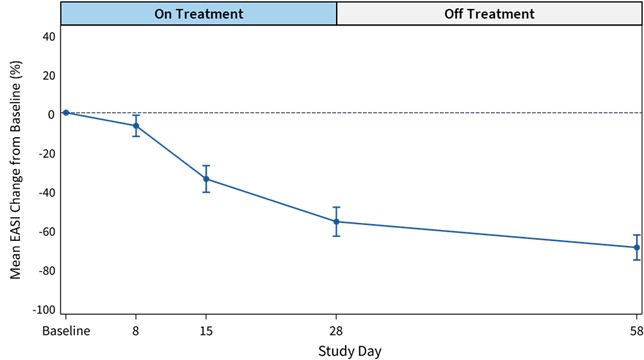
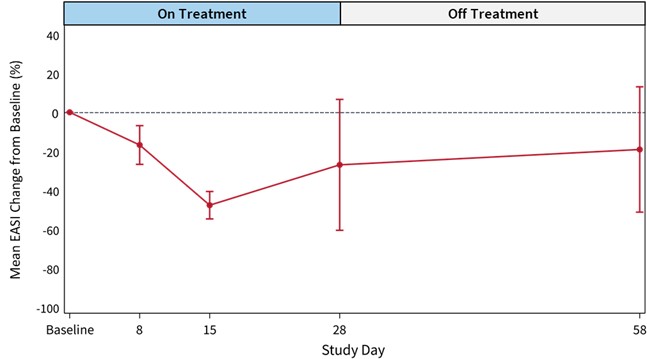
Cohort 1 Safety Data
No significant safety issues were observed. All the patients completed 28 days of dosing. One patient reported Grade 1 nausea that did not interfere with the subject receiving the full treatment course, and one patient developed COVID-19 on day 28 of treatment; that patient had an uneventful recovery. No clinically significant laboratory abnormalities were seen. See Table 3 below.
Table 3: Cohort 1 Safety | |
| | Soquelitinib | | Placebo | |
| | (N=12) | | (N=4) | |
Subjects with adverse events | | 2* | | 0 | |
Serious adverse events | | 0 | | 0 | |
Adverse events leading to study drug discontinuation | | 0 | | 0 | |
Adverse events leading to death | | 0 | | 0 | |
Treatment-related adverse events: | | | | | |
Nausea (Grade 1) | | 1 | | 0 | |
* Reported adverse events: Nausea (N=1) and Covid-19 (N=1); both resolved without any dose modification.
Serum Cytokine Changes
Relationships between reductions in certain cytokines with improvement in EASI scores were observed. Significant cytokines changes were seen for IL-5, IL-17, IL-31, IL-33, TSLP and a trend for TARC in EASI 50 responders (N=9) compared to non-responders (N=3). No such relationships were seen in the placebo group.
Cohort 2 Initial Efficacy and Safety Data
As of December 16, the Company has enrolled 12 patients in Cohort 2 of the trial (soquelitinib 200 mg oral once per day). As of December 7, Day 28 follow up data is available for three patients with efficacy results consistent with that seen in Cohort 1. No clinically significant laboratory abnormalities or treatment related adverse events have been reported in any of the patients enrolled in Cohort 2.
Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements, including statements related to the potential safety and efficacy of soquelitinib; expected cash proceeds from the exercise of warrants; and the Company’s conduct of, enrollment in and timing of clinical trials. All statements other than statements of historical fact contained in this Current Report on Form 8-K are forward-looking statements. These statements often include words such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate,” “seek,” “will,” “may” or similar expressions. Forward-looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond the Company’s control. The Company’s actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Quarterly Report on Form 10-Q for the three months ended September 30, 2024, filed with the Securities and Exchange Commission on November 12, 2024, as well as other documents that may be filed by the Company from time to time with the Securities and Exchange Commission. In particular, the following factors, among others, could cause results to differ materially from those expressed or implied by such forward-looking statements: the Company’s ability to demonstrate sufficient evidence of efficacy and safety in its clinical trials of its product candidates; the accuracy of the Company’s estimates relating to its ability to initiate and/or complete preclinical studies and clinical trials and release data from such studies and clinical trials; the results of preclinical studies and interim data from clinical trials not being predictive of future results; the Company’s ability to enroll sufficient numbers of patients in its clinical trials; the unpredictability of the regulatory process; regulatory developments in the United States and other foreign countries; the costs of clinical trials may exceed expectations; and the Company’s ability to raise additional capital. Although the