
Reimagining The Potential Of Human Enzyme Therapeutics Corporate Overview – September 2021 Exhibit 99.1 Company Logo

Forward Looking Statements ©2021 Aeglea BioTherapeutics. External 2 This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our current and future financial performance, business plans and objectives, current and future clinical and preclinical development activities, timing and success of our ongoing and planned clinical trials and related data, the timing of announcements, updates and results of our clinical trials and related data, our ability to obtain and maintain regulatory approval, the potential therapeutic benefits and economic value of our lead product candidate and our other product candidates, potential growth opportunities, financing plans, use and adequacy of financing plans, competitive position, industry environment and potential market opportunities. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors including, but not limited to, those related to the success, cost and timing of our product candidate development activities and ongoing and planned clinical trials; our plans to develop and commercialize targeted therapeutics, including our lead product candidate pegzilarginase and our other product candidates for the treatment of homocystinuria and cystinuria; the design, progress of patient enrollment and dosing in our clinical trials, the ability of our product candidates to achieve applicable endpoints in clinical trials, the safety profile of our product candidates in clinical trials, the potential for data from our current and future clinical trials to support a marketing application, as well as the timing of these events, the potential for preclinical studies to be predictive of current or future clinical trials, our ability to obtain funding for our operations, development and commercialization of our product candidates; the impact of the COVID-19 pandemic on our operations and clinical development activities, including on the timing of enrollment of our clinical trials; the timing of and our ability to obtain and maintain regulatory approvals; our ability to obtain regulatory approval for, and commercialize, pegzilarginase, and recognize milestone and royalty payments from our agreement with Immedica; the potential for expeditated development and review of pegzilarginase as of a result of its Breakthrough Therapy designation; the potential addressable markets of the our product candidates; the rate and degree of market acceptance and clinical utility of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; our commercialization, marketing and manufacturing capabilities and strategy; future agreements with third parties in connection with the commercialization of our product candidates; our expectations regarding our ability to obtain and maintain intellectual property protection; our dependence on third party manufacturers; our ability to develop our own commercial manufacturing facility; the success of competing therapies that are or may become available; our ability to attract and retain key scientific or management personnel; our ability to identify additional product candidates with significant commercial potential consistent with our commercial objectives; and our estimates regarding expenses, future revenue, capital requirements and needs for additional financing. Moreover, we operate in a very competitive and rapidly changing environment, and new risks may emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed herein may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Further information on these and other factors that could affect these forward-looking statements is contained in our most recent Quarterly Report on Form 10-Q filed with the U.S. Securities and Exchange Commission (SEC) and other reports filed by the SEC. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. We undertake no obligation to publicly update any forward-looking statements, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Company Logo

Addressing Metabolic Imbalances to Improve the Lives of Patients ©2021 Aeglea BioTherapeutics. External 3 Delivering Metabolic Harmony Aeglea BioTherapeutics (Nasdaq: AGLE) At Aeglea, we believe that every patient deserves a chance at a better life. We are committed to helping people with rare and devastating metabolic diseases who have limited treatment options because having a rare disease doesn’t mean that you are in the fight alone. Company Logo

Unlocking the Potential of Human Enzymes to Deliver Life-Changing Solutions for People with Rare Metabolic Diseases ©2021 Aeglea BioTherapeutics. External 4 PEGZILARGINASE IN ARGINASE 1 DEFICIENCY AGLE-177 IN HOMOCYSTINURIA ENZYME THERAPEUTICS PLATFORM Enhanced human arginase that enzymatically lowers arginine levels PEACE – pivotal Phase 3 trial Novel recombinant human enzyme that enzymatically lowers homocysteine levels Phase 1/2 trial Rare metabolic disease monogenic focus Most advanced program in Cystinuria Cash, cash equivalents and marketable securities as of June 30, 2021: $130.41 million (no debt) Expected funding runway: into 2023 1Includes $1.8mm of restricted cash
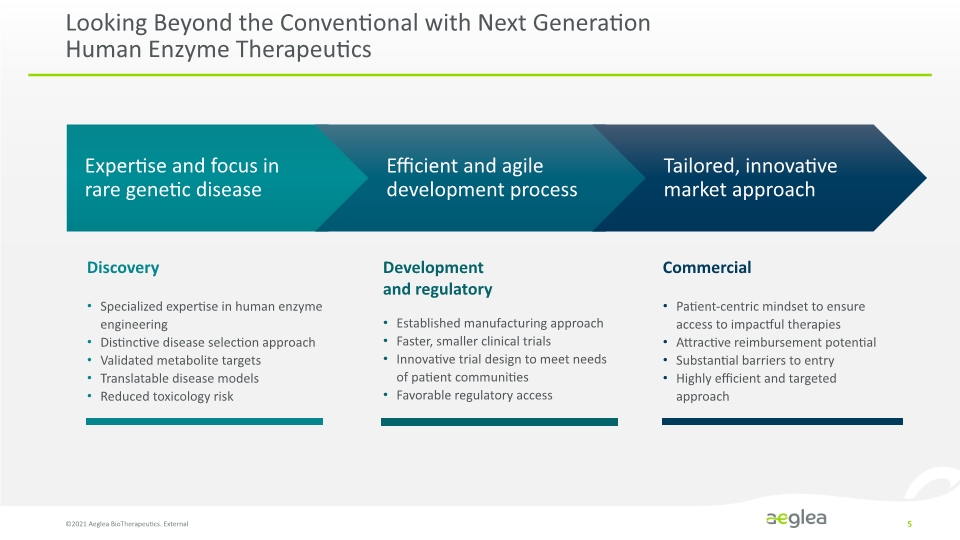
Looking Beyond the Conventional with Next Generation Human Enzyme Therapeutics ©2021 Aeglea BioTherapeutics. External 5 Specialized expertise in human enzyme engineering Distinctive disease selection approach Validated metabolite targets Translatable disease models Reduced toxicology risk Established manufacturing approach Faster, smaller clinical trials Innovative trial design to meet needs of patient communities Favorable regulatory access Patient-centric mindset to ensure access to impactful therapies Attractive reimbursement potential Substantial barriers to entry Highly efficient and targeted approach Discovery Development and regulatory Commercial Expertise and focus in rare genetic disease Efficient and agile development process Tailored, innovative market approach Company Logo
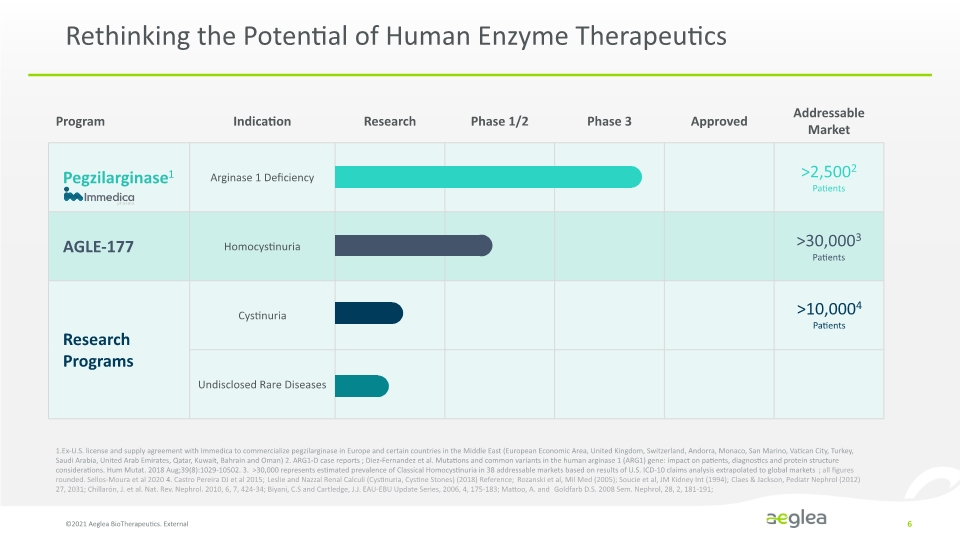
Rethinking the Potential of Human Enzyme Therapeutics ©2021 Aeglea BioTherapeutics. External 6 1.Ex-U.S. license and supply agreement with Immedica to commercialize pegzilarginase in Europe and certain countries in the Middle East (European Economic Area, United Kingdom, Switzerland, Andorra, Monaco, San Marino, Vatican City, Turkey, Saudi Arabia, United Arab Emirates, Qatar, Kuwait, Bahrain and Oman) 2. ARG1-D case reports ; Diez-Fernandez et al. Mutations and common variants in the human arginase 1 (ARG1) gene: impact on patients, diagnostics and protein structure considerations. Hum Mutat. 2018 Aug;39(8):1029-10502. 3. >30,000 represents estimated prevalence of Classical Homocystinuria in 38 addressable markets based on results of U.S. ICD-10 claims analysis extrapolated to global markets; all figures rounded. Sellos-Moura et al 2020 4. Castro Pereira DJ et al 2015; Leslie and Nazzal Renal Calculi (Cystinuria, Cystine Stones) (2018) Reference; Rozanski et al, Mil Med (2005); Soucie et al, JM Kidney Int (1994); Claes & Jackson, Pediatr Nephrol (2012) 27, 2031; Chillarón, J. et al. Nat. Rev. Nephrol. 2010, 6, 7, 424-34; Biyani, C.S and Cartledge, J.J. EAU-EBU Update Series, 2006, 4, 175-183; Mattoo, A. and Goldfarb D.S. 2008 Sem. Nephrol, 28, 2, 181-191; >10,0004 Patients Program Indication Research Phase 1/2 Phase 3 Approved Addressable Market Pegzilarginase1 Immedica Arginase 1 Deficiency >2,5002 Patients AGLE-177 Homocystinuria >30,0003 Patients Research Programs Cystinuria Undisclosed Rare Diseases Company Logo

