
Research and Development Day Virtual Webinar July 20, 2021 Exhibit 99.2

Forward Looking Statements
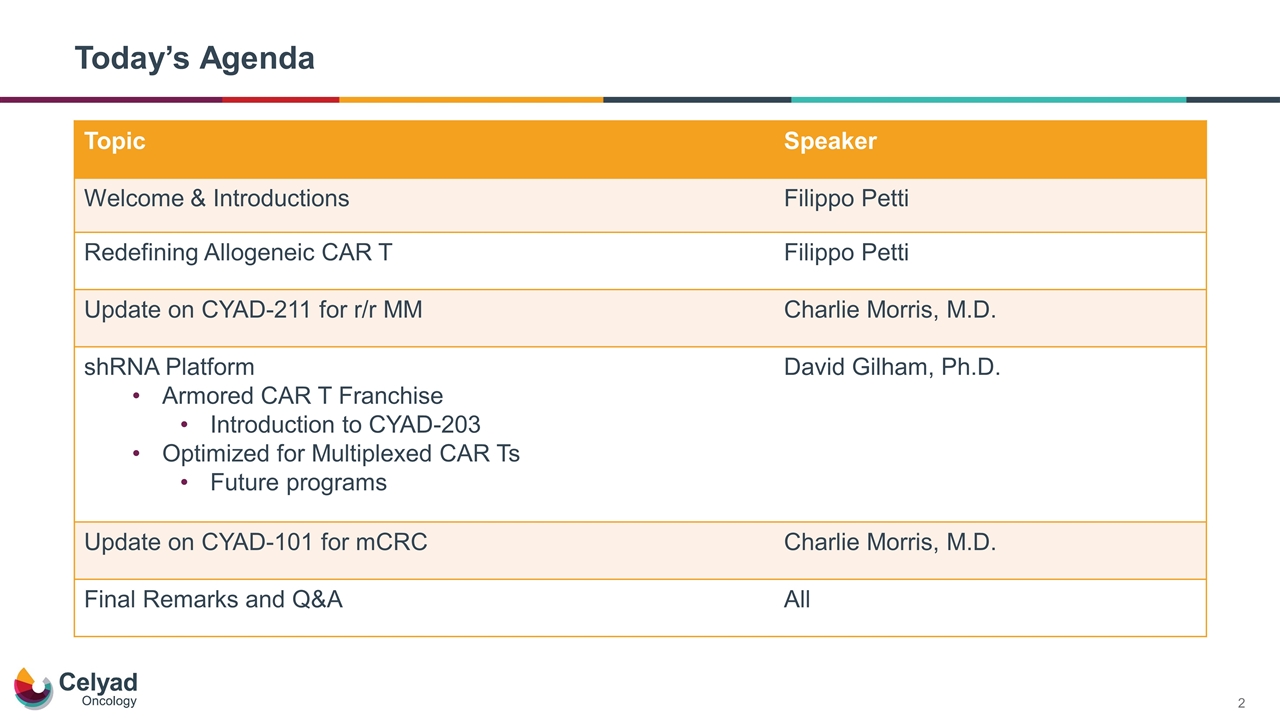
Today’s Agenda Topic Speaker Welcome & Introductions Filippo Petti Redefining Allogeneic CAR T Filippo Petti Update on CYAD-211 for r/r MM Charlie Morris, M.D. shRNA Platform Armored CAR T Franchise Introduction to CYAD-203 Optimized for Multiplexed CAR Ts Future programs David Gilham, Ph.D. Update on CYAD-101 for mCRC Charlie Morris, M.D. Final Remarks and Q&A All

Redefining Allogeneic CAR T
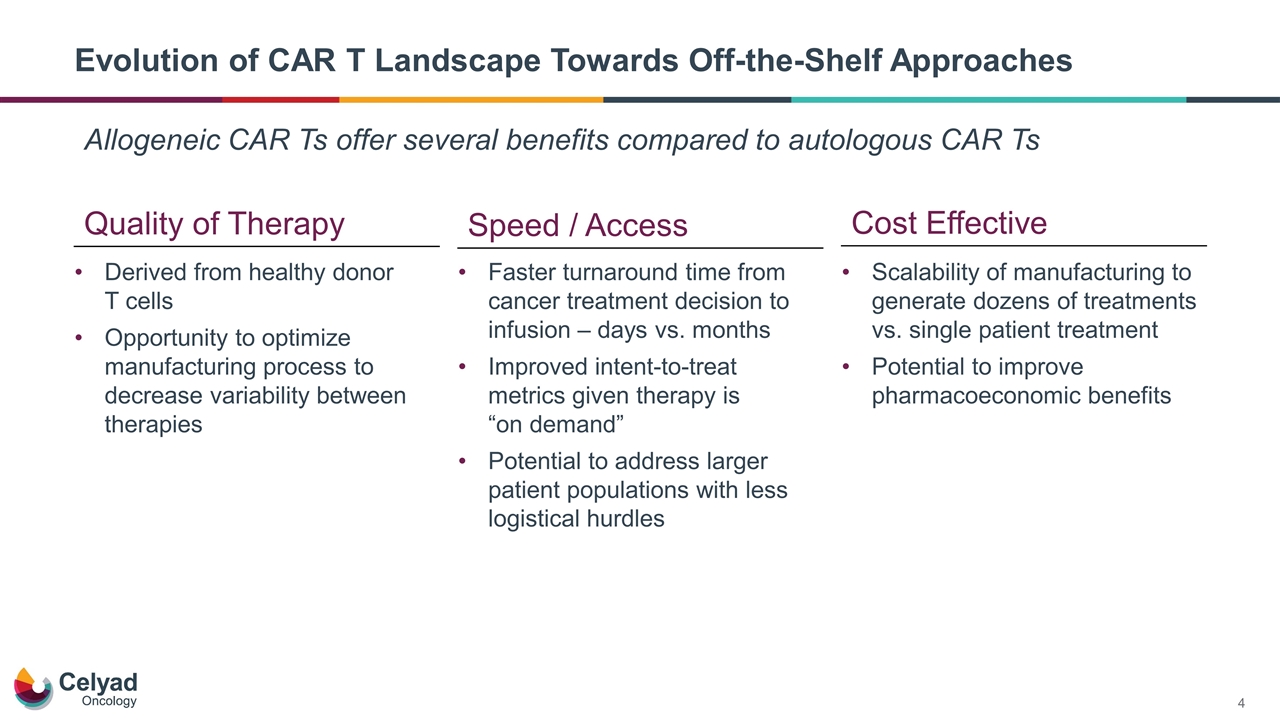
Evolution of CAR T Landscape Towards Off-the-Shelf Approaches Allogeneic CAR Ts offer several benefits compared to autologous CAR Ts Quality of Therapy Cost Effective Derived from healthy donor T cells Opportunity to optimize manufacturing process to decrease variability between therapies Speed / Access Faster turnaround time from cancer treatment decision to infusion – days vs. months Improved intent-to-treat metrics given therapy is “on demand” Potential to address larger patient populations with less logistical hurdles Scalability of manufacturing to generate dozens of treatments vs. single patient treatment Potential to improve pharmacoeconomic benefits
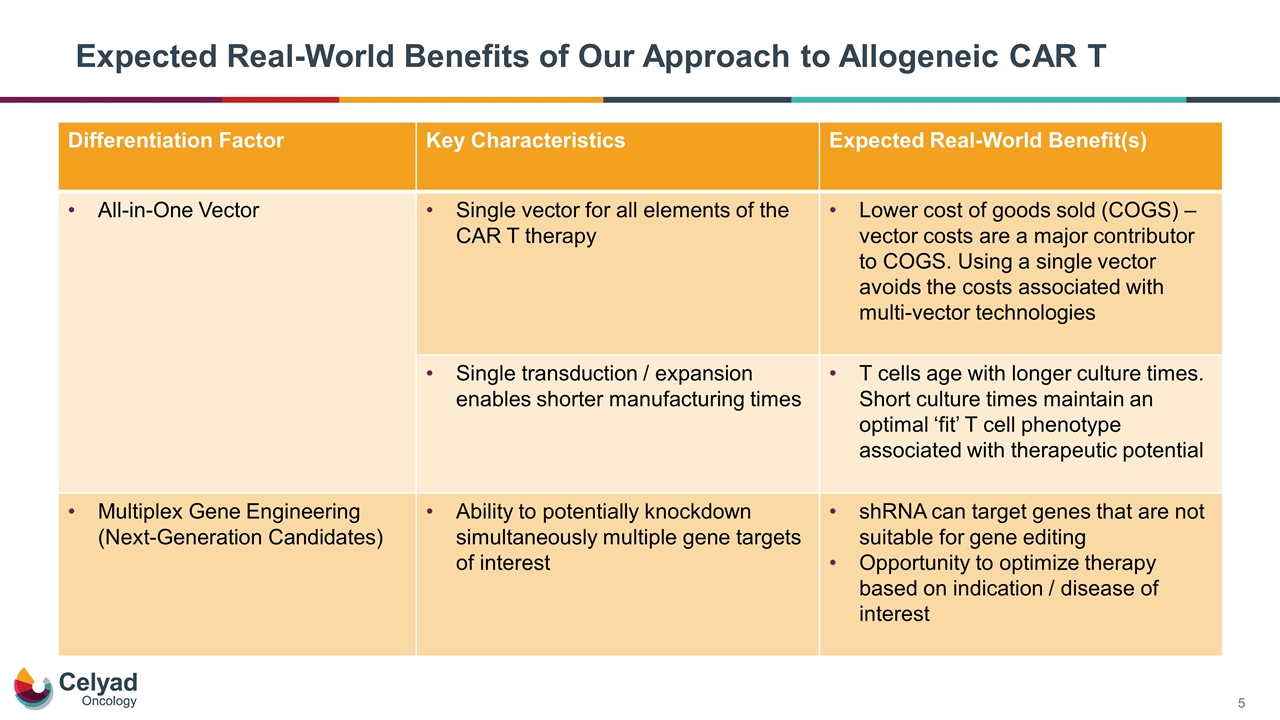
Expected Real-World Benefits of Our Approach to Allogeneic CAR T Differentiation Factor Key Characteristics Expected Real-World Benefit(s) All-in-One Vector Single vector for all elements of the CAR T therapy Lower cost of goods sold (COGS) – vector costs are a major contributor to COGS. Using a single vector avoids the costs associated with multi-vector technologies Single transduction / expansion enables shorter manufacturing times T cells age with longer culture times. Short culture times maintain an optimal ‘fit’ T cell phenotype associated with therapeutic potential Multiplex Gene Engineering (Next-Generation Candidates) Ability to potentially knockdown simultaneously multiple gene targets of interest shRNA can target genes that are not suitable for gene editing Opportunity to optimize therapy based on indication / disease of interest

Update on CYAD-211 for r/r MM
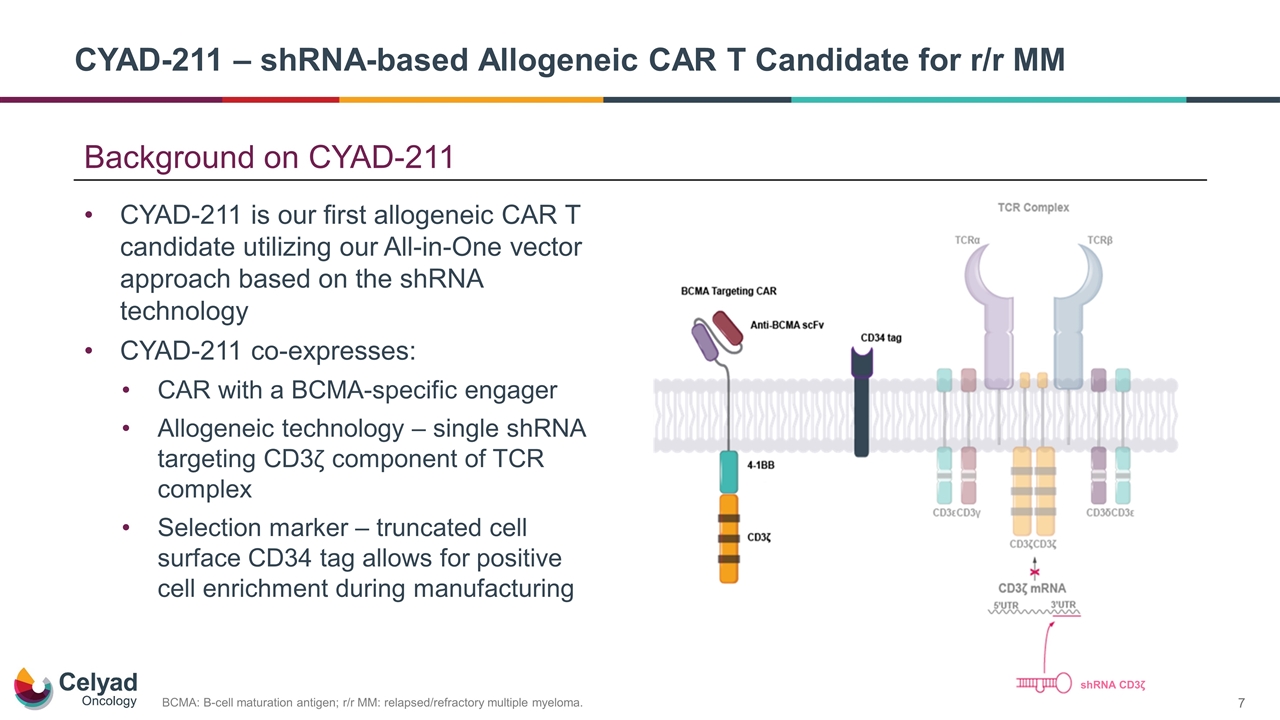
Background on CYAD-211 CYAD-211 is our first allogeneic CAR T candidate utilizing our All-in-One vector approach based on the shRNA technology CYAD-211 co-expresses: CAR with a BCMA-specific engager Allogeneic technology – single shRNA targeting CD3ζ component of TCR complex Selection marker – truncated cell surface CD34 tag allows for positive cell enrichment during manufacturing BCMA: B-cell maturation antigen; r/r MM: relapsed/refractory multiple myeloma. CYAD-211 – shRNA-based Allogeneic CAR T Candidate for r/r MM shRNA CD3ζ

Keys to establishing technology proof-of-concept No evidence of GvHD Cell kinetics Initial clinical activity shRNA as a Novel Allogeneic Technology for CAR T
Open-label, Phase 1 dose-escalation trial in relapsed or refractory multiple myeloma patients CYAD-211 – IMMUNICY-1 Phase 1 Trial Study Design (1) Patient Background Primary objective: safety and recommended dose of CYAD-211 Dose escalation: 30x106, 100x106 and 300x106 cells per infusion Standard preconditioning: Cyclophosphamide 300 mg/m² / Fludarabine 30 mg/m² (3 days) Eight patients enrolled across three dose levels Median prior lines of therapy: four Six of eight (75%) patients exposed to all three major MM drug classes (proteasome inhibitors, immunomodulatory drugs, and CD38 specific therapy) 1. NCT04613557 Safety, Activity and Cell Kinetics of CYAD-211 in Patients With Relapsed or Refractory Multiple Myeloma (IMMUNICY-1).
