
February 20, 2018 Phase 3 PALISADE Trial Top-Line Results Exhibit 99.2

Forward-Looking Statements This presentation contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, clinical development plans, anticipated milestones, product candidate benefits, potential market size, product adoption, market positioning, competitive strengths, product development, and other clinical, business and financial matters. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially. Risks and uncertainties include, but are not limited to, our limited operating history, our need for additional financing to achieve our goals, our dependence on our lead product AR101, the need for additional clinical testing of AR101, uncertainties relating to the regulatory process, uncertainties relating to the timing and operation of clinical trials, potential safety issues, possible lack of market acceptance of our product candidates, the intense competition in the biopharmaceutical industry, our dependence on exclusive third-party suppliers and manufacturers, and limitations on intellectual property protection. A further list and description of these risks, uncertainties and other factors can be found in our report on Form 10-Q filed on November 6, 2017. Copies of this filing are available online at www.sec.gov or www.aimmune.com. Any forward-looking statements made in this presentation speak only as of the date of the presentation. We do not undertake to update any forward-looking statements as a result of new information or future events or developments.
Phase 3 PALISADE Trial of AR101: Topline Data Overview PALISADE (Peanut ALlergy Oral Immunotherapy Study of AR101 for DEsensitization) was an international, multicenter, randomized, double-blind, placebo-controlled, Phase 3 study of AR101 in peanut-allergic individuals PALISADE had 554 randomized patients, of whom 496 were treated patients ages 4-17 (372 AR101-treated and 124 placebo-treated); 79.6% of patients completed AR101 treatment; 20.4% discontinued, 12.4% due to adverse events PALISADE met the primary efficacy endpoint; on an Intent-to-Treat (ITT) basis, 