
Transformative genome-edited therapies for patients ANTLER initial clinical data for CB-010 at �EHA 2022�June 10, 2022 Exhibit 99.2

Forward-looking statements All statements in this presentation, other than statements of historical facts, are forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements speak only as of the date of this presentation and are subject to a number of known and unknown risks, assumptions, uncertainties, and other factors that may cause the actual results, levels of activity, performance, or achievements of Caribou Biosciences, Inc. (the “Company,” “Caribou,” “we,” or “our”) to be materially different from those expressed or implied by any forward-looking statements. The words “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential,” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. All statements other than statements of historical facts contained in this presentation, including but not limited to any statements regarding the initiation, timing, progress, strategy, plans, objectives, expectations, and results of our product candidate preclinical studies, clinical trials, and research programs, including our expectations and timing regarding the initial clinical data from our ANTLER phase 1 clinical trial for our CB-010 product candidate; our ability to successfully develop our product candidates and to obtain and maintain regulatory approval for our product candidates; the number and type of diseases, indications, or applications we intend to pursue; the beneficial characteristics, safety, efficacy, therapeutic effects, and potential advantages of our product candidates; the expected timing or likelihood of regulatory filings and approval for our product candidates; and the sufficiency and anticipated use of our existing capital resources to fund our future operating expenses and capital expenditure requirements and needs for additional financing are forward-looking statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this presentation is given. This presentation discusses product candidates that are or will be under clinical investigation and that have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the therapeutic uses for which such product candidates are being or will be studied. As a result of many factors, including risks related to our limited operating history, history of net operating losses, financial position and our ability to raise additional capital as needed to fund our operations and product candidate development; uncertainties related to the initiation, cost, timing, and progress, and results of our current and future research and development programs, preclinical studies, and clinical trials; risks that initial or interim