
ACT-AD Topline Results Investor Webinar June 22, 2022 Exhibit 99.2

Forward Looking Statements © Athira Pharma, Inc. All Rights Reserved. This presentation and the accompanying oral commentary contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. Forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “expect,” “plan,” anticipate,” “believe,” “estimate,” “predict,” “intend,” “potential,” “would,” “continue,” “ongoing” or the negative of these terms or other comparable terminology. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our future financial performance, fosgonimeton as a potential treatment for Alzheimer's disease and other dementias; the potential learnings from the ACT-AD trial and their ability to inform and improve future clinical development plans; the anticipated reporting of data; business plans and objectives, timing and success of our planned development activities, our ability to obtain regulatory approval, the potential therapeutic benefits and economic value of our product candidates, potential growth opportunities, competitive position, industry environment and potential market opportunities, and the impact of the COVID-19 pandemic on our business and operations. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. These factors, together with those that are described in greater detail in our filings with the Securities and Exchange Commission (“SEC”) may cause our actual results, performance or achievements to differ materially and adversely from those anticipated or implied by our forward-looking statements. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this presentation, and although we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted a thorough inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and readers are cautioned not to unduly rely upon these statements. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. By attending or receiving this presentation you acknowledge that you will be solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of our business. This presentation contains estimates, projections and other information concerning market, industry and other data. We obtained this data from our own internal estimates and research and from academic and industry research, publications, surveys, and studies conducted by third parties, including governmental agencies. These data involve a number of assumptions and limitations, are subject to risks and uncertainties, and are subject to change based on various factors, including those discussed in our filings with the SEC. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us. While we believe such information is generally reliable, we have not independently verified any third-party information. The ACT-AD trial is supported by a grant from the National Institute on Aging of the National Institutes of Health under Award Number R01AG06268. The information presented in this press release is solely the responsibility of Athira and does not necessarily represent the official views of the National Institutes of Health. This presentation concerns drug candidates that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration. The drug candidates are currently limited by federal law to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated. We announce material information to the public through a variety of means, including filings with the SEC, press releases, public conference calls, our website (www.athira.com/), our investor relations website (investors.athira.com), and our news site (investors.athira.com/news-and-events/press-releases). We use these channels, as well as social media, including our Twitter account (@athirapharma) and Facebook page (https://www.facebook.com/athirapharmainc), to communicate with investors and the public about Athira, our products, and other matters. Therefore, we encourage investors, the media, and others interested in Athira to review the information we make public in these locations, as such information could be deemed to be material information. *** The ACT-AD trial is supported by a grant from the National Institute on Aging of the National Institutes of Health under Award Number R01AG06268. The information presented in this presentation is solely the responsibility of Athira and does not necessarily represent the official views of the National Institutes of Health.

Hans Moebius, M.D., Ph.D. Chief Medical Officer ACT-AD Topline Results © Athira Pharma, Inc. All Rights Reserved.
Proof of concept ACT-AD trial (N=77) provides important insights for further fosgonimeton development program Phase 1b study conducted with fosgonimeton as a monotherapy; Phase 2 allowed for add-on standard of care (AChEIs) to obtain safety data in a more representative real-world patient population Primary analysis not statistically significant; fosgonimeton suggests potentially beneficial treatment effect as a monotherapy By pre-specified analysis, fosgonimeton and background standard of care both showed positive treatment effects in ERP P300 latency and ADAS-Cog11 as monotherapies, but not in combination By post hoc analysis, the parallel ERP P300 latency (-28 ms) and ADAS-Cog11 (-3.3 points) signals appear more pronounced in fosgonimeton monotherapy Treatment with fosgonimeton was well tolerated, without typical CNS adverse effects, and safety profile was favorable Results will help inform LIFT-AD and optimize chances for success ACT-AD Executive Summary © Athira Pharma, Inc. All Rights Reserved.
