EXHIBIT 10.1
Confidential
Execution Copy
MATERIAL AND DATA TRANSFER, OPTION AND LICENSE AGREEMENT
THIS MATERIAL AND DATA TRANSFER, OPTION AND LICENSE AGREEMENT (this “Agreement”) entered into on this December 20, 2017 (the “Signature Date”) by and between NEOMED Institute, a not-for-profit corporation established under the Not-for-Profit Corporations Act (Canada), having an address at 7171 Frederick-Banting, Saint-Laurent, Quebec H4S 1Z9, Canada (“NEOMED”), and Artelo Biosciences, Inc., a Nevada corporation, having an address at 888 Prospect Street, Suite 210, La Jolla, California 92037, U.S.A. (“Artelo”) shall be effective as of the Effective Date (as defined in Section 1.9 below). Each of NEOMED and Artelo may be referred to herein as a “Party”, or jointly as the “Parties”.
WHEREAS, NEOMED owns or controls rights in and to its proprietary therapeutic compound NEO1940 (formally known as AZD1940);
WHEREAS, Artelo desires to obtain from NEOMED an exclusive option for an exclusive worldwide license to develop and commercialize products comprising or containing the NEO1940 compound;
WHEREAS, Artelo desires to obtain from NEOMED certain materials, data and information relating to the NEO1940 compound for evaluation, research and development use in order to assist Artelo in making the determination whether to exercise such exclusive option; and
WHEREAS, the Parties agree that in the event that Artelo exercises such exclusive option, NEOMED shall grant to Artelo such exclusive worldwide license, subject to and in accordance with the terms and conditions of this Agreement;
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants herein contained, the Parties agree as follows:
1. DEFINITIONS
1.1 “Affiliate” shall mean, with respect to any Person, any other Person which directly or indirectly controls, is controlled by, or is under common control with, such Person. A Person shall be regarded as in control of another Person if it owns, or directly or indirectly controls, more than fifty percent (50%) of the voting stock or other ownership interest of the other Person, or if it directly or indirectly possesses the power to direct or cause the direction of the management and policies of the other Person by any means whatsoever.
1.2 “Commercially Reasonable Efforts” shall mean, with respect to the efforts and resources to be expended by a Party, efforts and resources commensurate with the efforts and resources commonly used in the pharmaceutical or biotechnology industry by a company of comparable size in connection with the development or commercialization of pharmaceutical or biotechnology products that are of similar status, taking into account the proprietary position of the product (including intellectual property scope, subject matter and coverage), safety and efficacy, product profile, competitiveness of the marketplace, the regulatory status and approval process, anticipated or approved labeling, present and future market potential, the probable profitability of the applicable product (including pricing and reimbursement status achieved or likely to be achieved) and other relevant factors such as technical, legal, scientific or medical factors.
1.3 “Compound” shall mean NEOMED’s proprietary therapeutic compound NEO1940 (formerly known as AZD1940).
1.4 “Compound-Related Improvements” shall mean any and all IP Improvements relating solely to the Compound.
1.5 “Confidential Information” shall mean, with respect to a Party (“Discloser”), all information of any kind whatsoever, and all tangible and intangible embodiments thereof of any kind whatsoever, which is or was disclosed by or on behalf of such Party to the other Party (“Recipient”) in connection with this Agreement or the Confidentiality Agreement. Notwithstanding the foregoing, Confidential Information of a Party shall not include information which Recipient can establish by written documentation: (a) to have been publicly known prior to disclosure of such information by Discloser to Recipient, (b) to have become publicly known, without fault on the part of Recipient, subsequent to disclosure of such information by Discloser to Recipient, (c) to have been received by Recipient at any time from a source, other than Discloser, rightfully having possession of and the right to disclose such information without restriction, (d) to have been otherwise known by Recipient prior to disclosure of such information by Discloser to Recipient or (e) to have been independently developed by employees or agents of Recipient without access to or use of such information disclosed by Discloser to Recipient.
1.6 “Confidentiality Agreement” shall mean the Confidentiality Agreement between the Parties dated September 13, 2017.
1.7 “Control”, “Controls” or “Controlled” shall mean, with respect to any know-how, patents, copyrights, proprietary information or trade secrets, or other intellectual property rights (collectively, “Rights”), the legal authority or right (whether by ownership, license or otherwise) of a Party to grant a license or a sublicense of or under such Rights to the other Party, or to otherwise disclose such proprietary information or trade secrets to the other Party, without breaching the terms of any agreement with a Third Party, or misappropriating the proprietary information or trade secrets of a Third Party.
1.8 “Data” means all data, including, and not limited to, clinical study reports, clinical study protocols, clinical data, and historical clinical safety data that is necessary or useful to seek regulatory approval to market the Product, as well as all regulatory reports, regulatory communications and other regulatory information developed, received or prepared by or for Artelo for the Product, except for any and all patient information;
1.9 “Effective Date” shall mean January 2, 2018.
1.10 “FDA” shall mean the United States Food and Drug Administration or any successor agency thereto.
1.11 “FD&C Act” shall mean the United States Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 301 et seq.), as amended, and the rules and regulations promulgated thereunder.
1.12 “Field” shall mean all fields of use.
1.13 “First Commercial Sale” shall mean, with respect to a Product in any country, the first sale of such Product in such country to a Third Party for consideration. First Commercial Sale excludes any sale or other distribution of a Product for use in a clinical trial or other development activity, reasonable promotional use (including samples) prior to marketing approval or for reasonable compassionate use or on a named patient basis.
1.14 “Indication” shall mean each separate disease, disorder, illness, health condition, or interruption, cessation or disruption of a bodily function, system, tissue type or organ and all of its associated signs, symptoms, stages or progression (including precursor conditions) (“Condition”) for which a separate Regulatory Approval is required. Notwithstanding the foregoing, subpopulations or patients with a primary Condition, however stratified (including stratification by stages of progression, particular combinations of symptoms associated with the primary disease or condition, prior treatment courses, response to prior treatment, family history, clinical history, genotype, phenotype or other stratification) shall not be deemed to be separate Indications for the purposes of this Agreement. For clarity, different cancer types (breast, colon, lung, etc.) are each considered an Indication while stages of progression within a single cancer type are not considered an Indication under this definition.
1.15 “Improvement” shall mean, with respect to any intellectual property, any revision, modification, translation, abridgment, condensation, expansion, adaptation, addition, improvement, derivative work, update or upgrade to or from such intellectual property.
1.16 “IND” shall mean an Investigational New Drug Application, as defined in the FD&C Act, that is required to be filed with the FDA before beginning clinical testing of a Product in human subjects, or a comparable foreign filing.
1.17 “IP Improvement” shall mean any Improvement to any intellectual property covered by the Licensed IP Rights made by Artelo, its Affiliates or sublicensees after the date of the Option Exercise Notice.
1.18 “Know-How” shall mean all information and data that is not generally known (including, but not limited to, information and data regarding formulae, procedures, protocols, techniques, pharmacological, toxicological and clinical data and results and other results of experimentation and testing).
1.19 “Licensed IP Rights” shall mean, collectively, the Licensed Patent Rights, the Licensed Copyrights and the Licensed Know-How Rights.
1.20 “Licensed Copyrights” shall mean rights in works of authorship, including without limitation copyrights, whether registered or unregistered, arising under the laws of any jurisdiction anywhere in the world, including moral rights and mask work rights, and all registrations and applications for registration with respect thereto which are Controlled by NEOMED as of the Effective Date or at any time during the term of this Agreement and which are necessary or useful for Artelo and its Affiliates and sublicensees to use, develop, sell, or seek regulatory approval to market or otherwise exploit the Compound.
1.21 “Licensed Know-How” shall mean all Know-How which is Controlled by NEOMED as of the Effective Date or at any time during the term of this Agreement and which is necessary or useful for Artelo and its Affiliates and sublicensees to use, develop, sell, or seek regulatory approval to market or otherwise exploit the Compound.
1.22 “Licensed Know-How Rights” shall mean all trade secret, know-how and other intellectual property rights (other than Licensed Copyrights and Licensed Patent Rights) in the Licensed Know-How.
1.23 “Licensed Patent Rights” shall mean (a) all patent applications heretofore or hereafter filed or having legal force in any country within the Territory which claim any discovery or inventions relating to the Compound, or the process of manufacture or use thereof Controlled by NEOMED as of the Effective Date or at any times during the term of this Agreement, including without limitation those listed on Exhibit A attached hereto; (b) all patents that have issued or in the future issue from such patent applications, including utility, model and design patents and certificates of invention Controlled by NEOMED as of the Effective Date or at any times during the term of this Agreement, including without limitation those listed on Exhibit A attached hereto; and (c) all divisionals, continuations, continuations‑in‑part, reissues, renewals, extensions or additions to any such patent applications and patents.
1.24 “Major Market Countries” shall mean the United States, Canada, the United Kingdom, Germany, France, Spain, Italy and Japan.
1.25 “NDA” shall mean a new drug application or product license application or its equivalent filed with and accepted by the FDA after completion of human clinical trials to obtain marketing approval for any Product, or any comparable application filed with and accepted by the Regulatory Authority of a country other than the United States.
1.26 “Net Sales” shall mean, with respect to any Product, the invoiced sales price of such Product billed by Artelo, its Affiliates and sublicensees to Third Party customers, less reasonable and customary (a) credits, allowances, discounts and rebates to, and chargebacks from the account of, such independent customers for spoiled, damaged, out‑dated, rejected or returned Product; (b) prepaid actual freight and insurance costs incurred in transporting such Product to such customers separately invoiced and stated; (c) cash, quantity and trade discounts and other price reductions in amounts customary in the trade for quantity purchases; (d) sales, use, value‑added and other direct taxes incurred separately invoiced and borne by Artelo, its Affiliates and sublicensees; and (e) customs duties, surcharges and other governmental charges incurred in connection with the exportation or importation of such Product separately invoiced and borne by Artelo, its Affiliates and sublicensees. Product reasonably provided without charge in connection with research and development, clinical trials, compassionate use, humanitarian and charitable donations, indigent programs or for use as samples will be excluded from the computation of Net Sales.
1.27 “Option” shall have the meaning set forth in Section 3.1.
1.28 “Option Exercise Notice” shall have the meaning set forth in Section 3.2.
1.29 “Option Period” shall have the meaning set forth in Section 3.1.
1.30 “Person” shall mean an individual, corporation, partnership, limited liability company, trust, business trust, association, joint stock company, joint venture, pool, syndicate, sole proprietorship, unincorporated organization, governmental authority or any other form of entity not specifically listed herein.
1.31 “Phase II Clinical Study” shall mean a study of a Product in human patients to determine initial efficacy and dose range finding before embarking on Phase III Clinical Studies.
1.32 “Phase III Clinical Study” shall mean a study in human patients with a defined dose or a set of defined doses of a Product which study, if the defined end-points are met, is designed to ascertain or establish efficacy and safety of such Product in patients for the indication being studied for the purpose of preparing and submitting a Regulatory Approval Application to the competent Regulatory Authority in a country of the world.
1.33 “Product” shall mean any product containing or comprising the Compound and/or containing, prepared with, using or covered by Licensed IP Rights or any part thereof.
1.34 “Product-Related Improvements” shall mean any and all IP Improvements (other than Compound-Related Improvements) that are reasonably necessary to develop, make or sell Compound or Product.
1.35 “Regulatory Approval” shall mean any technical, medical and scientific license, registration, authorization or approval (including, without limitation, any approval of an NDA, supplement or amendment, pre- and post- approval, pricing approval, or labeling approval) of any national, supra-national, regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity, necessary for the commercial manufacture, distribution, marketing, promotion, offer for sale, use, import, export and sale of a Product in a regulatory jurisdiction.
1.36 “Regulatory Approval Application” shall mean an application submitted to the appropriate Regulatory Authority seeking Regulatory Approval of a Product for use in one or more therapeutic indications in a regulatory jurisdiction within the Territory.
1.37 “Regulatory Authority” shall mean any national, regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity in each country of the world involved in the granting of Regulatory Approval for the Product.
1.38 “Research Plan” shall mean the research plan attached hereto as Exhibit B attached hereto.
1.39 “Royalty Term” shall mean, with respect to each Product in each country, the period until the ten (10) year anniversary of the expiration of the last to expire Valid Claim in such country that would be infringed, or if such Valid Claim is a pending patent application, would be infringed if such application were to issue with the claims as then being prosecuted, as applicable to such Product in such country, but for the license granted by this Agreement. For the absence of doubt, as an example, if the last Valid Claim covering a Product in a country expires on January 1, 2025, the Royalty Term for such Product in such country expires on January 1, 2035.
1.40 “Technology Transfer Materials” means all information, materials, and documentation Controlled by NEOMED relating to the Compound which is reasonably necessary or useful (a) for Artelo’s evaluation, research and development use in order to assist Artelo in making the determination whether to exercise the Option, and (b) if Artelo exercises the Option, for Artelo’s use to research, develop and commercialize the Products, including (i) the quantity of Compound specified in the Research Plan; and (ii) any other data, information and documents Controlled by NEOMED relating to the Compound and known to NEOMED to be necessary or reasonably useful for Artelo to exercise its rights to perform any authorized activities under this Agreement.
1.41 “Territory” shall mean the entire world.
1.42 “Third Party” shall mean any Person other than NEOMED, Artelo and their respective Affiliates.
1.43 “Valid Claim” shall mean either (a) a claim of an issued and unexpired patent within the Licensed Patent Rights, which has not been held permanently revoked, unenforceable or invalid by a decision of a court or other governmental agency of competent jurisdiction, unappealable or unappealed within the time allowed for appeal, and which has not been admitted to be invalid or unenforceable through reissue or disclaimer or otherwise; or (b) a claim of a pending patent application within the Licensed Patent Rights.
2. MATERIAL AND DATA TRANSFER
2.1 Supply of Technology Transfer Materials.
2.1.1 Within thirty (30) days after the Effective Date, NEOMED, without additional consideration and at NEOMED’s sole cost, shall deliver to Artelo the Technology Transfer Materials set forth on Exhibit C in its possession and such quantity of Compound substance as mutually agreed to by the parties (“Required Compound Supply”).
2.1.2 If Artelo exercises the Option, promptly following the receipt of the Option Exercise Notice, NEOMED shall without additional consideration and at NEOMED’s sole cost, deliver to Artelo, without limitation, all Technology Transfer Materials and all remaining quantities of Compound substance in NEOMED’s possession. For the avoidance of doubt, NEOMED shall have no further obligation to provide Compound substance in addition to the quantity in NEOMED’s possession as of the Effective Date, and if Artelo requires additional supply of the Compound substance in addition to the such quantity of Compound substance in NEOMED’s possession, ARTELO will be responsible at its own cost for its sourcing, synthesis, and supply of such additional Compound substance.
2.1.3 If at any time during the term of this Agreement either Party discovers any additional Technology Transfer Materials (other than the Compound substance) which are Controlled by NEOMED but which were not provided by NEOMED to Artelo , or (ii) any updates to or new versions of any Technology Transfer Materials provided by NEOMED to Artelo pursuant to this Section 2.1 become available, NEOMED shall provide requested, updates to, or new versions of, as applicable, Technology Transfer Materials to Artelo, without additional consideration, promptly following the discovery thereof or as any such update or new version of such Technology Transfer Materials become available, as applicable.
2.2 Use of Technology Transfer Materials.
2.2.1 Unless and until Artelo exercises the Option, Artelo (a) shall use the Technology Transfer Materials solely to conduct the activities set forth in the Research Plan for evaluation, research and development purposes in order to assist Artelo in making the determination whether to exercise the Option, and (b) shall not file or prosecute in any country any patent application which claims or uses or purports to claim or use the Technology Transfer Materials or their use or anything resulting from their use.
