UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2016
OR
☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 001-36794
The Chemours Company
(Exact Name of Registrant as Specified in Its Charter)
Delaware |
| 46-4845564 |
(State or other Jurisdiction of Incorporation or Organization) |
| (I.R.S. Employer Identification No.) |
1007 Market Street, Wilmington, Delaware 19899
(Address of Principal Executive Offices)
Registrant’s Telephone Number: (302) 773-1000
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class |
| Name of Exchange on Which Registered |
Common Stock ($.01 par value) |
| New York Stock Exchange |
Securities are registered pursuant to Section 12(g) of the Act: None
Indicate by check mark whether the registrant is a well-known seasoned issuer (as defined in Rule 405 of the Securities Act). |
| Yes ☒ No ☐ | ||||
|
|
| ||||
Indicate by check mark whether the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. |
| Yes ☐ No ☒ | ||||
|
|
| ||||
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. |
| Yes ☒ No ☐ | ||||
|
|
| ||||
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). |
| Yes ☒ No ☐ | ||||
|
|
| ||||
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. |
| ☐ | ||||
|
|
| ||||
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer” and “large accelerated filer” in Rule 12b-2 of the Exchange Act. | ||||||
| ||||||
Large accelerated filer ☒ | Accelerated filer ☐ | Non-accelerated filer ☐ | Smaller reporting company ☐ | |||
|
|
| ||||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). |
| Yes ☐ No ☒ | ||||
The aggregate market value of common stock held by non-affiliates of the registrant as of June 30, 2016, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $1.5 billion. As of February 14, 2017, 183,153,218 shares of the company’s common stock, $0.01 par value, were outstanding.
Documents Incorporated by Reference
Portions of the registrant’s definitive proxy statement relating to its 2017 annual meeting of shareholders (2017 Proxy Statement) are incorporated by reference into Part III of this Annual Report on Form 10-K where indicated. The 2017 Proxy Statement will be filed with the U. S. Securities and Exchange Commission within 120 days after the end of the fiscal year to which this report relates.
Table of Contents
1
The Chemours Company
This section and other parts of this Annual Report on Form 10-K contain forward-looking statements, within the meaning of the federal securities law, that involve risks and uncertainties. Forward-looking statements provide current expectations of future events based on certain assumptions and include any statement that does not directly relate to any historical or current fact. The words “believe”, “expect”, “anticipate”, “plan”, “estimate”, “target”, “project” and similar expressions, among others, generally identify “forward-looking statements”, which speak only as of the date the statements were made. The matters discussed in these forward-looking statements are subject to risks, uncertainties and other factors that could cause actual results to differ materially from those set forth in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and in the Item 1A, “Risk Factors”.
Forward-looking statements are based on certain assumptions and expectations of future events which may not be accurate or realized. Forward-looking statements also involve risks and uncertainties, many of which are beyond Chemours’ control. Important factors that may materially affect such forward-looking statements and projections include:
| • | Fluctuations in energy and raw material prices; |
| • | Failure to develop and market new products and optimally manage product life cycles; |
| • | Our substantial indebtedness and availability of borrowing facilities, including access to our revolving credit facilities; |
| • | Uncertainty regarding the availability of additional financing in the future, and the terms of such financing; |
| • | Negative rating agency actions; |
| • | Significant litigation and environmental matters, including indemnifications we were required to assume; |
| • | Failure to appropriately manage process safety and product stewardship issues; |
| • | Changes in laws and regulations or political conditions; |
| • | Global economic and capital markets conditions, such as inflation, interest and currency exchange rates, and commodity prices, as well as regulatory requirements; |
| • | Currency related risks; |
| • | Business or supply disruptions and security threats, such as acts of sabotage, terrorism or war, weather events and natural disasters; |
| • | Ability to protect, defend and enforce Chemours’ intellectual property rights; |
| • | Increased competition and increasing consolidation of our core customers; |
| • | Changes in relationships with our significant customers and suppliers; |
| • | Significant or unanticipated expenses, including but not limited to litigation or legal settlement expenses; |
| • | Our ability to predict, identify and interpret changes in consumer preference and demand; |
| • | Our ability to realize the expected benefits of the Separation (as defined elsewhere in this Annual Report); |
| • | Our ability to complete potential divestitures or acquisitions and our ability to realize the expected benefits of divestitures or acquisitions if they are completed; |
| • | Our ability to deliver cost savings as anticipated, whether or not on the timelines proposed; |
| • | Our ability to pay a dividend and the amount of any such dividend declared; and |
| • | Disruptions in our information technology networks and systems. |
Additionally, there may be other risks and uncertainties that we are unable to identify at this time or that we do not currently expect to have a material impact on our business. The Company assumes no obligation to revise or update any forward-looking statement for any reason, except as required by law.
Unless the context otherwise requires, references herein to “The Chemours Company”, “The Chemours Company, LLC”, “Chemours”, “the Company”, “our company”, “we”, “us”, and “our” refer to The Chemours Company and its consolidated subsidiaries. References herein to “DuPont” refers to E.I. du Pont de Nemours and Company, a Delaware corporation, and its consolidated subsidiaries (other than Chemours and its consolidated subsidiaries), unless the context otherwise requires.
2
The Chemours Company
Overview
The Chemours Company is a leading global provider of performance chemicals. We began operating as an independent company on July 1, 2015 (the Separation Date) after separating from E. I. du Pont de Nemours and Company (DuPont) (the Separation). Our company is comprised of three reportable segments: Titanium Technologies, Fluoroproducts and Chemical Solutions. Our Titanium Technologies segment is the leading global producer of titanium dioxide (TiO2), a premium white pigment used to deliver whiteness, brightness, opacity and protection in a variety of applications. Our Fluoroproducts segment is a leading global provider of fluoroproducts, including refrigerants and industrial fluoropolymer resins. Our Chemical Solutions segment is a leading North American provider of industrial chemicals used in gold production, oil and gas, water treatment and other industries.
Effective prior to the opening of trading on the New York Stock Exchange (NYSE) on July 1, 2015, DuPont completed the separation of the businesses comprising DuPont’s Performance Chemicals reporting segment, and certain other assets and liabilities, into Chemours, a separate and distinct public company. The separation was completed by way of a distribution of all of the then-outstanding shares of common stock of Chemours through a dividend in kind of Chemours’ common stock (par value $0.01) to holders of DuPont common stock (par value $0.30) as of the close of business on June 23, 2015 (the Record Date) (the transaction is referred to herein as the Distribution).
On the Separation Date, each holder of DuPont’s common stock received one share of Chemours’ common stock for every five shares of DuPont’s common stock held on the Record Date. The Separation was completed pursuant to a separation agreement and other agreements with DuPont, including an employee matters agreement, a tax matters agreement, a transition services agreement and an intellectual property cross-license agreement. These agreements govern the relationship between Chemours and DuPont following the separation and provided for the allocation of various assets, liabilities, rights and obligations. These agreements also included arrangements for transition services provided by DuPont to Chemours that were substantially completed during 2016.
We operate 26 production facilities located in 10 countries and serve over 3,800 customers across a wide range of end markets in more than 130 countries. The following chart illustrates the global sales of our businesses for the years ended December 31, 2016, 2015, and 2014:

Chemours is committed to creating value for our customers through the reliable delivery of high quality products and services around the globe. We create value for customers and stockholders through (i) operational excellence and asset efficiency, which includes our commitment to safety and environmental stewardship, (ii) strong customer focus to produce innovative, high-performance products, (iii) focus on cash flow generation through optimization of our cost structure, and improvement in working capital and supply chain efficiencies through our transformation plan (described below), (iv) organic growth and inorganic expansions to current business and (v) creation of an organization that is committed to our corporate values of safety, customer appreciation, simplicity, collective entrepreneurship and integrity.
Many of Chemours’ commercial and industrial relationships span decades. Our customer base includes a diverse set of companies, many of which are leaders in their respective industries. Our sales are not materially dependent on any single customer. As of
3
The Chemours Company
December 31, 2016, no one individual customer balance represented more than five percent of Chemours’ total outstanding receivables balance and no one individual customer represented more than ten percent of our sales.
Chemours Five-Point Transformation Plan
Following the Separation, Chemours developed a Five-Point Transformation Plan to address changes to our organization, cost structure and portfolio of businesses. We have made considerable progress on our transformation plan throughout 2016, with additional cost reductions and growth targeted in 2017.
The objectives of our multi-year five-point transformation plan are to improve our financial performance, streamline and strengthen our portfolio and reduce our leverage by:
| 1. | Reducing our costs through a simpler business model; |
| 2. | Optimizing our portfolio to focus on our businesses where we have leading positions; |
| 3. | Growing our market positions where we have competitive advantages; |
| 4. | Refocusing our investments by concentrating our capital expenditures on our core businesses; and |
| 5. | Enhancing our organization to deliver our values and support our transformation to a higher-value chemistry company. |
Through cost reduction and growth, Chemours expects the transformation plan to deliver $500 million of incremental Adjusted EBITDA improvement over 2015 through 2017. Based on our anticipated cost reduction and growth initiatives, we expect that our cost savings of approximately $350 million and approximately $150 million in improvements from growth initiatives will also improve our pre-tax earnings by similar amounts over 2015 through 2017. These improvements will be partially offset by the impact of divestitures completed during 2016, unfavorable price and mix of fluoropolymer products and may also be impacted by market factors. For the year ended December 31, 2016, we had a pre-tax loss and Adjusted EBITDA of $11 million and $822 million, respectively, compared to a pre-tax loss of $188 million and adjusted EBITDA of $573 million for the year ended December 31, 2015. Our 2016 pre-tax loss includes net gain from divestitures of approximately $254 million offset by $335 million litigation accrual related to the PFOA MDL Settlement (see Item 3. Legal Proceedings, Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations, and Note 20 to the Consolidated Financial Statements included elsewhere in this Annual Report).
Through a combination of higher cash flow from operations, lower capital spending, and proceeds from asset sales, we anticipate reducing our leverage ratio (net debt to Adjusted EBITDA) to approximately three times by the end of 2017. As of December 31, 2016, our leverage ratio is approximately 3.3 times.
Adjusted EBITDA is a non-GAAP financial measure. For a discussion of our use of non-GAAP financial measures and reconciliations to the closest GAAP financial measures, see Item 7 - Management’s Discussion and Analysis of Financial Condition and Results of Operations - Non-GAAP Financial Measures.
Segments
In our Titanium Technologies segment, we have a long-standing history of delivering high-quality TiO2 pigment using our proprietary chloride technology. We are the largest global producer of TiO2, and our low-cost network of manufacturing facilities allows us to efficiently and cost-effectively serve our global customer base. During 2016, we further enhanced our operating cost advantage with the startup of our second production line at our Altamira, Mexico facility. Chemours is well positioned to remain one of the lowest cost TiO2 producers and continue to meet our customers’ growing needs around the world.
In our Fluoroproducts segment, we are one of two globally integrated producers making both fluorochemicals and fluoropolymers. In Fluorochemicals, we expect to see increased adoption of Opteon™, the world’s lowest global warming potential refrigerant, as governments around the world pass legislation that makes the use of low global warming potential refrigerants a requirement. Our fluoropolymers offerings provide customers with tailored products that have unique properties, including very high temperature resistance and high chemical resistance. We will continue to invest in research and development to remain a leader in these areas, and ensure that we are able to meet our customers’ needs as regulations change.
In our Chemical Solutions segment, we completed our strategic review of our portfolio in 2016, including the announced sales of the Beaumont Aniline facility, Clean & Disinfect business, and Sulfur products business, and ceased production at our Reactive Metals Solutions (RMS) facility in Niagara Falls, New York. We remain committed to retaining and improving our Mining Solutions
4
The Chemours Company
business (previously known as Cyanides business) and the product lines at our Belle, West Virginia site. We are investing in our Mining Solutions business during 2017 and 2018 to increase our capacity by approximately 50 percent. This additional capacity will allow us to serve the growing demand for sodium cyanide in the gold mining industry in the Americas.
We will maintain our commitment to responsible stewardship and safety for our employees, customers and the communities where we operate. Meeting and exceeding our customers’ expectations while conducting business in accordance with our high ethical standards will continue to be a primary focus for our company as we continue to transform Chemours into a higher-value chemistry company.
Additional information on our segments can be found in Management’s Discussion and Analysis of Financial Condition and Results of Operations and Note 24 to the Consolidated Financial Statements.
Titanium Technologies Segment
Segment Overview
The Chemours Titanium Technologies segment is the leading global manufacturer of titanium dioxide, or TiO2. TiO2 is a pigment used to deliver whiteness, opacity, brightness and protection from sunlight in applications such as architectural and industrial coatings, flexible and rigid plastic packaging, PVC window profiles, laminate papers, coated paper and coated paperboard used for packaging. We sell our TiO2 products under the Ti-Pure™ brand name to over 800 customers globally. We operate four TiO2 production facilities: two in the United States (U.S.), one in Mexico and one in Taiwan. In addition, we have a large-scale repackaging and distribution facility in Belgium and operate a mineral sands mining operation in Starke, Florida. In total, we have a TiO2 capacity of 1.25 million metric tons per year. In 2016, we expanded our TiO2 production facility in Altamira, Mexico. We plan to steadily ramp up production at Altamira, with full capacity of the new line reaching approximately 200,000 metric tons annually being achieved over the next few years.
Chemours is one of a limited number of producers operating a chloride process for the production of TiO2. We believe that our proprietary chloride technology enables us to operate plants at a much higher capacity than other chloride technology-based TiO2 producers, uniquely utilizing a broad spectrum of titanium-bearing ore feedstocks and achieving the highest unit margins in our industry. This technology, which is in use at all of our production facilities, provides us with one of the industry’s lowest manufacturing cost positions. Our research and development efforts focus on improving production processes and developing TiO2 grades that help our customers achieve optimal cost and product performance.
TiO2 demand is highly correlated to growth in the global residential housing, commercial construction and packaging markets. Industry demand for TiO2 is generally expected to be in line with global GDP growth, and can be cyclical due to economic and industry-specific market dynamics. We believe that the TiO2 demand grew above GDP growth rates in 2016 due to a pent-up demand created by destocking in 2015. We believe the market growth seen in 2016 was a combination of market growth and the return to more typical customer inventory levels. We expect TiO2 demand growth to return to approximate GDP growth rates in the long-term. Chemours’ future demand growth may be below average global GDP growth rates if our sales into developed markets outpaces our sales into emerging markets.
Our Titanium Technologies segment net sales by region for the years ended December 31, 2016, 2015, and 2014 is shown in the chart below:

5
The Chemours Company
We sell over 20 different grades of TiO2, with each pigment grade tailored for targeted applications. Our portfolio of premium performance TiO2 pigment grades provide end users with benefits beyond opacity, such as longer lasting performance, brighter colors and the brilliant whites achievable only through Chloride manufactured pigment.
We have operated a titanium mine in Starke, Florida since 1949. The mine provides us with access to a low cost source of domestic, high quality ilmenite ore feedstock and supplies less than ten percent of our ore feedstock consumption needs. Co-products of our mining operations, which comprised less than five percent of our total sales in Titanium Technologies in 2016, are zircon (zirconium silicate) and staurolite minerals. We are a major supplier of high quality calcined zircon in North America, primarily focused on the precision investment casting (PIC) industry, foundry and specialty applications, and ceramics. Our staurolite blasting abrasives, sold as Starblast, are used in steel preparation and maintenance, and paint removal.
Revenue and earnings performance in Titanium Technologies reflect the cyclical nature of the global TiO2 business. TiO2 pricing tends to move up and down in a cyclical manner depending in large part on global economic conditions. Following the global financial crisis in 2008, global economic recovery, resulting from the impact of government stimulus, resulted in strong customer demand for TiO2 compared to available supply. This drove TiO2 prices higher, ultimately reaching a historical peak in 2012. New industry capacity, stimulated by that period of strong demand between 2008-2012, came online at the same time that global GDP fell back to a 2-3 percent annual growth rate. This oversupply situation resulted in price declines, until early 2016. We believe the TiO2 industry has moved past the bottom of the cycle and has returned to a modest level of profitability. As described, Titanium Technologies has unique capabilities which deliver the industry’s best cost position, resulting in strong operating cash flow.
Industry Overview and Competitors
We estimate the worldwide demand for TiO2 in 2016 was approximately 5.7 million metric tons, of which 3.6 million metric tons were for premium performance pigments. Worldwide capacity in 2016 was estimated to be approximately 7.0 million metric tons. The products manufactured on this global capacity base are not fully substitutable due to pigment quality consistency and pigment product design. We believe that the utilization of the premium performance manufacturing base is considerably higher than that for general purpose - lower performance production.
Competition in the TiO2 pigment market is based primarily on product performance (both product design and quality consistency), supply capability and technical service. Our major competitors within higher performance pigments include: The National Titanium Dioxide Company, Ltd. (Cristal), Huntsman International LLC, Kronos Worldwide, Inc. and Tronox Limited.
Beyond multi-national suppliers, the other TiO2 pigment producers are very fragmented and mostly utilize the sulfate production process and compete in the general purpose – lower performance pigment market. In 2016, the combination of Sichuan Lomon and Henan Billions into the Lomon Billions entity demonstrated the consolidation of Chinese producers and created a large global producer representing approximately eight percent of global capacity. In the next one to three years, industry experts believe that there will be no new capacity added outside of China. Within China, the announced added effective capacity is expected to be somewhat offset by capacity shutdowns at marginal producers. Certain new capacity additions announced in China are based on a chloride technology.
Raw Materials
The primary raw materials used in the manufacture of TiO2 are titanium-bearing ores, chlorine, calcined petroleum coke and energy. We source titanium-bearing ores from a number of suppliers around the globe, who are primarily located in Australia and Africa. Our titanium mine in Starke, Florida supplies less than ten percent of our raw material needs. To ensure proper supply volume and to minimize pricing volatility, we generally enter into contracts in which volume is requirement-based and pricing is determined by a range of mechanisms structured to help us achieve competitive pricing relative to the market. We typically enter into a combination of long- and mid-term supply contracts and source our raw material from multiple suppliers across different regions and from multiple sites per supplier. Furthermore, we typically purchase multiple grades of ore from each supplier to limit our exposure to any single supplier for any single grade of ore in any given time period. Historically, we have not experienced any problems renewing such contracts for raw materials or securing our supply of titanium-bearing ores.
We play an active role in ore source development around the globe, especially for those ores which can only be used by Chemours, given the capability of our unique process technology. Supply chain flexibility allows for ore purchase and use optimization to manage short-term demand fluctuations and provides long-term competitive advantage. Our process technology and ability to use lower grade ilmenite ore gives us the flexibility to alter our ore mix to the lowest cost configuration based on sales, demand and projected ore pricing. Lastly, we have taken steps to optimize routes for distribution and increase storage capacity at our production facilities.
6
The Chemours Company
Transporting chlorine, one of our primary raw materials, can be costly. To reduce our expense and our need to transport chlorine, we have a chlor-alkali production facility run by a third party that is co-located at our New Johnsonville, Tennessee site. Calcined petroleum coke is an important raw material input to our process. We source calcined petroleum coke from well-established suppliers in North America and China, typically under contracts that run multiple years to facilitate material and logistics planning through the supply chain. Distribution efficiency is enhanced through use of bulk ocean, barge and rail transportation modes.
Energy is another key input cost into the TiO2 manufacturing process, representing approximately ten percent of the production cost. Chemours has access to natural gas based energy at our U.S. and Mexico TiO2 production facilities and our Florida minerals plant, supporting advantaged energy costs given the low cost shale gas in the U.S. Natural gas-based cogeneration of steam and electricity was recently extended as part of the major expansion at one of our TiO2 production facilities.
Sales, Marketing and Distribution
We sell the majority of our products through a direct sales force. We also utilize third-party sales agents and distributors to expand our reach. TiO2 represents a significant raw material cost for our customers and as a result, purchasing decisions are often made by our customers’ senior management team. Our sales organization works to develop and maintain close relationships with key decision makers in our value chain.
In addition, our sales and technical service teams work together to develop relationships with all layers of our customers’ organizations to ensure that we meet our customers’ commercial and technical requirements. When appropriate, we collaborate closely with customers to solve formulation or application problems by modifying product characteristics or developing new product grades.
To ensure an efficient distribution, we have a large fleet of railcars, which are predominantly used for outbound distribution of products in the U.S. and Canada. A dedicated logistics team, along with external partners, continually optimizes the assignment of our transportation equipment to product lines and geographic regions in order to maximize utilization and maintain an efficient supply chain.
Customers
Globally, we serve over 800 customers through our Titanium Technologies segment. In 2016, our ten largest Titanium Technologies customers accounted for approximately 30 percent of the segment’s sales. No single Titanium Technologies customer represented more than ten percent of our segment sales in 2016. Our larger customers in the U.S. and Europe are typically served through direct sales and tend to have medium- to long-term contracts with annual supply volume requirements and periodic price adjustment mechanisms. We serve our small- and mid-size customers through a combination of our direct sales and distribution network.
Our direct customers in Titanium Technologies are producers of decorative coatings, automotive and industrial coatings, polyolefin masterbatches, polyvinylchloride window profiles, engineering polymers, laminate paper, coatings paper and coated paperboard. We focus on developing long-term partnerships with key market participants in each of these sectors. We also deliver a high level of technical service to satisfy our customers’ specific needs, which helps us maintain strong customer relationships.
Seasonality
The demand for TiO2 is subject to seasonality due to the influence of weather conditions and holiday seasons on some of our applications, such as decorative coatings. As a result, our TiO2 sales volume is typically lowest in the first quarter, highest in the second and third quarters and moderate in the fourth quarter. This pattern applies to the entire TiO2 market, but may vary by region, country or application. It can also be altered by economic or other demand cycles.
Fluoroproducts Segment
Segment Overview
Our Fluoroproducts segment is a global leader in providing fluorine-based, advanced material solutions. The segment creates products that have unique properties such as high temperature resistance, high chemical resistance and unique di-electric properties for applications across a broad array of industries and applications. We are a global leader in providing fluoroproducts, such as refrigerants and industrial fluoropolymer resins and derivatives.
7
The Chemours Company
The manufacturing of fluoroproducts involves complex processes which include the use of highly corrosive and hazardous intermediates. We have an industry-leading safety culture and apply world-class technical expertise to ensure that our operations run safely and reliably. These capabilities, alongside our research and development expertise, allow us to continuously improve our process technology.
We sell fluoroproducts through two primary product groups: Fluorochemicals and Fluoropolymers.
Fluorochemicals products include refrigerants, air conditioning, foam expansion agents, propellants and fire extinguishants. We have held a leading position in the fluorochemicals market since the commercial introduction of Freon™ in 1930. Since the original chlorofluorocarbons (CFCs) based product was introduced, we have been at the forefront of new-technology research for lower global warming potential and ozone depleting products, leading to the development of hydrofluorocarbons (HFCs) and hydrochlorofluorocarbons (HCFCs). We have a leading position in HFC refrigerants under the brand name Freon™ and are a leader in the development of sustainable technologies like Opteon™, a line of low Global Warming Potential (GWP) hydrofluoroolefin (HFO) refrigerants, which also have a zero ozone depletion footprint. Opteon™ was jointly developed with Honeywell International, Inc., in response to the European Union’s (EU) Mobile Air Conditioning (MAC) Directive. This patented technology offers similar functionality to current HFC products but meets or exceeds currently mandated environmental standards and in some cases, provides energy efficiency benefits.
We led the industry in the Montreal-Protocol (1987) driven transition from CFCs to the lesser ozone depleting HCFCs and non-ozone depleting HFCs. In 1988, we committed to cease production of CFCs and started manufacturing non-ozone depleting HFCs in the early 1990s. Driven by new and emerging environmental legislations and standards currently being implemented across the U.S., Europe, Latin America and Japan, we have commercialized Opteon™. Over the years, regulation has pushed the industry to evolve and respond to environmental concerns. We will continue to invest in research and development to ensure that we remain a leader and are able to meet our customers’ needs as regulations change.
Fluorochemicals’ refrigerant sales fluctuate by season as sales in the first half of the year generally are slightly higher than sales in the second half of the year. However, Opteon™ sales into mobile air markets will be driven by automotive production, which may lead to less seasonality within Fluorochemicals overall.
Fluoropolymers products include various industrial resins, coatings, and other downstream products. We serve a wide range of industrial and end-user applications spanning from wearable electronics to automotive, network cables, pipe lining and gaskets, among others. Our products’ unique properties include corrosion resistance, non-stick adhesion, thermal stability, and extreme temperature resistance.
Our Fluoropolymers products are sold under the brand names Teflon™, Viton™, Krytox™, and Nafion™. Teflon™ coatings and additives are used in multiple end products including paints, fabrics, carpets, clothing, and other household applications. Teflon™ coatings, resins, additives and films are also used in a wide range of industrial products. Our fluoroelastomer products, sold under the Viton™ brand name, are used in automotive, consumer electronics, chemical processing, oil and gas, petroleum refining and transportation, and aircraft and aerospace applications. Our Krytox™ branded lubricants are used in a broad range of industrial applications, including bearings, electric motors, and gearboxes. We sell membranes under the brand name Nafion™, which are used in fuel cells, energy flow battery storage, transportation, stationary power, and medical tubing.
8
The Chemours Company
The Fluoroproducts segment’s net sales by region and product group for the years ended December 31, 2016, 2015, and 2014 are shown in the charts below:


Industry Overview and Competitors
Our Fluoroproducts segment competes against a broad variety of global manufacturers, including Honeywell, Arkema, Mexichem, Daikin, Solvay and Dyneon, as well as regional Chinese and Indian manufacturers. We have a leadership position in fluorine chemistry and materials science, a broad scope and scale of operations, market driven application development and deep customer knowledge.
Chemours has global leadership positions in the following fluoroproduct categories as set forth in the table below:
Fluoroproducts Leadership Positions | ||||||
Product Group |
| Position |
| Key Applications |
| Key Competitors |
Fluorochemicals |
| #1 Globally |
| Refrigeration and Air Conditioning |
| Honeywell, Arkema, Mexichem, Dongyue, Juhua |
Fluoropolymers |
| #1 Globally |
| Diversified industrial applications |
| Daikin, 3M, Solvay, Asahi Glass Company, Dongyue, Chenguang |
Fluoroproducts demand growth is generally in line with global GDP. Within Fluorochemicals, growth may be expected to be higher than GDP in situations where, for environmental reasons, regulatory drivers constrain the market or drive the market toward lower global warming alternatives. In Fluoropolymers, market growth is expected to be in line with GDP but influenced by increased competition and pricing pressure in some businesses.
9
The Chemours Company
Developed markets represent the largest fluoroproducts markets today. Global middle class growth and the increasing demand for expanding infrastructure, consumer electronics, telecommunications, automobiles, refrigerators and air conditioners are all key drivers of increased demand for various fluoroproducts.
Raw Materials
The primary raw materials required to support the Fluoroproducts segment are fluorspar, chlorinated organics, chlorinated inorganics, hydrofluoric acid and vinylidene fluoride. These are available in many countries and not concentrated in any particular region.
Our supply chains are designed for maximum competitiveness through favorable sourcing of key raw materials. Our contracts typically include terms that span from two to ten years, except for select resale purchases that are negotiated on a monthly basis. Most qualified Fluorspar sources have fixed contract prices or freely negotiated market-based pricing. Although the fluoroproduct industry has historically relied primarily on fluorspar exports from China, Chemours has diversified its sourcing through multiple geographic regions and suppliers to ensure a stable and cost competitive supply. Our current supply agreements are generally in effect for the next five years.
Sales, Marketing and Distribution
With more than 85 years of innovation and development in fluorine science, our technical, marketing and sales teams around the world have deep expertise in our products and their end-uses. We work with customers to select the appropriate fluoroproducts to meet their technical performance needs. We sell our products through direct channels and through resellers. Selling agreements vary by product line and markets served and include both spot pricing arrangements and contracts with a typical duration of one year.
We maintain a large fleet of railcars, tank trucks and containers to deliver our products and support our supply chain needs. For the portion of the fleet that is leased, related lease terms are usually staggered, which provides us with a competitive cost position as well as the ability to adjust the size of our fleet in response to changes in market conditions. A dedicated logistics team, along with external partners, continually optimizes the assignment of our transportation equipment to product lines and geographic regions in order to maximize utilization and flexibility of the supply chain.
Customers
We serve approximately 2,700 customers and distributors globally, and in many instances, these commercial relationships have been in place for decades. No single Fluoroproducts customer represented more than ten percent of the segment’s sales in 2016.
Seasonality
Seasonality in Fluorochemicals sales is mainly driven by increased demand for residential, commercial and automotive air conditioning in the spring. This demand peaks in the summer months and declines in the fall and winter. Commercial refrigeration demand is fairly steady throughout the year, but demand is slightly higher during the summer months. There is no significant seasonality for Fluoropolymers, as demand is relatively consistent throughout the year.
Chemical Solutions Segment
Segment Overview
Our Chemical Solutions segment comprises a portfolio of industrial chemical businesses primarily operating in the Americas. The Chemical Solutions segment’s products are used as important raw materials and catalysts for a diverse group of industries including, among others, gold production, oil and gas, water treatment, electronics and automotive. We are a leading provider of sodium cyanide in the Americas through our Mining Solutions business. Chemical Solutions generates value through the use of market leading manufacturing technology, safety performance, product stewardship, and differentiated logistics capabilities.
As part of our transformation plan announced in 2015, we conducted a strategic review of our Chemical Solutions segment. The process resulted in the divestiture of three assets and businesses, the shutdown of one business and the decision to retain the remaining businesses. Specifically, we sold our Aniline facility in Beaumont, Texas to The Dow Chemical Company in March 2016. We also sold our Sulfur Products business to Veolia in July 2016 and our Clean & Disinfect business to LANXESS Corporation (“Lanxess”) in August 2016. These divestitures resulted in gross proceeds of approximately $685 million in 2016. In addition, we ceased production at our RMS facility in Niagara Falls, New York in September 2016. The segment continues to include our mining solutions business
10
The Chemours Company
as well as the product lines at our Belle, West Virginia site, which include our Methylamines, Glycolic Acid and Vazo™ free radical initiators product lines.
Chemical Solutions operates at three dedicated production facilities in North America, which sells products and solutions through two primary product groups: Mining Solutions and Performance Chemicals & Intermediates. The Mining Solutions product group includes our sodium cyanide, hydrogen cyanide, and potassium cyanide product lines. We are the market leader in solid sodium cyanide production in the Americas, which is used primarily by the mining industry for gold and silver production. In the Performance Chemicals & Intermediates product group, we manufacture a wide variety of chemicals used in many different applications. Following the recent divestitures, Performance Chemicals & Intermediates is now comprised of our Methylamines, Glycolic Acid, and Vazo™ product lines. Our Performance Chemicals & Intermediates business is expected to generally grow in line with growth in global GDP.
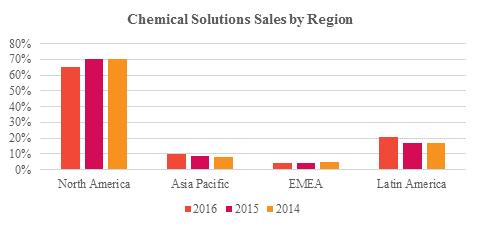
Chemical Solutions segment’s net sales by region and primary product groups for the years ended December 31, 2016, 2015, and 2014 are shown in the charts below.
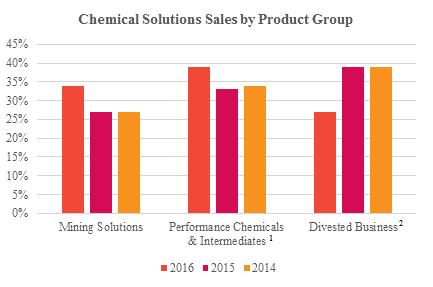
| 1 | 2015 and 2014 sales were recast to exclude sales from divested business. |
|
| 2 | Includes sales from our C&D business, Sulfur business and Aniline facility in Beaumont, TX, which were sold during 2016. |
|
Industry Overview and Competitors
The industrial and specialty chemicals produced by our Chemical Solutions segment are important raw materials for a wide range of industries and end markets. We hold a long standing reputation for high quality and the safe handling of hazardous products such as
11
The Chemours Company
sodium cyanide, methylamines and Vazo™. Our competitive cost positions in these products are the result of our process technology, manufacturing scale, efficient supply chain and proximity to large customers. Our Chemical Solutions segment also holds, and occasionally licenses, what we believe to be a leading process technologies for the production of hydrogen and sodium cyanide, which are used in industrial polymers and in gold production.
Chemours has global leadership positions in the following product categories:
Chemical Solutions Leadership Positions | ||||||
Product (Product Group) |
| Position |
| Key Applications |
| Key Competitors |
Mining Solutions 1 |
| #1 in Solid Sodium Cyanide in the Americas |
| Gold Production |
| Orica, Cyanco |
1 | Previously known as Cyanides business product group, which was renamed to Mining Solutions for the year ended December 31, 2016. |
Raw Materials
Key raw materials for Chemical Solutions include ammonia, methanol, natural gas, hydrogen and caustic soda. We source raw materials from global and regional suppliers where possible and maintain multiple supplier relationships to protect against supply disruptions and potential price increases. To further mitigate the risk of raw material availability and cost fluctuation, Chemical Solutions has also taken steps to optimize routes for distribution, lock in long-term contracts with key suppliers and increase the number of customer contracts with raw material price pass-through terms. We do not believe that the loss of any particular supplier would be material to our business.
Sales, Marketing and Distribution
Our technical, marketing and sales teams around the world have deep expertise with our products and their end markets. We predominantly sell directly to end-customers, although we also use a network of distributors for specific product lines and geographies. Sales may take place through either spot transactions or via long-term contracts.
Most of Chemical Solutions’ raw materials and products can be delivered by efficient bulk transportation. As such, we maintain a large fleet of railcars, tank trucks and containers to support our supply chain needs. For the portion of the fleet that is leased, related lease terms are usually staggered, which provides us with a competitive cost position as well as the ability to adjust the size of our container fleet in response to changes in market conditions. A dedicated logistics team, along with external partners, continually optimizes the assignment of our transportation equipment to product lines and geographic regions in order to maximize utilization and flexibility of the supply chain.
The strategic placement of our production facilities in locations designed to serve our key customer base in the Americas gives us robust distribution capabilities.
Customers
Our Chemical Solutions segment focuses on developing long-term partnerships with key market participants. Many of our commercial and industrial relationships have been in place for decades and are based on our proven value proposition of safely and reliably supplying our customers with the materials needed for their operations. Our reputation and long-term track record is a key competitive advantage as several of the products’ end users demand the highest level of excellence in safe manufacturing, distribution, handling and storage. Chemical Solutions has U.S. Department of Transportation Special Permits and Approvals in place for distribution of various materials associated with each of our business lines as required. Our Chemical Solutions segment serves over 400 customers globally. No single Chemical Solutions customer represented more than ten percent of the segment’s sales in 2016.
Seasonality
Our Chemical Solutions segment sales are subject to minimal seasonality.
Intellectual Property
Intellectual property, including trade secrets, certain patents, trademarks, copyrights, know-how and other proprietary rights, is a critical part of maintaining our technology leadership and competitive edge. Our business strategy is to file patent and trademark applications globally for proprietary new product and application development technologies. We hold many patents, particularly in
12
The Chemours Company
our Fluoroproducts segment, as described herein. These patents, including various patents that will expire from 2017 through 2034, in the aggregate, are believed to be of material importance to our business. However, we believe that no single patent (or related group of patents) is material in relation to our business as a whole. In addition, particularly in our Titanium Technologies segment, we hold significant intellectual property in the form of trade secrets and, while we believe that no single trade secret is material in relation to our combined business as a whole, we believe they are material in the aggregate. Unlike patents, trade secrets do not have a predetermined validity period, but are valid indefinitely, so long as their secrecy is maintained. We work actively on a global basis to create, protect and enforce our intellectual property rights. The protection afforded by these patents and trademarks varies based on country, scope of individual patent and trademark coverage, as well as the availability of legal remedies in each country. Although certain proprietary intellectual property rights are important to the success of our company, we do not believe that we are materially dependent on any particular patent or trademark. We believe that securing our intellectual property is critical to maintaining our technology leadership and our competitive position, especially with respect to new technologies or the extensions of existing technologies. Our proprietary process technology is also a source of incremental income through licensing arrangements.
Our Titanium Technologies segment in particular relies upon unpatented proprietary knowledge and continuing technological innovation and other trade secrets to develop and maintain our competitive position in this space. Our proprietary chloride production process is an important part of our technology and our business could be harmed if our trade secrets are not maintained in confidence. In our Titanium Technologies intellectual property portfolio, we consider our trademark Ti-Pure™ to be a valuable asset and have registered this trademark in a number of countries.
Our Fluoroproducts segment is the technology leader in the markets in which it participates. We have one of the largest patent portfolios in the fluorine derivatives industry. In our Fluoroproducts intellectual property portfolio, we consider our Freon™, Opteon™, Teflon™, Viton™, NafionTM and Krytox™ trademarks to be valuable assets.
Our Chemical Solutions segment is a manufacturing and application development technology leader in a majority of the markets in which it participates. Trade secrets are one of the key elements of our intellectual property security in Chemical Solutions as most of the segment’s manufacturing and application development technologies are no longer under patent coverage.
At separation, certain of our subsidiaries entered into an intellectual property cross-license agreement with DuPont, pursuant to which (i) DuPont has agreed to license to Chemours certain patents, know-how and technical information owned by DuPont or its affiliates and necessary or useful in Chemours’ business, and (ii) Chemours has agreed to license to DuPont certain patents owned by Chemours or its affiliates and necessary or useful in DuPont’s business. In most circumstances, the licenses are perpetual, irrevocable, sublicenseable (in connection with the party’s business), assignable (in connection with a sale of the applicable portion of a party’s business or assets, subject to certain exceptions) worldwide licenses in connection with the current operation of the businesses and, with respect to specified products and fields of use, future operation of such businesses, subject to certain limitations with respect to specified products and fields of use.
Research and Development
We perform research and development activities in all of our segments with the majority of our efforts focused in the Fluoroproducts segment. The Fluoroproducts segment efforts center on developing new sustainable fluorochemicals as well as determining new applications and formulations for fluoropolymers that meet customers’ technical requirements. In Titanium Technologies and Chemical Solutions, our efforts are focused on process technology to reduce cost and maintain safety and stewardship standards. The table below sets forth the last three years of research and development expense by segment:
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Titanium Technologies |
| $ | 27 |
|
| $ | 33 |
|
| $ | 47 |
|
Fluoroproducts |
|
| 46 |
|
|
| 50 |
|
|
| 79 |
|
Chemical Solutions |
|
| 7 |
|
|
| 14 |
|
|
| 17 |
|
Total |
| $ | 80 |
|
| $ | 97 |
|
| $ | 143 |
|
Backlog
In general, the Company does not manufacture its products against a backlog of orders and does not consider backlog to be a significant indicator of the level of future sales activity. Production and inventory levels are based on the level of incoming orders as well as projections of future demand. Therefore, the Company believes that backlog information is not material to understanding its
13
The Chemours Company
overall business and should not be considered a reliable indicator of the Company’s ability to achieve any particular level of revenue or financial performance.
Environmental Matters
Information related to environmental matters is included in several areas of this report: (1) Item 1A - Risk Factors, (2) Item 3 – Legal Proceedings – Environmental Proceedings, (3) Item 7 - Management’s Discussion and Analysis of Financial Condition and Results of Operations, and (4) Notes 3 and 20 to the Consolidated Financial Statements.
Available Information
Chemours is subject to the reporting requirements under the Securities Exchange Act of 1934. Consequently, the Company is required to file reports and information with the Securities and Exchange Commission (SEC), including reports on the following forms: annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934.
The public may read and copy any materials the Company files with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet site at http://www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
The Company’s annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports are also accessible on the Company’s website at http://www.chemours.com by clicking on the section labeled “Investor Relations”, then on “Filings & Reports” and then on “SEC Filings”. These reports are made available, without charge, as soon as is reasonably practicable after the Company files or furnishes them electronically with the SEC.
Employees
We have approximately 7,000 employees, approximately 21% of whom are represented by unions or works councils. Management believes that its relations with its employees and labor organizations are good. There have been no strikes or work stoppages in any of our locations in recent history.
The company’s operations could be affected by various risks, many of which are beyond our control. Based on current information, we believe that the following identifies the most significant risk factors that could affect our business, results of operations or financial condition. Past financial performance may not be a reliable indicator of future performance and historical trends should not be used to anticipate results or trends in future periods. See “Cautionary Statement Concerning Forward-Looking Statements” for more details.
Risks Related to Our Business
Conditions in the global economy and global capital markets may adversely affect our results of operations, financial condition, and cash flows.
Our business and operating results may in the future be adversely affected by global economic conditions, including instability in credit markets, declining consumer and business confidence, fluctuating commodity prices and interest rates, volatile exchange rates, and other challenges such as the changing financial regulatory environment that could affect the global economy. Our customers may experience deterioration of their businesses, cash flow shortages, and difficulty obtaining financing. As a result, existing or potential customers may delay or cancel plans to purchase products and may not be able to fulfill their obligations to us in a timely fashion. Further, suppliers could experience similar conditions, which could impact their ability to supply materials or otherwise fulfill their obligations to us. Because we have significant international operations, there are a large number of currency transactions that result from international sales, purchases, investments and borrowings. Also, our effective tax rate may fluctuate because of variability in geographic mix of earnings, changes in statutory rates, and taxes associated with repatriation of non-U.S. earnings. Future weakness in the global economy and failure to manage these risks could adversely affect our results of operations, financial condition and cash flows in future periods.
14
The Chemours Company
Market conditions, as well as global and regional economic downturns that adversely affect the demand for the end-use products that contain TiO2, fluoroproducts or our other products, could adversely affect the profitability of our operations and the prices at which we can sell our products, negatively impacting our financial results.
Our revenue and profitability is largely dependent on the TiO2 industry and the industries that are end users of our fluoroproducts. TiO2 and our fluoroproducts, such as refrigerants and resins, are used in many “quality of life” products for which demand historically has been linked to global, regional and local GDP and discretionary spending, which can be negatively impacted by regional and world events or economic conditions. Such events are likely to cause a decrease in demand for our products and, as a result, may have an adverse effect on our results of operations and financial condition. The future profitability of our operations, and cash flows generated by those operations, will also be affected by the available supply of our products in the market.
Our reported results could be adversely affected by currency exchange rates and currency devaluation could impair our competitiveness.
Due to our international operations, we transact in many foreign currencies, including but not limited to the Euro, Brazilian real, Mexican peso and Japanese yen. As a result, we are subject to the effects of changes in foreign currency exchange rates. During times of a strengthening U.S. dollar, our reported net revenues and operating income will be reduced because the local currency will be translated into fewer U.S. dollars. During periods of local economic crisis, local currencies may be devalued significantly against the U.S. dollar, potentially reducing our margin. For example, unfavorable movement in the Euro has negatively impacted our results of operations since the second half of 2014, and further decline of the Euro could affect future periods. Currently, Chemours does not hedge on a transactional basis. There can be no assurance that any hedging action in the future will lessen the adverse impact of a variation in currency rates. Also, actions to recover margins may result in lower volume and a weaker competitive position, which may have an adverse effect on our profitability. For example, in Titanium Technologies, a substantial portion of our manufacturing is located in the U.S. and Mexico, while our TiO2 is delivered to customers around the world. Furthermore, our ore cost is principally denominated in U.S. dollars. Accordingly, in periods when the U.S. dollar or Mexican Peso strengthen against other local currencies such as the Euro, our costs are higher relative to our competitors who operate largely outside of the United States, and the benefits we realize from having lower costs associated with our manufacturing process are reduced, impacting our profitability.
If we are unable to execute our cost reduction plans successfully, our total operating costs may be greater than expected, which may adversely affect our profitability.
We have announced a transformation plan that includes a number of cost saving measures. We have implemented a number of these measures and have realized a portion of the anticipated benefits. While we continue to search for opportunities to reduce our costs and expenses to improve operating profitability without jeopardizing the quality of our products or the effectiveness of our operations, our success in achieving targeted cost and expense reductions depends upon a number of factors such as timing of execution, market condition, and regulatory and local requirements and approvals. If we do not successfully execute on our cost reduction initiatives or if we experience delays in completing the implementation of these initiatives, our results of operations or financial condition could be adversely affected.
Our results of operations could be adversely affected by litigation and other commitments and contingencies.
We face risks arising from various unasserted and asserted legal claims, investigation and litigation matters, such as product liability, patent infringement, antitrust claims, and claims for third party property damage or personal injury stemming from alleged environmental actions (which may concern regulated or unregulated substances) or other torts, including, as discussed below, litigation related to the production and use of PFOA (collectively, perfluorooctanoic acids and its salts, including the ammonium salt) by DuPont prior to the separation. We have noted a nationwide trend in purported class actions against chemical manufacturers generally seeking relief such as medical monitoring, property damages, off-site remediation and punitive damages arising from alleged environmental actions (which may concern regulated or unregulated substances) or other torts without claiming present personal injuries. We also have noted a trend in public and private nuisance suits being filed on behalf of states, counties, cities and utilities alleging harm to the general public. Various factors or developments can lead to changes in current estimates of liabilities such as a final adverse judgment, significant settlement or changes in applicable law. A future adverse ruling or unfavorable development could result in future charges that could have a material adverse effect on us. An adverse outcome in any one or more of these matters could be material to our financial results and could adversely impact the value of any of our brands that are associated with any such matters. As discussed in more detail in Note 20 to the Consolidated Financial Statements, DuPont is the named defendant in approximately 3,500 lawsuits alleging that the respective plaintiffs were exposed to PFOA in drinking water as a result of DuPont’s use of PFOA at the Washington Works plant in Parkersburg, West Virginia. These personal injury lawsuits were consolidated in multi-district litigation in the United States District Court for the Southern District of Ohio (the “MDL”). As of December 31, 2016, three cases have gone to trial and resulted in a jury verdict in favor of the plaintiff, and several other cases have been settled. Although we,
15
The Chemours Company
through DuPont, are pursuing appeals of the cases that resulted in a jury verdict, there can be no assurance that any such appeal succeeds. On February 11, 2017, DuPont entered into an agreement in principle with plaintiffs’ counsel representing the MDL plaintiffs providing for a global settlement of all cases and claims in the MDL, including all filed and unfiled personal injury cases and claims that are part of the plaintiffs’ counsel’s claim inventory, as well as cases that have been tried to a jury verdict (the “MDL Settlement”). The total settlement amount is $670.7 million dollars in cash, half of which will be paid by Chemours and half paid by DuPont. DuPont’s payment would not be subject to indemnification or reimbursement by Chemours, and Chemours has accrued $335 million associated with this matter at December 31, 2016. In exchange for payment of the total settlement amount, DuPont and Chemours will receive a complete release of all claims by the settling plaintiffs. The MDL Settlement was entered into solely by way of compromise and settlement and is not in any way an admission of liability or fault by DuPont or Chemours. The MDL Settlement is not subject to court approval; however, the MDL Settlement may not proceed in certain conditions, including a walk-away right that enables DuPont to terminate the MDL Settlement if more than a specified number of plaintiffs determine not to participate. If the MDL Settlement does not proceed, any cases stayed or additional lawsuits may go to trial or appeal. An adverse ruling at trial or on appeal could result in us incurring additional costs and liabilities. There could also be new lawsuits filed related to DuPont’s use of PFOA, its manufacture of PFOA, or its customers use of DuPont products that may not be within the scope of the MDL Settlement. Any such new litigation could also result in us incurring additional costs and liabilities, which may be material to our financial results. If such litigation described above were to occur and if significant unfavorable outcomes in a number of cases were to result, losses, if incurred, in excess of amounts accrued at December 31, 2016 could, in the aggregate, have a material adverse effect on us.
In the ordinary course of business, we may make certain commitments, including representations, warranties and indemnities relating to current and past operations, including those related to divested businesses, and issue guarantees of third party obligations. Additionally, we are required to indemnify DuPont with regard to liabilities allocated to, or assumed by us under each of the separation agreement, the employee matters agreement, the tax matters agreement and the intellectual property cross-license agreement that were executed prior to the spin-off. These indemnification obligations to date have included defense costs associated with certain litigation matters as well as certain damages awards, settlements, and penalties. In connection with MDL Settlement mentioned above, DuPont and Chemours agreed, subject to and following the completion of the MDL Settlement, to a limited sharing of potential future PFOA liabilities (i.e., “indemnifiable losses,” as defined in the separation agreement between DuPont and Chemours) for a period of five years. During that five-year period, Chemours would annually pay future PFOA liabilities up to $25 million and, if such amount is exceeded, DuPont would pay any excess amount up to the next $25 million (which payment will not be subject to indemnification by Chemours), with Chemours annually bearing any further excess liabilities under the terms of the separation agreement. After the five-year period, this limited sharing agreement would expire, and Chemours’ indemnification obligations under the separation agreement would continue unchanged. Chemours has also agreed that, upon the MDL Settlement becoming effective, it will not contest its liability to DuPont under the separation agreement for PFOA liabilities on the basis of ostensible defenses generally applicable to the indemnification provisions under the separation agreement, including defenses relating to punitive damages, fines or penalties or attorneys’ fees, and waives any such defenses with respect to PFOA liabilities. Chemours has, however, retained defenses as to whether any particular PFOA claim is within the scope of the indemnification provisions of the separation agreement. As we are required to make payments, such payments could be significant and could exceed the amounts we have accrued with respect thereto, adversely affecting our results of operations. In addition, in the event that DuPont seeks indemnification for adverse trial rulings or outcomes, these indemnification claims could materially adversely affect our financial condition. Disputes between Chemours and DuPont many also arise with respect to indemnification matters including disputes based on matters of law or contract interpretation. If and to the extent these disputes arise, they could materially adversely affect us.
For further information about the Company’s litigation and other commitments and contingencies, see Item 3. Legal Proceedings and our Note 20 to the Consolidated Financial Statements included elsewhere in this Annual Report.
We are subject to extensive environmental, health and safety laws and regulations that may result in unanticipated loss or liability related to our current and past operations, which could reduce our profitability.
Our operations and production facilities are subject to extensive environmental and health and safety laws and regulations at national, international and local levels in numerous jurisdictions relating to pollution, protection of the environment, climate change, transporting and storing raw materials and finished products and storing and disposing of hazardous wastes. Such laws include, in the U.S., the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA, often referred to as Superfund), the Resource Conservation and Recovery Act (RCRA) and similar state and global laws for management and remediation of hazardous materials, the Clean Air Act (CAA) and the Clean Water Act, for protection of air and water resources, the Toxic Substances Control Act (TSCA), and in the EU, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH), for regulation of chemicals in commerce and reporting of potential known adverse effects and numerous local, state, federal and foreign laws and regulations governing materials transport and packaging. If we are found to be in violation of these laws or regulations, which may be subject to change based on legislative, scientific or other factors, we may incur substantial costs, including fines, damages, criminal or civil sanctions, remediation costs, reputational harm, loss of sales or market access, or experience interruptions in our operations. We
16
The Chemours Company
also may be subject to changes in our operations and production based on increased regulation or other changes to, or restrictions imposed by, any such additional regulations. In addition, the manner in which adopted regulations (including environmental regulations) are ultimately implemented may affect our products, the demand for and public perception of our products, the reputation of our brands, our market access and our results of operations. In the event of a catastrophic incident involving any of the raw materials we use or chemicals we produce, we could incur material costs as a result of addressing the consequences of such event and future reputational costs associated with any such event.
As a result of our operations, including the operations of divested businesses and certain discontinued operations, we could incur substantial costs, including remediation and restoration costs. The costs of complying with complex environmental laws and regulations, as well as internal voluntary programs, are significant and will continue to be significant for the foreseeable future. This includes costs we expect to continue to incur for environmental investigation and remediation activities at a number of our current or former sites and third-party disposal locations. However, the ultimate costs under environmental laws and the timing of these costs are difficult to accurately predict. While we establish accruals in accordance with generally accepted accounting principles, the ultimate actual costs and liabilities may vary from the accruals because the estimates on which the accruals are based depend on a number of factors (many of which are outside of our control), including the nature of the matter and any associated third-party claims, the complexity of the site, site geology, the nature and extent of contamination, the type of remedy, the outcome of discussions with regulatory agencies and other Potentially Responsible Parties (PRPs) at multi-party sites and the number and financial viability of other PRPs. See “Environmental Matters” within Item 7 - Management’s Discussion and Analysis (MD&A) of Financial Condition and Results of Operations for further information and Note 20 to the Consolidated Financial Statements included elsewhere in this Annual Report.
