As filed with the Securities and Exchange Commission on December 3, 2014
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-4
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
AVOGENX, INC.
(Exact name of registrant as specified in its charter)
| | | | | |
| Delaware | | 2834 | | 47-1806563 |
(State or other jurisdiction of incorporation or organization) | | (Primary Standard Industrial Classification Code Number) | | (I.R.S. Employer Identification No.) |
Avogenx, Inc.
6601 Six Forks Rd, Suite 140
Raleigh, North Carolina 27615
Telephone: (919) 480-1518
(Address, including zip code, and telephone number including area code, of registrant’s principal executive offices)
James Green
President and Chief Executive Officer
Avogenx, Inc.
6601 Six Forks Rd, Suite 140
Raleigh, North Carolina 27615
Telephone: (919) 480-1518
(name, address, including zip code, and telephone number, including area code, of agent for service)
copies to:
Darren L. Ofsink, Esq. Ofsink, LLC 230 Park Avenue, Suite 851 New York, New York 10169 Telephone: (646) 627-7326 Facsimile: (646) 224-9844 | | Heyward D. Armstrong, Esq. Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P. 150 Fayetteville Street, Suite 2300 Raleigh, North Carolina 27601 Telephone: (919) 821-1220 Facsimile: (919) 821-6800 |
Approximate date of commencement of proposed sale to the public: as soon as practicable after the effective date of this registration statement and all other conditions to the proposed transactions have been satisfied or waived as set forth in the merger agreement dated as of September 30, 2014 described herein.
If the securities being registered on this form are being offered in connection with the formation of a holding company and there is compliance with General Instruction G, check the following box. o
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act (check one):
| | | |
Large accelerated filer o | | Accelerated filer o |
Non-accelerated filer o (Do not check if a smaller reporting company) | | Smaller reporting company þ |
If applicable, place an X in the box to designate the appropriate rule provision relied upon in conducting this transaction:
Exchange Act Rule 13e-4(i) (Cross-Border Issuer Tender Offer) o
Exchange Act Rule 14d-1(d) (Cross-Border Third-Party Tender Offer) o
CALCULATION OF REGISTRATION FEE
| | | | | | | | | | | |
| | | | | | | | | | |
Title of each class of securities to be registered | Amount to be registered(1) | | Proposed maximum offering price per share | | | Proposed maximum aggregate offering price(2) | | | Amount of registration fee(3) | |
| Common Stock, par value $0.001 per share | 27,553,174 shares | | $ | 0.3902 | | | $ | 10,751,248.49 | | | $ | 1,249.30 | |
| | |
| (1) | Represents the maximum number of shares of common stock of the registrant (i) estimated to be issuable to existing holders of shares of common stock of Islet Sciences, Inc. upon conversion of their shares of common stock of Islet Sciences, Inc. into shares of common stock of the registrant and (ii) reserved for issuance upon exercise of outstanding warrants, stock options and other equity awards of Islet Sciences, Inc. which will be converted into warrants, stock options and other equity awards of the registrant, in each such case in connection with the holding company merger described herein, assuming a conversion rate of three shares of Islet Sciences, Inc. common stock for one share of Avogenx, Inc. common stock. In the event that the conversion rate in the holding company merger is greater than 3:1 (i.e., more than three of shares of Islet Sciences, Inc. common stock are exchanged for each share of Avogenx, Inc. common stock), the amount of common stock deemed to be covered by this registration statement shall be proportionately reduced. |
| (2) | The proposed maximum aggregate offering price of common stock of the registrant is based upon the market value of shares of common stock of Islet Sciences, Inc. in accordance with Rule 457(f)(1) and 457(c) under the Securities Act and is equal to the product of: (i) $0.3902, the average of the high and low prices per share of common stock of Islet Sciences, Inc. as reported by the Over-the-Counter Markets (“OTCQB”) on December 1, 2014 (as adjusted assuming a conversion rate in the holding company merger of 3:1 as described above) and (ii) 27,553,174, which represents the maximum number of shares to be registered by Avogenx, Inc. in connection with the holding company merger described herein (assuming a conversion rate in the holding company merger of 3:1 as described above). |
| (3) | Determined in accordance with Section 6(b) of the Securities Act at a rate equal to $116.20 per $1,000,000 of the proposed maximum aggregate offering price. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, or until the registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
THE INFORMATION IN THIS CONSENT SOLICITATION STATEMENT/PROSPECTUS IS NOT COMPLETE AND MAY BE CHANGED. WE MAY NOT OFFER THESE SECURITIES UNTIL THE REGISTRATION STATEMENT FILED WITH THE SECURITIES AND EXCHANGE COMMISSION IS EFFECTIVE. THIS CONSENT SOLICITATION STATEMENT/PROSPECTUS IS NOT AN OFFER TO SELL THESE SECURITIES AND IT IS NOT SOLICITING AN OFFER TO BUY THESE SECURITIES IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED.
SUBJECT TO COMPLETION—DATED _________, 2014
| CONSENT SOLICITATION STATEMENT OF ISLET SCIENCES, INC | | PROSPECTUS OF AVOGENX, INC. |
To Stockholders of Islet Sciences, Inc.:
As you may be aware, Islet Sciences, Inc., a Nevada corporation (“Islet Sciences”), entered into an Agreement and Plan of Merger, dated as of September 30, 2014 (the “Merger Agreement”), by and among Islet Sciences, Brighthaven Ventures, L.L.C., a North Carolina limited liability company (“BHV”), Avogenx, Inc., a Delaware corporation and a direct wholly owned subsidiary of Islet Sciences (“Holdco” or “Avogenx”), Islet Merger Sub, Inc., a Nevada corporation and a direct wholly owned subsidiary of Holdco (“Islet Merger Sub”), and each of the members of BHV (the “BHV Members”). Pursuant to the Merger Agreement, Islet Merger Sub will merge with and into Islet Sciences with Islet Sciences as the surviving entity (the “Merger”), and Avogenx will acquire all of the outstanding membership interests of BHV in consideration for the issuance of an aggregate of 30 million shares of common stock of Avogenx to the BHV Members, to be adjusted for the Exchange Ratio (as defined below), and additional milestone payments described below, which are based on the development of Remogliflozin (the “BHV Securities Exchange” and together with the Merger, the “Transactions”).
In connection with the Transactions, shares of Islet Sciences common stock outstanding immediately prior to the effective time of the Merger will be converted into the right to receive shares of Avogenx common stock at the conversion rate of not less than three and not more than 40 shares of Islet Sciences common stock for one share of Avogenx common stock, with the effective number of shares of Islet Sciences common stock convertible into one share of Avogenx common stock to be determined by the Islet Sciences board of directors prior to the effective time of the Merger (the “Exchange Ratio”). At November 24, 2014, 66,928,724 shares of common stock of Islet Sciences were outstanding. Upon completion of the Transactions, shares of common stock of Islet Sciences held by such holders will be cancelled. Avogenx intends to apply to quote its common stock, as successor to Islet Sciences, on the OTCQB and upon the completion of the Transactions, Avogenx common stock will trade under a new symbol.
The Islet Sciences board of directors has carefully considered the Merger and the terms of the Merger Agreement and has determined that the Merger and the Merger Agreement are fair, advisable and in the best interest of Islet Sciences and its stockholders. Accordingly, the Islet Sciences board of directors has approved the Merger and the Merger Agreement. However, the approval of Islet Sciences stockholders holding a majority of the outstanding Islet Sciences common stock is required for the Merger to close, and you are being sent this document to ask you to approve the Merger Agreement and the Merger by executing and returning the written consent furnished with this consent solicitation statement/prospectus.
Certain stockholders of Islet Sciences, representing approximately 36.8% of the outstanding shares of Islet Sciences common stock, have entered into a voting agreement with Islet Sciences under which they have agreed to execute and return consents with respect to their shares of Islet Sciences common stock approving the Merger Agreement and the Merger.
The Islet Sciences board of directors has set , 2014 as the record date for determining holders of Islet Sciences common stock entitled to execute and deliver written consents with respect to this solicitation. If you are a record holder of outstanding Islet Sciences common stock on that date, you are urged to complete, date and sign the enclosed written consent and promptly return it to Islet Sciences. See “Solicitation of Written Consents.”
We encourage you to read carefully this consent solicitation statement/prospectus in its entirety, including the section entitled “Risk Factors” beginning on page 11.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of the securities to be issued under this consent solicitation statement/prospectus, or determined if this consent solicitation statement/prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
This consent solicitation statement/prospectus is dated , 2014, and is first being mailed to Islet Sciences stockholders on or about , 2014.
| | | |
| | | | |
| | By: | /s/ James Green | |
| | | James Green | |
| | | President and Chief Executive Officer | |
| | | | |
Islet Sciences, Inc.
6601 Six Forks Rd, Suite 140
Raleigh, North Carolina 27615
Notice of Solicitation of Written Consent
To Stockholders of Islet Sciences, Inc.:
Pursuant to an Agreement and Plan of Merger, dated as of September 30, 2014 (the “Merger Agreement”), by and among Islet Sciences, Inc., a Nevada corporation (“Islet Sciences”), Brighthaven Ventures, L.L.C., a North Carolina limited liability company (“BHV”), Avogenx, Inc., a Delaware corporation and a direct wholly owned Subsidiary of Islet Sciences (“Holdco” or “Avogenx”), Islet Merger Sub, Inc., a Nevada corporation and a direct wholly owned subsidiary of Holdco (“Islet Merger Sub”), and each of the members of BHV (the “BHV Members”), Islet Merger Sub will merge with and into Islet Sciences with Islet Sciences as the surviving entity (the “Merger”), and Avogenx will acquire all of the outstanding membership interests of BHV in consideration for the issuance of an aggregate of 30 million shares of common stock of Avogenx to the BHV Members, to be adjusted for the Exchange Ratio (as defined below), and additional milestone payments described below, which are based on the development of BHV’s drug compound, Remogliflozin (the “BHV Securities Exchange” and together with the Merger, the “Transactions”). In connection with the Transactions, shares of Islet Sciences common stock outstanding immediately prior to the effective time of the Merger will be converted into the right to receive shares of Avogenx common stock at the conversion rate of not less than three and not more than 40 shares of Islet Sciences common stock for one share of Avogenx common stock, with the effective number of shares of Islet Sciences common stock convertible into one share of Avogenx common stock to be determined by the Islet Sciences board of directors prior to the effective time of the Merger (the “Exchange Ratio”). Following the completion of the Transactions, Islet Sciences and BHV will each be wholly owned subsidiaries of Avogenx.
This consent solicitation statement/prospectus is being delivered to you on behalf of the Islet Sciences board of directors to request that holders of Islet Sciences common stock as of the record date of , 2014 execute and return written consents to approve the Merger Agreement and the Merger.
This consent solicitation statement/prospectus describes the proposed Merger and the actions to be taken in connection with the Merger and provides additional information about the parties involved. Please give this information your careful attention. A copy of the Merger Agreement is attached as Annex A to this consent solicitation statement/prospectus.
A summary of the dissenter’s rights that may be available to you is described below under “Dissenter’s Rights.” Please note that if you wish to exercise dissenter’s rights you must not sign and return a written consent approving the Merger Agreement and the Merger. However, so long as you do not return a consent form at all, it is not necessary to affirmatively vote against or disapprove the Merger Agreement and the Merger. In addition, you must take all other steps necessary to perfect your dissenter’s rights.
The Islet Sciences board of directors has carefully considered the Merger and the terms of the Merger Agreement and has determined that the Merger and the Merger Agreement are fair, advisable and in the best interests of Islet Sciences and its stockholders.
Please complete, date and sign the written consent furnished with this consent solicitation statement/prospectus and return it promptly to Islet Sciences by one of the means described in “Solicitation of Written Consents.”
| | By Order of the Board of Directors, | |
| | | | |
| | | James Green | |
| | | President and Chief Executive Officer | |
| | | | |
TABLE OF CONTENTS
| | | Page |
| ADDITIONAL INFORMATION | | | | 1 |
| | |
| QUESTIONS AND ANSWERS | | 2 |
| | |
| SUMMARY | | 5 |
| | |
| SELECTED HISTORICAL FINANCIAL DATA OF ISLET SCIENCES | | 9 |
| | |
| SELECTED HISTORICAL FINANCIAL DATA OF BHV | | 9 |
| | |
| SELECTED UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION | | 10 |
| | |
| RISK FACTORS | | 11 |
| | |
| FORWARD-LOOKING STATEMENTS | | 17 |
| | |
| SOLICITATION OF WRITTEN CONSENTS | | 18 |
| | | |
| BACKGROUND OF THE TRANSACTIONS | | 19 |
| | |
| TERMS OF THE TRANSACTIONS | | 22 |
| | |
ISLET SCIENCES’ REASONS FOR THE TRANSACTIONS; RECOMMENDATION OF THE ISLET SCIENCES BOARD | | 32 |
| | |
| INTERESTS OF DIRECTORS AND EXECUTIVE OFFICERS OF ISLET SCIENCES IN THE TRANSACTIONS | | 34 |
| | |
| ACCOUNTING TREATMENT OF THE TRANSACTIONS | | 35 |
| | |
| MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES | | 35 |
| | |
| FINANCIAL STATEMENTS | | 37 |
| | |
| ISLET SCIENCES AND BHV UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION | | 37 |
| | |
| NOTES TO THE ISLET SCIENCES AND BHV UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION | | 41 |
| | |
| CERTAIN MARKET INFORMATION WITH RESPECT TO ISLET SCIENCES COMMON STOCK | | 43 |
| | |
| DIVIDEND POLICIES AND RESTRICTIONS | | 43 |
| | |
| BUSINESS OF ISLET SCIENCES | | 43 |
| | |
| BUSINESS OF BHV | | 52 |
| | |
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF ISLET SCIENCES | | 56 |
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF BHV | | 61 |
| | |
| SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN BENEFICIAL OWNERS OF ISLET SCIENCES | | 63 |
| | |
| SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN BENEFICIAL OWNERS OF BHV | | 65 |
| | |
| SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN BENEFICIAL OWNERS OF AVOGENX AFTER THE TRANSACTIONS | | 65 |
| | |
| EXECUTIVE OFFICERS AND DIRECTORS OF AVOGENX FOLLOWING THE TRANSACTIONS | | 67 |
| | |
| COMPENSATION OF EXECUTIVE OFFICERS AND DIRECTORS | | 69 |
| | |
| CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS, AND DIRECTOR INDEPENDENCE | | 72 |
| | |
| DESCRIPTION OF AVOGENX CAPITAL STOCK | | 72 |
| | | |
| COMPARISON OF RIGHTS OF STOCKHOLDERS | | 76 |
| | | |
| DISSENTER’S RIGHTS | | 82 |
| | |
| EXPERTS | | 83 |
| | |
| WHERE YOU CAN FIND MORE INFORMATION | | 83 |
| | |
| LEGAL MATTERS | | 83 |
| | |
| INDEX TO FINANCIAL STATEMENTS | | F-1 |
| | |
| ANNEX A – Agreement and Plan of Merger | | A-1 |
| | |
| ANNEX B – Voting Agreement | | B-1 |
| | |
| ANNEX C – Sections 92A.300 to 92A.500 of the Nevada Revised Statutes | | C-1 |
| | | |
| ANNEX D - Form of Written Consent | | D-1 |
ADDITIONAL INFORMATION
This document, which forms part of a registration statement on Form S-4 filed by Avogenx, Inc. (“Avogenx”) with the Securities and Exchange Commission (the “SEC”), constitutes a prospectus of Avogenx under Section 5 of the Securities Act of 1933, as amended (the “Securities Act”), with respect to the shares of Avogenx common stock to be issued to Islet Sciences, Inc. (“Islet Sciences”) stockholders pursuant to the Agreement and Plan of Merger, dated as of September 30, 2014 (the “Merger Agreement”), by and among Islet Sciences, Brighthaven Ventures, L.L.C. (“BHV”), Avogenx, Islet Merger Sub, Inc. (“Islet Merger Sub”), and each of the members of BHV (the “BHV Members”). This document also constitutes a consent solicitation statement of Islet Sciences with respect to the proposal to approve the Merger Agreement. Pursuant to the Merger Agreement, Islet Merger Sub will merge with and into Islet Sciences with Islet Sciences as the surviving entity (the “Merger”), and Avogenx will acquire all of the outstanding membership interests of BHV in consideration for the issuance of an aggregate of 30 million shares of common stock of Avogenx to the BHV Members, to be adjusted for the Exchange Ratio (as defined below), and additional milestone payments described below, which are based on the development of BHV’s drug compound, Remogliflozin (the “BHV Securities Exchange” and together with the Merger, the “Transactions”). In connection with the Transactions, shares of Islet Sciences common stock outstanding immediately prior to the effective time of the Merger will be converted into the right to receive shares of Avogenx common stock at the conversion rate of not less than three and not more than 40 shares of Islet Sciences common stock for one share of Avogenx common stock, with the effective number of shares of Islet Sciences common stock convertible into one share of Avogenx common stock to be determined by the Islet Sciences board of directors prior to the effective time of the Merger (the “Exchange Ratio”). Following the completion of the Transactions, Islet Sciences and BHV will each be wholly owned subsidiaries of Avogenx.
This consent solicitation statement/prospectus incorporates important business and financial information about Islet Sciences that is contained in documents filed with the SEC and that is not included in or delivered with this document. You may obtain this information without charge through the SEC’s website (www.sec.gov) or upon your written or oral request from Islet Sciences at 6601 Six Forks Rd, Suite 140, Raleigh, North Carolina 27615, Attn: Investor Relations, www.isletsciences.com, by emailing info@isletsciences.com or telephoning (919) 480-1518. To ensure timely delivery, any request should be made no later than , 2014. For additional details about where you can find information about Islet Sciences, see “Where You Can Find More Information.”
Information on the internet websites of Islet Sciences or BHV, or any subsidiary of Islet Sciences, is not part of this document. You should not rely on that information in deciding whether to approve the Merger Agreement and the Merger unless that information is in this document.
You should rely only on the information contained in this document. We have not authorized anyone to provide you with different information. This document is dated , 2014. You should not assume that information contained in this document is accurate as of any date other than that date. Neither the mailing of this document to Islet Sciences stockholders nor the issuance by Avogenx of common stock in the Merger will create any implication to the contrary.
This consent solicitation statement/prospectus does not constitute an offer to sell, or a solicitation of an offer to buy, any securities, or the solicitation of a consent, in any jurisdiction in which or from any person to whom it is unlawful to make any such offer or solicitation in such jurisdiction. Islet Sciences has supplied all information relating to Islet Sciences contained in this document, and BHV has supplied all information relating to BHV contained in this document.
QUESTIONS AND ANSWERS
The following are some questions that you, as a stockholder of Islet Sciences, may have regarding the Merger and the Merger Agreement, and brief answers to those questions. Islet Sciences urges you to read carefully the remainder of this consent solicitation statement/prospectus because the information in this section may not provide all the information that might be important to you with respect to the Merger. Additional important information is also contained in the annexes and exhibits to this consent solicitation statement/prospectus. See “Where You Can Find More Information.”
Q: What is the proposed transaction?
A: Islet Sciences has entered into an agreement with BHV, Avogenx, Islet Merger Sub, and the BHV Members, pursuant to which Islet Merger Sub will merge with and into Islet Sciences with Islet Sciences as the surviving entity, and Avogenx will acquire all of the outstanding membership interests of BHV in consideration for the issuance of an aggregate of 30 million shares of common stock of Avogenx to the BHV Members, to be adjusted for the Exchange Ratio, and additional milestone payments described below, which are based on the development of Remogliflozin. Following the completion of the Transactions, Islet Sciences and BHV will each be wholly owned subsidiaries of Avogenx.
The Merger Agreement is included as Annex A to this consent solicitation statement/prospectus. It is the legal document that governs the Transactions.
Q: Who is soliciting my written consent?
A: The Islet Sciences board of directors is providing these consent solicitation materials to you. These materials also constitute a prospectus with respect to the Avogenx common stock issuable to Islet Sciences stockholders and the BHV Members in connection with the Transactions.
Q: What am I being asked to approve?
A: You are being asked to approve the Merger Agreement and the Merger.
Q: Have the terms of the Transactions been evaluated by independent directors of Islet Sciences?
A: Yes. The independent members of the Islet Sciences board of directors, comprised of Dr. Eric Barnett and Dr. Michael Luther, considered and negotiated the terms and conditions of the Merger Agreement.
Q: Who is entitled to give a written consent?
A: The Islet Sciences board of directors has set , 2014 as the record date for determining holders of shares of Islet Sciences common stock entitled to execute and deliver written consents with respect to this solicitation. Holders of Islet Sciences common stock on the record date may (i) execute a written consent to approve the Merger Agreement (which is equivalent to a vote in favor of approval of the Merger Agreement), or (ii) choose not to execute the written consent (which is equivalent to a vote against approval of the Merger Agreement).
Q: What happens if I sell my shares of Islet Sciences common stock after the record date but before the Merger?
A: If you sell or otherwise transfer your shares of Islet Sciences common stock after the record date but before the consummation of the Merger, you will retain your right to execute a written consent to approve the Transactions (which is equivalent to a vote in favor of the proposal), or choose not to execute the written consent (which is equivalent to a vote against the proposal).
Q: What will I receive in the Merger?
A: Under the Merger Agreement, shares of Islet Sciences common stock outstanding immediately prior to the effective time of the Merger will be converted into the right to receive shares of Avogenx common stock at the conversion rate of not less than three and not more than 40 shares of Islet Sciences common stock for one share of Avogenx common stock, with the effective number of shares of Islet Sciences common stock convertible into one share of Avogenx common stock to be determined by the Islet Sciences board of directors prior to the effective time of the Merger. As of November 24, 2014, 66,928,724 shares of common stock of Islet Sciences were outstanding. Upon completion of the Merger, shares of common stock of Islet Sciences held by such holders will be cancelled.
Q: What is the recommendation of the Islet Sciences board of directors?
A: The Islet Sciences board of directors, with the directors who are the members of BHV abstaining from voting, has determined that the Merger Agreement and the Merger are advisable and fair to and in the best interests of Islet Sciences and its stockholders, and recommends that Islet Sciences’ stockholders approve the Merger Agreement and the Merger.
Q: What stockholder consent is required to approve the Merger?
A: We cannot complete the Merger unless Islet Sciences stockholders approve the Merger Agreement and the Merger. Approval of the Merger Agreement and the Merger require the approval of the holders of a majority of the outstanding shares of Islet Sciences common stock.
On September 30, 2014, Islet Sciences entered into a series of voting agreements, substantially in the form attached to this consent solicitation statement/prospectus as Annex B, with certain directors of Islet Sciences that own shares of Islet Sciences common stock and holders of more than 5% of Islet Sciences outstanding common stock, which collectively own approximately 36.8% of Islet Sciences’ outstanding common stock. Pursuant to these voting agreements, these stockholders agreed to deliver a written consent in favor of the approval of the Merger Agreement and the Merger following the delivery of this consent solicitation statement/prospectus upon effectiveness of the registration statement of which it is a part.
Q: How can I return my written consent?
A: If you hold shares of Islet Sciences common stock as of the record date for granting written consent and you wish to submit your consent, you must fill out the enclosed written consent, date and sign it, and promptly return it to Islet Sciences. Once you have completed, dated and signed your written consent, you may deliver it to Islet Sciences by emailing a .pdf copy of your written consent to info@isletsciences.com (Attention: Corporate Secretary) or by mailing your written consent to Islet Sciences, Inc. at 6601 Six Forks Rd, Suite 140, Raleigh, North Carolina 27615, Attention: Corporate Secretary. If you mail your written consent, please allow sufficient time for it to be delivered in advance of the delivery deadline discussed below. Islet Sciences will not be holding a stockholders’ meeting to consider this proposal, and therefore you will be unable to vote in person by attending a stockholders’ meeting.
Q: What happens if I do not return my written consent?
A: If you are a record holder of shares of Islet Sciences common stock and you do not return your written consent, that will have the same effect as a vote against the proposal to approve the Merger Agreement and the Merger.
Q: What is the deadline for returning my written consent?
A: The Islet Sciences board of directors has set , 2014 as the targeted final date for receipt of written consents, which is the date on which Islet Sciences expects to receive consents under the Voting Agreement. Islet Sciences reserves the right to extend the final date for receipt of written consents beyond , 2014. Any such extension may be made without notice to Islet Sciences stockholders. Once a sufficient number of consents to approve the Merger Agreement and the Merger have been received, the consent solicitation will conclude.
Q: If my shares of Islet Sciences common stock are held in “street name” by my broker or other nominee, will my broker or other nominee execute a written consent without instructions from me?
A: No. Your broker will not be able to execute a written consent without instructions from you. Please follow the procedure your broker provides to participate in the consent solicitation.
Q: What should I do if I receive more than one set of materials?
A: You may receive more than one set of materials for the written consent, including multiple copies of this consent solicitation statement/prospectus. For example, if you hold your shares of Islet Sciences common stock in more than one brokerage account, you will receive a separate instruction card for each brokerage account in which you hold shares. Please submit each separate instruction card or consent that you receive by following the instructions set forth in each separate instruction card or consent.
Q: Can I change or revoke my written consent?
A: Yes. If you are a record holder of shares of Islet Sciences common stock on the record date, you may change or revoke your consent at any time before the consents of a sufficient number of shares to approve the Merger Agreement and the Merger have been filed with the corporate secretary of Islet Sciences. If you wish to change or revoke your consent or prior revocation before that time, you may do so by sending in a new written consent with a later date by one of the means described in the section entitled “Solicitation of Written Consents—Submission of Consents,” or delivering a notice of revocation to the corporate secretary of Islet Sciences.
Q: Can I exercise dissenter’s rights?
A: If you are an Islet Sciences stockholder who does not approve the Merger Agreement via written consent, you may, by strictly complying with Sections 92A.300 to 92A.500 of the Nevada Revised Statutes, be entitled to the dissenter’s rights described therein. Sections 92A.300 to 92A.500 of the Nevada Revised Statutes are attached to this consent solicitation statement/prospectus as Annex C. Failure to follow precisely any of the statutory procedures set forth in Annex C may result in the loss or waiver of dissenter’s rights under Nevada law. Nevada law requires that, among other things, you send a demand for payment of fair value of your Islet shares to the surviving company in the Merger after receiving a notice from Islet Sciences that dissenter’s rights are available to you, which notice will be sent to non-consenting stockholders in the future. This consent solicitation/prospectus is not intended to constitute such a notice. Do not send in your demand prior to mailing of such notice because any demand for appraisal made prior to your receipt of such notice may not be effective to perfect your rights. See “Dissenter’s Rights.”
Q: What are the material United States federal income tax consequences of the Merger to the Islet Sciences stockholders?
A: The Merger is intended to qualify for U.S. federal income tax purposes as an exchange under Section 351 of the Internal Revenue Code of 1986, as amended (the “Code”). As a result, no gain or loss will be recognized by the stockholders of Islet Sciences as a result of the exchange of Islet Sciences shares for Avogenx shares pursuant to the Merger. In connection with the filing of the registration statement of which this document is a part, Anderson Bradshaw, PLLC has delivered an opinion to Islet Sciences that the exchange of Islet Sciences shares by the stockholders of Islet Sciences for Avogenx shares will qualify as an exchange under Section 351 of the Code and that no gain or loss recognition is incurred to the public stockholders of Islet Sciences. See “Material U.S. Federal Income Tax Consequences.”
Q: When does Islet Sciences expect to complete the Merger?
A: We anticipate that we will complete the Merger during the fourth quarter of our fiscal year ending April 30, 2014. However, we cannot assure you when or if the Merger will occur. We must first obtain the requisite approval of Islet Sciences stockholders and satisfy other conditions before we can complete the Merger.
Q: Should I send in my Islet Sciences stock certificate now?
A: No. Islet Sciences stockholders SHOULD NOT send in any stock certificates now. After the Transactions are completed, Avogenx will send you written instructions explaining how to exchange your Islet Science stock certificates.
Q: Whom should I contact if I have questions?
A: If you have questions about the Merger or the process for returning your written consent, or if you need additional copies of this document or a replacement written consent, please contact: Islet Sciences, Inc. at 6601 Six Forks Rd, Suite 140, Raleigh, North Carolina 27615, Attention: Corporate Secretary, Telephone: (919) 480-1518.
SUMMARY
This summary highlights selected information contained elsewhere in this consent solicitation statement/prospectus and does not contain all of the information that may be important to you. You should carefully read the entire consent solicitation statement/prospectus, including “Risk Factors” and the financial statements, before making an investment decision.
Unless the context requires otherwise, “we,” “us” and “our” refers to Avogenx, Inc., the issuer of the shares offered hereby, and its future wholly-owned subsidiary, Islet Sciences, as well as Islet Sciences’ direct and indirect subsidiaries. “Avogenx” refers solely to Avogenx, Inc., the issuer of the shares offered hereby. “Islet Sciences” refers to Islet Sciences, Inc., a Nevada corporation, our current public parent company individually or collectively with its subsidiaries not formed for purposes of the transactions described herein, as the context may require. “BHV” refers to Brighthaven Ventures, LLC, a North Carolina limited liability company, d/b/a BHV Pharma. When we express “beliefs” with respect to BHV, or its business, we have based those statements or beliefs on our general knowledge of the industries involved, the results of our due diligence investigations of BHV and other information provided to us by the management of BHV.
This consent solicitation statement/prospectus is being filed in contemplation of the Transactions, pursuant to which Islet Sciences will acquire BHV. This will be done pursuant to the Merger Agreement. Pursuant to the Merger Agreement, Islet Sciences has formed a wholly owned subsidiary, Avogenx, which has formed another wholly owned subsidiary, Islet Merger Sub.
Upon satisfaction or waiver of the conditions to the transactions specified in the Merger Agreement, (i) Islet Merger Sub will merge with and into Islet Sciences with Islet Sciences as the surviving entity, thus completing the Merger and (ii)Avogenx will acquire all of the outstanding membership interests of BHV in consideration for the issuance of an aggregate of 30 million shares of common stock of Avogenx to the BHV Members (to be adjusted for the Exchange Ratio) and additional milestone payments described below, which are based on the development of Remogliflozin (“Remo”), thus completing the BHV Securities Exchange. Following the completion of the Transactions, Islet Sciences and BHV will each be wholly owned subsidiaries of Avogenx.
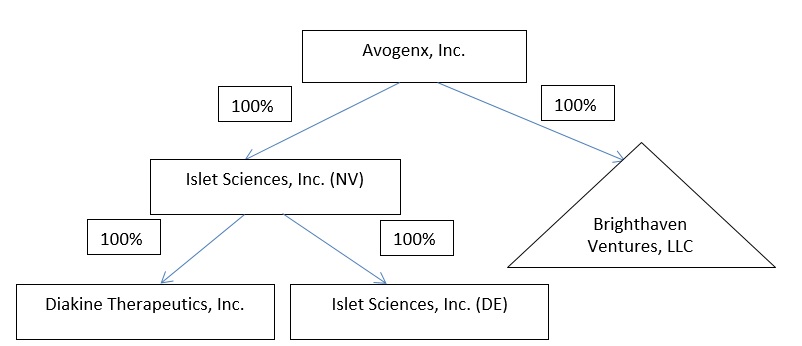
Therefore, the resulting structure will be as follows:
The Companies
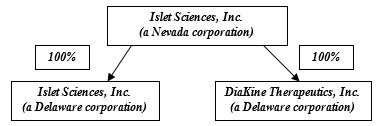
Islet Sciences, Inc.
6601 Six Forks Rd, Suite 140
Raleigh, NC 27615
(919) 480-1518
Islet Sciences is a biotechnology company engaged in the research, development, and commercialization of new medicines and technologies for the treatment of metabolic diseases and related indications where there is a significant medical need. The rising incidence of obesity is associated with many obesity-related health complications, including cardiovascular disease, diabetes, hyperlipidemia, hypertension, nonalcoholic fatty liver disease/steatohepatitis (NAFLD/NASH). This constellation is also recognized as the metabolic syndrome and is characterized by underlying insulin resistance. See “Comparative Review of Diets for the Metabolic Syndrome: Implications for Nonalcoholic Fatty Liver Disease,” The American Journal of Clinical Nutrition, Angela M. Zivkovic, J. Bruce German and Arun J. Sanyal (December 3, 2007). These various diseases have interrelated risk factors and markers, such that often treatment of one disease may allow new therapies and opportunities for treatment in one of these related indications.
Islet Sciences is developing the following therapeutic product candidates to address the needs of patients suffering from metabolic disease:
● | ISLT-P, an implantable suspension of encapsulated insulin-producing porcine islet cells, for the treatment of insulin-dependent diabetes; |
| ● | ISLT-2669, a novel lead IL-12 small molecule inhibitor selected for preclinical development for treatment of type 2 diabetes. This IL12 inhibitor blocks the auto-immune and inflammatory cascade initiated by IL-12 receptor activation; and |
| ● | ISLT-LSF Analogs, a library of small molecule lisofylline analogs that block inflammatory actions of cytokines that destroy insulin-producing beta cells, for diabetes and diabetes-related complications. |
Islet Sciences is also developing the following diagnostic product candidate to better enable patients and their physicians to understand disease diagnosis and progression.
| ● | ISLT-Bdx, a PCR based molecular diagnostic measuring hypomethylated beta cell-derived DNA as a biomarker of beta cell loss for the early detection of type 1 diabetes or onset of insulin dependent type 2 diabetes. |
Avogenx, Inc.
6601 Six Forks Rd, Suite 140
Raleigh, NC 27615
(919) 480-1518
Avogenx is a direct, wholly owned subsidiary of Islet Sciences formed solely to effect the Transactions and has not conducted any business. Pursuant to the Merger Agreement described in this consent solicitation statement/prospectus, Avogenx will, by operation of law, become the parent company of Islet Sciences and BHV, which will both survive as wholly owned subsidiaries of Avogenx.
Islet Merger Sub, Inc.
6601 Six Forks Rd, Suite 140
Raleigh, NC 27615
(919) 480-1518
Islet Merger Sub is a direct wholly owned subsidiary of Avogenx formed solely to effect the Transactions and has not conducted and will not conduct any business during any period of its existence. Pursuant to the Merger Agreement, Islet Merger Sub will merge with and into Islet Sciences with Islet Sciences continuing as the surviving corporation and a wholly owned subsidiary of Avogenx.
Brighthaven Ventures, LLC
6601 Six Forks Rd, Suite 140
Raleigh, NC 27615
(919) 480-1518
BHV is a privately held pharmaceutical company developing the SGLT2 inhibitor remogliflozin etabonate (“remogliflozin”) for type 2 diabetes and non-alcoholic steatohepatitis (“NASH”). Remogliflozin is currently in phase II clinical development.
The Transactions
In connection with reorganization of its holding company structure and to complete the acquisition of BHV, Islet Sciences has formed Avogenx, and Avogenx has formed Islet Merger Sub.
Upon satisfaction or waiver of the conditions to the Transactions, Islet Merger Sub will merge with and into Islet Sciences with Islet Sciences as the surviving entity. As a result of the Merger, Islet Sciences will, by operation of law, become a wholly owned subsidiary of Avogenx. The officers and directors of Avogenx at the effective time of the Merger will continue to be the officers and directors of Avogenx immediately after consummation of the Merger. Shares of Islet Sciences common stock outstanding immediately prior to consummation of the Merger will be converted into the right to receive shares of Avogenx common stock at the conversion rate of not less than three and not more than 40 shares of Islet Sciences common stock for one share of Avogenx common stock, with the effective number of shares of Islet Sciences common stock convertible into one share of Avogenx common stock to be determined by the Islet Sciences board of directors prior to the effective time of the Merger. We anticipate that Avogenx common stock will be quoted on the OTCQB and will trade under a new ticker symbol.
Upon satisfaction or waiver of the conditions to the Transactions specified in the Merger Agreement, Avogenx will acquire all of the outstanding membership interests of BHV in consideration for the issuance of an aggregate of 30 million shares of common stock of Avogenx to the BHV Members to be adjusted for the Exchange Ratio and additional milestone payments described below, which are based on the development of Remogliflozin. As a result of the BHV Securities Exchange, BHV will become a wholly-owned subsidiary of Avogenx.
Treatment of Islet Warrants and Options
Each outstanding Islet warrant, option or other right to acquire Islet Sciences common stock will upon closing of the Merger cease to represent a right to purchase shares of Islet Sciences common stock and will instead represent the right to purchase shares of Avogenx common stock equal to the number of shares of Islet Sciences common stock subject to such Islet warrant, option or other right to acquire Islet Sciences common stock immediately prior to the closing of the Merger, as adjusted for the Exchange Ratio. See “Terms of the Transactions— Islet Sciences Warrants and Stock Options.”
Ownership of Avogenx after the Transactions
Subject to the adjustment for the Exchange Ratio, thirty million shares of Avogenx common stock will be issuable to the BHV Members upon consummation of the BHV Securities Exchange. Based upon the 66,928,724 shares of Islet Sciences common stock outstanding on November 24, 2014, a total of 96,928,724 shares of Avogenx common stock would be outstanding after the Transactions, subject to the adjustment for the Exchange Ratio. Based on such shares outstanding on November 24, 2014, the shares issuable to the BHV Members in the BHV Securities Exchange would represent approximately 31.0% of the outstanding shares of Avogenx common stock immediately after the closing of the BHV Securities Exchange. The BHV Members can also receive additional milestone payments in Avogenx shares, as described below, which milestones are based on the development of Remogliflozin.
Recommendation of the Board of Directors of Islet
The Islet Sciences board of directors recommends that the Islet Sciences stockholders approve the Merger Agreement and the Merger by executing and delivering the written consent furnished with this consent solicitation/prospectus. The Islet board of directors believes the consideration to be received by the Islet Sciences stockholders is fair, advisable and in the best interests of Islet Sciences and its stockholders. See “Islet Sciences’ Reasons for the Acquisition of BHV; Recommendation of the Islet Sciences Board.”
Solicitation of Written Consents
Approval of the Merger Agreement and the Merger requires the approval of the holders of a majority of the outstanding shares of Islet Sciences common stock. See “Solicitation of Written Consents.”
Voting Agreement
On September 30, 2014, certain stockholders of Islet Sciences, who collectively hold approximately 36.8% of the outstanding shares of Islet Sciences common stock, entered into voting agreements with Islet Sciences pursuant to which they have agreed to execute and return consents with respect to their shares of Islet Sciences common stock approving the Merger Agreement and the Merger. See “Security Ownership of Management and Certain Beneficial Owners of Islet Sciences.”
Comparison of the Rights of Stockholders of Avogenx and Islet Sciences
If Islet Sciences stockholders approve the Merger Agreement and exchange their stock for Avogenx common stock, they will become Avogenx stockholders and will have rights different from those they currently have as Islet stockholders because Avogenx is a Delaware corporation and is subject to the requirements of Delaware law and its Certificate of Incorporation, which are different from the requirements that govern Islet Sciences as a Nevada corporation. See “Comparison of Rights of Stockholders.”
Conditions to Completion of the Transactions
The completion of the Transactions depends upon the satisfaction or waiver of a number of conditions which are described in more detail later in this consent solicitation statement/prospectus, including, among other things:
| | ● | | the absence of any legal prohibition on completion of the Transactions; |
| | ● | | the material accuracy, as of the date of the Merger Agreement and as of closing, of the representations and warranties made by the parties in the Merger Agreement and material compliance by the parties with their respective obligations under the Merger Agreement; |
| | ● | | the effectiveness of the registration statement of which this consent solicitation statement/prospectus is a part; |
| | ● | | the absence of any change since the date of the Merger Agreement that would reasonably be expected to have a Material Adverse Effect, subject to certain exceptions, upon either BHV or Islet Sciences. |
Dissenter’s Rights
Under the Nevada Revised Statutes (the “NRS”), if you do not wish to accept the consideration provided for in the Merger Agreement and the proposed Merger is consummated, you have the right (assuming all statutory conditions are met) to receive payment in cash for the fair value of your Islet Sciences common stock. To exercise your dissenter’s rights, you must (among other things) submit a written demand for payment to Islet Sciences, within 30 days after the date of delivery of a separate notice that will be mailed to non-consenting stockholders no later than 10 days after the effective date of the Merger. The demand for appraisal must not be sent prior to the date of such separate notice. Only the stockholder of record may submit a demand for payment; therefore, if your shares are not registered in your name, you will not be able to take these steps yourself, but must have them done by the record holder. To preserve your right to demand payment for the fair value of your shares, you must not submit a written consent in favor of approving the Merger Agreement, or otherwise consent to or vote FOR the approval of the Merger Agreement. However, so long as you do not return a consent form at all, it is not necessary to affirmatively vote against or disapprove the Merger Agreement. In addition, you must hold your shares continuously through the effective date of the Merger, meet other statutory conditions and take all other steps to perfect your dissenter’s right, which are not all listed here. A copy of the relevant statute, Sections 92A.300 to 92A.500 of the NRS, is appended to this consent solicitation statement/prospectus as Annex C for information purposes only. This consent solicitation statement/prospectus is not intended to constitute the notice of dissenter’s rights under Section 92A.430. See “Dissenter’s Rights.”
Material U.S. Federal Income Tax Consequences
Subject to the limitations and qualifications described in “Material U.S. Federal Income Tax Consequences” below, the exchange by U.S. holders of shares of Islet Sciences common stock for shares of Avogenx common stock pursuant to the Merger will constitute an exchange to which Section 351 of the Internal Revenue Code of 1986 (the “Code”) applies. As a result, no gain or loss will be recognized by the stockholders of Islet Sciences as a result of the exchange of Islet Sciences shares for Avogenx shares pursuant to the Merger. Your tax consequences will depend on your own situation. You should consult your tax advisor to fully understand the tax consequences of the Transactions to you.
In connection with the filing of the registration statement of which this document is a part, Anderson Bradshaw, PLLC has delivered an opinion to Islet Sciences that the exchange of Islet Sciences shares by the stockholders of Islet Sciences for Avogenx shares will qualify as an exchange under Section 351 of the Code and that no gain or loss recognition is incurred to the public stockholders of Islet Sciences.
Risk Factors
In evaluating the Merger and Merger Agreement, you should carefully read this consent solicitation statement/prospectus and the documents attached to this consent solicitation statement/prospectus, and especially consider the factors discussed in the section entitled “Risk Factors.”
SELECTED HISTORICAL FINANCIAL DATA OF ISLET SCIENCES
Annual Data
Set forth below is Islet Sciences’ selected historical consolidated financial and other operating data. Islet Sciences’ selected historical consolidated financial data and other data set forth below have been derived from its audited consolidated financial statements and should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations of Islet Sciences” and the Consolidated Financial Statements of Islet Sciences and Notes thereto set forth elsewhere in this consent solicitation statement/prospectus.
| | Years Ended April 30, | | Three Months Ended July 31, |
| | | 2014 | | | 2013 | | | 2014 | | | 2013 | |
| | | | | | | | | (Unaudited) | |
| | | | | | | | | | | | | |
| Statement of Operations Data: | | | | | | | | | | | | |
| Net sales | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| Loss from operations | | $ | (2,935,034 | ) | | $ | (12,122,474 | ) | | $ | (463,730 | ) | | $ | (1,292,623 | ) |
| Net loss per common share basic and diluted: | | $ | (0.05 | ) | | $ | (0.21 | ) | | $ | (0.01 | ) | | $ | (0.02 | ) |
| Net loss | | $ | (2,946,430 | ) | | $ | (11,702,599 | ) | | $ | (464,530 | ) | | $ | (1,294,854 | ) |
Weighted average common shares outstanding basic and diluted (in thousands) | | | 58,215 | | | | 54,512 | | | | 67,070 | | | | 56,715 | |
| | | | | | | | |
| Balance sheet data (at period end): | | | | | | | | | | | | | | | | |
| Total assets | | $ | 4,620,187 | | | $ | 3,642,889 | | | $ | 3,936,243 | | | $ | 3,631,814 | |
| Total liabilities | | $ | 4,343,161 | | | $ | 3,011,084 | | | $ | 3,783,763 | | | $ | 4,294,863 | |
| Other financial data: | | | | | | | | | | | | | | | | |
| Net cash used in operating activities | | $ | (972,928 | ) | | $ | (3,903,163 | ) | | $ | (614,121 | ) | | $ | (35,836 | ) |
| Net cash provided by (used in) investing activities | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| Net cash provided by (used in) financing activities | | $ | 2,110,719 | | | $ | 1,998,220 | | | $ | (69,823 | ) | | $ | 32,365 | |
SELECTED HISTORICAL FINANCIAL DATA OF BHV
Annual and Interim Data
Set forth below is BHV’s selected historical consolidated financial and other operating data. BHV’s selected historical consolidated financial data and other data set forth below have been derived from its audited consolidated financial statements and should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations of BHV” and the Consolidated Financial Statements of BHV and Notes thereto set forth elsewhere in this consent solicitation statement/prospectus.
| | | | | | | | | | | | | |
| | | Years Ended April 30, | | | Three Months Ended July 31, | |
| | | 2014 | | 2013 | | | 2014 | | | 2013 | |
| | | | | | | | (Unaudited) | |
| | | | | | | | | | | | |
| Statement of Operations Data: | | | | | | | | | | | | |
| Net sales | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| Loss from operations | | $ | (109,557 | ) | | $ | (96,977 | ) | | $ | (99,148 | ) | | $ | (16,468 | ) |
| Net loss | | $ | (125,520 | ) | | $ | (116,775 | ) | | $ | (102,448 | ) | | $ | (21,232 | ) |
| Balance sheet data (at period end): | | | | | | | | | | | | | | | | |
| Total assets | | $ | 15,906 | | | $ | 16,408 | | | $ | 12,587 | | | $ | 28,754 | |
| Total liabilities | | $ | 485,808 | | | $ | 360,790 | | | $ | 584,937 | | | $ | 391,458 | |
| Other financial data: | | | | | | | | | | | | | | | | |
| Net cash used in operating activities | | $ | (502 | ) | | $ | (36,562 | ) | | $ | (3,319 | ) | | $ | (21,232 | ) |
SELECTED UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
The following selected unaudited pro forma condensed combined financial information has been prepared to illustrate the effect of the acquisition of BHV and has been prepared for informational purposes only and should be read in conjunction with the unaudited pro forma condensed combined financial information and the accompanying notes thereto, contained elsewhere in this consent solicitation statement/prospectus. The selected unaudited pro forma condensed combined financial information is based upon the historical condensed consolidated financial statements of Islet Sciences and BHV and notes thereto and should be read in conjunction with the historical financial statements and the accompanying notes of Islet Sciences and BHV, each of which are contained in this consent solicitation statement/prospectus. The selected unaudited pro forma condensed combined financial information does not give effect to the proposed exchange ratio of between 3 and 40 shares of common stock of Islet Sciences for one shares of common stock of Avogenx.
The historical consolidated financial information has been adjusted in the selected unaudited pro forma combined financial statements to give effect to pro forma events that are (1) directly attributable to the acquisition of BHV, (2) factually supportable, and (3) with respect to the statement of operations, expected to have a continuing impact on the combined results of Islet Sciences and BHV. The following selected unaudited pro forma combined financial information depicts the combined balance sheet as of July 31, 2014 and the combined statement of operations for the three months ended July 31, 2014 and for the year ended April 30, 2014 as if the acquisition had occurred. The selected unaudited pro forma combined statement of operations has been prepared assuming the acquisition of BHV had been completed on May 1, 2013, the beginning of the earliest period presented. The selected unaudited pro forma combined balance sheet has been computed assuming the acquisition of BHV had been completed on July 31, 2014. The selected unaudited pro forma financial information has been adjusted with respect to certain aspects of the acquisition of BHV to reflect:
| | ● | | changes in assets and liabilities (as disclosed in more detail elsewhere in this consent solicitation statement/prospectus) to record their preliminary estimated fair values at the closing date of the acquisition and changes in certain expenses resulting therefrom; |
| | ● | | additional indebtedness, including, but not limited to, interest expense; and |
The selected unaudited pro forma combined financial information was prepared in accordance with the acquisition method of accounting under accounting principles generally accepted in the United States, or GAAP, and the regulations of the SEC, and is not necessarily indicative of the financial position or results of operations that would have occurred if the acquisition of BHV had been completed on the dates indicated, nor is it indicative of the future operating results or financial position of Islet Sciences and BHV. Assumptions and estimates underlying the pro forma adjustments are described in the notes accompanying the unaudited pro forma condensed combined financial information, which should be read in connection with the selected unaudited pro forma combined financial information. The accounting for the acquisition is dependent upon certain valuations and other studies that have yet to commence or progress to a stage where there is sufficient information for a definitive measurement. Due to the fact that the selected unaudited pro forma condensed combined financial information has been prepared based upon preliminary estimates, the final amounts recorded for the acquisition may differ materially from the information presented. These estimates are subject to change pending further review of the assets acquired and liabilities assumed.
The selected unaudited pro forma condensed combined statement of operations does not give effect to the legal and regulatory costs expected to be incurred by Islet Sciences and BHV in connection with the Transactions.
| | | Pro Forma Islet Sciences and BHV | |
| | | Year Ended April 30, 2014 | | | Three Months Ended July 31, 2014 | |
| | | | |
Summary of Net Revenue and Income (Loss): | | | | | | |
| Net revenue | | $ | — | | | $ | — | |
| Operating expenses: | | | | | | | | |
| Research and development | | | 656,319 | | | | 70,289 | |
| General and administrative | | | 2,294,686 | | | | 492,589 | |
| Impairment loss | | | 93,586 | | | | — | |
| | | | | | | | | |
| Total operating expenses | | | 3,044,591 | | | | 562,878 | |
| | | | | | | | | |
| Loss from operations | | | (3,044,591 | ) | | | (562,878 | ) |
| | | | | | | | | |
| Other income (expense): | | | | | | | | |
| Interest expense | | | (27,360 | ) | | | (4,100 | ) |
| | | | | | | | | |
| Total other income (expense) | | | (27,360 | ) | | | (4,100 | ) |
| | | | | | | | | |
| Loss before income taxes | | | (3,071,951 | ) | | | (566,978 | ) |
| | | | | | | | | |
| Net loss | | $ | (3,071,951 | ) | | $ | (566,978 | ) |
| | | | | | | | | |
| Basic and diluted net loss per share attributable to common stockholders | | $ | (0.03 | ) | | $ | (0.01 | ) |
| | | | | | | | | |
Outstanding Shares: | | | | | | | | |
| Weighted average shares used in the computation of basic and diluted net loss per share | | | 88,214,535 | | | | 97,069,789 | |
| | | | | | | | | |
Period-End Financial Position: | | | | | | | | |
| Total assets | | | | | | | $29,394,888 | |
| Total liabilities | | | | | | | $24,742,408 | |
| Stockholders’ equity | | | | | | | $4,652,480 | |
RISK FACTORS
You should carefully consider the following risks associated with the common stock covered hereby. Additional risks and uncertainties not presently known to us or which are not currently believed to be important also may adversely affect the Transactions and Avogenx following the Transactions.
We have a limited operating history and are a development stage company.
We started our operations in 2010. To date, we have had no sales and profits. We cannot assure investors that we will ever become or remain profitable. An investment in our securities is subject to all of the risks involved in a newly established business venture. Potential investors should be aware of the problems, delays, expenses, and difficulties experienced by companies in the early developmental stage, which generally include unanticipated problems and additional costs relating to the commencement of operations and implementation of a business plan.
Future funding requirements
To date, we have not generated any revenue from product sales. We do not know when, or if, we will generate any revenue from product sales. We do not expect to generate any revenue from product sales unless and until we obtain regulatory approval of our product candidates and our companion diagnostic products or any of our other product candidates. At the same time, we expect our expenses to increase in connection with our ongoing development activities, particularly as we continue the research, development and clinical trials of, and seek regulatory approval for, our product candidates and companion diagnostic. In addition, subject to obtaining regulatory approval of any of our product candidates and companion diagnostic, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. We anticipate that we will need significant additional funding, which may not be available to us on acceptable terms, or at all, and if not so available, may require us to delay, limit, reduce, or cease our operations.
Until such time that we can generate meaningful revenue from product sales, if ever, we expect to finance our operating activities through public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements, and other collaborations, strategic alliances and licensing arrangements or a combination of these approaches. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our common stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through government or other third-party funding, marketing and distribution arrangements or other collaborations, or strategic alliances or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us.
There are risks associated with our proposed acquisition of BHV.
On September 30 2014, Islet Sciences entered into the Merger Agreement with BHV. If all conditions to closing in the Merger Agreement are met, Islet Sciences will complete the acquisition of BHV, upon the effectiveness of the registration of which this consent solicitation statement/prospectus is a part, and the receipt of the requisite approval of Islet Sciences stockholders. The addition of BHV’s Remogliflozin to our pipeline is a key element of our strategy to transition into a clinical stage company and attract additional funding. If the Transactions are not completed or their completion is delayed, Islet Sciences’ strategy, results of operations and consolidated financial condition may be severely and adversely impacted.
Completion of the Transactions is subject to many conditions and if these conditions are not satisfied or, where permissible, waived, the merger will not be completed.
The obligations of Islet Sciences and BHV to complete the Transactions are subject to satisfaction or, where permissible, waiver of a number of conditions, including, among others: (i) Islet Sciences must have obtained stockholder approval of the Merger Agreement and the Merger, (ii) all authorizations, consents, orders or approvals of, or declarations or filings with, and all expirations of waiting periods required from, any governmental authority under applicable laws shall have been filed, have occurred or been obtained, and all such requisite regulatory approvals shall be in full force and effect, (iii) shares of Avogenx issuable pursuant to the Merger Agreement shall have been authorized for quotation on the OTCQB, (iv) the registration statement of which this consent solicitation statement/prospectus is a part shall have been declared effective, is not subject to any stop order and no proceeding for that purpose, and no similar proceeding with respect to this consent solicitation statement/prospectus, shall have been initiated or threatened in writing by the SEC or its staff, (v) no temporary restraining order, preliminary or permanent injunction or other order issued by any court of competent jurisdiction or other legal restraint or prohibition preventing the consummation of the Transactions shall be in effect. For a more complete summary of the conditions that must be satisfied (or, where permissible, waived) prior to completion of the Transactions, see “Terms of the Transactions – Terms of the Merger Agreement – Conditions to Completion of the Transactions.” There can be no assurance that the conditions to closing of the Transactions will be satisfied or, where permissible, waived, or that the Transactions will be completed. Further, it is possible that one or more of the conditions to closing the Transactions will not be met and that the party for whom the condition exists will waive the condition and the Transactions will be completed anyway.
Shares of Avogenx common stock to be received by Islet Sciences stockholders as a result of the Merger will have rights different from shares of the Islet Sciences common stock.
Upon completion of the Merger, the rights of former stockholders of Islet Sciences will be governed by Avogenx’s Certificate of Incorporation and By-Laws. The rights associated with Islet Sciences common stock are different from the rights associated with Avogenx common stock, especially because Islet Sciences is a Nevada corporation and Avogenx is a Delaware corporation. Please see the section entitled “Comparison of Rights of Stockholders” beginning on page 76 of this consent solicitation statement/prospectus for a discussion of the different rights associated with Avogenx common stock.
Islet Sciences will incur significant transaction and merger-related costs in connection with the Transactions.
Islet Sciences expects to incur a number of costs associated with the Transactions. The significant costs associated with the Transactions include, among others, professional fees and expenses, filing fees due in connection with filings required under applicable law and filing fees and printing and mailing costs for this consent solicitation statement/prospectus. Some of these costs have already been incurred or may be incurred regardless of whether the Transactions are consummated, including a portion of the professional fees and expenses and filing fees for this consent solicitation statement/prospectus.
The unaudited pro forma condensed combined financial statements included in this consent solicitation statement/prospectus are preliminary and the actual financial condition and results of operations of Avogenx after the Transactions may differ materially.
The unaudited pro forma combined financial statements in this consent solicitation statement/prospectus are presented for illustrative purposes only and are not necessarily indicative of what Avogenx’s actual financial condition or results or operations would have been had the Transactions been completed on the dates indicated. The unaudited pro forma condensed combined financial statements reflect adjustments, which are based upon preliminary estimates, to record the BHV identifiable assets acquired and liabilities assumed at fair value. The purchase price allocation reflected in this document is preliminary, and final allocation of the purchase price will be based upon the actual purchase price and the fair value of the assets and liabilities of BHV as of the date of the completion of the Transactions. Accordingly, the final acquisition accounting adjustments may differ materially from the pro forma adjustments reflected in this document. For more information, see “Islet Sciences and BHV Unaudited Pro Forma Condensed Combined Financial Information.”
If we cease to continue as an operational entity, due to lack of funding or otherwise, you may lose your entire investment.
Our current plans indicate that we will need substantial additional capital for research and development, including costs associated with developing our technology and conducting clinical trials of our product candidates, before we have any anticipated revenue generating products.
When we require additional funds, general market conditions or the then-current market price of our common stock may not support capital raising transactions such as additional public or private offerings of our common stock. If we require additional funds and we are unable to obtain them on a timely basis or on terms favorable to us, we may be required to scale back our development of new products, sell or license some or all of our technology or assets, or curtail or cease operations.
You may experience dilution of your ownership interests due to the future issuance of additional shares of our common stock.
Avogenx may in the future issue additional shares of common stock or other securities, resulting in the dilution of the ownership interests of its common stockholders. Avogenx is currently authorized to issue two hundred million shares of common stock and ten million shares of preferred stock with such designations, preferences and rights as determined by its board of directors. Issuance of additional shares of common stock may substantially dilute the ownership interests of Avogenx’s stockholders. Avogenx may also issue additional shares of its common stock or other securities that are convertible into or exercisable for common stock in connection with the hiring of personnel, future acquisitions, future public or private placements of our securities for capital raising purposes, or for other business purposes. Any such issuance would further dilute the interests of Avogenx’s stockholders.
There has been no active public market for our securities.
There has been no active public market for Islet Sciences common stock and may not be for Avogenx common stock. An active public market for our common stock may not develop or be sustained. The market price of our common stock may fluctuate significantly in response to factors, some of which are beyond our control. These factors include product liability claims or other litigation, the announcement of new pharmaceuticals or pharmaceutical enhancements by our competitors, developments concerning intellectual property rights and regulatory approvals, quarterly variations in competitors’ results of operations, changes in earnings estimates or recommendations by securities analysts, developments in our industry, and general market conditions and other factors, including factors unrelated to our operating performance.
Avogenx’s common stock is likely to be considered a "penny stock" and may be difficult to sell.
The SEC has adopted regulations which generally define “penny stock” to be an equity security that has a market or exercise price of less than $5.00 per share, subject to specific exemptions. The market price of Avogenx’s common stock is likely to be below $5.00 per share, as the Islet Sciences common stock is currently below this level, and therefore will be designated as a “penny stock” according to SEC rules. This designation requires any broker or dealer selling these securities to disclose certain information concerning the transaction, obtain a written agreement from the purchaser and determine that the purchaser is reasonably suitable to purchase the securities. These rules may restrict the ability of brokers or dealers to sell such shares and may affect the ability of investors to sell their shares and the ability of investors to buy shares. In addition, since Islet Sciences’ common stock is currently quoted on the OTC Bulletin Board, investors may find it difficult to obtain accurate quotations of the stock and may find few buyers to purchase the stock or a lack of market makers to support the stock price.
No assurance of future successful development.
Prospects for companies in the business of development of new drug products generally are uncertain given the nature of the industry and, accordingly, investments in such companies are highly speculative.
Our products may not be successfully developed or commercialized, which would harm us and force us to curtail our operations.
We may not be able to obtain regulatory approvals for product candidates we develop, to enter clinical trials for any of our product candidates, or to commercialize any products, on a timely basis or at all. If we are unable, for technological or other reasons, to complete the development, introduction or scale-up of manufacturing of potential products, or if our products do not achieve a significant level of market acceptance, we would be forced to curtail or cease operations.
Any product candidate we advance into clinical trials may cause undesirable side effects that could delay or prevent its regulatory approval or commercialization.
Undesirable side effects caused by any product candidate we advance into clinical trials could interrupt, delay or halt clinical trials and could result in the denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications. This, in turn, could prevent us from commercializing these or any other product candidate we advance into clinical trials.
Any one or a combination of these events could prevent us from achieving or maintaining market acceptance of the affected product or could substantially increase the costs and expenses of commercializing the product, which in turn could delay or prevent us from generating significant revenues from the sale of the product.
If we receive regulatory approval we will also be subject to ongoing FDA obligations and continued regulatory review such as continued safety reporting requirements, and we may also be subject to additional FDA post-marketing obligations. FDA and corresponding foreign regulatory requirements could adversely affect our ability to generate revenue and require additional expenditures to bring our products to market.
Any regulatory approvals that we receive for our products may also be subject to limitations on the indicated uses for which the product may be marketed or contain requirements for potentially costly post-marketing follow-up studies. In addition we or our third party manufacturers may be required to undergo a pre-approval inspection of manufacturing facilities by the FDA and foreign authorities before obtaining marketing approval and will be subject to periodic inspection by the FDA and corresponding foreign regulatory authorities under reciprocal agreements with the FDA. Such inspections may result in compliance issues that could prevent or delay marketing approval or require the expenditure of money or other resources to correct noncompliance.
If a regulatory agency discovers previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions on that product, our collaborators, or us, including requiring withdrawal of the product from the market. Our product candidates will also be subject to ongoing FDA requirements for the labeling, packaging, storage, advertising, promotion, record-keeping, and submission of safety and other post-market information on the drug. If our product candidates fail to comply with applicable regulatory requirements, a regulatory agency may:
| – | impose civil or criminal penalties; |
| – | withdraw regulatory approval; |
| – | suspend any ongoing clinical trials; |
| – | refuse to approve pending applications or supplements to approved applications filed by us or our collaborators; |
| – | impose restrictions on operations, including costly new manufacturing requirements; or |
| – | seize or detain products or require a product recall. |
Moreover, in order to market any products outside of the United States, we and our collaborators must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks described above regarding FDA approval in the United States. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others. Failure to obtain regulatory approval in other countries or any delay or setback in obtaining such approval could have the same adverse effects described above regarding FDA approval in the United States. As described above, such effects include the risk that our product candidates may not be approved for all indications requested, which could limit the uses of our product candidates and adversely impact potential royalties and product sales, and that such approval may be subject to limitations on the indicated uses for which the product may be marketed or require costly, post-marketing follow-up studies.
If we or our collaborators fail to comply with applicable domestic or foreign regulatory requirements, we and our collaborators may be subject to fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions, and criminal prosecution.
The transplantation of animal cells into humans involves risks which have resulted in additional FDA oversight and which in the future may result in additional regulation that may prevent or delay approval of our potential products and require additional expenditures to bring these products to market.
Our business involves the transplantation of animal cells into humans, a process known as xenotransplantation. Xenotransplantation poses a risk that viruses or other animal pathogens may be unintentionally transmitted to a human patient. The FDA will require testing to determine whether infectious agents, including porcine endogenous retroviruses, also known as PERV, are present in patients who have received cells, tissues, or organs from porcine sources. While PERV has not been shown to cause any disease in pigs, it is not known what effect, if any, PERV may have on humans.
Other companies are currently conducting clinical trials involving the transplantation of porcine cells into humans. The FDA requires lifelong monitoring of all recipients of xenotransplantation products. If PERV or any other virus or infectious agent is detected in tests or samples from these transplant recipients, the FDA may require that we not initiate or halt our clinical trials and perform additional tests to assess the risk of infection to potential patients. This could result in additional costs to us and delay in the trials of our products under development.
The FDA has published guidelines for development of xenotransplantation products and is continuing to monitor closely the development of such products to determine if additional guidelines are required as more data is obtained. Failure to comply with FDA guidelines may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval.
If we are unable to protect effectively our intellectual property, third parties may use our technology, which could impair our ability to compete in our markets.
Our success will depend on our ability to obtain and protect patents on our technology and to protect our trade secrets. The patents we currently license and any future patents we may obtain or license, may not afford meaningful protection for our technology and products. Others may challenge our patents and, as a result, our patents could be narrowed, invalidated, or unenforceable. In addition our current and future patent applications may not result in the issuance of patents in the United States or foreign countries. Competitors might develop products similar to ours that do not infringe our patents. In order to protect or enforce our patent rights, we may initiate interference proceedings, oppositions, or patent litigation against third parties, such as infringement suits. These lawsuits could be expensive, take significant time, and divert management's attention from other business concerns. The patent position of biotechnology firms generally is highly uncertain, involves complex legal and factual questions, and has recently been the subject of much litigation. No consistent policy has emerged from the U.S. Patent and Trademark Office or the courts regarding the breadth of claims allowed or the degree of protection afforded under biotechnology patents. In addition there is a substantial backlog of biotechnology patent applications at the U.S. Patent and Trademark Office, and the approval or rejection of patent applications may take several years.
In addition to patent protection we require our employees, consultants, advisors, and collaborators to execute confidentiality agreements. However, these agreements may not provide us with adequate protection against improper use or disclosure of confidential information. In addition, in some situations, these agreements may conflict with, or be subject to, the rights of third parties with whom our employees, consultants or advisors have prior employment or consulting relationships. Further, others may gain access to our trade secrets or independently develop substantially equivalent proprietary information and techniques.
Our success will depend partly on our ability to operate without infringing on or misappropriating the proprietary rights of others. If we are sued successfully for infringement or misappropriation of another’s proprietary rights, our ability to generate revenue could be substantially reduced or eliminated.
Any of our anticipated products may infringe patent and other proprietary rights of third parties. In addition, our competitors, many of which have substantially greater resources than us and have made significant investments in competing technologies or products, may seek to apply for and obtain patents that will prevent, limit, or interfere with our ability to make, use, and sell products either in the U.S. or international markets. Intellectual property litigation is costly and even if we prevail, the cost of such litigation could adversely affect our business, financial condition, and results of operations. In addition, litigation is time consuming and could divert management attention and resources away from our business. If we do not prevail in any litigation, we could be required to stop the infringing activity and/or pay substantial damages. Under some circumstances in the United States, these damages could be triple the actual damages the patent holder incurs. If we have supplied infringing products to third parties for marketing or licensed third parties to manufacture, use, or market infringing products, we may be obligated to indemnify these third parties for any damages they may be required to pay to the patent holder and for any losses the third parties may sustain themselves as the result of lost sales or damages paid to the patent holder.
If a third party holding rights under a patent successfully asserts an infringement claim with respect to any of our products, we may be prevented from manufacturing or marketing our infringing product in the country or countries covered by the patent we infringe, unless we can obtain a license from the patent holder. Any required license may not be available to us on acceptable terms, or at all. Some licenses may be non-exclusive, and therefore, our competitors may have access to the same technology licensed to us. If we fail to obtain a required license or are unable to design around a patent, we may be unable to market some of our anticipated products, which would adversely affect our ability to generate and grow revenues.
Some jurisdictions may require us to grant licenses to third parties. Such compulsory licenses could be extended to include some of our product candidates, which may limit our potential revenue opportunities.
Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition most countries limit the enforceability of patents against government agencies or government contractors. In these countries the patent owner may be limited to monetary relief and may be unable to enjoin infringement, which could materially diminish the value of the patent. Compulsory licensing of life-saving products is also becoming increasingly popular in developing countries, either through direct legislation or international initiatives. Such compulsory licenses could be extended to include some of our product candidates, which may limit our potential revenue opportunities.
We have no commercial production capability and we may encounter production problems or delays, which could result in lower revenues.
To date, we have not produced any commercially available products. To produce product to anticipated customer demand levels we will need to develop our commercial production capability and maintain adequate levels of inventory. We may not be able to produce sufficient quantities to meet market demand. We may not be able to maintain acceptable quality standards if we ramp up production. If we cannot achieve the required level and quality of production, we may need to outsource production or rely on licensing and other arrangements with third parties. We may not be able to successfully outsource our production or enter into licensing or other arrangements under acceptable terms with these third parties, which could adversely affect our business. Our inability to identify potential manufacturers, or to enter into or maintain agreements with them on acceptable terms, could delay or prevent the commercialization of our products, which would adversely affect our ability to generate revenues and could prevent us from achieving or maintaining profitability. In addition reliance on third-party manufacturers could reduce our gross margins and expose us to the risks inherent in relying on others. We may also encounter problems with production yields, shortages of qualified personnel, production costs, and the development of advanced manufacturing techniques and process controls.
We will be required to comply with good manufacturing practice requirements, and our failure to do so may subject us to fines and other penalties that will delay or prevent us from marketing and selling our products.
We, our collaborators, or other third party manufacturers of our products must comply with current good manufacturing practice, or cGMP, requirements demanded by customers and enforced by the FDA through its facilities inspection program. These requirements include quality control, quality assurance, and the maintenance of records and documentation. We, our collaborators, or other third party manufacturers of our products may be unable to comply with these cGMP requirements and with other FDA, state, and foreign regulatory requirements. These requirements may change over time and we, or third party manufacturers, may be unable to comply with the revised requirements. A failure to comply with these requirements may result in criminal and civil penalties, including fines, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval. If the safety of any quantities supplied by third-parties is compromised due to their failure to adhere to applicable laws or for other reasons, we may not be able to obtain regulatory approval for, or successfully commercialize, product candidates that we may develop.
We may incur substantial liabilities from any product liability claims, including claims made against third parties that we have agreed to indemnify. Our insurance coverage for those claims may be unavailable or inadequate.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials, and will face an even greater risk if we sell our product candidates commercially. An individual may bring a liability claim against us if one of our product candidates causes, or merely appears to have caused, an adverse effect or injury. These risks will exist even for products developed that may be cleared for commercial sale. If we cannot successfully defend ourselves against any product liability claims, we may incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in any one or a combination of the following:
| – | injury to our reputation; |
| – | withdrawal of clinical trial participants; |
| – | costs of related litigation; |
| – | substantial monetary awards to patients or other claimants; |
| – | decreased demand for our product candidates; |
| – | the inability to commercialize our product candidates. |
We intend to secure limited product liability insurance coverage, but we may not be able to obtain such insurance on acceptable terms with adequate coverage, or at reasonable or affordable costs. The amount of insurance coverage we obtain may not be adequate to protect us from all liabilities. We may not have sufficient resources to pay for any liabilities resulting from a claim beyond the limit of, or excluded from, our insurance coverage.
We use biological and hazardous materials in our business. If we are subject to claims relating to improper handling, storage, or disposal of these materials, our financial condition would suffer.
Our research and development processes involve the storage, use, and disposal of hazardous materials, including biological hazardous materials that could be dangerous to human health and safety or the environment. We are subject to federal, state, and local regulations governing the use, manufacture, storage, handling, and disposal of materials and waste products. Compliance with applicable environmental laws and regulations may be expensive, and current or future environmental laws and regulations may impair our product development efforts.
In the event of an accident we could be held liable for any damages that result and any liability could exceed the limits or fall outside the coverage of our insurance. We may not be able to maintain insurance on acceptable terms or at all. We could be required to incur significant costs to comply with current or future environmental laws and regulations.
If we experience significant fluctuations in our rate of anticipated growth and fail to balance our expenses with our revenue forecasts, our results could be harmed and our value may decline without advance notice.
Due to the unpredictability of new markets that we enter and deteriorating general economic and financial market conditions, we may not be able to accurately forecast our rate of growth. We plan our expense levels and investment on estimates of future revenue and future anticipated rate of growth. We may not be able to adjust our spending quickly enough if the rate of new or renewed orders falls short of our expectations. As a result, we expect that our revenues, operating results and cash flows may fluctuate significantly on a quarterly basis. We believe that period-to-period comparisons of our revenues, operating results and cash flows may not be meaningful and should not be relied upon as an indication of future performance.
Inability to manage growth
Although no assurance can be given, we contemplate that growth will occur as we implement our business strategies. We expect the expansion of our business to place a significant strain on our limited managerial, operational, and financial resources. We will be required to expand our operational and financial systems significantly and to expand, train, and manage our work force in order to manage the expansion of our operations. Our failure to fully integrate new employees into our operations could have a material adverse effect on our business, prospects, financial condition, and results of operations. Our ability to attract and retain highly skilled personnel in connection with our growth is critical to our operations and expansion. We face competition for these types of personnel from other biotechnology companies and more established organizations, many of which have significantly larger operations and greater financial, marketing, human, and other resources than we do. We may not be successful in attracting and retaining qualified personnel on a timely basis, on competitive terms, or at all. If we are not successful in attracting and retaining these personnel, our business, prospects, financial condition, and results of operations will be materially adversely affected.
Failure to achieve and maintain effective internal controls in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 could prevent us from producing reliable financial reports or identifying fraud. In addition, current and potential stockholders could lose confidence in our financial reporting, which could have an adverse effect on our stock price.
Effective internal controls are necessary for us to provide reliable financial reports and effectively prevent fraud, and a lack of effective controls could preclude us from accomplishing these critical functions. We are required to document and test our internal control procedures in order to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), which requires annual management assessments of the effectiveness of our internal controls over financial reporting.
If we fail to maintain the adequacy of our internal accounting controls, as such standards are modified, supplemented or amended from time to time, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404. Failure to achieve and maintain an effective internal control environment could cause investors to lose confidence in our reported financial information, which could have an adverse effect on our stock price.
FORWARD-LOOKING STATEMENTS
This consent solicitation statement/prospectus contains forward-looking statements. In addition, we and our representatives have made or may make forward-looking statements on telephone or conference calls, by webcasts or emails, in person, in presentations or written materials, or otherwise. These include statements about such matters as: expected future or targeted operational and financial performance; growth rates and future production and sales of products that incorporate or that are produced using Islet Sciences, Avogenx or BHV products; growth rates for, future prices and sales of, and demand for Islet Sciences, Avogenx or BHV products; financing and refinancing activities; investments and acquisitions that we have made or may make in the future and the performance of the businesses underlying such acquisitions and investments; employment and contributions of key personnel; employee relations; tax rates; capital expenditures and their impact on Islet Sciences, Avogenx or BHV; nature and timing of restructuring charges and payments; strategic plans and business projects; industry market conditions, the timing and magnitude of changes in such conditions and the impact thereof; strategic alliance, raw material and supply chain, technology development and collaboration, investment, acquisition, venture, operational, tax, financial and capital projects; legal proceedings; contingencies; consulting projects; potential offerings, sales and other actions regarding debt or equity securities of Islet Sciences, Avogenx or BHV; and costs, working capital, revenues, business opportunities, debt levels, cash flows, cost savings and reductions, margins, earnings and growth. The words “will,” “may,” “plan,” “estimate,” “project,” “believe,” “anticipate,” “expect,” “intend,” “should,” “would,” “could,” “target,” “goal,” “continue to,” “positioned to” and similar expressions, or the negatives thereof, identify some of these statements.
The expectations and targets of Islet Sciences, Avogenx or BHV are not predictors of actual performance and historically actual performance has deviated, often significantly, from expectations and targets. Actual future events and circumstances (including future results and trends) could differ materially, positively or negatively, from those set forth in these statements due to various factors. These factors include:
| | ● | | the possibility that challenging conditions or changes in the capital markets will limit Islet Sciences’, Avogenx’s or BHV’s ability to obtain financing for growth and other initiatives, on acceptable terms or at all; |
| | ● | | the possibility that the actual outcome of uncertainties associated with assumptions and estimates using judgment when applying critical accounting policies and preparing financial statements may have a material impact on Islet Sciences’, Avogenx’s or BHV’s results of operations or financial position; |
| | ● | | the possibility that Islet Sciences, Avogenx and BHV may be unable to protect their intellectual property or may infringe the intellectual property rights of others, resulting in damages, limitations on their ability to produce or sell products or limitations on their ability to prevent others from using that intellectual property to produce or sell products; |
| | ● | | the occurrence of unanticipated events or circumstances or changing interpretations and enforcement agendas relating to legal proceedings or compliance programs; |
| | ● | | the possibility that Islet Sciences or Avogenx may not achieve the earnings or other financial or operational metrics that it provides as guidance from time to time; |
| | ● | | the possibility that disclosure or internal controls may become inadequate because of changes in conditions or personnel, that the degree of compliance with policies and procedures related to those controls may deteriorate or that those controls may not operate effectively and may not prevent or detect misstatements or errors; |
| | ● | | the possibility that delays may occur in the financial statement closing process due to a change in internal control environment or personnel; |
| | ● | | the possibility that Islet Sciences will not be able to complete the Transactions in view of the various closing conditions described in this consent solicitation statement/prospectus; |
| | ● | | the possibility that Islet Sciences, Avogenx or BHV will not be able to achieve business plans and cost efficiencies and grow existing sales and volume profitably due to the high levels of competitive activity in the businesses on which the combined companies have chosen to focus; |
| | ● | | other risks and uncertainties, including those described elsewhere in Islet Sciences’ SEC filings, as well as future decisions by us. |
Occurrence of any of the events or circumstances described above could also have a material adverse effect on Islet Sciences’, Avogenx’s or BHV’s financial condition, results of operations, cash flows or the market price of Islet Sciences common stock or Avogenx common stock.
No assurance can be given that any future transaction about which forward-looking statements may be made will be completed or as to the timing or terms of any such transaction.
All subsequent written and oral forward-looking statements by or attributable to Islet Sciences, Avogenx or BHV or persons acting on their behalf are expressly qualified in their entirety by these factors. Except as otherwise required to be disclosed in periodic reports required to be filed by public companies with the SEC pursuant to the SEC’s rules, Islet Sciences, Avogenx and BHV undertake no duty to update these statements.
SOLICITATION OF WRITTEN CONSENTS
The Islet Sciences board of directors is providing these consent solicitation materials. Islet Sciences stockholders are being asked to approve the Merger Agreement and the Merger by executing and delivering the written consent furnished with this consent solicitation statement/prospectus.
Shares Entitled to Consent and Consent Required
Only Islet Sciences stockholders of record at the close of business on the record date of , 2014 will be notified of and be entitled to execute and deliver a written consent. On the record date, the outstanding securities of Islet Sciences eligible to consent with respect to approval of the Merger Agreement and the Merger consisted of shares of Islet Sciences common stock. Under Islet Sciences’ Articles of Incorporation and the NRS, each holder of Islet Sciences common stock is entitled to one vote for each share of common stock held of record.
Approval of the Merger Agreement and the Merger require the affirmative consent of the holders of a majority of the outstanding shares of Islet Sciences common stock.
On September 30, 2014, Islet Sciences entered into a series of voting agreements with certain directors of Islet Sciences that own shares of Islet Sciences common stock and holders of more than 5% of Islet Sciences outstanding common stock, which collectively own approximately 36.8% of Islet Sciences’ outstanding common stock. Pursuant to these voting agreements, these stockholders agreed to deliver a written consent in favor of the approval of the Merger Agreement and the Merger following the delivery of this consent solicitation statement/prospectus upon effectiveness of the registration statement of which it is a part. For additional information, see “Terms of the Transactions—Voting Agreement.”
Submission of Consents
You may consent to the proposal with respect to your shares by completing and signing the written consent furnished with this consent solicitation statement/prospectus and returning it to Islet Sciences.
If you hold shares of Islet Sciences common stock as of the record date and you wish to give your written consent, you must fill out the enclosed written consent, date and sign it, and promptly return it to Islet Sciences. Once you have completed, dated and signed the written consent, you may deliver it to Islet Sciences by emailing a .pdf copy of your written consent to info@isletsciences.com (Attention: Corporate Secretary) or by mailing your written consent to Islet Sciences, Inc. at 6601 Six Forks Rd, Suite 140, Raleigh, North Carolina 27615, Attention: Corporate Secretary. If you mail your written consent, please allow sufficient time for it to be delivered in advance of the delivery deadline discussed below. Islet Sciences will not be holding a stockholders’ meeting to consider this proposal, and therefore you will be unable to vote in person by attending a stockholders’ meeting.
The Islet Sciences board of directors has set , 2014 as the targeted final date for receipt of written consents. Islet Sciences reserves the right to extend the final date for receipt of written consents beyond , 2014. Any such extension may be made without notice to Islet Sciences stockholders. Once a sufficient number of consents to approve the Merger Agreement and the Merger have been received, the consent solicitation will conclude.
Executing Consents; Revocation of Consents
You may (i) execute a written consent to approve the Merger Agreement (which is equivalent to a vote FOR the approval of the Merger Agreement) or (ii) choose not to execute the written consent (which is equivalent to a vote AGAINST approval of the Merger Agreement). If you do not return your written consent, it will have the same effect as a vote against the approval of the Merger Agreement.
Your consent to the approval of the Merger Agreement and the Merger may be changed or revoked at any time before the consents of a sufficient number of shares to approve the Merger Agreement and the Merger have been filed with the corporate secretary of Islet Sciences. If you wish to change or revoke a previously given consent or prior revocation before that time, you may do so by delivering a notice of revocation to the corporate secretary of Islet Sciences or by delivering a new written consent with a later date.
Solicitation of Consents; Expenses
The expense of preparing, printing and mailing these consent solicitation materials is being borne by Islet Sciences. Officers and employees of Islet Sciences may solicit consents by telephone and personally, in addition to solicitation by mail. These persons will receive their regular salaries but no special compensation for soliciting consents.
BACKGROUND OF THE TRANSACTIONS
After initial preliminary discussions about the possibility of a business combination between Islet Sciences and BHV in 2012, the parties did not proceed with a transaction and discussions ended. On September 10, 2013, Cova Capital, LLC (“Cova”), Islet Sciences’ investment banker, on behalf of Islet Sciences, approached Dr. Wilkison to inquire whether Dr. Wilkison and Mr. Green would be interested in joining Islet Sciences in a management capacity. In addition, on September 10, 2013, at Cova's request, Islet Sciences and BHV entered into a confidentiality agreement related to a potential transaction regarding Remogliflozin.
On September 12, 2013, Mr. Green and Cova continued discussing the possibility of Mr. Green and Dr. Wilkison joining Islet Sciences, and Cova indicated that if they were to join, the parties should explore the possibility of jointly developing Remogliflozin. Mr. Green, Dr. Wilkison and Bentley Cheatham, BHV’s Chief Executive Officer, met to discuss Islet Sciences and to plan a due diligence process related to a potential transaction.
The BHV Members proceeded to conduct due diligence on Islet Sciences until the end of September 2013.
On September 30, 2013, Islet Sciences made an initial offer of terms for employment to Mr. Green and Dr. Wilkison. Over the next several weeks, Islet Sciences, Dr. Wilkison and Mr. Green had several telephone conferences to discuss terms of employment for Dr. Wilkison and Mr. Green, as well as a potential transaction to combine BHV and Islet Sciences.
In early October 2013, after multiple discussions between Dr. Wilkison, Mr. Green and representatives of Islet Sciences relating to potential employment of Dr. Wilkison and Mr. Green and Islet Sciences’ interest in Remogliflozin, BHV and Islet Sciences agreed to explore BHV licensing rights to Remogliflozin to Islet Sciences.
On October 2, 2013, the BHV Members and Islet Sciences’ scientific advisors and Cova held a conference call to discuss Remogliflozin. Later that day, BHV provided certain confidential information regarding Remogliflozin to Islet Sciences.
On October 3, 2013, the BHV Members and Islet Sciences’ scientific advisors and Cova held another conference call to discuss both the Islet pipeline and Remogliflozin.
On October 4, 2013, BHV provided Cova a draft of the employment agreement for Mr. Green and Dr. Wilkison, and also indicated that BHV would be sending a draft term sheet setting forth proposed terms of the potential transaction to license certain rights to Remogliflozin to Islet Sciences.
On October 8, 2013, BHV delivered a draft of the term sheet which included proposed terms for the license of Remogliflozin to Islet Sciences. After review of the term sheet by Islet Sciences with the assistance of counsel, the BHV Members and representatives of terms for the Islet Sciences held a conference call to discuss the term sheet. On the call, the parties agreed in principle to certain terms of the proposed transaction, and BHV committed to delivering a draft license agreement for Islet Sciences’ review.
On October 9, 2013, Islet Sciences’ counsel delivered drafts of revised employment agreements for Mr. Green and Dr. Wilkison to BHV.
On October 15, 2013, the BHV Members and representatives of Islet Sciences, including Cova, held a conference call to discuss Remogliflozin data.
On October 18, 2013, the BHV Members provided a draft license agreement to Islet Sciences. Over the next several days, representatives of BHV and Islet Sciences continued to negotiate terms of the proposed license agreement.
On October 22, 2013, BHV sent a revised draft of the license agreement for Remogliflozin to Islet Sciences.
The parties continued negotiating employment agreements for Mr. Green and Dr. Wilkison until October 25, 2013.
On October 25, 2013, the Islet Sciences board of directors approved the appointments of Mr. Green and Dr. Wilkison as Islet Sciences’ Chief Executive Officer and Chief Operating Officer, respectively. In addition, the Islet Sciences board of directors appointed Mr. Green to the Islet Sciences board of directors and Dr. Wilkison as a board observer. On October 30, 2013, Mr. Green and Dr. Wilkison accepted the appointments and executed their employment agreements with Islet Sciences.
After discussions between Cova and BHV during the last week of October 2013, BHV provided a revised draft of the license agreement to Islet Sciences that reflected the financial terms discussed on October 29, 2013.
From the end of October 2013 to December 2013, Islet Sciences and BHV continued discussion and negotiations of definitive transaction documents for the license of Remogliflozin to Islet Sciences from BHV.
During the latter part of December 2013 and the first two weeks of January 2014, BHV and its advisors, and Islet Sciences and its advisors, worked to finalize the license agreement, exchanging multiple drafts of the agreement.
On January 18, 2014, the Islet Sciences board of directors, without the participation of Mr. Green, consulted with a healthcare investment banker to review the terms of the proposed license from BHV. On January 20, 2014, Islet Sciences sent the then-current draft of the Remogliflozin license agreement to the investment banker for review.
On January 25, 2014, the Islet Sciences board of directors held a conference call, on which the investment banker advised the independent members of the Islet Sciences board of directors that the license structure could provide BHV and Islet Sciences with differing incentives, and alternatively suggested that an acquisition of the membership interests of BHV would be more favorable to Islet Sciences and its stockholders. Islet Sciences’ board of directors then proceeded to discuss with Mr. Green and Dr. Wilkison the potential structure of an acquisition of the membership interests of BHV on the same day. During a call, at Islet Science's request, the parties agreed to explore a merger structure but with the requirement that any non-cash payments made to the BHV Members would need to be structured as a tax-free exchange. The parties agreed to explore options for an upfront minority equity share grant plus additional milestone payments related to Remogliflozin. The BHV Members indicated a preference for milestone payments to be paid in cash. Islet Sciences indicated a preference to pay milestones by issuing stock, or, if Islet Sciences had sufficient capital at that time, cash. Islet Sciences indicated a desire to maintain the flexibility to avoid having to sell shares likely at a discount to market price, to investors who could freely trade their stock, as opposed to issuing shares to presumed insiders with an interest in the long-term success of Islet Sciences. The parties agreed to maintain flexibility for Islet Sciences to choose between cash or stock if it did not disrupt the overall tax treatment of the proposed transaction. Islet Sciences director, Dr. Eric Barnett, indicated that Islet Sciences would propose terms to BHV.
On January 27, 2014, Islet Sciences sent the BHV Members proposed acquisition terms for discussion.
On January 31, 2014, the Islet Sciences board of directors (without the participation of Mr. Green) consulted advisors regarding the tax treatment of the proposed structure.
On February 5, 2014, Dr. Barnett and Mr. Green had a conference call with the Islet Sciences’ advisors to discuss structuring of a tax efficient merger. Islet Sciences’ advisors recommended an exchange within the meaning of Section 351 of the Internal Revenue Code of 1986 that forms the basis for the current transaction.
On February 9, 2014, after telephone conferences between Dr. Barnett and the BHV Members, Islet Sciences and BHV agreed to the general terms for the proposed transaction, including the proposed acquisition by Islet Sciences of 100% of the issued and outstanding membership interests in BHV in exchange for Islet Sciences issuing 30 million shares of Islet Sciences common stock and the potential for up to $71 million of additional purchase price, based on the achievement of certain milestones.
On February 13, 2014, the Islet Sciences board of directors, with Mr. Green abstaining from voting, reviewed the general transaction terms and unanimously authorized certain independent members of the Islet Sciences board of directors to negotiate the terms of a binding letter of intent. The Islet Sciences board of directors, with Mr. Green abstaining from voting, also unanimously authorized the hiring of consultants to advise on structural issues related to the tax impact of the proposed transaction.
On February 19, 2014, Islet Sciences delivered a draft binding letter of intent to BHV outlining terms for the proposed Transactions.
Between February 20, 2014 and February 28, 2014, the parties engaged in due diligence regarding the proposed transaction, including numerous discussions about the structure of the proposed transaction from a tax perspective, and determined that the milestone payments should be paid in the form of Avogenx common stock in order to avoid triggering a taxable event for the BHV Members on the transfer of their membership interests in BHV to Avogenx.
On February 28, 2014, the Islet Sciences board of directors engaged an independent pharmaceutical business development executive to advise the board of directors on the reasonableness of the proposed transaction.
After considering alternative transaction structures that were not practicable for tax reasons, on March 5, 2014, Islet Sciences sent a draft of the binding letter of intent to BHV’s counsel for review and comment.
On March 7, 2014, after receiving a presentation from the independent pharmaceutical business development executive and advice from its advisors, the Islet Sciences board of directors, with Mr. Green abstaining from discussion and voting, unanimously approved entry into the binding letter of intent.
On March 12, 2014, Islet Sciences and BHV agreed to the terms of the Transactions and entered into a binding letter of intent for the acquisition of the outstanding membership interests of BHV.
On March 13, 2014, Islet Sciences and BHV issued a joint press release announcing entry into the binding letter of intent. On March 14, 2014, Islet Sciences filed a Form 8-K disclosing the entry into the binding letter of intent.
On April 11, 2014, the Islet board of directors appointed Dr. Wilkison as a director.
On April 24, 2014, Islet Sciences delivered an initial draft of the Merger Agreement and a related agreement providing registration rights to the BHV Members. Between this date and September 2014, Islet Sciences and BHV continued to negotiate the terms of the Merger Agreement and related transaction documents, with multiple discussions among the parties and their advisors.
At a meeting held on September 4, 2014, management of Islet Sciences reported to the Islet Sciences board of directors on due diligence findings and the status of negotiations with BHV. Mr. Green and Dr. Wilkison were present at the meeting to answer any questions from directors. The Islet Sciences board of directors, without the participation of Mr. Green and Dr. Wilkison, unanimously approved the Merger Agreement and the Transactions and recommended that the Islet Sciences stockholders approve the Merger Agreement and the Merger.
Between September 4 and September 30, 2014, the Merger Agreement and related documents were finalized, and the Merger Agreement was executed on September 30, 2014.
On October 1, 2014, Islet Sciences and BHV issued a joint press release announcing the execution of the Merger Agreement.
TERMS OF THE TRANSACTIONS
General
The following summary describes the material provisions of the Merger Agreement and is qualified in its entirety by reference to the complete text of the Merger Agreement, a copy of which is attached as Annex A to this consent solicitation statement/prospectus and which is incorporated herein by reference. The provisions of the Merger Agreement are extensive and not easily summarized. Accordingly, this summary may not contain all of the information about the Merger Agreement that is important to you. We encourage you to read the Merger Agreement carefully in its entirety for a more complete understanding of the Merger Agreement.
