
Wave Life Sciences Muscular Dystrophy Association Clinical and Scientific Conference Investor Update April 16, 2019 Exhibit 99.2

Forward-looking statements This document contains forward-looking statements. All statements other than statements of historical facts contained in this document, including statements regarding possible or assumed future results of operations, preclinical and clinical studies, business strategies, research and development plans, collaborations and partnerships, regulatory activities and timing thereof, competitive position, potential growth opportunities, use of proceeds and the effects of competition are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause the actual results, performance or achievements of Wave Life Sciences Ltd. (the “Company”) to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this presentation are only predictions. The Company has based these forward-looking statements largely on its current expectations and projections about future events and financial trends that it believes may affect the Company’s business, financial condition and results of operations. These forward-looking statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, including those listed under Risk Factors in the Company’s Form 10-K and other filings with the SEC, some of which cannot be predicted or quantified and some of which are beyond the Company’s control. The events and circumstances reflected in the Company’s forward-looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. Moreover, the Company operates in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that the Company may face. Except as required by applicable law, the Company does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.

Paul Bolno, MD, MBA President and CEO

Wave Life Sciences: Seeking to transform DMD treatment with stereopure exon skipping approach Exon 51: Wave’s lead development program in DMD High unmet need remains for ~13% of boys with DMD amenable to exon 51 skipping Developing stereopure exon therapies to be more potent than available treatments, without comprising safety Preclinical in vitro and in vivo models support ability to restore meaningful production of functional dystrophin protein Established US and EU regulatory paths Building a fully integrated genetic medicines company