clinical trial data will not ultimately be predictive of the safety and efficacy of our product candidates or that clinical outcomes may differ as more clinical data becomes available; our ability to obtain and maintain regulatory approval for our product candidates; risks that our product candidates, if approved, may not gain market acceptance due to negative public opinion and increased regulatory scrutiny of cell therapies involving genome editing; our ability to meet future regulatory standards with respect to our products; our ability to establish and/or maintain intellectual property rights covering our product candidates and genome-editing technology; risks of third parties asserting that our product candidates infringe their patents; developments related to our competitors and our industry; our reliance on third parties to conduct our clinical trials and manufacture our product candidates; the impact of COVID-19 and geopolitical events on our business and operations; and other risks described in greater detail in our filings with the Securities and Exchange Commission (the “SEC”), including the section titled “Risk Factors” of our Annual Report on Form 10-K for the year ended December 31, 2021, and other filings we make with the SEC; the events and circumstances reflected in our forward-looking statements may not be achieved or may not occur, and actual results could differ materially from those described in or implied by the forward-looking statements contained in this presentation. In light of the foregoing, you are urged not to rely on any forward-looking statement or third-party data in reaching any conclusion or making any investment decision about any securities of the Company. The forward-looking statements in this presentation are made only as of the date hereof. Except to the extent required by law, the Company assumes no obligation and does not intend to update any of these forward-looking statements after the date of this presentation or to conform these statements to actual results or revised expectations. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy any securities.

Introduction�Rachel Haurwitz, PhD�President & CEO�Caribou Biosciences, Inc.

Today’s guests Section Chief, New Drug Development Associate Professor, Department of Lymphoma/Myeloma The University of Texas MD Anderson Cancer Center Loretta J. Nastoupil, MD OHC hematologist, medical oncologist, blood and marrow transplant specialist Chair, Cellular Therapy, US Oncology Network OHC – Specialists in Cancer and Blood Disorders James H. Essell, MD

Aiming to set a new therapeutic bar for patients Our mission is to develop innovative, �transformative therapies for patients with devastating diseases through novel genome editing
