|
Exhibit 99.1 
|
Monthly injectables for chronic dog and cat pain
Clinical data and discussion
ACVIM Forum 2016
Welcome!
Introductions and overview
Agenda
Dr. Jürgen Horn Dr. med.vet, PhD, MBA
Overview
Prof. Duncan Lascelles BSc, BVSc, PhD, MRCVS, CertVA, DSAS(ST), DECVS, DACVS
Chronic pain—management & measurement (dogs and cats)
Dr. Colin Giles BVetMed, PhD, MRCVS
Ranevetmab – clinical data and background
Dr. Jane Eagleson BVSc, MVSc
NV-02 – clinical data and background
Forward-looking statements
This presentation and other presentations given today contain forward-looking statements. All statements, other than statements of historical facts, contained in these slides and the accompanying oral presentation, including statements regarding our future results of operations and financial position, business strategy, drug discovery, results of future studies, prospective products, product approvals, research and development costs, manufacturing facility operations and costs, timing and likelihood of success, regulatory approvals, plans and objectives of management for future operations, and future results of current and anticipated products, are forward-looking statements. These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. The words “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “goal,” “intend,” “may,” “might,” “objective,” “plan,” “potential,” “predict,” “project,” “positioned,” “seek,” “should,” “target,” “will,” “would,” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are based on current expectations, estimates, forecasts and projections about our business and the industry in which we operate and management’s beliefs and assumptions, are not guarantees of future performance or development and involve known and unknown risks, uncertainties, and other factors, including: our ability to become profitable; our expected uses of our capital; our ability to secure additional financing when needed on acceptable terms; our expectation regarding the safety and efficacy our lead product candidates; our ability to advance our lead product candidates through various stages of development, especially through pivotal safety and efficacy studies; our ability to develop future additional product candidates; our ability to obtain regulatory approval for our current or future product candidates under applicable regulatory requirements; our ability to discover, develop and manufacture biologics which involves relatively novel technology and an expensive and lengthy approval process with uncertain outcomes; our ability to successfully manufacture our own products, our reliance on third-party suppliers and distributors to supply and distribute our lead product candidates for us; the rate and degree of market acceptance and the successful commercial sale of our lead product candidates, if approved;
regulatory and legal developments in the United States and foreign countries; the success of competing platforms or therapies that are or become available; our ability to attract or retain key employees, advisors or consultants; our expectations and statements regarding the potential size, opportunity and growth potential of the companion animal therapeutics market, and our ability to effectively compete and achieve significant market penetration; our ability to obtain and maintain patent protection for our lead product candidates; the implementation of our business model and strategic plans for our business, products and technology; developments and growth relating to our industry; and uncertainties and assumptions described in greater detail in our annual report on Form 10-K, quarterly report on Form 10-Q and the other documents we have filed with the Securities and Exchange Commission (the “SEC”). We do not assume any obligation to update any forward-looking statements except as required by applicable law.
Certain information contained in this presentation concerning our industry and the markets in which we operate is based on information from our management estimates and primary market research commissioned by us, as well as from industry and general publications and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. This information, however, involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such information.
PETization, PETisation, Tevxen, BioNua, Nexvet, our logo and its other registered or common law trademarks, trade names or service marks appearing in this presentation are owned by us. Other trademarks, trade names or service marks appearing in this presentation are the property of their respective owners. Solely for convenience, our trademarks and tradenames appear without the ® or ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the right of the applicable licensor to these trademarks and tradenames.
100% biologics animal health company, focused on targets de-risked by late-stage human data
Unique target product profiles: a leap forward for animal health
Positive clinical studies (efficacy and safety) for lead program in chronic pain in dogs and cats
Clinically validated platform technology for interspecies mAb conversion, “PETization”
Global company located in Australia, Ireland and US. Multiple collaborations and partnerships with animal health companies
Vertically integrated research – clinical -manufacturing
Therapeutic focus areas
Nexvet is focused on monoclonal antibodies in four major therapeutic areas:
Pain Inflammation Allergy Immuno-oncology
Focus on chronic conditions with significant unmet medical need
Targets validated by late-stage human data
Chronic Pain (key target: NGF)
Chronic inflammation (key target: TNF)
Ranevetmab
NV-02 (cat)
Lead ID of new candidates based on validated human targets
Multiple mAb research candidates targeting TNF
Immuno-oncology (key target: PD-1)
PD-1 program f Others against targets validated in humans
PETization: a platform technology for rapid interspecies mAb design
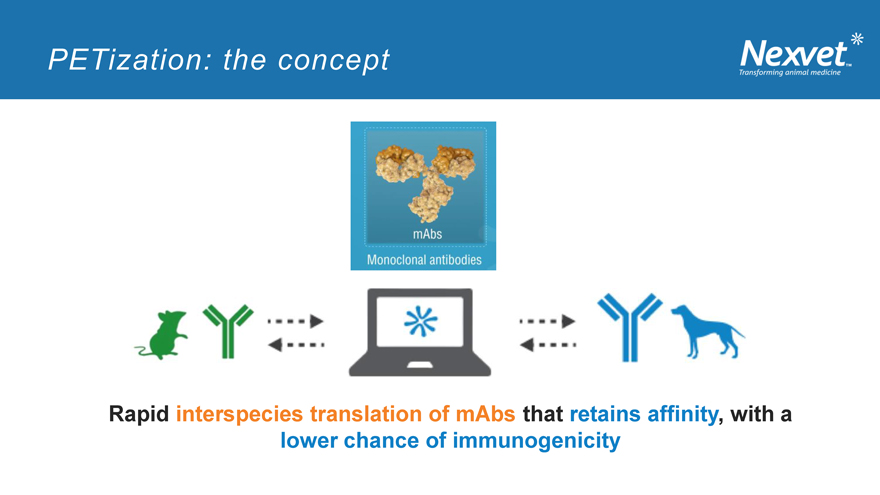
PETization: the concept
Rapid interspecies translation of mAbs that retains affinity, with a lower chance of immunogenicity
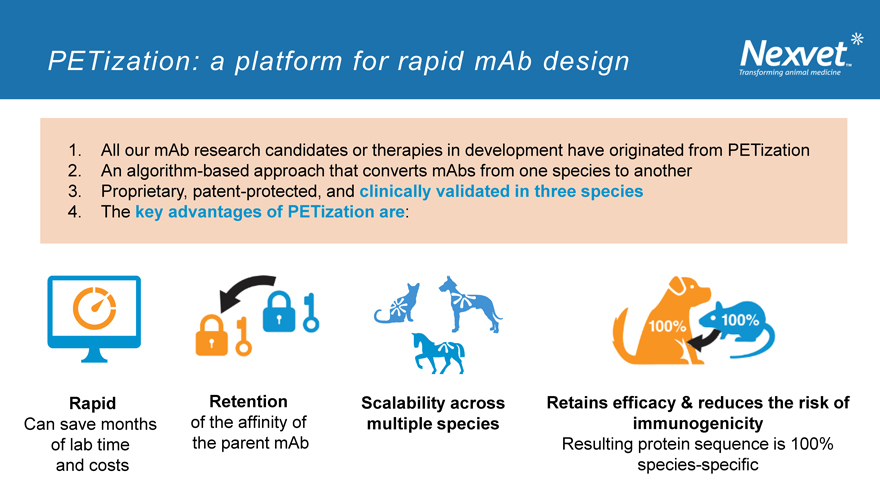
PETization: a platform for rapid mAb design
1. All our mAb research candidates or therapies in development have originated from PETization
2. An algorithm-based approach that converts mAbs from one species to another
3. Proprietary, patent-protected, and clinically validated in three species
4. The key advantages of PETization are:
Rapid Retention Scalability across Retains efficacy & reduces the risk of
Can save months of the affinity of multiple species immunogenicity
of lab time the parent mAb Resulting protein sequence is 100%
and costs species-specific
The time for pet biologics is now
Transforming platform: PETization
Nexvet: focused on delivering the therapeutic biologics revolution to veterinary care.
ultiple candidates ultiple clinical successes ractive efficacy and safety profiles Indications with significant medical need
Ranevetmab | a novel caninized anti-NGF monoclonal antibody for the control of pain associated with osteoarthritis in dogs Background and pivotal study results
Dr Colin Giles
Vice President – Clinical and Regulatory Affairs June 2016
Special note about therapies in development
The presentations made today include results of clinical trials for the following agents that are currently still in development and not approved for marketing: ranevetmab and NV-02.