There is also a risk that one or more of our key raw materials or one or more of our products may be found to have, or be characterized as having, a toxicological or health-related impact on the environment or on our customers or employees or unregulated emissions, which could potentially result in us incurring liability in connection with such characterization and the associated effects of any toxicological or health-related impact. If such a discovery or characterization occurs, we may incur increased costs in order to comply with new regulatory requirements or the relevant materials or products, including products of our customers incorporating our materials or products, may be recalled or banned. Changes in laws, science or regulations, or their interpretation, and our customers’ perception of such changes or interpretations may also affect the marketability of certain of our products.
The markets for many of our products have seasonally affected sales patterns.
The demand for TiO2, certain of our fluoroproducts and certain of our other products during a given year is subject to seasonal fluctuations. As a result of seasonal fluctuations, our operating cash flow may be negatively impacted due to demand fluctuations. In particular, because TiO2 is widely used in coatings, demand is higher in the painting seasons of spring and summer. Because certain fluoroproducts are used in refrigerants, such products are in higher demand in the spring and summer in the Northern Hemisphere. We may be adversely affected by anticipated or unanticipated changes in regional weather conditions. For example, poor weather conditions in a region can lead to an abbreviated painting season, which can depress consumer sales of paint products that use TiO2, which could have a negative effect on our cash position.
Failure to maintain effective internal controls could adversely affect our ability to meet our reporting requirements.
The Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”) requires, among other things, that we maintain effective internal control over financial reporting and disclosure controls and procedures. One key aspect of the Sarbanes-Oxley Act is that we must perform system and process evaluation and testing of our internal control over financial reporting to allow management and our independent registered public accounting firm to report on the effectiveness of our internal control over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act, with auditor attestation of the effectiveness of our internal controls. If we are not able to comply with the requirements of Section 404 in a timely manner, or if we or our independent registered public accounting firm identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our common shares could decline and we could be subject to penalties or investigations by the NYSE, the SEC or other regulatory authorities, which would require additional financial and management resources.
Effective internal controls are necessary for us to provide reasonable assurance with respect to our financial reports, and to effectively prevent fraud. Internal controls over financial reporting may not prevent or detect misstatements because of inherent limitations, including the possibility of human error, the circumvention or overriding of controls, or fraud. Therefore, even effective internal controls can provide only reasonable assurance with respect to the preparation and fair presentation of financial statements. If we cannot provide reasonable assurance with respect to our financial reports and effectively prevent fraud, our operating results could be harmed. In addition, if we fail to maintain the effectiveness of our internal controls, including any failure to implement required new or improved controls, or if we experience delay in the implementation of new or enhanced system, procedures and controls, or if we
17
The Chemours Company
experience difficulties in their implementation, our business and operating results could be harmed, we could fail to meet our reporting obligations, and there could be a material adverse effect on our stock price.
Effects of price fluctuations in energy and raw materials, our raw materials contracts and our inability to renew such contracts, could have a significant impact on our earnings.
Our manufacturing processes consume significant amounts of energy and raw materials, the costs of which are subject to worldwide supply and demand as well as other factors beyond our control. Variations in the cost of energy, which primarily reflect market prices for oil and natural gas, and for raw materials may significantly affect our operating results from period to period. Additionally, consolidation in the industries providing our raw materials may have an impact on the cost and availability of such materials. To the extent we do not have fixed price contracts with respect to specific raw materials, we have no control over the costs of raw materials and such costs may fluctuate widely for a variety of reasons, including changes in availability, major capacity additions or reductions, or significant facility operating problems.
When possible, we have purchased, and we plan to continue to purchase, raw materials, including titanium bearing ores and fluorspar, through negotiated medium- or long-term contracts to minimize the impact of price fluctuations. To the extent that we have been able to achieve favorable pricing in our existing negotiated long-term contracts, we may not be able to renew such contracts at the current prices, or at all, and this may adversely impact our cash flow from operations. However, to the extent that the prices of raw materials that we utilize significantly decline, we may be bound by the terms of our existing long-term contracts and obligated to purchase such raw materials at higher prices as compared to other market participants.
We attempt to offset the effects of higher energy and raw material costs through selling price increases, productivity improvements, and cost reduction programs. However, the outcome of these efforts is largely determined by existing competitive and economic conditions, and may be subject to a time delay between the increase in our raw materials costs and our ability to increase prices, which could vary significantly depending on the market served. If we are not able to fully offset the effects of higher energy or raw material costs, it could have a material adverse effect on our financial results.
Hazards associated with chemical manufacturing, storage and transportation could adversely affect our results of operations.
There are hazards associated with chemical manufacturing and the related storage and transportation of raw materials, products and wastes. These hazards could lead to an interruption or suspension of operations and have an adverse effect on the productivity and profitability of a particular manufacturing facility or on us as a whole. While we endeavor to provide adequate protection for the safe handling of these materials, issues could be created by various events, including natural disasters, severe weather events, acts of sabotage and performance by third parties, and as a result we could face the following potential hazards:
| • | piping and storage tank leaks and ruptures; |
| • | mechanical failure; |
| • | employee exposure to hazardous substances; and |
| • | chemical spills and other discharges or releases of toxic or hazardous substances or gases. |
These hazards may cause personal injury and loss of life, damage to property and contamination of the environment, which could lead to government fines and penalties, work stoppage injunctions, claims and lawsuits by injured persons, damage to our public reputation and brands, loss of sales and market access, customer dissatisfaction, and diminished product acceptance. If such actions are determined adversely to us or there is an associated economic impact to our business, we may have inadequate insurance or cash flow to offset any associated costs. Such outcomes could adversely affect our financial condition and results of operations.
The businesses in which we compete are highly competitive. This competition may adversely affect our results of operations and operating cash flows.
Each of the businesses in which we operate is highly competitive. Competition in the performance chemicals industry is based on a number of factors such as price, product quality and service. We face significant competition from major international and regional competitors. Additionally, our Titanium Technologies business competes with numerous regional producers, including producers in China, which have expanded their readily available production capacity during the previous five years. Additionally, the risk of substitution of Chinese producers by our customers could increase as they expand their use of chloride production technology. Some competitors have announced plans to expand their chloride capacity.
18
The Chemours Company
Our results of operations and financial condition could be seriously impacted by business disruptions and security breaches, including cybersecurity incidents.
Business and/or supply chain disruptions, plant downtime and/or power outages and information technology system and/or network disruptions, regardless of cause including acts of sabotage, employee error or other actions, geo-political activity, weather events and natural disasters could seriously harm our operations as well as the operations of our customers and suppliers. Failure to effectively prevent, detect and recover from security breaches, including attacks on information technology and infrastructure by hackers, viruses, breaches due to employee error or actions or other disruptions could result in misuse of our assets, business disruptions, loss of property including trade secrets and confidential business information, legal claims or proceedings, reporting errors, processing inefficiencies, negative media attention, loss of sales and interference with regulatory compliance. Like most major corporations, we have been and expect to be the target of industrial espionage, including cyber-attacks, from time to time. We have determined that these attacks have resulted, and could result in the future, in unauthorized parties gaining access to certain confidential business information, and have included the obtaining of trade secrets and proprietary information related to the chloride manufacturing process for TiO2 by third parties. Although we do not believe that we have experienced any material losses to date related to these breaches, there can be no assurance that we will not suffer any such losses in the future. We plan to actively manage the risks within our control that could lead to business disruptions and security breaches. As these threats continue to evolve, particularly around cybersecurity, we may be required to expend significant resources to enhance our control environment, processes, practices and other protective measures. Despite these efforts, such events could materially adversely affect our business, financial condition or results of operations.
If our intellectual property were compromised or copied by competitors, or if our competitors were to develop similar or superior intellectual property or technology, our results of operations could be negatively affected.
Intellectual property rights, including patents, trade secrets, confidential information, trademarks and tradenames are important to our business. We endeavor to protect our intellectual property rights in key jurisdictions in which our products are produced or used and in jurisdictions into which our products are imported. Our success depends to a significant degree upon our ability to protect and preserve our intellectual property rights. However, we may be unable to obtain protection for our intellectual property in key jurisdictions. Although we own and have applied for numerous patents and trademarks throughout the world, we may have to rely on judicial enforcement of our patents and other proprietary rights. Our patents and other intellectual property rights may be challenged, invalidated, circumvented, and rendered unenforceable or otherwise compromised. A failure to protect, defend or enforce our intellectual property could have an adverse effect on our financial condition and results of operations. Similarly, third parties may assert claims against us and our customers and distributors alleging our products infringe upon third party intellectual property rights.
We also rely materially upon unpatented proprietary technology, know-how and other trade secrets to maintain our competitive position. While we maintain policies to enter into confidentiality agreements with our employees and third parties to protect our proprietary expertise and other trade secrets, these agreements may not be enforceable or, even if legally enforceable, we may not have adequate remedies for breaches of such agreements. We also may not be able to readily detect breaches of such agreements. The failure of our patents or confidentiality agreements to protect our proprietary technology, know-how or trade secrets could result in significantly lower revenues, reduced profit margins or loss of market share.
If we must take legal action to protect, defend or enforce our intellectual property rights, any suits or proceedings could result in significant costs and diversion of resources and management’s attention, and we may not prevail in any such suits or proceedings. A failure to protect, defend or enforce our intellectual property rights could have an adverse effect on our financial condition and results of operations.
Restrictions under the intellectual property cross-license agreement could limit our ability to develop and commercialize certain products and/or prosecute, maintain and enforce certain intellectual property.
We depend to a certain extent on DuPont to prosecute, maintain and enforce certain of the intellectual property licensed under the intellectual property cross-license agreement. Specifically, DuPont is responsible for filing, prosecuting and maintaining patents that DuPont licenses to us. DuPont also has the first right to enforce such patents, trade secrets and the know-how licensed to us by DuPont. If DuPont fails to fulfill its obligations or chooses to not enforce the licensed patents, trade secrets or know-how under the intellectual property cross-license agreement, we may not be able to prevent competitors from making, using and selling competitive products (unless we are able to effectively exercise our secondary rights to enforce such patents, trade secrets and know-how).
In addition, our restrictions under the intellectual property cross-license agreement could limit our ability to develop and commercialize certain products. For example, the licenses granted to us under the agreement may not extend to all new products, services and businesses that we may enter in the future. These limitations and restrictions may make it more difficult, time consuming
19
The Chemours Company
or expensive for us to develop and commercialize certain new products and services, or may result in certain of our products or services being later to market than those of our competitors.
If we are unable to innovate and successfully introduce new products, or new technologies or processes reduce the demand for our products or the price at which we can sell products, our profitability could be adversely affected.
Our industries and the end-use markets into which we sell our products experience periodic technological change and product improvement. Our future growth will depend on our ability to gauge the direction of commercial and technological progress in key end-use markets and on our ability to fund and successfully develop, manufacture and market products in such changing end-use markets. We must continue to identify, develop and market innovative products or enhance existing products on a timely basis to maintain our profit margins and our competitive position. We may be unable to develop new products or technology, either alone or with third parties, or license intellectual property rights from third parties on a commercially competitive basis. If we fail to keep pace with the evolving technological innovations in our end-use markets on a competitive basis, including with respect to innovation with regard to the development of alternative uses for, or application of, products developed that utilize such end-use products, our financial condition and results of operations could be adversely affected. We cannot predict whether technological innovations will, in the future, result in a lower demand for our products or affect the competitiveness of our business. We may be required to invest significant resources to adapt to changing technologies, markets, competitive environments and laws and regulations. We cannot anticipate market acceptance of new products or future products. In addition, we may not achieve our expected benefits associated with new products developed to meet new laws or regulations if the implementation of such laws or regulations is delayed.
In connection with our separation, we were required to assume, and indemnify DuPont for, certain liabilities. As we are required to make payments pursuant to these indemnities to DuPont, we may need to divert cash to meet those obligations and our financial results could be negatively affected. In addition, DuPont’s obligation to indemnify us for certain liabilities may not be sufficient to insure us against the full amount of liabilities for which it will be allocated responsibility, and DuPont may not be able to satisfy its indemnification obligations in the future.
Pursuant to the separation agreement, the employee matters agreement, the tax matters agreement and the intellectual property cross-license agreement we entered into with DuPont prior to the spin-off, we were required to assume, and indemnify DuPont for, certain liabilities. These indemnification obligations to date have included, among other items, defense costs associated with certain litigation matters as well as certain damages awards, settlement amounts and penalties. In connection with MDL Settlement described above under “Our results of operations could be adversely affected by litigation and other commitments and contingencies”, DuPont and Chemours agreed, subject to and following the completion of the MDL Settlement, to a limited sharing of potential future PFOA liabilities (i.e., “indemnifiable losses,” as defined in the separation agreement between DuPont and Chemours) for a period of five years. During that five-year period, Chemours would annually pay future PFOA liabilities up to $25 million and, if such amount is exceeded, DuPont would pay any excess amount up to the next $25 million (which payment will not be subject to indemnification by Chemours), with Chemours annually bearing any further excess liabilities under the terms of the separation agreement. After the five-year period, this limited sharing agreement would expire, and Chemours’ indemnification obligations under the separation agreement would continue unchanged. Chemours has also agreed that, upon the MDL Settlement becoming effective, it will not contest its liability to DuPont under the separation agreement for PFOA liabilities on the basis of ostensible defenses generally applicable to the indemnification provisions under the separation agreement, including defenses relating to punitive damages, fines or penalties or attorneys’ fees, and waives any such defenses with respect to PFOA liabilities. Chemours has, however, retained defenses as to whether any particular PFOA claim is within the scope of the indemnification provisions of the separation agreement. Payments pursuant to these indemnities may be significant and could negatively impact our business, particularly indemnities relating to our actions that could impact the tax-free nature of the distribution. In addition, in the event that DuPont seeks indemnification for adverse trial rulings or outcomes, these indemnification claims could materially adversely affect our financial condition. Disputes between Chemours and DuPont may also arise with respect to indemnification matters, including disputes based on matter of law or contract interpretation. If and to the extent these disputes arise, they could materially adversely affect us.
Third parties could also seek to hold us responsible for any of the liabilities of the DuPont businesses. DuPont has agreed to indemnify us for such liabilities, but such indemnity from DuPont may not be sufficient to protect us against the full amount of such liabilities, and DuPont may not be able to fully satisfy its indemnification obligations. Moreover, even if we ultimately succeed in recovering from DuPont any amounts for which we are held liable, we may be temporarily required to bear these losses ourselves. Each of these risks could negatively affect our business, financial condition, results of operations and cash flows. See Note 20 to the Consolidated Financial Statements for further information.
20
The Chemours Company
In connection with our separation, we were required to enter into numerous separation-related and commercial agreements with our former parent company, DuPont, which may not reflect optimal or commercially beneficial terms to Chemours.
Commercial agreements we entered into with DuPont in connection with the separation were negotiated in the context of the separation while we were still a wholly-owned subsidiary of DuPont. Accordingly, during the period in which the terms of those agreements were negotiated, we did not have an independent board of directors or management independent of DuPont. Certain commercial agreements, having long terms and commercially advantageous cancellation and assignment rights to DuPont, may not include adjustments for changes in industry and market conditions. There is a risk that the pricing and other terms under these agreements may not be commercially beneficial and may not be able to be renegotiated in the future. The terms relate to, among other things, the allocation of assets, liabilities, rights and obligations, including the provision of products and services and the sharing and operation of property, manufacturing, office and laboratory sites, and other commercial rights and obligations between DuPont and us.
We may be unable to make, on a timely or cost-effective basis, the changes necessary to operate efficiently as an independent company.
There is a risk that, since separating from DuPont, we are more susceptible to market fluctuations and other adverse events than we would have been if we were still a part of DuPont’s organizational structure. As part of DuPont, we were able to enjoy certain benefits from DuPont’s operating diversity, purchasing power and opportunities to pursue integrated strategies with DuPont’s other businesses. As an independent, publicly traded company, we do not have similar diversity or integration opportunities and do not have similar purchasing power or access to capital markets. Additionally, as part of DuPont, we were able to leverage the DuPont historical market reputation and performance and brand identity to recruit and retain key personnel to run our business. As an independent, publicly traded company, we do not have the same historical market reputation and performance or brand identity as DuPont and it may be more difficult for us to recruit or retain such key personnel.
Our ability to make future strategic decisions regarding our manufacturing operations are subject to regulatory, environmental, political, legal and economic risks, and to certain extent may be subject to consents or cooperation from DuPont under the agreements entered into between us and DuPont as part of the separation. These could adversely affect our ability to execute our future strategic decisions and our results of operations and financial condition.
One of the ways we may improve our business is through the expansion or improvement of our existing facilities, such as the expansion of our Altamira TiO2 facility and the planned expansions for our OpteonTM refrigerant and our Mining Solutions facility. Construction of additions or modifications to facilities involves numerous regulatory, environmental, political, legal and economic uncertainties that are beyond our control. Such expansion or improvement projects may also require the expenditure of significant amounts of capital, and financing may not be available on economically acceptable terms or at all. As a result, these projects may not be completed on schedule, at the budgeted cost, or at all. Moreover, our revenue may not increase immediately upon the expenditure of funds on a particular project. As a result, we may not be able to realize our expected investment return, which could adversely affect our results of operations and financial condition.
We periodically assess our manufacturing operations in order to manufacture and distribute our products in the most efficient manner. Based on our assessments, we may make strategic decisions regarding our manufacturing operations such as capital improvements to modernize certain units, move manufacturing or distribution capabilities from one plant or facility to another plant or facility, discontinue manufacturing or distributing certain products or close or divest all or part of a manufacturing plant or facility, some of which have significant shared services and lease agreements with DuPont. These agreements may adversely impact our ability to take these strategic decisions regarding out manufacturing operations. Further, if such agreements are terminated or revised, we would have to assess and potentially adjust our manufacturing operations, the closure or divestiture of all or part of a manufacturing plant or facility that could result in future charges that could be significant.
21
The Chemours Company
Our customers, prospective customers, suppliers or other companies with whom we conduct business may need assurances that our financial stability is sufficient to satisfy their requirements for doing or continuing to do business with them.
Some of our customers, prospective customers, suppliers or other companies with whom we conduct business may need assurances that our financial stability is sufficient to satisfy their requirements for doing or continuing to do business with them, and may require us to provide additional credit support, such as letters of credit or other financial guarantees. Any failure of parties to be satisfied with our financial stability could have a material adverse effect on our business, financial condition, results of operations and cash flows.
We are a holding company that is dependent on cash flows from our operating subsidiaries to fund our debt obligations, capital expenditures and ongoing operations.
All of our operations are conducted and all of our assets are owned by our operating companies, which are our subsidiaries. We intend to continue to conduct our operations at the operating companies and any future subsidiaries. Consequently, our cash flow and our ability to meet our obligations or make cash distributions depends upon the cash flow of our operating companies and any future subsidiaries, and the payment of funds by our operating companies and any future subsidiaries in the form of dividends or otherwise. The ability of our operating companies and any future subsidiaries to make any payments to us depends on their earnings, the terms of their indebtedness, including the terms of any credit facilities, and legal restrictions regarding the transfer of funds.
Our debt is generally the exclusive obligation of The Chemours Company and our guarantor subsidiaries. Because a significant portion of our operations are conducted by nonguarantor subsidiaries, our cash flow and our ability to service indebtedness, including our ability to pay the interest on our debt when due and principal of such debt at maturity, are dependent to a large extent upon cash dividends and distributions or other transfers from such nonguarantor subsidiaries. Any payment of dividends, distributions, loans or advances by our nonguarantor subsidiaries to us could be subject to restrictions on dividends or repatriation of earnings under applicable local law, monetary transfer restrictions and foreign currency exchange regulations in the jurisdictions in which our subsidiaries operate, and any restrictions imposed by the current and future debt instruments of our nonguarantor subsidiaries. In addition, payments to us by our subsidiaries are contingent upon our subsidiaries’ earnings.
Our subsidiaries are separate legal entities and, except for our guarantor subsidiaries, have no obligation, contingent or otherwise, to pay any amounts due on our debt or to make any funds available for those amounts, whether by dividends, loans, distributions or other payments, and do not guarantee the payment of interest on, or principal of, our debt. Any right that we have to receive any assets of any of our subsidiaries that are not guarantors upon the liquidation or reorganization of any such subsidiary, and the consequent right of holders of notes to realize proceeds from the sale of their assets, will be structurally subordinated to the claims of that subsidiary’s creditors, including trade creditors and holders of debt issued by that subsidiary.
If our long-lived assets become impaired, we may be required to record a significant charge to earnings.
We have a significant amount of long-lived assets on our consolidated balance sheet. Under U.S. GAAP, we review our long-lived assets for impairment when events or changes in circumstances indicate the carrying value may not be recoverable. Factors that may be considered a change in circumstances, indicating that the carrying value of our long-lived assets may not be recoverable, include, but are not limited to, changes in the industries in which we operate, particularly the impact of a downturn in the global economy, as well as competition or other factors leading to reduction in expected long-term sales or profitability. We may be required to record a significant noncash charge in our financial statements during the period in which any impairment of our long-lived assets is determined, negatively impacting our results of operations.
Our failure to comply with the anti-corruption laws of the United States and various international jurisdictions could negatively impact our reputation and results of operations.
Doing business on a global basis requires us to comply with the laws and regulations of the U.S. government and those of various international and sub-national jurisdictions, and our failure to successfully comply with these rules and regulations may expose us to liabilities. These laws and regulations apply to companies, individual directors, officers, employees and agents, and may restrict our operations, trade practices, investment decisions and partnering activities. In particular, our international operations are subject to U.S. and foreign anti-corruption laws and regulations, such as the U.S. Foreign Corrupt Practices Act (the “FCPA”), the United Kingdom Bribery Act 2010 (the “Bribery Act”) as well as anti-corruption laws of the various jurisdictions in which we operate. The FCPA, the Bribery Act and other laws prohibit us and our officers, directors, employees and agents acting on our behalf from corruptly offering, promising, authorizing or providing anything of value to foreign officials for the purposes of influencing official decisions or obtaining or retaining business or otherwise obtaining favorable treatment. Our global operations may expose us to the risk of violating, or being accused of violating, the foregoing or other anti-corruption laws. Such violations could be punishable by criminal fines, imprisonment, civil penalties, disgorgement of profits, injunctions and exclusion from government contracts, as well as other
22
The Chemours Company
remedial measures. Investigations of alleged violations can be very expensive, disruptive, and damaging to our reputation. Although we have implemented anti-corruption policies and procedures since the separation, there can be no guarantee that these policies, procedures, and training will effectively prevent violations by our employees or representatives in the future. Additionally, we face a risk that our distributors and other business partners may violate the FCPA, the Bribery Act or similar laws or regulations. Such violations could expose us to FCPA and Bribery Act liability and/or our reputation may potentially be harmed by their violations and resulting sanctions and fines.
Risks Related to Our Indebtedness
Our significant indebtedness could adversely affect our financial condition, and we could have difficulty fulfilling our obligations under our indebtedness, which may have a material adverse effect on us.
As of December 31, 2016, we had approximately $3.6 billion of indebtedness. At December 31, 2016, together with the guarantors, we had approximately $1.4 billion of senior secured indebtedness outstanding, and had an additional $750 million of capacity under the Revolving Credit Facility, all of which would be senior secured indebtedness if drawn. Our significant level of indebtedness increases the risk that we may be unable to generate cash sufficient to pay amounts due in respect of our indebtedness. The level of our indebtedness could have other important consequences on our business, including:
| • | making it more difficult for us to satisfy our obligations with respect to indebtedness; |
| • | increasing our vulnerability to adverse changes in general economic, industry and competitive conditions; |
| • | requiring us to dedicate a significant portion of our cash flow from operations to make payments on our indebtedness, thereby reducing the availability of our cash flow to fund working capital and other general corporate purposes; |
| • | limiting our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; |
| • | restricting us from capitalizing on business opportunities; |
| • | placing us at a competitive disadvantage compared to our competitors that have less debt; |
| • | limiting our ability to borrow additional funds for working capital, acquisitions, debt service requirements, execution of our business strategy or other general corporate purposes; |
| • | limiting our ability to enter into certain commercial arrangements because of concerns of counterparty risks; and |
| • | limiting our ability to adjust to changing market conditions and placing us at a competitive disadvantage compared to our competitors that have less debt. |
The occurrence of any one or more of these circumstances could have a material adverse effect on us.
Despite our significant level of indebtedness, we may be able to incur substantially more debt and enter into other transactions which could further exacerbate the risks to our financial condition described above.
Notwithstanding our significant level of indebtedness, we may be able to incur significant additional indebtedness in the future, including additional secured indebtedness that would be effectively senior to the notes (including up to $750 million of available capacity under the Revolving Credit Facility). Although the indenture that governs the notes and the credit agreement that governs the Senior Secured Credit Facilities contain restrictions on our ability to incur additional indebtedness and to enter into certain types of other transactions, these restrictions are subject to a number of significant qualifications and exceptions. Additional indebtedness incurred in compliance with these restrictions, including secured indebtedness, could be substantial. These restrictions also do not prevent us from incurring obligations, such as trade payables, that do not constitute indebtedness as defined under our debt instruments. To the extent such new debt is added to our current debt levels, the substantial leverage risks described in the immediately preceding risk factor would increase.
We may need additional capital in the future and may not be able to obtain it on favorable terms.
Our industry is capital intensive, and we may require additional capital in the future to finance our growth and development, implement further marketing and sales activities, fund ongoing research and development activities and meet general working capital needs. Our capital requirements will depend on many factors, including acceptance of and demand for our products, the extent to which we invest in new technology and research and development projects, and the status and timing of these developments, as well as general availability of capital from debt and/or equity markets.
However, debt or equity financing may not be available to us on terms we find acceptable, if at all. Also, regardless of the terms of our debt or equity financing, our agreements and obligations under the tax matters agreement may limit our ability to issue stock. For a
23
The Chemours Company
more detailed discussion, see risk factor “We agreed to numerous restrictions to preserve the tax-free treatment of the transactions in the U.S., which may reduce our strategic and operating flexibility.” If we are unable to raise additional capital when needed, our financial condition could be materially and adversely affected.
Additionally, our failure to maintain the credit ratings on our debt securities, including the notes, could negatively affect our ability to access capital and could increase our interest expense on future indebtedness. We expect the credit rating agencies to periodically review our capital structure and the quality and stability of our earnings. Deterioration in our capital structure or the quality and stability of our earnings could result in a downgrade of the credit ratings on our debt securities. Any negative rating agency actions could constrain the capital available to us, reduce or eliminate available borrowing to us and could limit our access to and/or increase the cost of funding our operations. If, as a result, our ability to access capital when needed becomes constrained, our interest costs could increase, which could have material adverse effect on our results of operations, financial condition and cash flows.
Our variable rate indebtedness subjects us to interest rate risk, which could cause our indebtedness service obligations to increase significantly.
Our borrowings under the Senior Secured Credit Facilities are at variable rates and expose us to interest rate risk. As a result, if interest rates increase, our debt service obligations under the Senior Secured Credit Facilities or other variable rate debt would increase even though the amount borrowed remained the same, and our net income and cash flows, including cash available for servicing our indebtedness, would correspondingly decrease. As of December 31, 2016, we had approximately $1.4 billion of our outstanding debt at variable interest rates.
We may be unable to service our indebtedness, including the notes.
Our ability to make scheduled payments on and to refinance our indebtedness, including the notes, depends on and is subject to our financial and operating performance, which in turn is affected by general and regional economic, financial, competitive, business and other factors (many of which are beyond our control), including the availability of financing in the international banking and capital markets. We cannot be certain that our business will generate sufficient cash flow from operations or that future borrowings will be available to us in an amount sufficient to enable us to service our debt, including the notes, to refinance our debt or to fund our other liquidity needs.
If we are unable to meet our debt service obligations or to fund our other liquidity needs, we will need to restructure or refinance all or a portion of our debt, including the notes. Failure to successfully restructure or refinance our debt could cause us to default on our debt obligations and would impair our liquidity. Our ability to restructure or refinance our debt will depend on the condition of the capital markets and our financial condition at such time. Any refinancing of our indebtedness could be at higher interest rates and may require us to comply with more onerous covenants that could further restrict our business operations.
Moreover, in the event of a default of our debt service obligations, the holders of the applicable indebtedness, including the notes and the Senior Secured Credit Facilities, could elect to declare all the funds borrowed to be due and payable, together with accrued and unpaid interest. We cannot be certain that our assets or cash flows would be sufficient to fully repay borrowings under our outstanding debt instruments if accelerated upon an event of default. First, a default in our debt service obligations in respect of the notes would result in a cross default under the Senior Secured Credit Facilities. The foregoing would permit the lenders under the Revolving Credit Facility to terminate their commitments thereunder and cease making further loans, and would allow the lenders under the Senior Secured Credit Facilities to declare all loans immediately due and payable and to institute foreclosure proceedings against their collateral, which could force us into bankruptcy or liquidation. Second, any event of default or declaration of acceleration under the Senior Secured Credit Facilities or any other agreements relating to our outstanding indebtedness under which the total amount of outstanding indebtedness exceeds $100 million could also result in an event of default under the indenture governing the notes, and any event of default or declaration of acceleration under any other of our outstanding indebtedness may also contain a cross-default provision. Any such default, event of default or declaration of acceleration could materially and adversely affect our results of operation and financial condition.
24
The Chemours Company
The agreements governing our indebtedness restrict our current and future operations, particularly our ability to respond to changes or to take certain actions.
The agreements governing our indebtedness, including the notes, contain, and the agreements governing future indebtedness and future debt securities may contain, significant restrictive covenants and, in the case of the Revolving Credit Facility, financial maintenance covenants that will limit our operations, including our ability to engage in activities that may be in our long-term best interests. These restrictive covenants may limit us, and our restricted subsidiaries, from taking, or give rights to the holders of our indebtedness in the event of the following actions:
| • | incurring additional indebtedness and guaranteeing indebtedness; |
| • | paying dividends or making other distributions in respect of, or repurchasing or redeeming, our capital stock; |
| • | making acquisitions or other investments; |
| • | prepaying, redeeming or repurchasing certain indebtedness; |
| • | selling or otherwise disposing of assets; |
| • | selling stock of our subsidiaries; |
| • | incurring liens; |
| • | entering into transactions with affiliates; |
| • | entering into agreements restricting our subsidiaries’ ability to pay dividends; |
| • | entering into transactions that result in a change of control of us; and |
| • | consolidating, merging or selling all or substantially all of our assets. |
Our failure to comply with those covenants could result in an event of default that, if not cured or waived, could result in the acceleration of some or all of our indebtedness, which could lead us to bankruptcy, reorganization or insolvency.
Risks Related to the Separation
We may be unable to achieve some or all of the benefits that we expected to achieve from our separation from DuPont.
As an independent, publicly-traded company, we continue to, among other things, focus our financial and operational resources on our specific business, growth profile and strategic priorities, design and implement corporate strategies and policies targeted to our operational focus and strategic priorities, guide our processes and infrastructure to focus on our core strengths, implement and maintain a capital structure designed to meet our specific needs and more effectively respond to industry dynamics, all of which are benefits we expected to achieve from our separation. However, we may be unable to fully achieve some or all of these benefits. For example, in order to position ourselves for the separation and distribution, we undertook a series of strategic, structural and process realignment and restructuring actions within our operations. These actions may not provide the benefits we expected, and could lead to disruption of our operations, loss of, or inability to recruit, key personnel needed to operate and grow our businesses following the separation and distribution, weakening of our internal standards, controls or procedures and impairment of our key customer and supplier relationships. If we fail to achieve some or all of the benefits that we expected to achieve as an independent company, or do not achieve them in the time we expected, our business, financial condition and results of operations could be materially and adversely affected.
If the distribution, together with certain related transactions, were to fail to qualify for non-recognition treatment for U.S. federal income tax purposes, then we could be subject to significant tax and indemnification liability and stockholders receiving our common stock in the distribution could be subject to significant tax liability.
DuPont received a ruling from the IRS substantially to the effect that, among other things, the distribution qualified as a tax-free transaction under Section 355 and Section 368(a)(1)(D) of the Internal Revenue Code (the Code). The tax-free nature of the distribution was conditioned on the continued validity of the IRS Ruling, as well as on receipt of a tax opinion, in form and substance acceptable to DuPont, substantially to the effect that, among other things, the distribution would qualify as a tax-free transaction under Section 355 and Section 368(a)(1)(D) of the Code, and certain transactions related to the transfer of assets and liabilities to us in connection with the separation and distribution would not result in the recognition of any gain or loss to DuPont, us or our stockholders. The IRS Ruling and the tax opinion relied on certain facts, assumptions, and undertakings, and certain representations from DuPont and us, regarding the past and future conduct of both respective businesses and other matters, and the tax opinion relies on the IRS Ruling. Notwithstanding the IRS Ruling and the tax opinion, the IRS could determine that the distribution or such related transactions should be treated as a taxable transaction if it determines that any of these facts, assumptions, representations, or
25
The Chemours Company
undertakings were not correct, or that the distribution should be taxable for other reasons, including if the IRS were to disagree with the conclusions in the tax opinion that are not covered by the IRS Ruling.
If the distribution ultimately was determined to be taxable, then a stockholder of DuPont that received shares of our common stock in the distribution would be treated as having received a distribution of property in an amount equal to the fair market value of such shares on the distribution date and could incur significant income tax liabilities. Such distribution would be taxable to such stockholder as a dividend to the extent of DuPont’s current and accumulated earnings and profits. Any amount that exceeded DuPont’s earnings and profits would be treated first as a non-taxable return of capital to the extent of such stockholder’s tax basis in its shares of DuPont stock with any remaining amount being taxed as a capital gain. DuPont would recognize a taxable gain in an amount equal to the excess, if any, of the fair market value of the shares of our common stock held by DuPont on the distribution date over DuPont’s tax basis in such shares. In addition, if certain related transactions fail to qualify for tax-free treatment under U.S. federal, state and/or local tax law and/or foreign tax law, we and DuPont could incur significant tax liabilities under U.S. federal, state, local and/or foreign tax law.
Generally, taxes resulting from the failure of the separation and distribution or certain related transactions to qualify for non-recognition treatment under U.S. federal, state and/or local tax law and/or foreign tax law would be imposed on DuPont or DuPont’s stockholders and, under the tax matters agreement that we entered into with DuPont prior to the spin-off, DuPont is generally obligated to indemnify us against such taxes to the extent that we may be jointly, severally or secondarily liable for such taxes. However, under the terms of the tax matters agreement, we are also generally responsible for any taxes imposed on DuPont that arise from the failure of the distribution to qualify as tax-free for U.S. federal income tax purposes within the meaning of Section 355 of the Code or the failure of such related transactions to qualify for tax-free treatment, to the extent such failure to qualify is attributable to actions, events or transactions relating to our, or our affiliates’, stock, assets or business, or any breach of our or our affiliates’ representations, covenants or obligations under the tax matters agreement (or any other agreement we enter into in connection with the separation and distribution), the materials submitted to the IRS or other governmental authorities in connection with the request for the IRS Ruling or other tax rulings or the representation letter provided to counsel in connection with the tax opinion. Events triggering an indemnification obligation under the agreement include events occurring after the distribution that cause DuPont to recognize a gain under Section 355(e) of the Code. Such tax amounts could be significant. To the extent we are responsible for any liability under the tax matters agreement, there could be a material adverse impact on our business, financial condition, results of operations and cash flows in future reporting periods.
We are subject to continuing contingent tax-related liabilities of DuPont.
There are several significant areas where the liabilities of DuPont may become our obligations. For example, under the Code and the related rules and regulations, each corporation that was a member of DuPont’s consolidated tax reporting group during any taxable period or portion of any taxable period ending on or before the effective time of the distribution is jointly and severally liable for the U.S. federal income tax liability of the entire consolidated tax reporting group for such taxable period. In connection with the separation and distribution, we entered into a tax matters agreement with DuPont that allocates the responsibility for prior period taxes of DuPont’s consolidated tax reporting group between us and DuPont. If DuPont were unable to pay any prior period taxes for which it is responsible, however, we could be required to pay the entire amount of such taxes, and such amounts could be significant. Other provisions of federal, state, local, or foreign law may establish similar liability for other matters, including laws governing tax-qualified pension plans, as well as other contingent liabilities.
We agreed to numerous restrictions to preserve the tax-free treatment of the transactions in the U.S., which may reduce our strategic and operating flexibility.
Our ability to engage in significant equity transactions could be limited or restricted in order to preserve, for U.S. federal income tax purposes, the tax-free nature of the distribution by DuPont. Even if the distribution otherwise qualifies for tax-free treatment under Section 355 of the Code, the distribution may result in corporate-level taxable gain to DuPont under Sections 355(e) and 368(a)(1)(D) of the Code if 50 percent or more, by vote or value, of shares of our stock or DuPont’s stock are acquired or issued as part of a plan or series of related transactions that includes the distribution. The process for determining whether an acquisition or issuance triggering these provisions has occurred is complex, inherently factual and subject to interpretation of the facts and circumstances of a particular case. Any acquisitions or issuances of our stock or DuPont’s stock within a two-year period after the distribution generally are presumed to be part of such a plan, although we or DuPont, as applicable, may be able to rebut that presumption. Accordingly, under the tax matters agreement entered into prior to the spin-off, for the two-year period following the distribution, we are prohibited, except in certain circumstances, from:
| • | entering into any transaction resulting in the acquisition of 40 percent or more of our stock or substantially all of our assets, whether by merger or otherwise; |
26
The Chemours Company
| • | issuing equity securities beyond certain thresholds; |
| • | repurchasing our capital stock; or |
| • | ceasing to actively conduct our business. |
These restrictions may limit our ability to pursue certain strategic transactions or other transactions, including our transformation plans that we may believe to otherwise be in our best interests or that might increase the value of our business. In addition, under the tax matters agreement, we are required to indemnify DuPont against any such tax liabilities as a result of the acquisition of our stock or assets, even if we do not participate in or otherwise facilitate the acquisition.
Risks Related to Our Common Stock
Our stock price could become more volatile and investments could lose value.
The market price of our common stock and the number of shares traded each day has experienced significant fluctuations since our separation from DuPont and may continue to fluctuate significantly. The market price for our common stock may be affected by a number of factors, including, but not limited to:
| • | our quarterly or annual earnings, or those of other companies in our industry; |
| • | actual or anticipated fluctuations in our operating results; |
| • | changes in earnings estimates by securities analysts or our ability to meet those estimates or our earnings guidance; |
| • | anticipated or actual outcomes or resolutions of legal or other contingencies; |
| • | the operating and stock price performance of other comparable companies; |
| • | credit rating agency actions; |
| • | a change in our dividend or stock repurchase activities; |
| • | changes in rules or regulations applicable to our business; |
| • | the announcement of new products by us or our competitors; |
| • | overall market fluctuations and domestic and worldwide economic conditions; and |
| • | other factors described in these “Risk Factors” and elsewhere in this Form 10-K. |
A significant drop or rise in our stock price could expose us to costly and time-consuming litigation, which could result in substantial costs and divert management’s attention and resources, resulting in an adverse effect on our business.
We cannot guarantee the timing, amount, or payment of dividends on our common stock in the future.
The declaration, payment and amount of any dividend are subject to the sole discretion of our board of directors and, in the context of our financial policy, will depend upon many factors, including our financial condition and prospects, our capital requirements and access to capital markets, covenants associated with certain of our debt obligations, legal requirements and other factors that our board of directors may deem relevant, and there can be no assurances that we will continue to pay a dividend in the future.
A stockholder’s percentage of ownership in us may be diluted in the future.
A stockholder’s percentage ownership in us may be diluted because of equity issuances for acquisitions, capital market transactions or otherwise, including, without limitation, equity awards that we may be granting to our directors, officers and employees. Such issuances may have a dilutive effect on our earnings per share, which could adversely affect the market price of our common stock.
In addition, our amended and restated certificate of incorporation authorizes us to issue, without the approval of our stockholders, one or more classes or series of preferred stock having such designation, powers, preferences and relative, participating, optional and other special rights, including preferences over our common stock with respect to dividends and distributions, as our board of directors generally may determine. The terms of one or more classes or series of preferred stock could dilute the voting power or reduce the value of our common stock. For example, we could grant the holders of preferred stock the right to elect some number of our directors in all events or on the happening of specified events or to veto specified transactions. Similarly, the repurchase or redemption rights or liquidation preferences we could assign to holders of preferred stock could affect the residual value of our common stock.
27
The Chemours Company
Certain provisions in our amended and restated certificate of incorporation and amended and restated by-laws, and of Delaware law, may prevent or delay an acquisition of us, which could decrease the trading price of the common stock.
Our amended and restated certificate of incorporation and amended and restated by-laws contain, and Delaware law contains, provisions that are intended to deter coercive takeover practices and inadequate takeover bids by making such practices or bids unacceptably expensive to the bidder and to encourage prospective acquirers to negotiate with our board of directors rather than to attempt a hostile takeover. These provisions include, among others:
| • | the inability of our stockholders to act by written consent; |
| • | the limited ability of our stockholders to call a special meeting; |
| • | rules regarding how stockholders may present proposals or nominate directors for election at stockholder meetings; |
| • | the right of our board of directors to issue preferred stock without stockholder approval; |
| • | the ability of our directors, and not stockholders, to fill vacancies (including those resulting from an enlargement of the board of directors) on our board of directors; and |
| • | the requirement that stockholders holding at least 80 percent of our voting stock are required to amend certain provisions in our amended and restated certificate of incorporation and our amended and restated by-laws. |
In addition, we are subject to Section 203 of the Delaware General Corporations Law (the DGCL). Section 203 provides that, subject to limited exceptions, persons that (without prior board approval) acquire, or are affiliated with a person that acquires, more than 15 percent of the outstanding voting stock of a Delaware corporation shall not engage in any business combination with that corporation, including by merger, consolidation or acquisitions of additional shares, for a three-year period following the date on which that person or its affiliate becomes the holder of more than 15 percent of the corporation’s outstanding voting stock.
We believe these provisions will protect our stockholders from coercive or otherwise unfair takeover tactics by requiring potential acquirers to negotiate with our board of directors and by providing our board of directors with more time to assess any acquisition proposal. These provisions are not intended to make us immune from takeovers. However, these provisions will apply even if an acquisition proposal or offer may be considered beneficial by some stockholders and could delay or prevent an acquisition that our board of directors determines is not in our best interests and our stockholders’. These provisions may also prevent or discourage attempts to remove and replace incumbent directors.
Several of the agreements that we have entered into with DuPont require DuPont’s consent to any assignment by us of our rights and obligations, or a change of control of us, under the agreements. The consent rights set forth in these agreements might discourage, delay or prevent a change of control that a stockholder may consider favorable.
In addition, an acquisition or further issuance of our stock could trigger the application of Section 355(e) of the Code. Under the tax matters agreement executed prior to the spin-off, we would be required to indemnify DuPont for the tax imposed under Section 355(e) of the Code resulting from an acquisition or issuance of its stock, even if it did not participate in or otherwise facilitate the acquisition, and this indemnity obligation might discourage, delay or prevent a change of control that a stockholder may consider favorable. Please see the risk factor “If the distribution, together with certain related transactions, were to fail to qualify for non-recognition treatment for U.S. federal income tax purposes, then we could be subject to significant tax and indemnification liability and stockholders receiving our common stock in the distribution could be subject to significant tax liability.” for further information.
Item 1B. UNRESOLVED STAFF COMMENTS
None.
28
The Chemours Company
Chemours Production Facilities and Technical Centers
Our corporate headquarters is in Wilmington, Delaware, and we maintain a global network of production facilities and technical centers located in cost-effective and strategic locations. We also use contract manufacturing and joint venture partners in order to provide regional access or to lower manufacturing costs, as appropriate. The following chart lists our production facilities as of December 31, 2016:
Production Facilities | ||||||||
|
| Titanium Technologies |
| Fluoroproducts |
| Chemical Solutions |
| Shared Locations |
North America |
| DeLisle, MS New Johnsonville, TN Starke, FL (Mine) |
| El Dorado, AR 1 Elkton, MD 1 Louisville, KY Fayetteville, NC Deepwater, NJ Corpus Christi, TX LaPorte, TX 2 Washington, WV Maitland, Canada |
| Pascagoula, MS Memphis, TN
|
| Belle, WV 3 |
Europe, Middle East & Africa (EMEA) |
|
|
| Mechelen, Belgium Villers St. Paul, France 1 Dordrecht, Netherlands |
|
|
|
|
Latin America |
| Altamira, Mexico |
| Barra Mansa, Brazil 2 |
|
|
|
|
Asia Pacific |
| Kuan Yin, Taiwan |
| Changshu, China Shanghai, China 4 SiChuan, China 4 Chiba, Japan 4 Shimizu, Japan 4 |
|
|
|
|
1 | Site is leased from third party. |
2 | Site is leased from DuPont. |
3 | Shared facility between the Chemical Solutions and Fluoroproducts segments. |
4 | Sites with joint venture equity affiliates. |
We have technical centers and R&D facilities located at a number of our production facilities. We also maintain standalone technical centers to serve our customers and provide technical support. The following chart lists our standalone technical centers as of December 31, 2016:
Technical Centers | ||||||||
Region |
| Titanium Technologies |
| Fluoroproducts |
| Chemical Solutions |
| Shared Locations |
North America |
|
|
| Akron, OH 2 |
|
|
| Wilmington, DE (All Segments) 2, 3 |
EMEA |
| Kallo, Belgium 1 |
| Mechelen, Belgium 1 Meyrin, Switzerland 2 |
|
|
|
|
Latin America |
| Barra Mansa, Brazil 2 Mexico City, Mexico 1 |
|
|
|
|
|
|
Asia Pacific |
|
|
| Utsonomyia, Japan 2 |
|
|
| Shanghai, China 2 (All Segments) |
1 | Leased from third party. |
2 | Leased from DuPont. |
3 | There are two facilities at this location. |
29
The Chemours Company
Chemours’ plants and equipment are maintained and in good operating condition. Chemours believes it has sufficient production capacity for its primary products to meet demand in 2017. Properties are primarily owned by Chemours; however, certain properties are leased, as noted in the preceding tables.
Chemours recognizes that the security and safety of its operations are critical to its employees, community, and to the future of Chemours. Physical security measures have been combined with process safety measures, administrative procedures and emergency response preparedness into an integrated security plan. Prior to the separation, DuPont conducted vulnerability assessments at operating facilities in the U.S. and high priority sites worldwide and identified and implemented appropriate measures to protect these facilities from physical and cyber-attacks. Chemours intends to conduct similar vulnerability assessments periodically in the future. Chemours is partnering with carriers, including railroad, shipping and trucking companies, to secure chemicals in transit.
Legal Proceedings
The Company is subject to various legal proceedings, including, but not limited to, product liability, patent infringement, antitrust claims and claims for property damage or personal injury. Information regarding certain of these matters is set forth below and in Note 20 to the Consolidated Financial Statements.
Litigation
PFOA: Environmental and Litigation Proceedings
For purposes of this report, the term PFOA means collectively perfluorooctanoic acid and its salts, including the ammonium salt and does not distinguish between the two forms. Information related to this and other litigation matters is included in Note 20 to the Consolidated Financial Statements.
Environmental Proceedings
LaPorte Plant, LaPorte, Texas
The U.S. Environmental Protection Agency (EPA) conducted a multimedia inspection at the DuPont LaPorte facility in January 2008. DuPont, the EPA and the Department of Justice (DOJ) began discussions in the fall of 2011 relating to the management of certain materials in the facility’s waste water treatment system, hazardous waste management, flare and air emissions. These negotiations continue. Chemours operates a fluoroproducts production facility at this site.
Dordrecht, Netherlands
The Company has received requests from the Labor Inspectorate (ISZW), the local environmental agency (OZHZ) and the National Institute for Public Health and the Environment (RIVM) in the Netherlands for information and documents regarding the Dordrecht site’s operations. The Company has complied with the requests. We understand that some of the requests from OZHZ are part of a preliminary investigation initiated by a public prosecutor, although we have not received notice that it intends to pursue such action.
Item 4. MINE SAFETY DISCLOSURES
Information regarding mine safety and other regulatory actions at the Company’s surface mine in Starke, Florida is included in Exhibit 95 to this report.
EXECUTIVE OFFICERS OF THE REGISTRANT
The following is a list of the executive officers and a summary of their professional experience:
Mark P. Vergnano, age 59, serves as our President and Chief Executive Officer. Prior to joining Chemours, he held roles of increasing responsibility at E. I. du Pont de Nemours and Company. In October 2009, Mr. Vergnano was appointed Executive Vice President of DuPont and was responsible for multiple businesses and functions, including the businesses in the Chemours segment: DuPont Chemicals & Fluoroproducts and Titanium Technologies. In June 2006, he was named Group Vice President of DuPont Safety & Protection. In October 2005, he was named Vice President and General Manager - Surfaces and Building Innovations. In
30
The Chemours Company
February 2003, he was named Vice President and General Manager -Nonwovens. Prior to that, he had several assignments in manufacturing, technology, marketing, sales and business strategy. Mr. Vergnano joined DuPont in 1980 as a process engineer. Mr. Vergnano has served on the board of directors of the National Safety Council since 2007, the American Chemistry Council since 2015, and Johnson Controls International plc since 2016. He previously served on the board of directors of Johnson Controls, Inc. from 2011 to 2016.
Mark E. Newman, age 53, serves as our Senior Vice President and Chief Financial Officer. Mr. Newman joined Chemours in November 2014 from SunCoke Energy where he was SunCoke Energy’s Senior Vice President and Chief Financial Officer and led its financial, strategy, business development and information technology functions. Mr. Newman joined SunCoke’s leadership team in March 2011 to help drive SunCoke’s separation from its parent company, Sunoco, Inc. He led SunCoke through an initial public offering and championed a major restructuring of SunCoke, which resulted in the initial public offering of SunCoke Energy Partners in January 2013, creating the first coke-manufacturing master limited partnership. Prior to joining SunCoke, Mr. Newman served as Vice President Remarketing & Managing Director of SmartAuction, Ally Financial Inc. (previously General Motors Acceptance Corporation). Mr. Newman began his career at General Motors in 1986 as an Industrial Engineer and progressed through several financial and operational leadership roles within the global automaker, including Vice President and Chief Financial Officer of Shanghai General Motors Limited; Assistant Treasurer of General Motors Corporation; and North America Vice President and CFO.
E. Bryan Snell, age 60, serves as our President - Titanium Technologies. Mr. Snell was appointed President - Titanium Technologies in May 2015. Previously, he served as Planning Director - DuPont Performance Chemicals (2014-2015). Prior to that, he held leadership positions in DuPont Titanium Technologies, including Planning Director (2011-12 in Wilmington, DE and 2012-13 in Singapore) and Global Sales and Marketing Director (2008-2010). Mr. Snell served as Regional Operations Director - DuPont Coatings and Color Technologies Platform in 2007 and 2008. He was posted in Taiwan from 2002 to 2006, in the roles of Plant Manager -Kuan Yin Plant and Asia/Pacific Regional Director, DuPont Titanium Technologies. Mr. Snell joined DuPont in 1978 as a process engineer and has experience in nuclear and petrochemical operations, as well as sales, business strategy and M&A.
Paul Kirsch, age 53, serves as our President - Fluoroproducts. Mr. Kirsch joined Chemours in June 2016 from Henkel AG & Company, where he served as Senior Vice President of supply chain and operations for three years. Prior to that, he was President of the automotive, metals, and aerospace division of Henkel AG & Company KGaA. He also served as Co-Chairman of a Henkel-BASF joint venture. Before joining Henkel in 2009, Mr. Kirsch spent nearly 25 years in various engineering, operations, and business development roles of increasing responsibility within the automotive and telematics industries. He was Vice President of Hughes Telematics, where his responsibilities included business development, quality, and strategic planning. He also served as Vice President of XM Satellite Radio, where he was responsible for growing and running the automotive business of the Washington, DC–based firm. Mr. Kirsch started his career at Delphi in 1985, where he worked for nearly 19 years, in both regional and global roles ranging from product engineer to director of engineering for Asia Pacific to director of mergers and acquisitions, as well as general director of sales, marketing, and strategic planning.
Christian W. Siemer, age 58, serves as our President - Chemical Solutions. He moved to this role in July 2014. Mr. Siemer joined DuPont in 2010 as the Managing Director of Clean Technologies, a business unit of DuPont Sustainable Solutions focused on process technology development and licensing. He led the successful acquisition of MECS Inc., the global leader in technology for the production of sulfuric acid. Mr. Siemer began his career in 1980 with Stauffer Chemicals as a process engineer. Following Stauffer’s acquisition by ICI plc, Mr. Siemer moved through a range of commercial roles and overseas assignments managing portfolios of international industrial and specialty chemical businesses.
