
VIRTUAL INVESTOR EVENT EHA 2022 11 June 2022 Exhibit 99.1

This presentation and the accompanying oral presentation by Imago BioSciences, Inc. (“Imago,” the “Company,” “we,” “us” or similar terms) contains forward looking statements. These statements may relate to, but are not limited to, the results, conduct, progress and timing of Imago clinical trials, the regulatory approval path for bomedemstat and plans for future operations, as well as assumptions relating to the foregoing. Forward looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified. In some cases, you can identify forward looking statements by terminology such as “may,” “will,” “should,” “could,” “expect,” “plan,” anticipate,” “believe,” “estimate,” “predict,” “intend,” “potential,” “would,” “continue,” “ongoing” or the negative of these terms or other comparable terminology. You should not put undue reliance on any forward-looking statements. Forward looking statements should not be read as a guarantee of future performance or results and will not necessarily be accurate indications of the times at, or by, which such performance or results will be achieved, if at all. Forward looking statements are based on information available at the time those statements are made and management’s good faith beliefs and assumptions as of that time with respect to future events and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. In light of these risks and uncertainties, the forward-looking events and circumstances discussed in this presentation may not occur and actual results could differ materially from those anticipated or implied in the forward-looking statements. These risks and uncertainties are described in greater detail under the heading “Risk Factors” in the form 10K for the year ended December 31, 2022 and our subsequent quarterly reports that we have filed with the Securities and Exchange Commission (the “SEC”) and include, among others, risks relating to our limited operating history and lack of products for commercial sale; our significant losses since inception and for the foreseeable future; our need for substantial additional financing; our unpredictable operating results; our business’s dependence on development, regulatory approval and commercialization of our product candidates; difficulties in enrolling patients and risks of substantial delays in our clinical trials; our minimal control over product candidates in investigator-initiated clinical