
Aeglea Corporate Overview Exhibit 99.1

Forward Looking Statements This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our current and future financial performance, business plans and objectives, current and future clinical and preclinical development activities, timing and success of our ongoing and planned clinical trials and related data, the timing of announcements, updates and results of our clinical trials and related data, our ability to obtain and maintain regulatory approval, the potential therapeutic benefits and economic value of our lead product candidate and our other product candidates, potential growth opportunities, financing plans, use and adequacy of financing plans, competitive position, industry environment and potential market opportunities. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors including, but not limited to, those related to the success, cost and timing of our product candidate development activities and ongoing and planned clinical trials; our plans to develop and commercialize targeted therapeutics, including our lead product candidate pegzilarginase and our other product candidates homocystinuria and cystinuria; the design, progress of patient enrollment and dosing in our clinical trials, the ability of our product candidates to achieve applicable endpoints in clinical trials, the ability for patients who participate in the Phase 3 PEACE trial to participate in a long-term extension study, the safety profile of our product candidates in clinical trials, the potential for data from our current and future clinical trials to support a marketing application, as well as the timing of these events, the potential for preclinical studies to be predictive of current or future clinical trials, our ability to obtain funding for our operations, development and commercialization of our product candidates; the impact of the COVID-19 pandemic on our operations and clinical development activities; the timing of and our ability to obtain and maintain regulatory approvals; the potential for expeditated development and review of pegzilarginase as of a result of its Breakthrough Therapy designation; the potential addressable markets of the our product candidates; the rate and degree of market acceptance and clinical utility of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; our commercialization, marketing and manufacturing capabilities and strategy; future agreements with third parties in connection with the commercialization of our product candidates; our expectations regarding our ability to obtain and maintain intellectual property protection; our dependence on third party manufacturers; our ability to develop our own commercial manufacturing facility; the success of competing therapies that are or may become available; our ability to attract and retain key scientific or management personnel; our ability to identify additional product candidates with significant commercial potential consistent with our commercial objectives; and our estimates regarding expenses, future revenue, capital requirements and needs for additional financing. Moreover, we operate in a very competitive and rapidly changing environment, and new risks may emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed herein may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Further information on these and other factors that could affect these forward-looking statements is contained in our most recent Annual Report on Form 10-K filed with the U.S. Securities and Exchange Commission (SEC), the supplemental risk factor included in our Current Report on Form 8-K filed with the SEC on April 8, 2020 and other reports filed by the SEC. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. We undertake no obligation to publicly update any forward-looking statements, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.