2.2.2 Unless and until Artelo exercises the Option, promptly upon the expiration of the Option Period or earlier termination of this Agreement, Artelo shall return all remaining Technology Transfer Materials to NEOMED.
2.2.3 If Artelo’s activities conducted pursuant to the Research Plan prior to the exercise of the Option by Artelo result in any invention or discovery that constitutes an Improvement to the Technology Transfer Materials, such invention or discovery and all intellectual property rights therein shall be owned solely by NEOMED. Artelo shall promptly disclose each such invention or discovery to NEOMED. Artelo hereby assigns to NEOMED all right, title and interest in all such inventions and discoveries and all intellectual property rights therein (“Research Program IP”). Artelo shall perform such acts and sign and cause to be signed such documents (including assignments) necessary or useful to ensure that all rights in the Research Program IP vest with NEOMED at all times. For clarity, if Artelo exercises the Option, the Research Program IP shall be included in the Licensed IP Rights.
2.2.4 Artelo hereby acknowledges that the Technology Transfer Materials are experimental in nature and that they are provided “AS IS.” NEOMED MAKES NO REPRESENTATIONS OR WARRANTIES, EXPRESS OR IMPLIED, WITH RESPECT TO THE TECHNOLOGY TRANSFER MATERIALS OR THE USE THEREOF. Artelo shall comply with all laws and governmental rules, regulations and guidelines which are applicable to the Technology Transfer Materials or the use thereof, including without limitation biosafety procedures, and with any safety precautions accompanying the Technology Transfer Materials.
3. OPTION
3.1 Option Grant. For a period commencing on the Effective Date and subject to early termination, ending on the one (1) year anniversary of the date upon which NEOMED delivers the Required Compound Supply to Artelo (“Option Period”), Artelo shall have the sole and exclusive right, but not the obligation to receive an exclusive license under the Licensed IP Rights to research, develop, make, have made, use, offer for sale, sell, have sold and import Products and otherwise exploit the Licensed IP Rights in the Territory in the Field, subject to the terms and conditions of this Agreement (the “Option”). During the Option Period, NEOMED shall not, without Artelo’s prior written consent, directly or indirectly: (i) negotiate or enter into any agreement, arrangement or commitment according to which a Third Party is granted any right in the Territory under the Licensed IP Rights, (ii) take any action which may derogate from or conflict with, or refrain from taking any action which is necessary to preserve, the Option, or (iii) enter into any agreement, arrangement or commitment that would derogate from or conflict with the rights granted to Artelo under this Agreement.
3.2 Option Exercise. In order to exercise the Option, Artelo shall provide a written option exercise notice to NEOMED prior to the expiration of the Option Period (the “Option Exercise Notice”), and as of the date of the receipt of the Option Exercise Notice by NEOMED, the license grant to Artelo set forth in Section 4.1 shall automatically become effective. For the abundance of clarity, Artelo shall have the right to exercise the Option by providing the Option Exercise Notice to NEOMED at any point in time during the Option Period.
4. LICENSE GRANT
4.1 Licensed IP Rights. Conditional only upon Artelo serving the Option Exercise Notice and receipt of such Option Exercise Notice by NEOMED in accordance with Section 3.2, and subject to the terms and conditions of this Agreement, NEOMED hereby grants to Artelo an exclusive license under the Licensed IP Rights to research, develop, make, have made, use, offer for sale, sell, have sold and import Products and otherwise exploit the Licensed IP Rights in the Territory in the Field. During the term of this Agreement, NEOMED shall not enter into any agreement or otherwise license, grant, assign, transfer, convey or otherwise encumber or dispose any right, title or interest in, to or under any of the Licensed IP Rights, which agreement, license, grant, assignment, transfer, conveyance, encumbrance or disposition would conflict with the rights granted to Artelo hereunder.
4.2 Sublicenses. Artelo may grant sublicenses under the license in Section 4.1 to Third Parties, provided that each such sublicense agreement is consistent with the terms and conditions of this Agreement. Artelo shall remain directly jointly and severally responsible for each of its sublicensees’ compliance with this Agreement and shall promptly provide a copy of each such sublicense agreement to NEOMED, which sublicense agreement copy may be redacted, provided that no information necessary for NEOMED to verify the compliance of such sublicense agreement with this Agreement or to ascertain NEOMED’s rights with respect thereto may be redacted.
4.3 Extension to Affiliates. Artelo shall have the right to extend the rights and obligations granted in this Agreement to one or more of its Affiliates. All applicable terms and provisions of this Agreement shall apply to any such Affiliate to which this Agreement has been extended to the same extent as such terms and provisions apply to Artelo. Artelo shall remain directly, jointly and severally liable for any acts or omissions of its Affiliates, and Artelo hereby expressly waives any requirement that NEOMED exhaust any right, power or remedy, or proceed directly against such Affiliate, for any obligation or performance hereunder prior to proceeding directly against Artelo.
4.4 Rights Reserved. This Agreement shall not be interpreted or construed as granting Artelo any rights, expressed or implied, to any of the Licensed IP Rights, or other form of rights other than the rights specifically and expressly granted herein and in accordance with this Agreement.
4.5 Restriction. In consideration for the rights granted herein by NEOMED, Artelo undertakes not to license, acquire or develop any rights relating to a compound of the same chemical class as the Compound (other than the Compound) during the Royalty Term.
4.6 IP Improvements. Subject to Sections 12.6.2 and 12.6.3, Artelo shall retain all rights, titles and interests in and to the IP Improvements.
5. CONSIDERATION
5.1 Option Consideration.
5.1.1 Equity Grant. On the Effective Date, as partial consideration for the grant of the Option by NEOMED to Artelo and the supply of the Technology Transfer Materials, Artelo shall grant NEOMED 120,000 fully paid and non-assessable shares of Artelo’s common stock, subject to Artelo and NEOMED then executing a Common Stock Purchase Agreement in substantially the form attached hereto as Exhibit D (the “Purchase Agreement”). To the extent of any conflict between the terms of this Section 5.1.1 and the Purchase Agreement, the terms and conditions of the Purchase Agreement shall control and be determinative.
5.1.2 Cash Consideration. As partial consideration for the grant of the Option by NEOMED to Artelo and the supply of the Technology Transfer Materials, Artelo shall make the following payments to NEOMED: (a) Twenty-Five Thousand Dollars (US$25,000) due on the Effective Date, (b) Twenty-Five Thousand Dollars (US$25,000) due on April 1, 2018, (c) Fifty Thousand Dollars (US$50,000) due on July 1, 2018, and (d) One Hundred Thousand Dollars (US$100,000) due on October 1, 2018; provided that if any of the foregoing payments have not accrued and become due prior to the earlier to occur of (i) the exercise of the Option by Artelo, and (ii) termination of this Agreement by Artelo pursuant to Section 12.2 hereof, then Artelo’s payment obligations with respect to such payments that have not accrued and become due shall be extinguished and become null and void. If Artelo is unable to receive the Required Compound Supply within thirty (30) days after the Effective Date, then the due date for the payments owing under clauses (c) and (d) of this Section 5.1.2 shall be extended by the length of the period required for Artelo to receive the Required Compound Supply. Notwithstanding the foregoing, the due date for the payments owing under clauses (c) and (d) of this Section 5.1.2 will not be extended by more than sixty (60) days.
5.2 License Consideration.
5.2.1 Equity Grant. Conditional upon Artelo’s exercise of the Option, as partial consideration for the license granted to Artelo by NEOMED under Section 4.1, within ten (10) business days after the delivery of the Option Exercise Notice by Artelo to NEOMED, Artelo shall grant NEOMED a number of fully paid and non-assessable shares of Artelo’s common stock equal to two percent (2%) of Artelo’s Fully-Diluted Shares then outstanding, subject to Artelo and NEOMED then executing a Common Stock Purchase Agreement in substantially the form attached hereto as Exhibit E. For purposes of this Section 5.2.1, “Fully-Diluted Shares” shall mean, as of immediately prior to Artelo’s exercise of the Option, the sum of (a) the outstanding shares of common stock of Artelo; (b) the shares of common stock of Artelo directly or indirectly issuable upon conversion or exchange of all outstanding securities directly or indirectly convertible into or exchangeable for common stock of Artelo and the exercise of all outstanding options and warrants; and (c) the shares of common stock of Artelo reserved, but neither issued nor the subject of outstanding awards, under any equity incentive or similar plan of Artelo.
5.2.2 License Issuance Fee. Conditional upon Artelo’s exercise of the Option, as partial consideration for the license granted to Artelo by NEOMED under Section 4.1, within ten (10) business days after the delivery of the Option Exercise Notice by Artelo to NEOMED, Artelo shall pay to NEOMED a license issuance fee of One Million Five Hundred Thousand Dollars (US$1,500,000).
5.2.3 Royalties.
(a) Royalty Rates. Conditional upon Artelo’s exercise of the Option, as partial consideration for the license granted to Artelo by NEOMED under Section 4.1, during the Royalty Term, Artelo shall pay royalties to NEOMED on Net Sales of Products by Artelo, its Affiliates and sublicensees in the Territory as follows:
Cumulative Net Sales1 in a Calendar Year (“Annual Net Sales”) | Rate |
Portion of Annual Net Sales under or equal to $500,000,000 | 5% |
Portion of Annual Net Sales greater than $500,000,000 | 7.5% |
______________
| 1. | In calculating the Annual Net Sales for the purposes of determining the applicable royalty rate, Annual Net Sales shall include the cumulative Net Sales of Artelo, its Affiliates and sublicensees in a Calendar Year for all Products. |
(b) Royalty Payments. Royalties will be payable on a calendar quarter basis. Within sixty (60) days after the end of each calendar quarter following the First Commercial Sale of the first Product, Artelo shall deliver to NEOMED a report containing the following information for the prior calendar quarter on a Product-by-Product and country-by-country basis: (i) the gross sales associated with each Product sold by Artelo and its Affiliates; (ii) a calculation of Net Sales of each Products that are sold by Artelo and its Affiliates; and (iii) a calculation of payments due to NEOMED with respect to the foregoing. Concurrently with these reports, Artelo shall remit to NEOMED any payment due for the applicable calendar quarter on Net Sales of Artelo and its Affiliates. Within seventy-five (75) days after the end of each calendar quarter following the First Commercial Sale of the first Product, Artelo shall deliver to NEOMED a report containing the following information for the prior calendar quarter on a Product-by-Product and country-by-country basis: (i) the gross sales associated with each Product sold by Artelo’s sublicensees; (ii) a calculation of Net Sales of each Product that are sold by Artelo’s sublicensees; and (iii) a calculation of payments due to NEOMED with respect to the foregoing. Concurrently with these reports, Artelo shall remit to NEOMED any payment due for the applicable calendar quarter on Net Sales of Artelo’s sublicensees. If no royalties are due to NEOMED for such reporting period, the report shall so state.
(c) Royalty Adjustment. In the event that the Royalty Rates payable to NEOMED negatively impacts the ability of Artelo, its Affiliates and sublicensees to maximize the Net Sales or earn reasonable profit thereon during the Royalty Term, but after the expiration of the last to expire Valid Claim in the applicable country, then the Parties agree to negotiate in good faith an adjustment to the royalty rates applicable to such country.
(d) Combination Product. If a Product is sold in a combination product with other pharmaceutically active components, Net Sales, for purposes of royalty payments on the combination product in a country, shall be calculated by multiplying the Net Sales of that combination product by the fraction A/A+B, where A is the invoice price of the Product sold separately in such country and B is the invoice price of the other active components in such combination product in such country. If no such separate sales are made by Artelo, its Affiliates or sublicensees, Net Sales for royalty determination shall be calculated by multiplying Net Sales of the combination by the fraction C/(C+D), where C is the fully allocated cost of the Product in such country and D is the fully allocated cost of such other active components in such country. Notwithstanding the above, Artelo shall in all cases notify and provide supporting data to NEOMED and NEOMED shall have the right to review any Combination Product calculations.
(e) Third Party Royalties. If Artelo, its Affiliates or sublicensees are required to pay royalties to any Third Party in order to exercise its rights hereunder to develop, make, use, offer for sale, sell or import any Product, then Artelo shall have the right to credit up to fifty percent (50%) of such Third Party royalty actually paid against the royalties owing to NEOMED under Section 5.2.3(a) above with respect to sales of such Product; provided, however, that Artelo shall first inform NEOMED and not reduce the amount of the royalties paid to NEOMED under Section 5.2.3(a) above, with respect to sales of such Product, by more than fifty percent (50%).
5.2.4 Milestone Payments. Conditional upon Artelo’s exercise of the Option, as partial consideration for the license granted to Artelo by NEOMED under Section 4.1, Artelo shall pay to NEOMED the following milestone payments:
(a) Five Million Dollars (US$5,000,000) upon the acceptance of Regulatory Approval Application for a Product by a Regulatory Authority of a Major Market Country;
(b) Five Million Dollars (US$5,000,000) upon the First Commercial Sale of a Product in a Major Market Country;
(c) Five Million Dollars (US$5,000,000) upon the Regulatory Approval of a Product in a Major Market Country for each additional Indication following the Regulatory Approval of a Product in the same Major Market Country for the first Indication;
(d) Ten Million Dollars (US$10,000,000) upon Annual Net Sales reaching One Hundred Million Dollars (US$100,000,000);
(e) Twenty-Five Million Dollars (US$25,000,000) upon Annual Net Sales reaching Two Hundred Fifty Million Dollars (US$250,000,000);
(f) Fifty Million Dollars (US$50,000,000) upon Annual Net Sales reaching Five Hundred Million Dollars (US$500,000,000); and
(g) One Hundred Million Dollars (US$100,000,000) upon Annual Net Sales reaching One Billion Dollars (US$1,000,000,000).
Milestone payments owing by Artelo to NEOMED pursuant to Section 5.2.4(a) through (c) shall be payable by Artelo within thirty (30) days following the achievement of the corresponding milestone event. Milestone payments owing by Artelo to NEOMED pursuant to Section 5.2.4(d) through (g) shall be payable by Artelo within sixty (60) days following the achievement of the corresponding milestone event. For the avoidance of doubt, each milestone payment is only payable once, regardless of the number of times such milestone may be achieved by Artelo, its Affiliates and sublicensees, except that with respect to the milestone event set forth in Section 5.2.4(c), a corresponding milestone payment shall be due each time such milestone event is achieved. For the abundance of clarity, no milestone payment shall be due upon the Regulatory Approval of a Product for the first Indication in any country. For further clarity, all Annual Net Sales made by Artelo, its Affiliates and sublicensees prior to the expiration of the Royalty Term shall be considered in determining whether the milestone payments owing by Artelo to NEOMED pursuant to Section 5.2.4(d) through (g) become due.
Solely with respect to the milestone payment due under Section 5.2.4(a), payment shall be by any of the following, or a combination thereof, at the election of Artelo: (x) cash; or (y) issuance of shares of Artelo’s common stock which shall be valued at its Fair Market Value on the date of exercise. For purposes of this Section 5.2.4, “Fair Market Value” shall mean (i) if Artelo’s common stock is listed on any established stock exchange or a national market system, including without limitation the New York Stock Exchange, the NASDAQ Global Select Market, the NASDAQ Global Market or the NASDAQ Capital Market of The NASDAQ Stock Market, its Fair Market Value will be based on the five (5) day volume-weighted average price immediately preceding the milestone as quoted on such exchange or system on the day of determination, as reported in The Wall Street Journal or such other source as Artelo deems reliable; or (ii) in the absence of an established market for Artelo’s common stock, the Fair Market Value will be determined in good faith by Artelo’s board of directors provided that, the Fair Market Value will in no circumstances be greater than the price paid by a third party investor in the last private financing round in excess of $1,000,000 completed by Artelo.
