
2021 American Society of Hematology Meeting Update December 13, 2021 Exhibit 99.2

Forward Looking Statements
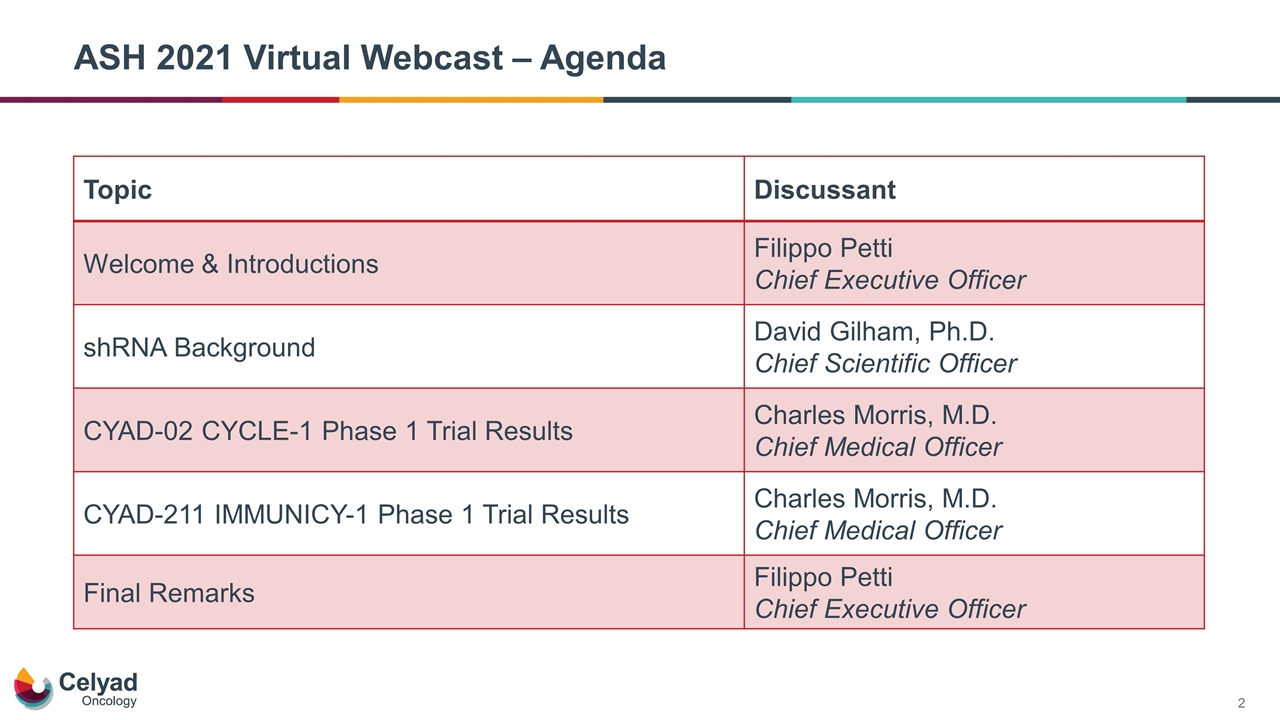
ASH 2021 Virtual Webcast – Agenda Topic Discussant Welcome & Introductions Filippo Petti Chief Executive Officer shRNA Background David Gilham, Ph.D. Chief Scientific Officer CYAD-02 CYCLE-1 Phase 1 Trial Results Charles Morris, M.D. Chief Medical Officer CYAD-211 IMMUNICY-1 Phase 1 Trial Results Charles Morris, M.D. Chief Medical Officer Final Remarks Filippo Petti Chief Executive Officer
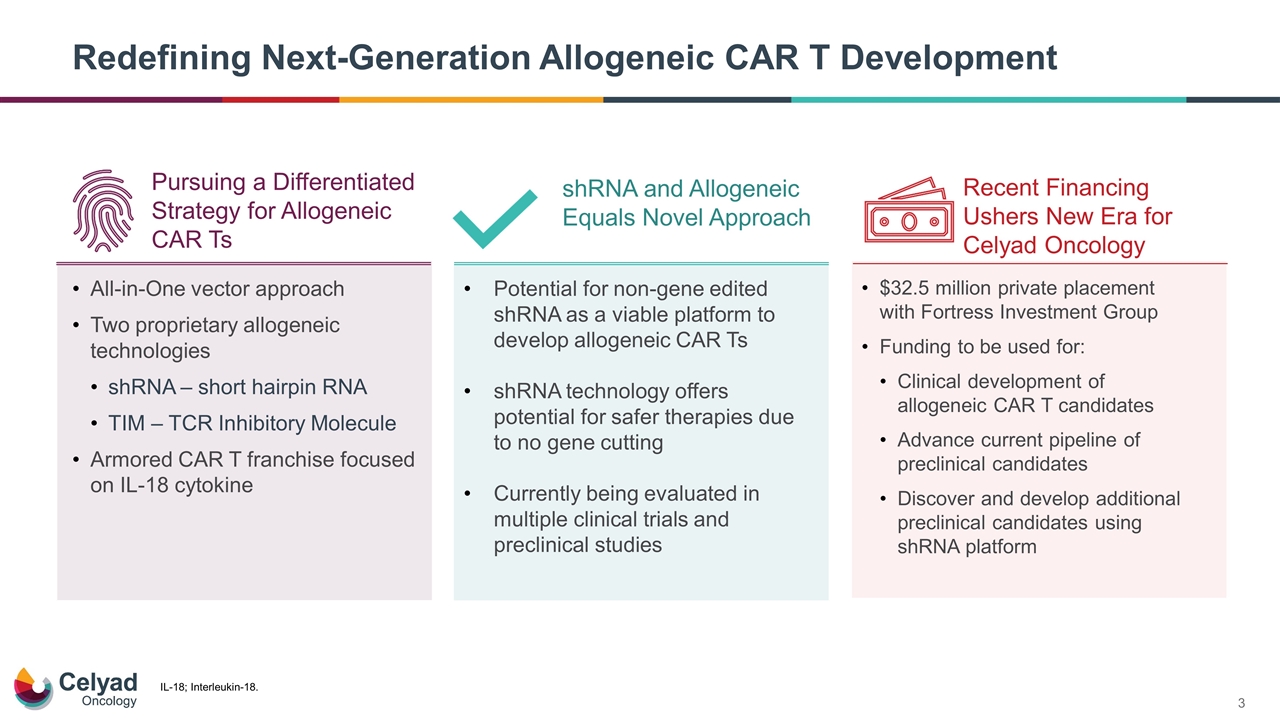
Redefining Next-Generation Allogeneic CAR T Development All-in-One vector approach Two proprietary allogeneic technologies shRNA – short hairpin RNA TIM – TCR Inhibitory Molecule Armored CAR T franchise focused on IL-18 cytokine Potential for non-gene edited shRNA as a viable platform to develop allogeneic CAR Ts shRNA technology offers potential for safer therapies due to no gene cutting Currently being evaluated in multiple clinical trials and preclinical studies $32.5 million private placement with Fortress Investment Group Funding to be used for: Clinical development of allogeneic CAR T candidates Advance current pipeline of preclinical candidates Discover and develop additional preclinical candidates using shRNA platform shRNA and Allogeneic Equals Novel Approach Recent Financing Ushers New Era for Celyad Oncology Pursuing a Differentiated Strategy for Allogeneic CAR Ts IL-18; Interleukin-18.

