As confidentially submitted to the Securities and Exchange Commission on December 6, 2017.
This draft registration statement has not been publicly filed with the Securities and Exchange Commission
and all information herein remains strictly confidential.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1 to
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Bioceres S.A.
(Exact name of Registrant as Specified in its Charter)
Not Applicable
(Translation of Registrant’s name into English)
Republic of Argentina | 2870 | Not Applicable |
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification No.) |
Bioceres S.A.
Ocampo 210 bis
Predio CCT, Rosario, Santa Fe, Argentina
Tel: +54 (341) 486-1100
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Cogency Global Inc.
10 E. 40th Street, 10th Floor
New York, NY 10016
Tel.: +1 (212) 947-7200
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Conrado Tenaglia, Esq. Matthew S. Poulter, Esq. Linklaters LLP 1345 Avenue of the Americas New York, New York 10105 Phone: +1 (212) 903-9000 Fax: +1 (212) 903-9100 | John R. Vetterli, Esq. White & Case LLP 1221 Avenue of the Americas New York, New York 10020 Phone: +1 (212) 819-8200 Fax: +1 (212) 354-8113 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933. Emerging growth company. ☒
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. o
CALCULATION OF REGISTRATION FEE
Title of each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price(1)(2)(3) | Amount of Registration Fee | ||||
Ordinary Shares, nominal value Ps.1 per share(4) | US$ | US$ | ||||
| (1) | Estimated solely for purposes of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. |
| (2) | Includes the aggregate offering price of additional ordinary shares the international underwriters have the option to purchase, if any. |
| (3) | Translated into dollars based on the exchange rate of Ps.17.32 per US$1.00 reported by the Central Bank of Argentina on September 30, 2017. |
| (4) | A separate registration statement on Form F-6 will be filed for the registration of American depositary shares issuable upon deposit of the ordinary shares registered hereby. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the Securities and Exchange Commission declares our registration statement effective. This preliminary prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED DECEMBER 6, 2017
PRELIMINARY PROSPECTUS
American Depositary Shares

Bioceres S.A.
Representing Ordinary Shares
We are offering American Depositary Shares, or ADSs. Each ADS represents ordinary shares. We refer to this offering of ADSs as the “international offering.” The ADSs may be evidenced by American Depositary Receipts, or ADRs. This is our initial public offering of our ADSs. We are concurrently offering ordinary shares in Argentina, which we refer to as the “Argentine offering,” through the Argentine placement agents under a Spanish-language offering document. We refer to the international offering and the Argentine offering together as the “global offering.” The closings of the international offering and the Argentine offering are conditioned upon each other. No public market has previously existed for our ADSs or ordinary shares. All of the ADSs and ordinary shares to be sold in the global offering are being sold by us.
Under Argentine law, all of our existing shareholders are entitled to preemptive and accretion rights to subscribe to our capital increase underlying the global offering and will have the opportunity to subscribe for newly issued ordinary shares at the same price as the shares offered and sold pursuant to the Argentine offering. Existing shareholders may assign their preferential subscription rights subject to applicable law. In order to facilitate the execution of the global offering, certain of our shareholders, representing % of our outstanding share capital, have assigned to AR Partners S.A., as exercise agent, their preemptive and accretion rights with respect to % of the shares to be issued pursuant to the rights. Concurrently with the global offering, we will conduct a preemptive and accretion rights offering in Argentina, or the Argentine Rights Offering. See “Rights Offering in Argentina.”
The estimated initial public offering price per ADS is between US$ and US$ . The estimated initial public offering price per ordinary share is between US$ and US$ .
We have applied to list our ADSs in the United States on the New York Stock Exchange, or NYSE, under the symbol “BIOX.” We have applied to list our ordinary shares in Argentina on the Bolsas y Mercados Argentinos S.A., or BYMA, under the symbol “BIOX.”
Investing in our ADSs involves a high degree of risk. Before buying any ADSs, you should carefully read the discussion of material risks of investing in our ADSs in “Risk Factors” beginning on page 29 of this prospectus.
We are an “emerging growth company” as defined under the federal securities laws and, as such, will be subject to reduced public company reporting requirements. See “Prospectus Summary—Implications of Being an Emerging Growth Company and a Foreign Private Issuer” for additional information.
Neither the U.S. Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
Per ADS | Total | |||||
Public offering price | US$ | US$ | ||||
Underwriting discounts and commissions(1) | US$ | US$ | ||||
Proceeds to Bioceres S.A., before expenses | US$ | US$ | ||||
| (1) | We have agreed to reimburse the underwriters for certain expenses. See “Underwriting.” |
Delivery of the ADSs is expected to be made on or about , 2017. We have granted the international underwriters an option for a period of 30 days to purchase an additional ADSs. If the international underwriters exercise the option in full, the total underwriting discounts and commissions payable by us will be US$ , and the total proceeds to us, before expenses, will be US$ .
Sole Global Coordinator | |||
Jefferies | |||
Joint book-running managers | |||
Jefferies | Piper Jaffray | ||
Prospectus dated , 2017.
TABLE OF CONTENTS
Page | |||
This prospectus has been prepared by us solely for use in connection with the proposed offering of ADSs in the United States and elsewhere outside Argentina. Neither we nor the international underwriters have authorized anyone to provide information different from that contained in this prospectus, any amendment or supplement to this prospectus or in any free writing prospectus prepared by us or on our behalf. When you make a decision about whether to invest in our ADSs, you should not rely upon any information other than the information in this prospectus and any free writing prospectus prepared by us or on our behalf. Neither the delivery of this prospectus nor the sale of our ADSs means that information contained in this prospectus is correct after the date of this prospectus. This prospectus is not an offer to sell or the solicitation of an offer to buy our ordinary shares or ADSs in any circumstances under which such offer or solicitation is unlawful. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or when any sale of ADSs occurs.
This prospectus includes statistical, market and industry data and forecasts which we have obtained from publicly available information and independent industry publications and reports that we believe to be reliable sources. These publicly available industry publications and reports generally state that they obtain their information from sources that they believe to be reliable, but they do not guarantee the accuracy or completeness of the information. Although we believe that these sources are reliable, we have not independently verified the information contained in such
i
publications. Certain estimates and forecasts involve uncertainties and risks and are subject to change based on various factors, including those discussed under the headings “Special Note Regarding Forward-Looking Statements” and “Risk Factors” in this prospectus.
Certain figures included in this prospectus have been subject to rounding adjustments. Accordingly, figures shown as totals in certain tables may not be an arithmetic aggregation of the figures that precede them.
This offering is being made in the United States and elsewhere outside of Argentina solely on the basis of the information contained in this prospectus. The concurrent offering of our ordinary shares is being made in Argentina by a prospectus in Spanish that will be submitted to the CNV. The prospectus for the Argentine offering, although in a different format in accordance with CNV regulations, contains substantially the same information as contained in this prospectus. The public offering of the ordinary shares in Argentina was authorized by the CNV pursuant to Resolution No. 17919, on December 4, 2015, subject to the fulfillment of certain requirements. The authorization by the CNV means only that the information requirements of the CNV have been satisfied. The CNV has not rendered an opinion with respect to the accuracy of the information contained in this prospectus. No offer or sale of ADSs may be made to the public in Argentina except in circumstances that do not constitute a public offer or distribution under Argentine laws and regulations.
IMPORTANT INFORMATION ABOUT FINANCIAL PRESENTATION
We present financial statements in accordance with IFRS as issued by the IASB and all financial information included in this prospectus is presented in accordance with IFRS as issued by the IASB, except as otherwise indicated. In particular, this prospectus contains certain non-IFRS financial measures which are described under “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Non-IFRS Financial Measures.”
The financial statements we have included in this prospectus consist of:
| ■ | the audited consolidated financial statements of Bioceres and its subsidiaries as of and for the six-month transition period ended June 30, 2017, or the Transition Period, and as of and for the fiscal years ended December 31, 2016 and 2015 and for the unaudited six-month period ended June 30, 2016, and the notes thereto; |
| ■ | the audited consolidated financial statements of Rizobacter as of and for the fiscal years ended June 30, 2017, 2016 and 2015, and the notes thereto; |
| ■ | the unaudited interim condensed consolidated financial statements of Bioceres and its subsidiaries as of September 30, 2017 and June 30, 2017 and for the three-month periods ended September 30, 2017 and 2016, and the notes thereto; and |
| ■ | the unaudited interim condensed consolidated financial statements of Rizobacter as of September 30, 2017 and June 30, 2017 and for the three-month periods ended September 30, 2017 and 2016, and the notes thereto. |
The financial information of Rizobacter: (i) is prepared on a standalone basis; (ii) does not reflect the purchase accounting adjustments recorded at the consolidated level of Bioceres after the acquisition; (iii) does not include any corporate and other expenses that should be allocated to Rizobacter; (iv) uses different segments than the Bioceres consolidated financial statements; (v) is for information purposes only and (vi) should not be viewed as a substitute for the consolidated financial statements of Bioceres.
On December 15, 2016, our shareholders approved a change in our fiscal year end from December 31 to June 30. As a result of this change, the Transition Period figures presented in our consolidated financial statements are not entirely comparable to the years ended December 31, 2016 and 2015 and we do not present financial statements for a separate historical period that is comparable to the Transition Period. Following the Transition Period, we will prepare annual financial statements for the fiscal years ending June 30, beginning with the fiscal year ended June 30, 2018.
Furthermore, the comparability of our results of operations is affected by the completion of our acquisition of Rizobacter, which was consummated on October 19, 2016. Our results of operations for periods prior to this date do not include the
ii
results of Rizobacter and therefore are not comparable to our results for periods after this date. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Note Regarding Comparability of Our Results of Operations.”
We have also included certain unaudited pro forma consolidated financial information derived by the application of pro forma adjustments with respect to our acquisition of Rizobacter to the historical consolidated financial information of Bioceres. See “Unaudited Pro Forma Consolidated Financial Information” and “Unaudited Pro Forma Condensed Combined Financial Information.”
The consolidated historical financial information of Rizobacter for the year ended June 30, 2017 is provided as additional information to permit readers to compare the more recent results of Rizobacter on a stand-alone basis.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
We make forward-looking statements in this prospectus that are subject to risks and uncertainties. These forward-looking statements include information about possible or assumed future results of our business, financial condition, results of operations, liquidity, anticipated growth strategies, anticipated trends in our industry, our potential growth opportunities, plans and objectives. In some cases, you can identify forward-looking statements by terminology such as “believe,” “may,” “might,” “will,” “consider,” “estimate,” “continue,” “anticipate,” “intend,” “target,” “project,” “contemplate,” “should,” “plan,” “expect,” “predict,” “potential,” or the negative of these terms or other similar terms or expressions. The statements we make regarding the following matters are forward-looking by their nature:
| ■ | our ability to develop and commercialize biotechnology products; |
| ■ | our ability to maintain our joint venture agreements with our current partners; |
| ■ | our ability to enter into new joint ventures and expand our technology sourcing and product development to new seed traits and crops; |
| ■ | our or our collaborators’ ability to develop commercial products that incorporate our seed traits and complete the regulatory approval process for such products; |
| ■ | our expectations regarding the commercial value of our key products in yield and abiotic stress and biotic stress; |
| ■ | our expectations regarding regulatory approval of products developed by us, our joint ventures and third-party collaborators; |
| ■ | our ability to adapt to continuous technological change in our industry; |
| ■ | our expectations that products containing our seed traits will be commercialized and we will earn royalties from the sales of such products; |
| ■ | our ability to maintain and recruit knowledgeable or specialized personnel and collaborators to perform our R&D work; |
| ■ | our expectations regarding the future growth of the global agricultural, agricultural biotechnology, biological-based chemical and agro-industrial biotechnology markets; |
| ■ | our ability to develop and exploit a proprietary channel for the sale of our biotechnology products; |
| ■ | our compliance with laws and regulations that impact our business and changes to such laws and regulations; |
| ■ | our ability to assemble, store, integrate and analyze significant amounts of public and proprietary data; and |
| ■ | our ability to protect our intellectual property through patents, PVP, trademarks, trade secret laws, confidentiality provisions, and licensing arrangements for the genes that we identify. |
The preceding list is not intended to be an exhaustive list of all of our forward-looking statements. The forward-looking statements are based on our beliefs, assumptions and expectations of future performance, taking into account the information currently available to us. These statements are only predictions based upon our current expectations and projections about future events. There are important factors that could cause our actual results, levels of activity,
iii
performance or achievements to differ materially from the results, levels of activity, performance or achievements expressed or implied by the forward-looking statements. In particular, you should consider the risks provided under “Risk Factors” in this prospectus.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that future results, levels of activity, performance and events and circumstances reflected in the forward-looking statements will be achieved or will occur. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus or to conform these statements to actual results or to changes in our expectations.
iv
Unless otherwise specified or the context requires otherwise in this prospectus:
| ■ | references to “we,” “us,” the “Company” and “our” refer to Bioceres S.A., together with our subsidiaries; |
| ■ | references to “US$” and “Dollars” are to U.S. Dollars, and references to “Ps.” and “Argentine pesos” are to Argentine pesos; |
| ■ | “AACREA” refers to the Argentine Association of Regional Consortiums for Agricultural Experimentation (Asociación Argentina de Consorcios Regionales de Experimentación Agrícola), an organization representing certain large farm operations in Latin America; |
| ■ | “AAPRESID” refers to the Argentine Association of No-Till Producers (La Asociación Argentina de Productores en Siembra Directa), an Argentine farmers’ organization with an international leadership position in the adoption of new agricultural technologies; |
| ■ | “ADRs” refers to American Depositary Receipts, which are certificates evidencing a specific number of ADSs, registered in the name of the holder of such ADSs; |
| ■ | “ADSs” refers to American Depositary Shares representing our ordinary shares; |
| ■ | “ADS Depositary” refers to Deutsche Bank Trust Company Americas; |
| ■ | “AFIP” refers to the Argentine Federal Administration of Public Revenue (Administración Federal de Ingresos Públicos); |
| ■ | “AGBM” refers to AGBM S.A., an industrial company that produces and commercializes chymosin from modified safflower seeds; |
| ■ | “amaranth” refers to a family of plants that includes species cultivated for a highly nutritious grain; |
| ■ | “APHIS” refers to the Animal and Plant Health Inspection Service of the USDA; |
| ■ | “Arcadia Biosciences” refers to Arcadia Biosciences, Inc., a U.S. company that commercializes agriculture-based technologies; |
| ■ | “Argentine Capital Markets Law” refers to Law No. 26,831, as amended and supplemented; |
| ■ | “Argentine Corporate Law” refers to Law No. 19,550, as amended and supplemented; |
| ■ | “Argentine PVP Law” refers to Law No. 20,247 of the Seeds and Phytogenetic Creations Law; |
| ■ | “BAF” refers to BAF Latam Trade Finance Fund B.V.; |
| ■ | “Bioceres Semillas” refers to our proprietary commercial channel for seeds, Bioceres Semillas S.A.; |
| ■ | “biopolymers” refer to polymers produced by living organisms; |
| ■ | “BRS” refers to Biotechnology Regulatory Services; |
| ■ | “BYMA” refers to the Argentine Stock Exchange (Bolsas y Mercados Argentinos S.A.); |
| ■ | “Central Bank” refers to the Central Bank of Argentina (Banco Central de la República de Argentina); |
| ■ | “Certified Public Accountant” means a member of an officially accredited professional body of accountants; |
| ■ | “chymosin” refers to an enzyme used in the production of cheese; |
| ■ | “CIBIC” refers to Cibic Centro de Diagnóstico Médico de Alta Complejidad S.A.; |
| ■ | “CNBS” refers to the Brazilian National Biosafety Council (Conselho Nacional de Biossegurança); |
| ■ | “CNV” refers to the Argentine Securities Commission (Comisión Nacional de Valores); |
| ■ | “CONABIA” refers to the Argentine National Advisory Commission of Agricultural Biotechnology (Comisión Nacional Asesora de Biotecnología Agropecuaria); |
v
| ■ | “CONICET” refers to the National Scientific and Technical Research Council of Argentina (Consejo Nacional de Investigaciones Científicas y Técnicas), the main organization in charge of the promotion of science and technology in Argentina and an entity run by the Argentine federal government; |
| ■ | “Convention” refers to the Convention on Biological Diversity, an international treaty that was adopted at the Earth Summit in Rio de Janeiro, Brazil in 1992; |
| ■ | “CPI” refers to consumer price index; |
| ■ | “crop protection technologies” refers to our technologies that are designed to control weeds, insects or diseases; |
| ■ | “CTNBio” refers to the Brazilian National Technical Commission on Biosafety (Comissão Técnica Nacional de Biossegurança); |
| ■ | “De Sangosse” refers to De Sangosse Argentina S.A.; |
| ■ | “Ditta Calza Clemente” refers to Ditta Calza Clemente S.R.L.; |
| ■ | “Don Mario” refers to Asociados Don Mario S.A.; |
| ■ | “Dow AgroSciences” refers to Dow AgroSciences LLC; |
| ■ | “DRS” refers to the Direct Registration System, a system administered by DTC, pursuant to which the depositary may register the ownership of uncertificated ADSs; |
| ■ | “DTC” refers to The Depositary Trust Company; |
| ■ | “EcoSeed” refers to our customized seed treatments that complement our seed traits and germplasms; |
| ■ | “Exchange Act” refers to the U.S. Securities Exchange Act of 1934, as amended; |
| ■ | “FAO” refers to the Food and Agriculture Organization of the UN; |
| ■ | “Florimond Desprez” refers to FD Admiral SAS, a French company specializing in wheat breeding, among other crops; |
| ■ | “FSA” refers to the U.S. Federal Seed Act; |
| ■ | “GAAP” means generally accepted accounting principles; |
| ■ | “GDP” means gross domestic product; |
| ■ | “germplasm” refers to the genetic resources of a particular species and contains the information for a species’ genetic makeup; |
| ■ | “glyphosate” refers to a non-selective herbicide used to kill weeds; |
| ■ | “GM” refers to genetically modified; |
| ■ | “GMO” refers to genetically modified organism; |
| ■ | “GMPO” refers to genetically modified plant organism; |
| ■ | “growers” refers to farmers, plantation owners, breeders and cultivators of different types of crops; |
| ■ | “HB4” refers to the yield improvement technology we co-own with CONICET and the National University of the Litoral (La Universidad Nacional del Litoral), including our modified HB4 gene traits in our 2012 HB4 patents (see “Business—Intellectual Property” for a description of our 2012 HB4 patents); |
| ■ | “Hahb 4” refers to a sunflower gene that confers drought tolerance in crops and to an element which activates that gene, which are the subjects of 2003 patents co-owned by CONICET and National University of the Litoral from whom we have an exclusive license to commercially exploit their rights in the 2003 Hahb 4 patents (see “Business—Intellectual Property” for a description of the 2003 Hahb 4 patents); |
| ■ | “Héritas” refers to Héritas S.A., a company formed in partnership with CIBIC to provide translational medicine services to the regional community, mainly in Argentina; |
vi
| ■ | “IAS 34” refers to International Accounting Standard 34, Interim Financial Reporting; |
| ■ | “IASB” refers to International Accounting Standards Board; |
| ■ | “ICSID” refers to the International Centre for Settlement of Investment Disputes; |
| ■ | “IFRS” refers to International Financial Reporting Standards as issued by the IASB; |
| ■ | “IMF” refers to the International Monetary Fund; |
| ■ | “INDEAR” refers to our technology sourcing and product development subsidiary, Instituto de Agrobiotecnologia Rosario S.A.; |
| ■ | “INDEC” refers to the Argentine National Institute of Statistics and Census (Instituto Nacional de Estadística y Censos); |
| ■ | “industrial enzymes” refers to enzymes that have industrial applications, such as chymosin; |
| ■ | “INMET” refers to our technology sourcing and product development subsidiary specialized in bacterial fermentation solutions, Ingenieria Metabolica S.A.; |
| ■ | “INTA” refers to the Argentine National Agricultural Technological Institute (Instituto Nacional de Tecnología Agropecuaria); |
| ■ | “ISAAA” refers to the International Service for the Acquisition of Agri-biotech Applications; |
| ■ | “JOBS Act” refers to the U.S. Jumpstart Our Business Startups Act of 2012; |
| ■ | “MG” refers to maturity group; |
| ■ | “microbial fermentation solutions” refers to genetically engineered Bacillus subtilis modified for the conversion of low-value organic carbon sources into higher value molecules, such as biofuels, biopolymers and ectoine; |
| ■ | “Momentive” refers to Momentive Performance Materials Inc.; |
| ■ | “Monsanto” refers to Monsanto Argentina, S.R.L.; |
| ■ | “MULC” refers to the Argentine Exchange Currency Market (Mercado Único y Libre de Cambio); |
| ■ | “NUE” refers to nutrient use efficiency; |
| ■ | “OECD” refers to the Organization for Economic Co-operation and Development; |
| ■ | “PCAOB” refers to the Public Company Accounting Oversight Board; |
| ■ | “PCT” refers to the Patent Cooperation Treaty of 1970, as amended and modified; |
| ■ | “PFIC” refers to a passive foreign investment company within the meaning of Section 1297 of the Internal Revenue Code of 1986, as amended; |
| ■ | “polyhydroxyalkanoates” refer to completely biodegradable biopolymers with plastic properties (similar to polypropylene); |
| ■ | “Porta” refers to Porta Hermanos S.A.; |
| ■ | “PVP” refers to plant variety protection; |
| ■ | “quality traits” refer to technologies designed to increase or decrease the content of a particular grain or forage component, thus improving its nutritional value or generating a downstream benefit; |
| ■ | “RASA Holding” refers to RASA Holding LLC, a Delaware limited liability company and our wholly owned holding company used to acquire the stake in Rizobacter; |
| ■ | “R&D” refers to research and development; |
| ■ | “reduced lignin trait” refers to a technology that provides higher than average quality forage; |
vii
| ■ | “Rizobacter” refers to Rizobacter Argentina S.A., an Argentine company that focuses on research, development, production and commercialization of biological agents used in agriculture; |
| ■ | “RNC” refers to the National Registry of Seed Varieties (Registro Nacional de Cultivares); |
| ■ | “RNCFS” refers to the National Registry of Trade and Control of Seeds (Registro Nacional del Comercio y Fiscalización de Semillas); |
| ■ | “S&W Seed” refers to S&W Seed Company, a U.S.-based producer of warm climate, high-yield alfalfa seed varieties, including varieties that can thrive in poor, saline soils, which recently announced the acquisition of DuPont Pioneer’s alfalfa business, expanding its operation to include dormant materials; |
| ■ | “SAMSA” refers to Servicios de Aguas de Misiones S.A. which focuses on grain production, processing and exports; |
| ■ | “San Cristóbal” refers to San Cristóbal Seguro de Retiro S.A.; |
| ■ | “Sarbanes-Oxley Act” refers to the U.S. Sarbanes-Oxley Act of 2002, as amended; |
| ■ | “SAV” refers to Argentina’s Secretariat of Value Added (Secretaría de Valor Agregado); |
| ■ | “Semya” refers to Semya S.A., our Argentina-based, intra-company joint venture with Rizobacter that conducts technology sourcing and product development to commercialize seed treatments and agricultural biological inputs for soybean, wheat, corn and alfalfa; |
| ■ | “SENASA” refers to Argentina’s National Food Safety and Quality Service (Servicio Nacional de Sanidad y Calidad Agroalimentaria); |
| ■ | “senescence” refers to the condition or process of deterioration with age; |
| ■ | “SIMI” refers to the Import Monitoring System (Sistema Integral de Monitoreo de Importaciones) imposed by the Argentine government in December 2015; |
| ■ | “soy glycerin” refers to raw glycerin extracted from soy oil in the biodiesel production process; |
| ■ | “SPC” refers to our technology for the production of bovine chymosin; |
| ■ | “Synertech” refers to Synertech Industrias S.A., Rizobacter’s Argentine-based joint venture with De Sangosse that produces and commercializes fertilizers based on microgranules that promote efficiency and accuracy; |
| ■ | “Syngenta” refers to Syngenta AG; |
| ■ | “TMG” refers to Tropical Melhoramento e Genética Ltda; |
| ■ | “transgenic products” refer to plants or other products that have had traits artificially introduced into them; |
| ■ | “Transition Period” means the six-month transition period ended June 30, 2017; |
| ■ | “TREF” refers to resistance to low temperature stress (Tolerancia/Resistencia a Estrés por Frío); |
| ■ | “Trigall Genetics” refers to Trigall Genetics S.A., our Uruguay-based joint venture with Florimond Desprez to develop and commercialize conventional wheat varieties as well as varieties with next-generation biotechnologies in South America; |
| ■ | “UN” refers to the United Nations; |
| ■ | “USDA” refers to the United States Department of Agriculture; |
| ■ | “U.S. EPA” refers to the United States Environmental Protection Agency; |
| ■ | “U.S. FDA” refers to the United States Food and Drug Administration; |
| ■ | “Valent Biosciences” refers to Valent Biosciences LLC; |
| ■ | “Verdeca” refers to Verdeca LLC, our U.S.-based joint venture with Arcadia Biosciences that develops and deregulates soybean varieties with next-generation agricultural technologies; |
viii
| ■ | “we” or “us” refer to Bioceres S.A. and its consolidated subsidiaries; |
| ■ | “WPI” refers to Wholesale Price Index (Índice de Precios Internos al por Mayor); |
| ■ | “WUE” refers to water use efficiency; |
| ■ | “yield improvement” refers to the increase in the production of a given crop; and |
| ■ | “YPF” refers to YPF Sociedad Anónima, the main Argentine oil company, including YPF Tecnología S.A., its R&D subsidiary. |
ix
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our ADSs. Before investing in our ADSs, you should read carefully this entire prospectus for a more complete understanding of our business and this offering, including the sections entitled “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements included elsewhere in this prospectus.
Overview
We are a fully-integrated provider of crop productivity solutions, including seeds, seed traits, seed treatments, biologicals, high-value adjuvants and fertilizers. Unlike most industry participants that specialize in a single technology, chemistry, product, condition or stage of plant development, we have developed a multi-discipline and multi-product platform capable of providing solutions throughout the entire crop cycle, from pre-planting to transportation and storage. Our platform is designed to cost-effectively bring high-value technologies to market through an open-architecture approach. See “—Our Business Model”. Our headquarters and primary operations are based in Argentina, which is our key end-market as well as one of the largest markets globally for GM crops. We leverage our relationship with our 308 shareholders, many of whom are industry leaders and key participants in our end-markets, to increase adoption of our products and technologies. More recently, we raised capital through financing from Monsanto and BAF, which we believe represents strategic validation of our business model as well as endorsement of our products.
As of September 30, 2017, we owned or licensed approximately 300 registered products and we owned or licensed, either exclusively or non-exclusively, approximately 200 patents and patent applications. In some instances, our licenses are limited in terms of duration, geography and/or field of use. For the three-month period ended September 30, 2017, we distributed over 3.4 million doses of inoculants, 1.8 million liters of adjuvants, 1.2 thousand tons of high value fertilizers as well as other agricultural inputs across Argentina, Brazil, Paraguay, China, India, the United States and Uruguay, among others. For the twelve-month period ended June 30, 2017, we distributed over 12.3 million doses of inoculants, seven million liters of adjuvants, three thousand tons of high value fertilizers as well as other agricultural inputs across 25 countries, including Argentina, Brazil, Paraguay, China, India, the United States and Uruguay, among others. Our pipeline of products includes fertilizers, inoculants, adjuvants, baits, crop protection solutions and seeds. Our pro forma net revenue, net loss and Adjusted EBITDA for the nine-month period ended September 30, 2017 were US$83.3 million, US$14.1 million and US$7.9 million, respectively. Our pro forma net revenue, net loss and Adjusted EBITDA (including that of Rizobacter) for the nine-month period ended September 30, 2016 were US$59.1 million, US$14.8 million and US$5.4 million, respectively. Our pro forma net revenue, net loss and Adjusted EBITDA (including that of Rizobacter) for the year ended December 31, 2016 were US$104.1 million, US$15.0 million and US$15.5 million, respectively. Our net revenue, net loss and Adjusted EBITDA for the year ended December 31, 2016 were US$44.3 million, US$5.2 million and US$6.4 million, respectively. Our net revenue, net loss and Adjusted EBITDA for the three-month period ended September 30, 2017 were US$35.0 million, US$2.9 million and US$5.6 million, respectively. Our pro forma net revenue, net loss and Adjusted EBITDA (including that of Rizobacter) for the three-month period ended September 30, 2016 were US$28.0 million, US$0.8 million and US$6.6 million, respectively. Our net revenue, net loss and Adjusted EBITDA for the Transition Period were US$48.3 million, US$11.2 million and US$2.3 million respectively. Our pro forma net revenue, net loss and Adjusted EBITDA (including that of Rizobacter) for the six-month period ended June 30, 2016 were US$31.1 million, US$14 million and US$1.2 million, respectively. Adjusted EBITDA is a non-IFRS financial measure. Net loss is the most directly comparable measure calculated in accordance with IFRS. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Non-IFRS Financial Measures” and “Risk Factors” for information regarding our use of Adjusted EBITDA and a reconciliation of net loss to Adjusted EBITDA.
Over the past 40 years, we have established a leadership position in sourcing, development, production and sales of biological products for some of the most globally prolific crops, including soy, corn, wheat and alfalfa. We sell
1
our products through a 90-person sales and marketing team and enjoy exceptional access to the end-user grower as a result of: (i) our strategic alliances with global leaders, such as Syngenta, Valent Biosciences, Dow AgroSciences, Don Mario and TMG; (ii) our shareholders, who collectively control significant agricultural land; and (iii) our longstanding relationships with dealers and distributors. Our customers include global blue-chip companies and industry leaders, large distributors, co-ops and dealers, as well as growers. Our leading infrastructure, the success of our platform and commanding presence in our key markets have made us the effective flagship agricultural solutions provider, as well as the natural partner for global conglomerates, in South America.
Our History
Bioceres was founded in 2001 by a leading group of growers in Argentina to address the demand for higher crop yield and productivity in a sustainable and environmentally conscious way. Since our founding, we have developed one of the leading fully integrated biotechnology platforms of its kind to source, validate, develop and commercialize agricultural technologies and products. We have strategically targeted some of the most globally prolific crops, namely, soy, wheat, alfalfa and corn, in one of the largest geographies for GM plants on a global scale.
In order to bring our products to market in an efficient and cost-effective manner, we have established multiple joint ventures, formed non-joint venture collaborations, as well as created and acquired multiple companies. Our joint ventures include partnerships with important industry participants, such as Florimond Desprez, De Sangosse and Arcadia Biosciences. Some of our non-joint venture collaborations include those with Dow AgroSciences, Momentive, Syngenta and Forage Genetics, among others. Of the companies we have acquired, the most significant was our 2016 acquisition of the controlling stake in Rizobacter S.A., a global leader in biological products and a pioneer in liquid inoculants. We expect to exercise a mandatory call option for 9.99% of Rizobacter upon the successful completion of this offering. In addition, we intend to incrementally increase our participation in Rizobacter as and when possible. In addition to its market leading position in biological and non-biological products, Rizobacter offers fertilizers, professional seed treatment services, and tolling or formulation services.
The graph below sets forth our history and track record of innovation through joint ventures and acquisitions:

| (1) | Patents include issued and pending patents, both licensed and proprietary. |
| (2) | INMET was formed in 2011 and officially launched in 2013. |
2
Our Operational and Organizational Structure
Bioceres is headquartered in Rosario, Argentina, where we operate our INDEAR facility. INDEAR houses a state-of-the-art R&D laboratory spanning over 40,000 square feet. Our main manufacturing and distribution facilities are located in Pergamino, Argentina. Our manufacturing facilities include an approximately 1.05 million gallon formulation plant, an approximately 24,000 gallon fermentation plant as well as packaging and logistics operations with over 375,000 square feet of warehouse space. We also recently inaugurated our new 250,000-square foot fertilizer facility and as part of our joint venture with De Sangosse.
We test and conduct trial runs of our key technologies at our main field station located in Pergamino, which also has processing capabilities for foundation seed. We also operate facilities in Cordoba, Argentina as well as international facilities in Brazil and Paraguay and have sales offices or representatives in nine countries. We believe we will continue to grow our dominant position in Argentina and that our leadership position will continue to attract interest in partnerships from global industry leaders seeking to develop and commercialize high-value crop productivity solutions in the large and attractive Argentine and South American markets. As of September 30, 2017, we had 500 full-time employees in the companies in which we have a majority ownership interest.
The following graphic provides a simplified chart of our structure:
Our Structure

| (1) | Reflects our minority investment in Chemotecnica. See “Business—Significant Transactions—Chemotecnica Investment.” |
| (2) | Reflects our syndicated ownership of Rizobacter, of which we currently control 50.01% and expect to increase to 60% upon the exercise of a mandatory call option for an additional 9.99% of Rizobacter. |
Our Business Model
Our business model is driven by three key pillars: technology sourcing, product development partnering, and production and market access:
Technology Sourcing. This pillar is focused on identifying and validating leading scientific research and developing technologies for multiple applications and/or global end-markets. We source and validate promising early stage technologies, which are usually financed through public grants and/or other capital efficient sources, and thereby
3
mitigate the associated high financial risks associated with such early stage discoveries. We currently have 30 products in the proof of concept phase and 11 products in the early development phase. The following subsidiaries support this pillar:
| ■ | INDEAR represents our R&D services arm and was formed through a strategic alliance with CONICET. |
| ■ | INMET was formed to develop and commercialize fermentation solutions based on bacterial metabolic engineering. |
Product Development Partnering. This pillar is focused on collaborating with strategic partners and creating joint ventures to develop validated technologies and products, and to bring these to market. We currently have four products in the advanced development and regulation phase and three products in the pre-launch phase. By co-funding projects, we further reduce our financial burden and risk from product development activities while also increasing our ability to develop multiple products. The following joint ventures support this pillar:
| ■ | Verdeca, our U.S.-based joint venture, was created to develop and bring soybean varieties with next-generation agricultural technologies to market. |
| ■ | Trigall Genetics, our Uruguay-based joint venture, that focuses on developing and commercializing conventional and next-generation biotechnology wheat varieties for the South American market. |
| ■ | Semya, an intra-company joint venture with Rizobacter, is dedicated to the EcoSeed initiative and focuses on researching and developing seed treatments, as well as agricultural biological input applications for soybean, wheat and alfalfa markets. |
| ■ | S&W Semillas is a joint venture that was formed to pursue development of alfalfa traits and varieties. |
Production and Market Access. This pillar is focused on leveraging our shareholder base of leading South American growers as well as proprietary sales channels for direct access to end consumers. By establishing multiple pathways to markets, we maximize our market reach and rate of technology adoption. The following subsidiaries support this pillar:
| ■ | Rizobacter, a global leader in soybean biological products and Argentina’s leading provider of bio-based solutions for the agricultural sector with a strong focus on crop nutrition and protection solutions. |
| ■ | Bioceres Semillas, our sales channel for seeds, with a primary crop focus on wheat and soybean. |
| ■ | Synertech, which was formed in partnership with De Sangosse with the goal of producing and commercializing micro-beaded fertilizers. |
| ■ | AGBM, which is our industrial enzymes company working to produce and commercialize chymosin and safflower industrial by-products from modified safflower seeds using our leading molecular farming technology. |
| ■ | Héritas, which was formed in partnership with CIBIC to provide translational medicine services to the regional community, predominantly in Argentina. |
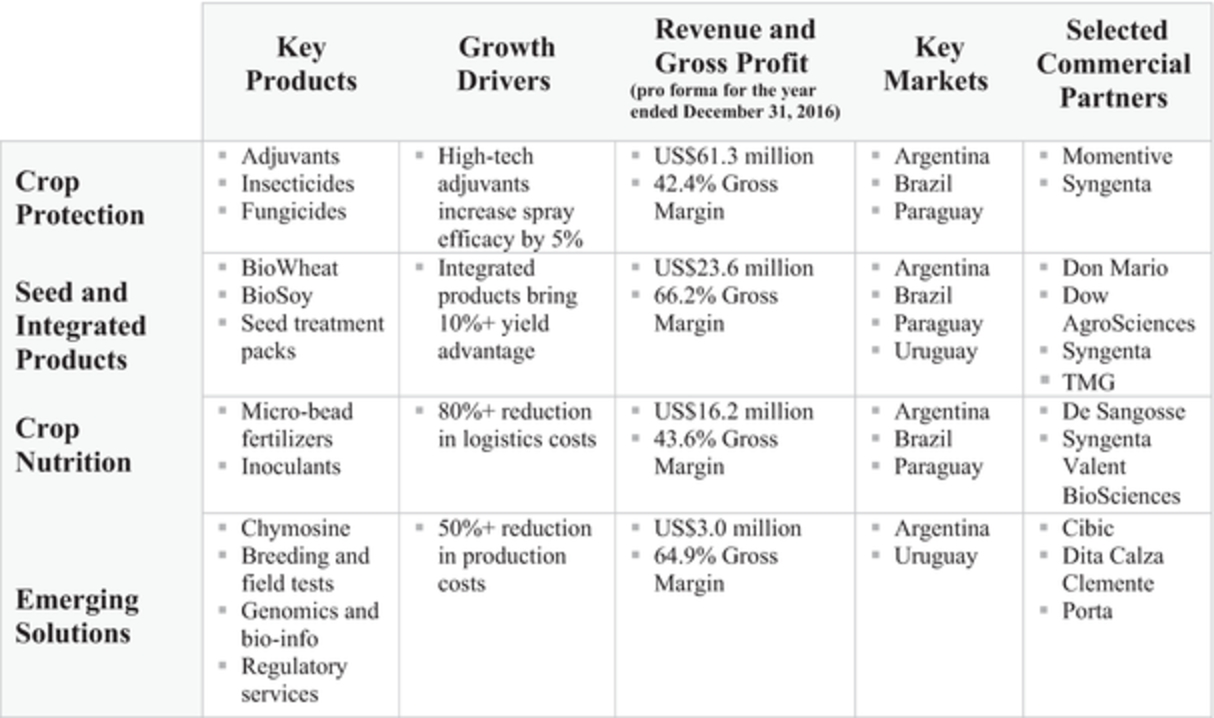
Our Segments and Key Products
We divide our business into the following four principal segments: crop protection, seed and integrated products, crop nutrition and emerging solutions.
Crop Protection. Our key crop protection products include adjuvants as well as seed-applied insecticides and fungicides.
| ■ | Adjuvants. Adjuvants are used in tank mixes to facilitate application and effectiveness of crop protection products. We produce the market leading silicone-based adjuvant, Silwet, and are currently developing microbially-enhanced adjuvants in partnership with other companies. |
| ■ | Insecticides and Fungicides. We offer a full range of chemical seed treatments tailored for specific crop and pest combinations. We are in the process of formulating and commercializing standalone chemical seed treatments, including fungicides and insecticides, in partnership with Syngenta, to reduce disease |
4
and pest pressure during crop establishment. Also, in partnership with De Sangosse, we offer a range of pest baits to effectively and safely control pests that are particularly harmful for harvested and stored grains or seeds. We hold a leading market position for such products, with an estimated 50% current market share. Furthermore, we are pursuing commercialization of biological fungicides formulated as seed treatments that control and restrict the growth of pathogenic agents in wheat and barley, as well as developing a microbial-based insecticide.
Seed and Integrated Products. The key products of this segment include seed traits, germplasms and seed treatments for healthier and higher yielding crops.
| ■ | Seed Traits. Our seed trait effort is primarily focused on improving plant yields by increasing plant tolerance to abiotic stress, such as drought or salinity. We also have a secondary focus on crop protection and quality traits. We gain access to these technologies by collaborating with the original developers or by co-developing new events with our partners. Our most advanced technology in the seed trait area, HB4, helps increase yield by an average 13% to 19% for multiple crops under various growing seasons and conditions, including sporadic drought episodes. HB4 is also able to provide this higher yield without adversely impacting yields in optimal growing conditions, which is a distinctive and important factor compared to other stress tolerance technologies. HB4 has been approved for use in soybean in Argentina and by the U.S. FDA. Submissions for approval for use of HB4 in wheat have been initiated in Argentina, Brazil, Uruguay and Paraguay, and we have also filed for approval of HB4 in soybean in China. |
| ■ | Germplasms. We currently breed germplasms for soybean and wheat and plan to advance our elite germplasms by delivering these technologies using our proprietary channels. Our soybean breeding program produces varieties that are registered or are in process of being registered in Argentina, Uruguay, Paraguay and South Africa. Our wheat breeding program is operated through our joint venture, Trigall Genetics, which has exclusive rights to the wheat breeding program of Florimond Desprez. In addition, we hold exclusive rights to all wheat varieties developed between 2003 and 2013 by the Argentine national breeding program at INTA. |
| ■ | Seed Treatments. Seed treatments comprise one of our core products and include Rizopacks, produced by our subsidiary Rizobacter in partnership with Syngenta Seedcare, which are the flagship soybean product with proprietary inoculants and fungicides. We also offer certain variations customized for peanut, beans and chickpea. In addition, we are pursuing the development of next-generation biologicals, particularly for seed treatments tailored for specific germplasms, seed traits and environment combinations. |
Crop Nutrition. Our main crop nutrition products include inoculants, biofertilizers and chemical-based fertilizers:
| ■ | Inoculants. Inoculants are broadly used nitrogen-fixing bacteria that promote growth of leguminous crops such as soybean and alfalfa. We hold a global leadership position in sales of soybean inoculants with approximately 21% market share per our internal projections. We are currently developing the next-generation of inoculants, including Bioinductor 2.0 and Extended-Shelf-Life products for professional seed treatment businesses. |
| ■ | Biofertilizers. Biofertilizers contain living microorganisms that colonize the interior of a plant and promote growth by increasing supply or availability of primary nutrients through the natural processes of nitrogen fixation, solubilizing phosphorus and stimulating plant growth through synthesis of growth-promoting substances. The combination of biologicals and chemical fertilizers can maximize crop yields while reducing environmental impact as a result of reduced use of chemicals. We are also in early stages of development for microbially-enhanced fertilizers for soybean, wheat, corn and chickpeas. |
| ■ | Chemical-Based Micro-Granulated Fertilizers. We produce and commercialize fertilizers based on chemically formulated microgranules. As these fertilizers can be applied next to the seed at planting, lower doses are needed than standard fertilizers, resulting in logistical efficiency and environmental benefits. Currently, our production is focused on Microstar PZ, a starter fertilizer that provides nitrogen, phosphorus, sulfur and zinc to different crops. |
5
Emerging Solutions. Our emerging solutions segment provides high value R&D, technical and advisory services to strategic partners and third parties. We also commercialize specialized products for a variety of end markets, including enzymes, such as chymosin, microbial fermentation solutions and translational medicine services.
| ■ | R&D Services. Our R&D services provide advanced biotechnology capabilities and specialized knowledge and expertise to facilitate validated technology sourcing and product development efforts from our industry collaborations. We also enter early-stage research collaboration arrangements with external research groups, most of which are funded through government grants. Our R&D services are provided through our two subsidiaries: INDEAR, which provides R&D services across a broad range of platforms, and INMET, which focuses on fermentation solutions using synthetic biology and metabolic engineering for application in agro-industrial solutions. |
| ■ | Agro-Industrial Biotechnology. We have developed a safflower-based molecular farming platform for industrial enzymes, which allows us to use harvested grains as an efficient way of storing enzymes prior to processing. Our most advanced molecular farming technology is SPC®, which is used to produce bovine chymosin, an enzyme used in the manufacturing of cheese. We are also developing solutions for producing biopolymers, such as PHA/PHB, biodiesel and ectoine from soybean glycerin based on bacillus fermentation, which we also use for cost-effective production of biological pest control agents. |
The following graphic sets forth the key products, growth drivers, revenue and gross profit, key markets and selected commercial partners for each of our segments:
Our Competitive Strengths
Our diversified platform generates revenues through multiple technologies, customers, distribution channels and end-markets, providing us with a profitable growth trajectory. Our key competitive strengths include:
Premier Agricultural Solutions Provider with the Flagship Position in Latin America. As the first non-governmental Latin America-based entity with a GMO event approved in a major global crop, we consider ourselves to be the pioneer in the agricultural biotechnology industry in Latin America. Our experience over the last 40 years has allowed us to become and maintain our position not only as a reference entity for governmental agencies and policy-makers, but
6
also as a leading choice for partnerships with global conglomerates. We have helped define regulations for gene editing and new breeding technologies as well as formulate intellectual property guidelines and legislation for our industry. We are a founding member of the Argentine Chamber of Biotechnology and one of a handful of selected companies collaborating with the Argentine Ministry of Science, Technology and Productive Innovation in the design of research grants aimed at our sector. We are a frequent and leading participant in all major forums dedicated to our industry and a prominent representative of our sector.
Proven Platform with a Successful Track-Record in Sourcing, Developing and Commercializing Key Biotechnologies. Over the last 40 years, we and our subsidiaries have created our proprietary platform for sourcing, validating, developing and bringing key technologies and products to commercialization.
We manage our sourcing efforts through our two key business divisions: INDEAR, which focuses on our technology and product development efforts and third party initiatives, and INMET, which focuses on metabolic engineering solutions for the conversion of agricultural feedstock into higher value molecules.
We source our technologies and products through various partnerships, collaborations and long standing relationships with research institutions and scientists. We are the strategic partner of various institutions including CONICET for the development of multiple GM trait leads, Danziger Innovations for the development of modified gene lines in soybeans as well as quality and protection traits and the University of Illinois for the development of herbicide tolerance technology for alfalfa and soybeans, among others. We have also entered into various collaborative product development agreements, including with: (a) Forage Genetics for enhanced alfalfa with herbicide resistance technology; (b) Dow AgroSciences for the development of new seed traits in soybeans; (c) Momentive for adjuvants; (d) Syngenta for new seed treatments; and (e) Valent BioSciences for microbials in the United States, among others.
We manage our product development via various joint ventures and partnerships with leading participants in the global agriculture sector. We focus our efforts on developing products and technologies that address the specific requirements and demands of our global customer base and for some of the highest demanded crops, such as soy, wheat and alfalfa, among others.
We operate a commercialization platform for agricultural biotechnology products in South America as well as other select global agricultural markets. We have access not only to the largest distributors, co-ops and dealers, but also to end customers through our well-established subsidiaries, divisions and partnerships. By selling our proven genetics, seed and seed treatments on a branded basis, we believe we are able to further strengthen our brand and grow our position in Latin America.
Capital-Efficient, Risk Mitigated Development Model. Development and regulatory approval for our products and technologies requires a highly evolved and complicated process that can last between 12 to 14 years. Furthermore, capital allocation requirements can be onerous due to the expensive research activities usually associated with life sciences research and the strict requirements for regulatory approval that are imposed on GM crops and technologies.
We believe that we have created a highly-competitive independent platform for developing such products and technologies in South America. We consider ourselves to be the go-to partner for advanced validation of promising research leads developed by local research institutions, most of which do not have the necessary capabilities for this purpose. As advanced validation initiatives are funded often by existing government programs, we are able to reduce our capital exposure at this high-risk stage of the R&D process.
Upon technology validation, we enter into joint ventures, partnerships and collaborative agreements with industry participants that agree on the merits of a new technology and pursue the business opportunity jointly with us. Partnering with others in this stage of the R&D process allows us to reduce our capital exposure while retaining a controlling interest in the product or technology under development.
7
We enjoy a competitive advantage in commercializing our products as we are able to leverage our strong industry relationships to bring our products to market faster than our competitors. We also facilitate the use of our technologies through licensing agreements and partnerships with global industry leaders, particularly in new markets with expanded regulatory requirements.
Patented and Well-Established High Impact Technologies and Products as well as a Robust Pipeline of New Products and Technologies at or Close to Commercialization Phase. Our patent and trademark portfolio for biologicals is amongst the most competitive in South America. As of the date of this prospectus, we have identified and sought patent protection in our capacity as either title holder or licensee, either as exclusive or non-exclusive licensee, to approximately 200 patents or patent applications. In some instances, our licenses are limited in terms of duration, geography and/or field of use. We usually seek patent protection in the largest global markets for our products and technologies, including, the United States, Brazil, Argentina, China, India, Mexico, Australia and certain other European and South American countries.
We have registrations in Argentina for 27 wheat, 18 soybean, 5 alfalfa, 4 corn and 2 safflower varieties and are also seeking registration for an additional 13 soybean, 2 wheat, 2 amaranth and 17 alfalfa varieties. Our portfolio also includes 42 trademarks and 4 trademark applications in Argentina, Brazil, the United States, and Uruguay for Bioceres, while our subsidiary Rizobacter has more than 355 trademarks and applications in Argentina and over 300 trademarks and applications globally.
We also have a robust and innovative portfolio of products and technologies for all stages of crop development across various chemistries, many of which are at or close to the commercialization phase, such as EcoSoy, EcoWheat and HarvXtra™ Alfalfa products.
Unique Ownership by Key Industry Influencers Leading to Early and Broad Adoption of Technologies and Products. Our current ownership structure is composed of 308 shareholders, including some of the largest farm operators, processors, distributors and commercial participants in the South American agricultural sector. Our shareholder structure also includes founding members of AAPRESID and leading members of AACREA. These unique relationships not only allow us to quickly bring our products to market and integrate our technologies into the broad market by creating a proprietary distribution and commercialization channel, but also provides us with a much desired early stage testing platform which allows us to receive direct market feedback early on in the testing process to vet and facilitate faster market penetration.
Highly Accomplished Management Team with a Unique Blend of Technical and Commercialization Experience as well as the Ability to Identify and Integrate Key Acquisitions. We believe we have a strong management team with a unique blend of executive, managerial, technical, commercialization and acquisition experience. We are able to leverage the experience of our management team not only to efficiently source and develop our technologies and products, but also to leverage their vast experience in commercial production, distribution, navigation of intellectual property requirements and inorganic acquisitions to strategically grow our company.
Our Growth Strategy
Our long-term growth strategy is based on an open-architecture approach to technology origination, identifying and accessing promising technologies from third parties, as well as forming strategic and capital-efficient partnerships that leverage each party’s strategic strengths and capabilities to more quickly bring innovations to market. Our near-term growth strategy includes the following:
Continue to Lead Development and Commercialization of New Agricultural Biotechnology Products in Existing and New Markets. We intend to build upon our diverse portfolio of crop productivity solutions by consolidating our position on biological assets, including microbial, seed traits and germplasm assets, and continuing to pursue an integrated approach in the development of superior yielding products. We intend to expand upon our direct reach to customers by offering additional high demand technologies, including digital farming solutions and direct-to-consumer retail, which we believe will facilitate the adoption and subsequent sales of our products as well as achieve efficiencies creating additional value opportunities.
8
Scale-up Production of Rizobacter Products to Accelerate Penetration in Local and Regional Crop Nutrition Markets. We have invested significant capital in future developments of specialty fertilizers and have recently completed the construction of our micro-beaded fertilizer facility in Pergamino, Argentina. The facility began operations in January 2017 and is expected to supply high-demand specialty fertilizers in Argentina and neighboring countries. Micro-beaded fertilizers place non-toxic formulations of macro- and micro-nutrients next to the planted seed allowing growers to significantly reduce application rates by as much as 75% to 80% on a weight basis and resulting in significant logistical as well as operational savings. Through our recent acquisition of Rizobacter, we also own Microstar, the leading brand in micro-granulated fertilizers.
Commercial Launch of Seed Traits and EcoSeed Products to Drive Penetration in Local and Regional Integrated Seed Market. Given the near-term commercialization opportunity that HB4 and other seed technologies represent, we plan to integrate these solutions into customized seed products that represent a superior value proposition in the current market, with an initial focus on Latin America. EcoWheat and EcoSoy seeds integrate the uniqueness of HB4 stress tolerance into locally-adapted germplasms, customized with a seed treatment solution prescribed for specific environments. We believe that the product differentiation provided by our unique and varied technologies increase the value of our products for EcoSeed customers and will drive significant growth in this segment of our business. In the medium-term, we expect royalties from HB4 licensees to represent a significant component of our revenues as this landmark technology is more broadly adopted through strategic partnerships and third-party channels. We also expect to obtain regulatory approval for the commercialization of the HarvXtra™ Alfalfa with Roundup Ready® technology developed by Forage Genetics International.
Expand our International Business by Accelerating Registration and Sales of Products Through Multiple Subsidiaries. We consider ourselves to be a global leader in the soybean biological market and have used this position to establish subsidiaries in Brazil, Paraguay, Bolivia, Uruguay, the United States, South Africa, and more recently, India, Colombia and France. We believe we can use our international footprint and sales force to continue to define our key brands by bringing our broader portfolio of crop productivity solutions to these markets. We expect international growth to be driven initially by continued growth in our historical biological business, as well as by incorporating high-value adjuvants and crop nutrition solutions in the future. In the medium-term, we expect to leverage our leading distribution network to bring our integrated seed products and other crop protection and nutrition solutions to all of our current and target markets.
Pursue Strategic Collaborations and Acquisitions in Key Markets. We intend to continue working with our collaboration partners to bring our products to customers in key markets. We also plan to continue pursuing acquisitions and in-licensing opportunities to gain access to validated and important later stage products and technologies that we believe to be a strategic fit for our business.
Further Develop Emerging Agro-Industrial Biotechnology Solutions. Through our investment in AGBM, we plan to scale-up our molecular farming business, by initially producing and commercializing chymosin, an enzyme used in cheese manufacturing. We intend to accelerate the cost-efficient development of commercial technologies that rely on metabolic engineering for bio-conversions of soy glycerin to produce biofuels, bio-chemicals and bioplastics as well as technologies for the production of new industrial enzymes through our safflower molecular farming platform. We believe that the ability to combine our capabilities in genomic and bio-informatics, synthetic biology, metabolic engineering and fermentation as well as unique relationships with some of the largest participants in the agricultural sector allows commercialization of technologies more quickly and efficiently and with lower risk than our competitors.
Global Industry Overview
We develop, produce and/or formulate: germplasm, seed traits, seed treatments, biological and micro-granulated fertilizers, specialty insecticides and fungicides, adjuvants and specialty enzymes. Our key geographical end-markets include Argentina, which is the third largest market for agricultural biotechnology products, Brazil and the rest of Latin America, the United States, China and India. We sell our products in more than 25 countries
9
globally. Our products and technologies have applicability across a wide variety of crops, including some of the most globally farmed crops such as corn, soy, alfalfa and wheat.
Demand for crop yields from agricultural lands are seeing a dramatic increase as a result of increasing global population, an expanding middle class, trend towards urbanization, decrease in agricultural land per capita, demand for reduced use of environmentally harmful chemicals and an increase in unfavorable weather patterns for farming. This demand cannot be met by conventional farming alone. Agricultural biotechnology products are the only current viable avenue available to meet this expected high demand in crop yields.
According to the USDA, global demand for grains increased by more than 57% from 1.4 billion metric tons in 1980 to 2.2 billion metric tons in 2010. Furthermore, according to the OECD and the FAO, this demand is expected to increase another 20% by 2020 reaching 2.6 billion metric tons. The increase in demand is primarily driven by population growth in developing countries and an expanding middle class. Also, according to the OECD and the FAO, global population is projected to increase from 7.3 billion in 2016 to 8.2 billion in 2026, with almost all of this increase occurring in developing countries. Furthermore, the OECD estimates that global middle-class population is expected to grow from 1.8 billion people in 2009 to 3.2 billion people by 2020 and 4.9 billion people by 2030. As household incomes rise, demand for protein-rich diets often increases and this drives additional demand for grains. The trend toward urbanization is also causing a large drop in arable land available per capita. The FAO estimates that ratio of arable land to population has declined by over 50% from 1962 to 2010. As a result of this, according to a report from Statista, the number of people fed per hectare is expected to increase by more than 100% from 2.3 to 5.6 people fed per hectare from 1960 to 2020.
The U.S. EPA has validated that more extreme temperature and precipitation can prevent crops from growing. Dealing with drought could become a challenge in areas, and although increased irrigation might be possible in some places, in other places water supplies may also be reduced, leaving less water available for irrigation when more is needed. According to the U.S. Global Change Research Program, climate disruptions to agricultural production have increased in the past 40 years and are projected to increase over the next 25 years. By mid-century and beyond, these impacts will be increasingly negative on most crops and livestock.
According to the ISAAA, conventional crop technology alone cannot address this immense demand or feed the increase in population. Sustainable approaches using the best of conventional crop technology, such as use of the best adapted germplasms, as well as the best of biotechnology are required in order to increase crop productivity enough to meet the growing demands associated with the increase in population. The last 20 years of commercialization of biotech crops has confirmed that biotech crops have and can deliver substantial agronomic, environmental, health, economic and social benefits. The rapid adoption of biotech crops reflects the multiple substantial benefits realized globally and in the last 20 years, with an accumulated 2 billion hectares of biotech crops grown commercially. Furthermore, in most countries, the adoption rate for biotech crops has reached over 90% for major products in principal markets in both developing and industrial companies.
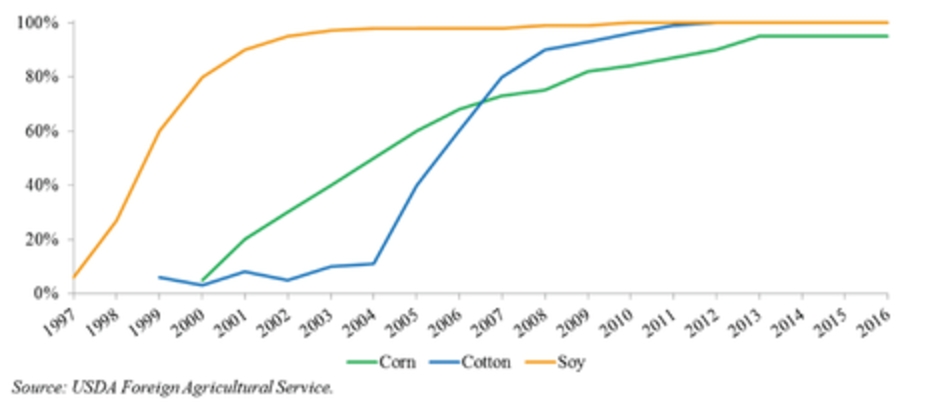
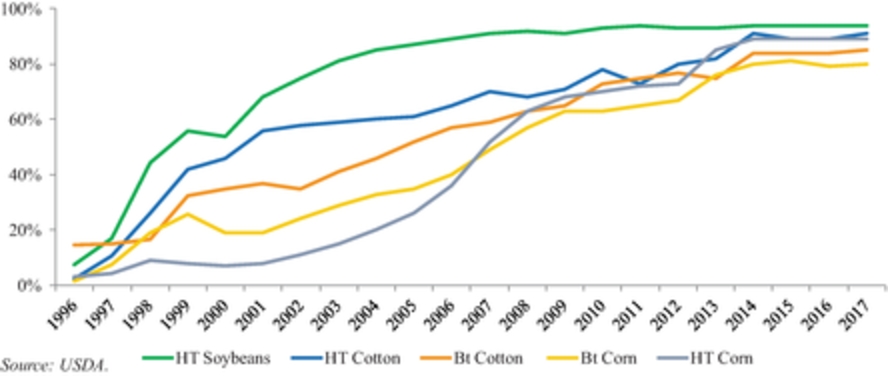
In 2016, according to the ISAAA, corn and soybeans represented a majority of the seed biotechnology market, making up approximately 87% of the global biotech seed market. The United States, Brazil and Argentina were the top planters of biotech seeds with more than 145 million hectares under production of biotech crops. Current adoption of GM varieties is above 90% for soybean, above 80% for corn and above 65% for cotton. In Argentina, approximately 24 million hectares of biotech crops were planted in 2016 with virtually 100% of soybean, 97% of corn and 95% of cotton hectares utilizing biotech varieties. Historically, the Argentine market has been quick to adopt biotechnology as a result of concentrated nature of farm groups as well as comfort with fast commercialization of new GM varieties. In the United States, which is the top producer of biotech seeds, 73 million hectares were planted using biotech crops. The United States has an established history of rapid adoption rates of GM crops, typically reaching 65% to 90% peak penetration in ten years driven by overall yield and productivity improvements of specific seed traits as well on-going consumer education and resulting acceptance.
10
The graph below reflects the adoption of rates of GM crops in Argentina for the periods indicated:
Adoption Rates of GM Crops in Argentina
In the United States, which is the top producer of biotech seeds, 73 million hectares were planted using biotech crops. The United States has an established history of rapid adoption rates of GM crops, typically reaching 65% to 90% peak penetration in ten years driven by overall yield and productivity improvements of specific seed traits as well on-going consumer education and resulting acceptance.
The graph below reflects the adoption rates of GM crops in the United States for the periods indicated:
Adoption Rates of GM Crops in the U.S.
The global seed market has grown an 85% in ten years up to US$37 billion in 2016 from US$20 billion in 2006 per a 2017 report by Phillips McDougall. Also, GM seeds have grown in prominence, representing only 29% (US$6 billion) of the global market in 2006 up to 55% (US$20 billion) in 2016. This increase of more than 330% in the market size of GM seeds underlines the increasing significance and need for agricultural biotechnology.
11
The graph below reflects the increase in the global seed market and the penetration of biotechnology crops for the periods indicated:
Global Seed Market & Penetration of Biotech Crops

Source: Phillips McDougall, 2017.
Syngenta, our joint venture partner and a global leader in crop protection, estimates that the global market for agrochemical products, including crop protection products doubled from 2000 to 2014, reaching an estimated size of US$63 billion. The Company, based on its research and market sources, also estimates this unprecedented growth to continue driven by introduction of new chemistries, which addressed many unmet agronomic challenges faced by growers, as well as need to address significant losses from abiotic stresses that could potentially be more than US$100 billion.
We believe that agricultural biotechnology and biologicals will continue to grow as the benefits of these technologies and products become more widely known and consumers appreciate the similar efficacy to conventional chemicals while also addressing other issues such as pest resistance and environmental safety.
Implications of Being an Emerging Growth Company and a Foreign Private Issuer
As a company with less than US$1.07 billion in revenue during our last fiscal year, we qualify as an “emerging growth company,” as defined in the JOBS Act. Section 107 of the JOBS Act provides that an emerging growth company may take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. Thus, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
An emerging growth company may also take advantage of reduced reporting requirements that are otherwise applicable to public companies. If we choose to take advantage of any of these reduced reporting burdens, the information we provide to shareholders may be different from that which you may receive from other public companies. These provisions include:
| ■ | a requirement to have only two years of audited financial statements in addition to any required interim financial statements and correspondingly reduced Management’s Discussion and Analysis of Financial Condition and Results of Operations disclosure; and |
12
| ■ | an exemption from the auditor attestation requirement under Section 404(b) of the Sarbanes-Oxley Act in the assessment of our internal control over financial reporting. |
We may take advantage of these provisions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company upon the earlier to occur of:
| ■ | the last day of our fiscal year during which we have total annual gross revenue of at least US$1.07 billion; |
| ■ | the date on which we have, during the previous three-year period, issued more than US$1.0 billion in non-convertible debt; or |
| ■ | the date on which we are deemed to be a “large accelerated filer” under the Securities Exchange Act of 1934, as amended, or the Exchange Act, which would occur if the market value of our shares that are held by non-affiliates exceeds US$700 million as of the last business day of our most recently completed second fiscal quarter. |
Once we cease to be an emerging growth company, we will not be entitled to the exemptions provided in the JOBS Act.
We are also considered a “foreign private issuer.” Even after we no longer qualify as an emerging growth company, as long as we qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
| ■ | the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations with respect to a security registered under the Exchange Act; |
| ■ | the requirement to comply with Regulation FD, which requires selective disclosure of material information; |
| ■ | the sections of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and |
| ■ | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K upon the occurrence of specified significant events. |
Both foreign private issuers and emerging growth companies are also exempt from certain more stringent executive compensation disclosure rules. Thus, even if we no longer qualify as an emerging growth company, but remain a foreign private issuer, we will continue to be exempt from the more stringent compensation disclosures required of companies that are neither an emerging growth company nor a foreign private issuer.
Corporate Information
Our principal executive offices are located at Ocampo 210 bis, Predio CCT, Rosario, Santa Fe, Argentina, and our telephone number is +54 341 486-1100. We were incorporated as a sociedad anónima under the laws of Argentina on April 11, 2002 for a duration of 50 years. Our agent for service of process in the United States is Cogency Global Inc., 10 E. 40th Street, 10th Floor, New York, NY 10016.
13
offering
We are offering up to ADSs (or ADSs if the international underwriters exercise their option to purchase additional ADSs in full) through the international underwriters in international offering.
We are offering up to ordinary shares in the Argentine offering.
Certain of the shares offered in the global offering are (i) shares that became available as a result of the decision of certain of our shareholders not to exercise their preemptive and accretion rights to subscribe to our capital increase underlying the global offering and the subsequent assignment to AR Partners S.A., as exercise agent, and (ii) additional shares that the international underwriters may acquire from us relating to preemptive rights not exercised by our shareholders.
See “Rights Offering in Argentina.”
14
15
16
concerning such companies’ relationship with shareholders and investors, without prejudice to the right of shareholders and investors to submit their claims (or challenge any arbitral award) to the competent courts of Argentina. For all matters relating to the deposit agreement and the ADSs, we will submit to the jurisdiction of the federal courts of the United States of America located in the City and County of New York, Borough of Manhattan. See “Description of Share Capital—Mandatory Public Offers Required Pursuant to Argentine Capital Markets Law and the CNV rules—Jurisdiction and Arbitration.”
The number of ordinary shares to be outstanding after the global offering is based on 25,644,300 ordinary shares to be outstanding prior to the commencement of this offering after the consummation of the Stock Split and includes shares issuable pursuant to the rights offering. See Notes 10 and 11 to our audited consolidated financial statements. The number of outstanding ordinary shares excludes:
| ■ | 929,040 ordinary shares issuable upon the exercise of stock options that have already been approved for issuance as of the date hereof under our stock option incentive plan and under individual option agreements that have been entered into with certain of our key employees and members of our board of directors, with an exercise price of US$7.91 per ordinary share; |
| ■ | 902,487 ordinary shares issuable upon the exercise of stock grants that have already been approved for issuance as of the date hereof under our stock grant incentive plan and under individual option agreements expected to be entered into with certain of our key employees and members of our board of directors; and |
| ■ | 696,473 ordinary shares reserved for future issuance, consisting of 334,960 ordinary shares issuable upon the exercise of future stock option awards under our stock option incentive plan and 361,513 ordinary shares issuable pursuant to future stock grants under our stock grant incentive plan, in each case authorized by our shareholders as of the date hereof. |
For further information on our equity incentive plans, see “Management—Equity Incentive Plans.”
Unless otherwise indicated, this prospectus:
| ■ | reflects the Stock Split of our ordinary shares effective immediately prior to the commencement of this offering; |
| ■ | assumes an initial public offering price of US$ per ADS, the midpoint of the price range set forth on the cover of this prospectus; and |
| ■ | assumes no exercise of the international underwriters’ option to purchase additional ADSs representing up to an additional ordinary shares. |
17
SUMMARY CONSOLIDATED HISTORICAL FINANCIAL AND OTHER INFORMATION
The following tables set forth, for the periods and dates indicated, certain summary historical financial information. You should read the following summary consolidated financial and other data in conjunction with “Selected Consolidated Historical Financial and Other Information,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the audited and unaudited financial statements and related notes included elsewhere in this prospectus. Historical results are not necessarily indicative of the results that may be expected in the future. Our consolidated financial statements have been prepared in accordance with IFRS as issued by IASB.
On December 16, 2016, our shareholders approved a change in our fiscal year end from December 31 to June 30. As a result of this change, the Transition Period figures presented in our consolidated financial statements are not entirely comparable to the years ended December 31, 2016 and 2015 and we do not present financial statements for a separate historical period that is comparable to the Transition Period. Following the Transition Period, we will prepare annual financial statements for fiscal years ending June 30, beginning with the fiscal year ended June 30, 2018.
Furthermore, the comparability of our results of operations is affected by the completion of our acquisition of Rizobacter, which was consummated on October 19, 2016. Our results of operations for periods prior to this date do not include the results of Rizobacter and therefore are not comparable to our results for periods after this date. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Note Regarding Comparability of Our Results of Operations.”
Summary of Consolidated Historical Financial Information of Bioceres
Consolidated statement of comprehensive income of Bioceres
The summary consolidated statements of comprehensive income data for Bioceres for the Transition Period, for the unaudited six-month period ended June 30, 2016 and for the years ended December 31, 2016 and 2015 are derived from our audited consolidated financial statements appearing elsewhere in this prospectus. The summary consolidated statements of comprehensive income data for Bioceres for the three-month periods ended September 30, 2017 and 2016 are derived from our unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus.
Bioceres | ||||||||||||||||||
Three-month period ended September 30, 2017 (unaudited) | Three-month period ended September 30, 2016(1) (unaudited) | Transition Period ended June 30, 2017 | Six-month period ended June 30, 2016(1) (unaudited) | Year ended December 31, | ||||||||||||||
2016(2) | 2015 | |||||||||||||||||
(US$, except share data) | ||||||||||||||||||
Total revenue | 34,986,505 | 1,963,513 | 48,341,121 | 2,538,399 | 44,349,263 | 10,195,884 | ||||||||||||
Crop protection | 16,795,948 | — | 31,191,970 | — | 21,493,419 | — | ||||||||||||
Seed and integrated products | 7,498,035 | 1,313,083 | 9,020,999 | 918,765 | 14,244,967 | 4,495,042 | ||||||||||||
Crop nutrition | 9,540,883 | — | 6,640,228 | — | 5,227,394 | — | ||||||||||||
Emerging solutions | 1,151,639 | 650,430 | 1,487,924 | 1,619,634 | 3,383,483 | 5,700,842 | ||||||||||||
Cost of sales | (20,195,931 | ) | (1,118,948 | ) | (30,185,446 | ) | (1,290,256 | ) | (31,600,998 | ) | (4,799,345 | ) | ||||||
Crop protection | (10,038,912 | ) | — | (22,641,887 | ) | — | (16,825,572 | ) | — | |||||||||
Seed and integrated products | (2,462,759 | ) | (855,682 | ) | (4,851,444 | ) | (790,671 | ) | (8,895,386 | ) | (3,733,701 | ) | ||||||
Crop nutrition | (7,509,962 | ) | — | (2,084,652 | ) | — | (4,819,455 | ) | — | |||||||||
Emerging solutions | (184,298 | ) | (263,266 | ) | (607,463 | ) | (499,585 | ) | (1,060,585 | ) | (1,065,644 | ) | ||||||
Research and development expenses | (1,725,645 | ) | (596,652 | ) | (3,601,624 | ) | (1,268,661 | ) | (2,860,771 | ) | (2,688,924 | ) | ||||||
18
Bioceres | ||||||||||||||||||
Three-month period ended September 30, 2017 (unaudited) | Three-month period ended September 30, 2016(1) (unaudited) | Transition Period ended June 30, 2017 | Six-month period ended June 30, 2016(1) (unaudited) | Year ended December 31, | ||||||||||||||
2016(2) | 2015 | |||||||||||||||||
(US$, except share data) | ||||||||||||||||||
Selling, general and administrative expenses | (9,993,183 | ) | (1,185,472 | ) | (17,324,407 | ) | (3,541,058 | ) | (12,906,021 | ) | (4,080,860 | ) | ||||||
Share of profit / (loss) joint ventures and associates | 84,857 | (238,100 | ) | (786,805 | ) | (455,181 | ) | (936,769 | ) | (1,553,002 | ) | |||||||
Other income / (expenses), net | (217,284 | ) | (18,135 | ) | 121,065 | — | 48,495 | — | ||||||||||
Operating profit / (loss) | 2,939,319 | 1,193,794 | (3,436,096 | ) | (4,016,757 | ) | (3,906,801 | ) | (2,926,267 | ) | ||||||||
Gain on previously held interest | — | — | — | — | 4,453,284 | — | ||||||||||||
Finance income | 2,042,066 | 381,327 | 2,136,265 | 790,814 | 1,006,953 | 1,509,736 | ||||||||||||
Finance costs | (9,268,419 | ) | (1,117,154 | ) | (14,945,495 | ) | (1,579,951 | ) | (10,923,378 | ) | (1,904,569 | ) | ||||||
Loss before income tax | (4,287,034 | ) | (1,929,621 | ) | (16,245,326 | ) | (4,805,894 | ) | (9,369,942 | ) | (3,321,100 | ) | ||||||
Income tax benefit / (expense) | 1,357,795 | 621,022 | 5,090,723 | 1,040,923 | 4,140,028 | (411,342 | ) | |||||||||||
Loss for the period / year | (2,929,239 | ) | (1,308,599 | ) | (11,154,603 | ) | (3,764,971 | ) | (5,229,914 | ) | (3,732,442 | ) | ||||||
Other comprehensive loss | (4,150,698 | ) | — | (2,581,500 | ) | — | (4,482,329 | ) | — | |||||||||
Total comprehensive loss(3) | (7,079,937 | ) | (1,308,599 | ) | (13,736,103 | ) | (3,764,971 | ) | (9,712,243 | ) | (3,732,442 | ) | ||||||
Loss per share(4) | ||||||||||||||||||
Basic loss per ordinary share | (0.1200 | ) | (0.0474 | ) | (0.3316 | ) | (0.1341 | ) | (0.1762 | ) | (0.1387 | ) | ||||||
Diluted loss per ordinary share of the parent | (0.1200 | ) | (0.0474 | ) | (0.3316 | ) | (0.1341 | ) | (0.1762 | ) | (0.1387 | ) | ||||||
Weighted average number of ordinary shares used in computing basic net loss per ordinary share | 25,546,550 | 25,615,265 | 25,536,100 | 25,593,700 | 25,592,104 | 25,534,542 | ||||||||||||
Weighted average number of ordinary shares used in computing diluted net loss per ordinary share | 25,546,550 | 25,615,265 | 25,536,100 | 25,593,700 | 25,292,104 | 25,534,542 | ||||||||||||
Non-IFRS measures: | ||||||||||||||||||
Adjusted EBITDA (unaudited)(5) | 5,566,129 | (739,683 | ) | 2,318,947 | (2,897,488 | ) | 6,390,481 | (2,376,297 | ) | |||||||||
| (1) | Consolidated statements of comprehensive income for the three-month period ended September 30, 2016 and the six-month period ended June 30, 2016 do not include the consolidated statements of comprehensive income of Rizobacter, control of which we assumed on October 19, 2016. |
| (2) | Consolidated results of our operations include results of operations of Rizobacter from October 19, 2016 to December 31, 2016 (the period beginning on the date whereupon we assumed control of Rizobacter following its acquisition by us). |
| (3) | Includes (i) exchange differences on translation of foreign operations from joint ventures, (ii) exchange differences on translation of foreign operations, (iii) revaluation of property, plant and equipment, net of tax from joint ventures and (iv) revaluation of property, plant and equipment, net of tax. |
| (4) | See Note 10 to our consolidated financial statements for an explanation of the Stock Split and the method used to calculate the basic and diluted earnings per share. |
| (5) | We define Adjusted EBITDA as profit / (loss) exclusive of financial income / (costs), income tax benefit / (expense), depreciation, amortization, share-based compensation and other items and charges. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Non-IFRS Financial Measures.” |
19
Bioceres | ||||||||||||||||||
Three-month period ended September 30, 2017 (unaudited) | Three-month period ended September 30, 2016 (unaudited) | Transition Period ended June 30, 2017 | Six-month period ended June 30, 2016 (unaudited) | Year ended December 31, | ||||||||||||||
2016 | 2015 | |||||||||||||||||
(US$) | ||||||||||||||||||
Reconciliation of Net Loss to Adjusted EBITDA: | ||||||||||||||||||
Loss for the period / year | (2,929,239 | ) | (1,308,599 | ) | (11,154,603 | ) | (3,764,971 | ) | (5,229,914 | ) | (3,732,442 | ) | ||||||
Income tax (benefit) / expense | (1,357,795 | ) | (621,022 | ) | (5,090,723 | ) | (1,040,923 | ) | (4,140,028 | ) | 411,342 | |||||||
Finance costs | 9,268,419 | 1,117,154 | 14,945,495 | 1,579,951 | 10,923,378 | 1,904,569 | ||||||||||||
Finance income | (2,042,066 | ) | (381,327 | ) | (2,136,265 | ) | (790,814 | ) | (1,006,953 | ) | (1,509,736 | ) | ||||||
Gain on previously held interest | — | — | — | — | (4,453,284 | ) | — | |||||||||||
Depreciation of property, plant and equipment | 771,724 | 146,061 | 1,524,709 | 256,492 | 1,074,733 | 456,444 | ||||||||||||
Amortization of intangible assets | 580,983 | 11,854 | 1,418,661 | 24,065 | 463,066 | 45,233 | ||||||||||||
Inventory purchase price allocation charge | 1,119,155 | — | 2,436,949 | — | 7,516,071 | — | ||||||||||||
Transaction expenses | — | — | — | 252,757 | 599,150 | — | ||||||||||||
Stock-based compensation charges | 154,948 | 296,196 | 374,724 | 585,955 | 644,261 | 48,293 | ||||||||||||
Adjusted EBITDA (unaudited) | 5,566,129 | (739,683 | ) | 2,318,947 | (2,897,488 | ) | 6,390,481 | (2,376,297 | ) | |||||||||
Unaudited pro forma consolidated statement of comprehensive income of Bioceres
The table below illustrates our results of operations for the 2017 periods indicated and the unaudited pro forma results of operations for the 2016 periods indicated. For more information, see “Unaudited Pro Forma Consolidated Financial Information.” The unaudited pro forma nine-month period ended September 30, 2016 is the sum of the unaudited pro forma three-month period ended September 30, 2016 and the unaudited six-month period ended June 30, 2016.
Bioceres | ||||||||||||||||||
Nine-month period ended September 30, | Three-month period ended September 30, | Transition Period ended June 30, 2017 | Six-month period ended June 30, 2016(1) | |||||||||||||||
2017 | 2016(1) | 2017 | 2016(1) | |||||||||||||||
(unaudited) | (unaudited) | (unaudited) | ||||||||||||||||
Total revenue | 83,327,626 | 59,088,148 | 34,986,505 | 27,977,571 | 48,341,121 | 31,110,577 | ||||||||||||
Crop protection | 47,987,918 | 29,597,533 | 16,795,948 | 12,142,498 | 31,191,970 | 17,455,035 | ||||||||||||
Seed and integrated products | 16,519,034 | 10,822,360 | 7,498,035 | 7,080,861 | 9,020,999 | 3,741,499 | ||||||||||||
Crop nutrition | 16,181,111 | 16,759,159 | 9,540,883 | 8,243,062 | 6,640,228 | 8,516,097 | ||||||||||||
Emerging solutions | 2,639,563 | 1,909,096 | 1,151,639 | 511,150 | 1,487,924 | 1,397,946 | ||||||||||||
Cost of sales | (50,381,377 | ) | (29,012,220 | ) | (20,195,931 | ) | (12,727,738 | ) | (30,185,446 | ) | (16,284,482 | ) | ||||||
Crop protection | (32,680,799 | ) | (14,136,216 | ) | (10,038,912 | ) | (5,765,825 | ) | (22,641,887 | ) | (8,370,392 | ) | ||||||
Seed and integrated products | (7,314,203 | ) | (3,162,513 | ) | (2,462,759 | ) | (2,376,759 | ) | (4,851,444 | ) | (785,754 | ) | ||||||
Crop nutrition | (9,594,614 | ) | (10,950,639 | ) | (7,509,962 | ) | (4,321,888 | ) | (2,084,652 | ) | (6,628,751 | ) | ||||||
Emerging solutions | (791,761 | ) | (762,851 | ) | (184,298 | ) | (263,266 | ) | (607,463 | ) | (499,585 | ) | ||||||
Research and development expenses | (5,327,269 | ) | (4,216,430 | ) | (1,725,645 | ) | (1,398,194 | ) | (3,601,624 | ) | (2,818,236 | ) | ||||||
Selling, general and administrative expenses | (27,317,590 | ) | (23,928,021 | ) | (9,993,183 | ) | (8,276,623 | ) | (17,324,407 | ) | (15,651,397 | ) | ||||||
Share of profit / (loss) of joint ventures and associates | (701,948 | ) | (1,027,756 | ) | 84,857 | (360,917 | ) | (786,805 | ) | (666,838 | ) | |||||||
Other income / (expenses), net | (96,219 | ) | 277,225 | (217,284 | ) | (41,834 | ) | 121,065 | 319,059 | |||||||||
Operating income / (loss) | (496,777 | ) | 1,180,947 | 2,939,319 | 5,172,265 | (3,436,096 | ) | (3,991,318 | ) | |||||||||
Finance income | 4,178,331 | 2,572,698 | 2,042,066 | 1,430,434 | 2,136,265 | 1,142,264 | ||||||||||||
Finance costs | (24,213,914 | ) | (23,984,209 | ) | (9,268,419 | ) | (7,769,807 | ) | (14,945,495 | ) | (16,214,402 | |||||||
Loss before income tax | (20,532,360 | ) | (20,230,564 | ) | (4,287,034 | ) | (1,167,108 | ) | (16,245,326 | ) | (19,063,456 | ) | ||||||
Income tax benefit | 6,448,518 | 5,422,960 | 1,357,795 | 357,944 | 5,090,723 | 5,065,016 | ||||||||||||
20
Bioceres | ||||||||||||||||||
Nine-month period ended September 30, | Three-month period ended September 30, | Transition Period ended June 30, 2017 | Six-month period ended June 30, 2016(1) | |||||||||||||||
2017 | 2016(1) | 2017 | 2016(1) | |||||||||||||||
(unaudited) | (unaudited) | (unaudited) | ||||||||||||||||
Loss for the period | (14,083,842 | ) | (14,807,604 | ) | (2,929,239 | ) | (809,164 | ) | (11,154,603 | ) | (13,998,440 | ) | ||||||
Attributable to: Equity holders of the parent | (11,534,333 | ) | (11,709,011 | ) | (3,066,809 | ) | (1,321,302 | ) | (8,467,524 | ) | (10,387,709 | ) | ||||||
Non controlling interest | (2,549,509 | ) | (3,098,593 | ) | 137,570 | 512,138 | (2,687,079 | ) | (3,610,731 | ) | ||||||||
Loss per share | ||||||||||||||||||
Basic loss per ordinary share | (0.4515 | ) | (0.4571 | ) | (0.1200 | ) | (0.0516 | ) | (0.3316 | ) | (0.4059 | ) | ||||||
Diluted loss per ordinary share of the parent | (0.4515 | ) | (0.4571 | ) | (0.1200 | ) | (0.0516 | ) | (0.3316 | ) | (0.4059 | ) | ||||||
Weighted average number of ordinary shares used in computing basic net loss per ordinary share(2) | 25,546,550 | 25,615,265 | 25,546,550 | 25,615,265 | 25,536,100 | 25,593,700 | ||||||||||||
Weighted average number of ordinary shares used in computing diluted net loss per ordinary share(2) | 25,546,550 | 25,615,265 | 25,546,550 | 25,615,265 | 25,536,100 | 25,593,700 | ||||||||||||
Non-IFRS measures | ||||||||||||||||||
Adjusted EBITDA (unaudited)(3) | 7,885,076 | 5,388,883 | 5,566,129 | 6,629,293 | 2,318,947 | (1,240,409 | ) | |||||||||||
| (1) | Unaudited Pro Forma Consolidated statements of comprehensive income for six-month period ended June 30, 2016 and the three-month and nine-month periods ended September 30, 2016 are presented as if the Acquisition had occurred on January 1, 2016. |
| (2) | See Note 10 to our consolidated financial statements for an explanation of the Stock Split and the method used to calculate the basic and diluted earnings per share. |
| (3) | We define Adjusted EBITDA as profit / (loss) exclusive of financial income / (costs), income tax benefit / (expense), depreciation, amortization, share-based compensation and other items and charges. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Non-IFRS Financial Measures.” |
| The table below provides a reconciliation of our pro forma loss for the periods to Adjusted EBITDA: |
Bioceres | ||||||||||||||||||
Nine-month period ended September 30, | Three-month period ended September 30, | Transition Period ended June 30, 2017 | Six-month period ended June 30, 2016 | |||||||||||||||
2017 | 2016 | 2017 | 2016 | |||||||||||||||
(unaudited) | (unaudited) | (unaudited) | ||||||||||||||||
(US$, except share data) | ||||||||||||||||||
Reconciliation of Net Loss to Adjusted EBITDA: | ||||||||||||||||||
Loss for the period | (14,083,842 | ) | (14,807,604 | ) | (2,929,239 | ) | (809,164 | ) | (11,154,603 | ) | (13,998,440 | ) | ||||||
Income tax (benefit) | (6,448,518 | ) | (5,422,960 | ) | (1,357,795 | ) | (357,944 | ) | (5,090,723 | ) | (5,065,016 | ) | ||||||
Finance costs | 24,213,914 | 23,984,209 | 9,268,419 | 7,769,807 | 14,945,495 | 16,214,402 | ||||||||||||
Finance income | (4,178,331 | ) | (2,572,698 | ) | (2,042,066 | ) | (1,430,434 | ) | (2,136,265 | ) | (1,142,264 | ) | ||||||
Depreciation of property, plant and equipment | 2,296,433 | 2,451,028 | 771,724 | 870,470 | 1,524,709 | 1,580,558 | ||||||||||||
Amortization of intangible assets | 1,999,644 | 874,756 | 580,983 | 290,361 | 1,418,661 | 584,395 | ||||||||||||
Inventory purchase price allocation charge | 3,556,104 | — | 1,119,155 | — | 2,436,949 | — | ||||||||||||
Transaction expenses | — | — | — | — | — | — | ||||||||||||
Stock-based compensation charges | 529,672 | 882,151 | 154,948 | 296,196 | 374,724 | 585,955 | ||||||||||||
Adjusted EBITDA (unaudited) | 7,885,076 | 5,388,883 | 5,566,129 | 6,629,293 | 2,318,947 | (1,240,409 | ) | |||||||||||
21
Consolidated statement of financial position data of Bioceres
The summary consolidated statements of financial position data for Bioceres as of September 30, 2017 are derived from our unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus.
Bioceres | |||||||||
As of September 30, 2017 | |||||||||
Actual | Pro Forma(1) | Pro Forma As Adjusted(2) | |||||||
(US$) | |||||||||
Cash and cash equivalents | 1,590,773 | 1,590,773 | |||||||
Working capital(3) | (28,153,131 | ) | 9,536,018 | ||||||
Total assets | 277,530,516 | 277,530,516 | |||||||
Total liabilities | 255,079,328 | 202,144,091 | |||||||
Total equity | 22,451,188 | 75,386,425 | |||||||
| (1) | The pro forma column as of September 30, 2017, reflects the impact of the following changes in the capital structure of Bioceres: |
| (i) | the mandatory conversion of the RASA Holding preferred shares (US$9.8 million are classified as non-controlling interests in the “total equity” line and US$2.8 million are classified as puttable instruments in the “total liabilities” line), |
| (ii) | the mandatory conversion of the Monsanto and BAF convertible loan (US$16.9 million is classified as convertible borrowings in current liabilities in the “working capital” line and the “total liabilities” line), and |
| (iii) | the voluntary conversion of the BAF loans into ordinary shares of Bioceres (US$33.2 million is classified as financed payment - acquisition of business in non-current liabilities in the “total liabilities” line). |
The instruments listed above are reflected in the unaudited pro forma column as converted into ordinary shares of Bioceres as if Bioceres had completed this offering on September 30, 2017. The pro forma column does not assume any proceeds from this offering.
| (2) | As further adjusted for this offering. |
| (3) | Working capital is defined as total current assets minus total current liabilities. |
Consolidated Statement of Cash Flows of Bioceres
The summary consolidated statements of cash flows for Bioceres for the Transition Period, for the unaudited six-month period ended June 30, 2016 and for the years ended December 31, 2016 and 2015 are derived from our audited consolidated financial statements appearing elsewhere in this prospectus.
Bioceres | ||||||||||||||||||
Three-month period ended September 30, | For the Transition Period ended June 30, 2017 | Six-month period ended June 30, 2016 | December 31, 2016 | December 31, 2015 | ||||||||||||||
2017 | 2016 | |||||||||||||||||
(unaudited) | (unaudited) | |||||||||||||||||
(US$) | ||||||||||||||||||
Net cash flows used in operating activities | (2,567,966 | ) | (1,801,001 | ) | (7,096,431 | ) | (7,346,974 | ) | (8,443,596 | ) | (3,044,790 | ) | ||||||
Net cash flows used in investing activities | (2,454,078 | ) | (808,875 | ) | (2,931,751 | ) | (4,050,699 | ) | (47,020,853 | ) | (2,873,523 | ) | ||||||
Net cash flows from financing activities | 4,492,934 | (944,050 | ) | 10,891,369 | 13,098,967 | 56,662,720 | 3,727,039 | |||||||||||
Net increase (decrease) in cash and cash equivalents | (529,110 | ) | (1,665,826 | ) | 863,187 | 1,701,294 | 1,198,271 | (2,191,274 | ) | |||||||||
22
Summary of Consolidated Historical Financial Information of Rizobacter
Consolidated statement of comprehensive income of Rizobacter
The consolidated statements of comprehensive income for the years ended June 30, 2017, 2016 and 2015 are derived from Rizobacter’s audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statements of comprehensive income for the three-month periods ended September 30, 2017 and 2016 are derived from Rizobacter’s unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus. However, this information: (i) has been prepared on a stand-alone basis; (ii) does not reflect the purchase accounting adjustments made at the consolidated level after the acquisition of Rizobacter; (iii) does not include any corporate and other expenses that should be allocated to Rizobacter; (iv) uses different segments than the Bioceres consolidated financial statements; (v) is provided for information purposes only and (vi) should not be viewed as a substitute for the consolidated financial statements of Bioceres subsequent to the date of the acquisition of Rizobacter. The consolidated statements of comprehensive income of Rizobacter for the year ended June 30, 2017 and for the three-month periods ended September 30, 2017 and 2016 are provided as additional information to permit readers to compare the more recent results of Rizobacter on a stand-alone basis.
Rizobacter | |||||||||||||||
Three-month period ended September 30, | Year ended June 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | 2015 | |||||||||||
(unaudited) | |||||||||||||||
(US$) | |||||||||||||||
Revenue | 32,852,813 | 26,153,338 | 112,296,212 | 93,405,678 | 99,163,146 | ||||||||||
Inoculants | 6,306,921 | 4,993,062 | 12,482,786 | 12,759,410 | 16,663,499 | ||||||||||
Seed therapics | 2,409,728 | 2,887,265 | 21,450,510 | 13,023,454 | 10,701,871 | ||||||||||
Adjuvants | 7,993,798 | 6,764,431 | 35,653,018 | 29,869,904 | 34,593,977 | ||||||||||
Packs | 6,515,983 | 5,767,778 | 23,111,291 | 20,843,223 | 24,497,906 | ||||||||||
Fertilizers and others | 9,626,383 | 5,740,802 | 19,598,607 | 16,909,687 | 12,705,893 | ||||||||||
Cost of sales | (18,060,896 | ) | (11,608,790 | ) | (58,838,471 | ) | (49,073,466 | ) | (47,357,049 | ) | |||||
Inoculants | (4,436,796 | ) | (1,730,079 | ) | (3,915,337 | ) | (7,936,177 | ) | (9,580,869 | ) | |||||
Seed therapics | (1,224,196 | ) | (1,482,593 | ) | (14,834,044 | ) | (8,949,443 | ) | (7,326,369 | ) | |||||
Adjuvants | (3,015,946 | ) | (2,863,643 | ) | (15,218,434 | ) | (10,678,992 | ) | (13,593,551 | ) | |||||
Packs | (1,554,859 | ) | (1,521,077 | ) | (6,789,019 | ) | (6,836,804 | ) | (6,621,439 | ) | |||||
Fertilizers and others | (7,829,099 | ) | (4,011,398 | ) | (18,081,636 | ) | (14,672,050 | ) | (10,234,821 | ) | |||||
Gross income | 14,791,917 | 14,544,548 | 53,457,741 | 44,332,212 | 51,806,097 | ||||||||||
Administrative expenses | (2,371,453 | ) | (2,125,642 | ) | (9,765,385 | ) | (8,363,830 | ) | (7,677,468 | ) | |||||
Distribution expenses | (5,262,815 | ) | (4,668,990 | ) | (19,502,749 | ) | (18,824,875 | ) | (20,102,818 | ) | |||||
Research expenses | (579,634 | ) | (533,601 | ) | (2,423,428 | ) | (2,254,885 | ) | (655,941 | ) | |||||
Other operating income and expenses, net | (175,320 | ) | (23,699 | ) | 49,654 | 446,739 | 158,731 | ||||||||
Operating income | 6,402,695 | 7,192,616 | 21,815,833 | 15,335,361 | 23,528,601 | ||||||||||
Financial income | 1,198,552 | 1,000,264 | 2,080,710 | 4,633,611 | 2,032,019 | ||||||||||
Financial costs | (5,719,583 | ) | (5,028,406 | ) | (19,450,815 | ) | (27,025,501 | ) | (15,944,826 | ) | |||||
Share of profit / (loss) of joint ventures and associates(1) | 297,939 | (232,404 | ) | (1,109,131 | ) | (848,948 | ) | (584,728 | ) | ||||||
Profit / (loss) before income tax | 2,179,603 | 2,932,070 | 3,336,596 | (7,905,477 | ) | 9,031,066 | |||||||||
Income tax benefit / (expense) | (489,761 | ) | (1,052,834 | ) | (1,789,654 | ) | 2,443,866 | (3,198,198 | ) | ||||||
Profit / (loss) for the year | 1,689,842 | 1,879,236 | 1,546,942 | (5,461,611 | ) | 5,832,868 | |||||||||
Other comprehensive income | (893,658 | ) | (365,644 | ) | 391,155 | (1,733,184 | ) | 7,976,923 | |||||||
Total comprehensive profit / (loss) | 796,184 | 1,513,593 | 1,938,097 | (7,194,795 | ) | 13,809,791 | |||||||||
Non-IFRS measures: | |||||||||||||||
Rizobacter Adjusted EBITDA (unaudited)(2) | 7,324,977 | 7,402,051 | 23,301,133 | 16,303,882 | 23,896,825 | ||||||||||
| (1) | Accounted for using the equity method. |
23
| (2) | We define Rizobacter Adjusted EBITDA as profit/(loss) exclusive of financial income/(costs), income tax benefit/(expense), depreciation, amortization, share-based compensation and other items and charges. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Non-IFRS Financial Measures.” |
The table below provides a reconciliation of Rizobacter’s net profit/(loss) for the year to Rizobacter Adjusted EBITDA:
Rizobacter | |||||||||||||||
Three-month period ended September 30, | Year ended June 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | 2015 | |||||||||||
(unaudited) | |||||||||||||||
(US$) | |||||||||||||||
Profit / (loss) for the year | 1,689,842 | 1,879,236 | 1,546,942 | (5,461,611 | ) | 5,832,868 | |||||||||
Income tax (benefit)/expense | 489,761 | 1,052,834 | 1,789,654 | (2,443,866 | ) | 3,198,198 | |||||||||
Finance costs | 5,719,583 | 5,028,406 | 19,450,815 | 27,025,501 | 15,944,826 | ||||||||||
Finance income | (1,198,551 | ) | (1,000,264 | ) | (2,080,709 | ) | (4,633,611 | ) | (2,032,019 | ) | |||||
Depreciation of property, plant and equipment | 469,397 | 431,272 | 2,038,380 | 1,761,478 | 920,271 | ||||||||||
Amortization of intangible assets | 154,945 | 10,567 | 556,051 | 55,991 | 32,681 | ||||||||||
Rizobacter Adjusted EBITDA (unaudited) | 7,324,977 | 7,402,051 | 23,301,133 | 16,303,882 | 23,896,825 | ||||||||||
For information regarding the comparability of the consolidated statement of comprehensive income of Rizobacter, see “Selected Consolidated Historical Financial and Other Information.”
Consolidated statement of financial position of Rizobacter
The summary consolidated statement of financial position data of Rizobacter as of September 30, 2017 is derived from Rizobacter’s unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus. However, this information: (i) has been prepared on a stand-alone basis; (ii) does not reflect the purchase accounting adjustments made at the consolidated level after the acquisition of Rizobacter; (iii) does not include any corporate and other expenses that should be allocated to Rizobacter; (iv) uses different segments than the Bioceres consolidated financial statements; (v) is provided for information purposes only and (vi) should not be viewed as a substitute for the consolidated financial statements of Bioceres subsequent to the date of the acquisition of Rizobacter. The summary consolidated statement of financial position of Rizobacter as of September 30, 2017 is provided as additional information to permit readers to compare the more recent results of Rizobacter on a stand-alone basis.
Rizobacter | |||
As of September 30, 2017 | |||
(US$) | |||
Cash and cash equivalents | 1,175,691 | ||
Working capital(1) | 21,512,143 | ||
Total assets | 151,232,478 | ||
Total liabilities | 127,465,411 | ||
Total equity | 23,767,067 | ||
| (1) | Working capital is defined as total current assets minus total current liabilities. |
For information regarding the comparability of the consolidated statement of financial position data of Rizobacter, see “Selected Consolidated Historical Financial and Other Information.”
24
Unaudited Pro Forma Consolidated Financial Information of Bioceres
The unaudited pro forma consolidated financial information has been derived by the application of pro forma adjustments to our historical consolidated financial information, which have been presented to give effect to the acquisition of Rizobacter, or the Acquisition, and certain other adjustments including the accounting effects of purchase price accounting and certain non-recurring transaction charges. The unaudited pro forma consolidated statements of income of Bioceres for the year ended December 31, 2016, for the six-month period ended June 30, 2016 and for the three-month and nine-month periods ended September 30, 2016 are presented as if the Acquisition had occurred on January 1, 2016.
You should read the information contained in this section in conjunction with “Unaudited Pro Forma Financial Information,” “Capitalization,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” our historical audited consolidated financial statements and the accompanying notes included elsewhere in this prospectus.
Unaudited Pro Forma Consolidated Statement of Income for the Twelve-Month Period Ended December 31, 2016:
Unaudited Pro Forma Consolidated Statement of Income for the Twelve-Month Period Ended December 31, 2016 | |||||||||||||||||
Historical Bioceres(1) | Historical Rizobacter(2) | Pro Forma Adjustments(3) | Pro Forma Combined(4) | ||||||||||||||
(all amounts expressed in US$, unless otherwise indicated) | |||||||||||||||||
Revenue | 43,587,834 | 60,070,278 | (360,968 | ) | 103,297,144 | ||||||||||||
Government grants | 761,429 | — | — | 761,429 | |||||||||||||
Total revenue | 44,349,263 | 60,070,278 | (360,968 | ) | 104,058,573 | ||||||||||||
Cost of sales | (31,600,998 | ) | (29,386,577 | ) | 7,518,256 | (53,469,319 | ) | ||||||||||
Research and development expenses | (2,860,771 | ) | (1,720,738 | ) | (893,891 | ) | (5,475,400 | ) | |||||||||
Selling, general and administrative expenses | (12,906,021 | ) | (20,520,583 | ) | (321,644 | ) | (33,748,248 | ) | |||||||||
Share of profit / (loss) of joint ventures and associates | (936,769 | ) | (730,365 | ) | 389,358 | (1,277,776 | ) | ||||||||||
Other income | 48,495 | 287,657 | — | 336,152 | |||||||||||||
Operating income / (loss) | (3,906,801 | ) | 7,999,672 | 6,331,111 | 10,423,982 | ||||||||||||
Gain on previously held interest | 4,453,284 | — | (4,453,284 | ) | — | ||||||||||||
Finance income | 1,006,953 | 1,433,352 | (102,369 | ) | 2,337,937 | ||||||||||||
Finance costs | (10,923,378 | ) | (17,293,817 | ) | (4,626,933 | ) | (32,844,128 | ) | |||||||||
Loss before income tax | (9,369,942 | ) | (7,860,792 | ) | (2,851,475 | ) | (20,082,209 | ) | |||||||||
Income tax benefit | 4,140,028 | 1,395,632 | (491,161 | ) | 5,044,499 | ||||||||||||
Loss for the year | (5,229,914 | ) | (6,465,160 | ) | (3,342,636 | ) | (15,037,710 | ) | |||||||||
Attributable to: | |||||||||||||||||
Equity holders of the parent | (4,508,061 | ) | (3,879,096 | ) | (4,828,174 | ) | (13,215,332 | ) | |||||||||
Non-controlling interest | (721,853 | ) | (2,586,064 | ) | 1,485,538 | (1,822,379 | ) | ||||||||||
Weighted-average number of shares outstanding | |||||||||||||||||
Basic | 25,592,104 | — | — | 25,592,104 | |||||||||||||
Diluted | 25,592,104 | — | — | 25,592,104 | |||||||||||||
Basic earnings per share attributable to equity holders of the parent | (0.18 | ) | — | — | (0.52 | ) | |||||||||||
Diluted earnings per share attributable to equity holders of the parent | (0.18 | ) | — | — | (0.52 | ) | |||||||||||
| (1) | Represents the historical consolidated statement of income of Bioceres for the twelve-month period ended December 31, 2016 and includes (i) Rizobacter’s results since its acquisition on October 19, 2016 and (ii) PPA-related accounting effects resulting from the revaluation of Rizobacter’s acquired inventories, property, plant and equipment and certain intangible assets, as well as certain consolidation-related eliminations. |
| (2) | Represents the historical statement of income of Rizobacter for the period from January 1, 2016 to October 18, 2016. |
25
| (3) | Represents the pro forma adjustments to reflect (i) the Rizobacter acquisition and (ii) the Semya consolidation and related eliminations for transactions occurred for the period from January 1, 2016 to October 18, 2016 between Rizobacter or Bioceres and Semya. |
| (4) | Represents the unaudited pro forma consolidated statement of income for the acquisition of Rizobacter under IFRS and reflects all adjustments described in note (3) above. See also the “Unaudited Pro Forma Consolidated Financial Information.” |
The table below provides a reconciliation of our loss for the year to Adjusted EBITDA included elsewhere in this prospectus:
Pro Forma Consolidated Statement of Income for the Twelve-Month Period Ended December 31, 2016 | |||
(unaudited) | |||
Reconciliation of Net Loss to Adjusted EBITDA: | |||
Loss for the period / year | (15,037,710 | ) | |
Income tax (benefit) / expense | (5,044,499 | ) | |
Finance costs | 32,844,128 | ||
Finance income | (2,337,937 | ) | |
Gain on previously held interest | — | ||
Depreciation of property, plant and equipment | 2,744,427 | ||
Amortization of intangible assets | 1,993,093 | ||
Inventory purchase price allocation charge | — | ||
Transaction expenses | — | ||
Stock-based compensation charges | 374,724 | ||
Adjusted EBITDA (unaudited) | 15,536,226 | ||
Unaudited Pro Forma Consolidated Statement of Income for the Six-Month Period Ended June 30, 2016:
Unaudited Pro Forma Consolidated Statement of Income for the Six-Month Period Ended June 30, 2016 | ||||||||||||
Historical Bioceres(1) | Historical Rizobacter(2) | Pro Forma Adjustments(3) | Pro Forma Combined(4) | |||||||||
(all amounts expressed in US$, unless otherwise indicated) | ||||||||||||
Revenue | 2,268,193 | 28,793,866 | (221,688 | ) | 30,840,371 | |||||||
Government grants | 270,206 | — | — | 270,206 | ||||||||
Total revenue | 2,538,399 | 28,793,866 | (221,688 | ) | 31,110,577 | |||||||
Cost of sales | (1,290,256 | ) | (14,994,226 | ) | — | (16,284,482 | ) | |||||
Research and development expenses | (1,268,661 | ) | (1,013,694 | ) | (535,881 | ) | (2,818,236 | ) | ||||
Selling, general and administrative expenses | (3,541,058 | ) | (11,517,410 | ) | (592,929 | ) | (15,651,397 | ) | ||||
Share of profit / (loss) of joint ventures and associates | (455,181 | ) | (491,429 | ) | 279,772 | (666,838 | ) | |||||
Other income | — | 319,059 | — | 319,059 | ||||||||
Operating income / (loss) | (4,016,757 | ) | 1,096,166 | (1,070,727 | ) | (3,991,318 | ) | |||||
Finance income | 790,814 | 404,975 | (53,525 | ) | 1,142,264 | |||||||
Finance costs | (1,579,951 | ) | (11,453,062 | ) | (3,181,389 | ) | (16,214,402 | ) | ||||
Loss before income tax | (4,805,894 | ) | (9,951,921 | ) | (4,305,641 | ) | (19,063,456 | ) | ||||
Income tax benefit | 1,040,923 | 2,478,057 | 1,546,036 | 5,065,016 | ||||||||
Loss for the period | (3,764,971 | ) | (7,473,864 | ) | (2,759,604 | ) | (13,998,440 | ) | ||||
Attributable to: | ||||||||||||
Equity holders of the parent | (3,435,546 | ) | (4,484,319 | ) | (2,467,844 | ) | (10,387,709 | ) | ||||
Non-controlling interest | (329,425 | ) | (2,989,546 | ) | (291,760 | ) | (3,610,731 | ) | ||||
26
Unaudited Pro Forma Consolidated Statement of Income for the Six-Month Period Ended June 30, 2016 | ||||||||||||
Historical Bioceres(1) | Historical Rizobacter(2) | Pro Forma Adjustments(3) | Pro Forma Combined(4) | |||||||||
(all amounts expressed in US$, unless otherwise indicated) | ||||||||||||
Weighted-average number of shares outstanding | ||||||||||||
Basic | 25,593,700 | — | — | 25,593,700 | ||||||||
Diluted | 25,593,700 | — | — | 25,593,700 | ||||||||
Basic earnings per share attributable to equity holders of the parent | (0.13 | ) | — | — | (0.41 | ) | ||||||
Diluted earnings per share attributable to equity holders of the parent | (0.13 | ) | — | — | (0.41 | ) | ||||||
| (1) | Represents the historical consolidated statement of income of Bioceres for the six-month period ended June 30, 2016. |
| (2) | Represents the historical statement of income of Rizobacter for the six-month period ended June 30, 2016. |
| (3) | Represents the pro forma adjustments to reflect (i) the effect of the Stock Split approved by our shareholders as if it had been completed as of January 1, 2016, (ii) the Acquisition and (iii) the Semya consolidation and related eliminations for transactions occurred for the period from January 1, 2016 to June 30, 2016 between Rizobacter or Bioceres and Semya. |
| (4) | Represents the unaudited pro forma consolidated statement of income for the Acquisition under IFRS and reflects all adjustments described in note (3) above. See also the “Unaudited Pro Forma Consolidated Financial Information.” |
The table below provides a reconciliation of our pro forma loss for the period to Adjusted EBITDA:
Pro Forma Consolidated Statement of Income for the Six-Month Period Ended June 30, 2016 | |||
(unaudited) | |||
Reconciliation of Net Loss to Adjusted EBITDA: | |||
Loss for the period / year | (13,998,440 | ) | |
Income tax (benefit) / expense | (5,065,016 | ) | |
Finance costs | 16,214,402 | ||
Finance income | (1,142,264 | ) | |
Gain on previously held interest | — | ||
Depreciation of property, plant and equipment | 1,580,558 | ||
Amortization of intangible assets | 584,395 | ||
Inventory purchase price allocation charge | — | ||
Transaction expenses | — | ||
Stock-based compensation charges | 585,955 | ||
Adjusted EBITDA (unaudited) | (1,240,409 | ) | |
27
Unaudited Pro Forma Consolidated Statement of Income for the Three-Month Period Ended September 30, 2016:
Unaudited Pro Forma Consolidated Statement of Income for the Three-Month Period Ended September 30, 2016 | ||||||||||||
Historical Bioceres(1) | Historical Rizobacter(2) | Pro Forma Adjustments(3) | Pro Forma Combined(4) | |||||||||
(all amounts expressed in US$, unless otherwise indicated) | ||||||||||||
Revenue | 1,846,295 | 26,153,338 | (139,280 | ) | 27,860,353 | |||||||
Government grants | 117,218 | — | — | 117,218 | ||||||||
Total revenue | 1,963,513 | 26,153,338 | (139,280 | ) | 27,977,571 | |||||||
Cost of sales | (1,118,948 | ) | (11,608,790 | ) | — | (12,727,738 | ) | |||||
Research and development expenses | (596,652 | ) | (533,601 | ) | (267,940 | ) | (1,398,194 | ) | ||||
Selling, general and administrative expenses | (1,185,472 | ) | (6,794,631 | ) | (296,520 | ) | (8,276,623 | ) | ||||
Share of profit / (loss) of joint ventures and associates | (238,100 | ) | (232,404 | ) | 109,587 | (360,917 | ) | |||||
Other expenses, net | (18,135 | ) | (23,699 | ) | — | (41,834 | ) | |||||
Operating income / (loss) | (1,193,794 | ) | 6,960,213 | (594,154 | ) | 5,172,265 | ||||||
Finance income | 381,327 | 1,000,264 | 48,844 | 1,430,434 | ||||||||
Finance costs | (1,117,154 | ) | (5,028,406 | ) | (1,624,247 | ) | (7,769,807 | ) | ||||
Income / (loss) before income tax | (1,929,621 | ) | 2,932,071 | (2,169,558 | ) | (1,167,108 | ) | |||||
Income tax benefit / (expense) | 621,022 | (1,052,834 | ) | 789,755 | 357,944 | |||||||
Profit / (loss) for the period | (1,308,599 | ) | 1,879,237 | (1,379,802 | ) | (809,164 | ) | |||||
Attributable to: | ||||||||||||
Equity holders of the parent | (1,214,922 | ) | 1,127,542 | (1,233,922 | ) | (1,321,302 | ) | |||||
Non-controlling interest | (93,677 | ) | 751,695 | (145,880 | ) | 512,138 | ||||||
Weighted-average number of shares outstanding | ||||||||||||
Basic | 25,615,265 | — | — | 25,615,265 | ||||||||
Diluted | 25,615,265 | — | — | 25,615,265 | ||||||||
Basic earnings per share attributable to equity holders of the parent | (0.05 | ) | — | — | (0.05 | ) | ||||||
Diluted earnings per share attributable to equity holders of the parent | (0.05 | ) | — | — | (0.05 | ) | ||||||
| (1) | Represents the historical consolidated statement of income of Bioceres for the three-month period ended September 30, 2016. |
| (2) | Represents the historical statement of income of Rizobacter for the three-month period ended September 30, 2016. |
| (3) | Represents the pro forma adjustments to reflect (i) the effect of the Stock Split approved by our shareholders as if it had been completed as of January 1, 2016, (ii) the Acquisition and (iii) the Semya consolidation and related eliminations for transactions occurred for the period from January 1, 2016 to September 30, 2016 between Rizobacter or Bioceres and Semya. |
| (4) | Represents the unaudited pro forma consolidated statement of income for the Acquisition under IFRS and reflects all adjustments described in note (3) above. See also the “Unaudited Pro Forma Consolidated Financial Information.” |
| The table below provides a reconciliation of our pro forma loss for the period to Adjusted EBITDA: |
Pro Forma Consolidated Statement of Income for the Three-Month Period Ended September 30, 2016 | |||
(unaudited) | |||
Reconciliation of Net Loss to Adjusted EBITDA: | |||
Loss for the period / year | (809,164 | ) | |
Income tax (benefit) / expense | (357,944 | ) | |
Finance costs | 7,769,807 | ||
Finance income | (1,430,434 | ) | |
Gain on previously held interest | — | ||
Depreciation of property, plant and equipment | 870,470 | ||
Amortization of intangible assets | 290,361 | ||
Inventory purchase price allocation charge | — | ||
Transaction expenses | — | ||
Stock-based compensation charges | 296,196 | ||
Adjusted EBITDA (unaudited) | 6,629,293 | ||
28
Investing in the ADSs involves a high degree of risk. You should consider carefully the following risks, together with all the other information in this prospectus, including our financial statements and notes thereto before you invest in our common stock. If any of the following risks actually materializes our operating results financial condition and liquidity could be materially adversely affected. As a result, the trading price of our common stock could decline and you could lose all or part of your investment.
Risks Related to Our Business and Industry
We may not be successful in developing marketable or commercial technologies.
Our success depends in part on our ability to identify and develop high-value crop productivity and agro-industrial technologies for use in commercial products. Through our technology sourcing and product development collaborations we commit substantial efforts and other resources to accomplish this. It may take several years, if at all, before many of our products complete the development process and become available for production and commercialization.
As of the date of this prospectus, many of our products have been commercialized by Rizobacter, including Rizoderma, crop protection products in the Maxim line and a variety of adjuvants and packs. There can be no assurance that our future crop productivity and agro-industrial technologies will be viable for commercial use, or that we will be able to generate revenues from those technologies, in a significant manner or at all. If seeds or other products that contain our seed traits or technology are unsuccessful in achieving their desired effect or otherwise fail to be commercialized, we will not receive revenues from our customers or royalty payments from the commercialization of the seed traits and technologies we develop, which could materially and adversely affect our business, financial condition, results of operations and growth strategy.
Seeds containing the seed traits or biological treatments that we develop may be unsuccessful or fail to achieve commercialization for any of the following reasons:
| ■ | our seed traits or biological treatments may not be successfully validated in the target crops; |
| ■ | our seed traits or biological treatments may not have the desired effect on the relevant crop sought by our end-market; |
| ■ | we or our joint ventures or collaborators may be unable to obtain the requisite regulatory approvals for the seeds containing our seed traits or for our biological treatments; |
| ■ | our competitors may launch competing or more effective seed traits, biological treatments or germplasms; |
| ■ | a market may not exist for seeds containing our seed traits or biological treatments or such products may not be commercially successful; |
| ■ | we may be unable to patent and/or obtain breeders’ rights or any other intellectual property rights on our traits and technologies in the necessary jurisdictions; |
| ■ | even if we obtain patent and/or breeders’ rights or any other intellectual property rights on our seed traits, such rights may be later challenged by competitors or other parties; and |
| ■ | even if we obtain patent and/or breeders’ rights or any other intellectual property rights on our seed traits, competitors may design competing products that do not infringe these intellectual property rights. |
Industrial enzymes, bacterial fermentation solutions and other agro-industrial biotech solutions that we develop may be unsuccessful or fail to achieve commercialization for any of the following reasons:
| ■ | we may not be able to effectively express or purify enzymes of interest in safflower grains; |
| ■ | even if we are able to express and purify enzymes of interest effectively using our safflower molecular farming technologies, these enzymes may not be effective for the industrial processes for which they were intended; |
29
| ■ | we or our collaborators may be unable to obtain the requisite regulatory approvals for safflower crop production, enzymes or by-products required or resulting from our molecular farming technologies or from the use of genetically engineered bacteria in the production of biofuels, biopolymers or higher value molecules; |
| ■ | we may not be able to effectively convert glycerin or other fermentable forms of carbon into biofuels, biopolymers or higher value molecules, such as ectoine, using our bacterial fermentation solutions; |
| ■ | we may not be able to effectively purify biopolymers and higher value molecules produced using our bacterial fermentation solutions; |
| ■ | our competitors may launch competing or more effective enzymes or agro-industrial biotech treatments or germplasms; |
| ■ | a market may not exist for our enzymes, safflower by-products, or agro-industrial biotech solutions or such enzymes and solutions may not be commercially successful; |
| ■ | we may be unable to seek and obtain adequate intellectual property protection for our molecular farming or agro-industrial biotech solutions in the necessary jurisdictions; |
| ■ | even if we obtain patent or other intellectual property protection for our molecular farming or agro-industrial biotech solutions, such protection may later be challenged by competitors or other parties; and |
| ■ | even if we obtain patent or other intellectual property protection for our molecular farming or agro-industrial biotech solutions, competitors may design competing solutions that do not infringe our intellectual property rights. |
Our business and the commercialization of our products currently in development are subject to various government regulations and we or our collaborators may be unable to obtain, or may face delays in obtaining, necessary regulatory approvals.
Our business is generally subject to two types of regulations: (i) those that apply to our operations and (ii) those that apply to products containing or based on our technology. We are responsible for applying for and maintaining the regulatory approvals necessary for our operations, particularly those covering our field trials, bio-safety evaluations and feed and food tests. Under the terms of our joint venture agreements, we and our joint venture partners are jointly responsible for obtaining and maintaining the regulatory approvals necessary for the commercialization of products that contain our seed traits and other technologies in the various relevant markets. As an operational matter, we generally lead these processes in Argentina through our subsidiary, INDEAR, and our international subsidiaries or our collaborators lead these efforts in the United States, China, Brazil, Paraguay, Uruguay and other international markets. In the future, we expect to seek regulatory approvals in other markets. Regulatory and legislative requirements affect the development, production and sale of our products, including the testing, commercializing and planting of seeds containing our biotechnology seed traits. Failure to receive such approvals or non-compliance with the applicable regulatory regime could adversely impact our operations and business strategy. Additionally, we may face difficulties in obtaining regulatory approvals in jurisdictions in which we have not previously operated or in which we have limited experience.
In most of our key target markets, including the United States, regulatory approvals must be received prior to the importation and commercialization of transgenic products. Regulatory regimes in some of our key target markets may be more onerous. For example, in Argentina, the federal government’s regulation of agricultural biotechnology is handled primarily by two agencies, the National Advisory Commission on Agricultural Biotechnology (Comisión Nacional Asesora de Biotecnología Agropecuaria), or CONABIA, which regulates activity related to biosafety, and the National Food Safety and Quality Service (Servicio Nacional de Sanidad y Calidad Agroalimentaria), or SENASA, which regulates activity related to food and feed safety. Additionally, the National Market Regulator (Dirección Nacional de Mercados) must conduct an economic evaluation. When products containing our seed traits or other technology reach large-scale field trials, bio-safety evaluations and commercial approval stages, if we, our joint ventures or other collaborators are unable to obtain the requisite regulatory approvals or if there is a delay in obtaining such approvals as a result of negative market perception, heightened regulatory standards or unfamiliarity with the applicable regulatory regime, such products will not be commercialized, which would negatively impact our business and results of operations.
30
Our EcoSeed business is dependent in large part on the success of a technology that we license and that remains subject to receipt of regulatory approval.
The majority of our biotechnology seed products currently under development incorporates HB4 technology. We expect that the sale of biotech seeds that contain HB4 technology, our EcoSeed business, will comprise an increasing and significant portion of our future revenues. As a result, our future growth and financial performance will largely depend on our ability to receive regulatory approval for and to commercialize our HB4 technology, and if this effort is unsuccessful we may not have the resources to pursue development of our other products and our business could be materially and adversely affected. We also depend on our continued exclusive use of the HB4 technology pursuant to the terms of licensing agreements with CONICET and the National University of the Litoral. We hold an exclusive license for further developments of HB4, which terminates on the expiration date of the last of the HB4 patents in 2033, unless terminated before such date in accordance with its terms. If this licensing agreement is declared unenforceable or invalid, we could lose access to one of our principal technologies and could become involved in a costly or time-consuming legal dispute.
We are party to funding agreements pursuant to which certain investors have a right to the majority of the payments we may receive in connection with the commercialization of our technologies in certain crops.
Between 2005 and 2007, we entered into agreements with various investors to obtain funding in the aggregate amount of US$1.0 million for research and early stage development of technology relating to a specific sunflower gene, Hahb 4, that is intended to promote drought tolerance in crops. The funding agreements grant the investors, in the aggregate, the right to receive 52.8% of the rights and royalties payable to us from the successful commercialization of the resulting technology with respect to soybean, wheat and corn. As of the date hereof, the promoter element of the technology developed in connection with our research and development of Hahb 4 is being incorporated into a leading soybean product that Verdeca is developing, which also incorporates our HB4 technology. In addition, the licenses of our HB4 technology that we have granted to other developers and our joint ventures with respect to certain crops include the Hahb 4 promoter element. Accordingly, we may have to pay third parties royalties otherwise due to us in the absence of these agreements and we may not receive the full economic benefit of the commercialization of certain of our technologies. In addition, the investors party to these funding arrangements may claim to be entitled to payments in addition to the royalties, which we believe are within the scope of such agreements. The investors may also dispute the allocation of revenue as it relates to the relative importance of our various technologies incorporated into a given product. We cannot be certain how a court would interpret any ambiguities regarding the scope of these funding agreements or other claims that may be raised by one or more investors pursuant to these funding agreements. Any dispute regarding these agreements could be costly and divert management’s attention from our operations, and if the investors are deemed to have rights to payments in excess of those we believe are applicable, our business, results of operations, cash flows and prospects would be materially and adversely affected. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Tabular Disclosure of Contractual Obligations,” “Business—Early Stage Technology Agreements” and “Certain Relationships and Related Party Transactions—Early Stage Technology Development Agreements.”
There are a limited number of prospective collaborators in the markets in which we operate.
Our R&D and commercialization activities are costly, time-intensive and require significant infrastructure and resources. Therefore, our business strategy involves entering into joint venture arrangements with global agricultural firms to leverage their resources, know-how and channels of distribution and into collaborations with research institutions and governmental agencies to facilitate our low-cost approach to R&D. The crop productivity and agro-industrial markets are highly consolidated and dominated by a relatively small number of large companies. Additionally, there are a limited number of researchers and research institutions focused on the technologies that we seek to develop and competition for entry into collaboration arrangements with them can be challenging. Due to the small number of companies in our markets and the small number of potential collaborators, there are limited opportunities for us to pursue additional joint ventures and collaborations with new partners and collaborators. We may cease to be attractive to prospective collaborators if our technology platform or track record is not perceived to be sufficiently developed or successful or if, in the case of prospective joint venture partners, such prospective partners view us as a competitor and choose not to collaborate with us. In addition, if we fail to develop or maintain our relationships with any of our existing collaborators, we could lose our opportunity to work with that collaborator and suffer a reputational risk that could impact our relationships with other collaborators in what is a relatively small industry community. If we are unable to enter into new
31
joint venture agreements or collaborations, we may face higher development costs than anticipated, greater difficulties in achieving commercialization, challenges in expanding our portfolio of technologies and distribution networks and commercial products, or other adverse impacts, which could have a material adverse effect on our business prospects.
The licenses that we grant to certain of the joint ventures in which we participate and to certain third parties are exclusive with respect to certain territories and/or crops, limiting our ability to use the licensed technology and future technologies either independently or with another partner.
The license we have agreed to grant to Verdeca would be exclusive with respect to HB4 soybean technology worldwide and, although we would be permitted to use this technology, we would be prohibited from licensing it to third parties. The license we granted to Trigall Genetics is exclusive with respect to HB4 wheat technology in Argentina, Brazil, Paraguay and Uruguay. We granted to S&W Semillas, our joint venture for production and commercialization of alfalfa HB4, an exclusive license to use our HB4 technology in Argentina, Brazil, Chile and Uruguay. Pursuant to the terms of the licenses in each of the above-mentioned joint ventures, we reserve the rights to use such technologies for research and non-commercial purposes. Each of the above-mentioned joint ventures grants us first negotiation rights for new technologies sourced after the formation of the joint ventures, within their respective field and territory. We are prohibited from independently using the technology we licensed to Trigall Genetics, Verdeca and S&W Semillas with respect to wheat, soybean and alfalfa, respectively, within their exclusive field and territories. As a result, we are, to a certain extent, dependent on the efforts of our joint ventures and licensees that hold or will hold exclusive licenses to commercialize our technologies in those fields and territories. These licenses are valid so long as the respective joint venture operates and can be recuperated by us upon joint venture dissolution. The restrictions imposed by these exclusive licenses limit our flexibility to commercialize our technology and expand our business, both of which could adversely affect our business, results of operations and prospects.
We granted an exclusive license to the Centro Integral de Biotecnología Aplicada (CIBA) from the San Pablo University of Tucumán to use our HB4 technology in the Argentine sugarcane industry. Although we would be permitted to use this technology for research and non-commercial purposes, we would be prohibited from licensing it to other third parties in the Argentine sugarcane industry.
Our product development cycle is lengthy and uncertain and we may never generate revenues or earn royalties on the sale of our products currently in development.
R&D in the crop productivity and agro-industrial biotech industries is expensive, complex, prolonged and uncertain. We may spend many years and dedicate significant financial and other resources developing products that may never generate revenues or come to market. Our process of developing and commercializing technologies involves several phases and can take several years from discovery to commercialization of a product. On average, it takes between five and 13 years to develop a product for our crop productivity-related segments and certain agro-industrial products dependent on GM plants, such as those based on our safflower molecular farming platform, and between three and five years to develop other agro-industrial products that rely on Bacillus subtilis fermentation. Some products will never reach the final stages of development.
Development of new or improved agricultural products involves risks of failure inherent in the development of products based on innovative and complex technologies. These risks include the possibility that:
| ■ | our products will fail to perform as expected in the field; |
| ■ | our products will not receive necessary regulatory permits and governmental clearances in the markets in which we intend to sell them; |
| ■ | our products may have adverse effects on consumers; |
| ■ | consumer preferences, which are unpredictable and can vary greatly, may change quickly, making our products no longer desirable; |
| ■ | our competitors develop new products that taste better or have other more appealing characteristics than our products; |
| ■ | our products will be viewed as too expensive by food companies or growers as compared to competitive products; |
32
| ■ | our products will be difficult to produce on a large scale or will not be economical to grow; |
| ■ | intellectual property and other proprietary rights of third parties will prevent us, our R&D partners, or our licensees from marketing and selling our products; |
| ■ | we may be unable to patent or otherwise obtain intellectual property protection for our discoveries in the necessary jurisdictions; |
| ■ | we or the customers that we sell our products to may be unable to fully develop or commercialize our products in a timely manner or at all; and |
| ■ | third parties may develop superior or equivalent products. |
We intend to continue to invest in R&D including additional and expanded field testing to validate potential products in real world conditions. Because of the long product development cycle and the complexities and uncertainties associated with biotech and agro-industrial technologies, there can be no assurance that we will ever generate significant revenues from the technologies or products that we are currently developing without significant delay, without the incurrence of unanticipated costs or at all.
We or our collaborators may fail to perform our respective contractual obligations and we may have disputes with our collaborators.
Pursuant to our joint venture agreements, other agreements with our joint venture partners and collaboration arrangements, we are required to provide R&D services over a particular period of time and meet other contractual obligations. If we fail to perform our obligations under these agreements, our collaborators’ obligations to us may be reduced and, in other cases, our collaborators may seek to dissolve the corresponding joint venture or terminate their agreements with us and, as a result, our anticipated revenues may decrease. In addition, the failure of any of our collaborators to perform their contractual obligations, due to financial hardship, disagreement under the relevant agreement or for any other reason, may hinder our research collaboration, development and commercialization activities, increase our costs and materially and adversely affect our results of operations. Because some of our intellectual property has been licensed to various joint ventures for use in several different fields, the interests of each of our partners in these joint ventures may not always be aligned. As a result, it is possible that potential disputes may arise between us and our partners.
Our ability to generate value from our joint ventures and research collaborations will depend on, among other things, our ability to work cooperatively with our collaborators for the discovery, development and commercialization of our technology and products and we may be unable to do so. We cannot be sure that the division of labor will be successful in aiding the commercialization of our products. Furthermore, the agreements governing our partnership and collaborations are complex and cover a range of future activities. The occurrence of any negative event with respect to the above matters or a dispute between us and our partners or collaborators could delay our development and commercialization efforts, and lead to the dissolution of the partnership or collaboration. If disagreements with a collaborator arise, any such dispute could be costly, time-consuming to resolve and distracting to our management. Such a dispute may also negatively affect our relationship with one or more of our other collaborators and may hinder our ability to enter into future collaboration agreements. Any of these occurrences could negatively impact our business and results of operations.
Our joint venture agreements or any partnerships that we may enter into in the future may not be successful, which could adversely affect our ability to develop and commercialize our product candidates.
We may seek partnerships or joint venture arrangements with third parties for the development or commercialization of our product candidates depending on the merits of retaining commercialization rights for ourselves as compared to entering into partnerships or joint venture arrangements. We will face, to the extent that we decide to enter into partnerships or joint venture agreements, significant competition in seeking appropriate partners. Moreover, partnerships or joint venture arrangements are complex and time-consuming to negotiate document implement and maintain. We may not be successful in our efforts to establish and implement partnerships, joint ventures, or other alternative arrangements
33
should we so chose to enter into such arrangements and any future partnerships or joint ventures that we enter into may not be successful. Furthermore, the terms of any partnerships, joint ventures, or other arrangements that we may establish may not be favorable to us.
The success of our R&D partnerships or joint venture arrangements will depend heavily on the efforts and activities of our partners. Our joint venture arrangements may present financial, managerial, and operational challenges, including potential disputes, liabilities, or contingencies and may involve risks not otherwise present when operating independently including:
| ■ | partners may have business interests, goals or cultures that are or become inconsistent with our business interests, goals or culture; |
| ■ | partners may have significant discretion in determining the efforts and resources that they will apply to partnerships or joint ventures; |
| ■ | partners may not pursue development and commercialization of our potential products or may elect not to continue or renew development or commercialization programs based on trial results, changes in their strategic focus due to the acquisition of competitive products, availability of funding or other external factors, such as a business combination that diverts resources or creates competing priorities; |
| ■ | partners may delay trials, provide insufficient funding for a trial program, stop a trial, abandon a product candidate, repeat or conduct new trials or require a new formulation of a product candidate for testing; |
| ■ | partners could independently develop, or develop with third parties, products that compete directly or indirectly with our products or product candidates; |
| ■ | a partner with marketing manufacturing and distribution rights to one or more products may not commit sufficient resources to or otherwise not perform satisfactorily in carrying out these activities; |
| ■ | we could grant exclusive rights to our partners that would prevent us from collaborating with others; |
| ■ | partners may not properly maintain or defend our intellectual property rights or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability; |
| ■ | we may incur liabilities or losses as a result of an action taken by the joint venture or our joint venture partners; |
| ■ | disputes may arise between us and a partner that causes the delay or termination of the research development or commercialization of our current or future products or that results in costly litigation or arbitration that diverts management attention and resources; |
| ■ | our joint venture partners may act contrary to our instructions, requests, policies or objectives, which could reduce our return on investment, harm our reputation or restrict our ability to run our business; |
| ■ | partnerships may be terminated, and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable current or future products; |
| ■ | partners may own or co-own intellectual property covering our products that results from our partnering with them and in such cases we would not have the exclusive right to develop or commercialize such intellectual property; and |
| ■ | a partner’s sales and marketing activities or other operations may not comply with applicable laws resulting in civil or criminal proceedings. |
The risks described above or the failure to continue any joint venture or joint development arrangement or to resolve disagreements with our current or future joint venture partners could materially and adversely affect our ability to transact the business that is the subject of such joint venture, which would in turn negatively affect our financial condition and results of operations.
34
We may experience difficulties in collecting payments or royalties to which we believe we are entitled.
We sell certain of our products to distributors through Rizobacter and Bioceres Semillas, our proprietary commercial channels for crop productivity technologies. We also often license the use of certain technology to collaborators and licensees who use or will use the intellectual property to develop and commercialize seeds with improved seed traits or agro-industrial products. Additionally, we may be entitled under applicable intellectual property laws in the countries in which we operate to the payment of royalties from end users who subsequently multiply and use our seed technology. In each case, we may not actually receive the payments or royalties to which we are entitled, due to failure or refusal of the responsible parties to pay the amounts due. Failure to receive amounts owed to us could have an adverse impact on our business.
In the case of royalty payments from licensees, we rely on the good faith of the licensees to report to us the sales they earn from these products and to accurately calculate the royalties, to which we are entitled, processes that may involve complicated and difficult calculations. Under existing agreements, we have the right to inspect the inventory and accounts of multipliers of our seeds and licensees of our technologies; however, we must also rely on the good faith of end users to accurately report to us the multiplication of our seeds and remit royalty payments due in respect of the same, which may be respected to varying degrees in different jurisdictions given the absence of contractual privity and prevailing market practice. Additionally, a licensee, collaborator or third party may use our intellectual property without our permission, dispute our ownership of certain intellectual property rights or argue that our intellectual property does not cover the joint venture’s marketed product. We seek to address these concerns in our contractual agreements, however, we may not have contractual arrangements with the party in question and/or such provisions may not be effective. If these provisions prove to be ineffective, we may not be able to achieve our objectives of generating significant revenues from crop productivity and agro-industrial products sales and royalties from our seed technologies and agro-industrial technologies. Furthermore, regardless of any resort to legal action, a dispute with an end-customer, a licensee or collaborator over intellectual property rights may damage our relationship with that licensee or collaborator, and may also harm our reputation in the industry.
We depend on our key personnel and research collaborators and we may be adversely affected if we are unable to attract and retain qualified scientific and business personnel.
Our business is dependent on our ability to recruit and maintain highly skilled and educated individuals through direct employment or collaboration arrangements, with expertise in a range of disciplines, including biology, chemistry, plant genetics, agronomics, mathematics programming and other subjects relevant to our business. Our ability to recruit such a work force depends in part on our ability to maintain our market leadership in agricultural biotech industry in Argentina and Latin America. Maintaining our ability to attract highly-skilled workers and leading scientific institutions depends in part on our ability to maintain a strong technology platform and state-of-the-art facilities, as well as our ability to consistently and successfully commercialize our technology. There can be no assurance that we will be able to maintain leading scientific capabilities or continue to successfully maintain advanced technology in the market.
Our success is also dependent to a significant degree upon the technical skills and continued service of certain members of our management team, in particular those of our CEO, Dr. Federico Trucco. Dr. Trucco has occupied several positions at Bioceres since 2005 and has vast experience and knowledge of our business, strategy and technologies. Furthermore, he has developed and maintained strong relationships with our original shareholders. The cessation of Dr. Trucco’s employment for any reason could have a material and negative impact on us. In addition, the number of qualified and highly educated personnel in Argentina, where the majority of our operations are located, is limited and competition for the services of such persons may be intense. Our inability to secure, retain or find replacements for key management and technical personnel could adversely affect our business and could have a material adverse effect on our business, operating results, financial condition and growth prospects.
We do not enter into non-compete agreements with our employees, and therefore we may be unable to prevent our competitors from benefiting from the expertise of our former employees.
We do not enter into non-compete agreements with our employees, which prevents us from limiting our key employees from joining our competitors or competing directly against us. As a result, we may be unable to prevent our competitors
35
from benefiting from the expertise of such employees. Direct competition by a former employee could materially adversely affect our business, results of operations and ability to capitalize on our proprietary information.
We may be adversely affected by global economic conditions.
Our ability to continue to develop and grow our business, build proprietary distribution channels and generate revenues from product sales and royalty payments may be adversely affected by global economic conditions in the future, including instability in credit markets, declining consumer and business confidence, fluctuating commodity prices and interest rates, volatile exchange rates and other challenges that could affect the global economy such as the changing financial regulatory environment. For example, our customers and licensees may experience deterioration of their businesses, cash flow shortages or difficulties obtaining financing, which could adversely affect the demand for our technologies, products and services. In addition, our earnings may be adversely affected by fluctuations in the price of certain commodities, such as grains, milk, meat, biofuels and biomaterials. If commodity prices are negatively impacted, the value of our products could be directly and negatively impacted. Additionally, growers’ incomes have historically been negatively affected by commodity prices. As a result, fluctuations in commodity prices could have an impact on growers’ purchasing decisions and negatively affect their ability and decisions to purchase our seeds or products that incorporate our proprietary technology. We cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
Our crop productivity business is highly seasonal and affected by factors beyond our control, which may cause our sales and operating results to fluctuate significantly.
The sale of products from our crop productivity-related segments is dependent upon planting and growing seasons, which vary from year to year, and are expected to result in both highly seasonal patterns and substantial fluctuations in quarterly sales and profitability. Weather conditions and natural disasters, such as heavy rains, hail, floods, freezing conditions, windstorms or fire, also affect decisions by our distributors, direct customers and end users about the types and amounts of products to use and the timing of harvesting and planting. Pergamino, where a large percentage of our operations are based, experienced intense flooding in late 2016. As we increase our sales in our current markets and expand into new markets in different geographies, it is possible that we may experience different seasonality patterns in our business. Disruptions may lead to delays in harvesting or planting by growers which can result in pushing orders to a future quarter, which could negatively affect results for the quarter in question and cause fluctuations in our operating results. Seasonal variations may be especially pronounced because our product lines are mainly sold in the Southern Hemisphere. Our seeds, biologicals and other crop input products sales tend to be comparatively low during the third and fourth quarters of our fiscal year, as soybean related sales peak in the second quarter. However, planting and growing seasons, climatic conditions and other variables on which sales of our products are dependent vary from year to year and quarter to quarter. As a result, we may experience substantial fluctuations in quarterly seed sales.