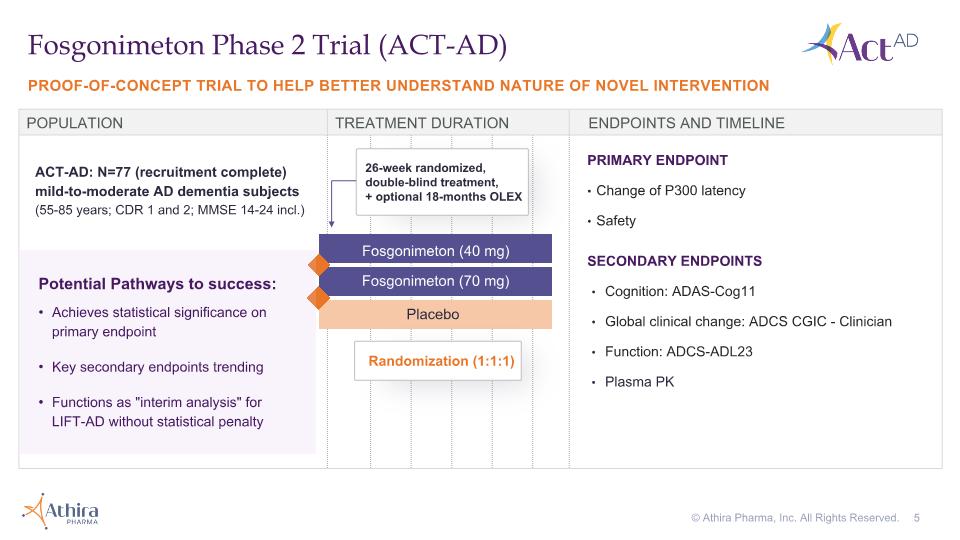
PROOF-OF-CONCEPT TRIAL TO HELP BETTER UNDERSTAND NATURE OF NOVEL INTERVENTION Fosgonimeton Phase 2 Trial (ACT-AD) POPULATION TREATMENT DURATION ENDPOINTS AND TIMELINE Fosgonimeton (70 mg) Placebo Fosgonimeton (40 mg) ACT-AD: N=77 (recruitment complete) mild-to-moderate AD dementia subjects �(55-85 years; CDR 1 and 2; MMSE 14-24 incl.) PRIMARY ENDPOINT Change of P300 latency Safety SECONDARY ENDPOINTS Cognition: ADAS-Cog11 Global clinical change: ADCS CGIC - Clinician Function: ADCS-ADL23 Plasma PK Potential Pathways to success: Achieves statistical significance on primary endpoint Key secondary endpoints trending Functions as "interim analysis" for LIFT-AD without statistical penalty Randomization (1:1:1) 26-week randomized, double-blind treatment, + optional 18-months OLEX © Athira Pharma, Inc. All Rights Reserved.
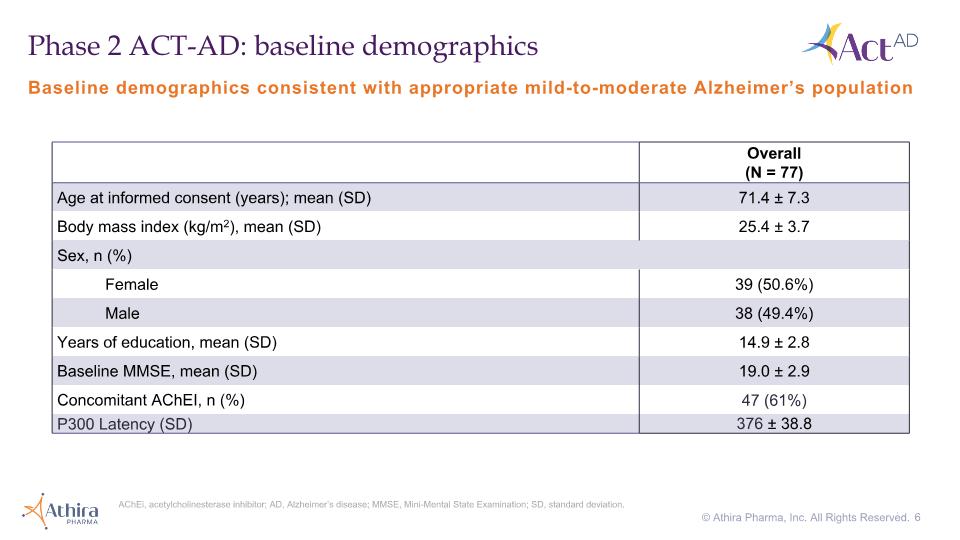
Overall (N = 77) Age at informed consent (years); mean (SD) 71.4 ± 7.3 Body mass index (kg/m2), mean (SD) 25.4 ± 3.7 Sex, n (%) Female 39 (50.6%) Male 38 (49.4%) Years of education, mean (SD) 14.9 ± 2.8 Baseline MMSE, mean (SD) 19.0 ± 2.9 Concomitant AChEI, n (%) 47 (61%) P300 Latency (SD) 376 ± 38.8 Baseline demographics consistent with appropriate mild-to-moderate Alzheimer’s population Phase 2 ACT-AD: baseline demographics AChEi, acetylcholinesterase inhibitor; AD, Alzheimer’s disease; MMSE, Mini-Mental State Examination; SD, standard deviation. . 6 © Athira Pharma, Inc. All Rights Reserved.
