
| Writer’s Direct Number | Writer’s E-mail Address |
| 212.756.2376 | eleazer.klein@srz.com |
February 17, 2017
VIA EDGAR AND ELECTRONIC MAIL
Daniel F. Duchovny Special Counsel, Office of Mergers and Acquisitions Division of Corporate Finance 100 F Street, NE U.S. Securities and Exchange Commission Washington, D.C. 20549 | | |
| | | Re: | Immunomedics, Inc. Definitive Additional Soliciting Materials Filed on February 10, 2017 by venBio Select Advisor LLC, et. al. Filed on February 14, 2017 by venBio Select Advisor LLC, et. al. File No. 000-12104 |
| | | | | | |
Dear Mr. Duchovny:
On behalf of venBio Select Advisor LLC (“venBio”) and the other filing persons of the venBio filings referenced herein (collectively, the “Filing Persons”), we are responding to your letters dated February 14, 2017 (the “February 14 SEC Comment Letter”) and February 15, 2017 (the “February 15 SEC Comment Letter,” collectively the “SEC Comment Letters”) in connection with the Filing Persons’ definitive additional materials on Schedule 14A filed on February 10, 2017 (the “February 10 Press Release”) and the Filing Persons’ definitive additional materials on Schedule 14A filed on February 14, 2017 (the “February 14 Press Release,” together with the February 10 Press Release, the “venBio Press Releases”) with respect to Immunomedics, Inc. (“Immunomedics” or the “Company”). We have reviewed the comments of the staff (the “Staff”) of the Securities and Exchange Commission (the “SEC,” or the “Commission”) and respond below. For your convenience, the comments are restated below in italics, with our responses following. In certain instances, the Staff’s comments have been grouped, reordered and numbered for ease of reference and in the interest of providing succinct responses to the Staff.
Definitive Additional Materials filed on February 10, 2017
| 1. | You must avoid issuing statements that directly or indirectly impugn the character, integrity or personal reputation or make charges of illegal, improper or immoral conduct without factual foundation. We note the statements listed below. Provide us supplementally, or disclose, the |
Daniel F. Duchovny, Esq. February 17, 2017 Page 2 | | |
factual foundation for your beliefs relating to the violation of the current directors’ fiduciary duties as well as generally acting in bad faith, among other statements or suggestions. In this regard, note that the factual foundation for such assertion must be reasonable. Refer to Rule 14a-9.
| (a) | • That the company is “blatantly spurning the will of stockholders” in announcing its agreement with Seattle Genetics. In this respect, address also how the will of the company’s security holders has manifested itself. |
• That security holders “lack confidence in current board and management.” In this respect, address also how the will of the company’s security holders has manifested itself.
• That the statements of the current board “cannot be trusted, and their credibility with stockholders has long ago been squandered.”
The Filing Persons, in the above referenced statements, have voiced their opinions and beliefs which are supported by reasonable factual basis and as such are fully compliant with the requirements of the proxy rules. As indicated in the venBio Press Releases, the Filing Persons believe that the will of the Company’s security holders has manifested itself through a desire for venBio’s slate of nominees (the “venBio Nominees”) to be elected to the board of directors of the Company (the “Board”), so a reconstituted Board can represent the best interests of the shareholders in reviewing and negotiating a strategic transaction that properly values the developmental drug IMMU-132, thus maximizing stockholder value and for a strategic transaction as significant as the Seattle Genetics Deal (defined below) to have been considered by a truly independent Board that has the vote of confidence of the stockholders.
The Filing Persons believe that the will of the Company’s stockholders was evidenced by the majority support for the venBio Nominees before the Company’s announcement of a transaction with Seattle Genetics, Inc. (“Seattle Genetics”) to license IMMU-132 (the “Seattle Genetics Deal”). As of February 9, 2017, merely one day before the announcement of the Seattle Genetics Deal, a majority of proxies for the Company’s outstanding shares had been submitted showing support in favor of the venBio Nominees, indicating strong support for stockholders to see a reconstituted Board prior to making any material decision regarding a strategic transactions such as the Seattle Genetics Deal. The Board’s proxy solicitors, much like the Filing Persons’ proxy solicitors, would have undoubtedly tracked the number of proxies tendered and would have reported to the Board that a majority of the Company’s shareholders as of that time had tendered proxies in support of all four venBio’s Nominees and if things remained the same a majority of the current Board would lose their seats. Accordingly, it was only natural for the Filing Persons to conclude that, given the Board’s inevitable knowledge of the voting results, which pointed towards a loss of a majority of the Board, along with the critical public comments of proxy advisors, shareholders and commentators, the incumbent Board knew they were likely a “lame duck” Board that no longer had a clear mandate from the stockholders to make vital decisions on their behalf especially where those decisions may not be undone by an incoming new Board to be voted into office by stockholders just days later.
Daniel F. Duchovny, Esq. February 17, 2017 Page 3 | | |
In fact, some of that support has been expressed publicly. The investment fund OrbiMed Advisors LLC, one of Immunomedics’s largest stockholders, publicly endorsed venBio’s campaign and the venBio Nominees in a press release issued on February 2, 2017, stating that “venBio’s proposal represents the superior path to improving the Company’s business and maximizing shareholder value.”
In addition, venBio received strong indications of support from all three major proxy advisory services such as Institutional Shareholder Services (“ISS”), Glass Lewis, and Egan Jones. Each of the foregoing advisory services recommended, in advance of the announcement of the Seattle Genetics Deal, that stockholders of the Company vote in favor of the venBio Nominees and for stockholders to forego a vote on the Company’s proxy card. As discussed further below in the Filing Persons’ response to Staff comment 2(b), at least two of the foregoing proxy advisors’ reports noted stockholder support for venBio across the spectrum of the Company’s stockholder base.