67.2% of AR101 patients ages 4–17 tolerated at least a 600-mg dose of peanut protein during the exit double-blind, placebo-controlled (DBPCFC) food challenge compared to 4.0% of placebo patients (p<0.00001), exceeding the pre-specified statistical threshold PALISADE provided evidence of an encouraging and expected tolerability profile for AR101
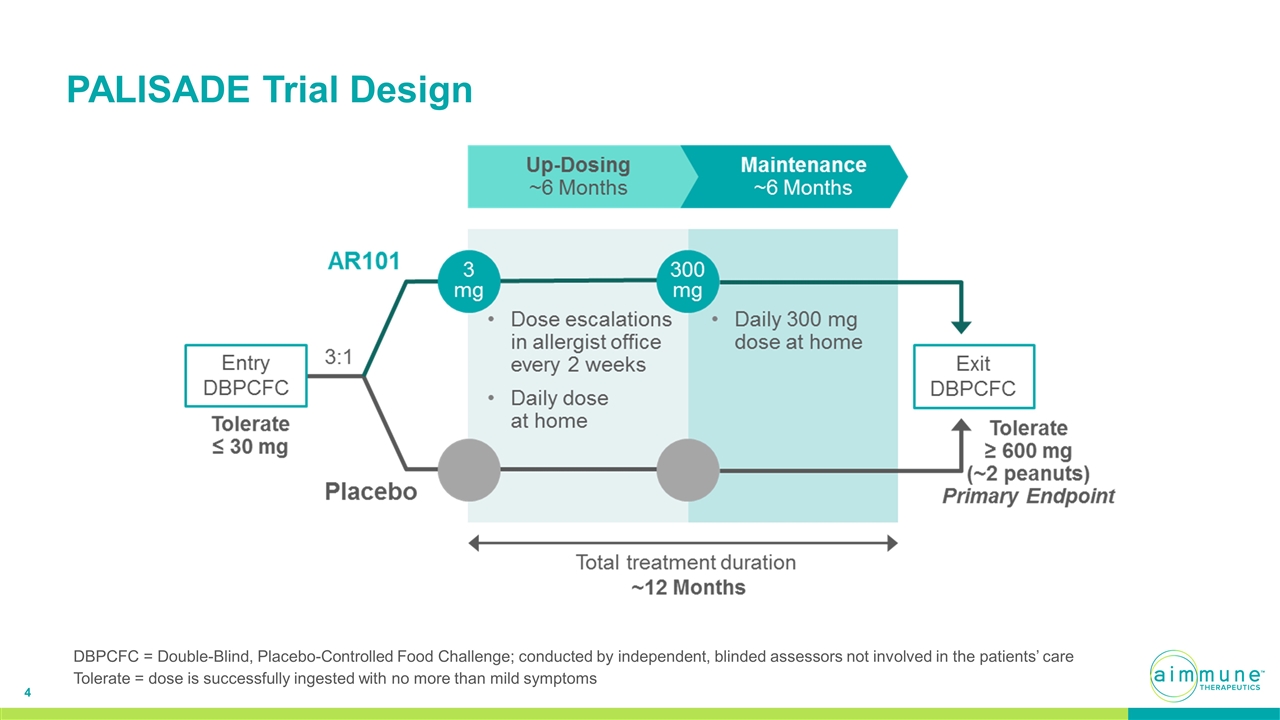
PALISADE Trial Design DBPCFC = Double-Blind, Placebo-Controlled Food Challenge; conducted by independent, blinded assessors not involved in the patients’ care Tolerate = dose is successfully ingested with no more than mild symptoms
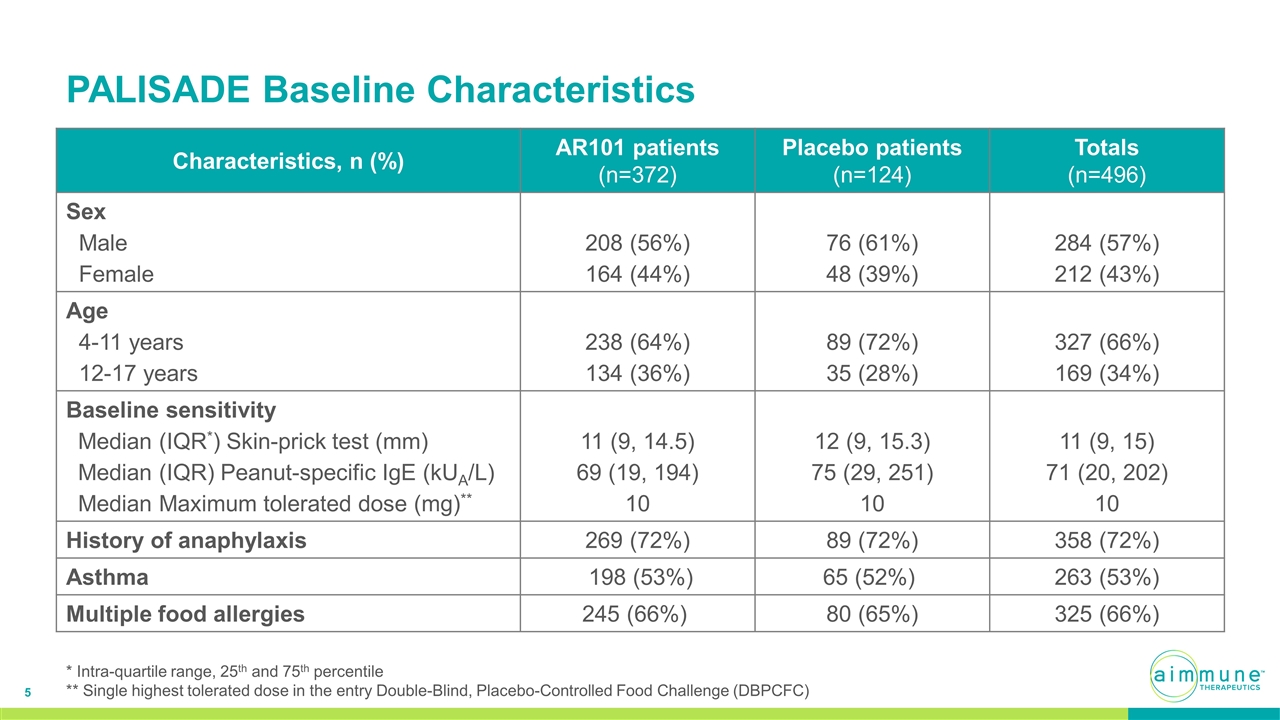
PALISADE Baseline Characteristics Characteristics, n (%) AR101 patients (n=372) Placebo patients (n=124) Totals (n=496) Sex Male Female 208 (56%) 164 (44%) 76 (61%) 48 (39%) 284 (57%) 212 (43%) Age 4-11 years 12-17 years 238 (64%) 134 (36%) 89 (72%) 35 (28%) 327 (66%) 169 (34%) Baseline sensitivity Median (IQR*) Skin-prick test (mm) Median (IQR) Peanut-specific IgE (kUA/L) Median Maximum tolerated dose (mg)** 11 (9, 14.5) 69 (19, 194) 10 12 (9, 15.3) 75 (29, 251) 10 11 (9, 15) 71 (20, 202) 10 History of anaphylaxis 269 (72%) 89 (72%) 358 (72%) Asthma 198 (53%) 65 (52%) 263 (53%) Multiple food allergies 245 (66%) 80 (65%) 325 (66%) * Intra-quartile range, 25th and 75th percentile ** Single highest tolerated dose in the entry Double-Blind, Placebo-Controlled Food Challenge (DBPCFC)
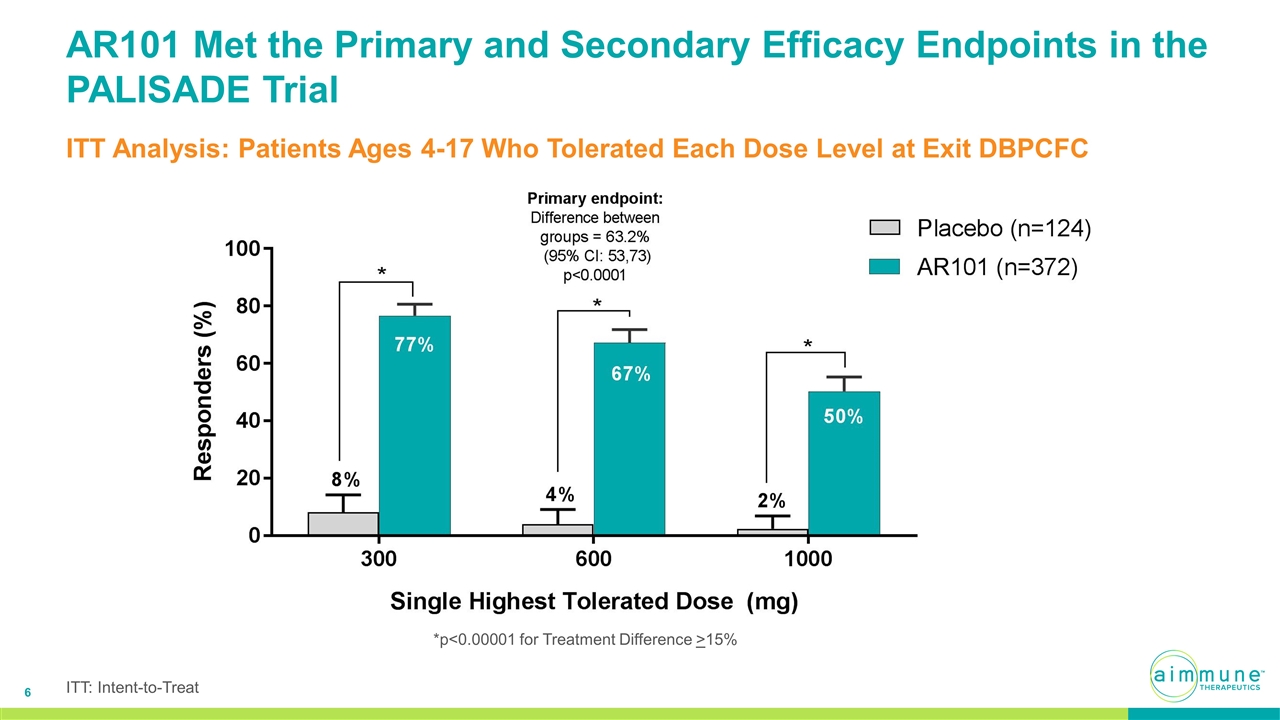
AR101 Met the Primary and Secondary Efficacy Endpoints in the PALISADE Trial ITT Analysis: Patients Ages 4-17 Who Tolerated Each Dose Level at Exit DBPCFC *p<0.00001 for Treatment Difference >15% ITT: Intent-to-Treat
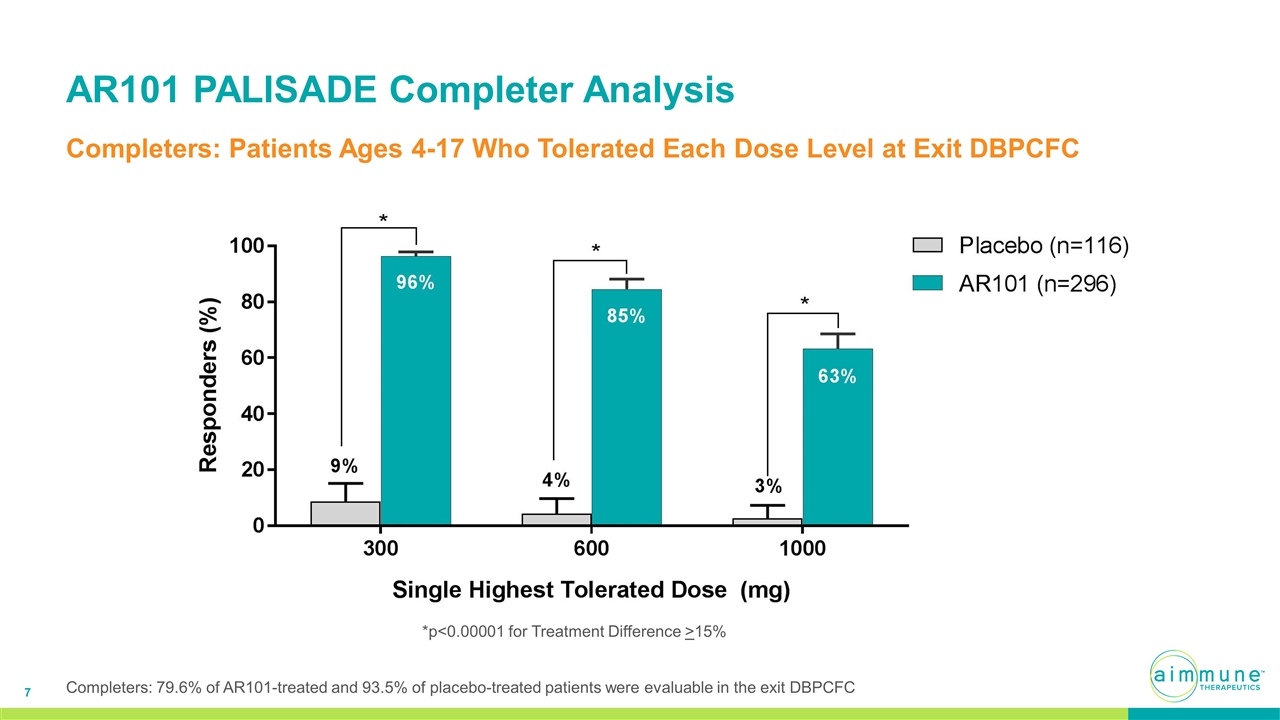
AR101 PALISADE Completer Analysis Completers: Patients Ages 4-17 Who Tolerated Each Dose Level at Exit DBPCFC *p<0.00001 for Treatment Difference >15% Completers: 79.6% of AR101-treated and 93.5% of placebo-treated patients were evaluable in the exit DBPCFC
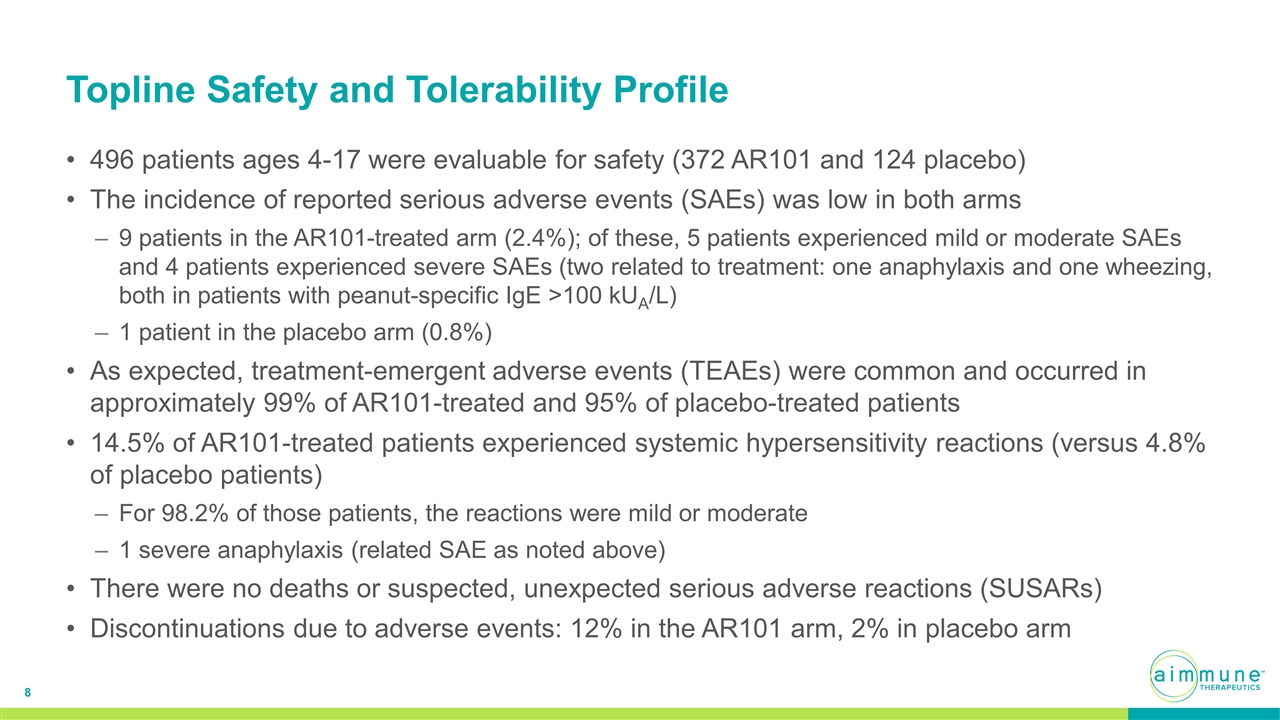