Overview and scientific background to ranevetmab
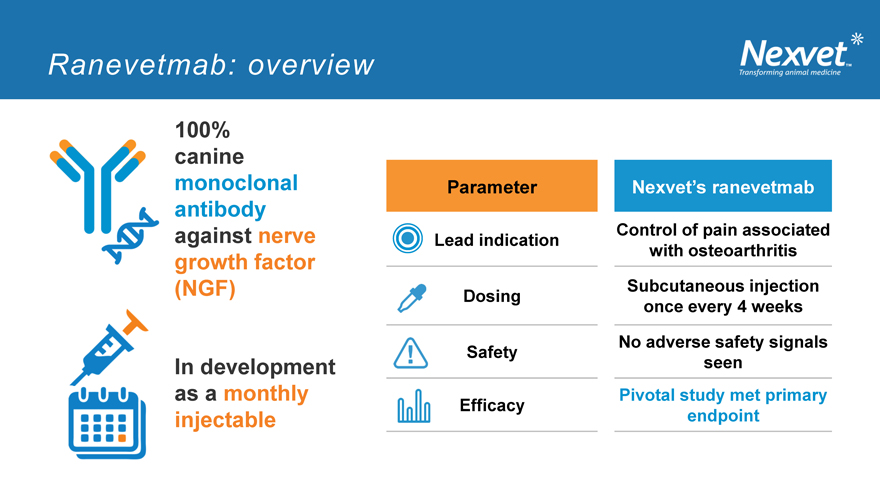
Ranevetmab: overview
100% canine monoclonal antibody against nerve growth factor
(NGF)
In development as a monthly injectable
Parameter Nexvet’s ranevetmab
Control of pain associated Lead indication with osteoarthritis
Subcutaneous injection Dosing once every 4 weeks
No adverse safety signals Safety seen Pivotal study met primary Efficacy endpoint
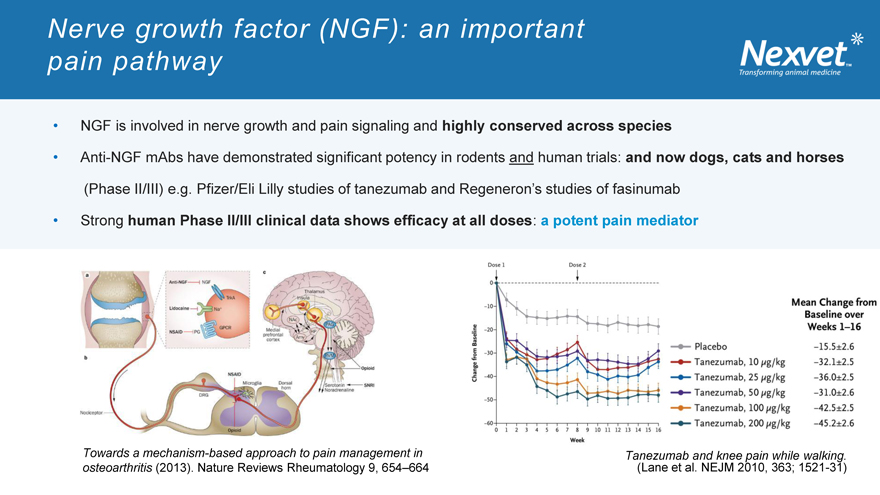
Nerve growth factor (NGF): an important pain pathway
NGF is involved in nerve growth and pain signaling and highly conserved across species
Anti-NGF mAbs have demonstrated significant potency in rodents and human trials: and now dogs, cats and horses (Phase II/III) e.g. Pfizer/Eli Lilly studies of tanezumab and Regeneron’s studies of fasinumab Strong human Phase II/III clinical data shows efficacy at all doses: a potent pain mediator
Towards a mechanism-based approach to pain management in osteoarthritis (2013). Nature Reviews Rheumatology 9, 654–664
Tanezumab and knee pain while walking.
(Lane et al. NEJM 2010, 363; 1521-31)
Ranevetmab: detailed description
Novel biotherapeutic veterinary medicine under clinical investigation for the control of pain associated with OA in dogs
Fully caninized“100% dog” monoclonal antibody that neutralizes nerve growth factor(NGF)
Produced as a secreted product from a genetically engineered CHO cell line, as a disulfide-linked, glycosylated, tetramer consisting of two identical 453 amino acid heavy gamma chains and two identical 217 amino acid kappa light chains.
Formulated as a sterile buffered solution of 2 mg/mL packaged in single-use glass vials for SC injection intended for administration to dogs only.
Under clinical investigation and currently NOT approved for use
mAbs
Monoclonal antibodies
Proof-of-concept data and pivotal study design
Earlier studies demonstrated:
Ranevetmab binds NGF with high affinity and is a potent inhibitor of canine NGF.
Ranevetmab does not recruit complement
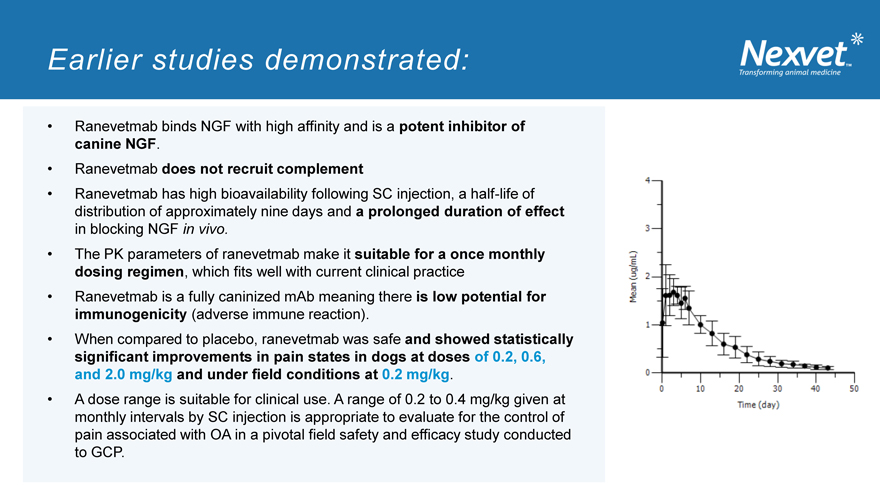
Ranevetmab has high bioavailability following SC injection, a half-life of distribution of approximately nine days and a prolonged duration of effect in blocking NGF in vivo.
The PK parameters of ranevetmab make it suitable for a once monthly dosing regimen, which fits well with current clinical practice
Ranevetmab is a fully caninized mAb meaning there is low potential for immunogenicity (adverse immune reaction).
When compared to placebo, ranevetmab was safe and showed statistically significant improvements in pain states in dogs at doses of 0.2, 0.6, and 2.0 mg/kg and under field conditions at 0.2 mg/kg.
A dose range is suitable for clinical use. A range of 0.2 to 0.4 mg/kg given at monthly intervals by SC injection is appropriate to evaluate for the control of pain associated with OA in a pivotal field safety and efficacy study conducted to GCP.
Pivotal study design
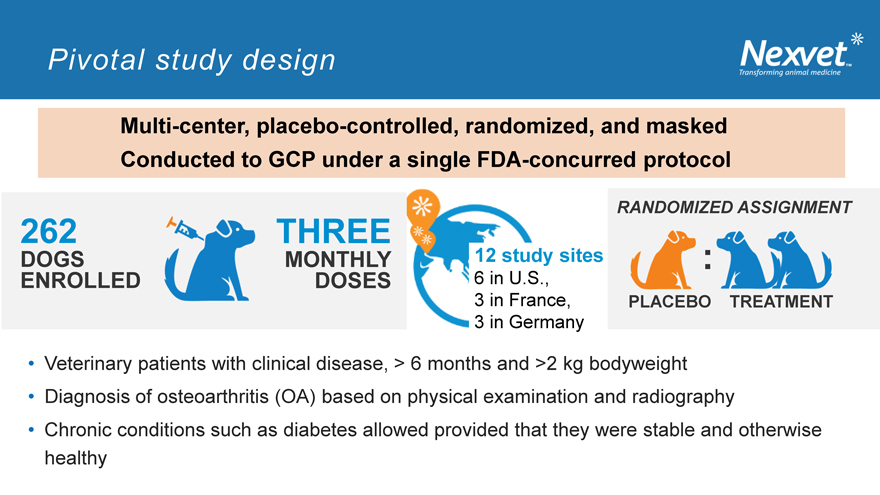
Multi-center, placebo-controlled, randomized, and masked Conducted to GCP under a single FDA-concurred protocol
262
DOGS ENROLLED
THREE
MONTHLY
DOSES
12 study sites
6 in U.S., 3 in France,
3 in Germany
RANDOMIZED ASSIGNMENT
:
PLACEBO TREATMENT
Veterinary patients with clinical disease, > 6 months and >2 kg bodyweight
