UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2022
OR
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-38443
Cogent Biosciences, Inc.
(Exact name of registrant as specified in its charter)
Delaware | | 46-5308248 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification Number) |
200 Cambridge Park Drive, Suite 2500 Cambridge, Massachusetts | | 02140 |
(Address of principal executive offices) | | (Zip code) |
(617) 945-5576
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
Common Stock, $0.001 Par Value | | COGT | | The Nasdaq Global Select Market |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ☐ | | Accelerated filer | ☐ |
Non-accelerated filer | ☒ | | Smaller reporting company | ☒ |
| | | Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of May 6, 2022, there were 45,819,266 shares of the registrant’s Common Stock, $0.001 par value per share, outstanding.
FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q contains forward-looking statements, which reflect our current views with respect to, among other things, our operations and financial performance. All statements other than statements of historical facts contained in this Quarterly Report on Form 10-Q, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth are forward-looking statements. These statements involve known and unknown risks, uncertainties, and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “may,” “should,” “expects,” “might,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential,” “seek,” “would” or “continue,” or the negative of these terms or other similar expressions. The forward looking statements in this Quarterly Report on Form 10-Q are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. These forward-looking statements speak only as of the date of this Quarterly Report on Form 10-Q and are subject to a number of risks, uncertainties and assumptions described in Item 1A. “Risk Factors” in our most recent Annual Report on Form 10-K. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Some of the key factors that could cause actual results to differ from our expectations include:
| • | the potential impacts of raising additional capital, including dilution to our existing stockholders, restrictions on our operations or requirements that we relinquish rights to our technologies or product candidates; |
| • | business interruptions resulting from the coronavirus disease (“COVID-19”) outbreak or similar public health crises, which could cause a disruption to the development of our product candidates and adversely impact our business; |
| • | the success, cost, and duration of our product development activities and clinical trials; |
| • | the timing of our planned regulatory submissions to the FDA for our bezuclastinib product candidate, also known as CGT9486; |
| • | our ability to obtain and maintain regulatory approval for our bezuclastinib product candidate and any other product candidates we may develop, and any related restrictions, limitations, and/or warnings in the label of an approved product candidate; |
| • | the potential for our identified research priorities to advance our bezuclastinib product candidate or for our teams to discover and develop additional product candidates; |
| • | the ability to license additional intellectual property rights relating to our bezuclastinib product candidate or future product candidates from third-parties and to comply with our existing or future license agreements and/or collaboration agreements; |
| • | our ability to commercialize our bezuclastinib product candidate and future product candidates in light of the intellectual property rights of others; |
| • | our ability to obtain funding for our operations, including funding necessary to complete further discovery, development and commercialization of our existing and future product candidates; |
| • | the scalability and commercial viability of our manufacturing methods and processes; |
| • | the commercialization of our product candidates, if approved; |
| • | our ability to attract collaborators with development, regulatory, and commercialization expertise; |
| • | future agreements with third parties in connection with the commercialization of our product candidates and any other approved product; |
| • | the size and growth potential of the markets for our product candidates, and our ability to serve those markets; |
| • | the rate and degree of market acceptance of our product candidates; |
| • | the pricing and reimbursement of our product candidates, if approved; |
| • | regulatory developments in the United States and foreign countries; |
i
| • | our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately; |
| • | the development and success of competing therapies that are or may be under development in clinical trials or become available commercially; |
| • | our ability to attract and retain key scientific and management personnel; |
| • | the accuracy of our estimates regarding expenses, future revenue, capital requirements, and needs for additional financing; |
| • | our use of the proceeds from the private placements, sales of our preferred stock and public offerings of our common stock from time to time; and |
| • | our expectations regarding our ability to obtain and maintain intellectual property protection for our bezuclastinib product candidate and future product candidates. |
While we may elect to update these forward-looking statements at some point in the future, whether as a result of any new information, future events, or otherwise, we have no current intention of doing so except to the extent required by applicable law.
ii
Cogent Biosciences, Inc.
Table of Contents
iii
PART I—FINANCIAL INFORMATION
Item 1. Financial Statements (Unaudited)
COGENT BIOSCIENCES, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share amounts)
(unaudited)
| | March 31, | | | December 31, | |
| | 2022 | | | 2021 | |
Assets | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 191,047 | | | $ | 219,684 | |
Prepaid expenses and other current assets | | | 3,686 | | | | 2,949 | |
Total current assets | | | 194,733 | | | | 222,633 | |
Operating lease, right-of-use asset | | | 3,197 | | | | 2,771 | |
Property and equipment, net | | | 2,078 | | | | 1,706 | |
Restricted cash | | | 1,255 | | | | 1,255 | |
Other assets | | | 5,606 | | | | 3,727 | |
Total assets | | $ | 206,869 | | | $ | 232,092 | |
Liabilities and Stockholders’ Equity | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 4,015 | | | $ | 3,483 | |
Accrued expenses and other current liabilities | | | 8,409 | | | | 8,210 | |
CVR liability (Note 3) | | | 3,060 | | | | 3,060 | |
Operating lease liability | | | 2,395 | | | | 2,324 | |
Total current liabilities | | | 17,879 | | | | 17,077 | |
Operating lease liability, net of current portion | | | 1,127 | | | | 831 | |
Total liabilities | | | 19,006 | | | | 17,908 | |
Commitments and contingencies (Note 7) | | | | | | | | |
Stockholders’ equity: | | | | | | | | |
Preferred stock, $0.001 par value; 9,000,000 shares authorized; 0 shares issued or outstanding | | | — | | | | — | |
Series A non-voting convertible preferred stock, $0.001 par value; 1,000,000 shares authorized; 95,334 and 103,289 shares issued and outstanding at March 31, 2022 and December 31, 2021, respectively | | | 78,400 | | | | 85,400 | |
Common stock, $0.001 par value; 150,000,000 shares authorized; 45,819,266 shares and 43,805,922 shares issued and outstanding at March 31, 2022 and December 31, 2021, respectively | | | 46 | | | | 44 | |
Additional paid-in capital | | | 411,024 | | | | 399,713 | |
Accumulated deficit | | | (301,607 | ) | | | (270,973 | ) |
Total stockholders’ equity | | | 187,863 | | | | 214,184 | |
Total liabilities and stockholders’ equity | | $ | 206,869 | | | $ | 232,092 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
1
COGENT BIOSCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(in thousands, except share and per share amounts)
(unaudited)
| | Three Months Ended March 31, | |
| | 2022 | | | 2021 | |
Operating expenses: | | | | | | | | |
Research and development | | | 25,470 | | | | 8,213 | |
General and administrative | | | 5,948 | | | | 4,587 | |
Total operating expenses | | | 31,418 | | | | 12,800 | |
Loss from operations | | | (31,418 | ) | | | (12,800 | ) |
Other income: | | | | | | | | |
Interest income | | | 107 | | | | 125 | |
Other income | | | 677 | | | | 604 | |
Change in fair value of CVR liability | | | — | | | | 343 | |
Total other income | | | 784 | | | | 1,072 | |
Net loss and comprehensive loss | | $ | (30,634 | ) | | $ | (11,728 | ) |
Net loss per share attributable to common stockholders, basic and diluted | | $ | (0.68 | ) | | $ | (0.34 | ) |
Weighted average common shares outstanding, basic and diluted | | | 45,105,923 | | | | 34,879,296 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
2
COGENT BIOSCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands, except share amounts)
(unaudited)
| | Series A Non-Voting | | | | | | | | | | | | | | | | | | | | | |
| | Convertible Preferred Stock | | | Common Stock | | | Additional Paid-in | | | Accumulated | | | Total Stockholders’ | |
| | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | Equity | |
Balances at December 31, 2021 | | | 103,289 | | | $ | 85,400 | | | | 43,805,922 | | | $ | 44 | | | $ | 399,713 | | | $ | (270,973 | ) | | $ | 214,184 | |
Conversion of Series A non-voting preferred stock into common stock | | | (7,955 | ) | | | (7,000 | ) | | | 1,988,750 | | | | 2 | | | | 6,998 | | | | — | | | | — | |
Issuance of common stock under Employee Stock Purchase Plan | | | — | | | | — | | | | 18,995 | | | | — | | | | 129 | | | | — | | | | 129 | |
Issuance of common stock upon exercise of stock options | | | — | | | | — | | | | 5,599 | | | | — | | | | 9 | | | | — | | | | 9 | |
Stock-based compensation expense | | | — | | | | — | | | | — | | | | — | | | | 4,175 | | | | — | | | | 4,175 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (30,634 | ) | | | (30,634 | ) |
Balances at March 31, 2022 | | | 95,334 | | | $ | 78,400 | | | | 45,819,266 | | | $ | 46 | | | $ | 411,024 | | | $ | (301,607 | ) | | $ | 187,863 | |
| | Series A Non-Voting | | | | | | | | | | | | | | | | | | | | | |
| | Convertible Preferred Stock | | | Common Stock | | | Additional Paid-in | | | Accumulated | | | Total Stockholders’ | |
| | Shares | | | Amount | | | Shares | | | Amount | | | Capital | | | Deficit | | | Equity | |
Balances at December 31, 2020 | | | 132,244 | | | $ | 110,881 | | | | 32,347,905 | | | $ | 32 | | | $ | 322,454 | | | $ | (198,700 | ) | | $ | 234,667 | |
Conversion of Series A non-voting preferred stock into common stock | | | (18,409 | ) | | | (16,200 | ) | | | 4,602,250 | | | | 5 | | | | 16,195 | | | | — | | | $ | — | |
Issuance of common stock to settle CVR liability | | | — | | | | — | | | | 212,429 | | | | — | | | | 2,043 | | | | — | | | $ | 2,043 | |
Issuance of common stock for services | | | — | | | | — | | | | 31,683 | | | | — | | | | 260 | | | | — | | | $ | 260 | |
Stock-based compensation expense | | | — | | | | — | | | | — | | | | — | | | | 1,521 | | | | — | | | $ | 1,521 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | (11,728 | ) | | $ | (11,728 | ) |
Balances at March 31, 2021 | | | 113,835 | | | | 94,681 | | | | 37,194,267 | | | | 37 | | | | 342,473 | | | | (210,428 | ) | | $ | 226,763 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
3
COGENT BIOSCIENCES, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
(unaudited)
| | Three Months Ended March 31, | |
| | 2022 | | | 2021 | |
Cash flows from operating activities: | | | | | | | | |
Net loss | | $ | (30,634 | ) | | $ | (11,728 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | |
Depreciation and amortization expense | | | 69 | | | | 17 | |
Stock-based compensation expense | | | 4,175 | | | | 1,781 | |
Change in fair value of CVR liability | | | — | | | | (343 | ) |
Changes in operating assets and liabilities: | | | | | | | | |
Prepaid expenses and other current assets | | | (737 | ) | | | 1,392 | |
Operating lease, right-of-use asset | | | 491 | | | | 446 | |
Other assets | | | (1,879 | ) | | | — | |
Accounts payable | | | 532 | | | | (417 | ) |
Accrued expenses and other current liabilities | | | 199 | | | | (2,029 | ) |
Operating lease liability | | | (550 | ) | | | (485 | ) |
Net cash used in operating activities | | | (28,334 | ) | | | (11,366 | ) |
Cash flows from investing activities: | | | | | | | | |
Purchases of property and equipment | | | (441 | ) | | | — | |
Net cash used in investing activities | | | (441 | ) | | | — | |
Cash flows from financing activities: | | | | | | | | |
Proceeds from issuance of stock from employee stock purchase plan | | | 129 | | | | — | |
Proceeds from issuance of common stock upon stock option exercises | | | 9 | | | | — | |
Payment to CVR Holders | | | — | | | | (85 | ) |
Net cash (used in) provided by financing activities | | | 138 | | | | (85 | ) |
Net decrease in cash, cash equivalents and restricted cash | | | (28,637 | ) | | | (11,451 | ) |
Cash, cash equivalents and restricted cash at beginning of period | | | 220,939 | | | | 243,445 | |
Cash, cash equivalents and restricted cash at end of period | | $ | 192,302 | | | $ | 231,994 | |
Supplemental disclosure of cash flow information: | | | | | | | | |
Right-of-use assets obtained in exchange for new operating lease liabilities | | $ | 917 | | | $ | — | |
Supplemental disclosure of noncash investing and financing information: | | | | | | | | |
Conversion of Series A non-voting convertible preferred stock into common stock | | $ | 7,000 | | | $ | 16,200 | |
Issuance of shares in partial settlement of CVR liability | | $ | — | | | $ | 2,043 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
4
COGENT BIOSCIENCES, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(unaudited)
1. Nature of the Business and Basis of Presentation
Cogent Biosciences, Inc. (“Cogent” or the “Company”) is a biotechnology company focused on developing precision therapies for genetically defined diseases. Cogent’s approach is to design rational precision therapies that treat the underlying cause of disease and improve the lives of patients. Cogent’s most advanced program is bezuclastinib, also known as CGT9486, a highly selective tyrosine kinase inhibitor designed to potently inhibit the KIT D816V mutation as well as other mutations in KIT exon 17. In the vast majority of cases, KIT D816V is responsible for driving Systemic Mastocytosis (“SM”), a serious disease caused by unchecked proliferation of mast cells. Exon 17 mutations are also found in patients with advanced gastrointestinal stromal tumors (“GIST”), a type of cancer with strong dependence on oncogenic KIT signaling. Bezuclastinib is a highly selective and potent KIT inhibitor with the potential to provide a new treatment option for these patient populations. In addition to bezuclastinib, the Company’s research team is developing a portfolio of novel targeted therapies to help patients fighting serious, genetically driven diseases, initially targeting FGFR2 and ErbB2. The Company was incorporated in March 2014 under the laws of the State of Delaware. On October 2, 2020 the Company filed an amendment to its certificate of incorporation to change its name from Unum Therapeutics Inc. to Cogent Biosciences, Inc. The name change became effective on October 6, 2020. In connection with the name change, the Company’s common stock began trading under the ticker symbol “COGT” and the new CUSIP for the Company’s common stock is 19240Q 201.
The Company is subject to risks and uncertainties common to early-stage companies in the biotechnology industry, including, but not limited to, development by competitors of new technological innovations, dependence on key personnel, protection of proprietary technology, the impact of COVID-19, compliance with government regulations and the ability to secure additional capital to fund operations. Product candidates currently under development will require significant additional research and development efforts, including extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel and infrastructure and extensive compliance-reporting capabilities. Even if the Company’s drug development efforts are successful, it is uncertain when, if ever, the Company will realize revenue from product sales.
The accompanying condensed consolidated financial statements have been prepared on the basis of continuity of operations, realization of assets and the satisfaction of liabilities and commitments in the ordinary course of business. The Company has incurred recurring losses since inception, including a net loss of $30.6 million for the three months ended March 31, 2022. As of March 31, 2022, the Company had an accumulated deficit of $301.6 million. The Company expects to continue to generate operating losses in the foreseeable future. As of the issuance date of the interim condensed consolidated financial statements, the Company expects that its cash and cash equivalents will be sufficient to fund its operating expenses and capital expenditure requirements for at least the next 12 months from issuance of the condensed consolidated financial statements.
The Company expects that it will continue to incur significant expenses in connection with its ongoing business activities. The Company will need to seek additional funding through equity offerings, debt financings, collaborations, licensing arrangements and other marketing and distribution arrangements, partnerships, joint ventures, combinations or divestitures of one or more of its assets or businesses. The Company may not be able to obtain financing on acceptable terms, or at all, and the Company may not be able to enter into collaborative arrangements or divest its assets. The terms of any financing may adversely affect the holdings or the rights of the Company’s stockholders. Arrangements with collaborators or others may require the Company to relinquish rights to certain of its technologies or product candidates. If the Company is unable to obtain funding, the Company could be forced to delay, reduce or eliminate its research and development programs or commercialization efforts, which could adversely affect its business prospects, or the Company may be unable to continue operations.
The Company’s condensed consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”).
2. Summary of Significant Accounting Policies
Unaudited Interim Financial Information
The consolidated balance sheet at December 31, 2021 was derived from audited financial statements but does not include all disclosures required by GAAP. The accompanying unaudited condensed consolidated financial statements as of March 31, 2022 and for the three months ended March 31, 2022 and 2021 have been prepared by the Company pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) for interim financial statements. Certain information and footnote disclosures normally included in the financial statements prepared in accordance with GAAP have been condensed or omitted pursuant to such rules and regulations. These condensed consolidated financial statements should be read in conjunction with the Company’s audited consolidated financial statements and the notes thereto for the year ended December 31, 2021 included in the Company’s Annual Report on Form 10-K on file with the SEC. In the opinion of management, all adjustments, consisting only of normal recurring adjustments necessary
5
for a fair statement of the Company’s financial position as of March 31, 2022 and results of operations for the three months ended March 31, 2022 and 2021 and cash flows for the three months ended March 31, 2022 and 2021 have been made. The Company’s results of operations for the three months ended March 31, 2022 are not necessarily indicative of the results of operations that may be expected for the year ending December 31, 2022.
Principles of Consolidation
The accompanying condensed consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries, Mono, Inc. and Kiq Bio LLC. All intercompany accounts and transactions have been eliminated.
Use of Estimates
The preparation of condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements and the reported amounts of revenue and expenses during the reporting periods. Significant estimates and assumptions reflected in these condensed consolidated financial statements include, but are not limited to, the accrual of research and development expenses, the valuation of the CVR liability and the valuation of stock-based awards. The Company bases its estimates on historical experience, known trends and other market-specific or other relevant factors that it believes to be reasonable under the circumstances. On an ongoing basis, management evaluates its estimates, as there are changes in circumstances, facts and experience. Actual results may differ from those estimates or assumptions.
Recently Adopted Accounting Pronouncements
In August 2020, the FASB issued ASU 2020-06 Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40) related to the measurement and disclosure requirements for convertible instruments and contracts in an entity’s own equity. The pronouncement simplifies and adds disclosure requirements for the accounting and measurement of convertible instruments and the settlement assessment for contracts in an entity’s own equity. The Company adopted ASU 2020-06 on January 1, 2022. The adoption of this guidance did not have a material impact on the Company’s condensed consolidated financial statements.
3. Fair Value of Financial Assets and Liabilities
The following tables present the Company’s fair value hierarchy for its financial assets and liabilities, which are measured at fair value on a recurring basis (in thousands):
| | Fair Value Measurements at March 31, 2022 Using: | |
| | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Liabilities: | | | | | | | | | | | | | | | | |
CVR Liability | | $ | — | | | $ | — | | | $ | 3,060 | | | $ | 3,060 | |
Total Liabilities | | $ | — | | | $ | — | | | $ | 3,060 | | | $ | 3,060 | |
| | Fair Value Measurements at December 31, 2021 Using: | |
| | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
Liabilities: | | | | | | | | | | | | | | | | |
CVR Liability | | $ | — | | | $ | — | | | $ | 3,060 | | | $ | 3,060 | |
Total Liabilities | | $ | — | | | $ | — | | | $ | 3,060 | | | $ | 3,060 | |
On July 6, 2020, the Company issued a non-transferrable contingent value right (“CVR”), which was distributed to stockholders of record as of the close of business on July 6, 2020, and prior to the issuance of any shares to acquire Kiq Bio LLC (“Kiq”) or sold to the Private Investment in Public Equity (“PIPE”) investors. Holders of the CVR are entitled to receive common shares and/or cash payments from proceeds received by the Company, if any, related to the disposition of its legacy cell therapy assets for a period of three years from July 2020. In accordance with the terms of the CVR agreement, the payment to CVR holders will be made in shares or cash, depending on the timing of the receipt of the sales proceeds by the Company. For sales proceeds received by the Company prior to
6
December 31, 2020, CVR holders were entitled to receive payment in the form of common shares of the Company. For sales proceeds received by the Company after December 31, 2020 and prior to July 2023, CVR holders are entitled to receive payment in cash.