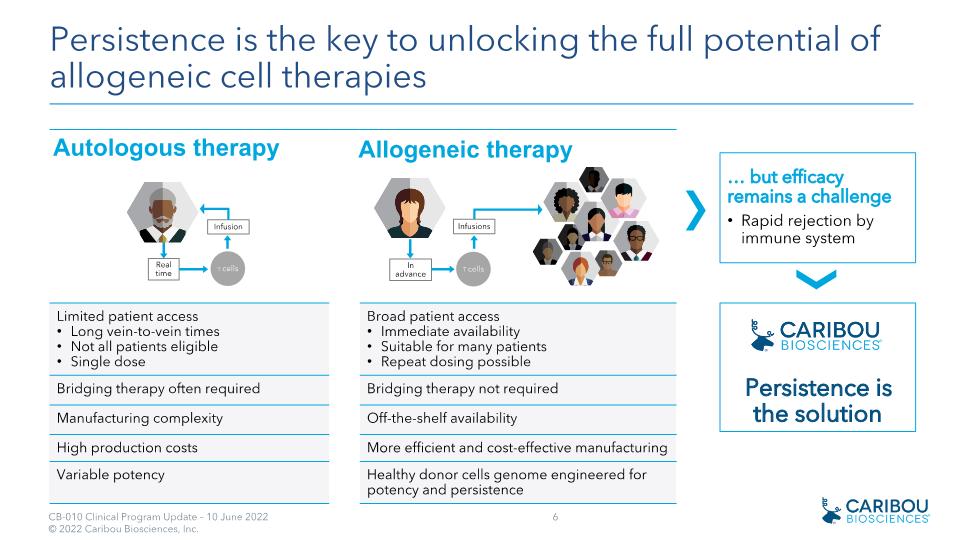
Limited patient access Long vein-to-vein times Not all patients eligible Single dose Broad patient access Immediate availability Suitable for many patients Repeat dosing possible Bridging therapy often required Bridging therapy not required Manufacturing complexity Off-the-shelf availability High production costs More efficient and cost-effective manufacturing Variable potency Healthy donor cells genome engineered for potency and persistence Allogeneic therapy Persistence is the key to unlocking the full potential of allogeneic cell therapies Autologous therapy … but efficacy remains a challenge Rapid rejection by immune system Persistence is the solution In�advance T cells Real time Infusion T cells Infusions
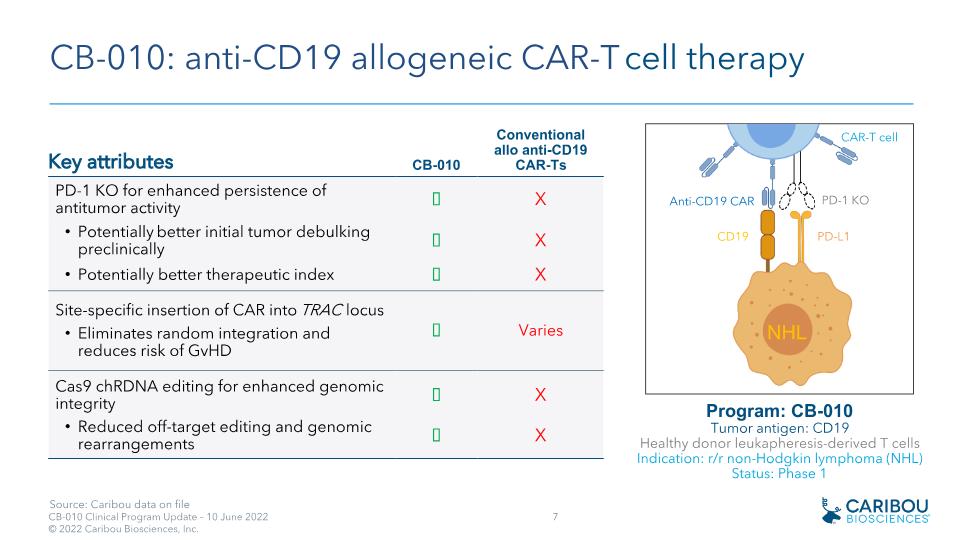
CB-010: anti-CD19 allogeneic CAR-T cell therapy Key attributes CB-010 Conventional �allo anti-CD19 CAR-Ts PD-1 KO for enhanced persistence of antitumor activity ✓ X Potentially better initial tumor debulking preclinically ✓ X Potentially better therapeutic index ✓ X Site-specific insertion of CAR into TRAC locus Eliminates random integration and reduces risk of GvHD ✓ Varies Cas9 chRDNA editing for enhanced genomic integrity ✓ X Reduced off-target editing and genomic rearrangements ✓ X Program: CB-010 Tumor antigen: CD19�Healthy donor leukapheresis-derived T cells Indication: r/r non-Hodgkin lymphoma (NHL)�Status: Phase 1 NHL CD19 Anti-CD19 CAR PD-L1 PD-1 KO CAR-T cell Source: Caribou data on file
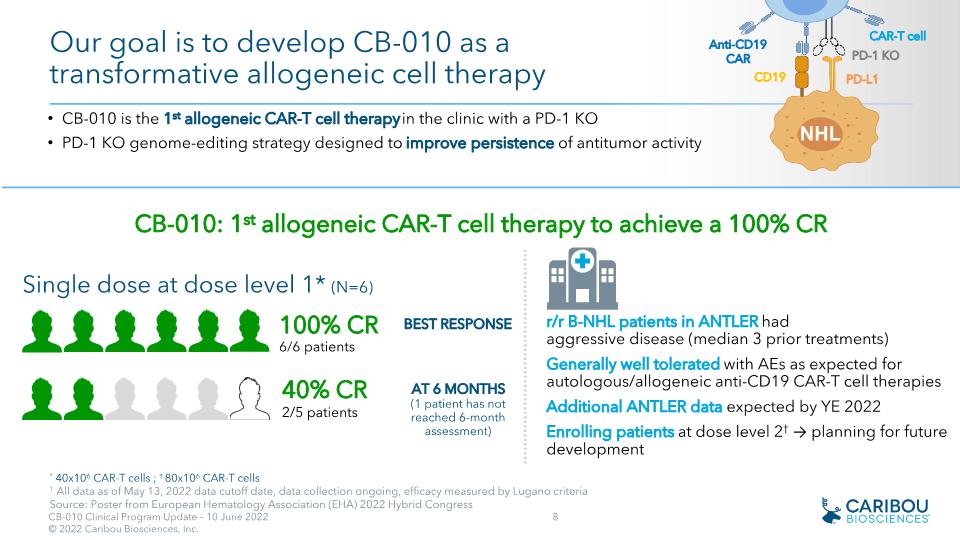
* 40x106 CAR-T cells ; † 80x106 CAR-T cells 1 All data as of May 13, 2022 data cutoff date, data collection ongoing, efficacy measured by Lugano criteria Source: Poster from European Hematology Association (EHA) 2022 Hybrid Congress r/r B-NHL patients in ANTLER had�aggressive disease (median 3 prior treatments) Generally well tolerated with AEs as expected for autologous/allogeneic anti-CD19 CAR-T cell therapies Additional ANTLER data expected by YE 2022 Enrolling patients at dose level 2† → planning for future development NHL CD19 Anti-CD19 CAR PD-L1 PD-1 KO CAR-T cell Our goal is to develop CB-010 as a transformative allogeneic cell therapy CB-010 is the 1st allogeneic CAR-T cell therapy in the clinic with a PD-1 KO PD-1 KO genome-editing strategy designed to improve persistence of antitumor activity 100% CR �6/6 patients CB-010: 1st allogeneic CAR-T cell therapy to achieve a 100% CR Single dose at dose level 1* (N=6) BEST RESPONSE AT 6 MONTHS (1 patient has not reached 6-month assessment) 40% CR �2/5 patients