Diagnosis of osteoarthritis (OA) based on physical examination and radiography
Chronic conditions such as diabetes allowed provided that they were stable and otherwise healthy
Ranevetmab pivotal study design: FDA-concurred protocol
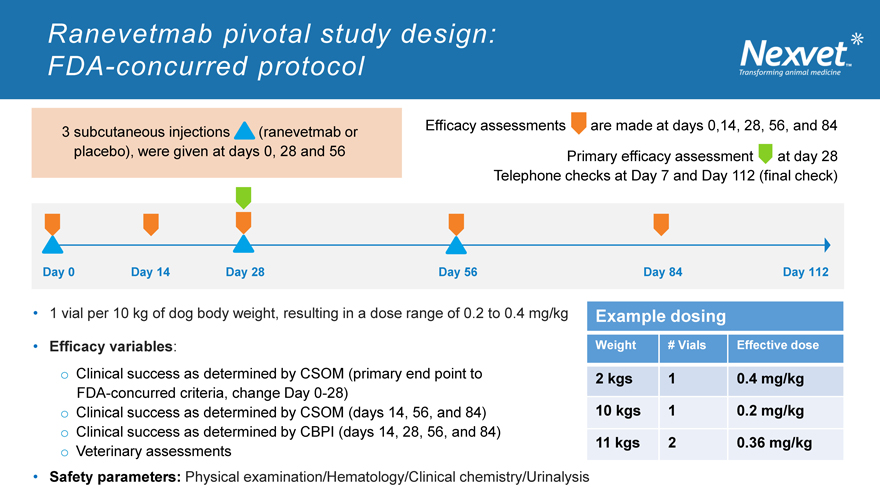
3 subcutaneous injections (ranevetmab or placebo), were given at days 0, 28 and 56
Efficacy assessments are made at days 0,14, 28, 56, and 84
Primary efficacy assessment at day 28
Telephone checks at Day 7 and Day 112 (final check)
1 vial per 10 kg of dog body weight, resulting in a dose range of 0.2 to 0.4 mg/kg
Efficacy variables: o Clinical success as determined by CSOM (primary end point to FDA-concurred criteria, change Day 0-28) o Clinical success as determined by CSOM (days 14, 56, and 84) o Clinical success as determined by CBPI (days 14, 28, 56, and 84) o Veterinary assessments
Safety parameters: Physical examination/Hematology/Clinical chemistry/Urinalysis
Example dosing
Weight # Vials Effective dose
2 kgs 1 0.4 mg/kg
10 kgs 1 0.2 mg/kg
11 kgs 2 0.36 mg/kg
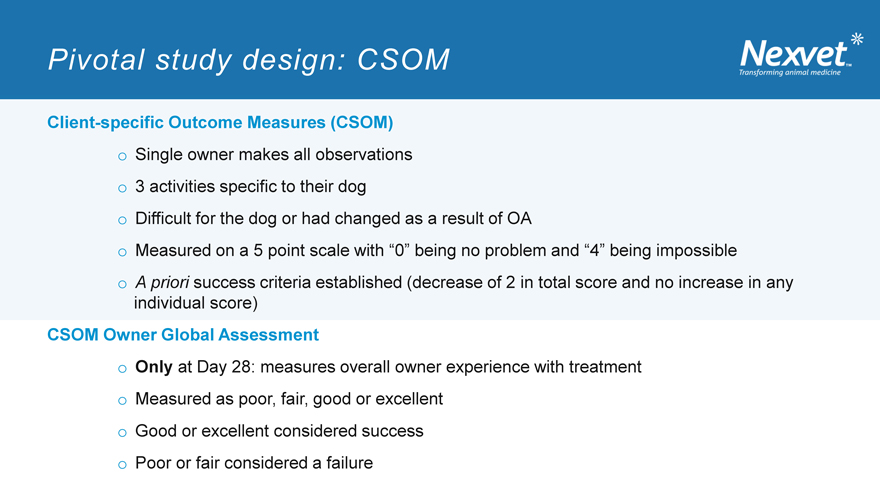
Pivotal study design: CSOM
Client-specific Outcome Measures (CSOM) o Single owner makes all observations o 3 activities specific to their dog o Difficult for the dog or had changed as a result of OA o Measured on a 5 point scale with “0” being no problem and “4” being impossible o A priori success criteria established (decrease of 2 in total score and no increase in any individual score)
CSOM Owner Global Assessment o Only at Day 28: measures overall owner experience with treatment o Measured as poor, fair, good or excellent o Good or excellent considered success o Poor or fair considered a failure
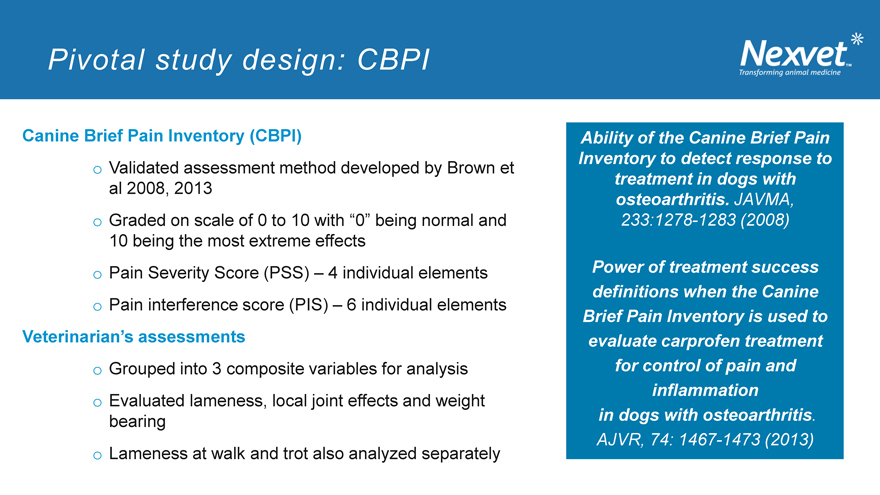
Pivotal study design: CBPI
Canine Brief Pain Inventory (CBPI) o Validated assessment method developed by Brown et al 2008, 2013 o Graded on scale of 0 to 10 with “0” being normal and
10 being the most extreme effects o Pain Severity Score (PSS) – 4 individual elements o Pain interference score (PIS) – 6 individual elements
Veterinarian’s assessments o Grouped into 3 composite variables for analysis o Evaluated lameness, local joint effects and weight bearing o Lameness at walk and trot also analyzed separately
Ability of the Canine Brief Pain Inventory to detect response to treatment in dogs with osteoarthritis. JAVMA,
233:1278-1283 (2008)
Power of treatment success definitions when the Canine Brief Pain Inventory is used to evaluate carprofen treatment for control of pain and inflammation in dogs with osteoarthritis.
AJVR, 74: 1467-1473 (2013)
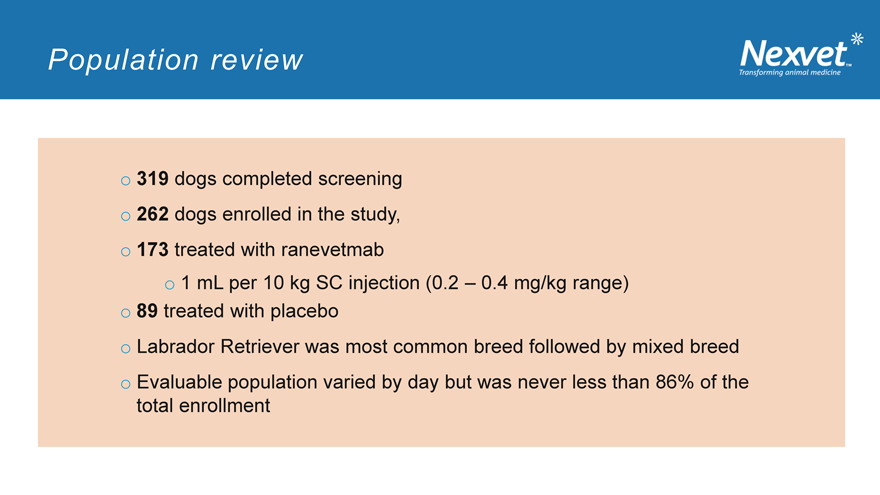
Population review
o 319 dogs completed screening o 262 dogs enrolled in the study, o 173 treated with ranevetmab o 1 mL per 10 kg SC injection (0.2 – 0.4 mg/kg range) o 89 treated with placebo o Labrador Retriever was most common breed followed by mixed breed o Evaluable population varied by day but was never less than 86% of the total enrollment
Ranevetmab pivotal safety and efficacy study results
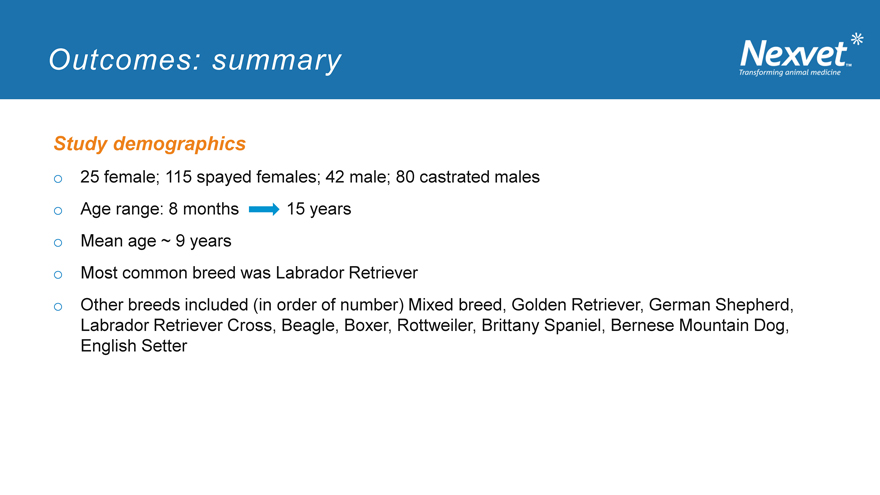
Outcomes: summary
Study demographics o 25 female; 115 spayed females; 42 male; 80 castrated males o Age range: 8 months 15 years o Mean age ~ 9 years o Most common breed was Labrador Retriever o Other breeds included (in order of number) Mixed breed, Golden Retriever, German Shepherd, Labrador Retriever Cross, Beagle, Boxer, Rottweiler, Brittany Spaniel, Bernese Mountain Dog, English Setter