Reimagining The Potential Of Human Enzyme Therapeutics For Patients With Rare Genetic Diseases Aeglea’s platform unlocks opportunities for innovative disease-modifying solutions in areas of high unmet medical need NASDAQ: AGLE Austin, Texas Cash, cash equivalents and marketable securities at March 31, 2020: $50.5 million1 (no debt) Includes $1.5M of restricted cash
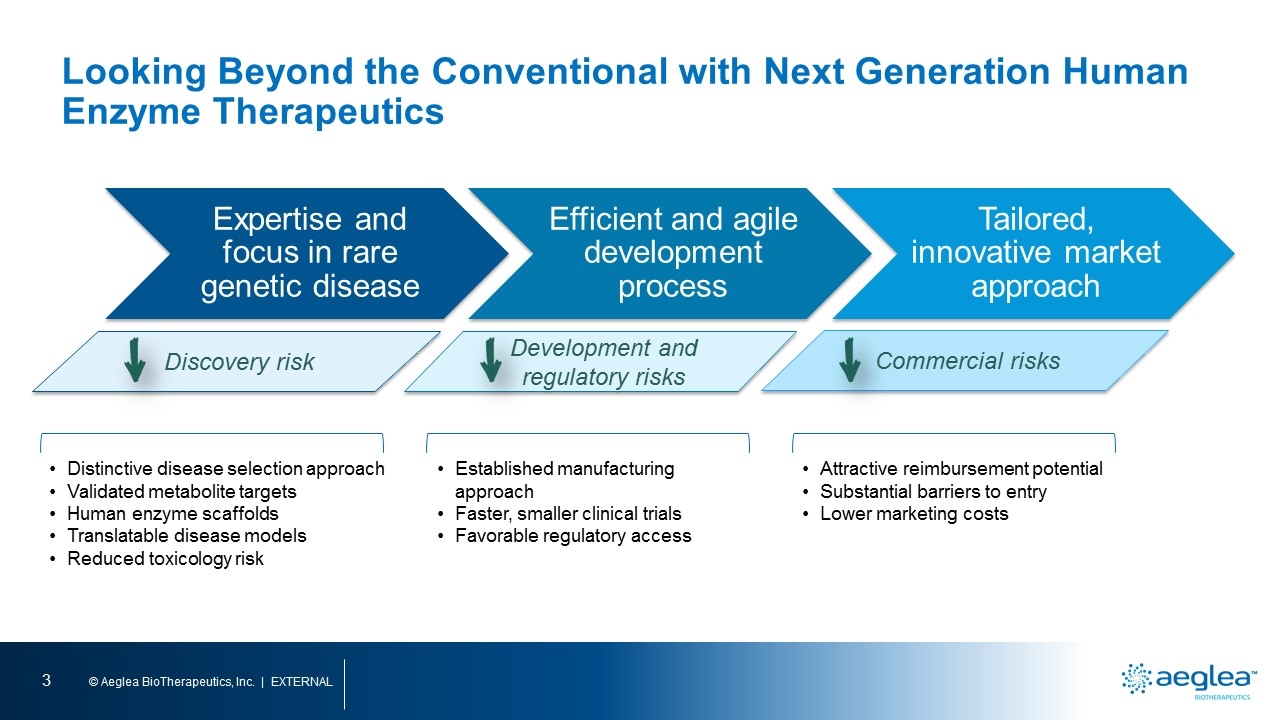
Looking Beyond the Conventional with Next Generation Human Enzyme Therapeutics Discovery risk Development and regulatory risks Commercial risks Distinctive disease selection approach Validated metabolite targets Human enzyme scaffolds Translatable disease models Reduced toxicology risk Established manufacturing approach Faster, smaller clinical trials Favorable regulatory access Attractive reimbursement potential Substantial barriers to entry Lower marketing costs Expertise and focus in rare genetic disease Efficient and agile development process Tailored, innovative market approach
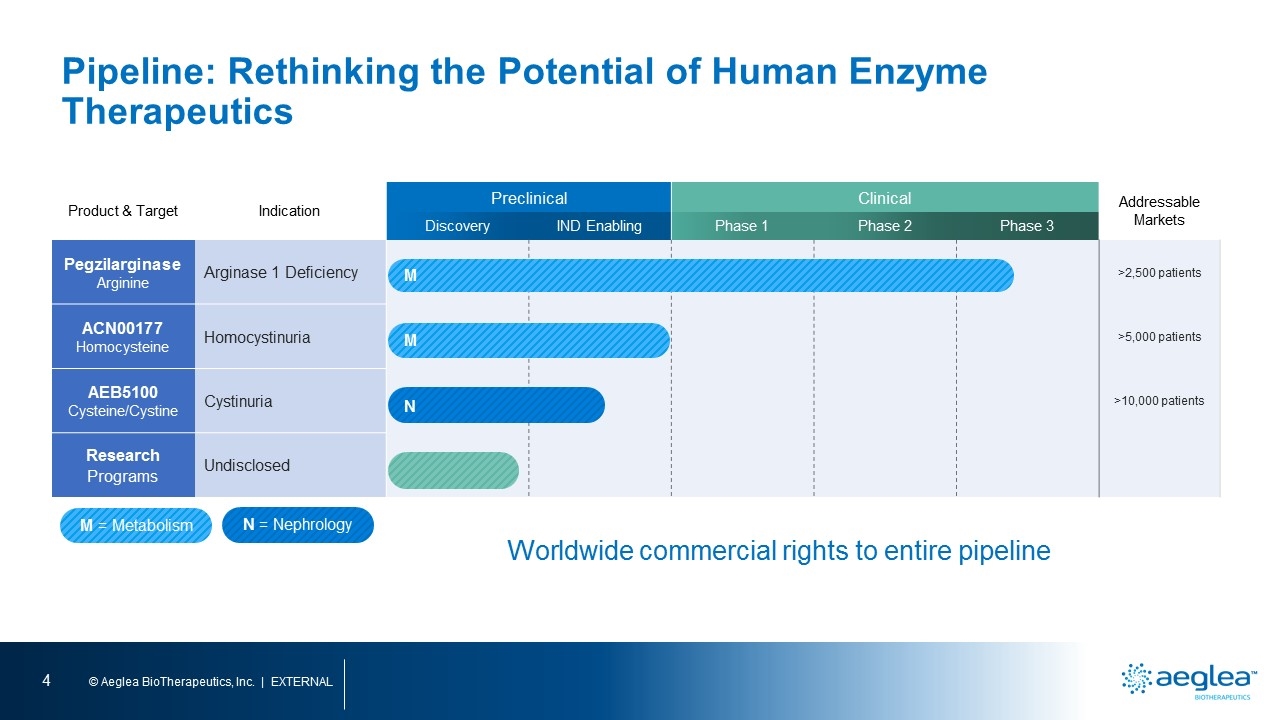
Pipeline: Rethinking the Potential of Human Enzyme Therapeutics Product & Target Indication Preclinical Clinical Addressable Markets Discovery IND Enabling Phase 1 Phase 2 Phase 3 Pegzilarginase Arginine Arginase 1 Deficiency >2,500 patients ACN00177 Homocysteine Homocystinuria >5,000 patients AEB5100 Cysteine/Cystine Cystinuria >10,000 patients Research Programs Undisclosed Worldwide commercial rights to entire pipeline M = Metabolism M M N N = Nephrology

Highly Experienced Leadership Team in Drug Discovery & Development Anthony G. Quinn, MD PhD Chief Executive Officer Leslie Sloan, PhD Chief Operating Officer Charles N. York II, MBA Chief Financial Officer Scott Rowlinson, PhD Vice President of Research Ravi M. Rao, MD PhD Chief Medical Officer Michael C. Hanley, MBA Chief Commercial Officer

Arginase 1 Deficiency A debilitating and progressive genetic disease with no effective therapeutic options, leaving patients with a considerable daily burden and early mortality Pegzilarginase: Lead Program
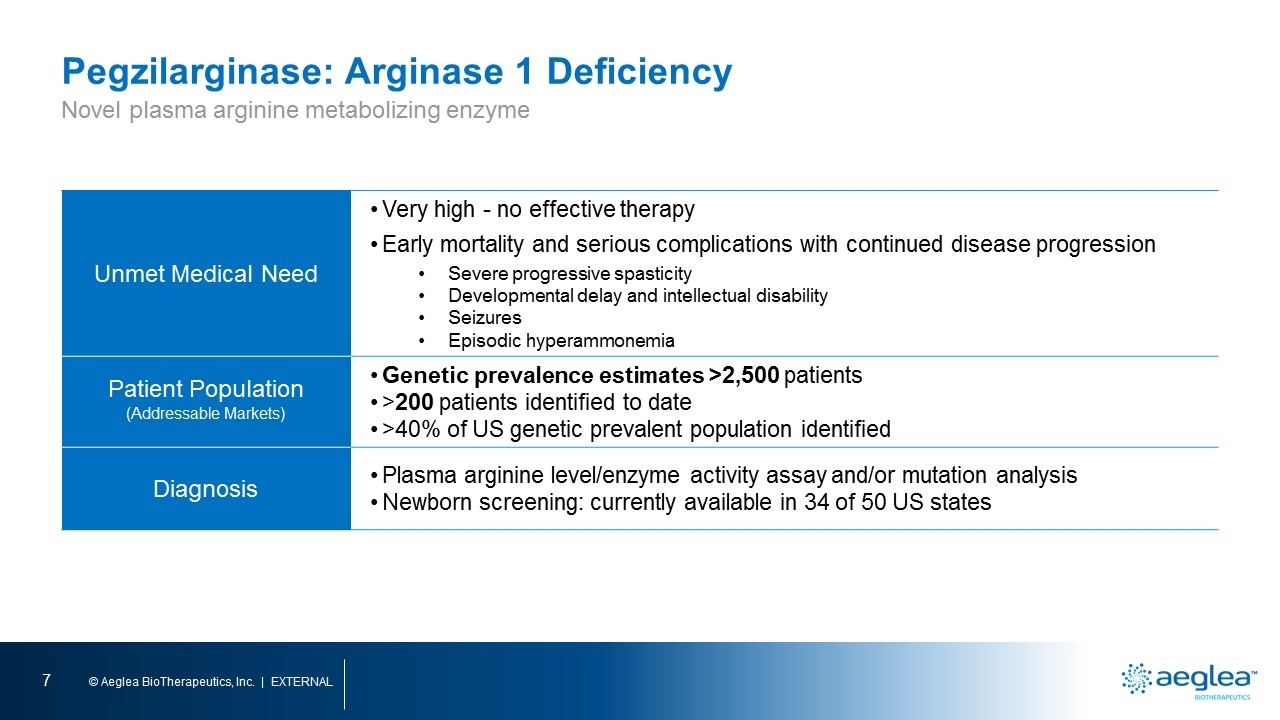
Pegzilarginase: Arginase 1 Deficiency Unmet Medical Need Very high - no effective therapy Early mortality and serious complications with continued disease progression Severe progressive spasticity Developmental delay and intellectual disability Seizures Episodic hyperammonemia Patient Population (Addressable Markets) Genetic prevalence estimates >2,500 patients >200 patients identified to date >40% of US genetic prevalent population identified Diagnosis Plasma arginine level/enzyme activity assay and/or mutation analysis Newborn screening: currently available in 34 of 50 US states Novel plasma arginine metabolizing enzyme
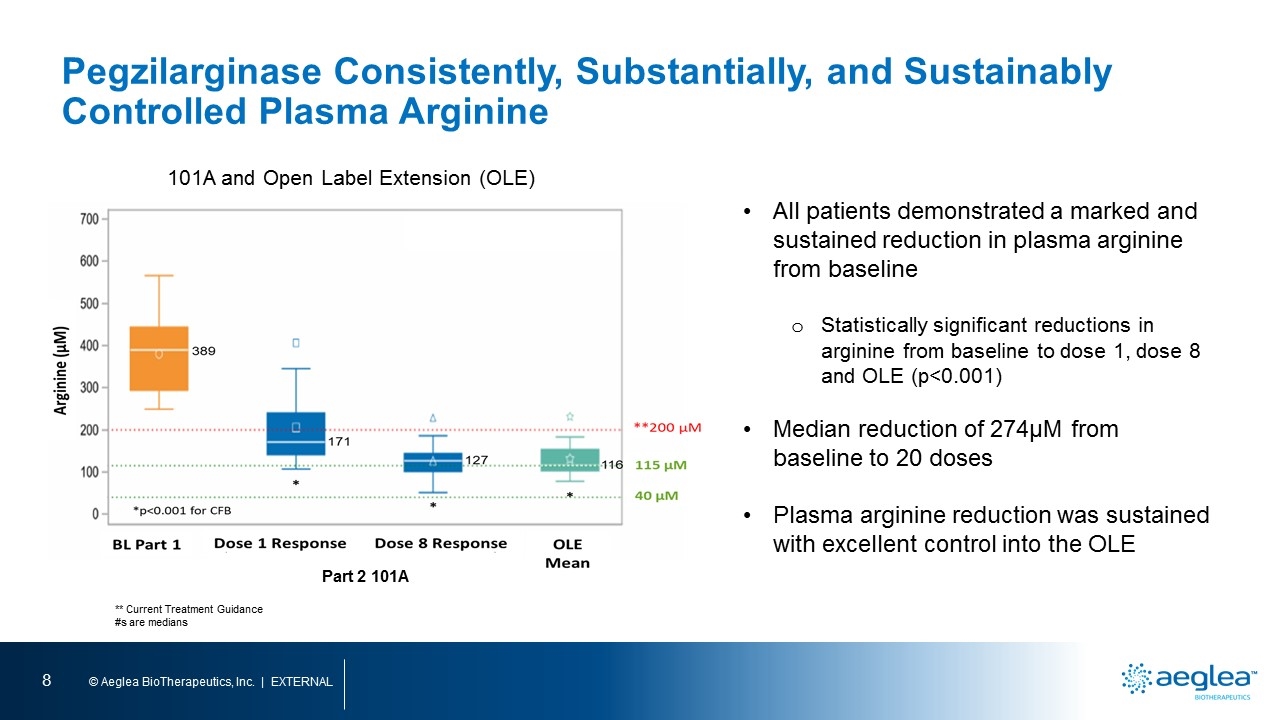
** Current Treatment Guidance #s are medians 101A and Open Label Extension (OLE) All patients demonstrated a marked and sustained reduction in plasma arginine from baseline Statistically significant reductions in arginine from baseline to dose 1, dose 8 and OLE (p˂0.001) Median reduction of 274µM from baseline to 20 doses Plasma arginine reduction was sustained with excellent control into the OLE Part 2 101A Pegzilarginase Consistently, Substantially, and Sustainably Controlled Plasma Arginine arginine (m) bl part 1 dose 1 response dose 8 response ole mean part 2 101A
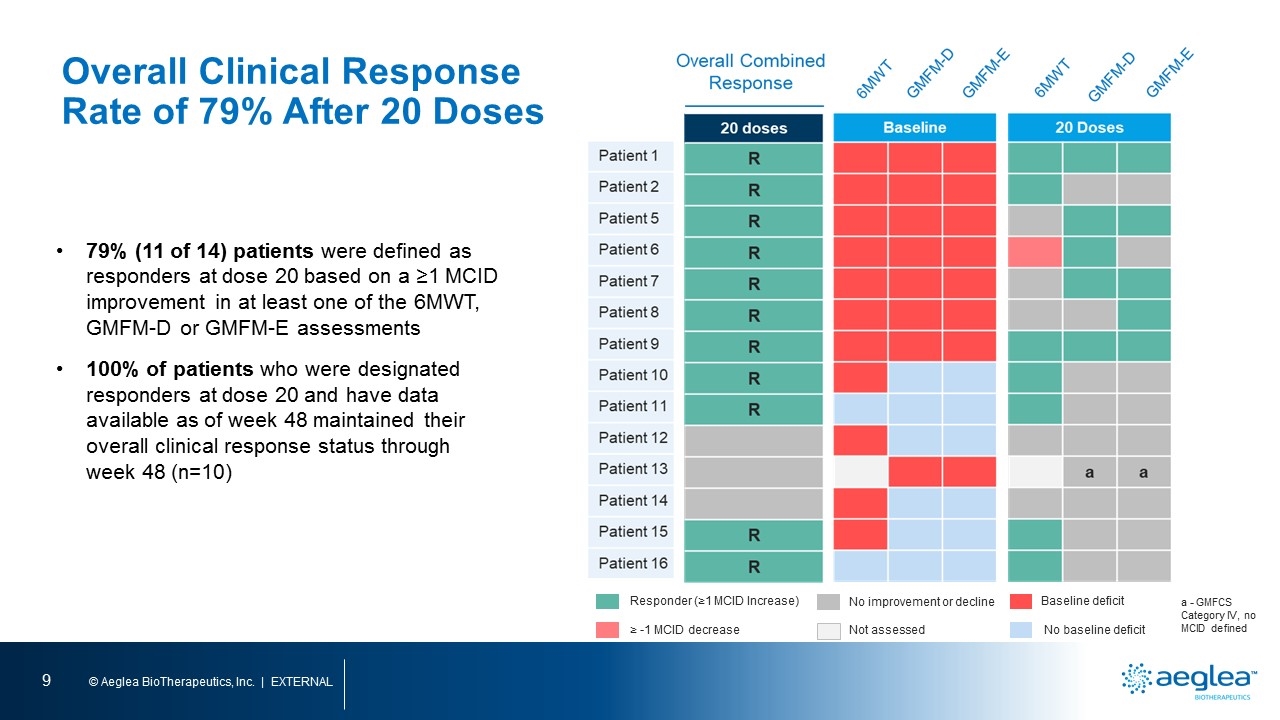