The Filing Persons also wish to note that on February 10, 2017, in response to the Seattle Genetics Deal, ISS issued a supplement to the ISS Report, stating that its recommendation for stockholders to vote in favor of the venBio Nominees has not changed and that:
The postponement of the annual meeting until after the closing of the go-shop period on Feb. 19 effectively prevents shareholders from electing a new board that could provide a second opinion as to whether the deal with Seattle Genetics represents the best alternative for shareholders. Although shareholders will be able to vote on the share issuance, the company indicated in a conference call that the transaction with Seattle Genetics is not contingent upon shareholder approval of the share issuance. (emphasis added)
ISS’s statement that the “go-shop” effectively prevents shareholders from electing a new board that could provide a second opinion as to whether the deal [. . .] represents the best alternative for shareholders” is a clear indication, in the Filing Persons’ mind, that the best interests of the stockholder franchise – the “will of shareholders” – has been negatively influenced by the Company’s action and a leading independent proxy service is concerned about the timing and implications of the current Board’s decision to enter into the Seattle Genetics Deal.
In addition to the above, the Filing Persons have taken stock of the negative comments made about the Seattle Genetics Deal on online message boards such as Silicon Investor, which the Filing Persons have good reason to believe (by virtue of the volume and content of the messages posted on such message boards) are frequented by investors in the Company and indicate both an interest in investors’ achieving value-optimization in a strategic deal involving IMMU-132 as well as a distrust of the Company’s current Board and management. Copied below is a sampling of comments posted to Silicon Investor on February 10, 2017 after the announcement of the Seattle Genetics Deal:
| · | “Market isn’t very impressed” |
| · | “Still need a new BOD: Gold.” |
| · | “Something is not right !!!!” |
| · | “A deal like this should easily double the stock price, especially with the high short ratio, they are getting 50% of present market cap in cash, something is wrong !!!” |
Daniel F. Duchovny, Esq. February 17, 2017 Page 4 | | |
| · | “Who are the other Companies in the hunt ? How can they blow this ? Other ?s Pre-Market high of $7.01/7.75 mill shares and going down since ? Something smells.” |
| · | “I feel the ‘shop around’ period until Feb 19 is window dressing. I don’t see other entities putting serious effort and resources to submit a higher bid knowing the SGEN has the right to match it.” |
| · | “I am worried why this is not higher than what it is. I understand as etc., but we have a partnership and 350 mil upfront and we are approaching our opening amount 4.30....:(” |
| · | “Looks like the investment community sees this as a desperation deal where IMMU gave away the crown jewel on the cheap. Almost 40,000,000 shares traded thus far and no traction to speak of.” |
As discussed further below in response to Staff comment 2(d), the Filing Persons believe that the foregoing comments strongly evidence that not only has the Company’s spurned the “will” of stockholders to see their investments optimized, but statements such as “Something is not right” and “Looks like the investment community sees this as a desperation deal [. . .] exhibits a lack of credibility in the current Board and management.
The Filing Persons’ concerns are further supported by unfavorable provisions in the Seattle Genetics Deal’, including granting Seattle Genetics shares and warrants in excess of the current shares available for issuance, which will have the effect of diluting the value of common stock held by existing stockholders. Moreover, while the Company’s stock price initially increased on announcement of the transaction, such an increase incorporated a mere 93 cent increase in the share price of the stock, when, under the terms of the Seattle Genetics Deal, the delivery of $250 million in cash consideration implied a $2.27 increase in share price, suggesting that investors were not entirely pleased with the deal and may lack confidence in the Company’s decision-making.
Finally, as noted in our previous response to the Staff’s comments on this proxy contest, dated February 3, 2017, the fact that the Company’s stock price had declined 62% since the end of June, when the Company was ejected from the prestigious ASCO Conference after it violated a data embargo, serves as strong evidence that stockholders “lack[ed] confidence in the current board and management” even before the announcement of the Seattle Genetics Deal. One such indication of a lack of confidence in the current board and management derives from the fact that on October 5, 2016, the Company issued a dilutive financing of $30,000,000 with 100% warrant coverage, while the stock was at its then lowest levels since June 2016. More than the financing itself, the Filing Persons believe that the Company’s actions of originally telling investors that the Company was not going to engage in dilutive financing was devastating to the share price, leading to a 24% share price decline in a single day while the NASDAQ Composite was up 0.5%, thus leading to a lack of trust in the Company’s board and management by stockholders.
The Filing Persons believe that the forcefulness of the foregoing market data, comments from proxy advisory services, and statements made by observers of the current proxy contest all supply a proper factual basis for the statement that the Company has not entered in a transaction that properly values IMMU-132, is taking action to undermine the future ability of the venBio Nominees to consider such a transaction, and that the current Board and management have acted in a manner that instills distrust and a lack of confidence in the Company’s stockholders, and that thus the Company is “blatantly spurning the will of stockholders” in entering into and announcing the Seattle Genetics Deal.
Daniel F. Duchovny, Esq. February 17, 2017 Page 5 | | |
| (b) | • That the current board is “afraid of losing proxy contest and is resorting to desperate measures…” |
• That the announcement of the agreement is “a blatant and shameful maneuver by the current Board and management to manipulate the outcome of the upcoming Annual Meeting and entrench themselves at the expense of stockholders’ best interests…”
• That the Board is employing “manipulative tactics.”
As discussed in the Filing Persons’ response to the Staff’s comment 1(a) above, the Filing Persons believe that a proper factual basis exists to conclude that the current Board was “afraid of losing the proxy contest,” that it was resorting to “desperate measures” or that announcing the Seattle Genetics Deal was “manipulative” based on the fact that, as of February 9, 2017, merely seven days before the 2016 annual meeting of stockholders, scheduled to be held on February 16, 2017 (the “Annual Meeting”), the venBio Nominees commanded a clear lead over the incumbent Board’s nominees with a majority of proxies delivered showing stockholders favored the venBio Nominees and that all major proxy advisory firms had recommended stockholders to vote in favor of the venBio Nominees.