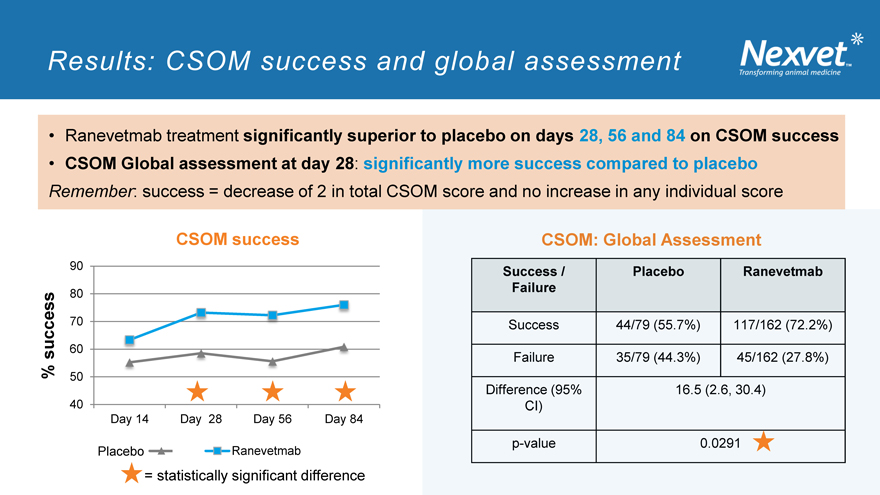
Results: CSOM success and global assessment
Ranevetmab treatment significantly superior to placebo on days 28, 56 and 84 on CSOM success
CSOM Global assessment at day 28: significantly more success compared to placebo
Remember: success = decrease of 2 in total CSOM score and no increase in any individual score
CSOM success
80
70
success 60
% 50 40
Day 14 Day 28 Day 56 Day 84
Placebo Ranevetmab
= statistically significant difference
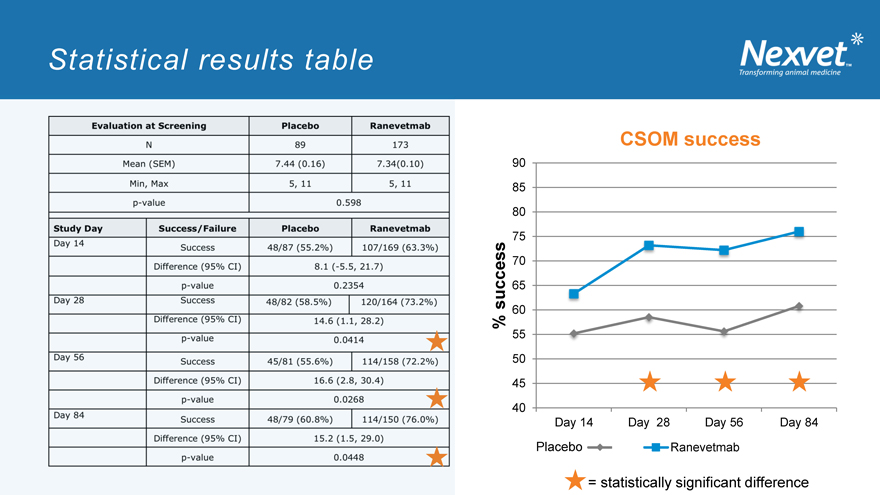
Statistical results table
Evaluation at Screening Placebo Ranevetmab
N 89 173
Mean (SEM) 7.44(0.16) 734(0.10)
Min, Max 5,11 5,11
p-value 0.598
Study Day Success/Failure Placebo Ranevetmab
Day 14 Success 48/87(55.2%) 107/169 (63.3%)
Difference (95% CI) 8.1 (-5.5, 21.7)
p-value 0.2354
Day 28 Success 48/82 (58.5%) 120/164 (73.2%)
Difference (95% CI) 14.6 (1.1, 28.2)
p-value 0.0414
Day 56 Success 45/81 (55.6%) 114/158 (72.2%)
Difference (95% CI) 16.6 (2.8, 30.4)
p-value 0.0268
Day 84 Success 48/79 (60.8%) 114/150 (76.0%)
Difference (95% CI) 15.2 (1.5, 29.0)
p-value 0.0448
CSOM success
% success
90 85 80 75 70 65 60 55 50 45 40
Day 14 Day 28 Day 56, Day 84
Placebo Ranevetmab
= statistically significant difference
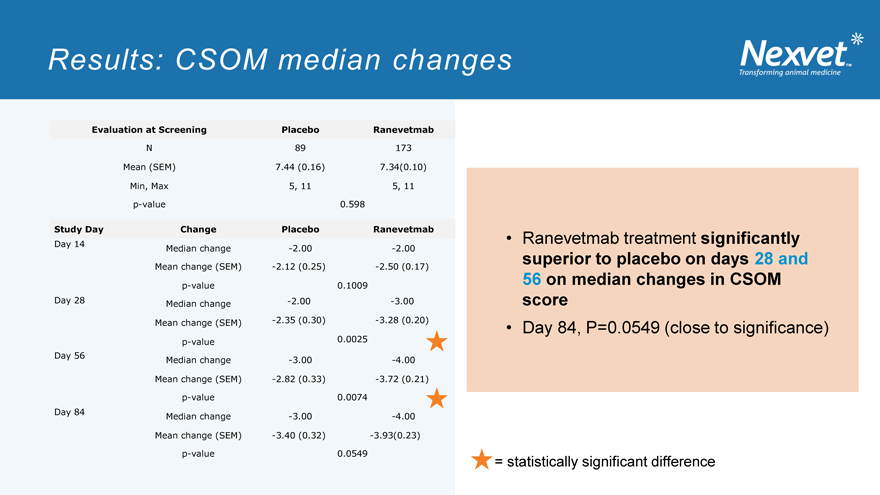
Results: CSOM median changes
Evaluation at Screening Placebo Ranevetmab
N 89 173 Mean (SEM) 7.44 (0.16) 7.34(0.10) Min, Max 5, 11 5, 11 p-value 0.598
Study Day Change Placebo Ranevetmab
Day 14
Median change -2.00 -2.00 Mean change (SEM) -2.12 (0.25) -2.50 (0.17) p-value 0.1009 Day 28 Median change -2.00 -3.00 Mean change (SEM) -2.35 (0.30) -3.28 (0.20) 0.0025 p-value Day 56 Median change -3.00 -4.00 Mean change (SEM) -2.82 (0.33) -3.72 (0.21) p-value 0.0074 Day 84 Median change -3.00 -4.00 Mean change (SEM) -3.40 (0.32) -3.93(0.23) p-value 0.0549
Ranevetmab treatment significantly superior to placebo on days 28 and 56 on median changes in CSOM score
Day 84, P=0.0549 (close to significance)
= statistically significant difference
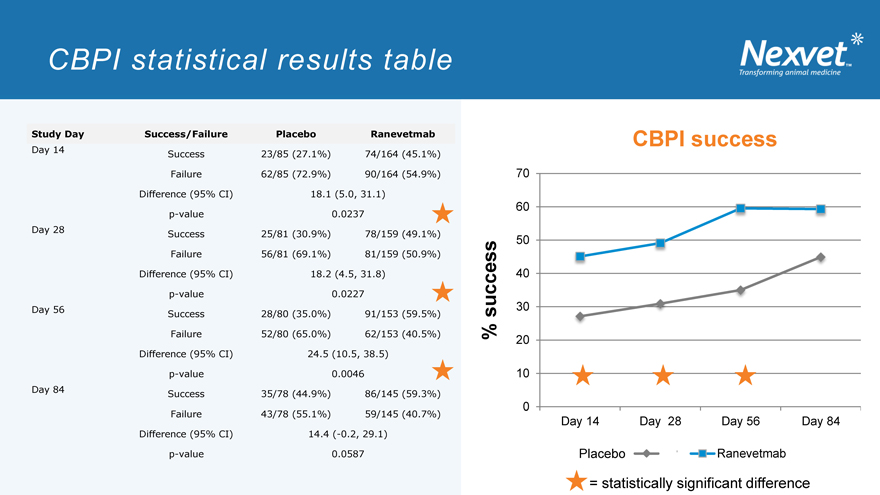
Results: CBPI successes
Ranevetmab treatment significantly superior to placebo on days 14, 28 and 56
(P<0.05) (day 84, P=0.059)
= statistically significant difference
CBPI success
60
50
40
success 30
% 20 10
0
Day 14 Day 28 Day 56 Day 84
Placebo Ranevetmab
CBPI statistical results table
Study Day Success/Failure Placebo Ranevetmab
Day 14 Success 23/85 (27.1%) 74/164 (45.1%) Failure 62/85 (72.9%) 90/164 (54.9%) Difference (95% CI) 18.1 (5.0, 31.1) p-value 0.0237 Day 28 Success 25/81 (30.9%) 78/159 (49.1%) Failure 56/81 (69.1%) 81/159 (50.9%) Difference (95% CI) 18.2 (4.5, 31.8) p-value 0.0227 Day 56 Success 28/80 (35.0%) 91/153 (59.5%) Failure 52/80 (65.0%) 62/153 (40.5%) Difference (95% CI) 24.5 (10.5, 38.5) p-value 0.0046 Day 84 Success 35/78 (44.9%) 86/145 (59.3%) Failure 43/78 (55.1%) 59/145 (40.7%) Difference (95% CI) 14.4 (-0.2, 29.1) p-value 0.0587
CBPI success
70 60 50
40
success 30 %
20
10
0
Day 14 Day 28 Day 56 Day 84
Placebo Ranevetmab
= statistically significant difference
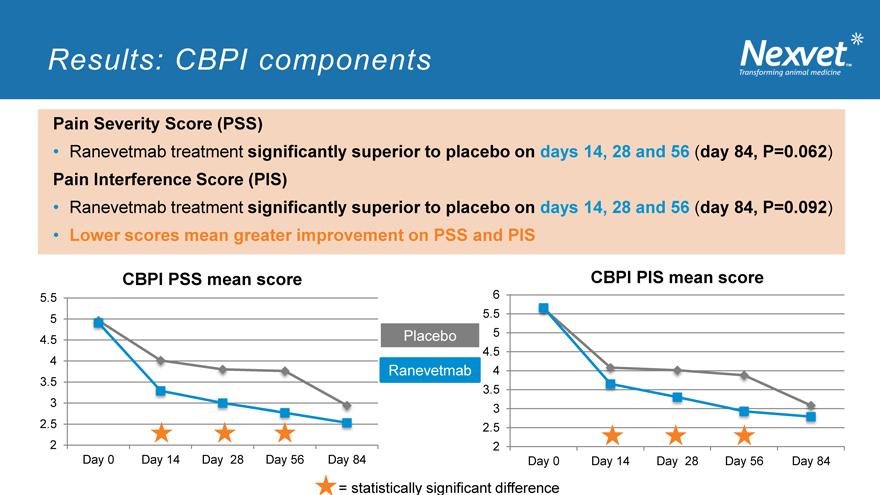
Results: CBPI components
Pain Severity Score (PSS)