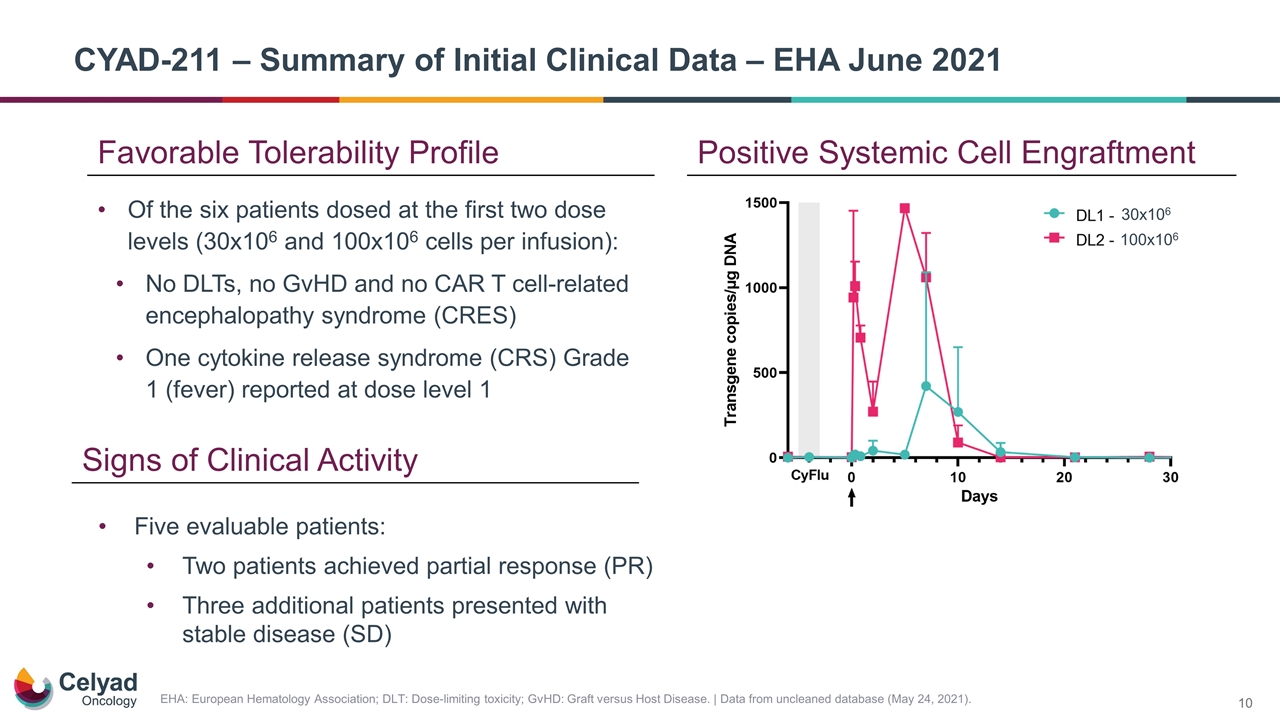
Positive Systemic Cell Engraftment EHA: European Hematology Association; DLT: Dose-limiting toxicity; GvHD: Graft versus Host Disease. | Data from uncleaned database (May 24, 2021). Of the six patients dosed at the first two dose levels (30x106 and 100x106 cells per infusion): No DLTs, no GvHD and no CAR T cell‑related encephalopathy syndrome (CRES) One cytokine release syndrome (CRS) Grade 1 (fever) reported at dose level 1 Favorable Tolerability Profile Five evaluable patients: Two patients achieved partial response (PR) Three additional patients presented with stable disease (SD) Signs of Clinical Activity CYAD-211 – Summary of Initial Clinical Data – EHA June 2021 30x106 100x106
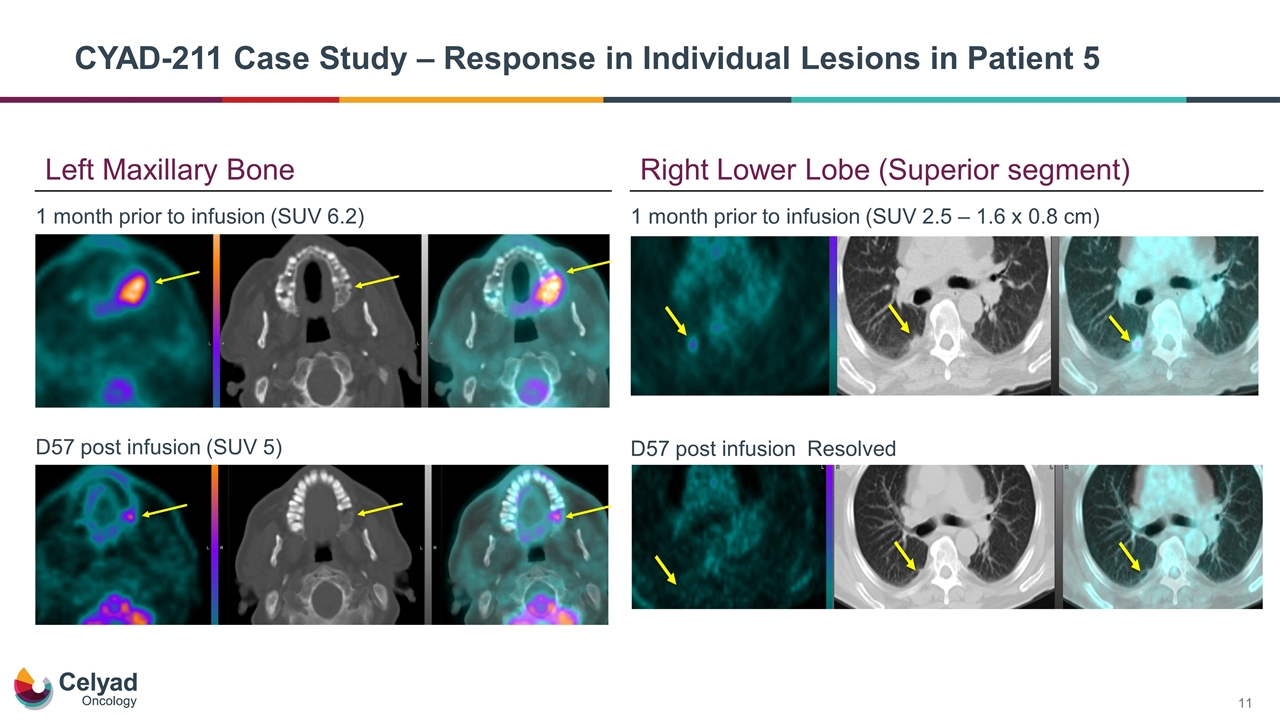
CYAD-211 Case Study – Response in Individual Lesions in Patient 5 1 month prior to infusion (SUV 6.2) D57 post infusion (SUV 5) 1 month prior to infusion (SUV 2.5 – 1.6 x 0.8 cm) D57 post infusion Resolved Left Maxillary Bone Right Lower Lobe (Superior segment)

Cell Kinetics Updated with First Patient at Dose Level 3 Duration and depth of lymphodepletion was variable in dose level 1 patients while patients in dose levels 2 and 3 showed similar lymphodepletion CYAD-211 cell levels detected by PCR-based methods in all patients Consistent with a dose dependent increase in cell engraftment No GVHD reported to date CYAD-211 cell levels detected by PCR-based methods in all patients Peripheral Blood Lymphocyte Count CYAD-211 Cell Engraftment DL: Dose level.

Keys to establishing technology proof-of-concept No evidence of GvHD Cell kinetics Initial clinical activity ü ü ü shRNA as a Novel Allogeneic Technology for CAR T
Enrollment ongoing in dose level 3 (300x106 cells per infusion) Plan to explore higher dose preconditioning regimens after dose level 3 Additional data from the dose escalation trial expected during second half 2021 Next Steps for CYAD-211 IMMUNICY-1 Trial

shRNA Platform – Armored CAR T Franchise
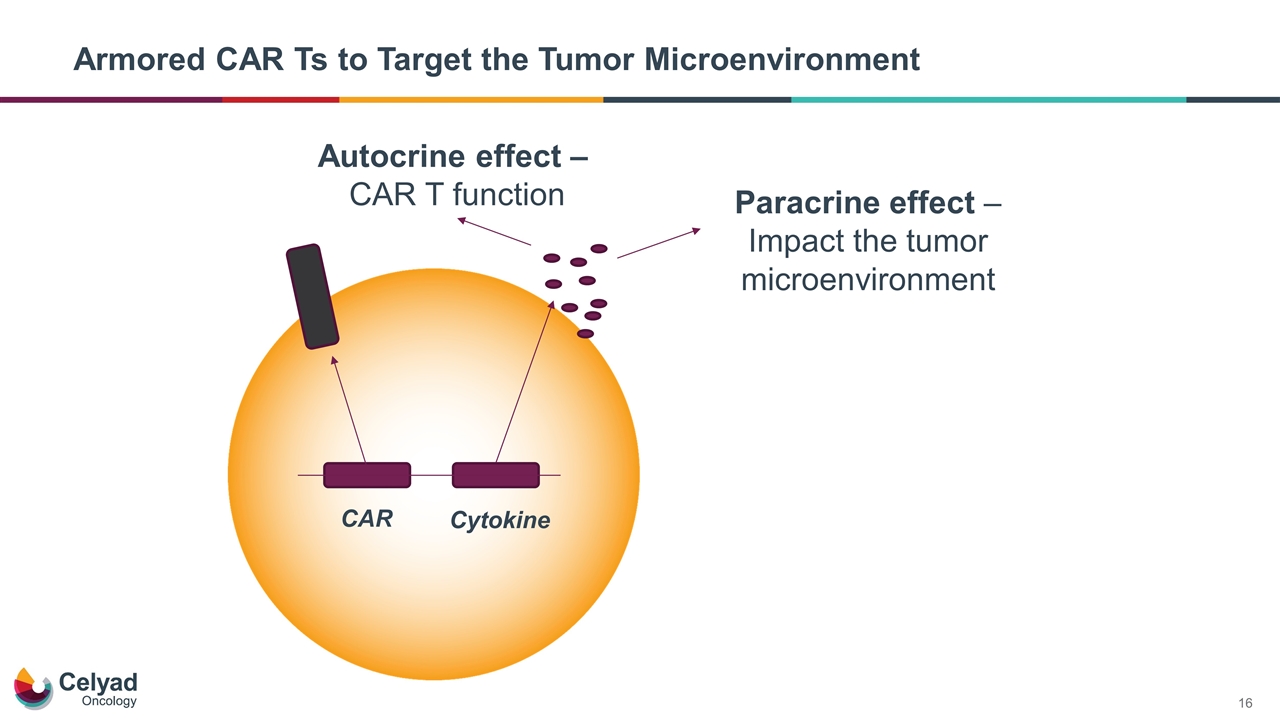
