
Exhibit 99.2 Initial Data from APEX Phase 2 Study of Bezuclastinib in Advanced Systemic Mastocytosis Investor Webcast Presented at the European Hematology Association Congress June 10, 2022

Forward Looking Statement and Risk Factors This presentation and the accompanying oral commentary contain forward-looking statements that involve risks, uncertainties and assumptions. If the risks or uncertainties ever materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by such forward looking statements. All statements other than statements of historical fact could be deemed forward-looking, including, but not limited to, any statements regarding the potential for bezuclastinib to provide meaningful clinical activity to systemic mastocytosis patients without the tolerability challenges seen with other available treatment options, the expectation to accelerate timelines and investment and provide another APEX clinical update by the end of 2022, the expectation to present SUMMIT clinical data in NonAdvSM patients in 2023, and the plan to continue enrolling patients in Part 1 of APEX to determine a recommended dose for use in Part 2 of the trial; any statements of the plans, strategies, and objectives of management for future operations, including our clinical development and commercialization plans; any projections of financial information; any statement about historical results that may suggest trends for our business; any statement of expectation or belief regarding future events; potential markets or market size, technology developments, our clinical product pipeline, clinical and pre-clinical data or the implications thereof, enforceability of our intellectual property rights, competitive strengths or our position within the industry; any statements regarding the anticipated benefits of our collaborations or other strategic transactions; and any statements of assumptions underlying any of the items mentioned. These statements are based on estimates and information available to us at the time of this presentation and are not guarantees of future performance. Actual results could differ materially from our current expectations as a result of many risks and uncertainties, including but not limited to, risks associated with: the potential impacts of raising additional capital, including dilution to our existing stockholders, restrictions on our operations or requirements that we relinquish rights to our technologies or product candidates; business interruptions resulting from the coronavirus disease outbreak or similar public health crises, which could cause a disruption to the development of our product candidates and adversely impact our business; our expected use of our existing cash and the net proceeds from this offering; the success, cost, and duration of our product development activities and clinical trials; the timing of our