The Company classifies the CVR as a liability on its condensed consolidated balance sheet. The fair value of the CVR liability was determined using the probability weighted discounted cash flow method to estimate future cash flows associated with the sale of the legacy cell therapy assets, including the Bolt-on Chimeric Receptor (“BOXR”) technology and Autologous Cell Therapy Industrial Automation (“ACTIA”) technology (collectively, the “BOXR Platform, Antibody-Coupled T cell Receptor (“ACTR”) technology and other fixed assets based on assumptions at the date of the CVR issuance and each subsequent quarterly period end, less certain permitted deductions. For sales proceeds received by the Company prior to December 31, 2020, the number of common shares to be received by CVR holders was determined by dividing the proceeds received by the Company by the closing price of the Company’s common stock on July 6, 2020 of $8.80. The closing price of the Company’s common stock at each measurement date through February 2021 was used to determine the fair value of the share payments included in the CVR liability. The liability measured at the date of CVR issuance was recorded as a common stock dividend, returning capital to the legacy stockholders of record as of the close of business on July 6, 2020. Changes in fair value of the liability are recognized as a component of Other income (expense) in the condensed consolidated statement of operations and comprehensive loss. The CVR liability was valued based on significant inputs not observable in the market, which represents a Level 3 measurement within the fair value hierarchy. On August 28, 2020, the Company sold the BOXR Platform and subsequently sold additional fixed assets, triggering a payment to CVR holders. In November 2020, the Company issued 707,938 shares of common stock in partial settlement of the CVR liability. In February 2021, the Company issued an additional 212,429 shares of common stock and paid $0.1 million in partial settlement of the CVR liability. Any settlement of the remaining CVR liability will be a cash settlement.
The following table sets forth a summary of the changes in the fair value of the Company’s CVR liability (in thousands):
Balance at December 31, 2020 | | $ | 5,531 | |
Change in fair value | | | (343 | ) |
CVR settlement | | | (2,128 | ) |
Balance at December 31, 2021 | | $ | 3,060 | |
Change in fair value | | | 0 | |
CVR settlement | | | 0 | |
Balance at March 31, 2022 | | $ | 3,060 | |
During the three months ended March 31, 2022 and 2021, there were 0 transfers between Level 1, Level 2 and Level 3.
4. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consisted of the following (in thousands):
| | March 31, 2022 | | | December 31, 2021 | |
Accrued employee compensation and benefits | | $ | 1,454 | | | $ | 3,389 | |
Accrued external research and development expense | | | 4,181 | | | | 1,953 | |
Accrued external manufacturing costs | | | 427 | | | | 1,556 | |
Accrued professional and consulting services | | | 2,095 | | | | 1,077 | |
Other | | | 252 | | | | 235 | |
Total | | $ | 8,409 | | | $ | 8,210 | |
5. Preferred Stock, Series A Non-Voting Convertible Preferred Stock and Common Stock
The Company’s authorized capital stock consists of 150,000,000 shares of common stock, par value $0.001 per share, and 10,000,000 shares of preferred stock, par value $0.001 per share, 1,000,000 of which are designated as Series A Preferred Stock and 9,000,000 of which shares of preferred stock are undesignated.
7
Series A Non-Voting Convertible Preferred Stock
On July 6, 2020, the Company filed a Certificate of Designation of Preferences, Rights and Limitations of the Series A Non-Voting Convertible Preferred Stock (“Series A Preferred Stock”) with the Secretary of State of the State of Delaware (the “Certificate of Designation”) in connection with the Merger and the PIPE. The Certificate of Designation provides for the issuance of shares of Series A Preferred Stock, par value $0.001 per share.
Holders of Series A Preferred Stock are entitled to receive dividends on shares of Series A Preferred Stock equal, on an as-if-converted-to-common-stock basis, and in the same form as dividends actually paid on shares of the common stock. Except as otherwise required by law, the Series A Preferred Stock does not have voting rights. However, as long as any shares of Series A Preferred Stock are outstanding, the Company will not, without the affirmative vote of the holders of a majority of the then outstanding shares of the Series A Preferred Stock, (a) alter or change adversely the powers, preferences or rights given to the Series A Preferred Stock, (b) alter or amend the Certificate of Designation, (c) amend its certificate of incorporation or other charter documents in any manner that adversely affects any rights of the holders of Series A Preferred Stock, (d) increase the number of authorized shares of Series A Preferred Stock, (e) prior to the stockholder approval of the Conversion Proposal or at any time while at least 40% of the originally issued Series A Preferred Stock remains issued and outstanding, consummate a Fundamental Transaction (as defined in the Certificate of Designation) or (f) enter into any agreement with respect to any of the foregoing. The Series A Preferred Stock does not have a preference upon any liquidation, dissolution or winding-up of the Company.
Each share of Series A Preferred Stock is convertible at any time at the option of the holder thereof, into 250 shares of common stock, subject to certain limitations, including that a holder of Series A Preferred Stock is prohibited from converting shares of Series A Preferred Stock into shares of common stock if, as a result of such conversion, such holder, together with its affiliates, would beneficially own more than a specified percentage (to be established by the holder between 4.9% and 19.9%) of the total number of shares of common stock issued and outstanding immediately after giving effect to such conversion. Cumulatively, through March 31, 2022, 67,991 shares of Series A Preferred Stock, or 41.6% of the issued Series A Preferred Stock, have been converted into 16,997,750 shares of common stock. The 95,334 shares of Series A Preferred Stock outstanding as of March 31, 2022 are convertible into 23,833,500 shares of common stock.
No other classes of preferred stock have been designated and 0 other preferred shares have been issued or are outstanding as of March 31, 2022.
Common Stock
Each share of common stock entitles the holder to one vote on all matters submitted to a vote of the Company’s stockholders. Common stockholders are not entitled to receive dividends, unless declared by the board of directors. In the event of the Company’s liquidation, dissolution or winding up, holders of the Company’s common stock will be entitled to share ratably in all assets remaining after payment of all debts and other liabilities and any liquidation preference of any outstanding preferred stock. The shares to be issued by us in this offering will be, when issued and paid for, validly issued, fully paid and non-assessable.
On February 8, 2021, the Company filed a shelf registration statement on Form S-3 with the SEC. The shelf registration statement allows the Company to sell from time-to-time up to $200.0 million of common stock, preferred stock, debt securities, warrants or units comprised of any combination of these securities, for its own account in one or more offerings. The terms of any offering under the shelf registration statement will be established at the time of such offering and will be described in a prospectus supplement filed with the SEC prior to the completion of any such offering.
Additionally, on February 8, 2021, pursuant to the Form S-3, the Company entered into a Sales Agreement (the “SVB Sales Agreement”) with SVB Leerink LLC (“SVB Leerink”), pursuant to which the Company may issue and sell, from time to time, shares of its common stock having an aggregate offering price of up to $75.0 million through SVB Leerink as the sales agent. Cumulatively, the Company has sold 3,954,900 shares of common stock under the SVB Sales Agreement with offering prices ranging between $9.25 and $10.30 per share for net proceeds of approximately $38.0 million. NaN shares were sold under the SVB Sales Agreement in the three months ended March 31, 2022.
6. Stock-Based Compensation
2018 Stock Option and Incentive Plan
The Company’s 2018 Stock Option and Incentive Plan, (the “2018 Plan”), which became effective on March 27, 2018, provides for the grant of incentive stock options, nonqualified stock options, stock appreciation rights, restricted stock units, restricted stock awards, unrestricted stock awards, cash-based awards and dividend equivalent rights. The number of shares initially reserved for issuance under the 2018 Plan was 700,180. Additionally, the shares of common stock that remained available for issuance under the previously outstanding 2015 Stock Incentive Plan (the “2015 Plan”) became available under the 2018 Plan. The number of shares
8
reserved for the 2018 Plan automatically increases on each January 1 by 4% of the number of shares of the Company’s common stock outstanding on the immediately preceding December 31 or a lesser number of shares determined by the Company’s board of directors. The number of authorized shares reserved for issuance under the 2018 Plan was increased by 1,752,237 shares effective as of January 1, 2022. The shares of common stock underlying any awards that are forfeited, canceled, held back upon exercise or settlement of an award to satisfy the exercise price or tax withholding, repurchased or are otherwise terminated by the Company under the 2018 Plan or the 2015 Plan will be added back to the shares of common stock available for issuance under the 2018 Plan.
On June 16, 2021, at the Company’s 2021 annual stockholder meeting, the Company’s stockholders approved the amendment and restatement of the 2018 Stock Plan to increase the number of shares of common stock issuable under the 2018 Plan by 6,000,000 shares. Upon stockholder approval, in accordance with ASC 718- Compensation- Stock Compensation, a grant date was established for accounting purposes with respect to 3,402,768 options previously granted to employees and non-employee directors during the year ended December 31, 2021, which were subject to stockholder approval of the amendment and restatement of the 2018 Plan.
As of March 31, 2022, 1,518,076 shares of common stock remain available for issuance under the 2018 Plan.
Inducement Plan
On October 22, 2020, the board of directors adopted the Cogent Biosciences, Inc. 2020 Inducement Plan (the “Inducement Plan”). The board of directors also adopted a form of non-qualified stock option agreement for use with the Inducement Plan. A total of 3,750,000 shares of common stock have been reserved for issuance under the Inducement Plan, subject to adjustment for stock dividends, stock splits, or other changes in Cogent’s common stock or capital structure. On November 5, 2020, the Company filed a Registration on Form S-8 related to the 3,750,000 shares of its common stock reserved for issuance under the Inducement Plan. As of March 31, 2022, 728,995 shares of common stock remain available for issuance under the Inducement Plan.