The overall level of seasonality in our business is difficult to evaluate as a result of our relatively early stage of development, our limited number of commercialized products, our expansion into new geographical territories, the introduction of new products and the timing of introductions of new products. It is possible that our business may be more seasonal or experience seasonality in different periods than anticipated. Other factors may also contribute to the unpredictability of our operating results, including the size and timing of significant distributor transactions, the delay or deferral of use of our commercial technology or products and the fiscal or quarterly budget cycles of our direct customers, distributors, licensees and end users. Customers may purchase large quantities of our products in a particular quarter to store and use over long periods of time or time their purchases to manage their inventories, which may cause significant fluctuations in our operating results for a particular quarter or year.
Our results of operations from our crop productivity and agro-industrial products may vary significantly from period to period due to circumstances beyond our control.
The crop productivity and agro-industrial markets are affected by various factors that make their operations relatively unpredictable from period to period. The development of our products may be adversely affected by circumstances beyond our control. For our crop productivity products, factors beyond our control include weather and climatic variations, such as droughts or heat stress, or other factors we are unable to identify. For example, if there were a prolonged or permanent disruption to the electricity, climate control or water supply operating systems in our
36
greenhouses or laboratories, the plants on which we are testing our seed traits and the samples we store in freezers, both of which are essential to our development activities, would be severely damaged or destroyed, adversely affecting our development activities and thereby our business and results of operations. We have experienced crop failures in the past for various reasons, which have resulted in re-start field trials and delays in achieving expected results.
For our agro-industrial products, factors that may affect the development of our products include an increase in cost of the raw materials for which we designed our technologies, such as raw glycerin. For example, if production of biodiesel were to decrease, glycerin would become scarce, which would increase our cost.
The crop productivity and agro-industrial markets are also vulnerable to crop disease and to pests, which may vary in severity and effect, depending on the stage of production at the time of infection or infestation, the type of treatment applied, climatic conditions and the risks associated with ongoing global climate change. The costs to control disease and other infestations vary depending on the severity of the damage and the extent of the plantings affected. Moreover, there can be no assurance that available technologies to control such infestations will continue to be effective. These infestations can also increase costs, decrease revenues and lead to additional charges to earnings, which may have a material adverse effect on our business, financial position and results of operations.
Any development or product failure we may experience or any inability to economically source necessary materials could result in increased cost of development of our crop productivity or agro-industrial products, which may negatively impact our business and results of operations.
Consumer and government resistance to GM crops may negatively affect our public image and reduce sales of seeds or other products containing our seed traits.
We are active in the field of biotech development of seeds and industrial applications, including GM seeds and bacteria and the successful commercialization of our products depends, in part, on public acceptance of genetically engineered agricultural products. Some consumers may reject foods and enzymes made from GM seeds and production of certain GM crops is prohibited in certain countries due to food safety and environmental concerns. Any increase in negative perceptions of GM crops, or more restrictive government regulations in response thereto, could have a negative effect on our business and may delay or impair the development and commercialization of our products.
The commercial success of our products may be adversely affected by claims that biotechnology plant products are unsafe for consumption or use, pose risks of damage to the environment, or create legal, social and ethical dilemmas. The high public profile of biotechnology in food production and food products and public attitudes about the safety and environmental hazards of, and ethical concerns over, genetic research and biotechnology plant products could negatively affect our public image and results of operations.
The prohibition of the production of certain GM crops in select countries and the current resistance from consumer groups to GM crops not only limits our access to such markets but also has the potential of spreading to and influencing the acceptance of products developed through biotechnology in other regions of the world and may also influence regulators in other countries to limit or ban production of GM crops, which could limit the commercial opportunities to exploit biotechnology. For example, in the United States, no product may be labelled as “organic” if it contains any GMOs. Additionally, some states in the United States are considering, and one state has passed a law relating to, mandatory labelling of GMO foods, which may carry a negative connotation for consumers and which could make it difficult and expensive for companies to use ingredients from GM crops and distribute products in compliance with the labelling requirements, each of which could in turn have an adverse impact on the sale of our GM seeds. In Argentina, a class action suit has been initiated against the national government and certain biotechnology companies, including us, requesting, among other changes, the mandatory labelling of GM foods and environmental protection of land use. As of the date of this prospectus, the plaintiffs’ request for an injunction against GMO approvals was rejected by the Federal Court of Appeals and an extraordinary appeal at the Supreme Court was filed, the practicable chances of success of which are low.
GM crops are grown principally in the United States, Brazil and Argentina where there are fewer restrictions on the production of GM crops. If these or other countries where GM crops are grown or where we engage in business activities enact laws or regulations that ban the production of such crops or make regulations more stringent, we could experience
37
a longer product development cycle for our products and may be forced to abandon projects related to certain crops or geographies, both of which would negatively affect our business and results of operations. Public attitudes towards ownership of genetic material and potential changes to laws regulating such ownership could weaken our intellectual property rights with respect to our genetic material and discourage R&D partners from supporting, developing or commercializing our products and technologies. Furthermore, any future labeling requirements could heighten these concerns and make consumers less likely to purchase food products containing gene-edited ingredients.
Competition in crop productivity and agro-industrial products is intense and requires continuous technological development.
We currently face significant direct and indirect competition in the markets in which we operate. The markets for crop productivity and agro-industrial products are intensely competitive and rapidly changing. Many companies engage in the development of crop productivity and agro-industrial products, and speed in commercializing a new product can be a significant competitive advantage. As an example, some of our competitors engage in research associated with discovery and therefore have R&D budgets allocated for crop productivity or agro-industrial products that are more significant than our own R&D budget and that cover more activities than those in which we engage. In addition, former collaborators, by virtue of having had access to our proprietary technology, may utilize this insight for their own development efforts.
In most segments of the crop productivity and agro-industrial markets, the number of products available to end-customers is steadily increasing as new products are introduced. We may be unable to compete successfully against our current and future competitors, which may result in price reductions, reduced margins and the inability to achieve market acceptance for products containing our seed traits and technology. In addition, many of our competitors have substantially greater financial, marketing, sales, distribution and technical resources than us and some of our competitors have more experience in R&D, regulatory matters, manufacturing and marketing. We anticipate increased competition in the future as new companies enter the market and new technologies become available. Programs to improve genetics and crop protection chemicals are generally concentrated within a relatively small number of large companies, while non-genetic approaches are underway with broader set of companies. Mergers and acquisitions in the plant science, specialty food ingredient and agricultural biotechnology seed and chemical industries may result in even more resources being concentrated among a smaller number of our competitors.
Our technology may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors, which will prevent or limit our ability to generate revenues from the commercialization of our seed traits and technology. At the same time, the expiration of patents covering existing products reduces the barriers to entry for competitors. Our ability to compete effectively and to achieve commercial success depends, in part, on our ability to control manufacturing and marketing costs; effectively price and market our products, successfully develop an effective marketing program and an efficient supply chain, develop new products with properties attractive to food manufacturers or growers and commercialize our products quickly without incurring major regulatory costs. We may not be successful in achieving these factors and any such failure may adversely affect our business, results of operations and financial condition.
Changes in laws and regulations to which we are subject, or to which we may become subject in the future, may materially increase our costs of operation, decrease our operating revenues and disrupt our business.
Laws and regulatory standards and procedures that impact our business are continuously changing. Responding to these changes and meeting existing and new requirements may be costly and burdensome. Changes in laws and regulations may occur that could:
| ■ | impair or eliminate our ability to source technology and develop our products, including validating our products through field trials and passing biosafety evaluations; |
| ■ | increase our compliance and other costs of doing business through increases in the cost to protect our intellectual property, including know-how, trade secrets and regulatory data, or increases in the cost to obtain the necessary regulatory approvals to commercialize and market the products we develop directly or jointly; |
| ■ | require significant product redesign or redevelopment; |
| ■ | render our seed traits and technology and products that incorporate them less profitable or less attractive compared to competing products; |
38
| ■ | reduce the amount of revenues we receive from government grants, licenses or other royalties; and |
| ■ | discourage us and other collaborators from offering, and end-markets from purchasing, products that incorporate our seed traits and technology. |
Any of these events could have a material adverse effect on our business, results of operations and financial condition. We believe we currently are in compliance with regulations related to growing GM crops in Argentina and other countries; however, if these regulations change, our validation trials and compliance efforts may become costly and burdensome.
Any changes in regulation in countries where GM crops are grown or exported into could result in our collaborators, other third parties or us being unable or unwilling to develop, commercialize or sell products that incorporate our seed traits or technology. In addition, we rely on various forms of intellectual property protection. Legislation and jurisprudence on intellectual property in the key markets where we seek protection, such as the United States, Brazil and Argentina, is evolving and changes in laws could affect our ability to obtain or maintain intellectual property protection for our products. Any changes to these existing laws and regulations may materially increase our costs, decrease our revenues and disrupt our business.
We generate revenue from government grants and rely on grants to fund our technology sourcing and product development activities. We cannot guarantee that we will continue to obtain government grants in the future, and our failure to do so for any reason could require us to change our operating model.
Together with our research collaborators, we apply for and seek to obtain research grants from governmental entities. The receipt of government grants is central to our strategy of minimizing our capital expenditures in connection with technology sourcing and product development and represents an important means of development of early-stage technology. Pursuant to such grants, the government directly pays for, or we are reimbursed for certain expenses incurred in connection with, our technology development activities. Additionally, a portion of our revenue is generated from payments to us or payments made directly to our suppliers in the form of government grants. Grant payments from government entities represented 0.5% of our total revenue as of September 30, 2017 and 1.7% of our total revenue for the year ended December 31, 2016. In those periods, all such payments were received from Argentine governmental entities.
Our ability to obtain grants and fund our technology sourcing and product development activities with funds received from government entities in the future depends upon the availability of funds under applicable government programs and approval of our applications to participate in such programs. The application process for these grants and other incentives is highly competitive. We may not be successful in obtaining any additional grants, loans or other incentives. The receipt of grant funds also subjects us to compliance with the specific grant requirements, including rigorous documentation requirements. Failure to comply with these requirements could lead to termination of these grant or difficulties in obtaining new grants, as well as an inability to receive reimbursement for our costs or a requirement to refund costs previously reimbursed.
Additionally, we are subject to audits in connection with our grant funds, which may subject us to penalties if we are not compliant with applicable requirements. An audit could result in a material adjustment to our results of operations and financial condition. In addition, serious reputational harm or significant adverse financial effects could occur if allegations of impropriety are made against us, even if we are ultimately found to have done no wrong.
Finally, there can be no assurance that the Argentine government, or the international bodies such as the Inter-American Development Bank and the World Bank that historically have provided the funding that the Argentine government has used to make research grants, will continue to provide grants or funding at current levels or at all. To the extent that our existing grants are terminated or modified or we are unsuccessful in securing government grants in the future, we may have to modify our business strategy and would lose a potential source of revenue and means of sourcing and developing new technology, which could adversely affect our results of operations and increase our costs.
39
Our substantial indebtedness could adversely affect our financial condition.
We have a significant amount of indebtedness. As of September 30, 2017 and June 30, 2017, our total indebtedness, including outstanding borrowings, puttable instruments, financing payments and contingent consideration from acquisitions, were US$179.3 million and US$172.9 million, respectively. Our high level of indebtedness could have important adverse consequences, including:
| ■ | limiting our ability to obtain additional financing to fund future working capital, capital expenditures, acquisitions or other general corporate requirements; |
| ■ | requiring a substantial portion of our cash flows to be dedicated to debt service payments instead of other purposes, thereby reducing the amount of cash flows available for working capital, capital expenditures, acquisitions and other general corporate purposes; |
| ■ | increasing our vulnerability to general adverse economic and industry conditions; |
| ■ | limiting our flexibility in planning for and reacting to changes in the industry in which we compete; |
| ■ | placing us at a disadvantage compared to other, less leveraged competitors; and |
| ■ | increasing the cost of borrowing. |
The occurrence of any of the above may negatively impact our business and results of operations.
Price increases and shortages of raw materials could adversely affect our results of operations.
Our results of operations may be affected by the availability and pricing of raw materials, principally materials needed to design our technologies, such as raw glycerin. Factors such as changes in the global or regional levels of supply and demand, weather conditions, seasonal fluctuations, shortages or interruptions, changes in global climates and government regulations could substantially impact the price of raw materials. To the extent we are unable to pass on increases in raw materials and energy prices to our customers, a substantial increase in raw material prices or a continued interruption in supply could have a material adverse effect on our financial condition and results of operations., our business, financial condition and results of operations could be materially adversely affected.
The overall agricultural industry is susceptible to commodity price changes and we, along with our food manufacturing customers and grower customers, are exposed to market risks from changes in commodity prices.
Changes in the prices of certain commodity products could result in higher overall cost along the agricultural supply chain, which may negatively affect our ability to commercialize our products We will be susceptible to changes in costs in the agricultural industry as a result of factors beyond our control, such as general economic conditions, seasonal fluctuations, weather conditions, demand, food safety concerns, product recalls and government regulations. As a result, we may not be able to anticipate or react to changing costs by adjusting our practices, which could cause our operating results to deteriorate.
We may be required to pay substantial damages as a result of product liability claims for which we do not have insurance.
Product liability claims are a commercial risk for our business, particularly as we are involved in the sale of commercial technology and the supply of biotechnological products, some of which may be shown in the future to be harmful to humans and the environment. We may be held liable if any product we develop, unsuitable during marketing, sale or consumption. We do not currently have insurance coverage for such claims. Courts have levied substantial damages in the United States and elsewhere against a number of companies in the agriculture industry in past years based upon claims for injuries allegedly caused by the use of their products. In addition, we may face product liability and similar claims involving cross-pollination of crops, which recently has affected other companies in our industry operating in the United States, and cross-contamination of GMO and non-GMO ingredients. In Argentina, there are no precedents for product liability cases in the agricultural industry related to transgenic or biotechnology products; however, there has been at least one product liability case related to the use of pesticides. There is a possibility that a products liability case could be filed against us in Argentina, in which case damages may be substantial albeit potentially smaller than those typically awarded in the United States. Product liability claims against us, our joint ventures or third-party licensees
40
selling products that contain our seed traits or technology or allegations of product liability relating to seeds or other products containing seed traits or technology developed by us could damage our reputation, harm our relationships with our collaborators and other business counterparties and materially and adversely affect our business, results of operations, financial condition and prospects.
Our operations are subject to various health and environmental risks associated with our use, handling and disposal of potentially toxic materials.
We are subject to numerous federal, state, local and foreign environmental, health and safety laws and regulations, including those governing laboratory procedures, the handling, use, storage, treatment, manufacture and disposal of hazardous materials and wastes, discharge of pollutants into the environment and human health and safety matters. As part of our technology sourcing and product development activities, we develop GMOs by inserting new genes into the genomes of certain plants and bacteria. Though we introduce these genes in order to improve plant traits, produce certain enzymes or higher value molecules, or engineer bacterial metabolism, we cannot always predict the effect that these genes may have on the organism. In some cases, the genes may render the organism poisonous or toxic, or they may cause the organism to develop other dangerous characteristics that could harm the organism’s surrounding environment. Furthermore, there is a risk that, when testing GMOs, the seeds or strains of these organisms may escape the laboratory, greenhouse, industrial facility or field in which they are being tested and contaminate nearby areas. Poisonous or toxic organisms may therefore be inadvertently introduced into the environment or possibly enter the food production system, harming the people and animals who come in contact with them. Our crop protection products, which include Rizoderma, adjutants, therapies, herbicides, fungicides and insecticides, among others, bear similar risks in the development stage.
We cannot eliminate the risk of contamination or discharge and any resultant injury from these materials. If these risks were to materialize, we could be subject to fines, liability, reputational harm or otherwise adverse effects on our business. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials, or may otherwise be required to remedy the contamination, and our liability may exceed any insurance coverage and our total assets Furthermore, compliance with environmental, health and safety laws and regulations may be expensive and may impair our R&D efforts. If we fail to comply with these requirements, we could incur substantial costs and liabilities, including civil or criminal fines and penalties, clean-up costs or capital expenditures for control equipment or operational changes necessary to achieve and maintain compliance In addition, we cannot predict the impact on our business of new or amended environmental, health and safety laws or regulations or any changes in the way existing and future laws and regulations are interpreted and enforced. These current or future laws and regulations may impair our research, development or production efforts.
The requirements of being a public company may strain our resources and distract our management, which could make it difficult to manage our business.
Following the completion of this offering, we will be required to comply with various regulatory and reporting requirements, including those required by the SEC and the CNV. Complying with these reporting and regulatory requirements will be time consuming, resulting in increased costs to us or other adverse consequences.
As a public company, we will be subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the requirements of the Sarbanes-Oxley Act as well as the Argentine securities law and CNV rules. These requirements may place a strain on our systems and resources. The Exchange Act requires that we file annual and current reports with respect to our business and financial condition. Likewise, CNV rules require that we make annual and quarterly filings and that we comply with disclosure obligations. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal controls over financial reporting. To maintain and improve the effectiveness of our disclosure controls and procedures, we will need to commit significant resources, hire additional staff and provide additional management oversight. We expect to implement additional procedures and processes for the purpose of addressing the standards and requirements applicable to public companies. These activities may divert management’s attention from other business concerns, which could have a material adverse effect on our business, financial condition, results of operations and cash flows.
41
As an “emerging growth company,” as defined in the JOBS Act, we may take advantage of certain temporary exemptions from various reporting requirements including, but not limited to, an exemption from compliance with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and the rules and regulations of the SEC thereunder. These exemptions will cease to apply by no later than the last day of our fiscal year following the fifth anniversary of the completion of this offering and we expect to incur additional expenses and devote increased management effort toward ensuring compliance with the additional reporting requirements that will apply when we cease to be an “emerging growth company.” We cannot predict or estimate the amount of additional costs we may incur as a result of becoming a public company or the timing of such costs.
We may require additional financing in the future and may not be able to obtain such financing on favorable terms, if at all, which could force us to delay, reduce or terminate some of our activities.
The process of developing and commercializing products is expensive, lengthy and risky and we expect to continue investing in our R&D services to identify new potential products for development. We may require additional capital to fund our technology sourcing and product development projects and to provide working capital to fund other aspects of our business. Although we believe that following this offering, our cash and cash equivalents and marketable securities will provide adequate resources to fund our operations, including technology sourcing and product development expenses, planned capital expenditures and working capital requirements for the foreseeable future, we may nevertheless need additional financing in the future, due to changes in our business strategy or the occurrence of unanticipated events. We may seek to issue equity securities or debt finance, which could result in dilution to our existing shareholders or subject us to restrictive covenants that limit our operating flexibility and require us to comply with certain financial ratios, respectively. Alternatively, we may not be able to raise sufficient additional funds on terms that are favorable to us, if at all. If we fail to raise the funds we require, our ability to fund our operations, take advantage of strategic opportunities, develop and commercialize products or technologies, or otherwise respond to competitive pressures could be significantly limited. In such an event, we may be forced to delay or terminate our development initiatives or the commercialization of our technology and products, curtail operations or grant licenses to our technology on terms that are not favorable to us. If adequate funds are not available, we may not be able to successfully execute our business strategy or continue our business.
Development and commercialization of our products may incur scrutiny under the Convention on Biological Diversity Treaty.
The Convention is an international treaty that was adopted at the Earth Summit in Rio de Janeiro, Brazil in 1992. The treaty provides that if a company uses genetic resources, such as an indigenous plant, from a participating country to develop a product, then such company must obtain the prior informed consent of the participating country and owes fair and equitable compensation to the participating country. Although the United States is not a participating country, most countries where we currently obtain or may obtain genetic resources in the future, including Argentina, have ratified the treaty and are currently participants in the Convention. We may fall under scrutiny of the Convention with respect to the development or commercialization of any of our products derived from genetic resources originating from any of the countries that are participants in the Convention. There can be no assurance that the government of a participating country will not assert that it is entitled to fair and equitable compensation from us. Such compensation, if demanded, may make commercialization of our products impracticable.
Our business strategy may change and the successful implementation of our business plan is uncertain.
As an emerging biotechnology company, we continually analyze our business plan and operations in the light of market conditions and developments. We currently generate a significant portion of our revenue from the sale of crop protection products. We anticipate that following the successful regulatory approval and commercialization of our technologies, including HB4, an increasing portion of our revenues will be generated by sales of seed and integrated products through our proprietary commercial channels and third party licensees, with incremental income projected to be generated by the joint ventures in which we participate. We face numerous challenges to completing the various steps necessary for the commercialization of our products and there can be no guarantee that we will be able to successfully commercialize our technologies. As a result of our continuous analyses of our crop productivity and agro-industrial solutions, we may decide to make substantial changes in our business plan and operations. Such modifications may also result from management’s belief that it has identified more economical or efficient means of achieving our objectives. Furthermore, such changes could relate to minor aspects of the business plan, such as the methods in which we sell our crop productivity and
42
agro-industrial solutions, or to key aspects of the plan, such as the type of technologies that we seek to commercialize. Changes to our business plan could result in material delays to the commercialization of our products.
Our failure to accurately forecast and manage inventory could result in an unexpected shortfall or surplus of products which could harm our business.
We are required to produce inventories of certain of our products (mainly seeds and biologicals) and we monitor our inventory levels based on our own projections of future demand. Because of the significant time it takes to produce commercial quantities of seeds, production decisions must be made well in advance of sales. An inaccurate forecast of demand for any seed variety can result in the unavailability of seeds in high demand. Such unavailability may depress sales volumes and adversely affect customer relationships. Conversely, an inaccurate forecast could also result in an over-supply of seeds which may increase costs, negatively impact cash flow, reduce the quality of inventory and ultimately create write-offs of inventory. The acquisition of Rizobacter has increased the scale of our sales operations and as a result increased the magnitude of these risks, the realization of which could have a material adverse effect on our business, results of operations and financial condition.
Disruption to our IT and operating system could adversely affect our reputation and have a material adverse effect on our business and results of operations.
Disruption or failure of our IT system due to technical reasons, natural disaster or other unanticipated catastrophic events, including power interruptions, storms, fires, floods, earthquakes, terrorist attacks and wars could significantly impair our ability to deliver data related to our projects to our collaborators on schedule and materially and adversely affect our relationships with our collaborators, our business and our results of operations. We expect to continue to develop our computational technologies and may need to update our IT system and storage capabilities. If our existing or future IT system does not function properly, or if the IT system proves incompatible with our new technologies, we could experience interruptions in data transmissions and slow response times, preventing us from completing routine research and business activities. Furthermore, we can provide no assurance that our current IT system is fully protected against third-party intrusions, viruses, hacker attacks, information or data theft or other similar threats.
Our business and operations would suffer in the event of computer system failures, cyber-attacks or a deficiency in our cyber-security.
Despite the implementation of security measures, our internal computer systems, and those of third Parties on which we rely, are vulnerable to damage from computer viruses, malware, natural disasters, terrorism war telecommunication and electrical failures, cyber-attacks or cyber-intrusions over the Internet, attachments to emails, persons inside our organization, or persons with access to systems inside our organization. The risk of a security breach or disruption, particularly through cyber-attacks or cyber-intrusion, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the number intensity and sophistication of attempted attacks and intrusions from around the world have increased. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our product development programs. For example, the loss of field trial data from completed or ongoing or planned field trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach was to result in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur material legal claims and liability, damage to our reputation, and the further development of our product candidates could be delayed.
Labor unions can request, and have requested, the unionization of some of our employees.
In December 2016 and March 2017, the Argentine Trade Union of Truck Drivers (Sindicato de Choferes de Camiones), or the SCC, and the Argentine Union of Rural Workers and Stevedores (Unión Argentina de Trabajadores Rurales y Estibadores), or UATRE, respectively requested the unionization of some employees of Rizobacter. With respect to the former, the SCC requested to unionize employees involved in logistics and operation of forklifts. UATRE requested to unionize workers engaged in the handling and storage of grain related to our seed treatment process undertaken seasonally. After negotiations, both SCC and UATRE came to an agreement with Rizobacter wherein Rizobacter agreed to
43
hire companies to carry out the operations covered by each union. Each company agreed to indemnify Rizobacter in relation to any subsequent claims by the workers registered with the SCC or the UATRE, as the case may be, without direct cause to Rizobacter.
If new union disputes arise, they may be time consuming and distracting to management. The occurrence of a union dispute could have a material and adverse effect on our costs and business, results of operations and financial condition.
We rely on third parties to grow our seeds. If these parties do not grow our seeds at a satisfactory quality, in a timely manner, in sufficient quantities or at an acceptable cost, our commercialization efforts could be delayed or otherwise negatively impacted.
We rely on affiliated and unaffiliated growers to grow the majority of our proprietary seed and to sell it to us at negotiated prices each year. Our current dependence upon others for the production of our seeds may adversely affect our ability to commercialize any products on a timely and competitive basis. If our growers decline to a significant degree to plant the acreage on which we rely, and if we cannot find other growers to plant the lost acreage, our inventory of seed could be insufficient to satisfy the needs of our customers. Furthermore, growers may refuse to grow our seeds for any reason, including deterioration in our business relationship or the existence of more favorable terms with other companies. For example, if a particular crop is paying a materially higher price than has been paid in the past, growers may decide to not grow our seeds in favor of receiving a higher return from an alternative crop planted on the same acreage. If third-party growers decline to grow our seeds or if they are unable to grow our seeds at acceptable quality levels, our business, results of operations and financial condition could materially decline.
Under Argentine law, if a company’s losses exceed its reserves and total equity as of the close of its fiscal year, such company’s shareholders may be required to make equity contributions to such company or the company may be forced to enter into a compulsory liquidation or winding up proceeding, if such capitalization does not happen.
Argentine law provides that if a company’s losses exceed its reserves and total equity as of the close of its fiscal year, its shareholders are required to make equity contributions to it sufficient to balance the company’s total equity position. In the event that a company is unable to restore positive total equity in this manner, the company will be required to enter into a compulsory liquidation or winding up proceeding under Argentine law. Although our total equity was US$29.0 million as of June 30, 2017, we presented negative equity attributable to equity holders of the parent which resulted from accounting effects related to the Rizobacter acquisition, including (i) a charge related to Rizobacter inventories acquired and sold post-acquisition that were revalued at acquisition due to the application of purchase price allocation, or PPA, accounting rules, (ii) currency exchange differences relating to Argentine peso-denominated assets and liabilities acquired and (iii) the classification of certain capital raises through convertible loans and puttable instruments as liabilities and through convertible preferred shares as non-controlling interest under IFRS. Although we had a negative total equity as of September 30, 2017, our consolidated total equity as of the same date was positive in the amount of US$22.5 million.
We expect to reverse our negative equity balance through the net proceeds from this offering and the expected conversion of certain financing instruments into ordinary shares.
We cannot assure you that by June 30, 2018 our negative total equity position will have been fully reversed or that we will not have negative total equity at the end of any future fiscal year, in which case the shareholders may be required to make equity contribution to the company or we may be obligated to enter into a compulsory liquidation or winding up proceeding under Argentine law if such capitalization does not happen. For further information see “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources—Projected Sources and Uses of Cash.”
We are subject to anti-corruption and anti-money laundering laws with respect to both our domestic and international operations, and non-compliance with such laws can subject us to criminal and civil liability and harm our business.
We are subject to the U.S. Foreign Corrupt Practices Act of 1977, as amended, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and possibly other anti-bribery and anti-money laundering laws in countries in which we conduct activities Anti-corruption laws are interpreted broadly and prohibit us and our collaborators from authorizing, offering, or directly or indirectly providing improper payments or benefits to
44
recipients in the public or Private sector. We or our collaborators may have direct and indirect interactions with government agencies and state-affiliated entities and universities in the course of our business We may also have certain matters come before public international organizations such as the UN. We use third-party collaborators, joint venture and strategic partners, law firms, and other representatives for regulatory compliance, patent registration, lobbying, deregulation advocacy, field testing, and other purposes in a variety of countries, including those that are known to present a high corruption risk such as India, China, and Latin American countries. We can be held liable for the corrupt or other illegal activities of these third-party collaborators, our employees, representatives, contractors, partners, and agents, even if we do not explicitly authorize such activities. In addition, although we have implemented policies and procedures to ensure compliance with anti-corruption and related laws, there can be no assurance that all of our employees, representatives, contractors, partners, or agents will comply with these laws at all times. Noncompliance with these laws could subject us to whistleblower complaints, investigations, sanctions, settlements, prosecution, other or injunctions, suspension and debarment from contracting with certain governments or other persons, the loss of export privileges, reputational harm, adverse media coverage, and other collateral consequences. If any subpoenas or investigations are launched, or governmental or other sanctions are imposed, or if we do not prevail in any possible civil or criminal litigation, our business, results of operations, and financial condition could be materially harmed. In addition, responding to any action will likely result in a materially significant diversion of management’s attention and resources and significant defense costs and other professional fees. Enforcement actions and sanctions could further harm our business, results of operations, and financial condition.
Risks Related to Our Intellectual Property
Agreements with our collaborators and third parties may not adequately prevent disclosure of trade secrets, know-how and other proprietary information, which could materially adversely affect our technology and harm our business.
We rely on a combination of intellectual property laws and other agreements with our collaborators and third parties to protect and otherwise seek to control access to, and distribution of, our proprietary information. These measures may not prevent disclosure, infringement or misappropriation of our confidential information. Our confidentiality and nondisclosure agreements or covenants may not be enforceable under applicable law and, even if they are enforceable, may be breached, and we may not have adequate remedies for such a breach that would effectively prevent the further dissemination of our confidential information or direct competition with us by a joint venture partner. We also have limited control over the protection of trade secrets used by our collaborators and could lose future trade secret protection if any unauthorized disclosure of such information occurs. Enforcement of any claim that a party illegally disclosed confidential information or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, others may independently discover our trade secrets and proprietary information, and in such cases we could not assert any trade secret rights against such parties. Laws regarding trade secret rights in certain markets where we operate may afford little or no protection of our trade secrets. If any of our trade secrets were to be disclosed to or independently developed by a competitor, or if we otherwise were to lose protection for our trade secrets or proprietary know-how, the value of this information may be greatly reduced and our business and competitive position could be harmed. Moreover, our collaborators may allege that we have disclosed their trade secrets or confidential information.
We may not be able to adequately protect our intellectual property rights throughout the world.
Our commercial success depends in part on our ability to obtain and maintain patent or other intellectual property protection and/or trade secrets protection for the technologies we develop and use. We are responsible for determining the jurisdictions in which patent protection will be pursued for our intellectual property. Filing, prosecuting, maintaining and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States are less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States and other countries in which we file for patent protection, such as Argentina, China, India, Brazil, Mexico and Australia. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products, and we may be unable to prevent such competitors from importing those infringing products into territories where we have patent protection but enforcement is not as strong due to the exhaustion of rights. These products may compete with our product candidates and our patents and other
45
intellectual property rights may not be effective or sufficient to prevent them from competing in those jurisdictions. In addition, competitors could use our patent disclosures and/or reverse engineer our trade-secret-protected products in order to produce competing products. Moreover, growers or others in the chain of commerce may raise legal challenges against our intellectual property rights or may infringe upon our intellectual property rights, including through means that may be difficult to prevent or detect. For example, in Argentina, growers may legally avoid paying royalties to the owners of intellectual property if they keep the seeds from their own harvests and plant them for personal use. Argentine legislation in respect of breeders’ rights includes a concept of a “farmer’s privilege,” which allows growers to use seeds obtained from their own harvests to be replanted on their own farm. According to the National Seed Institute of Argentina (Instituto Nacional de Semillas), the reserves of seeds kept for personal use has grown significantly in recent years, which may increase the likelihood that growers illegally claiming the privilege may use and/or sell GM seeds into the market without paying royalties owed to us.
The legal systems of certain countries, including China, where we have filed patent applications, have not historically favored the enforcement of patents or other intellectual property rights, which could hinder us from preventing the infringement of our patents or other intellectual property rights and result in substantial risks to us. Proceedings to enforce our patent rights in the United States or foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert patent infringement or other claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license from third parties.
Changes in Argentine and U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other biotech companies, our success is heavily dependent on intellectual property, including patents. Obtaining and enforcing biotech patents involves technological and legal complexity, and is costly, time consuming, and inherently uncertain. In this regard, the Argentine Patent Office (Instituto Nacional de Propiedad Intelectual) issued Regulation 283/15 with new guidelines for examining biotech inventions. These guidelines seriously restrict the patentability of several categories of inventions in the agricultural field. This restriction is already being followed in the practice of the Argentine Patent Office.
On September 2016, the Argentine Patent Office issued Regulation 56/16, under which the Argentine Patent Office will deem that any patent application whose examination had not begun by October 15, 2016 satisfies the substantive requirements of patentability (novelty, non-obviousness and industrial application); provided that a patent has been granted abroad for the same invention by a foreign patent office carrying out substantive examination in a country whose patent law has the same substantive requirements as Argentine law. This can result in prosecution times that are substantially shorter, and similar to those of the fastest jurisdictions. In particular, the patent office has applied this regulation to biotech cases as long as they are directed to matter that is not affected by the guidelines.
In addition, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. In a recent ruling (in re “Monsanto Technology LLC c/ Instituto Nacional de la Propiedad Industrial s/ Denegatoria de Patente”, case number CCF 8044/2007), Tribunal III of the Civil and Commercial Federal Court of Appeals of the City of Buenos Aires confirmed, by revoking a decision of a lower court, the rejection of a biotechnological patent application by the Argentine Patent Office, with the understanding that the invention should be protected as a PVP and not under a patent (the patent application was for a recombinant DNA molecule and a cell transformed by such molecule). Lack of inventive activity and non-patentable matters are also mentioned as grounds in this precedent. Although the Court of Appeals’ decision is now being reviewed by Argentine Supreme Court of Justice, this precedent may adversely affect patentability of the technologies we develop. Depending on decisions by the Argentine and U.S. Congresses, the federal courts in each country, the U.S. Patent and Trademark Office and the Argentine Patent Office, as well as the relevant authorities in other countries in which we hold patents, the laws and regulations governing patents could change in unpredictable ways that may weaken or undermine
46
our ability to obtain new patents or to enforce our existing patents and patents we might obtain in the future. During recent years, certain sectors of the Argentine agricultural industry have been requesting that the Argentine PVP Law is amended.
If we or one of our collaborators or licensees are sued for infringing the intellectual property rights of a third party, such litigation could be costly and time consuming and could prevent us or our collaborators or licensees from developing or commercializing products that incorporate our technology.
Our ability to generate significant revenues from our products depends on our and our joint ventures’ and licensees’ ability to develop, market and sell products and utilize our proprietary technologies without infringing the intellectual property and other rights of any third parties.
As the agricultural biotech industry continues to develop, we, our collaborators or licensees may become party to, or threatened with, litigation or other adverse proceedings regarding intellectual property or proprietary rights in our technology, processes, developed seed traits or seed treatments. Third parties may assert claims based on existing or future intellectual property rights and the outcome of any proceedings is subject to uncertainties that cannot be adequately quantified in advance. Any litigation proceedings could be costly and time consuming. A negative outcome from an intellectual property infringement suit could result in liability for monetary damages, require us to indemnify our licensees for damages arising from warranties we have made about the intellectual property we have licensed, which claims might not be subject to a cap, or treble damages and attorneys’ fees if we are found to have willfully infringed a patent. There is also no guarantee that we, our collaborators or licensees would be able to obtain a license under such infringed intellectual property on commercially reasonable terms or at all. A finding of infringement could prevent us, our collaborators or our licensees from developing, marketing or selling a product or force us to cease some or all of our business operations. Even if we are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel may be diverted as a result of these proceedings, which could have a material adverse effect on our operations. In some cases, our agreements with our collaborators or licensees might oblige us to pay for the enforcement of our owned or licensed intellectual property rights, even though our collaborators or licensees may be responsible for commercializing the potentially infringing products. Claims that we have misappropriated the confidential information or trade secrets of third parties could similarly have a negative impact on our business.
The value of our intellectual property could diminish due to technological developments or challenges by competitors, making our products less competitive.
Our intellectual property rights are important to the operation of our business and the commercialization of our crop productivity and agro-industrial products. We rely on a combination of patents, PVP, trademarks, trade secret laws, confidentiality provisions and licensing arrangements to establish and protect our intellectual property. However, the importance of technology development and intellectual property protection in the crop productivity and agro-industrial industries increases the risk that technological advances by others could render our products less competitive. Our business could be negatively affected by any of the following:
| ■ | our issued patents, PVP certificates and trademark registrations may be successfully challenged by our competitors; |
| ■ | we may be unable to obtain intellectual property licenses that are necessary or useful to our business on favorable terms, or at all; |
| ■ | new technology that is independently developed by others may supersede our technology and make our products less desirable or costlier in the marketplace; |
| ■ | competitors may design around our patent and/or PVP protections; and |
| ■ | competitors may reverse engineer our trade secret technologies. |
47
We may be required to pay royalties to employees who develop inventions that have been or will be commercialized by us, even if the rights to such inventions have been assigned to it, exclusive licenses have been granted to it and the employees have waived their rights to royalties or other additional compensation.
Under Argentine Patent and Utility Models Law No. 24,481 and Argentine Labor Law No. 20,744, which provide the legal framework related to ownership of inventions developed during an employer-employee relationship, the employer is awarded ownership of inventions when the employee was hired for the purpose of engaging in inventive discovery or when such invention otherwise derives from the knowledge acquired by virtue of the employee’s working for the employer. Depending on the nature and the scope of an employee’s contribution to an invention and the nature of his or her hiring, he or she may be entitled to additional compensation by the employer; however, the employer will still retain ownership rights on the conditions mentioned above. If an employee was hired for a purpose other than to engage in inventive discovery and he or she creates an invention that is not related to the employer’s processes, methods or business, the employee shall be the owner of the invention.
A significant portion of our employees are dedicated to activities that may be considered inventive. As a result, a significant portion of our employees execute confidentiality and ownership rights agreements upon commencement of employment whereby they agree to classify all work undertaken by them as engagement in inventive discovery, which grants us all ownership rights in inventions created while such employees are employed by us. If these assignments or exclusive licenses were deemed invalid or unenforceable, we could be required to pay royalties to our employees who have invented intellectual property that we have commercialized, which in turn may have a material adverse effect on our results of operations. In addition, if these assignments or exclusive licenses were deemed invalid or unenforceable, it is possible that our employees could assign or license to third parties their rights in any inventions created while employed by us. This could have a material adverse effect on our results of operations.
Risks Related to Our Acquisitions
Certain of the Rizobacter shares are subject to a judicial injunction.
We own 50.01% of Rizobacter’s capital stock through our subsidiary RASA Holding. Of the total shares of Rizobacter acquired by RASA Holding, 7.6 million shares (representing 19% of Rizobacter’s capital stock) are subject to a precautionary measure issued pursuant to an injunction that affects 44% of the total share capital of Rizobacter. The precautionary measure also covers 30% of the dividends distributed to such shares, directing such percentage of dividends into a judicially created escrow account. The precautionary measure relates to litigation among historical shareholders of Rizobacter arising from a disputed transfer of shares that occurred in 1995. Although the Supreme Court of Argentina ruled against certain of the litigating historical shareholders, such shareholders subsequently pursued other legal recourse—including the precautionary measure and non-innovative (medida de no innovar)—to further dispute the original transfer of shares.
We purchased our controlling stake in Rizobacter subject to the precautionary measure and associated ongoing litigation. Should such contingencies be lifted, we may be obligated to pay a contingent purchase price of US$17.3 million to certain selling shareholders of Rizobacter through RASA Holding. Conversely, should the court rule against the free transferability of the affected shares, we would be obligated to return certain shares, thereby reducing our equity participation in Rizobacter, and we would not be obligated to pay the abovementioned contingent purchase price. Given the Supreme Court of Argentina´s finding that the 1995 share transfer was valid, it is not likely or probable that our equity participation in Rizobacter will be affected and that we may be obligated to pay the contingent purchase price of US$17.3 million. The same Rizobacter shareholders who challenged the 1995 transfer requested an additional precautionary measure against the sellers and Rizobacter relating to the same disputed share transfer, in response to which Rizobacter filed a motion for reversal with an appeal, which is still pending of resolution as of the date of this prospectus. In a subsequent ruling, the Court of First Instance issued a new resolution (i) clarifying that Rizobacter does not require any judicial authorization to administer and to define the economic and financial plan of the company and (ii) limiting RASA Holding’s ability to voluntarily sell its participation in Rizobacter. RASA Holding’s ability to freely transfer the shares of Rizobacter may be restricted until the termination of the related litigation.
48
We may not be able to manage our growth successfully.
We expect that the acquisition of Rizobacter will expand our operations and that such expansion will continue. As we continue to grow, we must improve our operational, technical and managerial knowledge and compliance systems in order to effectively manage our operations across the expanded group. Failure to integrate, monitor and manage expanded operations could have a material adverse effect on our business, results of operations and financial condition.
Integration of Rizobacter involves certain risks that may have a material adverse effect on us.
We have engaged in acquisitions in the recent past, including the acquisition of Rizobacter in 2016, and may complete further mergers and acquisitions in the future as part of our growth strategy. We believe that these transactions will contribute to our continued growth and competitiveness.
Like any acquisition of companies and assets and the integration of such companies and assets, the acquisition of Rizobacter involves certain risks, including the risk that:
| ■ | integrating new networks, information systems, personnel, financial and accounting systems, risk and other management systems, financial planning and reporting, products and customer bases into our existing business may run into difficulties, cause us to incur unexpected costs and operating expenses and place additional demands on management time; |
| ■ | we may incur unexpected liabilities or contingencies relating to acquired businesses; |
| ■ | the expected operation and financial synergies and other benefits from such acquisitions may not be fully achieved; |
| ■ | the use of more cash or other financial resources on integration and implementation activities than expected; and |
| ■ | the use of more cash or other financial resources on integration and implementation activities than expected. |
If we fail to achieve the business growth opportunities, cost savings and other benefits it anticipates from mergers and acquisition transactions, or incur greater integration costs than it has estimated, our business, results of operations and financial condition may be materially and adversely affected.
We may acquire businesses or products, or form strategic alliances, in the future, and we may not realize the benefits of such acquisitions.
We plan to selectively partner, in-license or acquired key enabling technologies and businesses across our value chain that we believe will keep us on the cutting edge of our industry. We may not be able to identify appropriate targets or make acquisitions under satisfactory conditions, in particular, satisfactory price conditions. In addition, we may be unable to obtain the financing for these acquisitions under other purposes in the context of existing operations. If we acquire businesses with promising markets or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to successfully integrate them with our existing operations and company culture. We may encounter numerous difficulties in developing manufacturing and marketing any new products resulting from a strategic alliance or acquisition that delay or prevent us from realizing their expected benefits or enhancing our business We cannot assure you that, following any such acquisition, we will achieve the expected synergies to justify the transaction, which could have a material adverse effect on our business, financial conditions, earnings and prospects.
Risks Related to Operating in Latin America and Argentina
Latin America
Latin America has experienced, and may continue to experience, adverse economic or political conditions that may impact our business, financial condition and results of operations.
Our business is dependent to a certain extent upon the economic conditions prevalent in Argentina, as well as the other Latin American countries in which it currently operates, such as Uruguay, and in which it may seek to expand operations in the future, such as Brazil. Latin American countries have historically experienced uneven periods of economic growth,
49
recessions, periods of high inflation and economic instability. Recently, the economic growth rates of the economies of many Latin American countries have slowed and some have entered mild recessions. Additionally, economic and political developments in Latin America, including future economic changes or crises (such as inflation, currency devaluation or recession), government deadlock, political instability, terrorism, civil strife, changes in laws and regulations, restrictions on the repatriation of dividends or profits, expropriation or nationalization of property, restrictions on currency convertibility, volatility of the foreign exchange market and exchange controls could impact our operations and/or the market value of the ordinary shares and the ADSs and have a material adverse effect on our business, financial condition and results of operations.
Latin American governments have exercised, and continue to exercise, significant influence over the economies of the countries in which we operate, which could adversely affect our business, financial condition, results of operations and prospects.
Historically, governments in Latin America have frequently intervened in the economies of their respective countries and have occasionally made significant changes in policy and regulations. Governmental actions to control inflation and other policies and regulations have often involved, among others, price controls, currency devaluations, capital controls and tariffs. Our business, financial condition, results of operations and prospects may be adversely affected by the changes in government policies or regulations of Latin American governments, including:
| ■ | exchange rates and exchange control policies; |
| ■ | tariff and inflation control policies; |
| ■ | price control policies; |
| ■ | liquidity of domestic capital and lending markets; |
| ■ | tax policies, royalty and tax increases and retroactive tax claims; and |
| ■ | other political, diplomatic, social and economic developments in or affecting the countries in which we operate. |
Inflation and government measures to curb inflation in Latin America may adversely affect the economies in the countries in which we operate in Latin America, our business and results of operations.
Some of the countries in which we operate in Latin America have experienced, or are currently experiencing, high rates of inflation. The measures taken by the governments of some Latin American countries to control inflation have often included maintaining a tight monetary policy with high interest rates, thereby restricting the availability of credit and retarding economic growth. Inflation, measures to combat inflation and public speculation about possible additional actions have also contributed significantly to economic uncertainty in many of these countries and to heightened volatility in their securities markets.
Periods of higher inflation may also slow the growth rate of local economies. Inflation is also likely to increase some of our costs and expenses, which it may not be able to fully pass on to our customers. The occurrence of any of the above circumstances could adversely affect our operating margins and total revenues.
Argentina
Our business, results of operations and financial condition may be adversely affected by fluctuations in currency exchange rates, including between the U.S. dollar and the Argentine peso.
With the tightening of exchange controls beginning in late 2011, in particular with the introduction of measures that limited access to foreign currency by private companies and individuals (such as requiring an authorization of tax authorities to access the foreign currency exchange market), the implied exchange rate, increased significantly over the official exchange rate. Most foreign exchange restrictions have been lifted since December 2015 and, as a result, the substantial spread between the official exchange rate and the implied exchange rate derived from securities transactions has substantially decreased. See “Exchange Rates and Controls.”
In 2015, the peso depreciated 52.1% against the U.S. dollar primarily after the lifting of certain foreign exchange restrictions in the month of December. Since the devaluation in December 2015, the Central Bank has allowed the peso
50
to float and significantly limited interventions to those needed to ensure the orderly functioning of the MULC. In 2016, the peso depreciated 21.3% against the U.S. dollar. During the first seven months of 2017, the peso depreciated 7.2% against the U.S. dollar. As of September 30, 2017, the peso-U.S. dollar exchange rate was Ps.17.32 to US$1.00. We are unable to predict the future value of the peso against the U.S. dollar. If the peso continues to devalue, all or some of the negative effects on the Argentine economy related to such devaluation could reappear.
Our consolidated financial statements as included elsewhere herein are presented in U.S. dollars. Therefore, the resulting exchange differences arising from the translation of balances and transactions in Argentine pesos to U.S. dollars are recognized in the financial gain or expense item. Fluctuations in exchange rates relative to the U.S. dollar could impair the comparability of our results from period to period and could have a material adverse effect on our results of operations and financial condition. In addition, our results of operations and financial condition are affected by changes in the Argentine peso to U.S. dollar exchange rate because the majority of our operations are conducted in Argentina and, accordingly, a significant portion of our costs are incurred in Argentine pesos, while our revenues are primarily denominated in or influenced by U.S. dollars. Consequently, appreciation of the U.S. dollar relative to the Argentine peso, to the extent not offset by inflation in Argentina, could result in favorable variations in our operating margins and, conversely, appreciation of the Argentine peso against the U.S. dollar may raise our costs in U.S. dollars, which would increase the prices of our commercial technology, products and services to our customers, which, in turn, could adversely affect our business and results of operations and cause significant variability in our results of operations from period to period.
The devaluation of the Argentine peso has had a negative impact on the ability of certain Argentine businesses to honor their foreign currency-denominated debt and has also led to very high inflation and significantly reduced real wages. If the Argentine peso is further significantly devalued, the Argentine economy and our business could be adversely affected. Finally, certain restrictions on the purchase of foreign currency have given rise to the development of an implied rate of exchange. Significant variations in the comparative value of the Argentine peso to the U.S. dollar could adversely affect our business and results of operations.
Government intervention in the Argentine economy could adversely affect the economy and our financial condition and results of operations.
During recent years, the Argentine government increased its direct intervention in the economy, including through the implementation of regulation of market conditions, expropriations or nationalizations and price controls.
In April 2012, the Fernández de Kirchner administration decreed the removal of directors and senior officers of YPF, Argentina’s largest oil and gas company, which was controlled by the Spanish group Repsol, and submitted a bill to the Argentine Congress to expropriate shares held by Repsol representing 51% of the shares of YPF. The Argentine Congress approved the bill in May 2012 through the passage of Law No. 26,741, which declared the production, industrialization, transportation and marketing of hydrocarbons to be activities of public interest and fundamental policies of Argentina, and empowered the Argentine government to adopt any measures necessary to achieve self-sufficiency in hydrocarbon supply. In February 2014, the Argentine government and Repsol announced that they had reached an agreement on the terms of the compensation payable to Repsol for the expropriation of the YPF shares. Such compensation totaled US$5.0 billion (Ps.79.3 billion) payable by delivery of Argentine sovereign bonds with various maturities. The agreement, which was ratified by Law No. 26,932, settled the claim filed by Repsol with the ICSID.
In December 2012 and July 2013, the Argentine Congress established new regulations relating to domestic capital markets. The new regulations generally provide for increased intervention in the capital markets by the government, authorizing, for example, the CNV to appoint observers with the ability to veto the decisions of the board of directors of companies admitted to the public offering regime under certain circumstances and suspend the board of directors for a period of up to 180 days. In November 2017, the Argentine executive branch submitted a bill to the Argentine Congress to reform the current Argentine Capital Markets Law which, among other changes, proposes the abrogation of this power granted to the CNV and generally seeks to modernize the entire regulatory framework applicable to Argentine capital markets, incorporating current international practices to contribute to its development. However, as of the date of this prospectus, such bill has not yet been passed.
51
In September 2014, the Fernández de Kirchner administration enacted a law that enables the federal government to intervene in certain markets when it considers that any party to such market is trying to impose prices or supply restrictions in such market. This law applies to all economic processes linked to goods, facilities and services which, either directly or indirectly, satisfy basic needs of the population (so-called “basic needs goods”), and grants broad powers to the relevant enforcing agency to become involved in such processes. It also empowers the enforcing agency to order the sale, production, distribution and/or delivery of basic needs goods throughout the country in case of a shortage of supply.
In May 2016, the Argentine congress barred companies from laying off workers for a 180-day period in a law later vetoed by President Macri. The law has returned to the Argentine congress where it would need special majorities to override the veto.
Substantially all of our assets are located in Argentina. Therefore, we are subject to political uncertainties, including expropriation or nationalization of our business or assets, or subject to renegotiation or annulment of existing contracts and other similar risk, although the current administration has not taken an interventionist approach. In the future, intervention in the economy by the Argentine government may continue or increase, the occurrence of which may adversely affect Argentina’s economy and, in turn, our business, results of operations and financial condition. We cannot assure investors that these or other measures that may be adopted by the Argentine government in the future in response to social unrest, such as nationalizations, intervention by the CNV, forced renegotiations or modifications of existing contracts, new tax policies, price fixing, regulations and reforms affecting foreign trade and investments, will not have a material adverse effect on the Argentine economy and, consequently, will not adversely affect our business, results of operations and financial condition.
Political developments in Argentina could adversely affect the Argentine economy
Presidential and congressional elections in Argentina took place and a runoff election (ballotage) between the two leading presidential candidates was held on November 22, 2015, which resulted in Mr. Mauricio Macri being elected President of Argentina. The Macri administration assumed office on December 10, 2015.
Since assuming office, the Macri administration has announced and implemented several significant economic and policy reforms, including:
| ■ | INDEC reforms. In January 2016, based on its determination that INDEC had failed to produce reliable statistical information, the Macri administration declared the national statistical system and the INDEC in a state of administrative emergency. INDEC is implementing certain methodological reforms and adjusting certain macroeconomic statistics on the basis of these reforms which enabled a readjustment of Argentine duties towards the IMF. As of the date of this prospectus, INDEC has begun publishing certain revised data, including foreign trade and balance of payment statistics. On June 15, 2016, INDEC began publishing inflation rates. Using its new methodology for calculating the CPI, inflation from May to December 2016 was 15.8%. On June 29. 2017, INDEC also published revised GDP data for the years 2004 through 2015. |
| ■ | Agreement with holdout creditors. The Macri administration has settled the substantial majority of outstanding claims brought by holdout creditors and has issued sovereign bonds in the international financial markets passed by Congress through Law No. 27,249. Although the size of the claims involved has decreased significantly, litigation initiated by bondholders that have not accepted Argentina’s settlement offer continues in several jurisdictions. |
| ■ | Foreign exchange reforms. The Macri administration eliminated most foreign exchange restrictions, including certain currency controls, which were imposed by the previous administration. See “Exchange Rates and Controls.” |
| ■ | Foreign trade reforms. The Macri administration has eliminated export duties on wheat, corn, beef and other regional products, and reduced duties on soybeans by 5% from 35% to 30%. Furthermore, the 5% export duty on most industrial exports was eliminated. With respect to payments for imports of goods and services, the Macri administration announced the elimination of amount limitations for access to the Foreign Exchange Market for |
52
any new transactions as of December 17, 2015, and for existing debts for imports of goods and services as of April 22, 2016. On January 2, 2017, the federal government enacted a further reduction of the export duties rate set for soybean and soybean products, setting a monthly 0.5% cut on the export duties rate beginning on January 2018 and until December 2019.
| ■ | Fiscal policy. The Macri administration took steps to anchor fiscal accounts, reduce the primary fiscal deficit, eliminate subsidies, reorganize certain expenditures and generate increased revenue through a tax amnesty program. The fiscal deficit for 2016 was approximately 4.6% of GDP, 0.2% lower than expected; reducing fiscal deficit is one of the most important objectives for the administration in the coming years. |
| ■ | Correction of monetary imbalances. The Macri administration has adopted an inflation targeting regime in parallel with the floating exchange rate regime and set inflation targets for the next four years. The Central Bank has increased stabilization efforts to reduce excess monetary imbalances and raised peso interest rates to offset inflationary pressure. The Central Bank also announced inflation target ranges for 2017 (12% to 17%); 2018 (8% to 12%); and 2019 (3.5% to 6.5%). See “—Continuing high inflation may have a negative effect on the Argentine economy and our financial performance.” |
| ■ | Tax Amnesty Law. On June 29, 2016, the Argentine Congress passed law No. 27,260, which became effective on July 22, 2016 and provides for a tax amnesty regime and tax reform. This regime allowed individuals and entities to disclose undeclared assets both abroad and in Argentina, under the conditions set forth in the law and within a period extending from its effectiveness until March 31, 2017, without the need to repatriate such assets to Argentina and without penalty (other than charges described below) or the need to explain the source of the funds, among other benefits. The law also provides that there will be no charge on assets worth up to US$25,000, and a discounted applicable tax of 5% on property and assets worth up to US$80,000. Above that threshold, the applicable tax was 10% until the end of 2016 and 15% until the end of March 2017, when the amnesty window closed. |
| ■ | National Electricity State of Emergency and Reforms. Following years of very limited investment in the domestic energy sector, as well as a continued freeze on electrical power and natural gas tariffs since the 2001-2002 economic crisis, Argentina began to experience energy shortages in 2011. In response to the growing energy deficit left by the prior government, the Macri administration, upon assuming office, declared a state of emergency with respect to the national electrical power system, which will remain in effect until December 31, 2017. The state of emergency allows the federal government to take actions designed to ensure the supply of electrical power to the country, such as instructing the Ministry of Energy and Mining to design and implement, with the cooperation of all federal public entities, a coordinated program to guarantee the quality and security of the electrical power system. In addition, the Macri administration announced the elimination of certain energy subsidies and a substantial increase in electrical power rates. |
| ■ | Proposed New Capital Markets Law. The Macri administration is also focused on expanding and improving the capital markets. On November 17, 2016, the new administration submitted a bill to Argentine congress to reform the Argentine Capital Markets Law in order to foster the development of the local capital markets and attract investments. Though this proposed legislation has not been approved yet, it remains a key point in the agenda of the Macri administration. |
| ■ | Corporate Criminal Liability Bill (Proyecto de Ley de Responsabilidad Penal Empresaria). On November 8, 2017, the Argentine Congress passed a law which provides for the criminal liability of corporate entities when the following crimes are committed, directly or indirectly, with their intervention or on their behalf, interest or benefit: (a) local or international bribery and influence peddling, (b) negotiations that are incompatible with public office, (c) illegal payments made to public officials under the appearance of taxes or fees owed to the relevant government agency (concusión), (d) illegal enrichment of public officers and employees, and (e) producing knowingly false balance sheets and reports to cover up local or international bribery or influence peddling. Companies found liable for committing such crimes may be subject to various sanctions, including, among others, fines ranging from two to five times the “undue” benefit that was obtained or that could have been obtained through the actions incurred in breach of this regulation. Additionally, Companies found liable may forfeit assets obtained through the illegal actions. The law will become effective 90 days after its promulgation by the President of Argentina and published in the Argentine Official Gazette. |
53
| ■ | Amendment to Labor Risks Law. On February 15, 2017, the Argentine congress passed Law 27,348, which amends and complements Labor Risks Law No. 24,557, or the Labor Risks Law, and aims to reduce litigation arising from accidents at work. Under the new regime, prior to filing a lawsuit resulting from work-related accidents, affected workers must go through jurisdictional medical commissions, in order to assess the impact of any accident and to assign benefits provided for under the Labor Risks Law. |
| ■ | Draft Bill for Productive Financing. On November 13, 2017, the Macri Administration submitted to the Argentine congress a draft bill that aims to develop Argentina’s capital markets. The draft bill amends and updates the Argentine Capital Markets Law, the Mutual Funds Law and the Argentine Negotiable Obligations Law, among others. Furthermore, the bill amends certain tax provisions, regulates relating to derivatives and promotes a financial inclusion program. On November 22, 2017, the draft bill was passed by the Argentine congress and it has been sent to the Argentine senate. The draft bill is expected to be considered in extraordinary sessions prior to the end of 2017. |
| ■ | Labor and Social Security Reform Draft Bill. The Macri Administration recently announced a draft bill to reform labor and social security which was sent to the Argentine congress for debate on November 21, 2017. On November 29, 2017, the draft bill was passed by the Argentine senate, and sent to the Argentine congress the same day. The draft bill aims to improve competitiveness and efficiency of various sectors, increase employment, attract investment and reduce labor costs. |
| ■ | Tax Regime. On October 31, 2017, the Macri Administration announced its intention to submit a series of tax and social security reforms to the Argentine congress for consideration prior to the end of 2017. On November 15, 2017, a draft bill was sent to the Argentine congress including reforms which are intended to eliminate certain existing complexities and inefficiencies of the Argentine tax regime, reduce tax evasion, increase the coverage of income tax as applied to individuals and encourage investment while sustaining the Macri Administration’s medium- and long-term efforts aimed at restoring fiscal balance. The proposed reforms are part of a larger program announced by the Macri Administration to increase the competitiveness of the Argentine economy (including the reduction of the fiscal deficit) as well as employment and diminish poverty on a sustainable basis. |
Some of the measures proposed by the Macri administration may generate political and social opposition, which may in turn prevent the new government from adopting such measures as proposed. Political parties opposed to the Macri administration retained a majority of the seats in both chambers of the Argentine Congress in the last elections, which will require the Macri administration to seek political support from the opposition for its economic proposals. In October 2017, mid-term congressional elections were held in Argentina. Although President Macri’s governing coalition obtained the largest share of the votes at the national level, it continues without a majority in either chamber of congress. Such circumstances create further uncertainty in the ability of the Macri administration to pass legislation required to implement its proposals.
The fiscal, monetary and currency adjustments undertaken by the Macri administration may subdue growth in the short-term. For example, immediately after most of the foreign exchange controls were lifted on December 10, 2015, the dismantling of the multiple exchange regime resulted in the official peso exchange rate (available only for certain types of transactions) falling in value by 40.1%, as the peso-U.S. dollar exchange rate reached Ps.13.76 to US$1.00 on December 17, 2015. As of September 30, 2017, the peso-U.S. dollar exchange rate was Ps.17.32 to US$1.00.
As of the date of this prospectus, the impact that these measures and any future measures taken by the Macri administration will have on the Argentine economy cannot be predicted. The proposed deregulation could be disruptive to the economy and fail to benefit, or harm, our business. The failure of these measures to achieve their intended goals could adversely affect the Argentine economy and our business, financial condition and results of operations.
The credibility of several Argentine economic indexes has been called into question, which may lead to a lack of confidence in the Argentine economy and may in turn limit our ability to access the credit and capital markets
Since 2007, INDEC, which is the only institution in Argentina with the statutory authority to produce official national statistics, has experienced a process of institutional and methodological reforms that have given rise to controversy with respect to the reliability of the information that it produces including inflation, GDP and unemployment data. In spite of
54
the Macri administration’s recent reforms, the credibility of the CPI, as well as other indexes published by INDEC has been affected, with allegations that the inflation rate in Argentina and the other rates calculated by INDEC could be substantially different than as indicated in official reports.
Reports published by the IMF stated that their staff uses alternative measures of inflation for macroeconomic surveillance, including data produced by private sources, which have shown inflation rates considerably higher than those published by INDEC since 2007. The IMF has also censured Argentina for failing to make sufficient progress, as required under the Articles of Agreement of the IMF, in adopting remedial measures to address the quality of official data, including inflation and GDP data.
In February 2014, INDEC released a new inflation index, known as National Urban Consumer Price Index (Índice de Precios al Consumidor Nacional Urbano) that measures prices on goods across the country and replaces the previous index that only measured inflation in the urban sprawl of the City of Buenos Aires. Even though the new methodology brought inflation statistics closer to those estimated by private sources, material differences between recent official inflation data and private estimates remained during 2015.
However, during December 2015 and January 2016, the Macri administration declared the national statistical system and INDEC to be in a state of administrative emergency through December 31, 2016, and announced that INDEC would implement certain methodological reforms and adjust certain macroeconomic statistics on the basis of these reforms. Accordingly, the new head of INDEC announced the decision to temporarily suspend the publication of official data on prices, poverty, unemployment and GDP until a full review of the institution is completed. In the meantime, the Macri administration released an alternative CPI index based on data from the City of Buenos Aires and the province of San Luis. After implementing certain methodological reforms and adjusting certain macroeconomic statistics on the basis of these reforms, in June 2016 INDEC resumed its CPI publications and revised GDP data for the years 2004 through 2015. Among other adjustments, in calculating GDP for 2004, INDEC made changes to the composition of GDP that resulted in a downward adjustment of approximately 12% for that year. In calculating real GDP for subsequent years based on the revised 2004 GDP, INDEC used deflators that are consistent with its revised methodology to calculate inflation. By understating inflation in the past, INDEC had overstated growth in real terms. The adjustments made by INDEC resulted in a determination of real GDP growth for the period 2004-15 of 48.6%, as opposed to a 65% growth in real terms for the same period resulting from the information used prior to June 2016. As a consequence of these reforms, on November 9, 2016, the Executive Board of the IMF lifted its censure on Argentina, noting that Argentina had resumed the publication of data in a manner consistent with its obligations under the Articles of Agreement of the IMF. Still, uncertainty remains as to whether official data and measurement procedures sufficiently reflect inflation in the country, and what effect these reforms will have on the Argentine economy.
As of the date of this prospectus, the impact that these measures and other future measures taken by the Macri administration with respect to INDEC could have on the Argentine economy and investors’ perception of the country cannot be predicted.
Continuing high inflation may have a negative effect on the Argentine economy and on our financial performance
Inflation has, in the past, materially undermined the Argentine economy and the government’s ability to foster conditions that would permit stable growth. In recent years, Argentina has confronted inflationary pressures, evidenced by significantly higher fuel, energy and food prices, among other factors.
On January 8, 2016, Decree No. 55/2016 was issued by the federal government declaring a state of administrative emergency on the national statistical system and on INDEC, until December 31, 2016. INDEC ceased publishing statistical data until a rearrangement of its technical and administrative structure was finalized. After the process of reorganization, on June 15, 2016, INDEC began releasing official measurements of its primary indication of inflation, the CPI. INDEC reported that the monthly CPI increase in 2017 was 1.3% in January, 2.5% in February, 2.4% in March, 2.6% in April, 1.3% in May, 1.2% in June, 1.7% in July, 1.9% in August, 1.9% in September and 2.3% in October. INDEC has also published inflation figures for the WPI, for 2017, reporting monthly increases of 1.5% in January, 1.7% in February, 0.9% in March, 0.5% in April, 0.9% in May, 1.9% in June and 2.6% in July, 1.9% in August, 1.0% in September and 2.3% in October. The WPI for the year ended December 31, 2016 showed an annual increase of 34.5%. The WPI for the Transition Period showed an increase of 7.4%.
55
Inflation rates could escalate in the future, and there is uncertainty regarding the effects that the measures adopted, or that may be adopted in the future, by the Argentine government to control inflation may have. If inflation remains high or continues to rise, Argentina’s economy may be negatively impacted and our results of operations could be materially affected.
Argentina’s defaults with respect to the payment of its foreign debt could prevent the government and the private sector from accessing the international capital markets, which could adversely affect our financial condition, including its ability to obtain financing outside of Argentina.
Argentina’s 2001 sovereign default and its failure to fully restructure its sovereign debt and negotiate with the holdout creditors has limited and may continue to limit Argentina’s ability to access international financing. In 2005, Argentina completed the restructuring of a substantial portion of its indebtedness and settled all of its debt with the IMF. Additionally, in June 2010, Argentina completed the restructuring of a significant portion of the defaulted bonds that were not swapped in the 2005 restructuring. As a result of debt exchanges in 2005 and 2010, Argentina restructured approximately 91% of its defaulted debt that was eligible for restructuring. Holdout bondholders that declined to participate in the restructurings, however, filed lawsuits against Argentina in several countries, including the United States. Since late 2012, rulings from courts in the United States favorable to holdout bondholders exacerbated investors’ concerns about investing in the country.
In November 2012, the United States District Court for the Southern District of New York ratified the injunction order issued on February 23, 2012, which held that Argentina had violated the pari passu clause with respect to the bondholders that had not participated in the sovereign debt swaps in 2005 and 2010, and as a consequence was required pursuant to the District Court’s ruling to pay 100% of the amounts due to the plaintiffs together with the payment of the amounts due on the next maturity date to bondholders who had participated in the debt swaps. In June 2014, the U.S. Supreme Court denied Argentina’s appeal for certiorari of the Second Circuit Court of Appeals’ ruling affirming the District Court judgment. That same month, the District Court ruled that funds should not be delivered to the holders of restructured debt in the absence of a prior agreement with the holdout bondholders. In June 2015, the Second Circuit granted partial summary judgment to a group of “me-too” plaintiffs in 36 separate lawsuits, finding that, consistent with the previous ruling of such court, Argentina violated a pari passu clause in bonds issued to the “me-too” bondholders.
In February 2016, the Macri administration reached agreements in principle with certain holdout bondholders to settle these claims, which were subject to the approval of the Argentine Congress and the lifting of the pari passu injunctions. In March 2016, after the District Court agreed to vacate the pari passu injunctions subject to certain conditions, the Argentine Congress ratified these settlement agreements through Law No. 27,249 and repealed the so-called Lock Law No. 26,017 and the Sovereign Payment Law No. 26,984, which prohibited Argentina to offer to holdout bondholders more favorable terms than those offered in the 2005 and 2010 debt swaps. In recent months, the Argentine government has reached settlement agreements with holders of a significant portion of the defaulted bonds and has repaid the majority of the holdouts creditors with the proceeds from a US$16.5 billion international offering of 3-year, 5-year, 10-year and 30-year bonds on April 22, 2016. Through this offering, Argentina regained access to the international capital markets. Although the size of the claims involved has decreased significantly, litigation initiated by bondholders that have not accepted Argentina’s settlement offer continues in several jurisdictions.
Additionally, foreign shareholders of several Argentine companies have filed claims with ICSID, alleging that the emergency measures adopted by the Argentine government since the crisis in 2001 and 2002 differ from the just and equal treatment standards set forth in several bilateral investment treaties to which Argentina is a party. Many of these claims have been ruled against Argentina.
Holdout creditors litigation, as well as ICSID and other claims against the Argentine government, have resulted and may result in new material judgments against the government, lead to attachments of or injunctions relating to Argentina’s assets, or could bring Argentina in default of its other obligations, and such event may prevent Argentina from obtaining favorable terms or interest rates when accessing international capital markets or from accessing international financing at all. The termination of the injunctions issued by the U.S. courts preventing bondholders from receiving their interest payments on the bonds issued pursuant to the 2005 and 2010 exchange offers and the related subsequent events have paved the way for the Argentine government to regain access to the international capital markets. Nonetheless,
56
Argentina’s ability to obtain international or multilateral private financing or direct foreign investment may be limited, which may in turn impair its ability to implement reforms and public policies to foster economic growth. In addition, Argentina’s ongoing litigation with the remaining holdout creditors as well as ICSID and other claims against the Argentine government, or any future defaults by Argentina with its financial obligations, may prevent Argentine companies, such as us, from accessing the international capital markets or make the terms of any such transactions less favorable than those provided to companies in other countries in the region, potentially impacting our financial condition.
An increase in export and import duties and controls may have an adverse impact on our business.
Since 2002, the Argentine government has imposed duties on the exports of various primary and manufactured products. During the last ten years, such export taxes have undergone significant increases, reaching a maximum of 35% in the case of soybeans.
As of December 2015, the current administration has eliminated farm export duties on corn, wheat and other agricultural products such as rice and alfalfa, while soy export taxes were reduced by 5% to a 30% tariff for soybean and 27% for most soybean products.
On January 2, 2017, the current administration enacted a further reduction of the export duties rate set for soybean and soybean products. A monthly 0.5% cut on the export duties rate starting on January 2018 and until December 2019 has been set.
The government imposed the SIMI in December 2015. Under this new system, importers are required to submit certain information electronically through the SIMI application which, once approved, will be valid for 180 calendar days.
The Argentine government has also enacted an import licensing regime that includes automatic and non-automatic licensing for imports according to the tariff codes of the goods to be imported. Automatic import licensing implies that the importer must only get through the SIMI and any other certification related to the imported goods. Non-automatic licensing implies that the authorities also have a ten-day term to either approve or reasonably reject the import license requested due to its effect on local businesses, aside from the other import requirements that the goods may have (including SIMI and certifications).
Notwithstanding the above, we cannot make assurances or predictions that there will not be further increases in the export taxes or that other new export taxes or quotas will not be imposed. The imposition of new export taxes or quotas or a significant increase in existing export taxes or the application of export quotas or the imposition of regimes that aim to restrict or control imports and exports could adversely affect our financial condition or results of operations.
The implementation of future exchange controls and restrictions on capital inflows and outflows could limit the availability of international credit, adversely affecting the Argentine economy, and, as a result, our financial condition and results of operations.
Starting in 2001, and increasingly from 2011 until President Macri assumed office in December 2015, the Argentine government increased foreign exchange controls. Together with regulations established in 2012 that subjected certain foreign exchange transactions to prior approval by Argentine tax authorities or the Central Bank, the measures taken by the previous administration significantly curtailed access to the foreign exchange market by Argentine and non-Argentine individuals and private sector entities. In response, an unofficial U.S. dollar trading market developed in which the peso-U.S. dollar exchange rate differed substantially from the official peso-U.S. dollar exchange rate. See “Exchange Rates and Controls.”
In the past, the Argentine government also imposed informal restrictions, such as limitations on the ability of certain local companies and individuals to purchase foreign currency. These restrictions on foreign currency purchases started in October 2011 and tightened during 2012 through 2014 until the end of 2015. Informal restrictions may consist of de facto measures restricting local residents and companies from purchasing foreign currency through the foreign exchange market to make payments abroad, such as dividends, capital reductions, and payment for importation of goods and services.
57
In the future, the Argentine government could reinstate exchange controls, transfer restrictions, require repatriation through the MULC of proceeds raised through capital markets transactions conducted abroad or place restrictions on the movement of capital and take other measures in response to capital flight or a significant depreciation of the Argentine peso, all of which could limit our ability to access the international capital markets. Such measures could lead to political and social tensions and undermine the Argentine government’s public finances, as has occurred in the past, which could adversely affect Argentina’s economy and prospects for economic growth, which, in turn, could adversely affect our business and results of operations and the market value of our ordinary shares and the ADSs.
In addition, the Argentine government or the Central Bank may reinstate or impose new restrictions on the transfers of funds abroad, impairing our ability to make dividend payments to holders of the ADSs, which may adversely affect the market value of the ADSs.
Exchange controls which were in place in the previous administration affected the level of international reserves deposited with the Central Bank, which significantly decreased from US$47.4 billion (Ps.723.5 billion) as of November 1, 2011 to US$25.6 billion (Ps.332.9 billion) as of December 31, 2015, resulting in a reduced capacity of the Argentine government to intervene in the foreign exchange market and to provide access to such markets to private sector entities like us. As of September 30, 2017, the level of international reserves deposited with the Central Bank was US$50.2 billion (Ps.870.0 billion). Notwithstanding the measures adopted by the new administration, in the future the Argentine government could otherwise reduce the level of international reserves deposited with the Central Bank, which could lead to political and social tensions and undermine the Argentine government’s public finances, as has occurred in the past, which could adversely affect Argentina’s economy and prospects for economic growth.
The Argentine government may order salary increases for employees in the private sector, which could increase our operating costs and adversely affect our results of operations.
In the past, the Argentine government has passed laws, regulations and decrees requiring companies in the private sector to increase wages and provide specified benefits to employees and may do so again in the future. Argentine employers, both in the public and private sectors, have experienced significant pressure from their employees and labor organizations to increase wages and to provide additional employee benefits. Due to the high levels of inflation, employees and labor organizations are demanding significant wage increases. In August 2012, the Argentine government established a 25% increase in the minimum monthly salary to Ps. 2,875, effective as of February 2013. The Argentine government increased the minimum salary to Ps.3,300 in August 2013, to Ps.3,600 in January 2014, to Ps.4,400 in September 2014 and to Ps.4,716 in January 2015 to Ps.5,588 in August 2015, to 6,060 in January 2016, to Ps.6,810 in June 2016, to Ps.7,560 in September 2016, and to Ps.8,860 in September 2017. Recently, the INDEC published data regarding the evolution of salaries in the private and public sectors, which reflects approximately 32.91% and 32.58% salary increase in the private and public sectors, respectively, for the period from November 2015 through December 2016. As of August 31, 2017, labor unions agreed annual salary increases with employers’ associations of between 22% and 25%.
Due to high levels of inflation, employers in both the public and private sectors have historically experienced, and currently are experiencing, significant pressure from unions and their employees to further increase salaries. If, as a result of such measures, future salary increases in the Argentine peso exceed the pace of the devaluation of the Argentine peso, this could have a material and adverse effect on our costs and business, results of operations and financial condition.
Risks Related to our Ordinary Shares and the Offer
Our ADSs and ordinary shares have not previously been traded on stock exchanges and, therefore, an active and liquid market for the trading of our ADSs and the ordinary shares underlying the ADSs may not develop.
Before this global offering, our ordinary shares, including in the form of ADSs, were not traded on any stock exchange. In connection with this global offering, we will apply to list ADSs representing our ordinary shares on the NYSE and our ordinary shares on the BYMA. An active and liquid market for trading may not develop or, if developed, may not be able to maintain itself. The investment in marketable securities of issuers located in emerging countries, such as Argentina, usually represents higher levels of risk as compared to investments in securities issued in countries whose political and
58
economic situations are more stable, and in general, such investments are considered speculative in nature. Furthermore, if an active public market for our ADSs and ordinary shares does not develop on the NYSE and the BYMA following the completion of this global offering, the market price and liquidity of our ordinary shares, including in the form of ADSs, may be materially and adversely affected.
The initial public offering price for our ordinary shares and ADSs will be determined by negotiation between us and the underwriters based upon several factors, and the trading price of our ordinary shares and ADSs after this global offering may decline below the initial public offering price. As a result, investors may experience a significant decrease in the market price of our ordinary shares and ADSs.
Purchasers in this offering will experience immediate and substantial dilution in the book value of their investment, and may experience further dilution if our executives and directors exercise stock options granted to them or if we issue stock grants in the future.
The initial public offering price will be substantially higher than the net tangible book value per ADS immediately after this offering. Therefore, if you purchase ADSs in this offering, you will experience immediate and substantial dilution of approximately US$ per ADS in the price you pay for ADSs that represent our ordinary shares as compared to its net tangible book value as of September 30, 2017, assuming an initial public offering price of US$ per ADS, the midpoint of the price range set forth on the cover page of this prospectus. In addition, following this offering, purchasers in the international offering will have contributed approximately % of the total consideration paid by our equity holders to purchase ADSs, in exchange for acquiring approximately % of our total ordinary shares, including in the form of ADSs, as of September 30, 2017, assuming an initial public offering price of US$ per outstanding ordinary share, including in the form of ADSs, the midpoint of the price range set forth on the cover of this prospectus.
In April 2016, we entered into a convertible loan agreement with Monsanto (through Monsanto Argentina, S.R.L.) and BAF, as a financial investor, pursuant to which we issued promissory notes granting Monsanto and BAF participation rights to subscribe to a total nominal value of US$17.55 million of our ordinary shares in the event of a qualified or non-qualified financing and received US$15.0 million net proceeds, after applying a 17% subscription discount. The terms of the promissory notes dictate that after a two-year grace period, (i) the notes will begin to accrue interest at an 8% annual rate and (ii) the face amount of the promissory notes will be increased by an additional 10%. The promissory notes mature after the first of a qualified or unqualified financing event, such as this offering, or five years. The promissory notes are subject to mandatory conversion upon, among other events, a qualified financing event, such as an offering or private placement, the net proceeds of which exceed US$30 million (net of the Monsanto and BAF participation rights) and for which at least 30% of the offering is placed among new shareholders, and optional conversion upon a non-qualified public offering. The conversion price per share of the promissory note is equal to either the price per share of this offering or the price per share of a qualifying private placement. See “Participation Rights.” The loan agreement executed in connection with the promissory note contains customary representations, warranties and covenants. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Indebtedness — Promissory Notes to be Offset through Participation Rights.”
We also granted participation rights in October 2016 and May 2017 to the holders of preferred shares of RASA Holding for the subscription to ordinary shares in the event of a qualified or non-qualified financing. The participation rights become mandatory in the event of a qualified financing, such as a public offering, of at least US$50 million, net of proceeds received from holders of preferred shares of RASA Holding exercising their mandatory participation rights. The holders of preferred shares of RASA Holding shall be entitled to subscribe to the number of ordinary shares at the time of the qualified financing event in addition to the dividends accrued at that time, if any. As of June 30, 2017, there were 1,409,848 Class A preferred shares (excluding preferred dividends) held by third parties, which accrue an annual PIK (“pay in kind”) dividend coupon of 12%. Assuming that the Argentine offering occurs before December 31, 2017, the holders of preferred shares of RASA Holding will be entitled to subscribe to 1,982,970 ordinary shares at the offering. Proceeds will be used to acquire the remaining preferred shares of RASA Holding not currently owned. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations — Indebtedness — Promissory Notes to be Offset through Participation Rights.”
On December 17, 2014, shareholders representing 63.68% of our corporate capital approved the issuance of stock options exercisable in respect of up to 1,264,000 ordinary shares under our stock option incentive plan and stock grants
59
of up to 1,264,000 ordinary shares under our stock grant incentive plan. Of these stock options, our board authorized the issuance of stock options with respect to 929,040 ordinary shares under the stock option incentive plan, with an exercise price of US$7.91 per share, to certain of our executives, officers and directors with whom we had executed individual stock option agreements on December 16, 2015. Our board authorized the issuance of stock grants with respect to 902,487 ordinary shares under the stock grant incentive plan to certain of our executives, officers and directors. We expect our compensation committee to execute individual stock grant agreements in the second quarter of the current fiscal year.
We do not expect to declare any dividends in the foreseeable future.
We have never declared or paid any cash dividends on our ordinary shares, and we do not anticipate paying any cash dividends on our ordinary shares, including in the form of ADSs, in the foreseeable future. After the completion of this offering, we do not anticipate declaring any cash dividends to holders of our ADS, or the ordinary shares represented thereby, in the foreseeable future. We currently intend to retain all future earnings to fund the development and growth of our business. Any payment of future dividends will be at the proposal of our board of directors, and approval of shareholders, and will depend on, among other things, our earnings, financial condition, capital requirements, level of indebtedness, statutory and contractual restrictions applying to the payment of dividends and other considerations that the board of directors and shareholders deem relevant. Consequently, investors may need to rely on sales of their ADSs, and the ordinary shares represented thereby, after price appreciation, which may never occur, as the only way to realize any future gains on their investment. Investors seeking cash dividends should not purchase our ADSs.
As a foreign private issuer, we will not be subject to U.S. proxy rules and will be exempt from filing certain Exchange Act reports.
As a foreign private issuer, we will be exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors, and principal shareholders will be exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file annual and current reports and financial statements with the SEC as frequently or as promptly as domestic companies whose securities are registered under the Exchange Act, and we will generally be exempt from filing quarterly reports with the SEC under the Exchange Act.
In addition, we would lose our foreign private issuer status if a majority of our directors or executive officers are U.S. citizens or residents and we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. Although we have elected to comply with certain U.S. regulatory provisions, our loss of foreign private issuer status would make such provisions mandatory. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer may be significantly higher. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. We would have to present our financial statements under U.S. GAAP and may also be required to modify certain of our policies to comply with corporate governance practices applicable to U.S. domestic issuers. Such conversion and modifications will involve additional costs. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers.
We are an “emerging growth company” and we cannot be certain whether the reduced requirements applicable to emerging growth companies will make our ADSs less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we may take advantage of certain exemptions from various requirements that are applicable to other public companies that are not “emerging growth companies.” For so long as we remain an “emerging growth company,” we will not be subject to the provision of Section 404(b) of the Sarbanes-Oxley Act that requires that our independent registered public accounting firm to provide an attestation report on the effectiveness of our internal control over financial reporting. This may increase the risk that we fail to be aware of and remedy any material weaknesses or significant deficiencies in our internal control over financial reporting. We have also elected to include two years of audited consolidated financial statements and selected financial data. In general, these reduced reporting requirements may allow us to refrain from disclosing information that you may find important. We have irrevocably elected not to avail ourselves of the election to delay adopting new or revised accounting standards
60
until such time as those standards apply to private companies. Nevertheless, as a foreign private issuer that is an emerging growth company, we will not be required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act for up to five fiscal years after the date of completion of this offering. We will remain an emerging growth company until the earliest of: (a) the last day of our fiscal year during which we have total annual gross revenues of at least US$1.07 billion; (b) the last day of our fiscal year following the fifth anniversary of the completion of this offering; (c) the date on which we have, during the previous three-year period, issued more than US$1.0 billion in non-convertible debt; or (d) the date on which we are deemed to be a “large accelerated filer” under the Exchange Act. When we are no longer deemed to be an emerging growth company, we will not be entitled to the exemptions provided in the JOBS Act. We cannot predict if investors will find our ADSs less attractive as a result of our reliance on exemptions under the JOBS Act. If some investors find our ADSs less attractive as a result, there may be a less active trading market for our ADSs and our share price may be more volatile.
The market price of our ADSs could be negatively affected by future sales of our ADSs or ordinary shares.
After this offering, we will have ADSs and ordinary shares outstanding. If we or our shareholders sell substantial amounts of our ordinary shares or ADSs, either on the NYSE or on the BYMA, or if there is a public perception that these sales may occur in the future, the market price of our ADSs may decline. We, together with our directors, officers and substantially all of our shareholders, have agreed with the international underwriters of this offering not to sell any ordinary shares or ADSs, other than the ordinary shares represented by the ADSs offered through this prospectus, for a period of 180 days following the date of this prospectus. The international underwriters may, in their sole discretion, release all or any portion of our ordinary shares subject to these lock-up agreements for sale in public or private markets prior to the expiration of the lock-up period. The market price for our ADSs and ordinary shares could drop significantly when the restrictions on resale by our existing shareholders lapse or if the restrictions on resale are waived. A decline in the price of our securities could impede our ability to raise capital through the issuance of additional ADSs or other equity securities.
If we do not file or maintain a registration statement and no exemption from the Securities Act of 1933, or the Securities Act, registration is available, U.S. holders of ADSs may be unable to exercise preemptive rights granted to our holders of ordinary shares.
Under Argentine Corporate Law, if we issue new shares as part of a capital increase, our shareholders may have the right to subscribe to a proportional number of shares to maintain their existing ownership percentage. Rights to subscribe for shares in these circumstances are known as preemptive rights. In addition, shareholders are entitled to the right to subscribe for the unsubscribed shares remaining at the end of a preemptive rights offering on a pro rata basis, known as accretion rights. Upon the occurrence of any future increase in our capital stock, U.S. Persons (as defined in Regulation S under the Securities Act) holding our ordinary shares or ADSs may be unable to exercise preemptive and accretion rights granted to our holders of ordinary shares in connection with any future issuance of our ordinary shares unless a registration statement under the Securities Act is effective with respect to both the preemptive rights and the new ordinary shares, or an exemption from the registration requirements of the Securities Act is available. We are not obligated to file or maintain a registration statement relating to any preemptive rights offerings with respect to our ordinary shares, and we cannot assure you that we will file or maintain any such registration statement or that an exemption from registration will be available. Unless those ordinary shares or ADSs are registered or an exemption from registration applies, a U.S. holder of our ordinary shares or ADSs may receive only the net proceeds from those preemptive rights and accretion rights if those rights can be assigned by the ADS Depositary; if the rights cannot be sold, they will be allowed to lapse. Furthermore, the equity interest of holders of shares or ADSs located in the United States may be diluted proportionately upon future capital increases.
The preemptive and accretion rights granted to holders of our ordinary shares under Argentine law could limit the ways we can raise capital in the future.
We may seek additional financing in the future for a variety of reasons, including changes in our business strategy or the occurrence of unanticipated events. Because the Argentine Corporate Law provides shareholders preemptive rights and accretion rights, we may not be able to effectively utilize certain types of financing transactions, such as confidentially marketed public offerings or private investments in public equity, that are commonly used by U.S. domestic corporations
61
that do not afford their shareholders preemptive rights. Restrictions on the ways we can raise additional capital in the future that do not apply to most other U.S. public companies could adversely affect our financial condition and ability to compete in the markets in which we operate.
Our shareholders may be subject to liability for certain votes of their securities under Argentine law.
Although our shareholders are not liable for our obligations, shareholders, including beneficial owners of our shares who hold their shares in the form of ADSs, who willfully or negligently vote in favor of a resolution that is subsequently declared void by a court as contrary to Argentine Corporate Law, or our bylaws may be held jointly and severally liable for damages to us or to other third parties, including other shareholders. Additionally, shareholders and holders of ADSs who have a conflict of interest with us in respect of a particular transaction and do not abstain from voting on a relevant matter may be held liable for damages to us, but only to the extent such transaction would not have been approved without such shareholder’s vote. See “Description of Share Capital—Bylaws—Shareholders’ Liability.”
The imposition of future restrictions on transfers of foreign currency may interfere with the conversion of any future dividends or distributions from Argentine pesos into U.S. dollars and the remittance of U.S. dollars abroad.
Beginning in December 2001, the Argentine government implemented a number of monetary and foreign exchange control measures that included restrictions on the free disposition of funds deposited with banks and on the transfer of funds abroad without prior approval by the Central Bank, most of which have been lifted. See “Exchange Rates and Controls.”
Although the transfer of funds abroad by local companies in order to pay annual dividends to shareholders outside Argentina does not require formal approval by the Central Bank, in the past, the decrease in availability of U.S. dollars in Argentina has led the Argentine government to impose informal restrictions on certain local companies and individuals for purchasing foreign currency for the purpose of making payments abroad, such as dividends, capital reductions, and payment for importation of goods and services.
The imposition of future exchange controls could impair or prevent the conversion of anticipated dividends, distributions, or the proceeds from any sale of equity holdings in Argentina, as the case may be, from Argentine pesos into U.S. dollars and the remittance of the U.S. dollars abroad. These restrictions and controls could interfere with our ability to make distributions in U.S. dollars to us and thus our ability to pay dividends in the future. Additionally, if the exchange rate fluctuates significantly during a time when we cannot convert the foreign currency, we may lose some or all of the value of the dividend distribution or sale proceeds.
These restrictions and requirements could adversely affect our financial condition and the results of our operations, or the market price of our ordinary shares and ADSs.
You may face difficulties in exercising your voting rights or other rights relating to the ADSs.
ADS holders may only exercise certain of their rights relating to our underlying ordinary shares by providing voting instructions to the ADS Depositary in accordance with the ADS deposit agreement and custody agreement. Therefore, ADS holders may face difficulties in exercising their rights with respect to the underlying securities that would otherwise not exist if they held such securities directly.
For example, an ADS holder may not have sufficient or reasonable time to provide voting instructions to the ADS Depositary in accordance with the mechanisms set forth in the deposit agreement and custody agreement, and the ADS Depositary will not be held responsible for failure to deliver such instruction. The ability of ADS holders to hold us responsible for such failure may be limited. In addition, investors may need to be an owner of record to have standing to pursue certain actions against us. Any of these factors could substantially limit the ability of ADS holders to fully exercise their rights as shareholders.
62
Our status as a foreign private issuer allows us to follow alternate standards to the corporate governance standards of the NYSE, which may limit the protections afforded to investors.
We are a “foreign private issuer” within the meaning of the NYSE corporate governance standards. Under NYSE rules, a foreign private issuer may elect to comply with the practices of its home country and not comply with certain corporate governance requirements applicable to U.S. companies with securities listed on the exchange. We currently follow certain Argentine practices concerning corporate governance, including those required by the CNV rules related to our audit committee and the formation and composition of our board of directors and intend to continue to do so. Furthermore, we may in the future elect to follow Argentine standards with regard to other matters such as the formation and composition of our compensation and governance committees and separate sessions of independent directors. Accordingly, holders of our ADSs will not have the same protections afforded to shareholders of companies that are subject to all of the NYSE corporate governance requirements. For additional information, see the section entitled “Management—Corporate Governance Practices—Compliance with NYSE Standards.”
Changes in Argentine tax laws may adversely affect the tax treatment of our ordinary shares or ADSs.
Law No. 26,893 (enacted in 2013) established that the net gain resulting from the sale, exchange or other transfer of shares and other securities issued by Argentine companies by resident individuals in Argentina is subject to capital gains tax at a rate of 15%, unless those shares or other securities are listed in capital markets authorized by the CNV and/or have authorization for the public offering by the CNV.
In addition, capital gains of individuals or entities that are not residents of Argentina from the sale, exchange or other disposition of shares or other securities issued by Argentine companies would be subject to income tax, as the abovementioned exemption for shares is not applicable to non-resident beneficiaries. However, in transactions of this type between non-Argentine residents, the seller may opt to calculate the amount of the tax on the lower of 90% of the applicable rate (or 13.5%) on the sale price and 15% of the net gain; provided that the exception described above does not apply. The Argentine Income Tax Law No. 20,628, as amended, including Law No. 26,893, established that the non-resident of Argentine purchaser is responsible for paying the applicable capital gains tax. After almost four years without regulation on the withholding or payment mechanism applicable to the sale of shares exclusively between non-Argentine residents, on July 17, 2017, the AFIP issued the General Resolution 4094-E creating the payment mechanism for the withholding in transactions where both parties are not residents of Argentina, including all transactions completed since September 23, 2013. However, on July 19, 2017, the AFIP issued the General Resolution 4095-E which suspended the effects of the General Resolution 4094-E until January 16, 2018. Therefore, to the extent that the General Resolution 4094-E is not revoked or the suspension of its effects is not extended, the sale, exchange or other disposition of our ordinary shares and ADSs between parties that are not residents of Argentina would be subject to the capital gains tax described above. It is therefore possible that a capital gains tax is imposed on the sale, exchange or other disposition of our ordinary shares and ADSs between non-residents of Argentina. See “Dividend Policy” and “Taxation—Material Argentine Tax Considerations.”
Finally, following the amendments made by Law No. 26,893, and implementing Decree 2334/13, the tax treatment applicable to gains obtained by residents and non-residents of Argentina from the sale of ADSs is open to interpretation and may not be uniform under the amended Argentine Income Tax Law. Possible variations in the income source’s treatment of the ADSs may affect holders of ADSs residing both within and outside of Argentina. As of the date of this prospectus, there are no administrative or judicial decisions qualifying the ambiguity of the law as regards the source of income originated in the sale of ADSs.
The holders of our ordinary shares and the ADSs, are encouraged to consult with their tax advisors as to the particular Argentine income tax consequences. See “Dividend Policy” and “Taxation—Material Argentine Tax Considerations.”
If securities or industry analysts do not publish or cease publishing research or reports about us, our business, or our market, or if they change their recommendations regarding our stock adversely, our stock price and trading volume could decline.
The trading market for our common stock is influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. If any of the analysts who may cover us make adverse changes to their recommendation regarding our stock, or provide more favorable relative recommendations about our
63
competitors, our stock price would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
We have not yet determined whether our existing internal controls over financial reporting are effective to comply with Section 404 of the Sarbanes-Oxley Act, and we cannot provide any assurance that there are no material weaknesses or significant deficiencies in our existing internal controls.
Pursuant to Section 404 of the Sarbanes-Oxley Act, or Section 404, and the related rules adopted by the SEC and the PCAOB, starting with the second annual report that we file with the SEC after the consummation of this offering, our management will be required to report on the effectiveness of our internal control over financial reporting. In addition, once we no longer qualify as an “emerging growth company” under the JOBS Act and lose the ability to rely on the exemptions related thereto discussed above, our independent registered public accounting firm will also need to attest to the effectiveness of our internal control over financial reporting under Section 404 and PCAOB standards. We have not yet commenced the process of determining whether our existing internal controls over financial reporting are effective in accordance with a recognized internal control framework to comply with Section 404 and whether there are any material weaknesses or significant deficiencies in our existing internal controls. This process will require the investment of substantial time and resources, including by our Chief Financial Officer and other members of our senior management as well as our internal audit department. As a result, this process may divert internal resources and take a significant amount of time and effort. In addition, we cannot predict the outcome of this determination and whether we will need to implement remedial actions in order to implement effective internal control over financial reporting. The determination and any remedial actions required could result in us incurring additional costs that we did not anticipate. Irrespective of compliance with Section 404, any failure of our internal controls over financial reporting could have a material adverse effect on our stated results of operations, financial position and cash flows and harm our reputation. As a result, we may experience higher than anticipated operating expenses, as well as higher independent auditor fees during and after the implementation of these changes. If we are unable to implement any of the required changes to our internal control over financial reporting effectively or efficiently or are required to do so earlier than anticipated, it could adversely affect our operations, financial reporting and/or results of operations and could result in an adverse opinion on the effectiveness of internal controls over financial reporting from our independent auditors, if attestation of internal controls is required.
We are organized under the laws of Argentina and holders of our ADSs may find it difficult to enforce civil liabilities against us, our directors, officers and certain experts.
We are organized under the laws of Argentina. A significant portion of our and our subsidiaries’ assets are located outside the United States. Furthermore, all of our directors and officers and some advisors named in this prospectus reside in Argentina. Investors may not be able to effect service of process within the United States upon such persons or to enforce against them or us in United States courts judgments predicated upon the civil liability provisions of the federal securities laws of the United States. Likewise, it may also be difficult for an investor to enforce in U.S. courts judgments obtained against us or these persons in courts located in jurisdictions outside the United States, including judgments predicated upon the civil liability provisions of the U.S. federal securities laws. It may also be difficult for an investor to bring an original action in an Argentine court predicated upon the civil liability provisions of the U.S. federal securities laws against us or these persons.
Prior to any enforcement in Argentina, a judgment issued by a U.S. court will be subject to the requirements of Article 517 through 519 of the Argentine Federal Civil and Commercial Procedure Code if enforcement is sought before federal courts or courts with jurisdiction in commercial matters of the City of Buenos Aires. Those requirements are: (1) the judgment, which must be valid and final in the jurisdiction where rendered, was issued by a competent court in accordance with the Argentine principles regarding international jurisdiction and resulted from a personal action, or an in rem action with respect to personal property which was transferred to Argentine territory during or after the prosecution of the foreign action, (2) the defendant against whom enforcement of the judgment is sought was personally served with the summons and, in accordance with due process of law, was given an opportunity to defend against foreign action, (3) the authenticity of the judgment must be established in accordance with the requirements of Argentine law, (4) the judgement does not violate the principles of public policy of Argentine law and (5) the judgment is not contrary to a prior or simultaneous judgment of an Argentine court. Any document in a language other than Spanish, including, without
64
limitation, the foreign judgment and other documents related thereto, requires filing with the relevant court of a duly legalized translation by a sworn public translator into the Spanish language. See “Enforcement of Civil Liabilities.”
Pursuant to separate indemnification agreements, we indemnify our directors for and hold them harmless against all claims, actions, suits or proceedings brought against them, subject to limited exceptions. The rights and obligations among or between us and any of our current or former directors and officers are generally governed by the laws of Argentina and subject to the jurisdiction of the Argentine courts, unless such rights or obligations do not relate to or arise out of their capacities listed above.
The relative volatility and illiquidity of the Argentine securities markets may substantially limit your ability to sell the ordinary shares underlying our ADSs on the BYMA at the price and time you desire.
Investing in securities that trade in emerging markets, such as Argentina, often involves greater risk than investing in securities of issuers in the United States, and such investments are generally considered to be more speculative in nature. The Argentine securities market is substantially smaller, less liquid, more concentrated and can be more volatile than major securities markets in the United States, and is not as highly regulated or supervised as some of these other markets. There is also significantly greater concentration in the Argentine securities market than in major securities markets in the United States. The ten largest companies in terms of market capitalization represented approximately 57% of the aggregate market capitalization of the BYMA as of September 30, 2017. Accordingly, although you are entitled to withdraw our ordinary shares underlying the ADSs from the ADS Depositary at any time, your ability to sell such shares on the BYMA at a price and time at which you wish to do so may be substantially limited.
Non-Argentine companies that own our ordinary shares directly and not as ADSs may not be able to exercise their rights as shareholders unless they are registered in Argentina.
Under Argentine law, foreign companies that own shares in an Argentine corporation are required to register with the applicable Public Registry of Commerce in order to exercise certain shareholder rights, including voting rights. The registration requires the filing of corporate and accounting documents in order to demonstrate that the foreign shareholder is not a special purpose vehicle organized solely to conduct business in Argentina, is entitled to conduct business in its place of incorporation and meets certain foreign assets requirements. If you own our ordinary shares directly (rather than in the form of ADSs) and you are a non-Argentine company and you fail to register with the applicable Public Registry of Commerce, your ability to exercise your rights as a holder of our ordinary shares may be limited.
If we are a PFIC for U.S. federal income tax purposes for any taxable year, U.S. Holders of our ADSs could be subject to adverse U.S. federal income tax consequences.
If we were a PFIC within the meaning of Section 1297 of the Internal Revenue Code of 1986, as amended, for any taxable year during which a U.S. Holder (as defined in “Taxation—Material U.S. Federal Income Tax Considerations”) holds our ADSs or ordinary shares, certain adverse U.S. federal income tax consequences may apply to the U.S. Holder. We do not expect to be a PFIC for United States federal income tax purposes for our current taxable year. However, our possible status as a PFIC must be determined annually and therefore may be subject to change. This determination will depend on the composition of our income and assets, the market valuation of our assets (including, among others, our goodwill) from time to time, and our spending schedule for cash balances and the proceeds of the offering, as well as on the application of complex statutory and regulatory rules that are subject to potentially varying or changing interpretations. Accordingly, there can be no assurance that we will not be considered a PFIC for any taxable year. If we were a PFIC, U.S. Holders of our ADSs or ordinary shares may be subject to adverse U.S. federal income tax consequences, such as taxation at the highest marginal ordinary income tax rates on gains recognized on certain actual or deemed distributions, interest charges on certain taxes treated as deferred, and additional reporting requirements. See “Taxation—Material U.S. Federal Income Tax Considerations—Passive Foreign Investment Company Considerations.”
65
In January 2002, with the approval of the Public Emergency Law, Argentina declared a public emergency in its social, economic, administrative, financial and foreign exchange matters and authorized the Argentine executive branch to establish a system to determine the foreign exchange rate between the Argentine peso and foreign currencies and to issue foreign exchange-related rules and regulations.
On February 8, 2002, through Decree No. 260/2002, the Argentine executive branch established (i) the MULC, through which all foreign exchange transactions in foreign currency must be conducted, and (ii) that foreign exchange transactions in foreign currency must be conducted at the foreign exchange rate to be freely agreed upon among contracting parties, subject to the requirements and regulations imposed by the Central Bank. Since then, the executive branch and the Central Bank issued strict restrictions on the free purchase and sale of foreign currency and the inflow and outflow of foreign currency in and out of Argentina, including with certain exceptions, and without limitation: (i) restrictions to the purchase of foreign currency for investment or foreign portfolio investment purposes; (ii) the mandatory transfer into Argentina and sale through the MULC of the proceeds of the disbursement of foreign financial indebtedness, and the proceeds of the export of goods and services to foreign residents; (iii) the imposition of a 365 calendar day waiting period computed as from the date of settlement of the proceeds of the disbursements of foreign financial indebtedness in the MULC for the repayment of principal under such indebtedness; and (iv) the imposition of a mandatory deposit of an amount in U.S. dollars equal to 30% of the relevant amount transferred into Argentina in a registered and non-transferable and non-interest bearing account at an Argentine financial institution for a period of 365 calendar days.
In 2012 the Kirchner administration further significantly curtailed the access to the MULC subjecting certain foreign exchange transactions to the prior approval of the Argentine tax authority or the Central Bank. In response, an unofficial U.S. dollar trading market developed in which the Argentine peso-U.S. dollar exchange rate differed substantially from the official Argentine peso-U.S. dollar exchange rate.
In December 2015, in line with the economic reforms implemented by the newly elected Macri administration, the executive branch and the Central Bank eliminated a significant portion of the foreign exchange restrictions imposed in 2012, thereby reverting to the exchange controls regime in place prior to 2012 and easing some of the prior 2012 regime’s controls, including the reduction of the mandatory deposit to 0% and also reducing the mandatory waiting period from 365 to 120 calendar days, which was further reduced to no days in January 2017.
On August 8, 2016, the Argentine Central Bank introduced material changes to the foreign exchange regime and established a new foreign exchange regime by means of Communication “A” 6037 that significantly eases access to the MULC.
Furthermore, on May 19, 2017, the Central Bank issued Communication “A” 6244, which entered into effect on July 1, 2017 and was amended by Communication “A” 6312 dated August 30, 2017, and pursuant to which new regulations regarding access to the foreign exchange market were established, essentially abrogating all prior regulations on the matter. Pursuant to these regulations:
| ■ | The principle of a free foreign exchange market is established. |
| ■ | The obligation to carry out any exchange operation through an authorized entity is maintained. |
| ■ | The restrictions regarding hours to operate in the MULC are eliminated. |
| ■ | The obligation of Argentine residents to comply with the “Survey of foreign liabilities and debt issuances” (Communication “A” 3602 as supplemented) and the survey of direct investments (Communication “A” 4237 and complementary) are maintained, even if there had been no inflow of funds to the MULC and/or no future access to it for the operations to be declared. |
On November 1, 2017, the Argentine executive branch issued Decree No. 893/2017 (complemented by Communication “A” 6363 of the Central Bank dated November 10, 2017) pursuant to which foreign exchange restrictions related to exports of goods and services that continued to be in place (Mercado Único y Libre de Cambios) were eliminated, including the obligation of Argentine residents to transfer to Argentina and sell in the FX Market the proceeds of their exports of goods within the applicable deadline.
66
For a detailed description of all exchange restrictions and controls on capital inflows in effect as of the date hereof, investors may consult them on the website of legislative information of the Ministry of Justice and Human Rights or the Central Bank.
The following table sets forth the high, low, average and period-end exchange rates for the periods indicated, expressed in Argentine pesos per U.S. dollar and not adjusted for inflation. There can be no assurance that the Argentine peso will not further depreciate in the future. The exchange rates below should not be considered as representations that the Argentine peso amounts have been or could be converted into U.S. dollars at that rate or any other rate. The exchange rate reported by the Central Bank as of September 30, 2017 was Ps.17.32 to US$1.00.
Exchange Rate(1) Ps. per US$ | ||||||||||||
High(2) | Low(2) | Average(3) | Period End | |||||||||
Year Ended December 31, | ||||||||||||
2012 | 4.9173 | 4.3048 | 4.5515 | 4.9173 | ||||||||
2013 | 6.5180 | 4.9228 | 5.4789 | 6.5180 | ||||||||
2014 | 8.5555 | 6.5430 | 8.1188 | 8.5520 | ||||||||
2015 | 13.7633 | 8.5537 | 9.2689 | 13.0050 | ||||||||
2016 | 16.0392 | 13.0692 | 14.7794 | 15.8502 | ||||||||
Month Ended | ||||||||||||
June 2017 | 16.5985 | 15.8510 | 16.1166 | 16.5985 | ||||||||
July 2017 | 17.7642 | 16.6817 | 17.1690 | 17.6700 | ||||||||
August 2017 | 17.7833 | 17.0583 | 17.4165 | 17.3650 | ||||||||
September 2017 | 17.6108 | 16.9720 | 17.2465 | 17.3183 | ||||||||
October 2017 | 17.6775 | 17.3217 | 17.4528 | 17.6713 | ||||||||
November 2017 | 17.6703 | 17.3307 | 17.4925 | 17.3845 | ||||||||
December 2017 (through December 5, 2017) | 17.3513 | 17.2600 | 17.2907 | 17.3513 | ||||||||
Source: Central Bank.
| (1) | Central Bank reference exchange rates (Communication A 3500 of Central Bank). |
| (2) | Exchange rates are the actual low and high on a daily basis for each period. |
| (3) | The yearly average rate is calculated as the average of the exchange rates on the last day of each month during the period. The monthly average rate is calculated on a day-to-day basis for each month. |
67
We estimate that our net proceeds from the international offering will be approximately US$ million, or approximately US$ million if the international underwriters exercise their option to purchase additional ADSs in full, assuming the placement of all ordinary shares and ADSs offered at an initial public offering price of US$ per ordinary share and US$ per ADS (the midpoint of the range set forth on the cover page of this prospectus) and after deducting the underwriting discount and estimated offering expenses payable by us.
A US$1.00 increase (decrease) in the assumed initial public offering price of US$ per ordinary share and US$ per ADS would increase (decrease) the net proceeds to us from this offering, after deducting the underwriting discounts and estimated offering expenses payable by us, by approximately US$ million (assuming no exercise of the underwriters’ option to purchase additional ADSs). An increase (decrease) of 1,000,000 in the number of shares we are offering would increase (decrease) the net proceeds to us from this offering, after deducting the underwriting discount and estimated offering expenses payable by us, by approximately US$ million, assuming the assumed initial public offering price stays the same.
We intend to use the net proceeds of this offering (including any net proceeds from the underwriters’ exercise of their option to purchase additional ADSs) as follows:
| ■ | approximately US$ million to repay all or a portion of outstanding debt, which we expect to include the BAF Bridge Loans (as defined herein) with a balance of US$33.2 million as of September 30, 2017 and weighted average interest rate of 8.3% maturing in October 2018, in the event that BAF does not exercise certain participation rights that it holds; |
| ■ | approximately US$ million for investments in our seed and integrated products segment to commercially expand our EcoSeed business, including investments to develop next generation seed integrated products and in our commercial distribution network; |
| ■ | approximately US$ million investments in commercially expanding our agro-industrial biotech solutions business through continued technology sourcing and product development efforts; |
| ■ | approximately US$ million to exercise a mandatory call option for 9.99% of Rizobacter; and |
| ■ | the remainder for general corporate purposes and to fund working capital needs required to support the commercial expansion of our business segments. |
We may also use a portion of the net proceeds to acquire or invest in complementary products, technologies or businesses, although we currently have no agreements or binding commitments to complete any such transaction. The creation of a public market for our ordinary shares and ADSs will facilitate our ability to raise additional equity in the future and to use our ordinary shares and ADSs as a means of attracting and retaining key employees and as consideration for potential future acquisitions. We intend to use the net proceeds from this offering (including any net proceeds from the underwriters’ exercise of their option to purchase additional ADSs) primarily for working capital required to accelerate growth, capital expenditures, to repay debt and other general corporate purposes.
However, due to the uncertainties inherent in the product development process, it is difficult to estimate with certainty the exact amounts of the net proceeds from this offering that may be used for the above purposes. The amount and timing of our actual expenditures will depend upon numerous factors, including the results of our R&D efforts, the timing and success of our ongoing field tests or field tests we may commence in the future and the timing of regulatory submissions. As a result, our management will have broad discretion over the use of the net proceeds from this offering, and investors will be relying on our judgment regarding the application of the net proceeds. In addition, we might decide to postpone or not pursue certain activities or field tests if the net proceeds from this offering and our other sources of cash are less than expected.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, medium-term, investment-grade, interest-bearing instruments.
68
Since our inception, we have not declared or paid any cash or other form of dividends on our ordinary shares. We currently intend to retain any earnings for use in our business and do not intend, as of the date of this prospectus, to pay cash dividends on our ordinary shares for the foreseeable future. Dividends, if any, on our outstanding ordinary shares will be proposed by our board of directors and subject to the approval of our shareholders. Even if our shareholders decide to distribute dividends, the form, frequency and amount of such dividends will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that our board of directors and shareholders may deem relevant.
Our Dividend Policy
Under Argentine Corporate Law, the declaration and payment of dividends, subject to compliance with applicable Argentine Corporate Law (which dictates that dividends be paid only out of realized and net earnings (ganancias líquidas y realizadas) set forth in annual stand-alone financial statements presented in Argentine pesos), is determined by the annual shareholders’ meeting. The approval of dividends requires the affirmative vote of a majority of the shares entitled to vote at the meeting.
Under Argentine Corporate Law and our bylaws, we are required to maintain a legal reserve of 20% of our then-outstanding share capital, which legal reserve is not available for distribution to shareholders. Additionally, our annual net income must be allocated in the following order:
| ■ | to comply with the legal reserve requirement; |
| ■ | to pay the accrued fees of the members of the board of directors and supervisory committee; |
| ■ | to pay dividends on preferred shares, if any; |
| ■ | for voluntary or contingent reserves, as may be resolved from time to time by our shareholders at the annual ordinary shareholders’ meeting; and |
| ■ | the remainder of net income for the year may be distributed as dividends or as otherwise determined by our shareholders at the annual ordinary shareholders’ meeting. |
According to rules issued by the CNV, cash dividends must be paid to shareholders within 30 days of the resolution approving their distribution. In the case of share dividends, the relevant ordinary shares must be delivered to shareholders within three months of the annual ordinary shareholders’ meeting that approved the share dividend.
Payment of Dividends
In general, Argentine foreign exchange regulations grant access to the foreign exchange market for the purchase of foreign currency to pay dividends abroad to foreign shareholders or to a depositary for the benefit of the foreign holders of ADSs, provided that such dividends correspond to periods covered by approved annual audited financial statements and that our share capital is registered with the Central Bank. The shares underlying the ADSs are held in Argentina by Caja de Valores S.A., acting as the custodian agent for the ADS Depositary. The ADS Depositary will be the registered owner on the records of the registrar of our ordinary shares and will act as the registrar of our ADSs. We will inform the Central Bank of the amount of our ordinary shares held by foreign shareholders and the shares underlying the ADSs, and, therefore, should have access to the foreign exchange market to pay dividends with respect to our ordinary shares or ADSs, subject to certain structural restrictions as described further in “Risk Factors—Risks Related to Operating in Latin America and Argentina—Argentina—Argentine exchange controls on the acquisition of foreign currency and on transfers abroad and capital inflows have limited, and may continue to limit, the availability of international credit, access to capital markets and the ability to convert dividends from Argentine pesos to U.S. dollars” and “Risk Factors—Risks Related to Our ADSs and the Offering—Restrictions on transfers of foreign currency may interfere with the conversion of any future dividends or distributions from Argentine pesos into U.S. dollars and the remittance of U.S. dollars abroad.” Pursuant to the deposit agreement, holders of ADSs will be entitled to receive dividends, if any, declared with respect to the underlying ordinary shares represented by such ADSs to the same extent as the holders of the ordinary shares.
69
Payments of cash dividends and distributions, if any, will be made in Argentine pesos, although we reserve the right to pay in other currency. See “Risk Factors—Risks Related to Our ADSs and the Offering—We do not expect to declare any dividends in the foreseeable future.” The ADS Depositary will convert such dividends received by the depositary in Argentine pesos into U.S. dollars and pay such amount to holders of ADSs, net of any dividend distribution fees, depositary fees and expenses, currency conversion expenses, taxes or governmental charges, if any. In the event that the ADS Depositary is unable to convert immediately the Argentine currency received as dividends into U.S. dollars, the amount of U.S. dollars payable to holders of ADSs may be adversely affected by depreciation of the Argentine peso.
Contractual limitations on dividend payments
In October 2016, our subsidiary, Bioceres Inc., consummated a US$20 million loan facility, guaranteed by us and Rizobacter, with BAF, or the BAF US$20M Bridge Loan, as a bridge financing for our acquisition of Rizobacter. At the same time, we consummated a US$12 million loan facility as the borrower, guaranteed by Bioceres Inc. and Rizobacter, or the BAF US$12M Bridge Loan and together with the BAF US$20M Bridge Loan, the BAF Bridge Loans.
The BAF Bridge Loans contain restrictions on our ability, and on the ability of our subsidiaries Bioceres Inc. and Rizobacter, to issue dividends during the life of the loans, which depending on the participation of BAF in the offering may not extend beyond the date of the offering. For more information, see “Management’s Discussion and Analysis—Liquidity and Capital Resources—BAF Latam Trade Finance Fund B.V. Loan Facility Agreements.”
70
The following table sets forth our total capitalization, as of September 30, 2017:
| ■ | on an actual basis; |
| ■ | on a pro forma basis to reflect the exercise of the participation rights in the Argentine offering granted to (i) Monsanto and BAF in connection with the conversion of their loan instrument, (ii) holders of the preferred shares of RASA Holding and (iii) BAF under the BAF Bridge Loans; and |
| ■ | on a pro forma as adjusted basis to further reflect the offer and sale of (i) ADSs and ordinary shares, in the global offering at an assumed initial public offering price of US$ per ADS, which corresponds to the midpoint of the price range set forth on the cover page of this prospectus and (ii) ordinary shares to our existing shareholders who may exercise preemptive and accretion rights under Argentine law at a price of US$ per ordinary share (based on the exchange rate of Ps.17.32 per US$1.00 reported by the Central Bank on September 30, 2017). Based on this assumed offering price, we expect to receive total estimated net proceeds of approximately US$ (based on the exchange rate of Ps.17.32 per US$1.00 reported by the Central Bank on September 30, 2017, in respect of amounts sold to our existing shareholders), in each case, after deducting the application of the proceeds from the offering, estimated underwriting discounts and commissions and the estimated offering expenses payable by us. |
For more information specifically relating to the exercise of participation rights, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Rizobacter—Promissory Notes to be offset through Participation Rights—BAF Latam Trade Finance Fund B.V. Loan Facility Agreements.”
The prospective investor should read this information in conjunction with our consolidated financial statements and the related notes appearing at the end of this prospectus and the sections entitled “Use of Proceeds” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” as well as other financial information contained in this prospectus.
As of September 30, 2017 | |||||||||
Actual | Pro Forma(1) | Pro Forma as Adjusted(2) | |||||||
Short-term debt | 87,406,743 | ||||||||
Long-term debt (excluding current portions) | 91,661,345 | ||||||||
Total debt | 179,068,088 | ||||||||
Issued capital | 6,968,538 | ||||||||
Share premium and other reserves | 14,340,036 | ||||||||
Accumulated deficit and other reserves | (37,268,137 | ) | |||||||
Deficit attributable to equity holders of the parent | (15,959,563 | ) | |||||||
Equity attributable to non-controlling interests | 38,410,751 | ||||||||
Total capitalization | 201,519,276 | ||||||||
| (1) | The pro forma column reflects the mandatory conversion of the RASA Holding preferred shares (US$12.6 million), the mandatory conversion of the Monsanto and BAF convertible loan (US$16.6 million) and the voluntary conversion of the BAF Bridge Loans (US$33.2 million) into ordinary shares of the Company as if Bioceres had completed the IPO on September 30, 2017. For more information specifically relating to the exercise of participation rights, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Rizobacter—Promissory Notes to be offset through Participation Rights—BAF Latam Trade Finance Fund B.V. Loan Facility Agreements.” |
| (2) | A US$1.00 increase (decrease) in the assumed initial public offering price of US$ per ADS, which corresponds to the midpoint of the range set forth on the cover page of this prospectus, would increase (decrease) our as adjusted total shareholders’ equity and total capitalization by US$ million, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
71
The following is a brief summary of certain terms of the rights offering conducted in Argentina for our existing shareholders and has been included in this prospectus for information purposes only.
At an extraordinary shareholders’ meeting held on December 17, 2014, our shareholders approved (and at the shareholders’ meeting held on December 15, 2016 our shareholders ratified) a capital increase of up to 24,000,000 shares (after taking into account the Stock Split). The rights offering described below was carried out in furtherance of the approved capital increases.
Under Argentine law, our existing shareholders are entitled to preemptive rights to subscribe for shares subject to the capital increase underlying the global offering in a number sufficient to maintain their proportionate holdings in our total share capital. In addition, our existing shareholders have accretion rights, which allow them to subscribe for shares that are not otherwise subscribed for by other existing shareholders, in proportion to the percentage of shares for which the subscribing existing shareholders have exercised their preemptive rights. As a result, in connection with this global offering, on we commenced the rights offering in Argentina for our shareholders of record as of (the “Record Date”), who had the opportunity to subscribe for shares at the price of the shares offered and sold to the public in the global offering. The subscription period with respect to the rights offering will expire immediately prior to the announcement of the public offering price and the allocation of the shares and ADSs in the global offering. Our shareholders of record as of the Record Date had preemptive and accretion rights with respect to any shares that are available to the international underwriters under the option to purchase additional ADSs. New shareholders that purchase shares in the global offering will not have preemptive or accretion rights with respect to the shares to be sold pursuant to the option to purchase additional ADSs.
In order to facilitate the execution of the global offering, certain of our shareholders representing % of our total shares outstanding, assigned to AR Partners S.A., as exercise agent, substantially all of their preemptive and accretion rights in respect of the shares to be issued pursuant to the capital increase underlying the global offering. As a result, , or %, of the shares to be issued pursuant to our capital increase, will be available to be subscribed by holders of preemptive and accretion rights during the subscription period. A total of ordinary shares were subscribed pursuant to the rights offering, excluding shares subscribed to facilitate the global offering. Subject to closing conditions set forth in the international underwriting agreement, the international underwriters will instruct the exercise agent to exercise such preemptive rights (but not accretion rights) in order to facilitate the global offering.
The price determination for our shares in the global offering will be made by agreement between us and the international underwriters and Argentine placement agents based on the process for evaluating investor demand known as bookbuilding. The subscription price of the shares pursuant to the rights offering in Argentina will be the same as the price of the shares offered and sold to the public in the global offering, which will be determined upon the completion of the bookbuilding process and the expiration of the subscription period with respect to the rights offering. Our existing shareholders will not know the exact price per share at the time the shareholder commits to subscribe for the shares in the rights offering. However, a price range will be made available to such shareholders. Consequently, shareholders will not know the amount of dilution that could result from this offering. The shareholders exercising their accretion rights will only know how many shares it purchased in the rights offering after the offering price is determined.
If a shareholder elects to set forth a price per share in its subscription form, no shares will be allocated to that shareholder in the rights offering if the offering price exceeds the price per share indicated. If a shareholder elects to submit a subscription form without a purchase price or if the public offering price is at or below the purchase price indicated, then the shareholder will be bound and contractually obligated to pay for the shares at the public offering price. Shareholders participating in the rights offering will be obligated to pay for the subscription price of the shares allocated to them on a date determined by us, which may be any day between the pricing date and closing date of the global offering.
72
If you invest in our ADSs in this offering, your ownership interest will be immediately diluted to the extent of the difference between the initial public offering price per ADS and the net tangible book value per ADS after this offering. Our net tangible book value as of September 30, 2017 was Ps. per ordinary share and US$ per ADS. Net tangible book value per share and per ADS represents our total assets excluding our intangible assets less the amount of our total liabilities divided by 25,644,300, the total number of our ordinary shares outstanding as of September 30, 2017 adjusted in the case of the ADSs, to reflect the ratio of ordinary shares per ADS.
After giving effect to the sale of ADSs and ordinary shares in the global offering at an assumed initial public offering price of US$ per ADS, which corresponds to the midpoint of the initial public offering price range set forth on the cover page of this prospectus and (ii) ordinary shares to our existing shareholders who may exercise their preemptive and accretion rights under Argentine law at a price of US$ per ordinary share (based on the exchange rate of Ps.17.32 per US$1.00 reported by the Central Bank on September 30, 2017), and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, our net tangible book value on an adjusted basis as of September 30, 2017 would have been Ps. per ordinary share and US$ per ADS. This amount represents an immediate increase in net tangible book value of Ps. per ordinary share or US$ per ADS to our existing shareholders and an immediate decrease in net tangible book value of Ps. per ordinary share or US$ per ADS to new investors purchasing ADSs in this offering. We determine dilution by subtracting the as adjusted net tangible book value per ADS after this offering from the amount of cash that a new investor paid for an ADS.
The following table illustrates this dilution of US$ per ADS to purchasers of ADSs in this offering:
Per ADS | Percentage | |||||
Assumed initial public offering price | US$ | % | ||||
Net tangible book value as of September 30, 2017(1) | US$ | % | ||||
Increase attributable to this offering | US$ | % | ||||
As adjusted net tangible book value after this offering(2) | US$ | % | ||||
Dilution to new investors in this offering | US$ | % | ||||
| (1) | Net tangible book value per ADS represents our total assets excluding our intangible assets less the amount of our total liabilities divided by 25,644,300, the total number of our ordinary shares outstanding as of September 30, 2017, adjusted to reflect the ratio of ordinary shares per ADS. |
| (2) | As adjusted net tangible book value after this global offering corresponds to net tangible book value as of September 30, 2017, plus increase in net tangible book value attributable to this offering. |
A US$1.00 increase (decrease) in the assumed initial public offering price of US$ per ADS, which correspond to the midpoint of the range set forth on the cover page of this prospectus, would increase (decrease) our consolidated net tangible book value after this global offering by US$ million (Ps. million) and the dilution per ADS to new investors by US$ , after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us (based on the exchange rate of Ps.17.32 per US$1.00 reported by the Central Bank on September 30, 2017).
If the international underwriters exercise their over-allotment option in full in this offering, the as adjusted net tangible book value after the offering would be US$ per ADS, the increase in net tangible book value per share to existing shareholders would be US$ per ADS and the decrease in net tangible book value per share to new investors would be US$ per ADS at an assumed initial public offering price of US$ per ADS, which is the midpoint of the initial public offering price range set forth on the cover page of this prospectus (based on the exchange rate of Ps.17.32 per US$1.00 reported by the Central Bank on September 30, 2017).
On December 17, 2014, our shareholders approved the issuance of stock options exercisable in respect of up to 1,264,000 ordinary shares under our stock option incentive plan and stock grants of up to 1,264,000 ordinary shares under our stock grant incentive plan. Of such stock options, our board authorized the issuance of stock options with respect to 929,040 ordinary shares under the stock option incentive plan, with an exercise price of US$7.91 per share, to certain of our executives, officers and directors with whom we had executed individual stock option agreements on December 16, 2015. Our board authorized the issuance of stock grants with respect to 902,487 ordinary shares under the stock grant incentive plan to certain of our executives, officers and directors. We expect our compensation committee to execute individual stock grant agreements in the second quarter of the current fiscal year.
73
SELECTED CONSOLIDATED HISTORICAL FINANCIAL AND OTHER INFORMATION
The following tables set forth our selected consolidated financial data. You should read the following selected consolidated financial data in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes included elsewhere in this prospectus. Historical results are not necessarily indicative of the results that may be expected in the future. Our consolidated financial statements have been prepared in accordance with IFRS.
The consolidated statements of comprehensive income data for Bioceres for the Transition Period, for the unaudited six-month period ended June 30, 2016, for the years ended December 31, 2016 and 2015 and the summary consolidated statements of financial position data as of June 30, 2017 and as of December 31, 2016 and 2015 are derived from our audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statements of comprehensive income data for Bioceres for the three-month period ended September 30, 2017 and 2016 and the summary consolidated statements of financial position data as of September 30, 2017 are derived from our unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus.
The consolidated statements of comprehensive income data for Rizobacter for the years ended June 30, 2017, 2016 and 2015 and the summary consolidated statements of financial position data as of June 30, 2017, 2016 and 2015 are derived from Rizobacter’s audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statements of comprehensive income data for Rizobacter for the three-month periods ended September 30, 2017 and 2016 and the summary consolidated statements of financial position data as of September 30, 2017 are derived from Rizobacter’s unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus. This financial information (i) is prepared on a stand-alone basis, (ii) does not reflect the purchase accounting adjustments recorded at the consolidated level of Bioceres after the acquisition, (iii) does not include any corporate and other expenses that should be allocated to Rizobacter, (iv) uses different segments than the Bioceres consolidated financial statements, (v) is for information purposes only and (vi) should not be viewed as a substitute for the consolidated financial position of Bioceres at June 30, 2017.
74
Selected Consolidated Historical Financial Information of Bioceres
Consolidated statement of comprehensive income of Bioceres
The consolidated statements of comprehensive income for Bioceres for the Transition Period, for the unaudited six-month period ended June 30, 2016 and for the years ended December 31, 2016 and 2015 are derived from our audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statements of comprehensive income data for Bioceres for the three-month periods ended September 30, 2017 and 2016 are derived from our unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus.
Bioceres | ||||||||||||||||||
Three-month period ended September 30, | Three-month period ended September 30, | Transition Period ended June 30, | Six-month period ended June 30, | Year ended December 31, | ||||||||||||||
2017 (unaudited) | 2016(1) (unaudited) | 2017 | 2016(1) (unaudited) | 2016(2) | 2015 | |||||||||||||
(US$) | ||||||||||||||||||
Revenue | 34,828,443 | 1,846,295 | 48,068,098 | 2,268,193 | 43,587,834 | 8,965,310 | ||||||||||||
Government grants | 158,062 | 117,218 | 273,023 | 270,206 | 761,429 | 1,230,574 | ||||||||||||
Total revenue | 34,986,505 | 1,963,513 | 48,341,121 | 2,538,399 | 44,349,263 | 10,195,884 | ||||||||||||
Cost of sales | (20,195,931 | ) | (1,118,948 | ) | (30,185,446 | ) | (1,290,256 | ) | (31,600,998 | ) | (4,799,345 | ) | ||||||
Research and development expenses | (1,725,645 | ) | (596,652 | ) | (3,601,624 | ) | (1,268,661 | ) | (2,860,771 | ) | (2,688,924 | ) | ||||||
Selling, general and administrative expenses | (9,993,183 | ) | (1,185,472 | ) | (17,324,407 | ) | (3,541,058 | ) | (12,906,021 | ) | (4,080,860 | ) | ||||||
Share of profit / (loss) of joint ventures and associates | 84,857 | (238,100 | ) | (786,805 | ) | (455,181 | ) | (936,769 | ) | (1,553,002 | ) | |||||||
Other income and expenses, net | (217,284 | ) | (18,135 | ) | 121,065 | — | 48,495 | — | ||||||||||
Operating income / (loss) | 2,939,319 | (1,193,794 | ) | (3,436,096 | ) | (4,016,757 | ) | (3,906,801 | ) | (2,926,267 | ) | |||||||
Finance income | 2,042,066 | 381,327 | 2,136,265 | 790,814 | 1,006,953 | 1,509,736 | ||||||||||||
Finance costs | (9,268,419 | ) | (1,117,154 | ) | (14,945,495 | ) | (1,579,951 | ) | (10,923,378 | ) | (1,904,569 | ) | ||||||
Loss before income tax | (4,287,034 | ) | (1,929,621 | ) | (16,245,326 | ) | (4,805,894 | ) | (9,369,942 | ) | (3,321,100 | ) | ||||||
Income tax benefit | 1,357,795 | 621,022 | 5,090,723 | 1,040,923 | 4,140,028 | (411,342 | ) | |||||||||||
Loss for the period / year | (2,929,239 | ) | (1,308,599 | ) | (11,154,603 | ) | (3,764,971 | ) | (5,229,914 | ) | (3,732,442 | ) | ||||||
Other comprehensive income or loss | — | |||||||||||||||||
Items that may be subsequently reclassified to profit and loss | (4,150,698 | ) | — | (4,614,372 | ) | — | (4,482,329 | ) | — | |||||||||
Exchange differences on translation of foreign operations from joint ventures | (1,137,789 | ) | — | (1,265,831 | ) | — | (1,168,589 | ) | — | |||||||||
Exchange differences on translation of foreign operations | (3,012,909 | ) | — | (3,348,541 | ) | — | (3,313,740 | ) | — | |||||||||
Items that will not be subsequently reclassified to loss and profit | — | — | 2,032,872 | — | — | — | ||||||||||||
Revaluation of property, plant and equipment, net of tax from joint ventures | — | — | 189,025 | — | — | — | ||||||||||||
Revaluation of property, plant and equipment, net of tax | — | — | 1,843,847 | — | — | — | ||||||||||||
Total comprehensive loss(3) | (7,079,937 | ) | (1,308,599 | ) | (13,736,103 | ) | (3,764,971 | ) | (9,712,243 | ) | (3,732,442 | ) | ||||||
Profit / (loss) for the period / year attributable to: | ||||||||||||||||||
Equity holders of the parent | (3,066,809 | ) | (1,214,922 | ) | (8,467,524 | ) | (3,435,546 | ) | (4,508,061 | ) | (3,540,504 | ) | ||||||
Non-controlling interests | 137,570 | (93,677 | ) | (2,687,079 | ) | (329,425 | ) | (721,853 | ) | (191,938 | ) | |||||||
(2,929,239 | ) | (1,308,599 | ) | (11,154,603 | ) | (3,764,971 | ) | (5,229,914 | ) | (3,732,442 | ) | |||||||
Total comprehensive loss attributable to: | ||||||||||||||||||
Equity holders of the parent | (6,019,035 | ) | (1,214,922 | ) | (10,606,902 | ) | (3,435,546 | ) | (7,725,876 | ) | (3,540,504 | ) | ||||||
Non-controlling interests | (1,060,902 | ) | (93,677 | ) | (3,129,201 | ) | (329,425 | ) | (1,986,367 | ) | (191,938 | ) | ||||||
(7,079,937 | ) | (1,308,599 | ) | (13,736,103 | ) | (3,764,971 | ) | (9,712,243 | ) | (3,732,442 | ) | |||||||
75
Bioceres | ||||||||||||||||||
Three-month period ended September 30, | Three-month period ended September 30, | Transition Period ended June 30, | Six-month period ended June 30, | Year ended December 31, | ||||||||||||||
2017 (unaudited) | 2016(1) (unaudited) | 2017 | 2016(1) (unaudited) | 2016(2) | 2015 | |||||||||||||
(US$) | ||||||||||||||||||
Loss per share(4) | ||||||||||||||||||
Basic loss per ordinary share | (0.1200 | ) | (0.0474 | ) | (0.3316 | ) | (0.1342 | ) | (0.1762 | ) | (0.1387 | ) | ||||||
Diluted loss per ordinary share | (0.1200 | ) | (0.0474 | ) | (0.3316 | ) | (0.1342 | ) | (0.1762 | ) | (0.1387 | ) | ||||||
Weighted average number of ordinary shares used in computing basic net loss per ordinary share | 25,546,550 | 25,615,265 | 25,536,100 | 25,593,700 | 25,592,104 | 25,534,542 | ||||||||||||
Weighted average number of ordinary shares used in computing diluted net loss per ordinary share | 25,546,550 | 25,615,265 | 25,536,100 | 25,593,700 | 25,592,104 | 25,534,542 | ||||||||||||
Non-IFRS measures | ||||||||||||||||||
Adjusted EBITDA (unaudited)(5) | 5,566,129 | (739,683 | ) | 2,318,947 | (2,897,488 | ) | 6,390,481 | (2,376,297 | ) | |||||||||
| (1) | Consolidated statements of comprehensive income for the six-month period ended June 30, 2016 do not include the consolidated statements of comprehensive income of Rizobacter, control of which we assumed on October 19, 2016. |
| (2) | Consolidated results of our operations include results of operations of Rizobacter from October 19, 2016 to December 31, 2016 (the period beginning on the date whereupon we assumed control of Rizobacter following its acquisition by us). |
| (3) | Includes (i) exchange differences on translation of foreign operations from joint ventures, (ii) exchange differences on translation of foreign operations, (iii) revaluation of property, plant and equipment, net of tax from joint ventures and (iv) revaluation of property, plant and equipment, net of tax. |
| (4) | See Note 10 to our consolidated financial statements for an explanation of the Stock Split and the method used to calculate the basic and diluted earnings per share. |
| (5) | We define Adjusted EBITDA as profit/(loss) exclusive of financial income/(costs), income tax benefit/(expense), depreciation, amortization, share-based compensation and other items and charges. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Non-IFRS Financial Measures.” |
The table below provides a reconciliation of our loss for the period/year to Adjusted EBITDA:
Bioceres | ||||||||||||||||||
Three-month period ended September 30, | Three-month period ended September 30, | Transition Period ended June 30, | Six-month period ended June 30, | Year ended December 31, | ||||||||||||||
2017 (unaudited) | 2016 (unaudited) | 2017 | 2016 (unaudited) | 2016 | 2015 | |||||||||||||
(US$) | ||||||||||||||||||
Reconciliation of Net Loss to Adjusted EBITDA: | ||||||||||||||||||
Loss for the period / year | (2,929,239 | ) | (1,308,599 | ) | (11,154,603 | ) | (3,764,971 | ) | (5,229,914 | ) | (3,732,442 | ) | ||||||
Income tax (benefit) / expense | (1,357,795 | ) | (621,022 | ) | (5,090,723 | ) | (1,040,923 | ) | (4,140,028 | ) | 411,342 | |||||||
Finance costs | 9,268,419 | 1,117,154 | 14,945,495 | 1,579,951 | 10,923,378 | 1,904,569 | ||||||||||||
Finance income | (2,042,066 | ) | (381,327 | ) | (2,136,265 | ) | (790,814 | ) | (1,006,953 | ) | (1,509,736 | ) | ||||||
Gain on previously held interest | — | — | — | — | (4,453,284 | ) | — | |||||||||||
Depreciation of property, plant and equipment | 771,724 | 146,061 | 1,524,709 | 256,492 | 1,074,733 | 456,444 | ||||||||||||
Amortization of intangible assets | 580,983 | 11,854 | 1,418,661 | 24,065 | 463,066 | 45,233 | ||||||||||||
Inventory purchase price allocation charge | 1,119,155 | — | 2,436,949 | — | 7,516,071 | — | ||||||||||||
Transaction expenses | — | — | — | 252,757 | 599,150 | — | ||||||||||||
Stock-based compensation charges | 154,948 | 296,196 | 374,724 | 585,955 | 644,261 | 48,293 | ||||||||||||
Adjusted EBITDA (unaudited) | 5,566,129 | (739,683 | ) | 2,318,947 | (2,897,488 | ) | 6,390,481 | (2,376,297 | ) | |||||||||
76
Unaudited pro forma consolidated statement of comprehensive income of Bioceres
The table below illustrates our results of operations for the 2017 periods indicated and the unaudited pro forma results of operations for the 2016 periods indicated. For more information, see “Unaudited Pro Forma Consolidated Financial Information.” The unaudited pro forma nine-month period ended September 30, 2016 is the sum of the unaudited pro forma three-month period ended September 30, 2016 and the unaudited six-month period ended June 30, 2016.