Pegzilarginase for Arginase 1 Deficiency
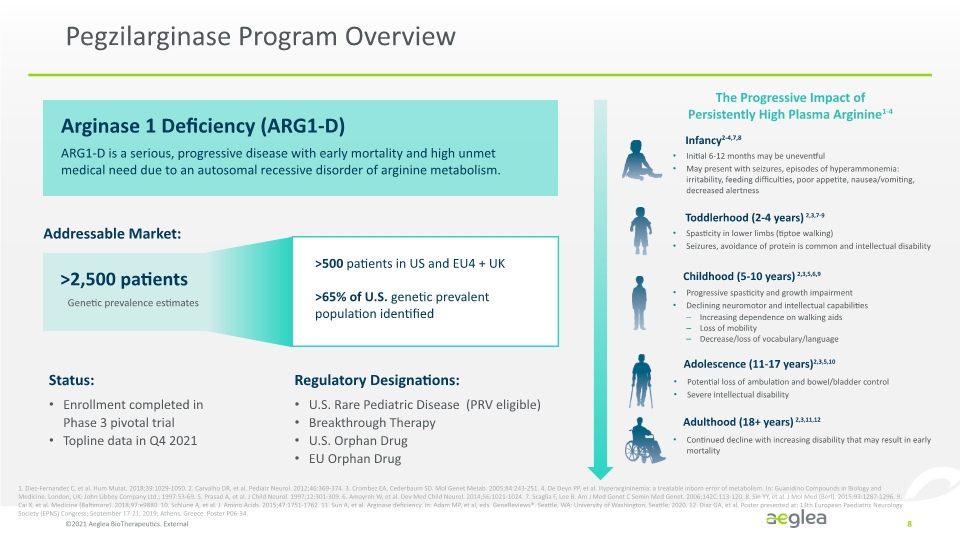
Pegzilarginase Program Overview ©2021 Aeglea BioTherapeutics. External 8 Arginase 1 Deficiency (ARG1-D) ARG1-D is a serious, progressive disease with early mortality and high unmet medical need due to an autosomal recessive disorder of arginine metabolism. Addressable Market: >2,500 patients Genetic prevalence estimates U.S. Rare Pediatric Disease (PRV eligible) Breakthrough Therapy U.S. Orphan Drug EU Orphan Drug Regulatory Designations: The Progressive Impact of Persistently High Plasma Arginine1-4 Infancy2-4,7,8 Initial 6-12 months may be uneventful May present with seizures, episodes of hyperammonemia: irritability, feeding difficulties, poor appetite, nausea/vomiting, decreased alertness >500 patients in US and EU4 + UK >65% of U.S. genetic prevalent population identified 1. Diez-Fernandez C, et al. Hum Mutat. 2018;39:1029-1050. 2. Carvalho DR, et al. Pediatr Neurol. 2012;46:369-374. 3. Crombez EA, Cederbaum SD. Mol Genet Metab. 2005;84:243-251. 4. De Deyn PP, et al. Hyperargininemia: a treatable inborn error of metabolism. In: Guanidino Compounds in Biology and Medicine. London, UK: John Libbey Company Ltd.; 1997:53-69. 5. Prasad A, et al. J Child Neurol. 1997;12:301-309. 6. Amayreh W, et al. Dev Med Child Neurol. 2014;56:1021-1024. 7. Scaglia F, Lee B. Am J Med Genet C Semin Med Genet. 2006;142C:113-120. 8. Sin YY, et al. J Mol Med (Berl). 2015;93:1287-1296. 9. Cai X, et al. Medicine (Baltimore). 2018;97:e9880. 10. Schlune A, et al. J. Amino Acids. 2015;47:1751-1762. 11. Sun A, et al. Arginase deficiency. In: Adam MP, et al, eds. GeneReviews®. Seattle, WA: University of Washington, Seattle; 2020. 12. Diaz GA, et al. Poster presented at: 13th European Paediatric Neurology Society (EPNS) Congress; September 17-21, 2019; Athens, Greece. Poster P06-34. Toddlerhood (2-4 years) 2,3,7-9 Spasticity in lower limbs (tiptoe walking) Seizures, avoidance of protein is common and intellectual disability Childhood (5-10 years) 2,3,5,6,9 Progressive spasticity and growth impairment Declining neuromotor and intellectual capabilities Increasing dependence on walking aids Loss of mobility Decrease/loss of vocabulary/language Adolescence (11-17 years)2,3,5,10 Potential loss of ambulation and bowel/bladder control Severe intellectual disability Adulthood (18+ years) 2,3,11,12 Continued decline with increasing disability that may result in early mortality Enrollment completed in Phase 3 pivotal trial Topline data in Q4 2021 Status: Company Logo