planned regulatory submissions to the FDA for our bezuclastinib product candidate, also known as CGT9486; our ability to obtain and maintain regulatory approval for our bezuclastinib product candidate and any other product candidates we may develop, and any related restrictions, limitations, and/or warnings in the label of an approved product candidate; the potential for our identified research priorities to advance our bezuclastinib product candidate or for our teams to discover and develop additional product candidates; the ability to license additional intellectual property rights relating to our bezuclastinib product candidate or future product candidates from third-parties and to comply with our existing or future license agreements and/or collaboration agreements; our ability to commercialize our bezuclastinib product candidate and future product candidates in light of the intellectual property rights of others; our ability to obtain funding for our operations, including funding necessary to complete further discovery, development and commercialization of our existing and future product candidates; the scalability and commercial viability of our manufacturing methods and processes; the commercialization of our product candidates, if approved; our ability to attract collaborators with development, regulatory, and commercialization expertise; future agreements with third parties in connection with the commercialization of our product candidates and any other approved product; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; the rate and degree of market acceptance of our product candidates; the pricing and reimbursement of our product candidates, if approved; regulatory developments in the United States and foreign countries; our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately; the development and success of competing therapies that are or may be under development in clinical trials or become available commercially; our ability to attract and retain key scientific and management personnel; the accuracy of our estimates regarding expenses, future revenue, capital requirements, and needs for additional financing; our use of the proceeds from the private placements, sales of our preferred stock and other public offerings of our common stock from time to time; and our expectations regarding our ability to obtain and maintain intellectual property protection for our bezuclastinib product candidate and future product candidates, among others. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to our business in general, see our most recent Annual Report on Form 10-K and our subsequent periodic reports filed from time to time with the Securities and Exchange Commission. Unless as required by law, we assume no obligation and do not intend to update these forward-looking statements or to conform these statements to actual results or to changes in our expectations. All of Cogent Biosciences, Inc. (“Cogent”) product candidates are investigational product candidates and their safety and efficacy have not yet been established. Cogent has not obtained marketing approval for any product, and there is no certainty that any marketing approvals will be obtained or as to the timelines on which they will be obtained. Any data pertaining to Cogent product candidates is interim data and may include investigator-reported interim data for which Cogent has not yet independently reviewed the source data. The interim data may not be representative of the final results that may be obtained in the corresponding trials and results from earlier trials may not be representative of results obtained in later trial or pivotal trials. 2
Webcast Agenda and Speakers Andrew Robbins Daniel J. DeAngelo, M.D., Ph.D. Jessica Sachs, M.D. President and Chief Executive Officer Chief of the Division of Leukemia Chief Medical Officer Dana-Farber Cancer Institute Introduction & Corporate Overview Andrew Robbins Review of Initial APEX Data with Bezuclastinib in Dr. Daniel DeAngelo Advanced Systemic Mastocytosis (ASM) patients Presentation Summary Andrew Robbins Andrew Robbins Q&A Dr. Jessica Sachs Dr. Daniel DeAngelo 3
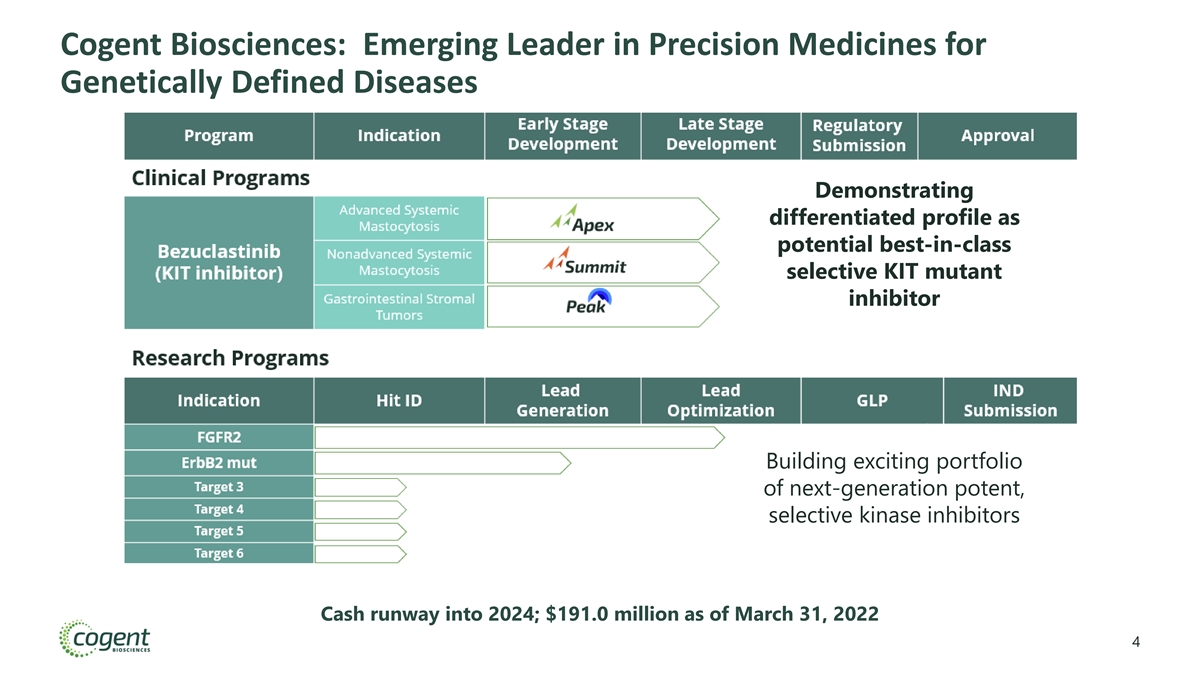
Cogent Biosciences: Emerging Leader in Precision Medicines for Genetically Defined Diseases Demonstrating differentiated profile as potential best-in-class selective KIT mutant inhibitor Building exciting portfolio of next-generation potent, selective kinase inhibitors Cash runway into 2024; $191.0 million as of March 31, 2022 4
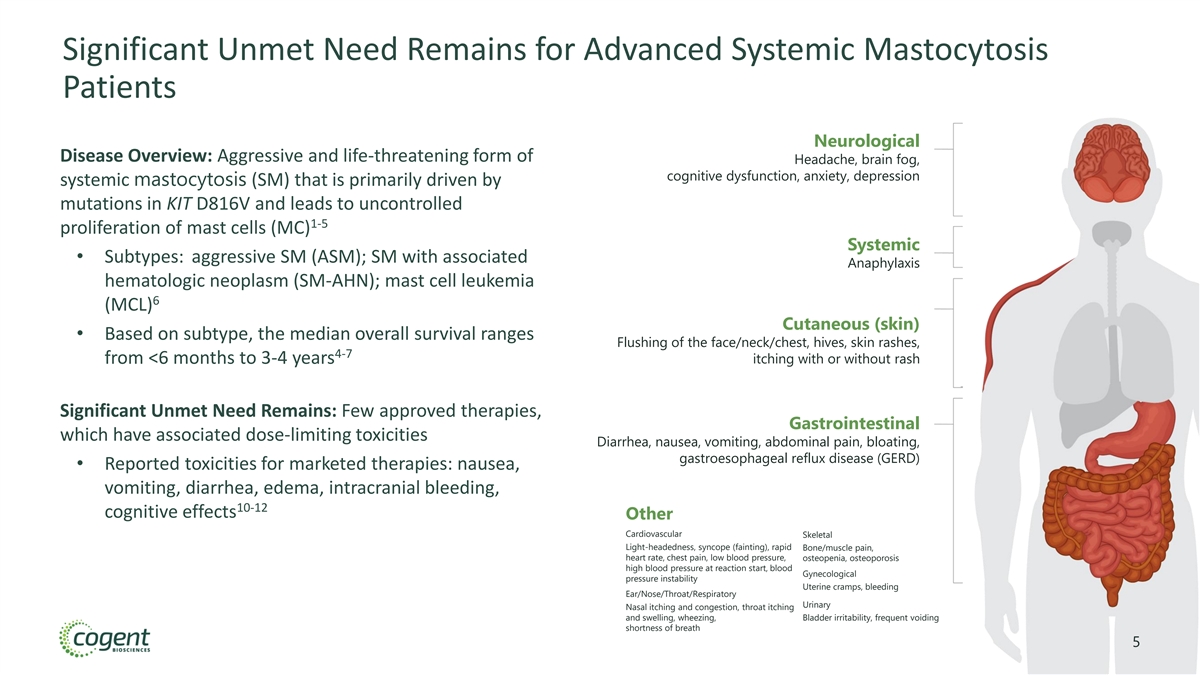
Significant Unmet Need Remains for Advanced Systemic Mastocytosis Patients Neurological Disease Overview: Aggressive and life-threatening form of Headache, brain fog, cognitive dysfunction, anxiety, depression systemic mastocytosis (SM) that is primarily driven by mutations in KIT D816V and leads to uncontrolled 1-5 proliferation of mast cells (MC) Systemic • Subtypes: aggressive SM (ASM); SM with associated Anaphylaxis hematologic neoplasm (SM-AHN); mast cell leukemia 6 (MCL) Cutaneous (skin) • Based on subtype, the median overall survival ranges Flushing of the face/neck/chest, hives, skin rashes, 4-7 from <6 months to 3-4 years itching with or without rash Significant Unmet Need Remains: Few approved therapies, Gastrointestinal which have associated dose-limiting toxicities Diarrhea, nausea, vomiting, abdominal pain, bloating, gastroesophageal reflux disease (GERD) • Reported toxicities for marketed therapies: nausea, vomiting, diarrhea, edema, intracranial bleeding, 10-12 cognitive effects Other Cardiovascular Skeletal Light-headedness, syncope (fainting), rapid Bone/muscle pain, heart rate, chest pain, low blood pressure, osteopenia, osteoporosis high blood pressure at reaction start, blood Gynecological pressure instability Uterine cramps, bleeding Ear/Nose/Throat/Respiratory Urinary Nasal itching and congestion, throat itching and swelling, wheezing, Bladder irritability, frequent voiding shortness of breath 5