David C. Shelton, age 53, serves as our Senior Vice President, General Counsel and Corporate Secretary. In 2011, Mr. Shelton was appointed Associate General Counsel, DuPont, and was responsible for the US Commercial team - the business lawyers and paralegals counseling all the DuPont business units with the exception of Agriculture. Mr. Shelton was the Commercial attorney to a variety of DuPont businesses including the Performance Materials platform, which he advised on international assignment in Geneva, and the businesses now comprising the DuPont Chemicals and Fluoroproducts business unit. Prior to that, Mr. Shelton advised the company on environmental and remediation matters as part of the environmental legal team. Mr. Shelton joined DuPont in 1996, after seven years in private practice as a litigator in Pennsylvania and New Jersey.
Beth Albright, age 50, serves as our Senior Vice President Human Resources. Mrs. Albright joined DuPont in October 2014 from Day & Zimmermann, where she held the position of Senior Vice-President Human Resources since May 2011. Prior to her experience at Day & Zimmermann, Mrs. Albright was the Global Vice President Human Resources for Tekni-Plex, which she joined in July 2009. She joined Rohm and Haas in 2000 and held various Human Resources supporting global businesses, technology, manufacturing and staff functions. In 1995 she joined FMC as site Human Resources manager at a manufacturing site and progressed into the corporate office. Mrs. Albright began her career with Fluor Daniel Construction in their Industrial Relations department in 1989.
31
The Chemours Company
Erich Parker, age 65, serves as our Vice President of Corporate Communications and Chief Brand Officer. Mr. Parker was appointed Creative Director and Global Director of Corporate Communications of DuPont in 2010. He led the initiative to develop corporate positioning and its creative expression through branded content and program sponsorship with large international media outlets. In 2008, Mr. Parker was appointed Communications Leader for DuPont’s Safety and Protection Platform. Prior to joining DuPont, Mr. Parker was principal of his own public relations and marketing communications firm based in Washington, D.C., and New York. Mr. Parker has also served as Executive Vice President of Association & Issues Management; Director of Communications for the American Academy of Actuaries; founding publisher and Executive Editor of the magazine Contingencies; and Public Affairs Aide for Renewable Energy to the Secretary of Energy, U.S. Department of Energy.
32
The Chemours Company
Item 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market for Registrant’s Common Equity and Related Stockholder Matters
The Company’s common stock is listed on the New York Stock Exchange, Inc. (symbol CC). The number of record holders of common stock was 54,322 at February 14, 2017.
Holders of the Company’s common stock are entitled to receive dividends when they are declared by the board of directors. Dividends on common stock are generally declared and paid on a quarterly basis. The Stock Transfer Agent and Registrar is Computershare Trust Company, N.A.
The Company’s stock began trading on July 1, 2015. The quarterly high and low trading stock prices and dividends per common share for 2016 and 2015 are shown below:
|
|
|
|
|
|
|
|
|
| Per Share |
|
| |
|
| Market Prices |
|
| Dividend |
|
| ||||||
|
| High |
|
| Low |
|
| Declared |
|
| |||
2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Fourth Quarter |
| $ | 27.29 |
|
| $ | 14.41 |
|
| $ | 0.03 |
|
|
Third Quarter |
|
| 16.08 |
|
|
| 5.82 |
|
|
| 0.03 |
|
|
Second Quarter |
|
| 10.83 |
|
|
| 6.99 |
|
|
| 0.03 |
|
|
First Quarter |
|
| 7.84 |
|
|
| 3.06 |
|
|
| 0.03 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Fourth Quarter |
| $ | 8.80 |
|
| $ | 4.58 |
|
| $ | 0.03 |
|
|
Third Quarter |
|
| 16.68 |
|
|
| 5.94 |
|
|
| 0.55 |
| (1) |
| 1 | Dividend was declared prior to our separation from DuPont and paid on September 11, 2015 to our stockholders of record as of August 3, 2015. |
|
Unregistered Sales of Equity Securities
Not Applicable.
Issuer Purchases of Equity Securities
Not Applicable.
33
The Chemours Company
The following graph presents the cumulative total shareholder return for the Company’s common stock compared with the Standard & Poor’s (S&P) MidCap 400, S&P MidCap 400 Chemical and S&P SmallCap 600 indices since our separation from DuPont on July 1, 2015.

The graph assumes that the values of Chemours’ common stock, the S&P MidCap 400 index, the S&P MidCap 400 Chemical index and the S&P SmallCap 600 index were each $100 on July 1, 2015, the date that Chemours’ common stock began “regular-way” trading on the New York Stock Exchange, and that all dividends were reinvested. On January 29, 2016, Chemours moved from the S&P MidCap 400 index to the S&P SmallCap 600 index. On January 3, 2017, Chemours moved from S&P SmallCap 600 index to the S&P MidCap 400 index.
34
The Chemours Company
Item 6. SELECTED HISTORICAL CONSOLIDATED FINANCIAL DATA
The following table presents Chemours’ selected historical consolidated financial data. The selected historical consolidated financial data as of December 31, 2016 and 2015 and for the years ended December 31, 2016, 2015 and 2014 are derived from audited information contained in Chemours’ consolidated financial statements included elsewhere in this Annual Report. The selected historical consolidated financial data as of and for the years ended December 31, 2013 and 2012 are derived from Chemours’ audited consolidated financial statements not included in this Annual Report.
The selected historical consolidated financial data for the periods ended December 31, 2012 through 2014 and for the first six months of the year ended December 31, 2015 include certain expenses of DuPont that were allocated to Chemours for certain corporate functions including information technology, research and development, finance, legal, insurance, compliance and human resources activities. These costs may not be representative of the future costs Chemours will incur as an independent, publicly traded company. In addition, Chemours’ historical financial information does not reflect changes that Chemours expects to experience in the future as a result of Chemours’ separation from DuPont, including changes in Chemours’ cost structure, personnel needs, tax structure, capital structure, financing and business operations. Consequently, the financial information included here may not necessarily reflect what Chemours’ financial position, results of operations and cash flows would have been had it been an independent, publicly traded company during the periods presented. Accordingly, these historical results should not be relied upon as an indicator of Chemours’ future performance.
For a better understanding, this section should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements and accompanying notes included elsewhere in this Annual Report on Form 10-K.
(Dollars in millions, except per share data) |
| Year Ended December 31, |
| |||||||||||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
|
| 2013 |
|
| 2012 |
| |||||
Summary of operations: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales |
| $ | 5,400 |
|
| $ | 5,717 |
|
| $ | 6,432 |
|
| $ | 6,859 |
|
| $ | 7,365 |
|
Restructuring and asset related charges, net |
|
| 170 |
|
|
| 333 |
|
|
| 21 |
|
|
| 2 |
|
|
| 36 |
|
(Loss) income before income taxes |
|
| (11 | ) |
|
| (188 | ) |
|
| 550 |
|
|
| 576 |
|
|
| 1,485 |
|
(Benefit from) provision for income taxes |
|
| (18 | ) |
|
| (98 | ) |
|
| 149 |
|
|
| 152 |
|
|
| 427 |
|
Net income (loss) attributable to Chemours |
|
| 7 |
|
|
| (90 | ) |
|
| 400 |
|
|
| 423 |
|
|
| 1,057 |
|
Basic earnings (loss) per share of common stock 1 |
|
| 0.04 |
|
|
| (0.50 | ) |
|
| 2.21 |
|
|
| 2.34 |
|
|
| 5.84 |
|
Diluted earnings (loss) per share of common stock 1 |
|
| 0.04 |
|
|
| (0.50 | ) |
|
| 2.21 |
|
|
| 2.34 |
|
|
| 5.84 |
|
Financial position at year end: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Working capital 2 |
|
| 782 |
|
|
| 835 |
|
|
| 543 |
|
|
| 474 |
|
|
| 601 |
|
Total assets |
|
| 6,060 |
|
|
| 6,298 |
|
|
| 5,959 |
|
|
| 5,580 |
|
|
| 5,309 |
|
Borrowings and capital lease obligations, net 3 |
|
| 3,544 |
|
|
| 3,954 |
|
|
| 1 |
|
|
| 1 |
|
|
| 1 |
|
General: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment |
|
| 338 |
|
|
| 519 |
|
|
| 604 |
|
|
| 438 |
|
|
| 432 |
|
Depreciation and amortization |
|
| 284 |
|
|
| 267 |
|
|
| 257 |
|
|
| 261 |
|
|
| 266 |
|
Dividends per common share 4 |
|
| 0.12 |
|
|
| 0.58 |
|
|
| — |
|
|
| — |
|
|
| — |
|
1 | For 2012-2014, pro forma earnings per share was calculated based on 180,996,833 shares of Chemours common stock that were distributed to DuPont shareholders on July 1, 2015. The same number of shares was used to calculate basic and diluted earnings per share since no Chemours equity awards were outstanding prior to the separation. |
2 | Current assets minus current liabilities. Current assets include cash and cash equivalents of $902 million and $366 million at December 31, 2016 and 2015, respectively. Years prior to 2015 do not include any cash, as Chemours’ needs were provided by its former parent, DuPont. |
3 | Amounts as of December 31, 2016 and 2015 include unamortized debt issuance costs and discount of $47 million and $60 million, respectively. |
4 | Dividends per common share for the year ended December 31, 2015 includes the following: |
| • | dividend of an aggregate amount of $100 million declared prior to separation by our then-board of directors (consisting of DuPont employees), which was paid on September 11, 2015 to our stockholders of record as of August 3, 2015, and |
| • | dividend of $0.03 per share declared after separation by our independent board of directors which was paid on December 14, 2015 to our stockholders of record as of November 13, 2015. |
35
The Chemours Company
Item 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Introduction
Management’s discussion and analysis, which we refer to as “MD&A”, of our results of operations and financial condition supplements the consolidated financial statements and related notes included elsewhere herein to help provide an understanding of our financial condition, changes in financial condition and results of our operations. The discussion and analysis presented below refer to and should be read in conjunction with the consolidated financial statements and the related notes included elsewhere in this Annual Report on Form 10-K.
Overview
Chemours is a leading global provider of performance chemicals that are key inputs in end-products and processes in a variety of industries. We deliver customized solutions with a wide range of industrial and specialty chemical products for markets including plastics and coatings, refrigeration and air conditioning, general industrial, mining and oil refining. Principal products include titanium dioxide, refrigerants, industrial fluoropolymer resins and a portfolio of mining and industrial chemicals including sodium cyanide.
Chemours manages and reports operating results through three reportable segments: Titanium Technologies, Fluoroproducts and Chemical Solutions. Our position with each of these businesses reflects the strong value proposition we provide to our customers based on our long history and reputation in the chemical industry for safety, quality and reliability.
On July 1, 2015, DuPont completed the previously announced spin-off of Chemours by distributing Chemours’ common stock, on a pro rata basis, to DuPont’s stockholders of record as of the close of business on June 23, 2015 (the Record Date). Each holder of DuPont common stock received one share of Chemours’ common stock for every five shares of DuPont’s common stock held on the Record Date. The Separation was completed pursuant to a separation agreement and several other agreements with DuPont, including an employee matters agreement, a tax matters agreement, a transition services agreement and an intellectual property cross-license agreement, each of which was filed with the SEC as an exhibit to our Current Report on Form 8-K on July 1, 2015. These agreements govern the relationship among Chemours and DuPont following the Separation and provide for the allocation of various assets, liabilities, rights and obligations. These agreements also include arrangements for transition services provided by DuPont to Chemours, which was substantially completed during 2016.
Basis of Presentation
Prior to July 1, 2015, Chemours operations were included in DuPont’s financial results in different legal forms, including but not limited to wholly-owned subsidiaries for which Chemours was the sole business, components of legal entities in which Chemours operated in conjunction with other DuPont businesses and a majority owned joint venture. For periods prior to July 1, 2015, the consolidated financial statements, included elsewhere in this Annual Report on Form 10-K, have been prepared from DuPont’s historical accounting records and are presented on a stand-alone basis as if the business operations had been conducted independently from DuPont. The consolidated financial statements include the historical operations, assets and liabilities of the legal entities that are considered to comprise the Chemours business, including certain environmental remediation and litigation obligations of DuPont and its subsidiaries that Chemours may be required to indemnify pursuant to the separation-related agreements executed prior to the Separation. All of the allocations and estimates in the consolidated financial statements prior to July 1, 2015 are based on assumptions that management believes are reasonable.
36
The Chemours Company
Transformation Plan
After the separation in 2015, Chemours announced a plan to transform the company by reducing structural costs, growing market positions, optimizing its portfolio, refocusing investments, and enhancing its organization. Chemours expects the transformation plan to deliver $500 million of incremental Adjusted EBITDA improvement over 2015 through 2017. Based on our anticipated cost reduction and growth initiatives, we expect that our cost savings of approximately $350 million and approximately $150 million in improvements from growth initiatives will also improve our pre-tax earnings by similar amounts through 2017. Through year-end 2016, we achieved approximately $200 million in cost savings, and we continue to implement additional cost reduction initiatives in order to realize our target of reducing structural costs. These improvements will be partially offset by the impact of divestitures completed during 2016 (discussed under “Chemical Solutions Portfolio Optimization Actions” below), unfavorable price and mix of fluoropolymer products, and may also be impacted by market factors. Results of our transformation actions are also discussed in the Results of Operations, Segment Reviews and Outlook sections of this MD&A.
Chemical Solutions Portfolio Optimization Actions
On June 13, 2016, the Company entered into an asset purchase agreement with Veolia North America (“Veolia”), pursuant to which Veolia agreed to acquire Chemours’ Sulfur Products business (“Sulfur business”) of its Chemical Solutions segment for a purchase price of $325 million in cash, subject to customary working capital and other adjustments, of which approximately $10 million was received in May 2016. The Company completed the sale on July 29, 2016 and received the remaining proceeds of approximately $311 million, net of estimated working capital adjustments.
On April 22, 2016, the Company entered into a Stock and Asset Purchase Agreement with Lanxess, pursuant to which Lanxess agreed to acquire Chemours’ Clean & Disinfect product line (the “C&D business”) by acquiring certain Chemours’ subsidiaries and assets comprising the C&D business for a purchase price of $230 million in cash, subject to customary working capital and other adjustments. The Company completed the sale on August 31, 2016 and received $223 million of cash, net of working capital adjustments and approximately $2 million of cash transferred.
On March 1, 2016, the Company completed the sale of its aniline facility in Beaumont, Texas to The Dow Chemical Company (Dow) for a cash proceeds of approximately $140 million. As part of this transaction, Chemours also entered into a supply agreement with an initial two-year term to supply Dow with its additional aniline requirements from Chemours’ Pascagoula, Mississippi production facility.
The Company expects to use the proceeds from the above sales to fund our future capital expenditures.
Settlement of PFOA MDL Litigation
As previously reported, approximately 3,500 lawsuits have been filed in various federal and state courts in Ohio and West Virginia alleging personal injury from exposure to perfluorooctanoic acid and its salts, including the ammonium salt (“PFOA”), in drinking water as a result of the historical manufacture or use of PFOA at the Washington Works plant outside Parkersburg, West Virginia. That plant was previously owned and/or operated by the performance chemicals segment of DuPont and is now owned and/or operated by Chemours. These personal injury lawsuits were consolidated in multi-district litigation in the United States District Court for the Southern District of Ohio (the “MDL”). See Item 3. Legal Proceedings and our Note 20 to the Consolidated Financial Statements included elsewhere in this Annual Report.
On February 11, 2017, DuPont entered into an agreement in principle with plaintiffs’ counsel representing the MDL plaintiffs providing for a global settlement of all cases and claims in the MDL, including all filed and unfiled personal injury cases and claims that are part of the plaintiffs’ counsel’s claim inventory, as well as cases that have been tried to a jury verdict (the “MDL Settlement”). The total settlement amount is $670.7 million dollars in cash, half of which will be paid by Chemours and half paid by DuPont. DuPont’s payment would not be subject to indemnification or reimbursement by Chemours, and Chemours has accrued $335 million associated with this matter at December 31, 2016. In exchange for payment of the total settlement amount, DuPont and Chemours will receive a complete release of all claims by the settling plaintiffs. The MDL Settlement was entered into solely by way of compromise and settlement and is not in any way an admission of liability or fault by DuPont or Chemours. The MDL Settlement is not subject to court approval; however, the MDL Settlement may not proceed in certain conditions, including a walk-away right that enables DuPont to terminate the MDL Settlement if more than a specified number of plaintiffs determine not to participate.
37
The Chemours Company
DuPont and Chemours have also agreed, subject to and following the completion of the MDL Settlement, to a limited sharing of potential future PFOA liabilities (i.e., “indemnifiable losses,” as defined in the separation agreement between DuPont and Chemours) for a period of five years. During that five-year period, Chemours would annually pay future PFOA liabilities up to $25 million and, if such amount is exceeded, DuPont would pay any excess amount up to the next $25 million (which payment will not be subject to indemnification by Chemours), with Chemours annually bearing any further excess liabilities under the terms of the separation agreement. After the five-year period, this limited sharing agreement would expire, and Chemours’ indemnification obligations under the separation agreement would continue unchanged. Chemours has also agreed that, upon the MDL Settlement becoming effective, it will not contest its liability to DuPont under the separation agreement for PFOA liabilities on the basis of ostensible defenses generally applicable to the indemnification provisions under the separation agreement, including defenses relating to punitive damages, fines or penalties or attorneys’ fees, and waives any such defenses with respect to PFOA liabilities. Chemours has, however, retained defenses as to whether any particular PFOA claim is within the scope of the indemnification provisions of the separation agreement.
Our Results and Business Highlights
Net sales for the year ended December 31, 2016 were $5.4 billion, a decrease of 6% from $5.7 billion for the year ended December 31, 2015. These decreases were driven primarily by the portfolio changes from divestitures in the Chemical Solutions segment and lower average selling price for TiO2, partially offset by volume increase in in the Fluoroproducts segment due to growth in OpteonTM and volume increase in Titanium Technologies segment.
We recognized net income of $7 million for the year ended December 31, 2016 compared with a net loss of $90 million for the year ended December 31, 2015. Our results for the year reflect $254 million of net gain on sale of assets and business in the Chemical Solutions segment, improvements from cost reductions initiatives and strong Fluoroproducts performance, offset by $335 million litigation accrual related to the PFOA MDL Settlement and $119 million of asset impairment charges in the Chemicals Solutions and Corporate and Other segments.
Our Adjusted EBITDA was $822 million for the year ended December 31, 2016 compared with $573 million for the year ended December 31, 2015. Our 2016 results reflect margin improvements in Titanium Technologies, improved profitability in Fluoroproducts, including growth in OpteonTM sales, and improvements from cost reductions initiatives, partially offset by the impact of divestitures within the Chemical Solutions segment.
Results of Operations
|
| Year Ended December 31, |
|
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
|
| |||
Net sales |
| $ | 5,400 |
|
| $ | 5,717 |
|
| $ | 6,432 |
|
|
Cost of goods sold |
|
| 4,290 |
|
|
| 4,762 |
|
|
| 5,072 |
|
|
Gross profit |
|
| 1,110 |
|
|
| 955 |
|
|
| 1,360 |
|
|
Selling, general and administrative expense 1 |
|
| 934 |
|
|
| 632 |
|
|
| 685 |
|
|
Research and development expense |
|
| 80 |
|
|
| 97 |
|
|
| 143 |
|
|
Restructuring and asset related charges, net |
|
| 170 |
|
|
| 333 |
|
|
| 21 |
|
|
Goodwill impairment |
|
| — |
|
|
| 25 |
|
|
| — |
|
|
Total expenses |
|
| 1,184 |
|
|
| 1,087 |
|
|
| 849 |
|
|
Equity in earnings of affiliates |
|
| 29 |
|
|
| 22 |
|
|
| 20 |
|
|
Interest expense, net |
|
| (213 | ) |
|
| (132 | ) |
|
| — |
|
|
Other income, net |
|
| 247 |
|
|
| 54 |
|
|
| 19 |
|
|
(Loss) income before income taxes |
|
| (11 | ) |
|
| (188 | ) |
|
| 550 |
|
|
(Benefit from) provision for income taxes |
|
| (18 | ) |
|
| (98 | ) |
|
| 149 |
|
|
Net income (loss) |
|
| 7 |
|
|
| (90 | ) |
|
| 401 |
|
|
Less: Net income attributable to noncontrolling interests |
|
| — |
|
|
| — |
|
|
| 1 |
|
|
Net income (loss) attributable to Chemours |
| $ | 7 |
|
| $ | (90 | ) |
| $ | 400 |
|
|
1 | Includes $335 million litigation accrual related to the PFOA MDL Settlement (see Note 20 to the Consolidated Financial Statements). |
Net sales
For the years ended December 31, 2016 and 2015: Net sales for the year ended December 31, 2016 were $5.4 billion, a decrease of approximately 6% compared to $5.7 billion for the year ended December 31, 2015. The decrease in net sales for the year ended
38
The Chemours Company
December 31, 2016 was driven by: i) portfolio changes from divestitures and lower selling prices resulting from the impact of lower raw materials costs on contractual pass-through terms in the Chemical Solutions segment and ii) lower selling prices for TiO2 year over year in the Titanium Technologies segment. These decreases were partially offset by improvements in the Fluoroproducts segment due to growth in Opteon™ and volume increase in Titanium Technologies due to higher demand in Europe and the United States.
For the years ended December 31, 2015 and 2014: Net sales for the year ended December 31, 2015 were of $5.7 billion, a decrease of approximately 11% compared to $6.4 billion for the year ended December 31, 2014. The decrease in net sales was primarily due to continued pressure on TiO2 prices and the negative impact of foreign currency, partially offset by price increases in Fluoroproducts and volume growth in Chemical Solutions portfolio.
The table below shows the impact of price, volume, currency and portfolio changes on net sales for the years ended December 31, 2016, 2015 and 2014.
|
| Year Ended December 31, |
| |||||
Change in net sales from prior period |
| 2016 |
|
| 2015 |
| ||
Price |
|
| (3 | )% |
|
| (5 | )% |
Volume |
|
| 2 | % |
|
| (1 | )% |
Currency |
|
| (1 | )% |
|
| (4 | )% |
Portfolio / Other |
|
| (4 | )% |
|
| (1 | )% |
Total Change |
|
| (6 | )% |
|
| (11 | )% |
For detailed discussion of net sales, see the Segment Review section of this MD&A.
Cost of goods sold
For the years ended December 31, 2016 and 2015: Cost of goods sold (COGS) decreased by 10% to $4.3 billion for the year ended December 31, 2016 compared to $4.8 billion for the year ended December 31, 2015. The decreases were primarily driven by lower operating costs, including lower raw materials and overhead costs, and improvement in plant utilization, which decreased COGS by approximately 7%, and portfolio changes in our Chemical Solutions segment, which also decreased COGS by approximately 4%. These decreases were partially offset by inventory write-downs in the Chemical Solutions segment of $10 million as a result of the reactive metals solution plant shutdown, $5 million assets write-off related to the Mining Solutions business of our Chemical Solutions segment, and approximately $10 million increase in performance related compensation accruals.
For the years ended December 31, 2015 and 2014: COGS decreased 6% during the year ended December 31, 2015 in comparison with the year ended December 31, 2014. Approximately 4% of the decrease was driven by lower production costs from lower costs of raw materials, lower employee benefits and the impact of global headcount reduction as a result of our transformation plan. The decrease was due to lower sales volume and mix, as well slightly favorable currency impact.
Selling, general and administrative expense
For the years ended December 31, 2016 and 2015: Selling, general and administrative (SG&A) expense increased 48% to $934 million for the year ended December 31, 2016 compared to $632 million for the year ended December 31, 2015. The increase in SG&A was primarily driven by the $335 million litigation accrual related to the PFOA MDL Settlement. In addition, the increase in SG&A includes an increase in performance related compensation accruals of approximately $36 million, higher transaction costs incurred primarily in connection with the sale of the C&D and Sulfur businesses of approximately $10 million and other legal settlements and related costs of $16 million. These increases were partially offset by lower pension costs and cost reduction initiatives, including the global workforce reduction and other initiatives in connection with the transformation plan, which contributed to an approximate 15% reduction in SG&A.
For the years ended December 31, 2015 and 2014: SG&A expense decreased 8% to $632 million for the year ended December 31, 2015 in comparison with the year ended December 31, 2014. This decrease is primarily driven by the cost reduction initiatives implemented during the year, such as the global workforce reduction and other initiatives in connection with the transformation plan, as well as lower employee benefits (including pension), slightly offset by $17 million of transactions, legal and other related charges and approximately $4 million higher stock-based compensation charges primarily related to the converted DuPont awards.
39
The Chemours Company
Research and development expense
For the years ended December 31, 2016 and 2015: Research and development (R&D) expense decreased by $17 million or 18% for the year ended December 31, 2016 in comparison with the year ended December 31, 2015. Reductions in R&D spend were primarily driven by decisions to focus on fewer higher return projects. The global workforce reduction initiative also impacted the R&D function and contributed to the overall decrease.
For the years ended December 31, 2015 and 2014: R&D expense decreased by $46 million or 32% for the year ended December 31, 2015 in comparison with the year ended December 31, 2014. Reductions in R&D spend were primarily driven by decisions to either delay or terminate projects following our separation from DuPont. The global workforce reduction initiative also impacted the R&D function and contributed to the decrease in R&D expense.
Restructuring and asset related charges, net
For the years ended December 31, 2016 and 2015: We recorded pre-tax charges of approximately $170 million in connection with the various restructuring activities implemented since 2015. This included $45 million of decommissioning, dismantling and other related charges, which consisted of $30 million related to the Edge Moor manufacturing plant closure in the Titanium Technology segment, $7 million related to the production lines shutdown in the Fluoroproducts segment, and $8 million related to the reactive metals solution (“RMS”) manufacturing facility plant closure in the Chemicals Solutions segment. Also, during 2016, we recorded $119 million of asset impairment charges, which consisted of $48 million related to the aniline facility in Pascagoula, Mississippi and $58 million asset impairment charges in connection with the sale of the Sulfur business, both of which are in the Chemical Solutions segment, and $13 million in connection with the sale of our corporate headquarters building in the U.S. included in Corporate and Other.
For the years ended December 31, 2015 and 2014: We recorded pre-tax charges of approximately $333 million for employee separation and other asset related charges in connection with various restructuring activities during the year. This cost included $112 million severance charges from our global workforce reduction, $140 million related to our capacity optimization in our Titanium Technologies segment, including the closure of our Edge Moor production facility, $21 million of Fluoroproducts restructuring activities, $57 million of restructuring relating to our Chemical Solutions segment, and impairment charges.
Refer to Note 6 to the Consolidated Financial Statements for additional information related to “Restructuring and asset related charges, net”.
Interest expense, net
For the years ended December 31, 2016 and 2015: We incurred interest expense of $213 million and $132 million for the years ended December 31, 2016 and 2015, respectively. Interest expense was higher in 2016 because the long-term debt was issued in May 2015 and was outstanding for the entirety of the 2016 fiscal year. In addition, we recorded approximately $4 million of non-cash write off of unamortized debt issuance costs attributable to the reduction in our revolver commitment in connection with the February 2016 amendment to our credit agreement. These increases were partially offset by approximately $10 million of net gain on debt extinguishment in 2016.
For the years ended December 31, 2015 and 2014: We incurred interest expense of $132 million for the year ended December 31, 2015 related to our financing transactions completed in May 2015 in connection with the separation. There was no comparable expense in 2014.
Refer to Note 19 to the Consolidated Financial Statements and the Liquidity and Capital Resources section of this MD&A for additional information related to our indebtedness.
Other income, net
For the years ended December 31, 2016 and 2015: For the year ended December 31, 2016, other income, net increased by $193 million when compared to the year ended December 31, 2015. This increase includes a $254 million gain on sale primarily in connection with the sale of our C&D business and the sale of our aniline facility in Beaumont, Texas, partially offset by foreign currency exchange losses of approximately $57 million driven by continued strengthening of the U.S. dollar primarily against the Mexican peso compared to a foreign currency exchange gain of $19 million in 2015, which was mainly driven by the net gain from foreign currency forward contracts.
40
The Chemours Company
For the years ended December 31, 2015 and 2014: For the year ended December 31, 2015 compared to the year ended December 31, 2014, other income, net increased by $35 million. This change is comprised of a $42 million gain on foreign exchange forward contracts, lower foreign currency exchange losses of approximately $23 million driven by the continued strengthening of the U.S. dollar versus the Mexican peso, the Euro and other currencies, and additional technology and licensing income of approximately $11 million. These increases were offset by a loss on sale of assets and businesses of $9 million in 2015 compared to the gain of $40 million recognized in 2014.
Refer to Note 8 to the Consolidated Financial Statements for details of “Other income, net”.
Provision for (benefit from) income taxes
For the years ended December 31, 2016 and 2015: For the years ended December 31, 2016 and 2015, Chemours recorded a tax benefit of $18 million with an effective income tax rate of 164% and $98 million with an effective tax rate of approximately 52%, respectively. The $80 million decrease in tax benefit and the corresponding change in the effective income tax rate were primarily due to a $50 million valuation allowance recorded on U.S. foreign tax credits, the Company’s geographical mix of earnings as well as the gain on the sale of assets and business, which resulted in tax expense and a corresponding change in the effective income tax rate for the year ended December 31, 2016 as compared to the same period in 2015; offset by the tax benefit on the $335 million PFOA MDL Settlement accrual (see Note 20 to the Consolidated Financial Statements for further information).
For the years ended December 31, 2015 and 2014: For the year ended December 31, 2015, Chemours recorded a tax benefit of $98 million with an effective income tax rate of approximately 52%. For the year ended December 31, 2014, Chemours recorded a tax provision of $149 million with an effective tax rate of approximately 27%. The $247 million decrease in the tax provision was related to the $738 million decrease in income before income taxes. This decrease in income before income taxes was primarily due to continued pressure on TiO2 prices, soft demand conditions for certain fluoropolymers products, and restructuring and asset impairment charges. Although the earnings in our foreign operations remained consistent for the years ended December 31, 2015 and 2014, the earnings in the U.S. were impacted by the aforementioned factors. The mix of geographical earnings resulted in the effective tax rates varying between 2015 and 2014.
Segment Reviews
The following table represents Chemours’ total consolidated Adjusted EBITDA by segment:
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Titanium Technologies |
| $ | 466 |
|
| $ | 326 |
|
| $ | 723 |
|
Fluoroproducts |
|
| 445 |
|
|
| 300 |
|
|
| 282 |
|
Chemical Solutions |
|
| 39 |
|
|
| 29 |
|
|
| 17 |
|
Corporate and Other |
|
| (128 | ) |
|
| (82 | ) |
|
| (146 | ) |
Total |
| $ | 822 |
|
| $ | 573 |
|
| $ | 876 |
|
Corporate costs and certain legal and environmental expenses that are not allocated to the segments and foreign exchange gains and losses are reflected in Corporate and Other.
Adjusted EBITDA represents our primary measure of segment performance and is defined as income (loss) before income taxes excluding the following:
| • | interest expense, depreciation and amortization, |
| • | non-operating pension and other postretirement employee benefit costs, which represent the component of net periodic pension costs (income) excluding service component, |
| • | exchange losses (gains) included in “other income, net” of the statement of operations, |
| • | employee separation, asset-related charges and other charges, net, |
| • | asset impairments, |
| • | losses (gains) on sale of business or assets, and |
| • | other items not considered indicative of our ongoing operational performance and expected to occur infrequently. |
41
The Chemours Company
A reconciliation of Adjusted EBITDA to net income (loss) for the years ended December 31, 2016, 2015 and 2014 is included in Non-GAAP Financial Measures in Item 7 and in Note 24 to the Consolidated Financial Statements.
Titanium Technologies
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Segment Net Sales |
| $ | 2,364 |
|
| $ | 2,392 |
|
| $ | 2,937 |
|
Adjusted EBITDA |
|
| 466 |
|
|
| 326 |
|
|
| 723 |
|
Adjusted EBITDA Margin |
|
| 20 | % |
|
| 14 | % |
|
| 25 | % |
|
| Year Ended December 31, |
| |||||
Change in segment net sales from prior period |
| 2016 |
|
| 2015 |
| ||
Price |
|
| (3 | )% |
|
| (12 | )% |
Volume |
|
| 2 | % |
|
| (2 | )% |
Currency |
|
| — | % |
|
| (5 | )% |
Portfolio / Other |
|
| — | % |
|
| — | % |
Total Change |
|
| (1 | )% |
|
| (19 | )% |
2016 versus 2015: Net sales decreased by $28 million or 1% for the year ended December 31, 2016 compared with the same period in 2015. The decrease in net sales was primarily due to lower average selling prices for TiO2 year over year, partially offset by an increase in TiO2 sales volume due to higher demand in Europe and the United States. Our sales volume in 2016 was in line with seasonal and historical trends.
Adjusted EBITDA increased by $140 million or 43% during the year ended December 31, 2016 in comparison with same period in 2015. The increase in Adjusted EBITDA was primarily driven by productivity improvement initiatives including the impact of the Edge Moor plant shut-down and global headcount reductions, which increased Adjusted EBITDA by approximately 69%. The productivity improvement initiatives resulted in lower raw materials and lower plant operating costs. The increase was partially offset by lower average selling price year-over-year, which decreased adjusted EBITDA by approximately 30%, and higher performance related compensation accruals.
2015 versus 2014: Net sales decreased by $545 million or 19% for the year ended December 31, 2015 compared with the same period in 2014, due primarily to lower selling prices and the continued unfavorable effect of foreign currency primarily against the Euro. Oversupply in the global titanium dioxide industry and weak demand continue to put downward pressure on pricing in all regions.
Adjusted EBITDA decreased by 55% during the year ended December 31, 2015 in comparison with same period in 2014. Adjusted EBITDA margin also decreased during the year ended December 31, 2015 in comparison with the same period in 2014. The decreases were primarily driven by lower sales and margin which contributed to an approximately 39% decrease in Adjusted EBITDA due to lower prices, and unfavorable effects of foreign currency which contributed to an approximately 19% decrease in Adjusted EBITDA. Partially offsetting these decreases were productivity improvement initiatives, which resulted in lower raw materials, energy and plant operating costs, as well as the impact of our cost reduction programs, which included certain Titanium Technology plant shut-downs and global headcount reductions.
Fluoroproducts
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Segment Net Sales |
| $ | 2,264 |
|
| $ | 2,230 |
|
| $ | 2,327 |
|
Adjusted EBITDA |
|
| 445 |
|
|
| 300 |
|
|
| 282 |
|
Adjusted EBITDA Margin |
|
| 20 | % |
|
| 13 | % |
|
| 12 | % |
42
The Chemours Company
|
| Year Ended December 31, |
| |||||
Change in segment net sales from prior period |
| 2016 |
|
| 2015 |
| ||
Price |
|
| (1 | )% |
|
| 2 | % |
Volume |
|
| 4 | % |
|
| — | % |
Currency |
|
| (1 | )% |
|
| (4 | )% |
Portfolio / Other |
|
| (1 | )% |
|
| (2 | )% |
Total Change |
|
| 1 | % |
|
| (4 | )% |
2016 versus 2015: Net sales increased $34 million or 2% for the year ended December 31, 2016 compared with the same period in 2015. Stronger demand for Opteon™ refrigerant in both Europe and the United States delivered a significant increase in volume over the prior year, partially offset by lower volume over the prior year due to the phase down of HCFCs refrigerants (i.e., Freon™) as stipulated by the Montreal Protocol and by lower selling prices for fluoropolymer products due to competitive pricing pressure. For the year ended December 31, 2016, unfavorable foreign currency impact, primarily against the Euro, Brazilian real and Mexican peso resulted in an overall decrease in net sales.
Adjusted EBITDA increased by $145 million or 48% for the year ended December 31, 2016 in comparison with same periods in 2015, due primarily to margin improvements and cost reductions from cost savings initiatives. Margin improvement from fluorochemicals sales, including growth in Opteon™ but excluding currency impact, contributed an increase of approximately 41% in the year ended December 31, 2016, and other cost reduction initiatives contributed an increase of approximately 20% for the year ended December 31, 2016. In addition, we incurred approximately $22 million or approximately 7% of costs in 2015 due to plant outages in certain of our manufacturing facilities in the United States that did not recur in 2016. These improvements were partially offset by lower selling price and unfavorable product mix of fluoropolymers, and higher performance related compensation accruals. Overall unfavorable currency in the year ended December 31, 2016 also resulted in a decrease in adjusted EBITDA by approximately 6%.
2015 versus 2014: Net sales decreased by $97 million or 4% for the year ended December 31, 2015 compared with the same period in 2014. Net sales were unfavorably impacted by foreign currency exchange rates, primarily related to the Euro, Brazilian real, and Japanese yen, and continued weaker demand for industrial resins. Favorable product mix with strong Opteon™ refrigerant adoption delivered increased prices and steady overall volumes over the prior year.
Adjusted EBITDA and adjusted EBITDA margin increased during the year ended December 31, 2015 in comparison with the same periods in 2014. Both increases were primarily due to product mix and cost reduction efforts including global headcount reductions during the second half of 2015.
Chemical Solutions
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Segment Net Sales |
| $ | 772 |
|
| $ | 1,095 |
|
| $ | 1,168 |
|
Adjusted EBITDA |
|
| 39 |
|
|
| 29 |
|
|
| 17 |
|
Adjusted EBITDA Margin |
|
| 5 | % |
|
| 3 | % |
|
| 1 | % |
|
| Year Ended December 31, |
| |||||
Change in segment net sales from prior period |
| 2016 |
|
| 2015 |
| ||
Price |
|
| (7 | )% |
|
| (5 | )% |
Volume |
|
| (3 | )% |
|
| 2 | % |
Currency |
|
| — | % |
|
| (3 | )% |
Portfolio / Other |
|
| (19 | )% |
|
| — | % |
Total Change |
|
| (29 | )% |
|
| (6 | )% |
2016 versus 2015: Net sales decreased by $323 million or 29% for the year ended December 31, 2016 compared with the same period in 2015. These decreases were due to a portfolio change resulting from the sale of our aniline facility in Beaumont, Texas, our C&D business and our Sulfur business, and the impact of RMS plant shutdown in September 2016. In addition, sales decreased due to lower selling prices resulting from the impact of lower raw materials costs on contractual pass-through terms, and lower sales volume substantially across all business units during the year except Sulfur.
43
The Chemours Company
Adjusted EBITDA and Adjusted EBITDA margin increased during the year ended December 31, 2016 in comparison with same period in 2015. Despite the decreases in net sales, Adjusted EBITDA and Adjusted EBITDA margin increased due primarily to the cost reduction efforts, including the global headcount reductions implemented in 2015, and improvement in plant operating costs.
2015 versus 2014: Net sales decreased by $73 million or 6%, for the year ended December 31, 2015 compared with the same period in 2014, primarily due to lower prices based on contractual pass-through terms, changes in the mix of products sold as well as the unfavorable impact of foreign currency exchange rates including the Mexican peso, Canadian dollar and the Euro. These decreases were partially offset by volume increases in cyanide and sulfur due to strong demand.
Adjusted EBITDA and Adjusted EBITDA margin increased during the year ended December 31, 2015 in comparison with same period in 2014. The slight increase in Adjusted EBITDA was driven primarily by lower R&D expense and cost reduction efforts, including the global headcount reductions, during the second half of 2015.
2017 Outlook
With our transformation plan on track, we are targeting an additional $150 million of structural costs reductions in 2017. These cost savings are expected to be generated from a combination of actions taken during 2016, including facilities closures, headcount reductions, and procurement and productivity enhancements, and additional actions that may be taken in 2017. Based on our anticipated cost reduction and growth initiatives, we continue to expect cost savings of approximately $350 million and approximately $150 million in improvements from growth initiatives that together will also improve our pre-tax earnings by similar amounts through 2017 over 2015. These improvements will be partially offset by the impact of divestitures completed during 2016, unfavorable price and mix of fluoropolymer products, and may also be impacted by market factors.
For 2017, we believe that those cost reductions from our transformation plan, along with growth from Opteon™, an improving pricing environment for TiO2, and the benefits of our newest line at our Altamira facility will be partially offset by headwinds in our Fluoroproducts segment and EBITDA lost from divestitures. Therefore, we expect our Adjusted EBITDA to be in line with our transformation plan goals. We expect our capital expenditures to be at approximately $450 million driven in large part by expenditures associated with our new Opteon™ plant under construction in Corpus Christi and our anticipated Mining Solutions expansion. Our outlook reflects our current visibility and expectations on market factors, such as currency movements, TiO2 pricing and end-market demand.
Liquidity and Capital Resources
Prior to the separation on July 1, 2015, transfers of cash to and from DuPont’s cash management system were reflected in DuPont Company Net Investment in the historical Consolidated Balance Sheets, Statements of Cash Flows and Statements of Changes in DuPont Company Net Investment. DuPont funded our cash needs through the date of the separation. Chemours has a historical pattern of seasonality, with working capital use of cash in the first half of the year, and a working capital source of cash in the second half of the year.
Chemours’ primary source of liquidity is cash generated from operations, available cash and borrowings under the debt financing arrangements as described below. We believe these sources are sufficient to fund our planned operations and to meet our interest, dividend and contractual obligations. Our financial policy seeks to deleverage by using free cash flow to repay outstanding borrowings, selectively invest for growth to enhance our portfolio including certain strategic capital investments, and return cash to shareholders through dividend payments.
Chemours’ operating cash flow generation is driven by, among other things, global economic conditions generally and the resulting impact on demand for our products, raw material and energy prices, and industry-specific issues, such as production capacity and utilization. Chemours has generated strong operating cash flow through various industry and economic cycles evidencing the operating strength of our businesses. Over the industry cycles in recent years, cash flows from operating activities increased in years leading up to the historical peak profitability achieved in 2011, and have decreased annually since that time. Despite the challenging market conditions in the TiO2 industry since the historical peak, we anticipate that through our cost reduction efforts and growth initiatives, our operations will provide sufficient liquidity to implement the transformation plan and support cash needs for the business.
While we were a wholly-owned subsidiary of DuPont, our then-board of directors, consisting of DuPont employees, declared a dividend of an aggregate amount of $100 million for the third quarter of 2015, which was paid on September 11, 2015 to our stockholders of record as of August 3, 2015. On September 1, 2015, our independent board of directors declared a dividend of $0.03
44
The Chemours Company
per share, which was paid on December 14, 2015 to our stockholders of record on November 13, 2015. During 2016, our board of directors also declared quarterly dividends of $0.03 per share, which were paid in each quarter during the year.
The separation agreements set forth a process to true-up cash and working capital transferred to us from DuPont at separation. In January 2016, Chemours and DuPont entered into an agreement, contingent upon the credit agreement amendment described herein, which provided for the extinguishment of payment obligations of cash and working capital true-ups previously contemplated in the separation agreements. As a result, Chemours was not required to make any payments to DuPont, nor did DuPont make any payments to Chemours related to the separation true-up mechanism. In addition, the agreement set forth an advance payment of approximately $190 million, which was paid to Chemours in February 2016, for certain specified goods and services that Chemours expects to provide to DuPont through mid-2017 under existing agreements with Chemours. Approximately $58 million of the prepayment amount remained outstanding as of December 31, 2016.
Over the next 12 months, Chemours expects to have significant interest, capital expenditure and restructuring payments. We expect to fund these payments through cash generated from operations, available cash and borrowings under the revolving credit facility. We anticipate that our operations and debt financing arrangements will provide sufficient liquidity over the next 12 months. The availability under our Revolving Credit Facility is subject to the last 12 months of our consolidated EBITDA as defined under the credit agreement.
As of December 31, 2016 and 2015, we had $678 million and $271 million, respectively, of cash and cash equivalents on our balance sheet held by our foreign subsidiaries, all of which is readily convertible into currencies used in our operations, including the U.S. dollar. Cash and earnings of our foreign subsidiaries are generally used to finance their operations and capital expenditures. At December 31, 2016 and 2015, management believed that sufficient liquidity was available in the United States, and it is our intention to indefinitely reinvest undistributed earnings of our foreign subsidiaries outside of the United States. No deferred tax liabilities have been recognized with regard to the approximately $678 million and $271 million of cash of our foreign subsidiaries as of December 31, 2016 and 2015, respectively, and undistributed earnings. The potential tax implications of the repatriation of unremitted earnings are driven by facts at the time of distribution, therefore, it is not practicable to estimate the income tax liabilities that might be incurred if such cash and earnings were repatriated to the United States.
Cash Flow
The following table sets forth a summary of the net cash provided by (used for) operating, investing and financing activities.
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Cash provided by operating activities |
| $ | 594 |
|
| $ | 182 |
|
| $ | 505 |
|
Cash provided by (used for) investing activities |
|
| 357 |
|
|
| (497 | ) |
|
| (560 | ) |
Cash (used for) provided by financing activities |
|
| (396 | ) |
|
| 687 |
|
|
| 55 |
|
Cash Provided by Operating Activities
Cash provided by operating activities improved by $412 million for the year ended December 31, 2016 compared to the same period in 2015, due primarily to overall improvements in the results of operations and working capital. In addition, we received an advance payment of $190 million from DuPont in February 2016 of which approximately $132 million was utilized during the year ended December 31, 2016. Partially offsetting these increases are interest payments in 2016 of approximately $220 million versus approximately $122 million in 2015, and restructuring payments of approximately $68 million in 2016 versus $39 million in 2015.
Cash provided by operating activities decreased by $323 million for the year ended December 31, 2015 compared with the same period in 2014, due to lower earnings than the prior year, payments on restructuring activities and interest payments on our 2015 financing transactions.
Cash Provided by (Used for) Investing Activities
Cash provided by investing activities the year ended December 31, 2016 includes the $140 million proceeds from the sale of our aniline facility in Beaumont, Texas, $321 million net proceeds from the sale of the Sulfur business and $223 million net proceeds from the sale of the C&D business (see Note 7 to the Consolidated Financial Statements for additional details), as well as $22 million proceeds from a sale of land in Repauno, New Jersey. These cash inflows were offset by capital expenditures during the period of $338 million. Our capital expenditures decreased by approximately $181 million when compared to the same period in 2015 due to
45
The Chemours Company
lower spending primarily from the completion of our Altamira plant expansion in April 2016 and no separation-related expenditures incurred during 2016.
Cash used for investing activities decreased $63 million for the year ended December 31, 2015 compared to the same period in 2014 primarily as a result of a $85 million decrease in capital expenditures of which $80 million relates to the expansion of Titanium Technologies’ Altamira plant in Mexico and approximately $50 million from other on-going and expansion activities, partially offset by increase in separation-related capital expenditures of $45 million. In addition, we realized approximately $42 million of net gain from foreign exchange contract settlements entered into in 2015 after the separation and no similar realized gains or losses were incurred prior to the separation. The decreases in cash used for investing activities are partially offset by incremental investments made to our unconsolidated affiliate in China and lower sales proceeds due to lesser business and asset sale activities during 2015.
Cash (Used for) Provided by Financing Activities
During 2016, utilizing our available cash, we repurchased and repaid a portion of our senior secured term loans with an aggregate principal amount of $105 million for $104 million in cash, a portion of our 2023 Notes with an aggregate principal amount of $192 million for $182 million in cash, and a portion of our Euro Notes with an aggregate principal amount of $73 million for $68 million in cash. These senior loan repurchases were in addition to our quarterly required repayments on the senior secured term loans equivalent to 1% per annum of its original principal. We also declared and paid approximately $22 million of dividends to our shareholders, equivalent to $0.12 per share.
Cash provided by financing activities increased by $632 million for the year ended December 31, 2015 compared to the same period in 2014, due primarily from the proceeds from our financing transactions offset by the net transfers to DuPont in connection with the separation. Through June 30, 2015, DuPont managed Chemours’ cash and financing arrangements and all excess cash generated through earnings was deemed remitted to DuPont and all sources of cash were deemed funded by DuPont. Prior to the separation on July 1, 2015, Chemours remitted approximately $3.4 billion to DuPont in the form of a dividend, using cash received from issuance of debt. See Note 4 to the Consolidated Financial Statements for additional information.
Current Assets
|
| December 31, |
|
| December 31, |
| ||
(Dollars in millions) |
| 2016 |
|
| 2015 |
| ||
Cash and cash equivalents |
| $ | 902 |
|
| $ | 366 |
|
Accounts and notes receivable - trade, net |
|
| 807 |
|
|
| 859 |
|
Inventories |
|
| 767 |
|
|
| 972 |
|
Prepaid expenses and other |
|
| 77 |
|
|
| 104 |
|
Total current assets |
| $ | 2,553 |
|
| $ | 2,301 |
|
Accounts and notes receivable - trade, net at December 31, 2016 decreased by $52 million compared to December 31, 2015 primarily due to lower sales in the fourth quarter of 2016 over fourth quarter of 2015, including impact of divested businesses, $22 million of accounts receivable disposed in connection with the sale of C&D and Sulfur businesses, and unfavorable currency translation of approximately $2 million.
Inventories at December 31, 2016 decreased $205 million compared to December 31, 2015. The decrease was due to the continued effort to reduce inventory on hand as well as due to the lower raw material and production costs. In addition, we recorded approximately $10 million of inventory write-down in the Chemical Solutions segment during 2016 as a result of the previously announced RMS restructuring, and approximately $17 million of inventory disposed in connection with the sale of our aniline facility in Beaumont, Texas, sale of C&D and Sulfur businesses, and approximately $23 million of unfavorable currency translation.
Prepaid expenses and other current assets at December 31, 2016 decreased compared to December 31, 2015 due to the sale of our aniline facility in Beaumont, Texas in February 2016, which was previously classified as assets held-for-sale for approximately $46 million and included in this account as of December 31, 2015.
46
The Chemours Company
|
| December 31, |
|
| December 31, |
| ||
(Dollars in millions) |
| 2016 |
|
| 2015 |
| ||
Accounts payable |
| $ | 884 |
|
| $ | 973 |
|
Short-term borrowings and current portion of long-term debt |
|
| 15 |
|
|
| 39 |
|
Other accrued liabilities |
|
| 872 |
|
|
| 454 |
|
Total current liabilities |
| $ | 1,771 |
|
| $ | 1,466 |
|
Accounts payable decreased by $89 million compared to December 31, 2015 due to lower inventories and timing of payments to vendors, as well as impact of divested businesses and favorable currency translation of approximately $20 million.
Other accrued liabilities increased primarily due to a $335 million litigation accrual related to the PFOA MDL Settlement. In addition, we received advance payment from DuPont in February 2016 and approximately $58 million of this liability remains outstanding as of December 31, 2016. Also, our performance related compensation accruals increased by approximately $42 million in line with the improvements in our business results.
Credit Facilities and Notes
On May 12, 2015, Chemours entered into certain financing transactions in connection with the Distribution and in recognition of the assets contributed to us by DuPont in anticipation of the separation. The proceeds from the financing transactions were used to fund a cash distribution to DuPont of $3.4 billion and a distribution in kind of Notes with an aggregate principal amount of $507 million. See Note 19 to the Consolidated Financial Statements for further discussion of these transactions.
The credit agreement provided for a seven-year senior secured term loan (the “Term Loan Facility”) in a principal amount of $1.5 billion repayable in equal quarterly installments at a rate of one percent of the original principal amount per year, with the balance payable on the final maturity date. The Term Loan Facility was issued with a $7 million original issue discount and bears interest at a rate of LIBOR plus 3.00%, with a 0.75% LIBOR floor. The proceeds from the Term Loan Facility were used to fund a portion of the distribution to DuPont, along with related fees and expenses.
Prior to an amendment in February 2016, the credit agreement also provided for a five-year $1.0 billion senior secured revolving credit facility (the “Revolving Credit Facility”). In February 2016, an amendment to the Revolving Credit Facility reduced the capacity to $750 million beginning in the first quarter of 2016 and amended certain covenants (see Debt Covenants discussion included herein). The proceeds of any loans made under the Revolving Credit Facility can be used to finance capital expenditures, acquisitions, working capital needs and for other general corporate purposes. Availability under the Revolving Credit Facility is subject to certain covenant limitations. At December 31, 2016, the facility had full borrowing capacity of $750 million, from which we have $132 million letters of credit issued and outstanding under this facility.
