On August 11, 2022, Allena Pharmaceuticals, Inc. (the “Company” or “Allena”), a biopharmaceutical company deploying its novel oral biologic platform to discover, develop and commercialize first-in-class, oral enzyme therapeutics for difficult-to-treat metabolic diseases, provided the following clinical and corporate update.
ALLN-346 Program Update
ALLN-346 is a potential first-in-class, non-absorbed, orally administered enzyme in development for the treatment of hyperuricemia and gout in the setting of advanced chronic kidney disease (CKD). In November 2021, the Company announced Fast Track Designation for ALLN-346 from the U.S. Food and Drug Administration (FDA).
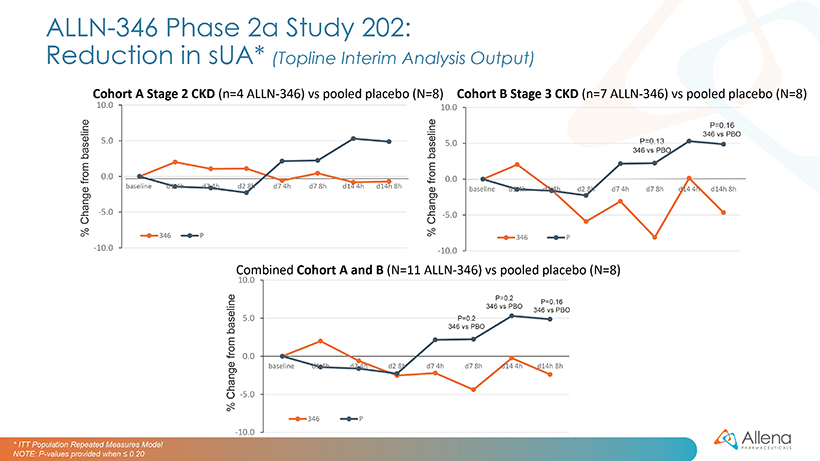
The ALLN-346 Phase 2a clinical program consists of two trials: Study 201, a one-week inpatient study conducted at a clinical pharmacology unit, and Study 202, a two-week outpatient study being conducted at 23 sites across the U.S. These studies follow previously reported Phase 1 studies, including both a single-ascending dose and multiple-ascending dose study in healthy volunteers. In both Phase 1 studies, ALLN-346 was well tolerated with no clinically significant safety signals and no dose-limiting toxicities observed in any cohort up to the highest administered dose.
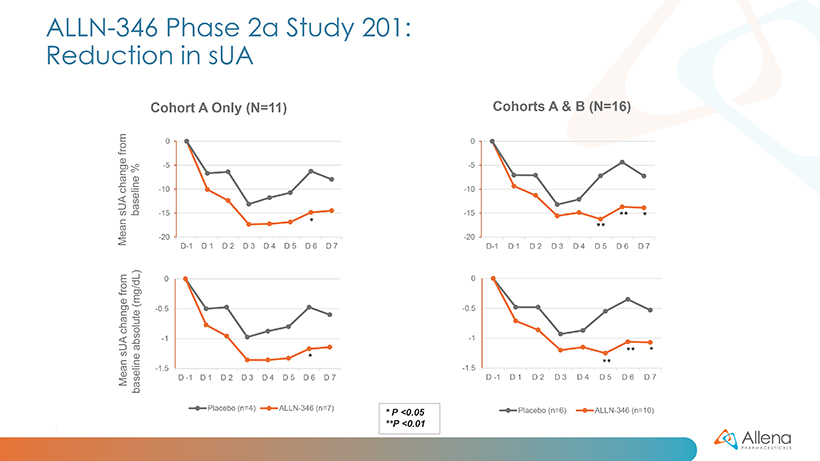
For Study 201, patients with hyperuricemia were randomized (2:1) to receive either five capsules of ALLN-346 or matching placebo three times daily for one week. The trial enrolled 16 patients (11 in cohort A and 5 in cohort B), the majority of whom had stage 2 CKD. Preliminary, topline data from the cohort A patients were reported in January 2022.
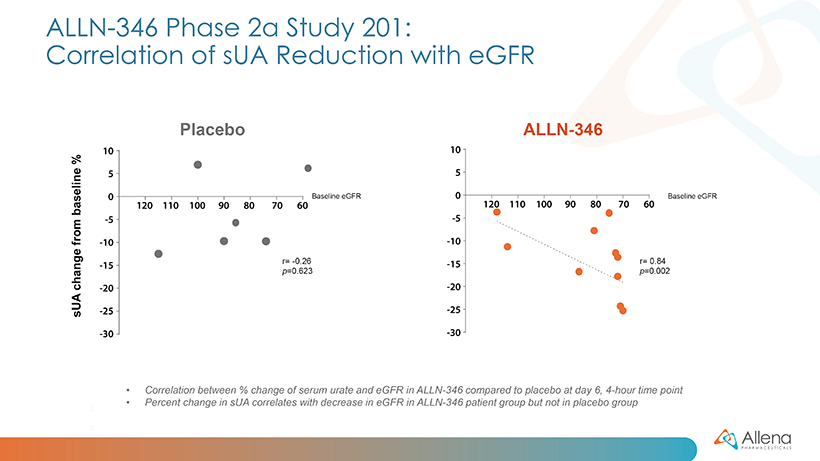
As announced in January, data from the first group of patients (cohort A) demonstrated a statistically significant reduction in serum uric acid (sUA) from baseline (p<0.05) in patients treated with ALLN-346 compared to placebo. The results from the additional five patients (cohort B) were consistent with those seen in the first 11 patients. In the full group of 16 patients, statistically significant reductions in sUA were seen from days five to seven (p<0.01 on days five and six, and p<0.05 on day seven). There was approximately a 15% reduction from baseline seen in sUA in patients treated with ALLN-346 vs. an approximately 8% reduction from baseline seen in the placebo group. The results also provide support for the potential GI-based mechanism of action of ALLN-346. Specifically, there was a positive correlation between the effect of ALLN-346 on sUA reduction and the degree of renal impairment as measured by estimated glomerular filtration rate (eGFR) (correlation coefficient r=0.84; p=0.002). In gout patients with advanced CKD, the intestinal tract becomes the primary route of elimination for urate, and ALLN-346 is specifically designed to capitalize on this physiologic adaptation by enhancing the breakdown and secretion of urate in the intestinal tract.