trials; uncertainties in the outcomes of our clinical studies; uncertainties in the regulatory review and approval of our product candidates if our pivotal studies are positive; potentially material changes to the interim, top-line and preliminary data from our clinical trials; potential undesirable effects of our product candidates and safety or supply issues with combination-use products; our potential inability to obtain and maintain orphan drug designation and delays in approvals despite Fast Track designation; risks related to clinical trials outside of the United States; our need to manufacture multiple batches of bomedemstat using a commercial current Good Manufacturing Process; risks related to COVID-19 or other pandemics, natural disasters and wars; risks related to competition; difficulties in expanding our organization and managing growth, attracting and retaining senior management and key scientific personnel and establishing sales and other commercialization functions; risks related to information technology system and cybersecurity; risks related to misconduct of our employees and independent contractors; risks related to hazardous materials and our compliance with environmental laws and regulations; risks related to litigation and other claims; risks related to reliance on third parties to conduct and support preclinical studies and clinical trials, and to manufacture our product candidates; risks related to third-party intellectual property infringement claims and our ability to protect our own intellectual property; risks related to governmental policies and regulations including with respect to drug prices and reimbursement, and changes thereof; risks related to our common stock; risks related to our public company, “emerging growth company” and “smaller reporting company” status; risks related to internal control over financial reporting. Except as required by law, Imago does not undertake any obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future developments or otherwise. This presentation contains statistical data, estimates and forecasts that are based on independent industry publications or other publicly available information, as well as other information based on our internal sources. This information involves many assumptions and limitations, and you are cautioned not to give undue weight to these estimates. We have not independently verified the accuracy or completeness of the data contained in these industry publications and other publicly available information. Accordingly, we make no representations as to the accuracy or completeness of that data nor do we undertake to update such data after the date of this presentation. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the platform and products of Imago. Disclaimer and Forward-Looking Statements