David Gilham, Ph.D. Chief Scientific Officer shRNA Background
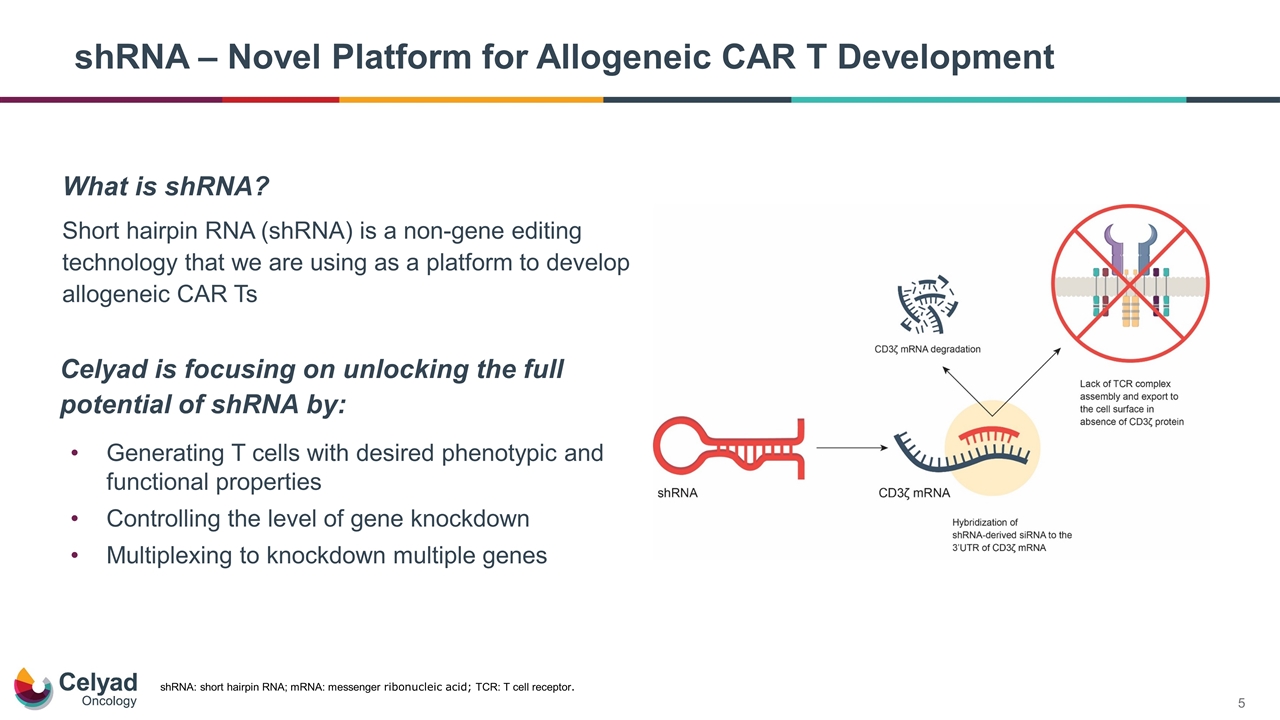
shRNA – Novel Platform for Allogeneic CAR T Development shRNA: short hairpin RNA; mRNA: messenger ribonucleic acid; TCR: T cell receptor. Generating T cells with desired phenotypic and functional properties Controlling the level of gene knockdown Multiplexing to knockdown multiple genes Celyad is focusing on unlocking the full potential of shRNA by: What is shRNA? Short hairpin RNA (shRNA) is a non-gene editing technology that we are using as a platform to develop allogeneic CAR Ts
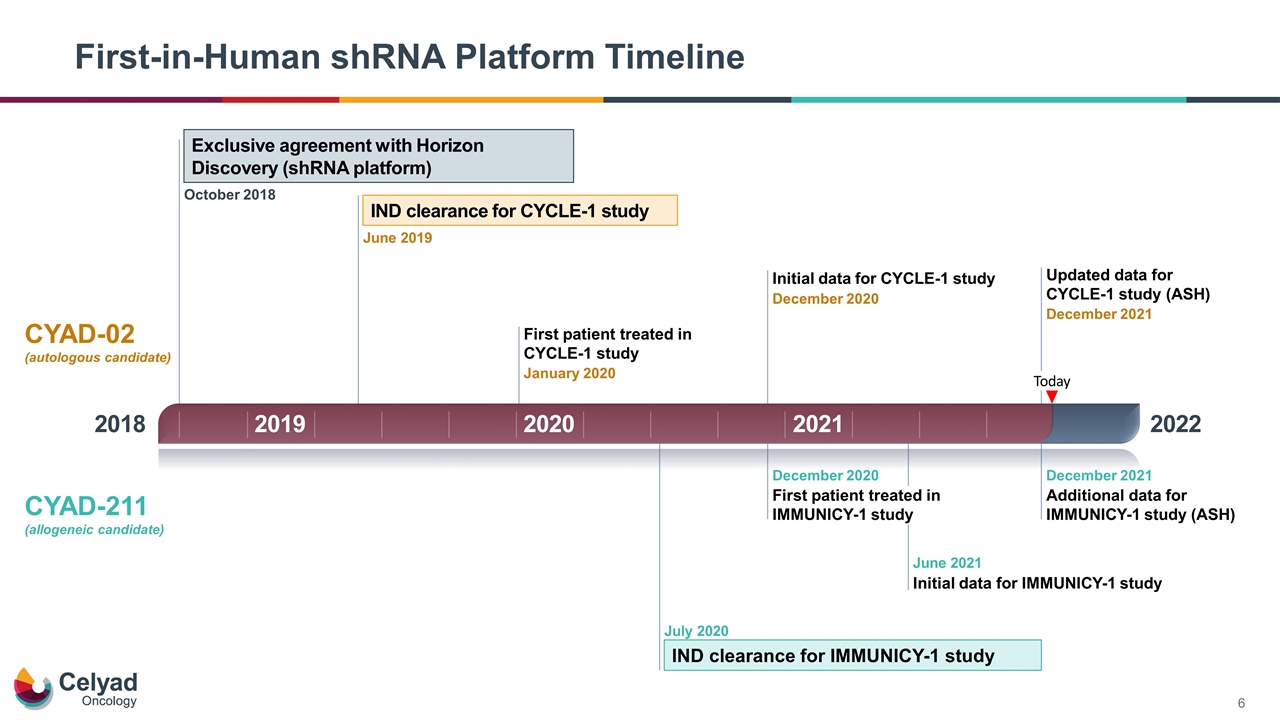
First-in-Human shRNA Platform Timeline 2018 2022 Today Exclusive agreement with Horizon Discovery (shRNA platform) October 2018 IND clearance for CYCLE-1 study June 2019 First patient treated in CYCLE-1 study January 2020 IND clearance for IMMUNICY-1 study July 2020 First patient treated in IMMUNICY-1 study December 2020 Initial data for CYCLE-1 study December 2020 Initial data for IMMUNICY-1 study June 2021 Updated data for CYCLE-1 study (ASH) December 2021 Additional data for IMMUNICY-1 study (ASH) December 2021 CYAD-02 (autologous candidate) CYAD-211 (allogeneic candidate) 2019 2020 2021
Potential Advantages of Non-Gene Edited Allogeneic CAR Ts Using shRNA No double strand breaks – expected minimal risk of genomic instability and translocation Not all allogeneic CAR Ts are created equal All-in-One vector – long clinical history including maintenance of transgenic payload integrity1,2 Level of gene expression can be titrated to the desired level through the choice of specific shRNA No target gene is inaccessible – genes that can be targeted by