Topline Safety and Tolerability Profile 496 patients ages 4-17 were evaluable for safety (372 AR101 and 124 placebo) The incidence of reported serious adverse events (SAEs) was low in both arms 9 patients in the AR101-treated arm (2.4%); of these, 5 patients experienced mild or moderate SAEs and 4 patients experienced severe SAEs (two related to treatment: one anaphylaxis and one wheezing, both in patients with peanut-specific IgE >100 kUA/L) 1 patient in the placebo arm (0.8%) As expected, treatment-emergent adverse events (TEAEs) were common and occurred in approximately 99% of AR101-treated and 95% of placebo-treated patients 14.5% of AR101-treated patients experienced systemic hypersensitivity reactions (versus 4.8% of placebo patients) For 98.2% of those patients, the reactions were mild or moderate 1 severe anaphylaxis (related SAE as noted above) There were no deaths or suspected, unexpected serious adverse reactions (SUSARs) Discontinuations due to adverse events: 12% in the AR101 arm, 2% in placebo arm
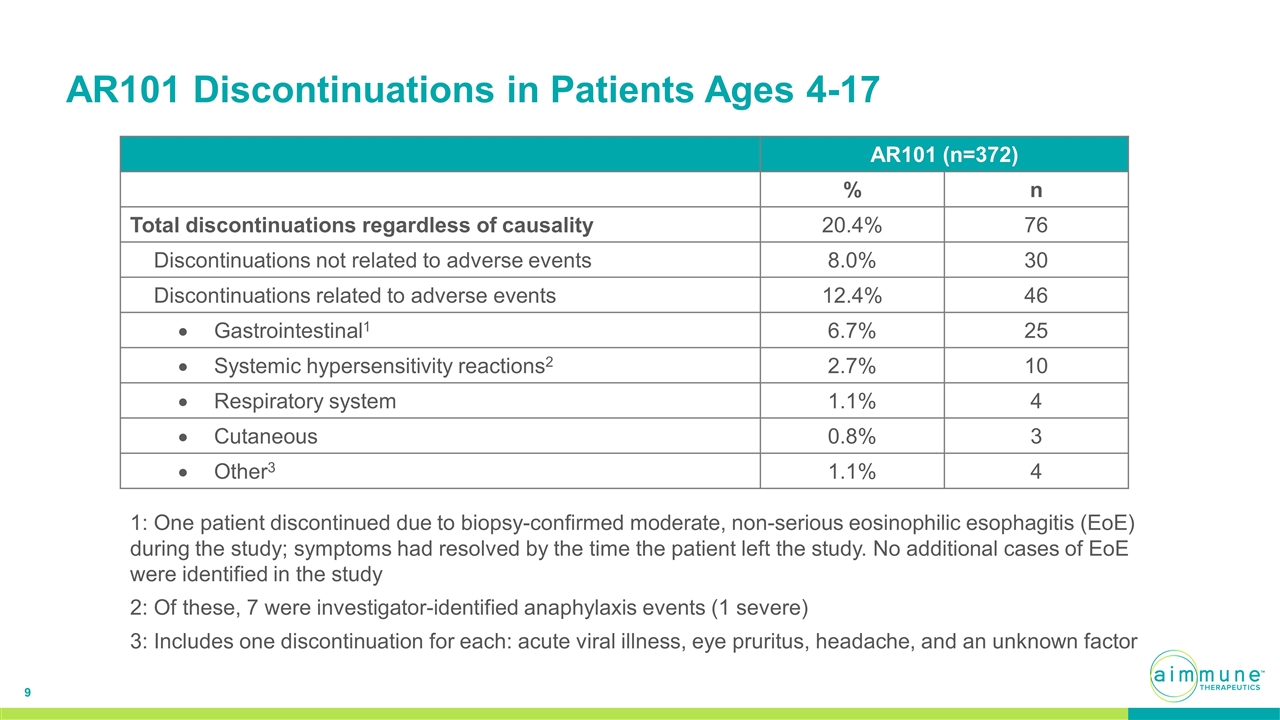