KEY AREAS: Arginase 1 Deficiency Patient Needs Not Adequately Addressed No treatments or approved therapies that effectively reduce arginine levels ©2021 Aeglea BioTherapeutics. External 9 Current standard of care: Severe dietary protein restriction Amino acid supplementation Ammonia scavengers Current disease management is inadequate: Limited effectiveness Challenging to maintain Doesn’t address non-dietary sources of arginine Delayed and un/misdiagnosed patients due to lack of disease awareness Patients not reaching target arginine levels with their current disease management strategy Ineffective treatment options result in disease progression and poor outcomes 1. 2. 3. Company Logo
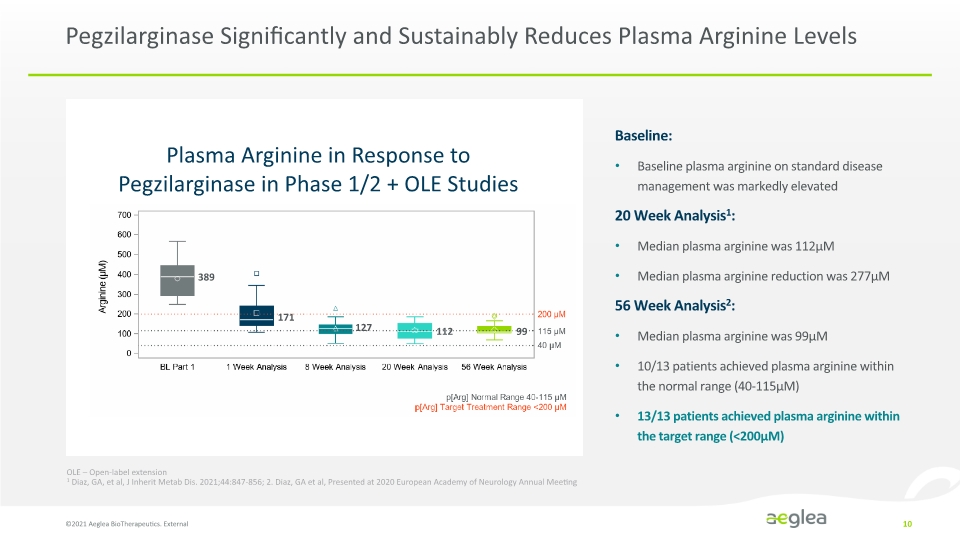
Pegzilarginase Significantly and Sustainably Reduces Plasma Arginine Levels ©2021 Aeglea BioTherapeutics. External 10 Plasma Arginine in Response to Pegzilarginase in Phase 1/2 + OLE Studies Baseline: Baseline plasma arginine on standard disease management was markedly elevated 20 Week Analysis1: Median plasma arginine was 112µM Median plasma arginine reduction was 277µM 56 Week Analysis2: Median plasma arginine was 99µM 10/13 patients achieved plasma arginine within the normal range (40-115µM) 13/13 patients achieved plasma arginine within the target range (<200µM) OLE – Open-label extension 1 Diaz, GA, et al, J Inherit Metab Dis. 2021;44:847-856; 2. Diaz, GA et al, Presented at 2020 European Academy of Neurology Annual Meeting 389 171 127 112 99 Line Chart Company Logo
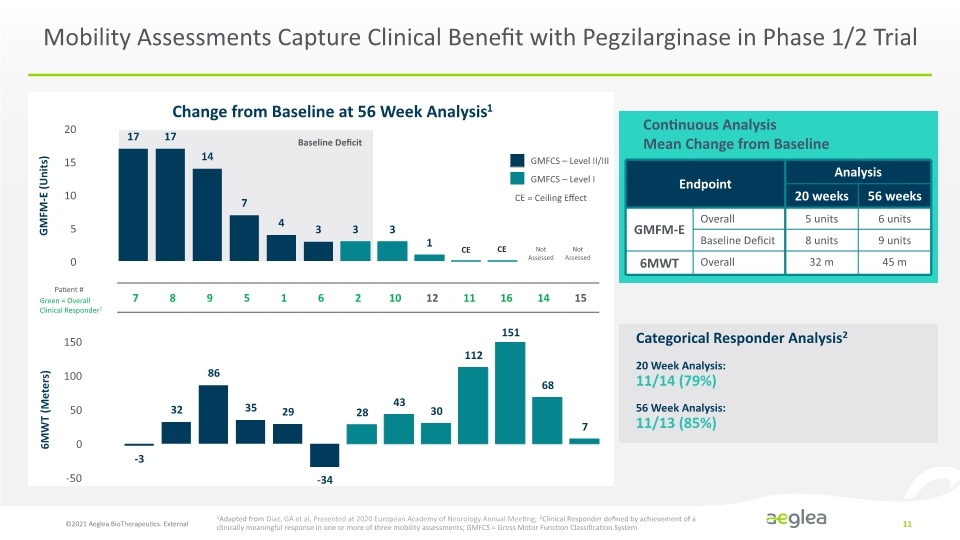
Mobility Assessments Capture Clinical Benefit with Pegzilarginase in Phase 1/2 Trial ©2021 Aeglea BioTherapeutics. External 11 Continuous Analysis Mean Change from Baseline GMFCS – Level II/III GMFCS – Level I CE = Ceiling Effect 20 Week Analysis: 11/14 (79%) 56 Week Analysis: 11/13 (85%) Categorical Responder Analysis2 1Adapted from Diaz, GA et al, Presented at 2020 European Academy of Neurology Annual Meeting; 2Clinical Responder defined by achievement of a clinically meaningful response in one or more of three mobility assessments; GMFCS = Gross Motor Function Classification System Change from Baseline at 56 Week Analysis1 Not Assessed Not Assessed CE CE Green = Overall Clinical Responder1 Patient # GMFM-E (Units) 6MWT (Meters) Baseline Deficit 151 Endpoint Analysis 20 weeks 56 weeks GMFM-E Overall 5 units 6 units Baseline Deficit 8 units 9 units 6MWT Overall 32 m 45 m Bar Chart Company Logo
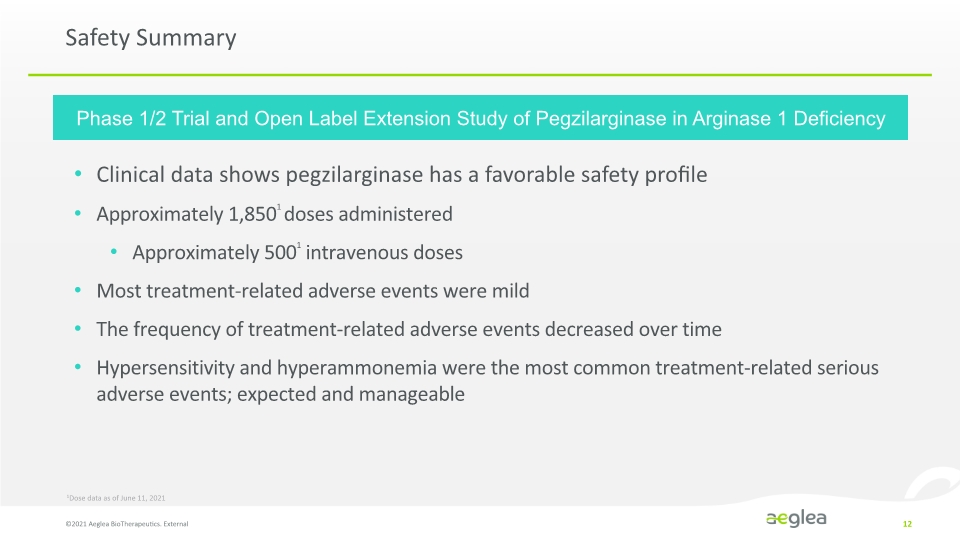
Safety Summary ©2021 Aeglea BioTherapeutics. External 12 Clinical data shows pegzilarginase has a favorable safety profile Approximately 1,8501 doses administered Approximately 5001 intravenous doses Most treatment-related adverse events were mild The frequency of treatment-related adverse events decreased over time Hypersensitivity and hyperammonemia were the most common treatment-related serious adverse events; expected and manageable 1Dose data as of June 11, 2021 Phase 1/2 Trial and Open Label Extension Study of Pegzilarginase in Arginase 1 Deficiency Company Logo
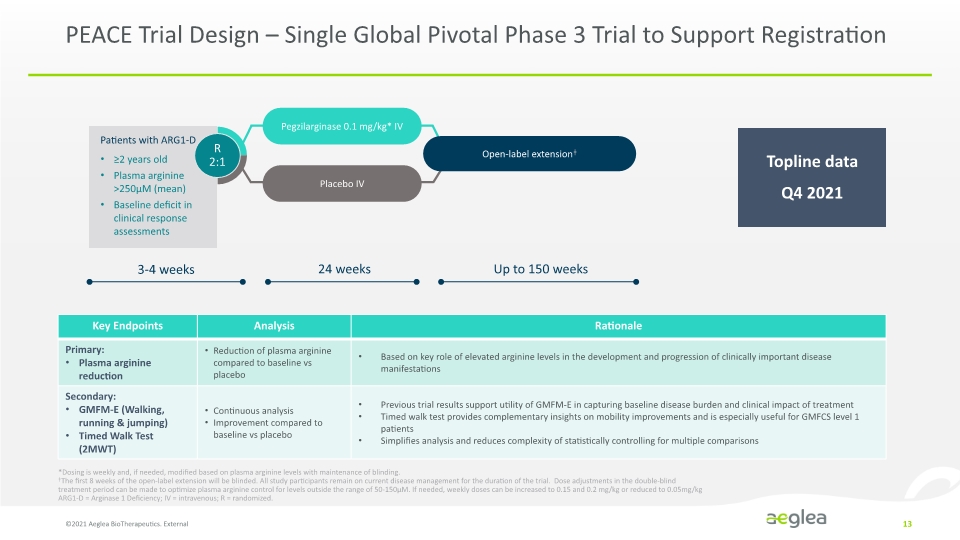
PEACE Trial Design – Single Global Pivotal Phase 3 Trial to Support Registration ©2021 Aeglea BioTherapeutics. External 13 *Dosing is weekly and, if needed, modified based on plasma arginine levels with maintenance of blinding. ǂThe first 8 weeks of the open-label extension will be blinded. All study participants remain on current disease management for the duration of the trial. Dose adjustments in the double-blind treatment period can be made to optimize plasma arginine control for levels outside the range of 50-150µM. If needed, weekly doses can be increased to 0.15 and 0.2 mg/kg or reduced to 0.05mg/kg ARG1-D = Arginase 1 Deficiency; IV = intravenous; R = randomized. Topline data Q4 2021 Patients with ARG1-D ≥2 years old Plasma arginine >250µM (mean) Baseline deficit in clinical response assessments R 2:1 Pegzilarginase 0.1 mg/kg* IV Placebo IV Open-label extensionǂ 3-4 weeks 24 weeks Up to 150 weeks Key Endpoints Analysis Rationale Primary: Plasma arginine reduction Reduction of plasma arginine compared to baseline vs placebo Based on key role of elevated arginine levels in the development and progression of clinically important disease manifestations Secondary: GMFM-E (Walking, running & jumping) Timed Walk Test (2MWT) Continuous analysis Improvement compared to baseline vs placebo Previous trial results support utility of GMFM-E in capturing baseline disease burden and clinical impact of treatment Timed walk test provides complementary insights on mobility improvements and is especially useful for GMFCS level 1 patients Simplifies analysis and reduces complexity of statistically controlling for multiple comparisons Company Logo

