
GENE THERAPIES AXO-MV-GMl for GMl Gangliosidosis Results from ongoing Phase 1/2 Dose Escalation Study • 6-Month Safety and Efficacy High-Dose Cohort • 12-Month Safety and Efficacy Low-Dose Cohort October 21, 2021 1 • • • Exhibit 99.3 1

AGENDA --------------------------------------------•• · · · · · · WELCOME Introduction & Opening Remarks Pavan Cheruvu, MD Chief Executive Officer Review of AXO-AAV-GM1 Phase 1/2 Clinical Trial Data Gavin Corcoran, MD, FACP Chief R&D Officer CLOSING REMARKS AND Q&A SESSION • *Dr. Gao serves as a paid advisor to Sio Gene Therapies Expert Perspective: Intravenous AAV9 Gene Therapy Guangping Gao, PhD* Chief AAV Scientific Advisor Director of the Horae Gene Therapy Center and Viral Vector Core at UMass Medical School GENE THERAPIES 2

FORWARD-LOOK! NG STATEMENTS ----------------------------------------•• · · · · · This presentation contains forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as "expect," "estimate," "may" and other similar expressions are intended to identify forward-looking statements. For example, all statements Sio makes regarding costs associated with its operating activities, funding requirements and/or runway to meet its upcoming clinical milestones, and timing and outcome of its upcoming clinical and manufacturing milestones are forward-looking. All forward-looking statements are based on estimates and assumptions by Sio's management that, although Sio believes to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that Sio expected. Such risks and uncertainties include, among others, the impact of the Covid-19 pandemic on our operations; the actual funds and/or runway required for our clinical and product development activities and anticipated upcoming milestones; actual costs related to our clinical and product development activities and our need to access additional capital resources prior to achieving any upcoming milestones; the initiation and conduct of preclinical studies and clinical trials; the availability of data from clinical trials; the development of a suspension-based manufacturing process for AXO-Lenti-PD; the scaling up of manufacturing, the expectations for regulatory submissions and approvals; the continued development of our gene therapy product candidates and platforms; Sio's scientific approach and general development progress; and the availability or commercial potential of Sio's product candidates. These statements are also subject to a number of material risks and uncertainties that are described in Sio's most recent Quarterly Report on Form 10-Qfiled with the Securities and Exchange Commission on August 12, 2021, as updated by its subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. Sio undertakes no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. GENE THERAPIES 3
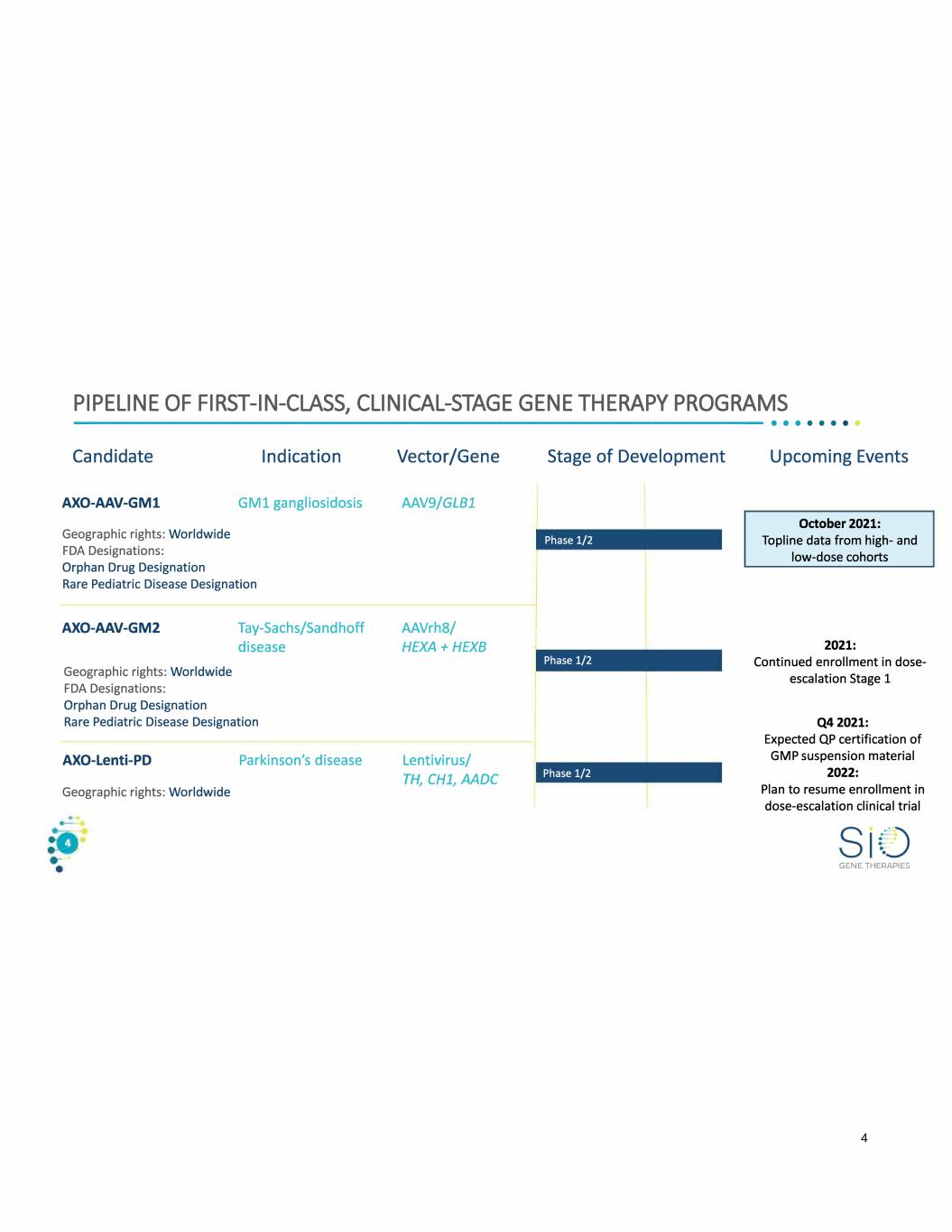
PIPELINE OF FIRST-IN-CLASS, CLINICAL-STAGE GENE THERAPY PROGRAMS -------------------------------------•• · · · · · · Candidate AXO-AAV-GM1 Geographic rights: Worldwide FDA Designations: Orphan Drug Designation Indication GM1 gangliosidosis Rare Pediatric Disease Designation AXO-AAV-GM2 Geographic rights: Worldwide FDA Designations: Orphan Drug Designation Tay-Sachs/Sandhoff disease Rare Pediatric Disease Designation AXO-Lenti-PD Parkinson's disease Geographic rights: Worldwide Vector/Gene AAV9/GLB1 AAVrh8/ HEXA+HEXB Lentivirus/ TH, CH1, AADC Stage of Development Phase 1/2 Phase 1/2 Phase 1/2 Upcoming Events October 2021: Topline data from high- and low-dose cohorts 2021: Continued enrollment in dose escalation Stage 1 Q42021: Expected QP certification of GMP suspension material 2022: Plan to resume enrollment in dose-escalation clinical trial GENE THERAPIES 4

TODAY'S PRESENTATION OF AXO-AAV-GMl CLINICAL TRIAL RESULTS -------------------------------•• · · · · · · KEY TAKEAWAYS • Most advanced gene therapy in development for GMl gangliosidosis with 10 patients dosed to date in Stage 1 of the clinical study (including 8 Type II patients and 2 Type I patients) • Encouraging safety and tolerability profile at both the low- and high-dose, with no SAEs attributable to gene therapy • Normalization of serum enzyme activity and GMl ganglioside in the CSF at the high dose at 6 months, and dose-dependent biomarker improvements observed • No overt disease progression in 4 out of 5 patients in the low-dose cohort at 12 months, and 2 out of 2 patients in the high-dose cohort at 6 months Clear dose response on key biomarkers of disease activity in serum and CSF Safety profile supports advancing clinical program to next steps of development GENE THERAPIES 5

