
Precision therapies for genetically defined diseases Corporate Presentation October 2020 Exhibit 99.2

Forward-Looking Statements and Risk Factors This presentation and the accompanying oral commentary contain forward-looking statements that involve risks, uncertainties and assumptions. If the risks or uncertainties ever materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by such forward looking statements. All statements other than statements of historical fact could be deemed forward-looking, including, but not limited to, any statements of the plans, strategies, and objectives of management for future operations, including our clinical development and commercialization plans; any projections of financial information; any statement about historical results that may suggest trends for our business; any statement of expectation or belief regarding future events; potential markets or market size, technology developments, our clinical product pipeline, clinical data or the implications thereof, enforceability of our intellectual property rights, competitive strengths or our position within the industry; any statements regarding the anticipated benefits of our collaborations or other strategic transactions; and any statements of assumptions underlying any of the items mentioned. These statement are based on estimates and information available to us at the time of this presentation and are not guarantees of future performance. Actual results could differ materially from our current expectations as a result of many risks and uncertainties, including but not limited to, risks associated with: the success, cost and timing of our product development activities and clinical trials; our ability to obtain regulatory approval for and to commercialize our product candidates; our ability to establish a commercially-viable manufacturing process and manufacturing infrastructure; regulatory requirements and regulatory developments; the effects of competition and technological advances; our dependence on third-party collaborators and other contractors in our research and development activities, including for the conduct of clinical trials and the manufacture of our product candidates; our ability to obtain, maintain, or protect intellectual property rights related to our product candidates; among others. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to our business in general, see our periodic filings filed from time to time with the Securities and Exchange Commission. Unless as required by law, we assume no obligation and do not intend to update these forward-looking statements or to conform these statements to actual results or to changes in our expectations. All of Cogent Biosciences (“Cogent”) product candidates are investigational product candidates and their safety and efficacy have not yet been established. Cogent has not obtained marketing approval for any product, and there is no certainty that any marketing approvals will be obtained or as to the timelines on which they will be obtained. Any data pertaining to Cogent product candidates is interim data and may include investigator-reported interim data for which Cogent has not yet independently reviewed the source data. The interim data may not be representative of the final results that may be obtained in the corresponding trials and results from earlier trials may not be representative of results obtained in later trial or pivotal trials.
Building a Rational Precision Therapy Company PLX9486, a potential best-in-class KIT D816V inhibitor, is a foundational program with promising clinical efficacy and safety in gastrointestinal stromal tumors (GIST) Accelerated timeline to proof-of-concept serum tryptase data in advanced systemic mastocytosis (ASM) and indolent systemic mastocytosis (ISM) Building a fully integrated company with an expanding product pipeline focused on genetically validated targets Attractive systemic mastocytosis commercial opportunity potentially adds another, larger indication for PLX9486 to pursue beyond GIST Focused on Addressing the True Underlying Drivers of Disease and Providing Real Hope for Patients

Lead Program PLX9486 is a Highly Selective and Potent KIT Mutant Inhibitor with a Potentially Best-in-Class Clinical Profile 11 months PFS shown in 18 heavily treatment-experienced GIST patients treated with PLX9486 in combination with sunitinib Single agent & combination activity and safety data in 50+ patients supports further clinical development KIT D816V inhibition supports future studies in mastocytosis; safety supports broad use PLX9486 Specifically targets KIT Exon 17 D816V Selective versus other targets including wild-type KIT, PDGFRα, VEGFR2, FLT3, FMS Worldwide rights to compound exclusively licensed from Plexxikon1 Patent protection through at least 20332 1 Plexxikon is eligible for mid- to high- single-digit royalties and additional development milestones. License includes rights to PLX0206, an additional selective KIT inhibitor in preclinical development 2 Does not include available patent term extension. 3 Based on a Phase 1/2 clinical trial including GIST patients conducted by Plexxikon Efficacy Data3 Safety Experience Potential Best-in-Class Profile
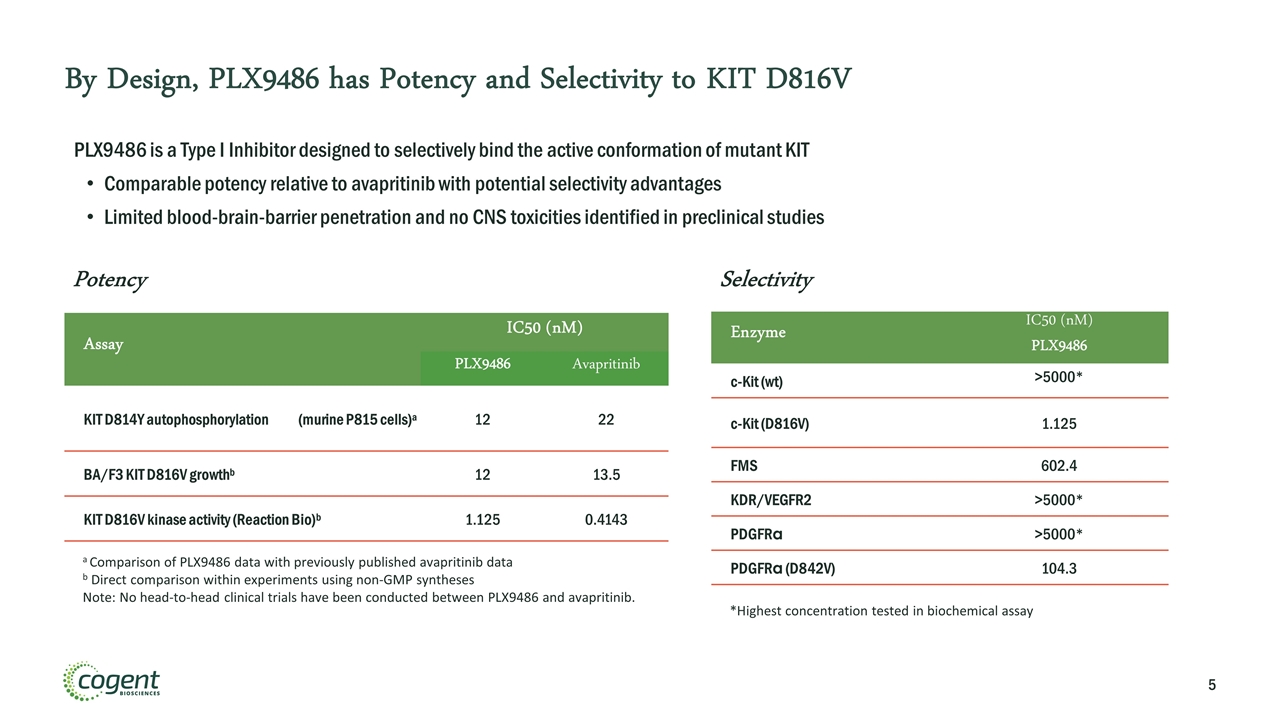
By Design, PLX9486 has Potency and Selectivity to KIT D816V PLX9486 is a Type I Inhibitor designed to selectively bind the active conformation of mutant KIT Comparable potency relative to avapritinib with potential selectivity advantages Limited blood-brain-barrier penetration and no CNS toxicities identified in preclinical studies Enzyme IC50 (nM) PLX9486 c-Kit (wt) >5000* c-Kit (D816V) 1.125 FMS 602.4 KDR/VEGFR2 >5000* PDGFRα >5000* PDGFRα (D842V) 104.3 *Highest concentration tested in biochemical assay Selectivity Assay IC50 (nM) PLX9486 Avapritinib KIT D814Y autophosphorylation (murine P815 cells)a 12 22 BA/F3 KIT D816V growthb 12 13.5 KIT D816V kinase activity (Reaction Bio)b 1.125 0.4143 Potency a Comparison of PLX9486 data with previously published avapritinib data b Direct comparison within experiments using non-GMP syntheses Note: No head-to-head clinical trials have been conducted between PLX9486 and avapritinib.