Attractive Commercial Opportunity for Pegzilarginase in ARG1-D ©2021 Aeglea BioTherapeutics. External 14 1 Licensing and supply agreement signed with Immedica PLC in March 2021; agreement covers countries in the European Economic Area and certain countries in the Middle East, United Kingdom, Switzerland, Andorra, Monaco, San Marino, Vatican City, Turkey, Saudi Arabia, United Arab Emirates, Qatar, Kuwait, Bahrain and Oman. Severe and progressive disease with early mortality Easily diagnosed with simple lab test or genetic testing No FDA-approved therapies to address underlying driver of disease Inadequate management options in severe protein-restricted diet, amino acid supplementation, and ammonia scavengers Suboptimal control of plasma arginine levels Poor patient compliance due to challenges with administration Significant Unmet Need Prevalence of >2,500 Patients in Key Global Markets United States >250 patients Western Europe and Middle East1 >900 patients Rest of World >1,350 patients (including 700 in LatAm, 400 in Asia Pacific) Company Logo
Building the Foundation for a Successful Launch and Enduring Commercial Presence ©2021 Aeglea BioTherapeutics. External 15 Patient Identification Accelerating the diagnosis and identification of patients through ongoing HCP engagement Disease Education and Awareness Driving HCP dialogue about the devastating and progressive nature of ARG1-D Access and Reimbursement Ensuring payer understanding of the significant clinical and economic burden of ARG1-D Organizational Readiness Establishing an internal mindset and infrastructure to meet the needs of the ARG1-D patient community
Engaging with HCP and Patient Communities to Accelerate Diagnosis, Broaden Awareness, and Deepen Understanding of ARG1-D ©2021 Aeglea BioTherapeutics. External 16 Continued patient identification momentum across key markets Results outpacing relevant benchmarks >65% of the estimated U.S. genetic prevalence identified Driving increased recognition and understanding of ARG1-D THINK ARGININE™: a disease awareness initiative with no-charge diagnostic testing Engagement with key institutions responsible for significant proportion of patients Ongoing scientific discourse to broaden awareness and deepen ARG1-D understanding and advocacy among HCP community Thoughtful engagement with the patient community to strengthen understanding of patient journey and unmet needs Patient Identification Disease Education and Awareness Company Logo

Laying the Groundwork to Transition Smoothly to Commercial Operations ©2021 Aeglea BioTherapeutics. External 17 Preliminary planning around patient services based on our deep understanding of the needs of the ARG1-D patient community Payer profiling to understand likely coverage policies at launch Initial payer dialogue providing key insight into disease understanding, educational opportunities, and management approaches Core commercial team in place with deep ultra-rare launch experience Commercial formulation on stability and distribution strategy in place High-touch patient services approach in development Commercial infrastructure built with pegzilarginase and earlier-stage pipeline programs in mind Access and Reimbursement Organizational Readiness Company Logo
Pegzilarginase Program - Key Highlights and Next Steps ©2021 Aeglea BioTherapeutics. External 18 Sustained control of plasma arginine 85% (11/13) of patients at the 56 week analysis were clinical responders Well tolerated and the rates of treatment-related adverse events decreased over time Primary endpoint: arginine reduction; secondary endpoints: clinical outcomes, safety and PK 32 patients, randomized 2:1 (pegzilarginase: placebo) 24 weeks dosing period Topline results in Q4 2021 Significant unmet medical need; no effective treatments currently available Estimated >2,500 patients worldwide with patient identification outpacing relevant benchmarks U.S. launch planning and efforts underway; building enduring commercial infrastructure Significant ex-U.S. opportunity: considerable progress in EU/Middle East with Immedica licensing agreement, retained rights in rest of world Phase 1/2 & OLE data demonstrates clinical impact PEACE: A single, global pivotal trial designed with input from FDA and EMA Opportunity Company Logo