SiU GENE THERAPIES GMl GANGLIOSIDOSIS DISEASE BACKGROUND . . .• rv . . l\ . . 6
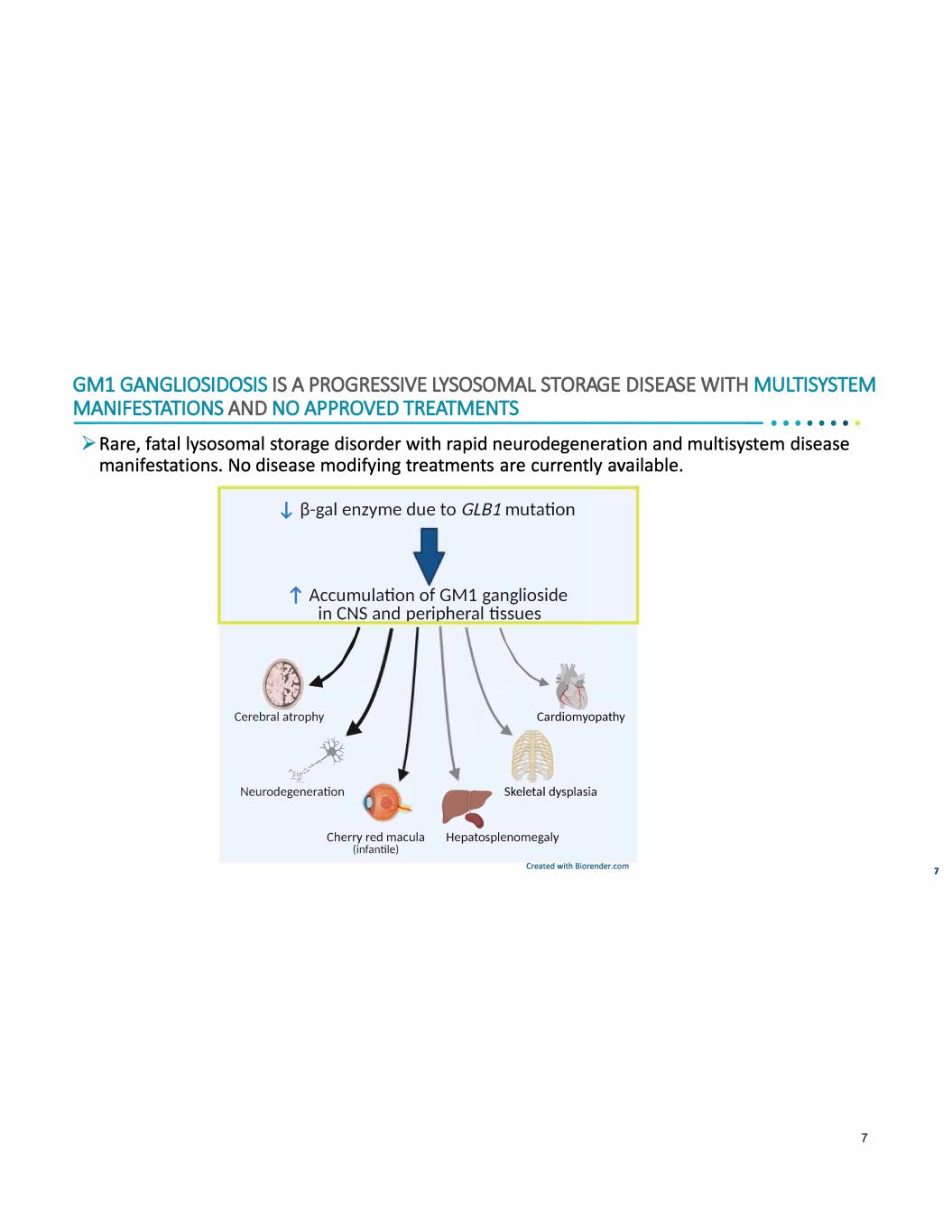
GMl GANGLIOSIDOSIS IS A PROGRESSIVE LYSOSOMAL STORAGE DISEASE WITH MULTISYSTEM MANIFESTATIONS AND NO APPROVED TREATMENTS -------------------------------•• · · · · · ► Rare, fatal lysosomal storage disorder with rapid neurodegeneration and multisystem disease manifestations. No disease modifying treatments are currently available. J, �-gal enzyme due to GLB1 mutation t Accumulation of GM1 ganglioside in CNS and peripheral tissues (Jj/ �- Cerebral atrophy Cardiomyopathy /� �-Jij-"7 Neurodegeneration Skeletal dysplasia Cherry red macula Hepatosplenomegaly (infantile) Created with Biorender.com 7 7
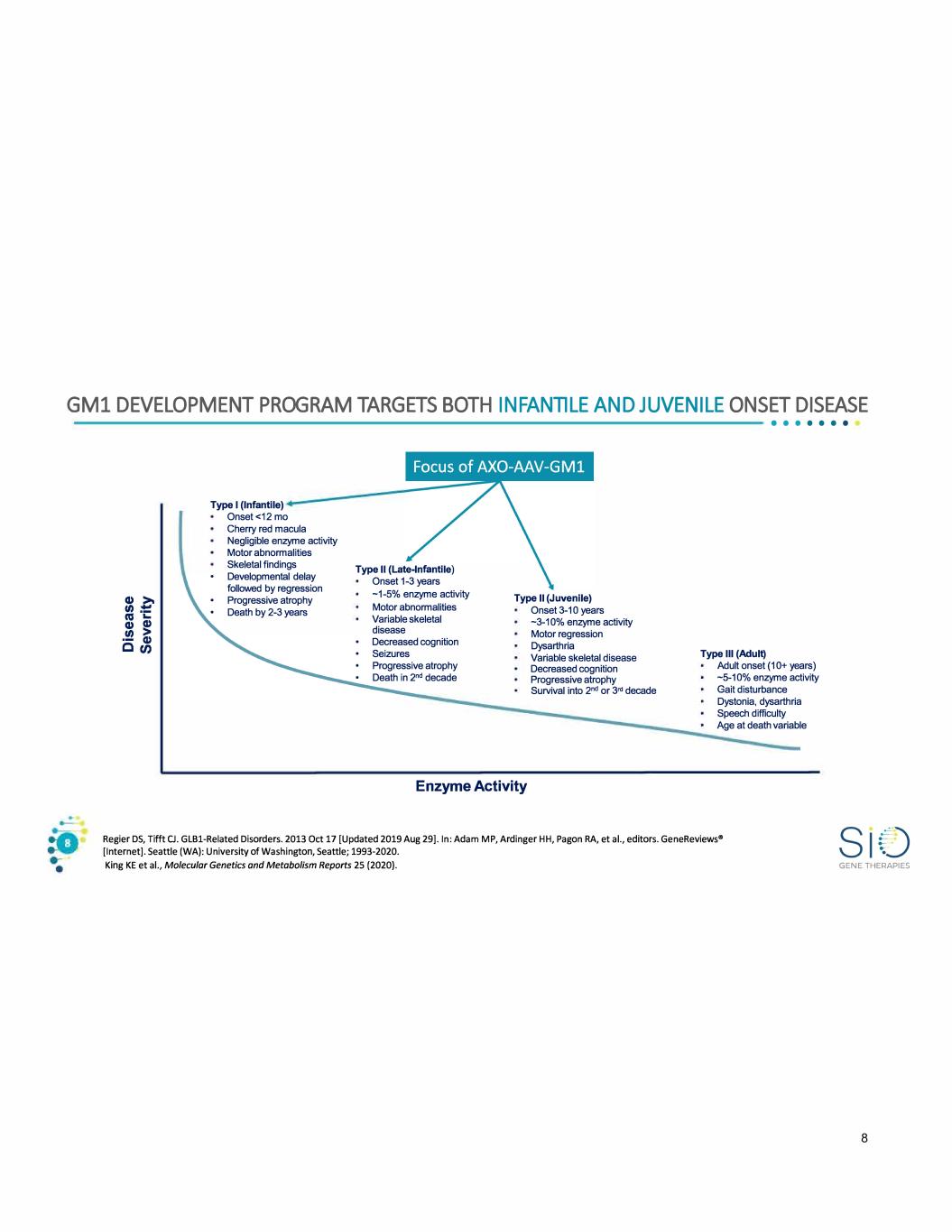
GM1 DEVELOPMENT PROGRAM TARGETS BOTH INFANTILE AND JUVENILE ONSET DISEASE • • • • • • • Type I (Infantile) Onset<12 mo Cherry red macula Negligible enzyme activity Motor abnormalities Skeletal findings Developmental delay followed by regression Progressive atrophy Death by 2-3 years Type II (Late-Infantile) Onset 1-3 years -1-5% enzyme activity Motor abnormalities Variable skeletal Type II (Juvenile) disease Decreased cognition Seizures Progressive atrophy Death in 2nd decade Enzyme Activity Onset 3-10 years -3-10% enzyme activity Motor regression Dysarthria Variable skeletal disease Decreased cognition Progressive atrophy Survival into 2nd or 3"' decade Type Ill (Adult) Adult onset ( 1 0+ years) -5-10% enzyme activity Gait disturbance Dystonia, dysarthria Speech difficulty Age at death variable Regier DS, Tifft CJ. GLBl-Related Disorders. 2013 Oct 17 [Updated 2019 Aug 29]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews• [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. King KE et al., Molecular Genetics and Metabolism Reports 25 (2020). GENE THERAPIES 8
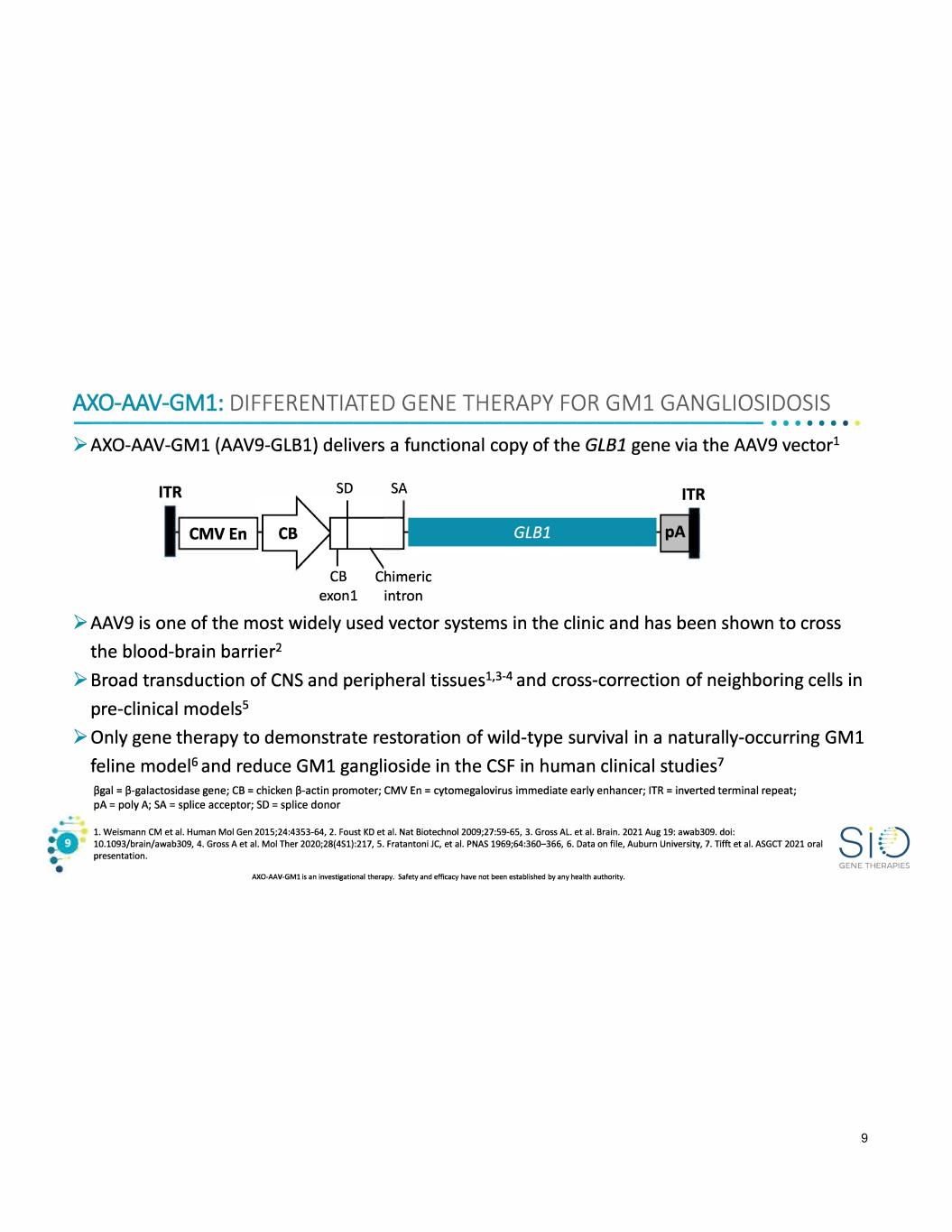
AXO-MV-GM1: DIFFERENTIATED GENE THERAPY FOR GM1 GANGLIOSIDOSIS -------------------------------------•• · · · · · ► AXO-AAV-GMl (AAV9-GLB1) delivers a functional copy of the GLB1 gene via the AAV9 vector1 ITR CMVEn SD SA CB Chimeric exonl intron ITR ► AAV9 is one of the most widely used vector systems in the clinic and has been shown to cross the blood-brain barrier2 ► Broad transduction of CNS and peripheral tissues1 , 3 - 4 and cross-correction of neighboring cells in pre-clinical models5 ► Only gene therapy to demonstrate restoration of wild-type survival in a naturally-occurring GMl feline model6 and reduce GMl ganglioside in the CSF in human clinical studies7 (3gal = (3-galactosidase gene; CB= chicken (3-actin promoter; CMV En = cytomegalovirus immediate early enhancer; ITR = inverted terminal repeat; pA = poly A; SA= splice acceptor; SD = splice donor 1. Weismann CM et al. Human Mol Gen 2015;24:4353-64, 2. Foust KD et al. Nat Biotechnol 2009;27:59-65, 3. Gross AL. et al. Brain. 2021 Aug 19: awab309. doi: 10.1093/brain/awab309, 4. Gross A et al. Mol Ther 2020;28(451):217, 5. Fratantoni JC, et al. PNAS 1969;64:360-366, 6. Data on file, Auburn University, 7. Tifft et al. A5GCT 2021 oral presentation. AXO-AAV-GMl is an investigational therapy. Safety and efficacy have not been established by any health authority. GENE THERAPIES 9

