Exhibit 99.2

CollPlant Corporate Presentation Nasdaq : CLGN February 2025

The statements made in this presentation speak only as of the date stated herein, and subsequent events and developments may cause our expectations and beliefs to change. Unless otherwise required by applicable securities laws, we do not intend, nor do we undertake any obligation, to update or revise any forward - looking statements contained in this presentation to reflect subsequent information, events, results or circumstances or otherwise. While we may elect to update these forward - looking statements publicly at some point in the future, we specifically disclaim any obligation to do so, whether as a result of new information, future events or otherwise, except as required by law. Certain statements in this presentation constitute "forward - looking statements" within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act and are usually identified by the use of words such as "anticipates," "believes," "estimates," "expects," "intends," "may," "plans," "projects," "seeks," "should," "will," and variations of such words or similar expressions. We intend these forward - looking statements to be covered by the safe harbor provisions for forward - looking statements contained in Section 27A of the Securities Act and Section 21E of the Securities Exchange Act and are making this statement for purposes of complying with those safe harbor provisions. These forward - looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those forward - looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward - looking statements and are expected to be affected by a variety of risks and factors that are beyond our control. Risks and uncertainties for our company include, but are not limited to: the Company’s history of significant losses and its need to raise additional capital and its inability to obtain additional capital on acceptable terms, or at all; the Company’s expectations regarding the timing and cost of commencing clinical trials with respect to tissues and organs which are based on its rhCollagen based Bioink and products for medical aesthetics; the Company’s ability to obtain favorable pre - clinical and clinical trial results; regulatory action with respect to rhCollagen based BioInkand medical aesthetics products including but not limited to acceptance of an application for marketing authorization, review and approval of such application, and, if approved, the scope of the approved indication and labeling; commercial success and market acceptance of the Company’s rhCollagen based products, in 3D bioprinting and medical aesthetics; the Company’s ability to establish sales and marketing capabilities or enter into agreements with third parties and its reliance on third party distributors and resellers; the Company’s ability to establish and maintain strategic partnerships and other corporate collaborations; the Company’s reliance on third parties to conduct some or all aspects of its product manufacturing; the scope of protection the Company is able to establish and maintain for intellectual property rights and the Company’s ability to operate its business without infringing the intellectual property rights of others; the overall global economic environment; the impact of competition and new technologies; general market, political, and economic conditions in the countries in which the Company operates; projected. capital expenditures and liquidity; changes in the Company’s strategy; and litigation and regulatory proceedings. Many of these factors that will determine actual results are beyond our ability to control or predict. For a discussion of the factors that may cause our actual results, performance or achievements to differ materially from any future results, performance or achievements expressed or implied in such forward - looking statements, see the “Risk Factors” section of included in our most recently filed Annual Report on Form 20 - F. Existing and prospective investors are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date hereof. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. 2 Forward - Looking Statements