Large, Yet Not Well Understood Population of SM Patients 16 Systemic Mastocytosis: Estimated prevalence in the U.S. is 20,000–30,000 patients NonAdvSM Significant unmet medical need Comprises upwards of for clinically active, well-tolerated 1 90% of all cases of SM treatment options for this patient population AdvSM 6
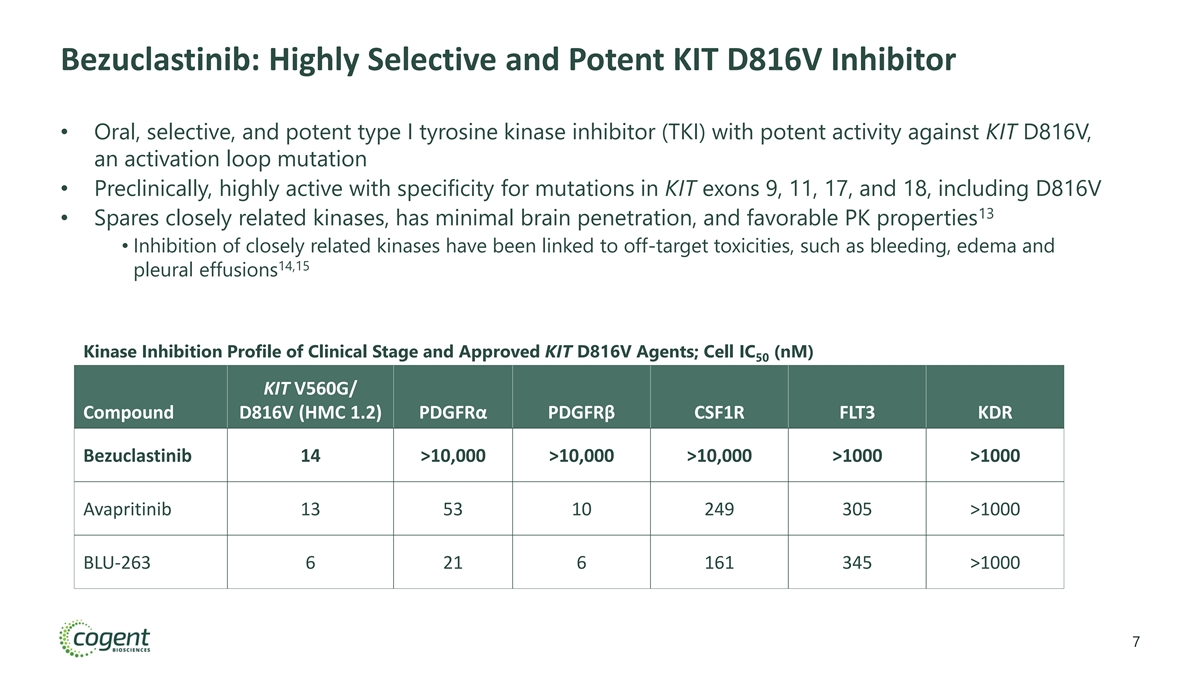
Bezuclastinib: Highly Selective and Potent KIT D816V Inhibitor • Oral, selective, and potent type I tyrosine kinase inhibitor (TKI) with potent activity against KIT D816V, an activation loop mutation • Preclinically, highly active with specificity for mutations in KIT exons 9, 11, 17, and 18, including D816V 13 • Spares closely related kinases, has minimal brain penetration, and favorable PK properties • Inhibition of closely related kinases have been linked to off-target toxicities, such as bleeding, edema and 14,15 pleural effusions Kinase Inhibition Profile of Clinical Stage and Approved KIT D816V Agents; Cell IC (nM) 50 KIT V560G/ Compound D816V (HMC 1.2) PDGFRα PDGFRβ CSF1R FLT3 KDR Bezuclastinib 14 >10,000 >10,000 >10,000 >1000 >1000 Avapritinib 13 53 10 249 305 >1000 BLU-263 6 21 6 161 345 >1000 7
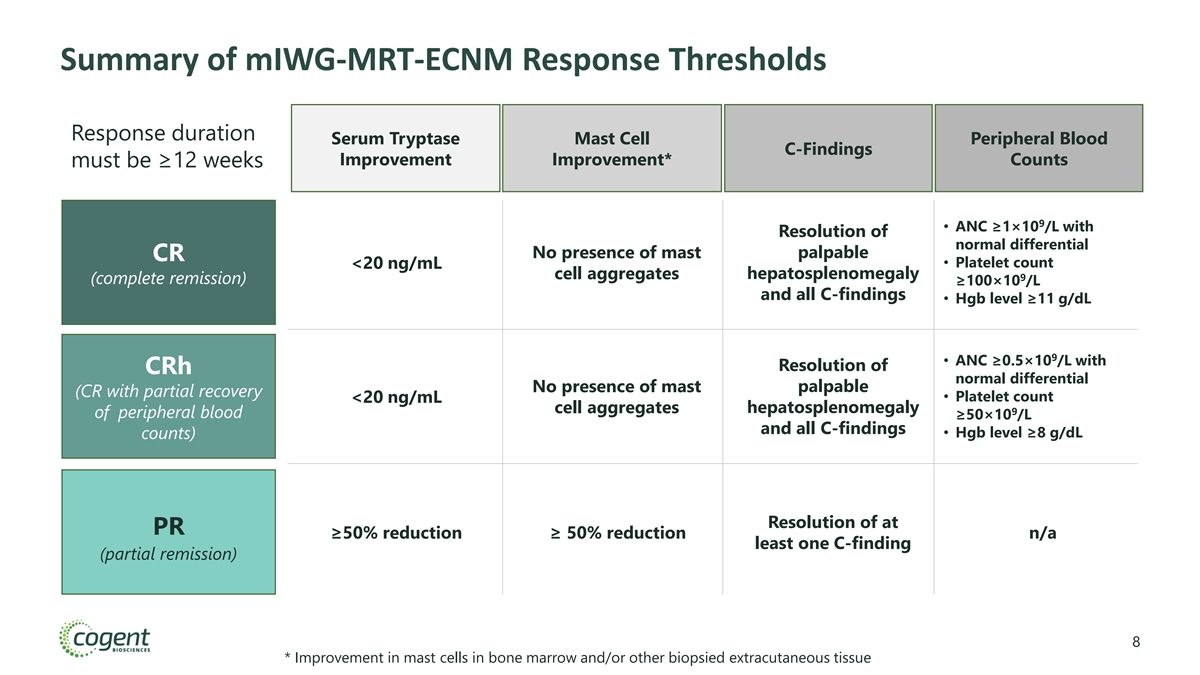
Summary of mIWG-MRT-ECNM Response Thresholds Response duration Serum Tryptase Mast Cell Peripheral Blood C-Findings Improvement Improvement* Counts must be ≥12 weeks 9 • ANC ≥1×10 /L with Resolution of normal differential No presence of mast palpable CR • Platelet count <20 ng/mL hepatosplenomegaly cell aggregates 9 (complete remission) ≥100×10 /L and all C-findings • Hgb level ≥11 g/dL 9 • ANC ≥0.5×10 /L with Resolution of CRh normal differential No presence of mast palpable (CR with partial recovery • Platelet count <20 ng/mL cell aggregates hepatosplenomegaly 9 of peripheral blood ≥50×10 /L and all C-findings • Hgb level ≥8 g/dL counts) Resolution of at PR ≥50% reduction ≥ 50% reduction n/a least one C-finding (partial remission) 8 * Improvement in mast cells in bone marrow and/or other biopsied extracutaneous tissue
A Phase 2 Study of Bezuclastinib (CGT9486), A Novel, Highly Selective, Potent KIT D816V Inhibitor, in Adults with Advanced Systemic Mastocytosis (Apex): Methods, Baseline Data, and Early Insights ABSTRACT CODE: P1049 EHA2022 HYBRID CONGRESS | VIENNA, AUSTRIA 10 JUNE 2022