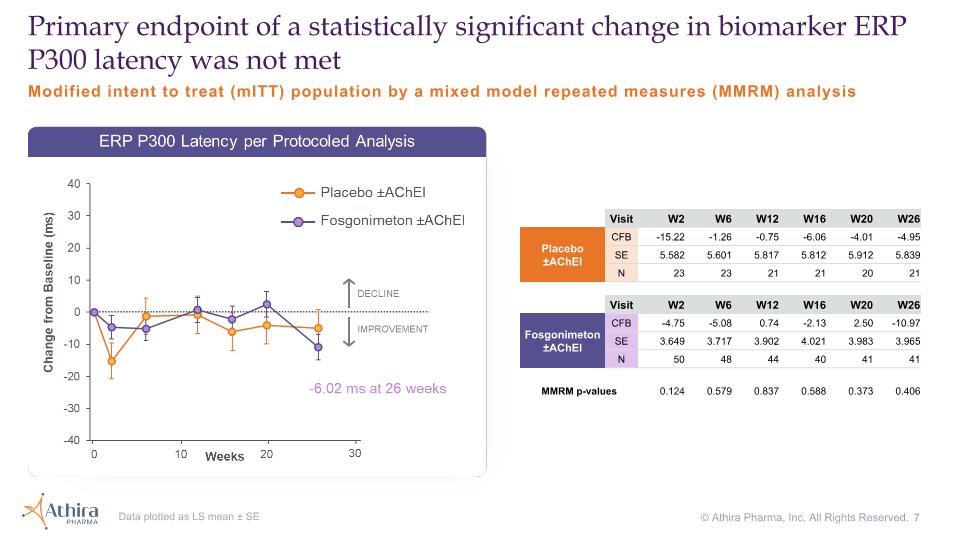
Modified intent to treat (mITT) population by a mixed model repeated measures (MMRM) analysis Primary endpoint of a statistically significant change in biomarker ERP P300 latency was not met Visit W2 W6 W12 W16 W20 W26 Placebo�±AChEI CFB -15.22 -1.26 -0.75 -6.06 -4.01 -4.95 SE 5.582 5.601 5.817 5.812 5.912 5.839 N 23 23 21 21 20 21 Visit W2 W6 W12 W16 W20 W26 Fosgonimeton ±AChEI CFB -4.75 -5.08 0.74 -2.13 2.50 -10.97 SE 3.649 3.717 3.902 4.021 3.983 3.965 N 50 48 44 40 41 41 MMRM p-values 0.124 0.579 0.837 0.588 0.373 0.406 Data plotted as LS mean ± SE © Athira Pharma, Inc. All Rights Reserved.