Ranevetmab treatment significantly superior to placebo on days 14, 28 and 56 (day 84, P=0.062) Pain Interference Score (PIS)
Ranevetmab treatment significantly superior to placebo on days 14, 28 and 56 (day 84, P=0.092)
Lower scores mean greater improvement on PSS and PIS
CBPI PSS mean score CBPI PIS mean score
5.5 6
5 5.5
Placebo 5
4.5
4.5
4
Ranevetmab 4
3.5
3.5 3 3
2.5 2.5
2 2
Day 0 Day 14 Day 28 Day 56 Day 84 Day 0 Day 14 Day 28 Day 56 Day 84
= statistically significant difference
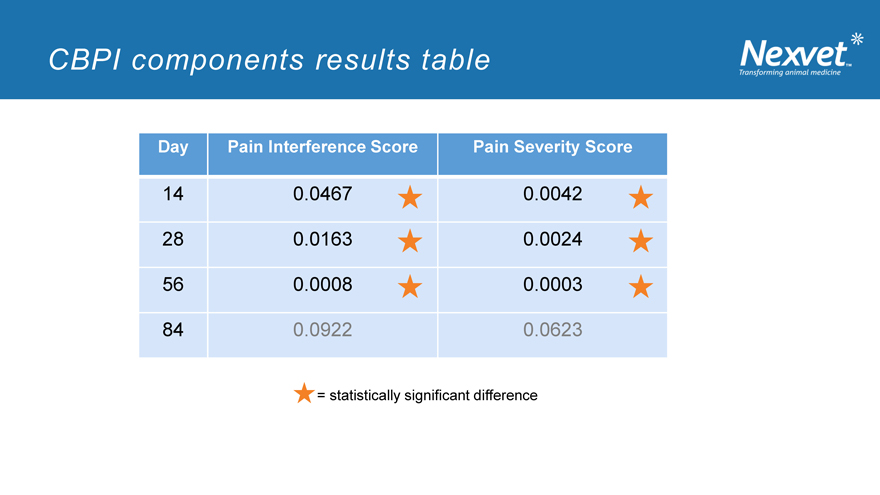
CBPI components results table
Day Pain Interference Score Pain Severity Score
14 0.0467 0.0042
28 0.0163 0.0024
56 0.0008 0.0003
84 0.0922 0.0623
= statistically significant difference
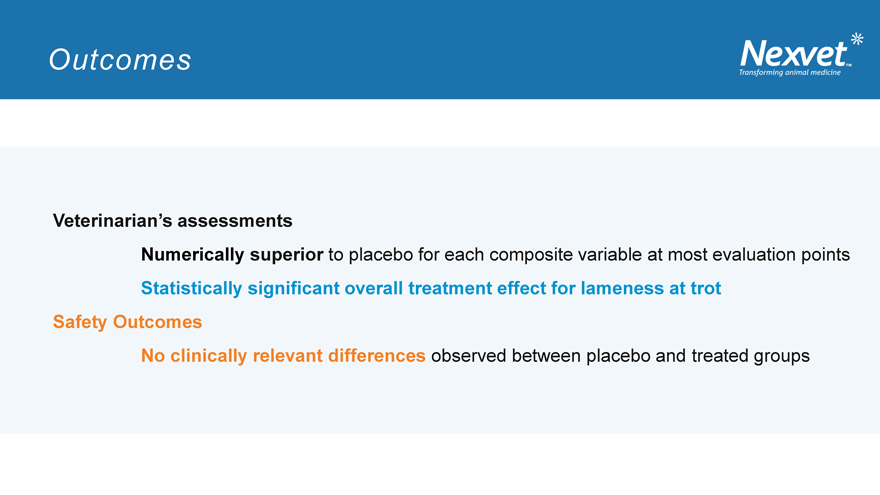
Outcomes
Veterinarian’s assessments
Numerically superior to placebo for each composite variable at most evaluation points
Statistically significant overall treatment effect for lameness at trot Safety Outcomes
No clinically relevant differences observed between placebo and treated groups
Final pivotal study conclusions
Ranevetmab administered to dogs at a dose range of 0.2 to 0.4 mg/kg is effective and safe for the treatment of pain associated with OA
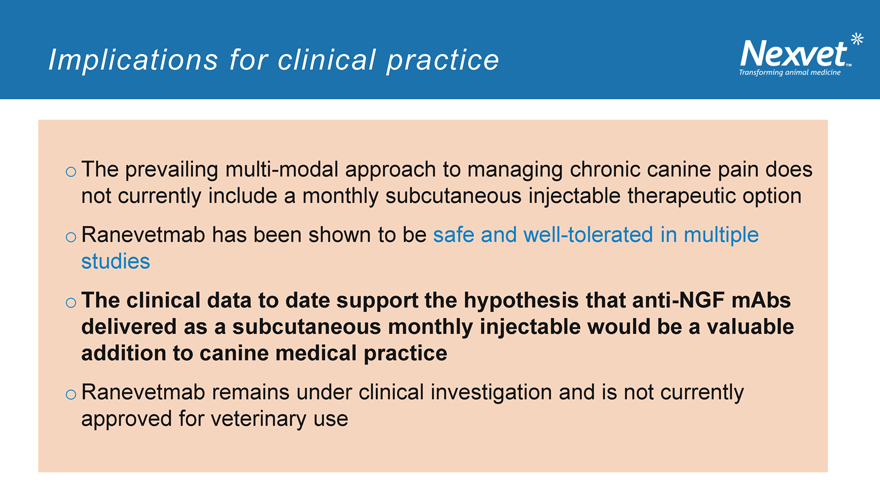
Implications for clinical practice
o The prevailing multi-modal approach to managing chronic canine pain does not currently include a monthly subcutaneous injectable therapeutic option o Ranevetmab has been shown to be safe and well-tolerated in multiple studies o The clinical data to date support the hypothesis that anti-NGF mAbs delivered as a subcutaneous monthly injectable would be a valuable addition to canine medical practice o Ranevetmab remains under clinical investigation and is not currently approved for veterinary use
NV-02 | a novel felinized anti-NGF monoclonal antibody for the control of pain associated with osteoarthritis in cats Background and pilot study results
Dr. Jane Eagleson June 2016
Background and clinical context for NV-02
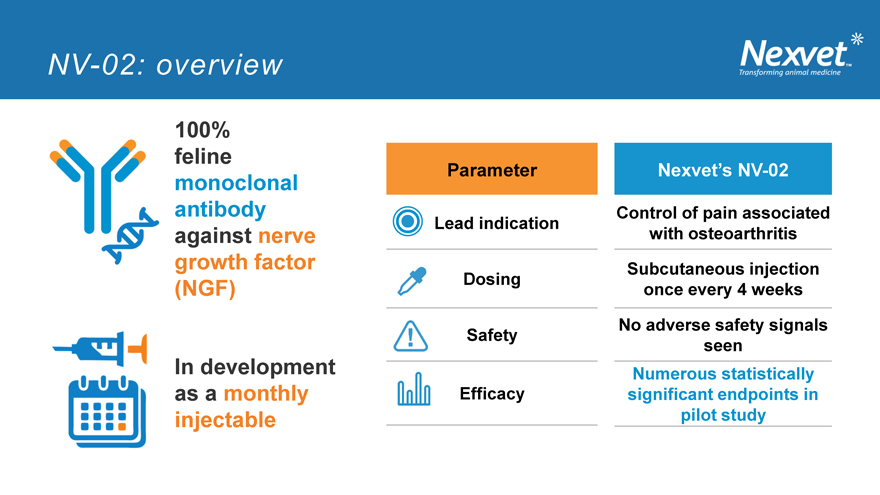
NV-02: overview
100% feline
Parameter Nexvet’s NV-02
monoclonal
antibody Control of pain associated Lead indication against nerve with osteoarthritis growth factor Dosing Subcutaneous injection (NGF) once every 4 weeks No adverse safety signals Safety seen In development Numerous statistically as a monthly Efficacy significant endpoints in injectable pilot study
NV-02: detailed description
USAN-approved nonproprietary name: frunevetmab
Novel biotherapeutic veterinary medicine under clinical investigation for the control of pain associated with OA in cats
Analogous cat therapy to ranevetmab in the dog
Fully felinized (“100% cat”) monoclonal antibody that neutralizes nerve growth factor (NGF)
Produced as a secreted product from a genetically engineered CHO cell line
Formulated as sterile buffered solution of 7 mg/mL packaged in single-use glass vials for SC injection intended for administration to cats only
Under clinical investigation and currently NOT approved for use
Pain relief in the cat: limited options
No FDA-approved therapies for the treatment of chronic pain in cats Safety concerns around chronic NSAID use in cats especially in the older population where incidence of chronic renal failure is high
NSAIDs only approved for short-term use in cats in the U.S.