79% (11 of 14) patients were defined as responders at dose 20 based on a ≥1 MCID improvement in at least one of the 6MWT, GMFM-D or GMFM-E assessments 100% of patients who were designated responders at dose 20 and have data available as of week 48 maintained their overall clinical response status through week 48 (n=10) Baseline deficit No baseline deficit Not assessed No improvement or decline Responder (≥1 MCID Increase) ≥ -1 MCID decrease a - GMFCS Category IV, no MCID defined Overall Clinical Response Rate of 79% After 20 Doses patient 1patient 2 patient 5 patient 6 patient 7 patient 8 patient 9 patient 10 patient 11 patient 12 patient 13 patient 14 patient 15 patient 16 6mwt gmfm-d gmfm-e 6mwt gmfm-d gmfm-e

Clinically Impactful Improvements with Pegzilarginase Patient 5: Baseline Patient 5: After 20 Doses Unable to cross legs and dependent on walking aid Able to cross legs and less dependent on walking aid Plasma arginine Baseline: 363 µM After 20 doses: 108.5 µM 108.5 µM = last 4 using PEACE criteria

Safety: Pegzilarginase Was Well Tolerated Hypersensitivity and hyperammonemia were the most common treatment-related SAEs, and were expected and manageable More than 650 doses were administered to 16 patients (mean of 41 doses per patient) More than 200 injections were administered to 10 patients subcutaneously Most treatment-related AEs were mild Treatment-related adverse events frequency decreased over time The majority of adverse events and all treatment-related SAEs were observed in the first trial (101A)
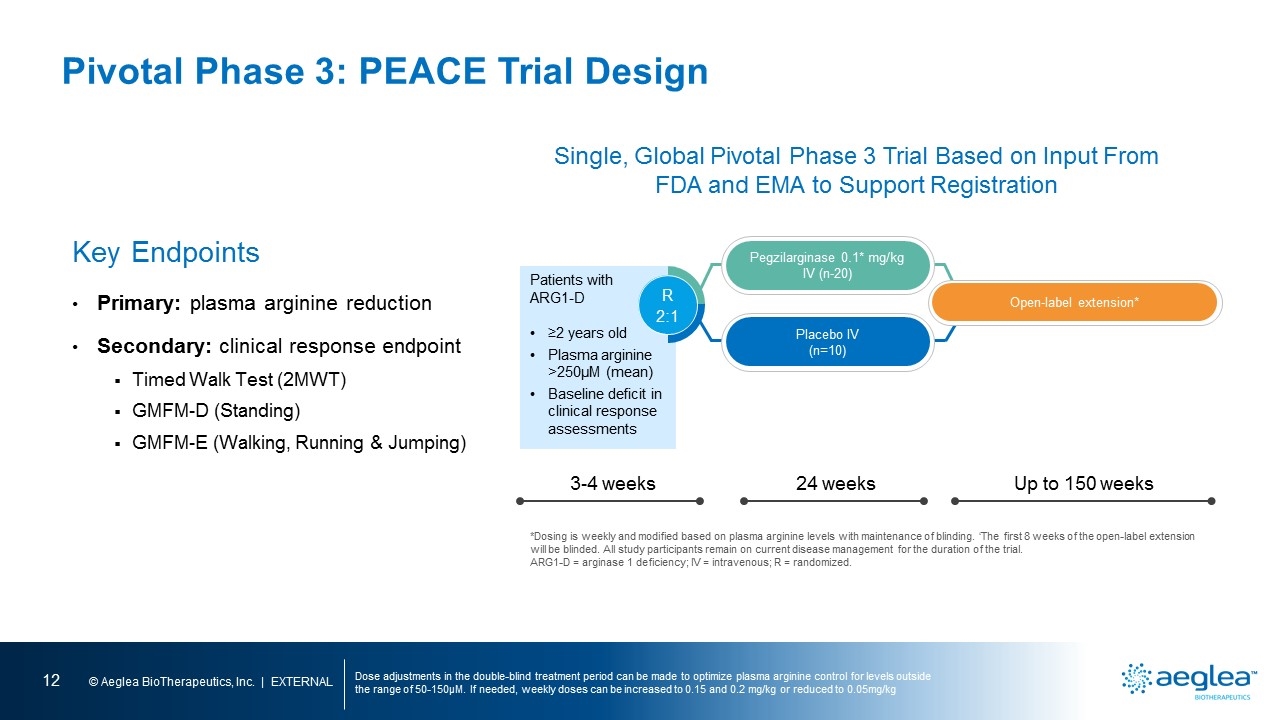
Pivotal Phase 3: PEACE Trial Design Dose adjustments in the double-blind treatment period can be made to optimize plasma arginine control for levels outside the range of 50-150µM. If needed, weekly doses can be increased to 0.15 and 0.2 mg/kg or reduced to 0.05mg/kg Key Endpoints Primary: plasma arginine reduction Secondary: clinical response endpoint Timed Walk Test (2MWT) GMFM-D (Standing) GMFM-E (Walking, Running & Jumping) *Dosing is weekly and modified based on plasma arginine levels with maintenance of blinding. ‘The first 8 weeks of the open-label extension will be blinded. All study participants remain on current disease management for the duration of the trial. ARG1-D = arginase 1 deficiency; IV = intravenous; R = randomized. Patients with ARG1-D ≥2 years old Plasma arginine >250µM (mean) Baseline deficit in clinical response assessments Placebo IV (n=10) R 2:1 Open-label extension* Pegzilarginase 0.1* mg/kg IV (n-20) 3-4 weeks 24 weeks Up to 150 weeks Single, Global Pivotal Phase 3 Trial Based on Input From FDA and EMA to Support Registration
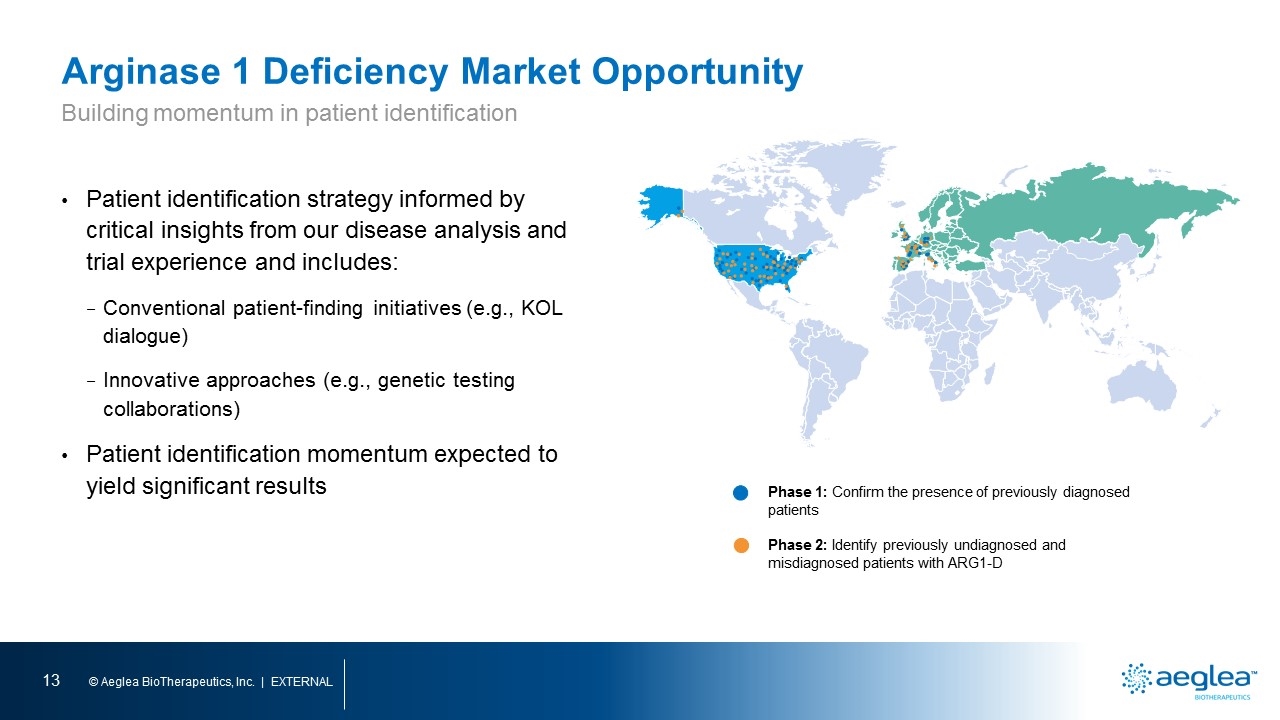
Arginase 1 Deficiency Market Opportunity Patient identification strategy informed by critical insights from our disease analysis and trial experience and includes: Conventional patient-finding initiatives (e.g., KOL dialogue) Innovative approaches (e.g., genetic testing collaborations) Patient identification momentum expected to yield significant results Phase 1: Confirm the presence of previously diagnosed patients Phase 2: Identify previously undiagnosed and misdiagnosed patients with ARG1-D Building momentum in patient identification
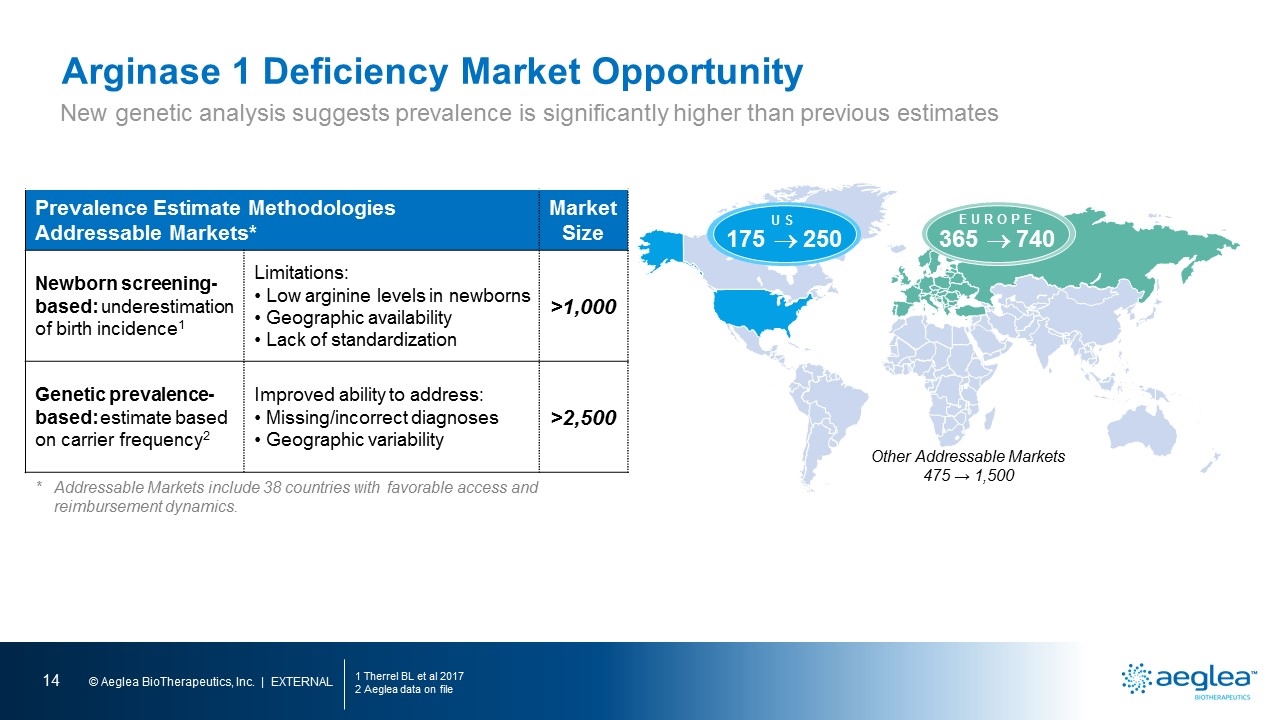