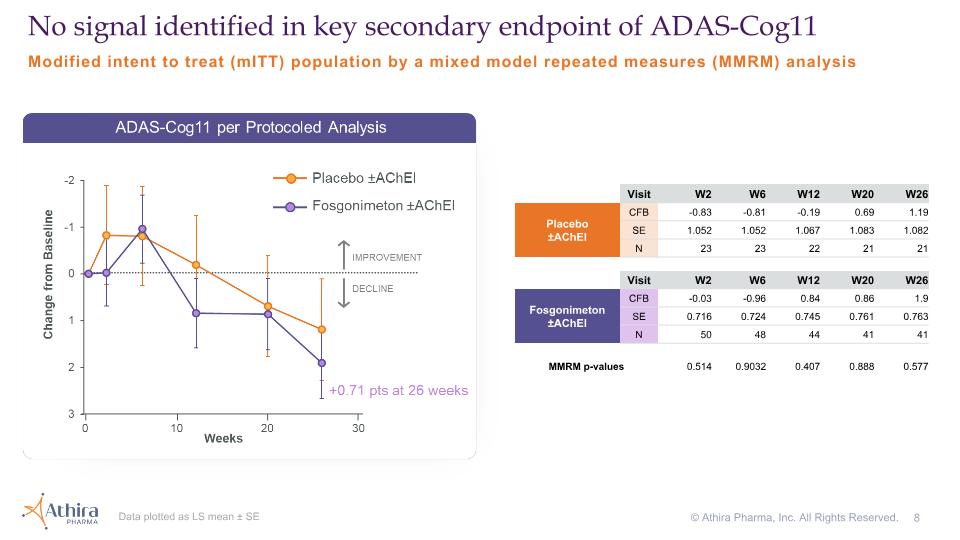
Modified intent to treat (mITT) population by a mixed model repeated measures (MMRM) analysis No signal identified in key secondary endpoint of ADAS-Cog11 Visit W2 W6 W12 W20 W26 Placebo�±AChEI CFB -0.83 -0.81 -0.19 0.69 1.19 SE 1.052 1.052 1.067 1.083 1.082 N 23 23 22 21 21 Visit W2 W6 W12 W20 W26 Fosgonimeton ±AChEI CFB -0.03 -0.96 0.84 0.86 1.9 SE 0.716 0.724 0.745 0.761 0.763 N 50 48 44 41 41 MMRM p-values 0.514 0.9032 0.407 0.888 0.577 Data plotted as LS mean ± SE © Athira Pharma, Inc. All Rights Reserved.
Pre-specified subgroup analysis suggested differential effects with concomitant standard of care Severity (mild or moderate) ApoE (carriers or non-carriers) AChEI use (+/- current use of acetylcholinesterase inhibitors including donepezil, rivastigmine, or galantamine) Analysis of the subgroups suggest AChEI use may impact outcomes No notable patterns observed in other subgroups to date Subgroup analysis was performed per protocoled SAP © Athira Pharma, Inc. All Rights Reserved.
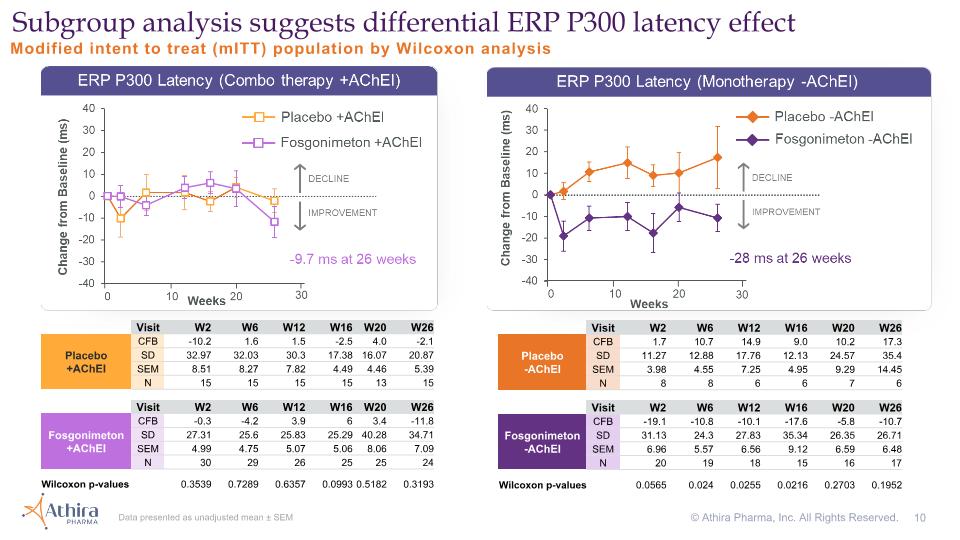
Modified intent to treat (mITT) population by Wilcoxon analysis Subgroup analysis suggests differential ERP P300 latency effect Data presented as unadjusted mean ± SEM Visit W2 W6 W12 W16 W20 W26 Placebo�+AChEI CFB -10.2 1.6 1.5 -2.5 4.0 -2.1 SD 32.97 32.03 30.3 17.38 16.07 20.87 SEM 8.51 8.27 7.82 4.49 4.46 5.39 N 15 15 15 15 13 15 Visit W2 W6 W12 W16 W20 W26 Fosgonimeton +AChEI CFB -0.3 -4.2 3.9 6 3.4 -11.8 SD 