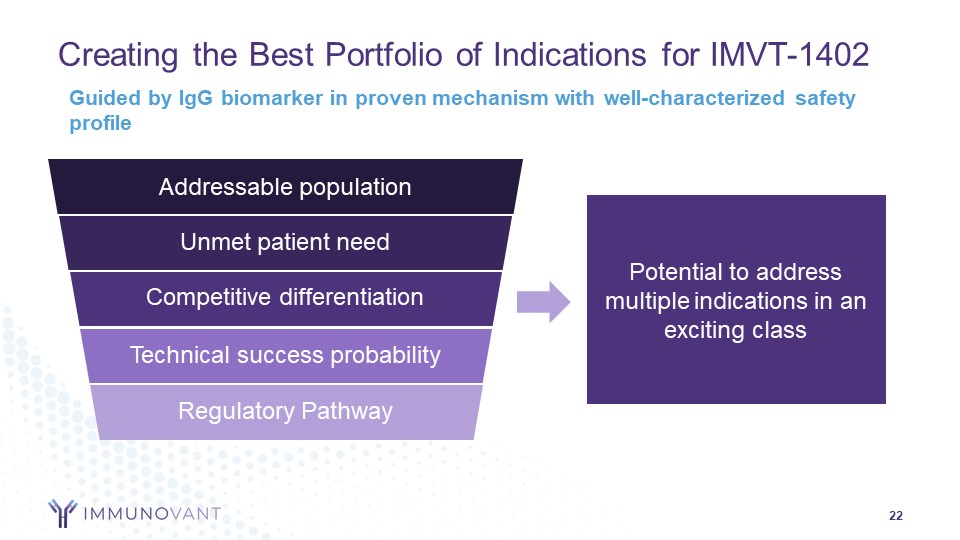
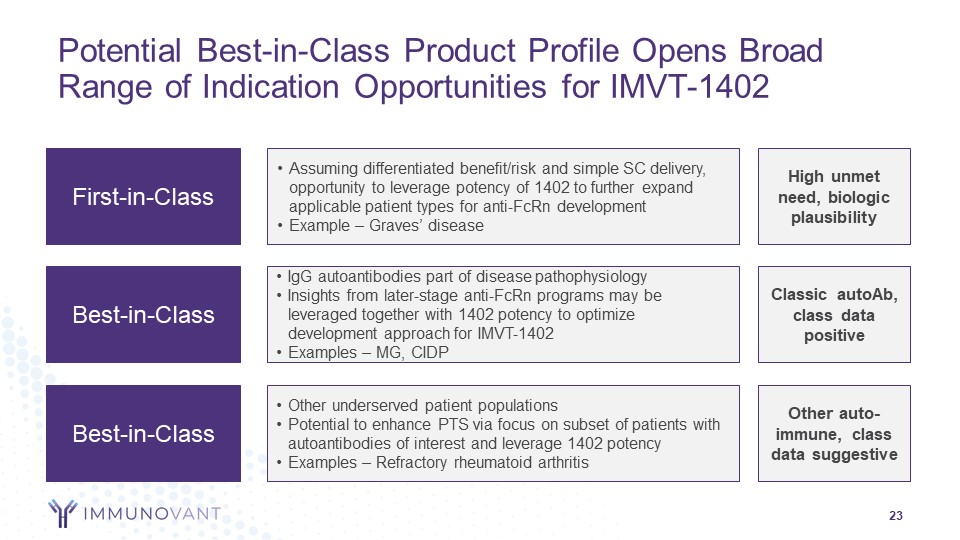
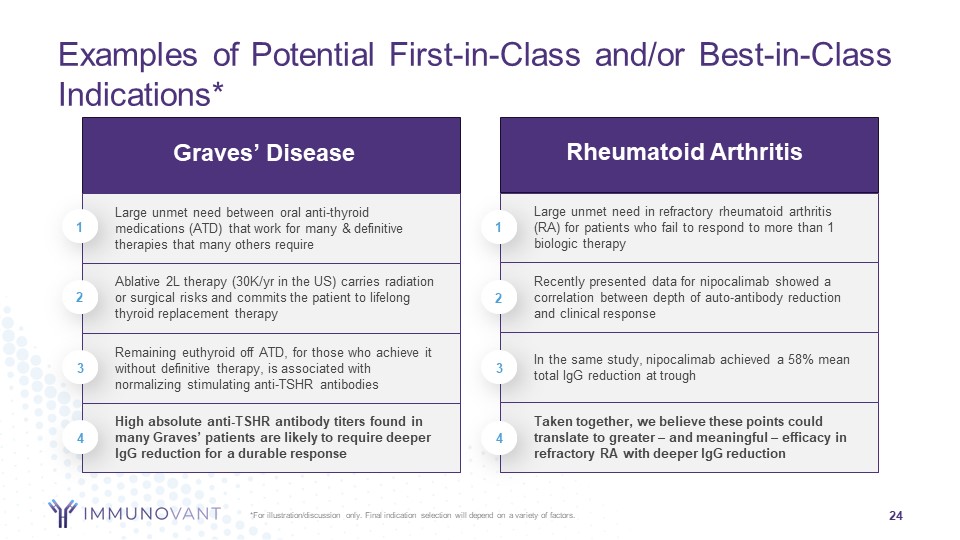
Forward-Looking Statements 2 This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as "can," “may,” “might,” “will,” “would,” “should,” “expect,” “believe,” “estimate,” “design,” “plan,” "intend," and other similar expressions are intended to identify forward-looking statements. Such forward looking statements include the timing and results of Immunovant’s clinical trials of IMVT–1402; expectations with respect to these planned clinical trials; the size and growth of the potential markets for Immunovant's product candidates and indication selections; Immunovant's beliefs regarding the potential benefits of IMVT-1402's unique product attributes including IMVT-1402's potential best-in-class Immunoglobulin G (IgG) reduction and tolerability and IMVT-1402's potential as a treatment for chronic inflammatory demyelinating polyneuropathy, Graves' disease, rheumatoid arthritis, and other autoimmune diseases; whether, if approved, IMVT-1402 will be successfully distributed, marketed, and commercialized; and Immunovant's expectations regarding the issuance and term of any pending patents. All forward-looking statements are based on estimates and assumptions by Immunovant’s management that, although Immunovant believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Immunovant expected. Such risks and uncertainties include, among others: initial results or other preliminary analyses or results of early clinical trials may not be predictive of final trial results or of the results of later clinical trials; results of animal studies may not be predictive of results in humans; the timing and availability of data from clinical trials; the timing of discussions with regulatory agencies, as well as regulatory submissions and potential approvals; the continued development of Immunovant’s product candidates, including the timing of the commencement of additional