
Revolutionizing Women’s Sexual and Reproductive Health Third Quarter 2021 Results Call November 15, 2021 NASDAQ: EVFM evofem.com © 2021 Evofem Biosciences, Inc. | For investor discussions only.

© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 2 Forward-Looking Statements This presentation contains forward looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 and other federal securities laws. In some cases, you can identify forward looking statements by terms such as “may,” ”will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “strategy,” “objective,” “designed,” “suggest,” “currently,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. Each of these forward-looking statements involves risks and uncertainties. Actual results may differ materially from those, express or implied, in these forward-looking statements. Factors that may cause differences between current expectations and actual results include, but are not limited to, the following: • The rate and degree of market acceptance of Phexxi® (lactic acid, citric acid and potassium bitartrate) vaginal gel • Evofem’s ability to successfully commercialize Phexxi in the United States and to enter into successful partnerships to commercialize Phexxi outside of the United States • Evofem’s ability to maintain and protect its intellectual property • Evofem’s ability to reduce its operating expenses, rely on existing cash reserves to fund its current development plans and operations, and to raise additional capital when needed • The success of Evofem’s ACA strategy • Evofem’s reliance on third-party providers, such as third-party manufacturers and clinical research organizations • The presence or absence of any adverse events or side effects relating to the use of Phexxi and EVO100 • The outcome, timing and success of Evofem’s clinical trials including EVOGUARD • Evofem’s ability to retain members of its management and other key personnel • General risks to the economy represented by spread and mutation of the COVID-19 virus • Evofem’s ability to obtain the necessary regulatory approvals for its product candidates and the timing of such approvals, and, • Any other risk factors detailed in Evofem’s filings from time to time with the U.S. Securities and Exchange Commission including, without limitation, the 10-K filed on March 12, 2020, 8-K filed on June 2, 2020 and subsequent filings. The forward looking statements in this presentation represent Evofem’s views only as of the date of this presentation, November 15, 2021, and Evofem expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Evofem’s expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based for any reason, except as required by law, even as new information becomes available or other events occur in the future. All forward-looking statements in this presentation are qualified in their entirety by this cautionary statement.

© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 3 Strong TRx Growth Continues 48% TRx INCREASE Q3 VS. Q2 27% MONTHLY TRx INCREASE FROM SEPT TO OCT 2021* 0 5,000 10,000 15,000 20,000 25,000 Q1 2021 Q2 2021 Q3 2021 0 2,500 5,000 7,500 10,000 12,500 Aug. 2021 Sept. 2021 Oct. 2021 Source(s): • TRx from Pharmacy (including Knipper) • 340b/VA data from Chargeback data from Cardinal 3PL • ~500 units/Rx from Carepoint Pharmacy * Through 29 October 2021 27%48%

© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 4 Q3 2021 Financial Results + Expected OpEx Reductions Gross revenues grew 29% Q3 vs. Q2 Net product sales: $1.7M Operating expenses: $45.1 million • R&D: $8.7 million - Phase 3 STI prevention trial • Selling & marketing: $30.5 million - Celebrity DTC campaign • G&A: $5.0 million Lower operating expenses expected going forward • Several million lower in Q4 vs. Q3 2021 • ~$50 million reduction in FY 2022 vs. FY 2021

© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 5 Balance Sheet Highlights $23.9 M for use in ongoing operations as of September 30, 2021 • $14.9 M cash and cash equivalents • $ 9.0 M restricted cash from the Adjuvant notes $10 M raised in October 2021 • Runway into Q1 2022
© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 6 Leading the Contraception Revolution
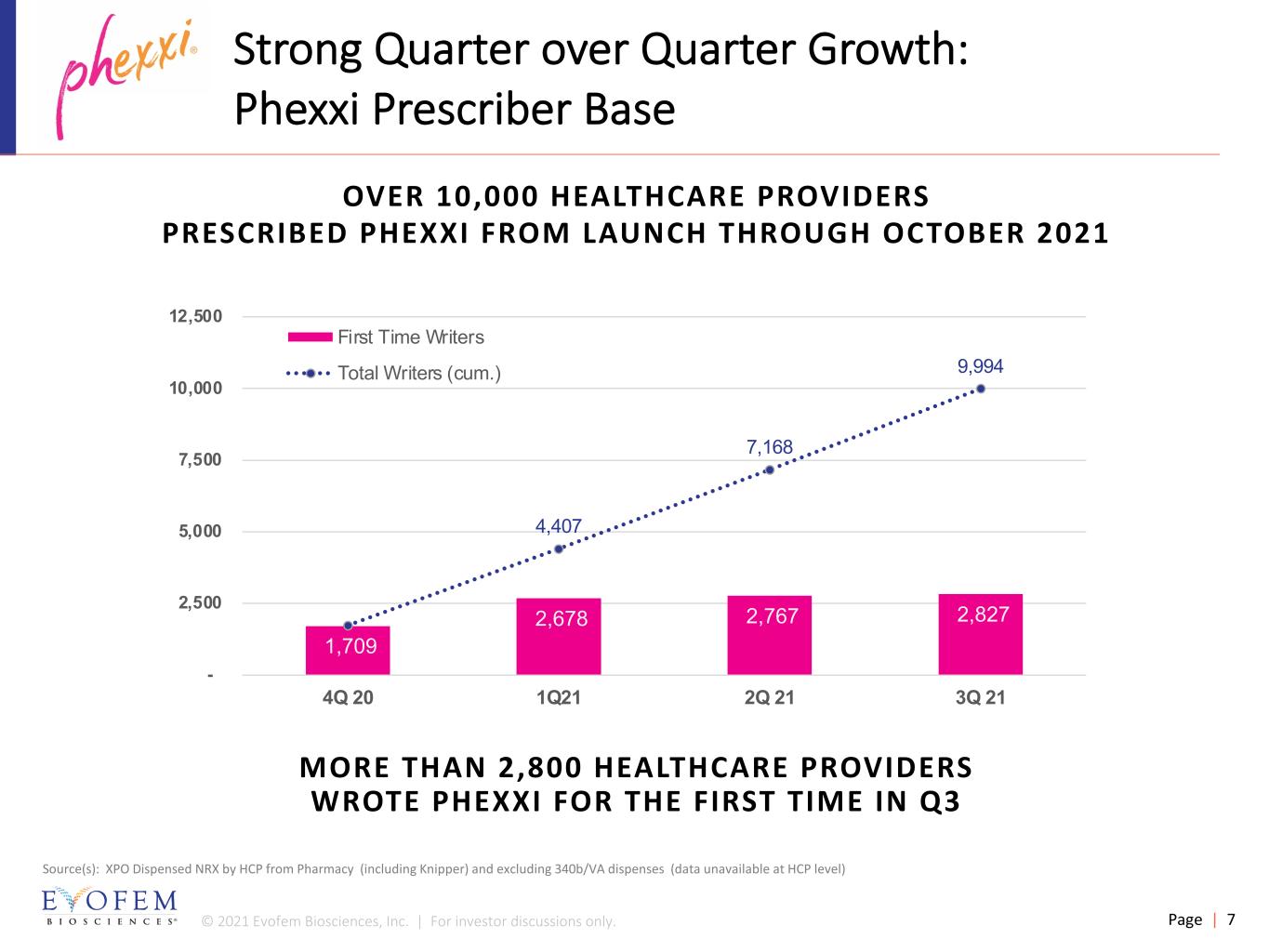
© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 7 Strong Quarter over Quarter Growth: Phexxi Prescriber Base 1,709 2,678 2,767 2,827 4,407 7,168 9,994 - 2,500 5,000 7,500 10,000 12,500 4Q 20 1Q21 2Q 21 3Q 21 First Time Writers Total Writers (cum.) MORE THAN 2,800 HEALTHCARE PROVIDERS WROTE PHEXXI FOR THE FIRST TIME IN Q3 Source(s): XPO Dispensed NRX by HCP from Pharmacy (including Knipper) and excluding 340b/VA dispenses (data unavailable at HCP level) OVER 10,000 HEALTHCARE PROVIDERS PRESCRIBED PHEXXI FROM LAUNCH THROUGH OCTOBER 2021
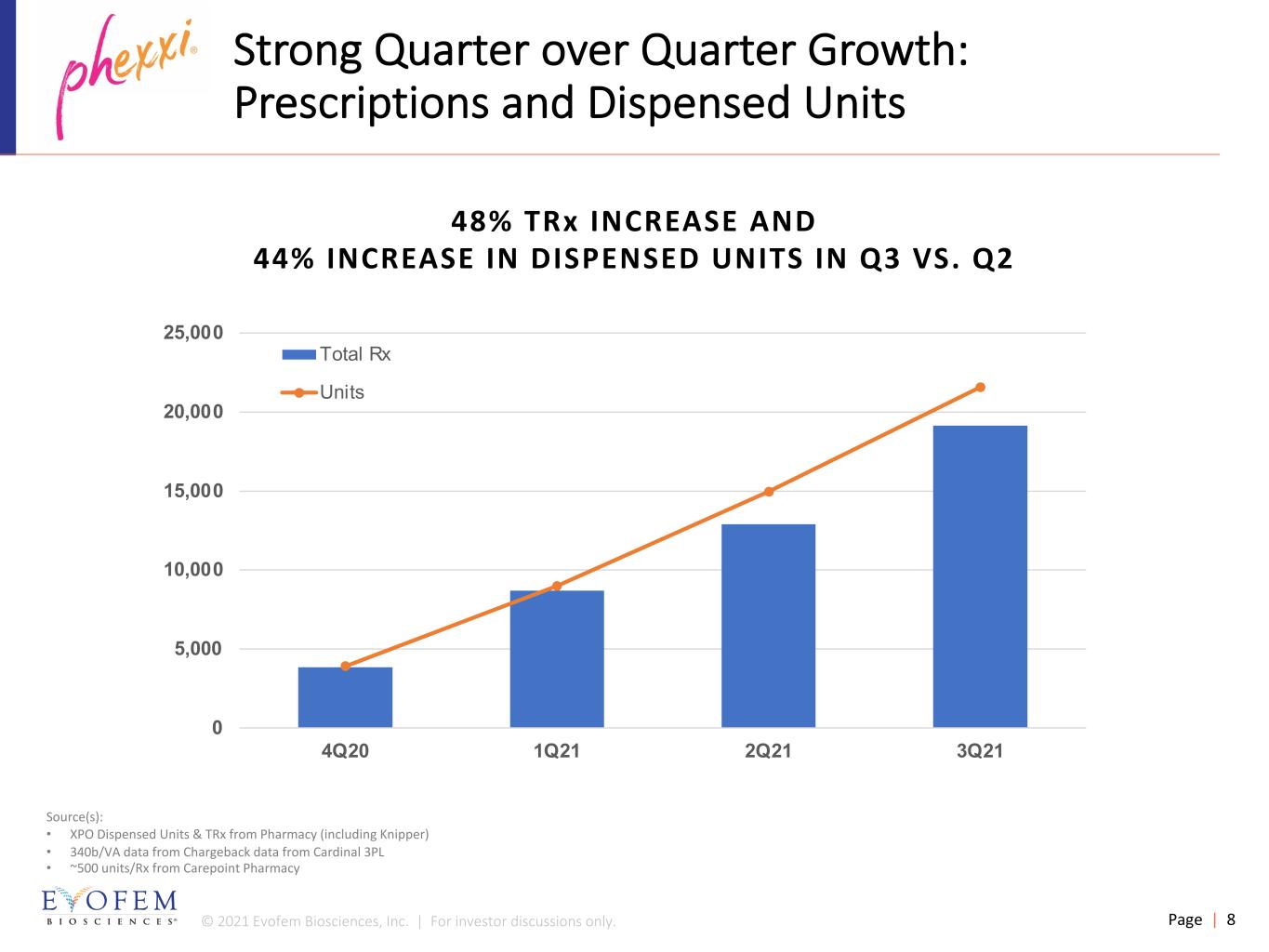
© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 8 Strong Quarter over Quarter Growth: Prescriptions and Dispensed Units 0 5,000 10,000 15,000 20,000 25,000 4Q20 1Q21 2Q21 3Q21 Total Rx Units Source(s): • XPO Dispensed Units & TRx from Pharmacy (including Knipper) • 340b/VA data from Chargeback data from Cardinal 3PL • ~500 units/Rx from Carepoint Pharmacy 48% TRx INCREASE AND 44% INCREASE IN DISPENSED UNITS IN Q3 VS. Q2