It is well known that delay of an annual meeting in the face of a proxy contest is a classic defense tactic to shareholder activism.1 Accordingly, the announcement of a delay of the Annual meeting until March 3, 2017, in conjunction with a major strategic transaction containing what the Filing Persons believes are unfavorable terms that do not maximize stockholder interests (as discussed further below), merely days before an already-delayed annual meeting for which several major proxy advisory firms had recommended against the incumbent Board, is a clear indication of the Board employing a defensive tactic to entrench themselves. It is therefore entirely justifiable, in the Filing Persons’ views, that the incumbent Board was acting “desperately” to quell stockholder frustration over the fact that IMMU-132 had not yet been licensed a strategic partner in an attempt to garner stockholder support before the Annual Meeting. Notably, in a press release issued on February 14, the Company characterized a legitimate lawsuit filed by the Filing Persons a “baseless, selfish and shameful lawsuit” as well as “a desperately self-interested act designed to prop up venBio’s attempt to take control of Immunomedics,” all without providing a shred of factual support for these disparaging statements that are presented as statements of fact rather than opinion or belief. Accordingly, it would be disingenuous to describe these actions as anything other than manipulative and desperate, especially since,
1 The Company’s own defense counsel has in effect admitted as much in an article authored by him and others.See, Stephen M. Gill, Kai Haakon E. Liekefett, and Leonard Wood, “Postponement,”Structural Defenses to Shareholder Activism,The Review of Sec. & Commodities Reg. Jun. 18, 2014, (“a board might prefer to delay either the upcoming stockholder meeting or the closing of the polls in a meeting already in progress, even if a quorum is present. The purpose in either case would be to solicit additional proxies.”),available at: http://www.law.harvard.edu/programs/corp_gov/shareholder-engagement-roundtable-2015-materials/vinson-elkins_structural-defenses-to-
shareholder-activism.pdf;See also, e.g.,Farrell, Maureen,Allergan’s Best Defense: Delay, Delay, Delay,Wall Street Journal,Jul. 21, 2014 (describing Allergan’s best defense in the face of an activist advocating for the target company to enter into a strategic transaction), available athttp://blogs.wsj.com/moneybeat/2014/07/21/allergans-best-defense-delay-delay-delay/.
Daniel F. Duchovny, Esq. February 17, 2017 Page 6 | | |
the burden is on the Board to show that they were reasonable actions not motivated by entrenchment.
| (c) | That the board’s explanation about the timing of the agreement with Seattle Genetics is “preposterous and clearly disingenuous…” |
As provided in a press release filed by the Company on February 10, 2017 under cover of Schedule 14A, the Company quotes Company CSO and board member Dr. David Goldenberg (“Dr. Goldenberg”) as remarking, “After a long period of interactions with many interested partnering candidates, and a considerable period of discussion with Seattle Genetics, we concluded that working with this group of successful business and marketing executives, clinicians and scientists would allow us to contribute our own scientific and clinical knowledge to them as they further develop IMMU-132 and bring it to commercialization.” Such a statement, among others, was made in light of the fact that the Board had entered into a definitive agreement with Seattle Genetics at the beginning of February, evidently without completing discussions with other interested parties.
As even noted by the Staff, the Company claimed least 18 other companies had entered into “advanced diligence” with Immunomedics, and for the Company to thus enter into a major transaction four business days before the Annual Meeting, where the stockholders would finally have the opportunity to elect an independent slate capable of re-evaluating the Seattle Genetics Deal renders the foregoing statement, along with other similar statements, disingenuous.2 Not only could the Company “contribute [its] own scientific and clinical knowledge” to one of these other companies, but, more significantly, there is no reason to believe that the Seattle Genetics Deal could not have waited a week to be signed, until after the results of the Annual Meeting were determined. There is no evidence to suggest that the value of IMMU-132, which is the asset at the center of the deal, would have changed from one week to the next, and thus the Filing Persons have no reason to believe that a reconstituted Board and Seattle Genetics could not have agreed to the same deal on the same terms after the Annual Meeting, thus making the Company’s claims about the timing of the Seattle Genetics Deal highly suspect and providing a sufficient basis for the Filing Persons to make claims attacking such statements about timing.
| (d) | That Seattle Genetics “appears to have aided and abetted the Board’s breach of its fiduciary duties…” |
The Filing Persons believe, based on the timing and nature of the Seattle Genetics Deal (as discussed in response to Staff comments 1(a) and (c) above and 2(d) below), that the entry into the Seattle Genetics Deal was a breach of the duty of loyalty of the incumbent Board under Delaware law. Under Delaware law, a claim for aiding and abetting a breach of fiduciary duty requires “(1) the existence of a fiduciary relationship, (2) a breach of the fiduciary’s duty, and (3) knowing participation in that breach.”3 The Board has a fiduciary relationship with the Company’s stockholders. As described elsewhere in this letter and in our complaint
3Carsanaro v. Bloodhound Techs., Inc., 65 A.3d 618, 643 (Del. Ch. 2013)
Daniel F. Duchovny, Esq. February 17, 2017 Page 7 | | |
filed with the Delaware Chancery Court, the Board’s decision to enter into the SGEN transaction was a breach of the Board’s fiduciary duties. Given the public nature of this proxy contest and venBio’s support by major proxy advisory firms and its garnering of 55% of the stockholder proxies in favor of the venBio Nominees as of February 9, 2017, a reasonable inference exists supporting the belief that Seattle Genetics knew that a proxy contest was ongoing and that strong indicators suggested venBio may have been successful in electing a majority of the Board at the Annual Meeting. In fact, it is reasonable to expect that as part of its due diligence Seattle Genetics would have required access to all information available to the Company regarding the likely outcome of the ongoing proxy contest. Serious and reasonable questions arise as to whether information regarding the potential outcome of the election contest may have affected the negotiations and ultimately the agreed upon terms of the transaction.