Bioceres | ||||||||||||||||||
Nine-month period ended September 30, | Three-month period ended September 30, | Transition Period ended June 30, | Six-month period ended June 30, | |||||||||||||||
2017 | 2016(1) | 2017 | 2016(1) | 2017 | 2016(1) | |||||||||||||
(unaudited) | (unaudited) (US$, except share data) | (unaudited) | ||||||||||||||||
Total revenue | 83,327,626 | 59,088,148 | 34,986,505 | 27,977,571 | 48,341,121 | 31,110,577 | ||||||||||||
Crop protection | 47,987,918 | 29,597,533 | 16,795,948 | 12,142,498 | 31,191,970 | 17,455,035 | ||||||||||||
Seed and integrated products | 16,519,034 | 10,822,360 | 7,498,035 | 7,080,861 | 9,020,999 | 3,741,499 | ||||||||||||
Crop nutrition | 16,181,111 | 16,759,159 | 9,540,883 | 8,243,062 | 6,640,228 | 8,516,097 | ||||||||||||
Emerging solutions | 2,639,563 | 1,909,096 | 1,151,639 | 511,150 | 1,487,924 | 1,397,946 | ||||||||||||
Cost of sales | (50,381,377 | ) | (29,012,220 | ) | (20,195,931 | ) | (12,727,738 | ) | (30,185,446 | ) | (16,284,482 | ) | ||||||
Crop protection | (32,680,799 | ) | (14,136,216 | ) | (10,038,912 | ) | (5,765,825 | ) | (22,641,887 | ) | (8,370,392 | ) | ||||||
Seed and integrated products | (7,314,203 | ) | (3,162,513 | ) | (2,462,759 | ) | (2,376,759 | ) | (4,851,444 | ) | (785,754 | ) | ||||||
Crop nutrition | (9,594,614 | ) | (10,950,639 | ) | (7,509,962 | ) | (4,321,888 | ) | (2,084,652 | ) | (6,628,751 | ) | ||||||
Emerging solutions | (791,761 | ) | (762,851 | ) | (184,298 | ) | (263,266 | ) | (607,463 | ) | (499,585 | ) | ||||||
Research and development expenses | (5,327,269 | ) | (4,216,430 | ) | (1,725,645 | ) | (1,398,194 | ) | (3,601,624 | ) | (2,818,236 | ) | ||||||
Selling, general and administrative expenses | (27,317,590 | ) | (23,928,021 | ) | (9,993,183 | ) | (8,276,623 | ) | (17,324,407 | ) | (15,651,397 | ) | ||||||
Share of profit / (loss) of joint ventures and associates | (701,948 | ) | (1,027,756 | ) | 84,857 | (360,917 | ) | (786,805 | ) | (666,838 | ) | |||||||
Other expenses, net | (96,219 | ) | 277,225 | (217,284 | ) | (41,834 | ) | 121,065 | 319,059 | |||||||||
Operating income / (loss) | (496,777 | ) | 1,180,947 | 2,939,319 | 5,172,265 | (3,436,096 | ) | (3,991,318 | ) | |||||||||
Finance income | 4,178,331 | 2,572,698 | 2,042,066 | 1,430,434 | 2,136,265 | 1,142,264 | ||||||||||||
Finance costs | (24,213,914 | ) | (23,984,209 | ) | (9,268,419 | ) | (7,769,807 | ) | (14,945,495 | ) | (16,214,402 | ) | ||||||
Loss before income tax | (20,532,360 | ) | (20,230,564 | ) | (4,287,034 | ) | (1,167,108 | ) | (16,245,326 | ) | (19,063,456 | ) | ||||||
Income tax benefit | 6,448,518 | 5,422,960 | 1,357,795 | 357,944 | 5,090,723 | 5,065,016 | ||||||||||||
Loss for the period | (14,083,842 | ) | (14,807,604 | ) | (2,929,239 | ) | (809,164 | ) | (11,154,603 | ) | (13,998,440 | ) | ||||||
Attributable to: Equity holders of the parent | (11,534,333 | ) | (11,709,011 | ) | (3,066,809 | ) | (1,321,302 | ) | (8,467,524 | ) | (10,387,709 | ) | ||||||
Non controlling interest | (2,549,509 | ) | (3,098,593 | ) | 137,570 | 512,138 | (2,687,079 | ) | (3,610,731 | ) | ||||||||
Loss per share | ||||||||||||||||||
Basic loss per ordinary share | (0.4515 | ) | (0.4571 | ) | (0.1200 | ) | (0.0516 | ) | (0.3316 | ) | (0.4059 | ) | ||||||
Diluted loss per ordinary share of the parent | (0.4515 | ) | (0.4571 | ) | (0.1200 | ) | (0.0516 | ) | (0.3316 | ) | (0.4059 | ) | ||||||
Weighted average number of ordinary shares used in computing basic net loss per ordinary share(3) | 25,546,550 | 25,615,265 | 25,546,550 | 25,615,265 | 25,536,100 | 25,593,700 | ||||||||||||
Weighted average number of ordinary shares used in computing diluted net loss per ordinary share(3) | 25,546,550 | 25,615,265 | 25,546,550 | 25,615,265 | 25,536,100 | 25,593,700 | ||||||||||||
Non-IFRS measures | ||||||||||||||||||
Adjusted EBITDA (unaudited)(4) | 7,885,076 | 5,388,883 | 5,566,129 | 6,629,293 | 2,318,947 | (1,240,409 | ) | |||||||||||
| (1) | Consolidated statements of comprehensive income for the three-month and nine-month periods ended September 30, 2016 do not include the consolidated statements of comprehensive income of Rizobacter, control of which we assumed on October 19, 2016. |
| (2) | Includes (i) exchange differences on translation of foreign operations from joint ventures, (ii) exchange differences on translation of foreign operations, (iii) revaluation of property, plant and equipment, net of tax from joint ventures and (iv) revaluation of property, plant and equipment, net of tax. |
| (3) | See Note 10 to our consolidated financial statements for an explanation of the Stock Split and the method used to calculate the basic and diluted earnings per share. |
| (4) | We define Adjusted EBITDA as profit/(loss) exclusive of financial income/(costs), income tax benefit/(expense), depreciation, amortization, share-based compensation and other items and charges. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Non-IFRS Financial Measures.” |
77
The table below provides a reconciliation of our loss for the periods to our Adjusted EBITDA:
Bioceres | ||||||||||||||||||
Nine-month period ended September 30, | Three-month period ended September 30, | Transition period ended June 30, | Six-month period ended June 30, | |||||||||||||||
2017 | 2016 | 2017 | 2016 | 2017 | 2016 | |||||||||||||
(unaudited) | (unaudited) | (unaudited) | ||||||||||||||||
(US$, except share data) | ||||||||||||||||||
Reconciliation of Net Loss to Adjusted EBITDA: | ||||||||||||||||||
Loss for the period | (14,083,842 | ) | (14,807,604 | ) | (2,929,239 | ) | (809,164 | ) | (11,154,603 | ) | (13,808,709 | ) | ||||||
Income tax (benefit) | (6,448,518 | ) | (5,422,960 | ) | (1,357,795 | ) | (357,944 | ) | (5,090,723 | ) | (4,962,853 | ) | ||||||
Finance costs | 24,213,914 | 23,984,209 | 9,268,419 | 7,769,807 | 14,945,495 | 15,922,509 | ||||||||||||
Finance income | (4,178,331 | ) | (2,572,698 | ) | (2,042,066 | ) | (1,430,434 | ) | (2,136,265 | ) | (1,142,264 | ) | ||||||
Depreciation of property, plant and equipment | 2,296,433 | 2,451,028 | 771,724 | 870,470 | 1,524,709 | 1,580,558 | ||||||||||||
Amortization of intangible assets | 1,999,644 | 874,756 | 580,983 | 290,361 | 1,418,661 | 584,395 | ||||||||||||
Inventory purchase price allocation charge | 3,556,104 | — | 1,119,155 | — | 2,436,949 | — | ||||||||||||
Transaction expenses | — | — | — | — | — | — | ||||||||||||
Stock-based compensation charges | 529,672 | 882,151 | 154,948 | 296,196 | 374,724 | 585,955 | ||||||||||||
Adjusted EBITDA (unaudited) | 7,885,076 | 5,388,883 | 5,566,129 | 6,629,293 | 2,318,947 | (1,240,409 | ) | |||||||||||
Consolidated statement of financial position of Bioceres
The consolidated statements of financial position for Bioceres as of June 30, 2017 and as of December 31, 2016 and 2015 are derived from our audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statement of financial position for Bioceres as of September 30, 2017 is derived from our unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus.
Bioceres | ||||||||||||
As of September 30, 2017 | As of June 30, 2017 | As of December 31, 2016 | As of December 31, 2015 | |||||||||
(unaudited) | ||||||||||||
(US$, except share data) | ||||||||||||
ASSETS | ||||||||||||
Current assets: | ||||||||||||
Cash and cash equivalents | 1,590,773 | 2,119,883 | 1,256,696 | 58,425 | ||||||||
Other financial assets | 4,450,887 | 4,526,960 | 4,554,474 | 844,000 | ||||||||
Trade receivables | 55,234,392 | 45,693,673 | 57,033,051 | 5,498,974 | ||||||||
Other receivables | 7,955,094 | 4,179,869 | 4,767,657 | 4,068,995 | ||||||||
Income and minimum presumed income taxes | 1,622,586 | 2,107,860 | — | — | ||||||||
Inventories | 36,871,630 | 31,723,752 | 33,157,565 | 3,060,041 | ||||||||
Total current assets | 107,725,362 | 90,351,997 | 100,769,443 | 13,530,435 | ||||||||
Non-current assets: | ||||||||||||
Other financial assets | 3,202,239 | 3,292,758 | 3,082,906 | — | ||||||||
Other receivables | 9,182,183 | 5,631,095 | 4,776,733 | 1,820,267 | ||||||||
Income and minimum presumed income taxes recoverable | 663,113 | 691,447 | 1,603,425 | 392,536 | ||||||||
Deferred tax assets | 8,315,318 | 7,272,648 | 5,077,851 | 1,379,201 | ||||||||
Investments in joint ventures | 28,124,407 | 28,977,935 | 29,433,063 | 299,276 | ||||||||
Property, plant and equipment | 48,571,389 | 51,110,617 | 51,738,436 | 4,767,810 | ||||||||
Intangible assets | 40,175,146 | 42,181,770 | 44,083,273 | 1,767,168 | ||||||||
Goodwill | 31,571,359 | 32,866,203 | 34,401,620 | 536,065 | ||||||||
Total non-current assets | 169,805,154 | 172,024,473 | 174,197,307 | 10,962,323 | ||||||||
Total assets | 277,530,516 | 262,376,470 | 274,966,750 | 24,492,758 | ||||||||
78
Bioceres | ||||||||||||
As of September 30, 2017 | As of June 30, 2017 | As of December 31, 2016 | As of December 31, 2015 | |||||||||
(unaudited) | ||||||||||||
(US$, except share data) | ||||||||||||
LIABILITIES | ||||||||||||
Current liabilities: | ||||||||||||
Trade and other payables | 39,070,197 | 23,748,712 | 37,050,214 | 7,091,742 | ||||||||
Borrowings | 60,310,507 | 51,020,481 | 65,847,948 | 2,215,282 | ||||||||
Employee benefits and social security | 6,008,683 | 5,570,209 | 4,019,556 | 376,691 | ||||||||
Deferred revenue and advances from customers | 2,584,436 | 1,256,975 | 944,273 | 667,373 | ||||||||
Income and minimum presumed income taxes payable | 271,386 | 223,187 | 1,878,657 | 68,366 | ||||||||
Government grants | 311,917 | 351,157 | 351,657 | 320,729 | ||||||||
Puttable instruments | 225,131 | — | — | — | ||||||||
Financed payment – Acquisition of business | 27,096,236 | 26,477,432 | 27,330,752 | — | ||||||||
Total current liabilities | 135,878,493 | 108,648,153 | 137,423,057 | 10,740,183 | ||||||||
Non-current liabilities | ||||||||||||
Borrowings | 36,568,583 | 41,106,995 | 11,898,062 | 815,501 | ||||||||
Government grants | 1,388,258 | 1,485,079 | 1,711,906 | 1,200,976 | ||||||||
Puttable instruments | 5,127,402 | 5,008,467 | 4,897,397 | 1,612,168 | ||||||||
Investments in joint ventures | 1,726,690 | 1,527,286 | 1,569,160 | 1,185,566 | ||||||||
Deferred tax liabilities | 22,786,161 | 24,620,369 | 25,247,390 | 407,708 | ||||||||
Provisions | 1,638,381 | 1,690,412 | 2,160,788 | 234,505 | ||||||||
Financed payment – Acquisition of business | 34,049,246 | 33,402,933 | 35,231,435 | — | ||||||||
Contingent consideration – Acquisition of business | 15,916,114 | 15,919,060 | 15,771,716 | — | ||||||||
Total non-current liabilities | 119,200,835 | 124,760,601 | 98,487,854 | 5,456,424 | ||||||||
Total liabilities | 255,079,328 | 233,408,754 | 235,910,911 | 16,196,607 | ||||||||
EQUITY | ||||||||||||
Issued capital | 6,968,538 | 6,968,538 | 6,968,538 | 6,968,538 | ||||||||
Share premium | 15,599,688 | 15,461,569 | 15,461,569 | 15,461,569 | ||||||||
Puttable instruments | (2,025,398 | ) | (2,025,398 | ) | (2,025,398 | ) | (1,423,386 | ) | ||||
Cost of own shares held by subsidiary | (456,480 | ) | (726,822 | ) | (726,822 | ) | — | |||||
Stock options | 1,222,226 | 1,067,278 | 692,554 | 48,293 | ||||||||
Accumulated losses | (28,958,718 | ) | (25,891,909 | ) | (17,424,385 | ) | (12,916,324 | ) | ||||
Revaluation of property, plant and equipment reserve | 1,219,600 | 1,219,600 | — | — | ||||||||
Foreign currency translation reserve | (9,529,019 | ) | (6,576,793 | ) | (3,217,815 | ) | — | |||||
Equity / (deficit) attributable to owners of the parent | (15,959,563 | ) | (10,503,937 | ) | (271,759 | ) | 8,138,690 | |||||
Non-controlling interests | 38,410,751 | 39,471,653 | 39,327,598 | 157,461 | ||||||||
Total equity | 22,451,188 | 28,967,716 | 39,055,839 | 8,296,151 | ||||||||
Total equity and liabilities | 277,530,516 | 262,376,470 | 274,966,750 | 24,492,758 | ||||||||
Weighted average number of ordinary shares | 25,546,550 | 25,536,100 | 25,592,104 | 25,534,542 | ||||||||
Declared dividends per share | — | — | — | — | ||||||||
79
Selected Consolidated Historical Financial Information of Rizobacter
Consolidated statement of comprehensive income of Rizobacter
The consolidated statements of comprehensive income for the years ended June 30, 2017, 2016 and 2015 are derived from Rizobacter’s audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statements of comprehensive income for the three-month periods ended September 30, 2017 and 2016 are derived from Rizobacter’s unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus. However, this information: (i) has been prepared on a stand-alone basis; (ii) does not reflect the purchase accounting adjustments made at the consolidated level after the acquisition of Rizobacter; (iii) does not include any corporate and other expenses that should be allocated to these financial statements; (iv) uses different segments from the Bioceres consolidated financial statements; (v) is for information purposes only and (vi) should not be viewed as a substitute for the consolidated financial statements of Bioceres subsequent to the date of the acquisition of Rizobacter. The consolidated statements of comprehensive income of Rizobacter for the year ended June 30, 2017 and the three-month periods ended September 30, 2017 and 2016 are provided as additional information to permit readers to compare the more recent results of Rizobacter on a stand-alone basis.
Rizobacter | |||||||||||||||
Three-month period ended September 30, | Year ended June 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | 2015 | |||||||||||
(Unaudited) | |||||||||||||||
(US$) | |||||||||||||||
Revenue | 32,852,813 | 26,153,338 | 112,296,212 | 93,405,678 | 99,163,146 | ||||||||||
Cost of sales | (18,080,896 | ) | (11,608,790 | ) | (58,838,471 | ) | (49,073,466 | ) | (47,357,049 | ) | |||||
Gross income | 14,791,917 | 14,544,548 | 53,457,741 | 44,332,212 | 51,806,097 | ||||||||||
Administrative expenses | (2,371,453 | ) | (2,125,642 | ) | (9,765,385 | ) | (8,363,830 | ) | (7,677,468 | ) | |||||
Distribution expenses | (5,262,815 | ) | (4,668,990 | ) | (19,502,749 | ) | (18,824,875 | ) | (20,102,818 | ) | |||||
Research expenses | (579,634 | ) | (533,601 | ) | (2,423,428 | ) | (2,254,885 | ) | (655,941 | ) | |||||
Other operating income and expenses, net | (175,320 | ) | (23,699 | ) | 49,654 | 446,739 | 158,731 | ||||||||
Operating income | 6,402,695 | 7,192,616 | 21,815,833 | 15,335,361 | 23,528,601 | ||||||||||
Financial income | 1,198,552 | 1,000,264 | 2,080,709 | 4,633,611 | 2,032,019 | ||||||||||
Financial costs | (5,719,583 | ) | (5,028,406 | ) | (19,450,815 | ) | (27,025,501 | ) | (15,944,826 | ) | |||||
Share of profit / (loss) of joint ventures and associates(1) | 297,939 | (232,404 | ) | (1,109,131 | ) | (848,948 | ) | (584,728 | ) | ||||||
Profit / (loss) before income tax | 2,179,603 | 2,932,070 | 3,336,596 | (7,905,477 | ) | 9,031,066 | |||||||||
Income tax benefit / (expense) | (489,761 | ) | (1,052,834 | ) | (1,789,654 | ) | 2,443,866 | (3,198,198 | ) | ||||||
Profit / (loss) for the year | 1,689,842 | 1,879,236 | 1,546,942 | (5,461,611 | ) | 5,832,868 | |||||||||
Profit / (loss) attributable to the equity holders of the parent company | 1,697,872 | 1,854,366 | 1,545,891 | (5,469,358 | ) | 5,834,608 | |||||||||
Profit / (loss) attributable to the non-controlling interests | (8,030 | ) | 24,870 | 1,051 | 7,747 | (1,740 | ) | ||||||||
1,689,842 | 1,879,236 | 1,546,942 | (5,461,611 | ) | 5,832,868 | ||||||||||
80
Rizobacter | |||||||||||||||
Three-month period ended September 30, | Year ended June 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | 2015 | |||||||||||
(Unaudited) | |||||||||||||||
(US$) | |||||||||||||||
Basic and diluted earnings / (loss) per share attributable to shareholders of the Company | 0.04 | 0.05 | 0.04 | (0.14 | ) | 0.15 | |||||||||
Other comprehensive income: | |||||||||||||||
Items that may be subsequently reclassified to profit and loss | |||||||||||||||
Exchange differences on translation of foreign operations | (893,658 | ) | (365,644 | ) | (1,865,399 | ) | (12,641,968 | ) | (2,790,627 | ) | |||||
Items that will not be subsequently reclassified to profit and loss | |||||||||||||||
Revaluation of property, plant and equipment, net of tax | — | — | 2,256,554 | 10,908,784 | 10,767,550 | ||||||||||
Other comprehensive income / (loss) | (893,658 | ) | (365,644 | ) | 391,155 | (1,733,184 | ) | 7,976,923 | |||||||
Total comprehensive income / (loss) | 796,184 | 1,513,593 | 1,938,097 | (7,194,795 | ) | 13,809,791 | |||||||||
Comprehensive income / (loss) attributable to: | |||||||||||||||
Equity holders of the parent company | 803,696 | 1,487,993 | 1,938,313 | (7,187,957 | ) | 13,803,650 | |||||||||
Non-controlling interests | (7,512 | ) | 25,603 | (216 | ) | (6,838 | ) | 6,141 | |||||||
796,184 | 1,513,593 | 1,938,097 | (7,194,795 | ) | 13,809,791 | ||||||||||
Non-IFRS measures: | |||||||||||||||
Rizobacter Adjusted EBITDA (unaudited)(2) | 7,324,977 | 7,402,051 | 23,301,133 | 16,303,882 | 23,896,825 | ||||||||||
| (1) | Accounted for using the equity method. |
| (2) | We define Rizobacter Adjusted EBITDA as profit/(loss) exclusive of financial income/(costs), income tax benefit/(expense), depreciation, amortization, share-based compensation and other items and charges. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Non-IFRS Financial Measures.” |
The table below provides a reconciliation of Rizobacter’s net profit/(loss) for the year to Rizobacter Adjusted EBITDA:
Rizobacter | |||||||||||||||
Three-month period ended September 30, | Year ended June 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | 2015 | |||||||||||
(Unaudited) | |||||||||||||||
(US$) | |||||||||||||||
Reconciliation of Net Loss to Rizobacter Adjusted EBITDA | |||||||||||||||
Net profit / (loss) for the year | 1,689,842 | 1,879,236 | 1,546,942 | (5,461,611 | ) | 5,832,868 | |||||||||
Income tax (benefit)/expense | 489,761 | 1,052,834 | 1,789,654 | (2,443,866 | ) | 3,198,198 | |||||||||
Finance costs | 5,719,583 | 5,028,406 | 19,450,815 | 27,025,501 | 15,944,826 | ||||||||||
Finance income | (1,198,551 | ) | (1,000,264 | ) | (2,080,709 | ) | (4,633,611 | ) | (2,032,019 | ) | |||||
Depreciation of property, plant and equipment | 469,397 | 431,272 | 2,038,380 | 1,761,478 | 920,271 | ||||||||||
Amortization of intangible assets | 154,945 | 10,567 | 556,051 | 55,991 | 32,681 | ||||||||||
Rizobacter Adjusted EBITDA (unaudited) | 7,324,977 | 7,402,051 | 23,301,133 | 16,303,882 | 23,896,825 | ||||||||||
81
The tables below show the adjustments to Rizobacter financial information needed for comparing information across periods:
Rizobacter | |||||||||||||||
Stand-alone for the twelve- month period ended June 30, 2017 | For the six- month period ended December 31, 2016 | For the six- month period ended June 30, 2017(1) | Reconciling items(2) | For the six- month period ended June 30, 2017(3) | |||||||||||
(US$) | |||||||||||||||
(a) | (b) | (a) – (b) | (c) | (a) – (b) + (c) | |||||||||||
Revenue | 112,296,212 | 67,837,770 | 44,458,442 | — | 44,458,442 | ||||||||||
Cost of sales | (58,838,471 | ) | (33,781,095 | ) | (25,057,376 | ) | (2,436,949 | ) | (27,494,324 | ) | |||||
Gross income / (loss) | 53,457,741 | 34,056,675 | 19,401,066 | (2,436,949 | ) | 16,964,117 | |||||||||
Operating income / (loss) | 21,815,833 | 17,450,809 | 4,365,024 | (3,543,008 | ) | 822,016 | |||||||||
Profit / (loss) before income tax | 3,336,596 | 8,302,065 | (4,965,470 | ) | (3,543,008 | ) | (8,508,478 | ) | |||||||
Income tax benefit / (expense) | (1,789,654 | ) | (2,903,336 | ) | 1,113,683 | 1,220,000 | 2,333,683 | ||||||||
Profit / (loss) | 1,546,942 | 5,398,729 | (3,851,786 | ) | (2,323,009 | ) | (6,174,795 | ) | |||||||
Total comprehensive income / (loss) | 1,938,097 | 3,208,815 | (1,270,717 | ) | (2,668,225 | ) | (3,938,942 | ) | |||||||
| (1) | Includes the Rizobacter’s stand-alone results of operations for the six-month period ended June 30, 2017. |
| (2) | This column includes the following PPA adjustments for the six-month period as of June 30, 2017, relating to the acquisition of Rizobacter: |
| i. | The cost of sales line includes a non-recurring incremental cost of US$2.4 million related to PPA adjustments for revalued inventories sold after the acquisition date. |
| ii. | The operating income line includes an incremental charge of depreciation for revalued property, plant and equipment of US$0.2 million, and an incremental charge of amortization for revalued intangible assets of US$0.9 million. |
| iii. | The income tax benefit / (expense) line includes the tax effects on the taxable adjustments included in the reconciling items column using an income tax rate of 35%. |
| (3) | This column represents the results of operations of Rizobacter as used for consolidation purposes of Bioceres. |
Rizobacter | |||||||||
Stand-alone for the three- month period ended September 30, 2017(1) | Reconciling items(2) | For the three- month period ended September 30, 2017(3) | |||||||
(US$) | |||||||||
(a) | (b) | (a) + (b) | |||||||
Revenue | 32,852,814 | — | 32,852,814 | ||||||
Cost of sales | (18,060,896 | ) | (1,119,155 | ) | (19,180,052 | ) | |||
Gross income / (loss) | 14,791,917 | (1,119,155 | ) | 13,672,762 | |||||
Operating income | 6,700,635 | (1,770,058 | ) | 4,930,577 | |||||
Profit / (loss) before income tax | 2,179,603 | (1,770,058 | ) | 409,545 | |||||
Income tax benefit / (expense) | (489,761 | ) | 602,860 | 113,099 | |||||
Profit / (loss) | 1,689,842 | (1,167,198 | ) | 522,644 | |||||
Total comprehensive income / (loss) | 796,184 | (1,167,198 | ) | (371,014 | ) | ||||
| (1) | Includes the Rizobacter’s stand-alone results of operations for the three-month period ended September 30, 2017. |
| (2) | This column includes the following PPA adjustments for the three-month period ended September 30, 2017, relating to the acquisition of Rizobacter: |
| (i) | The cost of sales line includes a non-recurring incremental cost of US$1.12 million related to PPA adjustments for revalued inventories sold after the acquisition date. |
| (ii) | The operating income line includes an incremental charge related for revalued property, plant and equipment of US$0.2 million, and an incremental charge of amortization for revalued intangible assets of US$0.4 million. |
| (iii) | The income tax benefit / (expense) line includes the tax effects on the taxable adjustments included in the reconciling items column using an income tax rate of 35%. |
| (3) | This column represents the results of operations of Rizobacter as used for consolidation purposes of Bioceres. |
82
Consolidated statement of financial position data of Rizobacter
The consolidated statements of financial position as of June 30, 2017, 2016 and 2015 are derived from Rizobacter’s audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statement of financial position as of September 30, 2017 is derived from Rizobacter’s unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus. However, this information: (i) has been prepared on a stand-alone basis; (ii) does not reflect the purchase accounting adjustments made at the consolidated level after the acquisition of Rizobacter; (iii) and does not include any corporate and other expenses that should be allocated to Rizobacter; (iv) uses different segments than the Bioceres consolidated financial statements; (v) is for information purposes only and (vi) should not be viewed as a substitute for the consolidated financial statements of Bioceres subsequent to the date of the acquisition of Rizobacter. The consolidated statements of financial position data of Rizobacter for the year ended June 30, 2017 and the three-month period ended September 30, 2017 are provided as additional information to permit readers to compare the more recent results of Rizobacter on a stand-alone basis.
Rizobacter | ||||||||||||
As of September 30, | As of June 30, | |||||||||||
2017 | 2017 | 2016 | 2015 | |||||||||
(Unaudited) | ||||||||||||
(US$) | ||||||||||||
ASSETS | ||||||||||||
Current assets: | ||||||||||||
Inventories | 33,981,195 | 27,574,042 | 23,187,382 | 31,608,911 | ||||||||
Trade and other receivables | 62,995,027 | 49,354,529 | 26,247,601 | 34,070,055 | ||||||||
Financial assets at fair value through profit or loss | 3,390 | 4,275 | 4,712 | 9,909 | ||||||||
Income tax credit | 1,209,792 | 1,701,381 | 1,800,997 | 676,727 | ||||||||
Other financial assets | 4,250,715 | 4,247,669 | — | — | ||||||||
Cash and cash equivalents | 1,175,691 | 940,895 | 1,030,067 | 3,671,219 | ||||||||
Total current assets | 103,616,410 | 83,822,791 | 52,270,759 | 70,036,821 | ||||||||
Non-current assets: | ||||||||||||
Property, plant and equipment | 36,889,065 | 38,604,264 | 40,197,525 | 35,955,482 | ||||||||
Intangible assets | 3,998,382 | 4,208,429 | 3,046,011 | 3,278,335 | ||||||||
Investment property | 870 | 906 | 999 | 1,653 | ||||||||
Investments in joint arrangements | 4,748,559 | 4,632,304 | 4,558,512 | 7,553,916 | ||||||||
Other financial assets | 128,471 | 92,805 | 14,669 | 25,637 | ||||||||
Trade and other receivables | 1,848,595 | 1,810,063 | 1,491,829 | 464,301 | ||||||||
Deferred tax assets | 2,125 | — | 8,813 | — | ||||||||
Total non-current assets | 47,616,068 | 49,348,771 | 49,318,358 | 47,279,324 | ||||||||
Total assets | 151,232,478 | 133,171,562 | 101,589,117 | 117,316,145 | ||||||||
LIABILITIES | ||||||||||||
Current liabilities: | ||||||||||||
Borrowings | 41,280,799 | 33,344,287 | 39,011,807 | 41,482,011 | ||||||||
Current tax liabilities | 77,748 | 29,789 | — | — | ||||||||
Trade and other payables | 40,745,720 | 26,817,961 | 18,577,552 | 24,760,679 | ||||||||
Total current liabilities | 82,104,267 | 60,192,037 | 57,589,359 | 66,242,690 | ||||||||
83
Rizobacter | ||||||||||||
As of September 30, | As of June 30, | |||||||||||
2017 | 2017 | 2016 | 2015 | |||||||||
(Unaudited) | ||||||||||||
(US$) | ||||||||||||
Non-current liabilities: | ||||||||||||
Borrowings | 36,462,304 | 40,450,586 | 13,225,810 | 12,377,904 | ||||||||
Provisions | 996,143 | 1,036,998 | 1,286,524 | 1,705,990 | ||||||||
Deferred tax liabilities | 7,627,799 | 8,234,885 | 6,925,457 | 5,130,246 | ||||||||
Trade and other payables | 274,898 | 286,173 | 80,824 | 56,362 | ||||||||
Total non-current liabilities | 45,361,144 | 50,008,642 | 21,518,615 | 19,270,502 | ||||||||
Total liabilities | 127,465,411 | 110,200,679 | 79,107,974 | 85,513,192 | ||||||||
EQUITY | ||||||||||||
Share capital and capital adjustments | 7,113,060 | 7,113,060 | 7,113,060 | 6,044,570 | ||||||||
Reserved profits and other equity components | 16,612,872 | 15,809,176 | 15,321,476 | 25,714,903 | ||||||||
Non-controlling interests | 41,135 | 48,647 | 46,607 | 43,480 | ||||||||
Total equity | 23,767,067 | 22,970,883 | 22,481,143 | 31,802,953 | ||||||||
Total liabilities and equity | 151,232,478 | 133,171,562 | 101,589,117 | 117,316,145 | ||||||||
The tables below show the adjustments to Rizobacter financial information needed for comparing information across periods:
Rizobacter | ||||||||||||
Stand-alone as of June 30, 2017(1) | Reconciling items(2) | Exhibition reclassifications | Pushed down as of June 30, 2017(3) | |||||||||
(US$) | ||||||||||||
(a) | (b) | (c) | (a) + (b) + (c) | |||||||||
Current assets | 83,822,791 | 2,379,324 | (3,152,239 | ) | 83,049,876 | |||||||
Non-current assets | 49,348,771 | 69,230,893 | (3,472 | ) | 118,576,191 | |||||||
Total assets | 133,171,562 | 71,610,217 | (3,155,711 | ) | 201,626,067 | |||||||
Current liabilities | 60,192,037 | — | (3,155,561 | ) | 57,036,475 | |||||||
Non-current liabilities | 50,008,642 | 15,595,895 | (150 | ) | 65,604,387 | |||||||
Total liabilities | 110,200,679 | 12,440,334 | (3,155,711 | ) | 119,485,302 | |||||||
Equity | 22,970,883 | 56,014,322 | — | 78,985,205 | ||||||||
Total liabilities and equity | 133,171,562 | 68,454,656 | (3,155,711 | ) | 198,470,506 | |||||||
| (1) | This column represents the financial position of Rizobacter on a stand-alone basis. |
| (2) | This column includes the following PPA adjustments related to the acquisition of Rizobacter: |
| i. | The non-current assets line mainly includes PPA valuation step-ups of US$7.4 million in revalued property, plant and equipment, US$34.7 million in revalued intangible assets and US$26.6 million in revalued investments in joint ventures. |
| ii. | The current assets line includes PPA valuation step-ups of US$2.4 million in remaining Rizobacter revalued inventories. |
| iii. | The non-current liabilities line includes PPA valuation step-ups of US$15.6 million in revalued deferred tax liabilities . |
| (3) | This column represents the financial position of Rizobacter as used for consolidation purposes of Bioceres. |
84
Rizobacter | |||||||||
Stand-alone as of September 30, 2017(1) | Reconciling items(2) | Pushed down as of September 30, 2017(3) | |||||||
(US$) | |||||||||
(a) | (b) | (a + b) | |||||||
Current assets | 103,616,410 | 1,163,360 | 104,779,770 | ||||||
Non-current assets | 47,616,068 | 61,760,440 | 109,376,508 | ||||||
Total assets | 151,232,478 | 62,923,800 | 214,156,278 | ||||||
Current liabilities | 82,104,267 | — | 82,104,267 | ||||||
Non-current liabilities | 45,361,144 | 14,376,944 | 59,738,088 | ||||||
Total liabilities | 127,465,411 | 14,376,944 | 141,842,355 | ||||||
Equity | 23,767,067 | 48,546,856 | 72,313,923 | ||||||
Total liabilities and equity | 151,232,478 | 62,923,800 | 214,156,278 | ||||||
| (1) | This column represents the financial position of Rizobacter on a stand-alone basis. |
| (2) | This column includes the following PPA adjustments related to the acquisition of Rizobacter: |
| (i) | The non-current assets line mainly includes PPA valuation step-ups of US$7.0 million in revalued property, plant and equipment, US$33.0 million in revalued intangible assets, US$21.5 million in revalued investments in joint ventures. |
| (ii) | The current assets line includes PPA valuation step-ups of US$1.2 million in remaining Rizobacter revalued inventories. |
| (iii) | The non-current liabilities line includes PPA valuation step-ups of US$14.4 million in revalued deferred tax liabilities. |
| (3) | This column represents the financial position of Rizobacter as used for consolidation purposes of Bioceres. |
85
Unaudited Pro Forma Consolidated Financial Information of Bioceres
The unaudited pro forma consolidated financial information has been derived by the application of pro forma adjustments to our historical consolidated financial information, which have been presented to give effect to the acquisition of Rizobacter, or the Acquisition, and certain other adjustments including the accounting effects of purchase price accounting and certain non-recurring transaction charges. The unaudited pro forma consolidated statements of income of Bioceres for the year ended December 31, 2016, for the six-month period ended June 30, 2016 and for the three-month and nine-month periods ended September 30, 2016 are presented as if the Acquisition had occurred on January 1, 2016.
You should read the information contained in this section in conjunction with “Unaudited Pro Forma Financial Information,” “Capitalization,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” our historical audited consolidated financial statements and the accompanying notes included elsewhere in this prospectus.
Unaudited Pro Forma Consolidated Statement of Income for the Twelve-Month Period Ended December 31, 2016:
Unaudited Pro Forma Consolidated Statement of Income for the Twelve-Month Period Ended December 31, 2016 | |||||||||||||||||
Historical Bioceres(1) | Historical Rizobacter(2) | Pro Forma Adjustments(3) | Pro Forma Combined(4) | ||||||||||||||
(all amounts expressed in US$, unless otherwise indicated) | |||||||||||||||||
Revenue | 43,587,834 | 60,070,278 | (360,968 | ) | 103,297,144 | ||||||||||||
Government grants | 761,429 | — | — | 761,429 | |||||||||||||
Total revenue | 44,349,263 | 60,070,278 | (360,968 | ) | 104,058,573 | ||||||||||||
Cost of sales | (31,600,998 | ) | (29,386,577 | ) | 7,518,256 | (53,469,319 | ) | ||||||||||
Research and development expenses | (2,860,771 | ) | (1,720,738 | ) | (893,891 | ) | (5,475,400 | ) | |||||||||
Selling, general and administrative expenses | (12,906,021 | ) | (20,520,583 | ) | (321,644 | ) | (33,748,248 | ) | |||||||||
Share of profit / (loss) of joint ventures and associates | (936,769 | ) | (730,365 | ) | 389,358 | (1,277,776 | ) | ||||||||||
Other income | 48,495 | 287,657 | — | 336,152 | |||||||||||||
Operating income / (loss) | (3,906,801 | ) | 7,999,672 | 6,331,111 | 10,423,982 | ||||||||||||
Gain on previously held interest | 4,453,284 | — | (4,453,284 | ) | — | ||||||||||||
Finance income | 1,006,953 | 1,433,352 | (102,369 | ) | 2,337,937 | ||||||||||||
Finance costs | (10,923,378 | ) | (17,293,817 | ) | (4,626,933 | ) | (32,844,128 | ) | |||||||||
Loss before income tax | (9,369,942 | ) | (7,860,792 | ) | (2,851,475 | ) | (20,082,209 | ) | |||||||||
Income tax benefit | 4,140,028 | 1,395,632 | (491,161 | ) | 5,044,499 | ||||||||||||
Loss for the year | (5,229,914 | ) | (6,465,160 | ) | (3,342,636 | ) | (15,037,710 | ) | |||||||||
Attributable to: | |||||||||||||||||
Equity holders of the parent | (4,508,061 | ) | (3,879,096 | ) | (4,828,174 | ) | (13,215,332 | ) | |||||||||
Non-controlling interest | (721,853 | ) | (2,586,064 | ) | 1,485,538 | (1,822,379 | ) | ||||||||||
Weighted-average number of shares outstanding | |||||||||||||||||
Basic | 25,592,104 | — | — | 25,592,104 | |||||||||||||
Diluted | 25,592,104 | — | — | 25,592,104 | |||||||||||||
Basic earnings per share attributable to equity holders of the parent | (0.18 | ) | — | — | (0.52 | ) | |||||||||||
Diluted earnings per share attributable to equity holders of the parent | (0.18 | ) | — | — | (0.52 | ) | |||||||||||
| (1) | Represents the historical consolidated statement of income of Bioceres for the twelve-month period ended December 31, 2016 and includes (i) Rizobacter’s results since its acquisition on October 19, 2016 and (ii) PPA-related accounting effects resulting from the revaluation of Rizobacter’s acquired inventories, property, plant and equipment and certain intangible assets, as well as certain consolidation-related eliminations. |
| (2) | Represents the historical statement of income of Rizobacter for the period from January 1, 2016 to October 18, 2016. |
| (3) | Represents the pro forma adjustments to reflect (i) the Rizobacter acquisition and (ii) the Semya consolidation and related eliminations for transactions occurred for the period from January 1, 2016 to October 18, 2016 between Rizobacter or Bioceres and Semya. |
86
| (4) | Represents the unaudited pro forma consolidated statement of income for the acquisition of Rizobacter under IFRS and reflects all adjustments described in note (3) above. See also the “Unaudited Pro Forma Consolidated Financial Information.” |
The table below provides a reconciliation of our loss for the year to Adjusted EBITDA included elsewhere in this prospectus:
Pro Forma Consolidated Statement of Income for the Twelve-Month Period Ended December 31, 2016 | |||
(unaudited) | |||
Reconciliation of Net Loss to Adjusted EBITDA: | |||
Loss for the period / year | (15,037,710 | ) | |
Income tax (benefit) / expense | (5,044,499 | ) | |
Finance costs | 32,844,128 | ||
Finance income | (2,337,937 | ) | |
Gain on previously held interest | — | ||
Depreciation of property, plant and equipment | 2,744,427 | ||
Amortization of intangible assets | 1,993,093 | ||
Inventory purchase price allocation charge | — | ||
Transaction expenses | — | ||
Stock-based compensation charges | 374,724 | ||
Adjusted EBITDA (unaudited) | 15,536,226 | ||
Unaudited Pro Forma Consolidated Statement of Income for the Six-Month Period Ended June 30, 2016:
Unaudited Pro Forma Consolidated Statement of Income for the Six-Month Period Ended June 30, 2016 | ||||||||||||
Historical Bioceres(1) | Historical Rizobacter(2) | Pro Forma Adjustments(3) | Pro Forma Combined(4) | |||||||||
(all amounts expressed in US$, unless otherwise indicated) | ||||||||||||
Revenue | 2,268,193 | 28,793,866 | (221,688 | ) | 30,840,371 | |||||||
Government grants | 270,206 | — | — | 270,206 | ||||||||
Total revenue | 2,538,399 | 28,793,866 | (221,688 | ) | 31,110,577 | |||||||
Cost of sales | (1,290,256 | ) | (14,994,226 | ) | — | (16,284,482 | ) | |||||
Research and development expenses | (1,268,661 | ) | (1,013,694 | ) | (535,881 | ) | (2,818,236 | ) | ||||
Selling, general and administrative expenses | (3,541,058 | ) | (11,517,410 | ) | (592,929 | ) | (15,651,397 | ) | ||||
Share of profit / (loss) of joint ventures and associates | (455,181 | ) | (491,429 | ) | 279,772 | (666,838 | ) | |||||
Other income | — | 319,059 | — | 319,059 | ||||||||
Operating income / (loss) | (4,016,757 | ) | 1,096,166 | (1,070,727 | ) | (3,991,318 | ) | |||||
Finance income | 790,814 | 404,975 | (53,525 | ) | 1,142,264 | |||||||
Finance costs | (1,579,951 | ) | (11,453,062 | ) | (3,181,389 | ) | (16,214,402 | ) | ||||
Loss before income tax | (4,805,894 | ) | (9,951,921 | ) | (4,305,641 | ) | (19,063,456 | ) | ||||
Income tax benefit | 1,040,923 | 2,478,057 | 1,546,036 | 5,065,016 | ||||||||
Loss for the period | (3,764,971 | ) | (7,473,864 | ) | (2,759,604 | ) | (13,998,440 | ) | ||||
Attributable to: | ||||||||||||
Equity holders of the parent | (3,435,546 | ) | (4,484,319 | ) | (2,467,844 | ) | (10,387,709 | ) | ||||
Non-controlling interest | (329,425 | ) | (2,989,546 | ) | (291,760 | ) | (3,610,731 | ) | ||||
Weighted-average number of shares outstanding | ||||||||||||
Basic | 25,337,763 | — | — | 25,593,700 | ||||||||
Diluted | 25,337,763 | — | — | 25,593,700 | ||||||||
87
Unaudited Pro Forma Consolidated Statement of Income for the Six-Month Period Ended June 30, 2016 | ||||||||||||
Historical Bioceres(1) | Historical Rizobacter(2) | Pro Forma Adjustments(3) | Pro Forma Combined(4) | |||||||||
(all amounts expressed in US$, unless otherwise indicated) | ||||||||||||
Basic earnings per share attributable to equity holders of the parent | (0.13 | ) | — | — | (0.41 | ) | ||||||
Diluted earnings per share attributable to equity holders of the parent | (0.13 | ) | — | — | (0.41 | ) | ||||||
| (1) | Represents the historical consolidated statement of income of Bioceres for the six-month period ended June 30, 2016. |
| (2) | Represents the historical statement of income of Rizobacter for the six-month period ended June 30, 2016. |
| (3) | Represents the pro forma adjustments to reflect (i) the effect of the Stock Split approved by our shareholders as if it had been completed as of January 1, 2016, (ii) the Acquisition and (iii) the Semya consolidation and related eliminations for transactions occurred for the period from January 1, 2016 to June 30, 2016 between Rizobacter or Bioceres and Semya. |
| (4) | Represents the unaudited pro forma consolidated statement of income for the Acquisition under IFRS and reflects all adjustments described in note (3) above. See also the “Unaudited Pro Forma Consolidated Financial Information.” |
The table below provides a reconciliation of our pro forma loss for the period to Adjusted EBITDA:
Pro Forma Consolidated Statement of Income for the Six-Month Period Ended June 30, 2016 | |||
(unaudited) | |||
Reconciliation of Net Loss to Adjusted EBITDA: | |||
Loss for the period / year | (13,998,440 | ) | |
Income tax (benefit) / expense | (5,065,016 | ) | |
Finance costs | 16,214,402 | ||
Finance income | (1,142,264 | ) | |
Gain on previously held interest | — | ||
Depreciation of property, plant and equipment | 1,580,558 | ||
Amortization of intangible assets | 584,395 | ||
Inventory purchase price allocation charge | — | ||
Transaction expenses | — | ||
Stock-based compensation charges | 585,955 | ||
Adjusted EBITDA (unaudited) | (1,240,409 | ) | |
88
Unaudited Pro Forma Consolidated Statement of Income for the Three-Month Period Ended September 30, 2016:
Unaudited Pro Forma Consolidated Statement of Income for the Three-Month Period Ended September 30, 2016 | ||||||||||||
Historical Bioceres(1) | Historical Rizobacter(2) | Pro Forma Adjustments(3) | Pro Forma Combined(4) | |||||||||
(all amounts expressed in US$, unless otherwise indicated) | ||||||||||||
Revenue | 1,846,295 | 26,153,338 | (139,280 | ) | 27,860,353 | |||||||
Government grants | 117,218 | — | — | 117,218 | ||||||||
Total revenue | 1,963,513 | 26,153,338 | (139,280 | ) | 27,977,571 | |||||||
Cost of sales | (1,118,948 | ) | (11,608,790 | ) | — | (12,727,738 | ) | |||||
Research and development expenses | (596,652 | ) | (533,601 | ) | (267,940 | ) | (1,398,194 | ) | ||||
Selling, general and administrative expenses | (1,185,472 | ) | (6,794,631 | ) | (296,520 | ) | (8,276,623 | ) | ||||
Share of profit / (loss) of joint ventures and associates | (238,100 | ) | (232,404 | ) | 109,587 | (360,917 | ) | |||||
Other expenses, net | (18,135 | ) | (23,699 | ) | — | (41,834 | ) | |||||
Operating income / (loss) | (1,193,794 | ) | 6,960,213 | (594,154 | ) | 5,172,265 | ||||||
Finance income | 381,327 | 1,000,264 | 48,844 | 1,430,434 | ||||||||
Finance costs | (1,117,154 | ) | (5,028,406 | ) | (1,624,247 | ) | (7,769,807 | ) | ||||
Loss before income tax | (1,929,621 | ) | 2,932,071 | (2,169,558 | ) | (1,021,161 | ) | |||||
Income tax benefit / (expense) | 621,022 | (1,052,834 | ) | 789,755 | 306,863 | |||||||
Profit / (loss) for the year | (1,308,599 | ) | 1,879,237 | (1,379,802 | ) | (714,299 | ) | |||||
Attributable to: | ||||||||||||
Equity holders of the parent | (1,214,922 | ) | 1,127,542 | (1,233,922 | ) | (1,321,302 | ) | |||||
Non-controlling interest | (93,677 | ) | 751,695 | (145,880 | ) | 512,138 | ||||||
Weighted-average number of shares outstanding | ||||||||||||
Basic | 25,615,265 | — | — | 25,615,265 | ||||||||
Diluted | 25,615,265 | — | — | 25,615,265 | ||||||||
Basic earnings per share attributable to equity holders of the parent | (0.05 | ) | — | — | (0.05 | ) | ||||||
Diluted earnings per share attributable to equity holders of the parent | (0.05 | ) | — | — | (0.05 | ) | ||||||
| (1) | Represents the historical consolidated statement of income of Bioceres for the three-month period ended September 30, 2016. |
| (2) | Represents the historical statement of income of Rizobacter for the three-month period ended September 30, 2016. |
| (3) | Represents the pro forma adjustments to reflect (i) the effect of the Stock Split approved by our shareholders as if it had been completed as of January 1, 2016, (ii) the Acquisition and (iii) the Semya consolidation and related eliminations for transactions occurred for the period from January 1, 2016 to September 30, 2016 between Rizobacter or Bioceres and Semya. |
| (4) | Represents the unaudited pro forma consolidated statement of income for the Acquisition under IFRS and reflects all adjustments described in note (3) above. See also the “Unaudited Pro Forma Consolidated Financial Information.” |
| The table below provides a reconciliation of our pro forma loss for the period to Adjusted EBITDA: |
Pro Forma Consolidated Statement of Income for the Three-Month Period Ended September 30, 2016 | |||
(unaudited) | |||
Reconciliation of Net Loss to Adjusted EBITDA: | |||
Loss for the period / year | (809,164 | ) | |
Income tax (benefit) / expense | (357,944 | ) | |
Finance costs | 7,769,807 | ||
Finance income | (1,430,434 | ) | |
Gain on previously held interest | — | ||
Depreciation of property, plant and equipment | 870,470 | ||
Amortization of intangible assets | 290,361 | ||
Inventory purchase price allocation charge | — | ||
Transaction expenses | — | ||
Stock-based compensation charges | 296,196 | ||
Adjusted EBITDA (unaudited) | 6,629,293 | ||
89
UNAUDITED PRO FORMA CONSOLIDATED FINANCIAL INFORMATION
The unaudited pro forma consolidated financial information has been derived by the application of pro forma adjustments to our historical consolidated financial information, which have been presented to give effect to the acquisition of Rizobacter, or the Acquisition, and certain other adjustments including the effect of the Stock Split approved by our shareholders, the accounting effects of purchase price accounting and certain non-recurring transaction charges. The unaudited pro forma consolidated statements of income of Bioceres for the year ended December 31, 2016, are presented as if the Acquisition had occurred on January 1, 2016.
Our historical financial information was derived from our audited consolidated financial statements as of and for the Transition Period and the years ended December 31, 2016 and 2015. The historical financial statements of Bioceres used in preparing the unaudited pro forma financial data should be read in conjunction with its historical financial statements and risk factors, all of which are included elsewhere herein.
The unaudited pro forma adjustments are based on estimates, available information and certain assumptions that Bioceres believes are reasonable. The unaudited pro forma adjustments and primary assumptions are described in the accompanying notes. The unaudited pro forma consolidated statement of income is being provided for illustrative purposes only and does not purport to represent what our results of operations or result for the period would have been if the Transactions had occurred on the dates indicated and are not intended to project our results of operations or results for any future period. Any of the factors underlying these estimates and assumptions may change or prove to be materially different and the estimates and assumptions may not be representative of facts that exist upon completion of the Acquisition.
Our historical financial information includes certain accounting effects related to the application of purchase price allocation rules, or PPA. Under the acquisition method of accounting, the assets and liabilities and any identifiable intangible assets being acquired are generally recorded at the respective fair values on the acquisition date. The fair values estimated as of Rizobacter’s acquisition date represent management’s best estimates based on available information and facts and circumstances in existence on the acquisition date. There may be differences between these preliminary estimates of fair value and the final acquisition accounting, which differences could have a material impact on the accompanying unaudited pro forma consolidated financial information and the consolidated company’s future results of operations and financial position.
You should read the information contained in this section in conjunction with “Unaudited Pro Forma Financial Information,” “Capitalization,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” our historical audited consolidated financial statements and the accompanying notes included elsewhere in this prospectus.
90
Unaudited Pro Forma Consolidated Statement of Income for the Twelve-Month Period Ended December 31, 2016:
I Historical Bioceres | II Bioceres Pro Forma Adjustments | Notes | III Sub-total Bioceres | IV Historical Rizobacter | V Pro Forma Adjustments Rizobacter’s Acquisition | Notes | VI Pro Forma Adjustments Eliminations | VII Pro Forma Combined | |||||||||||||||||||
(All amounts expressed in US$, unless otherwise indicated) | |||||||||||||||||||||||||||
Revenue | 43,587,834 | — | 43,587,834 | 60,070,278 | — | (360,968 | ) | 103,297,144 | |||||||||||||||||||
Government grants | 761,429 | — | 761,429 | — | — | — | 761,429 | ||||||||||||||||||||
Total revenue | 44,349,263 | — | 44,349,263 | 60,070,278 | — | (360,968 | ) | 104,058,573 | |||||||||||||||||||
Cost of sales | (31,600,998 | ) | — | (31,600,998 | ) | (29,386,577 | ) | 7,518,256 | — | (53,469,319 | ) | ||||||||||||||||
Research and development expenses | (2,860,771 | ) | — | (2,860,771 | ) | (1,720,738 | ) | (893,891 | ) | F | — | (5,475,400 | ) | ||||||||||||||
Selling, general and administrative expenses | (12,906,021 | ) | 599,150 | A | (12,306,871 | ) | (20,520,583 | ) | (910,756 | ) | F | (10,039 | ) | (33,748,248 | ) | ||||||||||||
Share of loss of joint ventures | (936,769 | ) | — | (936,769 | ) | (730,365 | ) | — | 389,358 | (1,277,776 | ) | ||||||||||||||||
Other income | 48,495 | — | 48,495 | 287,657 | — | — | 336,152 | ||||||||||||||||||||
Operating profit/(loss) | (3,906,801 | ) | 599,150 | (3,307,651 | ) | 7,999,672 | 5,713,610 | 18,351 | 10,423,982 | ||||||||||||||||||
Gain on previously held interest | 4,453,284 | (4,453,284 | ) | B | — | — | — | — | — | ||||||||||||||||||
Finance income | 1,006,953 | — | 1,006,953 | 1,433,352 | — | (102,369 | ) | 2,337,937 | |||||||||||||||||||
Finance costs | (10,923,378 | ) | 527,794 | C | (8,285,114 | ) | (17,293,817 | ) | (5,131,864 | ) | G | (22,863 | ) | (32,844,128 | ) | ||||||||||||
Loss before income tax | (9,369,942 | ) | (3,326,340 | ) | (10,395,584 | ) | (7,860,792 | ) | 581,746 | (106,881 | ) | (20,082,209 | ) | ||||||||||||||
Income tax benefit/(charge) | 4,140,028 | (394,430 | ) | D | 3,745,598 | 1,395,632 | (203,611 | ) | H | 106,881 | 5,044,499 | ||||||||||||||||
Profit/(Loss) for the Year | (5,229,914 | ) | (3,720,770 | ) | (8,950,684 | ) | (6,465,160 | ) | 378,135 | — | (15,037,710 | ) | |||||||||||||||
Attributable to: | |||||||||||||||||||||||||||
Equity holders of the parent | (4,508,061 | ) | (3,720,770 | ) | A | (8,228,831 | ) | (3,879,096 | ) | (1,107,404 | ) | — | (13,215,332 | ) | |||||||||||||
Non controlling interest | (721,853 | ) | — | A | (721,853 | ) | (2,586,064 | ) | 1,485,538 | — | (1,822,379 | ) | |||||||||||||||
Weighted-average number of shares outstanding | |||||||||||||||||||||||||||
Basic | 25,592,104 | — | 25,592,104 | — | — | — | 25,592,104 | ||||||||||||||||||||
Diluted | 25,592,104 | — | 25,592,104 | — | — | — | 25,592,104 | ||||||||||||||||||||
Basic earning per share attributable to equity holders of the parent | (0.18 | ) | — | (0.32 | ) | — | — | — | (0.52 | ) | |||||||||||||||||
Diluted earning per share attributable to equity holders of the parent | (0.18 | ) | — | (0.32 | ) | — | — | — | (0.52 | ) | |||||||||||||||||
91
Notes to the Pro Forma Consolidated Statement of Income for the Twelve-Month Period Ended December 31, 2016
| I. | This column represents the historical consolidated statement of income of Bioceres for the twelve-month period ended December 31, 2016. It includes Rizobacter’s results since its acquisition on October 19, 2016, and it also includes de PPA-related accounting effects resulting from the revaluation of Rizobacter’s acquired inventories, property, plant and equipment and certain intangible assets, as well as certain consolidation-related eliminations. |
| II. | This column represents the pro forma adjustments to reflect the results excluding certain non-recurring transaction charges and the effect of the Stock Split approved by our shareholders as if it had been completed as of January 1, 2016, as follows: |
| A. | These adjustments consist of non-recurring charges incurred by Bioceres directly attributed to the acquisition of Rizobacter that mainly include legal fees, due diligence expenses and valuation assistance services. |
| B. | This adjustment represents the non-recurring gain obtained on the equity interest previously held by Bioceres in Semya, as a result of the group acquiring full ownership of Semya due to the acquisition of Rizobacter. |
| C. | These adjustments consist of non-recurring charges that mainly include financial commissions incurred in relation to the funding of the Rizobacter acquisition. |
| D. | This adjustment represents the tax effects on the taxable pro forma adjustments included in column II using an income tax rate of 35%. |
| III. | This column represents the subtotal for the statement of income of Bioceres after the computation of the pro forma adjustments included in column II. |
| IV. | This column represents the historical statement of income of Rizobacter for the period from January 1, 2016 to October 18, 2016 (as Rizobacter’s results for the period from October 19, 2016 to December 31, 2016, are already incorporated in Bioceres historical consolidated financial statements presented in column I). |
| V. | This column shows the pro forma adjustments for the Rizobacter acquisition, as follows: |
| E. | This adjustment is made to eliminate the impact of inventory fair value adjustments recognized in Bioceres results that represent one-time adjustments directly attributed to the Rizobacter acquisition that will not have recurring significant ongoing impact in excess of one year. Therefore, the impact has been backed out of the pro forma financial results. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Effects of purchase price allocation.” |
| F. | This adjustment includes the incremental charge of depreciation and amortization for the period from January 1, 2016 to October 18, 2016, resulting from the higher depreciation of Rizobacter’s revalued property, plant and equipment and from the higher amortization of Rizobacter’s revalued intangible assets due to PPA accounting revaluation of Rizobacter’s assets (as the incremental depreciation and amortization charges corresponding to the period from October 19, 2016 to December 31, 2016 are already incorporated in Bioceres historical consolidated financial statements presented in column I). |
| G. | This adjustment includes the incremental financial costs related to the bridge financing facilities and acquisition deferred payments related to the acquisition of Rizobacter for the period from January 1, 2016 to October 18, 2016. |
| H. | This adjustment represents the tax effects on the taxable adjustments and charges included in column V using an income tax rate of 35%. |
92
| VI. | This column represents the Semya consolidation and related eliminations for transactions occurred for the period from January 1, 2016 to October 18, 2016 between Rizobacter or Bioceres and Semya. Semya became a subsidiary after Rizobacter’s acquisition on October 19, 2016 (as Semya consolidation and related eliminations for the period from October 19, 2016 to December 31, 2016 are already incorporated in Bioceres historical consolidated financial statements presented in column I). These include: |
| i. | The elimination of sales between Rizobacter and Bioceres invoiced to Semya during that period; |
| ii. | The inclusion of Semya’s general and administrative expenses, finance costs and income tax benefit; |
| iii. | The elimination of the share of loss of joint ventures relating to Semya recognized in Rizobacter and Bioceres; and |
| iv. | The elimination of financial income recognized in Bioceres for loans to Semya. |
| VII. | This column represents the unaudited pro forma consolidated statement of income for the acquisition of Rizobacter under IFRS and reflects all adjustments in columns I to VI above. |
| VIII. | Adjusted EBITDA is a non-IFRS measure. The table below provides a reconciliation of our loss for the year to Adjusted EBITDA: |
Reconciliation of Net Loss to Adjusted EBITDA: | |||
Loss for the period | (15,037,710 | ) | |
Income tax (benefit) / expense | (5,044,499 | ) | |
Finance costs | 32,844,128 | ||
Finance income | (2,337,937 | ) | |
Gain on previously held interest | — | ||
Depreciation of property, plant and equipment | 2,744,427 | ||
Amortization of intangible assets | 1,993,093 | ||
Inventory purchase price allocation charge | — | ||
Transaction expenses | — | ||
Stock-based compensation charges | 374,724 | ||
Adjusted EBITDA (unaudited) | 15,536,226 |
93
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
Overview
We are a fully-integrated provider of crop productivity solutions, including seeds, seed traits, seed treatments, biologicals, high-value adjuvants and fertilizers. We have developed a multi-discipline and multi-product platform capable of providing solutions throughout the entire crop cycle, from pre-planting to transportation and storage. Our headquarters and primary operations are based in Argentina, which is our key end-market as well as one of the largest markets globally for GM crops. We leverage our relationship with our 308 shareholders, many of whom are industry leaders and key participants in our end-markets, to increase adoption of our products and technologies.
As of September 30, 2017, we owned or licensed approximately 300 registered products and we owned or licensed, either exclusively or non-exclusively, approximately 200 patents and patent applications. For the twelve-month period ended June 30, 2017, we distributed over 12.3 million doses of inoculants, seven million liters of adjuvants, three thousand tons of high value fertilizers as well as other agricultural inputs across 25 countries, including Argentina, Brazil, Paraguay, China, India, the United States and Uruguay, among others. For the three-month period ended September 30, 2017, we distributed over 3.4 million doses of inoculants, 1.8 million liters of adjuvants, 1.2 thousand tons of high value fertilizers as well as other agricultural inputs across Argentina, Brazil, Paraguay, China, India, the United States and Uruguay, among others. Our pipeline of products includes fertilizers, inoculants, adjuvants, baits, crop protection solutions and seeds. Our pro forma net revenue, net loss and Adjusted EBITDA for the nine-month period ended September 30, 2017 were US$83.3 million, US$14.1 million and US$7.9 million, respectively. Our pro forma net revenue, net loss and Adjusted EBITDA (including that of Rizobacter) for the nine-month period ended September 30, 2016 were US$59.1 million, US$14.8 million and US$5.4 million, respectively. Our pro forma net revenue, net loss and Adjusted EBITDA (including that of Rizobacter) for the year ended December 31, 2016 were US$104.1 million, US$15.0 million and US$15.5 million, respectively. Our net revenue, net loss and Adjusted EBITDA for the year ended December 31, 2016 were US$44.3 million, US$5.2 million and US$6.4 million, respectively. Our net revenue, net loss and Adjusted EBITDA for the three-month period ended September 30, 2017 were US$35.0 million, US$2.9 million and US$5.6 million, respectively. Our pro forma net revenue, net loss and Adjusted EBITDA (including that of Rizobacter) for the three-month period ended September 30, 2016 were US$28.0 million, US$0.8 million and US$6.6 million, respectively. Our net revenue, net loss and Adjusted EBITDA for the Transition Period were US$48.3 million, US$11.2 million and US$2.3 million respectively. Our pro forma net revenue, net loss and Adjusted EBITDA (including that of Rizobacter) for the six-month period ended June 30, 2016 were US$31.1 million, US$14 million and US$1.2 million, respectively. Adjusted EBITDA is a non-IFRS financial measure. Net loss is the most directly comparable measure calculated in accordance with IFRS.
Our business model is driven by three key pillars: technology sourcing, product development partnering, and production and market access, and we divide our business into the following four principal segments: crop protection, seed and integrated products, crop nutrition and emerging solutions. Our diversified platform generates revenues through multiple technologies, customers, distribution channels and end-markets, providing us with a profitable growth trajectory. Our leading infrastructure, the success of our platform and commanding presence in our key markets have made us the effective flagship agricultural solutions provider, as well as the natural partner for global conglomerates, in South America.
Financial Presentation
The consolidated statements of comprehensive income data for Bioceres for the Transition Period, for the unaudited six-month period ended June 30, 2016 and for the years ended December 31, 2016 and 2015 and the summary consolidated statements of financial position data as of June 30, 2017 and as of December 31, 2016 and 2015 are derived from our audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statements of comprehensive income data for Bioceres for the three-month period ended September 30, 2017 and 2016 and the summary consolidated statements of financial position data as of September 30, 2017 are derived from our unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus.
94
The consolidated statements of comprehensive income data of Rizobacter for the years ended June 30, 2017, 2016 and 2015 and the summary consolidated statements of financial position data as of June 30, 2017, 2016 and 2015 are derived from Rizobacter’s audited consolidated financial statements appearing elsewhere in this prospectus. The consolidated statements of comprehensive income data for Rizobacter for the three-month periods ended September 30, 2017 and 2016 and the summary consolidated statements of financial position data as of September 30, 2017 are derived from Rizobacter’s unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus. This financial information is: (i) prepared on a stand-alone basis; (ii) does not reflect the purchase accounting adjustments recorded at the consolidated level of Bioceres after the acquisition; (iii) does not include any corporate and other expenses that should be allocated to Rizobacter; (iv) uses different segments than the Bioceres consolidated financial statements; (v) is for information purposes only and (vi) should not be viewed as a substitute for the consolidated financial position of Bioceres at June 30, 2017. The consolidated historical financial information of Rizobacter for the year ended June 30, 2017 and for the three-month period ended September 30, 2017 is provided as additional information to permit readers to compare the more recent results of Rizobacter on a stand-alone basis.
Our presentation currency is U.S. dollars. We account for our 50% equity interests in our joint ventures as equity method investments in our consolidated financial statements.
Rizobacter’s functional currency is the Argentine peso and its presentation currency is the U.S. dollar. Rizobacter accounts for its 50% equity interests in its joint ventures as equity method investments in its consolidated financial statements.
On December 16, 2016, the shareholders of Bioceres approved a change in our fiscal year end from December 31 to June 30. Following the Transition Period, our fiscal year will end on June 30 of each year. Due to this change, the Transition Period figures presented in our consolidated financial statements are not entirely comparable to the years ended December 31, 2016 and 2015.
We have applied the following standards and amendments for the first time for our annual reporting period commencing January 1, 2017:
| ■ | Recognition of Deferred Tax Assets for Unrealized Losses – Amendments to IAS 12; and |
| ■ | Disclosure Initiative – Amendments to IAS 7. |
The adoption of these amendments did not have any impact on the amounts recognized in prior periods. The amendments to IAS 7 require disclosure of changes in liabilities arising from financing activities.
As of June 30, 2017, our losses were higher than 50% of our capital and reserves. Under Argentine Corporate Law (Ley General de Sociedades), Section 206 establishes a mandatory reduction of capital when such a situation occurs. Additionally, even though our total consolidated equity was $28.9 million, we presented negative equity attributable to our equity holders. Section 94 (paragraph 5) of the Argentine Corporate Law states that when losses exceed a company’s capital and reserves balance at its annual fiscal close, such situation constitutes a cause for dissolution, and hence its shareholders shall take the necessary measures to capitalize the company. The six-month transition period ended June 30, 2017 presents negative equity which was mainly attributable as a result from the effects related to the Rizobacter acquisition, including (i) the step up charge included in the cost of sales of Rizobacter inventories acquired and sold post-acquisition that were revalued at acquisition due to the application of purchase price allocation, or PPA, accounting rules under IFRS, (ii) currency exchange differences relating to Argentine peso-denominated assets and liabilities acquired, (iii) the evolution of the fair value of assets and liabilities acquired, and (iv) the classification of certain capital raises through convertible loans and puttable instruments as liabilities and through convertible preferred shares as non-controlling interest under IFRS. The ongoing plan to reverse the negative equity balance is to capitalize the company through the capital increase approved by the Shareholder meetings held on December 17, 2014 and December 15, 2016 and placement of the newly issued shares in a public or private offering in Argentina and abroad, and the expected conversion of certain financing instruments into ordinary shares.
The division of segment information for Rizobacter corresponds in part to the segment reporting of Bioceres. The Rizobacter segment “Inoculants” fits within the “Crop Nutrition” segment of Bioceres, whereas the Rizobacter segment “Packs” makes up part of the “Seed and Integrated Products” segment of Bioceres. Both “Therapics” and “Adjuvants”
95
fall within the Bioceres segment “Crop Protection”. Rizobacter’s “Fertilizers and Others” segment, however, contains both fertilizers, which are included in “Crop Nutrition”, and a suite of other products that fall within Bioceres’ “Crop Protection” segment.
Factors Affecting Our Results of Operations
Our results of operations have been influenced and will continue to be influenced by the following factors:
Market demand for our products and services
Our sales and profitability are influenced by the demand for our crop productivity products, agro-industrial products and R&D services in the particular markets in which it operates.
Demand for our seed and integrated products, crop nutrition products and crop protection products is affected by the purchase decisions of our distributors and customers, which are typically driven by fluctuation in agricultural commodities prices and planting decisions, as well as externalities such as general market conditions, grower production decisions, commodity prices, operating costs and weather conditions. Demand for our R&D services is primarily affected by the number of R&D projects under development by our clients and the volume of technical services required by our joint venture partners and strategic collaborators, which is in turn driven by the quantity of joint venture partners we have and the number and scope of the strategic collaborations to which we are party. Demand for our agro-industrial products is affected by the purchase decisions of our enzyme clients and distributors, which are typically driven by demand for the end-product for which they are employed to produce, such as cheese in the case of chymosin we produce, as well as macroeconomic and other factors affecting the industries and companies employing our agro-industrial technologies and products.
Fluctuations in commodity prices
Our results of operations, particularly the demand and price for our seed and integrated products, crop production products and crop nutrition products, are affected by global agricultural commodities prices, such as grains, milk, meat, biofuels and biomaterials. Global prices of agricultural commodities vary in accordance with domestic and export market prices, which are primarily affected by the local and global demand for, and supply of, those commodities. Prices for agricultural commodities are also significantly influenced by speculative actions and by currency exchange rates, volatility in credit markets and fluctuation in consumer and business confidence. In addition, prices for agricultural commodities are affected by governmental programs and policies regarding agriculture, as well as general trade, fiscal and exchange control policies. Extrinsic factors, such as drought, floods, general weather conditions, disease and natural disasters may also affect agricultural commodities prices. Demand for agricultural commodities, such as wheat and soybeans, both for human consumption and as cattle feed, has generally increased with worldwide economic growth and prosperity.
Seasonality and weather conditions
Our revenues fluctuate depending on the timing of orders from our distributors and customers and on prevailing seed market prices, which influence the purchase decisions of growers, the end users of our seed and integrated products, crop protection products and crop nutrition products. Given the cyclicality of crop planting and harvesting and South America’s planting and growing seasons, which vary from year to year, our business is highly seasonal. This results in substantial fluctuations in quarterly sales and profitability. Generally, our sales are concentrated in the third and fourth quarters of each calendar year, when demand for our seed and integrated products, crop protection products and crop nutrition products increases as growers begin planting their fields. With our seed and integrated products business, we contract with growers and seed suppliers based upon our anticipated market demand. Generally, in our seed and integrated products business we stock the seed during the harvest season and ship from inventory throughout the year, with the objective of selling most of the inventory from the current year’s harvest before the next year’s, with our crop protection and our crop nutrition business following a similar cycle to the seed cycle. The impact of seasonality and the resulting fluctuations in quarterly results may be moderated as we achieve our international expansion plans for our seed business in geographies with contrasting seasons and climates. Milestone, royalty and license revenues are also likely to fluctuate from period to period given the seasonality of agriculture and time required to progress from one milestone to the next.
Our seed and integrated products, crop protection and crop nutrition businesses are also affected by unpredictable weather conditions such as heavy rains, hail, floods, freezing conditions, windstorms, drought or fire, as well as other
96
factors beyond our control, which may cause our sales and operating results to fluctuate significantly. In addition, disruptions that cause delays by growers in harvesting or planting can result in the movement of orders to a future quarter, which also causes fluctuations in our quarterly operating results. Finally, some of our customers and distributors order in bulk only one or two times a year, which may further cause our seed product revenues to fluctuate from period to period.
Stages of development of our products
Our results of operations will vary depending on the stage of development of our products and technologies. Some of our important technologies are currently in the early stages of development and our historical operating results are not indicative of the operating results we expect to experience in later stages of product development. As we are able to advance such technology through the development and regulatory phases to commercial launch, we expect our revenues and cash flows to increase. In particular, we expect our operating results prior to the time we fully launch and commercialize HB4 and related technologies included in our EcoSeed products to differ significantly from our operating results following such product launch.
As our seed and integrated products, crop protection and crop nutrition businesses continue to develop internationally, we expect to experience an increase in sales of fertilizers and inoculants from our crop nutrition solutions, adjuvants and other crop protection solutions and seed and integrated products as well as generating additional revenues from licensing fees from third parties and our joint venture partners, due to the introduction of our seed trait products to the global market, as these become commercially available, and to the acquisition of Rizobacter and its extensive distribution network. We will also continue to generate license fees from upfront payments and milestone payments that we receive from third parties pursuant to research and license agreements, as well as royalty fees from distributors and from growers who save harvested seeds that contain our technology and then use the seeds in subsequent harvests. We also expect to generate additional revenues from distribution fees that our joint venture partners pay to our proprietary distribution channels for selling seed and integrated products that incorporate our technologies.
Our emerging solutions segment currently generates income primarily with R&D and technical services fee income. The segment also generates other results through our participation in our joint venture AGBM that commercializes chymosin, an enzyme used in the cheese manufacturing industry, pursuant to contracts with third-party distributors and customers. We plan to generate additional revenues in the future by commercializing our Bacillus fermentation solutions for the production of biopolymers as well as other higher value molecules and compounds, particularly ag-biologicals that we can deploy in our integrated EcoSeed products, which are customized seed treatments for our trait-germplasm combinations.
Our costs are significantly impacted by the stage of development of our products and technologies, requiring, for example, expenditures in the research, development and regulatory phases of a product without corresponding flows of revenue until the time of commercial launch. Technology sourcing and product development expenses may fluctuate from period to period and may also increase significantly if we choose to accelerate certain technology sourcing and product development programs or if we elect to take a greater role in the regulatory and commercialization process with respect to one or more of our crop productivity technologies or products in development incorporating our crop productivity technologies.
Regulatory environment
Our results of operations will vary depending on the speed in which we are able to obtain regulatory approvals for our products and the cost and expense associated with gaining such approvals. The degree of regulation to which we are subject varies by activity and country. Our ability to sell our technologies and products depends on our obtaining and maintaining necessary authorizations, permits and regulatory approvals in the markets in which we operate. As of the date of this prospectus, we have obtained regulatory approval for our HB4 technology for soybeans in Argentina and the United States (pending USDA approval), and have been seeking to obtain regulatory approval for several technologies in Argentina and Uruguay, with plans to subsequently seek regulatory approvals either directly or through our joint ventures or collaborators in other large agricultural markets such as Brazil and the United States. Additionally, as we and our third-party collaborators develop new technologies, our results will be affected by our ability to achieve successful product launch and commercialization of approved technologies.
Effects of export taxes on our products
Following the economic and financial crisis experienced by Argentina in 2002, the Argentine government increased export taxes on agricultural products, mainly on soybean and its derivatives, wheat, rice and corn. However, as of
97
December 2015, the Macri administration has eliminated farm export duties on corn, wheat and other agricultural products such as rice and alfalfa, while soy export duties were reduced by 5% to a 30% tariff for soybean and a 27% tariff for most soybean products. On January 2, 2017, the current administration enacted a further reduction of the export duty rate set for soybean and soybean products, which set a monthly 0.5% cut on such export duty rates starting in January 2018 and continuing until December 2019. As local prices are determined taking into consideration the export parity reference, any change in export taxes would affect its financial results.
Effects of purchase price allocation
Since the Rizobacter acquisition on October 19, 2016, we have consolidated Rizobacter into our group’s accounting, and applied rules related to accounting purchase price allocation, or PPA, that have significantly impacted our balance sheet and results since the acquisition date. PPA rules required us to revalue certain assets at fair market value, including certain inventories, property, plant and equipment, intangible assets and investments in certain joint ventures, including Rizobacter’s 50% equity interests in Synertech and Semya (of which we control the remaining 50% equity interests). Our results of operations will be affected as the remaining revalued inventories are sold (recognizing a non-cash, non-recurring charge resulting in higher cost of goods sold related to the sale of those revalued inventories), the revalued property, plant and equipment is depreciated over its remaining useful life resulting in higher non-cash depreciation expenses, and the revalued intangible assets (including customer relationships, intellectual property and product registrations) are amortized over their remaining useful life resulting in higher non-cash amortization expenses.
As of December 31, 2016, our consolidated balance sheet included PPA valuation step-ups of US$4.9 million in remaining Rizobacter revalued inventories, US$8.1 million in revalued property, plant and equipment, US$38.5 million in revalued intangible assets, US$23.7 million in revalued investments in joint ventures and US$17.9 million in revalued deferred tax liabilities, as well as US$33.9 million of goodwill related to the Rizobacter acquisition. Our results of operations for the year ended December 31, 2016, were affected by the following PPA-related non-cash charges: (i) cost of sales included a non-recurring incremental cost of US$7.5 million related to PPA adjustments for revalued inventories sold since the acquisition date; (ii) R&D expenses and selling, general and administrative expenses of US$0.4 million related to incremental amortization charges from revalued intangible assets and property, plant and equipment. Those effects were partially offset by an income tax benefit related to PPA for US$2.8 million.
As of June 30, 2017, our consolidated balance sheet included PPA valuation step-ups of US$2.4 million in remaining Rizobacter revalued inventories, US$7.4 million in revalued property, plant and equipment, US$35.9 million in revalued intangible assets, US$22.4 million in revalued investments in joint ventures and US$16 million in revalued deferred tax liabilities, as well as US$32.3 million of goodwill related to the Rizobacter acquisition. Our results of operations for the Transition Period, were affected by the following PPA related non-cash charges: (i) cost of sales including a non-recurring incremental cost of US$2.4 million related to PPA adjustments for revalued inventories sold since the acquisition date; (ii) R&D expenses, including incremental depreciation charges of US$0.1 million related to revalued property, plant and equipment, and incremental amortization charges of US$0.4 million related to revalued intangible assets; (iii) selling, general and administrative expenses, including incremental depreciation charges of US$0.1 million related to revalued property, plant and equipment, and incremental amortization charges of US$0.5 million related to revalued intangible assets. Those effects were partially offset by an income tax benefit related to PPA for US$1.2 million.
As of September 30, 2017, our consolidated balance sheet included PPA valuation step-ups of US$1.1 million in remaining Rizobacter revalued inventories, US$7.0 million in revalued property, plant and equipment, US$34 million in revalued intangible assets, US$21.4 million in revalued investments in joint ventures and US$14.8 million in revalued deferred tax liabilities, as well as US$31.1 million of goodwill related to the Rizobacter acquisition. Our results of operations for the three-month period ended September 30, 2017, were affected by the following PPA related non-cash charges: (i) cost of sales including a non-recurring incremental cost of US$1.1 million related to PPA adjustments for revalued inventories sold during the three-month period; (ii) R&D expenses, including incremental depreciation charges of US$0.1 million related to revalued property, plant and equipment, and incremental amortization charges of US$0.2 million related to revalued intangible assets; and (iii) selling, general and administrative expenses, including incremental depreciation charges of US$0.1 million related to revalued property, plant and equipment, and incremental amortization charges of US$0.2 million related to revalued intangible assets. Those effects were partially offset by an income tax benefit related to PPA for US$0.6 million.
98
Macroeconomic conditions
Our financial condition and results of operations are affected by general macroeconomic conditions, fiscal policies and monetary measures in Argentina and other countries where we operate, including fluctuations in currency exchange rates, inflation and interest rates in those markets. Our results of operations are affected by the rate of depreciation or appreciation of the Argentine peso against the U.S. dollar, given that certain of our costs, assets and liabilities are denominated in Argentine pesos. Exchange differences resulting from the settlement of such transactions and from the translation at year-end exchange rates of monetary assets and liabilities are recognized in the finance gain or expense line. Our business, financial condition and results of operations may also be affected by inflation, since a significant proportion of our operating costs and expenses are incurred in Argentine pesos. High inflation rates could also affect our customers by adversely affecting their purchasing power and demand for our technologies, products and services. The impact of inflation in Argentina on our results of operations could be moderated as we undertake our international expansion plans in markets with lower inflation rates.
Note Regarding Comparability of Our Results of Operations
On December 15, 2016, our shareholders approved a change in our fiscal year end from December 31 to June 30. As a result of this change, the Transition Period figures presented in our consolidated financial statements are not entirely comparable to the years ended December 31, 2016 and 2015 and we do not present financial statements for a separate historical period that is comparable to the Transition Period. Following the Transition Period, we will prepare annual financial statements for fiscal years ending June 30, beginning with the fiscal year ended June 30, 2018.
The comparability of our results of operations is affected by the completion of the acquisition of Rizobacter consummated on October 19, 2016. Our results of operations for earlier periods that do not include Rizobacter may not be comparable to the results of a more recent period that reflects the results of such acquisition. See “Selected Consolidated Historical Financial and Other Information—Selected Consolidated Historical Financial Information of Rizobacter.”
Principal Components of our Results of Operations
Revenue
Revenues are generated by our four business segments: seed and integrated products, crop protection, crop nutrition, and emerging solutions.
Seed and integrated products. The seed and integrated products segment generates revenues primarily through sales of seeds and integrated products incorporating our proprietary or licensed technology to distributors and customers, as well as through receipt of royalty payments from distributors, through extended royalty payments from growers who save harvested seeds that contain our technology and then use the seeds in subsequent harvests, and through license fees in the form of upfront fees or milestone payments from third parties pursuant to license agreements. We generally recognize revenue from seed and integrated product sales upon delivery to third-party distributors or customers. We generally recognize distribution network royalty payments when the distributor reports the sales to us and recognizes extended royalty payment revenues when the non-governmental organization that manages the settlement system for extended royalty transactions, ARPOV (Asociación Argentina de Protección de las Obtenciones Vegetales), to which growers report and declare the amount of seed they save for future harvests, periodically settles and pays us the amount of extended royalties due and collected from growers. We generally recognize license fees as revenue when upfront fee payments are received for services rendered or when milestone fee related performance criteria are achieved. Milestones typically consist of significant stages of development for our seed traits in a potential commercial product, such as achievement of specific technological targets, completion of field trials, filing with regulatory agencies, completion of the regulatory process, and commercial launch of a product containing our seed traits.
Crop protection. The crop protection segment generates revenues primarily through sales of adjuvants, seed-applied insecticides and fungicides, acaricides and post-harvest pest control products. We generally recognize revenue from crop protection product sales upon delivery to third-party distributors or customers.
99
Crop nutrition. The crop nutrition segment generates revenues primarily through sales of inoculants, bioertilizers and micro-bead fertilizers. We generally recognize revenue from crop nutrition product sales upon delivery to third-party distributors or customers.
Emerging solutions. This segment currently generates revenues mainly through the provision of R&D and technical services to our joint venture partners and selected third parties pursuant to negotiated contractual arrangements as well as genomic sequencing and related data processing services. We generally recognize R&D services revenues as these services are provided. This segment also includes revenues and income generated by other products and services, including income generated by sales of chymosin enzyme through our joint venture AGBM, an enzyme used in the cheese manufacturing industry, pursuant to contracts with third-party distributors and customers. We generally recognize revenue from enzyme sales in AGBM upon delivery to our distributors or customers, and we account our participation in AGBM results through equity accounting.
Cost of sales
Cost of sales consists of direct costs related to the sale of our products and services. We generally recognize these costs when our products and services are delivered and recognized as revenue in each of our four business segments.
Seed and integrated products—Cost of sales for our seed and integrated products consists primarily of costs of purchasing third-party licensed seed products and raw materials including seeds, production services and costs relating to processing and packaging of our proprietary and licensed seed products and royalty and license payments we must make to our suppliers of seed products and licensors of technology.
Crop protection—Cost of sales for our crop protection products consists primarily of production costs incurred in manufacturing our adjuvants and other crop protection products and technologies, costs of purchasing third-party products, and costs incurred in delivering formulation services to third parties.
Crop nutrition—Cost of sales for our crop nutrition products consists primarily of production costs incurred in manufacturing our inoculants, biofertilizers and micro-bead fertilizers.
Emerging solutions—Cost of sales consists primarily of salaries and related personnel costs of our R&D employees related to delivered services, payments to third-party suppliers and the costs of disposable materials such as seeds, laboratory supplies, fertilizer, water and soil.
Research and development expenses
Research and development expenses consist of costs incurred in the discovery, development and testing of products that incorporate our seed traits and technologies, except for the capitalized portion of these costs. These expenses include employee salaries and benefits, fees paid to third-party research providers, scientists and professionals for product registration and approval, fees associated with licensed technology, chemicals and supplies, the pro rata cost of our facility for R&D purposes and other external expenses. R&D costs are generally expensed as incurred.
Selling, general and administrative expenses
Selling, general and administrative expenses consist primarily of salaries and benefits of our commercial and administrative employees; professional service fees (including consulting, legal, accounting and audit fees); expenses for advertising, marketing and promotional activities; shipping and handling costs; business development expenses; depreciation of our property, plant and equipment; and overhead and office administration costs. Selling, general and administrative expenses also include allowances for bad or doubtful accounts. Following the completion of this offering, selling, general and administrative expenses will include expenses incurred in connection with our public company obligations, including as result of compliance with the rules promulgated by the NYSE and the CNV. Selling, general and administrative expenses are generally expensed as incurred.
Share of profit or loss of joint ventures
Share of profit or loss of joint ventures consists of our participation in the profits or losses generated by our 50% equity interest in our unconsolidated joint ventures, Trigall Genetics, Verdeca, S&W Semillas and Synertech, our 49.99% equity interest in AGBM and our 33% equity interest in Héritas based on the equity method and the elimination of 50% of unrealized profit arising from transactions between us and our 50% joint ventures to the extent that those transactions generate costs and are capitalized in the accounts of our joint venture. We have not received any distributions from any of these unconsolidated joint venture entities since their inception.
100
Finance income
Finance income consists primarily of interest income charged to clients that purchase our products under deferred payment financing terms, foreign currency exchange gains on monetary assets and liabilities and gains from interests of financial assets measured at amortized cost using the effective interest method.
Finance costs
Finance costs consist primarily of interest on outstanding loans, borrowings and other payables, foreign currency exchange losses on monetary assets and liabilities, deferred offering costs related to our earlier IPO process and losses from interest of financial liabilities measured at amortized cost using the effective interest method.
Income tax benefit/(expense)
Income tax benefit consists of the credit for income tax and the variation in deferred tax registered in the statement of profit or loss and other comprehensive income. Our income tax provision has not been historically significant, as we have incurred losses since our inception. As of September 30, 2017, we had unused tax losses carryforwards of US$6.9 million, net of US$0.2 million of allowance for unused tax losses. These unused tax losses carryforwards in Argentina expire five years from the time of incurrence, and in the United States, 20 years. The statutory tax rate in Argentina was 35% for each of 2016 and 2015.
Results of Operations
For purposes of this comparison, the operating results of Rizobacter, the subsidiary we acquired in October 2016, are also shown separately on a stand-alone basis. The operating results of Rizobacter were consolidated with our results of operations as of October 19, 2016.
Bioceres
The table below illustrates our results of operations for the three-month periods ended September 30, 2017 and 2016.
Bioceres | ||||||
For the three-month period ended | ||||||
September 30, 2017 | September 30, 2016 | |||||
(unaudited) (US$) | ||||||
Total revenue | 34,986,505 | 1,963,513 | ||||
Crop protection | 16,795,948 | — | ||||
Seed and integrated products | 7,498,035 | 1,313,083 | ||||
Crop nutrition | 9,540,883 | — | ||||
Emerging solutions | 1,151,639 | 650,430 | ||||
Cost of sales | (20,195,931 | ) | (1,118,948 | ) | ||
Crop protection | (10,038,912 | ) | — | |||
Seed and integrated products | (2,462,759 | ) | (855,682 | ) | ||
Crop nutrition | (7,509,962 | ) | — | |||
Emerging solutions | (184,298 | ) | (263,266 | ) | ||
Research and development expenses | (1,725,645 | ) | (596,652 | ) | ||
Selling, general and administrative expenses | (9,993,183 | ) | (1,185,472 | ) | ||
Share of profit / (loss) of joint ventures and associates | 84,857 | (238,100 | ) | |||
Other expenses, net | (217,284 | ) | (18,135 | ) | ||
Operating income / (loss) | 2,939,319 | (1,193,794 | ) | |||
Finance income / (loss) | (2,042,066 | ) | 381,327 | |||
Finance costs | (9,268,419 | ) | (1,117,154 | ) | ||
101
Bioceres | ||||||
For the three-month period ended | ||||||
September 30, 2017 | September 30, 2016 | |||||
(unaudited) (US$) | ||||||
Loss before income tax | (4,287,034 | ) | (1,929,621 | ) | ||
Income tax benefit | 1,357,795 | 621,022 | ||||
Loss for the period/year | (2,929,239 | ) | (1,308,599 | ) | ||
Other comprehensive loss | (4,150,698 | ) | — | |||
Total comprehensive loss(1) | (7,079,937 | ) | (1,308,599 | ) | ||
Non-IFRS measures: | ||||||
Adjusted EBITDA (unaudited)(2) | 2,929,239 | 1,308,599 | ||||
| (1) | Includes exchange differences on translation of foreign operations. |
| (2) | Adjusted EBITDA is a non-IFRS measure. For a complete presentation of the reconciliation of Net Loss to Adjusted EBITDA, see the section entitled “—Non-IFRS Financial Measures.” |
Comparison of the three-month periods ended September 30, 2017 and 2016
Revenue
Revenue increased by 1,681.8%, or US$33.0 million, to US$35.0 million for the three-month period ended September 30, 2017 from US$2.0 million for the corresponding three-month period in 2016, primarily as a result of:
| ■ | the addition of Rizobacter’s revenues of US$32.9 million, including revenues during the three-month period ended September 30, 2017, of US$16.8 million from crop protection products, US$9.5 million from crop nutrition products and US$6.5 million from integrated products; and |
| ■ | an increase in R&D and technical fee income of US$0.5 million, mainly attributable to higher revenues generated from R&D and technical services rendered to third parties. |
The effect of these factors was partially offset by a decrease in seed sales of US$0.3 million, mainly attributable to lower sales of alfalfa and corn, partially offset by higher sales of soy.
Revenue by business segment
Crop Protection. Revenue was US$16.8 million for the three-month period ended September 30, 2017 compared to nil for the corresponding three-month period in 2016, primarily due to Rizobacter revenues recorded during the three-month period in 2017 compared to no Rizobacter revenues being recorded for the corresponding three-month period in 2016, as Rizobacter was not acquired until October 2016.
Seed and Integrated Products. Revenue increased by 471.0%, or US$6.2 million, to US$7.5 million for the three-month period ended September 30, 2017 compared to US$1.3 million for the corresponding three-month period in 2016, primarily due to integrated product sales of Rizobacter totaling US$6.5 million partially offset by a decrease in seed sales of US$0.3 million.
Crop Nutrition. Revenue was US$9.5 million for the three-month period ended September 30, 2017 compared to nil for the corresponding three-month period in 2016, primarily due to Rizobacter revenues recorded during the three-month period in 2017 compared to no Rizobacter revenues being recorded for the corresponding three-month period in 2016, as Rizobacter was not acquired until October 2016.
Emerging Solutions. Revenue increased by 77.1%, or US$0.5 million, to US$1.2 million for the three-month period ended September 30, 2017 compared to US$0.7 million for the corresponding three-month period in 2016, primarily due to higher revenues generated from R&D and technical services rendered to third parties.
102
Cost of sales
Cost of sales increased by 1,704.9%, or US$19.1 million, to US$20.2 million for the three-month period ended September 30, 2017 from US$1.1 million for the same three-month period in 2016, primarily as a result of the direct costs of Rizobacter products sold during the three-month period totaling US$19.2 million, which includes a non-recurring incremental cost of US$1.1 million related to PPA adjustments for revalued inventories sold during the three-month period, that was partially offset by lower cost of sales of US$0.1 million for our seeds and royalties and R&D services.
Cost of sales by business segment
Crop Protection. Cost of sales was US$10.0 million for the three-month period ended September 30, 2017 compared to nil for the corresponding three-month period in 2016, primarily due to Rizobacter sales costs recorded during the three-month period in 2017 compared to no Rizobacter sales costs being recorded for the corresponding three-month period in 2016, as Rizobacter was not acquired until October 2016.
Seed and Integrated Products. Cost of sales increased by 187.8%, or US$1.6 million, to US$2.5 million for the three-month period ended September 30, 2017 compared to US$0.9 million for the corresponding three-month period in 2016, primarily due to the direct costs of Rizobacter integrated products sold during the three-month period.
Crop Nutrition. Cost of sales was US$7.5 million for the three-month period ended September 30, 2017 compared to nil for the corresponding three-month period in 2016, primarily due to Rizobacter sales costs recorded during the three-month period in 2017 compared to no Rizobacter sales costs being recorded for the corresponding three-month period in 2016, as Rizobacter was not acquired until October 2016.
Emerging Solutions. Cost of sales decreased by 30.0%, or US$0.1 million, to US$0.2 million for the three-month period ended September 30, 2017 compared to US$0.3 million for the corresponding three-month period in 2016, primarily due to lower direct costs of R&D services rendered.
Research and development expenses
Research and development expenses increased by 189.2%, or US$1.1 million, to US$1.7 million for the three-month period ended September 30, 2017 from US$0.6 million for the corresponding three-month period in 2016, primarily as a result of increased R&D employee costs of US$0.6 million, a higher charge for amortization of intangible assets and for depreciation of property, plant and equipment of US$0.3 million and higher expenses in laboratory supplies and materials and professional fees of US$0.1 million.
Selling, general and administrative expenses
Selling, general and administrative expenses increased by 743.3%, or US$8.8 million, to US$10.0 million for the three-month period ended September 30, 2017 from US$1.2 million for the corresponding three-month period in 2016, primarily as a result of:
| ■ | an increase in employees’ salaries and related personnel costs of US$5.5 million, principally due to the administrative and commercial staff added by the acquisition of Rizobacter; |
| ■ | an increase of US$0.9 million in sales and other taxes mainly attributable to sales of Rizobacter; |
| ■ | an increase of US$0.4 million in charges for amortization of intangible assets and of US$0.2 million in charges for depreciation of property, plant and equipment, mainly due to the addition of Rizobacter’s intangible and fixed assets; |
| ■ | an increase of US$1.0 million in professional services fees; |
| ■ | an increase of US$0.6 million in publicity and advertising expenses; |
| ■ | an increase of US$0.4 million in charges for impairment of receivables; |
| ■ | an increase of US$0.4 million in import and export expenses; |
103
| ■ | an increase of US$0.3 million in freight expenses; |
| ■ | an increase of US$0.2 million in office supplies expenses; and |
| ■ | an increase of US$0.2 million in building maintenance expenses. |
Share of, profit (loss) of joint ventures and associates
Our share in the profit or loss of joint ventures and associates resulted in a profit of US$0.1 million for the three-month period ended September 30, 2017 compared to a loss of US$0.2 million for the corresponding three-month period in 2016, primarily as a result of profits and minor losses relating to our equity holdings in Synertech (through our subsidiary Rizobacter), AGBM, SW Semillas, partially offset by larger losses relating to our equity holdings in Trigall Genetics and Heritas.
Other expenses, net
Other expenses increased by US$0.2 million, to US$0.2 million for the three-month period ended September 30, 2017 from less than US$0.1 million for the corresponding three-month period in 2016, primarily as a result of minor expenses.
Operating income / (loss)
As a result of the foregoing, we recorded operating income of US$2.9 million for the three-month period ended September 30, 2017 compared to an operating loss of US$1.2 million for the corresponding three-month period in 2016.
Finance income
Finance income increased by 435.5%, or US$1.7 million, to US$2.0 million for the three-month period ended September 30, 2017 from US$0.4 million for the corresponding three-month period in 2016, primarily as a result of an increase in exchange differences of US$1.4 million, changes in fair value of financial assets or liabilities of US$0.2 million and higher interest income of US$0.1 million.
Finance costs
Finance costs increased by 729.6%, or US$8.2 million, to US$9.3 million for the three-month period ended September 30, 2017 from US$1.1 million for the corresponding three-month period in 2016, primarily as a result of higher interest expenses of US$4.2 million resulting mainly from interest charges added by Rizobacter, higher exchange differences of US$3.3 million, higher financial commissions from Rizobacter of US$0.4 million, and increases in fair value of financial assets or liabilities of US$0.2 million.
Loss before income tax
As a result of the foregoing, we recorded a loss before income tax of US$4.3 million for the three-month period ended September 30, 2017 compared to a loss before income tax of US$1.9 million for the corresponding three-month period in 2016.
Income tax benefit
Income tax benefit increased by 118.6%, or US$0.7 million, to US$1.4 million for the three-month period ended September 30, 2017 from US$0.6 million for the corresponding three-month period in 2016, primarily as a result of the higher loss incurred.
Loss for the period
As a result of the foregoing, loss for the period amounted to US$2.9 million for the three-month period ended September 30, 2017 compared to US$1.3 million for the corresponding three-month period in 2016.
104
Other comprehensive loss
Other comprehensive loss was US$4.1 million for the three-month period ended September 30, 2017 compared to nil for the corresponding three-month period in 2016, primarily as a result of exchange differences on translation of foreign operations of US$4.1 million.
Total comprehensive loss
As a result of the foregoing, we recorded a total comprehensive loss of US$7.1 million for the three-month period ended September 30, 2017 compared to a total comprehensive loss of US$1.3 million for the corresponding three-month period in 2016.
105
Comparison of the Three-Month Period Ended September 30, 2017 and the Unaudited Pro Forma Consolidated Statement of Income for the Three-Month Period Ended September 30, 2016
Unaudited Pro Forma Consolidated Financial Information of Bioceres
The table below illustrates our results of operations for the three-month period ended September 30, 2017, and the unaudited pro forma consolidated statement of income for three-month period ended September 30, 2016.
Bioceres | ||||||
For the three-month period ended | ||||||
September 30, 2017 | September 30, 2016(1) | |||||
(unaudited) | (unaudited proforma) | |||||
(US$) | ||||||
Total revenue | 34,986,505 | 27,977,571 | ||||
Crop protection | 16,795,948 | 12,142,498 | ||||
Seed and integrated products | 7,498,035 | 7,080,861 | ||||
Crop nutrition | 9,540,883 | 8,243,062 | ||||
Emerging solutions | 1,151,639 | 511,150 | ||||
Cost of sales | (20,195,931 | ) | (12,727,738 | ) | ||
Crop protection | (10,038,912 | ) | (5,765,825 | ) | ||
Seed and integrated products | (2,462,759 | ) | (2,376,759 | ) | ||
Crop nutrition | (7,509,962 | ) | (4,321,888 | ) | ||
Emerging solutions | (184,298 | ) | (263,266 | ) | ||
Research and development expenses | (1,725,645 | ) | (1,398,194 | ) | ||
Selling, general and administrative expenses | (9,993,183 | ) | (8,276,623 | ) | ||
Share of profit / (loss) of joint ventures and associates | 84,857 | (360,917 | ) | |||
Other expenses, net | (217,284 | ) | (41,834 | ) | ||
Operating loss | (2,939,319 | ) | (5,172,265 | ) | ||
Finance income | 2,042,066 | 1,430,434 | ||||
Finance costs | (9,268,419 | ) | (7,769,807 | ) | ||
Loss before income tax | (4,287,034 | ) | (1,167,108 | ) | ||
Income tax benefit | 1,357,795 | 357,944 | ||||
Loss for the period | (2,929,239 | ) | (809,164 | ) | ||
Non-IFRS measures: | ||||||
Adjusted EBITDA (unaudited)(2) | 5,566,129 | 6,629,293 | ||||
| (1) | Unaudited pro forma consolidated statement of income for three-month period ended September 30, 2017 include the consolidated statements of profit or loss of Rizobacter, as if the acquisition of Rizobacter had occurred on January 1, 2016. |
| (2) | Adjusted EBITDA is a non-IFRS measure. For a complete presentation of the reconciliation of Net Loss to Adjusted EBITDA, see the section entitled “—Reconciliation of Non-IFRS Financial Measures.” |
Comparison of the Three-Month Period Ended September 30, 2017 and the Unaudited Pro Forma Consolidated Statement of Income for the Three-Month Period Ended September 30, 2016
Revenue
Revenue increased by 25.1%, or US$7.0 million, to US$35.0 million for the three-month period ended September 30, 2017, from US$28.0 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, as a result of an increase in crop protection product sales of US$4.7 million, an increase in crop nutrition product sales of US$1.3 million, and an increase in emerging solutions sales of US$0.6 million and an increase in seed and integrated products sales of US$0.4 million.
106
Revenue by business segment
Crop Protection. Revenue increased by 38.3%, or US$4.7 million, to US$16.8 million for the three-month period ended September 30, 2017, compared to US$12.1 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, attributable mainly to an increase of US$3.4 million in sales of insecticides, fungicides and other products sales and an increase of US$1.2 million in sales of adjuvants.
Seed and Integrated Products. Revenue increased by 5.9%, or US$0.4 million, to US$7.5 million for the three-month period ended September 30, 2017, compared to US$7.1 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, attributable mainly to increased integrated product sales of Rizobacter totalling US$0.7 million and a decrease in seed sales and royalties of US$0.3 million.
Crop Nutrition. Revenue increased by 15.7%, or US$1.3 million, to US$9.5 million for the three-month period ended September 30, 2017, compared to US$8.2 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, primarily due to higher sales of inoculants totaling US$1.3 million.
Emerging Solutions. Revenue increased by 125.3%, or US$0.6 million, to US$1.1 million for the three-month period ended September 30, 2017, compared to US$0.5 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016.
Cost of sales
Cost of sales increased by 58.7%, or US$7.5 million, to US$20.2 million for the three-month period ended September 30, 2017, from US$12.7 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, primarily as a result of an increase of US$4.3 million in crop protection costs and US$3.2 million in crop nutrition costs as well as a non-recurring incremental cost of US$1.1 million related to PPA adjustments for revalued inventories sold during the three-month period ended September 30, 2017. The effect of these factors was partially offset by the lower cost of emerging solutions costs of less than US$0.1 million.
For the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, no higher costs have been estimated related to PPA adjustments.
Cost of sales by business segment
Crop Protection. Cost of sales increased by 74.1%, or US$4.3 million, to US$10.0 million for the three-month period ended September 30, 2017, compared to US$5.8 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, primarily due to an increase of US$3.5 million in insecticides, fungicides and other products and US$0.2 million in adjuvants costs as well as a non-recurring incremental cost of US$0.6 million related to PPA adjustments.
Seed and Integrated Products. Cost of sales increased by 3.6%, or less than US$0.1 million, to US$2.5 million for the three-month period ended September 30, 2017, compared to US$2.4 million for unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, primarily due to a non-recurring incremental cost from PPA adjustments of US$0.1 million.
Crop Nutrition. Cost of sales increased by 73.8%, or US$3.2 million, to US$7.5 million for the three-month period ended September 30, 2017, compared to US$4.3 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, primarily due to an increase of US$2.7 million in inoculants costs and a non-recurring incremental cost from PPA adjustments of US$0.4 million.
Emerging Solutions. Cost of sales decreased by 30.0%, or less than US$0.1 million, to US$0.2 million for the three-month period ended September 30, 2017, compared to US$0.3 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016.
107
Research and development expenses
Research and development expenses increased by 23.4%, or US$0.3 million, to US$1.7 million for the three-month period ended September 30, 2017, from US$1.4 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, primarily as a result of an increase of US$0.3 million in staff expenses and US$0.1 in laboratory supplies and materials, partially offset by a decrease of US$0.1 in other R&D expenses.
Selling, general and administrative expenses
Selling, general and administrative expenses increased by 20.7%, or US$1.7 million, to US$10.0 million for the three-month period ended September 30, 2017, from US$8.3 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, primarily as a result of an increase in employees’ salaries and related personnel costs of US$0.8 million, an increase of US$0.5 million in professional fees, an increase of US$0.2 million in sales taxes and other taxes, an increase of US$0.2 million in publicity and advertising and an increase of US$0.1 million in freight costs. The effect of these factors was primarily offset by the lower charges in impairment of receivables of US$0.3 million.
Share of profit / (loss) of joint ventures and associates
Our share in the profit or loss of joint ventures and associates increased by 123.5%, or US$0.4 million, to a profit of less than US$0.1 million for the three-month period ended September 30, 2017, from a loss of US$0.3 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, primarily as a result of higher charges relating to our equity holdings in Synertech and AGBM, partially offset by lower charges relating to our equity holdings in Trigall Genetics.
Other expenses, net
Other expenses increased by 419.4%, or US$0.2 million, to US$0.2 million for the three-month period ended September 30, 2017, from less than US$0.1 million for the corresponding unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016.
Operating loss
As a result of the foregoing, our operating loss decreased by 43.2%, or US$2.2 million, to US$2.9 million for the three-month period ended September 30, 2017, from US$5.2 million for the corresponding unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016.
Finance income
Finance income increased by 42.8%, or US$0.6 million, to US$2.0 million for the three-month period ended September 30, 2017, from US$1.4 million for the corresponding unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, primarily as a result of an increase of US$0.7 million in exchange differences.
Finance costs
Finance costs increased by 19.3%, or US$1.5 million, to US$9.3 million for the three-month period ended September 30, 2017, from US$7.8 million for the corresponding unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016, primarily as a result of an increase of US$1.7 million in exchange differences and an increase of US$0.2 million in financial commissions, partially offset by lower interest expenses and charges related from fair value of financial assets or liabilities of US$0.4 million.
108
Loss before income tax
As a result of the foregoing, we recorded a loss before income tax of US$4.3 million for the three-month period ended September 30, 2017, compared to a loss before income tax of US$1.2 million for the unaudited pro forma consolidated statement of income for the corresponding three-month period ended September 30, 2016.
Income tax benefit
Income tax benefit amounted to US$1.4 million for the three-month period ended September 30, 2017, compared to a benefit of US$0.4 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016.
Loss for the period
As a result of the foregoing, our loss amounted to US$2.9 million for the three-month period ended September 30, 2017, compared to US$0.8 million for the unaudited pro forma consolidated statement of income for the three-month period ended September 30, 2016.
Bioceres
The table below illustrates our results of operations for the Transition Period and the corresponding six-month period in 2016.
Bioceres | ||||||
For the Transition Period ended June 30, 2017 | For the six-month period ended June 30, 2016(1) (unaudited) | |||||
(US$) | ||||||
Total revenue | 48,341,121 | 2,538,399 | ||||
Crop protection | 31,191,970 | — | ||||
Seed and integrated products | 9,020,999 | 918,765 | ||||
Crop nutrition | 6,640,228 | — | ||||
Emerging solutions | 1,487,924 | 1,619,634 | ||||
Cost of sales | (30,185,446 | ) | (1,290,256 | ) | ||
Crop protection | (22,641,887 | ) | — | |||
Seed and integrated products | (4,851,444 | ) | (790,671 | ) | ||
Crop nutrition | (2,084,652 | ) | — | |||
Emerging solutions | (607,463 | ) | (499,585 | ) | ||
Research and development expenses | (3,601,624 | ) | (1,268,661 | ) | ||
Selling, general and administrative expenses | (17,324,407 | ) | (3,541,058 | ) | ||
Share of loss of joint ventures and associates | (786,805 | ) | (455,181 | ) | ||
Other income | 121,065 | — | ||||
Operating loss | (3,436,096 | ) | (4,016,757 | ) | ||
Finance income | 2,136,265 | 790,814 | ||||
Finance costs | (14,945,495 | ) | (1,579,951 | ) | ||
Loss before income tax | (16,245,326 | ) | (4,805,894 | ) | ||
Income tax benefit | 5,090,723 | 1,040,923 | ||||
Loss for the period | (11,154,603 | ) | (3,764,971 | ) | ||
Other comprehensive loss | (4,614,372 | ) | — | |||
Total comprehensive loss(2) | (13,736,103 | ) | (3,764,971 | ) | ||
Non-IFRS measures: | ||||||
Adjusted EBITDA (unaudited)(3) | 2,318,947 | (2,897,488 | ) | |||
| (1) | Consolidated statements of profit or loss and other comprehensive income for the six-month period ended June 30, 2016 do not include the consolidated statements of profit or loss and other comprehensive income of Rizobacter, control of which we assumed on October 19, 2016. |
109
| (2) | Includes (i) exchange differences on translation of foreign operations from joint ventures, (ii) exchange differences on translation of foreign operations, (iii) revaluation of property, plant and equipment, net of tax from joint ventures and (iv) revaluation of property, plant and equipment, net of tax. |
| (3) | Adjusted EBITDA is a non-IFRS measure. For a complete presentation of the reconciliation of Net Loss to Adjusted EBITDA, see the section entitled “—Non-IFRS Financial Measures.” |
Comparison of the Transition Period and the six-month period ended June 30, 2016
Revenue
Revenue increased by 1,804.4%, or US$45.8 million, to US$48.3 million for the Transition Period from US$2.5 million for the corresponding six-month period in 2016, primarily as a result of:
| ■ | the addition of Rizobacter’s revenues of US$44.5 million, including revenues during the Transition Period of US$31.2 million from crop protection products, US$6.6 million from integrated products and US$6.6 million from crop nutrition products; and |
| ■ | an increase in seed sales of US$1.5 million, mainly attributable to higher volumes of sales of wheat, soy and alfalfa. |
The effect of these factors was partially offset by a decrease in R&D and technical fee income of US$0.1 million, mainly attributable to lower revenues generated from R&D services rendered to third parties and to our joint venture partners as provided in our joint venture agreements.
Revenue by business segment
Crop Protection. Revenue was US$31.2 million for the Transition Period compared to nil for the six-month period in 2016, primarily due to Rizobacter revenues recorded during the Transition Period compared to no Rizobacter revenues being recorded for the corresponding six-month period in 2016, as Rizobacter was acquired in October 2016.
Seed and Integrated Products. Revenue increased by 881.9%, or US$8.1 million, to US$9.0 million for the Transition Period compared to US$0.9 million for the corresponding six-month period in 2016, primarily due to higher seed sales totaling US$1.5 million and integrated product sales totaling US$6.6 million of Rizobacter.
Crop Nutrition. Revenue was of US$6.6 million for the Transition Period compared to nil for the corresponding six-month period in 2016, primarily due to Rizobacter revenues recorded in the Transition Period compared to no Rizobacter revenues for the corresponding six-month period in 2016, as Rizobacter was acquired in October 2016.
Emerging Solutions. Revenue decreased by 8.1%, or US$0.1 million, to US$1.5 million for the Transition Period compared to US$1.6 million for the corresponding six-month period in 2016, primarily due to lower R&D and technical fee income mainly attributable to lower cost R&D services rendered to third parties and to our joint venture partners as provided in our joint venture agreements.
Cost of sales
Cost of sales increased by 2,239.5%, or US$28.9 million, to US$30.2 million for the Transition Period from US$1.3 million for the same six-month period in 2016, primarily as a result of the direct costs of Rizobacter products sold during the Transition Period totaling US$27.5 million, which includes a non-recurring incremental cost of US$2.4 million related to PPA adjustments for revalued inventories sold during the Transition Period and the higher cost of sales of US$1.3 million for our seed products and of US$0.1 million for our R&D services.
Cost of sales by business segment
Crop Protection. Cost of sales was US$22.6 million for the Transition Period compared to nil for the corresponding six-month period in 2016, primarily due to Rizobacter sales costs recorded during the Transition Period compared to no Rizobacter sales costs being recorded for the corresponding six-month period in 2016, as Rizobacter was acquired in October 2016.
110
Seed and Integrated Products. Cost of sales increased by 513.6%, or US$4.1 million, to US$4.9 million for the Transition Period compared to US$0.8 million for the corresponding six-month period in 2016, primarily due to higher cost of sales for our seed products totaling US$1.3 million and the direct costs of Rizobacter products sold during the Transition Period totaling US$2.8 million.
Crop Nutrition. Cost of sales was US$2.1 million for the Transition Period compared to nil for the corresponding six-month period in 2016, primarily due to Rizobacter sales costs recorded during the Transition Period compared to no Rizobacter sales costs being recorded for the corresponding six-month period in 2016, as Rizobacter was acquired in October 2016.
Emerging Solutions. Cost of sales increased by 21.6%, or US$0.1 million, to US$0.6 million for the Transition Period compared to US$0.5 million for the corresponding six-month period in 2016, primarily due to higher direct costs of R&D services rendered.
Research and development expenses
Research and development expenses increased by 183.9%, or US$2.3 million, to US$3.6 million for the Transition Period from US$1.3 million for the corresponding six-month period in 2016, primarily as a result of increased R&D employee costs, a higher charge for amortization of intangible assets and for depreciation of property, plant and equipment mainly due to the addition of Rizobacter’s fixed and intangible assets and higher expenses in laboratory supplies and materials.
Selling, general and administrative expenses
Selling, general and administrative expenses increased by 389.2%, or US$13.8 million, to US$17.3 million for the Transition Period from US$3.5 million for the corresponding six-month period in 2016, primarily as a result of:
| ■ | an increase in employees’ salaries and related personnel costs of US$7.3 million, principally due to the administrative staff added by the acquisition of Rizobacter; |
| ■ | an increase of US$1.6 million in sales and other taxes mainly attributable to sales of Rizobacter; |
| ■ | an increase of US$1.0 million in charges for amortization of intangible assets and of US$0.7 million in charges for depreciation of property, plant and equipment, mainly due to the addition of Rizobacter’s intangible and fixed assets; |
| ■ | an increase of US$0.9 million in freight expenses mainly attributable to Rizobacter; |
| ■ | an increase of US$0.5 million in office supplies expenses; |
| ■ | an increase of US$0.4 million in publicity and advertising expenses; and |
| ■ | an increase of US$0.3 million in professional services fees. |
The effect of these factors was partially offset by the lower impairment of receivables charges of US$0.2 million.
Share of loss of joint ventures and associates
The loss resulting from our share in the profit or loss of joint ventures and associates increased by 72.9%, or US$0.3 million, to US$0.8 million for the Transition Period from US$0.5 million for the corresponding six-month period in 2016, primarily as a result of higher charges relating to our equity holdings in Synertech (through our subsidiary Rizobacter) and AGBM, partially offset by lower charges relating to our equity holdings in in Trigall Genetics, Semya and S&W Semillas.
Other income
Other income increased to US$0.1 million for the Transition Period from nil for the corresponding six-month period in 2016, primarily as a result of the sale of equipment and other miscellaneous income.
111
Operating loss
As a result of the foregoing, operating loss decreased by 14.5%, or US$0.6 million, to US$3.4 million for the Transition Period from US$4.0 million for the corresponding six-month period in 2016.
Finance income
Finance income increased by 170.1%, or US$1.3 million, to US$2.1 million for the Transition Period from US$0.8 million for the corresponding six-month period in 2016, primarily as a result of an increase in exchange differences of US$1.3 million, higher interest income of US$0.2 million and present value adjustments of US$0.1 million, partially offset by changes in fair value of financial assets or liabilities of US$0.2 million.
Finance costs
Finance costs increased by 845.9%, or US$13.4 million, to US$14.9 million for the Transition Period from US$1.6 million for the corresponding six-month period in 2016, primarily as a result of higher interest expenses of US$8.1 million resulting mainly from interest charges added by Rizobacter, higher exchange differences of US$3.5 million, higher financial commissions from Rizobacter of US$1.1 million, and increases in fair value of financial assets or liabilities of US$1.2 million, partially offset by lower charges related to present value adjustments of US$0.5 million.
Loss before income tax
As a result of the foregoing, we recorded a loss before income tax of US$16.2 million for the Transition Period compared to a loss before income tax of US$4.8 million for the corresponding six-month period in 2016.
Income tax benefit
Income tax benefit amounted to US$5.1 million for the Transition Period compared to a benefit of US$1.0 million for the corresponding six-month period in 2016, primarily as a result of the higher loss incurred and fiscal gain obtained through the government program of promotion of scientific and technological development.
Loss for the period
As a result of the foregoing, loss for the period amounted to US$11.2 million for the Transition Period compared to US$3.8 million for the corresponding six-month period in 2016.
Other comprehensive loss
Other comprehensive loss amounted to US$4.6 million for the Transition Period compared to nil for the corresponding six-month period in 2016, primarily as a result of exchange differences on translation of foreign operations of US$4.6 million, partially offset by gains from revaluation of property, plant and equipment, net of tax for US$2.0 million.
Total comprehensive loss
As a result of the foregoing, we recorded a total comprehensive loss of US$13.7 million for the Transition Period compared to a total comprehensive loss of US$3.8 million for the corresponding six-month period in 2016.
Comparison of the Pro Forma Transition Period and the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016
The below comparison has been prepared to give pro forma effect to the Rizobacter acquisition for the six-month period ended June 30, 2016.
112
Unaudited Pro Forma Consolidated Financial Information of Bioceres
The table below illustrates our results of operations for the Transition Period and the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016.
Bioceres | ||||||
For the Transition Period ended June 30, 2017 | For the six-month period ended June 30, 2016(1) (unaudited pro forma) | |||||
(US$) | ||||||
Total revenue | 48,341,121 | 31,110,577 | ||||
Crop protection | 31,191,970 | 17,455,035 | ||||
Seed and integrated products | 9,020,999 | 3,741,499 | ||||
Crop nutrition | 6,640,228 | 8,516,097 | ||||
Emerging solutions | 1,487,924 | 1,397,946 | ||||
Cost of sales | (30,185,446 | ) | (16,284,482 | ) | ||
Crop protection | (22,641,887 | ) | (8,370,392 | ) | ||
Seed and integrated products | (4,851,444 | ) | (785,754 | ) | ||
Crop nutrition | (2,084,652 | ) | (6,628,751 | ) | ||
Emerging solutions | (607,463 | ) | (499,585 | ) | ||
Research and development expenses | (3,601,624 | ) | (2,818,236 | ) | ||
Selling, general and administrative expenses | (17,324,407 | ) | (15,651,397 | ) | ||
Share of loss of joint ventures and associates | (786,805 | ) | (666,838 | ) | ||
Other income | 121,065 | 319,059 | ||||
Operating loss | (3,436,096 | ) | (3,991,318 | ) | ||
Finance income | 2,136,265 | 1,142,264 | ||||
Finance costs | (14,945,495 | ) | (16,214,402 | ) | ||
Loss before income tax | (16,245,326 | ) | (19,063,456 | ) | ||
Income tax benefit | 5,090,723 | 5,065,016 | ||||
Loss for the period | (11,154,603 | ) | (13,998,440 | ) | ||
Non-IFRS measures: | ||||||
Adjusted EBITDA (unaudited)(2) | 2,318,947 | (1,240,409 | ) | |||
| (1) | Unaudited pro forma consolidated statement of income for six-month period ended June 30, 2016 includes the consolidated statements of profit or loss of Rizobacter, as if the acquisition of Rizobacter had occurred on January 1, 2016. |
| (2) | Adjusted EBITDA is a non-IFRS measure. For a complete presentation of the reconciliation of Net Loss to Adjusted EBITDA, see the section entitled “—Non-IFRS Financial Measures.” |
Comparison of the Transition Period and the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016
Revenue
Revenue increased by 55.4%, or US$17.2 million, to US$48.3 million for the Transition Period from US$31.1 million for the corresponding unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016, primarily as a result of an increase of US$13.7 million in crop protection product sales, an increase of US$5.3 million in seed and integrated products sales and an increase of US$0.1 million in emerging solutions sales, partially offset by a decrease in crop nutrition product sales of US$1.9 million.
113
Revenue by business segment
Crop Protection. Revenue increased by 78.7%, or US$13.7 million, to US$31.2 million for the Transition Period compared to US$17.5 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016, attributable mainly to an increase of US$5.3 million in sales of adjuvants and an increase of US$8.4 million in sales of insecticides and fungicides and other products sales.
Seed and Integrated Products. Revenue increased by 141.1%, or US$5.3 million, to US$9.0 million for the Transition Period compared to US$3.7 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016, attributable mainly to an increase of US$5.3 million in sales of adjuvants and an increase of US$8.4 million in sales of insecticides and fungicides and other products sales.
Crop Nutrition. Revenue decreased by 22.0%, or US$1.9 million, to US$6.6 million for the Transition Period compared to US$8.5 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016, primarily due to a decrease of US$1.4 million in sales of fertilizers and US$0.5 million in sales of inoculants.
Emerging Solutions. Revenue increased by 6.4%, or US$0.1 million, to US$1.5 million for the Transition Period compared to US$1.4 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016.
Cost of sales
Cost of sales increased by 85.4%, or US$13.9 million, to US$30.2 million for the Transition Period from US$16.3 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016, primarily as a result of an increase of US$12.3 in crop protection costs, US$4.0 in seeds and integrated product costs, US$0.1 in emerging solutions costs and a non-recurring incremental cost of US$2.4 million related to PPA adjustments for revalued inventories sold during the Transition Period, partially offset by the lower cost of crop nutrition products of US$4.7 million.
For the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016 no higher costs have been estimated related to PPA adjustments.
Cost of sales by business segment
Crop Protection. Cost of sales increased by 170.5%, or US$14.3 million, to US$22.6 million for the Transition Period compared to US$8.4 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016, primarily due to an increase of US$3.6 in adjuvants costs, US$8.7 in insecticides and fungicides and other products costs a non-recurring incremental cost of US$2.0 million related to PPA adjustments.
Seed and Integrated Products. Cost of sales increased by 517.4%, or US$4.1 million, to US$4.9 million for the Transition Period compared to US$0.8 million for the unaudited pro forma consolidated statement of income for six-month period ended June 30, 2016, primarily due to an increase of US$2.8 million in Rizobacter integrated products, including US$0.2 of a non-recurring incremental cost from PPA adjustment and an increase of US$1.3 million in seeds and royalties.
Crop Nutrition. Cost of sales decreased by 68.6%, or US$4.5 million, to US$2.1 million for the Transition Period compared to US$6.6 million for the unaudited pro forma consolidated statement of income for six-month period ended June 30, 2016, primarily due to a decrease of US$3.0 million in inoculants cost and US$ 1.8 million in fertilizers cost. The effect of these factors was partially offset by an increase of US$0.2 million in crop nutrition costs from a non-recurring incremental cost from PPA adjustments.
Emerging Solutions. Cost of sales increased by 21.6%, or US$0.1 million, to US$0.6 million for the Transition Period compared to US$0.5 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016.
114
Research and development expenses
Research and development expenses increased by 27.8%, or US$0.8 million, to US$3.6 million for the Transition Period from US$2.8 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016, as a result of an increase of US$0.4 million in staff expenses, US$0.2 million in laboratory supplies and materials, US$ 0.1 million in depreciation of property, plant and equipment and US$0.1 million in other R&D expenses.
Selling, general and administrative expenses
Selling, general and administrative expenses increased by 10.7%, or US$1.7 million, to US$17.3 million for the Transition Period from US$15.6 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016, primarily as a result of:
| ■ | an increase in employees’ salaries and related personnel costs of US$1.9 million; |
| ■ | an increase of US$0.7 million in charges for amortization of intangible assets and depreciation of property, plant and equipment; and |
| ■ | an increase of US$0.6 million in sales taxes and other taxes. |
The effect of these factors was partially offset by the lower charges of publicity, advertising and professional fees of US$1.5 million.
Share of loss of joint ventures and associates
The loss resulting from our share in the profit or loss of joint ventures and associates increased by 18.0%, or US$0.1 million, to US$0.8 million for the Transition Period from US$0.7 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016, primarily as a result of higher charges relating to our equity holdings in Synertech and AGBM, partially offset by lower charges relating to our equity holdings in Trigall Genetics and S&W Semillas.
Other income
Other income decreased by 62.1%, or US$0.2 million, to US$0.1 million for the three-month period ended September 30, 2017, from US$0.3 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016.
Operating loss
As a result of the foregoing, operating loss decreased by 13.9%, or US$0.6 million, to US$3.4 million for the Transition Period from US$4.0 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016.
Finance income
Finance income increased by 87.0%, or US$1.0 million, to US$2.1 million for the Transition Period from US$1.1 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016, primarily as a result of an increase in exchange differences of US$1.0 million.
Finance costs
Finance costs decreased by 7.8%, or US$1.3 million, to US$14.9 million for the Transition Period from US$16.2 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016, primarily as a result of higher interest expenses and charges related to fair value of financial assets or liabilities of US$2.8 million, partially offset by lower charges related to exchange differences and financial commissions of US$1.5 million.
115
Loss before income tax
As a result of the foregoing, we recorded a loss before income tax of US$16.2 million for the Transition Period compared to a loss before income tax of US$19.1 million for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016.
Income tax benefit
Income tax benefit amounted to US$5.1 million for the Transition Period and for the unaudited pro forma consolidated statement of income for the six-month period ended June 30, 2016.
Loss for the period
As a result of the foregoing, loss for the Transition Period amounted to US$11.1 million compared to US$14.0 million for the unaudited pro forma consolidated statement of income for six-month period ended June 30, 2016.
Comparison of the year ended December 31, 2016 and 2015
The table below illustrates our consolidated results of operations for the years ended December 31, 2016 and 2015.
Bioceres | ||||||
Year ended December 31, | ||||||
2016(1) | 2015 | |||||
(US$) | ||||||
Total revenue | 44,349,263 | 10,195,884 | ||||
Crop protection | 21,493,419 | — | ||||
Seed and integrated products | 14,244,967 | 4,495,042 | ||||
Crop nutrition | 5,227,394 | — | ||||
Emerging solutions | 3,383,483 | 5,700,842 | ||||
Cost of sales | (31,600,998 | ) | (4,799,345 | ) | ||
Crop protection | (16,825,572 | ) | — | |||
Seed and integrated products | (8,895,386 | ) | (3,733,701 | ) | ||
Crop nutrition | (4,819,455 | ) | — | |||
Emerging solutions | (1,060,585 | ) | (1,065,644 | ) | ||
Research and development expenses | (2,860,771 | ) | (2,688,924 | ) | ||
Selling, general and administrative expenses | (12,906,021 | ) | (4,080,860 | ) | ||
Share of loss of joint ventures and associates | (936,769 | ) | (1,553,002 | ) | ||
Other income | 48,495 | — | ||||
Operating loss | (3,906,801 | ) | (2,926,267 | ) | ||
Gain on previously held interest | 4,453,284 | — | ||||
Finance income | 1,006,953 | 1,509,736 | ||||
Finance costs | (10,923,378 | ) | (1,904,569 | ) | ||
Loss before income tax | (9,369,942 | ) | (3,321,100 | ) | ||
Income tax benefit / (expense) | 4,140,028 | (411,342 | ) | |||
Loss for the year | (5,229,914 | ) | (3,732,442 | ) | ||
Other comprehensive income | (4,482,329 | ) | — | |||
Total comprehensive loss(2) | (9,712,243 | ) | (3,732,442 | ) | ||
Non-IFRS measures: | ||||||
Adjusted EBITDA (unaudited)(3) | 6,390,481 | (2,376,297 | ) | |||
| (1) | Consolidated results of our operations include results of operations of Rizobacter from October 19, 2016 to December 31, 2016 (the period beginning on the date whereupon we assumed control of Rizobacter following its acquisition by us). |
| (2) | Includes (i) exchange differences on translation of foreign operations from joint ventures, (ii) exchange differences on translation of foreign operations, (iii) revaluation of property, plant and equipment, net of tax from joint ventures and (iv) revaluation of property, plant and equipment, net of tax. |
| (3) | Adjusted EBITDA is a non-IFRS measure. For a complete presentation of the reconciliation of Net Loss to Adjusted EBITDA, see the section entitled “—Non-IFRS Financial Measures.” |
116
Revenue
Revenues increased by 335.0%, or US$34.2 million, to US$44.3 million for 2016 from US$10.2 million for 2015, primarily as a result of:
| ■ | the addition of Rizobacter’s US$36.7 million of revenues since its acquisition on October 19, 2016; |
| ■ | a decrease of US$0.3 million in seed sales, mainly attributable to lower sales volumes of corn and alfalfa, partially offset by higher sales volumes of wheat and other crops; |
| ■ | a decrease of US$1.7 million in R&D and technical fee income, mainly attributable to lower R&D services rendered to third parties and to our joint venture partners as provided in our joint venture agreements, including US$0.7 million of services invoiced to Arcadia Biosciences in 2015 or R&D services rendered in relation to Verdeca in 2015 that did not occur in 2016; and |
| ■ | a decrease of US$0.1 million in enzyme sales mainly due to the transfer of our chymosin enzyme business to AGBM, a joint venture formed on November 1, 2016 and of which we own 49.9%, and due to executed sales in certain strains in 2015 that did not occur in 2016. |
Revenue by business segment
Crop Protection. Revenue was US$21.5 million for 2016 compared to nil for 2015, primarily as a result of the addition of Rizobacter since its acquisition on October 19, 2016.
Seed and Integrated Products. Revenue increased by 216.9%, or US$9.7 million, to US$14.2 million for 2016 compared to US$4.5 million for 2015, primarily due to the addition of US$10.0 million of Rizobacter revenues since its acquisition on October 19, 2016, partially offset by lower seed sales of US$0.3 million.
Crop Nutrition. Revenue was US$5.2 million for 2016 compared to nil for 2015, primarily as a result of the addition of Rizobacter since its acquisition on October 19, 2016.
Emerging Solutions. Revenue decreased by 40.6%, or US$2.3 million, to US$3.4 million for 2016 compared to US$5.7 million for 2015, primarily due to lower R&D services rendered to third parties and to our joint venture partners as provided in our joint venture agreements, and lower government grants received primarily as a result of decreased eligible R&D expenditures.
Cost of sales
Cost of sales increased by 558.4%, or US$26.8 million, to US$31.6 million for 2016 from US$4.8 million for 2015, primarily as a result of the direct costs of Rizobacter products sold since the acquisition on October 19, 2016, which includes a non-recurring incremental cost of US$7.5 million related to PPA adjustments for revalued inventories sold since the acquisition date, while the direct costs of Bioceres’ seed sales and R&D services sales remained unchanged from 2015 to 2016.
Cost of sales by business segment
Crop Protection. Cost of sales was US$16.8 million for 2016 compared to nil for 2015, primarily as a result of the addition of Rizobacter since its acquisition on October 19, 2016.
Seed and Integrated Products. Cost of sales increased by 138.2%, or US$5.2 million, to US$8.9 million for 2016 compared to US$3.7 million for 2015, primarily as a result of the direct costs of Rizobacter products sold since its acquisition on October 19, 2016, while the direct costs of seed sales remained unchanged from 2015 to 2016.
Crop Nutrition. Cost of sales was of US$4.8 million for 2016 compared to nil for 2015, primarily as a result of the addition of Rizobacter since its acquisition on October 19, 2016.
Emerging Solutions. Cost of sales decreased by 0.5%, or less than US$0.1 million, to US$1.1 million for 2016 from US$1.1 million in 2015, primarily as a result of services rendered with lower gross margin.
117
Research and development expenses
Research and development expenses increased by 6.4%, or US$0.2 to US$2.9 million for 2016 from US$2.7 million for 2015, primarily as a result of increased R&D employee costs, including newly granted stock options to key personnel, and a higher charge for depreciation of property, plant and equipment due to the addition of Rizobacter’s fixed assets since its acquisition on October 19, 2016, which increase was partially offset by lower laboratory supplies and technical service fee expenses.
Selling, general and administrative expenses
Selling, general and administrative expenses increased by 216.3%, or US$8.8 million, to US$12.9 million for 2016 from US$4.1 million for 2015, primarily as a result of:
| ■ | an increase of US$3.3 million in employees’ salaries and related personnel costs, principally due to the administrative staff added by the Rizobacter acquisition and to higher staff compensation including newly granted stock options; |
| ■ | an increase of US$1.5 million in professional services fees, principally since the Rizobacter acquisition, which includes US$0.6 million of non-recurring transaction-related expenses; |
| ■ | an increase of US$0.9 million in sales and other taxes mainly attributable to sales of Rizobacter added since its acquisition; |
| ■ | an increase of US$0.8 million in charges for amortization of intangible assets and depreciation of property, plant and equipment, mainly due to the addition of fixed and intangible assets as a result of the Rizobacter acquisition; |
| ■ | an increase of US$0.7 million in freight expenses; |
| ■ | an increase of US$0.3 million in publicity and advertising expenses; and |
| ■ | an increase of US$0.3 million in provisions. |
The effect of these factors was partially offset by US$0.1 million of lower storage costs and US$0.1 million of lower impairment of receivables charges.
Share of loss of joint ventures and associates
The loss resulting from our share in the loss of joint ventures and associates decreased by 39.7%, or US$0.6 million, to US$0.9 million for 2016 from a loss of US$1.6 million for 2015, primarily as a result of lower charges relating to our equity holdings in Trigall Genetics, Semya, S&W Semillas and, since the acquisition of Rizobacter, Synertech.
Other income
Other income amounted to less than US$0.1 million for 2016 compared to nil for 2015.
Operating loss
As a result of the foregoing, operating loss increased by 33.5%, or US$1.0 million, to US$3.9 million for 2016 from US$2.9 million for 2015.
Gain on previously held interest
The gain on previously held interest results from a non-recurring gain generated by the acquisition of control over Semya, of US$4.5 million for 2016 compared to nil for 2015, as a result of the acquisition of Rizobacter, which was our joint venture partner in Semya.
118
Finance income
Finance income decreased by 33.3%, or US$0.5 million to US$1 million for 2016 from US$1.5 million for 2015, primarily as a result of a decrease of US$0.2 million in exchange differences and US$1.3 million in present value adjustments, which was partially offset by changes in fair value of financial assets and liabilities of US$0.7 million and US$0.1 million of higher interest income generated by assets.
Finance costs
Finance costs increased by 473.5%, or US$9.0 million, to US$10.9 million for 2016 from US$1.9 million for 2015, primarily as a result of US$3.5 million of higher interest expenses resulting mainly from interest charges added by Rizobacter since its acquisition, US$1.5 million of higher exchange differences, US$1.1 million of higher financial commissions mainly related to Rizobacter since its acquisition, US$0.4 million of changes in fair value of financial assets and liabilities, US$0.4 million of higher present value adjustments and US$2.2 million of charges due to discontinuation of public offering expenses.
Loss before income tax
As a result of the foregoing, we recorded a loss before income tax of US$9.4 million for 2016 compared to a loss before income tax of US$3.3 million for 2015.
Income tax benefit / (expense)
Income tax benefit amounted to US$4.1 million for 2016 compared to an income tax expense of US$0.4 million for 2015. Although we recorded losses before income tax in each of 2016 and 2015, income tax arose in 2015 primarily because of differences between IFRS and Argentine tax law regarding the accounting treatment of certain items, such as the charge derived from our share of losses in joint ventures under IFRS, which is not deductible under Argentine tax law.
Other comprehensive loss
Other comprehensive loss amounted to US$4.5 million for 2016 compared to nil for 2015, primarily as a result of exchange differences on translation of foreign operations.
Total comprehensive loss
As a result of the foregoing, we recorded a total comprehensive loss of US$9.7 million for 2016 compared to a total comprehensive loss of US$3.7 million for 2015.
119
Rizobacter
Comparison of the three-month periods ended September 30, 2017 and 2016
The table below illustrates Rizobacter’s results of operations for the three-month periods ended September 30, 2017 and 2016.