5.2.5 Strategic Transaction Consideration. Conditional upon Artelo’s exercise of the Option, as partial consideration for the license granted to Artelo by NEOMED under Section 4.1, Artelo shall pay to NEOMED a percentage of the Strategic Transaction Consideration in addition to the consideration due under Sections 5.2.1, 5.2.2, 5.2.3 and 5.2.4 as follows:
(a) Thirty-five percent (35%) of the Strategic Transaction Consideration resulting from a Strategic Transaction consummated during the period from the date of the Option Exercise Notice until the one (1) year anniversary thereof;
(b) Twenty-five percent (25%) of the Strategic Transaction Consideration resulting from a Strategic Transaction consummated during the period from the one (1) year anniversary of the date of the Option Exercise Notice until the two (2) year anniversary of the date of the Option Exercise Notice; and
(c) Fifteen percent (15%) of the Strategic Transaction Consideration resulting from a Strategic Transaction consummated during the period from the two (2) year anniversary of the date of the Option Exercise Notice until the three (3) year anniversary of the date of the Option Exercise Notice.
Each Strategic Transaction Consideration payment owing by Artelo to NEOMED pursuant to this Section 5.2.5 shall be payable by Artelo within sixty (60) days following the receipt by Artelo of the corresponding Strategic Transaction Consideration. For clarity, no portion of any Strategic Transaction Consideration resulting from a Strategic Transaction consummated after the three (3) year anniversary of the date of the Option Exercise Notice shall be due to NEOMED.
As used herein, “Strategic Transaction” means either (x) a grant by Artelo of a sublicense under the Licensed IP Rights to a Third Party (“Sublicense”), or (y) a transaction or a series of related transactions that results in an acquisition of Artelo by a Third Party, including by way of merger, purchase of capital stock or purchase of assets or change of control or otherwise (“Acquisition”); and “Strategic Transaction Consideration” means any cash consideration and the fair market value of any non-cash consideration paid to Artelo by (x) any sublicensee in consideration for a grant of a Sublicense under the Licensed IP Rights, including, without limitation upfront payments and milestone payments, but expressly excluding (i) royalties for sales of products or services, (ii) payments for the performance of or reimbursement for research or development activities performed by or on behalf of Artelo, (iii) payments as reimbursement for patent-related costs, (iv) payments for grants of rights to technology other than Licensed IP Rights, (v) payments for the supply of products, services or materials to a sublicensee, or (y) any acquirer as consideration for the assignment of this Agreement pursuant to an Acquisition, provided, however, that if contemporaneously with an assignment of this Agreement pursuant to an Acquisition, Artelo also assigns or licenses to the assignee other intellectual property or any other assets of Artelo, then the Strategic Transaction Consideration shall be determined pro rata based on the fair market value of the Licensed IP Rights relative to the fair market value of such other intellectual property and assets. No payment shall be due to NEOMED on any Strategic Transaction Consideration received by Artelo from any of its Affiliates in consideration for a Sublicense (but excluding any Third Party that becomes an Affiliate of Artelo pursuant to the Acquisition).
6. ROYALTY REPORTS AND ACCOUNTING
6.1 Royalty Reports. During the term of this Agreement following the First Commercial Sale of a Product, Artelo shall furnish to NEOMED quarterly written reports showing in reasonably specific detail the calculation of royalties owing for the reporting period in accordance with Section 5.2.3(b). With respect to sales of Products invoiced in United States dollars, all amounts shall be expressed in United States dollars. With respect to sales of Products invoiced in a currency other than United States dollars, all amounts shall be expressed in the domestic currency of the party making the sale together with the United States dollar equivalent. The United States dollar equivalent shall be calculated using the average of the exchange rate (local currency per US$1) published in The Wall Street Journal, Western Edition, under the heading “Currency Trading” on the last business day of each month during the applicable calendar year. Artelo shall keep complete and accurate records in sufficient detail to enable the royalties and other payments payable hereunder to be determined.
6.2 Audits.
6.2.1 Upon the written request of NEOMED and not more than once in each calendar year, Artelo shall permit an independent certified public accounting firm of nationally recognized standing selected by NEOMED and reasonably acceptable to Artelo, at NEOMED’s expense, to have access during normal business hours to such of the records of Artelo as may be reasonably necessary to verify the accuracy of the royalty reports for any year ending not more than three (3) years prior to the date of such request. The accounting firm shall disclose to NEOMED only whether or not the reports are correct and the amount of any discrepancies. No other information shall be shared.
6.2.2 If such accounting firm concludes that additional payments were owed during such period, Artelo shall make such additional payments including a yearly interest rate of 12% to be computed daily, within thirty (30) days of the date NEOMED delivers to Artelo such accounting firm’s written report so concluding the fees and expenses charged by such accounting firm shall be paid by NEOMED; provided, if the audit correctly discloses that the amounts payable by Artelo for the audited period are five percent (5%) or more than the amounts actually paid by Artelo for such period, then Artelo shall pay the additional payments owed and the fees and expenses charged by such accounting firm.
6.2.3 NEOMED shall treat all financial information subject to review under this Section 6.2 as confidential, and shall cause its accounting firm to retain all such financial information in confidence under Section 10 below.
7. PAYMENTS
7.1 Payment Terms. Royalties shown to have accrued by each royalty report provided for under Section 6.1 shall be due on the date such royalty report is due. The method of payment shall be by check or wire transfer to an address or account specified in writing by NEOMED. Payment in whole or in part may be made in advance of such due date.
7.2 Exchange Control. If at any time legal restrictions prevent the prompt remittance of part or all royalties with respect to any country in the Territory where the Product is sold, Artelo shall have the right, in its sole discretion, to make such payments by depositing the amount thereof in local currency to NEOMED’s account in a bank or other depository institution in such country. If the royalty rate specified in this Agreement should exceed the permissible rate established in any country, the royalty rate for sales in such country shall be adjusted to the highest legally permissible or government-approved rate.
7.3 Withholding Taxes. Artelo shall be entitled to deduct the amount of any withholding taxes, value-added taxes or other taxes, levies or charges with respect to such amounts, other than United States taxes, payable by Artelo, its Affiliates or sublicensees, or any taxes required to be withheld by Artelo, its Affiliates or sublicensees, to the extent Artelo, its Affiliates or sublicensees pay to the appropriate governmental authority on behalf of NEOMED such taxes, levies or charges. Artelo shall use reasonable efforts to minimize any such taxes, levies or charges required to be withheld on behalf of NEOMED by Artelo, its Affiliates or sublicensees. Artelo promptly shall deliver to NEOMED proof of payment of all such taxes, levies and other charges, together with copies of all communications from or with such governmental authority with respect thereto.
8. DEVELOPMENT AND COMMERCIALIZATION OF PRODUCTS
8.1 Option Period. During the Option Period, prior to the exercise of the Option by Artelo, Artelo shall use Commercially Reasonable Efforts to conduct the activities set forth in the Research Plan at Artelo’s sole expense. Artelo shall further provide NEOMED with updates on the progress of the Research Plan for each quarterly period during the Option Period in the form of an update phone call promptly following the conclusion of each of the first two (2) months of each quarterly period, and a summary written report provided promptly following the conclusion of each quarterly period.
8.2 Following Option Exercise. Following the exercise of the Option by Artelo, Artelo shall use Commercially Reasonable Efforts to research, develop and commercialize Products in the Territory in accordance with this Section 8.2. The efforts of Artelo’s Affiliates and sublicensees shall be treated as the efforts of Artelo when evaluating Artelo’s compliance with the foregoing diligence obligations. Without limiting the generality of the foregoing, Artelo will be responsible for conducting all necessary studies, including safety studies and clinical trials that are necessary in connection with seeking Regulatory Approvals to market the Product in the Territory, at Artelo’s own cost and discretion.
8.2.1 Commercialization Efforts. Artelo shall use Commercially Reasonable Efforts to obtain Regulatory Approval of at least one Product in each of the Major Market Countries. Promptly following the Regulatory Approval of a Product in a country in the Territory, Artelo shall use Commercially Reasonable Efforts to launch the Product in such country. Marketing, distribution and sale of the Products in the Territory shall be the sole responsibility of Artelo, which shall have the sole right and discretion to make all decisions relating thereto. Following launch of a Product in a country, Artelo shall use Commercially Reasonable Efforts to market and sell such Product in such country. The efforts of Artelo’s Affiliates and sublicensees shall be treated as the efforts of Artelo when evaluating Artelo’s compliance with the foregoing diligence obligations.
8.2.2 Reporting. Artelo shall provide NEOMED with written reports detailing the activities of Artelo, its Affiliates and sublicensees with respect to the research and development of and pre-commercial launch activities for Products in the Field in the Territory, both as to activities conducted during the prior period and planned activities for the Compound and Products, in sufficient depth to enable NEOMED to reasonably assess Artelo’s compliance with its diligence obligations hereunder. Artelo shall present such report to NEOMED in conjunction with a meeting (either in person or by videoconference, as the Parties may agree) with Artelo’s personnel responsible for the conduct of such development (and, if applicable, pre-commercial launch activities) which personnel shall include at least one Artelo representative responsible for such development (and, if applicable, pre-commercial launch activities) at a level of vice president or higher. Such reports shall be made on a semi-annual basis (within sixty (60) days following the end of each six (6) month period ending June 30 and December 31) until the First Commercial Sale occurs. In addition to such reports, upon reasonable request of NEOMED, Artelo will provide written summary updates of its progress since the close of the period covered by the last report provided.
8.2.3 Specific Diligence Obligations. Without limiting the generality of Sections 8.1 and 8.2, Artelo shall:
(a) Submit to NEOMED, within one (1) year following the delivery of the Option Exercise Notice, a certification supported by reasonable documentation, that Artelo, its Affiliates and sublicensees (or any combination thereof) control sufficient resources to maintain the performance of the obligations under this Section 8.2;
(b) Use Commercially Reasonable Efforts to initiate, directly or through its Affiliates or sublicensees, a Phase II Clinical Study for a Product within three (3) years following the delivery of the Option Exercise Notice; and
(c) Use Commercially Reasonable Efforts to initiate, directly or through its Affiliates or sublicensees, a Phase III Clinical Study for a Product within five (5) years following the delivery of the Option Exercise Notice.
8.2.4 Regulatory Approvals and Regulatory Reporting. Artelo will be responsible for the preparation and filing of the Regulatory Approvals for the Products with the applicable Regulatory Authorities in the Territory. Artelo shall prepare and file the Regulatory Approval Applications for the Products with the Regulatory Authorities in its name and at its cost. Artelo shall file, in its own name and at its own expense, all other applications for any approvals required for any clinical study or other study or action necessary or desirable to obtain such Regulatory Approval. Artelo shall have the sole responsibility for communicating with any Regulatory Authority regarding any Regulatory Approval Application or any Regulatory Approval for the Products once granted or any such other applications. Artelo shall be responsible for filing, at its own expense, all reports required to be filed in order to maintain any Regulatory Approvals granted for the Products.
8.2.5 Product Labeling. Artelo shall be solely responsible for the preparing, updating and maintaining product labeling in connection with commercialization of the Products and in compliance with all applicable laws and regulations. Such labeling may include but is not limited to text and graphical contents of printed labels and labeling components, including but not necessarily limited to healthcare professional leaflets or inserts, patient leaflets or inserts, and cartons.
9. REPRESENTATIONS AND WARRANTIES
9.1 Mutual Representations and Warranties. Each Party hereby represents and warrants to the other Party as follows as of the Effective Date:
9.1.1 Corporate Existence. Such Party is a not-for-profit corporation or a corporation, as applicable, duly organized, validly existing and in good standing under the laws of the jurisdiction in which it is organized.
9.1.2 Authorization and Enforcement of Obligations. Such Party (a) has the organizational power and authority and the legal right to enter into this Agreement and to perform its obligations hereunder, and (b) has taken all necessary organizational action on its part to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder. This Agreement has been duly executed and delivered on behalf of such Party, and constitutes a legal, valid, binding obligation, enforceable against such Party in accordance with its terms.
9.1.3 No Consents. All necessary consents, approvals and authorizations of all governmental authorities and other Persons required to be obtained by such Party in connection with this Agreement have been obtained.
9.1.4 No Conflict. The execution and delivery of this Agreement and the performance of such Party’s obligations hereunder (a) do not conflict with or violate any requirement of applicable laws or regulations, and (b) do not conflict with, or constitute a default under, any contractual obligation of it.
9.2 Additional NEOMED Representations and Warranties. In addition, NEOMED hereby represents and warrants to Artelo that:
9.2.1 Each item of the Licensed Patent Rights set forth in Exhibit A (a) is subsisting and in full force and effect, (b) has not been abandoned or passed into the public domain and (c) is free and clear of any liens or encumbrances (other than payment obligations to one or more Third Party).
9.2.2 NEOMED has not transferred ownership of, or granted any license of or right to use, or authorized the retention of any rights to use or joint ownership of, any Licensed IP Rights to any Person in any manner that would conflict with the license granted to Artelo in Section 4.1.
9.2.3 NEOMED exclusively Controls the Licensed IP Rights and has all rights necessary to grant the licenses to Artelo hereunder.
9.2.4 No patent application or registration within the Licensed Patent Rights is the subject of any pending interference, opposition, cancellation or patent protest.
9.2.5 No Person has made any claim or allegation to NEOMED or its Affiliates in writing that such Person has any right or interest in, to or under the Licensed IP Rights.
9.2.6 Except as disclosed by NEOMED to Artelo prior to the Effective Date and summarized on Schedule 9.2.6 attached hereto, NEOMED has no knowledge of any facts, circumstances or information that (a) would render any Licensed Patent Right invalid or unenforceable or (b) would adversely affect any pending application for any Licensed Patent Right.
9.2.7 NEOMED has not misrepresented, or failed to disclose, and has no knowledge of any misrepresentation or failure to disclose, any material fact or circumstances in any application for any Licensed Patent Right that would constitute fraud or a misrepresentation with respect to such application or that would otherwise affect the validity or enforceability of any Licensed Patent Right.
9.2.8 All necessary registration, maintenance and renewal fees in connection with each item of the Licensed Patent Rights have been paid and to NEOMED’s knowledge all necessary documents and certificates in connection with such Licensed Patent Rights have been filed with the relevant patent or other authorities in the United States or foreign jurisdictions, as the case may be, for the purposes of maintaining such Licensed Patent Rights.
9.2.9 No claim or litigation has been brought against it or to its knowledge threatened by any Third Party alleging that (a) the Licensed Patent Rights are invalid or unenforceable or (b) the manufacture, sale, offer for sale, importation or exploitation of the Compound and/or any subject matter covered by the Licensed IP Rights infringe or misappropriate or would infringe or misappropriate any right of any Third Party.
9.3 Disclaimers and Limitations
9.3.1 SUBJECT TO THE WARRANTIES SET FORTH IN SECTION 9.2, THE RIGHTS TO THE LICENSED IP RIGHTS GRANTED HEREUNDER ARE PROVIDED TO ARTELO ON AN “AS IS” BASIS IN ALL OTHER RESPECTS.
9.3.2 EXCEPT AS EXPRESSLY PROVIDED FOR IN THIS ARTICLE 9, NEOMED DISCLAIMS ANY AND ALL REPRESENTATIONS AND WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, WITH RESPECT TO THE SUBJECT MATTER OF THIS AGREEMENT, INCLUDING, WITHOUT LIMITATION, WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR USE OR PURPOSE, NON-INFRINGEMENT AND ANY WARRANTY ARISING OUT OF PRIOR COURSE OF DEALING AND/OR USAGE OF TRADE.