Chemours’ obligations under the Term Loan Facility and Revolving Credit Facility (collectively, the Senior Secured Credit Facilities) are guaranteed on a senior secured basis by all of its material domestic subsidiaries, subject to certain agreed upon exceptions. The obligations under the Senior Secured Credit Facilities are also, subject to certain agreed upon exceptions, secured by a first priority lien on substantially all of Chemours and its material wholly-owned domestic subsidiaries’ assets, including 100% of the stock of domestic subsidiaries and 65% of the stock of certain foreign subsidiaries.
Additionally, on May 12, 2015, Chemours issued approximately $2,503 million aggregate principal of senior unsecured notes (the “Notes”) in a private placement. The 2023 notes (the “2023 Notes”) with an aggregate principal amount of $1,350 million bear interest at a rate of 6.625% per annum and will mature on May 15, 2023 with all principal paid at maturity. The 2025 notes (the “2025 Notes”) with an aggregate principal amount of $750 million bear interest at a rate of 7.000% per annum and will mature on May 15, 2025 with all principal paid at maturity. The 2023 euro notes (the “Euro Notes”) with an aggregate principal amount of €360 million bear interest at a rate of 6.125% per annum and will mature on May 15, 2023 with all principal paid at maturity. Interest on the Notes is payable semi-annually in cash in arrears on May 15 and November 15 of each year, which commenced on November 15, 2015. The Notes were offered in the U.S. to persons reasonably believed to be qualified institutional buyers in reliance on Rule 144A under the Securities Act, and outside the U.S. to non-U.S. persons in reliance on Regulation S under the Securities Act. In connection with the issuance of the Notes, Chemours entered into a registration rights agreement, in which Chemours agreed to file with the SEC a registration statement for the exchange of the Notes for new registered notes with identical terms. On March 18, 2016, Chemours filed a registration statement on Form S-4 with respect to the exchange offer. The registration statement was declared effective on
47
The Chemours Company
April 12, 2016, and the exchange offer was completed on May 19, 2016. In addition, on May 5, 2016, the Euro Notes were listed for trading on the Global Exchange Market of the Irish Stock Exchange.
Each series of Notes is or will be fully and unconditionally guaranteed, jointly and severally, by Chemours’ existing and future domestic subsidiaries that guarantee (the Guarantors) the Senior Secured Credit Facilities or that guarantee other indebtedness of Chemours or any guarantor in an aggregate principal amount in excess of $75 million (the Guarantees). The Notes are unsecured and unsubordinated obligations of Chemours. The Guarantees are unsecured and unsubordinated obligations of the Guarantors. The Notes rank equally in right of payment to all of Chemours’ existing and future unsecured unsubordinated debt and senior in right of payment to all of Chemours’ existing and future debt that is by its terms expressly subordinated in right of payment to the Notes. The Notes are subordinated to indebtedness under the Senior Secured Credit Facilities as well as any future secured debt to the extent of the value of the assets securing such debt. Chemours’ is obligated to offer to purchase the Notes at a price of (a) 101 percent of their principal amount, together with accrued and unpaid interest, if any, to the date of purchase, upon the occurrence of certain change of control events and (b) 100 percent of their principal amount, together with accrued and unpaid interest, if any, to the date of purchase, with the proceeds from certain asset dispositions. These restrictions and prohibitions are subject to certain qualifications and exceptions set forth in the Indenture, including without limitation, reinvestment rights with respect to the proceeds of asset dispositions. Chemours is permitted to redeem some or all of the 2023 Notes and Euro Notes by paying a “make-whole” premium prior to May 15, 2018, and on or after May 15, 2018 and thereafter at specified redemption prices. Chemours also may redeem some or all of the 2025 Notes on or after May 15, 2020 at specified redemption prices. Chemours also may redeem some or all of the 2023 Notes and Euro Notes by means other than a redemption, including tender offer and open market repurchases.
Debt Covenants
Chemours is subject to certain debt covenants that, among other things, limit Chemours and certain of Chemours’ subsidiaries to incur indebtedness, pay dividends or make other distributions, prepay, redeem or repurchase certain debt, make loans and investments, sell assets, incur liens, enter into transactions with affiliates and consolidate or merge. These covenants are subject to a number of exceptions and qualifications set forth in the respective agreements.
In December 2016, we entered into a third amendment to the credit agreement to change certain covenants and allow the Company to enter into a sale and leaseback transaction for its corporate headquarters building located in Wilmington, Delaware. These transactions are expected to be completed in the first quarter of 2017, and we expect to receive approximately $32 million proceeds. The amendment requires us to use the proceeds from the sale to repay a portion of the term loans.
In February 2016, we proactively pursued a second amendment to the credit agreement in order to ensure that we would retain adequate liquidity and sufficient cushion in the event of an unexpected, further significant decline in TiO2 pricing. The second amendment also provided further flexibility by allowing us to include, on a pro forma basis, future benefits of cost savings initiatives in the calculation of financial covenants that rely on consolidated EBITDA for an additional year, and increased the amount of the applicable cost savings benefits that could be utilized in the covenant calculation. In addition, the second amendment replaced the total net leverage ratio with the senior secured net leverage ratio and modified the minimum required levels of the interest expense coverage ratio. These changes were designed to allow us to have full access to the revolving credit facility, provide flexibility to execute our transformation plan through 2017 and provide additional cushion in the event of an unexpected, further significant decline in TiO2 pricing. Furthermore, the amendment reduced the size of the revolving credit facility by $250 million to $750 million. With the on-going efforts to improve working capital usage as a part of our transformation plan, we believe that $750 million of revolver access will be sufficient to meet our working capital and other cash needs over the next 12 months.
In September 2015, in connection with the Company’s transformation plan announced in August 2015, we undertook a first amendment to the credit agreement to allow pro forma inclusion of future benefits from cost savings initiatives in the calculation of financial covenants that rely on consolidated EBITDA beginning from the quarter ended September 30, 2015. Since the revolver availability in any quarter is determined by the cushion remaining in the financial maintenance covenants at the end of the previous quarter, this amendment increased our access to the revolving credit facility.
The credit agreement, as amended, contains financial covenants which, solely with respect to the revolving credit facility, require us not to exceed a maximum senior secured net leverage ratio of 3.50 to 1.00 each quarter through December 31, 2016, 3.00 to 1.00 through June 30, 2017 and further decreasing by 0.25 to 1.00 every subsequent six months to 2.00 to 1.00 by January 1, 2019 and thereafter. We are also required to maintain a minimum interest coverage ratio of 1.75 to 1.00 each quarter through June 30, 2017 and further increasing by 0.25 to 1.00 every subsequent six months to 3.00 to 1.00 by January 1, 2019 and thereafter. In addition, the credit agreement contains customary affirmative and negative covenants that, among other things, limit or restrict us and our subsidiaries’ ability, subject to certain exceptions, to incur liens, merge, consolidate or sell, transfer or lease assets, make investments, pay dividends, transact with subsidiaries and incur indebtedness. The credit agreement also contains customary representations and
48
The Chemours Company
warranties and events of default. The senior secured credit facilities and the senior unsecured notes contain events of default customary for these types of financings, including cross default and cross acceleration provisions to material indebtedness of Chemours. We were in compliance with our debt covenants as of December 31, 2016.
In the event of default under the revolving credit facility, our lenders under the revolving credit facility can terminate their commitments thereunder, cease making further revolving loans and accelerate outstanding revolving loans. This would allow the lenders under the revolving credit facility to declare the outstanding term loans to be immediately due and payable and to institute foreclosure proceedings against the collateral securing the credit facility, which could force us into bankruptcy or liquidation. Any event of default or declaration of acceleration under the credit agreement also may result in an event of default under the indenture governing the notes. Any such default, event of default or declaration of acceleration could materially and adversely affect our results of operations and financial condition. Please see the section titled “Risks Related to our Indebtedness” of the “Risk Factors” section for additional detail.
Maturities
Chemours has required principal payments related to the Term Loan Facility of $15 million in each year from 2017 to 2021, with the remaining balance due at maturity. Debt maturities related to the Term Loan Facility and the Notes in 2022 and beyond will be $3,513 million.
In addition, following the end of each fiscal year commencing on the year ended December 31, 2016, the Company is also required to make additional principal repayments, depending on leverage levels as defined in the credit agreement, equivalent to up to 50% of excess cash flow based on certain leverage targets with stepdowns to 25% and 0% as actual leverage decreases to below 3.00 to 1.00 leverage target.
Supplier Financing
In 2015, we entered into a global paying services agreement with a financial institution. Under this agreement, the financial institution acts as the paying agent for Chemours with respect to accounts payable due to our suppliers who elect to participate in the program. The agreement allows our suppliers to sell their receivables to the financial institution at the discretion of both parties on terms that are negotiated between them. Our obligations to our suppliers, including the amounts due and scheduled payment dates, are not impacted by our suppliers’ decisions to sell their receivables under this program. At December 31, 2016, the payment instructions from Chemours were $134 million. Pursuant to their agreement with the financial institution, certain suppliers may elect to get paid early at their discretion. The available capacity under this program can vary based on the number of investors participating in this program at any point of time.
Capital Expenditures
Our operations are capital intensive, requiring ongoing investment to upgrade or enhance existing operations and to meet environmental and operational regulations. Our capital requirements have consisted, and are expected to continue to consist, primarily of:
| • | ongoing capital expenditures, such as those required to maintain equipment reliability, the integrity and safety of our manufacturing sites and to comply with environmental regulations; |
| • | investments in our existing facilities to help support introduction of new products and de-bottleneck to expand capacity and grow our business; and |
| • | investment in projects to reduce future operating costs and enhance productivity. |
49
The Chemours Company
The following table summarizes ongoing and expansion capital expenditures (which includes environmental capital expenditures), as well as expenditures related to our separation from DuPont, for the years ended December 31, 2016, 2015 and 2014:
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Titanium Technologies |
| $ | 105 |
|
| $ | 255 |
|
| $ | 365 |
|
Fluoroproducts |
|
| 120 |
|
|
| 142 |
|
|
| 133 |
|
Chemical Solutions |
|
| 104 |
|
|
| 117 |
|
|
| 106 |
|
Corporate & Other |
|
| 9 |
|
|
| 5 |
|
|
| — |
|
Total Capital Expenditures 1 |
| $ | 338 |
|
| $ | 519 |
|
| $ | 604 |
|
| 1 | Includes separation-related capital expenditures of $66 million and $21 million for the years ended December 31, 2015 and 2014, respectively. |
|
Our capital expenditures, excluding separation-related spending, declined in 2016 as we finished the expansion of our Altamira production facility. We expect our capital expenditures in 2017 to be at approximately $450 million driven in large part by expenditures associated with our new Opteon™ plant under construction in Corpus Christi and our anticipated Mining Solutions expansion. We are targeting to return to approximately $350 million of capital expenditures per year following the completion of the new facilities. For further detail related to our environmental capital expenditures, please see the Environmental Matters section of this MD&A.
Contractual Obligations
Information related to the Company’s significant contractual obligations is summarized in the table below.
|
| Total at |
|
| Payments Due In |
| ||||||||||||||
(Dollars in millions) |
| December 31, 2016 |
|
| 2017 |
|
| 2018 - 2019 |
|
| 2020 - 2021 |
|
| 2022 and Beyond |
| |||||
Long-term debt obligations 1 |
| $ | 3,588 |
|
| $ | 15 |
|
| $ | 30 |
|
| $ | 30 |
|
| $ | 3,513 |
|
Interest payments on long-term debt obligations 1 |
|
| 1,339 |
|
|
| 200 |
|
|
| 398 |
|
|
| 396 |
|
|
| 345 |
|
Operating leases |
|
| 259 |
|
|
| 62 |
|
|
| 99 |
|
|
| 55 |
|
|
| 43 |
|
Purchase obligations 2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Raw material obligations |
|
| 1,244 |
|
|
| 97 |
|
|
| 153 |
|
|
| 144 |
|
|
| 850 |
|
Utility obligations |
|
| 673 |
|
|
| 98 |
|
|
| 143 |
|
|
| 129 |
|
|
| 303 |
|
Other |
|
| 341 |
|
|
| 89 |
|
|
| 128 |
|
|
| 87 |
|
|
| 37 |
|
Total purchase obligations |
|
| 2,258 |
|
|
| 284 |
|
|
| 424 |
|
|
| 360 |
|
|
| 1,190 |
|
Other liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Workers’ compensation |
|
| 36 |
|
|
| 6 |
|
|
| 15 |
|
|
| 7 |
|
|
| 8 |
|
Asset retirement obligations |
|
| 43 |
|
|
| 2 |
|
|
| 2 |
|
|
| — |
|
|
| 39 |
|
Environmental remediation |
|
| 278 |
|
|
| 71 |
|
|
| 104 |
|
|
| 55 |
|
|
| 48 |
|
Legal settlements 3 |
|
| 348 |
|
|
| 337 |
|
|
| 4 |
|
|
| 4 |
|
|
| 3 |
|
Employee separation costs |
|
| 34 |
|
|
| 31 |
|
|
| 3 |
|
|
| — |
|
|
| — |
|
Other 4 |
|
| 56 |
|
|
| 29 |
|
|
| 4 |
|
|
| 4 |
|
|
| 19 |
|
Total other liabilities |
|
| 795 |
|
|
| 476 |
|
|
| 132 |
|
|
| 70 |
|
|
| 117 |
|
Total contractual obligations 5 |
| $ | 8,239 |
|
| $ | 1,037 |
|
| $ | 1,083 |
|
| $ | 911 |
|
| $ | 5,208 |
|
| 1 | To calculate payments due for principal and interest, we assumed that interest rates, foreign currency exchange rates, and outstanding borrowings under credit facilities were unchanged from December 31, 2016 through maturity. |
| 2 | Represents enforceable and legally binding agreements to purchase goods or services that specify fixed or minimum quantities; fixed minimum or variable price provisions; and the approximate timing of the agreement. |
| 3 | Includes $335 million litigation accrual related to the PFOA MDL Settlement (see Note 20 to the Consolidated Financial Statements). |
| 4 | Includes expected contributions and benefits payments in excess of plan assets to be made to fund our pension and other long-term employee benefit plans. Actual payments will depend on several factors, including investment performance and discount rates, and may also be affected by changes in applicable local requirements. See Note 22 to the Consolidated Financial Statements for additional information. |
| 5 | Due to uncertainty regarding the completion of tax audits and possible outcomes, we are unable to determine the timing of payments related to unrecognized tax benefits. See Note 9 to the Consolidated Financial Statements for additional information. |
50
The Chemours Company
Off Balance Sheet Arrangements
Information with respect to Chemours’ guarantees is included in Note 20 to the Consolidated Financial Statements. Historically, Chemours has not made significant payments to satisfy guarantee obligations; however, Chemours believes it has the financial resources to satisfy these guarantees in the event required.
Recent Accounting Pronouncements
See Note 3 to the Consolidated Financial Statements included elsewhere in this Annual Report for a summary of recent accounting pronouncements.
Critical Accounting Policies and Estimates
Chemours’ significant accounting policies are more fully described in Note 3 to the Consolidated Financial Statements. Management believes that the application of these policies on a consistent basis enables the Company to provide the users of the financial statements with useful and reliable information about the Company’s operating results and financial condition.
The preparation of the consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts, including, but not limited to, receivable and inventory valuations, impairment of tangible and intangible assets, long-term employee benefit obligations, income taxes, restructuring liabilities, environmental matters, and litigation. Management’s estimates are based on historical experience, facts and circumstances available at the time and various other assumptions that are believed to be reasonable. The Company reviews these matters and reflects changes in estimates as appropriate. Management believes that the following represents some of the more critical judgment areas in the applications of the Company’s accounting policies which could have a material effect on the Company’s financial position, results of operations or cash flows.
Goodwill
Goodwill is tested for impairment at least annually on October 1; however, impairment tests are performed more frequently when events or changes in circumstances indicate that the asset may be impaired. Impairment exists when carrying value exceeds fair value. Goodwill is evaluated for impairment at the reporting unit level, which is defined as one level below our operating segments with the exception of Titanium Technologies, which is both an operating segment and a reporting unit. A reporting unit is the level at which discrete financial information is available and reviewed by business management on a regular basis.
The Company evaluates goodwill for impairment using a two-step process. The first step is utilizing a discounted cash flow methodology to calculate the fair value of its reporting units; and where market comparables are available, the Company includes EBITDA multiples as part of the reporting unit valuation analysis. Key assumptions used in the discounted cash flows include projected cash flows, growth rates, discount rates, tax rates and terminal values. Factors considered in developing cash flows and EBITDA projections include: 1) macroeconomic conditions; 2) industry and market considerations; 3) costs of raw materials, labor or other costs having a negative effect on earnings and cash flows; 4) overall financial performance; and 5) other relevant entity-specific events. The discount rate used represents the weighted average cost of capital for the reporting units considering the risks and uncertainty inherent in the cash flows of the reporting units and in the internally developed forecasts. The second step of the quantitative test is required if the first step of the quantitative test indicates a potential impairment. The second step is performed by comparing the implied fair value of a reporting unit’s goodwill with the carrying amount of its goodwill. If the carrying amount of goodwill is greater than its implied fair value, an impairment loss is recorded.
Based on the evaluation performed in 2016, no impairment of goodwill was recorded as the estimated fair value of each reporting unit, for which goodwill is recorded, substantially exceeded the reporting unit’s carrying amount, indicating that none of the Company’s goodwill was impaired. In 2015, in connection with the strategic evaluation of the Chemical Solutions portfolio and the resulting changes to the reporting units in the third quarter of 2015, the Chemical Solutions segments recorded a $25 million pre-tax impairment charge related to its Sulfur reporting unit. Sulfur reporting unit was disposed through the sale of its assets and business during 2016 (see Note 7 to the Consolidated Financial Statements for further details).
The determination of whether or not goodwill is impaired involves a significant level of judgment in the assumptions underlying the approach used to determine the estimated fair value of our reporting units. Chemours believes that assumptions and rates used in the impairment assessment are reasonable. However, these assumptions are judgmental and variations in any assumptions could result in materially different calculations of fair value. The Company will continue to evaluate goodwill on an annual basis as of October 1, and whenever events or changes in circumstances, such as significant adverse changes in operating results, market conditions or
51
The Chemours Company
changes in management’s business strategy, indicate that there may be a probable indicator of impairment. It is possible that the assumptions used by management related to the evaluation may change or that actual results may vary significantly from management’s estimates.
Long-lived Assets
Assessment of potential impairment of property, plant and equipment and other intangible assets is an integral part of Chemours’ normal ongoing review of operations. Chemours evaluates the carrying value of long-lived assets to be held and used when events or changes in circumstances indicate that the carrying value may not be recoverable. For purposes of recognition or measurement of an impairment loss, the assessment is performed on the asset or asset group at the level for which identifiable cash flows are largely independent of the cash flows of other groups of assets and liabilities. To determine the level at which the assessment is performed, Chemours considers factors such as revenue dependency, shared costs and the extent of vertical integration. The carrying value of a long-lived asset is considered impaired when the total projected undiscounted cash flows from the use and eventual disposition of asset or asset group are less than its carrying value. In that event, a loss is recognized based on the amount by which the carrying value exceeds the fair value of the long-lived asset. The fair value methodology used is an estimate of fair market value which is made based on prices of similar assets or other valuation methodologies including present value techniques. Long-lived assets to be disposed of other than by sale are classified as held for use until their disposal. Long-lived assets to be disposed of by sale are classified as held for sale and are reported at the lower of carrying amount or fair market value less cost to sell. Depreciation is discontinued for long-lived assets classified as held for sale.
Testing for potential impairment of these assets is significantly dependent on numerous assumptions and reflects management’s best estimates at a particular point in time. The dynamic economic environments in which Chemours’ segments operate, and key economic and business assumptions with respect to projected selling prices, market growth and inflation rates, can significantly affect the outcome of impairment tests. Estimates based on these assumptions may differ significantly from actual results. Changes in factors and assumptions used in assessing potential impairments can have a significant impact on the existence and magnitude of impairments, as well as the time in which such impairments are recognized. In addition, Chemours continually reviews its diverse portfolio of assets to ensure they are achieving their greatest potential and are aligned with Chemours’ growth strategy. Strategic decisions involving a particular group of assets may trigger an assessment of the recoverability of the related assets. Such an assessment could result in impairment losses. During 2016, Chemours recorded a $48 million pre-tax asset impairment of our Pascagoula Aniline facility, $58 million pre-tax asset impairment in connection with the sale of the Sulfur business and $13 million pre-tax asset impairment in connection with the sale of the Company’s corporate headquarters building. During 2015, Chemours recorded a $45 million pre-tax asset impairment of the RMS facility. All charges, except for the corporate headquarters building impairment (which is recorded in Corporate and Other), are recorded in the Chemical Solutions segment. Refer to Notes 7, 13 and 15 to the Consolidated Financial Statements for additional information related to these charges.
Environmental Liabilities and Expenditures
Chemours accrues for remediation activities when it is probable that a liability has been incurred and a reasonable estimate of the liability can be made. Where the available information is sufficient to estimate the amount of liability, that estimate has been used. Where the information is only sufficient to establish a range of probable liability and no point within the range is more likely than any other, the lower end of the range has been used. Estimated liabilities are determined based upon existing remediation laws and technologies. Inherent uncertainties exist in such evaluations primarily due to unknown environmental conditions, changing governmental regulations and legal standards regarding liability, and emerging remediation technologies. These accruals are adjusted periodically as remediation efforts progress and as additional technology, regulatory and legal information become available.
Environmental liabilities and expenditures include claims for matters that are liabilities of DuPont and its subsidiaries, that Chemours may be required to indemnify pursuant to the separation-related agreements executed prior to the separation. Accrued liabilities are undiscounted and do not include claims against third parties. These liabilities are included in “Other accrued liabilities” and “Other liabilities” in the Consolidated Balance Sheet.
Costs related to environmental remediation are charged to expense in the period incurred, in “Cost of goods sold” of the Consolidated Statement of Operations. Other environmental costs are also charged to expense in the period incurred, unless they increase the value of the property or reduce or prevent contamination from future operations, in which case, they are capitalized and amortized.
Litigation
Chemours accrues for litigation matters when it is probable that a liability has been incurred and the amount of the liability can be reasonably estimated. Litigation liabilities and expenditures included in the consolidated financial statements represent litigation
52
The Chemours Company
matters that are liabilities of DuPont and its subsidiaries, that Chemours may be required to indemnify pursuant to the separation-related agreements executed prior to the Distribution. Disputes between Chemours and DuPont may arise with respect to indemnification of these matters, including disputes based on matters of law or contract interpretation. If and to the extent these disputes arise, they could materially adversely affect Chemours’ results of operations. Legal costs such as outside counsel fees and expenses are charged to expense in the period services are received.
Income Taxes
The provision for income taxes is determined using the asset and liability approach of accounting for income taxes. Under this approach, deferred taxes represent the future tax consequences expected to occur when the reported amounts of assets and liabilities are recovered or paid. The provision for income taxes represents income taxes paid or payable for the current year plus the change in deferred taxes during the year. Deferred taxes result from differences between the financial and tax basis of Chemours’ assets and liabilities and are adjusted for changes in tax rates and tax laws when changes are enacted. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized. In evaluating the ability to realize deferred tax assets, the Company relies on, in order of increasing subjectivity, taxable income in prior carryback years, the future reversals of existing taxable temporary differences, tax planning strategies and forecasted taxable income using historical and projected future operating results.
The breadth of Chemours’ operations and the global complexity of tax regulations require assessments of uncertainties and judgments in estimating the taxes that Chemours will ultimately pay. The final taxes paid are dependent upon many factors, including negotiations with taxing authorities in various jurisdictions, outcomes of tax litigation and resolution of disputes arising from federal, state and international tax audits in the normal course of business. A liability for unrecognized tax benefits is recorded when management concludes that the likelihood of sustaining such positions upon examination by taxing authorities is less than “more likely than not”. It is Chemours’ policy to include accrued interest related to unrecognized tax benefits in other income, net and income tax related penalties to be included in the provision for income taxes.
Prior to July 1, 2015, income taxes as presented herein attribute current and deferred income taxes of DuPont to Chemours’ stand-alone financial statements in a manner that is systematic, rational, and consistent with the asset and liability method prescribed by Accounting Standards Codification 740, Income Taxes (ASC 740), issued by the Financial Accounting Standards Board (FASB). Accordingly, Chemours’ income tax provision was prepared following the separate return method. The separate return method applies ASC 740 to the stand-alone financial statements of each member of the consolidated group as if the group member were a separate taxpayer and a stand-alone enterprise. As a result, actual tax transactions included in the consolidated financial statements of DuPont may not be included in the separate combined financial statements of Chemours. Similarly, the tax treatment of certain items reflected in the separate combined financial statements of Chemours may not be reflected in the consolidated financial statements and tax returns of DuPont; therefore, such items as net operating losses, credit carryforwards, and valuation allowances may exist in the stand-alone financial statements that may or may not exist in DuPont’s consolidated financial statements.
The taxable income (loss) of various Chemours entities, prior to July 1, 2015, was included in DuPont’s consolidated tax returns, where applicable, in jurisdictions around the world. As such, separate income tax returns were not prepared for many Chemours’ entities. Consequently, income taxes currently payable are deemed to have been remitted to DuPont, in cash, in the period the liability arose and income taxes currently receivable are deemed to have been received from DuPont in the period that a refund could have been recognized by Chemours had Chemours been a separate taxpayer. As described in Note 2 to the Consolidated Financial Statements, the operations comprising Chemours are in various legal entities which have no direct ownership relationship. Consequently, no provision has been made for income taxes on unremitted earnings of subsidiaries and affiliates. Unremitted earnings of subsidiaries outside the U.S. are considered to be reinvested indefinitely.
Employee Benefits
The amounts recognized in the consolidated financial statements related to pension and other long-term employee benefits plans are determined from actuarial valuations. Inherent in these valuations are assumptions including expected return on plan assets, discount rates at which liabilities could have been settled, rate of increase in future compensation levels, and mortality rates. These assumptions are updated annually and are disclosed in Note 22 to the Consolidated Financial Statements. In accordance with U.S. GAAP, actual results that differed from the assumptions are accumulated and amortized over future periods and therefore, affect expense recognized and obligations recorded in future periods.
Chemours generally utilizes discount rates that are developed by matching the expected cash flows of each benefit plan to various yield curves constructed from a portfolio of high quality, fixed income instruments provided by the plan’s actuary as of the measurement date. As of December 31, 2016, the weighted average discount rate was 1.8%.
53
The Chemours Company
Expected long-term rate of return on assets is determined by performing a detailed analysis of historical and expected returns based on the strategic asset allocation of the underlying asset class applicable to each country. We also consider our historical experience with the pension fund asset performance. The expected long-term rate of return is an assumption and not what is expected to be earned in any one particular year. The weighted average long-term rate of return assumption used for determining net periodic pension expense for 2016 was 5.7%.
A 50 basis point increase in the discount rate would result in a decrease of approximately $6 million to the net periodic benefit cost for 2017, while a 50 basis point decrease in the discount rate would result in an increase of approximately $5 million. A 50 basis point increase in the expected return on asset assumption would result in a decrease of approximately $6 million to the net periodic benefit cost for 2017, while a 50 basis point decrease in the expected return on asset assumption would result in an increase of approximately $6 million.
Prior to separation, certain of Chemours’ employees participated in defined benefit pension and other post-employment benefit plans (the Plans) sponsored by DuPont and accounted for by DuPont in accordance with accounting guidance for defined benefit pension and other post-employment benefit plans. Substantially all expenses related to these plans were allocated in shared entities and reported within costs of goods sold, selling, general and administrative expenses and research and development expense in the Consolidated Statements of Operations. Chemours considered all plans to be part of a multi-employer plan with DuPont prior to January 1, 2015.
In connection with the spin-off, Chemours retained the existing Netherlands pension plan and an agreement was executed in 2015 to ensure continuance of the plan for both DuPont and Chemours employees and retirees. As a result of that agreement, Chemours now accounts for the Netherlands plan as a multiple employer plan. Additionally, in 2015, Chemours formed new pension plans in Taiwan, Germany, Belgium, Switzerland, Japan, Korea and Mexico that mirror the plans historically operated by DuPont in these countries. The new plans are accounted for under the single employer method.
Environmental Matters
Consistent with our Chemours values and our Environment, Health and Safety (EHS) Policy, Chemours is committed to preventing releases to the environment at our manufacturing sites to keep our people and communities safe and to be good stewards of the environment. Chemours is also subject to environmental laws and regulations relating to the protection of the environment. We believe that, as a general matter, our policies, standards and procedures are properly designed to prevent unreasonable risk of harm to people and the environment, and that our handling, manufacture, use and disposal of hazardous substances are in accordance with applicable environmental laws and regulations.
Environmental Expenses and Capital Expenditures
Chemours incurs costs for pollution abatement activities including waste collection and disposal, installation and maintenance of air pollution controls and wastewater treatment, emissions testing and monitoring and obtaining permits. Annual expenses charged to current operations include environmental operating costs and the increase in the remediation accrual (further described below), if any, during the period reported. We expect expenses in 2017 will be comparable or within the historical range.
Annual expenditures in the near future are also not expected to vary significantly from the expenditures incurred during the past few years. However, longer term, expenditures are subject to considerable uncertainty and may fluctuate significantly. In the U.S., additional capital expenditures are expected to be required over the next decade for treatment, storage and disposal facilities for solid and hazardous waste and for compliance with the Clean Air Act (CAA). Until all CAA regulatory requirements are established and known, considerable uncertainty will remain regarding estimates for future capital expenditures.
For the years ended December 31, 2016 and 2015, Chemours spent approximately $13 million and $27 million, respectively, on environmental capital projects either required by law or necessary to meet Chemours’ internal environmental objectives. We currently estimate expenditures for environmental-related capital projects to be approximately $18 million in 2017, which will be funded by our operating cash flows.
Management does not believe that the costs to comply with environmental requirements and the year over year changes, if any, in environmental expenses will have a material impact on Chemours’ financial position, results of operations or cash flows.
54
The Chemours Company
Mainly because of past operations, operations of predecessor companies or past disposal practices, we, like many other similar companies, have clean-up responsibilities, associated remediation costs and are subject to claims by other parties, including claims for matters that are liabilities of DuPont and its subsidiaries that Chemours may be required to indemnify pursuant to the separation-related agreements executed prior to the separation.
Chemours accrues for clean-up activities consistent with the policy as described in Note 3 to the Consolidated Financial Statements. Our environmental reserve includes estimated costs related to a number of sites for which it is probable that environmental remediation will be required, whether or not subject to enforcement activities, as well as those obligations that result from environmental laws such as the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA, often referred to as Superfund), the Resource Conservation and Recovery Act (RCRA) and similar state, federal and foreign laws. These laws require certain investigative, remediation and restoration activities at sites where Chemours conducts or once conducted operations or at sites where Chemours-generated waste was disposed. At December 31, 2016 and 2015, we recorded environmental remediation accruals of $278 and $297 million, respectively, relating to these matters which, in management’s opinion, is appropriate based on existing facts and circumstances. The following table summarizes the activities in our remediation accruals.
|
| December 31, |
| |||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
| ||
Balance at January 1 |
| $ | 297 |
|
| $ | 302 |
|
Remediation payments |
|
| (63 | ) |
|
| (43 | ) |
Increase in remediation accrual |
|
| 44 |
|
|
| 38 |
|
Currency translation adjustment |
|
| — |
|
|
| — |
|
Balance at December 31 |
| $ | 278 |
|
| $ | 297 |
|
Our liability covered approximately 212 sites. The table below reflects our estimated environmental liability by site category:
|
| December 31, 2016 |
|
| December 31, 2015 |
| ||||||||||
Site Category |
| Number of Sites |
|
| Remediation Accrual |
|
| Number of Sites |
|
| Remediation Accrual |
| ||||
Chemours-owned 1 |
|
| 29 |
|
| $ | 219 |
|
|
| 29 |
|
| $ | 231 |
|
Multi-Party Superfund / Non-Owned 2 |
|
| 86 |
|
|
| 59 |
|
|
| 88 |
|
|
| 66 |
|
Closed or settled |
|
| 97 |
|
|
| — |
|
|
| 95 |
|
|
| — |
|
Total |
|
| 212 |
|
| $ | 278 |
|
|
| 212 |
|
| $ | 297 |
|
| 1 | Includes remediation accrual of divested or sold sites where certain environmental obligations were retained by Chemours in accordance with the related sale agreements. |
|
| 2 | Sites not owned by Chemours, including sites previously owned by DuPont and sites owned by a third party where remediation obligations are imposed by Superfund Law such as CERCLA or similar state laws. |
|
As part of our legacy as a former subsidiary of DuPont, we are cleaning up historical impacts to soil and groundwater that have occurred in the past at the 29 sites that we own. These operating and former operating sites make up approximately 80% of our remediation reserve.
In addition, we inherited numerous clean-up obligations from our DuPont legacy in approximately 86 sites. We are meeting our obligations to clean-up those sites, including sites previously owned by DuPont and sites that Chemours or DuPont never owned or operated. The majority of these never-owned sites are multi-party Superfund sites that Chemours, through DuPont, has been notified of potential liability under CERCLA or similar state laws and which, in some cases, may represent a small fraction of the total waste that was allegedly disposed of at a site. These sites represent approximately 20% of our remediation reserve. Included in the 86 is approximately 35 inactive sites where there has been no known investigation, clean-up or monitoring activity and no remediation obligation is imposed or required; as such, no remediation accrual is recorded.
The remaining approximately 97 sites, which are either multi-party Superfund sites and other sites not owned by Chemours, are already closed, or settled, or for and which Chemours does not believe it has clean-up responsibility based on current information.
55
The Chemours Company
Our remediation portfolio is relatively mature, with many of our sites under active clean-up moving towards final completion. The below graph illustrates the number of remediation sites by site clean-up phase, and the remediation reserve by site clean-up phase as of December 31, 2016.
1 | The number of sites included in the chart do not include the 35 inactive sites where there has been no known investigation, clean-up or monitoring activity. |
As remediation efforts progress, sites move from the investigation phase (Investigation) to the active clean-up phase, and as construction is completed at active clean-ups (Active Remediation), those sites move to the ongoing maintenance and monitoring (OM&M) or closure phase. As final clean-ups for some significant sites are completed over the next several years, we expect our annual expenses related to these active sites to decline over time. The time-frame for a site to go through all phases of remediation (investigation and active clean-up) may take about 15 to 20 years, followed by several years of OM&M activities. Remediation activities, including OM&M activities, vary substantially in duration and cost from site to site. These activities, and their associated costs, depend on the mix of unique site characteristics, evolving remediation technologies, diverse regulatory requirements, as well as the presence or absence of other potentially responsible parties. In addition, for claims that Chemours may be required to indemnify DuPont pursuant to the separation-related agreements, Chemours, through DuPont, has limited available information for certain sites or is in the early stages of discussions with regulators. For these sites in particular there may be considerable variability between the clean-up activities that are currently being undertaken or planned and the ultimate actions that could be required. Therefore, considerable uncertainty exists with respect to environmental remediation costs and, under adverse changes in circumstances, although deemed remote, the potential liability may range up to approximately $535 million above the amount accrued at December 31, 2016. In general, uncertainty is greatest and the range of potential liability is widest in the investigation phase, and narrows over time as regulatory agencies approve site remedial plans, uncertainty is reduced and, ultimately, sites move into OM&M, where needed. As more sites advance from investigation to active clean-up to OM&M or closure, the upper end of the range of potential liability is expected to decrease over time.
Some remediation sites will achieve site closure and will require no further action to protect people and the environment and comply with laws and regulations. At certain sites, we expect that there will continue to be some level of remediation activity due to on-going monitoring and/or operations & maintenance of remedial systems. In addition, portfolio changes such as an acquisition or divestiture or notification as a potentially responsible party for a multi-party Superfund site could result in additional remediation activity and potentially additional accrual.
Management does not believe that any loss, in excess of amounts accrued, related to remediation activities at any individual site will have a material impact on our financial position, results of operations or cash flows at any given year, as such obligation can be satisfied or settled over many years.
56
The Chemours Company
While there are many remediation sites that contribute to the total environmental remediation accrual, the following sites are among the most significant:
|
| December 31, |
|
| |||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| ||
Beaumont, Texas |
| $ | 12 |
|
| $ | 12 |
|
|
Chambers Works, New Jersey |
|
| 24 |
|
|
| 20 |
|
|
East Chicago, Indiana |
|
| 20 |
|
|
| 19 |
|
|
Pompton Lakes, New Jersey |
|
| 77 |
|
|
| 87 |
|
|
USS Lead, East Chicago, Indiana |
|
| 21 |
|
|
| 15 |
|
|
All Other Sites |
|
| 124 |
|
|
| 144 |
|
|
Balance at December 31 |
| $ | 278 |
|
| $ | 297 |
|
|
The five sites listed above represent more than 50% of our reserve and we expect to spend, in aggregate, approximately $104 million over the next three years. For all other sites, we expect to spend approximately $71 million over the next three years.
Beaumont Works, Beaumont, Texas
Beaumont Works began operations in 1954 in Beaumont, Jefferson County, Texas. Over the years, Beaumont Works has produced a number of basic chemicals and elastomer products including acrylonitrile, ammonia, methanol, methyl methacrylate, caprolactam, Hypalon® synthetic rubber, Nordel® hydrocarbon rubber and blended tetraethyl lead with halo-carbon solvent/stabilizers. As of December 31, 2016, with sale of the aniline production unit to Dow in 2016, Chemours has no on-going manufacturing operations on the site. Dow and Lucite remain as long-term manufacturing tenants.
As site owner, Chemours remains responsible for remediation of historical chemical releases from past operations and is conducting this work under a RCRA hazardous waste post-closure permit and Compliance Plan (CP) issued by the State of Texas. The hazardous waste permit includes provisions to manage wastes and to investigate and mitigate releases. The CP is a component of the permit and includes mitigation and monitoring requirements, including a groundwater remediation system that was installed in 1991 to control chemical migration and protect adjacent water bodies. In addition, several solid waste management unit closures have been conducted and areas of past release addressed through interim measures to protect people and the environment. Over the years, extensive site studies have been completed and a final investigation report (Affected Property Assessment Report, or APAR, under the Texas Risk Reduction Program) for the entire site was approved by the State in 2014. Chemours is currently in the process of completing a remedial action plan (RAP) that will address all remaining historical solid waste management units and areas of concern identified in these studies, and expects to have this RAP approved in 2017.
The remediation accrual for Beaumont addresses remaining work identified in the RAP under review by the State as well as post-closure care and monitoring and on-going operation of the groundwater remediation system. A portion of the accrual also addresses an outstanding Natural Resource Damage claim by state and federal trustees directed to impacts on marshlands within the plant property.
Chambers Works, Deepwater, New Jersey
The Chambers Works complex is located on the eastern shore of the Delaware River in Deepwater, Salem County, New Jersey. The site comprises the former Carneys Point Works in the northern area and the Chambers Works manufacturing area in the southern area. Site operations began in 1892 when the former Carneys Point smokeless gunpowder plant was constructed at the northern end of Carneys Point. Site operations began in the manufacturing area around 1914 and included the manufacture of dyes, aromatics, elastomers, CFCs, and tetraethyl lead. Chemours continues to manufacture a variety of fluorochemicals and finished products at Chambers Works. In addition, three tenants operate processes at Chambers Works including steam/electricity generation, industrial gas production and the manufacture of intermediate chemicals. As a result of over 100 years of continuous industrial activity, site soils and groundwater have been impacted by chemical releases.
In response to identified groundwater contamination, a groundwater interceptor well system (IWS) was installed in 1970, which was designed to contain contaminated groundwater and restrict off-site migration. Additional remediation is being completed under a Federal RCRA Corrective Action Permit. The site has been studied extensively over the years and more than 25 remedial actions have been completed to date and engineering and institutional controls put in place to ensure protection of people and the environment.
Remaining work beyond continued operation of the IWS and groundwater monitoring includes completion of a site perimeter sheet pile barrier intended to more efficiently contain groundwater, completion of various targeted studies onsite and in adjacent water
57
The Chemours Company
bodies to close investigation data gaps, and selection and implementation of final remedies under RCRA Corrective Action for various solid waste management units and areas of concern not yet addressed through interim measures.
East Chicago, Indiana
East Chicago is a former manufacturing facility owned by Chemours in East Chicago, Lake County, Indiana. The approximately 440-acre site is bounded to the south by the East Branch of the Grand Calumet River, to the east and north by residential and commercial areas, and to the west by industrial areas, including a former lead processing facility. The inorganic chemicals unit on site produced various chloride, ammonia, and zinc products and inorganic agricultural chemicals beginning in 1892 until 1986. Organic chemical manufacturing began in 1944, consisting primarily of chlorofluorocarbons production. Current operations, including support activities, now cover 28 acres of the Site. The remaining business was sold to W.R. Grace Company (Grace) in early 2000, and Grace operates the unit as a tenant. Approximately 172 acres of the site were never developed and are managed by The Nature Conservancy for habitat preservation.
A comprehensive evaluation of soil and groundwater conditions at the Site was performed as part of the RCRA corrective action process. Studies of historical site impacts began in 1983 in response to preliminary CERCLA actions undertaken by EPA. USEPA eventually issued an Administrative Order on Consent for the Site in 1997. The order specified that remediation work be performed under RCRA Corrective Action authority. Work has proceeded under the RCRA Corrective Action process since that time.
Subsequent investigations included the preparation of initial environmental site assessments and multiple phases of investigation. In 2002, as an interim remedial measure, two 2,000-foot-long permeable reactive barrier treatment walls were installed along the northern property boundary to address migration of chemicals in groundwater. Since that time, the investigation process has been completed and approved by EPA and work is in progress to define the final remedy for the site.
Pompton Lakes, New Jersey
During the twentieth century, blasting caps, fuses and related materials were manufactured at Pompton Lakes, Passaic County, New Jersey. Operating activities at the site were ceased in the mid-1990s. Primary contaminants in the soil and sediments are lead and mercury. Ground water contaminants include volatile organic compounds. Under the authority of the EPA and NJDEP, remedial actions at the site are focused on investigating and cleaning up the area. Ground water monitoring at the site is on-going, and Chemours has installed and continues to install vapor mitigation systems at residences within the ground water plume. In addition, Chemours is further assessing ground water conditions. In June 2015, the EPA issued a modification to the site’s RCRA permit that requires Chemours to dredge mercury contamination from a 36-acre area of the lake and remove sediment from two other areas of the lake near the shoreline. The remediation activities commenced when permits and implementation plans were approved in May 2016.
U.S. Smelter and Lead Refinery, Inc. (USS Lead), East Chicago, Indiana
The USS Lead Superfund Site is located in the Calumet neighborhood of East Chicago, Lake County, Indiana. The site includes the former USS Lead facility along with nearby commercial, municipal and residential areas. The primary compounds of interest are lead and arsenic which may be found in soils within the impacted area. The U.S. Environmental Protection Agency (EPA) is directing and organizing remediation on this site, and Chemours is one of a number of parties working cooperatively with the EPA on the safe and timely completion of this work. DuPont’s former East Chicago manufacturing facility was located adjacent to the site, and DuPont assigned responsibility for the site to Chemours in the 2015 separation agreement.
The USS Lead site was listed on the National Priorities List (NPL) in 2009. To facilitate negotiations with potentially responsible parties, EPA divided the residential part of the USS Lead Superfund Site into three zones, referred to as Zone 1, Zone 2, and Zone 3. The division into three zones resulted in Atlantic Richfield Co. and DuPont entering into an agreement in 2014 with EPA and the State of Indiana to reimburse EPA’s costs to implement cleanup in Zone 1 and Zone 3. According to its website, EPA is continuing its efforts to identify additional parties who might be potentially responsible for the cleanup of Zone 2. Once it has concluded these efforts, EPA will engage in negotiations with all known viable and liable parties.
The environmental accrual is based on Record of Decision (“ROD”) and Statement of Work currently in place for Zones 1 and 3. EPA has announced its intent to reconsider the ROD for Zone 1 and the result of that review could increase or decrease Chemours’ future obligations. In addition, there is uncertainty in the outlook for Zone 2 given EPA’s stated objective to identify additional parties. As such, Chemours’ obligation for work in Zone 2 cannot be estimated at this time.
58
The Chemours Company
Chemours believes that climate change is an important global issue that presents risks and opportunities. Chemours continuously evaluates opportunities for existing and new product and service offerings in light of the anticipated demands of a low-carbon economy. Our new, low GWP products are anticipated to reduce greenhouse gas content of refrigerants by 90 million metric tons carbon dioxide equivalent in the U.S. and greater than 300 million metric tons worldwide by 2025.
We continue to monitor legislative and regulatory developments to control or limit greenhouse gas (GHG) emissions. Depending on the scope and content, changes could affect Chemours’ energy source and supply choices, as well as increase the cost of energy and raw materials derived from fossil fuels. Such efforts are also expected to provide the business community with greater certainty for the regulatory future, help guide investment decisions, and drive growth in demand for low-carbon and energy-efficient products, technologies, and services. Similarly, demand is expected to grow for products that facilitate adaptation to a changing climate.
Several of Chemours facilities in the EU are regulated under the EU Emissions Trading Scheme. In 2015, China announced a national cap and trade program to be implemented in 2017. Similarly, South Korea implemented its emission trading scheme on January 1, 2015. In the EU, U.S. and Japan, policy efforts to reduce the GHG emissions associated with gases used in refrigeration and air conditioning are creating market opportunities for new solutions to lower GHG emissions.
In May 2010, the EPA launched a phased-in scheme to regulate GHG emissions first from large stationary sources under the existing Clean Air Act permitting requirements administered by state and local authorities. As a result, large capital investments may be required to install Best Available Control Technology on major new or modified sources of GHG emissions. This type of GHG emissions regulation by the EPA, in the absence of or in addition to federal legislation, could result in more costly, less efficient facility-by-facility controls versus a federal program that incorporates policies that provide an economic balance that does not severely distort markets. In 2015, the EPA promulgated regulations for carbon dioxide emissions from new and reconstructed/modified Electric Generating Units (EGUs) and for carbon dioxide emissions from existing EGUs that would be based on individual state emission reduction programs. If these or similar regulations are enacted, they may affect the long term price and supply of electricity and natural gas and demand for products that contribute to energy efficiency and renewable energy. Chemours, as well as our suppliers and customers, could be in a competitive disadvantage by the added costs of complying with a variety of state-specific requirements. However, the precise impact of these regulations is uncertain due to the anticipated legal challenges to this regulatory approach.
PFOA
See discussion under “PFOA” in Note 20 to the Consolidated Financial Statements.
Non-GAAP Financial Measures
We prepare our financial statements in accordance with U.S. GAAP. To supplement our financial information presented in accordance with U.S. GAAP, we provide the following non-GAAP financial measures, “Adjusted EBITDA”, “Adjusted Net Income” and “Free Cash Flow”, in order to clarify and provide investors with a better understanding of the company’s performance when analyzing changes in our underlying business between reporting periods and provide for greater transparency with respect to supplemental information used by management in its financial and operational decision making. We utilize Adjusted EBITDA as the primary measure of segment profitability used by our Chief Operating Decision Maker (CODM).
Adjusted EBITDA is defined as income (loss) before taxes excluding the following:
| • | interest expense, depreciation and amortization, |
| • | non-operating pension and other postretirement employee benefit costs, which represent the components of net periodic pension costs (income) excluding service cost component, |
| • | exchange losses (gains) included “other income, net” of the statements of operations, |
| • | restructuring, asset-related charges and other charges, net, |
| • | asset impairments, |
| • | losses (gains) on sale of business or assets, and |
| • | other items not considered indicative of our ongoing operational performance and expected to occur infrequently. |
59
The Chemours Company
Adjusted net income (loss) is defined as net income (loss) attributable to Chemours adjusted for items excluded from Adjusted EBITDA except interest expense, depreciation and amortization, and certain provision for (benefit from) income taxes. Free Cash Flow is defined as cash provided by (used for) operating activities less cash used for purchases of property, plant and equipment as disclosed in the Consolidated Statements of Cash Flows.
We believe the presentation of these non-GAAP financial measures, when used in conjunction with GAAP financial measures, is a useful financial analysis tool that can assist investors in assessing the company’s operating performance and underlying prospects. This analysis should not be considered in isolation or as a substitute for analysis of our results as reported under GAAP. In the future, we may incur expenses similar to those eliminated in this presentation. Our presentation of Adjusted EBITDA, Adjusted Net Income and Free Cash Flow should not be construed as an inference that our future results will be unaffected by unusual or infrequently occurring items. The non-GAAP financial measures we use may be defined differently from measures with the same or similar names used by other companies. This analysis, as well as the other information provide in this annual report on Form 10-K, should be read in conjunction with the company’s financial statements and notes thereto included in this report.
The following table reconciles Adjusted EBITDA and Adjusted Net Income discussed above to net income (loss) attributable to Chemours for the periods presented:
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Net income (loss) attributable to Chemours |
| $ | 7 |
|
| $ | (90 | ) |
| $ | 400 |
|
Non-operating pension and other postretirement employee benefit (income) costs |
|
| (20 | ) |
|
| (3 | ) |
|
| 22 |
|
Exchange losses (gains) |
|
| 57 |
|
|
| (19 | ) |
|
| 66 |
|
Restructuring charges |
|
| 51 |
|
|
| 285 |
|
|
| 21 |
|
Asset related charges 1 |
|
| 124 |
|
|
| 73 |
|
|
| — |
|
(Gains) losses on sale of business or assets |
|
| (254 | ) |
|
| 9 |
|
|
| (40 | ) |
Transaction costs 2 |
|
| 19 |
|
|
| 9 |
|
|
| — |
|
Legal and other charges 3 |
|
| 359 |
|
|
| 8 |
|
|
| — |
|
Benefit from income taxes relating to reconciling items 4 |
|
| (156 | ) |
|
| (129 | ) |
|
| (16 | ) |
Adjusted Net Income |
|
| 187 |
|
|
| 143 |
|
|
| 453 |
|
Net income attributable to noncontrolling interests |
|
| — |
|
|
| — |
|
|
| 1 |
|
Interest expense, net |
|
| 213 |
|
|
| 132 |
|
|
| — |
|
Depreciation and amortization |
|
| 284 |
|
|
| 267 |
|
|
| 257 |
|
All remaining provision for income taxes 4 |
|
| 138 |
|
|
| 31 |
|
|
| 165 |
|
Adjusted EBITDA |
| $ | 822 |
|
| $ | 573 |
|
| $ | 876 |
|
1 | The year ended December 31, 2016 includes $48 million pre-tax asset impairment of our Pascagoula Aniline facility, $58 million pre-tax asset impairment in connection with the sale of the Sulfur business, $13 million pre-tax asset impairment in connection with the sale of the Company’s corporate headquarters building and other asset write-offs. The year ended December 31, 2015 includes $25 million of goodwill impairment and $45 million asset impairment of RMS facility. All charges, except for the corporate headquarters building impairment (which is recorded in Corporate and Other), are recorded in the Chemical Solutions segment. Refer to Notes 7, 13, 14 and 15 to the Consolidated Financial Statements for additional information related to these charges. |
|
2 | Includes accounting, legal and bankers transaction fees incurred related to the Company’s strategic initiatives, which includes pre-sale transaction costs incurred in connection with the sales of the C&D and Sulfur businesses (see Note 7 to the Consolidated Financial Statements). |
|
3 | Includes litigation settlements, water treatment accruals and $335 million litigation accrual related to the PFOA MDL Settlement (see Note 20 to the Consolidated Financial Statements), and lease termination charges. |
|
4 | Total (benefit from) provision for income taxes reconciles to the amount reported in the consolidated statement of operations for the years ended December 31, 2016, 2015 and 2014. |
|
60
The Chemours Company
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to changes in foreign currency exchange rates because of our global operations. As a result, we have assets, liabilities and cash flows denominated in a variety of foreign currencies. We are also exposed to changes in the prices of certain commodities that we use in production. Changes in these rates and commodity prices may have an impact on future cash flow and earnings. We manage these risks through normal operating and financing activities and, when deemed appropriate, through the use of derivative financial instruments. We do not enter into derivative financial instruments for trading or speculative purposes.
By using derivative instruments, we are subject to credit and market risk. The fair market value of the derivative instruments is determined by using valuation models whose inputs are derived using market observable inputs, and reflects the asset or liability position as of the end of each reporting period. When the fair value of a derivative contract is positive, the counterparty owes us, thus creating a receivable risk for us. We are exposed to counterparty credit risk in the event of non-performance by counterparties to our derivative agreements. We minimize counterparty credit (or repayment) risk by entering into transactions with major financial institutions of investment grade credit rating.