AGLE-177 in Homocystinuria
AGLE-177 Program Overview ©2021 Aeglea BioTherapeutics. External 20 PRV – Priority Review Voucher 1 >30,000 represents estimated prevalence of Classical Homocystinuria (including B6-responsive and B6-non-responsive) in 38 addressable markets. 2 Mudd et al 1985. 3 Kožich et al, 2020. 4 ~6,000-6,600 represents estimated prevalence of B6-non-responsive Homocystinuria in the U.S (~3,300) and the EU4 plus UK (~3,300) Enrollment ongoing in Phase 1/2 clinical trial Status: Homocystinuria (HCU): Cystathionine beta synthase (CBS) deficiency, also known as Classical Homocystinuria, is a serious and progressive metabolic disorder characterized by elevated levels the amino acid homocysteine. Addressable Market: >30,000 HCU patients1 Includes ~15,000 – 18,000 with B6-non-responsive disease 2,3 Significant U.S. & EU4 + UK opportunity with ~6,000-6,600 B6-non-responsive patients 4 Serious Disease Complications Eyes Lens dislocation, glaucoma, severe near-sightedness Nervous System Intellectual and developmental delays, behavioral abnormalities, seizures Vascular Life-threatening thrombotic events, heart attack, stroke Skeletal Long bone (Marfanoid) features, skeletal deformities, osteoporosis U.S. Orphan Drug Designation EU Orphan Drug Designation U.S. Rare Pediatric Disease Designation (PRV eligible) Regulatory Designations: Company Logo
Current Homocystinuria Treatments Have Limited Effectiveness and Create a Significant Burden for Patients ©2021 Aeglea BioTherapeutics. External 21 Patients intolerant of available adjunctive therapies due to either safety issues or other adverse reactions Therapy-resistant patients on standard disease management KEY AREAS: Patients who are at high risk of non-compliance which places them at increased risk of severe complications Unmet Need: Therapy that addresses both disease and treatment burden Current standard of care: B6 (pyridoxine) for responsive patients Low-methionine diet + amino acid supplements Betaine Current disease management is inadequate with an inability to control tHcy levels: Limited effectiveness Non-compliance Poor tolerability of amino acid supplementation and Betaine 1. 2. 3. tHcy – Total plasma homocysteine Company Logo
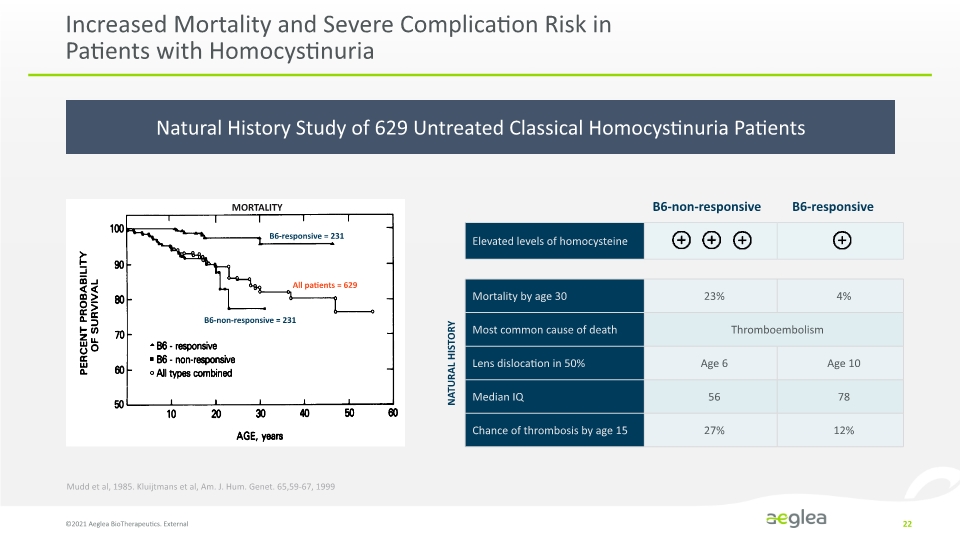
Natural History Study of 629 Untreated Classical Homocystinuria Patients Increased Mortality and Severe Complication Risk in Patients with Homocystinuria ©2021 Aeglea BioTherapeutics. External 22 B6-responsive = 231 All patients = 629 B6-non-responsive = 231 MORTALITY NATURAL HISTORY Mudd et al, 1985. Kluijtmans et al, Am. J. Hum. Genet. 65,59-67, 1999 B6-non-responsive B6-responsive Elevated levels of homocysteine Mortality by age 30 23% 4% Most common cause of death Thromboembolism Lens dislocation in 50% Age 6 Age 10 Median IQ 56 78 Chance of thrombosis by age 15 27% 12% Percent Probability of Survival 100 90 80 70 60 50 10 20 30 40 50 60 Age, years B6 – responsive B6 – non responsive All type combined Company Logo
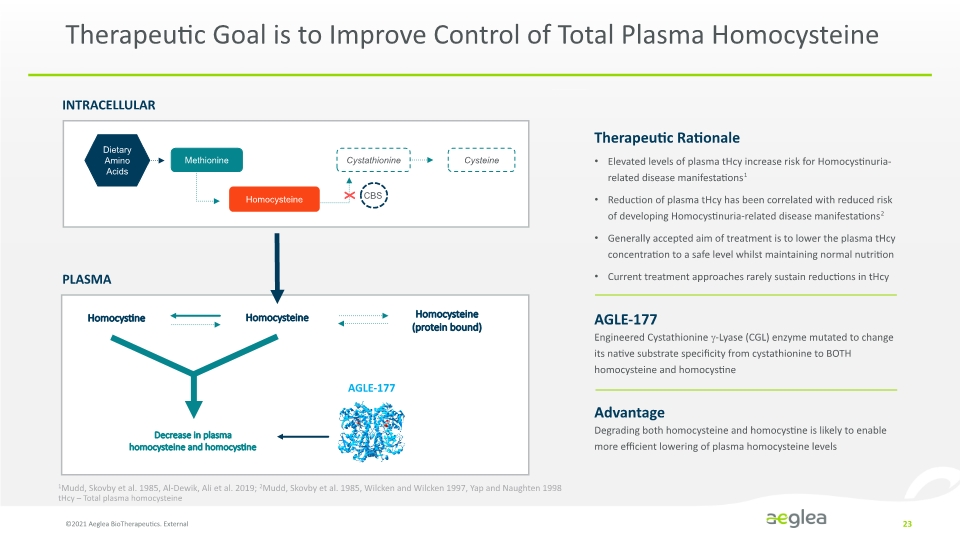
Therapeutic Goal is to Improve Control of Total Plasma Homocysteine ©2021 Aeglea BioTherapeutics. External 23 INTRACELLULAR PLASMA Homocysteine Homocystine AGLE-177 Homocysteine (protein bound) Decrease in plasma homocysteine and homocystine X Therapeutic Rationale Elevated levels of plasma tHcy increase risk for Homocystinuria-related disease manifestations1 Reduction of plasma tHcy has been correlated with reduced risk of developing Homocystinuria-related disease manifestations2 Generally accepted aim of treatment is to lower the plasma tHcy concentration to a safe level whilst maintaining normal nutrition Current treatment approaches rarely sustain reductions in tHcy AGLE-177 Engineered Cystathionine -Lyase (CGL) enzyme mutated to change its native substrate specificity from cystathionine to BOTH homocysteine and homocystine Advantage Degrading both homocysteine and homocystine is likely to enable more efficient lowering of plasma homocysteine levels 1Mudd, Skovby et al. 1985, Al-Dewik, Ali et al. 2019; 2Mudd, Skovby et al. 1985, Wilcken and Wilcken 1997, Yap and Naughten 1998 tHcy – Total plasma homocysteine Dietary Amino Acids Methionine Cystathionine Cysteine Homocysteine CBS Company Logo