With gratitude for patients, caregivers, investigators Clinicaltrials.gov NCT#04637763 MD Anderson Cancer Center, Houston Chao Family Comprehensive Cancer Center /�University of California Irvine, Orange Oncology Hematology Care, Cincinnati Baylor Chares A. Sammons Cancer Center, Dallas HonorHealth, Scottsdale University of California San Diego Moores Cancer Center, La Jolla Additional sites coming soon THANK YOU for your contributions toward Caribou’s mission to develop innovative, transformative therapies for patients with devastating diseases through novel genome editing

ANTLER Phase 1 trial initial data for CB-010�EHA 2022�Loretta J. Nastoupil, MD�Section Chief, New Drug Development�Associate Professor, Department of Lymphoma/Myeloma�The University of Texas MD Anderson Cancer Center

Disclosures LJN has received honorarium for participation in advisory boards from ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Genmab, Gilead/Kite, Janssen, MorphoSys, Novartis, and Takeda. LJN has received research support from BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Janssen, IGM Biosciences, Novartis, and Takeda. LJN serves on data safety monitoring boards for DeNovo, Genentech, MEI, and Takeda.
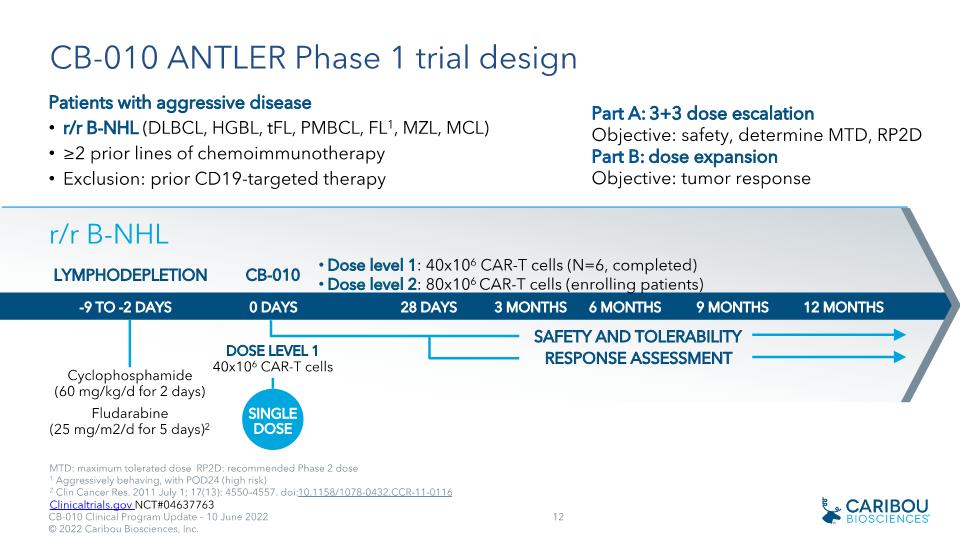
CB-010 ANTLER Phase 1 trial design Patients with aggressive disease r/r B-NHL (DLBCL, HGBL, tFL, PMBCL, FL1, MZL, MCL) ≥2 prior lines of chemoimmunotherapy Exclusion: prior CD19-targeted therapy MTD: maximum tolerated dose RP2D: recommended Phase 2 dose 1 Aggressively behaving, with POD24 (high risk) 2 Clin Cancer Res. 2011 July 1; 17(13): 4550–4557. doi:10.1158/1078-0432.CCR-11-0116 SAFETY AND TOLERABILITY 28 DAYS 3 months 6 months 9 months 12 MONTHS -9 TO -2 DAYS _ Cyclophosphamide �(60 mg/kg/d for 2 days) Fludarabine �(25 mg/m2/d for 5 days)2 LYMPHODEPLETION r/r B-NHL 0 DAYS DOSE LEVEL 1�40x106 CAR-T cells CB-010 SINGLE�DOSE Part A: 3+3 dose escalation Objective: safety, determine MTD, RP2D Part B: dose expansion Objective: tumor response Dose level 1: 40x106 CAR-T cells (N=6, completed) Dose level 2: 80x106 CAR-T cells (enrolling patients) RESPONSE ASSESSMENT Clinicaltrials.gov NCT#04637763
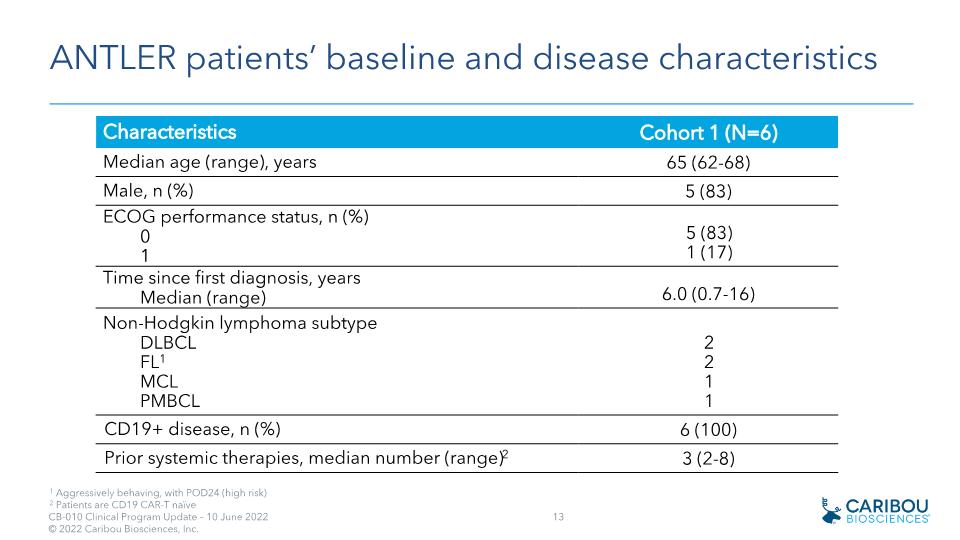
ANTLER patients’ baseline and disease characteristics 1 Aggressively behaving, with POD24 (high risk) 2 Patients are CD19 CAR-T naïve Characteristics Cohort 1 (N=6) Median age (range), years 65 (62-68) Male, n (%) 5 (83) ECOG performance status, n (%) 0 1 5 (83) 1 (17) Time since first diagnosis, years Median (range) 6.0 (0.7-16) Non-Hodgkin lymphoma subtype DLBCL FL1 MCL PMBCL 2 2 1 1 CD19+ disease, n (%) 6 (100) Prior systemic therapies, median number (range)2 3 (2-8)
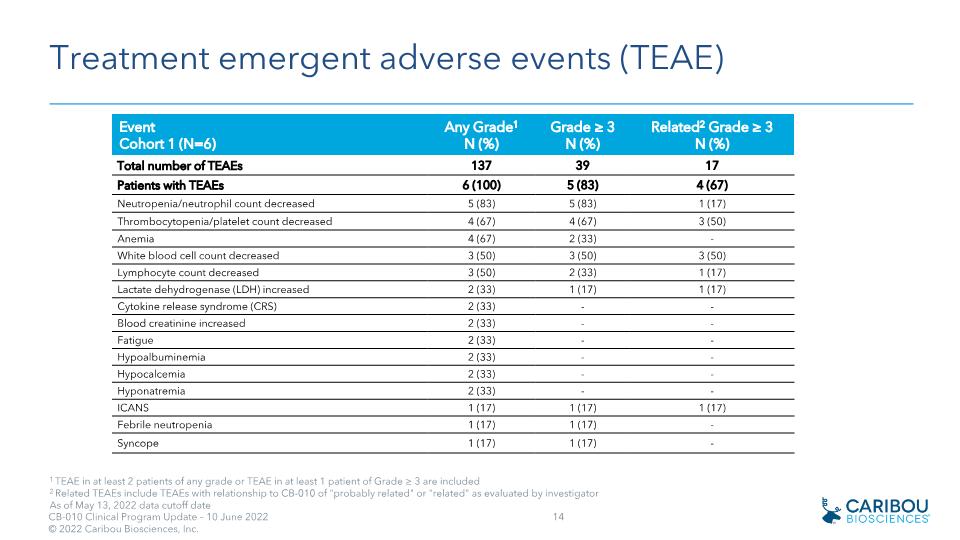