APEX: A Phase 2 Open-Label, Multicenter Clinical Study of Bezuclastinib in Patients with Advanced Systemic Mastocytosis PART 2: EXPANSION** KEY ENTRY CRITERIA PART 1: DOSE OPTIMIZATION* N=80 N=60 • Diagnosed with ASM, SM-AHN, or MCL per 50 mg BID AdvSM IWG evaluable (N=60) WHO 2016 100 mg BID Classification Selected R • Measurable disease per Dose 200 mg BID mIWG-MRT-ECNM AdvSM w/C-Findings not measurable 1:1:1:1 (IWG) confirmed by per IWG (N=20) 400 mg QD Eligibility Committee • No restrictions on prior therapy Primary Endpoint • Dose Optimization: Incidence of AEs/SAEs, laboratory changes, PK, biomarkers, ORR • Expansion: ORR (confirmed CR, CRh, PR and CI) per mIWG-MRT-ECNM and assessed by Central Response Review Committee Other Endpoints • Safety/Tolerability: Incidence of AEs leading to dose modification, changes in Patient Reported Outcomes (PROs) • Efficacy: DOR, TTR, PFS, OS, pure pathologic response • PK/PD: plasma concentration of bezuclastinib, serum tryptase, KIT D816V burden *Interim analysis (IA) when ~28 pts (~7pts/dose level) have completed Cycle 2 (C2) to enrich at promising dose levels 10 **Part 2 may be expanded based on Part 1 results and Regulatory Authority discussions
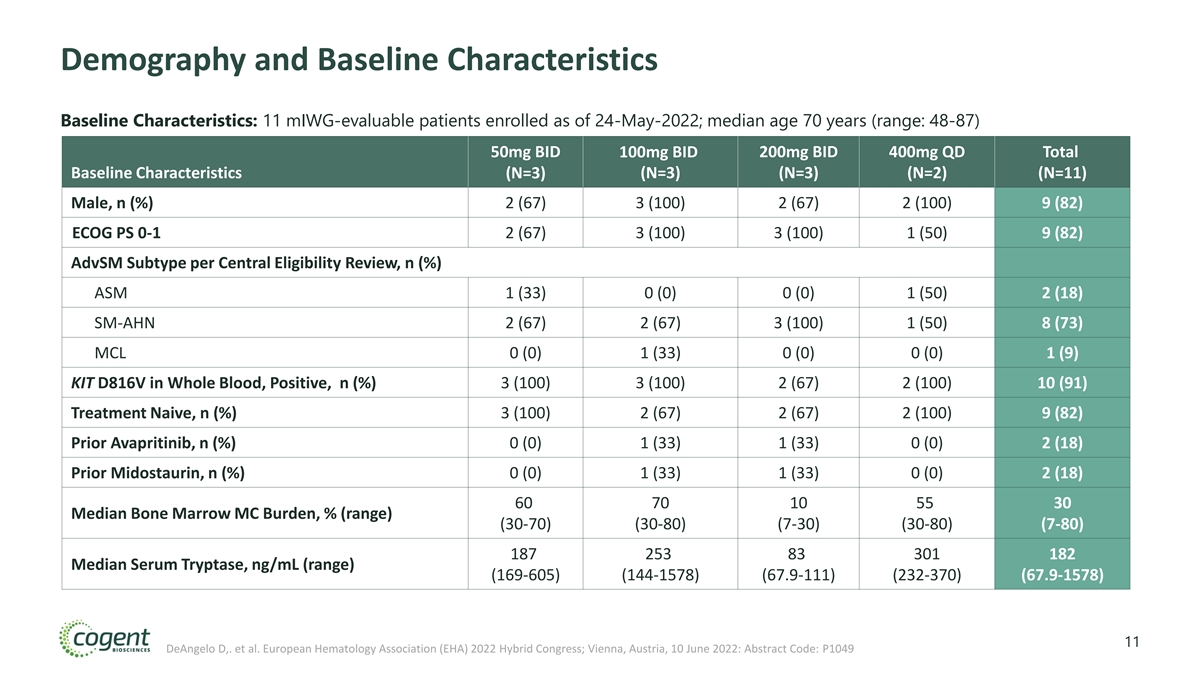
Demography and Baseline Characteristics Baseline Characteristics: 11 mIWG-evaluable patients enrolled as of 24-May-2022; median age 70 years (range: 48-87) 50mg BID 100mg BID 200mg BID 400mg QD Total Baseline Characteristics (N=3) (N=3) (N=3) (N=2) (N=11) Male, n (%) 2 (67) 3 (100) 2 (67) 2 (100) 9 (82) ECOG PS 0-1 2 (67) 3 (100) 3 (100) 1 (50) 9 (82) AdvSM Subtype per Central Eligibility Review, n (%) ASM 1 (33) 0 (0) 0 (0) 1 (50) 2 (18) SM-AHN 2 (67) 2 (67) 3 (100) 1 (50) 8 (73) MCL 0 (0) 1 (33) 0 (0) 0 (0) 1 (9) KIT D816V in Whole Blood, Positive, n (%) 3 (100) 3 (100) 2 (67) 2 (100) 10 (91) Treatment Naive, n (%) 3 (100) 2 (67) 2 (67) 2 (100) 9 (82) Prior Avapritinib, n (%) 0 (0) 1 (33) 1 (33) 0 (0) 2 (18) Prior Midostaurin, n (%) 0 (0) 1 (33) 1 (33) 0 (0) 2 (18) 60 70 10 55 30 Median Bone Marrow MC Burden, % (range) (30-70) (30-80) (7-30) (30-80) (7-80) 187 253 83 301 182 Median Serum Tryptase, ng/mL (range) (169-605) (144-1578) (67.9-111) (232-370) (67.9-1578) 11 DeAngelo D,. et al. European Hematology Association (EHA) 2022 Hybrid Congress; Vienna, Austria, 10 June 2022: Abstract Code: P1049

Summary of Safety and Tolerability • Majority of treatment emergent adverse events (TEAE) were of low-grade with one serious adverse event (SAE) and no Grade 4 events • No periorbital/peripheral edema, cognitive effects or intracranial bleeding reported • No treatment discontinuations; all patients remained on study* • Two patients dose reduced due to AEs; one re-escalated to randomized dose TEAE Occurring in >1 Patient and All Grade 3 Events TEAE Preferred Term, n (%) (N=11) Grade 1/2 Grade 3 Grade 4 Anemia 2 (18) 1 (9) 0 (0) Neutropenia 1 (9) 1 (9) 0 (0) Thrombocytopenia 2 (18) 0 (0) 0 (0) ‡ Diarrhea 0 (0) 1 (9) 0 (0) † Hypersensitivity 0 (0) 1 (9) 0 (0) ‡ Investigator assessed as not related to treatment † SAE of hypersensitivity (mediator flare); patient continued treatment and remains on study (See: Patient Summary; Case 2) * Subsequent to data cut-off, one SM-AHN patient with chronic myelomonocytic leukemia (CMML) transformed to acute myeloid leukemia (AML) and discontinued participation in the trial 12 Data as of: 24May2022 DeAngelo D,. et al. European Hematology Association (EHA) 2022 Hybrid Congress; Vienna, Austria, 10 June 2022: Abstract Code: P1049