9.3.3 WITHOUT LIMITING THE GENERALITY OF THE FOREGOING, NEOMED SPECIFICALLY DOES NOT MAKE ANY WARRANTIES OR REPRESENTATIONS EXPRESS OR IMPLIED, CONCERNING THE LICENSED IP RIGHTS OR OF THE COMPOUND OR PRODUCT OR THE SCOPE OF THE LICENSED PATENT RIGHTS. NEOMED MAKES NO REPRESENTATIONS, EXTENDS NO WARRANTIES, EXPRESS OR IMPLIED, AND ASSUMES NO LIABILITIES OR RESPONSIBILITIES WITH RESPECT TO THE USE, SALE OR OTHER DISPOSITION BY ARTELO, ITS AFFILIATES, ITS SUB-LICENSEES, ASSIGNEES OR END USERS OF THE COMPOUND OR THE PRODUCT MANUFACTURED OR DEVELOPED USING THE LICENSED IP RIGHTS. IN NO EVENT SHALL NEOMED BE LIABLE FOR ANY DIRECT, INDIRECT, INCIDENTAL, SPECIAL OR CONSEQUENTIAL DAMAGES, INCLUDING LOSS OF PROFITS, REVENUE, DATA OR USE, INCURRED BY ARTELO, ITS AFFILAITES, OR THE SUBLICENSEES OR BY ANY OTHER PERSON, WHETHER IN AN ACTION IN CONTRACT OR TORT, ARISING UNDER THIS AGREEMENT OR IN RELATION WITH THE LICENSED IP RIGHTS, THE COMPOUND OR THE PRODUCT, EVEN IF NEOMED OR ANY THIRD PARTY HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES.
10. CONFIDENTIALITY
10.1 Confidentiality Obligations. Recipient shall keep and hold Confidential Information of Discloser in strictest confidence, and shall not use such Confidential Information for any purpose, other than as may be reasonably necessary for the performance of its duties under this Agreement, without Discloser’s prior written consent. Recipient shall not disclose any such Confidential Information to any person or entity without Discloser’s prior written consent, except to its and its Affiliates’ employees, consultants and agents, as necessary for the sole purpose of performing Recipient’s duties hereunder, under written terms and conditions no less protective of the Confidential Information than the terms and conditions of this Section 10. The obligations of confidentiality under this Section 10 shall last until the applicable Confidential Information is no longer secret and confidential, through no fault of Recipient or any of its Affiliates or sublicensees, or until one of the exceptions in Section 1.5 applies to such Confidential Information, whichever occurs first.
10.2 Permitted Disclosures. Notwithstanding anything herein to the contrary, Recipient may disclose Confidential Information of Discloser to the extent necessary to: (a) comply with an applicable law, regulation of a governmental agency or order of a court of competent jurisdiction, (b) to disclose information to any governmental agency for purposes of obtaining approval to test or market a Product or (c) prosecute or defend litigation; provided that if Recipient is required by law or regulation to make any such disclosure of Discloser’s Confidential Information, it will give reasonable advance notice to Discloser of such disclosure requirement and will use commercially reasonable efforts to assist such Discloser to secure a protective order or confidential treatment of the Confidential Information required to be disclosed and will limit disclosure to such Confidential information required to be disclosed. In addition, notwithstanding anything herein to the contrary, Recipient may disclose Discloser’s Confidential Information to the extent (and only to the extent) such disclosure is reasonably necessary in the following instances: (i) in order for it to reasonably fulfill its obligations herein and to conduct its ordinary course of business, to its subcontractors, vendors, outside legal counsel, accountants and auditors under written obligations of confidentiality and non-use no less protective of the of the Confidential Information than the terms and conditions of this Section 10; (ii) in connection with prosecuting and enforcing intellectual property rights in connection with Recipient’s rights and obligations pursuant to this Agreement; and (iii) in connection with exercising its rights hereunder, to its Affiliates, potential and future bona fide collaborators (including sublicensees, potential and permitted acquirers or assignees and potential investment bankers, investors and lenders) under written obligations of confidentiality and non-use no less protective of the Confidential Information than the terms and conditions of this Section 10.
10.3 Confidential Terms. Each of the Parties agrees not to disclose to any Third Party the terms and conditions of this Agreement without the prior approval of the other party, except to advisors (including financial advisors, attorneys and accountants), under written obligations of confidentiality and non-use no less protective of the Confidential Information than the terms and conditions of this Section 10, to potential and existing bona fide investors, financing sources, merger or other business partners and acquirers, on a need to know basis. Notwithstanding the foregoing, the Parties agree to issue, on such date as reasonably requested by Artelo, a joint press release to announce the execution of this Agreement, in the form mutually agreed to by the parties; thereafter, each party may each disclose to third parties the information contained in such press release without the need for further approval by the other party.
10.4 SEC or Similar Filings. Either Party may disclose the terms of this Agreement and events related to the development or commercialization of Products (including the receipt of milestone payments) to the extent reasonably required to comply with applicable laws, rules and regulations, including, without limitation, the rules and regulations promulgated by the United States Securities and Exchange Commission comparable foreign regulator and self-regulatory organizations (such as securities exchanges).
10.5 Injunctive Relief Authorized. Each Party as a Recipient acknowledges and agrees that (a) the Confidential Information of Discloser is of a special, unique, unusual, extraordinary and intellectual character; (b) the unauthorized use or disclosure of any Confidential Information of Discloser would constitute a material breach of this Agreement; (c) the interests of Discloser in and to the Confidential Information would be irreparably injured by the unauthorized use or disclosure of such information; and (d) money damages would not be sufficient to compensate Discloser for any such unauthorized use or disclosure. Accordingly, Recipient agrees that, in addition to any other remedies available to Discloser at law, in equity or under this Agreement, Discloser shall be entitled to seek specific performance, injunctive relief and other equitable relief to prevent any actual or threatened use or disclosure of the Confidential Information of Discloser without obligation to post any bond.
11. PATENTS
11.1 Prosecution and Maintenance of Licensed Patent Rights.
11.1.1 Option Period.
(a) Unless and until Artelo provides NEOMED with the Option Exercise Notice pursuant to Section 3.2, NEOMED shall be responsible for the preparation, filing, prosecution and maintenance of the Licensed Patent Rights in accordance with the provisions of this Section 11.1.1.
(b) NEOMED will diligently prosecute and maintain the United States and foreign patent applications and patents within the Licensed Patent Rights, subject to Artelo’s reimbursement of all NEOMED’s costs under Section 11.1.1(d). NEOMED will have sole responsibility for retaining and instructing patent counsel. NEOMED will provide Artelo with copies of all proposed patent office filings and correspondence, sufficiently in advance of submitting same to the patent office, so that Artelo may comment upon such documentation. NEOMED will promptly provide Artelo with copies of all official patent office correspondence so that Artelo may be informed of the continuing prosecution, and Artelo agrees to keep this documentation confidential in accordance with Section 10. Artelo may comment upon such documentation, provided that, if Artelo has not commented upon such documentation in reasonable time for NEOMED to sufficiently consider Artelo’s comments prior to the deadline for filing a response with the relevant government patent office, NEOMED will be free to respond appropriately without consideration of Artelo’s comments.
(c) NEOMED will cooperate with Artelo in good faith to prepare or amend any patent application within Licensed Patent Rights to include claims reasonably requested by Artelo to protect the Products contemplated to be developed by Artelo under this Agreement.
(d) Artelo shall reimburse NEOMED for all its costs for preparing, filing, prosecuting, and maintaining all United States and foreign patent applications and patents under Licensed Patent Rights that are incurred during the period from the Effective Date until either the patent prosecution and maintenance responsibilities are transitioned to Artelo pursuant to Section 11.1.2 or this Agreement is terminated. Patent cost reimbursement payments are due within thirty (30) days after receipt of invoice from NEOMED.
11.1.2 Following Option Exercise. At such time as Artelo provides NEOMED with the Option Exercise Notice pursuant to Section 3.2, Artelo shall assume and shall be responsible for the preparation, filing, prosecution and maintenance of the Licensed Patent Rights, at Artelo’s sole expense. Promptly following NEOMED’s receipt of the Option Exercise Notice, and in any event within thirty (30) days thereafter, the Parties shall cooperate in good faith to execute a transition of the patent prosecution and maintenance responsibilities from NEOMED to Artelo. NEOMED shall cause its patent prosecution counsel to cooperate with Artelo’s patent prosecution counsel to ensure a smooth transition. Artelo will provide NEOMED with copies of all proposed patent office filings and correspondence, sufficiently in advance of submitting same to the patent office, so that NEOMED may comment upon such documentation. Artelo will promptly provide NEOMED with copies of all official patent office correspondence so that Artelo may be informed of the continuing prosecution. If Artelo elects not to file any patent application claiming any Licensed Patent Rights or otherwise abandon the prosecution and maintenance of any patent application or patent claiming any Licensed Patent Rights, then (a) Artelo shall provide NEOMED with reasonable notice of such decision so as to permit NEOMED to decide whether to assume such responsibilities (such notice shall be given no later than thirty (30) days prior to the next deadline to take any necessary action with the relevant patent office); and (b) NEOMED shall have the right but not the obligation to control the filing, prosecution and maintenance of such patent application or patent on such Licensed Patent Rights, at NEOMED’s sole cost.
11.2 Enforcement of Licensed Patent Rights.
11.2.1 Each Party shall notify the other Party of any infringement known to such Party of any Licensed Patent Rights by a Third Party and shall provide the other Party with the available evidence, if any, of such infringement.
11.2.2 Artelo, at its sole expense, shall have the first right to determine the appropriate course of action to enforce the Licensed Patent Rights or otherwise abate such infringement, to take (or refrain from taking) appropriate action to enforce the Licensed Patent Rights, to control any litigation or other enforcement action and, subject to NEOMED’s written consent, not to be unreasonably withheld, delayed or conditioned, to enter into, or permit, the settlement of any such litigation or other enforcement action with respect to the Licensed Patent Rights, and shall endeavor, in good faith, that the interests of NEOMED are protected in so doing. NEOMED agrees to cooperate reasonably with Artelo in any action to enforce the Licensed Patent Rights under this Section 11.2.2, provided that Artelo reimburses NEOMED promptly for any costs and expenses incurred by NEOMED in providing such assistance. Without limiting the generality of the foregoing, should Artelo elect to bring suit against an infringer of Licensed Patent Rights, NEOMED agrees to be joined as party plaintiff in any such suit, if deemed a necessary party, subject to Artelo’s reimbursement of NEOMED’s costs and expenses as set forth above.
11.2.3 If Artelo does not, within ninety (90) days of receipt of notice from NEOMED under Section 11.2.1, abate the infringement or file suit to enforce the Licensed Patent Rights , NEOMED shall have the right to take whatever action it deems appropriate to enforce the Licensed Patent Rights. The Party controlling any such enforcement action shall not settle the action or otherwise consent to an adverse judgment in such action that adversely affects the rights or interests of the non-controlling Party without the prior written consent of the other Party.
11.2.4 All monies recovered upon the final judgment or settlement of any such suit to enforce the Licensed Patent Rights pursuant to this Section 11.2 shall be first used to reimburse each Party for its costs and expenses incurred in connection with such suit pro rata, and the remainder, if any, shall be allocated as follows: (a) for any suit initiated and prosecuted by Artelo, the remainder shall be deemed Net Sales and allocated in accordance with Section 5.2.3, and (b) for any suit initiated and prosecuted by NEOMED, the remainder shall be remitted to NEOMED.
11.3 IP Improvements. Artelo shall be responsible for the preparation, filing, prosecution, maintenance and enforcement of the patent rights within the IP Improvements, at Artelo’s sole expense
11.4 Undertakings.
11.4.1 Artelo hereby undertakes not to challenge the validity of any of the Licensed IP Rights, nor institute any legal proceeding of any nature and kind against NEOMED, its Affiliates or sublicensees in respect of the Compounds, the Licensed IP Rights and/or Products relating to this Agreement, other than with respect to any uncured breach of this Agreement by NEOMED.
11.4.2 Artelo hereby agrees to comply with the patent marking laws and regulations in each country of the Territory in which any Product is sold by Artelo, its Affiliates or its sublicensees.
12. TERMINATION
12.1 Expiration. Subject to early termination pursuant to the provisions of Sections 12.2, 12.3, 12.4 and 12.5 below, this Agreement shall expire (a) if Artelo does not provide an Option Exercise Notice prior to the expiration of the Option Period, upon the expiration of the Option Period, or (b) if Artelo provides an Option Exercise Notice prior to the expiration of the Option Period, upon the expiration of Artelo’s obligation to pay royalties to NEOMED under Section 5.2.3 above. If this Agreement expires pursuant to clause (b) of this Section 12.1, then upon expiration of the Royalty Term, on a country-by-country basis, the licenses granted to Artelo by NEOMED under this Agreement shall be fully paid-up and irrevocable in the countries in which the Royalty Term has expired.
12.2 Termination by Artelo. Artelo may terminate this Agreement in its entirety, in its sole discretion, at any time effective (a) immediately, during the Option Period, or (b) sixty (60) days after NEOMED’s receipt of a written notice of termination provided by Artelo to NEOMED, following the date of the Option Exercise Notice.
12.3 Termination by NEOMED. NEOMED shall have the right to terminate this Agreement if Artelo does not exercise Commercially Reasonable Efforts to meet its obligations under Section 8.2.3(a), (b) or (c) in accordance with the following procedure:
(a) If Artelo fails to meet its obligations under Section 8.2.3(a), (b) or (c), the Parties shall promptly review the underlying reasons and agree on appropriate measures to enable Artelo to meet such obligations. Artelo shall use Commercially Reasonable Efforts to pursue implementation of such measures in good faith during a cure period of one hundred eighty (180) days. Should Artelo (i) fail to use Commercially Reasonable Efforts to pursue implementation of such agreed appropriate measures in good faith and (ii) as a result fails to meet its obligations under Section 8.2.3(b) or (c) prior to the expiration of the one hundred eighty (180) day cure period, NEOMED shall have the right to terminate this Agreement upon thirty (30) days’ written notice.
(b) Notwithstanding the above, if any such failure by Artelo was due to a material adverse event or any factors which could have not been reasonably foreseen by Artelo or which were outside the control of Artelo (including without limitation any action or failure to act by any Regulatory Authority or any other governmental authority, including without limitation the FDA or the U.S. Drug Enforcement Administration), then (i) Artelo shall notify NEOMED of such event or factors promptly upon becoming aware thereof and (ii) a new planning process will be triggered to determine revised targets taking into account the estimated impact of such events and/or factors promptly following such notice.
12.4 Termination for Cause. Either Party will have the right to terminate this Agreement in full upon delivery of written notice to the other Party in the event of any material breach by the other Party of any terms and conditions of this Agreement, provided, that such termination will not be effective if such breach has been cured within ninety (90) days after written notice thereof is given by the non-breaching Party to the breaching Party specifying in reasonable detail the nature of the alleged breach; provided further, however, that if the material breach is not reasonably capable of being cured within the ninety (90)-day cure period, and if the breaching party (a) proposes within such ninety (90)-day period a written plan to cure such breach that is reasonably acceptable to the non-breaching Party, and (b) makes good faith efforts to cure such default and to implement such written cure plan and reports at least monthly to the non-breaching Party in writing as to the status of such efforts and cure, then the non-breaching Party may not terminate this Agreement for so long as the breaching Party is diligently pursuing such cure in accordance with such plan. Further, if the material breach is disputed, then no termination pursuant to this Section 12.4 shall be effective prior to the resolution of such dispute in accordance with Section 14.3. All of the terms and conditions of this Agreement shall remain in full force and effect during the pendency of such dispute resolution process.