Foreign Currency Risks
Fluctuations in the value of the U.S. dollar compared to foreign currencies may impact Chemours’ earnings. In 2016 and 2015, Chemours entered into foreign currency forward contracts to minimize volatility in earnings related to the foreign exchange gains and losses resulting from remeasuring monetary assets and liabilities that Chemours holds which are denominated in non-functional currencies. These derivatives are stand-alone and have not been designated as a hedge. As of December 31, 2016, we had open foreign exchange forward contracts with an aggregate notional U.S. dollar equivalent of $518 million, the fair value of which amounted to $2 million of net unrealized loss. Chemours recognized a net loss of $15 million and a net gain of $42 million for the years ended December 31, 2016 and 2015, respectively.
Prior to 2015, Chemours participated in DuPont’s foreign currency hedging program to reduce earnings volatility associated with remeasurement of foreign currency denominated net monetary assets. DuPont formally documented the hedge relationships, including identification of the hedging instruments and hedged items, the risk management objectives and strategies for undertaking the hedge transactions, and the methodologies used to assess effectiveness and measure ineffectiveness. Realized gains and losses on derivative instruments of DuPont were allocated by DuPont to Chemours based on projected exposure. Chemours recognized its allocable share of the gains and losses on DuPont’s derivative financial instruments in earnings when the forecasted purchases occurred for natural gas hedges and when the forecasted sales occurred for foreign currency hedges. The impact of Chemours’ participation in the foreign currency hedging program was a gain of $4 million in 2014.
In a hypothetical adverse change in the market prices or rates that existed at December 31, 2016, a 10% increase in the U.S. dollar against our outstanding hedged contracts on foreign currencies, such as the Euro and Chinese yuan, at the currency exchange rates as of December 31, 2016 would increase our net loss by approximately $8 million, while a 10% depreciation of the U.S. Dollar against the same hedged currencies would decrease our net loss by approximately $9 million.
Beginning in July 2015, Chemours designated its €360 million Euro notes as a hedge of its net investments in certain of its international subsidiaries that use the Euro as functional currency in order to reduce the volatility in stockholders’ equity caused by the changes in foreign currency exchange rates of the Euro with respect to the U.S. dollar. Chemours uses the spot method to measure the effectiveness of the net investment hedge. Under this method, for each reporting period, the change in the carrying value of the Euro notes due to remeasurement of the effective portion is reported in accumulated other comprehensive loss in the Consolidated Balance Sheet and the remaining change in the carrying value of the ineffective portion, if any, is recognized in “Other income, net” in the Consolidated Statements of Operations. Chemours evaluates the effectiveness of its net investment hedge at the beginning of every quarter.
Chemours’ risk management programs and the underlying exposure are closely correlated, such that the potential loss in value for the risk management portfolio described above would be largely offset by change in the value of the underlying exposure. See Note 21 to the Consolidated Financial Statements for further information.
Concentration of Credit Risk
Chemours’ sales are not dependent on any single customer. As of December 31, 2016 and 2015, no individual customer balance represented more than five percent of Chemours’ total outstanding receivables balance. Credit risk associated with Chemours’ receivables balance is representative of the geographic, industry and customer diversity associated with Chemours’ global businesses.
61
The Chemours Company
As a result of our customer base being widely dispersed, we do not believe our exposure to credit-related losses related to our business as of December 31, 2016 and 2015 was material.
Chemours also maintains strong credit controls in evaluating and granting customer credit. As a result, it may require that customers provide some type of financial guarantee in certain circumstances. Length of terms for customer credit varies by industry and region.
Commodities Risk
A portion of our products and raw materials are commodities whose prices fluctuate as market supply and demand fundamentals change. Accordingly, product margins and the level of our profitability tend to fluctuate with the changes in the business cycle. Chemours tries to protect against such instability through various business strategies. These include provisions in sales contracts allowing us to pass on higher raw material costs through timely price increases and formula price contracts to transfer or share commodity price risk. Chemours did not have any commodity derivative instruments in place as of December 31, 2016 and 2015.
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The financial statements and supplementary data required by this Item are included herein, commencing on page F-1 of this Annual Report.
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
Item 9A. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures designed to provide reasonable assurance that information required to be disclosed in the Company’s reports filed or submitted under the Securities Exchange Act of 1934 (Exchange Act) is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission. These controls and procedures also provide reasonable assurance that information required to be disclosed in such reports is accumulated and communicated to management, including its Chief Executive Officer (CEO) and Chief Financial Officer (CFO), to allow timely decisions regarding required disclosures.
As of December 31, 2016, the Company’s CEO and CFO, together with management, conducted an evaluation of the effectiveness of the Company’s disclosure controls and procedures as defined in Rule 13a-15(e) under the Exchange Act. Based on that evaluation, the CEO and CFO have concluded that these disclosure controls and procedures are effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
There have been no changes in the Company’s internal control over financial reporting that occurred during the quarter ended December 31, 2016 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
The Company has completed its evaluation of its internal control over financial reporting and has concluded that the Company’s internal control over financial reporting was effective as of December 31, 2016 (see Management’s Report on Internal Control over Financial Reporting on page F-2).
None.
62
The Chemours Company
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Except for information concerning executive officers, which is included in Part I of this annual report under the caption “Executive Officers of the Registrant”, the information about the Company’s directors required by this Item 10 is contained under the caption “Proposal 1 - Election of Directors” in the Company’s definitive proxy statement for its 2017 annual meeting of stockholders (2017 Proxy Statement) which the Company anticipates filing with the Securities and Exchange Commission within 120 days after the end of the fiscal year to which this report relates, and is incorporated herein by reference.
Information regarding the Company’s Audit Committee, code of ethics, and compliance with Section 16(a) of the Exchange Act is contained in the 2017 Proxy Statement under the captions “Corporate Governance”, “Board Structure and Committee Composition” and “Section 16(a) Beneficial Ownership Reporting Compliance” and is incorporated herein by reference.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item 11 is contained in the 2017 Proxy Statement under the captions “Executive Compensation”, “Director Compensation”, “Compensation Committee Report” and “Compensation Committee Interlocks and Insider Participation” and is incorporated herein by reference.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by Item 12 and not otherwise set forth below is contained in the 2017 Proxy Statement under the caption “Security Ownership of Certain Beneficial Owners and Management” and is incorporated herein by reference.
Securities authorized for issuance under equity compensation plans as of December 31, 2016
(shares in thousands, except per share) |
|
|
|
|
|
|
|
|
|
|
|
|
Plan Category |
| Number of securities to be issued upon Exercise of Outstanding Options, Warrants and Rights 1 |
|
| Weighted Average Exercise Price of Outstanding Options, Warrants and Rights 2 |
|
| Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans 3 |
| |||
Equity compensation plans approved by security holders |
|
| 11,088 |
|
| $ | 13.72 |
|
|
| 7,806 |
|
1 | Includes outstanding stock options, restricted stock units, performance stock units granted under the Company’s Equity and Incentive Plan. |
2 | Represents the weighted average exercise price of the outstanding stock options only. No exercise price related to the restricted stock units and performance stock units. |
3 | Reflects shares available for issuance pursuant to the Equity and Incentive Plan approved by our former parent prior to separation while the Company was a wholly-owned subsidiary of DuPont (see Note 23 to Consolidated Financial Statements for further information). The maximum number of shares of stock reserved for the grant or settlement of awards under the plan shall be 13,500,000 plus the number of shares of stock of the converted DuPont awards. The aggregate number of shares of stock granted during any fiscal year to any single individual (other than with regard to converted DuPont awards) shall not exceed 3,000,000 shares. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
The information required by Item 13 is contained in the 2017 Proxy Statement under the captions “Director Independence” and “Certain Relationships and Transactions” and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by Item 14 is contained in the 2017 Proxy Statement under the captions “Proposal 3 - Ratification of Selection of Independent Registered Public Accounting Firm”, “Fees Paid to Independent Registered Public Accounting Firm” and “Audit Committee’s Pre-Approval Policies and Procedures” and is incorporated herein by reference.
63
The Chemours Company
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)(1) Financial Statements
See the Index to the Consolidated Financial Statements on page F-1 of this report.
(a)(2) Financial Statement Schedules
See Schedule II listed below.
Schedule II - Valuation and Qualifying Accounts
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Accounts Receivable - Allowance for Doubtful Accounts |
|
|
|
|
|
|
|
|
|
|
|
|
Balance at beginning of period |
| $ | 4 |
|
| $ | 4 |
|
| $ | 7 |
|
Additions charged to expenses |
|
| 1 |
|
|
| 1 |
|
|
| 1 |
|
Deductions from reserves 1 |
|
| — |
|
|
| — |
|
|
| (4 | ) |
Currency translation |
|
| — |
|
|
| (1 | ) |
|
| — |
|
Balance at end of period |
| $ | 5 |
|
| $ | 4 |
|
| $ | 4 |
|
Deferred Tax Assets - Valuation Allowance |
|
|
|
|
|
|
|
|
|
|
|
|
Balance at beginning of period |
| $ | — |
|
| $ | 36 |
|
| $ | 26 |
|
Net charges to income tax expense |
|
| 50 |
|
|
| — |
|
|
| 10 |
|
Release of valuation allowance 2 |
|
| — |
|
|
| (36 | ) |
|
| — |
|
Balance at end of period |
| $ | 50 |
|
| $ | — |
|
| $ | 36 |
|
| 1 | Bad debt write-offs were less than $1 for the years ended December 31, 2016 and 2015. |
|
| 2 | Release of the valuation allowance during 2015 was related to tax loss carryforward incurred prior to July 1, 2015 that is attributable to DuPont’s tax periods pursuant to the tax matters agreement. The adjustment was recorded in the “DuPont Company Net Investment” of the Consolidated Statements of Stockholders’ Equity for the year ended December 31, 2015. |
|
(a)(3) Exhibits
See the Exhibit List beginning on page 66 of this report.
The Company has elected not to include a Form 10-K summary under this Item 16.
64
The Chemours Company
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
THE CHEMOURS COMPANY | ||
(Registrant) | ||
|
|
|
Date: |
| February 17, 2017 |
|
|
|
By: |
| /s/ Mark E. Newman |
|
| Mark E. Newman |
|
| Senior Vice President and Chief Financial Officer |
|
| (As Duly Authorized Officer and Principal Financial Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated:
Signature |
| Title(s) |
| Date |
|
|
|
|
|
/s/ Mark P. Vergnano |
| President, Chief Executive Officer, and |
| February 17, 2017 |
Mark P. Vergnano |
| Director |
|
|
(Principal Executive Officer) | ||||
|
|
|
|
|
/s/ Mark E. Newman |
| Senior Vice President and Chief |
| February 17, 2017 |
Mark E. Newman |
| Financial Officer |
|
|
(Principal Financial Officer) | ||||
|
|
|
|
|
/s/ Amy P. Trojanowski |
| Vice President and Controller |
| February 17, 2017 |
Amy P. Trojanowski |
| (Principal Accounting Officer) |
|
|
|
|
|
|
|
/s/ Richard H. Brown |
| Chairman of the Board |
| February 17, 2017 |
Richard H. Brown |
|
|
|
|
|
|
|
|
|
/s/ Curtis V. Anastasio |
| Director |
| February 17, 2017 |
Curtis V. Anastasio |
|
|
|
|
|
|
|
|
|
/s/ Bradley J. Bell |
| Director |
| February 17, 2017 |
Bradley J. Bell |
|
|
|
|
|
|
|
|
|
/s/ Mary B. Cranston |
| Director |
| February 17, 2017 |
Mary B. Cranston |
|
|
|
|
|
|
|
|
|
/s/ Curtis J. Crawford |
| Director |
| February 17, 2017 |
Curtis J. Crawford |
|
|
|
|
|
|
|
|
|
/s/ Dawn L. Farrell |
| Director |
| February 17, 2017 |
Dawn L. Farrell |
|
|
|
|
|
|
|
|
|
/s/ Stephen D. Newlin |
| Director |
| February 17, 2017 |
Stephen D. Newlin |
|
|
|
|
65
The Chemours Company
Exhibit Number |
| Description |
|
|
|
2.1 |
| Separation Agreement by and between E. I. du Pont de Nemours and Company and the Chemours Company (incorporated by reference to Exhibit 2 to the Company’s Current Report on Form 8-K, as filed with the U.S. Securities and Exchange Commission on July 1, 2015). |
3.1 |
| Company’s Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to the Company’s Current Report on Form 8-K, as filed with the U.S. Securities and Exchange Commission on July 1, 2015). |
3.2 |
| Company’s Amended and Restated Bylaws (incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K, as filed with the U.S. Securities and Exchange Commission on July 1, 2015). |
10.1 |
| Second Amended and Restated Transition Services Agreement by and between E. I. du Pont de Nemours and Company and The Chemours Company (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K, as filed with the U.S. Securities and Exchange Commission on July 1, 2015). |
10.2 |
| Tax Matters Agreement by and between E. I. du Pont de Nemours and Company and The Chemours Company (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K, as filed with the U.S. Securities and Exchange Commission on July 1, 2015). |
10.3 |
| Employee Matters Agreement by and between E. I. du Pont de Nemours and Company and The Chemours Company (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K, as filed with the U.S. Securities and Exchange Commission on July 1, 2015). |
10.4 |
| Third Amended and Restated Intellectual Property Cross-License Agreement by and among E. I. du Pont de Nemours and Company, The Chemours Company FC and The Chemours Company TT, LLC (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K, as filed with the U.S. Securities and Exchange Commission on July 1, 2015). |
10.5* |
| Offer of Employment Letter between Mark E. Newman and E. I. du Pont de Nemours and Company, dated October 14, 2014 (incorporated by reference to Exhibit 10.5 to the Company’s Amendment No. 2 to Form 10, as filed with the U.S. Securities and Exchange Commission on April 21, 2015). |
10.6* |
| Offer of Employment Letter between Elizabeth Albright and E. I. du Pont de Nemours and Company, dated September 25, 2014 (incorporated by reference to Exhibit 10.6 to the Company’s Amendment No. 2 to Form 10, as filed with the U.S. Securities and Exchange Commission on April 21, 2015). |
10.7 |
| Indenture, dated May 12, 2015 by and among The Chemours Company, The Guarantors party thereto and U.S. Bank National Association, as Trustee, Elavon Financial Services Limited, as Registrar and Transfer Agent for the Euro Notes (incorporated by reference to Exhibit 10.7 to the Company’s Amendment No. 3 to Form 10, as filed with the U.S. Securities and Exchange Commission on May 13, 2015). |
10.8 |
| First Supplemental Indenture, dated May 12, 2015, by and among The Chemours Company, the Guarantors party thereto and U.S. Bank National Association, as Trustee (incorporated by reference to Exhibit 10.8 to the Company’s Amendment No. 3 to Form 10, as filed with the U.S. Securities and Exchange Commission on May 13, 2015). |
10.9 |
| Second Supplemental Indenture, dated May 12, 2015, by and among The Chemours Company, the Guarantors party thereto and U.S. Bank National Association, as Trustee (incorporated by reference to Exhibit 10.9 to the Company’s Amendment No. 3 to Form 10, as filed with the U.S. Securities and Exchange Commission on May 13, 2015). |
10.10 |
| Third Supplemental Indenture, dated May 12, 2015, by and among The Chemours Company, the Guarantors party thereto and U.S. Bank National Association, as Trustee, Elavon Financial Services Limited, UK Branch, as Paying Agent for the Euro Notes and Elavon Financial Services Limited, as Registrar and Transfer Agent for the Euro Notes (incorporated by reference to Exhibit 10.10 to the Company’s Amendment No. 3 to Form 10, as filed with the U.S. Securities and Exchange Commission on May 13, 2015). |
10.11 |
| 6.625% Notes due 2023 (included in Exhibit 10.8). |
10.12 |
| 7.000% Notes due 2025 (included in Exhibit 10.9). |
10.13 |
| 6.125% Notes due 2023 (included in Exhibit 10.10). |
66
The Chemours Company
67
The Chemours Company
Exhibit Number |
| Description |
|
|
|
| Letter Agreement dated January 28, 2016 by and between The Chemours Company and E. I. du Pont de Nemours and Company (incorporated by reference to Item 10.2 to the Company’s Current Report on Form 8-K, as filed with the U.S. Securities and Exchange Commission on February 23, 2016). | |
10.31* |
| Form of Option Award Terms under the Company’s Equity Incentive Plan for grantees located in the U.S. |
10.32* |
| Form of Option Award Terms under the Company’s Equity Incentive Plan for grantees located outside the U.S. |
10.33* |
| Form of Award Terms of Time-Vested Restricted Stock Units under the Company’s Equity Incentive Plan for grantees located in the U.S. |
10.34* |
| Form of Award Terms of Time-Vested Restricted Stock Units under the Company’s Equity Incentive Plan for grantees located outside the U.S. |
10.35* |
| Form of Award Terms of Performance Share Units under the Company’s Equity Incentive Plan. |
12.1 |
| Computation of Ratio of Earnings to Fixed Charges for the Company. |
21 |
| Subsidiaries of the Registrant |
23 |
| Consent of Independent Registered Public Accounting Firm |
31.1 |
| Rule 13a-14(a)/15d-14(a) Certification of the Company’s Principal Executive Officer. |
31.2 |
| Rule 13a-14(a)/15d-14(a) Certification of the Company’s Principal Financial Officer. |
32.1 |
| Section 1350 Certification of the company’s Principal Executive Officer. The information contained in this Exhibit shall not be deemed filed with the Securities and Exchange Commission nor incorporated by reference in any registration statement filed by the registrant under the Securities Act of 1933, as amended. |
32.2 |
| Section 1350 Certification of the company’s Principal Financial Officer. The information contained in this Exhibit shall not be deemed filed with the Securities and Exchange Commission nor incorporated by reference in any registration statement filed by the registrant under the Securities Act of 1933, as amended. |
95 |
| Mine Safety Disclosures |
101.INS |
| XBRL Instance Document |
101.SCH |
| XBRL Taxonomy Extension Schema Document |
101.CAL |
| XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF |
| XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB |
| XBRL Taxonomy Extension Label Linkbase Document |
101.PRE |
| XBRL Taxonomy Extension Presentation Linkbase Document |
|
* Management contract or compensatory plan or arrangement.
68
Index to the Consolidated Financial Statements
F-1
Management’s Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934. The company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The company’s internal control over financial reporting includes those policies and procedures that:
| i. | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; |
| ii. | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles and that receipts and expenditures of the company are being made only in accordance with authorization of management and directors of the company; and |
| iii. | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisitions, use or disposition of the company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of the company’s internal control over financial reporting as of December 31, 2016, based on criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework (2013). Based on its assessment and those criteria, management concluded that the company maintained effective internal control over financial reporting as of December 31, 2016.
PricewaterhouseCoopers LLP, an independent registered public accounting firm, has audited the effectiveness of the company’s internal control over financial reporting as of December 31, 2016, as stated in their report, which is presented on the following page.
/s/ Mark P. Vergnano |
| /s/ Mark E. Newman |
|
Mark P. Vergnano |
| Mark E. Newman |
|
President and Chief Executive Officer |
| Senior Vice President and Chief Financial Officer |
|
February 17, 2017
F-2
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of The Chemours Company:
In our opinion, the consolidated financial statements listed in the accompanying index present fairly, in all material respects, the financial position of The Chemours Company and its subsidiaries at December 31, 2016 and 2015, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2016 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion the financial statement schedule listed in the index appearing under Item 15(a)(2) presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company’s internal control over financial reporting based on our audits (which was an integrated audit in 2016). We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP |
Philadelphia, Pennsylvania |
February 17, 2017 |
F-3
Consolidated Statements of Operations
(Dollars in millions, except per share)
|
| Year Ended December 31, |
|
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
|
| |||
Net sales |
| $ | 5,400 |
|
| $ | 5,717 |
|
| $ | 6,432 |
|
|
Cost of goods sold |
|
| 4,290 |
|
|
| 4,762 |
|
|
| 5,072 |
|
|
Gross profit |
|
| 1,110 |
|
|
| 955 |
|
|
| 1,360 |
|
|
Selling, general and administrative expense |
|
| 934 |
|
|
| 632 |
|
|
| 685 |
|
|
Research and development expense |
|
| 80 |
|
|
| 97 |
|
|
| 143 |
|
|
Restructuring and asset related charges, net |
|
| 170 |
|
|
| 333 |
|
|
| 21 |
|
|
Goodwill impairment |
|
| — |
|
|
| 25 |
|
|
| — |
|
|
Total expenses |
|
| 1,184 |
|
|
| 1,087 |
|
|
| 849 |
|
|
Equity in earnings of affiliates |
|
| 29 |
|
|
| 22 |
|
|
| 20 |
|
|
Interest expense, net |
|
| (213 | ) |
|
| (132 | ) |
|
| — |
|
|
Other income, net |
|
| 247 |
|
|
| 54 |
|
|
| 19 |
|
|
(Loss) income before income taxes |
|
| (11 | ) |
|
| (188 | ) |
|
| 550 |
|
|
(Benefit from) provision for income taxes |
|
| (18 | ) |
|
| (98 | ) |
|
| 149 |
|
|
Net income (loss) |
|
| 7 |
|
|
| (90 | ) |
|
| 401 |
|
|
Less: Net income attributable to noncontrolling interests |
|
| — |
|
|
| — |
|
|
| 1 |
|
|
Net income (loss) attributable to Chemours |
| $ | 7 |
|
| $ | (90 | ) |
| $ | 400 |
|
|
Per share data |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic earnings (loss) per share of common stock |
| $ | 0.04 |
|
| $ | (0.50 | ) |
| $ | 2.21 |
| 1 |
Diluted earnings (loss) per share of common stock |
| $ | 0.04 |
|
| $ | (0.50 | ) |
| $ | 2.21 |
| 1 |
Dividends per share of common stock |
| $ | 0.12 |
|
| $ | 0.58 |
|
| N/A |
|
| |
1 | On July 1, 2015, E. I. du Pont de Nemours and Company distributed 180,966,833 shares of Chemours’ common stock to holders of its common stock. Basic and diluted earnings (loss) per common share for the year ended December 31, 2014 was calculated using the shares distributed on July 1, 2015. Refer to Note 10 for information regarding the calculation of basic and diluted earnings per share. |
See accompanying notes to the consolidated financial statements.
F-4
Consolidated Statements of Comprehensive Income (Loss)
(Dollars in millions)
|
| Year Ended December 31, |
| |||||||||||||||||||||||||||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||||||||||||||||||||||||||
|
| Pre-Tax |
|
| Tax |
|
| After-Tax |
|
| Pre-Tax |
|
| Tax |
|
| After-Tax |
|
| Pre-Tax |
|
| Tax |
|
| After-Tax |
| |||||||||
Net income (loss) |
| $ | (11 | ) |
| $ | 18 |
|
| $ | 7 |
|
| $ | (188 | ) |
| $ | 98 |
|
| $ | (90 | ) |
| $ | 550 |
|
| $ | (149 | ) |
| $ | 401 |
|
Other comprehensive income (loss): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized gain on net investment hedge |
|
| 14 |
|
|
| — |
|
|
| 14 |
|
|
| 8 |
|
|
| — |
|
|
| 8 |
|
|
| — |
|
|
| — |
|
|
| — |
|
Cumulative translation adjustments |
|
| (73 | ) |
|
| — |
|
|
| (73 | ) |
|
| (304 | ) |
|
| — |
|
|
| (304 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
Defined benefit plans, net: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
|
| (17 | ) |
|
| 5 |
|
|
| (12 | ) |
|
| (11 | ) |
|
| 1 |
|
|
| (10 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
Prior service credit |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 24 |
|
|
| (4 | ) |
|
| 20 |
|
|
| — |
|
|
| — |
|
|
| — |
|
Effect of foreign exchange rates |
|
| 15 |
|
|
| (3 | ) |
|
| 12 |
|
|
| 33 |
|
|
| (8 | ) |
|
| 25 |
|
|
| — |
|
|
| — |
|
|
| — |
|
Reclassifications to net income 1: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of prior service cost |
|
| (1 | ) |
|
| — |
|
|
| (1 | ) |
|
| 4 |
|
|
| — |
|
|
| 4 |
|
|
| — |
|
|
| — |
|
|
| — |
|
Amortization of loss |
|
| 23 | �� |
|
| (6 | ) |
|
| 17 |
|
|
| 16 |
|
|
| (3 | ) |
|
| 13 |
|
|
| — |
|
|
| — |
|
|
| — |
|
Settlements |
|
| 5 |
|
|
| (1 | ) |
|
| 4 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Curtailment gain |
|
| (2 | ) |
|
| — |
|
|
| (2 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Defined benefit plans, net |
|
| 23 |
|
|
| (5 | ) |
|
| 18 |
|
|
| 66 |
|
|
| (14 | ) |
|
| 52 |
|
|
| — |
|
|
| — |
|
|
| — |
|
Other comprehensive loss |
|
| (36 | ) |
|
| (5 | ) |
|
| (41 | ) |
|
| (230 | ) |
|
| (14 | ) |
|
| (244 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
Comprehensive (loss) income |
|
| (47 | ) |
|
| 13 |
|
|
| (34 | ) |
|
| (418 | ) |
|
| 84 |
|
|
| (334 | ) |
|
| 550 |
|
|
| (149 | ) |
|
| 401 |
|
Less: Comprehensive income attributable to noncontrolling interests |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 1 |
|
|
| — |
|
|
| 1 |
|
Comprehensive (loss) income attributable to Chemours |
| $ | (47 | ) |
| $ | 13 |
|
| $ | (34 | ) |
| $ | (418 | ) |
| $ | 84 |
|
| $ | (334 | ) |
| $ | 549 |
|
| $ | (149 | ) |
| $ | 400 |
|
1 | These other comprehensive income (loss) components are included in the computation of net periodic benefit costs. Refer to Note 22 for further information. |
See accompanying notes to the consolidated financial statements.
F-5
(Dollars in millions, except per share amount)
|
| December 31, 2016 |
|
| December 31, 2015 |
| ||
Assets |
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
| $ | 902 |
|
| $ | 366 |
|
Accounts and notes receivable - trade, net |
|
| 807 |
|
|
| 859 |
|
Inventories |
|
| 767 |
|
|
| 972 |
|
Prepaid expenses and other |
|
| 77 |
|
|
| 104 |
|
Total current assets |
|
| 2,553 |
|
|
| 2,301 |
|
Property, plant and equipment |
|
| 7,997 |
|
|
| 9,015 |
|
Less: Accumulated depreciation |
|
| (5,213 | ) |
|
| (5,838 | ) |
Net property, plant and equipment |
|
| 2,784 |
|
|
| 3,177 |
|
Goodwill and other intangible assets, net |
|
| 170 |
|
|
| 176 |
|
Investments in affiliates |
|
| 136 |
|
|
| 136 |
|
Other assets |
|
| 417 |
|
|
| 508 |
|
Total assets |
| $ | 6,060 |
|
| $ | 6,298 |
|
Liabilities and equity |
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
| $ | 884 |
|
| $ | 973 |
|
Short-term borrowings and current maturities of long-term debt |
|
| 15 |
|
|
| 39 |
|
Other accrued liabilities |
|
| 872 |
|
|
| 454 |
|
Total current liabilities |
|
| 1,771 |
|
|
| 1,466 |
|
Long-term debt, net |
|
| 3,529 |
|
|
| 3,915 |
|
Deferred income taxes |
|
| 132 |
|
|
| 234 |
|
Other liabilities |
|
| 524 |
|
|
| 553 |
|
Total liabilities |
|
| 5,956 |
|
|
| 6,168 |
|
Commitments and contingent liabilities |
|
|
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
|
|
Common stock (par value $0.01 per share; 810,000,000 shares authorized; shares issued and outstanding at December 31, 2016: 182,600,533 and 2015: 181,069,751) |
|
| 2 |
|
|
| 2 |
|
Additional paid-in capital |
|
| 789 |
|
|
| 775 |
|
Accumulated deficit |
|
| (114 | ) |
|
| (115 | ) |
Accumulated other comprehensive loss |
|
| (577 | ) |
|
| (536 | ) |
Total Chemours stockholders’ equity |
|
| 100 |
|
|
| 126 |
|
Noncontrolling interests |
|
| 4 |
|
|
| 4 |
|
Total equity |
|
| 104 |
|
|
| 130 |
|
Total liabilities and equity |
| $ | 6,060 |
|
| $ | 6,298 |
|
See accompanying notes to the consolidated financial statements.
F-6
Consolidated Statements of Stockholders’ Equity
Years ended December 31, 2016, 2015 and 2014
(Dollars in millions)
|
| Common Stock |
|
| DuPont Company Net |
|
| Additional Paid-In |
|
| Accumulated Other Comprehensive |
|
| Noncontrolling |
|
| Accumulated |
|
|
|
|
| ||||||||||
|
| Shares |
|
| Amount |
|
| Investment |
|
| Capital |
|
| Income (Loss) |
|
| Interests |
|
| Deficit |
|
| Total |
| ||||||||
Balance at December 31, 2013 |
|
| — |
|
| $ | — |
|
| $ | 3,195 |
|
| $ | — |
|
| $ | 19 |
|
| $ | 3 |
|
| $ | — |
|
| $ | 3,217 |
|
Net income |
|
| — |
|
|
| — |
|
|
| 400 |
|
|
| — |
|
|
| — |
|
|
| 1 |
|
|
| — |
|
|
| 401 |
|
Net transfers from DuPont |
|
| — |
|
|
| — |
|
|
| 55 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 55 |
|
Balance at December 31, 2014 |
|
| — |
|
|
| — |
|
|
| 3,650 |
|
|
| — |
|
|
| 19 |
|
|
| 4 |
|
|
| — |
|
|
| 3,673 |
|
Net income (loss) |
|
| — |
|
|
| — |
|
|
| 25 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (115 | ) |
|
| (90 | ) |
Other comprehensive loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (244 | ) |
|
| — |
|
|
| — |
|
|
| (244 | ) |
Issuance of common stock at separation |
|
| 180,966,833 |
|
|
| 2 |
|
|
| — |
|
|
| (2 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Common stock issued - compensation plans |
|
| 102,918 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Establishment of pension plans, net and related accumulated other comprehensive income (loss) |
|
| — |
|
|
| — |
|
|
| 268 |
|
|
| — |
|
|
| (311 | ) |
|
| — |
|
|
| — |
|
|
| (43 | ) |
Dividends |
|
| — |
|
|
| — |
|
|
| (100 | ) |
|
| (5 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (105 | ) |
Non-cash debt exchange |
|
| — |
|
|
| — |
|
|
| (507 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (507 | ) |
Cash provided at separation by DuPont |
|
| — |
|
|
| — |
|
|
| 247 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 247 |
|
Net transfers to DuPont |
|
| — |
|
|
| — |
|
|
| (3,583 | ) |
|
| 769 |
|
|
| — |
|
|
| — |
|
|
|
|
|
|
| (2,814 | ) |
Stock-based compensation expense |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 13 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 13 |
|
Balance at December 31, 2015 |
|
| 181,069,751 |
|
|
| 2 |
|
|
| — |
|
|
| 775 |
|
|
| (536 | ) |
|
| 4 |
|
|
| (115 | ) |
|
| 130 |
|
Net income |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 7 |
|
|
| 7 |
|
Common stock issued - compensation plans |
|
| 583,859 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Dividends |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (16 | ) |
|
| — |
|
|
| — |
|
|
| (6 | ) |
|
| (22 | ) |
Other comprehensive loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (41 | ) |
|
| — |
|
|
| — |
|
|
| (41 | ) |
Stock-based compensation expense |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 19 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 19 |
|
Exercise of stock options |
|
| 946,923 |
|
|
| — |
|
|
| — |
|
|
| 11 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 11 |
|
Balance at December 31, 2016 |
|
| 182,600,533 |
|
| $ | 2 |
|
| $ | — |
|
| $ | 789 |
|
| $ | (577 | ) |
| $ | 4 |
|
| $ | (114 | ) |
| $ | 104 |
|
See accompanying notes to the consolidated financial statements.
F-7
Consolidated Statements of Cash Flows
(Dollars in millions)
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) |
| $ | 7 |
|
| $ | (90 | ) |
| $ | 401 |
|
Adjustments to reconcile net income (loss) to cash provided by operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
| 284 |
|
|
| 267 |
|
|
| 257 |
|
Amortization of deferred financing costs and issuance discount |
|
| 20 |
|
|
| 8 |
|
|
| — |
|
Other operating charges and credits, net |
|
| 52 |
|
|
| 7 |
|
|
| 18 |
|
(Gain) loss on sale of assets and businesses |
|
| (254 | ) |
|
| 9 |
|
|
| (40 | ) |
Equity in earnings of affiliates, net of dividends received of $18, $23 and $19 |
|
| (12 | ) |
|
| — |
|
|
| 1 |
|
Deferred tax benefits |
|
| (111 | ) |
|
| (198 | ) |
|
| (22 | ) |
Asset related charges |
|
| 124 |
|
|
| 206 |
|
|
| — |
|
Decrease (increase) in operating assets: |
|
|
|
|
|
|
|
|
|
|
|
|
Accounts and notes receivable - trade, net |
|
| 5 |
|
|
| (64 | ) |
|
| 4 |
|
Inventories and other operating assets |
|
| 147 |
|
|
| 19 |
|
|
| (29 | ) |
Increase (decrease) in operating liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable and other operating liabilities |
|
| 332 |
|
|
| 18 |
|
|
| (85 | ) |
Cash provided by operating activities |
|
| 594 |
|
|
| 182 |
|
|
| 505 |
|
Investing activities |
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment |
|
| (338 | ) |
|
| (519 | ) |
|
| (604 | ) |
Proceeds from sales of assets, net |
|
| 708 |
|
|
| 12 |
|
|
| 32 |
|
Foreign exchange contract settlements |
|
| (12 | ) |
|
| 42 |
|
|
| — |
|
Investment in affiliates |
|
| (1 | ) |
|
| (32 | ) |
|
| (8 | ) |
Other investing activities |
|
| — |
|
|
| — |
|
|
| 20 |
|
Cash provided by (used for) investing activities |
|
| 357 |
|
|
| (497 | ) |
|
| (560 | ) |
Financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of debt, net |
|
| — |
|
|
| 3,491 |
|
|
| — |
|
Debt repayments |
|
| (381 | ) |
|
| (10 | ) |
|
| — |
|
Dividends paid |
|
| (22 | ) |
|
| (105 | ) |
|
| — |
|
Debt issuance costs |
|
| (4 | ) |
|
| (79 | ) |
|
| — |
|
Proceeds from exercised stock options |
|
| 11 |
|
|
| — |
|
|
| — |
|
Cash provided at separation by DuPont |
|
| — |
|
|
| 247 |
|
|
| — |
|
Net transfers (to) from DuPont |
|
| — |
|
|
| (2,857 | ) |
|
| 55 |
|
Cash (used for) provided by financing activities |
|
| (396 | ) |
|
| 687 |
|
|
| 55 |
|
Effect of exchange rate changes on cash |
|
| (19 | ) |
|
| (6 | ) |
|
| — |
|
Increase in cash and cash equivalents |
|
| 536 |
|
|
| 366 |
|
|
| — |
|
Cash and cash equivalents at beginning of year |
|
| 366 |
|
|
| — |
|
|
| — |
|
Cash and cash equivalents at end of year |
| $ | 902 |
|
| $ | 366 |
|
| $ | — |
|
SUPPLEMENTAL CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid during the year for: |
|
|
|
|
|
|
|
|
|
|
|
|
Interest, net of amounts capitalized |
| $ | 208 |
|
| $ | 103 |
|
| $ | — |
|
Income taxes, net of refunds |
| $ | 50 |
|
| $ | 53 |
|
| $ | — |
|
Non-cash change in property, plant and equipment included in accounts payable |
| $ | (12 | ) |
| $ | 45 |
|
| $ | (11 | ) |
See accompanying notes to the consolidated financial statements.
F-8
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Note 1. Background and Description of the Business
The Chemours Company (Chemours or the Company) delivers customized solutions with a wide range of industrial and specialty chemical products for markets including plastics and coatings, refrigeration and air conditioning, general industrial, mining and oil refining. Principal products include titanium dioxide (TiO2), refrigerants, industrial fluoropolymer resins, sodium cyanide and performance chemicals & intermediates. Chemours consists of three reportable segments: Titanium Technologies, Fluoroproducts and Chemical Solutions.
Chemours is globally operated with manufacturing facilities, sales centers, administrative offices and warehouses located throughout the world. Chemours’ operations are primarily located in the United States (U.S.), Canada, Mexico, Brazil, the Netherlands, Belgium, China, Taiwan, Japan, Switzerland, Singapore, Hong Kong, India and France. As of December 31, 2016, Chemours has 26 production facilities globally, five dedicated to Titanium Technologies, 18 dedicated to Fluoroproducts, two dedicated to Chemical Solutions and one that supports multiple Chemours segments.
Effective prior to the opening of trading on the New York Stock Exchange (NYSE) on July 1, 2015 (the Distribution Date), E. I. DuPont de Nemours and Company (DuPont) completed the previously announced separation of the businesses comprising DuPont’s Performance Chemicals reporting segment, and certain other assets and liabilities, into Chemours, a separate and distinct public company. The separation was completed by way of a distribution of all of the then-outstanding shares of common stock of Chemours through a dividend in kind of Chemours’ common stock (par value $0.01) to holders of DuPont common stock (par value $0.30) as of the close of business on June 23, 2015 (the Record Date) (the transaction referred to herein as the Distribution).
On the Distribution Date, each holder of DuPont’s common stock received one share of Chemours’ common stock for every five shares of DuPont’s common stock held on the Record Date. The separation was completed pursuant to a separation agreement and other agreements with DuPont, including an employee matters agreement, a tax matters agreement, a transition services agreement and an intellectual property cross-license agreement. These agreements govern the relationship between Chemours and DuPont following the separation and provided for the allocation of various assets, liabilities, rights and obligations. These agreements also include arrangements for transition services to be provided by DuPont to Chemours that were substantially completed during 2016.
Unless the context otherwise requires, references in these Notes to the Consolidated Financial Statements to “we”, “us”, “our”, “Chemours” and the “Company” refer to The Chemours Company and its consolidated subsidiaries after giving effect to the Distribution.
Note 2. Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the U.S. (GAAP). The notes that follow are an integral part of the consolidated financial statements.
Chemours did not operate as a separate, stand-alone entity for all periods included within these consolidated financial statements. Prior to the separation on July 1, 2015, Chemours operations were included in DuPont’s financial results in different legal forms, including but not limited to wholly-owned subsidiaries for which Chemours was the sole business, components of legal entities in which Chemours operated in conjunction with other DuPont businesses and a majority owned joint venture. For periods prior to July 1, 2015, the accompanying consolidated financial statements have been prepared from DuPont’s historical accounting records and are presented on a stand-alone basis as if the business operations had been conducted independently from DuPont. Prior to January 1, 2015, aside from a Japanese entity that is a dual-resident for U.S. federal income tax purposes, there was no direct ownership relationship among all the other various legal entities comprising Chemours. Prior to July 1, 2015, DuPont and its subsidiaries’ net investments in these operations is shown in lieu of Stockholders’ Equity in the consolidated financial statements. The consolidated financial statements include the historical operations, assets and liabilities of the legal entities that are considered to comprise the Chemours business, including certain environmental remediation and litigation obligations of DuPont and its subsidiaries that Chemours may be required to indemnify pursuant to the separation-related agreements executed prior to the spin-off.
All of the allocations and estimates in the consolidated financial statements prior to July 1, 2015 are based on assumptions that management believes are reasonable. Therefore, the results of operations and cash flows prior to July 1, 2015 included herein may not be indicative of the financial position, results of operations and cash flows of Chemours in the future or if Chemours had been a separate, stand-alone entity during the periods presented.
F-9
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
The net transfers from DuPont on the Consolidated Statements of Stockholders’ Equity include a non-cash contribution from DuPont of $109 for the year ended December 31, 2015. This non-cash contribution occurred during physical separation activities at shared production facilities in the U.S. prior to the separation and certain assets identified at separation. It was determined that assets previously managed by other DuPont businesses would be transferred to and managed by Chemours.
Comprehensive income as of December 31, 2016 includes an out of period adjustment of $31 million relating to 2015 cumulative translation adjustments with corresponding adjustment to other current assets. This adjustment is not material to the Company’s consolidated financial statements taken as a whole.
Note 3. Summary of Significant Accounting Policies
These consolidated financial statements have been prepared in accordance with GAAP. The significant accounting policies described below, together with the other notes that follow, are an integral part of the consolidated financial statements.
Preparation of Financial Statements
The preparation of the consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses, including allocations of costs as discussed above, during the reporting period. Management’s estimates are based on historical experience, facts and circumstances available at the time and various other assumptions that we believe are reasonable. Actual results could differ from those estimates.
Principles of Consolidation and Combination
The consolidated financial statements include the accounts Chemours and its subsidiaries, and entities in which a controlling interest is maintained. For those consolidated subsidiaries in which the Company’s ownership is less than 100%, the outside shareholders’ interests are shown as noncontrolling interests. Investments in companies in which Chemours, directly or indirectly, owns 20% to 50% of the voting stock or has the ability to exercise significant influence over operating and financial policies of the investee are accounting for using the equity method of accounting. As a result, Chemours’ share of the earnings or losses of such equity affiliates is included in the Consolidated Statements of Operations and Chemours’ share of such equity affiliates equity is included in the Consolidated Balance Sheets.
The financial statements for the periods prior to the separation on July 1, 2015 include the combined assets, liabilities, revenues, and expenses of Chemours. We eliminated all intercompany accounts and transactions in the preparation of the accompanying consolidated financial statements.
Revenue Recognition
Revenue is recognized when the earnings process is complete. Revenue for product sales is recognized when products are shipped to the customer in accordance with the terms of the agreement, when title and risk of loss have been transferred, collectability is reasonably assured and pricing is fixed or determinable. Revenue associated with advance payments are recorded as deferred revenue and are recognized as shipments are made and title, ownership and risk of loss pass to the customer. Accruals are made for sales returns and other allowances based on historical experience. Cash sales incentives are accounted for as a reduction in sales and noncash sales incentives are recorded as a charge to cost of goods sold at the time the revenue or selling expense, depending on the nature of the incentive, is recorded. Amounts billed to customers for shipping and handling fees are included in net sales and costs incurred by Chemours for the delivery of goods are classified as cost of goods sold in the Consolidated Statements of Operations. Taxes on revenue-producing transactions are excluded from net sales. Licensing and royalty income is recognized in accordance with agreed upon terms, when performance obligations are satisfied, the amount is fixed or determinable and collectability is reasonably assured.
Cash and Cash Equivalents
Cash and cash equivalents generally include cash, time deposits or highly liquid investments with original maturities of three months or less.
F-10
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Receivables and Allowance for Doubtful Accounts
Receivables are recognized net of an allowance for doubtful accounts. The allowance for doubtful accounts reflects the best estimate of losses inherent in Chemours’ accounts receivable portfolio determined on the basis of historical experience, specific allowances for known troubled accounts and other available evidence. Accounts receivable are written off when management determines that they are uncollectible.
Inventories
Chemours’ inventories are valued at the lower of cost or market. Inventories held at substantially all U.S. locations are valued using the last-in, first-out (LIFO) method. Inventories held outside the U.S. are determined by the average cost method. Elements of cost in inventories include raw materials, direct labor, and manufacturing overhead. Stores and supplies are valued at cost or market, whichever is lower; cost is generally determined by the average cost method. Approximately 48% and 54% of inventory is on a LIFO basis as of December 31, 2016 and 2015, respectively. The remainder is accounted for using the average cost method.
Property, Plant and Equipment
Property, plant and equipment is carried at cost and is depreciated using the straight-line method. Property, plant and equipment placed in service prior to 1995 is depreciated under the sum-of-the-years’ digits method or other substantially similar methods. Substantially all equipment and buildings are depreciated over useful lives ranging from 15 to 25 years. Capitalizable costs associated with computer software for internal use are amortized on a straight-line basis over five to seven years. When assets are surrendered, retired, sold or otherwise disposed of, their gross carrying values and related accumulated depreciation are removed from the balance sheet and included in determining gain or loss on such disposals.
Repair and maintenance costs that materially add to the value of the asset or prolong its useful life are capitalized and depreciated based on the extension to the useful life. Capitalized repair and maintenance costs are recorded on the Consolidated Balance Sheets in “Other assets”.
Goodwill and Other Intangible Assets
The excess of the purchase price over the estimated fair value of the net assets acquired, including identified intangibles, in a business combination is recorded as goodwill. Goodwill is tested for impairment annually on October 1; however, these tests are performed more frequently when events or changes in circumstances indicate that the asset may be impaired. Goodwill is evaluated for impairment at the reporting unit level, which is defined as one level below operating segment except for Titanium Technologies, which is both an operating segment and a reporting unit. A reporting unit is the level at which discrete financial information is available and reviewed by business management on a regular basis. The Company utilizes an income approach (or discounted cash flow method) and market approach to calculate the fair value of its reporting units.
Definite-lived intangible assets, such as purchased and licensed technology, patents, trademarks, and customer lists are amortized over their estimated useful lives, generally for periods ranging from five to 20 years. The reasonableness of the useful lives of these assets is continually evaluated.
Impairment of Long-Lived Assets
Chemours evaluates the carrying value of long-lived assets to be held and used when events or changes in circumstances indicate the carrying value may not be recoverable. For purposes of recognition or measurement of an impairment loss, the assessment is performed on the asset or asset group at the lowest level for which identifiable cash flows are largely independent of the cash flows of other groups of assets and liabilities. To determine the level at which the assessment is performed, Chemours considers factors such as revenue dependency, shared costs and the extent of vertical integration.
The carrying value of a long-lived asset is considered impaired when the total projected undiscounted cash flows from the use and eventual disposition of an asset or asset group are separately identifiable and are less than its carrying value. In that event, a loss is recognized based on the amount by which the carrying value exceeds the fair value of the long-lived asset. The fair value methodology used is an estimate of fair market value which is made based on prices of similar assets or other valuation methodologies including present value techniques. Long-lived assets to be disposed of other than by sale are classified as held for use until their
F-11
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
disposal. Long-lived assets to be disposed of by sale are classified as held for sale and are reported at the lower of carrying amount or fair market value less cost to sell. Depreciation is discontinued for long-lived assets classified as held for sale.
Research and Development
Research and development costs are expensed as incurred. Research and development expenses include costs (primarily consisting of employee costs, materials, contract services, research agreements, and other external spend) relating to the discovery and development of new products, enhancement of existing products and regulatory approval of new and existing products.
Environmental Liabilities and Expenditures
Chemours accrues for remediation activities when it is probable that a liability has been incurred and a reasonable estimate of the liability can be made. Where the available information is sufficient to estimate the amount of liability, that estimate has been used. Where the information is only sufficient to establish a range of probable liability and no point within the range is more likely than any other, the lower end of the range has been used. Estimated liabilities are determined based upon existing remediation laws and technologies. Inherent uncertainties exist in such evaluations primarily due to unknown environmental conditions, changing governmental regulations and legal standards regarding liability, and emerging remediation technologies. These accruals are adjusted periodically as remediation efforts progress and as additional technology, regulatory and legal information become available.
Environmental liabilities and expenditures included claims for matters that are liabilities of DuPont and its subsidiaries, that Chemours may be required to indemnify pursuant to the separation-related agreements executed prior to the separation. Accrued liabilities are undiscounted and do not include claims against third parties. These liabilities are included in “Other accrued liabilities” and “Other liabilities” in the Consolidated Balance Sheet.
Costs related to environmental remediation are charged to expense in the period incurred, in “Cost of goods sold” of the Consolidated Statement of Operations. Other environmental costs are also charged to expense in the period incurred, unless they increase the value of the property or reduce or prevent contamination from future operations, in which case they are capitalized and amortized.
Asset Retirement Obligations
Chemours records asset retirement obligations at fair value at the time the liability is incurred. Fair value is measured using expected future cash outflows discounted at Chemours’ credit-adjusted risk-free interest rate, which are considered level 3 inputs. Accretion expense is recognized as an operating expense classified within cost of goods sold on the Consolidated Income Statements using the credit-adjusted risk-free interest rate in effect when the liability was recognized. The associated asset retirement obligations are capitalized as part of the carrying amount of the long-lived asset and depreciated over the estimated remaining useful life of the asset, generally for periods ranging from two to 25 years.
Litigation
Chemours accrues for litigation matters when it is probable that a liability has been incurred and the amount of the liability can be reasonably estimated. Litigation liabilities and expenditures included in the consolidated financial statements represent litigation matters that are liabilities of DuPont and its subsidiaries, that Chemours may be required to indemnify pursuant to the separation-related agreements executed prior to the separation. Legal costs such as outside counsel fees and expenses are charged to expense in the period services are received.
Insurance
Chemours insures certain risks where permitted by law or regulation, including workers’ compensation, vehicle liability and employee related benefits. Liabilities associated with these risks are estimated in part by considering historical claims experience, demographic factors and other actuarial assumptions. For other risks, the Company uses a combination of insurance and self-insurance, reflecting comprehensive reviews of relevant risks. A receivable for an insurance recovery is generally recognized when the loss has occurred and collection is considered probable.
Prior to the separation, Chemours was a participant in DuPont’s self-insurance program where permitted by law or regulation, including workers’ compensation, vehicle liability and employee related benefits. Liabilities associated with these risks are estimated in part by considering historical claims experience, demographic factors, and other actuarial assumptions. For other risks, a
F-12
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
combination of insurance and self-insurance is used, reflecting comprehensive reviews of relevant risks. The annual cost was allocated to all of the participating businesses using methodologies deemed reasonable by management. All obligations pursuant to these plans have historically been obligations of DuPont. As such, these obligations were not included in the Consolidated Balance Sheets, with the exception of self-insurance liabilities related to workers compensation, vehicle liability and employee related benefits.
Defined Benefit Plans
We have defined benefit plans covering certain of our employees outside the U.S., which are generally required by local regulations. The benefits, which primarily relate to pension, are accrued over the employees’ service periods. We use actuarial methods and assumptions in the valuation of defined benefit obligations and the determination of net periodic pension income or expense. Differences between actual and expected results or changes in the value of defined benefit obligations and plan assets, if any, are not recognized in earnings as they occur but rather systematically over subsequent periods.
Stock-based Compensation
Chemours’ stock-based compensation consists of stock options, restricted share units (RSUs) and performance share units (PSUs) to employees and non-employee directors. Stock options are measured at fair value on the grant date or date of modification, as applicable. We recognize compensation expense on a straight-line basis over the requisite service period. The number of awards ultimately expected to vest is determined by use of an estimated forfeiture rate. The estimated forfeiture rate is based on historical data for the employee group awarded options and expected employee turnover rates, which management reevaluates each period.
Income Taxes
The provision for income taxes is determined using the asset and liability approach of accounting for income taxes. Under this approach, deferred taxes represent the future tax consequences expected to occur when the reported amounts of assets and liabilities are recovered or paid. The provision for income taxes represents income taxes paid or payable for the current year plus the change in deferred taxes during the year. Deferred taxes result from differences between the financial and tax basis of Chemours’ assets and liabilities and are adjusted for changes in tax rates and tax laws when changes are enacted. Valuation allowances are recorded to reduce deferred tax assets when it is more likely than not that a tax benefit will not be realized.
Chemours recognizes income tax positions that meet the more likely than not threshold and accrues interest related to unrecognized income tax positions, which is included in other income, net in our Consolidated Statements of Operations. Income tax related penalties are included in the provision for income taxes.
Chemours does not provide for income taxes on undistributed earnings of all foreign subsidiaries that are intended to be indefinitely reinvested.
Prior to separation, income taxes presented attributed current and deferred income taxes of DuPont to Chemours’ stand-alone financial statements in a manner that is systematic, rational, and consistent with the asset and liability method prescribed by ASC 740, Income Taxes (ASC 740). Accordingly, Chemours’ income tax provision was prepared following the separate return method. The separate return method applies ASC 740 to the stand-alone financial statements of each member of the consolidated group as if the group member were a separate taxpayer and a stand-alone enterprise.
Foreign Currency Translation
Chemours identifies its separate and distinct foreign entities and groups them into two categories: (1) extensions of the parent (U.S. dollar functional currency) and (2) self-contained (local functional currency). If a foreign entity does not align with either category, factors are evaluated and a judgment is made to determine the functional currency. Chemours changes the functional currency of its separate and distinct foreign entities only when significant changes in economic facts and circumstances indicate clearly that the functional currency has changed.