Safety and Tolerability of Suvodirsen (WVE-210201) in Patients With Duchenne Muscular Dystrophy: Results From a Phase 1 Clinical Trial Michael Panzara, MD, MPH Chief Medical Officer

Study Objective Primary Objective To evaluate the safety and tolerability of single ascending doses of suvodirsen in patients with Duchenne muscular dystrophy (DMD) Secondary Objective To assess the pharmacokinetic (PK) profile of suvodirsen after single-dose administration
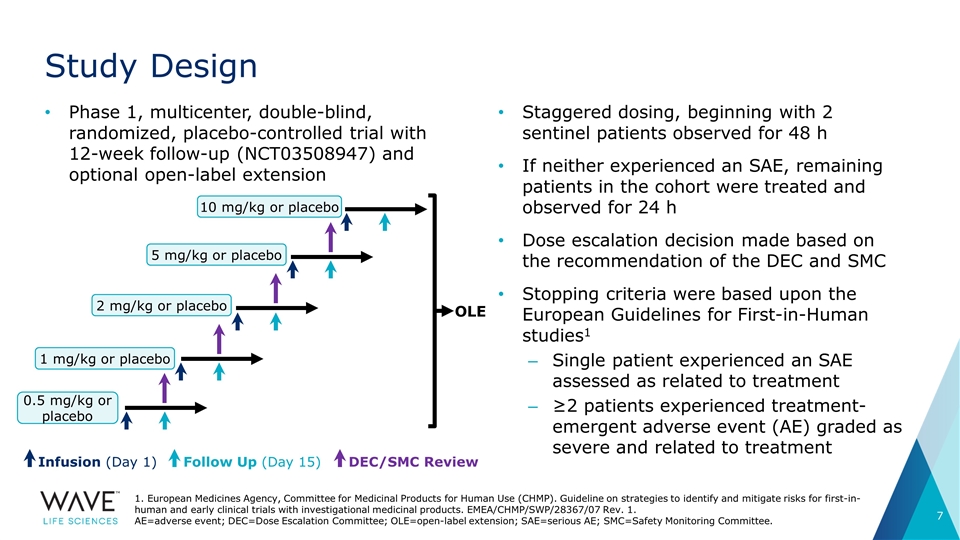
Phase 1, multicenter, double-blind, randomized, placebo-controlled trial with 12-week follow-up (NCT03508947) and optional open-label extension Study Design Staggered dosing, beginning with 2 sentinel patients observed for 48 h If neither experienced an SAE, remaining patients in the cohort were treated and observed for 24 h Dose escalation decision made based on the recommendation of the DEC and SMC Stopping criteria were based upon the European Guidelines for First-in-Human studies1 Single patient experienced an SAE assessed as related to treatment ≥2 patients experienced treatment-emergent adverse event (AE) graded as severe and related to treatment 1. European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on strategies to identify and mitigate risks for first-in-human and early clinical trials with investigational medicinal products. EMEA/CHMP/SWP/28367/07 Rev. 1. AE=adverse event; DEC=Dose Escalation Committee; OLE=open-label extension; SAE=serious AE; SMC=Safety Monitoring Committee. OLE 0.5 mg/kg or placebo 1 mg/kg or placebo 2 mg/kg or placebo 5 mg/kg or placebo 10 mg/kg or placebo Infusion (Day 1) Follow Up (Day 15) DEC/SMC Review
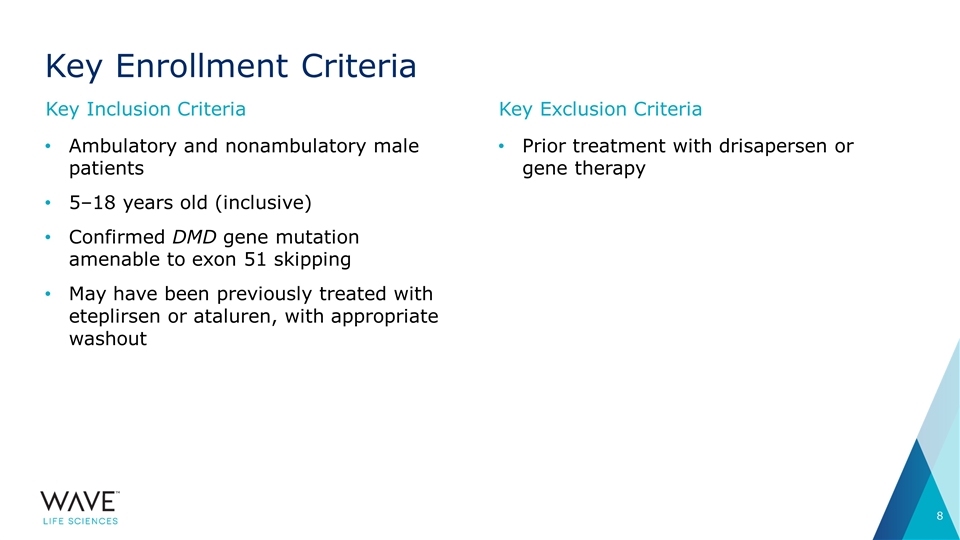
Key Enrollment Criteria Ambulatory and nonambulatory male patients 5–18 years old (inclusive) Confirmed DMD gene mutation amenable to exon 51 skipping May have been previously treated with eteplirsen or ataluren, with appropriate washout Prior treatment with drisapersen or gene therapy Key Inclusion Criteria Key Exclusion Criteria
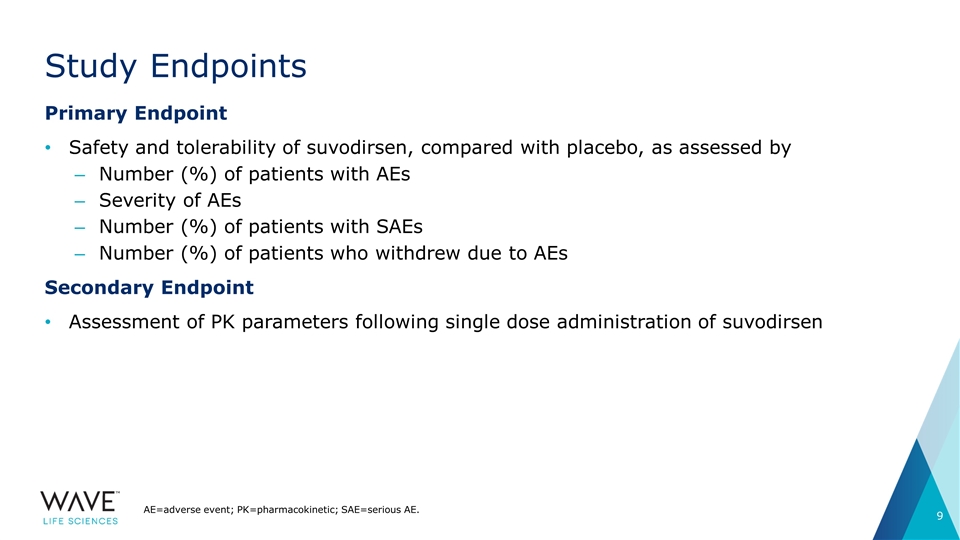
Study Endpoints Primary Endpoint Safety and tolerability of suvodirsen, compared with placebo, as assessed by Number (%) of patients with AEs Severity of AEs Number (%) of patients with SAEs Number (%) of patients who withdrew due to AEs Secondary Endpoint Assessment of PK parameters following single dose administration of suvodirsen AE=adverse event; PK=pharmacokinetic; SAE=serious AE.
