Exhibit 99.1

Corporate Presentation Nasdaq: NTEC September 2019

This presentation by Intec Pharma Ltd. (referred to as “we” or “our”) contains forward - looking statements about our expectations , beliefs and intentions regarding, among other things, our product development efforts, business, financial condition, results of operations, strategi es, plans and prospects. In addition, from time to time, we or our representatives have made or may make forward - looking statements, orally or in writing. F orward - looking statements can be identified by the use of forward - looking words such as “believe”, “expect”, “intend”, “plan“, “may“, “should“, “could“, “might“, “seek“, “target“, “will”, “project“, “forecast“, “continue” or “anticipate” or their negatives or variations of these words o r o ther comparable words or by the fact that these statements do not relate strictly to historical matters. For example, forward - looking statements are used in this presentation when we discuss the potential for multiple collaborations, the potential advantages our technology over our competitors, our future p lan s and our expected timeline of our development pipeline. Forward - looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward - looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause our actual results to differ materially from any future results expressed or implied by the fo rwa rd - looking statements. In addition, historical results or conclusions from scientific research and clinical studies do not guarantee that future result s w ould suggest similar conclusions or that historical results referred to herein would be interpreted similarly in light of additional research or o the rwise. Many factors could cause our actual activities or results to differ materially from the activities and results anticipated in forward - looking state ments, including, but not limited to, the following: our limited operating history and history of operating losses, our ability to continue as a going con cern, our ability to obtain