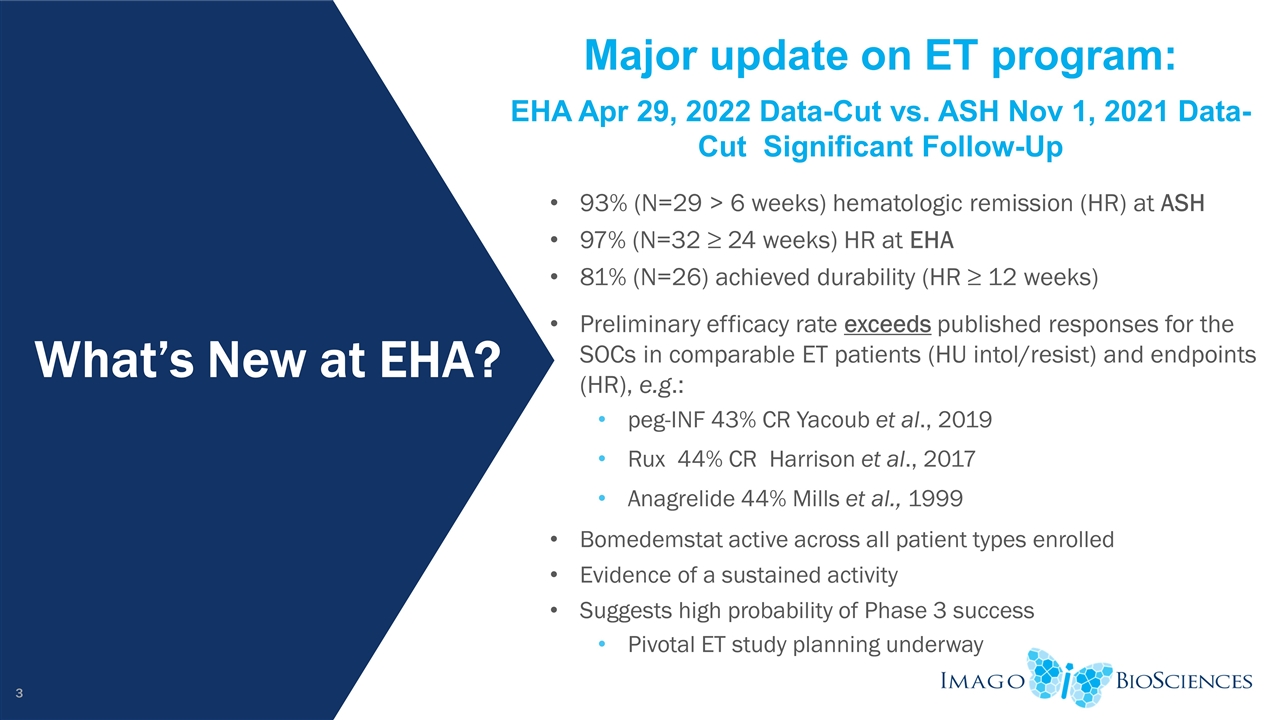
What’s New at EHA? Major update on ET program: EHA Apr 29, 2022 Data-Cut vs. ASH Nov 1, 2021 Data-Cut Significant Follow-Up 93% (N=29 > 6 weeks) hematologic remission (HR) at ASH 97% (N=32 ≥ 24 weeks) HR at EHA 81% (N=26) achieved durability (HR ≥ 12 weeks) Preliminary efficacy rate exceeds published responses for the SOCs in comparable ET patients (HU intol/resist) and endpoints (HR), e.g.: peg-INF 43% CR Yacoub et al., 2019 Rux 44% CR Harrison et al., 2017 Anagrelide 44% Mills et al., 1999 Bomedemstat active across all patient types enrolled Evidence of a sustained activity Suggests high probability of Phase 3 success Pivotal ET study planning underway
Introduction to Bomedemstat *IMG-7289-CTP-101, IMG-7289-CTP-102, CTP-202 and IMG-7289-CTP-201 Bomedemstat is an irreversible inhibitor of LSD1 Discovered by Imago – COM 2034+ PK and dose-response data support once-daily dosing No apparent penetration across the blood-brain barrier Bomedemstat has been evaluated in 200+ * patients with advanced myeloid malignancies
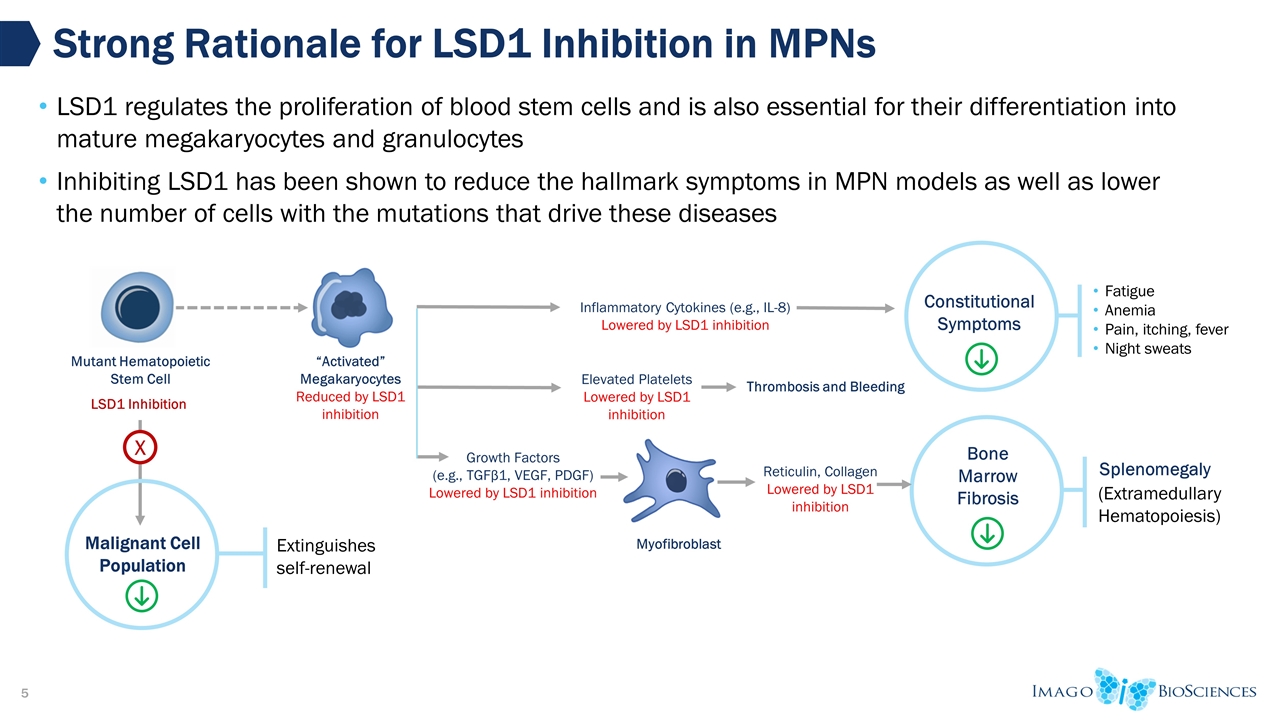
Malignant Cell Population Strong Rationale for LSD1 Inhibition in MPNs “Activated” Megakaryocytes Reduced by LSD1 inhibition Mutant Hematopoietic Stem Cell Reticulin, Collagen Lowered by LSD1 inhibition Inflammatory Cytokines (e.g., IL-8) Lowered by LSD1 inhibition Growth Factors (e.g., TGFβ1, VEGF, PDGF) Lowered by LSD1 inhibition LSD1 Inhibition Myofibroblast X Extinguishes self-renewal LSD1 regulates the proliferation of blood stem cells and is also essential for their differentiation into mature megakaryocytes and granulocytes Inhibiting LSD1 has been shown to reduce the hallmark symptoms in MPN models as well as lower the number of cells with the mutations that drive these diseases Constitutional Symptoms Fatigue Anemia Pain, itching, fever Night sweats Bone Marrow Fibrosis Splenomegaly (Extramedullary Hematopoiesis) Elevated Platelets Lowered by LSD1 inhibition Thrombosis and Bleeding
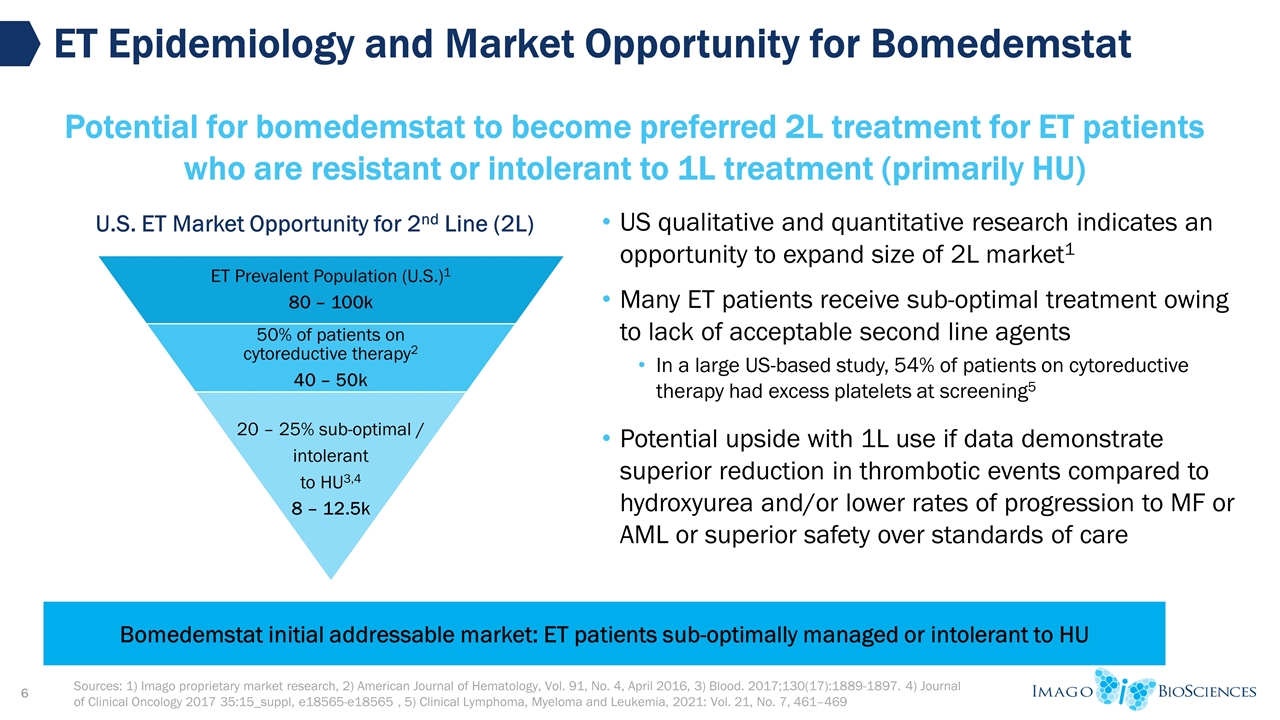