genetic knockout can be controlled with shRNA Multiplexed shRNA – targeting multiple genes simultaneously in the absence of reported genomic instability issues associated with multiplexed gene editing3 1 Marcucci et al. (2018) Mol Therapy doi: 10.1016/j.ymthe.2017.10.012 2 Scholler et al. (2012). Sci Trans Med. 10.1126/scitranslmed.3003761 3 Bother et al. (2020) The Crispr Journal (http://doi.org/10.1089/crispr.2019.0074)
Expected Real-World Benefits of Our Approach to Allogeneic CAR T Gene Knockdown Aims to Optimize Therapy Shorter Manufacturing Time Lower Cost of Goods Sold Ability to potentially simultaneously knockdown multiple gene targets of interest shRNA can target genes that are not suitable for gene editing, thereby potentially expanding therapies based on indication / disease of interest T cells age with longer culture times. Short culture times expected to maintain an optimal ‘fit’ T cell phenotype associated with therapeutic potential Vector costs are a major contributor to Cost of Goods Sold Using a single vector avoids the costs associated with multi-vector technologies

Technology continues to show promise as a platform No evidence of GvHD Cell kinetics Initial clinical activity shRNA as a Novel Allogeneic Technology for CAR T

Charles Morris, M.D. Chief Medical Officer CYAD-02 CYCLE-1 Trial Results
CYAD-02 – Next-Generation Autologous NKG2D CAR T CYAD-02 incorporates shRNA technology to silence the expression of the NKG2D ligands MICA and MICB Targeting MICA and MICB with a single shRNA leads to decrease of ligand expression on T cells and enhanced in vitro expansion compared to first-generation autologous NKG2D candidate, CYAD-01 Background on CYAD-02 NKG2D - natural killer group 2D receptor Building upon our first-generation autologous program