additional financing, our ability to successfully operate our business or execute our business plan, the timing and cost of o ur clinical trials, the completion and receiving favorable results in our clinical trials, our ability to obtain and maintain regulatory approval of our product ca ndidates, our ability to protect and maintain our intellectual property and licensing arrangements, our ability to develop, manufacture and commercialize our pro duct candidates, the risk of product liability claims, the availability of reimbursement, and the influence of extensive and costly government reg ula tion. We believe these forward - looking statements are reasonable; however, these statements are only current predictions and are subje ct to known and unknown risks, uncertainties and other factors that may cause our or our industry’s actual results, levels of activity, perfo rma nce or achievements to be materially different from those anticipated by the forward - looking statements. Given these uncertainties, you should not rely up on forward - looking statements as predictions of future events. All forward - looking statements attributable to us or persons acting on our behalf speak only as of the date of this presentation and are expressly qualified in their entirety by the cautionary statements included in this presentation. We undertake no obligations to update or revise forward - looking statements to reflect events or circumstances that arise after the date made or to reflect the occurrence of unanticipated even ts, except as required by applicable law. In evaluating forward - looking statements, you should consider these risks and uncertainties. More detailed infor mation about the risks and uncertainties affecting us is contained under the heading “Risk Factors” in the Annual Report and in our period filings w ith the SEC. The presentation contains information about investigation - stage drug products under development, which have not yet been approve d by the FDA for commercial distribution in the United States. All representations in this presentation are based upon investigations in certa in clinical and other research, but which accordingly should not be construed as general claims for the safety or efficacy of the products when used by patie nts . Forward Looking Statements 2
