAL OFFICER Wally Klemp Lori Bisson Dr. Robert Schwartz Landy Toth Trent Smith 21 Acquisition $550 Million Acquisition >$1 Billion Annual Revenue • Med device exit to AbbVie (Nasdaq: SOLY; $550 Million 12/21) • Four successful IPOs • ~$1 billion development and growth stage financing • 3 successful IPOs • Med device exit to AbbVie (Nasdaq: SOLY; $550 Million 12/21) • Deep life science/med tech experience • Inventor of Watchman TM ($1 billion revs; sold to Boston Sci) • Mayo Clinic • Dir. Minneapolis Heart Institute • Interventional cardiologist • Mechanical engineer/medical device expert • Commercialization of wearable and interventional diagnostic medical technologies • Med device exit to AbbVie (Nasdaq: SOLY; $550 Million 12/21) • SEC reporting background • Med tech background

22 Unleashing the Power of Nerve - Targeted Treatments Investment Summary Targeting Total Combined $100 Billion Market Opportunity Transforming the diagnosis and treatment of pain and diseases of the nervous system Ongoing human clinical trial with multiple near - term milestones Proven Team with Multiple Successful Exits

Company Investor / Media Relations Autonomix Medical, Inc. 21 Waterway Ave, Suite 300 The Woodlands, TX 77380 833 - 475 - 8247 autonomix@jtcir.com autonomix.com