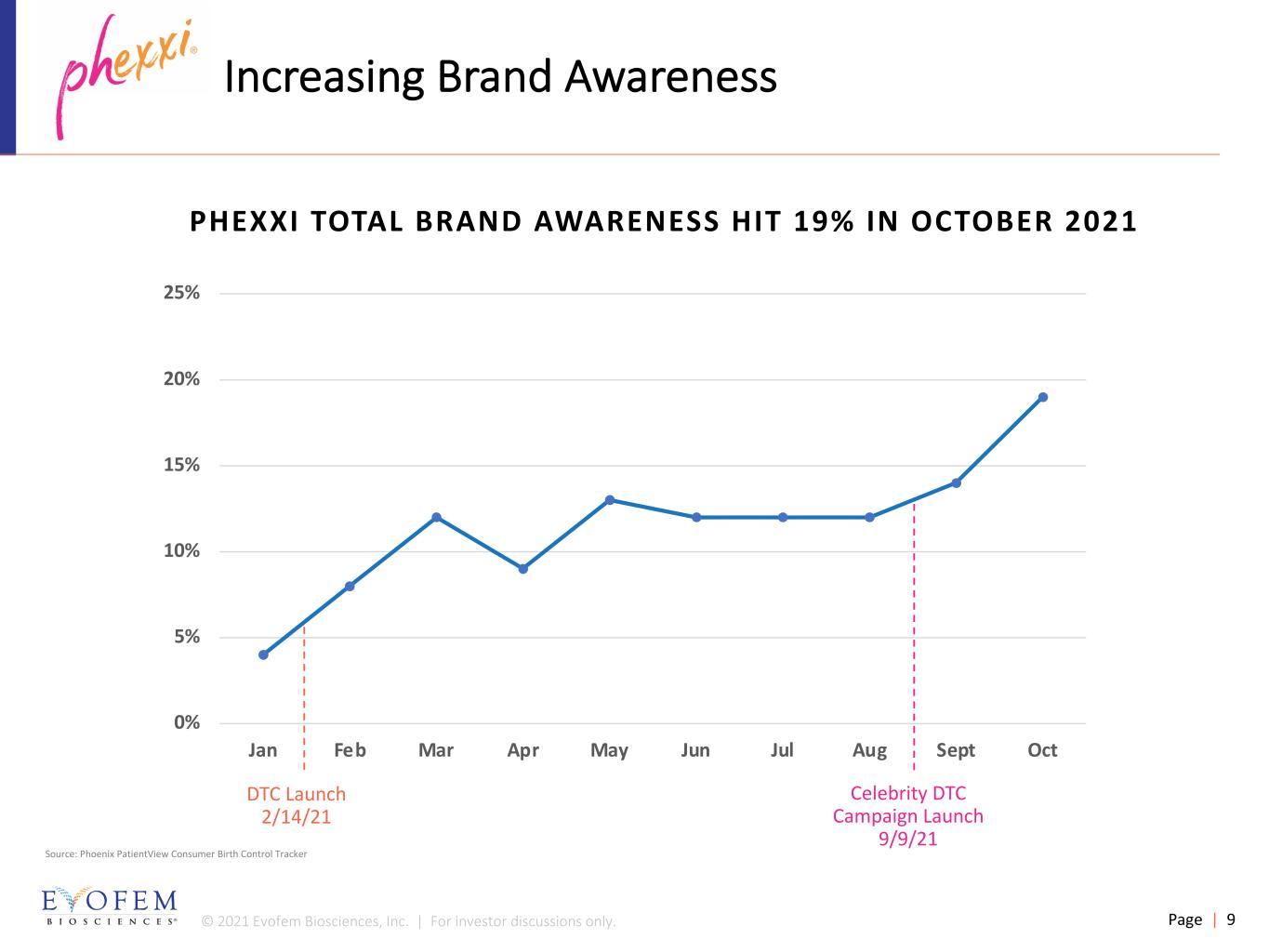
© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 9 Increasing Brand Awareness Source: Phoenix PatientView Consumer Birth Control Tracker PHEXXI TOTAL BRAND AWARENESS HIT 19% IN OCTOBER 2021 0% 5% 10% 15% 20% 25% Jan Feb Mar Apr May Jun Jul Aug Sept Oct DTC Launch 2/14/21 Celebrity DTC Campaign Launch 9/9/21
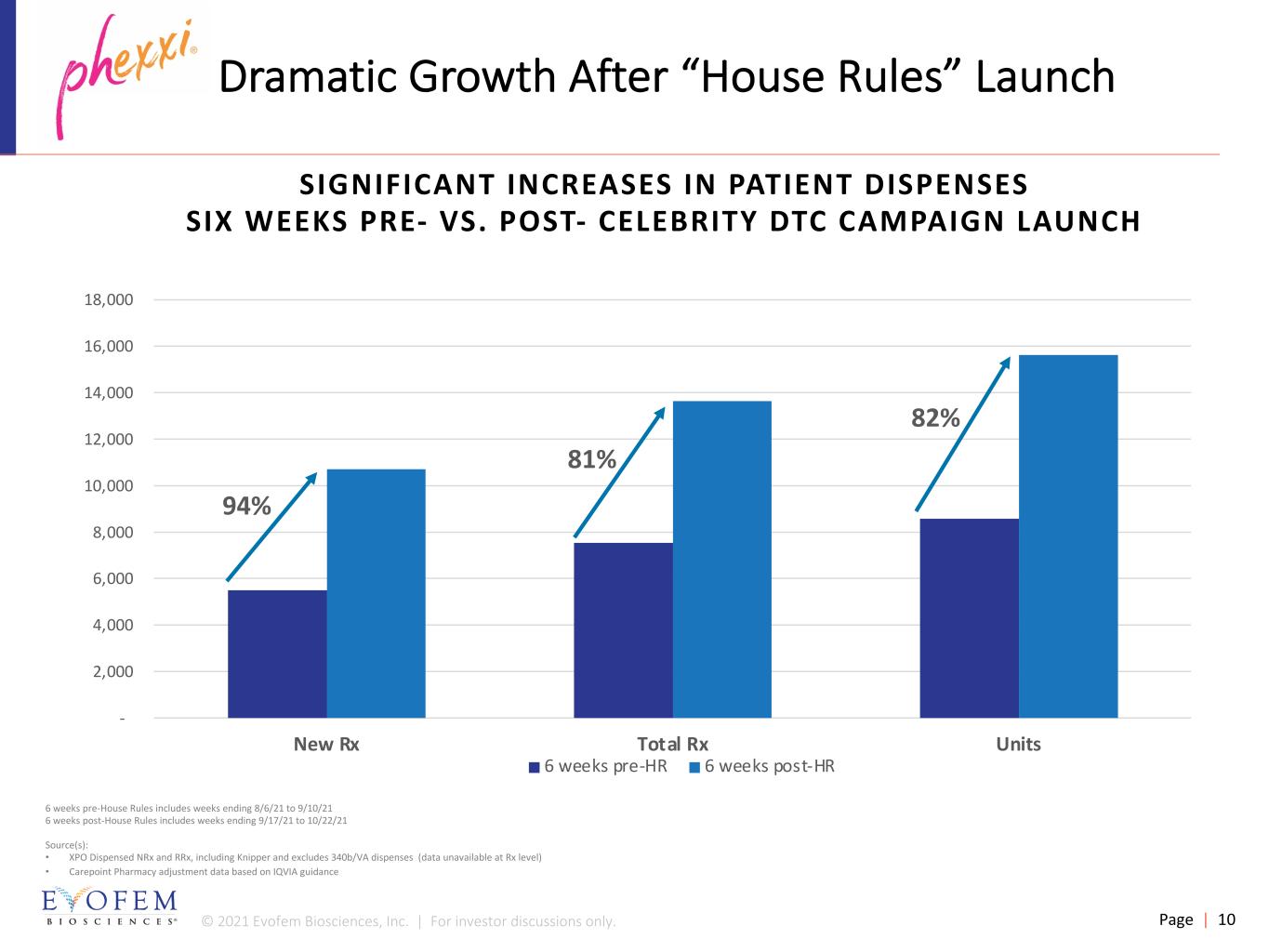
© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 10 - 2,000 4,000 6,000 8,000 10,000 12,000 14,000 16,000 18,000 New Rx Total Rx Units 6 weeks pre-HR 6 weeks post-HR Dramatic Growth After “House Rules” Launch 82% 6 weeks pre-House Rules includes weeks ending 8/6/21 to 9/10/21 6 weeks post-House Rules includes weeks ending 9/17/21 to 10/22/21 Source(s): • XPO Dispensed NRx and RRx, including Knipper and excludes 340b/VA dispenses (data unavailable at Rx level) • Carepoint Pharmacy adjustment data based on IQVIA guidance 81% 94% SIGNIFICANT INCREASES IN PATIENT DISPENSES SIX WEEKS PRE- VS. POST- CELEBRITY DTC CAMPAIGN LAUNCH

© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 11 ACA Strategy – Two Paths, Both a Win for Phexxi Goal: Guide revised to include new Vaginal pH Modulator category Update the Office of Women’s Health birth control guide Goal: Prevent OWH birth control guide from being used to limit contraceptive choices for women Update guidance from HRSA

© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 12 ACA Strategy 1: Update the OWH Birth Control Guide Goal: Guide revised to include new Vaginal pH Modulator category Update the Office of Women’s Health birth control guide Goal: Prevent OWH birth control guide from being used to limit contraceptive choices for women Update guidance from HRSA
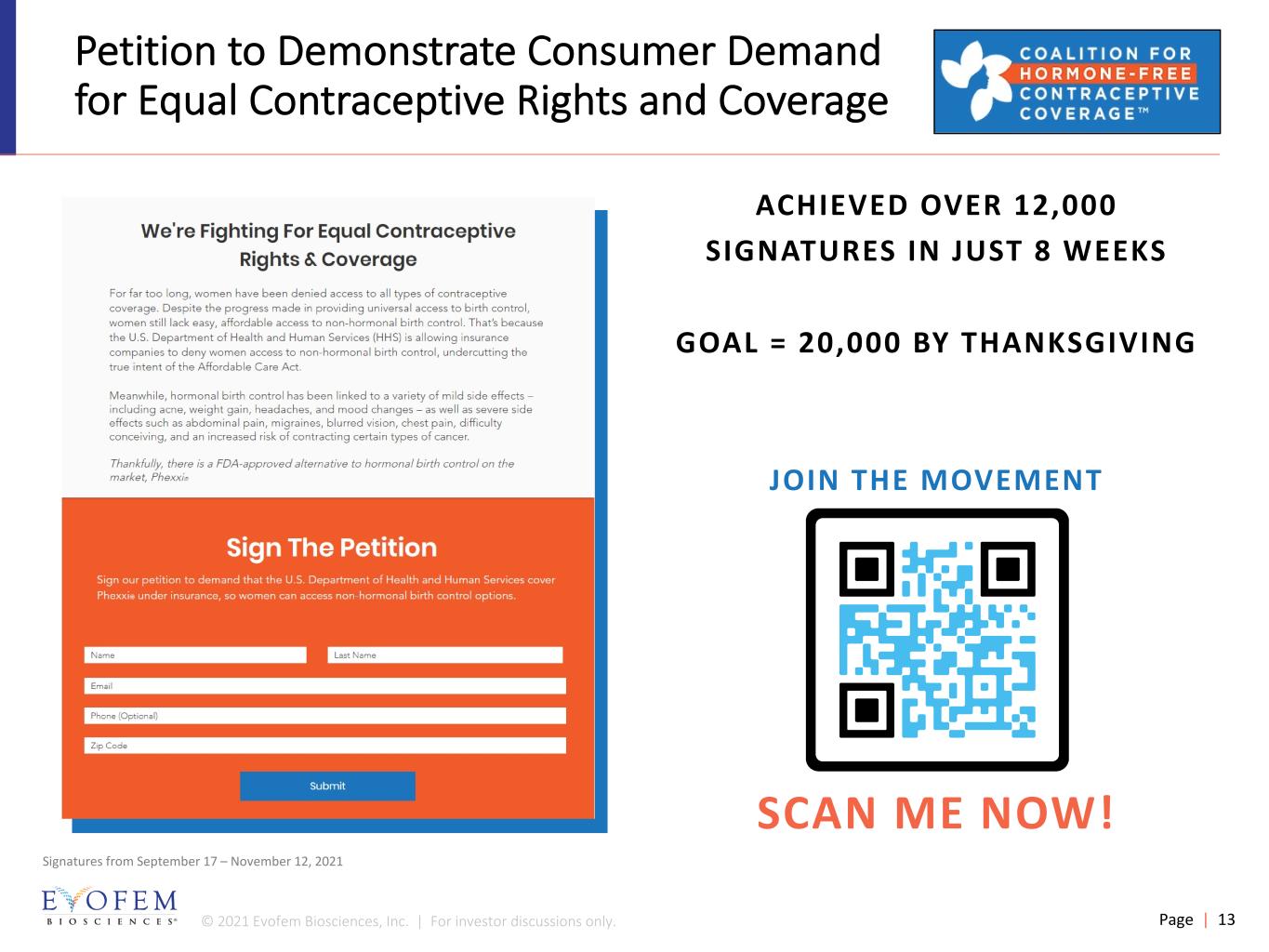
© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 13 Petition to Demonstrate Consumer Demand for Equal Contraceptive Rights and Coverage ACHIEVED OVER 12,000 SIGNATURES IN JUST 8 WEEKS GOAL = 20,000 BY THANKSGIVING JOIN THE MOVEMENT SCAN ME NOW! Signatures from September 17 – November 12, 2021

© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 14 ACA Strategy 2: Update Guidance from HRSA Goal: Guide revised to include new Vaginal pH Modulator category Update the Office of Women’s Health birth control guide Goal: Prevent OWH birth control guide from being used to limit contraceptive choices for women Update guidance from HRSA

© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 15 R&D: Prevention of Chlamydia & Gonorrhea 1. US Centers for Disease Control and Prevention (2019): CDC detailed fact sheet on gonorrhea and CDC detailed fact sheet on chlamydia 2. Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief. 2018; 327: 1-14. 3. US Centers for Disease Control and Prevention (2021): Sexually Transmitted Disease Surveillance 2019. https://www.cdc.gov/std/statistics/2019/default.htm EVO100 is investigational and safety and efficacy have not been established. Any sexually active person can be infected with chlamydia or gonorrhea1 72.2 million women in the U.S. alone2 1.8 million chlamydia cases & 616,392 gonorrhea cases reported in 20193 Chlamydia is the most common notifiable condition in the U.S.3
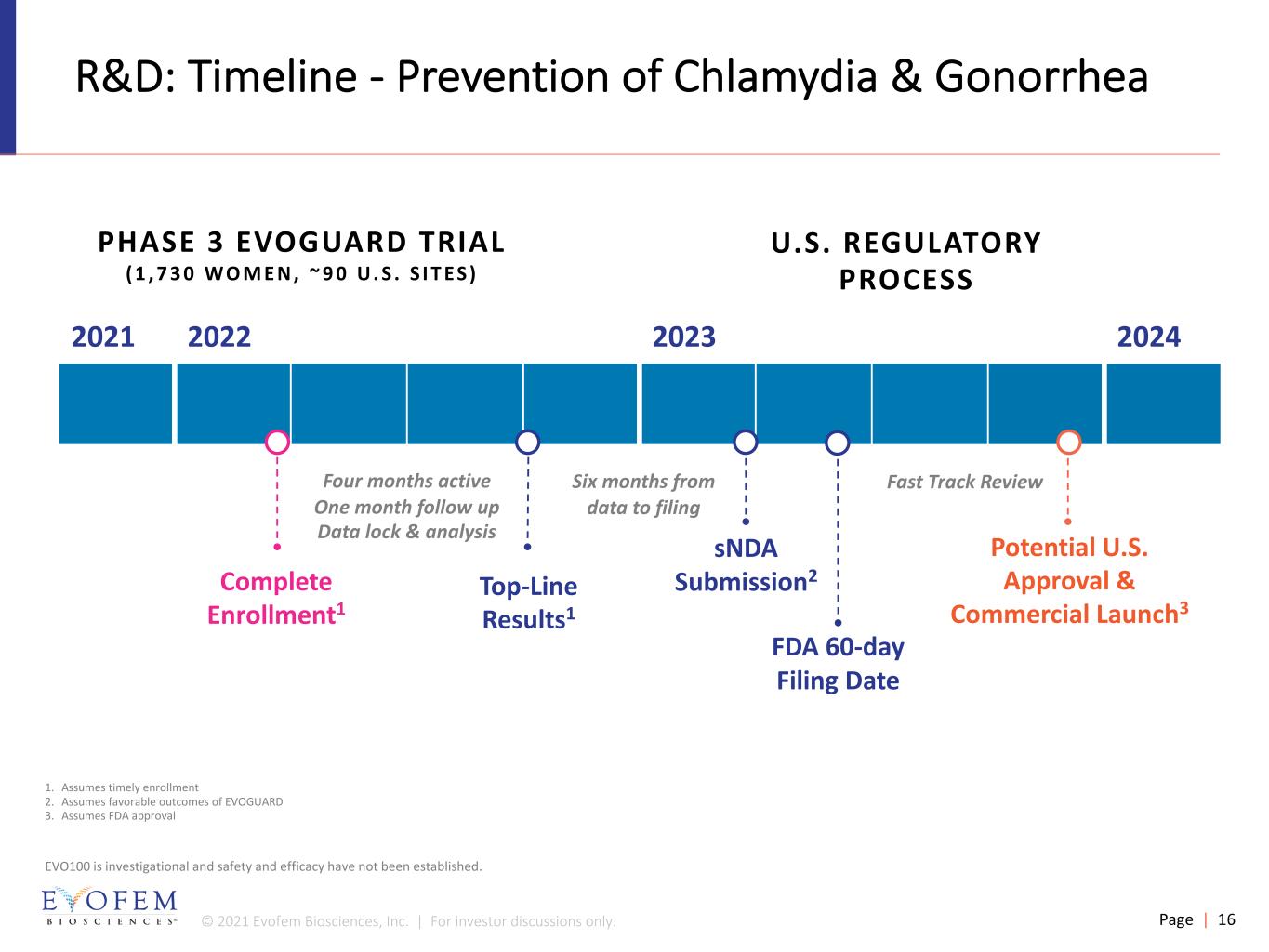
© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 16 R&D: Timeline - Prevention of Chlamydia & Gonorrhea Fast Track Review 2021 2022 2023 2024 Potential U.S. Approval & Commercial Launch3 sNDA Submission2Top-Line Results1 Complete Enrollment1 1. Assumes timely enrollment 2. Assumes favorable outcomes of EVOGUARD 3. Assumes FDA approval EVO100 is investigational and safety and efficacy have not been established. FDA 60-day Filing Date PHASE 3 EVOGUARD TRIAL ( 1 , 7 3 0 W O M E N , ~ 9 0 U . S . S I T E S ) U.S. REGULATORY PROCESS Four months active One month follow up Data lock & analysis Six months from data to filing

© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 17 Additional Value Creating Opportunities U.S.: leverage sales force • Detail synergistic product to OB/GYNs and allied HCPs • Offset sales force cost Outside U.S. • Secure partnership(s) for commercialization of Phexxi • Discussions ongoing with potential global and regional partners for key international markets • LMIC entries to fulfill Adjuvant Global Health Agreement • Submitted in Mexico; expected commercialization in 2023 under brand name Femidence™ Other strategic opportunities

Questions

© 2021 Evofem Biosciences, Inc. | For investor discussions only. Page | 19 Executing Our Key Initiatives Reduce operating expenses by ~$50 million in 2022 Increase access to Phexxi by spurring an update to the OWH chart or HRSA guidance Drive our STI prevention program forward Enter licensing agreements to get Phexxi into international markets Continue to increase Phexxi demand in the U.S.

1.858.550.1900 ir@evofem.com 12400 High Bluff Drive, Suite 600 San Diego, California 92130 NASDAQ: EVFM evofem.com



