Rizobacter | ||||||
Three-month period ended September 30, | ||||||
2017 | 2016 | |||||
(unaudited) | ||||||
Revenue | 32,852,813 | 26,153,338 | ||||
Inoculants | 6,306,921 | 4,993,062 | ||||
Seed therapics | 2,409,728 | 2,887,265 | ||||
Adjuvants | 7,993,798 | 6,764,431 | ||||
Packs | 6,515,983 | 5,767,778 | ||||
Fertilizers and Others | 9,626,383 | 5,740,802 | ||||
Cost of sales | (18,060,896 | ) | (11,608,790 | ) | ||
Inoculants | (4,436,796 | ) | (1,730,079 | ) | ||
Seed therapics | (1,224,196 | ) | (1,482,593 | ) | ||
Adjuvants | (3,015,946 | ) | (2,863,643 | ) | ||
Packs | (1,554,859 | ) | (1,521,077 | ) | ||
Fertilizers and Others | (7,829,099 | ) | (4,011,398 | ) | ||
Gross income | 14,791,917 | 14,544,548 | ||||
Administrative expenses | (2,371,453 | ) | (2,125,642 | ) | ||
Distribution expenses | (5,262,815 | ) | (4,668,990 | ) | ||
Research expenses | (579,634 | ) | (533,601 | ) | ||
Other operating expenses, net | (175,320 | ) | (23,699 | ) | ||
Operating income | 6,402,695 | 7,192,616 | ||||
Financial income | 1,198,552 | 1,000,264 | ||||
Financial costs | (5,719,583 | ) | (5,028,406 | ) | ||
Share of profit / (loss) of joint ventures and associates(1) | 297,939 | (232,404 | ) | |||
Profit / (loss) before income tax | 2,179,603 | 2,932,070 | ||||
Income tax (expense) | (489,761 | ) | (1,052,834 | ) | ||
Profit / (loss) for the year | 1,689,842 | 1,879,236 | ||||
Other comprehensive income / (loss) | (893,658 | ) | (365,644 | ) | ||
Total comprehensive income | 796,184 | 1,513,593 | ||||
Non-IFRS measures: | ||||||
Adjusted EBITDA (unaudited)(2) | 7,324,977 | 7,402,051 | ||||
| (1) | Accounted for using the equity method. |
| (2) | Adjusted EBITDA is a non-IFRS measure. For a complete presentation of the reconciliation of Net Loss to Adjusted EBITDA, see the section entitled “—Non-IFRS Financial Measures.” |
Revenue
Revenue increased by 25.6%, or US$6.7million, to US$32.9 million for the three-month period ended September 30, 2017 from US$26.2 million for the same three-month period in 2016, due to the increased demand from growers for more high quality technological products in order to improve crop profitability mainly in wheat, soybean and maize.
120
Revenue by business segment
Inoculants. Revenue increased by 26.3%, or US$1.3 million, to US$6.3 million for the three-month period ended September 30, 2017 compared to US$5.0 million for the same three-month period in 2016, primarily due to the substitution of lower value products for high value products, such as packs, at the beginning of the soybean season.
Seed Therapics. Revenue decreased by 16.5%, or US$0.5 million, to US$2.4 million for the three-month period ended September 30, 2017 compared to US$2.9 million for the same three-month period in 2016, primarily due to customer delays in making purchasing decisions.
Adjuvants. Revenue increased by 18.2%, or US$1.2 million, to US$8.0 million for the three-month period ended September 30, 2017 compared to US$6.7 million for the same three-month period in 2016, primarily due to an increased usage of crop protection products by customers at the beginning of the soybean and maize seasons.
Packs. Revenue increased by 12.9%, or US$0.8 million, to US$6.5 million for the three-month period ended September 30, 2017 compared to US$5.8 million for the same three-month period in 2016, due to the decision by many growers to use more higher value technological products due in order to increase crop profitability mainly in wheat, soybean and maize.
Fertilizers and Others. Revenue increased by 67.9%, or US$3.9 million, to US$9.6 million for the three-month period ended September 30, 2017 compared to US$5.7 million for the same three-month period in 2016, primarily due to increased usage for crop protection and crop nutrition products by growers at the beginning of the wheat, soybean and maize seasons.
Cost of sales
Cost of sales increased by 55.6%, or US$6.5 million, to US$18.1 million for the three-month period ended September 30, 2017 from US$11.6 million for the same three-month period in 2016, primarily as a result of higher product sales volumes, a change in the product mix, and cost increases mainly due to inflation that was not offset by exchange rate devaluation.
Cost of sales by business segment
Inoculants. Cost of sales increased by 156.4%, or US$ 2.7 million, to US$4.4 million for the three-month period ended September 30, 2017 compared to US$1.7 million for the same three-month period in 2016, primarily due to an increase sales volume, a change in the product mix and cost increases due to inflation.
Seed Therapics. Cost of sales decreased by 17.4%, or US$0.3million, to US$1.2 million for the three-month period ended September 30, 2017 compared to US$1.5 million for the same three-month period in 2016, primarily due to due to a change in the product mix and a decrease in sales volumes.
Adjuvants. Cost of sales increased by 5.3%, or US$0.2 million, to US$3.0 million for the three-month period ended September 30, 2017 compared to US$2.9 million for the same three-month period in 2016, primarily due to an increase in sales volume, a change in the product mix and cost increases due to inflation.
Packs. Cost of sales increased by 2.2%, or less than US$0.1 million, to US$1.5 million for the three-month period ended September 30, 2017 compared to US$1.5 million for the same three-month period in 2016, primarily due to a change in product mix and cost increases due to inflation.
Fertilizers and Others. Cost of sales increased by 95.1%, or US$3.8 million, to US$7.8 million for the three-month period ended September 30, 2017 compared to US$4.0 million for the same three-month period in 2016, primarily due to an increase in sales volume, a change in product mix and cost increases due to inflation.
121
Gross income
Gross income increased by 1.8%, or US$0.2 million, to US$14.7 million for the three-month period ended September 30, 2017 compared to US$14.5 million for the same three-month period in 2016, primarily due to an increase in sales volume, a change in product mix and cost increases due to inflation.
Gross income by business segment
Inoculants. As a result of the foregoing, gross income decreased by 42.7%, or US$1.4 million, to US$1.9 million for the three-month period ended September 30, 2017 compared to US$3.3 million for the same three-month period in 2016, primarily due to pricing pressures, cost increases and a change in product mix.
Seed Therapics. As a result of the foregoing, gross income decreased by 15.6%, or US$0.2 million, to US$1.2 million for the three-month period ended September 30, 2017 compared to US$1.4 million for the same three-month period in 2016, due to a decrease in sales volumes and changes in the mix of products.
Adjuvants. As a result of the foregoing, gross income increased by 27.6%, or US$1.1 million, to US$5.0 million for the three-month period ended June 30, 2017 compared to US$3.9 million for the same three-month period in 2016, mainly due to higher sales volumes and a change in product mix.
Packs. As a result of the foregoing, gross income increased by 16.8%, or US$0.7 million, to US$5.0 million for the three-month period ended September 30, 2017 compared to US$4.3 million for the same three-month period in 2016, mainly due to a change in product mix.
Fertilizers and Others. As a result of the foregoing, gross income increased by 3.9%, or less than US$0.1 million, to US$1.8 million for the three-month period ended September 30, 2017 compared to US$1.7 million for the same period in 2016.
Administrative expenses
Administrative expenses increased by 11.6%, or US$0.3 million, to US$2.4 million for the three-month period ended June 30, 2017 from US$2.1 million for the same three-month period in 2016, primarily as a result of inflation and staffing increases.
Distribution expenses
Distribution expenses increased by 12.7%, or US$0.6 million, to US$5.3 million the three-month period ended September 30, 2017 from US$4.7 million for the same three-month period in 2016, primarily as a result of increased investment in marketing and publicity, increased personnel costs due to inflation and business growth.
Research expenses
Research expenses increased by 8.7%, or less than US$0.1 million, to US$0.6 million for the three-month period ended September 30, 2017 from US$0.5 million for the same three-month period in 2016, primarily as a result of cost increases due to inflation.
Other operating expenses, net
Other operating income, net, increased by 640.0%, or US$0.2 million, to US$0.2 million the three-month period ended September 30, 2017 from less than US$0.1 million for the same three-month period in 2016, due to other expenses and inflation.
Operating income
As a result of the foregoing, operating income decreased by 10.9%, or US$0.8 million, to US$6.4 million for the three-month period ended September 30, 2017 from US$7.2 million for the same period in 2016.
122
Financial income
Financial income increased by 19.9%, or US$0.2 million, to US$1.2 million for the three-month period ended September 30, 2017 from US$1.0 million for the same period in 2016, primarily as a result of higher interest rates.
Financial costs
Financial costs increased by 13.7%, or US$0.7 million, to US$5.7 million for the three-month period ended September 30, 2017 from US$5.0 million for the same three-month period in 2016, primarily as a result of higher interest rates.
Share of profit / (loss) of joint ventures and associates
The profit resulting from Rizobacter’s share in the loss-profit of joint ventures and associates accounted for using the equity method increased by 228.0%, or US$0.5 million, to US$0.3 million for the three-month period ended September 30, 2017 from a loss of US$0.2 million for the same three-month period in 2016 as a result of increased profits in our joint venture Synertech.
Profit / (loss) before income tax
As a result of the foregoing, Rizobacter recorded a net profit before income tax of US$2.2 million for the three-month period ended September 30, 2017 compared to a net profit before income tax of US$2.9 million for the same three-month period in 2016.
Income tax (expense)
As a result of the foregoing, Rizobacter recorded a loss from income tax of US$0.5 million for the three-month period ended September 30, 2017 compared to a loss from income tax of US$1.1 million for the same three-month period in 2016.
Profit / (loss) for the period
As a result of the foregoing, Rizobacter recorded a profit of US$1.7 million for the three-month period ended September 30, 2017 compared to a profit of US$1.9 million for the same three-month period in 2016.
123
Comparison of the years ended June 30, 2017 and 2016
The table below illustrates Rizobacter’s results of operations for the years ended June 30, 2017 and 2016.
Rizobacter | ||||||
Year ended June 30, | ||||||
2017 | 2016 | |||||
Revenue | 112,296,212 | 93,405,678 | ||||
Inoculants | 12,482,786 | 12,759,410 | ||||
Seed therapics | 21,450,510 | 13,023,454 | ||||
Adjuvants | 35,653,018 | 29,869,904 | ||||
Packs | 23,111,291 | 20,843,223 | ||||
Fertilizers and others | 19,598,607 | 16,909,687 | ||||
Cost of sales | (58,838,471 | ) | (49,073,466 | ) | ||
Inoculants | (3,915,337 | ) | (7,936,177 | ) | ||
Seed therapics | (14,834,044 | ) | (8,949,443 | ) | ||
Adjuvants | (15,218,434 | ) | (10,678,992 | ) | ||
Packs | (6,789,019 | ) | (6,836,804 | ) | ||
Fertilizers and others | (18,081,636 | ) | (14,672,050 | ) | ||
Gross income | 53,457,741 | 44,332,212 | ||||
Administrative expenses | (9,765,385 | ) | (8,363,830 | ) | ||
Distribution expenses | (19,502,749 | ) | (18,824,875 | ) | ||
Research expenses | (2,423,428 | ) | (2,254,885 | ) | ||
Other operating income, net | 49,654 | 446,739 | ||||
Operating income | 21,815,833 | 15,335,361 | ||||
Financial income | 2,080,709 | 4,633,611 | ||||
Financial costs | (19,450,815 | ) | (27,025,501 | ) | ||
Share of loss of joint ventures and associates(1) | (1,109,131 | ) | (848,948 | ) | ||
Net profit / (loss) before income tax | 3,336,596 | (7,905,477 | ) | |||
Income tax (expense) / benefit | (1,789,654 | ) | 2,443,866 | |||
Net profit / (loss) for the year | 1,546,942 | (5,461,611 | ) | |||
Other comprehensive income / (loss) | 391,155 | (1,733,184 | ) | |||
Total comprehensive profit / (loss) | 1,938,097 | (7,194,795 | ) | |||
Non-IFRS measures: | ||||||
Adjusted EBITDA (unaudited)(2) | 23,301,133 | 16,303,882 | ||||
| (1) | Accounted for using the equity method. |
| (2) | Adjusted EBITDA is a non-IFRS measure. For a complete presentation of the reconciliation of Net Loss to Adjusted EBITDA, see the section entitled “—Non-IFRS Financial Measures.” |
Revenue
Revenue increased by 20.2%, or US$18.9 million, to US$112.3 million for the year ended June 30, 2017 from US$93.4 million for the same period in 2016, due to increased demand for crop protection and crop nutrition products from growers mainly in wheat, soy and maize, supported in part by the increased purchasing power of growers due to favorable public policies to the agricultural sector including reduced taxation.
124
Revenue by business segment
Inoculants. Revenue decreased by 2.2%, or US$0.3 million, to US$12.5 million for the year ended June 30, 2017 compared to US$12.8 million for the same period in 2016, primarily due to the substitution of low value products for high value products, such as packs.
Seed Therapics. Revenue increased by 64.7%, or US$8.4 million, to US$21.5 million for the year ended June 30, 2017 compared to US$13.0 million for the same period in 2016, primarily due to increased demand for and application of therapics on wheat and soybean crops due to diseases afflicting such crops as a result of certain climate conditions.
Adjuvants. Revenue increased by 19.4%, or US$5.8 million, to US$35.7 million for the year ended June 30, 2017 compared to US$29.9 million for the same period in 2016, primarily due to increased use of products of high technology and value and decreased consumer demand for and use of lower-tech products such as adjuvants.
Packs. Revenue increased by 10.9%, or US$2.3 million, to US$23.1 million for the year ended June 30, 2017 compared to US$20.8 million for the same period in 2016, primarily due to an increased investment by growers in high value products such as packs, replacing lower value products.
Fertilizers and Others. Revenue increased by 15.9%, or US$2.7 million, to US$19.6 million for the year ended June 30, 2017 compared to US$16.9 million for the same period in 2016, primarily due to this product line benefitting from the previously mentioned increase in demand for and investment in products of higher technology values.
Cost of sales
Cost of sales increased by 19.9%, or US$9.8 million, to US$58.8 million for the year ended June 30, 2017 from US$49.1 million for the same period in 2016, primarily as a result of higher sales volumes, a change in the mix of products sold and an increase in certain production costs due to cost inflation that was not offset by a corresponding devaluation of the Argentine peso.
Cost of sales by business segment
Inoculants. Cost of sales decreased by 50.7%, or US$4.0 million, to US$3.9 million for the year ended June 30, 2017 compared to US$7.9 million for the same period in 2016, primarily due to a decrease in the volume of sales and a differing mix of products.
Seed Therapics. Cost of sales increased by 65.8%, or US$5.9 million, to US$14.8 million for the year ended June 30, 2017 compared to US$8.9 million for the same period in 2016, primarily due to an increase in the volume of sales and a differing mix of products.
Adjuvants. Cost of sales in increased by 42.5%, or US$4.5 million, to US$15.2 million for the year ended June 30, 2017 compared to US$10.7 million for the same period in 2016, primarily due to an increase in the volume of sales and a differing mix of products.
Packs. Cost of sales decreased slightly by 0.7%, or less than US$0.1 million, to US$6.8 million for the year ended June 30, 2017 compared to US$6.8 million for the same period in 2016, primarily due to a differing mix of products.
Fertilizers and Others. Cost of sales increased by 23.2%, or US$3.4 million, to US$18.1 million for the year ended June 30, 2017 compared to US$14.7 million for the same period in 2016, primarily due to an increase in the volume of sales and a differing mix of products.
Gross income
Gross income increased by 20.6%, or US$9.1 million, to US$53.5 million for the year ended June 30, 2017 from US$44.3 million for the same period in 2016, primarily as a result of a change in consumer spending habits, which saw agricultural growers invest in more high-tech products, impacting the mix of products.
125
Gross income by business segment
Inoculants. As a result of the foregoing, gross income increased by 77.6%, or US$3.7 million, to US$8.6 million for the year ended June 30, 2017 compared to US$4.8 million for the same period in 2016.
Seed Therapics. As a result of the foregoing, gross income increased by 62.4%, or US$2.5 million, to US$6.6 million for the year ended June 30, 2017 compared to US$4.1 million for the same period in 2016.
Adjuvants. As a result of the foregoing, gross income increased by 6.5%, or US$1.2 million, to US$20.4 million for the year ended June 30, 2017 compared to US$19.2 million for the same period in 2016.
Packs. As a result of the foregoing, gross income increased by 16.5%, or US$2.3 million, to US$16.3 million for the year ended June 30, 2017 compared to US$14.0 million for the same period in 2016.
Fertilizers and Others. As a result of the foregoing, gross income decreased by 32.2%, or US$0.7 million, to US$1.5 million for the year ended June 30, 2017 compared to US$2.2 million for the same period in 2016.
Administrative expenses
Administrative expenses increased by 16.8%, or US$1.4 million, to US$9.8 million for the year ended June 30, 2017 from US$8.4 million for the same period in 2016, primarily as a result of an increase in staff and professional services costs and inflation in Argentina affecting staff and other administrative costs.
Distribution expenses
Distribution expenses increased by 3.6%, or US$0.7 million, to US$19.5 million the year ended June 30, 2017 from US$18.8 million for the same period in 2016, primarily as a result of increased investment in marketing and publicity, increased personnel costs due to inflation and business growth.
Research expenses
Research expenses increased by 7.5%, or US$0.2 million, to US$2.4 million for the year ended June 30, 2017 from US$2.3 million for the same period in 2016, primarily as a result of a general increase in research activities, coupled with the increased cost of salaries due to inflation.
Other operating income, net
Other operating income, net, decreased by 88.9%, or US$0.4 million, to less than US$0.1 million the year ended June 30, 2017 from US$0.4 million for the same period in 2016, primarily as a result of increased miscellaneous expenses, partially offset by other income.
Operating income
As a result of the foregoing, operating income increased by 42.3%, or US$6.5 million, to US$21.8 million for the year ended June 30, 2017 from US$15.3 million for the same period in 2016.
Financial income
Financial income decreased by 55.1%, or US$2.6 million, to US$2.1 million for the year ended June 30, 2017 from US$4.6 million for the same period in 2016, primarily as a result of an lower favorable variations in foreign exchange rates in relation to non-Argentine peso denominated assets, and the impact in lower interest rates for investments.
126
Financial costs
Financial costs decreased by 28.0%, or US$7.6 million, to US$19.5 million for the year ended June 30, 2017 from US$27.0 million for the same period in 2016, primarily as a result of reduced unfavorable variations in foreign exchange rates in relation to non-Argentine peso denominated liabilities, and the impact in lower interest rates for financing activities.
Share of loss of joint ventures and associates
The loss resulting from Rizobacter’s share in the loss of joint ventures and associates accounted for using the equity method increased by 30.6%, or US$0.3 million, to US$1.1 million for the year ended June 30, 2017 from US$0.8 million for the same period in 2016, primarily as a result of certain investments that have not started to generate profits, mainly with respect to the investment made in Synertech.
Profit / (loss) before income tax
As a result of the foregoing, Rizobacter recorded a net profit before income tax of US$3.3 million for the year ended June 30, 2017 compared to the net loss before income tax of US$7.9 million for the same period in 2016.
Income tax (expense) / benefit
Rizobacter recorded a loss from income tax of US$1.8 million for the year ended June 30, 2017 compared to a profit charge of US$2.4 million for the same period in 2016, primarily as a result of the pre-tax profit generated in the year ended June 30, 2017 compared to the pre-tax loss generated in the same period in 2016.
Profit / (loss) for the period
As a result of the foregoing, Rizobacter recorded a profit of US$1.5 million for the year ended June 30, 2017 compared to a loss of US$5.5 million for June 30, 2016.
127
Comparison of the years ended June 30, 2016 and 2015
The table below illustrates Rizobacter’s results of operations for the years ended June 30, 2016 and 2015.
Rizobacter | ||||||
Year ended June 30, | ||||||
2016 | 2015 | |||||
Revenue | 93,405,678 | 99,163,146 | ||||
Inoculants | 12,759,410 | 16,663,499 | ||||
Seed therapics | 13,023,454 | 10,701,871 | ||||
Adjuvants | 29,869,904 | 34,593,977 | ||||
Packs | 20,843,223 | 24,497,906 | ||||
Fertilizers and others | 16,909,687 | 12,705,893 | ||||
Cost of sales | (49,073,466 | ) | (47,357,049 | ) | ||
Inoculants | (7,936,177 | ) | (9,580,869 | ) | ||
Seed therapics | (8,949,443 | ) | (7,326,369 | ) | ||
Adjuvants | (10,678,992 | ) | (13,593,551 | ) | ||
Packs | (6,836,804 | ) | (6,621,439 | ) | ||
Fertilizers and others | (14,672,050 | ) | (10,234,821 | ) | ||
Gross income | 44,332,212 | 51,806,097 | ||||
Administrative expenses | (8,363,830 | ) | (7,677,468 | ) | ||
Distribution expenses | (18,824,875 | ) | (20,102,818 | ) | ||
Research expenses | (2,254,885 | ) | (655,941 | ) | ||
Other operating income, net | 446,739 | 158,731 | ||||
Operating income | 15,335,361 | 23,528,601 | ||||
Financial income | 4,633,611 | 2,032,019 | ||||
Financial costs | (27,025,501 | ) | (15,944,826 | ) | ||
Share of loss of joint ventures and associates(1) | (848,948 | ) | (584,728 | ) | ||
Net (loss) / profit before income tax | (7,905,477 | ) | 9,031,066 | |||
Income tax (expense) / benefit | 2,443,866 | (3,198,198 | ) | |||
Net (loss) / profit for the year | (5,461,611 | ) | 5,832,868 | |||
Other comprehensive loss | (1,733,184 | ) | (7,976,923 | ) | ||
Total comprehensive profit / (loss) | (7,194,795 | ) | (13,809,791 | ) | ||
Non-IFRS measures: | ||||||
Adjusted EBITDA (unaudited)(2) | 16,303,882 | 23,896,825 | ||||
| (1) | Accounted for using the equity method. |
| (2) | Adjusted EBITDA is a non-IFRS measure. For a complete presentation of the reconciliation of Net Loss to Adjusted EBITDA, see the section entitled “—Non-IFRS Financial Measures.” |
Revenue
Revenue decreased by 5.8%, or US$5.8 million, to US$93.4 million for the year ended June 30, 2016 from US$99.2 million for the same period in 2015, primarily as a result of a reduction of commodities prices during the fiscal year, adverse climactic conditions and currency exchange controls and import restrictions introduced in Argentina.
128
Revenue by business segment
Inoculants. Revenue decreased by 23.4%, or US$3.9 million, to US$12.8 million for the year ended June 30, 2016 compared to US$16.7 million for the same period in 2015, primarily due to decreased consumer investment by agricultural growers in certain crops and the substitution of low value products in lieu of high value products, such as inoculants, by such growers.
Seed Therapics. Revenue increased by 21.7%, or US$2.3 million, to US$13.0 million for the year ended June 30, 2016 compared to US$10.7 million for the same period in 2015, primarily due to increased demand for and application of therapics on wheat and soybean crops due to diseases afflicting such crops as a result of certain climate conditions.
Adjuvants. Revenue decreased by 13.7%, or US$4.7 million, to US$29.9 million for the year ended June 30, 2016 compared to US$34.6 million for the same period in 2015, primarily due to increased use of products of lower technology and value and decreased consumer demand for and use of high-tech products such as adjuvants.
Packs. Revenue decreased by 14.9%, or US$3.7 million, to US$20.8 million for the year ended June 30, 2016 compared to US$24.5 million for the same period in 2015, primarily due to decreased investment by agricultural growers in high value products such as packs, which have been replaced by lower value products.
Fertilizers and Others. Revenue increased by 33.1%, or US$4.2 million, to US$16.9 million for the year ended June 30, 2016 compared to US$12.7 million for the same period in 2015, primarily due to this product line benefitting from the previously mentioned decrease in demand for and investment in products of higher technology and value and increase in demand for products of lower value and margin, which shift in demand helped to partly compensate for the decrease in sales for the rest of the product lines.
Cost of sales
Cost of sales increased by 3.6%, or US$1.7 million, to US$49.1 million for the year ended June 30, 2016 from US$47.4 million for the same period in 2015, primarily as a result of an increase in the cost of the source products used in production, which was primarily due to increased inflation that was not offset by a corresponding devaluation of the Argentine peso. A portion of the increased cost of sales is also attributable to products rejected by customers.
Cost of sales by business segment
Inoculants. Cost of sales decreased by 17.2%, or US$1.6 million, to US$7.9 million for the year ended June 30, 2016 compared to US$9.6 million for the same period in 2015, primarily due to a decrease in the volume of sales and a differing mix of products.
Seed Therapics. Cost of sales increased by 22.2%, or US$1.6 million, to US$8.9 million for the year ended June 30, 2016 compared to US$7.3 million for the same period in 2015, primarily due to an increase in the volume of sales and a differing mix of products.
Adjuvants. Cost of sales decreased by 21.4%, or US$2.9 million, to US$10.7 million for the year ended June 30, 2016 compared to US$13.6 million for the same period in 2015, primarily due to a decrease in the volume of sales and a differing mix of products.
Packs. Cost of sales increased by 3.3%, or US$0.2 million, to US$6.8 million for the year ended June 30, 2016 compared to US$6.6 million for the same period in 2015, primarily due to a differing mix of products, partially offset by a decrease in the volume of sales.
Fertilizers and Others. Cost of sales increased by 43.4%, or US$4.4 million, to US$14.7 million for the year ended June 30, 2016 compared to US$10.2 million for the same period in 2015, primarily due to an increase in the volume of sales and a differing mix of products.
129
Gross income
Gross income decreased by 14.4%, or US$7.5 million, to US$44.3 million for the year ended June 30, 2016 from US$51.8 million for the same period in 2015, primarily as a result of a change in consumer spending habits, which saw agricultural growers invest in fewer high-tech products.
Gross income by business segment
Inoculants. As a result of the foregoing, gross income decreased by 31.9%, or US$2.3 million, to US$4.8 million for the year ended June 30, 2016 compared to US$7.1 million for the same period in 2015.
Seed Therapics. As a result of the foregoing, gross income increased by 20.7%, or US$0.7 million, to US$4.1 million for the year ended June 30, 2016 compared to US$3.4 million for the same period in 2015.
Adjuvants. As a result of the foregoing, gross income decreased by 8.6%, or US$1.8 million, to US$19.2 million for the year ended June 30, 2016 compared to US$21.0 million for the same period in 2015.
Packs. As a result of the foregoing, gross income decreased by 21.6%, or US$3.9 million, to US$14.0 million for the year ended June 30, 2016 compared to US$17.9 million for the same period in 2015.
Fertilizers and Others. As a result of the foregoing, gross income decreased by 9.4%, or US$0.2 million, to US$2.2 million for the year ended June 30, 2016 compared to US$2.5 million for the same period in 2015.
Administrative expenses
Administrative expenses increased by 8.9%, or US$0.7 million, to US$8.4 million for the year ended June 30, 2016 from US$7.7 million for the same period in 2015, primarily as a result of an increase in staff and professional services costs, which was primarily due to inflation in Argentina.
Distribution expenses
Distribution expenses decreased by 6.4%, or US$1.3 million, to US$18.8 million the year ended June 30, 2016 from US$20.1 million for the same period in 2015, primarily as a result of decreased investment in marketing and publicity, which was partially offset by the increased cost of salaries due to inflation.
Research expenses
Research expenses increased by 243.8%, or US$1.6 million, to US$2.3 million for the year ended June 30, 2016 from US$0.7 million for the same period in 2015, primarily as a result of a general increase in research, coupled with the increased cost of salaries due to inflation.
Other operating income, net
Other operating income, net, increased by 181.4%, or US$0.3 million, to US$0.4 million the year ended June 30, 2016 from US$0.2 million for the same period in 2015, primarily as a result of increased minor expenses, which was partially offset by other minor income.
Operating income
As a result of the foregoing, operating income decreased by 34.8%, or US$8.2 million, to US$15.3 million for the year ended June 30, 2016 from US$23.5 million for the same period in 2015.
Financial income
Financial income increased by 128.0%, or US$2.6 million, to US$4.6 million for the year ended June 30, 2016 from US$2.0 million for the same period in 2015, primarily as a result of an increase of favorable variations in foreign exchange rates in relation to non-Argentine peso denominated assets.
130
Finance costs
Financial costs increased by 69.5%, or US$11.1 million, to US$27.0 million for the year ended June 30, 2016 from US$15.9 million for the same period in 2015, primarily as a result of unfavorable variations in foreign exchange rates in relation to non-Argentine peso denominated liabilities.
Share of loss of joint ventures and associates
The loss resulting from Rizobacter’s share in the profit or loss of joint ventures accounted for using the equity method increased by 45.2%, or US$0.3 million, to US$0.8 million for the year ended June 30, 2016 from US$0.6 million for the same period in 2015, primarily as a result of certain investments that have not started to generate returns, particularly with respect to the investment made in Synertech.
Profit / (loss) before income tax
As a result of the foregoing, Rizobacter recorded a net loss before income tax of US$7.9 million for the year ended June 30, 2016 compared to a net profit before income tax of US$9.0 million for the same period in 2015.
Income tax (expense) / benefit
Rizobacter recorded a gain from income tax of US$2.4 million for the year ended June 30, 2016, from a charge of US$3.2 million for the same period in 2015, primarily as a result of the pre-tax loss generated in the year ended June 30, 2016 compared to the pre-tax gain generated in the same period in 2015.
Profit / (loss) for the period
As a result of the foregoing, Rizobacter recorded a loss of US$5.5 million for the year ended June 30, 2016 compared to a profit of US$5.8 million for June 30, 2015.
The tables below show the adjustments to Rizobacter financial information needed for comparing information across periods:
Rizobacter | |||||||||||||||
Stand-alone for the six-month period ended June 30, 2017 | For the twelve-month period ended December 31, 2016 | For the six-month period ended June 30, 2017(1) | Reconciling items(2) | For the six-month period ended June 30, 2017(3) | |||||||||||
(a) | (b) | (a) − (b) | (c) | (a) − (b) + (c) | |||||||||||
(US$) | |||||||||||||||
Revenue | 112,296,212 | 67,837,770 | 44,458,442 | — | 44,458,442 | ||||||||||
Cost of sales | (58,838,471 | ) | (33,781,095 | ) | (25,057,376 | ) | (2,436,949 | ) | (27,494,324 | ) | |||||
Gross income | 53,457,741 | 34,056,675 | 19,401,066 | (2,436,949 | ) | 16,964,117 | |||||||||
Operating income | 21,815,833 | 17,450,809 | 4,365,023 | (3,543,008 | ) | 822,015 | |||||||||
Profit / (loss) before income tax | 3,336,596 | 8,302,065 | (4,965,470 | ) | (3,543,008 | ) | (8,508,478 | ) | |||||||
Income tax benefit / (expense) | (1,789,654 | ) | (2,903,336 | ) | 1,113,683 | 1,220,000 | 2,333,683 | ||||||||
Profit / (loss) | 1,546,942 | 5,398,729 | (3,851,786 | ) | (2,323,009 | ) | (6,174,795 | ) | |||||||
Total comprehensive income / (loss) | 1,938,097 | 3,208,815 | (1,270,717 | ) | (2,668,225 | ) | (3,938,942 | ) | |||||||
| (1) | Includes the Rizobacter’s stand-alone results of operations for the six-month period ended June 30, 2017. |
| (2) | This column includes the following PPA adjustments for the six-month period as of June 30, 2017, relating to the acquisition of Rizobacter: |
| i. | The cost of sales line includes a non-recurring incremental cost of US$2.4 million related to PPA adjustments for revalued inventories sold after the acquisition date. |
| ii. | The operating income line includes an incremental charge of depreciation for revalued property, plan and equipment of US$0.2 million, and an incremental charge of amortization for revalued intangible assets of US$0.9 million. |
| iii. | The income tax benefit / (expense) line includes the tax effects on the taxabe adjustments included in the reconciling items column using an income tax rate of 35%. |
| (3) | This column represents the results of operations of Rizobacter as used for consolidation purposes of Bioceres. |
131
Rizobacter | |||||||||
Stand-alone for the three-month period ended September 30, 2017(1) | Reconciling items(2) | For the three-month period ended September 30, 2017(3) | |||||||
(US$) | |||||||||
(a) | (b) | (a) + (b) | |||||||
Revenue | 32,852,814 | — | 32,852,814 | ||||||
Cost of sales | (18,060,896 | ) | (1,119,155 | ) | (19,180,052 | ) | |||
Gross income | 14,791,917 | (1,119,155 | ) | 13,672,762 | |||||
Operating income | 6,700,635 | (1,770,058 | ) | 4,930,577 | |||||
Profit / (loss) before income tax | 2,179,603 | (1,770,058 | ) | 409,545 | |||||
Income tax benefit / (expense) | (489,761 | ) | 602,860 | 113,099 | |||||
Profit / (loss) | 1,689,842 | (1,167,198 | ) | 522,644 | |||||
Total comprehensive income / (loss) | 796,184 | (1,167,198 | ) | (371,014 | ) | ||||
| (1) | Includes the Rizobacter’s stand-alone results of operations for the three-month period ended September 30, 2017. |
| (2) | This column includes the following PPA adjustments for the three-month period ended September 30, 2017, relating to the acquisition of Rizobacter: |
| (i) | The cost of sales line includes a non-recurring incremental cost of US$1.12 million related to PPA adjustments for revalued inventories sold after the acquisition date. |
| (ii) | The operating income line includes an incremental charge related for revalued property, plant and equipment of US$0.2 million, and an incremental charge of amortization for revalued intangible assets of US$0.4 million. |
| (iii) | The income tax benefit / (expense) line includes the tax effects on the taxable adjustments included in the reconciling items column using an income tax rate of 35%. |
| (3) | This column represents the results of operations of Rizobacter as used for consolidation purposes of Bioceres. |
Non-IFRS Financial Measures
We supplement the use of IFRS financial measures in this prospectus with non-IFRS financial measures, including Adjusted EBITDA and Rizobacter Adjusted EBITDA. We define Adjusted EBITDA and Rizobacter Adjusted EBITDA as profit/(loss) exclusive of financial income/(costs), income tax benefit/(expense), depreciation, amortization, share-based compensation and other items and charges.
We believe that Adjusted EBITDA and Rizobacter Adjusted EBITDA provide useful supplemental information to investors about us and our results. Adjusted EBITDA and Rizobacter Adjusted EBITDA are among the measures used by our management team to evaluate our financial and operating performance and make day-to-day financial and operating decisions. In addition, Adjusted EBITDA and Rizobacter Adjusted EBITDA are frequently used by our competitors, rating agencies, securities analysts, investors and other parties to evaluate companies in our industry. We also believe that Adjusted EBITDA and Rizobacter Adjusted EBITDA are helpful to investors because they provide additional information about trends in our core operating performance prior to considering the impact of capital structure, depreciation, amortization and taxation on our results. Adjusted EBITDA and Rizobacter Adjusted EBITDA should not be considered in isolation or as substitutes for other measures of financial performance reported in accordance with IFRS. Adjusted EBITDA and Rizobacter Adjusted EBITDA have limitations as analytical tools, including:
| ■ | Adjusted EBITDA and Rizobacter Adjusted EBITDA do not reflect changes in, including cash requirements for, our working capital needs or contractual commitments; |
| ■ | Adjusted EBITDA and Rizobacter Adjusted EBITDA do not reflect our financial expenses, or the cash requirements to service interest or principal payments on our indebtedness, or interest income or other financial income; |
| ■ | Adjusted EBITDA and Rizobacter Adjusted EBITDA do not reflect our income tax expense or the cash requirements to pay our income taxes; |
| ■ | although depreciation and amortization are non-cash charges, the assets being depreciated or amortized often will need to be replaced in the future, and Adjusted EBITDA and Rizobacter Adjusted EBITDA do not reflect any cash requirements for the replacements; |
132
| ■ | although share-based compensation is a non-cash charge, Adjusted EBITDA and Rizobacter Adjusted EBITDA do not consider the potentially dilutive impact of share-based compensation; and |
| ■ | other companies may calculate Adjusted EBITDA differently, limiting its usefulness as a comparative measure. |
We compensate for the inherent limitations associated with using Adjusted EBITDA and Rizobacter Adjusted EBITDA through disclosure of these limitations, presentation of our consolidated financial statements in accordance with IFRS and reconciliation of Adjusted EBITDA and Rizobacter Adjusted EBITDA to the most directly comparable IFRS measure, income/(loss) for the period or year.
The table below provides a reconciliation of our loss for the period/year to Adjusted EBITDA:
Bioceres | ||||||||||||||||||||||||
Three-month period ended September 30 | Nine-month Period ended September 30, 2016 | Transition Period ended June 30, | Six-month period ended June 30, | Six-month Period ended June 30, 2016 | Year ended December 31, | |||||||||||||||||||
2017 | 2016 | 2017 | 2016 | 2016 | 2015 | |||||||||||||||||||
(unaudited) | (pro forma, unaudited) | (unaudited) | (pro forma, unaudited) | (unaudited) | (unaudited) | |||||||||||||||||||
(US$) | ||||||||||||||||||||||||
Reconciliation of Net Loss to Adjusted EBITDA: | ||||||||||||||||||||||||
Loss for the period / year | (2,929,239 | ) | (1,308,599 | ) | (14,807,604 | ) | (11,154,603 | ) | (3,764,971 | ) | (13,998,440 | ) | (5,229,914 | ) | (3,732,442 | ) | ||||||||
Income tax (benefit) / expense | (1,357,795 | ) | (621,022 | ) | (5,422,960 | ) | (5,090,723 | ) | (1,040,923 | ) | 5,065,016 | (4,140,028 | ) | 411,342 | ||||||||||
Finance costs | 9,268,419 | 1,117,154 | 23,984,209 | 14,945,495 | 1,579,951 | 16,214,402 | 10,923,378 | 1,904,569 | ||||||||||||||||
Finance income | (2,042,066 | ) | (381,327 | ) | (2,572,698 | ) | (2,136,265 | ) | (790,814 | ) | (1,142,264 | ) | (1,006,953 | ) | (1,509,736 | ) | ||||||||
Gain on previously held interest | — | — | — | — | — | — | (4,453,284 | ) | — | |||||||||||||||
Depreciation of property, plant and equipment | 771,724 | 146,061 | 2,451,028 | 1,524,709 | 256,492 | 1,580,558 | 1,074,733 | 456,444 | ||||||||||||||||
Amortization of intangible assets | 580,983 | 11,854 | 874,756 | 1,418,661 | 24,065 | 584,395 | 463,066 | 45,233 | ||||||||||||||||
Inventory purchase price allocation charge | 1,119,155 | — | — | 2,436,949 | — | — | 7,516,071 | — | ||||||||||||||||
Transaction expenses | — | 252,757 | — | — | 252,757 | — | 599,150 | — | ||||||||||||||||
Stock-based compensation charges | 154,948 | 296,196 | 882,151 | 375,724 | 585,955 | 585,955 | 644,261 | 48,283 | ||||||||||||||||
Adjusted EBITDA (unaudited) | 5,566,129 | (739,683 | ) | 5,388,883 | 2,318,947 | (2,897,488 | ) | 1,240,409 | 6,390,481 | (2,376,297 | ) | |||||||||||||
The table below provides a reconciliation of Rizobacter’s net profit/(loss) for the period/year to Rizobacter Adjusted EBITDA:
Rizobacter | |||||||||||||||
Three-month period ended September 30 | Year ended June 30, | ||||||||||||||
2017 | 2016 | 2017 | 2016 | 2015 | |||||||||||
(unaudited) | |||||||||||||||
(US$) | |||||||||||||||
Net profit / (loss) for the period / year | 1,689,842 | 1,879,236 | 1,546,942 | (5,461,611 | ) | 5,832,868 | |||||||||
Income tax (benefit) / expense | 489,761 | 1,052,834 | 1,789,654 | (2,443,866 | ) | 3,198,198 | |||||||||
Finance costs | 5,719,583 | 5,028,406 | 19,450,815 | 27,025,501 | 15,944,826 | ||||||||||
Finance income | (1,198,551 | ) | (1,000,264 | ) | (2,080,709 | ) | (4,633,611 | ) | 2,032,019 | ||||||
Depreciation of property, plant and equipment | 469,397 | 431,272 | 2,038,380 | 1,761,478 | 920,271 | ||||||||||
Amortization of intangible assets | 154,945 | 10,567 | 556,051 | 55,991 | 32,681 | ||||||||||
Rizobacter Adjusted EBITDA (unaudited) | 7,324,977 | 7,402,051 | 23,301,133 | 16,303,882 | 23,896,825 | ||||||||||
133
Liquidity and Capital Resources
Since our inception, we have funded our operations primarily with equity contributions from our shareholders; borrowings, including loans and credit facilities; income generated by our seed product sales, licensing fees and R&D service revenues; and payments from government grants. Our principal use of cash is to fund our operations, investments in intangible assets, expenditures in property, plant and equipment, working capital requirements and repayment of debt obligations.
As of September 30, 2017 and June 30, 2017, our cash and cash equivalents amounted to US$1.6 million and US$2.1 million, respectively. As of September 30, 2017 and June 30, 2017, we held other financial assets that amounted to US$4.5 million and US$4.5 million, respectively.
We believe that our existing cash and cash equivalents, cash inflows from revenue, current lines of credit and the net proceeds to us from this offering will be adequate to meet our anticipated cash needs for the next 12 months. In addition, we expect that the net proceeds from this offering will provide us with additional financial flexibility to execute our strategic objectives, including the possibility of expanding our businesses into new markets and making strategic investments and acquisitions.
Our ability to generate cash is subject to our performance, general economic conditions, industry trends and other factors. To the extent that funds from this offering, combined with existing cash and cash equivalents are insufficient to fund our future activities and requirements, we may need to raise additional funds through public or private equity or debt financing. If we issue equity securities in order to raise additional funds, substantial dilution to existing shareholders may occur. If we raise cash through the issuance of indebtedness, we may be subject to additional contractual restrictions on our business. We cannot assure the investor that we would be able to raise additional funds on favorable terms or at all.
Consolidated Statement of Cash Flows
Bioceres
The tables below illustrate our consolidated statement of cash flows for the periods indicated:
Bioceres | ||||||||||||
Three-month period ended September 30, | Nine-month period ended September 30, | |||||||||||
2017 | 2016 | 2017 | 2016 | |||||||||
(unaudited) (US$) | ||||||||||||
Net cash flows used in operating activities | (2,567,966 | ) | (1,801,001 | ) | (4,528,465 | ) | (9,147,975 | ) | ||||
Net cash flows used in investing activities | (2,454,078 | ) | (808,875 | ) | (5,385,829 | ) | (4,859,574 | ) | ||||
Net cash flows from financing activities | 4,492,934 | 944,050 | 15,384,303 | 14,043,017 | ||||||||
Net increase (decrease) in cash and cash equivalents | (529,110 | ) | (1,665,826 | ) | 5,470,009 | 35,468 | ||||||
Bioceres | ||||||||||||
For the Transition Period ended | For the six months ended | For the year ended | ||||||||||
June 30, 2017 | June 30, 2016 (unaudited) | December 31, 2016 | December 31, 2015 | |||||||||
(US$) | ||||||||||||
Net cash flows used in operating activities | (7,096,431 | ) | (7,346,974 | ) | (8,443,596 | ) | (3,044,790 | ) | ||||
Net cash flows used in investing activities | (2,931,751 | ) | (4,050,699 | ) | (47,020,853 | ) | (2,873,523 | ) | ||||
Net cash flows generated from financing activities | 10,891,369 | 13,098,967 | 56,662,720 | 3,727,039 | ||||||||
Net increase (decrease) in cash and cash equivalents | 863,187 | 1,701,294 | 1,198,271 | (2,191,274 | ) | |||||||
134
Net cash flows generated from operating activities
Cash used in operating activities for the three-month period ended September 30, 2017 amounted to US$2.6 million. Our loss of US$2.9 million, non-cash negative adjustments for income tax credit of US$1.3 million and a negative variation in net working capital of US$3.7 million, were partly offset by non-cash positive adjustments relating primarily to accrued financial charges of US$3.5 million, depreciation and amortization charges of US$1.3 million, charges of impairment of trade debtors of US$0.4 million and charges for stock based compensation of US$0.2 million.
Cash used in operating activities for the three-month period ended September 30, 2016 amounted to US$1.8 million. Our loss of US$1.3 million, non-cash negative adjustments for income tax credit of US$0.6 million and a negative variation in net working capital of US$0.6 million, were partly offset by non-cash positive adjustments relating primarily to depreciation and amortization charges of US$0.2 million, charges for stock based compensation of US$0.3 million and charges for our share in the results of joint ventures of US$0.2 million.
Cash used in operating activities for the Transition Period amounted to US$7.1 million. Our loss of US$11.2 million, non-cash negative adjustments for income tax credit of US$5.1 million and for a gain in the transfer of certain assets of US$0.7 million, and a negative variation in net working capital of US$0.4 million, were partly offset by non-cash positive adjustments relating primarily to accrued financial charges of US$6.3 million, depreciation and amortization charges of US$2.9 million, charges for our share in the results of joint ventures of US$0.8 million and charges for stock based compensation of US$0.4 million.
Cash used in operating activities for the six-month period ended June 30, 2016 amounted to US$7.3 million. Our loss of US$3.8 million, a non-cash negative adjustment for income tax credit of US$1 million, and working capital requirements of US$4 million relating primarily to reduced trade and other payables, were partly offset by non-cash positive adjustments relating primarily to charges for stock based compensation of US$0.6 million, charges for our share in the results of joint ventures of US$0.5 million and depreciation and amortization charges of US$0.3 million.
Cash used in operating activities for the year ended December 31, 2016 amounted to US$8.4 million. Our loss of US$5.2 million, non-cash negative adjustments for income tax credit of US$4.1 million, for a gain on the acquisition of Semya of US$4.5 million and for the transfer of certain assets of US$0.3 million, combined with working capital requirements of US$7.4 million relating primarily to higher trade and other receivables and reduced trade payables compensated partially by lower inventories, were partly offset by non-cash positive adjustments relating primarily to accrued financial charges of US$8.5 million, depreciation and amortization charges of US$1.5 million, charges for our share in the results of joint ventures of US$0.9 million, and charges for stock based compensation of US$0.6 million.
Cash used in operating activities for the year ended December 31, 2015 amounted to US$3.0 million. Our loss of US$3.7 million and working capital requirements of US$1.8 million relating primarily to higher inventories and other receivables partially compensated by higher trade and other payables, were partly offset by non-cash positive adjustments relating primarily to charges for our share in the results of joint ventures of US$1.6 million, depreciation and amortization charges of US$0.5 million and an income tax charge of US$0.4 million.
Net cash flows used in investing activities
Cash used in investing activities for the three-month period ended September 30, 2017 amounted to US$2.5 million and was primarily attributable to loans granted to join ventures of US$2.0 million, investments in property, plant and equipment of US$0.3 million and investments in intangible assets of US$0.1 million.
Cash used in investing activities for the three-month period ended September 30, 2016 amounted to US$0.8 million and was primarily attributable to investments in property, plant and equipment of US$0.7 million, investments in intangible assets of US$0.1 million and investments of US$0.1 million in the joint ventures in which we participate.
Cash used in investing activities for the Transition Period amounted to US$2.9 million and was primarily attributable to investments in intangible assets of US$1.5 million, investments in property, plant and equipment of US$0.8 million, and investments of US$0.6 million in the joint ventures in which we participate.
Cash used in investing activities for the transition period ended June 30, 2016 amounted to US$4.1 million and was primarily attributable to investments of US$3.4 million in short-term marketable securities, investments in property,
135
plant and equipment of US$0.5 million, investments in intangible assets of US$0.1 million, and investments of US$0.1 million in the joint ventures in which we participate.
Cash used in investing activities for the year ended December 31, 2016 amounted to US$47.0 million and was primarily attributable to the Rizobacter acquisition of US$41.1 million, short-term investments of US$3 million, investments in property, plant and equipment of US$1.9 million, investments in intangible assets of US$0.8 million and investments of US$0.2 million in the joint ventures in which we participate.
Cash used in investing activities for the year ended December 31, 2015 amounted to US$2.9 million and was primarily attributable to investments in joint ventures of US$2 million, investments in property, plant and equipment of US$0.6 million and investments in intangible assets of US$0.2 million.
Net cash flows generated from financing activities
Cash provided by financing activities for the three-month period ended September 30, 2017 amounted to US$4.5 million and consisted of proceeds of US$70.2 million raised from bank borrowings, partially offset by the repayment of bank borrowings for US$66.1 million and proceeds of US$0.4 million from the sale of own shares held by a subsidiary.
Cash provided by financing activities for the three-month period ended September 30, 2016 amounted to US$0.9 million and consisted of proceeds of US$1 million raised from bank borrowings, partially offset by the repayment of bank borrowings for US$0.1 million.
Cash provided by financing activities for the Transition Period amounted to US$10.9 million and consisted of proceeds of US$61.1 million raised from bank borrowings, partially offset by the repayment of bank borrowings for US$50.2 million.
Cash provided by financing activities for the transition period ended June 30, 2016 amounted to US$13.1 million and consisted of proceeds of US$15 million raised from bank borrowings, partially offset by repayments of bank borrowings for US$1.9 million.
Cash provided by financing activities for the year ended December 31, 2016 amounted to US$56.7 million and consisted of proceeds raised from facilities for US$35.6 million destined to fund the cash consideration of the Rizobacter acquisition, from bank borrowings for US$17.4 million, and from preferred shares issued by our subsidiary RASA Holding to private investors for US$5.5 million, partially offset by repayments of bank borrowings for US$1.8 million.
Cash provided by financing activities for the year ended December 31, 2015 amounted to US$3.7 million and consisted of proceeds raised from bank borrowings for US$2.3 million, and from capital contributions for US$2 million, partially offset by repayments of bank borrowings for US$0.5 million.
Indebtedness
As of September 30, 2017, our total outstanding borrowings were US$96.9 million, which consists of US$60.3 million of current borrowings, including US$24.4 million of the short-term portion of long-term loans, US$16.9 million in convertible borrowings, US$12.8 million in discounted checks and US$6.1 million of the short-term portion of corporate bonds and US$36.6 million of non-current borrowings, consisting of long-term loans and credit facilities.
Our borrowings denominated in Argentine pesos amounted to US$22.1 million, and include loans for US$17.9 million that bear fixed interest rates ranging from 1% to 26%, and loans for US$4.1 million that bear variable interest rates that ranged from 9% to 33%. Our borrowings denominated in currencies other than the Argentine peso amounted to US$74.8 million, and include loans for US$72.4 million that bear fixed interest rates ranging from 2.1% to 8.5%, and loans for US$2.4 million that bear variable interest rates around 10%. Of our total outstanding borrowings as of September 30, 2017, US$42.3 million was unsecured, US$35.4 was secured by certain receivables, by checks for US$12.8 million, by time deposits for US$4.5 million, by certain property, plant and equipment for US$1.0 million, by certain Argentine reciprocal guarantee companies for US$0.5 million and by certain equity shareholdings for US$0.4 million.
136
Additionally, as of September 30, 2017 we had liabilities for financed payments relating to the acquisition of Rizobacter of US$61.1 million, consisting of US$27.1 million of current financed payments and US$34.0 million of non-current financed payments.
Puttable instruments
As of September 30, 2017, our non-current liabilities included US$2.4 million and our current liabilities included US$0.2 million of puttable instruments relating to ordinary shares of Bioceres S.A. purchased by three investors, each of whom has the right to put the shares back to us for cash consideration, as further explained below, and US$2.8 million of puttable instruments relating to preferred shares of RASA Holding held by EQC Agrifund Ltd., or EQC.
On July 15, 2014 and November 21, 2014, respectively, San Cristóbal and YPF each purchased 128,900 ordinary shares of Bioceres S.A. in connection with our capital increase, for US$7.91 per share, equal to a total consideration of US$1,020,424 each, or US$2,040,848 total. Each entity concurrently signed a put option contract with Bioceres, Inc. providing each entity the right to sell such shares to Bioceres, Inc. for a price equal to US$7.91 per share plus 4.5% of annual interest from the date on which the agreement was signed. The option may be exercised incrementally each year for five years in an amount equal to 20.0% of our total shares beginning in July 2018 in the case of San Cristóbal and November 2016 in the case of YPF.
As of June 30, 2017, YPF had not provided notice of its decision to exercise the put option. A decision to exercise the put option requires at least 12 months’ prior notice in advance of the redemption date.
Further, on June 14, 2016, one of our shareholders, Gador S.A., accepted a put option offer for 129,100 shares of Bioceres S.A. that it holds. This offer gave Gador a right to sell those shares to Bioceres, Inc under the same terms as YPF and San Cristobal. The option may be exercised incrementally each year for five years for an amount equal to 20% of the total purchased shares as from June 2020.
On July 12, 2017, San Cristobal exercised its put option for the first tranche of 20% of its shareholding and therefore said share will be payable at one year since the notification of the exercise of the option with an annual interest of 4.5%.
On October 14, 2016, Bioceres Inc. granted EQC a put option, giving EQC a right to sell its holdings in shares of RASA Holding that EQC acquires in the event of a mandatory conversion of its holdings of preferred shares into ordinary shares of RASA Holding in five years, or in the case of a public offering by RASA Holding or Rizobacter.
In April 7, 2016, Bioceres Inc. granted BAF a put option, giving BAF a right to sell the shares of Bioceres that BAF acquires in the event of a financing event, as defined in the participation rights agreement.
In October 14, 2016, Bioceres Inc. granted BAF a put option, giving BAF a right to sell the shares of Bioceres and/or RASA Holding that BAF acquires in the event of a financing event, as defined in the participation rights agreement, and/or in the event of a mandatory conversion of its holdings of preferred shares into ordinary shares of RASA Holding in five years, or in the case of a public offering by RASA Holding or Rizobacter.
Promissory Notes to be Offset through Participation Rights
In April 2016, we entered into a convertible loan agreement with Monsanto and BAF, pursuant to which we issued promissory notes granting Monsanto and BAF participation rights to subscribe to a total nominal value of US$17.55 million of our ordinary shares in the event of a qualified or non-qualified financing and received US$15.0 million net proceeds, after applying a 17% subscription discount. The terms of the promissory notes dictate that after a two-year grace period, (i) the notes will begin to accrue interest at an 8% annual rate and (ii) the face amount of the promissory notes will be increased by an additional 10%. The promissory notes mature after the first of a qualified or unqualified financing event, such as this offering, or five years. The promissory notes are subject to mandatory conversion upon, among other events, a qualified financing event, such as an offering or private placement, the net proceeds of which exceed US$30 million, and optional conversion upon a non-qualified public offering. The conversion price per share of the promissory note is equal either to the price per share of this offering or the price per share of a qualifying private placement. The loan agreement executed in connection with the promissory note contains customary representations, warranties and covenants. For more information on the conversion rights of Monsanto and BAF pursuant to these promissory notes see Note 7.10 of the audited consolidated financial statements of Bioceres and its subsidiaries.
137
We also granted participation rights in October 2016 and May 2017 to the holders of preferred shares of RASA Holding for the subscription to ordinary shares in the event of a qualified or non-qualified financing. The participation rights become mandatory in the event of a qualified financing, such as this offering, of at least US$50 million received from holders of the preferred shares of RASA Holding in exercise of their mandatory participation rights. The holders of preferred shares of RASA Holding shall be entitled to subscribe to the number of ordinary shares resulting from their holding of preferred shares at the time of the qualified financing event plus the dividends accrued at that moment if any. As of June 30, 2017, there were 1,409,848 Class A preferred shares (excluding preferred dividends) held by third parties, which accrue an annual PIK (“pay in kind”) dividend coupon of 12%. Assuming that the Argentine offering occurs before December 31, 2017, the holders of preferred shares of RASA Holding will be entitled to subscribe to 1,982,970 ordinary shares at the offering. Proceeds from these investors will be used to acquire all of the preferred shares of RASA Holding not currently owned by us. Preemptive were suspended by our shareholders’ meeting dated December 15, 2016.
We granted voluntary participation rights to BAF in connection with each of the BAF Bridge Loans for the subscription of our ordinary shares for the total amount of US$32 million upon the occurrence of a qualified public offering, the proceeds from which would offset the amount owed by us under the BAF Bridge Loans used for the acquisition of Rizobacter. Preemptive rights in accordance with BAF’s participation rights were suspended in our shareholders’ meeting dated December 15, 2016.
BAF Latam Trade Finance Fund B.V. Loan Facility Agreements
In October 2016, our wholly-owned subsidiary, Bioceres Inc., consummated the BAF US$20M Bridge Loan as a bridge financing for our acquisition of Rizobacter. At the same time, we consummated the BAF US$12M Bridge Loan. The loans are secured by pledges of the shares of our indirectly wholly-owned subsidiary, RASA Holding. We pledged 800,000 preferred shares and Bioceres, Inc. pledged 400,000 ordinary shares of RASA Holding for the BAF US$12M Bridge Loan. Bioceres, Inc. pledged 2,000,000 preferred shares of RASA Holding for the BAF US$20M Bridge Loan.
The BAF US$12M Bridge Loan accrues interest at an annual rate of 8.0% and the BAF US$20M Bridge Loan accrues interest at an annual rate of 8.5%, each payable semi-annually. The BAF Bridge Loans matured after 12 months, but have since been extended through October 2018. The BAF Bridge Loans contemplate certain financial covenants which may limit our ability to incur additional debt, requiring each borrower to maintain a capitalization ratio of at least 0.10x during the life of the loan.
The proceeds of the BAF Bridge Loans were used to fund our acquisition of Rizobacter, through the subscription of preferred shares of RASA Holding, owner of 50.01% of Rizobacter’s capital stock.
In connection with each of the BAF Bridge Loans, we have executed participation rights agreements pursuant to which, upon the occurrence of a qualified public offering, BAF has the right to subscribe to purchase our ordinary shares at the placement price for an amount equal to the outstanding balance of the respective BAF Bridge Loans, which would result in the extinguishment of all of our obligations (including those of our subsidiaries) under the relevant BAF Bridge Loan if exercised.
Rizobacter Facility
We consummated a syndicated loan facility for up to US$45 million among Rizobacter and a group of banks including Banco de Galicia y Buenos Aires S.A., as administrative agent, or the Syndicated Loan Facility, with a first installment of US$22 million funded in March 2017 and a second installment of US$23 million funded in April 2017. The terms of the syndicated loan dictate a final maturity in 48 months, with quarterly interest payments at a 6.5% annual rate, and 13 quarterly principal repayments after a one-year grace period.
The proceeds of the Syndicated Loan Facility were used to fund repayment of Rizobacter’s short-term borrowings and working capital needs. The facility is guaranteed with a portion of Rizobacter’s cash flows, a US$4.3 million dedicated term deposit and Bioceres S.A guarantees. The Syndicated Loan Facility includes certain standard representations and warranties on behalf of Rizobacter and certain covenants, including limitations on the change of control, i.e., reduction of our shareholding in Rizobacter’s capital stock, and on dividends by Rizobacter and payments to shareholders of Rizobacter while the loan facility is effective. The Syndicated Loan Facility limits lending by Rizobacter in favor of any affiliate for an amount in excess of US$5 million. The facility also contemplates certain financial covenants which may limit Rizobacter’s ability to incur additional debt, requiring a ratio of financial debt to EBITDA of no more than 3x,
138
interest coverage ratios ranging from 1.2x to 2x over the course of the loan and limitations placed on the overall ratio of liabilities to assets ranging from 0.85x to 0.8x over the course of the loan.
Projected Sources and Uses of Cash
Historically, our principal sources of liquidity have consisted of shareholder’s equity capital contributions, equity-linked and convertible financings, debt borrowings, bank credit lines and facilities, and our operating cash flow. We anticipate that we will generate cash from projected growth in our operating cash flow as we execute our expansion plans, and from additional future equity, equity-linked and debt financings we may raise to optimize our capital structure and cost of capital.
We expect our operating cash flows to be positive in the foreseeable future as a result of growing revenue and profitability through expanding local and international sales of our current products, and of our pipeline’s new product launches, by:
| ■ | Commercially launching seed traits and EcoSeed products to drive penetration in local and regional seed markets. |
| ■ | Scaling up production of micro-beaded fertilizers to accelerate penetration in local and regional crop nutrition markets. |
| ■ | Expanding our international business by accelerating registration and sales of products through our multiple subsidiaries. |
| ■ | Developing and commercializing new agricultural biotechnology products and solutions in existing and new markets. |
Nevertheless, we cannot assure you that we will generate positive operating cash flow.
We note that, even though our total equity was US$22.4 million, we presented negative equity attributable to equity holders of the parent in the balance sheet as of September 30, 2017. This negative equity is primarily due to certain accounting effects related to the Rizobacter acquisition, including (i) an extraordinary charge included in the cost of sales of Rizobacter inventories acquired and sold post-acquisition that were revalued at acquisition due to the application of PPA accounting rules, (ii) currency exchange differences relating to Argentine peso-denominated assets and liabilities acquired, and (iii) the classification of certain capital raises through convertible loans and puttable instruments as liabilities and through convertible preferred shares as non-controlling interest under IFRS. We expect to reverse our negative equity balance through the net proceeds from this offering and the expected conversion of certain convertible instruments into ordinary shares. See “Use of Proceeds.”
Notwithstanding the above, even if as of September 30, 2017, the Company had a negative total equity, the Group’s consolidated total equity as of the same date was positive by US$23.6 million.
139
Tabular Disclosure of Contractual Obligations
The following table summarizes our contractual obligations as of June 30, 2017.
Up to one year | Between one and three years | Between three and five years | Subsequent years | Total | |||||||||||
Trade and other payables | 23,748,712 | — | — | — | 23,748,712 | ||||||||||
Borrowings(1) | 56,540,974 | 26,955,690 | 18,773,427 | — | 102,270,091 | ||||||||||
Employee benefits and social security | 5,570,209 | — | — | — | 5,570,209 | ||||||||||
Income and minimum presumed income taxes payable | 223,187 | — | — | — | 223,187 | ||||||||||
Financed payment – Business combination(1) | 27,819,832 | 36,277,550 | — | — | 64,097,332 | ||||||||||
Contingent consideration – Business combination(1) | — | 17,300,000 | — | — | 17,300,000 | ||||||||||
Puttable instruments(1) | — | 1,176,549 | 5,889,427 | 518,375 | 7,584,351 | ||||||||||
Total | 113,902,910 | 81,709,739 | 24,662,854 | 518,375 | 220,793,882 | ||||||||||
| (1) | Includes principal and prospective interest. |
We do not currently engage in, or have not engaged in for the periods presented, any off-balance sheet transactions, arrangements or obligations with unconsolidated entities or otherwise.
Between 2005 and 2007, we entered into agreements with various investors in order to obtain funding in the aggregate amount of US$1.0 million for research related to early stage technology for the development of technology relating to a specific gene from sunflower intended to promote drought tolerance in crops, which we refer to as Hahb 4. The agreements grant the investors in the aggregate the right to receive 52.8% of the rights and royalties payable to Bioceres S.A. from the successful commercialization of the resulting technology with respect to soybean, wheat and corn. As of the date hereof, a portion of the Hahb 4 technology relating to a promoter element is being incorporated into a leading soybean product under development by Verdeca that also incorporates our HB4 technology. In consideration for the exclusive license we agreed to grant to Verdeca to use and commercialize our technology with respect to soybeans, including technology relating to both Hahb 4 and HB4, Bioceres Inc. will receive from Verdeca 15% of the net revenue realized from the commercialization of those soybeans by Verdeca. Accordingly, upon Verdeca’s successful commercialization of soybean incorporating our technology, we will be contractually obliged to pay investors an aggregate of 52.8% of a portion of the royalty income that Bioceres S.A. receives from Verdeca based on the revenues derived from the commercialization of the Hahb 4 technology. As the royalty amounts payable from Verdeca are not exclusively in relation to Hahb 4 technology, we believe only a portion of the royalty income we receive from Verdeca will be subject to the investor royalty sharing rights described above. We may also be obligated to pay a percentage of future royalty income to the investors if we or our licensees commercialize wheat and corn products incorporating Hahb 4 technology in the future.
Critical Accounting Policies and Estimates
The preparation of our consolidated financial statements and related disclosures in accordance with IFRS requires our management to make certain judgments, estimates and assumptions regarding the future. Estimates and judgments are continually evaluated based on historical experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances. In the future, actual experience may differ from these judgments, estimates and assumptions. The judgments, estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year are discussed in our Consolidated Financial Statements.
In order to provide an understanding of the manner in which our management forms its judgments about future events, including the variables underlying our judgments, estimates and assumptions, we summarize our critical accounting policies in Note 6 to our Consolidated Financial Statements.
140
Quantitative and Qualitative Disclosure about Market Risk
Our board of directors has overall responsibility for establishing and monitoring our risk management objectives and policies. While it retains ultimate responsibility for risk management, it has delegated the day to day monitoring to our finance team to design and operate processes that ensure effective implementation of the risk management objectives and policies of our finance team, which periodically reports to the board on the evolution of the risk management activities and results. Our audit committee will also review the risk management policies and processes and report our findings to the board. The overall objective of the board is to set policies that seek to reduce risk as far as possible without unduly affecting our competitiveness and flexibility. We do not use market risk-sensitive instruments for trading or speculative purposes.
The risks and methods for managing the risks are reviewed regularly, in order to reflect changes in market conditions and our activities. Through training, management standards and procedures, we aim to develop a disciplined and constructive control environment in which all our employees understand their roles and obligations. The principal risks and uncertainties facing the business, set out below, do not appear in any particular order of potential materiality or probability of occurrence.
Currency risk
Foreign currency risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rate. Currency on foreign exchange risk arises when we enter into transactions denominated in a currency other than its functional currency. A significant part of our business activities is conducted in Argentine peso. However, some of our subsidiaries with Argentine peso as functional currency also have significant transactions denominated in U.S. dollars, mainly sales and financing activities.
Our policy is, where possible, to allow our entities to settle liabilities denominated in U.S. dollars with the cash generated from their own operations in U.S. dollars. We have liabilities denominated in U.S. Dollars in entities with Argentine peso as functional currency, which expose us to foreign currency exchange risks. Such risks are partially mitigated by our revenues, which are also partly denominated in U.S. Dollars (mainly exports) or Pesos but adjusted to reflect changes in U.S. Dollars.
We periodically evaluate the use of derivatives and other financial instruments to hedge our foreign exchange rate exposure, but do not have any exchange rate related financial instruments in place.
The table below sets forth our net exposure to currency risk as of September 30, and June 30, 2017 and December 31, 2016 and 2015:
September 30, | June 30, | December 31, | ||||||||||
2017 | 2017 | 2016 | 2015 | |||||||||
(US$) | ||||||||||||
Net USD foreign currency position | (70,487,329 | ) | (69,106,000 | ) | (47,511,149 | ) | 1,212,452 | |||||
We estimate that a devaluation of the Argentine peso against the U.S. dollar of 20% in the three-month period ended September 30, 2017, would have resulted in a net pre-tax loss of approximately US$14.1 million. We estimate that an appreciation of Argentine peso against the U.S. dollar of 20% would have resulted in a net pre-tax gain of approximately US$14.1 million.
Interest rate risk
Our financing costs may be affected by interest rate volatility. Borrowings under our interest rate management policy may be fixed or floating rate. We maintain adequate committed borrowing facilities and hold most of our financial assets primarily in cash or checks collected from customers that are readily convertible into known amounts of cash.
Our interest rate risk arises from long term borrowings. Borrowings issued at floating rates expose us to cash flow interest rate risk. Borrowings issued at fixed rates expose us to fair value interest rate risk.
We have not entered into derivative contracts to hedge this exposure.
141
Our debt consists of loans and of financing for the Acquisition, as set out below.
September 30, 2017 | June 30, 2017 | |||||
Carrying amount | ||||||
(US$) | ||||||
Fixed-rate instruments | ||||||
Current financial liabilities | 82,272,511 | 70,678,974 | ||||
Non-current financial liabilities | 69,260,873 | 73,798,769 | ||||
Variable-rate instruments | ||||||
Current financial liabilities | 5,134,232 | 6,818,938 | ||||
Non-current financial liabilities | 1,356,956 | 711,157 | ||||
We do not use derivative financial instruments to hedge our interest rate risk exposure.
Credit risk
Our credit risk is our risk of financial loss if a customer or counterparty fails to meet its contractual obligations, and derives mainly from trade receivables and other receivables generated by services and product sales, as well as from cash and deposits in financial institutions. We are also exposed to political and economic risk events, which may cause nonpayment of local and foreign currency obligations owed to us by customers, partners, contractors and/or suppliers.
We sell seed and integrated products, crop protection products and crop nutrition products, and offer emerging solutions that mainly include and offer R&D and technical services to a diverse base of customers. Our customers include multi-national and local agricultural companies, distributors, research and educational institutions and growers who purchase our seed products and R&D services. The type and class of customers may vary among our business segments.
Our finance function determines concentrations of credit risk by periodically monitoring the credit rating of existing customers and through monthly reviews of the trade receivables’ aging analysis. Based on our periodic monitoring the customers’ credit risk, customers are grouped according to their credit characteristics.
About 44% of our seed and integrated products segment sales for the three-month period ended September 30, 2017 were made to five well-known customers with good quality standing, with the top two customers representing 34% of such segment sales. In the crop protection segment, the top five customers represented 24% of the segment’s sales. In the crop nutrition segment, the top 5 customers represented 34% of the segment’s sales. In the emerging solutions segment, sales for services rendered to the top five customers represented 77% of segment sales (including grants) for the three-month period ended September 30, 2017.
Our policy is to manage credit exposure to counterparties through a process of credit rating. We perform credit evaluations of existing and new customers, and conduct a thorough credit check on every new customer before offering the customer transaction terms. Our examination includes collecting outside credit rating information, if available. Additionally, and even if there is no independent outside rating, we assess the credit quality of the customer taking into account our financial position, past experience, bank references and other factors. We prescribe a credit limit for each customer and examine such limits annually. Customers that do not meet our criteria for credit quality may do business with us on the basis of a prepayment or upon furnishing appropriate collateral. We may seek collateral and guarantees, as considered appropriate, for the credit profile of any customer.
Based on our periodic monitoring of customer credit risk, the customers are grouped according to a characterization of their credit, based on geographical location, industry, aging of receivables, maturity, and existence of past financial difficulties. Customers defined as “high risk” are added to a restricted customer list and are supervised by management. In the case of a doubtful debt, we record a provision for the amount of the debt less the value of the collateral provided and take measures to enforce upon the collateral.
We are also exposed to counterparty credit risk on cash and cash equivalent balances. We hold cash on deposit with a number of financial institutions. We manage our credit risk exposure by limiting individual deposits to clearly defined limits. We only deposit funds with high-quality banks and financial institutions.
142
Global Industry Overview
We develop, produce and/or formulate: germplasm, seed traits, seed treatments, biological and microgranulated fertilizers, specialty insecticides and fungicides, adjuvants and specialty enzymes. Our key geographical end-markets include Argentina, which is the third largest market for agricultural biotechnology products, Brazil and the rest of Latin America, the United States, China and India. We sell our products in more than 25 countries globally. Our products and technologies have applicability across a wide variety of crops, including some of the most globally farmed crops such as corn, soy, alfalfa and wheat.
Demand for crop yields from agricultural lands are seeing a dramatic increase as a result of increasing global population, an expanding middle class, trend towards urbanization, decrease in agricultural land per capita, demand for reduced use of environmentally harmful chemicals and an increase in unfavorable weather patterns for farming. This demand cannot be met by conventional farming alone. Agricultural biotechnology products are the only current viable avenue available to meet this expected high demand in crop yields.
According to the USDA, global demand for grains increased by more than 57% from 1.4 billion metric tons in 1980 to 2.2 billion metric tons in 2010. Furthermore, according to the OECD and the FAO, this demand is expected to increase another 20% by 2020 reaching 2.6 billion metric tons. The increase in demand is primarily driven by population growth in developing countries and an expanding middle class. Also, according to the OECD and the FAO, global population is projected to increase from 7.3 billion in 2016 to 8.2 billion in 2025, with almost all of this increase occurring in developing countries. Furthermore, the OECD estimates that global middle-class population is expected to grow from 1.8 billion people in 2009 to 3.2 billion people by 2020 and 4.9 billion people by 2030. As household incomes rise, demand for protein-rich diets often increases and this drives additional demand for grains. The trend toward urbanization is also causing a large drop in arable land available per capita. The FAO estimates that ratio of arable land to population has declined by over 50% from 1962 to 2010. As a result of this, according to a report from Statista, the number of people fed per hectare is expected to increase by 100% from 2.3 to 5.6 people fed per hectare from 1960 to 2020.
The chart below sets forth the arable land per person over periods indicated.
Arable Land per Person Over Time

In addition to reducing available land for farming, urbanization leads to a change in dietary tendencies. According to the ISAAA, the shift on the composition of diets towards more meat consumption has led to an increase in demand for feed grains. The transition also increases demand of open land for cattle raising and grazing, making arable land increasingly scarce. The finite availability of arable land has driven the growth in demand of high yielding agriculture products in order to supply the demand while utilizing less hectarage.
143
Due to the location of the remaining arable land worldwide and its uneven distribution, certain regions have been driven to produce a larger proportion part of the required supply. As these trends continue South America will present an interesting opportunity for developing and exporting crops, to meet growing demand. For example, according to the USDA, soybean demand is expected to grow over 4% in 2018, with Asia accounting for almost half of that demand and dependent on exports from other countries. According to the USDA Brazil accounted for over 40% of soybean exports over the last five years.
The U.S. EPA has validated that more extreme temperature and precipitation can prevent crops from growing. Dealing with drought could become a challenge in areas, and although increased irrigation might be possible in some places, in other places water supplies may also be reduced, leaving less water available for irrigation when more is needed. According to the U.S. Global Change Research Program, climate disruptions to agricultural production have increased in the past 40 years and are projected to increase over the next 25 years. By mid-century and beyond, these impacts will be increasingly negative on most crops and livestock.
These trends will continue to drive growth in the global agriculture sector. The USDA reported global demand for grains increased by more than 57% from 1.4 billion metric tons to 2.2 billion metric tons in 2010. Furthermore, according to the OECD and the FAO, this demand is expected to increase by an additional 20% by 2020 reaching 2.6 billion metric tons.
The agricultural sector is of key importance to Argentina. According to the 2017 CIA Factbook, 10.6% of the country’s GDP originates from agriculture and approximately 54% of land has a connection to the agricultural sector (arable land 13.9%; permanent crops 0.4%; permanent pasture 39.6%).
Due to the importance of the sector, the administration led by Mauricio Macri, in office since December 2015, has enacted favorable policies focused on growth for agricultural exports. On December 29, 2015, the government eliminated the export permit system known as the Register of Export Operations, or ROEs, for grains and oilseeds, along with significant reform to export taxes. ROEs were used as export declarations and were allotted based on discretionary government quotas causing restrictions on exports. The removal of these export restrictions and reduction in export taxes are expected to encourage higher production and further innovation. Per the USDA’s October 2017 report, Argentina is the third largest producer and exporter of soybeans and Argentine soybean exports are projected to increase from 7.0 million metric tons in October 2017 to 8.0 million metric tons in October 2018.
The by crop charts below set forth the harvest and production profile in Argentina:
Argentine Harvest and Production Profile
According to the ISAAA, conventional crop technology alone cannot address this immense demand or feed the increase in population. Sustainable approaches using the best of conventional crop technology, such as use of the best adapted germplasms, as well as the best of biotechnology are required in order to increase crop productivity enough to meet the growing demands associated with the increase in population. The last 20 years of commercialization of biotech crops has confirmed that biotech crops have and can deliver substantial agronomic, environmental, health, economic and social
144
benefits. The rapid adoption of biotech crops reflects the multiple substantial benefits realized globally and in the last 20 years, with an accumulated 2 billion hectares of biotech crops grown commercially. Furthermore, in most countries, the adoption rate for biotech crops has reached over 90% for major products in principal markets in both developing and industrial companies.
Sustainable approaches using top of the line conventional crop technology, such as use of the best adapted germplasms, as well as the best of biotechnology is required to meet crop productivity demands. The last 20 years of commercialization of biotech crops has confirmed that these have and can deliver substantial agronomic, environmental, health, economic and social benefits. More than 18 million growers in 26 countries have experienced the benefits of biotech crops, including increasing productivity, conserving biodiversity, ability to be self-sufficient on available arable land, mitigating negative impacts of climate change and overall improvement of their economic situation.
According to the ISAAA, in most countries adoption for biotech crops has reached over 90% for major products in principal markets in both developing and industrial countries. Globally accumulated hectarage of planted biotech crops have reached over 2 billion worldwide over a period of 20 years, with developing countries leading the growth over the last five years, accounting for 54% of the global biotech hectarage growth in 2015 and 2016.
According to the ISAAA, biotech crops were present in only 26 countries as of 2016, presenting a significant long-term growth opportunity in the sector as demand continues to rise. Approximately, 19 of the countries with biotech crops are considered developing markets. In these countries, the estimated yield gaps exceed 50%, presenting a significant opportunity for improvement. Large agricultural companies, as well as smaller independent research firms, have invested billions of dollars to identify and commercialize high-value seed traits to sell to growers. Given the ability of these products to differentiate through yield performance, these markets have demonstrated stronger growth in sales than conventional seed sales. Phillips McDougall, an industry consultant, estimates the market for GM seeds to be US$20 billion in 2016. The ISAAA states that 180 million hectares were planted with GM crops in 2016.
The map below sets forth the global biotechnology crop hectarage in 2016:
Global Biotech Crop Hectarage (2016)
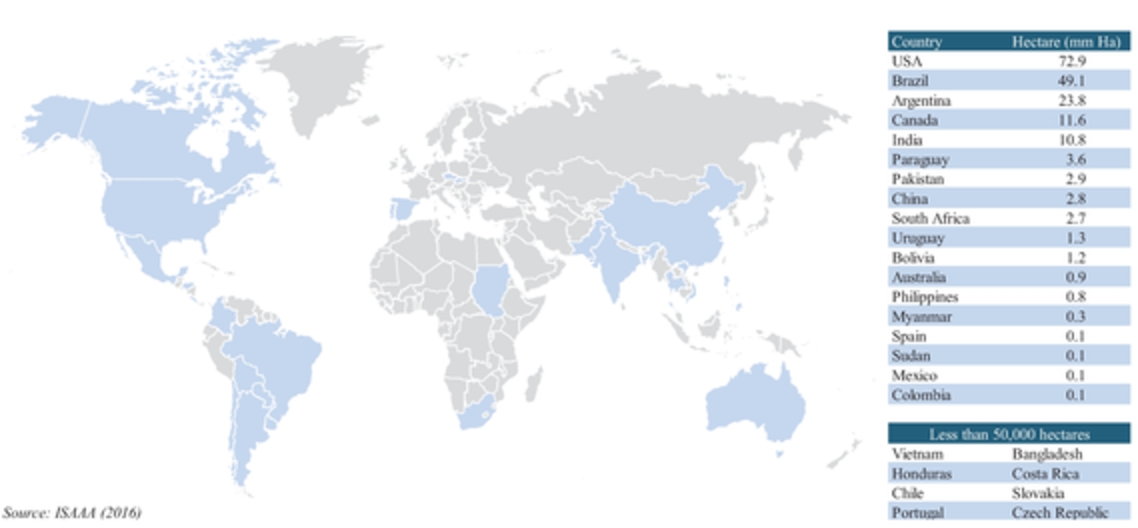
In 2016, according to the ISAAA, corn and soybeans represented a majority of the seed biotechnology market, making up approximately 87% of the global biotech seed market. The United States, Brazil and Argentina were the top planters of biotech seeds with more than 145 million hectares under production of biotech crops. Current adoption of GM varieties is above 90% for soybean, above 80% for corn and above 65% for cotton. In Argentina, approximately 24 million hectares of biotech crops were planted in 2016 with virtually 100% of soybean, 97% of corn and 95% of cotton hectares utilizing biotech varieties. Historically, the Argentine market has been quick to adopt biotechnology as a result of concentrated nature of farm groups as well as comfort with fast commercialization of new GM varieties. In the United
145
States, which is the top producer of biotech seeds, 73 million hectares were planted using biotech crops. The United States has an established history of rapid adoption rates of GM crops, typically reaching 65% to 90% peak penetration in ten years driven by overall yield and productivity improvements of specific seed traits as well on-going consumer education and resulting acceptance.
The chart below sets forth the global adoption rates for different crops in 2016:
Global Adoption Rates (2016)
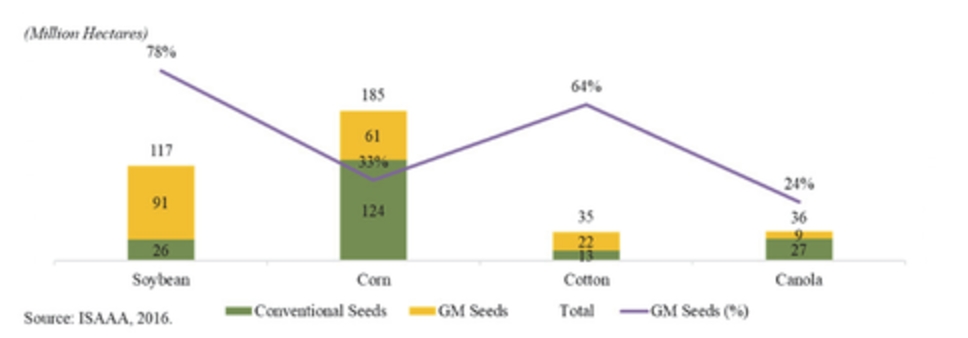
The most attractive trait in biotech seeds for growers is herbicide tolerance, which accounts for approximately 47% of all seeds used. However, demand for stacked seed traits is growing and accounted for 41% of seeds in 2016, up from 33% a year earlier. As more stacked traits seed varieties become available growers will shift towards these to increase profitability. In response to this shift, technology developers are currently focused on stacked traits seeds, which represented 83% of the total 251 of approved events.
The charts below set forth biotechnology crops by trait:
Global Area of Biotech Crops by Trait
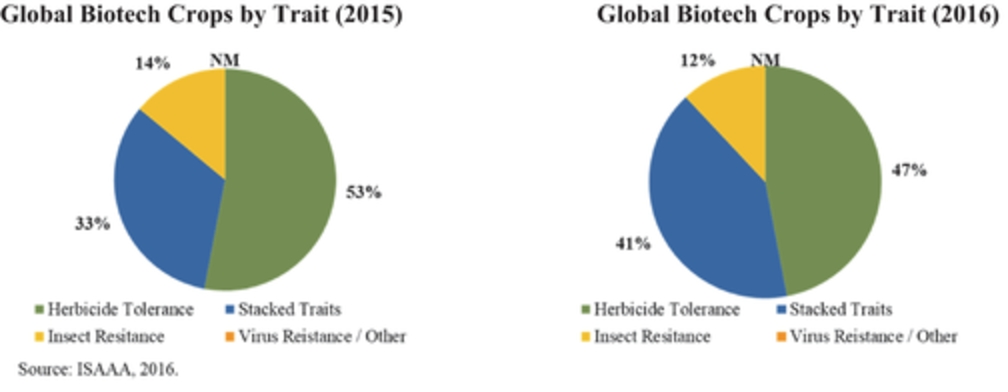
The aggregate economic benefits of biotech crops in the last twenty years account for on incremental US$167.8 billion to growers, according to the ISAAA. Over 50% of these gains were in developing countries and the United States, Argentina and India were the top three beneficiaries.
Based on its economic gain to growers and the rest of the agricultural supply chain, biotech crops have been the fastest adopted agricultural technology over the last twenty years, increasing productivity by an aggregate 574 million tons.
In the United States, the top producer of biotech seeds, 73 million hectares were planted using biotech crops. The United States has an established history of rapid adoption rates of GM crops, typically reaching 65% to 90% peak penetration in ten years driven by overall yield and productivity improvements of specific seed traits as well as on-going consumer education and broader acceptance.
146
Historically, the Argentine market has swiftly adopted biotechnology as a result of concentrated farm groups as well as comfort with the fast commercialization of new GM varieties. Adoption of GM crops in Argentina began in the mid 1990’s with the herbicide-tolerant soybean. The country is in an early adapter and is considered one of the six Founder Biotech Crop Counties alongside countries like the U.S., China and Canada.
As previously mentioned, Argentina is one of the top three countries in terms of global share of planted biotech seed hectares, with approximately 13% of total global planted hectarage. In 2016, approximately 24 million hectares of biotech crops were planted, comprised of 18.7 million hectares of soybean, 4.8 million of corn and 0.4 million of cotton accounting for virtually 100% of soybean, 97% of corn and 95% of cotton hectares utilizing biotech varieties.
The graph below reflects the adoption rates of GM crops in Argentina for the periods indicated:
Adoption Rates of GM Crops in Argentina

In the United States, which is the top producer of biotech seeds, 73 million hectares were planted using biotech crops. The United States has an established history of rapid adoption rates of GM crops, typically reaching 65% to 90% peak penetration in ten years driven by overall yield and productivity improvements of specific seed traits as well on-going consumer education and resulting acceptance.
The graph below reflects the adoption rates of GM crops in the United States for the periods indicated:
Adoption Rates of GM Crops in the U.S.

Due to this early adoption of technology and its leading position in the biotech market, Argentina has developed one of the first and most recognizable regulatory systems for Genetically Engineered (GE) events. CONABIA has been recognized by FAO as “Center of Reference for the Biosafety of GE Events”; since its creation CONABIA has reviewed over 1,500 permit applications. The Ministry of Agriculture remains committed to the technological development and improvements of bureaucratic processes for agricultural biotechnology. During 2012, the system was revamped to reduce approval time for new events to 24 months from 42 months, allowing for continued innovation and reduced bureaucracy in the system.
147
According to the USDA, although Argentina is a major producer and exporter of agricultural biotechnology products, it faces regulatory challenges in providing adequate protection of intellectual property, or IP, rights for agricultural biotechnology. Current regulation provides growers protection from repercussion if a seed is saved or replanted. Newly proposed legislation introduced in October 2016 looks to address company’s seed IP by allowing seed companies to attempt to collect under the Patent Law from non-exempt producers for up to three years after the initial purchase.
The global seed market has grown 85% in ten years up to US$37 billion in 2016 from US$20 billion in 2006 per a 2017 report by Phillips McDougall. Also, GM seeds have grown in prominence, representing only 29% (US$6 billion) of the global market in 2006 up to 55% (US$20 billion) in 2016. This increase of more than 330% in the market size of GM seeds underlines the increasing significance and need for agricultural biotechnology.
The graph below reflects the increase in the global seed market and the penetration of biotechnology crops for the periods indicated:
Global Seed Market & Penetration of Biotech Crops

Source: Phillips McDougall, 2017.
The graph below reflects global biotechnology sales by crop and area of biotechnology crops by country:
Global Biotech Sales by Crop and Area of Biotech Crops by Country
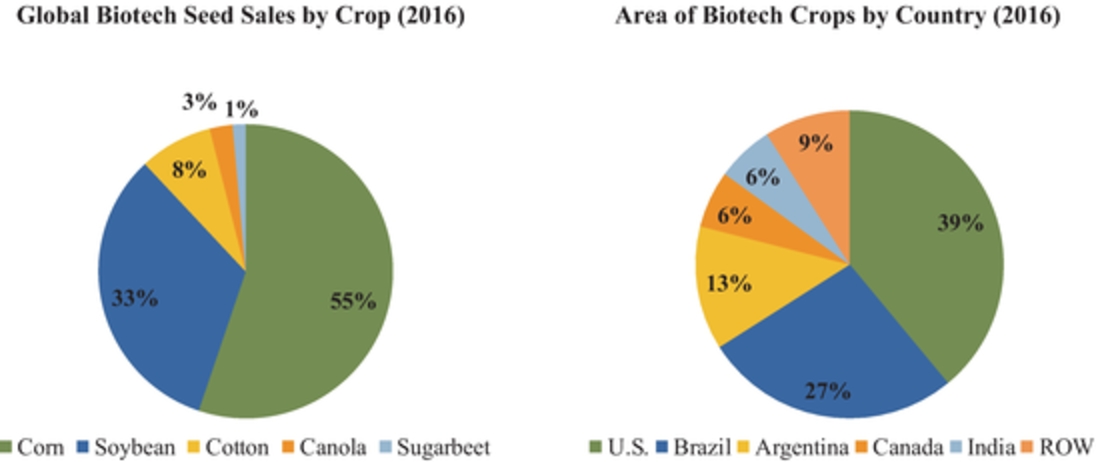
Source: ISAAA, 2016.
148
Syngenta, our joint venture partner and a global leader in crop protection, estimates that the global market for agrochemical products, including crop protection products, doubled from 2000 to 2014, reaching an estimated size of US$63 billion. The Company, based on its research and market sources, also estimates this unprecedented growth to continue driven by introduction of new chemistries, which addressed many unmet agronomic challenges faced by growers, as well as need to address significant losses from abiotic stresses that could potentially be more than US$100 billion.
We believe that agricultural biotechnology and biologicals will continue to grow as the benefits of these technologies and products become more widely known and consumers appreciate the similar efficacy to conventional chemicals while also addressing other issues such as pest resistance and environmental safety.
149
Overview
We are a fully-integrated provider of crop productivity solutions, including seeds, seed traits, seed treatments, biologicals, high-value adjuvants and fertilizers. Unlike most industry participants that specialize in a single technology, chemistry, product, condition or stage of plant development, we have developed a multi-discipline and multi-product platform capable of providing solutions throughout the entire crop cycle, from pre-planting to transportation and storage. Our platform is designed to cost-effectively bring high-value technologies to market through an open-architecture approach. See “—Our Business Model ”. Our headquarters and primary operations are based in Argentina, which is our key end-market as well as one of the largest markets globally for GM crops. We leverage our relationship with our 308 shareholders, many of whom are industry leaders and key participants in our end-markets, to increase adoption of our products and technologies. More recently, we raised capital through financing from Monsanto and BAF, which we believe represents strategic validation of our business model as well as endorsement of our products.
As of September 30, 2017, we owned or licensed approximately 300 registered products and we owned or licensed, either exclusively or non-exclusively, approximately 200 patents and patent applications. In some instances, our licenses are limited in terms of duration, geography and/or field of use. For the three-month period ended September 30, 2017, we distributed over 3.4 million doses of inoculants, 1.8 million liters of adjuvants, 1.2 thousand tons of high value fertilizers as well as other agricultural inputs across Argentina, Brazil, Paraguay, China, India, the United States and Uruguay, among others. For the twelve-month period ended June 30, 2017, we distributed over 12.3 million doses of inoculants, seven million liters of adjuvants, three thousand tons of high value fertilizers as well as other agricultural inputs across 25 countries, including Argentina, Brazil, Paraguay, China, India, the United States and Uruguay, among others. Our pipeline of products includes fertilizers, inoculants, adjuvants, baits, crop protection solutions and seeds. Our pro forma net revenue, net loss and Adjusted EBITDA for the nine-month period ended September 30, 2017 were US$83.3 million, US$14.1 million and US$7.9 million, respectively. Our pro forma net revenue, net loss and Adjusted EBITDA (including that of Rizobacter) for the nine-month period ended September 30, 2016 were US$59.1 million, US$14.8 million and US$5.4 million, respectively. Our pro forma net revenue, net loss and Adjusted EBITDA (including that of Rizobacter) for the year ended December 31, 2016 were US$104.1 million, US$15.0 million and US$15.5 million, respectively. Our net revenue, net loss and Adjusted EBITDA for the year ended December 31, 2016 were US$44.3 million, US$5.2 million and US$6.4 million, respectively. Our net revenue, net loss and Adjusted EBITDA for the three-month period ended September 30, 2017 were US$35.0 million, US$2.9 million and US$5.6 million, respectively. Our pro forma net revenue, net loss and Adjusted EBITDA (including that of Rizobacter) for the three-month period ended September 30, 2016 were US$28.0 million, US$0.8 million and US$6.6 million, respectively. Our net revenue, net loss and Adjusted EBITDA for the Transition Period were US$48.3 million, US$11.2 million and US$2.3 million respectively. Our pro forma net revenue, net loss and Adjusted EBITDA (including that of Rizobacter) for the six-month period ended June 30, 2016 were US$31.1 million, US$14 million and US$1.2 million, respectively. Adjusted EBITDA is a non-IFRS financial measure. Net loss is the most directly comparable measure calculated in accordance with IFRS. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Non-IFRS Financial Measures” and “Risk Factors” for information regarding our use of Adjusted EBITDA and a reconciliation of net loss to Adjusted EBITDA.
Over the past 40 years, we have established a leadership position in sourcing, development, production and sales of biological products for some of the most globally prolific crops, including soy, corn, wheat and alfalfa. We sell our products through a 90-person sales and marketing team and enjoy exceptional access to the end-user grower as a result of: (i) our strategic alliances with global leaders, such as Syngenta, Valent Biosciences, Dow AgroSciences, Don Mario and TMG; (ii) our shareholders, who collectively control significant agricultural land; and (iii) our longstanding relationships with dealers and distributors. Our customers include global blue-chip companies and industry leaders, large distributors, co-ops and dealers, as well as growers.
Our leading infrastructure, the success of our platform and commanding presence in our key markets have made us the effective flagship agricultural solutions provider, as well as the natural partner for global conglomerates, in South America.
Our History
Bioceres was founded in 2001 by a leading group of growers in Argentina to address the demand for higher crop yield and productivity in a sustainable and environmentally conscious way. Since our founding, we have developed one of the
150
leading fully integrated biotechnology platforms of its kind to source, validate, develop and commercialize agricultural technologies and products. We have strategically targeted some of the most globally prolific crops, namely, soy, wheat, alfalfa and corn, in one of the largest geographies for GM plants on a global scale.
In order to bring our products to market in an efficient and cost-effective manner, we have established multiple joint ventures, formed non-joint venture collaborations, as well as created and acquired multiple companies. Our joint ventures include partnerships with important industry participants, such as Florimond Desprez, De Sangosse and Arcadia Biosciences. Some of our non-joint venture collaborations include those with Dow AgroSciences, Momentive, Syngenta and Forage Genetics, among others. Of the companies we have acquired, the most significant was our 2016 acquisition of the controlling stake in Rizobacter S.A., a global leader in biological products and a pioneer in liquid inoculants. We expect to exercise a mandatory call option for 9.99% of Rizobacter upon the successful completion of this offering. Also, subject to the completion of this offering, we expect to acquire additional incremental amounts of Rizobacter. In addition to its market leading position in biological and non-biological products, Rizobacter offers fertilizers, professional seed treatment services, and tolling or formulation services.
The graph below sets forth our history and track record of innovation through joint ventures and acquisitions:

| (1) | Patents include issued and pending patents, both licensed and proprietary. |
| (2) | INMET was formed in 2011 and officially launched in 2013. |
Our Operational and Organizational Structure
Bioceres is headquartered in Rosario, Argentina, where we operate our INDEAR facility. INDEAR houses a state-of-the-art R&D laboratory spanning over 40,000 square feet. Our main manufacturing and distribution facilities are located in Pergamino, Argentina. Our manufacturing facilities include an approximately 1.05 million gallon formulation plant, an approximately 24,000 gallon fermentation plant as well as packaging and logistics operations with over 375,000 square feet of warehouse space. We also recently inaugurated our new 250,000-square foot fertilizer facility and as part of our joint venture with De Sangosse.
We test and conduct trial runs of our key technologies at our main field station located in Pergamino, which also has processing capabilities for foundation seed. We also operate facilities in Cordoba, Argentina as well as international facilities in Brazil and Paraguay and have sales offices or representatives in nine countries. We believe we will continue to grow our dominant position in Argentina and that our leadership position will continue to attract interest in partnerships from global industry leaders seeking to develop and commercialize high-value crop productivity solutions in the large and attractive Argentine and South American markets. As of September 30, 2017, we had 500 full-time employees in the companies in which we have a majority ownership interest.
151
The following graphic provides a simplified chart of our structure:
Our Structure

| (1) | Reflects our minority investment in Chemotecnica. See “Business—Significant Transactions—Chemotecnica Investment.” |
| (2) | Reflects our syndicated ownership of Rizobacter, of which we currently control 50.01% and expect to increase to 60% upon the exercise of a mandatory call option for an additional 9.99% of Rizobacter. |
152
Our Subsidiaries and Partnerships
In order to more quickly and cost-effectively deliver our products to market, we have created or acquired companies or joint ventures that allow us to capture additional value from our targeted technologies in each of the following three stages of our business model: technology sourcing, product development partnering, and production and market access. See “—Our Business Model,” The chart below sets forth the main companies and joint ventures within each of the three stages of our business model.

Technology Sourcing
This pillar is focused on identifying and validating leading scientific research and developing technologies for multiple applications and/or global end-markets. We source and validate promising early stage technologies, which are usually financed through public grants and/or other capital efficient sources, and thereby mitigate the associated high financial risks associated with such early stage discoveries. The following subsidiaries support this pillar:
| ■ | INDEAR represents our R&D services arm and was formed through a strategic alliance with CONICET. INDEAR is organized into seven core technology platforms: plant transformation and gene editing; molecular biology; breeding and testing; regulatory science; genomics and bioinformatics; protein technology and process engineering. These platforms support each phase of technology sourcing and product development pipeline. |
| ■ | INMET was formed to develop and commercialize fermentation solutions based on bacterial metabolic engineering. |
Product Development Partnering
This pillar is focused on collaborating with strategic partners and creating joint ventures to develop validated technologies and bring products, and to bring these to market. By co-funding projects, we further reduce our financial burden and risk from product development activities while also increasing our ability to develop multiple products. The following joint ventures support this pillar:
| ■ | Verdeca, our U.S.-based joint venture, was created to develop and bring soybean varieties with next-generation agricultural technologies to market. |
| ■ | Trigall Genetics, our Uruguay-based joint venture that focuses on developing and commercializing conventional and next-generation biotechnology wheat varieties for the South American market. |
153
| ■ | Semya, an intra-company joint venture with Rizobacter, is dedicated to the EcoSeed initiative and focuses on researching and developing seed treatments as well as agricultural biological input applications for soybean, wheat and alfalfa markets. |
| ■ | S&W Semillas is a joint venture formed to pursue development of alfalfa traits and varieties. |
Production and Market Access
This pillar is focused on leveraging our shareholder base of leading South American growers as well as proprietary sales channels for direct access to end consumers. By establishing multiple pathways to markets, we maximize our market reach and rate of technology adoption. The following subsidiaries support this pillar:
| ■ | Rizobacter, a global leader in soybean biological products and Argentina’s leading provider of bio-based solutions for the agricultural sector with a strong focus on crop nutrition and protection solutions. |
| ■ | Bioceres Semillas, our sales channel for seeds, with a primary crop focus on wheat and soybean. |
| ■ | Synertech, which was formed in partnership with De Sangosse with the goal of producing and commercializing micro-beaded fertilizers. |
| ■ | AGBM, which is our industrial enzymes company working to produce and commercialize chymosin and safflower industrial by-products from modified safflower seeds using our leading molecular farming technology. |
| ■ | Héritas, which was formed in partnership with CIBIC to provide translational medicine services to the regional community, predominantly in Argentina. |
Joint Ventures and Key Collaborators
Some of our main projects are conducted through joint ventures and key collaborations. The form of the collaborations depends on the nature and stage of development of the particular product candidate. We participate in joint ventures to develop certain technologies and maintain a diverse pipeline of products. When a joint venture successfully develops a product, we integrate such product into our commercial offering and license the technology to third-party channels through the joint-venture vehicle. We engage in non-joint venture collaborations to develop a single or otherwise limited product opportunity. We generate revenue from our non-joint venture collaborations primarily by licensing our technology for inclusion in end products, or for the use of our technologies in industrial processes. Finally, we have relationships with third parties who have product development capabilities or market presence outside of our core geographies or crops, to whom we license our technologies. For our corporate chart, see “—Overview—Our Operational and Organizational Structure.”
Joint Ventures and Unconsolidated Entities
Verdeca LLC
In February 2012, we formed a joint venture with Arcadia Biosciences through our U.S.-based subsidiary, Bioceres Inc. The resulting joint venture, in which we have a 50% equity interest, Verdeca, is engaged in the development and deregulation of soybean traits.
Our joint venture agreement provides for each of the joint venture partners to license our trait technologies to Verdeca for use in soybeans. Accordingly, we have agreed to grant an exclusive, worldwide, sublicensable license to Verdeca for our technologies, including HB4, for use in soybeans. The first product in the Verdeca pipeline is our HB4 trait.
In April 2015, Verdeca entered into an agreement with Dow AgroSciences to develop and commercialize innovative traits in soybeans, as well as to advance the ExZact gene editing platform.
Trigall Genetics S.A.
In December 2013, we formed a joint venture in Uruguay with Florimond Desprez through our U.S.-based subsidiary, Bioceres Inc. The resulting joint venture, Trigall Genetics, in which we have a 50% equity interest, is engaged in the development and deregulation of conventional and GM wheat varieties in Latin America. The first products in the Trigall Genetics pipeline are conventional wheat varieties that will be sold through our seed subsidiary, Bioceres Semillas, as well as through other Trigall Genetics licensees. Our first GM product is our HB4 trait, which is now in the advanced deregulation phase of development in Argentina and Uruguay, and will be licensed in Trigall Genetics finished wheat varieties.
154
Synertech Industrias S.A.
Synertech, acquired as part of the Rizobacter acquisition in 2016, was formed by Rizobacter in partnership with De Sangosse for production and commercialization of micro-beaded fertilizers. Rizobacter, together with De Sangosse, operates its own production plant for Synertech in Pergamino with a capacity to produce 50,000 tons of micro-beaded fertilizers annually.
S&W Semillas S.A.
S&W Semillas was formed in 2015 for the development of alfalfa traits and varieties. We selected S&W Seed as the partner in alfalfa traits due to our germplasm assets, our alfalfa seed production capabilities and our international leadership, primarily in non-dormant alfalfas.
Héritas S.A.
Héritas, launched in 2016, was formed in partnership with CIBIC to provide translational medicine services to the regional community, leveraging on INDEAR’s DNA sequencing and bioinformaticscapabilities.
AGBM S.A.
AGBM, launched in 2016, is our industrial company that produces and commercializes chymosin from modified safflower seeds as a molecular farming technology called SPC®. The industrial plant is located in Cordoba and is capable of processing 20,000 tons of safflower grain to produce two million liters of chymosin annually.
Semya S.A.
Semya was formed in 2014 in partnership with Rizobacter to create, research and develop biological products or their metabolites for agricultural and industrial use.
Announced Joint Ventures at Pre-Operational Stage
On January 21, 2016, INMET entered into an agency and services agreement with SenesTech Inc., a developer of proprietary technologies, for managing animal pest populations through fertility control that is based in Flagstaff, Arizona. The initial purpose of the collaboration is to seek Argentine regulatory approval for ContraPest®, SenesTech’s patent-protected technology for managing rodent pest populations through reducing fertility. Once regulatory clearance is obtained, INMET will sell and market ContraPest® in agricultural, residential and public spaces applications. Additionally, INMET will develop an alternative production method for Triptolide, the active ingredient used for the production of ContraPest®, among other uses.
Non-Joint Venture Collaborations
We principally engage in strategic non-joint venture collaborations for product development or with academic entities and internationally recognized research institutions with whom we collaborate in pre-competitive technology sourcing and early-stage research. We pay a percentage of profits to our non-joint venture collaborators according to the stage of development of the technology. In earlier stages, where our R&D costs are relatively low, we pay a comparatively higher percentage of profits, while in later stages of development, where our costs are relatively high, we pay a comparatively lower percentage of profits. Our collaborations that focus on product development are primarily driven by our commercial interest in a particular product that we hope to research and develop on a preliminary basis before including in our pipeline of products. Examples of our most important collaborations of this nature include: Monsanto Company and Forage Genetics International for the deregulation of HarvXtra™ Alfalfa with Roundup Ready® Technology in Argentina; DBN Biotechnology Center of China, for the development of crop protection technologies for soybean and corn; YPF for the development of technologies for the production of second-generation bioethanol; Eagle Seeds & Biotech Ltd. for breeding and field testing of conventional soybean varieties in India; and Sensako Pty Ltd. for field testing of soybean varieties in South Africa.
Rizobacter has a strategic alliance with Syngenta, one of the leading global companies in the research, development, production, marketing and sale of seed treatment products and solutions. Syngenta installed their Seed Care Institute in
155
Rizobacter’s principal facility located in Pergamino, for the research, development, production, marketing and sale of Syngenta’s seed treatment products and solutions in Argentina, including Maxim Integral, Maxim Evolution, Suren Plus, Rizopack® 420 Hc, Ekey Top, Funcion Pack and Cruiser Pack.
Rizobacter also engages in a strategic partnership with Momentive for the distribution and commercialization of Momentive’s well-known silicone spray adjuvant, Silwet, in Argentina, Bolivia, Uruguay, Mexico and Paraguay. Rizobacter additionally partners with Valent Biosciences, a global leader in developing, registering, manufacturing and commercializing biorational products in the areas of public health, forestry, crop protection, plant health, plant growth regulation and post-harvest treatments, focusing on the co-funding and joint research and development of Rizoderma, a biofungicide, and of next generation inoculant technologies. The two companies also appointed each other the exclusive manufacturer and distributor of the inoculant product in the United States and in Argentina and Brazil, respectively.
Our collaborations that focus on technology sourcing are primarily driven by our ability to accelerate, in a capital-efficient manner, the development of promising technology discovered by internationally recognized scientific groups or institutions. Examples of our most important collaborations of this nature include: CONICET and the National University of the Litoral in the creation of seed traits, with a focus on transcription factors for the development of drought-tolerant transgenic plants, including the grant to us of partial ownership of the HB4 patent families; Phytogene Pty Ltd., a wholly-owned subsidiary of Agriculture Victoria Services Pty Ltd., for the development of multiple gene leads in forages and other crops; and the University of Illinois in accessing herbicide tolerance technology for use in the fields of soybeans and alfalfa.
For non-joint venture collaborations, we pay a percentage of profits to our collaborators according to the stage of development of the technology. In earlier stages, when our R&D costs are relatively low, we pay a comparatively higher percentage of profits while in later stages of development, when our costs are relatively high, we pay a comparatively lower percentage of profits.
Third-Party Channels
We license our technologies to third parties with who have product development capabilities or market presence outside of our core geographic area and with whom we have developed and maintained strong relationships. For example, through Verdeca, we entered into a series of agreements with GDM Seeds (Grupo Don Mario) for the licensing of our biotechnology related to soy and have granted various licenses to TMG for our biotechnology related to soy in Brazil, Paraguay and Uruguay.
Significant Transactions
We have recently concluded the following three transactions of significance to our business model and trajectory:
Rizobacter Acquisition
On October 19, 2016, RASA Holding, our subsidiary incorporated in Delaware, acquired 20,004,000 shares of Rizobacter, an Argentine company located in Pergamino, Province of Buenos Aires, representing 50.01% of the outstanding capital stock. The total purchase price was US$57.3 million, of which US$42 million was paid in cash on the date of acquisition and the remainder was agreed to be paid through financing. In addition, a contingent payment of US$17.3 million may be payable to certain of the selling shareholders of Rizobacter subject to precautionary measures and associated ongoing litigation. See “Business — Legal Proceedings — Contingent Fee Payment of US$17.3 Million Related to Precautionary Measure (Medidas Cautelares) in Connection with Rizobacter Acquisition.”
Furthermore, we have a call option to purchase an additional 9.99% of the capital stock of Rizobacter within two years from the date of the acquisition. The call option will become irrevocable upon the acceptance by the holders of the 9.99% interest (i) after two years have passed from the date of the acquisition or (ii) in the event that we or our affiliates purchase a portion or all of the equity interests held by certain shareholders of Rizobacter. In the event that we fail to meet our obligations with respect to the call option agreement, we would be subject to a fee in the amount of US$15 million guaranteed by our subsidiaries, Bioceres Inc. and Indear S.A., payable to the holders of the 9.99%
156
interest. The acquisition of Rizobacter was approved by the Argentine Antitrust Commission (Comisión Nancional de Defensa de la Competencia or CNDC) on August 25, 2017. For more information on the Rizobacter Acquisition see Note 1.3 of our consolidated financial statements.
With 40 years of history, Rizobacter has developed a leading global position in soybean biologicals and a leading ag-input channel for high-value products in Argentina and neighboring countries. Prior to the Rizobacter acquisition, we developed a partnership relationship with Rizobacter through jointly-owned Semya, a product development initiative focused on identifying customized seed treatments for our EcoSeed products. The Rizobacter acquisition has allowed us to combine Rizobacter’s experience in microbials with our pre-existing pipeline of germplasm and trait assets and provides us a unique position on biological assets for key row crops, which represent one of the most difficult sets of assets to develop within the ag-input value chain, resulting in one of the highest-value segments within the sector. Additionally, Rizobacter’s 40-year commercial history in the ag-input market provides a unique platform that facilitates the launch of new products and the continued development of our pipeline of innovations.
Monsanto-Led Investment
In April 2016, we entered into a convertible loan agreement with Monsanto (through Monsanto Argentina, S.R.L.) and BAF, as a financial investor, pursuant to which we issued promissory notes granting Monsanto and BAF participation rights to subscribe to a total nominal value of US$17.55 million of our ordinary shares in a future qualified or non-qualified financing and received US$15 million net proceeds, after applying a 17% subscription discount. The terms of the promissory notes dictate that after a two-year grace period, (i) the notes will begin to accrue interest at an 8% annual rate and (ii) the face amount of the notes will be increased by an additional 10%. The notes mature after the first of a qualified or unqualified financing event, such as this offering, or five years. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations— Indebtedness — Promissory Notes to be Offset through Participation Rights.”
Monsanto is a global leader in seed biotechnology and other crop productivity solutions. We believe that the Monsanto-led investment represents an endorsement of our products and an initial strategic validation of our pipeline and business model.
BAF Loan Facility
In October 2016, our subsidiary, Bioceres Inc., consummated the BAF US$20M Bridge Loan as a bridge financing for our acquisition of Rizobacter. At the same time, we consummated the BAF US$12M Bridge Loan. The BAF US$12M Bridge Loan accrues interest at an annual rate of 8.0% and the BAF US$20M Bridge Loans accrues interest at an annual rate of 8.5%, each payable semi-annually. The BAF Bridge Loans were to mature after twelve months, but we have since extended them for an additional year, through October 2018.
The proceeds of the BAF Bridge Loans were used to fund our acquisition of Rizobacter, through the subscription of preferred shares of RASA Holding, owner of 50.01% of Rizobacter´s capital stock. The loans are secured by share pledges of RASA Holding, with 800,000 of our preferred shares and 400,000 ordinary shares owned by Bioceres Inc. pledged to secure the BAF US$12M Bridge Loan, and with 2,000,000 preferred shares of RASA Holding owned by Bioceres S.A. pledged to secure the BAF US$20M Bridge Loan. The BAF Bridge Loans include standard representations and warranties and certain covenants, including limitations on dividends. The BAF Bridge Loans contemplate certain financial covenants which may limit our ability to incur additional debt, requiring each borrower to maintain a capitalization ratio of at least 0.10x during the life of the loan.
In connection with each of the BAF Bridge Loans, we have executed participation rights agreements pursuant to which, upon the occurrence of a qualified public offering, BAF has the right to subscribe to purchase our ordinary shares at the placement price for an amount equal to the outstanding balance of the respective BAF Bridge Loans, which would result in the extinguishment of all of our obligations (including those of our subsidiary) under the relevant BAF Bridge Loan if exercised.
157
Chemotecnica Investment
On December 22, 2016, we completed the acquisition of 27.99% of the capital stock of Chemotecnica for US$3.1 million, as part of a consortium with SAMSA and Lartirigoyen & Cia S.A., from a group of eight sellers. We acquired 27.99% of Chemotecnica through the transfer of 354,061 preferred shares of RASA Holding to the sellers, and with the remaining purchase price of US$7,039,978 paid by SAMSA and Lartirigoyen & Cia S.A, the sellers acquired 765,987 preferred shares of RASA Holding (of which 389,061 shares were transferred on December 22, 2016, and the remaining 376,926 transferred in May 2017 using proceeds from the sale on terms equal to those of original investors). As a result of the transaction, the sellers own 1,120,048 preferred shares of RASA Holding, representing 23.19% of our outstanding preferred capital stock in this subsidiary.
Chemotecnica is an Argentine pioneer within the ag-chemical space, with an original focus on the synthesis and formulation of insecticides. Today the company produces a number of post-patent insecticides, herbicides and fungicides that complement our proprietary technologies. Chemotecnica operates directly with its long-established brands or by providing synthesis or formulation services to local and international companies operating in Argentina.
Our Business Model
Our business model is driven by three key pillars: technology sourcing, product development partnering and production and market access.
Technology Sourcing
The technology sourcing stage of our business model involves identifying and collaborating with leading academic and independent research institutions at the early stages of technology development. We search for collaborators who are pursuing innovative technological concepts that are consistent with our business strategy and have generated promising preliminary evidence, but have yet to be validated for their intended purposes. In these collaborations, we employ our advanced biotechnology capabilities and specialized know-how to leverage the technology discovery process already undertaken. Because our technology sourcing activities are mainly financed through public grants and other capital efficient sources meant to limit our financial exposure, we have created a team of specialized professionals that help our collaborations apply for and manage grant funding.
Product Development Partnering
The product development partnering stage of our business model involves identifying and collaborating with strategic partners and creating joint ventures to develop and bring products to market. We have created an extensive network of regional and international relationships in the agricultural sector from which we source partners for our product development initiatives. We employ an open-architecture approach to technology origination which involves identifying and accessing promising technologies from third parties, and forming strategic and capital-efficient partnerships that leverage each party’s capabilities to quickly bring innovations to market. By co-funding projects and leveraging the discovery efforts of leading global research institutions and scientists with whom we have developed and maintained strong relationships, we are able to reduce the risks and expenses associated with biotechnology discovery and development and increase our ability to develop multiple products. Upon technology validation, we partner with internationally-recognized entities that can provide co-funding, technology sourcing, intellectual property and market access for the development of our technologies into products. For more detail about the “proof of concept phase”, see “—Research & Development Process.” In selecting a partner, we look for internationally-recognized entities that can provide complementary funding, technology, sourcing, product development capabilities, intellectual property and market access.
Production and Market Access
The production and market access stage of our business model focuses on leveraging shareholder base and proprietary sales channels to access and establish multiple pathways to markets and maximize market reach and develop innovative technology. Once a technology obtains the required regulatory approvals, we, our joint ventures or our technology licensees commercialize products that employ such technology and sell them to end-users in domestic and international markets through shareholder base and proprietary sales channels. We also complement our direct sales efforts by licensing our technologies to other companies for inclusion in their products or production systems. This complementary approach seeks to widen the presence of our technologies in the market and increase our revenues.
158
The Technology Sourcing and Product Development Timeline and Process
The technology sourcing and product development process for our products and technology generally include the following phases: discovery, proof of concept, early development, advances development, pre-launch and product launch.
Below is a description of the relative cost, risk and approximate expected timing for each of the phases of technology sourcing and product development.

The development and integration of technologies into products that can be commercialized is a lengthy process, which varies depending on the complexity of the technology being developed and the type of crop involved. Furthermore, the length of the technology development process impacts the uncertainty of product development. For example, during the technology sourcing and product development process, a technology may fail to address the performance criteria required in order to advance to later development stages or the development of a certain technology may be affected by changes in the competitive landscape.
159
The below chart sets forth an annual estimated timeline for the development of our seed and agro-industrial biotechnology products based on the phases described above:

The estimated timeframes of phase duration are based on our experience and estimates. The phases may overlap during the product development cycle and the total development time for a particular product may be longer or shorter than the duration presented above depending on a range of factors including the type of crop and trait involved and the resources available or devoted to our development. For example, although the process for developing seed traits or biological seed treatments is relatively similar, the two differ significantly in terms of development timelines. Obtaining regulatory approval for GM, seeds is a far more comprehensive and lengthy process than for a biological seed treatment. Similarly, although our breeding programs and agro-industrial biotechnology solutions may have shorter or simpler phases than those described herein, we have used the industry consensus for seed-trait development phases to characterize our technology portfolio, which is generally divided into the following six phases.
Discovery
The first phase in the technology development process is discovery, or the identification of candidate genes or genetic systems, metabolites, or microorganisms, potentially capable of enhancing specified plant characteristics or enabling an agro-industrial biotech solution. For the most part, we rely on collaborators such as leading research institutions or private research groups to perform this early-stage discovery work and we then advance the work through our technological platforms and processes. We use the technology infrastructure of our subsidiaries, INDEAR and INMET, and our scientific teams and know-how to accelerate this process with our third-party collaborators, often through the use of government grants. It is at the discovery phase that we generally negotiate our rights with respect to the intellectual property generated by our third-party collaborators, which can include partial ownership and exclusive licenses for commercial development. The discovery phase typically lasts 18 months, although it may range from as few as six months for microbial solutions to as many as 36 months for plant GM traits.
Proof of Concept (Phase I)
Upon successful validation of the technologies in model systems (in vitro or in vivo), promising technologies graduate from discovery and are advanced to a phase referred to as “proof of concept.” In this phase, the technologies are integrated into, or tested in, target organisms to verify their efficacies using greenhouse trials, field trials, or both. In the case of solutions that require microbial fermentation, these technologies are validated at laboratory scale of between one to five liters of batch production.
160
The goal of the proof of concept phase is to validate a technology within the targeted organism before moving forward with technology escalation activities or extensive field validation, which minimizes risk of investment in technologies that may not prove viable. The proof of concept phase is typically conducted by us as part of our research collaboration with relevant groups and represents the last phase of our technology sourcing collaborations. We generally file for intellectual property protection at this stage. See “—Intellectual Property.” In our experience, the proof of concept phase typically lasts 36 months, although it may range from as few as six months for a microbial solution to as many as five years for plant GM traits.
Early Development (Phase II)
The goal of the early development phase is to identify the best use of a technology and to define our performance concept. Escalation tests are initiated in the early development phase of microbial-based solutions. Similarly, for GM traits, field tests are expanded to evaluate various permutations of a technology in multiple geographies and growing cycles.
At the end of the early development phase and before initiating the most capital-intensive stages of product development, we typically identify strategic partners for further development of our technologies. For collaborations involving multiple technologies within a pipeline, we often create new entities or joint ventures with our strategic partners. Examples of such joint ventures are Verdeca and Trigall Genetics, dedicated to soybean and wheat technologies, respectively. Single or limited product development collaborations are often structured using framework agreements. Examples of this type of collaboration include those we have with Forage Genetics International for HarvXtra™ Alfalfa with Roundup Ready® Technology, and our development efforts with YPF for SPCel and SPCel Max to produce enzymes for second generation biofuels, among others.
Advanced Development (Phase III)
In the advanced development phase, extensive tests are used to demonstrate the effectiveness of the technology for our intended purpose. In the case of GM traits, the process of obtaining regulatory approvals from government authorities is also initiated during this phase, and tests are performed to evaluate the potential environmental impact of modified plants. For solutions involving microbial fermentation, industrial-scale runs are conducted. In Argentina and some other countries in South America, we are primarily responsible for undertaking this phase of product development. Similarly, our strategic partners usually lead the advanced development and regulation activities in other markets in connection with the applicable contractual arrangements.
The advanced development phase typically lasts about 24 months, with some projects requiring substantial regulatory data taking as many as three to five years. For our molecular farming projects, where grain production will occur within Argentina, we may follow a simplified regulatory approach due to a special set of requirements, which requires less time than traditional GM regulatory approvals.
Pre-Launch (Phase IV)
The pre-launch phase involves finalizing the regulatory approval process and preparing for the launch and commercialization of the technology being developed. The range of activities in this phase includes, pre-commercial production, seed increases and product and solution testing with selected customers. Usually, a more detailed marketing strategy and preparation of marketing materials occur during this phase. In Argentina and some other countries, we are responsible for this phase of product development, while pre-launch activities in other markets are primarily undertaken by our strategic partners. The pre-launch phase may last up to 24 months.
Product Launch
In general, we, our joint ventures and/or our technology licensees carry out the launch and commercialization of the technology, which is the last phase of the technology sourcing and product development process. When we commercialize technology through collaboration partners or licensees, pursuant to the respective agreements, a successful product launch triggers royalty payments, which are generally calculated as a percentage of the net sales generated by the technology and captured upon commercialization. Typically, in this phase, revenues at launch are limited by our ability to make products available, especially when dealing with seed products that need multiple seasons of multiplication before they can satisfy demand.
161
Our Segments and Key Products
We divide our business into the following four principal segments: crop protection, seed and integrated products, crop nutrition and emerging solutions.
Crop Protection
Our key crop protection products include adjuvants as well as seed-applied insecticides and fungicides.
Adjuvants
Adjuvants are used in tank mixes to facilitate application and effectiveness of crop protection products. We produce the market leading silicone-based adjuvant, Silwet, and are currently developing microbially-enhanced adjuvants in partnership with other companies.
Insecticides and Fungicides
We offer a full range of chemical seed treatments tailored for specific crop and pest combinations. We are in the process of formulating and commercializing stand-alone chemical seed treatments, including fungicides and insecticides, in partnership with Syngenta, to reduce disease and pest pressure during crop establishment. Also, in partnership with De Sangosse, we offer a range of pest baits to effectively and safely control pests that are particularly harmful for harvested and stored grains or seeds. We hold a leading market position for such products, with an estimated 50% current market share. Furthermore, we are pursuing commercialization of biological fungicides formulated as seed treatments that control and restrict the growth of pathogenic agents in wheat and barley, as well as developing a microbial-based insecticide.
Seed and Integrated Products
The key products of this segment include seed traits, germplasms and seed treatments for healthier and higher yielding crops.
Seed Traits
Our seed trait effort is primarily focused on improving plant yields by increasing plant tolerance to abiotic stress, such as drought or salinity. We also have a secondary focus on crop protection and quality traits. We gain access to these technologies by collaborating with the original developers of the technologies or by co-developing new events with our partners. Our most advanced technology in the seed trait area, HB4 helps increase yield by an average of 13% to 19% for multiple crops under various growing seasons and conditions, including sporadic drought episodes. HB4 is also able to provide higher yield without adversely impacting yields in optimal growing conditions, which is a distinctive and important factor compared to other stress tolerance technologies. HB4 has been approved for use in soybean in Argentina and by the U.S. FDA. Submissions for approval for use of HB4 in wheat have been initiated in Argentina, Brazil, Uruguay and Paraguay.
We also have a secondary focus on crop protection and quality traits. We gain access to these technologies by collaborating with the original developers of the technologies or by co-developing new events with our partners.
Germplasms
We currently breed germplasms for soybean and wheat and we plan to advance our elite germplasms by delivering these technologies using proprietary channels. Our soybean breeding program produces varieties that are registered or are in the process of being registered in Argentina, Uruguay, Paraguay, and South Africa. Our wheat breeding program is operated through our joint venture, Trigall Genetics, which has exclusive rights to the breeding program of Florimond Desprez. In addition, we hold exclusive rights to all wheat varieties developed between 2003 and 2013 by the Argentine national breeding program at the INTA.
Seed Treatments
Seed treatments comprise one of our core products and include Rizopacks, produced by our subsidiary Rizobacter in partnership with Syngenta Seedcare, which are the flagship soybean product with proprietary inoculants and fungicides.
162
We also offer certain variations customized for peanut, beans and chickpea. In addition, we are pursuing the development of next-generation biologicals, particularly for seed treatments tailored for specific germplasms, seed traits and environment combinations.
Crop Nutrition
Our main crop nutrition products include inoculants, biofertilizers and chemical-based fertilizers.
Inoculants
Inoculants are broadly used nitrogen-fixing bacteria that promote growth of leguminous crops such as soybean and alfalfa. We hold a global leadership position in sales of soybean inoculants, with approximately 21% market share per our internal projections. We are currently developing the next-generation of inoculants, including Bioinductor 2.0 and Extended-Shelf-Life products for professional seed treatment businesses. Additionally, we are developing new biofertilizers, such as plant-growth promoting rizobacteria, for wheat, corn, chickpea and pea.
Biofertilizers
Biofertilizers contain living microorganisms that colonize the interior of a plant and promote growth by increasing supply or availability of primary nutrients through the natural processes of nitrogen fixation, solubilizing phosphorus and stimulating plant growth through synthesis of growth-promoting substances. The combination of biologicals and chemical fertilizers can maximize crop yields while reducing environmental impact as a result of reduced use of chemicals. We are also in early stages of development for microbially-enhanced fertilizers for soybean, wheat, corn and chickpeas.
Chemical-Based Micro-Granulated Fertilizers
We produce and commercialize fertilizers based on chemically formulated microgranules. As these fertilizers can be applied next to the seed at planting, lower doses are needed than standard fertilizers, resulting in logistical efficiency and environmental benefits. Currently, our production is focused on Microstar PZ, a starter fertilizer that provides nitrogen, phosphorus, sulfur and zinc to different crops.
Emerging Solutions
Our emerging solutions segment provides high value R&D, technical and advisory services to strategic partners and third parties. We also commercialize specialized products for a variety of end markets, including enzymes, such as chymosin, microbial fermentation solutions and translational medicine services.
R&D Services
Our R&D services provide advanced biotechnology capabilities and specialized knowledge and expertise to facilitate validated technology sourcing and product development efforts from our industry collaborations and, to a lesser extent, makes these platforms available to third parties. We also enter early-stage research collaboration arrangements with external research groups, most of which are funded through government grants. Our R&D services are provided through our two subsidiaries: INDEAR, which provides R&D services across a broad range of platforms, and INMET, which focuses on fermentation solutions using synthetic biology and metabolic engineering for application in agro-industrial solutions.
Agro-Industrial Biotechnology
We have developed a safflower-based molecular farming platform for industrial enzymes that, which allows us to use harvested grains as an efficient method of storing enzymes prior to processing. Our most advanced molecular farming technology is SPC®, which is used to produce bovine chymosin, an enzyme used in the manufacturing of cheese. We are also developing solutions for producing biopolymers, such as PHA/PHB, biodiesel and ectoine from soybean glycerin based on bacillus fermentation, which we also use for cost-effective production of biological pest control agents.
163
The following graphic sets forth the key products, growth drivers, revenue and gross profit, key markets and selected commercial partners for each of our segments:

Early Stage Technology Development Agreements
Between 2005 and 2007, we entered into agreements with various investors in order to obtain funding in the aggregate amount of US$1.0 million for research related to early stage technology for the development of technology relating to a specific gene from sunflower intended to promote drought tolerance in crops, which was refer to as Hahb 4. The agreements grant the investors in the aggregate the right to receive 52.8% of the rights and royalties payable to Bioceres S.A. from the successful commercialization of the resulting technology with respect to soybean, wheat and corn.
See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Tabular Disclosure of Contractual Obligations” and “Certain Relationships and Related Party Transactions—Early Stage Technology Development Agreements.”
164
Our Competitive Strengths
Our diversified platform generates revenues through multiple technologies, customers, distribution channels and end-markets, providing us with a profitable growth trajectory. Our key competitive strengths include:
Premier Agricultural Solutions Provider with the Flagship Position in Latin America.
As the first non-governmental Latin America-based entity with a GMO event approved in a major global crop, we consider ourselves to be the pioneer in the agricultural biotechnology industry in Latin America. Our experience over the past 40 years has allowed us to become and maintain our position not only as a reference entity for governmental agencies and policy-makers, but also as a leading choice for partnerships with global conglomerates. We have helped define regulations for gene editing and new breeding technologies as well as formulate intellectual property guidelines and legislation for our industry. We are a founding member of the Argentine Chamber of Biotechnology and one of a handful of selected companies collaborating with the Argentine Ministry of Science, Technology and Productive Innovation in the design of research grants aimed at our sector. We are a frequent and leading participant in all major forums dedicated to our industry and a prominent representative of our sector.
Proven Platform with a Successful Track-Record in Sourcing, Developing and Commercializing Key Biotechnologies.
Over the last 40 years, we and our subsidiaries have created our proprietary platform for sourcing, validating, developing and bringing key technologies and products to commercialization.
We manage our sourcing efforts through our two key business divisions: INDEAR, which focuses on our technology and product development efforts and third party initiatives, and INMET, which focuses on metabolic engineering solutions for the conversion of agricultural feedstock into higher value molecules.
We source our technologies and products through various partnerships, collaborations and long standing relationships with research institutions and scientists. We are the strategic partner of various institutions including CONICET for the development of multiple GM trait leads, Danziger Innovations for the development of modified gene lines in soybeans as well as quality and protection traits and the University of Illinois for the development of herbicide tolerance technology for alfalfa and soybeans, among others. We have also entered into various collaborative product development agreements including with: (a) Forage Genetics for enhanced alfalfa with herbicide resistance technology; (b) Dow AgroSciences for the development of new seed traits in soybeans; (c) Momentive for adjuvants; (d) Syngenta for new seed treatments; and (e) Valent BioSciences for the microbials in the United States, among others.
We manage our product development via various joint ventures and partnerships with leading participants in the global agriculture sector. We focus our efforts on developing products and technologies that address the specific requirements and demands of our global customer base and for some of the highest demanded crops, such as soy, wheat and alfalfa, among others.
We operate a commercialization platform for agricultural biotechnology products in South America as well as other select global agricultural markets. We have access not only to the largest distributors, co-ops and dealers, but also to end customers through our well-established subsidiaries, divisions and partnerships. By selling our proven genetics, seed and seed treatments on a branded basis, we believe we are able to further strengthen our brand and grow our position in Latin America.
Capital-Efficient, Risk Mitigated Development Model.
Development and regulatory approval for our products and technologies requires a highly evolved and complicated process that can last between 12 to 14 years. Furthermore, capital allocation requirements can be onerous due to the expensive discovery activities usually associated with life sciences research and the strict requirements for regulatory approval that are imposed on GM crops and technologies.
We believe that we have created a highly-competitive independent platform for developing such products and technologies in South America. We consider ourselves to be the go-to partner for advanced validation of promising research leads developed by local research institutions, most of which do not have the necessary capabilities for this purpose. As advanced validation initiatives are funded often by existing government programs, we are able to reduce our capital exposure at this high-risk stage of the R&D process.
165
Upon technology validation, we enter into joint ventures, partnerships and collaborative agreements with industry participants that agree on the merits of a new technology and pursue the business opportunity jointly with us. Partnering with others in this stage of the R&D process allows us to reduce our capital exposure while retaining a controlling interest in the product or technology under development.
We enjoy a competitive advantage in commercializing our products as we are able to leverage our strong industry relationships to bring our products to market faster than our competitors. We also facilitate the use of our technologies through licensing agreements and partnerships with global industry leaders, particularly in new markets with expanded regulatory requirements.
Patented and Well-Established High Impact Technologies and Products as well as a Robust Pipeline of New Products and Technologies at or Close to Commercialization Phase.
Our patent and trademark portfolio for plant-related biologicals is amongst the most competitive in South America. As of the date of this prospectus, we have identified and sought patent protection in our capacity as either title holder or licensee, either as exclusive or non-exclusive licensee, to approximately 200 patents or patent applications. In some instances, our licenses are limited in terms of duration, geography and/or field of use. We usually seek patent protection in the largest global markets for our products and technologies, including, the United States, Brazil, Argentina, China, India, Mexico, Australia and certain other European and South American countries.
We have registrations in Argentina for 27 wheat, 18 soybean, 5 alfalfa, 4 corn and 2 safflower varieties and are also seeking registration for an additional 13 soybean, 2 wheat, 2 amaranth and 17 alfalfa varieties. Our portfolio also includes 42 trademarks and 4 trademark applications in Argentina, Brazil, the United States, and Uruguay for Bioceres, while our subsidiary Rizobacter has more than 355 trademarks and applications in Argentina and 300 trademarks and applications globally.
We also have a robust and innovative portfolio of products and technologies for all stages of crop development across various chemistries, many of which are at or close to the commercialization phase, such as EcoSoy, EcoWheat and HarvXtra™ Alfalfa products.
Unique Ownership by Key Industry Influencers Leading to Early and Broad Adoption of Technologies and Products.
Our current ownership structure is composed of 308 shareholders, including some of the largest farm operators, processors, distributors and commercial participants in the South American agricultural sector. Our shareholder structure also includes founding members of AAPRESID and leading members of AACREA. These unique relationships not only allow us to quickly bring our products to market and integrate our technologies into the broad market by creating a proprietary distribution and commercialization channel, but also provides us with a much desired early stage testing platform which allows us to receive direct market feedback early on in the testing process to vet and facilitate faster market penetration.
Highly Accomplished Management Team with a Unique Blend of Technical and Commercialization Experience as well as the Ability to Identify and Integrate Key Acquisitions.
We believe we have a strong management team with a unique blend of executive, managerial, technical, commercialization and acquisition experience. We are able to leverage the experience of our management team not only to efficiently source and develop our technologies and products, but also to leverage their vast experience in commercial production, distribution, navigation of intellectual property requirements and inorganic acquisitions to strategically grow our company.
Our Growth Strategy
Our long-term growth strategy is based on an open-architecture approach to technology origination, identifying and accessing promising technologies from third parties, as well as forming strategic and capital-efficient partnerships that leverage each party’s strategic strengths and capabilities to more quickly bring innovations to market. Our near-term growth strategy includes the following:
Continue to Lead Development and Commercialization of New Agricultural Biotechnology Products in Existing and New Markets.
We intend to build upon our diverse portfolio of crop productivity solutions by consolidating our position on biological assets, including microbial, seed traits and germplasm assets, and continuing to pursue an integrated approach in the development of superior yielding products. We intend to expand upon our direct reach to customers by offering additional
166
high demand technologies, including digital farming solutions and direct-to-consumer retail, which we believe will facilitate the adoption and subsequent sales of our products as well as achieve efficiencies creating additional value opportunities.
Scale-up Production of Rizobacter Products to Accelerate Penetration in Local and Regional Crop Nutrition Markets.
We have invested significant capital in future developments of specialty fertilizers and have recently completed the construction of our micro-beaded fertilizer facility in Pergamino, Argentina. The facility began operations in January 2017 and is expected to supply high-demand specialty fertilizers in Argentina and neighboring countries. Micro-beaded fertilizers place non-toxic formulations of macro- and micro-nutrients next to the planted seed allowing growers to significantly reduce application rates by as much as 75% to 80% on a weight basis and resulting in significant logistical as well as operational savings. Through our recent acquisition of Rizobacter, we also own Microstar, the leading brand in micro-granulated fertilizers.
Commercial Launch of Seed Traits and EcoSeed Products to Drive Penetration in Local and Regional Integrated Seed Market.
Given the near-term commercialization opportunity that HB4 and other seed technologies represent, we plan to integrate these solutions into customized seed products that represent a superior value proposition in the current market, with an initial focus on Latin America. EcoWheat and EcoSoy seeds integrate the uniqueness of HB4 stress tolerance into locally-adapted germplasms, customized with a seed treatment solution prescribed for specific environments. We believe that the product differentiation provided by our unique and varied technologies increase the value of our products for EcoSeed customers and will drive significant growth in this segment of our business. In the medium-term, we expect royalties from HB4 licensees to represent a significant component of our revenues as this landmark technology is more broadly adopted through strategic partnerships and third-party channels. We also expect to obtain regulatory approval for the commercialization of the HarvXtra™ Alfalfa with Roundup Ready® technology developed by Forage Genetics International.
Expand our International Business by Accelerating Registration and Sales of Products Through Multiple Subsidiaries.
We consider ourselves to be a global leader in the soybean biological market and have used this position to establish subsidiaries in Brazil, Paraguay, Bolivia, Uruguay, the United States, South Africa, and more recently, India, Colombia and France. We believe we can use our international footprint and sales force to continue to define our key brands by bringing our broader portfolio of crop productivity solutions to these markets. We expect international growth to be driven initially by continued growth in our historical biological business, as well as by incorporating high-value adjuvants and crop nutrition solutions in the future. In the medium-term, we expect to leverage our leading distribution network to bring our integrated seed products and other crop protection and nutrition solutions to all of our current and target markets.
Pursue Strategic Collaborations and Acquisitions in Key Markets.
We intend to continue working with our collaboration partners to bring our products to customers in key markets. We also plan to continue pursuing acquisitions and in-licensing opportunities to gain access to validated and important later stage products and technologies that we believe to be a strategic fit for our business.
Further Develop Emerging Agro-Industrial Biotechnology Solutions.
Through our investment in AGBM, we plan to scale-up our molecular farming business, by initially producing and commercializing chymosin, an enzyme used in cheese manufacturing. We intend to accelerate the cost-efficient development of commercial technologies that rely on metabolic engineering for bio-conversions of soy glycerin to produce biofuels, bio-chemicals and bioplastics as well as technologies for the production of new industrial enzymes through our safflower molecular farming platform. We believe that the ability to combine our capabilities in genomic and bio-informatics, synthetic biology, metabolic engineering and fermentation as well as unique relationships with some of the largest participants in the agricultural sector allows commercialization of technologies more quickly and efficiently and with lower risk than our competitors.