During the periods covered by the consolidated financial statements, part of the Chemours business operated within foreign entities. For foreign entities where the U.S. dollar is the functional currency, all foreign currency-denominated asset and liability amounts are remeasured into U.S. dollars at end-of-period exchange rates, except for inventories; prepaid expenses; property, plant and equipment; goodwill and other intangible assets, which are remeasured at historical rates. Foreign currency-denominated income and expenses are remeasured at average exchange rates in effect during the period, except for expenses related to balance sheet amounts remeasured
F-13
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
at historical exchange rates. Exchange gains and losses arising from remeasurement of foreign currency-denominated monetary assets and liabilities are included in other income, net in the period in which they occur.
For foreign entities where the local currency is the functional currency, assets and liabilities denominated in local currencies are translated into U.S. dollars at end-of-period exchange rates and the resulting translation adjustments are reported as a component of accumulated other comprehensive (loss) income in equity. Assets and liabilities denominated in other than the functional currency are remeasured into the functional currency prior to translation into U.S. dollars and the resulting exchange gains or losses are included in income in the period in which they occur. Income and expenses are translated into U.S. dollars at average exchange rates in effect during the period.
Beginning in 2015, when the Chemours operations were legally and operationally separated within DuPont in anticipation of the separation, certain of Chemours foreign entities set their local currency as the functional currency.
Derivatives
Chemours enters into forward currency exchange contracts to minimize volatility in earnings related to the foreign exchange gains and losses resulting from remeasuring net monetary assets that Chemours holds which are denominated in non-functional currencies. Chemours does not hold or issue financial instruments for speculative or trading purposes. The derivative assets and liabilities are reported on a gross basis in the Consolidated Balance Sheets. All gains and losses resulting from the revaluation of the derivative assets and liabilities are recognized in other income, net in the Consolidated Statements of Operations during the period in which they occurred. Please refer to Note 21 for additional information.
Fair Value Measurement
Under the accounting for fair value measurements and disclosures, a fair value hierarchy was established that prioritizes the inputs to valuation techniques used to measure fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets and liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
Chemours uses the following valuation techniques to measure fair value for its assets and liabilities:
(a) Level 1—Quoted market prices in active markets for identical assets and liabilities;
(b) Level 2—Significant other observable inputs (e.g. quoted prices for similar items in active markets, quoted prices for identical or similar items in markets that are not active, inputs other than quoted prices that are observable, such as interest rate and yield curves, and market-corroborated inputs); and
(c) Level 3—Unobservable inputs for the asset or liability, which are valued based on management’s estimates of assumptions that market participants would use in pricing the asset or liability.
Recent Accounting Pronouncements
Accounting Guidance Issued Not Yet Adopted
In May 2014, the Financial Accounting Standards Board (FASB) issued ASU No. 2014-09, "Revenue from Contracts with Customers (Topic 606)." The objective of this standard update is to remove inconsistent practices with regard to revenue recognition between US GAAP and IFRS. The standard intends to improve comparability of revenue recognition practices across entities, industries, jurisdictions and capital markets. The provisions of ASU No. 2014-09 will be effective for interim and annual periods beginning after December 15, 2017, with early adoption permitted for annual periods beginning after December 15, 2016. The Company plans to adopt ASU 2014-09 as of January 1, 2018. Subsequent to the issuance of ASU No. 2014-09, the FASB has issued multiple updates in connection with Topic 606. These updates affect the guidance contained within ASU 2014-09 and will be assessed as part of the Company's revenue recognition project plan.
F-14
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
The Company’s project plan includes a three-phase approach to implementing this standard update. Phase one, the assessment phase, is currently in process and is expected to be completed in the second quarter of 2017. In connection with this initial phase, the Company is currently performing the following activities: conducting internal surveys of its businesses to initially identify a set of applicable revenue recognition changes related to the new standard update, holding revenue recognition workshops with sales and business unit finance leadership highlighting the impacts of the new standard update, and reviewing a representative sample of revenue arrangements across all businesses to assess and validate the impact of the new guidance. Once completed, the Company will pursue the second phase of the project, where the objectives will be to establish and document key accounting policies, assess disclosure, business process and control impacts, and determine an initial quantitative impact resulting from the new standard update. Lastly, phase three’s objectives will comprise of effectively implementing the new standard update and embedding the new accounting treatment into the Company’s business processes and controls to support the financial reporting requirements.
The Company is still evaluating the impact that the new standard update will have on the Company’s consolidated financial statements and will be unable to quantify its impact until the third phase of the project has been completed. The method of adoption has not yet been determined and is also not expected to be finalized until the third phase of the project plan has been completed.
In January 2017, the FASB issued ASU No. 2017-04, “Intangibles - Goodwill and Other (Topic 350): Simplifying the Test for Goodwill Impairment”, which eliminates the requirement to determine the fair value of individual assets and liabilities of a reporting unit to measure goodwill impairment. Under the amendments, goodwill impairment testing will be performed by comparing the fair value of the reporting unit with its carrying amount and recognizing an impairment charge for the amount by which the carrying amount exceeds the reporting unit’s fair value, but not to exceed the total amount of goodwill allocated to the reporting unit. The guidance is effective for annual and interim goodwill impairment tests in fiscal years beginning after December 15, 2019, and should be applied on a prospective basis. Early adoption is permitted for annual or interim goodwill impairment testing performed after January 1, 2017. The Company plans to adopt this guidance in 2017.
In August 2016, the FASB issued various updates to the Accounting Standards Update (ASU) 2016-15, “Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments”, which clarifies and amends certain cash receipts and cash payments presentation and classification in the statement of cash flows. The guidance is effective for public business entities for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. The amendments should be applied using a retrospective transition method (unless impractical to do so) to each period presented and earlier application is permitted. Chemours is currently evaluating the impact of adopting this guidance but does not expect the adoption will have a significant impact on its cash flows.
In February 2016, the FASB issued ASU No. 2016-02, “Leases (Topic 842)”, which supersedes the leases requirements in Topic 840. The core principle of Topic 842 is that a lessee should recognize the assets and liabilities that arise from leases in accordance with FASB Concepts Statement No. 6, Elements of Financial Statements, and, therefore, recognition of those lease assets and lease liabilities represents an improvement over previous GAAP, which did not require lease assets and lease liabilities to be recognized for most leases. A qualitative disclosure along with specific quantitative disclosures is required to provide enough information to supplement the amounts recorded in the financial statements so that users can understand more about the nature of an entity’s leasing activities. Lessees and lessors are required to recognize and measure leases at the beginning of the earliest period presented using a modified retrospective approach, which includes a number of optional practical expedients that entities may elect to apply. The amendments in this update are effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. Early application of the amendments in this update is permitted for all entities. Chemours is currently evaluating the impact of adopting this guidance on its financial position, results of operations and debt covenants.
Recently Adopted Accounting Guidance
In March 2016, the FASB issued ASU No. 2016-09, “Compensation - Stock Compensation (Topic 718)”. The update sets forth areas for simplification within several aspects of the accounting for shared-based payment transactions, including the income tax consequences, classification of awards as either equity or liabilities, and classification on the statement of cash flows. The amendments in this update are effective for fiscal periods, and interim periods within those fiscal years, beginning after December 15, 2016. Chemours adopted this guidance effective January 1, 2017 and the adoption did not have a significant impact on the Company’s financial position, results of operations and of cash flows.
In April 2015, the FASB issued ASU No. 2015-05, “Customer’s Accounting for Fees Paid in a Cloud Computing Arrangement”, which provides guidance about whether a cloud computing arrangement includes a software license. The customer should account for the software license element of the arrangement consistent with the acquisition of other software licenses. If the cloud computing
F-15
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
arrangement does not include a software license, the customer should account for the arrangement as a service contract. This guidance is effective for annual periods, including interim periods within those annual periods, beginning after December 15, 2015, and early adoption is permitted. Chemours adopted this guidance effective January 1, 2016 prospectively to all arrangements entered into or materially modified after the effective date. The adoption did not have a significant impact on our financial position or results of operations.
In February 2015, the FASB issued ASU No. 2015-02, “Consolidation (Topic 810): Amendments to the Consolidation Analysis”. The amendments modify the evaluation of whether limited partnerships and similar legal entities are variable interest entities (VIEs) or voting interest entities and eliminate the presumption that a general partner should consolidate a limited partnership. The amendment is effective for public business entities for fiscal years, and for interim periods within those fiscal years, beginning after December 15, 2015. Chemours adopted this guidance effective January 1, 2016 and the adoption did not change our consolidated entities, and therefore had no impact on the Company’s financial position, results of operations and cash flows.
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements – Going Concern”. The update requires management to evaluate whether there is substantial doubt about a company’s ability to continue as a going concern and to provide related footnote disclosures. The update is effective for the annual period ending after December 15, 2016, and for annual periods and interim periods thereafter. Early adoption is permitted. Chemours adopted the guidance effective December 31, 2016. No disclosure was considered necessary as of December 31, 2016 as a result of management’s evaluation.
Note 4. Relationship with DuPont and Related Entities
Prior to the separation, Chemours sold finished goods to DuPont and its non-Chemours businesses. Related party sales to DuPont recorded by Titanium Technologies, Fluoroproducts and Chemical Solutions for the year ended December 31, 2015 were $2, $34 and $21, respectively, and for the year ended December 31, 2014 were $0, $45 and $65, respectively. Subsequent to the separation, beginning on July 1, 2015, transactions with DuPont businesses were not considered related party transactions.
Also prior to the separation, DuPont incurred significant corporate costs for services provided to Chemours as well as other DuPont businesses. These costs included expenses for information systems, accounting, other financial services such as treasury and audit, purchasing, human resources, legal, facilities, engineering, corporate research and development, corporate stewardship, marketing and business analysis support. A portion of these costs benefited multiple or all DuPont businesses, including Chemours, and were allocated to Chemours and its reportable segments using methods based on proportionate formulas involving total costs or other various allocation methods that management considered consistent and reasonable. Other Chemours corporate costs are not allocated to the reportable segments and are reported in Corporate and Other.
The allocated leveraged functional service expenses and general corporate expenses included in the Consolidated Statements of Operations were $238 and $492 for the years December 31, 2015 and 2014, respectively, and were recorded within cost of goods sold, selling, general and administrative expense and research and development expense for $23, $205 and $10, respectively, for the year ended December 31, 2015, and $32, $411 and $49, respectively, for the year ended December 31, 2014. Subsequent to the separation on July 1, 2015, transactions with DuPont businesses were not considered related party transactions. Accordingly, no costs from DuPont were allocated to Chemours after July 1, 2015.
Cash Management and Financing
The separation agreement sets forth a process to true-up cash and working capital transferred to us from DuPont at separation. In January 2016, Chemours and DuPont entered into an agreement, contingent upon the credit agreement amendment (described in Note 19), which provided for the extinguishment of payment obligations of cash and working capital true-ups previously contemplated in the separation agreement. As a result, Chemours is no longer required to make any payments to DuPont, nor will DuPont make any payments to Chemours.
In addition, the agreement set forth an advance payment by DuPont of approximately $190, which Chemours received in February 2016, for certain specified goods and services that Chemours expects to provide to DuPont through mid-2017 under existing agreements with Chemours. The advance payment was recorded as deferred liability included in other accrued liabilities of the Consolidated Balance Sheets and approximately $58 remains outstanding as of December 31, 2016.
F-16
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
The tax matters agreement that Chemours and DuPont entered into governs the parties’ respective rights, responsibilities and obligations with respect to tax liabilities and benefits, tax attributes, the preparation and filing of tax returns, the control of audits and other tax proceedings and other matters regarding taxes. In general, under the agreement, DuPont is responsible for any U.S. federal, state and local taxes (and any related interest, penalties or audit adjustments) reportable on a consolidated, combined or unitary return that includes DuPont or any of its subsidiaries and Chemours and/or any of its subsidiaries for any periods or portions thereof ending on or prior to the date of the separation and Chemours is responsible for any U.S. federal, state, local and foreign taxes (and any related interest, penalties or audit adjustments) that are imposed on Chemours and/or any of its subsidiaries for all tax periods, whether before or after the date of the separation.
Note 5. Research and Development Expense
Research and development expense directly incurred by Chemours was $80, $87 and $94 for the years ended December 31, 2016, 2015 and 2014, respectively. Research and development expense for the years ended December 31, 2015 and 2014 includes $10 and $49, respectively, which represents an assignment of costs associated primarily with DuPont’s Corporate Central Research and Development long-term research activities. This assignment was based on the cost of research projects for which Chemours was determined to be the sponsor or co-sponsor. All research services previously provided by DuPont’s Central Research and Development to Chemours were specifically requested by Chemours, covered by service-level agreements and billed based on usage. DuPont research and development services were no longer used after the separation on July 1, 2015.
Note 6. Restructuring and Asset Related Charges, Net
For the years ended December 31, 2016, 2015 and 2014, Chemours recorded charges for employee separation and asset related charges as follows:
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Restructuring Related Charges: |
|
|
|
|
|
|
|
|
|
|
|
|
Employee Separation Charges |
| $ | 4 |
|
| $ | 137 |
|
| $ | 18 |
|
Decommissioning and other charges, net - Restructuring |
|
| 47 |
|
|
| 18 |
|
|
| — |
|
Asset Related Charges - Restructuring |
|
| — |
|
|
| 133 |
|
|
| 3 |
|
Total restructuring charges, net |
|
| 51 |
|
|
| 288 |
|
|
| 21 |
|
Asset Related Charges - Impairment 1 |
|
| 119 |
|
|
| 45 |
|
|
| — |
|
Total restructuring and asset related charges, net |
| $ | 170 |
|
| $ | 333 |
|
| $ | 21 |
|
| 1 | Impairment charges for the year ended December 31, 2016 include $48 of impairment charges related to the aniline facility in Pascagoula, Mississippi (see Note 13 for further information), $58 of impairment charges in connection with the sale of the Sulfur business (see Note 7 for further information), and $13 of impairment charges in connection with the sale of the Company’s corporate headquarters building (see Note 15 for further information). Impairment charges for the year ended December 31, 2015 represent asset impairment related to the reactive metals manufacturing facility (see Note 13 for further information). |
F-17
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
The charges related to the restructuring programs impacted segment earnings for the years ended December 31, 2016, 2015 and 2014 are as follows:
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Plant and product line closures 1 |
|
|
|
|
|
|
|
|
|
|
|
|
Titanium Technologies |
| $ | 30 |
|
| $ | 140 |
|
| $ | — |
|
Fluoroproducts |
|
| 7 |
|
|
| 24 |
|
|
| — |
|
Chemical Solutions |
|
| 8 |
|
|
| 12 |
|
|
| — |
|
Sub-total |
|
| 45 |
|
|
| 176 |
|
|
| — |
|
Global restructuring 2 |
|
|
|
|
|
|
|
|
|
|
|
|
Titanium Technologies |
|
| 2 |
|
|
| 33 |
|
|
| 3 |
|
Fluoroproducts |
|
| 4 |
|
|
| 54 |
|
|
| 16 |
|
Chemical Solutions |
|
| — |
|
|
| 25 |
|
|
| — |
|
Sub-total |
|
| 6 |
|
|
| 112 |
|
|
| 19 |
|
Total |
| $ | 51 |
|
| $ | 288 |
|
| $ | 19 |
|
| 1 | Includes charges related to employee separation, decommissioning and dismantling costs, and asset related charges in connection with the restructuring activities. |
| 2 | Includes approximately $24 related to corporate overhead functions that was allocated to the segments for the year ended December 31, 2015. |
Plant and product line closures
Titanium Technologies Plant and Product Line Closures: In August 2015, the Company announced the closure of its Edge Moor, Delaware manufacturing site located in the U.S. The Edge Moor plant produced TiO2 product for use in the paper industry and other applications where demand had steadily declined, resulting in underused capacity at the plant. In addition, the Company permanently shut down one underused TiO2 production line at its New Johnsonville, Tennessee plant. The Company stopped production at Edge Moor in September 2015 and immediately began decommissioning the plant. These actions resulted in the write-off of substantially all of the Edge Moor plant asset carrying value in 2015.
As a result, for the year ended December 31, 2015, the Company recorded charges of approximately $140, which consisted of employee separation costs of $11, property, plant and equipment and other asset impairment charges of $115, and decommission costs and other charges of $14. For the year ended December 31, 2016, the Company recorded additional charges of approximately $30, which relates to decommissioning, dismantling and removal activities. The Company substantially completed the dismantling and removal activities in January 2017.
Fluoroproducts Restructuring: Also, in August 2015, in an effort to improve the profitability of the Fluoroproducts segment, management approved the shutdown of certain production lines of the segment’s manufacturing facilities in the U.S. As a result, for the year ended December 31, 2015, the Company recorded restructuring charges of approximately $21, which consist of property, plant and equipment accelerated depreciation of $18, employee separation costs of $2, and decommissioning and other costs of $1. For the year ended December 31, 2016, the Company recorded additional charges of approximately $7, which relates to decommissioning, dismantling and removal activities. The Company expects to incur additional charges of approximately $3 for decommissioning, dismantling and removal costs in 2017, which will be expensed as incurred.
RMS Plant Closure: In the fourth quarter of 2015, the Company announced the completion of the strategic review of its RMS business and the decision to stop production at the Niagara Falls, New York site. The Niagara Falls plant has approximately 200 employees and contractors impacted by this action. The production stopped in September 2016 and the Company immediately began decommissioning the plant.
As a result, for the year ended December 31, 2015, the Company recorded approximately $12 of employee separation costs. For the year ended December 31, 2016, the Company recorded approximately $8, which consist of contract termination charges of $2 and decommissioning and other related charges of $6. Additional restructuring charges of approximately $9 for decommissioning and site redevelopment are expected to be incurred in 2017. Impairment of RMS related assets were recorded in the third quarter of 2015 (see Note 13 for further information).
F-18
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
In November 2015, Chemours announced an additional global workforce reduction of approximately 430 positions. This action is part of ongoing efforts to streamline and simplify the structure of the organization worldwide and to reduce costs. As a result of these actions, the Company recorded approximately $48 of employee separation costs during the fourth quarter of 2015. The associated headcount reductions were completed as of December 31, 2016, and all related payments are expected to be completed in 2017.
In June 2015, in light of continued weakness in the global titanium dioxide market cycle and continued foreign currency impacts due to the strengthening of the U.S. dollar, Chemours implemented a restructuring plan to reduce and simplify its cost structure. This plan resulted in a global workforce reduction of more than 430 positions. As a result, we recorded a pre-tax charge of $64 for employee separation costs in the year ended December 31, 2015. All actions associated with this charge were completed as of December 31, 2016.
In 2014, Chemours implemented a restructuring plan to increase productivity and recorded a pre-tax charge of $19 related to this initiative. The charge consisted of $16 related to employee separation costs and $3 for asset shut-down costs. All actions associated with this charge were completed as of December 31, 2015.
The following table shows the change in the employee separation related liability account associated with the restructuring programs:
|
| Titanium Technologies Site Closures |
|
| Fluoroproducts Lines Shutdown |
|
| Chemical Solutions Site Closures |
|
| 2015 Global Restructuring |
|
| Total |
| |||||
Year ended December 31, 2014 |
| $ | — |
|
| $ | — |
|
| $ | — |
|
| $ | — |
|
| $ | — |
|
Charges to income for the year ended December 31, 2015 1 |
|
| 11 |
|
|
| 2 |
|
|
| 12 |
|
|
| 112 |
|
|
| 137 |
|
Charges to liability accounts: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (39 | ) |
|
| (39 | ) |
Net currency translation and other adjustment 2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| — |
|
Year ended December 31, 2015 |
| $ | 11 |
|
| $ | 2 |
|
| $ | 12 |
|
| $ | 73 |
|
| $ | 98 |
|
Charges (credits) to income for the year ended December 31, 2016 1 |
|
| — |
|
|
| — |
|
|
| (2 | ) |
|
| 6 |
|
|
| 4 |
|
Charges to liability accounts: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments |
|
| (7 | ) |
|
| (1 | ) |
|
| (1 | ) |
|
| (59 | ) |
|
| (68 | ) |
Net currency translation and other adjustment 2 |
|
| — |
|
|
| — |
|
|
| (1 | ) |
|
| 1 |
|
|
| — |
|
Balance as of December 31, 2016 |
| $ | 4 |
|
| $ | 1 |
|
| $ | 8 |
|
| $ | 21 |
|
| $ | 34 |
|
1 | Due to unexpected resignations of certain employees at the Company’s Niagara site during 2016, approximately $2 of employee separation charges, related to the RMS plant closure, were reversed to income during the year ended December 31, 2016. |
2 | Amounts include net currency translation adjustment of less than $1 for the period presented and rounding differences. |
There are no significant outstanding liabilities related to the decommissioning and other restructuring related charges.
Note 7. Sales of Assets and Businesses
On June 13, 2016, the Company entered into an asset purchase agreement with Veolia North America (“Veolia”), pursuant to which Veolia agreed to acquire the Sulfur business of Chemours’ Chemical Solutions segment for a purchase price of $325 in cash, subject to customary working capital and other adjustments, of which approximately $10 was received in May 2016. The Company completed the sale and received the remaining proceeds of approximately $311 on July 29, 2016, net of estimated working capital adjustments. Prior to the completion of the sale, in the second quarter of 2016, the Company recorded an impairment loss of approximately $58 in “Restructuring and asset related charges, net” of the Consolidated Statement of Operations. When the sale was completed, the Company also recorded an additional pre-tax loss on sale of approximately $4, net of a benefit from contract adjustments, in “Other income, net” in the Consolidated Statement of Operations. The net book value of the assets and liabilities disposed were $342 and $11, respectively.
F-19
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
On April 22, 2016, the Company entered into a stock and asset purchase agreement with LANXESS Corporation, (“Lanxess”), pursuant to which Lanxess agreed to acquire the C&D business of Chemours’ Chemical Solutions segment by acquiring certain Chemours’ subsidiaries and assets for a purchase price of $230 in cash, subject to customary working capital and other adjustments. The Company completed the sale and received proceeds of $223 on August 31, 2016, net of working capital adjustments and approximately $2 of cash transferred. As a result, for the year ended December 31, 2016, the Company recorded a pre-tax gain of approximately $169 in “Other income, net” in the Consolidated Statement of Operations. The net book values of the assets and liabilities disposed were $48 (including goodwill of $13) and $6, respectively, and the Company incurred approximately $9 of transaction and other related charges.
In November 2015, the Company signed a definitive agreement to sell its aniline facility in Beaumont, Texas to The Dow Chemical Company (“Dow”), and the net book value of the related asset group (including goodwill) was classified as assets held-for-sale at December 31, 2015 included in “Prepaid expenses and other” of the Consolidated Balance Sheet. The transaction closed on March 1, 2016 and Chemours received $140 in cash from Dow. The net book value of the assets disposed was $41 (including goodwill of $4), and the Company incurred approximately $11 of transaction and other related charges. As a result of this transaction, for the year ended December 31, 2016, Chemours recognized a pre-tax gain in the Chemical Solutions segment of approximately $88 recorded in “Other income, net” in the Consolidated Statement of Operations.
The aggregate amount and major components of assets and liabilities disposed during the year ended December 31, 2016 are as follows:
|
| December 31, 2016 |
| |
Current assets: |
|
|
|
|
Accounts receivables |
| $ | 22 |
|
Inventories |
|
| 17 |
|
Total current assets |
|
| 39 |
|
Property, plant and equipment, net |
|
| 298 |
|
Goodwill |
|
| 17 |
|
Other assets |
|
| 136 |
|
Less: Impairment loss |
|
| (58 | ) |
Total non-current assets, net |
|
| 393 |
|
Total assets |
|
| 432 |
|
Accounts payable and accrued liabilities |
|
| 17 |
|
Total liabilities |
|
| 17 |
|
Net assets disposed |
| $ | 415 |
|
Note 8. Other Income (Expense), Net
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Leasing, contract services and miscellaneous income 1 |
| $ | 35 |
|
| $ | 25 |
|
| $ | 17 |
|
Royalty income 2 |
|
| 15 |
|
|
| 19 |
|
|
| 28 |
|
Gain (loss) on sale of assets and businesses 3 |
|
| 254 |
|
|
| (9 | ) |
|
| 40 |
|
Exchange (losses) gains, net 4 |
|
| (57 | ) |
|
| 19 |
|
|
| (66 | ) |
Total other income, net |
| $ | 247 |
|
| $ | 54 |
|
| $ | 19 |
|
| 1 | Miscellaneous income includes accrued interest related to unrecognized tax benefits. |
| 2 | Royalty income is primarily for technology and trademark licensing. |
| 3 | The twelve months ended December 31, 2016 includes a gain on sale of C&D business and aniline factory; and a loss on sale of Sulfur business. See Note 7 for further details. |
| 4 | Exchange losses, net includes gains and losses on foreign currency forward contracts. See Note 21 for additional information. |
F-20
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Current tax expense: |
|
|
|
|
|
|
|
|
|
|
|
|
U.S. federal |
| $ | — |
|
| $ | 37 |
| 1 | $ | 85 |
|
U.S. state and local |
|
| — |
|
|
| 1 |
| 1 |
| 13 |
|
International |
|
| 93 |
|
|
| 62 |
|
|
| 73 |
|
Total current tax expense |
|
| 93 |
|
|
| 100 |
|
|
| 171 |
|
Deferred tax (benefit) expense: |
|
|
|
|
|
|
|
|
|
|
|
|
U.S. federal |
|
| (101 | ) |
|
| (187 | ) |
|
| (20 | ) |
U.S. state and local |
|
| (17 | ) |
|
| (14 | ) |
|
| (3 | ) |
International |
|
| 7 |
|
|
| 3 |
|
|
| 1 |
|
Total deferred tax benefit |
|
| (111 | ) |
|
| (198 | ) |
|
| (22 | ) |
Total (benefit from) provision for income taxes |
| $ | (18 | ) |
| $ | (98 | ) |
| $ | 149 |
|
| 1 | Recorded pursuant to the tax matters agreement. |
The significant components of deferred tax assets and liabilities are as follows:
(Dollars in millions) |
| December 31, 2016 |
|
| December 31, 2015 |
| ||
Deferred tax assets: |
|
|
|
|
|
|
|
|
Environmental and other reserves |
| $ | 150 |
|
| $ | 158 |
|
Litigation reserves |
|
| 149 |
|
|
| 22 |
|
Stock compensation and accrued employee benefits |
|
| 35 |
|
|
| 42 |
|
Other assets and other accrued liabilities |
|
| 27 |
|
|
| 35 |
|
Tax loss carryforwards |
|
| 95 |
|
|
| 124 |
|
Total deferred tax assets |
|
| 456 |
|
|
| 381 |
|
Valuation allowance |
|
| (50 | ) |
|
| — |
|
Total deferred tax assets, net |
|
| 406 |
|
|
| 381 |
|
Deferred tax liabilities: |
|
|
|
|
|
|
|
|
Pension and other liabilities |
|
| (16 | ) |
|
| (7 | ) |
Property, plant and equipment |
|
| (441 | ) |
|
| (530 | ) |
Inventories and other assets |
|
| (40 | ) |
|
| (31 | ) |
Total deferred tax liabilities |
|
| (497 | ) |
|
| (568 | ) |
Net deferred tax liability |
| $ | (91 | ) |
| $ | (187 | ) |
F-21
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
An analysis of the Company’s effective tax rate is as follows:
|
| Year Ended December 31, |
| |||||||||||||||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||||||||||||||
(Dollars in millions) |
| $ |
|
| % |
|
| $ |
|
| % |
|
| $ |
|
| % |
| ||||||
Statutory U.S. federal income tax rate |
| $ | (4 | ) |
|
| 35.0 | % |
| $ | (66 | ) |
|
| 35.0 | % |
| $ | 192 |
|
|
| 35.0 | % |
State income taxes, net of federal benefit |
|
| (16 | ) |
|
| 150.4 | % |
|
| (10 | ) |
|
| 5.1 | % |
|
| 6 |
|
|
| 1.0 | % |
Lower effective tax rate on international operations-net |
|
| (61 | ) |
|
| 552.5 | % |
|
| (23 | ) |
|
| 12.0 | % |
|
| (53 | ) |
|
| (9.6 | )% |
Depletion |
|
| (6 | ) |
|
| 51.2 | % |
|
| (6 | ) |
|
| 3.4 | % |
|
| (8 | ) |
|
| (1.5 | )% |
Goodwill |
|
| 5 |
|
|
| (47.9 | )% |
|
| 6 |
|
|
| (3.2 | )% |
|
| — |
|
|
| — | % |
Exchange losses (gains) |
|
| 4 |
|
|
| (39.1 | )% |
|
| (1 | ) |
|
| 0.5 | % |
|
| 15 |
|
|
| 2.7 | % |
Provision to return and other adjustments |
|
| 6 |
|
|
| (57.9 | )% |
|
| — |
|
|
| — | % |
|
| — |
|
|
| — | % |
Permanent items |
|
| 3 |
|
|
| (27.3 | )% |
|
| 1 |
|
|
| (0.5 | )% |
|
| (5 | ) |
|
| (0.9 | )% |
Valuation allowance |
|
| 50 |
|
|
| (451.6 | )% |
|
| — |
| 1 |
| — | % |
|
| 11 |
|
|
| 2.0 | % |
Section 199 domestic manufacturing deduction |
|
| — |
|
|
| (— | )% |
|
| — |
|
|
| — | % |
|
| (4 | ) |
|
| (0.7 | )% |
Other, net |
|
| 1 |
|
|
| (1.7 | )% |
|
| 1 |
|
|
| (0.2 | )% |
|
| (5 | ) |
|
| (0.9 | )% |
Total effective tax rate |
| $ | (18 | ) |
|
| 163.6 | % |
| $ | (98 | ) |
|
| 52.1 | % |
| $ | 149 |
|
|
| 27.1 | % |
| 1 | Release of the valuation allowance during 2015 was related to tax loss carryforward incurred prior to July 1, 2015 that is attributable to DuPont’s tax periods pursuant to the tax matters agreement and did not impact the effective tax rate as the adjustment was recorded in the “DuPont Company Net Investment” of the Consolidated Statements of Stockholders’ Equity for the year ended December 31, 2015. |
|
(Loss) income before income taxes for U.S. and international operations was:
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
U.S. (including exports) |
| $ | (481 | ) |
| $ | (492 | ) |
| $ | 244 |
|
International |
|
| 470 |
|
|
| 304 |
|
|
| 306 |
|
Total (loss) income before income taxes |
| $ | (11 | ) |
| $ | (188 | ) |
| $ | 550 |
|
Chemours recorded a tax benefit of $18 for the year ended December 31, 2016, a tax benefit of $98 for the year ended December 31, 2015 and a tax provision of $149 for the year ended December 31, 2014. The $80 decrease in tax benefits and the corresponding change in the effective income tax rates were primarily due to a $50 valuation allowance recorded on U.S. foreign tax credits, the Company’s geographical mix of earnings as well as the gain on the sale of assets and business, which resulted in a tax expense and a corresponding change in the effective income tax rate for the year ended December 31, 2016 as compared to the same period in 2015; offset by the tax benefit on the $335 PFOA MDL Settlement accrual (see Note 20).
The decrease in state income tax provision and the corresponding decrease in the state effective tax rate, net of federal tax benefit, for the year ended December 31, 2016 when compared to 2015 and 2014 is due to the tax benefit recognized as a result of changes in state tax law which decreased apportionment in states where the Company has significant operations as well as the state benefit of the litigation accruals discussed above. The tax benefit from international operations is primarily driven by Chemours’ overall geographic mix of earnings as well as gains on foreign divestitures that were not subject to tax. In comparing the impact of foreign operations on our overall effective tax rate from 2015 to 2016, the significant driver of the change in the benefit is a reduction in the pre-tax loss within the US while income from foreign operations has increased over the prior period.
The Company recorded a valuation allowance of $50 as of December 31, 2016 on its U.S. foreign tax credits based on available positive and negative evidence. For the three years ended December 31, 2016, the Company has incurred pre-tax losses in the U.S., which limit the ability to consider other subjective evidence such as the Company’s projections for future growth. The amount of the foreign tax credits that are considered realizable, however, could be adjusted if estimates of future taxable income during the carryforward period increase or if objective negative evidence in the form of cumulative losses is no longer present. Exchange gains (losses) principally reflect the impact of non-taxable gains and losses resulting from remeasurement of foreign currency-denominated monetary assets and liabilities. Depletion represents the tax benefit from the percentage depletion deductions taken pursuant to
F-22
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Section 613 of the Code. Goodwill represents the tax effect of the non-deductible goodwill associated with asset sales that took place in 2016. In 2015, the goodwill adjustment was the tax impact of reallocations based on Chemours’ new business reporting units and impairment charges, as described in Note 14. In addition, Chemours is entitled to a domestic manufacturing deduction relating to income from certain qualifying domestic production activities pursuant to Section 199 of the Code in tax years 2014, as well as a one-time tax benefit recognized in 2014 relating to a tax accounting method change. Due to net operating losses in 2015 and 2016, the Company is not computing a benefit related to Section 199 in those years. Consistent with the discussion in Note 2, the pre-spin effective tax rate stated herein may not be indicative of the future effective tax rate of Chemours as a result of the separation from DuPont.
Under the tax laws of various jurisdictions in which the Company operates, deductions or credits that cannot be fully utilized for tax purposes during the current year may be carried forward or back, subject to statutory limitations, to reduce taxable income or taxes payable in the future or prior years. At December 31, 2016, the U.S federal and state tax losses are $42, which substantially expire in 2035. The Company also has U.S. foreign tax credit carryforwards of $50, of which $27 expire in 2025 and $23 expire in 2026, which are fully offset by a valuation allowance. Lastly, the Company has foreign net operating losses of $3, which substantially expire between 2025 and 2026.
At December 31, 2016, the Company deemed approximately $2,100 of unremitted earnings of subsidiaries outside the U.S. as indefinitely reinvested. No deferred tax liability has been recognized with regard to the remittance of such earnings. It is not practical to estimate the income tax liability that might be incurred if such earnings were remitted to the U.S.
Each year, Chemours and/or its subsidiaries, files income tax returns in the U.S. federal jurisdiction and various states and non-U.S. jurisdictions. The following significant jurisdictions’ tax returns are subject to examination by their respective taxing authorities in the open years listed:
Jurisdiction |
| Open Years |
| |
China |
|
| 2011 through 2016 |
|
Mexico |
|
| 2011 through 2016 |
|
Netherlands |
|
| 2012 through 2016 |
|
Taiwan |
|
| 2014 through 2016 |
|
United States |
|
| 2015 through 2016 |
|
Positions challenged by the taxing authorities may be settled or appealed by Chemours and/or DuPont in accordance with the tax matters agreement. As a result, income tax uncertainties are recognized in the Company’s consolidated financial statements in accordance with accounting for income taxes, when applicable. Although it is difficult to predict the timing, we estimate that approximately $6 of unrecognized income tax benefits, excluding the impact relating to accrued interest and penalties, could be resolved within the next twelve months as a result of an accounting method change request filed with the Internal Revenue Service in the fourth quarter of 2016. We are not aware of any other matters that would result in significant changes to the amount of unrecognized income tax benefits reflected in the Consolidated Balance Sheet as of December 31, 2016.
As previously discussed in Note 3, prior to the separation, Chemours was included in DuPont’s consolidated income tax returns, and Chemours’ income taxes for those periods are computed and reported herein under the “separate return method”. Use of the separate return method may result in differences when the sum of the amounts allocated to stand-alone tax provisions are compared with amounts presented in the consolidated financial statements. In that event, the related deferred tax assets and liabilities could be significantly different from those presented herein for these periods. Certain tax attributes, e.g. net operating loss carryforwards, which were actually reflected in DuPont’s consolidated financial statements may or may not exist at the stand-alone Chemours level. Chemours’ consolidated financial statements do not reflect any amounts due to DuPont for income tax related matters prior to separation as it is assumed that all such amounts due to DuPont were settled on December 31 of each year.
F-23
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
The following table shows the change in our unrecognized tax benefit.
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Total unrecognized tax benefits as of January 1 |
| $ | 7 |
|
| $ | 39 |
|
| $ | 26 |
|
Gross amounts of decreases in unrecognized tax benefits as a result of adjustments to tax provisions taken during the prior period |
|
| (1 | ) |
|
| — |
|
|
| (1 | ) |
Gross amounts of increases in unrecognized tax benefits as a result of tax positions taken during the current period |
|
| — |
|
|
| — |
|
|
| 15 |
|
Reduction to unrecognized tax benefits as a result of a lapse of the applicable statute of limitations |
|
| — |
|
|
| (32 | ) | 1 |
| (1 | ) |
Total unrecognized tax benefits as of December 31 |
| $ | 6 |
|
| $ | 7 |
|
| $ | 39 |
|
Total unrecognized tax benefits, if recognized, that would impact the effective tax rate |
| $ | — |
|
| $ | — |
|
| $ | 39 |
|
Total amount of interest and penalties recognized in the Consolidated Statements of Operations |
|
| — |
|
|
| 1 |
| 1 |
| 2 |
|
Total amount of interest and penalties recognized in the Consolidated Balance Sheets |
|
| — |
|
|
| — |
|
|
| 8 |
|
| 1 | Reduction to the unrecognized tax benefits represents DuPont’s responsibilities for uncertain income tax positions recorded prior to July 1, 2015 pursuant to the tax matters agreement. The reduction was recorded in “DuPont Company Net Investment” of the Consolidated Statements of Stockholders’ Equity for the year ended December 31, 2015. |
|
The following is a rollforward of the deferred tax asset valuation allowance for the years ended December 31, 2016, 2015 and 2014.
|
| Year Ended December 31, |
| |||||||||
(Dollars in millions) |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Balance at beginning of period |
| $ | — |
|
| $ | 36 |
|
| $ | 26 |
|
Net charges to income tax expense |
|
| 50 |
|
|
| — |
|
|
| 10 |
|
Release of valuation allowance 1 |
|
| — |
|
|
| (36 | ) |
|
| — |
|
Balance at end of period |
| $ | 50 |
|
| $ | — |
|
| $ | 36 |
|
| 1 | Release of the valuation allowance during 2015 was related to tax loss carryforward incurred prior to July 1, 2015 that is attributable to DuPont’s tax periods pursuant to the tax matters agreement. The adjustment was recorded in the “DuPont Company Net Investment” of the Consolidated Statements of Stockholders’ Equity for the year ended December 31, 2015. |
|
Note 10. Earnings Per Share of Common Stock
The table below shows a reconciliation of the numerator and denominator for basic and diluted earnings per share calculations for the periods indicated.
|
| Year Ended December 31, |
|
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
|
| |||
Numerator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss) attributable to Chemours |
| $ | 7 |
|
| $ | (90 | ) |
| $ | 400 |
|
|
Denominator: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of common shares outstanding- Basic |
|
| 181,621,422 |
|
|
| 180,993,623 |
|
|
| 180,966,833 |
| 1 |
Dilutive effect of the Company’s employee compensation plans 2 |
|
| 1,795,078 |
|
|
| — |
|
|
| — |
|
|
Weighted average number of common shares outstanding - Diluted 2 |
|
| 183,416,500 |
|
|
| 180,993,623 |
|
|
| 180,966,833 |
|
|
| 1 | For 2014, pro forma earnings per share was calculated based on 180,966,833 shares of Chemours common stock that were distributed to DuPont shareholders on July 1, 2015. |
|
F-24
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
The following average number of stock options were antidilutive and, therefore, were not included in the diluted earnings per share calculation:
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Average number of stock options |
|
| 5,820,499 |
|
|
| 8,358,894 |
|
|
| — |
|
Note 11. Accounts and Notes Receivable – Trade, Net
|
| December 31, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
Accounts receivable—trade, net 1 |
| $ | 742 |
|
| $ | 757 |
|
VAT, GST and other taxes 2 |
|
| 46 |
|
|
| 68 |
|
Leases receivable—current |
|
| — |
|
|
| 13 |
|
Other receivables 3 |
|
| 19 |
|
|
| 21 |
|
Total |
| $ | 807 |
|
| $ | 859 |
|
| 1 | Accounts receivable – trade is net of allowances of $5 and $4 as of December 31, 2016 and 2015, respectively. Allowances are equal to the estimated uncollectible amounts. |
|
| 2 | Value Added Tax (VAT) and Goods and Services Tax (GST). |
|
| 3 | Other receivables consist of notes receivable, advances and other deposits. |
|
Accounts and notes receivable are carried at amounts that approximate fair value. Bad debt expense was $7, $1 and $1 for the years ended December 31, 2016, 2015 and 2014, respectively.
Note 12. Inventories
|
| December 31, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
Finished products |
| $ | 532 |
|
| $ | 613 |
|
Semi-finished products |
|
| 150 |
|
|
| 172 |
|
Raw materials, stores and supplies |
|
| 285 |
|
|
| 433 |
|
Subtotal |
|
| 967 |
|
|
| 1,218 |
|
Adjustment of inventories to LIFO basis |
|
| (200 | ) |
|
| (246 | ) |
Total |
| $ | 767 |
|
| $ | 972 |
|
Inventory values, before LIFO adjustment, are generally determined by the average cost method, which approximates current cost. Inventories are valued using the LIFO method at substantially all of the U.S. locations, which comprised $465 and $657 or 48% and 54% of inventories before the LIFO adjustments at December 31, 2016 and December 31, 2015, respectively. The remainder of inventory held in international locations and certain U.S. locations is valued using the average cost method.
F-25
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Note 13. Property, Plant and Equipment
Chemours’ property, plant and equipment consisted of:
|
| December 31, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
Equipment |
| $ | 6,748 |
|
| $ | 7,327 |
|
Buildings |
|
| 814 |
|
|
| 737 |
|
Construction in progress |
|
| 293 |
|
|
| 804 |
|
Land |
|
| 106 |
|
|
| 111 |
|
Mineral rights |
|
| 36 |
|
|
| 36 |
|
Total |
|
| 7,997 |
|
|
| 9,015 |
|
Accumulated depreciation |
|
| (5,213 | ) |
|
| (5,838 | ) |
Net property, plant and equipment |
| $ | 2,784 |
|
| $ | 3,177 |
|
Depreciation expense amounted to $281, $264 and $254 for the years ended December 31, 2016, 2015 and 2014, respectively. Property, plant and equipment includes gross assets under capital leases of $5 and $7 at December 31, 2016 and 2015, respectively. Interest expense capitalized as part of property, plant and equipment was $18 and $21 for the years ended December 31, 2016 and 2015. Chemours did not incur interest in the year ended December 31, 2014.
During the third quarter of 2016, the Company evaluated the carrying value of its aniline manufacturing facility in Pascagoula, Mississippi for recoverability given current business plans. The evaluation performed indicated that the carrying amount of this asset group was not recoverable when compared to the expected undiscounted cash flows. Based on management’s assessment of the fair value of the asset group, the Company determined that the carrying value of the Pascagoula aniline asset group exceeded its fair value and as a result, a $48 pre-tax impairment charge was recorded in the Chemical Solutions segment, which represents an impairment of substantially all of the remaining net book value of the Pascagoula aniline asset group.
During the third quarter of 2015, in connection with the strategic evaluation of the Chemical Solutions portfolio, excluding cyanides, the Company determined that the carrying value of the RMS manufacturing facility of the Chemical Solutions segment may not be recoverable given the strategic decision to discontinue investment in the business. An impairment evaluation was performed which indicated that the carrying amount of this asset group in the U.S. was not recoverable when compared to the expected undiscounted cash flows. Based on management’s assessment of the fair value of the asset group, the Company determined that the carrying value of that asset group exceeded the fair value and as a result, a $45 pre-tax impairment charge was recorded in the Chemical Solutions segment, which represents an impairment of substantially all of the remaining net book value of the RMS asset group.
The fair value of the asset groups were determined using an income approach based on the present value of the estimated future cash flows. The key assumptions used included growth rates and cash flow projections, discount rate, tax rate and an estimated terminal value. The amount was recorded in “restructuring and asset related charges, net” in the Consolidated Statements of Operations. Refer to Note 6 for additional information.
F-26
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Note 14. Goodwill and Other Intangible Assets, Net
Goodwill: The following table summarizes changes in the carrying amount of goodwill by reportable segment:
|
| Titanium Technologies |
|
| Fluoroproducts |
|
| Chemical Solutions |
|
| Total |
| ||||
Balance as of December 31, 2014 |
| $ | 13 |
|
| $ | 85 |
|
| $ | 100 |
|
| $ | 198 |
|
Impairment charge |
|
| — |
|
|
| — |
|
|
| (25 | ) |
|
| (25 | ) |
Currency translation and other |
|
| — |
|
|
| — |
|
|
| (7 | ) |
|
| (7 | ) |
Balance as of December 31, 2015 |
| $ | 13 |
|
| $ | 85 |
|
| $ | 68 |
|
| $ | 166 |
|
Sale of business 1 |
|
| — |
|
|
| — |
|
|
| (13 | ) |
|
| (13 | ) |
Currency translation and other |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Balance as of December 31, 2016 |
| $ | 13 |
|
| $ | 85 |
|
| $ | 55 |
|
| $ | 153 |
|
| 1 | Represents goodwill disposed in connection with the sale of the C&D business (See Note 7). |
Accumulated impairment losses as of December 31, 2016, 2015 and 2014 included in goodwill are $25, $25 and $0, respectively.
The Company has three operating segments: Titanium Technologies, Fluoroproducts and Chemical Solutions (see Note 24). The Company defines its reporting units as one level below the operating segments except for Titanium Technologies, which is an operating segment and a reporting unit. The Company tested the goodwill attributable for each of reporting units within the operating segments for impairment as of October 1, 2016 and concluded that the estimated fair value of each reporting unit, for which goodwill is recorded, substantially exceeded the reporting unit’s carrying value, indicating that none of the Company’s goodwill was impaired.
In October 2016, the Fluoroproducts segment leader announced a new organizational structure change to better manage the business and drive accountability. The change in the organizational structure also changed the reporting units of Fluoroproducts segment to Fluorochemicals and Fluoropolymers effective November 1, 2016. Prior to this change, the Fluoroproducts segment’s reporting units were Fluorochemicals, Industrial Resins and Diversified Technologies, which were tested for annual impairment as of October 1, 2016. The reporting unit change did not result in any impairment based on a separate impairment evaluation performed at the effective date of the change, as estimated fair value of the new reporting unit substantially exceeded its carrying value.
In 2015, in connection with the strategic evaluation of the Chemical Solutions portfolio and the resulting changes to the reporting units in the third quarter of 2015, the Chemical Solutions segments recorded a $25 pre-tax impairment charge related to its Sulfur reporting unit. Sulfur reporting unit was disposed through the sale of its assets and business during 2016 (see Note 7).
The Company evaluates goodwill for impairment using a two-step process. The first step is utilizing a discounted cash flow methodology to calculate the fair value of its reporting units; and where market comparables are available, the Company considers EBITDA multiples as part of the reporting unit valuation analysis. Key assumptions used in the discounted cash flows include projected growth rates, discount rates, tax rates and terminal values. Factors considered in developing cash flows and EBITDA projections include: 1) macroeconomic conditions; 2) industry and market considerations; 3) costs of raw materials and labor or other costs; 4) overall financial performance; and 5) other relevant entity-specific events. The discount rate used represents the weighted average cost of capital for the reporting units considering the risks and uncertainty inherent in the cash flows of the reporting units and in the internally developed forecasts. The second step of the quantitative test is required if the first step of the quantitative test indicates a potential impairment. The second step is performed by comparing the implied fair value of a reporting unit’s goodwill with the carrying amount of its goodwill. If the carrying amount of goodwill is greater than its implied fair value, an impairment loss is recorded. The use of these unobservable inputs results in the fair value estimate being classified as a Level 3 asset measured at fair value on a nonrecurring basis subsequent to its original recognition.
The determination of whether or not goodwill is impaired involves a significant level of judgment in the assumptions underlying the approaches’ used to determine the estimated fair value of our reporting units. Chemours believes that assumptions and rates used in the impairment assessment are reasonable. However, these assumptions are judgmental and variations in any assumptions could result in materially different calculations of fair value. The Company will continue to evaluate goodwill on an annual basis as of October 1, and whenever events or changes in circumstances, such as significant adverse changes in operating results, market conditions or changes in management’s business strategy, indicate that there may be a probable indicator of impairment. It is possible that the
F-27
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
assumptions used by management related to the evaluation may change or that actual results may vary significantly from management’s estimates.
Intangible Assets, Net: The following table summarizes the gross carrying amounts and accumulated amortization of other intangible assets by major class:
|
| December 31, 2016 |
|
| December 31, 2015 |
| ||||||||||||||||||
|
| Cost |
|
| Accumulated Amortization |
|
| Net |
|
| Cost |
|
| Accumulated Amortization |
|
| Net |
| ||||||
Customer lists |
| $ | 9 |
|
| $ | (7 | ) |
| $ | 2 |
|
| $ | 10 |
|
| $ | (8 | ) |
| $ | 2 |
|
Patents |
|
| 19 |
|
|
| (18 | ) |
|
| 1 |
|
|
| 19 |
|
|
| (17 | ) |
|
| 2 |
|
Purchased trademarks |
|
| 5 |
|
|
| (2 | ) |
|
| 3 |
|
|
| 5 |
|
|
| (2 | ) |
|
| 3 |
|
Purchased and licensed technology |
|
| 3 |
|
|
| (2 | ) |
|
| 1 |
|
|
| 8 |
|
|
| (6 | ) |
|
| 2 |
|
Other 1 |
|
| 10 |
|
|
| — |
|
|
| 10 |
|
|
| 3 |
|
|
| (2 | ) |
|
| 1 |
|
Total |
| $ | 46 |
|
| $ | (29 | ) |
| $ | 17 |
|
| $ | 45 |
|
| $ | (35 | ) |
| $ | 10 |
|
| 1 | Represents non-cash favorable supply contracts acquired in connection with the sale of Sulfur business and recognized during the third quarter of 2016 based on the present value of the difference between their contractual cash flows and estimated cash flows had the contracts been executed at a determinable market price. These contract intangibles will be amortized to cost of goods sold over the remaining life of the supply contracts through 2021. |
The aggregate pre-tax amortization expense for definite-lived intangible assets was $3, $3 and $3 for the years ended December 31, 2016, 2015 and 2014, respectively. The estimated aggregate pretax amortization expense for 2017, 2018, 2019, 2020 and 2021 is $4, $3, $3, $3 and $1, respectively. Definite-lived intangible assets are amortized over their estimated useful lives, generally for periods ranging from 5 to 20 years. The reasonableness of the useful lives of these assets is continually evaluated. There are no indefinite-lived intangible assets.
Note 15. Other Assets
|
| December 31, 2016 |
|
| December 31, 2015 |
| ||
Leases receivable - non-current 1 |
| $ | — |
|
| $ | 125 |
|
Capitalized repair and maintenance costs |
|
| 145 |
|
|
| 149 |
|
Pension assets 2 |
|
| 159 |
|
|
| 138 |
|
Advances and deposits |
|
| — |
|
|
| 11 |
|
Deferred income taxes |
|
| 41 |
|
|
| 47 |
|
Asset held for sale |
|
| 29 |
|
|
| — |
|
Miscellaneous 3 |
|
| 43 |
|
|
| 38 |
|
Total |
| $ | 417 |
|
| $ | 508 |
|
| 1 | Relates to Sulfur business which was included in the assets disposed in 2016. See Direct Financing Leases below. |
| 2 | Pension assets represent the funded status of certain of the Company’s long-term employee benefit plans. |
| 3 | Miscellaneous includes deferred financing fees related to the Revolving Credit Facility of $13 and $19 as of December 31, 2016 and 2015, respectively. |
Asset Held for Sale
In December 2016, the Company’s corporate headquarters building located in Wilmington, Delaware, was classified as held for sale in connection with a sale agreement entered into in January 2017. The transaction is expected to be completed in the first quarter of 2017, and the Company expects to receive approximately $32 proceeds, subject to customary closing adjustments. As a result, the Company recorded approximately $13 pre-tax impairment charge for the year ended December 31, 2016. The Company intends to lease a portion of the building subject to the completion of the sale.
Direct Financing Leases
Prior to the sale of the Sulfur business, Chemours had constructed fixed assets on land that it leased from third parties at two of its facilities in the U.S. (Borderland and Morses Mill). Management has analyzed these arrangements and determined these assets
F-28
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
represent direct financing leases, whereby Chemours was the lessor of this equipment. Lease receivables were recorded, which represent the balance of the minimum future lease payments. The current portion of lease receivables was previously included in accounts and notes receivable - trade, net, as shown in Note 11 and the long-term portion was previously included in other assets, as shown above. These lease receivables were included in the sale of the Sulfur business that was completed in August 2016 (see Note 7).