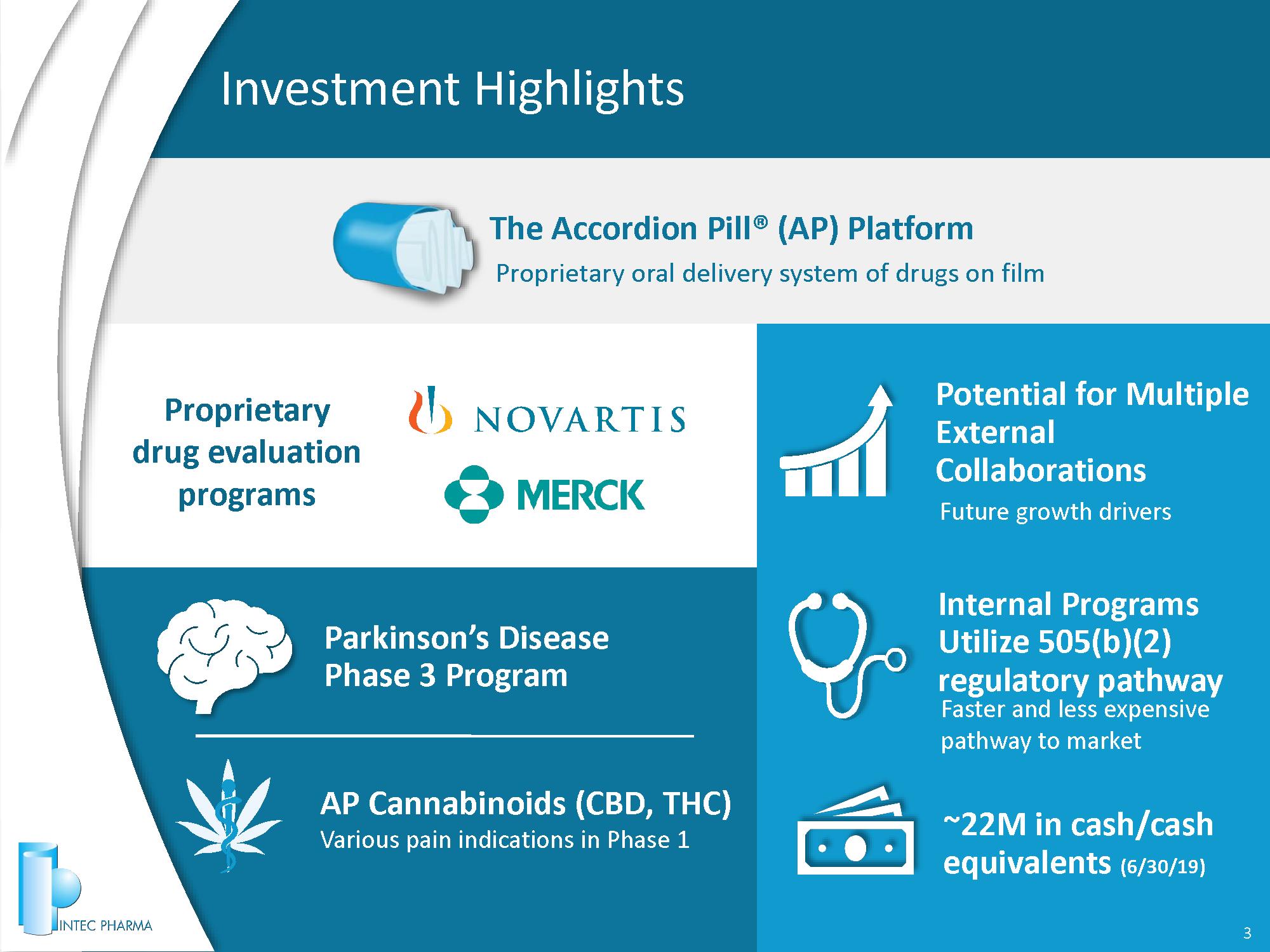
Investment Highlights 3 Parkinson’s Disease Phase 3 Program AP Cannabinoids (CBD, THC) Various pain indications in Phase 1 Proprietary drug evaluation programs Proprietary oral delivery system of drugs on film The Accordion Pill® (AP) Platform Potential for Multiple External Collaborations Future growth drivers ~22 M in cash/cash equivalents (6/30/19) Internal Programs Utilize 505(b)(2) regulatory pathway Faster and less expensive pathway to market
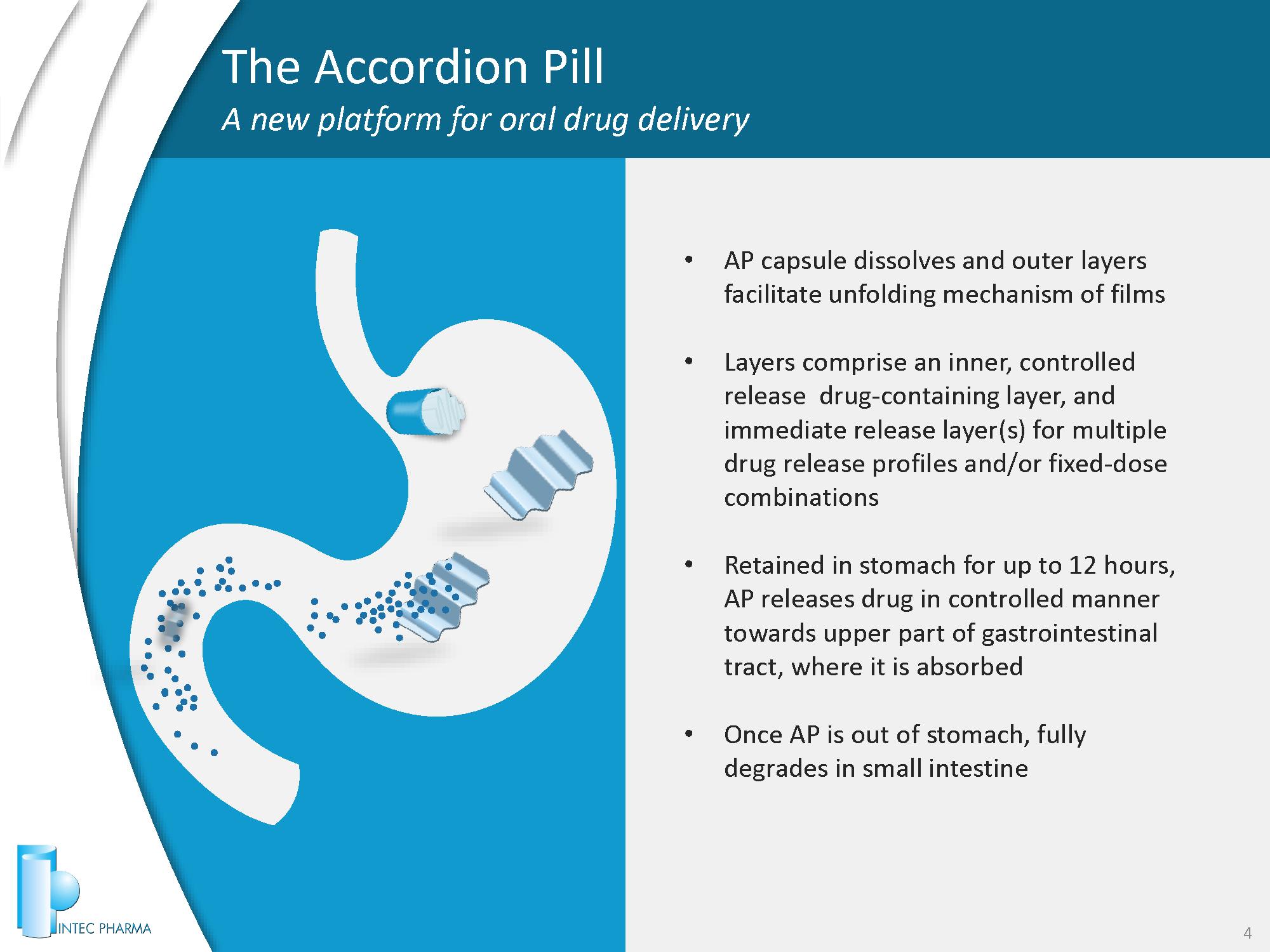
The Accordion Pill A new platform for oral drug delivery 4 • AP capsule dissolves and outer layers facilitate unfolding mechanism of films • Layers comprise an inner, controlled release drug - containing layer, and immediate release layer(s) for multiple drug release profiles and/or fixed - dose combinations • Retained in stomach for up to 12 hours, AP releases drug in controlled manner towards upper part of gastrointestinal tract, where it is absorbed • Once AP is out of stomach, fully degrades in small intestine
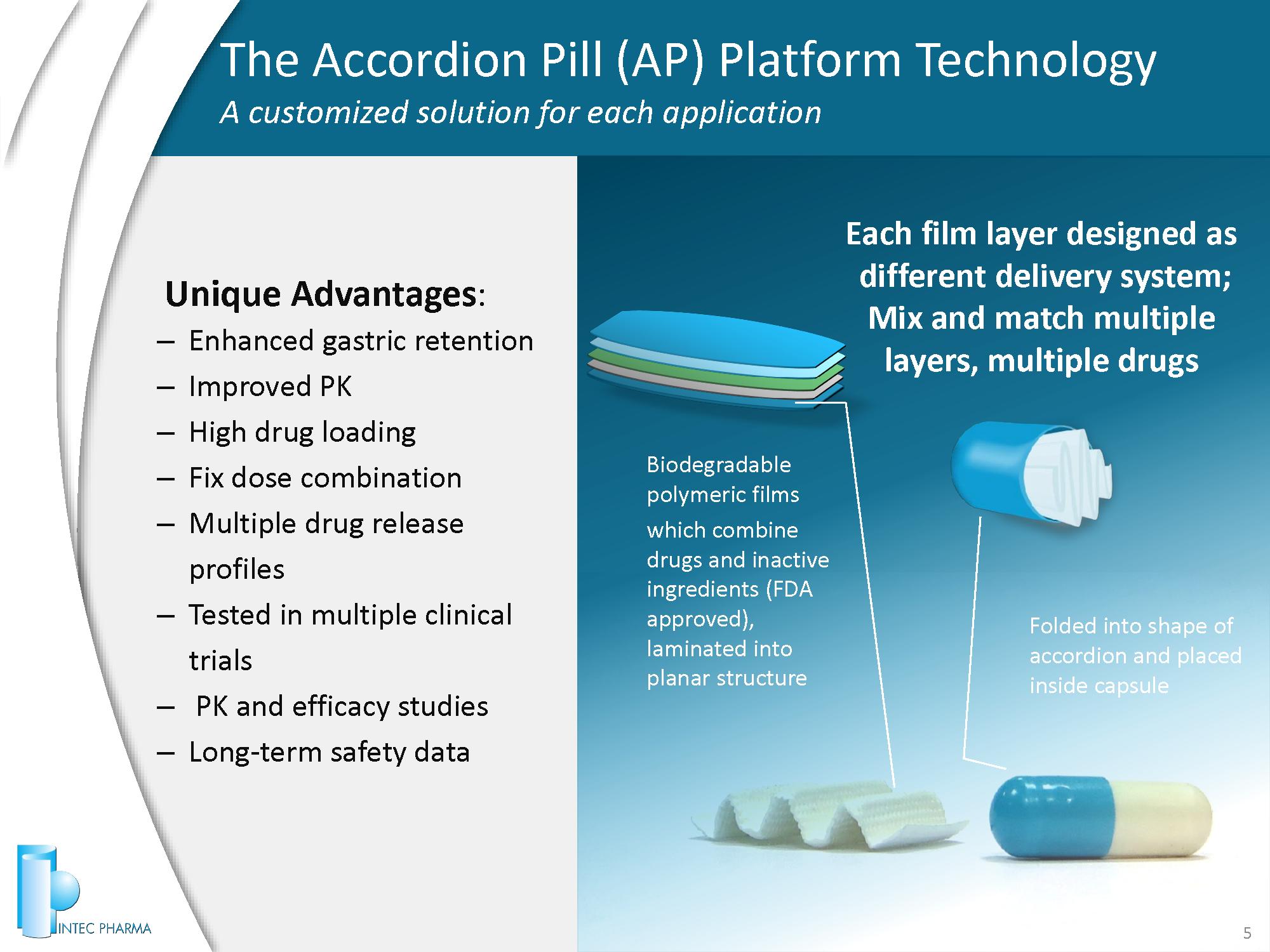
Each film layer designed as different delivery system; Mix and match multiple layers, multiple drugs 5 Biodegradable polymeric films which combine drugs and inactive ingredients (FDA approved), laminated into planar structure Unique Advantages : – Enhanced gastric retention – Improved PK – High drug loading – Fix dose combination – Multiple drug release profiles – Tested in multiple clinical trials – PK and efficacy studies – Long - term safety data Folded into shape of accordion and placed inside capsule The Accordion Pill (AP) Platform Technology A customized solution for each application

Business Strategy 6 Partner’s Proprietary Drugs In Development In - house Development Of Existing Drugs 505 (b) 2