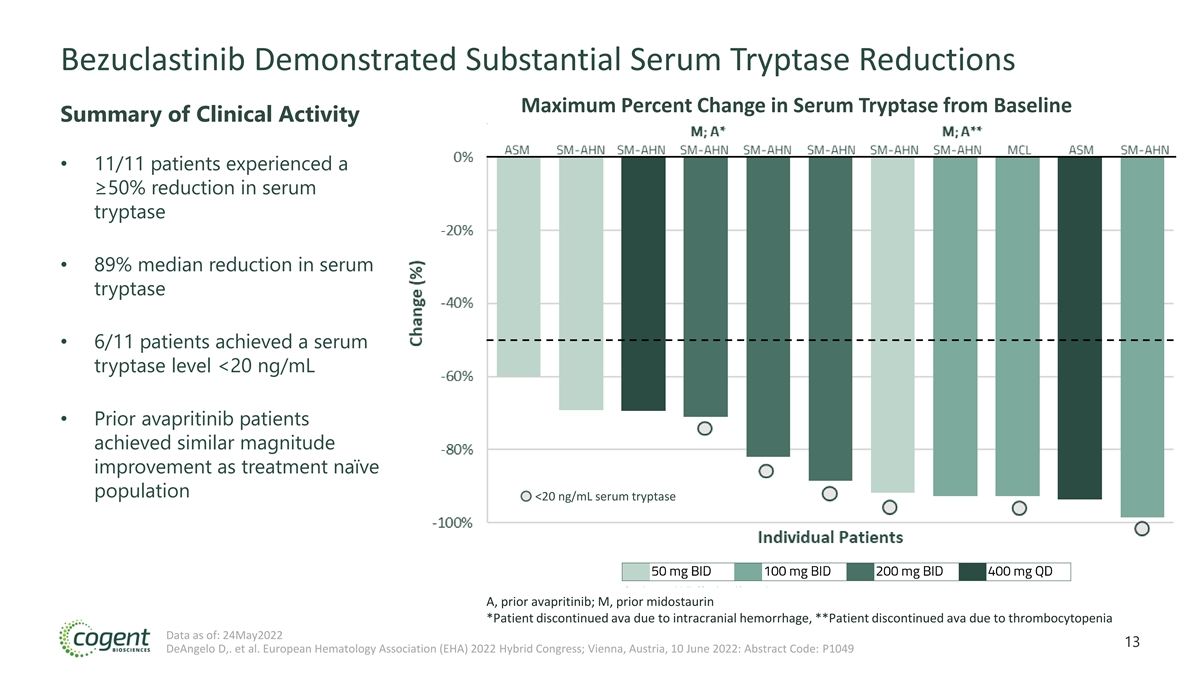
Bezuclastinib Demonstrated Substantial Serum Tryptase Reductions Maximum Percent Change in Serum Tryptase from Baseline Summary of Clinical Activity • 11/11 patients experienced a ≥50% reduction in serum tryptase • 89% median reduction in serum tryptase • 6/11 patients achieved a serum tryptase level <20 ng/mL • Prior avapritinib patients achieved similar magnitude improvement as treatment naïve population <20 ng/mL serum tryptase A, prior avapritinib; M, prior midostaurin *Patient discontinued ava due to intracranial hemorrhage, **Patient discontinued ava due to thrombocytopenia Data as of: 24May2022 13 DeAngelo D,. et al. European Hematology Association (EHA) 2022 Hybrid Congress; Vienna, Austria, 10 June 2022: Abstract Code: P1049
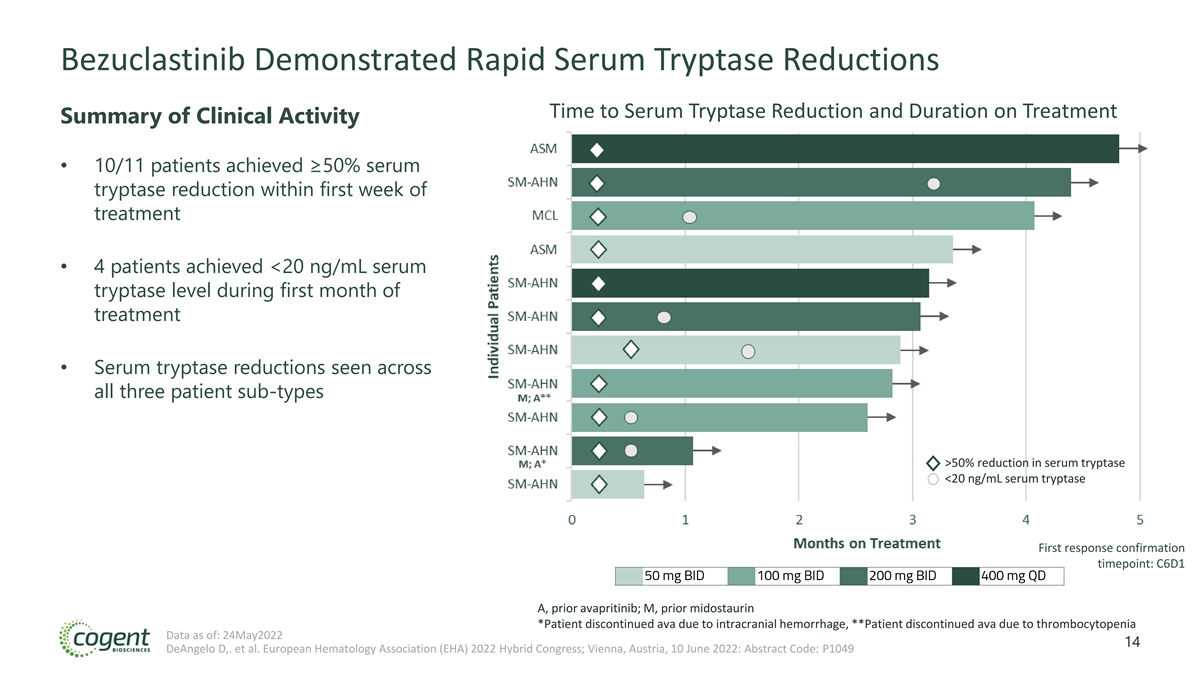
Bezuclastinib Demonstrated Rapid Serum Tryptase Reductions Time to Serum Tryptase Reduction and Duration on Treatment Summary of Clinical Activity • 10/11 patients achieved ≥50% serum tryptase reduction within first week of treatment • 4 patients achieved <20 ng/mL serum tryptase level during first month of treatment • Serum tryptase reductions seen across all three patient sub-types >50% reduction in serum tryptase <20 ng/mL serum tryptase First response confirmation timepoint: C6D1 A, prior avapritinib; M, prior midostaurin *Patient discontinued ava due to intracranial hemorrhage, **Patient discontinued ava due to thrombocytopenia Data as of: 24May2022 14 DeAngelo D,. et al. European Hematology Association (EHA) 2022 Hybrid Congress; Vienna, Austria, 10 June 2022: Abstract Code: P1049
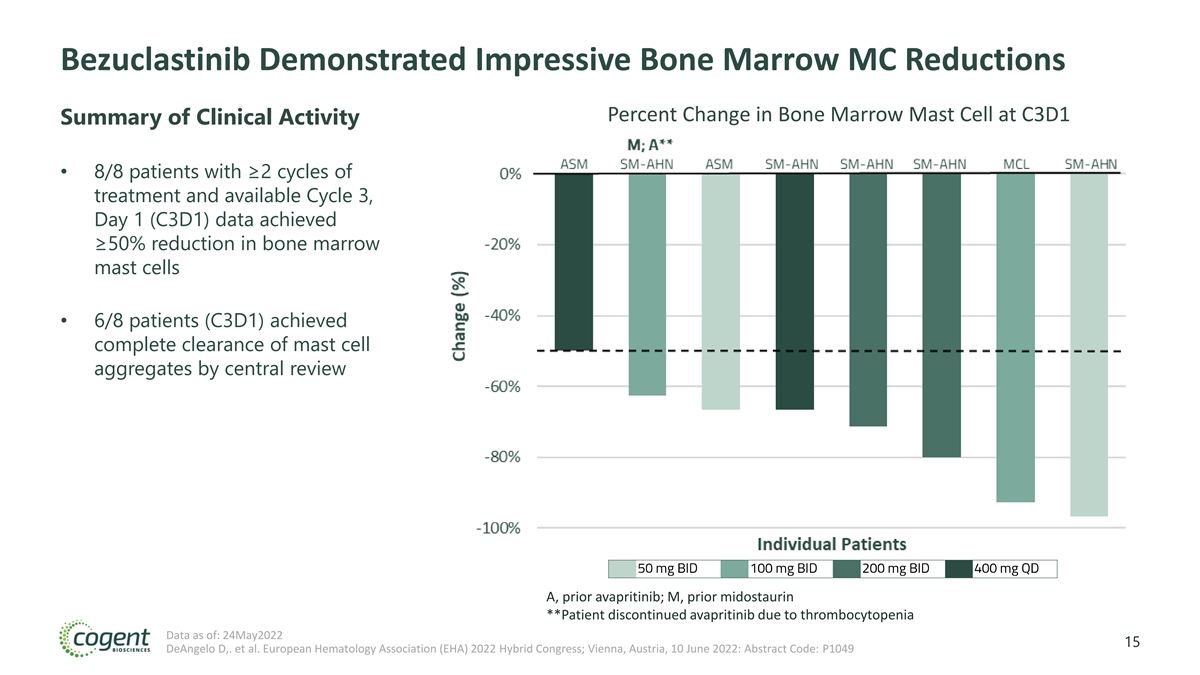
Bezuclastinib Demonstrated Impressive Bone Marrow MC Reductions Percent Change in Bone Marrow Mast Cell at C3D1 Summary of Clinical Activity • 8/8 patients with ≥2 cycles of treatment and available Cycle 3, Day 1 (C3D1) data achieved ≥50% reduction in bone marrow mast cells • 6/8 patients (C3D1) achieved complete clearance of mast cell aggregates by central review A, prior avapritinib; M, prior midostaurin **Patient discontinued avapritinib due to thrombocytopenia Data as of: 24May2022 15 DeAngelo D,. et al. European Hematology Association (EHA) 2022 Hybrid Congress; Vienna, Austria, 10 June 2022: Abstract Code: P1049
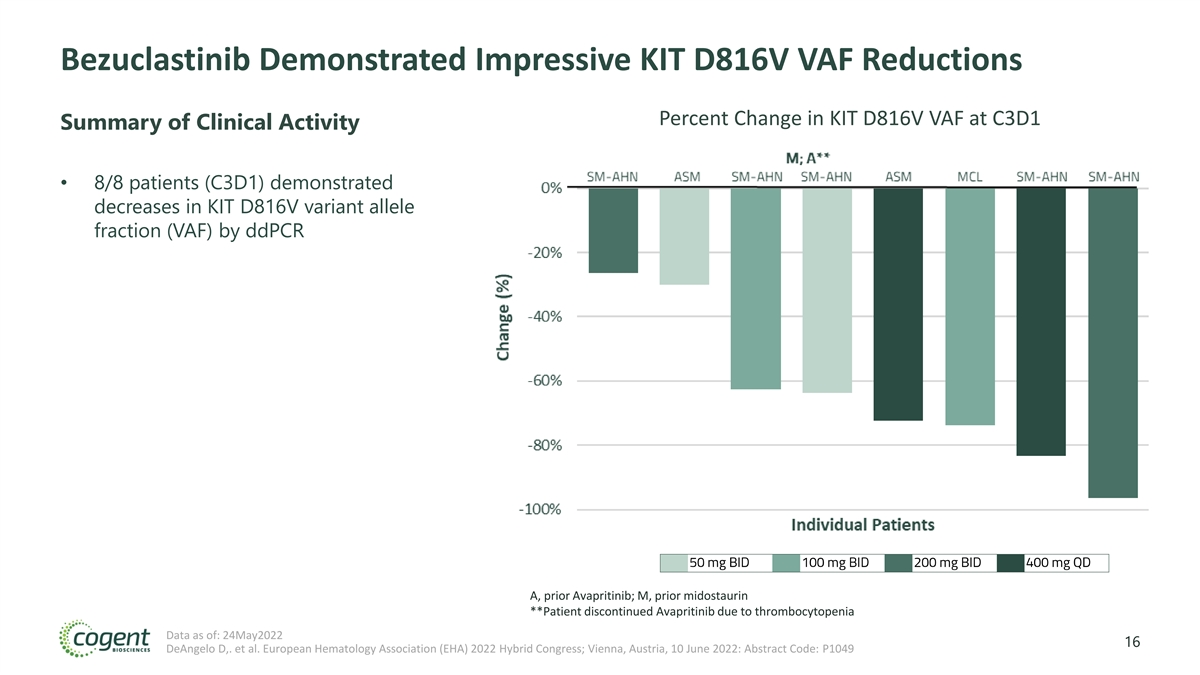