clinical trials; Immunovant’s scientific approach, clinical trial design, indication selection, and general development progress; future clinical trials may not confirm any safety, potency, or other product characteristics described or assumed in this presentation; any product candidate that Immunovant develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; Immunovant's pending composition of matter patent for IMVT-1402 may not be issued; Immunovant’s product candidates may not be beneficial to patients, or even if approved by regulatory authorities, successfully commercialized; the effect of global factors such as impacts from the post-COVID-19 environment, geopolitical tensions, and adverse macroeconomic conditions on Immunovant’s business operations and supply chains, including its clinical development plans and timelines; Immunovant’s business is heavily dependent on the successful development, regulatory approval and commercialization of batoclimab and IMVT-1402; Immunovant is at an early stage in development for IMVT-1402 and in various stages of clinical development for batoclimab; and Immunovant will require additional capital to fund its operations and advance batoclimab and IMVT-1402 through clinical development. These and other risks and uncertainties are more fully described in Immunovant’s periodic and other reports filed with the Securities and Exchange Commission (SEC), including in the section titled “Risk Factors” in Immunovant’s most recent Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2023, filed with the SEC on November 9, 2023, and Immunovant’s subsequent filings with the SEC. Any forward-looking statement speaks only as of the date on which it was made. Immunovant undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. All trademarks, trade names, service marks, and copyrights appearing in this presentation are the property of their respective owners. Dates used in this presentation refer to the applicable calendar year unless otherwise noted. 2