Subcutaneous Dosing Reduces Total Plasma Homocysteine and Improves Survival in Preclinical Model ©2021 Aeglea BioTherapeutics. External 24 AGLE-177 (10 mg/kg, BIW) AGLE-177 (3 mg.kg, BIW) AGLE-177 (1.0 mg/kg, BIW) Vehicle (BIW) Single SC Dose in CBS -/- Mouse Model1, 2 In Vivo Efficacy at Multiple SC Dose Levels1,3 AGLE-177 statistically significant reduction in total plasma homocysteine Improvement in survival following subcutaneous treatment in CBS-/- mouse model 1 Daige C. et al. Poster presented at ASHG 2020 Virtual Meeting. 2CBS -/- mice were dosed twice per week (BIW) starting at D10 with 0.81 mg/kg HED, AGLE-177 for 5 weeks to ensure survival. ADA development that impacts the PD response cannot be ruled out at this latter timepoint. Animals underwent a two-week washout of drug followed by a single subcutaneous (SC) injection of AGLE-177 and PD assessed (tHcy) over 1 week. 3 Animals dosed SC BIWx3. tHcy = total Homocysteine P < 0.05 by Manley Cox test AGLE-177 (1.0 mg/kg) AGLE-177 (10.0 mg/kg) Plasma tHcy 375 300 225 150 75 iHcy (µM) 0 24 48 72 96 120 144 168 Hours Begin Dosing Wean 0 50 100 Percent Survival (%) 10 20 30 40 50 60 70 Age (Days) Company Logo
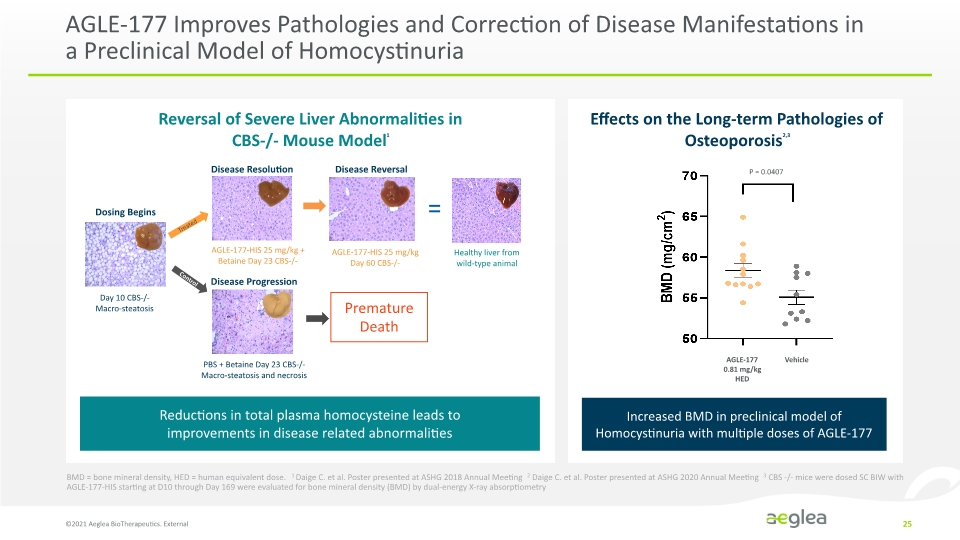
AGLE-177 Improves Pathologies and Correction of Disease Manifestations in a Preclinical Model of Homocystinuria ©2021 Aeglea BioTherapeutics. External 25 Disease Resolution Disease Reversal Dosing Begins Day 10 CBS-/- Macro-steatosis Disease Progression AGLE-177-HIS 25 mg/kg + Betaine Day 23 CBS-/- AGLE-177-HIS 25 mg/kg Day 60 CBS-/- PBS + Betaine Day 23 CBS-/- Macro-steatosis and necrosis Premature Death Healthy liver from wild-type animal Reversal of Severe Liver Abnormalities in CBS-/- Mouse Model1 Reductions in total plasma homocysteine leads to improvements in disease related abnormalities Effects on the Long-term Pathologies of Osteoporosis2,3 Increased BMD in preclinical model of Homocystinuria with multiple doses of AGLE-177 BMD = bone mineral density, HED = human equivalent dose. 1 Daige C. et al. Poster presented at ASHG 2018 Annual Meeting 2 Daige C. et al. Poster presented at ASHG 2020 Annual Meeting 3 CBS -/- mice were dosed SC BIW with AGLE-177-HIS starting at D10 through Day 169 were evaluated for bone mineral density (BMD) by dual-energy X-ray absorptiometry BMD (mg/cm2) 60 66 60 65 70 P – 0.0407 AGLE-177 081 mg/kg HED Vehicle
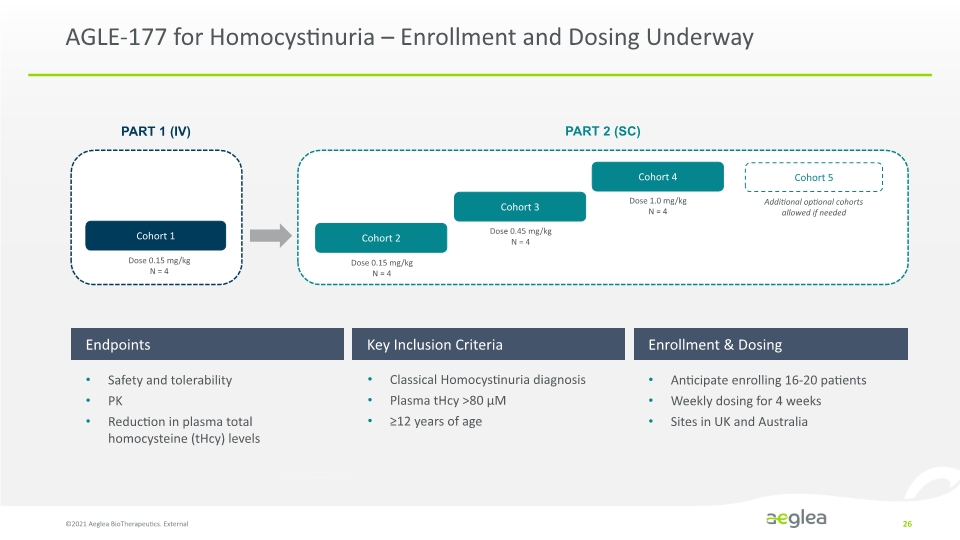
AGLE-177 for Homocystinuria – Enrollment and Dosing Underway ©2021 Aeglea BioTherapeutics. External 26 Endpoints Key Inclusion Criteria Enrollment & Dosing Safety and tolerability PK Reduction in plasma total homocysteine (tHcy) levels Classical Homocystinuria diagnosis Plasma tHcy >80 µM ≥12 years of age Anticipate enrolling 16-20 patients Weekly dosing for 4 weeks Sites in UK and Australia PART 1 (IV) Cohort 1 Dose 0.15 mg/kg N=4 PART 2 (SC) Cohort 2 0.15 mg/kg N=4 Cohort 3 0.45 mg/kg N=4 Cohort 4 1.0 mg/kg N=4 Cohort 5 0.15 Additional optional cohorts allowed if needed
AGLE-177 Program – Key Highlights ©2021 Aeglea BioTherapeutics. External 27 Limited effectiveness of SOC Limited effectiveness of lowering homocysteine, non-compliance and poor tolerability of low methionine diet and Betaine AGLE-177 is an engineered human enzyme designed to address the needs of this patient population which we anticipate will lower the harmful plasma homocysteine levels Includes ~15,000 – 18,000 with B6-non-responsive disease Significant U.S. & EU4 + UK opportunity with ~6,000-6,600 B6-non-responsive patients U.S. Orphan Drug Designation EU Orphan Drug Designation U.S. Rare Pediatric Disease Designation (PRV eligible) PRV – Priority Review Voucher Currently enrolling Phase 1/2 clinical trial ADDRESSABLE MARKET > 30,000 patients REGULATORY DESIFNATION AEGLEA’S APPROACH Company Logo