Meloxicam: single administration Robenacoxib: 3 days maximum
Off label use includes NSAIDs, gabapentin, amantadine, amitriptyline, corticosteroids
Veterinarian surveys indicate that safe and efficacious products are desired for treatment of osteoarthritis (OA)
92% of cats aged 6 months to 20 years had radiographic evidence of DJD (Lascelles,
2010)
Major Unmet Need in Chronic Cat Pain
Felinized anti-NGF mAb (NV-02) has potential to meet this need
Proof-of-concept data and pilot study design
Earlier studies demonstrated:
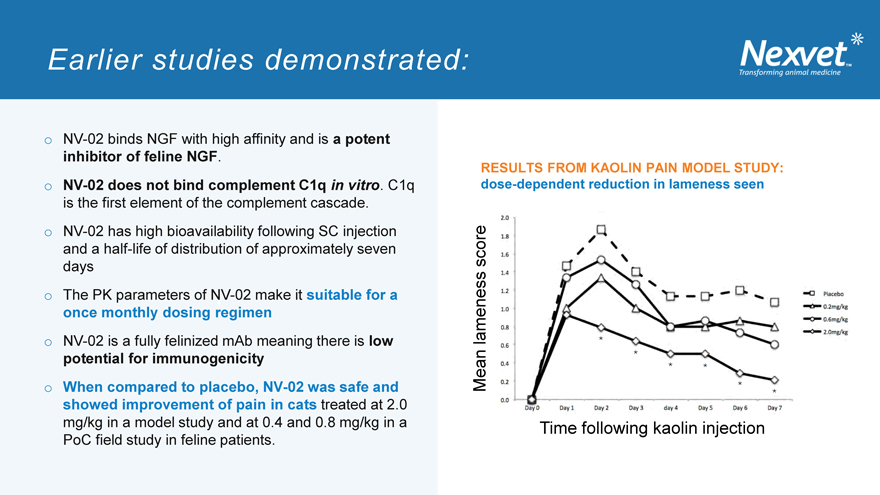
o NV-02 binds NGF with high affinity and is a potent inhibitor of feline NGF. o NV-02 does not bind complement C1q in vitro. C1q is the first element of the complement cascade. o NV-02 has high bioavailability following SC injection and a half-life of distribution of approximately seven days o The PK parameters of NV-02 I make it suitable for a once monthly dosing regimen o NV-02 is a fully felinized mAb meaning there is low potential for immunogenicity o When compared to placebo, NV-02 was safe and showed improvement of pain in cats treated at 2.0 mg/kg in a model study and at 0.4 and 0.8 mg/kg in a PoC field study in feline patients.
RESULTS FROM KAOLIN PAIN MODEL STUDY: dose-dependent reduction in lameness seen
score lameness Mean
Time following kaolin injection
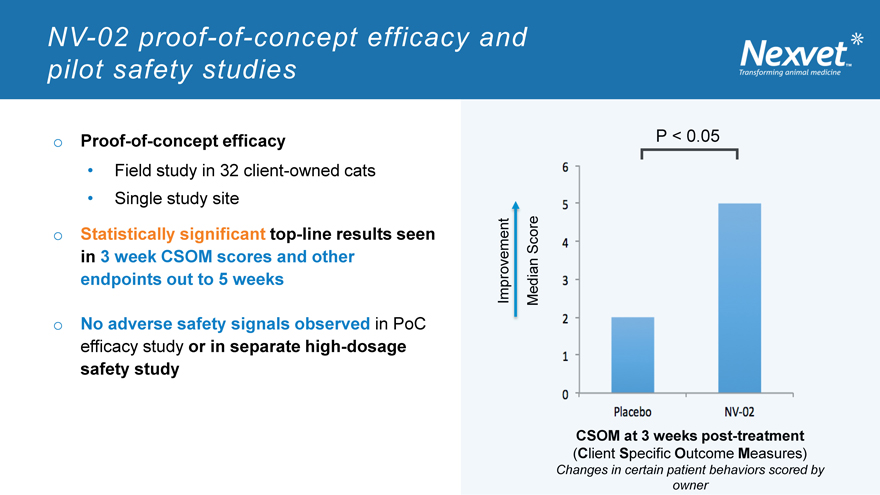
NV-02 proof-of-concept efficacy and pilot safety studies
o Proof-of-concept efficacy
Field study in 32 client-owned cats
Single study site
o Statistically significant top-line results seen in 3 week CSOM scores and other endpoints out to 5 weeks
o No adverse safety signals observed in PoC efficacy study or in separate high-dosage safety study
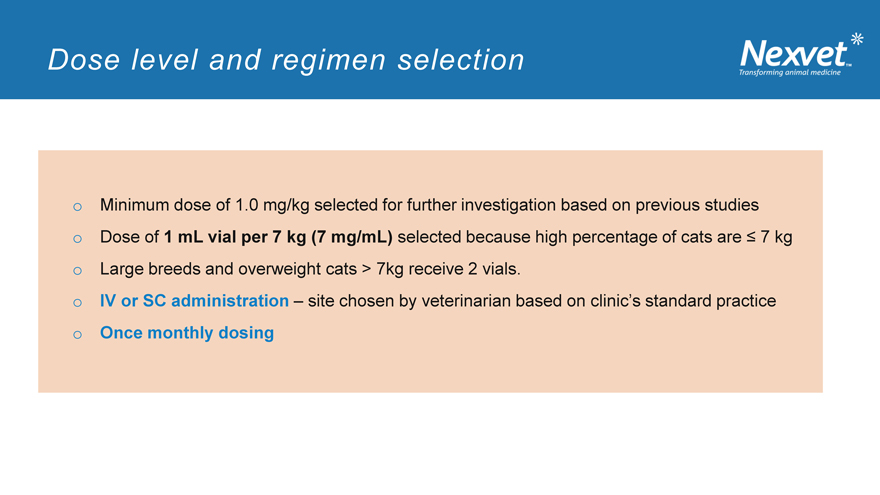
Dose level and regimen selection
o Minimum dose of 1.0 mg/kg selected for further investigation based on previous studies o Dose of 1 mL vial per 7 kg (7 mg/mL) selected because high percentage of cats are ? 7 kg o Large breeds and overweight cats > 7kg receive 2 vials. o IV or SC administration – site chosen by veterinarian based on clinic’s standard practice o Once monthly dosing
Larger exploratory study: the design
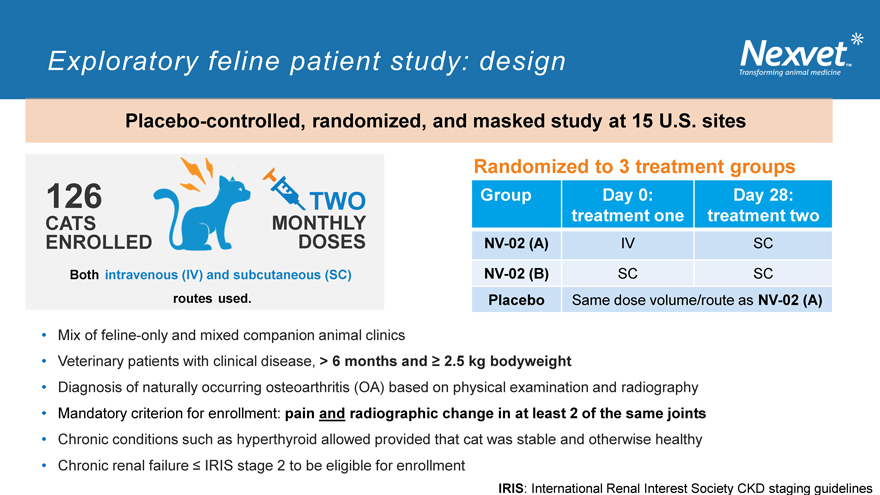
Exploratory feline patient study: design
Placebo-controlled, randomized, and masked study at 15 U.S. sites
Randomized to 3 treatment groups
126 TWO Group Day 0: Day 28: CATS MONTHLY treatment one treatment two
ENROLLED DOSES NV-02 (A) IV SC
Both intravenous (IV) and subcutaneous (SC) NV-02 (B) SC SC routes used. Placebo Same dose volume/route as NV-02 (A)
Mix of feline-only and mixed companion animal clinics
Veterinary patients with clinical disease, > 6 months and ? 2.5 kg bodyweight
Diagnosis of naturally occurring osteoarthritis (OA) based on physical examination and radiography
Mandatory criterion for enrollment: pain and radiographic change in at least 2 of the same joints
Chronic conditions such as hyperthyroid allowed provided that cat was stable and otherwise healthy
Chronic renal failure ? IRIS stage 2 to be eligible for enrollment
IRIS: International Renal Interest Society CKD staging guidelines
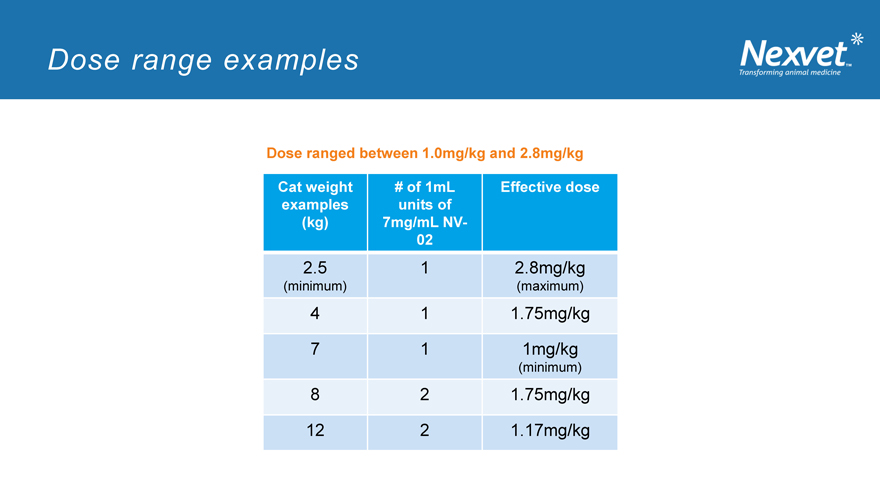
Dose range examples
Dose ranged between 1.0mg/kg and 2.8mg/kg
Cat weight # of 1mL Effective dose examples units of (kg) 7mg/mL NV-02
2.5 1 2.8mg/kg
(minimum) (maximum)
4 1 1.75mg/kg
7 1 1mg/kg
(minimum)
8 2 1.75mg/kg
12 2 1.17mg/kg
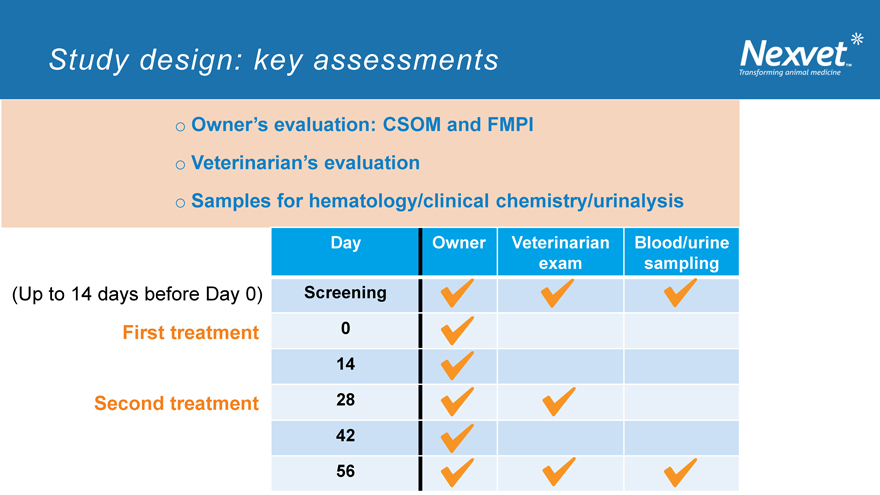
Study design: key assessments
o Owner’s evaluation: CSOM and FMPI o Veterinarian’s evaluation o Samples for hematology/clinical chemistry/urinalysis
Day Owner Veterinarian Blood/urine exam sampling
(Up to 14 days before Day 0) Screening
First treatment 0 14
Second treatment 28 42
56
Study design: CSOM owner assessment
CSOM: single owner makes all observations o Owner selects 3 activities specific to their cat that are difficult for the cat or have changed as a result of osteoarthritis
o Measured on a 5 point scale with “1” being no problem and “5” being impossible
o A priori success criteria established (decrease of 2 in total score and no increase in any individual score = a success)
CSOM Global Assessment
Measurement of overall owner experience with treatment.
