Exhibit 99.1


Immunobiology and Clinical Activity of CPI - 006, an Anti - CD73 Antibody with Immunomodulating Properties in a Phase 1/1b Trial in Advanced Cancers Luke JJ, Merchan J, Harshman LC, Marron T, Powderly J, Barve M, LoRusso P, Johnson M, Hotson A, Gittelman R, Munneke B, Buggy J, Willingham S, Piccione E, Mobasher M, Miller R

Disclosures Jason J. Luke : University of Pittsburgh Medical Center, Pittsburgh The following relationships exist related to this presentation: • Data and Safety Monitoring Board: TTC Oncology • Scientific Advisory Board: 7 Hills, Actym , Alphamab Oncology, Mavu , Pyxis, Springbank , Tempest • Consultancy : Abbvie , Akrevia , Array, Astellas, AstraZeneca, Bayer, Bristol - Myers Squibb, Compugen , EMD Serono, Ideaya , Immunocore , Incyte, Janssen, Leap, Merck, Mersana , Novartis, RefleXion , Silicon, Vividion • Research Support: (all to institution for clinical trials unless noted) AbbVie, Agios (IIT), Array (IIT), Astellas, Boston Biomedical, Bristol - Myers Squibb, CheckMate (SRA), Compugen , Corvus, EMD Serono, Evelo (SRA), Five Prime, FLX Bio, Genentech, Immatics , Immunocore , Incyte, Leap, MedImmune , Macrogenics , Necktar , Novartis, Palleon (SRA), Merck, Springbank , Tesaro , Tizona , Xencor • Travel : Akrevia , AstraZeneca, Bayer, Bristol - Myers Squibb, EMD Serono, Immunocore , Incyte, Janssen, Merck, Mersana , Novartis, RefleXion • Patents : (both provisional) Serial #15/612,657 (Cancer Immunotherapy), PCT/US18/36052 (Microbiome Biomarkers for Anti - PD - 1/PD - L1 Responsiveness: Diagnostic, Prognostic and Therapeutic Uses Thereof) Corvus Pharmaceutical Inc. is the sponsor of this study.
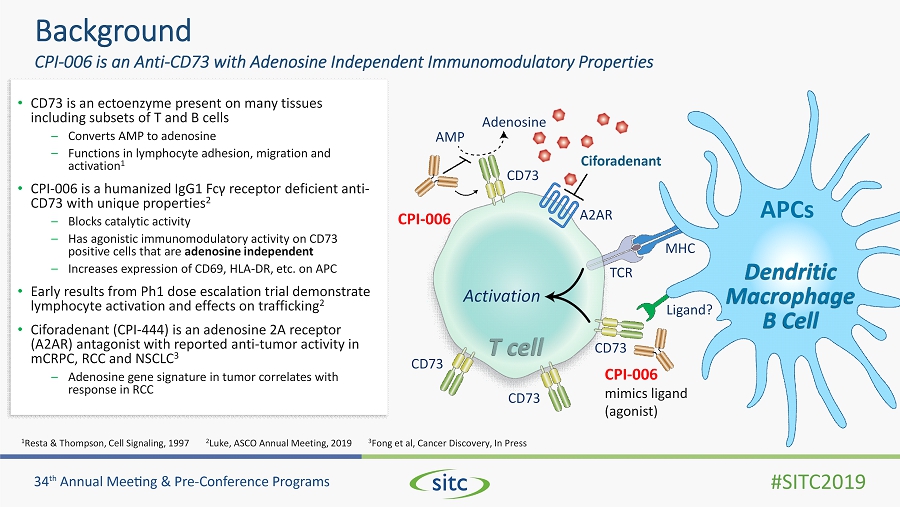
AMP Adenosine CD73 CD73 CD73 CD73 Activation Dendritic Macrophage B Cell MHC TCR Ligand? CPI - 006 mimics ligand (agonist) APCs A2AR CPI - 006 Ciforadenant T cell • CD73 is an ectoenzyme present on many tissues including subsets of T and B cells – Converts AMP to adenosine – Functions in lymphocyte adhesion, migration and activation 1 • CPI - 006 is a humanized IgG1 Fc γ receptor deficient anti - CD73 with unique properties 2 – Blocks catalytic activity – Has agonistic immunomodulatory activity on CD73 positive cells that are adenosine independent – Increases expression of CD69, HLA - DR, etc. on APC • Early results from Ph1 dose escalation trial demonstrate lymphocyte activation and effects on trafficking 2 • Ciforadenant (CPI - 444) is an adenosine 2A receptor (A2AR) antagonist with reported anti - tumor activity in mCRPC, RCC and NSCLC 3 – Adenosine gene signature in tumor correlates with response in RCC 1 Resta & Thompson, Cell Signaling, 1997 2 Luke, ASCO Annual Meeting, 2019 3 Fong et al, Cancer Discovery, In Press Background CPI - 006 is an Anti - CD73 with Adenosine Independent Immunomodulatory Properties
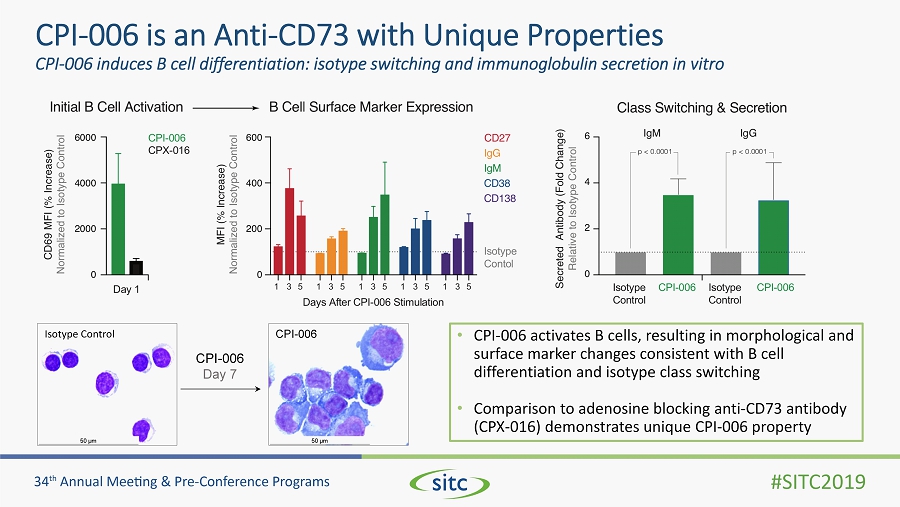