Sales and Marketing
Our business model is based on a multi-channel sales structure of (1) direct sales to distributors and end-users via our proprietary sales channels, and (2) non-exclusive licensing of commercial technology to third parties directly or via our joint ventures for incorporation into non-proprietary products.
167
Rizobacter
Rizobacter commercializes crop nutrition and crop protection products. With more than 750 distributors across Argentina, Rizobacter is positioned as the local leader for certain products, such as soybean inoculants, seed treatments, adjuvants, and pest baits where it accounts for 26%, 27%, 27% and 50% of the local market, respectively. Additionally, Rizobacter has more than 20 international distributors, increasing our market reach to Brazil, Paraguay and Uruguay.
Sales through the local channel account for 70% of our total sales whereas sales through the international channel account for 15% of our total sales. In addition to the distribution network sales, Rizobacter directly caters to other businesses, particularly large end-users. Large end-users include growers who have operations of over 10,000 hectares or seed companies that use Rizobacter products in professional seed treatments or for other needs. Sales made directly to companies account for 10% of our total sales and sales made directly to large end-users account for 15% of our total sales.
Our distribution network is composed of four main warehouses located in Pergamino, Necochea, Paraná and Rio IV. The map below sets forth Rizobacter’s distribution network in Argentina.
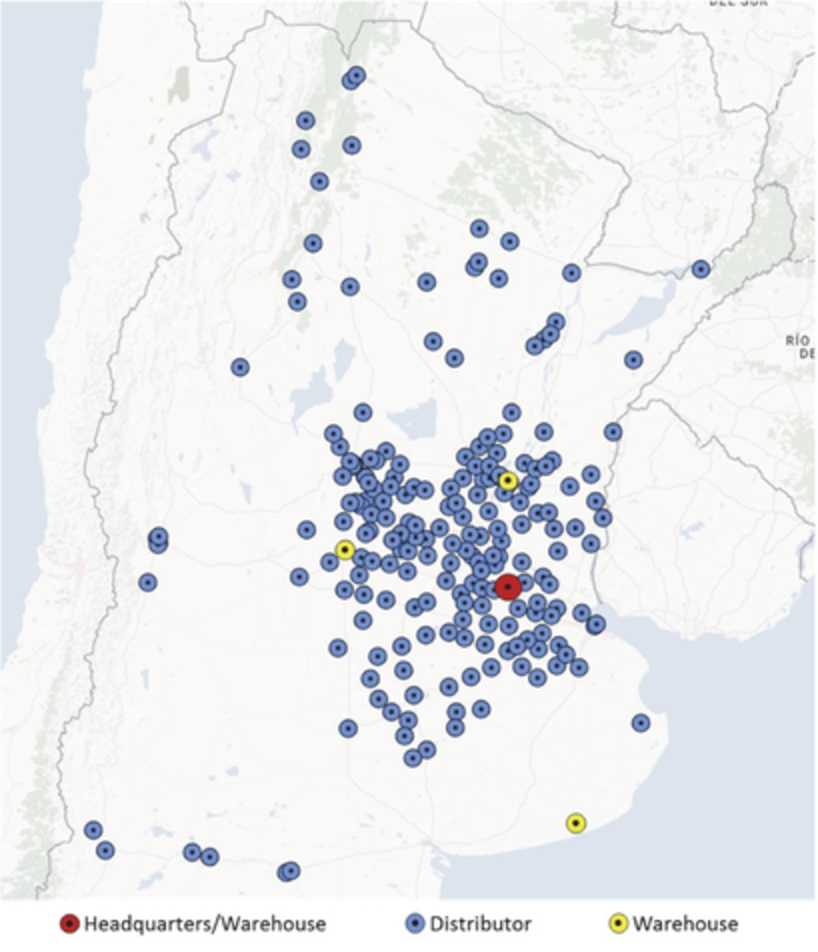
Bioceres Semillas
Bioceres Semillas is our proprietary commercial channel for seeds and integrated seed products, selling to a number of distributors and end-users under the Bioceres Semillas brand. As of 2017, Bioceres Semillas has been operated by Rizobacter, our recently acquired subsidiary. This proprietary channel also serves as a competitive driver for third-party non-exclusive licensees of our technology to seek a rapid path to market.
168
Synertech Industrias
Synertech is exclusively focused on the commercialization of micro-beaded fertilizers within the crop productivity segment to major regional distributors, including the distribution networks of both partners (Rizobacter and De Sangosse), taking advantage of both partners’ extensive distribution capabilities. Synertech targets different geographies, at a global level, for product commercialization, including but not limited to major Latin American markets.
AGBM
AGBM is an industrial company that produces and commercializes chymosin from modified safflower seeds. Production at full capacity can fulfil 10% of the global demand for chymosin at current consumption levels. AGBM has secured market access through Ditta Calza Clemente, a company based in Italy focusing on ingredients and additives for the dairy industry.
Héritas
Héritas provides translational medicine services in Argentina. Its market access is sustained and supported by the extensive customer portfolio managed by CIBIC, our partner in the project.
Third-Party Channels
We also rely on third-party channels for the commercialization of our proprietary technology and licenses, either directly or through our joint venture companies, to participants in the biotech seed and agro-industrial market. We license such technology to our joint venture partners and large companies active in the biotechnology and agro-industrial space, in each case for incorporation into non-proprietary products and subsequent sale to end-customers. Subsequent sales of any products incorporating our technology generate royalty income.
Third-party channels for commercialization and development of new soybean varieties in Argentina and Brazil include TMG and GDM seeds.
Customers and Contracts
Below is a description of our principal customers and contracts across our main segments.
Seed and Integrated Products
In the three-month period ended September 30, 2017, we sold our seed and integrated products to customers in Argentina and Uruguay. Our top five customers in our seed and integrated products segment represented 44% of our total revenue in this segment for the three-month period ended September 30, 2017. Sales to our grower-shareholders accounted for 3% of our total revenue in our seed and integrated products segment for the three-month period ended September 30, 2017. We currently generate revenues in our seed and integrated products segment through the sale of seed treatments, seed packs and seeds products to distributors and end-users. In the future, we intend to sell our seed and integrated products directly to our customers or to third parties as licensors of seed traits, germplasm and/or seed treatments for incorporation into non-proprietary products. Licensing arrangements for use of our HB4 technology provide for pre-commercial payments including, depending on the particular agreement, a technology access fee, milestone fees, and/or annual fees, as well as future commercial royalties based on the income generated by the technology. We expect that the commercialization of EcoSeed products containing our HB4 technology combined with the expected licensing arrangements for use of our HB4 technology in non-proprietary products will expand our seed and integrated products segment.
Crop Protection
In the three-month period ended September 30, 2017, sales from our crop protection segment were made to customers in Argentina, South Africa, Brazil, Paraguay, Bolivia and Uruguay. Our top five customers in our crop protection segment represented 24% of our total revenue in this segment for the three-month period ended September 30, 2017. Sales to our grower-shareholders accounted for 1% of our revenue in our crop protection segment for the three-month period ended September 30, 2017.
169
Crop Nutrition
In the three-month period ended September 30, 2017, sales from our crop nutrition segment were made in Argentina, Brazil, Uruguay, Paraguay and other international markets. Our top five customers in our crop nutrition segment represented 34% of our total revenue in this segment for the three-month period ended September 30, 2017. Sales to our grower-shareholders accounted for 0.1% of our revenue in our crop nutrition segment for the three-month period ended September 30, 2017.
Emerging Solutions
During the three-month period ended September 30, 2017, we sold R&D and technical services to a relatively limited number of clients primarily in Argentina, the United States, Uruguay, China and Canada, and received government grants in Argentina. Our top five emerging solutions customers represented 77% of our total emerging solutions segment revenue for the three-month period ended September 30, 2017. The provision of services to the joint ventures in which we participate accounted for 48% of our emerging solutions segment revenue (including government grants), while services invoiced to third parties represented 38% and government grants represented 14% for the three-month period ended September 30, 2017.
Competition
The market for agricultural biotechnology products is characterized by intense commercial and technological change and we face significant direct and indirect competition in each of our business segments. The crop productivity sector is highly competitive and includes large companies, such as Monsanto Company, Bayer, DuPont Pioneer, Dow Agrosciences, Novozymes, and Syngenta AG, as well as other smaller companies. In order to provide customers with the most advanced products, companies in the crop productivity sector must continuously invest substantial resources in the development of seeds, seed traits and agronomic methods and products. Large companies that have access to a broad range of germplasm as a platform for trait commercialization have a key competitive advantage in this sector. Despite Rizobacter’s leading position in the local microbial sector, it is a highly competitive market. In 2011, Novozymes acquisition of Nitragin positioned it as the second largest player in the sector, representing a 15% market share according to internal projections. Similarly, Bayer’s acquisition of Biagro in 2014 positioned it as the fourth largest player in the sector, representing a 7% market share according to internal projections. Other important competitors include Nova, Barenbrug and Becker Underwood, each of which represents a 11% or less market share according to internal projections.
Competition in the seed and integrated products sector extends to each of the components that we provide for our integrated products. We face competition for our seed traits from other companies, such as Evogene Ltd., that engage in R&D and license technology to customers. We also face competition for our proprietary germplasm from other seed producers who produce their own germplasm, such as Don Mario.
Our principal competitors in our R&D services are primarily other institutions that have sophisticated biotech services centers and present attractive opportunities to our third-party collaborators, joint venture partners and clients, however, due to the high costs associated with developing R&D services, we currently do not have any significant competitors in Argentina. On an international level, our competitors include research institutions and governmental entities that provide R&D services through biotechnology centers. The agro-industrial sector is also highly competitive and includes well-established and emerging companies in the industrial enzymes and metabolic engineering sectors, many of which have substantially greater resources than we do.
We believe that the key competitive drivers in the crop productivity industry are proven performance, customer support and value of the product, which encompasses the price of the seed as well as pre- and post-sale support to secure overall customer satisfaction. We believe that our long track record as a technology provider as established through the Rizobacter group of brands, our strong personal relationships with growers in Argentina, and our reputation for using advanced science and technology in the development of innovative products provide us with a strategic advantage in the markets in which we participate. As a result of the integrated and specialized products and services we offer, our principal competitors are, in many instances, some of our third-party collaborators, joint venture partners and clients. We believe that in order to stay competitive and maintain our leadership position we must continue to offer access to market-ready platforms to our third-party collaborators, joint venture partners and clients in order to achieve their R&D goals.
170
Intellectual Property
Our success depends in large part on our ability to obtain and maintain intellectual property and proprietary protection of our products and technology related to our business, defend and enforce our intellectual property rights (in particular our patent rights, plant variety, trademarks and trade secrets), preserve the confidentiality of our intellectual property and operate without infringing valid and enforceable intellectual property rights of others.
We seek to protect our proprietary products, technology and trade secrets, in part, by entering into confidential disclosure agreements with our employees, consultants and potential and actual third-party collaborators. By protecting our proprietary technologies we are able to offer our customers and partners unique products that they cannot obtain from our competitors and to limit our competitors from using technologies that we have developed or exclusively licensed from other parties.
Individual patent terms extend for varying periods of time, depending upon the date of filing of the patent application, the date of patent issuance, and the legal term of patents in the countries in which they are obtained. Our patents for the HB4 family expire in 2033, for the Coxc5-1 family in 2025 and for the HB10 family in 2026. Our patents for SPC®, the chymosin production in safflower seeds, expire in 2020. We are the exclusive licensees and non-exclusive licensees of the following patents: rGRF3 technology patents which expire in 2033; NUE technology patents which expire in 2030; WUE technology patents which expire in 2026; TREF technology patents which expire in 2028; herbicide resistant patents which expire in 2028; LXR®, a delayed senescence technology, which expires in 2026; HarvXtra™ Alfalfa with Roundup Ready® Technology, a reduced lignin technology, which expires in 2032; Intacta RR2 PRO technology which expires in 2032; and ZFP® technology which expires in 2034.
In 2003, we entered into an Hahb 4 research and development agreement with CONICET and the National University of the Litoral under which they became equal owners of the patents derived from their research activity regarding the Hahb 4 gene, which we refer to as the 2003 Hahb 4 patents. Under this agreement, that was subsequently amended in 2010, we financed the Hahb 4 research activities performed by CONICET and the National University of the Litoral in exchange for an exclusive license to commercially exploit their rights in the 2003 Hahb 4 patents. Other than the commercialization of a promoter element used in connection with our HB4 modified gene trait, we do not expect to commercialize the Hahb 4 technology or 2003 Hahb 4 patents. The Hahb 4 promoter element can be used to initiate expression of the modified HB4 gene.
In 2012, we entered into a separate agreement with CONICET and the National University of the Litoral for the ownership and licensing rights of additional patents for development of our modified HB4 gene trait, which we refer to as the 2012 HB4 patents. Pursuant to this agreement, we own 40% of the 2012 HB4 patents, and CONICET and the National University of the Litoral each own 30%. In addition, CONICET and the National University of the Litoral granted us an exclusive license to commercially exploit their respective rights in the 2012 HB4 patents. The license related to additional patents under the 2012 agreement will remain in force until the expiration of the last 2012 HB4 patent in 2033, unless terminated earlier in accordance with the terms of the agreement.
Our patents or patent applications generally relate to compositions of matter for DNA and protein sequences, plants, plant parts and enzymes, and methods of improving plants and bacteria. We continue to file new patent applications and the main countries in which we seek patent protection are in the United States, Brazil, Argentina, Australia, India, China, Mexico and certain other countries in South America and Europe.
As of the date of this prospectus, we, in our capacity as either title holder or licensee (either as exclusive or non-exclusive licensee), have approximately 200 patents and patent applications concerning technologies such as improved yield, drought tolerance, increased performance in high saline environments, NUE, WUE and TREF technologies, delayed senescence, herbicide resistance, reduced lignin technology and molecular farming technology for the production of chymosin in plants. Certain of these technologies are currently protected through our own or exclusively licensed approximately 30 patents and 20 patent applications. We have also licensed approximately 165 patent filings linked to seed traits such as NUE, WUE and TREF technologies, delayed senescence, reduced lignin technology, herbicide resistance, Intacta technology, ZFP® technology for use in our products. In some instances, our licenses are limited in terms of duration, geography and/or field of use.
171
The discovery phase of our R&D process is based largely on collaborations with governmental agencies and scientific institutions, such as CONICET, the University of Illinois, the National University of the Litoral and various research universities throughout the world. See “—Research & Development Process—Discovery.” After we determine that we have discovered a new trait, trait composition, industrial enzyme, or a production methodology, we file a PCT patent application under the PCT. The PCT application allows an applicant to file one single application to seek protection for an invention in 152 countries throughout the world.
Within 18 months of the PCT filing, we file national applications in the countries in which we would like to seek protection. The main PCT countries in which we file in addition to the United States include Brazil, Australia, India, China, Mexico and certain other countries in South America and Europe. For non-PCT countries such as Argentina, Uruguay and Paraguay, a national filing is made at the same time as the PCT filing or 12 months after the PCT filing.
We seek additional protection of our seed and germplasm intellectual property through PVP certificates, which preserve a variety owner’s exclusive rights to sell, reproduce, import, and export a plant variety and our seed. The duration of PVP protection varies among jurisdictions, and is 20 years from the time of issue in the United States and 20 and 15 years from the time of issue in Argentina and Brazil, respectively. As of the date of this prospectus, we do not have PVP certificates in the United States. In addition, in Argentina, we have received, as owner and/or as licensee, registrations with the RNC and the Registro Nacional de Propiedad de Cultivares for 27 wheat varieties, 18 soybean varieties, five alfalfa varieties, four corn varieties and two sunflower varieties, all of which are authorized for our marketing in Argentina. We are currently seeking registration with the RNC and the Registro Nacional de Propiedad de Cultivares for 13 soybean varieties, two wheat varieties, two amaranth varieties and 17 alfalfa varieties. We have also received the registration for seven soybean varieties and four wheat varieties in Uruguay and one soybean variety in South Africa.
We seek to protect our non-patent intellectual property such as know-how and regulatory data through contracts and confidentiality agreements. Know-how generated by the activities of our companies is protected by specific services agreements or employment agreements. Employment agreements include undertakings regarding confidentiality and assignment of inventions and discoveries. Our regulatory data is protected by standard confidentiality and data protection mechanisms. We have 42 trademarks and four trademark applications in Argentina, Brazil, the United States and Uruguay, including Bioceres, HB4, and SPC. Rizobacter has 355 trademarks and applications in Argentina and 300 trademarks and applications abroad, in Brazil, China, the United States, Uruguay, Turkey, Pakistan, Paraguay, Peru, Mexico, Colombia, Chile, Canada, Bolivia, South Africa, India and the EU, including RIZOBACTER, SIGNUM, RIZOSPRAY, RIZOFOS, RIZOLIQ, RIZOPACK, RIZODERMA, RIZOSTAR and RIZOOIL.
We will continue to file and prosecute patent, PVP certificate and trademark applications in the United States and foreign jurisdictions, as well as maintain trade secrets as is consistent with our business plan in an ongoing effort to protect our intellectual property.
Government Regulation
We are subject to agriculture, health and the environmental regulations in the countries in which we operate. We must obtain and comply with various permits and licenses from government authorities and municipalities in the jurisdictions in which we operate. The laws and regulations we are subject to will continue to evolve as there are advances in biotechnology and our other businesses. For further information on regulation, see “Regulation.”
172
Employees
As of September 30, 2017, we had 500 employees, of which 23.8% were involved in technology sourcing and product development. Our team’s expertise extends across multiple disciplines, including experts in biology, chemistry, plant genetics, agronomics, mathematics, computer science, process engineering and other related fields.
Our employees are located mainly in Argentina. The table below shows our employees by role as of the dates indicated and does not include employees of our research collaborators or joint venture partners.
As of September 30, | As of June 30, | As of December 31, | ||||||||||
2017 | 2017 | 2016 | 2015 | |||||||||
Management, administrative and sales | 381 | 377 | 353 | 45 | ||||||||
Research and development services | 119 | 119 | 124 | 80 | ||||||||
Total | 500 | 496 | 474 | 125 | ||||||||
Facilities
Our principal R&D facility is located in Rosario, Argentina and consists of 3,800 square meters (approximately 40,900 square feet) of office, laboratory and greenhouse space. CONICET assigned to us the right to use for a 30-year period the land on which our principal facility is located. We designed and built a facility on these premises for use by INDEAR. Upon expiration of the agreement in 2034, we must return the facility to CONICET. Additionally, we have a right of first offer to lease the facility if CONICET does not establish a specific use for the facility. Additional facilities are leased in Pergamino, including a 1,500 square meter seed processing facility as well as a 35-hectare (86-acre) field station.
Rizobacter’s main facilities are located in Pergamino and include a fermentation plant, laboratories and offices spanning 135,600 square feet; a warehouse of 253,500 square feet; and a tolling plant of 53,800 square feet. The 92,000-liter fermentation plant has a daily capacity of 200,000 doses of inoculants. Rizobacter also owns storage and manufacturing facilities in Brazil and Paraguay.
Synertech owns a 257,600 square-foot plant in Pergamino with a production capacity of 50,000 tons of micro-beaded fertilizers annually.
AGBM’s industrial facility is located in Cordoba City. It has a safflower-processing capacity of 6,000 metric tons per year, and can produce 2 million liters of commercial-level chymosin (up to 20% of the global market share) annually. The facility also includes 7,500 square feet of offices and laboratories, as well as two warehouses of 9,800 square feet.
Insurance
We maintain customary insurance policies that we consider to be in line with market practice and adequate for our business. Our principal insurance policies are personal injury (as mandated by Argentine labor law), civil liability, fire, theft, car, transport, credit, work injury risk and bond insurance entered into in connection with grants received. We do not maintain product liability insurance coverage.
Legal Proceedings
From time to time, we may become party to litigation or other legal proceedings that we consider to be a part of the ordinary course of our business. We are not currently involved in any legal proceedings that could reasonably be expected to have a material adverse effect on our business, prospects, financial condition or results of operations, other than the proceeding described below. As of the date of this prospectus, we are involved in one material legal proceeding, as described below, and we do not face any claims of possible intellectual property infringement. We may become involved in material legal proceedings in the future as part of the ordinary course of our business.
173
Contingent Fee Payment of US$17.3 Million Related to Precautionary Measure (Medidas Cautelares) in Connection with Rizobacter Acquisition.
Through our subsidiary RASA Holding we own 50.01% of Rizobacter’s capital stock. Of the total shares in Rizobacter acquired by our subsidiary RASA Holding, 7.6 million shares (representing 19% of Rizobacter’s capital stock) are subject to a precautionary measure issued by an injunction that affects 44% of the total capital stock of Rizobacter. The precautionary measure also covers 30% of the dividends distributed to such shares, directing such percentage of dividends into a judicially created escrow account. The precautionary measure relates to litigation among historical shareholders of Rizobacter arising from a disputed transfer of shares that occurred in 1995. In the event the contingencies are lifted, we may be obligated to pay a contingent fee payment of US$17.3 million to certain selling shareholders of Rizobacter through RASA Holding. Conversely, in the event that the court rules against the free transferability of the affected shares, we would not be obligated to pay the contingent fee payment but we may be obligated to return certain shares, thereby reducing our equity participation in Rizobacter. For further information on the precautionary measure, see “Risk Factors—Risks Related to Our Acquisitions—Certain of the Rizobacter shares are subject to a judicial injunction.”
174
Our business is subject to agricultural, health and environmental regulations. To operate, we must obtain various permits and licenses from government authorities and municipalities within the jurisdictions in which we operate, and we must maintain compliance with the terms of those permits, licenses and other government standards as required. These laws and regulations, particularly in relation to biotechnology, are not fully settled, but continue to evolve in order to keep pace with technological advances.
General
Phytosanitary Certification
Nearly all countries, including the United States, Argentina, Brazil and Uruguay, and many local jurisdictions, require phytosanitary certificates to import seed or plant materials. These certificates, issued by government agricultural inspectors where seeds or plants are produced or packaged, attest that seeds or plants are clean, free of prohibited impurities and have been tested for the presence of various pathogens that can be carried in or on the seeds or plant tissue. We obtain such certificates when necessary, including in connection with the use of our seeds for sample testing.
Hazardous Materials
Our laboratory and field activities inherently involve the use of potentially hazardous materials, which are subject to health, safety and environmental regulations. Our infrastructure, procedures and equipment are designed to meet our obligations under these regulations. We perform recurring internal and third-party audits and provide employees ongoing training and support, as required. All employees must comply with safety instructions and procedures, which are codified in our employment policies.
Regulation in Argentina
Regulation Relating to GMOs
The primary Argentine regulatory framework governing GMOs is established by Resolution No. 763/2011 issued by the former Argentine Ministry of Agriculture, Livestock and Fishing (Ministerio de Agricultura, Ganadería y Pesca) and supported by other ancillary regulations. Currently, we only develop plant GMOs, or GMPOs, and GM organisms at the experimental stage. Argentine law governs the following activities: (1) release into the environment GMPOs; (2) the production of seeds and/or biomass with genetically and regulated modified materials; and (3) authorization to commercialize GMO products. Activities in relation to laboratory greenhouses that develop GMPOs must also comply with applicable regulations. Resolution No. 763/2015 sets forth the procedures to be applied for determining whether a certain crop is to be considered GMPO.
On December 2016, INDEAR, our technology sourcing and product development subsidiary obtained the award of Excellence Through Stewardship (ETC) for stewardship, Incident Response Management, and Maintaining Plant Product Integrity: Laboratory, Containment Facility, and Confined Field Trials.
Approval from the Secretariat of Value Added (Secretaría de Agregado de Valor), or SVA, must be obtained before the release into the environment of any GMO for which commercial approval has not been obtained. Sanctions may be levied if GMOs are released without, or with inadequate, authorization.
Additionally, any entity seeking to experiment, import, export, produce, multiply or conduct any activity with GMPOs that have not yet been approved for commercialization in Argentina must be registered with the National Registry of Genetically Modified Plant Organisms. We are registered before this registry under registration No. 43.
Resolution No. 701/2011 sets forth the procedures for the submission and evaluation of the applications for experimental release of GMPOs that do not have commercial approval in Argentina. Resolution No. 318/2013 sets forth a different procedure for those cases that contain gene constructions identical or essentially similar to those incorporated to events that already have a risk evaluation.
The SVA has granted us the relevant authorization with respect to the following GMPOs: wheat, safflower, soybean, corn and alfalfa.
175
Resolution No. 17/13 regulates the release into the environment and for purposes other than experimentation of GM seeds and/or biomass (with regulated materials). This resolution applies to seeds which have not yet been authorized for commercialization in Argentina but are in advanced stages of experimentation. We have been granted the relevant authorization by the SAV with respect to GM safflower.
The SVA grants commercial approval of a GMPO based on the respective analysis carried out by specialized national agencies.
Notwithstanding any other sanction that may apply, including criminal, sanctions may be levied by the SVA if any release and/or commercialization of a GMO is made without the appropriate approvals and also if the unauthorized release and/or commercialization is undertaken by an entity that is not registered with the relevant registries. Sanctions may include revocation of permits and approvals. We are currently subject to the approval processes before the SVA relating to HB4 wheat, safflower and HB4 alfalfa, and we expect to obtain final commercialization approval by 2018.
Activities taking place in biosafety greenhouses or laboratories with regulated GMPOs must comply with the provisions of Resolution No. 241/12 and obtain special authorizaration. We have been granted this authorization by the SVA.
Seed and Plant Variety Registration
Argentine law aims to ensure the integral identity and quality of seeds purchased by agricultural producers and to protect the property of plant-related creations in accordance with the terms of the Argentine PVP Law, and its regulation, Decree No. 2183/91 and Law No. 24,376, which approves the International Convention for the Protection of New Varieties of Plants. In order to protect title to a seed variety and to be able to market it, the seed variety must be registered with the National Registry of Property of Seed Varieties (Registro Nacional de la Propiedad de Cultivares) and the RNC, respectively. A trader, in order to commercialize a seed variety, must be registered with the RNCFS. There are certain exceptions to protections.
We are registered with the RNCFS. In Argentina, we have received, as owner and/or as licensee, registrations for 27 wheat varieties, 18 soybean varieties, five alfalfa varieties, four corn varieties and two sunflower varieties, all of which are authorized for our marketing in Argentina. We are currently seeking registration with the RNC and the Registro Nacional de Propiedad de Cultivares for 13 soybean varieties, two wheat variety, two amaranth varieties and 17 alfalfa varieties. We have also received the registration for seven soybean varieties and four wheat varieties in Uruguay and one soybean variety in South Africa.
Regulation and Control of Agricultural Production
The quality of agricultural products is regulated and controlled by the SENASA, an entity within the Ministry of Agroindustry. In order to promote food safety, our INDEAR facility, and some of our products, such as our solution of safflower chymosin, are required to be registered with the Food Safety Agency of Santa Fe (Agencia Santafesina de Seguridad Alimentaria).
According to SENASA Resolution No. 492/2001, all commercial operators who export and/or import animals, vegetables, reproductive material, animal or vegetable products, by-products and/or their derivatives, and goods that contain animal or vegetable ingredients must be registered with the Registry of Importers and Exporters (Registro de Importadores y/o Exportadores) within the purview of SENASA. We are registered before this registry under registration No. 17933.
In addition, all exporters and importers, including us, must be registered with the Registry of Importers and Exporters (Registro de Importadores y/o Exportadores) administered by the AFIP. Companies carrying out agricultural export activities must comply with certain specific procedures established by Law No. 21,453 and related decrees.
Pursuant to Resolution No. 21-E/2017 of the Ministry of Agroindustry, any individual or company involved in the trade and industrialization of agri-food products in the markets for grains, livestock and dairy products and their by-products and/or derivatives shall be registered at the Registry of the Agro-Industrial Chain (Registro Único de Operadores de la Cadena Agroindustrial) within the Sub-Secretariat of Commercial Agriculture Control as its implementing authority. The Company is currently registered under registry No. 001938.
176
Registration with and authorization from the National Registry of Chemical Precursors (Registro Nacional de Precursores Químicos) is required when regulated substances and chemical products are used in the industrial process. All persons engaged in industrial activity in Argentina must be registered with the National Industrial Registry (Registro Industrial de la Nación).
Environmental Regulation for Agricultural operations involved in the development of GM crops
Our activities are subject to a number of national, provincial and municipal environmental regulations. The Argentine Constitution, as amended in 1994, provides that all Argentine inhabitants have the right to a healthy and balanced environment, fit for human development and have a duty to preserve the environment. Law No. 25,675 regulates the minimum standards for the achievement of a sustainable environment and the preservation and protection of biodiversity. Any project or activity capable of significantly degrading the environment or its components or which may adversely affect the quality of life, is subject to an environmental impact evaluation prior to its execution or performance. In addition, duties and obligations are triggered by any damage to the environment, generally requiring restoration of the environment to its former condition or, if that is not technically feasible, payment of compensation in lieu thereof.
Section 22 of Law No. 25,675 establishes that any person that conducts activities that may pose a risk to the environment must acquire environmental insurance in order to guarantee the payment of remedies for the potential damage deriving from such activities. The former Federal Environmental Secretariat has determined which activities must comply with this obligation in Annex I to Resolution No 177/07, as amended.
Pursuant to federal, provincial and/or local laws and regulations, permits are required and regulations are to be complied with in order to (1) generate, store, transport and dispose hazardous and pathogenic wastes, (2) use high pressure equipment, (3) use fuel tanks, (4) use chemical precursors, (5) use and dispose pesticides, agrochemical products and crop protection agents and (6) dispose liquid and gaseous effluents, among others. In addition, the use of public water is subject to the granting of permits by each provincial jurisdiction.
The violation of these laws and regulations may subject us to criminal, civil and administrative penalties, including remediation of the environment and reimbursement to third parties for damages resulting from the violation of environmental laws and regulations. Environmental, tort and criminal liability can be extended to managers, directors, statutory auditors and/or other officers who participate in our decision-making process.
Our laboratory and field activities inherently involve the use of potentially hazardous materials, which are subject to health, safety and environmental regulations. Our infrastructure, procedures and equipment are designed to meet our obligations under these regulations. We perform recurring internal and third-party audits and provide employees ongoing training and support to assist with complying with these regulations.
Regulation in the United States
The existing U.S. regulatory framework for biotechnology is based on the Coordinated Framework for Regulation of Biotechnology Products. Under the Coordinated Framework, three federal agencies – the USDA, the U.S. EPA and the U.S. FDA – share primary responsibility for regulating biotechnology. The USDA reviews biotechnology-derived applications, which contain or are produced using potential plant pests.
APHIS is the principal USDA agency involved in biotechnology regulation. APHIS is responsible for protecting U.S. agriculture against threats from pests and diseases. Under APHIS regulations, most GM plants are considered “regulated articles.” A “regulated article” is defined in APHIS regulations as “any organism which has been altered or produced through genetic engineering” if the donor organism, recipient organism, vector or vector agent is a “plant pest.” APHIS regulates field tests and interstate shipments of GM plants. APHIS must be notified prior to any interstate shipment or field test of these “regulated articles.” The Federal Plant Protection Act is the primary statute under which APHIS regulates agricultural biotechnology. Originally intended to prevent the introduction and interstate movement of plant pests, the Plant Pest Act has been adapted by APHIS to regulate GM plants so that they do not become “plant pests.” BRS, within APHIS, has direct oversight of the introduction (importation, interstate movement, or environmental release) of certain GM organisms.
177
Under the Federal Plant Protection Act, regulatory approval is required before the introduction, including the environmental release, interstate movement, and importation, of certain GM organisms, including our biotechnology products.
Regulation of Biotechnology Products
In the typical product development process for our biotechnology products, approval by APHIS through permitting or notification initially is required for field testing of a new product. The vast majority of new plant releases now require only a notification to APHIS. Eligibility criteria are characteristics of the regulated article, for example the plant, and the introduced genetic material. We believe that our technology as applied to core global crops will be eligible for notification based on present regulatory criteria and required performance standards. The performance standards are a set of six conditions that must be met in order to ensure that the regulated article is introduced in such a way that it is not inadvertently released beyond the area of proposed introduction, allowing it to persist in the environment. The performance standards include, among others, how the release will be conducted, actions taken to prevent the release of pollen or seed during shipping or from the test site and possible persistence in the environment, including compliance with the Endangered Species Act.
Within the notification process, applicants must provide information on the nature of the plant and introduced genes, descriptions of genetic modifications, size of the introduction, and origin and destination for movement or the location of a field test. Upon approval, notifications are valid for one year from the date of issue. Acknowledgement letters for notification of interstate movement are sent within 10 days of receipt of the complete notification. APHIS will send acknowledgement letters for notification of proposed importations and environmental releases within 30 days of receipt of the complete notification.
We have proceeded under the notification process for our HB4 soybean regulatory field trials, which are in advanced stages with the U.S. FDA. On August 10, 2017, the U.S. FDA completed its full review of the safety evaluation for HB4 soybeans, clearing it for use in human food and animal food (pending USDA approval).
If a plant does not meet the eligibility criteria for notification as is the case for our HB4 wheat, the applicant must follow the permitting process. This process involves a more comprehensive review than notification does. In addition to the data required in the notification process, field-test permit applicants must provide a detailed description of how they will perform the test. This description must include specific measures to reduce the risk of harm to other plants, and the organisms being tested therefore remain confined and they and their progeny may not persist after completion of the field test. Depending on the characteristics of the GMO, APHIS may impose additional measures and supplemental permit conditions. Permits may also be valid for one year or more from the date of issue. Issuance of a permit could take up to 120 days or longer depending on the need for an environmental assessment.
Upon the compilation of sufficient evidence that its GMOs pose no more of a plant pest risk than an organism’s conventional counterpart, applicants may petition APHIS to determine “non-regulated status” of that GM product. If the petition is approved by APHIS, the Federal Plant Protection Act does not apply and the GM product in question may then be introduced into the United States without any further APHIS regulatory approval. The petition process is a multi-year process that varies based on a number of factors, including the extent of the supporting information required, the nature and extent of review by APHIS, including the type and scope of the environmental review conducted, and the number and types of public comments received. Deregulation of a product is not a guaranteed outcome when a petition to deregulate a biotechnology plant is submitted to APHIS.
Since the petition process was first added to APHIS biotechnology regulations in 1992, the time it takes APHIS to reach a final decision has grown from six months on average to three to five years or more. The process for obtaining favorable action on petitions for non-regulated status, as well as permits for field testing, has become more complex and time-consuming in recent years. In November 2011, APHIS announced plans to improve the agency’s process for making determinations on petitions for non-regulated status for GM organisms.
Regulation of Seeds
We also are subject to the FSA, which regulates the interstate shipment of agricultural and vegetable seed. The FSA requires that seed shipped in interstate commerce be labeled with information that allows seed buyers to make informed choices and mandates that seed labeling information and advertisements pertaining to seed must be truthful. The FSA also helps promote uniformity among state laws and fair competition within the seed industry.
178
Regulation of Food and Feed Derived from the product
Although not required by act or regulation, the USDA and APHIS consider completion of the U.S. FDA consultation process when completing the deregulation process. All GM crops commercialized in the U.S. to date have completed this process. When a substance present in food derived from a GM crop is one that is already present at generally comparable or greater levels in currently consumed foods, there is unlikely to be a safety question sufficient to call into question the presumed “generally recognized as safe” status of such naturally occurring substances and warrant formal premarket review and approval by the U.S. FDA.
Environmental Regulation
Agricultural operations involved in the development of GM crops are also subject to a broad range of evolving environmental laws and regulations. These laws and regulations include the Clean Air Act, the Clean Water Act, the Resource Conservation and Recovery Act, the Federal Insecticide, Fungicide and Rodenticide Act and the Comprehensive Environmental Response, Compensation and Liability Act. These environmental laws and regulations are intended to address concerns related to air quality, storm water discharge and management and disposal of agricultural chemicals relating to seed treatment both for domestic and overseas varieties.
Compliance with these laws and related regulations is an ongoing process. Environmental concerns are, however, inherent in most agro-industrial operations, including those conducted by us, and the cost of compliance with environmental laws and regulations may be material.
Regulation in Brazil
The research, production and commercialization of GMOs in Brazil are regulated by Law No 11,105 (known as the Brazilian Biosafety Law) and related resolutions. In particular, Law No. 11,105 establishes certain safety standards and inspection mechanisms with respect to virtually all activities (such as research, commercialization, storage, transportation, release, consumption, discharge) involving GMOs and their byproducts, even in laboratory or contention regimes (without exposure to nature).
Pursuant to Law 11,105, activities involving GMOs (for instance, research and/or commercialization of GMOs) must obtain authorization from the CTNBio.
The CTNBio is part of the Brazilian Ministry of Science and Technology and Innovation, and is a multi-disciplinary board of several specialists from different fields of expertise and Brazilian Ministries. The CTNBio provides technical assistance and support for the formulation and implementation of biosafety policies with respect to GMOs and their byproducts and the promulgation of safety and technical standards regarding the authorization of research and commercialization of GMOs. In creating these standards, the CTNBio weighs factors such as the harmful effects that the particular GMO and its byproducts may have on animal, plant or human health, as well as any environmental risks.
As a general rule, in order to manipulate transgenic organisms in Brazil, each institution must: (i) obtain a Biosafety Quality Certificate, or BQC, which provides that the CTNBio accepts the institution’s attestation that the institution has appropriate infrastructure and technical, scientific and financial conditions to carry out the intended activities with GMO and its derivatives; and (ii) form an Internal Biosafety Committee, or CIBio, having a technical lead for each specific project.
Applications for a BQC must be made to the CTNBio by the CIBio itself, and must describe, among other information, the geographic areas that will be affected by the proposed experimental field trials. Such applications are reviewed on a monthly basis.
The CTNBio must also approve the commercial release of GMOs and their derivatives. As part of the approval process, applicants must identify and assess the potential adverse effects of the GMOs on humans, animals, plants or the environment and provide information regarding environmental and food safety risks. Applicants also must provide the CTNBio a plan for monitoring the GMO after its commercial release. The CTNBio will hold a public 30-day consultation prior to authorizing commercial release of a particular GMO. Lastly, prior to approval, the CNBS will analyze any socio-economic or national interest that may be impacted by approval of the particular GMO crop. In addition to obtaining authorization from the CTNBio, the applicant must comply with all other legal obligations related to the subject of the application. The CTNBio may revoke or suspend its authorization for commercial release if there is scientific evidence showing that there may be adverse effects to the environment or to human or animal health.
179
Executive Officers and Directors
Below is a list of names and a brief account of the business experience of the persons appointed, or to be appointed, to serve as our executive officers and directors prior to the consummation of this offering. There is no predetermined expiration for the term of the executive officers. The business address of each of the members of our board of directors and each of our executive officer is Ocampo 210 bis, Predio CCT, Rosario, Santa Fe, Argentina.
Our Executive Officers
Name | Age | Position | Current Position Held Since |
Federico Trucco, Ph.D. | 40 | Chief Executive Officer | June 2011 |
Enrique Lopez Lecube | 35 | Chief Financial Officer | December 2017 |
Claudio Marcelo Dunan, Ph.D. | 57 | Director of Strategy | December 2011 |
Gloria Montaron Estrada | 46 | General Counsel | March 2014 |
Gerónimo Watson | 39 | Chief Technology Officer | June 2014 |
Martín Vazquez, Ph.D. | 50 | Chief Scientific Officer | March 2014 |
Jorge Wagner | 48 | Chief Operating Officer | October 2016 |
The following are brief biographical descriptions of our executive officers.
Federico Trucco, Ph.D., has served as our chief executive officer since June 2011 and was appointed as a member of our board of directors in December 2014. He previously served in various positions at INDEAR including as general manager from 2009 to 2011, director of product development from 2008 to 2009 and research team leader of the Amaranth project from 2005 to 2009. Dr. Trucco also serves as president of Bioceres Inc. and S&W Semillas, manager of Verdeca, a director of RASA Holding, Heritas and Rizobacter Argentina, and vicepresident of SEMYA and AGBM. Dr. Trucco received a Ph.D. in crop sciences and a CBA from the University of Illinois, a master of science degree in plant pathology and weed science from the Colorado State University and a bachelor’s degree in biochemistry from the Louisiana State University.
Enrique López Lecoube has served as our chief financial officer since December 2017. He was appointed as executive member of the board of directors of Chemotécnica in December 2016. He has served as Manager for M&A and Corporate Finance at Lartirigoyen & Cia S.A. - a Glencore subsidiary - since 2016, where he has led transactions in the food, chemical and service industries and worked in the finance department to oversee 10 group entities. Previously, he worked as head trader from 2010 to 2016 at Lartirigoyen & Cia S.A. From 2008 to 2010, he worked at Cargill focusing on grain and oilseed. Mr. López Lecoube received an MBA from Kellogg of School of Management of Northwestern University and a degree as an industrial engineer from ITBA (Instituto Tecnológico de Buenos Aires).
Claudio Marcelo Dunan, Ph.D., has served as our director of strategy since December 2011. He was a founding member of Bioceres and previously served as our strategic planning consultant. Dr. Dunan previously served as general manager of Sintesis Quimica S.A. from 2009 to 2011 and has served in several senior management positions, including as country manager for Zeneca-Syngenta AG, Makteshim–Agan and the Punjab Group. Dr. Dunan received a Ph.D. in ecology from Colorado State University, as well as a bachelor’s degree in agronomy from the National University of Buenos Aires, Argentina.
Gloria Montaron Estrada has served as our general counsel since March 2014. She previously served as an attorney at Marval, O’Farrell & Mairal in Buenos Aires for 16 years, where her practice as centered around intellectual property, corporate and banking matters. Ms. Montaron also serves as legal director and secretary of Bioceres Inc., as secretary of RASA Holding, and as a director of INDEAR. She received an LLM in intellectual property from the University of Palermo, Buenos Aires and a degree in Law from the University of Buenos Aires, Argentina.
Gerónimo Watson has served as our chief technology officer since June 2014. He previously served in several positions at INDEAR, including as director of product development from 2011 to 2014, head of technology testing and field operations until 2011 and as a member of the Amaranth project starting in 2005. Mr. Watson also serves as a director of
180
Trigall and Verdeca and as chief technology officer of Bioceres Inc. Mr. Watson received a master’s degree in agronomy from the Kansas State University and a degree in agronomy from the Catholic University of Cordoba, Argentina.
Martín Vázquez, Ph.D., has served as our chief scientific officer since March 2014. He previously served in several positions at INDEAR, including as team leader in genomics and bioinformatics from 2009 to 2014 and as an external consultant for high-throughput genomic sequencing technologies from 2005 to 2009. Dr. Vázquez has served as an independent researcher at CONICET since 1999. He received a Ph.D. in biological science and a degree in biology from the University of Buenos Aires, Argentina.
Jorge Wagner has served as our chief operating officer since October 2016. He has also served as chief financial officer of Rizobacter since 2013. Prior to that, he served as regional chief financial officer (Paraguay, Uruguay, Argentina and Bolivia) of Syngenta Agro S.A. from 2010 to 2013. He received a CPA and an MBA from the University of Buenos Aires, Argentina.
The following table lists the current members of our board of directors:
Name | Age | Position | Current Position Held Since | Year of Expiration of Current Term |
Marcelo Adolfo Carrique(4) | 58 | Chairman | April 2017 | October 2019 |
Cintia Guillermina Castagnino(1) (2) (3) (4) (5) | 55 | Director | April 2017 | October 2019 |
Manuel Alberto Sobrado(1) (2) (4) (5) | 58 | Director | April 2017 | October 2019 |
Aimar Dimo(4) | 52 | Vice-Chairman | April 2017 | October 2019 |
Federico Trucco | 40 | Director | April 2017 | October 2019 |
Ignacio Lartirigoyen(1) (2) (4) (5) | 63 | Director | April 2017 | October 2019 |
Carlos Popik(3) (4) (5) | 72 | Director | April 2017 | October 2019 |
Santiago Sacerdote(4) (5) | 42 | Director | April 2017 | October 2019 |
Matías Hugo Kugler(3) (4) (5) | 52 | Director | September 2017 | October 2019 |
Jorge Carlos Joaquín Romagnoli | 63 | Alternate Director | April 2017 | October 2019 |
Hugo Osvaldo Ghio | 68 | Alternate Director | April 2017 | October 2019 |
Guillermo Cabrini | 68 | Alternate Director | April 2017 | October 2019 |
Victor Hugo Trucco | 73 | Alternate Director | April 2017 | October 2019 |
| (1) | Member of our audit committee. |
| (2) | Member of our compensation committee. |
| (3) | Member of our governance committee. |
| (4) | Independent director under the rules of CNV. |
| (5) | Independent director under the rules of NYSE. |
Brief descriptions of the biographical information of the members of the board of directors are presented below.
Marcelo Adolfo Carrique has served as a member of our board of directors and as our chairman since April 2017. He served as vice-chairman of the board of directors of INDEAR and has served on the board of directors of Berria Energia S.A. since 2014, Fullagro SRL since 2004 and Sorbona Combustibles S.A. since 1997. Mr. Carrique also serves as a director of INMET and Bioceres Inc. He has also served as chairman of the board of directors of Espartina S.A. since 2010 and previously served as president of AACREA. Mr Carrique received a postgraduate degree in economics from the Austral University in Buenos Aires, a postgraduate degree in business from the IAE Business School Universidad Austral and a degree in agronomy from the University of Buenos Aires.
Cintia Guillermina Castagnino has served as a member of our board of directors since April 2017. Ms. Castagnino also serves as a member of the board of directors of ACD S.A., Agropecuaria Don Juan S.A. and Cintia S.A. and has served as the president of San Ignacio de Alto S.A. since 2012 and Valeria S.A. since 2010. She has served as a director of several agricultural companies, including Bioceres Inc. and as an alternate director of INMET. Ms. Castagnino received a graduate degree in agribusiness from the Austral University in Buenos Aires and a degree in accounting and business management from the National University of Rosario, Argentina.
181
Manuel Alberto Sobrado has served as a member of our board of directors since April 2017. He has been executive director of Grupo Insud since 2006 and previously taught at the Faculty of Economic Sciences of the University of Buenos Aires, the Faculty of Economic Sciences of the Catholic University of Argentina and the Foundation of High Studies Dr. Perez Companc. Mr. Sobrado received degrees for completion of advanced professional training programs from the J.L. Kellogg Graduate School of Management, the Massachusetts Institute of Technology (MIT) and the Austral University in Argentina and received a degree in business administration from the University of Buenos Aires, Argentina.
Aimar Dimo has served as a member of our board of directors and as our vice-chairman since April 2017. He has served as chairman of the board of directors of INDEAR and INMET. He has served as chairman of the board of directors of Kiñewen S.A., Santa Angelina S.A. and Produsem, S.A. and as vice-chairman of Agro Matara S.A. and Kalpachay S.A.. In addition, Mr. Dimo has served as a partner at Dimku SRL and at AGROMAE and has served as a member of the executive committee of AAPRESID. He received a degree in agronomy from the National University of Rosario, Argentina.
Ignacio Lartirigoyen has served as a member of our board of directors since April 2017. He has served as the chairman of the board of directors of Lartirigoyen & Cia since 1997. In addition, Mr. Lartirigoyen has also served as chairman of the board of directors of Palma Horqueta S.A. since 2004, Pampa Bio S.A. since 2011, Agropecuaria Catrilo S.A. since 1998, Rotamar Agrícola Ganadera S.A. since 2002, Agropecuaria Los Tres Vascos S.A. since 2013 and Abiayala S.A. since 2001, and as vice-chairman of the board of directors of Lartirigoyen y Oromi S.A. since 2012. Mr. Lartirigoyen received a degree in agronomy from the University of Buenos Aires, Argentina.
Carlos Popik has served as a member of our board of directors since April 2017. He is the owner of Popiquen S.A. and has served as the chairman of its board of directors of since 1998. Mr. Popik has also served as a member of the Board of Investment of Aqua Capital Partners since 2011 and has occupied several positions at Monsanto including president of the board of directors in Argentina from 1991 to 2003, vice-chairman of the board of directors in Latin America from 1998 to 2003 and member of the advisory council of Monsanto in the U.S.. In addition, he has previously served as a member of the advisory board for agribusiness at the University of San Andrés. Mr. Popik attended the executive Program in Business Administration at Columbia University and holds a graduate degree in Agricultural Extension and a degree in agronomy from the National University of La Plata, Argentina.
Santiago Sacerdote has served as a member of our board of directors since April 2017. He serves as the general manager of Y-Tec (YPF Tecnología S.A.) and he is a member of its board of directors. In addition, Mr. Sacerdote served as the vice-chairman of technology affairs of CONICET from 2012 to 2015 and previously served at the Argentinian Industrial Union for six years. He received a master’s degree in Training for Political Action and Citizen Participation from the University of Francisco de Vitoria/ Rey Juan Carlos University of Spain and a degree in industrial engineering from the Buenos Aires Technology Institute, Argentina.
Matías Hugo Kugler has served as a member of our board of directors since September 2017. He serves as Managing Partner at Natal Agro S.R.L. He previously served as vice president of Tinto Holding S.A. from 2003 to 2014, as a managing partner at Emporio Agro from 2011 to 2014, as executive director of Bioceres Semillas from 2008 to 2010 and as chairman of the board of directors of Bioceres Semillas from 2010 to 2014. Mr. Kugler received a degree in agronomy from the University of Buenos Aires, Argentina.
Víctor Hugo Trucco. Mr. Trucco has been a board member since the inception of the Company and has served as an alternate director of our board of directors since April 2017. He previously served as our chairman from 2008 to 2011 and as our vice-chairman from 2012 to 2017. In addition, he is currently serving as an honorary President of AAPRESID as a director of Bioceres Inc. and an alternate director of INMET. Mr. Trucco received a Ph.D. and a degree in biochemistry from the National University of Rosario, Argentina.
Jorge Carlos Joaquín Romagnoli has served as an alternate director of our board of directors since April 2017. He has served as the chairman of the board of directors of Euksa S.A since 2010 and of Cordoba Sur S.A. and Julia del Sur S.A. since 2010. In addition, he has served as president of AAPRESID from 2004 to 2008 and serves as president of La Lucia S.A. and Kalpachay S.A. Mr. Romagnoli received a degree in agronomy from the National University of Northeast, Argentina.
182
Guillermo Cabrini has served as an alternate director of our board of directors since April 2017. He has served as chairman of La Madrugada since 2005. Mr. Cabrini received a degree in agronomy from the National University of Rosario, Argentina.
Hugo Osvaldo Guio has served as an alternate director of our board of directors since April 2017. Since 2010, he has served as chairman of the board of directors of Campo Fertil S.A., Paynaken S.A. and Aimará S.A., and as vice-chairman of the board of directors of Coleufú. He also serves as chairman of Bioceres Semillas S.A. In addition, Mr. Guio has served as the manager of Don Osvaldo since 1991. He received a degree in agronomy from the National University of Rosario, Argentina.
Board of Directors and Executive Officers
Under the Argentine Corporate Law and our bylaws, the management of our business is vested in our board of directors. Our alternate directors replace regular board members in the event of a vacancy and they do so in the order of their appointments. Our Chief Executive Officer is responsible for our day-to-day management. Our Chief Executive Officer is appointed by, and serves at the discretion of, our board of directors. Under our bylaws, our board of directors must consist of a minimum of three and a maximum of 13 directors and an equal or smaller number of alternate directors. Currently, our board of directors consists of nine directors and four alternate directors.
The board of directors is responsible for implementing any resolutions approved at the annual shareholders’ meetings and for fulfilling the assignments specifically entrusted to them by the shareholders. Under Argentine Corporate Law, directors must perform their duties with the loyalty and diligence of good businessmen. Directors are jointly and severally liable to the organization they represent, our shareholders and third parties for mismanagement, as defined in light of the criteria mentioned above, for violating any law, the corporate bylaws or rules, if any, and for any damage resulting from fraud, abuse of discretion or wrongdoing.
Section 271 of the Argentine General Companies Law allows directors to enter into agreements with the company that relate to such director’s activity and under arms’ length conditions. Agreements that do not satisfy any of the foregoing conditions must have prior approval of the board of directors (or the supervisory committee in the absence of board quorum), and must be notified to the shareholders at a shareholders’ meeting. If the shareholders reject the agreement, the directors or the members of the supervisory committee, as the case may be, shall be jointly and severally liable for any damages to the company that may result from such agreement. Agreements that do not satisfy the conditions described above and are rejected by the shareholders are null and void, without prejudice to the liability of the directors or members of the supervisory committee for any damages to the company.
Furthermore, Argentine Corporate Law requires that directors shall: (1) always inform the board of directors and the supervisory committee of the company of any conflict of interest in relation to a particular issue and refrain from participating in the discussion and voting on such issue; and (2) never participate in any activity that is in competition with the company, unless expressly approved by a shareholders’ meeting.
In general, a director will not be held liable for a decision of the board of directors, even if that director participated in the decision or had knowledge of the decision, if (i) there is written evidence of the director’s opposition to the decision and (ii) the director notifies the Supervisory Committee of such opposition. However, both conditions must be satisfied before the director may be deemed not to be liable before the board of directors, the supervisory committee or the shareholders or relevant authority or the commercial courts.
Furthermore, the Argentine Capital Markets Law also imposes on directors the duties of loyalty and diligence in the exercise of their roles.
Corporate Governance Practices
Compliance with NYSE Standards
We are a “foreign private issuer” within the meaning of the NYSE corporate governance standards. Under NYSE rules, a foreign private issuer may elect to comply with the practices of its home country and not comply with certain corporate governance requirements applicable to U.S. companies with securities listed on the exchange. We currently follow certain Argentine practices, including those required by the CNV rules, with regard to the following NYSE listing requirements:
183
| ■ | Audit committee. The NYSE rules require domestic listed companies to have an audit committee with a written charter that covers certain minimum specified duties. In addition, the audit committee must comply with Rule 10A-3 under the Exchange Act, or Rule 10A-3. Our audit committee has a written charter that complies with CNV rules and Rule 10A-3 to the extent permitted by Argentine law. Our reliance on the exemption for foreign private issuers under Rule 10A-3(c)(3) does not, in our opinion, materially adversely affect the ability of our audit committee to act independently and to satisfy the other requirements of Rule 10A-3. |
| ■ | Shareholder Approval. The NYSE rules require domestic listed companies to obtain shareholder approval for certain dilutive events, such as the establishment or amendment of certain equity-based compensation plans. In comparison, under Argentine law, any dilutive events, such as the issuance of equity-compensation plans are required to be approved by a general shareholders’ meeting. |
Audit Committee
Our audit committee consists of Manuel Alberto Sobrado, Cintia Guillermina Castagnino and Ignacio Lartirigoyen. Our board of directors has determined that each of the members of our audit committee is independent under the standards of the SEC, CNV and NYSE. Our board of directors has also determined that that each member is “financially literate” within the meaning of the rules of the NYSE, is an “audit committee financial expert” within the meaning of Item 407(d) of Regulation S-K under the Securities Act and has the of requisite business, financial or accounting experience as defined by the CNV and NYSE rules applicable to audit committee members. Our shareholders have also determined that each of the members of our audit committee is independent under CNV rules.
Pursuant to the Argentine Capital Markets Law, the audit committee has the following rights and duties, among others: (1) issuing an opinion on the independence of potential candidates for independent accountant; (2) supervising the performance of internal controls and administrative and accounting systems; and (3) issuing an opinion regarding any related party transactions and other transactions that may result in conflicts of interest. The audit committee must also prepare an annual plan for the fiscal year and report on the plan to the board of directors and the supervisory committee.
Our board of directors has adopted a written charter for our audit committee consistent with NYSE rules and applicable Argentine Corporate Law, which is available on our website.
Compensation Committee
Our compensation committee consists of Cintia Guillermina Castagnino, Manuel Alberto Sobrado and Ignacio Lartirigoyen. Our board of directors has determined that each of the members of our compensation committee is independent under the standards of the NYSE. Our shareholders have also determined that each of the members of our compensation committee is independent under CNV rules.
Our compensation committee has the following rights and duties, among others: (1) to formulate and establish the compensation policy for the board of directors, executive officers and management employees; (2) to administer and establish the guidelines for the allocation of Company shares and stock options to the board of directors, executive officers and management employees; (3) to conduct the performance evaluations of the executive officers and management of the Company and submit the performance evaluations to the board of directors; and (4) to identify and recommend to the Governance Committee persons that are qualified and eligible to serve as executive officers of the Company.
Our board of directors has adopted a written charter for our compensation committee consistent with NYSE rules and applicable Argentine Corporate Law, which is available on our website.
Governance Committee
Our nominating and corporate governance committee consists of Cintia Guillermina Castagnino, Carlos Popik and Matias Kugler. Our board of directors has determined that each of the members of our governance committee is independent under the standards of the NYSE. Our shareholders have also determined that each of the members of our governance committee is independent under CNV rules.
Our governance committee will (1) identify individuals qualified to become directors; (2) recommend to our board of directors nominees for each election of directors; (3) develop and recommend to our board of directors criteria for
184
selecting qualified director candidates; (4) consider committee member qualifications, appointment and removal; (5) recommend corporate governance guidelines applicable to us; and (6) provide oversight in the evaluation of our board of directors and each committee.
Our board of directors has adopted a written charter for our governance committee consistent with NYSE rules and applicable Argentine Corporate Law, which is available on our website.
Code of Ethics and Corporate Governance Guidelines
We have adopted a code of ethics applicable to all of our employees and officers, including our principal executive, financial and accounting officers and all persons performing similar functions which will take effect upon the closing of the offering and will comply with the rules of the NYSE and will be a “code of ethics” as defined in Item 16B of Form 20-F promulgated by the SEC. A copy of our code is available on our website. We expect that any amendments to such code, or any waivers of our requirements, will be disclosed on the website.
Our Board has also adopted governance guidelines, which complies with the rules of the NYSE, to provide a framework for performing its duties and responsibilities. This includes provisions on composition of the Board, guidelines for meetings, evaluation of executive officers and other best practices. A copy of these guidelines is available on our website.
Supervisory Committee
Pursuant to Argentine Corporate Law, Argentine Capital Markets Law and CNV rules, we are required to establish a supervisory committee (comisión fiscalizadora) composed only of lawyers, certified public accountants authorized to practice in Argentina and civil-law partnerships whose members are either lawyers or certified public accountants authorized to practice in Argentina. Our supervisory committee is separate from the committees of the board of directors and is composed of three members and three alternate members, none of whom are members of our board of directors. Both the members as well as the alternate members of the supervisory committee are appointed at a shareholders’ meeting for a three-year term.
The members of our supervisory committee are Humberto Domingo Santoni, Alberto Antonio Romano and Daniel Edmundo Juan Vigna and the alternate members are Carina Mariel Foglia, Pedro Figueroa Casas and Luis Antonio Gritti. The members and alternate members of our supervisory committee were elected at the shareholders’ meeting held on April 27, 2017.
The principal rights and duties of the members of the supervisory committee under Argentine Corporate Law are: (1) to review corporate management by examining accounts and documents whenever convenient, but at least every three months; (2) to verify, at the same time and frequency, amount of securities on hand and compliance with obligations; (3) to attend, without voting, the annual shareholders’ meetings and meetings of the board of directors; (4) to summon extraordinary shareholders’ meetings, if deemed necessary, and regular and special shareholders’ meetings should the board of directors not summon them; (5) to submit to the shareholders’ meetings a written report regarding the economic and financial condition of the company and to render an opinion about the report, the balance sheet and the income statements of the company and (6) to analyze the written claims of shareholders representing no less than 2% of the outstanding capital stock. When conducting these activities, the supervisory committee is prohibited from controlling the operations and from assessing the fairness of the decisions made by directors. The supervisory committee has unlimited access to corporate documents and other documents and accounts and it has the right to solicit any information that it deems necessary in order to perform our duties.
Below is a brief account of the business experience of the persons appointed, or to be appointed, to serve as members and alternate members of our supervisory committee prior to the consummation of this offering:
Humberto Domingo Santoni has been a member of our supervisory committee since December 2014. Mr. Santoni has been a partner at Vigna, Santoni & Associates since 1985. He also served as manager of the auditing department at Gamarra, Lattuca & Associates (currently Deloitte & Co.) from March 1979 to September 1985 and as an expert accountant for Rosario’s commercial and trade arbitration tribunal since 1989. Mr. Santoni holds a degree in accounting from the National University of Rosario.
185
Alberto Antonio Romano has been a member of our supervisory committee since December 2014. Mr. Romano has served as director of the corporate law department at the Austral University in Rosario since 2003, as a partner of Romera, Ongay, Romano, Castellani & Figueroa Casa Abogados since 1998, as president of the Corporate Law Institute since 2003 and as arbitrator at the Rosario Bar Association since 2006. Mr. Romano holds a doctorate in juridical sciences and a law degree from the Pontifical Catholic University of Argentina.
Daniel Edmundo Juan Vigna has been a member of our supervisory committee since December 2014. Mr. Vigna has been a partner at Vigna, Santoni & Associates since 1985. He has also served as a member of the Tax and Pensions committee of the Rosario Board of Trade since 1989. Mr Vigna holds a degree in accounting from the National University of Rosario.
Carina Mariel Foglia has served as an alternate member of our supervisory committee since December 2014. Ms. Foglia has been a partner at Vigna, Santoni & Associates since 1985. She has served as the head of practical training in accounting at the University of Latin American Education Center since 2003. Ms. Foglia holds a degree in accounting from the National University of Rosario.
Pedro Figueroa Casas has served as an alternate member of our supervisory committee since December 2014. Mr. Casas has been a partner at Romera, Ongay, Romano, Castellani & Figueroa Casas Abogados since 1998. Mr. Casas holds a degree in law from the Austral University of Rosario, Santa Fe.
Luis Antonio Gritti has served as an alternate member of our supervisory committee since December 2014. Mr. Gritti has been a partner at Gritti & Associates since 1986. He holds a degree in accounting from the National University of Rosario.
Family Relationships
As of the date of this prospectus, there were no family relationships among the members of our executive officers and board of directors, other than that of Mr. Victor Trucco and Dr. Federico Trucco, who are father and son.
Compensation Policies
For the period ended September 30, 2017 and 2016, we paid US$0.4 million and US$0.4 million, respectively, in compensation to our executive officers. During our annual ordinary shareholders’ meeting, which was held April 27, 2017, the members of the board of directors waived their rights to compensation for the fiscal year ended December 31, 2016, except for compensation to Dr. Federico Trucco, to whom we paid ARS 421,894.
Executive Officers
The compensation of our executive officers, if consistent with our then-effective compensation policy, requires the approval of our compensation committee, followed by our board of directors. Compensation of any such office holder that deviates from the then-effective compensation policy will also require shareholder approval.
Board of Directors
Under Argentine Corporate Law, if the compensation of the members of the board of directors is not established in the bylaws of the company, it should be determined by the shareholders’ meeting. The maximum amount of total compensation to the members of the board of directors, including wages and compensation for technical or administrative permanent activities, cannot exceed 25% of the earnings of the company. That amount should be limited to 5% when there is no distribution of dividends to shareholders and will be increased proportionally to the distribution. When one or more directors perform special commissions or technical or administrative activities, and there are no earnings to distribute, or they are reduced, the shareholders meeting may approve compensation in excess of the limits described above.
The compensation of our directors for each fiscal year is determined pursuant to Argentine Corporate Law, and taking into consideration whether the directors performed technical or administrative activities and our fiscal year results. Once the amounts are determined, they are considered at the shareholders’ meeting.
186
Benefits upon Termination of Employment
Neither we nor our subsidiaries, majority shareholders, customers or suppliers have entered into any agreement providing for benefits to any director upon termination of service.
Audit Committee, Compensation Committee, Governance Committee and Supervisory Committee
Our shareholders approved the creation of the audit committee and supervisory committees at the shareholders’ meeting held on December 17, 2014 and authorized our board to create any other committee that seems appropriate for the business of the company. Our board approved the creation of the compensation and corporate governance committee. Each committee will take effect upon the consummation of this offering. The remuneration for members of these committees will be determined by the shareholders at our shareholders’ meeting.
Equity Incentive Plans
On December 17, 2014, our shareholders approved the issuance of a stock option incentive plan exercisable in respect of up to 1,264,000 ordinary shares (considering the Stock Split) and a stock grant incentive plan permitting stock grants of up to 1,264,000 ordinary shares, or the Equity Incentive Plans or the Plans. Of such stock options, our board authorized the issuance of stock options with respect to 929,040 ordinary shares under the stock option incentive plan, with an exercise price of US$7.91 per share, to certain of our executives, officers and directors with whom we had executed individual stock option agreements with respect to 929,040 ordinary shares authorized to the designated officers with a vesting date of April 1, 2017, which will remain exercisable until two years after the vesting date. Our board also authorized the issuance of stock grants with respect to 902,487 ordinary shares under the stock grant incentive plan to certain of our executives, officers and directors. We expect our compensation committee to execute the individual stock grant agreements in the second quarter of the current fiscal year.
Purpose. We believe that the awards under the Equity Incentive Plans, or the Equity Incentive Awards, will promote our long-term growth and profitability by (1) providing key people with incentives to improve shareholder value and to contribute to growth and financial success through their future services, and (2) enabling us to attract, retain and reward the best available personnel.
Eligibility. Certain members of our board of directors, management and other critical personnel are eligible for Equity Incentive Awards under the Plans. Awards that are directed to members of the compensation committee of the Equity Incentive Plans require approval by our board of directors.
Administration. The Equity Incentive Plans approved by the shareholders are administered by the compensation committee consisting of three members of our board of directors, appointed for a three-year term by the board of directors. The committee has authority to grant the Equity Incentive Awards under the Plans in accordance with the terms and conditions of the Plans already approved by our board and to take any actions deemed necessary or advisable in connection therewith, including without limitation the authority to appoint the beneficiaries, determine the amount of options or shares to be granted, determine the price and timeframe for the exercise of the stock options and determine the issuance of stock grants and settling disputes involving the Equity Incentive Awards and the Plans.
187
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following discussion is a brief summary of certain material arrangements, agreements and transactions we have with related parties. We also engage in other transactions with related parties that we do not perceive as material.
Shareholders’ Agreement
On April 10, 2014, certain of our current shareholders, whom we refer to as the syndicated shareholders, entered into a shareholders’ agreement (Convenio de Accionistas), or the Shareholders’ Agreement. The Shareholders’ Agreement sets forth the commitment of the syndicated shareholders to syndicate their voting rights in our shareholders’ meetings in accordance with the decisions taken in the meetings of the syndicated shareholders. The Shareholders’ Agreement also restricts the ability of the syndicated shareholders to transfer and/or establish liens over their shares. In particular, syndicated shareholders who hold at least 3% of our outstanding share capital must require any potential purchaser of all or part of such syndicated shareholder’s shares to also purchase the ordinary shares held by the other syndicated shareholders in the potential purchase in the same proportion, and subject to the same terms and conditions as those offered to the selling shareholder. Additionally, the syndicated shareholders may not establish any liens, encumbrances or other rights in favor of third-parties that could adversely impact the rights of the other syndicated shareholders under the Shareholders’ Agreement. The Shareholders’ Agreement will terminate upon the effectiveness of this registration statement and the public offering authorization of our ordinary shares by the CNV.
In connection with the acquisition of Rizobacter by RASA Holding, RASA Holding has entered into an agreement with certain existing shareholders of Rizobacter, or the Rizobacter Shareholders’ Agreement. Through the Rizobacter Shareholders’ Agreement, the Company has control over a supermajority of 80% of the voting shares of Rizobacter. The Rizobacter Shareholders’ Agreement grants to RASA Holding (i) a right of first refusal for any transfer of shares owned by certain existing shareholders on the same terms as the relevant firm offer, (ii) the right to appoint a majority of the board of directors of Rizobacter, including the chairman., and the right to choose two of the three members of the Supervisory Committee of Rizobacter and (iii) the commitment of certain existing shareholders to vote in the shareholders meetings in accordance with the instructions of the Company. The Rizobacter Shareholders’ Agreement also restricts the existing shareholders’ ability to sell shares to third parties in accordance with the terms of the tag-along rights and drag-along rights for approved sales of shares to third parties as set forth therein.
Corporate Services Agreements
In connection with the operation of our businesses, Bioceres S.A. typically enters into services agreements with each of Bioceres Semillas, INMET and INDEAR. Below is a summary of these standard services agreements.
Term. Each contract term runs for a period of two years from the date of effectiveness. Once the initial two-year term is reached, the parties can agree to extend the term of the original contract for another two years and can do so indefinitely. The contracts between Bioceres and each of Bioceres Semillas, INDEAR and INMET were renewed in January 2017 for another two-year period.
Services. Each contract typically covers day-to-day operations and services, including administrative services, human resources, accounting, financial operations, supervision of personnel, management, communications, strategy for business development, as well as compliance with laws and regulations.
Compensation. In return for the services provided by Bioceres, each counterparty must pay Bioceres an amount equal to, in the case of INMET 10% of general and administrative costs incurred by Bioceres, and in the case of each INDEAR and Bioceres Semillas 30% of such costs. These fees are invoiced on a quarterly basis.
Intellectual Property. Under each contract, Bioceres will gain ownership over all intellectual property rights that arise from services rendered. The intellectual property rights include, but are not limited to, patents, trademarks, trade secrets and know how.
Confidentiality. The parties have agreed to keep the terms of each contract confidential, subject to certain exceptions, during the term of the contract and for a period of ten years after termination of the contract.
188
Indemnification. Bioceres has agreed to indemnify each counterparty for any liability that arises out of the performance of the services under each contract.
Research and Development Services Agreements
In connection with our R&D services, Bioceres, Bioceres Semillas and INMET typically enter into agreements with INDEAR for R&D services. Below is a summary of technology sourcing and product development contracts entered into between INDEAR and each of Bioceres, Bioceres Semillas and INMET.
Term. Each contract term runs for a period of two years from the date of effectiveness. Once the initial two-year term is reached, the parties can agree to extend the term of the original contract for another two years and can do so indefinitely. The contracts between INDEAR and each of Bioceres and Bioceres Semillas and INMET were renewed in January 2017 for another two-year period.
Services. INDEAR will provide R&D services.
Termination. INDEAR’s counterparty can terminate the R&D services contract at any time at its own discretion upon 30 days’ written notice. INDEAR will receive payment for services rendered until the termination of the contract by the counterparty. Further, INDEAR’s failure to perform any of its obligations under the contract gives the contracting party the unilateral right to terminate and require INDEAR to return all payments received under the applicable contract.
Compensation. In return for the R&D services provided by INDEAR, each counterparty will pay a sum equal to the costs of the services rendered plus 15% of such amount. These fees will be calculated in accordance with an itemized schedule and are invoiced on a quarterly basis.
Intellectual Property. Under each contract, INDEAR’s counterparties will gain ownership over all intellectual property rights that arise from services rendered. The intellectual property rights include, but are not limited to, patents, trademarks, plant variety rights, trade secrets, and any intellectual property rights that arise out of the R&D services.
Confidentiality. The parties have agreed to keep the terms of the contract confidential, subject to certain exceptions, during the term of the contract and for a period of five years after effectiveness and/or termination of the contract.
Indemnification. INDEAR has agreed to indemnify each of the counterparties for any liability that arises out of the performance of our R&D services.
Office and Laboratory Services Agreements
In connection with our day-to-day operations, Bioceres, Bioceres Semillas and INMET typically enter into agreements with INDEAR for the provision of office and laboratory services. Below is a summary of the office and laboratory services agreements between INDEAR and each of Bioceres, Bioceres Semillas and INMET.
Term. Each contract term runs for a period of two years from the date of effectiveness. Once the initial two-year term is reached, the parties can agree to extend the term of the original contract for another two years, and can do so indefinitely. The contracts between INDEAR and each of Bioceres, Bioceres Semillas and INMET were renewed in January 2017 for another two-year period.
Services. Each contract covers administrative expenses related to each counterparty’s use of INDEAR’s office and laboratory space.
Compensation. In return for the office and laboratory services provided by INDEAR, each counterparty will pay INDEAR an amount equal to the costs of the office services rendered. These fees are invoiced on a quarterly basis.
Early Stage Technology Development Agreements
Between 2005 and 2007, we entered into agreements with various investors in order to obtain funding in the aggregate amount of US$1.0 million for research related to early stage technology for the development of technology relating to a specific gene from sunflower intended to promote drought tolerance in crops, which we refer to as Hahb 4. The
189
agreements grant the investors in the aggregate the right to receive 52.8% of the rights and royalties payable to Bioceres S.A. from the successful commercialization of the resulting technology with respect to soybean, wheat and corn.
Trigall Genetics S.A. Loan Facility Agreement
In December 2013, Bioceres, Inc., a subsidiary of Bioceres S.A., entered into a revolving loan facility agreement with Trigall Genetics, as the borrower, acting as a joint lender with Florimond Desprez for a period of seven years ending on December 2020, up to a maximum amount of US$6 million. Responsibility for funding amounts drawn under facility is apportioned proportionally between Bioceres Inc. and Florimond Desprez up to US$3 million, respectively. The aggregate amount outstanding under the loan owed to Bioceres Inc. as of June 30, 2015 amounted to US$1.6 million. Trigall Genetics has no obligation to pay any interest on amounts owing under the facility.
Director and Officer Indemnification and Insurance
We have entered into indemnification agreements with each of our directors and executive officers, and we intend to purchase prior to the closing of the offering a policy of directors’ and officers’ liability insurance that will insure our directors and executive officers against the cost of defense, settlement or payment of a judgment under certain circumstances.
Policies and Procedures Regarding Related Party Transactions, and Director Independence
In reviewing all related party transactions, the board of directors or our authorized committee will consider the NYSE’s and the CNV’s governance standards related to independence determinations.
Based on the foregoing, most recently, the board determined that the following directors are independent under applicable NYSE standards: Marcelo Adolfo Carrique, Cintia Guillermina Castagnino, Matías Kugler, Manuel Alberto Sobrado, Aimar Dimo, Santiago Sacerdorte, Carlos Popik and Ignacio Lartirigoyen. The board also determined that the following directors are not independent under the applicable NYSE standards: Dr. Federico Trucco. We also describe below certain other transactions with our directors, executive officers and stockholders.
Furthermore, the shareholders’ meetings held on April 27, 2017 determined that the following directors are independent under applicable CNV rules: Marcelo Adolfo Carrique, Cintia Guillermina Castagnino, Aimar Dimo, Manuel Alberto Sobrado Santiago Sacerdorte, Carlos Popik and Ignacio Lartirigoyen. The shareholders’ meetings held on December 17, 2014 and April 24, 2015 also determined that Dr. Federico Trucco is not independent under the applicable CNV rules.
Espartina S.A.
In the three-year period ended in December 31, 2016 our subsidiary, Bioceres Semillas, bought seed products from Espartina S.A., an entity in which our President, Mr. Marcelo Carrique, is a partner and for which he serves as President. The total aggregate amount invoiced by Espartina S.A. in connection with seed sales during the three-year period ended December 31, 2016 amounted to US$0.3 million.
Produsem S.A.
In the three-year period ended in December 31, 2016 our subsidiary, Bioceres Semillas, sold seed products to Produsem S.A., an entity that is 92.2% indirectly owned by one of our directors, Mr. Aimar Dimo, in addition to the 2% directly owned by his wife, and for which he serves as president. The total aggregate amount we invoiced to Produsem S.A. in connection with seed sales during the three-year period ended December 31, 2016 amounted to US$1.8 million. In the Transition Period ended June 30, 2017, we invoiced Produsem a total amount of US$0.1 million. The total aggregate amount that Produsem S.A. invoiced Bioceres Semillas, in connection with seed sales during the three-year period ended December 31, 2016, amounted to US$1.6 million.
Santa Angelina S.A.
In the three-year period ended December 31, 2016 our subsidiary, Bioceres Semillas, sold seed products to Santa Angelina S.A., an entity that is 90% indirectly owned by one of our directors, Mr. Aimar Dimo, in addition to the 10%
190
directly owned by his wife, and for which he serves as president. The total aggregate amount we invoiced to Santa Angelina in connection with seed sales during the three-year period ended December 31, 2016 amounted to US$0.1 million. The total aggregate amount that Santa Angelina S.A. invoiced Bioceres Semillas, in connection with sawflower production during the three-year period ended December 31, 2016, amounted to less than US$0.1 million.
Kiñewen S.A.
In the three-year period ended December 31, 2016 our subsidiary, Bioceres Semillas, sold seed products to Kiñewen S.A., an entity that is indirectly owned by one of our directors, Mr. Aimar Dimo, and for which he serves as president. The total aggregate amount we invoiced to Kiñewen in connection with seed sales during the three-year period ended December 31, 2016 amounted to US$0.4 million. The total aggregate amount that Kiñewen S.A. invoiced Bioceres Semillas in connection with seed sales during the three-year period ended December 31, 2016, amounted US$0.5 million. In the Transition Period ended June 30, 2017, Kiñewen S.A. invoiced a total amount of US$0.1 million.
Lartirigoyen y CIA S.A.
In the three-year period ended December 31, 2016 our subsidiary, Bioceres Semillas, sold seed products to Lartirigoyen y CIA S.A. an entity in which one of our directors, Mr. Ignacio Lartirigoyen, is a partner and for which he serves as president. The total aggregate amount we invoiced to Lartirigoyen y CIA S.A. in connection with seed sales during the three-year period ended December 31, 2016 amounted to US$0.1 million. In the Transition Period ended June 30, 2017, we invoiced a total amount of less than US$0.1 million.
Chemotecnica S.A.
In the three-year period ended December 31, 2016 our subsidiary, Bioceres Semillas, sold seed products to Chemotecnica, an entity that is indirectly owned by one of our directors, Mr. Ignacio Lartirigoyen. The total aggregate amount we invoiced to Chemotecnica, in connection with seed sales during the three-year period ended December 31, 2016 amounted to less than US$0.1 million. In the Transition Period ended June 30, 2017, we invoiced Chemotecnica S.A. a total amount of less than US$0.1 million. The total aggregate amount that Chemotecnica invoiced Bioceres Semillas in connection with seed products during the three-year period ended December 31, 2016, amounted US$0.7 million. In the three-month period ended September 30, 2017, and in the Transition Period, Chemotecnica S.A. invoiced Rizobacter a total amount of US$0.1 million and less than US$0.1 million respectively.
Don Osvaldo S.A.
In the three-year period ended December 31, 2016, our subsidiary, Bioceres Semillas, sold seed products to Don Osvaldo S.A., an entity in which one of our directors, Mr. Hugo Ghio, is a partner and for which he serves as president. The total aggregate amount we invoiced to Don Osvaldo S.A. in connection with seed sales during the three-year period ended December 31, 2016 amounted to US$0.4 million. In the Transition Period ended June 30, 2017, we invoiced a total amount of US$0.1 million.
Indemnification Agreement
We have entered into indemnification agreements with each of our directors and executive officers. These agreements provide that the director or officer will be indemnified by us to the fullest extent permitted by law against liability and against all expenses reasonably incurred or paid by him or her in connection with any claim, action, suit or proceeding which he or she becomes involved as a party or otherwise by virtue of his or her being or having been such a director or officer of our company and against amounts paid or incurred by him or her in the settlement thereof. The agreements are subject to certain exceptions, including that no indemnification will be provided to any director or officer against any liability to us or our shareholder (i) by reason of intentional fraudulent conduct, dishonesty, willful misconduct, or gross negligence on the part of the director or officer; or (ii) by reason of payment made under an insurance policy or any third party that has no recourse against the indemnitee director or officer.
191
The following table sets forth information with respect to the beneficial ownership of our shares, including shares in the form of ADSs, as of September 30, 2017 and after this offering by:
| ■ | each of our directors, executive officers and members of the supervisory committee individually; and |
| ■ | all of our directors, executive officers and members of the supervisory committee as a group. |
No person or entity is known by us to own beneficially more than 5% of our outstanding shares. The beneficial ownership of ordinary shares is determined in accordance with the rules of the SEC and generally includes any ordinary shares over which a person exercises sole or shared voting or investment power, or the right to receive the economic benefit of ownership. There are no shares subject to options that are currently exercisable or exercisable within 60 days of the date hereof. The percentage of shares beneficially owned prior to the offering is based on 25,644,300 ordinary shares outstanding as of the completion of this offering, which assumes the Stock Split effective immediately prior to the commencement of this offering. The percentage of beneficial ownership of our ordinary shares after the offering is based on ordinary shares outstanding after the offering. We have also set forth below information known to us regarding any significant change in the percentage ownership of our ordinary shares by any major shareholders during the past three years.
As of September 30, 2017, we had three holders of record of our ordinary shares in the United States representing 0.96% of our outstanding ordinary shares.
All of our shareholders, including the shareholders listed below, have the same voting rights attached to their ordinary shares. See “Description of Share Capital—Bylaws—Voting Rights.” Neither our principal shareholders nor our directors and executive officers have different or special voting rights.
A description of any material relationship that our principal shareholders have had with us or any of our predecessors or affiliates within the past three years is included under “Certain Relationships and Related Party Transactions.”
Unless otherwise noted below, each shareholder’s address is c/o Bioceres S.A., Ocampo 210 bis, Predio CCT, Rosario, Santa Fe, Argentina.
Shares Beneficially Owned Prior to Offering | Shares Beneficially Owned After Offering (Assuming No Exercise of the Over-Allotment Option)(11) | Shares Beneficially Owned After Offering (Assuming Full Exercise of the Over-Allotment Option)(11) | ||||||||||||||||
Name of Beneficial Owner | Number | Percentage | Number | Percentage | Number | Percentage | ||||||||||||
Directors and Executive Officers | ||||||||||||||||||
Marcelo Adolfo Carrique | 288,000 | 1.12 | % | |||||||||||||||
Cintia Guillermina Castagnino(1) | 285,000 | 1.11 | % | |||||||||||||||
Manuel Alberto Sobrado | 15,000 | * | ||||||||||||||||
Aimar Dimo(2) | 772,800 | 3.01 | % | |||||||||||||||
Federico Trucco(3) | 192,000 | * | ||||||||||||||||
Ignacio Lartirigoyen(4) | 731,200 | 2.85 | % | |||||||||||||||
Carlos Popik(5) | 254,400 | * | ||||||||||||||||
Santiago Sacerdote | — | — | ||||||||||||||||
Matías Hugo Kugler | 57,600 | * | ||||||||||||||||
Victor Hugo Trucco | — | — | ||||||||||||||||
Jorge Joaquín Romagnoli(6) | 367,800 | 1.42 | % | |||||||||||||||
Guillermo Cabrini | 441,600 | 1.72 | % | |||||||||||||||
Hugo Osvaldo Ghio | 144,000 | * | ||||||||||||||||
192
Shares Beneficially Owned Prior to Offering | Shares Beneficially Owned After Offering (Assuming No Exercise of the Over-Allotment Option)(11) | Shares Beneficially Owned After Offering (Assuming Full Exercise of the Over-Allotment Option)(11) | ||||||||||||||||
Name of Beneficial Owner | Number | Percentage | Number | Percentage | Number | Percentage | ||||||||||||
Enrique López Lecoube | — | — | ||||||||||||||||
Claudio Dunan(7) | 101,800 | * | ||||||||||||||||
Gloria Montaron Estrada(8) | — | — | ||||||||||||||||
Gerónimo Watson(9) | 71,200 | * | ||||||||||||||||
Martín Vazquez(10) | 10,700 | * | ||||||||||||||||
Jorge Wagner | — | — | ||||||||||||||||
Supervisory Committee Members and Alternates | ||||||||||||||||||
Humberto Domingo Santoni | — | — | ||||||||||||||||
Alberto Antonio Romano | — | — | ||||||||||||||||
Daniel Edmundo Juan Vigna | — | — | ||||||||||||||||
Carina Mariel Foglia | — | — | ||||||||||||||||
Pedro Figueroa Casas | — | — | ||||||||||||||||
Luis Antonio Gritti | — | — | ||||||||||||||||
All directors, executive officers and members of supervisory committee as a group (24 persons) | 3,491,600 | 13.6 | % | |||||||||||||||
Principal Shareholders | ||||||||||||||||||
Villpen Corp S.A. | 1,152,000 | 4.49 | % | |||||||||||||||
Maria Raquel Bachiochi Rojas, , Maria Eugenia Bachiochi Rojas and Maria Celeste Bachiochi Rojas as co-ownership | 698,500 | 2.72 | % | |||||||||||||||
Y.P.F. S.A. | 608,900 | 2.37 | % | |||||||||||||||
Nuevo Banco de Santa Fe S.A. | 505,300 | 1.97 | % | |||||||||||||||
Lartirigoyen y CIA S.A. | 471,400 | 1.83 | % | |||||||||||||||
Guillermo Luis Cabrini | 441,600 | 1.72 | % | |||||||||||||||
Paula Elena Marra | 441,600 | 1.72 | % | |||||||||||||||
Maria Ferna Castellani | 352,100 | 1.37 | % | |||||||||||||||
Juan Diego Cabrini | 368,900 | 1.43 | % | |||||||||||||||
All other shareholders as a group (300 shareholders) | 20,972,900 | 81.78 | % | |||||||||||||||
| * | Represents beneficial ownership of less than 1%. |
| (1) | Includes 65,600 ordinary shares held by Cintia Guillermina Castagnino and includes 219,400 ordinary shares held by Roberto Atilio Peiretti (Cintia Castagnino’s spouse). |
| (2) | Includes 305,500 ordinary shares held by Aimar Dimo and includes 352,100 ordinary shares held by Maria Fernanda Castellani (Aimar Dimo’s spouse) and 115,200 ordinary shares held by Santa Angelina S.A. |
| (3) | Does not include 240,160 stock options previously authorized and granted as of April 1, 2017 at a price of US$7.91, with no expiration. |
| (4) | Includes 471,400 ordinary shares held by Lartirigoyen y CIA S.A., 202,200 ordinary shares held by Ignacio Lartirigoyen, 19,200 shares held by Ignacio Lartirigoyen (Ignacio Lartirigoyen’s son), 19,200 shares held by Marcos Lartirigoyen and 19,200 shares held by Rosario Lartirigoyen. |
| (5) | Includes 240,000 ordinary shares held by Carlos Popik and includes 14,400 ordinary shares held by Popiquen S.A. |
| (6) | Includes 183,900 shares held by Mrs. Viviana Isable Bilbao (Mr. Romagnoli’s spouse). |
| (7) | Does not include 50,560 stock options previously authorized and granted as of April 1, 2017 at a price of US$7.91, with no expiration. |
| (8) | Does not include 101,120 stock options previously authorized and granted as of April 1, 2017 at a price of US$7.91, with no expiration. |
| (9) | Does not include 120,080 stock options previously authorized and granted as of April 1, 2017 at a price of US$7.91, with no expiration. |
| (10) | Does not include 63,200 stock options previously authorized and granted as of April 1, 2017 at a price of US$7.91, with no expiration. |
| (11) | Includes shares issuable pursuant to the rights offering. |
193
Set forth below is certain information relating to our share capital, including brief summaries of certain provisions of our bylaws, Argentine Corporate Law and certain related laws and regulations of Argentina, all as in effect as at the date of this prospectus. The following summary description of our share capital does not purport to be complete and is qualified in our entirety by reference to our bylaws, Argentine Corporate Law and the provisions of other applicable Argentine laws and regulations, including the CNV and the BYMA rules.
Share Capital
We are a stock corporation (sociedad anónima) duly incorporated under the laws of Argentina on December 12, 2001 for a 50-year period and registered with the Public Registry of Commerce of the City of Rosario, Santa Fe Province on April 11, 2002, under No. 106 of Volume 83, Page 1,679 of Sociedades Anónimas.
On December 17, 2014, our shareholders approved (and on December 15, 2016, ratified) the capital increase issuing up to 24,000,000 (consisting of the Stock Split of our ordinary shares effective immediately prior to the commencement of this offering and after giving retroactive effect thereto, as resolved by our shareholders’ meeting of April 27, 2017) new ordinary book-entry shares, with a par value of Ps.1 each and the right to one vote per share, as well as the issuance of additional shares, if necessary, in the event that the international underwriters exercise their option to purchase additional ADSs.
All outstanding shares are fully paid as of the date of this prospectus. Our share capital as of September 30, 2017 consisted of Ps.25,644,300 represented by 25,644,300 ordinary, book-entry shares (reflecting the Stock Split), with a par value of Ps.1 each and the right to one vote per share. Under our bylaws, all shares will be ordinary, book-entry form, with a par value of Ps.1 per share and each entitled to one vote.
Bylaws
Corporate Purpose
Our bylaws provide that our corporate purpose is to participate in agricultural activities, such as the commercialization, production, multiplication, licensing, purchase, sale, importation, exportation and distribution of seeds, germplasm, and other resources and technologies related to bio-engineering, among others, as well as engage in any activity related to the research, development, production and administration of biotechnology and agricultural projects; to participate in industrial activities, including research, development, and production of synthetic biotechnology and metabolic engineering, as well as the selection, identification, multiplication, commercialization, import and export of these organisms and products; to provide services, such as R&D of biotechnological platforms in all of our fields of application, and other services related to the biotechnology industry; invest in national or foreign companies, private or partially state-owned; to subscribe, acquire or transfer shares, interest or securities, to form subsidiaries, merge, change of corporate form, restructuring or any kind of business combination with other companies, national or foreign; to provide guarantees to third parties; to invest in certificates of deposit in financial institutions and other financial instruments, such as private or public securities, certificates of investment in financial trusts or mutual funds; to invest in any kind of movable or real estate property, in biotechnology assets or in technologies related to food, health and/or agriculture and farming.
Shareholders’ Liability
Shareholders’ liability for the losses of a company is limited to their respective shareholding in the company. Under Argentine Corporate Law, however, shareholders who voted in favor of a resolution that is subsequently declared void by a court as contrary to Argentine law or a company’s bylaws (or regulation, if any) may be held jointly and severally liable for damages to such company, other shareholders or third parties resulting from such resolution. In addition, a shareholder who votes on a business transaction in which the shareholder’s interest conflicts with that of the company may be liable for damages under Argentine Corporate Law, but only if the transaction would not have been validly approved without such shareholder’s vote. See also “Risk Factors— Our shareholders may be subject to liability for certain votes of their securities under Argentine law.”
In addition, the shareholders are liable for damages inflicted on the company from the shareholders’ willful misconduct (dolo) or negligence (culpa). The shareholders are jointly and severally liable for any damages derived from any act of the
194
company that (a) conceals the prosecution of interests different from the interests of the company, or (b) constitute a mere resort for breaching the law, violating principles of public policy or good faith, or frustrating third parties’ rights.
Under the Argentine Bankruptcy Law No. 24,522, the bankruptcy of the company may be extended to its controlling shareholder(s) if it (a) used the company to perform acts in its own interest and in detriment of the company’s interest and disposed of the company’s assets as if they were of the controlling shareholder, all in fraud of the company’s creditors; or (b) who unlawfully diverted the company’s corporate interest subjecting it to a unified management in the interest of the controlling shareholder or its group; or (c) with respect to whom there is an indivisible confusion with the assets of the company, or a major part thereof, that impedes the clear delimitation of the assets and liabilities of each of such parties.
Voting Rights
Under our bylaws, each ordinary share entitles the holder thereof to one vote at any meeting of our shareholders. Under Argentine Corporate Law, a shareholder is required to abstain from voting on any resolution in which the holder’s direct or indirect interests conflict with, or are different from, that of the company. In the event that such shareholder votes on such resolution, and the relevant resolution would not have been approved without the shareholder’s vote, the resolution may be declared void by a court and the shareholder may be held liable for damages to the company, other shareholders and third parties. Argentine Corporate Law allows for cumulative voting to elect up to one third of vacant board positions. The remaining positions are elected using a plurality voting system.
Pursuant to Section 244 of the Argentine General Companies Law (“LGS”), all extraordinary shareholders’ meetings, whether convened on a first or second quorum call, require the affirmative vote of the majority of shares with right to vote in order to approve the following decisions: the voluntary winding up of the company in advance, transfer of the domicile of the company outside of Argentina, a fundamental change to the corporate purpose of the company, total or partial mandatory repayment by the shareholders of the paid in capital and a merger or a spin off, where we will not be the surviving entity. In such cases, the plurality of votes granted by a certain class of shares shall not be considered. Also, under Section 284 of the LGS, plurality of votes will not be applicable to the election and removal of statutory supervisors or members of the supervisory committee, provided that the LGS allows for the election of up to one third of vacant supervisory committee member positions through the cumulative voting system in terms similar to those described for the election of the members of the board of directors.
In accordance with Argentine Corporate Law, so long as we remain an entity authorized to publicly offer our shares, we cannot issue additional shares of any class of capital stock that could entitle the holder thereof to more than one vote per share. For more information regarding voting rights, see “–Shareholders’ Meetings.”
Registration Requirements of Foreign Companies Holding Ordinary Shares Directly
Under Argentine law, foreign companies that hold shares directly (and not in the form of ADSs) in an Argentine company must register with the public registry of commerce to exercise certain shareholder rights, including voting rights. In order to register with the public registry of commerce, the foreign company must: (1) file its corporate and accounting documents so as to show that it is not a special purpose vehicle organized solely to conduct business in Argentina, (2) verify that it is able to conduct business in its place of incorporation and (3) meet certain foreign asset requirements.
Redemption and Appraisal Rights
Our ordinary shares may be redeemed in connection with a reduction in capital by the vote of a majority of shareholders present at an extraordinary shareholders’ meeting. Any shares so redeemed must be canceled by us.
Whenever our shareholders approve a spin-off or merger in which it is not the surviving corporation, the dissolution prior to the expiration of the corporate term, a fundamental change in our corporate purpose, change of our domicile away from Argentina, voluntary withdrawal from public offering or delisting, our continuation in the case of mandatory delisting or cancelation of the public offering authorization, or a total or partial recapitalization following a mandatory reduction of our capital or liquidation, any shareholder that voted against such action that was approved or did not attend the meeting at which the decision was taken, may withdraw and receive the book value of our shares, determined on the basis of our latest balance sheet prepared or that should have been prepared in accordance with Argentine laws and regulations, provided that such shareholder exercises our appraisal rights within a determined period. Appraisal rights must be exercised within the five days of the extraordinary shareholders’ meeting at which the resolution was adopted, in the event that the dissenting shareholder voted against such resolution, or within 15 days of such meeting if the dissenting
195
shareholder did not attend the shareholders’ meeting and can prove that he was a shareholder on the date of such meeting. In the case of merger or spin-off, appraisal rights may not be exercised if the shares to be received as a result of such transaction are authorized for public offering or listed. Appraisal rights are extinguished if the resolution giving rise to such rights is revoked at another shareholders’ meeting held within 75 days of the meeting at which the resolution was adopted.
Payment on the appraisal rights must be made within one year of the date of the shareholders’ meeting at which the resolution was adopted, except in the case of our withdrawal, denial or voluntary retirement from the public offering regime, our delisting or any continuation of the withdrawal of the authorization to perform activities, in which case the payment period is reduced to 60 days from the date of the adjournment of the shareholders’ meeting or following the publication of the withdrawal, denial or approval of the voluntary retirement from the public offering regime.
Preemptive and Accretion Rights
Under the LGS, in the event of a capital increase, holders of existing ordinary shares of any given class have a preemptive right to subscribe for a number of shares of the same class, so that they may maintain the same proportion of shares in that class. In addition, shareholders are entitled to accretion rights which allow them to subscribe for shares that are not otherwise subscribed by other existing shareholders in proportion to the percentage of shares for which subscribing existing shareholders have exercised their preemptive rights. Shares not subscribed by the shareholders by virtue of their exercise of preemptive rights or accretion rights may be offered to third parties.
Preemptive rights and accretion rights may be waived only by each shareholder on a case-by-case basis. Additionally, the LGS permits shareholders at a special shareholders’ meeting to suspend or limit the preemptive rights relating to the issuance of new shares in specific and exceptional cases in which the interest of the company requires such action and, additionally, under the following specific conditions: (i) the suspension or limitation to exercise the preemptive rights is expressly included in the list of matters to be addressed at the shareholders’ meeting; and (ii) the shares to be issued are to be paid in-kind or in exchange for payment under preexisting obligations.
Furthermore, Article 12 of the Negotiable Obligations Law No. 23,576, as amended, permits shareholders at a special shareholders’ meeting to eliminate preemptive subscription rights for the subscription of convertible bonds under the conditions described above. According to such law, preemptive rights may also be eliminated in the event that a given company enters into an underwriting agreement with an agent for the placement of the bonds, by means of a shareholders resolution passed with an affirmative vote of at least 50% of the outstanding share capital with a right to exercise such preemptive rights, so long as votes against such resolution do not represent 5% or more of the share capital. This provision also applies to the issuance of warrants over shares of capital stock or other securities convertible into capital stock.
Holders of ADSs may be restricted in their ability to exercise preemptive rights if a registration statement under the Securities Act relating thereto has not been filed or is not effective or an exemption is not available. In addition, holders of ADSs wishing to exercise their preemptive rights in connection with our ordinary shares underlying their ADSs directly will have to request to the depositary of the ADSs the cancellation of their ADSs and the release and delivery of the underlying ordinary shares, for which purposes, holders of the ADSs will need to have a custody account with Caja de Valores, or another custody account in Argentina.
Under Section 194 of the LGS, the right to preemptive subscription must be exercised within 30 days following the announcement to the shareholders that they can exercise their rights. Such announcement must be published for a period of three days in the Official Gazette of the Republic of Argentina and in an Argentine newspaper of wide circulation. According to the LGS, companies admitted to the public offering regime may, upon authorization of an extraordinary shareholders’ meeting, reduce this period to 10 days.
In the case of companies admitted to the public offering regime, preemptive rights are exercisable during the 10 days following the last publication of notice to the shareholders in the Official Bulletin of the Province of Santa Fe, or the applicable newspaper for legal notices and an Argentine newspaper of wide circulation.
In accordance with LGS, as long as we continue to be an entity authorized to publicly offer our ordinary shares, we will not issue additional shares of any class that entitle the holder to more than one vote per share.
196
Liquidation Rights
In the case of our liquidation or dissolution, our assets will be applied to satisfy our outstanding liabilities and then proportionally distributed first among the holders of our preferred shares as per the terms of such preferred shares, if any. If any surplus remains, it will be proportionally distributed among holders of our ordinary shares.
Form and Transfer of Shares
Our current share capital is represented by book-entry shares. The registry for our shares is maintained by Caja de Valores S.A. in Argentina. Only those persons whose names appear on such share registry are recognized as owners of our ordinary shares. Transfers, encumbrances and liens on our shares must be registered in our share registry and are only enforceable against us and third parties from the moment registration takes place.
Shareholders’ Meetings
Shareholders’ meetings may be ordinary or extraordinary. We are required to convene and hold an ordinary meeting of shareholders within four months of the close of each fiscal year to consider the matters specified in the first two paragraphs of Section 234 of Argentine Corporate Law, such as the approval of our financial statements, allocation of net income for such fiscal year, approval of the reports of the board of directors and supervisory committee and election and remuneration of directors and members of the supervisory committee. Other matters which may be considered at an ordinary meeting convened and held at any time include the responsibility of directors and members of the supervisory committee, and capital increases without limit, according to our bylaws.
In addition, under the provisions of section 71 of the Argentine Capital Markets Law and due to our being a company authorized to publicly offer our shares, the ordinary shareholders’ meeting is to undertake (i) the transfer or encumbrance of all or a substantial part of our assets, other than in the ordinary course of business; and (ii) the execution of an administration or management agreement as it relates to our business and/or assets. The same applies to the approval of any other agreement pursuant to which the assets or services received by us are paid for, totally or partially, with a percentage of our income, results or profits, if such amount is substantial as it relates to our business or assets.
Extraordinary shareholders’ meetings may be convened at any time to consider matters beyond the authority of an ordinary meeting, including amendment of the bylaws; reduction and reimbursement of capital; redemption, reimbursement and amortization of shares; merger, transformation and dissolution of the company; appointment, removal and remuneration of liquidators; division; examination of accounts and any other matters related to management during the liquidation of the corporation, which may require a final approving resolution; limitation or suspension of preemptive rights pursuant to Section 197 of Argentine Corporate Law; issue of debentures and their conversion into shares; and issue of bonds.
Argentine Corporate Law provides that shareholders’ meetings may be called by our board of directors or by our supervisory committee or at the request of the holders of shares representing no less than 5% of the ordinary shares. Any meetings called at the request of shareholders must be held within 40 days after the request is made. Any shareholder may appoint any person as its duly authorized representative at a shareholders’ meeting, by granting a proxy.
Notice of shareholders’ meetings must be published for five days in the Official Bulletin of the Province of Santa Fe, in an Argentine newspaper of wide circulation and in the publications of Argentine exchanges or securities markets in which our shares are traded, at least 10 but not more than 30 days prior to the date on which the meeting is to be held. Such notice must include information regarding the type of meeting to be held, the date, time and place of such meeting, the agenda and any special requirements for the attendance of the shareholders in accordance with the bylaws. If a quorum is not available at such first call for the meeting, a notice for a second call for the meeting, which must be held within 30 days of the date on which the first meeting was called, must be published for three days, at least eight days before the date of the second call for the meeting. The above-described notices of shareholders’ meetings may be effected simultaneously for the second call for the meeting to be held on the same day as the first call whenever it is established in the bylaws, except in the case of companies offering their shares publicly, which can only call the first and second call for ordinary shareholders’ meetings simultaneously. Shareholders’ meetings may be validly held without notice if all shares of our outstanding share capital are present and resolutions are adopted by unanimous vote of such shares.
According to our bylaws, shareholders’ meetings may be conducted via electronic means.
197
Under Argentine Corporate Law and our bylaws, quorum for ordinary meetings of shareholders on first call is a majority of the shares entitled to vote, and action may be taken by the affirmative vote of an absolute majority of the shares present that are entitled to vote on such action. If a quorum is not available at the first call for the meeting, a second call for the meeting may be held at which action may be taken by the holders of an absolute majority of the shares present, regardless of the number of such shares. The quorum for an extraordinary shareholders’ meeting on first call is 60% of the shares entitled to vote, and if such quorum is not available, an extraordinary meeting following a second call may be held with the presence of any number of shares entitled to vote.
However, pursuant to Section 244 of the LGS, all shareholders’ meetings, whether convened on a first or second quorum call, require the affirmative vote of the majority of shares with right to vote in order to approve the following decisions: voluntary winding-up of the company, transfer of the domicile of the company outside of Argentina, fundamental change to the purpose of the company, total or partial mandatory repayment by the shareholders of the paid-in capital; and a merger or a spin-off, when our company will not be the surviving company. In such cases, the plurality of votes granted by a certain class of shares shall not be considered. Also, under Section 284 of the LGS, plurality of votes will not be applicable to the election of statutory supervisors or members of the supervisory committee, provided that the LGS allows for the election of up to one third of vacant supervisory committee member positions through the cumulative voting system in terms similar to those described for the election of the members of the board of directors.
Election of Directors, Quorum and Resolutions
Currently, the shareholders present at any annual ordinary meeting may determine the size of the board of directors, provided that there shall be no less than three and no more than 13. Any director so appointed will serve for up to three fiscal years and shall remain in its office until replaced.
Under our bylaws, quorum for board meetings is the majority of board members, and any action may be taken by the affirmative vote of an absolute majority of those present that are entitled to vote on such action, having the president double vote in the event of a tie.
End of Fiscal Year
Our fiscal year ends on June 30 of each year.
Share Capital of Our Subsidiaries
We own 96% of the equity interest and 98.82% of the voting rights of INDEAR.
We own 82.46% of the equity interest and 93.84% of the voting rights of Bioceres Semillas.
We own 30% of the equity interest and voting rights of INMET. In addition, through INDEAR, we indirectly own 28.8% of the equity interest and voting rights of INMET.
We, through our subsidiary INDEAR, own 33% of the equity interest and voting rights of Héritas and is entitled to acquire an additional 7% thereof.
We, through our subsidiary RASA Holdings, own 50.01% of the equity interest and voting rights of Rizobacter and are entitled to acquire an additional 9.99% thereof.
We, through our subsidiary Rizobacter, own 50% of the equity interest and voting rights of Synertech.
We own 28% of the equity interest and voting rights of Chemotecnica S.A.
We own 49.9% of the equity interest and voting rights of AGBM.
We own 50% of the equity interest and voting rights of S&W Semillas S.A.
We own 50% of the equity interest and voting rights of Semya. In addition, through RASA Holdings, we indirectly own 25% of the equity interest and voting rights of Semya, and would indirectly acquire an additional 5% in the event that our ownership of Rizobacter increases after exercise by us of our option to acquire an additional 9.99% of outstanding Rizobacter shares.
198
We own 100% of the equity interest and voting rights of Bioceres Inc. Through Bioceres Inc., we indirectly own 100% of the ordinary shares of RASA Holding, 50% of the share capital and voting rights of Verdeca, and 50% of the equity interest and voting rights of Trigall Genetics.
Mandatory Public Offers Required Pursuant to Argentine Capital Markets Law and the CNV rules
Mandatory Public Offer in the Case of Significant Acquisition of Our Capital Stock and Votes
Pursuant to the Argentine Capital Markets Law and the CNV rules, any person who directly or indirectly intends to acquire for value, whether acting individually or in conjunction with others, in a single transaction or a series of transactions over a period of 90 calendar days, a number of voting shares, stock warrants or stock options, convertible securities or other similar instruments issued by a company, which directly or indirectly give such person the right to subscribe, acquire or otherwise convert into voting shares, irrespective of how the transaction is carried out, an amount of shares which, when considered with such person’s existing interests in a company, equals a “significant interest” in the company’s voting capital stock and/or votes, must, within ten days after a firm decision to make an offer to acquire any such instruments is made, announce a public tender offer to acquire and/or swap securities in accordance with the procedure and scope established under the CNV rules. We refer to such an offer as an OPA.
According to the CNV rules, a “significant interest” means an interest equal to or greater than 35% and up to 50% of the voting capital stock and/or the votes of the company. When trying to achieve an interest equal to or greater than 35% of the voting capital stock and/or the votes of the company, provided that such acquisition implies a change of control of the company, the offer shall be made on a number of securities that would allow the purchaser to achieve, at least 50% of the voting capital stock of the company. When trying to reach a stake equal to or greater than 50% of the voting capital stock and/or the votes of the company, the offer shall be made on a number of shares that would allow the purchaser to reach 100% of the voting capital stock of the company.
The price offered in an OPA shall be determined by the offeror with the following exceptions:
| ■ | if the purchaser has purchased other securities related to the offering within the 90 days prior to the announcement of the offer, the price cannot be lower than the highest price the purchaser paid in such transactions; and |
| ■ | if the purchaser has obtained firm sale commitments from the controlling shareholder or other shareholders entitled to take part in the public offering, the price cannot be lower than the price provided for in such commitments. |
In order to determine the purchase price, the purchaser must also consider the following criteria, according to the CNV rules:
| ■ | the book value of the shares; |
| ■ | a valuation of the target company according to discounted cash flows or other applicable valuation criteria applicable to comparable business; and |
| ■ | the average price of the shares for the last six months before the “offer.” |
Based on certain interpretations of Argentine Capital Markets Law and the CNV rules, the average price of the shares for the last six months before the “offer” should be considered as a minimum price. Additionally, the price may be challenged by both the CNV and any offeree shareholder.
When the securities are acquired in breach of the obligation to make a public offer as stated herein, the CNV will declare such acquisition illegal and ineffective for administrative purposes and will carry out the auction of the shares acquired in breach, without prejudice of other applicable penalties.
Public Offers in the Case of Voluntary Withdrawal from the Public Offer and Listing System in Argentina
The Argentine Capital Markets Law and the CNV rules also provide that, when a company whose shares are publicly offered voluntarily agrees to withdraw from the public offer and listing system, the company must follow the procedures contemplated in CNV rules and must also launch a mandatory public offer to acquire the full amount of its shares and/or stock warrants or securities convertible into shares or stock options, in accordance with the provisions of the CNV rules.
199
The public offer need not be addressed to any shareholders who voted for withdrawal at the relevant shareholders’ meeting. The public offer may be made solely as a sale transaction, and payment thereunder must be made in cash.
The company’s own shares may be bought solely by using earned and net profits or freely-available cash reserves, provided that they are fully paid-up, and for the amortization or disposition thereof, within the term established in Section 221 of the LGS. The company must provide the CNV with proof of the company’s financial capacity to buy such shares as well as proof of the fact that the company’s financial soundness will not be adversely affected as a result of payment of the shares.
The price offered in the case of voluntary withdrawal from the public offer and listing system in Argentina must be fair and the following criteria must be taken into account for purposes of that fairness determination:
| ■ | the book value of the shares, taking into account a special balance sheet for withdrawal from the public offer and/or listing system; |
| ■ | the company’s value, in accordance with discounted cash flows and/or coefficients applicable to comparable companies or enterprises; |
| ■ | the company’s liquidation value; |
| ■ | average trading prices in the stock market where the shares were listed over the six months immediately preceding the request for withdrawal, irrespective of the number of sessions required for such trading; and |
| ■ | the amount of consideration previously offered, or the placement price of the new shares, if a public offer has been made in connection with the same shares or any new shares have been issued, as the case may be, in the last year before the date when an agreement is reached to submit a request for withdrawal. |
The price offered shall not be lower than the average trading price referred to above. The criteria for calculation of the price per share in the event of withdrawal from public offer and listing in Argentina are established in the Argentine Capital Markets Law and the CNV rules and may differ from the price that might arise from application of the Argentine Capital Markets Law in the event of a shareholder exercising his/her/its appraisal rights.
Mandatory or Voluntary Acquisition Public Offer in the Event of Almost Total Control (Squeeze Out)
If one person directly or indirectly owns 95% or more of the outstanding shares of a company whose shares are publicly offered in Argentina, any minority shareholder may require the controlling shareholder to launch a mandatory public offer for all the outstanding shares of the company. Conversely, a person who directly or indirectly owns 95% or more of the outstanding shares of a public company in Argentina may unilaterally make the decision to buy all of the outstanding shares of the company within six months of the date on which said person attains said 95% ownership of the company, and withdraw the company from the system for public offer and listing of shares. The price offered must be fair, in accordance with the criteria listed above and established in the Argentine Capital Markets Law and the CNV rules.
Jurisdiction and Arbitration
Pursuant to article 46 of the Argentine Capital Markets Law, companies whose shares are listed on any authorized market (including the BYMA), such as we intend our ordinary shares to be, are subject to the jurisdiction of the arbitration court of such authorized market (in this case, the Tribunal de Arbitraje General de la Bolsa de Comercio de Buenos Aires, or any successor thereof) for all matters concerning such companies’ relationship with shareholders and investors, without prejudice to the right of shareholders and investors to submit their claims (or challenge any arbitral award, as provided by Sections 758 and 760 of the Argentine Code of Civil and Commercial Procedure) to the competent courts of Argentina. In case that the applicable laws provide for the accumulation of claims related to the same subject matter, such accumulation will be subject to the jurisdiction of the judicial courts.
200
DESCRIPTION OF AMERICAN DEPOSITARY SHARES
American Depositary Shares
Deutsche Bank Trust Company Americas, as depositary, will register and deliver the ADSs. Each ADS will represent ownership of one ordinary share, deposited with the Buenos Aires, Argentina office of Deutsche Bank S.A., as custodian for the depositary. Each ADS will also represent ownership of any other securities, cash or other property which may be held by the depositary. The depositary’s corporate trust office at which the ADSs will be administered is located at 60 Wall Street, New York, NY 10005, USA. The principal executive office of the depositary is located at 60 Wall Street, New York, NY 10005, USA.
We have applied to list our ADSs in the United States on the NYSE under the symbol “BIOX.” We have applied to list our ordinary shares in Argentina on the BYMA.
The Direct Registration System, or DRS, is a system administered by The Depository Trust Company, or DTC, pursuant to which the depositary may register the ownership of uncertificated ADSs, which ownership shall be evidenced by periodic statements issued by the depositary to the ADS holders entitled thereto.
We will not treat ADS holders as our shareholders and accordingly, you, as an ADS holder, will not have shareholder rights. Argentine law governs shareholder rights. The depositary will be the holder of the ordinary shares underlying your ADSs. As a holder of ADSs, you will have ADS holder rights. A deposit agreement among us, the depositary and you, as an ADS holder, and the beneficial owners of ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. The laws of the State of New York govern the deposit agreement and the ADSs.
The following is a summary of the material provisions of the deposit agreement. For more complete information, you should read the entire deposit agreement and the form of American Depository Receipt. For directions on how to obtain copies of those documents, see “Where You Can Find Additional Information.”
Holding the ADSs
How will you hold your ADSs?
You may hold ADSs either (1) directly (a) by having an American Depository Receipt, or ADR, which is a certificate evidencing a specific number of ADSs, registered in your name, or (b) by holding ADSs in DRS, or (2) indirectly through your broker or other financial institution. If you hold ADSs directly, you are an ADS holder. This description assumes you hold your ADSs directly. ADSs will be issued directily through DRS, unless you specifically request certificated ADRs. If you hold the ADSs indirectly, you must rely on the procedures of your broker or other financial institution to assert the rights of ADS holders described in this section. You should consult with your broker or financial institution to find out what those procedures are.
Dividends and Other Distributions
How will you receive dividends and other distributions on the shares?
The depositary has agreed to pay to you the cash dividends or other distributions it or the custodian receives on ordinary shares or other deposited securities, after deducting its fees and expenses. You will receive these distributions in proportion to the number of ordinary shares your ADSs represent as of the record date, which will be as close as practicable to the record date for our ordinary shares, set by the depositary with respect to the ADSs.
| ■ | Cash. The depositary will convert or cause to be converted any cash dividend or other cash distribution we pay on the ordinary shares or any net proceeds from the sale of any ordinary shares, rights, securities or other entitlements under the terms of the deposit agreement into U.S. dollars if it can do so on a practicable basis, and can transfer the U.S. dollars to the United States and will distribute promptly the amount thus received. If the depositary shall determine in its judgment that such conversions or transfers are not practical or lawful or if any government approval or license is needed and cannot be obtained at a reasonable cost within a reasonable period or otherwise sought, the deposit agreement allows the depositary to distribute the foreign currency only to |
201
those ADS holders to whom it is possible to do so. It will hold or cause the custodian to hold the foreign currency it cannot convert for the account of the ADS holders who have not been paid and such funds will be held for the respective accounts of the ADS holders. It will not invest the foreign currency and it will not be liable for any interest for the respective accounts of the ADS holders.
| Before making a distribution, any taxes or other governmental charges, together with fees and expenses of the depositary, that must be paid, will be deducted. See “Taxation.” It will distribute only whole U.S. dollars and cents and will round down fractional cents to the nearest whole cent. If the exchange rates fluctuate during a time when the depositary cannot convert the foreign currency, you may lose some or all of the value of the distribution. |
| ■ | Shares. For any ordinary shares we distribute as a dividend or free distribution, either (1) the depositary will distribute additional ADSs representing such ordinary shares or (2) existing ADSs as of the applicable record date will represent rights and interests in the additional ordinary shares distributed, to the extent reasonably practicable and permissible under law, in either case, net of applicable fees, charges and expenses incurred by the depositary and taxes and/or other governmental charges. The depositary will only distribute whole ADSs. It will try to sell ordinary shares which would require it to deliver a fractional ADS and distribute the net proceeds in the same way as it does with cash. The depositary may sell a portion of the distributed ordinary shares sufficient to pay its fees and expenses, and any taxes and government charges, in connection with that distribution. |
| ■ | Elective Distributions in Cash or Shares. If we offer holders of our ordinary shares the option to receive dividends in either cash or shares, the depositary, after consultation with us and having received timely notice as described in the deposit agreement of such elective distribution by us, has discretion to determine to what extent such elective distribution will be made available to you as a holder of the ADSs. We must timely first instruct the depositary to make such elective distribution available to you and furnish it with satisfactory evidence that it is legal to do so. The depositary could decide it is not legal or reasonably practicable to make such elective distribution available to you. In such case, the depositary shall, on the basis of the same determination as is made in respect of the ordinary shares for which no election is made, distribute either cash in the same way as it does in a cash distribution, or additional ADSs representing ordinary shares in the same way as it does in a share distribution. The depositary is not obligated to make available to you a method to receive the elective dividend in shares rather than in ADSs. There can be no assurance that you will be given the opportunity to receive elective distributions on the same terms and conditions as the holders of ordinary shares. |
| ■ | Rights to Purchase Additional Shares. If we offer holders of our ordinary shares any rights to subscribe for additional shares, the depositary shall having received timely notice as described in the deposit agreement of such distribution by us, consult with us, and we must determine whether it is lawful and reasonably practicable to make these rights available to you. We must first instruct the depositary to make such rights available to you and furnish the depositary with satisfactory evidence that it is legal to do so. If the depositary decides it is not legal or reasonably practicable to make the rights available but that it is lawful and reasonably practicable to sell the rights, the depositary will endeavor to sell the rights and in a riskless principal capacity or otherwise, at such place and upon such terms (including public or private sale) as it may deem proper distribute the net proceeds in the same way as it does with cash. The depositary will allow rights that are not distributed or sold to lapse. In that case, you will receive no value for them. If the depositary makes rights available to you, it will establish procedures to distribute such rights and enable you to exercise the rights upon your payment of applicable fees, charges and expenses incurred by the depositary and taxes and/or other governmental charges. The Depositary shall not be obliged to make available to you a method to exercise such rights to subscribe for ordinary shares (rather than ADSs). U.S. securities laws may restrict transfers and cancellation of the ADSs represented by shares purchased upon exercise of rights. For example, you may not be able to trade these ADSs freely in the United States. There can be no assurance that you will be given the opportunity to exercise rights on the same terms and conditions as the holders of ordinary shares or be able to exercise such rights. |
202
| ■ | Other Distributions. Subject to receipt of timely notice, as described in the deposit agreement, from us with the request to make any such distribution available to you, and provided the depositary has determined such distribution is lawful and reasonably practicable and feasible and in accordance with the terms of the deposit agreement, the depositary will distribute to you anything else we distribute on deposited securities by any means it may deem practicable, upon your payment of applicable fees, charges and expenses incurred by the depositary and taxes and/or other governmental charges. If any of the conditions above are not met, the depositary will endeavor to sell, or cause to be sold, what we distributed and distribute the net proceeds in the same way as it does with cash; or, if it is unable to sell such property, the depositary may dispose of such property in any way it deems reasonably practicable under the circumstances for nominal or no consideration, such that you may have no rights to or arising from such property. |
The depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any ADS holders. We have no obligation to register ADSs, shares, rights or other securities under the Securities Act. We also have no obligation to take any other action to permit the distribution of ADSs, shares, rights or anything else to ADS holders. This means that you may not receive the distributions we make on our shares or any value for them if we and/or the depositary determines that it is illegal or not practicable for us or the depositary to make them available to you.
Deposit, Withdrawal and Cancellation
How are ADSs issued?
The depositary will deliver ADSs if you or your broker deposit ordinary shares or evidence of rights to receive ordinary shares with the custodian. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will register the appropriate number of ADSs in the names you request and will deliver the ADSs to or upon the order of the person or persons entitled thereto.
How do ADS holders cancel an American Depositary Share?
You may turn in your ADSs at the depositary’s principal office or by providing appropriate instructions to your broker. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will deliver the ordinary shares and any other deposited securities underlying the ADSs to you or a person you designate at the office of the custodian. Or, at your request, risk and expense, the depository will deliver the deposited securities at its principal offices, to the extent permitted by law.
How do ADS holders interchange between Certificated ADSs and Uncertificated ADSs?
You may surrender your ADR to the depositary for the purpose of exchanging your ADR for uncertificated ADSs. The depositary will cancel that ADR and will send you a statement confirming that you are the owner of uncertificated ADSs. Alternatively, upon receipt by the depositary of a proper instruction from a holder of uncertificated ADSs requesting the exchange of uncertificated ADSs for certificated ADSs, the depositary will execute and deliver to you an ADR evidencing those ADSs.
Voting Rights
How do you vote?
You may instruct the depositary to vote the ordinary shares or other deposited securities underlying your ADSs at any meeting at which you are entitled to vote pursuant to any applicable law, the provisions of the deposit agreement, the provisions of our constituent documents, and the provisions of or governing the deposited securities. Otherwise, you could exercise your right to vote directly if you withdraw the ordinary shares. However, you may not know about the meeting sufficiently enough in advance to withdraw the ordinary shares.
If we ask for your instructions and upon timely notice from us by regular, ordinary mail delivery, or by electronic transmission, as described in the deposit agreement, the depositary will notify you of the upcoming meeting at which you are entitled to vote pursuant to any applicable law, the provisions of the deposit agreement, the provisions of our constituent documents, and the provisions of or governing the deposited securities, and arrange to deliver our voting materials to you. The materials will include or reproduce (a) such notice of meeting or solicitation of consents or proxies; (b) a statement that the ADS holders at the close of business on the ADS record date will be entitled, subject to any applicable law, the provisions of the deposit agreement, the provisions of our constituent documents, and the provisions of or governing the deposited securities, to instruct the depositary as to the exercise of the voting rights, if any, pertaining
203
to the ordinary shares or other deposited securities represented by such holder’s ADSs; and (c) a brief statement as to the manner in which such instructions may be given. Voting instructions may be given only in respect of a number of ADSs representing an integral number of ordinary shares or other deposited securities. For instructions to be valid, the depositary must receive them in writing on or before the date specified. The depositary will try, as far as practicable, subject to applicable law, the provisions of the deposit agreement, the provisions of our constituent documents, and the provisions of or governing the deposited securities, to vote or to have its agents vote the ordinary shares or other deposited securities (in person or by proxy) as you instruct. The depositary will only vote or attempt to vote as you instruct. If we timely request the depositary to solicit your instructions but no instructions are received by the depositary from an owner with respect to any of the ordinary shares or deposited securities represented by the ADSs of that owner on or before the date established by the depositary for such purpose, such ordinary shares or deposited securities shall not be voted.
We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote the ordinary shares underlying your ADSs. In addition, there can be no assurance that ADS holders and beneficial owners generally, or any holder or beneficial owner in particular, will be given the opportunity to vote or cause the custodian to vote on the same terms and conditions as the holders of our ordinary shares.
The depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that you may not be able to exercise your right to vote and you may have no recourse if the ordinary shares underlying your ADSs are not voted as you requested.
In order to give you a reasonable opportunity to instruct the depositary as to the exercise of voting rights relating to deposited securities, if we request the depositary to act, we will give the depositary notice of any such meeting and details concerning the matters to be voted at least 30 days in advance of the meeting date.
Compliance with Regulations
Information Requests
Each ADS holder and beneficial owner shall (a) provide such information as we or the depositary may request pursuant to law, including, without limitation, relevant Argentine law, regulations issued by the CNV, any applicable law of the United States of America, our constituent documents, any resolutions of our board of directors adopted pursuant to such constituent documents, the requirements of any markets or exchanges upon which the ordinary shares, ADSs or ADRs are listed or traded, or to any requirements of any electronic book-entry system by which the ADSs or ADRs may be transferred, regarding the capacity in which they own or owned ADRs, the identity of any other persons then or previously interested in such ADRs and the nature of such interest, and any other applicable matters, (b) be bound by and subject to applicable provisions of the laws of Argentina, regulations issued by the CNV, our constituent documents, and the requirements of any markets or exchanges upon which the ADSs, ADRs or ordinary shares are listed or traded, or pursuant to any requirements of any electronic book-entry system by which the ADSs, ADRs or ordinary shares may be transferred, to the same extent as if such ADS holder or beneficial owner held ordinary shares directly, in each case irrespective of whether or not they are ADS holders or beneficial owners at the time such request is made.
Compliance with Argentine Law
ADS holders and beneficial owners shall comply with the obligations that shareholders have in Argentina including, without limitation, certain shareholding disclosure requirements included in the regulations issued by the CNV. Applicable laws and regulations may require ADS holders and beneficial owners to satisfy reporting requirements and obtain regulatory approvals in certain circumstances. ADS holders and beneficial owners are solely responsible for determining and complying with such reporting requirements and obtaining such approvals. ADS holders and beneficial owners shall provide us information related to their shareholdings which, we may request from time to time under Argentine laws, including, without limitation, the regulations issued by the CNV.
Ownership Restrictions
ADS holders and beneficial owners shall comply with any limitations on ownership of ordinary shares under our constituent documents or applicable Argentine law as if they held the number of shares their ADSs represent. We shall inform ADS holders, beneficial owners and the depositary of any such ownership restrictions in place from time to time.
204
Fees and Expenses
As an ADS holder, you will be required to pay the following service fees to the depositary bank and certain taxes and governmental charges (in addition to any applicable fees, expenses, taxes and other governmental charges payable on the deposited securities represented by any of your ADSs):
Service | Fees |
To any person to which ADSs are issued or to any person to which a distribution is made in respect of ADS distributions pursuant to share dividends or other free distributions of shares, bonus distributions, share splits or other distributions (except where converted to cash) | Up to US$0.05 per ADS issued |
Cancellation of ADSs, including the case of termination of the deposit agreement | Up to US$0.05 per ADS cancelled |
Distribution of cash dividends | Up to US$0.05 per ADS held |
Distribution of cash entitlements (other than cash dividends) and/or cash proceeds from the sale of rights, securities and other entitlements | Up to US$0.05 per ADS held |
Distribution of ADSs pursuant to exercise of rights | Up to US$0.05 per ADS held |
Distribution of securities other than ADSs or rights to purchase additional ADSs | Up to US$0.05 per ADS held |
Annual fee for depositary services | Up to US$0.05 per ADS held on the applicable record date(s) established by the depositary bank |
As an ADS holder, you will also be responsible to pay certain fees and expenses incurred by the depositary bank and certain taxes and governmental charges (in addition to any applicable fees, expenses, taxes and other governmental charges payable on the deposited securities represented by any of your ADSs) such as:
| ■ | Fees for the transfer and registration of ordinary shares charged by the registrar and transfer agent for the ordinary shares in Argentina (i.e., upon deposit and withdrawal of ordinary shares). |
| ■ | Expenses incurred for converting foreign currency into U.S. dollars. |
| ■ | Expenses for cable, telex and fax transmissions and for delivery of securities. |
| ■ | Taxes and duties upon the transfer of securities, including any applicable stamp duties, any stock transfer charges or withholding taxes (i.e., when ordinary shares are deposited or withdrawn from deposit). |
| ■ | Fees and expenses incurred in connection with the delivery or servicing of ordinary shares on deposit. |
| ■ | Fees and expenses incurred in connection with complying with exchange control regulations and other regulatory requirements applicable to ordinary shares, deposited securities, ADSs and ADRs. |
| ■ | Any applicable fees and penalties thereon. |
The depositary fees payable upon the issuance and cancellation of ADSs are typically paid to the depositary bank by the brokers (on behalf of their clients) receiving the newly issued ADSs from the depositary bank and by the brokers (on behalf of their clients) delivering the ADSs to the depositary bank for cancellation. The brokers in turn charge these fees to their clients. Depositary fees payable in connection with distributions of cash or securities to ADS holders and the depositary services fee are charged by the depositary bank to the holders of record of ADSs as of the applicable ADS record date.
The depositary fees payable for cash distributions are generally deducted from the cash being distributed or by selling a portion of distributable property to pay the fees. In the case of distributions other than cash (i.e., share dividends, rights), the depositary bank charges the applicable fee to the ADS record date holders concurrent with the distribution. In the case of ADSs registered in the name of the investor (whether certificated or uncertificated in direct registration), the
205
depositary bank sends invoices to the applicable record date ADS holders. In the case of ADSs held in brokerage and custodian accounts (via DTC), the depositary bank generally collects its fees through the systems provided by DTC (whose nominee is the registered holder of the ADSs held in DTC) from the brokers and custodians holding ADSs in their DTC accounts. The brokers and custodians who hold their clients’ ADSs in DTC accounts in turn charge their clients’ accounts the amount of the fees paid to the depositary banks.
In the event of refusal to pay the depositary fees, the depositary bank may, under the terms of the deposit agreement, refuse the requested service until payment is received or may set off the amount of the depositary fees from any distribution to be made to the ADS holder.
The depositary may make payments to us or reimburse us for certain costs and expenses, by making available a portion of ADS fees collected in respect of the ADR program or otherwise, upon such terms and conditions as we and the depositary agree from time to time.
Payment of Taxes
You will be responsible for any taxes or other governmental charges payable, or which become payable, on your ADSs or on the deposited securities represented by any of your ADSs. The depositary may refuse to register or transfer your ADSs or allow you to withdraw the deposited securities represented by your ADSs until such taxes or other charges are paid. It may apply payments owed to you or sell deposited securities represented by your ADSs to pay any taxes owed and you will remain liable for any deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number of ADSs to reflect the sale and pay to you any net proceeds, or send to you any property, remaining after it has paid the taxes. You agree to indemnify us, the depositary, the custodian and each of our and their respective agents, directors, employees and affiliates for, and hold each of them harmless from, any claims with respect to taxes (including applicable interest and penalties thereon) arising from any refund of taxes, reduced rate of withholding at source or other tax benefit obtained for you. You may be required from time to time, and in a timely manner, to file such proof of taxpayer status, residence and beneficial ownership (as applicable), to execute such certificates and to make such representations and warranties, or to provide any other information or documents, as the depositary may deem necessary or proper to fulfill its obligations under applicable law. Your obligations under this paragraph shall survive any transfer of ADRs, any surrender of ADRs and withdrawal of deposited securities or the termination of the deposit agreement.
Reclassifications, Recapitalizations and Mergers
If we: | Then: |
Change the nominal or par value of our ordinary shares | The cash, shares or other securities received by the depositary will become deposited securities. |
Reclassify, split up or consolidate any of the deposited securities | Each ADS will automatically represent its equal share of the new deposited securities. |
Distribute securities on the ordinary shares that are not distributed to you, or recapitalize, reorganize, merge, liquidate, sell all or substantially all of our assets, or take any similar action | The depositary may distribute some or all of the cash, shares or other securities it received. It may also deliver new ADSs or ask you to surrender your outstanding ADRs in exchange for new ADRs identifying the new deposited securities. |
206
Amendment and Termination
How may the deposit agreement be amended?
We may agree with the depositary to amend the deposit agreement and the form of ADR without your consent for any reason. If an amendment adds or increases fees or charges, except for taxes and other governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery charges or similar items, including expenses incurred in connection with foreign exchange control regulations and other charges specifically payable by ADS holders under the deposit agreement, or materially prejudices a substantial existing right of ADS holders, it will not become effective for outstanding ADSs until 30 days after the depositary notifies ADS holders of the amendment. At the time an amendment becomes effective, you are considered, by continuing to hold your ADSs, to agree to the amendment and to be bound by the ADRs and the deposit agreement as amended. If any new laws are adopted which would require the deposit agreement to be amended in order to comply therewith, we and the depositary may amend the deposit agreement in accordance with such laws, and such amendment may become effective before notice thereof is given to ADS holders.
How may the deposit agreement be terminated?
The depositary will terminate the deposit agreement if we ask it to do so, in which case the depositary will give notice to you at least 90 days prior to termination. The depositary may also terminate the deposit agreement if the depositary has told us that it would like to resign, or if we have removed the depositary, and in either case we have not appointed a new depositary within 90 days. In either such case, the depositary must notify you at least 30 days before termination.
After termination, the depositary and its agents will do the following under the deposit agreement but nothing else: collect distributions on the deposited securities, sell rights and other property and deliver ordinary shares and other deposited securities upon cancellation of ADSs after payment of any fees, charges, taxes or other governmental charges. Six months or more after the date of termination, the depositary may sell any remaining deposited securities by public or private sale. After that, the depositary will hold the money it received on the sale, as well as any other cash it is holding under the deposit agreement, for the pro rata benefit of the ADS holders that have not surrendered their ADSs. It will not invest the money and has no liability for interest. After such sale, the depositary’s only obligations will be to account for the money and other cash. After termination, we shall be discharged from all obligations under the deposit agreement except for our obligations to the depositary thereunder.
Books of Depositary
The depositary will maintain ADS holder records at its principal office. You may inspect such records at such office during regular business hours but solely for the purpose of communicating with other holders in the interest of business matters relating to our company, the ADRs and the deposit agreement.
The depositary will maintain facilities in the Borough of Manhattan, The City of New York to record and process the issuance, cancellation, combination, split-up and transfer of ADRs.
These facilities may be closed at any time or from time to time when such action is deemed necessary or advisable by the depositary in connection with the performance of its duties under the deposit agreement.
Limitations on Obligations and Liability
Limits on our Obligations and the Obligations of the Depositary and the Custodian; Limits on Liability to Holders of ADSs
The deposit agreement expressly limits our obligations and the obligations of the depositary and the custodian. It also limits our liability and the liability of the depositary. The depositary and the custodian:
| ■ | are only obligated to take the actions specifically set forth in the deposit agreement without gross negligence or willful misconduct; |
| ■ | are not liable if any of us or our respective controlling persons or agents are prevented or forbidden from, or subjected to any civil or criminal penalty or restraint on account of, or delayed in, doing or performing any act or thing required by the terms of the deposit agreement and any ADR, by reason of any provision of any present or future law or regulation of the United States or any state thereof, Argentina or any other country, or of any other governmental authority or regulatory authority or stock exchange, or on account of the possible criminal or civil penalties or restraint, or by reason of any provision, present or future, of our constituent documents or any |
207
provision of or governing any deposited securities, or by reason of any act of God or war or other circumstances beyond its control (including, without limitation, nationalization, expropriation, currency restrictions, work stoppage, strikes, civil unrest, revolutions, rebellions, explosions and computer failure);
| ■ | are not liable by reason of any exercise of, or failure to exercise, any discretion provided for in the deposit agreement or in our constituent documents or provisions of or governing deposited securities; |
| ■ | are not liable for any action or inaction of the depositary, the custodian or us or their or our respective controlling persons or agents in reliance upon the advice of or information from legal counsel, any person presenting ordinary shares for deposit or any other person believed by it in good faith to be competent to give such advice or information including, without limitation, in determining if a proposed distribution, action or transaction is lawful; |
| ■ | are not liable for the inability of any holder of ADSs to benefit from any distribution on deposited securities that is not made available to holders of ADSs under the terms of the deposit agreement; |
| ■ | are not liable for any indirect, special, consequential or punitive damages for any breach of the terms of the deposit agreement, or otherwise; |
| ■ | may rely upon any documents we believe in good faith to be genuine and to have been signed or presented by the proper party; |
| ■ | disclaim any liability for any action or inaction or inaction of any of us or our respective controlling persons or agents in reliance upon the advice of or information from legal counsel, accountants, any person presenting ordinary shares for deposit, holders and beneficial owners (or authorized representatives) of ADSs, or any person believed in good faith to be competent to give such advice or information; |
| ■ | disclaim any liability for inability of any holder to benefit from any distribution, offering, right or other benefit made available to holders of deposited securities but not made available to holders of ADS; and |
| ■ | are not liable for the price received in connection with any sale of securities, the timing thereof or any delay in action or omission to act nor shall either of them be responsible for any error or delay in action, omission to act, default or negligence on the part of any party retained in connection with any such sale or proposed sale. |
The depositary and any of its agents also disclaim any liability (i) for any failure to carry out any instructions to vote, the manner in which any vote is cast or the effect of any vote or failure to determine that any distribution or action may be lawful or reasonably practicable or for allowing any rights to lapse in accordance with the provisions of the deposit agreement, (ii) the failure or timeliness of any notice from us, the content of any information submitted to it by us for distribution to you or for any inaccuracy of any translation thereof, (iii) any investment risk associated with the acquisition of an interest in the deposited securities, the validity or worth of the deposited securities, the credit-worthiness of any third party, (iv) for any tax consequences that may result from ownership of ADSs, ordinary shares or deposited securities, or (vi) for any acts or omissions made by a successor depositary whether in connection with a previous act or omission of the depositary or in connection with any matter arising wholly after the removal or resignation of the depositary, provided that in connection with the issue out of which such potential liability arises the depositary performed its obligations without gross negligence or willful misconduct while it acted as depositary.