These facts provide, by a preponderance of available information, a factual basis for the Filing Persons to believe that Seattle Genetics was aware that the incumbent Board was acting in violation of their fiduciary duties by entering into the Seattle Genetics Deal and to state that Seattle Genetics “appears” to have aided such a breach by taking other action to interfere with the stockholders’ exercise of their franchise rights to elect new directors to the Board.
| (e) | That “the current Board has hastily given away their ‘crown jewel’…” |
As described in numerous filings with the SEC, IMMU-132 was the Company’s largest major asset, having being granted “Breakthrough Designation” by the FDA and showing compelling early data during its development, such as greater than average tumor shrinkage in tested patients and reductions in disease progression. The drug had enjoyed major clinical progress compared to its peers as well, had reached “Phase 2” testing by the Company, and was even touted by the Company in a press release issued on January 18, 2017, wherein one of the Company’s headlines read, “IMMU-132 Provides Opportunity for Significant Near-Term Value Creation.” These facts alone provide a reasonable basis for anyone to claim that IMMU-132 was a highly important to the Company and may be considered a “crown jewel.”
The Seattle Genetics Deal, as described by the Company and Seattle Genetics in its recent SEC filings, is a long-term partnership contract allowing Seattle Genetics to develop, manufacture, and commercialize IMMU-132 worldwide for an one-time, up-front payment of $250 million. This means that the Company has effectively sold a major asset without stockholder input or consent, by vote of a Board, which is comprised of a majority of directors appointed 4 weeks earlier, many of whom had reasonable basis to believe that they will not be reelected by shareholders at the upcoming annual meeting. In addition, the Board is comprised of interested directors such as Dr. Goldenberg, who stand to benefit personally from any such transaction through royalty payments for patented products under the terms of his employment agreement.
Such a sale may be properly characterized as “hastily giving away” the “crown jewel” because the Seattle Genetics Deal contains an extraordinarily short go-shop period of six business days (as discussed further below in response to Staff comment 2(a)) limited to potential partners that had previously engaged with the
2On a related note, we note that while the Company claimed that 18 companies entered into advanced diligence in its February 10, 2017 presentation, the Company has subsequently admitted that only “9 companies reach]ed an advance stage of diligence” in a court filing it made on February 15, 2017, further evidencing the Board’s attempts to manipulate stockholders.SeeImmunomedics Resp. at 4, venBio Select Advisor LLC v. Goldenberg et. al., C.A. No. 2017-0108-JTL (Del. Ch. Feb. 15, 2017).
Daniel F. Duchovny, Esq. February 17, 2017 Page 8 | | |
Company, making it not a true market check and highly unlikely that a better deal will be found or that another partner could put together a credible bid in that period, particularly when the Company has failed to provide sufficient details on the transaction. The right for Seattle Genetics to match any offer plainly disincentivizes potential partners as well, and, the only “better” deal that the Board could accept would have to be the same type of transaction, notwithstanding the Company’s own disclosures that most of the strategic options it was considering were looking to purchase the entire enterprise.
It is clear from the Company’s disclosure surrounding the Seattle Genetics Deal that the Board did not finish the strategic process discussions with several other highly interested parties in an effort to execute the transaction before the Annual Meeting—otherwise, it would have said so or the deal process would have lasted even longer, given that Greenhill reached out to 45 companies “post-Thanksgiving and gave guidance that it was looking for non-binding bids in late January or early February 2017.”4It would be highly doubtful for the Company to have fully completed a strategic process involving at least 18 companies that had entered into “advanced diligence” with the Company between the time Greenhill asked for non-binding bids and February 10, 2017. Accordingly, the incompleteness of this strategic process, coupled with the presence of deal terms that preclude the ability for a subsequent third-party bidder to present more favorable terms for licensing of IMMU-132 provides a clear factual basis for claiming that the Company has “hastily” entered into a transaction to “give away” its “crown jewel.”
| 2. | Each statement or assertion of opinion or belief must be clearly characterized as such, and a reasonable factual basis must exist for each such opinion or belief. Support for opinions or beliefs should be self-evident, disclosed in the proxy statement or provided to the staff on a supplemental basis. Provide support for your statement listed below: |
| (a) | That the “six business day ‘go-shop’ period is absurdly short and lacks credibility…” |
The Filing Persons believe that the six business day go-shop period lacks credibility for the reasons discussed in response to comment 1(e) above, which is, namely, that, the Board has only allowed for certain parties to bid during such period and that go-shop periods are uncommon for biotech licensing deals and are usually taken as an indication that negotiations were unfinished and the deal was rushed. Finally, the Filing Persons believe that the go-shop period lacks credibility due to its relative shortness compared to average go-shop provisions. In a study conducted by PracticalLaw, published September 30, 2016, encompassing merger agreements for acquisitions of 187 US reporting companies, “The most common length of the go-shop period in 2015 was30 or 35 days. This is similar to the study’s findings in each of the previous two years, in which 30 days was the most common length of time for the go-shop period”5(emphasis added). For ease of reference, the relevant portions of this study are attached hereto asExhibit A.
| (b) | That the proxy advisory firms’ reports highlight support from other security holders. In this respect, we note that you refer to multiple security holders but name only one. |
4See the Company’s investor presentation, filed with the SEC on February 10, 2017 under cover of Schedule 14A, describing how the Board engaged Greenhill & Co. to assist it in a strategic process in September 2016.
5The study is available on PLI’s website at http://us.practicallaw.com/w-003-2638?q=deal+study#a000005.