CAR Cytokine Autocrine effect – CAR T function Paracrine effect – Impact the tumor microenvironment Armored CAR Ts to Target the Tumor Microenvironment
Armored CARs Using Interleuken-18 (IL-18)
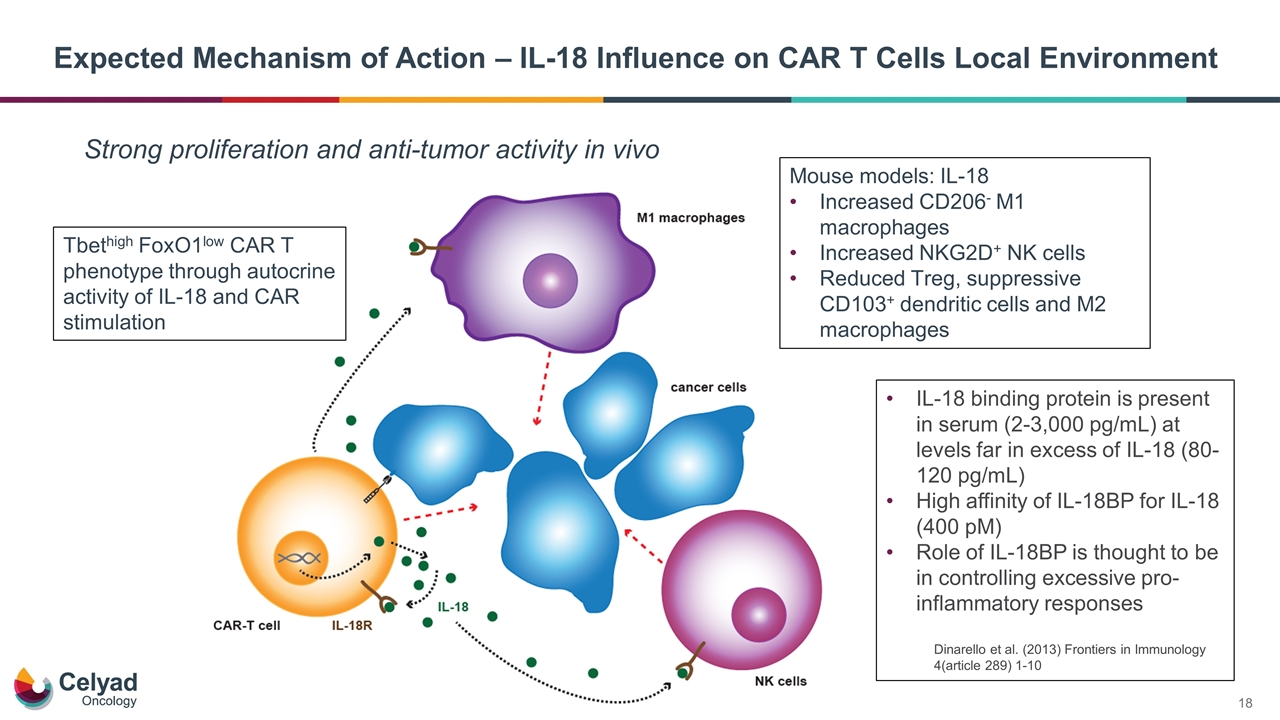
Expected Mechanism of Action – IL-18 Influence on CAR T Cells Local Environment Strong proliferation and anti-tumor activity in vivo Tbethigh FoxO1low CAR T phenotype through autocrine activity of IL-18 and CAR stimulation Mouse models: IL-18 Increased CD206- M1 macrophages Increased NKG2D+ NK cells Reduced Treg, suppressive CD103+ dendritic cells and M2 macrophages IL-18 binding protein is present in serum (2-3,000 pg/mL) at levels far in excess of IL-18 (80-120 pg/mL) High affinity of IL-18BP for IL-18 (400 pM) Role of IL-18BP is thought to be in controlling excessive pro-inflammatory responses Dinarello et al. (2013) Frontiers in Immunology 4(article 289) 1-10
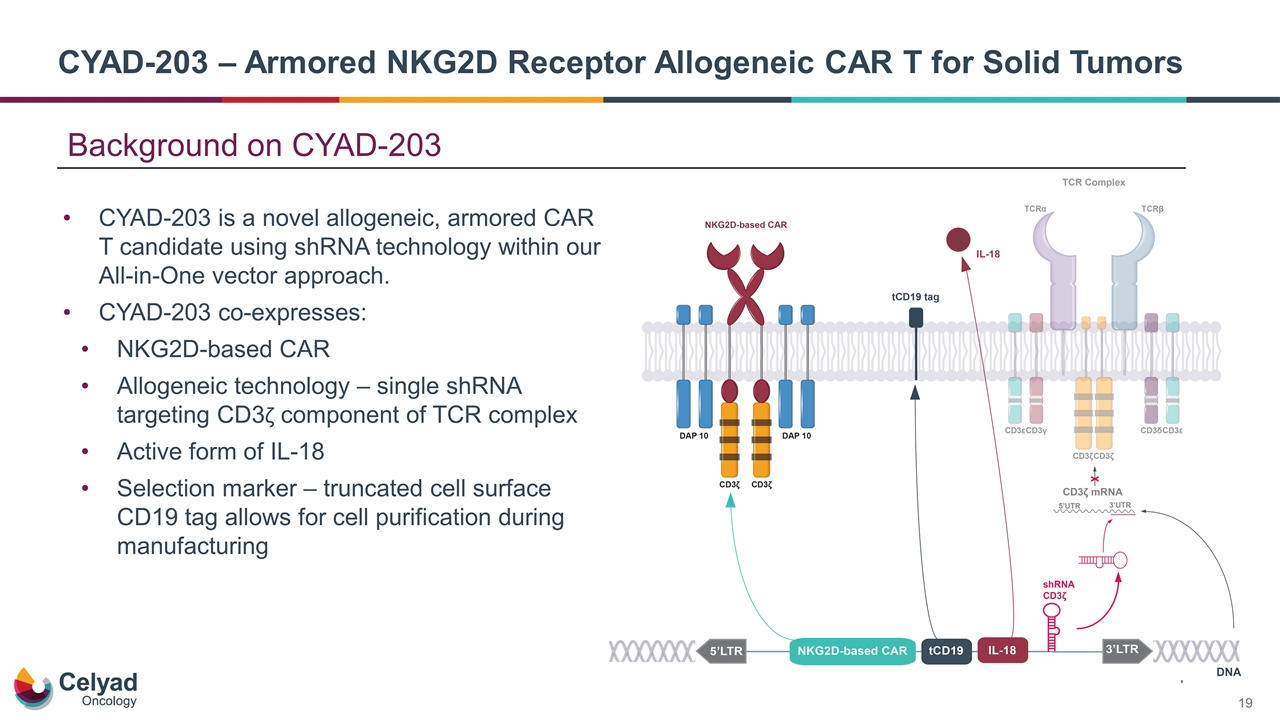
CYAD-203 – Armored NKG2D Receptor Allogeneic CAR T for Solid Tumors CYAD-203 is a novel allogeneic, armored CAR T candidate using shRNA technology within our All-in-One vector approach. CYAD-203 co-expresses: NKG2D-based CAR Allogeneic technology – single shRNA targeting CD3ζ component of TCR complex Active form of IL-18 Selection marker – truncated cell surface CD19 tag allows for cell purification during manufacturing Background on CYAD-203
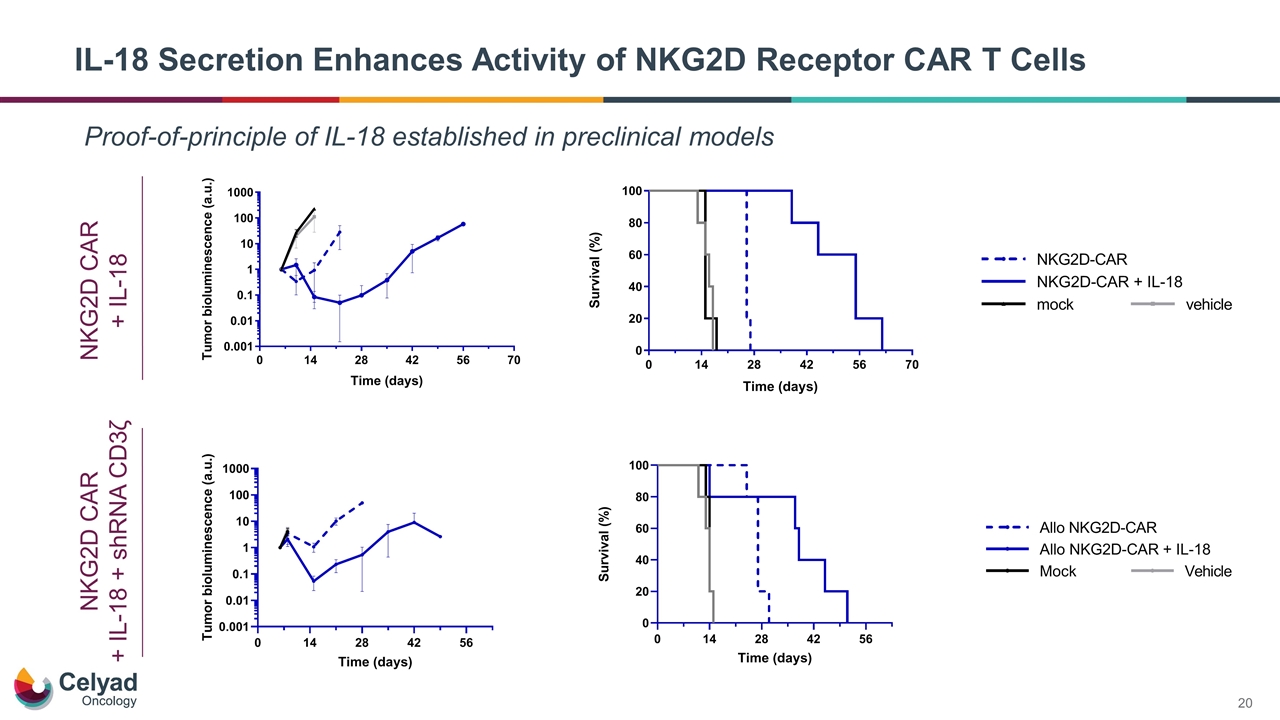
IL-18 Secretion Enhances Activity of NKG2D Receptor CAR T Cells Proof-of-principle of IL-18 established in preclinical models NKG2D CAR + IL-18 NKG2D CAR + IL-18 + shRNA CD3ζ
Next Steps for Armored CAR T Franchise Investigational New Drug (IND) enabling studies ongoing Anticipate submission of IND application in mid-2022 To our knowledge, CYAD-203 on track to be first IL-18 secreting allogeneic CAR T candidate Additional Armored CAR Ts CYAD-203 Program Currently evaluating the co-expression of IL-18 in multiple discovery stage next-generation, shRNA-based allogeneic CAR T candidates Opportunity to drive series of differentiated candidates for both solid tumors and hematological malignancies