2018 Employee Stock Purchase Plan
The Company’s 2018 Employee Stock Purchase Plan (the “ESPP”) became effective on March 28, 2018, at which time a total of 78,500 shares of common stock were reserved for issuance. In addition, the number of shares of common stock that may be issued under the ESPP automatically increases on each January 1 through January 1, 2027, by the least of (i) 125,000 shares of common stock, (ii) 1% of the number of shares of the Company’s common stock outstanding on the immediately preceding December 31 or (iii) such lesser number of shares as determined by the ESPP administrator. The number of authorized shares reserved for issuance under the ESPP was increased by 125,000 shares effective as of January 1, 2022. In January 2022, 18,995 shares were issued to employees under the ESPP. As of March 31, 2022, 442,924 shares remain available for issuance under the ESPP.
Stock-Based Compensation
The Company recorded stock-based compensation expense in the following expense categories of its condensed consolidated statements of operations and comprehensive loss (in thousands):
| | Three Months Ended March 31, | |
| | 2022 | | | 2021 | |
Research and development expenses | | $ | 1,924 | | | $ | 206 | |
General and administrative expenses | | | 2,251 | | | | 1,575 | |
Total | | $ | 4,175 | | | $ | 1,781 | |
As of March 31, 2022, total unrecognized compensation cost related to the unvested stock-based options was $54.6 million, which is expected to be recognized over a weighted average period of 3.19 years.
7. Commitments and Contingencies
Operating Leases
Corporate Headquarters- Cambridge, MA
The Company leases office and laboratory space in Cambridge, MA for its corporate headquarters under a non-cancelable operating lease (the “Cambridge Lease”) that expires in April 2023, with the Company’s option to extend for an additional five-year term. The Company has the right to terminate the lease in the event of the inability to use the space due to substantial damage while the lessor has the right to terminate the lease for tenant’s default of lease financial obligations. Per the terms of the Cambridge Lease, the Company does not have any residual value guarantees. This extension has not been considered in the determination of the lease liability
9
as the Company is not obligated to exercise its option and it is not reasonably certain that the option will be exercised. The lease payments include fixed lease payments that escalate over the term of the lease on an annual basis. The Cambridge Lease is a net lease, as the non-lease components (i.e. common area maintenance) are paid separately from rent based on actual costs incurred. Therefore, the non-lease component and related payments are not included in the right-of-use asset and liability and are reflected as an expense in the period incurred. The discount rate used in determining the lease liability represents the Company’s incremental borrowing rate as the rate implicit in the lease could not be readily determined.
On August 28, 2020, the Company amended the lease (the “Cambridge Lease Amendment”) resulting in increased annual rent payments. No other terms of the Cambridge Lease were changed. The Company determined that the lease modification did not grant an additional right of use and concluded that the modification was not a separate new lease, but rather that it should reassess and remeasure the right-of-use asset and lease liability on the effective date of the modification. The Company increased the right-of-use asset and operating lease liabilities by $0.9 million, respectively.
Concurrent with the Cambridge Lease Amendment and the BOXR sale, the Company entered into a sublease (the “Cambridge Sublease Agreement”) for a significant portion of the leased premises for the remaining term of the lease. Under the terms of the Cambridge Sublease Agreement, the sublessee leased approximately 70% of the facility and is responsible for the corresponding percentage of operating lease costs and variable lease costs. Variable lease costs include common area maintenance and other operating charges.
Research Facility- Boulder, CO
On July 6, 2021, the Company entered into a lease agreement (the “Original Lease”) pursuant to which the Company leases approximately 38,075 square feet (the “Initial Premises”) in Boulder, CO, which will include office and laboratory space. Subsequently, on March 29, 2022, the Company entered into the First Amendment to the lease agreement (the “First Amendment” and together with the Original Lease, the “Boulder Lease”) pursuant to which the Company leases approximately 6,582 square feet of additional office space on the second floor (the “Expansion Premises”).
The Company expects to incur net construction costs of $8.0 million to $10.0 million for the development of the Initial Premises at the Boulder location. Per the terms of the Original Lease, the landlord will contribute an aggregate of approximately $6.9 million toward the cost of landlord assets (the “Improvements”), as well as an additional amount of up to approximately $2.3 million in the form of a tenant improvement loan at an annual interest rate of 6%. Any monies borrowed under the tenant improvement loan are required to be repaid over the Boulder Lease term. Additionally, under the terms of the First Amendment, the landlord will provide an additional tenant improvement allowance (the “Additional Allowance”) of $0.6 million, of which $0.3 million will be used in the Initial Premises toward the cost of landlord assets. The remaining $0.3 million additional allowance is to be used for work to be performed in the Expansion Premises for the construction of lessee assets.
The Boulder Lease has an initial term of 12 years with the option to extend for three successive five-year terms. Boulder Lease payments will begin in June 2023 after an initial free rent period. Rent will be payable in equal monthly installments and subject to annual increases over the term. Additionally, the Company is responsible for reimbursing the landlord for its share of the building’s property taxes and operating expenses. The Boulder Lease is an operating lease. In connection with the Boulder Lease, the Company provided a cash security deposit to the landlord in an amount of $0.7 million which is recorded in Other Assets in the condensed consolidated balance sheet as of March 31, 2022.
The lease commencement date occurred for a portion of the Expansion Premises in March 2022 as the Company gained access to the space under the terms of the lease. The Company has recorded the right-of-use asset and lease liability for this lease component of $1.1 million as of the lease commencement date.
As of March 31, 2022, the Company has determined that it does not have control of the Initial Premises, as defined in ASC 842, during the construction period and as such, the accounting lease commencement date has not occurred as of March 31, 2022 and the Company will not record a right-of-use asset or lease liability for the Initial Premises until the accounting lease commencement date which is expected to be in the second quarter of 2022. The Company has determined the cost of Improvements during the construction period are lessor assets and considered a prepayment of lease under ASC 842. The Company has paid $1.1 million towards the construction of lessor assets, which is included in Other Assets in the condensed consolidated balance sheet as of March 31, 2022.
The elements of the lease expense, net of sublease income, were as follows (in thousands):
10
| | Three Months Ended March 31, 2022 | |
Lease cost | | | | |
Operating lease cost | | $ | 612 | |
Variable lease cost (1) | | | 261 | |
Sublease Income | | | (652 | ) |
Total lease cost | | $ | 221 | |
| | | | |
Other information | | | | |
Cash paid for amounts included in the measurement of lease liabilities | | $ | 873 | |
Weighted average remaining lease term | | | 4.25 | |
Weighted average discount rate | | | 8.93 | % |
(1) | The variable lease costs for the three months ended March 31, 2022 include common area maintenance and other operating charges. |
Future minimum lease payments under the Cambridge and Boulder operating leases commenced as of March 31, 2022 are as follows (in thousands):
Year Ending December 31, | | | | |
2022 (remaining 9 months) | | | 1,885 | |
2023 | | | 928 | |
2024 | | | 134 | |
2025 | | | 138 | |
2026 | | | 141 | |
Thereafter | | | 1,370 | |
Total future minimum lease payments | | | 4,596 | |
Less: imputed interest | | | 886 | |
Less: tenant improvement allowance receivable | | | 188 | |
Total operating lease liability | | $ | 3,522 | |
Included in the condensed consolidated balance sheet: | | | | |
Current operating lease liability | | $ | 2,395 | |
Operating lease liability, net of current portion | | | 1,127 | |
Total operating lease liability | | $ | 3,522 | |
Under the terms of the Cambridge Lease, the Company issued a $1.3 million letter of credit to the landlord as collateral for the leased facility. The underlying cash collateralizing this letter of credit has been classified as non-current restricted cash in the accompanying condensed consolidated balance sheets. This is a refundable deposit and not a lease payment. Under the terms of the Cambridge Sublease Agreement, the sublessee obtained a letter of credit for $1.3 million for the benefit of the Company. This has been excluded from the undiscounted cash flows above.
License Agreements
Plexxikon License Agreement
In July 2020, the Company obtained an exclusive, sublicensable, worldwide license (the “License Agreement”) to certain patents and other intellectual property rights to research, develop and commercialize bezuclastinib. Under the terms of the License Agreement, the Company is required to pay Plexxikon Inc. (“Plexxikon”) aggregate payments of up to $7.5 million upon the satisfaction of certain clinical milestones and up to $25.0 million upon the satisfaction of certain regulatory milestones. In April 2022, as a result of the Company’s review of the progression of the Peak study and discussions with Plexxikon, the first milestone clinical milestone was deemed to have been achieved, triggering payment of $2.5 million to Plexxikon in Q2 2022.
The Company is also required to pay Plexxikon tiered royalties ranging from a low-single digit percentage to a high-single digit percentage on annual net sales of products. These royalty obligations last on a product-by-product basis and country-by-country basis until the latest of (i) the date on which there is no validate claim of a licensed
11
Plexxikon patent covering a subject product in such country or (ii) the 10th anniversary of the date of the first commercial sale of the product in such country. In addition, if the Company sublicenses the rights under the License Agreement, the Company is required to pay a certain percentage of the sublicense revenue to Plexxikon ranging from mid-double digit percentages to mid-single digit percentages, depending on whether the sublicense is entered into prior to or after certain clinical trial events.
The license agreement will expire on a country-by-country and licensed product-by-licensed product basis until the later of the last to expire of the patents covering such licensed products or services or the 10-year anniversary of the date of first commercial sale of the licensed product in such country. The Company may terminate the license agreement within 30 days after written notice in the event of a material breach. The Company may also terminate the agreement upon written notice in the event of the Company’s bankruptcy, liquidation or insolvency. In addition, the Company has the right to terminate this agreement in its entirety at will upon 90 days’ advance written notice to Plexxikon.