AGENDA --------------------------------------------•• · · · · · · WELCOME Introduction & Opening Remarks Pavan Cheruvu, MD Chief Executive Officer Review of AXO-AAV-GM1 Phase 1/2 Clinical Trial Data Gavin Corcoran, MD, FACP Chief R&D Officer CLOSING REMARKS AND Q&A SESSION • *Dr. Gao serves as a paid advisor to Sio Gene Therapies Expert Perspective: Intravenous AAV9 Gene Therapy Guangping Gao, PhD* Chief AAV Scientific Advisor Director of the Horae Gene Therapy Center and Viral Vector Core at UMass Medical School GENE THERAPIES 10

GMl GANGLIOSIDOSIS DATA REVIEW OUTLINE -------------------------------•• · · · · · · The data review will focus on the safety, biomarkers, MR imaging and clinical outcomes. 0 0 e Study Design and Demographics Safety Summary Biomarker Data Volumetric Brain MRI Clinical Outcomes GENE THERAPIES 11

SiU GENE THERAPIES CLINICAL STUDY DESIGN AND BASELINE DEMOGRAPHICS . . .• rv . . l\ . . 12
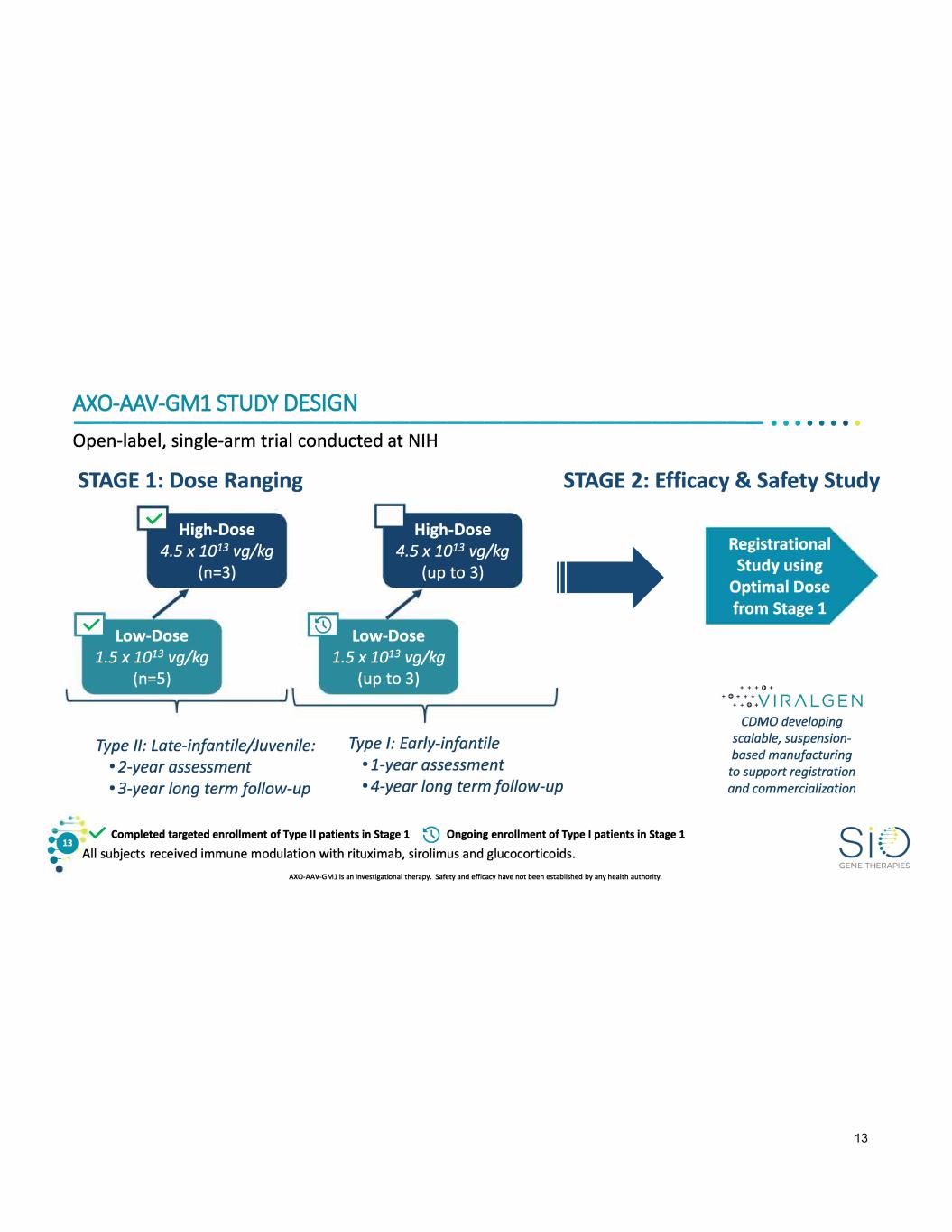
AXO-AAV-GMl STUDY DESIGN -------------------------------------•• · · · · · · Open-label, single-arm trial conducted at NIH STAGE 1: Dose Ranging STAGE 2: Efficacy & Safety Study m High-Dose 4.5 x 1013 vg/kg (n=3) l'J Low-Dose 1.5 x 1013 vg/kg (n=S) • High-Dose 4.5 x 1013 vg/kg (up to 3) a;] Low-Dose 1.5 x 1013 vg/kg (up to 3) Type II: Late-infantile/Juvenile: Type I: Early-infantile • 2-year assessment • 1-year assessment 11..+ • 3-year long term follow-up • 4-year long term follow-up :G, v Completed targeted enrollment of Type II patients in Stage 1 @ Ongoing enrollment of Type I patients in Stage 1 .-- All subjects received immune modulation with rituximab, sirolimus and glucocorticoids. AXO-AAV-GMl is an investigational therapy. Safety and efficacy have not been established by any health authority. Registrational Study using Optimal Dose from Stage 1 + + + 0 + + 0 ::0+.V I R /\ LG E N COMO developing scalable, suspension based manufacturing to support registration and commercialization GENE THERAPIES 13
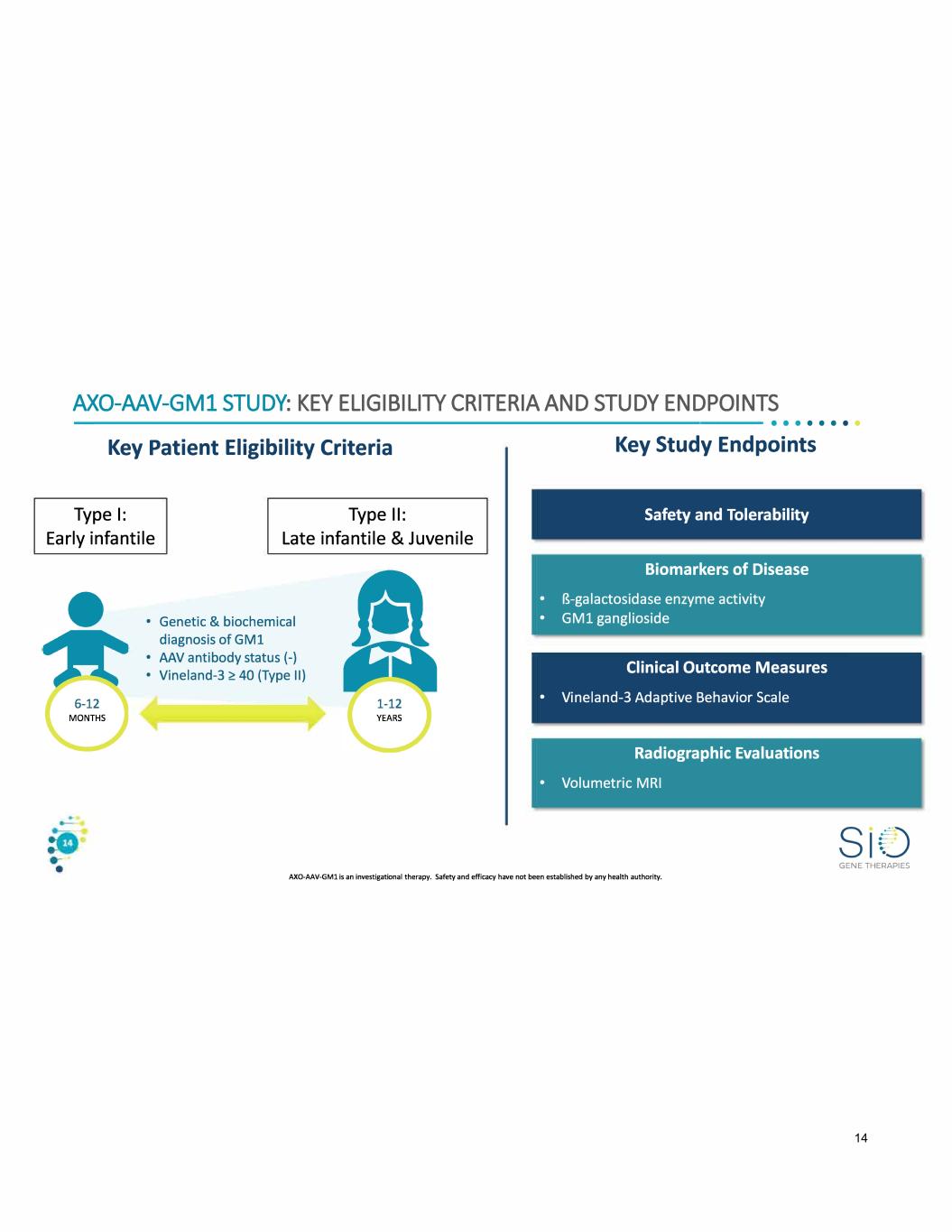
AXO-MV-GM1 STUDY: KEY ELIGIBILITY CRITERIA AND STUDY ENDPOINTS ---------------------------------•• · · · · · · Key Patient Eligibility Criteria Type I: Type II: Early infantile Late infantile & Juvenile • • Genetic & biochemical diagnosis of GM1 • AAV antibody status(-) • Vineland-3 <!: 40 (Type II) Key Study Endpoints Safety and Tolerability Biomarkers of Disease • B-galactosidase enzyme activity • GM1 ganglioside Clinical Outcome Measures • Vineland-3 Adaptive Behavior Scale Radiographic Evaluations • Volumetric MRI AXO-AAV-GMl is an investigational therapy. Safety and efficacy have not been established by any health authority. GENE THERAPIES 14
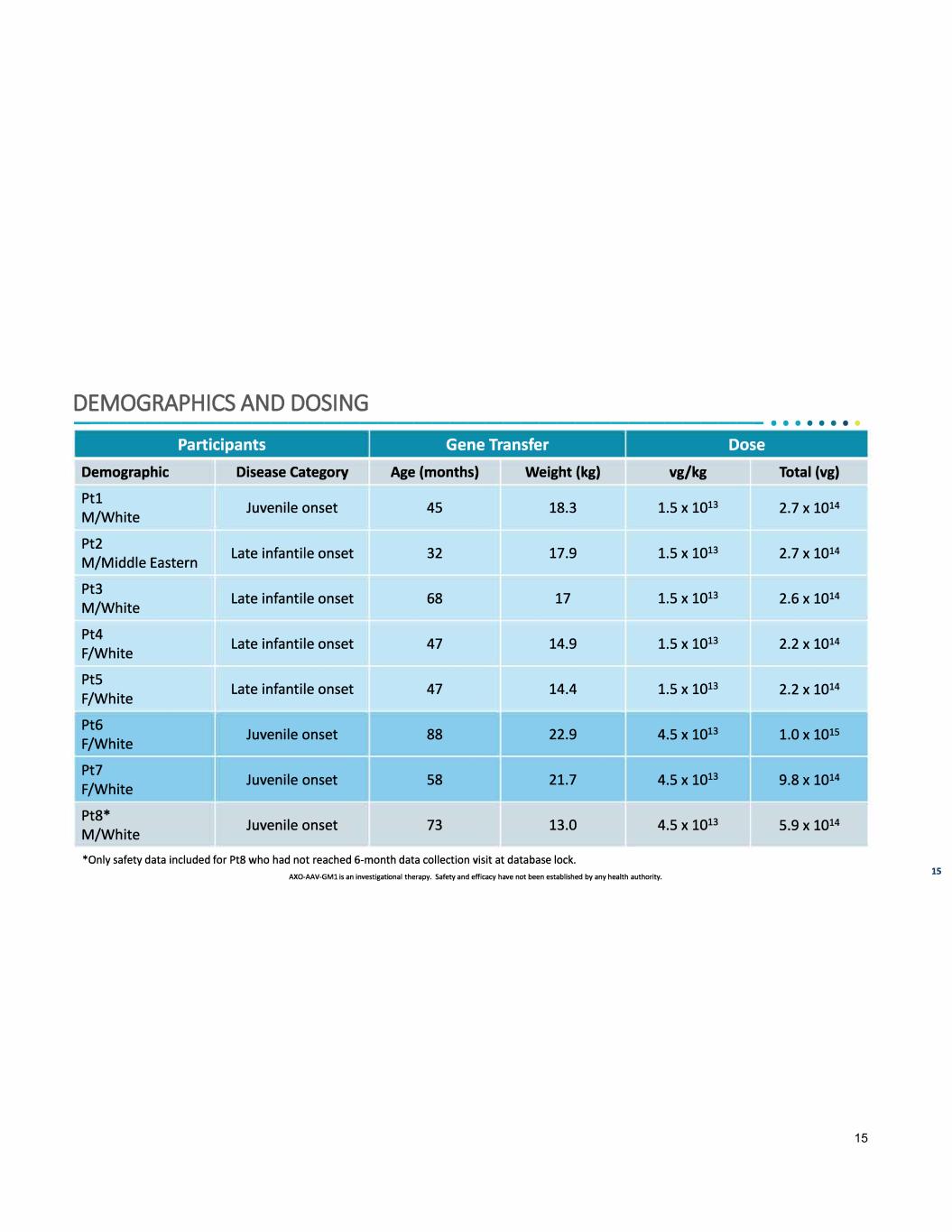
DEMOGRAPHICS AND DOSING --------------------------------------- • • · · · · · · Participants Gene Transfer Dose Demographic Disease Category Age (months) Weight (kg) vg/kg Total (vg) Ptl Juvenile onset 45 18.3 1.5 X 1013 2.7 X 1014 M/White Pt2 Late infantile onset 32 17.9 1.5 X 1013 2.7 X 1014 M/Middle Eastern Pt3 Late infantile onset 68 17 1.5 X 1013 2.6 X 1014 M/White Pt4 Late infantile onset 47 14.9 1.5 X 1013 2.2 X 1014 F/White Pt5 Late infantile onset 47 14.4 1.5 X 1013 2.2 X 1014 F/White Pt6 Juvenile onset 88 22.9 4.5 X 1013 1.0 X 1015 F/White Pt7 Juvenile onset 58 21.7 4.5 X 1013 9.8 X 1014 F/White Pt8* Juvenile onset 73 13.0 4.5 X 1013 5.9 X 1014 M/White - -- *Only safety data included for Pt8 who had not reached 6-month data collection visit at database lock. AXO-AAV-GMl is an investigational therapy. Safety and efficacy have not been established by any health authority. 15 15