CPI - 006 is an Anti - CD73 with Unique Properties CPI - 006 induces B cell differentiation: isotype switching and immunoglobulin secretion in vitro • CPI - 006 activates B cells, resulting in morphological and surface marker changes consistent with B cell differentiation and isotype class switching • Comparison to adenosine blocking anti - CD73 antibody (CPX - 016) demonstrates unique CPI - 006 property CPI - 006 Day 7 Isotype Control CPI - 006
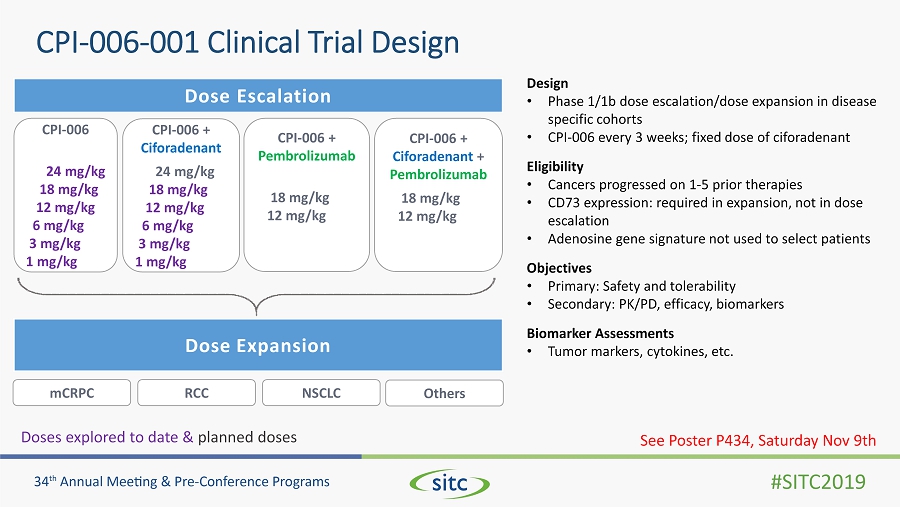
CPI - 006 - 001 Clinical Trial Design Design • Phase 1/1b dose escalation/dose expansion in disease specific cohorts • CPI - 006 every 3 weeks; fixed dose of ciforadenant Eligibility • Cancers progressed on 1 - 5 prior therapies • CD73 expression: required in expansion, not in dose escalation • Adenosine gene signature not used to select patients Objectives • Primary: Safety and tolerability • Secondary: PK/PD, efficacy, biomarkers Biomarker Assessments • Tumor markers, cytokines, etc. Dose Escalation Dose Expansion CPI - 006 24 mg/kg 18 mg/kg 12 mg/kg 6 mg/kg 3 mg/kg 1 mg/kg CPI - 006 + Ciforadenant 24 mg/kg 18 mg/kg 12 mg/kg 6 mg/kg 3 mg/kg 1 mg/kg CPI - 006 + Ciforadenant + Pembrolizumab 18 mg/kg 12 mg/kg CPI - 006 + Pembrolizumab 18 mg/kg 12 mg/kg See Poster P434, Saturday Nov 9th NSCLC mCRPC RCC Others Doses explored to date & planned doses