27.31 25.6 25.83 25.29 40.28 34.71 SEM 4.99 4.75 5.07 5.06 8.06 7.09 N 30 29 26 25 25 24 Wilcoxon p-values 0.3539 0.7289 0.6357 0.0993 0.5182 0.3193 Visit W2 W6 W12 W16 W20 W26 Placebo�-AChEI CFB 1.7 10.7 14.9 9.0 10.2 17.3 SD 11.27 12.88 17.76 12.13 24.57 35.4 SEM 3.98 4.55 7.25 4.95 9.29 14.45 N 8 8 6 6 7 6 Visit W2 W6 W12 W16 W20 W26 Fosgonimeton -AChEI CFB -19.1 -10.8 -10.1 -17.6 -5.8 -10.7 SD 31.13 24.3 27.83 35.34 26.35 26.71 SEM 6.96 5.57 6.56 9.12 6.59 6.48 N 20 19 18 15 16 17 Wilcoxon p-values 0.0565 0.024 0.0255 0.0216 0.2703 0.1952 © Athira Pharma, Inc. All Rights Reserved.
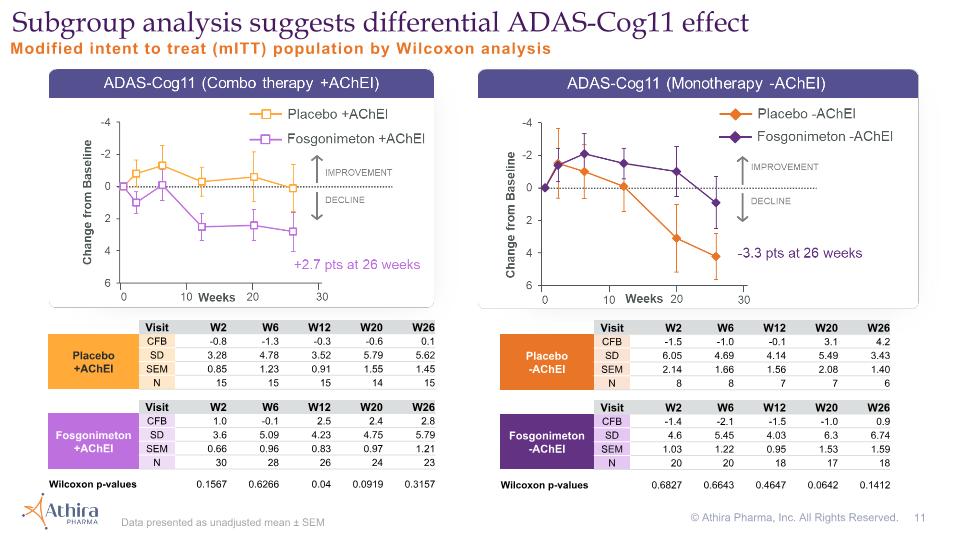
Modified intent to treat (mITT) population by Wilcoxon analysis Subgroup analysis suggests differential ADAS-Cog11 effect Visit W2 W6 W12 W20 W26 Placebo�+AChEI CFB -0.8 -1.3 -0.3 -0.6 0.1 SD 3.28 4.78 3.52 5.79 5.62 SEM 0.85 1.23 0.91 1.55 1.45 N 15 15 15 14 15 Visit W2 W6 W12 W20 W26 Fosgonimeton +AChEI CFB 1.0 -0.1 2.5 2.4 2.8 SD 3.6 5.09 4.23 4.75 5.79 SEM 0.66 0.96 0.83 0.97 1.21 N 30 28 26 24 23 Wilcoxon p-values 0.1567 0.6266 0.04 0.0919 0.3157 Visit W2 W6 W12 W20 W26 Placebo�-AChEI CFB -1.5 -1.0 -0.1 3.1 4.2 SD 6.05 4.69 4.14 5.49 3.43 SEM 2.14 1.66 1.56 2.08 1.40 N 8 8 7 7 6 Visit W2 W6 W12 W20 W26 Fosgonimeton -AChEI CFB -1.4 -2.1 -1.5 -1.0 0.9 SD 4.6 5.45 4.03 6.3 6.74 SEM 1.03 1.22 0.95 1.53 1.59 N 20 20 18 17 18 Wilcoxon p-values 0.6827 0.6643 0.4647 0.0642 0.1412 Data presented as unadjusted mean ± SEM © Athira Pharma, Inc. All Rights Reserved.