Bezuclastinib Demonstrated Impressive KIT D816V VAF Reductions Percent Change in KIT D816V VAF at C3D1 Summary of Clinical Activity • 8/8 patients (C3D1) demonstrated decreases in KIT D816V variant allele fraction (VAF) by ddPCR A, prior Avapritinib; M, prior midostaurin **Patient discontinued Avapritinib due to thrombocytopenia Data as of: 24May2022 16 DeAngelo D,. et al. European Hematology Association (EHA) 2022 Hybrid Congress; Vienna, Austria, 10 June 2022: Abstract Code: P1049
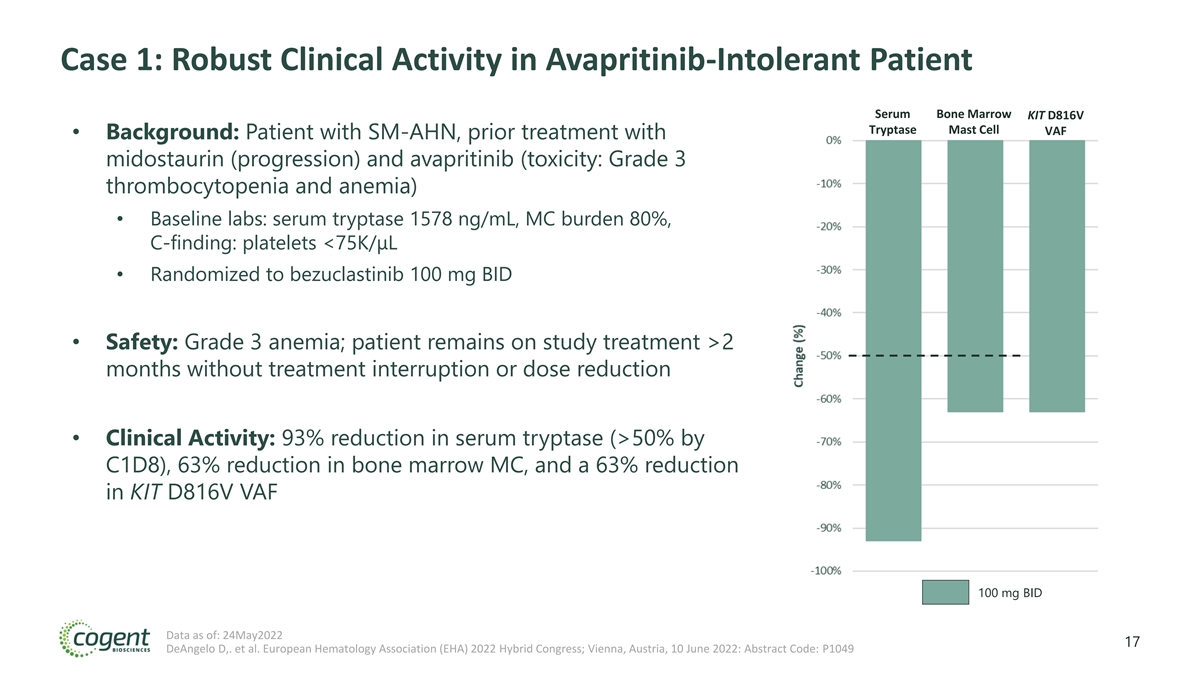
Case 1: Robust Clinical Activity in Avapritinib-Intolerant Patient Serum Bone Marrow KIT D816V Tryptase Mast Cell VAF • Background: Patient with SM-AHN, prior treatment with midostaurin (progression) and avapritinib (toxicity: Grade 3 thrombocytopenia and anemia) • Baseline labs: serum tryptase 1578 ng/mL, MC burden 80%, C-finding: platelets <75K/μL • Randomized to bezuclastinib 100 mg BID • Safety: Grade 3 anemia; patient remains on study treatment >2 months without treatment interruption or dose reduction • Clinical Activity: 93% reduction in serum tryptase (>50% by C1D8), 63% reduction in bone marrow MC, and a 63% reduction in KIT D816V VAF 100 mg BID Data as of: 24May2022 17 DeAngelo D,. et al. European Hematology Association (EHA) 2022 Hybrid Congress; Vienna, Austria, 10 June 2022: Abstract Code: P1049
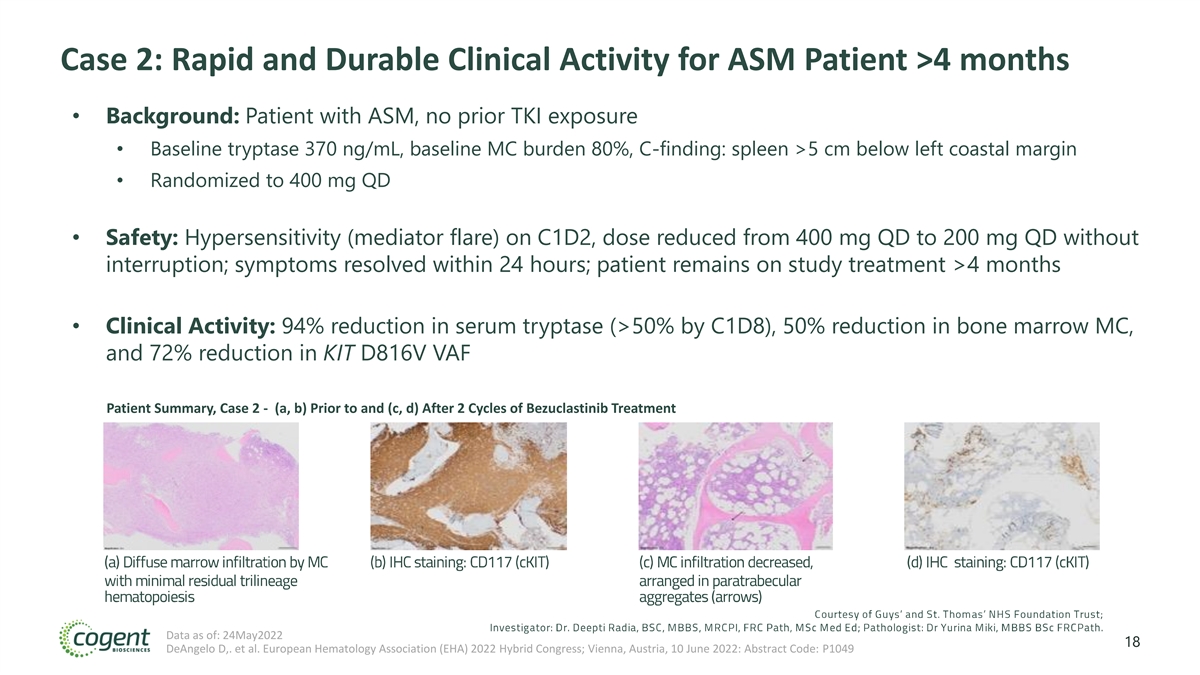
Case 2: Rapid and Durable Clinical Activity for ASM Patient >4 months • Background: Patient with ASM, no prior TKI exposure • Baseline tryptase 370 ng/mL, baseline MC burden 80%, C-finding: spleen >5 cm below left coastal margin • Randomized to 400 mg QD • Safety: Hypersensitivity (mediator flare) on C1D2, dose reduced from 400 mg QD to 200 mg QD without interruption; symptoms resolved within 24 hours; patient remains on study treatment >4 months • Clinical Activity: 94% reduction in serum tryptase (>50% by C1D8), 50% reduction in bone marrow MC, and 72% reduction in KIT D816V VAF Patient Summary, Case 2 - (a, b) Prior to and (c, d) After 2 Cycles of Bezuclastinib Treatment Data as of: 24May2022 18 DeAngelo D,. et al. European Hematology Association (EHA) 2022 Hybrid Congress; Vienna, Austria, 10 June 2022: Abstract Code: P1049
Bezuclastinib has Potential to Provide Meaningful Clinical Benefit • Bezuclastinib is a highly potent and selective tyrosine kinase inhibitor that targets the KIT D816V mutation, the primary driver of systemic mastocytosis • Bezuclastinib was generally well-tolerated across all dose levels o Majority of adverse events were Gr 1/2 and seen in no more than one patient, with one serious adverse event and no Grade 4 events reported o No reported periorbital/peripheral edema, cognitive effects or intracranial bleeding events o Hematological events are expected in this patient population with advanced hematologic disease, frequently presenting with baseline cytopenias related to underlying disease and/or prior therapy • Encouraging early signs of clinical activity demonstrated across all dose levels o Clinically meaningful reduction in