Treatment emergent adverse events (TEAE) Event Cohort 1 (N=6) Any Grade1 N (%) Grade ≥ 3 N (%) Related2 Grade ≥ 3 N (%) Total number of TEAEs 137 39 17 Patients with TEAEs 6 (100) 5 (83) 4 (67) Neutropenia/neutrophil count decreased 5 (83) 5 (83) 1 (17) Thrombocytopenia/platelet count decreased 4 (67) 4 (67) 3 (50) Anemia 4 (67) 2 (33) - White blood cell count decreased 3 (50) 3 (50) 3 (50) Lymphocyte count decreased 3 (50) 2 (33) 1 (17) Lactate dehydrogenase (LDH) increased 2 (33) 1 (17) 1 (17) Cytokine release syndrome (CRS) 2 (33) - - Blood creatinine increased 2 (33) - - Fatigue 2 (33) - - Hypoalbuminemia 2 (33) - - Hypocalcemia 2 (33) - - Hyponatremia 2 (33) - - ICANS 1 (17) 1 (17) 1 (17) Febrile neutropenia 1 (17) 1 (17) - Syncope 1 (17) 1 (17) - 1 TEAE in at least 2 patients of any grade or TEAE in at least 1 patient of Grade ≥ 3 are included 2 Related TEAEs include TEAEs with relationship to CB-010 of "probably related" or "related" as evaluated by investigator As of May 13, 2022 data cutoff date
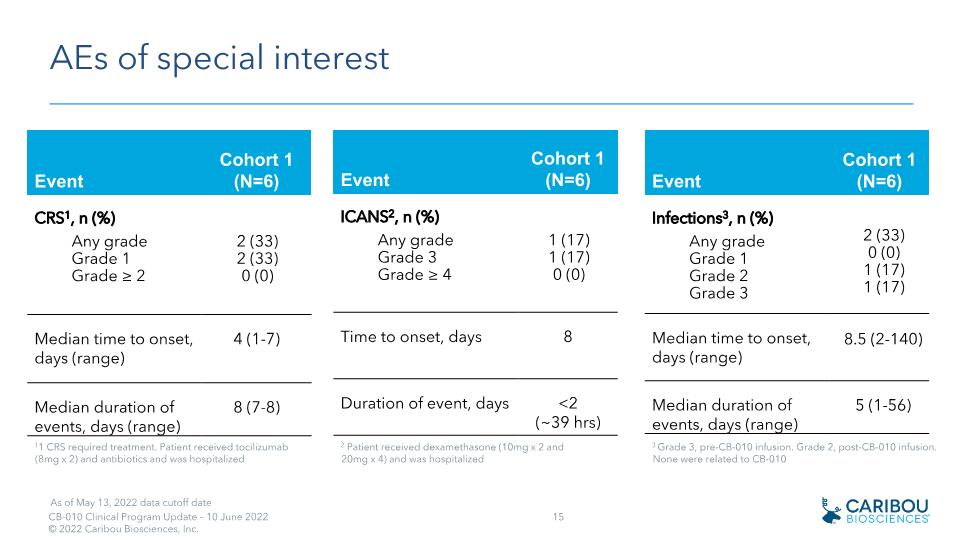
AEs of special interest Event Cohort 1 (N=6) Infections3, n (%) Any grade Grade 1 Grade 2 Grade 3 2 (33) 0 (0) 1 (17) 1 (17) Median time to onset, days (range) 8.5 (2-140) Median duration of events, days (range) 5 (1-56) 3 Grade 3, pre-CB-010 infusion. Grade 2, post-CB-010 infusion. None were related to CB-010 Event Cohort 1 (N=6) CRS1, n (%) Any grade Grade 1 Grade ≥ 2 2 (33) 2 (33) 0 (0) Median time to onset, days (range) 4 (1-7) Median duration of events, days (range) 8 (7-8) Event Cohort 1 (N=6) ICANS2, n (%) Any grade Grade 3 Grade ≥ 4 1 (17) 1 (17) 0 (0) Time to onset, days 8 Duration of event, days <2 (~39 hrs) 11 CRS required treatment. Patient received tocilizumab (8mg x 2) and antibiotics and was hospitalized 2 Patient received dexamethasone (10mg x 2 and 20mg x 4) and was hospitalized As of May 13, 2022 data cutoff date
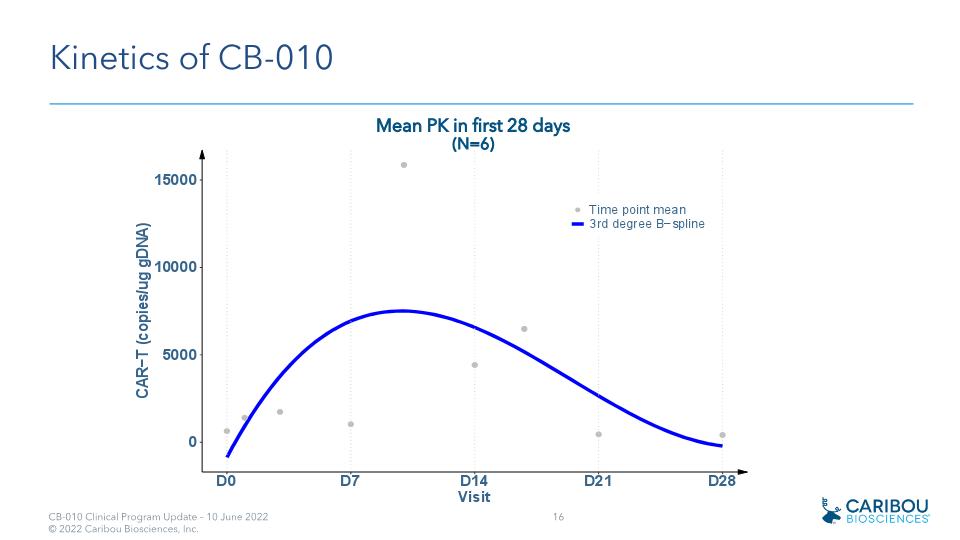
Kinetics of CB-010 Mean PK in first 28 days (N=6)
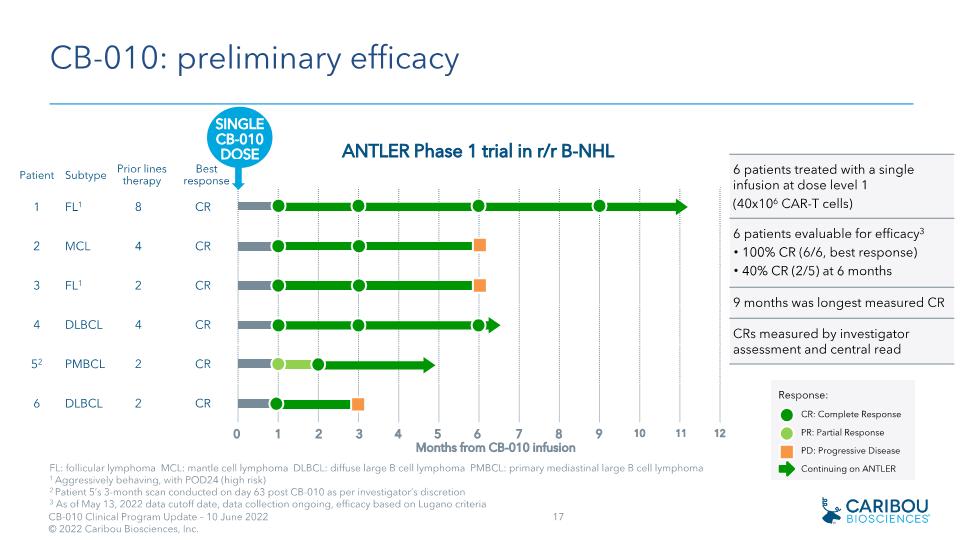
CB-010: preliminary efficacy 6 patients treated with a single infusion at dose level 1 (40x106 CAR-T cells) 6 patients evaluable for efficacy3 100% CR (6/6, best response) 40% CR (2/5) at 6 months 9 months was longest measured CR CRs measured by investigator assessment and central read FL: follicular lymphoma MCL: mantle cell lymphoma DLBCL: diffuse large B cell lymphoma PMBCL: primary mediastinal large B cell lymphoma 1 Aggressively behaving, with POD24 (high risk) 2 Patient 5’s 3-month scan conducted on day 63 post CB-010 as per investigator’s discretion 3 As of May 13, 2022 data cutoff date, data collection ongoing, efficacy based on Lugano criteria Patient Subtype Prior lines therapy Best response 1 FL1 8 CR 2 MCL 4 CR 3 FL1 2 CR 4 DLBCL 4 CR 52 PMBCL 2 CR 6 DLBCL 2 CR 0 1 2 3 4 5 6 7 8 10 Months from CB-010 infusion ANTLER Phase 1 trial in r/r B-NHL SINGLE�CB-010 DOSE Response: CR: Complete Response PR: Partial Response PD: Progressive Disease Continuing on ANTLER 9 11 12