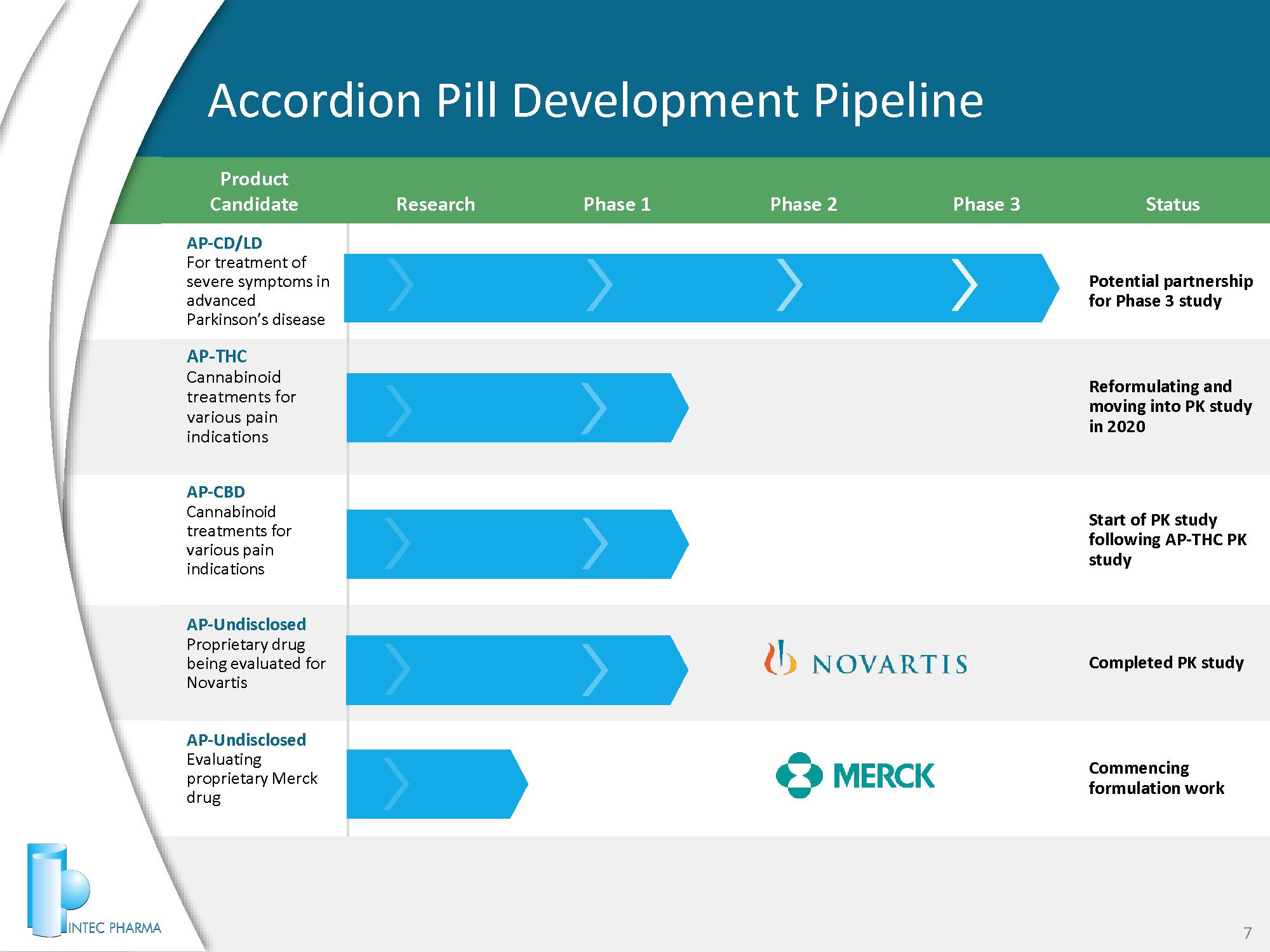
Product Candidate Research Phase 1 Phase 2 Phase 3 Status AP - CD/LD For treatment of severe symptoms in advanced Parkinson’s disease Potential partnership for Phase 3 study AP - THC Cannabinoid treatments for various pain indications Reformulating and moving into PK study in 2020 AP - CBD Cannabinoid treatments for various pain indications Start of PK study following AP - THC PK study AP - Undisclosed Proprietary drug being evaluated for Novartis Completed PK study AP - Undisclosed Evaluating proprietary Merck drug Commencing formulation work Accordion Pill Development Pipeline 7
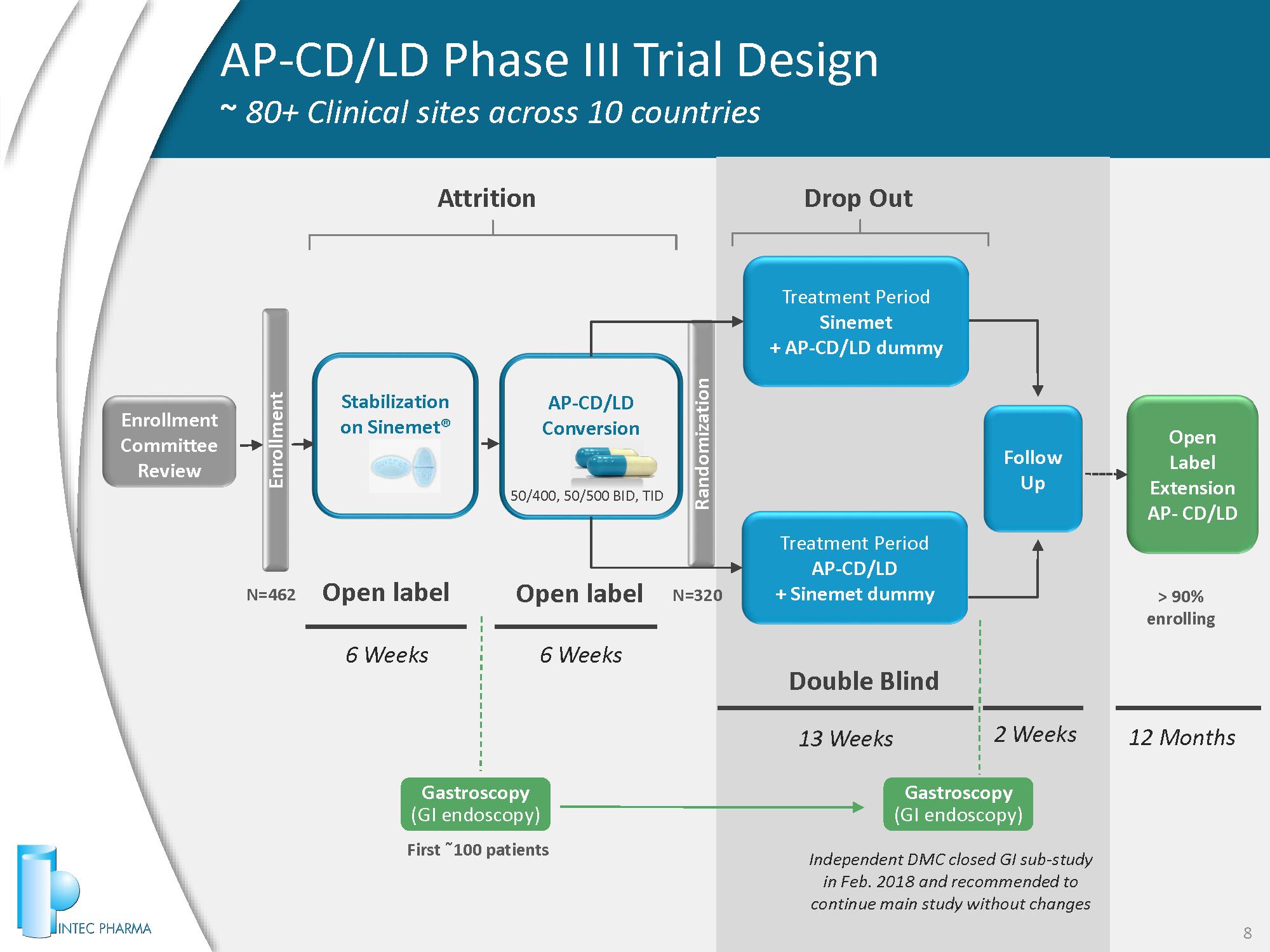
AP - CD/LD Phase III Trial Design ~ 80+ Clinical sites across 10 countries 8 6 Weeks 13 Weeks 2 Weeks 50 / 400 , 50 / 500 BID, TID Open label N= 462 Double Blind 12 Months 6 Weeks Stabilization on Sinemet® AP - CD/LD Conversion Enrollment Committee Review Treatment Period Sinemet + AP - CD/LD dummy Treatment Period AP - CD/LD + Sinemet dummy Follow Up Open Label Extension AP - CD/LD Randomization N= 320 Enrollment Attrition Open label Drop Out Gastroscopy (GI endoscopy) Gastroscopy (GI endoscopy) First ˜ 100 patients Independent DMC closed GI sub - study in Feb. 2018 and recommended to continue main study without changes > 90 % enrolling
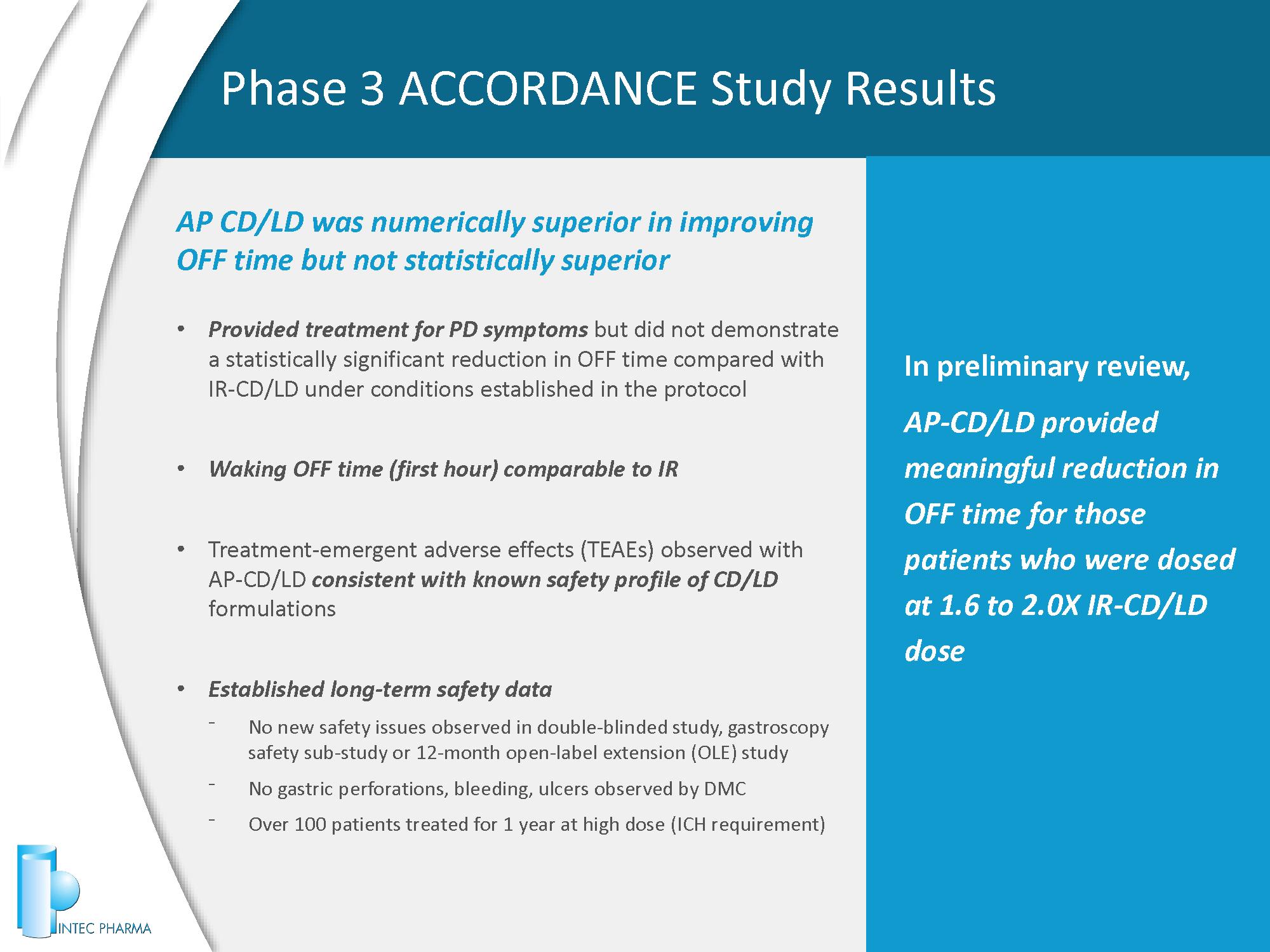
AP CD/LD was numerically superior in improving OFF time but not statistically superior • Provided treatment for PD symptoms but did not demonstrate a statistically significant reduction in OFF time compared with IR - CD/LD under conditions established in the protocol • Waking OFF time (first hour) comparable to IR • Treatment - emergent adverse effects (TEAEs) observed with AP - CD/LD consistent with known safety profile of CD/LD formulations • Established long - term safety data ⁻ No new safety issues observed in double - blinded study, gastroscopy safety sub - study or 12 - month open - label extension (OLE) study ⁻ No gastric perforations, bleeding, ulcers observed by DMC ⁻ Over 100 patients treated for 1 year at high dose (ICH requirement) Phase 3 ACCORDANCE Study Results 9 In preliminary review, AP - CD/LD provided meaningful reduction in OFF time for those patients who were dosed at 1.6 to 2.0X IR - CD/LD dose