Arginase 1 Deficiency Market Opportunity 1 Therrel BL et al 2017 2 Aeglea data on file Prevalence Estimate Methodologies Addressable Markets* Market Size Newborn screening- based: underestimation of birth incidence1 Limitations: Low arginine levels in newborns Geographic availability Lack of standardization >1,000 Genetic prevalence-based: estimate based on carrier frequency2 Improved ability to address: Missing/incorrect diagnoses Geographic variability >2,500 *Addressable Markets include 38 countries with favorable access and reimbursement dynamics. 175 ® 250 US 365 ® 740 EUROPE Other Addressable Markets 475 → 1,500 New genetic analysis suggests prevalence is significantly higher than previous estimates
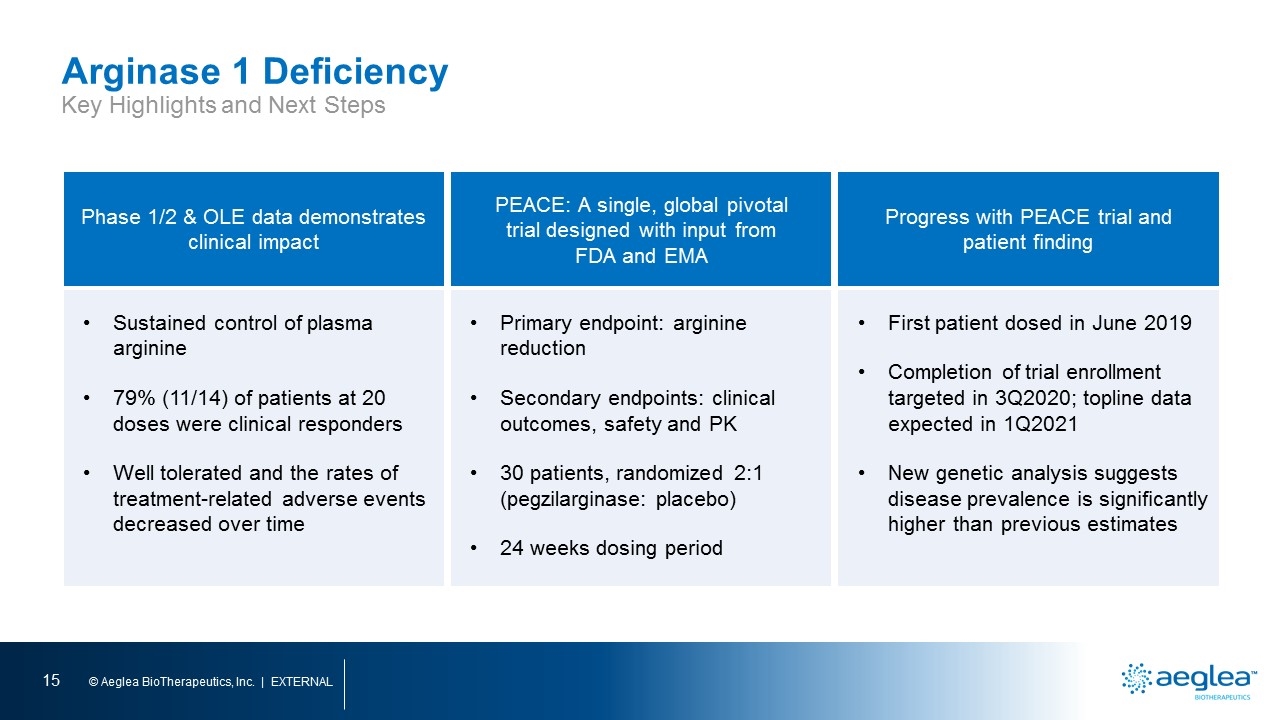
Arginase 1 Deficiency Key Highlights and Next Steps Phase 1/2 & OLE data demonstrates clinical impact PEACE: A single, global pivotal trial designed with input from FDA and EMA Progress with PEACE trial and patient finding Sustained control of plasma arginine 79% (11/14) of patients at 20 doses were clinical responders Well tolerated and the rates of treatment-related adverse events decreased over time Primary endpoint: arginine reduction Secondary endpoints: clinical outcomes, safety and PK 30 patients, randomized 2:1 (pegzilarginase: placebo) 24 weeks dosing period First patient dosed in June 2019 Completion of trial enrollment targeted in 3Q2020; topline data expected in 1Q2021 New genetic analysis suggests disease prevalence is significantly higher than previous estimates

ACN00177 Aeglea’s AEB4104 program targets homocystinuria due to cystathionine beta synthase (CBS) deficiency
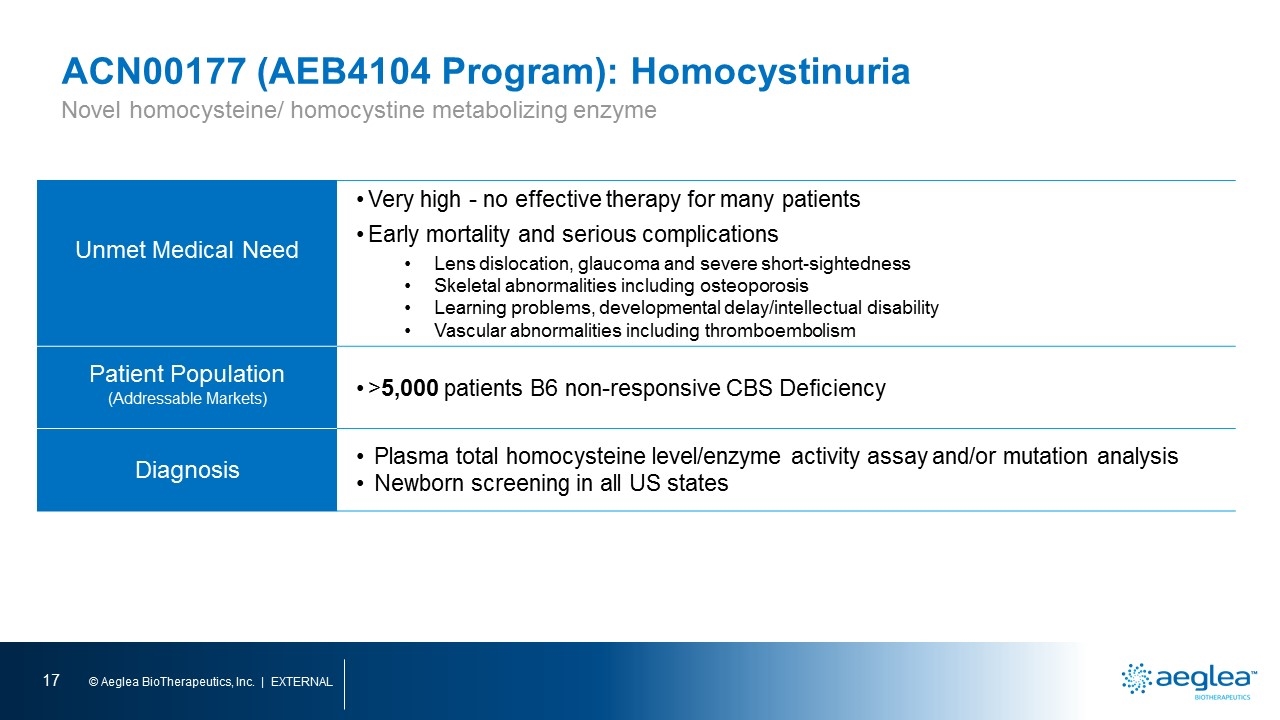
ACN00177 (AEB4104 Program): Homocystinuria Unmet Medical Need Very high - no effective therapy for many patients Early mortality and serious complications Lens dislocation, glaucoma and severe short-sightedness Skeletal abnormalities including osteoporosis Learning problems, developmental delay/intellectual disability Vascular abnormalities including thromboembolism Patient Population (Addressable Markets) >5,000 patients B6 non-responsive CBS Deficiency Diagnosis Plasma total homocysteine level/enzyme activity assay and/or mutation analysis Newborn screening in all US states Novel homocysteine/ homocystine metabolizing enzyme
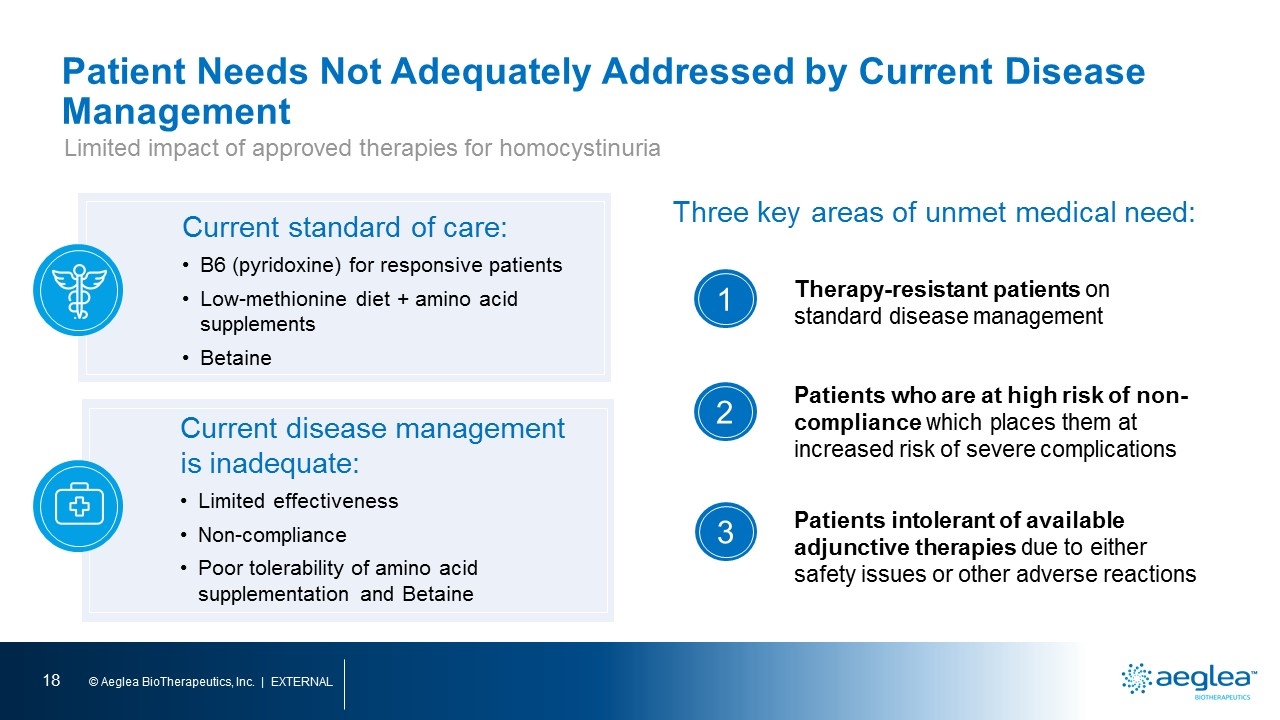
Patient Needs Not Adequately Addressed by Current Disease Management Limited impact of approved therapies for homocystinuria Current standard of care: B6 (pyridoxine) for responsive patients Low-methionine diet + amino acid supplements Betaine Current disease management is inadequate: Limited effectiveness Non-compliance Poor tolerability of amino acid supplementation and Betaine Therapy-resistant patients on standard disease management Patients who are at high risk of non-compliance which places them at increased risk of severe complications Patients intolerant of available adjunctive therapies due to either safety issues or other adverse reactions Three key areas of unmet medical need: 1 2 3
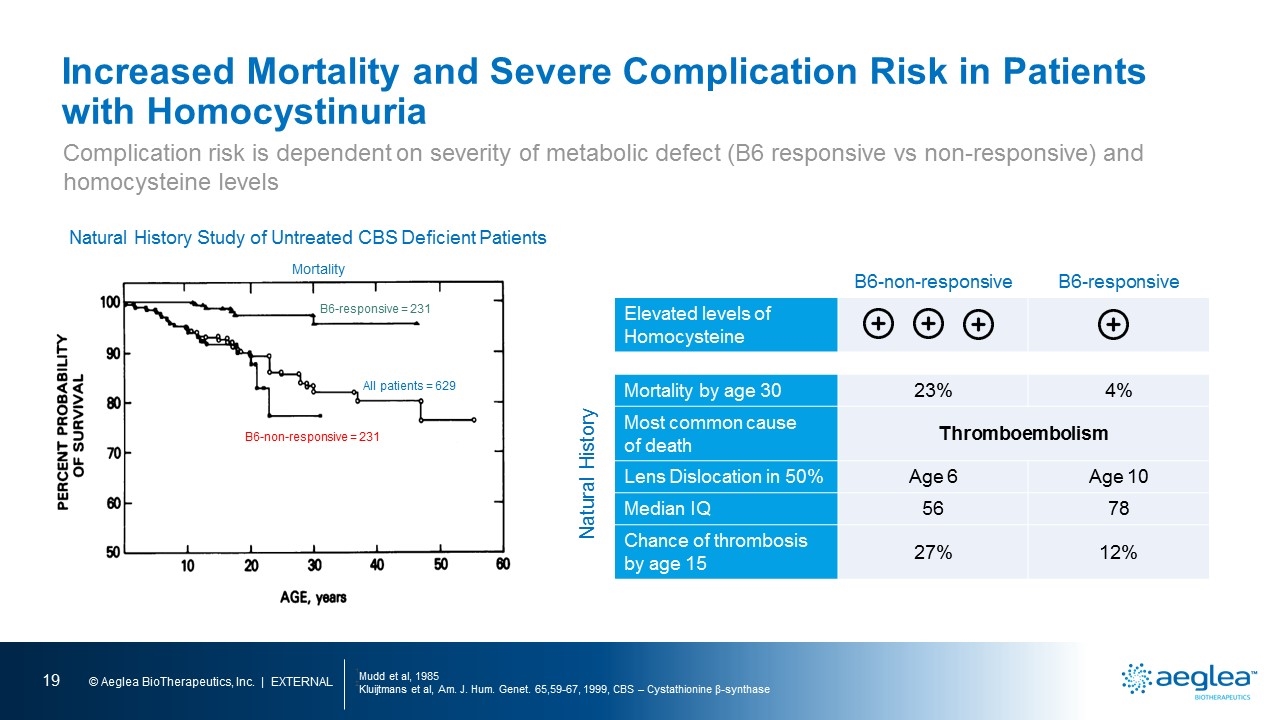
Increased Mortality and Severe Complication Risk in Patients with Homocystinuria 1Mudd et al, 1985 2Kluijtmans et al, Am. J. Hum. Genet. 65,59-67, 1999, CBS – Cystathionine β-synthase Natural History Study of Untreated CBS Deficient Patients Mortality B6-responsive = 231 All patients = 629 Complication risk is dependent on severity of metabolic defect (B6 responsive vs non-responsive) and homocysteine levels B6-non-responsive B6-responsive Elevated levels of Homocysteine Mortality by age 30 23% 4% Most common cause of death Thromboembolism Lens Dislocation in 50% Age 6 Age 10 Median IQ 56 78 Chance of thrombosis by age 15 27% 12% Natural History B6-non-responsive = 231 [line graph] percent probability of survival age, years