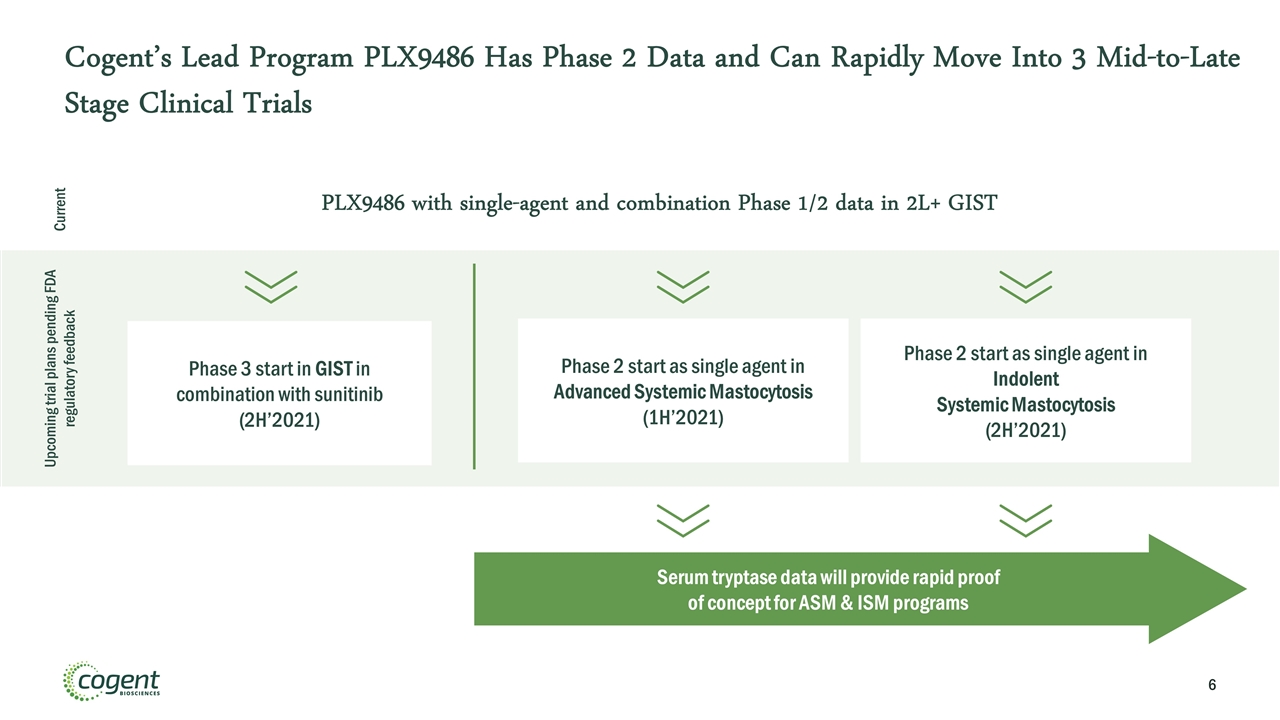
Cogent’s Lead Program PLX9486 Has Phase 2 Data and Can Rapidly Move Into 3 Mid-to-Late Stage Clinical Trials Phase 3 start in GIST in combination with sunitinib (2H’2021) Phase 2 start as single agent in Indolent Systemic Mastocytosis (2H’2021) Phase 2 start as single agent in Advanced Systemic Mastocytosis (1H’2021) PLX9486 with single-agent and combination Phase 1/2 data in 2L+ GIST Serum tryptase data will provide rapid proof of concept for ASM & ISM programs Current Upcoming trial plans pending FDA regulatory feedback

PLX9486 FUTURE CLINICAL PROGRAMS Advanced Systemic Mastocytosis (ASM) Indolent Systemic Mastocytosis (ISM)

Both Advanced SM and Indolent SM Are Primarily Driven by D816V Mutations The most common KIT mutation in patients with systemic mastocytosis, aspartate to valine at residue 816 (D816V), lies within the activation loop domain and causes a conformational change in the enzymatic pocket of the receptor This conformational change results in ligand independent constitutive activation of KIT and leads to increased proliferation

Advanced Systemic Mastocytosis Median survival of approximately ≤ 3.5 years FDA approved drug, Rydapt (Midostaurin), broad spectrum TKI, challenging tolerability Indolent and Smoldering Mastocytosis Poor quality of life No approved therapies: current treatments include H1 and H2 anti-histamines, mast cell stabilizers, leukotriene inhibitors Unmet Need in ASM and ISM Neurological Headache, brain fog, cognitive dysfunction, anxiety, depression Systemic Anaphylaxis Cutaneous (skin) Flushing of the face/neck/chest, hives, skin rashes, itching with or without rash Gastrointestinal Diarrhea, nausea, vomiting, abdominal pain, bloating, gastroesophageal reflux disease (GERD) Other Cardiovascular Light-headedness, syncope (fainting), rapid heart rate, chest pain, low blood pressure, high blood pressure at reaction start, blood pressure instability Ear/Nose/Throat/Respiratory Nasal itching and congestion, throat itching and swelling, wheezing, shortness of breath Skeletal Bone/muscle pain, osteopenia, osteoporosis Gynecological Uterine cramps, bleeding Urinary Bladder irritability, frequent voiding
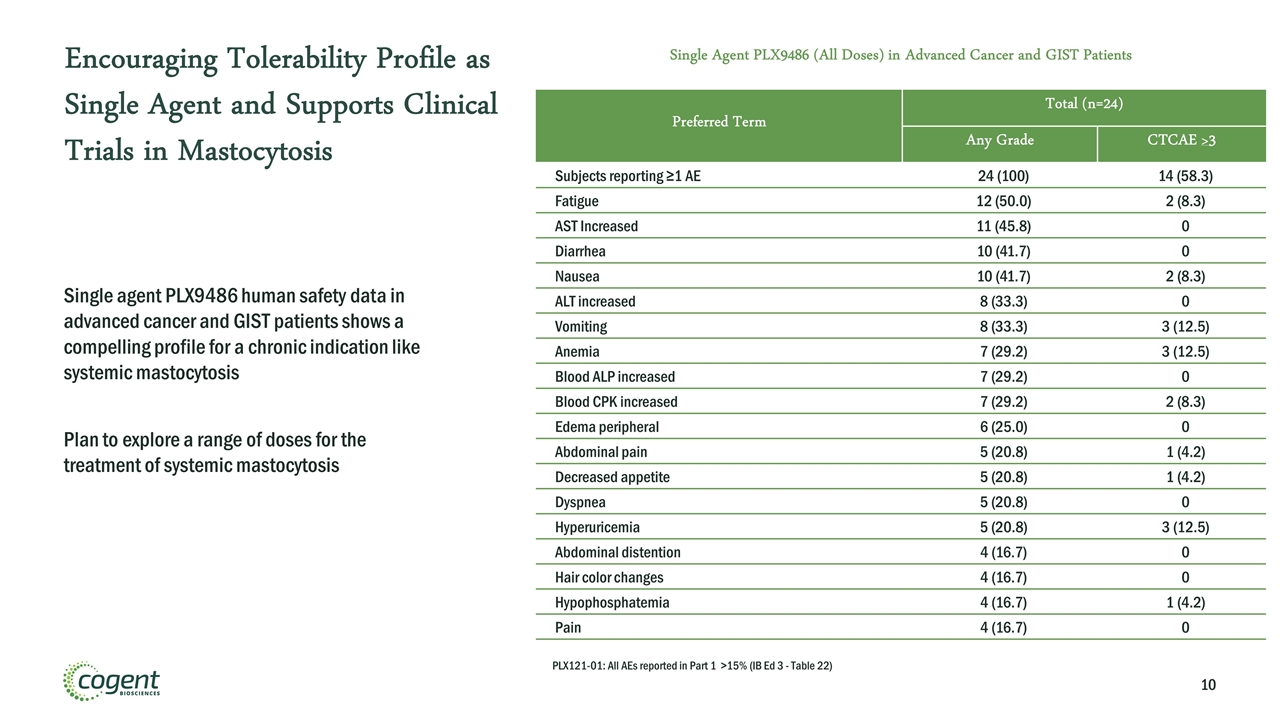
Preferred Term Total (n=24) Any Grade CTCAE >3 Subjects reporting ≥1 AE 24 (100) 14 (58.3) Fatigue 12 (50.0) 2 (8.3) AST Increased 11 (45.8) 0 Diarrhea 10 (41.7) 0 Nausea 10 (41.7) 2 (8.3) ALT increased 8 (33.3) 0 Vomiting 8 (33.3) 3 (12.5) Anemia 7 (29.2) 3 (12.5) Blood ALP increased 7 (29.2) 0 Blood CPK increased 7 (29.2) 2 (8.3) Edema peripheral 6 (25.0) 0 Abdominal pain 5 (20.8) 1 (4.2) Decreased appetite 5 (20.8) 1 (4.2) Dyspnea 5 (20.8) 0 Hyperuricemia 5 (20.8) 3 (12.5) Abdominal distention 4 (16.7) 0 Hair color changes 4 (16.7) 0 Hypophosphatemia 4 (16.7) 1 (4.2) Pain 4 (16.7) 0 Encouraging Tolerability Profile as Single Agent and Supports Clinical Trials in Mastocytosis Single Agent PLX9486 (All Doses) in Advanced Cancer and GIST Patients Single agent PLX9486 human safety data in advanced cancer and GIST patients shows a compelling profile for a chronic indication like systemic mastocytosis Plan to explore a range of doses for the treatment of systemic mastocytosis PLX121-01: All AEs reported in Part 1 >15% (IB Ed 3 - Table 22)
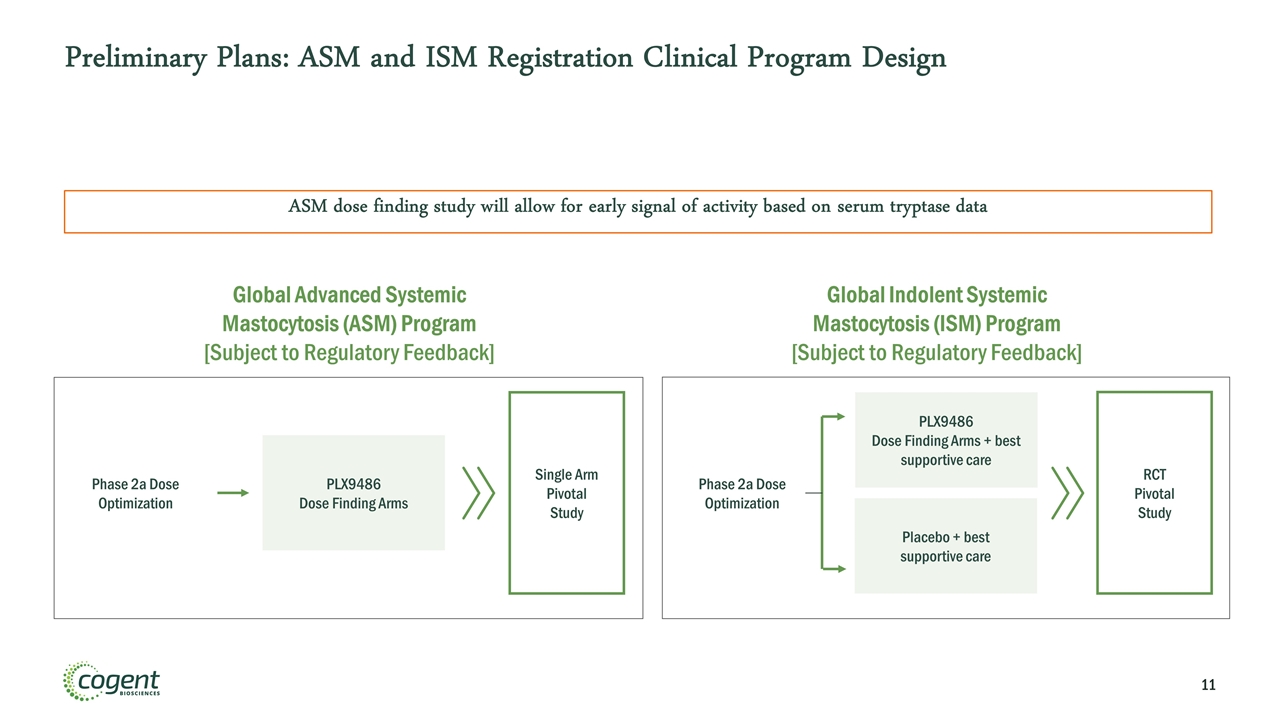
Preliminary Plans: ASM and ISM Registration Clinical Program Design Global Advanced Systemic Mastocytosis (ASM) Program [Subject to Regulatory Feedback] Global Indolent Systemic Mastocytosis (ISM) Program [Subject to Regulatory Feedback] PLX9486 Dose Finding Arms Single Arm Pivotal Study Phase 2a Dose Optimization PLX9486 Dose Finding Arms + best supportive care Placebo + best supportive care Phase 2a Dose Optimization RCT Pivotal Study ASM dose finding study will allow for early signal of activity based on serum tryptase data
PLX9486 Program: GIST
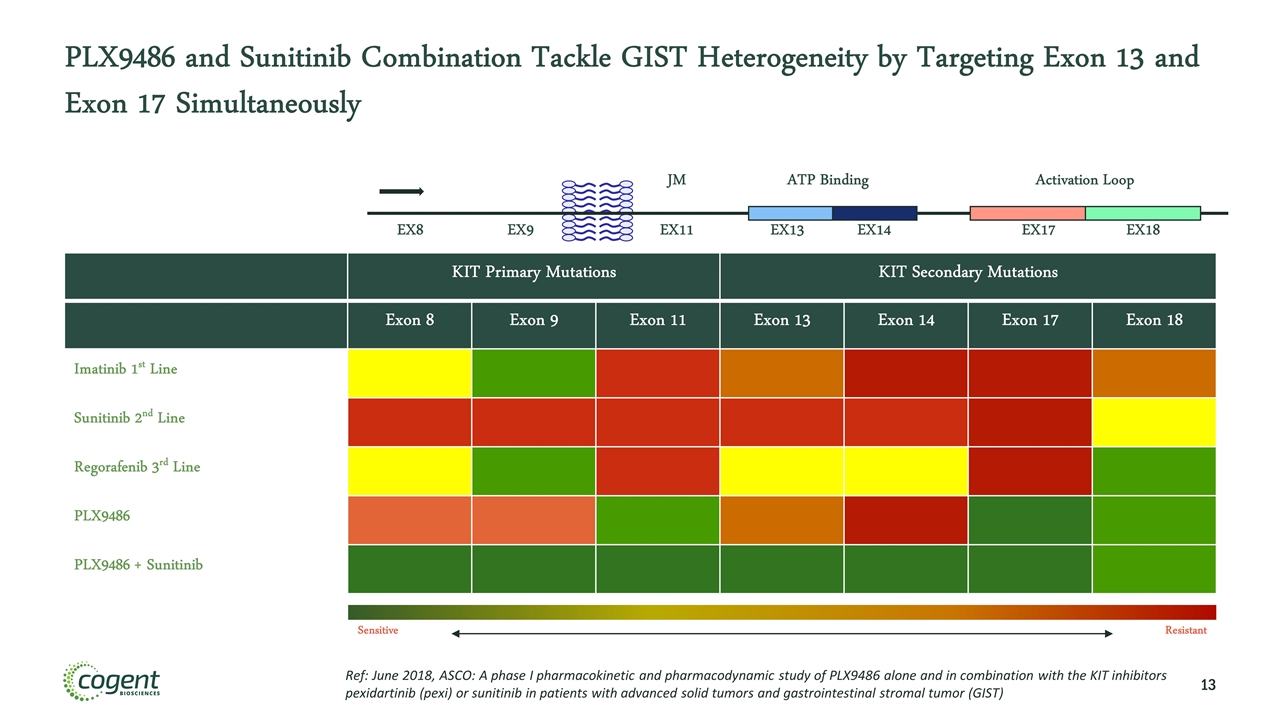