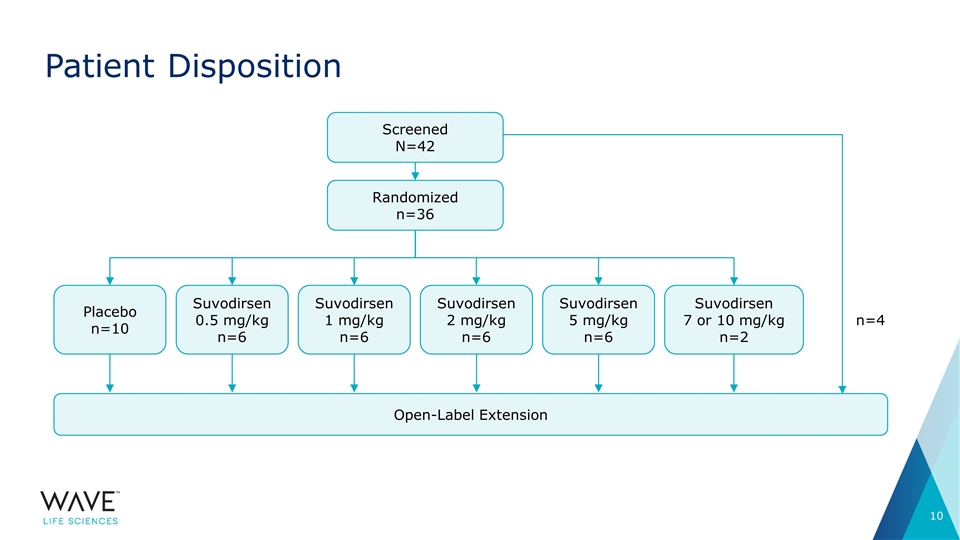
Patient Disposition Screened N=42 Randomized n=36 Suvodirsen1 mg/kg n=6 Suvodirsen2 mg/kg n=6 Suvodirsen 5 mg/kg n=6 Suvodirsen 0.5 mg/kg n=6 Placebo n=10 Open-Label Extension Suvodirsen 7 or 10 mg/kg n=2 n=4
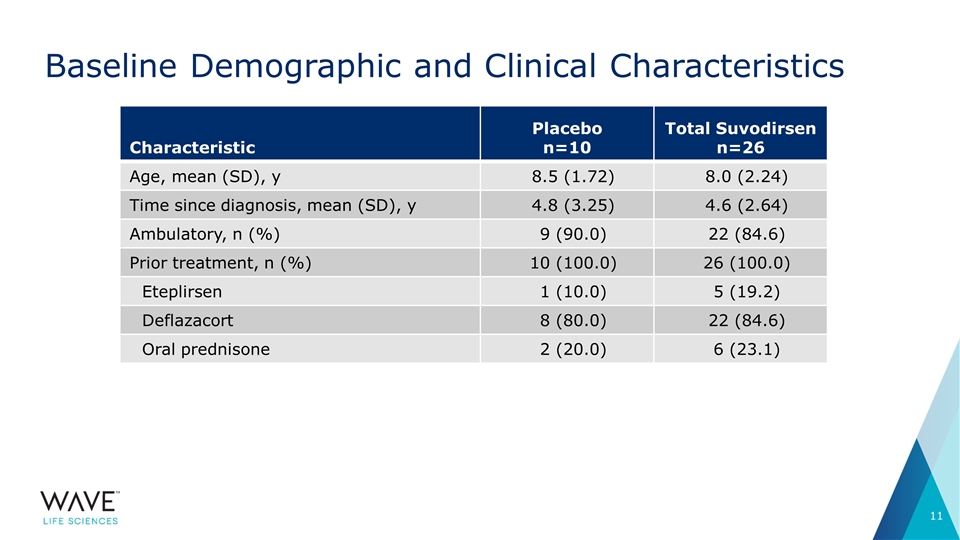
Baseline Demographic and Clinical Characteristics Characteristic Placebo n=10 Total Suvodirsen n=26 Age, mean (SD), y 8.5 (1.72) 8.0 (2.24) Time since diagnosis, mean (SD), y 4.8 (3.25) 4.6 (2.64) Ambulatory, n (%) 9 (90.0) 22 (84.6) Prior treatment, n (%) 10 (100.0) 26 (100.0) Eteplirsen 1 (10.0) 5 (19.2) Deflazacort 8 (80.0) 22 (84.6) Oral prednisone 2 (20.0) 6 (23.1)
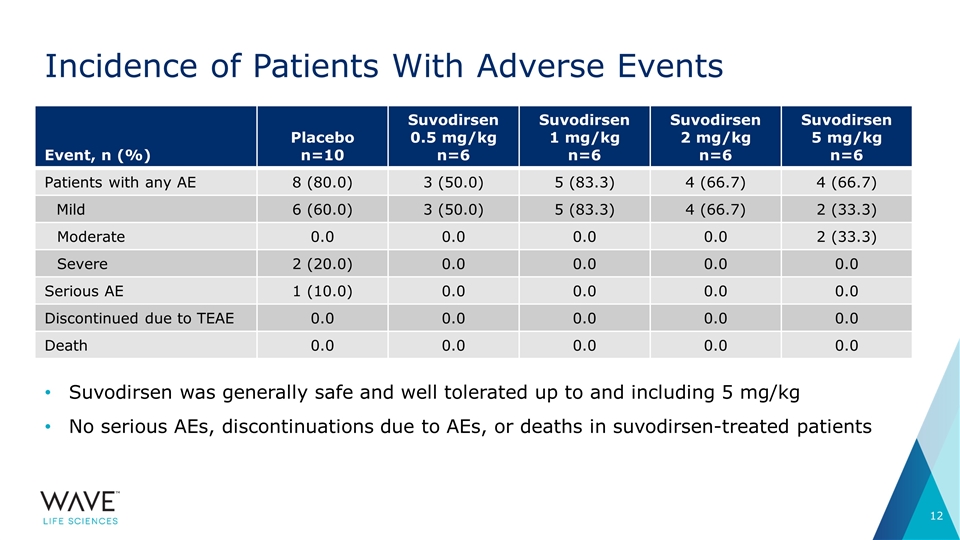
Incidence of Patients With Adverse Events Suvodirsen was generally safe and well tolerated up to and including 5 mg/kg No serious AEs, discontinuations due to AEs, or deaths in suvodirsen-treated patients Event, n (%) Placebo n=10 Suvodirsen 0.5 mg/kg n=6 Suvodirsen 1 mg/kg n=6 Suvodirsen 2 mg/kg n=6 Suvodirsen 5 mg/kg n=6 Patients with any AE 8 (80.0) 3 (50.0) 5 (83.3) 4 (66.7) 4 (66.7) Mild 6 (60.0) 3 (50.0) 5 (83.3) 4 (66.7) 2 (33.3) Moderate 0.0 0.0 0.0 0.0 2 (33.3) Severe 2 (20.0) 0.0 0.0 0.0 0.0 Serious AE 1 (10.0) 0.0 0.0 0.0 0.0 Discontinued due to TEAE 0.0 0.0 0.0 0.0 0.0 Death 0.0 0.0 0.0 0.0 0.0