shRNA Platform – Optimized for Multiplexed CAR Ts
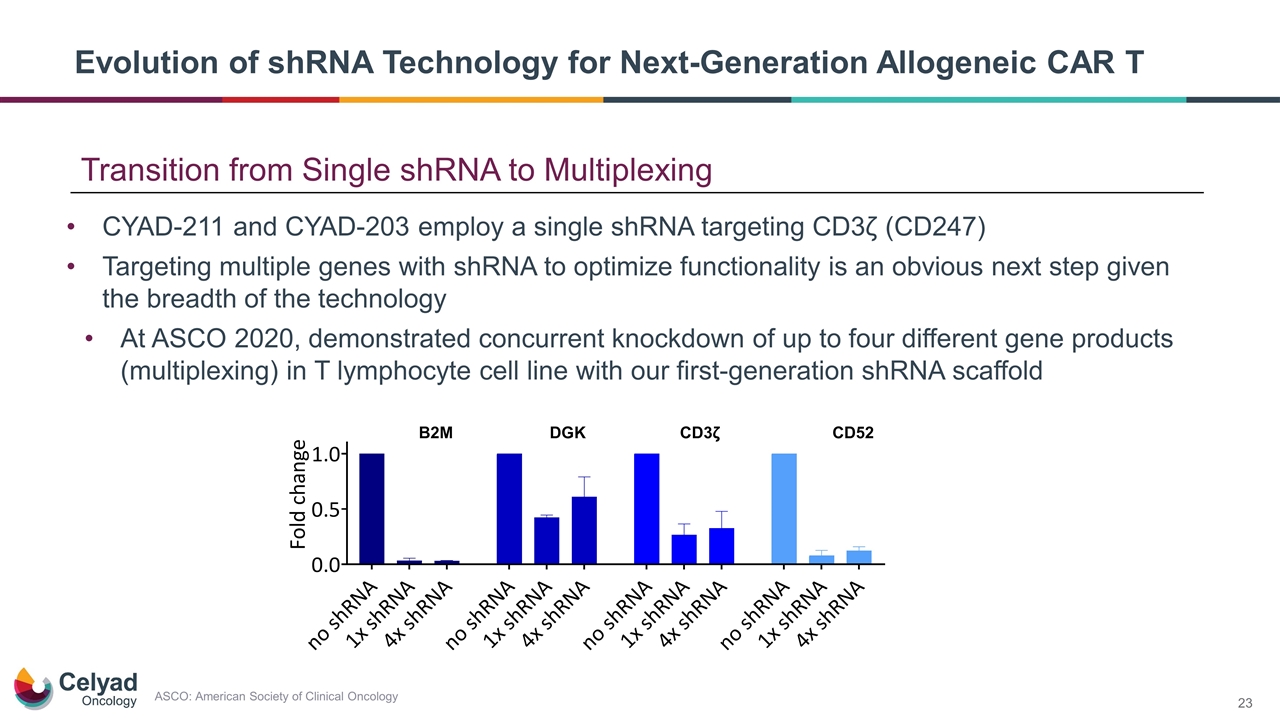
Evolution of shRNA Technology for Next-Generation Allogeneic CAR T CYAD-211 and CYAD-203 employ a single shRNA targeting CD3ζ (CD247) Targeting multiple genes with shRNA to optimize functionality is an obvious next step given the breadth of the technology At ASCO 2020, demonstrated concurrent knockdown of up to four different gene products (multiplexing) in T lymphocyte cell line with our first-generation shRNA scaffold Transition from Single shRNA to Multiplexing ASCO: American Society of Clinical Oncology
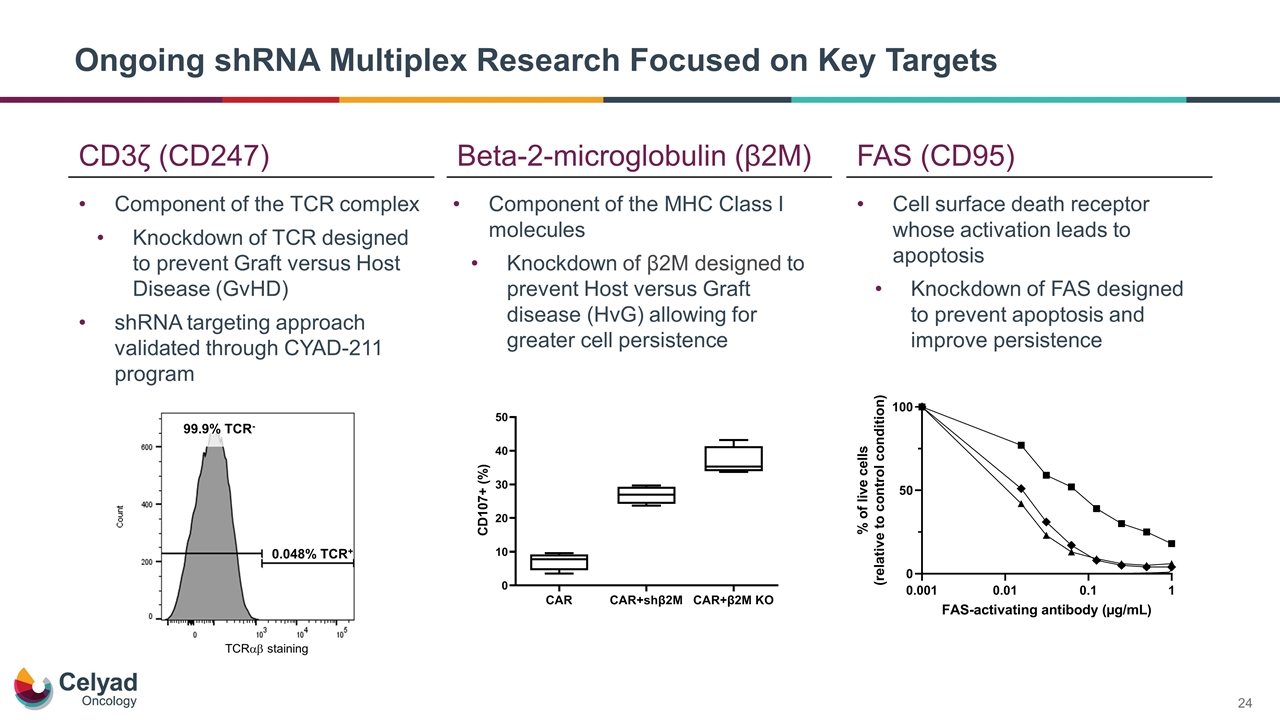
Ongoing shRNA Multiplex Research Focused on Key Targets CD3ζ (CD247) Beta-2-microglobulin (β2M) FAS (CD95) Component of the TCR complex Knockdown of TCR designed to prevent Graft versus Host Disease (GvHD) shRNA targeting approach validated through CYAD-211 program Component of the MHC Class I molecules Knockdown of β2M designed to prevent Host versus Graft disease (HvG) allowing for greater cell persistence Cell surface death receptor whose activation leads to apoptosis Knockdown of FAS designed to prevent apoptosis and improve persistence 0.048% TCR+ TCRab staining 99.9% TCR-
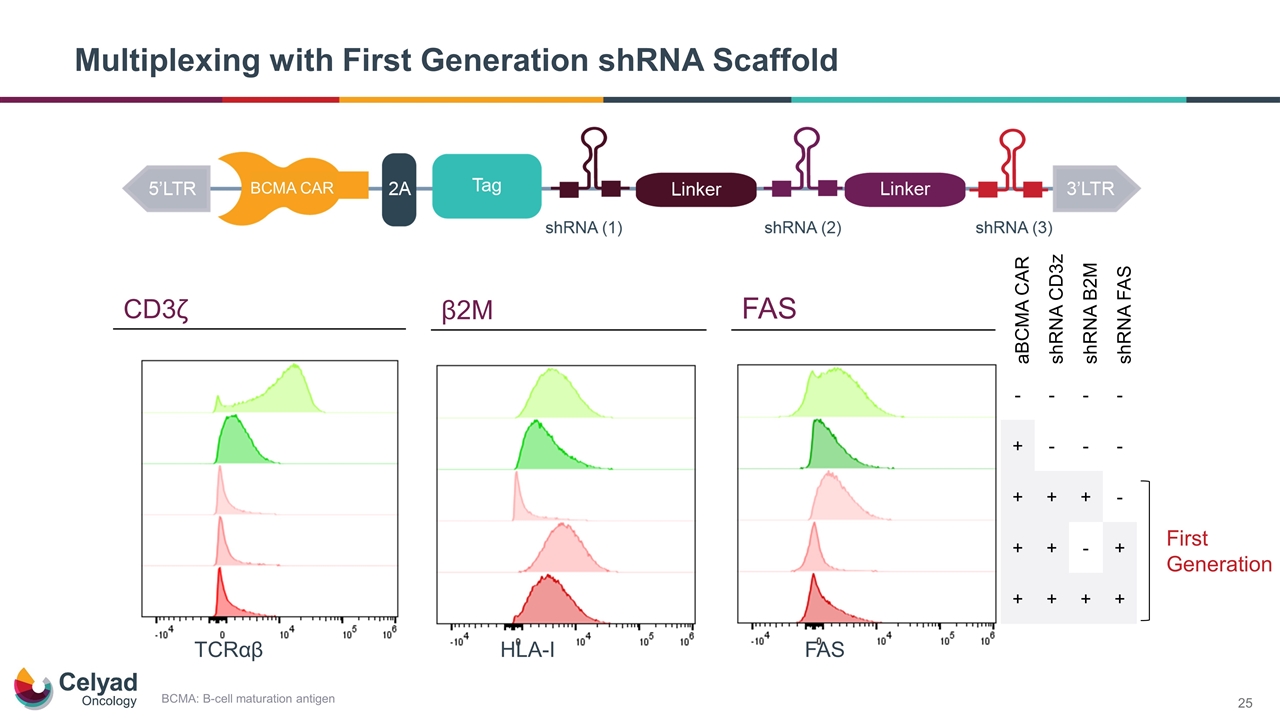
Multiplexing with First Generation shRNA Scaffold FAS HLA-I TCRαβ aBCMA CAR shRNA CD3z shRNA B2M shRNA FAS - - - - + - - - + + + - + + - + + + + + First Generation BCMA CAR CD3ζ β2M FAS BCMA: B-cell maturation antigen
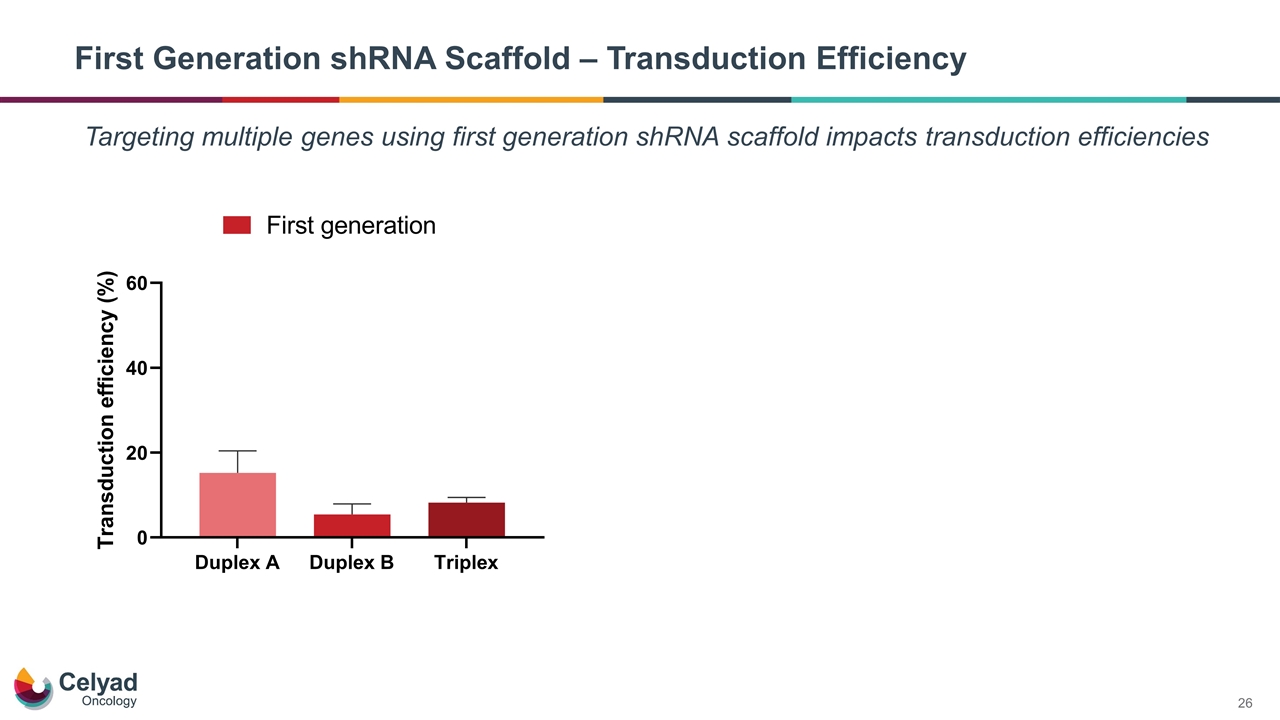
First Generation shRNA Scaffold – Transduction Efficiency Targeting multiple genes using first generation shRNA scaffold impacts transduction efficiencies
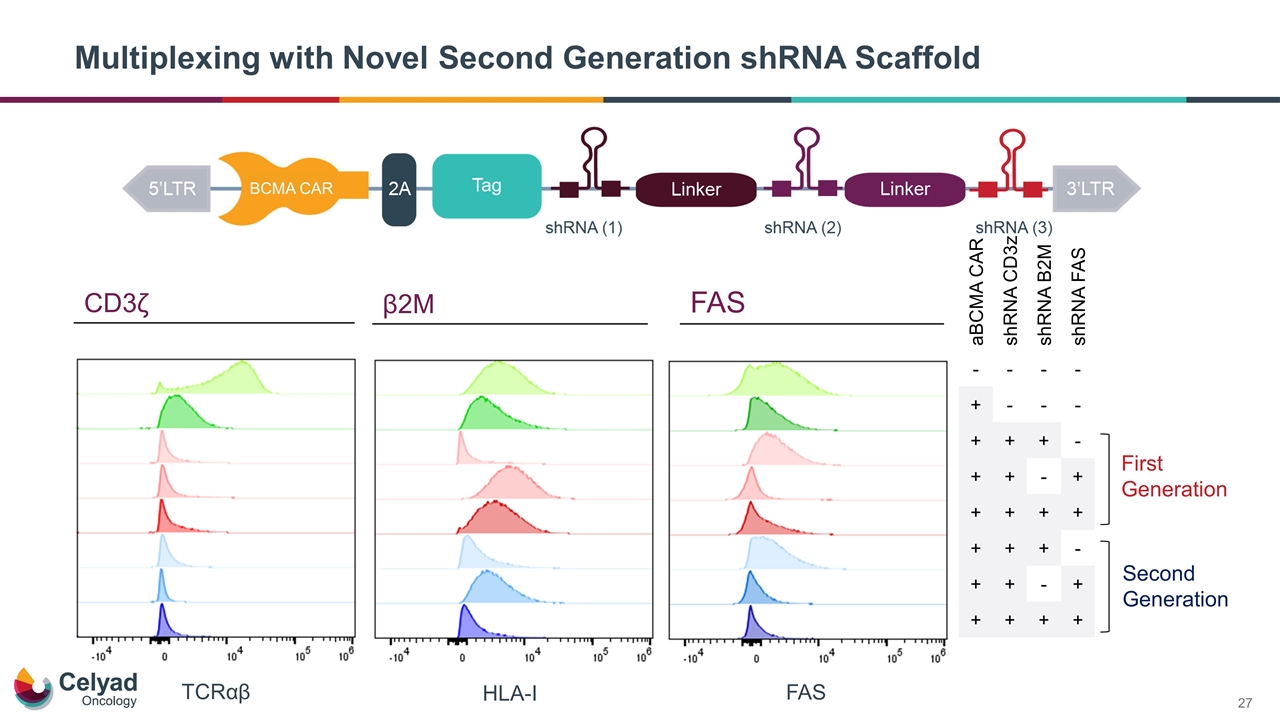
Multiplexing with Novel Second Generation shRNA Scaffold FAS HLA-I TCRαβ First Generation Second Generation aBCMA CAR shRNA CD3z shRNA B2M shRNA FAS - - - - + - - - + + + - + + - + + + + + + + + - + + - + + + + + CD3ζ β2M FAS BCMA CAR
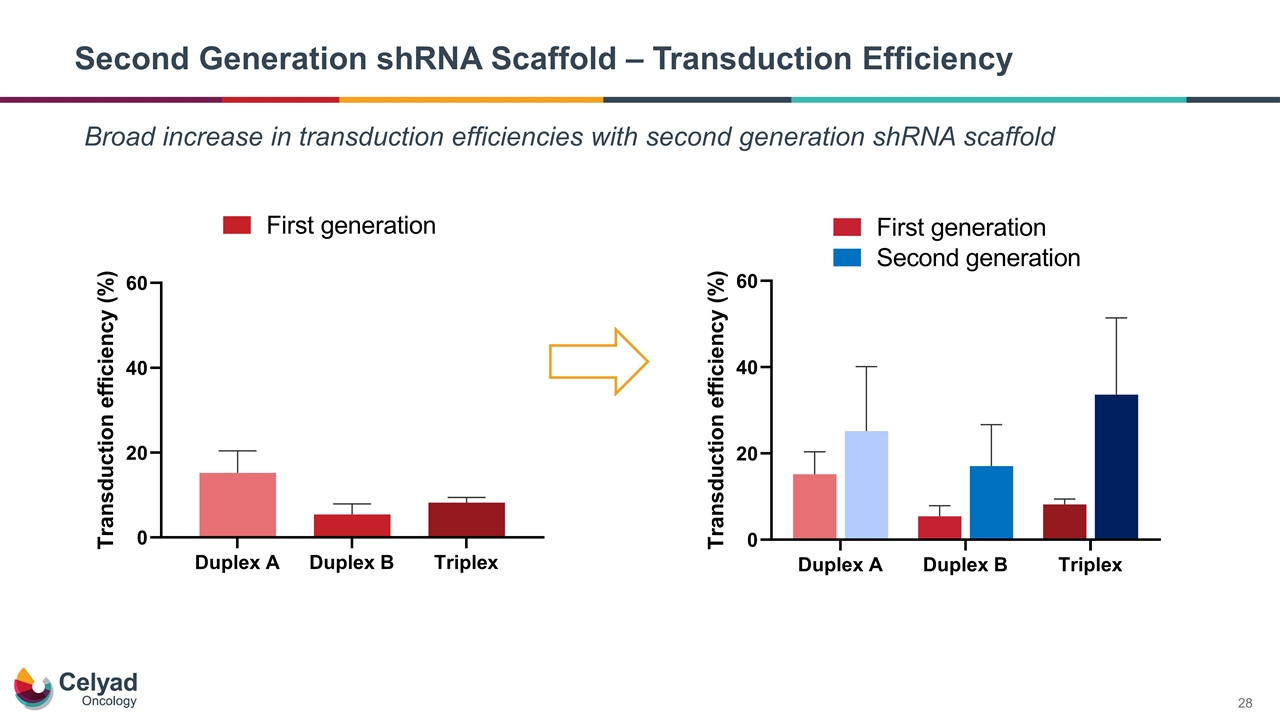