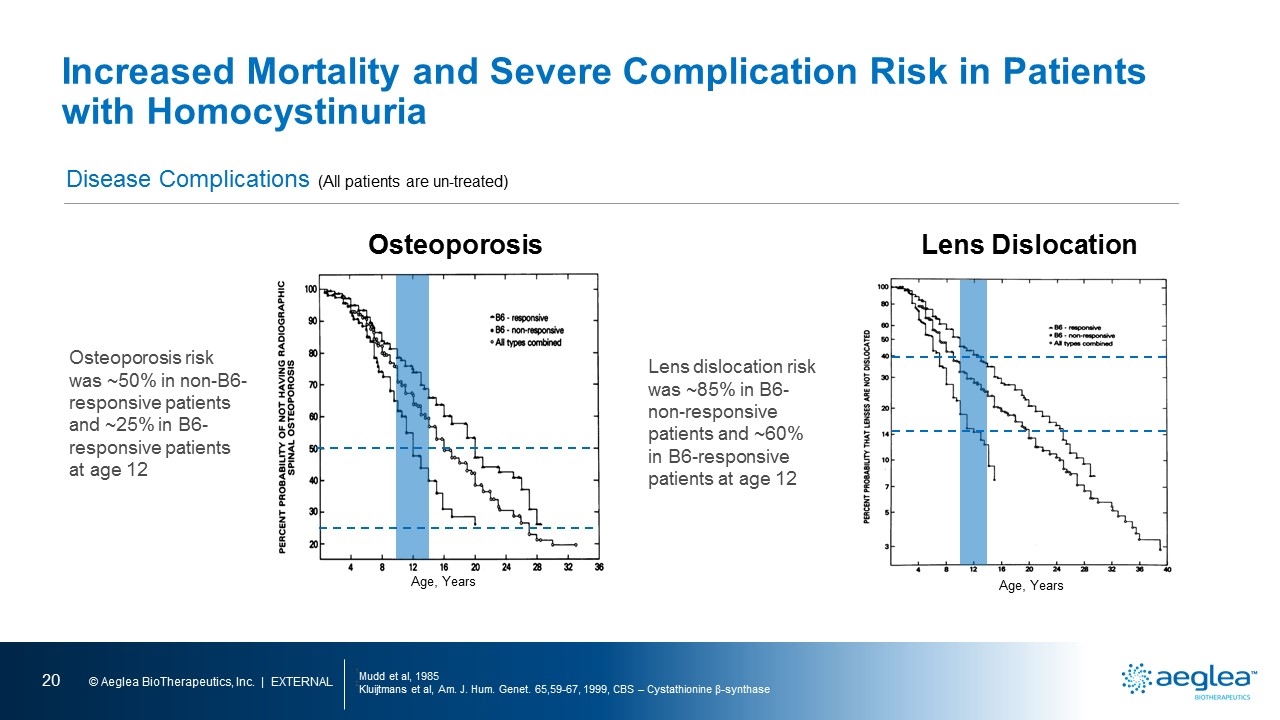
Increased Mortality and Severe Complication Risk in Patients with Homocystinuria 1Mudd et al, 1985 2Kluijtmans et al, Am. J. Hum. Genet. 65,59-67, 1999, CBS – Cystathionine β-synthase Disease Complications (All patients are un-treated) Osteoporosis risk was ~50% in non-B6-responsive patients and ~25% in B6-responsive patients at age 12 Age, Years Lens Dislocation Age, Years Osteoporosis Lens dislocation risk was ~85% in B6-non-responsive patients and ~60% in B6-responsive patients at age 12 Osteoporosis Lens Dislocation percent probability of not having radiographic spinal osteoporosis age, years percent probability that lenses are not dislocated age, years
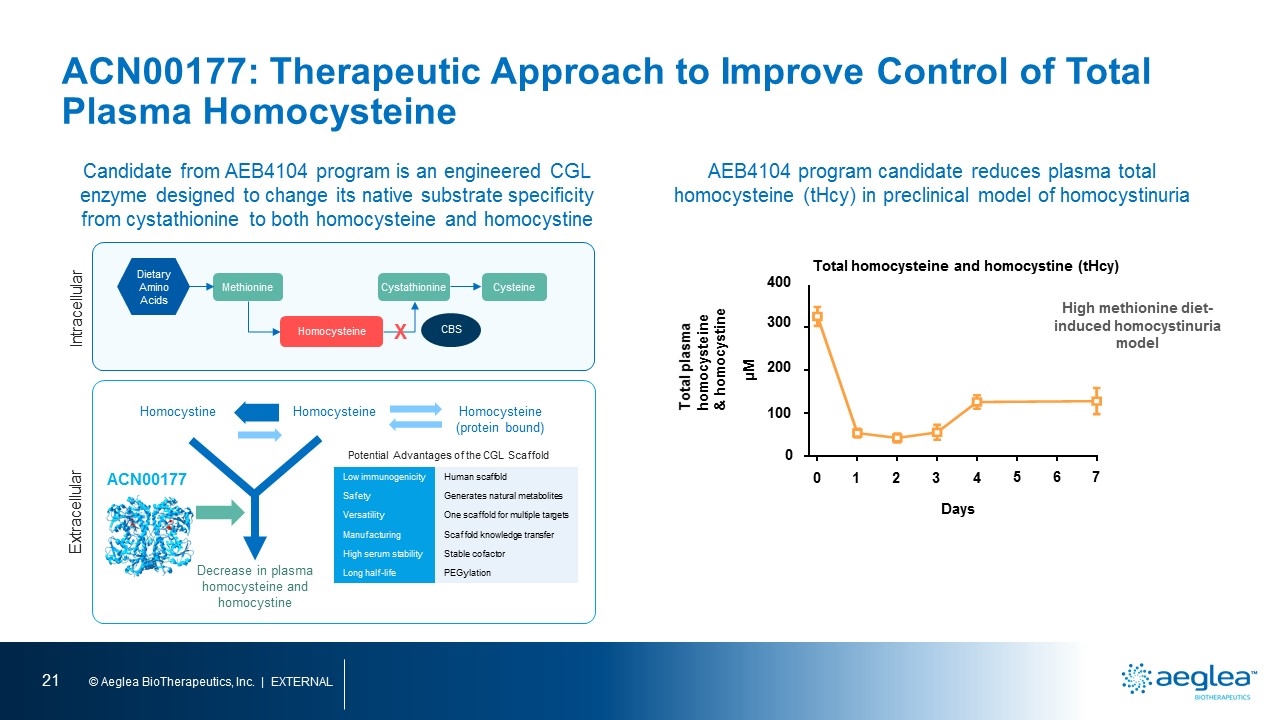
ACN00177: Therapeutic Approach to Improve Control of Total Plasma Homocysteine AEB4104 program candidate reduces plasma total homocysteine (tHcy) in preclinical model of homocystinuria Candidate from AEB4104 program is an engineered CGL enzyme designed to change its native substrate specificity from cystathionine to both homocysteine and homocystine Methionine Homocysteine Cystathionine Cysteine CBS Intracellular Extracellular Homocysteine Homocystine ACN00177 Homocysteine (protein bound) Potential Advantages of the CGL Scaffold Decrease in plasma homocysteine and homocystine Dietary Amino Acids Low immunogenicity Human scaffold Safety Generates natural metabolites Versatility One scaffold for multiple targets Manufacturing Scaffold knowledge transfer High serum stability Stable cofactor Long half-life PEGylation X Total plasma homocysteine & homocystine High methionine diet-induced homocystinuria model Total homocysteine and homocystine (tHcy) µM 400 300 200 100 0 0 1 2 3 4 5 6 7 Days
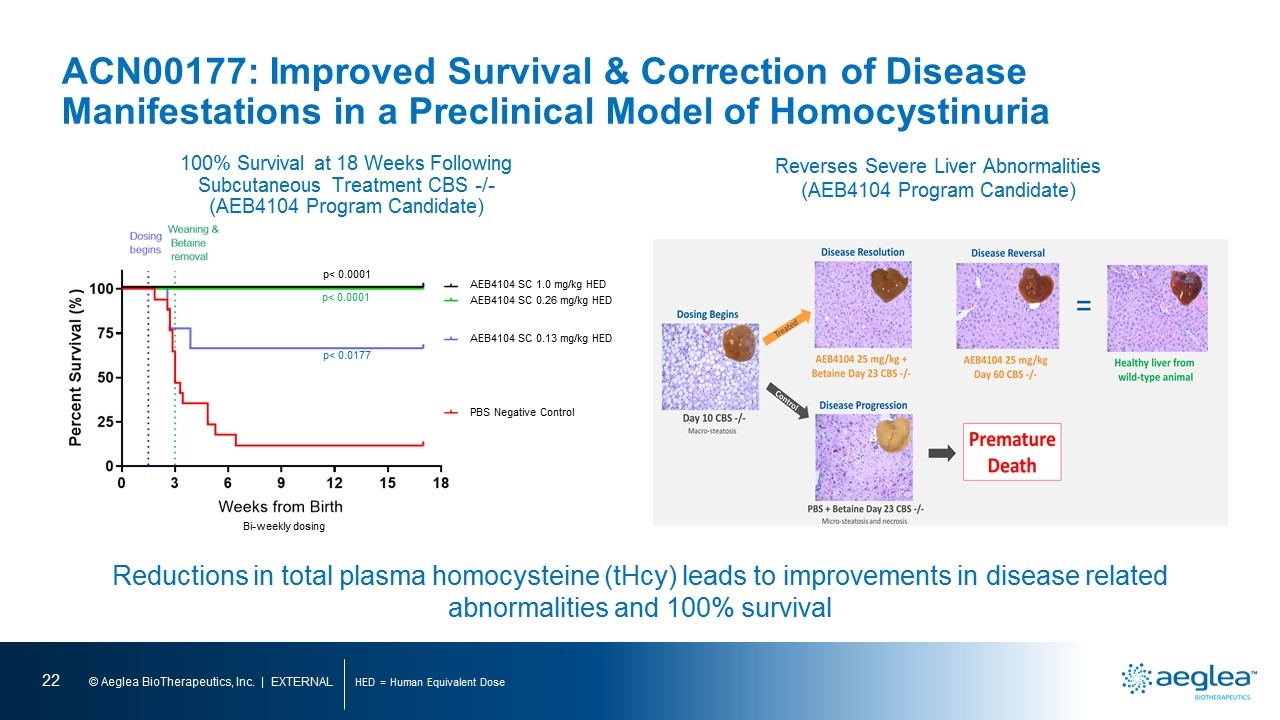
ACN00177: Improved Survival & Correction of Disease Manifestations in a Preclinical Model of Homocystinuria HED = Human Equivalent Dose 100% Survival at 18 Weeks Following Subcutaneous Treatment CBS -/- (AEB4104 Program Candidate) AEB4104 SC 1.0 mg/kg HED AEB4104 SC 0.26 mg/kg HED AEB4104 SC 0.13 mg/kg HED PBS Negative Control p< 0.0001 p< 0.0001 p< 0.0177 Bi-weekly dosing Reductions in total plasma homocysteine (tHcy) leads to improvements in disease related abnormalities and 100% survival Reverses Severe Liver Abnormalities (AEB4104 Program Candidate) percent survival (%) weeks from birth bi-weekly dosing begins day 10 cbs macro-steatosis disease resolution aeb4104 25 mg/kg betaine day 23 cbs disease reversal aeb4104 25 mg/kg day 60 cbs healthy liver from wild type animal disease progression pbs betaine day 23 cbs premature death
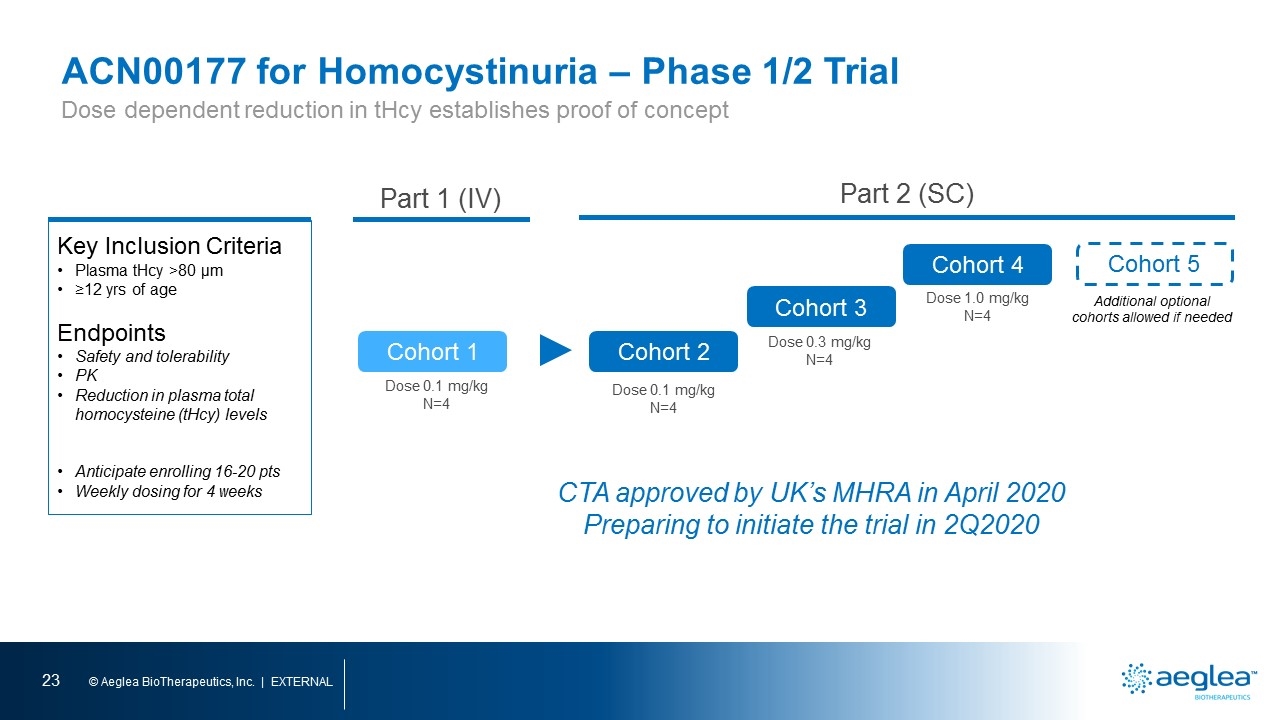
ACN00177 for Homocystinuria – Phase 1/2 Trial Key Inclusion Criteria Plasma tHcy >80 µm ≥12 yrs of age Endpoints Safety and tolerability PK Reduction in plasma total homocysteine (tHcy) levels Anticipate enrolling 16-20 pts Weekly dosing for 4 weeks Cohort 2 Cohort 3 Cohort 4 Part 1 (IV) Part 2 (SC) Dose 0.3 mg/kg N=4 Dose 1.0 mg/kg N=4 Dose 0.1 mg/kg N=4 Cohort 1 Dose 0.1 mg/kg N=4 Cohort 5 Additional optional cohorts allowed if needed CTA approved by UK’s MHRA in April 2020 Preparing to initiate the trial in 2Q2020 Dose dependent reduction in tHcy establishes proof of concept