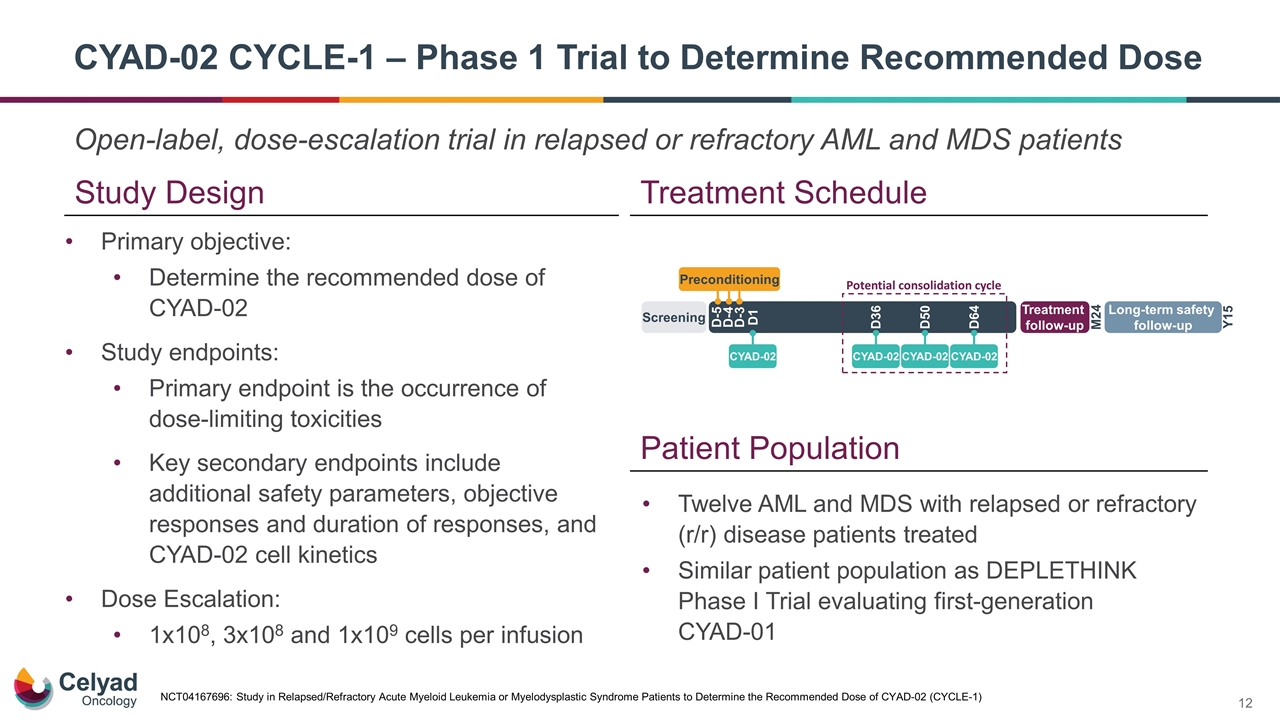
Open-label, dose-escalation trial in relapsed or refractory AML and MDS patients CYAD-02 CYCLE-1 – Phase 1 Trial to Determine Recommended Dose Study Design Treatment Schedule Twelve AML and MDS with relapsed or refractory (r/r) disease patients treated Similar patient population as DEPLETHINK Phase I Trial evaluating first-generation CYAD-01 Patient Population Primary objective: Determine the recommended dose of CYAD-02 Study endpoints: Primary endpoint is the occurrence of dose-limiting toxicities Key secondary endpoints include additional safety parameters, objective responses and duration of responses, and CYAD-02 cell kinetics Dose Escalation: 1x108, 3x108 and 1x109 cells per infusion NCT04167696: Study in Relapsed/Refractory Acute Myeloid Leukemia or Myelodysplastic Syndrome Patients to Determine the Recommended Dose of CYAD-02 (CYCLE-1)
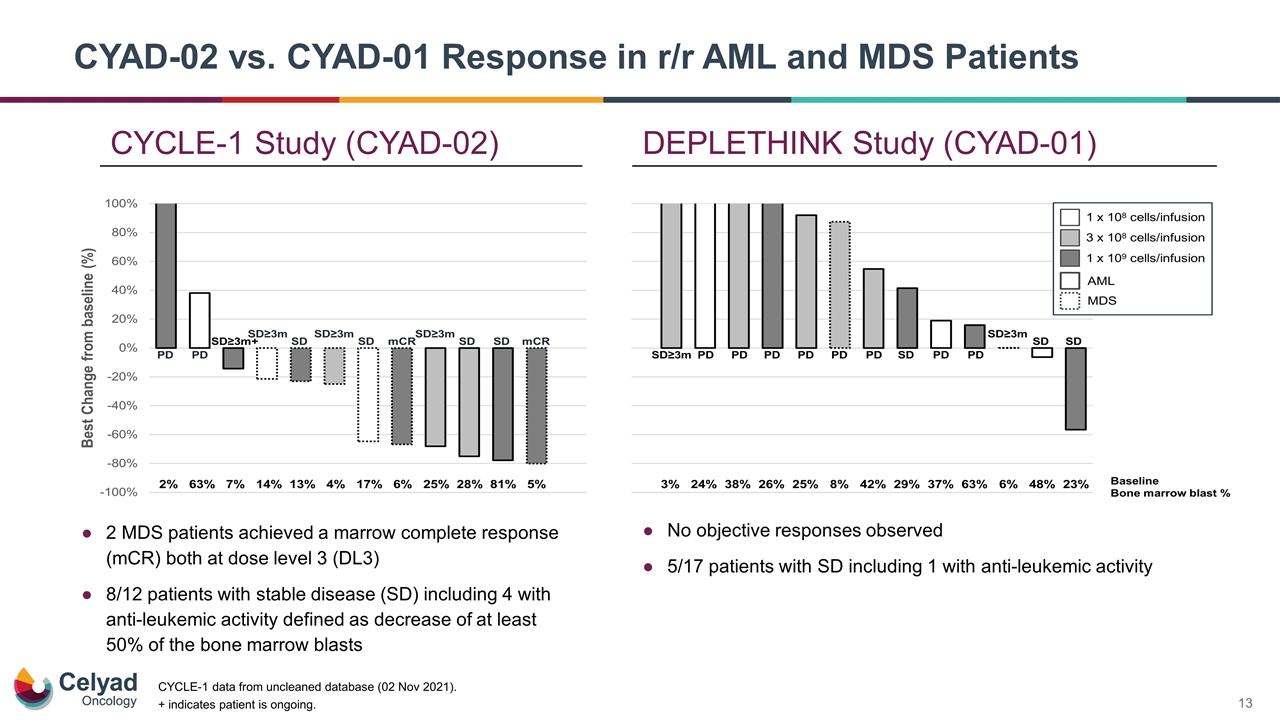
+ indicates patient is ongoing. No objective responses observed 5/17 patients with SD including 1 with anti-leukemic activity 2 MDS patients achieved a marrow complete response (mCR) both at dose level 3 (DL3) 8/12 patients with stable disease (SD) including 4 with anti-leukemic activity defined as decrease of at least 50% of the bone marrow blasts CYCLE-1 data from uncleaned database (02 Nov 2021). CYCLE-1 Study (CYAD-02) DEPLETHINK Study (CYAD-01) CYAD-02 vs. CYAD-01 Response in r/r AML and MDS Patients
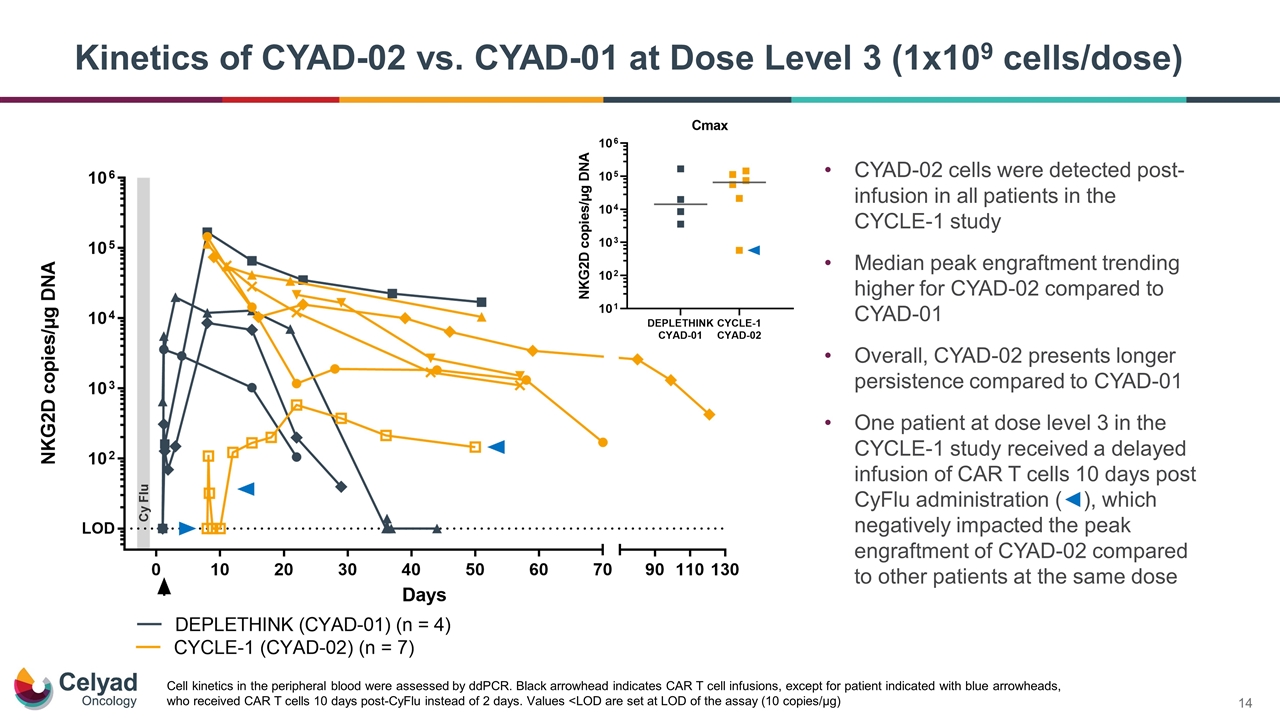