In addition, the deposit agreement provides that each party to the deposit agreement (including each holder, beneficial owner and holder of interests in the ADRs) irrevocably waives, to the fullest extent permitted by applicable law, any right it may have to a trial by jury in any lawsuit or proceeding against the depositary or our company related to our shares, the ADSs or the deposit agreement.
In the deposit agreement, we agree to indemnify the depositary under certain circumstances.
208
Requirements for Depositary Actions
Before the depositary will issue, deliver or register a transfer of an ADS, split-up, subdivide or combine ADSs, make a distribution on an ADS, or permit withdrawal of ordinary shares, the depositary may require:
| ■ | payment of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for the transfer of any ordinary shares or other deposited securities and payment of the applicable fees, expenses and charges of the depositary; |
| ■ | satisfactory proof of the identity and genuineness of any signature or any other matters contemplated in the deposit agreement; and |
| ■ | compliance with (A) any laws or governmental regulations relating to the execution and delivery of ADRs or ADSs or to the withdrawal or delivery of deposited securities and (B) such reasonable regulations and procedures as the depositary may establish, from time to time, consistent with the deposit agreement and applicable laws, including presentation of transfer documents. |
The depositary may refuse to issue and deliver ADSs or register transfers of ADSs generally when the register of the depositary or our transfer books are closed or at any time if the depositary or we determine that it is necessary or advisable to do so.
Your Right to Receive the Shares Underlying Your ADSs
You have the right to cancel your ADSs and withdraw the underlying ordinary shares at any time except:
| ■ | when temporary delays arise because: (1) the depositary has closed its transfer books or we have closed our transfer books; (2) the transfer of ordinary shares is blocked to permit voting at a shareholders’ meeting; or (3) we are paying a dividend on our ordinary shares; |
| ■ | when you owe money to pay fees, taxes and similar charges; |
| ■ | when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of ordinary shares or other deposited securities, or |
| ■ | other circumstances specifically contemplated by Section I.A.(l) of the General Instructions to Form F-6 (as such General Instructions may be amended from time to time); or |
| ■ | for any other reason if the depositary or we determine, in good faith, that it is necessary or advisable to prohibit withdrawals. |
The depositary shall not knowingly accept for deposit under the deposit agreement any ordinary shares or other deposited securities required to be registered under the provisions of the Securities Act, unless a registration statement is in effect as to such ordinary shares.
This right of withdrawal may not be limited by any other provision of the deposit agreement.
Direct Registration System
In the deposit agreement, all parties to the deposit agreement acknowledge that the DRS and Profile Modification System, or Profile, will apply to uncertificated ADSs upon acceptance thereof to DRS by DTC. DRS is the system administered by DTC pursuant to which the depositary may register the ownership of uncertificated ADSs, which ownership shall be evidenced by periodic statements issued by the depositary to the ADS holders entitled thereto. Profile is a required feature of DRS which allows a DTC participant, claiming to act on behalf of an ADS holder, to direct the depositary to register a transfer of those ADSs to DTC or its nominee and to deliver those ADSs to the DTC account of that DTC participant without receipt by the depositary of prior authorization from the ADS holder to register such transfer.
209
SHARES ELIGIBLE FOR FUTURE SALE
Future sales of substantial amounts of our shares in the public market, or the perception that such sales may occur, could adversely affect the prevailing market price of our shares. No prediction can be made as to the effect, if any, future sales of shares, or the availability of our shares for future sales, will have on the market price of our shares prevailing from time to time. The number of shares available for future sale in the public market is subject to legal and contractual restrictions, some of which are described below. The expiration of these restrictions will permit sales of substantial amounts of our shares in the public market, or could create the perception that these sales may occur, which could adversely affect the prevailing market price of our shares. These factors also could make it more difficult for us to raise funds through future offerings of our shares.
Eligibility of Restricted Shares for Sale in the U.S. Public Market
As a result of the lock-up agreements described below, the following indicates approximately when the ordinary shares that are not being sold in this offering, but which will be outstanding at the time this offering is complete, will be eligible for sale into the U.S. public market, under the provisions of Rule 144:
| ■ | on the date of this prospectus, shares, or % of our outstanding shares before this offering, will be eligible for resale, none of which are subject to volume, manner of sale and other limitations under Rule 144; and |
| ■ | 180 days after the date of this prospectus, the remaining shares will be eligible for resale, certain of which shares held by our affiliates, such as our officers and directors, will be subject to volume, manner of sale and other limitations under Rule 144. |
Lock-Up Agreements
We, our officers, directors and substantially all of our shareholders have agreed, subject to specified exceptions, not to directly or indirectly: offer to sell, pledge, announce the intention to sell, sell, contract to sell or lend, hypothecate or grant any security interest in, establish or sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, make any short sale, increase a put equivalent position, liquidate or decrease any call equivalent position or in any other way transfer or dispose of any ordinary shares or ADSs or any options or warrants or other rights to acquire ADSs or ordinary shares or any securities convertible into, exercisable or exchangeable for ordinary shares or ADSs whether now owned or hereafter acquired; enter into any swap, hedge or other arrangement or agreement that transfers, in whole or in part, any of the economic risk of ownership of the ordinary shares, ADSs or any securities convertible into, exercisable or exchangeable for ordinary shares or ADSs; make any demand for, or exercise any right with respect to, the registration of any ordinary shares or ADSs or any security convertible into or exercisable or exchangeable for ordinary shares or ADSs or cause to be filed a registration statement, prospectus or prospectus supplement (or an amendment or supplement thereto) with respect to any such registration; or file (or participate in the filing of) a registration statement or publicly disclose the intention to do any of the foregoing for a period of 180 days commencing on the date of this prospectus, without the prior written consent of the representatives of the international underwriters, which may withhold their consent in their sole discretion. This restriction terminates after the close of trading of the ADSs and including the 180th day after the date of this prospectus.
Rule 144
In general, under Rule 144 of the Securities Act, a person (or persons whose shares are aggregated) who is not deemed to have been an affiliate of ours at any time during the three months preceding a sale, and who has beneficially owned restricted securities within the meaning of Rule 144 for at least six months (including any period of consecutive ownership of preceding non-affiliated holders) would be entitled to sell those shares, subject only to the availability of current public information about us. A non-affiliated person who has beneficially owned restricted securities within the meaning of Rule 144 for at least one year would be entitled to sell those shares without regard to the provisions of Rule 144.
210
A person (or persons whose shares are aggregated) who is deemed to be an affiliate of ours and who has beneficially owned restricted securities within the meaning of Rule 144 for at least six months would be entitled to sell within any three-month period a number of shares that does not exceed the greater of 1% of the then outstanding shares of our ordinary shares or the average weekly trading volume of our ordinary shares, as represented by ADS, on NYSE during the four calendar weeks preceding such sale. Such sales are also subject to certain manner of sale provisions, notice requirements and the availability of current public information about us.
Equity Incentive Plans
See “Management—Equity Incentive Plans.”
211
The following description is not intended to constitute a complete analysis of all tax consequences relating to the acquisition, ownership and disposition of our ordinary shares. You are urged to consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences of the purchase, ownership and disposition of our ordinary shares that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
Material Argentine Tax Considerations
The following discussion contains a description of material Argentine tax matters based upon the tax laws of Argentina and regulations thereunder as of the date of this prospectus, and is subject to any subsequent change in Argentine laws and regulations which may come into effect after such date. This section is the opinion of the law firm Marval, O’Farrell & Mairal, insofar as it relates to matters of Argentine tax law, of the material Argentine tax considerations relating to the purchase, ownership and disposition of our ordinary shares or ADSs. This opinion summary does not purport to be a comprehensive description of all of the tax considerations that may be relevant to a holder of such securities. No assurance can be given that the courts or tax authorities responsible for the administration of the laws and regulations described in this prospectus will agree with this interpretation. Holders should carefully read “Risk Factors—Risks Related to Our ADSs and the Offering—Changes in the Argentine tax laws may adversely affect the tax treatment of our ordinary shares or ADSs” and consult their tax advisors regarding the tax treatment of our ordinary shares and ADSs. This discussion is also based upon the representations of the depositary and on the assumption that each obligation in the deposit agreement among us, Deutsche Bank Trust Company Americas, as depositary and the registered holders and beneficial owners of the ADSs, and any related documents, will be performed in accordance with its terms.
Taxation on dividends
As a general rule, dividends are exempt from income tax in respect of both Argentine and non-Argentine resident shareholders. However, dividends paid in excess of the Taxable Accumulated Income, as defined below, will be subject to an equalization tax at the rate of 35%, or the Equalization Tax, applicable on such excess and regarding both resident and non-resident in Argentina shareholders. This withholding rate might be lower if the holder of our ordinary shares is resident of a country which signed a treaty to avoid double taxation with Argentina, and meets all the substantial and formal requirements for such treaty to apply. Equalization Tax is applicable when dividends distributed are greater than the income determined according to the application of the Argentine Income Tax Law, accumulated at the fiscal year immediately preceding the year on which the distribution is made, referred to as “Taxable Accumulated Income.”
The Equalization Tax will be imposed as a withholding tax on the shareholder receiving the dividend. Dividend distribution made in property (other than cash) will be subject to the same tax rules as cash dividends. Stock dividends on fully paid shares (“acciones liberadas”) are not subject to Equalization Tax.
Capital gains
Gains derived from the transfer of shares, quotas and other equity interests, titles, bonds and other securities of Argentine companies are subject to Argentine income tax, regardless of the type of beneficiary who obtains the income.
Capital gains realized by Argentine corporate entities (in general, entities organized or incorporated under Argentine law, certain traders and intermediaries, local branches of non-Argentine entities, sole proprietorships and individuals carrying on certain commercial activities in Argentina), or the Argentine Entities, derived from the sale, exchange or other disposition of shares are subject to income tax at the rate of 35% on net profit. Losses from a previous fiscal year as a result of the disposition of shares can only be applied and compensated against net gains resulting from the same kind of transaction, and these losses can be carried forward for five fiscal years.
Capital gains realized by individuals residing in Argentina from the sale of shares and other securities is subject to income tax at a 15% rate on the capital gain, unless such securities were traded in stock markets and/or have public offering authorization, in which case an exemption applies. The implementing Decree 2334/2013 introduced a provision stating that the exemption only includes income derived from the sale of shares and other securities made through a stock exchange market duly authorized by the CNV.
It is not clear whether the term “includes” (as used in the implementing Decree 2334/2013) means that the exemption only refers to sales of securities made through a stock exchange market duly authorized by the CNV or whether the
212
implementing Decree 2334/2013 intended to clarify that such sales were just one of the possibilities that may be covered by the exemption (in addition to publicly offering authorized securities, as provided in the Argentine Income Tax Law). Certain qualified tax scholars have publicly opined that the exemption exclusively refers to sales of securities made through a stock exchange market duly authorized by the CNV.
Capital gains recognized by individuals or entities that are not residents of Argentina from the sale, exchange or other disposition of shares would be subject to capital gains tax, as the abovementioned exemption for shares is not applicable to non-resident beneficiaries. Therefore, the gain derived from the disposition of shares by non-residents is subject to Argentine income tax through an Income Tax Withholding to be performed by the buyer of the shares or securities at a rate of 15% either (i) on the net amount resulting from deducting from the sale price of the shares, the acquisition cost and the expenses incurred in Argentina necessary for obtaining, maintaining and conserving this asset, as well as the deductions admitted by the Argentine Income Tax Law or (ii) on the net presumed income provided by the Argentine Income Tax Law for this type of transaction (i.e., 90%), which results in an effective rate of 13.5% of the sales price.
Following the amendments made by Law No. 26,893, and its implementing Decree 2334/13, the tax treatment applicable to income obtained by non-Argentine resident beneficiaries from the sale of ADSs is not clearly established under the amended Argentine Income Tax Law. As of the date of this prospectus, there are no administrative or judicial decisions clarifying the treatment regarding the source of income originated in the sale of ADS. The possibly varying treatment of source of income could impact both Argentine resident holders as well as non-Argentine resident holders, therefore holders are encouraged to consult a tax advisor as to the particular Argentine income tax consequences derived from holding and disposing the ADSs.
In addition, in case the sale of the ordinary shares or the ADSs involves a non-Argentine resident, provided the sale of ADSs be deemed to give rise to Argentine source income as it was previously considered, please note that after almost four years without regulation regarding the mechanism for the payment of the income tax when the sale involves only non-resident parties in Argentina, on July 18, 2017, through General Resolution No. 4094-E, the AFIP regulated the mechanism for the payment of the capital gains tax. The aforementioned Resolution provides that: (i) in case the securities are sold by a non-Argentine resident through an Argentine stock exchange market, the broker of the non-Argentine resident seller would have to act as withholding agent of the income tax; (ii) in case the securities were sold by a non-Argentine resident, but not through an Argentine stock exchange market and there is an Argentine buyer involved, the Argentine buyer would have to act as withholding agent of the income tax; and (iii) when both the seller and the buyer are non-Argentine residents and the sale is not performed through an Argentine stock exchange market, the person liable to pay the tax shall be the buyer of the shares or securities being transferred.
However, by General Resolution No. 4095-E dated July 20, 2017, the AFIP resolved to suspend the effects of the General Resolution No. 4094-E until January 16, 2018. In accordance with Resolution No. 4095-E, such suspension would have been based on the need to adequate processes and systems by the participants in the market. As of the date of this prospectus supplements, it is uncertain if, once the 180 days are due, the General Resolution No. 4094-E will be applicable without modifications, if the terms of the General Resolution will be modified, if the term of suspension will be extended or if the bill to reform the Capital Markets Law will be passed into law, thus revoking General Resolution No. 4094-E.
Therefore, to the extent that the General Resolution 4094-E is not revoked or the suspension of its effects extended, the sale, exchange or other disposition of our ordinary shares between parties that are not residents of Argentina would be subject to the capital gains tax as described above but with mechanism in force to receive payments of such tax.
Holders of our ordinary shares or ADS are encouraged to consult a tax advisor as to the particular Argentine income tax consequences derived from holding and disposing of not only our ordinary shares but also the ADSs.
Personal assets tax
Argentine entities, such as us, have to pay the personal assets tax corresponding to Argentine domiciled individuals, foreign domiciled individuals and foreign domiciled entities for the holding of our shares at December 31 of each year. The applicable tax rate is 0.25% and is levied on the proportional net worth value (valor patrimonial proporcional), or the book value, of the shares arising from the last balance sheet of the Argentine entity calculated under Argentine GAAP. Pursuant to the Personal Assets Tax Law, the Argentine company is entitled to seek reimbursement of such paid tax from the applicable Argentine domiciled individuals and/or foreign domiciled shareholders by setting off the applicable tax
213
against any amount due to its shareholders or retaining the assets that give origin to the payment (i.e. the ordinary shares) or, under certain circumstances, waive its right under Argentine law to seek reimbursement from the shareholders.
In particular, it is debatable if the ADSs are subject to personal assets tax for non-Argentine residents; while the underlying assets (the ordinary shares) are subject to personal assets tax. Under this framework, holders are encouraged to consult a tax advisor as to the particular Argentine personal assets tax consequences derived from the holding of ADSs.
Value added tax
The sale, exchange or other disposition of our ordinary shares and ADSs, and the distribution of dividends in connection therewith are exempted from the value added tax.
Tax on debits and credits on Argentine bank accounts
Credits to and debits from bank accounts held at Argentine financial institutions, as well as certain cash payments, are subject to this tax, which is assessed at a general rate of 0.6%. There are also increased rates of 1.2% and reduced rates of 0.075% that may apply in certain cases. Owners of bank accounts subject to the general 0.6% rate may consider 34% of the tax paid upon credits to such bank accounts as a tax credit while taxpayers subject to the 1.2% rate may consider 17% of all tax paid upon credits to such bank accounts as a tax credit against income tax or tax on presumed minimum income. Recently, Law No. 27,264 increased the creditable portion of the tax to 100% for small-sized companies and to 50% to medium-sized companies registered as such.
Tax on minimum presumed income
Entities domiciled in Argentina are subject to this tax at the rate of 1% applicable over the total value of their taxable assets, to the extent it exceeds in the aggregate an amount of Ps.200,000. Specifically, the law establishes that banks, other financial institutions and insurance companies will consider a taxable base equal to 20% of the value of taxable assets. This tax shall be payable only to the extent the income tax determined for any fiscal year does not equal or exceed the amount owed under the tax on minimum presumed income. In such case, only the difference between the tax on minimum presumed income determined for such fiscal year and the income tax determined for that fiscal year shall be paid. Any tax on minimum presumed income paid will be applied as a credit toward income tax owed in the immediately-following 10 fiscal years. Please note that shares and other equity participations in entities subject to tax on minimum presumed income are exempt from this tax. Law 27,260 set forth that the tax on minimum presumed income is abrogated for tax years starting January 1, 2019.
Holders of ADSs or ordinary shares are encouraged to consult a tax advisor as to the particular minimum presumed income tax consequences.
Turnover tax
In addition, gross turnover tax could be applicable on the transfer of shares and on the perception of dividends to the extent such activity is conducted on a regular basis within an Argentine province or within the City of Buenos Aires, unless an exemption applies. For example, under the Tax Code of the City of Buenos Aires, any transactions with shares, as well as the perception of dividends are exempt from gross turnover tax. The Tax Code of the Province of Santa Fe exempts dividends from the Turnover Tax. Argentine holders of our ordinary shares or ADSs are encouraged to consult a tax advisor as to the particular Argentine turnover tax consequences derived from holding and disposing of our ordinary shares or ADS.
Regimes for the Collection of Provincial Tax Revenues on the Amounts Credited to Bank Accounts
Different tax authorities (i.e., City of Buenos Aires, Corrientes, Cordoba, Tucuman, Province of Buenos Aires and Salta, among others) have established collection regimes for gross turnover tax purposes applicable to those credits verified in accounts opened at financial entities, of any type and/or nature and including all branch offices, irrespective of territorial location. These regimes apply to those taxpayers included in the lists provided monthly by the tax authorities of each jurisdiction. The applicable rates may vary depending on the jurisdiction involved. Collections made under these regimes shall be considered as a payment on account of the turnover tax. Note that certain jurisdictions have excluded the application of these regimes on certain financial transactions.
Holders shall corroborate the existence of any exclusion to these regimes in accordance with the jurisdiction involved.
214
Stamp tax
Stamp tax is a local tax that is levied based on the formal execution of public or private instruments within Argentine provinces or in the City of Buenos Aires or that have effects in said jurisdictions, even if executed abroad. Documents subject to stamp tax include, among others, all types of contracts, notarial deeds and promissory notes. Each province and the City of Buenos Aires have its own stamp tax legislation. Stamp tax rates vary according to the jurisdiction and type of agreement involved. In certain jurisdictions, acts or instruments related to the negotiation of shares and other securities duly authorized for its public offering by the CNV are exempt from stamp tax. Holders of ADSs or ordinary shares are encouraged to consult a tax advisor as to the particular stamp tax consequences arising in the involved jurisdictions.
Other taxes
There are no Argentine federal inheritance or succession taxes applicable to the ownership, transfer or disposition of our shares, except for the provinces of Buenos Aires and Entre Ríos. In such jurisdictions, there is a tax on free transmission of assets, including inheritance, legacies, donations, etc. Since January 2011, the tax rates in the Province of Buenos Aires have been set between 4% and 21.925% according to the taxable base and the familial relationships. The province of Entre Ríos has similar tax rates. Free transmission of ADSs or ordinary shares could be subject to this tax in the province of Buenos Aires to the extent that the transmission is equal to or more than Ps.107,640 or Ps.448,500 in the case of parents, children and spouse were involved. As well, in the province of Entre Ríos the free transmission could be levied in case it is equal to or more than Ps.60,000 or Ps.250,000 in the case of parents, children and spouse.
Holders of ADSs or ordinary shares are encouraged to consult a tax advisor as to the particular tax consequences arising in the involved jurisdictions.
Incoming funds arising from Non-Cooperative Jurisdictions
According to the legal presumption under Section 18.1 of Law No. 11,683, as amended, incoming funds from jurisdictions not considered as cooperative for purposes of fiscal transparency will be deemed as an unjustified increase in net worth for the Argentine party, regardless of the nature of the operation involved. Unjustified increases in net worth are subject to the following taxes:
| ■ | income tax at a 35% rate would be assessed on 110% of the amount of the transfer; and |
| ■ | value added tax at 21% rate would be assessed on 110% of the amount of the transfer. |
Despite uncertainty revolving the interpretation of the concept of “incoming funds,” it should be understood to include any transfer of funds (i) from an account in a non-cooperative jurisdiction or from a bank account opened outside of a non-cooperative jurisdiction but owned by an entity located in a non-cooperative jurisdiction (ii) to a bank account located in Argentina or to a bank account opened outside of Argentina but owned by an Argentine tax resident.
The Argentine tax resident may rebut such legal presumption by duly evidencing before the Argentine Tax Authority that the funds arise from activities effectively performed by the Argentine taxpayer or by a third party in such jurisdictions, or that such funds have been previously declared.
Decree No. 589/2013 published in the Official Gazette on May 30, 2013 replaced Section 21.7 of the regulatory decree of the Income Tax Law, providing that, for the purposes set forth in the Income Tax Law and its regulatory decree, any reference to low tax or no tax countries should be understood as countries that are not deemed to cooperate for tax transparency purposes. Cooperating countries for tax transparency purposes are countries that enter into a tax disclosure agreement or convention with Argentina in order to avoid international double taxation and that include a broad information exchange clause, provided the information is actually disclosed. The white list of cooperative jurisdictions is currently available on the Argentine tax authority’s website.
Court Tax
In the case of litigation regarding the shares before a federal court of Argentina or the courts sitting in the City of Buenos Aires, a 3% court tax calculated on the basis of the claim shall be charged. Certain court and other taxes could be imposed on the amount of any claim brought before the courts of the relevant province.
Tax treaties
Argentina has signed tax treaties for the avoidance of double taxation with several countries, although there is currently no tax treaty or convention in effect between Argentina and the United States.
215
Recent developments
On November 15, 2017, a tax reform bill (or the “Tax Reform Bill”) was submitted to Argentine Congress by the Argentine executive branch. It is important to note that, pursuant to the current wording of the Tax Reform Bill (which be subject to further amendments during congressional debates), the Tax Reform Bill would amend the income tax law by establishing that:
| (i) | Capital gains derived from the sale or disposition of shares or depositary receipts obtained by Argentine resident individuals and undivided estates located in Argentina would be exempted from income tax provided the securities are traded on Argentine stock markets. Please note that further requisites apply for this exemption. In case the exemption does not apply, such capital gains would be subject to income tax at a rate of 15%. |
| (ii) | Non-Argentine residents would be exempt taxation on capital gains derived from the sale or other disposition of shares or depositary receipts provided that (i) the securities are traded in Argentine stock markets and (ii) to the extent such non-Argentine residents do not reside in nor channel their funds through non-cooperative jurisdictions. The non-cooperative tax jurisdiction list would be prepared by the Executive and published online. |
| (iii) | The aforementioned amendments would be enforced as of January 1, 2018. |
| (iv) | Dividends paid to Argentine resident individuals and non-Argentine residents would be subject to income tax at a rate of 7% for fiscal periods 2018 to 2020 and 13% from fiscal period 2020 and following. |
| (v) | The corporate income tax imposed upon Argentine legal entities would be reduced to 30% for fiscal periods commencing after January 1, 2018 through December 31, 2019, and to 25% for fiscal periods commencing on or after January 1, 2020. |
As of the date of this Offering Memorandum, the Tax Reform Bill has not yet been debated by Argentine Congress.
THE ABOVE OPINION IS NOT INTENDED TO BE A COMPLETE ANALYSIS OF ALL TAX CONSEQUENCES RELATING TO THE OWNERSHIP OR DISPOSITION OF SHARES OR ADSs. HOLDERS ARE ENCOURAGED TO CONSULT THEIR TAX ADVISERS CONCERNING THE TAX CONSEQUENCES ARISING IN EACH PARTICULAR CASE.
Material U.S. Federal Income Tax Considerations
The following is a discussion of the material U.S. federal income tax consequences of the acquisition, ownership and disposition of our ADSs or ordinary shares by a U.S. Holder (as defined below). This summary deals only with initial purchasers of our ADSs or ordinary shares that are U.S. Holders that will hold our ADSs or ordinary shares as capital assets. This discussion does not cover all aspects of U.S. federal income taxation that may be relevant to, or the actual tax effect that any of the matters described in this prospectus will have on, the acquisition, ownership or disposition of our ADSs or ordinary shares by particular investors, including consequences under the alternative minimum tax or the Medicare tax on net investment income, and does not address state, local, non-U.S. or other tax laws. This summary also does not address tax considerations applicable to investors that own (directly or indirectly) 5% or more of the total combined voting power of Bioceres, nor does this summary discuss all of the tax considerations that may be relevant to certain types of investors subject to special treatment under the U.S. federal income tax laws (such as financial institutions, insurance companies, individual retirement accounts and other tax-deferred accounts, tax-exempt organizations, dealers in securities or currencies, investors that will hold our ADSs or ordinary shares as part of straddles, hedging transactions or conversion transactions for U.S. federal income tax purposes, persons that have ceased to be U.S. citizens or lawful permanent residents of the United States, investors holding our ADSs or ordinary shares in connection with a trade or business conducted outside of the United States, U.S. expatriates or investors whose functional currency is not the U.S. dollar).
The term “U.S. Holder” means a beneficial owner of our ADSs or ordinary shares that is, for U.S. federal income tax purposes, (1) an individual citizen or resident of the United States, (2) a corporation created or organized under the laws of the United States, any state thereof or the District of Columbia, (3) an estate the income of which is subject to U.S. federal income tax without regard to its source or (4) a trust if a court within the United States is able to exercise primary
216
supervision over the administration of the trust and one or more U.S. persons have the authority to control all substantial decisions of the trust, or a valid election is in place to treat the trust as a U.S. person for U.S. federal income tax purposes.
The U.S. federal income tax treatment of a partner in an entity or arrangement treated as a partnership for U.S. federal income tax purposes that holds our ADSs or ordinary shares will depend on the status of the partner and the activities of the partnership. Prospective purchasers that are entities or arrangements treated as partnerships for U.S. federal income tax purposes should consult their tax advisers concerning the U.S. federal income tax consequences to them and their partners of the acquisition, ownership and disposition of our ADSs or ordinary shares by the partnership.
Except as otherwise noted, this summary assumes that we are not and will not become a PFIC for U.S. federal income tax purposes. However, our status as a PFIC must be determined annually and therefore may be subject to change. If we were to be a PFIC in any year, materially adverse consequences could result for U.S. Holders, as described below.
This summary is based on the tax laws of the United States, including the Internal Revenue Code of 1986, as amended, its legislative history, existing and proposed regulations thereunder, published rulings and court decisions, all as of the date of this prospectus and all subject to change at any time, possibly with retroactive effect.
ALL PROSPECTIVE PURCHASERS SHOULD CONSULT THEIR TAX ADVISERS AS TO THE PARTICULAR TAX CONSEQUENCES TO THEM OF ACQUIRING, OWNING, AND DISPOSING OF OUR ADSS OR ORDINARY SHARES, THE APPLICABILITY AND EFFECT OF STATE, LOCAL, NON-U.S. AND OTHER TAX LAWS AND POSSIBLE CHANGES IN TAX LAW.
In general, for U.S. federal income tax purposes, U.S. Holders that are beneficial owners of our ADSs should be treated as the beneficial owners of our ordinary shares represented by those ADSs. Accordingly, deposits or withdrawals of ordinary shares for ADSs should not be subject to U.S. federal income tax.
Dividends
General
We do not anticipate making any distributions on our ordinary shares, including in the form of ADSs, in the foreseeable future. If we were to make a distribution, then to the extent the distribution is paid out of our current or accumulated earnings and profits (as determined for U.S. federal income tax purposes), before reduction for any Argentine withholding tax paid by us with respect thereto, it generally will be taxable to a U.S. Holder as foreign source dividend income, and will not be eligible for the dividends received deduction allowed to corporations. Distributions in excess of our current and accumulated earnings and profits will be treated as a non-taxable return of capital to the extent of the U.S. Holder’s adjusted tax basis in our ADSs or ordinary shares and thereafter as capital gain. However, we do not maintain calculations of our earnings and profits in accordance with U.S. federal income tax accounting principles. U.S. Holders should therefore assume that any distribution by us with respect to our ordinary shares represented by the ADSs or ordinary shares will be reported as ordinary dividend income. U.S. Holders should consult their own tax advisers with respect to the appropriate U.S. federal income tax treatment of any distribution received from us.
Subject to certain exceptions for short-term and hedged positions, the amount of dividends received by certain U.S. Holders (including individuals) with respect to our ADSs will be subject to taxation at a reduced rate if the dividends represent “qualified dividend income.” Dividends received by U.S. Holders on ordinary shares will not be treated as “qualified dividend income.” Dividends paid on our ADSs should be treated as qualified dividend income if (1) our ADSs are readily tradable on an established securities market in the United States (such as the New York Stock Exchange, on which we have applied for the ADSs (but not the ordinary shares) to be listed) and (2) we were not in the year prior to the year in which the dividend was paid, and are not in the year in which the dividend is paid, a PFIC. Under current guidance issued by the IRS, our ADSs should qualify as readily tradable on an established securities market in the United States so long as they are listed on the New York Stock Exchange, but no assurances can be given that our ADSs will be or remain readily tradable under future guidance. See below for a discussion of the PFIC rules. U.S. Holders should consult their own tax advisors regarding the availability of the preferential dividend tax rate.
Dividends paid in Argentine pesos (or other non-U.S. currency) will be included in income in a U.S. dollar amount calculated by reference to the exchange rate in effect on the day the dividends are received by the ADS Depositary or, in the case of ordinary shares, the U.S. Holder, regardless of whether the pesos (or other non-U.S. currency) are in fact converted to U.S. dollars at that time. If dividends paid in Argentine pesos (or other non-U.S. currency) are converted
217
into U.S. dollars on the day they are received by the ADS Depositary or, in the case of ordinary shares, the U.S. Holder, U.S. Holders should not be required to recognize exchange gain or loss in respect of the conversion transaction relating to such dividend income. Gain or loss resulting from currency exchange fluctuations during the period from the date the dividend is received by the ADS Depositary or, in the case of ordinary shares, the U.S. Holder, through the date such payment is converted into U.S. dollars (or otherwise disposed of) should be U.S. source ordinary income or loss. U.S. Holders should consult their own tax advisors regarding the tax treatment of such exchange gain or loss.
Effect of Argentine Withholding Taxes
As discussed in “Taxation—Material Argentine Tax Considerations—Taxation on dividends,” under current law, payments of dividends by us to non-Argentine investors may be subject to Argentine withholding tax. For U.S. federal income tax purposes, U.S. Holders would be treated as having received the amount of Argentine taxes withheld by us, and as then having paid over the withheld taxes to the Argentine taxing authorities. As a result, the amount of dividend income included in gross income for U.S. federal income tax purposes by a U.S. Holder with respect to a payment of dividends may be greater than the amount of cash actually received (or receivable) by the U.S. Holder. U.S. Holders are encouraged to consult their tax advisors regarding the proper reporting of such dividend income and any Argentine foreign tax credits relating thereto to which they may be entitled.
A U.S. Holder generally will be entitled, subject to certain limitations, to a credit against its U.S. federal income tax liability, or a deduction in computing its U.S. federal taxable income, for Argentine income taxes withheld by us. The rules relating to computing foreign tax credits or deducting foreign taxes are extremely complex, and U.S. Holders are urged to consult their tax advisors regarding the availability of foreign tax credits with respect to any Argentine income taxes withheld from a dividend on our ordinary shares, including ordinary shares represented by the ADSs. The IRS has expressed concern that intermediaries in connection with depositary arrangements may be taking actions that are inconsistent with the claiming of foreign tax credits by U.S. persons who are holders of depositary shares. Accordingly, U.S. Holders should be aware that the discussion above regarding the availability of foreign tax credits for Argentine withholding tax on dividends paid with respect to our ordinary shares, including ordinary shares represented by ADSs could be affected by future action taken by the IRS.
Sale or Other Disposition
Upon a sale or other disposition of our ADSs or ordinary shares, a U.S. Holder generally will recognize capital gain or loss for U.S. federal income tax purposes equal to the difference, if any, between the amount realized (including the gross amount of the proceeds of the sale or other disposition before the deduction of Argentine tax) on the sale or other disposition and the U.S. Holder’s adjusted tax basis in our ADSs or ordinary shares, in each case as determined in U.S. dollars. This capital gain or loss will be long-term capital gain or loss if the U.S. Holder’s holding period in our ADSs or ordinary shares exceeds one year. Any gain or loss generally will be U.S. source. U.S. Holders should consult their own tax advisors about how to account for proceeds received on the sale or other disposition of ordinary shares that are not paid in U.S. dollars.
As discussed in “Taxation—Material Argentine Tax Considerations—Capital gains,” under current law gains derived by non-Argentine investors from the sale or other disposition of our ADSs or ordinary shares may be subject to Argentine income tax. If Argentine income tax is imposed on the seller due to the sale or other disposition of our ADSs or ordinary shares, the amount realized by a U.S. Holder will include the gross amount of the proceeds of such sale or other disposition before deduction of the Argentine income tax on such capital gain. The availability of U.S. foreign tax credits for these Argentine taxes is subject to various limitations and involves the application of rules that depend on a U.S. Holder’s particular circumstances. Prospective purchasers are urged to consult their tax advisers as to the availability of and limitations on any foreign tax credit with respect to this Argentine income tax.
Passive Foreign Investment Company Considerations
If we are a PFIC for any taxable year during which a U.S. Holder holds ADSs or ordinary shares, certain adverse U.S. federal income tax consequences may apply to the U.S. Holder. Based on the projected composition of our income and valuation of our assets, including goodwill, we do not expect to be a PFIC for our current taxable year. However, our possible PFIC status must be determined annually and depends on the composition of our income and assets, and the fair market value of our assets (including, among others, any less than 25% owned equity investments) from time to time, as well as on the application of complex statutory and regulatory rules that are subject to potentially varying or changing interpretations. Because we have valued our goodwill based on the projected market value of our equity, a
218
decrease in the price of our ADSs or ordinary shares may also result in our becoming a PFIC. The composition of our income and our assets will also be affected by how, and how quickly, we use the proceeds from this offering. Under circumstances where the cash is not deployed for active purposes, our risk of becoming a PFIC may increase. Accordingly, there can be no assurance that we will not be considered a PFIC for any taxable year. If we are a PFIC for any taxable year during which a U.S. Holder holds our ADSs or ordinary shares, such U.S. Holder will be subject to special tax rules discussed below.
In general, a non-U.S. corporation will be a PFIC in any taxable year in which, after taking into account the income and assets of the corporation and certain subsidiaries pursuant to applicable “look-through rules,” either: (i) at least 75% of its gross income is “passive income” or (ii) at least 50% of the average value of its assets is attributable to assets which produce passive income or are held for the production of passive income. For purposes of the PFIC rules, “passive income” generally includes, among other things, dividends, interest, and royalties. However, royalties derived in the “active conduct of a trade or business” and meeting specified criteria are generally excluded from the definition of passive income. The law is unclear as to what constitutes the active conduct of a trade or business and there are also other uncertainties regarding the criteria royalties must meet. Accordingly, there can be no assurance that we are not a PFIC or will not become a PFIC.
If we are a PFIC for any taxable year during which a U.S. Holder holds ADSs or ordinary shares, gain recognized by a U.S. Holder on a sale or other disposition of ADSs or ordinary shares would generally be allocated ratably over the U.S. Holder’s holding period for ADSs or ordinary shares, as applicable. The amounts allocated to the taxable year of the sale or other disposition and to any year before we became a PFIC would be taxed as ordinary income. The amount allocated to each other taxable year would be subject to U.S. federal income tax at the highest rate in effect in that year for individuals or corporations, as appropriate, and an interest charge would be imposed on the resulting U.S. federal income tax liability. The same treatment would generally apply to any distribution in respect of ADSs or ordinary shares to the extent the distribution exceeds 125% of the average of the annual distributions on ADSs or ordinary shares received by the U.S. Holder during the preceding three years or the U.S. Holder’s holding period, whichever is shorter.
Additionally, if we are a PFIC for a taxable year in which we pay a dividend or in the prior taxable year, the favorable rate discussed above with respect to dividends paid to certain non-corporate U.S. Holders would not apply.
Furthermore, if we are a PFIC with respect to a U.S. Holder for any taxable year, to the extent any of our subsidiaries are also PFICs, the U.S. Holder may be deemed to own shares in such lower-tier PFICs that are directly or indirectly owned by us in the proportion to which the value of ADSs or ordinary shares such U.S. Holder owns bears to the value of all of our shares, and the U.S. Holder may be subject to the tax consequences described above with respect to the shares of such lower-tier PFIC that such U.S. Holder would be deemed to own. As a result, if we are a PFIC and received a distribution from any lower-tier PFIC or if any shares in a lower-tier PFIC are disposed of (or deemed disposed of), a U.S. Holder may be subject to tax under the PFIC rules described above in the same manner as if the U.S. Holder had held its proportionate share of the lower-tier PFIC stock directly even though such U.S. Holder has not received the proceeds of the distribution or disposition directly. U.S. Holders should consult their tax advisers regarding the application of the PFIC rules to any of our subsidiaries.
Alternatively, if we are a PFIC and our ADSs are “regularly traded” on a “qualified exchange” (such as the New York Stock Exchange where ADSs are expected to be listed), a U.S. Holder could make a mark-to-market election as to such ADSs that would result in tax treatment different from the general tax treatment for PFICs described above. Because a mark-to-market election cannot be made for equity interests in any lower tier PFICs we may own, a U.S. Holder may continue to be subject to the PFIC rules with respect to any indirect investments held by us that are treated as an equity interest in a PFIC for U.S. federal income tax purposes. It should also be noted that it is intended that only ADSs and not the ordinary shares will be listed on the New York Stock Exchange. Consequently, a U.S. Holder of ordinary shares that are not represented by ADSs generally will not be eligible to make a mark-to-market election if we are or were to become a PFIC.
In some cases, a shareholder of a PFIC can avoid the interest charge and the other adverse PFIC consequences described above by making a “qualified electing fund” election (a “QEF election”) to be taxed currently on its share of the PFIC’s undistributed income. We do not, however, expect to provide to U.S. Holders the information regarding this income that would be necessary in order for a U.S. Holder to make a QEF election with respect to its ADSs or ordinary shares.
219
A U.S. Holder who owns, or who is treated as owning, PFIC stock during any taxable year in which we are classified as a PFIC may be required to file IRS Form 8621. Prospective purchasers should consult their tax advisers regarding the requirement to file IRS Form 8621 and the potential application of the PFIC regime.
Information Reporting and Backup Withholding
Payments of dividends on our ADSs or ordinary shares and other proceeds from the sale or other disposition of our ADSs or ordinary shares by a U.S. paying agent or other U.S. intermediary will be reported to the IRS and to the U.S. Holder as may be required under applicable regulations. Backup withholding may apply to these payments if the U.S. Holder fails to provide an accurate taxpayer identification number or certification of exempt status or fails to comply with applicable certification requirements. Certain U.S. Holders are not subject to backup withholding. U.S. Holders should consult their tax advisers about these rules and any other reporting obligations that may apply to the ownership or disposition of our ADSs or ordinary shares, including requirements related to the holding of certain “specified foreign financial assets.”
220
Subject to the terms and conditions set forth in the underwriting agreement, dated 2017, among us and Jefferies LLC and Piper Jaffray & Co., acting as the representatives of the international underwriters named below and the joint book-running managers of this offering, we have agreed to sell to the international underwriters, and each of the international underwriters has agreed, severally and not jointly, to purchase from us, the respective number of ADSs shown opposite its name below. Jefferies LLC is acting as sole global coordinator of this offering.
International Underwriters | Number of ADSs | ||
Jefferies LLC | |||
Piper Jaffray & Co. | |||
Total | |||
The underwriting agreement provides that the obligations of the several international underwriters are subject to certain conditions precedent such as the receipt by the international underwriters of officers’ certificates and legal opinions and approval of certain legal matters by their counsel. The underwriting agreement provides that the international underwriters will purchase all of the ADSs in the international offering if any of them are purchased. If an international underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting international underwriters may be increased or the underwriting agreement may be terminated. We have agreed to indemnify the international underwriters and certain of their controlling persons against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the international underwriters may be required to make in respect of those liabilities.
The international underwriters have advised us that, following the completion of the international offering, they currently intend to make a market in the ADSs as permitted by applicable laws and regulations. However, the international underwriters are not obligated to do so, and the international underwriters may discontinue any market-making activities at any time without notice in their sole discretion. Accordingly, no assurance can be given as to the liquidity of the trading market for the ADSs, that you will be able to sell any of the ADSs held by you at a particular time or that the prices that you receive when you sell will be favorable.
The international underwriters are offering the ADSs subject to their acceptance of the ADSs from us and subject to prior sale. The international underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part. In addition, the international underwriters have advised us that they do not intend to confirm sales to any account over which they exercise discretionary authority.
The offering of our ordinary shares in Argentina, or the Argentine Offering, will be concurrently carried out by the Argentine placement agents according to the Argentine placement agreement among us, AR Partners S.A. and BAF Securities S.A., the Argentine placement agents. Pursuant to the terms of the Argentine placement agreement, the Argentine placement agents shall use their best efforts to carry out the concurrent offering and sale by the Company of ordinary shares in Argentina, but have not undertaken any underwriting commitments in connection with the Argentine offering.
Under Argentine law, our existing shareholders are entitled to preemptive and accretion rights to subscribe to our capital increase underlying the global offering. In order to facilitate the execution of the global offering, certain of our shareholders, representing % of our total outstanding shares, have, pursuant to certain preemptive rights assignment agreements, assigned to AR Partners S.A., as exercise agent, substantially all of such shareholders’ preemptive and accretion rights in respect of the shares to be issued pursuant to the Argentine offering. Subject to the closing conditions set forth in the underwriting agreement, the representatives of the international underwriters shall instruct the exercise agent to exercise such preemptive rights (but not accretion rights) in order to facilitate the global offering. See “—Rights Offering in Argentina.”
221
Commission and Expenses
The international underwriters have advised us that they propose to offer the ADSs to the public initially at the public offering price set forth on the cover page of this prospectus and to certain dealers, which may include the international underwriters, at the same prices less a concession of not more than $ per ADS. The international underwriters may allow, and certain dealers may re-allow, a discount from the concession not in excess of $ per ADS to certain brokers and dealers. After the international offering, the initial public offering price, concession and reallowance to dealers may be reduced by the representatives. No such reduction will change the amount of proceeds to be received by us as set forth on the cover page of this prospectus.
The following table summarizes the public offering price, the underwriting discounts and commissions that we will pay to the international underwriters and the proceeds, before expenses, to us in connection with the international offering. Such amounts are shown assuming both no exercise and full exercise of the international underwriters’ option to purchase additional ADSs.
Per ADS | Total | |||||||||||
Without Option to Purchase Additional ADSs | With Option to Purchase Additional ADSs | Without Option to Purchase Additional ADSs | With Option to Purchase Additional ADSs | |||||||||
Public offering price | $ | $ | $ | $ | ||||||||
Underwriting discounts and commissions | $ | $ | $ | $ | ||||||||
Proceeds to us, before expenses | $ | $ | $ | $ | ||||||||
We estimate expenses payable by us in connection with this international offering, other than the underwriting discounts and commissions referred to above, will be approximately $ million. We have agreed to reimburse the international underwriters in an amount up to $ for counsel fees and expenses in connection with the clearance of this international offering with the Financial Industry Regulatory Authority, or FINRA. In accordance with FINRA Rule 5110, these reimbursed expenses are deemed underwriting compensation for this international offering.
The commission and expenses shall not include the securities to be allocated through the participation rights in the Argentine offering granted to (i) Monsanto and BAF in connection with the conversion of their loan instrument, (ii) holders of the Preferred Shares of RASA Holding and (iii) BAF under the BAF Bridge Loans in connection with the financing of the Company for the acquisition of Rizobacter.
Determination of Offering Price
Prior to this international offering, there has not been a public market for our ADSs. Consequently, the initial public offering price for our ADSs will be determined by negotiations between us and the representatives. Among the factors to be considered in these negotiations will be prevailing market conditions, our financial information, market valuations of other companies that we and the international underwriters believe to be comparable to us, estimates of our business potential, the present state of our development and other factors deemed relevant.
There can be no assurance that the initial public offering price of the ADSs, representing our ordinary shares, will correspond to the price at which the ADSs will trade in the public market subsequent to the international offering or that an active public market for the ADSs will develop and continue after the international offering.
Listing
We have applied to list our ordinary shares on the BYMA under the symbol “BIOX”, and we have applied to list our ADSs on the NYSE under the symbol “BIOX.”
222
Option to Purchase ADSs
We have granted to the international underwriters, an option, exercisable for 30 days from the date of this prospectus, to purchase, from time to time, in whole or in part, up to an aggregate of additional ADSs from us, at the public offering price set forth on the cover page of this prospectus, less the underwriting discounts and commissions. If the international underwriters exercise this option, each international underwriter will be obligated, subject to specified conditions, to purchase a number of additional ADSs proportionate to that underwriter’s initial purchase commitment as indicated in the table above. This option may be exercised only if the international underwriters sell more ADSs than the total number set forth on the cover page of this prospectus. Our shareholders of record as of the Record Date had preemptive and accretion rights with respect to any shares that are available to the international underwriters under the option to purchase additional ADSs.
No Sales of Similar Securities
We, our officers, directors and substantially all of our shareholders have agreed, subject to specified exceptions, not to directly or indirectly:
| ■ | offer to sell, pledge, announce the intention to sell, sell, contract to sell or lend, hypothecate or grant any security interest in, establish or sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, make any short sale, increase a put equivalent position, liquidate or decrease any call equivalent position or in any other way transfer or dispose of any ordinary shares or ADSs or any options or warrants or other rights to acquire ADSs or ordinary shares or any securities convertible into, exercisable or exchangeable for ordinary shares or ADSs whether now owned or hereafter acquired; |
| ■ | enter into any swap, hedge or other arrangement or agreement that transfers, in whole or in part, any of the economic risk of ownership of the ordinary shares, ADSs or any securities convertible into, exercisable or exchangeable for ordinary shares or ADSs; |
| ■ | make any demand for, or exercise any right with respect to, the registration of any ordinary shares or ADSs or any security convertible into or exercisable or exchangeable for ordinary shares or ADSs or cause to be filed a registration statement, prospectus or prospectus supplement (or an amendment or supplement thereto) with respect to any such registration; or |
| ■ | file (or participate in the filing of) a registration statement or publicly disclose the intention to do any of the foregoing for a period of 180 days commencing on the date of this prospectus, without the prior written consent of the representatives of the international underwriters, which may withhold their consent in their sole discretion |
This restriction terminates after the close of trading of the ADSs and including the 180th day after the date of this prospectus.
The representatives may, in their sole discretion and at any time or from time to time before the termination of the 180-day period, release all or any portion of the securities subject to lock-up agreements, provided that at least three business days before the effective date of any release or waiver of any lock-up restriction on the transfer of our shares by any of our directors or officers, the Representatives will notify us of the impending release or waiver and we will announce the impending release or waiver issuing a press release through a major news service, at least two business days before the effective date of the release or waiver, except where the release or waiver is effected solely to permit a transfer of securities that is not for consideration and where the transferee has agreed in writing to be bound by the same lock-up agreement terms in place for the transferor.
Stabilization
The international underwriters have advised us that, pursuant to Regulation M under the Exchange Act, certain persons participating in this international offering may engage in short sale transactions (which may involve either “covered” short sales or “naked” short sales), stabilizing transactions, syndicate covering transactions or the imposition of penalty bids in connection with this international offering. These activities may have the effect of stabilizing or maintaining the market price of our ADSs at a level above that which might otherwise prevail in the open market. Establishing short sale positions may involve either “covered” short sales or “naked” short sales.
223
“Covered” short sales are sales made in an amount not greater than the international underwriters’ option to purchase additional ADSs in this international offering. The international underwriters may close out any covered short position by either exercising their option to purchase additional ADSs or purchasing our ADSs in the open market. In determining the source of ADSs to close out the covered short position, the international underwriters will consider, among other things, the price of ADSs available for purchase in the open market as compared to the price at which they may purchase ADSs through the option to purchase additional ADSs.
“Naked” short sales are sales in excess of the option to purchase additional ADSs. The international underwriters must close out any naked short position by purchasing ADSs in the open market. A naked short position is more likely to be created if the international underwriters are concerned that there may be downward pressure on the price of our ADSs in the open market after pricing that could adversely affect investors who purchase in this international offering.
A stabilizing bid is a bid for the purchase of ADSs on behalf of the international underwriters for the purpose of fixing or maintaining the price of the ADSs. A syndicate covering transaction is the bid for or the purchase of ADSs on behalf of the international underwriters to reduce a short position incurred by the international underwriters in connection with the international offering. Similar to other purchase transactions, the international underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our ADSs or preventing or retarding a decline in the market price of our ADSs. As a result, the price of our ADSs may be higher than the price that might otherwise exist in the open market. A penalty bid is an arrangement permitting the international underwriters to reclaim the selling concession otherwise accruing to a syndicate member in connection with the international offering if the ADSs originally sold by such syndicate member are purchased in a syndicate covering transaction and therefore have not been effectively placed by such syndicate member.
Neither we, nor any of the international underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our ADSs. The international underwriters are not obligated to engage in these activities and, if commenced, any of the activities may be discontinued at any time.
Electronic Distribution
A prospectus in electronic format may be made available by e-mail or on the web sites or through online services maintained by one or more of the international underwriters or their affiliates. In those cases, prospective investors may view offering terms online and may be allowed to place orders online. The international underwriters may agree with us to allocate a specific number of ADSs for sale to online brokerage account holders. Any such allocation for online distributions will be made by the international underwriters on the same basis as other allocations. Other than the prospectus in electronic format, the information on the international underwriters’ web sites and any information contained in any other web site maintained by any of the international underwriters is not part of this prospectus, has not been approved and/or endorsed by us or the international underwriters and should not be relied upon by investors.
Other Activities and Relationships
The international underwriters and certain of their affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The international underwriters and certain of their affiliates have, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for us and our affiliates, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the international underwriters and certain of their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments issued by us and our affiliates. If the international underwriters or their respective affiliates have a lending relationship with us, they routinely hedge their credit exposure to us consistent with their customary risk management policies. The international underwriters and their respective affiliates may hedge such exposure by entering into transactions which consist of either the purchase of credit
224
default swaps or the creation of short positions in our securities or the securities of our affiliates, including potentially the ADSs offered hereby. Any such short positions could adversely affect future trading prices of the ADSs offered hereby. The international underwriters and certain of their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Disclaimers About Non-U.S. Jurisdictions
Each of the international underwriters may arrange to sell the ADSs offered hereby in certain jurisdictions outside the United States, either directly or through affiliates, where they are permitted to do so. Except as described elsewhere in this prospectus with respect to the public offering of ordinary shares in Argentina, no action has been taken in any jurisdiction (except in the United States) that would permit a public offering of our ordinary shares or ADSs, or the possession, circulation or distribution of this prospectus or any other material relating to us or our ordinary shares or ADSs in any jurisdiction where action for that purpose is required. Accordingly, neither the shares nor ADSs may not be offered or sold, directly or indirectly, and neither this prospectus nor any other offering material or advertisements in connection with our ordinary shares or ADSs may be distributed or published, in or from any country or jurisdiction, except in compliance with any applicable rules and regulations of any such country or jurisdiction.
Argentina
The Argentine public offering of the ordinary shares has been authorized by the CNV pursuant to Resolution No. 17919 dated December 4, 2015, subject to the fulfillment of certain requirements.
The ordinary shares may be offered directly to the public in Argentina only through the Argentine placement agents, which are authorized under the laws and regulations of Argentina to offer or sell securities to the public in Argentina. The offering of the ordinary shares in Argentina will be made by a substantially similar prospectus in Spanish and in accordance with CNV regulations.
Australia
No placement document, prospectus, product disclosure statement or other disclosure document has been lodged with the Australian Securities and Investments Commission (“ASIC”), in relation to the offering. This prospectus does not constitute a prospectus, product disclosure statement or other disclosure document under the Corporations Act 2001 (the “Corporations Act”), and does not purport to include the information required for a prospectus, product disclosure statement or other disclosure document under the Corporations Act. Accordingly, if you receive this prospectus in Australia:
| A. | You confirm and warrant that you are either: |
| ■ | a “sophisticated investor” (within the meaning of section 708(8)(a) or (b) of the Corporations Act); |
| ■ | a “sophisticated investor” under section 708(8)(c) or (d) of the Corporations Act and that you have provided an accountant’s certificate to the Company which complies with the requirements of section 708(8)(c)(i) or (ii) of the Corporations Act and related regulations before the offer has been made; |
| ■ | a person associated with the Company under Section 708(12) of the Corporations Act; |
| ■ | a “professional investors” (within the meaning of section 708(11) of the Corporations Act); or |
| ■ | otherwise pursuant to one or more exemptions contained in section 708 of the Corporations Act so that it is lawful to offer the shares without disclosure to investors under Chapter 6D of the Corporations Act. |
To the extent that you are unable to confirm or warrant that you are an exempt sophisticated investor, associated person or professional investor under the Corporations Act any offer made to you under this Offering Memorandum is void and incapable of acceptance.
| B. | You warrant and agree that you will offer any of the securities issued to you pursuant to this prospectus for sale in Australia in the period of 12 months after the date of allotment under the offering, except in circumstances where |
225
disclosure to investors under Chapter 6D of the Corporations Act would not be required pursuant to an exemption under section 708 of the Corporations Act or otherwise or where the offer is pursuant to a disclosure document which complies with Chapter 6D of the Corporations Act.
This prospectus contains general information only and does not take account of the investment objectives, financial situation or particular needs of any particular person. It does not contain any securities recommendations or financial product advice. Before making an investment decision, investors need to consider whether the information in this prospectus is appropriate to their needs, objectives and circumstances, and, if necessary, seek expert advice on those matters.
Brazil
The offer and sale of our ADSs will not be carried out by any means that would constitute a public offering in Brazil under Law 6,385, of December 7, 1976, as amended, and under Brazilian Securities Commission (Comissao de Valores Mobiliarios) Rule (instruçao) No. 400, of December 29, 2003, as amended. The offer and sale of the ADSs has not been and will not be registered with the Comissão de Valores Mobiliários in Brazil. Any representation to the contrary is untruthful and unlawful. Any public offering or distribution, as defined under Brazilian laws and regulations, of the ADSs in Brazil is not legal without such prior registration. Documents relating to the offering of the ADSs, as well as information contained therein, may not be supplied to the public in Brazil, as the offering of the ADSs is not a public offering of securities in Brazil, nor may they be used in connection with any offer for sale of the ADSs to the public in Brazil.
Any offer of our ADSs is addressed to the addressee personally, upon such addressee’s request and for its sole benefit, and is not to be transmitted to anyone else, to be relied upon by anyone else or for any other purpose either quoted or referred to in any other public or private document or to be filed with anyone without the international underwriters’ prior, express and written consent.
Canada
The ADSs may be sold only to purchasers in Canada purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the shares of common stock must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus supplement (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (“NI 33-105”), the underwriter is not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Cayman Islands
This prospectus does not constitute an invitation or offer to the public in the Cayman Islands of the ADSs, whether by way of sale or subscription. The international underwriters have not offered or sold, and will not offer or sell, directly or indirectly, any ADSs in the Cayman Islands.
Chile
The ADSs are not registered in the Securities Registry (Registro de Valores) or subject to the control of the Chilean Securities and Exchange Commission (Superintendencia de Valores y Seguros de Chile). This prospectus and other offering materials relating to the offer of the ADSs do not constitute a public offer of, or an invitation to subscribe for or
226
purchase, the ADSs in the Republic of Chile, other than to individually identified purchasers pursuant to a private offering within the meaning of Article 4 of the Chilean Securities Market Act (Ley de Mercado de Valores) (an offer that is not “addressed to the public at large or to a certain sector or specific group of the public”).
European Economic Area
In relation to each member state of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”), no offer of any securities which are the subject of the offering has been, or will be made to the public in that Relevant Member State, other than under the following exemptions under the Prospective Directive:
| ■ | to any legal entity which is a “qualified investor” as defined under the Prospectus Directive; |
| ■ | to fewer than 100, or, if the Relevant Member State has implemented the relevant provision of Directive 2010/73/EU, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representatives of the international underwriters or the international underwriters nominated by us for any such offer; or |
| ■ | in any other circumstances falling within Article 3(2) of the Prospectus Directive, |
provided that no such offer of securities described in this prospectus shall result in a requirement for the Company or any representative of the international underwriters to publish a prospectus pursuant to Article 3 of the Prospectus Directive, or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
We, the underwriter and our respective affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgments and agreements.
This prospectus has been prepared on the basis that any offer of shares in any Relevant Member State will be made pursuant to an exemption under the Prospectus Directive from the requirement to publish a prospectus for offers of shares. Accordingly any person making or intending to make an offer in that Relevant Member State of shares which are the subject of the offering contemplated in this prospectus may only do so in circumstances in which no obligation arises for us or any of the underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive in relation to such offer. Neither we nor the underwriter has authorized, nor do they authorize, the making of any offer of shares in circumstances in which an obligation arises for us or the underwriter to publish a prospectus for such offer.
For the purposes of this provision, the expression an “offer of securities to the public” in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe the securities, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State. The expression “Prospectus Directive” means Directive 2003/71/EC (as amended) and includes any relevant implementing measure in each Relevant Member State.
France
This prospectus (including any amendment, supplement or replacement thereto) is not being distributed in the context of a public offering in France within the meaning of Article L. 411-1 of the French Monetary and Financial Code (Code monétaire et financier).
Neither this prospectus nor any other offering material relating to the shares described in this prospectus has been nor will be submitted to the clearance procedures of the Autorité des Marchés Financiers or of the competent authority of another member state of the European Economic Area and notified to the Autorité des Marchés Financiers (the “AMF”). The shares have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
Neither this prospectus nor any other offering material relating to the shares has been or will be:
| ■ | released, issued, distributed or caused to be released, issued or distributed to the public in France; or |
| ■ | used in connection with any offer for subscription or sale of the shares to the public in France. |
227
Such offers, sales and distributions will be made in France only:
| ■ | to qualified investors (investisseurs qualifiés) and/or to a restricted circle of investors (cercle restreint d’investisseurs), in each case investing for their own account, all as defined in, and in accordance with articles L.411-2, D.411-1, D.411-2, D.734-1, D.744-1, D.754-1 and D.764-1 of the French Code monétaire et financier; |
| ■ | to investment services providers authorized to engage in portfolio management on behalf of third parties; or |
| ■ | in a transaction that, in accordance with article L.411-2-II-1°-or-2°-or 3° of the French Code monétaire et financier and article 211-2 of the General Regulations (Règlement Général) of the Autorité des Marchés Financiers, does not constitute a public offer (appel public à l’épargne). |
The shares may be resold directly or indirectly, only in compliance with articles L.411-1, L.411-2, L.412-1 and L.621-8 through L.621-8-3 of the French Code monétaire et financier.
Hong Kong
This prospectus has not been approved by or registered with the Securities and Futures Commission of Hong Kong or the Registrar of Companies of Hong Kong. No person may offer or sell in Hong Kong, by means of any document, any ordinary shares or ADSs other than (i) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance, or (ii) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No person may issue or have in its possession for the purposes of issue, whether in Hong Kong or elsewhere, any advertisement, invitation or document relating to the ADSs which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to ADSs which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
WARNING
The contents of this document have not been reviewed by any regulatory authority in Hong Kong. You are advised to exercise caution in relation to the offer. If you are in any doubt about any of the contents of this document, you should obtain independent professional advice.
Israel
This document does not constitute a prospectus under the Israeli Securities Law, 5728-1968, or the Securities Law, and has not been filed with or approved by the Israel Securities Authority. In Israel, this prospectus is being distributed only to, and is directed only at, and any offer of the ADSs is directed only at, (i) a limited number of persons in accordance with the Israeli Securities Law and (ii) investors listed in the first addendum, or the Addendum, to the Israeli Securities Law, consisting primarily of joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriters, venture capital funds, entities with equity in excess of NIS 50 million and “qualified individuals,” each as defined in the Addendum (as it may be amended from time to time), collectively referred to as qualified investors (in each case, purchasing for their own account or, where permitted under the Addendum, for the accounts of their clients who are investors listed in the Addendum). Qualified investors are required to submit written confirmation that they fall within the scope of the Addendum, are aware of the meaning of same and agree to it.
Japan
The ordinary shares and ADSs have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948 of Japan, as amended), or FIEL. The international underwriters have represented and agreed that they will not, directly or indirectly, offer or sell any ADSs in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for reoffer or resale, directly or indirectly, in Japan or to, or for the
228
benefit of, any resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the FIEL and any other applicable laws, regulations and ministerial guidelines of Japan.
Mexico
The ordinary shares and ADSs have not been registered with the National Securities’ Registry (Registro Nacional de Valores) maintained by the National Banking and Securities Commission (Comisión Nacional Bancaria y de Valores or CNBV), and may not be offered or sold publicly in Mexico.
This document is not intended to be publicly distributed to an undetermined person through mass media, nor to serve as an application for the registration of the securities in Mexico, nor as a prospectus for their public offering in said jurisdiction.
This document is addressed to you under a private offering exception contained in article 8 of the Securities Market Law (Ley del Mercado de Valores or LMV), for which you must comply with any of the following requirements:
| ■ | you are either an institutional or qualified investor for purposes of Mexican law; |
| ■ | you are a member of a group of less than 100 individually identified people to whom the ADSs are being offered directly and personally; or |
| ■ | you are an employee of the issuer and a beneficiary of an employees’ benefit plan of said issuer. |
The LMV and CNBV regulations (along with other laws applicable in Mexico) define institutional investors as Mexican and foreign banks, broker dealers, insurance and bond companies, bonded warehouses, financial leasing companies, factoring companies and investment funds, private pension and annuities funds and foreign pension and investment funds. Such regulations also define qualified investors as individuals and corporations which maintain during the previous year investments in securities for an amount equal or similar to 1.5 million Mexican Unidades de Inversión or “UDIS” (approximately US$330,000) or that have obtained during the previous two years a gross income of at least 500,000 UDIS (approximately US$110,000) per year.
Norway
This prospectus has not been produced in accordance with the prospectus requirements set out in the Norwegian Securities Trading Act 2007 nor in accordance with the prospectus requirements set out in the Norwegian Securities Fund Act 1981 as amended. This prospectus has not been approved or disapproved by, or registered with, the Oslo Stock Exchange, the Norwegian FSA or the Norwegian Registry of Business Enterprises.
This prospectus is only and exclusively addressed to the addressees and cannot be distributed, offered or presented, either directly or indirectly to other persons or entities domiciled in Norway.
People’s Republic of China
This prospectus may not be circulated or distributed in the PRC and our ADSs may not be offered or sold, and will not offer or sell to any person for re-offering or resale directly or indirectly to any resident of the PRC except pursuant to applicable laws and regulations of the PRC. For the purpose of this paragraph, PRC does not include Taiwan and the special administrative regions of Hong Kong and Macau.
Singapore
This prospectus has not been and will not be lodged or registered as a prospectus with the Monetary Authority of Singapore under the Securities and Futures Act, Chapter 289 of Singapore, or the SFA. Accordingly, no person may offer or sell such ADSs or cause such ADSs to be made the subject of an invitation for subscription or purchase, or circulate or distribute, this prospectus or any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of such ADSs, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the SFA, (ii) to a relevant person pursuant to Section 275(1) of the SFA, or (iii) to any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275 of the SFA, or otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
229
Where the ADSs are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| ■ | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| ■ | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, |
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the ADSs pursuant to an offer made under Section 275 of the SFA except:
| ■ | to an institutional investor or to a relevant person defined in Section 275(2) of the SFA or to any person arising from an offer referred to in Section 275(1A) or Section 276 (4)(i)(B) of the SFA; |
| ■ | where no consideration is or will be given for the transfer; |
| ■ | where the transfer is by operation of law; |
| ■ | as specified in Section 276(7) of the SFA; or |
as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore.
Spain
This offer of ADSs has not been and will not be registered with the Spanish National Securities Market Commission (Comisión Nacional del Mercado de Valores or CNMV) and, therefore, no ADSs may be offered, sold or distributed in any manner, nor may any resale of the ordinary be carried out in Spain except in circumstances which do not constitute a public offer of securities in Spain or are exempted from the obligation to publish a prospectus, as set forth in Spanish Securities Market Act (Ley 24/1988, de 28 de julio, del Mercado de Valores) and Royal Decree 1310/2005, of 4 November, and other applicable regulations, as amended from time to time, or otherwise without complying with all legal and regulatory requirements in relation thereto. Neither the prospectus nor any offering or advertising materials relating to the ADSs have been or will be registered with the CNMV and therefore they are not intended for the public offer of the ADSs in Spain.
Switzerland
The ADSs may not be offered to the public in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. This document constitutes neither a public offer in Switzerland nor a prospectus supplement in accordance with applicable legislation in Switzerland and may not be issued, distributed or published in Switzerland in a manner which would be deemed to constitute a public offer of the ADSs in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company or the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this dicument will not be filed with, and the offer of ADSs will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of ADSs has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (“CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of securities.
United Arab Emirates
This prospectus is not intended to constitute an offer, sale or delivery of shares or other securities under the laws of the United Arab Emirates, or the UAE. This offering, the ADSs and interests therein have not been approved or licensed by
230
the Central Bank of the UAE, Securities and Commodities Authority of the UAE and/or any other relevant licensing authority in the UAE including any licensing authority incorporated under the laws and regulations of any of the free zones established and operating in the territory of the UAE, in particular the Dubai Financial Services Authority, or DFSA, a regulatory authority of the Dubai International Financial Centre, or DIFC, and do not constitute a public offer of securities under the laws of the UAE, DIFC and/or any other free zone in accordance with the Commercial Companies Law, Federal Law No 8 of 1984 (as amended), DFSA Offered Securities Rules and NASDAQ Dubai Listing Rules, accordingly, or otherwise. The ADSs have not been and will not be registered under Federal Law No. 4 of 2000 Concerning the Emirates Securities and Commodities Authority and the Emirates Security and Commodity Exchange, or with the UAE Central Bank, the Dubai Financial Market, the Abu Dhabi Securities Market or with any other UAE exchange.
In relation to its use in the UAE or any of its free zones, this prospectus is strictly private and confidential and is being distributed to a limited number of investors who qualify as sophisticated investors under the relevant laws and regulations of the UAE or the free zone concerned and must not be provided to any person other than the original recipient, and may not be reproduced or used for any other purpose. The interests in the ADSs may not be offered or sold directly or indirectly to the public in the UAE and/or any of the free zones.
United Kingdom
This prospectus is only being distributed to, and is only directed at, persons in the United Kingdom that are qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive, that are also (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended, or the Order, and/or (ii) high net worth entities falling within Article 49(2)(a) to (d) of the Order and (iii) any other person to whom it may lawfully be communicated pursuant to the Order (each such person being referred to as a Relevant Person).
This prospectus and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom. Any person in the United Kingdom that is not a Relevant Person should not act or rely on this document or any of its contents. Any investment or investment activity to which this prospectus relates will only be available to, and will only be engaged with, Relevant Persons.
All applicable provisions of the Financial Services and Markets Act 2000 (as amended) must be complied with in respect to anything done by any person in relation to our common stock in, from or otherwise involving the United Kingdom.
231
We estimate that expense in connection with the offering, other than underwriting fees and commissions, will be as follows:
Expense | Amount |
Securities and Exchange Commission registration fee | |* |
Comisión Nacional de Valores fees | |* |
NYSE listing fee | |* |
Financial Industry Regulatory Authority filing fee | |* |
Printing and engraving expenses | |* |
Legal fees and expenses (including tax advice) | |* |
Accounting fees and expenses | |* |
Transfer agent and registrar fees | |* |
Miscellaneous fees and expenses | |* |
Total | US$ |* |
| * | To be completed by amendment |
All of the above expenses will be paid by Bioceres.
232
The validity of the ADSs and certain other matters of New York law will be passed upon by Linklaters LLP, New York, New York. The validity of the ordinary shares and certain other matters relating to Argentine law will be passed upon by Marval, O’Farrell & Mairal. White & Case LLP is U.S. counsel to the international underwriters in connection with this offering, and Bruchou, Fernández Madero & Lombardi is Argentine counsel to the international underwriters in connection with this offering and the Argentine placement agents in connection with the Argentine Offering.
233
The consolidated financial statements of Bioceres as of June 30, 2017, December 31, 2016 and 2015 and for the six-month period ended June 30, 2017 and for each of the two years in the period ended December 31, 2016 included in this Prospectus have been so included in reliance on the report of Price Waterhouse & Co. S.R.L., an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
The consolidated financial statements of Rizobacter as of June 30, 2016 and 2015 and for each of the two years in the period ended June 30, 2016 included in this Prospectus have been so included in reliance on the report of Price Waterhouse & Co. S.R.L., an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
234
ENFORCEMENT OF CIVIL LIABILITIES
We are incorporated under the laws of Argentina. Substantially all of our and our subsidiaries’ assets are located outside the United States. All of our directors and all our officers and certain advisors named in this prospectus reside in Argentina. As a result, it may not be possible for investors to effect service of process within the United States upon such persons or to enforce against them or us in United States courts judgments predicated upon the civil liability provisions of the federal securities laws of the United States.
We have been advised by our Argentine counsel, Marval, O’Farrell & Mairal, of the uncertainty in terms of extent and timeliness for enforceability of liabilities predicated solely upon the federal securities laws of the United States in original actions in Argentine courts as compared to actions brought in a United States or other non-Argentine court, as well as with respect to the enforceability of judgments of United States courts in Argentine courts that were obtained in actions against us, that were predicated upon the civil liability provisions of the federal securities laws of the United States and which will be subject to compliance with certain requirements under Argentine law mentioned below, including the condition that any such judgment does not violate Argentine public policy (orden público).
If enforcement of a judgment issued by a U.S. court is sought before federal courts or courts with jurisdiction in commercial matters of the City of Buenos Aires, such judgment will be recognized and enforced by the courts in Argentina, provided that the requirements set out in Articles 517 through 519 of the Argentine Federal Civil and Commercial Procedure Code (if enforcement is sought before the Argentine federal courts) are met. Such requirements are as follows: (1) the judgment, which must be valid and final in the jurisdiction where rendered, was issued by a competent court in accordance with the Argentine principles regarding international jurisdiction and resulted from a personal action, or an in rem action with respect to personal property which was transferred to Argentine territory during or after the prosecution of the foreign action, (2) the defendant against whom enforcement of the judgment is sought was personally served with the summons and, in accordance with due process of law, was given an opportunity to defend against foreign action, (3) the authenticity of the judgment must be established in accordance with the requirements of Argentine law, (4) does not violate the principles of public policy of Argentine law, and (5) the judgment is not contrary to a prior or simultaneous judgment of an Argentine court. Any document in a language other than Spanish (including, without limitation, the foreign judgment and other documents related thereto) requires filing with the relevant court of a duly legalized translation by a sworn public translator into the Spanish language.
Enforcement of foreign judgments before the provincial courts of the Province of Santa Fe would be recognized provided that the requirements of Articles 269 through 271 of the Procedure Code of the Province of Santa Fe (approved by Law No. 5,531, as amended) are met as follows: (1) the judgement must not invalidate the jurisdiction of the Argentine courts; (2) if the defendant was domiciled in Argentina, the judgment must have not been issued as a default judgment; (3) the judgment must be valid according to Argentine law and must not violate the principles of public policy (public order) in Argentina; (4) the judgement must be valid in the jurisdiction where rendered and its authenticity must be established in accordance with the requirements of Argentine law; (5) if the jurisdiction where the judgment was rendered includes any additional requirement to enforce judgements rendered in Argentina, the judgement must also comply with such additional requirements; and (6) judgements rendered in Argentina must be enforceable in the jurisdiction where the judgment was rendered.
The filing of claims with the Argentine judicial system is subject to the payment of a court tax to be paid by the person filing a claim. Such tax rate varies from one jurisdiction to another (the current court tax in the courts sitting in the City of Buenos Aires is levied at a rate of 3% of the amount claimed, in conformity with Article 2 of Argentine Law No. 23,898; the current court tax in the courts sitting in the Province of Santa Fe is also levied, for ordinary proceedings, at a rate of 3% of the amount claimed, in conformity with Articles 35 and 36 of Santa Fe Province Tax Law No. 3650. Furthermore, pursuant to Argentine Law No. 26,589 (as amended), certain mediation procedures must be exhausted prior to the initiation of lawsuits in Argentina (with the exception, among others, of bankruptcy and executory proceedings, which executory proceedings include the enforcement of foreign judgments, in which case mediation procedures remain optional for the plaintiff). A similar procedure, that is optional for the party trying to enforce a judgment obtained abroad in the Province of Santa Fe, may be followed as provided by Provincial Law No. 13,515 and its regulatory Decree No. 1747/2011 of the Province of Santa Fe.
235
Subject to compliance with Article 517 of the Argentine Federal Civil and Commercial Procedure Code or Article 269 of the Procedure Code of the Province of Santa Fe, as described above, a judgment against us or the persons described above and obtained outside Argentina would be enforceable in Argentina without reconsideration of the merits. Nevertheless, a judgment’s recognition and enforcement in Argentina may be denied by Argentine courts in light of the March 6, 2014 decision of the Supreme Court of Argentina in Claren Corporation vs. Estado Nacional. In that case, the plaintiff was a bondholder who sought to recognize a U.S. judgment against the Argentine federal government. The Supreme Court of Argentina held that enforcing such judgment violated Argentine law principles of public policy. It further stated that granting the enforcement would imply that a plaintiff, through an individual action filed before a foreign court, would be able to circumvent the public debt restructuring process set forth by the federal government through emergency legislation enacted in accordance with the Argentine Constitution.
Pursuant to Article 519 bis of the National Civil and Commercial Procedure Code, awards issued by foreign arbitral tribunals can be enforced in Argentina following the procedure established for the enforcement of foreign judgments, provided that: (i) the applicable requirements of Article 517 are met; (ii) the waiver by a foreign court of its jurisdiction is not prohibited by law; and (iii) the matter debated in the case may be subjected to arbitration.
We have been further advised by our Argentine counsel that the ability of a judgment creditor or the other persons named above to satisfy a judgment by attaching certain assets of ours, our directors, our executive officers and/or the advisors named in this prospectus, respectively, may be limited by provisions in Argentine law.
A plaintiff (whether Argentine or non-Argentine) residing outside Argentina during the course of litigation in Argentina may be required to provide a bond to guarantee court costs and legal fees incurred under such litigation, if the plaintiff owns no real property in Argentina that could secure such payment. The aforementioned bond should have a value at least sufficient to pay for court fees and defendant’s attorney fees in the corresponding litigation, as determined by the Argentine judge. This requirement does not apply to the enforcement of foreign judgments. See also “Risk Factors—Risks Related to Our ADSs and the Offering—We are organized under the laws of Argentina and holders of our ADSs may find it difficult to enforce civil liabilities against us, our directors, officers and certain experts.”
236
On April 13, 2000, the Argentine Congress passed Law No. 25,246 (as amended, among others, by Laws No. 26,087, 26,119, 26,268, 26,683 and 26,734, together referred to as the Anti-Money Laundering Law) which defines money laundering as a crime. Furthermore, the Anti-Money Laundering Law, which supersedes several sections of the Argentine criminal code, established severe penalties for anyone participating in any such criminal activity and created the Financial Information Unit (the FIU or UIF for its acronym in Spanish) which established an administrative criminal system.
Below is a summary of certain provisions regarding the anti-money laundering regime as set forth by the Anti-Money Laundering Law, as amended and supplemented by other rules and regulations, including regulations issued by the UIF, the Central Bank, the CNV and other regulatory entities. Investors are advised to consult their own legal counsel and to read the Anti-Money Laundering Law and its related regulations. The UIF is the agency responsible for the analysis, treatment and transmission of information, with the aim to prevent money laundering that may result from crimes and the financing of terrorism. The Argentine Criminal Code defines money laundering as a crime committed by any person who exchanges, transfers, manages, sells, levies, disguises or in any other way commercializes goods obtained through a crime with the possible consequence of making the original assets or the substitute thereof appear to come from a lawful source, provided that their value exceeds Ps.300,000 (or approximately US$17,231, using the September 30, 2017 exchange rate of Ps.17.32 to US$1.00 reported by the Central Bank), whether such amount results from one or more related transactions. The penalties established for such activity are as follows:
| ■ | imprisonment for three (3) to ten (10) years and fines of two (2) to ten (10) times the amount of the transaction; |
| ■ | the penalty provided in the precedent paragraph shall be increased by one third of the maximum and a half of the minimum, when (a) the person carries out the act on a regular basis or as a member of an association or organized group with the aim to continuously commit acts of a similar nature, and (b) the person is an officer of the government who carries out the act in the course of his or her duties; or |
| ■ | if the value of the assets does not exceed Ps.300,000, the penalty shall be imprisonment for a period ranging between six (6) months to three (3) years. |
The Argentine Criminal Code further punishes any person who receives money or other assets from a criminal source with the purpose of applying such funds to a transaction to make them appear to have come from a lawful source, with imprisonment for three to six years.
In line with internationally accepted practices, the Anti-Money Laundering Law does not merely assign responsibility for controlling these criminal transactions to government agencies, but also assigns certain duties to various private sector entities such as banks, stockbrokers, brokerage houses and insurance companies as legally bound reporting parties. These duties primarily consist of information capturing functions.
According to the Anti-Money Laundering Law, the following persons, among others, are subject to UIF reporting: (i) financial institutions and insurance companies; (ii) exchange agencies and individuals or legal entities authorized by the Central Bank to operate in the purchase and sale of foreign currency in the form of cash or checks drawn in foreign currency or by means of credit or debit cards or in the transfer of funds within Argentina or abroad; (iii) broker-dealers, companies managing investment funds, over-the-counter market agents, and intermediaries engaged in the purchase, lease, or borrowing of securities; (iv) armored transportation services companies and companies or concessionaires rendering postal services that carry out foreign currency transfers or remittance of different types of currency or notes; (v) governmental organizations, such as the Central Bank, the Argentine Tax Authority, the National Superintendent of Insurance (Superintendencia de Seguros de la Nación), the CNV and the Public Registry of the City of Buenos Aires; (vi) professionals in economics sciences and notaries public; and (vii) individuals and legal entities acting as trustees of any kind and individuals or legal entities related directly or indirectly to trust accounts, trustees and trustors under trust agreements.
Individuals and entities subject to the Anti-Money Laundering Law must comply with certain duties that include: (i) obtaining documentation from their customers that irrefutably evidences their identity, legal status, domicile, and other data stipulated in each case (so-called “know your customer” policies), comply with Resolution UIF No. 11/2011, as amended, with respect to politically exposed persons, check the client’s name against the list of terrorists and/or
237
terrorist organizations (Resolution UIF No. 29/2013) and request information about products to be used and the reasons therefore); (ii) reporting any suspicious event or transaction (which, according to the customary practices of the field involved as well as the experience and competence of the parties who have the duty to inform, are those transactions attempted or consummated that were previously identified as unusual transactions by the legally bound reporting party or have no economic or legal justification or relations with the customer’s risk or transactional profile or are unusually or unjustifiably complex, whether performed on a single occasion or repeatedly (regardless of the amount)); and (iii) abstaining from disclosing to customers or third parties any act performed in compliance with the Anti-Money Laundering Law. If a transaction is suspected to be money laundering, such reports shall be made by the legally bound reporting parties within 15 days from the date on which the operation is qualified as suspicious whenever the legally bound reporting party is subject to Resolution UIF No. 30/2017; and within the maximum term of 150 days from the day on which the transaction was made, for all legally bound reporting parties. If the legally bound reporting parties suspect terrorist financing, the term is reduced to a maximum of 48 (forty-eight) hours from the date on which the transaction was made. Within the framework of analysis of a suspicious transaction report, the aforementioned individuals and entities cannot refrain from disclosing to the UIF any information required from it by claiming that such information is subject to bank, stock market or professional secrecy, or legal or contractual confidentiality agreements. The AFIP shall only disclose to UIF the information in its possession when the suspected transaction report has been made by such entity and refers to the individuals or entities directly involved with the reported transaction. In all other cases the UIF shall request that the federal judge holding authority in the criminal matter order the AFIP to disclose the information in its possession. Additionally, in accordance with Resolution UIF No. 50/2011, as supplemented, among others, by Resolution UIF No. 460/2015, the Obligors and their respective compliance officers, have the obligation to register through www.argentina.gob.ar/uif in the Operations Reporting System, or ORS, of the UIF, as well as to submit in paper before the UIF’s reception desk, within 15 administrative working days as of registration in the ORS, all documentation supporting such registration.
Argentine financial institutions must comply with all applicable anti-money laundering regulations as provided by the Central Bank, the UIF, and, if applicable, the CNV. In this regard, in accordance with Resolution No. 229/2014 of the UIF, both the Central Bank and the CNV are considered “Specific Control Organs.” In such capacity, they must cooperate with the UIF in evaluating the compliance with the anti-money laundering proceedings by the legally bound reporting parties subject to their control. In that respect, they are entitled to supervise, monitor and inspect such entities, and if necessary, to implement certain corrective measures and actions. Resolution 121/2011 issued by the UIF, as amended and to which we refer as Resolution No. 121, is applicable to financial entities subject to Law No. 21,526, to entities subject to Law No. 18,924, as amended, and to individuals and legal entities authorized by the Central Bank to intervene in the purchase and sale of foreign currency through cash or checks issued in foreign currency or through the use of credit or payment cards, or in the transfer of funds within or outside the national territory. Resolution No. 30/2017 replaced Resolution No. 121 and entered into force on September 15, 2017. Notwithstanding as set forth below, the new resolution gives the legally bound reporting parties more time to comply with certain new obligations. Resolution No. 229/2011 of the UIF, as amended or supplemented by Resolutions No. 140/2012, 104/2016 and 4/2017 among others and to which we refer as Resolution No. 229, is applicable to brokers and brokerage firms, companies managing common investment funds, agents of the over-the-counter market, intermediaries in the purchase or leasing of securities affiliated with stock exchange entities with or without associated markets, and intermediary agents registered on forwards or option markets. Resolution No. 121 and Resolution No. 229 regulate, among other things, the obligation to collect documentation from customers as well as the terms, obligations and restrictions for compliance with the reporting duty regarding suspicious money laundering and terrorism financing transactions.
Resolution No. 229 set forth general guidelines in connection with the customer’s identification (including the distinction between occasional and regular customers), the information to be requested, the documentation to be filed and the procedures to detect and report suspicious transactions. Furthermore, the aforementioned resolutions established that these legally bound parties must implement a due diligence procedure based on risk approach in order to comply with the “know your customer” policies. Resolution No. 30 replaced Resolution No. 121, has been issued based on the standards of the Financial Action Task Force, or the FATF, with an approach based on risks. Pursuant to Resolution No. 30, financial entities will develop a risk identification methodology and evaluation to prevent risks according to their nature and activities. Resolution No. 30 contemplates a gradual implementation plan by which: (i) such new methodology should be developed by the legally bound reporting parties by December 31, 2017; (ii) a
238
technical report that reflects the results of such methodology should be issued by March 31, 2018; and (iii) the implementation of policies and procedures should be completed by June 30, 2018.
Likewise, in accordance with Resolution No. 04/2017 of the UIF, a special due diligence procedure (based on risk approach) has been established for the remote opening of special investment accounts. It is applicable to the obligated subjects included in subsections 1, 4 and 5 of article 20 of the Anti-Money Laundering Law.
The Central Bank and the CNV must also comply with anti-money laundering regulations set forth by the UIF, including reporting suspicious transactions. In particular, the Central Bank must comply with UIF Resolution No. 12/2011, as supplemented by, among other resolutions, Resolutions No. 1/2012 and No 92/2012, which, among other things, sets forth the Central Bank’s obligation to evaluate the anti-money laundering controls implemented by Argentine financial institutions (with the limitation of access to the reports and records of suspicious operations, which are, as explained above, confidential and subject only to the UIF’s supervision), and lists examples of what circumstances should be specifically considered in order to establish whether a particular transaction may be considered unusual and eventually qualified as suspicious.
Central Bank regulations require Argentine banks to take certain minimum precautions to prevent money laundering. Each institution must have an anti-money laundering committee, formed by a member of the board of directors and an officer responsible for anti-money laundering matters (oficial de cumplimiento). Additionally, each financial institution must appoint a member of the board of directors as the person responsible for (i) money laundering prevention, (ii) centralizing any information the Central Bank may require by its own initiative or at the request of any competent authority and (iii) reporting any suspicious transactions to the UIF. Notwithstanding the individual board member’s role as a liaison with the UIF, all board members have personal, joint, several and unlimited responsibility for the entity’s compliance with its reporting duties with the UIF. Furthermore, the board member will be responsible for the implementation, tracking and control of internal procedures to ensure compliance with the regulations in financial institutions and its subsidiaries.
In addition, pursuant to Communication “A” 5738 of the Central Bank (as amended and supplemented by Communication “A” 6090, among others), Argentine financial institutions must comply with certain additional “know your customer” policies. Pursuant to such Communication, under no circumstance may new commercial relationships be initiated if the “know your customer” policies and the risk management legal standards have not been complied with. Furthermore, in respect to the existing customers, if the “know your customer” policies could not be complied with, the Argentine financial institution must analyze the continuance of the commercial relationship with such client using a risk-based approach. In case the operations with the client are discontinued, the termination of the relationship must be implemented in accordance with Central Bank’s regulations for each type of product. Operations do not have to be discontinued when the “know your customer” policies are complied with in such period or when simplified due diligence procedures were implemented pursuant to applicable laws. Furthermore, pursuant to this Communication, Argentine financial entities must keep the documentation related to the discontinuance for ten years and include in their prevention manuals the detailed procedures to initiate and discontinue operations with customers in accordance with the above-mentioned additional “know your customer” policies implemented.
The CNV Rules (as amended in September 2013) include a specific chapter regarding the “Prevention of Money Laundering and the Financing of Terrorism” which states that the persons set forth therein (negotiation agents, clearing and settlement agents such as (such as stockbrokers), distribution and placement agents, manager and custody agents of collective investment funds, brokerage agents, collective depositary agents, issuers with respect to capital contributions, irrevocable capital contributions for future capital increases or significant loans that have been made in its benefit, specifically with respect to the identity of contributors and/or creditors and the origin and legality of the funds so contributed or loaned) are to be considered legally bound reporting parties under the Anti-Money Laundering Law and therefore must comply with all the laws and regulations in force in connection with anti-money laundering and terrorism financing, including resolutions issued by the UIF, presidential decrees referring to resolutions issued by the UN Security Council in connection with the fight against terrorism and the resolutions (and its annexes) issued by the Ministry of Foreign Affairs. Furthermore, CNV Rules impose certain restrictions in connection with payment arrangements (limiting, among other things, the cash amount that the entities set forth therein could receive or pay per day and per client, to Ps.1,000, or its equivalent in other currencies) and impose certain reporting obligations.
239
In addition, the CNV Rules establish that the above-mentioned entities shall only be able to carry out any transactions contemplated under the public offering system, when such transactions are carried out or ordered by persons organized, domiciled or residing in dominions, jurisdictions, territories or associated States included in the cooperating countries list contained in Executive Decree No. 589/2013, section 2(b). If such persons are not included in such list and, under their home jurisdiction, they qualify as registered intermediaries in an entity under control and supervision of a body that carries out similar functions to those carried out by the CNV, such persons will only be allowed to carry out such transactions by providing evidence indicating that the relevant securities and exchange commission in their home jurisdiction has signed a memorandum of understanding for cooperation and exchange of information with the CNV.
Recently, the “National Coordination Program for the Prevention of Asset Laundering and the Financing of Terrorism” was created by Executive Decree No. 360/2016 as an instrument of the Ministry of Justice and Human Rights. This Program was formed to reorganize, coordinate and strengthen the national system for the prevention of money laundering and the financing of terrorism, taking into consideration the specific risks that might have an impact on Argentine territory and the global demand for a more effective compliance with international obligations and recommendations established under UN Conventions and the standards of the Financial Action Task Force, or FATF. These duties will be performed and implemented by a National Coordinator appointed for this particular purpose. In addition, through the modification of applicable statutory rules, the Ministry of Justice and Human Rights was established as the federal government’s central authority in charge of the inter-institutional coordination among all public and private agencies and entities with competent jurisdiction on this matter, while the UIF will retain the ability to perform operating coordination activities at the national, provincial and municipal levels in relation to matters strictly inherent in its jurisdiction as a financial intelligence agency.
Through the issuance of Law No. 27,260 and its regulatory decree No. 895/2016, the UIF was given the authority to communicate information to other public entities and was empowered with intelligence or investigatory authority. This authority may only be exercised after a founded resolution of the President of the UIF, and providing that there is serious, precise and consistent evidence regarding the commission of any of the crimes under the Anti-Money Laundering Law. Such communications, shall include language regarding the transfer of the duty of confidentiality established under article 22 of the Anti-Money Laundering Law, which prescribes liability for those officers of a receiving entity that, by themselves or through another, reveal confidential information. The UIF will not exercise the power referred to in cases related to voluntary and exceptional statements made under Law No.27, 260.
Accordingly, in August 2016, the UIF issued Resolution No. 92/2016, which required those captured by the Resolution to implement a risk management system according to the “Sistema voluntario y excepcional de declaración de tenencia de moneda nacional, extranjera y demás bienes en el país y en el exterior” established under Law No. 27,260, for the purpose of reporting suspicious transactions carried out by clients up to March 31, 2017, arising from the fiscal sincerity regime. Such report must be duly substantiated and contain a description of the circumstances in which the transaction was deemed to be suspicious and should reveal an adequate analysis of the operation and the profile of the client (in this case, the requirements related to tax documentation are not necessary). Pursuant to Law No. 25,246 and Decree No. 2/2017 the UIF became a financially independent instrumentality of the Ministry of Finance.
For an extensive analysis of the money laundering regime in effect as of the date of this prospectus, investors should consult legal counsel and read Title XIII, Book 2 of the Argentine Criminal Code and any regulations issued by the UIF, the CNV and the Central Bank in their entirety. For such purposes, interested parties may visit the websites of the Argentine Ministry of Justice and Human Rights, at www.infoleg.gov.ar, the UIF, at https://www.argentina.gob.ar/uif, the CNV, at www.cnv.gob.ar, or the Central Bank, at www.bcra.gov.ar. The information found on such websites is not a part of this prospectus.
240
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We will file with the SEC a registration statement on Form F-1 under the Securities Act relating to this offering of our ordinary shares. This prospectus does not contain all of the information contained in the registration statement. The rules and regulations of the SEC allow us to omit certain information from this prospectus that is included in the registration statement. Statements made in this prospectus concerning the contents of any contract, agreement or other document are summaries of all material information about the documents summarized, but are not complete descriptions of all terms of these documents. If we filed any of these documents as an exhibit to the registration statement, you may read the document itself for a complete description of its terms.
You may read and copy the registration statement, including the related exhibits and schedules, and any document we file with the SEC without charge at the SEC’s public reference room at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Room 1580, Washington, DC 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference room. The SEC also maintains an Internet website that contains reports and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are also available to the public through the SEC’s website at http://www.sec.gov.
We are not currently subject to the informational requirements of the Exchange Act. Upon completion of this offering, we will be subject to the information reporting requirements of the Exchange Act that are applicable to foreign private issuers, and under those requirements will file reports with the SEC. Those other reports or other information may be inspected without charge at the locations described above. As a foreign private issuer, we will be exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders will be exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. However, we will file with the SEC, within four months after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm, and will submit to the SEC, on Form 6-K, unaudited quarterly financial information for the first three quarters of each fiscal year within 60 days after the end of each such quarter, or such applicable time as required by the SEC, as well as all the annual, quarterly and current financial and non-financial information we file with the CNV.
We maintain a corporate website at www.bioceres.com.ar. Information contained on, or that can be accessed through, our website does not constitute a part of this prospectus. We have included our website address in this prospectus solely for information purposes.
241
Interim Condensed Consolidated Financial Statements for Bioceres S.A. as of September 30, 2017 and June 30, 2017 and for the three-month periods ended on September 30, 2017 and 2016. | |||
Audited Consolidated Financial Statements for Bioceres S.A. and its subsidiaries as of and for the Transition Period ended June 30, 2017 and as of and for the fiscal years ended December 31, 2016 and 2015 and for the unaudited six-month period ended June 30, 2016. | |||
Interim Condensed Consolidated Financial Statements for Rizobacter Argentina S.A. as of September 30, 2017 and June 30, 2017 and for the three-month periods ended on September 30, 2017 and 2016. | |||
Audited Consolidated Financial Statements for Rizobacter Argentina S.A. as of and for the fiscal years ended June 30, 2017, 2016 and 2015. | |||
F-1
BIOCERES S.A.
INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
Interim Condensed Consolidated Financial Statements as of September 30, 2017 and June 30, 2017
and for the three-month period ended on September 30, 2017 and 2016
Amounts expressed in United States Dollars (“USD”)
Name: | BIOCERES S.A. | |
Legal Address: | Ocampo 210 Bis - Predio CCT Rosario – Province of Santa Fe - Argentina | |
Main activities of Bioceres S.A.: | Investments in other companies. Investments in projects and goods related to biotechnology and technologies related to food, agribusiness and healthcare industries. | |
Main activities of the Group controlled by Bioceres S.A.: | Sale of seeds and provision of technology sourcing and product development services in the agricultural biotechnology sector, including through joint ventures and third-party collaborations. | |
Registration before the Public Registry of Commerce: | Bylaws | April 11, 2002 |
Last amendment | July 31, 2015 | |
Bylaws expiry date: | April 11, 2052 | |
F-2
BIOCERES S.A.
CERTAIN DEFINED TERMS
ARS: Argentine peso (legal currency in Republic of Argentina)
Argentina: Republic of Argentina
ARG GAAP: Argentine Generally Accepted Accounting Principles
BAF: BAF Capital. Financial servicies.
Bioceres: Bioceres S.A. (Company incorporated in Republic of Argentina)
Bioceres, Inc.: Bioceres, Inc. (a wholly owned subsidiary of Bioceres S.A., incorporated in United States)
Bioceres Semillas: Bioceres Semillas S.A. (Subsidiary of Bioceres S.A., incorporated in Republic of Argentina)
BYMA: Bolsas y mercados argentinos S.A.
Chemotécnica: Chemoténica S.A. (Company incorporated in Republic of Argentina)
CNV: Comisión Nacional de Valores (Argentine Securities and Exchange Commission)
Company: Bioceres S.A. (Company incorporated in Republic of Argentina)
EPS: Earnings per share (IAS 33)
FACPCE: Federación Argentina de Consejos Profesionales de Ciencias Económicas (accounting and auditing standards setter in Argentina)
Group: Collectively, Bioceres S.A. and all its subsidiaries
Héritas: Héritas S.A (Company incorporated in Republic of Argentina)
IASB: International Accounting Standards Board (IFRS Standards Setter)
IFRIC: IFRS Interpretation Committee (or Interpretation issued by the IFRS Interpretation Committee, if preceded by a number)
INDEAR: Instituto de Agrobiotecnología Rosario S.A. (subsidiary of Bioceres S.A, incorporated in Republic of Argentina)
INMET: Ingeniería Metabólica S.A. (subsidiary of Bioceres S.A, incorporated in Republic of Argentina)
Management: collectively, the members of Management of Bioceres S.A.
IAS: International Accounting Standard
IFRS: International Financial Reporting Standards (or International Financial Reporting Standard, if preceded by a number)
NYSE: New York Stock Exchange
IPO: Initial Public Offering
NCI: non-controlling interest
RASA Holding: RASA Holding, LLC (a wholly owned subsidiary of Bioceres Inc., incorporated in United States)
R&D: Research and development
RT: Resolución Técnica (accounting and auditing standard issued by FACPCE)
Rizobacter: Rizobacter Argentina S.A. (Subsidiary of Bioceres S.A., incorporated in Republic of Argentina)
SEC: Securities and Exchange Commission
Semya: Semya S.A. (Company incorporated in Republic of Argentina)
Synertech: Synertech S.A. (Joint arrangement between Rizobacter S.A. and De Sangosse L.A., incorporated in Republic of Argentina)
S&W Semillas: S&W Semillas S.A. (Company incorporated in Republic of Argentina)
Trigall: Trigall Genetics S.A. (Company incorporated in Republic of Uruguay)
USD: United States Dollars
F-3
BIOCERES S.A.
INTERIM CONDENSED CONSOLIDATED STATEMENT OF FINANCIAL POSITION
At September 30, 2017 and June 30, 2017
(Amounts in USD)
Notes | Pro forma (Note 2.7) 09/30/2017 | 09/30/2017 | 06/30/2017 | |||||||||
(unaudited) | (unaudited) | |||||||||||
ASSETS | ||||||||||||
CURRENT ASSETS | ||||||||||||
Cash and cash equivalents | 4.1 | 1,590,773 | 1,590,773 | 2,119,883 | ||||||||
Other financial assets | 4.2 | 4,450,887 | 4,450,887 | 4,526,960 | ||||||||
Trade receivables | 4.3 | 55,234,392 | 55,234,392 | 45,693,673 | ||||||||
Other receivables | 4.4 | 7,955,094 | 7,955,094 | 4,179,869 | ||||||||
Income and minimum presumed income taxes recoverable | 1,622,586 | 1,622,586 | 2,107,860 | |||||||||
Inventories | 4.5 | 36,871,630 | 36,871,630 | 31,723,752 | ||||||||
Total current assets | 107,725,362 | 107,725,362 | 90,351,997 | |||||||||
NON-CURRENT ASSETS | ||||||||||||
Other financial assets | 4.2 | 3,202,239 | 3,202,239 | 3,292,758 | ||||||||
Other receivables | 4.4 | 9,182,183 | 9,182,183 | 5,631,095 | ||||||||
Income and minimum presumed income taxes recoverable | 663,113 | 663,113 | 691,447 | |||||||||
Deferred tax assets | 8,315,318 | 8,315,318 | 7,272,648 | |||||||||
Investments in joint ventures | 9 | 28,124,407 | 28,124,407 | 28,977,935 | ||||||||
Property, plant and equipment | 4.6 | 48,571,389 | 48,571,389 | 51,110,617 | ||||||||
Intangible assets | 4.7 | 40,175,146 | 40,175,146 | 42,181,770 | ||||||||
Goodwill | 4.8 | 31,571,359 | 31,571,359 | 32,866,203 | ||||||||
Total non-current assets | 169,805,154 | 169,805,154 | 172,024,473 | |||||||||
Total assets | 277,530,516 | 277,530,516 | 262,376,470 | |||||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-4
BIOCERES S.A.
INTERIM CONDENSED CONSOLIDATED STATEMENT OF FINANCIAL POSITION (Cont’d)
At September 30, 2017 and June 30, 2017
(Amounts in USD)
Notes | Pro forma (Note 2.7) 09/30/2017 | 09/30/2017 | 06/30/2017 | |||||||||
(unaudited) | (unaudited) | |||||||||||
LIABILITIES | ||||||||||||
CURRENT LIABILITIES | ||||||||||||
Trade and other payables | 4.9 | 39,070,197 | 39,070,197 | 23,748,712 | ||||||||
Borrowings | 4.10 | 43,376,781 | 60,310,507 | 51,020,481 | ||||||||
Employee benefits and social security | 4.11 | 6,008,683 | 6,008,683 | 5,570,209 | ||||||||
Deferred revenue and advances from customers | 4.12 | 2,584,436 | 2,584,436 | 1,256,975 | ||||||||
Income and minimum presumed income taxes payable | 271,386 | 271,386 | 223,187 | |||||||||
Government grants | 4.13 | 311,917 | 311,917 | 351,157 | ||||||||
Puttable instruments | 4.15 | 225,131 | 225,131 | — | ||||||||
Financed payment - Acquisition of business | 4.16 | 6,340,813 | 27,096,236 | 26,477,432 | ||||||||
Total current liabilities | 98,189,344 | 135,878,493 | 108,648,153 | |||||||||
NON-CURRENT LIABILITIES | ||||||||||||
Borrowings | 4.10 | 36,568,583 | 36,568,583 | 41,106,995 | ||||||||
Government grants | 4.13 | 1,388,258 | 1,388,258 | 1,485,079 | ||||||||
Puttable instruments | 4.15 | 2,338,909 | 5,127,402 | 5,008,467 | ||||||||
Investments in joint ventures | 9 | 1,726,690 | 1,726,690 | 1,527,286 | ||||||||
Deferred tax liabilities | 22,786,161 | 22,786,161 | 24,620,369 | |||||||||
Provisions | 4.14 | 1,638,381 | 1,638,381 | 1,690,412 | ||||||||
Financed payment - Acquisition of business | 4.16 | 21,591,651 | 34,049,246 | 33,402,933 | ||||||||
Contingent consideration - Acquisition of business | 4.17 | 15,916,114 | 15,916,114 | 15,919,060 | ||||||||
Total non-current liabilities | 103,954,747 | 119,200,835 | 124,760,601 | |||||||||
Total liabilities | 202,144,091 | 255,079,328 | 233,408,754 | |||||||||
EQUITY | ||||||||||||
Issued capital | 7.1 | 6,968,538 | 6,968,538 | 6,968,538 | ||||||||
Share premium | 7.1 | 78,294,470 | 15,599,688 | 15,461,569 | ||||||||
Puttable instruments | (2,025,398 | ) | (2,025,398 | ) | (2,025,398 | ) | ||||||
Cost of own shares held by subsidiary | 7.1 | (456,480 | ) | (456,480 | ) | (726,822 | ) | |||||
Stock options | 1,222,226 | 1,222,226 | 1,067,278 | |||||||||
Accumulated losses | (28,958,718 | ) | (28,958,718 | ) | (25,891,909 | ) | ||||||
Revaluation of property, plant and equipment reserve | 1,219,600 | 1,219,600 | 1,219,600 | |||||||||
Foreign currency translation reserve | (9,529,019 | ) | (9,529,019 | ) | (6,576,793 | ) | ||||||
Equity / (deficit) attributable to owners of the parent | 46,735,219 | (15,959,563 | ) | (10,503,937 | ) | |||||||
Non-controlling interests | 28,651,206 | 38,410,751 | 39,471,653 | |||||||||
Total equity | 75,386,425 | 22,451,188 | 28,967,716 | |||||||||
Total equity and liabilities | 277,530,516 | 277,530,516 | 262,376,470 | |||||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-5
BIOCERES S.A.
INTERIM CONDENSED CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME
For the three-month period ended September 30, 2017 and 2016
(Amounts in USD)
Notes | 09/30/2017 | 09/30/2016 | |||||
(unaudited) | |||||||
Revenue | 5.1 | 34,828,443 | 1,846,295 | ||||
Government grants | 4.13 | 158,062 | 117,218 | ||||
Total revenue | 34,986,505 | 1,963,513 | |||||
Cost of sales | 5.2 | (20,195,931 | ) | (1,118,948 | ) | ||
Research and development expenses | 5.3 | (1,725,645 | ) | (596,652 | ) | ||
Selling, general and administrative expenses | 5.4 | (9,993,183 | ) | (1,185,472 | ) | ||
Share of income / (loss) of joint ventures and associates | 9 | 84,857 | (238,100 | ) | |||
Other expenses, net | (217,284 | ) | (18,135 | ) | |||
Operating income / (loss) | 2,939,319 | (1,193,794 | ) | ||||
Finance income | 5.5 | 2,042,066 | 381,327 | ||||
Finance costs | 5.6 | (9,268,419 | ) | (1,117,154 | ) | ||
Loss before income tax | (4,287,034 | ) | (1,929,621 | ) | |||
Income tax benefit | 1,357,795 | 621,022 | |||||
Loss for the period | (2,929,239 | ) | (1,308,599 | ) | |||
Other comprehensive income or loss | |||||||
Items that may be subsequently reclassified to profit and loss | (4,150,698 | ) | — | ||||
Exchange differences on translation of foreign operations from joint ventures | (1,137,789 | ) | — | ||||
Exchange differences on translation of foreign operations | (3,012,909 | ) | — | ||||
Total comprehensive loss | (7,079,937 | ) | (1,308,599 | ) | |||
Profit / (loss) for the period attributable to: | |||||||
Equity holders of the parent | (3,066,809 | ) | (1,214,922 | ) | |||
Non-controlling interests | 137,570 | (93,677 | ) | ||||
(2,929,239 | ) | (1,308,599 | ) | ||||
Total comprehensive loss attributable to: | |||||||
Equity holders of the parent | (6,019,035 | ) | (1,214,922 | ) | |||
Non-controlling interests | (1,060,902 | ) | (93,677 | ) | |||
(7,079,937 | ) | (1,308,599 | ) | ||||
Earnings per share | |||||||
Basic loss attributable to ordinary equity holders of the parent | 6 | (0.1200 | ) | (0.0474 | ) | ||
Diluted loss attributable to ordinary equity holders of the parent | 6 | (0.1200 | ) | (0.0474 | ) | ||
The accompanying Notes are an integral part of these consolidated financial statements
F-6
BIOCERES S.A.
INTERIM CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
For the three-month period ended September 30, 2017 and 2016
(Amounts in USD)
Description | Attributable to the equity holders of the parent | Non- controlling Interests | Total equity | ||||||||||||||||||||||||||||||||||||
Shareholder`s contributions | Accumulated losses | Equity / (deficit) attributable to owners of the parent | |||||||||||||||||||||||||||||||||||||
Issued capital (Note 7.1) | Share premium | Puttable instruments | Cost of own shares held by subsidiary | Stock options | Subtotal | Accumulated losses | Foreign currency translation reserve | Revaluation of property, plant and equipment reserve | Subtotal | ||||||||||||||||||||||||||||||
06/30/2017 | 6,968,538 | 15,461,569 | (2,025,398 | ) | (726,822 | ) | 1,067,278 | 20,745,165 | (25,891,909 | ) | (6,576,793 | ) | 1,219,600 | (31,249,102 | ) | (10,503,937 | ) | 39,471,653 | 28,967,716 | ||||||||||||||||||||
Sale of own shares by subsidiary (Note 7.1) | — | 138,119 | — | 270,342 | — | 408,461 | — | — | — | — | 408,461 | — | 408,461 | ||||||||||||||||||||||||||
Stock options | — | — | — | — | 154,948 | 154,948 | — | — | — | — | 154,948 | — | 154,948 | ||||||||||||||||||||||||||
Loss for the period | — | — | — | — | — | — | (3,066,809 | ) | — | — | (3,066,809 | ) | (3,066,809 | ) | 137,570 | (2,929,239 | ) | ||||||||||||||||||||||
Other comprehensive loss | — | — | — | — | — | — | — | (2,952,226 | ) | — | (2,952,226 | ) | (2,952,226 | ) | (1,198,472 | ) | (4,150,698 | ) | |||||||||||||||||||||
09/30/2017 (unaudited) | 6,968,538 | 15,599,688 | (2,025,398 | ) | (456,480 | ) | 1,222,226 | 21,308,574 | (28,958,718 | ) | (9,529,019 | ) | 1,219,600 | (37,268,137 | ) | (15,959,563 | ) | 38,410,751 | 22,451,188 | ||||||||||||||||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-7
BIOCERES S.A.
INTERIM CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
For the three-month period ended September 30, 2017 and 2016
(Amounts in USD)
Description | Attributable to the equity holders of the parent | Non- controlling Interests | Total equity | ||||||||||||||||||||||||||||||
Shareholder’s contributions | Accumulated losses | Equity / (deficit) attributable to owners of the parent | |||||||||||||||||||||||||||||||
Issued capital (Note 7.1) | Share premium | Puttable instruments | Cost of own shares held by subsidiary | Stock options | Subtotal | Accumulated losses | Subtotal | ||||||||||||||||||||||||||
06/30/2016 (unaudited) | 6,968,538 | 15,461,569 | (2,237,956 | ) | (270,342 | ) | 634,248 | 20,556,057 | (16,351,870 | ) | (16,351,870 | ) | 4,204,187 | (185,754 | ) | 4,018,433 | |||||||||||||||||
Stock options | — | — | — | — | 296,196 | 296,196 | — | — | 296,196 | — | 296,196 | ||||||||||||||||||||||
Loss for the period | — | — | — | — | — | — | (1,214,922 | ) | (1,214,922 | ) | (1,214,922 | ) | (93,677 | ) | (1,308,599 | ) | |||||||||||||||||
09/30/2016 (unaudited) | 6,968,538 | 15,461,569 | (2,237,956 | ) | (270,342 | ) | 930,444 | 20,852,253 | (17,566,792 | ) | (17,566,792 | ) | 3,285,461 | (279,431 | ) | 3,006,030 | |||||||||||||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-8
BIOCERES S.A.
INTERIM CONDENSED CONSOLIDATED STATEMENT OF CASH FLOWS
For the three-month period ended September 30, 2017 and 2016
(Amounts in USD)
Notes | 09/30/2017 | 09/30/2016 | |||||||
(unaudited) | |||||||||
OPERATING ACTIVITIES | |||||||||
Loss for the period | (2,929,239 | ) | (1,308,599 | ) | |||||
Adjustments to reconcile profit / (loss) to net cash flows | |||||||||
Income tax (benefit) | (1,357,795 | ) | (621,022 | ) | |||||
Depreciation of property, plant and equipment | 4.6 | 771,724 | 146,061 | ||||||
Amortization of intangible assets | 4.7 | 580,983 | 11,854 | ||||||
Share of income/(loss) of joint ventures and associates | 9 | (84,857 | ) | 238,100 | |||||
Changes in fair value of financial assets | 166,592 | (236,500 | ) | ||||||
Provisions for contingencies | 4.18 | 14,571 | 14,009 | ||||||
Allowance for impairment of trade debtors | 381,551 | — | |||||||
Allowance for obsolescence | 19,923 | — | |||||||
Puttable instruments interests | 344,066 | 55,323 | |||||||
Stock option | 154,948 | 296,196 | |||||||
Gain or loss on sale of equipment and intangible assets | 121,768 | — | |||||||
Interests and exchange differences from borrowings | 1,929,311 | 223,727 | |||||||
Other financial results accrued | 1,061,107 | — | |||||||
Working capital adjustments | |||||||||
Trade receivables | (9,922,270 | ) | (994,937 | ) | |||||
Other receivables | (3,589,224 | ) | (495,895 | ) | |||||
Income and minimum presumed income taxes | (301,362 | ) | (34,614 | ) | |||||
Inventories | (5,167,801 | ) | 1,012,794 | ||||||
Trade and other payables | 13,608,164 | (531,802 | ) | ||||||
Employee benefits and social security | 438,474 | 200,728 | |||||||
Deferred revenue and advances from customers | 1,327,461 | 38,885 | |||||||
Government grants | (136,061 | ) | 184,691 | ||||||
Net cash flows used in operating activities | (2,567,966 | ) | (1,801,001 | ) | |||||
INVESTMENT ACTIVITIES | |||||||||
Investment in joint ventures and associates | 9 | — | (79,207 | ) | |||||
Proceeds from sale of property, plant and equipment | 30,417 | — | |||||||
Purchase of property, plant and equipment | 4.6 | (315,395 | ) | (661,295 | ) | ||||
Loans granted to joint ventures | (2,054,184 | ) | — | ||||||
Capitalized development expenditures | 4.7 | (111,513 | ) | (68,373 | ) | ||||
Purchase of intangible assets | 4.7 | (3,403 | ) | — | |||||
Net cash flows used in investing activities | (2,454,078 | ) | (808,875 | ) | |||||
FINANCING ACTIVITIES | |||||||||
Proceeds from borrowings | 70,194,350 | 1,000,000 | |||||||
Repayment of borrowings and interest payments | (69,467,436 | ) | (22,616 | ) | |||||
Net increase (decrease) bank overdraft and other short-term borrowings | 3,357,559 | (33,334 | ) | ||||||
Sale of own shares by subsidiary | 408,461 | — | |||||||
Net cash flows provided by financing activities | 4,492,934 | 944,050 | |||||||
Net decrease in cash and cash equivalents | (529,110 | ) | (1,665,826 | ) | |||||
Cash and cash equivalents as of beginning of the period | 4.1 | 2,119,883 | 1,759,719 | ||||||
Cash and cash equivalents as of the end of the period | 4.1 | 1,590,773 | 93,893 | ||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-9
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
Index
| 1. | General information. |
| 1.1 | The Group |
| 1.2. | Financial and economic situation |
| 2. | Accounting standards and basis of preparation |
| 2.1. | Basis of preparation |
| 2.2. | Authorization for the issue of the interim condensed Consolidated Financial Statements |
| 2.3. | Change in fiscal year-end |
| 2.4. | Changes in accounting polices |
| 2.5. | Changes in accounting estimates and judgments |
| 2.6. | Changes in subsidiaries |
| 2.7. | Unaudited pro forma consolidated statement of financial position presentation |
| 2.8. | New standards, amendments and interpretations issued by the IASB |
| 3. | Seasonality |
| 4. | Information about components of consolidated statements of financial position |
| 4.1. | Cash and cash equivalents |
| 4.2. | Other financial assets |
| 4.3. | Trade receivables |
| 4.4. | Other receivables |
| 4.5. | Inventories |
| 4.6. | Property, plant and equipment |
| 4.7. | Intangible assets |
| 4.8. | Goodwill |
| 4.9. | Trade and other payables |
| 4.10. | Borrowings |
| 4.11. | Employee benefits and social security |
| 4.12. | Deferred revenue and advances from customers |
| 4.13. | Government grants |
| 4.14. | Provisions |
| 4.15. | Puttable instruments |
| 4.16. | Financed payment - Acquisition of business |
| 4.17. | Contingent consideration - Acquisition of business |
| 4.18. | Changes in allowances and provisions |
| 5. | Information about components of consolidated statement of comprehensive income |
F-10
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
| 5.1. | Revenue |
| 5.2. | Cost of sales |
| 5.3. | R&D classified by nature |
| 5.4. | Expenses classified by nature and function |
| 5.5. | Finance income |
| 5.6. | Finance costs |
| 6. | Earnings per share |
| 7. | Information about consolidated components of equity |
| 7.1. | Shareholder’s contributions |
| 7.2. | Non-controlling interests |
| 8. | Cash flow information |
| 9. | Joint arrangements |
| 9.1. | Joint ventures |
| 9.2. | Joint operations |
| 10. | Segment information |
| 11. | Financial instruments–risk management |
| 12. | Shareholders and other related parties balances and transactions |
| 13. | Key management personnel compensation |
| 14. | Contingencies, commitments and restrictions on the distribution of profits |
| 15. | Events occurring after the reporting period |
F-11
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
1. GENERAL INFORMATION
1.1. The Group
Bioceres S.A. is a sociedad anónima incorporated under Argentine Corporations Law in the Province of Santa Fe, Republic of Argentina and domiciled at Ocampo 210 Bis - Predio CCT, in the City of Rosario, Province of Santa Fe, Argentina.
The Group is a fully-integrated agricultural biotechnology business leader in South America. The Group operates multiple technology platforms to develop and commercialize products that enhance crop productivity and expand feedstock applications in order to provide value to its customers around the world. See detail of the organization structure in Note 2.6 of the Consolidated Financial Statements as of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017, for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016 that has been authorized by the Board of Directors (“BOD” or the “Board”) of the Company at its meeting held on October 30, 2017 (hereinafter “June 30, 2017 consolidated financial statements”).
1.2. Financial and economic situation
The following section explains the major events that occurred in 2017 and 2016, which have impacted the current financial and economic condition of the Group.
Rizobacter acquisition
On October 19, 2016, Bioceres acquired a controlling equity interest in Rizobacter Argentina S.A., an Argentine company and global leader in soybean biological products. Rizobacter is Argentina’s leading provider of bio-based solutions for the agricultural sector with a strong focus on crop nutrition and crop protection solutions and an extensive network of international affiliates.
Rizobacter has a strategic partnership with the French company De Sangosse. As part of a joint venture, they formed Synertech, a company that produces and commercializes micro-beaded fertilizers. In 2016, Synertech opened a new factory for the production of such fertilizers, which required an investment of more than USD 30 million.
See detailed information about the acquisition in the consolidated financial statements as of June 30, 2017 consolidated financial statements.
The Group’s capital structure and financing of the Rizobacter acquisition
IPO transaction
On December 17, 2014, the Company’s shareholders authorized the Board of Directors to make an initial public offering (“IPO”) of new ordinary shares of the Company in Argentina with the CNV and with certain other foreign securities commissions.
On September 24, 2015, the SEC accepted the submission of a registration statement by Bioceres for the offering of the American Depositary Receipts (“ADRs”) to be listed on the NYSE, and on December 4, 2015, the CNV authorized Bioceres to make a public offering of ordinary shares in Argentina to be listed on BYMA upon compliance with certain requirements.
On December 15, 2016, the Company’s shareholders ratified the capital increase approved at an extraordinary shareholders’ meeting held on December 17, 2014 in connection with the relaunch of the proposed IPO.
In early 2017, the Company informed the SEC that it intended to continue the IPO process and submit a new registration statement in order to reflect the changes to Bioceres’ business, including with respect to the acquisition of
F-12
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
Rizobacter. At that point, the Company determined that the best course of action would be to request a withdrawal of the previous registration statement. On February 1, 2017, the Company requested the withdrawal of its prior registration Statement from the SEC.
As of June 30, 2017, the date on which the Group’s annual financial statements close, the Company’s losses were higher than 50% of its capital and reserves. Under Argentine corporate law (Ley general de sociedades), art. 206 establishes a mandatory reduction of capital when such situation happens. Additionally, even though total consolidated equity was USD 29 million, the Company presented negative equity attributable to equity holders of the parent. The art. 94 (inc. 5) of Argentine corporate law states that when losses exceed a company’s capital and reserves at its annual fiscal close, such situation constitutes cause for dissolution, and hence, its shareholders shall take the necessary measures to capitalize the company. The six-month transition period ended June 30, 2017 and the three-month period ended September 30, 2017 present negative equity which was primarily as a result of the effects related to the Rizobacter acquisition, including (i) the step up charge included in the cost of sales of Rizobacter inventories acquired and sold post-acquisition that were revalued at acquisition due to the application of purchase price allocation (“PPA”) accounting rules under IFRS, (ii) currency exchange differences relating to Argentine peso-denominated assets and liabilities acquired, (iii) the evolution of the fair value of assets and liabilities acquired and (iv) the classification of certain capital raises through convertible loans and puttable instruments as liabilities and through convertible preferred shares as non-controlling interest under IFRS. The ongoing plan to reverse the negative equity balance is to capitalize the company through the capital increase approved by the shareholders of the Company and placement of the newly issued shares in a public or private offering in Argentina and abroad and the expected conversion of certain financing instruments into ordinary shares of the company.
Bioceres is planning to meet the balances due under deferred payment obligations for the acquisition of Rizobacter and the loans received from BAF mainly with (i) funds from the sale of ordinary shares of Bioceres or the conversion of the debt convertible instruments; (ii) the additional sale of Class A preferred shares of RASA Holding (Bioceres holds Class A preferred shares for a total par value of USD 34.2 million, which are available for placement with private investors); (iii) funds from cash and bank balances and operating income generated in the ordinary course of business of the Group; and (iv) medium- and long-term structured bank financing. Furthermore, the proceeds from the IPO of Bioceres, if consummated, will generate additional funds that will enable the Group to optimize capital structure and financing costs.
Bioceres also granted participation rights to BAF for the subscription of shares in the Company in a public offering and/or a private capital increase if either occurs before the end of the second anniversary of the USD 12 million loan to Bioceres, the end of the first anniversary of the USD 20 million loan to Bioceres Inc. or the second anniversary if it is extended by the parties by mutual consent, as a mechanism for repayment of the loans granted.
On October 26, 2017, the Group renewed the BAF loans with maturity extending to October 2018.
In consequence, in view of the economic projections and financial plans devised by Management, these interim condensed consolidated financial statements have been prepared by applying going-concern principles, considering the Company's ability to continue with its ordinary course of business.
2. ACCOUNTING STANDARDS AND BASIS OF PREPARATION
2.1. Basis of preparation
These interim condensed consolidated financial statements have been prepared in accordance with IAS 34 as issued by IASB. They do not include all the information and disclosures that would be necessary in a complete set of financial statements and must be read in conjunction with the June 30, 2017 consolidated financial statements. In the opinion of the Company, the accompanying unaudited condensed consolidated financial statements contain all adjustments necessary for an accurate statement of its financial position as of September 30, 2017, and its results of operations and cash flows for the three-month period ended September 30, 2017 and 2016. The accounting policies adopted are consistent with those of the previous financial statements.
F-13
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
2.2. Authorization for the issue of the Interim condensed consolidated financial statements
These interim condensed consolidated financial statements of the Group as of September 30, 2017 and June 30, 2017 and for the three-month period ended September 30, 2017 and 2016 have been authorized by the Board of the Company at its meeting held on December 6, 2017. The historical earnings per share information in these financial statements was updated to reflect the stock split described in Note 10 that will be effective after the issuance of these financial statements (December 6, 2017) but before the effective date of the registration statement.
2.3. Change in fiscal year-end
On December 16, 2016, the Company’s shareholders approved an amendment to article 16 of its bylaws, thereby changing the fiscal year-end date to from December 31 to June 30 of each year.
2.4. Changes in accounting policies
The accounting policies adopted in the preparation of the Interim condensed consolidated financial statements are consistent with those adopted for the preparation of the June 30, 2017 consolidated financial statements.
2.5. Changes in accounting estimates and judgments
There were no significant changes in accounting estimates and judgments with respect to the June 30, 2017 consolidated financial statements.
2.6. Changes in subsidiaries
There were no changes in the composition of the Group's subsidiaries, nor in the share of its equity and votes with respect to the June 30, 2017 consolidated financial statements
2.7. Unaudited pro forma consolidated statement of financial position presentation
The unaudited pro forma consolidated statement of financial position presentation as of September 30, 2017 reflects the mandatory conversion of the RASA Holding preferred shares, the mandatory conversion of the Monsanto and BAF convertible loan and the voluntary conversion of the BAF loans into ordinary shares of the Company as if the Company had completed its IPO on September 30, 2017. These instruments are described in the June 30, 2017 consolidated financial statements.
Immediately prior to the IPO, the RASA Holding preferred shares (classified as non-controlling interests) will convert to ordinary shares of the Company at the applicable pre-defined conversion ratio. The pro forma balance sheet reflects the amount as of September 30, 2017 of the BAF loans and the Monsanto and BAF convertible loan (USD 33.2 million and USD 16.9 million respectively), both of which are convertible at the price set for the IPO. The conversion ratio depends on the price per share as set under the IPO. Consequently, the number of ordinary shares to be issued upon conversion will not be known until immediately prior to the IPO.
The unaudited pro forma consolidated statement of financial position does not assume any proceeds from the proposed IPO.
2.8. New standards, amendments and interpretations issued by the IASB
The standards and interpretations issued but not yet effective up to the date of issuance of the Group’s consolidated financial statements, which are or may be applicable to the Group, are disclosed below. The Group intends to adopt these standards and interpretations when they become effective, except as otherwise stated.
F-14
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
IFRS 9 – Financial Instruments (version 2014)
IFRS 15 – Revenue from Contracts with Customers
IFRS 16 – Leases
Amendments to IFRS 2 – Classification and measurement of share-based payment transactions
IFRIC 22 – Foreign currency transactions and advance consideration
Amendments to IAS 40 – Transfers of investment
IFRS 9 – Financial Instruments (version 2014)
In July 2014, the IASB issued the final version of “IFRS 9 Financial Instruments” which reflects all phases of the financial instruments project and replaces “IAS 39 Financial Instruments: Recognition and Measurement” and all previous versions of IFRS 9. The standard introduces new requirements for classification and measurement, impairment and hedge accounting. IFRS 9 is effective for annual periods beginning on or after January 1, 2018, with early application permitted. Retrospective application is required, but comparative information is not compulsory. Early application of previous versions of IFRS 9 (2009, 2010 and 2013) is permitted if the date of initial application is before February 1, 2015.
The Group previously elected to adopt IFRS 9 (version 2013) early.
The new measurement and classification requirements in IFRS 9 (version 2014) are not applicable to the Group’s business model for managing its financial assets and that model is not expected to change in the foreseeable future. The Group does not use hedge accounting.
Regarding the new impairment requirements in IFRS 9 (version 2014), the Group will have to change its impairment testing methodology for financial assets from an “incurred losses model” to the new “expected losses model.” Due to this change, the Group may need to recognize in the future certain impairment losses for financial assets generated from expected losses not yet incurred, which are not recognized under the current model.
The Group is currently assessing the impact of full IFRS 9, including impairment requirement and new disclosure requirements, and plans to adopt the new standard on the required effective date.
IFRS 15 – Revenue from Contracts with Customers
IFRS 15 was issued in May 2014 and establishes a new five-step model that will apply to revenue arising from contracts with customers. Under IFRS 15 revenue is recognized at an amount that reflects the consideration to which an entity expects to be entitled in exchange for transferring goods or services to a customer.
The principles in IFRS 15 provide a more structured approach to measuring and recognizing revenue.
The new revenue standard is applicable to all entities and will supersede all current revenue recognition requirements under IFRS. Either a full or modified retrospective application is required for annual periods beginning on or after January 1, 2018 with early adoption permitted.
The Group is currently assessing the impact of IFRS 15, including with respect to the new disclosure requirements, and plans to adopt the new standard on the required effective date.
Because of the above-mentioned factors, it is not possible at this moment to estimate the potential effects on the Group’s revenue recognition accounting policies, but it can be anticipated that disclosures about revenue shall be more extensive.
F-15
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
IFRS 16 - Leases
IFRS 16 was issued in January 2016. It will result in almost all leases being recognized on the balance sheet, as the distinction between operating and finance leases will be removed. Under the new standard, an asset (the right to use the leased item) and a financial liability to pay rentals are recognized. The only exceptions are short-term and low-value leases.
The standard will affect primarily the accounting for the Group’s operating leases. However, the Group has not yet assessed what adjustments, if any, are necessary due to the changes in the definition of the lease term and the different treatment of variable lease payments and of extension and termination options. It is therefore not yet possible to estimate the amount of right-of-use assets and lease liabilities that will have to be recognized when the new standard is adopted and how this may affect the Group’s profit or loss and classification of cash flows going forward.
The new standard will be effective for financial years commencing on or after January 1, 2019. At this stage, the Group does not intend to adopt the standard before its effective date.
The Group is currently assessing the impact of the new disclosure requirements and currently it is not possible to estimate the potential effects to the Group.
Amendments to IFRS 2 – Classification and measurement of share-based payment transactions
The standard was amended in June 2016 to clarify the measurement basis for cash-settled share-based payments and the accounting for modifications that change an award from cash-settled to equity-settled. It also introduces an exception to IFRS 2 principles by requiring an award to be treated as if it were wholly equity-settled where an employer is obliged to withhold an amount in respect of the employee’s tax obligation associated with a share-based payment and to pay that amount to the relevant tax authority. It is effective for annual periods beginning on or after January 1, 2018. The Group is currently analyzing the impact of its application on the Group’s operating results or financial position.
IFRIC 22 – Foreign currency transactions and advance consideration
IFRIC 22 was issued in December 2016. The interpretation addresses how to determine the date of the transaction for the purpose of determining the exchange rate to use on initial recognition of the related asset, expense or income related to an entity that has received or paid advance consideration in a foreign currency. The date of the transaction is the date on which an entity initially recognizes the non-monetary asset or non-monetary liability arising from the payment or receipt of such advance consideration. The standard is effective for annual periods beginning on January 1, 2018. The Group is currently analyzing the impact of its application on the Group’s operating results or financial position.
Amendments to IAS 40 – Transfers of investment
These modifications clarify when an entity must transfer properties, including properties under construction or development, or outside investment property. The modifications establish that a change in use occurs when the property complies with or fails to meet the definition of investment property and there is evidence of change in use. A simple change in Management's intentions about the use of the property does not provide evidence of change in use.
The changes to the standard will become effective for annual periods beginning on January 1, 2018, with advance application permitted. The modifications are applied prospectively to changes in use occurring after the beginning of the annual period in which the modifications are applied for the first time.
These amendments are not expected to have material impact on the Group.
F-16
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
3. SEASONALITY
The Group revenues fluctuate depending on the timing of orders from our distributors and customers and on prevailing seed market prices, which influence the purchase decisions of growers, the end users of seed and integrated products, crop protection products and crop nutrition products. Given the cyclicality of crop planting and harvesting and South America’s planting and growing seasons, which vary from year to year, our business is highly seasonal. This results in substantial fluctuations in quarterly sales and profitability. Generally, the Group sales are concentrated in the third and fourth quarters of each calendar year, when demand for seed and integrated products, crop protection products and crop nutrition products increases as growers begin planting their fields. With seed and integrated products business, the Group contract with growers and seed suppliers based upon our anticipated market demand. Generally, in seed and integrated products business we stock the seed during the harvest season and ship from inventory throughout the year, with the objective of selling most of the inventory from the current year’s harvest before the next year’s, with crop protection and crop nutrition business following a similar cycle to the seed cycle. The impact of seasonality and the resulting fluctuations in quarterly results may be moderated as we achieve our international expansion plans for seed business in geographies with contrasting seasons and climates. Milestone, royalty and license revenues are also likely to fluctuate from period to period given the seasonality of agriculture and time required to progress from one milestone to the next.
4. INFORMATION ABOUT COMPONENTS OF CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
4.1. Cash and cash equivalents
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Cash and banks | 1,590,773 | 2,119,883 | ||||
1,590,773 | 2,119,883 | |||||
4.2. Other financial assets
09/30/2017 | 06/30/2017 | |||||
Current | (unaudited) | |||||
Shares S&W Seed Company | 196,182 | 262,168 | ||||
Other marketable securities | 3,990 | 4,275 | ||||
Restricted short-term deposit | 4,250,715 | 4,260,517 | ||||
4,450,887 | 4,526,960 | |||||
Non-Current | ||||||
Chemotecnica shares | 3,077,220 | 3,203,427 | ||||
Other marketable securities | 125,019 | 89,331 | ||||
3,202,239 | 3,292,758 | |||||
As of September 30, 2017, the Company holds 62,461 S&W Shares which are valued at USD 196,182 based on the quoted share price of the S&W Shares on NASDAQ.
F-17
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
4.3. Trade receivables
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Trade debtors | 38,070,454 | 32,174,171 | ||||
Allowance for impairment of trade debtors | (1,254,391 | ) | (484,549 | ) | ||
Shareholders and other related parties (Note 12) | 3,754,728 | 4,217,840 | ||||
Allowance for impairment of related parties (Note 12) | (214,592 | ) | (206,196 | ) | ||
Allowance for return of goods | (391,130 | ) | (1,393,059 | ) | ||
Trade debtors - Joint ventures (Note 12) | 825,572 | 592,700 | ||||
Deferred checks | 14,440,692 | 10,792,766 | ||||
Deferred checks - Shareholders and other related parties (Note 12) | 3,059 | — | ||||
55,234,392 | 45,693,673 | |||||
Variations in the allowance for uncollectible trade receivables are reported in Note 4.18.
4.4. Other receivables
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Current | ||||||
Taxes | 2,878,678 | 1,962,361 | ||||
Insurance to be accrued | 19,244 | 15,217 | ||||
Other receivables - Shareholders and other related parties (Note 11) | 29,600 | 30,814 | ||||
Prepayments to suppliers | 1,996,727 | 1,153,188 | ||||
Prepayments to suppliers - Shareholders and other related parties (Note 11) | — | 98,167 | ||||
Reimbursements over exports | 193,942 | 151,106 | ||||
Government grants receivable | 44,258 | 76,868 | ||||
Other - Joint ventures (Note 12) | 203,842 | 9,654 | ||||
Deferred offering costs | 1,713,321 | — | ||||
Prepaid expenses and other receivables | 870,901 | 676,762 | ||||
Loans receivable | 4,581 | 5,732 | ||||
7,955,094 | 4,179,869 | |||||
Non-Current | ||||||
Taxes | 1,171,559 | 1,224,908 | ||||
Reimbursements over exports | 414,423 | 472,276 | ||||
Loans receivable - Joint ventures (Note 12) | 7,492,317 | 3,812,469 | ||||
Other receivables - Shareholders and other related parties (Note 12) | 102,827 | 107,045 | ||||
Other receivables - Miscellaneous | 1,057 | 14,397 | ||||
9,182,183 | 5,631,095 | |||||
F-18
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
4.5. Inventories
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Agrochemicals | 175,886 | 183,822 | ||||
Seeds and grains | 1,164,316 | 1,273,515 | ||||
Microbiological resale products | 15,460,715 | 13,749,668 | ||||
Microbiological products produced | 12,600,850 | 8,931,124 | ||||
Goods in transit | 321,261 | 482,185 | ||||
Supplies | 7,621,234 | 7,810,543 | ||||
Allowance for obsolescence | (472,632 | ) | (707,105 | ) | ||
36,871,630 | 31,723,752 | |||||
Variations in the allowance for obsolescence are reported in Note 4.18.