About CollPlant A regenerative and aesthetic medicine company Developing innovative technologies and products for tissue regeneration and organ manufacturing The Company's products and candidates are based on rhCollagen that is produced in genetically engineered tobacco plants Strategic agreement with global, top - tier pharmaceutical company AbbVie NASDAQ (CLGN), listed since 2018 3
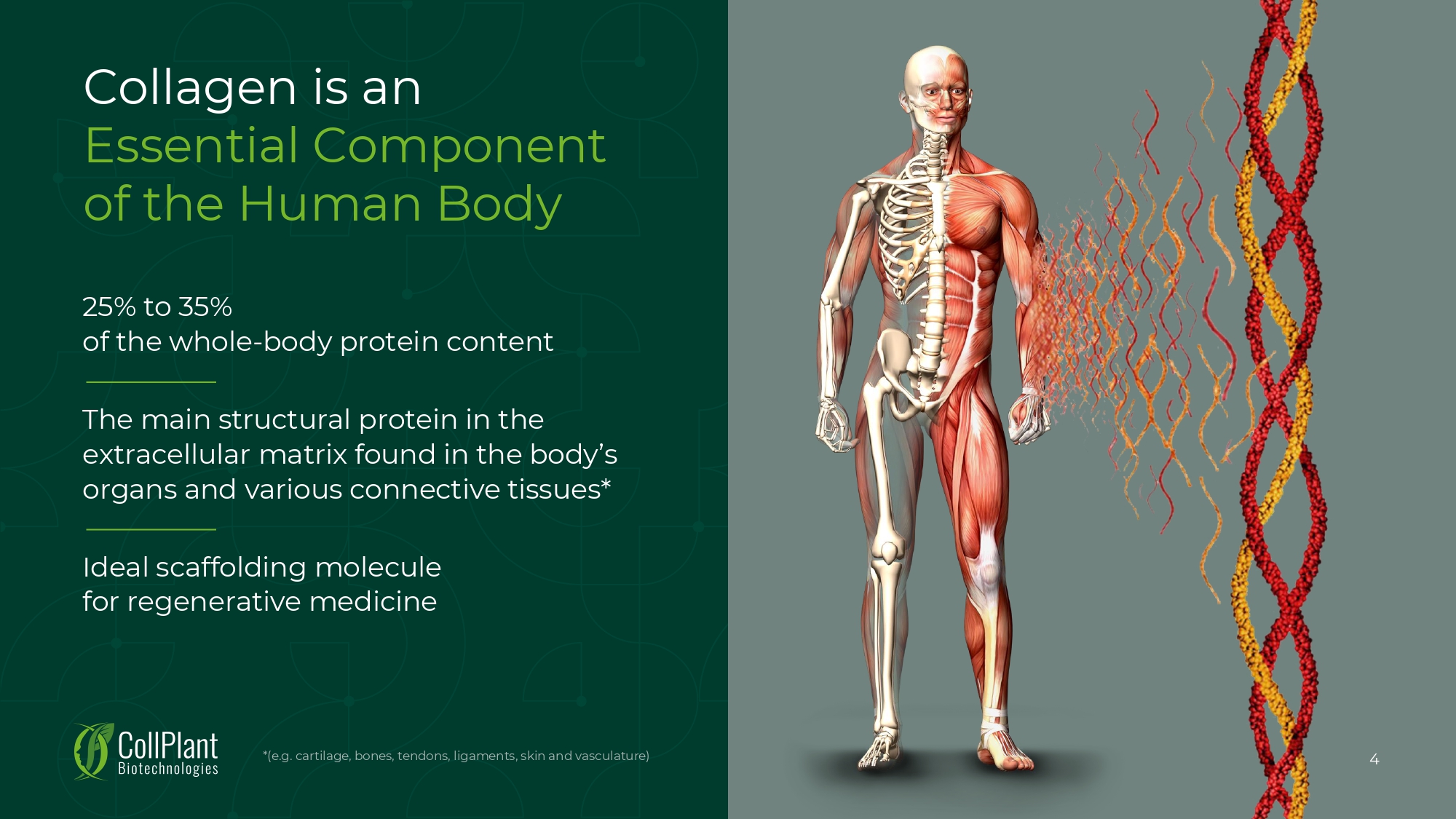
Collagen is an Essential Component of the Human Body 25% to 35% of the whole - body protein content The main structural protein in the extracellular matrix found in the body’s organs and various connective tissues* Ideal scaffolding molecule for regenerative medicine *(e.g. cartilage, bones, tendons, ligaments, skin and vasculature) 4
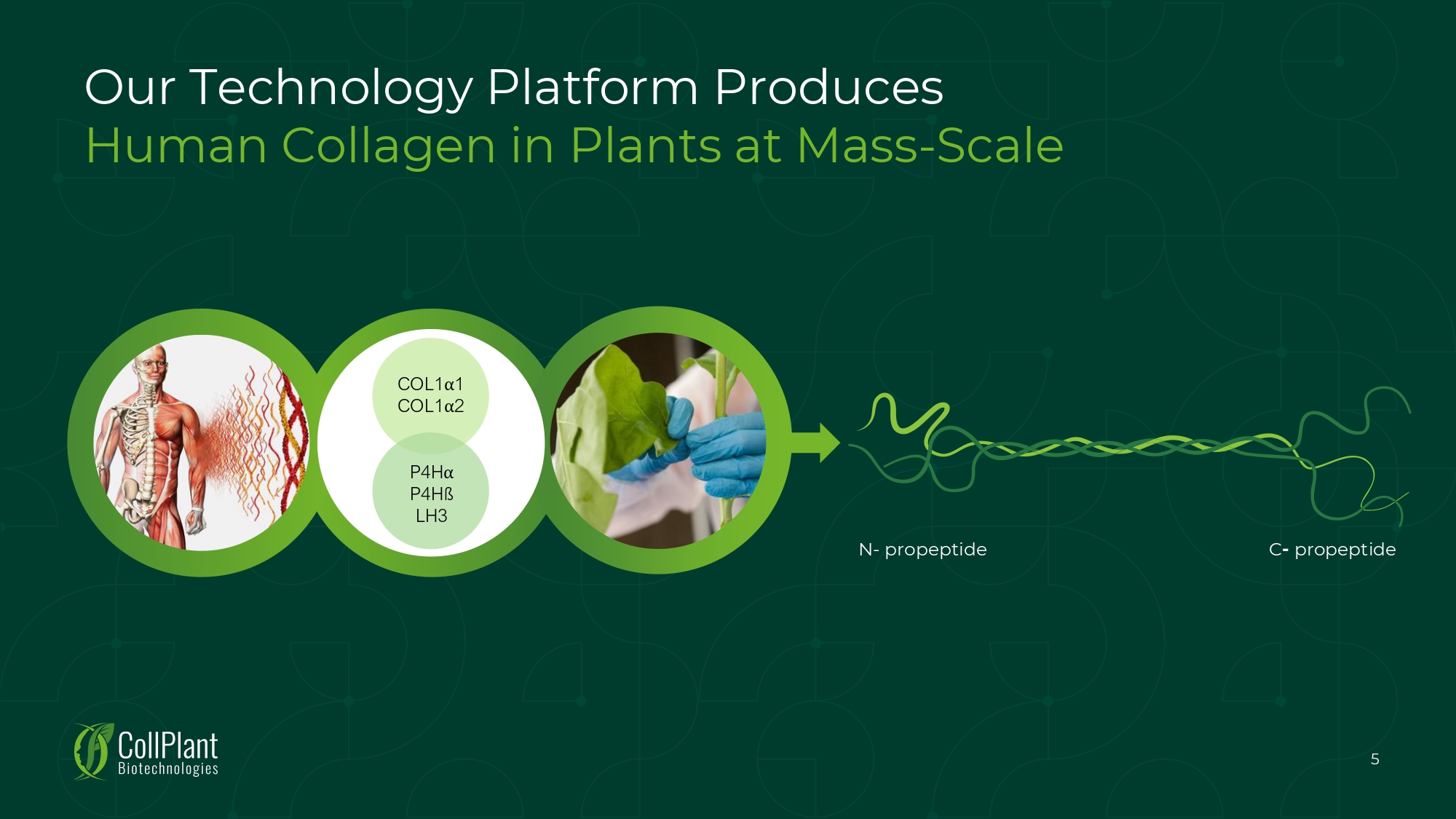
N - propeptide C - propeptide COL1 ੑ 1 COL1 ੑ 2 P 4 H ੑ P 4 Hß LH 3 5 Our Technology Platform Produces Human Collagen in Plants at Mass - Scale
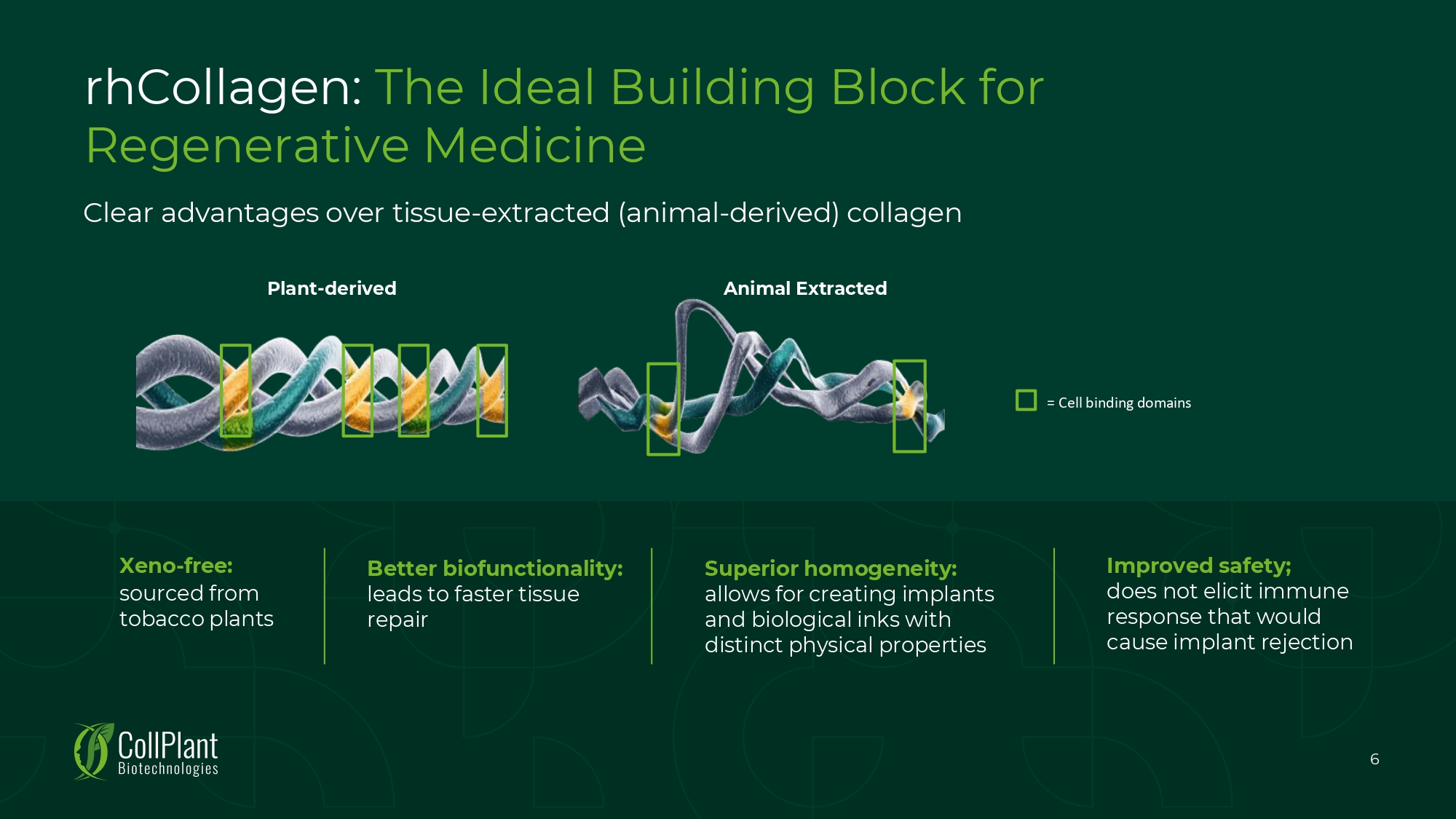
Animal Extracted Plant - derived Clear advantages over tissue - extracted (animal - derived) collagen rhCollagen: The Ideal Building Block for Regenerative Medicine Superior homogeneity: allows for creating implants and biological inks with distinct physical properties Improved safety; does not elicit immune response that would cause implant rejection Xeno - free: sourced from tobacco plants Better biofunctionality: leads to faster tissue repair = Cell binding domains 6
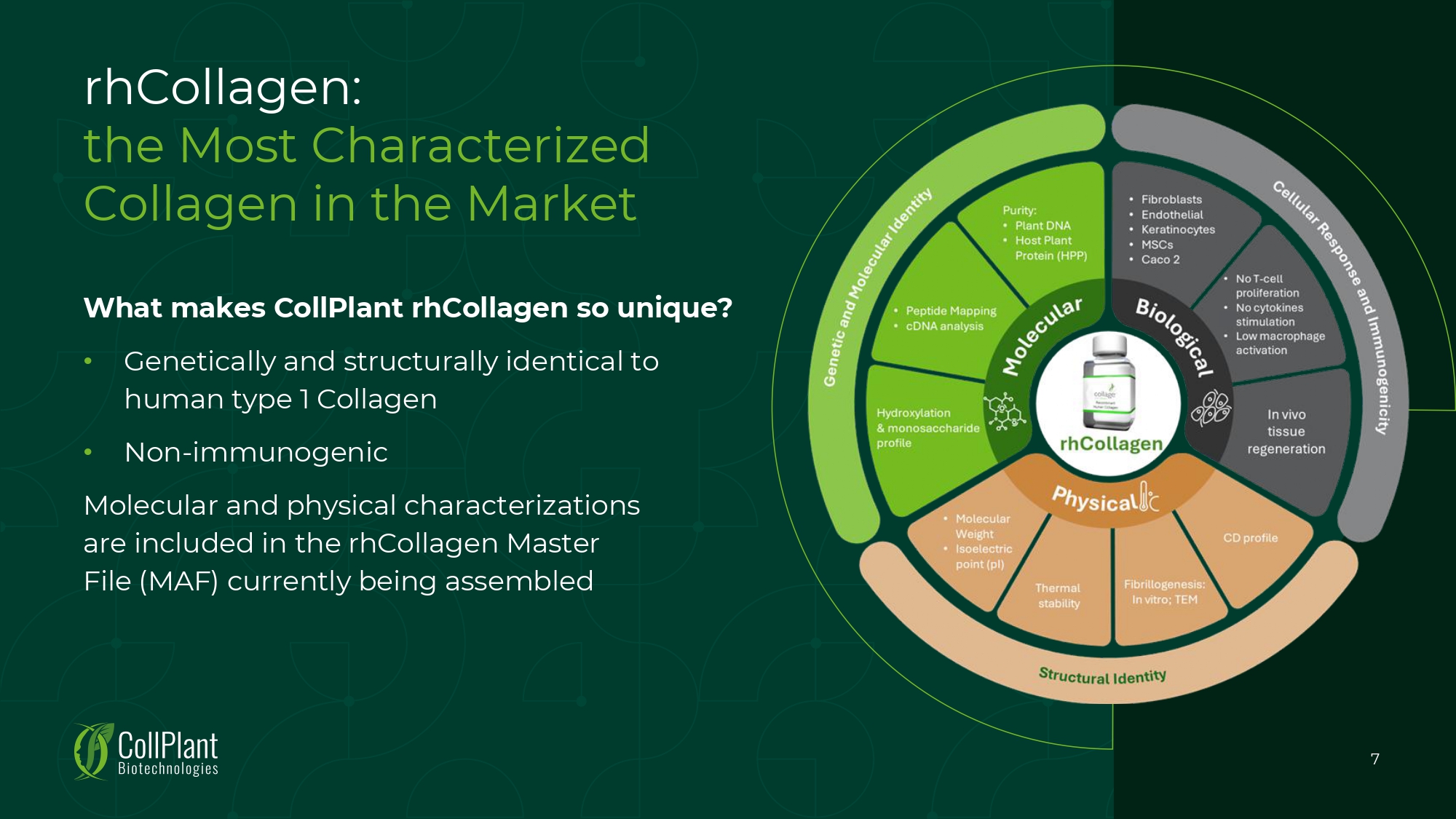
rhCollagen: the Most Characterized Collagen in the Market What makes CollPlant rhCollagen so unique? • Genetically and structurally identical to human type 1 Collagen • Non - immunogenic Molecular and physical characterizations are included in the rhCollagen Master File (MAF) currently being assembled 7
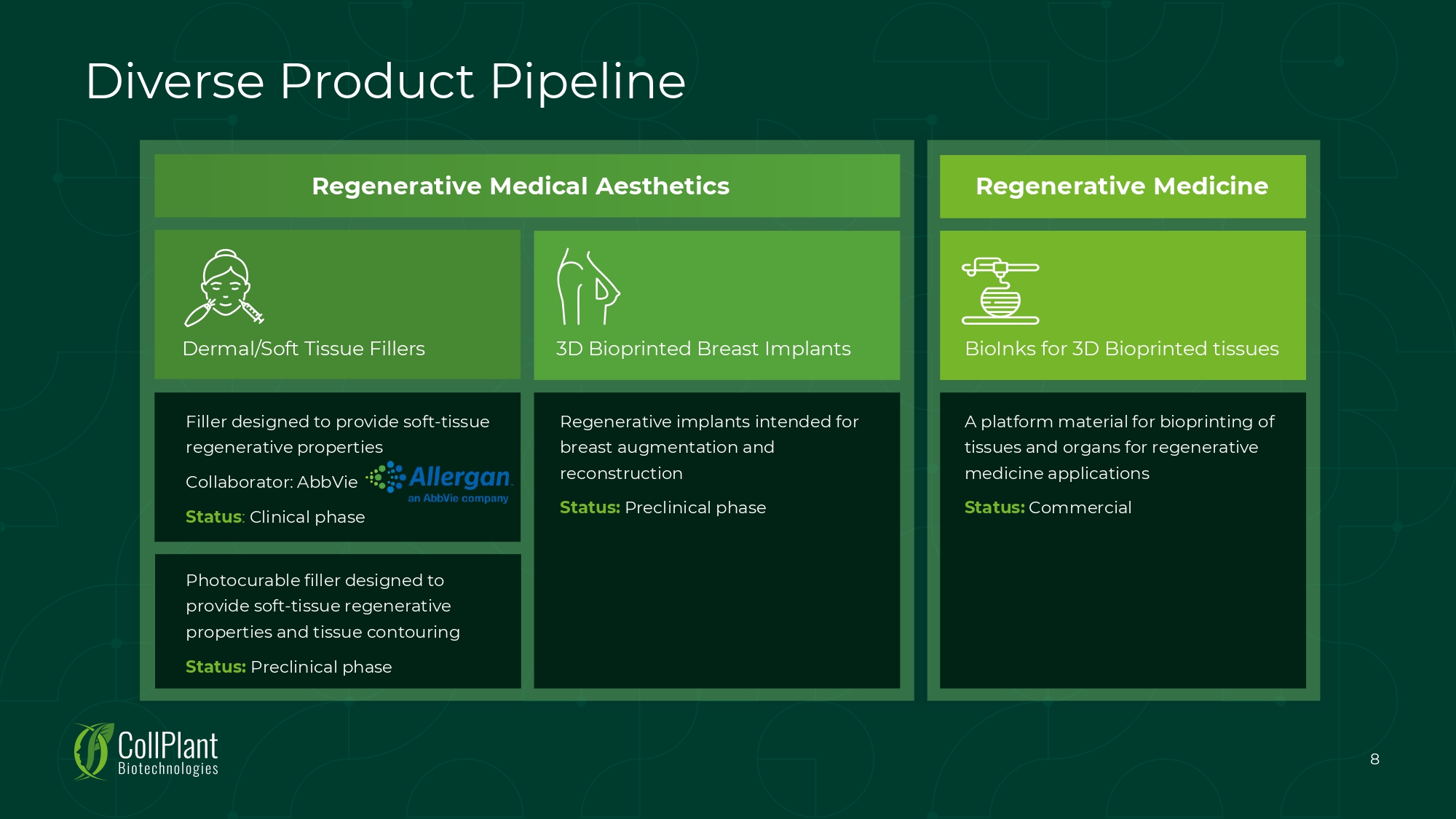
Filler designed to provide soft - tissue regenerative properties Collaborator: AbbVie Status : Clinical phase Dermal/Soft Tissue Fillers Regenerative implants intended for breast augmentation and reconstruction Status: Preclinical phase 3D Bioprinted Breast Implants Regenerative Medical Aesthetics Regenerative Medicine BioInks for 3D Bioprinted tissues A platform material for bioprinting of tissues and organs for regenerative medicine applications Status: Commercial Diverse Product Pipeline Photocurable filler designed to provide soft - tissue regenerative properties and tissue contouring Status: Preclinical phase 8

How We Are Applying Our rhCollagen: Areas of Focus 9 Status/Partner Commercial Clinical Preclinical Use Product Aesthetic medicine * Dermal/soft tissue fillers Injectable tissue fillers Dermal/soft tissue fillers Photocurable filler Regenerative medicine Breast reconstruction and augmentation Breast implants Tissues, organs, drug discovery and tissue modelling BioInks * AbbVie is collecting data and conducting a review of interim results from the first cohort of patients enrolled in the dermal and soft tissue filler clinical trials initiated in 2023 and next steps for the program are to be determined upon complete assessment.