Patient case study�James H. Essell, MD�OHC hematologist, medical oncologist, blood and �marrow transplant specialist�Chair, Cellular Therapy, US Oncology Network�OHC – Specialists in Cancer and Blood Disorders
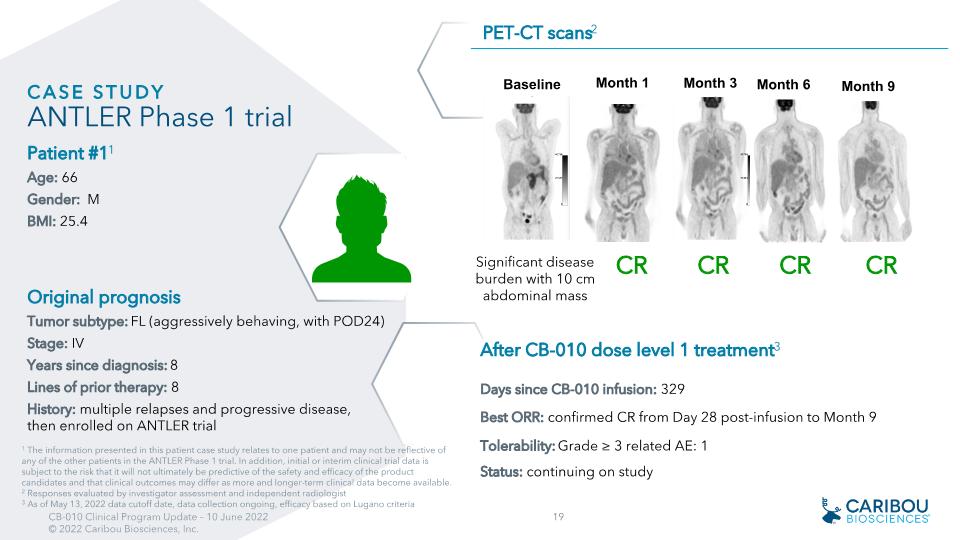
Original prognosis Tumor subtype: FL (aggressively behaving, with POD24) Stage: IV Years since diagnosis: 8 Lines of prior therapy: 8 History: multiple relapses and progressive disease, then enrolled on ANTLER trial PET-CT scans2 1 The information presented in this patient case study relates to one patient and may not be reflective of any of the other patients in the ANTLER Phase 1 trial. In addition, initial or interim clinical trial data is subject to the risk that it will not ultimately be predictive of the safety and efficacy of the product candidates and that clinical outcomes may differ as more and longer-term clinical data become available. 2 Responses evaluated by investigator assessment and independent radiologist 3 As of May 13, 2022 data cutoff date, data collection ongoing, efficacy based on Lugano criteria Significant disease burden with 10 cm abdominal mass CR CR CR CR CASE study�ANTLER Phase 1 trial Patient #11 Age: 66 Gender: M BMI: 25.4 After CB-010 dose level 1 treatment3 Days since CB-010 infusion: 329 Best ORR: confirmed CR from Day 28 post-infusion to Month 9 Tolerability: Grade ≥ 3 related AE: 1 Status: continuing on study Baseline Month 1 Month 3 VS = 3 Month 6 Month 9
Fireside chat

Fireside chat with Dr. Nastoupil and Dr. Essell Loretta J. Nastoupil, MD Section Chief, New Drug Development Associate Professor, Department of Lymphoma/Myeloma The University of Texas MD Anderson Cancer Center James H. Essell, MD OHC hematologist, medical oncologist, blood and marrow transplant specialist Chair, Cellular Therapy, US Oncology Network OHC – Specialists in Cancer and Blood Disorders Rachel Haurwitz, PhD President and CEO Caribou Biosciences
Q&A

Open to your questions Loretta J. Nastoupil, MD Section Chief, New Drug Development Associate Professor, Department of Lymphoma/Myeloma The University of Texas MD Anderson Cancer Center James H. Essell, MD OHC hematologist, medical oncologist, blood and marrow transplant specialist Chair, Cellular Therapy, US Oncology Network OHC – Specialists in Cancer and Blood Disorders Steve Kanner, PhD Chief Scientific Officer Rachel Haurwitz, PhD President and CEO Director Syed Rizvi, MD Chief Medical Officer Caribou Biosciences Caribou Biosciences Caribou Biosciences

Closing remarks�Rachel Haurwitz, PhD�President & CEO�Caribou Biosciences, Inc.