Study design: CSOM owner assessment
Example CSOM activities: o Full body stretching o Stair climbing o Jumping up to elevated feeding areas o Running while playing with other cat o Jumping off the bed in the morning o Whole body grooming
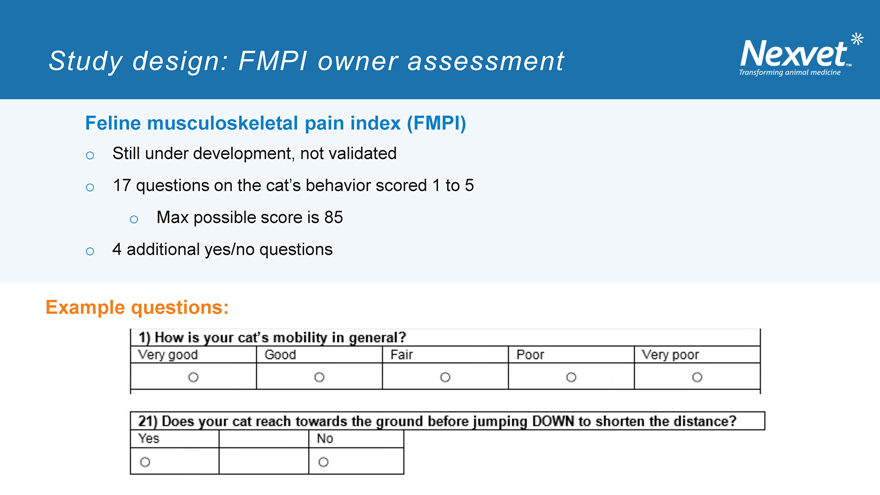
Study design: FMPI owner assessment
Feline musculoskeletal pain index (FMPI) o Still under development, not validated o 17 questions on the cat’s behavior scored 1 to 5 o Max possible score is 85 o 4 additional yes/no questions
Example questions:
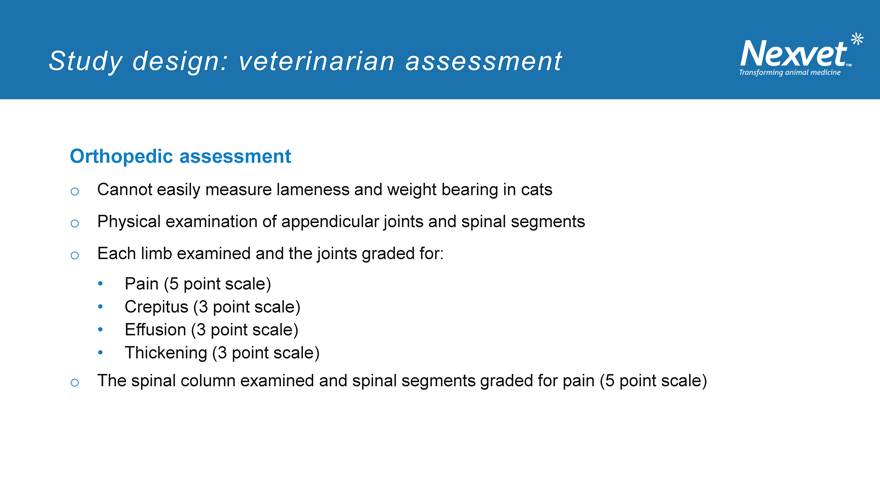
Study design: veterinarian assessment
Orthopedic assessment o Cannot easily measure lameness and weight bearing in cats o Physical examination of appendicular joints and spinal segments o Each limb examined and the joints graded for:
Pain (5 point scale)
Crepitus (3 point scale)
Effusion (3 point scale)
Thickening (3 point scale) o The spinal column examined and spinal segments graded for pain (5 point scale)
Exploratory study outcomes
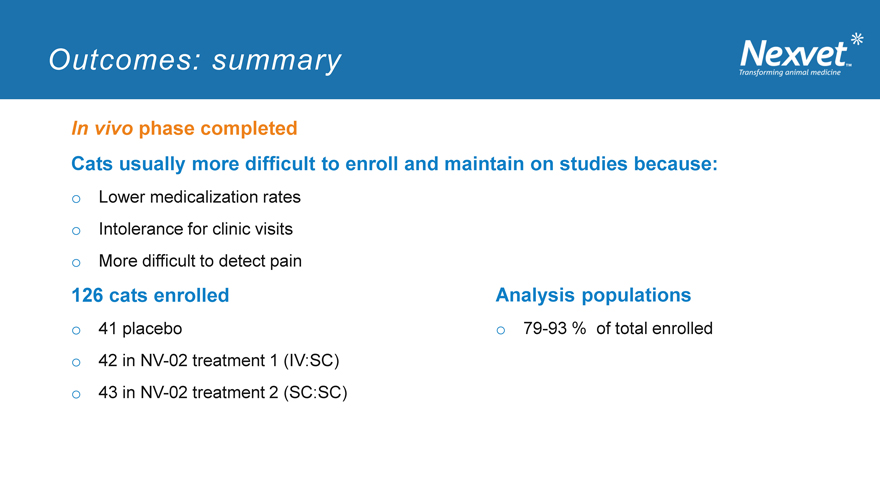
Outcomes: summary
In vivo phase completed
Cats usually more difficult to enroll and maintain on studies because: o Lower medicalization rates o Intolerance for clinic visits o More difficult to detect pain
126 cats enrolled Analysis populations o 41 placebo o 79-93 % of total enrolled o 42 in NV-02 treatment 1 (IV:SC) o 43 in NV-02 treatment 2 (SC:SC)
Outcomes: summary
Study demographics o 52 castrated males, 74 spayed females o Age range: 1 year, 0 months 21 years, 10 months o 108 cats weighed ? 7kg (1 vial/dose), 18 cats weighed > 7 kg (2 vials/dose) o Most common breed was non-pedigree domestic shorthair o Other breeds included Siamese, Ragdoll, Manx, Persian, Munchkin, Norwegian Forest Cat, Angora, Bombay, Maine Coon, Devon Rex, American Shorthair, British Shorthair
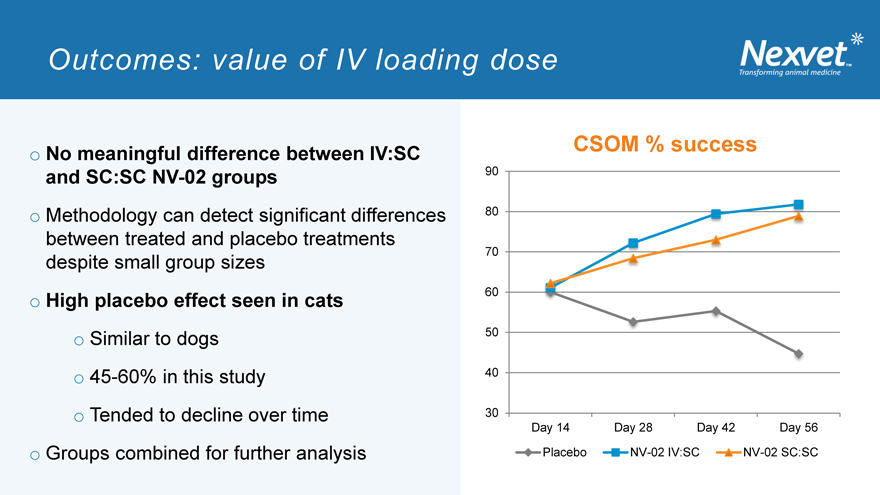
Outcomes: value of IV loading dose
o No meaningful difference between IV:SC and SC:SC NV-02 groups o Methodology can detect significant differences between treated and placebo treatments despite small group sizes o High placebo effect seen in cats o Similar to dogs o 45-60% in this study o Tended to decline over time o Groups combined for further analysis
CSOM % success
90 80 70 60 50 40
30
Day 14 Day 28 Day 42 Day 56
Placebo NV-02 IV:SC NV-02 SC:SC
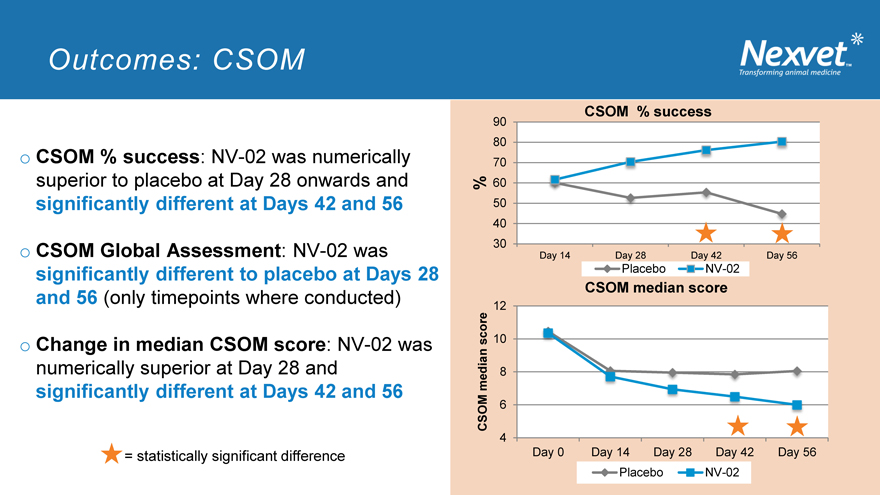
Outcomes: CSOM
o CSOM % success: NV-02 was numerically superior to placebo at Day 28 onwards and significantly different at Days 42 and 56
o CSOM Global Assessment: NV-02 was significantly different to placebo at Days 28 and 56 (only timepoints where conducted)
o Change in median CSOM score: NV-02 was numerically superior at Day 28 and significantly different at Days 42 and 56
= statistically significant difference
CSOM % success
90 80 70
% 60 50 40 30
Day 14 Day 28 Day 42 Day 56
Placebo NV-02
CSOM median score
12 score 10 median 8 CSOM 6 4
Day 0 Day 14 Day 28 Day 42 Day 56
Placebo NV-02
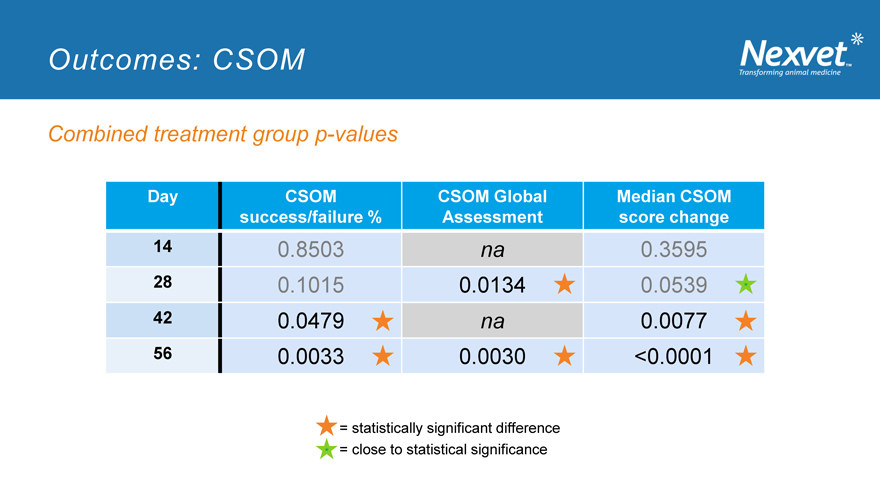
Outcomes: CSOM
