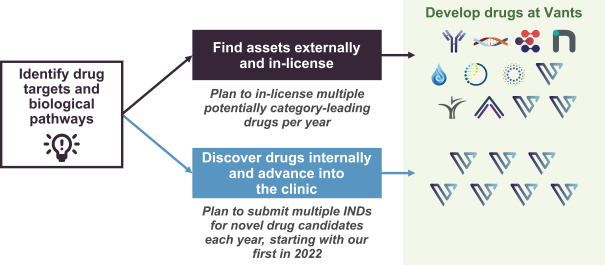
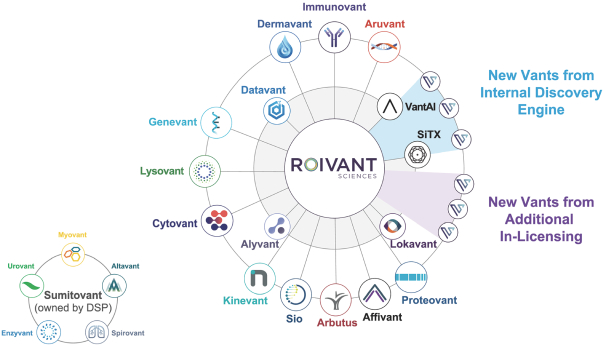
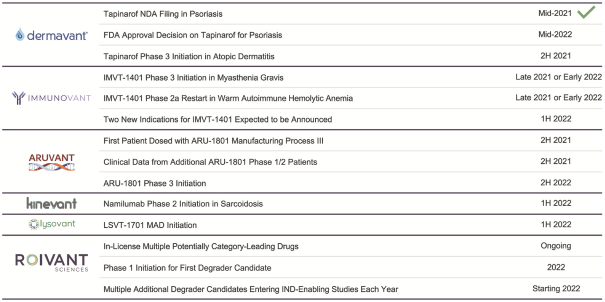
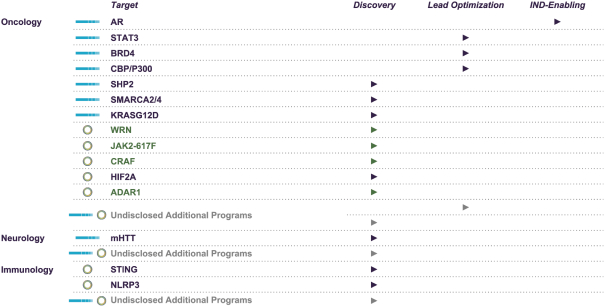
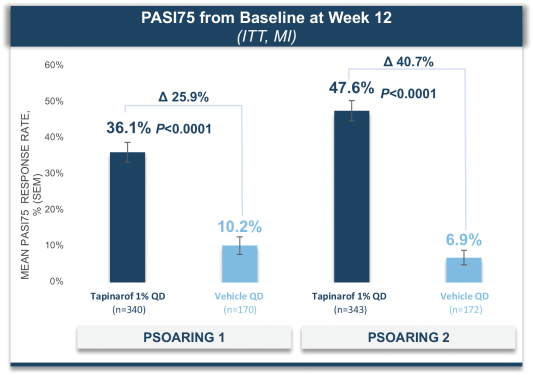
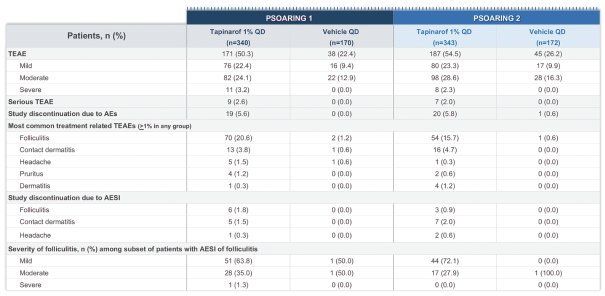
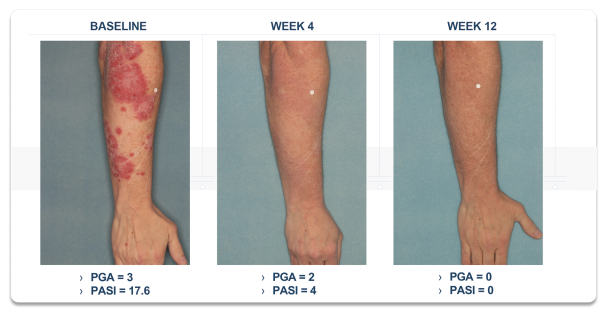
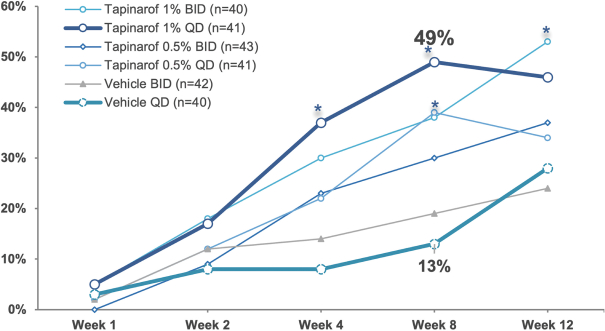
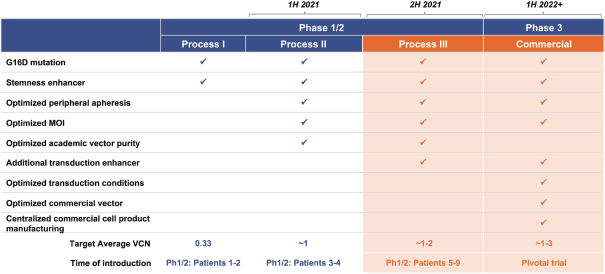
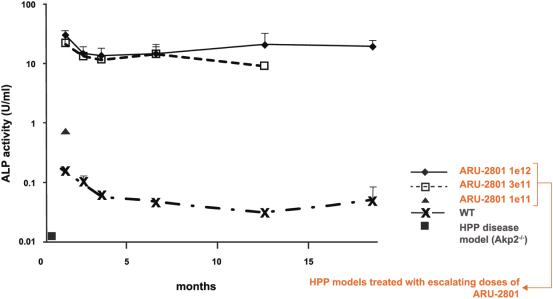
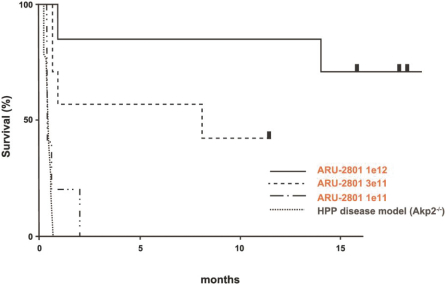
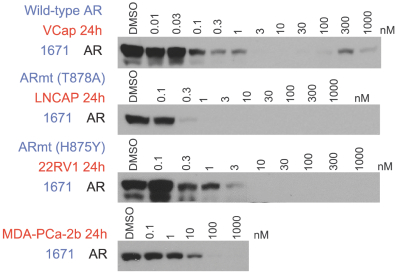
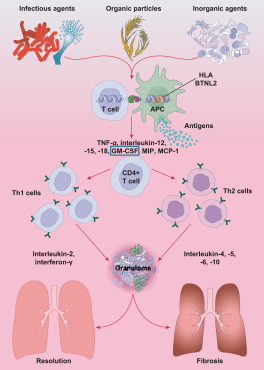
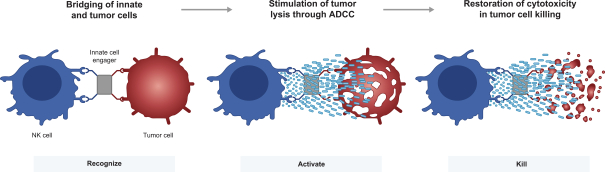
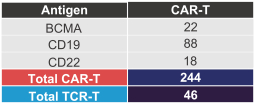
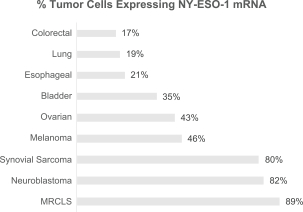
material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they are made, not misleading; provided that, notwithstanding the foregoing provisions of this Section 3.23, no representation or warranty is made by the Company or Merger Sub with respect to any information or statements included or incorporated by reference in the Registration Statement / Proxy Statement supplied by or on behalf of MAAC for use therein.
Section 3.24 Regulatory Compliance. Except as set forth in Section 3.24 of the Company Disclosure Schedules:
(a) Each Group Company conducts (and since January 1, 2019, has conducted) its business in accordance with all Public Health Laws applicable to such Group Company and is not in violation of any such Public Health Law or Order, except as is not and would not reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole.
(b) No Group Company has received, as of the date hereof, any written communication from the FDA or other Governmental Entity, including a warning, untitled or notice of violation letter or Form FDA-483, that alleges that any Group Company or the research, development, or manufacture of any Company Product is not in compliance with any Public Health Laws, except as is not and would not reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole.
(c) There is (and since January 1, 2019 there has been) no Proceeding pending or, to the Company’s knowledge, threatened against or involving any Group Company related to compliance with Public Health Laws, except as is not and would not reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole. The Group Companies do not have, and since January 1, 2019 have not had, any Liabilities for failure to comply with any Public Health Laws, except as is not and would not reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole.