AEB5100 Program Cystinuria
AEB5100 Program: Cystinuria Unmet Medical Need Very high - no effective therapy for many patients Serious complications and high treatment burden Severe, recurrent flank/abdominal pain and hematuria from an early age Multiple hospitalizations and invasive procedures including surgical interventions Increased risk of hypertension and chronic kidney failure Patient Population (Addressable Markets) >10,000 patients Diagnosis Urine cystine concentration and/or mutation analysis Novel cystine metabolizing enzyme
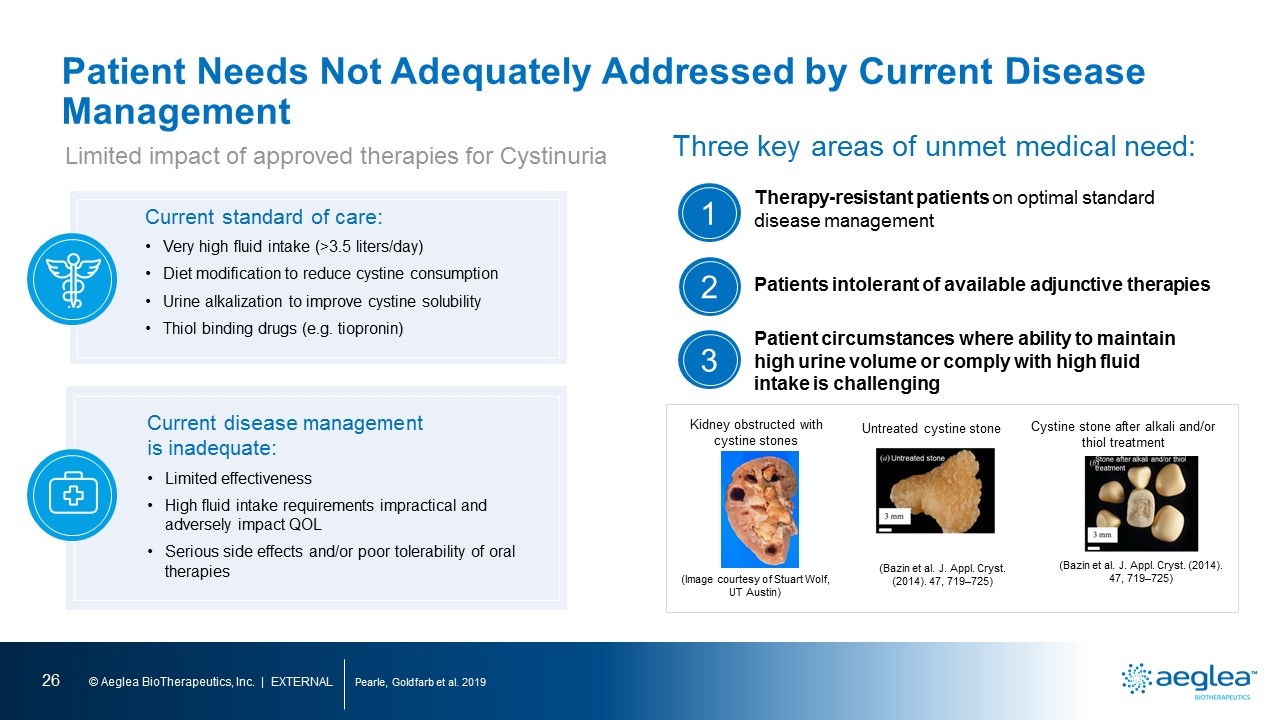
Patient Needs Not Adequately Addressed by Current Disease Management Pearle, Goldfarb et al. 2019 Limited impact of approved therapies for Cystinuria Kidney obstructed with cystine stones Untreated cystine stone Cystine stone after alkali and/or thiol treatment (Image courtesy of Stuart Wolf, UT Austin) (Bazin et al. J. Appl. Cryst. (2014). 47, 719–725) (Bazin et al. J. Appl. Cryst. (2014). 47, 719–725) Current standard of care: Very high fluid intake (>3.5 liters/day) Diet modification to reduce cystine consumption Urine alkalization to improve cystine solubility Thiol binding drugs (e.g. tiopronin) Current disease management is inadequate: Limited effectiveness High fluid intake requirements impractical and adversely impact QOL Serious side effects and/or poor tolerability of oral therapies Therapy-resistant patients on optimal standard disease management Patients intolerant of available adjunctive therapies Patient circumstances where ability to maintain high urine volume or comply with high fluid intake is challenging Three key areas of unmet medical need: 1 2 3
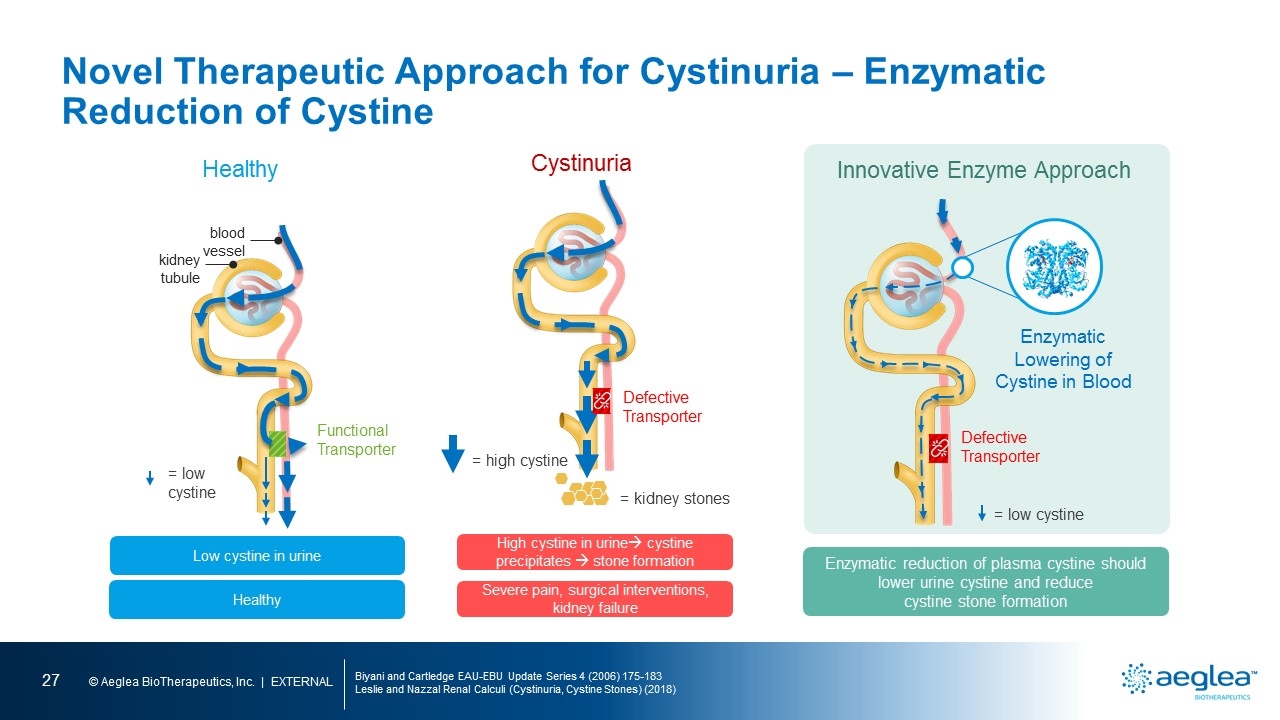
Novel Therapeutic Approach for Cystinuria – Enzymatic Reduction of Cystine Biyani and Cartledge EAU-EBU Update Series 4 (2006) 175-183 Leslie and Nazzal Renal Calculi (Cystinuria, Cystine Stones) (2018) Healthy Low cystine in urine Healthy Functional Transporter kidney tubule blood vessel = low cystine Innovative Enzyme Approach Enzymatic reduction of plasma cystine should lower urine cystine and reduce cystine stone formation Defective Transporter Enzymatic Lowering of Cystine in Blood = low cystine Cystinuria High cystine in urineà cystine precipitates à stone formation Severe pain, surgical interventions, kidney failure Defective Transporter = high cystine = kidney stones

There are no naturally occurring human enzymes that efficiently degrade cystine AEB5100 Program: Novel Human Enzyme Solution for Cystinuria Cramer SL et al, 2017 Nature Medicine 23:120-7 Creating Novel Cystine Degrading Activity1 ¥ total cysteine (µM) normalized to creatinine (mM) * p<0.005 ** p<0.05 Plasma Cystine & Cysteine Urine Cystine & Cysteine Protein Engineering Proof of Concept Determination in Cystinuria Disease Model Slc3a1 -/- mouse urine cystine crystals Before Dosing After Dosing increase catalytic activity increase serum stability improve manufacturability
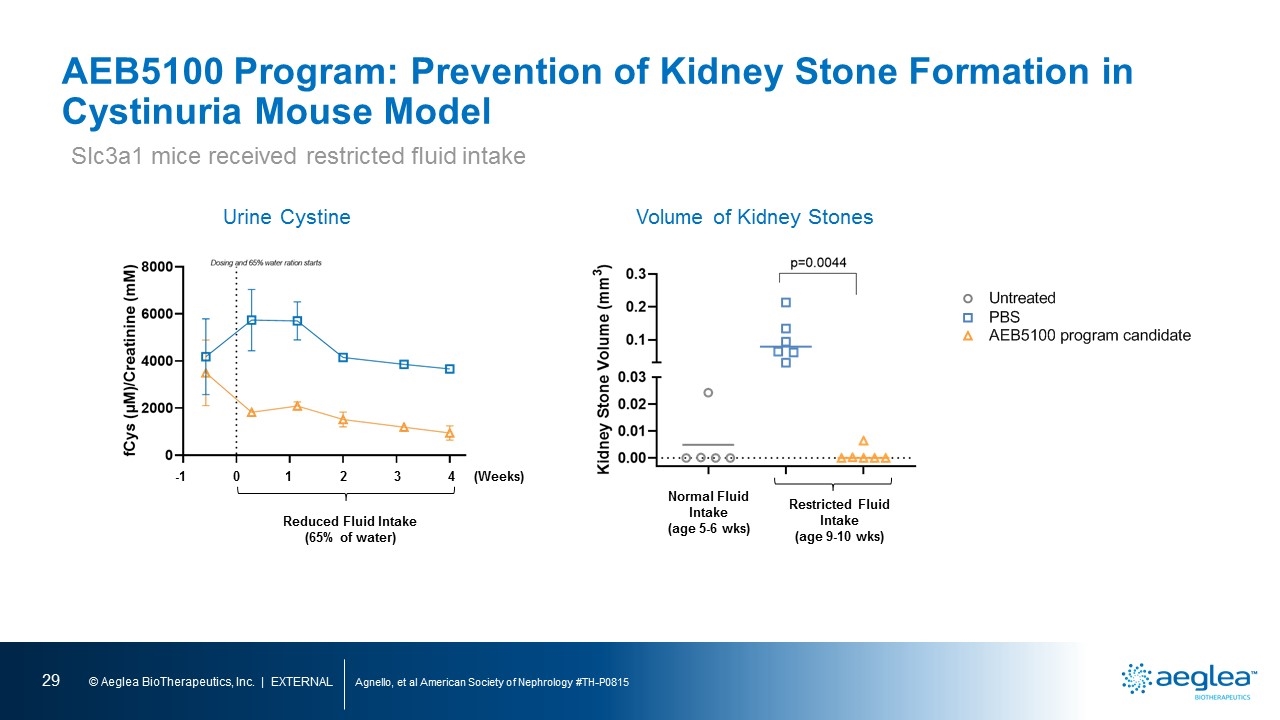
AEB5100 Program: Prevention of Kidney Stone Formation in Cystinuria Mouse Model Agnello, et al American Society of Nephrology #TH-P0815 Volume of Kidney Stones Slc3a1 mice received restricted fluid intake Urine Cystine 0 1 2 3 4 -1 (Weeks) Reduced Fluid Intake (65% of water) Normal Fluid Intake (age 5-6 wks) Restricted Fluid Intake (age 9-10 wks)
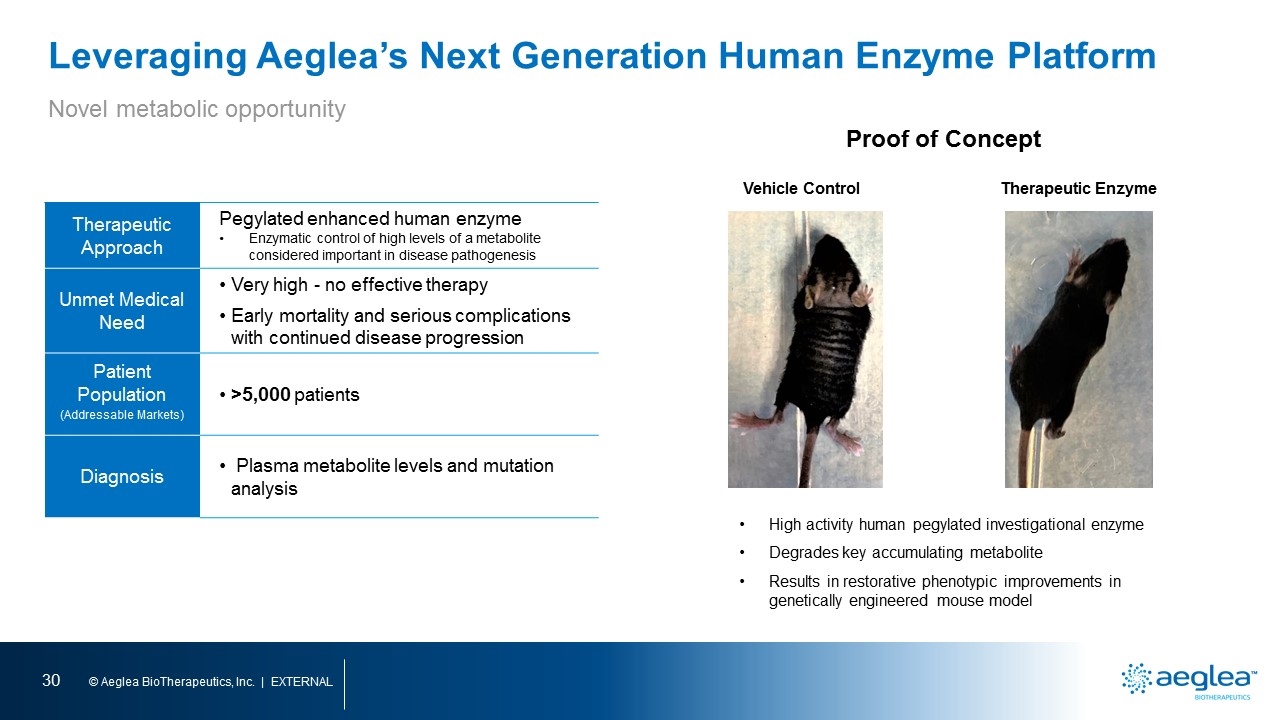
