The Company also reported the TRAEs for 99 patients who received 1,200 mg of RMC-9805 a day (1,200 mg once daily (n=60) or 600 mg twice daily (n=39)) as of the Data Cutoff Date (Table 2). The most common TRAEs observed were GI-related toxicities and rash. TRAEs of any grade led to dose reduction in approximately 4% of these patients. No TRAEs led to treatment discontinuation in these patients, and there were no treatment-related Grade 4 or 5 AEs or SAEs reported.
Table 2. RMC-9805-001: TRAEs for patients treated with 1,200 mg a day (1,200 mg once daily (n=60) or 600 mg twice daily (n=39))
| | | | | | | | |
Maximum Severity of TRAEs | | Grade 1 | | Grade 2 | | Grade 3 | | Any Grade |
TRAEs occurring in ≥10% of patients, n (%) | | | | | | | | |
Nausea | | 23 (23%) | | 4 (4%) | | 0 (0%) | | 27 (27%) |
Diarrhea | | 16 (16%) | | 4 (4%) | | 0 (0%) | | 20 (20%) |
Vomiting | | 13 (13%) | | 2 (2%) | | 0 (0%) | | 15 (15%) |
Rash‡ | | 10 (10%) | | 0 (0%) | | 0 (0%) | | 10 (10%) |
Other select TRAEs, n (%) | | | | | | | | |
ALT elevation | | 5 (5%) | | 0 (0%) | | 1 (1%) | | 6 (6%) |
AST elevation | | 3 (3%) | | 1 (1%) | | 0 (0%) | | 4 (4%) |
Stomatitis | | 0 (0%) | | 0 (0%) | | 0 (0%) | | 0 (0%) |
TRAEs leading to dose reduction, n (%) | | 4 (4%) | | 0 (0%) | | 0 (0%) | | 4 (4%) |
TRAEs leading to treatment discontinuation, n (%) | | 0 (0%) | | 0 (0%) | | 0 (0%) | | 0 (0%) |
| | ‡ | Includes preferred terms of dermatitis, dermatitis acneiform, dermatitis psoriasiform, eczema, erthyema, rash, rash erythematous, rash macular, rash maculo-papular, rash papular, rash pruritic and rash pustular. |
ALT, alanine transaminase; AST, aspartate transferase.
The Company also reported best percentage change in tumor size from baseline as of the Data Cutoff Date for patients with pancreatic ductal adenocarcinoma (“PDAC”) in the second-line or later (“2L+”) setting who received 1,200 mg a day (1,200 mg once daily (n=20) or 600 mg twice daily (n=20)) (Figure 1). For these patients who received a first dose of RMC-9805 at least 14 weeks prior to the Data Cutoff Date, the objective response rate (“ORR”) (including both confirmed and pending responses) was 30%, and the disease control rate (“DCR”) was 80%.
Figure 1. RMC-9805-001: Best percentage change in tumor size from baseline and response rates for 2L+ PDAC patients treated with 1,200 mg daily
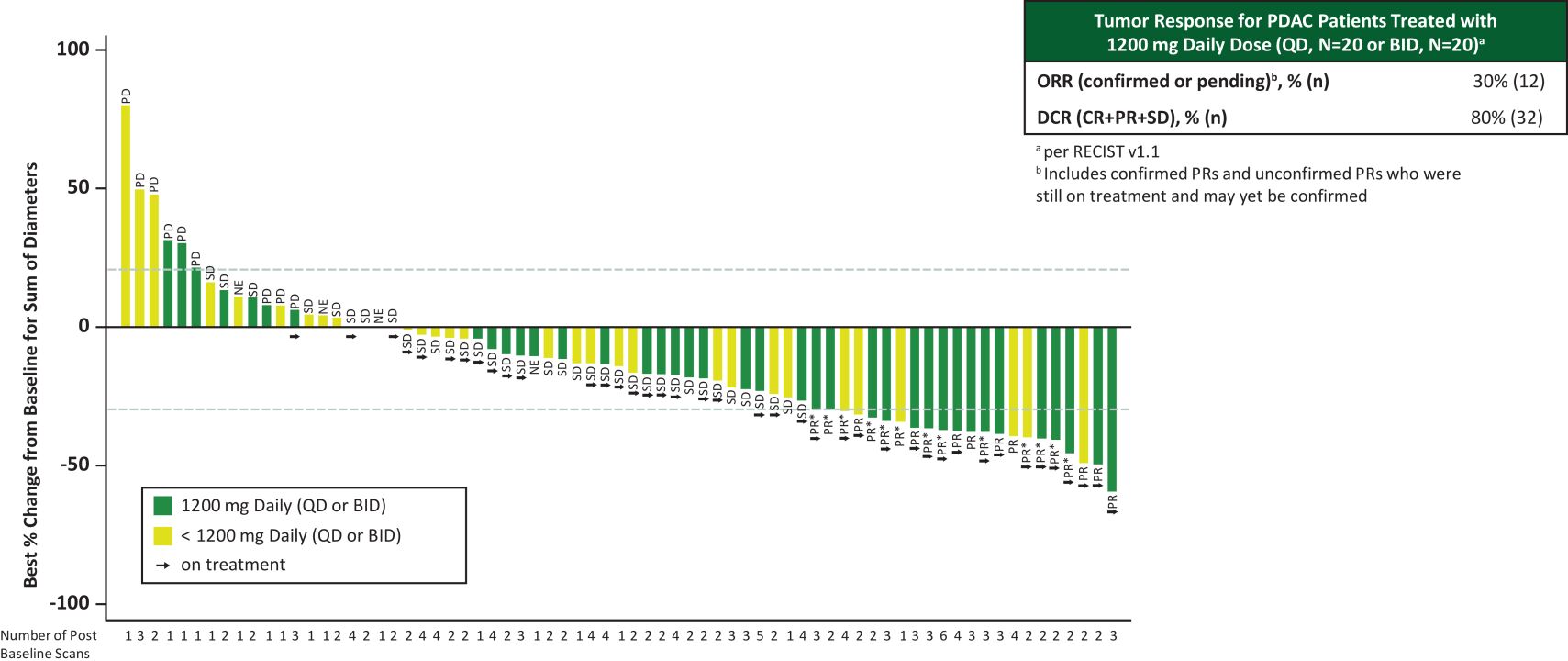
Data Cutoff Date of September 2, 2024
All treated patients with PDAC who received a first daily dose at least 14 weeks prior to Data Cutoff Date (applies to Waterfall plot and ORR table); 3 additional patients (n=2 at 1200 mg daily; n=1 at < 1200 mg daily) are not displayed on the Waterfall plot due to withdrawal of consent or clinical progression.
Among patients with a response (confirmed or unconfirmed), 55% of first response occurred after 2 months of RMC-9805 treatment (all dose levels).
CR, complete response; NE, not evaluable; PD, progressive disease; PR, partial response; PRu*, unconfirmed partial response; SD, stable disease; RECIST, Response Evaluation Criteria in Solid Tumors.