Second Generation shRNA Scaffold – Transduction Efficiency Broad increase in transduction efficiencies with second generation shRNA scaffold
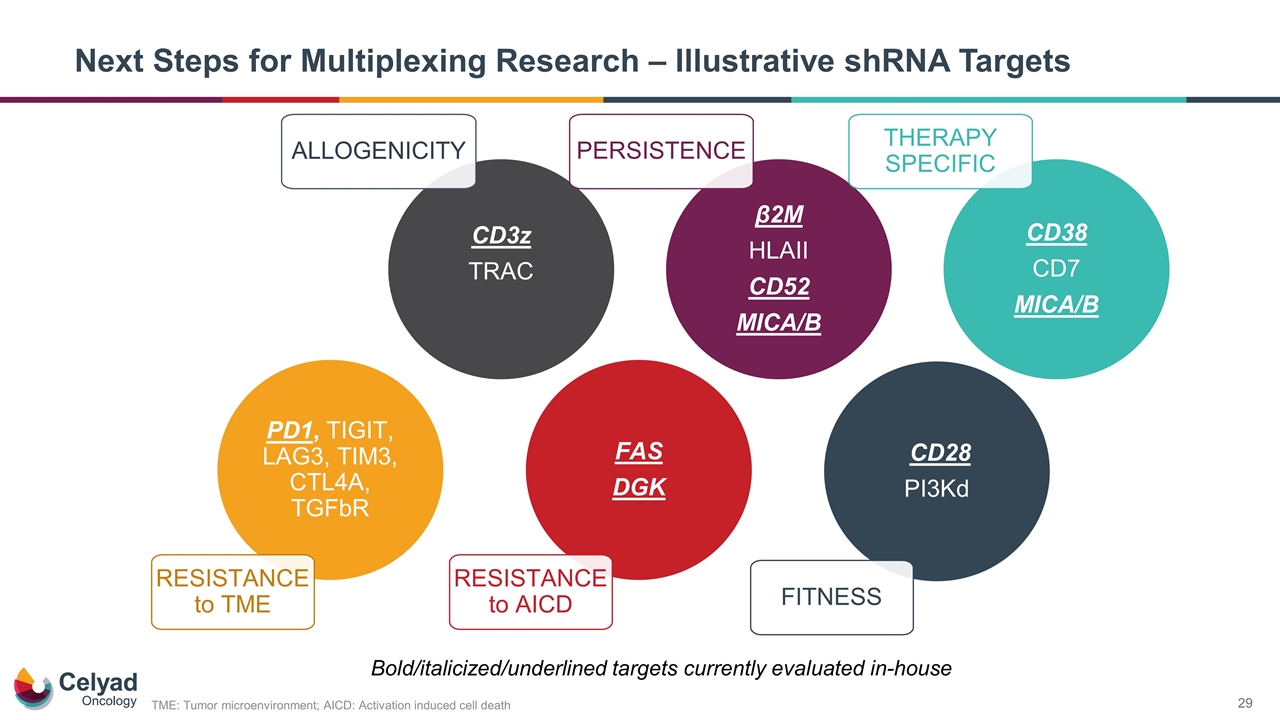
Next Steps for Multiplexing Research – Illustrative shRNA Targets CD3z TRAC β2M HLAII CD52 MICA/B CD28 PI3Kd PERSISTENCE FAS DGK PD1, TIGIT, LAG3, TIM3, CTL4A, TGFbR ALLOGENICITY FITNESS RESISTANCE to TME RESISTANCE to AICD CD38 CD7 MICA/B THERAPY SPECIFIC Bold/italicized/underlined targets currently evaluated in-house TME: Tumor microenvironment; AICD: Activation induced cell death

shRNA Platform – Future Programs
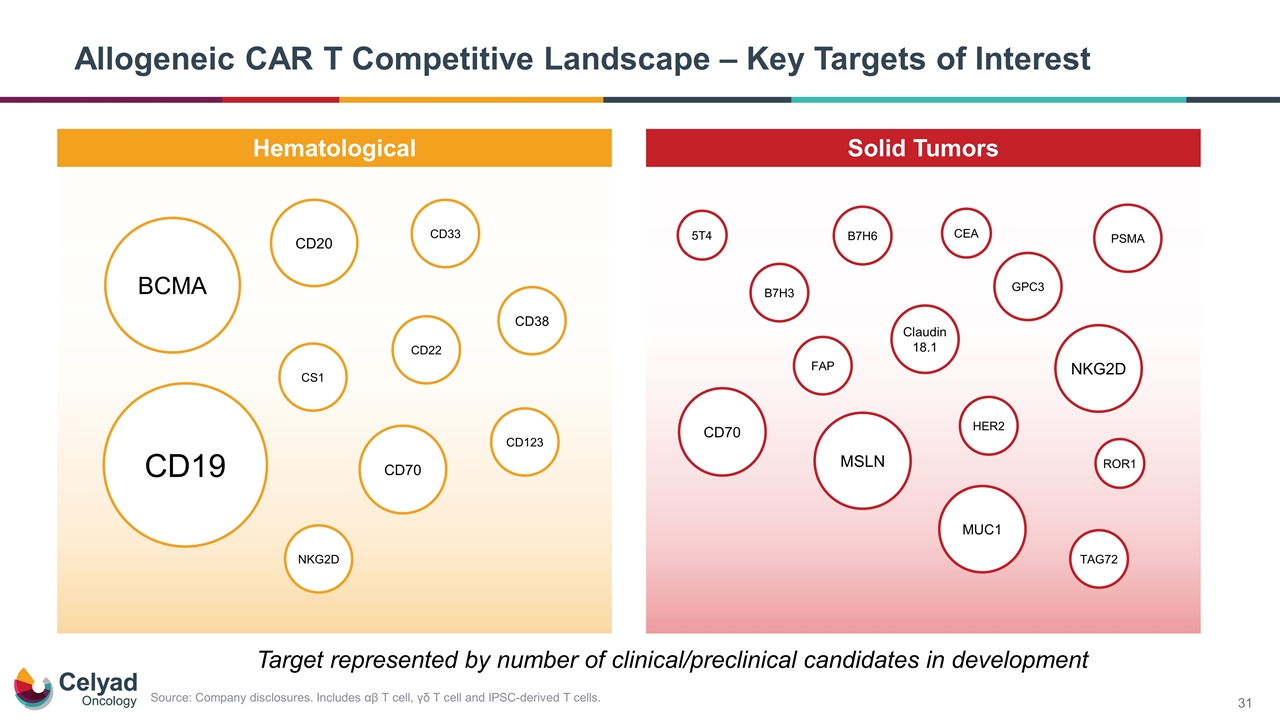
Allogeneic CAR T Competitive Landscape – Key Targets of Interest Hematological Solid Tumors CD123 CD20 PSMA HER2 CD70 GPC3 CD19 CS1 CD38 BCMA CD22 CD33 NKG2D MSLN MUC1 ROR1 Claudin 18.1 NKG2D B7H3 FAP TAG72 CD70 Target represented by number of clinical/preclinical candidates in development B7H6 5T4 CEA Source: Company disclosures. Includes αβ T cell, γδ T cell and IPSC-derived T cells.
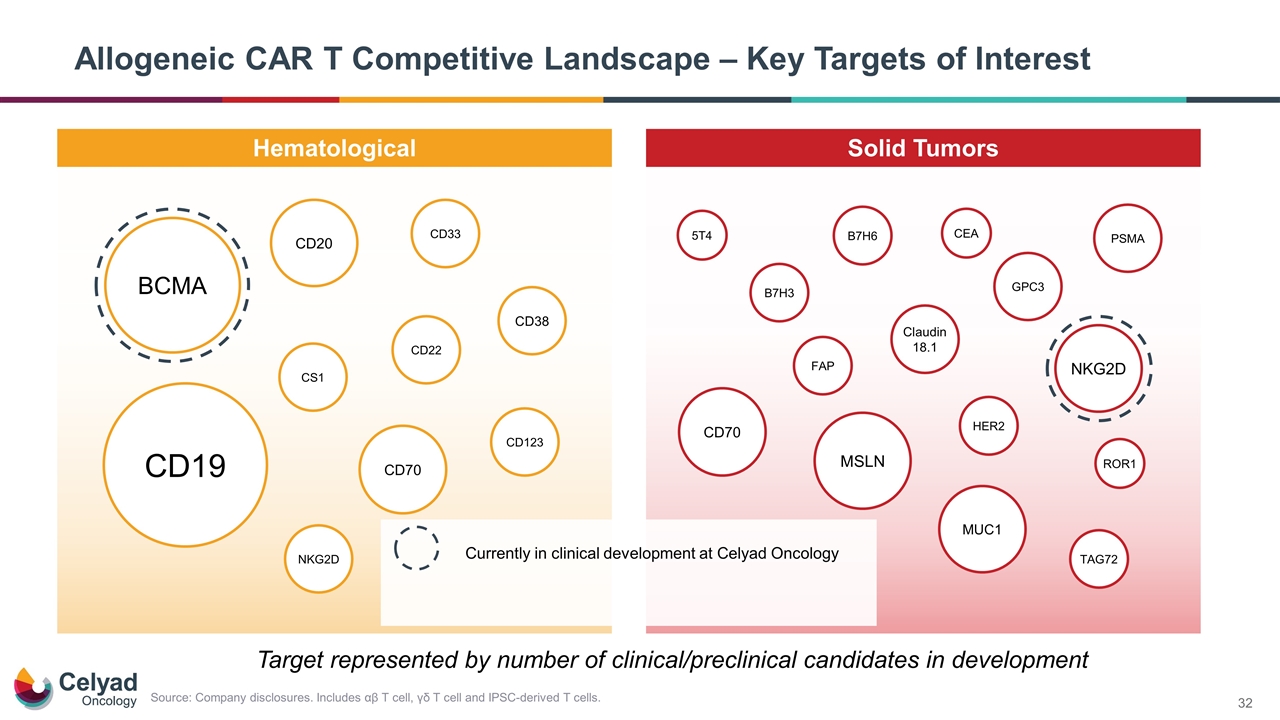
Allogeneic CAR T Competitive Landscape – Key Targets of Interest Hematological Solid Tumors CD123 CD20 PSMA HER2 CD70 GPC3 CD19 CS1 CD38 BCMA CD22 CD33 NKG2D MSLN MUC1 ROR1 Claudin 18.1 NKG2D B7H3 FAP TAG72 CD70 Target represented by number of clinical/preclinical candidates in development Source: Company disclosures. Includes αβ T cell, γδ T cell and IPSC-derived T cells. B7H6 5T4 CEA Currently in clinical development at Celyad Oncology
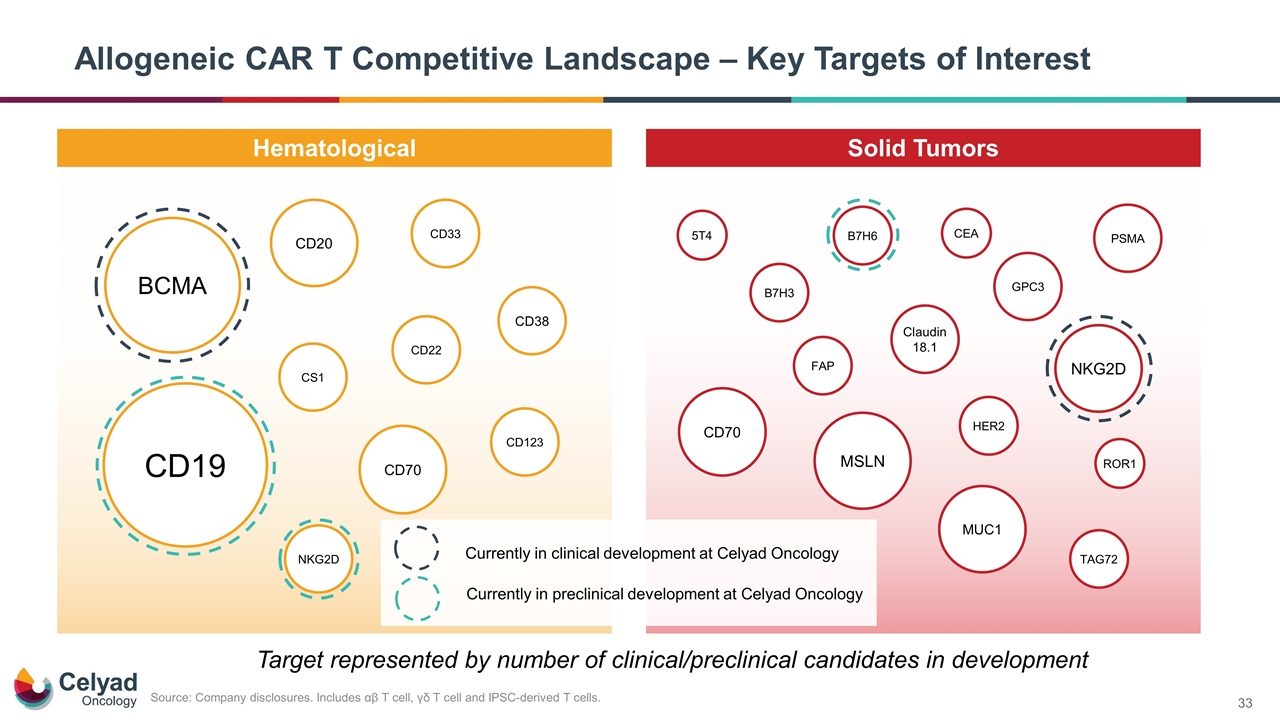
Allogeneic CAR T Competitive Landscape – Key Targets of Interest Hematological Solid Tumors CD123 CD20 PSMA HER2 CD70 GPC3 CD19 CS1 CD38 BCMA CD22 CD33 NKG2D MSLN MUC1 ROR1 Claudin 18.1 NKG2D B7H3 FAP TAG72 CD70 Target represented by number of clinical/preclinical candidates in development B7H6 5T4 CEA Currently in clinical development at Celyad Oncology Currently in preclinical development at Celyad Oncology Source: Company disclosures. Includes αβ T cell, γδ T cell and IPSC-derived T cells.