10 Dermal/Soft Tissue Fillers
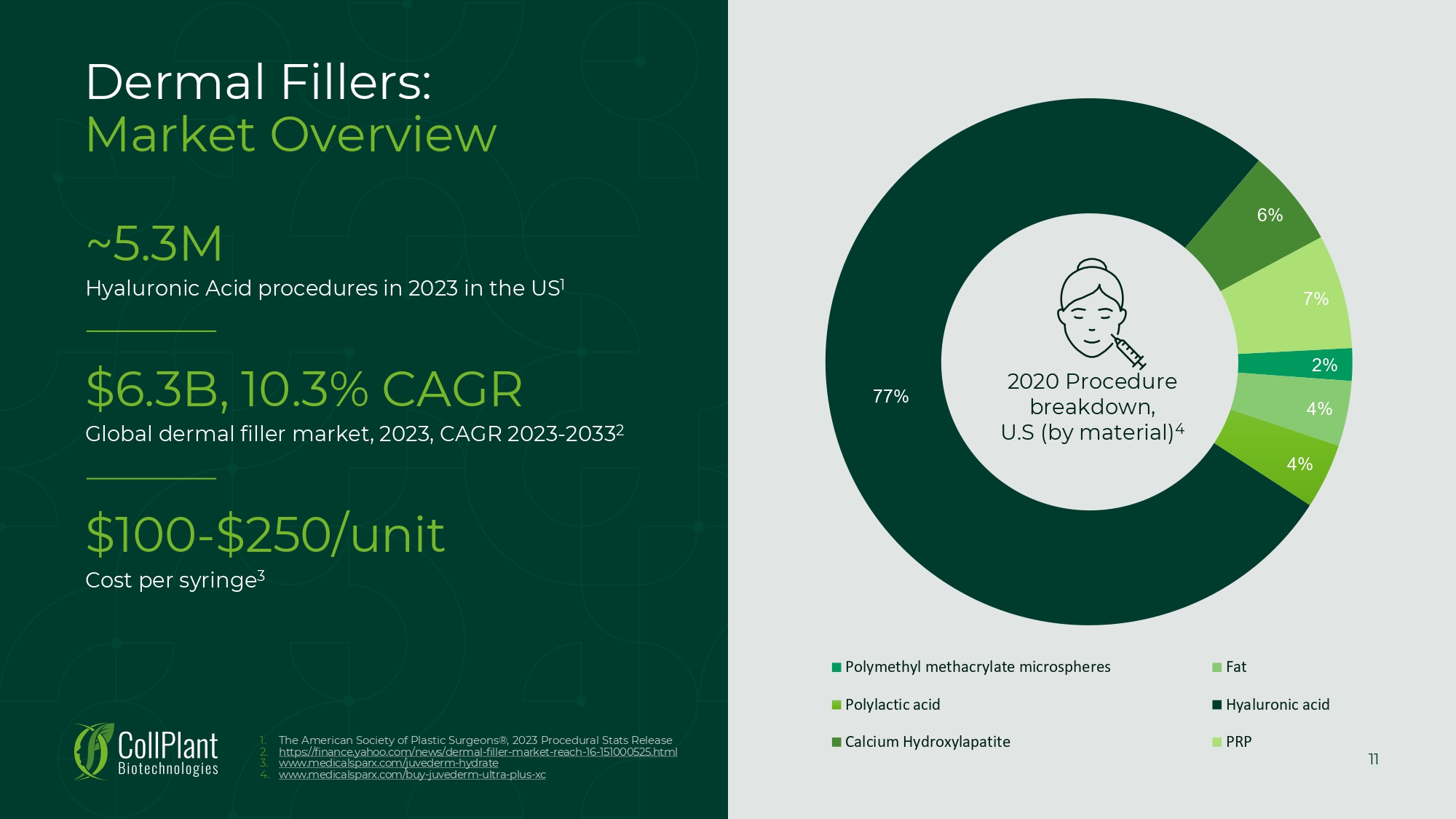
11 Dermal Fillers: Market Overview ~5.3M Hyaluronic Acid procedures in 2023 in the US 1 $6.3B, 10.3% CAGR Global dermal filler market, 2023, CAGR 2023 - 2033 2 $100 - $250/unit Cost per syringe 3 2% 4% 4% 77% 6% 7% Polymethyl methacrylate microspheres Fat Polylactic acid Hyaluronic acid Calcium Hydroxylapatite PRP 2020 Procedure breakdown, U.S (by material) 4 1. The American Society of Plastic Surgeons®, 2023 Procedural Stats Release 2. https://finance.yahoo.com/news/dermal - filler - market - reach - 16 - 151000525.htm l 3. www.medicalsparx.com/juvederm - hydrate 4. www.medicalsparx.com/buy - juvederm - ultra - plus - xc

Photocurable Regenerative Dermal Filler 12
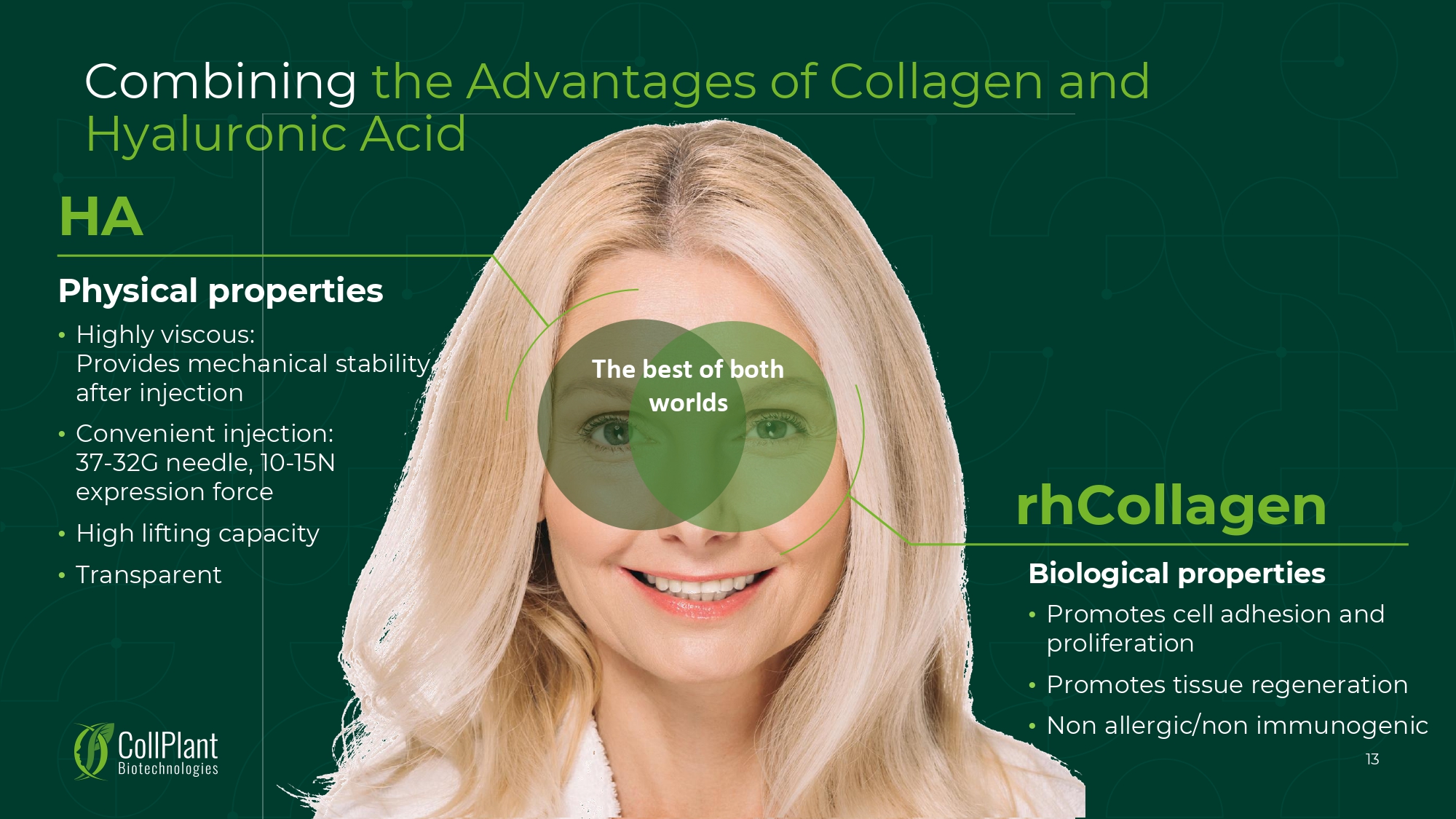
Combining the Advantages of Collagen and Hyaluronic Acid HA Physical properties • Highly viscous: Provides mechanical stability after injection • Convenient injection : 37 - 32 G needle, 10 - 15 N expression force • High lifting capacity • Transparent rhCollagen Biological properties • Promotes cell adhesion and proliferation • Promotes tissue regeneration • Non allergic/non immunogenic The best of both worlds 13
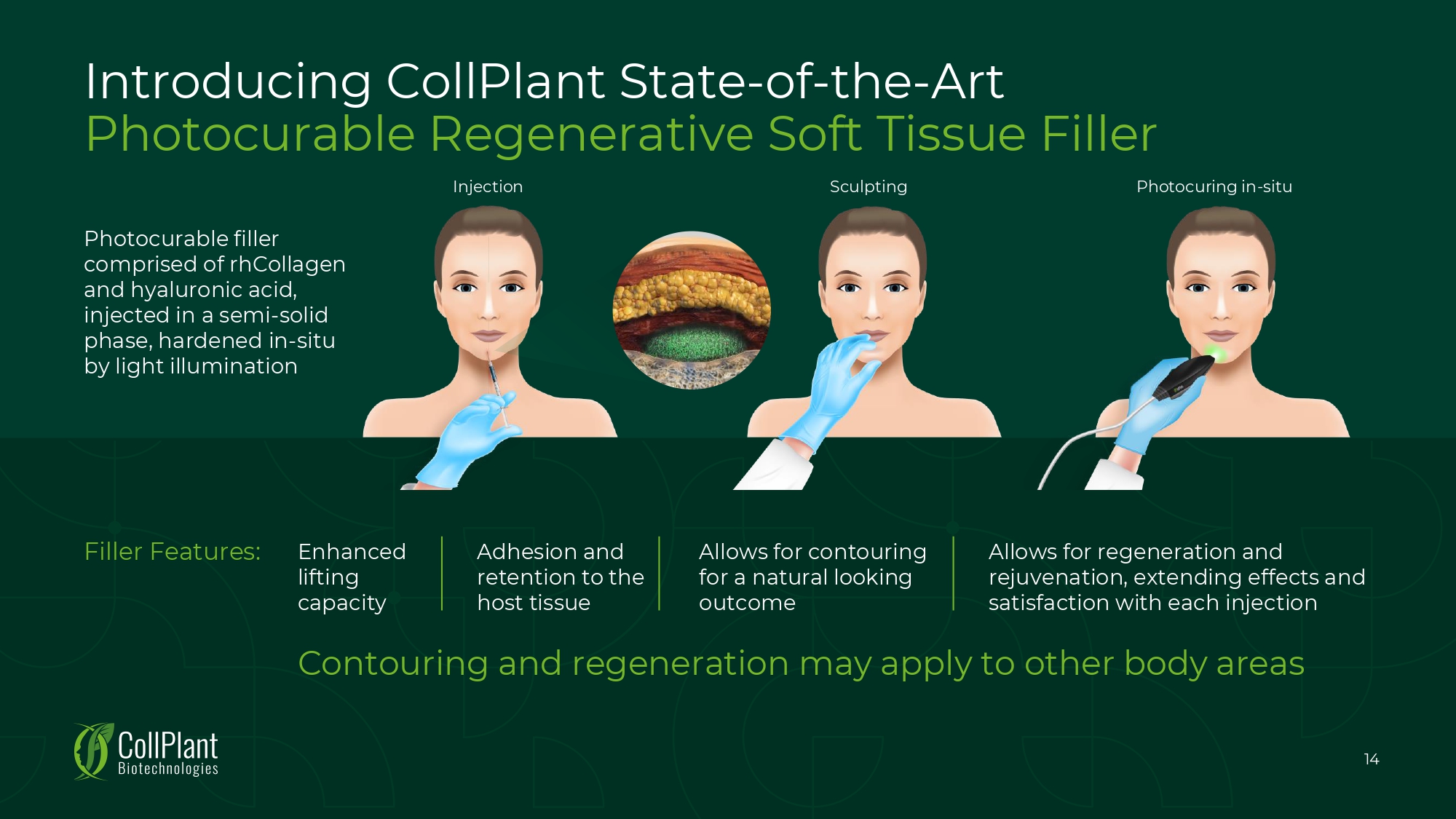
Introducing CollPlant State - of - the - Art Photocurable Regenerative Soft Tissue Filler Photocurable filler comprised of rhCollagen and hyaluronic acid, injected in a semi - solid phase, hardened in - situ by light illumination Filler Features: 14 Contouring and regeneration may apply to other body areas Allows for regeneration and rejuvenation, extending effects and satisfaction with each injection Enhanced lifting capacity Adhesion and retention to the host tissue Allows for contouring for a natural looking outcome Injection Sculpting Photocuring in - situ