Phase 3 ACCORDANCE Study Take Aways 10 Validated the Accordion Pill platform x Delivered levodopa and treated PD symptoms x Provided long - term safety data • Sub - optimal dosing – AP dose was capped at AP - CD/LD 50 / 500 mg TID; IR - CD/LD had no limit to daily doses – 1.6 to 2.0 ratio of AP - CD/LD to IR - CD/LD should be the goal – AP - CD/LD’s smoother PK allows for delivery of more LD with potentially fewer AEs – Future studies should include potential for additional doses and multiple pills per dose • Titration targets in protocol were sub - optimal
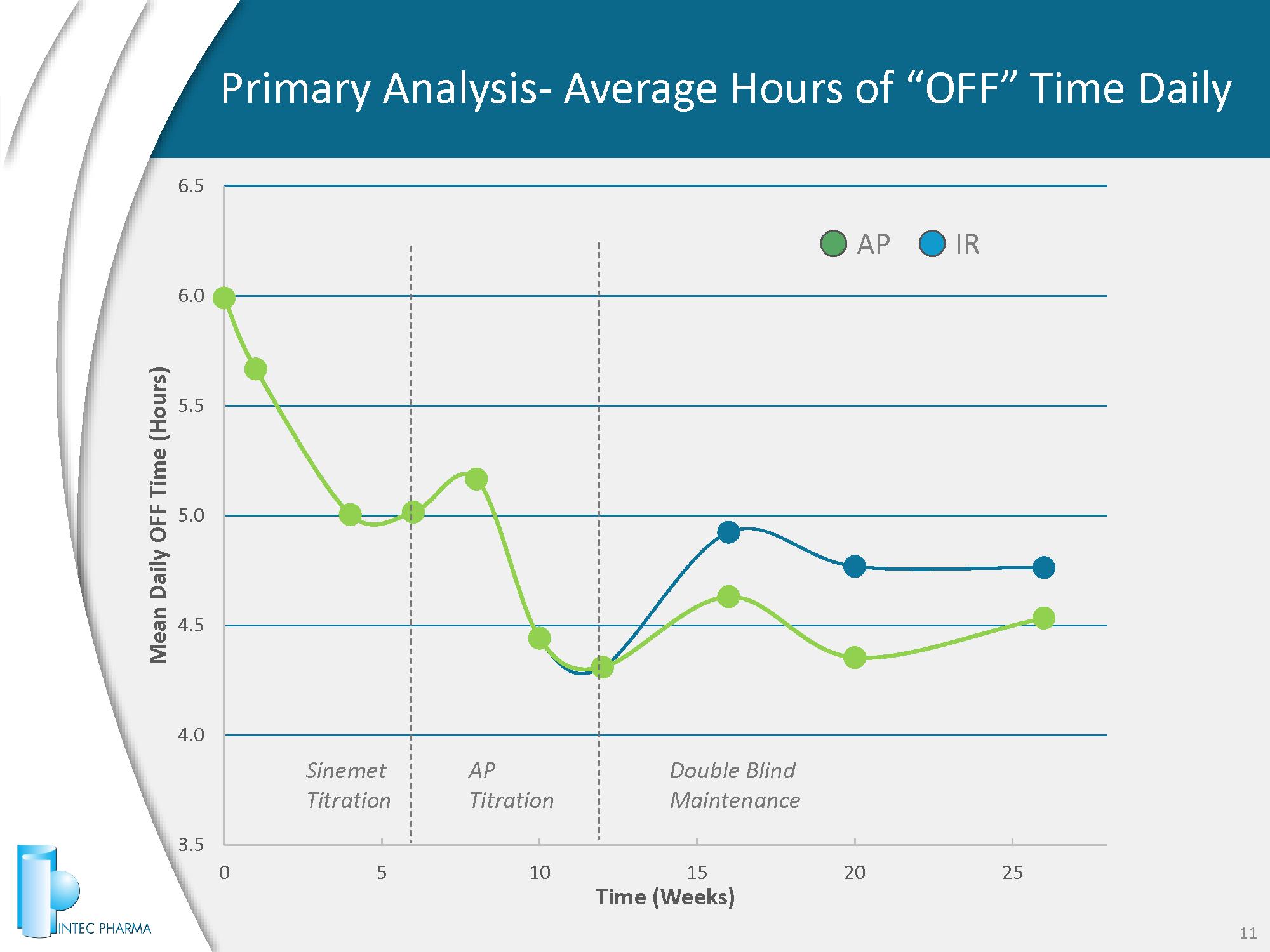
3.5 4.0 4.5 5.0 5.5 6.0 6.5 0 5 10 15 20 25 Mean Daily OFF Time (Hours) Time (Weeks) Primary Analysis - Average Hours of “OFF” Time Daily 11 AP IR Sinemet Titration AP Titration Double Blind Maintenance
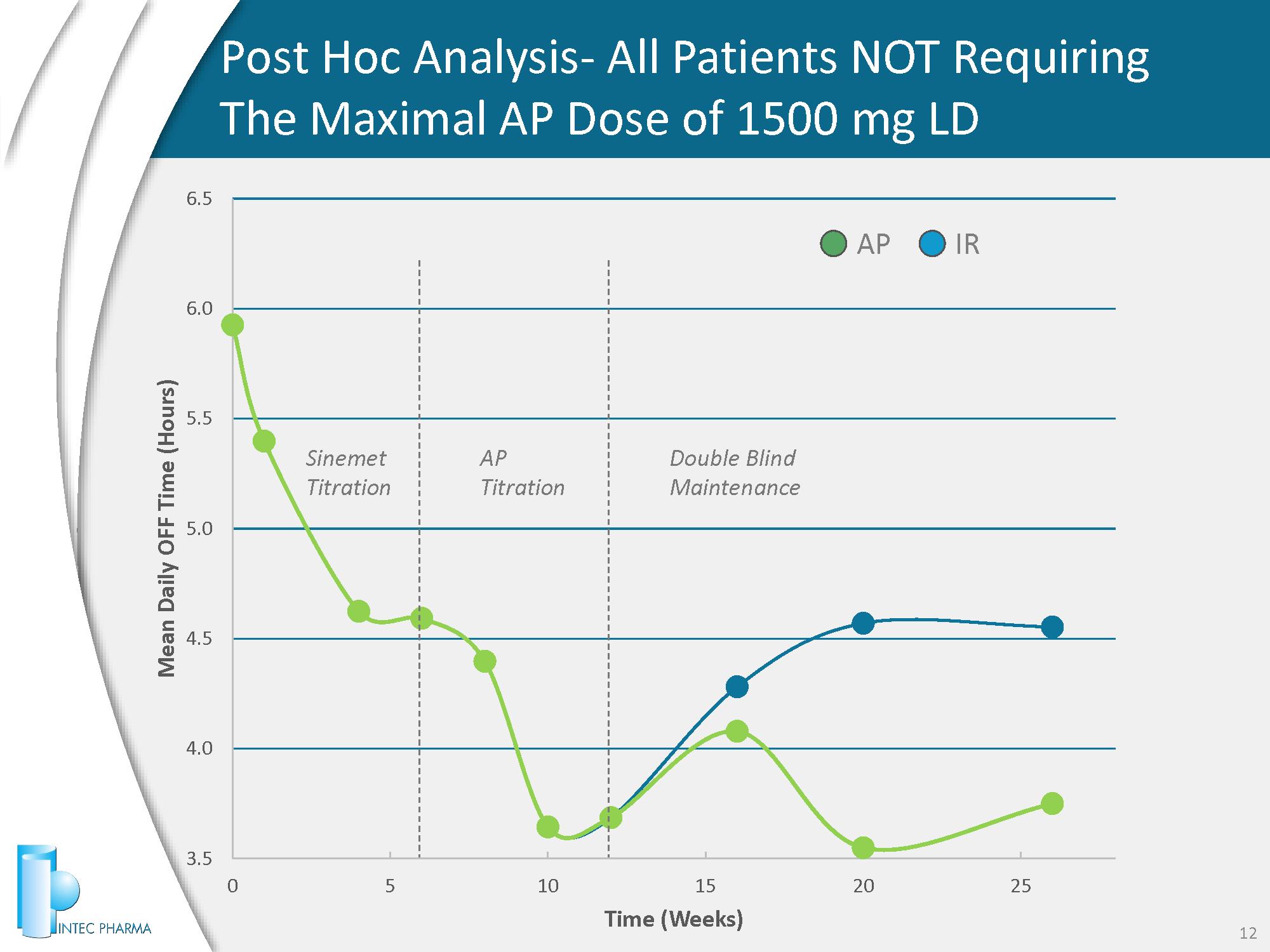
3.5 4.0 4.5 5.0 5.5 6.0 6.5 0 5 10 15 20 25 Mean Daily OFF Time (Hours) Time (Weeks) Post Hoc Analysis - All Patients NOT Requiring The Maximal AP Dose of 1500 mg LD 12 AP IR Sinemet Titration AP Titration Double Blind Maintenance
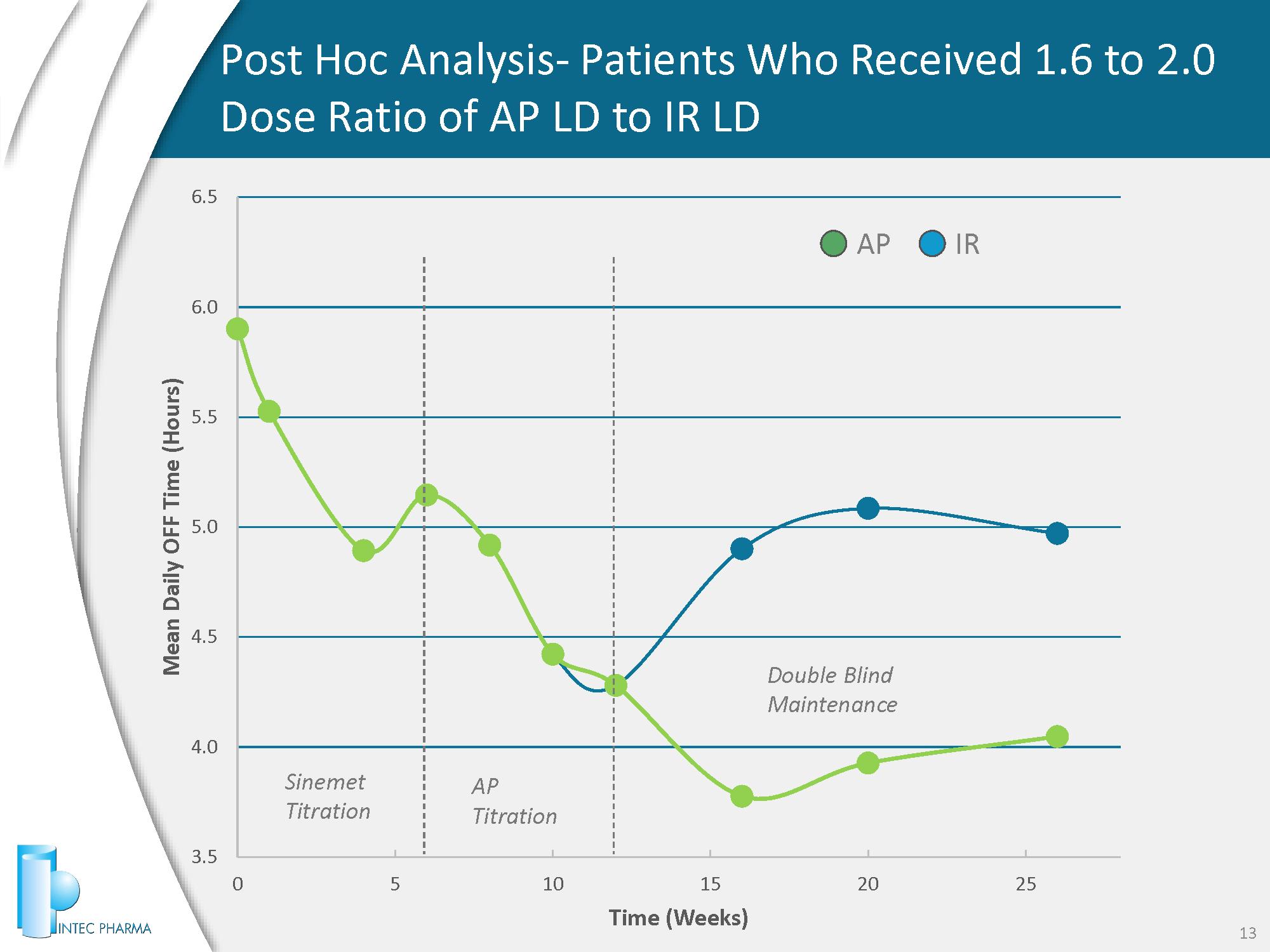
3.5 4.0 4.5 5.0 5.5 6.0 6.5 0 5 10 15 20 25 Mean Daily OFF Time (Hours) Time (Weeks) Post Hoc Analysis - Patients Who Received 1.6 to 2.0 Dose Ratio of AP LD to IR LD 13 AP IR Sinemet Titration AP Titration Double Blind Maintenance
Pre - Commercial Activities 14 Regulatory Market Access Rx Packaging Manufacturing Market Research

Commercial Scale Manufacturing • Partnership with LTS for commercial scale production of AP - CD/LD • Leading global formulation and film technology manufacturer • Commercial scale machine delivered in YE 18 • Qualification of AP commercial manufacturing system in 3 Q 19 • Validation, bridging and stability studies throughout 2019