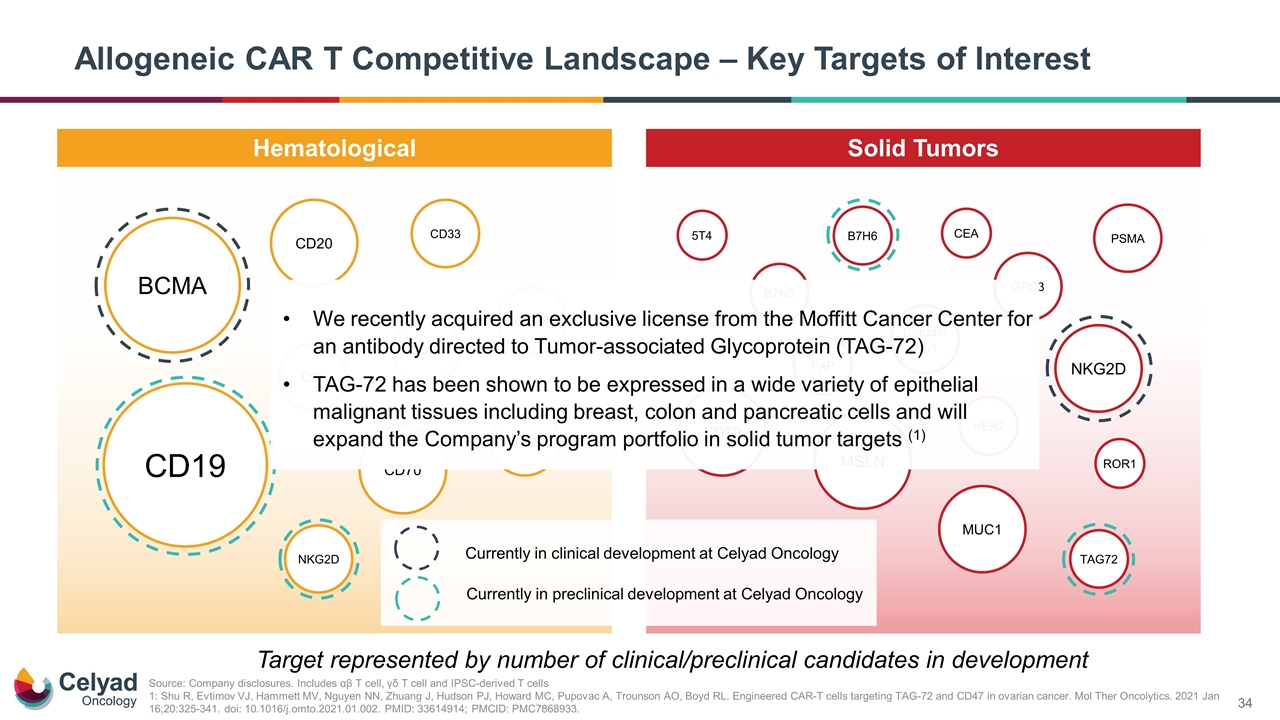
Allogeneic CAR T Competitive Landscape – Key Targets of Interest Hematological Solid Tumors CD123 CD20 PSMA HER2 CD70 GPC3 CD19 CS1 CD38 BCMA CD22 CD33 NKG2D MSLN MUC1 ROR1 Claudin 18.1 NKG2D B7H3 FAP TAG72 CD70 Target represented by number of clinical/preclinical candidates in development Source: Company disclosures. Includes αβ T cell, γδ T cell and IPSC-derived T cells 1: Shu R, Evtimov VJ, Hammett MV, Nguyen NN, Zhuang J, Hudson PJ, Howard MC, Pupovac A, Trounson AO, Boyd RL. Engineered CAR-T cells targeting TAG-72 and CD47 in ovarian cancer. Mol Ther Oncolytics. 2021 Jan 16;20:325-341. doi: 10.1016/j.omto.2021.01.002. PMID: 33614914; PMCID: PMC7868933. . B7H6 5T4 CEA Currently in clinical development at Celyad Oncology Currently in preclinical development at Celyad Oncology We recently acquired an exclusive license from the Moffitt Cancer Center for an antibody directed to Tumor-associated Glycoprotein (TAG-72) TAG-72 has been shown to be expressed in a wide variety of epithelial malignant tissues including breast, colon and pancreatic cells and will expand the Company’s program portfolio in solid tumor targets (1)

Update on CYAD-101 for mCRC
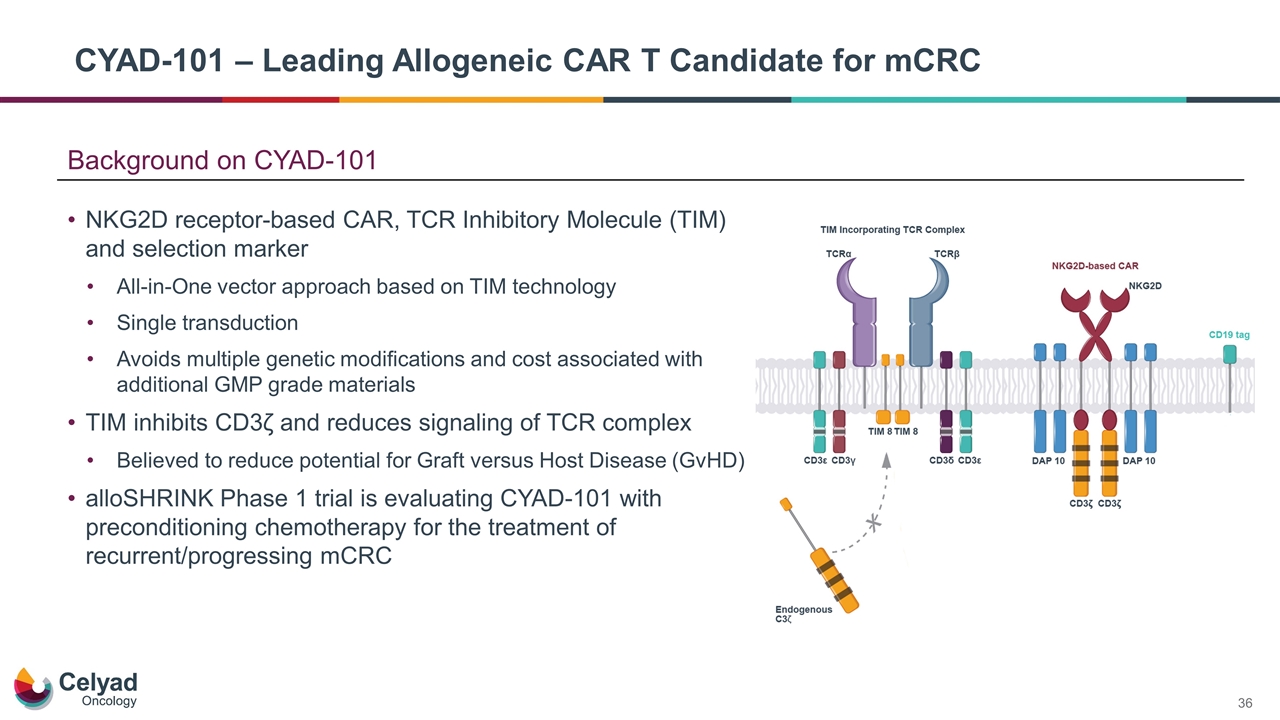
Background on CYAD-101 NKG2D receptor-based CAR, TCR Inhibitory Molecule (TIM) and selection marker All-in-One vector approach based on TIM technology Single transduction Avoids multiple genetic modifications and cost associated with additional GMP grade materials TIM inhibits CD3ζ and reduces signaling of TCR complex Believed to reduce potential for Graft versus Host Disease (GvHD) alloSHRINK Phase 1 trial is evaluating CYAD-101 with preconditioning chemotherapy for the treatment of recurrent/progressing mCRC CYAD-101 – Leading Allogeneic CAR T Candidate for mCRC
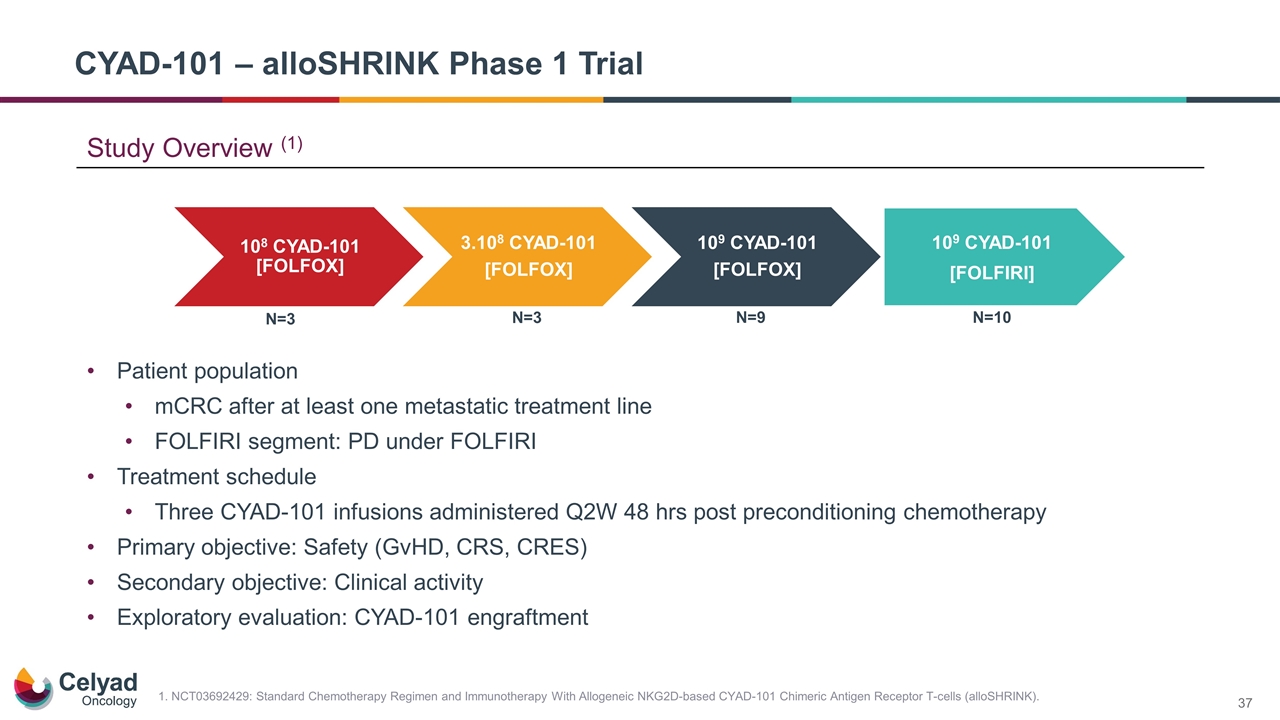
Patient population mCRC after at least one metastatic treatment line FOLFIRI segment: PD under FOLFIRI Treatment schedule Three CYAD-101 infusions administered Q2W 48 hrs post preconditioning chemotherapy Primary objective: Safety (GvHD, CRS, CRES) Secondary objective: Clinical activity Exploratory evaluation: CYAD-101 engraftment CYAD-101 – alloSHRINK Phase 1 Trial N=3 N=3 N=9 N=10 109 CYAD-101 [FOLFIRI] 1. NCT03692429: Standard Chemotherapy Regimen and Immunotherapy With Allogeneic NKG2D-based CYAD-101 Chimeric Antigen Receptor T-cells (alloSHRINK). Study Overview (1) 10 8 CYAD-101 [FOLFOX] 3.10 8 CYAD-101 [FOLFOX] 10 9 CYAD-101 [FOLFOX]
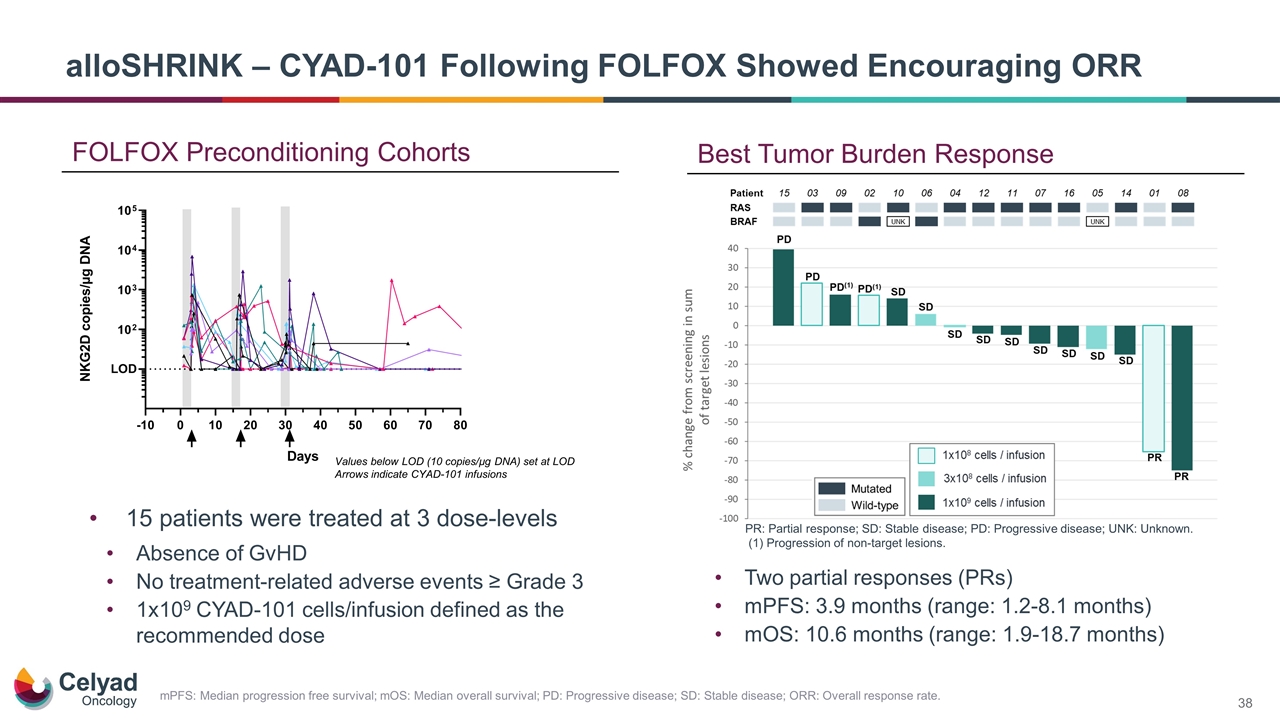
FOLFOX Preconditioning Cohorts Values below LOD (10 copies/µg DNA) set at LOD Arrows indicate CYAD-101 infusions alloSHRINK – CYAD-101 Following FOLFOX Showed Encouraging ORR 15 patients were treated at 3 dose-levels Absence of GvHD No treatment-related adverse events ≥ Grade 3 1x109 CYAD-101 cells/infusion defined as the recommended dose PR: Partial response; SD: Stable disease; PD: Progressive disease; UNK: Unknown. (1) Progression of non-target lesions. Best Tumor Burden Response Two partial responses (PRs) mPFS: 3.9 months (range: 1.2‑8.1 months) mOS: 10.6 months (range: 1.9‑18.7 months) mPFS: Median progression free survival; mOS: Median overall survival; PD: Progressive disease; SD: Stable disease; ORR: Overall response rate.