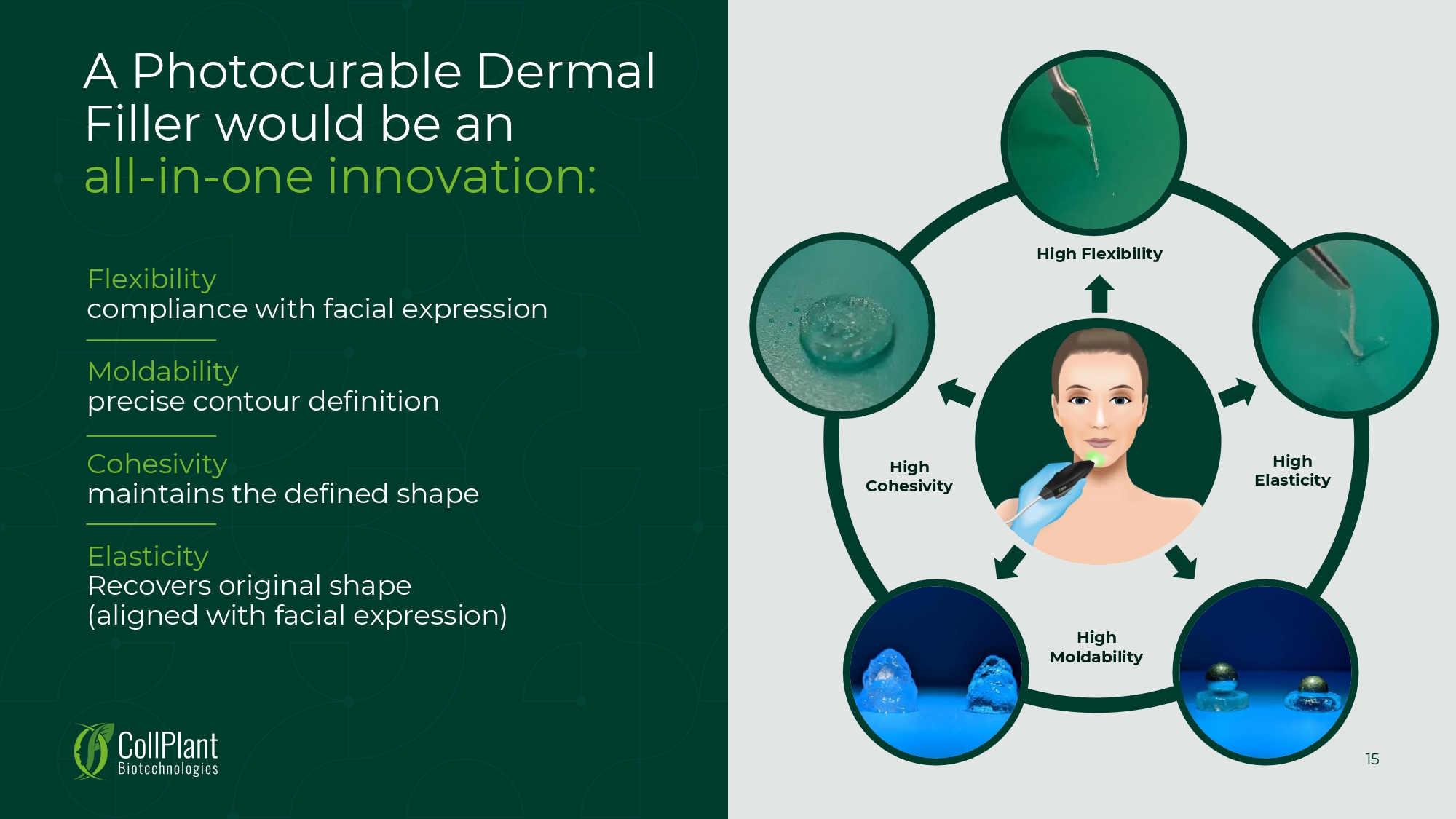
High Elasticity High Moldability High Cohesivity High Flexibility A Photocurable Dermal Filler would be an all - in - one innovation: Flexibility compliance with facial expression Moldability precise contour definition Cohesivity maintains the defined shape Elasticity Recovers original shape (aligned with facial expression) 15

16 KOL Testimonial* Dr. Jason Bloom MD, FACS https://www.bloomfacialplastics.com/ Full video at: https://www.youtube.com/watch?v=D114gdmrdek Use is experimental only at physician’s discretion. CollPlant’s photocurable filler is not yet FDA - approved. BLOOM FACIAL PLASTIC SURGERY • “The first injectable moldable implant” • “Provides cohesivity and structural support that far exceeds anything in the market” • “A customizable implant that can be implanted in the office with a minimally invasive procedure.” • “Huge advance in the dermal and soft tissue market, and facial plastic surgery” • “Long lasting results” • “Game changer for facial plastic surgery and soft tissue filler” * Following Use of Photocurable Dermal Filler

17 https:// www.luxurgerynyc.com/dr - shridharani/ Full video at: https://youtu.be/FvdcfO5sQTw Use is experimental only at physician’s discretion. CollPlant’s photocurable filler is not yet FDA - approved. KOL Testimonial Dr. Sachin M. Shridharani, MD, FACS Director - LUXURGERY (New York, NY) • “It changes the overall approach of long - lasting facial