12.5 Termination for Business Failure. Either Party will have the right to terminate this Agreement immediately upon written notice to the other Party in the event that (i) the other Party becomes insolvent, or (ii) a receiver, trustee, or custodian is appointed for the other Party, or (iii) the other Party makes an assignment for the benefit of creditors, or is involuntary liquidated or dissolved, and (iv) in the event of the occurrence of any action or event which is, comparable in law of one or more of the events described in this Section 12.5.
12.6 Effect of Expiration or Termination. Upon expiration of this Agreement pursuant to Section 12.1(a) (but not expiration of this Agreement pursuant to Section 12.1(b)) or any termination of this Agreement pursuant to Section 12.2 or 12.3 or any termination of this Agreement by NEOMED pursuant to 12.4 or 12.5:
12.6.1 Termination of Licenses. All rights and licenses granted to Artelo hereunder shall terminate.
12.6.2 Transfer of Data and Compound-Related Improvements. Artelo further agrees to transfer all Data and Compound-Related Improvements in its possession and control to NEOMED as soon as reasonably practicable.
12.6.3 Product-Related Improvements. To the extent requested in writing by NEOMED within thirty (30) days after the effective date of termination, the Parties shall negotiate, in good faith, an agreement on commercially reasonable terms, pursuant to which Artelo would grant to NEOMED a license under Artelo’s intellectual property rights in the Product-Related Improvements to develop, make, use, sell, offer for sale and import the Compound or a Product. Such negotiation shall take into consideration amounts invested by Artelo in the clinical development of the Compound and Products.
12.6.4 Confidential Information. Recipient shall, within thirty (30) days after the effective date of termination and at Recipient’s expense, return or destroy, at Discloser’s election, all Discloser Know-How and other Confidential Information of Discloser (provided that Recipient may keep one copy of such Confidential Information for archival purposes only and such additional copies of or any computer records or files containing such Confidential Information that have been created solely by Recipient’s automatic or routine archiving and back-up procedures, to the extent created and retained in a manner consistent with Recipient’s standard archiving and back-up procedures, but not for any other use or purpose, subject to an ongoing obligation of confidentiality in accordance with Section 10).
12.6.5 Sublicenses. If this Agreement is terminated pursuant to Section 12.2, 12.3, 12.4 or 12.5, all sublicenses that are granted by Artelo pursuant to this Agreement and in accordance with Section 4.2 where the sublicensee is in compliance with its sublicense agreement and this Agreement as of the date of such termination will remain in effect and will be assigned to NEOMED, provided that (a) such sublicensee agrees in writing to perform all obligations of Artelo hereunder, (b) a copy of the sublicense agreement subject of the assignment was provided to NEOMED prior to the assignment and not disapproved in writing by NEOMED within thirty (30) days, and (c) NEOMED will not be bound to perform any duties or obligations, or to grant any rights, set forth in any sublicense agreements that extend beyond the duties and obligations of NEOMED, or grant of rights by NEOMED, set forth in this Agreement.
12.7 Survival. Expiration or termination of this Agreement shall not relieve the parties of any obligation accruing prior to such expiration or termination, and the provisions of Sections 2.2.3, 2.2.4, 4.4, 4.6, 5.2.3, 6, 9.3, 10, 12.6, 12.7, 13 and 14, and such other Sections of this Agreement that are required to give effect to such Sections, shall survive the expiration or termination of this Agreement.
13. INDEMNIFICATION
13.1 Indemnification by Artelo. Artelo shall defend, indemnify and hold NEOMED and its (and its Affiliates’) members, directors, officers, employees and agents harmless from all losses, liabilities, damages and expenses (including reasonable attorneys’ fees and costs) (collectively, “Losses”) resulting from any claims, demands, actions and other proceedings including without limitation, for bodily injury (including death), to the extent resulting from Artelo’s or its Affiliates’ or sublicensees’ use of the Licensed IP Rights, any use or handling of the Compound substance or research, development or commercialization of the Compound or any Product under this Agreement except to the extent such Losses are subject to NEOMED’s indemnification obligations under Section 13.2 below.
13.2 Indemnification by NEOMED. NEOMED shall defend, indemnify and hold Artelo and its (and its’ Affiliates’) directors, officers, employees and agents harmless from all Losses resulting from any claims, demands, actions and other proceedings to the extent resulting from (a) the material breach of the representations and warranties made hereunder by NEOMED or its Affiliates or (b) any governmental charges, fines, penalties or similar amounts arising from any use or handling of the Compound substance by or on behalf of NEOMED or its Affiliates, except to the extent such Losses are subject to Artelo’s indemnification obligations under Section 13.1 above.
13.3 Procedure. The Party seeking indemnification (the “Indemnified Party”) promptly shall notify the other party (the “Indemnifying Party”) of any claim, demand, action or other proceeding for which the Indemnified Party intends to claim indemnification. The Indemnifying Party shall have the right to participate in, and to the extent the Indemnifying Party so desires jointly with any other indemnitor similarly noticed, to assume the defense thereof with counsel selected by the Indemnifying Party; provided, however, that the Indemnified Party shall have the right to retain its own counsel, with the fees and expenses to be paid by the Indemnified Party. The indemnity obligations under this Section 13 shall not apply to amounts paid in settlement of any claim, demand, action or other proceeding if such settlement is effected without the prior express written consent of the Indemnifying Party, which consent shall not be unreasonably withheld or delayed. The failure to deliver notice to the Indemnifying Party within a reasonable time after notice of any such claim or demand, or the commencement of any such action or other proceeding, if prejudicial to its ability to defend such claim, demand, action or other proceeding, shall relieve such Indemnifying Party of any liability to the Indemnified Party under this Section 13 with respect thereto, but the omission so to deliver notice to the Indemnifying Party shall not relieve it of any liability that it may have to the Indemnified Party other than under this Section 13. The Indemnifying Party may not settle or otherwise consent to an adverse judgment in any such claim, demand, action or other proceeding, that diminishes the rights or interests of, or places any obligations upon, the Indemnified Party without the prior express written consent of the Indemnified Party, which consent shall not be unreasonably withheld or delayed. The Indemnified Party, its employees and agents, shall reasonably cooperate with the Indemnifying Party and its legal representatives in the investigation of any claim, demand, action or other proceeding covered by this Section 13.
13.4 LIMITATION OF DAMAGES. IN NO EVENT SHALL EITHER PARTY BE LIABLE HEREUNDER TO THE OTHER PARTY FOR ANY PUNITIVE, INDIRECT, SPECIAL, INCIDENTAL OR CONSEQUENTIAL DAMAGES (INCLUDING LOST REVENUE, LOST PROFITS OR LOST SAVINGS) HOWEVER CAUSED AND UNDER ANY THEORY, EVEN IF IT HAS NOTICE OF THE POSSIBILITY OF SUCH DAMAGES. THE LIMITATIONS SET FORTH IN THIS SECTION 13.4 SHALL NOT APPLY WITH RESPECT TO (I) ANY BREACH OF SECTION 10 OR (II) THE INTENTIONAL MISCONDUCT OR GROSS NEGLIGENCE OF A PARTY. NOTHING IN THIS SECTION 13.4 IS INTENDED TO LIMIT OR RESTRICT THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF A PARTY UNDER THIS SECTION 13 WITH RESPECT TO ANY DAMAGES OWED OR PAID TO A THIRD PARTY IN CONNECTION WITH A THIRD PARTY CLAIM.
13.5 Insurance.
13.5.1 Artelo shall maintain at its own cost and at all times during the term of this Agreement policies of insurance consistent with normal business practices of prudent pharmaceutical companies similarly situated. Such insurance policies shall include, without limitation, commercial general liability insurance, including, without limitation, product liability, covering claims for damages because of bodily injury (including, without limitation, death), personal injury and property damage arising out of Artelo’s acts or omissions and including coverage for contractual liabilities. Without limiting the foregoing, no later than Artelo’s commencement of clinical trials in respect of any Product, Artelo shall obtain, and maintain in full force and effect, adequate clinical trials insurance, for claims arising out of or in connection with such clinical trials. In addition, no later than the commencement of commercial distribution of any Product by or on behalf of Artelo, Artelo shall obtain, and maintain in full force and effect, adequate general and product liability insurance for bodily injury and property damage claims.
13.5.2 The policies described in Section 13.5.1 above will be in such amounts and cover such risks as are reasonable and prudent for those types of policies, but shall in no event be less than, in the aggregate: (a) one million U.S. dollars (US$1,000,000) as of the Effective Date, (b) five million U.S. dollars (US$5,000,000) as of the date of the commencement of any clinical trial, and (c) ten million U.S. dollars (US$10,000,000) as of the date of the First Commercial Sale. Such policies will be written by insurance companies with an A.M. Best’s rating of A:VIII or higher (or if such policies are not subject to the Best rating, then by carriers who are reasonably acceptable to NEOMED). The foregoing policies will: (i) cover claims arising out of the performance of this Agreement that are made within a period of not less than six (6) years after the expiration or earlier termination of this Agreement; and (ii) be primary and non-contributory to any liability insurance carried by NEOMED, which insurance will be excess for claims and losses arising out of the performance of this Agreement. The policies described above will be specifically endorsed to list NEOMED as an additional insured (as long as such endorsement is allowed by Applicable Law), and Artelo will notify NEOMED at least thirty (30) days in advance of any cancellation or non-renewal of or material changes in of such insurance coverage. Artelo shall provide NEOMED with a valid, current certificate of insurance as evidence of the insurance required herein upon request. Maintenance of such insurance coverage will not relieve Artelo of any responsibility under this Agreement for damages in excess of insurance limits or otherwise.
14. MISCELLANEOUS
14.1 Notices. Any consent, notice or report required or permitted to be given or made under this Agreement by one of the parties to the other shall be in writing and addressed to such other party at its address indicated below, or to such other address as the addressee shall have last furnished in writing to the addressor for purposes of this Section 14.1, and shall be effective upon receipt by the addressee.
| If to NEOMED: | | The NEOMED Institute 7171 Frederick-Banting Saint-Laurent, Quebec H4S 1Z9, Canada Attention: Daniel Böck, VP Business Development |
| | | |
| If to Artelo: | | Artelo Biosciences, Inc. 888 Prospect Street, Suite 210 La Jolla, CA 92037 USA Attention : Gregory D. Gorgas, President & CEO |
14.2 Assignment. Except as otherwise expressly provided under this Agreement neither this Agreement nor any right or obligation hereunder may be assigned or otherwise transferred (whether voluntarily, by operation of law or otherwise), without the prior express written consent of the other party; provided, however, that either party may, without such consent, assign this Agreement and its rights and obligations hereunder (i) to an Affiliate or (ii) in connection with the transfer or sale of all or substantially all of its business relating to this Agreement, or in the event of its merger, consolidation, change in control or similar transaction. In addition, NEOMED shall have the right to hypothecate, pledge, assign in warranty or otherwise charge its payment rights hereunder in favour of a financial institution as security. This Agreement shall be binding upon and inure to the benefit of each party and its successors and permitted assigns. Any purported assignment or transfer in violation of this Section 14.2 shall be void.
14.3 Governing Law; Jurisdiction; Venue. This Agreement shall be governed by and construed in accordance with the laws of the State of New York, without regard to the conflicts of law principles thereof. For any legal action arising from or related to this Agreement, the Parties hereby irrevocably: (a) consent solely to personal jurisdiction of and exclusive venue in the state and federal courts located in New York County, New York, USA; (b) agree that such courts will be the sole courts utilized; and (c) hereby waive any jurisdictional or venue objections to such courts, including without limitation, forum non conveniens. If any dispute arises between the Parties in connection with this Agreement which leads to a proceeding to resolve such dispute, the prevailing Party in such proceeding will be entitled to receive its reasonable attorneys’ fees, expert witness fees and out-of-pocket costs incurred in connection with such proceeding, in addition to any other relief it may be awarded.
14.4 Entire Agreement. This Agreement contains the entire understanding of the Parties with respect to the subject matter hereof. There are no agreements, representations, warranties, covenants or undertakings with respect to the subject matter hereof other than those expressly set forth herein. All express or implied representations, agreements and understandings relating to such subject matter, either oral or written, heretofore made are expressly superseded by this Agreement, including, without limitation the Confidentiality Agreement.
14.5 Independent Contractors. Each Party hereby acknowledges that the Parties shall be independent contractors and that the relationship between the Parties shall not constitute a partnership, joint venture or agency. Neither Party shall have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on the other Party, without the prior consent of the other Party to do so.
14.6 Remedies. All remedies, either under this Agreement, by law, or otherwise afforded to any Party, shall be cumulative and not alternative.
14.7 Force Majeure. Neither Party shall be held liable or responsible to the other Party nor be deemed to have defaulted under or breached this Agreement for failure or delay in fulfilling or performing any term of this Agreement solely to the extent, and for so long as, such failure or delay is caused by or results from causes beyond the reasonable control of the affected Party including but not limited to fire, floods, embargoes, war, acts of war (whether war be declared or not), insurrections, riots, civil commotions, strikes, lockouts or other labor disturbances, acts of God or acts, omissions or delays in acting by any governmental authority or the other Party. If any such event continues for more than ninety (90) days, then such other Party shall have the right to terminate this Agreement upon thirty (30) days prior written notice to the affected Party.
14.8 Fees and Expenses. Each Party shall pay its own costs and expenses in connection with this Agreement and the transactions contemplated hereby (including the fees and expenses of its advisers, accountants and legal counsel).
14.9 Section 365(n) of the Bankruptcy Code. All rights and licenses granted under or pursuant to any section of this Agreement are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code, licenses of rights to “intellectual property” as defined under Section 101(35A) of the U.S. Bankruptcy Code to the extent permitted thereunder. Artelo shall retain and may fully exercise all of its rights and elections under the U.S. Bankruptcy Code. Upon the bankruptcy of NEOMED, Artelo shall further be entitled to a complete duplicate of (or complete access to, as appropriate) any such intellectual property (solely to the extent that such license in effect at such time), and such, if not already in its possession, shall be promptly delivered to Artelo, unless NEOMED elects to continue, and continues, to perform all of its obligations under this Agreement.
14.10 Further Assurances. At any time or from time to time after the date hereof, the Parties agree to cooperate with each other, and at the request of any other party, to execute and deliver any further instruments or documents and to take all such further action as the other Party may reasonably request in order to evidence or effectuate the consummation of the transactions contemplated hereby and to otherwise carry out the intent of the parties hereunder.
14.11 Interpretation. The captions to the Sections of this Agreement are not a part of this Agreement, but are included for convenience of reference and shall not affect its meaning or interpretation. In this Agreement: (i) the word “including,” “includes,” “included,” and “include” shall be deemed to be followed by the phrase “without limitation” or like expression; (ii) the singular shall include the plural and vice versa; (iii) masculine, feminine, and neuter pronouns and expressions shall be interchangeable; (iv) the words “hereof,” “herein,” “hereto,” “hereby,” “hereunder,” and derivative or similar words refer to this Agreement as an entirety and not solely to any particular provision of this Agreement; (v) each reference in this Agreement to a particular Section, appendix, schedule, or exhibit means a Section, appendix, schedule, or exhibit of or to this Agreement, unless another agreement is specified; (vi) “the word “will” shall be construed to have the same meaning and effect as the word “shall”; (vii) “or” is not disjunctive (i.e., it means “and/or”) unless the context clearly requires otherwise; (viii) references to any party or Person shall include its permitted successors or assigns; and (ix) whenever this Agreement refers to a number of days, such number shall refer to calendar days unless business days are specified; and business days means any day, except Saturday and Sunday, on which commercial banking institutions in New York, New York and Montreal, Quebec are open for business. This Agreement has been prepared jointly and shall not be strictly construed against either Party.