Patient Characteristics Total Patients N=40 CPI - 006 (N = 24) CPI - 006 + Ciforadenant (N=16) Age ( yrs ), median (range) 62 (46, 78) 67 (36, 86) Gender, male N (%) 18 (75) 12 (75) No. of prior therapies, median (range) 4 (1, 6) 4 (2, 7) Histologies N N Renal Cell Cancer 2 4 Non - small cell lung cancer 2 1 Prostate Cancer 5 1 Colorectal Cancer 7 5 Head and Neck Cancer 3 2 Pancreatic Cancer 3 3 Sarcoma 1 0 Bladder Cancer 1 0
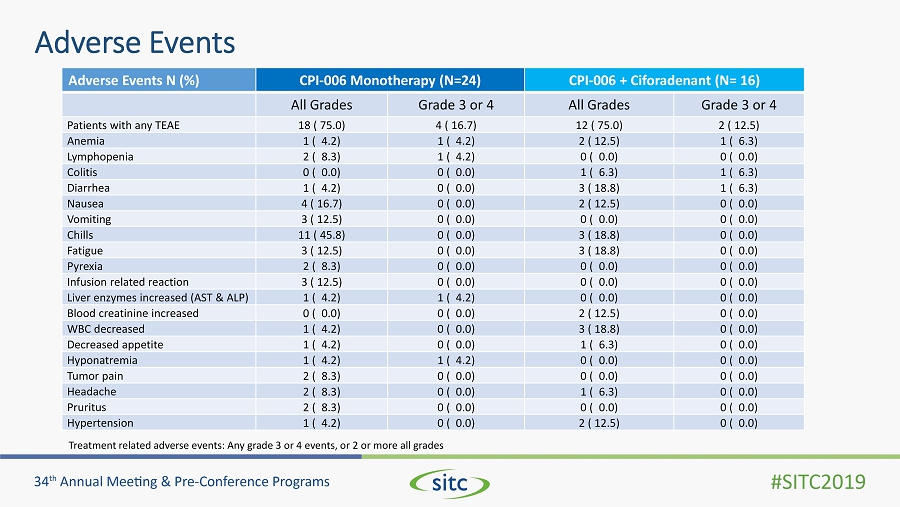
Adverse Events Adverse Events N (%) CPI - 006 Monotherapy (N=24) CPI - 006 + Ciforadenant (N= 16) All Grades Grade 3 or 4 All Grades Grade 3 or 4 Patients with any TEAE 18 ( 75.0) 4 ( 16.7) 12 ( 75.0) 2 ( 12.5) Anemia 1 ( 4.2) 1 ( 4.2) 2 ( 12.5) 1 ( 6.3) Lymphopenia 2 ( 8.3) 1 ( 4.2) 0 ( 0.0) 0 ( 0.0) Colitis 0 ( 0.0) 0 ( 0.0) 1 ( 6.3) 1 ( 6.3) Diarrhea 1 ( 4.2) 0 ( 0.0) 3 ( 18.8) 1 ( 6.3) Nausea 4 ( 16.7) 0 ( 0.0) 2 ( 12.5) 0 ( 0.0) Vomiting 3 ( 12.5) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Chills 11 ( 45.8) 0 ( 0.0) 3 ( 18.8) 0 ( 0.0) Fatigue 3 ( 12.5) 0 ( 0.0) 3 ( 18.8) 0 ( 0.0) Pyrexia 2 ( 8.3) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Infusion related reaction 3 ( 12.5) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Liver enzymes increased (AST & ALP) 1 ( 4.2) 1 ( 4.2) 0 ( 0.0) 0 ( 0.0) Blood creatinine increased 0 ( 0.0) 0 ( 0.0) 2 ( 12.5) 0 ( 0.0) WBC decreased 1 ( 4.2) 0 ( 0.0) 3 ( 18.8) 0 ( 0.0) Decreased appetite 1 ( 4.2) 0 ( 0.0) 1 ( 6.3) 0 ( 0.0) Hyponatremia 1 ( 4.2) 1 ( 4.2) 0 ( 0.0) 0 ( 0.0) Tumor pain 2 ( 8.3) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Headache 2 ( 8.3) 0 ( 0.0) 1 ( 6.3) 0 ( 0.0) Pruritus 2 ( 8.3) 0 ( 0.0) 0 ( 0.0) 0 ( 0.0) Hypertension 1 ( 4.2) 0 ( 0.0) 2 ( 12.5) 0 ( 0.0) Treatment related adverse events: Any grade 3 or 4 events, or 2 or more all grades
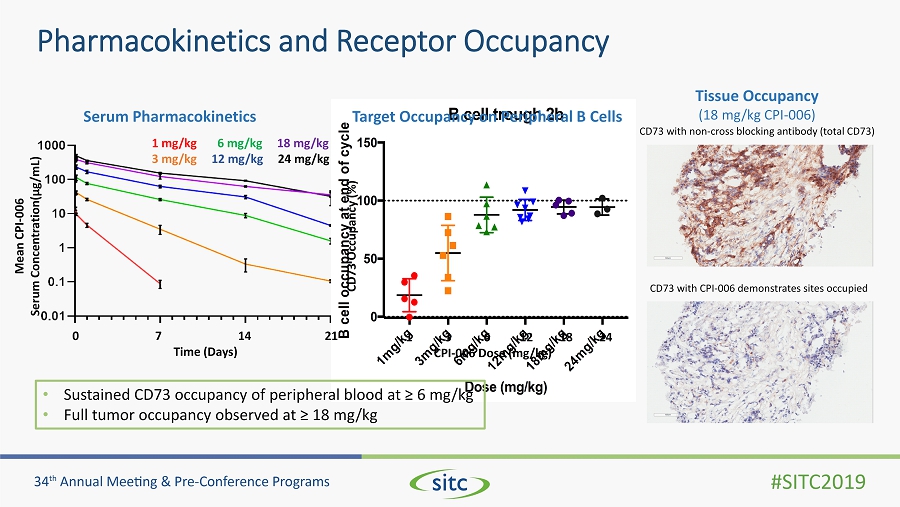
Pharmacokinetics and Receptor Occupancy 1 m g / k g 3 m g / k g 6 m g / k g 1 2 m g / k g 1 8 m g / k g 2 4 m g / k g 0 50 100 150 Dose (mg/kg) B c e l l o c c u p a n c y a t e n d o f c y c l e B cell trough 2b • Sustained CD73 occupancy of peripheral blood at ≥ 6 mg/kg • Full tumor occupancy observed at ≥ 18 mg/kg Serum Pharmacokinetics Target Occupancy on Peripheral B Cells 1 3 6 12 18 24 CPI - 006 Dose (mg/kg) Tissue Occupancy (18 mg/kg CPI - 006) CD73 with non - cross blocking antibody (total CD73) CD73 Occupancy (%) CD73 with CPI - 006 demonstrates sites occupied 0 7 14 21 Time (Days) Mean CPI - 006 Serum Concentration( μ g/mL) 1 mg/kg 6 mg/kg 18 mg/kg 3 mg/kg 12 mg/kg 24 mg/kg
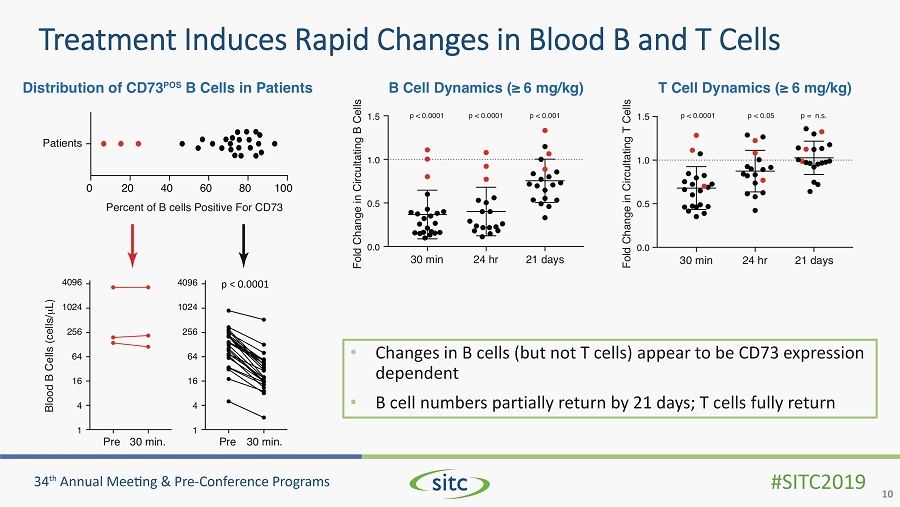
Treatment Induces Rapid Changes in Blood B and T Cells 10 • Changes in B cells (but not T cells) appear to be CD73 expression dependent • B cell numbers partially return by 21 days; T cells fully return p < 0.0001
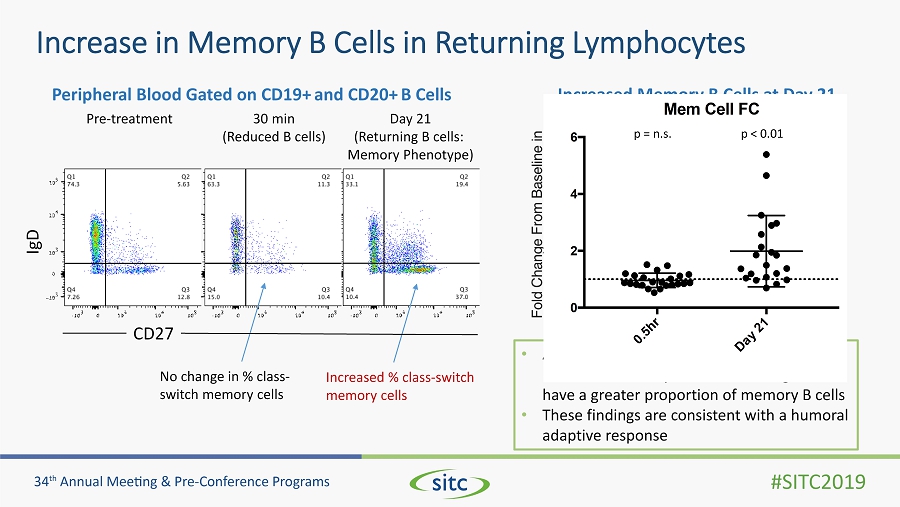