AR101 Discontinuations in Patients Ages 4-17 AR101 (n=372) % n Total discontinuations regardless of causality 20.4% 76 Discontinuations not related to adverse events 8.0% 30 Discontinuations related to adverse events 12.4% 46 Gastrointestinal1 6.7% 25 Systemic hypersensitivity reactions2 2.7% 10 Respiratory system 1.1% 4 Cutaneous 0.8% 3 Other3 1.1% 4 1: One patient discontinued due to biopsy-confirmed moderate, non-serious eosinophilic esophagitis (EoE) during the study; symptoms had resolved by the time the patient left the study. No additional cases of EoE were identified in the study 2: Of these, 7 were investigator-identified anaphylaxis events (1 severe) 3: Includes one discontinuation for each: acute viral illness, eye pruritus, headache, and an unknown factor
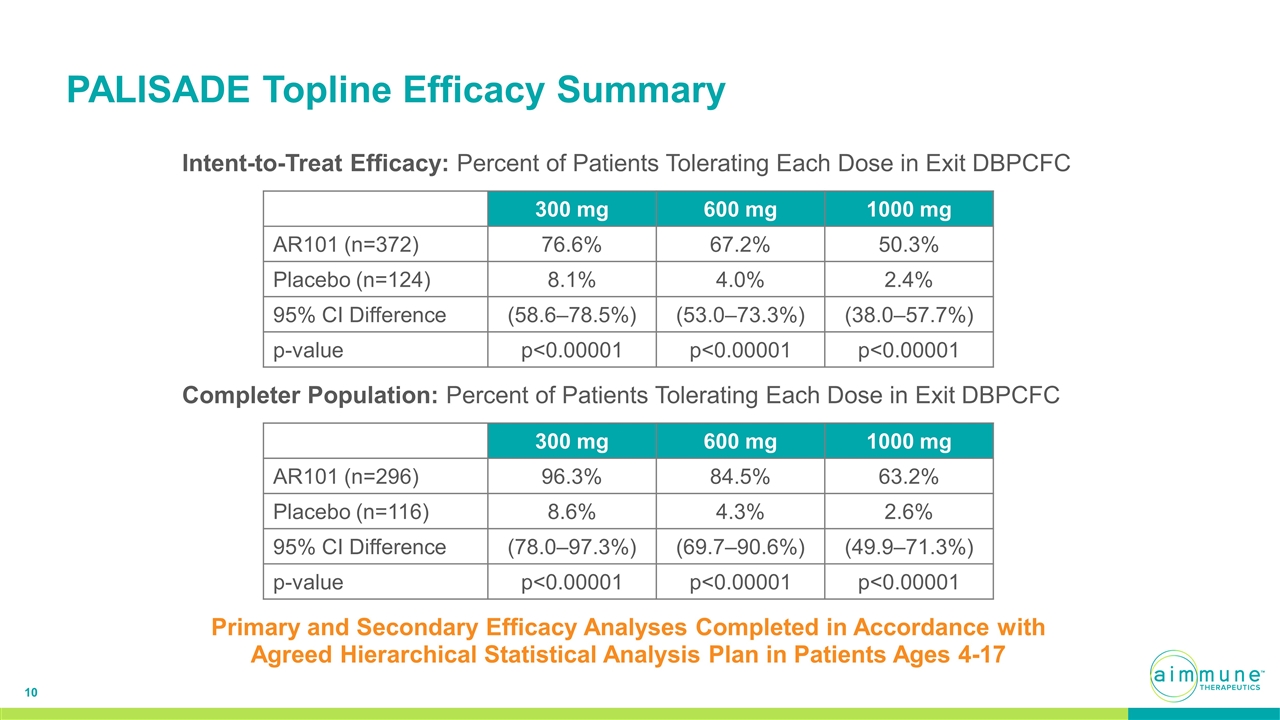
PALISADE Topline Efficacy Summary Primary and Secondary Efficacy Analyses Completed in Accordance with Agreed Hierarchical Statistical Analysis Plan in Patients Ages 4-17 300 mg 600 mg 1000 mg AR101 (n=372) 76.6% 67.2% 50.3% Placebo (n=124) 8.1% 4.0% 2.4% 95% CI Difference (58.6–78.5%) (53.0–73.3%) (38.0–57.7%) p-value