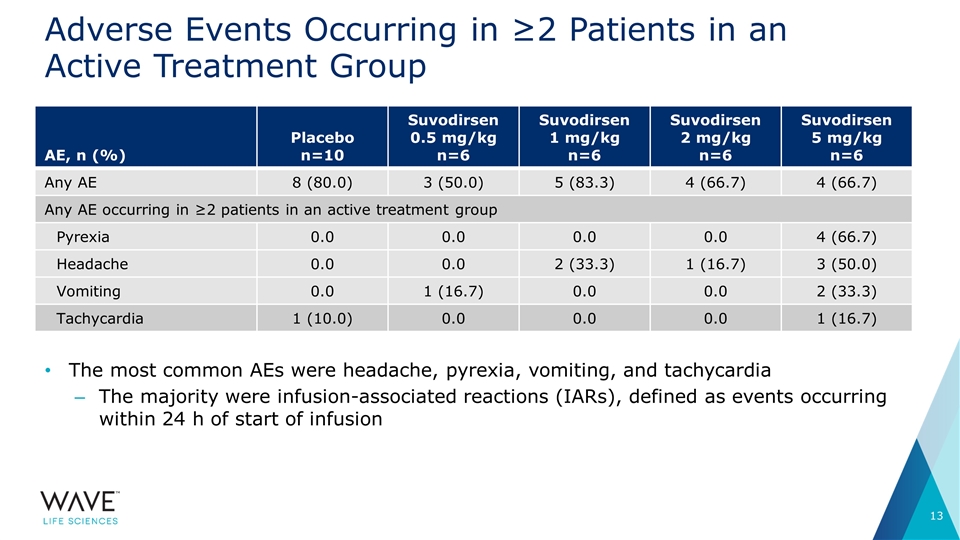
Adverse Events Occurring in ≥2 Patients in an Active Treatment Group AE, n (%) Placebo n=10 Suvodirsen 0.5 mg/kg n=6 Suvodirsen 1 mg/kg n=6 Suvodirsen 2 mg/kg n=6 Suvodirsen 5 mg/kg n=6 Any AE 8 (80.0) 3 (50.0) 5 (83.3) 4 (66.7) 4 (66.7) Any AE occurring in ≥2 patients in an active treatment group Pyrexia 0.0 0.0 0.0 0.0 4 (66.7) Headache 0.0 0.0 2 (33.3) 1 (16.7) 3 (50.0) Vomiting 0.0 1 (16.7) 0.0 0.0 2 (33.3) Tachycardia 1 (10.0) 0.0 0.0 0.0 1 (16.7) The most common AEs were headache, pyrexia, vomiting, and tachycardia The majority were infusion-associated reactions (IARs), defined as events occurring within 24 h of start of infusion
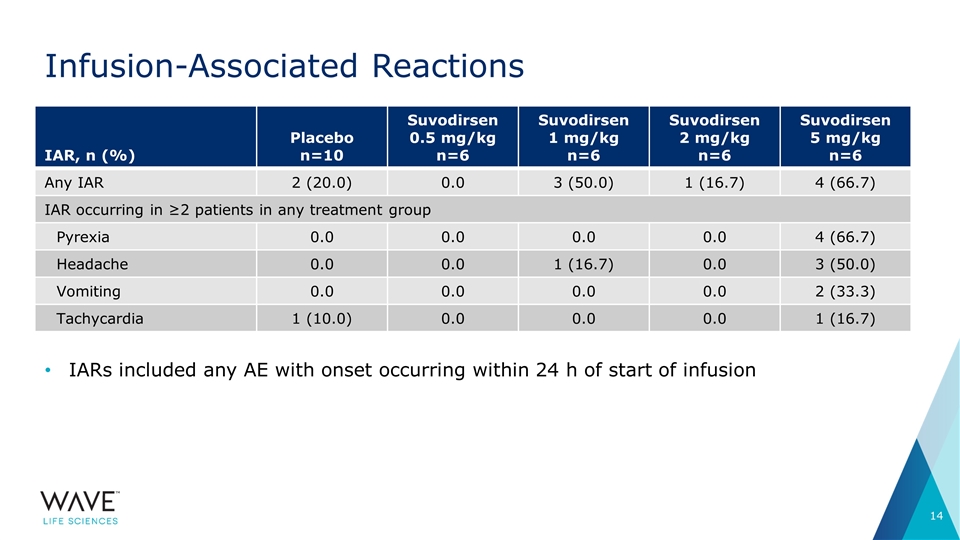
Infusion-Associated Reactions IAR, n (%) Placebo n=10 Suvodirsen 0.5 mg/kg n=6 Suvodirsen 1 mg/kg n=6 Suvodirsen 2 mg/kg n=6 Suvodirsen 5 mg/kg n=6 Any IAR 2 (20.0) 0.0 3 (50.0) 1 (16.7) 4 (66.7) IAR occurring in ≥2 patients in any treatment group Pyrexia 0.0 0.0 0.0 0.0 4 (66.7) Headache 0.0 0.0 1 (16.7) 0.0 3 (50.0) Vomiting 0.0 0.0 0.0 0.0 2 (33.3) Tachycardia 1 (10.0) 0.0 0.0 0.0 1 (16.7) IARs included any AE with onset occurring within 24 h of start of infusion
Dose Exploration Above 5 mg/kg Suvodirsen SMC endorsed exploration of a higher dose as planned per protocol A patient received 10 mg/kg infused over 1 hour Patient experienced pyrexia (39.6°C), headache, tachycardia, and vomiting 4 h after EOI Characterized by investigator as nonserious but severe Patient was treated with 2 doses of hydrocortisone and acetaminophen with resolution of symptoms A patient then received 7 mg/kg infused over 2 hours, in the setting of pretreatment with hydrocortisone and acetaminophen Patient experienced isolated pyrexia (39.5°C) with no other associated symptoms 6 hours after EOI Characterized by investigator as nonserious but severe Resolved with acetaminophen treatment IARs were associated with transient increases in hsCRP and complement factor Bb, with no change in complement C3 Predefined stopping criteria met; SMC reviewed and agreed dosing could proceed at 5 mg/kg EOI=end of infusion; hsCRP=high-sensitivity C-reactive protein; IAR=infusion-associated reaction; SMC=Safety Monitoring Committee.