Si0 GENE THERAPIES SAFETY SUMMARY Overview of adverse events Liver function tests 16

OVERALL SAFETY SUMMARY OF AXO-AAV-GM1 ----------------------------•• · · · · · · • The favorable safety profile in the low-dose and high-dose cohorts to date supports continued enrollment of patients in the AXO-AAV-GM1 study ► Generally safe and well tolerated ► Two Serious Adverse Events (SAEs) reported, both unrelated to the gene therapy • Bacterial sepsis due to PICC line infection • Focal seizures due to disease progression ► No liver-related adverse events had associated clinical sequelae, and none required clinical intervention • The DSMB endorsed continued enrollment per study protocol ► Two infantile-onset (Type I) subjects have now received the low dose AXO-AAV-GMl is an investigational therapy. Safety and efficacy have not been established by any health authority. GENE THERAPIES 17
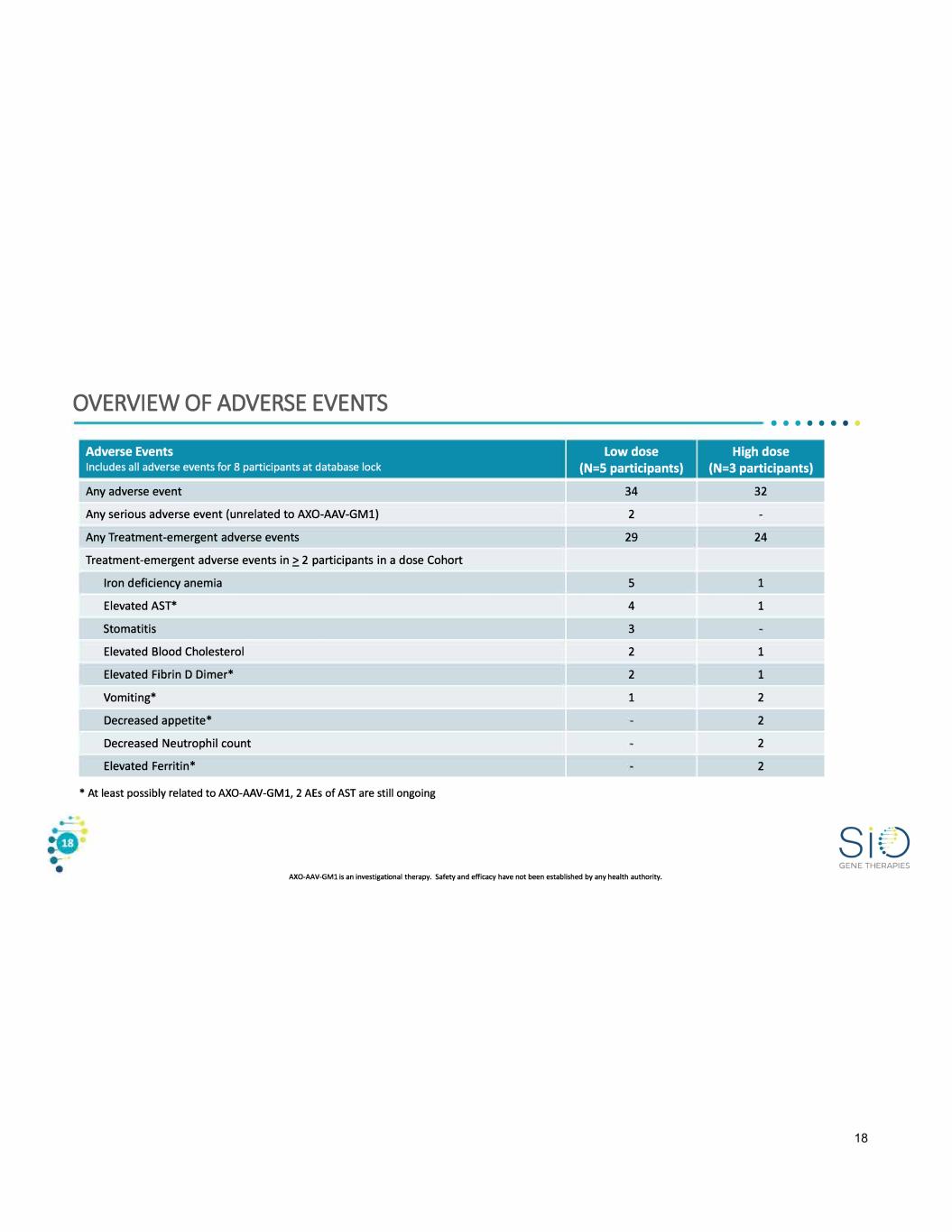
OVERVIEW OF ADVERSE EVENTS Adverse Events Includes all adverse events for 8 participants at database lock Any adverse event Any serious adverse event (unrelated to AXO-AAV-GMl) Any Treatment-emergent adverse events Treatment-emergent adverse events in?. 2 participants in a dose Cohort Iron deficiency anemia Elevated AST* Stomatitis Elevated Blood Cholesterol Elevated Fibrin D Dimer* Vomiting* Decreased appetite* Decreased Neutrophil count Elevated Ferritin* • At least possibly related to AXO-AAV-GMl, 2 AEs of AST are still ongoing Low dose (N=S participants) 34 2 29 5 4 3 2 2 1 AXO-AAV-GMl is an investigational therapy. Safety and efficacy have not been established by any health authority. • • • • • • • • High dose (N=3 participants) 32 24 1 1 1 1 2 2 2 2 GENE THERAPIES 18