14.12 Waivers. It is agreed that no delay or omission to exercise any right, power or remedy accruing to any Party, upon any breach, default or noncompliance by the other Party under this Agreement, shall impair any such right, power or remedy, nor shall it be construed to be a waiver of any such breach, default or noncompliance, or any acquiescence therein, or of or in any similar breach, default or noncompliance thereafter occurring. It is further agreed that any waiver, permit, consent or approval of any kind or character on the part of any party hereto of any breach, default or noncompliance under this Agreement or any waiver on such party’s part of any provisions or conditions of this Agreement, must be in writing and shall be effective only to the extent specifically set forth in such writing. The waiver by a Party of any right hereunder, or of any failure to perform or breach by the other Party hereunder, shall not be deemed a waiver of any other right hereunder or of any other breach or failure by the other Party hereunder whether of a similar nature or otherwise.
14.13 Severability. Whenever possible, each provision of this Agreement shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Agreement is held to be invalid, illegal or unenforceable in any respect under any applicable law or rule in any jurisdiction, such invalidity, illegality or unenforceability shall not affect any other provision or any other jurisdiction, but this Agreement shall be reformed, construed and enforced in such jurisdiction as if such invalid, illegal or unenforceable provision had never been contained herein.
14.14 Enforcement. Each Party hereto acknowledges that money damages would not be an adequate remedy in the event that any of the covenants or agreements in this Agreement are not performed by the Parties in accordance with its terms, and it is therefore agreed that in addition to and without limiting any other remedy or right each party may have, each party will have the right to an injunction, temporary restraining order or other equitable relief in any court of competent jurisdiction enjoining any such breach and enforcing specifically the terms and provisions hereof.
14.15 Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Counterparts may be delivered via facsimile, electronic mail (including pdf) or other transmission method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
[Signature Page Follows.]
Confidential
IN WITNESS WHEREOF, the parties have executed this Material and Data Transfer, Option and License Agreement as of the Effective Date.
| NEOMED INSTITUTE | |
| | | |
| By | /s/ Donald Olds | |
| | Donald Olds, President & CEO | |
| | | |
| | |
| ARTELO BIOSCIENCES, INC. | |
| | | |
| By | Gregory D. Gorgas | |
| | Gregory D. Gorgas, President & CEO | |
[Signature Page to Material and Data Transfer, Option and License Agreement]
EXHIBIT A
LICENSED PATENT RIGHTS
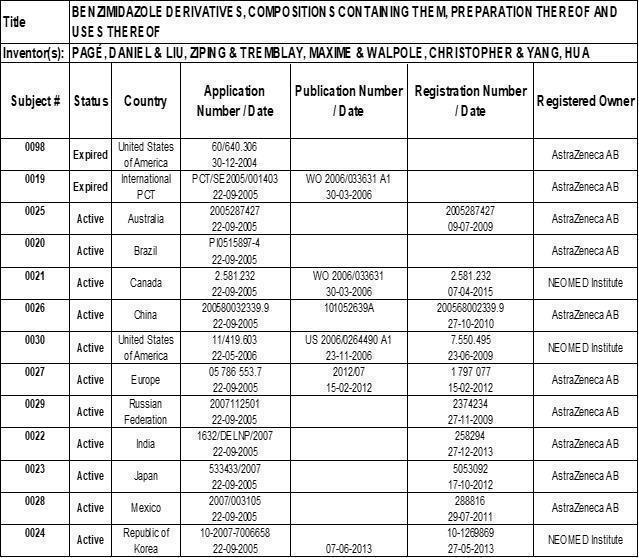

EXHIBIT B
RESEARCH PLAN
The team at Artelo has extensive experience in cancer drug development and commercialization. Artelo believes NEO1940 has promise as an anti-tumour therapy based upon the extensive literature on the anti-tumour effects of CB1 and CB2 agonists. NEO1940 has not been screened in any in-vivo or in-vitro cancer assays. The purpose of the Option Period is to provide Artelo, as well as the regulatory authorities and clinical ethics committees, with the appropriate pre-clinical data in cancer screens and models to provide confidence in the optimized; tumour type, dose and dosing schedule; as well as an understanding of the mechanisms by which NEO1940 exerts its anti-tumour effects. The pre-clinical work being undertaken is also driven at evaluating the potential for new intellectual property (i.e. method of use) that will protect NEO1940 as a direct anti-tumour drug. The following activities are anticipated to be conducted by Artelo under this Research Plan:
| · | Pre-clinical research – limited to in-vitro and in-vivo Oncology screening assays testing the Compound against several different Oncology cell lines and models. |
| | |
| · | Active Pharmaceutical Ingredient evaluation – limited to assessing the feasibility to provide Good Manufacturing Practice material for clinical evaluation either by clean-up of the current batch of the Compound substance or the synthesis of new batch of material. |
| | |
| · | IND Application (or European equivalent) – limited to opening an IND for the indication of testing of the Compound in cancer patients for the purposes of reducing tumour burden and/or extending Progression Free Survival. |
The pre-clinical evaluation will be divided into 2 defined milestones concluding with an Oncology specific scientific advisory board (SAB) to assess the data and confirm next steps.
| o | Milestone 1a – Determination that NEO1940 has anti-tumour activity in a minimum of 1 diverse histotype tumour line from a 12-panel screen. |
| | |
| o | Milestone 1b – Demonstrate that the anti-tumour activity is mediated via direct CB1/CB2 agonism. Provide evidence that the in-vitro activity is maintained in a non-2D cellular screen. |
| o | Milestone 2 – Determination of evidence of a >50% reduction in tumour growth or metastasis in a minimum of one xenograft model. Provide evidence that this effect is mediated by CB1/CB2 agonism. Provide guidance for clinical work on optimized dosing (high pulsed dose vs low chronic dose) and its utility on top of chemotherapy or radiotherapy. |
Best case estimates of the timeframe for the completion of work are as follows:
| · | Milestone 1a = 1 month |
| | |
| · | Milestone 1b = 2 months |
| | |
| · | Milestone 2 = 4 months |
| | |
| · | Total time = 7 months |
Worst case estimates of the timeframe for the completion of work are as follows:
| · | Milestone 1a = 1 month |
| | |
| · | Milestone 1b = 4 months |
| | |
| · | Milestone 2 = 7 months |
| | |
| · | Total time = 12 months |
The full schema for the estimated work under this Research Plan is shown in Figure. As some of the experiments cannot be accurately planned until we have data from the preceding experiments there is a potential best case that the entire work can be completed within 7 months.
Figure 1 – Pre-clinical work plan for NEO1940
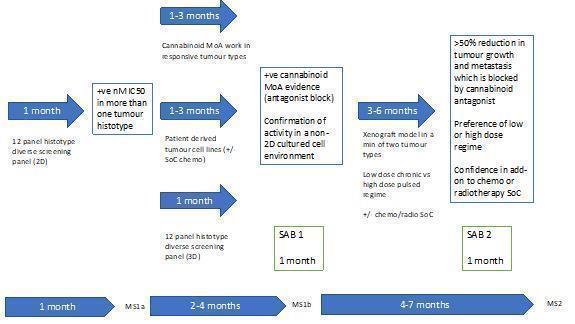
EXHIBIT C
TECHNOLOGY TRANSFER MATERIALS
| 1. | Full study data for the following studies: |
Study | | Details |
SAD1 (UK) | | First Time In Man, Oral Single Ascending Doses |
SAD (Japan) | | Pharmacokinetics of after administration of Oral Single Ascending Doses in Japanese Subjects |
POP1 (UK) | | Effects of a single oral dose on intradermal and topical capsaicin-evoked pain symptoms |
POP2 (USA) | | Analgesic efficacy of a single dose in patients undergoing impacted mandibular third molar extraction |
MAD (Sweden) | | Pharmacokinetics after administration of oral multiple ascending doses in adult subjects with chronic low back pain |
AZD1940/NEO1940 pre-clinical | | All preclinical studies undertaken by AstraZeneca, NEOMED and third parties |
| 2. | Copies of all patent files for the Licensed Patent Rights. |
| | |
| 3. | All necessary documentation for the use of Compound substance and manufacture of future batches. |
| | |
| 4. | Copies of all filings to Regulatory Authorities and any assistance that maybe necessary in regulatory interactions such as letter of access etc. |
| | |
| 5. | Details of any quality audits undertaken by NEOMED or its Affiliates, as well as applicable Third Parties, of clinical trials and investigator sites. |
| | |
| 6. | Copies of all Regulatory Authority audits. |
EXHIBIT D
PURCHASE AGREEMENT
ARTELO BIOSCIENCES, INC.
COMMON STOCK PURCHASE AGREEMENT
This Common Stock Purchase Agreement (this “Agreement”) is made as of January 2, 2018, by and between Artelo Biosciences, Inc., a Nevada corporation (the “Company”), and NEOMED Institute, a not-for-profit corporation established under the Not-for-Profit Corporations Act (Canada) (the “Purchaser”).
Reference is hereby made to the Material and Data Transfer, Option and License Agreement dated as of the date hereof by and between the Company and the Purchaser (the “Development Agreement”). Capitalized terms used in this Agreement that are not otherwise defined herein shall have the respective meanings assigned to them in the Development Agreement.
In consideration of the mutual covenants and representations set forth below, the Company and Purchaser agree as follows:
1. Issuance of the Shares. Subject to the terms and conditions of this Agreement, as partial consideration for the grant of the Option (as defined in the Development Agreement) by NEOMED to Artelo and the supply of the Technology Transfer Materials (as defined in the Development Agreement) and pursuant to the terms of Section 5.1.1 of the Development Agreement, the Company hereby issues to the Purchaser on the Closing (as defined below) One Hundred and Twenty Thousand (120,000) shares of the Company’s common stock (the “Shares”).
2. Closing. The purchase and sale of the Shares shall occur at a closing (the “Closing”) to be held on the date hereof.
3. Restrictions on Transfer.
A. Investment Representations and Legend Requirements. The Purchaser hereby makes the investment representations listed on Exhibit A to the Company as of the date of this Agreement, and agrees that such representations are incorporated into this Agreement by this reference, such that the Company may rely on them in issuing the Shares. Purchaser understands and agrees that the Company shall cause the legends set forth below, or substantially equivalent legends, to be placed upon any certificate(s) evidencing ownership of the Shares, together with any other legends that may be required by the Company or by applicable state or federal securities laws:
THE SECURITIES REPRESENTED HEREBY HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933 (THE “ACT”) AND MAY NOT BE OFFERED, SOLD OR OTHERWISE TRANSFERRED, PLEDGED OR HYPOTHECATED UNLESS AND UNTIL REGISTERED UNDER THE ACT OR, IN THE OPINION OF COUNSEL SATISFACTORY TO THE ISSUER OF THESE SECURITIES, SUCH OFFER, SALE OR TRANSFER, PLEDGE OR HYPOTHECATION OTHERWISE COMPLIES WITH THE ACT. |
THE SHARES REPRESENTED BY THIS CERTIFICATE ARE SUBJECT TO CERTAIN RESTRICTIONS ON TRANSFER IN THE EVENT OF A PUBLIC OFFERING AS SET FORTH IN THE COMMON STOCK PURCHASE AGREEMENT BETWEEN THE ISSUER AND THE ORIGINAL HOLDER OF THESE SHARES, A COPY OF WHICH MAY BE OBTAINED AT THE PRINCIPAL OFFICE OF THE ISSUER. SUCH TRANSFER RESTRICTIONS ARE BINDING ON TRANSFEREES OF THESE SHARES. |
B. Stop-Transfer Notices. Purchaser agrees that to ensure compliance with the restrictions referred to herein, the Company may issue appropriate “stop transfer” instructions to its transfer agent, if any, and that, if the Company transfers its own securities, it may make appropriate notations to the same effect in its own records.
C. Refusal to Transfer. The Company shall not be required (i) to transfer on its books any Shares that have been sold or otherwise transferred in violation of any of the provisions of this Agreement or (ii) to treat as owner of such Shares or to accord the right to vote or pay dividends to any purchaser or other transferee to whom such Shares shall have been so transferred.
D. Limitation on restriction: Notwithstanding anything contained in this Agreement (including Exhibit A), the Company acknowledges and agrees that the Purchaser has the right to transfer 20% of the shares to AstraZeneca at any time, provided that the terms and conditions of this agreement (including Exhibit A) equally apply to AstraZeneca.
4. Tax Consequences. The Purchaser has reviewed with the Purchaser’s own tax advisors the federal, state, local and foreign tax consequences of this investment and the transactions contemplated by this Agreement. The Purchaser is relying solely on such advisors and not on any statements or representations of the Company or any of its agents. The Purchaser understands that the Purchaser (and not the Company) shall be responsible for any tax liability that may arise as a result of the transactions contemplated by this Agreement.
5. Representations and Warranties of the Company
The Company hereby makes the following representations and warranties to the Purchaser as of the date of this Agreement and acknowledges that the Purchaser is relying on such representations and warranties in entering into this Agreement:
A. the Company is a corporation duly formed and validly existing under the laws of Nevada and has all necessary corporate power and authority to execute this Agreement and any certificate evidencing ownership of the Shares (the “Certificate”) and to offer, sell and issue the Shares, and to perform its obligations under this Agreement and the Certificate;
B. the authorized capital of the Company consists of 150,000,000 shares of common stock, of which 11,352,302 are currently issued and outstanding;
C. the Company has complied and will comply with all applicable corporate and securities laws and regulations in connection with the offer, sale and issuance of the Shares, and the execution, delivery and performance of this Agreement and the transactions contemplated by this Agreement will not result in any violation of or constitute either a default under any provision of its organizational documents or of any material agreement or instrument to which the Company is a party or by which the Company is bound, or any judgment, decree, order, law, statute, rule or regulation applicable to the Company;
D. this Agreement has been duly and validly authorized, executed and delivered by the Company and constitutes a legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms;
E. the issuance of the Shares has been duly authorized and approved by all requisite corporate, regulatory and other action and upon Closing the Shares will be validly issued as fully paid and non-assessable, and shall be free and clear of any liens, mortgages, charges, security interests, burdens, encumbrances and other restrictions or limitations of any kind whatsoever;
F. the Company is duly qualified to transact business in each jurisdiction where the nature and extent of its business and properties (both owned and leased) requires;
G. the Company has been conducting its business in the ordinary course in compliance with all applicable laws, rules and regulations, including but not limited to federal, state, municipal and local laws, rules and regulations of each jurisdiction in which it carries on business and is not in breach of any such laws, rules or regulations;
H. the Company does not have any information or knowledge of any fact not communicated to the Purchaser and relating to the business of the Company which, if known to the Purchaser, might reasonably be expected to have a materially adverse effect on the Company’s business or deter the Purchaser from entering into this Agreement or from completing the transactions contemplated hereby;
I. the corporate records of the Company are complete and accurate in all material respects and all corporate proceedings and actions reflected therein have been conducted or taken in compliance with all applicable laws and with the constating documents of the Company, and all transactions, reorganisations or other actions of the Company have been duly authorized by all necessary corporate action;
J. no other consents or approvals are required from any person whatsoever in connection with the transactions contemplated hereby nor are necessary on the part of the Company to authorise the execution and delivery of this Agreement or the consummation by the Company of the transactions contemplated hereby.
The Company agrees that the Purchaser is entitled to rely on the representations and warranties of the Company contained in this section 5 and the Company will indemnify and hold harmless the Purchaser for any loss, cost, expense, damage or any other liability the Purchaser may suffer as a result of a misrepresentation by the Company
6. General Provisions.
A. Choice of Law. This Agreement shall be governed by the internal substantive laws, but not the choice of law rules, of New York.
B. Integration. The Development Agreement and this Agreement, including all exhibits hereto, represent the entire agreement between the parties with respect to the issuance of the Shares to the Purchaser and supersedes and replaces any and all prior written or oral agreements regarding the subject matter of this Agreement including, but not limited to, any representations made during any interviews, relocation discussions or negotiations whether written or oral.