Indemnification Agreements
In the ordinary course of business, the Company may provide indemnification of varying scope and terms to vendors, lessors, business partners and other parties with respect to certain matters including, but not limited to, losses arising out of breach of such agreements or from intellectual property infringement claims made by third parties. In addition, the Company has entered into indemnification agreements with members of its board of directors and its executive officers that will require the Company, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service as directors or officers. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is, in many cases, unlimited. To date, the Company has not incurred any material costs as a result of such indemnifications. The Company is not aware of any claims under indemnification arrangements that will have a material effect on its financial position, results of operations or cash flows, and it has not accrued any liabilities related to such obligations in its condensed consolidated financial statements as of March 31, 2022 or its consolidated financial statements as of December 31, 2021.
Legal Proceedings
The Company is not currently party to any material legal proceedings. At each reporting date, the Company evaluates whether or not a potential loss amount or a potential range of loss is probable and reasonably estimable under the provisions of the authoritative guidance that addresses accounting for contingencies. The Company expenses as incurred the costs related to such legal proceedings.
8. Net Loss Per Share
Basic and diluted net loss per common share was calculated as follows (in thousands, except share and per share amounts):
| | Three Months Ended March 31, | |
| | 2022 | | | 2021 | |
Numerator: | | | | | | | | |
Net loss | | $ | (30,634 | ) | | $ | (11,728 | ) |
Net loss attributable to common stockholders | | $ | (30,634 | ) | | $ | (11,728 | ) |
Denominator: | | | | | | | | |
Weighted average common shares outstanding, basic and diluted | | | 45,105,923 | | | | 34,879,296 | |
Net loss per common share, basic and diluted | | $ | (0.68 | ) | | $ | (0.34 | ) |
The Company’s potential dilutive securities have been excluded from the computation of diluted net loss per share as the effect would be anti-dilutive and would result in a reduction to net loss per share. The Company excluded the following potential common shares, presented based on amounts outstanding at each period end, from the computation of diluted net loss per share attributable to common stockholders for the periods indicated above because including them would have had an anti-dilutive effect:
| | March 31, | |
| | 2022 | | | 2021 | |
Stock options to purchase common stock | | | 12,101,396 | | | | 4,154,361 | |
Series A Preferred Stock | | | 23,833,500 | | | | 28,458,750 | |
| | | 35,934,896 | | | | 32,613,111 | |
12
9. Retirement Plan
The Company has a defined-contribution plan under Section 401(k) of the Internal Revenue Code (the “401(k) Plan”). The 401(k) Plan covers all employees who meet defined minimum age and service requirements and allows participants to defer a portion of their annual compensation on a pre-tax basis. The 401(k) Plan allows for discretionary matching contributions of 100% of the first 4% of elective contributions, which vest immediately. Contributions under the plan were approximately $0.1 million for the three months ended March 31, 2022 and 2021, respectively.
10. Subsequent Events
Waltham lease agreement
On March 19, 2022, the Company and Cimpress USA Incorporated (the “Cimpress”) entered into a sublease agreement (the “Waltham Sublease”) pursuant to which the Company will sublease approximately 17,749 square feet of office space in Waltham, MA (the “Subleased Space”). The Waltham Sublease became effective on May 5, 2022, upon receiving landlord consent. The Waltham Sublease has a term of four years and four months, commencing June 1, 2022 and expiring September 30, 2026. The Company will pay Cimpress base rent at an initial rate of $42.50 per square foot per year. Rent will be payable in equal monthly installments and subject to $1.00 per square foot annual increases over the term. Additionally, the Company is responsible for reimbursing Cimpress for the Company’s share of the building’s property taxes and operating expenses.
Registration on Form S-3 and ATM Sales Agreement
On May 6, 2022, the Company filed a shelf registration statement on Form S-3 with the SEC. The shelf registration statement allows the Company to sell from time-to-time up to $300 million of common stock, preferred stock, debt securities, warrants or units comprised of any combination of these securities, for its own account in one or more offerings. The terms of any offering under the shelf registration statement will be established at the time of such offering and will be described in a prospectus supplement filed with the SEC prior to the completion of any such offering.
Additionally, on May 6, 2022, pursuant to the Form S-3, the Company entered into a Sales Agreement (the “Sales Agreement”) with Guggenheim Securities, LLC (“Guggenheim Securities”), pursuant to which the Company may issue and sell, from time to time, shares of its common stock having an aggregate offering price of up to $75 million through Guggenheim Securities as the sales agent.
On May 6, 2022, the Company filed an Amendment to its February 8, 2021 S-3 Registration Statement to terminate the effectiveness of the registration statement and to remove from registration all securities registered but not sold under the registration statement.
The Company terminated the existing SVB Sales Agreement, effective as of May 5, 2022. The Company will 0t incur any termination penalties as a result of the termination of the SVB Sales Agreement. No further sales will be made pursuant to the SVB Sales Agreement.
13
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our condensed consolidated financial statements and related notes appearing elsewhere in this Quarterly Report on Form 10-Q and our Annual Report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this Quarterly Report on Form 10-Q, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including but not limited to those set forth under the caption “Risk Factors” in this Quarterly Report on Form 10-Q and in our Annual Report on Form 10-K for the year ended December 31, 2021.
Overview
We are a biotechnology company focused on developing precision therapies for genetically defined diseases. Our approach is to design rational precision therapies that treat the underlying cause of disease and improve the lives of patients. Our most advanced program is bezuclastinib (also known as CGT9486), a selective tyrosine kinase inhibitor designed to potently inhibit the KIT D816V mutation as well as other mutations in KIT exon 17. In the vast majority of cases, KIT D816V is responsible for driving Systemic Mastocytosis (“SM”), a serious disease caused by unchecked proliferation of mast cells. Exon 17 mutations are also found in patients with advanced gastrointestinal stromal tumors (“GIST”), a type of cancer with strong dependence on oncogenic KIT signaling. Bezuclastinib is a highly selective and potent KIT inhibitor with the potential to provide a new treatment option for these patient populations. In addition to bezuclastinib, the Cogent Research Team is developing a portfolio of novel targeted therapies to help patients fighting serious, genetically driven diseases initially targeting FGFR2 and ErbB2.
Pipeline
Our current pipeline is below:
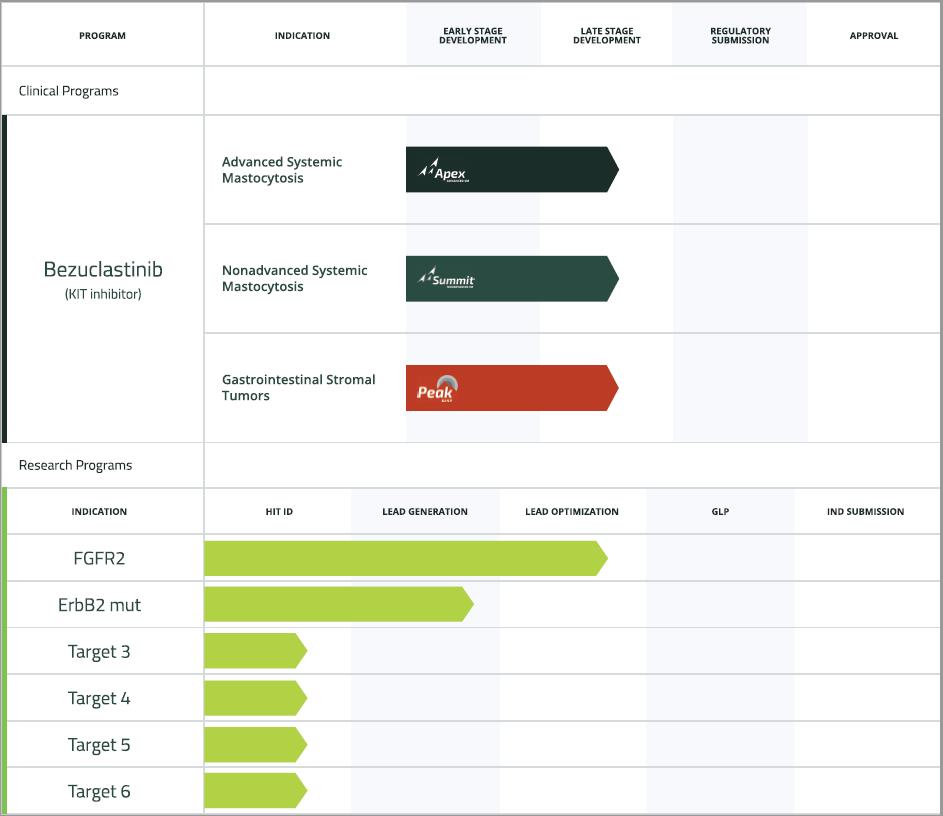
Bezuclastinib
Bezuclastinib has been studied in more than 50 advanced solid tumor and GIST patients in a Phase 1/2 clinical trial, with the vast majority of those patients living with advanced GIST. GIST is a disease frequently driven by KIT mutations, and resistance to currently available therapeutics is frequently associated with the emergence of other KIT mutations. Anti-tumor activity for bezuclastinib was
14
observed in both single agent and combination settings, including in combination with sunitinib, an approved treatment option for GIST patients. Clinical data from this trial have been published in the Journal of American Medical Association and have been presented at several scientific conferences, including most recently by Cogent at the 2020 annual Connective Tissue Oncology Society (“CTOS”) meeting, and previously by Plexxikon Inc. (“Plexxikon”), a member of the Daiichi Sankyo Group, at the 2018 annual American Society of Clinical Oncology meeting and the 2017 annual CTOS meeting. Within the group of 15 heavily pre-treated GIST patients who received the combination of bezuclastinib and sunitinib, and who had not received prior treatment with bezuclastinib, the confirmed objective response rate was twenty percent, including two partial responses and one complete response, while the estimated median progression free survival (“mPFS”) for this group was twelve months. Four subjects continued to receive bezuclastinib via individual patient INDs beyond the conclusion of the trial. In October 2021, we presented preclinical data in a virtual poster at the 2021 AACR-NCI-EORTC Virtual International Conference on Molecular Targets and Cancer Therapeutics that identified bezuclastinib as a differentiated, potent and selective KIT mutant inhibitor with unique selectivity for KIT D816V and minimal evidence of brain penetration that avoids targeting PDGFR isoforms. In April 2022, additional preclinical data presented at the 2022 American Associated for Cancer Research annual meeting (“AACR”) demonstrated that bezuclastinib potently inhibits A loop-mutations exquisitely selective against other closely related kinases, and differentiates bezuclastinib by its lack of brain penetration. These data support that bezuclastinib inhibits KIT downstream signaling and may drive tumor regressions at clinically achievable doses.