The Merger Agreement and this summary of its terms have been included with this consent solicitation statement/prospectus to provide you with information regarding the terms of the Merger Agreement and are not intended to modify or supplement any factual disclosures about Islet Sciences or Avogenx in our public reports filed with the SEC. In particular, the Merger Agreement and related summary are not intended to be, and should not be relied upon as, disclosures regarding any facts and circumstances relating to Islet Sciences, Avogenx or BHV. The representations and warranties contained in the Merger Agreement have been negotiated with the principal purpose of establishing the circumstances in which a party may have the right not to close the Transactions if the representations and warranties of the other party prove to be untrue due to a change of circumstances or otherwise, and allocate risk between the parties, rather than establishing matters as facts. The representations and warranties may also be subject to a contractual standard of materiality different from those generally applicable to public disclosures.
Structure of the Transactions
In connection with reorganization of its holding company structure, and to complete the acquisition of BHV, pursuant to the Merger Agreement and Section 92A.120 of the NRS, Islet Sciences has formed Avogenx, and Avogenx has formed Islet Merger Sub.
Upon satisfaction or waiver of the conditions to the Transactions, Islet Merger Sub will merge with and into Islet Sciences with Islet Sciences as the surviving entity. The officers and directors of Avogenx at the effective time of the Merger will continue to be the officers and directors of Avogenx immediately after consummation of the Merger. Shares of Islet Sciences common stock outstanding immediately prior to the effective time of the Merger will be converted into the right to receive shares of Avogenx common stock at the conversion rate of not less than three and not more than 40 shares of Islet Sciences common stock for one share of Avogenx common stock, with the effective number of shares of Islet Sciences common stock convertible into one share of Avogenx common stock to be determined by the Islet Sciences board of directors prior to the effective time of the Merger. Following the completion of the Transactions, Islet Sciences and BHV will each be wholly owned subsidiaries of Avogenx. Avogenx common stock will be quoted on the OTC Markets, Inc. OTCQB and will trade under a new symbol.
Upon satisfaction or waiver of the conditions to the Transactions, Avogenx will acquire all of the outstanding membership interests of BHV in consideration for the issuance of an aggregate of 30 million shares of common stock of Avogenx to the BHV Members to be adjusted for the Exchange Ratio and additional milestone payments described below, which are based on the development of Remogliflozin.
As a result of the BHV Securities Exchange, BHV will become a wholly owned subsidiary of Avogenx and our organizational structure will be as follows:
Islet Sciences Warrants and Stock Options
Upon consummation of the Merger, each warrant to purchase common stock of Islet Sciences then outstanding, whether or not then exercisable, will cease to represent a right to acquire shares of Islet Sciences common stock and will be converted automatically into a right to acquire shares of Avogenx common stock equal to the number of shares of Islet Sciences common stock subject to such Islet Sciences warrant immediately prior to the closing of the Merger, as adjusted for the Exchange Ratio. Upon consummation of the Merger, each stock option under any compensation or benefit agreement, plan or arrangement of Islet Sciences then outstanding, whether or not then vested or exercisable, will cease to represent a right to acquire shares of Islet Sciences common stock and will be converted automatically into a right to acquire shares of Avogenx common stock equal to the number of shares of Islet Sciences common stock subject to such Islet Sciences stock option immediately prior to the closing of the Merger, as adjusted for the Exchange Ratio. The exercise price per share of Avogenx common stock subject to any such Islet Sciences warrant, stock option or other right to acquire Islet Sciences common stock at and after the effective time of the Merger will be equal to the exercise price per share of Islet Sciences common stock subject to such Islet Sciences warrant, stock option or other right to acquire Islet Sciences common stock prior to the effective time of the Merger, as adjusted for the Exchange Ratio.
No Fractional Shares
Avogenx will not issue fractional shares of Avogenx common stock in connection with the Merger. Instead, all fractional shares of Avogenx common stock that a stockholder of Islet Sciences would otherwise be entitled to receive as a result of the Merger will be aggregated, and if a fractional share results from such aggregation, such fractional share shall be automatically rounded up to a whole share.
Dissenter’s Rights
The Merger Agreement provides that shares of Islet Sciences common stock which are held by stockholders that do not vote to approve the Merger Agreement and the Merger or consent thereto in writing and who properly demand payment of fair value of such shares pursuant to Section 92A.440 of the NRS shall not be converted into the right to receive shares of Avogenx common stock and instead shall be entitled to the rights provided under Sections 92A.300 to 92A.500 of the NRS which provide holders of Islet Sciences common stock with the ability to seek payment of fair value of their shares. A holder of record of Islet Sciences common stock who properly seeks payment of the fair value of such holder’s shares and complies with the applicable requirements of the NRS will forego the Merger consideration and instead be entitled to receive a cash payment equal to the fair value of his, her or its shares of Islet Sciences common stock. Stockholders who follow this procedure are referred to below and elsewhere in this consent solicitation/prospectus as dissenting stockholders. Within 30 days after receiving a properly executed payment demand, Islet Sciences will pay the holder what it determines to be the fair value of the shares, plus accrued interest (computed from the effective date of the Merger until the date of payment). If the holder believes that the amount Islet Sciences pays in exchange for the holder’s dissenting shares is less than the fair value of such shares or that the amount of accrued interest is not correctly determined, the holder can demand payment of the difference between his, her or its estimate and the amount of payment by Islet Sciences. The holder must make such demand within 30 days after Islet Sciences has made or offered payment; otherwise, the holder’s right to challenge calculation of fair value terminates.
If there is still disagreement about the fair market value within 60 days after Islet Sciences receives the holder’s demand, it will petition a Nevada district court to determine the fair value of the shares and the accrued interest. If the holder does not commence that legal action within the 60-day period, it will have to pay the amount demanded for all unsettled demands. All dissenters whose demands remain unsettled will be made parties to the proceeding, and are entitled to a judgment. Dissenting stockholders will not know the fair value at the time such holders must elect whether to demand payment. Failure to follow exactly the procedures specified under Nevada law will result in the loss of dissenter’s rights. A detailed description of the dissenter’s rights available to holders of Islet Sciences common stock and procedures required to exercise statutory dissenter’s rights is included in the section entitled “Dissenter’s Rights.”
Voting Agreements
On September 30, 2014, Islet Sciences entered into a series of voting agreements with certain directors of Islet Sciences that own shares of Islet Sciences common stock and holders of more than 5% of Islet Sciences outstanding common stock, which collectively own approximately 36.8% of Islet Sciences’ outstanding common stock. Pursuant to these voting agreements, these stockholders agreed to deliver a written consent in favor of the approval of the Merger Agreement and the Merger following the delivery of this consent solicitation statement/prospectus upon effectiveness of the registration statement of which it is a part.
Ownership of Avogenx after the Transactions
Subject to the adjustment for the Exchange Ratio, thirty million shares of Avogenx common stock are issuable to the BHV Members, upon consummation of the BHV Securities Exchange. Based upon the 66,928,724 shares of Islet Sciences common stock outstanding on November 24, 2014, a total of 96,928,724 shares of Avogenx common stock would be outstanding after the Transactions, subject to the adjustment for the Exchange Ratio. Based on such shares outstanding on November 24, 2014, the shares issuable to the BHV Members in the BHV Securities Exchange would represent approximately 31.0% of the outstanding shares of Avogenx common stock immediately after the closing of the BHV Securities Exchange. The BHV Members can also receive additional milestone payments in shares described below which are based on the development of Remogliflozin.
Terms of the Merger Agreement
Representations and Warranties
The Merger Agreement contains representations and warranties made by Islet Sciences, Avogenx, Islet Merger Sub, BHV and the BHV Members that are customary for transactions of this type. The current BHV Members are James Green and William Wilkison. Certain of these representations and warranties are qualified by “materiality” or “Material Adverse Effect.” For purposes of the Merger Agreement, “Material Adverse Effect” means with respect to any party to the Merger Agreement, any result, occurrence, fact, change, event or effect that has a material adverse effect on the financial condition, business or results of operations of such party and its subsidiaries, taken as a whole, provided, that in determining whether or not a Material Adverse Effect has occurred no change or event resulting from certain matters including any of the following will be taken into account:
| | ● | | changes in prevailing economic or market conditions or the securities, credit or financial markets in the United States or elsewhere (except to the extent any such change has a materially disproportionate effect on such entity and its subsidiaries relative to other similarly situated participants in the industries in which they operate); |
| | ● | | changes or events affecting the industries in which the affected party operates (except to the extent any such change or event has a materially disproportionate effect on such entity and its subsidiaries relative to other similarly situated participants in the industries in which they operate); |
| | ● | | any changes in generally accepted accounting principles, or other similar accounting requirements in foreign countries, applicable to such affected party and its subsidiaries, and which are not specific to the affected party; |
| | ● | | any changes, after the date of the Merger Agreement, in laws, rules or regulations of general applicability or interpretations thereof by any governmental entity; |
| | ● | | the announcement of the Merger Agreement or the Transactions; and |
| | ● | | any weather-related or other force majeure event. |
Except for those representations and warranties made by the BHV Members in the Merger Agreement, which by their terms apply or are to be performed in whole or in part after the effective time of the Transactions, the representations and warranties made in the Merger Agreement do not survive the effective time of the Transactions. The following is a brief summary of certain of the principal representations and warranties made by BHV and/or the BHV Members in the Merger Agreement:
| | ● | | (a) BHV has all requisite limited liability company power and authority to enter into the Merger Agreement and to consummate the Transactions, (b) BHV has duly executed the Merger Agreement and (c) the Merger Agreement is enforceable against BHV in accordance with its terms; |
| | ● | | the execution and delivery by BHV of the Merger Agreement and the consummation of the Transactions would not conflict with, or result in any violation of, or constitute a default under, or give rise to a right of termination, cancellation or acceleration of any obligation or the loss of a material benefit under, or the creation of a lien or encumbrance on any assets pursuant to any provision of its governing documents or the law under which it has been formed; |
| | ● | | BHV has disclosed in its disclosure letter to the Merger Agreement the holders of all of the issued and outstanding equity of BHV and except as otherwise disclosed, there are no options, warrants, rights, convertible or exchangeable securities, “phantom” stock rights, stock appreciation rights, stock-based performance units, commitments, contracts, arrangements or undertakings of any kind to which BHV is a party or by which BHV is bound obligating BHV to issue or sell any equity; |
| | ● | | BHV has good and valid title to all its properties and assets that are material to its business ; |
| | ● | | no agent, broker, investment banker, financial advisor or other firm or person is or will be entitled to any broker’s or finder’s fee or any other similar commission or fee in connection with any of the Transactions; |
| | ● | | each BHV Member owns free and clear of any and all liens, claims, encumbrances, preemptive rights, right of first refusal and adverse interests of any kind, the equity in BHV disclosed in the disclosure letter to the Merger Agreement; |
| | ● | | (a) all acts required to be taken by each BHV Member to enter into the Merger Agreement have been properly taken and (b) the Merger Agreement is enforceable against each BHV Member ; and |
| | ● | | the execution and delivery by each BHV Member of the Merger Agreement (a) would not require the consent of any third party or any governmental entity under any laws, (b) would not violate any laws applicable to such BHV Member and (c) would not violate or breach any contractual obligation to which such BHV Member is a party. |
Conduct of the Business of BHV Prior to Closing
In the Merger Agreement, BHV has agreed to conduct its business in the ordinary course consistent with past practices, to use its reasonable best efforts to preserve intact its current business organization, preserve its relationships with employees, customers, suppliers and others having business dealings with BHV, and to not engage in specified material transactions. In general, BHV must conduct its business in all material respects to keep substantially intact the business, properties and assets of BHV. Subject to certain limited exceptions described in the Merger Agreement or set forth in the BHV’s disclosure letter to the Merger Agreement, BHV has agreed that it will not, without the consent of Islet Sciences, during the period prior to the effective time of the Transactions thereunder:
| | ● | | authorize amendments to its organizational documents; |
| | ● | | declare, set aside or pay any dividends on, or make any other distributions in respect of any of its equity interests; |
| | ● | | split, combine or reclassify any of its equity interests; |
| | ● | | issue, deliver or sell, or authorize or propose the issuance, delivery or sale of, any equity interests or securities convertible into or exercisable or exchangeable for, or any rights, warrants or options to acquire, any such equity interests, or enter into any agreement with respect to any of the foregoing; |
| | ● | | repurchase, redeem or otherwise acquire or issue or sell any of its equity interests or any other securities convertible into or exercisable for any of its equity interests; |
| | ● | | acquire or agree to acquire, by merging or consolidating with, by purchasing a substantial equity interest in or a substantial portion of the assets of, by forming a partnership or joint venture with, or by any other manner, any business or any corporation, partnership, association or other business organization or division thereof or otherwise acquire or agree to acquire any assets that are material to BHV; |
| | ● | | grant any increases in compensation to any director or employee other than normal increases in base salary or wages in the ordinary course of business consistent with past practice for employees who are not directors of BHV or officers of BHV; |
| | ● | | grant any increase in, or accelerate the accrual rate, vesting or timing of payment or funding of, any compensation, severance, benefits or other rights of any director or employee of BHV; |
| | ● | | enter into any employment, consulting, severance or termination agreement with any director or employee; |
| | ● | | make any material tax election or any change in accounting methods; |
| | ● | | incur, create or assume any indebtedness for borrowed money (or modify any of the material terms of any such outstanding indebtedness), guarantee any such indebtedness or issue or sell any debt securities or warrants or rights to acquire any debt securities of BHV or guarantee any long-term debt securities of others; |
| | ● | | enter into any material agreement or make any material changes to any material agreement other than in the ordinary course of business; |
| | ● | | adopt a plan or agreement of complete or partial liquidation, dissolution or reorganization; |
| | ● | | sell, lease, assign, encumber or otherwise dispose of, or agree to sell, lease, assign, encumber or otherwise dispose of, any of its material assets; or |
| | ● | | authorize any of, or commit or agree to take any of, the foregoing actions. |
Exclusivity and Non-Solicitation
In the Merger Agreement, BHV has agreed that neither it nor any of its subsidiaries, nor their respective agents and representatives (including any investment banker, attorney or accountant retained by it or any of its subsidiaries) will, directly or indirectly:
| | ● | | initiate, solicit, encourage or knowingly facilitate any inquiries or the making of any proposal or offer with respect to the acquisition of BHV, or any of its equity or any material portion of its assets, by a third party; |
| | ● | | have any discussions with or provide any confidential information or data to any person relating to a proposal to acquire BHV, or engage in any negotiations concerning such proposal, or knowingly facilitate any effort or attempt to make or implement such proposal; or |
| | ● | | approve or recommend, or propose to approve or recommend, or execute or enter into, any letter of intent, agreement in principle, Merger Agreement, asset purchase or share exchange agreement, option agreement or other similar agreement relating to a proposal to acquire BHV. |
Conduct of the Business of Islet Sciences Prior to Closing
In the Merger Agreement, Islet Sciences has agreed to conduct its business in the ordinary course consistent with past practices, to use its reasonable best efforts to preserve intact its current business organization, preserve its relationships with employees, customers, suppliers and others having business dealings with Islet Sciences, and to not engage in specified material transactions. In general, Islet Sciences must conduct its business in all material respects to keep substantially intact the business, properties and assets of Islet Sciences. Subject to certain limited exceptions described in the Merger Agreement, Islet Sciences has agreed that it will not, without the consent of BHV, during the period prior to the effective time of the Transactions thereunder:
| | ● | | authorize any amendments to its organizational documents; |
| | ● | | declare, set aside or pay any dividends on, or make any other distributions in respect of any of its capital stock; |
| | ● | | split, combine or reclassify any of its equity interests; |
| | ● | | issue, deliver or sell, or authorize or propose the issuance, delivery or sale of, any shares of its capital stock of any class, any stock appreciation rights, or any securities convertible into or exercisable or exchangeable for, or any rights, warrants or options to acquire, any such shares, or enter into any agreement with respect to any of the foregoing, other than (i) the issuance of common stock or other equity rights or obligations under any Islet Sciences stock plan or employee benefit plans currently in effect or (ii) issuances by its wholly-owned subsidiary of its capital stock to its parent or to another wholly-owned subsidiary of Islet Sciences; |
| | ● | | acquire or agree to acquire, by merging or consolidating with, by purchasing a substantial equity interest in or a substantial portion of the assets of, by forming a partnership or joint venture with, or by any other manner, any business or any corporation, partnership, association or other business organization or division thereof or otherwise acquire or agree to acquire any assets that are material to Islet Sciences; |
| | ● | | grant any increases in compensation to any director or employee other than normal increases in base salary or wages in the ordinary course of business consistent with past practice for employees who are not directors of Islet Sciences or officers of Islet Sciences; |
| | ● | | grant any increase in, or accelerate the accrual rate, vesting or timing of payment or funding of, any compensation, severance, benefits or other rights of any director or employee of Islet Sciences; |
| | ● | | enter into any employment, consulting, severance or termination agreement with any director or employee; |
| | ● | | make any material tax election or any change in accounting methods; |
| | ● | | incur, create or assume any indebtedness for borrowed money (or modify any of the material terms of any such outstanding indebtedness), guarantee any such indebtedness or issue or sell any debt securities or warrants or rights to acquire any debt securities of Islet Sciences or guarantee any long-term debt securities of others; |
| | ● | | enter into any material agreement or make any material changes to any material agreement other than in the ordinary course of business; |
| | ● | | adopt a plan or agreement of complete or partial liquidation, dissolution or reorganization; |
| | ● | | sell, lease, assign, encumber or otherwise dispose of, or agree to sell, lease, assign, encumber or otherwise dispose of, any of its material assets; or |
| | ● | | authorize any of, or commit or agree to take any of, the foregoing actions. |
Reasonable Best Efforts to Close
Each of the parties to the Merger Agreement agreed to use its reasonable best efforts to take, or cause to be taken, all actions, and to do, or cause to be done, all things necessary, proper or advisable under the Merger Agreement and applicable laws to consummate the Transactions as soon as practicable after the date of the Merger Agreement, including:
| | ● | | preparing and filing as promptly as practicable all documentation to effect all necessary applications, notices, filings and other documents; and |
| | ● | | obtaining as promptly as practicable all requisite regulatory approvals and all other consents, waivers, orders, approvals, permits, rulings, authorizations and clearances necessary or advisable to be obtained from any third party or any governmental entity in order to consummate the Transactions or any of the other transactions contemplated by the Merger Agreement. |
Each of Islet Sciences and BHV agreed in the Merger Agreement to cooperate regarding, and keep the other reasonably apprised of, the status of, matters relating to the completion of the Transactions and (i) work cooperatively in connection with any filing or submission and in connection with any investigation or other inquiry, including any proceeding initiated by a private party and permit the other party to review in advance any proposed written communication to any governmental entity or private party, related to the Transactions, (ii) promptly inform the other party of the status of any of the matters contemplated thereby, including providing the other party with a copy of any written communication (or summary of oral communications) received by such party from, or given by such party to, any governmental entity and of any written communication (or summary of oral communications) received or given in connection with any proceeding by a private party, in each case regarding any of the Transactions, and (iii) consult with each other in advance to the extent practicable of any meeting or conference with any such governmental entity or, in connection with any proceeding by a private party, with any such other person, and to the extent permitted by any such governmental entity or other person, give the other party the opportunity to attend and participate in such meetings and conferences.
Director and Officer Indemnification Post-Closing
Islet Sciences agreed in the Merger Agreement that following consummation of the Transactions it would or would cause BHV and Avogenx to indemnify persons who were officers or managers of BHV prior to consummation of the Merger against all losses, claims, damages, costs, expenses, liabilities or judgments or amounts that are paid in settlement of or in connection with any claim, action, suit, proceeding or investigation based in whole or in part on or arising in whole or in part out of the fact that such person is or was a manager, officer or employee of BHV, and pertaining to any matter existing or occurring, or any acts or omissions occurring, at or prior to the effective time of the Transactions (including with respect to acts or omissions occurring in connection with the approval of the Merger Agreement and the consummation of the Transactions), whether asserted or claimed prior to, or at or after, the effective time of the Transactions, in each case to the fullest extent such persons are permitted by applicable law to be indemnified by, or have the right to advancement of expenses from, BHV as of the date of the Merger Agreement.
Board of Director Composition Post-Closing
Following the closing of the Transactions, the board of directors of Avogenx will consist of five directors pursuant to the terms of the Merger Agreement.
Public Announcements Prior to Closing
Islet Sciences and BHV agreed to use reasonable best efforts (i) to develop a joint communications plan, (ii) to ensure that all press releases and other public statements with respect to the Transactions shall be consistent with such joint communications plan, and (iii) except in respect of any announcement required by applicable law or by obligations pursuant to any listing agreement with or rules of any securities exchange in which it is impracticable to consult with each other as contemplated by the Merger Agreement, to consult with each other before issuing any press release or, to the extent practical, otherwise making any public statement with respect to the Merger Agreement or the Transactions.
Milestones
In addition to the 30 million shares of Avogenx common stock to be received by the BHV Members in the BHV Securities Exchange (as adjusted by the Exchange Ratio), Avogenx will issue to the BHV Members within five (5) business days of the occurrence of certain success-based development, regulatory and commercial milestones the number of shares of newly-issued Avogenx common stock equal in the aggregate to the (i) absolute value of the dollar amount applicable to each milestone, equal in the aggregate to $71,000,000, divided by (ii) the volume weighted average price of Avogenx common stock for the thirty (30) trading days preceding the occurrence of each milestone as reported by Bloomberg L.P. Each milestone will be deemed to have been achieved in the event that it is achieved by or on behalf of Avogenx or any of its affiliates, licensees or sub-licensees. There is no guarantee that any of the milestones will be met or that any of the corresponding payments will be made to the BHV Members.
Anti-dilution
If at any time following the date of the Merger Agreement Islet Sciences or Avogenx, as the case may be, issues additional shares of Islet Sciences common stock or Avogenx common stock, respectively, pursuant to or in connection with any agreement or arrangement that was in effect as of the date of the Merger Agreement or that results from or arises out of facts in existence as of the date of the Merger Agreement (other than any compensatory award pursuant to an Islet Sciences employee benefit plan or otherwise pursuant to any currently outstanding security exercisable for or convertible into Islet Sciences common stock or Avogenx common stock included in Islet Sciences’ most recent publicly filed financial statements), or any amendment, modification, termination, replacement or settlement of or in connection with any such agreement or arrangement (regardless of whether prior to, on or after the date hereof) (each, a “Dilutive Agreement”), Avogenx will be required to issue such number of shares of Avogenx common stock to each BHV Member as is necessary to prevent each BHV Member’s percentage ownership in Avogenx from being diluted (the “Anti-dilution Shares”). Any issuance(s) of Anti-dilution Shares will be made concurrently with the issuance(s) of shares pursuant to the Dilutive Agreement; provided, however, that if any issuance of shares pursuant to a Dilutive Agreement occurs prior to closing of the Merger, such related Anti-dilution Shares will be issued at the closing of the Merger.
Warrant Adjustment
If, during the period commencing immediately following the closing of the Merger and ending on the date of the issuance of milestone shares in connection with achievement of the final milestone under the Merger Agreement (the “BHV Warrant Period”), Avogenx issues any shares of Avogenx common stock pursuant to an outstanding BHV warrant (the “BHV Warrant Shares”) or makes any cash payment in connection with the termination of the an outstanding BHV warrant (a “BHV Warrant Payment”), the number of shares of Avogenx common stock remaining to be issued to the BHV Members as milestone shares pursuant to the Merger Agreement will be reduced by the Milestone Adjustment Amount. The “Milestone Adjustment Amount” will be equal to the following: (i) in the case of the issuance of Avogenx common stock pursuant to an outstanding BHV warrant during the BHV Warrant Period, the number of BHV Warrant Shares, or (ii) in the case of a BHV Warrant Payment pursuant to termination of an outstanding BHV warrant during the BHV Warrant Period, a number of shares of Avogenx common stock determined by dividing the BHV Warrant Payment by the weighted average price of Avogenx common stock for the thirty (30) trading days preceding the date the BHV Warrant Payment is made, subject in each case to the following: (A) the Milestone Adjustment Amount will be reduced by the number of shares of Avogenx common stock determined by dividing the value of any cash or other property received by Avogenx or any affiliate of Avogenx in connection with the issuance of the BHV Warrant Shares (e.g., any exercise price paid) by the closing price of Avogenx common stock on the date of payment; (B) in no event will the total Milestone Adjustment Amount be greater than the number of shares of Avogenx common stock to which the holder of an outstanding BHV warrant would have been entitled under the Merger Agreement had it exercised the BHV warrant prior to the closing of the Merger and been a party to the Merger Agreement; (C) the Milestone Adjustment Amount will be applied proportionally against any remaining milestones that may be achieved based on the relative dollar amounts of such milestones, provided that if the Milestone Adjustment Amount exceeds the number of shares that would otherwise have been issued based on the achievement of such milestone, the Milestone Adjustment Amount applicable to such milestone will be limited to such number of shares; and (D) Avogenx and Islet Sciences agree to use commercially reasonable efforts to minimize the size of any Milestone Adjustment Amount.
Certain Tax Matters
Each of Islet Sciences and Avogenx agreed in the Merger Agreement not to take or cause to be taken any action, or knowingly fail to take or cause to be taken any action, which action or failure to act could reasonably be expected to prevent the exchange of BHV membership interests and Islet Sciences common stock for Avogenx common stock pursuant to the Transactions from qualifying, for United States federal income tax purposes, as an exchange described in Section 351 of the Code and the Treasury Regulations promulgated thereunder.
Conditions to Completion of the Transactions
The respective obligation of each party to the Merger Agreement to effect the Transactions is subject to the satisfaction or waiver on or prior to the closing date of the Transactions of the following conditions:
| | ● | | Islet Sciences must have obtained stockholder approval of the Merger Agreement; |
| | ● | | all authorizations, consents, orders or approvals of, or declarations or filings with, and all expirations of waiting periods required from, any governmental authority under applicable laws shall have been filed, have occurred or been obtained, and all such requisite regulatory approvals shall be in full force and effect; |
| | ● | | shares of Avogenx issuable pursuant to the Merger Agreement shall have been authorized for quotation on the OTCQB; |
| | ● | | the registration statement of which this consent solicitation statement/prospectus is a part shall have been declared effective, is not subject to any stop order and no proceeding for that purpose, and no similar proceeding with respect to this consent solicitation statement/prospectus, shall have been initiated or threatened in writing by the SEC or its staff; and |
| | ● | | no temporary restraining order, preliminary or permanent injunction or other order issued by any court of competent jurisdiction or other legal restraint or prohibition preventing the consummation of the Transactions shall be in effect. There shall not be any action taken, or any law, rule, regulation or order enacted, entered, enforced or deemed applicable to the Transactions, by any governmental entity of competent jurisdiction that makes the consummation of the Transactions illegal. |
| | ● | | Avogenx and the BHV Members shall have entered into the investor rights agreement in the form set forth in the Merger Agreement (the “Investor Rights Agreement”); |
| | ● | | Islet Sciences shall have received an opinion from Anderson Bradshaw, PLLC, in form and substance reasonably satisfactory to each of Islet Sciences and BHV to the effect that, on the basis of facts, representations and assumptions set forth or referred to in such opinion, (i) the formation of Avogenx and Islet Merger Sub in anticipation of the Merger will be treated for federal income tax purposes as an exchange described in Section 351 of the Code as an exchange, and as such, these formations will be treated as non-taxable capitalization and will be disregarded for tax purposes, (ii) the conversion of Islet Sciences securities by the securityholders of Islet Sciences for Avogenx securities will qualify as an exchange under Section 351 of the Code, and the provisions of Section 368(a)(1)(E) of the Code will apply to this conversion such that the Merger will qualify as a tax-free reorganization, and (iii) the Islet Sciences securityholders’ basis in their Islet securities will transfer to and be considered as carryover basis in the Avogenx securities received as a result of the conversion; and |
| | ● | | BHV shall have received an opinion from BDO USA, LLP, in form and substance reasonably satisfactory to each of Islet and BHV to the effect that, on the basis of facts, representations and assumptions set forth or referred to in such opinion, the BHV Securities Exchange (including the milestone payments) should be treated for U.S. federal income tax purposes as an exchange described in Section 351 of the Code. |
In addition, the obligation of Islet Sciences is subject to the following conditions:
| | ● | | the representations and warranties of BHV contained in the Merger Agreement shall be true and correct as of the date of the Merger Agreement and as of the closing date of the Transactions as if made at and as of that time (except for representations and warranties made only as of a specified date, which shall be true and correct as of the specified date), except to the extent where the failures of any such representations and warranties to be so true and correct, in the aggregate, have not had, and would not reasonably be expected to have, a Material Adverse Effect on BHV; |
| | ● | | BHV shall have performed in all material respects all obligations required to be performed by it under the Merger Agreement at or prior to the closing date of the Transactions; |
| | ● | | there shall not have been any action with respect to the Transactions taken since the date of the Merger Agreement by any court or other governmental entity (which action has not been vacated or reversed), or any law, injunction, order or decree enacted, promulgated or issued with respect to the Transactions by any court or other governmental entity (which law, injunction, order or decree remains in effect), that would reasonably be expected to result in a judgment that would have any of the following effects: (i) challenging or seeking to make illegal, to delay materially or otherwise to restrain or prohibit the consummations of the Transactions, (ii) seeking to restrain or prohibit Islet Sciences’ or Avogenx’s ownership or operation (or that of its respective subsidiaries or affiliates) of all or any material portion of the business or assets of BHV and its subsidiaries, taken as a whole, or of Islet Sciences and its subsidiaries, taken as a whole, or (iii) seeking to compel Islet Sciences or Avogenx or any of their respective subsidiaries to sell, hold separate or otherwise disposing of any of its or BHV’s business or assets or materially restricting the conduct its or BHV’s business if doing so would, individually or in the aggregate, reasonably be expected to result in a Material Adverse Effect on BHV; |
| | ● | | the BHV Members shall have delivered to Islet Sciences assignment agreements assigning the ownership of the BHV membership interests to Avogenx; and |
| | ● | | From the date of the Merger Agreement until closing of the Merger, there must not have been, with respect to BHV, any Material Adverse Effect or any event, change or effect that would, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect. |
In addition, the obligation of BHV is subject to the following conditions:
| | ● | | the representations and warranties of Islet Sciences contained in the Merger Agreement shall be true and correct as of the date of the Merger Agreement and as of the closing date of the Transactions as if made at and as of that time (except for representations and warranties made only as of a specified date, which shall be true and correct as of the specified date), except to the extent where the failures of any such representations and warranties to be so true and correct, in the aggregate, have not had, and would not reasonably be expected to have, a Material Adverse Effect on Islet Sciences; |
| | ● | | Islet Sciences shall have performed in all material respects all obligations required to be performed by it under the Merger Agreement at or prior to the closing date of the Transactions; and |
| | ● | | From the date of the Merger Agreement until closing of the Merger, there must not have been, with respect to Islet Sciences, any Material Adverse Effect or any event, change or effect that would, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect. |
Termination
The Merger Agreement provides that it may be terminated and the Transactions may be abandoned at any time before the closing:
| | ● | | by the mutual written agreement of Islet Sciences and BHV; |
| | ● | | by BHV or Islet Sciences, upon written notice to the other party, if a governmental entity of competent jurisdiction that must grant a requisite regulatory approval has denied approval of the Merger or the BHV Securities Exchange and such denial has become final and non-appealable; or any governmental entity of competent jurisdiction shall have issued an order, decree or ruling or taken any other action permanently restraining, enjoining or otherwise prohibiting the Merger or BHV Securities Exchange, and such order, decree, ruling or other action has become final and non-appealable, provided, however, that the right to terminate the Merger Agreement shall not be available to any party whose failure to comply with the closing conditions of the Merger Agreement has been the cause of, or resulted in, such action; |
| | ● | | by BHV or Islet Sciences, upon written notice to the other party, if there shall have been a breach by the other party of any of the covenants or agreements, or failure to be true of any of the representations or warranties, set forth in the Merger Agreement on the part of such other party, which breach, or failure to be true, either individually or in the aggregate, would make representations and warranties made by such party cease to be true and correct or result in failure of such party to perform its obligations under the Merger Agreement, as the case may be, and which breach, or failure to be true, has not been cured within thirty (30) days following written notice thereof to the affected party or, by its nature, cannot be cured within such time period; |
| | ● | | by either Islet Sciences or BHV, if the Transactions have not been consummated by April 30, 2015; or |
| | ● | | by BHV, if Islet Sciences enters into, or announces the entry into, an agreement for a transaction to effect, a merger, reorganization, share exchange, consolidation, business combination, recapitalization, liquidation, dissolution or similar transaction involving it or any of its significant subsidiaries or any purchase or sale of 50% or more of the consolidated assets (including stock of its subsidiaries) of it and its subsidiaries, taken as a whole, or any purchase or sale of, or tender or exchange offer for, its voting securities that, if consummated, would result in any person (or the stockholders of such person) beneficially owning securities representing 50% or more of its total voting power (or of the surviving parent entity in such transaction) or any of its significant subsidiaries. |
Expenses
Pursuant to the Merger Agreement, whether or not the Transactions are consummated, all costs and expenses incurred by any party to the Merger Agreement in connection with the Merger Agreement and the Transactions will be borne by Islet Sciences or Avogenx or, to the extent previously paid by BHV or any BHV Member, reimbursed to BHV or such BHV Member.
In addition, Islet Sciences and Avogenx will indemnify and reimburse each BHV Member with respect to any taxes he may incur as a result of (i) the application of Section 357(c) of the Code, any applicable treasury regulations promulgated thereunder and any similar or successor provisions of the Code or treasury regulations promulgated thereunder (and any corresponding or resulting state or local taxes) resulting from the liabilities of BHV assumed by Avogenx exceeding the adjusted tax basis of the assets of BHV that are transferred to Avogenx in connection with the Transactions, (ii) the payment by Islet Sciences or Avogenx of costs and expenses incurred by BHV or such BHV Member in connection with the Merger Agreement and (iii) any payments made by Islet Sciences or Avogenx to such BHV Member pursuant to these indemnification and reimbursement provisions.
Amendments and Waivers
Any provision of the Merger Agreement may be amended or waived prior to the effective time if such amendment or waiver is in writing and is signed, in the case of an amendment, by each party thereto or, in the case of a waiver, by each party against whom the waiver is to be effective.
Description of the Investor Rights Agreement
Pursuant to the Merger Agreement, at the closing of the Transactions, Avogenx and the BHV Members will enter into the Investor Rights Agreement in the form attached to the Merger Agreement. The Investor Rights Agreement will contain director nomination rights and registration rights with respect to shares of Avogenx common stock issued to BHV Members which are described below. It will also contain preemptive rights of the BHV Members with respect to securities of Avogenx common stock issuable in the future.
Registration Rights
Avogenx will grant registration rights to the BHV Members, which will include an unlimited number of demand registrations exercisable at any time when there is no effective registration statement with respect to all of the outstanding shares of Avogenx issued to the BHV Members in the BHV Securities Exchange, and not all of such shares can be resold without registration and without volume restrictions pursuant to Rule 144, and piggyback rights on certain registrations by Avogenx (whether for its own account or for the account of other stockholders). The Investor Rights Agreement will contain customary registration procedures and indemnification provisions relating to the registration rights, and Avogenx will agree to pay all expenses (other than commissions, discounts and stock transfer taxes) relating to such registrations. The registration rights provisions (other than the indemnification provisions) terminate on the date that the BHV Members no longer beneficially own any registrable securities.
Board Nomination Rights
Following the Transactions, and provided the BHV Members continue to hold in the aggregate at least 10% of outstanding shares of Avogenx common stock, the BHV Members shall have the right to designate two persons for nomination for election to the board of directors of Avogenx. As long as the BHV Members have the right to designate to the Avogenx Board their nominees, the Board may not expand its size beyond the number set forth in the Merger Agreement, which is five directors, including without limitation by creation of a vacancy under applicable law, without the approval of each designee of the BHV Members.
Participation Rights
For so long as the BHV Members continue to beneficially own, directly or indirectly, in the aggregate at least 10% of the outstanding shares of Avogenx common stock, Avogenx shall not issue or sell any capital stock or securities convertible into or exchangeable for capital stock of Avogenx in a transaction with the primary purpose of raising capital for Avogenx, unless Avogenx first submits a written offer to each of the BHV Members to permit them to participate in the purchase of such securities on the same terms and conditions, including price, as proposed by Avogenx in connection with such issuance or sale.
Material U.S. Federal Income Tax Consequences
Subject to the limitations and qualifications described in “Material U.S. Federal Income Tax Consequences” below, the exchange by U.S. holders of shares of Islet Sciences common stock for shares of Avogenx common stock pursuant to the Merger will constitute an exchange to which Section 351 of the Code applies. As a result, no gain or loss will be recognized by the stockholders of Islet Sciences as a result of the exchange of Islet Sciences shares for Avogenx shares pursuant to the Merger. Your tax consequences will depend on your own situation. You should consult your tax advisor to fully understand the tax consequences of the Transactions to you.
In connection with the filing of the registration statement of which this consent solicitation statement/prospectus is a part, Anderson Bradshaw, PLLC has delivered an opinion to Islet Sciences that the exchange of Islet Sciences shares by the stockholders of Islet Sciences for Avogenx shares will qualify as an exchange under Section 351 of the Code and that no gain or loss recognition is incurred to the public stockholders of Islet Sciences.
ISLET SCIENCES’ REASONS FOR THE TRANSACTIONS; RECOMMENDATION OF THE ISLET SCIENCES BOARD
On September 4, 2014, the Islet Sciences board of directors, at a meeting, approved, with the directors who are the members of BHV abstaining from voting, the Merger Agreement and the Transactions and recommended that the Islet Sciences stockholders approve the Merger Agreement and the Merger.
In considering Islet Sciences’ reasons for the Transactions, you should be aware that Islet Sciences’ directors may have interests in the Merger and the Transactions that may be different from, or in addition to, the interests of Islet Sciences’ stockholders generally. These interests are described in “Interests of Directors and Executive Officers of Islet Sciences in the Transactions.”
In reaching its decisions to approve the Transactions, including the acquisition of BHV, the Islet Sciences board of directors consulted with Islet Sciences’ management, as well as with legal and business advisors, and considered a number of factors, including the following:
| | ● | | Strategic, commercial and financial rationales for the acquisition of BHV. |
| | ● | | Its knowledge of Islet Sciences’ business, operations, financial condition, earnings and prospects. |
| | ● | | The knowledge of BHV’s business, operations, financial condition, earnings and prospects which the Islet Sciences board of directors and Islet Sciences’ management gained as a result of Islet Sciences’ due diligence review of BHV. |
| | ● | | The prevailing macroeconomic conditions, and the economic environment of the industries in which Islet Sciences and BHV operate. |
| | ● | | The fact that BHV has a program in advanced stage development for type 2 diabetes and NASH. |
| | ● | | The fact that BHV has an experienced management team which should assist in the integration of BHV’s operations into Islet Sciences’ business. |
| | ● | | The fact that the ability to use Islet Sciences common stock as the consideration to be delivered to the BHV Members would exclude Islet Sciences’ need for any financing for this transaction and preserve financing capacity for other corporate purposes. |
Risks
In addition to the factors described above, the Islet Sciences board of directors identified and considered a variety of risks and potentially negative factors concerning the acquisition of BHV, including:
| | ● | | The possibility that the acquisition may not be completed, or that completion of the acquisition may be unduly delayed, for reasons beyond the control of Islet Sciences or BHV. |
| | ● | | The difficulties of integrating the business, including implementing and maintaining uniform standards, controls, procedures and policies at the businesses to be acquired. |
| | ● | | The anticipated amount and timing of capital expenditures for the acquired businesses. |
| | ● | | The risks of the type and nature described under “Risk Factors” beginning on page 11 of this consent solicitation statement/prospectus, and the matters discussed under “Forward-Looking Statements” beginning on page 17 of this consent solicitation statement/prospectus. |
In view of the wide variety of factors considered in connection with its evaluation of each acquisition and the complexity of those matters, the Islet Sciences board of directors did not find it useful to, and did not attempt to, quantify, rank or otherwise assign relative weights to these factors.
In addition, the Islet Sciences board of directors did not undertake to make any specific determination as to whether any particular factor, or any aspect of any particular factor, was favorable or unfavorable to its ultimate determination, but rather the Islet Sciences board of directors conducted an overall analysis of the factors applicable to the acquisition of BHV, including discussions with Islet Sciences’ management and legal and business. In considering the factors described above, individual members of the Islet Sciences board of directors may have given different weight to different factors.
Consultant Engaged by the Islet Sciences Board of Directors
The Islet Sciences board of directors engaged Jonathan Berlent in March 2014 to review certain available information regarding Remo and its potential market value in order to aid the directors in determining whether the consideration to be paid by Islet Sciences for BHV in the BHV Securities Exchange is reasonable. Mr. Berlent was, at the time, a Vice President of Business Development at Tris Pharma, Inc., a privately held pharmaceutical company. He has 10 years of experience as an executive of pharmaceutical companies in such areas as over-the-counter and brand drugs, sustained-release and drug delivery technologies, novel dosage forms, CNS/pain/abuse deterrence, and orphan drugs. Prior to that, Mr. Berlent worked for 12 years in the banking industry and held various leadership positions, including Vice President and Head Trader in the capital markets division of Bank of America. The Islet Sciences board of directors selected Mr. Berlent to provide his analysis of Remo based on his experience within the relevant market.
On March 7, 2014, Mr. Berlent made a presentation to the Islet Sciences board of directors during a board teleconference.
Mr. Berlent’s conclusions were provided to the Islet Sciences board of directors (in its capacity as such) for its information in connection with its evaluation of the consideration to be paid by Islet Sciences for BHV in the BHV Securities Exchange from a financial point of view to Islet Sciences and did not address any other aspect of the Transactions, including the relative merits of the Transactions as compared to alternative transactions or strategies that might be available to Islet Sciences or the underlying business decision of Islet Sciences to proceed with the Transactions. Mr. Berlent’s conclusions spoke only as of the date they were delivered and do not constitute advice or a recommendation to any stockholder as to how such stockholder should vote or act on any matter relating to the Transactions or otherwise. Mr. Berlent expressed no opinion as to what the value of Avogenx common stock actually will be when issued pursuant to the Transactions or the price at which Avogenx common stock will trade at any time.
No limitations were imposed by the Islet Sciences board of directors on the scope of Mr. Berlent’s investigation or the procedures to be followed by Mr. Berlent in reaching his conclusions. Mr. Berlent did not recommend to Islet Sciences or its board of directors or management any consideration payable under the Merger Agreement or any of the terms or conditions of the Merger Agreement, each of which was determined through negotiations between Islet Sciences and BHV. Mr. Berlent’s conclusions were based on the comparative analysis described below.
Mr. Berlent's presentation was given in reliance on information and representations made or given by Islet Sciences and, BHV, and their respective officers, and on certain information from recognized independent sources. Mr. Berlent did not independently verify the information concerning BHV, nor other data which Mr. Berlent considered in his review and, for purposes of his presentation, Mr. Berlent assumed and relied upon the accuracy and completeness of all such information and data. No review of the intellectual property estate or potential issuance of patents was undertaken.
With respect to estimates, forecasts or projections of BHV’s future financial performance reviewed with the Islet Sciences board of directors, or obtained from public sources, Mr. Berlent assumed that such estimates, forecasts and projections were reasonably prepared on the basis reflecting the best available estimates, forecasts and projections available to BHV and Islet Sciences and Mr. Berlent, and that they provided a reasonable basis upon which Mr. Berlent could form his conclusions. The estimates, forecasts and projections were not prepared with the expectation of public disclosure and are based on numerous variables and assumptions that are inherently uncertain, including, without limitation, factors related to general economic and competitive conditions. Accordingly, actual results could vary significantly from those set forth in such estimates, forecasts and projections.
Mr. Berlent reviewed confidential and non-confidential information concerning the development of Remo, projected costs of such development and projected revenues from sales of Remo, and financial terms of the BHV acquisition. Using publicly available information, Mr. Berlent also compared Remo to drugs with a similar mechanism of action that have been approved by the FDA, namely Invokana® and Farxiga®.
Based on the foregoing, Mr. Berlent formed the following conclusions, and relayed such conclusions to the Islet Sciences board of directors on March 6, 2014:
| – | The Remo development program is consistent with a development program of a similar compound in a similar stage; |
| – | While Remo has safety concerns similar to other compounds in the same class of drugs, it is presented as comparably less symptomatic with potentially lower incidence of adverse events with respect to Invokana® and Farxiga®; |
| – | Clinical efficacy as presented by BHV presents Remo as potential best in class therapy when compared to Invokana® and Farxiga® with respect to reduction of HbA1C; |
| – | The estimated cumulative probability of success factor of Remo is within industry norms; |
| – | Total estimated development costs of Remo are considered reasonable; |
| – | The estimated development and launch timeline of Remo is reasonable and consistent with the current development stage of the compound; |
| – | Projected global peak sales for base case, upside and downside scenarios represent reasonable assumptions; |
| – | The financial deal terms are within the bounds of comparable deal structures associated with pharmaceutical assets of the same stage, and consistent with projections for a single indication; |
| – | The current market landscape and prevailing market dynamics for both the diabetes market in general and the competitive landscape for drug therapies going forward as presented by BHV are consistent with current knowledge; and |
| – | It would be reasonable for the Islet Sciences board of directors to proceed with the acquisition of BHV. |
Mr. Berlent received a fee totaling $3,375 prior to the execution of the Merger Agreement. The fee was not contingent upon any conclusion that Mr. Berlent may reach or upon completion of the BHV Securities Exchange.
Mr. Berlent has not had any material relationship with Islet Sciences or BHV during the past two years in which compensation was received or was intended to be received as a result of the relationship between Mr. Berlent and Islet Sciences or BHV.
INTERESTS OF DIRECTORS AND EXECUTIVE OFFICERS OF ISLET SCIENCES IN THE TRANSACTIONS
The directors and executive officers of Islet Sciences have interests in the Transactions that may be different from, or in addition to, the interests of Islet Sciences stockholders generally. The Islet Sciences board of directors was aware of these interests and considered them, among other matters, in approving the Merger Agreement and the Transactions and in determining to recommend to Islet Sciences stockholders to approve the Merger Agreement and the Merger.
Islet Sciences current executive officers and directors, James Green and William Wilkison, are also sole members of BHV and as such will benefit from the BHV Securities Exchange. Under the Merger Agreement, the BHV Members will receive in the aggregate 30,000,000 shares Avogenx common stock (as adjusted by the Exchange Ratio) upon the closing of the Transactions, and will be entitled to certain success-based milestone payments equal in the aggregate to $71,000,000 payable in shares of Avogenx common stock. See “Terms of the Transactions – Terms of the Merger Agreement.” There is no guarantee that any of the milestones will be met or that any of the corresponding payments will be made to the BHV Members.
Pursuant to the Merger Agreement, at the closing of the Transactions, Avogenx and James Green and William Wilkison, as the BHV Members, will enter into an Investor Rights Agreement, which will contain director nomination rights and registration rights with respect to shares of Avogenx common stock issued to the BHV Members. It will also contain preemptive rights of the BHV Members with respect to securities of Avogenx common stock issuable in the future. See “Terms of the Transactions – Description of the Investor Rights Agreement.”
Islet Sciences’ current Chief Financial Officer, Steve Delmar, is entitled to a conditional grant of 100,000 common stock options of Islet Sciences upon the effective date of the registration statement of which this consent solicitation statement/prospectus is a part.
ACCOUNTING TREATMENT OF THE TRANSACTIONS
We will account for the BHV Securities Exchange using the acquisition method of accounting under Accounting Standards Codification Topic 805, Business Combinations. The acquisition method of accounting requires that all assets acquired and liabilities assumed of the acquired businesses, including tangible property and equipment, intangible assets, and contingent liabilities, be recorded at their fair value on the acquisition date. The excess of the consideration transferred (purchase price) over the fair value of the net assets acquired will be recognized as goodwill.
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES
The following discussion summarizes the material U.S. federal income tax consequences of the Transactions that are expected to apply generally to U.S. holders (as defined below) of Islet Sciences common stock. For purposes of this discussion, a “U.S. holder” is a beneficial owner of a share of Islet Sciences common stock that is:
| | ● | | a citizen or individual resident of the United States; |
| | ● | | a corporation or other entity taxable as a corporation, created or organized in or under the laws of the United States or any political subdivision thereof; |
| | ● | | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| | ● | | a trust if it (1) is subject to the primary supervision of a court within the United States and one or more United States persons has the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable United States Treasury regulations to be treated as a United States person. |
This discussion is based on the Code, applicable Treasury regulations, administrative interpretations and court decisions as in effect as of the date of this consent solicitation statement/prospectus, all of which may change, possibly with retroactive effect. This discussion assumes that the Transactions will be completed in accordance with the terms of the Merger Agreement. No ruling has been or will be sought from the Internal Revenue Service (the “IRS”) as to the U.S. federal income tax consequences of the Transactions, and the following summary is not binding on the IRS or the courts. As a result, the IRS could adopt a contrary position, and such a contrary position could be sustained by a court.
This discussion only addresses U.S. holders who hold shares of Islet Sciences common stock (and will hold shares of Avogenx common stock) as capital assets and does not purport to be a complete analysis of all potential tax effects of the Transactions. In addition, this discussion does not address the tax consequences of transactions effectuated prior to or after the Transactions (whether or not such transactions occur in connection with the Transactions), including, without limitation, any exercise of an option or warrant to acquire Islet Sciences common stock or the acquisition or disposition of shares of Islet Sciences common stock other than pursuant to the Transactions. It does not address the U.S. federal income tax considerations applicable to holders of options or warrants to purchase Islet Sciences common stock, or holders of debt instruments convertible into Islet Sciences common stock. It also does not address all aspects of U.S. federal income taxation that may be important to a U.S. holder of Islet Sciences common stock in light of that holder’s particular circumstances or to a U.S. holder subject to special rules, such as:
| | ● | | U.S. holders subject to special treatment under U.S. federal income tax laws (for example, brokers or dealers in securities, financial institutions, mutual funds, insurance companies, or tax-exempt organizations); |
| | ● | | a U.S. holder that holds Islet Sciences common stock as part of a hedge, appreciated financial position, straddle, conversion transaction or other risk reduction strategy; |
| | ● | | a U.S. holder whose functional currency for U.S. federal income tax purposes is not the U.S. dollar; |
| | ● | | a U.S. holder that is a partnership or other entity classified as a partnership for U.S. federal income tax purposes; |
| | ● | | a U.S. holder that holds Islet Sciences common stock through a pass-through entity; |
| | ● | | a U.S. holder liable for the alternative minimum tax; |
| | ● | | a U.S. holder who acquired Islet Sciences common stock pursuant to the exercise of options or rights or otherwise as compensation or through a tax-qualified retirement plan; or |
| | ● | | a U.S. holder who actually or constructively owns an interest in BHV. |
This discussion of material U.S. federal income tax consequences is not a complete analysis or description of all potential U.S. federal income tax consequences of the Transactions. This discussion does not address tax consequences that may vary with, or are contingent on, individual circumstances. In addition, it does not address any non-income tax or any foreign, state or local tax consequences of the Transactions, or the consequences under any proposed Treasury regulations that have not taken effect as of the date of this consent solicitation statement/prospectus. Accordingly, we strongly urge each Islet Sciences stockholder to consult his or her own tax advisor to determine the particular U.S. federal, state or local or foreign income or other tax consequences to him or her of the Transactions.
Consequences of Exchange to U.S. Holders
The exchange by U.S. holders of shares of Islet Sciences common stock for shares of Avogenx common stock pursuant to the Merger will be treated as an exchange described in Section 351 of the Code, or as a reorganization within the meaning of Section 368(a) of the Code, or both. A U.S. holder will not recognize any gain or loss for U.S. federal income tax purposes upon its exchange of shares of Islet Sciences common stock for shares of Avogenx common stock. Such holder will have a tax basis in the Avogenx common stock received in the Merger equal to the tax basis of the Islet Sciences common stock surrendered therefor (assuming that no election to reduce such basis is made). The holding period for Avogenx common stock received in the Merger will include the holding period for the Islet Sciences common stock surrendered therefor.
Consequences to Islet Sciences and Avogenx
Neither Islet Sciences nor Avogenx will recognize any gain or loss for U.S. federal income tax purposes as a result of the exchange of Islet Sciences shares for Avogenx shares pursuant to the Merger. In addition, no gain or loss will be recognized by Islet Sciences or Avogenx as a result of the issuance of shares of Avogenx common stock in exchange for the equity interests in BHV pursuant to the BHV Securities Exchange.
Information on Transactions to Be Filed with Islet Sciences Stockholders’ Returns
A U.S. holder that qualifies as a “significant transferor” and certain U.S. stockholders of a foreign corporation that qualifies as a significant transferor will be required to attach statements to their tax returns for the year in which the Transactions are consummated that contain the information listed in Treasury Regulation Section 1.351-3. A significant transferor includes a person that transfers property to a corporation and receives stock of the transferee corporation in an exchange described in Section 351 of the Code if, immediately after the exchange, such person owns at least five percent (by vote or value) of the total outstanding stock of the transferee corporation and the stock owned by such person is publicly traded. The statement must include the significant transferor’s tax basis in and the fair market value of the Islet Sciences common stock that it exchanges for Avogenx common stock.
FINANCIAL STATEMENTS
See the Index to Financial Statements on page F-1 of this consent solicitation statement/prospectus for a complete list of the historical financial statements of Islet Sciences and BHV included in this consent solicitation statement/prospectus.
ISLET SCIENCES AND BHV UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
The unaudited pro forma condensed combined balance sheet combines the historical consolidated balance sheets of Islet Sciences and BHV as if the acquisition of BHV took place on July 31, 2014.
The unaudited pro forma condensed combined statements of operations for the three months ended July 31, 2014 and for the year ended April 30, 2014 combine the historical consolidated statements of operations for Islet Sciences and BHV as if the acquisition of BHV took place on May 1, 2013, the beginning of the earliest period presented.
The historical condensed consolidated financial information has been adjusted in the unaudited condensed combined financial statements to give effect to pro forma events that are (1) directly attributable to the acquisition of BHV; (2) factually supportable; and (3) with respect to the statement of operations, expected to have a continuing impact on the combined company’s results. The pro forma adjustments are described in the accompanying footnotes.
The unaudited pro forma condensed combined financial information was based on and should be read in conjunction with the following historical consolidated financial statements and accompanying notes of Islet Sciences and BHV for the applicable periods, which are included in this document:
| | ● | | Separate unaudited financial statements of Islet Sciences as of and for the three months ended July 31, 2014; |
| | ● | | Separate unaudited financial statements of BHV as of and for the three months ended July 31, 2014; |
| | ● | | Separate historical audited financial statements of Islet Sciences as of and for the year ended April 30, 2014; and |
| | ● | | Separate historical audited financial statements of BHV as of and for the year ended April 30, 2014. |
The unaudited pro forma condensed combined financial information is presented for informational purposes only and is not intended to represent or be indicative of the combined results of operations or financial position that we would have reported had the acquisition been completed as of the date and for the periods presented, and should not be taken as representative of our consolidated results of operations or financial condition following completion of the acquisition of BHV. In addition, the unaudited pro forma condensed combined financial information is not intended to project the future financial position or results of operations of the combined company. There were no material transactions between Islet Sciences and BHV during the periods presented that are required to be eliminated.
The unaudited pro forma condensed combined financial information was prepared using the acquisition method of accounting under GAAP. Islet Sciences has been treated as the acquirer. The acquisition accounting is dependent upon certain valuations and other studies that have yet to commence or progress to a stage where there is sufficient information for a definitive measurement. Accordingly, the pro forma adjustments are preliminary and have been made solely for the purpose of providing unaudited condensed combined financial information. Differences between these preliminary management estimates (for example estimates as to the value of acquired intangibles) and the final acquisition accounting will occur, and these differences could have a material impact on the accompanying unaudited pro forma condensed combined financial statements and the combined company’s future results of operations and financial position.
The unaudited pro forma combined financial information does not reflect any cost savings, operational synergies or revenue enhancements that the combined company may achieve as a result of the acquisition or the costs to combine the operations or the costs necessary to achieve cost savings, operating synergies and revenue enhancements.
Islet Sciences and BHV
Pro Forma Unaudited Condensed Combined Statement of Operations
For the Year Ended April 30, 2014
| | | Islet Sciences Historical | | | BHV Historical | | | Pro Forma Adjustments | | | Pro Forma Combined | |
| | | | |
| Net revenue | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development | | | 646,250 | | | | 10,069 | | | | — | | | | 656,319 | |
| General and administrative | | | 2,195,198 | | | | 99,488 | | | | — | | | | 2,294,686 | |
| Impairment loss | | | 93,586 | | | | — | | | | — | | | | 93,586 | |
| | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 2,935,034 | | | | 109,557 | | | | — | | | | 3,044,591 | |
| | | | | | | | | | | | | | | | | |
| Loss from operations | | | (2,935,034 | ) | | | (109,557 | ) | | | — | | | | (3,044,591 | ) |
| | | | | | | | | | | | | | | | | |
| Other income (expense): | | | | | | | | | | | | | | | | |
| Interest expense | | | (11,397 | ) | | | (15,963) | | | | — | | | | (27,360 | ) |
| | | | | | | | | | | | | | | | | |
| Total other income (expense) | | | (11,397) | | | | (15,963 | ) | | | — | | | | (27,360) | |
| | | | | | | | | | | | | | | | | |
| Net loss | | $ | (2,946,431 | ) | | $ | (125,520 | ) | | $ | — | | | $ | (3,071,951 | ) |
| | | | | | | | | | | | | | | | | |
| Basic and diluted net loss per share attributable to common stockholders | | $ | (0.05 | ) | | | | | | $ | — | | | $ | (0.03 | ) |
| | | | | | | | | | | | | | | | | |
| Weighted average shares used in the computation of basic and diluted net loss per share | | | 58,214,535 | | | | | | | | 30,000,000 | | | | 88,214,535 | |
See the accompanying notes to the unaudited pro forma condensed combined financial statements which are an integral part of these statements. The pro forma adjustments are explained in “Note 3. Pro Forma and Acquisition Accounting Adjustments.”
Islet Sciences and BHV
Pro Forma Unaudited Condensed Combined Statement of Operations
For the Three Months Ended July 31, 2014
| | | Islet Sciences Historical | | | BHV Historical | | | Pro Forma Adjustments | | | Pro Forma Combined | |
| | | | |
| Net revenue | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| Operating expenses: | | | | | | | | | | | | | | | | |
| Research and development | | | 58,331 | | | | 11,958 | | | | — | | | | 70,289 | |
| General and administrative | | | 405,399 | | | | 87,190 | | | | — | | | | 492,589 | |
| | | | | | | | | | | | | | | | | |
| Total operating expenses | | | 463,730 | | | | 99,148 | | | | — | | | | 562,878 | |
| | | | | | | | | | | | | | | | | |
| Loss from operations | | | (463,730 | ) | | | (99,148 | ) | | | — | | | | (562,878 | ) |
| | | | | | | | | | | | | | | | | |
| Other income (expense): | | | | | | | | | | | | | | | | |
| Interest expense | | | (800 | ) | | | (3,300 | ) | | | — | | | | (4,100 | ) |
| | | | | | | | | | | | | | | | | |
| Loss before income taxes | | | (464,530 | ) | | | (102,448 | ) | | | — | | | | (566,978 | ) |
| | | | | | | | | | | | | | | | | |
| Net loss | | $ | (464,530 | ) | | $ | (102,448 | ) | | $ | — | | | $ | (566,978 | ) |
| | | | | | | | | | | | | | | | | |
| Basic and diluted net loss per share | | $ | (0.01 | ) | | | | | | $ | — | | | $ | (0.01 | ) |
| | | | | | | | | | | | | | | | | |
| Weighted average shares used in the computation of basic and diluted net loss per share | | | 67,069,789 | | | | | | | | 30,000,000 | | | | 97,069,789 | |
See the accompanying notes to the unaudited pro forma condensed combined financial statements which are an integral part of these statements. The pro forma adjustments are explained in “Note 3. Pro Forma and Acquisition Accounting Adjustments.”
Islet Sciences and BHV
Pro Forma Unaudited Condensed Combined Balance Sheet
As of July 31, 2014
| | | | | | | | | | | | | | | |
| | | Islet Sciences Historical | | | BHV Historical | | | Pro Forma Adjustments | | | | | Pro Forma Combined | |
| Assets | | | | | | | | | | | | | | |
| Current assets: | | | | | | | | | | | | | | |
| Cash | | $ | 457,436 | | | $ | 12,587 | | | $ | — | | | | | $ | 470,023 | |
| Inventory and prepaid expenses | | | 700 | | | | — | | | | 140,900 | | A | | | | 141,600 | |
| | | | | | | | | | | | | | | | | | | |
| Total current assets | | | 458,136 | | | | 12,587 | | | | 140,900 | | | | | | 611,623 | |
| | | | | | | | | | | | | | | | | | | |
| Goodwill | | | 2,111,107 | | | | — | | | | — | | | | | | 2,111,107 | |
| In-Process Research and Development | | | 1,367,000 | | | | — | | | | 25,305,158 | | B | | | | 26,672,158 | |
| | | | | | | | | | | | | | | | | | | |
| Total assets | | $ | 3,936,243 | | | $ | 12,587 | | | $ | 25,446,058 | | | | | $ | 29,394,888 | |
| | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | |
| Current liabilities: | | | | | | | | | | | | | | | | | | |
| Accounts payable & accrued expenses | | $ | 3,184,945 | | | $ | 272,248 | | | $ | — | | | | | $ | 3,457,193 | |
| Related party payable | | | 51,818 | | | | — | | | | — | | | | | | 51,818 | |
| Notes payable | | | — | | | | 312,689 | | | | — | | | | | | 312,689 | |
| | | | | | | | | | | | | | | | | | | |
| Total current liabilities | | | 3,236,763 | | | | 584,937 | | | | | | | | | | 3,821,700 | |
| | | | | | | | | | | | | | | | | | | |
| Contingent liability—former BHV unit holders | | | — | | | | — | | | | 20,373,708 | | C | | | | 20,373,708 | |
| Deferred income taxes | | | 547,000 | | | | — | | | | — | | | | | | 547,000 | |
| | | | | | | | | | | | | | | | | | | |
| Total liabilities | | | 3,783,763 | | | | 584,937 | | | | 20,373,708 | | | | | | 24,742,408 | |
Stockholders’ equity (deficit): | | | | | | | | | | | | | | | | | | |
| Common stock | | | 66,929 | | | | 41,656 | | | | 30,000 | | D | | | | 96,929 | |
| | | | | | | | | | | | (41,656 | ) | E | | | | | |
| Additional paid-in capital | | | 20,938,814 | | | | — | | | | 4,470,000 | | D | | | | 25,408,814 | |
| Accumulated deficit | | | (20,853,263 | ) | | | (614,006 | ) | | | 614,006 | | E | | | | (20,853,263 | ) |
| | | | | | | | | | | | | | | | | | | |
| Total stockholders’ equity (deficit) | | | 152,480 | | | | (572,350 | ) | | | 5,072,350 | | | | | | 4,652,480 | |
| | | | | | | | | | | | | | | | | | | |
| Total liabilities and stockholders’ equity (deficit) | | $ | 3,936,243 | | | $ | 12,587 | | | $ | 25,446,058 | | | | | $ | 29,394,888 | |
See the accompanying notes to the unaudited pro forma condensed combined financial statements which are an integral part of these statements. The pro forma adjustments are explained in “Note 3. Pro Forma and Acquisition Accounting Adjustments.”
NOTES TO THE ISLET SCIENCES AND BHV UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
On September 30, 2014, Islet Sciences and BHV entered into the Merger Agreement pursuant to which Islet Merger Sub will merge with and into Islet Sciences with Islet Sciences as the surviving entity, and Avogenx will acquire all of the outstanding membership interests of BHV in consideration for the issuance of an aggregate of 30 million shares of common stock of Avogenx to the BHV Members, to be adjusted for the Exchange Ratio, and additional milestone payments described below, which are based on the development of BHV’s drug compound. In connection with the Transactions, shares of Islet Sciences common stock outstanding immediately prior to the effective time of the Merger will be converted into the right to receive shares of Avogenx common stock at the Exchange Ratio. The pro forma condensed combined financial information does not take into consideration the Exchange Ratio. Following the completion of the Transactions, Islet Sciences and BHV will each be wholly owned subsidiaries of Avogenx.
The preliminary estimated total purchase price of the proposed merger is as follows:
| Initial consideration | | $ | 4,500,000 | |
| Contingent consideration payable | | | 20,373,708 | |
| | | | | |
| Total consideration | | $ | 24,873,708 | |
The initial consideration consists of 30,000,000 shares of Avogenx common stock, subject to the Exchange Ratio, valued at the closing price on October 20, 2014 of $0.15 per share. Avogenx will issue to the BHV Members, upon the occurrence of certain development, regulatory and commercial milestones associated with the remogliflozin etabonate program, a number of shares of newly-issued Avogenx common stock equal in the aggregate to the (i) absolute dollar value of the applicable milestone, divided by (ii) the volume weighted average price of Avogenx common stock, as reported by Bloomberg L.P., for the thirty (30) trading days preceding the occurrence of each milestone. The contingent consideration cash flows have been probability weighted and discounted over the projected milestone periods to determine a fair value of the milestones at the time of acquisition. The first potential milestone is not expected to be reached until 2016. The aggregate value of all potential milestones is $71 million. There is no guarantee that any of the milestones will be achieved.
Under the acquisition method of accounting, the total purchase price is allocated to the acquired tangible and intangible assets and assumed liabilities of BHV based on their estimated fair values as of the closing of the Transactions.
A preliminary allocation of the total preliminary estimated purchase price, as shown above, to the acquired assets and assumed liabilities of BHV based on the estimated fair values as of July 31, 2014 is as follows:
| | | Preliminary Allocation of Purchase Price | |
| Cash, cash equivalents and marketable securities | | $ | 12,587 | |
| Active pharmaceutical ingredient inventory | | | 140,900 | |
| Intangible assets: in-process research and development | | | 25,305,158 | |
| Current liabilities | | | (272,248 | ) |
| Notes payable | | | (312,689 | ) |
| | | |
| Total | | $ | 24,873,708 | |
The allocation of the estimated purchase price is preliminary because the proposed BHV Securities Exchange have not yet been completed. The purchase price allocation will remain preliminary until Avogenx’s management determines the fair values of assets acquired and liabilities assumed. The final determination of the purchase price allocation is anticipated to be completed as soon as practicable after completion of the Transactions and will be based on the fair values of the assets acquired and liabilities assumed as of the closing date. The final amounts allocated to assets acquired and liabilities assumed could differ significantly from the amounts presented in the unaudited pro forma condensed combined financial statements.
Given the significant uncertainty concerning the ultimate achievement of product development, Islet Sciences has estimated the fair value of the acquired identifiable intangible assets using a probability-weighted scenario approach, employing income approaches in each scenario. These estimates are based on a preliminary valuation and are subject to further review by management at the close of the transaction.
Material assumptions used to preliminarily value the BHV product assets included:
| | ● | | The forcasted BHV product revenues and associated royalties due to Kissei, as well as an appropriate discount rate giving consideration to the market forecast risk involved; |
| | ● | | Material assumptions used to preliminarily value the Remogliflozin development project included: |
| | | | - The forecasted future development costs, product revenues and net income, graduated success probabilities by phase of development, and the appropriate discount rate given consideration to the market and forecast risk involved. |
The estimated fair value of the contingent payment liabilities to the BHV Members was determined using the same probability, cash flow, sale proceeds and timing assumptions referenced above, with consideration also given to the specific timing of the payments to the BHV Members pursuant to the terms of the Merger Agreement.
| 3. | Pro Forma and Acquisition Accounting Adjustments |
The unaudited pro forma condensed combined financial statements include pro forma adjustments to give effect to certain significant capital transactions of Islet Sciences occurring as a direct result of the proposed Merger, the acquisition of BHV by Islet Sciences for accounting purposes.
The unaudited pro forma condensed combined financial statements do not reflect the effect of the proposed Exchange Ratio of between 3 and 40.
The pro forma adjustments are as follows:
| | (A) | To reflect the fair market value of usable active pharmaceutical ingredients inventory of Remogliflozin Etabonate. |
| | (B) | To reflect the estimated values of in-process research and development and intangible assets associated with the development projects related to and license of Remogliflozin Etabonate. The estimated fair value of the in-process research and development and intangibles were determined using the same probability, cash flow, sale proceeds and timing assumptions used to estimate the contingent consideration payable to the BHV unit holders. |
| | (C) | To reflect the estimated value of the contingent consideration cash flows which have been probability weighted and discounted over the projected milestone periods to determine a fair value of the milestones at the time of acquisition. The milestones are tied to certain development, regulatory and commercial milestones associated with the remogliflozin etabonate program. The first potential milestone is not expected to be reached until 2016. The aggregate value of all potential milestones is $71 million. There is no guarantee that any of the milestones will be achieved. |
| | (D) | Avogenx will acquire all of the outstanding membership interests of BHV in consideration for the issuance of an aggregate of 30 million shares of common stock of Avogenx to the BHV Members, to be adjusted for the Exchange Ratio, valued at the closing stock price of $0.138 on November 24, 2014. |
| | (E) | To reflect the elimination of the historical common stock and accumulated deficit of BHV. |
CERTAIN MARKET INFORMATION WITH RESPECT TO ISLET SCIENCES COMMON STOCK
Islet Sciences common stock is quoted on the OTCQB under the trading symbol “ISLT,” and upon approval of our application for quotation, Avogenx common stock will be quoted on the OTCQB under a new trading symbol. The closing sales price of Islet Sciences common stock was $0.138 on November 24, 2014. The closing sales price of Islet Sciences common stock was $0.46 on March 13, 2014 (the day prior to the public announcement of the Transactions). The following table sets forth, for the periods indicated, the high and low closing sales price per share for Islet Sciences common stock as reported by the OTCQB.
| Fiscal Year ended April 30, | | High | | | Low | |
| 2015 | | | | | | |
| November 1 through December 2, 2014 | | $ | 0.20 | | | $ | 0.10 | |
| Quarter Ended October 31, 2014 | | | 0.31 | | | | 0.13 | |
| Quarter Ended July 31, 2014 | | | 0.30 | | | | 0.14 | |
| | | | | | | | | |
| 2014 | | | | | | |
| Quarter Ended April 30, 2014 | | $ | 0.50 | | | $ | 0.29 | |
| Quarter Ended January 31, 2014 | | | 0.98 | | | | 0.18 | |
| Quarter Ended October 31, 2013 | | | 0.51 | | | | 0.20 | |
| Quarter Ended July 31, 2013 | | | 0.51 | | | | 0.18 | |
| | | | | | | | | |
| 2013 | | | | | | | | |
| Quarter Ended April 30, 2013 | | $ | 4.75 | | | $ | 0.15 | |
| Quarter Ended January 31, 2013 | | | 5.00 | | | | 1.99 | |
| Quarter Ended October 31, 2012 | | | 3.10 | | | | 3.00 | |
| Quarter Ended July 31, 2012 | | | 6.00 | | | | 3.00 | |
At November 24, 2014 there were 318 stockholders of record of Islet Sciences common stock.
DIVIDEND POLICIES AND RESTRICTIONS
We currently intend to retain any future earnings to finance the development and growth of our business and do not anticipate paying cash dividends on our common stock in the foreseeable future, but will review this policy as circumstances dictate. If in the future we are able to pay dividends and determine it is in our best interest to do so, such dividends will be paid at the discretion of our board of directors after taking into account various factors, including our financial condition, operating results, capital requirements, restrictions contained in any future financing instruments and other factors our board of directors deems relevant.
BUSINESS OF ISLET SCIENCES
Corporate History
Islet Sciences, Inc. (the “Islet Sciences”) was incorporated under the name One E-Commerce Corporation on September 14, 1994 in the State of Nevada. Effective February 23, 2012, the company changed its name to Islet Sciences, Inc.
On December 30, 2011, Islet Sciences entered into an Agreement and Plan of Merger with ONCE, Inc., a Delaware corporation which was wholly-owned by Islet Sciences, and Islet Sciences, Inc., a Delaware corporation (“ISI”). Pursuant to the merger agreement, on December 30, 2011, ONCE, Inc. was merged with and into ISI, the holders of common stock of ISI received an aggregate of 38,005.87 shares of Islet Sciences’ Series B preferred stock, $.001 par value per share (“Series B Preferred”) in exchange for the cancellation of all of the shares of common stock of ISI formerly owned by them, and the holders of Series A preferred stock of ISI received an aggregate of 1,173 shares of Islet Sciences’ Series A preferred stock, $.001 par value per share (“Series A Preferred”) in exchange for the cancellation of all of the shares of Series A preferred stock of ISI formerly owned by them.
Effective February 23, 2012, Islet Sciences completed a 1-for-45 reverse stock split (the “Reverse Split”) of shares of its common stock. Upon effectiveness of the Reverse Split, shares of Series A Preferred were automatically converted into an aggregate of 1,173,000 shares of common stock at a conversion ratio of one share of Series A Preferred for one thousand shares of common stock, and shares of Series B Preferred were automatically converted into an aggregate of 38,005,870 shares of common stock, at a conversion ratio of one share of Series B Preferred for one thousand shares of common stock. Upon conversion of Series A Preferred, the holders received warrants to purchase an aggregate of 586,500 shares of Islet Sciences common stock at an exercise price of $1.00 per share.
Acquisition of DiaKine Therapeutics, Inc.
On March 14, 2012, Islet Sciences completed its acquisition of DiaKine Therapeutics, Inc., a Delaware corporation (“DTI”), whereby Islet Sciences issued to the DTI stockholders an aggregate of 200,000 shares of its newly designated shares of Series C convertible preferred stock, par value $0.001 per share (“Series C Preferred”), in exchange for all issued and outstanding shares of DTI. Islet Sciences also issued to DTI 100,000 shares of its common stock for no additional consideration in satisfaction of DTI’s liabilities outstanding at the closing under the agreement. As a result, DTI became a wholly-owned subsidiary of Islet Sciences. Subsequent to the closing of the DTI acquisition, the preferred stock was converted into 2,000,000 shares of common stock.
DTI’s compounds are small-molecule drugs that block the destructive, inflammatory actions of immune agents called cytokines. Cytokines have been shown in numerous studies to be components in the inflammation pathway that destroy insulin producing beta cells found in the pancreatic islets – a hallmark of type 1 diabetes and Latent Autoimmune Diabetes of Adults (LADA). Additionally, there is evidence that lipotoxicity, glucotoxicity, and other inflammatory factors induces progressive beta cell drop out in type 2 diabetes.
Acquisition of BHV
On September 30, 2014 Islet Sciences entered into the Merger Agreement with BHV. Under the Merger Agreement, in exchange for 100% ownership of BHV, Avogenx will issue 30 million shares of its common stock to the BHV Members, to be adjusted for the Exchange Ratio. Additional shares of common stock will be issued upon successful completion of development, regulatory and commercial milestones associated with the Remogliflozin program. James Green and William Wilkison, the BHV Members, are the Chief Executive Officer and the Chief Operating Officer of Islet Sciences, respectively.
Islet Sciences’ Corporate Structure
Below is Islet Sciences’ current corporate structure:
Our Business
Overview
We are a biotechnology company engaged in the research, development, and commercialization of new medicines and technologies for the treatment of metabolic diseases and related indications where there is a significant medical need. The rising incidence of obesity is associated with many obesity-related health complications, including cardiovascular disease, diabetes, hyperlipidemia, hypertension, nonalcoholic fatty liver disease/steatohepatitis (NAFLD/NASH). This constellation is also recognized as the metabolic syndrome and is characterized by underlying insulin resistance. These various diseases have interrelated risk factors and markers, such that often treatment of one disease may allow new therapies and opportunities for treatment in one of these related indications. Our focused effort to develop new therapies and related diagnostics for metabolic related diseases establishes us as a recognized leader in a large and growing market.
Metabolism is the ability of the human body and its organs to process energy derived from the intake of nutrition. Metabolic diseases typically involve a derangement or malfunction of the normal processing of such metabolism. Some of the more prevalent metabolic diseases include obesity (excessive storage of nutrients); diabetes (loss of storage capability); NAFLD/NASH (excessive storage of lipids and fibrotic accumulation in the liver); dyslipidemia (inability to process fat from the blood); hypertension (excessive elevation of blood pressure).
We are developing the following therapeutic product candidates to address the needs of patients suffering from metabolic disease:
| ● | ISLT-P, an implantable suspension of encapsulated insulin-producing porcine islet cells, for the treatment of insulin-dependent diabetes; |
| ● | ISLT-2669, a novel lead IL-12 small molecule inhibitor selected for preclinical development for treatment of type 2 diabetes. This IL12 inhibitor blocks the auto-immune and inflammatory cascade initiated by IL-12 receptor activation; and |
| ● | ISLT-LSF Analogs, a library of small molecule lisofylline analogs that block inflammatory actions of cytokines that destroy insulin-producing beta cells, for diabetes and diabetes-related complications. |
We are also developing the following diagnostic product candidate to better enable patients and their physicians to understand disease diagnosis and progression.
| ● | ISLT-Bdx, a PCR based molecular diagnostic measuring hypomethylated beta cell-derived DNA as a biomarker of beta cell loss for the early detection of type 1 diabetes or onset of insulin dependent type 2 diabetes; |
ISLT-P is an implantable suspension of encapsulated insulin-producing porcine islet cells, for the treatment of insulin-dependent diabetes. We believe that ISLT-P has significantly more commercial potential than human-to-human (allotransplantation) islet replacement approaches, due to the high cost and inherently limited supply of human islets. These implanted porcine islets are expected to produce insulin in response to increases in blood glucose, replacing the function of the patient’s destroyed pancreatic islets. Our near term development plans include initiation of an IND-enabling second species preclinical safety and efficacy study. After successful filing of an investigational new drug (“IND”) application, we anticipate initiating an open label phase I / II clinical study in patients with type 1 diabetes.
We anticipate that ISLT-B, our PCR based molecular diagnostic measuring beta-cell loss, can be the first commercially available diagnostic capable of recognizing active beta cell loss through measurement of hypomethalated beta-cell DNA circulating in bodily fluids. The diagnostic has been shown to predict the onset of type 1 diabetes as early as 625 days in advance of definitive diagnosis in populations with familial predisposition to type 1 diabetes. Early detection may provide for more effective disease management. We believe the diagnostic may also prove useful in measuring the progression of type 2 diabetics into insulin dependent type 2 diabetics. Our near term development strategy for ISLT-Bdx is the analysis of clinical serum samples from prospective type I diabetics and type II diabetics in clinical trials to confirm the ability of the assay to predict the onset of diabetes. In addition, we are establishing a state-of-the-art laboratory that will be certified under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, to perform diagnostic testing of patient serum samples. We are dedicated to further validating and commercializing our diagnostic capabilities and making our diagnostic services available to all patients who need them through sales and distribution partnerships.
ISLT-2669 is a first-in-class immune-modulating IL-12 inhibitor shown to protect insulin-producing beta-cells from cytokines responsible for cell destruction. IL-12 is a pro-inflammatory cytokine important for immune responses leading to type 1 diabetes and atherosclerosis and involves the JAK/STAT4 signaling system to induce genes linked to chronic inflammatory disorders. It has been demonstrated that hyperglycemia, obesity and diabetes markedly increase IL-12/STAT4 expression in key tissues. We identified this compound from our proprietary library of orally bioavailable immune modulators. These compounds were screened in vitro for their ability to provide beta cell protection against cytokines and preservation of insulin secretion. From this initial screening effort, we identified ISLT-2669 as a potent inhibitor of STAT4 activation. ISLT-2669 had an EC50~10nM for inhibition of cytokine-induced apoptosis of a pancreatic beta cell line and produced insulin secretion responses very similar to LSF in vitro. Importantly, basal insulin secretion was not stimulated by increasing concentrations of ISLT-2669. This compound was also very stable in rat liver microsomes and showed little inhibitory effects on various CYP enzymes. The compound appeared to have good oral bioavailability (F=77%) and a rapid tmax (~30 min). An additional study in a rat model of NASH showed that ISLT-2669 may directly reduce liver fibrosis.
We continue to create and screen another series of small molecule analogs of lisofylline (“LSF”). LSF is a novel compound shown to have anti-inflammatory properties and has been the subject of three clinical studies. In these trials, LSF was well tolerated with no subject experiencing a serious adverse event. IL-12 and STAT 4 activation are important pathways linked to inflammatory damage to insulin producing cells. It has been shown that LSF and LSF analogs reduce activation of IL-12/STAT4, leading to preservation of islet function and viability and reduction of insulin resistance. IL-12 is made directly in insulin producing beta cells of the pancreas and can directly lead to reduced insulin secretion and cell death while Stat4 deletion reduces insulin resistance. In the Phase I/II trial, LSF significantly reduced the plasma levels of several major inflammatory cytokines linked to autoimmunity. LSF however has poor pharmaceutical characteristics. Therefore, these next generation compounds have better oral bioavailability and efficacy than LSF, offering the promise of providing a new therapeutic approach to treat type 1 or type 2 diabetes. We are developing a structure/activity relationship assay as well as creating new LSF analogs based on our previously generated data.
We currently intend to develop and commercialize these product candidates ourselves or in conjunction with appropriate business partners.
Market Opportunity
Diabetes is a chronic disease that occurs when the pancreas does not produce enough insulin or when the body cannot effectively use the insulin it produces. Insulin is a hormone secreted by beta-cells in the pancreas to regulate blood sugar. Hyperglycemia (high blood sugar) is a common effect of uncontrolled diabetes and over time leads to serious damage to many of the body's systems, especially the nerves and blood vessels. According to the World Health Organization, 347 million people worldwide have diabetes with an estimated 3.4 million deaths attributable to the disease in 2010. The American Diabetes Association (“ADA”) estimates that 29 million Americans have diabetes, 9.3% of the population, with another 86 million prediabetics that account for a $176 billion direct medical expenditure. Also according to the ADA, diabetes represents the 7th leading cause of death in the United States. Standard & Poor's has estimated the annual market for diabetes medications will hit $58 billion by 2018, from about $35 billion today (http://www.fiercepharma.com/special-reports/10-top-selling-diabetes-drugs-2012).
Type 1 diabetes (previously known as insulin-dependent or juvenile diabetes) is characterized by an autoimmune attack leading to destruction of insulin producing pancreatic beta-cells. The islets of Langerhans are invaded by mononuclear cells in an inflammatory reaction termed ‘insulitis’, leading to loss of up to 70% -80% of β cells by the time of onset of clinical symptoms (9). The destruction of β cells commences years before diagnosis. The result is an inability by the pancreas to produce insulin thereby requiring daily administration of exogenous insulin. The cause of type 1 diabetes is unknown, it is not preventable and there is currently no cure for the disease. Type 2 diabetes (formerly known as adult-onset diabetes) results from the body’s ineffective use of insulin. Type 2 diabetes comprises 90% of the global diabetic population and is largely the result of excess body weight and reduced physical activity.
Over time, diabetes can lead to complications in heart, blood vessels, eyes, kidneys, and nerves. Diabetes is associated with increased risk of heart disease and stroke. 50% of people with diabetes die of cardiovascular disease (primarily heart disease and stroke) (Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001, 44 Suppl 2:S14–S21.). Combined with diabetic induced reductions in blood flow, neuropathy (nerve damage) in the feet increases incidence rates of foot ulcers, infections and limb amputation. Diabetic retinopathy is a common cause of blindness and is the result of long-term accumulated damage to blood vessels in the retina. Diabetes is among the leading causes of kidney failure (Global status report on noncommunicable diseases 2010. Geneva, World Health Organization, 2011.).
The overall risk of dying among people with diabetes is at least double the risk of their peers without diabetes (Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care, 2005, 28(9):2130–2135). The overall risk of dying among people with diabetes is at least double the risk of their peers without diabetes (Roglic G, Unwin N, Bennett PH, Mathers C, Tuomilehto J, Nag S et al. The burden of mortality attributable to diabetes: realistic estimates for the year 2000. Diabetes Care, 2005, 28(9):2130–2135).
Our Strategy
Our strategy is to transition into a clinical-stage growth company and become a leading innovator, developer and provider of therapeutics and diagnostics for patients suffering from metabolic related disease. As part of that transition we will continue to rebrand our company to better reflect our broader focus on metabolic related disease. We believe that completion of the acquisition of BHV can successfully complete our transition into a clinical stage growth company. We intend to seek additional investment in our company to support the development of our pipeline of therapeutic and diagnostic product candidates. Additionally, we intend to develop strategic relationships with other companies in order to accelerate, where possible, product development and to develop distribution channels for our product candidates. The key elements of our strategy are as follows:
| ● | Validate and commercialize ISLT-Bdx for type 1 diabetes using a CLIA certified diagnostic laboratory and a sales and distribution partner. Once the commercial market is established and validation for type 1 diabetes complete, we expect to bring forward a diagnostic product for type 2 diabetes; |
| ● | Continue preclinical development of ISLT-2669 and LSF analogs for diabetes and other autoimmune and/or inflammatory related diseases |
| ● | Advance ISLT-P into a second species IND enabling study targeting T1DM |
| ● | Form development and commercialization partnerships where appropriate; and |
| ● | Selectively acquire rights to and develop complementary product candidates. |
Intellectual Property
Islet Sciences seeks patents and other intellectual property rights to protect and preserve its proprietary technology and our right to capitalize on the results of its research and development activities. Islet Sciences is pursuing patents in the US and in foreign countries. Islet Sciences may also rely on trade secrets, know-how, continuing technological innovations and licensing opportunities to provide competitive advantages for its eventual products in various markets and to accelerate new product introductions.
Below is a list of the US patent applications held by Islet Sciences.
Islet Sciences, Inc.
| Title | | Patent Application Number | | Status |
| | | | | |
| Ex Vivo Maturation of Islet Cells | | W02103/049693 | | Pending |
| | | US/14,346,454 | | |
| | | PCT/US2012/058087 | | |
| | | | | | |
| Compositions And Methods For Detecting Hypo-Methylated DNA In Body Fluids | | PCT/US2014/020431 | | Pending National Phase begins September 2015 |
The maturation patent comprises a novel method for isolating islets cells from a porcine animal model in which the pancreas is excised, intact, from the porcine animal model which is then severed into a series of appropriate sizes and volume. The isolated and severed exocrine tissue and islet cells are then treated in-vitro with a non-specific collagenase enzyme exposure for a specific period that results in a partial digestion of the exocrine tissue comprising islets cells that are embedded within and protected by the partial digested exocrine tissue. It is the partial digestion followed by in vitro maturation that allows islets to function and be able to survive long term. In addition, the present invention further comprises a novel method for maturing a plurality of isolated porcine islet cells that are functional and can respond to glucose stimulation early in the maturation process.
The international patent application PCT/US2014/010431 provides for methods of detecting hypomethylated DNA in a process similar to that described for the beta cell loss diagnostic but in bodily fluids such as saliva and urine as opposed to blood/serum. The obvious advantage is the non-invasive nature of this testing.
Patent Applications that are licensed by Islet Sciences, Inc. from Yale University
| Title | | Patent Application Number | | Status |
| | | | | |
| Compositions and Methods of Diagnosing Diseases and Disorders associated with b cell death | | EP 2723901 | | Pending |
| | | US 14/127,906 | | |
| | | | | |
| These applications correspond to WO2012/178007 | | | | |
The family of patent applications corresponding to WO2012/178007 and PCT/US2013/028862 relate to compositions and methods for detecting cell death by detecting β-cell insulin gene DNA in a biological sample based on the discovery that the presence of extrapancreatic hypomethylated β-cell DNA is indicative of β-cell death. Thus, in one embodiment, the invention is a method of detecting hypomethylated β-cell insulin DNA in a biological sample of a subject including the steps of: obtaining a biological sample from the subject, where the biological sample is obtained from outside of the subject's pancreas, and where the biological sample contains β-cell insulin DNA; determining the methylation status of at least one of the CpG dinucleotides in the β-cell insulin DNA, where when at least one of the CpG dinucleotides in the β-cell insulin DNA is determined to be unmethylated, the hypomethylated β-cell insulin DNA is detected.
DiaKine Therapeutics, Inc.
DiaKine has licensed the rights for next generation small molecule immune modulator-anti-inflammatory drugs from the University of Virginia Patent Foundation.
US Patents licensed by DTI from the University of Virginia Patent Foundation
| Title | | Patent Application Number | | Status |
| | | | | |
| Lisofylline analogs and methods for use | | US 8,481,580 US 13/928691 | | Patent Pending |
| | | | | |
| Lisofylline analogs and methods for use | | US 13/477,613 | | Pending |
Foreign Patents and Patent Applications that are licensed by DTI from the University of Virginia Patent Foundation
| Title | | Patent Application Number | | Status |
| | | | | |
| Lysofylline Analogs and Methods for Use | | WO2007027719A2 | | Pending |
There can be no assurance that Islet Sciences will succeed in obtaining any patent protection from its pending patent applications. No assurance can be given that any patent will be issued or that the scope of any patent protection will exclude competitors or that any patent, if issued, will be held valid if subsequently challenged. There can be no assurance that any steps Islet Sciences takes in this regard will be adequate to deter misappropriation of its proprietary rights or independent third parties developing functionally equivalent products. Despite precautions, unauthorized parties may attempt to engineer, reverse engineer, copy, or obtain and use Islet Sciences’ products or other information. Although management believes that Islet Sciences’ products do not infringe on the intellectual property rights of others, there can be no assurance that an infringement claim will not be asserted in the future. The prosecution or defense of any intellectual property litigation can be extremely expensive and would place a material burden upon Islet Sciences’ working capital.
License and Supply Agreements
On May 2, 2012, Islet Sciences entered into a license agreement with the Yale University (“Yale”). Under the agreement, Islet Sciences received exclusive license to technology directed to compositions and methods for detecting cell death by detecting β-cell insulin gene DNA in a biological sample based on the discovery that the presence of extrapancreatic hypomethylated β-cell DNA is indicative of β-cell death and supports our efforts with the ISLT-BDx which is patented by Yale. In consideration of the license granted under the agreement, Islet Sciences paid Yale a license issue royalty of $10,000 (plus a $10,000 annual renewal fee) and issued 20,000 shares of its common stock, and agreed to pay certain milestones royalties by issuing an aggregate of 160,000 shares of common stock. Islet Sciences also agreed to pay to Yale a royalty on net sales. The agreement will expire automatically, on a country-by-country basis, on the date on which the last of the claims of the subject patents expires. The agreement can be terminated by Yale if Islet Sciences defaults on its obligations under the agreement and fails to cure such default within 60 days of a written notice by the university. Islet Sciences can terminate the agreement upon a six month notice subject to payment of all amounts due Yale under the agreement.
