- SYRE Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Spyre Therapeutics (SYRE) 8-KRegulation FD Disclosure
Filed: 19 Apr 16, 12:00am
Exhibit 99.1
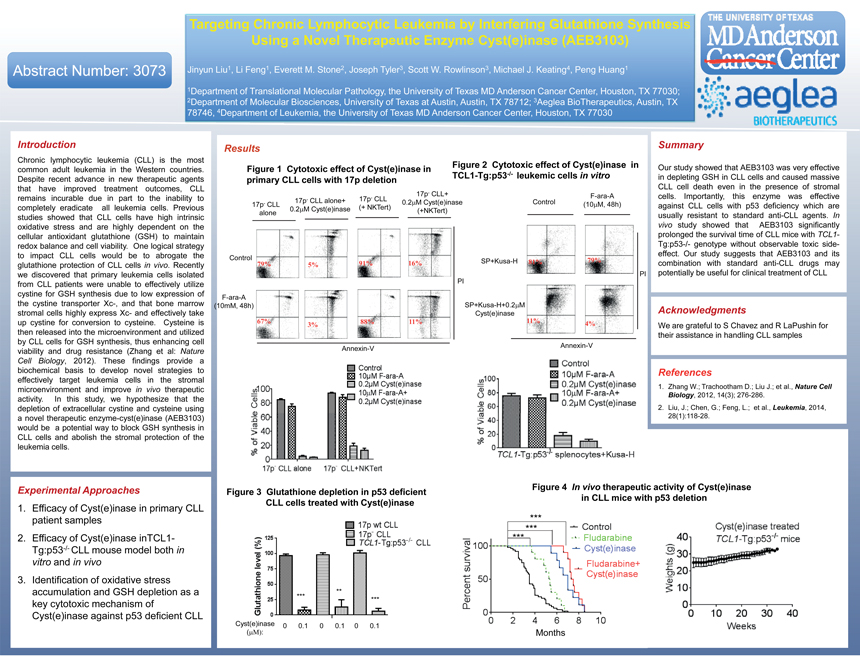
Targeting Chronic Lymphocytic Leukemia by Interfering Glutathione Synthesis Using a Novel Therapeutic Enzyme Cyst(e)inase (AEB3103) Abstract Number: 3073 Jinyun Liu1, Li Feng1, Everett M. Stone2, Joseph Tyler3, Scott W. Rowlinson3, Michael J. Keating4, Peng Huang1 1 , , , 2Department of Molecular Biosciences, University of Texas at Austin, Austin, TX 78712; 3Aeglea BioTherapeutics, Austin, TX 78746, 4Department of Leukemia, the University of Texas MD Anderson Cancer Center, Houston, TX 77030 Introduction Results Summary Chronic lymphocytic leukemia (CLL) is the most Figure 2 Cytotoxic effect of Cyst(e)inase in . TCL1-Tg:p53-/- leukemic cells in vitro in depleting GSH in CLL cells and caused massive Despite recent advance in new therapeutic agents primary CLL cells with 17p deletion CLL cell death even in the presence of stromal that have improved treatment outcomes, CLL 17p- CLL+ F-ara-A cells. Importantly, this enzyme was effective remains incurable due in part to the inability to 17p- CLL alone+ 17p- CLL 17p- CLL 0.2M Cyst(e)inase Control (10M, 48h) completely eradicate all leukemia cells. Previous 0.2M Cyst(e)inase (+ NKTert) against CLL cells with p53 deficiency which are alone (+NKTert) studies showed that CLL cells have high intrinsic usually resistant to standard anti-CLL agents. In oxidative stress and are highly dependent on the vivo study showed that AEB3103 significantly cellular antioxidant glutathione (GSH) to maintain prolonged the survival time of CLL mice with TCL1-redox balance and cell viability. One logical strategy Tg:p53-/- genotype without observable toxic side-to impact CLL cells would be to abrogate the effect. Our study suggests that AEB3103 and its Control 79% 79% 91% 16% SP+Kusa-H 81% combination with standard anti-CLL drugs may glutathione protection of CLL cells in vivo. Recently 5% we discovered that primary leukemia cells isolated PI potentially be useful for clinical treatment of CLL from CLL patients were unable to effectively utilize PI cystine for GSH synthesis due to low expression of F-ara-A the cystine transporter Xc-, and that bone marrow (10mM, 48h) SP+Kusa-H+0.2M stromal cells highly express Xc- and effectively take Cyst(e)inase Acknowledgments up cystine for conversion to cysteine. Cysteine is 67% 88% 11% 11% 4% 3% We are grateful to S Chavez and R LaPushin for their assistance in handling CLL samples by CLL cells for GSH synthesis, thus enhancing cell Annexin-V Annexin-V viability and drug resistance (Zhang et al: Nature Cell Biology, 2012). These findings provide a biochemical basis to develop novel strategies to References effectively target leukemia cells in the stromal 1. Zhang W.; Trachootham D.; Liu J.; et al., Nature Cell microenvironment and improve in vivo therapeutic Biology, 2012, 14(3); 276-286. activity. In this study, we hypothesize that the depletion of extracellular cystine and cysteine using 2. Liu, J.; Chen, G.; Feng, L.; et al., Leukemia, 2014, a novel therapeutic enzyme-cyst(e)inase (AEB3103) 28(1):118-28. would be a potential way to block GSH synthesis in CLL cells and abolish the stromal protection of the leukemia cells. Figure 4 In vivo therapeutic activity of Cyst(e)inase in CLL mice with p53 deletion CLL cells treated with Cyst(e)inase 1. Efficacy of Cyst(e)inase in primary CLL patient samples *** 2. Efficacy of Cyst(e)inase inTCL1- ****** Tg:p53-/- CLL mouse model both in vitro and in vivo 3. Identification of oxidative stress accumulation and GSH depletion as a ** *** key cytotoxic mechanism of *** Cyst(e)inase against p53 deficient CLL Cyst(e)inase 0 0.1 0 0.1 0 0.1 (: Months