Daniel F. Duchovny, Esq. February 17, 2017 Page 9 | | |
Each of the ISS Proxy Analysis & Benchmark Policy Voting Recommendations, published on February 3, 2017 (the “ISS Report”) and the Glass Lewis “proxy paper,” published on February 6, 2017 (the “Glass Lewis Report”) refers to support for venBio by numerous stockholders. The ISS Report states, “[m]any shareholders – including OrbiMed, one of the company’s largest holders, which has publicly stated its support for the venBio slate – are rightfully skeptical of the current board’s ability to objectively and independently assess the company’s available options and to pursue a strategy that is in their best interest.” In addition, the Glass Lewis Report States, “An additional investment fund, OrbiMed Advisors LLC (“OrbiMed”), has publicly endorsed venBio’s campaign and slate of nominees. OrbiMed purportedly represents clients holding shares and convertible securities representing approximately 9.1% of the outstanding shares of the Company on a fully-converted basis as of December 31, 2016. Additionally, a large contingent of retail shareholders have expressed support for venBio and its nominees.” Given that at least two major proxy advisory firms have mentioned broader shareholder support for the venBio Nominees, the Filing Persons respectfully submit that a proper factual basis exists for the statement that that advisory firms’ reports highlight support from other security holders. For ease of reference, the relevant portions of the ISS Report and Glass Lewis Report are attached herein asExhibit B.
| (c) | That the market’s reaction to the announcement of the agreement has been “unenthusiastic.” |
In response to the Staff’s foregoing comment, and in the interest of brevity, the Filing Persons refer the Staff to paragraphs 5 through 8 of its response to Staff comment 1(a) and incorporate such response by reference herein.
| (d) | That the agreement is “poor.” |
In response to the Staff’s foregoing comment, and in the interest of brevity, the Filing Persons respectfully refer the Staff to its response to Staff comment 1(e) and incorporate such response by reference herein. In addition, the Filing Persons note that the Seattle Genetics Deal involves an immediate $15 million equity investment by Seattle Genetics to acquire 2.8% of Immunomedics stock, and grants Seattle Genetics warrants to exercise within three years for up to 9.9% of the Company’s stock, both at below-market prices, which further dilutes the value of stock of existing stockholders. Moreover, the Filing Persons note that Company’s market capitalization is estimated to be roughly $600 million after the closing of the transaction, which is small compared to other pharmaceutical companies that leveraged their respective crown jewels to achieve market capitalization in the billions. Recent transactions involving similar and lower-stage pharmaceuticals have been priced much higher:
Daniel F. Duchovny, Esq. February 17, 2017 Page 10 | | |
| Year | Buyer | Seller | Developmental Phase | Value ($M) |
| 2015 | Medivation | BioMarin | Early-stage testing | 410 |
| 2015 | AstraZeneca | Acerta | Early-stage testing | 4,000 |
| 2016 | AbbVie | Stemcentrx | Early-stage testing | 5,800 |
| 2015 | Shire | Dyax | Late-stage testing | 5,900 |
| 2016 | Jazz | Celator | Pre-registration | 1,500 |
| 2017 | Takeda | Ariad | Commercial/Registration | 5,200 |
| 2017 | Seattle Genetics | Immunomedics | Pre-registration | 250 |
Given that IMMU-132 is in Phase 2 testing and is being licensed out for a fraction of the price that other pharmaceuticals were licensed-out at earlier stages of development provides a solid basis along for the Filing Persons to claim that the Seattle Genetics Deal is “poor.”
| (e) | Your statement that “A proper governance process would allow stockholders to vote on the composition of the Board first and then allow the newly elected Board to make material decisions regarding the fate of the company after the election.” In this respect, provide us support for what is a “proper governance process” and explain why the current board needs, in your view, to defer to a future board, whatever its composition. |
A proper governance process entails that the Company be managed by aduly elected board of directors that effectively and honestly fulfills its fiduciary duties to shareholders. InBlasius Indus., Inc. v. Atlas Corp., 564 A.2d 651, 659 (Del. Ch. 1988), the Delaware Chancery Court noted, “[t]he shareholder franchise is the ideological underpinning upon which the legitimacy of directorial power rests.” The Court further explained that the shareholder franchise “is critical to the theory that legitimates the exercise of power by some (directors and officers) over vast aggregations of property that they do not own.” In other words, a board that is not elected by stockholders lacks the legitimacy to exercise vast power over a corporation’s property. Other Delaware cases reinforce this, as the Court noted inMM Cos. v. Liquid Audio, Inc., 813 A.2d 1118, 1127 (Del. 2002), that “the power of managing the corporate enterprise is vested in the shareholders’duly elected representatives” (emphasis added). The current Board, which is comprised of a majority of unelected directors, simply lacks legitimacy until elected by shareholders. As the need for legitimacy underpins the entire shareholder franchise, the Board should have deferred to the newly elected Board to make material decisions regarding the fate of the Company.
| (f) | That you have received “overwhelming support” including the support of the proxy advisory firms. |
In response to the Staff’s foregoing comment, and in the interest of brevity, the Filing Persons respectfully refer the Staff to paragraphs 3 through 5 of its response to Staff comment 1(a) and its response to Staff comment 2(b) and wish to incorporate such response by reference herein.