SiU GENE THERAPIES BIOMARKER DATA Enzyme activity in serum GMl ganglioside in CSF Urine oligosaccharides . . . . .... .• rv . . l\ . . 19
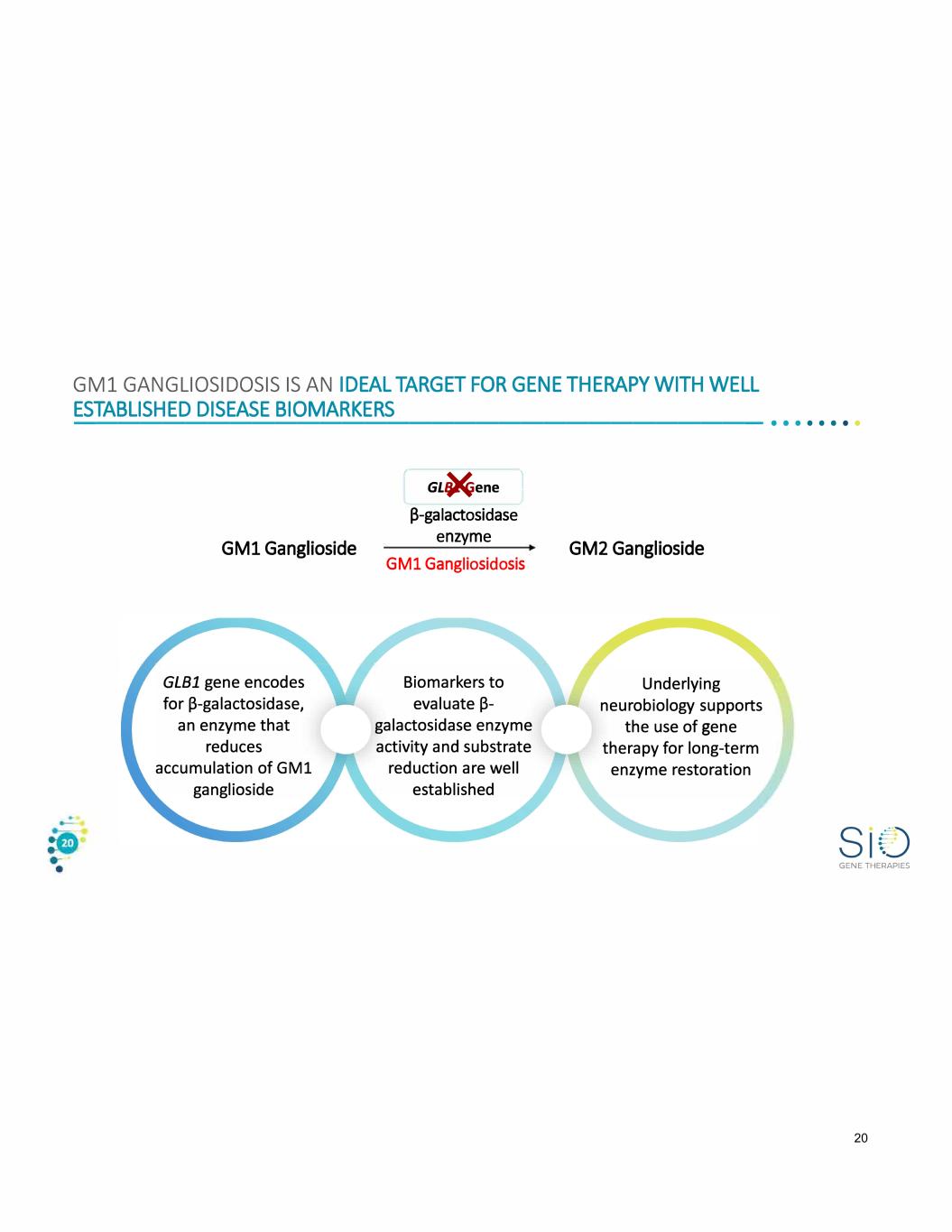
GMl GANGLIOSIDOSIS IS AN IDEAL TARGET FOR GENE THERAPY WITH WELL ESTABLISHED DISEASE BIOMARKERS -------------------------------•• · · · · · · GMl Ganglioside GLB1 gene encodes for 13-galactosidase, an enzyme that reduces accumulation of GMl ganglioside 1 GL�ene l P-galactosidase enzyme GMl Gangliosidosis Biomarkers to evaluate 13- galactosidase enzyme activity and substrate reduction are well established GM2 Ganglioside Underlying neurobiology supports the use of gene therapy for long-term enzyme restoration GENE THERAPIES 20
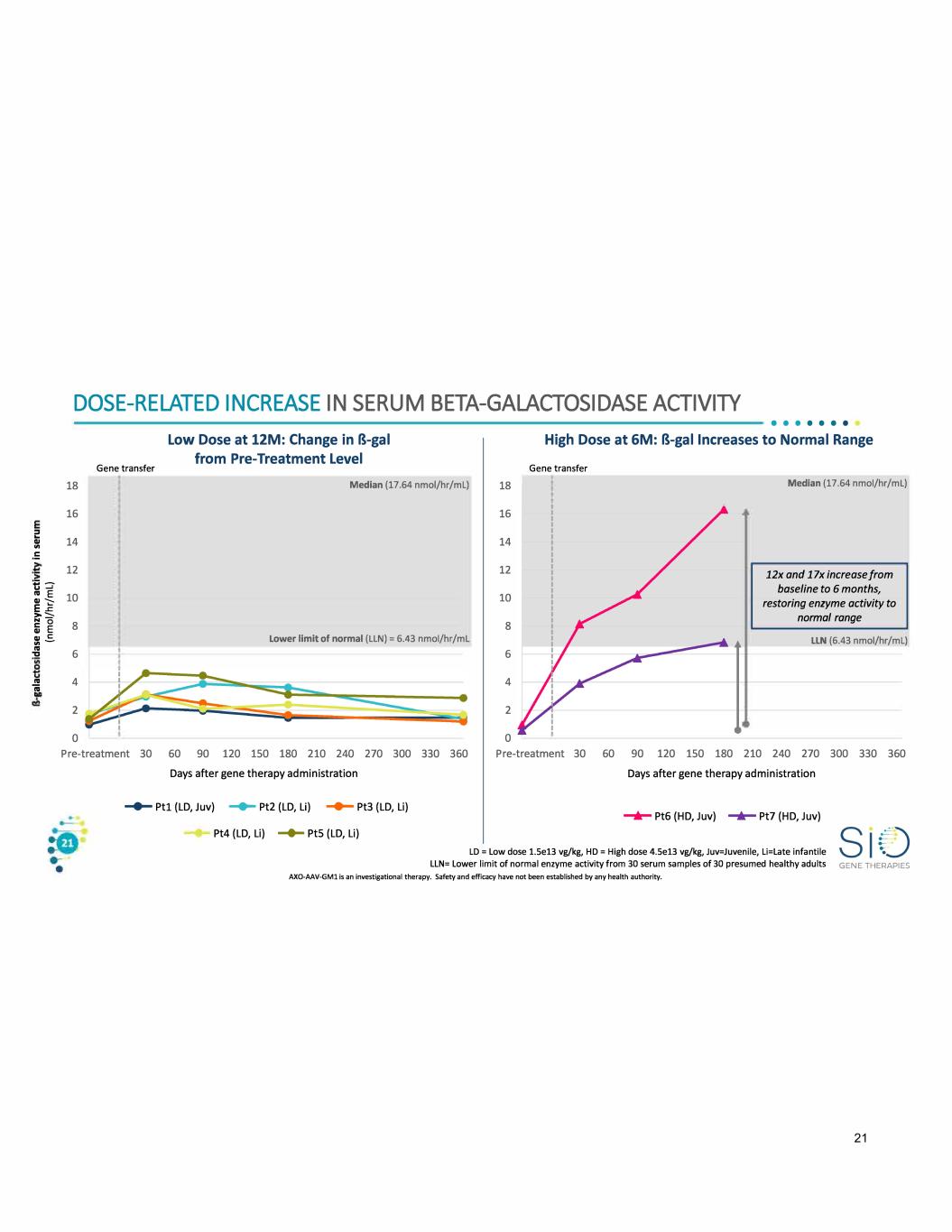
2 I!( .!: ti :::. DOSE-RELATED INCREASE IN SERUM BETA-GALACTOSIDASE ACTIVllY 18 16 14 12 Gene transfer Low Dose at 12M: Change in B-gal from Pre-Treatment Level Median (17.64 nmol/hr/ml) • • • • • • • High Dose at 6M: B-gal Increases to Normal Range Gene transfer Median (17.64 nmol/hr/ml) : -S 10 E ..c: >::::,. 18 16 14 12 10 12x and 1 lx increase from baseline to 6 months, restoring enzyme activity to normal range i � .. C: .. - .. "Cl .. ii 8 lower limit of normal (LLN) = 6.43 nmol/hr/ml 6 4 2 0 8 6 4 2 0 llN (6.43 nmol/hr/ml) Pre-treatment 30 60 90 120 150 180 210 240 270 300 330 360 Pre-treatment 30 60 90 120 150 180 210 240 270 300 330 360 Days after gene therapy administration Days after gene therapy administration - Ptl (LD, Juv) ....... Pt2 (LD, Li) - Pt3 (LD, Li) Pt4 (LD, Li) -+- Pt5 {LD, Li) -+- Pt6 (HD, Juv) -+- Pt7 (HD, Juv) LD = Low dose 1.Se13 vg/kg, HD = High dose 4.Se13 vg/kg, Juv=Juvenile, Li=Late infantile LlN= lower limit of normal enzyme activity from 30 serum samples of 30 presumed healthy adults AXO-AAV-GMl is an investigational therapy. Safety and efficacy have not been established by any health authority. GENE THERAPIES 21
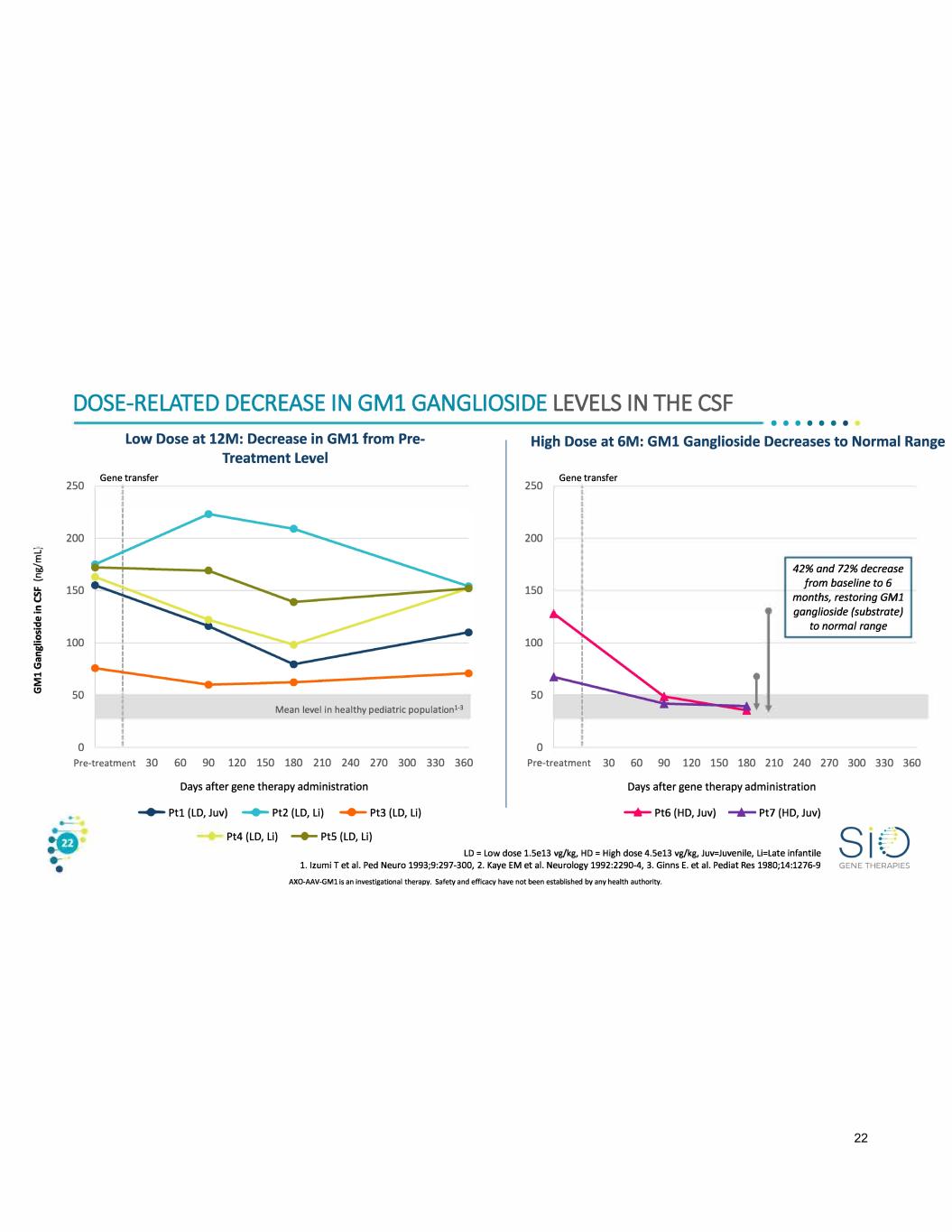
DOSE-RELATED DECREASE IN GMl GANGLIOSIDE LEVELS IN THE CSF 250 200 150 100 so 0 Low Dose at 12M: Decrease in GM1 from Pre Treatment Level Gene transfer Mean level in healthy pediatric population1•3 • • • • • • • High Dose at 6M: GM1 Ganglioside Decreases to Normal Range 250 200 150 100 so 0 Gene transfer 42% and 72% decrease from baseline to 6 months, restoring GMl ganglioside (substrate) to normal range Pre-treatment 