On July 23, 2012, Islet Sciences entered into a licensing agreement with the Winthrop University Hospital (“Winthrop”) to license certain patents and technology directed to compositions and methods for detecting cell death by detecting β-cell insulin gene DNA in a biological sample based on the discovery that the presence of extrapancreatic hypomethylated β-cell DNA is indicative of β-cell death and supports our efforts with the ISLT-BDx. In consideration of the license granted under the agreement, Islet Sciences agreed to pay to Winthrop a license issue royalty of $10,000 (plus a $10,000 annual renewal fee) and issue 20,000 shares of its common stock, and to pay certain milestones royalties by issuing an aggregate of 160,000 shares of common stock. Islet Sciences also agreed to pay to Winthrop a royalty on net sales. The agreement will expire automatically, on a country-by-country basis, on the date on which the last of the claims of the subject patents expires. It can be terminated by Winthrop if Islet Sciences defaults on its obligations under the agreement and fails to cure such default within 60 days of a written notice by the university. Islet Sciences can terminate the agreement upon a six month notice subject to payment of all amounts due Winthrop under the agreement. Islet Sciences is currently in default regarding its payment obligations under the foregoing agreements and is in active discussions with Winthrop University Hospital. On September 24, 2014, the parties terminated the agreement with no payment obligations.
On January 10, 2012, Islet Sciences entered into a letter agreement with Progenitor Cell Therapy, LLC (“PCT”), a subsidiary of NeoStem, Inc. (“NeoStem”), which was amended by an agreement dated May 15, 2012 by and between Islet Sciences and NeoStem, PCT’s parent company. Under the agreement, PCT was to, among other things, generate protocols, develop procedures for testing and quality control and manufacturing to support the development and submission of an investigational new drug application to the FDA for Islet Sciences’ encapsulated porcine islet cells for the treatment of diabetes. The Letter Agreement estimated the duration of services to span approximately six months beginning on January 17, 2012. The Letter Agreement required the parties to enter into a mutually agreeable Services Agreement that would more particularly set forth the services to be provided by PCT and include customary representations, warranties, covenants, indemnities and agreements between the parties. The Services Agreement was never entered into by the parties. As compensation for the services of PCT, Islet Sciences agreed to pay to PCT a non-refundable monthly fee of $63,000 and a non-refundable monthly charge of between $33,000 and $54,000. NeoStem was entitled to receive shares and warrants of Islet Sciences’ common stock, as well as additional shares for no consideration so that NeoStem’s ownership was not less than 1% of outstanding shares on a fully diluted basis. PCT was also given the exclusive right for a period of ten years to manufacturer any product involved in the services to be provided under the agreement. With respect to commercial production of such products, PCT will be entitled to a royalty on gross sales and a percentage of sublicensing fees, royalties, milestone fees or profit sharing payments.
On February 7, 2013, the services had not yet been completed and Islet Sciences provided to NeoStem Inc. notice of termination of the agreement with PCT dated January 10, 2012, as amended by the agreement dated May 15, 2012 by and between Islet Sciences and NeoStem.
On April 25, 2014, LLC, PCT filed a lawsuit against Islet Sciences in the United States District Court for the District of New Jersey (Case No. 2:14-cv-02658-SDW-MCA). PCT’s complaint asserts various claims, including breach of contract and unjust enrichment, based on the alleged failure of Islet Sciences to pay for services and goods provided by PCT under a January 10, 2012 letter agreement. See “Legal Proceedings” below.
On July 23, 2012, Islet Sciences entered into a long-term supply agreement with Spring Point Project, a source animal facility to purchase pigs for use in Islet Sciences’ xenotransplantation research. On August 12, 2013, Islet Sciences received from Spring Point Project a notice of termination of the supply agreement, effective November 10, 2013, unless Islet Sciences cures the default before that date. The default was not cured and the agreement with Spring Point Project has been terminated.
In August 2012, Islet Sciences entered into an agreement with the Regents of the University of California, Los Angles, (“UCLA”), whereby UCLA was provided work dealing with small molecule mediated porcine islet proliferation. Work under this agreement will be performed for a period of six months with an estimated cost of $23,100. Islet Sciences had continued to work with UCLA on a month to month basis following the six month period. On February 25, 2014, Islet Sciences terminated its licensing agreement with UCLA.
In May 2013, Islet Sciences entered into a sales and services agreement with the Regents of the University of California, Irvine (“UCI”) to provide materials consisting of isolated islets to be supplied to the Spring Point Project. Islet Sciences terminated this agreement on March 24, 2014 for settlement payments totaling $150,000 on total liabilities of approximately $305,000 for expenses incurred in this and last fiscal year.
In September 2013, Islet Sciences entered into a consulting agreement with American Capital Ventures, Inc. to provide consulting services related to investor relations and corporate communications. Islet Sciences terminated this agreement on January 23, 2014.
Competition
The biotechnology and pharmaceutical industries are highly competitive and characterized by rapid technological change. We face significant competition in each of the aspects of our business from other pharmaceutical and biotechnology companies. Many of our competitors have substantially greater research and development capabilities and financial, scientific, marketing and human resources than we do. As a result, our competitors may succeed in developing products earlier than we do, obtaining approvals from the Food and Drug Administration or FDA or other regulatory agencies for those products more rapidly than we do, or developing products that are more effective than those we propose to develop. Similarly, our collaborators face similar competition from other competitors who may succeed in developing products more quickly, or developing products that are more effective, than those developed by our collaborators. Any products that we may develop or discover are likely to be in highly competitive markets.
Government Regulation
Overview
The development and commercialization of our products will be subject to extensive regulation in the U.S. by a number of regulatory authorities, including the FDA, and by comparable regulatory authorities in foreign countries. These regulatory authorities and other federal, state and local entities will regulate, among other things, the preclinical and clinical testing, safety, effectiveness, approval, manufacturing, labeling, packaging, export, storage, recordkeeping, adverse event reporting, and promotion and advertising of our products. We will require FDA approval of our products in the United States, including a review of the manufacturing processes and facilities used to produce our products, before we may market the products in the U.S.
ISLT-P will be classified as combination biological and device products by the CBER (Center for Biologicals Evaluation and Research), another division within the FDA. The islets comprise the biological component and the microencapsulation material comprises the device component. The FDA Center for Biologics (“CBER”) has jurisdiction, meaning the ISLT-P product will be reviewed primarily as a biological product. Finally, ISLT-Bdx ISLTDX will be subject to approval by the Medical Device division of the FDA.
Clinical Trial Process
For the ISLT-P and ISLT-2669 and LSF inhibitory analogs, the development of a therapeutic product for human use under applicable laws and regulations is a multi-step process. First, in vitro and/or animal testing must be conducted in a manner consistent with good laboratory practices to establish the potential safety and effectiveness of the experimental product with regard to a given disease. Before human clinical trials may begin for new drugs and biologics, an IND application containing, among other things, preclinical data, chemistry, manufacturing and control information, an investigative plan, must be submitted to the FDA. The Clinical trials following approval of an IND will also require the approval and oversight by an Institutional Review Board (“IRB”) to assure proper patient protection. Clinical trials of certain medical devices generally require the same sort of submission in the form of an application for an investigational device exemption (“IDE”). We believe that the submission of an IND will be adequate for ISLT-P, and that a separate IDE submission will not be required for either product. Once a trial begins, changes to the investigational product or study protocol may require prior approval before implementation. There can be no assurance that submission of an IND application or an IDE will result in the ability to commence clinical trials. In addition, the FDA may place a clinical trial on hold or terminate it at any phase if, among other reasons, it concludes that clinical subjects are being exposed to an unacceptable health risk. Finally, pursuant to the FDA's Bioresearch Monitoring (“BIMO”) program, the FDA conducts on-site inspections and data audits of the conduct and reporting of all FDA-regulated research. The FDA's BIMO compliance programs address inspections of non-clinical testing labs, clinical investigators, clinical trial sponsors/monitors and IRBs.
Clinical trials of pharmaceuticals or biologics typically involve three phases, although those phases can overlap.
● Phase I is conducted to evaluate the basic safety, metabolism, and pharmacology, and pharmacokinetics, and to identify potential side effects with escalating doses, the maximum tolerated dose of the experimental product in humans, and if possible, to begin to evaluate various routes, dosages, and schedules of product administration. These studies are often conducted in healthy subjects. Phase I trials are not intended to find early indications of effectiveness; however, it is not uncommon to evaluate these endpoints.
● Phase I/II clinical trials are conducted to evaluate safety and initial efficacy indications in the patient population afflicted with a specific disease or condition for which the product is intended for use.
● Phase II clinical trials are conducted in groups of patients afflicted with a specific disease or condition for which the product is intended for use in order to further test safety, begin evaluating effectiveness, optimize dosage amounts and determine dose schedules and routes of administration.
● Phase III studies are usually randomized, double blind studies testing for product safety and effectiveness in an expanded patient population in order to evaluate the overall risk/benefit relationship of the product and to provide an adequate basis for product labeling. These studies also may compare the safety and effectiveness of the product with currently available products.
Employees
As of November 24, 2014, Islet Sciences had 3 full-time and 2 part time employees. Islet Sciences also utilizes the services of consultants and advisors on an appropriate as needed basis.
Emerging Growth Company
Islet Sciences is an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”), and may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to, not being required to comply with the auditor attestation requirements of section 404(b) of the Sarbanes-Oxley Act, and exemptions from the requirements of Sections 14A(a) and (b) of the Securities Exchange Act of 1934 to hold a nonbinding advisory vote of stockholders on executive compensation and any golden parachute payments not previously approved.
Islet Sciences has elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(1) of the JOBS Act. This election allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. As a result of this election, our financial statements may not be comparable to companies that comply with public company effective dates.
We will remain an “emerging growth company” until the earliest of (1) the last day of the fiscal year during which our revenues exceed $1 billion, (2) the date on which we issue more than $1 billion in non-convertible debt in a three year period, (3) the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement filed pursuant to the Securities Act of 1933, as amended, or (4) when the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter.
To the extent that we continue to qualify as a “smaller reporting company,” as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, after we cease to qualify as an emerging growth company, certain of the exemptions available to us as an emerging growth company may continue to be available to us as a smaller reporting company, including: (1) not being required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes Oxley Act; (2) scaled executive compensation disclosures; and (3) the requirement to provide only two years of audited financial statements, instead of three years.
Properties
Our principal place of business is at 6601 Six Forks Rd, Suite 140 Raleigh, NC 27615.
Legal Proceedings
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business. We are currently not aware of any such legal proceedings or claims that we believe will have a material adverse effect on our business, financial condition or operating results.
In April 2012, Sand Dollar Partners, LLC, (“Sand Dollar”), a stockholder of Islet Sciences, filed a complaint in the Superior Court of Arizona, Pima County against, among other parties, Islet Sciences, Inc. a Delaware corporation (“ISI”), our wholly-owned subsidiary, John Steel, our former CEO and director, and Jonathan Lakey. In 2010, Sand Dollar invested $357,000 in ISI through the purchase of a convertible promissory note which was converted into 3,591,729 shares of Islet Sciences’ common stock. The plaintiff contends that it was entitled to issuance of additional shares and nomination of one board member.
On October 25, 2013, Islet Sciences entered into a settlement agreement with Sand Dollar. At a hearing on February 21, 2014, Islet Sciences and Sand Dollar agreed to amend the settlement agreement whereby Sand Dollar placed in escrow all of Islet Sciences’ common stock it held and retained a broker dealer to sell sufficient shares to receive $500,000 in cash and to pay fees to the broker dealer. Additionally, Islet Sciences agreed to issue 130,000 warrants that will vest over the next three years and make a $30,000 payment on May 16, 2014. Islet Sciences issued these warrants and made the $30,000 payment on May 16, 2014 fully completing the settlement and will receive back the remaining 1,344,529 shares of common stock. The broker dealer sold 2,247,200 shares of Islet Sciences’ escrowed stock to settle the obligation and Islet Sciences received back the remaining 1,344,529 shares of its common stock.
In July 2012, a complaint was filed against Islet Sciences, and John Steel in the United States District Court for the District of Utah, Central Division for infringement and misappropriation of a patent. The plaintiffs contend that they were the actual purchasers of the MicroIslet patent out of MicroIslet’s bankruptcy proceedings in 2009 and that the respective intellectual property rights have been never assigned to either ISI or Islet Sciences. As a result, they allege that Islet Sciences’ claim to the ownership of the MicroIslet patent based on the assignment of the patent by its founders is baseless. The complaint sought monetary damages including punitive damages of at least $12 million, costs, attorneys’ fees, and declaratory judgment. On January 8, 2013 the Court dismissed the plaintiff’s action for lack of recoverable damages. Between March and May of 2013, a settlement agreement was negotiated, and partly implemented, between the plaintiffs and defendants in the litigation. The settlement failed because a third party defendant was unable to deliver a portion of his shares pursuant to the settlement agreement. Islet Sciences fully complied with its obligations under the settlement agreement but the plaintiff repudiated the settlement nonetheless.
After the settlement failed, on July 3, 2013, plaintiffs filed their complaint, which Islet Sciences successfully moved to dismiss and plaintiffs filed an amended pleading. Islet Sciences moved to dismiss the amended complaint. Instead of hearing the motion to dismiss, the court is now considering whether the 2013 failed settlement agreement is enforceable. We believe the plaintiffs’ claims to be without merit and will continue to vigorously defend against this action.
On April 25, 2014, Progenitor Cell Therapy, LLC (“PCT”), a subsidiary of NeoStem, Inc. (“NeoStem”), filed a lawsuit against Islet Sciences in the United States District Court for the District of New Jersey (Case No. 2:14-cv-02658-SDW-MCA). PCT’s complaint asserts various claims, including breach of contract and unjust enrichment, based on the alleged failure of Islet Sciences to pay for services and goods provided by PCT under a January 10, 2012 letter agreement. PCT seeks an unspecified amount of compensatory and other damages, plus interest and costs. Islet Sciences filed an answer to the complaint on June 24, 2014 denying all liability, including on the grounds that the January 10, 2012 letter agreement is unenforceable and PCT failed to provide the goods and services it stated it would provide. In connection with its answer to PCT’s complaint, Islet Sciences filed a counterclaim against PCT and a third-party complaint against NeoStem to seek, among other things, (i) a declaration that the January 10, 2012 letter agreement is unenforceable, (ii) monetary damages and (iii) rescission of equity securities Islet Sciences previously issued to NeoStem. Islet Sciences intends to vigorously defend the claims by PCT and prosecute its claims against PCT and NeoStem. As the lawsuit is at an early stage, Islet Sciences cannot at this time estimate the possible loss or range of loss, if any, that may result from this lawsuit.
On October 22, 2014, a stockholder of Islet Sciences holding more than 5% of its outstanding common stock threatened a legal action against Islet Sciences alleging material misrepresentations by Islet Sciences’ management in connection with his investment in Islet Sciences common stock and demanded rescission of his investment. Islet Sciences believes such allegations to be without merit and intends to vigorously defend against a formal legal action should the stockholder institute such a proceeding.
BHV is a privately-held clinical stage pharmaceutical company focused on the development of Remo, a novel new chemical entity being developed for the treatment of type 2 diabetes (“T2DM”) and non-alcoholic steatohepatitis (“NASH”). BHV was organized as a North Carolina limited liability company in 2009 with the purpose of advancing the development of Remo. In 2010, BHV executed a license for the exclusive world-wide rights to Remo, excluding the territories of Japan, Korea and Taiwan. BHV is headquartered in Research Triangle Park, North Carolina. The founders, William Wilkison, Ph.D. and James Green, remain the sole members of BHV.
In August 2014, BHV granted an exclusive license to LIBBS North America, LLC (“LIBBS”), a wholly-owned subsidiary of LIBBS Farmacêutica Ltda. of Brazil, for the development and commercialization of Remo for all human therapeutic, palliative, and prophylactic uses in certain countries in Latin America, including Brazil. In exchange for these rights, LIBBS is required to make a payment to BHV upon BHV’s submission of the study protocol for BHV’s currently-planned phase IIb study of Remo for type 2 diabetes to the FDA and approval of the protocol by the responsible Institutional Review Board, and to make profit-sharing and royalty payments to BHV based on sales of Remo in LIBBS’s exclusive territory. LIBBS also agreed to pay for the cost of BHV’s currently-planned phase IIb study of Remo for type 2 diabetes, up to an agreed maximum amount. LIBBS is required to use commercially reasonable efforts to develop and commercialize Remo in LIBBS’s exclusive territory at LIBBS’s cost.
Remo is a highly selective phase II sodium glucose co-transporter 2 (“SGLT2”) inhibitor that demonstrates best-in-class efficacy and tolerability profiles. SGLT2 inhibitors are an important new class of metabolic disease compounds showing robust insulin-independent HbA1c lowering and weight loss, which work by inhibiting reabsorption of glucose in the kidney. SGLT2 inhibitors are expected to be the only oral diabetes therapy to safely provide glycemic control with significant weight loss.
Remo was discovered and developed preclinically by Kissei Pharmaceuticals (“Kissei”) of Japan. In 2002, Kissei licensed its SGLT2 program to GlaxoSmithKline (“GSK”) where Dr. Wilkison was a member of the project team. Remo emerged as the lead candidate in 2005. GSK performed fifteen phase I, three phase IIa, and two phase IIb clinical trials before terminating its license to the program in mid-2009 and returning all rights and interests to Kissei. The BHV team, recognizing the unique attributes of Remo, has developed a formulation of Remo which they predict will yield the efficacy and safety necessary to commercially differentiate from other SGLT2 inhibitors.
The reformulation of Remo is complete and was administered in a clinical study measuring pharmacodynamics and pharmacokinetics in health volunteers. Remo is now positioned for a confirmatory Phase IIb trial. In addition to being a once-daily oral treatment for T2DM, BHV believes Remo may be an effective treatment for type 1 diabetes and fatty liver disease/non-alcoholic steatohepatitis (NASH).
SGLT2 Mechanism of Action
In the kidney, glucose is filtered in the glomerulus and is reabsorbed through active transport mechanisms in the proximal tubule. Both sodium-glucose co-transporters, SGLT1 and SGLT2, are responsible for glucose reabsorption. SGLT2 is expressed exclusively in the proximal tubule and accounts for approximately 90% of glucose reabsorption. SGLT1, which is also expressed in GI and cardiac tissues, among others, accounts for the remaining approximate 10% of reabsorption. SGLT1 inhibition is known to cause significant GI distress; its effect on cardiac tissue is not completely known but it has been demonstrated that SGLT1 plays an active metabolic role in cardiac tissue.
Treating hyperglycemia with drugs that block renal glucose reabsorption through the SGLT2 transporter represents a novel approach to the treatment of type 2 diabetes. In non-diabetics, plasma glucose is filtered by the kidneys, but virtually all of it is reabsorbed. Inhibiting functional SGLT2 in the kidney reduces glucose reabsorption and therefore, more excess blood glucose is excreted into the urine. Although this approach does not directly target glucose metabolism, it does promote lowering of circulating glucose, weight loss and indirectly improves many features of the condition, including increased hepatic glucose output and pancreatic beta-cell failure.
Remo, as well as other SGLT2 inhibitors, are efficacious in reversing insulin resistance. However, Remo is unique among other SGLT2 inhibitors in that the Remo molecule conveys a significant anti-oxidant activity. Therefore, it is the combination of these two activities that will be suitable for the treatment of NAFLD/NASH.
Pre-Clinical Summary
The DMPK package, as well as long term safety studies in rodents and dog, including two-year oncology studies, are complete. Toxicology studies in dog (52 week), rat (26 week) and mouse (13 week) demonstrate a very wide therapeutic window. Multiple diabetic rodent models also demonstrated significant plasma glucose, as well as reduction of fatty liver disease and weight loss.
Clinical Summary
Remo has been administered in sixteen phase I, three phase IIa, and two phase IIb clinical studies. Approximately 850 subjects, including 496 subjects with T2DM, have been dosed with Remo. Remo has demonstrated dose-related glycosuria consistent with SGLT2 inhibition with best in class lowering of HbA1c, plasma glucose, and body weight observed in subjects with T2DM over 12 weeks of treatment. Remo has also proven to be well tolerated with low incidences of urogenital infections over 12 weeks of treatment in subjects with T2DM.
Phase I Clinical Studies
Remo has proven to be safe and well tolerated. Fifteen phase I studies, with doses up to 4000 mg/day, have been performed in order to measure the PK, safety and drug-drug interaction of Remo. Remogliflozin etabonate (prodrug) was rapidly absorbed and extensively converted to its active entity, Remogliflozin. Drug exposures were proportional to the dose administered and there were no apparent age or sex effect on the PK. There were no clinically significant changes in vital signs or ECGs and a thorough QtC study showed no effects of the drug on cardiac function. Subjects with mild and moderate renal impairment did not demonstrate any decrease in pharmacodynamic efficacy (as measured by UGE) or alteration in plasma PK. Drug-drug interaction studies with ketaconazole, bupropion, and metformin demonstrated no adverse effects of Remo on these compounds or vice versa.
Phase IIa Clinical Studies
Three phase IIa studies were conducted in subjects with T2DM. One study (KG219017) demonstrated that Remo caused FPG lowering in type 2 diabetics. All patients taking Remo also had clinically significant increases in urinary glucose excretion. Another study (KGW108201) utilizing echo MRI demonstrated that the weight loss observed with patients taking Remo was due to adipose (fat) loss and not fluid or lean mass loss. A third study (KG2110243) demonstrated that Remo was safe and effective when co-dosed with metformin. Fifty poorly controlled T2DM subjects taking metformin were treated with Remo for 13 days. The weighted mean glucose and insulin values following an oral glucose tolerance test (75 g glucose) were decreased in all treatment groups.
Phase IIb Clinical Studies
Two 12-week phase IIb studies of Remo were completed. The studies were multi-center, randomized, double blind, placebo-controlled, parallel-group, and dose-ranging with pioglitazone as an active comparator. The primary objective of both studies was to determine the dose response and efficacy of a range of doses of Remo versus placebo on the change from baseline in HbA1c over 12 weeks. Secondary objectives included additional markers of glycemic control, body weight and waist circumference. In addition, the safety and tolerability of Remo were characterized. Efficacy measurements included HbA1c, fasting plasma glucose, weight change and blood pressure. Safety measurements included clinical chemistry, UTI, GFI, and incidence of hypoglycemia. HbA1c entry criterion for both studies was ≥7.0% to ≤9.5%. In all doses, there were clinically significant reductions in HbA1c compared to placebo. The decreases in HbA1c were rapid, and were seen as early as Week 4 and continuing through to Week 12.
Study KG2110375 (“Remo QD”) was conducted to determine the dose response and efficacy of a range of once-daily doses with 252 subjects randomized across 14 countries. Clinically significant decreases from baseline in FPG were observed at Week 12 compared with placebo in all Remo once daily doses but were not as robust as the twice daily doses. Changes in FPG were seen as early as Week 2 and were maintained throughout to Week 12. In addition, the 24-hour UGE level increased from baseline in all remogliflozin etabonate treatment groups. Statistically significant decreases in body weight from baseline compared with placebo were observed at Week 12.
Study KG2105255 (“Remo BID”) was conducted to determine the dose response and efficacy of a range of twice-daily doses with 336 subjects randomized across 18 countries. A clinically significant dose response for the change from baseline in HbA1c at week 12 and significant decreases in HbA1c versus placebo were seen for all Remo doses. The decrease in HbA1c was generally rapid, beginning as early as Week 4. Significant dose-related decreases from baseline in FPG were observed at week 12 in all Remo doses compared with placebo, with changes seen as early as week 2. Similarly, 24-hour urinary glucose excretion (“UGE”) increased from baseline in all Remo treatment groups at week 12. Statistically significant decreases in body weight from baseline compared with placebo were observed at week 12.
Remogliflozin etabonate was efficacious for Hba1c lowering in both twice daily and once daily dosing, although the magnitude of decrease in Hba1c was greater with twice daily dosing. The compound also caused weight loss in both studies. Remogliflozin was well tolerated in both studies, with low incidences of hypoglycemia, urinary tract infection and genital infection.
Phase IIb Safety Profile
In both the QD and BID phase IIb studies, Remo was safe and well tolerated with no drug related SAEs. The overall rate of AEs across the treatment groups was low and the AE’s were all considered mild. The incidence of reported hypoglycemic events was low and were mild to moderate in intensity. There was also a low incidence of renal/urinary AEs. There were no significant differences from baseline in ECG. There were no significant changes in clinical chemistry parameters related to bone metabolism.
BHV Development Strategy
BHV’s development strategy is to pursue a commercially differentiated product profile through a non-obvious formulation of remo. BHV’s objective is to develop Remo in a once daily dosage form with a pharmacokinetic profile that maintains the lower incidence of urogenital infections and no increase in LDLc seen with once-daily dosing while maintaining the best-in-class Hba1c lowering and weight loss seen with twice-daily dosing. The rationale for this strategy is revealed with a better understanding of Remo’s chemistry relative to the biology of SGLT2 inhibition. Further understanding of the chemistry of competing molecules in the class relative to their effect on the biology provides BHV’s development strategy with a defensible competitive advantage unique to Remo.
SGLT2 inhibitors in development have proven to effectively improve glycemic control in diabetic patients. However due to the competitive inhibitory effect, an SGLT2 inhibitor is maximally effective at elevated plasma glucose concentrations. Therefore, SGLT2 inhibitors work best during postprandial glucose excursions. Glucose levels tend to peak around 1 hour after the start of a meal and typically return to pre-prandial levels within 2–3 hours. So maintaining plasma concentrations of an SGLT2 inhibitor that maximally blocks renal glucose resorption during mealtime glucose excursions should result in optimal plasma glucose control while otherwise providing a minimal effect on UGE during times of steady-state blood glucose. This approach is very similar to the treatment of postprandial glucose excursions with acarbose or other alpha-glucosidase inhibitors.
Diabetics are known to be more susceptible to urogenital fungal infections than non-diabetics. SGLT2 inhibitors have been known to cause increased incidences of urogenital funfal infections in these subjects. While the exact cause has not been well characterized in the literature, it is thought to be due, in part, to increased urinary glucose excretion that creates an ideal environment for microbial growth. Another cause of urogenital infections may be nighttime incontinence. It has been shown that nighttime incontinence (leakage) is more predictive of urogenital infection in diabetics than daytime incontinence. Nighttime inhibition of SGLT2 also leads to mild increases in nighttime incontinence by increasing the amount of urinary glucose in the bladder and subsequently causing increased fluid accumulation due to the osmotic pressure of the glucose. Therefore, by extension, BHV believes, and has data supporting this position, that nighttime inhibition of SGLT2 leads to the increase incidences of urogenital infections.
Remo is an O-aryl glucoside, which typically has a shorter half-life than the other C-aryl glucoside SGLT2 inhibitors. Once-daily dosing using solely the instant release formulation limited Remo’s ability to significantly inhibit SGLT2 late in the day (i.e., blunt the dinner postprandial glucose excursion), thus reducing the upper end of HbA1c lowering and weight loss relative to the demonstrated effects of Remo dosed twice-daily. Even with its shorter half-life, Remo dosed once-daily demonstrates primary efficacy endpoints comparable to competing molecules. However when dosed twice-daily, Remo clearly demonstrated superior relative efficacy. So if inhibiting SGLT2 during periods of normal or reduced blood glucose concentration does not provide additional clinical benefit, and overnight inhibition of the SGLT2 transporter promotes urogenital infections, the ideal SGLT2 inhibitor would be one that provides sufficient plasma coverage during the largest daytime glucose incursions without having an inhibitory effect overnight. This suggests that a formulation of Remo that effectively inhibits SGLT2 during the three major glucose incursions associated with meals but limits drug exposure to the waking hours would deliver an ideal combination of the superior efficacy demonstrated with Remo dosed BID with the low rate of urogenital infections demonstrated with Remo dosed QD. Given the long half-life of C-aryl glucosides, BHV does not believe the competitor SGLT2 inhibitors can achieve this 24 hour exposure pattern.
BHV believes this ideal pattern of SGLT2 inhibition is viable and easily achievable using a bi-phasic formulation of Remogliflozin. BHV’s development plan is to couple a dose of instant release (“IR”) Remo with a dose of delayed release (“DR”) Remo in a once daily dosage form. BHV also believes this formulation will deliver superior differentiation on HbA1c lowering, weight loss, UTI and GFI.
A bi-phasic formulation of Remogliflozin etabonate will provide an improved effect on daytime urinary glucose excretion that will lead to greater decreases in fasting plasma glucose and HbA1c, plus greater calorie excretion and weight loss. This formulation will also reduce nighttime SGLT2 inhibition during the sleep/fasting period resulting in reduced drug related incidence of urogenital infections, as well as adverse effects on bone and lipid metabolism.
Once daily dosing of remogliflozin etabonate leads to no significant changes in LDL-c. This is in contrast to what is observed with twice daily dosing of Remo (and other long half-life SGLT2 inhibitors). The major difference between these dosing regimens (once daily versus twice daily) is overnight inhibition of SGLT2 in the twice daily study and lack of overnight inhibition in the once-daily study. Therefore, BHV believes overnight inhibition of SGLT2 likely plays a role in the observed increases in LDL-c with SGLT2 inhibitors that inhibit the transporter overnight. This effect is likely due to a carbohydrate deprived state during sleep (with overnight inhibition of SGLT2) leading to mobilization and metabolism of VLDL into LDLc.
Type 2 Diabetes Overview
Type 2 diabetes is a progressive metabolic disorder characterized by insulin insensitivity, relative insulin deficiency, consequent hyperglycemia and increased incidence of cardiovascular complications. T2DM, sometimes referred to as adult-onset diabetes, accounts for ~90% of all diagnosed cases of diabetes and is a lifelong disease for which there is no known cure. Patients are typically treated using a combination of prescribed exercise, dietary modification and drug therapies. It is estimated that patients with diabetes have medical expenditures that are over 2 times higher than those without diabetes. Total healthcare related costs for the treatment of diabetes are estimated at $175 billion with $117 billion attributable to direct medical costs and $58 billion from indirect costs such as disability payments, lost productivity, and premature death. Currently available diabetes treatments have many drawbacks, including tolerability issues, weight gain, and loss of efficacy over time (diabetes therapies are typically taken in combination to help prolong their effectiveness). There remains a vast unmet need for new oral, insulin independent treatments for patients with T2DM.
NASH/NAFLD
In parallel with the increasing incidence of obesity and insulin resistance in the general population is the occurrence of fatty liver. Non-Alcoholic Fatty Liver Disease (“NAFLD”) is a chronic hepatic disorder that may affect up to 25% of the general population; including a significant portion of pediatric cases. NAFLD can progress to Nonalcoholic steatohepatitis (“NASH”) which is thought to affect 2%-5% of the U.S. population.
NASH is characterized by increased fibrosis, cirrhosis and chronic inflammation which can lead to end-stage liver disease. Current predictions estimate 40% of the population will be obese by 2025, and that NAFLD/NASH will become the leading cause of liver disease and liver transplantation. Even now the demand for livers suitable for transplant and non-transplantable livers for research far exceeds availability, leaving an urgent need for alternative human cell-based models.
NAFLD/NASH is a common, often “silent” liver disease. It resembles alcoholic liver disease, but occurs even in people who drink little or no alcohol. The major feature of NAFLD/NASH is fat in the liver, along with inflammation and damage. Most people with NAFLD/NASH generally feel well and are not aware of their liver problem. Left untreated NAFLD/NASH leads to liver fibrosis and ultimately cirrhosis of the liver, in which the liver is permanently damaged and no longer able to function properly. While the underlying reason for NAFLD/NASH is not known, two key factors thought to be responsible include insulin resistance and oxidative stress inside liver cells.
Although NAFLD/NASH has become more common, its underlying cause is still not clear. It most often occurs in persons who are middle-aged and overweight or obese. Many patients with NAFLD/NASH have elevated blood lipids, such as cholesterol and triglycerides, and many have diabetes or pre-diabetes, but not every obese person or every patient with diabetes has NAFLD/NASH. Furthermore, some patients with NAFLD/NASH are not obese, do not have diabetes, and have normal blood cholesterol and lipids. NAFLD/NASH can occur without any apparent risk factor and can even occur in children. Thus, NAFLD/NASH is not simply obesity that affects the liver. While the underlying reason for the liver injury that causes NASH is not known, several factors are possible candidates:
| ● | release of toxic inflammatory proteins by fat cells (cytokines) |
| ● | oxidative stress (deterioration of cells) inside liver cells |
Currently, no specific therapies for NAFLD/NASH exist. The most important recommendations given to persons with this disease are to reduce their weight (if obese or overweight), follow a balanced and healthy diet, increase physical activity and avoid alcohol. Experimental approaches under evaluation in patients with NAFLD/NASH include antioxidants, such as vitamin E, selenium, and betaine. These medications act by reducing the oxidative stress that appears to increase inside the liver in patients with NAFLD/NASH. Another experimental approach to treating NAFLD/NASH is the use of newer antidiabetic medications—even in persons without diabetes. Most patients with NAFLD/NASH have insulin resistance, meaning that the insulin normally present in the bloodstream is less effective for them in controlling blood glucose and fatty acids in the blood than it is for people who do not have NAFLD/NASH. The newer antidiabetic medications make the body more sensitive to insulin and may help reduce liver injury in patients with NAFLD/NASH.
An ideal therapy would combine the anti-oxidant effect with reductions in insulin resistance. Remogliflozin has clinically validated anti-diabetic activity with demonstrated improvements in insulin resistance and reductions in liver triglyceride content in a fatty liver disease mouse model. Additionally, BHV has recently established that Remogliflozin has significant anti-oxidant activity 10 fold greater than Canagliflozin (Cana) and Dapagliflozin (Dapa). Therefore Remogliflozin would be an ideal molecule to develop to address this large unmet medical need.
Intellectual Property
Below is a list of the US patent applications held by BHV.
| Title | | Patent (Application) Number | | Status | | Ownership |
| | | | | | | |
| Glucopyranosyloxypyrazole derivatives, medicinal compositions containing the same and intermediates in the production thereof | | US 6,972,283; 7,056,892; 7,115,575; | | Patent | | Licensed |
| | | | | | | |
| Glucopyranosyloxypyrazole Derivates and Use Thereof in Medicines | | US 7,084,123; 7,393,838; 7,465,713 | | Patent | | Licensed |
| | | | | | | |
| Progression Inhibitor For Disease Attributed To Abnormal Accumulation Of Liver Fat | | US 12/511,654 | | Pending | | Licensed |
| | | WO2006/009149 | | | | |
| | | | | | | |
| Process For Production Of Glucopyranosyloxypyrazole Derivative | | US 8,022,192 | | Patent | | Licensed |
| | | WO2006/098413 | | | | |
| | | | | | | |
| Combination immediate/delayed release delivery system for short half-life pharmaceuticals including remogliflozin | | PCT/US2011/043143 | | Pending | | Owned |
| | | WO2012/006398 | | | | |
The first two sets of issued patents relate to composition of matter patents for glucopyranosyloxypyrazole derivatives representing remogliflozin (the active circulating species) and remogliflozin etabonate (the prodrug) which have inhibitory activity in human SGLT2 and are useful as agents for the prevention or treatment of diabetes, diabetic complications or obesity, and to pharmaceutical compositions comprising the same and intermediates thereof. The patent application US 12/511,654 and WO2006/0091149 provides a pharmaceutical composition characterized by containing a sodium/glucose cotransporter 2 inhibitor as an active ingredient. This pharmaceutical composition is capable of inhibiting any abnormal accumulation of fat in the liver and is highly suitable for use as a progression inhibitor for not only general fatty liver, but also non-alcoholic fatty liver (NAFL), non-alcoholic steatohepatitis (NASH), hyperalimentation-induced fatty liver, diabetic fatty liver, alcohol-induced fatty liver or toxic fatty liver. US 8,022,192 and WO2006/098413 discloses a process for producing a glucopyranosyloxypyrazole derivative, such as remogliflozin etabonate, which is useful as a prophylactic or therapeutic agent for a disease induced by hyperglycemia, such as diabetes, a diabetes complication and obesity. This process is quite useful as the process for producing a pharmaceutical. The family of patent applications corresponding to WO2012/006398 (as well as South African patent ZA 2013/00877) relate to a combination immediate/delayed release delivery system for compounds which have short half-lives, such as the antidiabetic remogliflozin etabonate. This delivery system provide a dosage form that has two distinct phases of release, a formulation that promotes immediate release of the compound upon ingestion and another formulation which delays the release of the compound so that a once-daily dosing regimen of remogliflozin etabonate may be achieved while providing effective control of plasma glucose and minimizing the nighttime exposure of this compound. Methods for forming the so-described immediate/delayed release delivery system and using such delivery system for treating diabetes are also provided.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF ISLET SCIENCES
CERTAIN STATEMENTS IN THIS REPORT, INCLUDING STATEMENTS IN THE FOLLOWING DISCUSSION, ARE WHAT ARE KNOWN AS “FORWARD-LOOKING STATEMENTS”, WHICH ARE BASICALLY STATEMENTS ABOUT THE FUTURE. FOR THAT REASON, THESE STATEMENTS INVOLVE RISK AND UNCERTAINTY SINCE NO ONE CAN ACCURATELY PREDICT THE FUTURE. WORDS SUCH AS “PLANS”, “INTENDS”, “WILL”, “HOPES”, “SEEKS”, “ANTICIPATES”, “EXPECTS” AND THE LIKE OFTEN IDENTIFY SUCH FORWARD-LOOKING STATEMENTS, BUT ARE NOT THE ONLY INDICATION THAT A STATEMENT IS A FORWARD-LOOKING STATEMENT. SUCH FORWARD-LOOKING STATEMENTS INCLUDE STATEMENTS CONCERNING OUR PLANS AND OBJECTIVES WITH RESPECT TO THE PRESENT AND FUTURE OPERATIONS OF THE COMPANY, AND STATEMENTS WHICH EXPRESS OR IMPLY THAT SUCH PRESENT AND FUTURE OPERATIONS WILL OR MAY PRODUCE REVENUES, INCOME OR PROFITS. NUMEROUS FACTORS AND FUTURE EVENTS COULD CAUSE THE COMPANY TO CHANGE SUCH PLANS AND OBJECTIVES OR FAIL TO SUCCESSFULLY IMPLEMENT SUCH PLANS OR ACHIEVE SUCH OBJECTIVES, OR CAUSE SUCH PRESENT AND FUTURE OPERATIONS TO FAIL TO PRODUCE REVENUES, INCOME OR PROFITS. THEREFORE, THE READER IS ADVISED THAT THE FOLLOWING DISCUSSION SHOULD BE CONSIDERED IN LIGHT OF THE DISCUSSION OF RISKS AND OTHER FACTORS CONTAINED IN THIS CONSENT SOLICITATION STATEMENT/PROSPECTUS AND IN THE COMPANY'S OTHER FILINGS WITH THE SECURITIES AND EXCHANGE COMMISSION. NO STATEMENTS CONTAINED IN THE FOLLOWING DISCUSSION SHOULD BE CONSTRUED AS A GUARANTEE OR ASSURANCE OF FUTURE PERFORMANCE OR FUTURE RESULTS.
Overview
Islet Sciences is a biotechnology company engaged in the research, development, and commercialization of new medicines and technologies for the treatment of metabolic diseases and related indications where there is a significant measurable unmet medical need. The rising incidence of obesity is associated with many obesity-related health complications, including cardiovascular disease, diabetes, hyperlipidemia, hypertension, nonalcoholic fatty liver disease/steatohepatitis (NAFLD/NASH). This constellation is also recognized as the metabolic syndrome and is characterized by underlying insulin resistance.
Metabolism is the ability of the human body and its organs to process energy derived from the intake of nutrition. Metabolic diseases typically involve a derangement or malfunction of the normal processing of such metabolism. Some of the more prevalent metabolic diseases include obesity (excessive storage of nutrients); diabetes (loss of storage capability); NAFLD/NASH (excessive storage of lipids and fibrotic accumulation in the liver); dyslipidemia (inability to process fat from the blood); hypertension (excessive elevation of blood pressure). Since the primary risk factor for metabolic disease is often tied to obesity, the primary patient populations tend be concentrated in first-world nations where there is broad access to healthcare. However, due to the influx of “Western” diets and more sedentary lifestyles, more and more developing nations, such as China, India, and Brazil are seeing significant increases in the number of patients being diagnosed with metabolic disease.
We are developing the following therapeutic product candidates to address the needs of patients suffering from metabolic disease:
| ● | ISLT-P, an implantable suspension of encapsulated insulin-producing porcine islet cells, for the treatment of insulin-dependent diabetes; |
| ● | ISLT-2669, a novel lead IL-12 small molecule inhibitor selected for preclinical development for treatment of type 2 diabetes. This IL12 inhibitor blocks the auto-immune and inflammatory cascade initiated by IL-12 receptor activation; and |
| ● | ISLT-LSF Analogs, a library of lisofylline small molecule analogs that block inflammatory actions of cytokines that destroy insulin-producing beta cells, for diabetes and diabetes-related complications. |
We are also developing the following diagnostic product candidate to better enable patients and their physicians to understand disease diagnosis and progression.
| ● | ISLT-Bdx, a PCR based molecular diagnostic measuring hypomethylated beta cell-derived DNA as a biomarker of beta cell loss for the early detection of type 1 diabetes or onset of insulin dependent type 2 diabetes; |
We intend to develop these technologies in partnership with large pharmaceutical or biotechnology companies.
Going Concern
The financial statements included elsewhere in this consent solicitation statement/prospectus have been prepared assuming we will continue as a going concern. We have incurred operating losses and negative operating cash flows through July 31, 2014, and as of that date our cash position was $457,436 and our current liabilities were approximately $3.2 million. We have incurred net losses of approximately $2.9 million and $464,530, and negative operating cash flows of approximately $1.0 million and $614,121 for the fiscal year ended April 30, 2014 and for the three months ended July 31, 2014, respectively. Currently, management has projected that additional cash will be needed to allow us to continue our operations through April 30, 2015. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Our future cash requirements will depend on many factors, including continued scientific progress in our research and development programs, the scope and results of pre-clinical and clinical trials, the time and costs involved in obtaining regulatory approvals, the costs involved in filing, prosecuting and enforcing patents, competing technological and market developments, and the cost of product commercialization. We do not expect to generate a positive cash flow from operations at least until the commercial launch of our first product and possibly later given the expected spending for research and development programs and the cost of commercializing product candidates. During fiscal year ended April 30, 2014, we completed private placements of equity securities for aggregate gross proceeds of approximately $2.0 million. Although we have had success in raising this capital, there cannot be any assurance that this success will continue in future periods which is necessary to fund our future capital requirements, research and operations.
Results of Operations
Three Months Ended July 31, 2014 and 2013
There were no revenues for the three months ended July 31, 2014 and 2013.
During the three months ended July 31, 2014, general and administrative expenses totaled $405,399 compared to $518,439 for the three months ended July 31, 2013. The primary reason for the reduction in general and administrative expenses was due to the reduced operating activity during the quarter. General and administrative expenses for the three months ended July 31, 2014 included approximately $271,000 for professional fees, approximately $57,000 for stock compensation for the Board of Directors and employees, and approximately $69,000 for employee payroll and benefits. General and administrative expenses for the three months ended July 31, 2013 included approximately $221,000 for professional fees, approximately $99,000 for stock compensation for the Board of Directors, and approximately $81,000 for consulting fees.
During the three months ended July 31, 2014, research and development expenses totaled $58,331 compared to $774,184, for the three months ended July 31, 2013. The primary reason for the $715,853 decrease in research and development expenses was the substantial completion or curtailment of various supply agreements, studies and various agreements with universities totaling approximately $482,000 in reduced expenses. Also, there was a reduction of expenses for subcontractors and compensation for the Scientific Advisory Board of approximately $234,000.
Fiscal Years Ended April 30, 2014 and 2013
There were no revenues for the fiscal years ended April 30, 2014 and 2013.
During the fiscal year ended April 30, 2014, general and administrative expenses totaled approximately $2.2 million, compared to approximately $8.1 million for the fiscal year ended April 30, 2013. Beginning in October 2013, management implemented a strategy to significantly reduce expenses, raise additional interim capital and to add additional product candidates by signing a binding letter of intent to acquire BHV. As a result of this plan and other actions, the primary reason for the $5.9 million decrease in general and administrative expenses was due to a reduction of expense of $2.4 million associated with stock issuance in the settlement with a placement agent, a reduction of stock compensation of $2.2 million and a reduction in travel related expenses of $0.5 million.
During the fiscal year ended April 30, 2014, research and development expenses totaled approximately $0.6 million, compared to approximately $4.1 million for the fiscal year ended April 30, 2013. The primary reason for the $3.5 million decrease in research and development expenses was the substantial completion or curtailment of various supply agreements, studies and subcontractors such as the $1.4 million decrease in the Progenitor Cell Therapy agreement to provide the protocols, procedures, systems, equipment, testing, quality controls, and manufacturing and distribution services to support the development and commercialization of Islet Sciences’ encapsulated porcine islet cells for the treatment of diabetes. There was also a $0.7 million decrease in the Spring Point Project, a source animal facility to purchase pigs for use in Islet Sciences’ xenotransplantation research. Additionally, Islet Sciences completed or cancelled various agreements with universities and subcontractors decreasing research and development expenses by an additional $0.9 million.
Liquidity and Capital Resources
We have historically financed our operations primarily through the issuance of common stock and debt and by relying on other financing. We have not generated revenues from sales of products and have had losses since inception. We anticipate that we will incur substantial additional operating losses in future years as we progress in our research and development programs. We do not expect to produce revenues from product sales for the foreseeable future so our revenues will be limited to research grants or licensing agreements we are able to obtain.
We will need significant additional funding to fully implement our strategy, either through equity or debt financings or partnering arrangements, or we will be forced to curtail or cease some or all of our operations. As of July 31, 2014, we had $457,436 cash on hand. Islet Sciences has no available or negotiated credit facilities with third party lenders.
Operating Activities
During the three month periods ending July 31, 2014 and 2013, cash used in operating activities was $614,121 and $35,836, respectively. The increase of cash used in operating activities is primarily attributable to a higher net loss and a reduction of accounts payable.
During the fiscal years ended April 30, 2014 and 2013, cash used in operating activities was approximately $1.0 million and approximately $3.9 million, respectively. The decrease in use of cash is primarily attributable to a decrease in our research and development efforts and overall general operating expenses which resulted in net losses of approximately $3.0 million offset by the accumulation of approximately $1.0 million of payables for delayed payments to our vendors due to our cash management, non-cash expenses from the issuance of common stock issued as compensation and for services of approximately $0.8 million.
Financing Activities
Islet Sciences has financed its operating activities primarily from the proceeds of private placements of common stock. During the three months ended July 31, 2014, there was no cash provided by financing activities from the private placement of common stock.
During the fiscal year ended April 30, 2014, cash provided by financing activities was approximately $2.1 million compared to approximately $2.0 million during the fiscal year ended April 30, 2013. Of the total net cash provided by financing activities, approximately $2.0 million was from the net proceeds from the private placement of common stock that has been issued at year end.
Critical Accounting Policies
Our significant accounting policies are disclosed in Note 2 to our audited consolidated financial statements included in this consent solicitation statement/prospectus. Certain of our policies require the application of management judgment in making estimates and assumptions which affect the amounts reported in the consolidated financial statements and disclosures made in the accompanying notes. Those estimates and assumptions are based on historical experience and various other factors deemed to be applicable and reasonable under the circumstances. The use of judgment in determining such estimates and assumptions is by nature, subject to a degree of uncertainty. Accordingly, actual results could differ from the estimates made.
Intangible Assets
Intangible assets represents a patent acquired from a third party, which is recorded at cost and amortized over the remaining life of the patent. This patent was fully impaired and written off to expense during the year ended April 30, 2014. Intangible assets also include the purchase of DiaKine Therapeutics, Inc. patent portfolio and know-how as in-process research and development. The intangible assets with estimable useful lives are amortized on a straight line basis over their respective estimated useful lives to their estimated residual values. This method of amortization approximates the expected future cash flow generated from their use. Definite lived intangibles are reviewed for impairment in accordance with FASB ASC 360, Property, Plant and Equipment.
Goodwill
Goodwill represents the excess of the purchase price over the estimated fair value of the identifiable assets acquired and liabilities assumed in business acquisitions. Goodwill is reviewed at least annually for impairment in the fourth quarter of the fiscal year, at Islet Sciences level, which is the sole reporting unit, and at any other time at which events occur or circumstances indicate that the carrying amount of goodwill may exceed its fair value. Such indicators would include a significant reduction in Islet Sciences’ market capitalization, a decrease in operating results or a deterioration in Islet Sciences’ financial position.
Research and Development
Research and development expenses consist of expenses incurred in performing research and development activities including salaries and benefits, facilities and other overhead expenses, contract services and other outside expenses. Research and development costs are charged to operations when incurred.
Stock Based Compensation
Stock awards
FASB ASC 718, Compensation-Stock Compensation (FASB ASC 718), requires the fair value of all share-based payments to employees, including grants of stock options, to be recognized in the statement of operations over the requisite service period.FASB ASC-718 requires all share based payments to employees, including grants of employee stock option, to be recognized in operating results as compensation expense based on fair value over the requisite service period of the awards. Islet Sciences determines the fair value of share-based awards using the Black-Scholes option-pricing model which uses both historical and current market data to estimate fair value. The method incorporates various assumptions such as the risk-free interest rate, expected volatility, expected dividend yield, expected forfeiture rate and expected life of the options. As allowed by FASB ASC 718, for companies with a short period of publicly traded stock history, Islet Sciences’ estimate of expected volatility is based on the average volatilities of a sampling of three companies with similar attributes to Islet Sciences, including industry, stage of life cycle, size and financial leverage. The risk-free rate for periods within the contractual life of the stock options is based on the U.S. Treasury yield curve in effect at the time of grant valuation. The expected term of options granted is derived using the “simplified method” which computes expected term as the average of the sum of the vesting term plus the contract term as historically Islet Sciences had limited activity surrounding its options. Islet Sciences has not issued stock options for nonemployees.
Warrants
Warrants granted to service providers are normally valued at the fair value of the instrument on the date of the grant (grant date) and are recognized in the statement of operations over the requisite service period or when they vest. When the requisite service period precedes the grant date and a market condition exists in the warrant, Islet Sciences values the warrant using the Black-Scholes option-pricing model. Warrants issued in connection with capital raises are normally valued at the fair value of the instrument on the date of the grant (grant date) and valued for disclosure purposes if they meet all the criteria under FASB ASC 718. Islet Sciences values these warrant using the Black-Scholes Method as well. As allowed by FASB ASC 718, for companies with a short period of publicly traded stock history, Islet Sciences’ estimate of expected volatility is based on the average volatilities of a sampling of companies with similar attributes to Islet Sciences, including industry, stage of life cycle, size and financial leverage. The risk-free rate for periods within the contractual life of the warrant is based on the U.S. Treasury yield curve in effect at the time of grant valuation.
Off-Balance Sheet Arrangements
We do not have off-balance sheet arrangements, financings, or other relationships with unconsolidated entities or other persons, also known as "special purpose entities" (SPEs).
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION OF BHV
SPECIAL NOTE OF CAUTION REGARDING FORWARD-LOOKING STATEMENTS
CERTAIN STATEMENTS IN THIS REPORT, INCLUDING STATEMENTS IN THE FOLLOWING DISCUSSION, ARE WHAT ARE KNOWN AS "FORWARD-LOOKING STATEMENTS", WHICH ARE BASICALLY STATEMENTS ABOUT THE FUTURE. FOR THAT REASON, THESE STATEMENTS INVOLVE RISK AND UNCERTAINTY SINCE NO ONE CAN ACCURATELY PREDICT THE FUTURE. WORDS SUCH AS “PLANS”, “INTENDS”, “WILL”, “HOPES”, “SEEKS”, “ANTICIPATES”, “EXPECTS“ AND THE LIKE OFTEN IDENTIFY SUCH FORWARD-LOOKING STATEMENTS, BUT ARE NOT THE ONLY INDICATION THAT A STATEMENT IS A FORWARD-LOOKING STATEMENT. SUCH FORWARD-LOOKING STATEMENTS INCLUDE STATEMENTS CONCERNING BHV’S PLANS AND OBJECTIVES WITH RESPECT TO THE PRESENT AND FUTURE OPERATIONS OF BHV, AND STATEMENTS WHICH EXPRESS OR IMPLY THAT SUCH PRESENT AND FUTURE OPERATIONS WILL OR MAY PRODUCE REVENUES, INCOME OR PROFITS. NUMEROUS FACTORS AND FUTURE EVENTS COULD CAUSE BHV TO CHANGE SUCH PLANS AND OBJECTIVES OR FAIL TO SUCCESSFULLY IMPLEMENT SUCH PLANS OR ACHIEVE SUCH OBJECTIVES, OR CAUSE SUCH PRESENT AND FUTURE OPERATIONS TO FAIL TO PRODUCE REVENUES, INCOME OR PROFITS. THEREFORE, THE READER IS ADVISED THAT THE FOLLOWING DISCUSSION SHOULD BE CONSIDERED IN LIGHT OF THE DISCUSSION OF RISKS AND OTHER FACTORS CONTAINED IN THIS CONSENT SOLICITATION STATEMENT/PROSPECTUS. NO STATEMENTS CONTAINED IN THE FOLLOWING DISCUSSION SHOULD BE CONSTRUED AS A GUARANTEE OR ASSURANCE OF FUTURE PERFORMANCE OR FUTURE RESULTS.
Overview
BHV is a clinical stage pharmaceutical company focused on the development of Remogliflozin. Remogliflozin is a new chemical entity in phase IIb development for the treatment of type 2 diabetes (“T2DM”) and non-alcoholic steatohepatitis (“NASH”). Remogliflozin is a selective sodium glucose co-transporter 2 (“SGLT2”) inhibitor that is expected to emerge as a best in class molecule. SGLT2 inhibitors are an important new class of metabolic disease compounds which work by inhibiting reabsorption of glucose in the kidney and provide robust insulin-independent HbA1c lowering and weight loss. SGLT2 inhibitors are the only oral diabetes therapy to safely provide glycemic control with significant weight loss through an insulin independent mechanism of action.
Remogliflozin has been administered in sixteen phase I, three phase IIa, and two phase IIb clinical studies. Approximately 850 human subjects, including 496 subjects with T2DM, have been dosed with Remogliflozin. Remogliflozin has demonstrated dose-related glycosuria consistent with SGLT2 inhibition with best in class lowering of HbA1c, plasma glucose, and body weight observed in subjects with T2DM over 12 weeks of treatment. Remogliflozin has also proven to be well tolerated with low incidences of urogenital infections over 12 weeks of treatment in subjects with T2DM. BHV’s near term development plans call for one phase IIb study of Remogliflozin for type 2 diabetes and another phase IIb study for NASH. BHV may also pursue additional clinical studies of Remogliflozin for type 1 diabetes.
Going Concern
The financial statements included elsewhere in this consent solicitation statement/prospectus have been prepared assuming BHV will continue as a going concern. BHV has incurred operating losses and negative operating cash flows since inception. As of July 31, 2014, BHV’s cash position was approximately $13,000 and its current liabilities were approximately $585,000. BHV incurred a net loss of $125,520 for the fiscal year ended April 30, 2014, $102,447 for the quarter ended July 31, 2014, and has a loss accumulated during the development stage totaling $614,005. BHV’s operations to date have been limited to organizing and staffing the company, business planning, raising capital and acquiring and developing its pipeline. To date, BHV has not generated any revenues and has financed its operations primarily with grants and by borrowings through its promissory notes. Since inception, BHV has generated recurring operating losses. These losses have resulted principally from costs incurred in connection with clinical trials, consulting fees and general and administrative expenses, including planning for the clinical trials of Remogliflozin.
BHV’s future cash requirements will depend on many factors, including continued scientific progress in its research and development programs, the scope and results of clinical trials, the time and costs involved in obtaining regulatory approvals, the costs involved in filing, prosecuting and enforcing patents, competing technological and market developments, and the cost of product commercialization. BHV expects to continue to incur operating losses for the next several years as it pursues the clinical development and commercialization of Remogliflozin and works to develop additional product candidates through BHV’s research and development programs. As a result, BHV may be required to seek to fund its operations through public or private equity or debt financings or other sources, such as collaborations and government contracts. BHV’s failure to raise capital as and when needed would have a negative impact on its financial condition and its ability to pursue its business strategies. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Results of Operations
Three Months Ended July 31, 2014 and 2013
There were no revenues for the three months ended July 31, 2014 and 2013.
During the three months ended July 31, 2014, general and administrative expenses totaled approximately $87,000, compared to approximately $14,000 for the quarter ended July 31, 2013. The primary reason for the $73,000 increase in general and administrative expenses was due to an increase of expenses associated with legal fees incurred in connection with the Transactions.
During the three months ended July 31, 2014, research and development expenses totaled approximately $12,000, compared to approximately $2,500 for the quarter ended July 31, 2013. The primary reason for the $9,500 increase in research and development expenses was the receipt of reimbursements received from Kissei for costs related to the maintenance of the investigational new drug application with the FDA for Remogliflozin.
Fiscal Years Ended April 30, 2014 and 2013
There were no revenues for the fiscal years ended April 30, 2014 and 2013.
During the fiscal year ended April 30, 2014, general and administrative expenses totaled approximately $99,000, compared to approximately $67,000 for the fiscal year ended April 30, 2013. The primary reason for the $32,000 increase in general and administrative expenses was due to an increase in legal expenses.
During the fiscal year ended April 30, 2014, research and development expenses totaled approximately $10,000, compared to approximately $30,000 for the fiscal year ended April 30, 2013. The primary reason for the $20,000 decrease in research and development expenses was the reduction in consulting fees related to the maintenance of the investigational new drug application with the FDA for Remogliflozin.
Liquidity and Capital Resources
BHV has historically financed its operations primarily through grants and by borrowing through its promissory notes. BHV has not generated revenues from sales of products and has had losses since inception.
BHV will need significant additional funding to fully implement its strategy, either through equity or debt financings or partnering arrangements, or BHV will be forced to curtail or cease some or all of our operations. As of July 31, 2014, BHV had $12,587 cash on hand. BHV has no available or negotiated credit facilities with third party lenders.
In January 2010, BHV signed an unsecured promissory note agreement with the North Carolina Biotechnology Center (the “Center”), an entity primarily funded by the State of North Carolina, to borrow $28,407. The original note was due in one lump sum in January 2013 and had an interest rate of 4.25%. The note was renegotiated and the maturity date for the unpaid principal and accrued interest of $31,554 was extended to January 11, 2014. In November 2010, BHV issued a second unsecured promissory note to the Center to borrow $250,000. The original note was due November 2013 and had an interest rate of 4.25%. The note was renegotiated and the maturity date for the unpaid principal and accrued interest of $277,836 was extended through an amendment to the promissory note. The note, as amended, bears interest at a rate of 4.25% per annum, compounded annually and required payment of $125,000 on or before January 15, 2014 and any remaining amount of unpaid principal and accrued interest on or before January 15, 2015. No payments on this note have been made to-date. In conjunction with the second note, BHV issued the Center a warrant to purchase 12,500 units of BHV exercisable at any time. The unit exercise price is $5 per unit and the warrant expires November 15, 2020.
In August 2014, BHV granted an exclusive license to LIBBS, a wholly-owned subsidiary of LIBBS Farmacêutica Ltda. of Brazil, for the development and commercialization of Remo for all human therapeutic, palliative, and prophylactic uses in certain countries in Latin America, including Brazil. In exchange for these rights, LIBBS is required to make a payment to BHV upon BHV’s submission of the study protocol for BHV’s currently-planned phase IIb study of Remo for type 2 diabetes to the FDA and approval of the protocol by the responsible Institutional Review Board, and to make profit-sharing and royalty payments to BHV based on sales of Remo in LIBBS’s exclusive territory. LIBBS also agreed to pay for the cost of BHV’s currently-planned phase IIb study of Remo for type 2 diabetes up to an agreed maximum amount. LIBBS is required to use commercially reasonable efforts to develop and commercialize Remo in LIBBS’s exclusive territory at LIBBS’s cost.
Operating Activities
During the three months ending July 31, 2014 and 2013 cash (used in) provided by operating activities was ($3,319) and $9,436, respectively. The decrease in cash is primarily attributable to the losses incurred offset by an increase in accounts payable and accrued expenses.
During the fiscal years ended April 30, 2014 and 2013, cash used in operating activities was approximately $500 and approximately $37,000, respectively. The decrease in use of cash is primarily attributable to an increase in accrued expenses.
Financing and Investing Activities
No cash was provided from financing or investing activities for the three months ended July 31, 2014 and 2013, and for the fiscal years ended April 30, 2014 and 2013.
Critical Accounting Policies
BHV’s significant accounting policies are disclosed in Note 1 to its financial statements. Certain of its policies require the application of management’s judgment in making estimates and assumptions which affect the amounts reported in the financial statements and disclosures made in the accompanying notes. Those estimates and assumptions are based on historical experience and various other factors deemed to be applicable and reasonable under the circumstances. The use of judgment in determining such estimates and assumptions is by nature, subject to a degree of uncertainty. Accordingly, actual results could differ from the estimates made.
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN BENEFICIAL OWNERS OF ISLET SCIENCES
The following table sets forth information regarding beneficial ownership of our common stock as of November 24, 2014 by (i) any person or group with more than 5% of any class of voting securities, (ii) each director, (iii) our named executive officers, and (iv) all of our executive officers and directors as a group. Unless otherwise specified, the address of each of the officers and directors set forth below is in care of Islet Sciences, Inc., 6601 Six Forks Rd., Suite 140, Raleigh, North Carolina 27615. Except as indicated in the footnotes to this table and subject to applicable community property laws, the persons named in the table to our knowledge have sole voting and investment power with respect to all shares of securities shown as beneficially owned by them.
Name | | Office | | Shares Beneficially Owned (1) | | | Percent of Class (2) | |
| | | | | | | | | |
| Executive Officers and Directors | | | | | | | | |
| | | | | | | | | | | |
James Green(3) | | CEO and Director | | | 1,100,000 | | | | 1.6 | % |
| | | | | | | | | | | |
William Wilkison, Ph.D.(3) | | COO and Director | | | 1,100,000 | | | | 1.6 | % |
| | | | | | | | | | | |
Joel D. Perlin(4) | | Vice-President and Director | | | 5,515,000 | | | | 8.1 | % |
| | | | | | | | | | | |
| Dr. Michael Luther | | Director | | | 50,000 | | | | 0.1 | % |
| | | | | | | | | | | |
| Eric Barnett | | Director | | | 70,000 | | | | 0.1 | % |
| | | | | | | | | | | |
John Steel(6) | | Director, Former CEO | | | 7,233,127 | | | | 10.7 | % |
| | | | | | | | | | | |
Richard Egan(5) | | Former CFO | | | 250,000 | | | | 0.4 | % |
| | | | | | | | | | | |
Michael Early(7) | | Former CEO | | | 0 | | | | 0 | |
| | | | | | | | | | | |
| All executive officers and directors as a group | | | | | 15,318,127 | | | | 21.8 | % |
| | | | | | | | | | | |
| 5% Security Holders | | | | | | | | | | |
| | | | | | | | | | | |
Charles Dupont 13740 Nob Avenue Belmar, California 92014 | | | | | 5,980,685 | | | | 8.9 | % |
| | | | | | | | | | | |
Andrew K. Boszhardt C/O Great Oaks Capital - 660 Madison Avenue New York, NY 10065 | | | | | 5,066,666 | | | | 7.4 | % |
| | | | | | | | | | | |
Richard Schoninger (8) 31 West 11th St., Apt 4A New York, NY 10011 | | | | | 7,200,000 | | | | 10.5 | % |
| | | | | | | | | | | |
_________________
| (1) | | Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. |
| | | |
| (2) | | Based on 66,928,724 shares of Islet Sciences’ common stock outstanding as of November 24, 2014. |
| (3) | | James Green and Dr. William Wilkison were issued options to purchase 1,500,000 shares of Islet Sciences’ common stock at $0.265 per share as part of their employment agreement with Islet Sciences. Mr. Green and Dr. Wilkison have 1,100,000 options each currently exercisable or exercisable within 60 days of November 24, 2014. |
| | | |
| (4) | | Includes shares and warrants held by entities affiliated with or controlled by Mr. Perlin as follows: |
| | | 2,640,000 shares held by H.S. Perlin Co., Inc., Defined Benefit Pension Plan |
| | | 2,001,875 shares of common stock and 440,000 warrants held by The Perlin Family Trust (DTD 12/27/95) |
| | | 433,125 shares held by Joel Perlin. |
| | | |
| (5) | | Richard Egan resigned as Treasurer and Chief Financial Officer of Islet Sciences as of May 22, 2014. |
| | | |
| (6) | | John Steel resigned as the CEO of Islet Sciences on July 1, 2013. |
| (7) | | Michael Early resigned as the CEO of Islet Sciences on July 30, 2013. |
| (8) | | Includes 1,600,000 shares of common stock issuable upon exercise of warrants. |
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN BENEFICIAL OWNERS OF BHV
The following table sets forth information regarding beneficial ownership of BHV’s membership interests as of November 24, 2014. Unless otherwise specified, the address of each of the members of BHV set forth below is in care of BHV, 6601 Six Forks Rd., Suite 140, Raleigh, North Carolina 27615. Except as indicated in the footnotes to this table and subject to applicable community property laws, the persons named in the table to our knowledge have sole voting and investment power with respect to all membership interest shown as beneficially owned by them.
Name | | Common Membership Units Owned | | | Percent of Class | |
| | | | | | | | | |
James Green (1) | | | 2,000,000 | | | | 50 | % |
| | | | | | | | | |
William Wilkison, Ph.D. (1) | | | 2,000,000 | | | | 50 | % |
| | | | | | | | | |
Bentley Cheatham, Ph.D. (2) | | | - | | | | - | |
| | | | | | | | | |
| All executive officers and members as a group | | | - | | | | - | |
_________________
| (1) | | James Green and Dr. William Wilkison are the sole managing members of BHV. |
| | | |
| (2) | | Bentley Cheatham, Ph.D. was appointed as the Chief Executive Officer of BHV on October 30, 2013. |
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN BENEFICIAL OWNERS OF AVOGENX AFTER THE TRANSACTIONS
The following table sets forth information regarding beneficial ownership of our common stock immediately following the closing of the Transactions by (i) any person or group with more than 5% of any class of voting securities, (ii) each director, (iii) our named executive officers, and (iv) all of our executive officers and directors as a group. Unless otherwise specified, the address of each of the officers and directors set forth below is in care of the Company, 6601 Six Forks Rd., Suite 140, Raleigh, North Carolina 27615. Except as indicated in the footnotes to this table and subject to applicable community property laws, the persons named in the table to our knowledge have sole voting and investment power with respect to all shares of securities shown as beneficially owned by them. The numbers of shares of common stock presented in this table are not adjusted for the Exchange Ratio, which will not be determined until prior to the effective time of the Merger.
Name | | Office | | Shares Beneficially Owned (1) | | | Percent of Class (2) | |
| | | | | | | | | |
| Executive Officers and Directors | | | | | | | | |
| | | | | | | | | | | |
James Green(3) | | CEO and Director | | | 16,100,000 | | | | 16.4 | % |
| | | | | | | | | | | |
William Wilkison, Ph.D.(3) | | COO and Director | | | 16,100,000 | | | | 16.4 | % |
| | | | | | | | | | | |
Joel D. Perlin(4) | | Vice-President and Director | | | 5,515,000 | | | | 5.6 | % |
| Eric Barnett | Director | | | 70,000 | | | | 0.1 | % |
| | | | | | | | | | |
| Dr. Michael Luther | Director | | | 50,000 | | | | 0.1 | % |
| | | | | | | | | | |
John Steel(6) | Director, Former CEO | | | 7,233,127 | | | | 7.4 | % |
| | | | | | | | | | |
Richard Egan(5) | Former CFO | | | 250,000 | | | | 0.3 | % |
| | | | | | | | | | |
Michael Early(7) | Former CEO | | | 0 | | | | 0 | |
| | | | | | | | | | |
| All executive officers and directors as a group | | | | 45,318,127 | | | | 45.2 | % |
| | | | | | | | | | |
| 5% Securities Holders | | | | | | | | | |
| | | | | | | | | | |
Charles Dupont 13740 Nob Avenue Belmar, California 92014 | | | | 5,980,685 | | | | 6.2 | % |
| | | | | | | | | | |
Andrew K. Boszhardt C/O Great Oaks Capital - 660 Madison Avenue New York, NY 10065 | | | | 5,066,666 | | | | 5.2 | % |
| | | | | | | | | | |
Richard Schoninger(8) 31 West 11th St., Apt 4A New York, NY 10011 | | | | 7,200,000 | | | | 7.2 | % |
| | | | | | | | | | |
_________________
| (1) | | Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. |
| | | |
| (2) | | Assuming 96,928,724 shares of Avogenx common stock outstanding immediately following the closing of the Transactions, based on the 66,928,724 shares of Islet Sciences common stock outstanding on November 24, 2014 plus the 30,000,000 shares to be issued to the BHV Members in connection with the BHV Securities Exchange. |
| (3) | | James Green and Dr. William Wilkison were issued options to purchase 1,500,000 shares of Islet Sciences’ common stock at $0.265 per share as part of their employment agreement with Islet Sciences. Mr. Green and Dr. Wilkison have 1,100,000 options each currently exercisable or exercisable within 60 days of November 24, 2014. Also includes 15,000,000 shares issued as part of the Transaction to each Mr. Green and Mr. Wilkison. |
| (4) | | Includes shares and warrants held by entities affiliated with or controlled by Mr. Perlin as follows: |
| | | 2,640,000 shares held by H.S. Perlin Co., Inc., Defined Benefit Pension Plan |
| | | 2,001,875 shares of common stock and 440,000 warrants held by The Perlin Family Trust (DTD 12/27/95) |
| | | 433,125 shares held by Joel Perlin. |
| (5) | | Richard Egan resigned as Treasurer and Chief Financial Officer of Islet Sciences as of May 22, 2014. |
| | | |
| (6) | | John Steel resigned as the CEO of Islet Sciences on July 1, 2013. |
| (7) | | Michael Early resigned as the CEO of Islet Sciences on July 30, 2013. |
| (8) | | Includes 1,600,000 shares of common stock issuable upon exercise of warrants. |
EXECUTIVE OFFICERS AND DIRECTORS OF AVOGENX FOLLOWING THE TRANSACTIONS
The following table lists the names, ages and positions as of November 24, 2014 of individuals currently expected to serve as directors and executive officers of Avogenx upon consummation of the Transactions:
| Name | | Age | | Position |
| | | | | |
| James Green | | 45 | | President, Chief Executive Officer and Chairman |
| | | | | |
| William Wilkison, Ph.D. | | 53 | | Chief Operating Officer and Director |
| | | | | |
| Steve Delmar | | 58 | | Chief Financial Officer |
| | | | | |
| Joel D. Perlin | | 68 | | Director |
| | | | | |
| Dr. Michael Luther, Ph.D. | | 58 | | Director |
| | | | | |
| Dr. Eric Barnett | | 51 | | Director |
James Green joined Islet Sciences as CEO in October 2013 and in January 2014 was appointed to the position of Chairman. Mr. Green joined Islet from BHV where he served as Chairman and CEO. BHV is a clinical stage pharmaceutical company focused on developing novel therapeutics for metabolic diseases. Prior to BHV, James spent several years at GlaxoSmithKline (“GSK”) in business development and finance roles. During that time he was responsible for license, acquisition, divestiture, and collaboration agreements related to preclinical and clinical development programs as well as equity investments in portfolio companies and special projects related to corporate growth. Prior to GSK, Mr. Green advised boards and management teams on mergers and acquisitions, financings, and other strategic alternatives while at Citigroup, Bank of America, and Ernst & Young Corporate Finance. Mr. Green was also a trustee for corporate, municipal, and securitized bond issues while at BNY Mellon. In addition, Mr. Green served as a director for the Pharmacogenomics for Every Nation Initiative, board observer for Nuada Pharmaceuticals Inc. and Golden Helix, Inc. and as member of the Animal Care and Use Committee at the Duke University Medical Center. Mr. Green earned a bachelor of business administration from the University of North Florida and an MBA with a concentration in corporate finance from the Katz Graduate School of Business at The University of Pittsburgh.
William Wilkison, Ph.D. joined Islet Sciences as Chief Operating Officer in October 2013 and was appointed as the Corporate Secretary in January 2014 and was appointed as a director of the Company in April 2014. Since 2009, Dr. Wilkison has been at BHV where he was a founder, director, and Chief Scientific Officer since its inception. BHV is a clinical stage pharmaceutical company focused on developing novel therapeutics for metabolic diseases. Prior to founding BHV in 2009, Dr. Wilkison spent several years at GSK in business development roles where he was responsible for managing key relationships between GSK and partner companies and participating on therapeutic project teams. Prior to GSK, Dr. Wilkison was a founder and Chief Operating Officer at Artecel Sciences, Inc. an adipose-derived stem cell discovery company. Artecel Sciences, Inc. was spun off from Zen-Bio, Inc. a biotech company founded by Dr. Wilkison, focused on the development and commercialization of human adipose cells. Prior to Zen-Bio, Dr. Wilkison spent six years at Glaxo/GlaxoWellcome studying metabolic disease and was responsible for target/lead identification and development. Dr. Wilkison has published numerous peer-reviewed manuscripts in the metabolic disease area and is an inventor on 17 approved patents. Dr. Wilkison holds a Ph.D. from Duke University Medical Center and did his postdoctoral work at Harvard Medical School.
Steve Delmar joined Islet Sciences as Chief Financial Officer in 2014 and brings more than 35 years of financial and operational executive leadership experience to the company, serving most recently as Director of Business Operations at Computer Sciences Corporation (CSC). Prior to his role at CSC, Mr. Delmar served as Chief Financial Officer of Integrity Management Consulting, a provider of major systems acquisition and program management support services to government agencies. He also served as Senior Vice President and Chief Financial Officer of ACE*COMM Corporation, a publicly held telecommunications company where he successfully completed multiple acquisitions and facilitated the sale of the company to a private equity investor. Mr. Delmar spent 22 years at Microlog Corporation, a publicly held internet software and services telecommunications company, including 15 years as an executive officer, serving most recently as Chief Financial Officer. While at Microlog, Steve successfully led the initial public offering and secondary offerings as well as multiple acquisitions. Steve earned a Bachelor of Science in Accounting from Clemson University and is a member of the AICPA.
Joel D. Perlin has been the President of H.S. Perlin Co., Inc. since 2002. After his graduation from San Diego State University in 1969, he pursued a career in the gold and rare coin industry. He has since become a renowned professional numismatist and a recognized expert in international gold trade and advisory. For more than 40 years, he has been instrumental in developing personalized investment portfolios using both rare coins and U.S. gold coins for high net worth investors, corporate pension plans and financially private investors. Mr. Perlin received his B.S. degree in marketing from San Diego State University in 1969.
Dr. Eric Barnett, Executive Vice President at Piedmont Pharmaceuticals, is a doctor of medicine, and chartered accountant. At Piedmont Pharmaceuticals, Eric is responsible for Business Development and Marketing, to develop and execute the company’s long-term growth strategies including launching new brand initiatives. Dr. Barnett was previously Vice President at GSK and member of the clinical executive committee where he was responsible for financial capital management of the global research and development clinical portfolio. After an early career in medicine, Dr. Barnett joined a predecessor of PricewaterhouseCoopers (“PwC”) in London where he was a member of an advisory team instrumental in streamlining the United Kingdom’s National Health Service. After his work with PwC and prior to joining GSK, Eric was the CFO at one of London’s National Health Service hospital groups. Dr. Barnett earned his medical degree at the University of Bristol in Bristol, England. He also is a qualified chartered accountant (PwC alumnus) and a member of the Institute of Chartered Accountants of England and Wales.
Dr. Michael Luther is Senior Vice President of Discovery and Development at Albany Molecular Research, Inc. (“AMRI”). At AMRI, Dr. Luther is responsible for the strategic, operational, and business development activities for AMRI’s global discovery and development divisions including scientific oversight as well as P/L responsibility. Prior to his current position at AMRI, Dr. Luther was Corporate Vice President of Global Discovery Research Services at Charles River Labs. Dr. Luther has also previously served as the founding President of the David H. Murdock Research Institute, Vice President and Site Head at Merck-Frosst, and Vice President of High Throughput Biology at GSK. Dr. Luther earned a Ph.D. in biophysical chemistry from Washington & St. Louis University School of Medicine and was a MDA Post-Doctoral Fellow at the Salk Institute. Dr. Luther also holds a Master of Business Administration from the Duke University Fuqua School of Business.
The directors of Avogenx will hold their positions on the board of directors until our next annual meeting of stockholders, and until their successors have been qualified after being elected or appointed or until their earlier resignation or removal. Officers serve at the discretion of the board of directors.
There are no family relationships among our directors and executive officers. Other than the Investor Rights Agreement to be entered into in connection with the closing of the Transactions, which provides James Green and Dr. William Wilkison with the right to appoint two directors to the Avogenx board of directors, there is no arrangement or understanding between or among our executive officers and directors pursuant to which any director or officer was or is to be selected as a director or officer, and there is no arrangement, plan or understanding as to whether non-management stockholders will exercise their voting rights to continue to elect the current board of directors. See “Terms of the Transactions – Description of the Investor Rights Agreement.”
Our directors and executive officers have not, during the past ten years:
| | ● | had any bankruptcy petition filed by or against any business of which was a general partner or executive officer, either at the time of the bankruptcy or within two years prior to that time, |
| | ● | been convicted in a criminal proceeding and is not subject to a pending criminal proceeding, |
| | ● | been subject to any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities, futures, commodities or banking activities; or |
| | ● | been found by a court of competent jurisdiction (in a civil action), the Securities Exchange Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended or vacate |
COMPENSATION OF EXECUTIVE OFFICERS AND DIRECTORS
The following is a summary of the compensation paid for services rendered in all capacities to Islet Sciences and BHV for the fiscal years indicated, by individuals who are expected to serve as executive officers of Avogenx following the consummation of the Transactions.
Summary Compensation Table
| Name and Position | | Year | | Salary ($) | | | Bonus ($) | | | Option Awards1 ($) | | | All Other Compensation ($) | | | Total ($) | |
| | | | | | | | | | | | | | | | | | |
James Green, CEO2 | | 2014 | | | 12,500 | | | | 62,500 | | | | 205,815 | | | | - | | | | 280,815 | |
William Wilkison, COO2 | | 2014 | | | 12,500 | | | | 62,500 | | | | 205,815 | | | | - | | | | 280,815 | |
Steve Delmar, CFO3 | | 2014 | | | - | | | | - | | | | - | | | | - | | | | - | |
| (1) | Represents the aggregate grant date fair value of awards granted during the year computed in accordance with Financial Accounting Standards Board ASC Topic 718, Compensation — Stock Compensation. The assumptions made in determining the fair values of our stock and option awards are set forth in Note 2 to Islet Sciences’ audited consolidated financial statements included in this consent solicitation statement/prospectus. |
| (2) | James Green was appointed the President and Chief Executive Officer of Islet Sciences on October 30, 2013. Dr. William Wilkison was appointed the Chief Operating Officer of Islet Sciences on October 30, 2013. Compensation for Mr. Green and Dr. Wilkison disclosed in the table above represents compensation received from Islet Sciences. Mr. Green and Dr. Wilkison did not receive any compensation from BHV during fiscal 2014. |
| (3) | Steve Delmar was appointed as Islet Sciences’ Treasurer and Chief Financial Officer on May 22, 2014. |
Overview
Following the consummation of the Transactions, we intend to provide individuals who are expected to serve as our executive officers with a competitive base salary that is in line with their roles and responsibilities when compared to peer companies of comparable size in similar locations.
Employment Agreements
James Green
On October 30, 2013, Mr. James Green accepted Islet Sciences’ appointment as the President and Chief Executive Officer and Director of Islet Sciences, and Islet Sciences entered into an employment agreement with Mr. Green, dated October 30, 2013. Mr. Green is compensated as follows:
| 1. | Initially, no annual salary until Islet Sciences’ financial condition improves to allow sufficient cash flow; |
| 2. | Bonus of $12,500 per each month of service payable within 10 days following the completion by Islet Sciences of a financing with proceeds of at least $1,500,000; |
| 3. | Options to purchase 1,500,000 shares of the Common Stock of Islet Sciences, exercisable at $0.265 per share, as follows: (i) 500,000 shares vesting on the 91st day from the option grant date, (ii) 1,000,000 shares vesting in equal installments of 200,000 on the last day of each 90 day period starting from the 91st day after the option grant date. If Mr. Green’s employment terminates on account of a termination by the company without cause, or a termination by executive for good reason or in conjunction with a change of control, then Islet Sciences agrees that all outstanding unvested stock options shall immediately be 100% vested upon the date that his employment terminates. |
Dr. William Wilkison
On October 30, 2013, Dr. William Wilkison accepted Islet Sciences’ appointment as the Chief Operating Officer and as an Islet Sciences board of directors’ observer, and Islet Sciences entered into an employment agreement with Dr. Wilkison dated October 30, 2013. On April 11, 2014, Dr. Wilkison was appointed a director of Islet Sciences. Dr. Wilkison is compensated as follows:
| 1. | Initially, no annual salary until Islet Sciences’ financial condition improves to allow sufficient cash flow; |
| 2. | Bonus of $12,500 per each month of service payable within 10 days following the completion by Islet Sciences of a financing with proceeds of at least $1,500,000; |
| 3. | Options to purchase 1,500,000 shares of the Common Stock of Islet Sciences, exercisable at $0.265 per share, as follows: (i) 500,000 shares vesting on the 91st day from the option grant date, (ii) 1,000,000 shares vesting in equal installments of 200,000 on the last day of each 90 day period starting from the 91st day after the option grant date. If Dr. Wilkison’s employment terminates on account of a termination by the company without cause, or a termination by executive for good reason, or in the event of a change of control, then Islet Sciences agrees that all outstanding unvested stock options shall immediately be 100% vested upon the date that his employment terminates. |
On May 22, 2014, the Islet Sciences board of directors approved the following changes to the compensation for Mr. Green and Dr. Wilkison: (i) combined annual salary budget of $300,000 payable monthly pro rata (up to $25,000 per month) to be divided between Mr. Green and Dr. Wilkison at the discretion of the CEO; the salary budget will be increased to $500,000 upon raising of additional capital by Islet Sciences and approval by the board of directors of a respective corporate budget, and (ii) full health insurance benefits.
Steven Delmar
On May 22, 2014, Mr. Delmar was appointed as Islet Sciences’ Treasurer and Chief Financial Officer. Mr. Delmar will receive an initial annual salary of $100,000, a grant of 300,000 stock options to purchase common shares of Islet Sciences, a conditional grant of 100,000 stock options upon the effective date of this S-4 filing and full health insurance benefits.
Outstanding Equity Awards at 2014 Fiscal Year End
The following table reflects the unexercised options, stock that has not vested and equity incentive plan awards for individuals who are expected to serve as our named executive officers following the consummation of the Transactions outstanding as of the end of the fiscal year ended April 30, 2014:
| | | Option Awards | | |
Name | | Number of Securities Underlying Unexercised Options (#) Exercisable | | | Number of Securities Underlying Unexercised Options (#) Unexercisable | | | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | | | Option Exercise Price ($) | | Option Expiration Date |
| James Green | | | 700,000 | (1) | | | 800,000 | | | | — | | | $ | 0.265 | | 3/31/2020 |
| William Wilkison | | | 700,000 | (1) | | | 800,000 | | | | — | | | $ | 0.265 | | 3/31/2020 |
| (1) | James Green and Dr. William Wilkison were issued options to purchase 1,500,000 shares of Islet Sciences’ common stock at $0.265 per share as part of their employment agreement with Islet Sciences. As of April 30, 2014, both Mr. Green and Dr. Wilkison have vested 700,000 options each. |
Additional Narrative Disclosure
We currently have no plans that provide for the payment of retirement benefits, or benefits that will be paid primarily following retirement, including, but not limited to, tax qualified defined benefit plans, supplemental executive retirement plans, tax qualified defined contribution plans and non-qualified defined contribution plans.
2014 Director Compensation
The following table reflects the compensation of the persons who are expected to serve as directors of Avogenx following the consummation of the Transactions (other than the named executive officers) including director fees, consulting fees and scientific advisory board fees for Islet Sciences’ fiscal year ended April 30, 2014:
| | | Fees Earned or Paid in Cash | | | Stock Awards | | | Total | |
| Name of Director | | ($) | | | ($)(1) | | | ($) | |
| Joel D. Perlin | | | - | | | | 92,813 | | | | 92,813 | |
| Dr. Michael Luther | | | - | | | | 20,000 | | | | 20,000 | |
| Dr. Eric Barnett | | | - | | | | 22,400 | | | | 22,400 | |
| (1) | The amounts in this column represent the aggregate grant date fair value of awards granted during the year computed in accordance with Financial Accounting Standards Board ASC Topic 718, Compensation — Stock Compensation. The assumptions made in determining the fair values of our stock awards are set forth in Note 2 to Islet Sciences’ audited consolidated financial statements included in this consent solicitation statement/prospectus. |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The following disclosure includes discussion of related party transactions with persons who are expected to serve as directors or officers of Avogenx following the consummation of the Transactions:
Transactions with related persons
During the year ended April 30, 2014, one of Islet Sciences’ directors loaned Islet Sciences a total of $74,531 at a 6.5% interest rate. A promissory note was issued for this amount. Islet Sciences repaid to this director $15,000 during the fiscal year ended April 30, 2014, and $20,000 during the three months ended July 31, 2014, which included accrued interest. The remaining balance at July 31, 2014 was $43,343.
Mr. James Green, President and Chief Executive Officer, and Dr. William Wilkison, Chief Operating Officer, of Islet Sciences and Avogenx are the sole members of BHV.
Other than the transactions discussed in this section and the Transactions, which are described elsewhere in this consent solicitation statement/prospectus, there have been no related party transactions, or any other transactions or relationships required to be disclosed pursuant to Item 404 of Regulation S-K. Islet Sciences is currently not a subsidiary of any company.
The Islet Sciences board of directors conducts an appropriate review of and oversees all related party transactions on a continuing basis and reviews potential conflict of interest situations where appropriate. The board of directors has not adopted formal standards to apply when it reviews, approves or ratifies any related party transaction. However, the board of directors believes that the related party transactions are fair and reasonable to Islet Sciences and on terms comparable to those reasonably expected to be agreed to with independent third parties for the same goods and/or services at the time they are authorized by the board of directors.
Director Independence
Islet Sciences board of directors, in its business judgment, has made an affirmative determination that Michael Luther qualifies as an independent director pursuant to the definition of “independent director” under the Rules of NASDAQ, Marketplace Rule 5605(a)(2). Islet Sciences’ common stock is, and Avogenx’s common stock is intended to be, quoted on the OTCQB and not listed on a national securities exchange. Therefore, neither the SEC nor the Nasdaq Listing Rules requiring board/committee members to be independent are applicable to us.
DESCRIPTION OF AVOGENX CAPITAL STOCK
The following description is only a summary of the material provisions of Avogenx’s Certificate of Incorporation and Avogenx’s By-Laws and specified provisions of Delaware law, in each case to the extent that they relate to Avogenx common stock and preferred stock. This summary does not purport to be complete and is subject to, and is qualified in its entirety by reference to, all of the provisions of each of these documents. These documents may be amended from time to time. Each of these documents (other than such provisions of Delaware law) has been filed as an exhibit to the registration statement of which this consent solicitation statement/prospectus is a part. You should read each of these documents because they, not this description, define your rights as stockholders.
General
Avogenx’s authorized capital stock consists of 200,000,000 shares of common stock, par value $.001 per share, and 10,000,000 shares of preferred stock, par value $.001 per share.
Common Stock
The holders of shares of Avogenx common stock are entitled to one vote per share held on all matters submitted to a vote at a meeting of stockholders. Each stockholder may exercise such vote either in person or by proxy. Stockholders are not entitled to cumulate their votes for the election of directors, which means that, subject to such rights as may be granted to the holders of shares of preferred stock, the holders of more than 50% of the shares of common stock voting for the election of directors are able to elect all of the directors and the holders of the remaining shares of common stock will not be able to elect any director.
Subject to preferences to which holders of shares of preferred stock may be entitled, the holders of shares of Avogenx common stock are entitled to receive ratably such dividends, if any, as may be declared from time to time by Avogenx’s board of directors out of funds (including cash, securities and other property) legally available therefor. Subject to the prior rights of creditors and to preferences to which holders of shares of preferred stock may be entitled, the holders of shares of Avogenx common stock are entitled to receive ratably all of Avogenx’s assets (including cash, securities and other property) distributed upon a liquidation, dissolution or winding up of Avogenx.
The holders of shares of Avogenx common stock do not have any preemptive, subscription, redemption or sinking fund rights (other than the preferred share purchase rights).
The outstanding shares of Avogenx common stock will be duly authorized, validly issued, fully paid and non-assessable. This means that you will have paid the full purchase price for your shares and you will not be assessed any additional amount for your shares.
The outstanding shares of Avogenx common stock will be, upon official notice of issuance, quoted on the OTCQB under a new symbol. The transfer agent and registrar for Avogenx common stock is Interwest Transfer Co., Inc.
Preferred Stock
Avogenx’s board of directors has the authority to issue shares of preferred stock in one or more series and to fix all rights, preferences, privileges and powers thereof, and all qualifications, limitations and restrictions thereon, without approval of stockholders, including:
| | ● | | the designation of each series; |
| | ● | | number of shares in each series; |
| | ● | | the optional and mandatory repurchase and redemption rights of each series (including sinking fund and share retirement provisions), if any; |
| | ● | | the liquidation preference of each series (including liquidation premiums and treatment of a merger, asset sale and other business combination as a liquidation), if any; |
| | ● | | mandatory and optional conversion and exchange rights of each series (including anti-dilution protection), if any; |
| | ● | | the restrictions on authorization or issuance of shares in the same series or any other series; |
| | ● | | the relative ranking (as to dividends, distributions, redemption, repurchase, liquidation preferences and other rights) of each series in respect of each other series and the common stock, if any; |
| | ● | | the preemptive and subscription rights of each series, if any; and |
| | ● | | the voting rights of each series (including class voting rights, director nomination and election rights, cumulative voting rights and disproportionate voting rights as relative to the common stock or each other series), if any. |
All shares of preferred stock must be identical, except that the following relative rights and preferences may vary between different series:
| | ● | | the number of shares constituting such series, and the designation thereof to distinguish the shares of such series from the shares of all other series; |
| | ● | | the rate of dividend, the time of payment and the dates from which dividends shall be cumulative, and the extent of participation rights, if any; |
| | ● | | any right to vote with holders of shares of any other series or class, the number of votes per share and any right to vote as a class, either generally or as a condition to specified corporate action; |
| | ● | | the price at and the terms and conditions on which shares may be redeemed; |
| | ● | | the amount payable upon shares in the event of involuntary liquidation; |
| | ● | | the amount payable upon shares in the event of voluntary liquidation; |
| | ● | | sinking fund provisions for the redemption or purchase of shares; and |
| | ● | | the terms and conditions on which shares may be converted, if the shares of any series are issued with the privilege of conversion. |
Such rights, privileges, preferences, powers, qualifications, limitations and restrictions will be set forth in a certificate of designations adopted by Avogenx’s board of directors and filed with the Secretary of State of the State of Delaware, whereupon it will become part of Avogenx’s Certificate of Incorporation.