Conclusions Suvodirsen was generally safe and well tolerated at doses up to and including 5 mg/kg Most common AEs were infusion-associated Mild to moderate in intensity Associated with transient increases in hsCRP and complement factor Bb Resolved with symptomatic treatment Increased severity at doses above 5 mg/kg Results from this first-in-human trial support the initiation of the global Phase 2/3 efficacy and safety trial of suvodirsen in patients with DMD amenable to exon 51 skipping (DYSTANCE 51) AE=adverse event; DMD=Duchenne muscular dystrophy; hsCRP=high-sensitivity C-reactive protein; IAR=infusion-associated reaction

DYSTANCE 51: A Phase 2/3 Clinical Trial of Suvodirsen in Patients with Duchenne Muscular Dystrophy
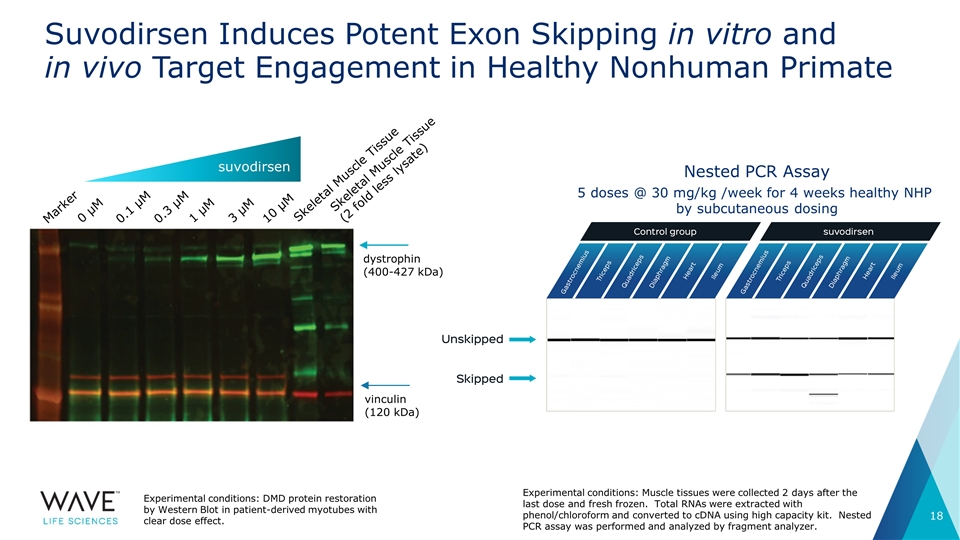
Suvodirsen Induces Potent Exon Skipping in vitro and in vivo Target Engagement in Healthy Nonhuman Primate 5 doses @ 30 mg/kg /week for 4 weeks healthy NHP by subcutaneous dosing Nested PCR Assay Marker 0 µM Skeletal Muscle Tissue (2 fold less lysate) 0.1 µM 0.3 µM 1 µM 3 µM 10 µM Skeletal Muscle Tissue dystrophin (400-427 kDa) vinculin (120 kDa) suvodirsen Experimental conditions: DMD protein restoration by Western Blot in patient-derived myotubes with clear dose effect. Experimental conditions: Muscle tissues were collected 2 days after the last dose and fresh frozen. Total RNAs were extracted with phenol/chloroform and converted to cDNA using high capacity kit. Nested PCR assay was performed and analyzed by fragment analyzer.
Preclinical and Clinical Data Used to Support Final Dose Selection in OLE and DYSTANCE 51 In vitro studies of suvodirsen In vivo study of suvodirsen target engagement in normal monkey Multiple in vitro and in vivo studies in mdx23 mouse Human PK data from Phase 1 study of suvodirsen Both tolerability data and PK profile Conclusions: Preclinical and clinical data support determination that doses of 3 mg/kg and 4.5* mg/kg are likely to provide meaningful levels of skipping and potential dystrophin restoration with differences between their tolerability profiles Note: 4.5 mg/kg dose in DYSTANCE 51 provides approximately the same amount of active ingredient as the 5 mg/kg dose in the Phase 1 clinical trial