Human Enzyme Platform
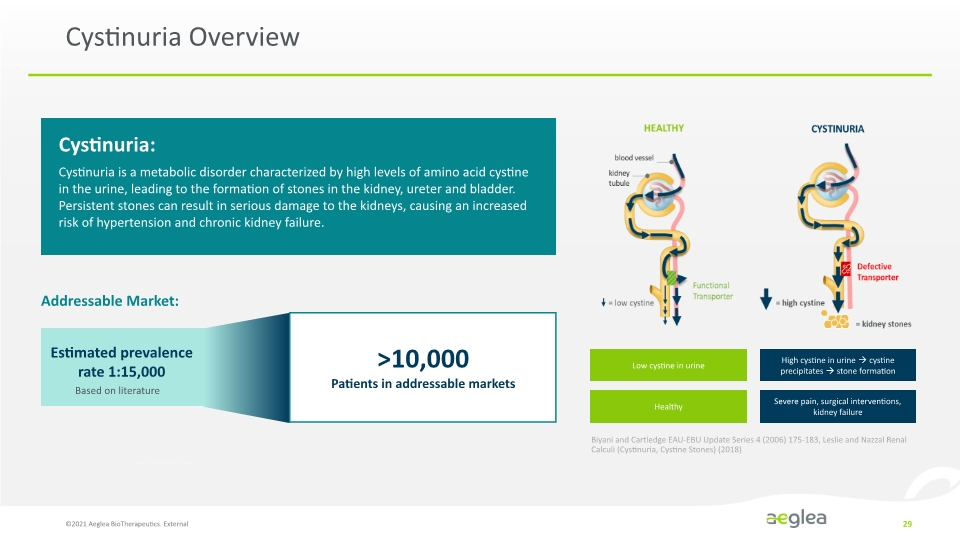
Cystinuria Overview ©2021 Aeglea BioTherapeutics. External 29 Cystinuria: Cystinuria is a metabolic disorder characterized by high levels of amino acid cystine in the urine, leading to the formation of stones in the kidney, ureter and bladder. Persistent stones can result in serious damage to the kidneys, causing an increased risk of hypertension and chronic kidney failure. Addressable Market: Estimated prevalence rate 1:15,000 Based on literature >10,000 Patients in addressable markets Low cystine in urine Healthy High cystine in urine cystine precipitates stone formation Severe pain, surgical interventions, kidney failure Biyani and Cartledge EAU-EBU Update Series 4 (2006) 175-183, Leslie and Nazzal Renal Calculi (Cystinuria, Cystine Stones) (2018) HEALTHY blood vessel kidney tubule low cystine CYSINURIA Functional Transporter high cystine Defective Transporter kidney stones
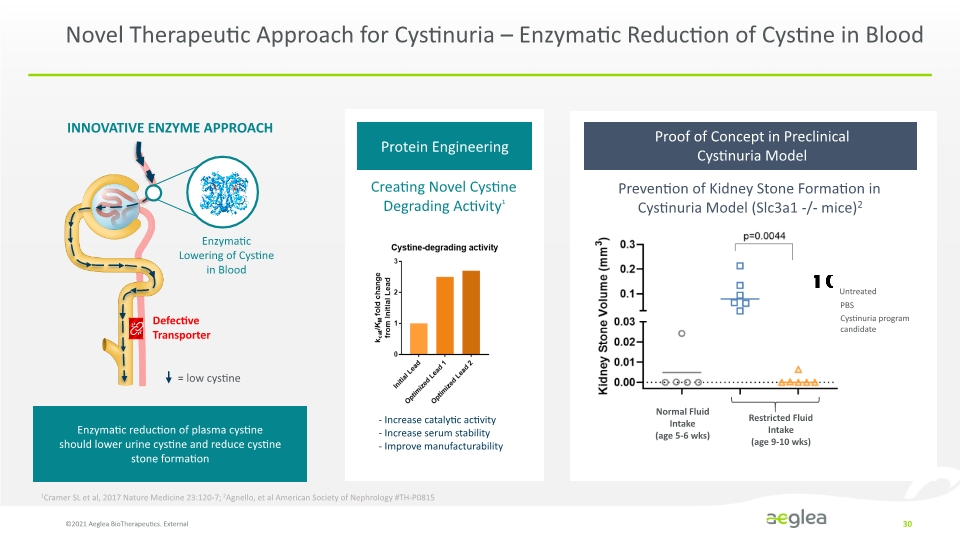
Novel Therapeutic Approach for Cystinuria – Enzymatic Reduction of Cystine in Blood ©2021 Aeglea BioTherapeutics. External 30 INNOVATIVE ENZYME APPROACH Enzymatic reduction of plasma cystine should lower urine cystine and reduce cystine stone formation Creating Novel Cystine Degrading Activity1 - Increase catalytic activity - Increase serum stability - Improve manufacturability Normal Fluid Intake (age 5-6 wks) Restricted Fluid Intake (age 9-10 wks) Prevention of Kidney Stone Formation in Cystinuria Model (Slc3a1 -/- mice)2 1Cramer SL et al, 2017 Nature Medicine 23:120-7; 2Agnello, et al American Society of Nephrology #TH-P0815 Enzymatic Lowering of Cystine in Blood Defective Transporter low cystine Cystine degrading activity Initial Lead Optimized Lead 1 Optimized Lead 2 from Initial Lead total change 0 1 2 3 0.00 0.01 0.02 0.03 0.1 0.2 0.3 Kidney Stone Volume (mm2) Normal Fluid intake (age 5-6 wks) Restricted Fluid intake age 9-10 wks Untreated PBS Cystinuria program candidate p=0.0044

Leveraging Aeglea’s Next Generation Human Enzyme Platform ©2021 Aeglea BioTherapeutics. External 31 High activity human pegylated investigational enzyme Degrades key accumulating metabolite Results in restorative phenotypic improvements in genetically engineered mouse model PROOF OF CONCEPT Vehicle Control Therapeutic Enzyme Therapeutic Approach Pegylated enhanced human enzyme Enzymatic control of high levels of a metabolite considered important in disease pathogenesis Unmet Medical Need Very high - no effective therapy Early mortality and serious complications with continued disease progression Patient Population (Addressable Markets) >5,000 patients Diagnosis Plasma metabolite levels and mutation analysis

Summary
Strong Momentum with Near-Term Catalysts ©2021 Aeglea BioTherapeutics. External 33 ACCOMPLISHMENTS 2021 MILESTONES Pegzilarginase: ARG1-D Enrollment complete Ex-U.S. license and supply agreement with Immedica Topline pivotal Phase 3 data: Q4 2021 Pegzilarginase: ARG1-D PEACE enrollment complete Ex-U.S. license and supply agreement with Immedica to commercialize pegzilarginase in Europe and several Middle East countries Phase 1/2 & OLE efficacy data at 56 week analysis: Statistically significant arginine reduction vs baseline (p<0.001) Durable high clinical responder rate, 85% Breakthrough Therapy Designation (BTD) PRV eligible: Rare Pediatric Disease Designation (RPD) Subcutaneous dosing; all eligible patients in OLE AGLE-177: Homocystinuria First patient dosed in Phase 1/2 trial U.S. and EU Orphan Designations PRV eligible: Rare Pediatric Disease Designation (RPD) Preclinical proof of concept: Plasma homocysteine levels reduction improves disease-related abnormalities Novel engineered human enzyme designed to degrade both homocysteine and homocystine Cystinuria Program Innovative human enzyme-based solution for disease management Proof of concept: Lowering of plasma cystine lowers urine cystine levels and reduces kidney stone formation in a preclinical disease model AGLE-177: Homocystinuria Patient enrollment and dosing underway

Financial Summary ©2021 Aeglea BioTherapeutics. External 34 Cash, cash equivalents and marketable securities as of June 30, 2021: $130.4 million1 (no debt) As of July 30, 2021, 65.7 million2 common shares and pre-funded warrants outstanding Expected funding runway: into 2023 1Includes $1.8mm of restricted. 2Includes 49.1 million common shares outstanding and 16.6 million pre-funded warrants. 3Includes 12.0 million of license revenue and $1.7 million of development fee revenue. $ Millions Revenue 1 R&D Expense Net Loss Three Months Ended 6/30/2021 $13.7$ 13.6 $6.8 $6.8 Six Months Ended $ 13.7 $25.4 $13.2 $ 25.0 Company Logo
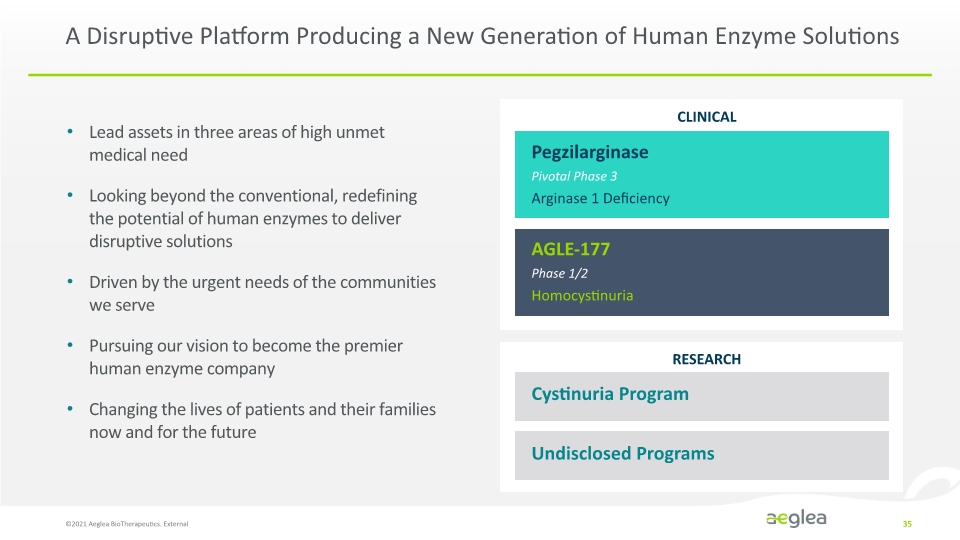
A Disruptive Platform Producing a New Generation of Human Enzyme Solutions ©2021 Aeglea BioTherapeutics. External 35 Lead assets in three areas of high unmet medical need Looking beyond the conventional, redefining the potential of human enzymes to deliver disruptive solutions Driven by the urgent needs of the communities we serve Pursuing our vision to become the premier human enzyme company Changing the lives of patients and their families now and for the future CLINICAL RESEARCH Pegzilarginase Pivotal Phase 3 Arginase 1 Deficiency AGLE-177 Phase 1/2 Homocystinuria Cystinuria Program Undisclosed Programs