Leveraging Aeglea’s Next Generation Human Enzyme Platform Therapeutic Approach Pegylated enhanced human enzyme Enzymatic control of high levels of a metabolite considered important in disease pathogenesis Unmet Medical Need Very high - no effective therapy Early mortality and serious complications with continued disease progression Patient Population (Addressable Markets) >5,000 patients Diagnosis Plasma metabolite levels and mutation analysis Vehicle Control Therapeutic Enzyme High activity human pegylated investigational enzyme Degrades key accumulating metabolite Results in restorative phenotypic improvements in genetically engineered mouse model Proof of Concept Novel metabolic opportunity
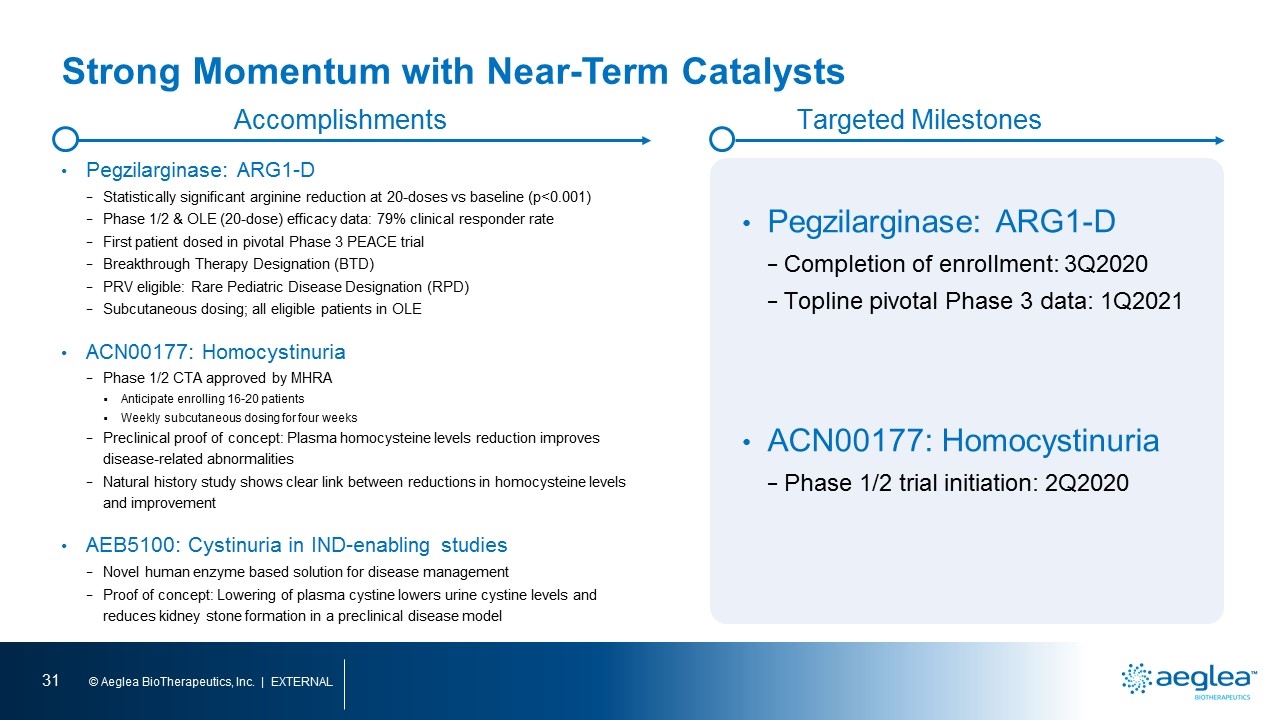
Strong Momentum with Near-Term Catalysts Pegzilarginase: ARG1-D Statistically significant arginine reduction at 20-doses vs baseline (p<0.001) Phase 1/2 & OLE (20-dose) efficacy data: 79% clinical responder rate First patient dosed in pivotal Phase 3 PEACE trial Breakthrough Therapy Designation (BTD) PRV eligible: Rare Pediatric Disease Designation (RPD) Subcutaneous dosing; all eligible patients in OLE ACN00177: Homocystinuria Phase 1/2 CTA approved by MHRA Anticipate enrolling 16-20 patients Weekly subcutaneous dosing for four weeks Preclinical proof of concept: Plasma homocysteine levels reduction improves disease-related abnormalities Natural history study shows clear link between reductions in homocysteine levels and improvement AEB5100: Cystinuria in IND-enabling studies Novel human enzyme based solution for disease management Proof of concept: Lowering of plasma cystine lowers urine cystine levels and reduces kidney stone formation in a preclinical disease model Accomplishments Targeted Milestones Pegzilarginase: ARG1-D Completion of enrollment: 3Q2020 Topline pivotal Phase 3 data: 1Q2021 ACN00177: Homocystinuria Phase 1/2 trial initiation: 2Q2020
Homocystinuria Metabolism Nephrology Cystinuria Pegzilarginase Pivotal Phase 3 ACN00177 AEB5100 Arginase 1 Deficiency A Disruptive Platform Producing Next-Generation Human Enzyme Solutions Lead assets in three areas of high unmet medical need Looking beyond the conventional, redefining the potential of human enzymes to deliver disruptive solutions Driven by the urgent needs of the communities we serve Pursuing our vision to become the premier human enzyme company Changing the lives of patients and their families now and for the future
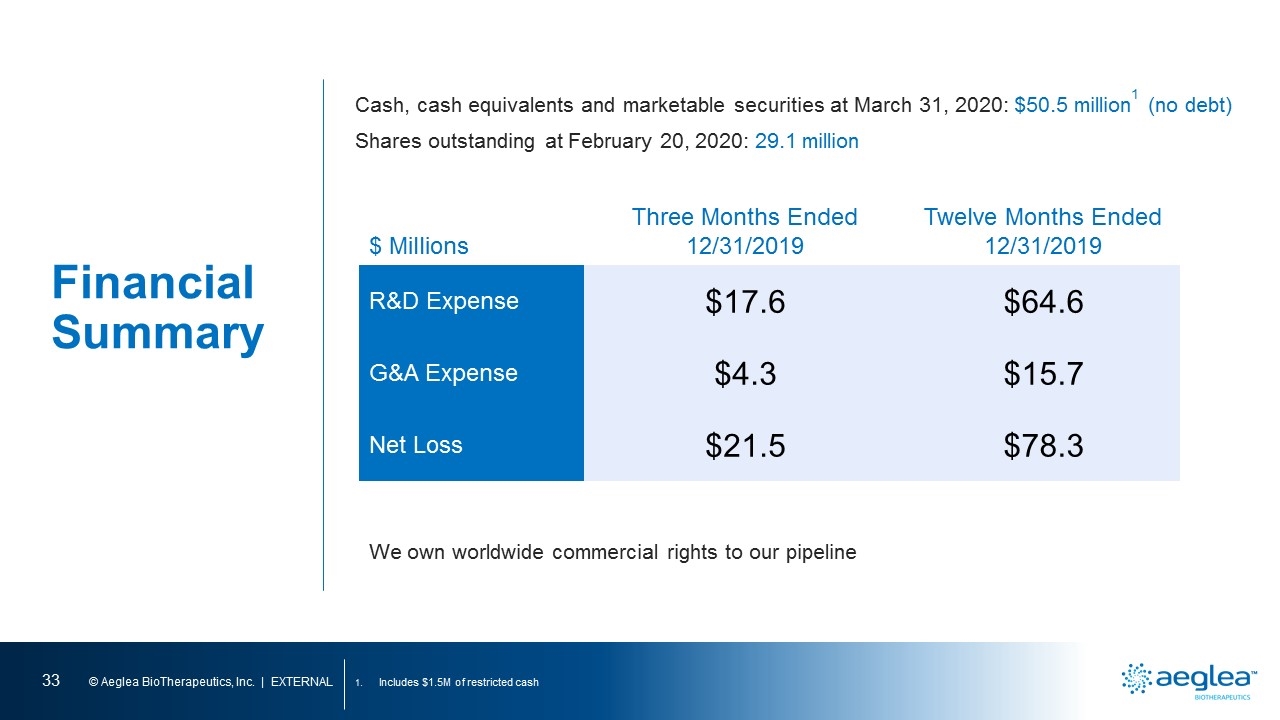
Financial Summary Includes $1.5M of restricted cash $ Millions Three Months Ended 12/31/2019 Twelve Months Ended 12/31/2019 R&D Expense $17.6 $64.6 G&A Expense $4.3 $15.7 Net Loss $21.5 $78.3 Cash, cash equivalents and marketable securities at March 31, 2020: $50.5 million1 (no debt) Shares outstanding at February 20, 2020: 29.1 million We own worldwide commercial rights to our pipeline

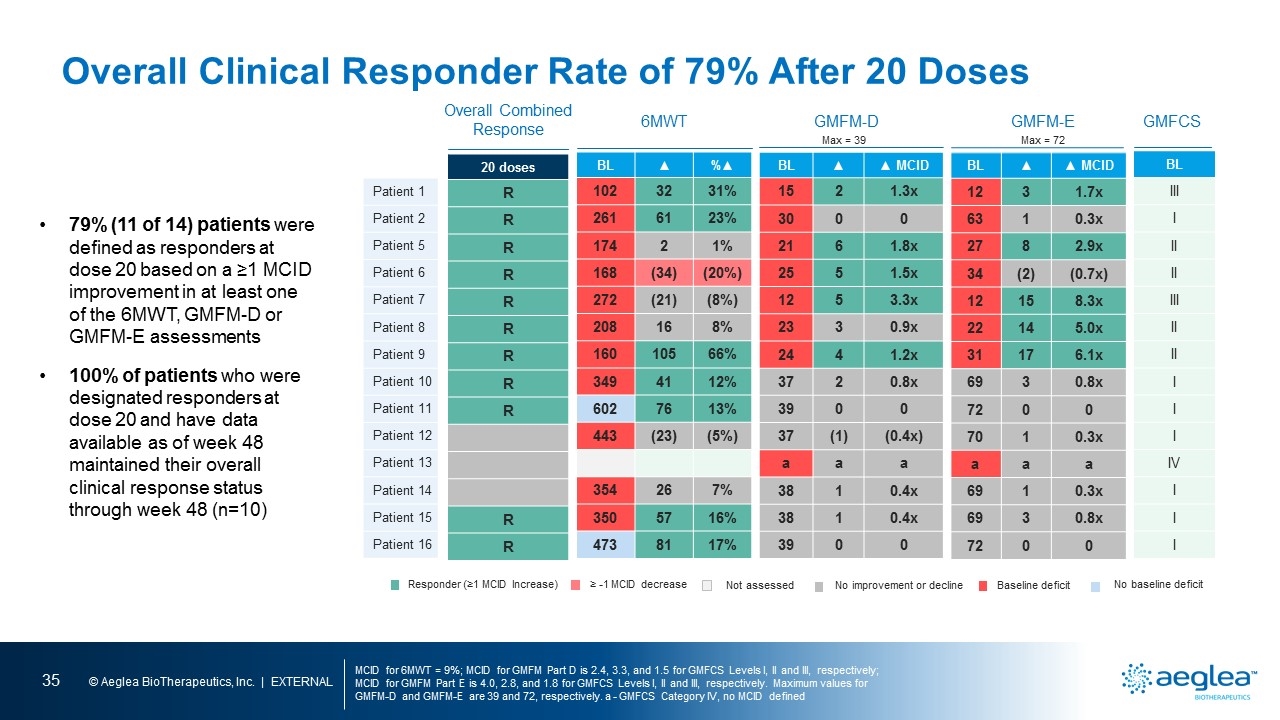
Overall Clinical Responder Rate of 79% After 20 Doses MCID for 6MWT = 9%; MCID for GMFM Part D is 2.4, 3.3, and 1.5 for GMFCS Levels I, II and III, respectively; MCID for GMFM Part E is 4.0, 2.8, and 1.8 for GMFCS Levels I, II and III, respectively. Maximum values for GMFM-D and GMFM-E are 39 and 72, respectively. a - GMFCS Category IV, no MCID defined Patient 1 Patient 2 Patient 5 Patient 6 Patient 7 Patient 8 Patient 9 Patient 10 Patient 11 Patient 12 Patient 13 Patient 14 Patient 15 Patient 16 Overall Combined Response BL ▲ %▲ 102 32 31% 261 61 23% 174 2 1% 168 (34) (20%) 272 (21) (8%) 208 16 8% 160 