US qualitative and quantitative research indicates an opportunity to expand size of 2L market1 Many ET patients receive sub-optimal treatment owing to lack of acceptable second line agents In a large US-based study, 54% of patients on cytoreductive therapy had excess platelets at screening5 Potential upside with 1L use if data demonstrate superior reduction in thrombotic events compared to hydroxyurea and/or lower rates of progression to MF or AML or superior safety over standards of care Sources: 1) Imago proprietary market research, 2) American Journal of Hematology, Vol. 91, No. 4, April 2016, 3) Blood. 2017;130(17):1889-1897. 4) Journal of Clinical Oncology 2017 35:15_suppl, e18565-e18565 , 5) Clinical Lymphoma, Myeloma and Leukemia, 2021: Vol. 21, No. 7, 461–469 ET Epidemiology and Market Opportunity for Bomedemstat Potential for bomedemstat to become preferred 2L treatment for ET patients who are resistant or intolerant to 1L treatment (primarily HU) U.S. ET Market Opportunity for 2nd Line (2L) Bomedemstat initial addressable market: ET patients sub-optimally managed or intolerant to HU ET Prevalent Population (U.S.) 1 80 – 100k 50% of patients on cytoreductive therapy 2 40 – 50k 20 – 25% sub-optimal / intolerant to HU 3,4 8 – 12.5k
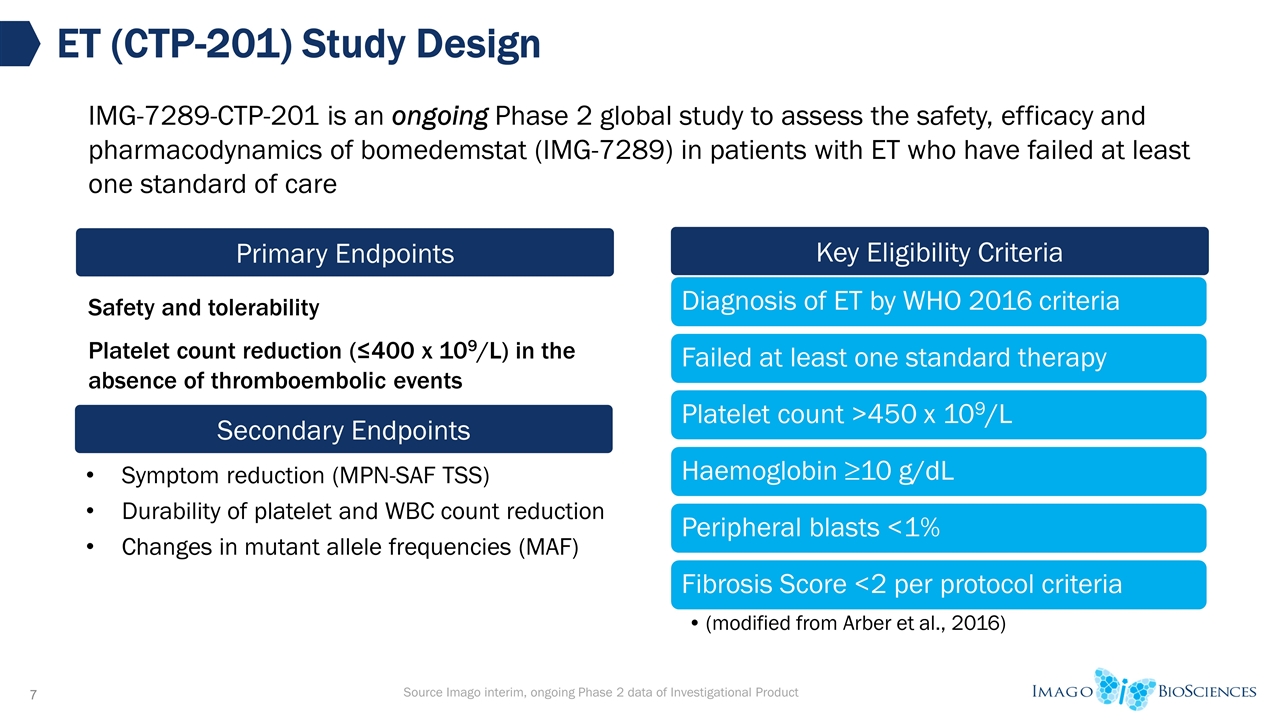
Safety and tolerability Platelet count reduction (≤400 x 109/L) in the absence of thromboembolic events ET (CTP-201) Study Design Primary Endpoints Key Eligibility Criteria Secondary Endpoints Symptom reduction (MPN-SAF TSS) Durability of platelet and WBC count reduction Changes in mutant allele frequencies (MAF) IMG-7289-CTP-201 is an ongoing Phase 2 global study to assess the safety, efficacy and pharmacodynamics of bomedemstat (IMG-7289) in patients with ET who have failed at least one standard of care Source Imago interim, ongoing Phase 2 data of Investigational Product Diagnosis of ET by WHO 2016 criteria Failed at least one standard therapy Platelet count >450 x 10 9 /L Haemoglobin ≥10 g/dL Peripheral blasts <1% Fibrosis Score <2 per protocol criteria (modified from Arber et al., 2016)
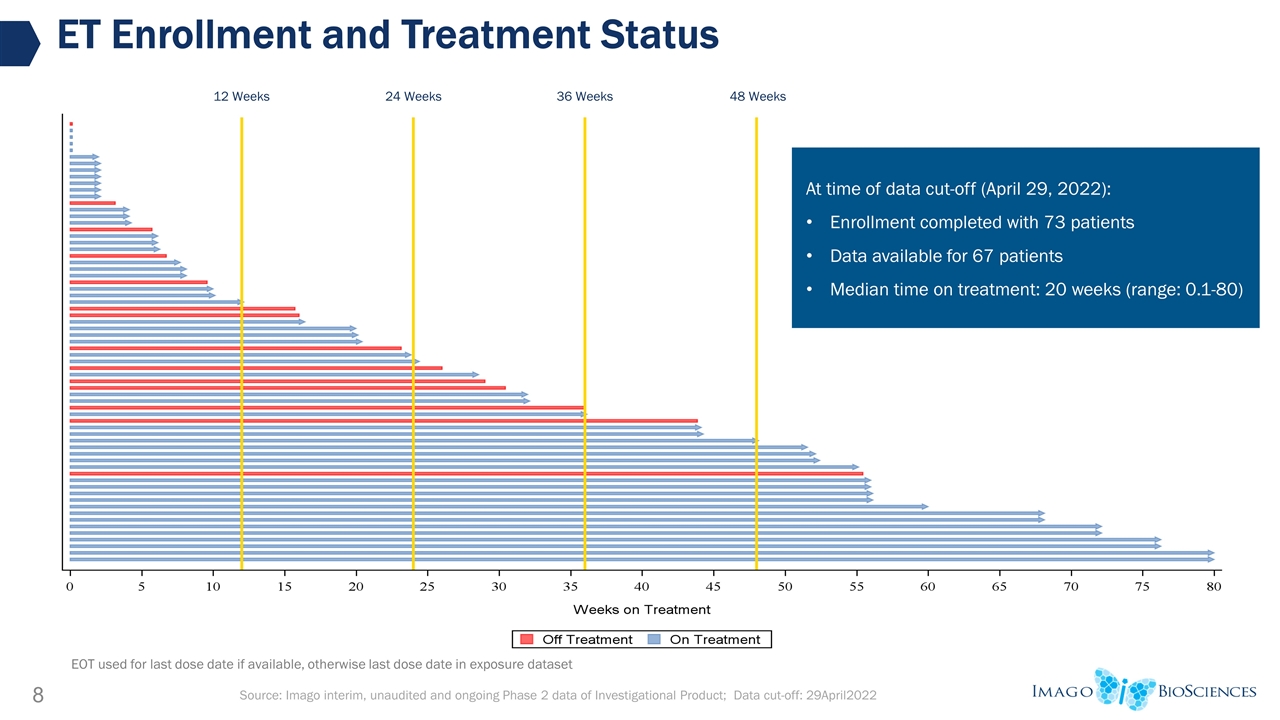
ET Enrollment and Treatment Status 12 Weeks 24 Weeks 48 Weeks 36 Weeks Source: Imago interim, unaudited and ongoing Phase 2 data of Investigational Product; Data cut-off: 29April2022 EOT used for last dose date if available, otherwise last dose date in exposure dataset At time of data cut-off (April 29, 2022): Enrollment completed with 73 patients Data available for 67 patients Median time on treatment: 20 weeks (range: 0.1-80)