30 60 90 120 150 180 210 240 270 300 330 360 Pre-treatment 30 60 90 120 150 180 210 240 270 300 330 360 Days after gene therapy administration ....... Ptl (LD, Juv) ....... Pt2 (LD, Li) ....... Pt3 (LD, Li) Pt4 (LD, Li) -+- PtS (LD, Li) Days after gene therapy administration ...,._ Pt6 (HD, Juv) -+- Pt7 (HD, Juv) LD = Low dose 1.Se13 vg/kg, HD= High dose 4.Se13 vg/kg, Juv=Juvenile, Li=Late infantile 1. Izumi T et al. Ped Neuro 1993;9:297-300, 2. Kaye EM et al. Neurology 1992:2290-4, 3. Ginns E. et al. Pediat Res 1980;14:1276-9 AXO-AAV-GMl is an investigational therapy. Safety and efficacy have not been established by any health authority. GENE THERAPIES 22
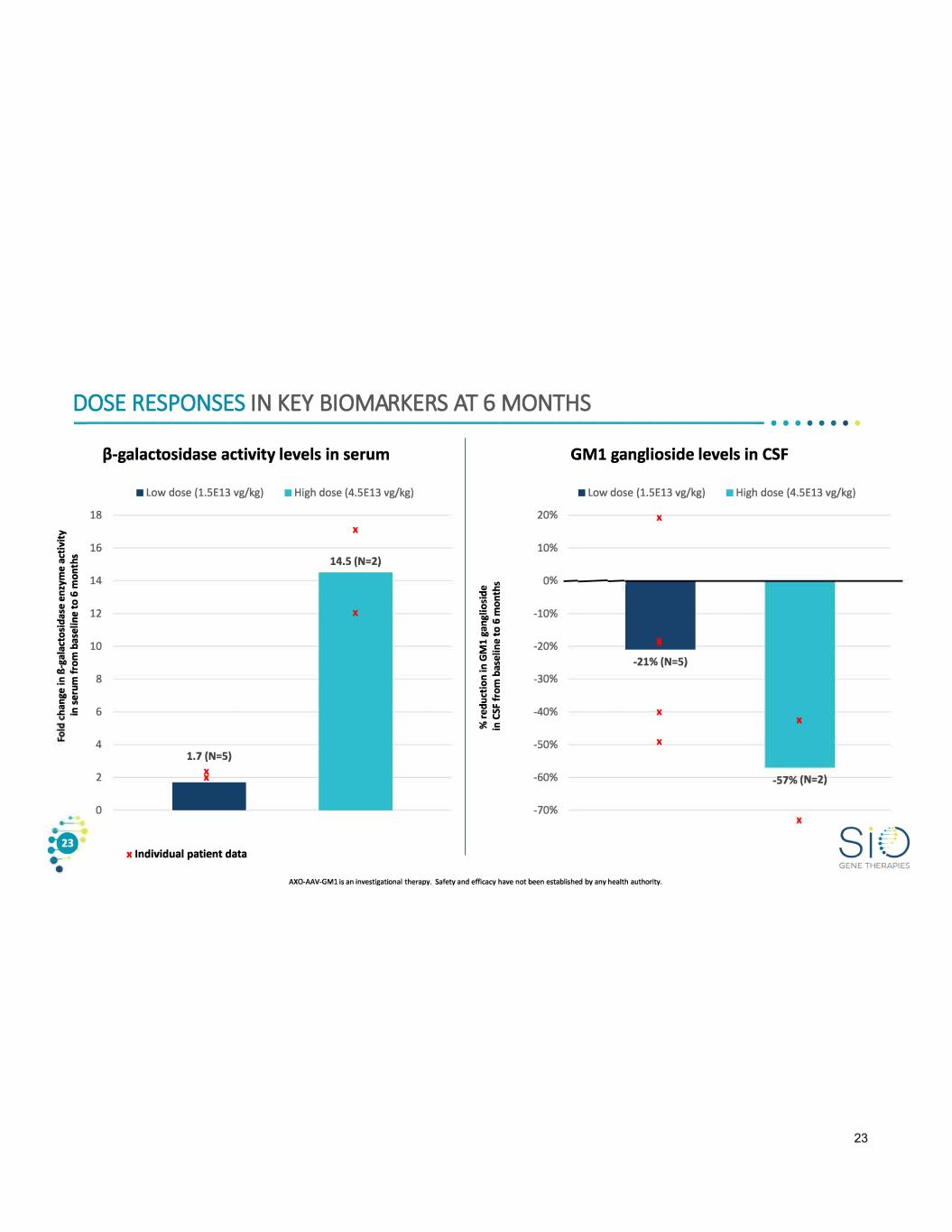
DOSE RESPONSES IN KEY BIOMARKERS AT 6 MONTHS ------------------------------------------------- • • · · · · · · (3-galactosidase activity levels in serum GMl ganglioside levels in CSF ■ Low dose {1.SE13 vg/kg) ■ High dose (4.SE13 vg/kg) ■ Low dose {1.SE13 vg/kg) ■ High dose (4.SE13 vg/kg) 18 20% X � X :! 16 tl "' 14.S (N=2).. ..c e& 14�E 10% 0%---- C ID .. 0 .. .. 12 ii -10% ·;;;� 0 "' tl .. 10 .. ..0 -20% ;;; E .. 0 ca J:: -21%(N=S) .!: E 8 -30% .. ::, �� IV C 6 ..c - -40% X X 4 -50% X 1.7 (N=S) 2 X X 0 - -60% -57%{N=2) -70% :G x Individual patient data GENE THERAPIES AXO-AAV-GMl is an investigational therapy. Safety and efficacy have not been established by any health authority. 23
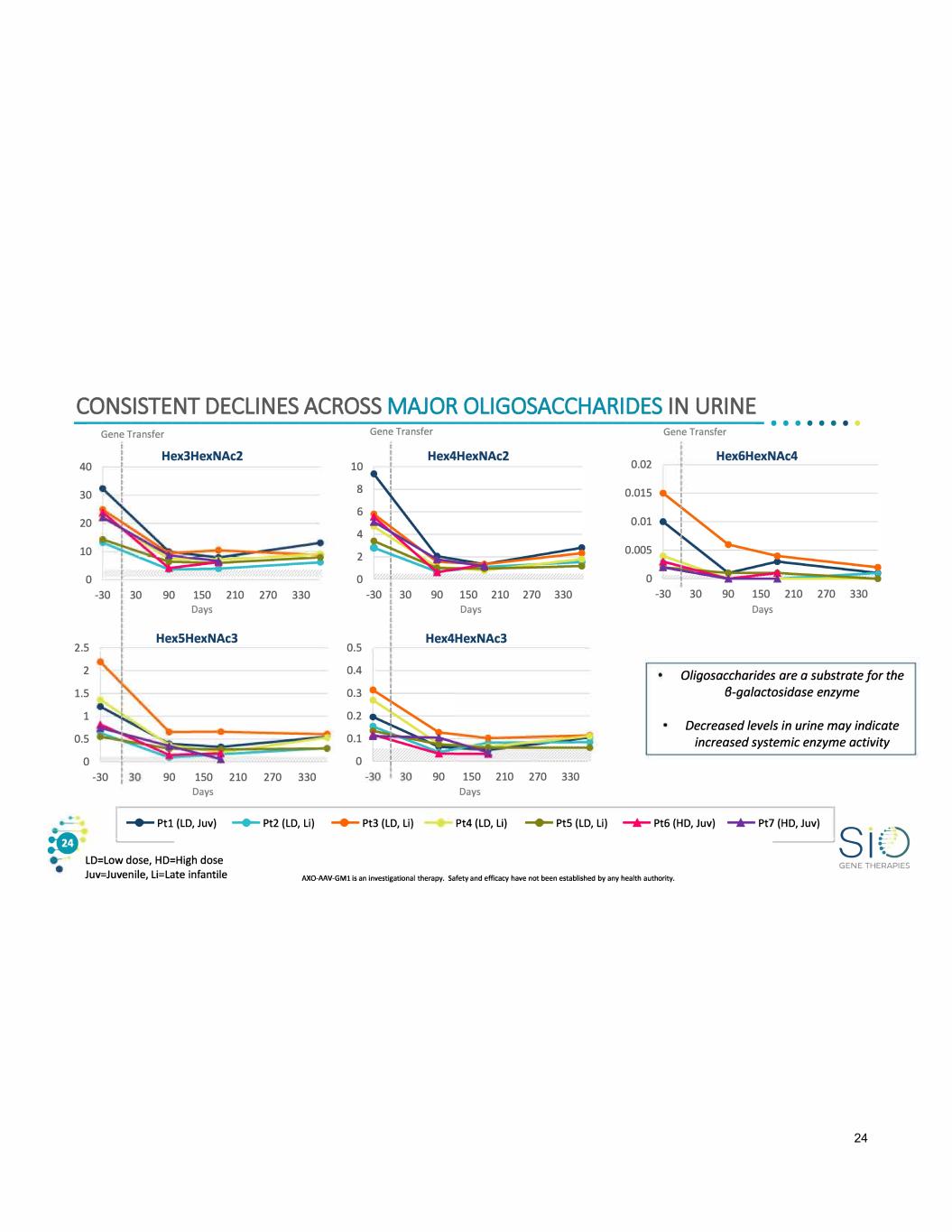
CONSISTENT DECLINES ACROSS MAJOR OLIGOSACCHARIDES IN URINE ------------------------------------------------- • • · · · · · · 40 30 20 10 0 2.5 2 1.5 1 0.5 0 :G Gene Transfer Gene Transfer Gene Transfer -30 -30 Hex3HexNAc2 10 8 6 4 2 0 30 90 150 210 270 330 -30 Days Hex5HexNAc3 0.5 0.4 0.3 0.2 0.1 0 90 150 210 270 330 Days Hex4HexNAc2 30 90 150 210 Days Hex4HexNAc3 90 150 210 Days 270 270 330 330 0.02 0.015 0.01 0.005 0 Hex6HexNAc4 -30 30 90 150 210 270 330 Days Oligosaccharides are a substrate for the 8-galactosidase enzyme Decreased levels in urine may indicate increased systemic enzyme activity -+- Ptl (LD, Juv) -- Pt2 (LD, Li) -+- Pt3 (LD, Li) Pt4 (LD, Li) -- Pt5 (LD, Li) _.,_ Pt6 (HD, Juv) -+- Pt7 (HD, Juv) �-----------S1fJ LD=Low dose, HD=High dose Juv=Juvenile, Li=Late infantile GENE THERAPIES AXO-MV-GM1 is an investigational therapy. Safety and efficacy have not been established by any health authority. 24