Increase in Memory B Cells in Returning Lymphocytes Pre - treatment 30 min (Reduced B cells) Day 21 (Returning B cells: Memory Phenotype) Increased % class - switch memory cells No change in % class - switch memory cells CD27 IgD • At 0.5 hours no change in proportion of naïve and memory B cells; returning cells have a greater proportion of memory B cells • These findings are consistent with a humoral adaptive response Peripheral Blood Gated on CD19+ and CD20+ B Cells Increased Memory B Cells at Day 21 0 . 5 h r D a y 2 1 0 2 4 6 Mem Cell FC p = n.s . p < 0.01
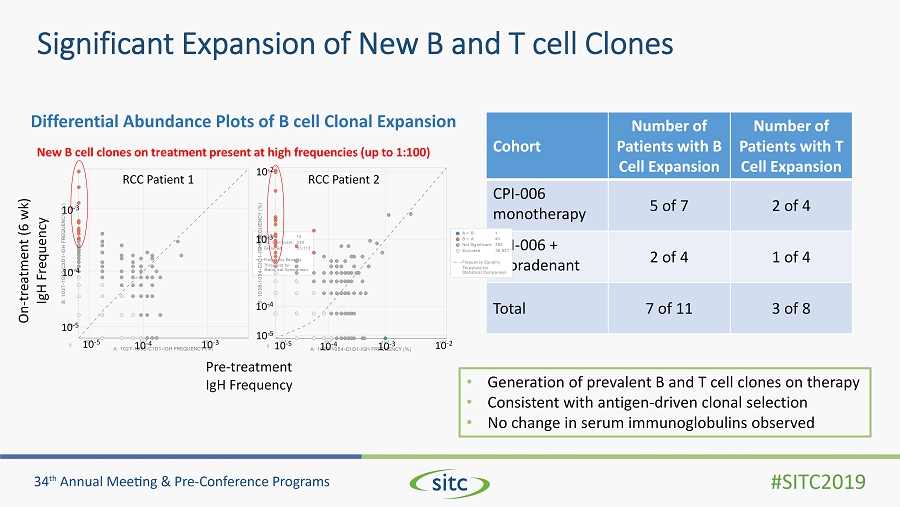
Significant Expansion of New B and T cell Clones Differential Abundance Plots of B cell Clonal Expansion Cohort Number of Patients with B Cell Expansion Number of Patients with T Cell Expansion CPI - 006 monotherapy 5 of 7 2 of 4 CPI - 006 + ciforadenant 2 of 4 1 of 4 Total 7 of 11 3 of 8 10 -5 10 -4 10 -3 A: 1027-1033-C1D1-IGH FREQUENCY (%) 10 -5 10 -4 10 -3 B : 1 0 2 7 - 1 0 3 3 - C 3 D 1 - I G H F R E Q U E N C Y ( % ) 0 B > A: 14 Not Significant: 239 Excluded: 83,113 Frequency Equality Threshold for Statistical Comparison Pre - treatment IgH Frequency On - treatment (6 wk ) IgH Frequency 10 - 5 10 - 4 10 - 3 10 - 5 10 - 4 10 - 3 • Generation of prevalent B and T cell clones on therapy • Consistent with antigen - driven clonal selection • No change in serum immunoglobulins observed 10 -5 10 -4 10 -3 10 -2 A: 1038-1054-C1D1-IGH FREQUENCY (%) 10 -5 10 -4 10 -3 10 -2 B : 1 