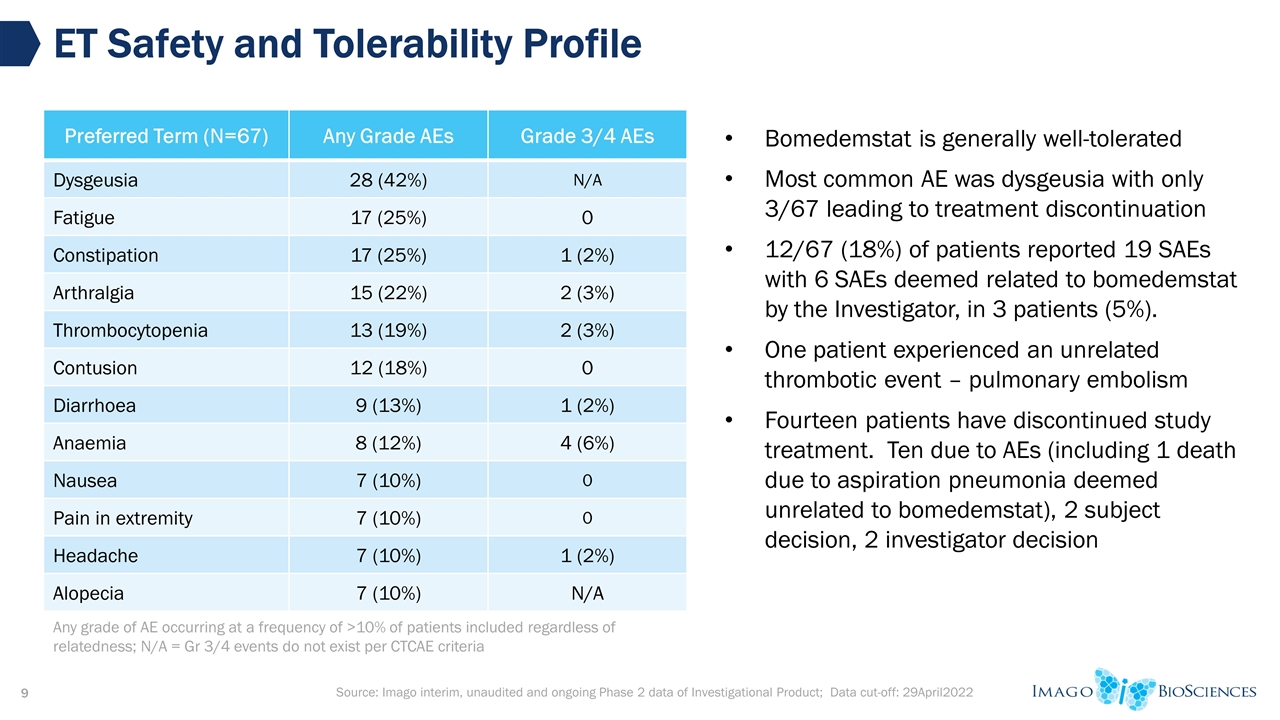
Source: Imago interim, unaudited and ongoing Phase 2 data of Investigational Product; Data cut-off: 29April2022 ET Safety and Tolerability Profile Preferred Term (N=67) Any Grade AEs Grade 3/4 AEs Dysgeusia 28 (42%) N/A Fatigue 17 (25%) 0 Constipation 17 (25%) 1 (2%) Arthralgia 15 (22%) 2 (3%) Thrombocytopenia 13 (19%) 2 (3%) Contusion 12 (18%) 0 Diarrhoea 9 (13%) 1 (2%) Anaemia 8 (12%) 4 (6%) Nausea 7 (10%) 0 Pain in extremity 7 (10%) 0 Headache 7 (10%) 1 (2%) Alopecia 7 (10%) N/A Bomedemstat is generally well-tolerated Most common AE was dysgeusia with only 3/67 leading to treatment discontinuation 12/67 (18%) of patients reported 19 SAEs with 6 SAEs deemed related to bomedemstat by the Investigator, in 3 patients (5%). One patient experienced an unrelated thrombotic event – pulmonary embolism Fourteen patients have discontinued study treatment. Ten due to AEs (including 1 death due to aspiration pneumonia deemed unrelated to bomedemstat), 2 subject decision, 2 investigator decision Any grade of AE occurring at a frequency of >10% of patients included regardless of relatedness; N/A = Gr 3/4 events do not exist per CTCAE criteria
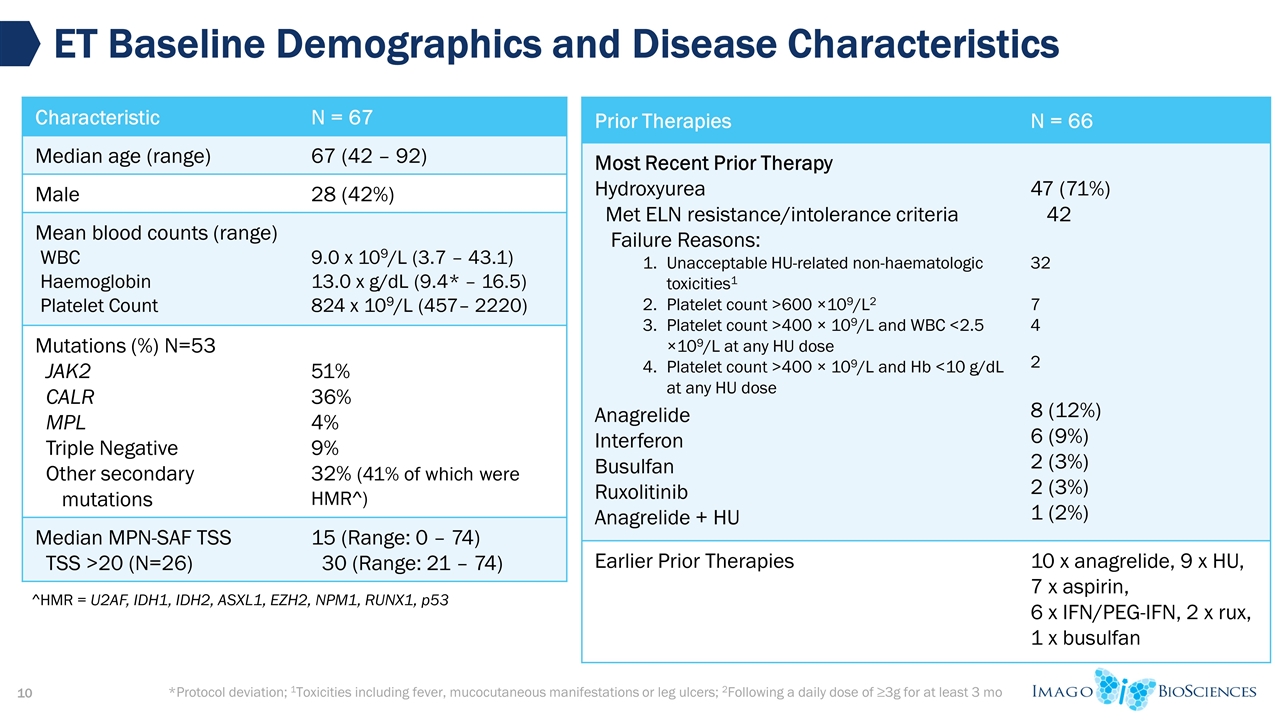
*Protocol deviation; 1Toxicities including fever, mucocutaneous manifestations or leg ulcers; 2Following a daily dose of ≥3g for at least 3 mo ET Baseline Demographics and Disease Characteristics Characteristic N = 67 Median age (range) 67 (42 – 92) Male 28 (42%) Mean blood counts (range) WBC Haemoglobin Platelet Count 9.0 x 109/L (3.7 – 43.1) 13.0 x g/dL (9.4* – 16.5) 824 x 109/L (457– 2220) Mutations (%) N=53 JAK2 CALR MPL Triple Negative Other secondary mutations 51% 36% 4% 9% 32% (41% of which were HMR^) Median MPN-SAF TSS TSS >20 (N=26) 15 (Range: 0 – 74) 30 (Range: 21 – 74) Prior Therapies N = 66 Most Recent Prior Therapy Hydroxyurea Met ELN resistance/intolerance criteria Failure Reasons: Unacceptable HU-related non-haematologic toxicities1 Platelet count >600 ×109/L2 Platelet count >400 × 109/L and WBC <2.5 ×109/L at any HU dose Platelet count >400 × 109/L and Hb <10 g/dL at any HU dose Anagrelide Interferon Busulfan Ruxolitinib Anagrelide + HU 47 (71%) 42 32 7 4 2 8 (12%) 6 (9%) 2 (3%) 2 (3%) 1 (2%) Earlier Prior Therapies 10 x anagrelide, 9 x HU, 7 x aspirin, 6 x IFN/PEG-IFN, 2 x rux, 1 x busulfan ^HMR = U2AF, IDH1, IDH2, ASXL1, EZH2, NPM1, RUNX1, p53