We initiated PEAK, a randomized open-label, global Phase 3 clinical trial in the fourth quarter of 2021. The PEAK study is designed to evaluate the safety, tolerability, and efficacy of bezuclastinib in combination with sunitinib compared to sunitinib alone in patients with locally advanced, unresectable or metastatic GIST who have received prior treatment with imatinib. The FDA has granted orphan drug designation to bezuclastinib for the treatment of GIST.
In addition to continuing the development of bezuclastinib in GIST patients, we are pursuing development of the compound in patients living with Advanced Systemic Mastocytosis (“AdvSM”) and Non-Advanced Systemic Mastocytosis (“Non-AdvSM”). The vast majority of AdvSM and Non-AdvSM patients have a KIT D816V mutation. Patients with AdvSM have a significantly diminished lifespan with a median survival of less than 3.5 years. For patients with Non-AdvSM, there are no available approved therapies, and while their lifespan is not impacted by the disease, these patients suffer from a poor quality of life and new treatment options are badly needed. Emerging clinical data for other kinase inhibitors with activity against KIT D816V have shown that the disease is highly sensitive to inhibition of the target. Bezuclastinib was specifically designed to selectively inhibit KIT mutations on exon 17, including KIT D816V, and we have expanded the clinical development program to include clinical trials in SM patients.
In the second quarter of 2021, we initiated APEX, a Phase 2 clinical study of bezuclastinib in patients with AdvSM. APEX is an open-label, global, multicenter study evaluating the safety, efficacy, pharmacokinetic, and pharmacodynamic profiles of bezuclastinib. We expect to report initial clinical data the European Hematology Association Annual Congress during the first half of 2022, including safety and tolerability data from patients across each dose cohort, as well as bezuclastinib’s impact on serum tryptase levels, a validated biomarker of mast cell activity.
In the fourth quarter of 2021, we initiated SUMMIT, a randomized, double-blind, placebo-controlled, global Phase 2 clinical trial. The study is designed to evaluate the safety and efficacy of bezuclastinib in patients with moderate to severe Indolent Systemic Mastocytosis or Smoldering Systemic Mastocytosis.
In November 2021, through a partnership with Serán Biosciences, we announced the development of an updated formulation of bezuclastinib. This formulation is expected to reduce the number of daily tablets, improving the overall patient experience, and is initially being used in our PEAK study.
Worldwide rights to develop and commercialize bezuclastinib are exclusively licensed from Plexxikon. Under the terms of the license agreement, Plexxikon received an upfront payment and is eligible for additional development milestones of up to $7.5 million upon the satisfaction of certain clinical milestones and up to $25.0 million upon the satisfaction of certain regulatory milestones. In April 2022, as a result of our review of the progression of the Peak study and discussions with Plexxikon, the first clinical milestone was deemed to have been achieved, triggering a payment of $2.5 million to Plexxikon in Q2 2022.
Patents protecting bezuclastinib include composition of matter claims which have issued in the US and other key territories and provide exclusivity through 2033 and potentially beyond through patent term extensions.
Research programs
During the second quarter of 2021, we announced the formation of the Cogent Research Team, a highly experienced discovery and research group. Based in Boulder, Colorado, the Cogent Research Team is focused on pioneering best-in-class, small molecule therapeutics to expand our pipeline and deliver novel precision therapies for patients living with unmet medical needs. Our research team is building a pipeline of small molecule inhibitors, with our first efforts aimed toward targeting currently undrugged mutations in FGFR. FGFR mutations are well-established oncogenic drivers in multiple diseases, but approved medicines fail to capture the full landscape of FGFR altered tumor types, with FGFR1-mediated hyperphosphatemia serving as the most common dose-limiting toxicity for pan-FGFR inhibitors. Based on preclinical data presented at AACR in April 2022, our FGFR program has the potential to both spare
15
FGFR1 inhibition, avoiding related toxicity, as well as potently cover the relevant molecular brake and gatekeeper mutations associated with this target. Additionally, we see an opportunity to provide a more robust molecular response compared to existing therapies. We are advancing a potent, selective FGFR2 inhibitor program toward candidate selection later this year and expect to file this first internally developed Investigational New Drug application (IND) in the second half of 2023. We are also advancing our novel, non-exon 20 ErbB2 mutant program, which is focused on actionable and underserved mutations in a variety of solid tumor indications.
Since our inception in 2014, we have focused significant efforts and financial resources on establishing and protecting our intellectual property portfolio, conducting research and development of our product candidates, manufacturing drug product material for use in preclinical studies and clinical trials, staffing our company, and raising capital. We do not have any products approved for sale and have not generated any revenue from product sales. Our ability to generate product revenue sufficient to achieve profitability will depend heavily on the successful development and eventual commercialization of one or more of our product candidates. Our net losses were $30.6 million for the three months ended March 31, 2022 compared to net losses of $11.7 million for the three months ended March 31, 2021. As of March 31, 2022, we had an accumulated deficit of $301.6 million. We expect to continue to incur significant expenses and operating losses for at least the next several years. We expect that our expenses and capital requirements will increase substantially in connection with our ongoing activities, particularly if and as we:
| • | initiate and increase enrollment for our existing and planned clinical trials for our product candidates; |
| • | continue to discover and develop additional product candidates, including through the creation of our research team in Boulder, CO, and build out our lab facility in Boulder, CO; |
| • | acquire or in-license other product candidates and technologies; |
| • | maintain, expand, and protect our intellectual property portfolio; |
| • | hire additional research, clinical, scientific, and commercial personnel; |
| • | establish a commercial manufacturing source and secure supply chain capacity sufficient to provide commercial quantities of any product candidates for which we may obtain regulatory approval; |
| • | seek regulatory approvals for any product candidates that successfully complete clinical trials; |
| • | establish a sales, marketing, and distribution infrastructure to commercialize any products for which we may obtain regulatory approval; and |
| • | add operational, financial, and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts. |
We will not generate revenue from product sales unless and until we successfully complete clinical development and obtain regulatory approval for our product candidates. If we obtain regulatory approval for any of our product candidates and do not enter into a commercialization partnership, we expect to incur significant expenses related to developing our internal commercialization capability to support product sales, marketing, and distribution.
As a result, we will need substantial additional funding to support our continuing operations and pursue our growth strategy. Until such time as we can generate significant revenue from product sales, if ever, we expect to finance our operations through a combination of equity offerings, debt financings, collaborations, strategic alliances, and marketing, distribution, or licensing arrangements. We may be unable to raise additional funds or enter into such other agreements or arrangements when needed on favorable terms, or at all. If we fail to raise capital or enter into such agreements as, and when, needed, we may have to significantly delay, scale back, or discontinue the development and commercialization of one or more of our product candidates.
Because of the numerous risks and uncertainties associated with pharmaceutical product development, we are unable to accurately predict the timing or amount of increased expenses or when, or if, we will be able to achieve or maintain profitability. Even if we are able to generate product sales, we may not become profitable. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce or terminate our operations.
As of March 31, 2022, we had cash and cash equivalents of $191.0 million. Based on our current plans, we expect that our current cash and cash equivalents will be sufficient to fund our operating expenses and capital expenditure requirements into 2024.
The COVID-19 Pandemic
In March 2020, the World Health Organization declared the outbreak of a novel strain of coronavirus, or COVID-19, as a pandemic, which has spread throughout the United States and worldwide. We could be materially and adversely affected by the risks, or the public perception of the risks, related to an epidemic, pandemic, outbreak, or other public health crisis, such as the recent outbreak of COVID-19 or variants thereof. We continue to monitor the pandemic and have taken steps to identify and mitigate the adverse impacts on, and risks to, our business posed by its spread and actions taken by governmental and health authorities to address the COVID-19
16
pandemic. The spread of COVID-19 has caused us to modify our business practices, including implementing a work-from-home policy for all employees who are able to perform their duties remotely and restricting all nonessential travel, and we expect to continue to take actions as may be required or recommended by government authorities or as we determine are in the best interests of our employees, the patients we serve and other business partners in light of COVID-19. Given the fluidity of the COVID-19 pandemic however, we do not yet know the full extent of the potential impact of COVID-19 on our business operations. The ultimate extent of the impact of any epidemic, pandemic, outbreak, or other public health crisis on our business, financial condition and results of operations will depend on future developments, which are highly uncertain and cannot be predicted, including new information that may emerge concerning the severity of such epidemic, pandemic, outbreak, or other public health crisis and actions taken to contain or prevent the further spread, among others. Accordingly, we cannot predict with certainty the extent to which our business, financial condition and results of operations will be affected. We will continue to work diligently with our partners and stakeholders to continue advancing our product candidate under regulatory review as well as in our clinical studies to the extent safe to do so for patients, caregivers and healthcare practitioners, and ensuring the continuity of our manufacturing and supply chain.
Components of Our Results of Operations
Operating Expenses
Research and Development Expenses
Research and development expenses consist primarily of costs incurred for our research activities, including our drug discovery efforts, and the development of our product candidates, which include:
| • | expenses incurred in connection with the discovery, preclinical and clinical development of our product candidates, including under agreements with third parties, such as consultants, contractors and contract research organizations (“CROs”); |
| • | the cost of manufacturing drug products for use in our preclinical studies and clinical trials, including under agreements with third parties, such as consultants, contractors and contract manufacturing organizations (“CMOs”); |
| • | employee-related expenses, including salaries, related benefits and stock-based compensation expense for employees engaged in research and development functions; |
| • | laboratory supplies and animal care; |
| • | facilities, depreciation and other expenses, which include direct and allocated expenses for rent and maintenance of facilities and insurance; and |
| • | payments made under third-party licensing agreements. |
We expense research and development costs as incurred. Advance payments that we make for goods or services to be received in the future for use in research and development activities are recorded as prepaid expenses. The prepaid amounts are expensed as the related goods are delivered or the services are performed.