(d) All preclinical studies, tests, and research being conducted by or on behalf of any Group Company or with respect to any Company Product are being, and, in each case, at since January 1, 2019 have been, to the extent applicable, conducted in compliance with all applicable Laws, including the good laboratory practice regulations set forth at 21 C.F.R. Part 58, except as is not and would not reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole. Except as is not and would not reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole, all clinical studies, tests, and research being conducted by or on behalf of the Company or with respect to any Company Product are being and, in each case, at all times have been, conducted in compliance with all applicable Laws and with good clinical practice, as defined or recognized by FDA, such as in the guidance document E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1), and applicable provisions of the FDCA and implementing regulations at 21 C.F.R. Parts 50, 54, 56, and 312. Except as is not and would not reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole, no preclinical or clinical trial conducted by or on behalf of the Company or with respect to any Company Product has been terminated or suspended prior to completion for safety, data integrity, or non-compliance reasons, and neither the FDA nor any other Governmental Entity or regulatory authority, clinical investigator or institutional review board that has or had jurisdiction over or participated in any such clinical trial has initiated or threatened in writing to initiate, any action to terminate, delay, suspend or modify any such ongoing preclinical or clinical trial, or, to the Company’s knowledge, to disqualify, restrict or debar any preclinical or clinical investigator or other person involved in any such preclinical or clinical trial.
(e) To the Company’s knowledge, as of the date hereof, no information, condition or circumstance exists that could reasonably be expected to materially adversely affect the acceptance, or the subsequent approval, of any filing, application or request for approval of any Company Product.
(f) Except as is not and would not reasonably be expected to be, individually or in the aggregate, material to the Group Companies, taken as a whole, since January 1, 2019, none of the Group Companies or, to
A-39