C. Notices. Any notice, demand, offer, request or other communication required or permitted to be given by either the Company or the Purchaser pursuant to the terms of this Agreement shall be in writing and shall be deemed effectively given the earlier of (i) when received, (ii) when delivered personally, (iii) one business day after being delivered by facsimile (with receipt of appropriate confirmation), (iv) one business day after being deposited with an overnight courier service or (v) four days after being deposited in the U.S. mail, First Class with postage prepaid and return receipt requested, and addressed to the parties at the addresses provided to the Company (which the Company agrees to disclose to the other parties upon request) or such other address as a party may request by notifying the other in writing.
D. Successors. Any successor to the Company (whether direct or indirect and whether by purchase, merger, consolidation, liquidation or otherwise) to all or substantially all of the Company’s business and/or assets shall assume the obligations under this Agreement and agree expressly to perform the obligations under this Agreement in the same manner and to the same extent as the Company would be required to perform such obligations in the absence of a succession. For all purposes under this Agreement, the term “Company” shall include any successor to the Company’s business and/or assets which executes and delivers the assumption agreement described in this section or which becomes bound by the terms of this Agreement by operation of law. Subject to the restrictions on transfer set forth in this Agreement, this Agreement shall be binding upon Purchaser and its executors, administrators, successors and assigns.
E. Assignment; Transfers. Except as set forth in this Agreement and subject to Section 3D above, this Agreement, and any and all rights, duties and obligations hereunder, shall not be assigned, transferred, delegated or sublicensed by the Purchaser without the prior written consent of the Company. Any attempt by the Purchaser without such consent to assign, transfer, delegate or sublicense any rights, duties or obligations that arise under this Agreement shall be void. Except as set forth in this Agreement, any transfers in violation of any restriction upon transfer contained in any section of this Agreement shall be void, unless such restriction is waived in accordance with the terms of this Agreement.
F. Amendment and Waiver. Any term of the Agreement may be amended and the observance of any term of the Agreement may be waived (either generally or in a particular instance and either retroactively or prospectively), only with the written consent of the Company and the Purchaser. Any amendment or waiver effected in accordance with this Section 5.F shall be binding upon each holder of any Shares at the time outstanding, each future holder of all such securities and the Company
G. Either party’s failure to enforce any provision of this Agreement shall not in any way be construed as a waiver of any such provision, nor prevent that party from thereafter enforcing any other provision of this Agreement. The rights granted both parties hereunder are cumulative and shall not constitute a waiver of either party’s right to assert any other legal remedy available to it.
H. Purchaser Investment Representations and Further Documents. Each Party agrees upon request to execute any further documents or instruments necessary or reasonably desirable to carry out the purposes or intent of this Agreement, including (but not limited to) the applicable exhibits and attachments to this Agreement.
I. Severability. Should any provision of this Agreement be found to be illegal or unenforceable, the other provisions shall nevertheless remain effective and shall remain enforceable to the greatest extent permitted by law.
J. Rights as Stockholder. Subject to the terms and conditions of this Agreement, Purchaser shall have all of the rights of a stockholder of the Company with respect to the Shares from and after the date that Purchaser delivers a fully executed copy of this Agreement (including the applicable exhibits and attachments to this Agreement) to the Company, and until such time as Purchaser disposes of the Shares in accordance with this Agreement. Upon such transfer, Purchaser shall have no further rights as a holder of the Shares so purchased except (in the case of a transfer to the Company) the right to receive payment for the Shares so purchased in accordance with the provisions of this Agreement, and Purchaser shall forthwith cause the certificate(s) evidencing the Shares so purchased to be surrendered to the Company for transfer or cancellation.
K. Adjustment for Stock Split. All references to the number of Shares in this Agreement shall be adjusted to reflect any stock split, stock dividend or other change in the Shares which may be made after the date of this Agreement.
L. Counterparts. This Agreement may be executed in one or more counterparts, each of which will be deemed an original, but all of which together will constitute one and the same agreement. Facsimile copies of signed signature pages shall be binding originals.
M. Reliance on Counsel and Advisors. The parties acknowledge that Wilson Sonsini Goodrich & Rosati, Professional Corporation, is representing only the Company in this transaction. The parties acknowledge that they have had the opportunity to review this Agreement, including all attachments hereto, and the transactions contemplated by this Agreement with their own legal counsel, tax advisors and other advisors. The parties are relying solely on their own counsel and advisors and not on any statements or representations of the Company or its agents for legal or other advice with respect to the transactions contemplated by this Agreement.
(signature page follows)
The parties represent that they have read this Agreement in its entirety, have had an opportunity to obtain the advice of counsel prior to executing this Agreement and fully understand this Agreement. The Purchaser agrees to notify the Company of any change in his or her address below.
NEOMED INSTITUTE | | | ARTELO BIOSCIENCES, INC. | |
| | | | | |
| /s/ Donald Olds | | | /s/ Gregory D. Gorgas | |
| Signature | | | Signature | |
| | | | |
Donald Olds | | | Gregory D. Gorgas | |
Print Name | | | Print Name | |
| | | | |
President and Chief Executive Officer | | | President and Chief Executive Officer | |
Print Title | | | Print Title | |
| | | | |
Address: | | | | |
| | | | |
7171 Frederick-Banting Saint-Laurent, Quebec, Canada H4S 1Z9 | | | | |
Artelo Biosciences, Inc.
(Signature Page to Common Stock Purchase Agreement)
EXHIBIT A
INVESTMENT REPRESENTATION STATEMENT
PURCHASER | : | NEOMED Institute |
COMPANY | : | Artelo Biosciences, Inc. |
| | |
SECURITY | : | Common Stock |
AMOUNT | : | 120,000 shares |
DATE | : | January 2, 2018 |
| | |
In connection with the purchase of the above-listed shares, the undersigned Purchaser represents to the Company as follows:
1. The Company may rely on these representations. It understands that the Company’s sale of the shares to it has not been registered under the Securities Act of 1933, as amended (the “Securities Act”), because the Company believes, relying in part on my representations in this document, that an exemption from such registration requirement is available for such sale. It understands that the availability of this exemption depends upon the representations it is making to the Company in this document being true and correct.
2. It is purchasing for investment. It is purchasing the shares solely for investment purposes, and not for further distribution. Its entire legal and beneficial ownership interest in the shares is being purchased and shall be held solely for its account. Subject to Section 3D it is not a party to, and does not presently intend to enter into, any contract or other arrangement with any other person or entity involving the resale, transfer, grant of participation with respect to or other distribution of any of the shares. Its investment intent is not limited to their present intention to hold the shares for the minimum capital gains period specified under any applicable tax law, for a deferred sale, for a specified increase or decrease in the market price of the shares, or for any other fixed period in the future.
3. It can protect their own interests. It can properly evaluate the merits and risks of an investment in the shares and can protect its own interests in this regard, whether by reason of its own business and financial expertise, the business and financial expertise of certain professional advisors unaffiliated with the Company with whom it has consulted, or its preexisting business or personal relationship with the Company or any of its officers, directors or controlling persons.
4. It is informed about the Company. It is sufficiently aware of the Company’s business affairs and financial condition to reach an informed and knowledgeable decision to acquire the shares. It has had opportunity to discuss the plans, operations and financial condition of the Company with its officers, directors or controlling persons, and have received all information they deem appropriate for assessing the risk of an investment in the shares.
5. It recognizes the economic risk. It realizes that the purchase of the shares involves a high degree of risk, and that the Company’s future prospects are uncertain. It is able to hold the shares indefinitely if required, and is able to bear the loss of their entire investment in the shares.
6. It knows that the shares are restricted securities. It understands that the shares are “restricted securities” in that the Company’s sale of the shares to them has not been registered under the Securities Act in reliance upon an exemption for non-public offerings. In this regard, it also understands and agrees that:
A. It must hold the shares indefinitely, unless any subsequent proposed resale by them is registered under the Securities Act, or unless an exemption from registration is otherwise available (such as Rule 144);
B. the Company is under no obligation to register any subsequent proposed resale of the shares by it; and
C. the certificate evidencing the shares will be imprinted with a legend which prohibits the transfer of the shares unless such transfer is registered or such registration is not required in the opinion of counsel for the Company.
7. It is familiar with Rule 144. It is familiar with Rule 144 adopted under the Securities Act, which in some circumstances permits limited public resales of “restricted securities” like the shares acquired from an issuer in a non-public offering. It understands that its ability to sell the shares under Rule 144 in the future is uncertain, and may depend upon, among other things: (i) the availability of certain current public information about the Company; and (ii) the resale occurring more than a specified period after their purchase and full payment (within the meaning of Rule 144) for the shares; and (iii) the Company’s former or current status as a “shell company”.
8. It knows that Rule 144 may never be available. It understands that the requirements of Rule 144 may never be met, and that the shares may never be saleable under the rule. It further understands that at the time it wishes to sell the shares, there may be no public market for the Company’s stock upon which to make such a sale, or the current public information requirements of Rule 144 may not be satisfied, either of which may preclude them from selling the shares under Rule 144 even if the relevant holding period had been satisfied.
9. It knows that it is subject to further restrictions on resale. It understands that in the event Rule 144 is not available to it, any future proposed sale of any of the shares by it will not be possible without prior registration under the Securities Act, compliance with some other registration exemption (which may or may not be available), or each of the following: (i) their written notice to the Company containing detailed information regarding the proposed sale, (ii) their providing an opinion of its counsel to the effect that such sale will not require registration, and (iii) the Company notifying them in writing that its counsel concurs in such opinion or that the Company otherwise consents to the proposed sale or consent. It understands that neither the Company nor its counsel is obligated to provide it with any such opinion. It understands that although Rule 144 is not exclusive, the Staff of the SEC has stated that persons proposing to sell private placement securities other than in a registered offering or pursuant to Rule 144 will have a substantial burden of proof in establishing that an exemption from registration is available for such offers or sales, and that such persons and their respective brokers who participate in such transactions do so at their own risk.
10. It knows that it may have tax liability due to the uncertain value of the shares. It understands that the Internal Revenue Service may successfully assert that the value of the shares on the date of their purchase is substantially greater than the appraisal of the Board of Directors. It understands that any additional value ascribed to the shares by such an IRS determination will constitute ordinary income to it as of the purchase date, and that any additional taxes and interest due as a result will be their sole responsibility payable only by it, and that the Company need not and will not reimburse it for that tax liability. It understands that if such additional value represents more than 25% of their gross income for the year in which the value of the shares is taxable, the IRS will have 6 years from the due date for filing the return (or the actual filing date of the return if filed thereafter) within which to assess them the additional tax and interest due.
11. Principal Place of Business. The address of their principal place of business is set forth on the signature page below.
12. No “bad actor” disqualification events. Neither it nor any person that would be deemed a beneficial owner of the shares (in accordance with Rule 506(d) of the Securities Act) is subject to any of the “bad actor” disqualifications described in Rule 506(d)(1)(i) through (viii) under the Securities Act, except as set forth in Rule 506(d)(2)(ii) or (iii) or (d)(3) under the Securities Act and disclosed, reasonably in advance of the purchase or acquisition of the shares, in writing in reasonable detail to the Company.
By signing below, it acknowledges its agreement with each of the statements contained in this Investment Representation Statement as of the date first set forth above, and its intent for the Company to rely on such statements in issuing the shares to it.
| | NEOMED INSTITUTE | |
| | | |
| | |
| Purchaser’s Signature | |
| | | |
| | |
| Name Donald Olds | |
| | |
| | |
| | Title President and Chief Executive Officer | |
| | |
Address: 7171 Frederick-Banting Saint-Laurent, Quebec, Canada H4S 1Z9 | | |
EXHIBIT E
COMMON STOCK PURCHASE AGREEMENT
ARTELO BIOSCIENCES, INC.
COMMON STOCK PURCHASE AGREEMENT
This Common Stock Purchase Agreement (this “Agreement”) is made as of [____], by and between Artelo Biosciences, Inc., a Nevada corporation (the “Company”), and NEOMED Institute, a not-for-profit corporation established under the Not-for-Profit Corporations Act (Canada) (the “Purchaser”).
Reference is hereby made to the Material and Data Transfer, Option and License Agreement dated as of the date hereof by and between the Company and the Purchaser (the “Development Agreement”). Capitalized terms used in this Agreement that are not otherwise defined herein shall have the respective meanings assigned to them in the Development Agreement.
In consideration of the mutual covenants and representations set forth below, the Company and Purchaser agree as follows:
1. Issuance of the Shares. Subject to the terms and conditions of this Agreement, partial consideration for the license granted to the Company by Purchaser under Section 4.1 of the Development Agreement and pursuant to the terms of Section 5.2.1 of the Development Agreement, the Company hereby issues to the Purchaser on the Closing (as defined below) [___]1 shares of the Company’s common stock (the “Shares”).
2. Closing. The purchase and sale of the Shares shall occur at a closing (the “Closing”) to be held on the date hereof.
3. Restrictions on Transfer.
A. Investment Representations and Legend Requirements. The Purchaser hereby makes the investment representations listed on Exhibit A to the Company as of the date of this Agreement, and agrees that such representations are incorporated into this Agreement by this reference, such that the Company may rely on them in issuing the Shares. Purchaser understands and agrees that the Company shall cause the legends set forth below, or substantially equivalent legends, to be placed upon any certificate(s) evidencing ownership of the Shares, together with any other legends that may be required by the Company or by applicable state or federal securities laws:
| THE SECURITIES REPRESENTED HEREBY HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933 (THE “ACT”) AND MAY NOT BE OFFERED, SOLD OR OTHERWISE TRANSFERRED, PLEDGED OR HYPOTHECATED UNLESS AND UNTIL REGISTERED UNDER THE ACT OR, IN THE OPINION OF COUNSEL SATISFACTORY TO THE ISSUER OF THESE SECURITIES, SUCH OFFER, SALE OR TRANSFER, PLEDGE OR HYPOTHECATION OTHERWISE COMPLIES WITH THE ACT. | |
________________
1 Equal to 2% of Artelo’s Fully Diluted Shares. “Fully-Diluted Shares” shall mean, as of immediately prior to Artelo’s exercise of the Option, the sum of (a) the outstanding shares of common stock of Artelo; (b) the shares of common stock of Artelo directly or indirectly issuable upon conversion or exchange of all outstanding securities directly or indirectly convertible into or exchangeable for common stock of Artelo and the exercise of all outstanding options and warrants; and (c) the shares of common stock of Artelo reserved, but neither issued nor the subject of outstanding awards, under any equity incentive or similar plan of Artelo.
| THE SHARES REPRESENTED BY THIS CERTIFICATE ARE SUBJECT TO CERTAIN RESTRICTIONS ON TRANSFER IN THE EVENT OF A PUBLIC OFFERING AS SET FORTH IN THE COMMON STOCK PURCHASE AGREEMENT BETWEEN THE ISSUER AND THE ORIGINAL HOLDER OF THESE SHARES, A COPY OF WHICH MAY BE OBTAINED AT THE PRINCIPAL OFFICE OF THE ISSUER. SUCH TRANSFER RESTRICTIONS ARE BINDING ON TRANSFEREES OF THESE SHARES. | |
B. Stop-Transfer Notices. Purchaser agrees that to ensure compliance with the restrictions referred to herein, the Company may issue appropriate “stop transfer” instructions to its transfer agent, if any, and that, if the Company transfers its own securities, it may make appropriate notations to the same effect in its own records.
C. Refusal to Transfer. The Company shall not be required (i) to transfer on its books any Shares that have been sold or otherwise transferred in violation of any of the provisions of this Agreement or (ii) to treat as owner of such Shares or to accord the right to vote or pay dividends to any purchaser or other transferee to whom such Shares shall have been so transferred.