CYAD-02 cells were detected post-infusion in all patients in the CYCLE-1 study Median peak engraftment trending higher for CYAD-02 compared to CYAD-01 Overall, CYAD-02 presents longer persistence compared to CYAD-01 One patient at dose level 3 in the CYCLE-1 study received a delayed infusion of CAR T cells 10 days post CyFlu administration (◄), which negatively impacted the peak engraftment of CYAD-02 compared to other patients at the same dose Kinetics of CYAD-02 vs. CYAD-01 at Dose Level 3 (1x109 cells/dose) Cell kinetics in the peripheral blood were assessed by ddPCR. Black arrowhead indicates CAR T cell infusions, except for patient indicated with blue arrowheads, who received CAR T cells 10 days post-CyFlu instead of 2 days. Values <LOD are set at LOD of the assay (10 copies/µg)
CYAD-02 CYCLE-1 Summary Data support the use of shRNA platform for CAR T development Single shRNA can target two independent genes to enhance the phenotype of the CAR T cells Overall good tolerability profile of CYAD-02 post CyFlu preconditioning Two objective responses (mCR) at dose level 3 leading to 33% objective response rate and anti-leukemic activity in 50% of patients The knockdown of MICA/MICB with shRNA appears to have a positive contribution to the initial clinical activity of CYAD-02 as compared to first generation autologous NKG2D CAR T, CYAD-01 Trending higher engraftment of CYAD-02 vs. CYAD-01, potentially associated with the knockdown of MICA/MICB and reduced fratricide in vivo The lack of IL-7/IL-15 induction following preconditioning can be a limiting factor to the homeostatic proliferation of CAR T cells Likely related to the biology of myeloid malignancies A factor for consideration for further clinical development in these specific diseases