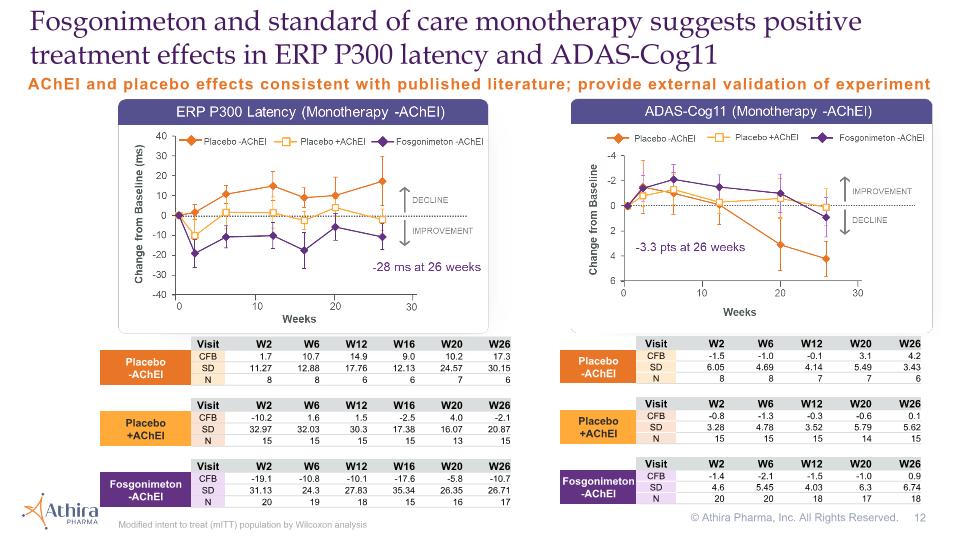
AChEI and placebo effects consistent with published literature; provide external validation of experiment Fosgonimeton and standard of care monotherapy suggests positive treatment effects in ERP P300 latency and ADAS-Cog11 Modified intent to treat (mITT) population by Wilcoxon analysis © Athira Pharma, Inc. All Rights Reserved. Visit W2 W6 W12 W20 W26 Placebo�-AChEI CFB -1.5 -1.0 -0.1 3.1 4.2 SD 6.05 4.69 4.14 5.49 3.43 N 8 8 7 7 6 Visit W2 W6 W12 W20 W26 Placebo�+AChEI CFB -0.8 -1.3 -0.3 -0.6 0.1 SD 3.28 4.78 3.52 5.79 5.62 N 15 15 15 14 15 Visit W2 W6 W12 W20 W26 Fosgonimeton -AChEI CFB -1.4 -2.1 -1.5 -1.0 0.9 SD 4.6 5.45 4.03 6.3 6.74 N 20 20 18 17 18 Visit W2 W6 W12 W16 W20 W26 Placebo�-AChEI CFB 1.7 10.7 14.9 9.0 10.2 17.3 SD 11.27 12.88 17.76 12.13 24.57 30.15 N 8 8 6 6 7 6 Visit W2 W6 W12 W16 W20 W26 Placebo�+AChEI CFB -10.2 1.6 1.5 -2.5 4.0 -2.1 SD 32.97 32.03 30.3 17.38 16.07 20.87 N 15 15 15 15 13 15 Visit W2 W6 W12 W16 W20 W26 Fosgonimeton -AChEI CFB -19.1 -10.8 -10.1 -17.6 -5.8 -10.7 SD 31.13 24.3 27.83 35.34 26.35 26.71 N 20 19 18 15 16 17
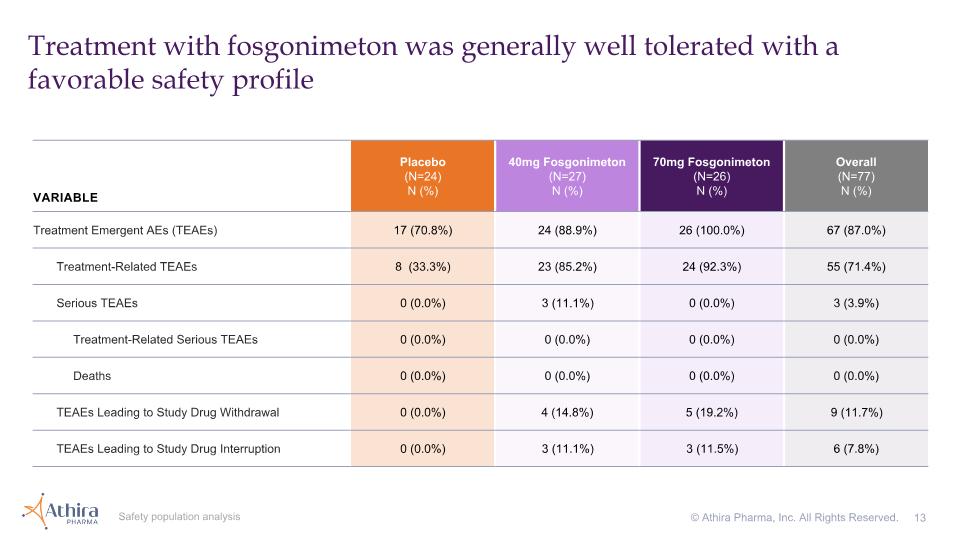
Treatment with fosgonimeton was generally well tolerated with a favorable safety profile Safety population analysis © Athira Pharma, Inc. All Rights Reserved. VARIABLE Placebo (N=24) N (%) 40mg Fosgonimeton (N=27) N (%) 70mg Fosgonimeton (N=26) N (%) Overall (N=77) N (%) Treatment Emergent AEs (TEAEs) 17 (70.8%) 24 (88.9%) 26 (100.0%) 67 (87.0%) Treatment-Related TEAEs 8 (33.3%) 23 (85.2%) 24 (92.3%) 55 (71.4%) Serious TEAEs 0 (0.0%) 3 (11.1%) 0 (0.0%) 3 (3.9%) Treatment-Related Serious