Partnering PD Program for Late Stage Development and Commercialization 16 The Opportunity • ACCORDANCE results validate the AP platform and provide long - term safety data • Responder analysis and subset analyses provide key insights for future study design and dosing • Commercial scale manufacturing secured and can provide clinical supply for Phase 3 study • Continued unmet need for a better baseline LD • Large market opportunity between $ 200 - $ 500 million Next Steps • Implement formal process to seek potential partner • Continue commercial manufacturing activities at LTS • Complete required regulatory submissions and reports • Prepare and design additional trial protocols required for submission
Cannabis and Pain Relief 17 • Medical cannabis market has growing acceptance and regulatory path forward • Pain relief is most commonly cited reason for medical use of cannabis • Endocannabinoid system is associated with mechanisms of pain reduction Challenges : • Hydrophobic • Insoluble • Poorly bioavailable • Short duration of effect • Delayed onset • Dose variability • Adverse events (i.e., psychotropic excursions) that correlate with peak levels Current Treatment Drawbacks
Single - center, single - dose, randomized, 3 - way crossover study compared PK, safety and tolerability of AP - CBD/THC with Sativex ® in 21 normal healthy volunteers Phase I Topline Results with AP - CBD/THC 18 • Accordion Pill delivered CBD and THC with significant improvements in exposure compared with Sativex ‒ Increased exposure of CBD by 290% to 330% ‒ Increased exposure of THC by 25% to 50% • Reduction in THC metabolite, the AP - CBD/THC avoided some of the first - pass metabolism of THC in the liver ‒ Approximately 25% - 29% less than Sativex • AP - THC/CBD safe and well tolerated – No serious AE’s reported Sativex® is a commercially available oral buccal spray containing CBD and THC and is a registered trade mark of GW Pharmaceuticals