Certain of our direct research and development expenses are tracked on a program-by-program basis and consist of costs, such as fees paid to consultants, contractors, CMOs, and CROs in connection with our discovery, preclinical and clinical development activities. We do not allocate employee costs, costs associated with the manufacture of bezuclastinib, costs associated with our discovery efforts, laboratory supplies, and facilities, including depreciation or other indirect costs, to specific product development programs because these costs are deployed across multiple product development programs and, as such, are not separately classified.
Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect that our research and development expenses will increase substantially in connection with our planned clinical and preclinical development activities in the near term and in the future. At this time, we cannot reasonably estimate or know the nature, timing, and costs of the efforts that will be necessary to complete the preclinical and clinical development of any of our product candidates. The successful development and commercialization of our product candidates is highly uncertain. This is due to the numerous risks and uncertainties associated with product development and commercialization, including the following:
| • | the timing and progress of our preclinical and clinical development activities; |
| • | the number and scope of preclinical and clinical programs we decide to pursue; |
| • | the progress of the development efforts of parties with whom we have entered, or may enter, into collaboration arrangements; |
| • | our ability to maintain our current research and development programs and to establish new ones; |
17
| • | our ability to establish new licensing or collaboration arrangements; |
| • | the future productivity of our research team in Boulder, CO and its ability to discover new product candidates and build our pipeline; |
| • | the successful completion of clinical trials with safety, tolerability, and efficacy profiles that are satisfactory to the FDA or any comparable foreign regulatory authority; |
| • | the receipt of regulatory approvals from applicable regulatory authorities; |
| • | the success in establishing and operating a manufacturing facility, or securing manufacturing supply through relationships with third parties; |
| • | our ability to obtain and maintain patents, trade secret protection, and regulatory exclusivity, both in the United States and internationally; |
| • | our ability to protect our rights in our intellectual property portfolio; |
| • | the commercialization of our product candidates, if and when approved; |
| • | the acceptance of our product candidates, if approved, by patients, the medical community, and third-party payors; |
| • | competition with other products; and |
| • | a continued acceptable safety profile of our therapies following approval. |
A change in the outcome of any of these variables with respect to the development of any of our product candidates could significantly change the costs and timing associated with the development of that product candidate. We may never succeed in obtaining regulatory approval for any of our product candidates.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and related costs, including stock-based compensation, for personnel in executive, finance, and administrative functions. General and administrative expenses also include direct and allocated facility-related costs as well as professional fees for legal, patent, consulting, investor and public relations, accounting, and audit services. We anticipate that our general and administrative expenses will increase in the future as a result of the costs associated with the expansion of operations to support our on-going discovery, preclinical and clinical activities.
Other Income
Interest Income
Interest income consists of interest earned on our cash equivalents balances. Our interest income has not been significant due to low interest rates on invested balances.
Other Income
Other income consists of miscellaneous income and expense unrelated to our core operations, primarily income from subleasing a portion of our headquarters facilities.
Change in Fair Value of the CVR liability
This consists of changes in the fair value of the CVR liability.
Income Taxes
Since our inception, we have not recorded any current or deferred tax benefit for the net losses we have incurred in each year or for our research and development tax credits generated, as we believe, based upon the weight of available evidence, that it is more likely than not that our net operating loss carryforwards and tax credits will not be realized. Accordingly, a full valuation allowance has been established against the deferred tax assets as of December 31, 2021. We reevaluate the utilization of net operating loss carryforwards and tax credits at each reporting period. As of December 31, 2021, we had U.S. federal and state net operating loss carryforwards of $128.8 million and $47.1 million, respectively, which may be available to offset future income tax liabilities and begin to expire in 2035. Of the federal net operating loss carryforwards at December 31, 2021, $125.5 million is available to be carried forward indefinitely but
18
can only offset 80% of taxable income per year. As of December 31, 2021, we also had U.S. federal and state research and development tax credit carryforwards of $3.1 million and $0.8 million, respectively, which may be available to offset future income tax liabilities and begin to expire in 2040 and 2035, respectively.
Utilization of the U.S. federal and state net operating loss carryforwards and research and development tax credit carryforwards may be subject to annual limitation under Section 382 of the Internal Revenue Code of 1986, and corresponding provisions of state law, due to ownership changes that have occurred previously or that could occur in the future. These ownership changes may limit the amount of carryforwards that can be utilized annually to offset future taxable income. In general, an ownership change, as defined by Section 382, results from transactions increasing the ownership of certain stockholders or public groups in the stock of a corporation by more than 50% over a three-year period.
We have recorded a full valuation allowance against our net deferred tax assets at each balance sheet date.
Results of Operations
Comparison of the Three Months Ended March 31, 2022 and 2021
The following table summarizes our results of operations for the three months ended March 31, 2022 and 2021:
| | Three Months Ended March 31, | | | | | |
| | 2022 | | | 2021 | | | Change | |
| | (in thousands) | | | | | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | | 25,470 | | | | 8,213 | | | | 17,257 | |
General and administrative | | | 5,948 | | | | 4,587 | | | | 1,361 | |
Total operating expenses | | | 31,418 | | | | 12,800 | | | | 18,618 | |
Loss from operations | | | (31,418 | ) | | | (12,800 | ) | | | (18,618 | ) |
Other income: | | | | | | | | | | | | |
Interest income | | | 107 | | | | 125 | | | | (18 | ) |
Other income | | | 677 | | | | 604 | | | | 73 | |
Change in fair value of CVR liability | | | — | | | | 343 | | | | (343 | ) |
Total other income | | | 784 | | | | 1,072 | | | | (288 | ) |
Net loss | | $ | (30,634 | ) | | $ | (11,728 | ) | | $ | (18,906 | ) |
Research and Development Expenses
The following table summarizes our research and development expenses for the three months ended March 31, 2022 and 2021:
| | Three Months Ended March 31, | | | | | |
| | 2022 | | | 2021 | | | Change | |
| | (in thousands) | | | | | |
Direct external research and development expenses: | | | | | | | | | | | | |
Bezuclastinib | | $ | 13,460 | | | $ | 5,847 | | | | 7,613 | |
Preclinical research and discovery | | | 2,369 | | | | — | | | | 2,369 | |
Unallocated expenses: | | | | | | | | | | | | |
Personnel related (including stock-based compensation) | | | 7,651 | | | | 1,469 | | | | 6,182 | |
Laboratory supplies, facility related and other | | | 1,990 | | | | 897 | | | | 1,093 | |
Total research and development expenses | | $ | 25,470 | | | $ | 8,213 | | | $ | 17,257 | |
Total research and development expense increased by $17.3 million for the three months ended March 31, 2022 compared to the three months ended March 31, 2021 and the increase was driven by higher external research and development costs associated with the manufacture and development of bezuclastinib, including costs associated with the APEX, SUMMIT and PEAK, and the development of the research pipeline. Additionally, there was an increase in unallocated expenses driven by higher personnel costs due to an increase in headcount and increased lab supplies and other facilities costs to support the build-out of the research team.
19
General and Administrative Expenses
General and administrative expenses for the three months ended March 31, 2022 were $5.9 million, compared to $4.6 million for the three months ended March 31, 2021. The increase in general and administrative expenses was primarily due to higher personnel costs driven by an increase in headcount.
Interest Income
Interest income for the three months ended March 31, 2022 and March 31, 2021 was $0.1 million, respectively. The impact of lower average invested balances in the current year was partially offset by higher interest rates in the current year compared to the prior period.
Other Income
Other income, net was $0.7 million in the three months ended March 31, 2022, compared to $0.6 million for the three months ended March 31, 2021. Other income represents sublease income recognized resulting from the sublease of a portion of our leased office space.
Change in Fair Value of CVR Liability
There was no change in fair value of the CVR liability for the three months ended March 31, 2022. Any settlement of the remaining liability will be a cash settlement.
Liquidity and Capital Resources
We have incurred certain costs related to the COVID-19 outbreak as a result of taking necessary precautions for essential personnel to operate safely both in person as well as remotely. Costs incurred include items like incremental payroll costs, consulting support, IT infrastructure and facilities related costs. The estimated impact of COVID-19 is currently unknown. The final impact may vary based on the duration of the current social and economic conditions. To the extent the COVID-19 pandemic continues, it may materially impact our financial condition, liquidity or results of operations in the future. We do not currently believe the accumulated costs will present a material impact to our financial liquidity or position.
Since our inception, we have incurred significant operating losses. We have generated limited revenue to date from funding arrangements with our former collaboration partner. We have not yet commercialized any of our product candidates and we do not expect to generate revenue from sales of any product candidates for several years, if at all. We have historically funded our operations primarily through the public offering and private placement of our securities and consideration received from our collaborative agreements.
On July 6, 2020, the Company completed its asset acquisition of Kiq Bio LLC (“Kiq”) (the “Kiq Acquisition”), in accordance with the terms of the Agreement and Plan of Merger (the “Merger Agreement”), signed and closed on July 6, 2020. Under the terms of the Merger Agreement, at the closing of the Merger, the Company issued the securityholders of Kiq 1,558,975 shares of common stock and 44,687 shares of Series A Non-Voting Convertible Preferred Stock (“Series A Preferred Stock”). On July 9, 2020, the Company completed a Private Investment in Public Equity (“PIPE”) of 118,638 Series A Non-Voting Convertible Preferred Stock to new and existing investors in exchange for net proceeds of $98.9 million, after deducting commissions and offering costs. Cumulatively, through March 31, 2022, 67,991 shares of Series A Preferred Stock, or 41.6% of the issued Series A Preferred Stock, have been converted into 16,997,750 shares of common stock. The 95,334 shares of Series A Preferred Stock outstanding as of March 31, 2022 are convertible into 23,833,500 shares of common stock, for total common shares outstanding, on an as-converted basis, of 69,652,766.