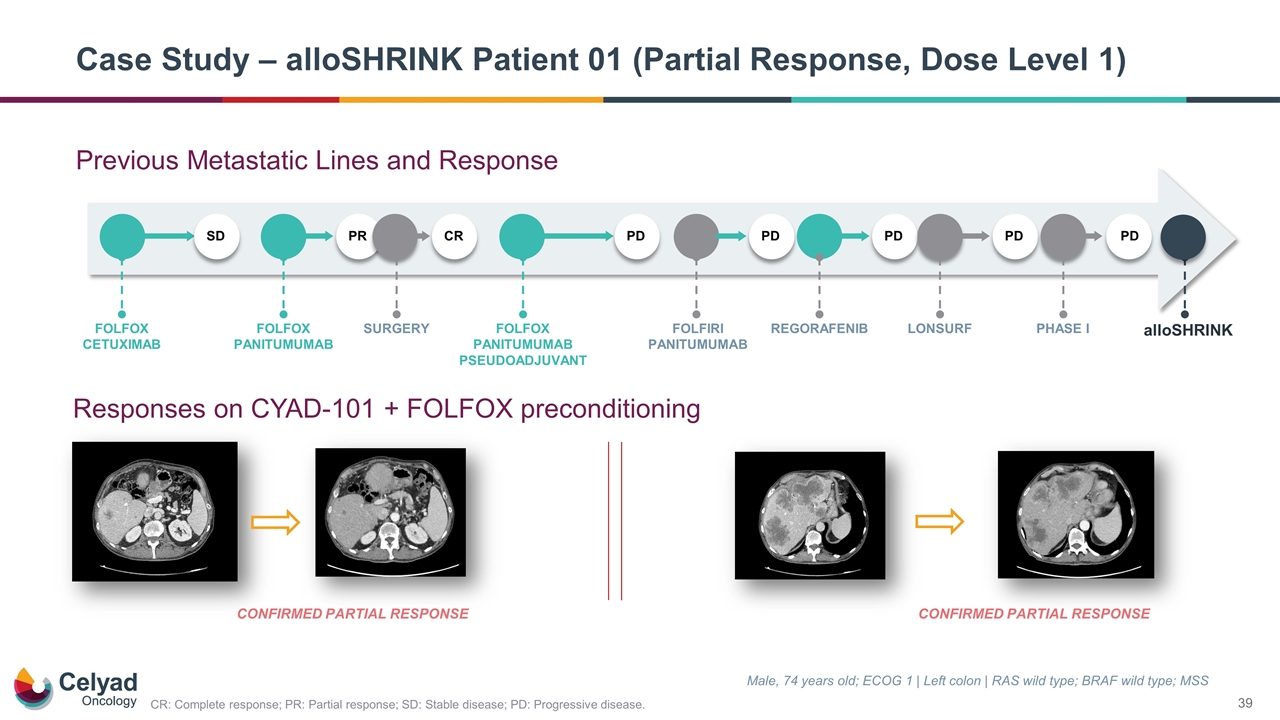
Previous Metastatic Lines and Response Case Study – alloSHRINK Patient 01 (Partial Response, Dose Level 1) Male, 74 years old; ECOG 1 | Left colon | RAS wild type; BRAF wild type; MSS CONFIRMED PARTIAL RESPONSE Responses on CYAD-101 + FOLFOX preconditioning SD FOLFOX CETUXIMAB PR FOLFOX PANITUMUMAB CR SURGERY PD FOLFOX PANITUMUMAB PSEUDOADJUVANT PD FOLFIRI PANITUMUMAB PD REGORAFENIB PD LONSURF PD PHASE I alloSHRINK CONFIRMED PARTIAL RESPONSE CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease.
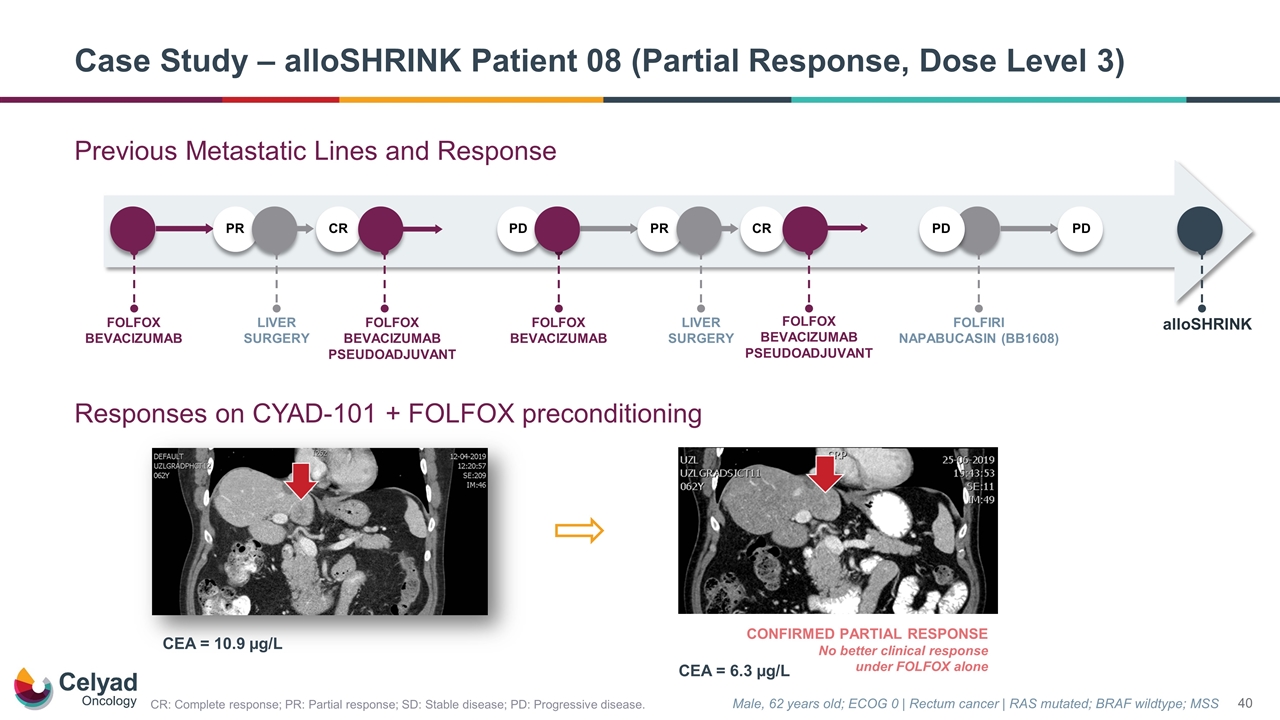
CONFIRMED PARTIAL RESPONSE No better clinical response under FOLFOX alone Male, 62 years old; ECOG 0 | Rectum cancer | RAS mutated; BRAF wildtype; MSS CEA = 10.9 µg/L CEA = 6.3 µg/L Case Study – alloSHRINK Patient 08 (Partial Response, Dose Level 3) Previous Metastatic Lines and Response Responses on CYAD-101 + FOLFOX preconditioning alloSHRINK FOLFOX BEVACIZUMAB PD PD FOLFIRI NAPABUCASIN (BB1608) PD PR LIVER SURGERY CR PR FOLFOX BEVACIZUMAB LIVER SURGERY CR FOLFOX BEVACIZUMAB PSEUDOADJUVANT FOLFOX BEVACIZUMAB PSEUDOADJUVANT CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease.
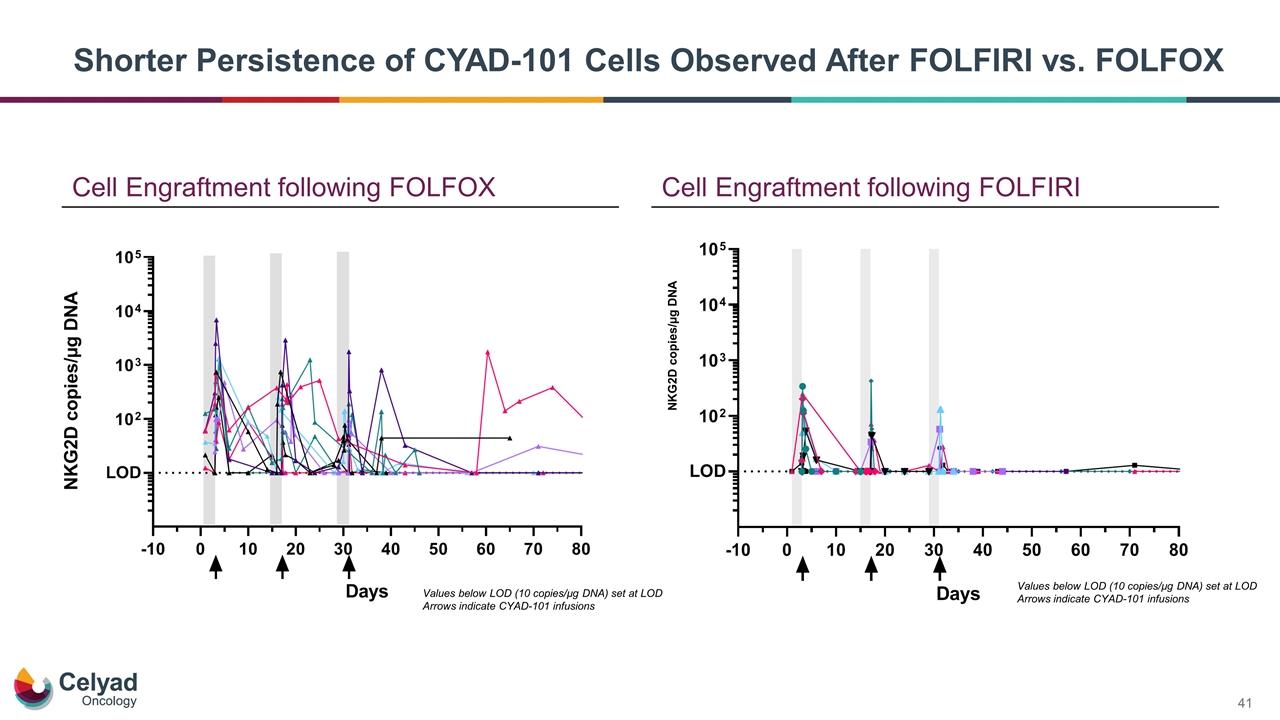
Cell Engraftment following FOLFIRI NKG2D copies/µg DNA Cell Engraftment following FOLFOX Values below LOD (10 copies/µg DNA) set at LOD Arrows indicate CYAD-101 infusions Values below LOD (10 copies/µg DNA) set at LOD Arrows indicate CYAD-101 infusions Shorter Persistence of CYAD-101 Cells Observed After FOLFIRI vs. FOLFOX
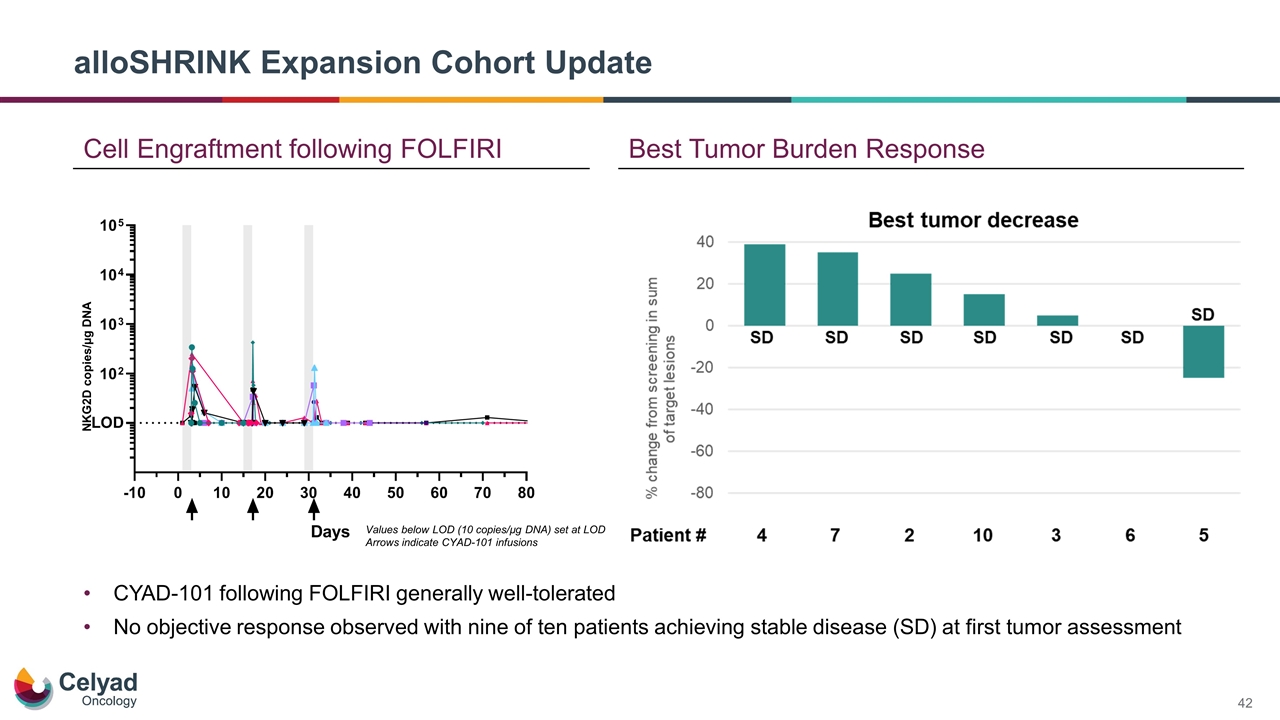
alloSHRINK Expansion Cohort Update Best Tumor Burden Response Cell Engraftment following FOLFIRI NKG2D copies/µg DNA Values below LOD (10 copies/µg DNA) set at LOD Arrows indicate CYAD-101 infusions CYAD-101 following FOLFIRI generally well-tolerated No objective response observed with nine of ten patients achieving stable disease (SD) at first tumor assessment
No relevant differences between CYAD-101 batches used CYAD-101 cells may be impacted (lysis) by irinotecan metabolite In vitro testing is ongoing to investigate lead hypothesis Cell Engraftment following FOLFIRI Cell Engraftment following FOLFOX Shorter Persistence of CYAD-101 Cells Observed After FOLFIRI vs. FOLFOX Values below LOD (10 copies/µg DNA) set at LOD Arrows indicate CYAD-101 infusions NKG2D copies/µg DNA Values below LOD (10 copies/µg DNA) set at LOD Arrows indicate CYAD-101 infusions Irinotecan pharmacokinetics-pharmacodynamics: the clinical relevance of prolonged exposure to SN-38 RHJ Mathijssen, J Verweij, WJ Loos, P de Bruijn, K Nooster and A parreboom - British Journal of Cancer (2002) 87, 144-150 - ©2002 Cancer Research UK British Journal of Cancer (2002) 87, 144-150 Cancer Research UK. All rights reserved 007-920/02 www.bjcancer.com
