Exhibit 99.1

NeuroBo Pharmaceuticals’ Novel GLP1R and GCGR Dual Agonist, DA-1726, Shown to
Elicit Superior Weight Loss Efficacy Compared to Semaglutide and Tirzepatide
in Preclinical Models
Preclinical Data Also Show DA-1726 Effectively Reduces Body Weight and Glycemic Control
Data Presented in One ePoster Theater Discussion and Two General Poster Presentations at the American Diabetes Association 83rd Scientific Sessions
BOSTON, June 27, 2023 – NeuroBo Pharmaceuticals, Inc. (Nasdaq: NRBO), a clinical-stage biotechnology company on a quest to transform cardiometabolic diseases, today announced that DA-1726, a novel oxyntomodulin (OXM) analogue functioning as a glucagon-like peptide-1 receptor (GLP1R) and glucagon receptor (GCGR) dual agonist, had been shown to elicit superior weight loss efficacy compared to Semaglutide (SEMA) and Tirzepatide (TIR) in preclinical testing. This, and other data, was presented in one ePoster theater discussion and two general poster presentations at the American Diabetes Association’s 83rd Scientific Sessions, held June 23-26, 2023.
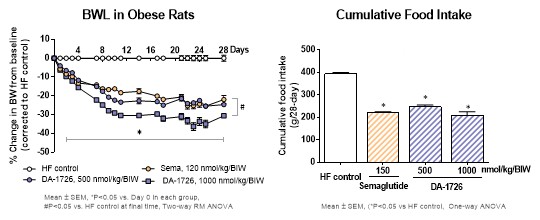
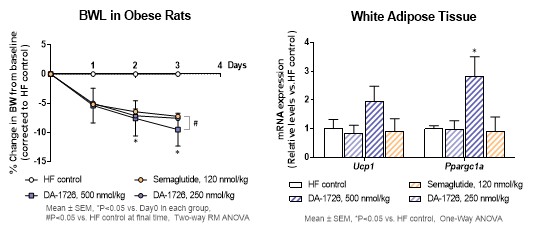
“DA-1726, a long acting OXM analog that binds and activates both GLP-1 and glucagon receptors using a well understood mechanism, has shown weight loss efficacy, despite similar or higher food intake, in a dose-dependent manner, that is better than SEMA in diet-induced obese (DIO) rats (32.6% for DA-1726 at a high dose compared to 24.0% for SEMA, p<0.05),” stated Yuna Chae, DA-1726 Project Manager, Dong-A ST Research Center. “Additionally, the high-dose DA-1726 increased the expression of the thermogenic-related gene (Ucp1) in white adipose tissues, supporting increased energy expenditure, and the gene expression in white adipose tissue was increased. This suggests that major genetic changes related to energy expenditure are directly related to weight loss. As a result, we believe that DA-1726 will have similar, superior weight loss effects in humans utilizing its novel mechanism of action.”
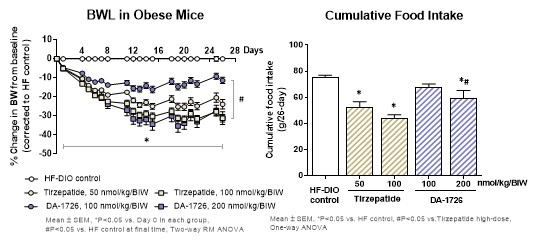
“In preclinical mice models, DA-1726 also showed the ability to improve plasma glucose insulin and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) as compared to Cotadutide (COTA), another OXM analog. Importantly, DA-1726 showed superior plasma triglyceride (TG) reduction and a similar reduction of total cholesterol (T-CHO) compared to COTA. In a comparative preclinical mouse study with GLP1R/glucose-dependent insulinotropic polypeptide receptor (GIPR) dual agonist, Tirzepatide (TIR), DA-1726 showed similar efficacy on weight loss, despite consuming more food. However, DA-1726 was more efficacious in improving plasma metabolic parameters such as glucose, TG, and T-CHO compared to TIR, indicating differential metabolic effects caused by GCGR agonism. Taken together, these data suggest that DA-1726 is a well-balanced GLP-1 receptor and glucagon receptor dual agonist and we anticipate that DA-1726 will have effective weight loss and glycemic control in humans,” concluded Ms. Chae.
"DA-1726 is a long-acting, novel peptide drug candidate in preclinical development with therapeutic promise for obesity and NASH, in preparation for a phase 1 clinical trial for obesity,” stated Joe Hooker, Interim President and Chief Executive Officer of NeuroBo. “Presenting two general posters and one ePoster theater discussion at this important scientific meeting provided us the ability to highlight positive preclinical data for DA-1726 as a potential treatment for obesity, and data showing superior effectiveness as compared to competitor peptide therapeutics including SEMA. During the second half of this year, our