serum tryptase levels, reductions in MC burden, and KIT D816V VAF in all evaluable patients across doses tested o With median 89%, all patients achieved ≥50% serum tryptase reduction; all C3D1 patients achieved ≥50% bone marrow MC reductions o Patients treated with prior KIT inhibitors, including avapritinib, demonstrated similar magnitude reductions across serum tryptase, MC burden, and KIT D816V VAF 19 DeAngelo D,. et al. European Hematology Association (EHA) 2022 Hybrid Congress; Vienna, Austria, 10 June 2022: Abstract Code: P1049

Investor Webcast Summary CogentBio.com Cogent Biosciences, Inc. | 200 Cambridge Park Drive Suite 2500 | Cambridge, MA 02140 USA
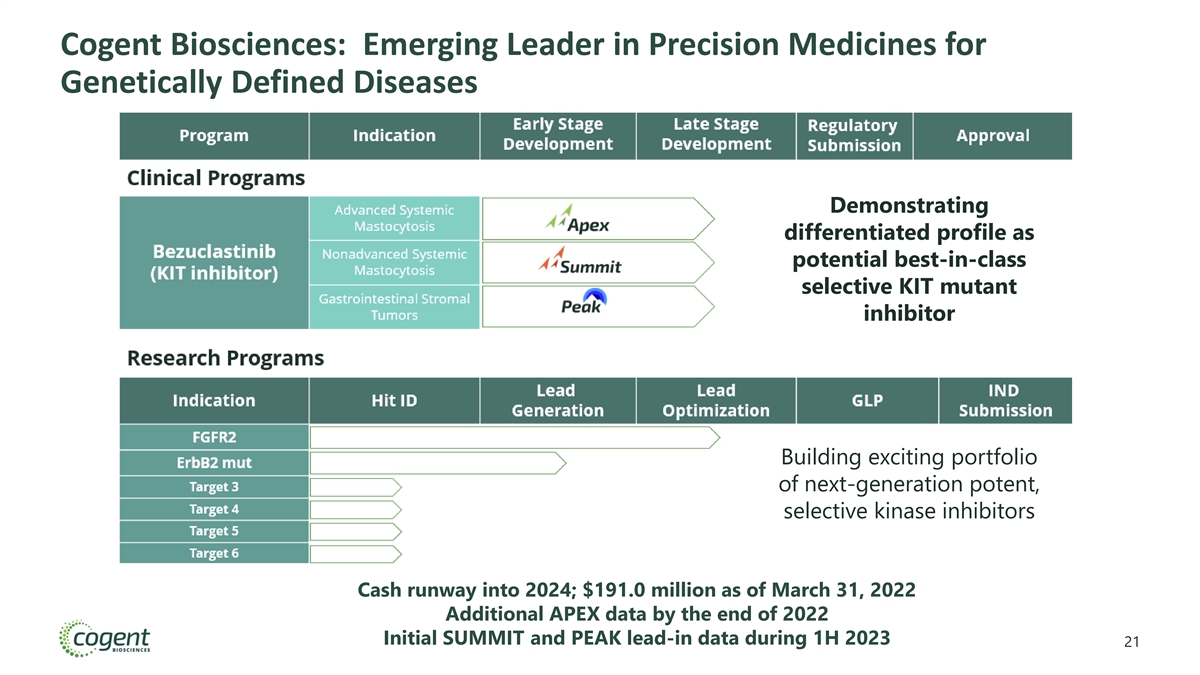
Cogent Biosciences: Emerging Leader in Precision Medicines for Genetically Defined Diseases Demonstrating Demonstrating differentiated profile as differentiated profile as potential best-in-class potential best-in-class selective KIT mutant selective KIT mutant inhibitor inhibitor Building exciting portfolio Building exciting portfolio of next-generation potent, of next-generation potent, selective kinase inhibitors selective kinase inhibitors Cash runway into 2024; $191.0 million as of March 31, 2022 Additional APEX data by the end of 2022 Initial SUMMIT and PEAK lead-in data during 1H 2023 21

Questions & Answers CogentBio.com Cogent Biosciences, Inc. | 200 Cambridge Park Drive Suite 2500 | Cambridge, MA 02140 USA

Appendix CogentBio.com Cogent Biosciences, Inc. | 200 Cambridge Park Drive Suite 2500 | Cambridge, MA 02140 USA

References + Disclosures References: 1. Garcia-Montero AC, et al. Blood. 2006;108(7):2366-72 2. Jara-Acevedo M, et al. Mod Pathol. 2015;28(8):1138-49 3. Vaes M, et al. Front Med (Lausanne). 2017;4:110 4. Pardanani A. Am J Hematol. 2019;94(3):363-77 5. Gotlib J, et al. J Natl Compr Canc Netw. 2018;16(12):1500-37 6. NCCN: SM. J Natl Compr Canc Netw Version 2.2019:16, 2 7. Shomali W, Gotlib J. Hematology Am Soc Hematol Educ Program. 2018;2018(1):127-36 8. Jennings S, et al. J Allergy Clin Immunol Pract. 2014;2(1):70-6 9. Rossignol J, et al. F1000Res. 2019;8 10. Magliacane D, et al. Transl Med UniSa. 2014;8:65-74 11. RYDAPT [US Prescribing Information]. East Hanover, NJ: Novartis Pharmaceuticals; 2017 12. AVYAKIT [US Prescribing Information]. Cambridge, MA: Blueprint Medicines Corporation; 2021 13. Guarnieri et al, AACR Annual Meeting 2022; poster presentation:147 14. Das A, et al. Crit Rev Oncol Hematol. 2021 Jan;157:103186 15. Je Y, et al. Lancet Oncol. 2009 Oct;10(10):967-74 16. Coltoff A, Mascarenhas J., 2019. Disclosures: Miguel Piris Villaespesa: Research Funding: Novartis; Advisory Boards/Consulting/Honoraria: Novartis, Blueprint Medicines; Amanda Pilla, Ben Exter, Hina A. Jolin, Zamaneh Mikhak: Employees of Cogent Biosciences Funding: Study funded and managed by Cogent Biosciences 24