On February 8, 2021, we filed a shelf registration statement on Form S-3 with the SEC. The shelf registration statement allows us to sell from time-to-time up to $200.0 million of common stock, preferred stock, debt securities, warrants or units comprised of any combination of these securities, for our own account in one or more offerings. The terms of any offering under the shelf registration statement will be established at the time of such offering and will be described in a prospectus supplement filed with the SEC prior to the completion of any such offering.
20
Additionally, on February 8, 2021, pursuant to the Form S-3, we entered into a Sales Agreement (the “SVB Sales Agreement”) with SVB Leerink LLC (“SVB Leerink”), pursuant to which we may issue and sell, from time to time, shares of our common stock having an aggregate offering price of up to $75.0 million through SVB Leerink as the sales agent. Cumulatively, through March 31, 2022, 3,954,900 shares have been sold under the SVB Sales Agreement for net proceeds of approximately $38.0 million. During the three months ended March 31, 2022, no shares have been sold under the SVB Sales Agreement.
On May 6, 2022, we filed a shelf registration statement on Form S-3 with the SEC. The shelf registration statement allows us to sell from time-to-time up to $300 million of common stock, preferred stock, debt securities, warrants or units comprised of any combination of these securities, for our own account in one or more offerings. The terms of any offering under the shelf registration statement will be established at the time of such offering and will be described in a prospectus supplement filed with the SEC prior to the completion of any such offering.
Additionally, on May 6, 2022, pursuant to the Form S-3, we entered into a Sales Agreement (the “Sales Agreement”) with Guggenheim Securities, LLC (“Guggenheim Securities”), pursuant to which we may issue and sell, from time to time, shares of our common stock having an aggregate offering price of up to $75 million through Guggenheim Securities as the sales agent.
On May 6, 2022, the Company filed an Amendment to its February 8, 2021 S-3 Registration Statement to terminate the effectiveness of the registration statement and to remove from registration all securities registered but not sold under the registration statement.
We terminated our existing SVB Sales Agreement, effective as of May 5, 2022. We will not incur any termination penalties as a result of the termination of the SVB Sales Agreement. No further sales will be made pursuant to the SVB Sales Agreement.
As of March 31, 2022, we had cash and cash equivalents of $191.0 million, which we believe will be sufficient to fund our operating expenses and capital expenditure requirements into 2024.
Cash Flows
The following table summarizes our sources and uses of cash for each of the periods presented:
| | Three Months Ended March 31, | |
| | 2022 | | | 2021 | |
| | (in thousands) | |
Cash used in operating activities | | $ | (28,334 | ) | | $ | (11,366 | ) |
Cash used in investing activities | | | (441 | ) | | | — | |
Net cash (used in) provided by financing activities | | | 138 | | | | (85 | ) |
Net decrease in cash, cash equivalents and restricted cash | | $ | (28,637 | ) | | $ | (11,451 | ) |
Operating Activities
During the three months ended March 31, 2022, operating activities used $28.3 million of cash, primarily resulting from our net loss of $30.6 million and changes in our operating assets and liabilities of $1.9 million, partially offset by net cash provided by net noncash charges of $4.2 million. Net cash used in changes in our operating assets and liabilities for the three months ended March 31, 2022 consisted primarily of a $1.9 million increase in other assets, a $0.7 million increase in prepaid expenses and other current assets, and a $0.6 million decrease in the operating lease liability, partially offset by a $0.7 million increase in accounts payable and accrued expenses and other current liabilities and a $0.5 million decrease in the right-of-use asset.
During the three months ended March 31, 2021, operating activities used $11.4 million of cash, primarily resulting from our net loss of $11.7 million and from net cash used by changes in our operating assets and liabilities of $1.1 million, partially offset by net non-cash charges of $1.4 million. Net cash used by changes in our operating assets and liabilities for the three months ended March 31, 2021 consisted primarily of a $2.4 million decrease in accounts payable and accrued expenses and other current liabilities, and a $0.5 million decrease in operating lease liabilities, partially offset by a $1.4 million decrease in prepaid expenses and other current assets and a $0.5 million decrease in the right-of-use asset.
Investing Activities
During the three months ended March 31, 2022, net cash used in investing activities was $0.4 million, consisting of purchases of property and equipment. There were no investing activities for the three months ended March 31, 2021.
21
Financing Activities
During the three months ended March 31, 2022, net cash provided by financing activities was $0.1 million, which consisted of the proceeds from the issuance of common stock under the Employee Stock Purchase Plan and proceeds from the issuance of common stock upon stock option exercises.
During the three months ended March 31, 2021, net cash used by financing activities was $0.1 million which consisted of the partial settlement of the CVR obligation.
Funding Requirements
We expect our expenses to increase in connection with our ongoing activities, particularly as we advance the clinical development of our current and any future product candidates and conduct additional research, development and preclinical activities. The timing and amount of our operating expenditures will depend largely on:
| • | the initiation, progress, timing, and completion of preclinical studies and clinical trials for our current and future potential product candidates, including the impact of COVID-19 on our ongoing and planned research and development efforts; |
| • | any delay in our regulatory filings for our product candidates and any adverse development or perceived adverse development with respect to the applicable regulatory authority’s review of such filings, including without limitation the FDA’s issuance of a “refusal to file” letter or a request for additional information; |
| • | adverse results or delays in clinical trials; |
| • | our decision to initiate a clinical trial, not to initiate a clinical trial, or to terminate an existing clinical trial; |
| • | adverse regulatory decisions, including failure to receive regulatory approval of our product candidates; |
| • | changes in laws or regulations applicable to our products, including but not limited to clinical trial requirements for approvals; |
| • | adverse developments concerning our manufacturers; |
| • | our inability to obtain adequate product supply for any approved product or our inability to do so at acceptable prices; |
| • | our inability to establish collaborations, if desired or needed; |
| • | our failure to commercialize our product candidates; |
| • | the cost and timing of completion of the build out of our new office and laboratory facility in Boulder, CO; |
| • | additions or departures of key scientific or management personnel; |
| • | unanticipated serious safety concerns related to the use of our product candidates; and |
| • | the impact of COVID-19 on the operations of key governmental agencies, such as the FDA, which may delay the development of our current product candidates or any future product candidates. |
Based on our current plans, we believe that our existing cash and cash equivalents of $191.0 million as of March 31, 2022 will enable us to fund our operating expenses and capital expenditure requirements into 2024. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect. The Company will require additional funding to complete the critical activities planned to support ongoing research and development programs.
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our operations through a combination of equity offerings, debt financings, collaborations, strategic alliances, and marketing, distribution, or licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, existing ownership interests will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of common stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making acquisitions or capital expenditures, or declaring dividends. If we raise additional funds through collaborations, strategic alliances, or marketing, distribution, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or drug candidates, or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, limit, reduce, or terminate our research, product development, or future commercialization efforts, or grant rights to develop and market drug candidates that we would otherwise prefer to develop and market ourselves.
22
Critical Accounting Estimates
There have been no material changes in our critical accounting policies during the three months ended March 31, 2022, as compared to those described under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Significant Judgments and Estimates” in our Annual Report on Form 10-K.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
Recently Issued Accounting Pronouncements
A description of recently issued accounting pronouncements that may potentially impact our financial position and results of operations is disclosed in Note 2 to our condensed consolidated financial statements appearing elsewhere in this Quarterly Report.
Emerging Growth Company Status
The Jumpstart Our Business Startups Act of 2012 (“JOBS Act”) permits an “emerging growth company” such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies until those standards would otherwise apply to private companies. We have irrevocably elected to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards when they are required to be adopted by public companies that are not emerging growth companies.
23
Item 3. Quantitative and Qualitative Disclosures about Market Risk.
We are a smaller reporting company, as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended, for this reporting period and are not required to provide the information required under this item.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and President and our Chief Financial Officer (our principal executive officer and principal financial officer, respectively), evaluated the effectiveness of our disclosure controls and procedures as of March 31, 2022. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of March 31, 2022, our Chief Executive Officer and our Chief Financial Officer concluded that, as of such date, our disclosure controls and procedures were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
No change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) occurred during the three months ended March 31, 2022 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
24
PART II—OTHER INFORMATION
Item 1. Legal Proceedings.
We are not currently subject to any material legal proceedings. From time to time, we may be subject to various legal proceedings and claims that arise in the ordinary course of our business activities. Regardless of the outcome, litigation can have a material adverse effect on us because of defense and settlement costs, diversion of management resources, and other factors.
Item 1A. Risk Factors.
There have been no material changes from our risk factors described in our Annual Report on Form 10-K for the year ended December 31, 2021, as filed with the SEC on March 15, 2022. The risks described in our Form 10-K are not the only risks facing our Company. Additional risk and uncertainties not currently known to us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition and/or operating results.
Item 2. Recent Sales of Unregistered Securities and Use of Proceeds
None.
Item 3. Defaults Upon Senior Securities
Not applicable.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
None.
25
Item 6. Exhibits.
† | The certifications attached as Exhibits 32.1 and 32.2 that accompany this Quarterly Report on Form 10-Q are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of Cogent Biosciences, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Quarterly Report on Form 10-Q, irrespective of any general incorporation language contained in such filing. |
26
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| COGENT BIOSCIENCES, INC. |
| | |
Date: May 10, 2022 | By: | /s/ Andrew Robbins |
| | Andrew Robbins |
| | President and Chief Executive Officer |
| | (Principal Executive Officer) |
| | |
Date: May 10, 2022 | By: | /s/ John Green |
| | John Green |
| | Chief Financial Officer |
| | (Principal Accounting and Financial Officer) |
27