To the extent that applicable law or the applicable certificate of designations provides that holders of shares of a series of preferred stock are entitled to voting rights, each holder shall be entitled to vote ratably (relative to each other such holder) on all matters submitted to a vote of such holders. Each holder may exercise such vote either in person or by proxy.
Subject to preferences to which holders of shares of any other series of preferred stock may be entitled and to the extent that the applicable certificate of designations so provides, the holders of shares of a series of preferred stock shall be entitled to receive ratably (relative to each other such holder) such dividends, if any, as may be declared from time to time in respect of shares of such series by Avogenx’s board of directors out of funds (including cash, securities and other property) legally available therefor. Subject to the prior rights of creditors and to preferences to which holders of shares of any other series of preferred stock may be entitled and to the extent that the applicable certificate of designations so provides, the holders of shares of a series of preferred stock are entitled to receive ratably (relative to each other such holder) Avogenx’s assets (including cash, securities and other property) distributed upon a liquidation, dissolution or winding up.
Certain Effects of Authorized and Unissued Stock
The unissued shares of authorized capital stock may be issued for a variety of proper corporate purposes, including acquisitions, compensation and incentive plans, and future public or private offerings to raise additional capital. One of the effects of the existence of such unissued shares may be to enable Avogenx’s board of directors to discourage or prevent a potential acquisition or takeover (by means of a tender or exchange offer, proxy contest or otherwise) and thereby to protect the continuity of Avogenx’s management. The issuance of shares of preferred stock, whether or not related to any acquisition or takeover attempt, may adversely affect the rights of the holders of shares of Avogenx common stock.
Certain Charter and Statutory Provisions
Certain provisions of Avogenx’s Certificate of Incorporation, Avogenx’s By-Laws and Delaware law may:
| | ● | | limit the ultimate liability of Avogenx’s directors and executive officers for breaches of certain of their duties to Avogenx and its stockholders; and |
| | ● | | discourage or prevent a potential acquisition or takeover (by means of a proxy contest, tender or exchange offer, or otherwise) and consideration of stockholder proposals (such as proposals regarding reorganization, restructuring, liquidation or sale of all or a substantial part of Avogenx’s assets). |
Elimination of Director Liability
Under Delaware law, directors of a Delaware corporation can generally be held liable for certain acts and omissions in connection with the performance of their duties to the corporation and its stockholders. As permitted by Delaware law, however, Avogenx’s Certificate of Incorporation contains a provision eliminating the liability of directors for monetary damages for breaches of their duties to Avogenx and its stockholders. This provision does not, however, eliminate liability for:
| | ● | | breaches of the duty of loyalty to Avogenx and its stockholders; |
| | ● | | acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
| | ● | | transactions from which improper personal benefit is derived; and |
| | ● | | unlawful declaration of dividends or repurchases or redemptions of shares of capital stock. |
This provision applies to officers only if they are directors and are acting in their capacity as directors. Although the issue has not been determined by any court, this provision may have no effect on claims arising under federal securities laws. This provision does not eliminate the duty of care, but only eliminates liability for monetary damages for breaches of such duty under various circumstances. Accordingly, this provision has no effect on the availability of equitable remedies, such as an injunction or rescission, based upon a breach of the duty of care. Equitable remedies may not, however, be wholly effective to remedy the injury caused by any such breach.
Indemnification of Directors and Officers
Avogenx’s Certificate of Incorporation provides that to the fullest extent permitted by Section 145 of the DGCL, Avogenx will indemnify any and all persons whom it has power to indemnify under the DGCL from and against any and all of the expenses, liabilities or other matters referred to in or covered by Section 145 of the DGCL, and will advance expenses to any and all such persons to the fullest extent permitted by the DGCL. The indemnification and advancement provided for in Avogenx’s Certificate of Incorporation will not be deemed exclusive under any Bylaw, agreement, vote of stockholders or disinterested directors or otherwise, and will continue as to a person who has ceased to be a director, officer, employee or agent and shall inure to the benefit of the heirs, executors and administrators of such a person.
Islet Sciences currently maintains a policy providing up to $3.0 million of insurance to its directors and officers against certain losses and expenses arising out of certain claims, including claims arising in connection with the Transactions.
Other Provisions
Avogenx’s Certificate of Incorporation and By-Laws provide that:
| | ● | | the number of members of Avogenx’s board of directors shall consist of that number (not less than one with the initial board of directors consisting of five persons) as may be fixed from time to time by our board of directors; |
| | ● | | except as otherwise required by Delaware law, directors (other than those elected by the holders of shares of preferred stock, if any) can be removed only for cause by either the affirmative vote of holders of the majority of the voting power of all then outstanding shares of Avogenx capital stock entitled to vote generally for the election of directors (the “Voting Stock”) or by the board of directors; and |
| | ● | | except as otherwise required by Delaware law, in the interim between annual meetings of stockholders or of special meetings of stockholders called for the election of directors and/or for the removal of one or more directors and for the filling of any vacancy in that connection, a vacancy on the board of directors, including a vacancy created by an increase in the authorized number of directors or resulting from the removal of directors, may be filled only by a majority vote of the directors then in office (and not by the stockholders unless no directors are then in office). |
In addition, Avogenx’s Certificate of Incorporation and By-Laws provide that:
| | ● | | stockholders are not permitted to call a special meeting of stockholders or to require the board of directors or officers to call such a special meeting; and |
| | ● | | only the Board of Directors acting upon (but only upon) the affirmative vote of that number of directors who would constitute at least a majority of the members of the board of directors if there were no vacancies, the President or the Chief Executive Officer are permitted to call such a special meeting. |
These provisions, taken together, prevent stockholders from forcing consideration by the stockholders of stockholder proposals over the opposition of the board of directors, except at an annual meeting.
Avogenx’s By-Laws provide that notice of nominations for the election of directors to be made at, and business to be brought before, an annual or special meeting of stockholders by a stockholder must be received by our Secretary not earlier than 150 days, and not later than 120 days, prior to the first anniversary of the immediately preceding year's annual meeting; provided, however, that in the event that no annual meeting was held in the previous year or the annual meeting is called for a date that is more than 30 days earlier or more than 60 days later than such anniversary date, notice by the stockholder in order to be timely must be so delivered or received not later than 10 days following the earlier of (i) the day on which public disclosure of the date of such annual meeting is first made, and (ii) the receipt by such stockholder of actual notice of the date of such annual meeting. A notice regarding any nomination must contain detailed information regarding the stockholder making the nomination and each nominee. A notice regarding any business to be brought before the meeting must contain detailed information regarding the business to be so brought, the reasons for conducting such business at the meeting, the stockholder proposing such business and any material interest of such stockholder in such business. Although such provisions do not give the board of directors any power to approve or disapprove stockholder nominations or proposals, they have the effect of precluding a contest for the election of directors or the consideration of stockholder proposals if the procedures established by Avogenx’s By-Laws are not complied with and may have the effect of discouraging a stockholder from conducting a contest or making a proposal.
Amendments
Avogenx’s Certificate of Incorporation provides that the affirmative vote of the holders of the majority of the total voting power of all outstanding shares will be required to amend, modify or repeal any provision of the Certificate of Incorporation, provided, however, that certain provisions require the affirmative vote of holders of not less than two-thirds of the total voting power of all outstanding shares. Avogenx’s Certificate of Incorporation provides that its board of directors is able to amend, modify or repeal its By-Laws.
COMPARISON OF RIGHTS OF STOCKHOLDERS
This section of the consent solicitation statement/prospectus describes the material differences between the rights of holders of Islet Sciences capital stock and holders of Avogenx common stock. While we believe that the description covers the material differences between the two, this summary may not contain all of the information that is important to you. You should carefully read this entire document and the other documents we refer to for a more complete understanding of the differences between being a stockholder of Islet Sciences and being a stockholder of Avogenx.
Upon consummation of the Merger, the holders of issued and outstanding Islet Sciences common stock will be entitled to receive Avogenx common stock. The rights of the holders of Avogenx common stock are governed by Avogenx’s Certificate of Incorporation, Avogenx’s By-Laws and the DGCL, while the rights of holders of Islet Sciences common stock are generally governed by Islet Sciences’ articles of incorporation, Islet Sciences’ bylaws and the NRS.
Although it is impractical to compare all aspects in which Avogenx’s and Islet Sciences’ governing documents differ with respect to rights of stockholders, the following is a brief discussion summarizing certain differences between them.
| | | | | |
| | | Avogenx Stockholder Rights | | Islet Sciences Stockholder Rights |
| Authorized Capital Stock | | The authorized capital stock of Avogenx consists of 200 million shares of common stock, par value $0.001 per share, and 10 million shares of preferred stock, par value $0.001 per share. | | The authorized capital stock of Islet Sciences consists of 100 million shares of common stock, par value $0.001 per share, and 10 million shares of preferred stock, par value $0.001 per share. |
| | | |
| Number of Directors | | Avogenx’s By-Laws state that the Avogenx board of directors will consist of not less than one director, with the specific number determined by the board or stockholders, with the initial board consisting of five directors. Currently, there are five directors. | | Islet Sciences’ bylaws state that the Islet Sciences board of directors will consist of not less than one director, with the specific number determined by the board. Currently, there are six directors. |
| | | | | |
| Rights of Holders of Preferred Stock to Elect Directors | | No shares of preferred stock are outstanding. | | No shares of preferred stock are authorized or outstanding. |
| | | |
| Vacancy of Directors | | Under Avogenx’s By-Laws, vacancies and newly created directorships, resulting from an increase in the number of directors by action of the board, may be filled by the majority of the remaining directors, even if less than a quorum, or by the sole remaining director. The director elected to fill a vacancy will serve until the next annual meeting of stockholders and until such director’s successor has been elected and qualified, or until such director’s earlier death, resignation or removal from office. | | Under Islet Sciences bylaws, vacancies and newly created directorships resulting from an increase in the number of directors may be filled by the majority of the directors then in office, even if less than a quorum, or by the sole remaining director. |
| | | |
| | | | | |
| | | |
| Stockholder Nomination of Directors | | Under Avogenx’s By-Laws, nominations for the election of directors may be made by the Avogenx board or a committee appointed by the board or a stockholder of record entitled to vote on the matter. | | Under Islet Sciences’ bylaws, all matters submitted to a meeting of the stockholders will be decided by the vote of the holders, present in person or by proxy, of a majority of Islet Sciences’ issued and outstanding stock, unless the question is one upon which, by express provision of law or the certificate of incorporation or bylaws, a different vote is required, in which case such express provision shall govern and control the decision of such question. |
| | | |
| | | Such stockholders may nominate directors for election if notice of intent to nominate a director and information regarding that nominee is received no later than close of business on the 120th day nor earlier than close of business on the 150th day prior to the first anniversary of the preceding year’s annual meeting (or if the date of the annual meeting is more than 30 days before or 60 days after such anniversary date, then not later than the 10th day following the earlier of the day on which public disclosure of the date of the annual meeting was made and the receipt by such stockholder of actual notice of the date of such annual meeting). | | |
| | | |
| | | Avogenx’s By-Laws require that the stockholder notice contain certain information about the stockholder and its nominee(s). | | |
| | | |
| Removal of Directors | | Directors may be removed for cause by the vote of the majority of outstanding common shares or by the board of directors. | | Under the NRS, directors may be removed with or without cause by vote of the holders of record, present in person or by proxy, of not less than two-thirds of Islet Sciences issued and outstanding capital stock. |
| | | |
| Stockholder Action without a Meeting | | Under Avogenx’s By-Laws, any action that may be taken by stockholders at a meeting may be decided by written consent of the stockholders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted; provided that prompt notice be given to those stockholders of the taking of such action without a meeting that have not consented in writing. | | Under Islet Sciences’ bylaws, any action that may be taken by stockholders at a meeting may be decided by written consent of the holders of outstanding stock having not less than the minimum number of votes that would be necessary to take such action at a meeting at which all shares entitled to vote thereon were present and voted. Prompt, written notice of the action taken by means of any such consent which is other than unanimous shall be given to those stockholders who have not consented in writing. |
| | | | | |
| Special Meetings of Stockholders | | Under Avogenx’s By-Laws, the majority of the whole board of directors, the president or the chief executive officer may call special meetings of stockholders for any purpose. | | Under Islet Sciences’ bylaws, the chairman or the president may call a special meeting of stockholders for any purpose and the president or secretary may call a special meeting of the stockholders if directed by the board of directors or requested in writing by the holders of at least majority of the stock. |
| | | |
| Amendment of Certificate of Incorporation | | Under Avogenx’s Certificate of Incorporation, the provision relating to the company’s name, purpose and capital structure may be amended or repealed by the majority of the outstanding shares of common stock entitled to vote. All other provisions of the Certificate of Incorporation may be amended, altered or repealed only by the vote of at least two-thirds of the outstanding shares of Avogenx common stock. | | Under Islet Sciences’ articles of incorporation, they can be amended by the vote of a majority of the stock entitled to vote. |
| | | |
| Amendment of Bylaws | | The Avogenx board of directors, by a majority of the whole board, and holders of a majority of the outstanding shares entitled to vote may amend Avogenx’s By-Laws. | | The Islet Sciences stockholders acting by majority vote can adopt, amend or repeal the bylaws. The board also has the power to adopt, amend or repeal the bylaws if such power is conferred upon the board in the articles of incorporation. |
| | | |
| Quorum | | The holders of one third of the shares entitled to vote will constitute a quorum at a meeting of stockholders for the transaction of any business. | | The holders of a majority of the outstanding stock will constitute a quorum. |
| | | |
| Voting Rights | | Each holder of Avogenx common stock is entitled to one vote for each share held of record. Directors are elected by a plurality of the votes cast. Other stockholder actions generally require the affirmative vote of a majority of the votes cast. | | Each holder of Islet Sciences common stock is entitled to one vote for each share held. Under Islet Sciences bylaws, all matters submitted to a meeting of stockholders will be decided by a vote of the holders, present in person or by proxy, of a majority of the outstanding stock, except as otherwise provided by law. |
| | | | | |
| Dividends | | Under the DGCL, Avogenx may declare and pay dividends either out of its surplus or, if there is no surplus, out of its net profits for the fiscal year in which the dividend is declared and/or the preceding year, in the discretion of Avogenx’s board of directors. | | Under the NRS, a board of directors may authorize and Islet Sciences may make distributions to its stockholders, including distributions on shares that are partially paid. However, no distribution may be made if, after giving effect to such distribution: (a) the corporation would not be able to pay its debts as they become due in the usual course of business; or (b) Islet Sciences’ total assets would be less than the sum of its total liabilities plus the amount that would be needed, if the corporation were to be dissolved at the time of distribution, to satisfy the preferential rights upon dissolution of stockholders whose preferential rights are superior to those receiving the distribution. |
| | | |
| Indemnification of Officers and Directors | | Under Avogenx’s Certificate of Incorporation, Avogenx must indemnify officers and directors to the full extent permitted under the DGCL. Avogenx will advance to a current or former director or officer expenses incurred in defending any action for which the right of indemnification is applicable. | | Under Islet Sciences’ bylaws, Islet Sciences must indemnify officers and directors against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by them in connection with an action, suit or proceeding, whether civil, criminal, administrative or investigative suit or proceeding if the person acted in good faith and in a manner which he or she reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe the conduct was unlawful. Islet Sciences will advance to a current or former director or officer expenses incurred in defending any action for which the right of indemnification is applicable upon receipt of an undertaking by or on behalf of such director or officer to repay such amount if it shall ultimately be determined that such person is not entitled to be indemnified by the company. |
| | | | | |
| Limitations on the Liability of Directors | | Avogenx’s Certificate of Incorporation provides that Avogenx’s directors are protected from personal liability for breaches of fiduciary duties except (i) for any breach of the director’s duty of loyalty to Avogenx or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) for an unlawful payment of dividends or unlawful purchase or redemption of stock or (iv) for any transaction from which the director derived an improper personal benefit. | | Islet Sciences’ certificate of incorporation provides that no director of Islet Sciences shall be personally liable to Islet Sciences or its stockholders for monetary damages for breach of fiduciary duty as a director, except (i) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, or (ii) for an unlawful payment of dividends. |
| | | |
| Notice of Stockholder Meetings | | Under Avogenx’s By-Laws, notice must be delivered to each stockholder entitled to notice of or to vote at the meeting not less than ten or more than sixty days before the meeting. | | Under Islet Sciences’ bylaws, notice must be delivered to each stockholder entitled to notice of or to vote at the meeting not less than ten or more than sixty days before the meeting. |
| | | |
| Stockholder Proposals | | Under Avogenx’s By-Laws, stockholders entitled to vote on the matter may bring business before annual meetings, provided that the business is a proper matter for stockholder action under Delaware law, the advance notice and information requirements of Avogenx’s By-Laws are met, and the business is specified in the notice of meeting given to stockholders. | | Islet Sciences’ bylaws do not have a similar provision. |
| | | |
| | | For annual meetings, notice of such business containing the information required by Avogenx’s By-Laws must be given to Avogenx no later than close of business on the 120th day nor earlier than close of business on the 150th day prior to the first anniversary of the preceding year’s annual meeting (or if the date of the annual meeting is more than 30 days before or 60 days after such anniversary date, then not later than the 10th day following the earlier of the day on which public disclosure of the date of the annual meeting was made and the receipt by such stockholder of actual notice of the date of such annual meeting). | | |
| | | | | |
| Business Combinations with Interested Stockholders | | Avogenx has opted out of section 203 of the DGCL which governs business combinations with interested stockholders. | | Islet Sciences has not opted out of sections 78.411 to 78.444 of the NRS which govern business combinations with interested stockholders. |
| Exclusivity Forum | | Under Avogenx’s By-Laws, unless Avogenx consents in writing to the selection of an alternative forum, the United States District Court for the Eastern District of North Carolina or, if such court lacks jurisdiction, any North Carolina state court that has jurisdiction, will be the exclusive forum for derivative actions or proceedings brought on behalf of Avogenx, actions for breach of fiduciary duties owed by directors, officers or other employees of Avogenx to Avogenx or its stockholders, actions asserting a claim against Avogenx or any director, officer or other employee of Avogenx arising under the DGCL or Avogenx’s Certificate of Incorporation or By-Laws or actions asserting a claim against Avogenx or any director, officer or other employee of Avogenx governed by the internal affairs doctrine. | | Islet Sciences’ bylaws do not have a forum selection provision. |
| | | | | |
DISSENTER’S RIGHTS
Under the NRS, as more fully described below, if you do not wish to accept shares of Avogenx common stock in exchange for your shares of Islet Sciences common stock and the Merger is consummated, you have the right to dissent and to receive payment in cash for the fair value of your Islet Sciences common stock together with interest, if any, to be paid upon the amount determined to be fair value. These rights are known as dissenter’s rights. Islet Sciences stockholders who elect to exercise dissenter’s rights must not vote in favor of or consent in writing to the proposal to approve the Merger Agreement and the Merger and must comply with the provisions of Sections 92A.300 to 92A.500 of the NRS to perfect their rights. Strict compliance with the statutory procedures in Sections 92A.300 to 92A.500 of the NRS is required. A holder of Islet Sciences common stock that wishes to exercise dissenter’s rights, or preserve the ability to do so, must not sign and deliver a written consent approving the Merger Agreement and the Merger.
This section is intended as a brief summary of the material provisions of the Nevada statutory procedures that a stockholder must follow to seek and perfect dissenter’s rights. This summary, however, is not a complete statement of all applicable requirements, and it is qualified in its entirety by reference to Sections 92A.300 to 92A.500 of the NRS, the full text of which appears in Annex C to this consent solicitation statement/prospectus. The following summary does not constitute any legal or other advice, nor does it constitute a recommendation that stockholders exercise their dissenter’s rights under Sections 92A.300 to 92A.500 of the NRS.
Sections 92A.300 to 92A.500 of the NRS require that, where a merger agreement is approved by written consent of stockholders in lieu of a meeting, certain stockholders must be given notice that dissenter’s rights are available. The notice must be provided no later than 10 days after the effective date of the merger. Only those Islet Sciences stockholders who did not submit a consent in favor of the proposal to approve the Merger Agreement and the Merger and who have otherwise complied with Sections 92A.300 to 92A.500 of the NRS are entitled to receive such notice. The dissenter’s notice specifies where you must send your payment demand and where and when you must deposit your stock certificates, if any, among other information. The date by which Islet Sciences must receive the demand for payment may not be less than 30 or more than 60 days after the date the notice is delivered.
Failure to demand payment in the proper form or deposit your certificates as described in the dissenter’s notice will terminate your right to receive payment for your shares pursuant to the Nevada’s dissenter’s rights statutes. Your rights as a stockholder will continue until those rights are cancelled or modified by the completion of the Merger.
A stockholder of record may assert dissenter’s rights as to fewer than all of the shares registered in his or her name only if the stockholder of record dissents with respect to all shares of the class or series beneficially owned by any one person and notifies Islet Sciences in writing of the name and address of each person on whose behalf the stockholder of record asserts dissenter’s rights. The rights of a partial dissenter are determined as if the shares as to which the partial dissenter dissents and his or her other shares were registered in the names of different stockholders.
If shares of Islet Sciences common stock are owned of record by a person other than the beneficial owner, including a broker, fiduciary (such as a trustee, guardian or custodian) or other nominee, a beneficial stockholder may assert dissenter’s rights as to shares held on his or her behalf only if the beneficial stockholder:
(a) Submits to Islet Sciences the written consent of the stockholder of record to the dissent not later than the time the beneficial stockholder asserts dissenter’s rights; and
(b) Does so with respect to all shares of which he or she is the beneficial stockholder or over which he or she has power to direct the vote.
Within 30 days after receiving your properly executed payment demand, Islet Sciences will pay you what we determine to be the fair value of your shares, plus accrued interest (computed from the effective date of the Merger until the date of payment). We will enclose the following with the payment:
| | ● | our balance sheet as of the end of a fiscal year ended not more than 16 months before the date of payment, an income statement for that year, a statement of changes in stockholders’ equity for that year, and the latest available interim financial statements, if any; |
| | ● | an explanation of how we estimated the fair value of the shares and how the interest was calculated; |
| | ● | information regarding your right to challenge the estimated fair value; and |
| | ● | a copy of the Nevada’s dissenter’s rights statutes. |
We may elect to withhold payment from you if you became the beneficial owner of the shares on or after the date set forth in the dissenter’s notice. If we withhold payment, after the consummation of the Merger, we will estimate the fair value of the shares, plus accrued interest, and offer to pay this amount to you in full satisfaction of your demand. The offer will contain a statement of our estimate of the fair value, an explanation of how the interest was calculated, and a statement of dissenter’s rights to demand payment under Nevada law Section 92A.480.
If you believe that the amount Islet Sciences pays in exchange for your dissenting shares is less than the fair value of your shares or that the interest is not correctly determined, you can demand payment of the difference between your estimate and Islet Sciences’. You must make such demand within 30 days after Islet Sciences have made or offered payment; otherwise, your right to challenge calculation of fair value terminates.
If there is still disagreement about the fair market value within 60 days after Islet Sciences receives your demand, Islet Sciences will petition a Nevada district court to determine the fair value of the shares and the accrued interest. If Islet Sciences does not commence that legal action within the 60-day period, it will have to pay the amount demanded for all unsettled demands. All dissenters whose demands remain unsettled will be made parties to the proceeding, and are entitled to a judgment for either:
| | ● | the amount of the fair value of the shares, plus interest, in excess of the amount we paid; or |
| | ● | the fair value, plus accrued interest, of the after-acquired shares for which we withheld payment. |
Islet Sciences will pay the costs and expenses of the court proceeding, unless the court finds the dissenters acted arbitrarily, vexatious, or in bad faith. If the court makes such a finding, the court may assess costs including reasonable fees of counsel and experts, against such dissenters.
Failure to follow the steps required by Nevada law Sections 92A.400 through 92A.480 for perfecting dissenter’s rights may result in the loss of those rights. If dissenter’s rights are not perfected, you will be entitled to receive the consideration receivable with respect to those shares in accordance with the Merger Agreement. In view of the complexity of the provisions of Nevada’s dissenter’s rights statute, you should consult your own legal advisor.
EXPERTS
The consolidated financial statements of Islet Sciences at April 30, 2014 and 2013 and for each of the years in the two-year period ended April 30, 2014 included in this consent solicitation statement/prospectus have been so included in reliance on the report of PKF, Certified Public Accountants, APC, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
The consolidated financial statements of BHV at April 30, 2014 and 2013 and for the years then ended have been included herein in reliance upon the reports of PKF, Certified Public Accountants, APC, an independent registered public accounting firm, given on the authority of said firm as experts in accounting and auditing. Certain United States federal income tax consequences relating to the merger will be passed upon for Avogenx by Anderson Bradshaw, PLLC.
WHERE YOU CAN FIND MORE INFORMATION
Islet Sciences files annual, quarterly and special reports and other information with the SEC under the Exchange Act. You may read and copy this information at the SEC’s Public Reference Room, 100 F Street, N.E., Washington, D.C. 20549, or at the SEC’s public reference rooms in New York, New York or Chicago, Illinois. You may also obtain copies of this information by mail from the Public Reference Section of the SEC, 100 F Street, N.E., Washington, D.C. 20549, at prescribed rates. The SEC also maintains an Internet website that has reports, proxy statements and other information about issuers, like Islet Sciences, that make electronic filings with the SEC. The address of that site is www.sec.gov.
Avogenx filed a registration statement on Form S-4 to register with the SEC the Avogenx common stock to be issued to Islet Sciences stockholders in exchange for their Islet Sciences common stock. This consent solicitation statement/prospectus is a part of that registration statement and constitutes a prospectus of Avogenx. The registration statement contains more information than this consent solicitation statement/prospectus regarding Avogenx and the securities, including exhibits and schedules. You can obtain a copy of the registration statement from the SEC at any address listed above or from the SEC’s web site.
You may also find more information by visiting the Islet Sciences website at www.isletsciences.com. No information provided on this website is incorporated into this consent solicitation statement/prospectus or registration statement.
LEGAL MATTERS
Ofsink, LLC, counsel to Avogenx, will pass on the validity of the Avogenx common stock to be issued to Islet Sciences stockholders in exchange for their Islet Sciences common stock. The member and an employee of Ofsink, LLC hold in the aggregate 216,287 shares of Islet Sciences common stock.
INDEX TO FINANCIAL STATEMENTS
| | | |
| | |
| Islet Sciences, Inc. | | |
| | | |
| Unaudited Financial Statements | | |
| Condensed Consolidated Balance Sheets at July 31, 2014 and April 30, 2014 | | F-2 |
| Condensed Consolidated Statements of Operations for the three months ended July 31, 2014 and 2013 | | F-3 |
| Condensed Consolidated Statements of Cash Flows for the three months ended July 31, 2014 and 2013 | | F-4 |
| Notes to the Condensed Consolidated Financial Statements | | F-5 |
| | | |
| Audited Financial Statements | | |
| Report of Independent Registered Public Accounting Firm | | F-12 |
| Consolidated Balance Sheets at April 30, 2014 and 2013 | | F-13 |
| Consolidated Statements of Operations for the years ended April 30, 2014 and 2013 and for the period from May 4, 2010 (inception) through April 30, 2014 | | F-14 |
| Consolidated Statements of Stockholders’ Equity for the years ended April 30, 2014 and 2013 and for the period from May 4, 2010 (inception) through April 30, 2014 | | F-15 |
| Consolidated Statements of Cash Flows for the years ended April 30, 2014 and 2013 and for the period from May 4, 2010 (inception) through April 30, 2014 | | F-16 |
| Notes to the Consolidated Financial Statements | | F-17 |
| | |
| Brighthaven Ventures, LLC d/b/a BHV Pharma | | |
| | | |
| Unaudited Financial Statements | | |
| Balance Sheets at July 31, 2014 and April 30, 2014 | | F-36 |
| Statements of Operations for the three months ended July 31, 2014 and 2013 and for the period from August 26, 2009 (inception) through July 31, 2014 | | F-37 |
| Statements of Cash Flows for the three months ended July 31, 2014 and 2013 and for the period from August 26, 2009 (inception) through July 31, 2014 | | F-39 |
| Notes to the Financial Statements | | F-40 |
| | | |
| Audited Financial Statements | | |
| Report of Independent Registered Public Accounting Firm | | F-45 |
| Balance Sheets at April 30, 2014 and 2013 | | F-46 |
| Statements of Operations for the years ended April 30, 2014 and 2013 and for the period from August 26, 2009 (inception) through April 30, 2014 | | F-47 |
| Statements of Members’ Equity (Deficit) for the years ended April 30, 2014 and 2013 and for the period from August 26, 2009 (inception) through April 30, 2014 | | F-48 |
| Statements of Cash Flows for the years ended April 30, 2014 and 2013 and for the period from August 26, 2009 (inception) through April 30, 2014 | | F-49 |
| Notes to the Financial Statements | | F-50 |
Islet Sciences, Inc. and Subsidiaries
| | | July 31, | | | April 30, | |
| | | 2014 | | | 2014 | |
| | | Unaudited | | | Audited | |
| ASSETS | | | | | | |
| CURRENT ASSETS | | | | | | |
| Cash | | $ | 457,436 | | | $ | 1,141,380 | |
| Prepaid expenses | | | 700 | | | | 700 | |
| Total current assets | | | 458,136 | | | | 1,142,080 | |
| OTHER ASSETS | | | | | | | | |
| Intangible asset (Note 3) | | | 1,367,000 | | | | 1,367,000 | |
| Goodwill (Note 3) | | | 2,111,107 | | | | 2,111,107 | |
| Total other assets | | | 3,478,107 | | | | 3,478,107 | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 3,936,243 | | | $ | 4,620,187 | |
| | | | | | | | | |
| LIABILITIES & STOCKHOLDERS' EQUITY | | | | | | | | |
| CURRENT LIABILITIES | | | | | | | | |
| Accounts payable | | $ | 3,042,768 | | | $ | 3,271,174 | |
| Accrued stock compensation expenses (Note 4) | | | 142,177 | | | | 433,346 | |
| Notes payable - related parties | | | 51,818 | | | | 91,641 | |
| Total current liabilities | | | 3,236,763 | | | | 3,796,161 | |
| | | | | | | | | |
| Deferred income taxes | | | 547,000 | | | | 547,000 | |
| Total liabilities | | | 3,783,763 | | | | 4,343,161 | |
| | | | | | | | | |
| Commitments and Contingencies (Note 5) | | | | | | | | |
| | | | | | | | | |
| STOCKHOLDERS' EQUITY | | | | | | | | |
| Preferred stock, $0.001 par value, 10,000,000 shares authorized; no shares | | | | | | | | |
| issued and outstanding at July 31, 2014 and April 30, 2014 | | | - | | | | - | |
| Common stock, $0.001 par value, 100,000,000 shares authorized; | | | | | | | | |
| 66,928,724 and 67,516,253 shares issued and outstanding at July 31, 2014 and April 30, 2014, respectively | | | 66,929 | | | | 67,517 | |
| Additional paid-in capital | | | 20,938,814 | | | | 20,598,242 | |
| Accumulated deficit | | | (20,853,263 | ) | | | (20,388,733 | ) |
| Total stockholders' equity | | | 152,480 | | | | 277,026 | |
| TOTAL LIABILITIES & STOCKHOLDERS' EQUITY | | $ | 3,936,243 | | | $ | 4,620,187 | |
See notes to condensed consolidated financial statements
Islet Sciences, Inc. and Subsidiaries
| | | Three months ended July 31, | |
| | | 2014 | | | 2013 | |
| REVENUE | | $ | - | | | $ | - | |
| | | | | | | | | |
| OPERATING EXPENSES | | | | | | | | |
| General and administrative | | | 405,399 | | | | 518,439 | |
| Research and development | | | 58,331 | | | | 774,184 | |
| Total operating expenses | | | 463,730 | | | | 1,292,623 | |
| | | | | | | | | |
| LOSS FROM OPERATIONS | | | (463,730 | ) | | | (1,292,623 | ) |
| | | | | | | | | |
| OTHER INCOME (EXPENSE) | | | | | | | | |
| Interest expense | | | (800 | ) | | | (2,231 | ) |
| Total other expense | | | (800 | ) | | | (2,231 | ) |
| | | | | | | | | |
| LOSS BEFORE INCOME TAXES | | | (464,530 | ) | | | (1,294,854 | ) |
| | | | | | | | | |
| INCOME TAX EXPENSE | | | - | | | | - | |
| | | | | | | | | |
| NET LOSS | | $ | (464,530 | ) | | $ | (1,294,854 | ) |
| | | | | | | | | |
| NET LOSS PER COMMON SHARE, | | | | | | | | |
| BASIC AND DILUTED | | $ | (0.01 | ) | | $ | (0.02 | ) |
| WEIGHTED AVERAGE NUMBER OF | | | | | | | | |
| COMMON SHARES OUTSTANDING BASIC AND DILUTED | | | 67,069,789 | | | | 56,715,117 | |
See notes to condensed consolidated financial statements
Islet Sciences, Inc. and Subsidiaries
| | | Three months ended July 31, | |
| | | 2014 | | | 2013 | |
| | | | | | | |
| Cash flows from operating activities: | | | | | | |
| Net loss | | $ | (464,530 | ) | | $ | (1,294,854 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Stock based compensation for services and other | | | 78,815 | | | | 239,000 | |
| Derivative liability | | | - | | | | 24,283 | |
| Amortization of intangible asset | | | - | | | | 7,601 | |
| Change in operating assets and liabilities: | | | | | | | | |
| Accounts payable | | | (228,406 | ) | | | 988,134 | |
| | | | | | | | | |
| Net cash used in operating activities | | | (614,121 | ) | | | (35,836 | ) |
| | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | |
| Net cash provided by investing activities | | | - | | | | - | |
| | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
| Proceeds from notes payable - related parties | | | - | | | | 32,362 | |
| Payments on notes payable - related parties | | | (39,823 | ) | | | - | |
| Repurchase of stock | | | (30,000 | ) | | | - | |
| Net cash (used in) provided by financing activities | | | (69,823 | ) | | | 32,362 | |
| | | | | | | | | |
| Net decrease in cash | | | (683,944 | ) | | | (3,474 | ) |
| | | | | | | | | |
| Cash at beginning period | | | 1,141,380 | | | | 3,589 | |
| | | | | | | | | |
| Cash at end period | | $ | 457,436 | | | $ | 115 | |
| | | | | | | | | |
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | | | | | |
| Cash paid during the period for: | | | | | | | | |
| Interest | | $ | 800 | | | $ | - | |
| Income taxes | | $ | - | | | $ | - | |
| | | | | | | | | |
| SUPPLEMENTAL DISCLOSURE OF NON-CASH INVESTING AND FINANCING INFORMATION: | |
| Shares issued for settlement of accrued expenses | | $ | 313,444 | | | $ | - | |
See notes to condensed consolidated financial statements
Islet Sciences, Inc. and Subsidiaries
Unaudited Interim financial statements
The accompanying unaudited interim condensed consolidated financial statements have been prepared by the Islet Sciences, Inc. pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”). In the opinion of management, all adjustments (which include only normal recurring adjustments except as noted in management’s discussion and analysis of financial condition and results of operations) necessary to present fairly the financial position, results of operations and changes in cash flows have been made.
Certain information and footnote disclosures normally included in financial statements prepared in accordance with accounting principles generally accepted in the United States of America have been condensed or omitted. It is suggested that these financial statements be read in conjunction with the 2014 financial statements and notes thereto included within the report on Form 10-K filed with the SEC on July 28, 2014. The results of operations for the three months ended July 31, 2014, are not necessarily indicative of the operating results for the full year.
NOTE 1. DESCRIPTION OF BUSINESS
Description of Business
We are a biotechnology company engaged in the research, development, and commercialization of new medicines and technologies for the treatment of metabolic disease and related indications where there is significant measurable unmet medical need. The rising incidence of obesity is associated with many obesity-related health complications, including cardiovascular disease, diabetes, hyperlipidemia, hypertension, nonalcoholic fatty liver disease/steatohepatitis (NAFLD/NASH). This constellation is also recognized as the metabolic syndrome and is characterized by underlying insulin resistance. These various diseases have interrelated risk factors and markers, such that often treatment of one disease may allow new therapies and opportunities for treatment in one of these related indications. Our focused effort to develop new therapies and related diagnostics for metabolic related diseases establishes us as a recognized leader in a large and growing market.
In March 2014, the Company announced it had signed a binding letter of intent to enter into a merger agreement with and acquire Brighthaven Ventures, LLC d/b/a BHV Pharma (“BHV”) a privately held pharmaceutical company developing the SGLT2 inhibitor remogliflozin etabonate (“remogliflozin”) for type 2 diabetes and non-alcoholic steatohepatitis (“NASH”). Remogliflozin is currently in phase II clinical development.
Islet Sciences was incorporated under the name One E-Commerce Corporation on September 14, 1994 in the State of Nevada. Effective February 23, 2012, the Company changed its name to Islet Sciences, Inc. On March 14, 2012, Islet Sciences acquired DiaKine Therapeutics, Inc., a Delaware corporation (“DTI”). Islet Sciences together with its subsidiaries, Islet Sciences Inc., a Delaware corporation (“ISI”), and DTI are referred to as the Company.
Going Concern
The condensed consolidated financial statements have been prepared assuming the Company will continue as a going concern. This contemplates the continuity of operations, realization of assets, and liquidation of liabilities in the normal course of business. Since inception, the Company has incurred operating losses of $20,853,263. As of July 31, 2014, the Company had cash of $457,436. Further, the Company has incurred net losses of $464,530 and negative operating cash flows of $614,121 for the three months ended July 31, 2014. The condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
The Company’s future cash requirements will depend on many factors, including continued scientific progress in its research and development programs, the scope and results of pre-clinical and clinical trials, the time and costs involved in obtaining regulatory approvals, the costs involved in filing, prosecuting and enforcing patents, competing technological and market developments, and the cost of product commercialization. The Company does not expect to generate a positive cash flow from operations at least until the commercial launch of its first product and possibly later given the expected spending for research and development programs and the cost of commercializing product candidates. The Company’s continued operations will depend on its ability to raise funds through various potential sources such as debt and equity financing. There can be no assurance that such capital will be available on favorable terms or at all. If the Company is unable to raise additional capital, the Company will likely be forced to curtail its desired development activities, which would delay the development of its product candidates.
Merger with BHV Pharma
In March 2014, the Company announced it had signed a binding letter of intent to enter into a merger agreement with and acquire Brighthaven Ventures, LLC, a North Carolina limited liability company, d/b/a BHV Pharma (“BHV”) a privately held pharmaceutical company developing the SGLT2 inhibitor remogliflozin etabonate (“remogliflozin”) for type 2 diabetes and non-alcoholic steatohepatitis (“NASH”). Remogliflozin is currently in phase II clinical development. In exchange for 100% ownership of BHV, the Company will issue 30 million shares of its common stock to the holders of BHV units. Additional shares of common stock will be issued upon successful completion of development, regulatory and commercial milestones associated with the remogliflozin program. James Green and William Wilkison, the current members of BHV, are the Chief Executive Officer ("CEO") and the Chief Operating Officer ("COO"), of the Company, respectively, and are the sole members of BHV.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying condensed consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”).
The accompanying condensed consolidated financial statements include the accounts of Islet Sciences and its wholly-owned subsidiaries, ISI and DTI. All significant intercompany balances have been eliminated.
Use of Estimates
The preparation of condensed consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates include the recoverability of long-lived assets, the valuation of intangible assets and goodwill, the valuation of common stock, warrants and stock options and the valuation of deferred tax assets. Actual results could differ from those estimates.
Concentration of Credit Risk
The Company maintains its cash balances at a credit-worthy financial institution and management believes the risk of loss of cash balances to be low. The Company’s cash balances were not fully insured at July 31, 2014.
Intangible Assets
Intangible assets represent a patent acquired from a third party, which is recorded at cost and amortized over the remaining life of the patent. This patent was fully impaired and written off during the year ended April 30, 2014. Intangible assets also include the purchase of DiaKine Therapeutics, Inc. patent portfolio and know-how as in-process research and development (“IPR&D”). IPR&D has an indefinite life and is not amortized until completion and development of the project, at which time the IPR&D becomes an amortizable asset. If the related project is not completed in a timely manner or the project is terminated or abandoned, the Company may have an impairment related to the IPR&D, calculated as the excess of the asset’s carrying value over its fair value. The intangible assets with estimable useful lives are amortized on a straight line basis over their respective estimated useful lives to their estimated residual values. This method of amortization approximates the expected future cash flow generated from their use. Definite lived intangibles are reviewed for impairment in accordance with FASB ASC 360, Property, Plant and Equipment (FASB ASC 360).
Goodwill
Goodwill represents the excess of the purchase price over the estimated fair value of the identifiable assets acquired and liabilities assumed in business acquisitions. Goodwill is reviewed at least annually for impairment in the fourth quarter of the fiscal year, at the Company level, which is the sole reporting unit, and at any other time at which events occur or circumstances indicate that the carrying amount of goodwill may exceed its fair value. Such indicators would include a significant reduction in the Company’s market capitalization, a decrease in operating results or deterioration in the Company’s financial position.
Impairment of Long-Lived Assets
The Company applies the provisions of FASB ASC 360-10, Property, Plant and Equipment (FASB ASC 360-10), where applicable to all long lived assets. FASB ASC 360-10 addresses accounting and reporting for impairment and disposal of long-lived assets. The Company periodically evaluates the carrying value of long-lived assets to be held and used in accordance with FASB ASC 360-10. FASB ASC 360-10 requires impairment losses to be recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amounts. In that event, a loss is recognized based on the amount by which the carrying amount exceeds the fair market value of the long-lived assets. Loss on long-lived assets to be disposed of is determined in a similar manner, except that fair market values are reduced for the cost of disposal.
Loss Per Share Data
Basic loss per share is calculated based on the weighted average common shares outstanding during the period. Diluted earnings per share also give effect to the dilutive effect of restricted common stock and warrants. The Company does not present diluted earnings per share for years in which it incurred net losses as the effect is anti-dilutive.
At July 31, 2014, there are no unvested shares of restricted common stock and warrants to exercise 12,430,798 shares of common stock were outstanding, but were not included in the computation of diluted earnings per share as their effect would be anti-dilutive. At July 31, 2013, 1,368,750 unvested shares of restricted common stock and warrants to exercise 6,366,794 shares of common stock were outstanding, but were not included in the computation of diluted earnings per share as their effect would be anti-dilutive
Research and Development
Research and development expenses consist of expenses incurred in performing research and development activities including salaries and benefits, facilities and other overhead expenses, contract services and other outside expenses. Research and development costs are charged to operations when incurred.
Stock Based Compensation
Stock awards
FASB ASC 718, Compensation-Stock Compensation (FASB ASC 718), requires the fair value of all share-based payments to employees, including grants of stock options, to be recognized in the condensed consolidated statement of operations over the requisite service period.FASB ASC 718 requires all share based payments to employees, including grants of employee stock option, to be recognized in operating results as compensation expense based on fair value over the requisite service period of the awards. The Company determines the fair value of share-based awards using the Black-Scholes option-pricing model which uses both historical and current market data to estimate fair value. The method incorporates various assumptions such as the risk-free interest rate, expected volatility, expected dividend yield, expected forfeiture rate and expected life of the options. As allowed by FASB ASC 718, for companies with a short period of publicly traded stock history, the Company’s estimate of expected volatility is based on the average volatilities of a sampling of three companies with similar attributes to the Company, including industry, stage of life cycle, size and financial leverage. The risk-free rate for periods within the contractual life of the stock options is based on the U.S. Treasury yield curve in effect at the time of grant valuation. The expected term of options granted is derived using the “simplified method” which computes expected term as the average of the sum of the vesting term plus the contract term as historically the Company had limited activity surrounding its options. The Company has not issued stock options for nonemployees.
Warrants
Warrants granted to service providers are normally valued at the fair value of the instrument on the date of the grant (grant date) and are recognized in the condensed consolidated statement of operations over the requisite service period or when they vest. When the requisite service period precedes the grant date and a market condition exists in the warrant, the Company values the warrant using the Black-Scholes option pricing model. Warrants issued in connection with capital raises are normally valued at the fair value of the instrument on the date of the grant (grant date) and valued for disclosure purposes if they meet all the criteria under FASB ASC 718. The Company values these warrant using the Black-Scholes option pricing model as well. As allowed by FASB ASC 718, for companies with a short period of publicly traded stock history, the Company’s estimate of expected volatility is based on the average volatilities of a sampling of three companies with similar attributes to the Company, including industry, stage of life cycle, size and financial leverage. The risk-free rate for periods within the contractual life of the warrant is based on the U.S. Treasury yield curve in effect at the time of grant valuation.
Segment Reporting
The Company currently operates in a single operating segment. In addition, financial results are prepared and reviewed by management as a single operating segment. The Company continually evaluates its operating activities and the method utilized by management to evaluate such activities and will report on a segment basis if and when appropriate to do so.
Reclassification
Certain prior period balances have been reclassified to conform to current period presentation.
Recent Pronouncements
In May 2014, the FASB issued ASU No. 2014-09 “Revenue from Contracts with Customers,” which will supercede nearly all existing revenue recognition guidance under GAAP. ASU No. 2014-09 provides that an entity recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This update also requires additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgements and changes in judgements, and assets recognized from costs incurred to obtain or fulfill a contract. ASU No. 2014-09 allows for either full retrospective or modified retrospective adoption and will become efective for the Company in the first quarter of 2018. The Company is a development stage entity and will evaluate the effects of this update on its Condensed consolidated financial statements when it generates revenues.
In June 2014, the FASB issued ASU No. 2014-10, which eliminated certain financial reporting requirements of companies previously identified as “Development Stage Entities” (Topic 915). The amendments in this ASU simplify accounting guidance by removing all incremental financial reporting requirements for development stage entities. The amendments also reduce data maintenance and, for those entities subject to audit, audit costs by eliminating the requirement for development stage entities to present inception-to-date information in the statements of income, cash flows, and shareholder equity. Early application of each of the amendments is permitted for any annual reporting period or interim period for which the entity’s financial statements have not yet been issued (public business entities) or made available for issuance (other entities). Upon adoption, entities will no longer present or disclose any information required by Topic 915. The Company has adopted this standard in the current quarter.
In August 2014, the FASB issued ASU No. 2014-15, Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concen ("ASU 2014-15"). ASU 2014-15 will explicitly require management to assess an entity's ability to continue as a going concern, and to provide related footnote disclosure in certain circumstances. The new standard will be effective for all entities in the first annual period ending after December 15, 2016. Earlier adoption is permitted. The Company is currently evaluating the impact of the adoption of ASU 2014-15.
NOTE 3. INTANGIBLE ASSET AND GOODWILL
On March 14, 2012, the Company acquired the IPR&D from DiaKine Therapeutics, Inc. As of July 31, 2014, the $1.4 million of acquired IPR&D is classified as indefinite life asset and is not being amortized. In conjunction with this acquisition, the Company recognized $2.1 million of goodwill. The Company has not identified any triggering events at July 31, 2014 that would require additional analysis for potential impairment of its intangible assets and goodwill.
NOTE 4. STOCKHOLDERS’ EQUITY
On July 31, 2014, an aggregate of 123,750 shares of its common stock issuable to members of the Board of Directors fully vested. The Company valued these shares based on the July 31, 2014 share price of $0.18 per share, or $22,275, and recorded it to accrued stock compensation expense until the common stock is issued. The Company has included these costs as general and administrative expenses within the condensed consolidated statements of operations for the three months ended July 31, 2014.
On June 4, 2014, the Company issued 14,500 shares of common stock to a consultant pursuant to a settlement agreement. The Company valued these shares at $48,000 and were included in accrued stock compensation expense at April 30, 2014.
On May 9, 2014, the Company issued 742,500 shares of common stock, previously vested and included in accrued stock compensation expense, to the members of the Board of Directors as part of the approved compensation plan.
On October 30, 2013, as part of the employee agreements with the new CEO and COO, the Board of Directors granted each of them stock options to purchase 1.5 million shares of Company common stock at an exercise price of $0.265. The stock options will vest as follows: (i) 500,000 shares vesting on the 91st day from the option grant date, (ii) 1,000,000 shares vesting in equal installments of 200,000 on the last day of each 90 day period starting from the 91st day after the option grant date. For the three months ended July 30, 2014, 200,000 stock options vested for each the CEO and COO. The Company recorded stock-based compensation of $54,886 for the vested stock options as general and administrative expenses within the condensed consolidated statements of operations for the three months ended July 31, 2014.
On May 22, 2014, as part of the employee agreement with the new Chief Financial Officer, the Board of Directors granted stock options to purchase 300,000 shares of Company common stock at an exercise price of $0.23. The stock options will vest over 48 months. The Company recorded stock-based compensation of $1,655 for the vested stock options as general and administrative expenses within the condensed consolidated statements of operations for the three months ended July 31, 2014.
NOTE 5. COMMITMENTS AND CONTINGENCIES
Licenses
On May 2, 2012, the Company, entered into a license agreement with the Yale University (“Yale”). Under the agreement, the Company received exclusive license to the technology patented by Yale. In consideration of the license granted under the agreement, the Company paid Yale a license issue royalty of $10,000 (plus a $10,000 annual renewal fee) and issued 20,000 shares of its common stock, and agreed to pay certain milestones royalties by issuing an aggregate of 160,000 shares of common stock. The Company also agreed to pay to Yale a royalty on net sales. The agreement will expire automatically, on a country-by-country basis, on the date on which the last of the claims of the subject patents expires. The agreement can be terminated by Yale if the Company defaults on its obligations under the agreement and fails to cure such default within 60 days of a written notice by Yale. The Company can terminate the agreement upon a six month notice subject to payment of all amounts due to Yale under the agreement. During the three months ended July 31, 2014 and 2013, the Company expensed approximately $0 and $39,000, respectively, within the consolidated statements of operations.
On July 23, 2012, the Company entered into a licensing agreement with the Winthrop University Hospital (“Winthrop”) to license certain patents and technology. In consideration of the license granted under the agreement, the Company agreed to pay to Winthrop a license issue royalty of $10,000 (plus a $10,000 annual renewal fee) and issue 20,000 shares of its common stock, and to pay certain milestones royalties by issuing an aggregate of 160,000 shares of common stock. The Company also agreed to pay to Winthrop a royalty on net sales. The agreement will expire automatically, on a country-by-country basis, on the date on which the last of the claims of the subject patents expires. It can be terminated by Winthrop if the Company defaults on its obligations under the agreement and fails to cure such default within 60 days of a written notice by the university. The Company can terminate the agreement upon a six month notice subject to payment of all amounts due Winthrop under the agreement. During the three months ended July 31, 2014 and 2013, the Company expensed as research and development expense within the consolidated statements of operations of approximately $0 and $62,000 respectively, related to this contract. The Company is currently in default regarding its payment obligations under the foregoing license and research agreements.
Related Party Transactions
The Company borrowed $25,880 from its former CEO. Promissory notes were issued for these amounts. Repayments of $5,000 were made during the three months ended July 31, 2014. The remaining balance at July 31, 2014 was $8,475.
During the year ended April 30, 2014, one of the Company’s Board Members loaned the Company a total of $74,531 at a 6.5% interest rate. A promissory note was issued for this amount. Repayments of $20,000 were made during the three months ended July 31, 2014, which included $800 of accrued interest. The remaining balance at July 31, 2014 was $43,343.
A contractor of the Company loaned the Company $15,623. Payments of $15,623 have been made during the three months ended July 31, 2014. The remaining balance at July 31, 2014 was $0.
Litigation
In April 2012, Sand Dollar Partners, LLC, a shareholder of the Company filed a complaint in the Superior Court of Arizona, Pima County against, among other parties, ISI, our wholly-owned subsidiary, John Steel, our former CEO and director, and Jonathan Lakey, our former director. In 2010, Sand Dollar invested $357,000 in ISI through the purchase of a convertible promissory note which was converted into 3,591,729 shares of the Company’s common stock. The plaintiff contends that it was entitled to issuance of additional shares and nomination of one board member.
On October 25, 2013, the Company entered into a settlement agreement with Sand Dollar Partners, LLC. At a hearing on February 21, 2014, the Company and Sand Dollar agreed to amend the settlement agreement whereby, Sand Dollar placed in escrow all of the Company’s common stock it held and retained a broker dealer to sell sufficient shares to receive $500,000 in cash and to pay fees to the broker dealer. Additionally, the Company agreed to issue 130,000 warrants that will vest over the next three years and make a $30,000 payment on May 15, 2014. The Company issued these warrants and made the $30,000 payment on May 15, 2014 fully completing the settlement. The broker dealer sold 2,247,200 shares of the Company’s escrowed stock to settle the obligation and the Company has received back the remaining 1,344,529 shares of its common stock. The sale of these shares and the return of the remaining shares had no impact on the condensed consolidated financial statements.
On April 25, 2014, Progenitor Cell Therapy, LLC (“PCT”), filed a lawsuit against the Company in the United States District Court for the District of New Jersey (Case No. 2:14-cv-02658-SDW-MCA). PCT’s complaint asserts various claims, including breach of contract and unjust enrichment, based on the alleged failure of the Company to pay for services and goods provided by PCT under a January 10, 2012 letter agreement. PCT seeks an unspecified amount of compensatory and other damages, plus interest and costs. The Company filed an answer to the complaint on June 24, 2014 denying all liability, including on the grounds that the January 10, 2012 letter agreement is unenforceable and PCT failed to provide the goods and services it stated it would provide. In connection with its answer to PCT’s complaint, the Company filed a counterclaim against PCT and a third-party complaint against NeoStem to seek, among other things, (i) a declaration that the January 10, 2012 letter agreement is unenforceable, (ii) monetary damages and (iii) rescission of equity securities the Company previously issued to NeoStem. The Company intends to vigorously defend the claims by PCT and prosecute its claims against PCT and NeoStem. As the lawsuit is at an early stage, the Company cannot at this time estimate the possible loss or range of loss, if any, that may result from this lawsuit.
In July 2012, a complaint was filed against the Company and John Steel in the United States District Court for the District of Utah, Central Division for infringement and misappropriation of a patent. The plaintiffs contend that they were the actual purchasers of the MicroIslet patent out of MicroIslet’s bankruptcy proceedings in 2009 and that the respective intellectual property rights have been never assigned to either ISI or the Company. As a result, they allege that the Company’s claim to the ownership of the MicroIslet patent based on the assignment of the patent by its founders is baseless. The complaint sought monetary damages including punitive damages of at least $12 million, costs, attorneys' fees, and declaratory judgment. On January 8, 2013, the Court dismissed the plaintiff’s action for lack of recoverable damages. The plaintiffs refiled their claim and the Company has filed a motion with the Court for dismissal. The Company believes the plaintiffs’ claims to be without merit and will continue to vigorously defend against this action and has determined that it is unlikely any damages will be paid.
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders of
Islet Sciences, Inc. and Subsidiaries
We have audited the accompanying consolidated balance sheets of Islet Sciences, Inc. and Subsidiaries (A Development Stage Company) (the “Company”) as of April 30, 2014 and 2013 and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the two years then ended, and for the period from May 4, 2010 (Inception) through April 30, 2014. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We have conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal controls over financial reporting. Our audit includes consideration of internal controls over financial reporting as a basis for designing audit procedures that are appropriate in the circumstance, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Islet Sciences, Inc. and Subsidiaries (A Development Stage Company) as of April 30, 2014 and 2013, and the results of its operations and cash flows for each of the two years then ended, and for the period from May 4, 2010 (Inception) through April 30, 2014, in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has incurred losses since inception resulting in an accumulated deficit of approximately $20.4 million as of April 30, 2014 and further losses are anticipated in the development of its business that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| | | | |
| July 28, 2014 | | /s/ PKF | |
| San Diego, California | | PKF | |
| | | Certified Public Accountants | |
| | | A Professional Corporation | |
Islet Sciences, Inc. and Subsidiaries
(A Development Stage Company)
Consolidated Balance Sheets
| | | April 30, | | | April 30, | |
| | | 2014 | | | 2013 | |
| | | | | | | |
| ASSETS | | | | | | |
| CURRENT ASSETS | | | | | | |
| Cash | | $ | 1,141,380 | | | $ | 3,589 | |
| Prepaid expenses | | | 700 | | | | 50,000 | |
| Advance to related party | | | - | | | | 2,405 | |
| Total current assets | | | 1,142,080 | | | | 55,994 | |
| OTHER ASSETS | | | | | | | | |
| Intangible assets, net (Note 3) | | | 1,367,000 | | | | 1,475,788 | |
| Goodwill (Note 3) | | | 2,111,107 | | | | 2,111,107 | |
| Total other assets | | | 3,478,107 | | | | 3,586,895 | |
| | | | | | | | | |
| TOTAL ASSETS | | $ | 4,620,187 | | | $ | 3,642,889 | |
| | | | | | | | | |
| LIABILITIES & STOCKHOLDERS' EQUITY | | | | | | | | |
| CURRENT LIABILITIES | | | | | | | | |
| Accounts payable | | $ | 3,271,174 | | | $ | 2,233,314 | |
| Subscribed shares - not issued | | | - | | | | 96,600 | |
| Accrued stock compensation expenses (Note 4) | | | 433,346 | | | | 63,702 | |
| Notes payable - related parties | | | 91,641 | | | | 11,880 | |
| Derivative liability | | | - | | | | 58,588 | |
| Total current liabilities | | | 3,796,161 | | | | 2,464,084 | |
| | | | | | | | | |
| Deferred income taxes | | | 547,000 | | | | 547,000 | |
| Total liabilities | | | 4,343,161 | | | | 3,011,084 | |
| | | | | | | | | |
| Commitments and Contingencies (Note 5) | | | | | | | | |
| | | | | | | | | |
| STOCKHOLDERS' EQUITY | | | | | | | | |
| Preferred stock, $0.001 par value, 10,000,000 shares authorized; no shares | | | | | | | | |
| issued and outstanding at April 30, 2014 and April 30, 2013 | | | - | | | | - | |
| Common stock, $0.001 par value, 100,000,000 shares authorized; | | | | | | | | |
| 67,516,253 and 56,715,117 shares issued and outstanding at April 30, 2014 and April 30, 2013, respectively | | | 67,517 | | | | 56,716 | |
| Additional paid-in capital | | | 20,598,242 | | | | 18,017,392 | |
| Deficit accumulated during the developments stage | | | (20,388,733 | ) | | | (17,442,303 | ) |
| Total stockholders' equity | | | 277,026 | | | | 631,805 | |
| | | | | | | | | |
| TOTAL LIABILITIES & STOCKHOLDERS' EQUITY | | $ | 4,620,187 | | | $ | 3,642,889 | |
See accompanying notes to the consolidated financial statements
Islet Sciences, Inc. and Subsidiaries
(A Development Stage Company)
Consolidated Statements of Operations
| | | | | | | | | For the period from May 4, 2010 (Inception) through | |
| | | Year ended April 30, | | | April 30, | |
| | | 2014 | | | 2013 | | | 2014 | |
| REVENUE | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | |
| OPERATING EXPENSES | | | | | | | | | | | | |
| General and administrative | | | 2,195,198 | | | | 8,058,684 | | | | 12,562,136 | |
| Research and development | | | 646,250 | | | | 4,063,790 | | | | 6,795,160 | |
| Impairment loss | | | 93,586 | | | | - | | | | 93,586 | |
| Total operating expenses | | | 2,935,034 | | | | 12,122,474 | | | | 19,450,882 | |
| | | | | | | | | | | | | |
| LOSS FROM OPERATIONS | | | (2,935,034 | ) | | | (12,122,474 | ) | | | (19,450,882 | ) |
| | | | | | | | | | | | | |
| OTHER INCOME (EXPENSE) | | | | | | | | | | | | |
| Other income | | | - | | | | 430,000 | | | | 430,000 | |
| Other expenses | | | - | | | | - | | | | (1,345,710 | ) |
| Interest expense | | | (11,397 | ) | | | (10,125 | ) | | | (22,143 | ) |
| Total other expense | | | (11,397 | ) | | | 419,875 | | | | (937,853 | ) |
| | | | | | | | | | | | | |
| LOSS BEFORE INCOME TAXES | | | (2,946,430 | ) | | | (11,702,599 | ) | | | (20,388,734 | ) |
| | | | | | | | | | | | | |
| INCOME TAX EXPENSE | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | |
| NET LOSS | | $ | (2,946,430 | ) | | $ | (11,702,599 | ) | | $ | (20,388,734 | ) |
| | | | | | | | | | | | | |
| NET LOSS PER COMMON SHARE, | | | | | | | | | | | | |
| BASIC AND DILUTED | | $ | (0.05 | ) | | $ | (0.21 | ) | | | | |
| WEIGHTED AVERAGE NUMBER OF | | | | | | | | | | | | |
| COMMON SHARES OUTSTANDING BASIC AND DILUTED | | | 58,214,535 | | | | 54,511,585 | | | | | |
See accompanying notes to the consolidated financial statements
Islet Sciences, Inc. and Subsidiaries
(A Development Stage Company)
Consolidated Statements of Stockholders' Equity
| | | Series A Preferred | | | Series B Preferred | | | Series C Preferred | | | Common Stock | | | | | | Additional | | | | | | Total | |
| | | Number of | | | | | | Number of | | | | | | Number of | | | | | | Number of | | | | | | Paid-In | | | Development | | | Shareholders’ | |
| | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Stage | | | Equity/(Deficit) | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, May 4, 2010 | | | - | | | $ | - | | | | - | | | $ | - | | | | - | | | $ | - | | | | - | | | $ | - | | | $ | - | | | $ | - | | | $ | - | |
| Issuance of founders' common stock | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 13,430,000 | | | | 13,430 | | | | (13,430 | ) | | | - | | | | - | |
| Issuance of common stock in exchange for intangible asset | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 3,000,000 | | | | 3,000 | | | | 197,000 | | | | - | | | | 200,000 | |
| Issuance of common stock to reduce accounts payable – related party | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 100,000 | | | | 100 | | | | 9,900 | | | | - | | | | 10,000 | |
| Conversion of debt and accrued interest into common stock | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 3,591,729 | | | | 3,592 | | | | 353,408 | | | | - | | | | 357,000 | |
| Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | | | | | | | 7,031 | | | | - | | | | 7,031 | |
| Issuance of common stock for services | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 1,200,000 | | | | 1,200 | | | | 118,800 | | | | - | | | | 120,000 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (701,566 | ) | | | (701,566 | ) |
| Balance, April 30, 2011 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 21,321,729 | | | | 21,322 | | | | 672,709 | | | | (701,566 | ) | | | (7,535 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Sale of common stock at $0.10/share | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 8,090,000 | | | | 8,090 | | | | 780,910 | | | | - | | | | 789,000 | |
| Issuance of common stock for services | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 4,931,668 | | | | 4,932 | | | | 488,236 | | | | - | | | | 493,168 | |
| Sale of common stock at $0.12/share | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 2,474,997 | | | | 2,475 | | | | 287,525 | | | | - | | | | 290,000 | |
| Issuance of common stock for purchase consideration in reverse merger | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 1,187,476 | | | | 1,187 | | | | 533,178 | | | | - | | | | 534,365 | |
| Conversion of shares during merger | | | - | | | | - | | | | 38,006 | | | | 38 | | | | - | | | | - | | | | (38,005,870 | ) | | | (38,006 | ) | | | 37,968 | | | | - | | | | - | |
| Assumption of One E Commerce Shares upon reverse merger | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 187,063 | | | | 187 | | | | (187 | ) | | | - | | | | - | |
| Sale of preferred A shares at $450/share | | | 1,173 | | | | 1 | | | | - | | | | - | | | | - | | | | - | | | | | | | | | | | | 464,638 | | | | - | | | | 464,639 | |
| Purchase of Diakine with shares | | | - | | | | - | | | | - | | | | - | | | | 200,000 | | | | 200 | | | | 100,000 | | | | 100 | | | | 2,829,523 | | | | - | | | | 2,829,823 | |
| Conversion of shares upon completion of merger with One E Commerce | | | (1,173 | ) | | | (1 | ) | | | (38,006 | ) | | | (38 | ) | | | - | | | | - | | | | 39,178,870 | | | | 39,179 | | | | (39,140 | ) | | | - | | | | - | |
| Automatic conversion of shares | | | - | | | | - | | | | - | | | | - | | | | (200,000 | ) | | | (200 | ) | | | 2,000,000 | | | | 2,000 | | | | (1,800 | ) | | | - | | | | - | |
| Sale of common stock at $0.45/share | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 3,118,922 | | | | 3,119 | | | | 1,346,876 | | | | - | | | | 1,349,995 | |
| Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 4,761 | | | | - | | | | 4,761 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | | | | | (5,038,138 | ) | | | (5,038,138 | ) |
| Balance, April 30, 2012 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 44,584,855 | | | | 44,585 | | | | 7,405,197 | | | | (5,739,704 | ) | | | 1,710,078 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Sale of common stock at $0.45 a share | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 7,041,667 | | | | 7,042 | | | | 3,008,405 | | | | - | | | | 3,015,447 | |
| Issuance of common shares for services | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 4,563,855 | | | | 4,564 | | | | 6,685,689 | | | | - | | | | 6,690,253 | |
| Issuance of common stock for purchase consideration in reverse merger and settlement of litigation | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 524,740 | | | | 525 | | | | 734,112 | | | | - | | | | 734,637 | |
| Issuance of warrants for services | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 183,989 | | | | - | | | | 183,989 | |
| Net loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (11,702,599 | ) | | | (11,702,599 | ) |
| Balance, April 30, 2013 | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 56,715,117 | | | | 56,716 | | | | 18,017,392 | | | | (17,442,303 | ) | | | 631,805 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Sale of common stock at $0.41 a share | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 233,333 | | | | 233 | | | | 96,367 | | | | - | | | | 96,600 | |
| Stock-based compensation | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 192,100 | | | | - | | | | 192,100 | |
| Sale of common stock at $0.25 a share | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 9,210,000 | | | | 9,210 | | | | 2,021,090 | | | | - | | | | 2,030,300 | |
| Issuance of common shares for services | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 1,357,803 | | | | 1,358 | | | | 251,345 | | | | - | | | | 252,703 | |
| Issuance of warrants for settlement of litigation | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | 19,948 | | | | - | | | | 19,948 | |
| Net Loss | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | | | | (2,946,430 | ) | | | (2,946,430 | ) |
| Total Balance, April 30, 2014 | | | - | | | $ | - | | | | - | | | $ | - | | | | - | | | $ | - | | | | 67,516,253 | | | $ | 67,517 | | | $ | 20,598,242 | | | $ | (20,388,733 | ) | | $ | 277,026 | |
See accompanying notes to the consolidated financial statements
Islet Sciences, Inc. and Subsidiaries
(A Development Stage Company)
Consolidated Statements of Cash Flows
| | | | | | | | | For the period | |
| | | | | | | | | from May 4, 2010 | |
| | | | | | | | | (Inception) | |
| | | Year ended April 30, | | | through April 30, | |
| | | 2014 | | | 2013 | | | 2014 | |
| | | | | | | | | | |
| Cash flows from operating activities: | | | | | | | | | |
| Net loss | | $ | (2,946,430 | ) | | $ | (11,702,599 | ) | | $ | (20,388,734 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | |
| Equity issued for acquisition of One E-Commerce Corporation | | | - | | | | - | | | | 534,365 | |
| Equity issued for payment of accounts payable - related party | | | - | | | | - | | | | 10,000 | |
| Stock based compensation for services and other | | | 459,393 | | | | 5,635,053 | | | | 6,622,805 | |
| Derivative liabilities | | | (58,588 | ) | | | 72,174 | | | | 838,461 | |
| Accrued stock compensation expenses | | | 373,644 | | | | 63,702 | | | | 1,572,711 | |
| Amortization of intangible asset | | | 15,202 | | | | 30,404 | | | | 106,414 | |
| Impairment loss | | | 93,586 | | | | - | | | | 93,586 | |
| Change in operating assets and liabilities: | | | | | | | | | | | | |
| Advances to related party | | | 2,405 | | | | (2,405 | ) | | | - | |
| Prepaid expense | | | 50,000 | | | | (50,000 | ) | | | - | |
| Accounts payable | | | 1,037,860 | | | | 2,064,736 | | | | 3,191,622 | |
| Accounts payable - related party | | | - | | | | (14,228 | ) | | | (20,000 | ) |
| | | | | | | | | | | | | |
| Net cash used in operating activities | | | (972,928 | ) | | | (3,903,163 | ) | | | (7,438,770 | ) |
| | | | | | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | | | | | |
| Net cash provided by investing activities | | | - | | | | - | | | | - | |
| | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | |
| Proceeds from issuance of stock | | | 1,950,958 | | | | 1,891,182 | | | | 6,548,375 | |
| Subscribed shares - not issued | | | 80,000 | | | | 96,600 | | | | 1,584,865 | |
| Proceeds from notes payable - related parties | | | 107,165 | | | | 13,500 | | | | 507,666 | |
| Payments on notes payable - related parties | | | (27,404 | ) | | | (3,062 | ) | | | (60,756 | ) |
| | | | | | | | | | | | | |
| Net cash provided by financing activities | | | 2,110,719 | | | | 1,998,220 | | | | 8,580,150 | |
| | | | | | | | | | | | | |
| Net increase (decrease) in cash | | | 1,137,791 | | | | (1,904,943 | ) | | | 1,141,380 | |
| | | | | | | | | | | | | |
| Cash at beginning period | | | 3,589 | | | | 1,908,532 | | | | - | |
| | | | | | | | | | | | | |
| Cash at end period | | $ | 1,141,380 | | | $ | 3,589 | | | $ | 1,141,380 | |
| | | | | | | | | | | | | |
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | | | | | | | | | |
| Cash paid during the period for: | | | | | | | | | | | | |
| Interest | | $ | - | | | $ | - | | | $ | - | |
| Income taxes | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | |
| SUPPLEMENTAL DISCLOSURE OF NON-CASH INVESTING AND FINANCING INFORMATION: | | | | | |
| Shares issued for settlement of accrued expenses | | $ | 4,000 | | | $ | 1,135,365 | | | $ | 1,139,365 | |
| Shares issued for settlement of derivative liabilities | | $ | - | | | $ | 838,461 | | | $ | 838,461 | |
| Common stock issued for subscribed shares liability | | $ | 177,300 | | | $ | 1,124,265 | | | $ | 1,504,865 | |
| Shares isssued to escrow as security for legal expenses | | $ | 700 | | | $ | - | | | $ | 700 | |
| Shares issued for acquisition of Diakine Therapeutics, Inc. | | $ | - | | | $ | - | | | $ | 2,829,823 | |
| Net liabilities assumed in acquisition of Diakine Theapeutics, Inc. | | $ | - | | | $ | - | | | $ | 101,284 | |
| Deferred income tax liability and goodwill associated with the acquisition of Diakine Therapeutics, Inc. | | $ | - | | | $ | - | | | $ | 547,000 | |
| Common stock issued in exchange for convertible notes | | $ | - | | | $ | - | | | $ | 357,000 | |
| Common stock issued in exchange for intangible asset | | $ | - | | | $ | - | | | $ | 200,000 | |
| Common stock issued in exchange for accounts payable - related party | | $ | - | | | $ | - | | | $ | 10,000 | |
See accompanying notes to the consolidated financial statements
ISLET SCIENCES, INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1. DESCRIPTION OF BUSINESS AND GOING CONCERN
Description of Business
We are a biotechnology company engaged in the research, development, and commercialization of new medicines and technologies for the treatment of metabolic disease and related indications where there is significant measurable unmet medical need. The rising incidence of obesity is associated with many obesity-related health complications, including cardiovascular disease, diabetes, hyperlipidemia, hypertension, nonalcoholic fatty liver disease/steatohepatitis (NAFLD/NASH). This constellation is also recognized as the metabolic syndrome and is characterized by underlying insulin resistance. These various diseases have interrelated risk factors and markers, such that often treatment of one disease may allow new therapies and opportunities for treatment in one of these related indications. Our focused effort to develop new therapies and related diagnostics for metabolic related diseases establishes us as a recognized leader in a large and growing market.
In March 2014, the Company announced it had signed a binding letter of intent to enter into a merger agreement with and acquire Brighthaven Ventures, LLC d/b/a BHV Pharma (“BHV”) a privately held pharmaceutical company developing the SGLT2 inhibitor remogliflozin etabonate (“remogliflozin”) for type 2 diabetes and non-alcoholic steatohepatitis (“NASH”). Remogliflozin is currently in phase II clinical development. The Company anticipates it will sign the merger agreement within the next thirty days.
Islet Sciences was incorporated under the name One E-Commerce Corporation on September 14, 1994 in the State of Nevada. Effective February 23, 2012, the Company changed its name to Islet Sciences, Inc. On March 14, 2012, Islet Sciences acquired DiaKine Therapeutics, Inc., a Delaware corporation (“DTI”). Islet Sciences together with its subsidiaries, Islet Sciences Inc., a Delaware corporation (“ISI”), and DTI are referred to as the Company.
Going Concern
The consolidated financial statements have been prepared assuming the Company will continue as a going concern. This contemplates the continuity of operations, realization of assets, and liquidation of liabilities in the normal course of business. Since inception, the Company has incurred operating losses of $20,388,734 and has had negative operating cash flows of $7,438,771. As of April 30, 2014, the Company had cash of $1,141,380. Further, the Company has incurred net losses of $2,946,430 and negative operating cash flows of $972,928 for the fiscal year ended April 30, 2014. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
The Company’s future cash requirements will depend on many factors, including continued scientific progress in its research and development programs, the scope and results of pre-clinical and clinical trials, the time and costs involved in obtaining regulatory approvals, the costs involved in filing, prosecuting and enforcing patents, competing technological and market developments, and the cost of product commercialization. The Company does not expect to generate a positive cash flow from operations at least until the commercial launch of its first product and possibly later given the expected spending for research and development programs and the cost of commercializing product candidates. The Company’s continued operations will depend on its ability to raise funds through various potential sources such as debt and equity financing. There can be no assurance that such capital will be available on favorable terms or at all. If the Company is unable to raise additional capital, the Company will likely be forced to curtail its desired development activities, which would delay the development of its product candidates.
Reverse Merger Agreement
On September 15, 2011, Mr. John Welch, shareholder, director and Chief Executive Officer of One-E Commerce Corporation, entered into a stock purchase agreement, pursuant to which Mr. Welch sold to ISI, an aggregate of 9,902,180 shares of One E-Commerce Corporation common stock, which shares then represented approximately 54.06% of the issued and outstanding shares of common stock, and certain convertible promissory notes in the aggregate principal amount of $514,458 and accrued interest, previously issued by One E-Commerce Corporation, for an aggregate purchase price of $250,000. Additionally, under the stock purchase agreement, ISI agreed to cause One E-Commerce Corporation to enter into a reverse merger transaction at a future date whereby the Company was to acquire all of the outstanding equity interests of ISI in consideration for the issuance of its shares to the shareholders of ISI (“Reverse Merger Transaction”). The closing that took place on September 22, 2011, resulted in the change of control of One E-Commerce Corporation. Immediately after the closing, the shares together with the notes, acquired by ISI comprised 54.06% of the then issued and outstanding common stock of the One-E Commence Corporation on a non-diluted basis and 82.8% on a fully-diluted basis.
On December 30, 2011, ONCE, Inc., a Delaware corporation wholly-owned by the Company (the “Merger Sub”), ISI and Islet Sciences consummated the Reverse Merger Transaction, whereby the Merger Sub was merged with and into ISI, and the holders of common stock of ISI received an aggregate of 38,005.87 shares of Islet Sciences’ Series B preferred stock, $0.001 par value per share (“Series B Preferred”) in exchange for the cancellation of all of the shares of common stock of ISI formerly owned by them, and the holders of Series A preferred stock of ISI received an aggregate of 1,173 shares of the Series A preferred stock, $0.001 par value per share (“Series A Preferred”) in exchange for the cancellation of all of the shares of Series A preferred stock of ISI formerly owned by them. The issuance of Series A and B Preferred, with each share of preferred stock having voting rights equal to 1,000 shares of the Company’s common stock, resulted in ISI’s shareholders having obtained control of the combined Company. ISI is deemed to be the accounting acquirer (legal acquiree) and One E-Commerce Corporation to be the accounting acquiree (legal acquirer). The financial statements before the date of the Reverse Merger Transaction are those of ISI with the results of the Company being consolidated from the date of the Reverse Merger Transaction. The equity section and earnings per share have been retroactively restated to reflect the reverse acquisition. The merger of a private operating company into a non-operating public shell corporation with nominal net assets is considered to be a capital transaction, in substance, rather than a business combination, for accounting purposes. Accordingly, ISI treated this transaction as a capital transaction without recording goodwill or adjusting any of its other assets or liabilities. The consideration in the amount of $1,345,710 for the Company, consisting of $250,000 paid in cash and $1,095,710 paid in the form of common stock, was recorded as another expense item in the accompanying consolidated statements of operations during the year ended April 30, 2012. Effective February 23, 2012, Islet Sciences completed a 1-for-45 reverse stock split of its issued and outstanding common stock. Upon effectiveness of the reverse stock split, all outstanding shares of Series A Preferred and Series B Preferred were converted into common shares based on their respective conversion ratios. In connection with the closing of the Reverse Merger Transaction, ISI agreed to cancel the shares and the outstanding notes of One E-Commerce Corporation purchased from Mr. Welch, and the interest accrued thereon effective upon the effectiveness of the reverse stock split.
NOTE 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). All shares and per share amounts in these consolidated financial statements and notes thereto have been retroactively adjusted to give effect to the reverse stock split.
The accompanying consolidated financial statements include the accounts of Islet Sciences and its wholly-owned subsidiaries, ISI and DTI. All significant intercompany balances have been eliminated.
The Company's planned principal operations have not yet commenced. Accordingly, the Company's activities have been accounted for as those of a development stage enterprise in accordance with Financial Accounting Standards Board ("FASB") Accounting Standards Codification (“ASC”) 915-10, Accounting and Reporting by Development Stage Enterprises (FASB ASC 915-10). All losses since inception have been considered as part of the Company's development stage activities.
Emerging Growth Company
The Company is an “emerging growth company” and has elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(1) of the JOBS Act. This election allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies.
Use of Estimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates include the recoverability of long-lived assets, the valuation of intangible assets and goodwill, the valuation of common stock, warrants and stock options and the valuation of deferred tax assets. Actual results could differ from those estimates.
Concentration of Credit Risk
The Company maintains its cash balances at a credit-worthy financial institution and management believes the risk of loss of cash balances to be low. The Company’s cash balances were not fully insured at April 30, 2014.
Intangible Assets
Intangible assets represent a patent acquired from a third party, which is recorded at cost and amortized over the remaining life of the patent. This patent was fully impaired and written off during the year ended April 30, 2014. Intangible assets also include the purchase of DiaKine Therapeutics, Inc. patent portfolio and know-how as in-process research and development (“IPR&D”). IPR&D has an indefinite life and is not amortized until completion and development of the project, at which time the IPR&D becomes an amortizable asset. If the related project is not completed in a timely manner or the project is terminated or abandoned, the Company may have an impairment related to the IPR&D, calculated as the excess of the asset’s carrying value over its fair value. The intangible assets with estimable useful lives are amortized on a straight line basis over their respective estimated useful lives to their estimated residual values. This method of amortization approximates the expected future cash flow generated from their use. Definite lived intangibles are reviewed for impairment in accordance with FASB ASC 360, Property, Plant and Equipment (FASB ASC 360).
Goodwill
Goodwill represents the excess of the purchase price over the estimated fair value of the identifiable assets acquired and liabilities assumed in business acquisitions. Goodwill is reviewed at least annually for impairment in the fourth quarter of the fiscal year, at the Company level, which is the sole reporting unit, and at any other time at which events occur or circumstances indicate that the carrying amount of goodwill may exceed its fair value. Such indicators would include a significant reduction in the Company’s market capitalization, a decrease in operating results or a deterioration in the Company’s consolidated financial position.
Impairment of Long-Lived Assets
The Company applies the provisions of FASB ASC 360-10, Property, Plant and Equipment (FASB ASC 360-10), where applicable to all long lived assets. FASB ASC 360-10 addresses accounting and reporting for impairment and disposal of long-lived assets. The Company periodically evaluates the carrying value of long-lived assets to be held and used in accordance with FASB ASC 360-10. FASB ASC 360-10 requires impairment losses to be recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amounts. In that event, a loss is recognized based on the amount by which the carrying amount exceeds the fair market value of the long-lived assets. Loss on long-lived assets to be disposed of is determined in a similar manner, except that fair market values are reduced for the cost of disposal.
Loss Per Share Data
Basic loss per share is calculated based on the weighted average common shares outstanding during the period. Diluted earnings per share also give effect to the dilutive effect of restricted stock. The Company does not present diluted earnings per share for years in which it incurred net losses as the effect is anti-dilutive.
At April 30, 2014, 123,750 unvested shares of restricted common stock, warrants to exercise 12,430,798 shares of common stock and 3,000,000 shares of stock options were outstanding, but were not included in the computation of diluted earnings per share as their effect would be anti-dilutive. At April 30, 2013, 2,275,625 unvested shares of restricted common stock and warrants to exercise 6,366,798 shares of common stock were outstanding, but were not included in the computation of diluted earnings per share as their effect would be anti-dilutive.
Fair Value of Financial Instruments
The Company adopted FASB ASC 820, Fair Value Measurements and Disclosures (FASB ASC 820), which provides a framework for measuring fair value under GAAP. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The standard also expands disclosures about instruments measured at fair value and establishes a fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The standard describes three levels of inputs that may be used to measure fair value:
Level 1 — Quoted prices for identical assets and liabilities in active markets;
Level 2 — Quoted prices for similar assets and liabilities in active markets; quoted prices for identical or similar assets and liabilities in markets that are not active; and model-derived valuations in which all significant inputs and significant value drivers are observable in active markets.
Level 3 — Valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable.
Research and Development
Research and development expenses consist of expenses incurred in performing research and development activities including salaries and benefits, facilities and other overhead expenses, contract services and other outside expenses. Research and development costs are charged to operations when incurred.
Stock Based Compensation
Stock awards
FASB ASC 718, Compensation-Stock Compensation (FASB ASC 718), requires the fair value of all share-based payments to employees, including grants of stock options, to be recognized in the statement of operations over the requisite service period. FASB ASC 718 requires all share based payments to employees, including grants of employee stock option, to be recognized in operating results as compensation expense based on fair value over the requisite service period of the awards. The Company determines the fair value of share-based awards using the Black-Scholes option-pricing model which uses both historical and current market data to estimate fair value. The method incorporates various assumptions such as the risk-free interest rate, expected volatility, expected dividend yield, expected forfeiture rate and expected life of the options. As allowed by FASB ASC 718, for companies with a short period of publicly traded stock history, the Company’s estimate of expected volatility is based on the average volatilities of a sampling of three companies with similar attributes to the Company, including industry, stage of life cycle, size and financial leverage. The risk-free rate for periods within the contractual life of the stock options is based on the U.S. Treasury yield curve in effect at the time of grant valuation. The expected term of options granted is derived using the “simplified method” which computes expected term as the average of the sum of the vesting term plus the contract term as historically the Company had limited activity surrounding its options. The Company has not issued stock options for nonemployees.
The Company used the following assumptions in valuing the stock options issued:
| | | April 30, | | | April 30, | |
| | | 2014 | | | 2013 | |
| Price per share of common stock | | $ | 0.25 | | | $ | - | |
| Exercise price per share | | $ | 0.265 | | | $ | - | |
| Expected volatility | | | 75.5 | % | | | - | % |
| Risk-free interest rate | | | 1.30 | % | | | - | % |
| Dividend yield | | | - | | | | - | |
Warrants
Warrants granted to service providers are normally valued at the fair value of the instrument on the date of the grant (grant date) and are recognized in the consolidated statement of operations over the requisite service period or when they vest. When the requisite service period precedes the grant date and a market condition exists in the warrant, the Company values the warrant using the Black-Scholes option pricing model. Warrants issued in connection with capital raises are normally valued at the fair value of the instrument on the date of the grant (grant date) and valued for disclosure purposes if they meet all the criteria under FASB ASC 718. The Company values these warrant using the Black-Scholes option pricing model as well. As allowed by FASB ASC 718, for companies with a short period of publicly traded stock history, the Company’s estimate of expected volatility is based on the average volatilities of a sampling of three companies with similar attributes to the Company, including industry, stage of life cycle, size and financial leverage. The risk-free rate for periods within the contractual life of the warrant is based on the U.S. Treasury yield curve in effect at the time of grant valuation.
The Company used the following assumptions in valuing the warrants issued:
| | | April 30, | | | April 30, | |
| | | 2014 | | | 2013 | |
| Price per share of common stock | | $ | 0.18 - $0.98 | | | $ | 0.45 - $1.40 | |
| Exercise price per share | | $ | 0.25 - $0.50 | | | $ | 0.45 - $2.00 | |
| Expected volatility | | | 79.1 - 97.1 | % | | | 80.0 - 102.7 | % |
| Risk-free interest rate | | | 0.65 - 1.67 | % | | | 0.65 - 1.30 | % |
| Dividend yield | | | - | | | | - | |
Income Taxes
The Company accounts for income taxes under an asset and liability approach. This process involves calculating the temporary and permanent differences between the carrying amounts of the assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The temporary differences result in deferred tax assets and liabilities, which would be recorded on the Company’s consolidated balance sheets in accordance with FASB ASC 740, Income Taxes, which established financial accounting and reporting standards for the effect of income taxes. The Company must assess the likelihood that its deferred tax assets will be recovered from future taxable income and, to the extent the Company believes that recovery is not likely, the Company must establish a valuation allowance. Changes in the Company’s valuation allowance in a period are recorded through the income tax provision on the consolidated statements of operations.
FASB ASC 740 clarifies the accounting for uncertainty in income taxes recognized in an entity’s consolidated financial statements and prescribes a recognition threshold and measurement attributes for financial statement disclosure of tax positions taken or expected to be taken on a tax return. Under FASB ASC 740-10, the impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more-likely-than-not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. Additionally, FASB ASC 740 provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. As a result of the implementation of FASB ASC 740, the Company recognized no material adjustment in the liability for unrecognized income tax benefits.
The Company’s policy is to record interest and penalties on uncertain tax positions as income tax expense. As of April 30, 2014 and 2013, the Company has no accrued interest or penalties related to uncertain tax positions.
Segment Reporting
The Company currently operates in a single operating segment. In addition, financial results are prepared and reviewed by management as a single operating segment. The Company continually evaluates its operating activities and the method utilized by management to evaluate such activities and will report on a segment basis if and when appropriate to do so.
Recent Pronouncements
In May 2014, the FASB issued ASU No. 2014-09 “Revenue from Contracts with Customers,” which will supersede nearly all existing revenue recognition guidance under GAAP. ASU No. 2014-09 provides that an entity recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This update also requires additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments, and assets recognized from costs incurred to obtain or fulfill a contract. ASU No. 2014-09 allows for either full retrospective or modified retrospective adoption and will become effective for the Company in the first quarter of 2018. The Company is a development stage entity and will evaluate the effects of this update on its consolidated financial statements when it generates revenues.
In June 2014, the FASB issued ASU No. 2014-10, which eliminated certain financial reporting requirements of companies previously identified as “Development Stage Entities” (Topic 915). The amendments in this ASU simplify accounting guidance by removing all incremental financial reporting requirements for development stage entities. The amendments also reduce data maintenance and, for those entities subject to audit, audit costs by eliminating the requirement for development stage entities to present inception-to-date information in the statements of income, cash flows, and shareholder equity. Early application of each of the amendments is permitted for any annual reporting period or interim period for which the entity’s financial statements have not yet been issued (public business entities) or made available for issuance (other entities). Upon adoption, entities will no longer present or disclose any information required by Topic 915. The Company has adopted this standard and will commence in future presentations.
Other Income
In the year ended April 30, 2013, the Company received an indemnification payment of $430,000 from a third party in connection with an agreement to indemnify the Company against litigation and other expenses incurred in connection with certain litigation.
NOTE 3. INTANGIBLE ASSETS AND GOODWILL
On May 4, 2010, the Company was assigned the intellectual property rights for a patent that was issued on December 1, 1999. The rights to this patent were purchased out of the bankruptcy proceedings of MicroIslet, Inc. for $200,000 and then assigned to ISI in exchange for the issuance of 3,000,000 shares of common stock.
On March 14, 2012, the Company acquired the IPR&D from DiaKine Therapeutics, Inc. As of April 30, 2014, the $1.3 million of acquired IPR&D is classified as indefinite life asset and is not being amortized. In conjunction with this acquisition, the Company recognized $2.1 million of goodwill. Initially, the residual purchase price of $1,564,107 was recorded as goodwill related to the acquisition of DTI. However, none of the goodwill recognized or amortization of the IPR&D acquired is expected to be deductible for income tax purposes. Accordingly, in conjunction with the valuation of intangible assets acquired, it was determined that a deferred income tax liability of $547,000 was required to reflect the book to tax differences of the acquisition. This same amount was added to the goodwill balance. The Company performed its annual impairment test of goodwill during the fiscal fourth quarter using an analysis of the present value of future discounted cash flows for its valuation. The Company evaluated its goodwill for impairment during the year ended April 30, 2014 and determined that the fair value of the reporting unit is in excess of its carrying value including goodwill.
The following is a summary of goodwill and intangible assets for the years ended April 30, 2014 and 2013:
| | | April 30, 2014 | | | April 30, 2013 | |
| | | Gross | | | | | | | | | Net | | | Gross | | | | | | Net | |
| | | Carrying | | | Accumulated | | | | | | Carrying | | | Carrying | | | Accumulated | | | Carrying | |
| | | Amount | | | Amortization | | | Impairment | | | Amount | | | Amount | | | Amortization | | | Amount | |
| Identifiable intangibles: | | | | | | | | | | | | | | | | | | | | | |
| Patents | | $ | 200,000 | | | $ | (106,414 | ) | | $ | (93,586 | ) | | $ | - | | | $ | 200,000 | | | $ | (91,212 | ) | | $ | 108,788 | |
| In-process technology | | | 1,367,000 | | | | - | | | | - | | | | 1,367,000 | | | | 1,367,000 | | | | - | | | | 1,367,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Subtotal of identifiable intangibles | | | 1,567,000 | | | | (106,414 | ) | | | (93,586 | ) | | | 1,367,000 | | | | 1,567,000 | | | | (91,212 | ) | | | 1,475,788 | |
| Goodwill | | | 2,111,107 | | | | - | | | | - | | | | 2,111,107 | | | | 2,111,107 | | | | - | | | | 2,111,107 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total goodwill and intangibles | | $ | 3,678,107 | | | $ | (106,414 | ) | | $ | (93,586 | ) | | $ | 3,478,107 | | | $ | 3,678,107 | | | $ | (91,212 | ) | | $ | 3,586,895 | |
The patent is being amortized based on the remaining life of the patent, which was 6.5 years at May 4, 2010, the date of assignment. For the year ended April 30, 2014, the amount amortized to expense was $15,202 and the Company did not expect to realize economic benefit from the claims associated with this intellectual property, therefore, the Company incurred an impairment of this asset of $93,586 during the year ended April 30, 2014. For the year ended April 30, 2013, the amount amortized to expense was $30,404.