4.6. Property, plant and equipment
Property, plant and equipment as of September 30, 2017 and June 30, 2017 and for the three-month period ended September 30, 2017 and 2016 included the following:
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Gross carrying amount | 54,139,103 | 56,128,677 | ||||
Accumulated depreciation | (5,567,714 | ) | (5,018,060 | ) | ||
Net carrying amount | 48,571,389 | 51,110,617 | ||||
Net carrying amount for each class of assets is as follows:
Class | Net carrying amount 09/30/2017 (unaudited) | Net carrying amount 06/30/2017 | ||||
Research instruments | 87,825 | 103,938 | ||||
Office equipment | 324,030 | 348,480 | ||||
Vehicles | 1,751,717 | 1,937,219 | ||||
Equipment and computer software | 316,765 | 344,642 | ||||
Fixtures and fittings | 4,791,702 | 4,887,918 | ||||
Machinery and equipment | 8,688,521 | 9,252,633 | ||||
Land and buildings | 28,935,572 | 30,103,117 | ||||
Building on third lands | 3,087,242 | 3,262,201 | ||||
Buildings in progress | 588,015 | 870,469 | ||||
Total | 48,571,389 | 51,110,617 | ||||
F-19
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
Gross carrying amount as of September 30, 2017 is as follows:
Gross carrying amount | ||||||||||||||||||
Class | As of the beginning of period | Additions | Transfers | Disposals | Foreign Currency Translation | As of the end of period | ||||||||||||
Research instruments | 535,313 | — | — | — | (21,090 | ) | 514,223 | |||||||||||
Office equipment | 499,502 | 5,633 | — | (4,799 | ) | (16,353 | ) | 483,983 | ||||||||||
Vehicles | 2,361,244 | 107,828 | — | (82,747 | ) | (64,315 | ) | 2,322,010 | ||||||||||
Equipment and computer software | 586,530 | 21,179 | 3,801 | — | (19,309 | ) | 592,201 | |||||||||||
Fixtures and fittings | 5,668,633 | 281 | 187,649 | — | (221,810 | ) | 5,634,753 | |||||||||||
Machinery and equipment | 10,407,725 | 52,150 | — | (25,990 | ) | (405,603 | ) | 10,028,282 | ||||||||||
Land and buildings | 30,931,226 | 50,659 | 94,496 | — | (1,200,631 | ) | 29,875,750 | |||||||||||
Building on third lands | 4,268,035 | — | — | — | (168,149 | ) | 4,099,886 | |||||||||||
Buildings in progress | 870,469 | 77,665 | (285,946 | ) | (46,113 | ) | (28,060 | ) | 588,015 | |||||||||
Total 09/30/2017 (unaudited) | 56,128,677 | 315,395 | — | (159,649 | ) | (2,145,320 | ) | 54,139,103 | ||||||||||
Accumulated depreciation as of September 30, 2017 is as follows:
Depreciation | |||||||||||||||
Class | Accumulated as of beginning of period | Disposals | of the period | Foreign Currency Translation | Accumulated as of end of period | ||||||||||
Research instruments | 431,375 | — | 11,984 | (16,961 | ) | 426,398 | |||||||||
Office equipment | 151,022 | — | 14,431 | (5,500 | ) | 159,953 | |||||||||
Vehicles | 424,025 | (37,881 | ) | 193,340 | (9,191 | ) | 570,293 | ||||||||
Equipment and computer software | 241,888 | — | 40,175 | (6,627 | ) | 275,436 | |||||||||
Fixtures and fittings | 780,715 | — | 92,784 | (30,448 | ) | 843,051 | |||||||||
Machinery and equipment | 1,155,092 | — | 228,734 | (44,065 | ) | 1,339,761 | |||||||||
Land and buildings | 828,109 | — | 143,966 | (31,897 | ) | 940,178 | |||||||||
Building on third lands | 1,005,834 | — | 46,310 | (39,500 | ) | 1,012,644 | |||||||||
Buildings in progress | — | — | — | — | — | ||||||||||
Total 09/30/2017 (unaudited) | 5,018,060 | (37,881 | ) | 771,724 | (184,189 | ) | 5,567,714 | ||||||||
F-20
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
Gross carrying amount as of September 30, 2016 is as follows:
Gross carrying amount | |||||||||
Class | As of the beginning of period | Additions | As of the end of period | ||||||
Research instruments | 476,366 | 89,257 | 565,623 | ||||||
Office equipment | 199,119 | 60,647 | 259,766 | ||||||
Vehicles | 144,975 | 97,722 | 242,697 | ||||||
Vehicles on finance lease | 65,544 | — | 65,544 | ||||||
Equipment and computer software | 257,031 | 7,320 | 264,351 | ||||||
Fixtures and fittings | 890,436 | 147,138 | 1,037,574 | ||||||
Machinery and equipment | 1,357,566 | 217,729 | 1,575,295 | ||||||
Building on third lands | 4,423,745 | 41,482 | 4,465,227 | ||||||
Total 09/30/2016 (unaudited) | 7,814,782 | 661,295 | 8,476,077 | ||||||
Accumulated depreciation as of September 30, 2016 is as follows:
Depreciation | |||||||||
Class | Accumulated as of beginning of period | of the period | Accumulated as of end of period | ||||||
Research instruments | 403,056 | 14,332 | 417,388 | ||||||
Office equipment | 111,925 | 5,761 | 117,686 | ||||||
Vehicles | 138,262 | 6,549 | 144,811 | ||||||
Vehicles on finance lease | 40,418 | 3,278 | 43,696 | ||||||
Equipment and computer software | 238,716 | 3,955 | 242,671 | ||||||
Fixtures and fittings | 502,537 | 25,302 | 527,839 | ||||||
Machinery and equipment | 474,508 | 38,126 | 512,634 | ||||||
Building on third lands | 857,785 | 48,758 | 906,543 | ||||||
Total 09/30/2016 (unaudited) | 2,767,207 | 146,061 | 2,913,268 | ||||||
The depreciation charge is included in Notes 5.3 and 5.4.
The Group has no commitments to purchase property, plant and equipment items.
4.7. Intangible assets
Intangible assets as of September 30, 2017 and June 30, 2017 and for the three-month period ended September 30, 2017 and 2016 included the following:
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Gross carrying amount | 42,551,427 | 44,054,864 | ||||
Accumulated amortization | (2,376,281 | ) | (1,873,094 | ) | ||
Net carrying amount | 40,175,146 | 42,181,770 | ||||
F-21
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
Net carrying amount of each class of intangible assets is as follows:
Class | Net carrying amount 09/30/2017 (unaudited) | Net carrying amount 06/30/2017 | ||||
Seed and integrated products | ||||||
Amaranth ALS | 128,310 | 132,848 | ||||
Soybean HB4 | 707,670 | 609,091 | ||||
Alfalfa Genuity Harv Xstra | 134,951 | 140,486 | ||||
Integrated products Semya | 2,184,381 | 2,273,970 | ||||
Crop nutrition | ||||||
Microbiológiy products | 3,374,793 | 3,491,268 | ||||
Other intangible assets | ||||||
Trademarks and patents | 9,820,169 | 10,402,765 | ||||
Software | 1,320,805 | 1,470,507 | ||||
Customer loyalty | 22,504,067 | 23,660,835 | ||||
Total | 40,175,146 | 42,181,770 | ||||
Gross carrying amount as of September 30, 3017 is as follows:
Gross carrying amount | |||||||||||||||
Class | As of the beginning of period | Additions | Disposals | Foreign Currency Translation | As of the end of period | ||||||||||
Seed and integrated products | |||||||||||||||
Amaranth ALS | 132,848 | 693 | — | (5,231 | ) | 128,310 | |||||||||
Soybean HB4 | 609,091 | — | — | 98,579 | 707,670 | ||||||||||
Alfalfa Genuity Harv Xstra | 140,486 | — | — | (5,535 | ) | 134,951 | |||||||||
Integrated products Semya | 2,273,970 | — | — | (89,589 | ) | 2,184,381 | |||||||||
Crop nutrition | |||||||||||||||
Microbiológiy products | 3,782,239 | 110,820 | — | (148,707 | ) | 3,744,352 | |||||||||
Other intangible assets | |||||||||||||||
Trademarks and patents | 10,906,960 | — | — | (429,707 | ) | 10,477,253 | |||||||||
Software | 1,893,787 | 3,403 | (5,579 | ) | (74,616 | ) | 1,816,995 | ||||||||
Customer loyalty | 24,315,483 | — | — | (957,968 | ) | 23,357,515 | |||||||||
Total 09/30/2017 (unaudited) | 44,054,864 | 114,916 | (5,579 | ) | (1,612,774 | ) | 42,551,427 | ||||||||
F-22
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
Accumulated amortization as of September 30, 2017 is as follows:
Amortization | |||||||||||||||
Class | Accumulated as of beginning of period | Disposals | of the period | Foreign Currency Translation | Accumulated as of the end of period | ||||||||||
Seed and integrated products | |||||||||||||||
Amaranth ALS | — | — | — | — | — | ||||||||||
Soybean HB4 | — | — | — | — | — | ||||||||||
Alfalfa Genuity Harv Xstra | — | — | — | — | — | ||||||||||
Integrated products Semya | — | — | — | — | — | ||||||||||
Crop nutrition | |||||||||||||||
Microbiológiy products | 290,971 | — | 89,805 | (11,217 | ) | 369,559 | |||||||||
Other intangible assets | |||||||||||||||
Trademarks and patents | 504,195 | — | 172,282 | (19,393 | ) | 657,084 | |||||||||
Software | 423,280 | (5,579 | ) | 94,919 | (16,430 | ) | 496,190 | ||||||||
Customer loyalty | 654,648 | — | 223,977 | (25,177 | ) | 853,448 | |||||||||
Total 09/30/2017 (unaudited) | 1,873,094 | (5,579 | ) | 580,983 | (72,217 | ) | 2,376,281 | ||||||||
Gross carrying amount as of September 30, 2016 is as follows:
Gross carrying amount | |||||||||
Class | As of the beginning of period | Additions | As of the end of period | ||||||
Seed and integrated products | |||||||||
Amaranth ALS | 136,586 | 1,158 | 137,744 | ||||||
Soybean HB4 | 471,167 | — | 471,167 | ||||||
Alfalfa Genuity Harv Xstra | 135,945 | 529 | 136,474 | ||||||
Emerging solutions | |||||||||
SPC Molecular Farming Platform | 1,091,507 | 61,810 | 1,153,317 | ||||||
Other intangible assets | |||||||||
Certification ISO Standards | 28,366 | — | 28,366 | ||||||
Trademarks and patents | 104,864 | — | 104,864 | ||||||
Software | 39,181 | 4,876 | 44,057 | ||||||
Total 09/30/2016 (unaudited) | 2,007,616 | 68,373 | 2,075,989 | ||||||
F-23
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
Accumulated amortization as of September 30, 2016 is as follows:
Amortization | |||||||||
Class | Accumulated as of beginning of period | of the period | Accumulated as of the end of period | ||||||
Seed and integrated products | |||||||||
Amaranth ALS | — | — | — | ||||||
Soybean HB4 | — | — | — | ||||||
Alfalfa Genuity Harv Xstra | — | — | — | ||||||
Emerging solutions | |||||||||
SPC Molecular Farming Platform | 88,120 | 6,294 | 94,414 | ||||||
Other intangible assets | |||||||||
Certification ISO Standards | 28,366 | — | 28,366 | ||||||
Trademarks and patents | 38,562 | 4,143 | 42,705 | ||||||
Software | 37,828 | 1,417 | 39,245 | ||||||
Total 09/30/2016 (unaudited) | 192,876 | 11,854 | 204,730 | ||||||
The depreciation charge is included in Notes 5.3 and 5.4.
There are no intangible assets whose use has been restricted or which have been delivered as a guarantee. The Group has not assumed any commitments to acquire new intangibles. There have been no intangible assets impairment indicators.
4.8. Goodwill
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Rizobacter | 24,091,263 | 25,079,324 | ||||
Semya | 6,988,134 | 7,274,740 | ||||
Indear | 491,962 | 512,139 | ||||
Total | 31,571,359 | 32,866,203 | ||||
The variations in goodwill occurred during the period ended September 30, 2017 correspond to translation differences. There have been no goodwill impairment indicators.
4.9. Trade and other payables
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Trade creditors | 31,674,950 | 20,656,681 | ||||
Shareholders and other related parties (Note 12) | 6,286,487 | 2,113,149 | ||||
Trade and other payables - Miscellaneous | 157,949 | 134,273 | ||||
Other - Joint ventures (Note 12) | 350,541 | 427,092 | ||||
Taxes | 600,270 | 417,517 | ||||
39,070,197 | 23,748,712 | |||||
F-24
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
4.10. Borrowings
09/30/2017 | 06/30/2017 | |||||
Current | (unaudited) | |||||
Bank overdraft | 642,785 | 357,643 | ||||
Bank borrowings | 23,462,869 | 19,219,577 | ||||
Corporate bonds | 6,136,687 | 4,644,621 | ||||
Convertible borrowings | 16,933,726 | 16,598,470 | ||||
Discount checks | 12,805,898 | 9,733,481 | ||||
Finance lease | 328,542 | 466,689 | ||||
60,310,507 | 51,020,481 | |||||
Non-current | ||||||
Corporate bonds | — | 3,889,874 | ||||
Bank borrowings | 35,981,144 | 36,538,128 | ||||
Finance lease | 587,439 | 678,993 | ||||
36,568,583 | 41,106,995 | |||||
The group has met the capital and interest installments whose maturity was effective in the three-month period ended September 30, 2017.
The group has no covenant requirement as of September 30, 2017. The Group has complied with these covenants as of June 2017.
4.11. Employee benefits and social security
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Salaries and social security | 1,585,709 | 2,273,306 | ||||
Staff incentives and vacations | 4,422,974 | 3,296,903 | ||||
6,008,683 | 5,570,209 | |||||
4.12. Deferred revenue and advances from customers
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Licenses and services to be accrued | 76,987 | 59,895 | ||||
Advances from customers | 2,507,449 | 1,197,080 | ||||
2,584,436 | 1,256,975 | |||||
F-25
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
4.13. Government grants
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
At the beginning of the period | 1,836,236 | 2,063,563 | ||||
Received during the period | 94,213 | 129,471 | ||||
Currency conversion difference | (72,212 | ) | (83,775 | ) | ||
Released to the statement of profit or loss | (158,062 | ) | (273,023 | ) | ||
At the end of period | 1,700,175 | 1,836,236 | ||||
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Current | 311,917 | 351,157 | ||||
Non-current | 1,388,258 | 1,485,079 | ||||
Total | 1,700,175 | 1,836,236 | ||||
There are neither unfulfilled conditions nor other contingencies attached to government grants or government assistance.
4.14. Provisions
06/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Provisions for contingencies | 1,638,381 | 1,690,412 | ||||
1,638,381 | 1,690,412 | |||||
The roll forward of the provision is in Note 4.18.
4.15. Puttable instruments
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Current | ||||||
Puttable shares (Note 12) | 225,131 | — | ||||
225,131 | — | |||||
Non-current | ||||||
Puttable shares (Note 12) | 2,338,909 | 2,508,467 | ||||
Puttable preferred shares | 2,788,493 | 2,500,000 | ||||
5,127,402 | 5,008,467 | |||||
F-26
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
4.16. Financed payment – Acquisition of business
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Current | ||||||
BAF loans | 20,755,423 | 20,257,452 | ||||
Financed payment to sellers | 6,340,813 | 6,219,980 | ||||
27,096,236 | 26,477,432 | |||||
Non-current | ||||||
BAF loans | 12,457,595 | 12,222,740 | ||||
Financed payment to sellers | 7,805,352 | 7,656,611 | ||||
Purchase option | 13,786,299 | 13,523,582 | ||||
34,049,246 | 33,402,933 | |||||
In October 2017, the first due payments of “Financed payment to sellers” have been canceled. For the cancellation of these, the Group obtained a loan with Banco Santander Río for USD 4.5M at an annual rate of 4% and maturing at 120 days. However, the seller, International Property Services Corp (“IPS”), notified the Group to refrain from transferring the amount due until IPS can inform the Group of a new bank account number as set forth in the Shares Purchase Agreement (“SPA”). This stop payment requested by IPS does not constitute a breach of or default under the applicable SPA.
On October 26, 2017, the Group has renewed the maturity of BAF loans until October 2018.
4.17. Contingent consideration – Acquisition of business
09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Conditional payment on acquisition of business | 15,916,114 | 15,919,060 | ||||
15,916,114 | 15,919,060 | |||||
The Group has not been notified of any progress with respect to the precautionary measure on which the contingent payment is subject. There were no changes in the management evaluation regarding the precautionary measure which is supported by the Supreme Court ruling. See details in the June 30, 2017 consolidated financial statements
4.18. Changes in allowances and provisions
Item | 06/30/2017 | Additions | Uses and reversals | Currency conversion difference | 09/30/2017 | ||||||||||
DEDUCTED FROM ASSETS | |||||||||||||||
Allowance for impairment of trade debtors | (690,745 | ) | (381,551 | ) | — | (396,687 | ) | (1,468,983 | ) | ||||||
Allowance for obsolescence | (707,105 | ) | (19,923 | ) | 212,632 | 41,764 | (472,632 | ) | |||||||
Allowance for unused tax losses | (176,990 | ) | — | — | 6,973 | (170,017 | ) | ||||||||
Total deducted from assets | (1,574,840 | ) | (401,474 | ) | 212,632 | (347,950 | ) | (2,111,632 | ) | ||||||
INCLUDED IN LIABILITIES | |||||||||||||||
Provisions for contingencies | (1,690,412 | ) | (14,571 | ) | — | 66,602 | (1,638,381 | ) | |||||||
Total included in liabilities | (1,690,412 | ) | (14,571 | ) | — | 66,602 | (1,638,381 | ) | |||||||
F-27
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
Item | 06/30/2017 | Additions | Uses and reversals | Currency conversion difference | 09/30/2017 | ||||||||||
Total | (3,265,252 | ) | (416,045 | ) | 212,632 | (281,348 | ) | (3,750,013 | ) | ||||||
DEDUCTED FROM ASSETS | |||||||||||||||
Allowance for impairment of trade debtors | (357,506 | ) | — | — | — | (357,506 | ) | ||||||||
Allowance for obsolescence | (167,989 | ) | — | 58,824 | — | (109,165 | ) | ||||||||
Allowance for unused tax losses | (144,277 | ) | — | 2,829 | — | (141,448 | ) | ||||||||
Total deducted from assets | (669,772 | ) | — | 61,653 | — | (608,119 | ) | ||||||||
INCLUDED IN LIABILITIES | |||||||||||||||
Provisions for contingencies | (231,768 | ) | (14,009 | ) | — | — | (245,777 | ) | |||||||
Total included in liabilities | (231,768 | ) | (14,009 | ) | — | — | (245,777 | ) | |||||||
Total | (901,540 | ) | (14,009 | ) | 61,653 | — | (853,896 | ) | |||||||
5. INFORMATION ABOUT COMPONENTS OF CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME
5.1. Revenue
Period ended | ||||||
09/30/2017 | 09/30/2016 | |||||
(unaudited) | ||||||
Sale of goods | 33,707,643 | 1,192,754 | ||||
Rendering of services | 993,577 | 533,212 | ||||
Royalties | 127,223 | 116,616 | ||||
Licenses | — | 3,713 | ||||
34,828,443 | 1,846,295 | |||||
5.2. Cost of sales
Item | 09/30/2017 | 09/30/2016 | ||||
(unaudited) | ||||||
Inventory as of the beginning of the period | 31,723,752 | 3,569,371 | ||||
Purchases of the period | 23,651,795 | 33,815 | ||||
Production costs (Note 5.4) | 2,924,657 | 413,642 | ||||
Currency conversion difference | (1,232,643 | ) | — | |||
Subtotal | 57,067,561 | 4,016,828 | ||||
Inventory as of the end of the period (Note 4.5) | (36,871,630 | ) | (2,897,880 | ) | ||
Cost of sales | 20,195,931 | 1,118,948 | ||||
F-28
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
5.3. R&D classified by nature
Item | Research and development expenses 09/30/2017 (unaudited) | Research and development expenses 09/30/2016 (unaudited) | ||||
Rent and other office expenses | 12,339 | 18,606 | ||||
Amortization intangible assets | 197,963 | 9,821 | ||||
Analysis and storage | 125 | 149 | ||||
Import and export expenses | 4,228 | — | ||||
Depreciation property, plant and equipment | 150,633 | 43,923 | ||||
Freight | 181 | — | ||||
Staff related expenses | 8,675 | 11,338 | ||||
Maintenance | 18,827 | 7,046 | ||||
Supplies and materials | 218,787 | 128,321 | ||||
Mobility and travel | 68,344 | 27,374 | ||||
Employee benefits and social security | 820,248 | 283,034 | ||||
Telephone and communications | 5,995 | 3,742 | ||||
Based incentive stock options | 59,040 | 20,736 | ||||
Professional fees and outsourced services | 87,388 | 23,543 | ||||
Office supplies | 16,542 | 1,429 | ||||
Sealed and certifications | 1,807 | 105 | ||||
Insurance | 6,026 | 282 | ||||
Licenses & Patents | 14,231 | 9,308 | ||||
Security expenses | 3,494 | 2,033 | ||||
Energy and fuel | 16,217 | 5,862 | ||||
Miscellaneous | 14,555 | — | ||||
Total | 1,725,645 | 596,652 | ||||
F-29
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
5.4. Expenses classified by nature and function
Item | Production costs | Selling, general and administrative expenses | Total 09/30/2017 (unaudited) | ||||||
Rent and other office expenses | 1,053 | 6,370 | 7,423 | ||||||
Amortization intangible assets | — | 383,020 | 383,020 | ||||||
Analysis and storage | 28,279 | 3,941 | 32,220 | ||||||
Commissions and rights | — | 114,173 | 114,173 | ||||||
Contingencies | — | 14,571 | 14,571 | ||||||
Bank expenses and commissions | — | 35,020 | 35,020 | ||||||
Import and export expenses | 24,683 | 365,667 | 390,350 | ||||||
Depreciation property, plant and equipment | 285,518 | 335,573 | 621,091 | ||||||
Impairment of receivables | — | 381,551 | 381,551 | ||||||
Freight | 52,111 | 413,591 | 465,702 | ||||||
Staff related expenses | 123 | 20,704 | 20,827 | ||||||
Maintenance | 251,591 | 191,629 | 443,220 | ||||||
Supplies and materials | 73,299 | 2,764 | 76,063 | ||||||
Mobility and travel | 12,517 | 266,857 | 279,374 | ||||||
Publicity and advertising | — | 605,995 | 605,995 | ||||||
Employee benefits and social security | 2,004,266 | 3,995,344 | 5,999,610 | ||||||
Telephone and communications | — | 19,557 | 19,557 | ||||||
Based incentive stock options | — | 95,908 | 95,908 | ||||||
Professional fees and outsourced services | — | 1,128,024 | 1,128,024 | ||||||
Office supplies | 9,230 | 166,715 | 175,945 | ||||||
Sealed and certifications | 37 | 2,792 | 2,829 | ||||||
Insurance | 26,531 | 146,027 | 172,558 | ||||||
Royalties | 49,108 | — | 49,108 | ||||||
Obsolescence | 19,923 | — | 19,923 | ||||||
Taxes | 6,789 | 885,940 | 892,729 | ||||||
Security expenses | 3,543 | 6,604 | 10,147 | ||||||
Energy and fuel | 52,186 | 143,970 | 196,156 | ||||||
Miscellaneous | 23,870 | 260,876 | 284,746 | ||||||
Total | 2,924,657 | 9,993,183 | 12,917,840 | ||||||
F-30
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
5.4. Expenses classified by nature and function (Cont’d)
Item | Production costs | Selling, general and administrative expenses | Total 09/30/2016 (unaudited) | ||||||
Rent and other office expenses | 2,534 | 3,707 | 6,241 | ||||||
Amortization intangible assets | — | 2,033 | 2,033 | ||||||
Analysis and storage | 20,226 | 12,263 | 32,489 | ||||||
Commissions and rights | — | 20,037 | 20,037 | ||||||
Contingencies | — | 14,009 | 14,009 | ||||||
Bank expenses and commissions | — | 45,587 | 45,587 | ||||||
Depreciation property, plant and equipment | 6,693 | 95,445 | 102,138 | ||||||
Freight | 605 | 67,145 | 67,750 | ||||||
Staff related expenses | 423 | 4,500 | 4,923 | ||||||
Maintenance | 13,401 | 30,778 | 44,179 | ||||||
Supplies and materials | 94,899 | — | 94,899 | ||||||
Mobility and travel | 12,280 | 32,916 | 45,196 | ||||||
Publicity and advertising | — | 35,863 | 35,863 | ||||||
Employee benefits and social security | 113,927 | 352,024 | 465,951 | ||||||
Telephone and communications | 829 | 9,123 | 9,952 | ||||||
Professional fees and outsourced services | 23,996 | 83,090 | 107,086 | ||||||
Based incentive stock options | — | 275,460 | 275,460 | ||||||
Office supplies | 5 | 1,094 | 1,099 | ||||||
Sealed and certifications | 74 | 27,879 | 27,953 | ||||||
Insurance | 199 | 8,218 | 8,417 | ||||||
Royalties | 117,344 | — | 117,344 | ||||||
Taxes | — | 18,795 | 18,795 | ||||||
Security expenses | 2,033 | 4,065 | 6,098 | ||||||
Energy and fuel | 4,174 | 8,349 | 12,523 | ||||||
Miscellaneous | — | 33,092 | 33,092 | ||||||
Total | 413,642 | 1,185,472 | 1,599,114 | ||||||
5.5. Finance income
Period ended | ||||||
09/30/2017 | 09/30/2016 | |||||
(unaudited) | ||||||
Interest generated by assets | 126,181 | 56,108 | ||||
Changes in fair value of financial assets or liabilities | 443,520 | 282,853 | ||||
Exchange differences | 1,472,365 | 42,366 | ||||
2,042,066 | 381,327 | |||||
F-31
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
5.6. Finance costs
Period ended | ||||||
09/30/2017 | 09/30/2016 | |||||
(unaudited) | ||||||
Exchange differences | (3,920,131 | ) | (637,166 | ) | ||
Interest generated by liabilities | (4,318,716 | ) | (91,738 | ) | ||
Financial commissions | (481,308 | ) | (52,458 | ) | ||
Changes in fair value of financial assets or liabilities | (548,264 | ) | (335,792 | ) | ||
(9,268,419 | ) | (1,117,154 | ) | |||
6. EARNINGS PER SHARE
The numerators and denominators used in the calculation of basic EPS and diluted EPS are presented below:
Period ended | ||||||
09/30/2017 | 09/30/2016 | |||||
Numerator | (unaudited) | |||||
Loss for the period / year (basic EPS) | (3,066,809 | ) | (1,214,922 | ) | ||
Loss for the period / year (diluted EPS) | (3,066,809 | ) | (1,214,922 | ) | ||
Denominator | ||||||
Weighted average number of shares (basic EPS) | 25,546,550 | 25,615,265 | ||||
Weighted average number of shares (diluted EPS) | 25,546,550 | 25,615,265 | ||||
On December 17, 2014, the Company’s shareholders authorized: (i) to effect a 1-to-50 stock split of the Company’s ordinary shares; and (ii) a capital increase issuing up to 12,000,000 new ordinary book-entry shares, with a par value of ARS 2 per share and the right to one vote per share, including shares for the possible over-allotment of the IPO, of which CNV authorized 6 million shares.
On April 27, 2017, shareholders approved a 1-to-100 stock split of the Company’s common stock that will be effective prior to the effectiveness of the public offering. Therefore, the capital increase will result in issuing up to 24,000,000 shares with a par value of ARS 1 per share and the right to one vote per share.
The effect of the share split was considered retrospectively in the EPS calculations. The denominators used in the EPS calculation are based on the assumption of the effectiveness of the split.
Some financial instruments were not included in the EPS calculations for the three-month period ended September 30, 2017 and 2016 because they were anti-dilutive securities in that period. Those instruments, which were listed in the June 30, 2017 consolidated financial statements, might dilute the EPS in the future.
There are neither ordinary shares transactions nor potential ordinary share transactions that have occurred after September 30, 2017 that would have changed significantly the number of ordinary shares or potential ordinary shares outstanding at the end of the reporting period.
The probable future issue of shares related to the authorized capital increase of 24,000,000 (after stock split of 1-to-100) shares for the IPO would have changed significantly the number of ordinary shares outstanding at the end of the reporting period if those shares had been issued before September 30, 2017. The future issue of shares related to the “stock options” and “stock grants” arrangements above mentioned could potentially dilute EPS in the future.
F-32
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
7. INFORMATION ABOUT CONSOLIDATED COMPONENTS OF EQUITY
7.1. Shareholder’s contributions
The authorized capital as of the end of each period is as follows:
Period | Authorized Capital (ARS) | Authorized Capital (number of shares) | ||||
09/30/2016 | 25,644,300 | 25,644,300 | ||||
09/30/2017 | 25,644,300 | 25,644,300 | ||||
The issuance of 24,000,000 (after stock split of 1-to-100) shares is not included in the above numbers. The effect of the share split was considered retrospectively in the EPS calculations and in the number of outstanding shares.
Shares outstanding as of the end of each period are as follows:
Period | Number of shares | Class | Face value | Voting right (per share) | Number of shares issued and fully paid | Number of shares issued and not paid | ||||||||||||
09/30/2016 | 25,644,300 | Ordinary | 1 | 1 | 25,644,300 | — | ||||||||||||
09/30/2017 | 25,644,300 | Ordinary | 1 | 1 | 25,644,300 | — | ||||||||||||
The issuance of 24,000,000 (after stock split of 1-to-100) shares is not included in the above numbers. The effect of the share split was considered retrospectively in the EPS calculations and in the number of outstanding shares.
There is a description of share capital as of the end of each year/period in the table below:
Period | Capital issued (ARS) | Capital issued and fully paid (ARS) | Capital issued and not paid (ARS) | ||||||
09/30/2016 | 25,644,300 | 25,644,300 | — | ||||||
09/30/2017 | 25,644,300 | 25,644,300 | — | ||||||
Puttable shares, which are fully paid, shall be included as of September 30, 2017 and 2016. Further detail is in note 7.16 of the June 30, 2017 consolidated financial statements.
The reconciliation of the number of shares outstanding at the beginning and at the end of each reporting period is as follows:
Period | Number of shares outstanding | Capital issued | ||||
06/30/2017 | 25,536,100 | 25,536,100 | ||||
Sale of own shares by subsidiary | 50,600 | 50,600 | ||||
09/30/2017 | 25,586,700 | 25,586,700 | ||||
F-33
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
Period | Number of shares outstanding | Capital issued | ||||
06/30/2016 | 25,593,700 | 25,593,700 | ||||
09/30/2016 | 25,593,700 | 25,593,700 | ||||
The issuance of 24,000,000 (after stock split of 1-to-100) shares is not included in the above numbers. The effect of the share split was considered retrospectively in the EPS calculations and in the number of outstanding shares.
The amounts included in “Share Premium” are the amounts subscribed for share capital in excess of par value (net of transaction costs).
On September 11, 2017, the Group sold, through its subsidiary Bioceres Semillas S.A., 50,600 shares that were received from a shareholder in lieu of the payment of a commercial debt held with that subsidiary. The transaction amount of the sale was ARS 7,050,029 (equivalent to USD 408,461) and was accounted as an equity transaction.
7.2. Non-controlling interests
As of September 30, 2017, there were 1,409,848 preferred shares of RASA Holding in the possession of third parties outside the Group.
On October 14, 2017, the Group authorized the issuance of 579,600 Series A Preferred Shares of RASA Holding to the holders of Series A Preferred Shares whose anniversary year occurs in 2017, so as to pay dividends in the amounts. 119,276 of those shares correspond to the non-controlling interests and will be delivered between October and December depending on the moment of initial subscription.
The number of shares to be subscribed and issued upon the mandatory conversion will be determined by converting at the applicable pre-defined conversion ratio.
8. CASH FLOW INFORMATION
Significant non-cash transactions related to investing and financing activities are as follows:
Period ended | ||||||
09/30/2017 | 09/30/2016 | |||||
(unaudited) | ||||||
Financing activities | ||||||
Deferred offering costs in accounts payable | (1,713,321 | ) | ||||
(1,713,321 | ) | — | ||||
F-34
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
9. JOINT ARRANGEMENTS
9.1. Joint ventures
Period ended 09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Assets | ||||||
S&W Semillas | 89,951 | 95,968 | ||||
A.G.B.M. | 1,498,603 | 1,483,208 | ||||
Synertech Industrias | 26,044,896 | 26,844,410 | ||||
Héritas | 490,957 | 554,349 | ||||
28,124,407 | 28,977,935 | |||||
Period ended 09/30/2017 | 06/30/2017 | |||||
(unaudited) | ||||||
Liabilities | ||||||
Trigall Genetics | 1,726,690 | 1,527,286 | ||||
1,726,690 | 1,527,286 | |||||
Period ended | ||||||
09/30/2017 | 09/30/2016 | |||||
(unaudited) | ||||||
Share of income / (loss) of joint ventures and associates | ||||||
Trigall Genetics | (199,403 | ) | (132,674 | ) | ||
Semya | — | (54,793 | ) | |||
S&W Semillas | (2,237 | ) | (50,633 | ) | ||
AGBM | 73,852 | — | ||||
Synertech Industrias | 257,380 | — | ||||
Héritas | (44,735 | ) | — | |||
84,857 | (238,100 | ) | ||||
Period ended | ||||||
09/30/2017 | 09/30/2016 | |||||
(unaudited) | ||||||
As of the beginning of the period | 27,450,649 | (1,258,667 | ) | |||
Monetary contributions | — | 79,207 | ||||
Foreign currency translation | (1,137,789 | ) | — | |||
Share of income / (loss) | 84,857 | (238,100 | ) | |||
As of the end of the period | 26,397,717 | (1,417,560 | ) | |||
F-35
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
9.2. Joint operations
During the three-month period ended September 30, 2017, the Group has not signed new collaborative agreements.
10. SEGMENT INFORMATION
The following tables present information with respect to the Group’s reporting segments:
Three-month period ended September 30, 2017 (unaudited) | Seed and integrated products | Crop protection | Crop nutrition | Emerging solutions | Consolidated | ||||||||||
Revenues | |||||||||||||||
Sale of goods | 7,370,812 | 16,795,948 | 9,540,883 | — | 33,707,643 | ||||||||||
Rendering of services | — | — | — | 993,577 | 993,577 | ||||||||||
Royalties | 127,223 | — | — | — | 127,223 | ||||||||||
Licenses | — | — | — | — | — | ||||||||||
Government grants | |||||||||||||||
Grants | — | — | — | 158,062 | 158,062 | ||||||||||
Total revenue | 7,498,035 | 16,795,948 | 9,540,883 | 1,151,639 | 34,986,505 | ||||||||||
Cost of sales | (2,462,759 | ) | (10,038,912 | ) | (7,509,962 | ) | (184,298 | ) | (20,195,931 | ) | |||||
Gross margin per segment | 5,035,276 | 6,757,036 | 2,030,921 | 967,341 | 14,790,574 | ||||||||||
% | 67 | % | 40 | % | 21 | % | 84 | % | 42 | % | |||||
Other expenses, net | — | — | — | — | (217,284 | ) | |||||||||
Operating costs | — | — | — | — | (11,718,828 | ) | |||||||||
Share of income of joint ventures and associates | — | — | — | — | 84,857 | ||||||||||
Financial results | — | — | — | — | (7,226,353 | ) | |||||||||
Loss before income tax | — | — | — | — | (4,287,034 | ) | |||||||||
Income tax benefit | — | — | — | — | 1,357,795 | ||||||||||
Loss | — | — | — | — | (2,929,239 | ) | |||||||||
Revenues | |||||||||||||||
Sale of goods | 1,192,754 | — | — | — | 1,192,754 | ||||||||||
Rendering of services | — | — | — | 533,212 | 533,212 | ||||||||||
Royalties | 116,616 | — | — | — | 116,616 | ||||||||||
Licenses | 3,713 | — | — | — | 3,713 | ||||||||||
Government grants | |||||||||||||||
Grants | — | — | — | 117,218 | 117,218 | ||||||||||
Total revenue | 1,313,083 | — | — | 650,430 | 1,963,513 | ||||||||||
Cost of sales | (855,682 | ) | — | — | (263,266 | ) | (1,118,948 | ) | |||||||
Gross margin per segment | 457,401 | — | — | 387,164 | 844,565 | ||||||||||
% | 35 | % | 60 | % | 43 | % | |||||||||
Other expenses, net | — | — | — | — | (18,135 | ) | |||||||||
Operating costs | — | — | — | — | (1,782,124 | ) | |||||||||
Share of loss of joint ventures | — | — | — | — | (238,099 | ) | |||||||||
Net financing costs | — | — | — | — | (735,828 | ) | |||||||||
Loss before income tax | — | — | — | — | (1,929,621 | ) | |||||||||
Income tax benefit | — | — | — | — | 621,022 | ||||||||||
Loss | — | — | — | — | (1,308,599 | ) | |||||||||
F-36
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
Revenue by products
09/30/2017 | 09/30/2016 | |||||
Seed and integrated products | 7,498,035 | 1,313,083 | ||||
Seeds, royalties & licenses | 982,052 | 1,313,083 | ||||
Packs | 6,515,983 | — | ||||
Crop protection | 16,795,948 | — | ||||
Adjuvants | 7,993,798 | — | ||||
Insecticides & fungicides | 2,409,728 | — | ||||
Other | 6,392,422 | — | ||||
Crop nutrition | 9,540,883 | — | ||||
Inoculants | 6,306,921 | — | ||||
Fertilizers | 3,233,962 | — | ||||
Emerging solutions | 1,151,639 | 650,430 | ||||
Research & development | 1,151,639 | 650,430 | ||||
Total revenue | 34,986,505 | 1,963,513 | ||||
11. FINANCIAL INSTRUMENTS – RISK MANAGEMENT
The following tables show additional information required under IFRS 7 on the financial assets and liabilities recorded as of September 30, 2017 and June 30, 2017.
Amortized cost | Mandatorily measured at fair value through profit or loss | |||||||||||
Financial asset | 09/30/2017 | 06/30/2017 | 09/30/2017 | 06/30/2017 | ||||||||
Cash and cash equivalents | 1,590,773 | 2,119,883 | — | — | ||||||||
Other financial assets | 4,250,715 | 4,260,517 | 3,402,411 | 3,559,201 | ||||||||
Trade receivables | 55,234,392 | 45,693,673 | — | — | ||||||||
Other receivables(*) | 12,537,084 | 7,882,847 | — | — | ||||||||
Total | 73,612,694 | 59,956,920 | 3,402,411 | 3,559,201 | ||||||||
| (*) | Does not include advances and deferred expenses. |
Amortized cost | Mandatorily measured at fair value through profit or loss | |||||||||||
Financial liability | 09/30/2017 | 06/30/2017 | 09/30/2017 | 06/30/2017 | ||||||||
Trade Payables and other payables | 39,070,197 | 23,748,712 | — | — | ||||||||
Borrowings | 96,879,090 | 92,127,476 | — | — | ||||||||
Financed payment - Acquisition of business | 61,145,482 | 59,880,365 | — | — | ||||||||
Puttable Instruments | 2,564,040 | 2,508,467 | 2,788,493 | 2,500,000 | ||||||||
Total | 199,658,809 | 178,265,020 | 2,788,493 | 2,500,000 | ||||||||
F-37
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
Financial instruments measured at fair value
Fair value by hierarchy
Measurement at fair value at 09/30/2017 | Level 1 | Level 2 | Level 3 | ||||||
Financial assets at fair value | |||||||||
Other financial assets | 200,172 | — | 3,202,239 | ||||||
Financial liabilities valued at fair value | |||||||||
Puttable Instruments | — | 2,788,493 | — | ||||||
Measurement at fair value at 06/30/2017 | Level 1 | Level 2 | Level 3 | ||||||
Financial assets at fair value | |||||||||
Other financial assets | 266,443 | — | 3,292,758 | ||||||
Financial liabilities valued at fair value | |||||||||
Puttable Instruments | — | 2,500,000 | — | ||||||
Estimation of fair value
The fair value of marketable securities is calculated using the market approach using quoted prices in active markets for identical assets (Level 1 of the fair value hierarchy).
The income approach method used for the valuation of the participation of Chemotécnica requires the use of assumptions, including: sales projections, EBITDA, rate marginal of taxes, weighted average cost of capital and discount rate. Management has developed these assumptions on the basis of historical knowledge of the business and the projected financial information of the Company analyzed. These assumptions may vary depending on the occurrence of future events, perceptions of different market participants and other factors outside the control of the Company, and such variations may be significant in comparison to estimated values. The sensitivity analysis for the discounted cash flows was based on a 13.5% reduction in the discount rate or a 10% reduction in future cash flows. Those decreases in isolation would have increased/decreased the amount of the financial asset in USD 2.0 million and USD 0.6 million, respectively.
The Group’s financial liabilities, which were not traded in an active market were determined using valuation techniques that maximize the use of available market information, and thus rely as little as possible on specific estimates of the entity. The rates applicable to similar loans in the market (Level 2 of the fair value hierarchy) were compared.
The Group’s policy is to recognize transfers between different categories of the fair value hierarchy at the time they occur or when there are changes in the circumstances that cause the transfer.
There were no transfers between levels of the fair value hierarchy. There were no changes in economic or business circumstances affecting fair value.
Financial instruments not measured at fair value
The financial instruments not measured at fair value include cash and cash equivalents, trade accounts receivable, other accounts receivable, trade payables and other debts, loans, financed payments and instruments with a put option.
The carrying value of financial instruments not measured at fair value does not differ significantly from their fair value.
Management estimates that the carrying value of the financial instruments measured at amortized cost approximates their fair value.
F-38
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
Financial risks
There were no changes to the financial risks disclosed in the June 30, 2017 consolidated financial statements, except for:
a) Liquidity risk
The following table sets out the contractual maturities of financial liabilities:
As of September 30, 2017 | Up to 3 months | 3 to 12 months | Between one and three years | Between three and five years | Subsequent years | ||||||||||
Trade Payables and other payables | 32,428,819 | 6,641,378 | — | — | — | ||||||||||
Borrowings | 10,045,470 | 53,798,490 | 40,058,026 | 10,540 | — | ||||||||||
Financed payment - Acquisition of business(*) | 25,734,999 | 15,417,500 | 8,812,500 | — | — | ||||||||||
Contingent consideration - Acquisition of business | — | — | 17,300,000 | — | — | ||||||||||
Puttable instruments | — | 240,820 | 1,473,205 | 5,963,688 | 268,788 | ||||||||||
Total | 68,209,288 | 76,098,188 | 67,643,731 | 5,974,228 | 268,788 | ||||||||||
| (*) | On October 26, 2017, the Group has renewed the maturity of USD 20.85 million short term BAF loans until October 2018. This financial statement as of September 30, 2017 does not reflect the amended terms of the debt. |
The current action being carried out by the Group for planning to meet payments are explained in Note 1.
b) Currency risk
The table below sets forth our significant net exposure to currency risk as of September 30, 2017 and June 30, 2017.
Net foreign currency position | 09/30/2017 | 06/30/2017 | ||||
Amount expressed in USD | (70,487,329 | ) | (69,106,000 | ) | ||
Considering only this net currency exposure at September 30, 2017, if an Argentine peso/U.S. dollar revaluation or depreciation in relation to other foreign currencies, with the remaining variables remaining constant, would have a positive or a negative impact on comprehensive income as a result of foreign exchange gains or losses.
We estimate that a devaluation of the Argentine peso against the U.S. dollar of 20% during the three-month period ended September 30, 2017 would have resulted in a net pre-tax loss of approximately USD 14.1 million. We estimate that an appreciation of the Argentine peso against the U.S. dollar of 20% during the same period would have resulted in a net pre-tax gain of approximately USD 14.1 million.
c) Interest rate risk
The Group’s debt composition, consisting of the loans and the payment financed for the acquisition of Rizobacter, is set out below.
09/30/2017 | |||
Carrying amount | |||
Fixed-rate instruments | |||
Current financial liabilities | (82,272,511 | ) | |
Non-current financial liabilities | (69,260,873 | ) | |
Variable-rate instruments | |||
Current financial liabilities | (5,134,232 | ) | |
Non-current financial liabilities | (1,356,956 | ) | |
F-39
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
During the three-month period ended September 30, 2017, an increase (decrease) in 1 percentage point in the rate related to the portion of financial liabilities with variable interest rate and considering all other variables would have resulted in an increase (decrease) in the loss for the period of USD 0.2 million.
12. SHAREHOLDERS AND OTHER RELATED PARTIES BALANCES AND TRANSACTIONS
During the three-month periods ended September 30, 2017 and 2016, the transactions between the Group and related parties, and the related balances owed by and to them, are as follows:
Amount of the transactions | |||||||
Party | Transaction type | 09/30/2017 | 09/30/2016 | ||||
Joint ventures | R&D sales and services | 549,077 | 1,217,550 | ||||
Joint ventures | Purchases of goods and services | 4,200,135 | — | ||||
Joint ventures | Equity contributions | — | 79,207 | ||||
Joint ventures | Loans granted | 2,054,184 | — | ||||
Joint ventures | Interest gain | 38,459 | 27,852 | ||||
Key management personnel | Salaries, social security benefits and benefits | 345,435 | 361,989 | ||||
Shareholders and other related parties | Sale of own shares by subsidiary | 408,461 | — | ||||
Shareholders and other related parties | Sales of goods and services | 341,584 | 154,825 | ||||
Shareholders and other related parties | Purchases of goods and services | (777,882 | ) | (298,678 | ) | ||
Total | 7,159,453 | 1,542,745 | |||||
Amounts receivable from related parties
Amounts receivable from related parties | |||||||
Party | Transaction type | 09/30/2017 | 06/30/2017 | ||||
Shareholders and other related parties | Trade receivables | 3,754,728 | 4,217,840 | ||||
Shareholders and other related parties | Allowance for impairment | (214,592 | ) | (206,196 | ) | ||
Shareholders and other related parties | Deferred checks | 3,059 | — | ||||
Shareholders and other related parties | Other accounts receivable | 132,427 | 137,859 | ||||
Shareholders and other related parties | Advances to suppliers | — | 98,167 | ||||
Joint ventures | Trade receivables | 825,572 | 592,700 | ||||
Joint ventures | Other accounts receivable | 203,842 | 9,654 | ||||
Joint ventures | Loans and other receivables | 7,492,317 | 3,812,469 | ||||
Total | 12,197,353 | 8,662,493 | |||||
Amounts payable to related parties
Amounts payable to related parties | |||||||
Party | Transaction type | 09/30/2017 | 06/30/2017 | ||||
Key management personnel | Salaries, social security benefits and benefits | 78,066 | 74,893 | ||||
Shareholders and other related parties | Trade payables | 6,286,487 | 2,113,149 | ||||
Shareholders and other related parties | Puttable instruments | 2,564,040 | 2,508,467 | ||||
Joint ventures | Other accounts payables | 350,541 | 427,092 | ||||
Total | 9,279,134 | 5,123,601 | |||||
F-40
BIOCERES S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in USD, except otherwise indicated)
13. KEY MANAGEMENT PERSONNEL COMPENSATION
The compensation of directors and other members of key management personnel, including social contributions and other benefits, was as follows for the three-month period ended September 30, 2017, 2016:
Period ended | ||||||
09/30/2017 | 09/30/2016 | |||||
(unaudited) | (unaudited) | |||||
Salaries, social security and other benefits | 229,487 | 140,345 | ||||
Incentives based on stock options | 115,948 | 221,644 | ||||
Total | 345,435 | 361,989 | ||||
14. CONTINGENCIES, COMMITMENTS AND RESTRICTIONS ON THE DISTRIBUTION OF PROFITS
In June 2017, Rizobacter granted a pledge of a fixed-term certificate at Banco Galicia for the syndicated loan for an amount of USD 4,260,517. On September 11, 2017, the fixed-term certificate was updated for an amount of USD 4,250,715.
There were no other material changes to the contingencies, commitments and restrictions on the distribution of profits disclosed in the June 30, 2017 consolidated financial statements.
15. EVENTS OCCURRING AFTER THE REPORTING PERIOD
On October 26, 2017, the Group has renewed the maturity of BAF loans until October 2018.
In October 2017, the first due payments of “Financed payment to sellers” have been canceled. For the cancellation of these, the Group obtained a loan with Banco Santander Río for USD 4.5M at an annual rate of 4% and maturing at 120 days. However, the seller, International Property Services Corp (“IPS”), notified the Group to refrain from transferring the due amount until IPS can inform the Group of a new bank account number as set forth in the Shares Purchase Agreement (“SPA”). This stop payment requested by IPS does not constitute a breach of or default under the applicable SPA.
On October 14, 2017, the Group authorized the issuance of 579,600 Series A Preferred Shares of RASA Holding to the holders of Series A Preferred Shares whose anniversary year occurs in 2017, so as to pay dividends on the amounts. 119,276 of those shares correspond to the non-controlling interests and will be delivered between October and December depending on the moment of initial subscription.
In addition to those mentioned above, after September 30, 2017, there have been no other situations or circumstances that may require significant adjustments to the consolidated financial statements or require disclosure in the Notes thereto.
The Group analyzed transactions that occurred after September 30, 2017 until the date of approval of these consolidated financial statements.
F-41
Report of Independent Registered Public Accounting Firm
The stock split described in Note 10 to the consolidated financial statements has not been consummated at December 6, 2017. When it has been consummated, we will be in a position to furnish the following report.
Price Waterhouse & Co. S.R.L. | |
/s/ GABRIEL MARCELO PERRONE | |
Gabriel Marcelo Perrone | |
Partner | |
Rosario, Argentina | |
December 6, 2017 |
“Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Bioceres S.A.
In our opinion, the accompanying consolidated statement of financial position and the related consolidated statements of comprehensive income, changes in equity and cash flows present fairly, in all material respects, the financial position of Bioceres S.A. and its subsidiaries at June 30, 2017, December 2016 and 2015, and the results of their operations and their cash flows for the six-month transition period ended June 30, 2017 and the years ended December 2016 and 2015 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
Rosario, Argentina
December 6, 2017, except for the effects of the stock split described in Note 10, as to which the date is , ”
F-42
BIOCERES S.A.
CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period
ended June 30, 2016
Transition Fiscal Year Nº 16
Started at January 1, 2017 and ended June 30, 2017
Amounts expressed in United States Dollars (“USD”)
Name: | BIOCERES S.A. | |
Legal Address: | Ocampo 210 Bis - Predio CCT Rosario – Province of Santa Fe - Argentina | |
Main activities of Bioceres S.A.: | Investments in other companies. Investments in projects and goods related to biotechnology and technologies related to food, agribusiness and healthcare industries. | |
Main activities of the Group controlled by Bioceres S.A.: | Sale of seeds and provision of technology sourcing and product development services in the agricultural biotechnology sector, including through joint ventures and third-party collaborations. | |
Registration before the Public Registry of Commerce: | Bylaws | April 11, 2002 |
Last amendment | July 31, 2015 | |
Bylaws expiry date: | April 11, 2052 | |
F-43
BIOCERES S.A.
CERTAIN DEFINED TERMS
AFIP: Administración Federal de Ingresos Públicos (Argentine tax authority)
ARS: Argentine peso (legal currency in Republic of Argentina)
ARAI: Aportes reembolsables a instituciones (“Reimbursable contributions to institutions”)
Arcadia: Arcadia Biosciences, Inc. (Company incorporated in United States)
Argentina: Republic of Argentina
Argentine Capital Markets Law: Argentine Law 26,831 as amended and supplemented
Argentine General Corporations´ Law: Argentine Law 19,550 as amended and supplemented
ARG GAAP: Argentine Generally Accepted Accounting Principles
BAF: BAF Capital. Fianancial servicies.
BCRA: Banco Central de la República Argentina (Central Bank of Republic of Argentina)
BICE: Banco de Inversión y Comercio Exterior (Bank of Investment and Foreign Trade)
Bioceres: Bioceres S.A. (Company incorporated in Republic of Argentina)
Bioceres, Inc.: Bioceres, Inc. (a wholly owned subsidiary of Bioceres S.A., incorporated in United States)
Bioceres Semillas: Bioceres Semillas S.A. (Subsidiary of Bioceres S.A., incorporated in Republic of Argentina)
CAPM: Capital Assets Pricing Model
CERIDER: Centro Regional de Investigación y Desarrollo Rosario (Argentina)
CGU: Cash Generating Unit (as defined in IAS 36)
Chemotécnica: Chemoténica S.A. (Company incorporated in Republic of Argentina)
CNV: Comisión Nacional de Valores (Argentine Securities and Exchange Commission)
Company: Bioceres S.A. (Company incorporated in Republic of Argentina)
CONICET: Consejo Nacional de Investigaciones Científicas y Técnicas (National Scientific and Technical Research Council of Argentina)
DDJJ: Declaración jurada (Tax return)
EBITDA: Earnings Before Interest, Taxes, Depreciation and Amortization
EMBI: Emerging Markets Bond Index
EQC: EQC Agrifund Ltd
EPS: Earnings per share (IAS 33)
FASB: Financial Accounting Standards Board (US GAAP Standards Setter)
FACPCE: Federación Argentina de Consejos Profesionales de Ciencias Económicas (accounting and auditing standards setter in Argentina)
FONTAR: Fondo Tecnológico Argentino (Argentine Technology Fund)
France: Republic of France
Gador: Gador S.A. (Company incorporated in Republic of Argentina)
GM: genetically modified
Group: Collectivelly, Bioceres S.A. and all its subsidiaries
GDM Seeds: company incorporated in United States, subsidiary of Asociados Don Mario S.A. (a company incorporated in Argentina)
Héritas: Héritas S.A (Company incorporated in Republic of Argentina)
IASB: International Accounting Standards Board (IFRS Standards Setter)
IFRIC: IFRS Interpretation Committee (or Interpretation issued by the IFRS Interpretation Committee, if preceded by a number)
INDEAR: Instituto de Agrobiotecnología Rosario S.A. (subsidiary of Bioceres S.A, incorporated in Republic of Argentina)
INMET: Ingeniería Metabólica S.A. (subsidiary of Bioceres S.A, incorporated in Republic of Argentina)
INTA: Instituto Nacional de Tecnología Agropecuaria (Nacional Institute of Agricultural Technology of Argentina)
Lartirigoyen: Lartirigoyen y Cía S.A.
LSE: London Stock Exchange.
Management: collectively, the members of Management of Bioceres S.A.
Merval: Mercado de Valores de Buenos Aires S.A. (Buenos Aires Stock Market)
MULC: Mercado Único Libre de Cambios de Argentina (free exchange market)
F-44
BIOCERES S.A.
CERTAIN DEFINED TERMS
IAS: International Accounting Standard
IFRS: International Financial Reporting Standards (or International Financial Reporting Standard, if preceded by a number)
NYSE: New York Stock Exchange
IPO: Initial Public Offering
NCI: non-controlling interest
RASA Holding: RASA Holding, LLC (a wholly owned subsidiary of Bioceres Inc., incorporated in United States)
R&D: Research and development
RT: Resolución Técnica (accounting and auditing standard issued by FACPCE)
Rizobacter: Rizobacter Argentina S.A. (Subsidiary of Bioceres S.A., incorporated in Republic of Argentina)
San Cristóbal: San Cristóbal Seguros de Retiro S.A.
SAMSA: Sucesión de Antonio Moreno S.A.C.A.I.F.E.I.
SEC: Securities and Exchange Commission
Semya: Semya S.A. (Company incorporated in Republic of Argentina)
SGR: (“Reciprocal Guarantee Company”, regulated by Argentine Law 24,467)
Synertech: Synertech S.A. (Joint arrangement between Rizobacter S.A. and De Sangosse L.A., incorporated in Republic of Argentina)
S&W: S&W Seed Company (Company incorporated in California, USA)
S&W Semillas: S&W Semillas S.A. (Company incorporated in Republic of Argentina)
Trigall: Trigall Genetics S.A. (Company incorporated in Republic of Uruguay)
TS&PD: Technology Sourcing and Product Development
Uruguay: Republic of Uruguay
USD: United States Dollars
U.S. FDA: Food and Drug Administration of United States of America
Verdeca: Verdeca LLC (Company incorporated in the United States)
WACC: Weighted Average Cost of Capital
YPF: YPF S.A. (public company incorporated in Argentina)
F-45
BIOCERES S.A.
CONSOLIDATED STATEMENT OF FINANCIAL POSITION
At June 30, 2017, December 31, 2016 and 2015
(Amounts in USD)
Notes | 06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||||
ASSETS | ||||||||||||
CURRENT ASSETS | ||||||||||||
Cash and cash equivalents | 7.1 | 2,119,883 | 1,256,696 | 58,425 | ||||||||
Other financial assets | 7.2 | 4,526,960 | 4,554,474 | 844,000 | ||||||||
Trade receivables | 7.3 | 45,693,673 | 57,033,051 | 5,498,974 | ||||||||
Other receivables | 7.4 | 4,179,869 | 4,767,657 | 4,068,995 | ||||||||
Income and minimum presumed income taxes recoverable | 9 | 2,107,860 | — | — | ||||||||
Inventories | 7.5 | 31,723,752 | 33,157,565 | 3,060,041 | ||||||||
Total current assets | 90,351,997 | 100,769,443 | 13,530,435 | |||||||||
�� | ||||||||||||
NON-CURRENT ASSETS | ||||||||||||
Other financial assets | 7.2 | 3,292,758 | 3,082,906 | — | ||||||||
Other receivables | 7.4 | 5,631,095 | 4,776,733 | 1,820,267 | ||||||||
Income and minimum presumed income taxes recoverable | 9 | 691,447 | 1,603,425 | 392,536 | ||||||||
Deferred tax assets | 9 | 7,272,648 | 5,077,851 | 1,379,201 | ||||||||
Investments in joint ventures | 13 | 28,977,935 | 29,433,063 | 299,276 | ||||||||
Property, plant and equipment | 7.6 | 51,110,617 | 51,738,436 | 4,767,810 | ||||||||
Intangible assets | 7.7 | 42,181,770 | 44,083,273 | 1,767,168 | ||||||||
Goodwill | 7.8 | 32,866,203 | 34,401,620 | 536,065 | ||||||||
Total-non current assets | 172,024,473 | 174,197,307 | 10,962,323 | |||||||||
Total assets | 262,376,470 | 274,966,750 | 24,492,758 | |||||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-46
BIOCERES S.A.
CONSOLIDATED STATEMENT OF FINANCIAL POSITION (Cont’d)
At June 30, 2017, December 31, 2016 and 2015
(Amounts in USD)
Notes | 06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||||
LIABILITIES | ||||||||||||
CURRENT LIABILITIES | ||||||||||||
Trade and other payables | 7.9 | 23,748,712 | 37,050,214 | 7,091,742 | ||||||||
Borrowings | 7.10 | 51,020,481 | 65,847,948 | 2,215,282 | ||||||||
Employee benefits and social security | 7.11 | 5,570,209 | 4,019,556 | 376,691 | ||||||||
Deferred revenue and advances from customers | 7.12 | 1,256,975 | 944,273 | 667,373 | ||||||||
Income and minimum presumed income taxes payable | 9 | 223,187 | 1,878,657 | 68,366 | ||||||||
Government grants | 7.13 | 351,157 | 351,657 | 320,729 | ||||||||
Financed payment - Acquisition of business | 7.16 | 26,477,432 | 27,330,752 | — | ||||||||
Total-current liabilities | 108,648,153 | 137,423,057 | 10,740,183 | |||||||||
NON-CURRENT LIABILITIES | ||||||||||||
Borrowings | 7.10 | 41,106,995 | 11,898,062 | 815,501 | ||||||||
Government grants | 7.13 | 1,485,079 | 1,711,906 | 1,200,976 | ||||||||
Puttable instruments | 7.15 | 5,008,467 | 4,897,397 | 1,612,168 | ||||||||
Investments in joint ventures | 13 | 1,527,286 | 1,569,160 | 1,185,566 | ||||||||
Deferred tax liabilities | 9 | 24,620,369 | 25,247,390 | 407,708 | ||||||||
Provisions | 7.14 | 1,690,412 | 2,160,788 | 234,505 | ||||||||
Financed payment - Acquisition of business | 7.16 | 33,402,933 | 35,231,435 | — | ||||||||
Contingent consideration - Acquisition of business | 7.17 | 15,919,060 | 15,771,716 | — | ||||||||
Total non-current liabilities | 124,760,601 | 98,487,854 | 5,456,424 | |||||||||
Total liabilities | 233,408,754 | 235,910,911 | 16,196,607 | |||||||||
EQUITY | ||||||||||||
Issued capital | 11.1 | 6,968,538 | 6,968,538 | 6,968,538 | ||||||||
Share premium | 15,461,569 | 15,461,569 | 15,461,569 | |||||||||
Puttable instruments | (2,025,398 | ) | (2,025,398 | ) | (1,423,386 | ) | ||||||
Cost of own shares held by subsidiary | (726,822 | ) | (726,822 | ) | — | |||||||
Stock options | 1,067,278 | 692,554 | 48,293 | |||||||||
Accumulated losses | (25,891,909 | ) | (17,424,385 | ) | (12,916,324 | ) | ||||||
Revaluation of property, plant and equipment reserve | 1,219,600 | — | — | |||||||||
Foreign currency translation reserve | (6,576,793 | ) | (3,217,815 | ) | — | |||||||
Equity / (deficit) attributable to owners of the parent | (10,503,937 | ) | (271,759 | ) | 8,138,690 | |||||||
Non-controlling interests | 39,471,653 | 39,327,598 | 157,461 | |||||||||
Total equity | 28,967,716 | 39,055,839 | 8,296,151 | |||||||||
Total equity and liabilities | 262,376,470 | 274,966,750 | 24,492,758 | |||||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-47
BIOCERES S.A.
CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME
For the six-month transition period ended June 2017, for the years ended December 31, 2016,
2015 and for the six-month period ended June 2016
(Amounts in USD)
Notes | 06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||||
(unaudited) | |||||||||||||||
Revenue | 8.1 | 48,068,098 | 2,268,193 | 43,587,834 | 8,965,310 | ||||||||||
Government grants | 7.13 | 273,023 | 270,206 | 761,429 | 1,230,574 | ||||||||||
Total revenue | 48,341,121 | 2,538,399 | 44,349,263 | 10,195,884 | |||||||||||
Cost of sales | 8.2 | (30,185,446 | ) | (1,290,256 | ) | (31,600,998 | ) | (4,799,345 | ) | ||||||
Research and development expenses | 8.3 | (3,601,624 | ) | (1,268,661 | ) | (2,860,771 | ) | (2,688,924 | ) | ||||||
Selling, general and administrative expenses | 8.4 | (17,324,407 | ) | (3,541,058 | ) | (12,906,021 | ) | (4,080,860 | ) | ||||||
Share of loss of joint ventures | 13 | (786,805 | ) | (455,181 | ) | (936,769 | ) | (1,553,022 | ) | ||||||
Other income | 121,065 | — | 48,495 | — | |||||||||||
Operating loss | (3,436,096 | ) | (4,016,757 | ) | (3,906,801 | ) | (2,926,267 | ) | |||||||
Gain on previously held interest | 13 | — | — | 4,453,284 | — | ||||||||||
Finance income | 8.5 | 2,136,265 | 790,814 | 1,006,953 | 1,509,736 | ||||||||||
Finance costs | 8.6 | (14,945,495 | ) | (1,579,951 | ) | (10,923,378 | ) | (1,904,569 | ) | ||||||
Loss before income tax | (16,245,326 | ) | (4,805,894 | ) | (9,369,942 | ) | (3,321,100 | ) | |||||||
Income tax benefit / (expense) | 9 | 5,090,723 | 1,040,923 | 4,140,028 | (411,342 | ) | |||||||||
Loss for the period / year | (11,154,603 | ) | (3,764,971 | ) | (5,229,914 | ) | (3,732,442 | ) | |||||||
Other comprehensive income or loss | |||||||||||||||
Items that may be subsequently reclassified to profit and loss | (4,614,372 | ) | — | (4,482,329 | ) | — | |||||||||
Exchange differences on translation of foreign operations from joint ventures | (1,265,831 | ) | — | (1,168,589 | ) | — | |||||||||
Exchange differences on translation of foreign operations | (3,348,541 | ) | — | (3,313,740 | ) | — | |||||||||
Items that will not be subsequently reclassified to loss and profit | 2,032,872 | — | — | — | |||||||||||
Revaluation of property, plant and equipment, net of tax from joint ventures | 189,025 | — | — | — | |||||||||||
Revaluation of property, plant and equipment, net of tax | 1,843,847 | — | — | — | |||||||||||
Total comprehensive loss | (13,736,103 | ) | (3,764,971 | ) | (9,712,243 | ) | (3,732,442 | ) | |||||||
Profit / (loss) for the period / year attributable to: | |||||||||||||||
Equity holders of the parent | (8,467,524 | ) | (3,435,546 | ) | (4,508,061 | ) | (3,540,504 | ) | |||||||
Non-controlling interests | (2,687,079 | ) | (329,425 | ) | (721,853 | ) | (191,938 | ) | |||||||
(11,154,603 | ) | (3,764,971 | ) | (5,229,914 | ) | (3,732,442 | ) | ||||||||
Total comprehensive income / (loss) attributable to: | |||||||||||||||
Equity holders of the parent | (10,606,902 | ) | (3,435,546 | ) | (7,725,876 | ) | (3,540,504 | ) | |||||||
Non-controlling interests | (3,129,201 | ) | (329,425 | ) | (1,986,367 | ) | (191,938 | ) | |||||||
(13,736,103 | ) | (3,764,971 | ) | (9,712,243 | ) | (3,732,442 | ) | ||||||||
Earnings Per Share | |||||||||||||||
Basic loss attributable to ordinary equity holders of the parent | 10 | (0.33 | ) | (0.13 | ) | (0.17 | ) | (0.13 | ) | ||||||
Diluted loss attributable to ordinary equity holders of the parent | 10 | (0.33 | ) | (0.13 | ) | (0.17 | ) | (0.13 | ) | ||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-48
BIOCERES S.A.
CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
For the six-month transition period ended June 30, 2017, for the years ended December 31, 2016 and 2015
and for the six-month period ended June 30, 2016
(Amounts in USD)
Description | Attributable to the equity holders of the parent | Non- controlling Interests | Total equity | ||||||||||||||||||||||||||||||||||||
Shareholder’s contributions | Accumulated losses | Equity / (deficit) attributable to owners of the parent | |||||||||||||||||||||||||||||||||||||
Issued capital (Note 11.1) | Share premium | Puttable instruments | Cost of own shares held by subsidiary | Stock options | Subtotal | Accumulated losses | Foreign currency translation reserve | Revaluation of property, plant and equipment reserve | Subtotal | ||||||||||||||||||||||||||||||
12/31/2016 | 6,968,538 | 15,461,569 | (2,025,398 | ) | (726,822 | ) | 692,554 | 20,370,441 | (17,424,385 | ) | (3,217,815 | ) | — | (20,642,200 | ) | (271,759 | ) | 39,327,598 | 39,055,839 | ||||||||||||||||||||
Stock options | — | — | — | — | 374,724 | 374,724 | — | — | — | — | 374,724 | — | 374,724 | ||||||||||||||||||||||||||
Cash dividends distributed by subsidiary | — | — | — | — | — | — | — | — | — | — | — | (4,359 | ) | (4,359 | ) | ||||||||||||||||||||||||
Sale of preferred stocks to non controlling interest (Note 11.2) | — | — | — | — | — | — | — | — | — | — | — | 3,277,615 | 3,277,615 | ||||||||||||||||||||||||||
Loss for the period | — | — | — | — | — | — | (8,467,524 | ) | — | — | (8,467,524 | ) | (8,467,524 | ) | (2,687,079 | ) | (11,154,603 | ) | |||||||||||||||||||||
Other comprehensive income or loss | — | — | — | — | — | — | — | (3,358,978 | ) | 1,219,600 | (2,139,378 | ) | (2,139,378 | ) | (442,122 | ) | (2,581,500 | ) | |||||||||||||||||||||
06/30/2017 | 6,968,538 | 15,461,569 | (2,025,398 | ) | (726,822 | ) | 1,067,278 | 20,745,165 | (25,891,909 | ) | (6,576,793 | ) | 1,219,600 | (31,249,102 | ) | (10,503,937 | ) | 39,471,653 | 28,967,716 | ||||||||||||||||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-49
BIOCERES S.A.
CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
For the six-month transition period ended June 30, 2017, for the years ended December 31, 2016 and 2015
and for the six-month period ended June 30, 2016
(Amounts in USD)
Description | Attributable to the equity holders of the parent | Non- controlling Interests | Total equity | ||||||||||||||||||||||||||||||
Shareholder’s contributions | Accumulated losses | Equity / (deficit) attributable to owners of the parent | |||||||||||||||||||||||||||||||
Issued capital (Note 11.1) | Share premium | Puttable instruments | Cost of own shares held by subsidiary | Stock options | Subtotal | Accumulated losses | Subtotal | ||||||||||||||||||||||||||
12/31/2015 | 6,968,538 | 15,461,569 | (1,423,386 | ) | — | 48,293 | 21,055,014 | (12,916,324 | ) | (12,916,324 | ) | 8,138,690 | 157,461 | 8,296,151 | |||||||||||||||||||
Puttable instruments | — | (814,570 | ) | — | — | (814,570 | ) | — | — | (814,570 | ) | — | (814,570 | ) | |||||||||||||||||||
Reception of own shares by subsidiary (Note 11.1) | — | — | — | (270,342 | ) | — | (270,342 | ) | — | — | (270,342 | ) | — | (270,342 | ) | ||||||||||||||||||
Stock options | — | — | — | — | 585,955 | 585,955 | — | — | 585,955 | — | 585,955 | ||||||||||||||||||||||
Cash dividends distributed by subsidiary | — | — | — | — | — | — | — | — | — | (13,790 | ) | (13,790 | ) | ||||||||||||||||||||
Loss for the period | — | — | — | — | — | — | (3,435,546 | ) | (3,435,546 | ) | (3,435,546 | ) | (329,425 | ) | (3,764,971 | ) | |||||||||||||||||
06/30/2016 (unaudited) | 6,968,538 | 15,461,569 | (2,237,956 | ) | (270,342 | ) | 634,248 | 20,556,057 | (16,351,870 | ) | (16,351,870 | ) | 4,204,187 | (185,754 | ) | 4,018,433 | |||||||||||||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-50
BIOCERES S.A.
CONSOLIDATED STATEMENT OF CHANGES IN EQUITY (Cont’d)
For the six-month transition period ended June 30, 2017, for the years ended December 31, 2016 and 2015
and for the six-month period ended June 30, 2016
(Amounts in USD)
Description | Attributable to the equity holders of the parent | Non- controlling Interests | Total equity | |||||||||||||||||||||||||||||||||
Shareholder’s contributions | Accumulated losses | Equity / (deficit) attributable to owners of the parent | ||||||||||||||||||||||||||||||||||
Issued capital (Note 11.1) | Share premium | Puttable instruments | Cost of own shares held by subsidiary | Stock options | Subtotal | Accumulated losses | Foreign currency translation reserve | Subtotal | ||||||||||||||||||||||||||||
12/31/2015 | 6,968,538 | 15,461,569 | (1,423,386 | ) | — | 48,293 | 21,055,014 | (12,916,324 | ) | — | (12,916,324 | ) | 8,138,690 | 157,461 | 8,296,151 | |||||||||||||||||||||
Issue of puttable instruments | — | — | (814,570 | ) | — | — | (814,570 | ) | — | — | — | (814,570 | ) | — | (814,570 | ) | ||||||||||||||||||||
Reclassification of puttable instruments (Note 11.1) | — | — | 212,558 | — | — | 212,558 | — | — | — | 212,558 | — | 212,558 | ||||||||||||||||||||||||
Own shares held by subsidiary (Note 11.1) | — | — | — | (726,822 | ) | — | (726,822 | ) | — | — | — | (726,822 | ) | — | (726,822 | ) | ||||||||||||||||||||
Stock options | — | — | — | — | 644,261 | 644,261 | — | — | — | 644,261 | — | 644,261 | ||||||||||||||||||||||||
Cash dividends distributed by subsidiary | — | — | — | — | — | — | — | — | — | — | (13,790 | ) | (13,790 | ) | ||||||||||||||||||||||
Issurance of preferred shares (note 11.2) | — | — | — | — | — | — | — | — | — | — | 6,481,930 | 6,481,930 | ||||||||||||||||||||||||
Non-controlling Interests - Acquisition of business | — | — | — | — | — | — | — | — | — | — | 34,688,364 | 34,688,364 | ||||||||||||||||||||||||
Loss for the year | — | — | — | — | — | — | (4,508,061 | ) | — | (4,508,061 | ) | (4,508,061 | ) | (721,853 | ) | (5,229,914 | ) | |||||||||||||||||||
Other Comprehensive loss | — | — | — | — | — | — | — | (3,217,815 | ) | (3,217,815 | ) | (3,217,815 | ) | (1,264,514 | ) | (4,482,329 | ) | |||||||||||||||||||
12/31/2016 | 6,968,538 | 15,461,569 | (2,025,398 | ) | (726,822 | ) | 692,554 | 20,370,441 | (17,424,385 | ) | (3,217,815 | ) | (20,642,200 | ) | (271,759 | ) | 39,327,598 | 39,055,839 | ||||||||||||||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-51
BIOCERES S.A.
CONSOLIDATED STATEMENT OF CHANGES IN EQUITY (Cont’d)
For the six-month transition period ended June 30, 2017, for the years ended December 31, 2016 and 2015
and for the six-month period ended June 30, 2016
(Amounts in USD)
Description | Attributable to the equity holders of the parent | Non- controlling Interests | Total equity | |||||||||||||||||||||||||||
Shareholder’s contributions | Accumulated losses | Equity / (deficit) attributable to owners of the parent | ||||||||||||||||||||||||||||
Issued capital (Note 11.1) | Share premium | Puttable instruments | Stock options | Subtotal | Accumulated losses | Subtotal | ||||||||||||||||||||||||
12/31/2014 | 6,924,146 | 12,497,731 | (1,627,471 | ) | — | 17,794,406 | (9,375,820 | ) | (9,375,820 | ) | 8,418,586 | 349,399 | 8,767,985 | |||||||||||||||||
Issue of share capital (note 11.1) | 44,392 | 2,963,838 | — | — | 3,008,230 | — | — | 3,008,230 | — | 3,008,230 | ||||||||||||||||||||
Reclassification of puttable instruments (Note 11.1) | — | — | 204,085 | — | 204,085 | — | — | 204,085 | — | 204,085 | ||||||||||||||||||||
Stock options | — | — | — | 48,293 | 48,293 | — | — | 48,293 | — | 48,293 | ||||||||||||||||||||
Loss for the year | — | — | — | — | — | (3,540,504 | ) | (3,540,504 | ) | (3,540,504 | ) | (191,938 | ) | (3,732,442 | ) | |||||||||||||||
12/31/2015 | 6,968,538 | 15,461,569 | (1,423,386 | ) | 48,293 | 21,055,014 | (12,916,324 | ) | (12,916,324 | ) | 8,138,690 | 157,461 | 8,296,151 | |||||||||||||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-52
BIOCERES S.A.
CONSOLIDATED STATEMENT OF CASH FLOWS
For the six-month transition period ended June 30, 2017, for for the six-month period ended June 30, 2016,
and for the years ended December 31, 2016 and 2015
(Amounts in USD)
Notes | 06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||||
(unaudited) | |||||||||||||||
OPERATING ACTIVITIES | |||||||||||||||
Loss for the period / year | (11,154,603 | ) | (3,764,971 | ) | (5,229,914 | ) | (3,732,442 | ) | |||||||
Adjustments to reconcile profit / (loss) to net cash flows | |||||||||||||||
Income tax | 9 | (5,090,723 | ) | (1,040,923 | ) | (4,140,028 | ) | 411,342 | |||||||
Depreciation of property, plant and equipment | 7.6 | 1,524,709 | 256,492 | 1,074,733 | 456,444 | ||||||||||
Amortization of intangible assets | 7.7 | 1,418,661 | 24,065 | 463,066 | 45,233 | ||||||||||
Share of loss of joint ventures | 13 | 786,805 | 455,181 | 936,769 | 1,553,022 | ||||||||||
Gain on previously held interest | — | — | (4,453,284 | ) | — | ||||||||||
Changes in fair value of financial assets | (116,138 | ) | (178,000 | ) | 2,371,338 | 155,841 | |||||||||
Provisions for contingencies | (396,897 | ) | (2,737 | ) | 343,569 | (16,677 | ) | ||||||||
Allowance for impairment of trade debtors | 271,986 | 6,724 | 140,386 | 181,972 | |||||||||||
Allowance for obsolescence | (2,211 | ) | — | 982,351 | 63,427 | ||||||||||
Puttable instruments interests | 111,070 | 73,664 | 183,217 | 148,120 | |||||||||||
Stock option | 374,724 | 585,955 | 644,261 | 48,293 | |||||||||||
Gain on sale of equipment and intangible assets | (712,885 | ) | — | (289,345 | ) | — | |||||||||
Interests and exchange differences from borrowings | 4,611,889 | 242,526 | 5,240,451 | (552,655 | ) | ||||||||||
Other financial results accrued | 1,707,095 | — | 670,097 | — | |||||||||||
Working capital adjustments | |||||||||||||||
Trade receivables | 11,067,392 | 1,637,239 | (11,019,803 | ) | 272,937 | ||||||||||
Other receivables | (649,588 | ) | (1,056,695 | ) | (4,385,512 | ) | (3,176,718 | ) | |||||||
Income and minimum presumed income taxes | (618,267 | ) | (9,666 | ) | (726,555 | ) | (246,244 | ) | |||||||
Inventories | 1,436,024 | (509,329 | ) | 9,766,899 | (2,152,030 | ) | |||||||||
Trade and other payables | (13,301,502 | ) | (4,353,315 | ) | (5,473,994 | ) | 2,640,222 | ||||||||
Employee benefits and social security | 1,550,653 | (197,744 | ) | 3,642,865 | 80,474 | ||||||||||
Deferred revenue and advances from customers | 312,702 | 79,943 | 276,900 | 420,700 | |||||||||||
Other tax payables | — | 5,344 | (3,921 | ) | (120,894 | ) | |||||||||
Government grants | (227,327 | ) | 399,273 | 541,858 | 474,843 | ||||||||||
Net cash flows used in operating activities | (7,096,431 | ) | (7,346,974 | ) | (8,443,596 | ) | (3,044,790 | ) | |||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-53
BIOCERES S.A.
CONSOLIDATED STATEMENT OF CASH FLOWS (Cont’d)
For the six-month transition period ended June 2017, for the six-month period ended June 30, 2016,
for the years ended December 31, 2016 and 2015
(Amounts in USD)
Notes | 06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||||
(unaudited) | |||||||||||||||
INVESTMENT ACTIVITIES | |||||||||||||||
Acquisition of business | — | — | (41,129,533 | ) | — | ||||||||||
Investment in joint ventures and associates | 13 | (617,236 | ) | (82,804 | ) | (162,012 | ) | (2,031,409 | ) | ||||||
Short-term investments | (66,200 | ) | (3,360,000 | ) | (3,259,704 | ) | — | ||||||||
Interest received on short-term investments | — | — | 261,471 | — | |||||||||||
Purchase of property, plant and equipment | 7.6 | (760,072 | ) | (536,257 | ) | (1,919,881 | ) | (640,743 | ) | ||||||
Capitalized development expenditures | 7.7 | (374,202 | ) | (71,638 | ) | (390,940 | ) | (194,276 | ) | ||||||
Purchase of intangible assets | 7.7 | (1,114,041 | ) | — | (420,254 | ) | (7,095 | ) | |||||||
Net cash flows used in investing activities | (2,931,751 | ) | (4,050,699 | ) | (47,020,853 | ) | (2,873,523 | ) | |||||||
FINANCING ACTIVITIES | |||||||||||||||
Procceeds from borrowings for the acquisition of business | — | — | 35,608,943 | — | |||||||||||
Proceeds from other borrowings | 61,098,344 | 15,000,000 | 17,375,000 | 992,456 | |||||||||||
Repayment of borrowings and interest payments | (47,145,503 | ) | (329,793 | ) | (495,396 | ) | (539,123 | ) | |||||||
Net increase (decrease) bank overdraft and other short-term borrowings | (3,057,113 | ) | (1,557,450 | ) | (1,332,036 | ) | 1,265,317 | ||||||||
Proceeds from the issuarance of puttable instruments | — | — | 2,500,000 | — | |||||||||||
Proceeds from the issuarance of preferred shares | — | — | 3,019,999 | — | |||||||||||
Sale of preferred stocks | |||||||||||||||
Cash dividends distributed by subsidiary | 11.1 | (4,359 | ) | (13,790 | ) | (13,790 | ) | — | |||||||
Capital contribution | — | — | — | 2,008,389 | |||||||||||
Net cash flows provided by financing activities | 10,891,369 | 13,098,967 | 56,662,720 | 3,727,039 | |||||||||||
Net increase/ (decrease) in cash and cash equivalents | 863,187 | 1,701,294 | 1,198,271 | (2,191,274 | ) | ||||||||||
Cash and cash equivalents as of beginning of the year | 7.1 | 1,256,696 | 58,425 | 58,425 | 2,249,699 | ||||||||||
Cash and cash equivalents as of the end of the year | 7.1 | 2,119,883 | 1,759,719 | 1,256,696 | 58,425 | ||||||||||
The accompanying Notes are an integral part of these consolidated financial statements
F-54
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Index
| 1. | General information. |
| 1.1. | The Group |
| 1.2. | Financial and economic situation |
| 1.3. | Acquisition of Rizobacter |
| 2. | Accounting standards and basis of preparation |
| 2.1. | Statement of compliance with IFRS as issued by IASB |
| 2.2. | Authorization for the issue of the Consolidated Financial Statements |
| 2.3. | Change in fiscal year-end |
| 2.4. | Basis of measurement |
| 2.5. | Functional currency and presentation currency |
| 2.6. | Subsidiaries and consolidation |
| 3. | Changes in Accounting Policies and estimates |
| 4. | New standards, amendments and interpretations issued by the IASB |
| 4.1. | New and amended standards adopted by the Group |
| 4.2. | New standards and interpretations not yet adopted |
| 5. | Summary of significant accounting policies |
| 5.1. | Cash and cash equivalents |
| 5.2. | Financial assets |
| 5.3. | Deferred offering costs |
| 5.4. | Inventories |
| 5.5. | Business combinations |
| 5.6. | Impairment of non-financial assets (excluding inventories and deferred tax assets) |
| 5.7. | Joint arrangements |
| 5.8. | Property, plant and equipment |
| 5.9. | Leased assets |
| 5.10. | Intangible assets |
| 5.11. | Financial liabilities |
| 5.12. | Employee benefits |
| 5.13. | Provisions |
| 5.14. | Shareholder’s contributions |
| 5.15. | Revenue recognition |
F-55
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
| 5.16. | Government grants |
| 5.17. | Borrowing costs |
| 5.18. | Income tax and minimum presumed income tax |
| 5.19. | Share-based payments |
| 6. | Critical accounting judgments and estimates |
| 6.1. | Critical judgments |
| 6.2. | Critical estimates |
| 7. | Information about components of consolidated statements of financial position |
| 7.1. | Cash and cash equivalents |
| 7.2. | Financial assets at fair value through profit or loss |
| 7.3. | Trade receivables |
| 7.4. | Other receivables |
| 7.5. | Inventories |
| 7.6. | Property, plant and equipment |
| 7.7. | Intangible assets |
| 7.8. | Goodwill |
| 7.9. | Trade and other payables |
| 7.10. | Borrowings |
| 7.11. | Employee benefits and social security |
| 7.12. | Deferred revenue and advances from customers |
| 7.13. | Government grants |
| 7.14. | Provisions |
| 7.15. | Puttable instruments |
| 7.16. | Financed payment - Acquisition of business |
| 7.17. | Contingent consideration - Acquisition of business |
| 7.18. | Changes in allowances and provisions |
| 8. | Information about components of consolidated statement of comprehensive income |
| 8.1. | Revenue |
| 8.2. | Cost of sales |
| 8.3. | R&D classified by nature |
| 8.4. | Expenses classified by nature and function |
| 8.5. | Finance income |
| 8.6. | Finance costs |
F-56
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
| 9. | Income tax and minimum presumed income tax |
| 10. | Earnings per share |
| 11. | Information about consolidated components of equity |
| 11.1. | Shareholder’s contributions |
| 11.2. | Non-controlling interests |
| 12. | Cash flow information |
| 13. | Joint arrangements |
| 13.1. | Joint ventures |
| 13.2. | Joint operations |
| 14. | Segment information |
| 15. | Financial instruments- risk management |
| 15.1. | Principal financial instruments |
| 15.2. | Financial instruments by category |
| 15.3. | Financial instruments measured at fair value |
| 15.4. | Financial instruments not measured at fair value |
| 15.5. | General objectives, policies and processes |
| 16. | Leases |
| 16.1. | Finance lease - lessee |
| 16.2. | Operating lease - lessee |
| 17. | Shareholders and other related parties balances and transactions |
| 18. | Key management personnel compensation |
| 19. | Share-based payments |
| 20. | Contingencies, commitments and restrictions on the distribution of profits |
| 21. | Events occurring after the reporting period |
F-57
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
1. GENERAL INFORMATION
1.1. The Group
Bioceres S.A. is a sociedad anónima incorporated under the Argentine Corporations Law in the Province of Santa Fe, Republic of Argentina and domiciled at Ocampo 210 Bis - Predio CCT, in the City of Rosario, Province of Santa Fe, Argentina.
The Group is a fully-integrated agricultural biotechnology business with a leadership position in the South America region. The Group operates multiple technology platforms to develop and commercialize products that enhance crop productivity and expand feedstock applications in order to provide value to its customers around the world. See detail of the organization structure in Note 2.6.
1.2. Financial and economic situation
The following section explains the major events that occurred in 2017 and 2016, which have impacted the current financial and economic condition of the Group.
Rizobacter acquisition
On October 19, 2016, Bioceres acquired a controlling equity interest in Rizobacter Argentina S.A., an Argentine company and global leader in soybean biological products. Rizobacter is Argentina’s leading provider of bio-based solutions for the agricultural sector with a strong focus on crop nutrition and crop protection solutions and an extensive network of international affiliates.
Rizobacter has a strategic partnership with the French company De Sangosse. As part of a joint venture, they formed Synertech, a company that produces and commercializes micro-beaded fertilizers. In 2016, Synertech opened a new factory for the production of such fertilizers, which required an investment of more than USD 30 million.
See detailed information about the acquisition in Note 1.3.
The Group’s capital structure and financing of the Rizobacter acquisition
IPO transaction
On December 17, 2014, the Company’s shareholders authorized the Board of Directors (BOD) to make an initial public offering (IPO) of new ordinary shares of the Company in Argentina with the CNV and with certain other foreign securities commissions.
On September 24, 2015, the SEC accepted the submission of a registration statement by Bioceres for the offering of the American Depositary Receipts (ADRs) to be listed the NYSE, and on December 4, 2015, the CNV authorized Bioceres to make a public offering of ordinary shares in Argentina to be listed on Merval upon compliance with certain requirements.
On December 15, 2016, the Company’s shareholders ratified the capital increase approved at an extraordinary shareholders’ meeting held on December 17, 2014 in connection with the relaunch of the proposed IPO.
In early 2017, the Company informed to the SEC that it intended to continue the IPO process and submit a new registration statement in order to reflect the changes to Bioceres’s business, including with respect to the acquisition of Rizobacter. At that point, the Company determined that the best course of action would be to request a withdrawal of the previous registration statement. On February 1, 2017, the Company requested the withdrawal of its prior registration statement from the SEC.
F-58
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Financing of the Rizobacter acquisition
On October 14, 2016, RASA Holding LLC, a subsidiary of Bioceres, authorized the issuance of 8,053,500 Class A preferred shares with a par value of USD 10 per share to raise funds for the USD 42 million necessary to finance the cash consideration required for the acquisition of 60% (50.01% plus an option for a further 9.99%) of the capital stock of Rizobacter in October 2016, of which 4,830,000 Class A preferred shares were subscribed at a price of USD 8.696 per share.
The Class A preferred shares in RASA Holding accrue an annual “pay in kind” (PIK) dividend coupon of 12% that accumulates as principal over a term of 5 years, and carry (i) a mandatory participation right for the subscription to ordinary shares of Bioceres as part of a qualified public offering (i.e., an offering that is consummated for at least USD 50 million net of the proceeds received from the holders of the RASA Holding Class A preferred shares), and to participate in an optional manner in the case of a non-qualified public offering and/or a private capital increase occurring between December 17, 2017 and October 14, 2021 and (ii) a mandatory conversion right for holders of the RASA Holding Class A preferred shares to convert such preferred shares into common shares of RASA Holding after 5 years, or in the case of a public offering made by RASA Holding or Rizobacter.
In October 2016, Bioceres subscribed Class A preferred shares for USD 39.5 million with the funds raised with the two loans granted by BAF for a total of USD 32 million, the loan from Banco Mariva for USD 3 million, the loan from Garruchos S.A. for USD 4 million and with its own funds for USD 0.5 million (see Acquisition of Rizobacter in Note 1.3), while a third-party investor subscribed the rest of the Class A preferred shares for USD 2.5 million. In December 2016, upon completion of the acquisition of a non-controlling equity interest in Chemotécnica (Note 7.2), the Sellers of Chemotécnica received Class A preferred shares from Bioceres for a total of USD 6.5 million, in exchange for 27.99% of Chemotécnica for USD 3.1 million, plus USD 3.4 million in cash (received by the Sellers upon the first payment from Lartirigoyen and SAMSA made in December 2016). These funds were applied to the full repayment of the Banco Mariva loan and a portion of the Garruchos loan.
In May 2017, the Sellers of Chemotécnica agreed to subscribe an additional USD 3.3 million of Class A preferred shares for payment in cash (resulting from the second and last payment from Lartirigoyen and SAMSA); Bioceres applied these funds to the repayment of the balance due to Garruchos.
As of December 31, 2016, there were 1,032,922 Class A preferred shares (excluding preferred dividends) held by third parties valued at USD 8.696 per share, representing a total nominal value of USD 8,981,931, of which 745,422 shares are included in equity for USD 6,481,930 and 287,500 shares are presented as puttable instruments in non-current liabilities for USD 2,500,000.
As of June 30, 2017, there were 1,409,848 Class A preferred shares (excluding preferred dividends) held by third parties valued at USD 8.696 per share, representing a total nominal of USD 12,259,545, of which 1,122,348 shares are included in equity for USD 9,759,545 and 287,500 shares are presented as puttable instruments in non-current liabilities for USD 2,500,000.
In March 2017, Rizobacter consummated a USD 45 million syndicated loan agreement with Banco Galicia as the syndicated loan underwriting entity, for the purpose of refinancing the short-term liabilities and financing the working capital of Rizobacter. The first installment of USD 22 million was funded in March 2017, while the second and final installment of USD 23 million was funded in April 2017. The syndicated loan has a 4-year maturity, will accrue interest at an annual rate of 6.5% (payable on a quarterly basis) and the principal of which will be amortized in equal quarterly installments as from the 12th month after the transaction date, counted as from the disbursement date. The loan may be repaid before maturity, at Rizobacter’s choice.
As of June 30, 2017, the date of that the Group’s annual financial statements close, the Company’s losses were higher than 50% of its capital and reserves. Under Argentine corporate law (Ley general de sociedades), art. 206 establishes a
F-59
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
mandatory reduction of capital when such situation happens. Additionally, even though total consolidated equity was $30 million, the Company presented negative equity attributable to equity holders of the parent. The art. 94 (inc. 5) of the Argentine corporate law states that when losses exceed a company’s capital and reserves at its annual fiscal close, such situation constitutes cause for dissolution, and hence, its shareholders shall take the necessary measures to capitalize the company. The six-month transition period ended June 30, 2017 presents negative equity which was primarily as a result of the effects related to the Rizobacter acquisition, including (i) the step up charge included in the cost of sales of Rizobacter inventories acquired and sold post-acquisition that were revalued at acquisition due to the application of purchase price allocation, or PPA, accounting rules under IFRS, (ii) currency exchange differences relating to Argentine peso-denominated assets and liabilities acquired, (iii) the evolution of the fair value of assets and liabilities acquired, and (iv) the classification of certain capital raises through convertible loans and puttable instruments as liabilities and through convertible preferred shares as non-controlling interest under IFRS. The ongoing plan to reverse the negative equity balance is to capitalize the company through the capital increase approved by the shareholders of the Company and placement of the newly issued shares in a public or private offering in Argentina and abroad, and the expected conversion of certain financing instruments into ordinary shares of the company.
Bioceres is planning to meet payment of the balances due under deferred payment obligations for the acquisition of Rizobacter and the loans received from BAF mainly with (i) funds from the sale of ordinary shares of Bioceres or conversion of the debt convertible instruments; (ii) the additional sale of Class A preferred shares of RASA Holding in its possession (Bioceres holds Class A preferred shares for a total par value of USD 34.2 million, which are available for placement with private investors); (iii) with funds from cash and banks balances and operating income generated in the ordinary course of business of the Group; and (iv) with medium- and long-term structured bank financing. Furthermore, the proceeds from the IPO of Bioceres, if consummated, will generate additional funds and will enable the Group to optimize capital structure and financing costs.
Bioceres also granted participation rights to BAF for the subscription of shares in the Company in a public offering and/or a private capital increase if either occurs before the end of the second anniversary of the USD 12 million loan to Bioceres or the end of the first anniversary of the USD 20 million loan to Bioceres Inc. or the second anniversary if it is extended by the parties by mutual consent, as a mechanism for repayment of the loans granted.
In October 2017 the Group extended the maturity of BAF loans until October 2018.
In consequence, in view of the economic projections and financial plans devised by Management, these financial statements have been prepared applying going-concern principles, considering the Company’s ability to continue with its ordinary course of business.
1.3 Acquisition of Rizobacter
On October 19, 2016, RASA Holding, a subsidiary of Bioceres, acquired 20,004,000 shares of Rizobacter representing 50.01% of the outstanding capital stock plus an option for a further 9.99%
The total purchase price was USD 57.3 millon, of which USD 42 millon was paid in cash on the date of acquisition and the remainder was paid through financing. Additionally, a contingent payment of USD 17.3 millons will become due if charges associated with certain shares are released.
F-60
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
A payment structure was agreed for this acquisition subject to the following conditions:
Total consideration | USD | ||
Cash payment(a) | 42,000,001 | ||
Financed payment(b) | 15,300,000 | ||
Conditional payment(c) | 17,300,000 | ||
Call option(d) | 14,985,000 | ||
Discount for present value(e) | (5,984,496 | ) | |
Subtotal | 83,600,505 | ||
Bioceres shares held by Rizobacter | (456,480 | ) | |
Total consideration | 83,144,025 | ||
| (a) | Cash payment: A cash payment for USD 42 million was agreed to be made on the transaction date. The net cash amount paid, less the cash and cash equivalent balances of the companies whose shares were purchased, was USD 41.1 million. |
| (b) | Financed payment: A deferred payment was agreed with certain selling shareholders of Rizobacter, pursuant to the terms of which payment is to be made in five (5) non-interest bearing installments, consisting of a payment of USD 3.54 million within 12 months from the transaction date, and subsequent payments for USD 2.94 million, within 18, 24, 30 and 36 months, for a total of USD 15.3 million. |
On October 2017, the first due payments have been canceled. For the cancellation of these, the Group obtained a loan with Banco Santander Río for USD 4.5M at an annual rate of 4% and maturing at 120 days.
| (c) | Conditional payment: A contingent purchase of price of USD 17.3 million may be payable to certain of the Sellers, as a portion of the equity interest acquired by RASA Holding (19% of Rizobacter’s capital stock) is subject to a precautionary measure issued pursuant to an injunction that affects 44% of the total capital stock of Rizobacter. The precautionary measure also covers 30% of the dividends distributed to such shares, directing such percentage of dividends into a judicially created escrow account. |
The precautionary measure relates to litigation among historical shareholders of Rizobacter arising from a disputed transfer of shares that occurred in 1995. Although the Supreme Court of Argentina ruled against certain of the litigating historical shareholders, such shareholders subsequently pursued other legal remedies (including the precautionary measure) to further dispute the original transfer of shares.
The Group purchased our controlling stake interest in Rizobacter subject to the ongoing precautionary measure and associated ongoing litigation. Should such contingencies be lifted, the Group may be obligated to pay a contingent purchase price of US$17.3 million to certain selling shareholders of Rizobacter through RASA Holding. Conversely, should the court rule against the free transferability of the affected shares, we the Group would be obligated to return certain shares, thereby reducing our equity participation in Rizobacter, and the Group would not be obligated to pay the abovementioned contingent purchase price. Given the Supreme Court of Argentina’s finding that the 1995 share transfer was valid, it is not likely or probable that our equity participation of Rizobacter will be affected. However, as the Group hold voting rights for 80% of Rizobacter, such a reduction in its equity participation might not impact their control of Rizobacter.
| (d) | Call option: Bioceres has the right to purchase a further 9.99% of the capital stock of Rizobacter within two (2) years from the date of acquisition. Once two (2) years have elapsed, or if Bioceres and/or its affiliates purchase all or a portion of the capital stock of Rizobacter from certain shareholders, the call option will become irrevocable upon acceptance by the shareholders, and the Group will have the obligation to purchase that percentage if such sellers so require it within 60 calendar days following either of the two above-mentioned events. |
The fair value of the purchase option was estimated based on a 100% probability that the option will be exercised within two years of the acquisition date.
| (e) | The discount annual rate for present value was estimated at 8%. It was based on the incremental borrowing rate available to the Group at the time of the acquisition considering the term and conditions of the payments. The discount annual rate for present value was applied to the financed payment, the conditional payment and the call option, taking into consideration the estimated time of the payments and the certainity of the call option being exercised and the conditional payment being made. |
Goodwill is mainly generated by the synergies between Rizobacter and the rest of the Group and by intangible assets not separately recognizable. Goodwill is not deductible for tax purposes.
F-61
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The acquired assets, the assumed liabilities and the value of minority interests, recognized at the transaction date (October 19, 2016), are as follows:
Fair value of assets acquired and liabilities assumed | USD | ||
Cash and cash equivalents | 870,468 | ||
Financial assets at fair value through profit or loss | 8,819 | ||
Trade receivables | 40,925,002 | ||
Other receivables | 1,972,842 | ||
Income and minimum presumed income taxes recoverable | 1,244,419 | ||
Inventories | 40,846,774 | ||
Investments in joint ventures and associates | 29,000,000 | ||
Property, plant and equipment | 48,222,848 | ||
Intangible assets | 44,976,359 | ||
Trade and other payables | (35,432,466 | ) | |
Other tax payables | (3,921 | ) | |
Borrowings | (55,051,665 | ) | |
Deferred tax liabilities | (29,028,245 | ) | |
Provisions | (1,372,086 | ) | |
Total net assets identified | 87,179,148 | ||
Acquisition of control over Semya | (4,317,619 | ) | |
Non-controlling interest in acquired companies | (34,688,362 | ) | |
Goodwill | 34,970,858 | ||
Total consideration | 83,144,025 | ||
Acquired trade receivables
The fair value of acquired trade receivables at the acquisition date was USD 40,925,002. The gross contractual amount for trade receivables due was USD 43,552,899 of which USD 2,627,897 was expected to be uncollectible. The group determined that the carrying value of Rizobacter trade receivables did not differ signicantly from the fair value.
Acquired inventory
The fair value of acquired inventory at the acquisition date was USD 40,846,774. The fair value of finished goods inventory (approximately USD 31.9 million) was measured by determining net realisable value (estimated selling prices of inventory, less the sum of (i) costs of disposal and (ii) a reasonable profit allowance for the selling effort) as this represents an exit price. The fair value of raw materials (aprox USD 8.9 million) was measured by determining the replacement cost.
Financing of the cash payment
The cash payment of USD 42 million was financed from the following sources:
| 1. | BAF loan to Bioceres for USD 12 million. The proceeds of this loan were used to fund the Company’s acquisition of Rizobacter through the subscription of preferred shares of RASA Holding. |
| 2. | Loan from BAF to Bioceres Inc. for USD 20 million. With the proceeds raised from this loan, Bioceres Inc. subscribed to the preferred shares in RASA Holding and RASA Holding purchased shares in Rizobacter. |
| 3. | Loan from Banco Mariva S.A. to Bioceres for USD 3 million. This loan was set to mature after a period of 180 days, accruing interest at an annual rate of 4.5%. However, this loan was repaid in full in December 2016 in advance of |
F-62
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
its maturity with the proceeds from the sale of Class A preferred shares in RASA Holding, owned by Bioceres, which were acquired and paid up by the selling shareholders of Chemotécnica (Note 7.2) with the proceeds from the sale of their equity interests.
| 4. | Loan granted by Garruchos to Bioceres S.A. for USD 4 million. This loan was repaid in full with the proceeds from the sale of Class A preferred shares in RASA Holding owned by Bioceres, which were acquired and paid up by the selling shareholders of Chemotécnica (Note 7.2) with the proceeds from the sale of their equity interests. |
| 5. | Preferred shares in RASA Holding acquired and paid up by a third-party investor for USD 2.5 million. |
| 6. | Bioceres’ own funds for USD 0.5 million. |
Transaction costs incurred in the acquisition include the following:
Transaction costs | |||
Professional fees(a) | 599,150 | ||
Financial commissions(b) | 527,794 | ||
Total transaction costs | 1,126,944 | ||
| (a) | Included in selling, general and administrative expenses for the year closed as of December 31, 2016 |
| (b) | Included in finance cost for the period closed as of December 31, 2016 |
Indemnity – Settlement agreement
On December 7, 2016, RASA Holding notified the Sellers of a undisclosed liability in connection with the settlement offers Nos. 1-A/2016, 2-A/2016, 3-A/2016, 4-A/2016 and 5-A/2016 (each dated October 18, 2016) relating to an undisclosed labor relation claim made by a third party against Rizobacter. The labor claim is considered a hidden liability pursuant to the representations and warranties made by the Sellers to RASA Holding in connection with the above-mentioned offers.
Rizobacter reached a settlement agreement for ARS 2,476,610 (USD 159,781) payable in 5 (five) consecutive monthly installments, the first of which would be in the amount of ARS 476,610 and the remaining four of which shall be in the amount of ARS 500,000 each. The settlement agreement was confirmed on March 3, 2017 by the Obligatory Labor Mediation Service (SECLO) of the Argentine Department of Labor, Employment and Social Security, under the terms of Section 15 of the Employment Contract Law.
The Group discounted 50.01% of the amount paid as hidden liability at the payment of the first installment of the Financed Payment scheduled for October 19, 2017, pursuant to the offsetting clause envisaged in the tender offers. According to the Indemnity clause established on the offers, the Group may withhold and/or offset against any receivable that might be held by any of the sellers with any amounts owed to the Group by the sellers under the offers.
Pro-forma information
Rizobacter contributed revenues of USD 36,739,497 and a net loss of USD 799,080 to the Group for the period from October 20 to December 31, 2016.
If the acquisition had occurred on January 1, 2016, consolidated pro forma revenue and loss for the year ended December 31, 2016 would have been USD 103,297,144 and USD 14,759,837, respectively
Argentine Antitrust Commission (Comisión Nacional de Defensa de la Competencia or “CNDC”) – Acquisition of Rizobacter
On October 26, 2016, RASA Holding, together with the Sellers of Rizobacter, notified the CNDC of the acquisition of the controlling interest in Rizobacter by RASA Holding. On August 25, 2017, the Argentine Secretary of Commerce of the
F-63
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Ministry of Production, supported by the favorable opinion provided by the CNDC, approved the acquisition of 50.01% of the capital stock of Rizobacter by Bioceres through its subsidiary, RASA Holding, pursuant to resolution N° 645/2017.
2. ACCOUNTING STANDARDS AND BASIS OF PREPARATION
2.1. Statement of compliance with IFRS as issued by IASB
The consolidated financial statements of the Group have been prepared and are presented in accordance with IFRS as issued by IASB following the accounting policies as set forth and summarized in Note 5.
2.2. Authorization for the issue of the Consolidated Financial Statements
These consolidated financial statements of the Group as of June 30, 2017, December 31, 2016, 2015, for the transition period ended June 30, 2017, for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016 have been authorized to be filed with the SEC by the Board of the Company at its meeting held on December 6, 2017. The information on historical earnings per share in these financial statements was updated to reflect the stock split described in Note 10 that will be effective after the issuance of these financial statements (December 6, 2017), but before the effective date of the registration statement.
2.3. Change in fiscal year-end
On December 16, 2016, the Company’s shareholders approved the amendment of article 16 of the bylaws, thereby changing the fiscal year-end date to from December 31 to June 30 of each year. As a result of the change in the Company’s fiscal year end, figures presented in these financial statements in connection with the six-month Transition Period are not entirely comparable to the fiscal years ended December 31, 2016 and 2015.
2.4. Basis of measurement
The consolidated financial statements of the Group have been prepared using:
| ■ | Going Concern Basis of Accounting, considering the conclusion of the assessment made by the Group’s Management about the ability of the Company and its subsidiaries to continue as a going concern, in accordance with the requirements of paragraph 25 of IAS 1; |
| ■ | Accrual Basis of Accounting (except for cash flows information). Under this basis of accounting, the effects of transactions and other events are recognized as they occur, even when there are no cash flows. |
2.5. Functional currency and presentation currency
| ■ | Functional currency |
Items included in the financial statements of each of the Group’s entities are measured using the currency of the primary economic market in which the entity operates (i.e., “the functional currency”).
The acquisition of Rizobacter generated synergies and other changes in the Group’s operations that, along with other changes in the business, triggered the reassessment of some of the Group companies’ functional currency. Management concluded that the Argentine Peso shall be the functional currency of all of the Group’s Argentine entities, including Bioceres, as from January 1, 2017. The group accounted for this change prospectively.
IAS 29 “Financial reporting in hyperinflationary economies” requires that the financial statements of an entity whose functional currency is the currency of a hyperinflationary economy with high inflation, whether they are based on the historical cost method or the current cost method, be stated in terms of the measuring unit current at the closing date of
F-64
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
the reporting period. For such purpose, the inflation produced from the acquisition date or the revaluation date, as applicable, must be computed in non-monetary items. To standard for concluding whether an economy is a hyperinflationary economy details a series of factors to be considered, including, but not limited to, a cumulative inflation rate over a three year period that approaches or exceeds 100%. Taking into consideration the inconsistency of Argentine inflation data published by the National Institute of Statistics and Census, the current downward trend of the level of inflation and the fact that other indicators are insufficient to reach a definite conclusion, there is insufficient evidence to conclude that Argentina’s economy is a hyperinflationary economy as of June 30, 2017. Therefore, the restatement criteria established in IAS 29 have not been applied with respect to the transition period.
Although the Argentine economy does not meet the necessary and objective conditions to qualify as a hyperinflationary economy in accordance with IAS 29, it is important to note that certain macroeconomic variables affecting the Group’s business, including the costs of salaries and supplies, have experienced significant annual variations, which must be taken into account when evaluating and interpreting the Group’s financial position and results of operations in these financial statements.
| ■ | Presentation currency |
The consolidated financial statements of the Group are presented in USD, which is the presentation currency.
| ■ | Foreign currency |
Transactions entered into by Group entities in a currency other than their functional currency are recorded at the relevant exchange rates as of the date upon which such transactions occur. Foreign currency monetary assets and liabilities are translated at the prevailing exchanges rates as of the final day of each reporting period. Exchange differences arising on the retranslation of unsettled monetary assets and liabilities are recognized immediately in profit or loss, except for foreign currency borrowings qualifying as a hedge of a net investment in a foreign operation for which exchange differences are recognized in other comprehensive income and accumulated in the foreign exchange reserve along with the exchange differences arising on the retranslation of the foreign operation. Upon the disposal of a foreign operation, the cumulative exchange differences recognized in the foreign exchange reserve relating to such operation up to the date of disposal are transferred to the consolidated statement of profit or loss and other comprehensive income as part of the profit or loss taking place upon such disposal.
2.6. Subsidiaries and consolidation
| ■ | Control |
Where the Company holds a controlling interest in another entity, such entity is classified as a subsidiary. The Company exercises control over such an entity if all three of the following elements are present: (i) the Company has the power to direct or cause the direction of the management and policies of the entity; the Company is exposed to the variable returns of such entity; and (iii) the Company has power to affect the variabity of such returns. Control is reassessed whenever facts and circumstances indicate that there may be a change in any of these elements of control.
De-facto control exists in situations where the Company has the practical ability to direct the relevant activities of an entity without holding the majority of the voting rights. In determining whether de facto control exists, the Company considers all relevant facts and circumstances, including:
| - | The relative share of the Company’s voting rights with respect both the size and dispersion of other parties who hold voting rights; |
| - | Substantive potential voting rights held by the Company and by other parties; |
| - | Other contractual arrangements; and |
| - | Historic patterns in voting attendance. |
F-65
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
| ■ | Consolidation method |
The consolidated financial statements present the results of the Group as if they formed a single entity. Intercompany transactions and balances between entities within the Group are therefore eliminated in full.
The consolidated financial statements incorporate the results of business combinations using the acquisition method. In the statement of financial position, an acquiree’s identifiable assets, liabilities and contingent liabilities are initially recognized at their fair values as of the acquisition date. The results of acquired operations are included in the consolidated statement of profit or loss and comprehensive income from the date on which the business combination is completed, and they are deconsolidated from the date on which control ceases.
| ■ | Subsidiaries |
The subsidiaries of the Group, all of which have been included in the consolidated financial statements of the Group, are as follows:
Name | Principal activities | Country of incorporation and principal place of business | Ref | % Equity interest | ||
06/30/2017 | 12/31/2016 | 12/31/2015 | ||||
Bioceres Semillas S.A. | Seed Business | Argentina | a | 82.46% | 82.46% | 82.46% |
Instituto de Agrobiotecnología Rosario S.A. | Research and development | Argentina | b | 96.00% | 96.00% | 96.00% |
Ingeniería Metabólica S.A. | Metabolic Engineering | Argentina | c | 58.80% | 58.80% | 58.80% |
Semya S.A. | Microbiology Business | Argentina | c | 80.00% | 80.00% | — |
Rizobacter Argentina S.A. | Microbiology Business | Argentina | d | 60.00% | 60.00% | — |
RASA Holding, LLC | Investment in subsidiaries | United States | c | 100.00% | 100.00% | — |
Bioceres, Inc. | Investment in Joint Ventures | United States | c | 100.00% | 100.00% | 100.00% |
Rizobacter do Brasil Ltda. | Selling of agricultural inputs | Brazil | e | 59.94% | 59.94% | — |
Rizobacter del Paraguay S.A. | Selling of agricultural inputs | Paraguay | e | 57.00% | 57.00% | — |
Rizobacter Uruguay | Selling of agricultural inputs | Uruguay | e | 59.40% | 59.40% | — |
Rizobacter South Africa | Selling of agricultural inputs | South Africa | e | 57.00% | 57.00% | — |
Comer. Agrop. Rizobacter de Bolivia S.A. | Selling of agricultural inputs | Bolivia | e | 57.00% | 57.00% | — |
Rizobacter USA, LLC | Selling of agricultural inputs | United States | e | 60.00% | 60.00% | — |
Indrasa Biotecnología S.A. | Research and development | Argentina | e/f | 31.50% | 31.50% | — |
Synertech SAS | Research and development | France | e. | 60.00% | — | |
The Group holds a majority share of the voting rights in all of its subsidiaries.
Bioceres, the parent company of the Group, holds the equity interest in each of the subsidiaries with the exception of INMET, RASA Holding, SEMYA and Rizobacter (including the Rizobacter subsidiaries) . In the case of INMET, Bioceres directly holds 30% and indirectly holds 28.8% (through INDEAR’s direct holding of 30% of the equity interest in INMET) of the equity interest in INMET. Bioceres, Inc holds a 100% equity interest in RASA Holding, and RASA Holding
F-66
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
holds 60% (50.01% plus a call option for a further 9.99%) equity interest in Rizobacter. Bioceres directly holds 50% and indirectly holds a further 30% (through Rizobacter) of the equity interest in Semya. Semya was a joint venture between Bioceres and Rizobacter until Bioceres’ acquisition of Rizobacter on October 19, 2016, at which point Semya became a subsidiary within the Group.
| a) | The voting rights attributable to Bioceres and its non-controlling interest in Bioceres Semillas were 93.84% and 6.16%, respectively, as of December 31, 2016 and 2015. |
| b) | The voting rights attributable to Bioceres and its non-controlling interest in INDEAR were 98.82% and 1.18%, respectively, as of December 31, 2016 and 2015. |
| c) | The percentage of the voting rights attributable to Bioceres is the same as the percentage of its equity interest as set forth in the table above. |
| d) | RASA Holding entered into a shareholders’ agreement with certain existing shareholders of Rizobacter pursuant to which Bioceres was granted control of 80% of the voting rights of Rizobacter. |
| e) | Indirect interests held through Rizobacter. The indirect equity interest participation included in this table was the 60% of the direct equity interest participation that Rizobacter owns in each entity. |
| f) | Rizobacter directly holds a 52.5% equity interest in Indrasa Biotecnología S.A. and an equal percentage of the voting rights thereof. The Group indirectly holds a 31.5% equity interest in Indrasa Biotecnología S.A through Rizobacter’s direct interest therein. |
3. CHANGES IN ACCOUNTING POLICIES AND ESTIMATES
There were no changes in accounting policies or estimates which could have a material effect on the consolidated financial statements for the six-month transition period ended June 30, 2017 and fiscal years ended December 31, 2016 and 2015, or which could have an effect on future fiscal years, nor were there any major changes in the estimates of the reported amounts in the latest interim financial statements except for as described in Note 5.8 (adopting the land and building revaluation method).
4. NEW STANDARDS, AMENDMENTS AND INTERPRETATIONS ISSUED BY THE IASB
4.1. New and amended standards adopted by the Group
The Group has applied the following standards and amendments for the first time in the six-month transition period commencing January 1, 2017:
| - | Recognition of Deferred Tax Assets for Unrealized Losses – Amendments to IAS 12, and |
| - | Disclosure initiative – amendments to IAS 7. |
The adoption of these amendments did not have any impact on the amounts recognized in prior periods.
The amendments to IAS 7 require disclosure of changes in liabilities arising from financing activities (Note 12).
F-67
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
4.2. New standards and interpretations not yet adopted
The standards and interpretations issued, but not yet effective up to the date of issuance of the Group’s consolidated financial statements, which are or may be applicable to the Group, are disclosed below. The Group intends to adopt these standards and interpretations when they become effective, except otherwise stated.
IFRS 9 – Financial Instruments (version 2014)
IFRS 15 – Revenue from Contracts with Customers
IFRS 16 – Leases
Amendments to IFRS 2 – Classification and measurement of share-based payment transactions
IFRIC 22 – Foreign currency transactions and advance consideration
Amendments to IAS 40 – Transfers of investment
IFRS 9 – Financial Instruments (version 2014)
In July 2014, the IASB issued the final version of “IFRS 9 Financial Instruments” which reflects all phases of the financial instruments project and replaces “IAS 39 Financial Instruments: Recognition and Measurement” and all previous versions of IFRS 9. The standard introduces new requirements for classification and measurement, impairment, and hedge accounting. IFRS 9 is effective for annual periods beginning on or after January 1, 2018, with early application permitted. Retrospective application is required, but comparative information is not compulsory. Early application of previous versions of IFRS 9 (2009, 2010 and 2013) is permitted if the date of initial application is before February 1, 2015.
The Group previously elected to adopt IFRS 9 (version 2013) early.
The new measurement and classification requirements in IFRS 9 (version 2014) are not applicable to the Group’s business model for managing its financial assets and that model is not expected to change in the foreseeable future. The Group does not use hedge accounting.
Regarding the new impairment requirements in IFRS 9 (version 2014), the Group will have to change its impairment testing methodology for financial assets from an “incurred losses model” to the new “expected losses model.” Due to this change, the Group may need to recognize in the future certain impairment losses for financial assets generated from expected losses not yet incurred, which losses are not recognized under the current model.
IFRS 15 – Revenue from Contracts with Costumers
IFRS 15 was issued in May 2014 and establishes a new five-step model that will apply to revenue arising from contracts with customers. Under IFRS 15 revenue is recognized at an amount that reflects the consideration to which an entity expects to be entitled in exchange for transferring goods or services to a customer.
The principles in IFRS 15 provide a more structured approach to measuring and recognizing revenue.
The new revenue standard is applicable to all entities and will supersede all current revenue recognition requirements under IFRS. Either a full or modified retrospective application is required for annual periods beginning on or after January 1, 2018 with early adoption permitted.
The Group is currently assessing the impact of IFRS 15, including with respect to the new disclosure requirements, and plans to adopt the new standard on the required effective date.
Because of the above-mentioned factors, it is not possible at this moment to estimate the potential effects in the Group`s revenue recognition accounting policies, but it can be anticipated that disclosures about revenue shall be more extensive.
F-68
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
IFRS 16 - Leases
IFRS 16 was issued in January 2016. It will result in almost all leases being recognized on the balance sheet, as the distinction between operating and finance leases will be removed. Under the new standard, an asset (the right to use the leased item) and a financial liability to pay rentals are recognized. The only exceptions are short-term and low-value leases.
The accounting for lessors will not significantly change.
The standard will affect primarily the accounting for the Group’s operating leases. However, the Group has not yet assessed what adjustments, if any, are necessary due to the change in the definition of the lease term and the different treatment of variable lease payments and of extension and termination options. It is therefore not yet possible to estimate the amount of right-of-use assets and lease liabilities that will have to be recognized on adoption of the new standard and how this may affect the Group’s profit or loss and classification of cash flows going forward.
The new standard will be effective for financial years commencing on or after January 1, 2019. At this stage, the Group does not intend to adopt the standard before its effective date.
The Group is currently assessing the impact of the new disclosure requirements and currently it is not possible to estimate the potential effects to the Group.
Amendments to IFRS 2 – Classification and measurement of share-based payment transactions
The standard was amended in June 2016 to clarify the measurement basis for cash-settled share-based payments and the accounting for modifications that change an award from cash-settled to equity-settled. It also introduces an exception to IFRS 2 principles by requiring an award to be treated as if it was wholly equity-settled where an employer is obliged to withhold an amount in respect of the employee’s tax obligation associated with a share-based payment and to pay that amount to the relevant tax authority. It is effective for annual periods beginning on or after January 1, 2018. The Group is currently analyzing the impact of its application on the Group’s operating results or financial position.
IFRIC 22 – Foreign currency transactions and advance consideration
IFRIC 22 was issued in December 2016. The interpretation addresses how to determine the date of the transaction for the purpose of determining the exchange rate to use on initial recognition of the related asset, expense or income related to an entity that has received or paid advance consideration in a foreign currency. The date of the transaction is the date on which an entity initially recognizes the non-monetary asset or non-monetary liability arising from the payment or receipt of such advance consideration. The standard is effective for annual periods beginning on January 1, 2018. The Group is currently analyzing the impact of its application on the Group’s operating results or financial position.
Amendments to IAS 40 – Transfers of investment
These modifications clarify when an entity must transfer properties, including properties under construction or development, or outside investment property. The modifications establish that a change in use occurs when the property complies with or fails to meet the definition of investment property and there is evidence of change in use. A simple change in Management’s intentions about the use of the property does not provide evidence of change in use.
The changes to the standard will become effective for annual periods beginning on January 1, 2018, with advance application permitted. The modifications are applied prospectively to changes in use occurring after the beginning of the annual period in which the modifications are applied for the first time.
These amendments are not expected to have material impact on the Group.
F-69
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
5. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
5.1. Cash and cash equivalents
For the purposes of the statements of financial position and statements of cash flows, cash and cash equivalents include cash on hand and in banks and short-term highly liquid investments (original maturity of less than 90 days). In the consolidated statements of financial position, bank overdrafts are included in borrowings within current liabilities.
5.2. Financial assets
The Group early adopted IFRS 9 (version 2013) early.
The Group measures its financial assets at initial recognition at fair value.
The Group classifies its financial assets as financial assets measured at amortized cost (using the effective interest method) on the basis of both:
| - | The Group’s business model for managing the financial assets; and |
| - | The contractual cash flows characteristics of the financial asset. |
The Group has not irrevocably designated a financial asset as measured at fair value through profit or loss to eliminate or significantly reduce a measurement or recognition inconsistency.
Financial assets at fair value through profit or loss are measured at fair value through profit and loss due to the business model used in their negotiation and/or the contractual characteristics of their cash flows.
The Group does not apply hedge accounting.
Estimates
The Group makes estimates of uncollectibility of its recorded receivables. Management analyzes trade account receivables in accordance with conventional criteria, adjusting the amount through a charge of an allowance for bad debts upon recognition of the inability of third parties to afford their financial obligations to the Group. Management specifically analyzes the accounts receivable, the historical bad debts, solvency of customers, current economic trends and the changes to the payment conditions of customers to assess the adequate allowance for bad debts.
5.3. Deferred offering costs
Deferred offering costs consist primarily of direct incremental accounting and legal fees related to the Company’s proposed IPO of its common shares that is expected to take place after the effectiveness of the prospectus filed with the CNV and equivalents institutions abroad.
As of the year ended December 31, 2016, deferred offering costs were recognized as finance costs because they are referred to the earlier IPO process that needed to be updated with Rizobacter acquisition. Capitalization of expenses related to the new process is expected in subsequent periods, which once the Company’s IPO is completed, will be reclassified as a deduction from the proceeds of the offering to be included in equity.
F-70
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
5.4. Inventories
Inventories are recognized at cost initially and subsequently at the lower of cost and net realizable value. Cost comprises all costs of purchase and conversion as well as other costs incurred in bringing the inventories to their present location and condition.
Weighted average cost is used to determine the cost of ordinarily interchangeable items.
Estimates
The Group assesses the recoverability of inventories considering their sale price, whether the inventories are damaged and whether they have become obsolete in whole or in part.
Net realizable value is the sale price estimated to be attained in the ordinary course of business, less costs of completion and other selling expenses.
The Group sets up an allowance for obsolescence or slow moving inventories in relation to finished and in-process products. The allowance for obsolescence or slow moving inventories is recognized for finished products and in-process products based on an analysis by Management of the aging of inventory stocks.
5.5. Business combinations
The Group applies the acquisition method to account for business combinations. The acquisition cost is measured as the aggregate of the consideration transferred for the acquisition of a subsidiary, which is measured at fair value at the acquisition date, and the amount of any non-controlling interest in such subsidiary. The Group recognizes any non-controlling interest in a subsidiary at the non-controlling interest’s proportionate share of the recognized amounts of subsidiary’s identifiable net assets. The acquisition related costs are expensed as incurred.
Any contingent consideration to be transferred by the Group is recognized at fair value at the acquisition date. The contingent consideration is classified as an asset or liability that is a financial instrument under IFRS 9 is measured at fair value through profit or loss.
Goodwill is initially measured at cost, which is the excess of the aggregate of the consideration transferred and the amount of the non-controlling interest and any previous interest carried over the net identifiable assets acquired and liabilities assumed.
After initial recognition, goodwill is measured at cost less any accumulated impairment losses. For the purpose of impairment testing, goodwill acquired in a business combination is, as of the acquisition date, allocated to each of the cash-generating units of the Group that is expected to benefit from the synergies of the combination, without considering whether other assets or liabilities of the subsidiary are allocated to those units.
Any impairment in the carrying value is recognized in the consolidated statement of comprehensive income. In the case of acquisitions in stages, prior to the write-off of the previously held equity interest in the subsidiary, said interest is re-measured at fair value as of the date of acquisition of control over the subsidiary. The result of the re-measurement at fair value is recognized in profit or loss.
When a seller in a business combination has contractually agreed to indemnify the Group for the result of a contingency or uncertainty related to the entirety or a portion of an asset or liability, the Group recognizes an indemnification asset. The indemnification asset is measured on the same basis as the indemnification item. At the end of each period, the Group measures the indemnification assets recognized at the acquisition date on the same basis as the indemnified liability, subject to any contractual limitation on the amount and, for an indemnification asset that is not periodically
F-71
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
measured at fair value, based on Management’s assessment of the recoverability of the indemnification asset. The Group derecognizes the indemnification asset when it collects or sells it, or when it loses the right over it.
Judgment
As indicated in Note 1, the Group entered into a purchase option contract with certain sellers of Rizobacter to acquire a further 9.99% interest, which option is effective until October 19, 2018. As stated in paragraphs B89 and B90 of IFRS 10, the Group must evaluate whether or not the purchase option grants it, in substance, rights to returns from its involvement with Rizobacter.
If the equity interest currently gives the Group rights to returns from its involvement with Rizobacter, then the proportion of returns allocated to the controlling entity should consider the possible exercise of the voting rights that currently entitle the Group to those returns. Otherwise, the possible exercise of the voting rights should not be considered and only the current voting rights are to be considered.
To perform this analysis, the Group has considered that the purchase option contract exposes the Group to returns from the change in the value of the shares (due to the fixed price of the option) and that dividends are not significant in relation to the returns from the equity interest, since the Group has the power to restrict dividend payments to equity holders in Rizobacter. Based on this analysis, the Group has concluded that the purchase option contract grants it, in substance, rights to returns from its involvement with Rizobacter and computed those possible voting rights when determining the proportion of returns allocated to the controlling entity.
Estimates
The contingent consideration for the acquisition of Rizobacter has been valued at fair value. The determination of fair value of the contingent consideration is based on an estimate of the weighted average present value of the probability of the expected cash flows. The main assumptions considered in determining fair value relate to the applicable discount rate and to the expected timing of the expected cash flows.
When the Group acquired control over Rizobacter, it also acquired control over Semya, which was previously a joint venture between Bioceres and Rizobacter that was measured through the equity method. As required by paragraph 42 of IFRS 3, the Group re-measured the fair value of its previous equity interest in Semya at acquisition date. The determination of fair value of Semya at the acquisition date is based on the application of a future cash flow present value technique. The main assumptions considered in determining fair value relate to the applicable discount rate and to the projections of revenue from the launch of seed treatment packs.
5.6. Impairment of non-financial assets (excluding inventories and deferred tax assets)
Impairment tests on goodwill and intangible assets not yet available for use are undertaken annually at the end of the reporting period. Other non-financial assets are subject to impairment tests whenever events or changes in circumstances indicate that their carrying amount may not be recoverable. Where the carrying value of an asset exceeds its recoverable amount (i.e. the higher of value in use and fair value less costs to sell), the asset is written down accordingly.
Where it is not possible to estimate the recoverable amount of an individual asset, the impairment test is carried out on the smallest group of assets to which it belongs for which there are separately identifiable cash flows (its Cash Generating Unit or CGU). Goodwill is allocated on initial recognition to each of the Group’s CGUs that are expected to benefit from a business combination that gives rise to the goodwill.
Impairment charges are included in profit or loss, except to the extent they reverse gains previously recognized in other comprehensive income. An impairment loss recognized for goodwill is not reversed.
F-72
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Estimate
Impairment testing of goodwill and intangible assets not yet available for use requires the use of significant assumptions for the estimation of future cash flows and the determination of discount rates. The significant assumptions and the determination of discount rates for the impairment testing of goodwill are further explained in note 7.8.
5.7. Joint arrangements
An associate is an entity over which the Group exerts significant influence. Significant influence is the power to participate in financial and operating policy decision-making at such entity, but it does not involve control or joint control over those policies.
The Group is a party to a joint arrangement when there is a contractual arrangement that confers joint control over the relevant activities of the arrangement to the Group and at least one other party. Joint control is assessed under the same principles as control over subsidiaries.
The Group classifies its interests in joint arrangements as either:
| - | Joint ventures: where the group has rights to only the net assets of the joint arrangement; or |
| - | Joint operations: where the group has both the rights to the assets and obligations for the liabilities of the joint arrangement. |
In assessing the classification of interests in joint arrangements, the Group considers:
| - | The structure of the joint arrangement; |
| - | The legal form of joint arrangements structured through a separate vehicle; |
| - | The contractual terms of the joint arrangement agreement; and |
| - | Any other facts and circumstances (including any other contractual arrangements). |
The Group accounts for its interests in joint ventures using the equity method, where the Group’s share of post-acquisition profits and losses and other comprehensive income is recognized in the consolidated statement of profit and loss and other comprehensive income.
Losses in excess of the Group’s investment in the joint venture are recognized only to the extent that the Group has incurred legal or constructive obligations or made payments on behalf of the joint venture.
Profits and losses arising on transactions between the Group and its joint ventures are recognized only to the extent of unrelated investors’ interests in the joint venture. The Group’s share in a joint venture’s profits and losses resulting from a transaction is eliminated against the carrying amount of investment in the joint venture through the line “share of profit (or loss) of joint ventures” in the consolidated statements of profit or loss and other comprehensive income.
Any premium paid for an investment in a joint venture above the fair value of the Group’s share of the identifiable assets, liabilities and contingent liabilities acquired is capitalized and included in the carrying amount of the investment in the joint venture. Where there is objective evidence that the investment in a joint venture has been impaired, the carrying amount of the investment is tested for impairment in the same way as other non-financial assets.
F-73
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
When the Group loses significant influence in an associate or joint control over a joint venture, it measures and recognizes any investment held at fair value. Any difference between the carrying amount of the associate or joint venture when losing significant influence or joint control and the fair value of the held investment and sale revenue are recognized in profit or loss.
The Group accounts for its interests in joint operations by recognizing its share of assets, liabilities, revenues and expenses in accordance with its contractually conferred rights and obligations.
For all joint arrangements structured in separate vehicles the Group must assess the substance of the joint arrangement in determining whether it is classified as a joint venture or joint operation. This assessment requires the Group to consider whether it has rights to the joint arrangement’s net assets (in which case it is classified as a joint venture), or rights to and obligations for specific assets, liabilities, expenses, and revenues (in which case it is classified as a joint operation).
Upon consideration of the factors mentioned above, the Group has determined that all of its joint arrangements structured through separate vehicles only give it rights to the net assets and are therefore classified as joint ventures (Note 13.1).
Estimates
There is considerable uncertainty regarding Management’s estimates of the Group’s ability to recover the carrying amounts of the investments in joint ventures, since such estimates depend on the joint ventures’ ability to generate sufficient funds to complete the development projects, the future outcome of the project deregulation process and the amounts and timing of the cash flows from projects, among other future events.
Management assesses whether there are impairment indicators and, if any, it performs a recoverability analysis.
Management estimates of the recoverability of these investments represent the best estimate based on available evidence, the existing facts and circumstances, using reasonable and provable assumptions in the cash flow projections.
Therefore, the consolidated financial statements do not include adjustments that would be required if the Group were unable to recover the carrying amount of the above-mentioned assets by generating sufficient economic benefits in the future.
When the Group acquired control of Rizobacter, it also acquired the joint control of Synertech. Therefore, the investment in Synertech was added at the time of initial recognition of the acquisition at fair value. The determination of the fair value of Synertech at the acquisition date is based on the application of a future cash flow present value technique. The main assumptions considered in determining fair value relate to the applicable discount rate and to the projections of higher revenue from sales of micro-granulated fertilizers.
5.8. Property, plant and equipment
Property, plant and equipment items are initially recognized at cost. In addition to the purchase price, cost also includes costs directly attributable to such property, plant and equipment items. There are no unavoidable costs with respect to dismantling and removing items. The cost of property, plant and equipment items acquired in a business combination is their fair value at the acquisition date.
F-74
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Depreciation is calculated using the straight-line method to allocate the property, plant or equipment items’ cost or revalued amounts, net of their residual values, over their estimated useful lives or, in the case of leasehold improvements and certain leased plant and equipment, the shorter lease term as follows:
Research instruments: 3 to 10 years
Office equipment: 5 to 10 years
Vehicles: 5 years
Computer equipment and software: 3 years
Fixture and fittings: 10 years
Machinery and equipment: 5 to 10 years
Buildings on third parties property: The lease term
Buildings: 50 years
Useful lives and depreciation methods are reviewed every year as required by IAS 16.
Assets under items Land and Buildings, are accounted for at fair value arising from the last revaluation performed, applying the revaluation model indicated by IAS 16. This policy was adopted by the Group as of the transition period ended June 30, 2017.
Revaluations are performed on a regular basis, when there are signs that the book value differs significantly from that to be determined using the fair value at the end of the reporting year.
To obtain fair values, the existence of an active market is considered for the assets in their current status. For those assets for which an active market in their current status exists, the fair values were determined based on their market values. For the remaining cases, the market values of comparable new assets are analysed, applying a discount based on the status and wear of each asset and considering the characteristics of each of the revalued assets (for example, improvements made, maintenance status, level of productivity, use, etc.)
Estimates
The Group carries certain classes of property, plant and equipment under the revaluation model under IAS 16. The revaluation model requires that the Group carry property, plant and equipment at revalued amounts, being fair value at the date of revaluation less any subsequent accumulated depreciation and any subsequent accumulated impairment losses. IAS 16 requires that the Group carry out these revaluations with sufficient regularity so that the carrying amounts of its property, plant and equipment do not differ materially from that which would be determined using fair value at the end of a reporting period. The determination of fair value at the date of revaluation requires judgments, estimates and assumptions based on market conditions prevailing at the time of any such revaluation. Changes to any of the Group’s judgments, estimates or assumptions or to the market conditions subsequent to a revaluation will result in changes to the fair value of property, plant and equipment.
The Group prepares the corresponding revaluations on a regular basis taking into account the work of independent appraisers. The Group uses different valuation techniques depending on the class of property being valued. Generally, the Group determines the fair value of its industrial buildings and warehouses based on a depreciated replacement cost approach. The Group determines the fair value of its land based on active market prices adjusted, if necessary, for differences in the nature, location or condition of the specific asset. If this information is not available, the Group may use alternative valuation methods, such as recent prices in less active markets.
Property valuation is a significant area of estimation uncertainty. Fair values are prepared regularly by Management, taking into account independent valuations. The determination of fair value for the different classes of property, plant and equipment is sensitive to the selection of various significant assumptions and estimates. Changes in those
F-75
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
significant assumptions and estimates could materially affect the determination of the revalued amounts of property, plant and equipment. The Group utilizes historical experience, market information and other internal information to determine and/or review the appropriate revalued amounts.
The following are the most significant assumptions used in the preparation of the revalued amounts for its classes of property, plant and equipment:
| ■ | Land: The Group generally uses the market price of a square meter of land for the same or similar location as the most significant assumption to determine the revalued amount. The Group typically uses comparable land sales in the same location to assess appropriateness of the value of its land. A 10% increase or decrease in the market price of land could have a significant impact on the revalued amount of its land. |
| ■ | Industrial buildings and warehouses: The Group generally determines the construction cost of a new asset and then the Group adjusts it for normal wear and tear. Construction prices may include, but are not limited to, construction materials, labor costs, installation and assembly costs, site preparation, professional fees and applicable taxes. Construction costs may differ significantly from year to year and are subject to macroeconomic changes in the economy where the Group operates, such as the impact of inflation and foreign exchange rates. The construction cost of its industrial buildings and warehouses is determined on a US dollar per constructed square meter basis, while the construction cost of its mills, facilities and grain storage facilities is determined by reference to their total capacity measured in tons milled or stored, respectively. A 5% increase or decrease in the construction costs relating to such assets could have a significant impact on their revalued amounts. A 5% variation in the estimate of normal wear and tear could also have a significant impact on their revalued amounts. |
Increases in the carrying amounts arising on revaluation of land and buildings are recognized, net of tax, in other comprehensive income and accumulated in reserves in shareholders’ equity. To the extent that the increase reverses a decrease previously recognized in profit or loss, the increase is first recognized in profit or loss. Decreases that reverse previous increases of the same asset are first recognized in other comprehensive income to the extent of the remaining surplus attributable to the asset; all other decreases are charged to profit or loss.
5.9. Leased assets
Where substantially all of the risks and rewards incidental to ownership of a leased asset have been transferred to the Group (a “finance lease”), the asset is treated as if it had been purchased outright. The amount initially recognized as an asset is the lower of the fair value of the leased property and the present value of the minimum lease payments payable over the term of the lease. The corresponding lease commitment is shown as a liability. Lease payments are categorized as capital or interest. The interest element is charged to the consolidated statement of profit or loss and other comprehensive income over the period of the lease and is calculated so that it represents a constant proportion of the lease liability. The capital element reduces the balance owed to the lessor.
Where substantially all of the risks and rewards incidental to ownership are not transferred to the Group (an “operating lease”), the total rentals payable under the lease are charged to the consolidated statement of profit or loss and other comprehensive income on a straight-line basis over the lease term.
The aggregate benefit of lease incentives is recognized as a reduction of the rental expense over the lease term on a straight-line basis.
5.10. Intangible assets
| ■ | Externally acquired intangible assets |
Externally acquired intangible assets are initially recognized at cost and subsequently amortized on a straight-line basis over their useful economic lives.
F-76
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Intangible assets acquired from third parties have an estimated useful life as follows (in years):
Software: 3 years
Trademarks and patents: 5 years
Certification ISO Standards: 3 years
Useful lives and amortization methods are reviewed every year as required by IAS 38.
| ■ | Internally generated intangible assets (development costs) |
Expenditure on internally developed products is capitalized if it can be demonstrated that:
| - | It is technically feasible to develop the product for it to be sold; |
| - | Adequate resources are available to complete the development; |
| - | There is an intention to complete and sell the product; |
| - | The Group is able to sell the product; |
| - | Sale of the product will generate future economic benefits; and |
| - | Expenditure on the project can be measured reliably. |
Development expenditure not satisfying the above criteria and expenditure on the research phase of internal projects are recognized in the consolidated statement of profit or loss and other comprehensive income as incurred (Note 8.3).
Capitalized development costs are amortized using the straight-line method over the periods the Group expects to benefit from selling the products developed (Note 7.7).
Useful lives and amortization methods are reviewed every year as required by IAS 38.