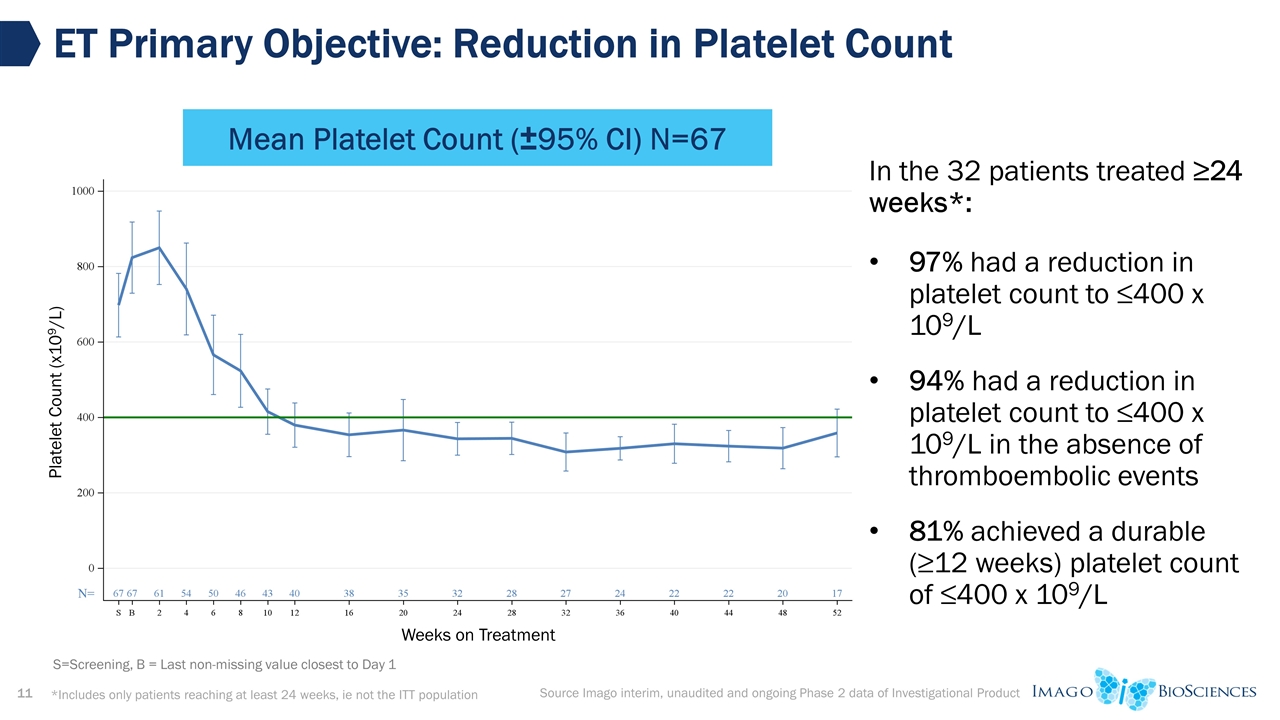
ET Primary Objective: Reduction in Platelet Count Source Imago interim, unaudited and ongoing Phase 2 data of Investigational Product Mean Platelet Count (±95% CI) N=67 *Includes only patients reaching at least 24 weeks, ie not the ITT population S=Screening, B = Last non-missing value closest to Day 1 Weeks on Treatment Platelet Count (x109/L) In the 32 patients treated ≥24 weeks*: 97% had a reduction in platelet count to ≤400 x 109/L 94% had a reduction in platelet count to ≤400 x 109/L in the absence of thromboembolic events 81% achieved a durable (≥12 weeks) platelet count of ≤400 x 109/L
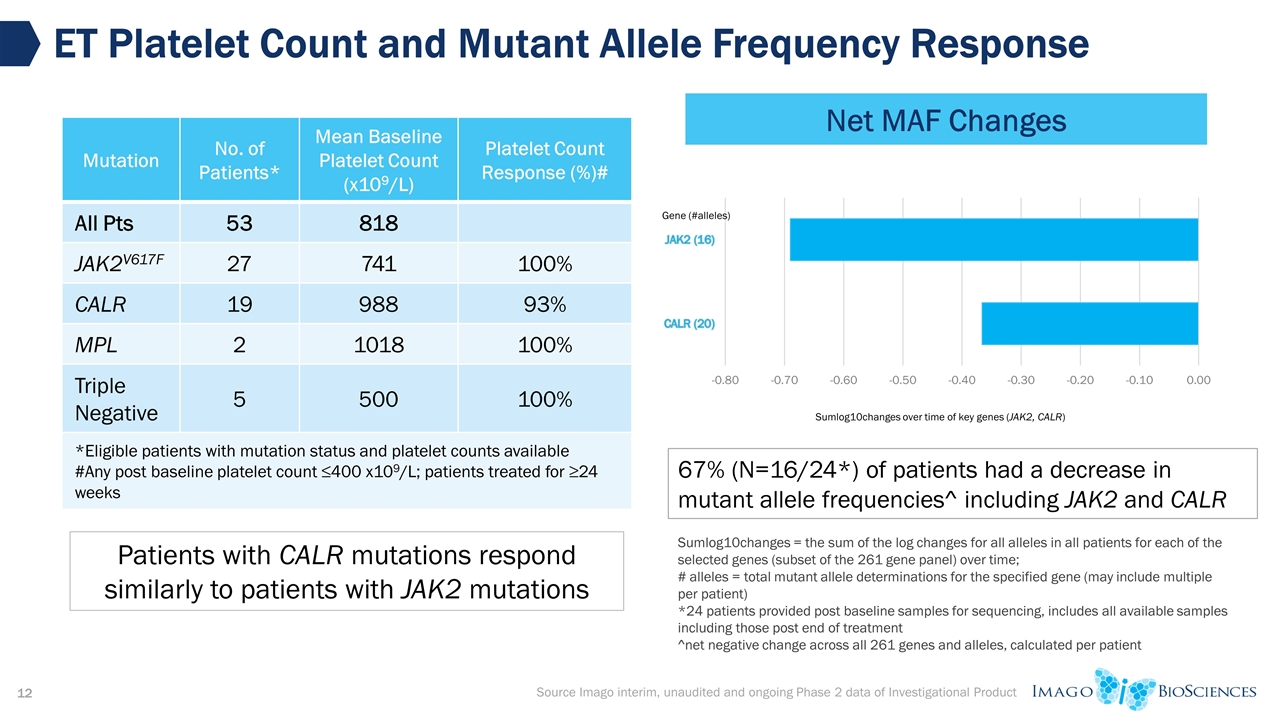
ET Platelet Count and Mutant Allele Frequency Response Source Imago interim, unaudited and ongoing Phase 2 data of Investigational Product Mutation No. of Patients* Mean Baseline Platelet Count (x109/L) Platelet Count Response (%)# All Pts 53 818 JAK2V617F 27 741 100% CALR 19 988 93% MPL 2 1018 100% Triple Negative 5 500 100% *Eligible patients with mutation status and platelet counts available #Any post baseline platelet count ≤400 x109/L; patients treated for ≥24 weeks Patients with CALR mutations respond similarly to patients with JAK2 mutations Sumlog10changes = the sum of the log changes for all alleles in all patients for each of the selected genes (subset of the 261 gene panel) over time; # alleles = total mutant allele determinations for the specified gene (may include multiple per patient) *24 patients provided post baseline samples for sequencing, includes all available samples including those post end of treatment ^net negative change across all 261 genes and alleles, calculated per patient 67% (N=16/24*) of patients had a decrease in mutant allele frequencies^ including JAK2 and CALR Net MAF Changes
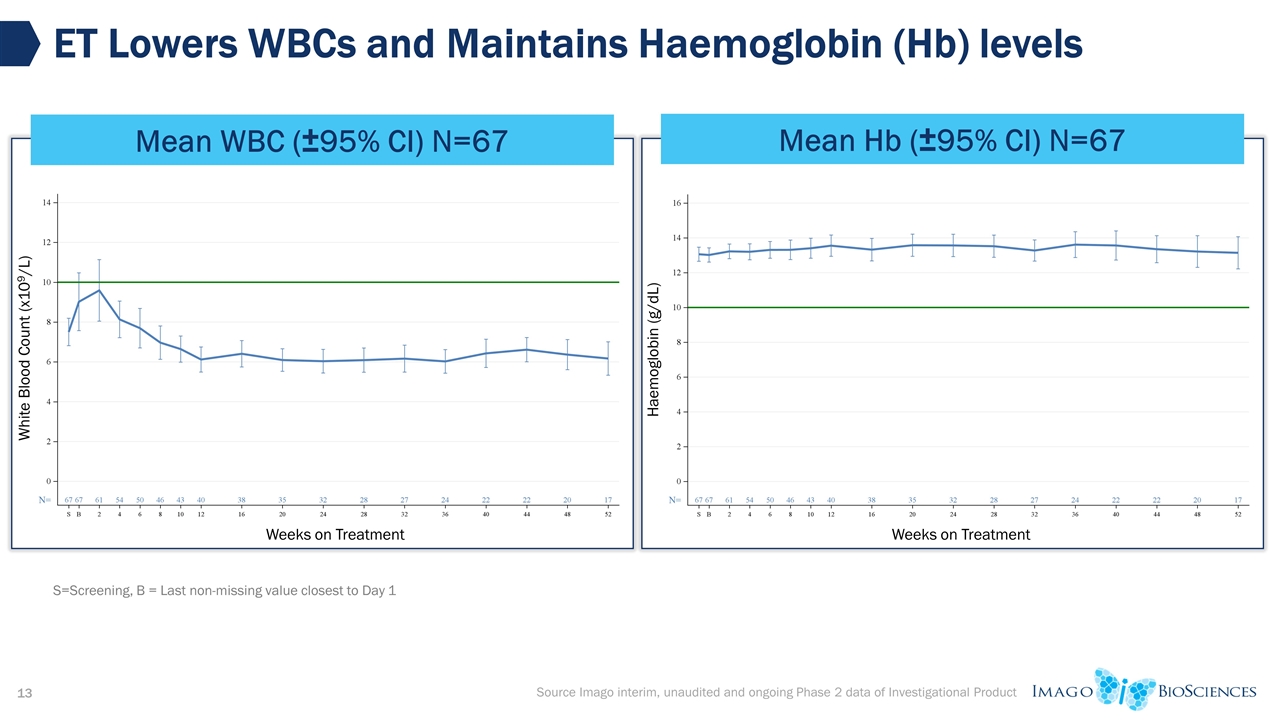
ET Lowers WBCs and Maintains Haemoglobin (Hb) levels Mean WBC (±95% CI) N=67 Source Imago interim, unaudited and ongoing Phase 2 data of Investigational Product Mean Hb (±95% CI) N=67 S=Screening, B = Last non-missing value closest to Day 1 Weeks on Treatment White Blood Count (x109/L) Weeks on Treatment Haemoglobin (g/dL)
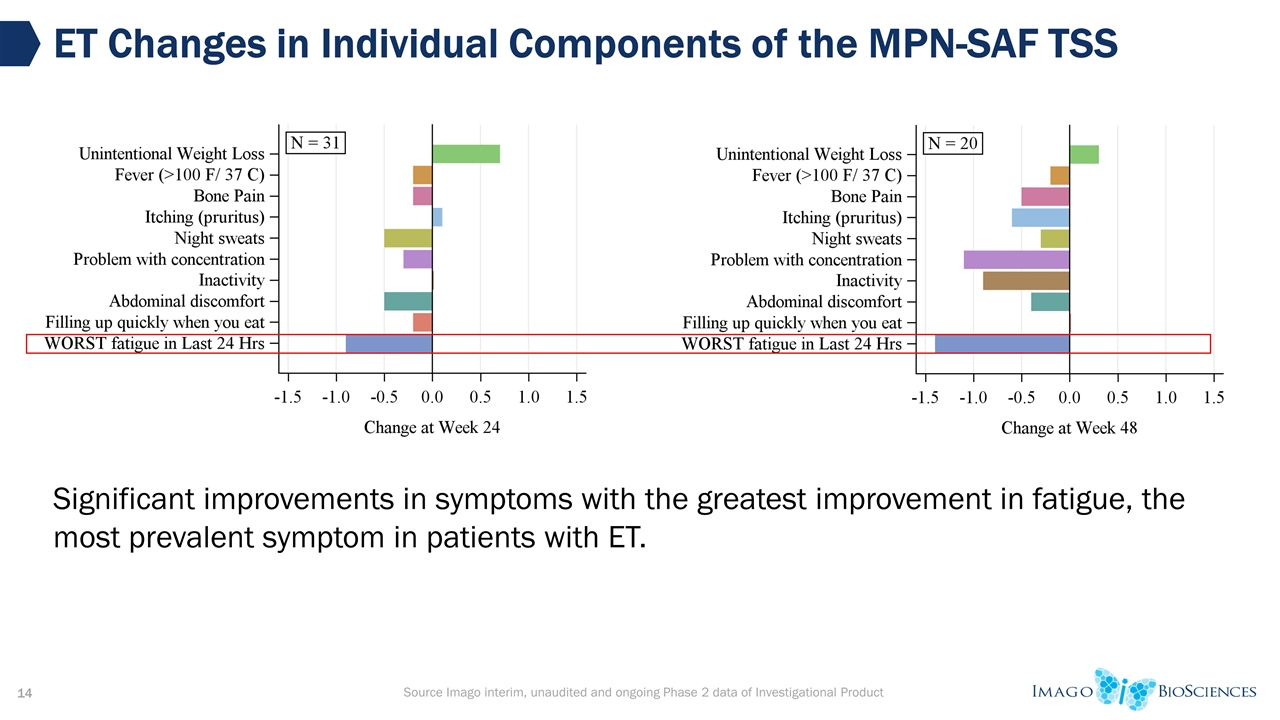
Source Imago interim, unaudited and ongoing Phase 2 data of Investigational Product ET Changes in Individual Components of the MPN-SAF TSS Significant improvements in symptoms with the greatest improvement in fatigue, the most prevalent symptom in patients with ET.