NOTE 4. STOCKHOLDERS’ EQUITY
As of April 30, 2014, the Company had 10,000,000 authorized shares of preferred stock, par value $0.001 per share; no shares were issued and outstanding at April 30, 2014 and 2013. Shares of Series A and Series B preferred stock issued at the closing of the Reverse Merger Transaction were automatically converted, upon the effectiveness of the reverse split on February 23, 2012, into common stock at a conversion rate of one thousands shares of common stock for one share of preferred stock. On March 14, 2012, the Company issued shares of Series C Preferred stock at $0.001 par value, in connection with the DTI acquisition. These shares were converted on April 26, 2012 into common stock at a conversion rate of ten shares of common stock for one share of preferred stock. At the conversion of all issued and outstanding shares of Series A, Series B and Series C preferred stock, designations of all three series of preferred stock were cancelled.
As of April 30, 2014, the Company had 100,000,000 authorized shares of common stock, par value $0.001 per share. Holders of common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. Holders of common stock are entitled to receive dividends ratably, if any, as may be declared by the Board of Directors out of legally available funds, subject to any preferential dividend rights of any outstanding preferred stock. Upon liquidation, dissolution or winding up, the holders of common stock are entitled to receive ratably net assets available after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock.
Holders of common stock have no preemptive, subscription, redemption or conversion rights. The outstanding shares of common stock are fully paid and non-assessable. The rights, preferences and privileges of holders of the common stock are subject to, and may be adversely affected by, the rights of holders of shares of any series of preferred stock.
Grants
At the inception, the Company issued 13,430,000 founder shares of common stock in consideration for intellectual property assignments related to the Company’s purpose, mission and contributions to the business plan and strategy.
On May 4, 2010, the Company issued 3,000,000 shares of common stock in consideration for the assignment of the intellectual property rights purchased from a company in bankruptcy proceedings for $200,000.
In May 2010, the Company issued a convertible note payable to an investor, in the amount of $357,000. In September 2010, the note payable was converted to 3,591,729 shares of common stock.
In July 2010, a Scientific Advisor Board member, and in October 2010, the CFO, received a restricted stock grant of 100,000 shares of common stock for services provided to the Company. The shares vest 50% on the first anniversary of the date of the agreement and the remaining shares vest on the second anniversary of the date of the agreement. For the years ended April 30, 2012 and 2011, the Company recognized stock-based compensation expense of $7,031, and $4,761, respectively. In June 2012, the grant was replaced with a grant of 300,000 shares of common stock with 50% vesting at the first anniversary date and 50% vesting at the second anniversary date. The shares were not issued as of April 30, 2014. The share price of the shares issued was valued at $0.10 per share, which equated to the then share price of common stock being sold to other investors.
In March 2011, as part of a consultant agreement for consulting services to be provided for a one year period, the Company issued 1,200,000 shares of common stock. The common shares were valued at $120,000 at the grant date, and were fully expensed as stock-based compensation upon issuance as the shares were not subject to forfeiture. The share price of the shares issued was valued at $0.10 per share, which equated to the then share price of common stock being sold to other investors.
In April 2011, the Company converted an accounts payable balance with a related party in the amount of $10,000 into 100,000 shares of common stock.
From June to September of 2011, the Company consummated a private placement of its common stock to certain accredited investors whereby the Company issued 8,090,000 shares of common stock for cash at a purchase price of $0.10 per share and 2,474,997 shares of common stock for a cash purchase price of $0.12 per share for total gross proceeds of $1,106,000. Originally, the shares sold were those of ISI’s common stock, which were ultimately converted into common stock of Islet Sciences as a result of the Reverse Merger Transaction and following the reverse stock split of Islet Sciences’ common stock.
In October 2011, the Company granted Mr. John Steel, the Company’s CEO, 1,190,000 shares of common stock, for the services that he has provided in previous periods as part of his employment agreement; 866,668 shares of common stock, as a signing bonus as part of his employment agreement; and 866,668 shares of common stock, as a deferred stock compensation with a vesting schedule of 50% on the first anniversary date and 50% on the second anniversary date. The total number of shares issued was 2,056,668 and were valued at $205,667 at the grant date, and was expensed ratably over the corresponding service period. The share price of the shares issued was valued at $0.10 per share, which equated to the then share price of shares being sold to other investors. The Company has agreed to issue Mr. Steel and additional 200,000 of common shares upon the completion of the contemplated DiaKine Therapeutics, Inc. acquisition. For the year ending April 30, 2012, the Company accrued $280,000 as a stock-based compensation expense related to the 200,000 shares which were to be granted as the result of the DiaKine Therapeutics, Inc. purchase. The shares were valued at $1.40 per share based on the valuation performed at the acquisition date and were issued during the year ended April 30, 2013, except for 433,334 shares which were forfeited because Mr. Steel resigned as the CEO of the Company on July 1, 2013 before these shares vested.
In October 2011, the Board of Directors granted certain consultants to the Company 2,775,000 shares of common stock as part of consultant agreements for services to be provided for multiple year periods. For the year ended April 30, 2012, the Company recognized $277,542 of stock-based compensation expense related to these grants. The share price of the shares issued was valued at $0.10 per share, which equated to the then share price of common stock being sold to other investors.
In October 2011, the Board of Directors allocated 400,000 shares of common stock for future issuance to members of the Company’s Scientific Advisory Board once Scientific Advisory Board members have been selected, other than Dr. Lakey. The members of the Scientific Advisory Board will have a term of two years.
In December 2011, the Company consummated a private placement of its preferred stock to certain accredited investors whereby the Company issued 1,173 shares of Series A preferred stock for cash at a purchase price of $450 per share for total net proceeds of $464,640. Originally, the shares sold were those of ISI’s Series A preferred stock, which were ultimately converted into common stock of Islet Sciences as a result of the Reverse Merger Transaction and following the reverse stock split of Islet Sciences’ common stock. In conjunction with the issuance of preferred stock, each share of issued preferred stock included the right to receive a warrant to purchase 500 shares of common stock of Islet Sciences at a price of $1.00 per share upon conversion of the preferred stock. The warrants were valued at approximately $147,000.
As per the DTI acquisition agreement, the Company was required to issue to Mr. John Welch shares of common stock representing 3% of the outstanding shares, after giving effect to the issuance of shares of common stock in the financing. On December 30, 2011, the Company issued 1,187,476 shares of common stock valued at $534,365 and was included in other expenses. During the year ended April 30, 2012, the Company computed the remaining number of shares issuable under the anti-dilutive provision of the contract in the amount of 375,398 shares, valued at $525,557 and are included as derivative liabilities in the consolidated balance sheets. The shares were valued at $1.40 per common share based on the stock valuation performed at the acquisition date. On June 6, 2012, the Company issued 375,398 shares of common stock to John Welch pursuant to the DTI acquisition.
On March 14, 2012, in exchange for all issued and outstanding stock of DTI, the Company issued to DTI shareholders 200,000 shares of Series C Preferred Stock with each share of preferred stock convertible into ten shares of the Company’s common stock. The Company also issued 100,000 shares of its common stock as part of the acquisition. On April 24, 2012, the Company issued a total of 2,000,000 shares of common stock to holders of 200,000 shares Series C Preferred Stock upon conversion of Series C Preferred Stock.
In March and April of 2012, the Company consummated a private placement of its common stock at a per share price of $0.45 pursuant to a series of subscription agreements with a number of accredited investors including one of the Company’s directors. The investors in the private placement were also issued, for no additional consideration, warrants to purchase shares of the Company’s common stock. The warrants granted the subscribers the right to purchase a number of shares of common stock, par value $.001 per share, of the Company’s common stock equal to fifty percent (50%) of the number of shares of common stock subscribed for. The warrants have an initial exercise price equal to $1.00 per share and shall be exercisable for a five year period. The warrants were valued at approximately $392,000 based on the assumptions used in Note 2. In April 2012, 3,118,922 shares of common stock and 1,559,461 warrants were issued for total net proceeds of $1,349,995. The remaining shares of common stock and warrants were subsequently issued in May 2012 and were part of additional capital raises on May 2, 2012 and May 9, 2012 described below.
On April 1, 2012, as compensation for future services, the CFO was awarded a grant of 150,000 shares of common stock which shall be subject to forfeiture if his consulting engagement is terminated before January 31, 2013. These shares vested during the year ended April 30, 2013 and were valued at $210,000, which was based on a previous appraisal at a share price of $1.40 and were included as general and administrative expenses in the consolidated statements of operations for the year ended April 30, 2013.
In May 2, 2012, the Company issued an aggregate of 4,552,222 shares of common stock at $0.45 per share and warrants to purchase 2,276,111 shares of common stock at an exercise price of $1.00 per share in connection with two closings of the private placement of its common stock of $2,048,500 that occurred in April 2012. The warrants were valued at approximately $492,000 based on the assumptions used in Note 2.
On May 9, 2012, the Company consummated a private placement of an aggregate of 1,711,667 shares its common stock at a per share price of $0.45, for gross proceeds of $770,250 pursuant to a series of subscription agreements with a number of accredited investors. The investors in the private placement were also issued for no additional consideration, warrants to purchase 855,833 shares of common stock at an exercise price of $1.00 per share. The warrants were valued at approximately $185,000 based on the assumptions used in Note 2.
In May 2012, the Company, entered into a license agreement with the Yale University (“Yale”). As part of this agreement, the Company issued 20,000 shares of its common stock valued at $28,000 which is included in the consolidated statement of operations for the year ended April 30, 2013.
On May 23, 2012, the Company issued a total of 270,000 shares of common stock as compensation to its employee and consultants valued at $378,000 of which 200,000 shares (or $280,000). The shares issued were valued using a previous appraisal of $1.40 per share.
On June 21, 2012, the Company consummated a private placement of an aggregate of 717,778 shares of common stock at a per share price of $0.45, for gross proceeds of $323,000 pursuant to a series of subscription agreements with a number of accredited investors. The investors in the private placement were also issued for no additional consideration warrants to purchase 358,889 shares of common stock at an exercise price of $1.00 per share. The warrants were valued at approximately $78,000 based on the assumptions used in Note 2.
In August 2012, the Company agreed to assume certain liabilities in connection with claims and liens on certain patents acquired for approximately $17,000 in cash and issued 144,342 shares of its common stock. The shares issued were valued using a previous appraisal of $1.40 per share or approximately $202,000. These costs have been expensed to general and administrative expenses during the year ended April 30, 2013.
In August 2012, the Company issued 1,750,000 shares of its common stock to one of its placement agents pursuant to an agreement dated as of April 21, 2011, as amended on June 29, 2012. The initial performance conditions in the agreement were not met by the placement agent, however, both parties agreed to settle and amend the agreement by issuing a total of 1,750,000 shares of the Company’s common stock to the placement agent. These shares were issued and the expense in the amount of $2,450,000 during the year ended April 30, 2013, which was based on a share price of $1.40 based on a stock valuation performed, is included as general and administrative expense in the consolidated statements of operations for the year ended April 30, 2013.
In August of 2012, the Company issued 3,000 shares of common stock to The Regents of the University of California as part of an exclusive licensing agreement. The Company valued these shares at approximately $4,200 and included the cost as research and development expense within the consolidated statements of operations for the year ended April 30, 2013.
In August 2012, the Company issued 60,000 shares of common stock that were previously designated as subscribed shares – not issued. The Company initially received proceeds of approximately $27,000 in December 2011. The investor was also issued for no additional consideration, a warrant to purchase 30,000 shares of common stock at an exercise price of $1.00 per share. The warrant was valued at approximately $6,000 based on the assumptions used in Note 2.
In September 2012, the Company issued an aggregate of 309,375 shares of its common stock to members of the Board of Directors which were considered to be vested. The Company valued these shares based on an earlier appraisal of $1.40 per share or approximately $433,000, and included the costs as general and administrative expenses within the consolidated statements of operations for the year ended April 30, 2013.
In October 15, 2012, the Board of Directors approved a grant of 250,000 shares of common stock to Dr. Jonathan Lakey for his role in the ongoing process with Yale University, PCT, and Spring Point Project which vested on January 31, 2013. These shares were issued and the expense in the amount of $350,000, which was based on a previous appraisal at a share price of $1.40, is included as research and development expenses in the consolidated statements of operations for the year ended April 30, 2013.
On October 19, 2012, the Company issued 375,000 shares of its common stock to a consultant to provide financial services. The shares are not subject to any vesting conditions or forfeiture. These shares were issued in the amount of $525,000, which was based on a previous appraisal at a share price of $1.40 and is included as general and administrative expenses in the consolidated statements of operations for the year ended April 30, 2013.
On November 21, 2012, the Company issued 20,000 shares of common stock to Winthrop University Hospital as part of the agreement to license certain patents and technology. These shares were valued at an earlier appraisal of $1.40 per share or $28,000. The Company accounted for these costs as research and development expenses within the consolidated statements of operations for the year ended April 30, 2013.
On December 19, 2012, the Company issued a total of 433,334 shares of common stock as compensation to its CEO, John Steel, valued at $606,667 based on a previous appraisal at a share price of $1.40 and included in general and administrative expenses within the consolidated statements of operations for the year ended April 30, 2013.
On December 26, 2012, an aggregate of 309,375 shares of its common stock issuable to members of the Board of Directors fully vested. These shares were issued during the year ended April 30, 2013 in the amount of approximately $433,000, which was based on a previous appraisal at a share price of $1.40 and is included as general and administrative expenses in the consolidated statements of operations for the year ended April 30, 2013.
On January 25, 2013, the Company issued 30,000 shares to a consultant to provide investor relations services. The shares are not subject to any vesting conditions or forfeiture. These shares were expensed at a value of $44,000. An additional $4,000 was accrued for the balance of the amount due under this agreement. A total of $48,000 is included in general and administrative expenses within the consolidated statements of operations for these services for the year ended April 30, 2013.
On January 28, 2013, the Company received net proceeds of $96,600 for the sale of 70,000 shares of common stock at $1.40 per share as part of a private offering. The Company had not issued these shares as of April 30, 2013, and has recorded these proceeds as subscribed shares not issued at April 30, 2013. On November 11, 2013, the Company issued these common shares.
On February 5, 2013, the Company issued 20,000 shares of common stock to certain related parties (Jerry L. Nadler, David Taylor-Fishwick, Anca Dobrian and Swarup Chalrabarti - “12LO Licensors”) as part of an exclusive licensing agreement. The Company valued these shares using a previous appraisal of $1.40 per share or $28,000 and included the cost as research and development expense within the consolidated statements of operations for the year ended April 30, 2013.
On March 19, 2013, an aggregate of 247,500 shares of its common stock issuable to members of the Board of Directors fully vested. The Company valued these shares based on the April 30, 2013 share price of $0.23 per share, or $56,925, and recorded it to accrued expense until the common stock is issued. The Company has included the costs as general and administrative expenses within the consolidated statements of operations for the year ended April 30, 2013. On April 10, 2014, the Company issued 61,875 common shares to one of its Board Members.
On April 1, 2013, the Company issued 5,000 shares of common stock to Southridge Partners II. LLP as part of a settlement agreement dated April 1, 2013 regarding an investment made by Southridge Partners II, LLP in the Company. The Company valued these shares at $7,000 which was based on a previous appraisal at a share price of $1.40 and is included as general and administrative expenses in the consolidated statements of operations for the year ended April 30, 2013.
At April 30, 2013, the Company had an indemnification agreement with a third-party, whereby, the Company received $80,000 in cash for shares of common stock of the Company that were to be provided by the same third party. On October 31, 2013, the Company was notified that the third-party did not transfer shares of Company common stock in accordance with the terms of the agreement. The Board of Directors has approved the issuance of Company common stock to fulfill the requirements of the indemnification agreement. On November 19, 2013, the Company issued 320,000 shares of common stock to satisfy its subscribed shares valued at $80,000. The Company has recorded $80,000 in general and administrative expense within the consolidated statements of operations for the year ended April 30, 2014.
During the year ended April 30, 2013, the Company initially issued 400,000 shares as part of its agreement with PCT along with a warrant to purchase 350,000 shares at an exercise price of $1.00 per share. This initial issuance was valued at approximately $855,000 and accrued at April 30, 2012. In addition and as part of the anti-dilution provision, the Company issued an additional 223,771 shares of common stock to maintain PCT’s 1% ownership of the Company. These additional issuances were valued at an earlier appraisal of $1.40 per share or approximately $314,000.
On July 31, 2013, an aggregate of 247,500 shares of its common stock issuable to members of the Board of Directors fully vested. The Company valued these shares based on the July 31, 2013 share price of $0.40 per share, or $99,000, and recorded it to accrued stock compensation expense until the common stock is issued. The Company has included these costs as general and administrative expenses within the consolidated statements of operations for the year ended April 30, 2014. On April 10, 2014, the Company issued 61,875 common shares to one of its Board Members.
On July 31, 2013, an aggregate of 350,000 shares of its common stock issuable to members of the Scientific Advisory Board fully vested. The Company valued these shares based on the July 31, 2013 share price of $0.40 per share, or $140,000, and recorded it to accrued stock compensation expense until the common stock is issued. The Company has included these costs as research and development expenses within the consolidated statements of operations for the year ended April 30, 2014. On April 1, 2014, the Company issued 200,000 of common shares to the Scientific Advisory Board Members.
On August 20, 2013, an aggregate of 200,000 shares of its common stock issuable to members of the Scientific Advisory Board fully vested. The Company valued these shares based on the October 31, 2013 share price of $0.40 per share, or $80,000. The Company has included these costs as research and development expenses within the consolidated statements of operations for the year ended April 30, 2014. On April 1, 2014, the Company issued 200,000 of common shares to the Scientific Advisory Board Members.
On October 30, 2013, as part of the employee agreements with the new CEO and COO, the Board of Directors granted each of them stock options to purchase 1.5 million shares of Company common stock at an exercise price of $0.265. The stock options will vest as follows: (i) 500,000 shares vesting on the 91st day from the option grant date, (ii) 1,000,000 shares vesting in equal installments of 200,000 on the last day of each 90 day period starting from the 91st day after the option grant date. At April 30, 2014, 700,000 stock options vested for each the CEO and COO. The Company recorded stock-based compensation of $192,100 for the vested stock options as general and administrative expenses within the consolidated statements of operations for the year ended April 30, 2014.
On October 31, 2013, an aggregate of 185,625 shares of its common stock issuable to members of the Board of Directors fully vested. The Company valued these shares based on the October 31, 2013 share price of $0.25 per share, or $46,406, and recorded it to accrued stock compensation expense until the common stock is issued. The Company has included these costs as general and administrative expenses within the consolidated statements of operations for the year ended April 30, 2014.
Beginning in December 2013 and concluding in March 2014, the Company received net proceeds of $1,950,300 for the sale of 8,890,000 shares of Company common stock as part of a private placement to multiple investors. The investors in the private placement were also issued for no additional consideration warrants to purchase 4,445,000 shares of Company common stock at an exercise price of $0.35 per share. The warrants were valued at approximately $1,302,000 based on the assumptions used in Note 2. The Company issued these common stock shares during the fourth quarter ending April 30, 2014. Additionally, the Company is obligated to issue 1,489,000 warrants at an exercise price of $0.25 to the investment banker for the private placement. These warrants were valued at approximately $266,000, however, as the cost of these warrants would have been a capital raise cost, there was no financial impact to the Company for the issuance of these warrants.
On January 8, 2014, the Company issued 700,000 shares of Company common stock as part of a collateral agreement for the benefit of the legal firms that are representing the Company in the Sand Dollar Partners, LLC complaint in the Superior Court of Arizona and the complaint that was filed against the Company and John Steel for infringement and misappropriation of MicroIslet patent in the United States District Court for the District of Utah, Central Division (see Note 6). These shares are to be held in escrow to secure payment of the legal fees incurred in the defense of the complaint. Since these shares are being held in escrow until a future date to potentially satisfy the Company’s obligations, the Company has recorded these shares at par value or $700 to prepaid expenses. If common stock shares are issued from escrow to pay the legal fees, the common stock will be valued on the date of settlement. Legal fees incurred relating to this matter are included in accounts payable on the accompanying consolidated balance sheet. On May 16, 2014, the Company completed the settlement with Sand Dollar Partners, LLC. (see Note 6).
On January 24, 2014, the Board of Directors approved the termination of the existing Scientific Advisory Board (“SAB”). The SAB members will not be entitled to the remaining 550,000 unvested shares.
On January 31, 2014, an aggregate of 185,625 shares of its common stock issuable to members of the Board of Directors fully vested. The Company valued these shares based on the January 31, 2014 share price of $0.60 per share, or $111,375, and recorded it to accrued stock compensation expense until the common stock is issued. The Company has included these costs as general and administrative expenses within the consolidated statements of operations for the year ended April 30, 2014.
On February 10, 2014, as part of a settlement agreement the Company issued 25,000 shares of its common stock to American Capital Ventures. The Company valued these shares based on the February 10, 2014 share price of $0.60 per share, or $15,000, The Company has included these costs as general and administrative expenses within the consolidated statements of operations for the year ended April 30, 2014.
On February 13, 2014, as part of an agreement for Dr. Eric Barnett to join the Board of Directors the Company granted 70,000 shares of its common stock issuable and fully vested. The Company valued these shares based on the February 13, 2014 share price of $0.32 per share, or $22,400. The Company has included these costs as general and administrative expenses within the consolidated statements of operations for the year ended April 30, 2014. On March 5, 2014 the Company issued these common shares.
On March 12, 2014, as part of compensation to join the Company’s Board of Directors the Company granted 50,000 shares of its common stock issuable and fully vested to Dr. Michael Luther. These shares based on the February 13, 2014 share price of $0.40 per share, or $20,000, and recorded it to accrued stock compensation expense until the common stock is issued. The Company has included these costs as general and administrative expenses within the consolidated statements of operations for the year ended April 30, 2014.
On April 30, 2014, as part of settling a consulting agreement, the Company granted 14,500 shares of its common stock issuable and fully vested to a consultant. The Company valued these shares at $48,000, and recorded it to accrued stock compensation expense until the common stock is issued. The Company has included these costs as general and administrative expenses within the consolidated statements of operations for the year ended April 30, 2014.
On April 30, 2014, an aggregate of 123,750 shares of its common stock issuable to members of the Board of Directors fully vested. The Company valued these shares based on the April 30, 2014 share price of $0.30 per share, or $37,125, and recorded it to accrued stock compensation expense until the common stock is issued. The Company has included these costs as general and administrative expenses within the consolidated statements of operations for the year ended April 30, 2014.
The following table summarizes information regarding the warrants outstanding as of April 30, 2014 and 2013:
| | | Number of Warrants | | | Weighted Average Exercise Price | | | Expiry Dates | |
| Warrants at April 30, 2011 | | | - | | | $ | - | | | n/a | |
| Granted | | | 2,145,961 | | | $ | 1.00 | | | February 2017 to March 2017 | |
| Expired | | | - | | | $ | - | | | n/a | |
| Warrants at April 30, 2012 | | | 2,145,961 | | | $ | 1.00 | | | February 2017 to March 2017 | |
| Granted | | | 4,420,837 | | | $ | 1.08 | | | April 2017 to January 2018 | |
| Expired | | | (200,000 | ) | | $ | 2.00 | | | August 20, 2017 | |
| Warrants at April 30, 2013 | | | 6,366,798 | | | $ | 1.02 | | | February 2017 to January 2018 | |
| Granted | | | 6,064,000 | | | $ | 0.33 | | | September 2015 to June 2019 | |
| Expired | | | - | | | $ | - | | | n/a | |
| Warrants at April 30, 2014 | | | 12,430,798 | | | $ | 0.68 | | | September 2015 to June 2019 | |
On October 30, 2013, the Company issued 3,000,000 Nonqualified Stock Options as part of the employment agreements for James Green, CEO and William Wilkison Ph.D, COO. These options were issued with a strike price of $0.265. Options vest over eighteen months from the grant date and expire six and one half years from grant date.
The following table summarizes information regarding the stock options outstanding as of April 30, 2014 and 2013:
| | | Number of Stock Options | | | Weighted Average Exercise Price | | | Weighted Average Contractual Life in Years | |
| Stock Options at April 30, 2013 | | | - | | | $ | - | | | | - | |
| Granted | | | 3,000,000 | | | $ | 0.265 | | | | 6.5 | |
| Exercised | | | - | | | $ | - | | | | - | |
| Expired or canceled | | | - | | | $ | - | | | | - | |
| Stock Options at April 30, 2014 | | | 3,000,000 | | | $ | 0.265 | | | | 6.0 | |
| Options Exercisable | | | 1,400,000 | | | $ | 0.265 | | | | 6.0 | |
The weighted average fair value of options granted during fiscal 2014 was $0.14. The intrinsic value of options outstanding, vested and exercisable, and exercised is $0. Stock-based compensation expense is $192,100 for the year ended April 30, 2014.
The following table summarizes information regarding non-vested stock options as of April 30, 2014 and 2013.
| | | Number of Stock Options | | | Stock Options Weighted Average Grant Date Fair Value | |
| Nonvested options at April 30, 2013 | | | - | | | $ | - | |
| Granted | | | 3,000,000 | | | $ | 0.14 | |
| Vested/Issued | | | (1,400,000 | ) | | $ | 0.14 | |
| Forfeited | | | - | | | $ | - | |
| Nonvested stock options at April 30, 2014 | | | 1,600,000 | | | $ | 0.14 | |
At April 30, 2014, total compensation costs related to non-vested stock option awards not yet recognized totaled $219,430. The weighted average period over which this amount is expected to be recognized is one year.
NOTE 5. COMMITMENTS AND CONTINGENCIES
Contracts
On January 10, 2012, the Company entered into an agreement with Progenitor Cell Therapy, LLC (PCT), a subsidiary of NeoStem, Inc. (NeoStem), which was amended by an agreement dated May 15, 2012 by and between the Company and NeoStem, PCTs parent company. Under the agreements, PCT will be providing the protocols, procedures, systems, equipment, testing, quality controls, and manufacturing and distribution services to support the development and commercialization of the Companys encapsulated porcine islet cells for the treatment of diabetes. As compensation for the services of PCT, the Company agreed to pay to PCT a non-refundable monthly fee of $63,000 and a non-refundable monthly charge of between $33,000 and $54,000. NeoStem was entitled to receive shares and warrants of the Companys common stock, as well as additional shares for no consideration so that NeoStems ownership is not less than 1% of outstanding shares on a fully diluted basis. PCT has the right for a period of ten years to be the exclusive manufacturer of any product involved in the services to be provided under the agreement. With respect to commercial production of such products, PCT will be entitled to a royalty of 2.85% of gross sales and 5% of any sublicensing fees, royalties, milestone fees or profit sharing payments. On February 7, 2013, the Company provided to NeoStem Inc. notice of termination of the agreement with Progenitor Cell Therapy, Inc. dated January 10, 2012, as amended by the agreement dated May 15, 2012 by and between the Company and NeoStem. During the years ended April 30, 2014, and 2013, the Company expensed as research and development expense within the consolidated statements of operations of approximately $0 and $936,000, respectively, related to this contract.
On July 23, 2012, the Company entered into a long-term supply agreement with Spring Point Project, a source animal facility to purchase pigs for use in the Company’s xenotransplantation research. During the years ended April 30, 2014 and 2013, the Company expensed for the year as research and development expense within the consolidated statements of operations of approximately $350,000 and $971,000, respectively, related to this contract. On August 12, 2013, the Company received from Spring Point Project a notice of termination of the supply agreement, effective November 10, 2013, unless the Company cured the default before that date. The default was not cured and the agreement with Spring Point Project has been terminated.
In August 2012, the Company entered into an agreement with the Regents of the University of California, Los Angles, (“UCLA’), whereby UCLA will provide work dealing with small molecule mediated porcine islet proliferation. The Company had a net credit to research and development expense of approximately $43,000 for the year ended April 30, 2014 within the consolidated statements of operations related to this contract. On February 25, 2014, the Company terminated its agreement with UCLA. The Company incurred $71,000 of expense during the fiscal year ending April 30, 2013.
In May 2013, the Company entered into a sales and services agreement with the Regents of the University of California, Irvine (“UCI”) to provide materials consisting of isolated islets to be supplied to the Spring Point Project. For the year ended April 30, 2014, the Company expensed as research and development expense within the consolidated statements of operations of approximately $110,000, related to this contract. The Company terminated this agreement on March 24, 2014 for settlement payments totaling $150,000 on total liabilities of approximately $305,000 for expenses incurred in this and last fiscal year. The Company incurred $276,000 of expense during the fiscal year ending April 30, 2013.
In September 2013, the Company entered into a consulting agreement with American Capital Ventures, Inc. to provide consulting services for implementation of the Company’s corporate and business development plan and to plan review and create corporate communications. On September 12, 2013, 200,000 shares vested and were valued at $0.29 per share, or $58,000. The Company terminated this agreement on January 23, 2014 and agreed to issue 25,000 shares valued at $0.60 per share of common stock as part of the termination agreement. The Company issued the shares on February 11, 2014 and expensed $15,000 related to this consulting agreement within the consolidated statements of operations for the year ended April 30, 2014.
Licenses
On May 2, 2012, the Company, entered into a license agreement with the Yale University (“Yale”). Under the agreement, the Company received exclusive license to the technology patented by Yale. In consideration of the license granted under the agreement, the Company paid Yale a license issue royalty of $10,000 (plus a $10,000 annual renewal fee) and issued 20,000 shares of its common stock, and agreed to pay certain milestones royalties by issuing an aggregate of 160,000 shares of common stock. The Company also agreed to pay to Yale a royalty on net sales. The agreement will expire automatically, on a country-by-country basis, on the date on which the last of the claims of the subject patents expires. The agreement can be terminated by Yale if the Company defaults on its obligations under the agreement and fails to cure such default within 60 days of a written notice by Yale. The Company can terminate the agreement upon a six month notice subject to payment of all amounts due to Yale under the agreement. During years ended April 30, 2014 and 2013, the Company expensed approximately $100,000 net a credit of approximately $156,510 for current and past year services and $144,000, respectively, within the consolidated statements of operations.
On July 23, 2012, the Company entered into a licensing agreement with the Winthrop University Hospital (“Winthrop”) to license certain patents and technology. In consideration of the license granted under the agreement, the Company agreed to pay to Winthrop a license issue royalty of $10,000 (plus a $10,000 annual renewal fee) and issue 20,000 shares of its common stock, and to pay certain milestones royalties by issuing an aggregate of 160,000 shares of common stock. The Company also agreed to pay to Winthrop a royalty on net sales. The agreement will expire automatically, on a country-by-country basis, on the date on which the last of the claims of the subject patents expires. It can be terminated by Winthrop if the Company defaults on its obligations under the agreement and fails to cure such default within 60 days of a written notice by the university. The Company can terminate the agreement upon a six month notice subject to payment of all amounts due Winthrop under the agreement. During the year ended April 30, 2014 and 2013, the Company expensed as research and development expense within the consolidated statements of operations of approximately $62,000 and $200,000 respectively, related to this contract. The Company is currently in default regarding its payment obligations under the foregoing license and research agreements.
On January 22, 2014, the Company terminated its licensing agreement dated, July 25, 2012, with The Regents of The University of California, Los Angeles.
Legal Proceedings
In April 2012, Sand Dollar Partners, LLC, a shareholder of the Company filed a complaint in the Superior Court of Arizona, Pima County against, among other parties, ISI, our wholly-owned subsidiary, John Steel, our former CEO and director, and Jonathan Lakey, our former director. In 2010, Sand Dollar invested $357,000 in ISI through the purchase of a convertible promissory note which was converted into 3,591,729 shares of the Company’s common stock. The plaintiff contends that it was entitled to issuance of additional shares and nomination of one board member.
On October 25, 2013, the Company entered into a settlement agreement with Sand Dollar Partners, LLC. At a hearing on February 21, 2014, the Company and Sand Dollar agreed to amend the settlement agreement whereby, Sand Dollar placed in escrow all of the Company’s common stock it held and retained a broker dealer to sell sufficient shares to receive $500,000 in cash and to pay fees to the broker dealer. Additionally, the Company agreed to issue 130,000 warrants that will vest over the next three years and make a $30,000 payment on May 15, 2014. The Company issued these warrants and made the $30,000 payment on May 15, 2014 fully completing the settlement. The broker dealer sold 2,247,200 shares of the Company’s escrowed stock to settle the obligation and the Company will receive back the remaining 1,344,529 shares of its common stock. The sale of these shares and the return of the remaining shares had no impact on the consolidated financial statements.
On April 25, 2014, PCT, filed a lawsuit against the Company in the United States District Court for the District of New Jersey (Case No. 2:14-cv-02658-SDW-MCA). PCT’s complaint asserts various claims, including breach of contract and unjust enrichment, based on the alleged failure of the Company to pay for services and goods provided by PCT under a January 10, 2012 letter agreement. PCT seeks an unspecified amount of compensatory and other damages, plus interest and costs. The Company filed an answer to the complaint on June 24, 2014 denying all liability, including on the grounds that the January 10, 2012 letter agreement is unenforceable and PCT failed to provide the goods and services it stated it would provide. In connection with its answer to PCT’s complaint, the Company filed a counterclaim against PCT and a third-party complaint against NeoStem to seek, among other things, (i) a declaration that the January 10, 2012 letter agreement is unenforceable, (ii) monetary damages and (iii) rescission of equity securities the Company previously issued to NeoStem. The Company intends to vigorously defend the claims by PCT and prosecute its claims against PCT and NeoStem. As the lawsuit is at an early stage, the Company cannot at this time estimate the possible loss or range of loss, if any, that may result from this lawsuit.
In July 2012, a complaint was filed against the Company and John Steel in the United States District Court for the District of Utah, Central Division for infringement and misappropriation of a patent. The plaintiffs contend that they were the actual purchasers of the MicroIslet patent out of MicroIslet’s bankruptcy proceedings in 2009 and that the respective intellectual property rights have been never assigned to either ISI or the Company. As a result, they allege that the Company’s claim to the ownership of the MicroIslet patent based on the assignment of the patent by its founders is baseless. The complaint sought monetary damages including punitive damages of at least $12 million, costs, attorneys' fees, and declaratory judgment. On January 8, 2013, the Court dismissed the plaintiff’s action for lack of recoverable damages. The plaintiffs refiled their claim and the Company has filed a motion with the Court for dismissal. The Company believes the plaintiffs’ claims to be without merit and will continue to vigorously defend against this action and has determined that it is unlikely any damages will be paid.
NOTE 6. RELATED PARTY TRANSACTIONS NOT INCLUDED ELSEWHERE
The Company borrowed $25,880 from its former CEO. Promissory notes were issued for these amounts. Repayments of $12,405 were made during the year. The remaining balance at April 30, 2014 is $13,475, repayments of $5,000 were made subsequent to year end. The balance at April 30, 2013 was $11,880.
During the year ended April 30, 2014, one of the Company’s Board Members loaned the Company a total of $74,531 at a 6.5% interest rate. A promissory note was issued for this amount. Repayments of $15,000 were made during the year. The remaining balance at April 30, 2014 was $62,542, repayments of $20,000 were made subsequent to year end.
A contractor of the Company loaned the Company $15,624. No repayments have been made as of April 30, 2014.
On May 10, 2010, the Company entered into a consulting agreement with John Steel to provide executive management services to the Company for a monthly fee of $15,000. On July 1, 2013, as part of Mr. Steel’s separation agreement, Mr. Steel and the Company entered into a new consulting agreement, whereby, Mr. Steel will provide business development services to the Company as may be directed by the CEO. Under the consulting agreement, Mr. Steel will receive a monthly consulting fee of $15,000. The consulting agreement expired on June 30, 2014. The outstanding amounts due to John Steel for his consulting services is approximately $208,000 and $18,000 at April 30, 2014 and 2013, respectively, which is included in accounts payable on the consolidated balance sheets.
NOTE 7. INCOME TAXES
Temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes give rise to the Company's deferred income taxes. The components of the Company's deferred tax assets as of April 30 are as follows:
| | | 2014 | | | 2013 | |
| Deferred Tax Assets: | | | | | | |
| Net operating loss carryforwards | | $ | 7,288,600 | | | $ | 6,133,500 | |
| Tax credits | | | 355,400 | | | | 428,200 | |
| Accrued expenses | | | - | | | | 42,500 | |
| Derivatives | | | - | | | | 23,300 | |
| Share-based compensation | | | 178,500 | | | | 2,000 | |
| Depreciation and amortization | | | 62,900 | | | | 19,600 | |
| Total deferred tax asset | | | 7,885,400 | | | | 6,649,100 | |
| | | | | | | | | |
| Valuation Allowance | | | (7,885,400 | ) | | | (6,649,100 | ) |
| | | | | | | | | |
| Deferred Tax Liabilities - Intangibles | | | (547,000 | ) | | | (547,000 | ) |
| | | | | | | | | |
| | | | (547,000 | ) | | | (547,000 | ) |
| | | | | | | | | |
| Total deferred tax liability | | $ | (547,000 | ) | | $ | (547,000 | ) |
FASB ASC 740, Income Taxes, requires that a valuation allowance be established when it is more likely than not that its net deferred tax asset will not be realized. In determining whether a valuation allowance is required, a company must take into account all positive and negative evidence with regard to the utilization of a deferred tax asset. FASB ASC 740 further states that it is difficult to conclude that a valuation allowance is not needed when there is negative evidence such as cumulative losses in recent years.
The Company plans to continue to provide a full valuation allowance on future tax benefits until it can sustain an appropriate level of profitability and until such time, the Company would not expect to recognize any significant tax benefits in its future results of operations. The valuation allowance increased by $1,236,300 and $4,304,000 for the years ended April 30, 2014 and 2013, respectively.
As of April 30, 2014, the Company had net operating loss carry forwards for Federal and state income tax purposes of approximately $18,270,000 and $18,457,000, respectively. These Federal and state net operating loss carry forwards will begin to expire in 2031. The Company had research and development credits carry forwards for Federal and state income tax purposes of approximately $284,000 and $72,000 respectively. Pursuant to the Internal Revenue Code, Sections 382 and 383, use of the Company’s net operating loss and credit carry forward could be limited if a cumulative change in ownership of more than 50% occurs within a three-year period. This analysis has not been performed as of April 30, 2014. Currently, the 2010 through 2013 tax years are open and subject to examination.
The provision for income taxes differs from the amount computed by applying the US statutory Federal income tax rate of 34% to income before income taxes. The reconciliation of statutory and effective taxes for the years ended April 30, 2014 and 2013 are presented below:
| | | 2014 | | | 2013 | |
| | | | | | | |
| Provision computed at statutory rate | | $ | (1,005,000 | ) | | $ | (3,929,000 | ) |
| State income tax, net of Federal tax benefit | | | (172,000 | ) | | | (674,000 | ) |
| Research and development credit | | | (18,000 | ) | | | (325,000 | ) |
| Change in valuation allowance | | | 1,236,300 | | | | 4,304,000 | |
| Permanent differences | | | 13,000 | | | | 625,000 | |
| Other | | | (54,300 | ) | | | (1,000 | ) |
| | | | | | | | | |
| Income tax expense | | $ | - | | | $ | - | |
NOTE 8. SUBSEQUENT EVENTS
On May 22, 2014, Mr. Delmar was appointed as the Company’s Chief Financial Officer. Mr. Delmar will receive, a grant of 300,000 stock options to purchase common shares of the Company and a conditional grant of 100,000 stock options upon the effective date of an S-4 filing.
On May 9, 2014, the Company issued 742,500 common shares to the members of the Board of Directors as part of the approved compensation plan.
On June 4, 2014, the Company issued 14,500 common shares to a consultant part of a settlement agreement.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Balance Sheets
July 31, 2014
| | | July 31, 2014 | | | April 30, 2014 | |
| | | Unaudited | | | Audited | |
| | | | | | | |
| Assets | | | | | | |
| | | | | | | |
| Current assets: | | | | | | |
| Cash | | $ | 12,587 | | | $ | 15,906 | |
| | | | | | | | | |
| Total assets | | $ | 12,587 | | | $ | 15,906 | |
| | | | | | | | | |
| Liabilities and Members’ Capital (Deficit) | | | | | | | | |
| | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 56,473 | | | $ | 17,851 | |
| Accrued expenses | | | 215,775 | | | | 158,567 | |
| Notes payable | | | 312,689 | | | | 309,390 | |
| | | | | | | | | |
| Total current liabilities | | | 584,937 | | | | 485,808 | |
| | | | | | | | | |
| Commitments and contingencies (Note 4) | | | | | | | | |
| | | | | | | | | |
| Members' capital (deficit): | | | | | | | | |
| Member capital (4,000,000 units authorized and issued) | | | 41,656 | | | | 41,656 | |
| Deficit accumulated during the development stage | | | (614,006 | ) | | | (511,558 | ) |
| | | | | | | | | |
| Total members’ capital (deficit) | | | (572,350 | ) | | | (469,902 | ) |
| | | | | | | | | |
| Total liabilities and members' capital (deficit) | | $ | 12,587 | | | $ | 15,906 | |
See accompanying notes to the financial statements.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Statements of Operations
Unaudited
| | | | | | | | | For the period | |
| | | | | | | | | From August 26, 2009 | |
| | | For the quarter ended | | | (inception) through | |
| | | July 31, 2014 | | | July 31, 2013 | | | July 31, 2014 | |
| | | | | | | | | | |
| Revenue | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | |
| General and administrative | | | 87,190 | | | | 13,951 | | | | 359,373 | |
| Research and development | | | 11,958 | | | | 2,517 | | | | 429,751 | |
| | | | | | | | | | | | | |
| Total operating expenses | | | 99,148 | | | | 16,468 | | | | 789,124 | |
| | | | | | | | | | | | | |
| Loss from operations | | | (99,148 | ) | | | (16,468 | ) | | | (789,124 | ) |
| | | | | | | | | | | | | |
| Other (expense) income: | | | | | | | | | | | | |
| Grant income | | | - | | | | - | | | | 236,350 | |
| Interest expense | | | (3,300 | ) | | | (4,764 | ) | | | (61,232 | ) |
| | | | | | | | | | | | | |
| Total other (expense) income | | | (3,300 | ) | | | (4,764 | ) | | | 175,118 | |
| | | | | | | | | | | | | |
| Net loss | | $ | (102,448 | ) | | $ | (21,232 | ) | | $ | (614,006 | ) |
See accompanying notes to the financial statements.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Statements of Cash Flows
Unaudited
| | | | | | | | | For the period | |
| | | | | | | | | From August 29, 2009 | |
| | | For the quarters ended, | | | (Inception) through | |
| | | July 31, 2014 | | | July 31, 2013 | | | July 31, 2014 | |
| Cash flows from operating activities: | | | | | | | | | |
| Net loss | | $ | (102,448 | ) | | $ | (21,232 | ) | | $ | (614,006 | ) |
| Adjustments to reconcile net loss to net cash (used in) provided by operating activities: | | | | | | | | | | | | |
| Amortization of debt discount | | | - | | | | - | | | | 20,000 | |
| Accrued interest | | | 3,300 | | | | 2,958 | | | | 34,283 | |
| Change in operating assets and liabilities: | | | | | | | | | | | | |
| Accounts payable | | | 38,622 | | | | - | | | | 56,473 | |
| Accrued expenses | | | 57,208 | | | | 27,710 | | | | 215,775 | |
| | | | | | | | | | | | | |
| Net cash (used in) provided by operating activities | | | (3,319 | ) | | | 9,436 | | | | (287,476 | ) |
| | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | |
| Net contributions by members | | | - | | | | - | | | | 21,656 | |
| Proceeds from notes payable | | | - | | | | - | | | | 278,407 | |
| Net cash provided by financing activities | | | - | | | | - | | | | 300,063 | |
| | | | | | | | | | | | | |
| Net increase (decrease) in cash | | | (3,319 | ) | | | 9,436 | | | | 12,587 | |
| | | | | | | | | | | | | |
| Cash at beginning of period | | | 15,906 | | | | 16,408 | | | | - | |
| | | | | | | | | | | | | |
| Cash at end of period | | $ | 12,587 | | | | 25,844 | | | $ | 12,587 | |
| | | | | | | | | | | | | |
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Cash paid during the period for: | | | | | | | | | | | | |
| Interest | | $ | - | | | $ | - | | | $ | - | |
| Income taxes | | $ | - | | | $ | - | | | $ | - | |
See accompanying notes to the financial statements.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Notes to the Financial Statements
Note 1 – Description of Business and Summary of Significant Accounting Policies
Description of Business
Brighthaven Ventures, LLC d/b/a BHV Pharma (referred to in these financial statements as the “Company”, “we”, “our” and “us”) is a privately-held clinical stage pharmaceutical company focused on the development of Remogliflozin Etabonate (“Remo” or “BHV091009”). Remo is a new chemical entity in phase IIb development for the treatment of type 2 diabetes (“T2DM”) and non-alcoholic steatohepatitis (“NASH”). Remo is a selective sodium glucose co-transporter 2 (“SGLT2”) inhibitor that is expected to emerge as a best in class molecule. SGLT2 inhibitors are an important new class of metabolic disease compounds which work by inhibiting reabsorption of glucose in the kidney and provide robust insulin-independent HbA1c lowering and weight loss. SGLT2 inhibitors are the only oral diabetes therapy to safely provide glycemic control with significant weight loss through an insulin independent mechanism of action.
Remo has been administered in sixteen phase II, three phase IIa, and two phase IIb clinical studies. Approximately 850 human subjects, including 496 subjects with T2DM, have been dosed with Remo. Remo has demonstrated dose-related glycosuria consistent with SGLT2 inhibition with best in class lowering of HbA1c, plasma glucose, and body weight observed in subjects with T2DM over 12 weeks of treatment. Remo has also proven to be well tolerated with low incidences of urogenital infections over 12 weeks of treatment in subjects with T2DM. Our near term development plans call for one phase IIb study of remo for type 2 diabetes and another phase IIb study for NASH. We may also pursue additional clinical studies of Remo for type 1 diabetes. We were organized under the laws of the State of North Carolina on August 26, 2009. Our principal executive offices are located in Raleigh, North Carolina.
Limited Liability Company
The debts, obligations, and liabilities of the Company, whether arising in contracts, tort or otherwise, shall be solely the debts, obligations, and liabilities of the Company, and the members of the Company shall not be obligated personally for any such debts, obligations, or liabilities of the Company solely by reason of being members of the Company. Except as otherwise provided by the North Carolina Limited Liability Company Act, the Company will continue as a limited liability company until such time as the managing members decides the advantages of the limited liability company structure are no more.
| Development Stage Company | |
As of July 31, 2014, we have devoted substantially all of our efforts to product development, raising capital and building infrastructure and have not realized revenues from any planned principal operations. Accordingly, we are considered to be in the development stage.
Basis of Accounting
The accompanying financial statements have been prepared in accordance with generally accepted accounting principles in the United States (“GAAP”).
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the accompanying financial statements. Significant estimates made in preparing these financial statements relate to debt and other contingencies. Actual results could differ from those estimates.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Notes to the Financial Statements
Note 1 – Description of Business and Summary of Significant Accounting Policies
(continued)
Cash
Cash consists of cash and short-term investments with original maturities of 90 days or less.
Concentration of Credit Risk
Financial instruments that potentially subject us to a concentration of credit risk principally consist of cash. We maintain our cash balances at what we believe are high credit-quality financial institutions. At times, balances at one financial institution may exceed federally insured limits of $250,000.
Research and Development Costs
We expense research and development costs as incurred. Research and development expenses are comprised of costs incurred in performing research and development activities, including professional service costs, contracted services, license agreements and other outside costs.
Grant Income
Grant income stems from the Company’s participation in local, state incentive programs and is recognized as other income. Grant income, employee tax credits from the State of North Carolina and incentive income are all recorded when received. There was a grant payment received in the amount of $236,350 for the year ended April 30, 2011. No other grant income has been received for any of the other years.
Income Taxes
The income or loss of the Company for federal income tax reporting purposes is includable in the income tax returns of the Members. In general, no recognition has been given to income taxes in the accompanying financial statements.
The Company reviews and evaluates tax positions within its major jurisdictions and determines whether or not there are uncertain tax positions that require financial statement recognition. The Company recognizes uncertain tax positions if it is more likely than not to be sustained upon examination by the applicable taxing authority, including the resolution of any related appeals or litigation processes, based on technical merits of the position. Tax benefits are measured as the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement, which could result in the Company recording a tax liability that would reduce members’ equity.
As of July 31, 2014, no reserves for uncertain tax positions were required to have been recorded for uncertainty in income taxes for any of the Company’s open tax years. The Company is not subject to examination by U.S. federal and state tax authorities for the tax years before 2011. The Company’s policy is to recognize interest and penalties on unrecognized tax benefits in income tax expense within the statement of operations.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Notes to the Financial Statements
Note 1 – Description of Business and Summary of Significant Accounting Policies
(continued)
Going Concern
We have incurred a net loss of $102,447 for the quarter ended July 31, 2014, and have a loss accumulated during the development stage totaling $614,006. Our operations to date have been limited to organizing and staffing our company, business planning, raising capital and acquiring and developing our pipeline. To date, we have not generated any revenues and have financed our operations primarily with grants and by borrowings through our promissory notes. Since inception, the Company has generated recurring operating losses. These losses have resulted principally from costs incurred in connection with clinical trials, consulting fees and general and administrative expenses, including planning for the clinical trials of BHV091009. We expect to continue to incur operating losses for the next several years as we pursue the clinical development and commercialization of BHV091009 and work to develop additional product candidates through our research and development programs. As a result, we will seek to fund our operations through public or private equity or debt financings or other sources, such as collaborations and government contracts. Our failure to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategies. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Fair Value of Financial Instruments
The carrying value of our cash, accounts payable and accrued expenses approximates fair value primarily because of the short maturities of these instruments.
Recent Accounting Pronouncements
Management does not believe that any recently issued accounting pronouncements would have a material effect on the accompanying consolidated financial statements.
Note 2 – Accrued Expenses
Accrued expenses consisted of the following:
| | | July 31, 2014 | | | April 30, 2014 | |
| | | Unaudited | | | Audited | |
| Accrued interest on notes payable | | $ | 10,248 | | | $ | 6,949 | |
| Accrued liabilities | | | 205,527 | | | | 151,618 | |
| Total accrued expenses | | $ | 215,775 | | | $ | 158,567 | |
| | | | | | | | | |
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Notes to the Financial Statements
Note 3 – Notes Payable
In January 2010, we issued an unsecured promissory note agreement with the North Carolina Biotechnology Center (the “Center”), an entity primarily funded by the State of North Carolina, to borrow $28,407. The original note was due January 2013 and had an interest rate of 4.25%. The note was renegotiated and the unpaid principal and accrued interest of $31,554 was extended through a new note to January 11, 2014. The new note bears interest at a rate of 4.25% per annum, compounded annually. The Center typically provides these loans to early stage biotechnology companies across the state to support company inception, research and growth and define the scope of work necessary for the Company to complete.
In November 2010, we issued a second unsecured promissory note agreement with the Center to borrow $250,000. The original note was due November 2013 and had an interest rate of 4.25%. The note was renegotiated and the unpaid principal and accrued interest of $277,836 was extended through a new note to January 15, 2015. The new note bears interest at a rate of 4.25% per annum, compounds annually and requires annual payments of $125,000 at January 15, 2014 and 2015. No payments on this note have been made to-date. In conjunction with the second note, we issued the Center a warrant to purchase 12,500 units of the Company exercisable at any time. The unit exercise price is $5 per share and the warrant expires November 15, 2020. The fair value of the warrant was allocated from the gross proceeds of the note with the respective discount amortized to interest expense over the original term of the note using the effective interest method. The warrant was valued at $20,000 using the Black-Scholes option pricing model and was treated as debt discount on the note payable. The valuation of the warrant was performed using inputs requiring us to make assumptions about the Company when issued. Alternative probabilities would have resulted in increases or decreases in the value of the conversion feature. Interest expense for the quarters ended July 31, 2014 and 2013 were $3,299 and $4,764, respectively.
Note 4 – Commitments and Contingencies
License Agreement
In October 2009 and as amended from time to time, we entered into an “Option Agreement” with Kissei Pharmaceuticals Co., Ltd., a Japanese pharmaceutical company, (“Kissei”), to license KGT-1650 (remogliflozin) and KGT-1681 (remogliflozin etabonate). The Option Agreement was to be commensurate and in conjunction with a license agreement, which was signed in December 2010 (see below).
In February 2014, we amended the Option Agreement to pay $733,000 of a previously agreed research and development expense to Kissei in two installments: the first installment of $200,000 due within a year of a proposed agreement between the Company and another entity becoming effective; and the second of $533,000 due at the “End-of-Phase II Meeting” with the U.S. Food and Drug Administration (“FDA”), as defined.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Notes to the Financial Statements
Note 4 – Commitments and Contingencies (continued)
As noted previously, in December 2010 and as amended from time to time, we entered into a world-wide exclusive license agreement for the development and commercialization of KGT-1650 and KGT-1681 and such other compounds included in the portfolio with Kissei. The agreement covers the development and commercialization of KGT-1650 and KGT-1681 globally, other than in Japan, Korea and Taiwan for the treatment, palliation or prevention of human disease.
Under this license agreement, the Company could be required to pay up to a total of $25 million upon achieving certain milestones, including the dosing of the first patient in the first phase III clinical trial, the submission of regulatory applications and certain regulatory approvals, in addition to royalties payable on commercial sales, if any occur. From inception through July 31, 2014, we have not paid or accrued for any such payments resulting from the license agreement and no milestones have been reached.
Note 5 – Subsequent Events
On September 30, 2014, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Islet Sciences, Inc. and its soon to be parent, Avogenx, Inc. (”Avogenx”). Pursuant to the Merger Agreement, Avogenx will acquire all of the outstanding membership interests of the Company in consideration for the issuance of an aggregate of 30 million shares of common stock of Avogenx (subject to adjustment pursuant to the Merger Agreement), and additional milestone payments in the form of additional shares of common stock of Avogenx in an aggregate amount of up to $71 million within five business days of the occurrence of certain milestones which are based on the development of our main compound, Remo.
In August 2014, we granted an exclusive license to LIBBS North America, LLC (“LIBBS”), a wholly-owned subsidiary of LIBBS Farmacêutica Ltda. of Brazil, for the development and ccommercialization of Remo for all human therapeutic, palliative, and prophylactic uses in certain countries in Latin America, including Brazil. In exchange for these rights, LIBBS is required to make a payment to us upon our submission of the study protocol for our currently-planned phase IIb study of Remo for type 2 diabetes to the FDA and approval of the protocol by the responsible Institutional Review Board, and to make profit-sharing and royalty payments to us based on sales of Remo in LIBBS’s exclusive territory. LIBBS also agreed to pay for the cost of our currently-planned phase IIb study of Remo for type 2 diabetes, up to an agreed maximum amount. LIBBS is required to use commercially reasonable efforts to develop and commercialize Remo in LIBBS’s exclusive territory at LIBBS’s cost.
We evaluated events subsequent to July 31, 2014 to assess the need for potential recognition or disclosure. Such events were evaluated through November 19, 2014, the date the financials were available to be issued.
Report of Independent Registered Public Accounting Firm
Board of Directors and Members of
Brighthaven Ventures, LLC d/b/a BHV Pharma
We have audited the accompanying balance sheets of Brighthaven Ventures, LLC d/b/a BHV Pharma (A Development Stage Company) (the “Company”) as of April 30, 2014 and 2013, and the related statements of operations, members’ capital (deficit), and cash flows for years ended April 30, 2014 and 2013, and for the period August 26, 2009 (Inception) through April 30, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We have conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal controls over financial reporting. Our audit includes consideration of internal controls over financial reporting as a basis for designing audit procedures that are appropriate in the circumstance, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Brighthaven Ventures, LLC d/b/a BHV Pharma (A Development Stage Company) as of April 30, 2014 and 2013, and the results of its operations and cash flows for the years ended April 30, 2014 and 2013, and for the period from August 26, 2009 (Inception) through April 30, 2014, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has incurred losses since inception resulting in an deficit accumulated during the development stage of approximately $512,000 as of April 30, 2014 and further losses are anticipated in the development of its business that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| | /s/ PKF | |
| November 19, 2014 | PKF |
| San Diego, California | Certified Public Accountants |
| | A Professional Corporation |
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Balance Sheets
| | | April 30, 2014 | | | April 30, 2013 | |
| | | | | | | |
| ASSETS | | | | | | |
| | | | | | | |
| Current assets: | | | | | | |
| Cash | | $ | 15,906 | | | $ | 16,408 | |
| | | | | | | | | |
| Total assets | | $ | 15,906 | | | $ | 16,408 | |
| | | | | | | | | |
| LIABILITIES AND MEMBERS' CAPITAL (DEFICIT) | | | | | | | | |
| | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 17,851 | | | $ | 15,508 | |
| Accrued expenses | | | 158,567 | | | | 70,486 | |
| Notes payable | | | 309,390 | | | | 274,796 | |
| | | | | | | | | |
| Total current liabilities | | | 485,808 | | | | 360,790 | |
| | | | | | | | | |
| Commitments and contingencies (Note 4) | | | | | | | | |
| | | | | | | | | |
| Members' capital (deficit): | | | | | | | | |
| Member capital (4,000,000 units authorized and issued) | | | 41,656 | | | | 41,656 | |
| Deficit accumulated during the development stage | | | (511,558 | ) | | | (386,038 | ) |
| | | | | | | | | |
| Total members' capital (deficit) | | | (469,902 | ) | | | (344,382 | ) |
| | | | | | | | | |
| Total liabilities and members' capital (deficit) | | $ | 15,906 | | | $ | 16,408 | |
See accompanying notes to the financial statements.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Statements of Operations
| | | | | | | | | For the period | |
| | | | | | | | | from August 26, 2009 | |
| | | For the years ended | | | | | | (Inception) through | |
| | | April 30, 2014 | | | April 30, 2013 | | | April 30, 2014 | |
| | | | | | | | | | |
| Revenue | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | |
| Operating expenses: | | | | | | | | | | | | |
| General and administrative | | | 99,488 | | | | 67,113 | | | | 272,183 | |
| Reseach and development | | | 10,069 | | | | 29,864 | | | | 417,793 | |
| | | | | | | | | | | | | |
| Total operating expenses | | | 109,557 | | | | 96,977 | | | | 689,976 | |
| | | | | | | | | | | | | |
| Loss from operations | | | (109,557 | ) | | | (96,977 | ) | | | (689,976 | ) |
| | | | | | | | | | | | | |
| Other (expense) income: | | | | | | | | | | | | |
| Grant income | | | - | | | | - | | | | 236,350 | |
| Interest expense | | | (15,963 | ) | | | (19,798 | ) | | | (57,932 | ) |
| | | | | | | | | | | | | |
| Total other (expense) income | | | (15,963 | ) | | | (19,798 | ) | | | 178,418 | |
| | | | | | | | | | | | | |
| Net loss | | $ | (125,520 | ) | | $ | (116,775 | ) | | $ | (511,558 | ) |
See accompanying notes to the financial statements.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Statements of Changes in Members’ Capital (Deficit)
| | | Units | | | Members' Capital | | | Deficit Accumulated During the Development Stage | | | Total Members' Capital (Deficit) | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| Balance at August 26, 2009 (Inception) | | | 4,000,000 | | | $ | - | | | $ | - | | | $ | - | |
| | | | | | | | | | | | | | | | | |
| Contributions by members | | | - | | | | 23,745 | | | | - | | | | 23,745 | |
| Net loss | | | - | | | | - | | | | (15,436 | ) | | | (15,436 | ) |
| | | | | | | | | | | | | | | | | |
| Balance at April 30, 2010 | | | 4,000,000 | | | | 23,745 | | | | (15,436 | ) | | | 8,309 | |
| | | | | | | | | | | | | | | | | |
| Value of warrant issued with | | | | | | | | | | | | | | | | |
| note payable | | | - | | | | 20,000 | | | | - | | | | 20,000 | |
| Distributions to members | | | - | | | | (1,769 | ) | | | - | | | | (1,769 | ) |
| Net loss | | | - | | | | - | | | | (19,994 | ) | | | (19,994 | ) |
| | | | | | | | | | | | | | | | | |
| Balance at April 30, 2011 | | | 4,000,000 | | | | 41,976 | | | | (35,430 | ) | | | 6,546 | |
| | | | | | | | | | | | | | | | | |
| Distributions to members | | | - | | | | (320 | ) | | | - | | | | (320 | ) |
| Net loss | | | - | | | | - | | | | (233,833 | ) | | | (233,833 | ) |
| | | | | | | | | | | | | | | | | |
| Balance at April 30, 2012 | | | 4,000,000 | | | | 41,656 | | | | (269,263 | ) | | | (227,607 | ) |
| | | | | | | | | | | | | | | | | |
| Net loss | | | - | | | | - | | | | (116,775 | ) | | | (116,775 | ) |
| | | | | | | | | | | | | | | | | |
| Balance at April 30, 2013 | | | 4,000,000 | | | | 41,656 | | | | (386,038 | ) | | | (344,382 | ) |
| | | | | | | | | | | | | | | | | |
| Net loss | | | - | | | | - | | | | (125,520 | ) | | | (125,520 | ) |
| | | | | | | | | | | | | | | | | |
| Balance at April 30, 2014 | | | 4,000,000 | | | $ | 41,656 | | | $ | (511,558 | ) | | $ | (469,902 | ) |
See accompanying notes to the financial statements.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Statements of Cash Flows
| | | | | | | | | For the period | |
| | | | | | | | | from August 29, 2009 | |
| | | For the years ended | | | (Inception) through | |
| | | April 30, 2014 | | | April 30, 2013 | | | April 30, 2014 | |
| | | | | | | | | | |
| Cash flows from operating activities: | | | | | | | | | |
| Net loss | | $ | (125,520 | ) | | $ | (116,775 | ) | | $ | (511,558 | ) |
| Adjustments to reconcile net loss to net cash | | | | | | | | | |
| used in operating activities: | | | | | | | | | | | | |
| Amortization of debt discount | | | 3,611 | | | | 6,667 | | | | 20,000 | |
| Accrued interest | | | 30,983 | | | | - | | | | 30,983 | |
| Change in operating assets and liabilities: | | | | | | | | | |
| Accounts payable | | | 2,343 | | | | 15,508 | | | | 17,851 | |
| Accrued expenses | | | 88,081 | | | | 58,038 | | | | 158,567 | |
| | | | | | | | | | | | | |
| Net cash used in operating activities | | | (502 | ) | | | (36,562 | ) | | | (284,157 | ) |
| | | | | | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | | | | | |
| Net contributions by members | | | - | | | | - | | | | 21,656 | |
| Proceeds from notes payable | | | - | | | | - | | | | 278,407 | |
| | | | | | | | | | | | | |
| Net cash provided by financing activities | | | - | | | | - | | | | 300,063 | |
| | | | | | | | | | | | | |
| Net increase (decrease) in cash | | | (502 | ) | | | (36,562 | ) | | | 15,906 | |
| | | | | | | | | | | | | |
| Cash at beginning of period | | | 16,408 | | | | 52,970 | | | | - | |
| | | | | | | | | | | | | |
| Cash at end of period | | $ | 15,906 | | | $ | 16,408 | | | $ | 15,906 | |
| | | | | | | | | | | | | |
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | | | | | |
| | | | | | | | | | | | | |
| Cash paid during the period for: | | | | | | | | | | | | |
| Interest | | $ | - | | | $ | - | | | $ | - | |
| Income taxes | | $ | - | | | $ | - | | | $ | - | |
See accompanying notes to the financial statements.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Notes to the Financial Statements
Note 1 – Description of Business and Summary of Significant Accounting Policies
Description of Business
Brighthaven Ventures, LLC d/b/a BHV Pharma (referred to in these financial statements as the “Company”, “we”, “our” and “us”) is a privately-held clinical stage pharmaceutical company focused on the development of Remogliflozin Etabonate (“Remo” or “BHV091009”). Remo is a new chemical entity in phase IIb development for the treatment of type 2 diabetes (“T2DM”) and non-alcoholic steatohepatitis (“NASH”). Remo is a selective sodium glucose co-transporter 2 (“SGLT2”) inhibitor that is expected to emerge as a best in class molecule. SGLT2 inhibitors are an important new class of metabolic disease compounds which work by inhibiting reabsorption of glucose in the kidney and provide robust insulin-independent HbA1c lowering and weight loss. SGLT2 inhibitors are the only oral diabetes therapy to safely provide glycemic control with significant weight loss through an insulin independent mechanism of action.
Remo has been administered in sixteen phase I, three phase IIa, and two phase IIb clinical studies. Approximately 850 human subjects, including 496 subjects with T2DM, have been dosed with Remo. Remo has demonstrated dose-related glycosuria consistent with SGLT2 inhibition with best in class lowering of HbA1c, plasma glucose, and body weight observed in subjects with T2DM over 12 weeks of treatment. Remo has also proven to be well tolerated with low incidences of urogenital infections over 12 weeks of treatment in subjects with T2DM. Our near term development plans call for one phase IIb study of remo for type 2 diabetes and another phase IIb study for NASH. We may also pursue additional clinical studies of Remo for type 1 diabetes. We were organized under the laws of the State of North Carolina on August 26, 2009. Our principal executive offices are located in Raleigh, North Carolina.
Limited Liability Company
The debts, obligations, and liabilities of the Company, whether arising in contracts, tort or otherwise, shall be solely the debts, obligations, and liabilities of the Company, and the members of the Company shall not be obligated personally for any such debts, obligations, or liabilities of the Company solely by reason of being members of the Company. Except as otherwise provided by the North Carolina Limited Liability Company Act, the Company will continue as a limited liability company until such time as the managing members decides the advantages of the limited liability company structure are no more.
| Development Stage Company |
As of April 30, 2014, we have devoted substantially all of our efforts to product development, raising capital and building infrastructure and have not realized revenues from any planned principal operations. Accordingly, we are considered to be in the development stage.
Basis of Accounting
The accompanying financial statements have been prepared in accordance with generally accepted accounting principles in the United States (“GAAP”).
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the accompanying financial statements. Significant estimates made in preparing these financial statements relate to debt and other contingencies. Actual results could differ from those estimates.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Notes to the Financial Statements
Note 1 – Description of Business and Summary of Significant Accounting Policies
(continued)
Cash
Cash consists of cash and short-term investments with original maturities of 90 days or less.
Concentration of Credit Risk
Financial instruments that potentially subject us to a concentration of credit risk principally consist of cash. We maintain our cash balances at what we believe are high credit-quality financial institutions. At times, balances at one financial institution may exceed federally insured limits of $250,000.
Research and Development Costs
We expense research and development costs as incurred. Research and development expenses are comprised of costs incurred in performing research and development activities, including professional service costs, contracted services, license agreements and other outside costs.
Grant Income
Grant income stems from the Company’s participation in local, state incentive programs and is recognized as other income. Grant income, employee tax credits from the State of North Carolina and incentive income are all recorded when received. There was a grant payment received in the amount of $236,350 for the year ended April 30, 2011. No other grant income has been received for any of the other years.
Income Taxes
The income or loss of the Company for federal income tax reporting purposes is includable in the income tax returns of the Members. In general, no recognition has been given to income taxes in the accompanying financial statements.
The Company reviews and evaluates tax positions within its major jurisdictions and determines whether or not there are uncertain tax positions that require financial statement recognition. The Company recognizes uncertain tax positions if it is more likely than not to be sustained upon examination by the applicable taxing authority, including the resolution of any related appeals or litigation processes, based on technical merits of the position. Tax benefits are measured as the largest amount of benefit that is greater than fifty percent likely of being realized upon ultimate settlement, which could result in the Company recording a tax liability that would reduce members’ equity.
As of April 30, 2014, no reserves for uncertain tax positions were required to have been recorded for uncertainty in income taxes for any of the Company’s open tax years. The Company is not subject to examination by U.S. federal and state tax authorities for the tax years before 2011. The Company’s policy is to recognize interest and penalties on unrecognized tax benefits in income tax expense within the statement of operations.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Notes to the Financial Statements
Note 1 – Description of Business and Summary of Significant Accounting Policies
(continued)
Going Concern
We have incurred a net loss of $125,520 for the year ended April 30, 2014, and have a loss accumulated during the development stage totaling $511,558. Our operations to date have been limited to organizing and staffing our company, business planning, raising capital and acquiring and developing our pipeline. To date, we have not generated any revenues and have financed our operations primarily with grants and by borrowings through our promissory notes. Since inception, the Company has generated recurring operating losses. These losses have resulted principally from costs incurred in connection with clinical trials, consulting fees and general and administrative expenses, including planning for the clinical trials of BHV091009. We expect to continue to incur operating losses for the next several years as we pursue the clinical development and commercialization of BHV091009 and work to develop additional product candidates through our research and development programs. As a result, we will seek to fund our operations through public or private equity or debt financings or other sources, such as collaborations and government contracts. Our failure to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategies. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Fair Value of Financial Instruments
The carrying value of our cash, accounts payable and accrued expenses approximates fair value primarily because of the short maturities of these instruments.
Recent Accounting Pronouncements
In June 2014, the FASB issued ASU No. 2014-10, Development Stage Entities, or ASU No. 2014-10, which eliminated certain financial reporting requirements of companies previously identified as development stage entities (Topic 915). The amendments in this ASU simplify accounting guidance by removing all incremental financial reporting requirements for development stage entities. The amendments also reduce data maintenance and, for those entities subject to audit, audit costs by eliminating the requirement for development stage entities to present inception-to-date information in the statements of income, cash flows, and stockholder equity. For public entities, these amendments begin to be effective for periods after December 31, 2014. Early application of each of the amendments is permitted for any annual reporting period or interim period for which the entity’s financial statements have not yet been issued (public business entities) or made available for issuance (other entities). Upon adoption, entities will no longer present or disclose any information required by Topic 915. We will adopt this standard in future presentations. The financial impact on the Company is expected to be negligible.
In August 2014, the FASB issued Accounting Standards Update, or ASU, No. 2014-15 Presentation of Financial Statements – Going Concern, or ASU No. 2014-15, which provides guidance on how management will evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date the financial statements are issued. ASU No. 2014-15 will become effective in 2016, although early adoption is allowed. We are currently evaluating this guidance and expect the financial impact on the Company to be negligible.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Notes to the Financial Statements
Note 2 – Accrued Expenses
Accrued expenses consisted of the following:
| | | April 30, 2014 | | | April 30, 2013 | |
| | | | | | | |
| Accrued interest on notes payable | | $ | 6,949 | | | $ | 25,580 | |
| Accrued liabilities | | | 151,618 | | | | 44,906 | |
| Total accrued expenses | | $ | 158,567 | | | $ | 70,486 | |
Note 3 – Notes Payable
In January 2010, we issued an unsecured promissory note agreement with the North Carolina Biotechnology Center (the “Center”), an entity primarily funded by the State of North Carolina, to borrow $28,407. The original note was due January 2013 and had an interest rate of 4.25%. The note was renegotiated and the unpaid principal and accrued interest of $31,554 was extended through a new note to January 11, 2014. The new note bears interest at a rate of 4.25% per annum, compounded annually. The Center typically provides these loans to early stage biotechnology companies across the state to support company inception, research and growth and define the scope of work necessary for the Company to complete.
In November 2010, we issued a second unsecured promissory note agreement with the Center to borrow $250,000. The original note was due November 2013 and had an interest rate of 4.25%. The note was renegotiated and the unpaid principal and accrued interest of $277,836 was extended through a new note to January 15, 2015. The new note bears interest at a rate of 4.25% per annum, compounds annually and requires annual payments of $125,000 at January 15, 2014 and 2015. No payments on this note have been made to-date. In conjunction with the second note, we issued the Center a warrant to purchase 12,500 units of the Company exercisable at any time. The unit exercise price is $5 per share and the warrant expires November 15, 2020. The fair value of the warrant was allocated from the gross proceeds of the note with the respective discount amortized to interest expense over the original term of the note using the effective interest method. The warrant was valued at $20,000 using the Black-Scholes option pricing model and was treated as debt discount on the note payable. The valuation of the warrant was performed using inputs requiring us to make assumptions about the Company when issued. Alternative probabilities would have resulted in increases or decreases in the value of the conversion feature. We amortized $3,611 and $6,667 of the discount to interest expense during the years ended April 30, 2014 and 2013, respectively and $20,000 from August 26, 2009 (inception) through April 30, 2014.
BRIGHTHAVEN VENTURES, LLC d/b/a BHV PHARMA
(A Development Stage Company)
Notes to the Financial Statements
Note 4 – Commitments and Contingencies
License Agreement
In October 2009 and as amended from time to time, we entered into an “Option Agreement” with Kissei Pharmaceuticals Co., Ltd., a Japanese pharmaceutical company, (“Kissei”), to license KGT-1650 (remogliflozin) and KGT-1681 (remogliflozin etabonate). The Option Agreement was to be commensurate and in conjunction with a license agreement, which was signed in December 2010 (see below).
In February 2014, we amended the Option Agreement to pay $733,000 of a previously agreed research and development expense to Kissei in two installments: the first installment of $200,000 due within a year of a proposed agreement between the Company and another entity becoming effective; and the second of $533,000 due at the “End-of-Phase II Meeting” with the U.S. Food and Drug Administration (“FDA”), as defined.
As noted previously, in December 2010 and as amended from time to time, we entered into a world-wide exclusive license agreement for the development and commercialization of KGT-1650 and KGT-1681 and such other compounds included in the portfolio with Kissei. The agreement covers the development and commercialization of KGT-1650 and KGT-1681 globally, other than in Japan, Korea and Taiwan for the treatment, palliation or prevention of human disease.
Under this license agreement, the Company could be required to pay up to a total of $25 million upon achieving certain milestones, including the dosing of the first patient in the first phase III clinical trial, the submission of regulatory applications and certain regulatory approvals, in addition to royalties payable on commercial sales, if any occur. From inception through April 30, 2014, we have not paid or accrued for any such payments resulting from the license agreement and no milestones have been reached.
Note 5 – Subsequent Events
On September 30, 2014, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Islet Sciences, Inc. and its soon to be parent, Avogenx, Inc.(”Avogenx”). Pursuant to the Merger Agreement, Avogenx will acquire all of the outstanding membership interests of the Company in consideration for the issuance of an aggregate of 30 million shares of common stock of Avogenx (subject to adjustment pursuant to the Merger Agreement), and additional milestone payments in the form of additional shares of common stock of Avogenx in an aggregate amount of up to $71 million within five business days of the occurrence of certain milestones which are based on the development of our main compound, Remo.
In August 2014, we granted an exclusive license to LIBBS North America, LLC (“LIBBS”), a wholly-owned subsidiary of LIBBS Farmacêutica Ltda. of Brazil, for the development and ccommercialization of Remo for all human therapeutic, palliative, and prophylactic uses in certain countries in Latin America, including Brazil. In exchange for these rights, LIBBS is required to make a payment to us upon our submission of the study protocol for our currently-planned phase IIb study of Remo for type 2 diabetes to the FDA and approval of the protocol by the responsible Institutional Review Board, and to make profit-sharing and royalty payments to us based on sales of Remo in LIBBS’s exclusive territory. LIBBS also agreed to pay for the cost of our currently-planned phase IIb study of Remo for type 2 diabetes, up to an agreed maximum amount. LIBBS is required to use commercially reasonable efforts to develop and commercialize Remo in LIBBS’s exclusive territory at LIBBS’s cost.
We evaluated events subsequent to April 30, 2014 to assess the need for potential recognition or disclosure. Such events were evaluated through November 19, 2014, the date the financials were available to be issued.
ANNEX A
AGREEMENT AND PLAN OF MERGER
dated as of
September 30, 2014
among
ISLET SCIENCES, INC.,
BRIGHTHAVEN VENTURES, L.L.C.,
AVOGENX, INC.,
ISLET MERGER SUB, INC.,
and
THE MEMBERS OF BRIGHTHAVEN VENTURES, L.L.C.
TABLE OF CONTENTS
| ARTICLE I DEFINITIONS | | 4 |
| ARTICLE II THE ACQUISITIONS | | 9 |
| | 2.1 | Organization of Holdco. | | 9 |
| | 2.2 | Organization of Islet Merger Sub. | | 9 |
| | 2.3 | The Acquisitions. | | 9 |
| | 2.4 | Effective Time of the Acquisitions. | | 9 |
| | 2.5 | Closing. | | 9 |
| | 2.6 | Charters and Bylaws of the Surviving Corporations and Holdco. | | 9 |
| | 2.7 | Directors. | | 10 |
| | 2.8 | Officers. | | 10 |
| ARTICLE III EFFECTS OF THE ACQUISITIONS | | 10 |
| | 3.1 | Conversion of Islet Securities. | | 10 |
| | 3.2 | Islet Warrants, Options and Other Stock-Based Awards. | | 10 |
| | 3.3 | Dissenting Shares. | | 11 |
| | 3.4 | Exchange of BHV Membership Interests. | | 11 |
| ARTICLE IV REPRESENTATIONS AND WARRANTIES | | 11 |
| | 4.1 | Representations and Warranties of BHV and BHV Members. | | 11 |
| | 4.2 | Representations and Warranties of BHV Members. | | 14 |
| | 4.3 | Representations and Warranties of Islet, Holdco and Islet Merger Sub. | | 15 |
| ARTICLE V COVENANTS RELATING TO CONDUCT OF BUSINESS | | 18 |
| | 5.1 | Covenants of BHV. | | 18 |
| | 5.2 | Covenants of Islet. | | 20 |
| | 5.3 | Advice of Changes; Government Filings. | | 21 |
| | 5.4 | Control of Other Party’s Business. | | 21 |
| ARTICLE VI ADDITIONAL AGREEMENTS | | 21 |
| | 6.1 | Preparation of Consent Solicitation Statement/Prospectus/Form S-4; Islet Lock-Up Agreements. | | 21 |
| | 6.2 | Access to Information; Confidentiality. | | 22 |
| | 6.3 | Reasonable Best Efforts. | | 22 |
| | 6.4 | Acquisition Proposals. | | 23 |
| | 6.5 | OTCQB Quotation. | | 23 |
| | 6.6 | Section 16 Matters. | | 23 |
| | 6.7 | Fees and Expenses; Taxes. | | 23 |
| | 6.8 | Governance. | | 23 |
| | 6.9 | Indemnification. | | 24 |
| | 6.1 | Public Announcements. | | 24 |
| | 6.11 | Milestones. | | 24 |
| | 6.12 | Anti-dilution. | | 26 |
| | 6.13 | Additional Agreements. | | 26 |
| ARTICLE VII CONDITIONS PRECEDENT | | 26 |
| | 7.1 | Conditions to Each Party’s Obligation to Effect the Merger. | | 26 |
| | 7.2 | Conditions to Obligations of Islet. | | 27 |
| | 7.3 | Conditions to Obligations of BHV. | | 28 |
| ARTICLE VIII TERMINATION AND AMENDMENT | | 28 |
| | 8.1 | Termination. | | 28 |
| | 8.2 | Effect of Termination. | | 28 |
| | 8.3 | Amendment. | | 28 |
| | 8.4 | Extension; Waiver. | | 29 |
| ARTICLE IX GENERAL PROVISIONS | | 29 |
| | 9.1 | Non-survival of Representations, Warranties and Agreements. | | 29 |
| | 9.2 | Notices. | | 29 |
| | 9.3 | Interpretation. | | 30 |
| | 9.4 | Counterparts. | | 30 |
| | 9.5 | Entire Agreement; No Third Party Beneficiaries. | | 31 |
| | 9.6 | Governing Law. | | 31 |
| | 9.7 | Severability. | | 31 |
| | 9.8 | Assignment. | | 31 |
| | 9.9 | Submission to Jurisdiction. | | 31 |
| | 9.10 | Prior Company Counsel; Non Transfer of Attorney-Client Privilege. | | 31 |
| | 9.11 | Enforcement. | | 32 |
| | 9.12 | WAIVER OF JURY TRIAL. | | 32 |
| Exhibit A | Form of Avogenx, Inc. Certificate of Incorporation |
| Exhibit B | Form of Avogenx, Inc. By-laws |
| Exhibit C | Form of Investor Rights Agreement |
| Exhibit D | Form of Membership Interest Assignment Agreement |
| | |
| Schedule 2.3(b) | Allocation Schedule |
| Schedule 6.11(a) | Milestone Payment Schedule |
AGREEMENT AND PLAN OF MERGER
AGREEMENT AND PLAN OF MERGER dated as of September 30, 2014 (this “Agreement”) is by and among Islet Sciences, Inc., a Nevada corporation (“Islet”), Brighthaven Ventures, L.L.C., a North Carolina limited liability company (“BHV”), Avogenx, Inc., a Delaware corporation and a direct wholly owned Subsidiary of Islet (“Holdco”), Islet Merger Sub, Inc., a Nevada corporation and a direct wholly owned Subsidiary of Holdco (“Islet Merger Sub”), and each of the members of BHV (the “BHV Members”).
WHEREAS, (i) each of Islet, Holdco and Islet Merger Sub desire, following the satisfaction or waiver of the conditions set forth in ARTICLE VII, to effect the Islet Merger upon the terms and conditions set forth in this Agreement whereby Islet Merger Sub shall be merged with and into Islet, with Islet as the surviving entity in the Islet Merger and Islet Surviving Corporation becoming a wholly owned Subsidiary of Holdco, and each share of Islet Common Stock issued and outstanding immediately prior to the Initial Effective Time shall be converted into the right to receive shares of validly issued, fully paid and non-assessable shares of Holdco Common Stock at the conversion rate of between three (3) and forty (40) shares of Islet Common Stock for one (1) share of Holdco Common Stock, and (ii) immediately following consummation of the Islet Merger, each of BHV and Holdco desire, following the satisfaction or waiver of the conditions set forth in ARTICLE VII, to effect the BHV Securities Exchange upon the terms and conditions set forth in this Agreement, whereby the BHV Members shall sell, transfer, convey, assign and deliver to Holdco 100% of the membership interests of BHV (the “BHV Membership Interests”) in exchange for shares of Holdco Common Stock, and BHV becoming a wholly owned Subsidiary of Holdco;
WHEREAS, the Board of Directors of each of Islet and Holdco has approved, and deems it advisable and in the best interests of their respective shareholders to consummate, the Islet Merger and BHV Securities Exchange;
WHEREAS, Islet will seek the affirmative consent of a majority of the outstanding shares of Islet Common Stock to approve this Agreement (the “Required Islet Consent”);
WHEREAS, the BHV Members own 100% of the BHV Membership Interests and have approved the BHV Securities Exchange;
WHEREAS, Islet, Holdco, Islet Merger Sub, BHV and the BHV Members desire to make certain representations, warranties and agreements in connection with the Acquisitions and also to prescribe various conditions to the Acquisitions; and
WHEREAS, it is intended that, for United States federal income tax purposes, the exchange of BHV Membership Interests and Islet Common Stock for Holdco Common Stock pursuant to the Acquisitions shall qualify as an exchange described in Section 351 of the Code and the Treasury Regulations promulgated thereunder.
NOW, THEREFORE, in consideration of the foregoing and the respective representations, warranties, covenants and agreements set forth herein, the parties agree as follows:
ARTICLE I
DEFINITIONS
Whenever used in this Agreement, unless otherwise clearly indicated by the context, the terms defined below shall have the following meanings:
“Acquisition Proposal” shall have the meaning set forth in Section 6.4.
“Acquisitions” shall have the meaning set forth in Section 2.3(b).
“Agreement” shall have the meaning set forth in the preamble.
“Anti-dilution Shares” shall have the meaning set forth in Section 6.12.
“BHV” shall have the meaning set forth in the preamble.
“BHV Articles of Organization” shall have the meaning set forth in Section 4.1(a).
“BHV Contracts” shall have the meaning set forth in Section 4.1(i).
“BHV Disclosure Letter” shall have the meaning set forth in Section 4.1.
“BHV Indemnified Parties” shall have the meaning set forth in Section 6.9(a).
“BHV Intellectual Property” shall have the meaning set forth in Section 4.1(o).
“BHV Members” shall have the meaning set forth in the preamble.
“BHV Membership Interests” shall have the meaning set forth in the recitals.
“BHV Operating Agreement” shall have the meaning set forth in Section 4.1(a).
“BHV Permits” shall have the meaning set forth in Section 4.1(f).
“BHV Securities Exchange” shall have the meaning set forth in Section 2.3(b).
“BHV Warrant” means that certain warrant to purchase BHV Membership Interests, dated November 16, 2010, as such warrant may be amended, modified or replaced, including any termination agreement in connection therewith.
“BHV Warrant Payment” shall have the meaning set forth in Section 6.11(h).
“BHV Warrant Period” shall have the meaning set forth in Section 6.11(h).
“BHV Warrant Shares” shall have the meaning set forth in Section 6.11(h).
“Closing” shall have the meaning set forth in Section 2.5.
“Closing Date” shall have the meaning set forth in Section 2.5.
“Code” shall mean the Internal Revenue Code of 1986, as amended.
“Commercially Reasonable Efforts” means such efforts and resources that a reasonable pharmaceutical development company would use to develop other compounds and products having comparable commercial potential, stage of development, medical/scientific, technical and regulatory profile, and intellectual property protection. For the avoidance of doubt and solely for purposes of this definition, in no event shall Holdco or its Affiliates take any action or omit to take any action which action or omission is intended to materially hinder or delay the achievement of any Milestone.
“Compound” means KGT-1650 (remogliflozin) and/or KGT-1681 (remogliflozin etabonate).
“Compound Change of Control” means any transaction (including a sale of assets, merger, sale of stock or other equity interests) pursuant to which the Kissei Agreement or any right thereunder, or any portion of the BHV Intellectual Property or know-how or trade secrets of BHV, is sold to or acquired by, directly or indirectly, any person other than Holdco or its Affiliates.
“Confidentiality Agreement” shall have the meaning set forth in Section 6.2.
“Consent Solicitation Statement/Prospectus” shall have the meaning set forth in Section 6.1(a).
“Conversion Rate” shall have the meaning set forth in Section 3.1(a).
“Dilutive Issuance” shall have the meaning set forth in Section 6.12.
“Dissenting Shares” shall have the meaning set forth in Section 3.3.
“Effective Time” shall have the meaning set forth in Section 2.4(b).
“Employee Benefit Plan” shall mean any employee benefit plan, program, policy, practice, or other arrangement providing benefits to any current or former employee, officer or director of the entity in question or any beneficiary or dependent thereof that is sponsored or maintained by the entity in question or to which such entity contributes or is obligated to contribute, whether or not written, including any employee welfare benefit plan within the meaning of Section 3(1) of ERISA, any employee pension benefit plan within the meaning of Section 3(2) of ERISA (whether or not such plan is subject to ERISA) and any bonus, incentive, deferred compensation, vacation, insurance, stock purchase, stock option, equity award, severance, employment, change of control or fringe benefit plan, program or agreement.
“Employment Agreement” shall mean a contract, offer letter or agreement of the entity in question with or addressed to any individual who is rendering or has rendered services thereto as an employee, director or consultant pursuant to which such entity has any actual or contingent liability or obligation to provide compensation and/or benefits in consideration for past, present or future services.
“ERISA” shall mean the Employee Retirement Income Security Act of 1974, as amended, and the regulations promulgated thereunder.
“ERISA Affiliate” shall mean, with respect to any entity, trade or business, any other entity, trade or business that is a member of a group described in Section 414(b), (c), (m) or (o) of the Code or Section 4001(b)(1) of ERISA that includes the first entity, trade or business, or that is a member of the same “controlled group” as the first entity, trade or business pursuant to Section 4001(a)(14) of ERISA.
“EU” means the European Union, as it may be constituted from time to time.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Field” means the treatment, palliation or prevention of human disease, including but not limited to diabetes in human beings.
“Form S-4” shall have the meaning set forth in Section 6.1(a).
“GAAP” shall mean generally accepted accounting principles in the United States.
“Governmental Entity” shall mean any court, administrative agency or commission or other governmental authority or instrumentality, domestic or foreign, or self-regulatory organization.
“Holdco” shall have the meaning set forth in the preamble.
“Holdco Common Stock” shall mean shares of Holdco common stock, par value $0.001 per share.
“Indemnified Parties” shall have the meaning set forth in Section 6.9(b).
“Infringe” shall have the meaning set forth in Section 4.1(o).
“Initial Effective Time” shall have the meaning set forth in Section 2.4(a).
“Injunction” shall have the meaning set forth in Section 7.1(f).
“Insiders” shall have the meaning set forth in Section 6.6.
“Islet” shall have the meaning set forth in the preamble.
“Islet Board Approval” shall have the meaning set forth in Section 4.3(n).
“Islet By-laws” shall have the meaning set forth in Section 2.6(a).
“Islet Certificate of Merger” shall have the meaning set forth in Section 2.4(a).
“Islet Charter” shall have the meaning set forth in Section 2.6(a).
“Islet Common Stock” shall have the meaning set forth in Section 3.1(a).
“Islet Contracts” shall have the meaning set forth in Section 4.3(j).
“Islet Disclosure Letter” shall have the meaning set forth in Section 4.3.
“Islet Indemnified Parties” shall have the meaning set forth in Section 6.9(b).
“Islet Merger” shall have the meaning set forth in Section 2.3(a).
“Islet Merger Sub” shall have the meaning set forth in the preamble.
“Islet Permits” shall have the meaning set forth in Section 4.3(g).
“Islet SEC Documents” shall have the meaning set forth in Section 4.3(d).
“Islet Stock Option” shall have the meaning set forth in Section 3.2(a).
“Islet Surviving Corporation” shall have the meaning set forth in Section 2.3(a).
“Kissei” means Kissei Pharmaceutical Co., Ltd.
“Kissei Agreement” means the Exclusive License Agreement between BHV and Kissei dated December 1, 2010, as amended.
“Liens” shall have the meaning set forth in Section 4.3(b)(ii).