0 3 8 - 1 0 5 4 - C 3 D 1 - I G H F R E Q U E N C Y ( % ) 0 A > B: 1 B > A: 40 Not Significant: 380 Excluded: 36,937 Frequency Equality Threshold for Statistical Comparison 10 - 5 10 - 4 10 - 3 10 - 5 10 - 4 10 - 3 10 - 2 10 - 2 New B cell clones on treatment present at high frequencies (up to 1:100) RCC Patient 1 RCC Patient 2
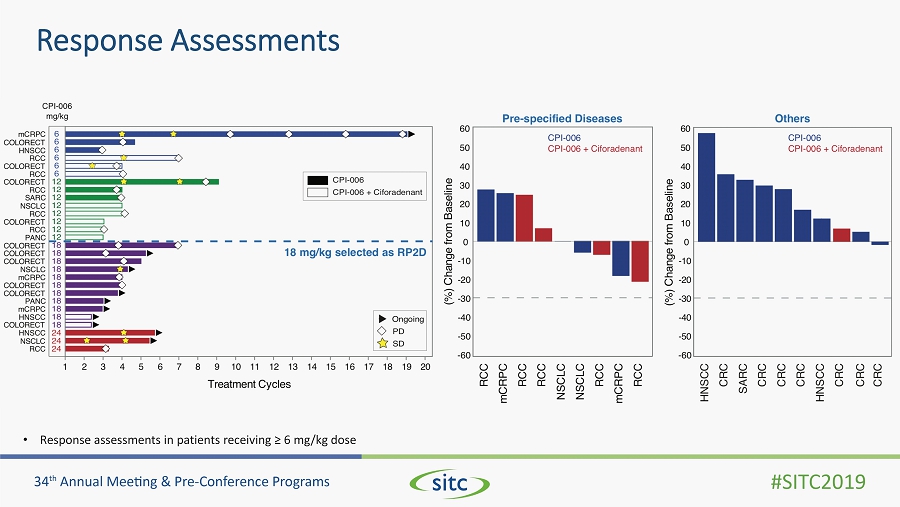
Response Assessments • Response assessments in patients receiving ≥ 6 mg/kg dose
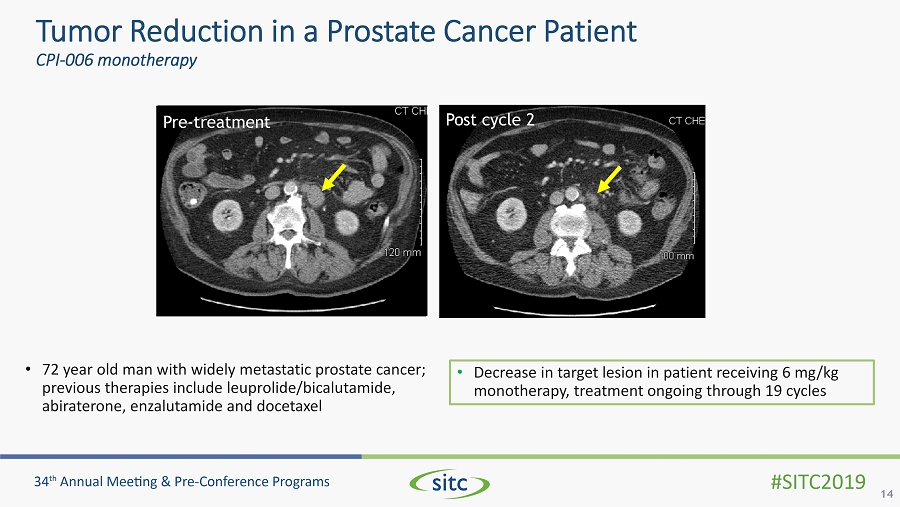
Tumor Reduction in a Prostate Cancer Patient CPI - 006 monotherapy 14 • Decrease in target lesion in patient receiving 6 mg/kg monotherapy, treatment ongoing through 19 cycles Pre - treatment Post cycle 2 • 72 year old man with widely metastatic prostate cancer; previous therapies include leuprolide/bicalutamide, abiraterone, enzalutamide and docetaxel
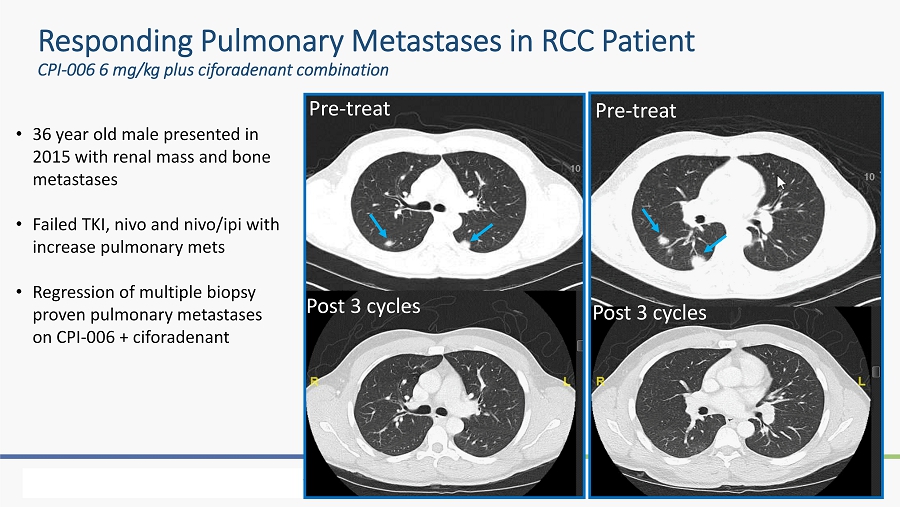
Responding Pulmonary Metastases in RCC Patient CPI - 006 6 mg/kg plus ciforadenant combination • 36 year old male presented in 2015 with renal mass and bone metastases • Failed TKI, nivo and nivo / ipi with increase pulmonary mets • Regression of multiple biopsy proven pulmonary metastases on CPI - 006 + ciforadenant Pre - treat Post 3 cycles Pre - treat Post 3 cycles
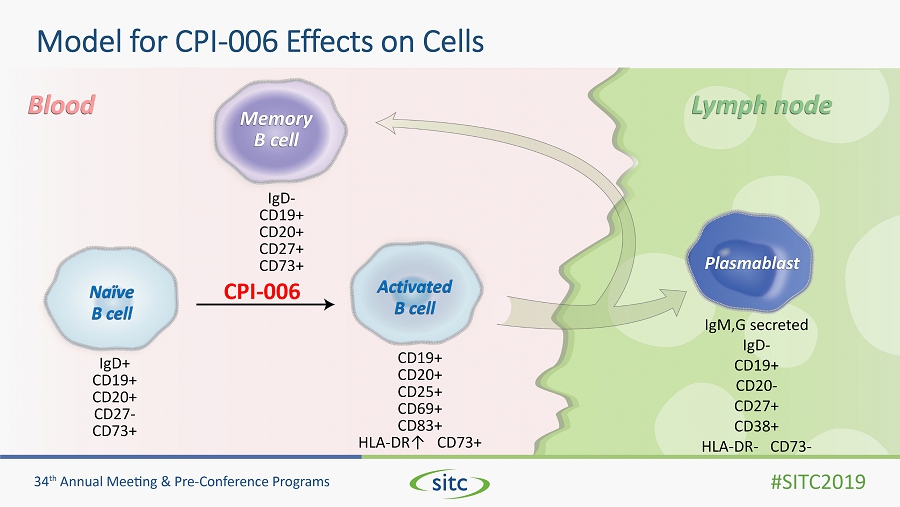