Conclusions of the ET Study Bomedemstat (IMG-7289) is generally well tolerated in patients with ET Majority of AEs were low-grade Bomedemstat as monotherapy demonstrates significant clinical activity: Normalization of platelet and WBC counts while maintaining hemoglobin Symptomatic improvement for some patients with significant MPN symptoms Mutant CALR and JAK2 clones are sensitive to bomedemstat with majority of patients experiencing a decrease in MAF Development Plans EOP2 meeting planned for 2H 2022 Planning for pivotal study in ET is underway
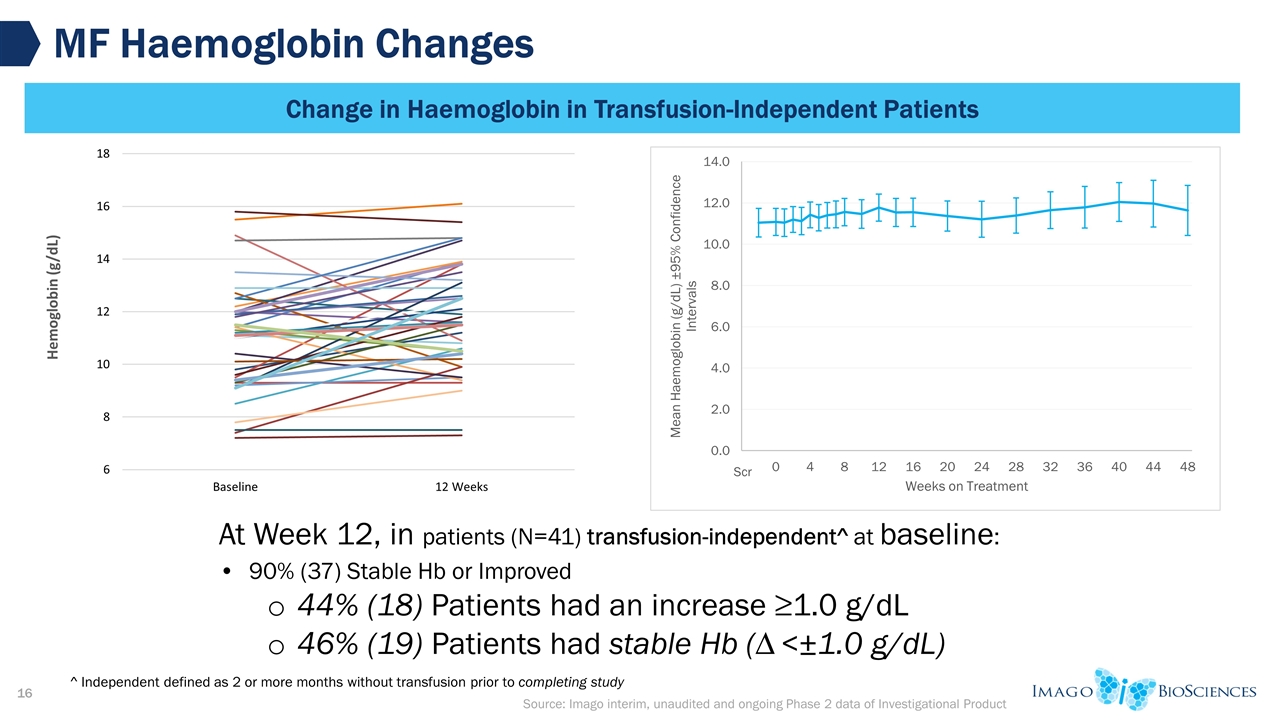
Source: Imago interim, unaudited and ongoing Phase 2 data of Investigational Product MF Haemoglobin Changes Change in Haemoglobin in Transfusion-Independent Patients At Week 12, in patients (N=41) transfusion-independent^ at baseline: 90% (37) Stable Hb or Improved 44% (18) Patients had an increase ≥1.0 g/dL 46% (19) Patients had stable Hb (∆ <±1.0 g/dL) ^ Independent defined as 2 or more months without transfusion prior to completing study
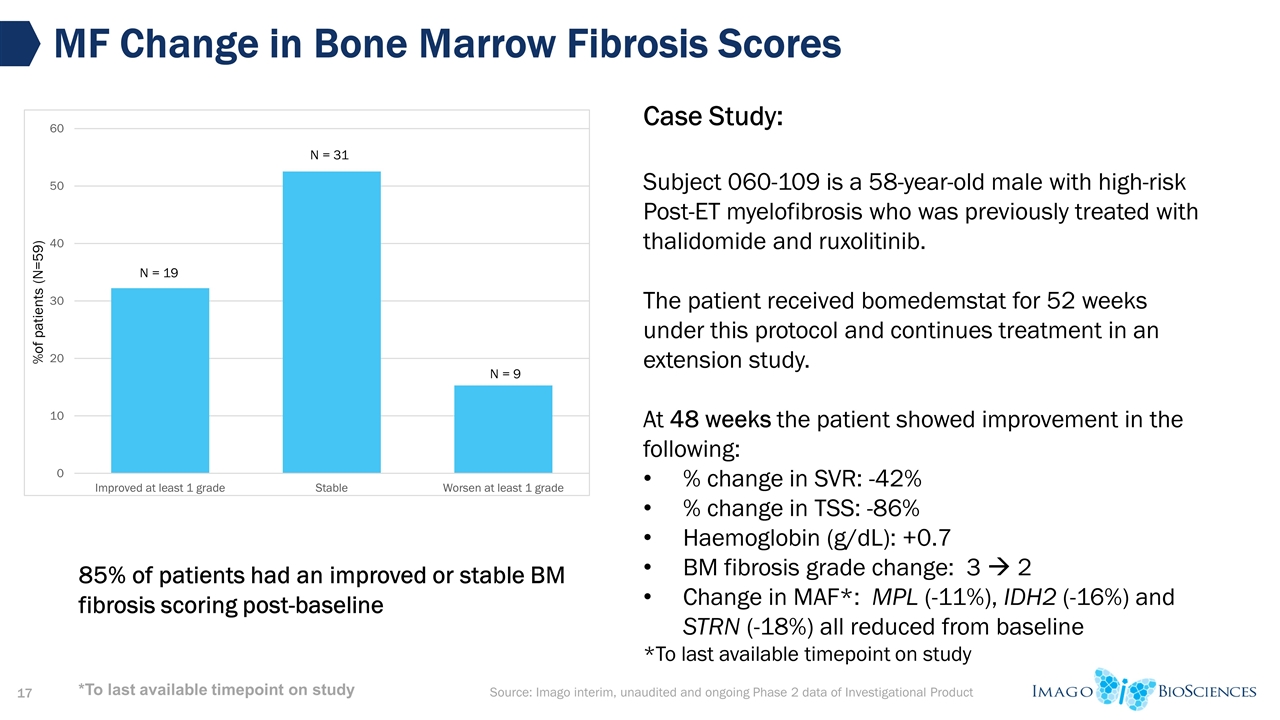
Source: Imago interim, unaudited and ongoing Phase 2 data of Investigational Product MF Change in Bone Marrow Fibrosis Scores Case Study: Subject 060-109 is a 58-year-old male with high-risk Post-ET myelofibrosis who was previously treated with thalidomide and ruxolitinib. The patient received bomedemstat for 52 weeks under this protocol and continues treatment in an extension study. At 48 weeks the patient showed improvement in the following: % change in SVR: -42% % change in TSS: -86% Haemoglobin (g/dL): +0.7 BM fibrosis grade change: 3 à 2 Change in MAF*: MPL (-11%), IDH2 (-16%) and STRN (-18%) all reduced from baseline *To last available timepoint on study 85% of patients had an improved or stable BM fibrosis scoring post-baseline *To last available timepoint on study
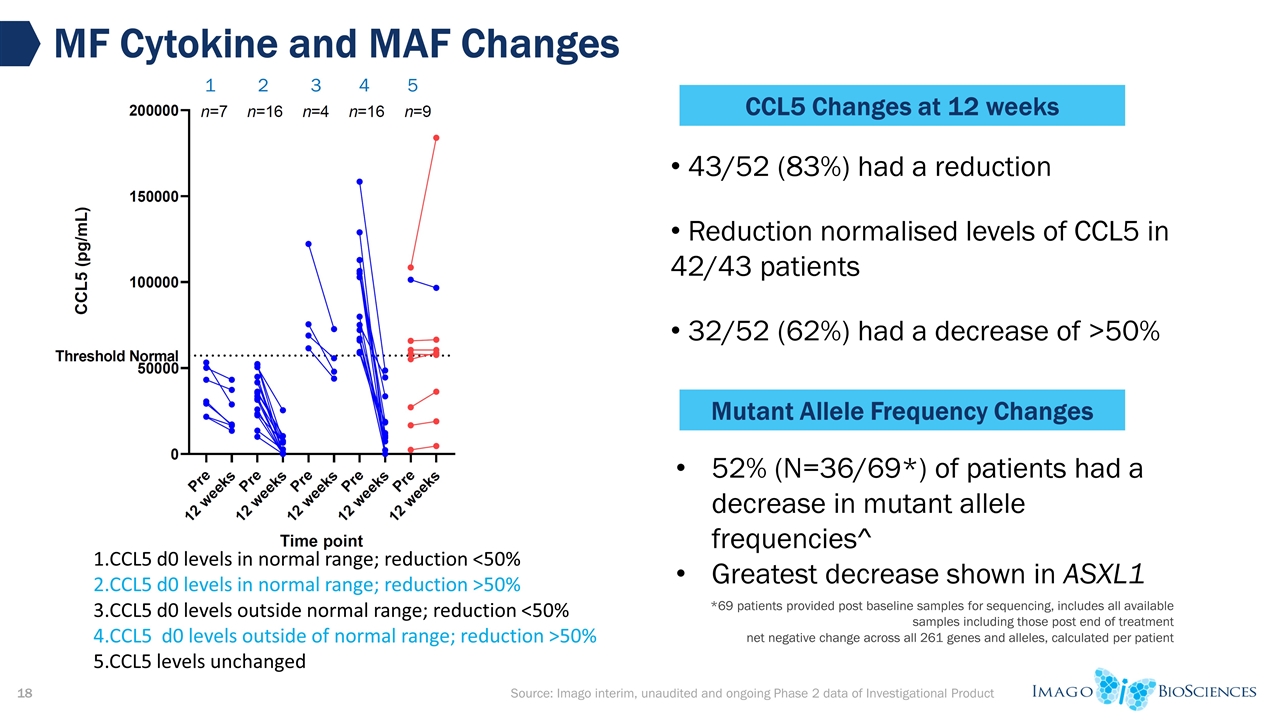
*69 patients provided post baseline samples for sequencing, includes all available samples including those post end of treatment net negative change across all 261 genes and alleles, calculated per patient MF Cytokine and MAF Changes CCL5 d0 levels in normal range; reduction <50% CCL5 d0 levels in normal range; reduction >50% CCL5 d0 levels outside normal range; reduction <50% CCL5 d0 levels outside of normal range; reduction >50% 5.CCL5 levels unchanged 43/52 (83%) had a reduction Reduction normalised levels of CCL5 in 42/43 patients 32/52 (62%) had a decrease of >50% CCL5 Changes at 12 weeks 1 2 3 4 5 Mutant Allele Frequency Changes 52% (N=36/69*) of patients had a decrease in mutant allele frequencies^ Greatest decrease shown in ASXL1 Source: Imago interim, unaudited and ongoing Phase 2 data of Investigational Product