PLX9486 and Sunitinib Combination Tackle GIST Heterogeneity by Targeting Exon 13 and Exon 17 Simultaneously KIT Primary Mutations KIT Secondary Mutations Exon 8 Exon 9 Exon 11 Exon 13 Exon 14 Exon 17 Exon 18 Imatinib 1st Line Sunitinib 2nd Line Regorafenib 3rd Line PLX9486 PLX9486 + Sunitinib EX8 EX9 EX11 EX13 EX14 EX18 EX17 JM ATP Binding Activation Loop Sensitive Resistant Ref: June 2018, ASCO: A phase I pharmacokinetic and pharmacodynamic study of PLX9486 alone and in combination with the KIT inhibitors pexidartinib (pexi) or sunitinib in patients with advanced solid tumors and gastrointestinal stromal tumor (GIST)
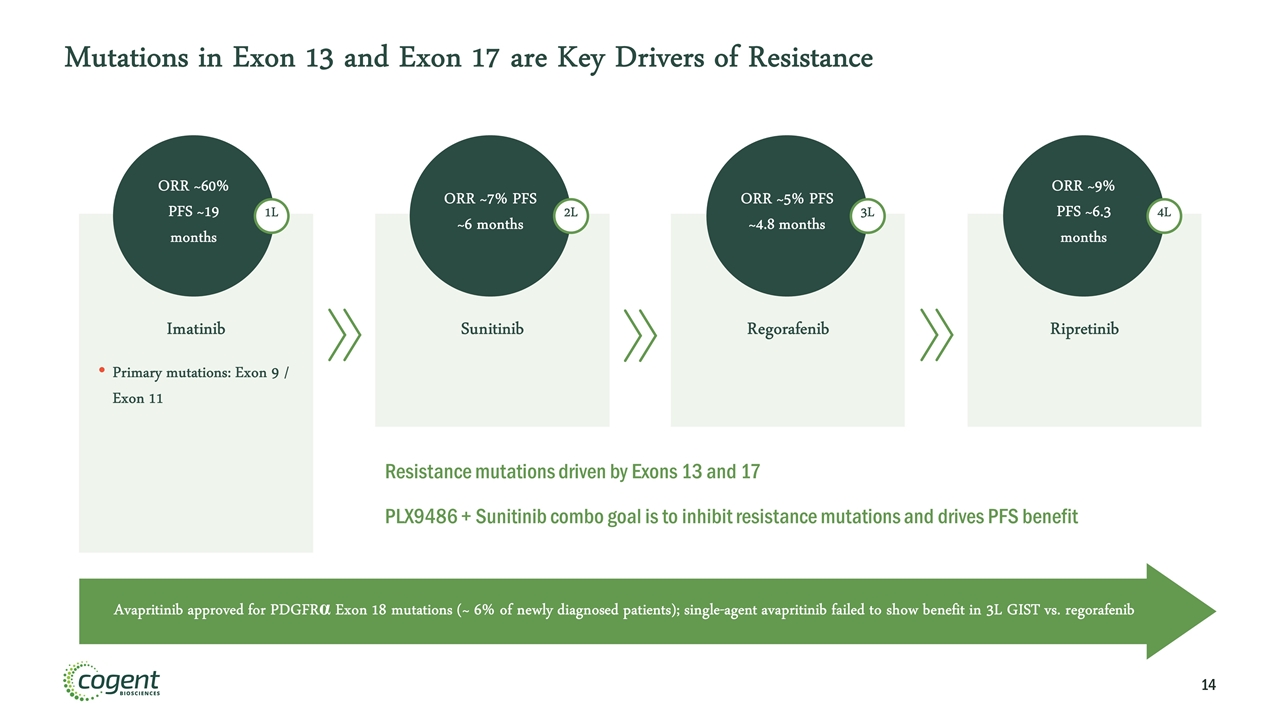
Mutations in Exon 13 and Exon 17 are Key Drivers of Resistance Imatinib Primary mutations: Exon 9 / Exon 11 Sunitinib Regorafenib Ripretinib ORR ~60% PFS ~19 months ORR ~7% PFS ~6 months ORR ~5% PFS ~4.8 months ORR ~9% PFS ~6.3 months 1L 2L 3L 4L Avapritinib approved for PDGFRα Exon 18 mutations (~ 6% of newly diagnosed patients); single-agent avapritinib failed to show benefit in 3L GIST vs. regorafenib Resistance mutations driven by Exons 13 and 17 PLX9486 + Sunitinib combo goal is to inhibit resistance mutations and drives PFS benefit
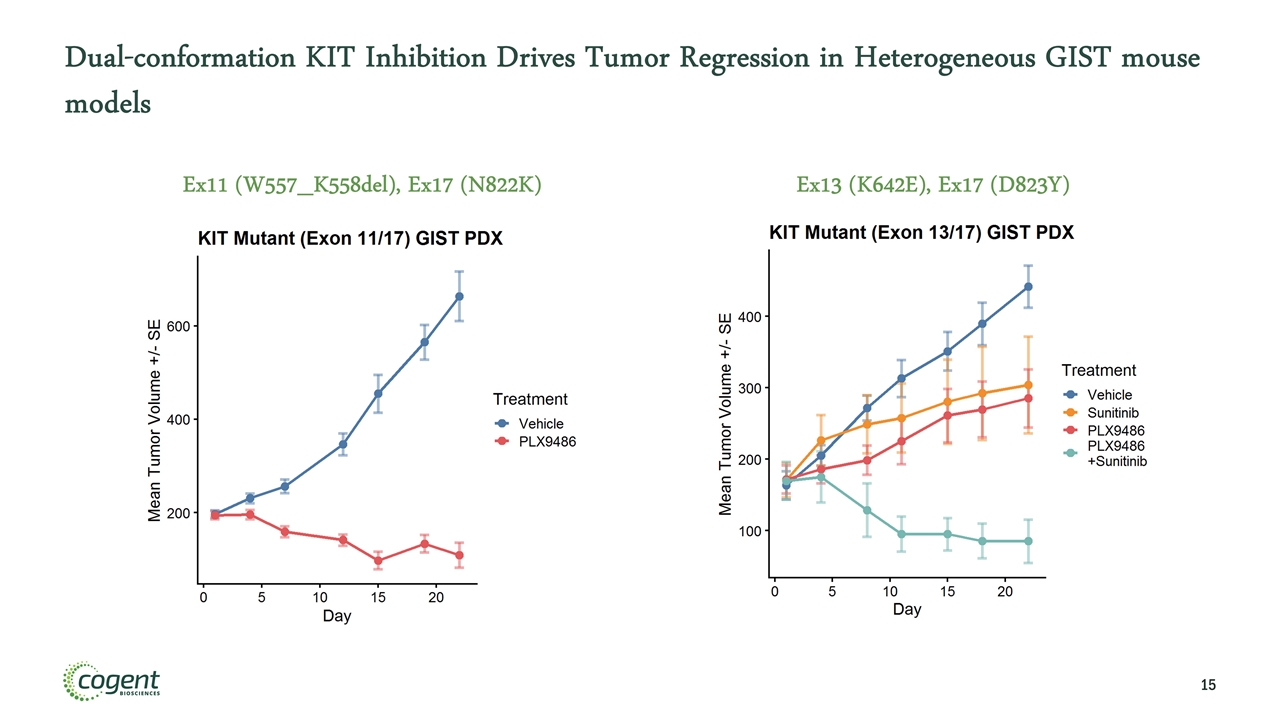
Dual-conformation KIT Inhibition Drives Tumor Regression in Heterogeneous GIST mouse models Ex11 (W557_K558del), Ex17 (N822K) Ex13 (K642E), Ex17 (D823Y)
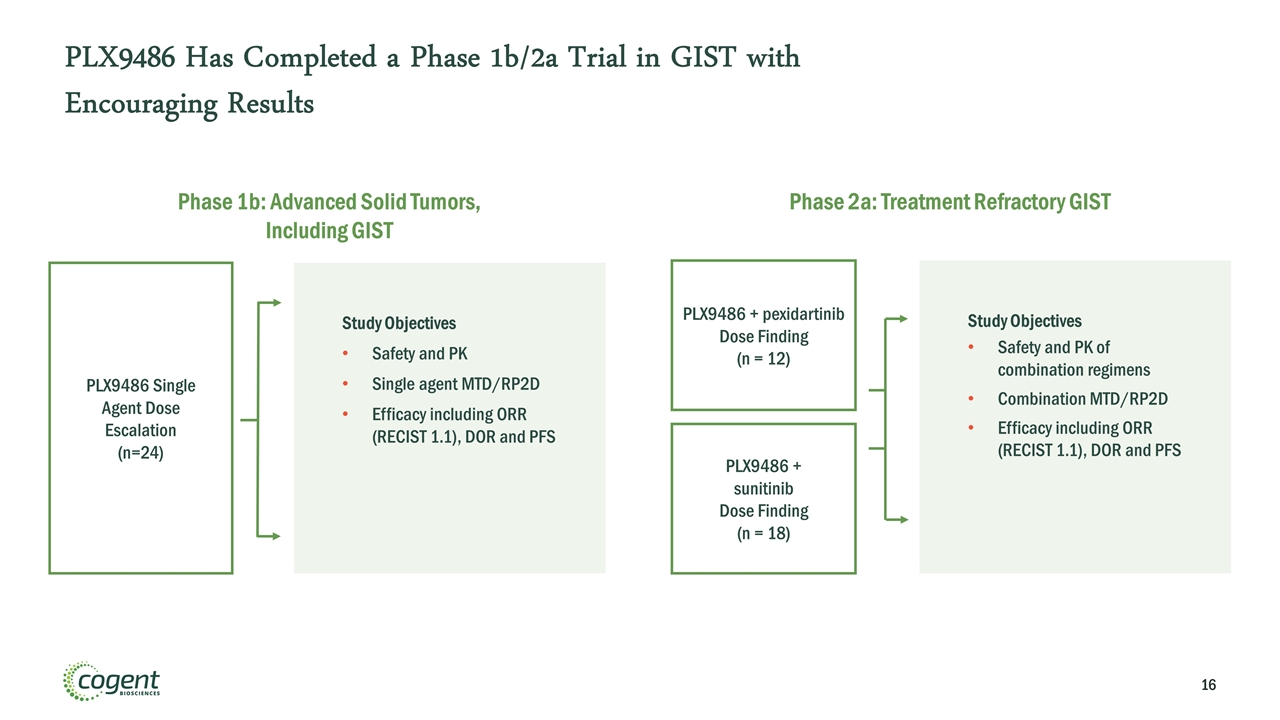