Model for CPI - 006 Effects on Cells CPI - 006 Memory B cell Naïve B cell Activated B cell Plasmablast Lymph node Blood IgD + CD19+ CD20+ CD27 - CD73+ IgD - CD19+ CD20+ CD27+ CD73+ CD19+ CD20+ CD25+ CD69+ CD83+ HLA - DR↑ CD73+ IgM,G secreted IgD - CD19+ CD20 - CD27+ CD38+ HLA - DR - CD73 -
Conclusions • CPI - 006 has novel immunomodulatory activities: • Induces differentiation of B cells, class switching, secretion of immunoglobulin (in vitro), and generation of memory B cells • Increases expression of CD69 and other markers consistent with increased antigen presentation by APCs • The optimum and well tolerated dose of CPI - 006 is 18 mg/kg • Treatment with CPI - 006 induces redistribution of T and B cells with an increase in returning memory B cells and expansion of new B cell clones • Changes in lymphocytes are consistent with induction of adaptive humoral immunity • Tumor regression observed in RCC and prostate • Treatment with CPI - 006 may represent an opportunity to identify novel anti - tumor antibodies

Acknowledgements • The patients and their families • Participating Centers and Investigators : Dana Farber Cancer Institute, Medical College of Wisconsin, Monash Health, Mount Sinai Icahn School of Medicine, University of California San Francisco Medical Center, University of Chicago, University of Miami, University of Oklahoma, Yale University, Carolina BioOncology Institute, City of Hope, Mary Crowley Cancer Research, Roswell Park Cancer Institute, Sarah Cannon Research Institute • Colleagues at Corvus

