Daniel F. Duchovny, Esq. February 17, 2017 Page 11 | | |
| 3. | We note your disclosure that, at the annual meeting, the current board is facing a “likely defeat.” With a view toward revised disclosure, please tell us the basis for such belief. Note Rule 14a-9 and note (d) therein. |
The Filing Persons note that this was a comment on a predicted outcome, not a claim of victory or loss, and that federal case law on Rule 14a-9 note (d) “supports the position that predictions regarding the results of a solicitation are not materially misleading or otherwise actionable under rule 14a-9.”Management Assistance Inc. v. Edelman, 584 F. Supp. 1016, 1020 (S.D.N.Y. 1984). The Court noted:
InKennecott Copper Corp. v. Curtiss-Wright Corp., 449 F. Supp. 951, 960 (S.D.N.Y.)aff’d in part andrev’d in part on other grounds, 584 F.2d 1195 (2d Cir. 1978), Judge MacMahon found that a prediction of victory made at a press conference was “an expectable exclamation of confidence that would not divert a reasonable shareholder from the task of coolly determining how best to vote his shares in light of the opposing platforms.” The judge distinguished claims of sure victory as potential violations of the rule. The court inJewelcor Incorporated v. Pearlman, 397 F. Supp. 221, 242, 249 (S.D.N.Y. 1975), went even further, ruling that a statement made by a proxy soliciting firm that 61 percent of the shareholders had voted for a proposal, when in fact no vote had been taken, was insufficient to establish a violation of section 14(a) and was not materially misleading. The statement allegedly made by [the defendant] does not claim a present victory; it merely predicts what the results will be sometime in the future.
The statement alleged by defendants’ is distinguishable from the statement made inGould v. American Hawaiian Steamship Company, 331 F. Supp. 981, 990-92 (D.Del. 1971), that 64 percent of the shareholders were contractually committed to vote in the defendants’ favor. The court in that case found that the statement falsely created the impression that the election was a forgone conclusion, causing the average shareholder not to bother to give the proxy statement careful consideration. That statement was actionable under the rule. The statement alleged here creates no such impression. It states that the management group will win, but does not suggest that management must win. Thus, it is not a claim regarding the results of a solicitation and is not proscribed by rule 14a-9.
The statement made by the Filing Persons did not suggest that the Filing Persons must win nor did it claim a present victory. It was a simple exclamation of confidence based on the proxies submitted to date and accordingly is not a violation of Rule 14a-9. Note (d) clearly contemplates a situation where a claim of victory is made before the meeting is held and deters persons from voting. The Filing Persons’ expression of a probability would not reasonably be expected to deter a stockholder from voting, and, as noted below in response to Staff comment 6, the Filing Person’s definitive proxy statement made explicit that proxies were revocable until the date of the Annual Meeting. Instead, given the facts of this situation, we believe the Filing Persons’ statement highlighted the importance of stockholders’ votes. Although not required to, the Filing Persons nonetheless issued a revised disclosure via a February 14, 2017 press release noting that “as of the day before the deal announcement, venBio’s lead in the stockholder vote was becoming increasingly clear…”
Daniel F. Duchovny, Esq. February 17, 2017 Page 12 | | |
This statement simply presents the facts as they existed at a certain time before the meeting and are not claims of the results of a meeting.
Definitive Additional Materials filed on February 14, 2017
| 4. | Refer to your statement that you believe “an elected, not appointed, board should have the opportunity to review deal.” Revise your disclosure to clarify that some current directors were elected, not appointed. |
The Filing Persons note that the February 14 Press Release further expounds upon this statement in the fourth bullet point and discloses that “[t]he majority of the current Board was appointed and was never elected by stockholders,” making it clear that some directors were elected.
| 5. | You must avoid issuing statements that directly or indirectly impugn the character, integrity or personal reputation or make charges of illegal, improper or immoral conduct without factual foundation. Provide us supplementally, or disclose, the factual foundation for the statements listed below. In this regard, note that the factual foundation for such assertion must be reasonable. Refer to Rule 14a-9. |
| · | That “postponement [of the annual meeting] indicates the board is desperately trying to entrench itself and fears losing proxy fight.” |
| · | That the current board “has no standing to make a deal.” |
| · | That the company is “in violation of Delaware law by not holding a stockholder vote in more than 13 months.” |
| · | That the board “lacks …legitimacy.” |
In response to the Staff’s foregoing comments regarding the Board “acting desperately,” the Board “lacking standing,” and the Board lacking “legitimacy,” and in the interest of brevity, the Filing Persons respectfully refer the Staff to its response to paragraphs 5 through 8 of its response to Staff comment 1(a) and its response to Staff comment 1(b) and wish to incorporate such responses by reference herein.
In response to the Staff’s comment regarding statements made regarding Delaware law, the Delaware Chancery Court inMFC Bancorp Ltd. v. Equidyne Corp., 844 A.2d 1015 (Del. Ch. 2003) stated DGCL section 211 “requires a corporation to do more than merely designate the date of its annual meeting before thethirteen-month deadline; rather, § 211 requires that the corporation hold its annual meeting before the statutory timeframe expires” (emphasis added). Given that the Company has exceeded such a thirteen-month deadline, it is fully reasonable for the Filing Persons to state that the Company has violated the tenants of Delaware law in this regard.
| 6. | Refer to your statement that the day before the agreement with Seattle Genetics was announced 55% of the proxies submitted were in your favor. Revise your disclosure to clarify that all proxies are revocable until the time of the meeting and that the results prior to the meeting may not be the same or similar to the results at the meeting. |
Daniel F. Duchovny, Esq. February 17, 2017 Page 13 | | |
The Filing Persons respectfully note that the Filing Persons have mailed to securities holders as of the record date, January 24, 2017, a definitive proxy containing the foregoing requested information and that the Filing Persons have included a legend at the end of all written soliciting materials directing stockholders to the definitive proxy statement and that it is available on the SEC’s website, free-of-charge.6
Nonetheless to assuage the Staff’s concerns, should the Filing Persons disclose any proxy totals in any additional solicitations or republish the quoted solicitation prior to the Annual Meeting, the Filing Persons will include the requested disclosures.