805 Las Cimas Parkway Suite 100 Austin, TX 78746 aeglea.com
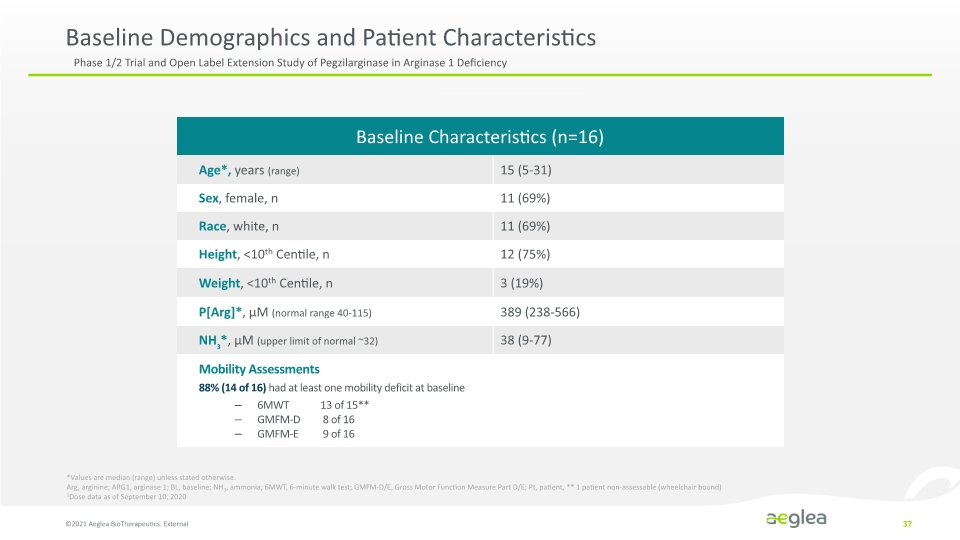
Baseline Demographics and Patient Characteristics ©2021 Aeglea BioTherapeutics. External 37 *Values are median (range) unless stated otherwise. Arg, arginine; ARG1, arginase 1; BL, baseline; NH3, ammonia; 6MWT, 6-minute walk test; GMFM-D/E, Gross Motor Function Measure Part D/E; Pt, patient, ** 1 patient non-assessable (wheelchair bound) 1Dose data as of September 10, 2020 Phase 1/2 Trial and Open Label Extension Study of Pegzilarginase in Arginase 1 Deficiency Baseline Characteristic (n=16) Age Sex Race Height<10th Centile, n Weight, <10th Centile,n P (Age) uM (normal range 40-115) HN3*, uM (Upper limit of normal 32) Mobility Assessments 88% (14+16) had at least one mobility at baseline – 6MWT 13 of 15** – GMFM-D 8of 16 – GMFM-E 9 of 16 15 (5-31) 11 (69%) 11 (69%) 12 (75%) 3 (19%) 389 (238-566) 38 (9-77)
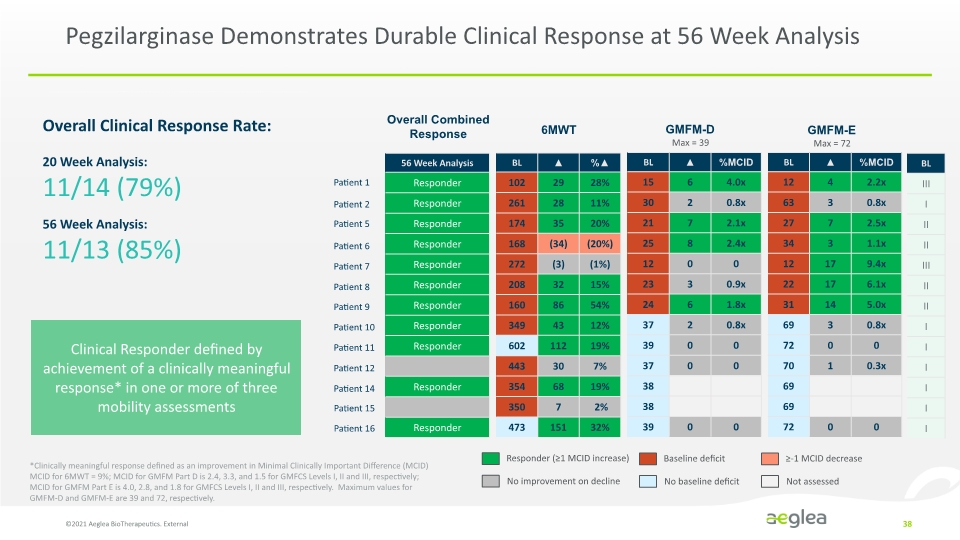
Pegzilarginase Demonstrates Durable Clinical Response at 56 Week Analysis Overall Combined Response Responder (≥1 MCID increase) No improvement on decline Baseline deficit No baseline deficit ≥-1 MCID decrease Not assessed Patient 1 Patient 2 Patient 5 Patient 6 Patient 7 Patient 8 Patient 9 Patient 10 Patient 11 Patient 12 Patient 14 Patient 15 Patient 16 6MWT GMFM-D Max = 39 GMFM-E Max = 72 ©2021 Aeglea BioTherapeutics. External 38 Clinical Responder defined by achievement of a clinically meaningful response* in one or more of three mobility assessments 20 Week Analysis: 11/14 (79%) 56 Week Analysis: 11/13 (85%) Overall Clinical Response Rate: *Clinically meaningful response defined as an improvement in Minimal Clinically Important Difference (MCID) MCID for 6MWT = 9%; MCID for GMFM Part D is 2.4, 3.3, and 1.5 for GMFCS Levels I, II and III, respectively; MCID for GMFM Part E is 4.0, 2.8, and 1.8 for GMFCS Levels I, II and III, respectively. Maximum values for GMFM-D and GMFM-E are 39 and 72, respectively. 56 Week Analysis Responder Responder Responder Responder Responder Responder Responder Responder Responder Responder Responder BL 102 261 174 168 22 208 160 349 602 443 354 350 473 29 28 35 (34) (3) 32 86 43 112 30 68 7 151 % 28% 11% 20% (20%) (1%) 15% 54% 12% 18% 7% 19% 7% 19% 2% 32% BL 15 30 21 25 12 23 24 37 39 37 38 38 39 6 2 7 8 0 3 6 2 0 0 0 % MCID 4.0x 0.8x 2.1x 2.4x 0 0.9x 1.8x 0.8x 0 0 0 BL 12 63 27 34 12 22 31 69 72 70 69 69 72 4 3 7 3 17 17 14 3 0 1 0 %MCID 2.2x 0.8x 2.5x 1.1x 9.4x 6.1x 5.0x 0.8x 0 0.3x 0 BL lll l ll ll lll ll ll l l l l l l

Designing Enzyme Therapeutics - Pegzilarginase ©2021 Aeglea BioTherapeutics. External 39 Improved Catalytic Activity & Serum Stability Reduced Circulating Arginine levels Substituted Metal Cofactor (Mn2+ replaced with Co2+ ) Pegzilarginase Catalytic activity 1000 800 600 400 200 0 Kact KM (uM) 818 44 Pegzilarginse PRGylated native human arginase 1 T ½ (h r) 20 30 40 Serum 37 + Plasma arginine levels in cynomologus monkey Pre dose arginine plasma levels 0 10 100 10 50 100 150 200 Lowest limit if detection Time (hours) PEGylated native human arginase l (single dose, 1 mg/kg) Pegzilarginase (single dose, 1 mg/kg)






































