Exhibit 99.2
PROSPECTUS SUPPLEMENT SUMMARY
This summary highlights information contained elsewhere or incorporated by reference in this prospectus supplement and the accompanying prospectus. This summary does not contain all of the information that you should consider before deciding to invest in our ordinary shares. You should read this entire prospectus supplement and the accompanying prospectus carefully, including the “Risk Factors” section contained in this prospectus supplement, our consolidated financial statements and the related notes thereto and the other documents and information incorporated by reference in this prospectus supplement and the accompanying prospectus.
Overview
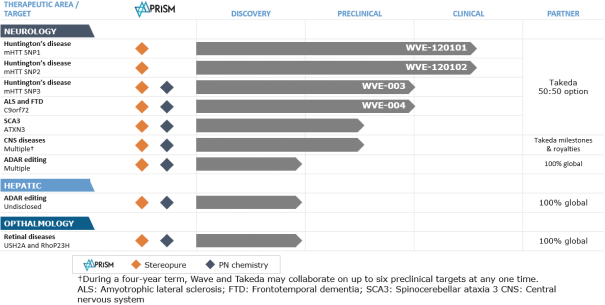
We are a clinical-stage genetic medicines company committed to delivering life-changing treatments for people battling devastating diseases. Using PRISM, our proprietary discovery and drug development platform that enables the precise design, optimization and production of novel stereopure oligonucleotides, we aspire to develop best in class medicines for genetically defined diseases with a high degree of unmet need.
Nucleic acid therapeutics, including oligonucleotides, are a growing and innovative class of drugs comprised of a sequence of nucleotides that are linked together by a backbone of chemical bonds. We are initially developing oligonucleotides that target the ribonucleic acid (“RNA”) to either reduce the expression of disease-promoting proteins or transform the production of dysfunctional mutant proteins into the production of functional proteins. RNA is a critical molecule that can adopt complex three-dimensional structures and affect various cellular functions. By intervening at the RNA level, we have the potential to address diseases that have historically been difficult to treat with small molecules or biologics. The mechanisms that we are currently using to target RNA with our oligonucleotides include RNase H-mediated RNA degradation, Ago2-mediated RNA interference (“RNAi”), exon-skipping, and ADAR (adenosine deaminases acting on RNA)-mediated RNA editing. Oligonucleotides have additional advantages as a therapeutic class including the ability to target multiple tissue types, often without the need for a delivery vehicle, and the ability to modulate the frequency of dosing to ensure broad distribution within tissues. Oligonucleotides also have well-established manufacturing processes and validated test methods based on decades of improvements.
The oligonucleotides we are developing with PRISM are stereopure. A stereopure oligonucleotide is comprised of molecules with atoms precisely arranged in three-dimensional orientations at each linkage. We believe that controlling the stereochemistry of each backbone position will optimize the pharmacological profile of our oligonucleotides by maximizing the potential therapeutic benefit while minimizing the potential for side effects and safety risks. The stereopure oligonucleotides we are developing differ from the mixture-based oligonucleotides currently on the market or in development by others. Our preclinical studies have demonstrated that our stereopure oligonucleotides may achieve superior pharmacological properties compared with mixture-based oligonucleotides. Through our work in developing stereopure oligonucleotides, we have created and continue to evolve PRISM, our proprietary discovery and drug development platform.
PRISM enables us to target genetically defined diseases with stereopure oligonucleotides across multiple therapeutic modalities. PRISM combines our unique ability to construct stereopure oligonucleotides with a deep understanding of how the interplay among oligonucleotide sequence, chemistry and backbone stereochemistry impacts key pharmacological properties. By exploring these interactions through iterative analysis of in vitro and in vivo outcomes and artificial intelligence-driven predictive modeling, we continue to define design principles that we deploy across programs to rapidly develop and manufacture clinical candidates that meet pre-defined product profiles.
Our lead clinical development programs are focused in genetic diseases within neurology. Our most advanced stereopure therapeutic candidates in development, WVE-120101 and WVE-120102, are designed to selectively target mutant huntingtin (“mHTT”) and spare wild-type, or healthy, huntingtin (“wtHTT”) for the treatment of Huntington’s disease (“HD”). WVE-120101 and WVE-120102 are currently being studied in two
1