sculpting” • “Transforming an injectable filler into a solid - like gel” • “It forms an elastic, cohesive and integrated piece” • “Increased G’, cohesivity, longevity, tissue regeneration” • “Compatible for off - the - face areas” • “Nothing like this is observed with available fillers” * Following Use of Photocurable Dermal Filler
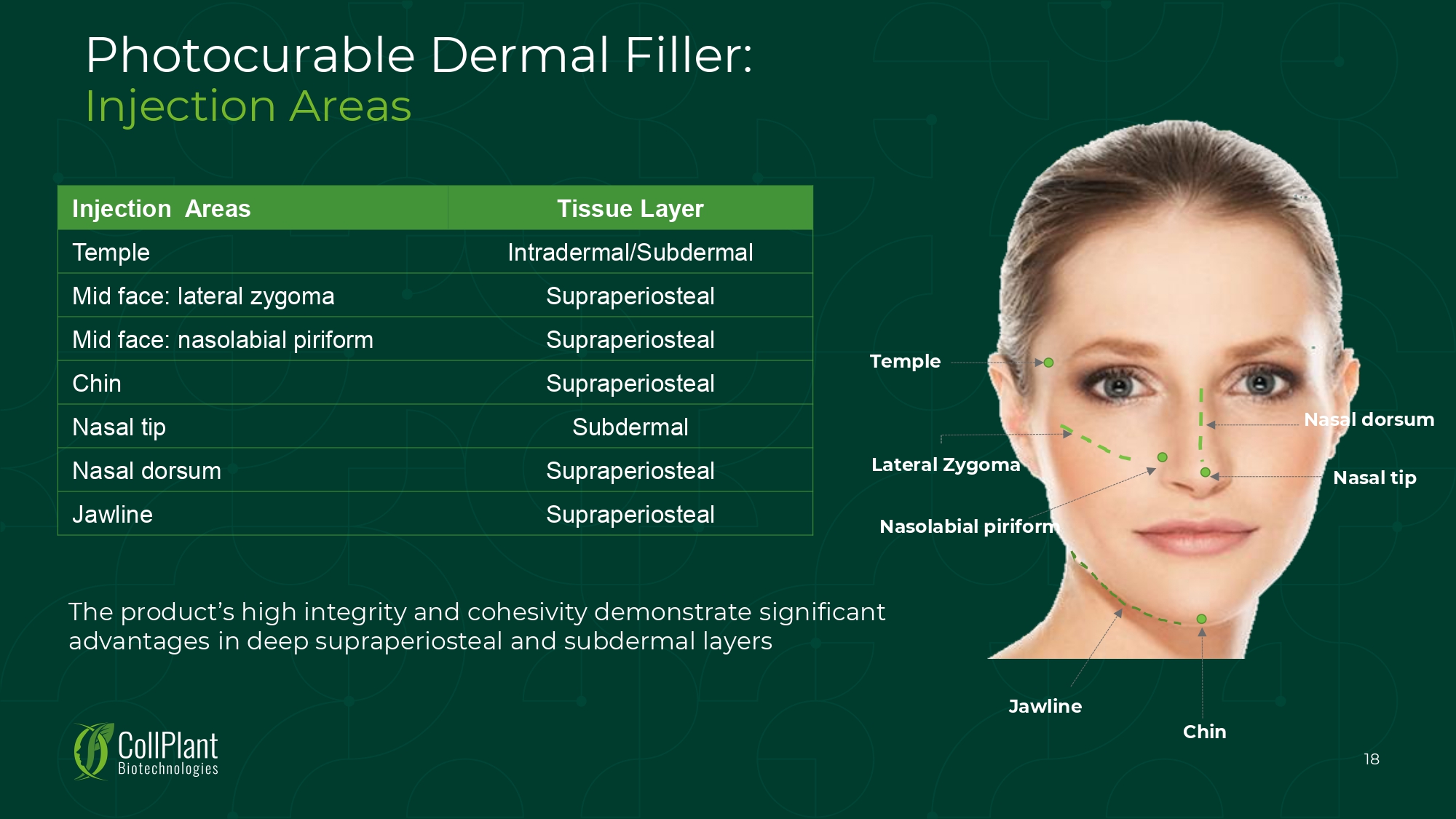
Photocurable Dermal Filler: Injection Areas Nasolabial piriform Lateral Zygoma Temple 18 Nasal tip Nasal dorsum Jawline Chin Tissue Layer Injection Areas Intradermal/Subdermal Temple Supraperiosteal Mid face: lateral zygoma Supraperiosteal Mid face: nasolabial piriform Supraperiosteal Chin Subdermal Nasal tip Supraperiosteal Nasal dorsum Supraperiosteal Jawline The product’s high integrity and cohesivity demonstrate significant advantages in deep supraperiosteal and subdermal layers
3D - bioprinted Regenerative Breast Implants 19
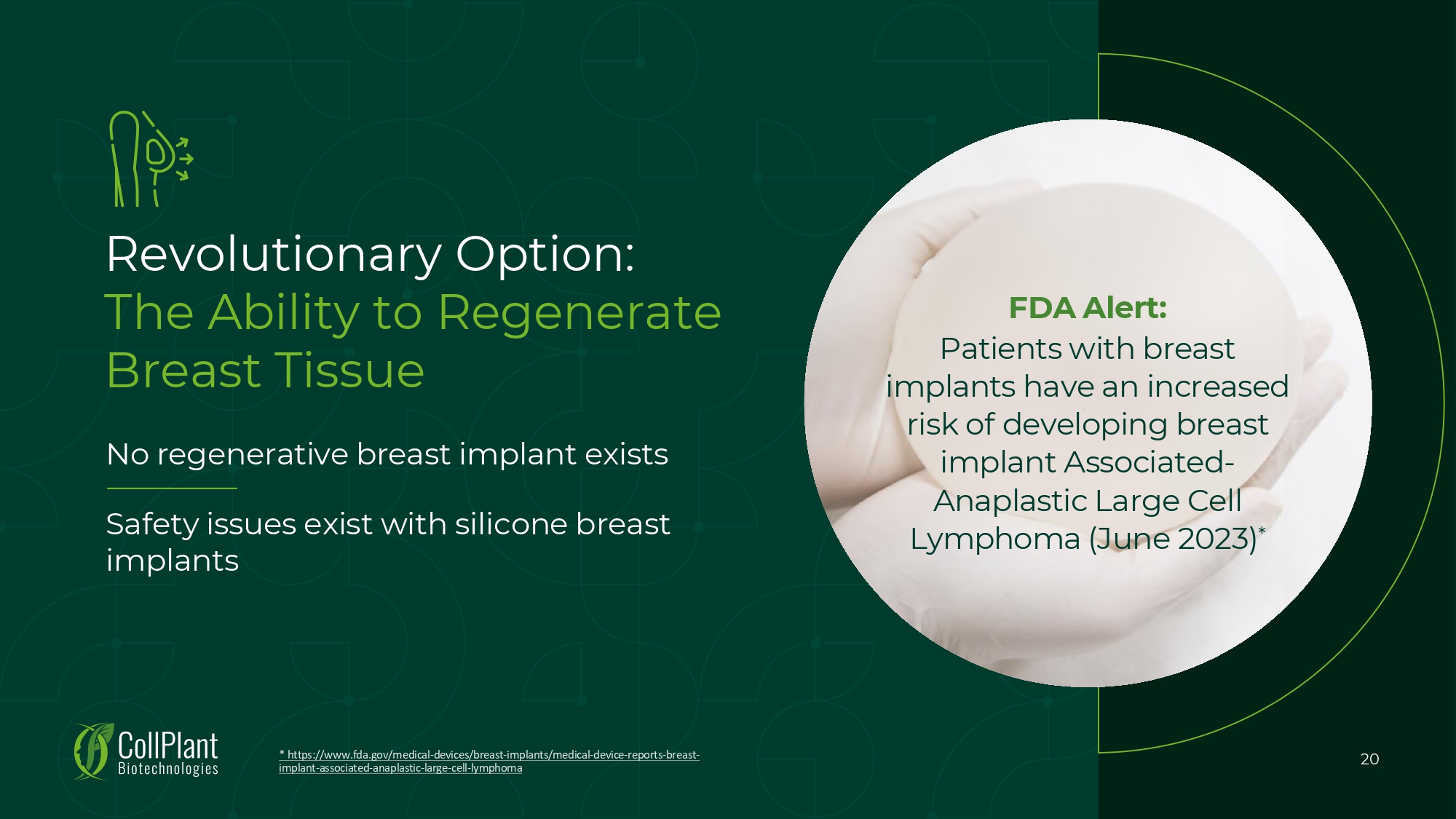
Revolutionary Option: The Ability to Regenerate Breast Tissue No regenerative breast implant exists * https://www.fda.gov/medical - devices/breast - implants/medical - device - reports - breast - implant - associated - anaplastic - large - cell - lymphoma 20 Safety issues exist with silicone breast implants FDA Alert: Patients with breast implants have an increased risk of developing breast implant Associated - Anaplastic Large Cell Lymphoma (June 2023) *
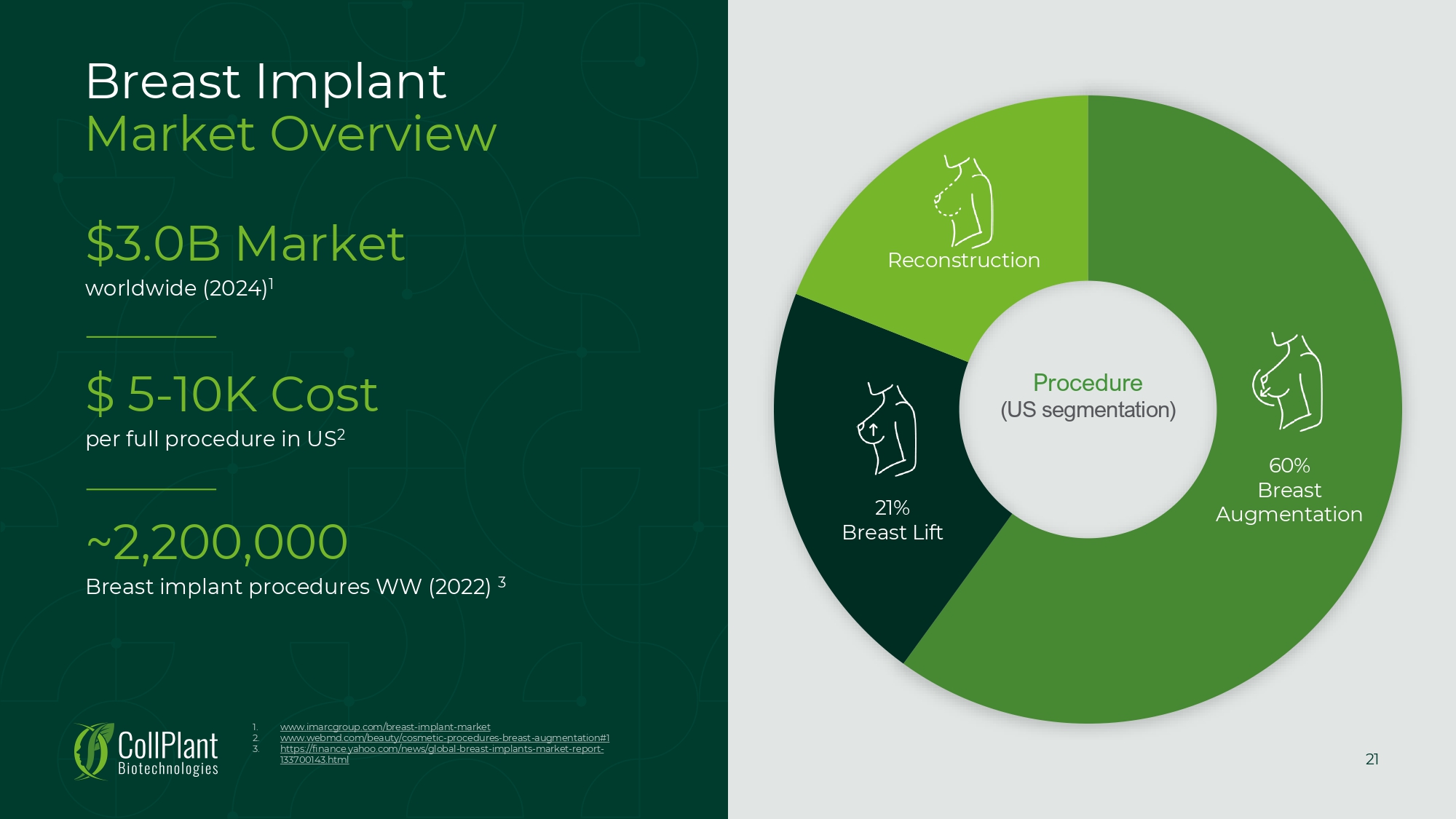