Charles Morris, M.D. Chief Medical Officer CYAD-211 IMMUNICY-1 Phase 1 Trial Update
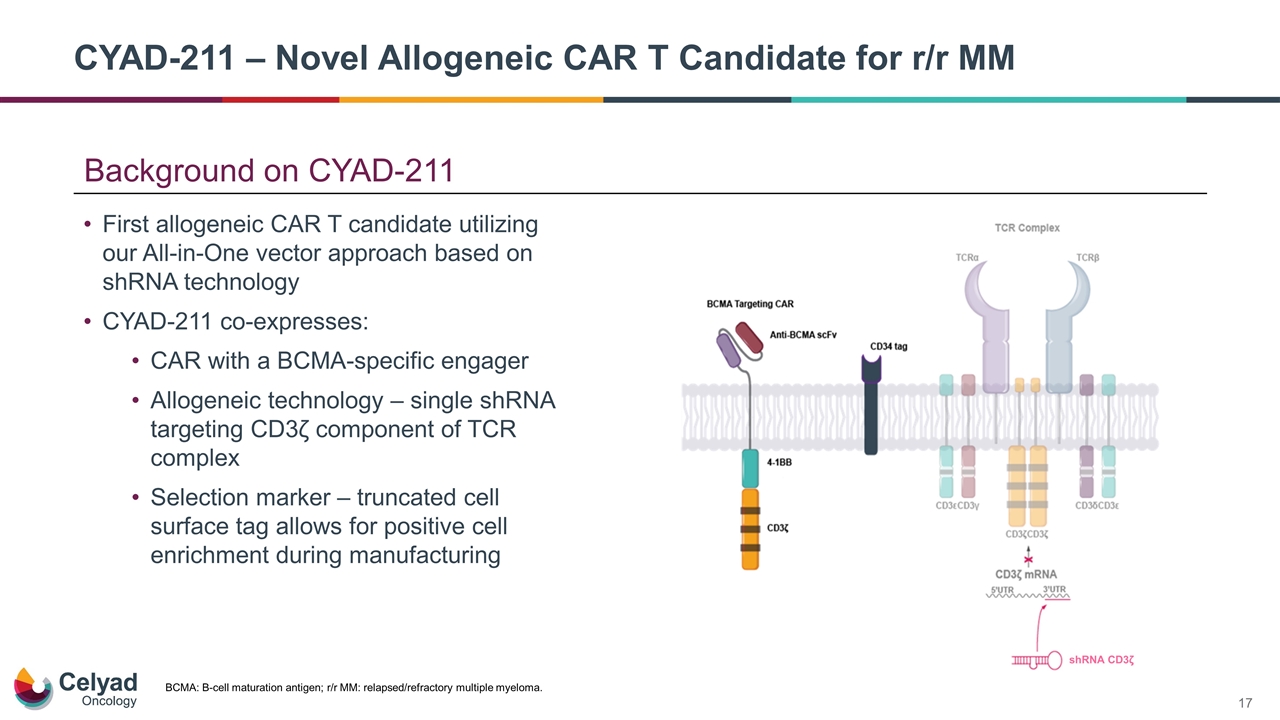
Background on CYAD-211 First allogeneic CAR T candidate utilizing our All-in-One vector approach based on shRNA technology CYAD-211 co-expresses: CAR with a BCMA-specific engager Allogeneic technology – single shRNA targeting CD3ζ component of TCR complex Selection marker – truncated cell surface tag allows for positive cell enrichment during manufacturing BCMA: B-cell maturation antigen; r/r MM: relapsed/refractory multiple myeloma. CYAD-211 – Novel Allogeneic CAR T Candidate for r/r MM shRNA CD3ζ
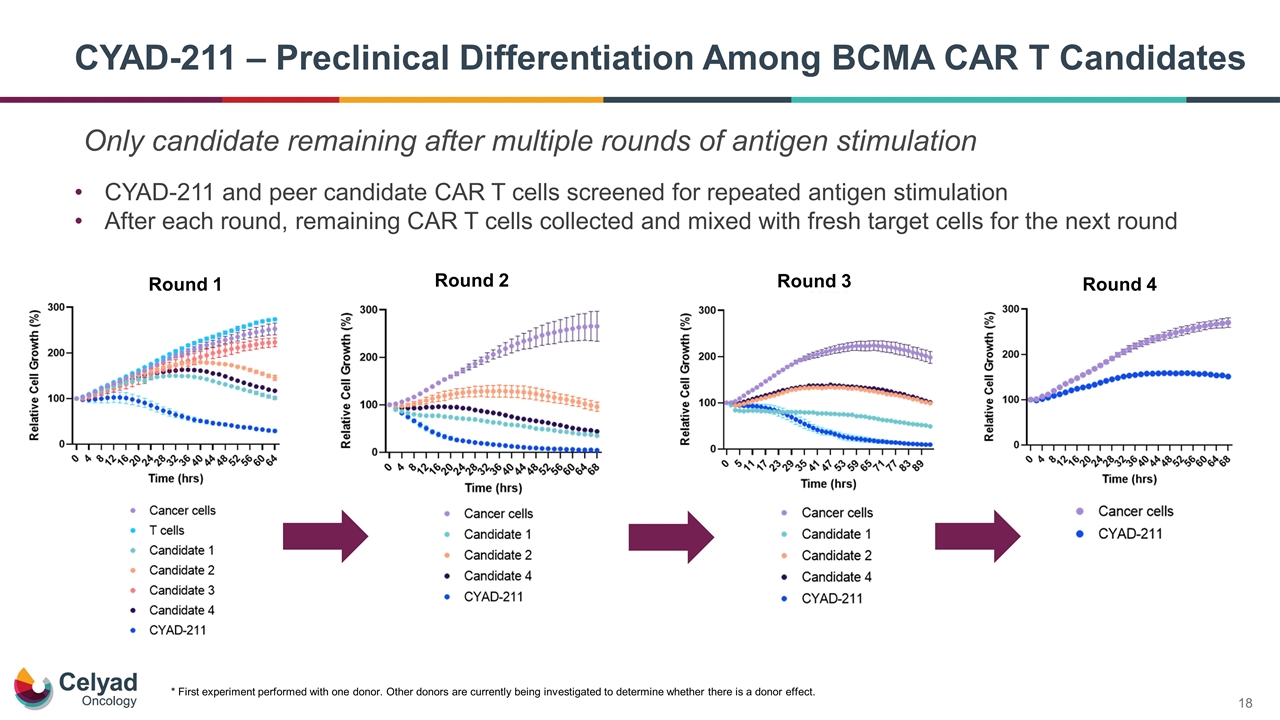
CYAD-211 – Preclinical Differentiation Among BCMA CAR T Candidates * First experiment performed with one donor. Other donors are currently being investigated to determine whether there is a donor effect. CYAD-211 and peer candidate CAR T cells screened for repeated antigen stimulation After each round, remaining CAR T cells collected and mixed with fresh target cells for the next round Round 1 Round 2 Round 3 Round 4 Only candidate remaining after multiple rounds of antigen stimulation
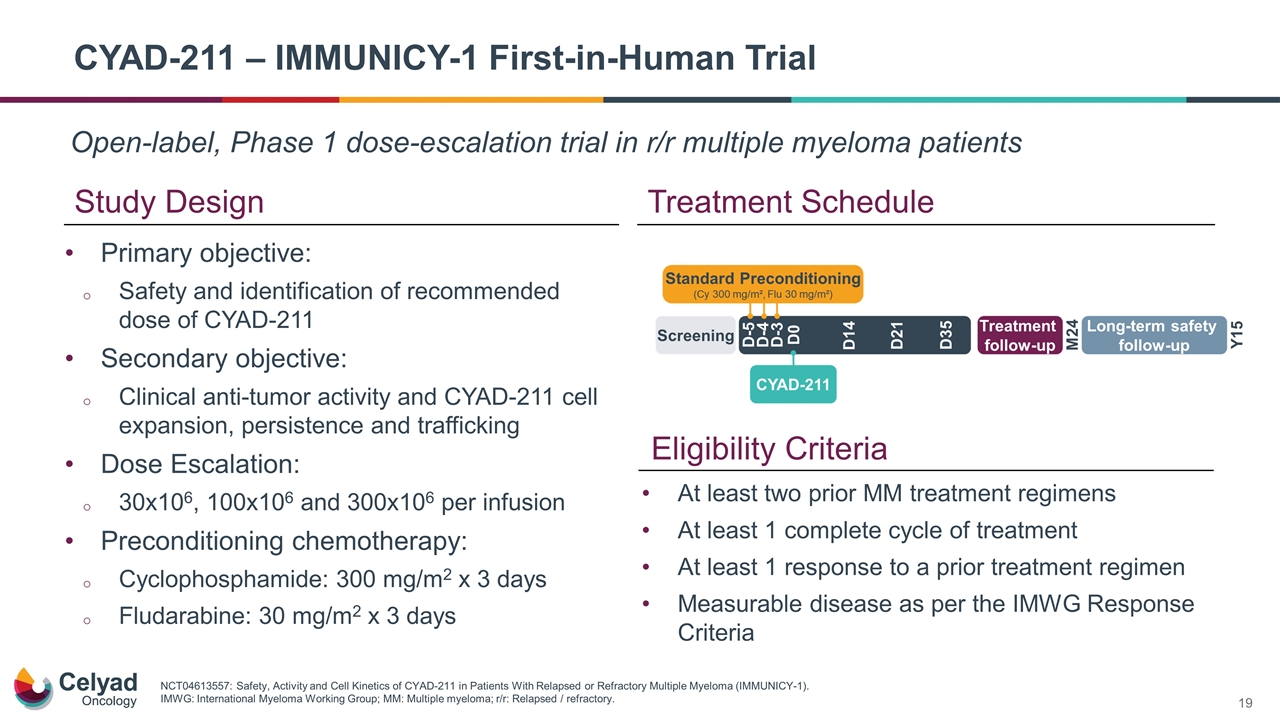
Open-label, Phase 1 dose-escalation trial in r/r multiple myeloma patients CYAD-211 – IMMUNICY-1 First-in-Human Trial Study Design Treatment Schedule Primary objective: Safety and identification of recommended dose of CYAD-211 Secondary objective: Clinical anti-tumor activity and CYAD-211 cell expansion, persistence and trafficking Dose Escalation: 30x106, 100x106 and 300x106 per infusion Preconditioning chemotherapy: Cyclophosphamide: 300 mg/m2 x 3 days Fludarabine: 30 mg/m2 x 3 days NCT04613557: Safety, Activity and Cell