TEAEs 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) Deaths 0 (0.0%) 0 (0.0%) 0 (0.0%) 0 (0.0%) TEAEs Leading to Study Drug Withdrawal 0 (0.0%) 4 (14.8%) 5 (19.2%) 9 (11.7%) TEAEs Leading to Study Drug Interruption 0 (0.0%) 3 (11.1%) 3 (11.5%) 6 (7.8%)
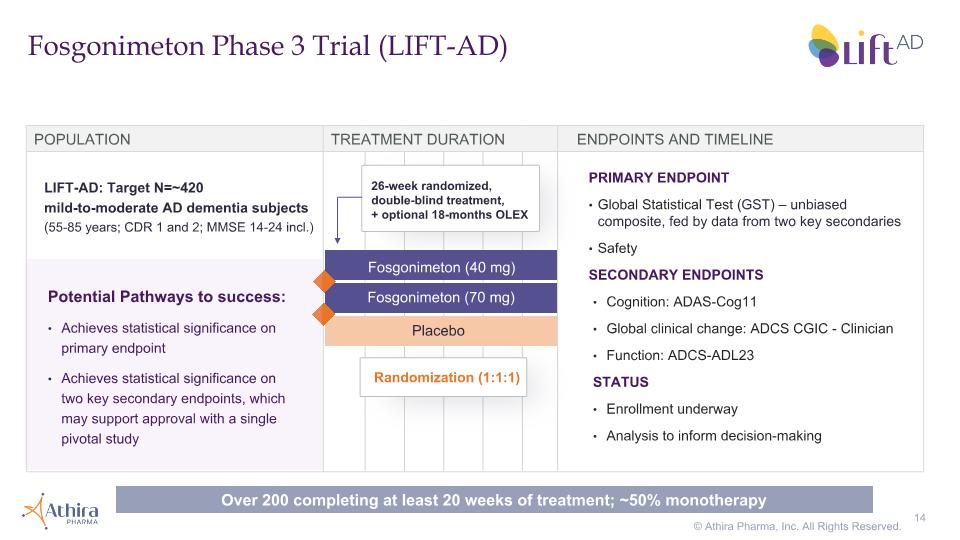
Fosgonimeton Phase 3 Trial (LIFT-AD) POPULATION TREATMENT DURATION ENDPOINTS AND TIMELINE Fosgonimeton (70 mg) Placebo Fosgonimeton (40 mg) LIFT-AD: Target N=~420 mild-to-moderate AD dementia subjects �(55-85 years; CDR 1 and 2; MMSE 14-24 incl.) PRIMARY ENDPOINT Global Statistical Test (GST) – unbiased composite, fed by data from two key secondaries Safety SECONDARY ENDPOINTS Cognition: ADAS-Cog11 Global clinical change: ADCS CGIC - Clinician Function: ADCS-ADL23 STATUS Enrollment underway Analysis to inform decision-making Potential Pathways to success: Achieves statistical significance on primary endpoint Achieves statistical significance on two key secondary endpoints, which may support approval with a single pivotal study Randomization (1:1:1) 26-week randomized, double-blind treatment, + optional 18-months OLEX Over 200 completing at least 20 weeks of treatment; ~50% monotherapy © Athira Pharma, Inc. All Rights Reserved.
This first small interventional trial with the positive HGF/MET modulator fosgonimeton supports its potential in Alzheimer’s disease Primary analysis not statistically significant; fosgonimeton suggests potentially beneficial treatment effect as a monotherapy By pre-specified analysis, background AChEIs and fosgonimeton both showed positive treatment effects in P300 latency and ADAS-Cog11 as