21 Breast Implant Market Overview 1. 2. 3. www.imarcgroup.com/breast - implant - market www.webmd.com/beauty/cosmetic - procedures - breast - augmentation# 1 https://finance.yahoo.com/news/global - breast - implants - market - report - 133700143.html 60% Breast Augmentation 21% Breast Lift Reconstruction Procedure (US segmentation) $3.0B Market worldwide (2024) 1 $ 5 - 10K Cost per full procedure in US 2 ~2,200,000 Breast implant procedures WW (2022) 3
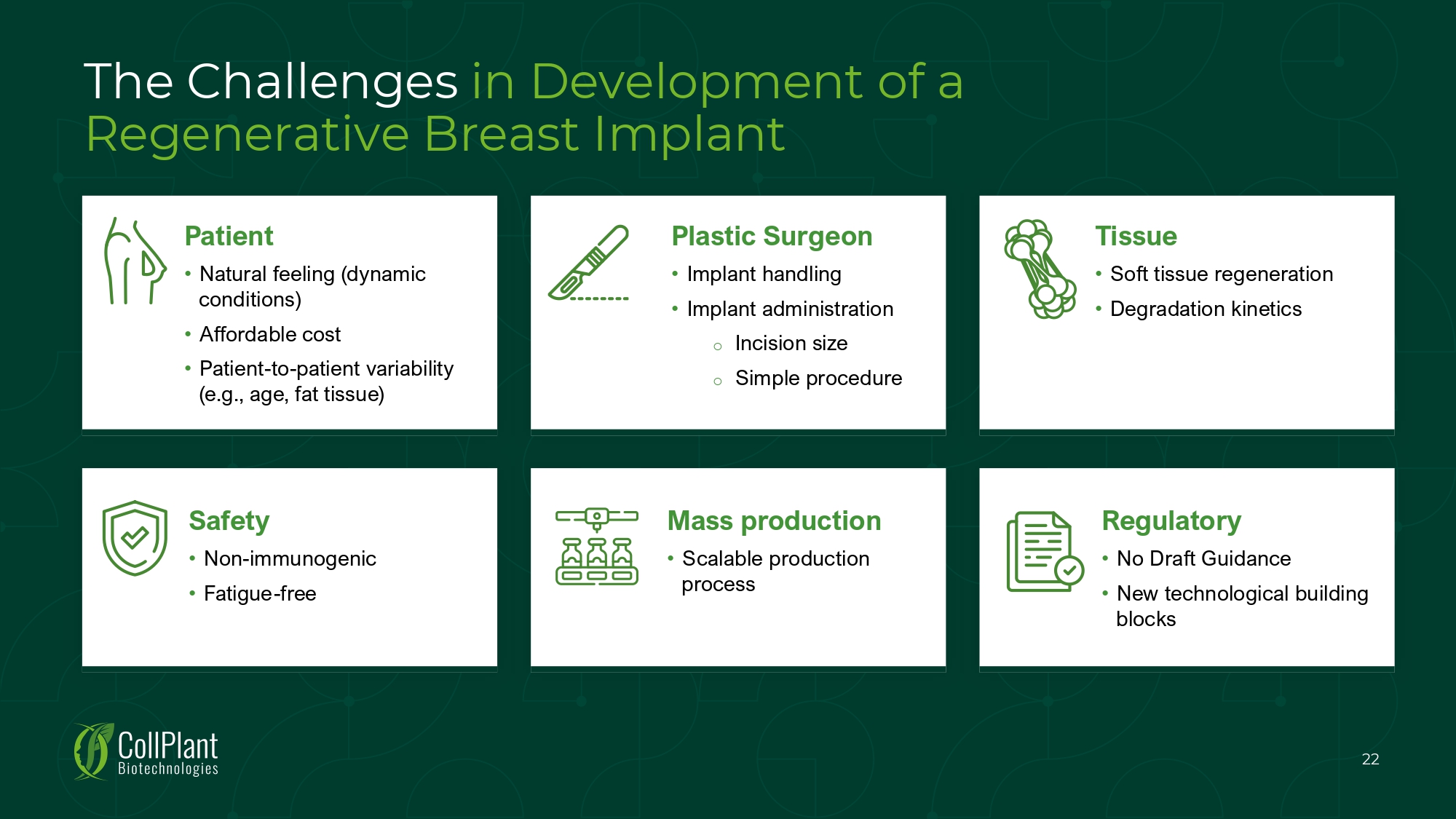
The Challenges in Development of a Regenerative Breast Implant Patient • Natural feeling (dynamic conditions) • Affordable cost • Patient - to - patient variability (e.g., age, fat tissue) Plastic Surgeon • Implant handling • Implant administration o Incision size o Simple procedure Tissue • Soft tissue regeneration • Degradation kinetics Safety • Non - immunogenic • Fatigue - free Mass production • Scalable production process Regulatory • No Draft Guidance • New technological building blocks 22
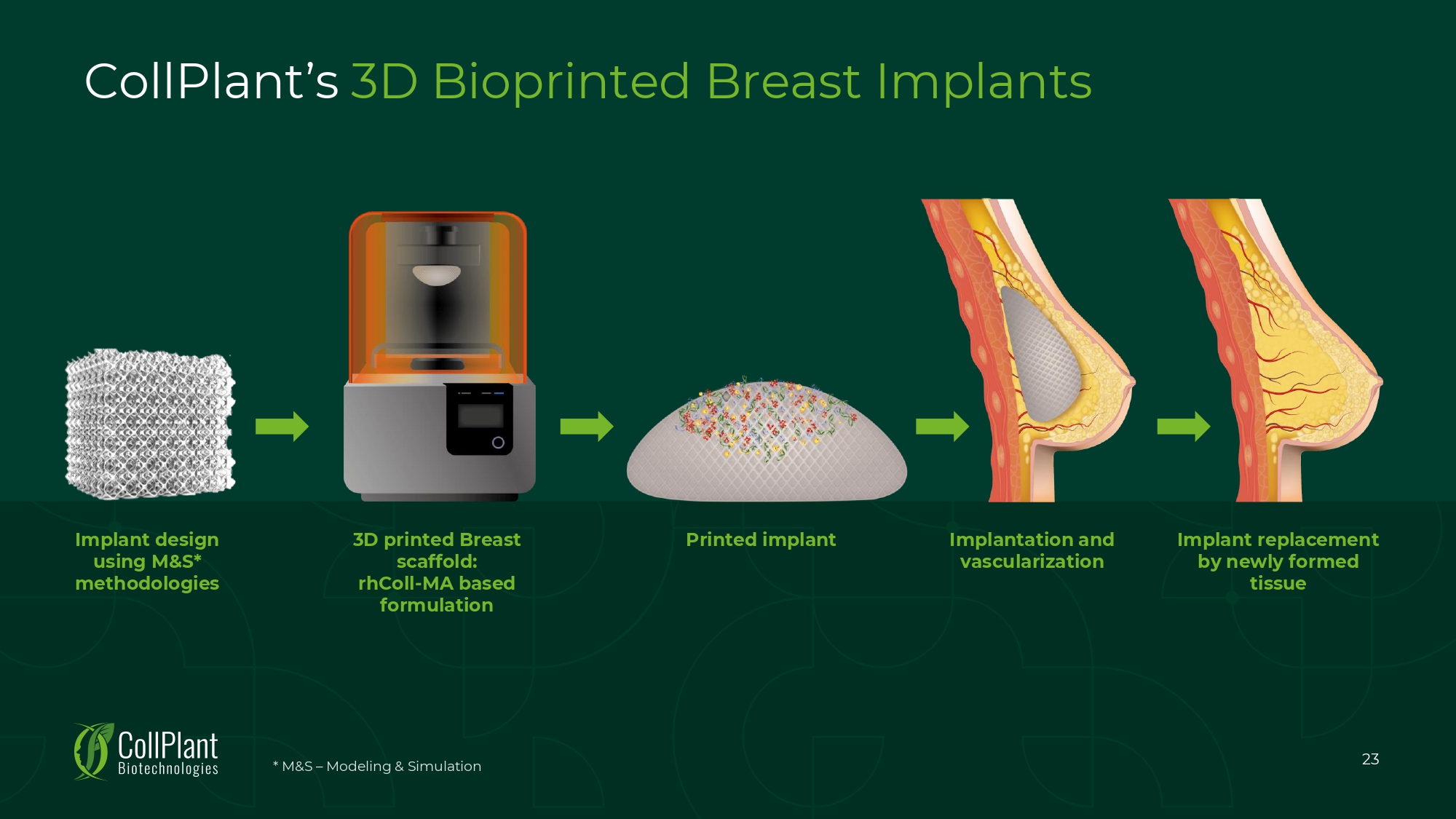
CollPlant’s 3D Bioprinted Breast Implants 3D printed Breast scaffold: rhColl - MA based formulation Printed implant Implantation and vascularization Implant replacement by newly formed tissue Implant design using M&S* methodologies 23 * M&S – Modeling & Simulation
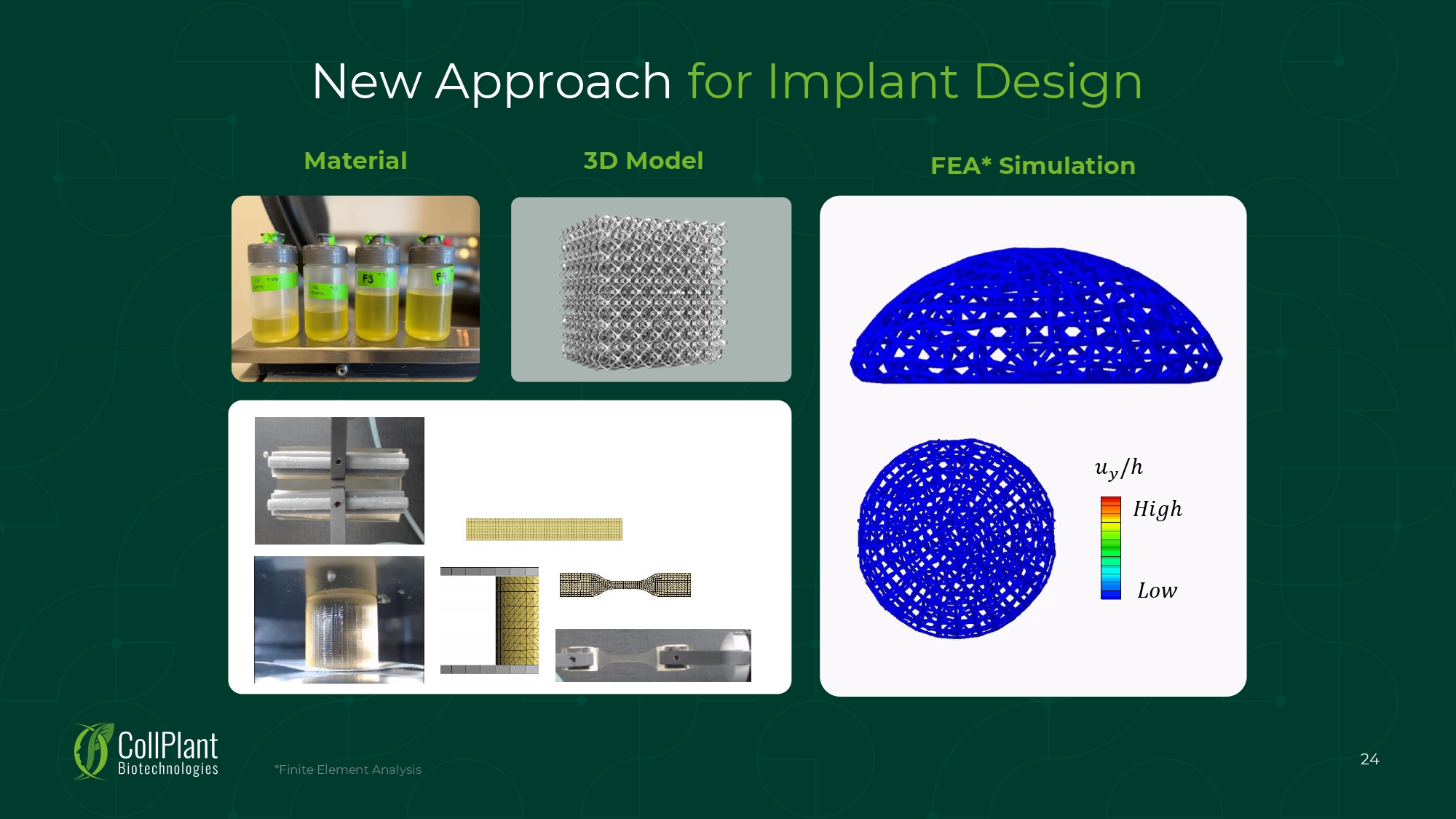
New Approach for Implant Design ݑ ݕ /ℎ ܪ ݅ ݃ ℎ ܮ ݓ Material 3D Model 24 *Finite Element Analysis FEA* Simulation
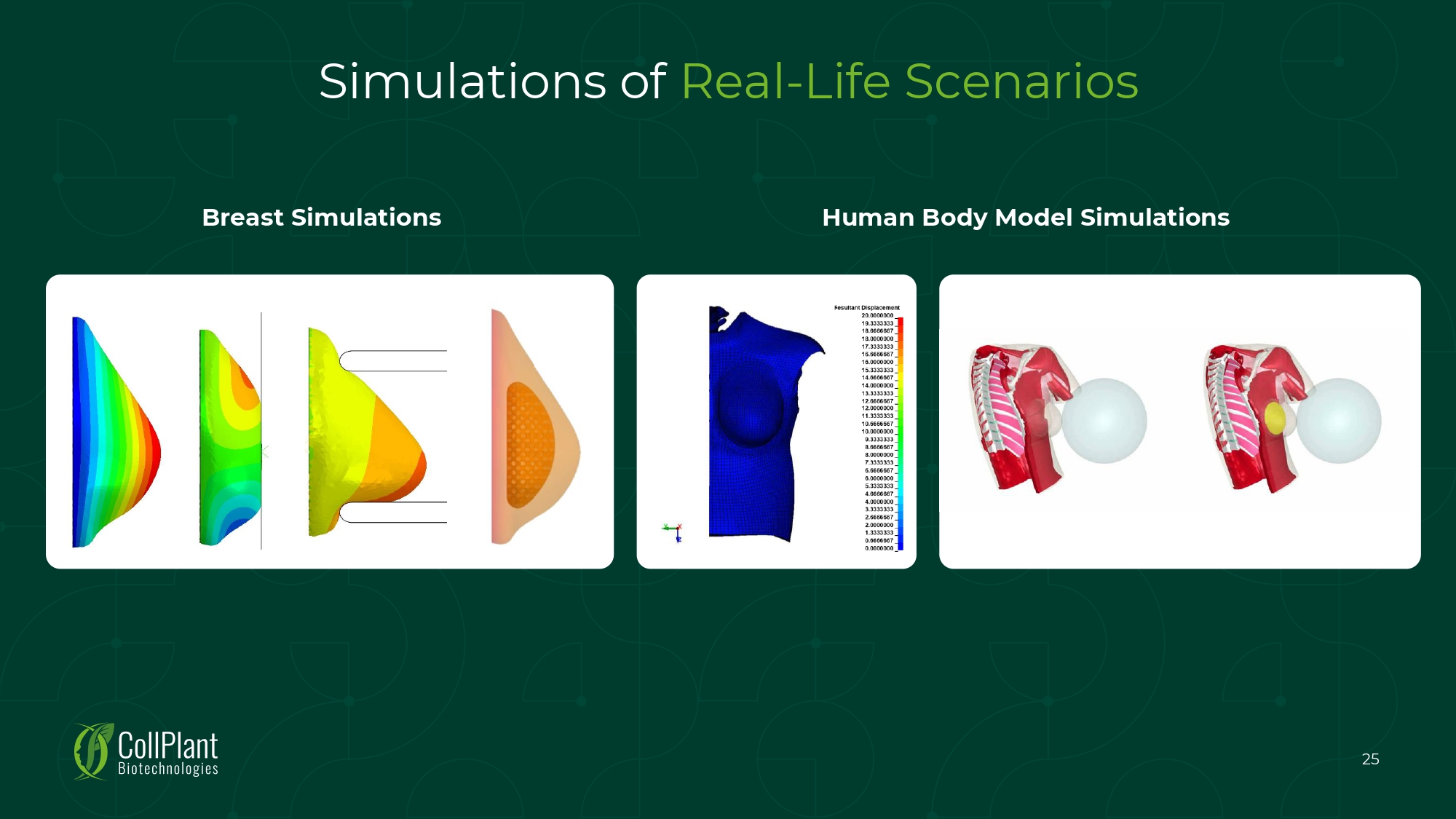
Simulations of Real - Life Scenarios Breast Simulations Human Body Model Simulations 25

3D Bioprinting of a Regenerative Breast Implant with rhCollagen BioInk 26
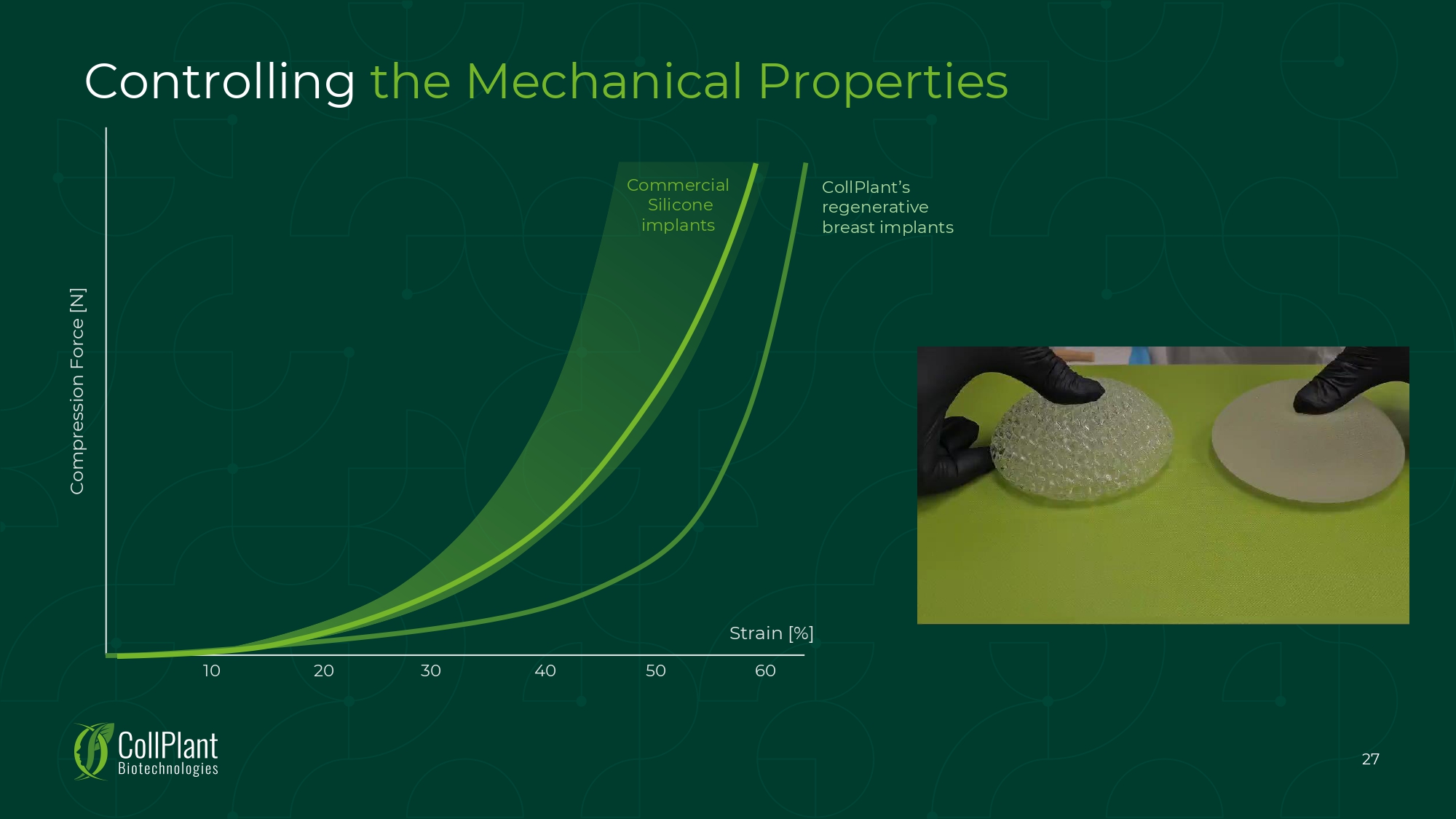
Controlling the Mechanical Properties Compression Force [N] Strain [%] Commercial Silicone implants CollPlant’s regenerative breast implants 10 20 30 40 50 60 27