| 7. | Each statement or assertion of opinion or belief must be clearly characterized as such, and a reasonable factual basis must exist for each such opinion or belief. Support for opinions or beliefs should be self-evident, disclosed in the proxy statement or provided to the staff on a supplemental basis. Provide support for your statement listed below: |
| (a) | That the support of the three proxy advisory firms for a dissident’s entire slate is “extremely rare.” |
The Filing Persons note that news publications have in the past described the fact that just two proxy advisors firms endorsed an entire activist board slate as rare— for instance, in a proxy contest involving Darden Restaurants, Inc., two out of three major proxy advisory services endorsed the stockholder activist while a the third proxy advisor did not endorse the activist’s slate, and even in that instance, a legal journalist described such support as “unprecedented.”7Even in that proxy fight, the third proxy advisory firm did not endorse the dissident slate.
| (b) | Your repeated references to the “rush” with which the company acted in entering into and announcing the Seattle Genetics agreement. We note that the company has publicly described process lasting over months and including many other parties besides Seattle Genetics. |
In response to the Staff’s foregoing comment, and in the interest of brevity, the Filing Persons respectfully refer the Staff to paragraphs 3 and 4 of its response to Staff comment 1(e) and wish to incorporate such response by reference herein.
| (c) | Your disclosure relating to the continued willingness of Seattle Genetics to enter into the current agreement at a date later than it did. |
6As an additional matter, the Filing Persons note that, while Rule 14a-12 explicitly requires, “a prominent legend in clear, plain language advising security holders to read the proxy statement when it is available because it contains important information,” such language is not present in Rule 14a-6 with respect to definitive additional materials published after the filing of a definitive proxy statement, and that the Filing Persons have elected to include such a legend in the spirit of full and fair disclosure to stockholders.
7Karlee Weinmann, In Premier Proxy Fight Of 2014, Momentum Sidelines Darden,Law 360, Oct. 2, 2014, available at https://www.law360.com/articles/582829/in-premier-proxy-fight-of-2014-momentum-sidelines-darden.
Daniel F. Duchovny, Esq. February 17, 2017 Page 14 | | |
In response to the Staff’s foregoing comment, and in the interest of brevity, the Filing Persons respectfully refer the Staff to paragraphs 2 of its response to Staff comment 1(c) and wish to incorporate such response by reference herein. In addition, the Filing Persons note that in the February 14 Press Release, the Filing Persons state that the deal terms are “favorable” towards Seattle Genetics. Accordingly, given that the Seattle Genetics had had a deal on the table as of the week before the Annual Meeting scheduled for February 16, 2017, the fact that the major asset at the center of the deal would have still been valuable a week after the Annual Meeting, and that the terms of the deal were favorable towards Seattle Genetics, a proper factual basis exists for the Filing Persons to make the foregoing statements.
| (d) | • That the Bristol Myers and AbbVie agreements are similar to the company’s agreement with Seattle Genetics. |
• That the amount of Seattle Genetics’ upfront payment of $250 million is “paltry.”
In response to the Staff’s foregoing comments, and in the interest of brevity, the Filing Persons respectfully refer the Staff to its response to Staff comment 2(d), and in particular, the chart that the Filing Persons have provided comparing the terms of the Seattle Genetics Deal to AbbVie. In each of these instances, including with respect to Bristol Myers, the asset at the center of such a deal, Five Prime, was similar to IMMU-132 in that it was an immuno-oncology therapeutic drug being licensed to a larger, more prominent pharmaceutical Company for advanced testing and distribution.
More specifically, the Bristol Meyers deal was very similar to the Immunomedics deal in that both drugs at the center of each deal are considered by the industry to be “pipelines in a pill”— in other words, they will support multiple cancer indications. Secondly, similar to Seattle Genetics Deal, the Bristol Myers deal involved a global out license arrangement in which a biologic drug was being investigated for the treatment of cancer. Additionally, Bristol Meyers has an existing franchise in cancer drugs and thus the deal was aimed towards creating synergies, which is just as Seattle Genetics has advertised it may be able to do with IMMU-132. However, in the instant case, IMMU-132 is much farther along than Five Prime— whereas Immunomedics claims to be able to go in front of the FDA this year, the industry does not know what the FDA registration pathway will look like due to a lack of available data about the drug. At the time of the deal, Five Prime had treated only a handful of cancer patients with their drug, and the data has still not been presented, making IMMU-132 much more advanced than Five Prime’s molecule.
With respect to AbbVie’s purchase of Stemcentryx, the agreement provided for the purchase of the entire company (Stemcentryx), but the only clinical candidate Stemcentryx had at the time was Rova-T, an oncology drug considered the centerpiece of the deal, which makes the deal very similar to the Seattle Genetics Deal. Like IMMU-132, Rova-T is an antibody drug conjugate and, similar to Five Prime discussed above, is thought of as a “pipeline in a pill.” In addition, as in the Bristol Myers deal, AbbVie has an existing oncology franchise into which it can add Rova-T. However, just like in the Bristol Myers deal, Rova-T is much less advanced in its development process than IMMU-132.[8]
Daniel F. Duchovny, Esq. February 17, 2017 Page 15 | | |
However, just like in the Bristol Myers deal, Rova-T is much less advanced in its development process than IMMU-132.8 Additionally, Rova-T is also targeting a much smaller population than IMMU-132 that requires a very specific marker for a patient to benefit, while IMMU is very broad in its action.
In light of the foregoing, it is perfectly acceptable for the Filing Persons to have drawn comparisons between the Seattle Genetics Deal and the Bristol Myers and AbbVie deals and to use such deals as a basis for comparison in stating that the Seattle Genetics Deal was unfavorable in comparison to such deals.
| 8. | We note the statement made by Mr. Aghazadeh that the Seattle Genetics agreement was announced “before [the current board] lost the vote and their board seats.” With a view toward revised disclosure, please tell us the basis for such belief. Note Rule 14a-9 and note (d) therein. |
In response to the Staff’s foregoing comment, and in the interest of brevity, the Filing Persons respectfully refer the Staff to its response to Staff comment 3 and wish to incorporate such response by reference herein.