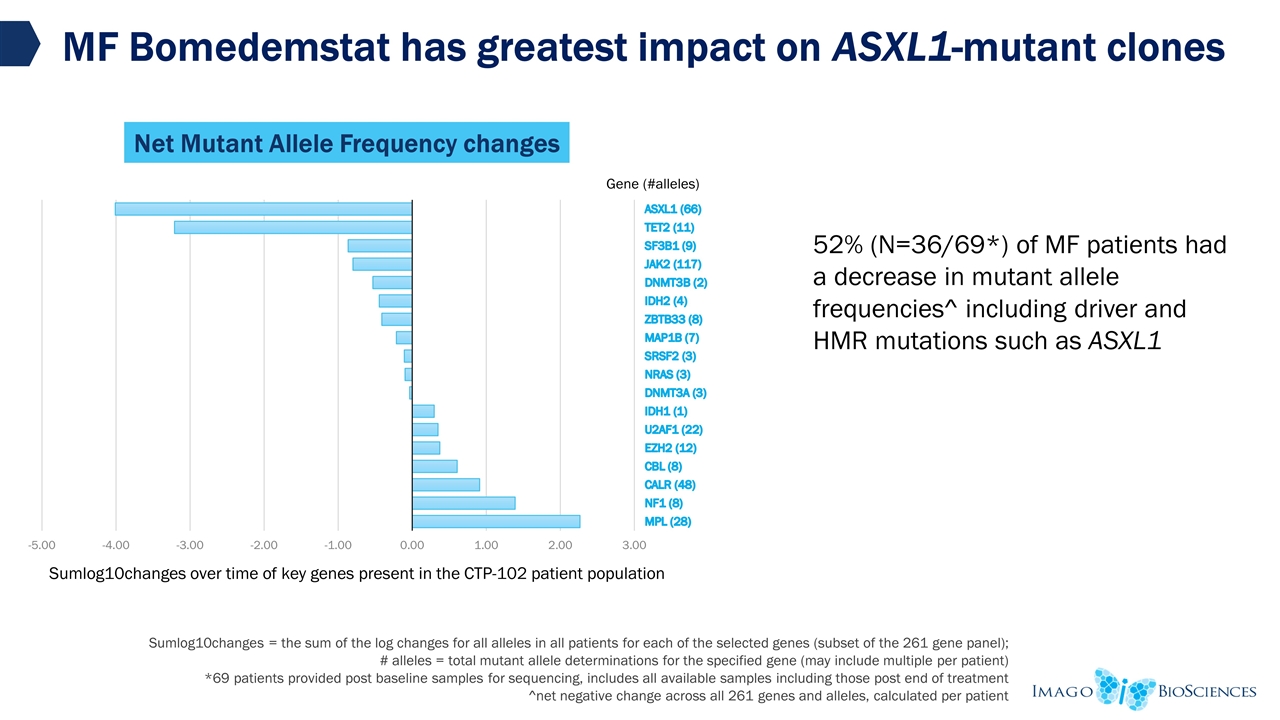
MF Bomedemstat has greatest impact on ASXL1-mutant clones Gene SumLog10Changes #cases Sumlog10changes = the sum of the log changes for all alleles in all patients for each of the selected genes (subset of the 261 gene panel); # alleles = total mutant allele determinations for the specified gene (may include multiple per patient) *69 patients provided post baseline samples for sequencing, includes all available samples including those post end of treatment ^net negative change across all 261 genes and alleles, calculated per patient Source: Imago interim, unaudited and ongoing Phase 2 data of Investigational Product 52% (N=36/69*) of MF patients had a decrease in mutant allele frequencies^ including driver and HMR mutations such as ASXL1 Net Mutant Allele Frequency changes
Overall Clinical Activity of Bomedemstat Bomedemstat (IMG-7289) is generally well tolerated in all patients 200+ patients with AML, MDS, MF and ET treated to date Majority of AEs have been low-grade; few related SAEs Bomedemstat as monotherapy demonstrates significant clinical activity: Dosing titration allows targeting specific platelet counts in MF and ET Clinical improvement in a majority of MPN patients No progression to AML in a high-risk MF patient population Potential use for the treatment of PV Potential for use in combinations for hematologic neoplasms and solid tumors
CTP-101 – R/R AML/MDS study completed CTP-102 Advanced MF – on-study patients all enrolled in Extension study (CTP-202) after 1 year CTP-103 Food Effect – completed CTP-104 Mass Balance – completed CTP-105 DDI – FPFV planned for 2H2022 CTP-201 2L ET – fully enrolled, ongoing – on-study patients enrolling in CTP-202 after 1 year CTP-203 2L PV – FPFV estimated TBD CTP-301 Pivotal 2L ET -- FPFV planned for initiation 1H2023 Investigator Initiated Trials (IIT) PV – ongoing SCLC combination with atezolizumab – ongoing MF combination with ruxolitinib – FPFV planned for 2H2022 AML combination with venetoclax – FPFV planned for 2H2022 Development History and Plans
Conclusions Bomedemstat has been generally well-tolerated in MF and ET patients Shown high level of activity on platelets, WBCs, and symptom improvement The P3 patient population will be very similar to those in the P2 Alignment with the FDA, subject to EOP2 meeting, on the composite primary endpoint for P3 EOP2 meeting with FDA planned for 2H22 Pivotal study for ET planned Cash runway into 2025 We believe bomedemstat could be a platform for MPN treatment

THANK YOU






