105 66% 349 41 12% 602 76 13% 443 (23) (5%) 354 26 7% 350 57 16% 473 81 17% BL ▲ ▲ MCID 15 2 1.3x 30 0 0 21 6 1.8x 25 5 1.5x 12 5 3.3x 23 3 0.9x 24 4 1.2x 37 2 0.8x 39 0 0 37 (1) (0.4x) a a a 38 1 0.4x 38 1 0.4x 39 0 0 20 doses R R R R R R R R R R R BL III I II II III II II I I I IV I I I Baseline deficit No baseline deficit Not assessed No improvement or decline Responder (≥1 MCID Increase) ≥ -1 MCID decrease 6MWT BL ▲ ▲ MCID 12 3 1.7x 63 1 0.3x 27 8 2.9x 34 (2) (0.7x) 12 15 8.3x 22 14 5.0x 31 17 6.1x 69 3 0.8x 72 0 0 70 1 0.3x a a a 69 1 0.3x 69 3 0.8x 72 0 0 GMFM-D GMFM-E GMFCS Max = 39 Max = 72 79% (11 of 14) patients were defined as responders at dose 20 based on a ≥1 MCID improvement in at least one of the 6MWT, GMFM-D or GMFM-E assessments 100% of patients who were designated responders at dose 20 and have data available as of week 48 maintained their overall clinical response status through week 48 (n=10)
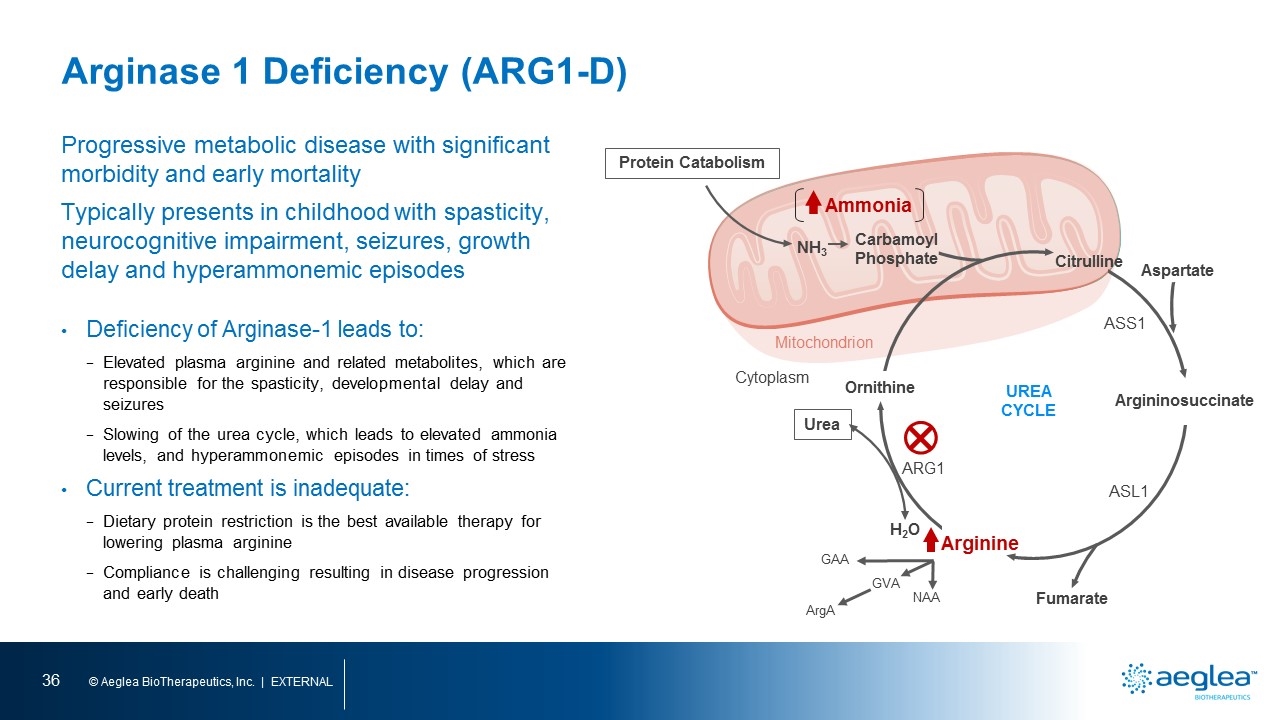
Arginase 1 Deficiency (ARG1-D) Progressive metabolic disease with significant morbidity and early mortality Typically presents in childhood with spasticity, neurocognitive impairment, seizures, growth delay and hyperammonemic episodes Deficiency of Arginase-1 leads to: Elevated plasma arginine and related metabolites, which are responsible for the spasticity, developmental delay and seizures Slowing of the urea cycle, which leads to elevated ammonia levels, and hyperammonemic episodes in times of stress Current treatment is inadequate: Dietary protein restriction is the best available therapy for lowering plasma arginine Compliance is challenging resulting in disease progression and early death Ammonia NH3 Carbamoyl Phosphate Mitochondrion Citrulline Argininosuccinate Fumarate Arginine H2O Urea Ornithine UREA CYCLE ARG1 ASL1 ASS1 Cytoplasm Protein Catabolism Aspartate GAA GVA ArgA NAA
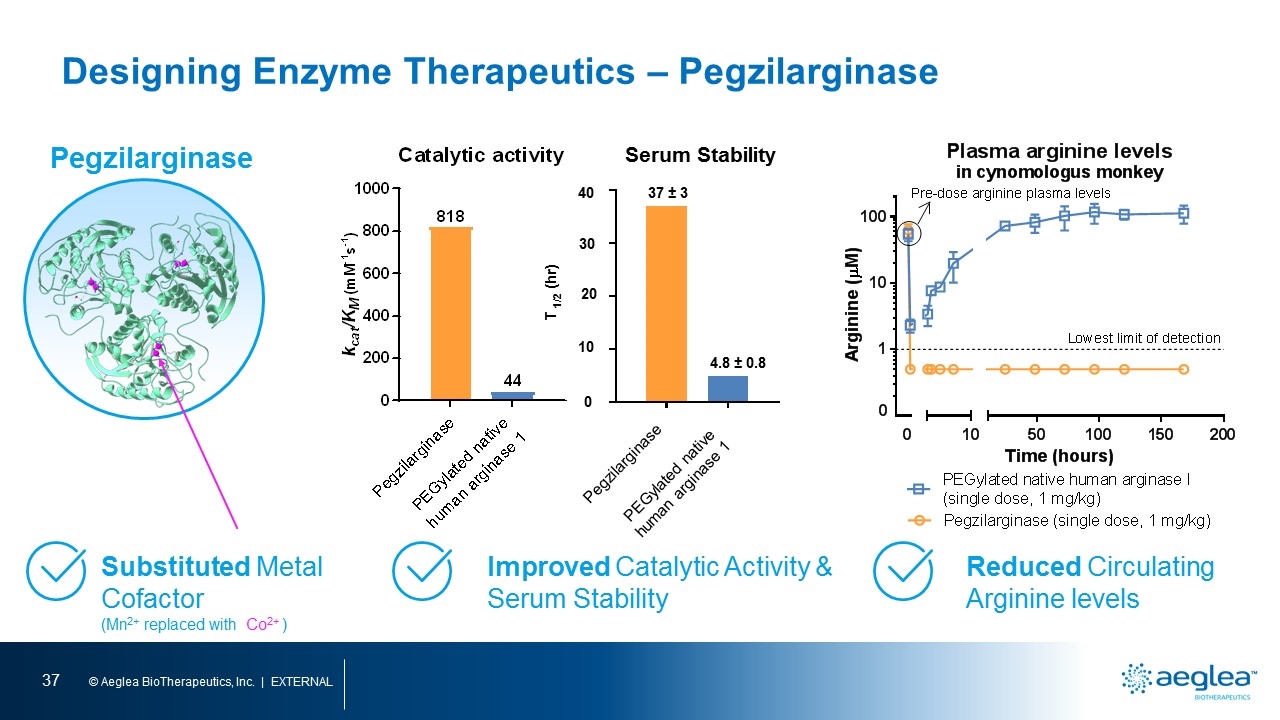
Designing Enzyme Therapeutics – Pegzilarginase Improved Catalytic Activity & Serum Stability Reduced Circulating Arginine levels Pegzilarginase Substituted Metal Cofactor (Mn2+ replaced with Co2+ ) Serum Stability 37 ± 3 4.8 ± 0.8 Pegzilarginase PEGylated native human arginase 1 T 1/2 (hr) 40 30 20 10 0
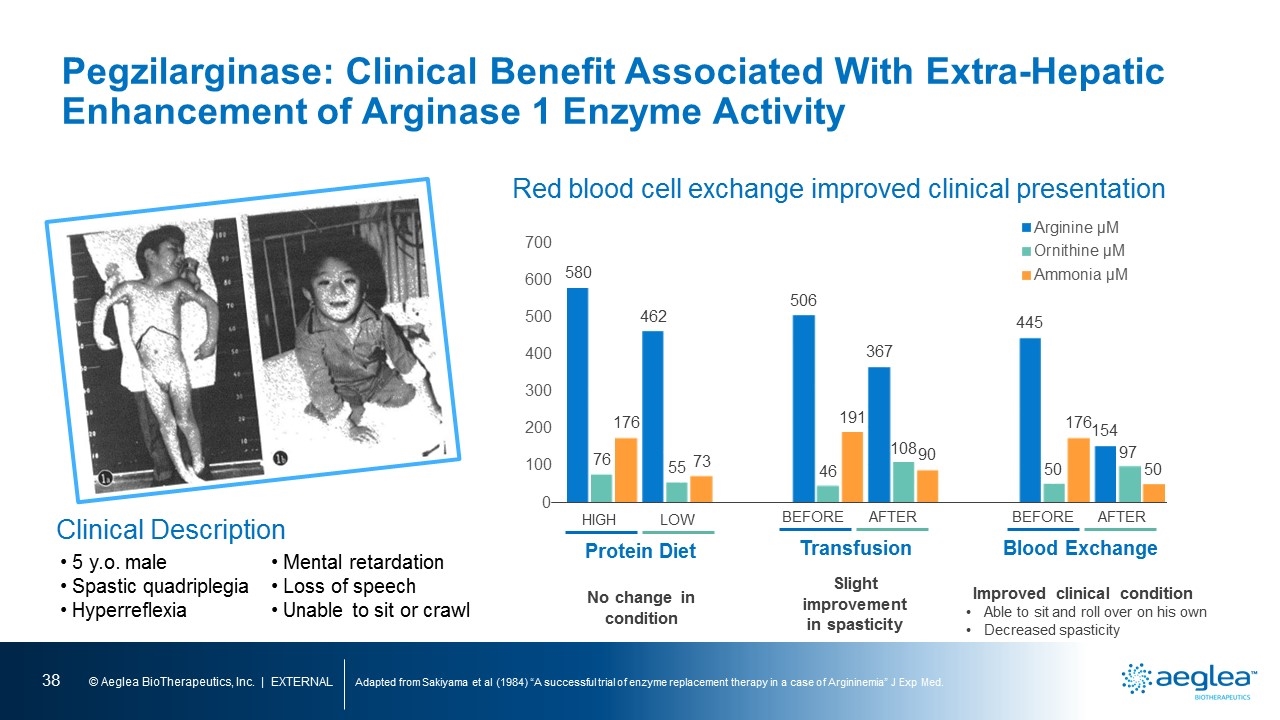
Pegzilarginase: Clinical Benefit Associated With Extra-Hepatic Enhancement of Arginase 1 Enzyme Activity Adapted from Sakiyama et al (1984) “A successful trial of enzyme replacement therapy in a case of Argininemia” J Exp Med. Red blood cell exchange improved clinical presentation Improved clinical condition Able to sit and roll over on his own Decreased spasticity Blood Exchange BEFORE AFTER No change in condition Protein Diet HIGH LOW Slight improvement in spasticity Transfusion BEFORE AFTER Clinical Description 5 y.o. male Spastic quadriplegia Hyperreflexia Mental retardation Loss of speech Unable to sit or crawl [bar graph] [bar graph] [bar graph]
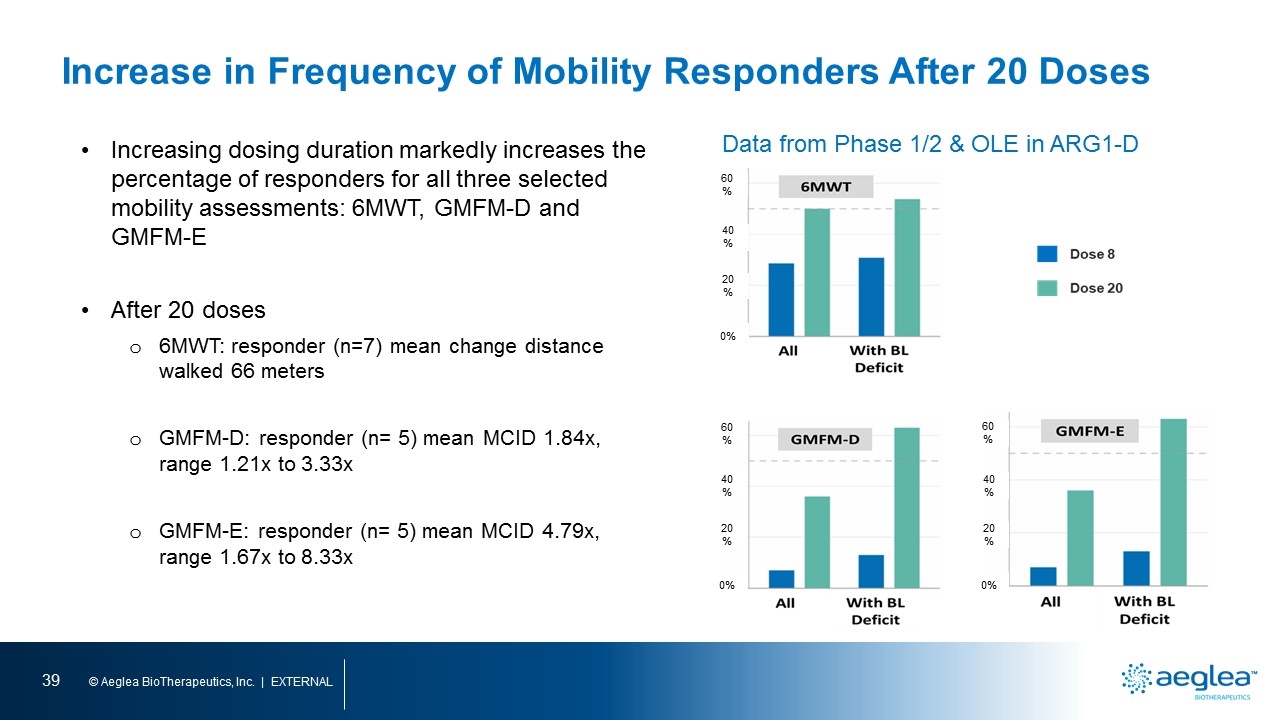
Increasing dosing duration markedly increases the percentage of responders for all three selected mobility assessments: 6MWT, GMFM-D and GMFM-E After 20 doses 6MWT: responder (n=7) mean change distance walked 66 meters GMFM-D: responder (n= 5) mean MCID 1.84x, range 1.21x to 3.33x GMFM-E: responder (n= 5) mean MCID 4.79x, range 1.67x to 8.33x 60% 40% 20% 0% 60% 40% 20% 0% 60% 40% 20% 0% Data from Phase 1/2 & OLE in ARG1-D Increase in Frequency of Mobility Responders After 20 Doses [bar graph] [bar graph] [bar graph]







