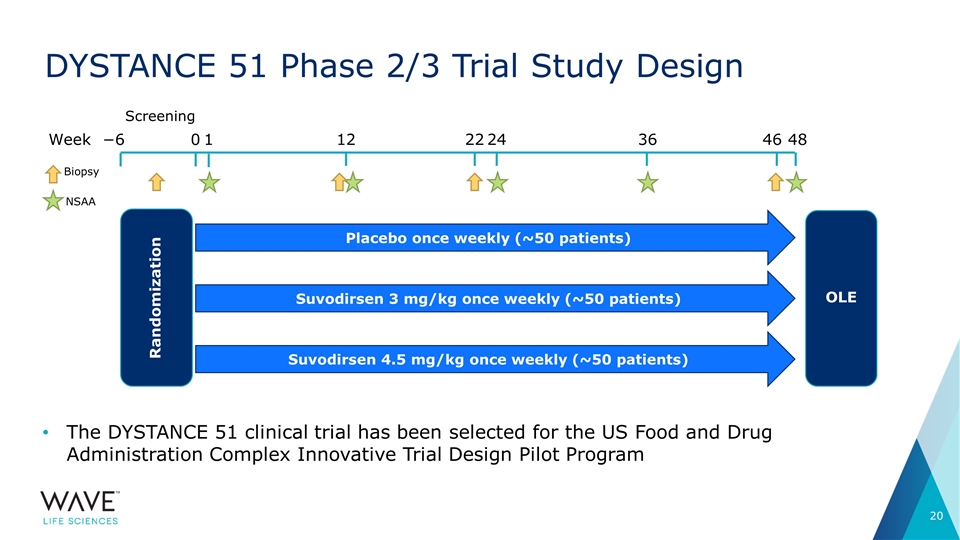
DYSTANCE 51 Phase 2/3 Trial Study Design The DYSTANCE 51 clinical trial has been selected for the US Food and Drug Administration Complex Innovative Trial Design Pilot Program 0 12 24 Week Screening −6 Suvodirsen 3 mg/kg once weekly (~50 patients) Placebo once weekly (~50 patients) Suvodirsen 4.5 mg/kg once weekly (~50 patients) OLE Randomization 1 36 48 22 46 Biopsy NSAA
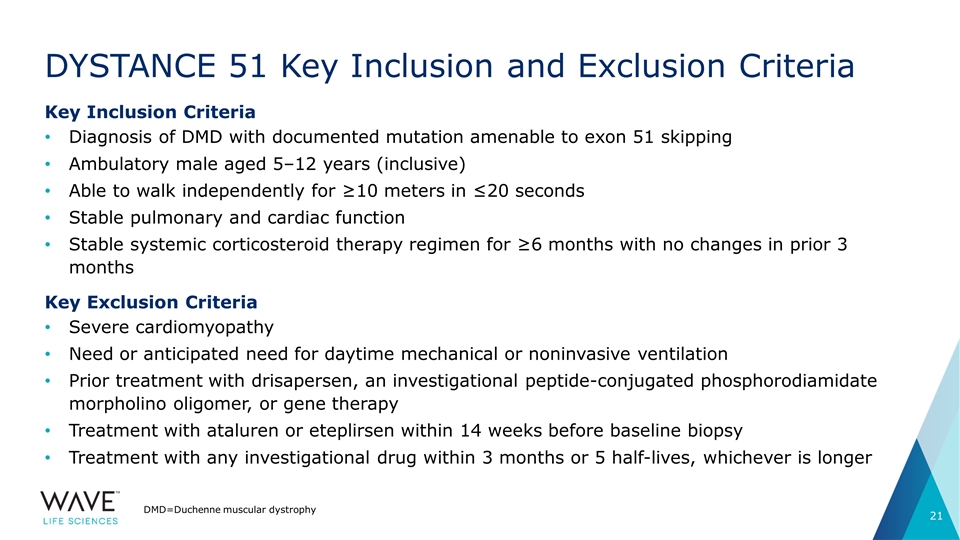
DYSTANCE 51 Key Inclusion and Exclusion Criteria Key Inclusion Criteria Diagnosis of DMD with documented mutation amenable to exon 51 skipping Ambulatory male aged 5–12 years (inclusive) Able to walk independently for ≥10 meters in ≤20 seconds Stable pulmonary and cardiac function Stable systemic corticosteroid therapy regimen for ≥6 months with no changes in prior 3 months Key Exclusion Criteria Severe cardiomyopathy Need or anticipated need for daytime mechanical or noninvasive ventilation Prior treatment with drisapersen, an investigational peptide-conjugated phosphorodiamidate morpholino oligomer, or gene therapy Treatment with ataluren or eteplirsen within 14 weeks before baseline biopsy Treatment with any investigational drug within 3 months or 5 half-lives, whichever is longer DMD=Duchenne muscular dystrophy
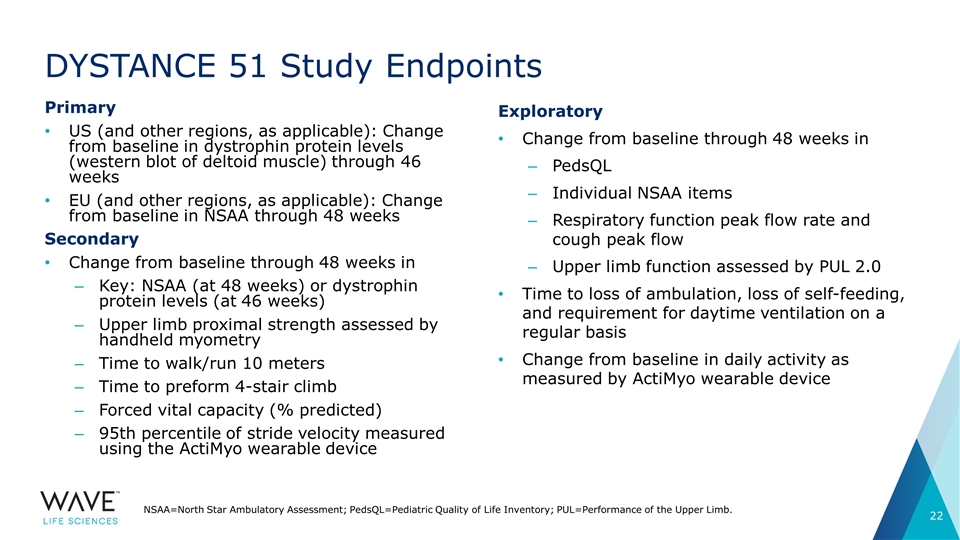
DYSTANCE 51 Study Endpoints Primary US (and other regions, as applicable): Change from baseline in dystrophin protein levels (western blot of deltoid muscle) through 46 weeks EU (and other regions, as applicable): Change from baseline in NSAA through 48 weeks Secondary Change from baseline through 48 weeks in Key: NSAA (at 48 weeks) or dystrophin protein levels (at 46 weeks) Upper limb proximal strength assessed by handheld myometry Time to walk/run 10 meters Time to preform 4-stair climb Forced vital capacity (% predicted) 95th percentile of stride velocity measured using the ActiMyo wearable device NSAA=North Star Ambulatory Assessment; PedsQL=Pediatric Quality of Life Inventory; PUL=Performance of the Upper Limb. Exploratory Change from baseline through 48 weeks in PedsQL Individual NSAA items Respiratory function peak flow rate and cough peak flow Upper limb function assessed by PUL 2.0 Time to loss of ambulation, loss of self-feeding, and requirement for daytime ventilation on a regular basis Change from baseline in daily activity as measured by ActiMyo wearable device