“Material Adverse Effect” shall mean, with respect to any party hereto, any result, occurrence, fact, change, event or effect that has a material adverse effect on the financial condition, businesses or results of operations of such party and its Subsidiaries taken as a whole; provided that, for purposes of this Agreement the following shall not be deemed to have a “material adverse effect”: any change or event caused by or resulting from (A) changes in prevailing economic or market conditions or the securities, credit or financial markets in the United States or elsewhere (except to the extent any such change has a materially disproportionate effect on such entity and its Subsidiaries relative to other similarly situated participants in the industries in which they operate), (B) changes or events affecting the industries in which the affected party operates (except to the extent any such change or event has a materially disproportionate effect on such entity and its Subsidiaries relative to other similarly situated participants in the industries in which they operate), (C) changes in GAAP, or other similar accounting requirements in foreign countries, applicable to such affected party and its Subsidiaries, and which are not specific to the affected party, (D) changes, after the date hereof, in laws, rules or regulations of general applicability or interpretations thereof by any Governmental Entity, (E) the announcement of this Agreement or the transactions contemplated by this Agreement, or (F) any weather-related or other force majeure event.
“Milestone” shall have the meaning set forth in Section 6.11(a).
“Milestone Adjustment Amount” shall have the meaning set forth in Section 6.11(h).
“Milestone Shares” shall have the meaning set forth in Section 6.11(a).
“Multiemployer Plan” shall mean any “multiemployer plan” within the meaning of Section 4001(a)(3) of ERISA.
“NCLLCA” shall mean the North Carolina Limited Liability Company Act.
“NRS” shall mean the Nevada Revised Statutes.
“Outside Date” shall have the meaning set forth in Section 8.1(d).
“Patent” means all patents and patent applications, including provisional and priority filings.
“Prior BHV Counsel” shall have the meaning set forth in Section 9.10(a).
“Proceedings” shall have the meaning set forth in Section 4.1(h)(iv).
“Required Islet Consent” shall have the meaning set forth in the recitals.
“Requisite Regulatory Approvals” shall have the meaning set forth in Section 7.1(c).
“RSUs” shall have the meaning set forth in Section 3.2(b).
“SEC” shall mean the Securities and Exchange Commission.
“Section 16 Information” shall have the meaning set forth in Section 6.6.
“Securities Act” shall mean the Securities Act of 1933, as amended.
“Significant Subsidiary” shall mean any Subsidiary of a person that constitutes a Significant Subsidiary of such person within the meaning of Rule 1-02 of Regulation S-X of the SEC.
“Subsidiary” shall mean with respect to any person, any corporation, partnership, trust, limited liability company or other non-corporate business enterprise in which such person (or another Subsidiary of such person) holds stock or other ownership interests representing (A) more that 50% of the voting power of all outstanding stock or ownership interests of such entity or (B) the right to receive more than 50% of the net assets of such entity available for distribution to the holders of outstanding stock or ownership interests upon a liquidation or dissolution of such entity.
“Tax” or “Taxes” shall mean all federal, state, local, and foreign income, excise, gross receipts, ad valorem, profits, gains, property, capital, sales, transfer, use, license, payroll, employment, social security, severance, unemployment, withholding, duties, excise, windfall profits, intangibles, franchise, backup withholding, value added, alternative or add-on minimum, estimated and other taxes, charges, levies or like assessments imposed by any Governmental Entity together with all penalties and additions to tax and interest thereon.
“Tax Return” shall mean any return, declaration, report, claim for refund, or information return or statement relating to Taxes, including any schedule or attachment thereto, and including any amendment thereof, supplied to, or required to be supplied to, a Governmental Entity.
“Territory” means all countries of the world, except for Japan, Korea, and Taiwan.
“Violation” shall have the meaning set forth in Section 4.1(c)(ii).
ARTICLE II
THE ACQUISITIONS
| 2.1 | Organization of Holdco. Islet has caused Holdco to be organized under the laws of the State of Delaware and owns all of the capital stock of Holdco. |
| 2.2 | Organization of Islet Merger Sub. Islet has caused Holdco to organize, and Holdco has organized, Islet Merger Sub under the laws of the State of Nevada and Holdco owns all of the capital stock of Islet Merger Sub. |
| (a) | At the Initial Effective Time, Islet Merger Sub shall be merged with and into Islet (the “Islet Merger”). Islet will be the surviving corporation in the Islet Merger (the “Islet Surviving Corporation”), and the separate existence of Islet Merger Sub shall cease. As a result of the Islet Merger, Islet shall become a wholly owned Subsidiary of Holdco. From and after the Initial Effective Time, the Islet Surviving Corporation shall possess all the rights, powers, privileges and franchises and be subject to all of the obligations, liabilities and duties of Islet and Islet Merger Sub, all as provided under the NRS. |
| (b) | At the Effective Time, the BHV Members shall sell, transfer, convey, assign and deliver to Holdco all of the BHV Membership Interests free and clear of all Liens in exchange for an aggregate of Thirty Million (30,000,000) shares of Holdco Common Stock (as adjusted for the Conversion Rate) and the right to receive additional shares of Holdco Common Stock pursuant to Section 6.11 and Section 6.12 of this Agreement. All shares of Holdco Common Stock issued to the BHV Members pursuant to this Agreement shall be issued and delivered based on the percentages set forth on Schedule 2.3(b) (the “BHV Securities Exchange” and together with the Islet Merger, the “Acquisitions”). As a result of the BHV Securities Exchange, BHV shall become a wholly owned Subsidiary of Holdco. |
| 2.4 | Effective Time of the Acquisitions. Subject to the provisions of this Agreement, on the Closing Date, the parties shall (and shall cause their Subsidiaries to) cause the following to occur: |
| (a) | Islet Merger Sub and Islet shall execute and deliver for filing a certificate of merger (the “Islet Certificate of Merger”) to the Secretary of State of the State of Nevada, in such form and manner provided in the NRS, and shall make all other filings or recordings required under the NRS to effect the Islet Merger. The Islet Merger shall become effective upon the filing with the Secretary of State of the State of Nevada of the Islet Certificate of Merger (such time of filing, the “Initial Effective Time”). |
| (b) | Immediately following the Initial Effective Time, BHV shall make all filings or recordings required under the NCLLCA, if any, to effect the BHV Securities Exchange. The BHV Securities Exchange shall become effective immediately following the Initial Effective Time (such time, the “Effective Time”). |
| 2.5 | Closing. The closing of the Acquisitions (the “Closing”) will take place at 10:00 a.m., New York City time, on the date (the “Closing Date”) that is the second business day after the satisfaction or waiver (subject to applicable law) of the conditions set forth in ARTICLE VII (excluding conditions that, by their terms, are to be satisfied on the Closing Date, but subject to the satisfaction or waiver of such conditions), unless another time or date is agreed to in writing. The Closing shall be held at such place or places as may be designated by Islet, unless another place is agreed to in writing. |
| 2.6 | Charters and Bylaws of the Surviving Corporations and Holdco. |
| (a) | At the Initial Effective Time, the Articles of Incorporation, as amended, of Islet (the “Islet Charter”) and By-laws, as amended, of Islet (the “Islet By-laws”) shall be amended so as to read in their entirety as the certificate of incorporation and by-laws of Islet Merger Sub as in effect immediately prior to the Initial Effective Time, except for the incorporator and except that the Islet Surviving Corporation shall be named “Islet Sciences, Inc.” |
| (b) | The certificate of incorporation and by-laws of Holdco immediately following the Initial Effective Time shall be in the form set forth in Exhibit A and Exhibit B, respectively. |
| 2.7 | Directors. The directors of Islet Merger Sub at the Initial Effective Time shall be the initial directors of the Islet Surviving Corporation, each to hold office in accordance with the articles of incorporation and by-laws of the Islet Surviving Corporation until such director’s successor is duly elected and qualified. The directors of Holdco at the Initial Effective Time shall continue to be the directors of Holdco following the Initial Effective Time, each to hold office in accordance with the certificate of incorporation and by-laws of Holdco until such director’s successor is duly elected and qualified. |
| 2.8 | Officers. The officers of Islet Merger Sub at the Initial Effective Time shall be the initial officers of the Islet Surviving Corporation, each to hold office in accordance with the articles of incorporation and by-laws of the Islet Surviving Corporation until such officer’s successor is appointed and has qualified, subject to earlier termination by removal or resignation. The officers of Holdco at the Initial Effective Time shall continue to be the officers of Holdco following the Initial Effective Time, each to hold office in accordance with the certificate of incorporation and by-laws of Holdco until such officer’s successor is appointed and has qualified, subject to earlier termination by removal or resignation. |
ARTICLE III
EFFECTS OF THE ACQUISITIONS
| 3.1 | Conversion of Islet Securities. At the Initial Effective Time, by virtue of the Islet Merger and without any action on the part of Holdco, Islet Merger Sub, Islet or the holders of any of the following securities, the following shall occur: |
| (a) | Conversion of Islet Common Stock. Shares of common stock, par value $0.001 per share, of Islet (“Islet Common Stock”) issued and outstanding immediately prior to the Initial Effective Time shall be converted into the right to receive shares of validly issued, fully paid and non-assessable shares of Holdco Common Stock at the conversion rate of not less than three (3) and not more than forty (40) shares of Islet Common Stock for one (1) share of Holdco Common Stock, with the effective number of shares of Islet Common Stock convertible into one (1) share of Holdco Common Stock to be determined by the Board of Directors of Islet (the “Conversion Rate”). No fractional shares of Holdco Common Stock shall be issued pursuant to this Agreement. All fractional shares of Holdco Common Stock that a holder of shares of Islet Common Stock would otherwise be entitled to receive pursuant to the Islet Merger shall be aggregated, and if a fractional share results from such aggregation, such fractional share shall be automatically rounded up to a whole share. |
| (b) | Conversion of Islet Merger Sub Stock. Each share of common stock of Islet Merger Sub issued and outstanding immediately prior to the Initial Effective Time shall be converted into one fully paid and non-assessable share of Islet Common Stock, following which the Islet Surviving Corporation shall become a wholly-owned Subsidiary of Holdco. |
| (c) | Cancellation of Holdco Common Stock Held by Islet. Each share of Holdco Common Stock held by Islet immediately prior to the Initial Effective Time shall be cancelled, and no consideration shall be paid with respect thereto. |
| (d) | Treatment of Islet Certificates and Islet Book-Entry Shares. All of the shares of Islet Common Stock converted into the right to receive Holdco Common Stock pursuant to this Section 3.1 shall cease to be outstanding and shall be cancelled and retired and shall cease to exist and, as of the Initial Effective Time, the holders of Islet Common Stock shall be deemed to have received shares of Holdco Common Stock (without the requirement for the surrender of any certificate previously representing any such shares of Islet Common Stock or issuance of new certificates representing Holdco Common Stock), with each certificate representing shares of Islet Common Stock prior to the Initial Effective Time being deemed to represent shares of Holdco Common Stock and with shares of Islet Common Stock represented by book-entry immediately prior to the Initial Effective Time being deemed to represent automatically shares of Holdco Common Stock, each as adjusted for the Conversion Rate. |
| 3.2 | Islet Warrants, Options and Other Stock-Based Awards. The Board of Directors of Islet or the appropriate committee thereof shall take all action necessary so that: |
| (a) | Each warrant (“Islet Warrant”), option or other right to acquire Islet Common Stock (“Islet Stock Option”) which is outstanding immediately prior to the Initial Effective Time (whether vested or unvested) shall, as of the Initial Effective Time, cease to represent a right to purchase shares of Islet Common Stock and shall instead represent the right to purchase shares of Holdco Common Stock equal to the number of shares of Islet Common Stock subject to such Islet Warrant or Islet Stock Option immediately prior to the Initial Effective Time, as adjusted for the Conversion Rate. The exercise price per share of Holdco Common Stock subject to any such Islet Warrant or Islet Stock Option at and after the Initial Effective Time shall be equal to the exercise price per share of Islet Common Stock subject to such Islet Warrant or Islet Stock Option prior to the Initial Effective Time, as adjusted for the Conversion Rate. |
| (b) | Each restricted stock unit, deferred stock unit or phantom unit in respect of a share of Islet Common Stock (the “RSUs”) which is outstanding (including any RSUs held in participant accounts under any employee benefit or compensation plan or arrangement of Islet) immediately prior to the Initial Effective Time shall, as of the Initial Effective Time, be converted into a number of restricted stock units in respect of shares of Holdco Common Stock, equal to the number of shares underlying RSUs held by the grantee immediately prior to the Initial Effective Time, as adjusted for the Conversion Rate. |
| 3.3 | Dissenting Shares. Notwithstanding any other provision of this Agreement to the contrary, shares of Islet Common Stock that are outstanding immediately prior to the Initial Effective Time and which are held by stockholders that shall not have voted in favor of the Acquisitions or consented thereto in writing and who shall have properly demanded and are entitled to dissenters’ rights for such shares in accordance with Sections 92A.300 to 92A.500 of the NRS (collectively, the “Dissenting Shares”) shall not be converted into shares of Holdco Common Stock. Such stockholders instead shall only be entitled to receive payment of the appraised value of such shares of Islet Common Stock held by them in accordance with the provisions of Sections 92A.300 to 92A.500 of the NRS, except that all Dissenting Shares held by stockholders who shall have failed to perfect or who effectively shall have waived, withdrawn, or otherwise are not entitled to, the dissenters’ right with respect to such shares of Islet Common Stock under Sections 92A.300 to 92A.500 of the NRS shall thereupon be deemed to have been canceled and converted into, as of the Initial Effective Time, shares of Holdco Common Stock in the manner provided in Section 3.1. |
| 3.4 | Exchange of BHV Membership Interests. At the Effective Time, the BHV Membership Interests shall be exchanged for Thirty Million (30,000,000) shares of Holdco Common Stock (as adjusted for the Conversion Rate) and the right to receive additional shares of Holdco Common Stock pursuant to Section 6.11 and Section 6.12 of this Agreement, following which BHV shall become a wholly-owned Subsidiary of Holdco. |
ARTICLE IV
REPRESENTATIONS AND WARRANTIES
| 4.1 | Representations and Warranties of BHV and BHV Members. Except with respect to any subsection of this Section 4.1, as set forth in the correspondingly identified subsection of the disclosure letter delivered by BHV to Islet concurrently herewith (the “BHV Disclosure Letter”) (it being understood by the parties that any information disclosed in one subsection of the BHV Disclosure Letter shall be deemed to be disclosed for purposes of each other subsection of the BHV Disclosure Letter to which the relevance of such information is reasonably apparent), BHV and the BHV Members represent and warrant to Islet as follows: |
| (a) | Organization, Standing and Power. BHV is a limited liability company duly organized, validly existing and, if applicable, in good standing under the laws of its jurisdiction of organization, has all requisite power and authority to own, lease and operate its properties and to carry on its business as now being conducted, and is duly qualified and, if applicable, in good standing to do business in each jurisdiction in which the nature of its business or the ownership or leasing of its properties makes such qualification necessary, in each case, other than as would not, either individually or in the aggregate, reasonably be expected to have a Material Adverse Effect on BHV. The Articles of Organization of BHV (the “BHV Articles of Organization”) and the Operating Agreement of BHV (the “BHV Operating Agreement”), copies of which have been made available to Islet, are true, complete and correct copies of such documents as in effect on the date hereof. |
| (b) | Capital Structure. As of the date of this Agreement, the authorized, issued and outstanding BHV Membership Interests are set forth in the BHV Disclosure Letter. No other BHV Membership Interests are issued, reserved for issuance or outstanding. Except as set forth in the BHV Disclosure Letter, as of the date of this Agreement, there are no options, warrants, rights, convertible or exchangeable securities, “phantom” stock rights, stock appreciation rights, stock-based performance units, commitments, contracts, arrangements or undertakings of any kind to which BHV is a party or by which BHV is bound (i) obligating BHV to issue, deliver or sell, or cause to be issued, delivered or sold, additional BHV Membership Interests or other equity interests in, or any security convertible or exercisable for or exchangeable into any BHV Membership Interests or other equity interest in, BHV, (ii) obligating BHV to issue, grant, extend or enter into any such option, warrant, call, right, security, commitment, contract, arrangement or undertaking or (iii) that give any person the right to receive any economic benefit or right similar to or derived from the economic benefits and rights occurring to holders of the BHV Membership Interests. |
(i)BHV has all requisite limited liability company power and authority to enter into this Agreement and to consummate the transactions contemplated hereby. The execution and delivery of this Agreement and the consummation of the transactions contemplated hereby have been duly authorized by all necessary limited liability company action on the part of BHV. This Agreement has been duly executed and delivered by BHV and constitutes a valid and binding obligation of BHV, enforceable against BHV in accordance with its terms, subject to bankruptcy, insolvency, fraudulent transfer, reorganization, moratorium and similar laws of general applicability relating to or affecting creditors’ rights and to general equitable principles.
(ii)The execution and delivery of this Agreement does not, and the consummation of the transactions contemplated hereby will not, (A) conflict with, or result in any violation of, or constitute a default (with or without notice or lapse of time, or both) under, or give rise to a right of termination, cancellation or acceleration of any obligation or the loss of a material benefit under, or the creation of a Lien (any such conflict, violation, default, right of termination, cancellation or acceleration, loss or creation, a “Violation”) pursuant to, any provision of the BHV Articles of Organization, BHV Operating Agreement or equivalent governing documents of any Significant Subsidiary of BHV, or (B) subject to obtaining or making the consents, approvals, orders, authorizations, registrations, declarations and filings referred to in Section 4.1(c)(iii) below, result in any Violation of any loan or credit agreement, note, mortgage, indenture, lease, or other agreement, obligation, instrument, permit, concession, franchise, license, judgment, order, decree, statute, law, ordinance, rule or regulation applicable to BHV or any Subsidiary of BHV or their respective properties or assets, which Violation, individually or in the aggregate, would reasonably be expected to (A) have a Material Adverse Effect on BHV or (B) prevent, materially delay or materially impede BHV’s ability to perform its obligations hereunder or to consummate the transactions contemplated hereby.
(iii)No consent, approval, order or authorization of, or registration, declaration or filing with any Governmental Entity is required by or with respect to BHV or any Subsidiary of BHV in connection with the execution and delivery of this Agreement by BHV or the consummation by BHV of the transactions contemplated hereby, the failure to make or obtain that, individually or in the aggregate, would reasonably be expected to (x) have a Material Adverse Effect on BHV or (y) prevent, materially delay or materially impede BHV’s ability to perform its obligations hereunder or to consummate the transactions contemplated hereby.
| (d) | Financial Statements. The financial statements of BHV as of and for the year ended April 30, 2014 that have been provided to Islet fairly present in all material respects the financial position of BHV and the results of operations, changes in members’ equity and cash flows of BHV as of and for the year ended April 30, 2014. |
| (e) | Undisclosed Liabilities. Except for (i) those liabilities that are appropriately reflected or reserved for in the audited financial statements of BHV for the fiscal year ended April 30, 2014, (ii) liabilities incurred since April 30, 2014 in the ordinary course of business consistent with past practice, (iii) those liabilities that are disclosed in the BHV Disclosure Letter, (iv) liabilities that, individually or in the aggregate, have not had, and would not reasonably be expected to have, a Material Adverse Effect on BHV, (v) liabilities incurred pursuant to the transactions contemplated by, or permitted by, this Agreement, and (vi) liabilities or obligations discharged or paid in full prior to the date hereof in the ordinary course of business consistent with past practice, BHV does not have, any liabilities or obligations of any nature whatsoever (whether accrued, absolute, contingent or otherwise). |
| (f) | Compliance with Applicable Laws. BHV holds all permits, certificates, licenses, variances, exemptions, orders and approvals of all Governmental Entities that are material to the operation of the businesses of BHV, taken as a whole (the “BHV Permits”), and BHV is and for the two years preceding the date hereof has been in compliance with the terms of the BHV Permits and all applicable laws and regulations, except where the failure so to hold or comply with any such BHV Permits and applicable laws and regulations would not reasonably be expected to have a Material Adverse Effect on BHV. The businesses of BHV are not being, and during the two years preceding the date hereof have not been, conducted in violation of any law, ordinance (including zoning) or regulation of any Governmental Entity, except for violations that, individually or in the aggregate, do not have, and would not reasonably be expected to have, a Material Adverse Effect on BHV. No investigation by any Governmental Entity with respect to BHV is pending or, to the knowledge of BHV, threatened, other than, in each case, those the outcome of which, individually or in the aggregate, would not reasonably be expected to have a Material Adverse Effect on BHV. |
| (g) | Legal Proceedings. There is no claim, suit, action, investigation or other demand or proceeding (whether judicial, arbitral, administrative or other) pending or, to the knowledge of BHV or a BHV Member, threatened, against or affecting BHV, any Subsidiary of BHV or any of their respective assets, rights or properties that would reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect on BHV or could prevent, enjoin, alter or delay any of the transactions contemplated by this Agreement, nor is there any judgment, decree, Injunction, rule or order of any Governmental Entity or arbitrator outstanding against BHV, any Subsidiary of BHV or any of their respective assets, rights or properties having or that would reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect on BHV or on Holdco after the Effective Time, or could prevent, enjoin, alter or delay any of the transactions contemplated by this Agreement. |
| (h) | Taxes. Except as would not reasonably be expected, individually or in the aggregate, to have a Material Adverse Effect on BHV: |
(i)BHV has duly and timely filed (including all applicable extensions) all Tax Returns required to be filed by it on or prior to the date of this Agreement (all such Tax Returns being true, correct and complete in all respects), has timely paid all Taxes shown thereon as arising and has duly and timely paid all Taxes that are due and payable or claimed to be due from it by federal, state, foreign or local taxing authorities other than Taxes that are being contested in good faith, which have not been finally determined;
(ii)BHV has withheld and paid over to the relevant taxing authority all Taxes required to have been withheld and paid in connection with payments to employees, independent contractors, creditors, shareholders or other third parties;
(iii)BHV has withheld and paid over to the relevant taxing authority all sales, use or other Taxes collected with respect to payments received from customers or other third parties;
(iv)BHV has not received written notice of any proposed or threatened proceeding, examination, investigation, audit or administrative or judicial proceeding (“Proceedings”) relating to tax matters against, or with respect to any Taxes of, BHV, and no such Proceedings are currently pending;
(v)No deficiencies for any Taxes have been proposed, asserted or assessed in writing against BHV that have not been finally resolved and paid in full;
(vi)BHV has not granted any extension or waiver of the limitation period applicable to any Tax that remains in effect;
(vii)No claim has been made in writing by any Governmental Entity in a jurisdiction in which BHV or any of its Subsidiaries does not file a Tax Return that BHV is or may be subject to taxation by such jurisdiction;
(viii)There are no Liens for Taxes (other than statutory Liens for Taxes not yet due and payable or the amount or validity of which is being contested in good faith by appropriate Proceedings) upon any of the assets of BHV;
(ix)BHV is not a party to or is bound by any Tax sharing, allocation, or indemnification agreement or arrangement (other than such an agreement or arrangement exclusively between or among BHV and other than customary tax indemnifications contained in credit or similar agreements), under which Holdco could be liable, following the Effective Time, for the Tax liability of another entity;
(x)BHV (A) has not been a member of a group filing a consolidated, combined or unitary Tax Return (other than a group the common parent of which was BHV) or (B) does not have any liability for the Taxes of any person (other than BHV) under Treasury Regulation Section 1.1502-6 (or any similar provision of state, local or foreign law), as a transferee or successor, by contract or otherwise;
(xi)BHV has not been, within the past two years or otherwise as part of a “plan (or series of related transactions)” within the meaning of Section 355(e) of the Code of which the Acquisitions are also a part, a “distributing corporation” or a “controlled corporation” (within the meaning of Section 355(a)(1)(A) of the Code) in a distribution of stock intending to qualify for tax-free treatment under Section 355 of the Code;
(xii)BHV has not participated in a “reportable transaction” within the meaning of Treasury Regulation Section 1.6011-4(b)(1); and
(xiii)As of the date of this Agreement, BHV has not taken or agreed to take any action, and does not know of any fact, agreement, plan or other circumstance, that could reasonably be expected to prevent the exchange of BHV Membership Interests and Islet Common Stock for Holdco Common Stock pursuant to the Acquisitions from qualifying, for United States federal income tax purposes, as an exchange described in Section 351 of the Code and the Treasury Regulations promulgated thereunder.
| (i) | Certain Agreements. Except for this Agreement, or as set forth in the BHV Disclosure Letter, as of the date hereof, BHV is not a party to or bound by any contract, arrangement, commitment or understanding (i) with respect to the employment of any directors or executive officers, (ii) that is a “material contract” (as such term is defined in Item 601(b)(10) of Regulation S-K of the SEC), (iii) that limits the ability of BHV to compete in any line of business, in any geographic area or with any person, or that requires referrals of business and, in each case, which limitation or requirement would reasonably be expected to be material to BHV, (iv) that relates to the sale of products by BHV which have not been performed by BHV and for which a performance obligation that is material to BHV would remain after the Effective Time, (v) in the case of an Employee Benefit Plan of BHV, any of the benefits of which will be increased, or the vesting of the benefits of which will be accelerated, by the occurrence of any of the transactions contemplated by this Agreement, or the value of any of the benefits of which will be calculated on the basis of any of the transactions contemplated by this Agreement, or (vi) that would reasonably be expected to prevent, materially delay or materially impede the consummation of any of the transactions contemplated by this Agreement. All contracts, arrangements, commitments or understandings of the type described in this Section 4.1(i) (collectively referred to herein as the “BHV Contracts”) are valid and in full force and effect, except to the extent they have previously expired in accordance with their terms or if the failure to be in full force and effect, individually or in the aggregate, would not reasonably be expected to have a Material Adverse Effect on BHV. BHV has not, and to the knowledge of BHV, none of the other parties thereto have, violated any provision of, or committed or failed to perform any act, and no event or condition exists, which with or without notice, lapse of time or both would constitute a default under the provisions of, any BHV Contract, except in each case for those violations and defaults that, individually or in the aggregate, would not reasonably be expected to result in a Material Adverse Effect on BHV. |
| (j) | Benefit Plans. None of BHV or its Subsidiaries or any of their respective ERISA Affiliates maintains, contributes to, or has an obligation to contribute to, nor has BHV or its Subsidiaries or any of their respective ERISA Affiliates ever maintained, contributed to, or had an obligation to contribute to, any Employee Benefit Plan, nor has BHV or any of its Subsidiaries or any officer or director of BHV or any of its Subsidiaries taken any action directly or indirectly to obligate BHV or any of its Subsidiaries to institute any such Employee Benefit Plan. |
| (k) | Subsidiaries. Section 4.1(k) of the BHV Disclosure Letter includes all the Subsidiaries of BHV. All of the shares of capital stock or other equity interests of each of the Subsidiaries held by BHV, if any, or by another Subsidiary of BHV are fully paid and non-assessable and are owned by BHV or a Subsidiary of BHV free and clear of any Lien. |
| (l) | Absence of Certain Changes or Events. Since April 30, 2014, (i) BHV has conducted its business in the ordinary course consistent in all material respects with their past practices and (ii) there has not been any change, circumstance or event that has had, or would reasonably be expected to have, a Material Adverse Effect on BHV. |
| (m) | BHV Approval. BHV and the BHV Members have taken all necessary steps to approve this Agreement and the transactions contemplated hereby. To the knowledge of BHV, no “moratorium,” “control share,” “fair price” or other anti-takeover law or regulation is applicable to this Agreement, the BHV Securities Exchange, or the other transactions contemplated hereby. |
| (n) | Properties. BHV (i) has good and valid title to all its properties and assets that are material to its business, free and clear of all Liens, except (1) statutory Liens securing payments not yet due, and (2) such imperfections or irregularities of title, claims, easements, covenants and other Liens or restrictions as do not affect in any material respect the current use of the properties or assets subject thereto or affected thereby or otherwise impair in any material respect the business operations at such properties, and (ii) is the lessee of all leasehold estates that are material to its business (except for leases that have expired by their terms since the date thereof or been assigned, terminated or otherwise disposed of in the ordinary course of business consistent with past practice) and is in possession of the properties purported to be leased thereunder, and each such lease is valid without any material default thereunder by the lessee or, to BHV’s knowledge, the lessor. |
| (o) | BHV Intellectual Property. Set forth in the BHV Disclosure Letter are all Patents and registered or applied-for copyrights, trademarks, service marks, domain names and trade names owned or licensed by BHV as of the date hereof (including the registration numbers of any registrations or the serial numbers of any applications for registration of any of the foregoing), and an indication of which are owned or licensed by BHV (collectively, the “BHV Intellectual Property”). Except as set forth in the BHV Disclosure Letter or as would not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect on BHV, (i) BHV owns free and clear of all Liens, or has a valid license to use, all BHV Intellectual Property necessary to carry on its business as currently conducted and to make, use and sell a product containing the Compound in the Field in the Territory, (ii) the BHV Intellectual Property does not, to the knowledge of BHV, infringe, misappropriate or otherwise violate (“Infringe”) the intellectual property rights of third parties and is not, to the knowledge of BHV, being Infringed by any third parties, (iii) to the knowledge of BHV, the BHV Intellectual Property that is registered, and BHV’s rights in the BHV Intellectual Property, are not invalid or unenforceable, (iv) BHV has taken reasonable actions to protect and maintain the BHV Intellectual Property, including BHV Intellectual Property that is confidential in nature, and (v) there are no claims, suits or other actions, and to the knowledge of BHV, no claim, suit or other action is threatened in writing, that seek to limit or challenge the validity, enforceability, ownership, or right to use, sell or license the BHV Intellectual Property. The representations and set forth in the foregoing clauses (ii) and (v) are the only representations and warranties of BHV regarding infringement, misappropriation or violation of intellectual property rights, and no other representations or warranties in this Section 4.1 shall be deemed to apply to such matters. |
| (p) | Kissei Agreement. The Kissei Agreement is valid and in full force and effect. Neither BHV nor any affiliate of BHV, and to the knowledge of BHV, none of the other parties thereto have, violated any provision of, or committed or failed to perform any act, and no event or condition exists, which with or without notice, lapse of time or both would constitute a default under the provisions of, the Kissei Agreement, except in each case for those violations and defaults that, individually or in the aggregate, would not reasonably be expected to result in a Material Adverse Effect on BHV or which have been cured. |
| (q) | Information Supplied. None of the information supplied or to be supplied by BHV for inclusion or incorporation by reference in (i) the Form S-4 will, at the time the Form S-4 is filed with the SEC and at the time it becomes effective under the Securities Act, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading and (ii) the Consent Solicitation Statement/Prospectus will, at the date of mailing to Islet shareholders, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. No representation or warranty is made by BHV with respect to statements made or incorporated by reference therein based on information supplied by Islet for inclusion or incorporation by reference in the Form S-4 and Consent Solicitation Statement/Prospectus. |
| (r) | No Other Representations or Warranties. Except for the representations and warranties made by BHV in this Section 4.1, neither BHV, the BHV Members, nor any other person, makes any express or implied representation or warranty with respect to BHV, BHV’s Subsidiaries, or their respective businesses, operations, assets, liabilities, conditions (financial or otherwise) or prospects, and BHV and the BHV Members hereby disclaim any such other representations or warranties. |
| 4.2 | Representations and Warranties of BHV Members. Except with respect to any subsection of this Section 4.2, as set forth in the correspondingly identified subsection of the BHV Disclosure Letter (it being understood by the parties that any information disclosed in one subsection of the BHV Disclosure Letter shall be deemed to be disclosed for purposes of each other subsection of the BHV Disclosure Letter to which the relevance of such information is reasonably apparent), each BHV Member, severally and not jointly, represents and warrants to Islet, with respect to such BHV Member, as follows: |
| (a) | Related-Party Transactions. Except for passive ownership of less than five percent (5%) of the outstanding stock of any publicly traded entity, such BHV Member does not own, directly or indirectly, any interest in, and is not an officer, director, employee or consultant of or otherwise receive remuneration from, (i) any business that competes, directly or indirectly, with BHV or its affiliates, or (ii) any lessor, lessee, customer or supplier of BHV. Such BHV Member does not have any personal interest in any tangible or intangible assets or real or personal property used in or pertaining to the business of BHV. |
| (b) | Brokers or Finders. No agent, broker, investment banker, financial advisor or other firm or person is or will be entitled to any broker’s or finder’s fee or any other similar commission or fee in connection with any of the transactions contemplated by this Agreement. |
| (c) | Title to BHV Membership Interest. Such BHV Member owns his BHV Membership Interests free and clear of any and all Liens. Upon registering of Holdco as the new owner of such BHV Membership Interests in the register of BHV, Holdco will receive good and valid title to such BHV Membership Interests, free and clear of all Liens. The BHV Membership Interests set forth in the BHV Disclosure Letter are and will be at Closing, all of the BHV Membership Interests. |
| (d) | Power and Authority. All acts required to be taken by such BHV Member to enter into this Agreement have been properly taken. This Agreement constitutes a legal, valid and binding obligation of such BHV Member, enforceable against such BHV Member in accordance with the terms hereof, subject to bankruptcy, insolvency, fraudulent transfer, reorganization, moratorium and similar laws of general applicability relating to or affecting creditors’ rights and to general equitable principles. |
| (e) | No Conflicts. Such BHV Member has the requisite power and authority to enter into this Agreement and to consummate the transactions contemplated hereby and otherwise to carry out his obligations hereunder. No consent, approval or agreement of any individual or entity is required to be obtained by such BHV Member in connection with the execution and performance by such BHV Member of this Agreement or the execution and performance by such BHV Member of any agreements, instruments or other obligations entered into in connection with this Agreement. The execution and delivery of this Agreement by such BHV Member and the performance by such BHV Member of his obligations hereunder in accordance with the terms hereof: (i) will not require the consent of any third party or any Governmental Entity under any laws; (ii) will not violate any laws applicable to such BHV Member; and (iii) will not violate or breach any contractual obligation to which such BHV Member is a party. |
| (f) | Purchase Entirely for Own Account. The Holdco Common Stock to be acquired by such BHV Member hereunder will be acquired for investment for his own account, and not with a view to the resale or distribution of any part thereof, and such BHV Member has no present intention of selling or otherwise distributing such Holdco Common Stock except in compliance with applicable securities laws. |
| (g) | Available Information. Such BHV Member has reviewed all Islet filings made with the SEC under the Exchange Act since January 1, 2013, and is familiar with the risks and disclosures made therein. Such BHV Member has such knowledge and experience in financial and business matters that he is capable of evaluating the merits and risks of an investment in Holdco. |
| (h) | No Other Representations or Warranties. Except for the representations and warranties made by the BHV Members in this Section 4.2, neither BHV, the BHV Members, nor any other person, makes any express or implied representation or warranty with respect to the BHV Members, and BHV and the BHV Members hereby disclaim any such other representations or warranties. |
| 4.3 | Representations and Warranties of Islet, Holdco and Islet Merger Sub. Except (a) with respect to any subsection of this Section 4.3, as set forth in the correspondingly identified subsection of the disclosure letter delivered by Islet, Holdco and Islet Merger Sub to BHV concurrently herewith (the “Islet Disclosure Letter”) (it being understood by the parties that any information disclosed in one subsection of the Islet Disclosure Letter shall be deemed to be disclosed for purposes of each other subsection of the Islet Disclosure Letter to which the relevance of such information is reasonably apparent) or (b) as disclosed in the Islet SEC Documents filed prior to the date hereof (excluding, in each case, any disclosures set forth in any risk factor section, in any section relating to forward looking statements and any other disclosures included therein to the extent that they are cautionary, predictive, or forward looking in nature), Islet, Holdco and Islet Merger Sub jointly represent and warrant to BHV as follows: |
| (a) | Organization, Standing and Power. |
(i)Each of Islet and the Significant Subsidiaries of Islet is a corporation or other entity duly organized, validly existing and, if applicable, in good standing under the laws of its jurisdiction of incorporation, has all requisite power and authority to own, lease and operate its properties and to carry on its business as now being conducted, and is duly qualified and, if applicable, in good standing to do business in each jurisdiction in which the nature of its business or the ownership or leasing of its properties makes such qualification necessary, in each case, other than as would not, either individually or in the aggregate, reasonably be expected to have a Material Adverse Effect on Islet.
(ii)Each of Holdco and the Islet Merger Sub is a corporation duly organized, validly existing and in good standing in its jurisdiction of incorporation. Since their respective dates of incorporation, none of Holdco or the Islet Merger Sub has carried on any business, incurred any liability or conducted any operations other than the execution of this Agreement, the performance of its obligations hereunder and matters ancillary thereto.
(i)As of the date of this Agreement, the authorized, issued and outstanding capital structure of Islet is set forth in the Islet Disclosure Letter. No other shares of capital stock of Islet are issued, reserved for issuance or outstanding. Except as set forth in the Islet Disclosure Letter, as of the date of this Agreement, there are no options, warrants, rights, convertible or exchangeable securities, “phantom” stock rights, stock appreciation rights, stock-based performance units, commitments, contracts, arrangements or undertakings of any kind to which Islet is a party or by which Islet is bound (A) obligating Islet to issue, deliver or sell, or cause to be issued, delivered or sold, additional shares of capital stock or other equity interests in, or any security convertible or exercisable for or exchangeable into any shares of capital stock or other equity interest in, Islet, (B) obligating Islet to issue, grant, extend or enter into any such option, warrant, call, right, security, commitment, contract, arrangement or undertaking or (C) that give any person the right to receive any economic benefit or right similar to or derived from the economic benefits and rights occurring to holders of the shares of capital stock of Islet.
(ii)As of the date of this Agreement, the authorized capital stock of Holdco consists of 10 million shares of preferred stock, par value $0.001 per share, and 200 million shares of Holdco Common Stock, of which one share has been issued to Islet, which share of Holdco Common Stock is validly issued, fully paid and non-assessable, and is owned directly by Islet free and clear of any liens (statutory or other), pledges, charges, encumbrances and security interests whatsoever (“Liens”), and is the only share of Holdco capital stock issued and outstanding. The authorized capital stock of Islet Merger Sub consists of 100 shares of common stock, par value $0.001 per share, all of which have been validly issued, fully paid and non-assessable, and are owned directly by Holdco free and clear of any Liens. Holdco owns all of the capital stock of Islet Merger Sub. All shares of Holdco Common Stock issued pursuant to ARTICLE III at the Initial Effective Time are validly issued, fully paid and non-assessable, and free and clear of any Liens.
(i)Islet, Holdco and the Islet Merger Sub have all requisite corporate power and authority to enter into this Agreement and to consummate the transactions contemplated hereby, subject in the case of the Islet Merger to the approval of this Agreement by the Required Islet Consent. The execution and delivery of this Agreement and the consummation of the transactions contemplated hereby have been duly authorized by all necessary corporate action, including without limitation, any required shareholder approval, on the part of each of Islet, Holdco and the Islet Merger Sub, subject in the case of the Islet Merger to the approval of this Agreement by the Required Islet Consent. This Agreement has been duly executed and delivered by each of Islet, Holdco and the Islet Merger Sub and constitutes a valid and binding obligation of each of Islet, Holdco and the Islet Merger Sub, enforceable against each of Islet, Holdco and the Islet Merger Sub in accordance with its terms, subject to bankruptcy, insolvency, fraudulent transfer, reorganization, moratorium and similar laws of general applicability relating to or affecting creditors’ rights and to general equitable principles.
(ii)The execution and delivery of this Agreement does not, and the consummation of the transactions contemplated hereby will not, (A) result in any Violation pursuant to any provision of the Islet Charter, the Islet By-laws or equivalent governing documents of any Significant Subsidiary of Islet, Holdco or the Islet Merger Sub, or (B) subject to obtaining or making the consents, approvals, orders, authorizations, registrations, declarations and filings referred to in Section 4.3(c)(iii) below, result in any Violation of any loan or credit agreement, note, mortgage, indenture, lease, obligation, instrument, permit, concession, franchise, license, judgment, order, decree, statute, law, ordinance, rule or regulation applicable to Islet, Holdco, or the Islet Merger Sub or any Subsidiary of Islet, Holdco or the Islet Merger Sub or any of their respective properties or assets which Violation, individually or in the aggregate, would reasonably be expected to (x) have a Material Adverse Effect on Islet, Holdco or the Islet Merger Sub or (y) prevent, materially delay or materially impede Islet’s, Holdco’s or the Islet Merger Sub’ ability to perform their respective obligations hereunder or to consummate the transactions contemplated hereby.
(iii)No consent, approval, order or authorization of, or registration, declaration or filing with, any Governmental Entity is required by or with respect to Islet, Holdco or the Islet Merger Sub or any Subsidiary of Islet, Holdco or the Islet Merger Sub in connection with the execution and delivery of this Agreement by Islet, Holdco and the Islet Merger Sub or the consummation by Islet, Holdco or the Islet Merger Sub of the transactions contemplated hereby, the failure to make or obtain that, individually or in the aggregate, would reasonably be expected to (x) have a Material Adverse Effect on Islet, Holdco or the Islet Merger Sub or (y) prevent, materially delay or materially impede Islet’s, Holdco’s or the Islet Merger Sub’s ability to perform its obligations hereunder or to consummate the transactions contemplated hereby, except for (A) the filing with the SEC of Form S-4 and Consent Solicitation Statement/Prospectus, (B) such filings and approvals as are required to be made or obtained under the securities or blue sky laws of various states in connection with the transactions contemplated by this Agreement, (C) the filing of the Islet Certificate of Merger with the Secretary of State of the State of Nevada, and (D) the quotation of the Holdco Common Stock on the OTCQB.
| (d) | SEC Documents. Except as set forth in the Islet Disclosure Letter, Islet has filed all required reports, schedules, registration statements and other documents with the SEC since January 1, 2013 (the “Islet SEC Documents”). Except as set forth in the Islet Disclosure Letter, during the twelve (12) months presiding the date hereof, Islet has timely filed all required Islet SEC Documents with the SEC. As of their respective dates of filing with the SEC (or, if amended or superseded by a filing prior to the date hereof, as of the date of such filing), the Islet SEC Documents complied in all material respects with the requirements of the Securities Act or the Exchange Act, as the case may be, and the rules and regulations of the SEC thereunder applicable to such Islet SEC Documents, and none of the Islet SEC Documents when filed, contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading. The financial statements of Islet included in the Islet SEC Documents complied as to form, as of their respective dates of filing with the SEC, in all material respects with all the published rules and regulations of the SEC with respect thereto (except, in the case of unaudited statements, as permitted by Form 10-Q of the SEC), have been prepared in accordance with GAAP applied on a consistent basis during the periods involved (except as may be disclosed therein) and fairly present in all material respects the consolidated financial position of Islet and its consolidated Subsidiaries and the consolidated results of operations, changes in shareholders’ equity and cash flows of such companies as of the dates and for the periods shown. As of the date hereof, there are no outstanding written comments from the SEC with respect to any of the Islet SEC Documents. |
| (e) | Undisclosed Liabilities. Except for (i) those liabilities that are appropriately reflected or reserved for in the consolidated financial statements of Islet included in its Annual Report on Form 10-K for the fiscal year ended April 30, 2014, as filed with the SEC prior to the date hereof, (ii) liabilities incurred since April 30, 2014 in the ordinary course of business consistent with past practice, (iii) liabilities that, individually or in the aggregate, have not had, and would not reasonably be expected to have, a Material Adverse Effect on Islet, (iv) liabilities incurred pursuant to the transactions contemplated by, or permitted by, this Agreement, and (v) liabilities or obligations discharged or paid in full prior to the date hereof in the ordinary course of business consistent with past practice, Islet and its Subsidiaries do not have, any liabilities or obligations of any nature whatsoever (whether accrued, absolute, contingent or otherwise) that are required to be reflected in Islet’s financial statements in accordance with GAAP. |
| (f) | Information Supplied. None of the information supplied or to be supplied by Islet for inclusion or incorporation by reference in (i) the Form S-4 will, at the time the Form S-4 is filed with the SEC and at the time it becomes effective under the Securities Act, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading and (ii) the Consent Solicitation Statement/Prospectus will, at the date of mailing to Islet shareholders, contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not misleading. The Form S-4 and Consent Solicitation Statement/Prospectus will comply as to form in all material respects with the requirements of the Securities Act, the Exchange Act and the rules and regulations of the SEC thereunder. No representation or warranty is made by Islet with respect to statements made or incorporated by reference therein based on information supplied by BHV for inclusion or incorporation by reference in the Form S-4 and Consent Solicitation Statement/Prospectus. |
| (g) | Compliance with Applicable Laws and Reporting Requirements. Islet and its Subsidiaries hold all permits, licenses, variances, exemptions, orders and approvals of all Governmental Entities that are material to the operation of the businesses of Islet and its Subsidiaries, taken as a whole (the “Islet Permits”), and Islet and its Subsidiaries are and for the two years preceding the date hereof have been in compliance with the terms of the Islet Permits and all applicable laws and regulations, except where the failure so to hold or comply with any such Islet Permits and applicable laws and regulations would not reasonably be expected to have a Material Adverse Effect on Islet. The businesses of Islet and its Subsidiaries are not being, and for the two years preceding the date hereof have not been, conducted in violation of any law, ordinance (including zoning) or regulation of any Governmental Entity (including the Sarbanes-Oxley Act of 2002), except for violations that, individually or in the aggregate, do not have, and would not reasonably be expected to have, a Material Adverse Effect on Islet. No investigation by any Governmental Entity with respect to Islet or any of its Subsidiaries is pending or, to the knowledge of Islet, threatened, other than, in each case, those the outcome of which, individually or in the aggregate, would not reasonably be expected to have a Material Adverse Effect on Islet. |
| (h) | Legal Proceedings. Except as set forth in the Islet Disclosure Letter, there is no claim, suit, action, investigation or other demand or proceeding (whether judicial, arbitral, administrative or other) pending or, to the knowledge of Islet, threatened against or affecting Islet, any Subsidiary of Islet or any of their respective assets, rights or properties that would reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect on Islet or could prevent, enjoin, alter or delay any of the transactions contemplated by this Agreement, nor is there any judgment, decree, Injunction, rule or order of any Governmental Entity or arbitrator outstanding against Islet, any Subsidiary of Islet or any of their respective assets, rights or properties having or that would reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect on Islet or on Holdco after the Effective Time, or could prevent, enjoin, alter or delay any of the transactions contemplated by this Agreement. |
| (i) | Taxes. Except as would not reasonably be expected, individually or in the aggregate, to have a Material Adverse Effect on Islet: |
(i)Each of Islet and its Subsidiaries has duly and timely filed (including all applicable extensions) all Tax Returns required to be filed by it on or prior to the date of this Agreement (all such Tax Returns being true, correct and complete in all respects), has timely paid all Taxes shown thereon as arising and has duly and timely paid all Taxes that are due and payable or claimed to be due from it by federal, state, foreign or local taxing authorities other than Taxes that are being contested in good faith, which have not been finally determined, and have been adequately reserved against in accordance with GAAP on Islet’s most recent consolidated financial statements;
(ii)There are no disputes, audits, examinations or Proceedings pending, or claims asserted in writing, for Taxes or assessments upon Islet or any of its Subsidiaries for which Islet does not have reserves that are adequate under GAAP;
(iii)Neither Islet nor any of its Subsidiaries has participated in a “listed transaction” within the meaning of Treasury Regulation Section 1.6011-4(b)(2);
(iv)As of the date of this Agreement, neither Islet nor any of its Subsidiaries has taken or agreed to take any action or knows of any fact, agreement, plan or other circumstance that could reasonably be expected to prevent the exchange of BHV Membership Interests and Islet Common Stock for Holdco Common Stock pursuant to the Acquisitions from qualifying, for United States federal income tax purposes, as an exchange described in Section 351 of the Code and the Treasury Regulations promulgated thereunder;
(v)Neither Islet nor any of its Subsidiaries has granted any extension or waiver of the limitation period applicable to any Tax that remains in effect;
(vi)There are no Liens for Taxes (other than statutory Liens for Taxes not yet due and payable or the amount or validity of which is being contested in good faith by appropriate Proceedings) upon any of the assets of Islet or its Subsidiaries;
(vii)Neither Islet nor any of its Subsidiaries has constituted either a “distributing corporation” or a “controlled corporation” in a distribution of stock intended to qualify for tax-free treatment under Section 355 of the Code in the five years prior to the date of this Agreement; and
(viii)Neither Islet nor any of its Subsidiaries has any material liability for Taxes of any person (other than Islet and its Subsidiaries) under Treasury Regulation Section 1.1502-6 (or any similar provision of state, local or foreign law), as a transferee or successor, by contract or otherwise.
| (j) | Certain Agreements. Except for this Agreement, or as set forth in the Islet Disclosure Letter or as attached to Islet’s latest annual report on Form 10-K as an exhibit, Islet is not a party to or bound by any contract, arrangement, commitment or understanding (i) with respect to the election, appointment, employment or compensation of any directors or executive officers, (ii) that is a “material contract” (as such term is defined in Item 601(b)(10) of Regulation S-K of the SEC), (iii) that limits the ability of Islet to compete in any line of business, in any geographic area or with any person, or that requires referrals of business and, in each case, which limitation or requirement would reasonably be expected to be material to Islet, (iv) that relates to the sale of products by Islet which have not been performed by Islet and for which a performance obligation that is material to Islet would remain after the Effective Time, (v) in the case of an Employee Benefit Plan of Islet, any of the benefits of which will be increased, or the vesting of the benefits of which will be accelerated, by the occurrence of any of the transactions contemplated by this Agreement, or the value of any of the benefits of which will be calculated on the basis of any of the transactions contemplated by this Agreement, or (vi) that would reasonably be expected to prevent, materially delay or materially impede the consummation of any of the transactions contemplated by this Agreement. All contracts, arrangements, commitments or understandings of the type described in this Section 4.3(i)(v) (collectively referred to herein as the “Islet Contracts”) are valid and in full force and effect, except to the extent they have previously expired in accordance with their terms or if the failure to be in full force and effect, individually or in the aggregate, would not reasonably be expected to have a Material Adverse Effect on Islet. Islet has not, and to the knowledge of Islet, none of the other parties thereto have, violated any provision of, or committed or failed to perform any act, and no event or condition exists, which with or without notice, lapse of time or both would constitute a default under the provisions of, any Islet Contract, except in each case for those violations and defaults that, individually or in the aggregate, would not reasonably be expected to result in a Material Adverse Effect on Islet. |
| (k) | Subsidiaries. Exhibit 21.1 to Islet’s Annual Report on Form 10-K for the fiscal year ended April 30, 2014 includes all the Subsidiaries of Islet that are Significant Subsidiaries. All of the shares of capital stock of each of the Subsidiaries held by Islet or by another Subsidiary of Islet have been validly issued, are fully paid and non-assessable and are owned by Islet or a Subsidiary of Islet free and clear of any Lien. |
| (l) | Absence of Certain Changes or Events. Since April 30, 2014, (i) Islet and its Subsidiaries have conducted their respective businesses in the ordinary course consistent in all material respects with their past practices and (ii) there has not been any change, circumstance or event that has had, or would reasonably be expected to have, a Material Adverse Effect on Islet. |
| (m) | Properties. Except as set forth in Section 4.3(m) of the Islet Disclosure Letter, Islet (i) has good and valid title to all its properties and assets that are material to its business, free and clear of all Liens, except (1) statutory Liens securing payments not yet due, and (2) such imperfections or irregularities of title, claims, easements, covenants and other Liens or restrictions as do not affect in any material respect the current use of the properties or assets subject thereto or affected thereby or otherwise impair in any material respect the business operations at such properties, and (ii) is the lessee of all leasehold estates that are material to its business (except for leases that have expired by their terms since the date thereof or been assigned, terminated or otherwise disposed of in the ordinary course of business consistent with past practice) and is in possession of the properties purported to be leased thereunder, and each such lease is valid without any material default thereunder by the lessee or, to Islet’s knowledge, the lessor. |
| (n) | Board Approval. The Board of Directors of Islet, by resolutions duly adopted by unanimous written consent or vote of those voting at a meeting duly called and held (the “Islet Board Approval”), has approved this Agreement and the transactions contemplated hereby. |
| (o) | Consent Required.The Required Islet Consent is the only vote of the holders of any class or series of Islet capital stock necessary to approve this Agreement and the transactions contemplated hereby. |
| (p) | Brokers or Finders. No agent, broker, investment banker, financial advisor or other firm or person is or will be entitled to any broker’s or finder’s fee or any other similar commission or fee in connection with any of the transactions contemplated by this Agreement. |
| (q) | No Other Representations or Warranties. Except for the representations and warranties made by Islet, Holdco and Islet Merger Sub in this Section 4.3, neither Islet, Holdco and Islet Merger Sub, nor any other person, makes any express or implied representation or warranty with respect to Islet, Holdco and Islet Merger Sub, and Islet, Holdco and Islet Merger Sub hereby disclaim any such other representations or warranties. |
ARTICLE V
COVENANTS RELATING TO CONDUCT OF BUSINESS
| 5.1 | Covenants of BHV. During the period from the date hereof and continuing until the Effective Time, BHV agrees as to itself and its Subsidiaries that, except as expressly permitted by this Agreement, as set forth on Section 5.1 of the BHV Disclosure Letter, or to the extent that Islet shall otherwise consent in writing, which consent shall not be arbitrarily withheld or arbitrarily delayed: |
| (a) | Ordinary Course. BHV and its Subsidiaries shall carry on their respective businesses in the usual, regular and ordinary course consistent with past practice and use its reasonable best efforts to preserve intact their present business organizations, maintain their rights, franchises, licenses and other authorizations issued by Governmental Entities and preserve their relationships with employees, customers, suppliers and others having business dealings with them to the end that their goodwill and ongoing businesses shall not be impaired in any material respect at the Effective Time. BHV shall not, nor shall it permit any of its Subsidiaries to, (i) enter into any new material line of business, (ii) change its or its Subsidiaries’ operating policies in any respect that is material to BHV, except as required by law or by policies imposed by a Governmental Entity, (iii) incur or commit to any capital expenditures or any obligations or liabilities in connection therewith other than capital expenditures and obligations or liabilities incurred or committed to in the ordinary course of business consistent with past practice and in any event not in excess of the amount set forth in Section 5.1(a) of the BHV Disclosure Letter, in the aggregate, or (iv) except in the ordinary course of business consistent with past practice, enter into any agreement that would constitute a BHV Contract had such agreement been in effect on the date hereof or terminate or make any material change to any BHV Contract, including, but not limited to the Kissei Agreement. |
| (b) | Distributions; Changes in Equity. BHV shall not, nor shall it permit any of its Subsidiaries to, or propose to, (i) declare or pay any dividends on or make any distributions in respect of any of its capital stock or membership interests, as applicable, (ii) split, combine or reclassify any of its capital stock or membership interests, as applicable, or issue or authorize or propose the issuance or authorization of any other securities in respect of, in lieu of or in substitution for, shares of its capital stock or its membership interests, as applicable, or (iii) repurchase, redeem or otherwise acquire, or permit any Subsidiary to redeem, purchase or otherwise acquire, any shares of its capital stock or its membership interests or any securities convertible into or exercisable for any shares of its capital or any of its membership interests, as applicable. |
| (c) | Issuance of Securities. Except as set forth in this Agreement, BHV shall not, nor shall it permit any of its Subsidiaries to, issue, deliver or sell, or authorize or propose the issuance, delivery or sale of, any shares of its capital stock of any class, any stock appreciation rights, or any securities convertible into or exercisable or exchangeable for, or any rights, warrants or options to acquire, any such shares, or enter into any agreement with respect to any of the foregoing. |
| (d) | Governing Documents, Etc. BHV shall not amend or propose to amend the BHV Articles of Organization or the BHV Operating Agreement or enter into, or permit any Subsidiary to enter into, a plan of consolidation, merger or reorganization with any person. |
| (e) | No Acquisitions. BHV shall not, and shall not permit any of its Subsidiaries to, acquire or agree to acquire, by merging or consolidating with, by purchasing a substantial equity interest in or a substantial portion of the assets of, by forming a partnership or joint venture with, or by any other manner, any business or any corporation, partnership, association or other business organization or division thereof or otherwise acquire or agree to acquire any assets that are material to BHV. |
| (f) | No Dispositions. BHV shall not, and shall not permit any of its Subsidiaries to, sell, lease, assign, encumber or otherwise dispose of, or agree to sell, lease, assign, encumber or otherwise dispose of, any of its material assets. |
| (g) | Indebtedness. BHV shall not, and shall not permit any of its Subsidiaries to, incur, create or assume any indebtedness for borrowed money (or modify any of the material terms of any such outstanding indebtedness), guarantee any such indebtedness or issue or sell any debt securities or warrants or rights to acquire any debt securities of BHV or guarantee any long-term debt securities of others. |
| (h) | Accounting Methods; Tax Matters. BHV shall not change its methods of accounting in effect as of the date of this Agreement, except as required by changes in law as concurred with by BHV’s independent auditors. BHV shall not, and shall not permit any of its Subsidiaries to, make, change or revoke any material Tax election, change an annual Tax accounting period, adopt or change any material Tax accounting method, file any material amended Tax Return, enter into any closing agreement with respect to a material amount of Taxes, settle any material Tax claim or assessment or surrender any right to claim a refund of a material amount of Taxes. |
| (i) | Tax-Free Qualification. BHV shall not, and shall not permit any of its Subsidiaries to, take or cause to be taken any action, or knowingly fail to take or cause to be taken any action, which action or failure to act could reasonably be expected to prevent the exchange of BHV Membership Interests and Islet Common Stock for Holdco Common Stock pursuant to the Acquisitions from qualifying, for United States federal income tax purposes, as an exchange described in Section 351 of the Code and the Treasury Regulations promulgated thereunder. |
| (j) | Compensation and Benefit Plans. Except as required by applicable law, BHV shall not and shall not permit its Subsidiaries to: (i) increase the wages, salaries, or incentive compensation or incentive compensation opportunities of any director or employee of BHV other than normal increases in base salary or wages in the ordinary course of business consistent with past practice for employees who are not directors of BHV or officers of BHV (ii) increase or accelerate the accrual rate, vesting or timing of payment or funding of, any compensation, severance, benefits or other rights of any director or employee of BHV or otherwise pay any amount to which any director or employee of BHV is not entitled, (iii) establish, adopt, or become a party to any new employment, severance or consulting agreement or any employee benefit or compensation plan, program, commitment or agreement or amend, suspend or terminate any Employee Benefit Plan or benefits, (iv) modify any option or other right to acquire BHV Membership Interests or other equity-based award, (v) make any discretionary contributions or payments to any trust or other funding vehicle or pay any discretionary premiums in respect of benefits under any Employee Benefit Plan or Employment Agreement or (vi) establish, adopt, enter into, amend, suspend or terminate any collective bargaining agreement, except as required by the terms of any collective bargaining agreement in effect on the date hereof. |
| (k) | No Liquidation. BHV shall not, and shall not permit any of its Subsidiaries to, adopt a plan of complete or partial liquidation or resolutions providing for or authorizing such a liquidation or a dissolution, restructuring, recapitalization or reorganization. |
| (l) | Litigation. BHV shall not, and shall not permit any of its Subsidiaries to, initiate, settle or compromise any material litigation. |
| (m) | No Restrictions on Business. BHV shall not, and shall not permit any of its Subsidiaries to, enter into or otherwise become party to any contract, arrangement, commitment or understanding that will restrict or limit, in any material respect, the ability of Holdco, BHV or Islet or any of their respective Subsidiaries from conducting, from and after the Closing, any of their businesses in any geographical area, other than any contract, arrangement, commitment or understanding terminable in full (including the restrictions and limitations on conduct of business) on notice of not more than forty-five (45) days by Holdco or a Subsidiary thereof without the incurrence of any liability (including an incurrence of an obligation to make any payment of any amount in respect of such termination). |
| (n) | Insurance. BHV shall not purchase any policies of directors’ and officers’ liability insurance. |
| (o) | Other Agreements. BHV shall not, and shall not permit any of its Subsidiaries to, agree to, or make any commitment to, take, or authorize, any of the actions prohibited by this Section 5.1 or any action that would make any representation or warranty of BHV in this Agreement untrue or incorrect in any material respect. |
| 5.2 | Covenants of Islet. During the period from the date hereof and continuing until the Effective Time, Islet agrees as to itself and its Subsidiaries that, except as expressly permitted by this Agreement, as set forth on Section 5.2 of the Islet Disclosure Letter, or to the extent that BHV shall otherwise consent in writing, which consent shall not be arbitrarily withheld or arbitrarily delayed: |
| (a) | Ordinary Course. Islet and its Subsidiaries shall carry on their respective businesses in the usual, regular and ordinary course consistent with past practice and use their reasonable best efforts to preserve intact their present business organizations, maintain their rights, franchises, licenses and other authorizations issued by Governmental Entities and preserve their relationships with employees, customers, suppliers and others having business dealings with them to the end that their goodwill and ongoing businesses shall not be impaired in any material respect at the Effective Time. Islet shall not, nor shall it permit any of its Subsidiaries to, (i) enter into any new material line of business, (ii) change its or its Subsidiaries’ operating policies in any respect that is material to Islet, except as required by law or by policies imposed by a Governmental Entity, (iii) incur or commit to any capital expenditures or any obligations or liabilities in connection therewith other than capital expenditures and obligations or liabilities incurred or committed to in the ordinary course of business consistent with past practice and in any event not in excess of the amount set forth in Section 5.2(a) of the Islet Disclosure Letter, in the aggregate, or (iv) except in the ordinary course of business consistent with past practice, enter into any agreement that would constitute a Islet Contract had such agreement been in effect on the date hereof or terminate or make any material change to any Islet Contract. |
| (b) | Dividends; Changes in Stock. Islet shall not, nor shall it permit any of its Subsidiaries to, or propose to (i) declare or pay any dividends on or make other distributions in respect of any of its capital stock, or (ii) split, combine or reclassify any of its capital stock or issue or authorize or propose the issuance or authorization of any other securities in respect of, in lieu of or in substitution for, shares of its capital stock (except for any split, combination or reclassification of capital stock of a wholly owned Subsidiary of Islet or any issuance or authorization or proposal to issue or authorize any securities of a wholly owned Subsidiary of Islet to Islet or another wholly owned Subsidiary of Islet). |
| (c) | Issuance of Securities. Islet shall not, nor shall it permit any of its Subsidiaries to, issue, deliver or sell, or authorize or propose the issuance, delivery or sale of, any shares of its capital stock of any class, any stock appreciation rights, or any securities convertible into or exercisable or exchangeable for, or any rights, warrants or options to acquire, any such shares, or enter into any agreement with respect to any of the foregoing, other than (i) the issuance of Islet Common Stock or other equity rights or obligations under any Employee Benefit Plans of Islet currently in effect or (ii) issuances by a wholly owned Subsidiary of its capital stock to its parent or to another wholly owned Subsidiary of Islet. |
| (d) | Governing Documents, Etc. Islet shall not amend or propose to amend the Islet Charter or the Islet Bylaws or enter into, or permit any Subsidiary to enter into, a plan of consolidation, merger or reorganization with any person except as provided for hereunder. |
| (e) | No Acquisitions. Islet shall not, and shall not permit any of its Subsidiaries to, acquire or agree to acquire, by merging or consolidating with, by purchasing a substantial equity interest in or a substantial portion of the assets of, by forming a partnership or joint venture with, or by any other manner, any business or any corporation, partnership, association or other business organization or division thereof or otherwise acquire or agree to acquire any assets that are material to Islet. |
| (f) | No Dispositions. Islet shall not, and shall not permit any of its Subsidiaries to, sell, lease, assign, encumber or otherwise dispose of, or agree to sell, lease, assign, encumber or otherwise dispose of, any of its material assets. |
| (g) | Indebtedness. Islet shall not, and shall not permit any of its Subsidiaries to, incur, create or assume any indebtedness for borrowed money (or modify any of the material terms of any such outstanding indebtedness), guarantee any such indebtedness or issue or sell any debt securities or warrants or rights to acquire any debt securities of Islet or guarantee any long-term debt securities of others. |
| (h) | Accounting Methods; Tax Matters. Islet shall not change its methods of accounting in effect as of the date of this Agreement, except as required by the SEC, by changes in GAAP or changes in law as concurred with by Islet’s independent auditors. Islet shall not, and shall not permit any of its Subsidiaries to, make, change or revoke any material Tax election, change an annual Tax accounting period, adopt or change any material Tax accounting method, file any material amended Tax Return, enter into any closing agreement with respect to a material amount of Taxes, settle any material Tax claim or assessment or surrender any right to claim a refund of a material amount of Taxes. |
| (i) | Section 351 Qualification. Islet shall not, and shall not permit Holdco, Islet Merger Sub or any of its other Subsidiaries to, take or cause to be taken any action, or knowingly fail to take or cause to be taken any action, which action or failure to act could reasonably be expected to prevent the exchange of BHV Membership Interests and Islet Common Stock for Holdco Common Stock pursuant to the Acquisitions from qualifying, for United States federal income tax purposes, as an exchange described in Section 351 of the Code and the Treasury Regulations promulgated thereunder. |
| (j) | Compensation and Benefit Plans. Except as required by applicable law, Islet shall not and shall not permit its Subsidiaries to: (i) increase the wages, salaries, or incentive compensation or incentive compensation opportunities of any director or employee of Islet other than normal increases in base salary or wages in the ordinary course of business or as provided for in any applicable Employment Agreement and is consistent with past practice for employees who are not directors of Islet or officers of Islet (ii) increase or accelerate the accrual rate, vesting or timing of payment or funding of, any compensation, severance, benefits or other rights of any director or employee of Islet or otherwise pay any amount to which any director or employee of Islet is not entitled, except otherwise provided for in any applicable Employment Agreement, (iii) establish, adopt, or become a party to any new employment, severance or consulting agreement or any employee benefit or compensation plan, program, commitment or agreement or amend, suspend or terminate any Employee Benefit Plan or Employment Agreement, (iv) modify any option or other right to acquire Islet Common Stock or other equity-based award, (v) make any discretionary contributions or payments to any trust or other funding vehicle or pay any discretionary premiums in respect of benefits under any Employee Benefit Plan, except for such contributions or payments as may be provided for in any applicable Employment Agreements, or (vi) establish, adopt, enter into, amend, suspend or terminate any collective bargaining agreement, except as required by the terms of any collective bargaining agreement in effect on the date hereof. |
| (k) | No Liquidation. Islet shall not adopt a plan of complete or partial liquidation or resolutions providing for or authorizing such a liquidation or a dissolution, restructuring, recapitalization or reorganization. |
| (l) | Litigation. Without the consent of BHV, which shall not be unreasonably withheld or denied, Islet shall not, and shall not permit any of its Subsidiaries to, initiate, settle or compromise any material litigation. |
| (m) | Insurance. Islet shall use commercially reasonable efforts to maintain insurance at levels substantially comparable to levels existing as of the date hereof. |
| (n) | Other Agreements. Islet shall not, and shall not permit any of its Subsidiaries to, agree to, or make any commitment to, take, or authorize, any of the actions prohibited by this Section 5.2 or any action that would make any representation or warranty of Islet, Holdco or Islet Merger Sub in this Agreement untrue or incorrect in any material respect. |
| 5.3 | Advice of Changes; Government Filings. Each party shall confer on a regular and frequent basis with the other, report on operational matters and promptly advise the other orally and in writing of any change or event having, or that would reasonably be expected to have, a Material Adverse Effect on such party or that would cause or constitute a material breach of any of the representations, warranties or covenants of such party contained herein; provided, however, that any noncompliance with the foregoing shall not constitute the failure to be satisfied of a condition set forth in ARTICLE VII or give rise to any right of termination under ARTICLE VIII unless the underlying breach shall independently constitute such a failure or give rise to such a right. BHV and Islet shall file all reports required to be filed by each of them (or their Subsidiaries) with the SEC on a timely basis between the date hereof and the Effective Time (which shall include available extensions) and shall deliver to the other party copies of all such reports promptly after the same are filed. Each of BHV and Islet shall have the right to review in advance, and to the extent practicable each will consult with the other, in each case subject to applicable laws relating to the exchange of information, with respect to, any filing made with, or written materials submitted to, any third party or any Governmental Entity in connection with the transactions contemplated by this Agreement. In exercising the foregoing right, each of the parties agrees to act reasonably and as promptly as practicable. Each party agrees that it will consult with the other party with respect to the obtaining of all permits, consents, approvals and authorizations of all third parties and Governmental Entities necessary or advisable to consummate the transactions contemplated by this Agreement, and each party will keep the other party apprised of the status of matters relating to completion of the transactions contemplated hereby. |
| 5.4 | Control of Other Party’s Business. Nothing contained in this Agreement shall give Islet, directly or indirectly, the right to control or direct the operations of BHV prior to the Effective Time. Prior to the Effective Time, BHV shall exercise, consistent with the terms and conditions of this Agreement, complete control and supervision over its and its Subsidiaries’ respective operations. |
ARTICLE VI
ADDITIONAL AGREEMENTS
| 6.1 | Preparation of Consent Solicitation Statement/Prospectus/Form S-4; Islet Lock-Up Agreements. |
| (a) | As promptly as practicable following the date of this Agreement, Holdco, Islet and BHV shall prepare and file with the SEC a registration statement on Form S-4 to register the issuance of Holdco Common Stock in the Acquisitions to the holders of Islet Common Stock and to the BHV Members (such Form S-4, and any amendments or supplements thereto, the “Form S-4”) in which a consent solicitation statement relating to the Required Islet Consent will be included (the “Consent Solicitation Statement/Prospectus”). Each of Islet and BHV shall use reasonable best efforts to have the Form S-4 declared effective under the Securities Act as promptly as practicable after such filing. Islet shall use reasonable best efforts to cause the Consent Solicitation Statement/Prospectus to be mailed to the holders of Islet Common Stock as promptly as practicable after the Form S-4 is declared effective. |
| (b) | If at any time prior to the Effective Time there shall occur (i) any event with respect to BHV, or with respect to other information supplied by BHV for inclusion in the Form S-4 or the Consent Solicitation Statement/Prospectus or (ii) any event with respect to Islet, or with respect to information supplied by Islet for inclusion in the Form S-4 or the Consent Solicitation Statement/Prospectus, which event is required to be described in an amendment of, or a supplement to, the Form S-4 or the Consent Solicitation Statement/Prospectus, such event shall be so described, and such amendment or supplement shall be promptly filed with the SEC. No filing of, or amendment or supplement to, the Form S-4 or Consent Solicitation Statement/Prospectus will be made by Holdco or Islet without first providing BHV with a reasonable opportunity to review and comment thereon. |
| (c) | Each of Holdco, Islet and BHV shall promptly notify each other of the receipt of any comments from the SEC or its staff or any other appropriate government official and of any requests by the SEC or its staff or any other appropriate government official for amendments or supplements to any of the filings with the SEC in connection with the Acquisitions and other transactions contemplated hereby or for additional information and shall supply each other with copies of all correspondence between BHV or any of its representatives, Holdco or any of its representatives, or Islet or any of its representatives, as the case may be, on the one hand, and the SEC or its staff or any other appropriate government official, on the other hand, with respect thereto. Holdco, Islet and BHV shall use their respective reasonable best efforts to respond to any comments of the SEC with respect to the Form S-4 or the Consent Solicitation Statement/Prospectus as promptly as practicable. Holdco, Islet and BHV shall cooperate with each other and provide to each other all information necessary in order to prepare the Form S-4 or the Consent Solicitation Statement/Prospectus, and shall provide promptly to each other party any information such party may obtain that could necessitate amending any such document. |
| (d) | Holdco shall file the tax opinions described in Section 7.1(g) with the Form S-4, or with an amendment thereto, as may be required by applicable law, SEC regulations and/or SEC Staff Legal Bulletin No. 19. |
| (e) | Islet shall use reasonable best efforts to (i) take all lawful action to enter into lock-up agreements with all of its executive officers, directors, affiliates, founders and their family members, and greater than 5% shareholders requiring such person and entities to consent to approval of this Agreement, the Islet Merger and the transactions contemplated hereby and (ii) solicit the consent of its shareholders for the Required Islet Consent. |
| 6.2 | Access to Information; Confidentiality. Subject to the Agreement, dated as of September 10, 2013 between Islet and BHV (the “Confidentiality Agreement”), and subject to applicable law, upon reasonable notice, BHV shall, and shall cause its Subsidiaries to, afford to Islet and to the officers, employees, accountants, counsel, financial advisors and other representatives of Islet, reasonable access during normal business hours during the period prior to the Effective Time to all its respective properties, books, contracts, commitments, personnel and records and, during such period, BHV shall, and shall cause each of its Subsidiaries to, furnish promptly to Islet (a) a copy of each material report, schedule, registration statement and other document filed by it with any Governmental Entity and (b) all other information concerning its business, properties and personnel as Islet may reasonably request. Subject to the Confidentiality Agreement, and subject to applicable law, Islet shall, and shall cause its Subsidiaries to, afford to BHV and to the officers, employees, accountants, counsel, financial advisors and other representatives of Islet, reasonable access during the period prior to the Effective Time to all information concerning its business, properties and personnel as BHV may reasonably request. No review pursuant to this Section 6.2 shall affect any representation or warranty given by BHV to Islet. Islet and BHV will hold, and will cause its respective officers, employees, accountants, counsel, financial advisors and other representatives and affiliates to hold, any nonpublic information in accordance with the terms of the Confidentiality Agreement. Any such investigation pursuant to this Section 6.2 shall be conducted in such a manner as not to interfere unreasonably with the business or operations of Islet or BHV, as the case may be. Neither party nor any of its Subsidiaries shall be required to provide access to or to disclose information where such access or disclosure would violate or prejudice the rights of its customers, jeopardize the attorney-client privilege of the institution in possession or control of such information or contravene any law, rule, regulation, order, judgment, decree or binding agreement entered into prior to the date hereof; provided, however, that in the event that either party or any of its Subsidiaries relies on this sentence to withhold access or disclosure, such party shall, to the extent permitted by law and the protection of such attorney-client privilege, notify the other party of the nature of the withheld information. |
| 6.3 | Reasonable Best Efforts. |
| (a) | Subject to the terms and conditions of this Agreement, each party will use its reasonable best efforts to take, or cause to be taken, all actions and to do, or cause to be done, all things necessary, proper or advisable under this Agreement and applicable laws, rules and regulations to consummate the Acquisitions and the other transactions contemplated by this Agreement as soon as practicable after the date hereof, including preparing and filing as promptly as practicable all documentation to effect all necessary applications, notices, filings and other documents and to obtain as promptly as practicable all Requisite Regulatory Approvals and all other consents, waivers, orders, approvals, permits, rulings, authorizations and clearances necessary or advisable to be obtained from any third party or any Governmental Entity in order to consummate the Acquisitions or any of the other transactions contemplated by this Agreement. |
| (b) | Each of Islet and BHV shall, in connection with the efforts referenced in Section 6.3(a), use its reasonable best efforts to (i) cooperate in all respects with each other in connection with any filing or submission and in connection with any investigation or other inquiry, including any proceeding initiated by a private party and permit the other party to review in advance any proposed written communication to any Governmental Entity or private party, related to the transactions contemplated by this Agreement, (ii) promptly inform the other party of the status of any of the matters contemplated hereby, including providing the other party with a copy of any written communication (or summary of oral communications) received by such party from, or given by such party to, any Governmental Entity and of any written communication (or summary of oral communications) received or given in connection with any proceeding by a private party, in each case regarding any of the transactions contemplated hereby, and (iii) consult with each other in advance to the extent practicable of any meeting or conference with any such Governmental Entity or, in connection with any proceeding by a private party, with any such other person, and to the extent permitted by any such Governmental Entity or other person, give the other party the opportunity to attend and participate in such meetings and conferences. |
| (c) | In furtherance and not in limitation of the covenants of the parties contained in this Section 6.3, (i) if (1) any objections are asserted with respect to the transactions contemplated hereby under any law, rule, regulation, order or decree, (2) any administrative or judicial action or proceeding is instituted (or threatened to be instituted) by any Governmental Entity or private party challenging the Acquisitions or the other transactions contemplated hereby as violative of any law, rule, regulation, order or decree or that would otherwise prevent, materially delay or materially impede the consummation of the Acquisitions or the other transactions contemplated hereby, or (3) any law, rule, regulation, order or decree is enacted, entered, promulgated or enforced by a Governmental Entity that would make the Acquisitions or the other transactions contemplated hereby illegal or would otherwise prevent, materially delay or materially impede the consummation of the Acquisitions or the other transactions contemplated hereby, then (ii) Islet shall use its reasonable best efforts to resolve any such objections, actions or Proceedings so as to permit the consummation of the transactions contemplated by this Agreement by the date set forth in Section 8.1(c). Notwithstanding the foregoing or any other provision in this Agreement to the contrary, nothing in this Section 6.3 shall require, or be deemed to require the taking by Islet of any such action that (1) is not conditional on the consummation of the Acquisitions or (2) would reasonably be expected to result in a Material Adverse Effect on BHV. |
| (d) | In furtherance and not in limitation of the covenants of the parties contained in this Section 6.3, if any of the events specified in Section 6.3(c)(i)(2) or (3) occurs, then each of Islet and BHV shall cooperate in all respects with each other and use its reasonable best efforts, subject to Section 6.3(c), to contest and resist any such administrative or judicial action or proceeding and to have vacated, lifted, reversed or overturned any judgment, Injunction or other decree or order, whether temporary, preliminary or permanent, that is in effect and that prevents, materially delays or materially impedes the consummation of the Acquisitions or the other transactions contemplated by this Agreement and to have such law, rule, regulation, order or decree repealed, rescinded or made inapplicable so as to permit consummation of the transactions contemplated by this Agreement, and each of Islet and BHV shall use its reasonable best efforts to defend, at Islet’s cost and expense, any such administrative or judicial actions or Proceedings. |
| (e) | Notwithstanding the foregoing or any other provision of this Agreement, nothing in this Section 6.3 shall limit a party’s right to terminate this Agreement pursuant to Section 8.1(b) or 8.1(c) so long as such party has up to then complied with its obligations under this Section 6.3. |
| (f) | Each of BHV and Islet and their respective Boards of Directors or Board of Managers, as applicable, shall, if any “moratorium,” “control share,” “fair price” or other anti-takeover law or regulation becomes applicable to this Agreement, the Acquisitions , or any other transactions contemplated hereby, use all reasonable best efforts to ensure that the Acquisitions and the other transactions contemplated by this Agreement may be consummated as promptly as practicable on the terms contemplated hereby and otherwise to minimize the effect of such law or regulation on this Agreement, the Acquisitions and the other transactions contemplated hereby. |
| 6.4 | Acquisition Proposals. BHV agrees that neither it nor any of its Subsidiaries (nor any of the employees or directors of it or its Subsidiaries), nor their respective agents and representatives (including any investment banker, attorney or accountant retained by it or any of its Subsidiaries) shall, directly or indirectly, (a) initiate, solicit, encourage or knowingly facilitate any inquiries or the making of any proposal or offer with respect to, or a transaction to effect, a merger, reorganization, share exchange, consolidation, business combination, recapitalization, liquidation, dissolution or similar transaction involving it or any of its Significant Subsidiaries (other than any such transaction permitted by Section 5.1(e) or (f)) or any purchase or sale of 50% or more of the consolidated assets (including stock of its Subsidiaries) of it and its Subsidiaries, taken as a whole, or any purchase or sale of, or tender or exchange offer for, its voting securities that, if consummated, would result in any person (or the shareholders of such person) beneficially owning securities representing 50% or more of its total voting power (or of the surviving parent entity in such transaction) or any of its Significant Subsidiaries (any such proposal, offer or transaction (other than a proposal or offer made by Islet or an affiliate thereof) being hereinafter referred to as an “Acquisition Proposal”), (b) have any discussions with or provide any confidential information or data to any person relating to an Acquisition Proposal, or engage in any negotiations concerning an Acquisition Proposal, or knowingly facilitate any effort or attempt to make or implement an Acquisition Proposal, or (c) approve or recommend, or propose to approve or recommend, or execute or enter into, any letter of intent, agreement in principle, merger agreement, asset purchase or share exchange agreement, option agreement or other similar agreement related to any Acquisition Proposal or propose or agree to do any of the foregoing. |
| 6.5 | OTCQB Quotation. Holdco and Islet shall use reasonable best efforts to cause (a) the shares of Holdco Common Stock to be issued in the Acquisitions and (b) the shares of Holdco Common Stock to be reserved for issuance upon the exercise of Holdco Options, to be approved for quotation on the OTCQB, subject to official notice of issuance, prior to the Closing Date. |
| 6.6 | Section 16 Matters. Assuming that BHV and Islet deliver to Holdco the Section 16 Information reasonably in advance of the Effective Time, the Board of Directors of Holdco, or a committee of Non-Employee Directors thereof (as such term is defined for purposes of Rule 16b-3(d) under the Exchange Act), shall reasonably promptly thereafter and in any event prior to the Effective Time adopt a resolution providing that the receipt by the Insiders of BHV and Islet of Holdco Common Stock in exchange for shares of BHV Membership Interests or shares of Islet Common Stock, as the case may be, in each case pursuant to the transactions contemplated hereby and to the extent such securities are listed in the Section 16 Information provided by BHV and Islet to Holdco prior to the Effective Time, is intended to be exempt from liability pursuant to Section 16(b) under the Exchange Act such that any such receipt shall be so exempt. “Section 16 Information” shall mean information accurate in all material respects regarding the Insiders of a person, the number of shares of the capital stock held by each such Insider, and the number and description of options, stock appreciation rights, restricted shares and other stock-based awards held by each such Insider. “Insiders,” with respect to a person, shall mean those officers and directors of such person who are subject to the reporting requirements of Section 16(a) of the Exchange Act and who are listed in the Section 16 Information. |
| 6.7 | Fees and Expenses; Taxes. |
| (a) | Whether or not the Acquisitions are consummated, all costs and expenses incurred by any party hereto in connection with this Agreement and the transactions contemplated hereby shall be paid by Islet or Holdco or, to the extent previously paid by BHV or any BHV Member, reimbursed to BHV or such BHV Member. The parties agree to treat the liabilities of BHV and of each BHV Member with respect to such expenses as being assumed by Holdco under Section 357(c)(1) of the Code. |
| (b) | Islet and Holdco shall indemnify and reimburse each BHV Member with respect to any Taxes he may incur as a result of (i) the application of Section 357(c) of the Code, any applicable Treasury Regulations promulgated thereunder and any similar or successor provisions of the Code or Treasury Regulations promulgated thereunder (and any corresponding or resulting state or local Taxes) resulting from the liabilities of BHV assumed by Holdco exceeding the adjusted tax basis of the assets of BHV that are transferred to Holdco in connection with the Acquisitions, (ii) the payment by Islet or Holdco of costs and expenses incurred by BHV or such BHV Member in connection with this Agreement as contemplated by Section 6.7(a) and (iii) any payments made by Islet or Holdco to such BHV Member pursuant to this Section 6.7(b). For purposes of this Section 6.7(b), each BHV Member shall be deemed to have incurred Taxes on any income or gain required to be recognized by such BHV Member pursuant to Section 357(c) of the Code, any applicable Treasury Regulations promulgated thereunder and any similar or successor provisions of the Code or Treasury Regulations promulgated thereunder (and any corresponding or resulting state or local Taxes) and on any payment made to such BHV Member under this Section 6.7(b) at the highest marginal federal and North Carolina income tax rates applicable to individuals, regardless of the extent to which such income, gain or payment actually increases the liability of such BHV Member for Taxes. |
| 6.8 | Governance. Holdco shall cause the number of directors that will comprise the full Board of Directors of Holdco on the business day immediately following the Closing Date to be 5 and to consist of the following persons: James Green, William Wilkison, Eric Barnett, Michael Luther, and Joel Perlin. |
| (a) | From and after the Effective Time, Holdco shall, or shall cause BHV and its Subsidiaries to, to the fullest extent permitted by applicable law, indemnify, defend and hold harmless, and provide advancement of expenses to, each person who is now, or has been at any time prior to the date hereof or who becomes prior to the Effective Time, an officer or manager of BHV (the “BHV Indemnified Parties”) against all losses, claims, damages, costs, expenses, liabilities or judgments or amounts that are paid in settlement of or in connection with any claim, action, suit, proceeding or investigation based in whole or in part on or arising in whole or in part out of the fact that such person is or was a manager, officer or employee of BHV or any Subsidiary of BHV, and pertaining to any matter existing or occurring, or any acts or omissions occurring, at or prior to the Effective Time (including with respect to acts or omissions occurring in connection with the approval of this Agreement and the consummation of the transactions contemplated hereby), whether asserted or claimed prior to, or at or after, the Effective Time, in each case to the fullest extent such persons are permitted by applicable law to be indemnified by, or have the right to advancement of expenses from, BHV as of the date hereof. |
| (b) | From and after the Initial Effective Time, Holdco shall, or shall cause the Islet Surviving Corporation and its Subsidiaries to, to the fullest extent permitted by applicable law, indemnify, defend and hold harmless, and provide advancement of expenses to, each person who is now, or has been at any time prior to the date hereof or who becomes prior to the Effective Time, an officer or director of Islet or any of its Subsidiaries (the “Islet Indemnified Parties” and, together with the BHV Indemnified Parties, the “Indemnified Parties”) against all losses, claims, damages, costs, expenses, liabilities or judgments or amounts that are paid in settlement of or in connection with any claim, action, suit, proceeding or investigation based in whole or in part on or arising in whole or in part out of the fact that such person is or was a director, officer or employee of Islet or any Subsidiary of Islet, and pertaining to any matter existing or occurring, or any acts or omissions occurring, at or prior to the Initial Effective Time, whether asserted or claimed prior to, or at or after, the Initial Effective Time, in each case to the fullest extent such persons are permitted by applicable law to be indemnified by, or have the right to advancement of expenses from, Islet as of the date hereof. |
| (c) | Holdco shall have the right to defend each Indemnified Party in any proceeding which may give rise to the payment of indemnifiable amounts hereunder; provided, however, that Holdco shall notify such Indemnified Party of any such decision to defend within twenty (20) business days of receipt of notice of any such proceeding, and, provided, further, that Holdco shall not, without the prior written consent of such Indemnified Party, consent to the entry of any judgment against such Indemnified Party or enter into any settlement or compromise which (i) includes an admission of fault of such Indemnified Party or (ii) does not include, as an unconditional term thereof, the full release of such Indemnified Party from all liability in respect of such proceeding, which release shall be in form and substance reasonably satisfactory to such Indemnified Party. Notwithstanding the foregoing, if in a proceeding to which an Indemnified Party is a party by reason of the Indemnified Party’s service as a director, manager, officer or employee of BHV, Islet or any of their respective Subsidiaries, (i) a conflict of interest or potential conflict of interest exists between such Indemnified Party and Holdco (including as a result of the existence of separate defenses or counterclaims that would make such representation inappropriate), or (ii) if Holdco fails to assume the defense of such proceeding in a timely manner, such Indemnified Party shall be entitled to be represented by separate legal counsel of such Indemnified Party’s choice at the expense of Holdco; provided, however, that Holdco shall not be liable for any settlement effected without its prior written consent (which consent shall not be arbitrarily withheld, conditioned or delayed). |
| (d) | Holdco shall pay (as incurred) all expenses, including reasonable fees and expenses of counsel, that an Indemnified Party may incur in enforcing the indemnity and other obligations provided for in this Section 6.9. |
| (e) | If Holdco or any of its successors or assigns (i) consolidates with or merges into any other person and shall not be the continuing or surviving corporation or entity of such consolidation or merger, or (ii) transfers or conveys all or substantially all of its properties and assets to any person, then, and in each such case, to the extent necessary, proper provision shall be made so that the successors and assigns of Holdco, as the case may be, shall assume the obligations set forth in this Section 6.9. |
| (f) | The provisions of this Section 6.9(f) are intended to be for the benefit of, and shall be enforceable by, each BHV Indemnified Party and Islet Indemnified Party and their respective heirs and representatives and are in addition to, and not in substitution for, any other rights to indemnification or contribution that any such person may have by contract or otherwise. |
| 6.10 | Public Announcements. Islet and BHV shall use reasonable best efforts (i) to develop a joint communications plan, (ii) to ensure that all press releases and other public statements with respect to the transactions contemplated hereby shall be consistent with such joint communications plan, and (iii) except in respect of any announcement required by applicable law or by obligations pursuant to any listing agreement with or rules of any securities exchange in which it is impracticable to consult with each other as contemplated by this clause (iii), to consult with each other before issuing any press release or, to the extent practical, otherwise making any public statement with respect to this Agreement or the transactions contemplated hereby. |
| (a) | In addition to the Thirty Million (30,000,000) shares of Holdco Common Stock (as adjusted for the Conversion Rate) to be received by the BHV Members in the BHV Securities Exchange, Holdco shall issue to the BHV Members, based on the percentages set forth on Schedule 2.3(b), within five (5) business days of the occurrence of the corresponding events set forth on Schedule 6.11(a) (each a “Milestone”) the number of shares of newly-issued Holdco Common Stock equal to the (i) absolute value of the dollar amount set forth next to such Milestone on Schedule 6.11(a), equal in the aggregate to $71,000,000, divided by (ii) the volume weighted average price of Holdco Common Stock for the thirty (30) trading days preceding the occurrence of each Milestone as reported by Bloomberg L.P. (the “Milestone Shares”), subject to adjustment based on Section 6.11(h). For the avoidance of doubt, a Milestone shall be deemed to have been achieved in the event that it is achieved by or on behalf of Holdco or any of its affiliates, licensees or sub-licensees. |
| (b) | In the event that a later Milestone (i.e., any applicable Milestone other than Milestone #1) is achieved and one or more of the earlier Milestones (i.e., any applicable Milestone other than the final Milestone) were not achieved or were not paid prior to the later Milestone being achieved, Holdco shall issue the Milestone Shares corresponding to each of such unachieved and/or unpaid earlier Milestones together with Milestone Shares to be issued for such later Milestone. |
| (c) | In the event that at the time of any Milestone, the Holdco Common Stock is not listed and traded on a national securities exchange or quoted in the over the counter markets on the OTCQB, the market price of the Holdco Common Stock shall be determined by a nationally recognized independent third party with expertise in valuing companies similar to Holdco appointed by the Holdco Board of Directors using their reasonable judgment. The Milestone Shares, when issued, shall be validly issued, fully paid and non-assessable, and free and clear of any Liens. |
| (d) | Holdco shall (i) use, and cause its affiliates to use, Commercially Reasonable Efforts to promptly achieve the Milestones; (ii) maintain, and cause its affiliates to maintain, the assets and properties of BHV and continuously use them in furtherance of achievement of the Milestones; and (iii) provide sufficient capital and personnel resources for the development of the Compound in order to optimize the opportunity for rapid achievement of the Milestones. |
| (e) | Should James T. Green or William Wilkison cease to be the Chief Executive Officer or the Chief Operating Officer of Holdco, respectively, until all Milestones have been achieved and all payments pursuant to Section 6.11(a) have been made, Holdco shall provide, and cause its affiliates to provide, the BHV Members with a written summary on the last day of each calendar quarter describing in reasonable detail the status of achieving each Milestone. |
| (f) | Upon the occurrence of either (i) a Compound Change of Control or (ii) Holdco’s violation of any term or condition of this Section 6.11, payments of all Milestone Shares under Section 6.11(a) shall become immediately due and payable in full without regard to the completion of any Milestone; provided, however, that with respect to a violation of Sections 6.11(a) and 6.11(e), payments of Milestone Shares shall only become immediately due and payable pursuant to this Section 6.11(f) upon Holdco’s failure to cure such violation within fifteen (15) days after receiving a notice of violation from a BHV Member. |
| (g) | If (i) James T. Green or William Wilkison is terminated from their respective roles as Chief Executive Officer or Chief Operating Officer of Holdco, other than for Cause (as such term is defined in their respective Employment Agreements) or (ii) Mr. Green or Dr. Wilkison terminates his respective Employment Agreement for Good Reason (as such term is defined therein), payment of the Milestone Shares related to the next Milestone that has not yet been achieved under Section 6.11(a) shall become immediately due and payable in full, without regard to the completion of such Milestone. |
| (h) | If, during the period commencing immediately following the Closing and ending on the date of the issuance of Milestone Shares in connection with achievement of the last Milestone under this Section 6.11 (the “BHV Warrant Period”), Holdco issues any shares of Holdco Common Stock pursuant to the BHV Warrant (the “BHV Warrant Shares”) or makes any cash payment in connection with the termination of the BHV Warrant (a “BHV Warrant Payment”), the number of shares of Holdco Common Stock remaining to be issued to the BHV Members as Milestone Shares pursuant to this Section 6.11 shall be reduced by the Milestone Adjustment Amount. The “Milestone Adjustment Amount” shall be equal to the following: (i) in the case of the issuance of Holdco Common Stock pursuant to the BHV Warrant during the BHV Warrant Period, the number of BHV Warrant Shares, or (ii) in the case of a BHV Warrant Payment pursuant to termination of the BHV Warrant during the BHV Warrant Period, a number of shares of Holdco Common Stock determined by dividing the BHV Warrant Payment by the weighted average price of Holdco Common Stock for the thirty (30) trading days preceding the date the BHV Warrant Payment is made, subject in each case to the following: (A) the Milestone Adjustment Amount shall be reduced by the number of shares of Holdco Common Stock determined by dividing the value of any cash or other property received by Holdco or any Affiliate of Holdco in connection with the issuance of the BHV Warrant Shares (e.g., any exercise price paid) by the closing price of Holdco Common Stock on the date of payment; (B) in no event shall the total Milestone Adjustment Amount be greater than the number of shares of Holdco Common Stock to which the holder of the BHV Warrant would have been entitled under this Agreement had it exercised the BHV Warrant prior to Closing and been a party hereto; (C) the Milestone Adjustment Amount shall be applied proportionally against any remaining Milestones that may be achieved under this Section 6.11 based on the relative dollar amounts of such Milestones as set forth in Schedule 6.11(a), provided that if the Milestone Adjustment Amount exceeds the number of shares that would otherwise have been issued based on the achievement of such Milestone, the Milestone Adjustment Amount applicable to such Milestone shall be limited to such number of shares; and (D) Holdco and Islet agree to use commercially reasonable efforts to minimize the size of any Milestone Adjustment Amount. This Section 6.11(h) constitutes the exclusive remedy for Holdco, Islet and their respective Affiliates in connection with all matters relating to the BHV Warrant. In furtherance and not in limitation of the foregoing, (1) if any BHV Warrant Shares are issued or any BHV Warrant Payment is made following the expiration of the BHV Warrant Period, there shall be no Milestone Adjustment Amount and the BHV Members will have no liability in connection with such issuance or payment; (2) if a Milestone to which all or a portion of the Milestone Adjustment Amount is allocated pursuant to clause (C) of the preceding sentence is ultimately not achieved, such Milestone Adjustment Amount shall nevertheless be deemed satisfied; and (3) in no event will any Milestone Shares actually issued to the BHV Members under this Section 6.11 be subject to redemption or forfeiture in connection with any issuance of BHV Warrant Shares, any BHV Warrant Payment or otherwise. |
| (i) | The rights of the BHV Members under this Section 6.11 shall not be represented by a certificate or other instrument and, until issuance of Holdco Common Stock pursuant to the provisions of this Section 6.11, shall not represent an ownership interest in Holdco or entitle the BHV Members to any rights common to any holder of any equity security of Holdco. |
| 6.12 | Anti-dilution. If at any time following the date of this Agreement Islet or Holdco, as the case may be, issues additional shares of Islet Common Stock or Holdco Common Stock, respectively, pursuant to or in connection with any agreement or arrangement that is in effect as of the date hereof or that results from or arises out of facts in existence as of the date hereof (other than any compensatory award pursuant to an Islet Employee Benefit Plan or otherwise pursuant to any currently outstanding security exercisable for or convertible into Islet Common Stock or Holdco Common Stock included in Islet’s most recent publicly filed financial statements), or any amendment, modification, termination, replacement or settlement of or in connection with any such agreement or arrangement (regardless of whether prior to, on or after the date hereof) (each, a “Dilutive Agreement”), Holdco shall be required to issue such number of shares of Holdco Common Stock to each BHV Member as is necessary to prevent each BHV Member’s percentage ownership in Holdco from being diluted (the “Anti-dilution Shares”). Any issuance(s) of Anti-dilution Shares shall be made concurrently with the issuance(s) of shares pursuant to the Dilutive Agreement; provided, however, that if any issuance of shares pursuant to a Dilutive Agreement occurs prior to Closing, such related Anti-dilution Shares shall be issued at Closing. In connection with each issuance of Anti-dilution Shares, if any, each BHV Member hereby represents and warrants that, as of the date of this Agreement, he is an “accredited investor” within the meaning of Rule 501 under the Securities Act, and further acknowledges and agrees as follows: |
| (a) | the Anti-dilution Shares issuable to such BHV Member hereunder will be issued by reason of a specific exemption from the registration provisions of the Securities Act which depends upon, among other things, the bona fide nature of the investment intent and the accuracy of the BHV Member’s representations as expressed herein; |
| (b) | the Anti-dilution Shares issuable to such BHV Member hereunder will be characterized as “restricted securities” under the Securities Act inasmuch as this Agreement contemplates that, if acquired by the BHV Member pursuant hereto, such shares would be acquired in a transaction not involving a public offering. Such BHV Member further acknowledges that the Anti-dilution Shares may not be resold without registration under the Securities Act or the existence of an exemption therefrom. Such BHV Member represents that he is familiar with Rule 144 promulgated under the Securities Act, as presently in effect, and understands the resale limitations imposed thereby and by the Securities Act; and |
| (c) | the Anti-dilution Shares issuable to such BHV Member hereunder will bear the following legend or another legend that is similar to the following: |
THESE SECURITIES HAVE NOT BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), AND, ACCORDINGLY, MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS AS EVIDENCED BY A LEGAL OPINION OF COUNSEL TO SUCH EFFECT, THE SUBSTANCE OF WHICH SHALL BE REASONABLY ACCEPTABLE TO THE COMPANY.