Note 16. Accounts Payable
|
| December 31, 2016 |
|
| December 31, 2015 |
| ||
Trade payables |
| $ | 858 |
|
| $ | 945 |
|
VAT and other payables |
|
| 26 |
|
|
| 28 |
|
Total |
| $ | 884 |
|
| $ | 973 |
|
Note 17. Other Accrued Liabilities
|
| December 31, 2016 |
|
| December 31, 2015 |
| ||
Compensation and other employee-related costs |
| $ | 154 |
|
| $ | 109 |
|
Employee separation costs 1 |
|
| 31 |
|
|
| 76 |
|
Accrued litigation 2 |
|
| 342 |
|
|
| 11 |
|
Environmental remediation 2 |
|
| 71 |
|
|
| 68 |
|
Income taxes |
|
| 39 |
|
|
| 32 |
|
Customer rebates |
|
| 53 |
|
|
| 53 |
|
Deferred revenue 3 |
|
| 76 |
|
|
| 20 |
|
Accrued interest |
|
| 21 |
|
|
| 21 |
|
Miscellaneous 4 |
|
| 85 |
|
|
| 64 |
|
Total |
| $ | 872 |
|
| $ | 454 |
|
| 1 | See Note 6 for further information. |
|
| 2 | Accrued litigation includes $335 litigation accrual related to the PFOA MDL Settlement. See Note 20 for further discussion of accrued litigation and environmental remediation. |
|
| 3 | Deferred revenue includes $58 prepayment by DuPont for specified goods and services, which Chemours expects to provide through mid-2017. |
|
| 4 | Miscellaneous primarily includes accrued utility expenses, property taxes, an accrued indemnification liability, asset retirement obligations (see Note 20) and other miscellaneous expenses. |
|
Note 18. Other Liabilities
|
| December 31, 2016 |
|
| December 31, 2015 |
| ||
Environmental remediation 1 |
| $ | 208 |
|
| $ | 223 |
|
Employee-related costs 2 |
|
| 113 |
|
|
| 108 |
|
Employee separation costs 3 |
|
| 3 |
|
|
| 23 |
|
Accrued litigation 1 |
|
| 53 |
|
|
| 58 |
|
Asset retirement obligations 1 |
|
| 41 |
|
|
| 41 |
|
Deferred revenue |
|
| 5 |
|
|
| 11 |
|
Miscellaneous 4 |
|
| 101 |
|
|
| 89 |
|
Total |
| $ | 524 |
|
| $ | 553 |
|
| 1 | See Note 20 for further details on environmental remediation, asset retirement obligations and accrued litigation. |
|
| 2 | See Note 22 for further details on long-term employee benefits. |
|
| 3 | See Note 6 for further information. |
|
| 4 | Miscellaneous primarily includes an accrued indemnification liability. |
|
F-29
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Long-term debt was comprised of the following at December 31, 2016 and 2015:
|
| December 31, 2016 |
|
| December 31, 2015 |
| ||
Long-term debt: |
|
|
|
|
|
|
|
|
Senior secured term loan |
| $ | 1,372 |
|
| $ | 1,493 |
|
Senior unsecured notes: |
|
|
|
|
|
|
|
|
6.625%, due May 2023 |
|
| 1,158 |
|
|
| 1,350 |
|
7.00%, due May 2025 |
|
| 750 |
|
|
| 750 |
|
6.125%, due May 2023 (€295 at December 31, 2016; €360 at December 31, 2015) |
|
| 308 |
|
|
| 395 |
|
Other |
|
| 3 |
|
|
| 26 |
|
Total |
|
| 3,591 |
|
|
| 4,014 |
|
Less: Unamortized issue discount on senior secured term loan |
|
| 5 |
|
|
| 7 |
|
Less: Unamortized debt issuance costs |
|
| 42 |
|
|
| 53 |
|
Less: Short-term borrowings and current maturities |
|
| 15 |
|
|
| 39 |
|
Long-term debt, net |
| $ | 3,529 |
|
| $ | 3,915 |
|
Senior Secured Credit Facilities
On May 12, 2015, Chemours entered into a credit agreement that provides for a seven-year senior secured term loan (the Term Loan Facility) in a principal amount of $1,500 repayable in equal quarterly installments at a rate of one percent of the original principal amount per year, with the balance payable on the final maturity date. The Term Loan Facility was issued with a $7 original issue discount and bears interest at a rate of LIBOR plus 3.00%, with a 0.75% LIBOR floor. The proceeds from the Term Loan Facility were used to fund a portion of the distribution to DuPont, along with related fees and expenses.
The credit agreement also provided for a five-year senior secured revolving credit facility (the Revolving Credit Facility), which has been reduced to $750 as part of the amendment completed on February 19, 2016 (discussed below). The proceeds of any loans made under the Revolving Credit Facility can be used for capital expenditures, acquisitions, working capital needs and other general corporate purposes. No borrowings were outstanding under our Revolving Credit Facility but had $132 and $129 in letters of credit issued and outstanding under this facility at December 31, 2016 and 2015, respectively. The Revolving Credit Facility bears variable interest of a range based on our total net leverage ratio between (a) 0.50% and 1.25% for base rate loans and (b) 1.50% and 2.25% for LIBOR loans. The applicable margins were 1.00% for base rate loans and 2.00% for LIBOR loans as of December 31, 2016 and 1.25% for base rate loans and 2.25% for LIBOR loans as of December 31, 2015. In addition, we are required to pay a commitment fee on the average daily unused amount of the Revolving Credit Facility at a rate based on our total net leverage ratio, between 0.20% and 0.35%. As of December 31, 2016 and 2015, commitment fees were assessed at a rate of 0.30% and 0.35%, respectively.
In September 2015, in connection with the Company’s transformation plan announced in August 2015, Chemours and its Revolving Credit Facility lenders entered into an amendment to the Revolving Credit Facility that modified the consolidated EBITDA definition in the covenant calculation to include pro forma benefits from future cost savings initiatives in the calculation of financial covenants that rely on consolidated EBITDA beginning from the quarter ended September 30, 2015. Since the revolver availability in any quarter is determined by the cushion remaining in the financial maintenance covenants at the end of the previous quarter, this amendment increased the Company’s access to the revolving credit facility.
In February 2016, Chemours and its Revolving Credit Facility lenders entered into a second amendment to the Revolving Credit Facility that (a) replaced the total net leverage ratio financial covenant with senior secured net leverage ratio; (b) reduced the minimum required levels of interest expense coverage ratio covenant; (c) increased the limits and extended the time horizon for inclusion of pro forma benefits of announced cost reduction initiatives into consolidated EBITDA definition for the purposes of calculating financial maintenance covenants; and (d) reduced the revolver availability from $1,000 to $750. As a result of this amendment, the Company recorded a charge of approximately $4 to write off a proportionate amount of unamortized debt issuance costs attributable to the reduction in revolver commitment, which was included in “Interest expense, net”.
F-30
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
In December 2016, Chemours entered into a third amendment to the credit agreement to change certain covenants and allow the Company to enter into a sale and leaseback transaction for the sale of its corporate headquarters building located in Wilmington, Delaware. The amendment requires the Company to use the proceeds from sale to repay portion of the term loans. These transactions are expected to be completed in the first quarter of 2017, and the Company expects to receive approximately $32 proceeds, subject to customary closing adjustments.
Fees and expenses incurred in connection with the amendments were approximately $3 and $1 for the years ended December 31, 2016 and 2015, respectively, which were primarily capitalized in “Other assets” of the Consolidated Balance Sheets and will be amortized to interest expense on a straight-line basis over the remaining term of the Revolving Credit Facility.
The credit agreement, as amended, contains financial covenants which, solely with respect to the Revolving Credit Facility, require Chemours not to exceed a maximum senior secured net leverage ratio of 3.50 to 1.00 each quarter through December 31, 2016, 3.00 to 1.00 through June 30, 2017 and further decreasing by 0.25 to 1.00 every subsequent six months to 2.00 to 1.00 by January 1, 2019 and thereafter. Chemours is also required to maintain a minimum interest coverage ratio of 1.75 to 1.00 each quarter through June 30, 2017 and further increasing by 0.25 to 1.00 every subsequent six months to 3.00 to 1.00 by January 1, 2019 and thereafter. In addition, the credit agreement contains customary affirmative and negative covenants that, among other things, limit or restrict Chemours and its subsidiaries’ ability, subject to certain exceptions, to incur liens, merge, consolidate or sell, transfer or lease assets, make investments, pay dividends, transact with subsidiaries and incur indebtedness. The credit agreement also contains customary representations and warranties and events of default. Chemours was in compliance with its debt covenants as of December 31, 2016.
Chemours’ obligations under the senior secured credit facilities are guaranteed on a senior secured basis by all of its material domestic subsidiaries, subject to certain agreed upon exceptions. The obligations under the senior secured credit facilities are also, subject to certain agreed upon exceptions, secured by a first priority lien on substantially all of Chemours and its material wholly-owned domestic subsidiaries’ assets, including 100% of the stock of domestic subsidiaries and 65% of the stock of certain foreign subsidiaries.
Senior Unsecured Notes
On May 12, 2015, Chemours issued senior unsecured notes (the “Notes”) with an aggregate principal of approximately $2,503 in a private placement, which comprise of $1,350 aggregate principal amount issued at an interest rate of 6.625% per annum and will mature on May 15, 2023 (the “2023 Notes”), $750 aggregate principal amount issued at an interest rate of 7.000% per annum and will mature on May 15, 2025 (the “2025 Notes”) and €360 aggregate principal amount issued at an interest rate of 6.125% and will mature on May 15, 2023 (the “Euro Notes”). The Notes require payment of principal at maturity and interest semi-annually in cash and in arrears on May 15 and November 15 of each year.
The proceeds from the Notes were used to fund the cash and in-kind distributions to DuPont and to pay related fees and expenses. The in-kind distribution to DuPont of $507 aggregate principal amount of Chemours 2025 Notes were exchanged by DuPont with third parties for certain DuPont notes.
In connection with the issuance of the Notes, Chemours entered into a registration rights agreement, in which Chemours agreed to file with the SEC, a registration statement for the exchange of the Notes for new registered notes with identical terms. On March 18, 2016, Chemours filed a registration statement on Form S-4 with respect to the exchange offer. The registration statement was declared effective on April 12, 2016, and the exchange offer was completed on May 19, 2016. In addition, on May 5, 2016, the Euro Notes were listed for trading on the Global Exchange Market of the Irish Stock Exchange.
Each series of Notes is or will be fully and unconditionally guaranteed, jointly and severally, by Chemours’ existing and future domestic subsidiaries that guarantee (the Guarantors) the Senior Secured Credit Facilities or that guarantee other indebtedness of Chemours or any guarantor in an aggregate principal amount in excess of $75 (the Guarantees). The Notes are unsecured and unsubordinated obligations of Chemours. The Guarantees are unsecured and unsubordinated obligations of the Guarantors. The Notes rank equally in right of payment to all of Chemours’ existing and future unsecured unsubordinated debt and senior in right of payment to all of Chemours’ existing and future debt that is by its terms expressly subordinated in right of payment to the Notes. The Notes are subordinated to indebtedness under the Senior Secured Credit Facilities as well as any future secured debt to the extent of the value of the assets securing such debt. Chemours’ is obligated to offer to purchase the Notes at a price of (a) 101 percent of their principal amount, together with accrued and unpaid interest, if any, to the date of purchase, upon the occurrence of certain change of control events and (b) 100 percent of their principal amount, together with accrued and unpaid interest, if any, to the date of purchase, with the proceeds from certain asset dispositions. These restrictions and prohibitions are subject to certain qualifications and exceptions set
F-31
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
forth in the Indenture, including without limitation, reinvestment rights with respect to the proceeds of asset dispositions. Chemours is permitted to redeem some or all of the 2023 Notes and Euro Notes by paying a “make-whole” premium prior to May 15, 2018, and on or after May 15, 2018 and thereafter at specified redemption prices. Chemours may redeem some or all of the 2025 Notes on or after May 15, 2020 at specified redemption prices. Chemours may also redeem some or all of the 2023 Notes and Euro Notes by means other than a redemption, including tender offer and open market repurchases.
Term Loans and Notes Repayments
During the year ended December 31, 2016, the Company repurchased or repaid portions of its senior secured term loans (“Term Loans”), 2023 Notes and Euro Notes with aggregate principal and cash payment amounts as follows:
|
| Year Ended December 31, 2016 |
| |||||
|
| Aggregate Principal |
|
| Cash Payment |
| ||
Term Loans 1 |
| $ | 105 |
|
| $ | 104 |
|
2023 Notes |
|
| 192 |
|
|
| 182 |
|
Euro Notes |
|
| 73 |
|
|
| 68 |
|
|
| $ | 370 |
|
| $ | 354 |
|
1 The Term Loans aggregate principal amounts exclude the required quarterly installment repayments equivalent to $15 per year.
For the year ended December 31, 2016, we recorded in “Interest expense, net” of the Consolidated Statements of Operations a net gain on extinguishment of debt of $10, net of approximately $5 charges related to the write-off of deferred financing costs associated with the extinguished debt.
Maturities
Chemours has required quarterly principal payments related to the Term Loan Facility equivalent to 1.00% per annum through March 2022, with the balance due at maturity. Term Loan principal maturities over the next five years are $15 in each year from 2017 to 2021. Debt maturities related to the Term Loan Facility and the Notes in 2022 and beyond will be $3,513.
In addition, following the end of each fiscal year commencing on the year ended December 31, 2016, the Company is also required to make additional principal repayments, depending on leverage levels as defined in the credit agreement, equivalent to up to 50% of excess cash flow based on certain leverage targets with stepdowns to 25% and 0% as actual leverage decreases to below 3.00 to 1.00 leverage target.
Debt Fair Value
The fair values of the Term Loan Facility, the 2023 notes, the 2025 notes and the 2023 Euro notes at December 31, 2016 were approximately $1,370, $1,149, $739 and $305, respectively. The estimated fair values of the Term Loan Facility and the Notes are based on quotes received from third party brokers, and are classified as Level 2 in the fair value hierarchy.
Note 20. Commitments and Contingent Liabilities
Guarantees
| (a) | Obligations for Equity Affiliates and Others |
Chemours has directly guaranteed various obligations of customers, suppliers and other third parties. At December 31, 2016 and December 31, 2015, Chemours had directly guaranteed less than $1 and $8 of such obligations, respectively. These represent the maximum potential amount of future (undiscounted) payments that Chemours could be required to make under the guarantees in the event of default by the guaranteed parties. No amounts were accrued at December 31, 2016 and 2015.
Chemours assesses the payment and performance risk by assigning default rates based on the duration of the guarantees. These default rates are assigned based on the external credit rating of the counterparty or through internal credit analysis and historical default
F-32
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
history for counterparties that do not have published credit ratings. For counterparties without an external rating or available credit history, a cumulative average default rate is used.
| (b) | Operating Leases |
Chemours uses various leased facilities and equipment in its operations. The terms for these leased assets vary depending on the lease agreement. Future minimum lease payments (including residual value guarantee amounts) under non-cancelable operating leases are $62, $53, $46, $23 and $31 for the years ended December 31, 2017, 2018, 2019, 2020 and 2021, respectively, and $43 for the years thereafter. Net rental expense under operating leases was $68, $83 and $75 during the years ended December 31, 2016, 2015 and 2014, respectively.
Asset Retirement Obligations
Chemours has recorded asset retirement obligations primarily associated with closure, reclamation and removal costs for mining operations related to the production of TiO2 in the Titanium Technologies segment. A summary of the changes in asset retirement obligations is as follows:
(Dollars in millions) |
| Year Ended December 31, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
Beginning balance |
| $ | 42 |
|
| $ | 43 |
|
Accretion expense |
|
| 2 |
|
|
| 1 |
|
Settlements/payments |
|
| (1 | ) |
|
| (2 | ) |
Ending balance |
| $ | 43 |
|
| $ | 42 |
|
Current portion |
| $ | 2 |
|
| $ | 1 |
|
Non-current portion |
| $ | 41 |
|
| $ | 41 |
|
Litigation
In addition to the matters discussed below, Chemours, by virtue of its status as a subsidiary of DuPont prior to the separation, is subject to or required under the separation-related agreements executed prior to the separation to indemnify DuPont against various pending legal proceedings arising out of the normal course of the Chemours business including product liability, intellectual property, commercial, environmental and antitrust lawsuits. It is not possible to predict the outcome of these various proceedings. Except for the PFOA litigation for which a separate assessment is provided in this Note, while management believes it is reasonably possible that Chemours could incur losses in excess of the amounts accrued, if any, for the aforementioned proceedings, it does not believe any such loss would have a material impact on Chemours’ consolidated financial position, results of operations or liquidity. With respect to the litigation matters discussed below, including PFOA multi-district litigation (“MDL”), management’s estimate of the probability of loss in excess of the amounts accrued, if any, is addressed individually for each matter. In the event that DuPont seeks indemnification for adverse trial rulings or outcomes for any such matter relating to PFOA, these indemnification claims could materially adversely affect Chemours’ financial condition. Disputes between Chemours and DuPont may also arise with respect to indemnification matters, including disputes based on matters of law or contract interpretation. If and to the extent these disputes arise, they could materially adversely affect Chemours.
| (a) | Asbestos |
At December 31, 2016 and 2015, there were approximately 1,900 lawsuits and 2,200 lawsuits, respectively, pending against DuPont alleging personal injury from exposure to asbestos. These cases are pending in state and federal court in numerous jurisdictions in the U.S. and are individually set for trial. A small number of cases are pending outside the U.S. Most of the actions were brought by contractors who worked at sites between 1950 and the 1990s. A small number of cases involve similar allegations by DuPont employees. A limited number of the cases were brought by household members of contractors or DuPont employees. Finally, certain lawsuits allege personal injury as a result of exposure to DuPont products.
At December 31, 2016 and 2015, Chemours had an accrual of $41 and $44 related to this matter, respectively. Chemours reviews this estimate and related assumptions quarterly. Management believes that the likelihood is remote that Chemours would incur losses in excess of the amounts accrued in connection with this matter.
F-33
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
In the separation, DuPont assigned its Benzene docket to Chemours. At December 31, 2016 and 2015, there are 27 and 29 cases pending against DuPont alleging benzene-related illnesses. These cases consist of premises matters involving contractors and deceased former employees who claim exposure to benzene while working at DuPont sites primarily in the 1960s through the 1980s, and product liability claims based on alleged exposure to benzene found in trace amounts in aromatic hydrocarbon solvents used to manufacture DuPont products, such as paints, thinners and reducers.
Through DuPont, Chemours has received a claim by Phillips66 for indemnity and defense for three matters arising at a former DuPont / Conoco Texas site. Phillips66 seeks reimbursement for its settlement and fees in one matter and assumption of the defense in two matters.
A benzene case (Hood v. DuPont) was tried to a verdict in Texas state court on October 20, 2015. Plaintiffs alleged that Mr. Hood’s Acute Myelogenous Leukemia (AML) was the result of 24 years of occupational exposure to trace benzene found in DuPont automotive paint products and that DuPont negligently failed to warn him that its paints, reducers and thinners contained benzene that could cause cancer or leukemia. The jury found in the Plaintiffs favor awarding $6.9 in compensatory damages and $1.5 in punitive damages. In March 2016, acting on the Company’s motion, the Court struck the punitive award. Through DuPont, Chemours has filed an appeal on the remaining award based upon substantial errors made at the trial court level. Plaintiffs have filed a cross appeal.
Management believes that a loss is reasonably possible related to these matters; however, given the evaluation of each Benzene matter is highly fact driven and impacted by disease, exposure and other factors, a range of such losses cannot be reasonably estimated at this time.
| (c) | PFOA |
Prior to the fourth quarter of 2014, the performance chemicals segment of DuPont made PFOA (collectively, perfluorooctanoic acids and its salts, including the ammonium salt) at its Fayetteville plant (Fayetteville, North Carolina) and used PFOA as a processing aid in the manufacture of fluoropolymers and fluoroelastomers at certain sites including: Washington Works (Parkersburg, West Virginia), Chambers Works (Deepwater, New Jersey), Dordrecht Works (Netherlands), Changshu Works (China), and Shimizu (Japan). These sites are now owned and/or operated by Chemours.
Chemours recorded accruals of $349 and $20 related to the PFOA matters discussed below at December 31, 2016 and 2015, respectively. In the fourth quarter of 2016, the Company recorded an approximately $335 accrual related to the PFOA MDL settlement, which is further discussed below.
The accruals also include charges related to DuPont’s obligations under agreements with the U.S. Environmental Protection Agency (EPA) and voluntary commitments to the New Jersey Department of Environmental Protection (NJDEP). These obligations and voluntary commitments include surveying, sampling and testing drinking water in and around certain company sites offering treatment or an alternative supply of drinking water if tests indicate the presence of PFOA in drinking water at or greater than the national Health Advisory. A provisional health advisory level was set in 2009 at 0.4 parts per billion (ppb) that includes PFOA in drinking water. In May 2016, the EPA announced a health advisory level of 0.07 ppb that includes PFOA in drinking water. As a result, we recorded an additional $4 in the second quarter of 2016 based on management’s best estimate of the impact of the new health advisory level on the company’s obligations to the EPA, which have expanded the testing and water supply commitments previously established. Based on prior testing, the Company has initiated additional testing and treatment in certain additional locations in and around Chambers Works and Washington Works plants. The Company will continue to work with the EPA regarding the extent of work that may be required with respect to these matters.
Drinking Water Actions
In August 2001, a class action, captioned Leach v. DuPont, was filed in West Virginia state court alleging that residents living near the Washington Works facility had suffered, or may suffer, deleterious health effects from exposure to PFOA in drinking water.
DuPont and attorneys for the class reached a settlement in 2004 that binds about 80,000 residents. In 2005, DuPont paid the plaintiffs’ attorneys’ fees and expenses of $23 and made a payment of $70, which class counsel designated to fund a community health project. Chemours, through DuPont, funded a series of health studies which were completed in October 2012 by an independent science panel of experts (the C8 Science Panel). The studies were conducted in communities exposed to PFOA to evaluate available scientific
F-34
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
evidence on whether any probable link exists, as defined in the settlement agreement, between exposure to PFOA and human disease. The C8 Science Panel found probable links, as defined in the settlement agreement, between exposure to PFOA and pregnancy-induced hypertension, including preeclampsia, kidney cancer, testicular cancer, thyroid disease, ulcerative colitis and diagnosed high cholesterol.
In May 2013, a panel of three independent medical doctors released its initial recommendations for screening and diagnostic testing of eligible class members. In September 2014, the medical panel recommended follow-up screening and diagnostic testing three years after initial testing, based on individual results. The medical panel has not communicated its anticipated schedule for completion of its protocol. Through DuPont, Chemours is obligated to fund up to $235 for a medical monitoring program for eligible class members and, in addition, administrative cost associated with the program, including class counsel fees. In January 2012, Chemours, through DuPont, put $1 in an escrow account to fund medical monitoring as required by the settlement agreement. The court-appointed Director of Medical Monitoring has established the program to implement the medical panel’s recommendations and the registration process, as well as eligibility screening, is ongoing. Diagnostic screening and testing has begun and associated payments to service providers are being disbursed from the escrow account. As of December 31, 2016, less than $1 has been disbursed from the escrow account related to medical monitoring.
In addition, under the settlement agreement, DuPont must continue to provide water treatment designed to reduce the level of PFOA in water to six area water districts and private well users. At separation, this obligation was assigned to Chemours, which is included in the accrual amounts recorded as of December 31, 2016.
Class members may pursue personal injury claims against DuPont only for those human diseases for which the C8 Science Panel determined a probable link exists. At December 31, 2016 and 2015, there were approximately 3,500 lawsuits filed in various federal and state courts in Ohio and West Virginia, an increase of approximately 600 over year end 2014. These lawsuits are consolidated in an MDL in Ohio federal court. Based on the information currently available to the Company, the majority of the lawsuits allege personal injury claims associated with high cholesterol and thyroid disease from exposure to PFOA in drinking water. There are 30 lawsuits alleging wrongful death.
Although the majority of the plaintiffs in the MDL allege multiple diseases, the table below approximates the number of plaintiffs in each of the six probable link disease categories.
Alleged Injury |
| Approximate Number of Plaintiffs |
| |
Kidney cancer |
|
| 200 |
|
Testicular cancer |
|
| 70 |
|
Ulcerative colitis |
|
| 300 |
|
Preeclampsia |
|
| 200 |
|
Thyroid disease |
|
| 1,430 |
|
High cholesterol |
|
| 1,340 |
|
In the third quarter of 2014, six plaintiffs from the MDL were selected for individual bellwether trials.
Settlement of MDL between DuPont and MDL Plaintiffs
On February 11, 2017, DuPont entered into an agreement in principle with plaintiffs’ counsel representing the MDL plaintiffs providing for a global settlement of all cases and claims in the MDL, including all filed and unfiled personal injury cases and claims that are part of the plaintiffs’ counsel’s claim inventory, as well as cases that have been tried to a jury verdict (the “MDL Settlement”). The total settlement amount is $670.7 million dollars in cash, half of which will be paid by Chemours and half paid by DuPont. DuPont’s payment would not be subject to indemnification or reimbursement by Chemours, and Chemours has accrued $335 million associated with this matter at December 31, 2016. In exchange for payment of the total settlement amount, DuPont and Chemours will receive a complete release of all claims by the settling plaintiffs. The MDL Settlement was entered into solely by way of compromise and settlement and is not in any way an admission of liability or fault by DuPont or Chemours. The MDL Settlement is not subject to court approval; however, the MDL Settlement may not proceed in certain conditions, including a walk-away right that enables DuPont to terminate the MDL Settlement if more than a specified number of plaintiffs determine not to participate. In connection with the MDL Settlement, DuPont and MDL plaintiffs’ counsel have sought a stay (the “Stay of MDL Litigation”) of all judicial proceedings related to this action in the federal district court and in the U.S. Court of Appeals for the Sixth Circuit. If the MDL Settlement is terminated or otherwise does not proceed, additional lawsuits may go to trial or appeal.
F-35
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Litigation and Procedural Posture Prior to Stay of MDL Litigation
All six bellwether cases in the MDL have now been tried, resolved, appealed or otherwise addressed. Two bellwether cases have been tried. The first case (Bartlett v. DuPont / kidney cancer) was tried to a verdict in October 2015. The jury found in favor of the plaintiff, awarding $1.1 in damages for negligence and $0.5 for emotional distress. The jury found that DuPont’s conduct did not warrant punitive damages. A second case (Freeman v. DuPont / testicular cancer) was tried to verdict in July 2016. The jury found in favor of the plaintiff awarding $5.1 in compensatory damages and $0.5 in punitive damages and attorneys’ fees. Plaintiff’s counsel alleges that they are entitled to at least $6.9 in attorneys’ fees and costs for the Freeman trial. Absent the Stay of MDL Litigation, the Court would make a determination after post-trial submissions by the parties. The Court’s determination would be subject to appeal. Court rulings made before and during both trials resulted in several significant grounds for appeal and an appeal to the Sixth Circuit has been filed for the first case. Oral argument on the appeal of the first case was held in December 2016. The Company, through DuPont, is pursuing post-trial motions and appeals for the second case.
Three bellwether PFOA cases were settled in 2016 as trial approached. These cases (Wolf v. DuPont / ulcerative colitis, Dowdy v. DuPont / kidney cancer, Baker v. DuPont / kidney cancer) were settled for amounts well below the incremental cost of preparing for trials. To date, the settlements have been individually and in aggregate immaterial to the Company. The final case (Pugh v. DuPont / ulcerative colitis) was removed from the bellwethers when it was determined that the plaintiff did not suffer from the alleged disease.
The trial court announced that, starting in May 2017, 40 individual plaintiff trials will be scheduled for a 12-month period. Following the conclusion of the six bellwether cases, on July 19, 2016, the court moved two of the 40 matters (Vigneron v. DuPont / testicular cancer and Moody v. DuPont / testicular cancer) forward and set the cases for trial on November 14, 2016 and January 17, 2017, respectively. The trial court’s multi-year plan pertains only to the approximately 270 cases claiming cancer. Based on the current plan, the remaining cases, comprising approximately 93% of the docket, will remain inactive.
The November 2016 trial (Vigneron v. DuPont / testicular cancer) resulted in a verdict in favor of the Plaintiff for $2 in compensatory damages, $10.5 in punitive damages, and attorney’s fees and costs. Absent the Stay of MDL Litigation, the Company, through DuPont, will pursue post-trial motions and appeals. The January 2017 trial (Moody v. DuPont / testicular cancer) commenced in the first quarter of 2017 but was stayed pending the MDL Settlement and its implementation.
A confidential mediation process was established by the court early in this MDL.
Chemours, through DuPont, denies the allegations in these lawsuits and is defending itself vigorously. The MDL Settlement was entered into solely by way of compromise and settlement and is not in any way an admission of liability or fault by DuPont or Chemours. No other claims in the MDL have been settled or resolved during the periods presented.
Settlement between DuPont and Chemours related to MDL
DuPont and Chemours have also agreed, subject to and following the completion of the MDL Settlement, to a limited sharing of potential future PFOA liabilities (i.e., “indemnifiable losses,” as defined in the separation agreement between DuPont and Chemours) for a period of five years. During that five-year period, Chemours would annually pay future PFOA liabilities up to $25 million and, if such amount is exceeded, DuPont would pay any excess amount up to the next $25 million (which payment will not be subject to indemnification by Chemours), with Chemours annually bearing any further excess liabilities under the terms of the separation agreement. After the five-year period, this limited sharing agreement would expire, and Chemours’ indemnification obligations under the separation agreement would continue unchanged. Chemours has also agreed that, upon the MDL Settlement becoming effective, it will not contest its liability to DuPont under the separation agreement for PFOA liabilities on the basis of ostensible defenses generally applicable to the indemnification provisions under the separation agreement, including defenses relating to punitive damages, fines or penalties or attorneys’ fees, and waives any such defenses with respect to PFOA liabilities. Chemours has, however, retained defenses as to whether any particular PFOA claim is within the scope of the indemnification provisions of the separation agreement.
PFOA Summary
While it is probable that the Company will incur costs related to the medical monitoring program discussed above, such costs cannot be reasonably estimated due to uncertainties surrounding the level of participation by eligible class members and the scope of testing.
Chemours has accrued $335 million associated with the MDL Settlement at December 31, 2016.
F-36
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
If the MDL Settlement does not proceed, any cases stayed or additional lawsuits may go to trial or appeal. An adverse ruling at trial or on appeal could result in our incurring additional costs and liabilities, which are difficult to estimate beyond accrued amounts and involve significant uncertainty due to the uniqueness of the individual MDL plaintiff’s claims and the defenses to those claims, both as to potential liability and damages on an individual claim basis, and numerous unsettled legal issues, among other factors, such as general versus specific causation, lack of specific fact discovery allowed to date on vast majority of the cases, lack of validation of basic facts associated with plaintiffs and related claims, and the three cases tried to verdict to date did not inform of the many salient facts and legal issues needed for assessment of the other cases. The appellate courts will rule on matters that have been tried. The Company believes there are strong common and individual grounds for appealing these verdicts and, if any such verdict is overturned, any subsequent verdict relying on such overturned ruling would likely also be overturned. The trials and appeals of the MDL matters will occur over the course of many years. Significant unfavorable outcomes in a number of cases in the MDL could have a material adverse effect on Chemours’ consolidated financial position, results of operations or cash flows.
There could also be new lawsuits filed related to DuPont’s use of PFOA, its manufacture of PFOA, or its customers use of DuPont products that may not be within the scope of the MDL Settlement. Any such new litigation could also result in Chemours incurring additional costs and liabilities. Management believes it is reasonably possible that the Company could incur losses related to other PFOA matters in excess of amounts accrued but any such losses are not estimable at this time.
| (d) | U.S. Smelter and Lead Refinery, Inc. |
Five lawsuits, including two putative class actions, were filed against DuPont by area residents concerning the U.S. Smelter and Lead Refinery multi-party Superfund site in East Chicago, Indiana. Three of the lawsuits allege that Chemours is now responsible for DuPont environmental liabilities. The lawsuits include allegations for personal injury damages, damages under the Comprehensive Environmental Response Compensation and Liability Act (“CERCLA”) and damages under the Fair Housing Act (“FHA”). At separation, DuPont assigned Chemours its former plant site, which is located south of the residential portion of the Superfund area, and its responsibility for the environmental remediation at the Superfund site. DuPont has requested that Chemours defend and indemnify it, and Chemours has agreed to do so under a reservation of rights. Management believes a loss is reasonably possible but not estimable at this time.
Environmental
Chemours, by virtue of its status as a subsidiary of DuPont prior to the separation, is subject to contingencies pursuant to environmental laws and regulations that in the future may require further action to correct the effects on the environment of prior disposal practices or releases of chemical substances by Chemours or other parties. Chemours accrues for environmental remediation activities consistent with the policy set forth in Note 3. Much of this liability results from the Comprehensive Environmental Response, Compensation and Liability Act (CERCLA, often referred to as Superfund), RCRA and similar state and global laws. These laws require Chemours to undertake certain investigative, remediation and restoration activities at sites where Chemours conducts or once conducted operations or at sites where Chemours-generated waste was disposed. The accrual also includes estimated costs related to a number of sites identified for which it is probable that environmental remediation will be required, but which are not currently the subject of enforcement activities.
At December 31, 2016 and 2015, the Consolidated Balance Sheets included a liability relating to these matters of $278 and $297, respectively, which, in management’s opinion, is appropriate based on existing facts and circumstances. The time-frame for a site to go through all phases of remediation (investigation and active clean-up) may take about 15 to 20 years, followed by several years of on-going maintenance and monitoring (“OM&M”) activities. Remediation activities, including OM&M activities, vary substantially in duration and cost from site to site. These activities, and their associated costs, depend on the mix of unique site characteristics, evolving remediation technologies, diverse regulatory requirements, as well as the presence or absence of other potentially responsible parties. In addition, for claims that Chemours may be required to indemnify DuPont pursuant to the separation-related agreements, Chemours, through DuPont, has limited available information for certain sites or is in the early stages of discussions with regulators. For these sites in particular there may be considerable variability between the clean-up activities that are currently being undertaken or planned and the ultimate actions that could be required. Therefore, considerable uncertainty exists with respect to environmental remediation costs and, under adverse changes in circumstances, although deemed remote, the potential liability may range up to approximately $535 above the amount accrued at December 31, 2016.
For the years ended December 31, 2016, 2015, and 2014, Chemours incurred environmental remediation expenses of $44, $38, and $59, respectively.
F-37
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Based on existing facts and circumstances, management does not believe that any loss, in excess of amounts accrued, related to remediation activities at any individual site will have a material impact on the Company’s financial position, results of operations or cash flows at any given year, as such obligation can be satisfied or settled over many years.
Note 21. Financial Instruments
Derivative Instruments
Objectives and Strategies for Holding Derivative Instruments
In the ordinary course of business, Chemours enters into contractual arrangements (derivatives) to reduce its exposure to foreign currency risks. The Company has established a derivative program to be utilized for financial risk management. This program reflects varying levels of exposure coverage and time horizons based on an assessment of risk. The derivative program has procedures consistent with Chemours’ financial risk management policies and guidelines.
Foreign Currency Forward Contracts
Chemours uses foreign currency forward contracts to reduce its net exposure, by currency, related to non-functional currency-denominated monetary assets and liabilities of its operations so that exchange gains and losses resulting from exchange rate changes are minimized. These derivative instruments are not part of a cash flow hedge program or a fair value hedge program, and have not been designated as a hedge. Although all of the forward contracts are subject to an enforceable master netting agreement, Chemours has elected to present the derivative assets and liabilities on a gross basis in the Consolidated Balance Sheets. No collateral has been required for these contracts. All gains and losses resulting from the revaluation of the derivative assets and liabilities are recognized in other income, net in the Consolidated Statements of Operations during the period in which they occurred.
At December 31, 2016, there were 45 forward exchange currency contracts outstanding with an aggregate gross notional value of $518. Chemours recognized a net loss of $15 for the year ended December 31, 2016 and a net gain of $42 for the year ended December 31, 2015, which is recorded in “other income, net” in the Consolidated Statements of Operations.
Net Investment Hedge - Foreign Currency Borrowings
Beginning on July 1, 2015, Chemours designated its €360 million Euro notes (see Note 19) as a hedge of its net investments in certain of its international subsidiaries that use the Euro as functional currency in order to reduce the volatility in stockholders’ equity caused by the changes in foreign currency exchange rates of the Euro with respect to the U.S. Dollar. Chemours uses the spot method to measure the effectiveness of the net investment hedge. Under this method, for each reporting period, the change in the carrying value of the Euro notes due to remeasurement of the effective portion is reported in accumulated other comprehensive loss in the Consolidated Balance Sheet and the remaining change in the carrying value of the ineffective portion, if any, is recognized in other income, net in the Consolidated Statements of Operations. Chemours evaluates the effectiveness of its net investment hedge quarterly at the beginning of each quarter. Chemours did not record any ineffectiveness for the year ended December 31, 2016. The Company recognized a gain of $14 and $8 for the years ended December 31, 2016 and 2015 on its net investment hedges within accumulated other comprehensive income.
F-38
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Fair Value of Derivative Instruments
The table below presents the fair value of Chemours’ derivative assets and liabilities within the fair value hierarchy, as described in Note 3 to the Consolidated Financial Statements.
|
|
|
| Fair Value Using Level 2 Inputs |
| |||||
|
| Balance Sheet Location |
| December 31, 2016 |
|
| December 31, 2015 |
| ||
Asset derivatives: |
|
|
|
|
|
|
|
|
|
|
Foreign currency forward contracts |
| Accounts and notes receivable - trade, net |
| $ | 2 |
|
| $ | 2 |
|
Total asset derivatives |
|
|
| $ | 2 |
|
| $ | 2 |
|
Liability derivatives: |
|
|
|
|
|
|
|
|
|
|
Foreign currency forward contracts |
| Other accrued liabilities |
| $ | 4 |
|
| $ | 2 |
|
Total liability derivatives |
|
|
| $ | 4 |
|
| $ | 2 |
|
We classify our foreign currency forward contracts in Level 2 as the valuation inputs are based on quoted prices and market observable data of similar instruments. For derivative assets and liabilities, standard industry models are used to calculate the fair value of the various financial instruments based on significant observable market inputs, such as foreign exchange rates and implied volatilities obtained from various market sources. Market inputs are obtained from well-established and recognized vendors of market data and subjected to tolerance and quality checks.
Note 22. Long-Term Employee Benefits
Plans Covering Employees in the U.S.
Chemours sponsors a variety of employee benefit plans which cover substantially all U.S. employees. Prior to July 1, 2015, U.S. employees generally participated in DuPont’s primary pension plan, the Retirement Savings Plan and certain other long-term employee benefit plans. In conjunction with the separation on July 1, 2015, Chemours employees stopped participating in DuPont plans and became participants in newly established Chemours plans. DuPont retained all liabilities related to its U.S. plans post-separation.
On July 1, 2015, Chemours established a defined contribution plan, similar in design to the DuPont Retirement Savings plan, which covered all eligible U.S. employees. The purpose of the Plan is to encourage employees to save for their future retirement needs. The plan is a tax qualified contributory profit sharing plan, with cash or deferred arrangement, and any eligible employee of Chemours may participate. Chemours matches 100% of the first 6% of the employee’s contribution election. Chemours may also provide an additional discretionary retirement savings contribution to eligible employees’ compensation. The amount of this contribution, if any, is at the sole discretion of the Company. The plan’s matching contributions vest immediately upon contribution. The discretionary contribution vests for employees with at least three years of service.
In lieu of a defined benefit plan like DuPont’s primary pension plan, Chemours provides an enhanced 401(k) contribution for employees who previously participated in DuPont’s pension plan. The enhanced benefits consist of an additional contribution of 1% to 7% of the employee’s eligible compensation depending on the employee’s length of service with DuPont at the time of separation. The plan will continue for a period up to 2019, subject to early termination.
Plans Covering Employees Outside the U.S.
Pension coverage for employees of Chemours non-U.S. subsidiaries is provided, to the extent deemed appropriate, through separate plans established after separation and comparable to the DuPont plans in those countries. Obligations under such plans are funded by depositing funds with trustees, covered by insurance contracts or are unfunded.
Participation in the Plans
Prior to July 1, 2015, Chemours participated in DuPont’s U.S. and non-U.S. plans, except for the plans in the Netherlands and Taiwan, as though they were participants in a multi-employer plan with the other businesses of DuPont. The following table presents the
F-39
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
multi-employer pension expense allocated by DuPont to Chemours for the plans in which Chemours participated prior to separation. The allocation of cost was based on active employee headcount and is included in the Consolidated Statement of Operations. These amounts do not represent cash payments to DuPont or DuPont’s plans.
|
| EIN / Pension |
| Year Ended December 31, |
| |||||||||
Plan Name |
| Number |
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
DuPont Pension and Retirement Plan (U.S.) |
| 51-0014090/001 |
| $ | — |
|
| $ | 48 |
|
| $ | 51 |
|
All other U.S. and non-U.S. Plans |
|
|
|
| — |
|
|
| 5 |
|
|
| (1 | ) |
Single and Multiple Employer Plans
Beginning in the first quarter of 2015, Chemours has accounted for the plans covering its employees in the Netherlands and Taiwan as a multiple employer plan and a single employer plan, respectively. In the third quarter of 2015, in connection with the separation, additional plans in Germany, Belgium, Japan, Korea, Mexico and Switzerland were established. As of December 31, 2015, these plans were all accounted for as single employer plans.
The net periodic benefit costs for the pension and amounts recognized in other comprehensive income for the years ended December 31, 2016, 2015 and 2014 were as follows:
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Net periodic pension cost (income): |
|
|
|
|
|
|
|
|
|
|
|
|
Service cost |
| $ | 14 |
|
| $ | 16 |
|
| $ | — |
|
Interest cost |
|
| 19 |
|
|
| 19 |
|
|
| — |
|
Expected return on plan assets |
|
| (63 | ) |
|
| (83 | ) |
|
| — |
|
Amortization of actuarial loss |
|
| 23 |
|
|
| 16 |
|
|
| — |
|
Amortization of prior service (credit) cost |
|
| (1 | ) |
|
| 4 |
|
|
| — |
|
Curtailment gain |
|
| (2 | ) |
|
| — |
|
|
| — |
|
Settlement loss |
|
| 5 |
|
|
| — |
|
|
| — |
|
Net periodic pension income |
| $ | (5 | ) |
| $ | (28 | ) |
| $ | — |
|
Changes in plan assets and benefit obligations recognized in other comprehensive loss (income): |
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
| $ | 17 |
|
| $ | 11 |
|
| $ | — |
|
Amortization of actuarial loss |
|
| (28 | ) |
|
| (16 | ) |
|
| — |
|
Prior service credit |
|
| — |
|
|
| (24 | ) |
|
| — |
|
Amortization of prior service credit (cost) and curtailment gain |
|
| 3 |
|
|
| (4 | ) |
|
| — |
|
Effect of foreign exchange rates |
|
| (15 | ) |
|
| (33 | ) |
|
| — |
|
Total benefit recognized in other comprehensive income |
| $ | (23 | ) |
| $ | (66 | ) |
| $ | — |
|
Total recognized in net periodic pension income and other comprehensive income |
| $ | (28 | ) |
| $ | (94 | ) |
| $ | — |
|
The pre-tax amounts recognized in accumulated other comprehensive loss are summarized below:
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Net loss |
| $ | 336 |
|
| $ | 363 |
|
| $ | — |
|
Prior service credit |
|
| (11 | ) |
|
| (16 | ) |
|
| — |
|
Total amount recognized in accumulated other comprehensive loss |
| $ | 325 |
|
| $ | 347 |
|
| $ | — |
|
The estimated pre-tax net loss and prior service credit for the defined benefit pension plans that will be amortized from accumulated other comprehensive income (loss) into net periodic benefit cost during 2017 are $16 and $2, respectively.
F-40
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Summarized information on the Company’s pension benefit plans is as follows:
|
| 2016 |
|
| 2015 |
| ||
Change in benefit obligation |
|
|
|
|
|
|
|
|
Benefit obligation at beginning of year |
| $ | 1,103 |
|
| $ | — |
|
Assumption and establishment of pension plans |
|
| — |
|
|
| 1,332 |
|
Service cost |
|
| 14 |
|
|
| 16 |
|
Interest cost |
|
| 19 |
|
|
| 19 |
|
Plan participants’ contributions |
|
| 2 |
|
|
| 2 |
|
Actuarial loss (gain) |
|
| 69 |
|
|
| (76 | ) |
Benefits paid |
|
| (36 | ) |
|
| (39 | ) |
Plan Amendments |
|
| — |
|
|
| (24 | ) |
Curtailments |
|
| (3 | ) |
|
| — |
|
Settlements & transfers |
|
| (12 | ) |
|
| (6 | ) |
Other events |
|
| (2 | ) |
|
| — |
|
Currency translation |
|
| (49 | ) |
|
| (121 | ) |
Benefit obligation at end of year |
|
| 1,105 |
|
|
| 1,103 |
|
Change in plan assets |
|
|
|
|
|
|
|
|
Fair value of plan assets at beginning of year |
|
| 1,137 |
|
|
| — |
|
Assumption and establishment of pension plans |
|
| — |
|
|
| 1,297 |
|
Actual return on plan assets |
|
| 113 |
|
|
| (7 | ) |
Employer contributions |
|
| 16 |
|
|
| 16 |
|
Plan participants’ contributions |
|
| 2 |
|
|
| 2 |
|
Benefits paid |
|
| (36 | ) |
|
| (39 | ) |
Settlements & transfers |
|
| (12 | ) |
|
| (6 | ) |
Currency translation |
|
| (51 | ) |
|
| (126 | ) |
Fair value of plan assets at end of year |
|
| 1,169 |
|
|
| 1,137 |
|
Funded status at end of year |
| $ | 64 |
|
| $ | 34 |
|
The net amounts recognized in the Consolidated Balance Sheet as of December 31, 2016 and 2015 consist of:
|
| 2016 |
|
| 2015 |
| ||
Noncurrent assets |
| $ | 159 |
|
| $ | 138 |
|
Current liabilities |
|
| (1 | ) |
|
| (2 | ) |
Noncurrent liabilities |
|
| (94 | ) |
|
| (102 | ) |
Net amount recognized |
| $ | 64 |
|
| $ | 34 |
|
The accumulated benefit obligation for all pension plans was $1,042 and $1,030 as of December 31, 2016 and 2015, respectively.
The following information relates to pension plans with projected and accumulated benefit obligations in excess of the fair value of plan assets at December 31, 2016 and 2015:
Pension plans with projected benefit obligation in excess of plan assets at December 31, |
| 2016 |
|
| 2015 |
| ||
Projected benefit obligation |
| $ | 183 |
|
| $ | 194 |
|
Accumulated benefit obligation |
|
| 152 |
|
|
| 158 |
|
Fair value of plan assets |
|
| 87 |
|
|
| 93 |
|
F-41
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Pension plans with accumulated benefit obligation in excess of plan assets at December 31, |
| 2016 |
|
| 2015 |
| ||
Projected benefit obligation |
| $ | 179 |
|
| $ | 190 |
|
Accumulated benefit obligation |
|
| 151 |
|
|
| 157 |
|
Fair value of plan assets |
|
| 84 |
|
|
| 90 |
|
Assumptions
The Company generally utilizes discount rates that are developed by matching the expected cash flows of each benefit plan to various yield curves constructed from a portfolio of high quality, fixed income instruments provided by the plan’s actuary as of the measurement date. The expected rate of return on assets reflects economic assumptions applicable to each country.
The following assumptions have been used to determine the benefit obligations and net benefit cost:
Weighted average assumptions used to determine benefit obligations at December 31, |
| 2016 |
|
| 2015 |
| ||
Discount rate |
|
| 1.8 | % |
|
| 2.4 | % |
Rate of compensation increase1 |
|
| 2.5 | % |
|
| 2.6 | % |
| 1 | The rate of compensation increase represents the single annual effective salary increase that an average plan participant would receive during the participant’s entire career at Chemours. |
Weighted average assumptions used to determine net benefit cost for the years ended December 31, |
| 2016 |
|
| 2015 |
| ||
Discount rate |
|
| 2.4 | % |
|
| 1.7 | % |
Rate of compensation increase |
|
| 2.5 | % |
|
| 3.9 | % |
Expected return on plan assets |
|
| 5.7 | % |
|
| 7.2 | % |
Plan Assets
Each pension plan’s assets are invested through a master trust fund. The strategic asset allocation for the trust fund is selected by management, reflecting the results of comprehensive asset and liability modeling. Chemours establishes strategic asset allocation percentage targets and appropriate benchmarks for significant asset classes with the aim of achieving a prudent balance between return and risk. Strategic asset allocations in countries are selected in accordance with the laws and practices of those countries.
The weighted average target allocation for Chemours’ pension plan assets is summarized as follows:
|
| 2016 |
|
| 2015 |
| ||
Cash and cash equivalents |
|
| 2.5 | % |
|
| 2.7 | % |
U.S. and non-U.S. equity securities |
|
| 41.6 | % |
|
| 42.3 | % |
Fixed income securities |
|
| 55.9 | % |
|
| 55.0 | % |
Total |
|
| 100.0 | % |
|
| 100.0 | % |
Fixed income securities include corporate issued, government issued and asset backed securities. Corporate debt investments encompass a range of credit risk and industry diversification.
Fair value calculations may not be indicative of net realizable value or reflective of future fair values. Furthermore, although Chemours believes its valuation methods are appropriate and consistent with other market participants, the use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different fair value measurement at the reporting date.
F-42
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
The table below presents the fair values of Chemours’ pension assets by level within the fair value hierarchy, as described in Note 3, as of December 31, 2016 and 2015.
|
| Fair Value Measurements at December 31, 2016 |
| |||||||||
|
| Total |
|
| Level 1 |
|
| Level 2 |
| |||
Asset Category: |
|
|
|
|
|
|
|
|
|
|
|
|
Debt - government issued |
| $ | 433 |
|
| $ | 8 |
|
| $ | 425 |
|
Debt - corporate issued |
|
| 142 |
|
|
| 76 |
|
|
| 66 |
|
Debt - asset backed |
|
| 42 |
|
|
| 25 |
|
|
| 17 |
|
U.S. and non U.S. equities |
|
| 502 |
|
|
| 28 |
|
|
| 474 |
|
Derivatives - asset position |
|
| 3 |
|
|
| — |
|
|
| 3 |
|
Derivatives - liability position |
|
| (32 | ) |
|
| — |
|
|
| (32 | ) |
Cash and cash equivalents |
|
| 77 |
|
|
| 77 |
|
|
| — |
|
Other |
|
| 7 |
|
|
| — |
|
|
| 7 |
|
|
|
| 1,174 |
|
| $ | 214 |
|
| $ | 960 |
|
Pension trust payables, net 1 |
|
| (5 | ) |
|
|
|
|
|
|
|
|
Total |
| $ | 1,169 |
|
|
|
|
|
|
|
|
|
| 1 | Payables are primarily for investment securities purchased. |
|
| Fair Value Measurements at December 31, 2015 |
| |||||||||
|
| Total |
|
| Level 1 |
|
| Level 2 |
| |||
Asset Category: |
|
|
|
|
|
|
|
|
|
|
|
|
Debt - government issued |
| $ | 465 |
|
| $ | 7 |
|
| $ | 458 |
|
Debt - corporate issued |
|
| 148 |
|
|
| 60 |
|
|
| 88 |
|
Debt - asset backed |
|
| 33 |
|
|
| — |
|
|
| 33 |
|
U.S. and non U.S. equities |
|
| 460 |
|
|
| 37 |
|
|
| 423 |
|
Derivatives - asset position |
|
| 4 |
|
|
| — |
|
|
| 4 |
|
Derivatives - liability position |
|
| (16 | ) |
|
| — |
|
|
| (16 | ) |
Cash and cash equivalents |
|
| 40 |
|
|
| 40 |
|
|
| — |
|
Other |
|
| 6 |
|
|
| 4 |
|
|
| 2 |
|
|
|
| 1,140 |
|
| $ | 148 |
|
| $ | 992 |
|
Pension trust payables, net 1 |
|
| (3 | ) |
|
|
|
|
|
|
|
|
Total |
| $ | 1,137 |
|
|
|
|
|
|
|
|
|
| 1 | Payables are primarily for investment securities purchased. |
For pension plan assets classified as Level 1, total fair value is either the price of the most recent trade at the time of the market close or the official close price, as defined by the exchange on which the asset is most actively traded on the last trading day of the period, multiplied by the number of units held without consideration of transaction costs.