Kinetics of CYAD-211 in Patients With Relapsed or Refractory Multiple Myeloma (IMMUNICY-1). IMWG: International Myeloma Working Group; MM: Multiple myeloma; r/r: Relapsed / refractory. Eligibility Criteria At least two prior MM treatment regimens At least 1 complete cycle of treatment At least 1 response to a prior treatment regimen Measurable disease as per the IMWG Response Criteria
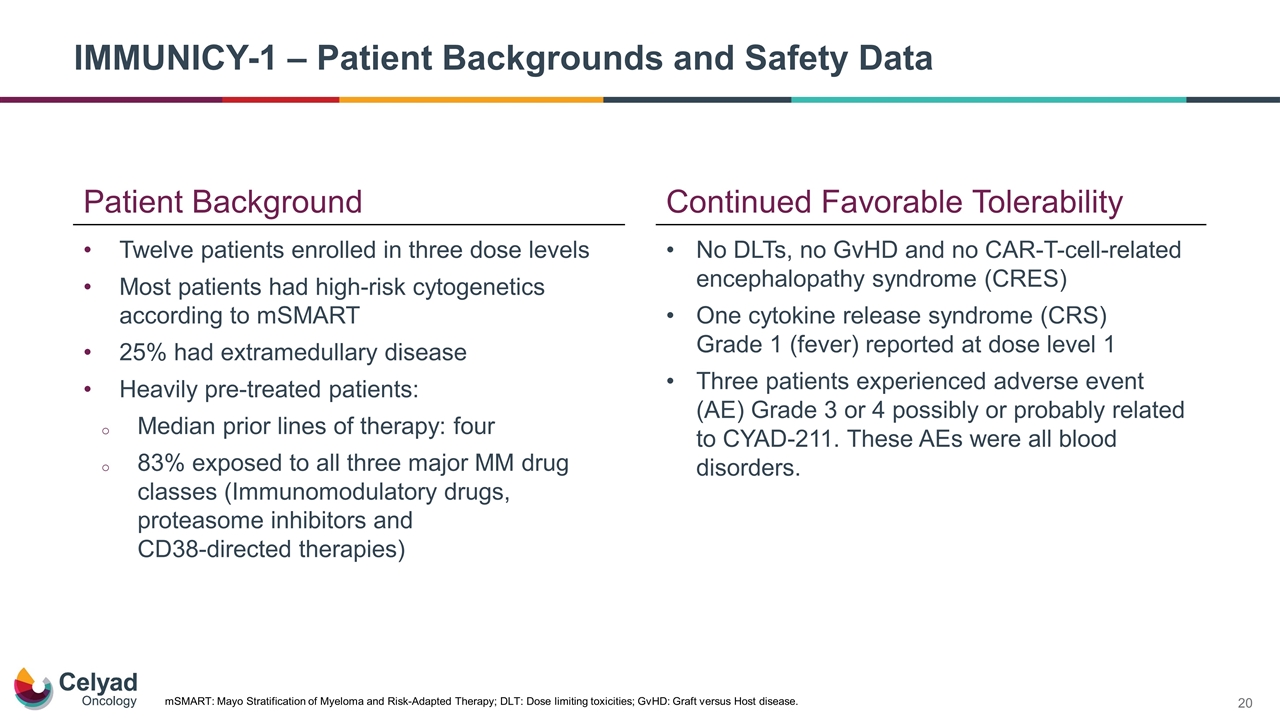
IMMUNICY-1 – Patient Backgrounds and Safety Data Twelve patients enrolled in three dose levels Most patients had high-risk cytogenetics according to mSMART 25% had extramedullary disease Heavily pre-treated patients: Median prior lines of therapy: four 83% exposed to all three major MM drug classes (Immunomodulatory drugs, proteasome inhibitors and CD38-directed therapies) mSMART: Mayo Stratification of Myeloma and Risk-Adapted Therapy; DLT: Dose limiting toxicities; GvHD: Graft versus Host disease. Continued Favorable Tolerability No DLTs, no GvHD and no CAR‑T‑cell‑related encephalopathy syndrome (CRES) One cytokine release syndrome (CRS) Grade 1 (fever) reported at dose level 1 Three patients experienced adverse event (AE) Grade 3 or 4 possibly or probably related to CYAD-211. These AEs were all blood disorders. Patient Background
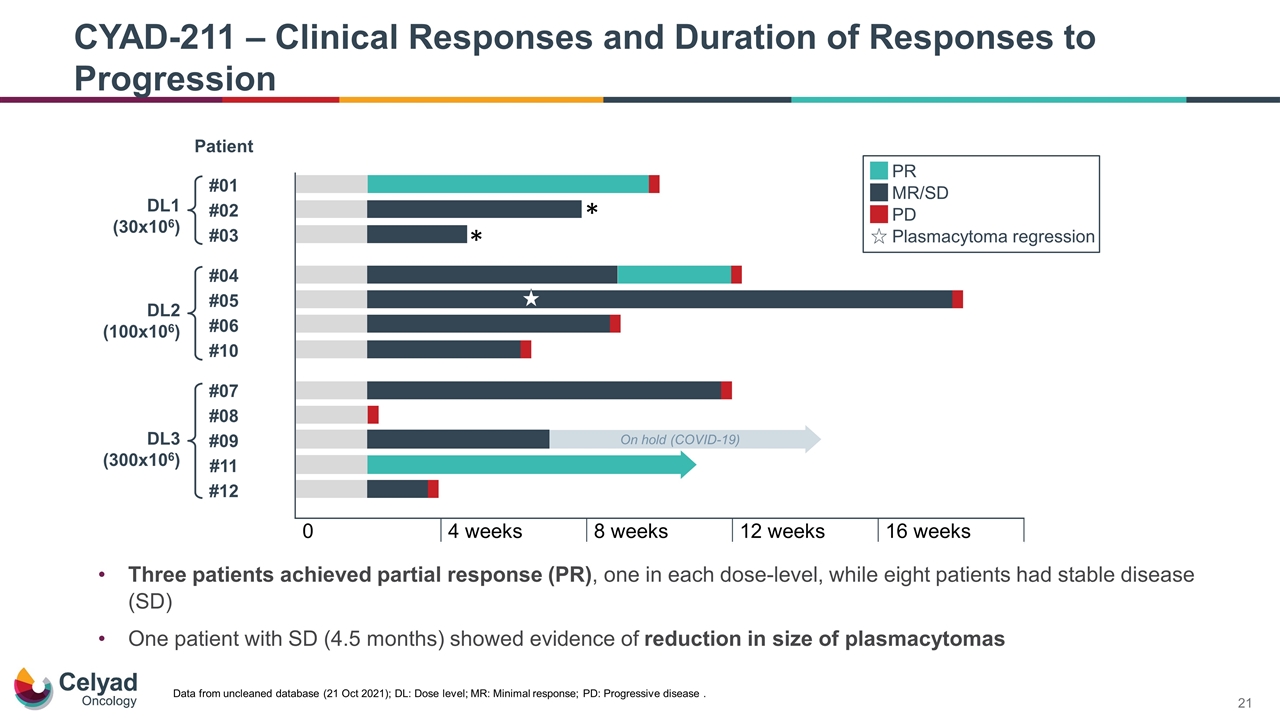
Three patients achieved partial response (PR), one in each dose-level, while eight patients had stable disease (SD) One patient with SD (4.5 months) showed evidence of reduction in size of plasmacytomas CYAD-211 – Clinical Responses and Duration of Responses to Progression Data from uncleaned database (21 Oct 2021); DL: Dose level; MR: Minimal response; PD: Progressive disease .