Initial ANTLER data are an important step toward validating Caribou’s chRDNA genome-editing platform 100% CR rate1 (6/6, best response), 40% CR rate1 (2/5) at 6 months from a single dose of CB-010 at dose level 1 1st allogeneic CAR-T cell therapy to achieve 100% CR rate, best response Promising initial safety profile Currently enrolling patients in ANTLER Phase 1 trial at dose level 2 Additional ANTLER data expected by YE 2022 Goal to develop CB-010 as an allogeneic cell therapy that can meaningfully rival autologous cell therapies to reach broader groups of patients globally who need off-the-shelf cell therapy CB-010 is Caribou’s lead program and part of a pipeline of precision genome-edited allogeneic CAR-T and CAR-NK cell therapies Experienced team and capital2 to execute on our mission 1 As of May 13, 2022 data cutoff date, data collection ongoing, efficacy based on Lugano criteria 2 $391M in cash, cash equivalents, and marketable securities as of March 31, 2022
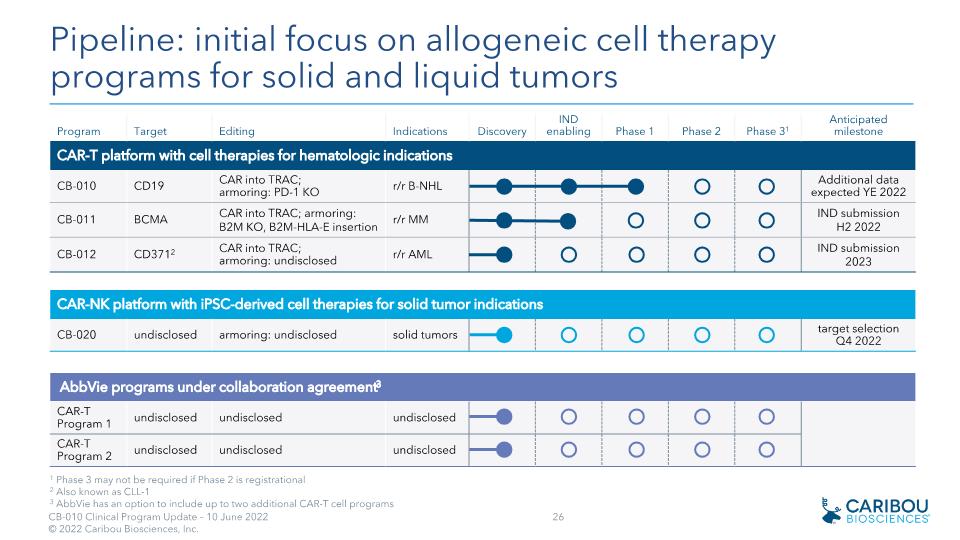
Program Target Editing Indications Discovery IND enabling Phase 1 Phase 2 Phase 31 Anticipated�milestone CAR-T platform with cell therapies for hematologic indications CB-010 CD19 CAR into TRAC;�armoring: PD-1 KO r/r B-NHL Additional data expected YE 2022 CB-011 BCMA CAR into TRAC; armoring: B2M KO, B2M-HLA-E insertion r/r MM IND submission H2 2022 CB-012 CD3712 CAR into TRAC; armoring: undisclosed r/r AML IND submission 2023 CAR-NK platform with iPSC-derived cell therapies for solid tumor indications CB-020 undisclosed armoring: undisclosed solid tumors target selection Q4 2022 AbbVie programs under collaboration agreement3 CAR-T Program 1 undisclosed undisclosed undisclosed CAR-T Program 2 undisclosed undisclosed undisclosed Collaboration with Pipeline: initial focus on allogeneic cell therapy programs for solid and liquid tumors 1 Phase 3 may not be required if Phase 2 is registrational 2 Also known as CLL-1 3 AbbVie has an option to include up to two additional CAR-T cell programs

cariboubio.com�info@cariboubio.com Thank you
Appendix