Accordion Pill CBD and THC Next Steps 19 • Evaluate THC alone and CBD alone for pain states • Reformulation of AP - THC following initial PK study results • Phase 1 PK study of AP - THC planned for 2020 Exploring the individual components will provide additional indications to pursue potential indication targets: • Cancer pain • Opioid - sparing pain relief

Partners’ Proprietary Drugs in Development 20 20 • Explore using the Accordion Pill platform for a proprietary Novartis compound • Developing Accordion Pill to meet Novartis criteria x Achieved technical specifications in vitro (YE 18 ) x Conducted Phase 1 PK study ( 1 H 19 ) x Phase 1 PK data to Novartis ( 7 / 19 ) • Novartis has option to enter negotiations for a potential licensing agreement for employing Accordion Pill technology Feasibility and Option Agreement Research Collaboration • Explore using the Accordion Pill platform for a proprietary Merck compound • Develop a custom - designed Accordion Pill to meet Merck criteria • Test technical specification in vitro • Merck decides to advance to Phase 1 PK study • Merck has option to negotiate a licensing agreement

Potential for Enhancing Delivery and Retention 21 • Many drugs fail to reach the market due to poor bioavailability • ~ 40 % of top 200 major market drugs are poorly soluble (BCS Class II or Class IV ( 1 ) ) • Historically, ~ 90 % of new chemical entities in development, were either BCS Class II or Class IV drugs ( 2 ) • Demand for novel, bioavailability enhancement solutions has grown significantly (1) AAPS J. 2009 Dec; 11(4): 740 - 746. (2) Drug Development & Delivery . • PK issues of drug X include only 30 % absorption and lack of dose proportionality above approved marketed dose (X mg) • AP significantly extended absorption phase of drug • Approximately 100 % increase in bioavailability with AP at Xmg • Dose proportionality obtained with AP at 2 x dose vs. 1 x dose Accordion Pill with Poorly Soluble Drugs: PK Clinical Study
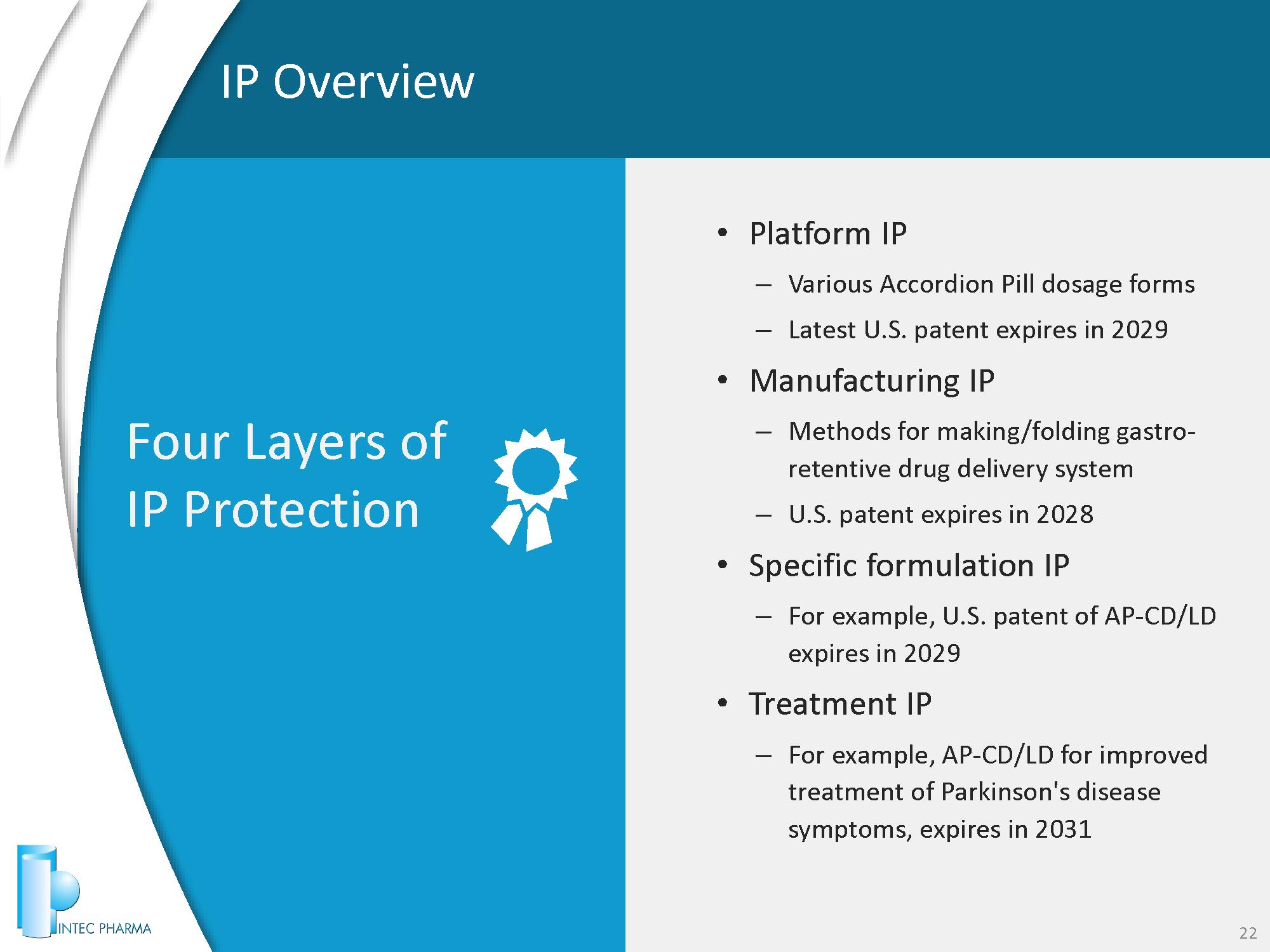