| 6.13 | Additional Agreements. In case at any time after the Effective Time any further action is necessary or desirable to carry out the purposes of this Agreement or to vest Holdco or the surviving corporation of the Acquisitions with full title to all properties, assets, rights, approvals, immunities and franchises of either of the constituent corporations of the Islet Merger and the BHV Securities Exchange, the proper officers and directors of each party to this Agreement shall use reasonable best efforts to take all such necessary action. |
ARTICLE VII
CONDITIONS PRECEDENT
| 7.1 | Conditions to Each Party’s Obligation to Effect the Merger. The respective obligation of each party to effect the Islet Merger or BHV Securities Exchange, as applicable, shall be subject to the satisfaction prior to the Closing Date of the following conditions: |
| (a) | Shareholder Approval. Islet shall have obtained the Required Islet Consent. |
| (b) | Quotation. The shares of Holdco Common Stock to be issued in the Acquisitions and Holdco Common Stock to be reserved for issuance in connection with the Acquisitions shall have been authorized for quotation on the OTCQB, upon official notice of issuance. |
| (c) | Requisite Regulatory Approvals. Except as would not, individually or in the aggregate, reasonably be expected to result in a Material Adverse Effect on BHV, the Islet Surviving Corporation or Holdco, (i) all authorizations, consents, orders or approvals of, or declarations or filings with, and all expirations of waiting periods required from, any Governmental Entity shall have been filed, have occurred or been obtained (all such permits, approvals, filings and consents and the lapse of all such waiting periods being referred to as the “Requisite Regulatory Approvals”), and (ii) all such Requisite Regulatory Approvals referred to in clause (A) shall be in full force and effect. |
| (d) | Form S-4. The Form S-4 shall have been declared effective under the Securities Act and shall not be the subject of any stop order, and no Proceeding for that purpose, and no similar Proceeding with respect to the Consent Solicitation Statement/Prospectus, shall have been initiated or threatened in writing by the SEC or its staff. |
| (e) | Investor Rights. Holdco and the BHV Members shall have entered into the Investor Rights Agreement in the form set forth in Exhibit C. |
| (f) | No Injunctions or Restraints; Illegality. No temporary restraining order, preliminary or permanent injunction or other order issued by any court of competent jurisdiction or other legal restraint or prohibition (an “Injunction”) preventing the consummation of the Acquisitions shall be in effect. There shall not be any action taken, or any law, rule, regulation or order enacted, entered, enforced or deemed applicable to the Acquisitions, by any Governmental Entity of competent jurisdiction that makes the consummation of the Acquisitions illegal. |
| (i) | Islet shall have received an opinion from Anderson Bradshaw, PLLC, in form and substance reasonably satisfactory to each of Islet and BHV, dated the Closing Date, to the effect that, on the basis of facts, representations and assumptions set forth or referred to in such opinion, (i) the formation of Holdco and Islet Merger Sub in anticipation of the Islet Merger will be treated for federal income tax purposes as an exchange described in Section 351 of the Code as an exchange, and as such, these formations will be treated as non-taxable capitalization and will be disregarded for tax purposes, (ii) the conversion of Islet securities by the securityholders of Islet for Holdco securities as described in Sections 3.1 and 3.2 of this Agreement will qualify as an exchange under Section 351 of the Code, and the provisions of Section 368(a)(1)(E) of the Code will apply to this conversion such that the Islet Merger will qualify as a tax-free reorganization, and (iii) the Islet securityholders’ basis in their Islet securities will transfer to and be considered as carryover basis in the Holdco securities received as a result of the conversion. In rendering such opinion, Anderson Bradshaw, PLLC shall be entitled to rely upon customary representations and assumptions provided by Holdco, Islet, BHV and others that Anderson Bradshaw, PLLC reasonably deems relevant. |
| (ii) | BHV shall have received an opinion from BDO USA, LLP, in form and substance reasonably satisfactory to each of Islet and BHV, dated the Closing Date, to the effect that, on the basis of facts, representations and assumptions set forth or referred to in such opinion, the exchange of BHV Membership Interests for Holdco Common Stock (including the shares of Holdco Common Stock to be received by the BHV Members pursuant to Section 6.11 of this Agreement) should be treated for U.S. federal income tax purposes as an exchange described in Section 351 of the Code. In rendering such opinion, BDO USA, LLP shall be entitled to rely upon customary representations and assumptions provided by Holdco, Islet, BHV and others that BDO USA, LLP reasonably deems relevant. |
| 7.2 | Conditions to Obligations of Islet. The obligation of Islet to effect the Islet Merger is subject to the satisfaction of the following conditions unless waived by Islet: |
| (a) | Representations and Warranties. The representations and warranties of BHV contained in this Agreement shall be true and correct as of the date hereof and as of the Closing Date as if made at and as of that time (except for representations and warranties made only as of a specified date, which shall be true and correct as of the specified date), except to the extent where the failures of any such representations and warranties to be so true and correct, in the aggregate, have not had, and would not reasonably be expected to have, a Material Adverse Effect on BHV. Islet shall have received a certificate signed on behalf of BHV by the Managers of BHV to such effect. |
| (b) | Performance of Obligations of BHV. BHV shall have performed in all material respects all obligations required to be performed by it under this Agreement at or prior to the Closing Date, and Islet shall have received a certificate signed on behalf of BHV by the Managers of BHV to such effect. |
| (c) | Absence of Legal Restraint. There shall not have been any action with respect to the Acquisitions taken since the date of this Agreement by any court or other Governmental Entity (which action has not been vacated or reversed), or any law, Injunction, order or decree enacted, promulgated or issued with respect to the Acquisitions by any court or other Governmental Entity (which law, Injunction, order or decree remains in effect), that would reasonably be expected to result in a judgment that would have any of the following effects: (i) challenging or seeking to make illegal, to delay materially or otherwise to restrain or prohibit the consummations of the Acquisitions, (ii) seeking to restrain or prohibit Islet’s or Holdco’s ownership or operation (or that of its respective Subsidiaries or affiliates) of all or any material portion of the business or assets of BHV and its Subsidiaries, taken as a whole, or of Islet and its Subsidiaries, taken as a whole, or (iii) seeking to compel Islet or Holdco or any of their respective Subsidiaries to sell, hold separate or otherwise disposing of any of its or BHV’s business or assets or materially restricting the conduct its or BHV’s business if doing so would, individually or in the aggregate, reasonably be expected to result in a Material Adverse Effect on BHV. |
| (d) | BHV Assignments. The BHV Members shall have delivered to Islet assignment agreements assigning the ownership of the BHV Membership Interests to Holdco in the form set forth in Exhibit D fully executed by each BHV Member. |
| (e) | BHV Material Adverse Effect. Since the date of this Agreement, there shall not have been, with respect to BHV, any Material Adverse Effect or any event, change or effect that would, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect. |
| 7.3 | Conditions to Obligations of BHV. The obligation of BHV to effect the BHV Securities Exchange is subject to the satisfaction of the following conditions unless waived by BHV: |
| (a) | Representations and Warranties. The representations and warranties of Islet contained in this Agreement shall be true and correct as of the date hereof and as of the Closing Date as if made at and as of that time (except for representations and warranties made only as of a specified date, which shall be true and correct as of the specified date), except to the extent where the failures of any such representations and warranties to be so true and correct, in the aggregate, have not had, and would not reasonably be expected to have, a Material Adverse Effect on Islet. BHV shall have received a certificate signed on behalf of Islet by the Chief Financial Officer of Islet to such effect. |
| (b) | Performance of Obligations of Islet. Islet shall have performed in all material respects all obligations required to be performed by it under this Agreement at or prior to the Closing Date, and BHV shall have received a certificate signed on behalf of Islet by the Chief Financial Officer of Islet to such effect. |
| (c) | Islet Material Adverse Effect. Since the date of this Agreement, there shall not have been, with respect to Islet, any Material Adverse Effect or any event, change or effect that would, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect. |
ARTICLE VIII
TERMINATION AND AMENDMENT
| 8.1 | Termination. This Agreement may be terminated at any time prior to the Effective Time, by action taken or authorized by the Board of Directors or Board of Managers of the terminating party or parties: |
| (a) | by mutual consent of Islet and BHV in a written instrument; |
| (b) | by either Islet or BHV, upon written notice to the other party, if a Governmental Entity of competent jurisdiction that must grant a Requisite Regulatory Approval has denied approval of the Islet Merger or BHV Securities Exchange and such denial has become final and non-appealable; or any Governmental Entity of competent jurisdiction shall have issued an order, decree or ruling or taken any other action permanently restraining, enjoining or otherwise prohibiting the Islet Merger or BHV Securities Exchange, and such order, decree, ruling or other action has become final and non-appealable; provided, however, that the right to terminate this Agreement under this Section 8.1(b) shall not be available to any party whose failure to comply with Section 6.3 or any other provision of this Agreement has been the cause of, or resulted in, such action; |
| (c) | by either Islet or BHV, upon written notice to the other party, if there shall have been a breach by the other party of any of the covenants or agreements, or failure to be true of any of the representations or warranties, set forth in this Agreement on the part of such other party, which breach, or failure to be true, either individually or in the aggregate, would result in, if occurring or continuing on the Closing Date, the failure of the condition set forth in Section 7.2(a) or (b) or Section 7.3(a) or (b), as the case may be, and which breach, or failure to be true, has not been cured within thirty (30) days following written notice thereof to the affected party or, by its nature, cannot be cured within such time period. |
| (d) | by either Islet or BHV, if the transactions contemplated by this Agreement shall not have been consummated by April 30, 2015 (the “Outside Date”), provided, however, that the right to terminate this Agreement pursuant to this Section 8.1(d) shall not be available to Islet or BHV if its action or failure to act constitutes a material breach or violation of any of its covenants, agreements or other obligations hereunder and such material breach or violation has been the principal cause of or directly resulted in (i) the failure to satisfy the conditions to the obligations of the terminating party to consummate the transactions contemplated by this Agreement prior to the Outside Date or (ii) the failure of the Closing to occur by the Outside Date. |
| (e) | by BHV, if Islet enters into, or announces the entry into, an agreement for a transaction to effect, a merger, reorganization, share exchange, consolidation, business combination, recapitalization, liquidation, dissolution or similar transaction involving it or any of its Significant Subsidiaries (other than any such transaction permitted by Section 5.2(e) or (f)) or any purchase or sale of 50% or more of the consolidated assets (including stock of its Subsidiaries) of it and its Subsidiaries, taken as a whole, or any purchase or sale of, or tender or exchange offer for, its voting securities that, if consummated, would result in any person (or the shareholders of such person) beneficially owning securities representing 50% or more of its total voting power (or of the surviving parent entity in such transaction) or any of its Significant Subsidiaries. |
| 8.2 | Effect of Termination. In the event of termination of this Agreement by either BHV or Islet as provided in Section 8.1, this Agreement shall forthwith become void, and there shall be no liability or obligation on the part of Islet or BHV or their respective officers or directors, except with respect to Section 6.2 (Access to Information; Confidentiality), Section 6.7 (Fees and Expenses; Taxes), this Section 8.2 (Effect of Termination), and ARTICLE IX (General Provisions), which shall survive such termination and except that no party shall be relieved or released from any liabilities or damages arising out of its willful and material breach of this Agreement. |
| 8.3 | Amendment. This Agreement may only be amended modified or supplemented by an agreement in writing signed by each party hereto. |
| 8.4 | Extension; Waiver. At any time prior to the Effective Time, the parties, by action taken or authorized by their respective Board of Directors or Board of Managers, as applicable, may, to the extent permitted by applicable law, (a) extend the time for the performance of any of the obligations or other acts of the other party, (b) waive any inaccuracies in the representations and warranties contained herein or in any document delivered pursuant hereto, and (c) waive compliance with any of the agreements or conditions contained herein. Any agreement on the part of a party to any such extension or waiver shall be valid only if set forth in a written instrument signed on behalf of such party. The failure of a party to assert any of its rights under this Agreement or otherwise shall not constitute a waiver of those rights. No single or partial exercise of any right, remedy, power or privilege hereunder shall preclude any other or further exercise thereof or the exercise of any other right, remedy, power or privilege. Any waiver shall be effective only in the specific instance and for the specific purpose for which given and shall not constitute a waiver to any subsequent or other exercise of any right, remedy, power or privilege hereunder. |
ARTICLE IX
GENERAL PROVISIONS
| 9.1 | Non-survival of Representations, Warranties and Agreements. None of the representations, warranties, covenants and agreements in this Agreement or in any instrument delivered pursuant to this Agreement, including any rights arising out of any breach of such representations, warranties, covenants and agreements, shall survive the Effective Time, except for those covenants and agreements that by their terms apply or are to be performed in whole or in part after the Effective Time, specifically including, but not limited to, the agreements set forth in Section 6.11. |
| 9.2 | Notices. All notices, requests, consents, claims, demands, waivers and other communications hereunder shall be in writing and shall be deemed to have been given (a) when delivered by hand (with written confirmation of receipt), (b) when received by the addressee if sent by a nationally recognized overnight courier (with confirmation of delivery), (c) on the date sent by e-mail (with confirmation of receipt) if sent during normal business hours of the recipient, and on the next business day if sent after normal business hours of the recipient or (d) on the third day after the date mailed, by certified or registered mail, return receipt requested, postage prepaid. Such communications must be sent to the respective parties at the following addresses or e-mail addresses (or at such other address or e-mail address for a party as shall be specified for such purpose in a notice given in accordance with this Section 9.2). |
(a) if to Islet, Holdco, or Islet Merger Sub, to:
Islet Sciences, Inc.
8601 Six Forks Rd., Suite 400
Raleigh, North Carolina 27615
Attention: Chief Financial Officer
E-mail: steve@isletsciences.com
with a copy to:
Ofsink, LLC
230 Park Avenue, Suite 851
New York, New York 10169
Attention: Darren L. Ofsink, Esq.
E-mail: dofsink@golawintl.com
(b) if to BHV, to:
Brighthaven Ventures, L.L.C.
8601 Six Forks Rd., Suite 400
Raleigh, North Carolina 27615
Attention: James Green
E-mail: greenjt@hotmail.com
with a copy to:
Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P.
2300 Wells Fargo Capitol Center
Post Office Box 2611
Raleigh, North Carolina 27602-2611
Attention: Heyward D. Armstrong, Esq.
E-mail: harmstrong@smithlaw.com
and
(b) if to a BHV Member, to:
James T. Green
8601 Six Forks Rd., Suite 400
Raleigh, North Carolina 27615
E-mail: greenjt@hotmail.com
William Wilkison
8601 Six Forks Rd., Suite 400
Raleigh, North Carolina 27615
E-mail: bill_wilkison@hotmail.com
with a copy to:
Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P.
2300 Wells Fargo Capitol Center
Post Office Box 2611
Raleigh, North Carolina 27602-2611
Attention: Heyward D. Armstrong, Esq.
E-mail: harmstrong@smithlaw.com
| 9.3 | Interpretation. When a reference is made in this Agreement to Sections, Exhibits or Schedules, such reference shall be to a Section of or Exhibit or Schedule to this Agreement unless otherwise indicated. The headings contained in this Agreement are for reference purposes only and shall not affect in any way the meaning or interpretation of this Agreement. Whenever the words “include,” “includes” or “including” are used in this Agreement, they shall be deemed to be followed by the words “without limitation.” The phrase “made available” in this Agreement shall mean that the information referred to has been made available by the party to whom such information is to be made available. The phrases “herein,” “hereof,” “hereunder” and words of similar import shall be deemed to refer to this Agreement as a whole, including the Exhibits and Schedules hereto, and not to any particular provision of this Agreement. The word “or” shall be inclusive and not exclusive. Any pronoun shall include the corresponding masculine, feminine and neuter forms. The phrases “known” or “knowledge” mean, with respect to any party to this Agreement, the actual knowledge of such party if an individual or, if such party is an entity, the actual knowledge of such entity’s executive officers who have been involved in the negotiation of this Agreement on behalf of such party. The term “affiliate” has the meaning given to it in Rule 12b-2 of the Exchange Act, and the term “person” has the meaning given to it in Sections 3(a)(9) and 13(d)(3) of the Exchange Act. |
| 9.4 | Counterparts. This Agreement may be executed in counterparts, each of which shall be considered one and the same agreement and this Agreement shall become effective when a counterpart signed by each party shall be delivered to the other party, it being understood that both parties need not sign the same counterpart. Facsimile execution and facsimile or electronic delivery of this Agreement is legal, valid and binding for all purposes. |
| 9.5 | Entire Agreement; No Third Party Beneficiaries. This Agreement (including the documents and the instruments referred to herein) (a) constitutes the entire agreement and supersedes all prior agreements and understandings, both written and oral, between the parties with respect to the subject matter hereof, other than the Confidentiality Agreement, which shall survive the execution and delivery of this Agreement in accordance with their terms and (b) except as provided in Sections 6.9(a) and (b) (which are intended for the benefit of only the persons specifically named therein), is not intended to confer upon any person other than the parties any rights or remedies hereunder. |
| 9.6 | Governing Law. This Agreement shall be governed by, and construed in accordance with, the laws of the State of Delaware, without regard to any applicable conflicts of law. |
| 9.7 | Severability. Any term or provision of this Agreement that is invalid or unenforceable in any jurisdiction shall, as to that jurisdiction, be ineffective to the extent of such invalidity or unenforceability and, unless the effect of such invalidity or unenforceability would prevent the parties from realizing the major portion of the economic benefits of the Acquisitions that they currently anticipate obtaining therefrom, shall not render invalid or unenforceable the remaining terms and provisions of this Agreement or affect the validity or enforceability of any of the terms or provisions of this Agreement in any other jurisdiction. If any provision of this Agreement is so broad as to be unenforceable, the provision shall be interpreted to be only so broad as is enforceable. |
| 9.8 | Assignment. Neither this Agreement nor any of their respective rights, interests or obligations may be assigned by Islet, Holdco or Islet Merger Sub (whether by operation of law or otherwise) without the prior written consent of the BHV Members, and any attempt to make any such assignment without such consent shall be null and void. The BHV Members may assign any of their respective rights, interests or obligations hereunder, without the prior written consent of Islet, Holdco or Islet Merger Sub; provided, however, that the rights of the BHV Members under Section 6.11 shall not be assignable except by operation of law. Subject to the foregoing, this Agreement will be binding upon, inure to the benefit of and be enforceable by the parties and their respective successors and permitted assigns. |
| 9.9 | Submission to Jurisdiction. Each party irrevocably submits to the jurisdiction of (a) any court sitting in New Castle County, Delaware, including the Delaware Court of Chancery, and (b) the United States District Court for the District of Delaware, for the purposes of any suit, action or other proceeding arising out of this Agreement or any transaction contemplated hereby. Each party agrees to commence any action, suit or proceeding relating hereto either in the United States District Court for the District of Delaware or, if such suit, action or other proceeding may not be brought in such court for reasons of subject matter jurisdiction, in any court sitting in New Castle County, Delaware. Each party irrevocably and unconditionally waives any objection to the laying of venue of any action, suit or proceeding arising out of this Agreement or any transaction contemplated hereby in (i) any court sitting in New Castle County, Delaware, including the Delaware Court of Chancery, or (ii) the United States District Court for the District of Delaware, and hereby further irrevocably and unconditionally waives and agrees not to plead or claim in any such court that any such action, suit or proceeding brought in any such court has been brought in an inconvenient forum. Each party further irrevocably consents to the service of process out of any of the aforementioned courts in any such suit, action or other proceeding by the mailing of copies thereof by mail to such party at its address set forth in this Agreement, such service of process to be effective upon acknowledgment of receipt of such registered mail; provided that nothing in this Section 9.9 shall affect the right of any party to serve legal process in any other manner permitted by law. The consent to jurisdiction set forth in this Section 9.9 shall not constitute a general consent to service of process in the State of Delaware and shall have no effect for any purpose except as provided in this Section 9.9. The parties agree that a final judgment in any such suit, action or proceeding shall be conclusive and may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by law. |
| 9.10 | Prior Company Counsel; Non Transfer of Attorney-Client Privilege. |
| (a) | Each of Islet, Holdco and Islet Merger Sub hereby acknowledges that Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P. prior to the Closing (the “Prior BHV Counsel”) represented BHV and the BHV Members. Each of Islet, Holdco and Islet Merger Sub agrees that to the extent that Holdco, through its acquisition of BHV and the BHV Membership Interests, acquires any right to treat the Prior BHV Counsel as Holdco’s counsel or former counsel, it will not, solely as a result thereof, take any action to disqualify the Prior BHV Counsel from acting and continuing to act as counsel to the BHV Members in connection with any matters related to this Agreement or in the event of a dispute hereunder. |
| (b) | Islet, Holdco and Islet Merger Sub further agree that, as to all communications among Prior BHV Counsel, BHV and any BHV Member, to the extent such communications are specifically related to the negotiation, documentation and consummation of the transactions contemplated by this Agreement, the attorney-client privilege and the expectation of client confidences belong to the BHV Members and shall not pass to or be claimed by Islet, Holdco, Islet Merger Sub or BHV after the Closing Date. Accordingly, Islet, Holdco, Islet Merger Sub and BHV after the Closing Date shall not, and shall not have any right to, own, retain, access or use any such privileged communications or the files of Prior BHV Counsel relating to such engagement from and after the Closing Date. Additionally, BHV, prior to the Effective Time, or the BHV Members, within thirty (30) days following the Closing Date, may transfer or copy electronic or hard copy files and records (or portions thereof) of BHV containing such privileged communications to one or more locations designated by the BHV Members that are not controlled by BHV and, if applicable, BHV shall cooperate with the BHV Members to cause any remaining copies of such privileged communications held by BHV to thereafter be deleted or destroyed. If, following the Closing Date, Holdco, Islet Merger Sub or BHV discover any additional files and records (or portions thereof) containing such privileged communications remaining with BHV, Holdco, Islet Merger Sub or BHV, as applicable, promptly shall notify the BHV Members and shall follow the direction of the BHV Members with respect to transfer or destruction of such files and records. None of Islet, Holdco, Islet Merger Sub or BHV may assert or contend that the attorney-client privilege was waived by the discovery after the Closing Date, by Islet, Holdco, Islet Merger Sub or BHV, of additional files or records (or portions thereof) containing such communications protected by the attorney-client privilege. All other privileged attorney-client communications and client confidences among BHV and its counsel, including Prior BHV Counsel, to the extent such communications are not specifically related to the transactions contemplated by this Agreement, shall pass and belong solely to BHV following the Closing. In addition and notwithstanding the foregoing, in the event that a dispute arises between Islet, Holdco, Islet Merger Sub or BHV and a third-party other than a party to this Agreement after the Closing, BHV may assert the attorney-client privilege to prevent disclosure to a third-party of such confidential communications that are specifically related to the transactions contemplated by this Agreement; provided, however, that BHV may not waive the attorney-client privilege regarding such communications without the express written consent of the BHV Members (which consent shall not be unreasonably withheld, conditioned or delayed). |
| 9.11 | Enforcement. The parties agree that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed in accordance with their specific terms on a timely basis or were otherwise breached. It is accordingly agreed that the parties shall be entitled to an Injunction or other equitable relief to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement in any court identified in the Section above, this being in addition to any other remedy to which they are entitled at law or in equity. |
| 9.12 | WAIVER OF JURY TRIAL. EACH OF THE PARTIES HEREBY WAIVES TRIAL BY JURY IN ANY JUDICIAL PROCEEDING DIRECTLY INVOLVING ANY MATTERS (WHETHER SOUNDING IN TORT, CONTRACT OR OTHERWISE) IN ANY WAY ARISING OUT OF, RELATED TO, OR CONNECTED WITH THIS AGREEMENT OR ANY TRANSACTION CONTEMPLATED HEREBY. |
[Remainder of this Page Intentionally Left Blank]
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be signed by their respective officers thereunto duly authorized, all as of the date first set forth above.
| | ISLET SCIENCES, INC. | |
| | | | |
| | By: | /s/ Steven Delmar | |
| | | Steven Delmar | |
| | | CFO | |
| | | | |
| | BRIGHTHAVEN VENTURES, L.L.C. | |
| | | | |
| | By: | /s/ Bentley Cheatham | |
| | | Bentley Cheatham | |
| | | CEO | |
| | | | |
| | AVOGENX, INC. | |
| | | | |
| | By: | /s/ Steven Delmar | |
| | | | |
| | | CFO | |
| | | | |
| | ISLET MERGER SUB, INC. | |
| | | | |
| | By: | /s/ Steven Delmar | |
| | | | |
| | | CFO | |
| | | | |
| | BHV MEMBERS: | |
| | | | |
| | | /s/ James T. Green | |
| | | James T. Green | |
| | | | |
| | | /s/William Wilkison | |
| | | William Wilkison | |
| | | | |
Exhibit A
CERTIFICATE OF INCORPORATION
OF
AVOGENX, INC.
FIRST: The name of the corporation (hereinafter called the “Corporation”) is Avogenx, Inc.
SECOND: The address of the registered office of the Corporation in the State of Delaware is 1811 Silverside Road in the City of Wilmington, zip code 19810, New Castle County; and the name of the registered agent of the Corporation at such address is Vcorp Services, LLC.
THIRD: The purpose of the Corporation is to engage in any lawful act or activity for which corporations may be organized under the Delaware General Corporation Law.
FOURTH: (a) The total number of shares of stock which the Corporation shall have authority to issue is 210,000,000 shares, of which 200,000,000 shares shall be Common Stock of the par value of $.001 per share and 10,000,000 shares shall be Preferred Stock of the par value of $.001 per share, issuable in series.
(b) The designations, preferences, privileges and voting powers of each class of stock of the Corporation, and the restrictions and qualifications thereof, shall be as follows:
A. The Preferred Stock. The Board of Directors is vested with authority, to the extent permitted by the laws of Delaware, to issue the Preferred Stock from time to time in one or more series, each series to have such relative rights, preferences and limitations as shall be determined by the Board of Directors. All shares of the Preferred Stock shall be identical except to the following relative rights and preferences as to which there may be variations between different series:
(1)The number of shares constituting such series, and the designation thereof to distinguish the shares of such series from the shares of all other series;
(2)The rate of dividend, the time of payment and the dates from which dividends shall be cumulative, and the extent of participation rights, if any:
(3)Any right to vote with holders of shares of any other series or class, the number of votes per share and any right to vote as a class, either generally or as a condition to specified corporate action;
(4)The price at and the terms and conditions on which shares may be redeemed;
(5)The amount payable upon shares in the event of involuntary liquidation;
(6)The amount payable upon shares in the event of voluntary liquidation;
(7)Sinking fund provisions for the redemption or purchase of shares;
(8)The terms and conditions on which shares may be converted, if the shares of any series are issued with the privilege of conversion.
Prior to the issuance of any shares of Preferred Stock, the Board of Directors shall have established such series by adopting a resolution or resolutions setting forth the designation and number of shares of the series and the voting powers, designations, preferences and relative, participating, optional, or other rights, if any, and the qualifications, limitations or restrictions thereof, if any, to the extent permitted by the provisions hereof, and the Corporation shall have filed, in the office of the Secretary of State of the State of Delaware, a certificate setting forth a copy of such resolution or resolutions.
B. The Common Stock. Subject to the preferences, privileges and voting powers, and the restrictions and qualifications thereof, of the Preferred Stock, the holders of the common stock shall have and possess all rights appertaining to capital stock of the Corporation. Holders of common stock shall have one vote for each share held.
FIFTH: The Corporation is to have perpetual existence.
SIXTH: For the management of the business and for the conduct of the affairs of the Corporation, and in further definition, limitation and regulation of the powers of the Corporation and of its directors and of its stockholders or any class thereof, as the case may be, it is further provided:
1. The management of the business and the conduct of the affairs of the Corporation, including the election of the Chairman of the Board of Directors, if any, the President, the Treasurer, the Secretary, and other principal officers of the Corporation, shall be vested in its Board of Directors. The number of directors which shall constitute the whole Board of Directors shall be fixed by, or in the manner provided in, the Bylaws. The phrase “whole Board” and the phrase “total number of directors” shall be deemed to have the same meaning, to wit, the total number of directors which the Corporation would have if there were no vacancies. No election of directors need be by written ballot. The initial Board of Directors shall be appointed by the incorporator.
2. The original Bylaws of the Corporation shall be adopted by the incorporator. Thereafter, the power to make, alter, or repeal the Bylaws, and to adopt any new Bylaw, shall be vested in the Board of Directors.
3. Whenever the Corporation shall be authorized to issue only one class of stock, each outstanding share shall entitle the holder thereof to notice of, and the right to vote at, any meetings of stockholders. Whenever the Corporation shall be authorized to issue more than one class of stock, no outstanding share of any class of stock which is denied voting power under the provisions of the certificate of incorporation shall entitle the holder thereof to the right to vote at any meeting of the stockholders except as the provisions of paragraph (b)(2) of Section 242 of the Delaware General Corporation Law shall otherwise require; provided, that no share of any such class which is otherwise denied voting power shall entitle the holder thereof to vote upon the increase or decrease in the number of authorized shares of said class.
SEVENTH: (a) The Corporation shall, to the fullest extent permitted by Section 145 of the Delaware General Corporation Law, as the same may be amended and supplemented, indemnify any and all persons whom it shall have power to indemnify under said section from and against any and all of the expenses, liabilities or other matters referred to in or covered by said section, and shall advance expenses to any and all such persons to the fullest extent permitted by said section, and the indemnification and advancement provided for herein shall not be deemed exclusive under any Bylaw, agreement, vote of stockholders or disinterested directors or otherwise, both as to action in his official capacity and as to action in another capacity while holding such office, and shall continue as to a person who has ceased to be a director, officer, employee or agent and shall inure to the benefit of the heirs, executors and administrators of such a person.
(b) The Corporation shall have the power to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the Corporation, or is or was serving at the request of the Corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against him and incurred by him in any such capacity, or arising out of his status as such, whether or not the Corporation would have the power to indemnify him against such liability under Section 145 of the Delaware General Corporation Law.
EIGHTH: No director shall be personally liable to the Corporation or its stockholders
for monetary damages for any breach of fiduciary duty as director, occurring on or after the effective date of this provision, provided that this provision shall not eliminate or limit the liability of a director (i) for any breach of the director’s duty of loyalty to the Corporation or its stockholders, (ii) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (iii) under Section 174 of the Delaware General Corporation Law, or (iv) for any transaction from which the director derived an improper personal benefit. Furthermore, notwithstanding the foregoing provision, if the Delaware General Corporation Law is amended or enacted to permit further limitation or elimination of the personal liability of directors, the personal liability of the Corporation’s directors shall be limited or eliminated to the fullest extent permitted by applicable law. No amendment to, modification of or repeal of this Article EIGHTH shall apply to or have any effect on the liability or alleged liability of any director of the Corporation for or with respect to any acts or omissions of such director occurring prior to such amendment.
NINTH: From time to time any of the provisions of this certificate of incorporation may be amended, altered or repealed, and other provisions authorized by the laws of the State of Delaware at the time in force may be added or inserted in the manner and at the time prescribed by said laws; provided, however, that the provisions set forth in Articles FIFTH, SIXTH, SEVENTH, EIGHTH, and NINTH may not be repealed or amended in any respect unless such repeal or amendment is approved by the affirmative vote of the holders of not less than two-thirds of the total voting power of all outstanding shares of voting stock of this Corporation. All rights at any time conferred upon the stockholders of the Corporation by this certificate of incorporation are granted subject to the provisions of this Article NINTH.
TENTH: The name and mailing address of the incorporator is Islet Sciences, Inc., a Nevada corporation, 8601 Six Forks Rd, Suite 400, Raleigh, NC 27615.
ELEVENTH: The Corporation shall not be governed by or subject to Section 203 of the Delaware General Corporation Law.
IN WITNESS WHEREOF, the undersigned, being the incorporator hereinbefore named, has caused this certificate to be executed this 3rd day of September 2014.
ISLET SCIENCES, INC.
Incorporator
By: /s/ James Green
Name: James Green
Title: Chief Executive Officer
Exhibit B
BY-LAWS
OF
AVOGENX, INC.
(A Delaware Corporation)
ARTICLE I
STOCKHOLDERS
Section 1. STOCK CERTIFICATES; UNCERTIFICATED SHARES. The shares of the corporation shall be represented by certificates, provided that the Board of Directors may provide by resolution or resolutions that some or all of any or all classes or series of its stock shall be uncertificated shares. Every holder of stock of the corporation represented by certificates shall be entitled to have a certificate, in such form as may be prescribed by law and by the Board of Directors, representing the number of shares held by such holder registered in certificate form. Each such certificate shall be signed in a manner that complies with Section 158 of the General Corporation Law of the State of Delaware. Each certificate for shares of stock which are subject to any restriction on transfer pursuant to the Certificate of Incorporation, these By-laws, applicable securities laws or any agreement among any number of stockholders or among such holders and the corporation shall have conspicuously noted on the face or back of the certificate either the full text of the restriction or a statement of the existence of such restriction.
If the corporation shall be authorized to issue more than one class of stock or more than one series of any class, the powers, designations, preferences and relative participating, optional or other special rights of each class of stock or series thereof and the qualifications, limitations or restrictions of such preferences and/or rights shall be set forth in full or summarized on the face or back of each certificate representing shares of such class or series of stock, provided that in lieu of the foregoing requirements there may be set forth on the face or back of each certificate representing shares of such class or series of stock a statement that the corporation will furnish without charge to each stockholder who so requests a copy of the full text of the powers, designations, preferences and relative, participating, optional or other special rights of each class of stock or series thereof and the qualifications, limitations or restrictions of such preferences and/or rights.
Within a reasonable time after the issuance or transfer of uncertificated stock, the corporation shall send to the registered owner thereof a written notice containing the information required to be set forth or stated on certificates pursuant to Sections 151, 202(a) or 218 (a) of the General Corporation Law of the State of Delaware or, with respect to Section 151 of General Corporation Law of the State of Delaware, a statement that the corporation will furnish without charge to each stockholder who so requests the powers, designations, preferences and relative participating, optional or other special rights of each class of stock or series thereof and the qualifications, limitations or restrictions of such preferences and/or rights.
The issue, transfer, conversion and registration of shares of stock of the corporation shall be governed by such other regulations as the Board of Directors may establish.
Section 2. FRACTIONAL SHARE INTERESTS. To the extent designated by the President or any Vice President or the Treasurer of the corporation, the corporation may issue fractional shares, but shall not otherwise be required to issue fractions of a share.
Section 3. STOCK TRANSFERS. Shares of stock of the corporation shall be transferable in the manner prescribed by law and in these By-laws. Transfers of shares of stock of the corporation shall be made only on the books of the corporation or by transfer agents designated to transfer shares of stock of the corporation. Subject to applicable law, shares of stock represented by certificates shall be transferred only on the books of the corporation by the surrender to the corporation or its transfer agent of the certificate representing such shares properly endorsed or accompanied by a written assignment or power of attorney properly executed, and with such proof of authority or the authenticity of signature as the corporation or its transfer agent may reasonably require. Except as may be otherwise required by law, by the Certificate of Incorporation or by these By-laws, the corporation shall be entitled to treat the record holder of stock as shown on its books as the owner of such stock for all purposes, including the payment of dividends and the right to vote with respect to such stock, regardless of any transfer, pledge or other disposition of such stock until the shares have been transferred on the books of the corporation in accordance with the requirements of these By-laws.
Section 4. RECORD DATE.
(a)RECORD DATE FOR STOCKHOLDER MEETINGS. For the purpose of determining the stockholders entitled to notice of or to vote at any meeting of stockholders or any adjournment thereof, the directors may fix, in advance, a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted by the Board of Directors, and which record date shall not be more than sixty (60) days nor less than ten (10) days before the date of such meeting. If no record date is fixed, the record date for determining stockholders entitled to notice of or to vote at a meeting of stockholders shall be at the close of business on the day next preceding the day on which notice is given, or, if notice is waived, at the close of business on the day next preceding the day on which the meeting is held. A determination of stockholders of record entitled to notice of or to vote at any meeting of stockholders shall apply to any adjournment of the meeting; provided, however, that the Board of Directors may fix a new record date for the adjourned meeting.
(b)RECORD DATE FOR PAYMENTS OF DIVIDENDS AND DISTRIBUTIONS. In order that the Corporation may determine the stockholders entitled to receive payment of any dividend or other distribution or allotment of any rights or the stockholders entitled to exercise any rights in respect of any change, conversion, or exchange of stock or for the purpose of any other lawful action, the Board of Directors may fix a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted, and which record date shall be not more than sixty (60) days prior to such action. If no record date is fixed, the record date for determining stockholders for any such purpose shall be at the close of business on the day on which the Board of Directors adopts the resolution relating thereto.
(c)RECORD DATE FOR CORPORATE ACTIONS BY WRITTEN CONSENT.
(i) Notwithstanding Section 4(a) and Section 4(b) of Article I of these By-laws, the record date for determining stockholders entitled to express consent to corporate action in writing without a meeting shall be as fixed by the Board of Directors or as otherwise established under this Section 4(c). Any person seeking to have the stockholders authorize or take corporate action by written consent without a meeting shall, by written notice addressed to the Secretary and delivered to the Corporation, request that a record date be fixed for such purpose. The Board of Directors may fix a record date for such purpose which shall be no more than ten (10) days after the date upon which the resolution fixing the record date is adopted by the Board and shall not precede the date such resolution is adopted. If the Board of Directors fails within ten (10) days after the Corporation receives such notice to fix a record date for such purpose, the record date shall be the day on which the first written consent is delivered to the Corporation in the manner described in Section 4(c)(ii) below unless prior action by the Board of Directors is required under the General Corporation Law of the State of Delaware, in which event the record date shall be at the close of business on the day on which the Board of Directors adopts the resolution taking such prior action.
(ii) (A) Every written consent purporting to take or authorizing the taking of corporate action and/or related revocations (each such written consent and related revocation is referred to in this Section 4(c)(ii) of Article I of the By-laws as a “Consent”) shall bear the date of signature of each stockholder who signs the Consent, and no Consent shall be effective to take the corporate action referred to therein unless, within sixty (60) days of the earliest dated Consent delivered in the manner required by this Section 4(c)(ii), Consents signed by a sufficient number of stockholders to take such action are so delivered to the Corporation.
(B) A Consent shall be delivered to the Corporation by delivery to its registered office in the State of Delaware, its principal place of business, or an officer or agent of the Corporation having custody of the book in which proceedings of meetings of stockholders are recorded. Delivery to the Corporation’s registered office shall be made by hand or by certified or registered mail, return receipt requested.
(C) In the event of the delivery to the Corporation of a Consent, the Secretary of the Corporation shall provide for the safe-keeping of such Consent and shall promptly conduct such ministerial review of the sufficiency of the Consents and of the validity of the action to be taken by stockholder consent as he deems necessary or appropriate, including, without limitation, whether the holders of a number of shares having the requisite voting power to authorize or take the action specified in the Consent have given consent.
Section 5. MEANING OF CERTAIN TERMS. As used herein in respect of the right to notice of a meeting of stockholders or a waiver thereof or to participate or vote thereat or to consent or dissent in writing in lieu of a meeting, as the case may be, the term “share” or “shares” or “share of stock” or “shares of stock” or “stockholder” or “stockholders” refers to an outstanding share or shares of stock and to a holder or holders of record of outstanding shares of stock when the corporation is authorized to issue only one class of shares of stock, and said reference is also intended to include any outstanding share or shares of stock and any holder or holders of record of outstanding shares of stock of any class upon which or upon whom the certificate of incorporation confers such rights where there are two or more classes or series of shares of stock or upon which or upon whom the General Corporation Law confers such rights notwithstanding that the certificate of incorporation may provide for more than one class or series of shares of stock, one or more of which are limited or denied such rights thereunder; provided, however, that no such right shall vest in the event of an increase or a decrease in the authorized number of shares of stock of any class or series which is otherwise denied voting rights under the provisions of the certificate of incorporation.
Section 6. STOCKHOLDER MEETINGS.
(a) TIME. All meetings of stockholders shall be held on the date and at the time fixed by the directors.
(b) PLACE. Annual meeting and special meetings shall be held at such place, within or without the State of Delaware, as the directors may, from time to time fix. Whenever the directors shall fail to fix such place, the meeting shall be held at the registered office of the corporation in the State of Delaware.
(c) CALL. Annual meetings and special meetings may be called only by the Board of Directors acting upon the affirmative vote of that number of directors who would constitute at least a majority of the members of our Board of Directors if there were no vacancies, the President or the Chief Executive Officer.
(d) NOTICE OR WAIVER OF NOTICE. Written notice of all meetings shall be given, stating the place, date, and hour of the meeting and stating the place within the city or other municipality or community at which the list of stockholders of the corporation may be examined. The notice of an annual meeting shall state that the meeting is called for the election of directors and for the transaction of other business which may properly come before the meeting, and shall, (if any other action which could be taken at a special meeting is to be taken at such annual meeting) state the purpose or purposes. The notice of a special meeting shall in all instances state the purpose or purposes for which the meeting is called. The notice of any meeting shall also include, or be accompanied by, any additional statements, information, or documents prescribed by the General Corporation Law. Except as otherwise provided by the General Corporation Law, a copy of the notice of any meeting shall be given, personally or by mail, not less than ten (10) days nor more than sixty (60) days before the date of the meeting, unless the lapse of the prescribed period of time shall have been waived, and directed to each stockholder at his record address or at such other address which he may have furnished by request in writing to the Secretary of the corporation. Notice by mail shall be deemed to be given when deposited, with postage thereon prepaid, in the United States mail. Without limiting the manner by which notice otherwise may be given effectively to stockholders, notice of meetings may be given to stockholders by means of electronic transmission in accordance with applicable law. If a meeting is adjourned to another time, not more than thirty days hence, and/or to another place, and if an announcement of the adjourned time and/or place is made at the meeting, it shall not be necessary to give notice of the adjourned meeting unless the directors, after adjournment, fix a new record date for the adjourned meeting. Notice need not be given to any stockholder who submits a written waiver of notice signed by him before or after the time stated therein. Attendance of a stockholder at a meeting of stockholders shall constitute a waiver of notice of such meeting, except when the stockholder attends the meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. Neither the business to be transacted at, nor the purpose of, any regular or special meeting of the stockholders need be specified in any written waiver of notice.
(e) STOCKHOLDER LIST. The officer who has charge of the stock ledger of the corporation shall prepare and make, at least ten days before every meeting of stockholders, a complete list of the stockholders, arranged in alphabetical order, and showing the address of each stockholder and the number of shares registered in the name of each stockholder. Such list shall be open to the examination of any stockholder, for any purpose germane to the meeting, during ordinary business hours, for a period of at least ten days prior to the meeting, either at a place within the city or other municipality or community where the meeting is to be held, which place shall be specified in the notice of the meeting, or if not so specified, at the place where the meeting is to be held. The list shall also be produced and kept at the time and place of the meeting during the whole time thereof, and may be inspected by any stockholder who is present. The stock ledger shall be the only evidence as to who are the stockholders entitled to examine the stock ledger, the list required by this section or the books of the corporation, or to vote at any meeting of stockholders.
(f) CONDUCT OF MEETING. Meetings of the stockholders shall be presided over by one of the following officers in the order of seniority and if present and acting - the Chairman of the Board, if any, the Vice-Chairman of the Board, if any, the President, a Vice-President, or, if none of the foregoing is in office and present and acting, by a chairman to be chosen by the stockholders. The Secretary of the corporation, or in his absence, an Assistant Secretary, shall act as secretary of every meeting, but if neither the Secretary nor an Assistant Secretary is present the Chairman of the meeting shall appoint a secretary of the meeting.
(g) PROXY REPRESENTATION. Every stockholder may authorize another person or persons to act for him by proxy in all matters in which a stockholder is entitled to participate, whether by waiving notice of any meeting, voting or participating at a meeting, or expressing consent or dissent without a meeting. Every proxy must be signed by the stockholder or by his attorney-in-fact. No proxy shall be voted or acted upon after three years from its date unless such proxy provides for a longer period. A duly executed proxy shall be irrevocable if it states that it is irrevocable and, if, and only as long as, it is coupled with an interest sufficient in law to support an irrevocable power. A proxy may be made irrevocable regardless of whether the interest with which it is coupled is an interest in the stock itself or an interest in the corporation generally.
(h) INSPECTORS. The directors, in advance of any meeting, may, but need not, appoint one or more inspectors of election to act at the meeting or any adjournment thereof. If any inspector or inspectors are not appointed, the person presiding at the meeting may, but need not, appoint one or more inspectors. In case any person who may be appointed as an inspector fails to appear or act, the vacancy may be filled by appointment made by the directors in advance of the meeting or at the meeting by the person presiding thereat. Each inspector, if any, before entering upon the discharge of his duties, shall take and sign an oath faithfully to execute the duties of inspector at such meeting with strict impartiality and according to the best of his ability. The inspectors, if any, shall determine the number of shares of stock outstanding and the voting power of each, the shares of stock represented at the meeting, the existence of a quorum, the validity and effect of proxies, and shall receive votes, ballots or consents, hear and determine all challenges and questions arising in connection with the right to vote, count and tabulate all votes, ballots or consents, determine the result, and do such acts as are proper to conduct the election or vote with fairness to all stockholders. On request of the person presiding at the meeting, the inspector or inspectors, if any, shall make a report in writing of any challenge, question, or matter determined by him or them and execute a certificate of any fact found by him or them. No person who is a candidate for office at an election may serve as an inspector at such election.
(i) QUORUM. The holders of one third of the outstanding shares of stock shall constitute a quorum at a meeting of stockholders for the transaction of any business. A quorum, once established, shall not be broken by the subsequent withdrawal of enough votes to leave less than a quorum. The stockholders present may adjourn the meeting despite the absence of a quorum.
(j) VOTING. Each share of stock shall entitle the holder thereof to one vote. In the election of directors, a plurality of the votes cast shall elect. Any other action shall be authorized by a majority of the votes cast except where the General Corporation Law prescribes a different percentage of votes and/or a different exercise of voting power. In the election of directors, and for any other action, voting need not be by ballot.
(k) NOTICE OF STOCKHOLDER PROPOSALS.
(1) At any annual meeting of stockholders of the Corporation, only such business shall be conducted as shall have been properly brought before the meeting. To be properly brought before an annual meeting, business must be (i) specified in the notice of meeting (or any supplement thereto) given by or at the direction of the Board of Directors, (ii) otherwise properly brought before the meeting by or at the direction of the Board of Directors, or (iii) otherwise properly and timely brought before the meeting by any stockholder of the Corporation in compliance with the notice procedures and other provisions of this Section 6(k).
(2) For business to be properly brought before an annual meeting by a stockholder, such business, as determined by the Chairman of the Board or such other person as is presiding over the meeting, must be a proper subject for stockholder action under the General Corporation Law of the State of Delaware, and such stockholder (i) must be a stockholder of record on the date of the giving of the notice provided for in this Section 6(k) and on the record date for the determination of stockholders entitled to vote at such annual meeting, (ii) must be entitled to vote at such annual meeting, and (iii) must comply with the notice procedures set forth in this Section 6(k). In addition to any other applicable requirements, for business to be properly brought before an annual meeting by a stockholder, such stockholder must have given timely notice thereof in proper written form to the Secretary of the Corporation.
(3) To be timely, a stockholder's notice must be delivered to, or mailed and received by, the Secretary at the principal executive offices of the Corporation not earlier than the one hundred fiftieth (150th) calendar day, and not later than the close of business on the one hundred twentieth (120th) calendar day, prior to the first anniversary of the immediately preceding year's annual meeting of stockholders; provided, however, that in the event that no annual meeting was held in the previous year or the annual meeting is called for a date that is more than thirty (30) calendar days earlier or more than sixty (60) calendar days later than such anniversary date, notice by the stockholder in order to be timely must be so delivered or received not later than the tenth (10th) calendar day following the earlier of (i) the day on which public disclosure of the date of such annual meeting is first made, and (ii) the receipt by such stockholder of actual notice of the date of such annual meeting. For purposes of this Section 6(k) of these By-laws, public disclosure shall be deemed to include a disclosure made in a press release reported by the Dow Jones News Services, Reuters, Associated Press or a comparable national news service, in a document filed by the Corporation with the Securities and Exchange Commission pursuant to Section 13, 14 or 15(d) of the Exchange Act, or in a notice pursuant to the applicable rules of an exchange on which the securities of the Corporation are listed. In no event shall the public announcement of a postponement of the mailing of the notice for such annual meeting or of an adjournment or postponement of the annual meeting to a later date or time commence a new time period for the giving of a stockholder’s notice as described above.
(4) To be in proper written form, a stockholder's notice to the Secretary shall set forth in writing, as to each matter the stockholder proposes to bring before the annual meeting (i) a brief description of the business desired to be brought before the annual meeting, including the text of the proposal or business and the text of any resolutions proposed for consideration, (ii) the reasons for conducting such business at the annual meeting, (iii) the name and record address, as they appear on the Corporation's stock ledger, of such stockholder and the name and address of any Stockholder Associated Person (as defined below), (iv) the class and series and number of shares of each class and series of capital stock of the Corporation which are owned beneficially and/or of record by such stockholder and/or any Stockholder Associated Person, and the date or dates such shares were acquired and the investment intent of such acquisition (which information shall be supplemented not later than ten (10) calendar days after the record date for the meeting to disclose such ownership as of the record date), (v) a description of all arrangements or understandings between such stockholder and/or any Stockholder Associated Person, and any other person or persons (naming such person or persons) in connection with the proposal of such business by such stockholder, (vi) any material interest of such stockholder and/or any Stockholder Associated Person in such business, individually or in the aggregate, including any anticipated benefit to the stockholder or any Stockholder Associated Person therefrom, (vii) a representation from the stockholder as to whether the stockholder or any Stockholder Associated Person intends or is part of a group which intends (1) to deliver a proxy statement and/or form of proxy to holders of at least the percentage of the Corporation’s outstanding capital stock required to approve or adopt the proposal and/or (2) otherwise to solicit proxies in support of such proposal, (viii) a representation that such stockholder is a holder of record of stock of the Corporation entitled to vote at such meeting, that such stockholder intends to vote such stock at such meeting, and that such stockholder intends to appear in person or by proxy at the annual meeting to bring such business before the meeting, (ix) whether and the extent to which any hedging transaction has been engaged in by or on behalf of such stockholder or any Stockholder Associated Person with respect to any shares of stock of the Corporation, without regard to whether such transaction is required to be reported on a Schedule 13d in accordance with the Exchange Act, (x) whether and the extent to which any agreement, arrangement or understanding has been made, the effect or intent of which is to increase or decrease the voting power of such stockholder or such Stockholder Associated Person with respect to any shares of the capital stock of the Corporation, without regard to whether such transaction is required to be reported on a Schedule 13d in accordance with the Exchange Act, (xi) in the event that such business includes a proposal to amend the Certificate of Incorporation and/or the By-laws of the Corporation, the language of the proposed amendment, and (xii) such other information regarding each matter of business to be proposed by such stockholder, regarding the stockholder in his or her capacity as a proponent of a stockholder proposal, or regarding any Stockholder Associated Person, as would be required to be included in a proxy statement or other filings required to be made in connection with solicitations of proxies pursuant to Section 14 of the Exchange Act (or pursuant to any law or statute replacing such section), and the rules and regulations promulgated thereunder.
(5) If the information submitted pursuant to this Section 6(k) by any stockholder proposing business for consideration at an annual meeting shall be inaccurate to any material extent, such information may be deemed not to have been provided in accordance with this Section 6(k). Upon written request by the Secretary, the Board of Directors or any committee thereof, any stockholder proposing business for consideration at an annual meeting shall provide, within seven business days of delivery of such request (or such other period as may be specified in such request), written verification, satisfactory in the discretion of the Board of Directors, any committee thereof or any authorized officer of the Corporation, to demonstrate the accuracy of any information submitted by the stockholder pursuant to this Section 6(k). If a stockholder fails to provide such written verification within such period, the information as to which written verification was requested may be deemed not to have been provided in accordance with this Section 6(k).
(6) For purposes of this Section 6(k) and Section 6(l) of these By-laws, the following definitions shall be applicable:
(i) beneficial ownership in the Corporation’s capital stock shall include, in addition to the definition of beneficial ownership contained in Rule 13d-3 of the Exchange Act (or any successor rule or regulation), any direct or indirect pecuniary interest in the Corporation’s capital stock,
(ii) business day shall mean any day other than a Saturday, a Sunday or a day on which banking institutions in the State of New York are authorized or obligated by law or executive order to close,
(iii) close of business shall mean 5:00 p.m., Eastern Time,
(iv) hedging of the Corporation’s capital stock shall mean any transaction or series of transactions that has been entered into, or any other agreement, arrangement or understanding (including, but not limited to, any borrowing or lending of shares or any short interest) that has been made, the effect or intent of which is to mitigate loss to or manage the risk or benefit of share price changes with respect to any shares of the capital stock of the Corporation,
(v) pecuniary interest in the Corporation’s capital stock shall include, but not be limited to, the opportunity, directly or indirectly, to profit or share in any profit derived from a transaction in the Corporation’s capital stock,
(vi) indirect pecuniary interest in the Corporation’s capital stock shall include, but not be limited to, (a) any derivative instrument which includes the opportunity, directly or indirectly, to profit or share in any profit derived from any increase in the value of the Corporation’s capital stock, including a person’s right to acquire the Corporation’s capital stock through the exercise or conversion of any derivative instrument, whether or not presently exercisable, (b) a general partner’s proportionate interest in the Corporation’s capital stock held by a general or limited partnership, (c) a person’s right to dividends that is separated or separable from the Corporation’s capital stock, (d) shares of the Corporation’s capital stock held by members of a person’s immediate family, and (e) a person’s interest in the Corporation’s capital stock that is held by a trust,
(vii) derivative instrument shall include, but not be limited to, any option, warrant, convertible security, stock appreciation right, or similar right with an exercise or conversion privilege or a settlement payment or mechanism at a price related to the value of the Corporation’s capital stock, or similar instrument with a value derived in whole or in part from the value of the Corporation’s capital stock, whether or not such instrument or right shall be subject to settlement in the Corporation’s capital stock or otherwise,
(viii) short interest in the Corporation’s capital stock shall mean that the person, directly or indirectly, through any contract, arrangement, understanding, relationship or otherwise, has the opportunity to profit or share in any profit derived from any decrease in the value of the Corporation’s capital stock, and
(ix) Stockholder Associated Person of any stockholder shall mean (a) any person controlling, directly or indirectly, or acting in concert with, such stockholder, (b) any beneficial owner of shares of stock of the Corporation owned of record or beneficially by such stockholder, and (c) any person controlling, controlled by or under common control with such Stockholder Associated Person.
(7) No business shall be conducted at the annual meeting of stockholders except business brought before the annual meeting in accordance with the procedures set forth in this Section 6(k).
(8) Except as otherwise required by law, the Certificate of Incorporation or these By-laws, the Chairman of the Board or other person presiding at an annual meeting shall have the power and duty (i) to determine whether any business proposed to be brought before the meeting was properly brought before the meeting in accordance with the procedures set forth in this Section 6(k), including whether the stockholder or the Stockholder Associated Person, if any, on whose behalf the proposal is made solicited (or is part of a group which solicited) or did not so solicit, as the case may be, proxies in support of such stockholder’s proposal in compliance with such stockholder’s representation as required by this Section 6(k), and (ii) if any proposed business was not brought in compliance with this Section 6(k), to declare that such proposal is defective and shall be disregarded.
(9) In addition to the provisions of this Section 6(k), a stockholder shall also comply with all applicable requirements of state law and all applicable requirements of the Exchange Act, and the rules and regulations thereunder, with respect to the matters set forth herein.
(10) Nothing in this Section 6(k) shall be deemed to affect any rights (i) of stockholders to request inclusion of proposals in the Corporation’s proxy statement pursuant to Rule 14a-8 under the Exchange Act, or (ii) of the holders of any series of preferred stock to elect directors pursuant to any applicable provisions of the Certificate of Incorporation.
(11) Notwithstanding anything in this Section 6(k) to the contrary, a stockholder intending to nominate one or more persons for election as a director at an annual meeting must comply with Section 6(l) of these by-laws for any such nomination to be properly brought before such meeting.
(12) Notwithstanding any other provision of these By-laws, and notwithstanding the fact that a lesser percentage may be specified by law, any amendment, alteration, repeal or rescission of, or the adoption of any provisions inconsistent with, this Section 6(k), Section 6(l) and/or Section 6(m) shall require either (i) the affirmative vote of not less than 66.67% of the directors then in office, or (ii) the affirmative vote of the holders of the Corporation’s capital stock representing not less than 66.67% of the votes which all stockholders would be entitled to cast at any election of directors held at a meeting of the stockholders called for that purpose (provided that notice of such proposed amendment, alteration, repeal or rescission is included in the notice of such meeting, which shall also include, without limitation, the text of any such proposed amendment or alteration and/or any resolution calling therefor or for any repeal or rescission).
(l) NOTICE OF NOMINATIONS BY STOCKHOLDERS.
(1) Subject to the rights of the holders of any class or series of stock having (x) special voting or nomination rights, or (y) a preference over the Common Stock as to dividends or upon liquidation, dissolution or winding up, nominations for the election of directors may be made (i) by or at the direction of the Board of Directors or a committee appointed by the Board of Directors, or (ii) by any stockholder of the Corporation (a) who is a stockholder of record on the date of the giving of the notice provided for in this Section 6(l), on the record date for the determination of the stockholders entitled to vote at such meeting and at the time of the annual meeting of stockholders, (b) who is entitled to vote at the meeting for the election of directors, and (c) who complies with the notice procedures set forth in this Section 6(l). In addition to any other applicable requirements, for a nomination to be made by a stockholder, such stockholder must have given timely notice thereof in proper written form to the Secretary of the Corporation.
(2) To be timely, a stockholder's notice of nomination must be delivered to, or mailed and received by, the Secretary at the principal executive offices of the Corporation not earlier than the one hundred fiftieth (150th) calendar day, and not later than the close of business on the one hundred twentieth (120th) calendar day, prior to the first anniversary of the immediately preceding year's annual meeting; provided, however, that in the event that no annual meeting was held in the previous year or the annual meeting is called for a date that is more than thirty (30) calendar days earlier or more than sixty (60) calendar days later than such anniversary date, notice by the stockholder in order to be timely must be so delivered or received not later than the tenth (10th) calendar day following the earlier of (i) the day on which public disclosure of the date of such annual meeting is first made, and (ii) the receipt by such stockholder of actual notice of the date of such annual meeting. For purposes of this Section 6(l), public disclosure shall be deemed to include a disclosure made in a press release reported by the Dow Jones News Services, Reuters, Associated Press or a comparable national news service, in a document filed by the Corporation with the Securities and Exchange Commission pursuant to Section 13, 14 or 15(d) of the Exchange Act, or in a notice pursuant to the applicable rules of an exchange on which the securities of the Corporation are listed. In no event shall the public announcement of a postponement of the mailing of the notice for such annual meeting or of an adjournment or postponement of the annual meeting to a later date or time commence a new time period for the giving of a stockholder’s notice as described above.
(3) To be in proper written form, a stockholder's notice of nomination to the Secretary shall set forth in writing:
(i) as to each person whom the stockholder proposes to nominate for election or reelection as a director (a) the name, age, business address and residence address of the person, (b) the principal occupation and employment of the person, (c) the class and series and number of shares of each class and series of capital stock of the Corporation which are owned beneficially or of record by the person (which information shall be supplemented not later than ten (10) calendar days after the record date for the meeting to disclose such ownership as of the record date), (d) the person’s executed written consent to being named in the proxy statement, if any, as a nominee and to serving as a director if elected, (e) any other information relating to the person that would be required to be disclosed in a proxy statement or other filings required to be made in connection with solicitations of proxies for election of directors, or is otherwise required, pursuant to Section 14 of the Exchange Act (or pursuant to any law or statute replacing such section), and the rules and regulations promulgated thereunder, (f) a representation from the stockholder as to whether the stockholder or any Stockholder Associated Person intends or is part of a group which intends (1) to deliver a proxy statement and/or form of proxy to holders of at least the percentage of the Corporation’s outstanding capital stock required to elect the person proposed as a nominee and/or (2) otherwise to solicit proxies in support of the election of such person, and (g) a written statement executed by the person acknowledging that, as a director of the Corporation, he or she will owe fiduciary duties, under the General Corporation Law of the State of Delaware, exclusively to the Corporation and its stockholders and no fiduciary duties to any specific stockholder or group of stockholders; and
(ii) as to the stockholder giving the notice (a) the name and record address of such stockholder, as they appear on the Corporation's stock ledger, and the name and address of any Stockholder Associated Person (as defined below), (b) the class and series and number of shares of each class and series of capital stock of the Corporation which are owned beneficially and/or of record by such stockholder and/or any Stockholder Associated Person, and the date or dates such shares were acquired and the investment intent of such acquisition (which information shall be supplemented not later than ten (10) calendar days after the record date for the meeting to disclose such ownership as of the record date), (c) a description of all arrangements or understandings between such stockholder and/or any Stockholder Associated Person and each proposed nominee and any other person or persons (naming such person or persons) pursuant to which the nomination(s) are to be made by such stockholder, (d) any material interest of such stockholder and/or any Stockholder Associated Person in the election of such proposed nominee, individually or in the aggregate, including any anticipated benefit to the stockholder or any Stockholder Associated Person therefrom, (e) a representation that such stockholder is a holder of record of stock of the Corporation entitled to vote at such meeting and that such stockholder intends to appear in person or by proxy at the meeting to nominate the person or persons named in its notice, (f) whether and the extent to which any hedging transaction has been engaged in by or on behalf of such stockholder or any Stockholder Associated Person with respect to any shares of stock of the Corporation, without regard to whether such transaction is required to be reported on a Schedule 13d in accordance with the Exchange Act, (g) whether and the extent to which any agreement, arrangement or understanding has been made, the effect or intent of which is to increase or decrease the voting power of such stockholder or such Stockholder Associated Person with respect to any shares of the capital stock of the Corporation, without regard to whether such transaction is required to be reported on a Schedule 13d in accordance with the Exchange Act, and (h) any other information relating to such stockholder, in his or her capacity as a proponent of a stockholder nomination, or any Stockholder Associated Person that would be required to be disclosed in a proxy statement or other filings required to be made in connection with solicitations of proxies for election of directors, or is otherwise required, pursuant to Section 14 of the Exchange Act (or pursuant to any law or statute replacing such section) and the rules and regulations promulgated thereunder.
(4) In addition to the information required above, the Corporation may require any proposed nominee to furnish such other information as it may reasonably require to determine the eligibility of such proposed nominee to serve as a director of the Corporation.
(5) If the information submitted pursuant to this Section 6(l) by any stockholder proposing a nominee for election as a director at an annual meeting shall be inaccurate to any material extent, such information may be deemed not to have been provided in accordance with this Section 6(l). Upon written request by the Secretary, the Board of Directors or any committee thereof, any stockholder proposing a nominee for election as a director at an annual meeting shall provide, within seven business days of delivery of such request (or such other period as may be specified in such request), written verification, satisfactory in the discretion of the Board of Directors, any committee thereof or any authorized officer of the Corporation, to demonstrate the accuracy of any information submitted by the stockholder pursuant to this Section 6(l). If a stockholder fails to provide such written verification within such period, the information as to which written verification was requested may be deemed not to have been provided in accordance with this Section 6(l).
(6) Notwithstanding anything in these By-laws to the contrary, no person shall be eligible for election at an annual meeting as a director of the Corporation unless nominated in accordance with the procedures set forth in this Section 6(l).
(7) Except as otherwise required by law, the Certificate of Incorporation or these By-laws, the Chairman of the Board or other person presiding at an annual meeting shall have the power and duty (i) to determine whether any nomination proposed to be brought before the meeting was properly made in accordance with the procedures set forth in this Section 6(l), including whether the stockholder or the Stockholder Associated Person, if any, on whose behalf the nomination is made solicited (or is part of a group which solicited) or did not so solicit, as the case may be, proxies in support of such stockholder’s nominee in compliance with such stockholder’s representation as required by this Section 6(l), and (ii) if any proposed nomination was not made in compliance with this Section 6(l) to declare that such defective nomination is null and void and shall be disregarded.
(8) Notwithstanding anything in this Section 6(l) to the contrary, in the event that the number of directors to be elected to the Board of Directors at an annual meeting of the stockholders is increased and there is no public disclosure, naming all of the nominees for directors or specifying the size of the increased Board of Directors, by the Corporation at least ninety (90) calendar days prior to the first anniversary of the date of the immediately preceding annual meeting, a stockholder’s notice required by this Section 6(l) shall also be considered timely, but only with respect to nominees for any new positions created by such increase, if it shall be delivered to, or mailed and received by, the Secretary at the principal executive offices of the Corporation not later than the close of business on the tenth (10th) calendar day following the earlier of the day that the stockholder first received actual notice of such increase and the day on which such public disclosure is first made by the Corporation.
(9) In addition to the provisions of this Section 6(l), a stockholder shall also comply with all applicable requirements of state law and all applicable requirements of the Exchange Act, and the rules and regulations thereunder, with respect to the matters set forth herein.
(m) SPECIAL MEETINGS OF STOCKHOLDERS.
(1) Only such business shall be conducted at a special meeting of stockholders as shall have been brought before the meeting pursuant to the Corporation's notice of meeting.
(2) Nominations of persons for election to the Board of Directors may be made at a special meeting of stockholders at which directors are to be elected (i) pursuant to the Corporation's notice of meeting, (ii) by or at the direction of the Board of Directors, or (iii) provided that the Board of Directors has determined that directors shall be elected at such special meeting, by any stockholder of the Corporation who (a) is a stockholder of record at the time of giving of notice provided for in Section 6(l) of these By-laws, (b) is a stockholder of record on the record date for the determination of the stockholders entitled to vote at such special meeting, (c) is a stockholder of record at the time of such special meeting, and (d) complies with the notice procedures set forth in this Section 6(m).
(3) In the event the Corporation calls a special meeting of stockholders for the purpose of electing one or more directors to the Board of Directors, a stockholder who complies with Section 6(m)(2) of these By-laws may nominate a person or persons (as the case may be) for election to such position as specified in the Corporation's notice of meeting if they give timely notice thereof in proper written form to the Secretary of the Corporation as provided hereinafter.
(4) To be timely and in proper form, the stockholder’s notice of nomination with respect to a special meeting must comply with Section 6(l)(3) of these By-laws and must be delivered to the Secretary of the Corporation at the principal executive office of the Corporation not later than the close of business on the tenth (10th) calendar day following the earlier of the day that the stockholder first received actual notice of the date of the special meeting and the nominees proposed by the Board of Directors to be elected at such meeting and the day on which such public disclosure is first made by the Corporation. In no event shall the public announcement of a postponement of the mailing of the notice for such special meeting or of an adjournment or postponement of the special meeting to a later date or time commence a new time period for the giving of a stockholder’s notice as described above.
(5) If the information submitted pursuant to this Section 6(m) by any stockholder proposing a nominee for election as a director at a special meeting shall be inaccurate to any material extent, such information may be deemed not to have been provided in accordance with this Section 6(m). Upon written request by the Secretary, the Board of Directors or any committee thereof, any stockholder proposing a nominee for election as a director at a special meeting shall provide, within seven business days of delivery of such request (or such other period as may be specified in such request), written verification, satisfactory in the discretion of the Board of Directors, any committee thereof or any authorized officer of the Corporation, to demonstrate the accuracy of any information submitted by the stockholder pursuant to this Section 6(m). If a stockholder fails to provide such written verification within such period, the information as to which written verification was requested may be deemed not to have been provided in accordance with this Section 6(m).
(6) Notwithstanding anything in these By-laws to the contrary, no person shall be eligible for election at a special meeting as a director of the Corporation unless nominated in accordance with the procedures set forth in this Section 6(m). The Chairman of the Board or other person presiding at a special meeting shall have the power and duty to determine whether any nomination proposed to be brought before a special meeting was properly made in accordance with the procedures set forth in this Section 6(m) and, if any proposed nomination was not made in compliance with this Section 6(m), or if the stockholder solicits proxies in support of such proposed nomination without having made the representation required by this Section 6(m), to declare that such defective nomination is null and void and shall be disregarded.
(7) In addition to the provisions of this Section 6(m), a stockholder shall also comply with all applicable requirements of state law and all applicable requirements of the Exchange Act, and the rules and regulations thereunder, with respect to the matters set forth herein.
(n) CONDUCT OF MEETING.
(1) At every meeting of stockholders, the Chairman of the Board, if there be one, shall serve as chairman of the meeting and conduct the meeting or, in the case of a vacancy in the office or absence of the Chairman of the Board, one of the following officers present shall serve as chairman of the meeting and conduct the meeting in the order stated: the President, the Vice Chairman of the Board, if there be one or, if there be more than one, the Vice Chairmen in order of seniority, the Executive Vice Presidents in their order of seniority, or, in the absence of such officers, a chairman chosen by the stockholders entitled to cast a majority of the votes which all stockholders present in person or by proxy are entitled to cast, shall serve as chairman.
(2) The Secretary, or, in the Secretary's absence, an Assistant Secretary, or in the absence of both the Secretary and Assistant Secretaries, a person appointed by the chairman of the meeting shall serve as secretary of the meeting. In the event that the Secretary presides at a meeting of the stockholders, an Assistant Secretary shall record the minutes of the meeting.
(3) At any meeting of stockholders, the time of the opening and the closing of the polls for each matter upon which the stockholder will vote at a meeting, the order of business and all other matters of procedure shall be determined by the chairman of the meeting.
(4) The chairman of the meeting may prescribe such rules, regulations and procedures and take such action as, in the discretion of such chairman, are appropriate for the proper conduct of the meeting. Such rules, regulations and procedures, whether adopted by the Board of Directors or prescribed by the chairman of the meeting, may include, without limitation (i) the establishment of an agenda for the meeting, (ii) restricting admission to the time set for the commencement of the meeting, (iii) limiting attendance at the meeting to stockholders of record of the Corporation entitled to vote at the meeting, their duly authorized proxies or other such persons as the chairman of the meeting may determine, (iv) limiting participation at the meeting on any matter to stockholders of record of the Corporation entitled to vote on such matter, their duly authorized proxies or other such persons as the chairman of the meeting may determine to recognize and, as a condition to recognizing any such participant, requiring such participant to provide the Chairman with evidence of his or her name and affiliation, whether her or she is a stockholder or a proxy for a stockholder, and the class and series and number of shares of each class and series of capital stock of the Corporation which are owned beneficially and/or of record by such stockholder, (v) restricting entry to the meeting after the time fixed for the commencement thereof, (vi) limiting the time allotted to questions or comments by participants, (vii) determining when the polls should be opened and closed for voting, (viii) taking such actions as are necessary or appropriate to maintain order, decorum, safety and security at the meeting, (ix) removing any stockholder who refuses to comply with meeting procedures, rules or guidelines as established by the chairman of the meeting, (x) recessing or adjourning the meeting to a later date, time and place announced at the meeting by the chairman, and (xi) complying with any state and local laws and regulations concerning safety and security.
(5) Unless otherwise determined by the chairman of the meeting, meetings of stockholders shall not be required to be held in accordance with the rules of parliamentary procedure.
Section 7. STOCKHOLDER ACTION WITHOUT MEETINGS. Any action required by the General Corporation Law to be taken at any annual or special meeting of stockholders, or any action which may be taken at any annual or special meeting of stockholders, may be taken without a meeting, without prior notice and without a vote, if a consent in writing, setting forth the action so taken, shall be signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted. Prompt notice of the taking of the corporate action without a meeting by less than unanimous written consent shall be given to those stockholders who have not consented in writing.
Section 8. EXCLUSIVE FORUM FOR CERTAIN DISPUTES. Unless the corporation consents in writing to the selection of an alternative forum, the United States District Court for the Eastern District of North Carolina or, if such court lacks jurisdiction, any North Carolina state court that has jurisdiction, shall, to the fullest extent permitted by law, be the sole and exclusive forum for (a) any derivative action or proceeding brought on behalf of the corporation, (b) any action asserting a claim of breach of a fiduciary duty owed by any director, officer or other employee of the corporation to the corporation or the corporation’s stockholders, (c) any action asserting a claim arising pursuant to any provision of the General Corporation Law of the State of Delaware, and (d) any action asserting a claim governed by the internal affairs doctrine. Any person or entity purchasing or otherwise acquiring or holding any interest in shares of capital stock of the corporation shall be deemed to have notice of and consented to the provisions of this Section 8.
ARTICLE II
DIRECTORS
Section 1. FUNCTIONS AND DEFINITION. The business and affairs of the corporation shall be managed by the Board of Directors of the corporation. The Board of Directors shall have authority to fix the compensation of the members thereof. The use of the phrase “whole board” herein refers to the total number of directors which the corporation would have if there were no vacancies.
Section 2. QUALIFICATIONS AND NUMBER. A director need not be a stockholder, a citizen of the United States, or a resident of the State of Delaware. Except as may otherwise be provided in the Certificate of Incorporation, the number of directors which shall constitute the whole Board of Directors shall be determined from time to time by resolution of the stockholders or the Board of Directors, but in no event shall be less than one (1). The initial Board of Directors shall consist of five persons. Each director shall hold office until such Director’s death, resignation, retirement, removal, disqualification or such director’s successor is elected and qualified. Directors need not be residents of the State of Delaware or stockholders of the corporation.
Section 3. ELECTION AND TERM. The first Board of Directors, unless the members thereof shall have been named in the certificate of incorporation, shall be elected by the incorporator or incorporators and shall hold office until first annual meeting of stockholders and until their successors are elected and qualified or until their earlier resignation or removal. Any director may resign at any time upon written notice to the corporation. Thereafter, directors who are elected at an annual meeting of stockholders, and directors who are elected in the interim to fill vacancies and newly created directorships, shall hold office until the next annual meeting, resignation or removal. Except as the General Corporation Law of the State of Delaware may otherwise require, in the interim between annual meetings of stockholders or of special meetings of stockholders called for the election of directors and/or for the removal of one or more directors and for the filling of any vacancy in that connection, newly created directorships and any vacancies in the Board of Directors, including unfilled vacancies resulting from the removal of directors, may be filled only by the vote of a majority of the remaining directors then in office, although less than a quorum, or by the sole remaining director.
Section 4. MEETINGS.
(a) TIME. Meetings shall be held at such time as the Board shall fix, except that the first meeting of the Board after an election of a class of directors shall be held as soon after such election as the directors in office may conveniently assemble.
(b) PLACE. Meeting shall be held at such place within or without the State of Delaware as shall be fixed by the Board.
(c) CALL. No call shall be required for regular meetings for which the time and place have been fixed. Special meetings may be called by or at the direction of the Chairman of the Board, if any, the Vice-Chairman of the Board, if any, or the President, or of a majority of the directors in office.
(d) NOTICE OR ACTUAL OR CONSTRUCTIVE WAIVER. No notice shall be required for regular meetings for which the time and place have been fixed. Written, oral, or any other mode of notice of the time and place shall be given for special meetings in sufficient time for the convenient assembly of the directors thereat. Notice need not be given to any director or to any member of a committee of directors who submits a written waiver of notice signed by him before or after the time stated therein. Attendance of any such person at a meeting shall constitute a waiver of notice of such meeting, except when he attends a meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. Neither the business to be transacted at, nor the purpose of, any regular or special meeting of the directors need be specified in any written waiver of notice.
(e) QUORUM AND ACTION. A majority of the whole Board shall constitute a quorum except when a vacancy or vacancies prevents such majority, whereupon a majority of the directors in office shall constitute a quorum, provided, that such majority shall constitute at least one-third of the whole Board. A majority of the directors present, whether or not a quorum is present, may adjourn a meeting to another time and place. Except as herein otherwise provided, and except as otherwise provided by the General Corporation Law, the vote of the majority of the directors present at a meeting at which a quorum is present shall be the act of the Board. The quorum and voting provisions herein stated shall not be construed as conflicting with any provisions of the General Corporation Law and these By-Laws which govern a meeting of directors held to fill vacancies and newly created directorships in the Board or action of disinterested directors.
(f) CHAIRMAN OF THE MEETING. The Chairman of the Board, if any and if present and acting, shall preside at all meetings. Otherwise, the Vice-Chairman of the Board, if any and if present and acting, or the President, if present and acting, or any other director chosen by the Board, shall preside.
Section 5. REMOVAL OF DIRECTORS. Any or all of the directors may be removed for cause by either the stockholders or Board of Directors.
Section 6. COMMITTEES. The Board of Directors may, by resolution passed by a majority of the whole Board, designate one or more committees, each committee to consist of two or more of the directors of the corporation. The Board may designate one or more directors as alternate members of any committee, who may replace any absent or disqualified member at any meeting of the committee. In the absence or disqualification of any member of any such committee or committees, the member or members thereof present at any meeting and not disqualified from voting, whether or not he or they constitute a quorum, may unanimously appoint another member of the Board of Directors to act at the meeting in the place of any such absent or disqualified member. Any such committee, to the extent provided in the resolution of the Board, shall have and may exercise the powers and authority of the Board of Directors in the management of the business and affairs of the corporation with the exception of any authority the delegation of which is prohibited by Section 141 of the General Corporation Law, and may authorize the seal of the corporation to be affixed to all papers which may require it.
Section 7. INFORMAL ACTION. Any member or members of the Board of Directors or of any committee designated by the Board, may participate in a meeting of the Board, or any such committee, as the case may be, by means of conference telephone or similar communications equipment by means of which all persons participating in the meeting can hear each other. Any action required or permitted to be taken at any meeting of the Board of Directors or any committee thereof may be taken without a meeting if all members of the Board or committee, as the case may be, consent thereto in writing or by electronic transmission, and the writing or electronic transmission is filed with the minutes of proceedings of the Board or committee.
ARTICLE III
OFFICERS
The directors shall elect a President, a Secretary, and a Treasurer, and may elect a Chairman of the Board of Directors, a Vice-Chairman thereof, and one or more Vice-Presidents, Assistant Secretaries, and Assistant Treasurers, and may elect or appoint such other officers and agents as are desired. The President may but need not be a director. Any number of offices may be held by the same person.
Unless otherwise provided in the resolution of election or appointment, each officer shall hold office until his successor is elected and qualified or until his earlier resignation or removal. Any officer may resign at any time upon written notice to the corporation.
Officers shall have the powers and duties defined in the resolutions appointing them; provided, that the Secretary shall record all proceedings of the meetings or of the written actions of the stockholders and of the directors, and any committee thereof, in a book to be kept for that purpose.
The Board of Directors may remove any officer for cause or without cause.
ARTICLE IV
CORPORATE SEAL
The corporate seal shall be in such form as the Board of Directors shall prescribe.
ARTICLE V
FISCAL YEAR
The fiscal year of the corporation shall begin on May 1 and end on April 30 of each year, subject to change by the Board of Directors.
ARTICLE VI
CONTROL OVER BY-LAWS
These by-laws may be amended, altered, changed, adopted and repealed or new by-laws adopted by the Board of Directors. The stockholders may make additional by-laws and may alter and repeal any by-laws whether such by-laws were originally adopted by them or otherwise.
Exhibit C
INVESTOR RIGHTS AGREEMENT
This Investor Rights Agreement (this “Agreement”) is made and entered into as of ___________, by and among Avogenx, Inc., a Delaware corporation (“Holdco”), and the shareholders of Holdco signatory hereto (each a “Shareholder” and collectively, the “Shareholders”).
This Agreement is made in connection with the Agreement and Plan of Merger, dated as of ___________, 2014 by and among Islet Sciences, Inc., a Nevada corporation, Brighthaven Ventures, L.L.C., a North Carolina limited liability company, Holdco, Islet Merger Sub, Inc., a Nevada corporation and a direct wholly-owned subsidiary of Holdco, and the Shareholders (the “Merger Agreement”).
In consideration of the mutual covenants and agreements contained herein and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, Holdco and the Shareholders hereby agree as follows:
1.Definitions. Capitalized terms used and not otherwise defined herein that are defined in the Merger Agreement will have the respective meanings given such terms in the Merger Agreement. As used in this Agreement, the following terms have the respective meanings set forth in this Section 1:
“Advice” has the meaning set forth in Section 9(c).
“Additional Securities” means capital stock of Holdco, or securities convertible into or exchangeable for capital stock of Holdco.
“Agreement” has the meaning set forth in the Preamble.
“Board” has the meaning set forth in Section 7(a).
“Commission” means the Securities and Exchange Commission.
“Commission Guidance” means any publicly available written or oral guidance, comments, requirements or requests of the Commission staff, provided that any such oral guidance, comments, requirements, or requests are reduced to writing by the Commission.
“Common Stock” means the common stock, par value per share of $0.001, of Holdco.
“Covered Transaction” means the sale for cash of shares of any Additional Securities, where the primary purpose of such offering is to raise capital for Holdco. For the avoidance of doubt, the term “Covered Transaction” will not apply to the issuance of (i) capital stock of Holdco, or securities convertible into or exchangeable for capital stock of Holdco, to directors, officers or employees of Holdco pursuant to an employee benefit plan, incentive award program or other bona fide compensation arrangement or (ii) capital stock of Holdco issued as consideration in a merger or acquisition transaction, other extraordinary business combination, joint venture or strategic transaction approved by the Board.
“Delaware Courts” means any court sitting in New Castle County, Delaware, including the Delaware Court of Chancery and the United States District Court for the District of Delaware.
“Demand Notice” has the meaning set forth in Section 2(a).
“Demand Registration” has the meaning set forth in Section 2(a).
“Demand Registration Statement” has the meaning set forth in Section 2(a).
“Effective Date” means, as to a Registration Statement, the date on which such Registration Statement is first declared effective by the Commission.
“Effectiveness Period” means, as to any Registration Statement required to be filed pursuant to this Agreement, the period commencing on the Effective Date of such Registration Statement and ending on the earliest to occur of (a) the second anniversary of such Effective Date, (b) such time as all of the Registrable Securities covered by such Registration Statement have been publicly sold by the Shareholder of the Registrable Securities included therein, or (c) such time as all of the Registrable Securities covered by such Registration Statement may be sold by the Shareholder without volume restrictions pursuant to Rule 144, in each case as determined by the counsel to Holdco pursuant to a written opinion letter to such effect, addressed and acceptable to Holdco.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Holdco” has the meaning set forth in the Preamble.
“Indemnified Party” has the meaning set forth in Section 5(c).
“Indemnifying Party” has the meaning set forth in Section 5(c).
“Losses” has the meaning set forth in Section 5(a).
“Merger Agreement” has the meaning set forth in the Preamble.
“Participation Offer” has the meaning set forth in Section 8(a).
“Piggyback Holders” has the meaning set forth in Section 2(b).
“Piggyback Notice” has the meaning set forth in Section 2(b).
“Piggyback Registration” has the meaning set forth in Section 2(b).
“Piggyback Registration Statement” has the meaning set forth in Section 2(b).
“Piggyback Request” has the meaning set forth in Section 2(b).
“Proceeding” means an action, claim, suit, investigation or proceeding (including, without limitation, an investigation or partial proceeding, such as a deposition), whether commenced or threatened.
“Prospectus” means the prospectus included in a Registration Statement (including, without limitation, a prospectus that includes any information previously omitted from a prospectus filed as part of an effective Registration Statement in reliance upon Rule 430A promulgated under the Securities Act), as amended or supplemented by any prospectus supplement, with respect to the terms of the offering of any portion of the Registrable Securities covered by a Registration Statement, and all other amendments and supplements to the Prospectus, including post-effective amendments, and all material incorporated by reference or deemed to be incorporated by reference in such Prospectus.
“Registrable Securities” means (i) the Shares, and (ii) any securities issued or issuable upon any stock split, dividend or other distribution, recapitalization or similar event, or any exercise price adjustment with respect to any of the securities referenced in (i) or (ii) above; provided, however, that following such time as any of the securities described in clauses (i) or (ii) above have been sold by a Shareholder pursuant to a Registration Statement or Rule 144, then such securities shall cease to be considered “Registrable Securities” for purposes of this Agreement.
“Registration Statement” means a registration statement filed in accordance with Sections 2(a) or 2(b) and any additional registration statements required to be filed under this Agreement, including in each case the Prospectus, amendments and supplements to such registration statements or Prospectus, including pre- and post-effective amendments, all exhibits thereto, and all material incorporated by reference or deemed to be incorporated by reference therein.
“Revoking Shareholders” has the meaning set forth in Section 2(a).