Worldwide NHL incidence2 B-NHL 5-year post-diagnosis survival rates3 1 National Cancer Institute, Leukemia & Lymphoma Society, Lymphoma Research Foundation 2 Evaluate Pharma, May 2022, www.evaluate.com 3 Cancer Research U.K. Diffuse large B cell lymphoma (DLBCL) Follicular lymphoma (FL) Marginal zone lymphoma (MZL) Mantle cell lymphoma (MCL) Other NHL is the most common hematologic malignancy in the U.S. Mature B cell lymphomas (B-NHL) are 80-85% of all NHL cases ~34% of B-NHL cases are considered relapsed or refractory (r/r)1 Current autologous CAR-T cell therapies have limited patient access with complex manufacturing and high production costs US incidence1: ~82,900 EU incidence: ~101,000 China incidence: ~97,500 Japan incidence: ~33,600 B-NHL subtypes1 r/r B-NHL: high unmet need globally for �off-the-shelf cell therapy
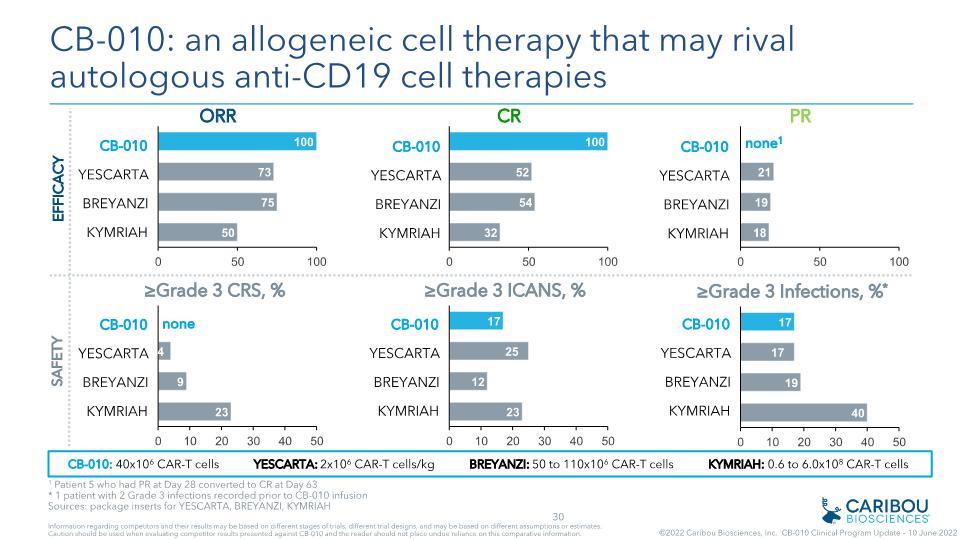
CB-010 YESCARTA BREYANZI KYMRIAH none CB-010: an allogeneic cell therapy that may rival autologous anti-CD19 cell therapies EFFICACY SAFETY CB-010: 40x106 CAR-T cells YESCARTA: 2x106 CAR-T cells/kg BREYANZI: 50 to 110x106 CAR-T cells KYMRIAH: 0.6 to 6.0x108 CAR-T cells CB-010 YESCARTA BREYANZI KYMRIAH CB-010 YESCARTA BREYANZI KYMRIAH CB-010 YESCARTA BREYANZI KYMRIAH CB-010 YESCARTA BREYANZI KYMRIAH CB-010 YESCARTA BREYANZI KYMRIAH none1 1 Patient 5 who had PR at Day 28 converted to CR at Day 63 * 1 patient with 2 Grade 3 infections recorded prior to CB-010 infusion Sources: package inserts for YESCARTA, BREYANZI, KYMRIAH Information regarding competitors and their results may be based on different stages of trials, different trial designs, and may be based on different assumptions or estimates. Caution should be used when evaluating competitor results presented against CB-010 and the reader should not place undue reliance on this comparative information. ©2022 Caribou Biosciences, Inc. CB-010 Clinical Program Update – 10 June 2022
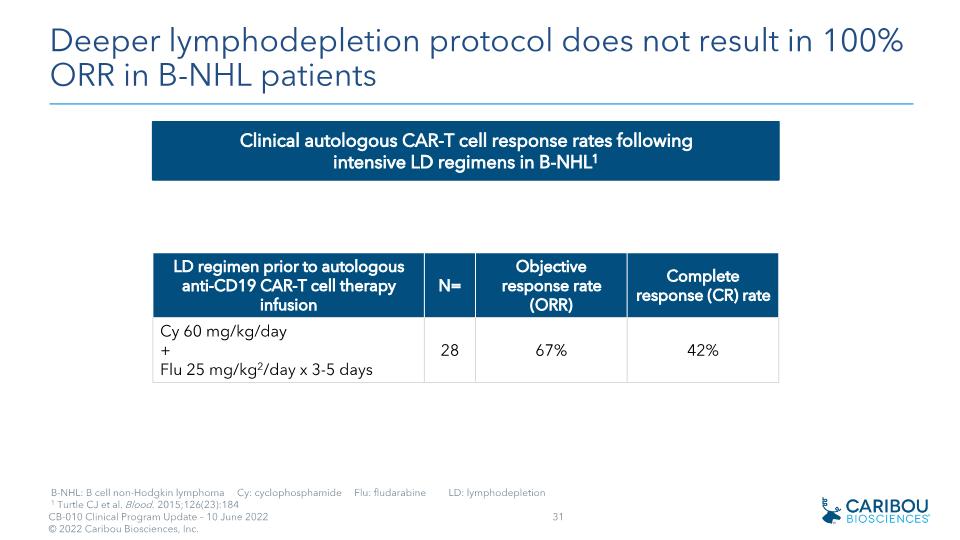
Deeper lymphodepletion protocol does not result in 100% ORR in B-NHL patients B-NHL: B cell non-Hodgkin lymphoma Cy: cyclophosphamide Flu: fludarabine LD: lymphodepletion 1 Turtle CJ et al. Blood. 2015;126(23):184 LD regimen prior to autologous anti-CD19 CAR-T cell therapy infusion N= Objective response rate (ORR) Complete response (CR) rate Cy 60 mg/kg/day +�Flu 25 mg/kg2/day x 3-5 days 28 67% 42% Clinical autologous CAR-T cell response rates following intensive LD regimens in B-NHL1






