IP Overview 22 • Platform IP – Various Accordion Pill dosage forms – Latest U.S. patent expires in 2029 • Manufacturing IP – Methods for making/folding gastro - retentive drug delivery system – U.S. patent expires in 2028 • Specific formulation IP – For example, U.S. patent of AP - CD/LD expires in 2029 • Treatment IP – For example, AP - CD/LD for improved treatment of Parkinson's disease symptoms, expires in 2031 Four Layers of IP Protection
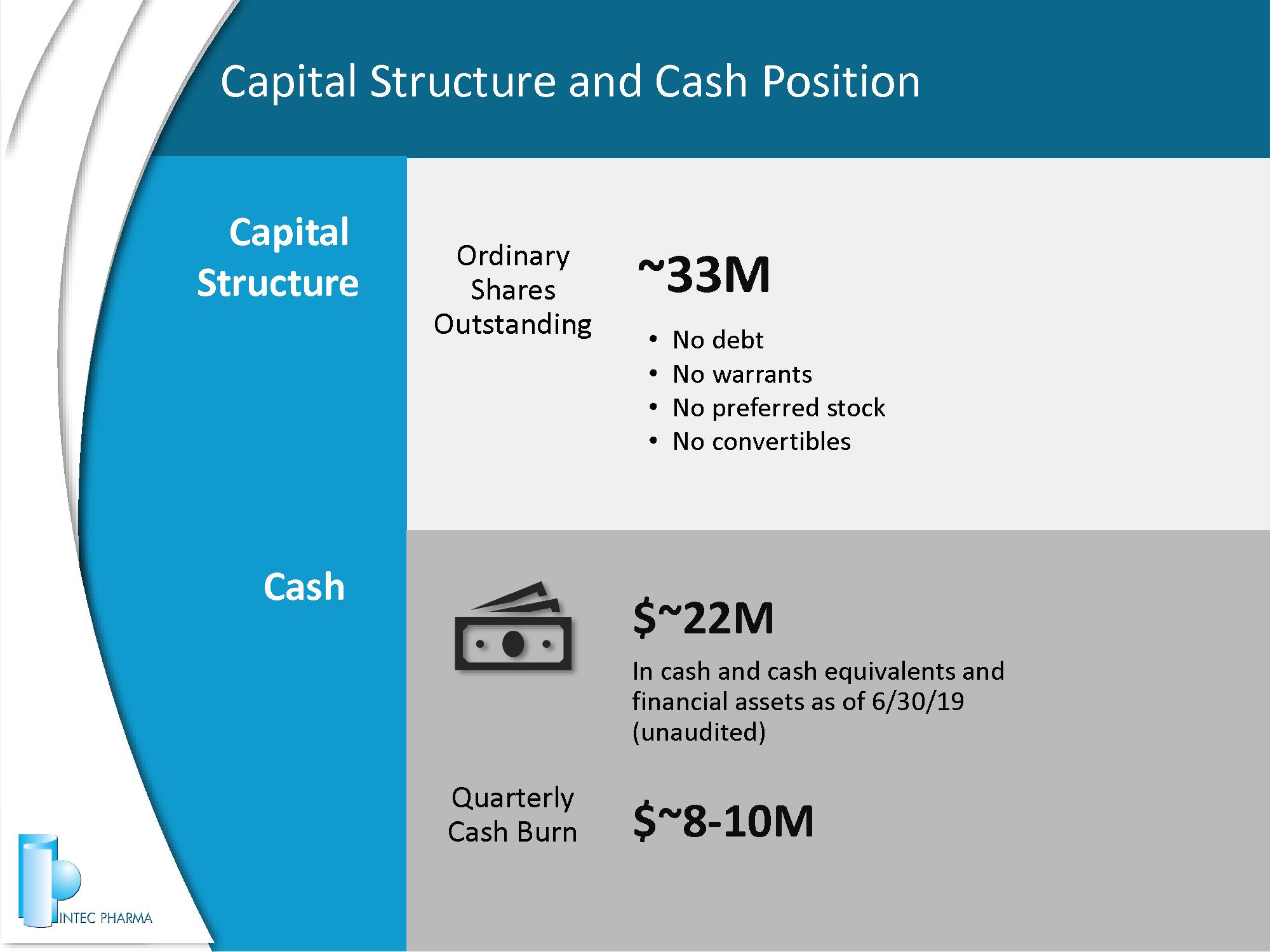
Capital Structure and Cash Position 23 ~ 33 M Ordinary Shares Outstanding Capital Structure Cash $~ 22 M In cash and cash equivalents and financial assets as of 6/30/19 (unaudited) $~ 8 - 10 M Quarterly Cash Burn • No debt • No warrants • No preferred stock • No convertibles

Experienced Board of Directors 24 John W. Kozarich, Ph.D. Chairman Gil Bianco Director Hila Karah Director Anthony J. Maddaluna Director Roger J. Pomerantz, M.D. Director Isaac Silberman Director Sulam Financial Holdings Company W. Brad Hayes Director

Accomplishments and Milestones 25 Recent Upcoming x Completed qualification studies of commercial manufacturing with LTS x Reported top - line data from Phase 3 study of AP - CD/LD in Parkinson’s disease did not meet primary endpoint x Completed PK studies for Novartis’ proprietary AP x Expanded pipeline with addition of Merck research collaboration • Novartis decision on commercial agreement for AP of its proprietary compound ( 2 H 2019 ) • Potential partnership for AP - CD/LD Phase 3 development program in PD • Continue pre - commercialization activities in Parkinson’s disease • Advance AP - THC into new PK study • Identify new AP applications -- proprietary & 505 b( 2 )
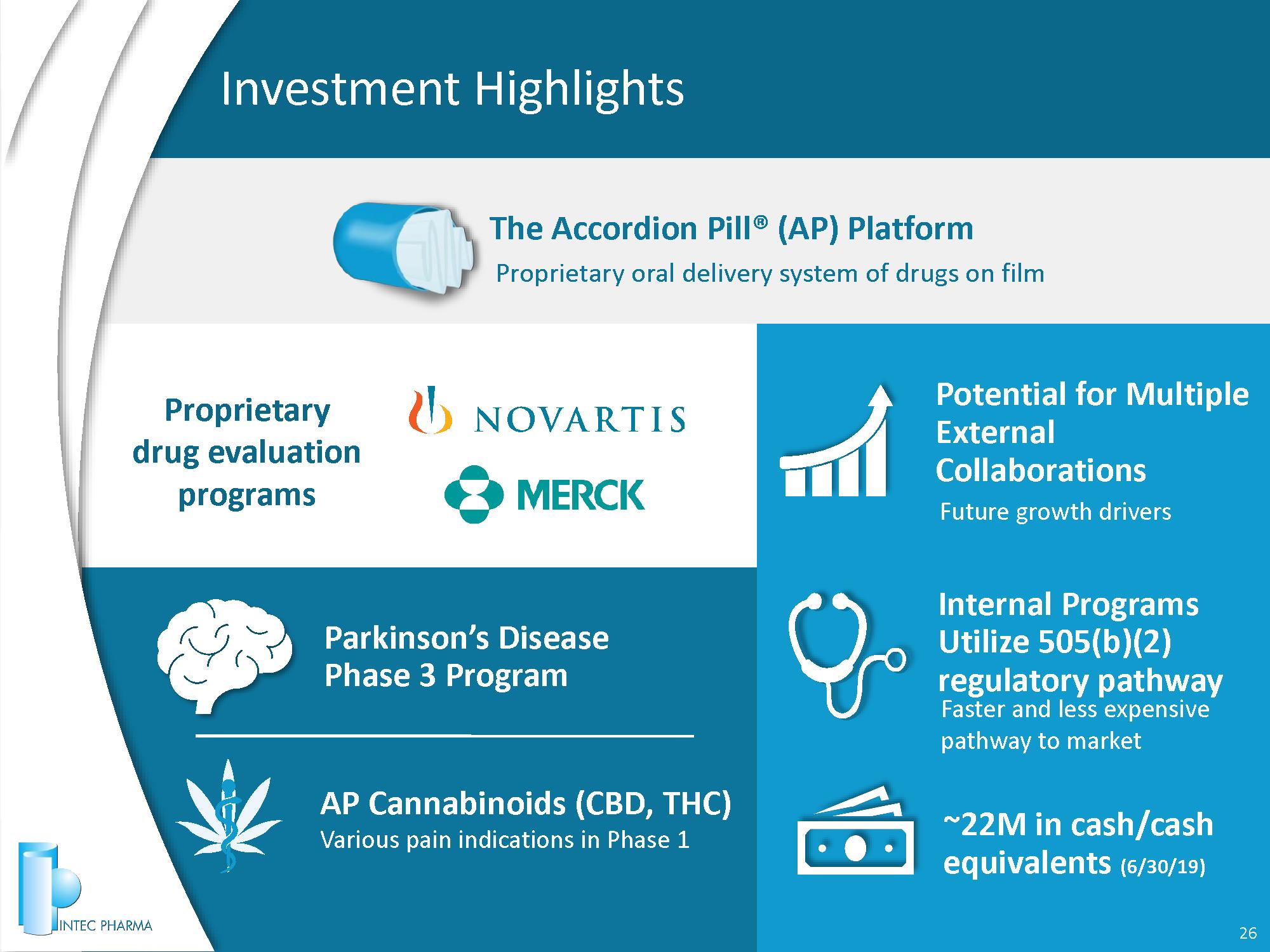
Investment Highlights 26 Parkinson’s Disease Phase 3 Program AP Cannabinoids (CBD, THC) Various pain indications in Phase 1 Proprietary drug evaluation programs Proprietary oral delivery system of drugs on film The Accordion Pill® (AP) Platform Potential for Multiple External Collaborations Future growth drivers ~ 22 M in cash/cash equivalents ( 6 / 30 / 19 ) Internal Programs Utilize 505(b)(2) regulatory pathway Faster and less expensive pathway to market

Thank you www.intecpharma.com


