For pension benefit plan assets classified as Level 2, where the security is frequently traded in less active markets, fair value is based on the closing price at the end of the period; where the security is less frequently traded, fair value is based on the price a dealer would pay for the security or similar securities, adjusted for any terms specific to that asset or liability. Market inputs are obtained from well-established and recognized vendors of market data and subjected to tolerance and quality checks. For derivative assets and liabilities, standard industry models are used to calculate the fair value of the various financial instruments based on significant observable market inputs, such as foreign exchange rates, commodity prices, swap rates, interest rates and implied volatilities obtained from various market sources.
F-43
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Defined Benefit Plan
DuPont contributed, on behalf of Chemours, $35 to its pension plans other than the principal U.S. pension plan in 2014. DuPont contributed, on behalf of Chemours, $66 to its other long-term employee benefit plans in 2014. DuPont contributed, on behalf of Chemours, $38 in the first half of 2015 to its pension and other long-term benefit plans and Chemours contributed $8 during 2015 to its pension plans. Chemours contributed $16 during 2016. Chemours expects to contribute $15 to its pension plans in 2017.
Estimated future benefit payments
The following benefit payments are expected to be paid over the next five years and the five years thereafter as of December 31, 2016:
2017 |
| $ | 52 |
|
2018 |
|
| 40 |
|
2019 |
|
| 42 |
|
2020 |
|
| 43 |
|
2021 |
|
| 43 |
|
2022 - 2026 |
|
| 232 |
|
Defined Contribution Plan
DuPont’s contributions to the plan on behalf of Chemours were allocated in the amounts of $52 for the year ended December 31, 2014. In addition, DuPont contributed on behalf of Chemours about $26 to its defined contribution plans for the first half of 2015. From July 1 to December 31, 2015, Chemours contributed $28 to its defined contribution plan. For the year ended December 31, 2016, Chemours contributed $44 to its defined contribution plan.
Note 23. Stock-based Compensation
Total stock-based compensation cost included in the Consolidated Statements of Operations was $20, $17 and $7 for the years ended December 31, 2016, 2015 and 2014, respectively. The income tax benefits related to stock-based compensation arrangements were $8, $7 and $3 for the years ended December 31, 2016, 2015 and 2014, respectively.
Stock-based compensation expense in prior years and until separation on July 1, 2015 was allocated to Chemours based on the portion of DuPont’s incentive stock program in which Chemours employees participated. Adopted at separation, the Chemours Company Equity and Incentive Plan grants certain employees, independent contractors, or non-employee directors of the Company different forms of awards, including stock options, restricted share units (RSUs) and performance share units (PSUs). The equity and incentive plan has maximum shares reserve of 13,500,000 for the grant of equity awards plus the number of shares of converted awards (described below). As of December 31, 2016, 7,806,040 shares of equity and incentive plan reserve are still available for grants. Chemours Compensation Committee determines the long-term incentive mix, including stock options PSU and RSU, and may authorize new grants annually.
In accordance with the employee matters agreement between DuPont and Chemours, certain executives and employees were entitled to receive equity compensation awards of Chemours in replacement of previously outstanding awards granted under various DuPont stock incentive plans prior to the separation. In connection with the spin-off, these awards were converted into new Chemours equity awards using a formula designed to preserve the intrinsic value of the awards immediately prior to the July 1, 2015 spin-off. At the date of conversion, total intrinsic value of the converted options was $18. As a result of the conversion of these awards, we recorded an approximate $3 incremental charge in the third quarter of 2015. The terms and conditions of the DuPont awards were replicated and as necessary, adjusted to ensure that the vesting schedule and economic value of the awards was unchanged by the conversion. Subject to vesting condition of the award, a retirement eligible employee retains any granted awards upon retirement provided the employee has rendered at least six months of service following the grant date.
F-44
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Chemours granted non-qualified options to employees in July 2015 representing replacement of previously granted performance stock unit awards at DuPont. The July 2015 grant will cliff vest March 1, 2018 and expire 10 years from date of grant. Other than those options, Chemours’ expense related to stock options was entirely related to options granted to replace outstanding option awards from DuPont that were converted to Chemours options on July 1, 2015. During 2016, Chemours granted non-qualified options to certain of its employees, which will serially vest over a three-year period and expire 10 years from the date of grant.
The fair value related to stock options granted was determined using Black-Scholes option pricing model and the weighted average assumptions are shown in the table below:
|
| Year Ended December 31, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
Risk-free interest rate |
|
| 1.46 | % |
|
| 1.50 | % |
Expected term (years) |
|
| 6.00 |
|
|
| 5.40 |
|
Volatility |
|
| 60.00 | % |
|
| 42.00 | % |
Dividend yield |
|
| 2.14 | % |
|
| 6.90 | % |
Fair value per stock option |
| $ | 3.41 |
|
| $ | 3.17 |
|
The Company determined the dividend yield by dividing the expected annual dividend on the Company’s stock by the option exercise price. A historical daily measurement of volatility is determined based on Chemours peer companies’ average volatility adjusted for the Company’s debt leverage. The risk-free interest rate is determined by reference to the yield on an outstanding U.S. Treasury note with a term equal to the expected life of the option granted. The expected term is determined using a simplified approach, calculated as the midpoint between the vesting period and the contractual life of the award. After the separation, the simplified approach was used due to the Company’s lack of historical experience upon which to estimate the expected lives of the options.
The following table summarizes Chemours stock option activity for the year ended December 31, 2016.
|
| Number of Shares (in thousands) |
|
| Weighted Average Exercise Price (per share) |
|
| Weighted Average Remaining Contractual Term (years) |
|
| Aggregate Intrinsic Value (in thousands) |
| ||||
Outstanding, December 31, 2015 |
|
| 8,284 |
|
| $ | 14.66 |
|
|
| 4.82 |
|
| $ | — |
|
Granted |
|
| 1,435 |
|
|
| 5.73 |
|
|
|
|
|
|
|
|
|
Exercised |
|
| (947 | ) |
|
| 12.17 |
|
|
|
|
|
|
|
|
|
Forfeited |
|
| (447 | ) |
|
| 15.53 |
|
|
|
|
|
|
|
|
|
Expired |
|
| (356 | ) |
|
| 5.82 |
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2016 |
|
| 7,969 |
|
| $ | 13.72 |
|
|
| 5.08 |
|
| $ | 66,668 |
|
Exercisable, December 31, 2016 |
|
| 3,912 |
|
| $ | 14.04 |
|
|
| 3.25 |
|
| $ | 31,487 |
|
The aggregate intrinsic values in the table above represent the total pre-tax intrinsic value (the difference between the Company’s closing stock price on the last trading day of December 31, 2015 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their in-the-money options at quarter end. The amount changes based on the fair market value of the Company’s stock. Total intrinsic value of options exercised for year ended December 31, 2015 was insignificant.
As of December 31, 2016, there was $3,762 of unrecognized stock-based compensation expense related to stock options that is expected to be recognized over a weighted average period of 1.64 years.
Restricted Share Units
At the time of separation, in accordance with the employee matters agreement, the Company issued RSUs that serially vest over a three-year period and, upon vesting, convert one-for-one to Chemours common stock to replace similar DuPont awards. Under the
F-45
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
existing awards, a retirement eligible employee retains any granted awards upon retirement provided the employee has rendered at least six months of service following the grant date. Additional RSUs were also granted to key senior management employees with a performance condition. These RSUs vest on the third anniversary of the date of grant subject to the satisfaction of the performance condition. The fair value of all stock-settled RSUs is based upon the market price of the underlying common stock as of the grant date.
Non-vested awards of RSUs, both with and without performance feature, as of December 31, 2016 are shown below. The weighted average grant date fair value of RSUs granted and converted during 2016 was $6.20.
|
| Number of Shares (in thousands) |
|
| Weighted Average Grant Date Fair Value (per share) |
| ||
Nonvested, December 31, 2015 |
|
| 2,349 |
|
| $ | 14.87 |
|
Granted |
|
| 1,003 |
|
|
| 6.20 |
|
Vested |
|
| (829 | ) |
|
| 14.74 |
|
Forfeited |
|
| (207 | ) |
|
| 15.09 |
|
Nonvested, December 31, 2016 |
|
| 2,316 |
|
| $ | 11.23 |
|
As of December 31, 2016, there was $12,128 of unrecognized stock-based compensation expense related to RSUs that is expected to be recognized over a weighted average period of 1.54 years.
Performance Share Units
During 2016, Chemours issued PSUs to key senior management employees which, vest and convert one-for-one to Chemours’ common stock to the extent specified performance goals, including certain market-based conditions, are met over the three year performance period specified in the grant, subject to exceptions. Each grantee is granted a target award of PSUs, and may earn between 0% and 200% of the target amount depending on the Company’s performance against the performance goals. During the year ended December 31, 2016, the Company recorded stock-based compensation related to PSUs as a component of selling, general and administrative expense of approximately $2. There were no PSUs granted prior to 2016.
The following table provides compensation costs for stock-based compensation related to PSUs:
|
| Number of Shares (in thousands) |
|
| Weighted Average Grant Date Fair Value (per share) |
| ||
Nonvested, December 31, 2015 |
|
| — |
|
| $ | — |
|
Granted |
|
| 825 |
|
|
| 6.10 |
|
Vested |
|
| — |
|
|
| — |
|
Forfeited |
|
| (22 | ) |
|
| 6.10 |
|
Nonvested, December 31, 2016 |
|
| 803 |
|
| $ | 6.10 |
|
A portion of the fair value of PSUs was estimated at the grant date based on the probability of satisfying the market-based conditions associated with the PSUs using the Monte Carlo valuation method, which assesses probabilities of various outcomes of market conditions. The other portion of the fair value of the PSUs is based on the fair market value of the Company’s stock at the grant date, regardless of whether the market-based condition is satisfied. The per unit weighted average fair value at the date of grant for PSUs granted during the period ended December 31, 2016 was $6.10. The fair value of each PSU grant is amortized monthly into compensation expense on a straight-line basis over their respective vesting periods over 36 months. The accrual of compensation costs is based on our estimate of the final expected value of the award, and is adjusted as required for the portion based on the performance-based condition. The Company assumes that forfeitures will be minimal, and recognizes forfeitures as they occur, which results in a reduction in compensation expense. As the payout of PSUs includes dividend equivalents, no separate dividend yield assumption is required in calculating the fair value of the PSUs.
F-46
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Note 24. Geographic and Segment Information
Geographic Information
|
| For and As of the Year Ended December 31, |
| |||||||||||||||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||||||||||||||
|
| Net Sales 1 |
|
| Net Property, Plant and Equipment |
|
| Net Sales 1 |
|
| Net Property, Plant and Equipment |
|
| Net Sales 1 |
|
| Net Property, Plant and Equipment |
| ||||||
North America 2 |
| $ | 2,288 |
|
| $ | 1,861 |
|
| $ | 2,570 |
|
| $ | 2,184 |
|
| $ | 2,759 |
|
| $ | 2,273 |
|
Asia Pacific |
|
| 1,315 |
|
|
| 129 |
|
|
| 1,393 |
|
|
| 136 |
|
|
| 1,548 |
|
|
| 140 |
|
EMEA 3 |
|
| 1,081 |
|
|
| 278 |
|
|
| 977 |
|
|
| 308 |
|
|
| 1,190 |
|
|
| 372 |
|
Latin America 4 |
|
| 716 |
|
|
| 516 |
|
|
| 777 |
|
|
| 549 |
|
|
| 935 |
|
|
| 523 |
|
Total |
| $ | 5,400 |
|
| $ | 2,784 |
|
| $ | 5,717 |
|
| $ | 3,177 |
|
| $ | 6,432 |
|
| $ | 3,308 |
|
1 | Net sales are attributed to countries based on customer location. |
2 | Includes net sales in Canada of $125, $140 and $147 in 2016, 2015 and 2014, respectively. Includes net property, plant and equipment in Canada of $11, $13 and $14 in 2016, 2015 and 2014, respectively. |
3 | EMEA includes Europe, Middle East and Africa. |
4 | Latin America includes Mexico. |
Segment Information
Chemours’ operations are classified into three segments namely: Titanium Technologies, Fluoroproducts and Chemical Solutions. Corporate costs and certain legal and environmental expenses that are not aligned with the segments and foreign exchange gains and losses are reflected in Corporate and Other.
The Titanium Technologies segment is the leading global producer of TiO2, a premium white pigment used to deliver opacity. The Fluoroproducts segment is a leading global provider of fluoroproducts, such as refrigerants and industrial fluoropolymer resins. The Chemical Solutions segment is a leading North American provider of industrial and specialty chemicals, which includes cyanides, sulfur products and performance chemicals and intermediates, used in gold production, oil refining, agriculture, industrial polymers and other industries. Chemours operates globally in substantially all of its product lines.
Certain products are transferred between segments on a basis intended to reflect, as nearly as practicable, the market value of the products. These product transfers were limited and were not significant for each of the periods presented. Depreciation and amortization includes depreciation on research and development facilities and amortization of other intangible assets, excluding write-down of assets. Segment net assets includes net working capital, net property, plant and equipment, and other noncurrent operating assets and liabilities of the segment. This is the measure of segment assets reviewed by the Chief Operating Decision Maker (“CODM”).
Adjusted EBITDA is the primary measure of segment profitability used by the CODM and is defined as income (loss) before income taxes excluding the following:
| • | interest expense, depreciation and amortization, |
| • | non-operating pension and other postretirement employee benefit costs, which represent the components of net periodic costs (income) excluding service cost component, |
| • | exchange losses (gains) included in “other income, net” of the statement of operations, |
| • | restructuring, asset-related charges and other charges, net, |
| • | asset impairments, |
| • | losses (gains) on sale of business or assets, and |
| • | other items not considered indicative of our ongoing operational performance and expected to occur infrequently. |
F-47
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
All periods presented reflect the current definition of Adjusted EBITDA.
Year Ended December 31, |
| Titanium Technologies |
|
| Fluoroproducts |
|
| Chemical Solutions |
|
| Corporate and Other |
|
| Total |
| |||||
2016 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales to external customers |
| $ | 2,364 |
|
| $ | 2,264 |
|
| $ | 772 |
|
| $ | — |
|
| $ | 5,400 |
|
Adjusted EBITDA |
|
| 466 |
|
|
| 445 |
|
|
| 39 |
|
|
| (128 | ) |
|
| 822 |
|
Depreciation and amortization |
|
| 119 |
|
|
| 101 |
|
|
| 30 |
|
|
| 34 |
|
|
| 284 |
|
Equity in earnings of affiliates |
|
| — |
|
|
| 26 |
|
|
| — |
|
|
| 3 |
|
|
| 29 |
|
Net assets |
|
| 1,513 |
|
|
| 1,400 |
|
|
| 292 |
|
|
| (3,101 | ) |
|
| 104 |
|
Investments in affiliates |
|
| — |
|
|
| 116 |
|
|
| — |
|
|
| 20 |
|
|
| 136 |
|
Purchases of plant, property and equipment |
|
| 105 |
|
|
| 120 |
|
|
| 104 |
|
|
| 9 |
|
|
| 338 |
|
2015 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales to external customers |
| $ | 2,392 |
|
| $ | 2,230 |
|
| $ | 1,095 |
|
| $ | — |
|
| $ | 5,717 |
|
Adjusted EBITDA |
|
| 326 |
|
|
| 300 |
|
|
| 29 |
|
|
| (82 | ) |
|
| 573 |
|
Depreciation and amortization |
|
| 125 |
|
|
| 88 |
|
|
| 52 |
|
|
| 2 |
|
|
| 267 |
|
Equity in earnings of affiliates |
|
| — |
|
|
| 21 |
|
|
| — |
|
|
| 1 |
|
|
| 22 |
|
Net assets |
|
| 1,659 |
|
|
| 1,567 |
|
|
| 839 |
|
|
| (3,935 | ) |
|
| 130 |
|
Investments in affiliates |
|
| — |
|
|
| 127 |
|
|
| — |
|
|
| 9 |
|
|
| 136 |
|
Purchases of plant, property and equipment |
|
| 255 |
|
|
| 142 |
|
|
| 117 |
|
|
| 5 |
|
|
| 519 |
|
2014 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net sales to external customers |
| $ | 2,937 |
|
| $ | 2,327 |
|
| $ | 1,168 |
|
| $ | — |
|
| $ | 6,432 |
|
Adjusted EBITDA |
|
| 723 |
|
|
| 282 |
|
|
| 17 |
|
|
| (146 | ) |
|
| 876 |
|
Depreciation and amortization |
|
| 125 |
|
|
| 83 |
|
|
| 48 |
|
|
| 1 |
|
|
| 257 |
|
Equity in earnings of affiliates |
|
| — |
|
|
| 20 |
|
|
| — |
|
|
| — |
|
|
| 20 |
|
Net assets |
|
| 1,748 |
|
|
| 1,480 |
|
|
| 782 |
|
|
| (337 | ) |
|
| 3,673 |
|
Investments in affiliates |
|
| — |
|
|
| 124 |
|
|
| — |
|
|
| — |
|
|
| 124 |
|
Purchases of plant, property and equipment |
|
| 365 |
|
|
| 133 |
|
|
| 106 |
|
|
| — |
|
|
| 604 |
|
Total Adjusted EBITDA reconciles to total consolidated net (loss) income in the Consolidated Statements of Operations as follows:
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
(Loss) income before income taxes |
| $ | (11 | ) |
| $ | (188 | ) |
| $ | 550 |
|
Interest expense, net |
|
| 213 |
|
|
| 132 |
|
|
| — |
|
Depreciation and amortization |
|
| 284 |
|
|
| 267 |
|
|
| 257 |
|
Non-operating pension and other postretirement employee benefit (income) costs |
|
| (20 | ) |
|
| (3 | ) |
|
| 22 |
|
Exchange losses (gains) |
|
| 57 |
|
|
| (19 | ) |
|
| 66 |
|
Restructuring charges |
|
| 51 |
|
|
| 285 |
|
|
| 21 |
|
Asset related charges1 |
|
| 124 |
|
|
| 73 |
|
|
| — |
|
(Gains) losses on sale of assets and businesses |
|
| (254 | ) |
|
| 9 |
|
|
| (40 | ) |
Transaction costs2 |
|
| 19 |
|
|
| 9 |
|
|
| — |
|
Legal and other charges3 |
|
| 359 |
|
|
| 8 |
|
|
| — |
|
Adjusted EBITDA |
| $ | 822 |
|
| $ | 573 |
|
| $ | 876 |
|
1 | The year ended December 31, 2016 includes $48 pre-tax asset impairment of our Pascagoula Aniline facility (see Note 13), $58 pre-tax asset impairment in connection with the sale of the Sulfur business (see Note 7), $13 pre-tax asset impairment in connection with the sale of the Company’s corporate headquarters building (see Note 15) and other asset write-offs. The year ended December 31, 2015 includes $25 of goodwill impairment (see Note 14) and $45 asset impairment of RMS facility (see Note 13). All charges, except for the corporate headquarters building impairment (which is included in Corporate and Other), are recorded in the Chemical Solutions segment. |
|
2 | Includes accounting, legal and bankers transaction fees incurred related to the Company’s strategic initiatives, which includes pre-sale transaction costs incurred in connection with the sales of the C&D and Sulfur businesses (see Note 7). |
|
F-48
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
3 | Includes litigation settlements, water treatment accruals and $335 litigation accrual related to the PFOA MDL Settlement (see Note 20), and lease termination charges. |
|
Net sales to external customers by product group were as follows:
|
| Year Ended December 31, |
| |||||||||
|
| 2016 |
|
| 2015 |
|
| 2014 |
| |||
Titanium dioxide |
| $ | 2,364 |
|
| $ | 2,392 |
|
| $ | 2,937 |
|
Fluoropolymers |
|
| 1,171 |
|
|
| 1,246 |
|
|
| 1,326 |
|
Fluorochemicals |
|
| 1,093 |
|
|
| 984 |
|
|
| 1,001 |
|
Performance chemicals and intermediates |
|
| 383 |
|
|
| 551 |
|
|
| 621 |
|
Mining solutions 1 |
|
| 262 |
|
|
| 301 |
|
|
| 314 |
|
Sulfur |
|
| 127 |
|
|
| 243 |
|
|
| 233 |
|
Total net sales to external customers |
| $ | 5,400 |
|
| $ | 5,717 |
|
| $ | 6,432 |
|
1 | Previously known as Cyanides product group, which was renamed to Mining Solutions effective for the year ended December 31, 2016. |
Note 25. Accumulated Other Comprehensive Income (Loss)
The components of accumulated other comprehensive income (loss), net of income taxes, consisted of:
|
| Currency Translation Adjustment |
|
| Net Investment Hedge |
|
| Employee Benefits |
|
| Total |
| ||||
Balance at December 31, 2013 |
| $ | 19 |
|
| $ | — |
|
| $ | — |
|
| $ | 19 |
|
Other comprehensive income |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Balance at December 31, 2014 |
|
| 19 |
|
|
| — |
|
|
| — |
|
|
| 19 |
|
Assumption and establishment of pension plans, net |
|
| — |
|
|
| — |
|
|
| (311 | ) |
|
| (311 | ) |
Other comprehensive (loss) income |
|
| (304 | ) |
|
| 8 |
|
|
| 52 |
|
|
| (244 | ) |
Balance at December 31, 2015 |
|
| (285 | ) |
|
| 8 |
|
|
| (259 | ) |
|
| (536 | ) |
Other comprehensive (loss) income |
|
| (73 | ) |
|
| 14 |
|
|
| 18 |
|
|
| (41 | ) |
Balance at December 31, 2016 |
| $ | (358 | ) |
| $ | 22 |
|
| $ | (241 | ) |
| $ | (577 | ) |
Note 26. Quarterly Financial Data (Unaudited)
The following is a summary of the quarterly results of operations for the years ended December 31, 2016 and 2015.
|
| For the three months ended |
|
|
|
|
| |||||||||||||
2016 |
| March 31 |
|
| June 30 |
|
| September 30 |
|
| December 31 |
|
| Full Year |
| |||||
Net Sales |
| $ | 1,297 |
|
| $ | 1,383 |
|
| $ | 1,398 |
|
| $ | 1,322 |
|
| $ | 5,400 |
|
Cost of goods sold |
|
| 1,095 |
|
|
| 1,116 |
|
|
| 1,056 |
|
|
| 1,024 |
|
|
| 4,290 |
|
Income (loss) before income taxes |
|
| 70 |
|
|
| (41 | ) |
|
| 234 |
|
|
| (273 | ) |
|
| (11 | ) |
Net income (loss) |
|
| 51 |
|
|
| (18 | ) |
|
| 204 |
|
|
| (230 | ) |
|
| 7 |
|
Net income (loss) attributable to Chemours |
|
| 51 |
|
|
| (18 | ) |
|
| 204 |
|
|
| (230 | ) |
|
| 7 |
|
Basic earnings (loss) per share |
|
| 0.28 |
|
|
| (0.10 | ) |
|
| 1.12 |
|
|
| (1.26 | ) |
|
| 0.04 |
|
Diluted earnings (loss) per share |
|
| 0.28 |
|
|
| (0.10 | ) |
|
| 1.11 |
|
|
| (1.26 | ) |
|
| 0.04 |
|
F-49
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
| For the three months ended |
|
|
|
|
| ||||||||||||||
2015 |
| March 31 |
|
| June 30 |
|
| September 30 |
|
| December 31 |
|
| Full Year |
| |||||
Net Sales |
| $ | 1,363 |
|
| $ | 1,508 |
|
| $ | 1,486 |
|
| $ | 1,360 |
|
| $ | 5,717 |
|
Cost of goods sold |
|
| 1,111 |
|
|
| 1,282 |
|
|
| 1,222 |
|
|
| 1,147 |
|
|
| 4,762 |
|
Income (loss) before income taxes |
|
| 58 |
|
|
| (18 | ) |
|
| (107 | ) |
|
| (121 | ) |
|
| (188 | ) |
Net income (loss) |
|
| 43 |
|
|
| (18 | ) |
|
| (29 | ) |
|
| (86 | ) |
|
| (90 | ) |
Net income (loss) attributable to Chemours |
|
| 43 |
|
|
| (18 | ) |
|
| (29 | ) |
|
| (86 | ) |
|
| (90 | ) |
Basic earnings (loss) per share 1 |
|
| 0.24 |
|
|
| (0.10 | ) |
|
| (0.16 | ) |
|
| (0.48 | ) |
|
| (0.50 | ) |
Diluted earnings (loss) per share 1 |
|
| 0.24 |
|
|
| (0.10 | ) |
|
| (0.16 | ) |
|
| (0.48 | ) |
|
| (0.50 | ) |
1 | On July 1, 2015, E. I. du Pont de Nemours and Company distributed 180,966,833 shares of Chemours’ common stock to holders of its common stock. Basic and diluted earnings (loss) per common share for all periods prior to July 1, 2015 were calculated using the shares distributed on July 1, 2015. Refer to Note 10 for information regarding the calculation of basic and diluted earnings per share. |
* | The summation of the quarterly data may not foot due to rounding. |
Note 27. Guarantor Condensed Consolidating Financial Information
The following guarantor financial information is included in accordance with Rule 3-10 of Regulation S-X (Rule 3-10) in connection with the issuance of the Notes by The Chemours Company (the “Parent Issuer”). The Notes are fully and unconditionally guaranteed, jointly and severally, on a senior unsecured unsubordinated basis, in each case, subject to certain exceptions, by the Parent Issuer and by certain subsidiaries (together, the “Guarantor Subsidiaries”). Each of the Guarantor Subsidiaries is 100% owned by the Company. None of the other subsidiaries of the Company, either direct or indirect, guarantee the Notes (together, the “Non-Guarantor Subsidiaries”). The Guarantor Subsidiaries, excluding the Parent Issuer, will be automatically released from those guarantees upon the occurrence of certain customary release provisions.
The following condensed consolidating financial information is presented to comply with the Company’s requirements under Rule 3-10:
| • | the Consolidating Statements of Comprehensive Income (Loss) for the years ended December 31, 2016, 2015 and 2014; |
| • | the Consolidating Balance Sheets as of December 31, 2016 and 2015; |
| • | the Consolidating Statements of Cash Flows for the years ended December 31, 2016, 2015 and 2014. |
As discussed in Note 2, Chemours did not operate as a separate, stand-alone entity for the full period covered by consolidated financial statements. Prior to our spin-off on July 1, 2015, Chemours operations were included in DuPont’s financial results in different legal forms, including but not limited to wholly-owned subsidiaries for which Chemours was the sole business, components of legal entities in which Chemours operated in conjunction with other DuPont businesses and a majority owned joint venture. For periods prior to July 1, 2015, the condensed consolidated financial information has been prepared from DuPont’s historical accounting records and are presented on a stand-alone basis as if the business operations had been conducted independently from DuPont.
The condensed consolidating financial information is presented using the equity method of accounting for the Company’s investments in 100% owned subsidiaries. Under the equity method, the investments in subsidiaries are recorded at cost and adjusted for our share of the subsidiaries cumulative results of operations, capital contributions, distributions and other equity changes. The elimination entries principally eliminate investments in subsidiaries and intercompany balances and transactions. The financial information in this footnote should be read in conjunction with the consolidated financial statements presented and other notes related thereto contained in this Annual Report.
As discussed in Note 7, the Company entered into a stock and asset purchase agreement with Lanxess, pursuant to which Lanxess acquired the Company’s C&D business comprise of certain assets and subsidiaries of the Company, including International Dioxide, Inc., which was a guarantor subsidiary.
F-50
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Condensed Consolidating Statements of Comprehensive Income (Loss)
| Year Ended December 31, 2016 |
| |||||||||||||||||
| Parent Issuer |
|
| Guarantor Subsidiaries |
|
| Non-Guarantor Subsidiaries |
|
| Eliminations and Adjustments |
|
| Consolidated |
| |||||
Net sales | $ | — |
|
| $ | 3,749 |
|
| $ | 3,222 |
|
| $ | (1,571 | ) |
| $ | 5,400 |
|
Cost of goods sold |
| — |
|
|
| 3,218 |
|
|
| 2,615 |
|
|
| (1,543 | ) |
|
| 4,290 |
|
Gross profit |
| — |
|
|
| 531 |
|
|
| 607 |
|
|
| (28 | ) |
|
| 1,110 |
|
Selling, general and administrative expenses |
| 21 |
|
|
| 794 |
|
|
| 139 |
|
|
| (20 | ) |
|
| 934 |
|
Research and development expense |
| — |
|
|
| 77 |
|
|
| 3 |
|
|
| — |
|
|
| 80 |
|
Restructuring and asset related charges, net |
| — |
|
|
| 168 |
|
|
| 2 |
|
|
| — |
|
|
| 170 |
|
Total expenses |
| 21 |
|
|
| 1,039 |
|
|
| 144 |
|
|
| (20 | ) |
|
| 1,184 |
|
Equity in earnings of affiliates |
| — |
|
|
| 4 |
|
|
| 25 |
|
|
| — |
|
|
| 29 |
|
Equity in earnings of subsidiaries |
| 100 |
|
|
| — |
|
|
| — |
|
|
| (100 | ) |
|
| — |
|
Interest (expense) income, net |
| (211 | ) |
|
| (3 | ) |
|
| 1 |
|
|
| — |
|
|
| (213 | ) |
Intercompany interest income (expense), net |
| 60 |
|
|
| 4 |
|
|
| (64 | ) |
|
| — |
|
|
| — |
|
Other income, net |
| 20 |
|
|
| 193 |
|
|
| 54 |
|
|
| (20 | ) |
|
| 247 |
|
(Loss) income before income taxes |
| (52 | ) |
|
| (310 | ) |
|
| 479 |
|
|
| (128 | ) |
|
| (11 | ) |
(Benefit from) provision for income taxes |
| (59 | ) |
|
| (52 | ) |
|
| 100 |
|
|
| (7 | ) |
|
| (18 | ) |
Net income (loss) |
| 7 |
|
|
| (258 | ) |
|
| 379 |
|
|
| (121 | ) |
|
| 7 |
|
Less: Net income attributable to noncontrolling interests |
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Net income (loss) attributable to Chemours | $ | 7 |
|
| $ | (258 | ) |
| $ | 379 |
|
| $ | (121 | ) |
| $ | 7 |
|
Comprehensive (loss) income attributable to Chemours | $ | (34 | ) |
| $ | (255 | ) |
| $ | 321 |
|
| $ | (66 | ) |
| $ | (34 | ) |
F-51
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Condensed Consolidating Statements of Comprehensive Income (Loss)
| Year Ended December 31, 2015 |
| |||||||||||||||||
| Parent Issuer |
|
| Guarantor Subsidiaries |
|
| Non-Guarantor Subsidiaries |
|
| Eliminations and Adjustments |
|
| Consolidated |
| |||||
Net sales | $ | — |
|
| $ | 4,044 |
|
| $ | 3,269 |
|
| $ | (1,596 | ) |
| $ | 5,717 |
|
Cost of goods sold |
| — |
|
|
| 3,708 |
|
|
| 2,650 |
|
|
| (1,596 | ) |
|
| 4,762 |
|
Gross profit |
| — |
|
|
| 336 |
|
|
| 619 |
|
|
| — |
|
|
| 955 |
|
Selling, general and administrative expenses |
| 15 |
|
|
| 426 |
|
|
| 204 |
|
|
| (13 | ) |
|
| 632 |
|
Research and development expense |
| — |
|
|
| 95 |
|
|
| 2 |
|
|
| — |
|
|
| 97 |
|
Restructuring and asset related charges, net |
| — |
|
|
| 295 |
|
|
| 38 |
|
|
| — |
|
|
| 333 |
|
Goodwill impairment |
| — |
|
|
| 25 |
|
|
| — |
|
|
| — |
|
|
| 25 |
|
Total expenses |
| 15 |
|
|
| 841 |
|
|
| 244 |
|
|
| (13 | ) |
|
| 1,087 |
|
Equity in earnings of affiliates |
| — |
|
|
| 1 |
|
|
| 21 |
|
|
| — |
|
|
| 22 |
|
Equity in (net loss) earnings of subsidiaries |
| (47 | ) |
|
| — |
|
|
| — |
|
|
| 47 |
|
|
| — |
|
Interest expense, net |
| (131 | ) |
|
| (1 | ) |
|
| — |
|
|
| — |
|
|
| (132 | ) |
Intercompany interest income (expense), net |
| 44 |
|
|
| — |
|
|
| (44 | ) |
|
| — |
|
|
| — |
|
Other income (expense), net |
| 13 |
|
|
| 92 |
|
|
| (31 | ) |
|
| (20 | ) |
|
| 54 |
|
(Loss) income before income taxes |
| (136 | ) |
|
| (413 | ) |
|
| 321 |
|
|
| 40 |
|
|
| (188 | ) |
(Benefit from) provision for income taxes |
| (46 | ) |
|
| (89 | ) |
|
| 40 |
|
|
| (3 | ) |
|
| (98 | ) |
Net (loss) income |
| (90 | ) |
|
| (324 | ) |
|
| 281 |
|
|
| 43 |
|
|
| (90 | ) |
Less: Net income attributable to noncontrolling interests |
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Net (loss) income attributable to Chemours | $ | (90 | ) |
| $ | (324 | ) |
| $ | 281 |
|
| $ | 43 |
|
| $ | (90 | ) |
Comprehensive (loss) income attributable to Chemours | $ | (334 | ) |
| $ | (324 | ) |
| $ | 29 |
|
| $ | 295 |
|
| $ | (334 | ) |
F-52
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Condensed Consolidating Statements of Comprehensive Income (Loss)
| Year Ended December 31, 2014 |
| |||||||||||||||||
| Parent Issuer |
|
| Guarantor Subsidiaries |
|
| Non-Guarantor Subsidiaries |
|
| Eliminations and Adjustments |
|
| Consolidated |
| |||||
Net sales | $ | — |
|
| $ | 4,593 |
|
| $ | 3,722 |
|
| $ | (1,883 | ) |
| $ | 6,432 |
|
Cost of goods sold |
| — |
|
|
| 3,863 |
|
|
| 3,093 |
|
|
| (1,884 | ) |
|
| 5,072 |
|
Gross profit |
| — |
|
|
| 730 |
|
|
| 629 |
|
|
| 1 |
|
|
| 1,360 |
|
Selling, general and administrative expenses |
| — |
|
|
| 429 |
|
|
| 256 |
|
|
| — |
|
|
| 685 |
|
Research and development expense |
| — |
|
|
| 127 |
|
|
| 16 |
|
|
| — |
|
|
| 143 |
|
Restructuring and asset related charges, net |
| — |
|
|
| 11 |
|
|
| 10 |
|
|
| — |
|
|
| 21 |
|
Total expenses |
| — |
|
|
| 567 |
|
|
| 282 |
|
|
| — |
|
|
| 849 |
|
Equity in earnings of affiliates |
| — |
|
|
| — |
|
|
| 20 |
|
|
| — |
|
|
| 20 |
|
Equity in earnings of subsidiaries |
| 400 |
|
|
| — |
|
|
| — |
|
|
| (400 | ) |
|
| — |
|
Other income (expense), net |
| — |
|
|
| 80 |
|
|
| (61 | ) |
|
| — |
|
|
| 19 |
|
Income before income taxes |
| 400 |
|
|
| 243 |
|
|
| 306 |
|
|
| (399 | ) |
|
| 550 |
|
Provision for income taxes |
| — |
|
|
| 75 |
|
|
| 76 |
|
|
| (2 | ) |
|
| 149 |
|
Net income |
| 400 |
|
|
| 168 |
|
|
| 230 |
|
|
| (397 | ) |
|
| 401 |
|
Less: Net income attributable to noncontrolling interests |
| — |
|
|
| — |
|
|
| 1 |
|
|
| — |
|
|
| 1 |
|
Net income attributable to Chemours | $ | 400 |
|
| $ | 168 |
|
| $ | 229 |
|
| $ | (397 | ) |
| $ | 400 |
|
Comprehensive income attributable to Chemours | $ | 400 |
|
| $ | 168 |
|
| $ | 229 |
|
| $ | (397 | ) |
| $ | 400 |
|
F-53
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Condensed Consolidating Balance Sheets
| Year Ended December 31, 2016 |
| |||||||||||||||||
| Parent Issuer |
|
| Guarantor Subsidiaries |
|
| Non-Guarantor Subsidiaries |
|
| Eliminations and Adjustments |
|
| Consolidated |
| |||||
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents | $ | — |
|
| $ | 224 |
|
| $ | 678 |
|
| $ | — |
|
| $ | 902 |
|
Accounts and notes receivable - trade, net |
| — |
|
|
| 299 |
|
|
| 508 |
|
|
| — |
|
|
| 807 |
|
Intercompany receivable |
| 3 |
|
|
| 1,050 |
|
|
| 46 |
|
|
| (1,099 | ) |
|
| — |
|
Inventories |
| — |
|
|
| 341 |
|
|
| 476 |
|
|
| (50 | ) |
|
| 767 |
|
Prepaid expenses and other |
| — |
|
|
| 38 |
|
|
| 32 |
|
|
| 7 |
|
|
| 77 |
|
Total current assets |
| 3 |
|
|
| 1,952 |
|
|
| 1,740 |
|
|
| (1,142 | ) |
|
| 2,553 |
|
Property, plant and equipment |
| — |
|
|
| 6,136 |
|
|
| 1,861 |
|
|
| — |
|
|
| 7,997 |
|
Less: Accumulated depreciation |
| — |
|
|
| (4,285 | ) |
|
| (928 | ) |
|
| — |
|
|
| (5,213 | ) |
Net property, plant and equipment |
| — |
|
|
| 1,851 |
|
|
| 933 |
|
|
| — |
|
|
| 2,784 |
|
Goodwill and other intangible assets, net |
| — |
|
|
| 156 |
|
|
| 14 |
|
|
| — |
|
|
| 170 |
|
Investments in affiliates |
| — |
|
|
| — |
|
|
| 136 |
|
|
| — |
|
|
| 136 |
|
Investment in subsidiaries |
| 3,258 |
|
|
| — |
|
|
| — |
|
|
| (3,258 | ) |
|
| — |
|
Intercompany notes receivable |
| 1,150 |
|
|
| — |
|
|
| — |
|
|
| (1,150 | ) |
|
| — |
|
Other assets |
| 13 |
|
|
| 178 |
|
|
| 226 |
|
|
| — |
|
|
| 417 |
|
Total assets | $ | 4,424 |
|
| $ | 4,137 |
|
| $ | 3,049 |
|
| $ | (5,550 | ) |
| $ | 6,060 |
|
Liabilities and equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable | $ | — |
|
| $ | 573 |
|
| $ | 311 |
|
| $ | — |
|
| $ | 884 |
|
Short-term borrowings and current maturities of long-term debt |
| 15 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 15 |
|
Intercompany payable |
| 762 |
|
|
| 46 |
|
|
| 291 |
|
|
| (1,099 | ) |
|
| — |
|
Other accrued liabilities |
| 21 |
|
|
| 718 |
|
|
| 133 |
|
|
| — |
|
|
| 872 |
|
Total current liabilities |
| 798 |
|
|
| 1,337 |
|
|
| 735 |
|
|
| (1,099 | ) |
|
| 1,771 |
|
Long-term debt, net |
| 3,526 |
|
|
| 3 |
|
|
| — |
|
|
| — |
|
|
| 3,529 |
|
Intercompany notes payable |
| — |
|
|
| — |
|
|
| 1,150 |
|
|
| (1,150 | ) |
|
| — |
|
Deferred income taxes |
| — |
|
|
| 59 |
|
|
| 73 |
|
|
| — |
|
|
| 132 |
|
Other liabilities |
| — |
|
|
| 428 |
|
|
| 96 |
|
|
| — |
|
|
| 524 |
|
Total liabilities |
| 4,324 |
|
|
| 1,827 |
|
|
| 2,054 |
|
|
| (2,249 | ) |
|
| 5,956 |
|
Commitments and contingent liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Chemours stockholders’ equity |
| 100 |
|
|
| 2,310 |
|
|
| 991 |
|
|
| (3,301 | ) |
|
| 100 |
|
Noncontrolling interests |
| — |
|
|
| — |
|
|
| 4 |
|
|
| — |
|
|
| 4 |
|
Total equity |
| 100 |
|
|
| 2,310 |
|
|
| 995 |
|
|
| (3,301 | ) |
|
| 104 |
|
Total liabilities and equity | $ | 4,424 |
|
| $ | 4,137 |
|
| $ | 3,049 |
|
| $ | (5,550 | ) |
| $ | 6,060 |
|
F-54
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Condensed Consolidating Balance Sheets
| Year Ended December 31, 2015 |
| |||||||||||||||||
| Parent Issuer |
|
| Guarantor Subsidiaries |
|
| Non-Guarantor Subsidiaries |
|
| Eliminations and Adjustments |
|
| Consolidated |
| |||||
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents | $ | — |
|
| $ | 95 |
|
| $ | 271 |
|
| $ | — |
|
| $ | 366 |
|
Accounts and notes receivable - trade, net |
| — |
|
|
| 344 |
|
|
| 515 |
|
|
| — |
|
|
| 859 |
|
Intercompany receivable |
| 3 |
|
|
| 459 |
|
|
| 54 |
|
|
| (516 | ) |
|
| — |
|
Inventories |
| — |
|
|
| 493 |
|
|
| 501 |
|
|
| (22 | ) |
|
| 972 |
|
Prepaid expenses and other |
| — |
|
|
| 49 |
|
|
| 52 |
|
|
| 3 |
|
|
| 104 |
|
Total current assets |
| 3 |
|
|
| 1,440 |
|
|
| 1,393 |
|
|
| (535 | ) |
|
| 2,301 |
|
Property, plant and equipment |
| — |
|
|
| 7,070 |
|
|
| 1,945 |
|
|
| — |
|
|
| 9,015 |
|
Less: Accumulated depreciation |
| — |
|
|
| (4,899 | ) |
|
| (939 | ) |
|
| — |
|
|
| (5,838 | ) |
Net property, plant and equipment |
| — |
|
|
| 2,171 |
|
|
| 1,006 |
|
|
| — |
|
|
| 3,177 |
|
Goodwill and other intangible assets, net |
| — |
|
|
| 151 |
|
|
| 25 |
|
|
| — |
|
|
| 176 |
|
Investments in affiliates |
| — |
|
|
| 9 |
|
|
| 127 |
|
|
| — |
|
|
| 136 |
|
Investment in subsidiaries |
| 3,105 |
|
|
| — |
|
|
| — |
|
|
| (3,105 | ) |
|
| — |
|
Intercompany notes receivable |
| 1,150 |
|
|
| — |
|
|
| — |
|
|
| (1,150 | ) |
|
| — |
|
Other assets |
| 19 |
|
|
| 275 |
|
|
| 214 |
|
|
| — |
|
|
| 508 |
|
Total assets | $ | 4,277 |
|
| $ | 4,046 |
|
| $ | 2,765 |
|
| $ | (4,790 | ) |
| $ | 6,298 |
|
Liabilities and equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable | $ | — |
|
| $ | 637 |
|
| $ | 336 |
|
| $ | — |
|
| $ | 973 |
|
Short-term borrowings and current maturities of long-term debt |
| 15 |
|
|
| 24 |
|
|
| — |
|
|
| — |
|
|
| 39 |
|
Intercompany payable |
| 202 |
|
|
| 54 |
|
|
| 260 |
|
|
| (516 | ) |
|
| — |
|
Other accrued liabilities |
| 21 |
|
|
| 287 |
|
|
| 146 |
|
|
| - |
|
|
| 454 |
|
Total current liabilities |
| 238 |
|
|
| 1,002 |
|
|
| 742 |
|
|
| (516 | ) |
|
| 1,466 |
|
Long-term debt, net |
| 3,913 |
|
|
| 2 |
|
|
| — |
|
|
| - |
|
|
| 3,915 |
|
Intercompany notes payable |
| — |
|
|
| — |
|
|
| 1,150 |
|
|
| (1,150 | ) |
|
| - |
|
Deferred income taxes |
| — |
|
|
| 173 |
|
|
| 61 |
|
|
| — |
|
|
| 234 |
|
Other liabilities |
| — |
|
|
| 456 |
|
|
| 97 |
|
|
| — |
|
|
| 553 |
|
Total liabilities |
| 4,151 |
|
|
| 1,633 |
|
|
| 2,050 |
|
|
| (1,666 | ) |
|
| 6,168 |
|
Commitments and contingent liabilities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Chemours stockholders’ equity |
| 126 |
|
|
| 2,413 |
|
|
| 711 |
|
|
| (3,124 | ) |
|
| 126 |
|
Noncontrolling interests |
| — |
|
|
| — |
|
|
| 4 |
|
|
| — |
|
|
| 4 |
|
Total equity |
| 126 |
|
|
| 2,413 |
|
|
| 715 |
|
|
| (3,124 | ) |
|
| 130 |
|
Total liabilities and equity | $ | 4,277 |
|
| $ | 4,046 |
|
| $ | 2,765 |
|
| $ | (4,790 | ) |
| $ | 6,298 |
|
F-55
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Condensed Consolidating Statements of Cash Flows
| Year Ended December 31, 2016 |
| |||||||||||||||||
| Parent Issuer |
|
| Guarantor Subsidiaries |
|
| Non-Guarantor Subsidiaries |
|
| Eliminations and Adjustments |
|
| Consolidated |
| |||||
Operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash (used for) provided by operating activities | $ | (176 | ) |
| $ | 355 |
|
| $ | 415 |
|
| $ | — |
|
| $ | 594 |
|
Investing activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment |
| — |
|
|
| (233 | ) |
|
| (105 | ) |
|
| — |
|
|
| (338 | ) |
Proceeds from sales of assets, net |
| — |
|
|
| 591 |
|
|
| 117 |
|
|
| — |
|
|
| 708 |
|
Intercompany investing activities |
| — |
|
|
| (560 | ) |
|
| — |
|
|
| 560 |
|
|
| — |
|
Foreign exchange contract settlements |
| — |
|
|
| (12 | ) |
|
| — |
|
|
| — |
|
|
| (12 | ) |
Investment in affiliates |
| — |
|
|
| — |
|
|
| (1 | ) |
|
| — |
|
|
| (1 | ) |
Cash (used for) provided by investing activities |
| — |
|
|
| (214 | ) |
|
| 11 |
|
|
| 560 |
|
|
| 357 |
|
Financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intercompany short-term borrowings, net | $ | 560 |
|
| $ | — |
|
| $ | — |
|
| $ | (560 | ) |
| $ | — |
|
Debt repayments |
| (369 | ) |
|
| (12 | ) |
|
| — |
|
|
| — |
|
|
| (381 | ) |
Dividends paid |
| (22 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (22 | ) |
Debt issuance costs |
| (4 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (4 | ) |
Proceeds from issuance of stock options |
| 11 |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| 11 |
|
Cash provided by (used for) financing activities |
| 176 |
|
|
| (12 | ) |
|
| — |
|
|
| (560 | ) |
|
| (396 | ) |
Effect of exchange rate changes on cash |
| — |
|
|
| — |
|
|
| (19 | ) |
|
| — |
|
|
| (19 | ) |
Increase in cash and cash equivalents |
| — |
|
|
| 129 |
|
|
| 407 |
|
|
| — |
|
|
| 536 |
|
Cash and cash equivalents at beginning of year |
| — |
|
|
| 95 |
|
|
| 271 |
|
|
| — |
|
|
| 366 |
|
Cash and cash equivalents at end of year | $ | — |
|
| $ | 224 |
|
| $ | 678 |
|
| $ | — |
|
| $ | 902 |
|
F-56
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Condensed Consolidating Statements of Cash Flows
| Year Ended December 31, 2015 |
| |||||||||||||||||
| Parent Issuer |
|
| Guarantor Subsidiaries |
|
| Non-Guarantor Subsidiaries |
|
| Eliminations and Adjustments |
|
| Consolidated |
| |||||
Operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash (used for) provided by operating activities | $ | (119 | ) |
| $ | 171 |
|
| $ | 121 |
|
| $ | 9 |
|
| $ | 182 |
|
Investing activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment |
| — |
|
|
| (292 | ) |
|
| (227 | ) |
|
| — |
|
|
| (519 | ) |
Proceeds from sales of assets, net |
| — |
|
|
| 6 |
|
|
| 6 |
|
|
| — |
|
|
| 12 |
|
Intercompany investing activities |
| — |
|
|
| (202 | ) |
|
| — |
|
|
| 202 |
|
|
| — |
|
Foreign exchange contract settlements |
| — |
|
|
| 42 |
|
|
| — |
|
|
| — |
|
|
| 42 |
|
Investment in affiliates |
| — |
|
|
| — |
|
|
| (32 | ) |
|
| — |
|
|
| (32 | ) |
Cash used for investing activities |
| — |
|
|
| (446 | ) |
|
| (253 | ) |
|
| 202 |
|
|
| (497 | ) |
Financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of debt, net |
| 3,489 |
|
|
| 2 |
|
|
| — |
|
|
| — |
|
|
| 3,491 |
|
Intercompany short-term borrowings, net |
| 202 |
|
|
| — |
|
|
| — |
|
|
| (202 | ) |
|
| - |
|
Debt repayments |
| (8 | ) |
|
| (2 | ) |
|
| — |
|
|
| — |
|
|
| (10 | ) |
Dividends paid |
| (105 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (105 | ) |
Debt issuance costs |
| (79 | ) |
|
| — |
|
|
| — |
|
|
| — |
|
|
| (79 | ) |
Cash provided at separation by DuPont |
| — |
|
|
| 87 |
|
|
| 160 |
|
|
| — |
|
|
| 247 |
|
Net transfers (to) from DuPont |
| (3,380 | ) |
|
| 283 |
|
|
| 249 |
|
|
| (9 | ) |
|
| (2,857 | ) |
Cash provided by financing activities |
| 119 |
|
|
| 370 |
|
|
| 409 |
|
|
| (211 | ) |
|
| 687 |
|
Effect of exchange rate changes on cash |
| — |
|
|
| — |
|
|
| (6 | ) |
|
| — |
|
|
| (6 | ) |
Increase in cash and cash equivalents |
| — |
|
|
| 95 |
|
|
| 271 |
|
|
| — |
|
|
| 366 |
|
Cash and cash equivalents at beginning of year |
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Cash and cash equivalents at end of year | $ | — |
|
| $ | 95 |
|
| $ | 271 |
|
| $ | — |
|
| $ | 366 |
|
F-57
The Chemours Company
Notes to the Consolidated Financial Statements
(Dollars in millions, except per share)
Condensed Consolidating Statements of Cash Flows
| Year Ended December 31, 2014 |
| |||||||||||||||||
| Parent Issuer |
|
| Guarantor Subsidiaries |
|
| Non-Guarantor Subsidiaries |
|
| Eliminations and Adjustments |
|
| Consolidated |
| |||||
Operating activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash provided by operating activities | $ | — |
|
| $ | 302 |
|
| $ | 208 |
|
| $ | (5 | ) |
| $ | 505 |
|
Investing activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant and equipment |
| — |
|
|
| (287 | ) |
|
| (317 | ) |
|
| — |
|
|
| (604 | ) |
Proceeds from sales of assets, net |
| — |
|
|
| 30 |
|
|
| 2 |
|
|
| — |
|
|
| 32 |
|
Investment in affiliates |
| — |
|
|
| - |
|
|
| (8 | ) |
|
| — |
|
|
| (8 | ) |
Other investing activities |
| — |
|
|
| 20 |
|
|
| — |
|
|
| — |
|
|
| 20 |
|
Cash used for investing activities |
| — |
|
|
| (237 | ) |
|
| (323 | ) |
|
| — |
|
|
| (560 | ) |
Financing activities |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net transfers (to) from DuPont |
| — |
|
|
| (65 | ) |
|
| 115 |
|
|
| 5 |
|
|
| 55 |
|
Cash (used for) provided by financing activities |
| — |
|
|
| (65 | ) |
|
| 115 |
|
|
| 5 |
|
|
| 55 |
|
Effect of exchange rate changes on cash |
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Increase in cash and cash equivalents |
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Cash and cash equivalents at beginning of year |
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
Cash and cash equivalents at end of year | $ | — |
|
| $ | — |
|
| $ | — |
|
| $ | — |
|
| $ | — |
|
F-58