“Rule 144” means Rule 144 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same effect as such Rule.
“Rule 415” means Rule 415 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same effect as such Rule.
“Rule 424” means Rule 424 promulgated by the Commission pursuant to the Securities Act, as such Rule may be amended from time to time, or any similar rule or regulation hereafter adopted by the Commission having substantially the same effect as such Rule.
“Securities Act” means the Securities Act of 1933, as amended.
“Shareholders” has the meaning set forth in the Preamble.
“Shareholder Designees” has the meaning set forth in Section 7(a).
“Shares” means the shares of Common Stock issuable to the Shareholders pursuant to the Merger Agreement.
2.Registration Rights.
(a)Demand Registration. If at any time (a) there is no effective Registration Statement with respect to all of the outstanding Registrable Securities and (b) not all of the outstanding Registrable Securities may be sold without registration and without volume restrictions pursuant to Rule 144, then the Shareholders may make a written demand for registration (a “Demand Registration” and the Registration Statement to be filed pursuant to such Demand Registration, the “Demand Registration Statement”) under the Securities Act of the sale of all or part of its Registrable Securities on Form S-1 or, if Holdco is so eligible, on Form S-3. Any request for a Demand Registration shall specify the number of shares (or other amount) of Registrable Securities proposed to be sold and the intended method(s) of distribution thereof (such written demand, a “Demand Notice”). Holdco shall use its best efforts to file such Demand Registration Statement within forty five (45) days after receiving the Demand Notice. Any Shareholder that has requested its Registrable Securities be included in a Demand Registration pursuant to this Section 2(a) may withdraw its Registrable Securities from such Demand Registration at any time prior to the effectiveness of the applicable Demand Registration Statement; provided, however, that a Demand Registration in its entirety may only be withdrawn with the consent of all the Shareholders (collectively, the “Revoking Shareholders”). Upon receipt of a notice to withdraw such Demand Registration, Holdco shall cease all efforts to secure effectiveness of the applicable Demand Registration Statement, and each of the Revoking Shareholders shall pay or reimburse Holdco for its pro rata share (based on the number of securities such Shareholder sought to register, as compared to the total number of securities of the Revoking Shareholders) of all registration expenses incurred by Holdco in connection with such Demand Registration.
(b)Piggyback Registration. Whenever Holdco proposes (other than pursuant to a Demand Notice) to register any of its equity securities under the Securities Act (whether for Holdco’s own account or for the account of any other person) other than in connection with a registration relating either to the sale of securities to participants in a Holdco stock option, stock purchase or similar benefit plan or pursuant to a Rule 145 transaction, including, without limitation, on Form S-4 or Form S-8, and the registration form to be used may be used for the registration of Registrable Securities (a “Piggyback Registration” and the Registration Statement to be filed pursuant to such Piggyback Registration, the “Piggyback Registration Statement”), Holdco shall give prompt, written notice to each of the Shareholders of its intention to effect such a registration (the “Piggyback Notice”), which Piggyback Notice shall offer each such Shareholder the opportunity to register on the same terms and conditions all or part of such Shareholder’s Registrable Securities. Any request from a Shareholder for a Piggyback Registration shall specify the number of shares (or other amount) of Registrable Securities proposed to be sold and the intended method(s) of distribution thereof (such request, a “Piggyback Request”). Holdco shall use its reasonable best efforts to include in such registration all Registrable Securities with respect to which Holdco has received Piggyback Requests within five (5) business days after the actual date of receipt of the Piggyback Notice by the last Shareholders to have received the subject Piggyback Notice (the “Piggyback Holders”).
(c)Notwithstanding anything to the contrary contained in this Section 2, in the event the Commission informs Holdco that all of the Registrable Securities cannot, as a result of the application of Rule 415, be registered for resale as a secondary offering on a single Registration Statement, Holdco agrees to promptly inform each of the Shareholders thereof and (i) use its commercially reasonable efforts to file amendments to the Registration Statement as required by the Commission and/or (ii) withdraw the Registration Statement and file a new Registration Statement, in either case covering the maximum number of Registrable Securities permitted to be registered by the Commission, on Form S-3 or such other form available to register for resale the Registrable Securities as a secondary offering; provided, however, that prior to filing such amendment or new Registration Statement, Holdco shall be obligated to use its commercially reasonable efforts to advocate with the Commission for the registration of all of the Registrable Securities in accordance with applicable Commission Guidance, including without limitation, Interpretive Response 612.09 of the Commission’s Securities Act Rules Compliance and Disclosure Interpretations; provided, that in no event may Holdco name any Shareholder as an underwriter without such Shareholder’s prior written consent. Notwithstanding any other provision of this Agreement, if any Commission Guidance sets forth a limitation of the number of Registrable Securities permitted to be registered on a particular Registration Statement as a secondary offering (and notwithstanding that Holdco used diligent efforts to advocate with the Commission for the registration of all or a greater number of Registrable Securities), unless otherwise directed in writing by a Shareholder as to its Registrable Securities, Holdco shall reduce the number of shares to be included by all Shareholders on a pro rata basis (based upon the number of Registrable Securities otherwise required to be included for each Shareholder) unless the inclusion of shares by one or more Shareholders is resulting in the Commission’s “by or on behalf of the company” offering position, in which event the shares held by such Shareholders shall be the only shares subject to reduction (and if by more than one Shareholder on a pro rata basis or on such other basis as would result in the exclusion of the least number of shares by all such Shareholders). If in addition to the Registrable Securities, the Registration Statement covers shares of Holdco securities that are not Registrable Securities, the Registrable Securities shall be given priority over such other securities so that in making any reduction required by the Commission, Holdco shall first reduce or eliminate the shares that are not Registrable Securities. In the event Holdco amends the Registration Statement or files a new Registration Statement, as the case may be, under clauses (i) or (ii) above, Holdco will use its commercially reasonable efforts to file with the Commission, as promptly as allowed by Commission or Commission Guidance, one or more Registration Statements on Form S-3 or such other form available to register for resale those Registrable Securities that were not registered for resale on the initial Registration Statement, as amended, or the new Registration Statement
3.Registration Procedures.
In connection with Holdco’s registration obligations hereunder, Holdco shall:
(a)(i) Prepare and file with the Commission such Registration Statements in order to register for resale under the Securities Act all Registrable Securities subject to a Demand Notice or Piggyback Request, such amendments, including post-effective amendments as may be necessary to keep the Registration Statement continuously effective as to the applicable Registrable Securities for its Effectiveness Period; (ii) cause the related Prospectus to be amended or supplemented by any required Prospectus supplement, and as so supplemented or amended to be filed pursuant to Rule 424; (iii) respond as promptly as reasonably possible to any comments received from the Commission with respect to each Registration Statement or any amendment thereto; and (iv) comply in all material respects with the provisions of the Securities Act and the Exchange Act with respect to the Registration Statement(s) and the disposition of all Registrable Securities covered by each Registration Statement, provided that as promptly as practicable before filing a Registration Statement or prospectus or any amendments or supplements thereto, Holdco shall furnish copies of all such documents proposed to be filed to one counsel selected by the Shareholders, which documents shall be subject to the review and reasonable comments of such counsel.
(b) Notify the Shareholders as promptly as reasonably possible (i)(A) when a Prospectus or any Prospectus supplement or post-effective amendment to a Registration Statement is proposed to be filed; (B) when the Commission notifies Holdco whether there will be a “review” of such Registration Statement and whenever the Commission comments in writing on such Registration Statement; and (C) with respect to each Registration Statement or any post-effective amendment, when the same has become effective; (ii) of any request by the Commission or any other Federal or state governmental authority for amendments or supplements to a Registration Statement or Prospectus or for additional information; (iii) of the issuance by the Commission of any stop order suspending the effectiveness of a Registration Statement covering any or all of the Registrable Securities or the initiation of any Proceedings for that purpose; (iv) of the receipt by Holdco of any notification with respect to the suspension of the qualification or exemption from qualification of any of the Registrable Securities for sale in any jurisdiction, or the initiation or threatening of any Proceeding for such purpose; and (v) of the occurrence of any event or passage of time that makes the financial statements included in a Registration Statement ineligible for inclusion therein or any statement made in such Registration Statement or Prospectus or any document incorporated or deemed to be incorporated therein by reference untrue in any material respect or that requires any revisions to such Registration Statement, Prospectus or other documents so that, in the case of such Registration Statement or the Prospectus, as the case may be, it will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading.
(c) Use its reasonable best efforts to avoid the issuance of, or, if issued, obtain the withdrawal of (i) any order suspending the effectiveness of a Registration Statement, or (ii) any suspension of the qualification (or exemption from qualification) of any of the Registrable Securities for sale in any jurisdiction, at the earliest practicable moment.
(d) Prior to any public offering of Registrable Securities, register or qualify such Registrable Securities for offer and sale under the securities or Blue Sky laws of all jurisdictions within the United States as any Shareholder may request, to keep each such registration or qualification (or exemption therefrom) effective during the Effectiveness Period and to do any and all other acts or things necessary or advisable to enable the disposition in such jurisdictions of the Registrable Securities covered by the Registration Statement(s).
(e) Cooperate with the Shareholder to facilitate the timely preparation and delivery of certificates representing Registrable Securities to be delivered to a transferee pursuant to the Registration Statement(s), which certificates shall be free, to the extent permitted by the Merger Agreement and applicable law, of all restrictive legends, and to enable such Registrable Securities to be in such denominations and registered in such names as any such Shareholder may request.
(f) Upon the occurrence of any event contemplated by Section 3(b)(v), as promptly as reasonably possible, prepare a supplement or amendment, including a post-effective amendment, to the affected Registration Statements or a supplement to the related Prospectus or any document incorporated or deemed to be incorporated therein by reference, and file any other required document so that, as thereafter delivered, no Registration Statement nor any Prospectus will contain an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading.
(g) Cause the Registrable Securities covered by a Registration Statement to be listed on each securities exchange or automated quotation system on which the Common Stock is then listed for trading.
4.Registration Expenses. Subject to Section 2(a), all fees and expenses incidental to Holdco’s performance of or compliance with this Agreement (other than underwriting or brokerage fees, discounts and commissions and transfer taxes, if any, paid with respect to Registrable Securities sold by the Shareholders) shall be borne by Holdco whether or not any Registrable Securities are sold pursuant to a Registration Statement. The fees and expenses referred to in the foregoing sentence shall include, without limitation, (i) all registration and filing fees (including, without limitation, fees and expenses (A) with respect to filings required to be made with any securities exchange or automated quotation system on which the Common Stock is then listed for trading, and (B) in compliance with applicable state securities or Blue Sky laws), (ii) printing expenses (including, without limitation, expenses of printing certificates for Registrable Securities and of printing prospectuses if the printing of prospectuses is reasonably requested by the holders of a majority of the Registrable Securities included in the Registration Statement), (iii) messenger, telephone and delivery expenses, (iv) fees and disbursements of counsel for Holdco, (v) Securities Act liability insurance, if Holdco so desires such insurance, (vi) fees and expenses of all other persons retained by Holdco in connection with the consummation of the transactions contemplated by this Agreement, and (vii) the reasonable fees and disbursements of one counsel selected by the Shareholders. In addition, Holdco shall be responsible for all of its internal expenses incurred in connection with the consummation of the transactions contemplated by this Agreement (including, without limitation, all salaries and expenses of its officers and employees performing legal or accounting duties), the expense of any annual audit and the fees and expenses incurred in connection with the listing of the Registrable Securities on any securities exchange as required hereunder.
5.Indemnification.
(a) Indemnification by Holdco. Holdco shall, notwithstanding any termination of this Agreement, indemnify and hold harmless each Shareholder, the officers, directors, agents, investment advisors, partners, members and employees of each of them, each person who controls any such Shareholder (within the meaning of Section 15 of the Securities Act or Section 20 of the Exchange Act) and the officers, directors, agents and employees of each such controlling person, to the fullest extent permitted by applicable law, from and against any and all losses, claims, damages, liabilities, costs (including, without limitation, reasonable costs of preparation and reasonable attorneys’ fees) and expenses (collectively, “Losses”), as incurred, arising out of or relating to (i) any violations by Holdco of the Securities Act, Exchange Act or any state securities law or any rule or regulation thereunder in connection with the performance of its obligations to register securities under this Agreement or (ii) any untrue or alleged untrue statement of a material fact contained in any Registration Statement, any Prospectus or any form of prospectus or in any amendment or supplement thereto or in any preliminary prospectus, or arising out of or relating to any omission or alleged omission of a material fact required to be stated therein or necessary to make the statements therein (in the case of any Prospectus or form of prospectus or supplement thereto, in light of the circumstances under which they were made) not misleading, except to the extent, but only to the extent, that (1) such untrue statements or omissions are based solely upon information regarding such Shareholder furnished in writing to Holdco by such Shareholder expressly for use therein, or to the extent that such information relates to such Shareholder or such Shareholder’s proposed method of distribution of Registrable Securities and was reviewed and expressly approved in writing by such Shareholder expressly for use in the Registration Statement, such Prospectus or such form of Prospectus or in any amendment or supplement thereto or (2) in the case of an occurrence of an event of the type specified in Section 3(b)(ii)-(v), the use by such Shareholder of an outdated or defective Prospectus after Holdco has notified such Shareholder in writing that the Prospectus is outdated or defective and prior to the receipt by such Shareholder of the Advice or an amended or supplemented Prospectus, but only if and to the extent that following the receipt of the Advice or the amended or supplemented Prospectus the misstatement or omission giving rise to such Loss would have been corrected. Holdco shall notify the Shareholder promptly of the institution, threat or assertion of any Proceeding of which Holdco is aware in connection with the transactions contemplated by this Agreement.
(b) Indemnification by Shareholder. In connection with any Registration Statement in which a holder of Registrable Securities is participating, each such Shareholder shall, severally and not jointly, indemnify and hold harmless Holdco, its directors, officers, agents and employees, each person who controls Holdco (within the meaning of Section 15 of the Securities Act and Section 20 of the Exchange Act), and the directors, officers, agents or employees of such controlling persons, to the fullest extent permitted by applicable law, from and against all Losses, as incurred, arising solely out of or based solely upon: (x) such Shareholder’s failure to comply with the prospectus delivery requirements of the Securities Act or (y) any untrue statement of a material fact contained in any Registration Statement, any Prospectus, or any form of prospectus, or in any amendment or supplement thereto, or arising solely out of or based solely upon any omission of a material fact required to be stated therein or necessary to make the statements therein not misleading to the extent, but only to the extent that, (1) such untrue statements or omissions are based solely upon information regarding such Shareholder furnished in writing to Holdco by such Shareholder expressly for use therein, or to the extent that such information relates to such Shareholder or such Shareholder’s proposed method of distribution of Registrable Securities and was reviewed and expressly approved in writing by such Shareholder expressly for use in the Registration Statement (it being understood that the Shareholder has approved Annex A hereto for this purpose), such Prospectus or such form of Prospectus or in any amendment or supplement thereto or (2) in the case of an occurrence of an event of the type specified in Section 3(b)(ii)-(v), the use by such Shareholder of an outdated or defective Prospectus after Holdco has notified such Shareholder in writing that the Prospectus is outdated or defective and prior to the receipt by such Shareholder of an Advice or an amended or supplemented Prospectus, but only if and to the extent that following the receipt of the Advice or the amended or supplemented Prospectus the misstatement or omission giving rise to such Loss would have been corrected. In no event shall the liability of any selling Shareholder hereunder be greater in amount than the dollar amount of the net proceeds received by such Shareholder upon the sale of the Registrable Securities giving rise to such indemnification obligation. In addition, no Shareholder shall have an indemnification obligation under this Section 5 from or against any Losses to the extent the untrue statement, omission, or allegation thereof upon which such Losses are based was made in any Prospectus used after such time as such Shareholder advised Holdco that the filing of a post-effective amendment or supplement thereto was required.
(c) Conduct of Indemnification Proceedings. If any Proceeding shall be brought or asserted against any person entitled to indemnity hereunder (an “Indemnified Party”), such Indemnified Party shall within ten (10) days notify the person from whom indemnity is sought (the “Indemnifying Party”) in writing, and the Indemnifying Party shall assume the defense thereof, including the employment of counsel reasonably satisfactory to the Indemnified Party and the payment of all fees and expenses incurred in connection with defense thereof; provided, that the failure of any Indemnified Party to give such notice shall not relieve the Indemnifying Party of its obligations or liabilities pursuant to this Agreement, except (and only) to the extent that it shall be finally determined by a court of competent jurisdiction (which determination is not subject to appeal or further review) that such failure shall have proximately and materially adversely prejudiced the Indemnifying Party.
An Indemnified Party shall have the right to employ separate counsel in any such Proceeding and to participate in the defense thereof, but the fees and expenses of such counsel shall be at the expense of such Indemnified Party or Parties unless: (1) the Indemnifying Party has agreed in writing to pay such fees and expenses; (2) the Indemnifying Party shall have failed promptly to assume the defense of such Proceeding and to employ counsel reasonably satisfactory to such Indemnified Party in any such Proceeding; or (3) the named parties to any such Proceeding (including any impleaded parties) include both such Indemnified Party and the Indemnifying Party, and such Indemnified Party shall have been advised by counsel that a conflict of interest is likely to exist if the same counsel were to represent such Indemnified Party and the Indemnifying Party (in which case, if such Indemnified Party notifies the Indemnifying Party in writing that it elects to employ separate counsel at the expense of the Indemnifying Party, the Indemnifying Party shall not have the right to assume the defense thereof and such counsel shall be at the expense of the Indemnifying Party). The Indemnifying Party shall not be liable for any settlement of any such Proceeding effected without its written consent, which consent shall not be unreasonably withheld. No Indemnifying Party shall, without the prior written consent of the Indemnified Party, effect any settlement of any pending Proceeding in respect of which any Indemnified Party is a party, unless such settlement includes an unconditional release of such Indemnified Party from all liability on claims that are the subject matter of such Proceeding.
All fees and expenses of the Indemnified Party (including reasonable fees and expenses to the extent incurred in connection with investigating or preparing to defend such Proceeding in a manner not inconsistent with this Section) shall be paid to the Indemnified Party, as incurred, within twenty (20) business days of written notice thereof to the Indemnifying Party (regardless of whether it is ultimately determined that an Indemnified Party is not entitled to indemnification hereunder; provided, that the Indemnifying Party may require such Indemnified Party to undertake to reimburse all such fees and expenses to the extent it is finally judicially determined that such Indemnified Party is not entitled to indemnification hereunder).
(d) Contribution. If a claim for indemnification under Section 5(a) or 5(b) is unavailable to an Indemnified Party (by reason of public policy or otherwise), then each Indemnifying Party, in lieu of indemnifying such Indemnified Party, shall contribute to the amount paid or payable by such Indemnified Party as a result of such Losses, in such proportion as is appropriate to reflect the relative fault of the Indemnifying Party and Indemnified Party in connection with the actions, statements or omissions that resulted in such Losses as well as any other relevant equitable considerations. The relative fault of such Indemnifying Party and Indemnified Party shall be determined by reference to, among other things, whether any action in question, including any untrue or alleged untrue statement of a material fact or omission or alleged omission of a material fact, has been taken or made by, or relates to information supplied by, such Indemnifying Party or Indemnified Party, and the parties’ relative intent, knowledge, access to information and opportunity to correct or prevent such action, statement or omission. The amount paid or payable by a party as a result of any Losses shall be deemed to include, subject to the limitations set forth in Section 5(c), any reasonable attorneys’ or other reasonable fees or expenses incurred by such party in connection with any Proceeding to the extent such party would have been indemnified for such fees or expenses if the indemnification provided for in this Section was available to such party in accordance with its terms.
The parties hereto agree that it would not be just and equitable if contribution pursuant to this Section 5(d) were determined by pro rata allocation or by any other method of allocation that does not take into account the equitable considerations referred to in the immediately preceding paragraph. Notwithstanding the provisions of this Section 5(d), no Shareholder shall be required to contribute, in the aggregate, any amount in excess of the amount by which the proceeds actually received by such Shareholder from the sale of the Registrable Securities subject to the Proceeding exceeds the amount of any damages that such Shareholder has otherwise been required to pay by reason of such untrue or alleged untrue statement or omission or alleged omission pursuant to Section 5(c).
The indemnity and contribution agreements contained in this Section are in addition to any liability that the Indemnifying Parties may have to the Indemnified Parties.
6. Rule 144. Holdco hereby agrees as follows: Holdco shall use its reasonable best efforts to make and keep current public information available, as those terms are understood and defined in Rule 144 under the Securities Act, at all times; Holdco shall use its reasonable best efforts to file with the Commission in a timely manner all reports and other documents as the Commission may prescribe under Section 13(a) or 15(d) of the Exchange Act; and Holdco shall furnish to each holder of Registrable Securities forthwith upon request (i) a written statement by Holdco as to its compliance with the reporting requirements of Rule 144 and of the Securities Act and the Exchange Act, (ii) a copy of the most recent annual or quarterly report of Holdco and (iii) such other reports and documents so filed as a holder of Registrable Securities may reasonably request to avail itself of any rule or regulation of the Commission allowing a holder of Registrable Securities to sell any such securities without registration.
7. Board of Directors.
(a)Holdco shall take all actions reasonably necessary such that as of the date of this Agreement, the Board of Directors of Holdco (the “Board”) shall consist of the number of directors as set forth in the Merger Agreement. For so long as the Shareholders continue to beneficially own (as determined under Section 13(d) of the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder), directly or indirectly, in the aggregate at least 10% of the outstanding shares of Holdco Common Stock, the Shareholders shall have the right to designate two persons for nomination for election to the Board. Persons designated by the Shareholders in accordance with the foregoing sentence shall be referred to as the “Shareholder Designees.” The remaining members of the Board shall be nominated by the Board or a committee of the Board.
(b) At each meeting of the shareholders of Holdco at which directors of Holdco are to be elected, Holdco shall nominate for election, and recommend that the shareholders of Holdco elect, to the Board each Shareholder Designee, provided, however, that notwithstanding anything to the contrary in this Agreement, Holdco (and its Board) shall be under no obligation to recommend to the shareholders or vote in favor of a Shareholder Designee to the extent that the Board (or a committee of the Board) determines in good faith that the nomination or recommendation of such nominee by the Board would reasonably be expected to violate the Board’s duties under applicable law because (i) such nominee is unfit to serve as a director of Holdco or (ii) service by such nominee as a director would reasonably be expected to violate applicable law. It is understood that the Board has determined that each of the Shareholders is eligible to serve as Shareholder Designees as of the date of this Agreement through the date of Holdco’s next annual meeting of shareholders.
(c) As long as the Shareholders have the right to designate to the Board the Shareholder Designees as specified in Section 7(a), the Board may not expand the size of the Board beyond such number as set forth in Section 7(a), including without limitation by creation of a vacancy under applicable law, without the approval of each Shareholder Designee.
8.Preemptive Rights.
(a) Participation Right. For so long as the Shareholders continue to beneficially own (as determined under Section 13(d) of the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder), directly or indirectly, in the aggregate at least 10% of the outstanding shares of Holdco Common Stock, Holdco shall not issue or sell any Additional Securities in a Covered Transaction, unless Holdco first submits a written offer (the “Participation Offer”) to each of the Shareholders to permit them to participate in the purchase of such Additional Securities on the same terms and conditions, including price, as proposed by Holdco in connection with such issuance or sale.
(b) Participation Procedures. In the event that Holdco proposes to undertake an issuance of Additional Securities, it shall send each Shareholder a Participation Offer describing the type of Additional Securities, the price and the general terms upon which Holdco proposes to issue such Additional Securities. Each Shareholder shall have fifteen (15) business days from the date of such Participation Offer to agree to purchase or otherwise acquire, at the price and on the terms specified in the Participation Offer, up to that portion of such Additional Securities as set forth in Section 8(c) by giving written notice to Holdco and stating therein the quantity of Additional Securities to be purchased (not to exceed such Shareholder’s portion of such Additional Securities as set forth in Section 8(c)). In the event that a Shareholder fails to exercise in full these preemptive rights within such fifteen (15) business day period, then Holdco shall have ninety (90) days thereafter to sell the Additional Securities with respect to which the Shareholder’s preemptive rights hereunder were not exercised, at a price and upon general terms not more favorable to the purchasers thereof than specified in the Participation Offer delivered to each Shareholder. In the event that Holdco has not issued and sold the Additional Securities within such ninety (90) day period, then Holdco shall not thereafter issue or sell any Additional Securities without again first making the Participation Offer required by this Section 8.
(c) Extent of Participation. The number of securities that may be purchased by any Shareholder upon receipt of a Participation Offer shall be equal to the amount determined by multiplying the total number of securities Holdco proposes to sell by the ratio of (i) the shares of Common Stock of Holdco then owned by such Shareholder or issuable (directly or indirectly) upon conversion and/or exercise, as applicable, of any other derivative security then held by such Shareholder, to (ii) all of the issued and outstanding shares of Common Stock of Holdco determined on a fully-diluted basis, including shares of Common Stock issuable upon conversion of any outstanding securities convertible into Common Stock.
9. Miscellaneous.
(a) Remedies. In the event of a breach by Holdco or by a Shareholder, of any of their obligations under this Agreement, each Shareholder or Holdco, as the case may be, in addition to being entitled to exercise all rights granted by law and under this Agreement, including recovery of damages, will be entitled to specific performance of its rights under this Agreement. Holdco and each Shareholder agree that monetary damages would not provide adequate compensation for any Losses incurred by reason of a breach by it of any of the provisions of this Agreement and hereby further agrees that, in the event of any action for specific performance in respect of such breach, it shall waive the defense that a remedy at law would be adequate.
(b) Compliance. Each Shareholder covenants and agrees that it will comply with the prospectus delivery requirements of the Securities Act as applicable to it in connection with sales of Registrable Securities pursuant to the Registration Statement.
(c) Discontinued Disposition. Each Shareholder agrees by its acquisition of such Registrable Securities that, upon receipt of a notice from Holdco of the occurrence of any event of the kind described in Section 3(b), such Shareholder will forthwith discontinue disposition of such Registrable Securities under the Registration Statement until such Shareholder’s receipt of the copies of the supplemented Prospectus and/or amended Registration Statement or until it is advised in writing (the “Advice”) by Holdco that the use of the applicable Prospectus may be resumed, and, in either case, has received copies of any additional or supplemental filings that are incorporated or deemed to be incorporated by reference in such Prospectus or Registration Statement. Holdco may provide appropriate stop orders to enforce the provisions of this paragraph.
(d) Amendments and Waivers. The provisions of this Agreement, including the provisions of this Section 9(d), may not be amended, modified or supplemented, and waivers or consents to departures from the provisions hereof may not be given, unless the same shall be in writing and signed by Holdco and the Shareholders.
(e) Notices. All notices, requests, consents, claims, demands, waivers and other communications hereunder shall be in writing and shall be deemed to have been given (a) when delivered by hand (with written confirmation of receipt), (b) when received by the addressee if sent by a nationally recognized overnight courier (with confirmation of delivery), (c) on the date sent by e-mail (with confirmation of receipt) if sent during normal business hours of the recipient, and on the next business day if sent after normal business hours of the recipient or (d) on the third day after the date mailed, by certified or registered mail, return receipt requested, postage prepaid. Such communications must be sent to the respective parties at the following addresses or e-mail addresses (or at such other address or e-mail address for a party as shall be specified for such purpose in a notice given in accordance with this Section 9(e)):
| | If to Holdco: | 8601 Six Forks Rd., Suite 400 |
| | | Raleigh, North Carolina 27615 |
| | | E-mail: steve@isletsciences.com |
| | | Attention: Chief Financial Officer |
| | With a copy to: | Ofsink LLC |
| | 230 Park Avenue, Suite 851 |
| | New York, NY 10169 |
| | E-mail: dofsink@golawintl.com |
| | Attention: Darren Ofsink, Esq. |
| | If to a Shareholder: | James T. Green |
| | 8601 Six Forks Rd., Suite 400 |
| | Raleigh, North Carolina 27615 |
| | E-mail: greenjt@hotmail.com |
| | |
| | William Wilkison |
| | 8601 Six Forks Rd., Suite 400 |
| | Raleigh, North Carolina 27615 |
| | E-mail: bill_wilkison@hotmail.com |
| | |
| | With a copy to: | Smith, Anderson, Blount, Dorsett, Mitchell & Jernigan, L.L.P. |
| | 2300 Wells Fargo Capitol Center |
| | Post Office Box 2611 |
| | Raleigh, North Carolina 27602-2611 |
| | E-mail: harmstrong@smithlaw.com |
| | Attention: Heyward D. Armstrong, Esq. |
(f) Successors and Assigns. This Agreement shall be enforceable by and inure to the benefit of and be binding upon the parties hereto and their respective successors and permitted assigns. Holdco may not assign its rights or obligations hereunder without the prior written consent of each Shareholder.
(g) Execution and Counterparts. This Agreement may be executed in any number of counterparts, each of which when so executed shall be deemed to be an original and, all of which taken together shall constitute one and the same Agreement. In the event that any signature is delivered by facsimile transmission, such signature shall create a valid binding obligation of the party executing (or on whose behalf such signature is executed) the same with the same force and effect as if such facsimile signature were the original thereof.
(h) Governing Law. All questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be governed by and construed and enforced in accordance with the internal laws of the State of Delaware, without regard to the principles of conflicts of law thereof. Each party agrees that all Proceedings concerning the interpretations, enforcement and defense of the transactions contemplated by this Agreement (whether brought against a party hereto or its respective Affiliates, employees or agents) will be commenced in the Delaware Courts. Each party hereto hereby irrevocably submits to the exclusive jurisdiction of the Delaware Courts for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any Proceeding, any claim that it is not personally subject to the jurisdiction of any Delaware Court, or that such Proceeding has been commenced in an improper or inconvenient forum. Each party hereto hereby irrevocably waives personal service of process and consents to process being served in any such Proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. Each party hereto hereby irrevocably waives, to the fullest extent permitted by applicable law, any and all right to trial by jury in any Proceeding arising out of or relating to this Agreement or the transactions contemplated hereby. If either party shall commence a Proceeding to enforce any provisions of this Agreement, then the prevailing party in such Proceeding shall be reimbursed by the other party for its attorney’s fees and other costs and expenses incurred with the investigation, preparation and prosecution of such Proceeding.
(i) Cumulative Remedies. The remedies provided herein are cumulative and not exclusive of any remedies provided by law.
(j) Severability. If any term, provision, covenant or restriction of this Agreement is held by a court of competent jurisdiction to be invalid, illegal, void or unenforceable, the remainder of the terms, provisions, covenants and restrictions set forth herein shall remain in full force and effect and shall in no way be affected, impaired or invalidated, and the parties hereto shall use their reasonable efforts to find and employ an alternative means to achieve the same or substantially the same result as that contemplated by such term, provision, covenant or restriction. It is hereby stipulated and declared to be the intention of the parties that they would have executed the remaining terms, provisions, covenants and restrictions without including any of such that may be hereafter declared invalid, illegal, void or unenforceable.
(k) Headings. The headings in this Agreement are for convenience of reference only and shall not limit or otherwise affect the meaning hereof.
(l) Independent Nature of Shareholders’ Obligations and Rights. The obligations of each Shareholder under this Agreement are several and not joint with the obligations of each other Shareholder, and no Shareholder shall be responsible in any way for the performance of the obligations of any other Shareholder under this Agreement. Nothing contained herein or in any documents or instruments referred to herein, and no action taken by any Shareholder pursuant thereto, shall be deemed to constitute the Shareholders as a partnership, an association, a joint venture or any other kind of entity, or create a presumption that the Shareholders are in any way acting in concert or as a group with respect to such obligations or the transactions contemplated by this Agreement or the Merger Agreement. Each Shareholder acknowledges that no other Shareholder will be acting as agent of such Shareholder in enforcing its rights under this Agreement. Each Shareholder shall be entitled to independently protect and enforce its rights, including without limitation the rights arising out of this Agreement, and it shall not be necessary for any other Shareholder to be joined as an additional party in any Proceeding for such purpose.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK
SIGNATURE PAGES TO FOLLOW]
IN WITNESS WHEREOF, the parties have executed this Investor Rights Agreement as of the date first written above.
| | AVOGENX, INC. | |
| | | | |
| | By: | /s/ | |
| | | Name | |
| | | Title | |
| | | | |
| | SHAREHOLDERS | |
| | | | |
| | | | |
| | | James T. Green | |
| | | | |
| | | | |
| | | William Wilkison | |
| | | | |
Exhibit D
ASSIGNMENT OF MEMBERSHIP INTERESTS
THIS ASSIGNMENT OF MEMBERSHIP INTERESTS (this “Assignment”) is made by [James T. Green/William Wilkison] (the “Assignor”), to Avogenx, Inc., a Delaware corporation (“Holdco”). Capitalized terms used but not defined herein have the meanings respectively assigned to them in that certain Agreement and Plan of Merger (the “Merger Agreement”) dated as of September __, 2014, by and among Islet Sciences, Inc., a Nevada corporation, Brighthaven Ventures, L.L.C., a North Carolina limited liability company (“BHV”), Holdco, Islet Merger Sub, Inc., a Nevada corporation and a direct wholly owned subsidiary of Holdco, and the Assignor and [William Wilkison/James T. Green] as members of BHV (the “BHV Members”).
The Assignor is a member of BHV, and owns fifty percent (50%) of the membership interests in BHV (the “Assigned Interest”).
Pursuant to the Merger Agreement, the BHV Members agreed to sell, transfer, convey, assign and deliver to Holdco all of the BHV Membership Interests (as defined in the Merger Agreement) on the terms and conditions set forth in the Merger Agreement.
Therefore, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, and intending to be legally bound, the Assignor hereby sells, transfers, assigns, conveys and delivers unto Holdco and its successors and assigns, all right, title and interest in and to the Assigned Interest, being fifty percent (50%) of the total membership interests in BHV.
[signature page follows]
[Signature page to Assignment of Membership Interests]
IN WITNESS WHEREOF, the Assignor has executed this Assignment as of the date first written above.
| | ASSIGNOR | |
| | | | |
| | | | |
| | | [James T. Green/William Wilkison] | |
| | | Title | |
| | | | |
Holdco acknowledges receipt of the Assigned Membership Interest.
ACCEPTED BY:
AVOGENX, INC.
By:
Name:
Title:
A-59
ANNEX B
VOTING AGREEMENT
This Voting Agreement (this “Agreement”) is made and entered into as of ___________, 2014, by and between Islet Sciences, Inc., a Nevada corporation (“Company”) and the person whose name appears on the signature page hereto (“Stockholder”).
RECITALS
A. On _________, 2014, Brighthaven Ventures, L.L.C., a North Carolina limited liability company (“BHV”), each of the members of BHV (the “BHV Members”), Avogenx, Inc., a Delaware corporation and a wholly owned subsidiary of Company (“Holdco”), Islet Merger Sub, Inc., a Nevada corporation and a direct wholly owned subsidiary of Holdco (“Merger Sub”), and Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) that, among other things, provides for (i) the merger of Merger Sub with and into the Company, with the Company as the surviving entity in the merger becoming a wholly owned subsidiary of Holdco, and each share of the Company Common Stock issued and outstanding immediately prior to the effective time of the merger to be converted into the right to receive validly issued, fully paid and non-assessable shares of Holdco common stock (the “Islet Merger”) immediately followed by (ii) the exchange of membership interests of BHV by the BHV Members for shares of Holdco common stock, with BHV becoming a wholly owned subsidiary of Holdco (together with the Islet Merger, the “Acquisitions”).
B. Stockholder agrees to enter into this Agreement with respect to each share of common stock, par value $0.001 per share, of the Company (the “Company Common Stock”) that the Stockholder owns, beneficially (as defined in Rule 13d-3 under the Securities Exchange Act) or of record, and any additional shares of Company Common Stock that Stockholder may hereinafter purchase or acquire (collectively, the “Shares”).
C. Stockholder is the beneficial or record owner, and has either sole or shared voting power over, such number of shares of Company Common Stock as is indicated on Schedule A attached hereto.
D. Company desires that Stockholder agree, and Stockholder is willing to agree, on terms and conditions set forth herein, not to Transfer (as defined below) any of the Shares, and to vote all of the Shares in a manner so as to facilitate consummation of the Acquisitions.
NOW, THEREFORE, in consideration of the foregoing and the respective representations, warranties, covenants and agreements set forth below and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto, intending to be legally bound, do hereby agree as follows:
1. Definitions. Capitalized terms used but not otherwise defined herein shall have the respective meanings ascribed to such terms in the Merger Agreement. When used in this Agreement, the following terms in all of their tenses, cases and correlative forms shall have the meanings assigned to them in this Section 1 or elsewhere in this Agreement.
“Expiration Time” shall mean the earliest to occur of (a) the Effective Time and (b) such date and time as the Merger Agreement shall be terminated validly pursuant to its terms.
“Transfer” shall mean any direct or indirect offer, sale, assignment, encumbrance, pledge, hypothecation, disposition, loan or other transfer (by operation of Law or otherwise), either voluntary or involuntary, or entry into any contract, option or other arrangement or understanding with respect to any offer, sale, assignment, encumbrance, pledge, hypothecation, disposition, loan or other transfer (by operation of Law or otherwise), excluding, for the avoidance of doubt, entry into this Agreement.
2. Agreement to Retain Shares.
2.1 No Transfer and Encumbrance of Shares. Until the Expiration Time, Stockholder agrees not to (a) Transfer any Shares, (b) deposit any Shares into a voting trust, (c) enter into a voting agreement or arrangement with respect to any Shares, (d) grant any proxy or power of attorney with respect to any Shares (other than pursuant to this Agreement); provided that Stockholder may Transfer Shares to any Affiliate of Stockholder if the transferee evidences in a writing reasonably satisfactory to Company such transferee’s agreement to be bound by and subject to the terms and provisions hereof to the same effect as Stockholder.
2.2 Additional Purchases. Stockholder agrees that any Company Common Stock that Stockholder purchases or acquires, or with respect to which Stockholder acquires sole voting power prior to the Expiration Time shall be subject to the terms and conditions of this Agreement and shall constitute Shares for all purposes under this Agreement.
2.3 Unpermitted Transfers. Any Transfer or attempted Transfer of Shares in violation of this Section 2 shall, to the fullest extent permitted by Law, be null and void ab initio.
3. Voting/ Proxy/Voting Trusts.
3.1 Agreement to Vote. Stockholder agrees during the term of this Agreement to vote the Shares or execute a written consent or consents if stockholders of the Company are requested to vote their shares through the execution of a written consent in lieu of meeting (a form of which is attached hereto as Exhibit A), and to cause any holder of record to so vote the Shares or execute a written consent: (a) in favor of the Merger Agreement and the Acquisitions at every meeting, or in connection with any action by written consent, of stockholders of the Company at which such matters are considered and at every adjournment or postponement thereof; (b) against (1) any Acquisition Proposal, other than the Acquisitions, (2) any action, proposal, transaction or agreement which could reasonably be expected to result in a breach of any covenant, representation or warranty or any other obligation or agreement of the Company under the Merger Agreement or of Stockholder under this Agreement and (3) any action, proposal, transaction or agreement that could reasonably be expected to impede, interfere with, delay, discourage, adversely affect or inhibit the timely consummation of the Acquisitions or the fulfillment of Holdco, the Merger Sub’s or the Company’s conditions under the Merger Agreement or change in any manner the voting rights of any class of shares of the Company (including any amendment to the Islet Charter or the Islet By-laws). Stockholder shall not enter into any tender, voting or other agreement, or grant a proxy or power of attorney, with respect to the Shares that is inconsistent with this Agreement or otherwise take any other action with respect to the Shares that would in any way restrict, limit or interfere with the performance of Stockholder’s obligations hereunder or the transactions contemplated hereby, including the approval of the Merger Agreement and the Acquisitions.
3.2 No Voting Trusts or Other Arrangements. Stockholder agrees that Stockholder will not, and will not permit any entity under Stockholder’s control to, deposit any of the Shares in a voting trust, grant any proxies with respect to the Shares or subject any of the Shares to any arrangement with respect to the voting of the Shares, other than agreements entered into with the Company.
4. Representations and Warranties of the Stockholders. Stockholder hereby represents and warrants to the Company as follows:
4.1 Due Authority. Stockholder has the full power and authority to make, enter into and carry out the terms of this Agreement. This Agreement has been duly and validly executed and delivered by Stockholder and constitutes a valid and binding agreement of Stockholder enforceable against it in accordance with its terms, except to the extent enforceability may be limited by the effect of applicable bankruptcy, reorganization, insolvency, moratorium or other Laws affecting the enforcement of creditors’ rights generally and the effect of general principles of equity, regardless of whether such enforceability is considered in a proceeding at Law or in equity.
4.2 Ownership of the Shares. As of the date hereof, Stockholder (a) is the beneficial (as defined in Rule 13d-3 under the Exchange Act) or record owner of the Shares set forth on Schedule A, free and clear of any and all Liens, other than those created by this Agreement, and (b) has sole voting power over each of the Shares. As of the date hereof, Stockholder does not own, beneficially or of record, any capital stock or other voting securities of the Company other than the Shares set forth on Schedule A. As of the date hereof, Stockholder does not own, beneficially (as defined in Rule 13d-3 under the Exchange Act) or of record, any rights to purchase or acquire any shares of capital stock of the Company, except as set forth on Schedule A.
4.3 No Conflict; Consents.
(a) The execution and delivery of this Agreement by Stockholder does not, and the performance by Stockholder of the obligations under this Agreement and the compliance by Stockholder with any provisions hereof do not and will not: (a) conflict with or violate any Laws applicable to Stockholder, or (b) result in any material breach of or constitute a material default (or an event that with notice or lapse of time or both would become a material default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, or result in the creation of a Lien on any of the Shares pursuant to any note, bond, mortgage, indenture, contract, agreement, lease, license, permit, franchise or other instrument or obligation to which Stockholder is a party or by which Stockholder is bound, except for any of the foregoing as could not reasonably be expected, either individually or in the aggregate, to impair the ability of Stockholder to perform its obligations hereunder or to consummate the transactions contemplated hereby on a timely basis.
(b) No consent, approval, order or authorization of, or registration, declaration or filing with, any governmental authority or any other Person, including without limitation a spouse under “community property” or other similar laws, is required by or with respect to Stockholder in connection with the execution and delivery of this Agreement or the consummation by Stockholder of the transactions contemplated hereby.
4.4 Absence of Litigation. As of the date hereof, there is no legal action pending against, or, to the knowledge of Stockholder, threatened against or affecting Stockholder that could reasonably be expected to materially impair or materially adversely affect the ability of Stockholder to perform Stockholder’s obligations hereunder or to consummate the transactions contemplated hereby on a timely basis.
5. Termination. This Agreement shall terminate and shall have no further force or effect immediately as of and following the Expiration Time.
6. Fiduciary Duties. The covenants and agreements set forth herein shall not prevent any of Stockholders’ designees serving on the board of directors of the Company from taking any action, subject to the provisions of the Merger Agreement, while acting in such designee’s capacity as a director of the Company. Stockholder is entering into this Agreement solely in its capacity as the beneficial (as defined in Rule 13d-3 under the Exchange Act) owner or record holder of the Shares.
7. No Ownership Interest. Nothing contained in this Agreement shall be deemed to vest in the Company any direct or indirect ownership or incidence of ownership of or with respect to the Shares. All rights, ownership and economic benefits of and relating to the Shares shall remain vested in and belong to the Stockholder, and Company shall have no authority to direct Stockholder in the voting or disposition of any of the Shares, except as otherwise provided herein.
8. Miscellaneous.
8.1 Severability. If any term or other provision of this Agreement is determined to be invalid, illegal or incapable of being enforced by any rule of Law or public policy, all other conditions and provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of the transactions contemplated hereby is not affected in any manner materially adverse to any party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Agreement so as to effect the original intent of the parties as closely as possible to the fullest extent permitted by applicable law in an acceptable manner to the end that the transactions contemplated hereby are fulfilled to the extent possible.
8.2 Binding Effect and Assignment. This Agreement and all of the provisions hereof shall be binding upon and inure to the benefit of the parties hereto and their respective successors and permitted assigns.
8.3 Amendments and Modifications. This Agreement may not be modified, amended, altered or supplemented except upon the execution and delivery of a written agreement executed by all of the parties hereto.
8.4 Specific Performance; Injunctive Relief. The parties hereto agree that irreparable damage would occur in the event any provision of this Agreement was not performed in accordance with the terms hereof or was otherwise breached. It is accordingly agreed that the parties shall be entitled to specific relief hereunder, including, without limitation, an injunction or injunctions to prevent and enjoin breaches of the provisions of this Agreement and to enforce specifically the terms and provisions hereof, in the Delaware Court of Chancery and any state appellate court therefrom within the State of Delaware (unless the Delaware Court of Chancery shall decline to accept jurisdiction over a particular matter, in which case, in any federal court within the State of Delaware), in addition to any other remedy to which they may be entitled at Law or in equity. Any requirements for the securing or posting of any bond with respect to any such remedy are hereby waived.
8.5 Notices. All notices, requests, claims, consents, demands and other communications under this Agreement shall be in writing and shall be deemed given if delivered personally, sent by overnight courier (providing proof of delivery) to the parties or sent by e-mail of a .pdf attachment (providing confirmation of transmission) at the following addresses (or at such other address for a party as shall be specified by like notice):
(i) if to the Stockholder, to the address set forth on Schedule A
(ii) if to Company, to:
| | | |
| | Name: | Islet Sciences, Inc. |
| | Address: | 8601 Six Forks Rd., Suite 400 |
| | | Raleigh, North Carolina 27615 |
| | E-mail: | info@isletsciences.com |
| | Attention: | James Green |
with a copy to (which shall not be considered notice):
| | | |
| | Name: | Ofsink, LLC |
| | Address: | 230 Park Avenue, Suite 851 |
| | | New York, New York 10169 |
| | E-mail: | dofsink@golawintl.com |
| | Attention: | Darren Ofsink, Esq. |
Or to such other address as any party may have furnished to the other in writing in accordance herewith, except that notices of change of address shall be effective upon receipt.
8.6 APPLICABLE LAW; JURISDICTION OF DISPUTES. THIS AGREEMENT SHALL BE GOVERNED BY AND CONSTRUED AND ENFORCED IN ACCORDANCE WITH THE INTERNAL LAWS OF THE STATE OF DELAWARE WITHOUT GIVING EFFECT TO THE PRINCIPLES OF CONFLICTS OF LAW THEREOF. IN THE EVENT ANY PARTY TO THIS AGREEMENT COMMENCES ANY LITIGATION, PROCEEDING OR OTHER LEGAL ACTION IN CONNECTION WITH OR RELATING TO NEGOTIATION AND EXPLORATION WITH RESPECT TO OR ENTERING INTO OF THIS AGREEMENT OR ANY MATTERS DESCRIBED OR CONTEMPLATED HEREIN, THE PARTIES TO THIS AGREEMENT HEREBY (A) AGREE THAT ANY SUCH LITIGATION, PROCEEDING OR OTHER LEGAL ACTION SHALL BE INSTITUTED EXCLUSIVELY IN A COURT OF COMPETENT JURISDICTION LOCATED WITHIN THE STATE OF DELAWARE, WHETHER A STATE OR FEDERAL COURT; (B) AGREE THAT IN THE EVENT OF ANY SUCH LITIGATION, PROCEEDING OR ACTION, SUCH PARTIES WILL CONSENT AND SUBMIT TO PERSONAL JURISDICTION IN ANY SUCH COURT DESCRIBED IN CLAUSE (A) OF THIS SECTION 8.6AND TO SERVICE OF PROCESS UPON THEM IN ACCORDANCE WITH THE RULES AND STATUTES GOVERNING SERVICE OF PROCESS; (C) AGREE TO WAIVE TO THE FULL EXTENT PERMITTED BY LAW ANY OBJECTION THAT THEY MAY NOW OR HEREAFTER HAVE TO THE VENUE OF ANY SUCH LITIGATION, PROCEEDING OR ACTION IN ANY SUCH COURT OR THAT ANY SUCH LITIGATION, PROCEEDING OR ACTION WAS BROUGHT IN AN INCONVENIENT FORUM; (D) AGREE AS AN ALTERNATIVE METHOD OF SERVICE TO SERVICE OF PROCESS IN ANY LEGAL PROCEEDING BY MAILING OF COPIES THEREOF TO SUCH PARTY AT ITS ADDRESS SET FORTH IN SECTION 8.5 FOR COMMUNICATIONS TO SUCH PARTY; (E) AGREE THAT ANY SERVICE MADE AS PROVIDED HEREIN SHALL BE EFFECTIVE AND BINDING SERVICE IN EVERY RESPECT; AND (F) AGREE THAT NOTHING HEREIN SHALL AFFECT THE RIGHTS OF ANY PARTY TO EFFECT SERVICE OF PROCESS IN ANY OTHER MANNER PERMITTED BY LAW.
8.7 WAIVER OF JURY TRIAL. EACH PARTY ACKNOWLEDGES AND AGREES THAT ANY CONTROVERSY WHICH MAY ARISE UNDER THIS AGREEMENT IS LIKELY TO INVOLVE COMPLICATED AND DIFFICULT ISSUES OF FACT AND LAW, AND THEREFORE, EACH SUCH PARTY HEREBY IRREVOCABLY AND UNCONDITIONALLY WAIVES ANY RIGHT SUCH PARTY MAY OTHERWISE HAVE TO A TRIAL BY JURY IN RESPECT OF ANY LITIGATION DIRECTLY OR INDIRECTLY ARISING OUT OF OR RELATING TO THE NEGOTIATION, EXPLORATION, DUE DILIGENCE WITH RESPECT TO OR ENTERING INTO OF THIS AGREEMENT, OR THE TRANSACTIONS CONTEMPLATED BY THIS AGREEMENT. EACH PARTY CERTIFIES AND ACKNOWLEDGES THAT (A) NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (B) EACH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF THIS WAIVER, (C) EACH PARTY MAKES THIS WAIVER VOLUNTARILY, AND (D) EACH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS AND CERTIFICATIONS IN THIS SECTION 8.7.
8.8 Entire Agreement. This Agreement contains the entire understanding of the parties in respect of the subject matter hereof, and supersedes all prior negotiations and understandings between the parties with respect to such subject matter.
8.9 Counterparts. This Agreement may be executed in several counterparts, each of which shall be an original, but all of which together shall constitute one and the same agreement. The signature pages hereto in facsimile copy or other electronic means, including e-mail attachment, shall be deemed an original for all purposes.
8.10 Effect of Headings. The section headings herein are for convenience only and shall not affect the construction of interpretation of this Agreement.
8.11 No Agreement Until Executed. Irrespective of negotiations among the parties or the exchanging of drafts of this Agreement, this Agreement shall not constitute or be deemed to evidence a contract, agreement, arrangement or understanding between the parties hereto unless and until this Agreement is executed and delivered by all parties hereto.
8.12 Legal Representation. This Agreement was negotiated by the parties with the benefit of legal representation and any rule of construction or interpretation otherwise requiring this Agreement to be construed or interpreted against any party shall not apply to any construction or interpretation thereof.
8.13 Expenses. All costs and expenses incurred in connection with this Agreement shall be paid by the party incurring such cost or expense.
8.14 No Recourse. Notwithstanding anything to the contrary contained herein or otherwise, this Agreement may only be enforced against, and any claims or causes of action that may be based upon, arise out of or relate to this Agreement, or the negotiation, execution or performance of this Agreement or the transactions contemplated hereby, may only be made against the entities and Persons that are expressly identified as parties to this Agreement in their capacities as such and no former, current or future stockholders, equity holders, controlling persons, directors, officers, employees, general or limited partners, members, managers, agents or Affiliates of any party hereto, or any former, current or future direct or indirect stockholder, equity holder, controlling person, director, officer, employee, general or limited partner, member, manager, agent or Affiliate of any of the foregoing (each, a “Non-Recourse Party”) shall have any liability for any obligations or liabilities of the parties to this Agreement or for any claim (whether in tort, contract or otherwise) based on, in respect of, or by reason of, the transactions contemplated hereby or in respect of any oral representations made or alleged to be made in connection herewith. Without limiting the rights of any party against the other parties hereto, in no event shall any party or any of its Affiliates seek to enforce this Agreement against, make any claims for breach of this Agreement against, or seek to recover monetary damages from, any Non-Recourse Party.
[Signature page follows]
IN WITNESS WHEREOF, the parties have caused this Agreement to be duly executed on the date and year first above written.
| | Islet Sciences, Inc. | |
| | | | |
| | By: | /s/ | |
| | | James Green | |
| | | Chief Executive Officer | |
| | | | |
Signature Page to Voting Agreement
STOCKHOLDER:
| | IF AN ENTITY: | |
| | | | |
| | | | |
| | | Name | |
| | | | |
| | By: | /s/ | |
| | | Name | |
| | | Title | |
| | | | |
| | IF AN INDIVIDUAL: | |
| | | | |
| | | | |
| | | Name | |
| | | | |
| | | | |
| | | | |
| | | (Signature) | |
| | | | |
| | | | |
Signature Page to Voting Agreement
Schedule A
| Address of Stockholder | | | Number of Shares of Company Common Stock | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
Exhibit A
FORM OF WRITTEN CONSENT
ISLET SCIENCES, INC.
This Written Consent is solicited by the Board of Directors of Islet Sciences, Inc.
Please return this consent no later than pm (Eastern Standard Time) on , 2014, which is the date that the Company Board of Directors expects to receive consents under the Voting Agreement.
The undersigned, being a holder of record of common stock, par value $0.001, of Islet Sciences, Inc., a Nevada corporation (the “Company”), on , 2014, hereby: (i) acknowledges receipt of the consent solicitation statement/prospectus of the Company, which is part of a registration statement on Form S-4 (No. 333- ______________), and (ii) consents, by virtue of this written consent without a meeting, with respect to each share of Company common stock that the undersigned holds beneficially or of record (which number is set forth below), to the merger of Islet Merger Sub, Inc., a Nevada corporation (“Merger Sub”), with and into the Company, with the Company as the surviving entity in the merger becoming a wholly owned subsidiary of Avogenx, Inc., a Delaware corporation (“Holdco”), and each share of the Company common stock issued and outstanding immediately prior to the effective time of the merger to be converted into the right to receive validly issued, fully paid and non-assessable shares of Holdco common stock, and adoption and approval of the Agreement and Plan of Merger by and among the Company, Holdco, Merger Sub, Brighthaven Ventures, L.L.C., a North Carolina limited liability company (“BHV”), and each of the members of BHV, dated as of __________, 2014, and the transactions contemplated thereby.
IMPORTANT: PLEASE DATE AND SIGN THE CONSENT BELOW. If held in joint tenancy, all persons must sign. When signing as attorney, trustee, executor, administrator, guardian or corporate officer, please give full title as such. If shares are held by a corporation, please sign the full corporate name by president or other authorized officer. If shares are held by a partnership or other entity, please sign the full partnership or other entity name by authorized person. Please execute, date, sign and return this Written Consent promptly to the Company by emailing a .pdf copy of the Written Consent to info@isletsciences.com (Attention: James Green, Chief Executive Officer), or by mailing this Written Consent to Islet Sciences, Inc. at 8601 Six Forks Rd., Suite 400, Raleigh, North Carolina 27615, Attention: James Green, Chief Executive Officer.
| | | |
IF AN INDIVIDUAL: By: (duly authorized signature) Name: (please print or type full name) Title: (please print or type full title) | | IF AN ENTITY: (please print or type complete name of entity) By: (duly authorized signature) Name: (please print or type full name) Title: (please print or type full title) |
| | |
| Date: ________________ , 2014 | | Date: ______________ , 2014 |
Number of shares held beneficially and of record:
B-9
ANNEX C
Sections 92A.300 to 92A.500 of the Nevada Revised Statutes
NRS 92A.300 Definitions. As used in NRS 92A.300 to 92A.500, inclusive, unless the context otherwise requires, the words and terms defined in NRS 92A.305 to 92A.335, inclusive, have the meanings ascribed to them in those sections.
NRS 92A.305 “Beneficial stockholder” defined. “Beneficial stockholder” means a person who is a beneficial owner of shares held in a voting trust or by a nominee as the stockholder of record.
NRS 92A.310 “Corporate action” defined. “Corporate action” means the action of a domestic corporation.
NRS 92A.315 “Dissenter” defined. “Dissenter” means a stockholder who is entitled to dissent from a domestic corporation’s action under NRS 92A.380 and who exercises that right when and in the manner required by NRS 92A.400 to 92A.480, inclusive.
NRS 92A.320 “Fair value” defined. “Fair value,” with respect to a dissenter’s shares, means the value of the shares determined: 1. Immediately before the effectuation of the corporate action to which the dissenter objects, excluding any appreciation or depreciation in anticipation of the corporate action unless exclusion would be inequitable;
2. Using customary and current valuation concepts and techniques generally employed for similar businesses in the context of the transaction requiring appraisal; and
3. Without discounting for lack of marketability or minority status.
NRS 92A.325 “Stockholder” defined. “Stockholder” means a stockholder of record or a beneficial stockholder of a domestic corporation.
NRS 92A.330 “Stockholder of record” defined. “Stockholder of record” means the person in whose name shares are registered in the records of a domestic corporation or the beneficial owner of shares to the extent of the rights granted by a nominee’s certificate on file with the domestic corporation.
NRS 92A.335 “Subject corporation” defined. “Subject corporation” means the domestic corporation which is the issuer of the shares held by a dissenter before the corporate action creating the dissenter’s rights becomes effective or the surviving or acquiring entity of that issuer after the corporate action becomes effective.
NRS 92A.340 Computation of interest. Interest payable pursuant to NRS 92A.300 to 92A.500, inclusive, must be computed from the effective date of the action until the date of payment, at the rate of interest most recently established pursuant to NRS 99.040.
NRS 92A.350 Rights of dissenting partner of domestic limited partnership. A partnership agreement of a domestic limited partnership or, unless otherwise provided in the partnership agreement, an agreement of merger or exchange, may provide that contractual rights with respect to the partnership interest of a dissenting general or limited partner of a domestic limited partnership are available for any class or group of partnership interests in connection with any merger or exchange in which the domestic limited partnership is a constituent entity.
NRS 92A.360 Rights of dissenting member of domestic limited-liability company. The articles of organization or operating agreement of a domestic limited-liability company or, unless otherwise provided in the articles of organization or operating agreement, an agreement of merger or exchange, may provide that contractual rights with respect to the interest of a dissenting member are available in connection with any merger or exchange in which the domestic limited-liability company is a constituent entity.
NRS 92A.370 Rights of dissenting member of domestic nonprofit corporation.
1. Except as otherwise provided in subsection 2, and unless otherwise provided in the articles or bylaws, any member of any constituent domestic nonprofit corporation who voted against the merger may, without prior notice, but within 30 days after the effective date of the merger, resign from membership and is thereby excused from all contractual obligations to the constituent or surviving corporations which did not occur before the member’s resignation and is thereby entitled to those rights, if any, which would have existed if there had been no merger and the membership had been terminated or the member had been expelled.
2. Unless otherwise provided in its articles of incorporation or bylaws, no member of a domestic nonprofit corporation, including, but not limited to, a cooperative corporation, which supplies services described in chapter 704 of NRS to its members only, and no person who is a member of a domestic nonprofit corporation as a condition of or by reason of the ownership of an interest in real property, may resign and dissent pursuant to subsection 1.
NRS 92A.380 Right of stockholder to dissent from certain corporate actions and to obtain payment for shares. 1. Except as otherwise provided in NRS 92A.370 and 92A.390, any stockholder is entitled to dissent from, and obtain payment of the fair value of the stockholder’s shares in the event of any of the following corporate actions:
(a) Consummation of a plan of merger to which the domestic corporation is a constituent entity:
(1) If approval by the stockholders is required for the merger by NRS 92A.120 to 92A.160, inclusive, or the articles of incorporation, regardless of whether the stockholder is entitled to vote on the plan of merger; or
(2) If the domestic corporation is a subsidiary and is merged with its parent pursuant to NRS 92A.180.
(b) Consummation of a plan of conversion to which the domestic corporation is a constituent entity as the corporation whose subject owner’s interests will be converted.
(c) Consummation of a plan of exchange to which the domestic corporation is a constituent entity as the corporation whose subject owner’s interests will be acquired, if the stockholder’s shares are to be acquired in the plan of exchange.
(d) Any corporate action taken pursuant to a vote of the stockholders to the extent that the articles of incorporation, bylaws or a resolution of the board of directors provides that voting or nonvoting stockholders are entitled to dissent and obtain payment for their shares.
(e) Accordance of full voting rights to control shares, as defined in NRS 78.3784, only to the extent provided for pursuant to NRS 78.3793.
(f) Any corporate action not described in this subsection that will result in the stockholder receiving money or scrip instead of fractional shares except where the stockholder would not be entitled to receive such payment pursuant to NRS 78.205, 78.2055 or 78.207.
2. A stockholder who is entitled to dissent and obtain payment pursuant to NRS 92A.300 to 92A.500, inclusive, may not challenge the corporate action creating the entitlement unless the action is unlawful or fraudulent with respect to the stockholder or the domestic corporation.
3. From and after the effective date of any corporate action described in subsection 1, no stockholder who has exercised the right to dissent pursuant to NRS 92A.300 to 92A.500, inclusive, is entitled to vote his or her shares for any purpose or to receive payment of dividends or any other distributions on shares. This subsection does not apply to dividends or other distributions payable to stockholders on a date before the effective date of any corporate action from which the stockholder has dissented.
NRS 92A.390 Limitations on right of dissent: Stockholders of certain classes or series; action of stockholders not required for plan of merger. 1. There is no right of dissent with respect to a plan of merger, conversion or exchange in favor of stockholders of any class or series which is:
(a) A covered security under section 18(b)(1)(A) or (B) of the Securities Act of 1933, 15 U.S.C. § 77r(b)(1)(A) or (B), as amended;
(b) Traded in an organized market and has at least 2,000 stockholders and a market value of at least $20,000,000, exclusive of the value of such shares held by the corporation’s subsidiaries, senior executives, directors and beneficial stockholders owning more than 10 percent of such shares; or (c) Issued by an open end management investment company registered with the Securities and Exchange Commission under the Investment Company Act of 1940 and which may be redeemed at the option of the holder at net asset value, unless the articles of incorporation of the corporation issuing the class or series provide otherwise.
2. The applicability of subsection 1 must be determined as of:
(a) The record date fixed to determine the stockholders entitled to receive notice of and to vote at the meeting of stockholders to act upon the corporate action requiring dissenter’s rights; or
(b) The day before the effective date of such corporate action if there is no meeting of stockholders.
3. Subsection 1 is not applicable and dissenter’s rights are available pursuant to NRS 92A.380 for the holders of any class or series of shares who are required by the terms of the corporate action requiring dissenter’s rights to accept for such shares anything other than cash or shares of any class or any series of shares of any corporation, or any other proprietary interest of any other entity, that satisfies the standards set forth in subsection 1 at the time the corporate action becomes effective.
4. There is no right of dissent for any holders of stock of the surviving domestic corporation if the plan of merger does not require action of the stockholders of the surviving domestic corporation under NRS 92A.130.
5. There is no right of dissent for any holders of stock of the parent domestic corporation if the plan of merger does not require action of the stockholders of the parent domestic corporation under NRS 92A.180.
NRS 92A.400 Limitations on right of dissent: Assertion as to portions only to shares registered to stockholder; assertion by beneficial stockholder. 1. A stockholder of record may assert dissenter’s rights as to fewer than all of the shares registered in his or her name only if the stockholder of record dissents with respect to all shares of the class or series beneficially owned by any one person and notifies the subject corporation in writing of the name and address of each person on whose behalf the stockholder of record asserts dissenter’s rights. The rights of a partial dissenter under this subsection are determined as if the shares as to which the partial dissenter dissents and his or her other shares were registered in the names of different stockholders.
2. A beneficial stockholder may assert dissenter’s rights as to shares held on his or her behalf only if the beneficial stockholder:
(a) Submits to the subject corporation the written consent of the stockholder of record to the dissent not later than the time the beneficial stockholder asserts dissenter’s rights; and
(b) Does so with respect to all shares of which he or she is the beneficial stockholder or over which he or she has power to direct the vote.
NRS 92A.410 Notification of stockholders regarding right of dissent. 1. If a proposed corporate action creating dissenters’ rights is submitted to a vote at a stockholders’ meeting, the notice of the meeting must state that stockholders are, are not or may be entitled to assert dissenters’ rights under NRS 92A.300 to 92A.500, inclusive. If the domestic corporation concludes that dissenter’s rights are or may be available, a copy of NRS 92A.300 to 92A.500, inclusive, must accompany the meeting notice sent to those record stockholders entitled to exercise dissenter’s rights.
2. If the corporate action creating dissenters’ rights is taken by written consent of the stockholders or without a vote of the stockholders, the domestic corporation shall notify in writing all stockholders entitled to assert dissenters’ rights that the action was taken and send them the dissenter’s notice described in NRS 92A.430.
NRS 92A.420 Prerequisites to demand for payment for shares. 1. If a proposed corporate action creating dissenters’ rights is submitted to a vote at a stockholders’ meeting, a stockholder who wishes to assert dissenter’s rights with respect to any class or series of shares:
(a) Must deliver to the subject corporation, before the vote is taken, written notice of the stockholder’s intent to demand payment for his or her shares if the proposed action is effectuated; and
(b) Must not vote, or cause or permit to be voted, any of his or her shares of such class or series in favor of the proposed action.
2. If a proposed corporate action creating dissenters’ rights is taken by written consent of the stockholders, a stockholder who wishes to assert dissenters’ rights with respect to any class or series of shares must not consent to or approve the proposed corporate action with respect to such class or series. 3. A stockholder who does not satisfy the requirements of subsection 1 or 2 and NRS 92A.400 is not entitled to payment for his or her shares under this chapter.
NRS 92A.430 Dissenter’s notice: Delivery to stockholders entitled to assert rights; contents. 1. The subject corporation shall deliver a written dissenter’s notice to all stockholders entitled to assert dissenters’ rights.
2. The dissenter’s notice must be sent no later than 10 days after the effective date of the corporate action specified in NRS 92A.380, and must:
(a) State where the demand for payment must be sent and where and when certificates, if any, for shares must be deposited;
(b) Inform the holders of shares not represented by certificates to what extent the transfer of the shares will be restricted after the demand for payment is received;
(c) Supply a form for demanding payment that includes the date of the first announcement to the news media or to the stockholders of the terms of the proposed action and requires that the person asserting dissenter’s rights certify whether or not the person acquired beneficial ownership of the shares before that date;
(d) Set a date by which the subject corporation must receive the demand for payment, which may not be less than 30 nor more than 60 days after the date the notice is delivered and state that the stockholder shall be deemed to have waived the right to demand payment with respect to the shares unless the form is received by the subject corporation by such specified date; and
(e) Be accompanied by a copy of NRS 92A.300 to 92A.500, inclusive.
NRS 92A.440 Demand for payment and deposit of certificates; loss of rights of stockholder; withdrawal from appraisal process. 1. A stockholder who receives a dissenter’s notice pursuant to NRS 92A.430 and who wishes to exercise dissenter’s rights must:
(a) Demand payment;
(b) Certify whether the stockholder or the beneficial owner on whose behalf he or she is dissenting, as the case may be, acquired beneficial ownership of the shares before the date required to be set forth in the dissenter’s notice for this certification; and
(c) Deposit the stockholder’s certificates, if any, in accordance with the terms of the notice.
2. If a stockholder fails to make the certification required by paragraph (b) of subsection 1, the subject corporation may elect to treat the stockholder’s shares as after-acquired shares under NRS 92A.470.
3. Once a stockholder deposits that stockholder’s certificates or, in the case of uncertified shares makes demand for payment, that stockholder loses all rights as a stockholder, unless the stockholder withdraws pursuant to subsection 4.
4. A stockholder who has complied with subsection 1 may nevertheless decline to exercise dissenter’s rights and withdraw from the appraisal process by so notifying the subject corporation in writing by the date set forth in the dissenter’s notice pursuant to NRS 92A.430. A stockholder who fails to so withdraw from the appraisal process may not thereafter withdraw without the subject corporation’s written consent.
5. The stockholder who does not demand payment or deposit his or her certificates where required, each by the date set forth in the dissenter’s notice, is not entitled to payment for his or her shares under this chapter.
NRS 92A.450 Uncertificated shares: Authority to restrict transfer after demand for payment. The subject corporation may restrict the transfer of shares not represented by a certificate from the date the demand for their payment is received.
NRS 92A.460 Payment for shares: General requirements. 1. Except as otherwise provided in NRS 92A.470, within 30 days after receipt of a demand for payment, the subject corporation shall pay in cash to each dissenter who complied with NRS 92A.440 the amount the subject corporation estimates to be the fair value of the dissenter’s shares, plus accrued interest. The obligation of the subject corporation under this subsection may be enforced by the district court:
(a) Of the county where the subject corporation’s principal office is located;
(b) If the subject corporation’s principal office is not located in this State, in the county in which the corporation’s registered office is located; or (c) At the election of any dissenter residing or having its principal or registered office in this State, of the county where the dissenter resides or has its principal or registered office.
The court shall dispose of the complaint promptly.
2. The payment must be accompanied by:
(a) The subject corporation’s balance sheet as of the end of a fiscal year ending not more than 16 months before the date of payment, a statement of income for that year, a statement of changes in the stockholders’ equity for that year or, where such financial statements are not reasonably available, then such reasonably equivalent financial information and the latest available quarterly financial statements, if any;
(b) A statement of the subject corporation’s estimate of the fair value of the shares; and
(c) A statement of the dissenter’s rights to demand payment under NRS 92A.480 and that if any such stockholder does not do so within the period specified, such stockholder shall be deemed to have accepted such payment in full satisfaction of the corporation’s obligations under this chapter.
NRS 92A.470 Withholding payment for shares acquired on or after date of dissenter’s notice: General requirements. 1. A subject corporation may elect to withhold payment from a dissenter unless the dissenter was the beneficial owner of the shares before the date set forth in the dissenter’s notice as the first date of any announcement to the news media or to the stockholders of the terms of the proposed action.
2. To the extent the subject corporation elects to withhold payment, within 30 days after receipt of a demand for payment, the subject corporation shall notify the dissenters described in subsection 1:
(a) Of the information required by paragraph (a) of subsection 2 of NRS 92A.460;
(b) Of the subject corporation’s estimate of fair value pursuant to paragraph (b) of subsection 2 of NRS 92A.460;
(c) That they may accept the subject corporation’s estimate of fair value, plus interest, in full satisfaction of their demands or demand appraisal under NRS 92A.480;
(d) That those stockholders who wish to accept such an offer must so notify the subject corporation of their acceptance of the offer within 30 days after receipt of such offer; and
(e) That those stockholders who do not satisfy the requirements for demanding appraisal under NRS 92A.480 shall be deemed to have accepted the subject corporation’s offer.
3. Within 10 days after receiving the stockholder’s acceptance pursuant to subsection 2, the subject corporation shall pay in cash the amount offered under paragraph (b) of subsection 2 to each stockholder who agreed to accept the subject corporation’s offer in full satisfaction of the stockholder’s demand.
4. Within 40 days after sending the notice described in subsection 2, the subject corporation shall pay in cash the amount offered under paragraph (b) of subsection 2 to each stockholder described in paragraph (e) of subsection 2.
NRS 92A.480 Dissenter’s estimate of fair value: Notification of subject corporation; demand for payment of estimate. 1. A dissenter paid pursuant to NRS 92A.460 who is dissatisfied with the amount of the payment may notify the subject corporation in writing of the dissenter’s own estimate of the fair value of his or her shares and the amount of interest due, and demand payment of such estimate, less any payment pursuant to NRS 92A.460. A dissenter offered payment pursuant to NRS 92A.470 who is dissatisfied with the offer may reject the offer pursuant to NRS 92A.470 and demand payment of the fair value of his or her shares and interest due.
2. A dissenter waives the right to demand payment pursuant to this section unless the dissenter notifies the subject corporation of his or her demand to be paid the dissenter’s stated estimate of fair value plus interest under subsection 1 in writing within 30 days after receiving the subject corporation’s payment or offer of payment under NRS 92A.460 or 92A.470 and is entitled only to the payment made or offered.
NRS 92A.490 Legal proceeding to determine fair value: Duties of subject corporation; powers of court; rights of dissenter. 1. If a demand for payment remains unsettled, the subject corporation shall commence a proceeding within 60 days after receiving the demand and petition the court to determine the fair value of the shares and accrued interest. If the subject corporation does not commence the proceeding within the 60-day period, it shall pay each dissenter whose demand remains unsettled the amount demanded by each dissenter pursuant to NRS 92A.480 plus interest. 2. A subject corporation shall commence the proceeding in the district court of the county where its principal office is located in this State. If the principal office of the subject corporation is not located in the State, it shall commence the proceeding in the county where the principal office of the domestic corporation merged with or whose shares were acquired by the foreign entity was located. If the principal office of the subject corporation and the domestic corporation merged with or whose shares were acquired is not located in this State, the subject corporation shall commence the proceeding in the district court in the county in which the corporation’s registered office is located.
3. The subject corporation shall make all dissenters, whether or not residents of Nevada, whose demands remain unsettled, parties to the proceeding as in an action against their shares. All parties must be served with a copy of the petition. Nonresidents may be served by registered or certified mail or by publication as provided by law.
4. The jurisdiction of the court in which the proceeding is commenced under subsection 2 is plenary and exclusive. The court may appoint one or more persons as appraisers to receive evidence and recommend a decision on the question of fair value. The appraisers have the powers described in the order appointing them, or any amendment thereto. The dissenters are entitled to the same discovery rights as parties in other civil proceedings.
5. Each dissenter who is made a party to the proceeding is entitled to a judgment:
(a) For the amount, if any, by which the court finds the fair value of the dissenter’s shares, plus interest, exceeds the amount paid by the subject corporation; or
(b) For the fair value, plus accrued interest, of the dissenter’s after-acquired shares for which the subject corporation elected to withhold payment pursuant to NRS 92A.470.
NRS 92A.500 Assessment of costs and fees in certain legal proceedings. 1. The court in a proceeding to determine fair value shall determine all of the costs of the proceeding, including the reasonable compensation and expenses of any appraisers appointed by the court. The court shall assess the costs against the subject corporation, except that the court may assess costs against all or some of the dissenters, in amounts the court finds equitable, to the extent the court finds the dissenters acted arbitrarily, vexatiously or not in good faith in demanding payment.
2. The court may also assess the fees and expenses of the counsel and experts for the respective parties, in amounts the court finds equitable:
(a) Against the subject corporation and in favor of all dissenters if the court finds the subject corporation did not substantially comply with the requirements of NRS 92A.300 to 92A.500, inclusive; or
(b) Against either the subject corporation or a dissenter in favor of any other party, if the court finds that the party against whom the fees and expenses are assessed acted arbitrarily, vexatiously or not in good faith with respect to the rights provided by NRS 92A.300 to 92A.500, inclusive.
3. If the court finds that the services of counsel for any dissenter were of substantial benefit to other dissenters similarly situated, and that the fees for those services should not be assessed against the subject corporation, the court may award to those counsel reasonable fees to be paid out of the amounts awarded to the dissenters who were benefited.
4. In a proceeding commenced pursuant to NRS 92A.460, the court may assess the costs against the subject corporation, except that the court may assess costs against all or some of the dissenters who are parties to the proceeding, in amounts the court finds equitable, to the extent the court finds that such parties did not act in good faith in instituting the proceeding.
5. To the extent the subject corporation fails to make a required payment pursuant to NRS 92A.460, 92A.470 or 92A.480, the dissenter may bring a cause of action directly for the amount owed and, to the extent the dissenter prevails, is entitled to recover all expenses of the suit.
6. This section does not preclude any party in a proceeding commenced pursuant to NRS 92A.460 or 92A.490 from applying the provisions of N.R.C.P. 68 or NRS 17.115.
Annex D
FORM OF WRITTEN CONSENT
ISLET SCIENCES, INC.
This Written Consent is solicited by the Board of Directors of Islet Sciences, Inc.
Please return this consent no later than pm (Eastern Time) on , 201_, which is the date that the Company Board of Directors expects to receive consents under the Voting Agreement.
The undersigned, being a holder of record of common stock, par value $0.001, of Islet Sciences, Inc., a Nevada corporation (the “Company”), on , 201_, hereby: (i) acknowledges receipt of the consent solicitation statement/prospectus of the Company, which is part of a registration statement on Form S-4 (No. 333- ______________), and (ii) by virtue of this written consent without a meeting, with respect to each share of Company common stock that the undersigned holds beneficially or of record (which number is set forth below), consents to the approval of, and hereby approves, the Agreement and Plan of Merger by and among the Company, Avogenx, Inc., a Delaware corporation (“Holdco”), Islet Merger Sub, Inc., a Nevada corporation (“Merger Sub”), Brighthaven Ventures, L.L.C., a North Carolina limited liability company (“BHV”), and each of the members of BHV, dated as of September 30, 2014, including without limitation the merger of Merger Sub with and into the Company, with the Company as the surviving entity in the merger becoming a wholly owned subsidiary of Avogenx.
IMPORTANT: PLEASE DATE AND SIGN THE CONSENT BELOW. If held in joint tenancy, all persons must sign. When signing as attorney, trustee, executor, administrator, guardian or corporate officer, please give full title as such. If shares are held by a corporation, please sign the full corporate name by president or other authorized officer. If shares are held by a partnership or other entity, please sign the full partnership or other entity name by authorized person. Please execute, date, sign and return this Written Consent promptly to the Company by emailing a .pdf copy of the Written Consent to info@isletsciences.com (Attention: Corporate Secretary), or by mailing this Written Consent to Islet Sciences, Inc. at 6601 Six Forks Rd, Suite 140, Raleigh, North Carolina 27615, Attention: Corporate Secretary.
IF AN INDIVIDUAL: By: (duly authorized signature) Name: (please print or type full name) Title: (please print or type full title) | | IF AN ENTITY: (please print or type complete name of entity) By: (duly authorized signature) Name: (please print or type full name) Title: (please print or type full title) |
| | |
Date: , 201_ | | Date: , 201_ |
Number of shares held beneficially and of record:
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Indemnification and Insurance
Islet Sciences maintains director’s and officer’s liability insurance policies which indemnify directors and officers for certain losses arising from claims by reason of a wrongful act, as defined therein, under certain circumstances. Directors and officers insured under the policies include directors and officers of our subsidiaries, including the registrant. Following the Merger, we expect to maintain similar insurance.
We expect to enter into indemnification agreements with our directors and officers. Pursuant to these agreements, each director and officer will be, to the fullest extent permitted by law, entitled to advancement of expenses and to indemnifications for all expenses, damages, judgments, fines, penalties, ERISA excise taxes and amounts paid in settlement (including all interest, assessments and other charges paid or payable therewith) resulting from any claim against such person arising from the fact that such person is or was a director or officer or is or was serving at our request as a director, officer, employee, trustee, agent or fiduciary of another entity or by reason of anything done or not done by such director or officer in any such capacity.
Under Delaware law, directors of a Delaware corporation can generally be held liable for certain acts and omissions in connection with the performance of their duties to the corporation and its stockholders. As permitted by Delaware law, however, our Certificate of Incorporation contains a provision eliminating the liability of directors for monetary damages for breaches of their duties to us and our stockholders. This provision does not, however, eliminate liability for:
| | ● | | breaches of duty of loyalty to us and our stockholders; |
| | ● | | acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
| | ● | | transactions from which improper personal benefit is derived; and |
| | ● | | unlawful declaration of dividends or repurchases or redemptions of shares of capital stock. |
This provision applies to officers only if they are directors and are acting in their capacity as directors. Although the issue has not been determined by any court, this provision may have no effect on claims arising under federal securities laws. This provision does not eliminate the duty of care, but only eliminates liability for monetary damages for breaches of such duty under various circumstances. Accordingly, this provision has no effect on the availability of equitable remedies, such as an injunction or rescission, based upon a breach of the duty of care. Equitable remedies may not, however, be wholly effective to remedy the injury caused by any such breach.
Avogenx’s Certificate of Incorporation provides that to the fullest extent permitted by Section 145 of the DGCL, Avogenx will indemnify any and all persons whom it has power to indemnify under the DGCL from and against any and all of the expenses, liabilities or other matters referred to in or covered by Section 145 of the DGCL, and will advance expenses to any and all such persons to the fullest extent permitted by the DGCL. The indemnification and advancement provided for in Avogenx’s Certificate of Incorporation will not be deemed exclusive under any Bylaw, agreement, vote of stockholders or disinterested directors or otherwise, and will continue as to a person who has ceased to be a director, officer, employee or agent and shall inure to the benefit of the heirs, executors and administrators of such a person.
The provisions of the documents filed as described above refer to or are based upon Sections 145 and 102(b) of the General Corporation Law of the State of Delaware (the “DGCL”).
Section 145 of the DGCL (“Section 145”) provides, among other things, that a Delaware corporation may indemnify any person who was, is or is threatened to be made, party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person is or was an officer, director, employee or agent of such corporation or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was unlawful. A Delaware corporation may indemnify any persons who were or are a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person is or was a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit, provided such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests, provided further that no indemnification is permitted without judicial approval if the officer, director, employee or agent is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against the expenses (including attorneys’ fees) which such officer or director has actually and reasonably incurred.
Section 145 further authorizes a corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or enterprise, against any liability asserted against such person and incurred by such person in any such capacity, or arising out of his or her status as such, whether or not the corporation would otherwise have the power to indemnify such person under Section 145.
Section 102(b)(7) of the DGCL allows a corporation to provide in its certificate of incorporation that a director of the corporation will not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except where the director breached the duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit.
Exhibits and Financial Statement Schedules
(a) Exhibits
See Exhibit Index.
(b) Financial Statement Schedules
Not applicable.
(c) Reports, Opinions or Appraisals
See Section (a) above.
Undertakings
The undersigned Registrant hereby undertakes as follows: that prior to any public reoffering of the securities registered hereunder through use of a prospectus which is a part of this Registration Statement, by any person or party who is deemed to be an underwriter within the meaning of the Rule 145(c), the issuer undertakes that such reoffering prospectus will contain the information called for by the applicable registration form with respect to reofferings by persons who may be deemed underwriters, in addition to the information called for by the other items of the applicable form.
The undersigned Registrant undertakes that every prospectus (i) that is filed pursuant to the immediately preceding paragraph, or (ii) that purports to meet the requirements of Section 10(a)(3) of the Securities Act of 1933 and is used in connection with an offering of securities subject to Rule 415, will be filed as a part of an amendment to the Registration Statement and will not be used until such amendment is effective, and that, for purposes of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers, and controlling persons of the Registrant pursuant to the foregoing, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer, or controlling person of the Registrant in the successful defense of any action, suit, or proceeding) is asserted by such director, officer, or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes to respond to requests for information that is incorporated by reference into the prospectus pursuant to Item 4 of this Registration Statement, within one business day of receipt of such request, and to send the incorporated documents by first class mail or other equally prompt means. This includes information contained in documents filed subsequent to the effective date of the Registration Statement through the date of responding to the request.
The undersigned registrant hereby undertakes to supply by means of a post-effective amendment all information concerning a transaction, and the company being acquired involved therein, that was not the subject of and included in the registration statement when it became effective.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized on December 2, 2014.
| | AVOGENX, INC. | |
| | | | |
| Date | By: | /s/ James Green | |
| | | James Green | |
| | | President, Chief Executive Officer and Director (Principal Executive Officer) | |
| | | | |
POWER OF ATTORNEY AND SIGNATURES
KNOW ALL MEN BY THESE PRESENTS, that each individual whose signature appears below hereby constitutes and appoints the Chief Executive Officer, the President, the General Counsel, the Secretary, the Assistant Secretary, the Chief Financial Officer, the Treasurer and the Assistant Treasurer, now or hereafter serving, of Avogenx, Inc., and each of them individually, his or her true and lawful agent, proxy and attorney-in-fact, with full power of substitution and resubstitution, for him or her and in his name, place and stead, in any and all capacities, to (i) act on, sign and file with the Securities and Exchange Commission any and all amendments to this registration statement (which includes any additional registration statement under Rule 462(b)) together with all schedules and exhibits thereto, (ii) act on, sign and file with the Securities and Exchange Commission any and all exhibits to this registration statement and any and all exhibits and schedules thereto, (iii) act on, sign and file any and all such certificates, applications, registration statements, notices, reports, instruments, agreements and other documents necessary or appropriate in connection with the registration or qualification under foreign and state securities laws of the securities described in this registration statement or any amendment thereto, or obtain an exemption therefrom, in connection with the offerings described therein and (iv) take any and all such actions which may be necessary or appropriate in connection therewith, granting unto such agents, proxies and attorneys-in-fact, and each of them individually, full power and authority to do and perform each and every act and thing necessary or appropriate to be done, as fully for all intents and purposes as he might or could do in person, and hereby approving, ratifying and confirming all that such agents, proxies and attorneys-in-fact, any of them or any of his or their substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed below by the following persons in the capacities and on the dates indicated.
| | | | | |
| | | | |
| | | |
James Green | | President, Chief Executive Officer and Director (Principal Executive Officer) | | December 2, 2014 |
| | | |
Steven Delmar | | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | | |
| | | |
Joel Perlin | | Director | | |
| | | | | |
William Wilkison | | Director | | |
Michael Luther | | Director | | |
| /S/ Eric Barnett Eric Barnett | | Director | | |
EXHIBIT INDEX
Exhibit Number | | Description |
| | | |
| 2.1 | | Agreement and Plan of Merger by and among Islet Sciences, Brighthaven Ventures, L.L.C., Avogenx, Inc., Islet Merger Sub, Inc., and each of the members of BHV (included as Annex A to the consent solicitation statement/prospectus forming a part of this Registration Statement) |
| | | |
| 2.2 | | Agreement and Plan of Merger of Islet Sciences (1) |
| | | |
| 3.1 | | Articles of Incorporation of Islet Sciences (2) |
| | | |
| 3.2 | | Certificate of Amendment to Articles of Incorporation of Islet Sciences dated March 30, 1999 (2) |
| | | |
| 3.3 | | Certificate of Amendment to Articles of Incorporation of Islet Sciences dated February 16, 2012 (2) |
| | | |
| 3.4 | | Amended and Restated By-laws of Islet Sciences (2) |
| | | |
| 4.1 | | Certificate of Designations of Preferences, Rights and Limitations of Series A Convertible Preferred Stock of Islet Sciences (1) |
| | | |
| 4.2 | | Certificate of Designations of Preferences, Rights and Limitations of Series B Convertible Preferred Stock of Islet Sciences (1) |
| | | |
| 4.3 | | Certificate of Designations of Preferences, Rights and Limitations of Series C Convertible Preferred Stock of Islet Sciences (3) |
| | | |
| 4.4 | | Form of Warrant of Islet Sciences (4) |
| | | |
| 4.5 | | Form of Warrant issued by Islet Sciences to NeoStem Inc. (2) |
| | | |
| 4.6 | | Specimen of Common Stock Certificate of Islet Sciences (2) |
| | | |
| 5.1 | | Opinion of Ofsink, LLC as to the legality of shares to be issued* |
| | | |
| 8.1 | | Opinion of Anderson Bradshaw, PLLC regarding certain federal income tax matters |
| | | |
| 10.1 | | Stock Purchase Agreement by and between Islet Sciences and John Welch (1) |
| | | |
| 10.2 | | Agreement dated January 10, 2012 by and between ISI and Progenitor Cell Therapy (5) |
| | | |
| 10.3 | | Share Exchange Agreement dated February 23, 2012 by and among Islet Sciences, ISI and DiaKine Therapeutics, Inc. (6) |
| | | |
| 10.4 | | Form of Lock-Up Agreement (6) |
| | | |
| 10.5 | | Employment Agreement dated March 1, 2012 by and between Islet Sciences and John Steel (2) |
| | | |
| 10.6 | | Consulting Agreement dated April 1, 2012 by and between Islet Sciences and Richard Egan (2) |
| | | |
| 10.7 | | Form of Subscription Agreement of Islet Sciences (4) |
| | | |
| 10.8 | | Long-Term Supply Agreement dated July 23, 2012 by and between Islet Sciences and Spring Point Project (7) |
| | | |
| 10.9 | | Separation Agreement and Release dated July 1, 2013 by and between Islet Sciences and John Steel (8) |
| | | |
| 10.10 | | Consulting Agreement dated July 1, 2013 by and between Islet Sciences and John Steel (8) |
| | | |
| 10.11 | | Employment Agreement dated October 30, 2013 by and between Islet Sciences and James Green (9) |
| | | |
| 10.12 | | Employment Agreement dated October 30, 2013 by and between Islet Sciences and Dr. William Wilkison (9) |
| | | |
| 10.13 | | Nonqualified Stock Option Agreement dated October 30, 2013 by and between Islet Sciences and James Green (9) |
| | | |
| 10.14 | | Nonqualified Stock Option Agreement dated October 30, 2013 by and between Islet Sciences and Dr. William Wilkison (9) |
| | | |
| 21.1 | | List of Subsidiaries |
| | | |
| 23.1 | | Consent of PKF, independent registered public accounting firm of Islet Sciences |
| | | |
| 23.2 | | Consent of PKF, independent registered public accounting firm of BHV |
| | | |
| 23.3 | | Consent of Ofsink, LLC (included in Exhibit 5.1)* |
| | | |
| 23.4 | | Consent of Anderson Bradshaw, PLLC (included in Exhibit 8.1) |
| | | |
| 24.1 | | Power of Attorney (included in signature page of this Registration Statement) |
| | | |
| 99.1 | | Form of Consent for holders of Islet Sciences common stock (inclueded as Annex D to the consent solicitation statement prospectus forming a part of this registration statement) |
| | | |
| 99.2 | | Consent of Jonathan Berlent |
_______________________
| * | To be filed by amendment. |
| (1) | Incorporated by reference to Islet Sciences’ Current Report on Form 8-K filed with the SEC on January 6, 2012. |
| (2) | Incorporated by reference to Islet Sciences’ Annual Report on Form 10-K filed with the SEC on July 30, 2012. |
| (3) | Incorporated by reference to Islet Sciences’ Current Report on Form 8-K filed with the SEC on March 16, 2012. |
| (4) | Incorporated by reference to Islet Sciences’ Current Report on Form 8-K filed with the SEC on March 21, 2012. |
| (5) | Incorporated by reference to Islet Sciences’ Current Report on Form 8-K filed with the SEC on January 13, 2012. |
| (6) | Incorporated by reference to Islet Sciences’ Current Report on Form 8-K filed with the SEC on February 29, 2012. |
| (7) | Incorporated by reference to Islet Sciences’ Current Report on Form 8-K filed with the SEC on July 27, 2012. |
| (8) | Incorporated by reference to Islet Sciences’ Current Report on Form 8-K filed with the SEC on July 16, 2013. |
| (9) | Incorporated by reference to Islet Sciences’ Current Report on Form 8-K filed with the SEC on October 31, 2013. |