Conclusions Suvodirsen was generally safe and well tolerated at doses up to and including 5 mg/kg Most common AEs were infusion-associated Mild to moderate in intensity Associated with transient changes in hsCRP and complement factor Bb Resolved with symptomatic treatment Increased severity at doses above 5 mg/kg Based on in vitro and in vivo preclinical studies and the Phase 1 clinical results, Wave selected 3 and 4.5 mg/kg for its planned Phase 2/3 clinical trial of suvodirsen Results from this first-in-human trial support the initiation of the global Phase 2/3 efficacy and safety trial of suvodirsen in patients with DMD amenable to exon 51 skipping (DYSTANCE 51) AE=adverse event; DMD=Duchenne muscular dystrophy; hsCRP=high-sensitivity C-reactive protein; IAR=infusion-associated reaction Note: 4.5 mg/kg dose in DYSTANCE 51 provides approximately the same amount of active ingredient as the 5 mg/kg dose in the Phase 1 clinical trial
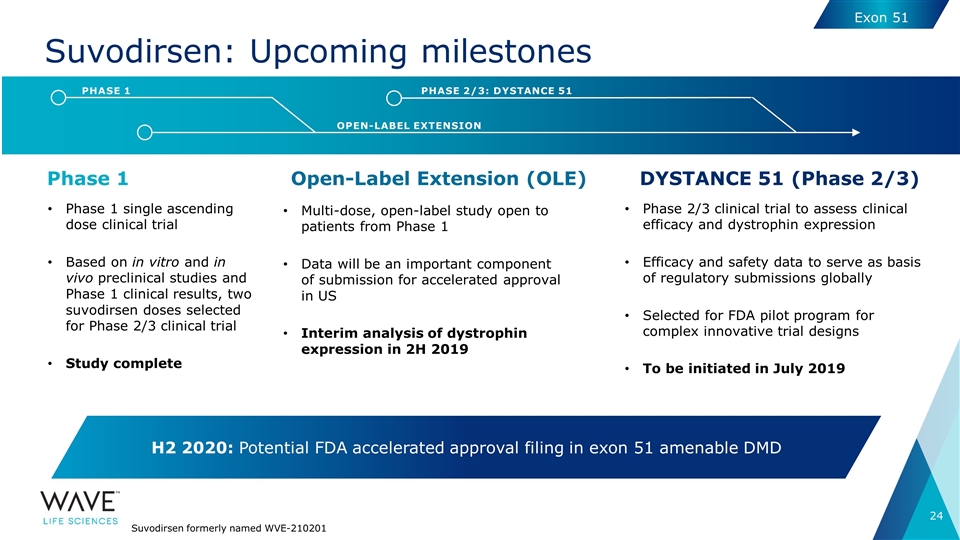
Suvodirsen: Upcoming milestones DYSTANCE 51 (Phase 2/3) Open-Label Extension (OLE) Phase 1 Phase 1 single ascending dose clinical trial Based on in vitro and in vivo preclinical studies and Phase 1 clinical results, two suvodirsen doses selected for Phase 2/3 clinical trial Study complete Multi-dose, open-label study open to patients from Phase 1 Data will be an important component of submission for accelerated approval in US Interim analysis of dystrophin expression in 2H 2019 Phase 2/3 clinical trial to assess clinical efficacy and dystrophin expression Efficacy and safety data to serve as basis of regulatory submissions globally Selected for FDA pilot program for complex innovative trial designs To be initiated in July 2019 OPEN-LABEL EXTENSION PHASE 1 PHASE 2/3: DYSTANCE 51 H2 2020: Potential FDA accelerated approval filing in exon 51 amenable DMD Suvodirsen formerly named WVE-210201 Exon 51

Wave Life Sciences Muscular Dystrophy Association Clinical and Scientific Conference Investor Update April 16, 2019
