p<0.00001 p<0.00001 p<0.00001 300 mg 600 mg 1000 mg AR101 (n=296) 96.3% 84.5% 63.2% Placebo (n=116) 8.6% 4.3% 2.6% 95% CI Difference (78.0–97.3%) (69.7–90.6%) (49.9–71.3%) p-value p<0.00001 p<0.00001 p<0.00001 Intent-to-Treat Efficacy: Percent of Patients Tolerating Each Dose in Exit DBPCFC Completer Population: Percent of Patients Tolerating Each Dose in Exit DBPCFC
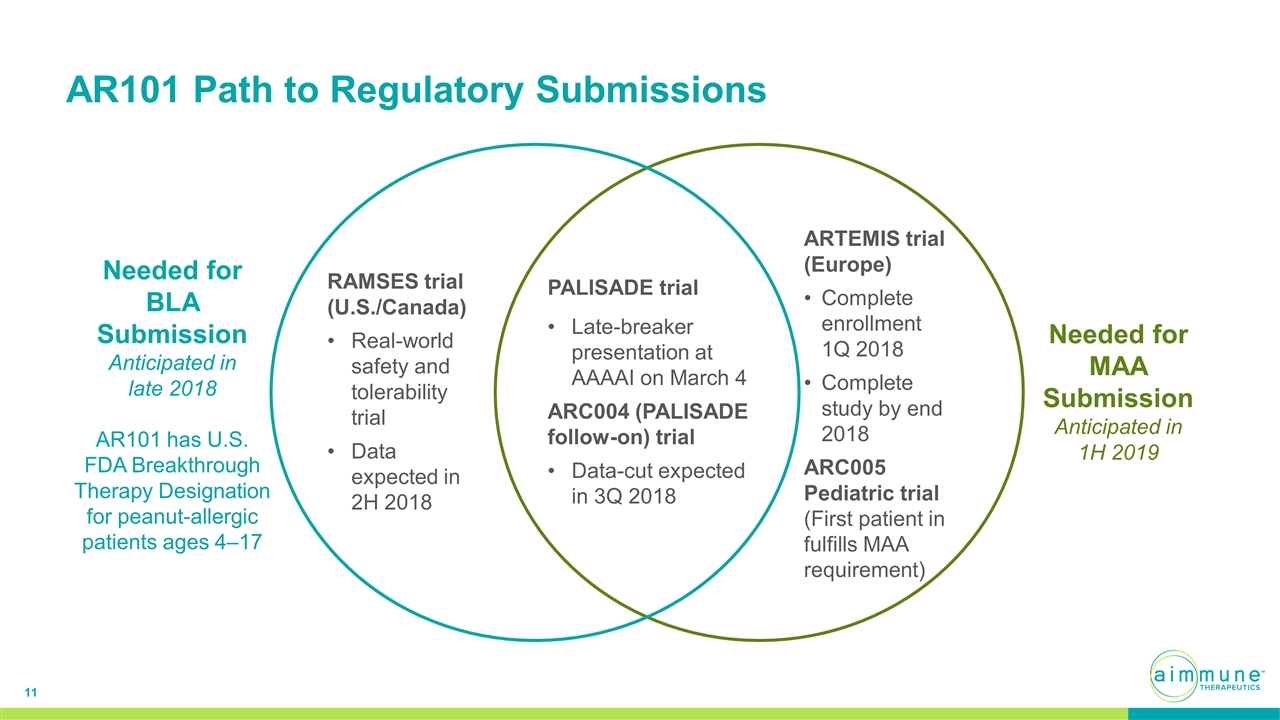
AR101 Path to Regulatory Submissions Needed for BLA Submission Anticipated in late 2018 AR101 has U.S. FDA Breakthrough Therapy Designation for peanut-allergic patients ages 4–17 Needed for MAA Submission Anticipated in 1H 2019 PALISADE trial Late-breaker presentation at AAAAI on March 4 ARC004 (PALISADE follow-on) trial Data-cut expected in 3Q 2018 ARTEMIS trial (Europe) Complete enrollment 1Q 2018 Complete study by end 2018 ARC005 Pediatric trial (First patient in fulfills MAA requirement) RAMSES trial (U.S./Canada) Real-world safety and tolerability trial Data expected in 2H 2018

Appendix
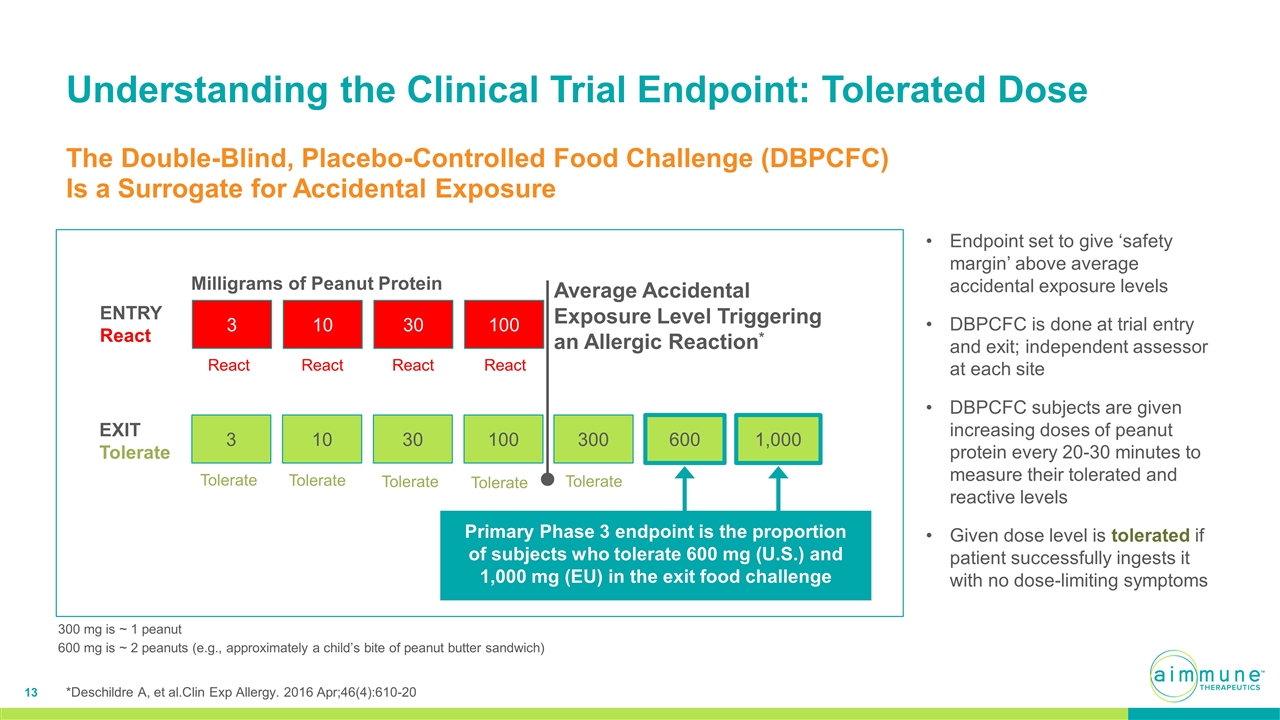
Understanding the Clinical Trial Endpoint: Tolerated Dose The Double-Blind, Placebo-Controlled Food Challenge (DBPCFC) Is a Surrogate for Accidental Exposure *Deschildre A, et al.Clin Exp Allergy. 2016 Apr;46(4):610-20 Milligrams of Peanut Protein ENTRY React EXIT Tolerate 3 10 30 100 300 600 3 10 30 100 1,000 React Endpoint set to give ‘safety margin’ above average accidental exposure levels DBPCFC is done at trial entry and exit; independent assessor at each site DBPCFC subjects are given increasing doses of peanut protein every 20-30 minutes to measure their tolerated and reactive levels Given dose level is tolerated if patient successfully ingests it with no dose-limiting symptoms React React React Tolerate Tolerate Tolerate Tolerate Tolerate Average Accidental Exposure Level Triggering an Allergic Reaction* 300 mg is ~ 1 peanut 600 mg is ~ 2 peanuts (e.g., approximately a child’s bite of peanut butter sandwich) Primary Phase 3 endpoint is the proportion of subjects who tolerate 600 mg (U.S.) and 1,000 mg (EU) in the exit food challenge