mCRC: Metastatic colorectal cancer; MoA: Mechanism of action; TME: Tumor microenvironment KEYNOTE-B79 Trial – CYAD-101 with KEYTRUDA® in Refractory mCRC alloSHRINK Highlights Expected Complementary MoAs Encouraging clinical activity observed in incurable mCRC patients Therapy well tolerated. No evidence of GvHD Evidence of new T-cell clones entering the hyper-expanded TCR repertoire post-treatment with CYAD-101 Modulation of the endogenous immune response NKG2D CAR T cells may convert the TME from immunosuppressive to immunostimulatory Anti-PD1 blocks the co-inhibitory interaction of cancer cells with immune cells and restores the immune response The anti-PD1 therapy, KEYTRUDA® (pembrolizumab), could enhance the CYAD-101-sculpted TME, thereby driving a more durable anti-tumor response KEYNOTE-B79 Trial
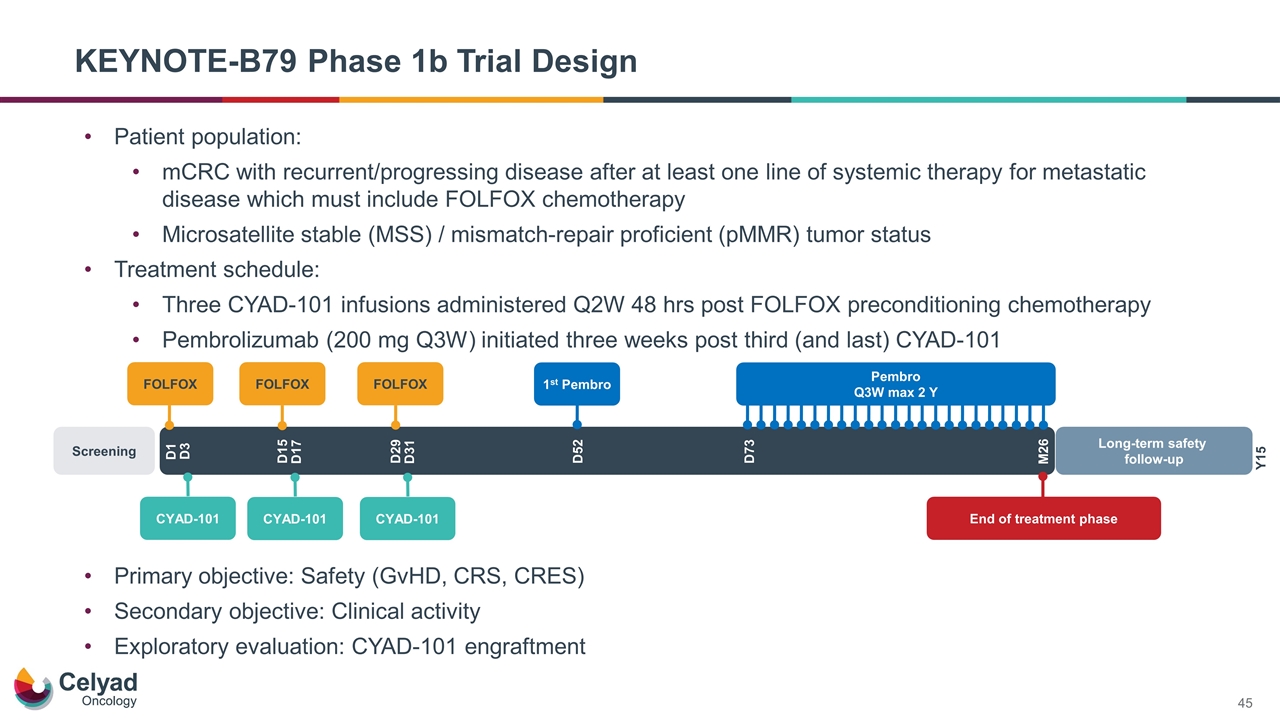
KEYNOTE-B79 Phase 1b Trial Design Primary objective: Safety (GvHD, CRS, CRES) Secondary objective: Clinical activity Exploratory evaluation: CYAD-101 engraftment FOLFOX Screening D1 Long-term safety follow-up Y15 D15 D29 D52 D73 M26 D3 D17 D31 FOLFOX FOLFOX CYAD-101 End of treatment phase CYAD-101 CYAD-101 1st Pembro Pembro Q3W max 2 Y Patient population: mCRC with recurrent/progressing disease after at least one line of systemic therapy for metastatic disease which must include FOLFOX chemotherapy Microsatellite stable (MSS) / mismatch-repair proficient (pMMR) tumor status Treatment schedule: Three CYAD-101 infusions administered Q2W 48 hrs post FOLFOX preconditioning chemotherapy Pembrolizumab (200 mg Q3W) initiated three weeks post third (and last) CYAD-101
alloSHRINK Phase 1 KEYNOTE-B79 Phase 1b Next Steps for CYAD-101 Program Continue to monitor patients Analyze SN-38 serum levels in study patients Evaluate sensitivity of CYAD-101 cells to SN-38 in vitro Regulatory process for review of protocol complete in U.S. and Belgium Study initiation by early fourth quarter 2021

Final Remarks and Q&A Company proprietary material – do not copy or distribute
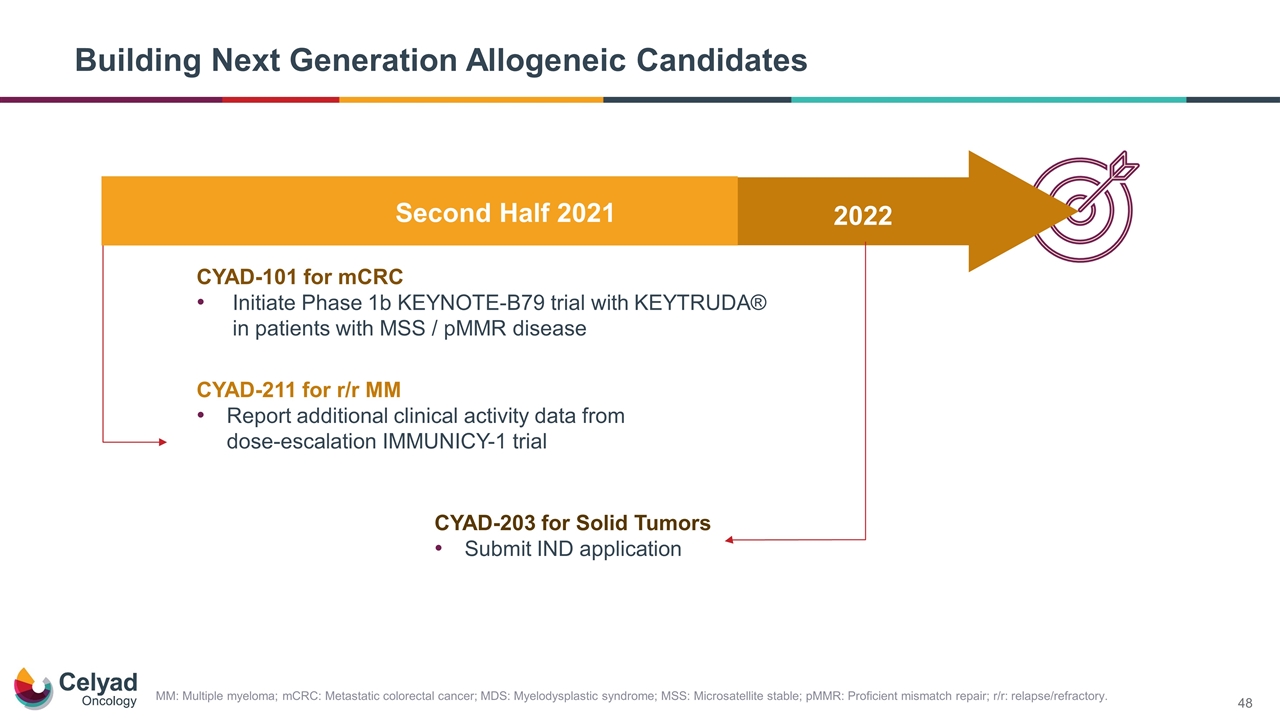
Building Next Generation Allogeneic Candidates MM: Multiple myeloma; mCRC: Metastatic colorectal cancer; MDS: Myelodysplastic syndrome; MSS: Microsatellite stable; pMMR: Proficient mismatch repair; r/r: relapse/refractory. Second Half 2021 2022 CYAD-211 for r/r MM Report additional clinical activity data from dose-escalation IMMUNICY-1 trial CYAD-101 for mCRC Initiate Phase 1b KEYNOTE-B79 trial with KEYTRUDA® in patients with MSS / pMMR disease CYAD-203 for Solid Tumors Submit IND application
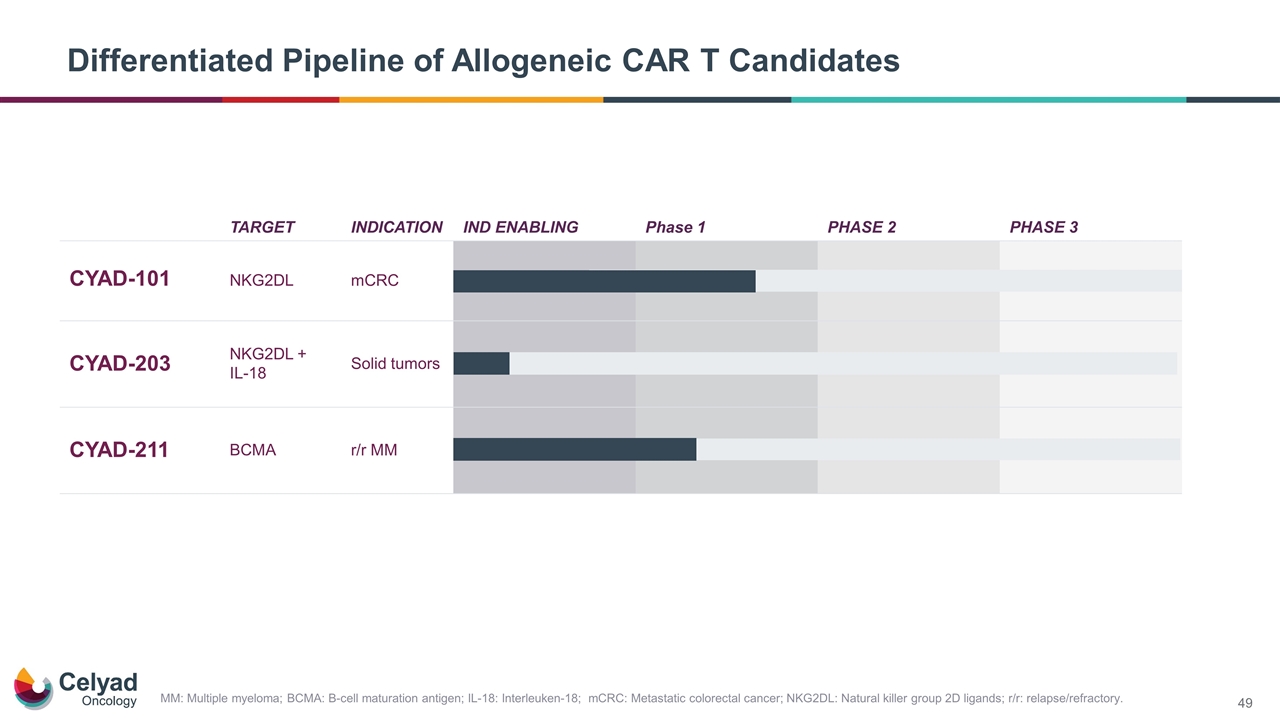
Differentiated Pipeline of Allogeneic CAR T Candidates TARGET INDICATION IND ENABLING Phase 1 PHASE 2 PHASE 3 CYAD-101 NKG2DL mCRC CYAD-203 NKG2DL + IL-18 Solid tumors CYAD-211 BCMA r/r MM MM: Multiple myeloma; BCMA: B-cell maturation antigen; IL-18: Interleuken-18; mCRC: Metastatic colorectal cancer; NKG2DL: Natural killer group 2D ligands; r/r: relapse/refractory.

Research and Development Day Virtual Webinar July 20, 2021


















