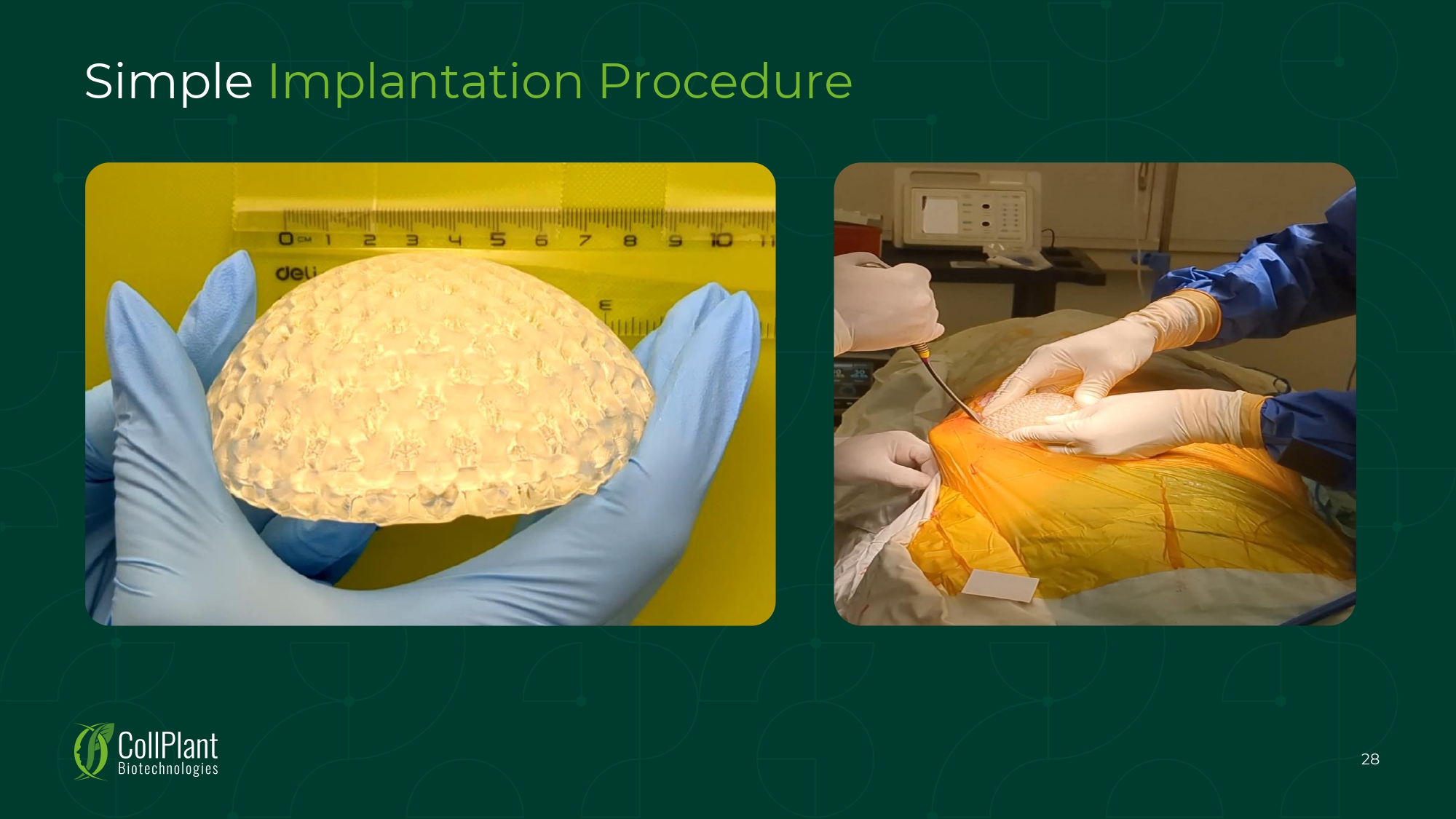
Simple Implantation Procedure 28
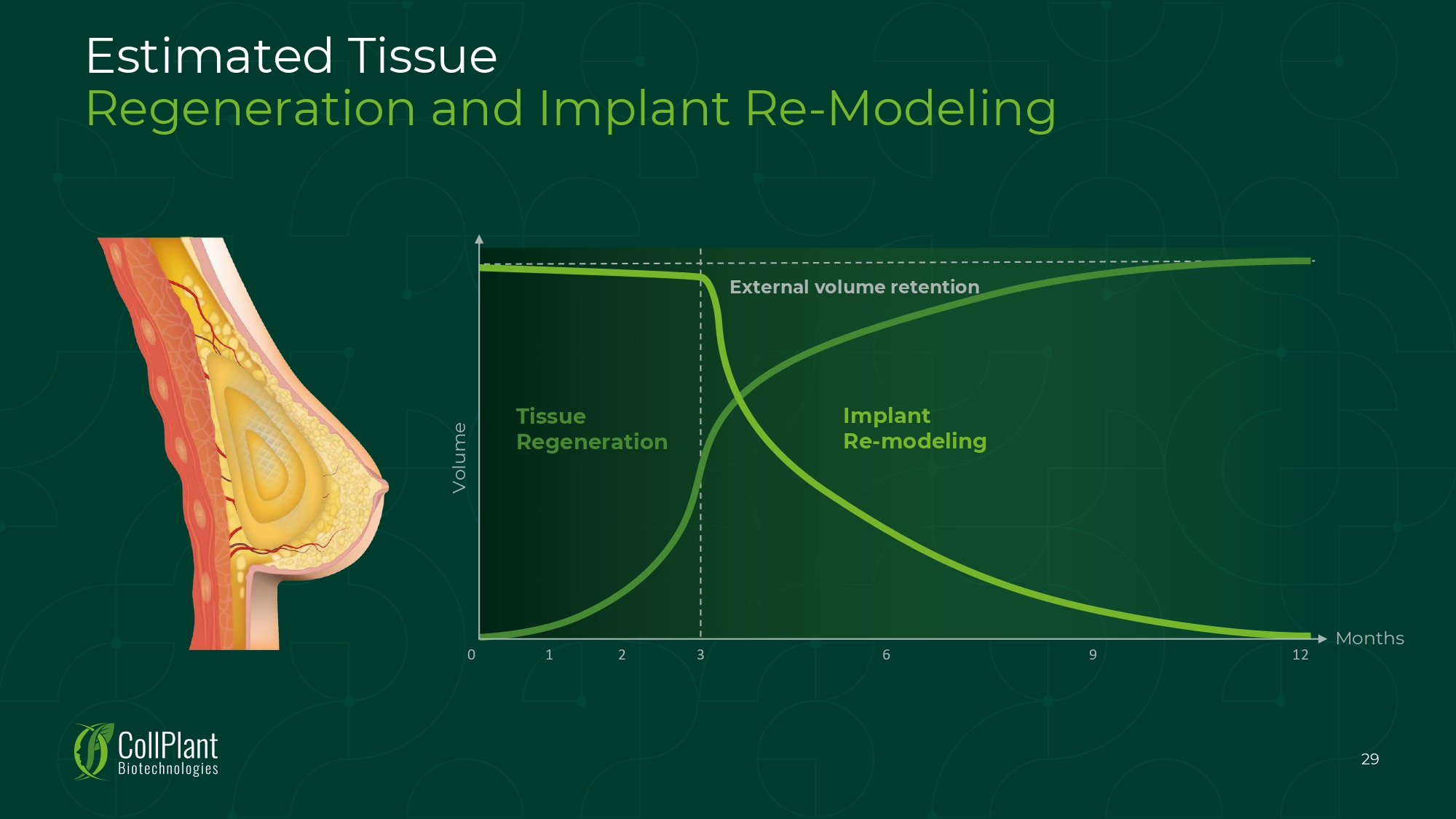
Estimated Tissue Regeneration and Implant Re - Modeling 0 12 Months Volume 3 6 9 1 2 Tissue Regeneration Implant Re - modeling External volume retention 29
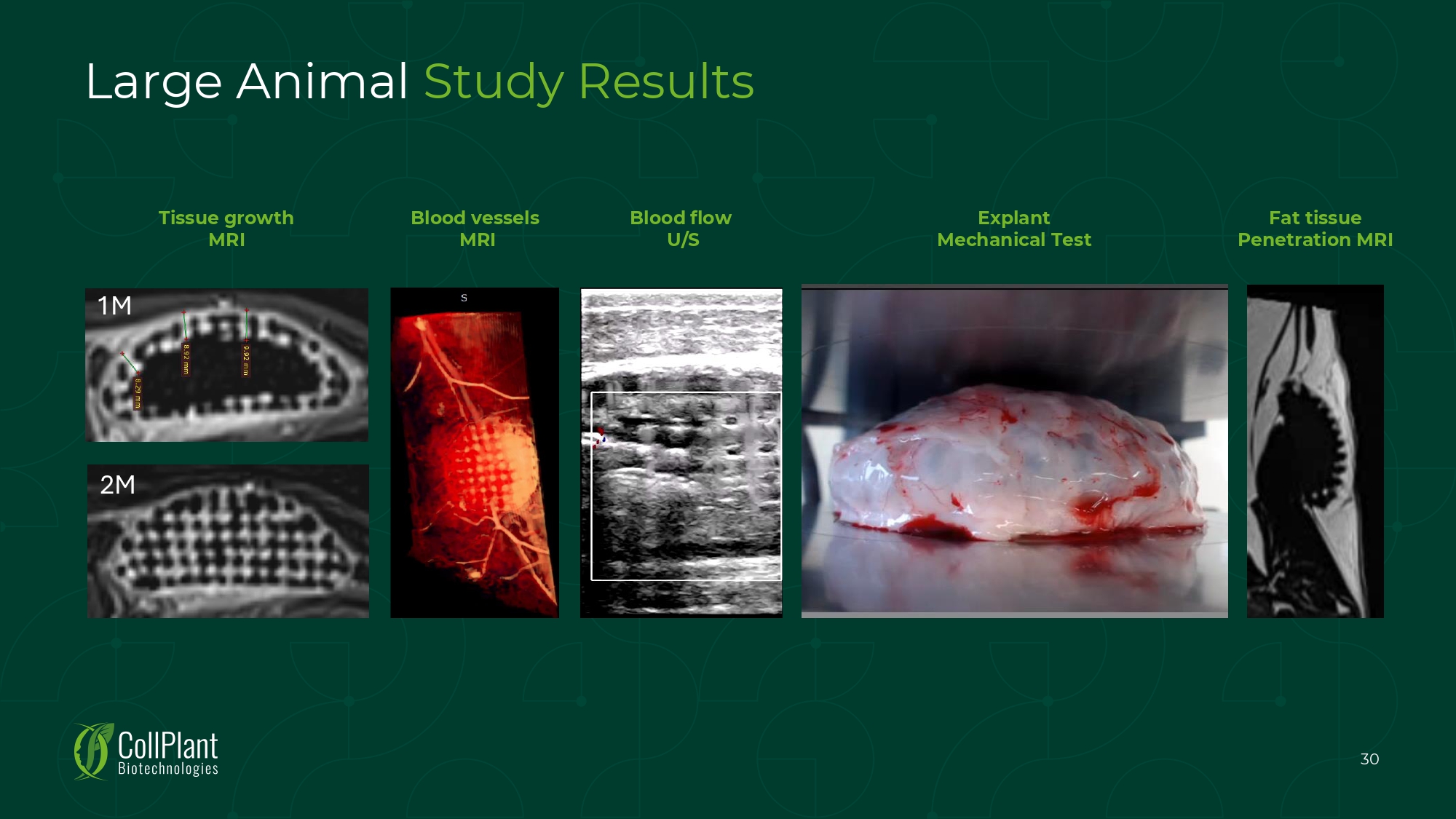
Explant Mechanical Test Fat tissue Penetration MRI 30 Blood flow U/S Blood vessels MRI Tissue growth MRI 1M 2M Large Animal Study Results

rhCollagen - based Bioink Platform for Biofabrication Animal - free: excellent safety profile, non immunogenic Optimal rheology at room temperature Cytocompatible, Biofunctional Compatible with major printing technologies Mass production - consistency robustness High homogeneity reproducibility 31

Seasoned Management Team with Engineering, Pharmaceutical, Device and Life Sciences Experience Yehiel Tal CEO Regentis Biomaterials ProChon Biotech Kulicke & Soffa Industries Eran Rotem Deputy CEO & CFO Tefron, CFO (NYSE,TASE) Healthcare Tech., CFO (NASDAQ) & Gamida, E&Y Elana Gazal, PhD VP R&D Neuroderm (now Mitsubishi Tanabe) Waters IS Foamix (now Wyne) Beckman Coulter Oren Fahimipoor VP Operations Omrix Biopharmaceuticals (J&J) Teva Pharmaceutical Industries Ltd Philippe Bensimon PharmD VP RA/QA/CA Maquet Getinge 3M Medical Hadas Dreiher - Horowitz VP HR Elbit Systems Teva Pharmaceutical Industries Ltd Mul - T - Lock 32