Combined treatment group p-values
Day CSOM CSOM Global Median CSOM success/failure % Assessment score change
14 0.8503 na 0.3595
28 0.1015 0.0134 0.0539
42 0.0479 na 0.0077
56 0.0033 0.0030 <0.0001
= statistically significant difference = close to statistical significance
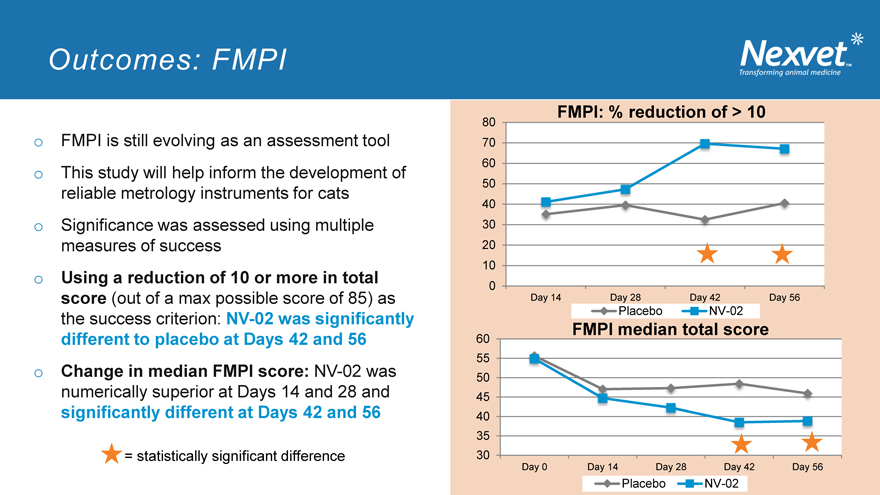
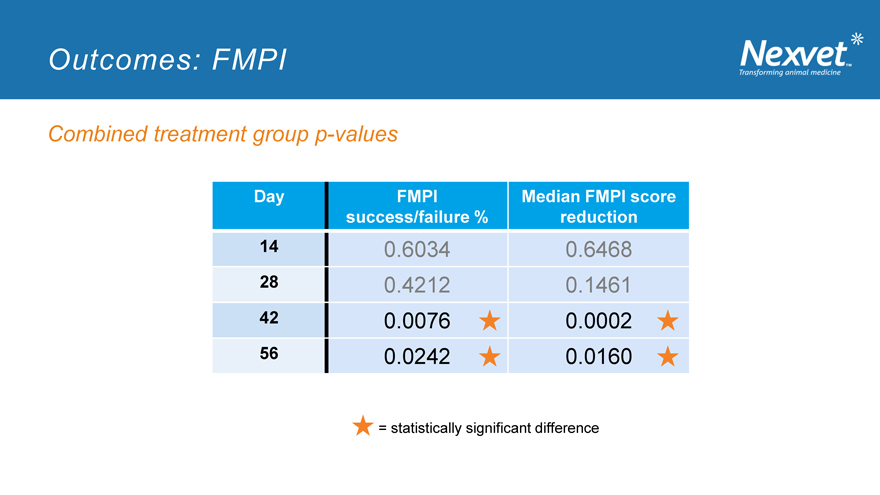
Outcomes: FMPI
o FMPI is still evolving as an assessment tool o This study will help inform the development of reliable metrology instruments for cats o Significance was assessed using multiple measures of success o Using a reduction of 10 or more in total score (out of a max possible score of 85) as the success criterion: NV-02 was significantly different to placebo at Days 42 and 56 o Change in median FMPI score: NV-02 was numerically superior at Days 14 and 28 and significantly different at Days 42 and 56
= statistically significant difference
FMPI: % reduction of > 10
80 70 60 50 40 30 20
10
0
Day 14 Day 28 Day 42 Day 56
Placebo NV-02
FMPI median total score
60 55 50
45
40 35 30
Day 0 Day 14 Day 28 Day 42 Day 56
Placebo NV-02
Outcomes: FMPI
Combined treatment group p-values
Day FMPI Median FMPI score success/failure % reduction
14 0.6034 0.6468
28 0.4212 0.1461
42 0.0076 0.0002
56 0.0242 0.0160
= statistically significant difference
Other outcomes
Additional FMPI “yes/no” questions o No consistent significant differences between groups o May not be appropriate for measuring efficacy for treatment of pain in the cat
Orthopedic (veterinarian’s) assessment o No significant differences between groups o Palpation and measures of joint health such as crepitus, effusion and swelling may not be appropriate for measuring efficacy for treatment of pain in the cat
Safety Assessments
Analyses not completed but no clinical relevant differences between groups were apparent during the in vivo phase
No adverse safety signals seen
Conclusions
o NV-02 has potential as a monthly injectable for treatment of pain due to osteoarthritis in cats o No adverse safety signal has been detected to date o Dose of 1.0 – 2.8 mg/kg administered once a month by SC injection is suitable for use in a pivotal efficacy and field safety study in feline patients.
This study is planned to commence in 2H 2016.
Implications for clinical practice
o A monthly subcutaneous injectable for chronic pain would be a novel modality for feline care o Currently no approved drugs for long-term use in the U.S. and most existing treatments come with safety concerns or may have frequent oral dosing requirements that impact compliance o The sparse ‘toolbox’ for addressing long-term feline pain does not meet the significant need: osteoarthritic cats are a significant but under-treated population o NV-02 has been shown to be safe and well-tolerated in multiple studies o The clinical data to date support the hypothesis that anti-NGF mAbs delivered as a subcutaneous monthly injectable would be a valuable addition to feline medical practice
Nexvet
Transforming animal medicine