SiU GENE THERAPIES VOLUMETRIC BRAIN MRI FINDINGS . . .• rv . . l\ . . 25
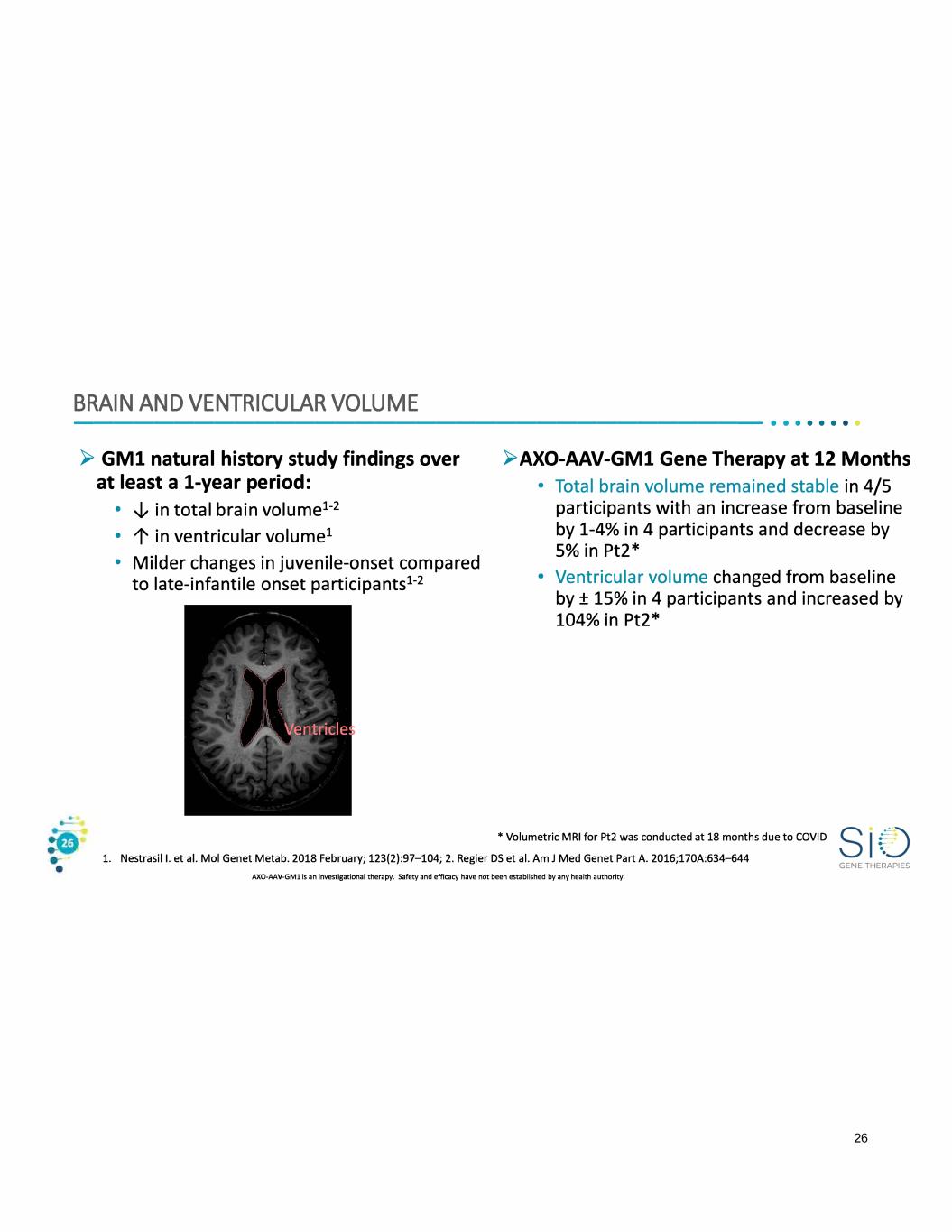
BRAIN AND VENTRICULAR VOLUME ----------------------------------•• · · · · · · ► GMl natural history study findings over at least a 1-year period: • -J,, in total brain volume1 - 2 • 1' in ventricular volume1 • Milder changes in juvenile-onset compared to late-infantile onset participants1 - 2 ► AXO-AAV-GMl Gene Therapy at 12 Months • Total brain volume remained stable in 4/5 participants with an increase from baseline by 1-4% in 4 participants and decrease by 5% in Pt2* • Ventricular volume changed from baseline by± 15% in 4 participants and increased by 104% in Pt2* • Volumetric MRI for Pt2 was conducted at 18 months due to COVID 1. Nestrasil I. et al. Mal Genet Metab. 2018 February; 123(2):97-104; 2. Regier DS et al. Am J Med Genet Part A. 2016;170A:634-644 GENE THERAPIES AXO-AAV-GMl is an investigational therapy. Safety and efficacy have not been established by any health authority. 26