Our Goals through 2025 Dermal fillers: Continue to advance development Commercial - sized breast implants: Expand studies in large animals to generate additional safety and efficacy data in support of commercialization ESG: Continue operations in line with Climate Action, energy efficiency, sustainable agriculture, waste management, talent engagement, sustainable sourcing rhCollagen - based bioinks: Continue to expand our commercial portfolio of Collaborations and Partnerships: Form new collaborations with industry leaders to co - develop therapeutics and medical applications using our rhCollagen 33

Investment Summary Pioneering Proprietary, Plant - Based Technology Platform The only commercially viable technology that can produce truly human collagen at mass scale and without reliance on animal tissue Addressing Multi - billion - dollar markets Innovative rhCollagen technology initially focused on medical aesthetic applications; differentiated, transformative, next - generation soft tissue filler is regenerative Broadly applicable, clinically validated technology Ideal building block/scaffolding molecule for regenerative medicine that has clear benefits over tissue - extracted collagen and the potential to create first - in - class products, extend product life cycles and expand applications in other areas of medicine Strategic agreement with global top - tier pharmaceutical company AbbVie Allows for up to $103M in milestone payments plus additional royalties on sales Highly seasoned management team With experience spanning the areas of bioengineering, biomaterials, broad life sciences, pharmaceuticals and devices In - house, sustainable production capabilities In - house cGMP production facility that utilizes proprietary manufacturing processes [under environmentally responsible guidelines] 34

Thank you!


