PLX9486 Has Completed a Phase 1b/2a Trial in GIST with Encouraging Results Phase 1b: Advanced Solid Tumors, Including GIST PLX9486 Single Agent Dose Escalation (n=24) Study Objectives Safety and PK Single agent MTD/RP2D Efficacy including ORR (RECIST 1.1), DOR and PFS Phase 2a: Treatment Refractory GIST PLX9486 + pexidartinib Dose Finding (n = 12) PLX9486 + sunitinib Dose Finding (n = 18) Study Objectives Safety and PK of combination regimens Combination MTD/RP2D Efficacy including ORR (RECIST 1.1), DOR and PFS
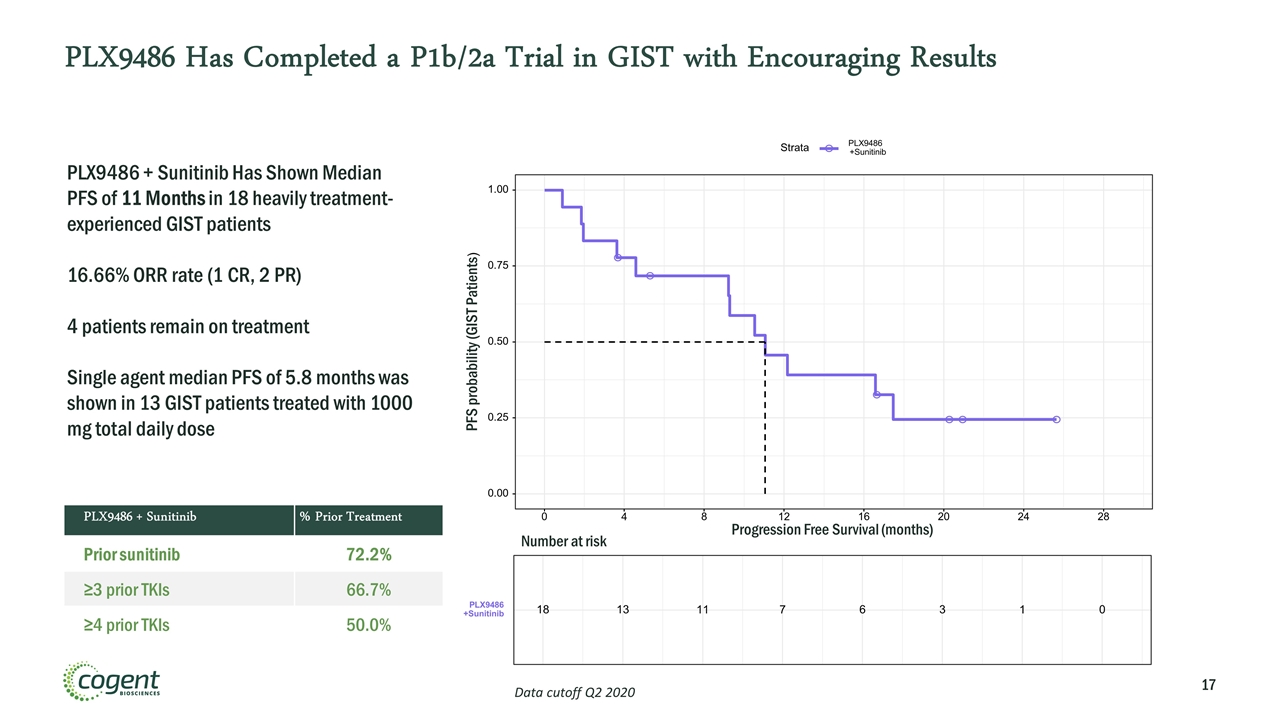
PLX9486 Has Completed a P1b/2a Trial in GIST with Encouraging Results 0.00 0.25 0.50 0.75 1.00 0 4 8 12 16 20 24 28 Progression Free Survival (months) PFS probability (GIST Patients) Strata PLX9486 +Sunitinib 18 13 11 7 6 3 1 0 PLX9486 +Sunitinib Number at risk PLX9486 + Sunitinib Has Shown Median PFS of 11 Months in 18 heavily treatment-experienced GIST patients 16.66% ORR rate (1 CR, 2 PR) 4 patients remain on treatment Single agent median PFS of 5.8 months was shown in 13 GIST patients treated with 1000 mg total daily dose Data cutoff Q2 2020 PLX9486 + Sunitinib % Prior Treatment Prior sunitinib 72.2% ≥3 prior TKIs 66.7% ≥4 prior TKIs 50.0%
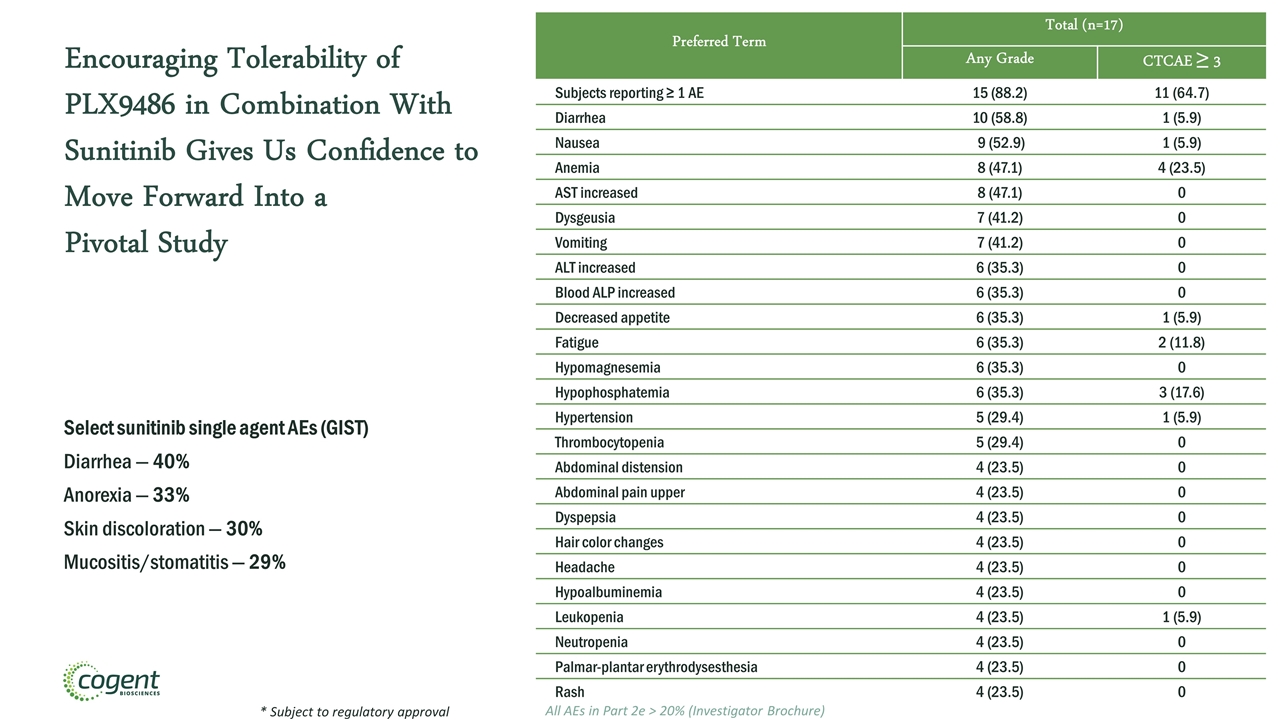