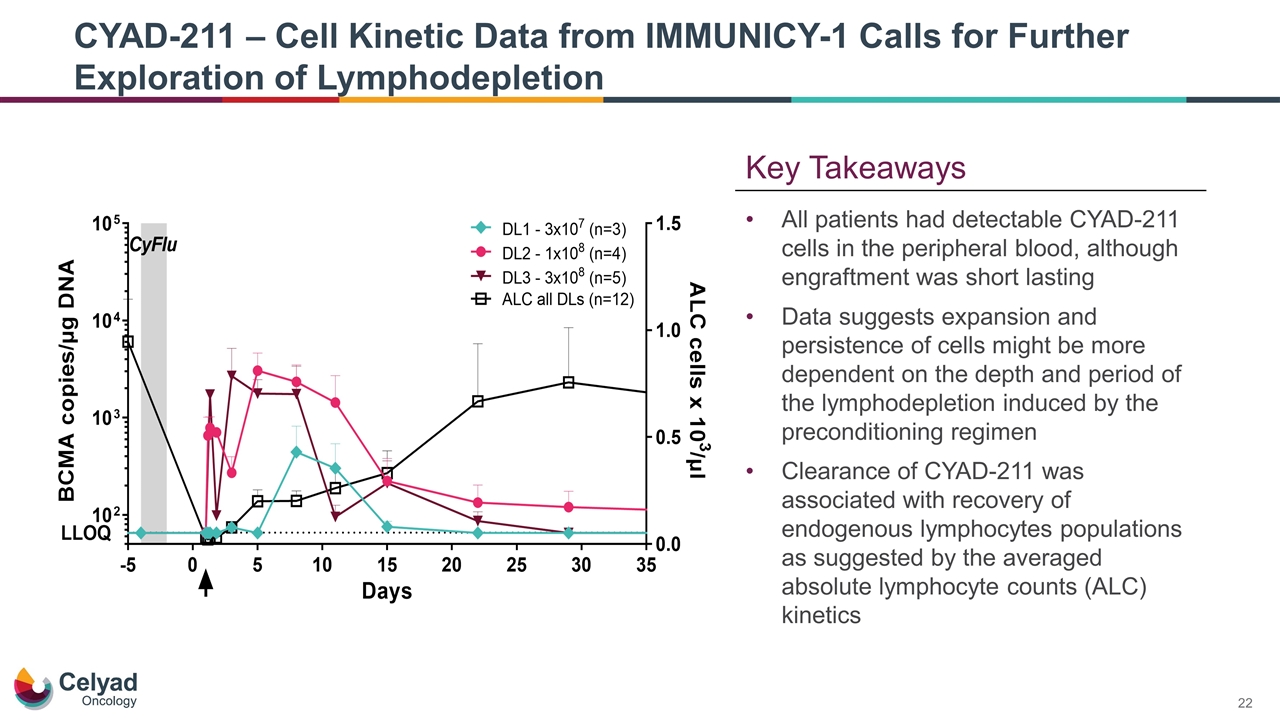
CYAD-211 – Cell Kinetic Data from IMMUNICY-1 Calls for Further Exploration of Lymphodepletion All patients had detectable CYAD-211 cells in the peripheral blood, although engraftment was short lasting Data suggests expansion and persistence of cells might be more dependent on the depth and period of the lymphodepletion induced by the preconditioning regimen Clearance of CYAD-211 was associated with recovery of endogenous lymphocytes populations as suggested by the averaged absolute lymphocyte counts (ALC) kinetics Key Takeaways
Data and observations support the potential of shRNA allogeneic therapies as a feasible approach to CAR T that may overcome challenges associated with autologous and gene edited CAR Ts Three patients achieved partial response (one in each dose level) Eight patients had stable disease One patient with stable disease showed evidence of reduction in plasmacytomas Favorable tolerability profile observed for CYAD-211 across all dose levels CYAD-211 IMMUNICY-1 Summary

Filippo Petti Chief Executive Officer Final Remarks

Technology continues to show promise as a platform shRNA as a Novel Allogeneic Technology for CAR T No evidence of GvHD Cell kinetics Initial clinical activity ü ü ü
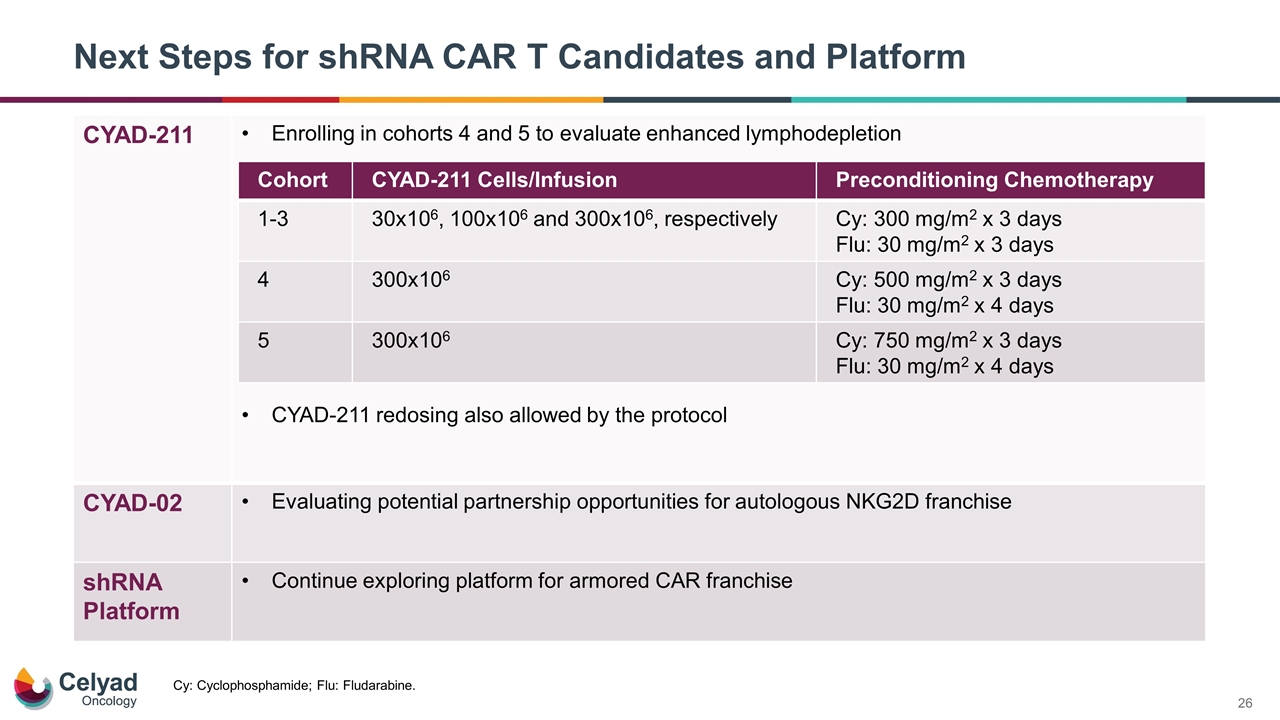
Next Steps for shRNA CAR T Candidates and Platform CYAD-211 Enrolling in cohorts 4 and 5 to evaluate enhanced lymphodepletion CYAD-211 redosing also allowed by the protocol CYAD-02 Evaluating potential partnership opportunities for autologous NKG2D franchise shRNA Platform Continue exploring platform for armored CAR franchise Cohort CYAD-211 Cells/Infusion Preconditioning Chemotherapy 1-3 30x106, 100x106 and 300x106, respectively Cy: 300 mg/m2 x 3 days Flu: 30 mg/m2 x 3 days 4 300x106 Cy: 500 mg/m2 x 3 days Flu: 30 mg/m2 x 4 days 5 300x106 Cy: 750 mg/m2 x 3 days Flu: 30 mg/m2 x 4 days Cy: Cyclophosphamide; Flu: Fludarabine.

2021 American Society of Hematology Meeting Update December 13, 2021



