SiU GENE THERAPIES CLINICAL OUTCOMES Vineland-3 Subscales 27

GROSS AND FINE MOTOR SKILLS 190 130 70 10 ,I. Gross Motor Skills j,. Late-infantile -------- ---- --- --- ,, _,, � � -e- G-- 12 24 36 48 60 72 84 Juvenile -- - 96 190 130 70 -€)0 10 108 120 �- Fine Motor Skills Late-infantile --- ,, ... --_.,,-- -- - - -- o-e-e 0 • • • • • • • • ---- --- - - G------€)0 12 24 36 48 60 72 84 Juvenile 96 108 120 190 ...... ----- 190 130 - � -------� 70 10 ....... Ptl (LD, Juv) - Pt2 (LD, Li) -+- Pt3 {LD, Li) - - - - Median Normal GSV LD=Low dose, HD=High dose, Juv=Juvenile, Li=Late infantile ----- - ___ .,, 130� l:s:'1 70 10 Age (months) Pt4 {LD, Li) - PtS {LD, Li) ........ Pt6 {HD, Juv) -+- Pt7 {HD, Juv) --e- Natural History Patients Vineland-3 Evaluation points: Baseline, 6 months, 12 months AXO-AAV-GMl is an investigational therapy. Safety and efficacy have not been established by any health authority. 28 28
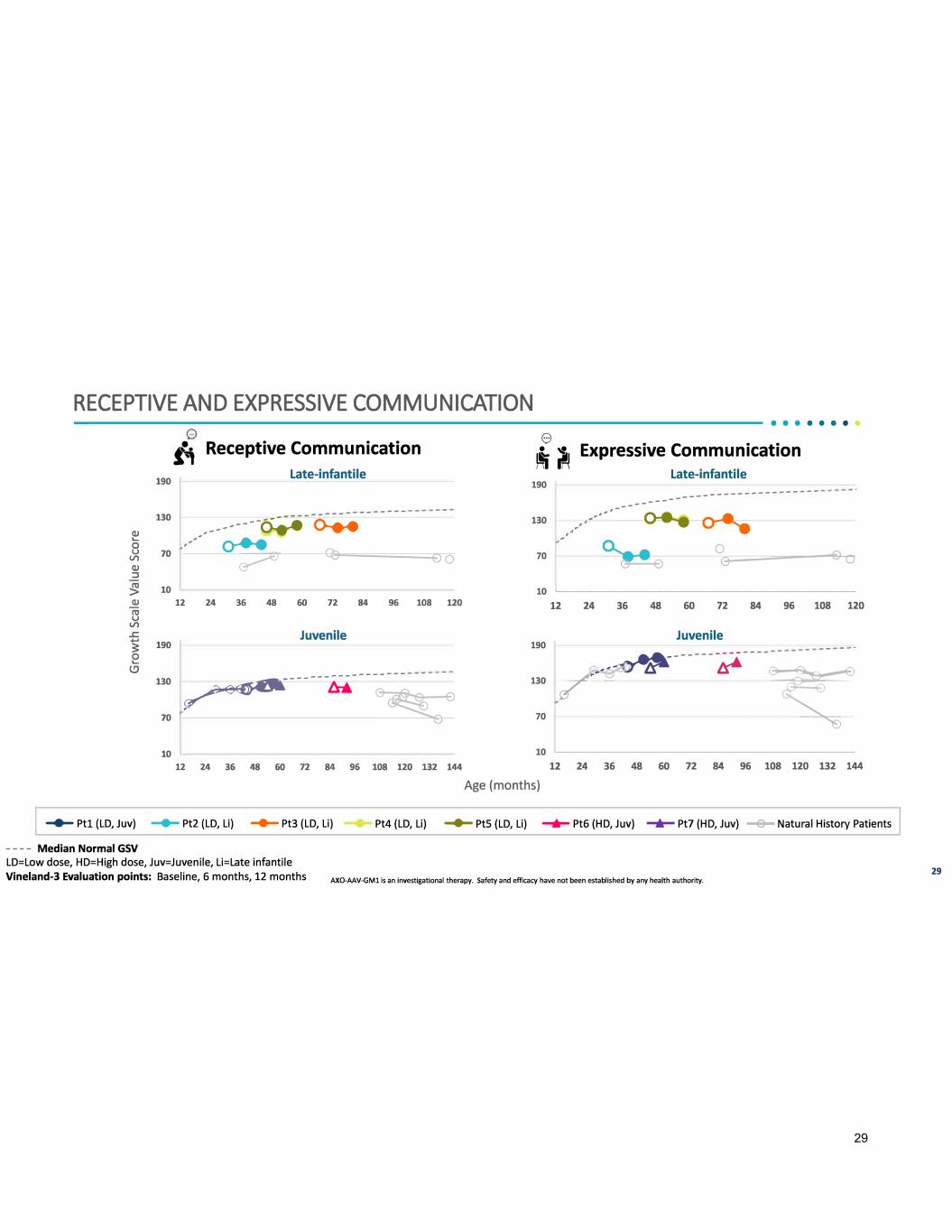
RECEPTIVE AND EXPRESSIVE COMMUNICATION --------------------------------------------••······ {; Receptive Communication 190 Late-infantile 130 ----------------- --------- -� 0--e-e ,-' o-e-e 70 � 10 190 Juvenile ----- -------- --- 130 � - /:rJ;. 70 10 0 � i/ Expressive Communication 190 130 70 ,• ,, .. ... .,. .. ,,' - -- -- -- - Late-infantile -- --- ---- ------ ------ - 0 e--�------4e��o 10 '-------------------- u � � � � n M % � rn Juvenile 190 � ------ � ---- � 130 ✓ � � 70 � 10 u � � � � n M % � rn m � Age (months) ....... Ptl (LD, Juv) - Pt2 (LD, Li) -+- Pt3 {LD, Li) Pt4 {LD, Li) - PtS {LD, Li) ........ Pt6 {HD, Juv) -+- Pt7 {HD, Juv) --e- Natural History Patients - - - - Median Normal GSV LD=Low dose, HD=High dose, Juv=Juvenile, Li=Late infantile Vineland-3 Evaluation points: Baseline, 6 months, 12 months AXO-AAV-GMl Is an investlgatlonal therapy. Safety and efficacy have not been established by any health authority. 29 29
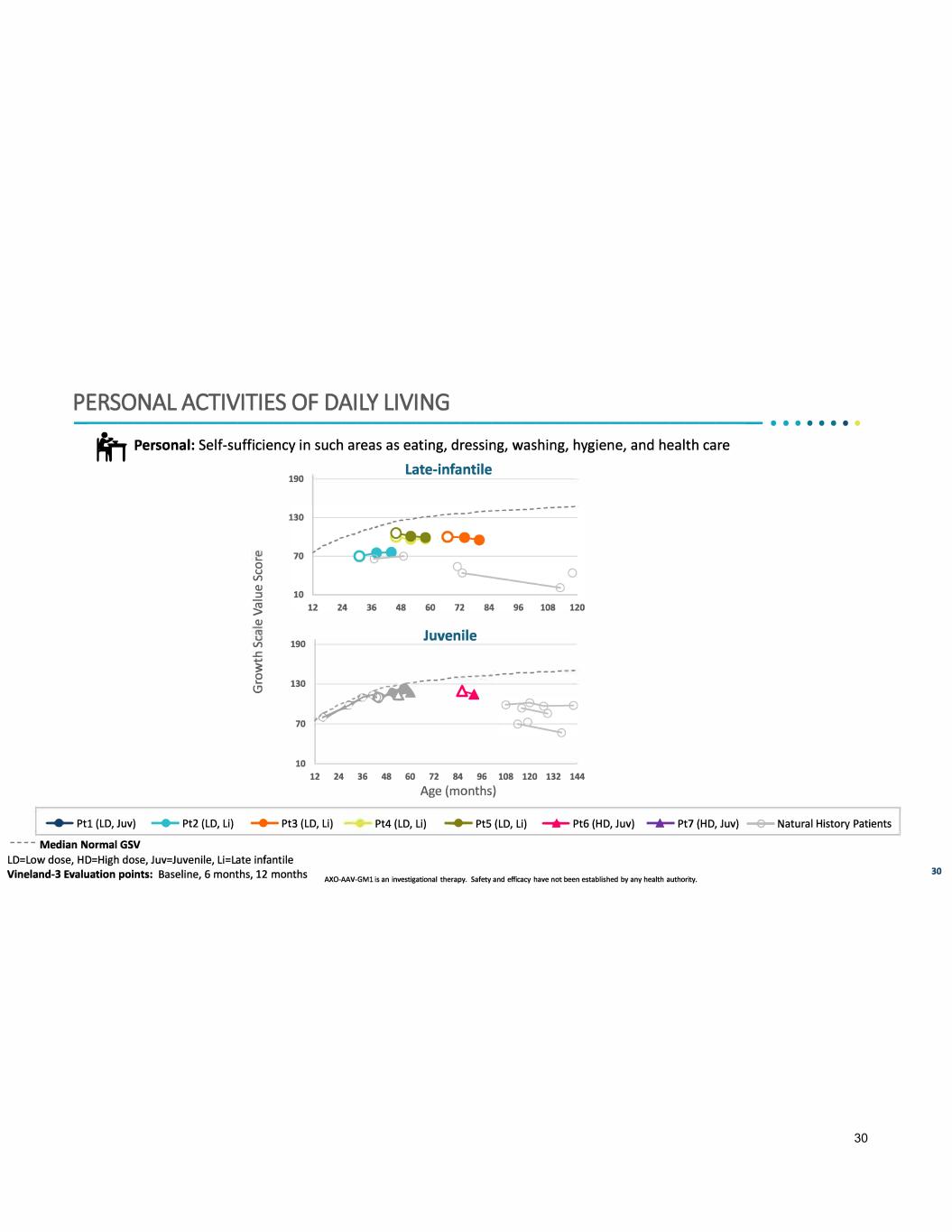
PERSONAL ACTIVITIES OF DAILY LIVING ----------------------------------------------•• · · · · · · t,- Personal: Self-sufficiency in such areas as eating, dressing, washing, hygiene, and health care Late-infantile 0 u Vl CJ) ::, � CJ) Vl 190 190 Juvenile ---------- � e l.!) 130 � ----- A& 70 10 12 24 36 48 60 72 84 96 108 120 132 144 Age (months) ....... Ptl (LD, Juv) - Pt2 (LD, Li) -+- Pt3 {LD, Li) Pt4 {LD, Li) - PtS {LD, Li) ........ Pt6 {HD, Juv) -+- Pt7 {HD, Juv) --e- Natural History Patients - - - - Median Normal GSV LD=Low dose, HD=High dose, Juv=Juvenile, Li=Late infantile Vineland-3 Evaluation points: Baseline, 6 months, 12 months AXO-AAV-GMl is an investigational therapy. Safety and efficacy have not been established by any health authority. 30 30
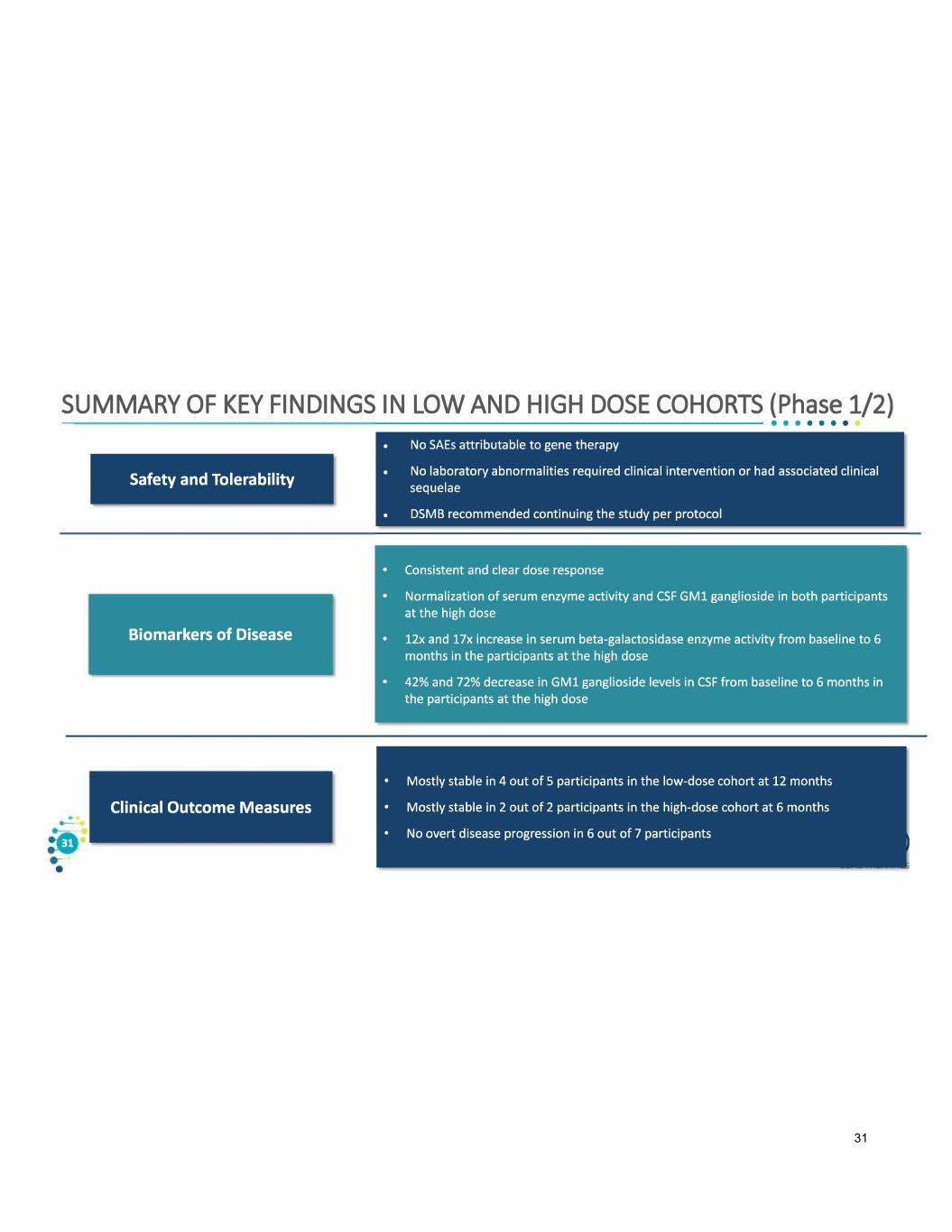
SUMMARY OF KEY FINDINGS IN LOW AND HIGH DOSE COHORTS (Phase 1/2) Safety and Tolerability Biomarkers of Disease Clinical Outcome Measures 31

AGENDA --------------------------------------------•• · · · · · · WELCOME Introduction & Opening Remarks Pavan Cheruvu, MD Chief Executive Officer Review of AXO-AAV-GM1 Phase 1/2 Clinical Trial Data Gavin Corcoran, MD, FACP Chief R&D Officer CLOSING REMARKS AND Q&A SESSION • *Dr. Gao serves as a paid advisor to Sio Gene Therapies Expert Perspective: Intravenous GM1 Gene Therapy Guangping Gao, PhD Chief AAV Scientific Advisor Director of the Horae Gene Therapy Center and Viral Vector Core at UMass Medical School GENE THERAPIES 32

EXPERT PERSPECTIVE: Rationale for intravenous AAV9 for the treatment of GM1 gangliosidosis ------------------------------------------•• · · · · · · Published results with AXO-AAV-GMl in a GMl feline model • AAV9 outperforms other AAV vector serotypes in head-to-head studies in GM1 feline model1 • IV administration achieves broad biodistribution1 • 2 • Restored brain anatomy and improved survival and functional outcomes1 • 2 Well-characterized AAV9 Vector System • AAV9, developed and characterized by Dr. Gao, is one of the most widely used gene therapy capsids in clinical development3 • Recent experience with IV AAV9 in SMA (where >1400 children have been dosed) provides strong rationale for safety and treatment effect in the CNS4 Threshold Phenomenon • Dose-dependent clinical outcomes were demonstrated in SMA at two distinct IV dose levels, once gene therapy reached a sufficiently high level, forming the basis for moving into a pivotal study5 1. Gross A et al. Mal Ther 2020;28(4S1):217, 2. Gross AL. et al. Brain. 2021 Aug 19:awab309. doi: 10.1093/brain/awab309, 3. Kuzmin et al., Nature s I f.� Reviews Drug Discovery. 2021, Vol 20, March 2021. 4. Findings from RESTORE registry of Zolgensma• presented at 2021 Muscular Dystrophy Association Conference, 5. Zolgensma® Prescribing Information, May 2021. GENE THERAPIES 33

AGENDA ------------------------------------------•• · · · · · · WELCOME Introduction & Opening Remarks Pavan Cheruvu, MD Chief Executive Officer Review of AXO-AAV-GM1 Phase 1/2 Clinical Trial Data Gavin Corcoran, MD, FACP Chief R&D Officer CLOSING REMARKS AND Q&A SESSION Expert Perspective: Intravenous GM1 Gene Therapy Guangping Gao, PhD Chief AAV Scientific Advisor Director of the Horae Gene Therapy Center and Viral Vector Core at UMass Medical School GENE THERAPIES 34

EXECUTING AGAINST AXO-MV-GM1 PROGRAM PRIORITIES --------------------------------•• · · · · · · l!/ May 2021: Presented 6-month biomarker data from low-dose cohort at ASGCT 2021 conference � October 2021: Reported interim clinical data from Stage 1 at ESGCT 2021 conference □ 1H 2022: Expect to provide data update from Stage 1 of the study, including both Type I and Type II patients, at future scientific conferences □ 1H 2022: Expect to engage with the FDA to review Stage 1 data and discuss next steps for clinical development GENE THERAPIES 35

GENE THERAPIES AXO-MV-GMl for GMl Gangliosidosis Results from ongoing Phase 1/2 Dose Escalation Study • 6-Month Safety and Efficacy High-Dose Cohort • 12-Month Safety and Efficacy Low-Dose Cohort October 21, 2021 36 • • • 36



