Encouraging Tolerability of PLX9486 in Combination With Sunitinib Gives Us Confidence to Move Forward Into a Pivotal Study Select sunitinib single agent AEs (GIST) Diarrhea — 40% Anorexia — 33% Skin discoloration — 30% Mucositis/stomatitis — 29% Preferred Term Total (n=17) Any Grade CTCAE ≥ 3 Subjects reporting ≥ 1 AE 15 (88.2) 11 (64.7) Diarrhea 10 (58.8) 1 (5.9) Nausea 9 (52.9) 1 (5.9) Anemia 8 (47.1) 4 (23.5) AST increased 8 (47.1) 0 Dysgeusia 7 (41.2) 0 Vomiting 7 (41.2) 0 ALT increased 6 (35.3) 0 Blood ALP increased 6 (35.3) 0 Decreased appetite 6 (35.3) 1 (5.9) Fatigue 6 (35.3) 2 (11.8) Hypomagnesemia 6 (35.3) 0 Hypophosphatemia 6 (35.3) 3 (17.6) Hypertension 5 (29.4) 1 (5.9) Thrombocytopenia 5 (29.4) 0 Abdominal distension 4 (23.5) 0 Abdominal pain upper 4 (23.5) 0 Dyspepsia 4 (23.5) 0 Hair color changes 4 (23.5) 0 Headache 4 (23.5) 0 Hypoalbuminemia 4 (23.5) 0 Leukopenia 4 (23.5) 1 (5.9) Neutropenia 4 (23.5) 0 Palmar-plantar erythrodysesthesia 4 (23.5) 0 Rash 4 (23.5) 0 * Subject to regulatory approval All AEs in Part 2e > 20% (Investigator Brochure)
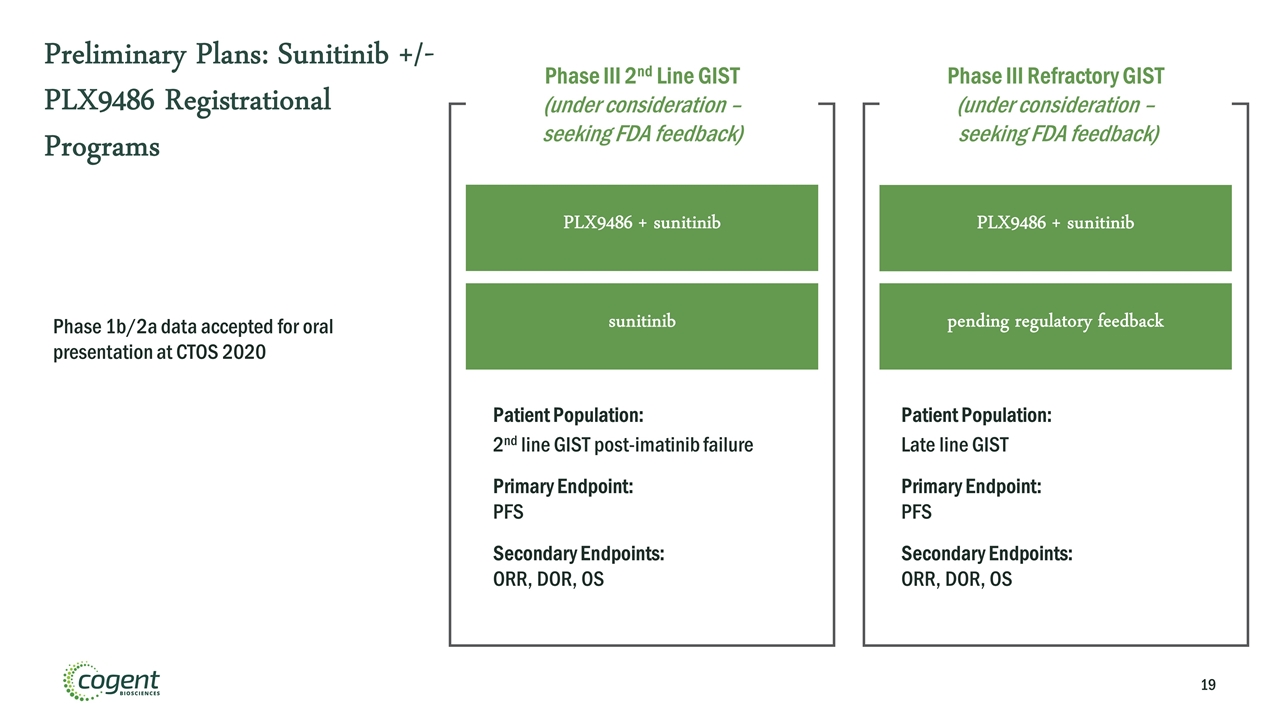
Preliminary Plans: Sunitinib +/- PLX9486 Registrational Programs Phase 1b/2a data accepted for oral presentation at CTOS 2020 PLX9486 + sunitinib Phase III Refractory GIST (under consideration – seeking FDA feedback) Patient Population: Late line GIST Primary Endpoint: PFS Secondary Endpoints: ORR, DOR, OS PLX9486 + sunitinib Patient Population: 2nd line GIST post-imatinib failure Primary Endpoint: PFS Secondary Endpoints: ORR, DOR, OS Phase III 2nd Line GIST (under consideration – seeking FDA feedback) sunitinib pending regulatory feedback
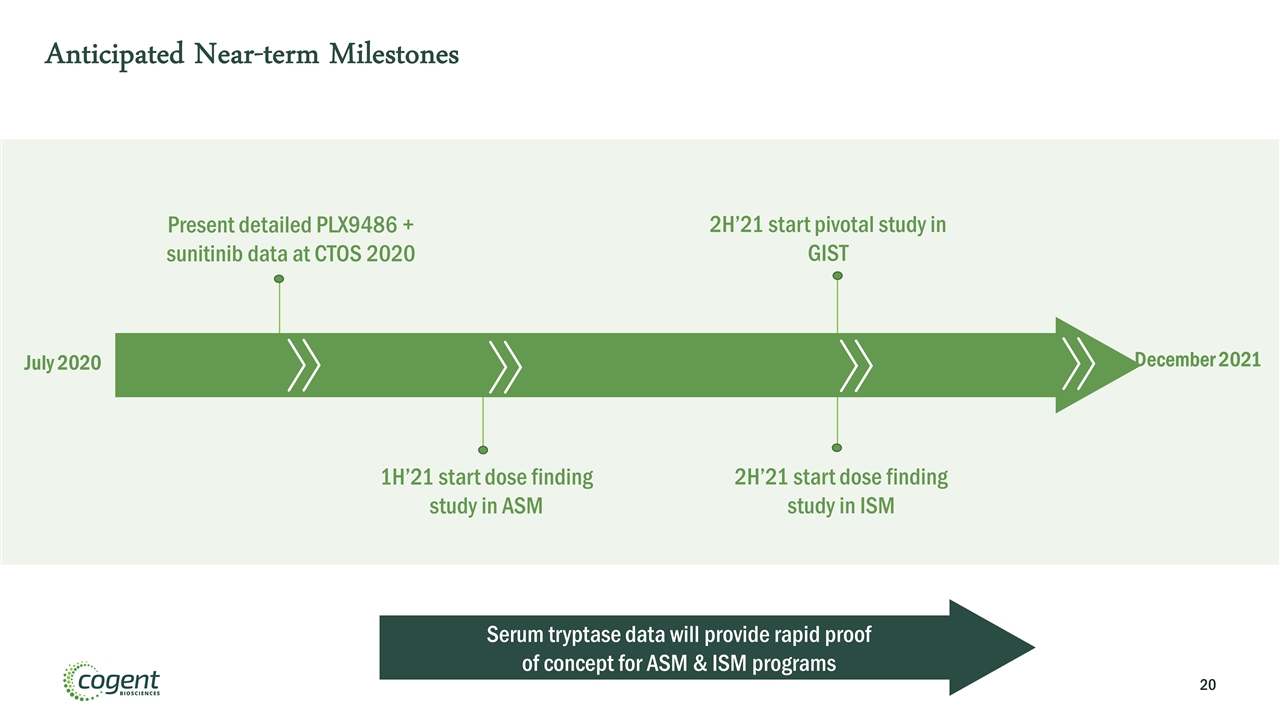
July 2020 Present detailed PLX9486 + sunitinib data at CTOS 2020 1H’21 start dose finding study in ASM December 2021 2H’21 start pivotal study in GIST Anticipated Near-term Milestones 2H’21 start dose finding study in ISM Serum tryptase data will provide rapid proof of concept for ASM & ISM programs
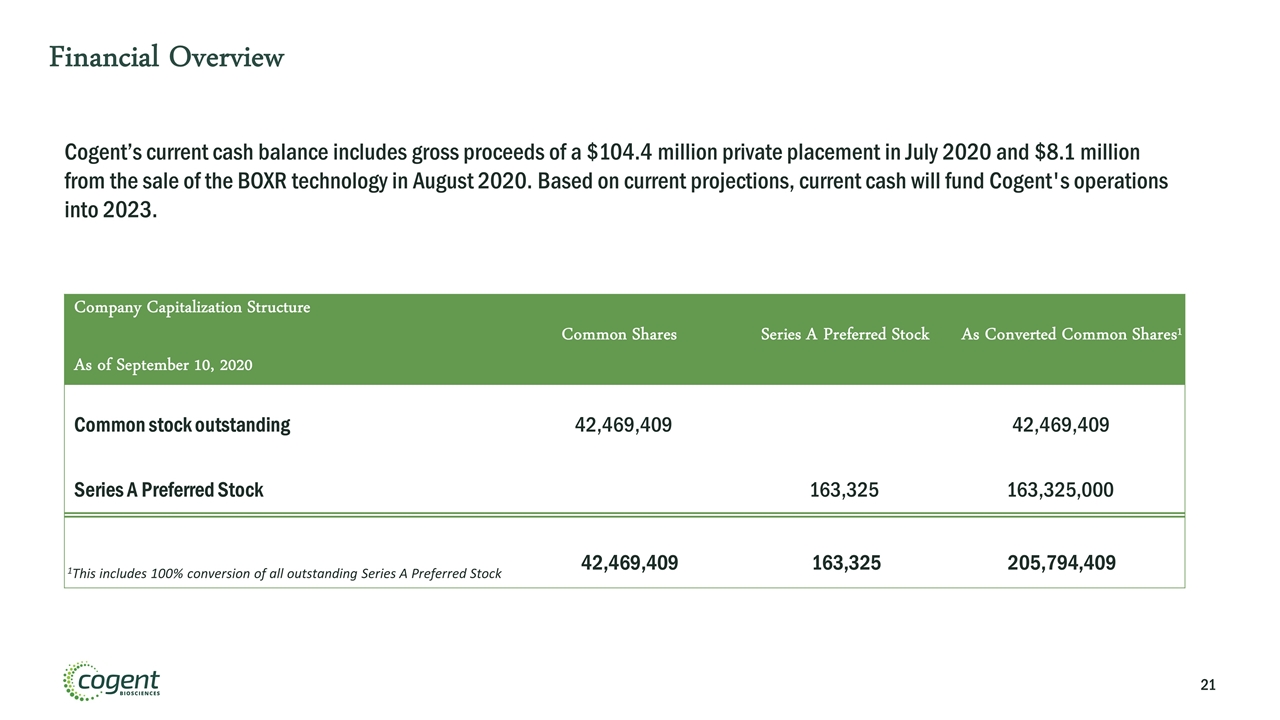
Financial Overview Cogent’s current cash balance includes gross proceeds of a $104.4 million private placement in July 2020 and $8.1 million from the sale of the BOXR technology in August 2020. Based on current projections, current cash will fund Cogent's operations into 2023. Company Capitalization Structure As of September 10, 2020 Common Shares Series A Preferred Stock As Converted Common Shares1 Common stock outstanding 42,469,409 42,469,409 Series A Preferred Stock 163,325 163,325,000 42,469,409 163,325 205,794,409 1This includes 100% conversion of all outstanding Series A Preferred Stock

Thank You CogentBio.com Cogent Biosciences Inc. | 200 Cambridge Park Drive Suite 2500 | Cambridge, MA 02140 USA





