monotherapies, but not in combination By post hoc analysis, the parallel P300 latency (-28 ms) and ADAS-Cog11 (-3.3 points) signal appears more pronounced in fosgonimeton monotherapy Treatment with fosgonimeton was well tolerated, without typical CNS adverse effects, and safety profile was favorable Full analysis is ongoing The results will inform the optimization of the parallel Phase 3 LIFT-AD study, with over 200 completing at least 20 weeks of treatment; ~50% monotherapy Will seek advice from experts, advisors, and regulators on how to expeditiously analyze and potentially adapt the LIFT-AD study Recruitment to LIFT-AD and the transitions to the Open Label Extension study will continue ACT-AD Conclusion © Athira Pharma, Inc. All Rights Reserved.

Mark Litton, Ph.D. Chief Executive Officer Conclusion © Athira Pharma, Inc. All Rights Reserved.
ACT-AD results to benefit LIFT-AD probability of success ACT-AD is the first study to show potential cognitive improvement with ADAS-Cog11 in AD patients by positive modulation of the HGF/MET receptor by fosgonimeton ACT-AD outcomes support our conviction that we have a unique opportunity to make a positive difference for patients suffering from neurodegenerative diseases ACT-AD results are important data that warrant further evaluation and inform how best to optimize the parallel LIFT-AD study Planned near term LIFT-AD analysis will also inform decision-making Closing: Fosgonimeton has potential in Alzheimer’s Disease © Athira Pharma, Inc. All Rights Reserved. Strong balance sheet to support fosgonimeton development program through key inflection points
