* * *
We hope our comments above satisfy the Staffs concerns. Should you have any questions or comments, or require any further information with respect to the foregoing Staff comment, please do not hesitate to call me at (212) 756-2376.
Very truly yours,
/s/ Eleazer Klein
Eleazer Klein
Encls.
8Rova-T, at the time of the Abbvie deal, had only been used to treat approximately 75 patients, only 30 of whom would be expected to benefit.
Exhibit A
Practical Law Case Study
Deal Protections and Remedies: A Study of Public Merger Agreements in 2015
Shop Terms
The provisions of the go-shop right are distinguishable primarily along two lines:
| · | The length of the initial period for the target company to solicit competing bids. |
| · | The treatment of “Excluded Parties”—those bidders who have submitted acquisition proposals during the go-shop period that could develop into superior offers—at the end of the go-shop period. |
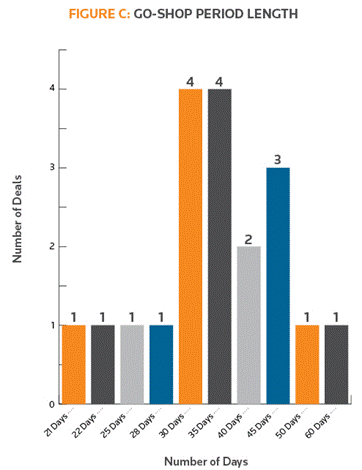
Figure C shows the length of the go-shop period for each of the 19 go-shops in this year’s study sample. The most common length of the go-shop period in 2015 was 30 or 35 days. This is similar to the study’s findings in each of the previous two years, in which 30 days was the most common length of time for the go-shop period.
A long go-shop period, however, is not the only the measure of the utility of the go-shop right. Some agreements permit the target company to continue its negotiations with competing bidders identified during the go-shop period even after the go-shop’s expiration. Others, however, place a cap on the length of that follow-up period.
Other agreements are distinguishable on the basis of whether a lower break-up fee is payable only if a superior proposal is agreed to during the go-shop period. This is a stricter formulation of the go-shop break-up fee incentive; more common is for the agreement to provide a grace period following the end of the go-shop for the target company to enter into the third-party agreement and capture the lower fee.
Exhibit B
ISS Report Excerpt
Governance Concerns
A board’s main responsibility is to oversee management’s strategy and its execution. Having a chairman who selects his wife as CEO creates significant conflicts of interest, especially since Goldenberg is also a C-level executive at the company. As an example of how these conflicts of interest play out in real life, Goldenberg, as the company’s Chief Scientific Officer and Chief Patent Officer, was apparently responsible for the presentation made at an oncology conference in April which resulted in the company’s ejection from ASCO’s conference a few months later. Missteps such as this raise questions about the board’s ability to exercise proper oversight or accountability, as this task would typically be conducted by the company’s CEO (Goldenberg’s wife) or the board, over which Goldenberg serves as Chairman.
Moreover, the fact that Goldenberg is listed as the primary inventor of every new intellectual property in a company with 143 professionals, including 33 clinical and developmental researchers, appears highly unusual, particularly in light of compensation schemes which allow Goldenberg to receive a percentage of the company’s revenues (sales or royalties) when he is listed as an inventor on the patent.
The company claims it has addressed investors’ main concerns, noting that it has appointed four new directors to the board, that Goldenberg will step down as Chairman, and that a search for a new CEO has already been initiated. The problems with this picture are twofold. First, the fact that the board only moved to fix its governance problems after the dissident started its campaign suggests a reactive response, as opposed to a carefully considered refreshment. Second, considering the long history of execution missteps and conflicts of interest, Goldenberg and Sullivan’s leadership of this refreshment process appears to undermine its sincerity. Many shareholders – including OrbiMed, one of the company’s largest holders, which has publicly stated its support for the venBio slate – are rightfully skeptical of the current board’s ability to objectively and independently assess the company’s available options and to pursue a strategy that is in their best interest.
Glass Lewis Report Excerpt
SUMMARY
The 2016 annual meeting of shareholders of Immunomedics, Inc. (“Immunomedics” or the “Company”) involves a contested election of directors. On the WHITE proxy card, the incumbent board of Immunomedics has nominated seven candidates for election to the board (the “Management Nominees”, being Messrs. Aryeh, Cox, Forrester, Goldenberg, Markison and Oliver and Ms. Sullivan) to serve for a one-year period each, expiring at the next annual meeting of shareholders. Meanwhile, on the GOLD proxy card, venBio Select Advisor LLC (“venBio” or the “Dissident”) has nominated four candidates for election to the board (the “Dissident Nominees”, being Messrs. Aghazadeh, Canute, Hutt and Islam) in opposition to the Management Nominees. If elected, the Dissident Nominees would serve for a one-year period each, expiring at the next annual meeting of shareholders. Furthermore, venBio is recommending that shareholders oppose Proposals 2 and 3, regarding the Company’s advisory resolution on executive compensation (say-on-pay) and the resolution requesting authorization of a further 40,000,000 shares of common stock, respectively.
As of the January 24, 2017 record date for the annual meeting, venBio held approximately 9.9% of the outstanding shares of the Company. venBio has been a shareholder of the Company for approximately one year, having first acquired an equity stake in February 2016. An additional investment fund, OrbiMed Advisors LLC (“OrbiMed”), has publicly endorsed venBio’s campaign and slate of nominees. OrbiMed purportedly represents clients holding shares and convertible securities representing approximately 9.1% of the outstanding shares of the Company on a fully-converted basis as of December 31, 2016. Additionally, a large contingent of retail shareholders have expressed support for venBio and its nominees.