D. Limitation on restriction: Notwithstanding anything contained in this Agreement (including Exhibit A), the Company acknowledges and agrees that the Purchaser has the right to transfer 20% of the shares to AstraZeneca at any time provided that the terms and conditions of this Agreement (including Exhibit A) equally apply to AstraZeneca.
4. Tax Consequences. The Purchaser has reviewed with the Purchaser’s own tax advisors the federal, state, local and foreign tax consequences of this investment and the transactions contemplated by this Agreement. The Purchaser is relying solely on such advisors and not on any statements or representations of the Company or any of its agents. The Purchaser understands that the Purchaser (and not the Company) shall be responsible for any tax liability that may arise as a result of the transactions contemplated by this Agreement.
5. Representations and Warranties of the Company
The Company hereby makes the following representations and warranties to the Purchaser as of the date of this Agreement and acknowledges that the Purchaser is relying on such representations and warranties in entering into this Agreement:
A. the Company is a corporation duly formed and validly existing under the laws of Nevada and has all necessary corporate power and authority to execute this Agreement and any certificate evidencing ownership of the Shares (the “Certificate”) and to offer, sell and issue the Shares, and to perform its obligations under this Agreement and the Certificate;
B. the authorized capital of the Company consists of 150,000,000 shares of common stock, of which 11,352,302 are currently issued and outstanding;
C. the Company has complied and will comply with all applicable corporate and securities laws and regulations in connection with the offer, sale and issuance of the Shares, and the execution, delivery and performance of this Agreement and the transactions contemplated by this Agreement will not result in any violation of or constitute either a default under any provision of its organizational documents or of any material agreement or instrument to which the Company is a party or by which the Company is bound, or any judgment, decree, order, law, statute, rule or regulation applicable to the Company;
D. this Agreement has been duly and validly authorized, executed and delivered by the Company and constitutes a legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms;
E. the issuance of the Shares has been duly authorized and approved by all requisite corporate, regulatory and other action and upon Closing the Shares will be validly issued as fully paid and non-assessable, and shall be free and clear of any liens, mortgages, charges, security interests, burdens, encumbrances and other restrictions or limitations of any kind whatsoever;
F. the Company is duly qualified to transact business in each jurisdiction where the nature and extent of its business and properties (both owned and leased) requires;
G. the Company has been conducting its business in the ordinary course in compliance with all applicable laws, rules and regulations, including but not limited to federal, state, municipal and local laws, rules and regulations of each jurisdiction in which it carries on business and is not in breach of any such laws, rules or regulations;
H. the Company does not have any information or knowledge of any fact not communicated to the Purchaser and relating to the business of the Company which, if known to the Purchaser, might reasonably be expected to have a materially adverse effect on the Company’s business or deter the Purchaser from entering into this Agreement or from completing the transactions contemplated hereby;
I. the corporate records of the Company are complete and accurate in all material respects and all corporate proceedings and actions reflected therein have been conducted or taken in compliance with all applicable laws and with the constating documents of the Company, and all transactions, reorganisations or other actions of the Company have been duly authorized by all necessary corporate action;
J. no other consents or approvals are required from any person whatsoever in connection with the transactions contemplated hereby nor are necessary on the part of the Company to authorise the execution and delivery of this Agreement or the consummation by the Company of the transactions contemplated hereby.
The Company agrees that the Purchaser is entitled to rely on the representations and warranties of the Company contained in this section 5 and the Company will indemnify and hold harmless the Purchaser for any loss, cost, expense, damage or any other liability the Purchaser may suffer as a result of a misrepresentation by the Company
6. General Provisions.
A. Choice of Law. This Agreement shall be governed by the internal substantive laws, but not the choice of law rules, of New York.
B. Integration. The Development Agreement and this Agreement, including all exhibits hereto, represent the entire agreement between the parties with respect to the issuance of the Shares to the Purchaser and supersedes and replaces any and all prior written or oral agreements regarding the subject matter of this Agreement including, but not limited to, any representations made during any interviews, relocation discussions or negotiations whether written or oral.
C. Notices. Any notice, demand, offer, request or other communication required or permitted to be given by either the Company or the Purchaser pursuant to the terms of this Agreement shall be in writing and shall be deemed effectively given the earlier of (i) when received, (ii) when delivered personally, (iii) one business day after being delivered by facsimile (with receipt of appropriate confirmation), (iv) one business day after being deposited with an overnight courier service or (v) four days after being deposited in the U.S. mail, First Class with postage prepaid and return receipt requested, and addressed to the parties at the addresses provided to the Company (which the Company agrees to disclose to the other parties upon request) or such other address as a party may request by notifying the other in writing.
D. Successors. Any successor to the Company (whether direct or indirect and whether by purchase, merger, consolidation, liquidation or otherwise) to all or substantially all of the Company’s business and/or assets shall assume the obligations under this Agreement and agree expressly to perform the obligations under this Agreement in the same manner and to the same extent as the Company would be required to perform such obligations in the absence of a succession. For all purposes under this Agreement, the term “Company” shall include any successor to the Company’s business and/or assets which executes and delivers the assumption agreement described in this section or which becomes bound by the terms of this Agreement by operation of law. Subject to the restrictions on transfer set forth in this Agreement, this Agreement shall be binding upon Purchaser and its heirs, executors, administrators, successors and assigns.
E. Assignment; Transfers. Except as set forth in this Agreement and subject to Section 3D above, this Agreement, and any and all rights, duties and obligations hereunder, shall not be assigned, transferred, delegated or sublicensed by the Purchaser without the prior written consent of the Company. Any attempt by the Purchaser without such consent to assign, transfer, delegate or sublicense any rights, duties or obligations that arise under this Agreement shall be void. Except as set forth in this Agreement, any transfers in violation of any restriction upon transfer contained in any section of this Agreement shall be void, unless such restriction is waived in accordance with the terms of this Agreement.
F. Amendment and Waiver. Any term of the Agreement may be amended and the observance of any term of the Agreement may be waived (either generally or in a particular instance and either retroactively or prospectively), only with the written consent of the Company and the Purchaser. Any amendment or waiver effected in accordance with this Section 5.F shall be binding upon each holder of any Shares at the time outstanding, each future holder of all such securities and the Company
G. Either party’s failure to enforce any provision of this Agreement shall not in any way be construed as a waiver of any such provision, nor prevent that party from thereafter enforcing any other provision of this Agreement. The rights granted both parties hereunder are cumulative and shall not constitute a waiver of either party’s right to assert any other legal remedy available to it.
H. Purchaser Investment Representations and Further Documents. Each Party agrees upon request to execute any further documents or instruments necessary or reasonably desirable to carry out the purposes or intent of this Agreement, including (but not limited to) the applicable exhibits and attachments to this Agreement.
I. Severability. Should any provision of this Agreement be found to be illegal or unenforceable, the other provisions shall nevertheless remain effective and shall remain enforceable to the greatest extent permitted by law.
J. Rights as Stockholder. Subject to the terms and conditions of this Agreement, Purchaser shall have all of the rights of a stockholder of the Company with respect to the Shares from and after the date that Purchaser delivers a fully executed copy of this Agreement (including the applicable exhibits and attachments to this Agreement) to the Company, and until such time as Purchaser disposes of the Shares in accordance with this Agreement. Upon such transfer, Purchaser shall have no further rights as a holder of the Shares so purchased except (in the case of a transfer to the Company) the right to receive payment for the Shares so purchased in accordance with the provisions of this Agreement, and Purchaser shall forthwith cause the certificate(s) evidencing the Shares so purchased to be surrendered to the Company for transfer or cancellation.
K. Adjustment for Stock Split. All references to the number of Shares in this Agreement shall be adjusted to reflect any stock split, stock dividend or other change in the Shares which may be made after the date of this Agreement.
L. Counterparts. This Agreement may be executed in one or more counterparts, each of which will be deemed an original, but all of which together will constitute one and the same agreement. Facsimile copies of signed signature pages shall be binding originals.
M. Reliance on Counsel and Advisors. The parties acknowledge that Wilson Sonsini Goodrich & Rosati, Professional Corporation, is representing only the Company in this transaction. The parties acknowledge that they have had the opportunity to review this Agreement, including all attachments hereto, and the transactions contemplated by this Agreement with their own legal counsel, tax advisors and other advisors. The parties are relying solely on their own counsel and advisors and not on any statements or representations of the Company or its agents for legal or other advice with respect to the transactions contemplated by this Agreement.
(signature page follows)
The parties represent that they have read this Agreement in its entirety, have had an opportunity to obtain the advice of counsel prior to executing this Agreement and fully understand this Agreement. The Purchaser agrees to notify the Company of any change in his or her address below.
| NEOMED INSTITUTE | | ARTELO BIOSCIENCES, INC. | |
| | | |
| | | |
| Signature | | Signature | |
| | | |
Donald Olds | | Gregory D. Gorgas | |
Print Name | | Print Name | |
| | | |
President and Chief Executive Officer | | President and Chief Executive Officer | |
Print Title | | Print Title | |
Address:
7171 Frederick-Banting
Saint-Laurent, Quebec, Canada
H4S 1Z9
Artelo Biosciences, Inc.
(Signature Page to Common Stock Purchase Agreement)
EXHIBIT A
INVESTMENT REPRESENTATION STATEMENT
PURCHASER | : | NEOMED Institute |
COMPANY | : | Artelo Biosciences, Inc. |
| | |
SECURITY | : | Common Stock |
AMOUNT | : | [______] shares |
DATE | : | [______] |
| | |
| | |
In connection with the purchase of the above-listed shares, the undersigned purchaser represents to the Company as follows:
13. The Company may rely on these representations. It understands that the Company’s sale of the shares to it has not been registered under the Securities Act of 1933, as amended (the “Securities Act”), because the Company believes, relying in part on my representations in this document, that an exemption from such registration requirement is available for such sale. It understands that the availability of this exemption depends upon the representations it is making to the Company in this document being true and correct.
14. It is purchasing for investment. It is purchasing the shares solely for investment purposes, and not for further distribution. Its entire legal and beneficial ownership interest in the shares is being purchased and shall be held solely for its account. Subject to Section 3D it is not a party to, and do not presently intend to enter into, any contract or other arrangement with any other person or entity involving the resale, transfer, grant of participation with respect to or other distribution of any of the shares. Its investment intent is not limited to their present intention to hold the shares for the minimum capital gains period specified under any applicable tax law, for a deferred sale, for a specified increase or decrease in the market price of the shares, or for any other fixed period in the future.
15. It can protect their own interests. It can properly evaluate the merits and risks of an investment in the shares and can protect its own interests in this regard, whether by reason of its own business and financial expertise, the business and financial expertise of certain professional advisors unaffiliated with the Company with whom it has consulted, or its preexisting business or personal relationship with the Company or any of its officers, directors or controlling persons.
16. It is informed about the Company. It is sufficiently aware of the Company’s business affairs and financial condition to reach an informed and knowledgeable decision to acquire the shares. It has had opportunity to discuss the plans, operations and financial condition of the Company with its officers, directors or controlling persons, and have received all information they deem appropriate for assessing the risk of an investment in the shares.
17. It recognizes the economic risk. It realizes that the purchase of the shares involves a high degree of risk, and that the Company’s future prospects are uncertain. It is able to hold the shares indefinitely if required, and is able to bear the loss of their entire investment in the shares.
18. It knows that the shares are restricted securities. It understands that the shares are “restricted securities” in that the Company’s sale of the shares to them has not been registered under the Securities Act in reliance upon an exemption for non-public offerings. In this regard, it also understands and agrees that:
D. It must hold the shares indefinitely, unless any subsequent proposed resale by them is registered under the Securities Act, or unless an exemption from registration is otherwise available (such as Rule 144);
E. the Company is under no obligation to register any subsequent proposed resale of the shares by it; and
F. the certificate evidencing the shares will be imprinted with a legend which prohibits the transfer of the shares unless such transfer is registered or such registration is not required in the opinion of counsel for the Company.
19. It is familiar with Rule 144. It is familiar with Rule 144 adopted under the Securities Act, which in some circumstances permits limited public resales of “restricted securities” like the shares acquired from an issuer in a non-public offering. It understands that its ability to sell the shares under Rule 144 in the future is uncertain, and may depend upon, among other things: (i) the availability of certain current public information about the Company; and (ii) the resale occurring more than a specified period after their purchase and full payment (within the meaning of Rule 144) for the shares; and (iii) the Company’s former or current status as a “shell company”.
20. It knows that Rule 144 may never be available. It understands that the requirements of Rule 144 may never be met, and that the shares may never be saleable under the rule. It further understands that at the time it wishes to sell the shares, there may be no public market for the Company’s stock upon which to make such a sale, or the current public information requirements of Rule 144 may not be satisfied, either of which may preclude them from selling the shares under Rule 144 even if the relevant holding period had been satisfied.
21. It knows that it is subject to further restrictions on resale. It understands that in the event Rule 144 is not available to it, any future proposed sale of any of the shares by it will not be possible without prior registration under the Securities Act, compliance with some other registration exemption (which may or may not be available), or each of the following: (i) their written notice to the Company containing detailed information regarding the proposed sale, (ii) their providing an opinion of its counsel to the effect that such sale will not require registration, and (iii) the Company notifying them in writing that its counsel concurs in such opinion or that the Company otherwise consents to the proposed sale or consent. It understands that neither the Company nor its counsel is obligated to provide them with any such opinion. They understand that although Rule 144 is not exclusive, the Staff of the SEC has stated that persons proposing to sell private placement securities other than in a registered offering or pursuant to Rule 144 will have a substantial burden of proof in establishing that an exemption from registration is available for such offers or sales, and that such persons and their respective brokers who participate in such transactions do so at their own risk.
22. It knows that it may have tax liability due to the uncertain value of the shares. It understands that the Internal Revenue Service may successfully assert that the value of the shares on the date of their purchase is substantially greater than appraisal of the Board of Directors. It understands that any additional value ascribed to the shares by such an IRS determination will constitute ordinary income to it as of the purchase date, and that any additional taxes and interest due as a result will be their sole responsibility payable only by it, and that the Company need not and will not reimburse it for that tax liability. It understands that if such additional value represents more than 25% of their gross income for the year in which the value of the shares is taxable, the IRS will have 6 years from the due date for filing the return (or the actual filing date of the return if filed thereafter) within which to assess them the additional tax and interest due.
23. Principal Place of Business. The address of their principal place of business is set forth on the signature page below.
24. No “bad actor” disqualification events. Neither it nor any person that would be deemed a beneficial owner of the shares (in accordance with Rule 506(d) of the Securities Act) is subject to any of the “bad actor” disqualifications described in Rule 506(d)(1)(i) through (viii) under the Securities Act, except as set forth in Rule 506(d)(2)(ii) or (iii) or (d)(3) under the Securities Act and disclosed, reasonably in advance of the purchase or acquisition of the shares, in writing in reasonable detail to the Company.
By signing below, it acknowledges its agreement with each of the statements contained in this Investment Representation Statement as of the date first set forth above, and its intent for the Company to rely on such statements in issuing the shares to it.
| | NEOMED INSTITUTE | |
| | | |
| | |
| Purchaser’s Signature | |
| | | |
| | |
| | Name Donald Olds | |
| | |
| | |
| Title President and Chief Executive Officer | |
| | |
Address: 7171 Frederick-Banting Saint-Laurent, Quebec, Canada H4S 1Z9 | | |
SCHEDULE 9.2.6
WARRANTY DISCLOSURE
| 1. | With respect to priority right assignment of application US 60/3640,306: NEOMED has the following documents: |
| a) | Undated assignment from the inventors to AstraZeneca AB (Assignment A); and |
| | |
| b) | Assignment with execution date from the inventors to AstraZeneca AB that is after the filing date of PCT/SE2005/001403 (Assignment B). |