The research and development process can be divided into several discrete steps or phases, which generally begin with discovery, validation and development and end with regulatory approval and commercial launch. The process for developing seed traits is relatively similar for both GM and non-GM traits. However, the two differ significantly in later phases of development. For example, obtaining regulatory approval for GM seeds is a far more comprehensive and lengthy process than for non-GM seeds. Although breeding programs and industrial biotechnology solutions may have shorter or simpler phases than those described below, the Group has used the industry consensus for seed-trait development phases to characterize its technology portfolios, which is generally divided into the following six phases:
| i) | Discovery: The first phase in the technology development process is the discovery or identification of candidate genes or genetic systems, metabolites, or microorganisms potentially capable of enhancing specified plant characteristics or enabling an agro-industrial biotech solution. In the Group’s experience, the discovery phase typically lasts 18 months, though it may range from as few as six months for microbial solutions to as many as 36 months for plant GM traits. |
| ii) | Proof of concept: Upon successful validation of the technologies in model systems (in vitro or in vivo), promising technologies graduate from discovery and are advanced to the proof of concept phase. The goal of this phase is to validate a technology within the targeted organism before moving forward with technology escalation activities or extensive field validation. In the Group’s experience, the proof of concept phase typically lasts 36 months, though it may range from as few as six months for a microbial solution to as many as four years for plant GM traits. |
F-77
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
| iii) | Early development: In this phase, field tests commenced in the proof of concept phase are expanded to evaluate various permutations of a technology in multiple geographies and growing cycles, as well as other characteristics in order to optimize the technology’s performance in the targeted organisms. The goal of the early development phase is to identify the best mode of use of a technology to define its performance concept. The early development phase typically lasts 24 months. |
| iv) | Advanced development and deregulation: In this phase, extensive field tests are used to demonstrate the effectiveness of the technology for its intended purpose. In the case of GM traits, the process of obtaining regulatory approvals from government authorities is also initiated during this phase, and tests are performed to evaluate the potential environmental impact of modified plants. For solutions involving microbial fermentation, industrial-scale runs are conducted. In Argentina and some other countries in South America, the Group is primarily responsible for undertaking this phase of product development. Similarly, the Group’s strategic partners usually lead the advanced development and deregulation activities in other markets pursuant to the applicable contractual arrangements. The advanced development and deregulation phase typically lasts about 24 months, with some projects requiring substantial regulatory data lasting as many as three years. For molecular farming projects, where grain production will occur within Argentina, the Group may follow a simplified regulatory approach, which requires less time than traditional GM regulatory approvals. |
| v) | Pre-launch: This phase involves finalizing the regulatory approval process and preparing for the launch and commercialization of the technology. The range of activities in this phase includes seed increases, pre-commercial production, and product and solution testing with selected customers. Usually, a more detailed marketing strategy and preparation of marketing materials occur during this phase. The pre-launch phase may last up to 24 months. |
| vi) | Product launch: In general, this phase, which is the last milestone of the research and development process, is carried out by the Group, the joint ventures and/or the Group’s technology licensees. When technology is commercialized through the joint ventures or technology licensees, a successful product launch will trigger royalty payments to the Group, which are generally calculated as a percentage of the net sales realized by the technology and captured upon commercialization. |
Demonstrability of technical feasibility generally occurs when the project reaches the “advanced development and deregulation” phase because at this stage success is considered to be probable.
| ■ | Intangible assets acquired in a business combination |
Intangible assets acquired in a business combination and recognized separately from goodwill are initially recognized at acquisition date fair value (which is considered as their cost). After initial recognition, those assets are measured at cost less accumulated amortization and accumulated impairment losses in the same manner as intangible assets acquired separately.
| - | Product registration: In accordance with regulations set by certain regulatory agencies such as the National Agri-Food Safety and Quality Service (SENASA), Rizobacter has been required to register products with regulatory authorities to be able to sell them both in the domestic and international markets (jointly referred to as "Product Registration"). Some of the registered products have been developed by third parties. |
| - | Brand: Rizobacter offers a wide variety of proprietary and third-party products, which are commercialized under the Rizobacter brand name. This intangible has been designated with an indefinite useful life. |
| - | Customer loyalty: Rizobacter sales to distributors of agrochemicals and to special accounts, mainly large retailers and wholesalers, whether inside or outside the Argentine territory are included. They are recognized at their fair value at the date of acquisition and are subsequently amortized on a straight-line based on the timing of projected cash flows of the clients over their estimated useful lives. |
F-78
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Intangible assets acquired in a business combination have an estimated useful life as follows (in years):
Product registration: 5 years
Customer loyalty: 26 years
The useful life of customer loyalty intangibles is estimated to be 26 years based on Rizobacter historical churn rate and is amortized on a straight-line basis over the period.
Estimates
The Group acquired certain intangible assets from Rizobacter in a business combination. To value those intangible assets, valuation techniques generally accepted in the market were applied, based mainly on the revenue approach (such as excess earnings, relief from royalty, and with or without), considering the characteristics of the assets to be valued and available information to estimate their acquisition date fair value. Application of these valuation techniques requires the use of several assumptions related to future cash flows and the discount rate.
5.11. Financial liabilities
The Group adopted IFRS 9 (version 2013) early.
The Group measures its financial liabilities at initial recognition at fair value.
The Group classifies all its financial liabilities as financial liabilities measured at amortized cost (using the effective interest rate method), except for the following liabilities that are measured at fair value: (a) Certain hybrid contracts that include embedded derivatives and for which the option of paragraph 4.3.5 of IFRS 9 was used to designate the hybrid contract as a whole at fair value through profit or loss; (b) the contingent consideration for the acquisition of Rizobacter; and (c) embedded derivatives.
In the case of hybrid contracts that were designated as a whole at fair value through profit or loss, the amount of the change in fair value of the financial liability that is attributable to changes in credit risk attaching to that liability is disclosed in other comprehensive income. The rest of the change in fair value of the liability is recognized in income.
In the case of the other financial liabilities measured at fair value, the change in fair value is charged to income.
The Group does not apply hedge accounting.
Estimates
The Group has designated certain hybrid contracts as a whole at fair value. Management of the Group periodically evaluates the appropriate valuation techniques and data used in the fair value measurement and estimation of changes in fair value derived from changes in credit risk. In estimating the fair value of those financial liabilities, the Group uses observable market inputs as far as possible.
Information about the valuation techniques and significant assumptions used is detailed in Note 15.
5.12. Employee benefits
Employee benefits are expected to be settled wholly within 12 months after the end of the reporting period and are presented as current liabilities.
The accounting policies related to incentive payments based on the stock options are detailed in Note 5.19
F-79
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
5.13. Provisions
The Group has recognized provisions for liabilities of uncertain timing or amount. The provision is measured at the best estimate of the expenditure required to settle the obligation at the end of the reporting period, discounted at a pre-tax rate reflecting current market assessments of the time value of money and risks specific to the liability.
Contingent liabilities acquired in a business combination are measured initially at fair value at the acquisition date. At period end, those contingent liabilities are measured at the higher of the amount that would be recognized in accordance with IAS 37 or the amount initially recognized.
5.14. Shareholder’s contributions
| ■ | Share capital |
Financial instruments issued by the Group are classified as equity only to the extent that they do not meet the definition of a financial liability.
The Group’s ordinary shares are classified as equity instruments, except for the puttable shares which are compound financial instruments. Puttable shares are segregated into separate components of equity instruments and puttable instruments, the latter of which is classified as a financial liability in accordance with IAS 32 (Note 7.16).
The shares classified as equity instruments are measured at nominal value.
| ■ | Share premium |
The shares classified as equity instruments are measured at nominal value.
| ■ | Treasury shares |
Consideration paid or received for the purchase or sale of treasury shares is recognized directly in equity. The cost of treasury shares held is presented as a separate reserve. Any excess of the consideration received on the sale of treasury shares over the weighted average cost of the shares sold is credited to a share premium for treasury shares.
5.15. Revenue recognition
Revenue is measured at fair value of consideration received or receivable.
| ■ | Sale of goods |
Revenue from the sale of goods is recognized when all the following conditions have been satisfied:
| (a) | the Group has transferred to the buyer the significant risks and rewards of ownership of the goods; |
| (b) | the Group retains neither continuing managerial involvement to the degree usually associated with ownership nor effective control over the goods sold; |
| (c) | the amount of revenue can be measured reliably; |
| (d) | it is probable that the economic benefits associated with the transaction will flow to the Group; and |
| (e) | the costs incurred or to be incurred in respect of the transaction can be measured reliably. |
F-80
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
In the case of sales made with where delivery is delayed at the buyer’s request but the buyer assumes ownership and accepts the invoice, revenue is recognized when the buyer assumes ownership, provided that:
| - | It must be probable that delivery will take place; |
| - | The goods must be on hand, identified and be ready for delivery to the buyer at the time the sale is recognized |
| - | The buyer must specifically acknowledge the deferred delivery instructions; and |
| - | The usual payment terms must apply. |
No revenue is recognized when there is only an intention to purchase or produce the goods in time for delivery.
| ■ | Rendering of services |
When the outcome of a transaction involving the rendering of services can be estimated reliably, revenue associated with the transaction is recognized by reference to the stage of completion of the transaction at the end of the reporting period. The outcome of a transaction can be estimated reliably when all the following conditions are satisfied:
| (a) | the amount of revenue can be measured reliably; |
| (b) | it is probable that the economic benefits associated with the transaction will flow to the entity; |
| (c) | the stage of completion of the transaction at the end of the reporting period can be measured reliably; and |
| (d) | the costs incurred for the transaction and the costs to complete the transaction can be measured reliably. |
When the outcome of the transaction involving the rendering of services cannot be estimated reliably, revenue is recognized only to the extent of the expenses recognized that are recoverable.
The stage of completion for research and development services is generally determined on the basis of internal records of execution of the performed tasks of the respective work plan.
For practical purposes, when services are performed by an indeterminate number of acts over a specified period of time, revenue is recognized on a straight-line basis over the specified period unless there is evidence that some other method better represents the stage of completion.
When a specific act is much more significant than any other acts, the recognition of revenue is postponed until the significant act is executed.
| ■ | Licenses and royalties |
Licenses and royalties are recognized when it is probable that the economic benefits associated with the transaction will flow to the Group; and the amount of revenue can be measured reliably.
Fees and royalties paid for the use of the Group’s assets are normally recognized in accordance with the substance of the agreement.
When a licensee has the right to use certain technology for a specified period of time, revenue is recognized on a straight-line basis over the life of the agreement.
An assignment of rights for a fixed fee or non-refundable guarantee under a non-cancellable contract which permits the licensee to exploit those rights freely and the licensor has no remaining obligations to perform is, in substance, a sale. In such cases, revenue is recognized at the time of sale.
F-81
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
In some cases, whether or not a license fee or royalty will be received is contingent on the occurrence of a future event. In such cases, revenue is recognized only when it is probable that the fee or royalty will be received, which is normally when the event has occurred.
5.16. Government grants
Government grants are recognized where there is reasonable assurance that the grant will be received and all attached conditions will be complied with. When the grant relates to an expense item, it is recognized as income on a systematic basis over the periods that the related costs, for which it is intended to compensate, are expensed. When the grant relates to an asset, it is recognized as income in equal amounts over the expected useful life of the related asset. Management elected this accounting policy because the Group determined it better shows the financial effect of government grants in the consolidated financial statements.
When the Group receives grants of non-monetary assets, the asset and the grant are recorded at nominal amounts and released to profit or loss over the expected useful life of the asset.
The difference between the money obtained under government loans at subsidized rates and the carrying amount of those loans is treated as a government grant, in accordance with IAS 20.
5.17. Borrowing costs
Borrowing costs, either generic or specific, attributable to the acquisition, construction or production of assets that necessarily take a substantial period of time to get ready for their intended use or sale (qualifying assets) are included in the cost of the assets until the moment that they are substantially ready for use or sale. Income earned on the temporary investments of funds generated in specific borrowings still pending use in the qualifying assets, are deducted from the total of financing costs potentially eligible for capitalization.
All other loan costs are recognized under financial costs, through profit and loss.
5.18. Income tax and minimum presumed income tax
Deferred tax assets and liabilities are recognized where the carrying amount of an asset or liability in the consolidated statement of financial position differs from its tax base, except for differences arising on:
| - | The initial recognition of goodwill; |
| - | The initial recognition of an asset or liability in a transaction which is not a business combination and at the time of the transaction affects neither accounting or taxable profit; and |
Investments in subsidiaries and jointly controlled entities where the Group is able to control the timing of the reversal of the difference and it is probable that the difference will not reverse in the foreseeable future.
Recognition of deferred tax assets is restricted to those instances where it is probable that taxable profit will be available against which the difference can be utilized.
The amount of the asset or liability is determined using tax rates that have been enacted or substantively enacted by the end of the reporting period and are expected to apply when the deferred tax liabilities / (assets) are settled / (recovered).
F-82
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Deferred tax assets and liabilities are offset when the Group has a legally enforceable right to offset current tax assets and liabilities and the deferred tax assets and liabilities relate to taxes levied by the same tax authority on either:
| - | The same taxable entity within the Group; or |
| - | Different entities within the Group which intend either to settle current tax assets and liabilities on a net basis, or to realize the assets and settle the liabilities simultaneously, in each future period in which significant amounts of deferred tax assets or liabilities are expected to be settled or recovered. |
Minimum presumed income tax applies to assets of the entities domiciled in Argentina. The tax is only applicable if the total value of the assets is above ARS 200,000 at the end of the fiscal year, and is levied at a rate of 1% on the total value of such assets. The amount of the tax paid on minimum presumed income tax is allowed as a credit toward income tax. Furthermore, to the extent that this tax cannot be credited against normal corporate income tax, it may be carried forward as a credit for the following ten years. Shares and other capital participations in the stock capital of entities subject to the minimum presumed income tax are exempted from the tax on minimum presumed income.
The Group determined on the basis of current jurisprudence that the aforementioned tax is not applicable in the transition period ended June 30, 2017, as the Group has both accounting and fiscal losses.
5.19. Share-based payments
Certain executives and directors at the Company and its subsidiaries are granted incentives in the form of options to purchase Company shares as consideration for services.
The cost of these share-based transactions is determined based on their fair value at the date upon which such incentives are granted using a valuation model that is appropriate in the circumstances.
This cost is recognized as an expense together with an increase in equity throughout the period in which the service or performance conditions are satisfied (i.e., the vesting period). The accumulated expense recorded in connection with these transactions at the end of each year until the vesting date reflects the time elapsed between the vesting period and Management’s best estimate of the number of equity instruments that will vest. The charge to income/loss for the period represents the variation in the accumulated expense recorded between the beginning and the end of the year.
Non-market related service and performance conditions are not taken into account when determining the grant date fair value of the equity instruments, but the probability that the conditions are fulfilled is assessed as part of Management’s best estimate of the number of equity instruments that will vest. Market-related performance conditions are reflected in the grant date fair value. Any other conditions related to equity-settled share-based payment transactions but without a service requirement are considered as non-vesting conditions. Non-vesting conditions are reflected in the fair value of the equity instruments and are charged to income/loss immediately unless there are service and/or performance conditions as well.
No amount is recognized for transactions that will not vest because non-market related performance conditions and/or service conditions were not satisfied. When transactions include market-related conditions or non-vesting conditions, the transactions are considered to be vested, irrespective of whether a market-related condition or the non-vesting condition is satisfied, provided that all the other performance and/or service conditions are met.
When the terms and conditions of an equity-settled share-based payment transaction are modified, the minimum expense recognized is the grant date fair value, unmodified, provided that the original terms have been complied with. An additional expense, measured at the date of modification, is recognized for any modification that increases the total fair value of the share-based payment transaction, or is otherwise beneficial to the employee.
F-83
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
When the transaction is settled by the Company or by the counterparty, any remainder of the fair value is charged to income immediately.
The dilutive effect of current options is considered in the calculation of the diluted earnings per share.
Estimates
The estimate of the fair value of equity-settled share-based payment transactions requires a determination to be made of the most adequate option pricing model to apply depending on the terms and conditions of the arrangement. This estimate also requires a determination of those factors most appropriate to the pricing model, including the expected life of the option and the expected volatility of the share price upon the basis of which hypotheses are made. The Group measures the fair value of these transactions at the grant date applying the Black-Scholes formula adjusted to consider the possible dilutive effect of the future exercise of the share options granted on their estimated fair value at grant date, as established in paragraph B41 of IFRS 2. The hypotheses used for the estimate of the fair value of these transactions are disclosed in Note 19 and will not necessarily take place in the future.
6. CRITICAL ACCOUNTING JUDGMENTS AND ESTIMATES
The Group makes certain estimates and assumptions regarding the future. Estimates and judgments are continually evaluated based on historical experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances. In the future, actual experience may differ from these estimates and assumptions. The estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year are discussed below.
6.1. Critical judgments
| - | Determination of the percentage of equity interest in Rizobacter (Note 5.5) |
| - | Determination that the Group does not have significant influence over Chemotécnica (Note 7.2) |
6.2. Critical estimates
| - | Estimate of the trade receivables impairment provision (Note 5.2) |
| - | Estimate of the inventory obsolescence allowance (Note 5.4) |
| - | Fair value of contingent consideration for business combination (Note 5.5) |
| - | Fair value of the investment in Semya in a step acquisition (Note 5.5) |
| - | Capitalization and impairment testing of development costs (Notes 5.6 and 7.7) |
| - | Impairment of goodwill (Notes 5.6 and 7.8) |
| - | Recoverability of investments in joint ventures (Note 5.7) |
| - | Fair value of the investment in Synertech (Note 5.7) |
| - | Fair value of land and buildings (Note 5.8) |
| - | Identification and fair value of identifiable intangible assets arising in a business combination (Note 5.10) |
F-84
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
| - | Fair value of financial liabilities measured at fair value through profit or loss (Notes 5.11 and 15) |
| - | Share-based payments (Notes 5.19 and 19) |
| - | Recognition and recoverability of deferred tax assets and credit for minimum presumed income tax (Note 9) |
7. INFORMATION ABOUT COMPONENTS OF CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
7.1. Cash and cash equivalents
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Cash and banks | 2,119,883 | 1,256,696 | 58,425 | ||||||
2,119,883 | 1,256,696 | 58,425 | |||||||
7.2. Other financial assets
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Current | |||||||||
Shares S&W Seed Company | 262,168 | 920,000 | 844,000 | ||||||
Government securities | — | 3,630,000 | — | ||||||
Other marketable securities | 4,275 | 4,474 | — | ||||||
Restricted short-term deposit | 4,260,517 | — | — | ||||||
4,526,960 | 4,554,474 | 844,000 | |||||||
Non-Current | |||||||||
Chemotécnica shares | 3,203,427 | 3,078,917 | — | ||||||
Other marketable securities | 89,331 | 3,989 | — | ||||||
3,292,758 | 3,082,906 | — | |||||||
On December 22, 2016, Bioceres, Lartirigoyen and SAMSA acquired 27.99%, 32% and 32%, respectively, of the capital stock of Chemotécnica, a company engaged in the synthesis and formulation of crop protection products, with a long track-record in the Argentine market.
Bioceres owns 27,99% of the capital stock and voting rights of Chemotécnica. Due to Chemotécnica’s shareholder structure and the application of the voting scheme in the Argentine Entities Law, Bioceres does not have, in principle, the ability to appoint a member of the board of directors or to exert significant influence on Chemotécnica by other means. Therefore, the Group has classified this investment as a financial asset at fair value through profit or loss.
On April 20, 2015, the Company consummated a swap of 200,000 shares in S&W Seed Company, a US-based company engaged in cultivation, processing and commercialization of alfalfa and stevia that is listed for trading on Nasdaq with a par value of USD 0.001 per share (hereinafter, the “S&W Shares”) for 1,263 shares of its own, with a par value of ARS 100, and a final value of USD 791.64 per share (hereinafter, the “Bioceres Shares” and together with the S&W Shares, the “Stock Swap”).
As of June 30, 2017, the Company holds 62,461 S&W Shares which are valued at USD 262,168 based on the quoted share price of the S&W Shares on NASDAQ.
F-85
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
As of December 31, 2016, Government securities amounted to USD 3,630,000 were valued as detailed below:
Detail | Code | Number of securities | Closing value in USD | ||||||
BONAR 24 | AY24D | 30,000 | 121.00 | ||||||
As of December 31, 2016, those securities were pledged as collateral for a line of credit from Banco Patagonia for a principal amount of USD 2,375,000. As of June 30, 2017, the loan had been repaid, the guarantee had been released and the securities had been sold.
7.3. Trade receivables
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Trade debtors | 32,174,171 | 43,446,425 | 1,230,342 | ||||||
Allowance for impairment of trade debtors | (484,549 | ) | (210,336 | ) | (256,508 | ) | |||
Shareholders and other related parties (Note 17) | 4,217,840 | 1,868,899 | 2,002,887 | ||||||
Allowance for impairment of related parties (Note 17) | (206,196 | ) | (135,938 | ) | (94,274 | ) | |||
Allowance for return of goods | (1,393,059 | ) | (658,958 | ) | — | ||||
Trade debtors - Joint ventures (Note 17) | 592,700 | 593,941 | 1,280,822 | ||||||
Deferred checks | 10,792,766 | 11,929,078 | 615,728 | ||||||
Deferred checks - Shareholders and other related parties (Note 17) | — | 59,090 | 719,977 | ||||||
Miscellaneous | — | 140,850 | — | ||||||
45,693,673 | 57,033,051 | 5,498,974 | |||||||
Variations in the allowance for uncollectible trade receivables are reported in Note 7.18.
Secured receivables are detailed in Note 20.
F-86
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
7.4. Other receivables
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Current | |||||||||
Taxes | 1,962,361 | 752,304 | 322,373 | ||||||
Insurance to be accrued | 15,217 | 7,602 | 7,703 | ||||||
Other receivables - Shareholders and other related parties (Note 17) | 30,814 | 1,386,470 | 36,206 | ||||||
Prepayments to suppliers | 1,153,188 | 1,082,185 | 689,115 | ||||||
Prepayments to suppliers - Shareholders and other related parties (Note 17) | 98,167 | 453,399 | 470,414 | ||||||
Reimbursements over exports | 151,106 | 133,301 | — | ||||||
Government grants receivable | 76,868 | 301,866 | 264,075 | ||||||
Other - Joint ventures (Note 17) | 9,654 | 3,873 | 2,800 | ||||||
Deferred offering costs (Note 5.3) | — | — | 1,948,605 | ||||||
Preferred shares - RASA Holding | — | 383,274 | — | ||||||
Prepaid expenses and other receivables | 676,762 | 255,549 | 136,864 | ||||||
Loans receivable | 5,732 | 7,834 | 190,840 | ||||||
4,179,869 | 4,767,657 | 4,068,995 | |||||||
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Non-Current | |||||||||
Taxes | 1,224,908 | 808,293 | 308,120 | ||||||
Reimbursements over exports | 472,276 | 245,047 | 21,645 | ||||||
Loans receivable - Joint ventures (Note 17) | 3,812,469 | 3,601,925 | 1,383,134 | ||||||
Other receivables - Shareholders and other related parties (Note 17) | 107,045 | 107,368 | 107,368 | ||||||
Other receivables - Miscellaneous | 14,397 | 14,100 | — | ||||||
5,631,095 | 4,776,733 | 1,820,267 | |||||||
7.5. Inventories
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Agrochemicals | 183,822 | — | 381,203 | ||||||
Finished seeds and grains | 873,866 | 1,041,777 | 1,793,220 | ||||||
Seeds and grains in production | 399,649 | 1,031,150 | 70,163 | ||||||
Microbiological resale products | 13,749,668 | 8,756,902 | — | ||||||
Microbiological products produced | 8,931,124 | 15,322,755 | — | ||||||
Goods in transit | 482,185 | 1,768,160 | 736,895 | ||||||
Safflower seeds | — | 74,726 | 45,953 | ||||||
Supplies | 7,810,543 | 5,735,215 | 200,596 | ||||||
Miscellaneous | — | 166,379 | — | ||||||
Allowance for obsolescence | (707,105 | ) | (739,499 | ) | (167,989 | ) | |||
31,723,752 | 33,157,565 | 3,060,041 | |||||||
The roll-forward of allowance for obsolescence is in Note 7.18.
F-87
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
7.6. Property, plant and equipment
Property, plant and equipment as of June 30, 2017 and December 31, 2016 and 2015 included the following:
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Gross carrying amount | 56,128,677 | 55,344,034 | 7,278,525 | ||||||
Accumulated depreciation | (5,018,060 | ) | (3,605,598 | ) | (2,510,715 | ) | |||
Net carrying amount | 51,110,617 | 51,738,436 | 4,767,810 | ||||||
1. Net carrying amount for each class of assets is as follows:
Class | Net Carrying amount 06/30/2017 | Net Carrying amount 12/31/2016 | Net Carrying amount 12/31/2015 | ||||||
Research instruments | 103,938 | 131,213 | 80,160 | ||||||
Office equipment | 348,480 | 346,889 | 96,659 | ||||||
Vehicles | 1,937,219 | 2,095,312 | 41,718 | ||||||
Equipment and computer software | 344,642 | 410,222 | 34,611 | ||||||
Fixtures and fittings | 4,887,918 | 5,190,597 | 402,956 | ||||||
Machinery and equipments | 9,252,633 | 10,046,845 | 928,089 | ||||||
Land and buildings | 30,103,117 | 29,584,854 | — | ||||||
Building on third lands | 3,262,201 | 3,511,818 | 3,183,617 | ||||||
Buildings in progress | 870,469 | 420,686 | — | ||||||
Total | 51,110,617 | 51,738,436 | 4,767,810 | ||||||
2. Gross carrying amount as of June 30, 2017 is as follows:
Gross carrying amount | |||||||||||||||||||||
Class | As of the beginning of period | Additions | Transfers | Disposals | Foreign Currency Translation | Revaluation | As of the end of period | ||||||||||||||
Research instruments | 565,623 | 1,793 | — | (7,131 | ) | (24,972 | ) | — | 535,313 | ||||||||||||
Office equipment | 494,700 | 34,001 | — | (7,473 | ) | (21,726 | ) | — | 499,502 | ||||||||||||
Vehicles | 2,299,762 | 367,585 | — | (211,504 | ) | (94,599 | ) | — | 2,361,244 | ||||||||||||
Equipment and computer software | 722,934 | 38,567 | — | (148,148 | ) | (26,823 | ) | — | 586,530 | ||||||||||||
Fixtures and fittings | 5,811,335 | — | 127,475 | (4,420 | ) | (265,757 | ) | — | 5,668,633 | ||||||||||||
Machinery and equipments | 10,801,961 | 32,163 | 73,717 | (12,896 | ) | (487,220 | ) | — | 10,407,725 | ||||||||||||
Land and buildings | 29,759,833 | 23,896 | (428,844 | ) | (208,102 | ) | (1,459,156 | ) | 3,243,599 | 30,931,226 | |||||||||||
Building on third lands | 4,467,200 | 677 | — | (449 | ) | (199,393 | ) | — | 4,268,035 | ||||||||||||
Buildings in progress | 420,686 | 261,390 | 227,652 | — | (39,259 | ) | — | 870,469 | |||||||||||||
Total 06/30/2017 | 55,344,034 | 760,072 | — | (600,123 | ) | (2,618,905 | ) | 3,243,599 | 56,128,677 | ||||||||||||
F-88
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
3. Accumulated depreciation as of June 30, 2017 is as follows:
Depreciation | ||||||||||||||||||
Class | Accumulated as of beginning of period | Disposals | of the period | Foreign Currency Translation | Revaluation | Accumulated as of end of period | ||||||||||||
Research instruments | 434,410 | (7,131 | ) | 24,365 | (20,269 | ) | — | 431,375 | ||||||||||
Office equipment | 147,811 | (7,473 | ) | 18,145 | (7,461 | ) | — | 151,022 | ||||||||||
Vehicles | 204,450 | (114,914 | ) | 345,389 | (10,900 | ) | — | 424,025 | ||||||||||
Equipment and computer software | 312,712 | (146,639 | ) | 86,860 | (11,045 | ) | — | 241,888 | ||||||||||
Fixtures and fittings | 620,738 | (1,360 | ) | 197,606 | (36,269 | ) | — | 780,715 | ||||||||||
Machinery and equipments | 755,116 | (12,896 | ) | 470,131 | (57,259 | ) | — | 1,155,092 | ||||||||||
Land and buildings | 174,979 | — | 283,658 | (42,978 | ) | 412,450 | 828,109 | |||||||||||
Building on third lands | 955,382 | (449 | ) | 98,555 | (47,654 | ) | — | 1,005,834 | ||||||||||
Buildings in progress | — | — | — | — | — | — | ||||||||||||
Total 06/30/2017 | 3,605,598 | (290,862 | ) | 1,524,709 | (233,835 | ) | 412,450 | 5,018,060 | ||||||||||
4. Gross carrying amount as of December 31, 2016 is as follows:
Gross carrying amount | ||||||||||||||||||
Class | As of the beginning of year | Additions | Additions from PPA | Disposals | Foreign Currency Translation | As of the end of year | ||||||||||||
Research instruments | 455,580 | 110,043 | — | — | — | 565,623 | ||||||||||||
Office equipment | 199,073 | 70,967 | 234,221 | — | (9,561 | ) | 494,700 | |||||||||||
Vehicles | 210,519 | 338,774 | 1,878,400 | (49,179 | ) | (78,752 | ) | 2,299,762 | ||||||||||
Equipment and computer software | 251,219 | 39,425 | 450,785 | — | (18,495 | ) | 722,934 | |||||||||||
Fixtures and fittings | 863,809 | 297,696 | 4,848,110 | — | (198,280 | ) | 5,811,335 | |||||||||||
Machinery and equipments | 1,332,040 | 352,023 | 9,533,037 | (29,280 | ) | (385,859 | ) | 10,801,961 | ||||||||||
Land and buildings | — | 210,038 | 30,792,604 | — | (1,242,809 | ) | 29,759,833 | |||||||||||
Building on third lands | 3,966,285 | 500,915 | — | — | — | 4,467,200 | ||||||||||||
Buildings in progress | — | — | 485,690 | (54,852 | ) | (10,152 | ) | 420,686 | ||||||||||
Total 12/31/2016 | 7,278,525 | 1,919,881 | 48,222,847 | (133,311 | ) | (1,943,908 | ) | 55,344,034 | ||||||||||
F-89
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
5. Accumulated depreciation as of December 31, 2016 is as follows:
Depreciation | |||||||||||||||
Class | Accumulated as of beginning of year | Disposals | of the year | Foreign Currency Translation | Accumulated as of end of year | ||||||||||
Research instruments | 375,420 | — | 58,990 | — | 434,410 | ||||||||||
Office equipment | 102,414 | — | 39,940 | 5,457 | 147,811 | ||||||||||
Vehicles | 168,801 | (49,179 | ) | 86,191 | (1,363 | ) | 204,450 | ||||||||
Equipment and computer software | 216,609 | — | 89,802 | 6,301 | 312,712 | ||||||||||
Fixtures and fittings | 460,853 | — | 246,298 | (86,413 | ) | 620,738 | |||||||||
Machinery and equipments | 403,950 | (7,076 | ) | 238,659 | 119,583 | 755,116 | |||||||||
Land and buildings | — | — | 142,139 | 32,840 | 174,979 | ||||||||||
Building on third lands | 782,668 | — | 172,714 | — | 955,382 | ||||||||||
Buildings in progress | — | — | — | — | — | ||||||||||
Total 12/31/2016 | 2,510,715 | (56,255 | ) | 1,074,733 | 76,405 | 3,605,598 | |||||||||
6. Gross carrying amount as of December 31, 2015 is as follows:
Gross carrying amount | ||||||||||||
Class | As of the beginning of year | Additions | Disposals | As of the end of year | ||||||||
Research instruments | 438,177 | 17,403 | — | 455,580 | ||||||||
Office equipment | 192,010 | 7,063 | — | 199,073 | ||||||||
Vehicles | 210,519 | — | — | 210,519 | ||||||||
Equipment and computer software | 222,489 | 28,731 | — | 251,220 | ||||||||
Fixtures and fittings | 829,928 | 33,881 | — | 863,809 | ||||||||
Machinery and equipments | 1,120,071 | 211,968 | — | 1,332,039 | ||||||||
Building on third lands | 3,624,588 | 341,697 | — | 3,966,285 | ||||||||
Total 12/31/2015 | 6,637,782 | 640,743 | — | 7,278,525 | ||||||||
7. Accumulated depreciation as of December 31, 2015 is as follows:
Depreciation | ||||||||||||
Class | Accumulated as of beginning of year | Disposals | of the year | Accumulated as of end of year | ||||||||
Research instruments | 328,985 | — | 46,435 | 375,420 | ||||||||
Office equipment | 83,424 | — | 18,990 | 102,414 | ||||||||
Vehicles | 138,750 | — | 30,051 | 168,801 | ||||||||
Equipment and computer software | 199,058 | — | 17,551 | 216,609 | ||||||||
Fixtures and fittings | 377,910 | — | 82,943 | 460,853 | ||||||||
Machinery and equipments | 280,436 | — | 123,514 | 403,950 | ||||||||
Building on third lands | 645,708 | — | 136,960 | 782,668 | ||||||||
Total 12/31/2015 | 2,054,271 | — | 456,444 | 2,510,715 | ||||||||
F-90
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
On November 30, 2004, a joint arrangement was entered into by INDEAR and CONICET for the purpose of coordinating the cooperation of scientific and technologic research activities of mutual interest, particularly with respect to agricultural biotechnology.
In order to best achieve the objectives of the arrangement, CONICET ceded the use of its land in ex-CERIDER (currently CCT- Rosario) to INDEAR for free for 30 years (expiring in 2034), and INDEAR committed to the construction of a building on that land to carry out activities of research and development. INDEAR shall surrender the building and its improvements to CONICET when the joint arrangement is over, while the equipment and furniture will remain the property of INDEAR.
The joint arrangement has a term of 30 years since the signing date, with possibility of renegotiating an extension of the joint arrangement in case CONICET decides against the property for its own and exclusive use. INDEAR has a right of first offer over third parties in relation to the future use of the property. The building is included in property, plant and equipment as “buildings on third parties’ property.” It shall be depreciated over the 30-year term of the contract with CONICET.
See further explanation of this joint arrangement in Note 13.2.
The depreciation charge is included in Notes 8.3 and 8.4.
The Group has no commitments to purchase property, plant and equipment items.
A detail of restricted assets is provided in Note 20.
Revaluation of property, plant and equipment
At a minimum, the Group updates their assessment of the fair value of its land and buildings at the end of each reporting year (after the revaluation policy was adopted), taking into account the most recent independent valuations and market data. Valuations were performed at June 30, 2017. Management determined the property, plant and equipment’s value within a range of reasonable fair value estimates.
All resulting fair value estimates for properties are included in level 3. An explanation of each level is provided in Note 15.2.
An increase of the rate per square meter unobservable input results in an increase in fair value measurement. On the other hand, an increase in the level of wear and tear assumed results in a decrease in the fair value figures.
The following are the carrying amounts that would have been recognized had the assets been carried under the cost model.
Class of property | Revalued amount | Value at cost | ||||
At June 30, 2017 | ||||||
Land and buildings | 30,103,117 | 28,229,649 | ||||
F-91
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
7.7. Intangible assets
Intangible assets as of June 30, 2017, December 31, 2016 and 2015 included the following:
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Gross carrying amount | 44,054,864 | 44,667,927 | 1,935,978 | ||||||
Accumulated amortization | (1,873,094 | ) | (584,654 | ) | (168,810 | ) | |||
Net carrying amount | 42,181,770 | 44,083,273 | 1,767,168 | ||||||
Seed and integrated products
The Group’s seed and integrated product activities concentrate primarily on the development and commercialization of seeds and technologies and products that increase yield per hectare, with a focus on providing seed and integrated crop protection and crop nutrition technologies designed to control weeds, insects or diseases, enhance quality traits of the seeds produced and improve nutritional value and other benefits. The Group has sought to develop integrated products that combine three distinct and complementary components, seed traits, germplasms and seed treatments—in order to deliver a superior agronomic experience to customers.
The Group’s technologies which are capitalized based on an advanced stage of development (phase 4 or higher), are set forth in the table below:
Crop | Technologies | R&D Phase | Entity | |||
Germplasm | Protection | Yield | Quality | |||
Soybean | MG III-VIII(1) | GT(2) | HB4 | — | Advanced Develop. | Bioceres INC |
Wheat | Spring / Winter | GluT(2) | HB4 | — | Advanced Develop. | Trigall Genetics(4) (Note 13.1) |
Alfalfa | Non-dorm(1) | Genuity(2) | — | HarvXtra(3) | Advanced Develop. | Bioceres |
Amaranth | A.cruentus(1) | ALS(2) | — | — | Pre-launch | INDEAR |
Notes:
| (1) | Soybean germplasms are categorized by maturity groups (MG) from III to VIII. Non-dormant germplasms are alfalfa elite breeding materials without winter dormancy. A. cruentus germplasms are amaranth varieties of the A. cruentus species. |
| (2) | GT means glyphosate tolerance. GluT means glufosinate tolerance. Genuity is the glyphosate tolerance technology developed by Monsanto and Forage Genetics International for alfalfa. ALS means ALS-inhibitor herbicide tolerance. |
| (3) | HarvXtra is the reduced lignin technology in alfalfa developed by Monsanto and Forage Genetics International, the de-regulation of which is under development by Bioceres in Argentina. LXR is a delayed-senescence technology used in multiple crops, shown to improve quality and forage productivity in alfalfa. |
| (4) | Included in Trigall’s financial statements. Reflected in the consolidated financial statements through the equity method investment. |
The soybean HB4 technology was approved by the Argentine Ministry of Agriculture, Livestock and Fishing on October 1, 2015, under Resolution N° 397/15.
The U.S. FDA completed its full review of the safety evaluation for HB4 soybeans, clearing it for use in human food and animal food (pending USDA approval) on August 10, 2017.
F-92
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The following have been recorded as intangibles, as a result of Semya business combination in stages and the fair value measurement of its assets and liabilities:
| - | Soybean EcoSeed Pack |
| - | The purpose of this project is to develop high value added biological products for the treatment of soybean seeds. Development of these products achieves biotechnological, germ-plasm and bio-inoculants synergies. |
| - | Wheat EcoSeed Pack The purpose of this project is to develop high value added biological products for the treatment of wheat seeds. Development of these products achieves biotechnological, germ-plasm and bio-inoculants synergies. |
| - | Bio-fungicides The purpose of this project is to produce high value added biological products for the treatment of seeds, focusing on the capacity to control diseases associated with seeds from wheat and soybean crops. |
We are leveraging our germplasm assets, particularly in soybean and wheat, in the development of our integrated EcoSeedPack products, our customized seed treatments that complement our traits and germplasms.
Crop nutrition
The Group’s crop nutrition activities include the development of and investment in microbiological products that have been incorporated as part of the integration of Rizobacter into the Group, including the following microbiological assets incorporated as intangible assets measured at fair value:
| - | TOP Technologies The TOP Osmo Protection Technology promotes high metabolic and physiological performance of bacteria and ensures bacterial survival and concentration in seeds and packages, reducing the impact of fungicides and insecticides on bacteria and substantially improving inoculants performance and their incidence in crop yields. |
| - | Signum Technologies Signum bio-inductor generates molecular signals which early activate bacterial and plant metabolic processes, thus maximizing the development of leguminous plants. Furthermore, it stimulates the interrelation with different soil beneficial microorganisms that provide additional advantages to inoculation, activates mechanisms of resistance to abiotic stress factors (low temperatures, droughts and soil acidity), and induces defensive responses in the interaction with harmful microorganisms. |
| - | LLI Technologies This technology marks a turning point in inoculation. The time of disposal to maintain living bacteria on seeds, a prerequisite for an effective nodulation. Ready-to-use (RTU) seed allows to reduce costs, simplify the sowing operation, minimize the risks normally associated with on farm treatments, and achieve the precise positioning of bacteria through the inoculant. This treatment provides for a higher number of healthier plants with outstanding root development to reach the soybean crop’s full yield potential. |
Other intangible assets
Other intangible assets identified in the business combination with Rizobacter:
| - | Product registration: In accordance with regulations set by certain regulatory agencies such as the National Agri-Food Safety and Quality Service (SENASA), Rizobacter has been required to register products with regulatory authorities to be able to sell them both in the domestic and international markets (jointly referred to as “Product Registration”). Some of the registered products have been developed by third parties. |
F-93
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
| - | Brand: Rizobacter offers a wide variety of proprietary and third-party products, which are commercialized under the Rizobacter brand name. This intangible has been designated with an indefinite useful life. |
| - | Customer loyalty: Rizobacter’s sales to distributors of agrochemicals and to special accounts, mainly large retailers and wholesalers, whether inside or outside the Argentine territory are included. They are recognized at their fair value at the date of acquisition and are subsequently amortized on a straight-line based on the timing of projected cash flows of the clients over their estimated useful lives. |
The Group’s technologies included in the molecular farming platform, which are capitalized based on an advanced stage of development (phase 4 or higher), are set forth in the table below:
Product or solution | R&D phase | Entity | ||||
SPC molecular farming platform | Launch | AGBM | ||||
SPC is a technology for the production of bovine chymosin, an enzyme used in the manufacturing of cheese transferred from Indear to AGBM, which is a joint arrangement of Bioceres and Porta Hnos S.A.
On September 6, 2016, AGBM industrial plant was opened for the production and commercialization of chymosin.
The industrial plant is situated in the city of Córdoba and has a production capacity of 2 million liters of chymosin per year, representing approximately 15% of the global market.
Those research and development expenses that were not capitalized - because they had not fulfilled the requirements of IAS 38 - are described in Note 8.3.
The amortization charge has been included in Notes 8.3 and 8.4.
1. Net carrying amount of each class of intangible assets is as follows:
Class | Net Carrying amount 06/30/2017 | Net Carrying amount 12/31/2016 | Net Carrying amount 12/31/2015 | ||||||
Seed and integrated products | |||||||||
Amaranth ALS | 132,848 | 138,558 | 131,858 | ||||||
Soybean HB4 | 609,091 | 471,167 | 471,167 | ||||||
Alfalfa Genuity Harv Xstra | 140,486 | 146,099 | 122,530 | ||||||
Integrated products Semya | 2,273,970 | 2,380,203 | — | ||||||
Crop nutrition | |||||||||
Microbiology products | 3,491,268 | 3,625,827 | — | ||||||
Other intangible assets | |||||||||
SPC Molecular Farming Platform | — | — | 962,481 | ||||||
Certification ISO Standards | — | — | — | ||||||
Trademarks and patents | 10,402,765 | 11,265,235 | 74,590 | ||||||
Software | 1,470,507 | 800,530 | 4,542 | ||||||
Customer loyalty | 23,660,835 | 25,255,654 | — | ||||||
Total 06/30/2017 | 42,181,770 | 44,083,273 | 1,767,168 | ||||||
F-94
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
2. Gross carrying amount as of June 30, 3017 is as follows:
Gross carrying amount | |||||||||||||||
Class | As of the beginning of period | Additions | Disposals | Foreign Currency Translation | As of the end of period | ||||||||||
Seed and integrated products | |||||||||||||||
Amaranth ALS | 138,558 | 500 | — | (6,210 | ) | 132,848 | |||||||||
Soybean HB4 | 471,167 | 167,514 | — | (29,590 | ) | 609,091 | |||||||||
Alfalfa Genuity Harv Xstra | 146,099 | 956 | — | (6,569 | ) | 140,486 | |||||||||
Integrated products Semya | 2,380,203 | — | — | (106,233 | ) | 2,273,970 | |||||||||
Crop nutrition | |||||||||||||||
Microbiology products | 3,755,094 | 205,232 | — | (178,087 | ) | 3,782,239 | |||||||||
Other intangible assets | |||||||||||||||
Certification ISO Standards | 28,366 | — | (28,366 | ) | — | — | |||||||||
Trademarks and patents | 11,421,239 | — | (4,769 | ) | (509,510 | ) | 10,906,960 | ||||||||
Software | 875,767 | 1,114,041 | — | (96,021 | ) | 1,893,787 | |||||||||
Customer loyalty | 25,451,434 | — | — | (1,135,951 | ) | 24,315,483 | |||||||||
Total 06/30/2017 | 44,667,927 | 1,488,243 | (33,135 | ) | (2,068,171 | ) | 44,054,864 | ||||||||
3. Accumulated amortization as of June 30, 2017 is as follows:
Amortization | |||||||||||||||
Class | Accumulated as of beginning of period | Disposals | of the period | Foreign Currency Translation | Accumulated as of the end of period | ||||||||||
Seed and integrated products | |||||||||||||||
Amaranth ALS | — | — | — | — | — | ||||||||||
Soybean HB4 | — | — | — | — | — | ||||||||||
Alfalfa Genuity Harv Xstra | — | — | — | — | — | ||||||||||
Integrated products Semya | — | — | — | — | |||||||||||
Crop nutrition | |||||||||||||||
Microbiology products | 129,267 | — | 176,494 | (14,790 | ) | 290,971 | |||||||||
Other intangible assets | |||||||||||||||
Certification ISO Standards | 28,366 | (28,366 | ) | — | — | — | |||||||||
Trademarks and patents | 156,004 | (4,769 | ) | 379,051 | (26,091 | ) | 504,195 | ||||||||
Software | 75,237 | — | 370,326 | (22,283 | ) | 423,280 | |||||||||
Customer loyalty | 195,780 | — | 492,790 | (33,922 | ) | 654,648 | |||||||||
Total 06/30/2017 | 584,654 | (33,135 | ) | 1,418,661 | (97,086 | ) | 1,873,094 | ||||||||
F-95
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
4. Gross carrying amount as of December 31, 3016 is as follows:
Gross carrying amount | ||||||||||||||||||
Class | As of the beginning of year | Additions | Additions from PPA | Disposals | Foreign Currency Translation | As of the end of year | ||||||||||||
Seed and integrated products | ||||||||||||||||||
Amaranth ALS | 131,858 | 6,700 | — | — | — | 138,558 | ||||||||||||
Soybean HB4 | 471,167 | — | — | — | — | 471,167 | ||||||||||||
Alfalfa Genuity Harv Xstra | 122,530 | 23,569 | — | — | — | 146,099 | ||||||||||||
Integrated products Semya | — | — | 2,380,203 | — | — | 2,380,203 | ||||||||||||
Crop nutrition | ||||||||||||||||||
Microbiology products | — | 175,527 | 3,733,981 | — | (154,414 | ) | 3,755,094 | |||||||||||
Other intangible assets | ||||||||||||||||||
SPC Molecular Farming Platform | 1,038,012 | 185,144 | — | (1,223,156 | ) | — | — | |||||||||||
Certification ISO Standards | 28,366 | — | — | — | — | 28,366 | ||||||||||||
Trademarks and patents | 104,864 | — | 11,896,495 | (99,454 | ) | (480,666 | ) | 11,421,239 | ||||||||||
Software | 39,181 | 420,254 | 442,607 | — | (26,275 | ) | 875,767 | |||||||||||
Customer loyalty | — | — | 26,523,073 | — | (1,071,639 | ) | 25,451,434 | |||||||||||
Total 12/31/2016 | 1,935,978 | 811,194 | 44,976,359 | (1,322,610 | ) | (1,732,994 | ) | 44,667,927 | ||||||||||
5. Accumulated amortization as of December 31, 2016 is as follows:
Amortization | |||||||||||||||
Class | Accumulated as of beginning of year | Disposals | of the period | Foreign Currency Translation | Accumulated as of the end of year | ||||||||||
Seed and integrated products | |||||||||||||||
Amaranth ALS | — | — | — | — | — | ||||||||||
Soybean HB4 | — | — | — | — | — | ||||||||||
Alfalfa Genuity Harv Xstra | — | — | — | — | — | ||||||||||
Integrated products Semya | — | — | — | — | — | ||||||||||
Crop nutrition | |||||||||||||||
Microbiology products | — | — | 21,316 | 107,951 | 129,267 | ||||||||||
Other intangible assets | |||||||||||||||
SPC Molecular Farming Platform | 75,531 | (98,610 | ) | 23,079 | — | — | |||||||||
Certification ISO Standards | 28,366 | — | — | — | 28,366 | ||||||||||
Trademarks and patents | 30,274 | (40,018 | ) | 168,853 | (3,105 | ) | 156,004 | ||||||||
Software | 34,639 | — | 50,001 | (9,403 | ) | 75,237 | |||||||||
Customer loyalty | — | 199,817 | (4,037 | ) | 195,780 | ||||||||||
Total 12/31/2016 | 168,810 | (138,628 | ) | 463,066 | 91,406 | 584,654 | |||||||||
F-96
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
6. Gross carrying amount as of December 31, 2015 is as follows
Gross carrying amount | ||||||||||||
Class | As of the beginning of year | Additions | Disposals | As of the end of year | ||||||||
Seed and integrated products | ||||||||||||
Amaranth ALS | 119,859 | 11,999 | — | 131,858 | ||||||||
Soybean HB4 | 471,167 | — | — | 471,167 | ||||||||
Alfalfa Genuity Harv Xstra | 83,990 | 38,540 | — | 122,530 | ||||||||
Other intangible assets | ||||||||||||
SPC Molecular Farming Platform | 894,275 | 143,737 | — | 1,038,012 | ||||||||
Certification ISO Standards | 28,366 | — | — | 28,366 | ||||||||
Trademarks and patents | 104,864 | — | — | 104,864 | ||||||||
Software | 32,086 | 7,095 | — | 39,181 | ||||||||
Total 12/31/2015 | 1,734,607 | 201,371 | — | 1,935,978 | ||||||||
7. Accumulated amortization as of December 31, 2015 is as follows:
Amortization | ||||||||||||
Class | Accumulated as of beginning of year | Disposals | of the period | Accumulated as of the end of year | ||||||||
Seed and integrated products | ||||||||||||
Amaranth ALS | — | — | — | — | ||||||||
Soybean HB4 | — | — | — | — | ||||||||
Alfalfa Genuity Harv Xstra | — | — | — | — | ||||||||
Other intangible assets | ||||||||||||
SPC Molecular Farming Platform | 50,354 | — | 25,177 | 75,531 | ||||||||
Certification ISO Standards | 28,366 | — | — | 28,366 | ||||||||
Trademarks and patents | 13,698 | — | 16,576 | 30,274 | ||||||||
Software | 31,159 | — | 3,480 | 34,639 | ||||||||
Total 12/31/2015 | 123,577 | — | 45,233 | 168,810 | ||||||||
There are no intangible assets whose use has been restricted or which have been delivered as a guarantee. The Group has not assumed any commitments to acquire new intangibles.
Estimates
There is an inherent material uncertainty related to Management’s estimation of the ability of the Group to recover the carrying amounts of internally generated intangible assets related to biotechnology projects because it is dependent upon Group’s ability to raise sufficient funds to complete the projects development, the future outcome of the regulatory process, and the timing and amount of the future cash flows generated by the projects, among other future events.
Management’s estimations about the demonstrability of the recognition criteria for these assets and the subsequent recoverability represent the best estimate that can be made based on all the available evidence, existing facts and circumstances and using reasonable and supportable assumptions in cash flow projections. Therefore, the consolidated
F-97
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
financial statements do not include any adjustments that would result if the Group were unable to recover the carrying amount of the above-mentioned assets through the generation of enough future economic benefits.
7.8. Goodwill
The Group is required to test whether goodwill has suffered any impairment on an annual basis. The recoverable amount is determined based on value in use calculations. The use of this method requires the estimation of future cash flows and the determination of a discount rate in order to calculate the present value of the cash flows.
After the business combinations that occurred in 2016, goodwill has been generated for Rizobacter and Semya CGUs.
| - | Rizobacter CGU: |
This CGU is composed of all revenues collected through Rizobacter from the production and sale of proprietary and third-party products, both in the domestic and international markets, except for Synertech and Semya operations. Additionally, Rizobacter generates revenue from the formulation, fragmentation and resale of third party products.
Among the main groups of products are i) microbiological products (bio-inductors/inoculants, biological fertilizers and bio-controllers); ii) crop and seed protection (treatments, adjuvants, baits, stored grains and seed treatment); and iii) crop nutrition (fertilizers). Packs are generally a combination of a microbiological product (bio-inductors/inoculants) with a crop and seed protection product (treatments).
The Rizobacter CGU goodwill was determined to be ARS 415,815,187 (USD 25,079,324 as of June 30, 2017) has been determined of.
| - | Semya CGU: |
This CGU is basically composed of expected revenue from the sale of R&D intensive products of the company Semya S.A.
These technologies seek to increase crop productivity, reduce the environmental impact and improve efficiency in the use of resources. The main products under development are Soybean EcoSeed Pack (HB4 Soybean), Wheat EcoSeed Pack (HB4 Wheat) and bio fungicides for soybean and wheat, which are expected to be commercialized as from 2018.
A value of addition of Semya CGU goodwill has been determined in the amount of ARS 115,741,863 (USD 7,274,740 as of June 30, 2017).
The rest of the variations in goodwill occurred during the six-month transition period ended June 30, 2017 correspond to translation differences. There have been no goodwill impairment indicators.
Carrying amount of goodwill as of June 30, 2017, December 31, 2016 and 2015 is as follows:
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Rizobacter | 25,079,324 | 26,250,959 | — | ||||||
Semya | 7,274,740 | 7,614,596 | — | ||||||
Indear | 512,139 | 536,065 | 536,065 | ||||||
Total | 32,866,203 | 34,401,620 | 536,065 | ||||||
F-98
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The Rizobacter brand intangible with an indefinite useful life has been allocated to the Rizobacter CGU. For the rest of the CGUs there are no allocated intangible assets with an indefinite useful life.
Management has made the estimates considering the cash flow projections projected by the management of Rizobacter and third-party valuation reports on the assets, intangible assets and liabilities assumed.
The key assumptions utilized are the following:
Key assumption | Management’s approach |
Discount rate | The discount rate used ranges between 15% and 17%, depending on the CGU. The weighted average cost of capital (“WACC”) rate has been estimated based on the market capital structure. For the cost of debt, the indebtedness cost of each CGU was taken. For the cost of equity, the discount rate is estimated based on the Capital Asset Pricing Model (CAPM). The value assigned is consistent with external sources of information. |
Budgeted market share of joint ventures and other customers | The projected revenue from the products and services of each CGU has been estimated by Rizobacter’s management based on market penetration data for comparable products and technologies and on future expectations of foreseen economic and market conditions. The value assigned is consistent with external sources of information. |
Budgeted product prices | The prices estimated in the revenue projections are based on current and projected market prices for the products and services of each CGU The value assigned is consistent with external sources of information. |
Growth rate used to extrapolate future cash flow projections to terminal period | The growth rate used to extrapolate the future cash flow projections to terminal period is 2%. The value assigned is consistent with external sources of information. |
Management believes that any reasonably possible change in any of these key assumptions would not cause the aggregate carrying amount of the CGU to exceed its recoverable amount.
7.9. Trade and other payables
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Trade creditors | 20,656,681 | 33,684,076 | 4,841,647 | ||||||
Shareholders and other related parties (Note 17) | 2,113,149 | 1,570,497 | 2,146,162 | ||||||
Trade and other payables - Miscellaneous | 134,273 | 127,728 | 32,096 | ||||||
Other - Joint ventures | 427,092 | 6,705 | 6,185 | ||||||
Tax | 417,517 | 1,661,208 | 65,652 | ||||||
23,748,712 | 37,050,214 | 7,091,742 | |||||||
F-99
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
7.10. Borrowings
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Current | |||||||||
Bank overdraft | 357,643 | 1,364,359 | 852,435 | ||||||
Foreign trade financing | — | 11,787,834 | — | ||||||
Bank borrowings | 19,219,577 | 21,263,032 | 808,778 | ||||||
Corporate bonds | 4,644,621 | 4,627,296 | — | ||||||
Convertible borrowings | 16,598,470 | 16,215,931 | — | ||||||
Discount checks | 9,733,481 | 10,131,883 | 493,677 | ||||||
Loans with key management personnel | — | — | 53,833 | ||||||
Finance lease | 466,689 | 457,613 | 6,559 | ||||||
51,020,481 | 65,847,948 | 2,215,282 | |||||||
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Non-Current | |||||||||
Corporate bonds | 3,889,874 | 6,273,911 | — | ||||||
Bank borrowings | 36,538,128 | 4,799,797 | 811,497 | ||||||
Finance lease | 678,993 | 824,354 | 4,005 | ||||||
41,106,995 | 11,898,062 | 815,501 | |||||||
Assets granted as collateral for repayment of the secured loans are detailed in Notes 7.2 and 7.6.
Further information about finance leases is in Note 16.
Convertible loan
On April 5, 2016, the Company entered into a USD 15 million loan agreement with two investors (Monsanto Argentina S.R.L. and BAF Latam Trade Finance Fund B.V. Monsanto Argentina S.R.L. invested USD 7 million through a contribution in Argentine pesos equivalent to ARS 102.76 million, while BAF Latam Trade Finance Fund B.V. invested USD 8 million through a contribution in US dollars. The loan grants mandatory participation rights to each investor to subscribe to a total nominal value of USD 17.5 million of ordinary shares of the Company in a qualified public offering (i.e., an offering that is consummated for at least USD 30 million net of the nominal value of the convertible loan and pursuant to which at least 30% of the shares are placed among new shareholders), and optional participation rights in the case of a non-qualified public offering, for a total nominal value of USD 17.55 million.
The convertible loan will expire upon the earlier of its conversion into shares upon the exercise of the participation rights or five years. If the conversion into shares does not occur before the end of the second anniversary of the loan agreement, the Group will pay interest at an annual rate of 8% as from the third anniversary, and the loan nominal value will be increased by 10%. The conversion of the loan into shares by means of the participation rights will take place at the subscription price.
F-100
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Corporate Bonds (CB) - Rizobacter Debt
a) Issuance of corporate bonds (Principal Market)
With the goal of consolidating its financial situation and obtaining funds for investments and working capital, on March 31, 2015 the Shareholders’ Meeting of Rizobacter approved the establishment of a global program of corporate bonds (“CB”) in the Argentine principal market (“the Program”) for the issue of one or more series of simple CB through public offering, for their future listing on stock exchanges and other markets, up to a revolving outstanding amount of USD 40,000,000 or the equivalent in other currencies, or a lower amount to be determined by the Board of Directors, with a maximum term of five years.
With the goal of consolidating its financial situation and obtaining funds for investments and working capital, on March 31, 2015 the shareholders of Rizobacter approved the establishment of a global corporate bonds (“CB”) program in the Argentine principal market for the issuance of one or more series of simple CB through public offering, for their future listing on exchanges and other markets, up to a revolving outstanding amount of USD 40,000,000 or the equivalent in other currencies, or a lower amount to be determined by the Board of Directors with a maximum term of five years (“the Program”).
Series I: On August 13, 2015, CNV approved the Program and the issue of Series I of the Negotiable Obligations for USD 10 million (increasable to a maximum of USD 17 million or the equivalent in Argentine pesos), through Resolution N° 17737, according to the main terms and conditions summarized in the Prospectus Supplement dated August 13, 2015, which Prospectus Supplement was published in the Daily Gazette of the Buenos Aires Stock Exchange and the Rosario Stock Exchange on the same date.
Series I (USD)
Amount of the Issue: USD 7,786,327
Date of Issue and Subscription: August 31, 2015
Applicable rate: 6% annual nominal rate
Maturity: August 31, 2018
Initial Exchange rate: ARS 9.28 / USD
Amortization: The principal will be amortized in five semiannual installments as from the twelfth month following the Date of Issue. The first four installments will be identical, each representing 17.50% of the principal amount issued, and the last installment will be equal to 30% of the principal amount issued.
Date of Payment of Services: Interest will be paid on a quarterly basis at a fixed annual nominal rate of 6%. If any service payment date is not a business day, payment will be made on the immediately subsequent business day without any interest accruing on this payment in respect of the days that elapse from the date of payment to the actual payment date.
Series I (Class I – Pesos)
Amount of the Issue: ARS 84,115,789 (equivalent to USD 5,592,805)
Date of Issue and Subscription: August 31, 2015
Applicable rate (initial): 26.50% annual nominal rate
Maturity: August 31, 2018
F-101
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Amortization: The principal will be amortized in 5 semiannual installments as from the twelfth month following the Date of Issue. The first four installments will be identical, each representing 17.50% of the principal amount issued, and the last will be equivalent to 30% of the principal amount issued.
Date of Payment of Services: Interest will be paid on a quarterly basis at a mixed annual nominal rate as follows: (a) from the Date of Issue up to an including the expiry of the ninth (9) month, interest will accrue at the Fixed Rate for NOs Series I Class I Pesos, and (b) from the start of the tenth (10) month up to the Maturity Date, interest will accrue at a Variable Rate equal to the Reference Rate plus a Differential Rate of 550 (five hundred and fifty) points. The Fixed Rate for NOs Series I Class I Pesos cannot exceed 35%. The Variable Rate for CB Series I Class I Pesos cannot be lower than an annual nominal 16% rate or exceed a 32% annual nominal rate. On June 15, 2015 the National Insurance Superintendency (“SSN”) issued Communication No. 4568 establishing that CB Series I Class I Pesos constitute productive investments under the framework of paragraph k) of item 35.8.1 of the General Regulation Governing Insurance Activities (Resolution SSN No. 21.523/1992).
Series I (Class II – Pesos)
Amount of the Issue: ARS 1,377,882 (equivalent to USD 91,614)
Date of Issue and Subscription: August 31, 2015
Applicable rate (initial): 27% annual nominal rate
Maturity: August 31, 2018
Amortization: The principal will be amortized in five semiannual installments as from the twelfth month following the Date of Issue. The first four installments will be identical, each representing 17.50% of the principal amount issued, and the last installment will be equivalent to 30% of the principal amount issued.
Date of Payment of Services: Interest will be paid on a quarterly basis at a mixed annual nominal rate as follows: (a) from the Date of Issue up to an including the expiry of the ninth (9) month, interest will accrue at the Fixed Rate for CB Series I Class II Pesos, and (b) from the start of the tenth (10) month up to the Maturity Date, interest will accrue at a Variable Rate equal to the Reference Rate plus a Differential Rate of 550 (five hundred and fifty) points.
In compliance with the law governing Negotiable Obligations, the net funds obtained from the issue of each Series and/or Class of Negotiable Obligations will be used by the Company for one or more of the following purposes: (i) as working capital (provided that the funds obtained for any such purposes must be subscribed in the country); (ii) to refinance liabilities; (iii) to fund investments in tangible assets located in the country; and/or (iv) for the subscription of capital contributions in controlled or related companies of the Company (the proceeds from the subscription will be applied exclusively to the destinations specified in article 36 of the Law governing Negotiable Obligations).
On February 28, 2017, the capital service N° 2 and the interest service N° 6 of the Negotiable Obligations corresponding to Series I Class I Pesos, Series I Class II Pesos and Series I Class Dollar were paid.
b) Syndicated loan
With the objective of financing working capital and improving its capital structure, Rizobacter consummated a USD 45 million syndicated loan with Banco de Galicia y Buenos Aires S.A. as administrator, together with Banco Santander Río S.A., Banco BBVA Francés S.A., Banco Ciudad de Buenos Aires, Banco Provincia de Córdoba S.A.., Banco Hipotecario S.A. and Banco Mariva S.A. acting as lenders. This loan was funded in two disbursements, the first of which was made on March 15, 2017 for the amount of USD 22 million, and the second of which was made on April 25, 2017 for the amount of USD 23 million.
F-102
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Amount: USD 45 million
Amortization: The principal will be amortized in 13 quarterly installments as from the twelfth month following the Date of Issue.
Applicable rate: 6.50%
Collaterals: Cash and short-term bank deposits collaterals and Bioceres guarantees.
Covenants: Under the terms of the syndicated loan, Rizobacter is required to comply with the following financial covenants:
Restrictions on assets dispositions
Restrictions on the payment of dividends
Restriction on loans to related parties, including Joint Ventures (USD 5 million per entitiy)
Maintenance the following ratios:
| - | Net Debt to EBITDA ratio must be less than 3x, |
| - | EBITDA to interest ratio must be more than (i) 1.2x for 2017, (ii) 1.5x for 2018 and (iii) 2x for the years 2019 and 2020, and |
| - | Liabilities to assets ratio less than (i) 0.85x for 2017, (ii) 0.825 for 2018 and (iii) 0.8 for 2019 and 2020 |
The Group has complied with these covenants as of June 2017.
7.11. Employee benefits and social security
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Salaries and social security | 2,273,306 | 3,242,094 | 244,841 | ||||||
Staff incentives and vacations | 3,296,903 | 777,462 | 131,850 | ||||||
5,570,209 | 4,019,556 | 376,691 | |||||||
7.12. Deferred revenue and advances from customers
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Licenses and services to be accrued | 59,895 | 65,399 | 56,712 | ||||||
Advances from customers - Joint ventures (Note 13) | — | — | 337,728 | ||||||
Advances from customers | 1,197,080 | 878,874 | 272,933 | ||||||
1,256,975 | 944,273 | 667,373 | |||||||
F-103
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
7.13. Government grants
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
As of January 1 | 2,063,563 | 1,521,705 | 1,046,862 | ||||||
Received during the period | 129,471 | 1,303,287 | 1,705,417 | ||||||
Currency conversion difference | (83,775 | ) | — | — | |||||
Released to the statement of profit or loss | (273,023 | ) | (761,429 | ) | (1,230,574 | ) | |||
At the end of period / year | 1,836,236 | 2,063,563 | 1,521,705 | ||||||
The Group receives government grants to fund research and development projects, some of which are related to the acquisition of property, plant and equipment while others are related to payment for certain expenses like salaries or inputs. Grants are generally implemented through direct payments to the supplier, delivery of cash or loans at subsidized rates.
In addition, the Group receives government assistance other than grants, including the right to use, for no valuable consideration, the Rosario Technological and Scientific Center (CCT) for a 30-year period (Note 7.6) and the provision of CONICET scientists through the “Researchers at Companies” program, whereby certain researchers registered as employees of CONICET develop R&D work for the Group while their remuneration is paid by CONICET (except for additional compensation equivalent to 5% of the CONICENT employees’ remuneration, which amount is borne by the Group).
There are neither unfulfilled conditions nor other contingencies attaching to government grants or government assistance.
7.14. Provisions
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Non-Current | |||||||||
Provisions for contingencies | 1,690,412 | 2,160,788 | 234,505 | ||||||
1,690,412 | 2,160,788 | 234,505 | |||||||
The Group has recorded a provision for probable administrative, judicial and out-of-court proceedings that could arise in the ordinary course of business, based on a prudent criterion according to its professional advisors and on Management’s assessment of the best estimate of the amount of possible claims. These potential claims are not likely to have a material impact on the results of the Group’s operations, its cash flow or financial position.
Management considers that the objective evidence is not enough to determine the date of the eventual cash outflow due to a lack of experience in any similar cases. However, the provision was classified under current or non-current liabilities, applying the best prudent criterion based on Management’s estimates.
There are no expected reimbursements related to the provisions.
The roll forward of the provision is in Note 7.18.
In order to assess the need for provisions and disclosures in its consolidated financial statements, Management considers the following factors: (i) nature of the claim and potential level of damages in the jurisdiction in which the claim has been brought; (ii) the progress of the eventual case; (iii) the opinions or views of tax and legal advisers; (iv) experience in similar cases; and (v) any decision of the Group’s management as to how it will respond to the eventual claim.
F-104
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
7.15. Puttable instruments
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Puttable shares (Note 17) | 2,508,467 | 2,397,397 | 1,612,168 | ||||||
Puttable preferred shares | 2,500,000 | 2,500,000 | — | ||||||
5,008,467 | 4,897,397 | 1,612,168 | |||||||
Puttable Shares
As of June 30, 2017, December 31, 2016, and 2015, the Group had non-current liabilities of USD 2,508,467, USD 2,397,397 and USD 1,612,168, respectively, consisting of puttable instruments relating to ordinary shares of Bioceres S.A. purchased by three investors, each of which has the right to put the shares back to the Group for cash consideration.
On July 15, 2014 and November 21, 2014, respectively, San Cristóbal and YPF each purchased 1,289 shares of Bioceres (equivalent to 128,900 shares after the 1-to-100 share split authorized by shareholders on April 27, 2017) in connection with the Company’s capital increase at a value per share of USD 791.64 (equivalent to USD 7.92 per share after the above-referenced share split), for total consideration of USD 1,020,424 each. Each entity concurrently signed a put option contract with Bioceres, Inc. providing each entity the right to sell such shares to Bioceres, Inc. for a price equivalent to USD 791.64 per share (equivalent to USD 7.92 after the 1-to-100 share split authorized by shareholders on April 27, 2017) plus 4.5% of interest from the period between the date on which the agreement was signed and the date on which the option is exercised.
The option can be exercised incrementally each year in an amount equal to 20% of the total acquired shares beginning in July 2018 in the case of San Cristóbal and November 2016 in the case of YPF.
At least 12 months’ prior notice in advance of the redemption date must be given of the decision to exercise the put option.
As of November 21, 2016 and 2015, YPF had not given notice of its decision to exercise the option for the tranches corresponding to 40% of the shares (20% per year), and thus, the amounts of USD 212,558, equivalent to ARS 3,336,412 and USD 204,085, equivalent to ARS 1,964,827, respectively, have been reclassified from liabilities to equity. The reclassified amount are equivalent to the carrying value at the date of reclassification of the liability recognized for 40% of the shares whose put option was not exercised.
Further, on June 14, 2016, Gador S.A. accepted a put option offer for 1,291 Bioceres, Inc. shares (equivalent to 129,100 shares after the 1-to-100 share split authorized by shareholders on April 27, 2017) in relation to its equity interest in Bioceres S.A. This offer gave Gador a right to sell those shares to Bioceres, Inc under the same terms as YPF and San Cristóbal. The option may be exercised incrementally each year for an amount equal to 20% of the total purchased shares as from June 2018.
On July 12, 2017, San Cristóbal notified the Group of its intent to exercise its put option for the first tranche of 20% of its shares and therefore said share of USD 222,452 has not been reclassified to equity and will be payable one year after the notification of the exercise of the option with an interest of 4.5%.
The holders of the shares have the right to put the shares back to the Group for cash.
The financial instruments are considered to be compound financial instruments because there are both equity (share of profit and loss) and liability (redemption amount, including interest) components. The difference between the fair value at initial recognition of the liability component and the cash received is considered an equity component. The liability component is considered a puttable instrument classified as a non-current financial liability.
F-105
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The puttable instrument component does not meet the exceptions of paragraph 16 A of IAS 32 because not all financial instruments in the most subordinated class of shares are puttable, and the expected cash flows attributable to the puttable shares are fixed.
The fair value of the liability component is the present value of the contractual cash flows which consist of the interest payment and the redemption amount of USD 791.64 per share (equivalent to USD 7.92 after the 1-to-100 share split authorized by shareholders on April 27, 2017), discounted using a market rate of interest applicable to a comparable instrument (regarding credit quality, cash flows and terms) but without the conversion option. Management considers that an appropriate market interest rate is 9%. Fair value of component liability at initial recognition is USD 1,627,470 and the equity component is USD 413,377.
Puttable preferred shares
Bioceres Inc. granted EQC a Put Option, giving EQC a right to sell its holdings in the preferred shares of RASA Holding that EQC acquires in the event of a mandatory conversion of its holdings of such preferred shares into ordinary shares of RASA Holding in 5 years’ time, or in the case of a public offering by RASA Holding or Rizobacter. The price of the Put Option is the nominal value of the preferred shares of RASA Holding or USD 10 nominal value.
7.16. Financed payment – Acquisition of business
Current | 06/30/2017 | 12/31/2016 | 12/31/2015 | ||||||
Garruchos S.A. loan | — | 4,029,918 | — | ||||||
BAF loans | 20,257,452 | 20,123,027 | — | ||||||
Financed payment to sellers | 6,219,980 | 3,177,807 | — | ||||||
26,477,432 | 27,330,752 | — | |||||||
Non-current | |||||||||
BAF loans | 12,222,740 | 12,199,765 | — | ||||||
Financed payment to sellers | 7,656,611 | 10,018,597 | — | ||||||
Purchase option | 13,523,582 | 13,013,073 | — | ||||||
33,402,933 | 35,231,435 | — | |||||||
Garruchos S.A. loan
The Group obtained a USD 4 million loan from Garruchos S.A. in October 2016 for the purpose of financing a portion of the cash payment for the acquisition of Rizobacter. The term of the loan was 180 days with interest to accrue at an annual rate 3.5%. As of June 30, 2017, the loan had already been repaid in full.
BAF loans
The disbursements from BAF also formed part of the financing of the cash payment for the acquisition of Rizobacter, according to Note 1. In this case, the proceeds of the loans were provided two companies:
| - | Bioceres S.A.: Principal in the amount of USD 12 million was granted to the Company, which amount was payable in 12 months (which may be extended at the Company’s choice for a further 12 months), accruing interest payable semi-annually at a per annum rate of 8%. |
| - | Bioceres, Inc: Principal in the amount of USD 20 million was granted to the company, which amount was payable in 12 months (which may be extended at BAF’s choice for a further 12 months), accruing interest payable semi-annually at a per annum rate of 8.5%. |
F-106
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
On October 2017, the Group has extended the maturity of both BAF loans until October 2018.
These are revolving loans where the Group may repay the amounts due and apply for new disbursements, provided that the amounts disbursed as requested and not yet paid do not exceed the maximum amount of the line of credit.
Upon the occurrence of a qualified public offering, BAF has the right to convert the loan balance into Bioceres ordinary shares through the exercise of certain participation rights, at the placement price.
Financing payment to sellers
RASA Holding entered into an agreement with the selling shareholders of Rizobacter which entitled such selling shareholders to receive deferred payment in five non-interest bearing installments, consisting of a payment of USD 3.6 million to be made 12 months from the transaction date, and the subsequent payments of USD 2.9 million to be paid within 18, 24, 30 and 36 months for a total of USD 15.3 million representing at June 30, 2017 USD 13,876,591.
Bioceres became jointly and severally liable for and the principal payor of the financed amount agreed with sellers as a guarantee.
Purchase option
As mentioned in Note 1, the Group subscribed to an option to purchase a further 9.99% of Rizobacter for a nominal value of USD 14.9 million. Bioceres has the right to exercise this option over a term of two years. As from the second anniversary, or if Bioceres and/or its affiliates purchase a portion or all of the equity interests held by certain shareholders, Bioceres will have the obligation to purchase the percentage if the Sellers so require it.
7.17. Contingent consideration- Acquisition of business
As mentioned in Note 1, of the total shares in Rizobacter acquired by our subsidiary RASA Holding, 7.6 million of such shares (which shares represent 19% of Rizobacter’s capital stock) fall within a precautionary measure issued by an injunction that affects 44% of the total capital of Rizobacter. The precautionary measure also covers 30% of the dividends distributed to such shares, directing such percentage of dividends into a judicially created escrow account. The precautionary measure relates to litigation among historical shareholders of Rizobacter arising from a disputed transfer of shares that occurred in 1995.
The Supreme Court of Argentina ruled against certain of the historical shareholders. However, such shareholders have subsequently pursued other legal actions (including the precautionary measure) to further dispute the original transfer of shares, despite the Supreme Court’s ruling. We purchased our controlling stake in Rizobacter subject to the ongoing precautionary measure and associated litigation. Should such contingencies be lifted, we may be obligated to pay a contingent purchase price of $17.3 million to certain selling shareholders of Rizobacter through RASA Holding. Conversely, should the court rule against the free transferability of the affected shares, we would be obligated to return certain shares, thereby reducing our equity participation in Rizobacter, and we would not be obligated to pay the abovementioned contingent purchase price. Given the Supreme Court of Argentina’s finding that the share transfer in 1995 was valid, it is not likely or probable that our control of Rizobacter will be affected (which conclusion is supported by the Supreme Court ruling).
F-107
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
7.18. Changes in allowances and provisions
Item | 12/31/2016 | Additions | Uses and reversals | Currency conversion difference | 06/30/2017 | ||||||||||
DEDUCTED FROM ASSETS | |||||||||||||||
Allowance for impairment of trade debtors | (346,274 | ) | (357,860 | ) | 85,874 | (72,485 | ) | (690,745 | ) | ||||||
Allowance for obsolescence | (739,499 | ) | — | 2,211 | 30,183 | (707,105 | ) | ||||||||
Allowance for unused tax losses | (238,416 | ) | — | 53,520 | 7,906 | (176,990 | ) | ||||||||
Total deducted from assets | (1,324,189 | ) | (357,860 | ) | 141,605 | (34,396 | ) | (1,574,840 | ) | ||||||
INCLUDED IN LIABILITIES | |||||||||||||||
Provisions for contingencies | (2,160,788 | ) | (72,504 | ) | 469,401 | 73,479 | (1,690,412 | ) | |||||||
Total included in liabilities | (2,160,788 | ) | (72,504 | ) | 469,401 | 73,479 | (1,690,412 | ) | |||||||
Total | (3,484,977 | ) | (430,364 | ) | 611,006 | 39,083 | (3,265,252 | ) | |||||||
Item | 12/31/2015 | Additions | Additions from PPA | Uses and reversals | Currency conversion difference | 12/31/2016 | ||||||||||||
DEDUCTED FROM ASSETS | ||||||||||||||||||
Allowance for impairment of trade debtors | (350,782 | ) | (140,386 | ) | — | — | 144,894 | (346,274 | ) | |||||||||
Allowance for obsolescence | (167,989 | ) | (982,351 | ) | — | — | 410,841 | (739,499 | ) | |||||||||
Allowance for unused tax losses | (246,835 | ) | (59,524 | ) | — | 67,511 | 432 | (238,416 | ) | |||||||||
Total deducted from assets | (765,606 | ) | (1,182,261 | ) | — | 67,511 | 556,167 | (1,324,189 | ) | |||||||||
INCLUDED IN LIABILITIES | ||||||||||||||||||
Provisions for contingencies | (234,505 | ) | (343,569 | ) | (1,372,086 | ) | — | (210,628 | ) | (2,160,788 | ) | |||||||
Total included in liabilities | (234,505 | ) | (343,569.00 | ) | (1,372,086 | ) | — | (210,628 | ) | (2,160,788 | ) | |||||||
Total | (1,000,111 | ) | (1,525,830 | ) | (1,372,086 | ) | 67,511 | 345,539 | (3,484,977 | ) | ||||||||
Item | 12/31/2014 | Additions | Amounts used and reversals | 12/31/2015 | ||||||||
DEDUCTED FROM ASSETS | ||||||||||||
Allowance for impairment of trade debtors | (168,810 | ) | (241,947 | ) | 59,975 | (350,782 | ) | |||||
Allowance for obsolescence | (104,562 | ) | (101,874 | ) | 38,447 | (167,989 | ) | |||||
Allowance for unused tax losses | (110,103 | ) | (136,732 | ) | — | (246,835 | ) | |||||
Total deducted from assets | (383,475 | ) | (480,553 | ) | 98,422 | (765,606 | ) | |||||
INCLUDED IN LIABILITIES | ||||||||||||
Provisions for contingencies | (251,182 | ) | — | 16,677 | (234,505 | ) | ||||||
Total included in liabilities | (251,182 | ) | — | 16,677 | (234,505 | ) | ||||||
Total | (634,657 | ) | (480,553 | ) | 115,099 | (1,000,111 | ) | |||||
F-108
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
8. INFORMATION ABOUT COMPONENTS OF CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME
8.1. Revenue
Period / year ended | ||||||||||||
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
(unaudited) | ||||||||||||
Sale of goods | 46,743,539 | 864,487 | 39,863,923 | 4,183,428 | ||||||||
Rendering of services | 1,214,901 | 1,313,192 | 3,358,694 | 4,010,326 | ||||||||
Initial services to joint ventures | — | — | — | 293,412 | ||||||||
Royalties | 102,178 | 82,500 | 349,760 | 354,118 | ||||||||
Licenses | 7,480 | 8,014 | 15,457 | 124,026 | ||||||||
48,068,098 | 2,268,193 | 43,587,834 | 8,965,310 | |||||||||
The joint ventures S&W Seeds and Semya, respectively, were administratively launched during the fiscal year ended December 31, 2015, so the Group has recognized revenue from ordinary activities for R&D services performed until the administrative launch of those joint ventures, including the services performed in prior years, because until that time, the requirements of paragraph 20 of IAS 18 to recognize revenue from ordinary activities had not been fulfilled. Initial services to joint ventures include the services provided before the creation of the respective joint venture. Such revenue from ordinary activities has been included in the Emerging Solutions segment (Note 14).
8.2. Cost of sales
Item | 06/30/2017 | 06/30/2016 (unaudited) | 12/31/2016 | 12/31/2015 | ||||||||
Inventory as of the beginning of the year | 33,157,565 | 3,060,042 | 3,060,041 | 967,220 | ||||||||
Additions from business combination | — | — | 40,846,774 | — | ||||||||
Purchases of the year | 24,360,587 | 1,189,808 | 17,307,320 | 5,348,897 | ||||||||
Production costs (Note 8.4) | 5,939,771 | 609,777 | 4,768,595 | 1,543,269 | ||||||||
Currency conversion difference | (1,548,725 | ) | — | (1,224,167 | ) | — | ||||||
Subtotal | 61,909,198 | 4,859,627 | 64,758,563 | 7,859,386 | ||||||||
Inventory as of the end of the period / year (Note 7.4) | (31,723,752 | ) | (3,569,371 | ) | (33,157,565 | ) | (3,060,041 | ) | ||||
Cost of sales | 30,185,446 | 1,290,256 | 31,600,998 | 4,799,345 | ||||||||
F-109
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
8.3. R&D classified by nature
Item | Research and development expenses. 06/30/2017 | Research and development expenses. 06/30/2016 (unaudited) | Research and development expenses. 12/31/2016 | Research and development expenses. 12/31/2015 | ||||||||
Rent and other office expenses | 22,783 | 11,700 | 48,584 | 48,693 | ||||||||
Amortization intangible assets | 435,462 | 21,495 | 38,234 | 40,728 | ||||||||
Analysis and storage | — | 1,093 | 899 | 860 | ||||||||
Import and export expenses | 14,165 | — | 1,398 | — | ||||||||
Depreciation property, plant and equipment | 287,312 | 77,503 | 185,697 | 112,127 | ||||||||
Freight | 73,275 | 487 | 622 | 3,250 | ||||||||
Staff related expenses | 21,031 | 15,126 | 33,448 | 37,552 | ||||||||
Maintenance | 42,251 | 15,122 | 37,329 | 15,436 | ||||||||
Supplies and materials | 457,438 | 237,972 | 440,774 | 634,656 | ||||||||
Mobility and travel | 118,538 | 59,622 | 127,527 | 107,347 | ||||||||
Employee benefits and social security | 1,323,128 | 455,417 | 1,326,910 | 1,208,859 | ||||||||
Telephone and communications | 12,592 | 6,944 | 18,593 | 15,756 | ||||||||
Based incentive stock options | 117,356 | 183,838 | 131,320 | — | ||||||||
Professional fees and outsourced services | 180,417 | 90,724 | 138,227 | 255,734 | ||||||||
Office supplies | 60,531 | 1,215 | 42,089 | 5,787 | ||||||||
Sealed and certifications | 148 | 87 | 629 | 6,876 | ||||||||
Insurance | 19,229 | 547 | 7,776 | 4,603 | ||||||||
Licenses & Patents | 32,829 | 76,946 | 101,361 | 162,079 | ||||||||
Security expenses | 5,188 | 5,988 | 10,899 | 13,125 | ||||||||
Energy and fuel | 69,231 | 6,835 | 42,161 | 15,456 | ||||||||
Miscellaneous | 308,720 | — | 126,294 | — | ||||||||
Total | 3,601,624 | 1,268,661 | 2,860,771 | 2,688,924 | ||||||||
Period / year ended | ||||||||||||
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
(unaudited) | ||||||||||||
R&D Capitalized (Note 7.6) | 374,202 | 71,638 | 390,940 | 194,277 | ||||||||
R&D profit and loss | 3,601,624 | 1,268,661 | 2,860,771 | 2,688,924 | ||||||||
Total | 3,975,826 | 1,340,299 | 3,251,711 | 2,883,201 | ||||||||
% of total revenue | 8.22 | % | 52.80 | % | 7.33 | % | 28.28 | % | ||||
F-110
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
8.4. Expenses classified by nature and function
Item | Production costs | Selling, general and administrative expenses | Total 06/30/2017 | ||||||
Rent and other office expenses | 1,950 | 15,544 | 17,494 | ||||||
Amortization intangible assets | — | 983,199 | 983,199 | ||||||
Analysis and storage | 20,745 | 46,781 | 67,526 | ||||||
Commissions and rights | — | 258,513 | 258,513 | ||||||
Contingencies | — | 72,504 | 72,504 | ||||||
Bank expenses and commissions | — | 168,617 | 168,617 | ||||||
Import and export expenses | 76,818 | — | 76,818 | ||||||
Depreciation property, plant and equipment | 397,812 | 839,585 | 1,237,397 | ||||||
Impairment of receivables | — | 357,860 | 357,860 | ||||||
Freight | 117,472 | 934,946 | 1,052,418 | ||||||
Staff related expenses | 582 | 12,829 | 13,411 | ||||||
Maintenance | 427,769 | 348,751 | 776,520 | ||||||
Supplies and materials | 463,757 | — | 463,757 | ||||||
Mobility and travel | 26,862 | 795,332 | 822,194 | ||||||
Publicity and advertising | — | 498,626 | 498,626 | ||||||
Employee benefits and social security | 3,697,763 | 7,336,000 | 11,033,763 | ||||||
Telephone and communications | — | 27,671 | 27,671 | ||||||
Based incentive stock options | — | 257,765 | 257,765 | ||||||
Professional fees and outsourced services | 26,519 | 1,269,123 | 1,295,642 | ||||||
Office supplies | 3,056 | 465,318 | 468,374 | ||||||
Sealed and certifications | 51 | 17,232 | 17,283 | ||||||
Insurance | 70,981 | 302,721 | 373,702 | ||||||
Royalties | 60,052 | — | 60,052 | ||||||
Taxes | 23,850 | 1,769,382 | 1,793,232 | ||||||
Security expenses | 5,188 | 10,377 | 15,565 | ||||||
Energy and fuel | 204,825 | 297,595 | 502,420 | ||||||
Miscellaneous | 313,719 | 238,136 | 551,855 | ||||||
Total | 5,939,771 | 17,324,407 | 23,264,178 | ||||||
F-111
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
8.4. Expenses classified by nature and function (Cont’d)
Item | Production costs (unaudited) | Selling, general and administrative expenses (unaudited) | Total 06/30/2016 (unaudited) | ||||||
Rent and other office expenses | 473 | 15,725 | 16,198 | ||||||
Amortization intangible assets | — | 2,570 | 2,570 | ||||||
Analysis and storage | 45,443 | 45,363 | 90,806 | ||||||
Commissions and rights | — | 10,309 | 10,309 | ||||||
Bank expenses and commissions | — | 283,198 | 283,198 | ||||||
Depreciation property, plant and equipment | 11,958 | 167,031 | 178,989 | ||||||
Impairment of receivables | — | 549,173 | 549,173 | ||||||
Freight | 2,083 | 43,060 | 45,143 | ||||||
Staff related expenses | 716 | 10,485 | 11,201 | ||||||
Maintenance | 20,577 | 40,803 | 61,380 | ||||||
Supplies and materials | 155,367 | — | 155,367 | ||||||
Mobility and travel | 10,719 | 103,556 | 114,275 | ||||||
Publicity and advertising | — | 69,522 | 69,522 | ||||||
Employee benefits and social security | 283,854 | 589,190 | 873,044 | ||||||
Tax provisions | — | 27,517 | 27,517 | ||||||
Telephone and communications | 1,939 | 16,731 | 18,670 | ||||||
Professional fees and outsourced services | 5,240 | 923,516 | 928,756 | ||||||
Based incentive stock options | — | 402,117 | 402,117 | ||||||
Office supplies | — | 2,257 | 2,257 | ||||||
Sealed and certifications | 77 | 10,637 | 10,714 | ||||||
Insurance | 398 | 16,116 | 16,514 | ||||||
Royalties | 58,110 | — | 58,110 | ||||||
Taxes | — | 153,444 | 153,444 | ||||||
Security expenses | 5,988 | 11,976 | 17,964 | ||||||
Energy and fuel | 6,835 | 13,670 | 20,505 | ||||||
Miscellaneous | — | 33,092 | 33,092 | ||||||
Total | 609,777 | 3,541,058 | 4,150,835 | ||||||
F-112
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
8.4. Expenses classified by nature and function (Cont’d)
Item | Production costs | Selling, general and administrative expenses | Total 12/31/2016 | ||||||
Rent and other office expenses | 2,997 | 31,983 | 34,980 | ||||||
Amortization intangible assets | — | 424,832 | 424,832 | ||||||
Analysis and storage | 67,052 | 71,126 | 138,178 | ||||||
Commissions and rights | 34,897 | 94,631 | 129,528 | ||||||
Contingencies | — | 343,569 | 343,569 | ||||||
Bank expenses and commissions | — | 464,145 | 464,145 | ||||||
Import and export expenses | 21,435 | 232,402 | 253,837 | ||||||
Depreciation property, plant and equipment | 267,001 | 622,035 | 889,036 | ||||||
Impairment of receivables | — | 140,386 | 140,386 | ||||||
Freight | 36,326 | 753,362 | 789,688 | ||||||
Staff related expenses | 1,269 | 21,123 | 22,392 | ||||||
Maintenance | 39,208 | 221,754 | 260,962 | ||||||
Supplies and materials | 343,147 | 381 | 343,528 | ||||||
Mobility and travel | 36,035 | 399,557 | 435,592 | ||||||
Publicity and advertising | — | 460,586 | 460,586 | ||||||
Employee benefits and social security | 2,335,063 | 4,011,021 | 6,346,084 | ||||||
Telephone and communications | 2,869 | 36,380 | 39,249 | ||||||
Based incentive stock options | — | 512,940 | 512,940 | ||||||
Professional fees and outsourced services | 47,081 | 1,986,546 | 2,033,627 | ||||||
Office supplies | 5,818 | 162,132 | 167,950 | ||||||
Sealed and certifications | 268 | 43,619 | 43,887 | ||||||
Insurance | 809 | 101,487 | 102,296 | ||||||
Royalties | 371,026 | — | 371,026 | ||||||
Obsolescence | 982,351 | — | 982,351 | ||||||
Taxes | 11,967 | 1,235,871 | 1,247,838 | ||||||
Security expenses | 10,899 | 21,798 | 32,697 | ||||||
Energy and fuel | 106,521 | 165,221 | 271,742 | ||||||
Miscellaneous | 44,556 | 347,134 | 391,690 | ||||||
Total | 4,768,595 | 12,906,021 | 17,674,616 | ||||||
F-113
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
8.4. Expenses classified by nature and function (Cont’d)
Item | Production costs | Selling, general and administrative expenses | Total 12/31/2015 | ||||||
Rent and other office expenses | 430 | 41,484 | 41,914 | ||||||
Amortization intangible assets | — | 4,505 | 4,505 | ||||||
Analysis and storage | 14,502 | 122,842 | 137,344 | ||||||
Commissions and rights | 1,021 | 24,109 | 25,130 | ||||||
Bank expenses and commissions | — | 307,885 | 307,885 | ||||||
Depreciation property, plant and equipment | 57,228 | 287,089 | 344,317 | ||||||
Impairment of receivables | — | 241,947 | 241,947 | ||||||
Freight | 29,587 | 66,715 | 96,302 | ||||||
Staff related expenses | 4,352 | 53,798 | 58,150 | ||||||
Maintenance | 5,453 | 94,613 | 100,066 | ||||||
Supplies and materials | 253,464 | 3,794 | 257,258 | ||||||
Mobility and travel | 26,602 | 202,051 | 228,653 | ||||||
Publicity and advertising | — | 195,878 | 195,878 | ||||||
Employee benefits and social security | 602,140 | 1,390,220 | 1,992,360 | ||||||
Based incentive stock options | 42,284 | 42,284 | |||||||
Telephone and communications | 1,382 | 44,163 | 45,545 | ||||||
Professional fees and outsourced services | 62,732 | 447,107 | 509,839 | ||||||
Office supplies | 445 | 7,518 | 7,963 | ||||||
Sealed and certifications | 1,112 | 23,883 | 24,995 | ||||||
Insurance | 1,808 | 20,776 | 22,584 | ||||||
Royalties | 350,556 | — | 350,556 | ||||||
Obsolescence | 101,874 | — | 101,874 | ||||||
Taxes | — | 355,109 | 355,109 | ||||||
Security expenses | 13,125 | 24,720 | 37,845 | ||||||
Energy and fuel | 15,456 | 27,590 | 43,046 | ||||||
Miscellaneous | — | 50,780 | 50,780 | ||||||
Total 12/31/2015 | 1,543,269 | 4,080,860 | 5,624,129 | ||||||
F-114
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
8.5. Finance Income
Period / year ended | ||||||||||||
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
(unaudited) | ||||||||||||
Interest generated by assets | 286,106 | 86,185 | 247,087 | 158,234 | ||||||||
Changes in fair value of financial assets or liabilities | 336,954 | 502,163 | 730,278 | 174,610 | ||||||||
Exchange differences | 1,513,205 | 202,466 | 29,588 | 1,176,892 | ||||||||
2,136,265 | 790,814 | 1,006,953 | 1,509,736 | |||||||||
8.6. Finance Costs
Period / year ended | ||||||||||||
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
(unaudited) | ||||||||||||
Exchange differences | (3,746,801 | ) | (273,079 | ) | (1,513,732 | ) | — | |||||
Interest generated by liabilities | (8,557,971 | ) | (417,535 | ) | (4,804,652 | ) | (1,341,746 | ) | ||||
Financial commissions | (1,096,700 | ) | — | (1,062,767 | ) | — | ||||||
Changes in fair value of financial assets or liabilities | (1,544,023 | ) | (889,337 | ) | (1,316,697 | ) | (562,823 | ) | ||||
Discontinuation of public offering expenses | — | — | (2,225,530 | ) | — | |||||||
(14,945,495 | ) | (1,579,951 | ) | (10,923,378 | ) | (1,904,569 | ) | |||||
9. INCOME TAX AND MINIMUM PRESUMED INCOME TAX
The balances of income tax and minimum presumed income tax recoverable and payable are as follows:
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Current assets | |||||||||
Income tax | 1,409,152 | — | — | ||||||
Minimum presumed income tax | 698,708 | — | — | ||||||
2,107,860 | — | — | |||||||
Non-current assets | |||||||||
Income tax | 95,620 | 337,581 | 185,886 | ||||||
Minimum presumed income tax | 595,827 | 1,265,844 | 206,650 | ||||||
691,447 | 1,603,425 | 392,536 | |||||||
Current liabilities | |||||||||
Minimum presumed income tax | 184,434 | 277,453 | 35,571 | ||||||
Income tax | 38,753 | 1,601,204 | 32,795 | ||||||
223,187 | 1,878,657 | 68,366 | |||||||
F-115
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The breakdown of items making up deferred tax assets and liabilities as of June 30, 2017, December 31, 2016 and 2015 is as follows:
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Deferred tax assets | |||||||||
Unused tax losses | 6,903,193 | 4,229,575 | 1,308,731 | ||||||
Other credits | 257,361 | 212,899 | 316,200 | ||||||
Other | 5,859 | 6,133 | 699 | ||||||
Research and development | 385,170 | 569,120 | 83,992 | ||||||
Short-term investments | (129,580 | ) | (126,040 | ) | (58,453 | ) | |||
Property, plant and equipment depreciation | (941,063 | ) | (121,929 | ) | (132,082 | ) | |||
Allowance for impairment of receivables | 119,533 | 105,569 | 118,720 | ||||||
Goverment grants | 616,380 | 267,421 | 234,949 | ||||||
Other provisions | 92,964 | — | — | ||||||
Inventories | 139,821 | 173,519 | (246,720 | ) | |||||
Total deferred tax assets | 7,449,638 | 5,316,267 | 1,626,036 | ||||||
Allowance for unused tax losses | (176,990 | ) | (238,416 | ) | (246,835 | ) | |||
Total Deferred Tax Assets Net | 7,272,648 | 5,077,851 | 1,379,201 | ||||||
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Deferred tax liabilities | |||||||||
Unused tax losses | — | 95,265 | 175,951 | ||||||
Discounted value monetary assets and liabilities | 117,775 | 220,536 | (21,751 | ) | |||||
Research and development | (13,429,296 | ) | (14,363,148 | ) | (87,739 | ) | |||
Property, plant and equipment depreciation | (12,895,965 | ) | (13,663,466 | ) | (813,426 | ) | |||
Provision for impairment of receivables | 829,095 | 810,640 | 1,493 | ||||||
Inventories | 367,682 | 518,401 | (18,261 | ) | |||||
Other provisions | 439,240 | 744,267 | 58,379 | ||||||
Borrowings | (39,257 | ) | (27,484 | ) | — | ||||
Other | (9,643 | ) | (9,728 | ) | — | ||||
Goverment grants | — | 427,327 | 297,646 | ||||||
Total deferred tax liabilities | (24,620,369 | ) | (25,247,390 | ) | (407,708 | ) | |||
F-116
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The roll forward of deferred tax assets and liabilities as of June 30, 2017, December 31, 2016 and 2015 are as follows:
Balance | Differences between the provision and the tax return | Income tax provision | Balance | Business combination | Prescription | Differences between the provision and the tax return | Income tax provision | Conversion difference | Balance | Exposure correction Semya | Balance | |||||||||||||||||||||||||
12/31/2014 | 12/31/2015 | 12/31/2015 | 12/31/2015 | 12/31/2016 | 12/31/2016 | 12/31/2016 | 12/31/2016 | 12/31/2016 | 12/31/2016 | 12/31/2016 | 12/31/2016 | |||||||||||||||||||||||||
Deferred tax assets | ||||||||||||||||||||||||||||||||||||
Unused tax losses | 1,487,615 | (14,837 | ) | (164,047 | ) | 1,308,731 | — | (67,511 | ) | 146,959 | 2,640,782 | 89,097 | 4,118,058 | 111,517 | 4,229,575 | |||||||||||||||||||||
Other credits | — | — | 316,200 | 316,200 | — | — | — | (146,674 | ) | (7,827 | ) | 161,699 | 51,200 | 212,899 | ||||||||||||||||||||||
Other | — | — | 699 | 699 | 32,725 | — | — | (45,245 | ) | 17,954 | 6,133 | — | 6,133 | |||||||||||||||||||||||
Research and development | 24,545 | — | 59,447 | 83,992 | — | — | (42 | ) | (80,644 | ) | (5,548 | ) | (2,242 | ) | 571,362 | 569,120 | ||||||||||||||||||||
Short-term investments | — | — | (58,453 | ) | (58,453 | ) | — | — | — | (64,174 | ) | (3,413 | ) | (126,040 | ) | — | (126,040 | ) | ||||||||||||||||||
Property,Plant and equipment depreciation | (57,281 | ) | — | (74,801 | ) | (132,082 | ) | — | — | — | 5,845 | 4,308 | (121,929 | ) | — | (121,929 | ) | |||||||||||||||||||
Allowance for impairment of receivables | 28,148 | — | 90,572 | 118,720 | — | — | — | (12,494 | ) | (657 | ) | 105,569 | — | 105,569 | ||||||||||||||||||||||
Goverment grants | 139,514 | — | 95,435 | 234,949 | — | — | — | 30,841 | 1,631 | 267,421 | — | 267,421 | ||||||||||||||||||||||||
Inventories | 88,462 | — | (335,182 | ) | (246,720 | ) | — | — | — | 399,258 | 20,981 | 173,519 | — | 173,519 | ||||||||||||||||||||||
Deferred revenue and advances from customers | 12,250 | — | (12,250 | ) | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||
Total deferred tax assets | 1,723,253 | (14,837 | ) | (82,380 | ) | 1,626,036 | 32,725 | (67,511 | ) | 146,917 | 2,727,495 | 116,526 | 4,582,188 | 734,079 | 5,316,267 | |||||||||||||||||||||
Allowance for unused tax losses | (110,103 | ) | — | (136,732 | ) | (246,835 | ) | — | 67,511 | — | (59,524 | ) | 432 | (238,416 | ) | — | (238,416 | ) | ||||||||||||||||||
Total deferred tax assets net | 1,613,150 | (14,837 | ) | (219,112 | ) | 1,379,201 | 32,725 | — | 146,917 | 2,667,971 | 116,958 | 4,343,772 | 734,079 | 5,077,851 | ||||||||||||||||||||||
F-117
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Balance | Differences between the provision and the tax return | Income tax provision | Balance | Business combination | Prescription | Differences between the provision and the tax return | Income tax provision | Conversion difference | Balance | Exposure correction Semya | Balance | |||||||||||||||||||||||||
12/31/2014 | 12/31/2015 | 12/31/2015 | 12/31/2015 | 12/31/2016 | 12/31/2016 | 12/31/2016 | 12/31/2016 | 12/31/2016 | 12/31/2016 | 12/31/2016 | 12/31/2016 | |||||||||||||||||||||||||
Deferred tax liabilities | ||||||||||||||||||||||||||||||||||||
Unused tax losses | — | — | 175,951 | 175,951 | 198,669 | — | (10,214 | ) | (164,811 | ) | 7,187 | 206,782 | (111,517 | ) | 95,265 | |||||||||||||||||||||
Discounted value monetary assets and liabilities | (78,827 | ) | — | 57,076 | (21,751 | ) | 134,567 | — | — | 168,835 | (9,915 | ) | 271,736 | (51,200 | ) | 220,536 | ||||||||||||||||||||
Research and development | 179,306 | — | (267,045 | ) | (87,739 | ) | (14,546,055 | ) | — | — | 275,854 | 566,154 | (13,791,786 | ) | (571,362 | ) | (14,363,148 | ) | ||||||||||||||||||
Property, plant and equipment depreciation | (798,495 | ) | — | (14,931 | ) | (813,426 | ) | (13,256,350 | ) | — | — | (130,183 | ) | 536,493 | (13,663,466 | ) | — | (13,663,466 | ) | |||||||||||||||||
Allowance for impairment of receivables | — | — | 1,493 | 1,493 | 836,032 | — | — | 4,433 | (31,318 | ) | 810,640 | — | 810,640 | |||||||||||||||||||||||
Inventories | (5,157 | ) | — | (13,104 | ) | (18,261 | ) | (2,926,468 | ) | — | — | 3,344,023 | 119,107 | 518,401 | — | 518,401 | ||||||||||||||||||||
Other provisions | — | — | 58,379 | 58,379 | 545,804 | — | — | 160,661 | (20,577 | ) | 744,267 | — | 744,267 | |||||||||||||||||||||||
Borrowings | — | — | — | — | (28,639 | ) | — | — | — | 1,155 | (27,484 | ) | — | (27,484 | ) | |||||||||||||||||||||
Other | — | — | — | — | (18,531 | ) | — | — | 13,610 | (4,807 | ) | (9,728 | ) | — | (9,728 | ) | ||||||||||||||||||||
Goverment grants | 244,387 | — | 53,259 | 297,646 | — | — | — | 123,205 | 6,476 | 427,327 | — | 427,327 | ||||||||||||||||||||||||
Total deferred tax liabilities | (458,786 | ) | — | 51,078 | (407,708 | ) | (29,060,971 | ) | — | (10,214 | ) | 3,795,627 | 1,169,955 | (24,513,311 | ) | (734,079 | ) | (25,247,390 | ) | |||||||||||||||||
Net deferred tax liabilities | 1,154,364 | (14,837 | ) | (168,034 | ) | 971,493 | (29,028,246 | ) | — | 136,703 | 6,463,598 | 1,286,913 | (20,169,539 | ) | — | (20,169,539 | ) | |||||||||||||||||||
F-118
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Balance | Transfer from deferred tax liabilities | Prescription of tax losses | Income tax provision | Conversion difference | Balance | |||||||||||||
12/31/2016 | 12/31/2016 | 06/30/2017 | ||||||||||||||||
Deferred tax assets | ||||||||||||||||||
Unused tax losses | 4,229,575 | 95,265 | (53,520 | ) | 2,822,165 | (190,292 | ) | 6,903,193 | ||||||||||
Other credits | 212,899 | 113,874 | — | (54,836 | ) | (14,576 | ) | 257,361 | ||||||||||
Other | 6,133 | — | — | — | (274 | ) | 5,859 | |||||||||||
Research and development | 569,120 | 79,825 | — | (234,809 | ) | (28,966 | ) | 385,170 | ||||||||||
Short-term investments | (126,040 | ) | — | — | (9,166 | ) | 5,626 | (129,580 | ) | |||||||||
Property, plant and equipment depreciation | (121,929 | ) | (962,713 | ) | — | 95,168 | 48,411 | (941,063 | ) | |||||||||
Allowance for impairment of receivables | 105,569 | 6,159 | — | 12,791 | (4,986 | ) | 119,533 | |||||||||||
Other provisions | — | 87,940 | — | 8,949 | (3,925 | ) | 92,964 | |||||||||||
Goverment grants | 267,421 | 427,327 | — | (47,360 | ) | (31,008 | ) | 616,380 | ||||||||||
Inventories | 173,519 | (909 | ) | — | (25,076 | ) | (7,713 | ) | 139,821 | |||||||||
Deferred revenue and advances from customers | — | — | — | — | — | — | ||||||||||||
Total deferred tax assets | 5,316,267 | (153,232 | ) | (53,520 | ) | 2,567,826 | (227,703 | ) | 7,449,638 | |||||||||
Allowance for unused tax losses | (238,416 | ) | — | 53,520 | — | 7,906 | (176,990 | ) | ||||||||||
Total deferred tax assets net | 5,077,851 | (153,232 | ) | — | 2,567,826 | (219,797 | ) | 7,272,648 | ||||||||||
Balance | Transfer from deferred tax liabilities | Imputed to equity | Income tax provision | Conversion difference | Balance | |||||||||||||
12/31/2016 | 12/31/2016 | 06/30/2017 | ||||||||||||||||
Deferred tax liabilities | ||||||||||||||||||
Unused tax losses | 95,265 | (95,265 | ) | — | — | — | — | |||||||||||
Discounted value monetary assets and liabilities | 220,536 | (113,874 | ) | — | 15,874 | (4,761 | ) | 117,775 | ||||||||||
Research and development | (14,363,148 | ) | (79,825 | ) | — | 369,055 | 644,622 | (13,429,296 | ) | |||||||||
Property, plant and equipment depreciation | (13,663,466 | ) | 962,713 | (936,029 | ) | 173,956 | 566,861 | (12,895,965 | ) | |||||||||
Allowance for impairment of receivables | 810,640 | (6,159 | ) | — | 60,520 | (35,906 | ) | 829,095 | ||||||||||
Inventories | 518,401 | 909 | — | (128,450 | ) | (23,178 | ) | 367,682 | ||||||||||
Other provisions | 744,267 | (87,940 | ) | — | (187,793 | ) | (29,294 | ) | 439,240 | |||||||||
Borrowings | (27,484 | ) | — | — | (13,002 | ) | 1,229 | (39,257 | ) | |||||||||
Other | (9,728 | ) | — | — | (348 | ) | 433 | (9,643 | ) | |||||||||
Goverment grants | 427,327 | (427,327 | ) | — | — | — | — | |||||||||||
Total deferred tax liabilities | (25,247,390 | ) | 153,232 | (936,029 | ) | 289,812 | 1,120,006 | (24,620,369 | ) | |||||||||
Net deferred tax liabilities | (20,169,539 | ) | — | (936,029 | ) | 2,857,638 | 900,209 | (17,347,721 | ) | |||||||||
F-119
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The following table provides a reconciliation of the statutory tax rate to the effective tax rate. As the operations of the company and its Argentine subsidiaries are the most significant source of profit or loss before tax, the following reconciliation has been prepared using the Argentine statutory tax rate:
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Loss before income tax | (16,245,326 | ) | (9,369,942 | ) | (3,321,100 | ) | |||
Tax rate | 35 | % | 35 | % | 35 | % | |||
Income tax charge by applying | |||||||||
Tax rate to profit / (loss) before tax | 5,685,864 | 3,279,480 | 1,162,385 | ||||||
Share of profit or loss of joint ventures | (275,230 | ) | (245,269 | ) | 115,893 | ||||
Put instruments charge | (36,241 | ) | (60,809 | ) | (51,842 | ) | |||
Stock options charge | (136,492 | ) | (225,661 | ) | (6,366 | ) | |||
Others | (252,640 | ) | 406,270 | (8,196 | ) | ||||
Change in the fair value of Semya | — | 1,497,432 | — | ||||||
Unrecognized Deferred tax assets Rasa Holding | — | (378,714 | ) | — | |||||
Allowance for unused tax losses | — | (59,524 | ) | (146,177 | ) | ||||
Allowance for Tax on minimum presumed income | — | (17,159 | ) | (44,601 | ) | ||||
Differences between the provision and the tax return | 12,106 | 157,131 | (43,038 | ) | |||||
CTA and exchange difference | 93,356 | (213,149 | ) | (1,389,400 | ) | ||||
Income tax benefit / (expense) | 5,090,723 | 4,140,028 | (411,342 | ) | |||||
The charge for income tax charged directly to profit or loss includes the following:
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
(Unaudited) | ||||||||||||
Current tax expense | 2,256,799 | — | 25,200,813 | (61,701 | ) | |||||||
Differences between the provision and the tax return | 12,106 | (10,830 | ) | 157,131 | (43,038 | ) | ||||||
Allowance for unused tax losses | — | — | (62,693 | ) | (246,835 | ) | ||||||
Allowance for income tax | — | — | (14,191 | ) | (15,047 | ) | ||||||
Deferred tax | 2,821,818 | 1,051,753 | (21,141,032 | ) | (44,721 | ) | ||||||
5,090,723 | 1,040,923 | 4,140,028 | (411,342 | ) | ||||||||
F-120
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The amount and expiry date of carry forward tax losses as of June 30, 2017 is as follows:
Fiscal year | Unused Tax credit (tax rate) | Unused Tax Losses | Prescription | Country | ||||
2012 | 79,744 | 227,839 | 2017 | Argentina | ||||
2012 | 97,412 | 278,320 | 2032 | United States | ||||
2013 | 66,882 | 191,092 | 2018 | Argentina | ||||
2013 | 594,910 | 1,699,743 | 2033 | United States | ||||
2014 | 100,828 | 288,081 | 2019 | Argentina | ||||
2015 | 357,051 | 1,020,147 | 2020 | Argentina | ||||
2015 | 2,762 | 7,892 | 2035 | United States | ||||
2016 | 1,445,177 | 4,129,077 | 2021 | Argentina | ||||
2016 | 630,563 | 1,801,609 | 2036 | United States | ||||
2017 | 1,823,489 | 5,209,972 | 2022 | Argentina | ||||
2017 | 1,704,375 | 4,869,642 | 2037 | United States | ||||
6,903,193 | 19,723,414 | |||||||
The amount of tax losses for the fiscal year ended on June 30, 2017 is an estimate of the amount to be presented in the tax return.
The amount and expiry date of unused tax credits of Argentina minimum presumed income tax as of June 30, 2017 is as follows:
Fiscal year | Amount | Prescription | ||
2007 | 8,437 | 2017 | ||
2008 | 5,718 | 2018 | ||
2009 | 6,709 | 2019 | ||
2010 | 10,036 | 2020 | ||
2011 | 14,366 | 2021 | ||
2012 | 22,197 | 2022 | ||
2013 | 25,161 | 2023 | ||
2014 | 70,563 | 2024 | ||
2015 | 167,057 | 2025 | ||
2016 | 986,285 | 2026 | ||
Total | 1,316,529 | |||
Allowance for unused tax credits | (21,994 | ) | ||
Total | 1,294,535 | |||
The amount of tax credit of minimum presumed income tax for the fiscal year ended on June 30, 2017 is an estimate of the amount to be presented in the tax return.
F-121
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Estimates
There is an inherent material uncertainty related to Management’s estimation of the ability of the Group to use the deferred tax assets (both carryforward of unused tax losses and deductible temporary differences) and the credit of minimum presumed income tax because their future utilization depends on the generation of enough future taxable income by the entities within the Group during the periods in which those temporary differences are deductible or when the unused tax losses can be used.
Based on the projections of future taxable income for the periods in which the deferred tax assets are deductible, the Group’s management estimates that, except for the part of deferred tax asset for which an allowance has been recognized, it is probable that the entities within the Group can utilize those deferred tax assets, which depends, among other factors, on the success of the current projects of agricultural biotechnology, the future market price of commodities and the market share of the entities within the Group.
The estimates of Management about the demonstrability of the recognition criteria for these deferred tax assets and their subsequent recoverability represent the best estimate that can be made based on all the available evidence, existing facts and circumstances and the use of reasonable and supportable assumptions in the projections of future taxable income. Therefore, the consolidated financial statements do not include adjustments that could result if the entities within the Group would not be able to recover the deferred tax assets through the generation of enough future taxable income.
10. EARNINGS PER SHARE
The numerators and denominators used in the calculation of basic EPS and diluted EPS are presented below:
Period ended | ||||||||||||
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
(unaudited) | ||||||||||||
Numerator | ||||||||||||
Loss for the period / year (basic EPS) | (8,467,524 | ) | (3,435,546 | ) | (4,508,061 | ) | (3,540,504 | ) | ||||
Loss for the period / year (diluted EPS) | (8,467,524 | ) | (3,435,546 | ) | (4,508,061 | ) | (3,540,504 | ) | ||||
Denominator | ||||||||||||
Weighted average number of shares (basic EPS) | 25,536,100 | 25,593,700 | 25,592,104 | 25,534,542 | ||||||||
Weighted average number of shares (diluted EPS) | 25,536,100 | 25,593,700 | 25,592,104 | 25,534,542 | ||||||||
On December 17, 2014, the Company’s shareholders authorized: (i) to effect a 1-to-50 stock split of the Company’s ordinary shares, and (ii) a capital increase issuing up to 12,000,000 new ordinary book-entry shares, with a par value of ARS 2 per share and the right to one vote per share, including shares for the possible over-allotment of the IPO, of which CNV authorized 6 million shares.
On April 27, 2017, shareholders approved a 1-to-100 stock split of the Company’s common stock that will be effective prior to the effctiveness of the public offering. Therefore, the capital increase will result in issuing up to 24,000,000 shares with a par value of ARS 1 per and the right to one vote per share.
The effect of the share split was considered retrospectively in the EPS calculations. The denominators used in the EPS calculation are based on the assumption of the effectiveness of the share split.
F-122
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The 24,000,000 new ordinary book-entry shares exclude:
| ■ | 929,040 ordinary shares issuable upon the exercise of stock options as of the date hereof under share option agreements to be entered into with certain of the Company’s key employees and members of its board of directors at an exercise price of USD 7.91 per ordinary share (Note 19); and |
| ■ | 2,193,040 ordinary shares reserved for future issuance, consisting of 334,960 ordinary shares issuable upon the exercise of future stock option awards and 1,264,000 ordinary shares issuable pursuant to future stock grants, in each case authorized by the Company’s shareholders as of the date hereof. |
Some financial instruments were not included in the GPA calculations for the transition period ended June 30, 2017 and the fiscal years ended December 31, 2016 and 2015 because they were anti-dilutive securities in that period. Those instruments, which are listed as follows, might dilute the EPS in the future:
| - | Instruments with a put option (Note 7.17) |
| - | Stock options (Note 19) |
| - | Malbec loan (Note 7.10): issued on April 8, 2016 |
| - | BAF loans (Note 7.17): issued on October 14, 2016 |
| - | RASA Holding preferred shares (Note 11.2): issued on October 14, 2016. |
There are neither ordinary shares transactions nor potential ordinary shares transactions that have occurred after June 30, 2017 that would have changed significantly the number of ordinary shares or potential ordinary shares outstanding at the end of the reporting period.
The probable future issue of shares related to the authorized capital increase of 24,000,000 (after the stock split of 1-to-100) shares for the IPO would have changed significantly the number of ordinary shares outstanding at the end of the reporting period if those shares had been issued before June 30, 2017. The future issue of shares related to the “stock options” and “stock grants” arrangements above mentioned could potentially dilute EPS in the future.
11. INFORMATION ABOUT CONSOLIDATED COMPONENTS OF EQUITY
11.1. Shareholder’s contributions
The authorized capital as of the end of each year is as follows:
Year | Authorized Capital (ARS) | Authorized Capital (number of shares) | ||||
12/31/2015 | 25,644,300 | 25,644,300 | ||||
12/31/2016 | 25,644,300 | 25,644,300 | ||||
06/30/2017 | 25,644,300 | 25,644,300 | ||||
The issuance of 24,000,000 (after stock split of 1-to-100) shares is not included in the above numbers because they are conditional to the materialization of the public offering. The effect of the share split was considered retrospectively in the EPS calculations and in the number of outstanding shares.
F-123
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Shares outstanding as of the end of each year are as follows:
Year | Number of shares | Class | Face value | Voting right (per share) | Number of shares issued and fully paid | Number of shares issued and not paid | ||||||||||
12/31/2015 | 25,644,300 | Ordinary | 1 | 1 | 25,644,300 | — | ||||||||||
12/31/2016 | 25,644,300 | Ordinary | 1 | 1 | 25,644,300 | — | ||||||||||
06/30/2017 | 25,644,300 | Ordinary | 1 | 1 | 25,644,300 | — | ||||||||||
The issue of 24,000,000 (after stock split of 1-to-100) shares are not included in the above numbers. The effect of the share split was considered retrospectively in the EPS calculations and in the number of outstanding shares.
There is a description of share capital as of the end of each year in the table below:
Year | Capital issued (ARS) | Capital issued and fully paid (ARS) | Capital issued and not paid (ARS) | ||||||
12/31/2015 | 25,644,300 | 25,644,300 | — | ||||||
12/31/2016 | 25,644,300 | 25,644,300 | — | ||||||
06/30/2017 | 25,644,300 | 25,644,300 | — | ||||||
Puttable shares, which are fully paid, as of June 30, 2017, December 31, 2016 and 2015 are included. Further detail is in Note 7.16.
The reconciliation of the number of shares outstanding at the beginning and at the end of each reporting period is as follows:
Period | Number of shares outstanding | Capital issued | ||||
12/31/2015 | 25,644,300 | 25,644,300 | ||||
Receptions of shares by subsidiaries | (50,600 | ) | (50,600 | ) | ||
Receptions of shares by subsidiaries on business combination | (57,600 | ) | (57,600 | ) | ||
Reclassification of shares (puttable) | (103,300 | ) | (103,300 | ) | ||
Reclassification of shares (non - puttable) | 103,300 | 103,300 | ||||
12/31/2016 | 25,536,100 | 25,536,100 | ||||
06/30/2017 | 25,536,100 | 25,536,100 | ||||
The issuance of 24,000,000 (after stock split of 1-to-100) shares of the Company are not included in the above numbers. The effect of the share split was considered retrospectively in the EPS calculations and in the number of outstanding shares.
The amounts included in “Share Premium” are the amounts subscribed for share capital in excess of par value (net of transaction costs).
As of November 21, 2016 and 2015, YPF had not given notice of its decision to exercise its option for the first two tranches corresponding to 40% of the Company’s ordinary shares and thus, USD 212,510 (equivalent to ARS 3,336,412) and USD 204,085 (equivalent to ARS 1,964,827), respectively, have been reclassified from liabilities to equity (allocated to the additional paid-in capital because it corresponds to the contributions in excess of the par value of the shares). The reclassified amounts correspond to the carrying value at the date of reclassification of the liability recognized for 40% of the shares whose put option was not exercised (Note 7.15).
F-124
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
On July 12, 2017, San Cristóbal notified the Group of its intent to exercise its put option for the first tranche of 20% of its shares and therefore said share of USD 222,452 has not been reclassified to equity and will be payable one year after notification of the exercise of the option with an interest of 4.5% payable.
On June 14, 2016, Gador entered into a put option contract with Bioceres, Inc. in relation to 129,100 shares in the Company. The option may be exercised incrementally each year for an amount equal to 20% of the total purchased shares, and the first redemption date will be June 14, 2019. Gador must notify the Group of any decision to exercise the put option at least 12 months in advance of the redemption date (Note 7.15).
On March 15, 2016, the Group received, through its subsidiary Bioceres Semillas S.A., 50,600 shares from a shareholder in lieu of the payment of a commercial debt held with that subsidiary. The transaction cost was ARS 3,946,993. As of the date of issuance of these financial statements, these shares had been sold.
On October 19, 2016, the Group acquired control over Rizobacter. At the time of its acquisition, Rizobacter held 57,600 shares in the Company.
11.2. Non-controlling interests
The two subsidiaries whose non-controlling interest is material as of June 30, 2017 and December 31, 2016 are:
a) Rizobacter Argentina
Name | Country | % of equity interest and votes of the NCI | |||||||
06/30/2017 | 12/31/2016 | ||||||||
Rizobacter Argentina S.A. | Argentina | 40 | % | 40 | % | ||||
Below is a detail of the summarized financial information of Rizobacter, prepared in accordance with IFRS, and modified due to fair value adjustments at the acquisition date and differences in accounting policies. The information is presented prior to eliminations between that subsidiary and other Group companies. Only the profit and loss since October 19, 2016, the date of the Rizobacter acquisition, have been disclosed.
Summary financial statements: | Rizobacter | |||||
06/30/2017 | 12/31/2016 | |||||
Current assets | 86,202,115 | 90,827,000 | ||||
Non-current assets | 113,906,651 | 120,428,870 | ||||
Total assets | 200,108,766 | 211,255,870 | ||||
Current liabilities | 60,192,036 | 86,958,449 | ||||
Non-current liabilities | 65,604,538 | 38,308,346 | ||||
Total liabilities | 125,796,574 | 125,266,795 | ||||
Equity attributable to controlling interest | 74,266,985 | 85,913,069 | ||||
Equity attributable to non-controlling interest | 45,208 | 76,006 | ||||
Total equity | 74,312,193 | 85,989,075 | ||||
Total liabilities and equity | 200,108,767 | 211,255,870 | ||||
F-125
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Summary statements of comprehensive income or loss | Rizobacter | |||||
Six-month period ended 06/30/2017 | Period from 10/19/16 - 12/31/2016 | |||||
Revenues | 44,458,442 | 36,739,496 | ||||
Cost of sales | (27,494,324 | ) | (26,818,086 | ) | ||
Gross margin | 16,964,118 | 9,921,409 | ||||
Research and development expenses | (1,711,089 | ) | (555,676 | ) | ||
Selling, general and administrative expenses | (14,501,080 | ) | (6,936,425 | ) | ||
Share of profit (or loss) of joint ventures and associates | (610,994 | ) | (161,436 | ) | ||
Other income | 53,928 | — | ||||
Operating profit | 194,883 | 2,267,872 | ||||
Financial results | (8,619,703 | ) | (4,014,402 | ) | ||
Loss before taxes | (8,424,820 | ) | (1,746,530 | ) | ||
Income tax benefit (expense) | 2,333,310 | (1,836,968 | ) | |||
Loss for the period / year | (6,091,510 | ) | (3,583,498 | ) | ||
Exchange differences on translation of foreign operations | 202,981 | (898,790 | ) | |||
Revaluation of property, plant and equipment, net of tax | 2,032,872 | — | ||||
Total comprehensive loss | (3,855,657 | ) | (4,482,288 | ) | ||
There were no dividends paid to Rizobacter non-controlling interest (NCI) during the period from October 19, 2016 to June 30, 2017.
b) RASA Holding preferred shares
On October 14, 2016, RASA Holding issued 4,830,000 Class A preferred shares, with a par value of USD 10 per share and a subscription price of USD 8.696 per share, in order to raise USD 42 million to finance the cash payment required for the completion of the acquisition of Rizobacter.
The Class A preferred shares in RASA Holding accrue an annual “pay in kind” (PIK) dividend coupon of 12% that accumulates as principal over a maturity term of five years, and carry (i) a mandatory participation right in the subscription to ordinary shares of Bioceres as part of a qualified public offering (i.e., an offering that is consummated for at least USD 50 million net of the proceeds received from the holders of the RASA Holding Class A preferred shares), and to participate in an optional manner in the case of a non-qualified public offering and/or a private capital increase occurring between December 17, 2017 and October 14, 2021; and (ii) a mandatory conversion right for holders of the RASA Holding Class A preferred shares to convert such preferred shares into common shares of RASA Holding after five years or in the case of a public offering made by RASA Holding or of Rizobacter.
In addition, this agreement grants liquidity preference rights to the Class A preferred shares, which give the holders a right to a cash option that can be exercised in the following situations: (i) sale or substantial transfer of the assets of
F-126
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
RASA Holding or Rizobacter; (ii) mergers or reorganizations in which RASA Holding is involved; (iii) change of control of RASA Holding; or (iv) liquidation or dissolution of RASA Holding.
During the current period, 376,926 preferred shares were acquired and paid for by the selling shareholders of Chemotécnica, and the amount collected was used to pay the Garruchos S.A. loans. As of June 30, 2017, there were 1,409,848 preferred shares in the possession of third parties outside the Group. The number of shares to be subscribed and issued upon the mandatory conversion will be determined by converting at the applicable pre-defined conversion ratio.
As there is no contractual obligation to pay cash in any situation, liquidity preferences are under the control of the Group and the agreement establishes the delivery of a fixed number of its own equity instruments for a fixed amount of cash, preferred shares in possession of third parties outside the Group are classified as non-controlling interest in the consolidated financial statements.
12. CASH FLOW INFORMATION
Significant non-cash transactions related to investing and financing activities are as follows:
Period ended | ||||||||||||
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
(unaudited) | ||||||||||||
Investment Activities | ||||||||||||
S&W Seed Company stocks trading | — | — | — | (999,841 | ) | |||||||
Cash dividends distributed by subsidiary | 4,359 | 13,790 | 13,790 | — | ||||||||
Investment in joint ventures | 1,022,146 | — | 33,629,300 | — | ||||||||
1,026,505 | 13,790 | 33,643,090 | (999,841 | ) | ||||||||
Period ended | ||||||||||||
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
(unaudited) | ||||||||||||
Financing Activities | ||||||||||||
Transfer of preferred shares of subsidiary | (3,277,615 | ) | — | (3,078,917 | ) | — | ||||||
Receivables from preferred shares sales | (383,014 | ) | — | 383,014 | — | |||||||
Payment of loans with preferred shares | 3,660,629 | — | — | — | ||||||||
S&W Seed Company stocks trading | — | — | — | 999,841 | ||||||||
Cost of own shares held by subsidiary | — | (270,342 | ) | (726,822 | ) | — | ||||||
Issue of puttable instruments | — | (814,570 | ) | (814,570 | ) | — | ||||||
Reclassification of puttable instruments | — | — | 212,558 | 204,085 | ||||||||
— | (1,084,912 | ) | (4,024,737 | ) | 1,203,926 | |||||||
As of June 30, 2017, the investment in joint ventures is due to Synertech. It corresponds to an irrevocable contribution of land made by Rizobacter and revaluation of property plant and equipment.
The transfer of preferred shares of subsidiary was used to pay Garruchos S.A. loans jointly with the receivables from preferred stocks sales made in December 2016 for the acquisition of Chemotecnica S.A.
F-127
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
As of December 31, 2016, the investment in joint ventures is due to:
| - | Synertech for USD 30,550,383, corresponding to the addition of the joint venture after the business combination. |
| - | AGBM for USD 1,550,383 arising from the capitalization of the balance receivable and results of the transfer to that affiliate of the SPC farming molecular platform intangible. |
The receipt of company’s own shares derives from the collection by Bioceres Semillas of commercial balances with Bioceres S.A. shares for USD 726,822, and after the business combination occurred in the fiscal year because the acquire holds shares in Bioceres S.A. within its assets.
The issuance and reclassification of instruments with a put option are explained in Note 7.16.
The stock swap with S&W Seed Company is explained in Note 7.2.
The amendments to IAS 7 require the following disclosure of changes in liabilities arising from financing activities:
Financial liabilities | ||||||||||||
Borrowings | Financed payment - Acquisition of business | Contingent consideration - Acquisition of business | Total | |||||||||
As of 31 December, 2016 | 77,746,010 | 62,562,187 | 15,771,716 | 156,079,913 | ||||||||
Payments or cash income | 20,076,584 | — | — | 20,076,584 | ||||||||
Interest payment | (7,858,245 | ) | (1,322,611 | ) | — | (9,180,856 | ) | |||||
Financial results | 2,163,127 | 2,301,418 | 147,344 | 4,611,889 | ||||||||
Payment of loans with preferred shares | — | (3,660,629 | ) | — | (3,660,629 | ) | ||||||
As of 30 June, 2017 | 92,127,476 | 59,880,365 | 15,919,060 | 167,926,901 | ||||||||
13. JOINT ARRANGEMENTS
13.1. Joint Ventures
Investments in joint ventures and related companies | 06/30/2017 | 12/31/2016 | 12/31/2015 | ||||||
Liabilities | |||||||||
Trigall Genetics | 1,527,286 | 1,569,160 | 1,185,566 | ||||||
1,527,286 | 1,569,160 | 1,185,566 | |||||||
Investments in joint ventures and related companies | 06/30/2017 | 12/31/2016 | 12/31/2015 | ||||||
Assets | |||||||||
Semya | — | — | 59,015 | ||||||
S&W Semillas | 95,968 | 95,888 | 240,261 | ||||||
A.G.B.M. | 1,483,208 | 1,661,080 | — | ||||||
Synertech Industrias | 26,844,410 | 27,676,095 | — | ||||||
Héritas | 554,349 | — | — | ||||||
F-128
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Investments in joint ventures and related companies | 06/30/2017 | 12/31/2016 | 12/31/2015 | ||||||
28,977,935 | 29,433,063 | 299,276 | |||||||
Period / Year ended | ||||||||||||
Share of profit or loss of joint ventures and related companies | 06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | ||||||||
(unaudited) | ||||||||||||
Trigall Genetics(i) | (38,081 | ) | (226,330 | ) | (545,606 | ) | (858,158 | ) | ||||
Semya(iii) | — | (139,886 | ) | (202,171 | ) | (489,691 | ) | |||||
S&W Semillas(iv) | 4,575 | (88,965 | ) | (144,373 | ) | (205,173 | ) | |||||
AGBM(v) | (108,936 | ) | — | 110,697 | — | |||||||
Synertech Industrias(vi) | (596,854 | ) | — | (155,316 | ) | — | ||||||
Héritas(vii) | (47,509 | ) | — | — | — | |||||||
(786,805 | ) | (455,181 | ) | (936,769 | ) | (1,553,022 | ) | |||||
Period / Year ended | ||||||||||||
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
(unaudited) | ||||||||||||
As of the beginning of the period / year | 27,863,903 | (886,290 | ) | (886,290 | ) | (1,364,677 | ) | |||||
Monetary contributions | 617,236 | 82,804 | 162,012 | 2,031,409 | ||||||||
Non-monetary contributions | 833,121 | — | 1,550,383 | — | ||||||||
Revaluation of property plant and equipment | 189,025 | — | — | — | ||||||||
Business combinations - Synertch | — | — | 29,000,000 | — | ||||||||
Reclassification of Semya for business combination | — | — | 143,156 | — | ||||||||
Foreign currency translation | (1,265,831 | ) | — | (1,168,589 | ) | — | ||||||
Share of profit or loss | (786,805 | ) | (455,181 | ) | (936,769 | ) | (1,553,022 | ) | ||||
As the end of the period / year | 27,450,649 | (1,258,667 | ) | 27,863,903 | (886,290 | ) | ||||||
(i) Trigall Genetics S.A
This joint venture was formed in December 2013 with Florimond Desprez (a company from France with an internationally recognized track record in wheat breeding) through Bioceres, Inc. Equity interest is equally shared between both participants.
Trigall is a separately structured vehicle incorporated and operating in Uruguay. The primary activity of Trigall is being engaged in the development and deregulation of conventional and GM wheat varieties in Latin America, which is in line with the Group’s core business.
This joint arrangement provides for each of the joint venture partners to exclusively license its trait and germplasm technologies to Trigall for use in wheat. The Group granted an exclusive, sub licensable license to Trigall for certain of Group`s technologies, including HB4, for use in wheat.
Both the Group and Florimond Desprez have agreed to make loans to Trigall in accordance with each party`s ownership interest in the joint venture to provide it funds needed for its operation and growth.
F-129
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The first products in Trigall`s pipeline are conventional wheat varieties that will be sold through Bioceres Semillas, as well as through other Trigall licensees. The first Group`s GM product is HB4, which is now in the advanced development and deregulation phase. Trigall is currently seeking to add other market channel partners in the region.
The contractual arrangement provides the Group with only the rights to the net assets of the joint arrangement. The rights to the assets and obligation for liabilities of the joint arrangement rest primarily with Trigall. Under IFRS 11 this joint arrangement is classified as a joint venture and has been included in the consolidated financial statements using the equity method. The shares of Trigall are not quoted.
Summarized financial information in relation to the joint venture is presented below:
Summary statements of financial position: | Trigall Genetics S.A. | ||||||||
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
CURRENT ASSETS | |||||||||
Cash and cash equivalents | 9,758 | 9,088 | 5,269 | ||||||
Trade receivables | 51,041 | 30,000 | — | ||||||
Other receivables | 11,096 | 6,462 | 8,467 | ||||||
Income and minimum presumed income taxes recoverable | — | 36,976 | 22,595 | ||||||
NON-CURRENT ASSETS | |||||||||
Intangible assets | 7,580,170 | 6,892,810 | 5,304,979 | ||||||
Total current assets | 7,652,065 | 6,975,336 | 5,341,310 | ||||||
Trigall Genetics S.A. | |||||||||
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
CURRENT LIABILITIES | |||||||||
Trade and other payables | 546,072 | 728,817 | 2,000 | ||||||
Income and minimum presumed income taxes payable | 4,183 | 44,199 | 27,249 | ||||||
Borrowings | — | — | — | ||||||
NON-CURRENT LIABILITIES | |||||||||
Trade and other payables | 26,339 | 26,331 | 1,122,721 | ||||||
Borrowings | 5,213,328 | 4,442,988 | 2,739,539 | ||||||
Income and minimum presumed income taxes payable | 12,974 | 24,417 | 59,340 | ||||||
Deferred tax liabilities | 606,480 | 784,006 | 515,404 | ||||||
TOTAL LIABILITIES | 6,409,376 | 6,050,758 | 4,466,253 | ||||||
Equity before loss for the period / year | 1,245,036 | 1,199,081 | 1,287,365 | ||||||
Loss for the period / year | (2,347 | ) | (274,503 | ) | (412,308 | ) | |||
EQUITY | 1,242,689 | 924,578 | 875,057 | ||||||
TOTAL EQUITY AND LIABILITIES | 7,652,065 | 6,975,336 | 5,341,310 | ||||||
F-130
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Statement of comprehensive Income or loss | Trigall Genetics S.A. | |||||||||||
Period / year ended | ||||||||||||
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
(unaudited) | ||||||||||||
Revenue | 21,041 | 30,000 | 30,000 | — | ||||||||
Administrative, selling and distribution expenses | (57,342 | ) | (167,623 | ) | (85,752 | ) | (76,106 | ) | ||||
Finance income | 16,977 | 87,860 | 74,267 | 235,709 | ||||||||
Profit / (loss) before income tax | (19,324 | ) | (49,763 | ) | 18,515 | 159,603 | ||||||
Income tax benefit / (expense) | 16,977 | — | (293,018 | ) | (571,911 | ) | ||||||
Profit / (loss) | (2,347 | ) | (49,763 | ) | (274,503 | ) | (412,308 | ) | ||||
Total comprehensive income loss | (2,347 | ) | (49,763 | ) | (274,503 | ) | (412,308 | ) | ||||
As of June 30, 2017, December 31, 2016 and 2015, the Group recognized in the consolidated financial statements a negative carrying amount of the investment in Trigall for USD 1,527,286, USD 1,569,160 and USD 1,185,566, respectively. The negative carrying amount is generated from the elimination of the Group’s share (50%) of unrealized profit included in the intangible assets of Trigall.
There are no significant restrictions on the ability of the joint venture to transfer funds to the Group in the form of cash dividends, or to repay loans or advances made by the Group, except for the obligation to establish a legal reserve for 5% of the profit for the year until reaching 20% of the capital.
(ii) Verdeca LLC
This joint venture was formed in February 2012 with Arcadia (an USA based company that develops agricultural biotechnologies) through Bioceres, Inc. Equity interest is equally shared between both participants.
Verdeca LLC is a separately structured vehicle incorporated and operating in USA. The primary activity of Verdeca is the development and deregulation of soybean traits with second generation technology, which is in line with the Group’s core business.
This joint arrangement provides for each of the joint venture partners to license its trait technologies to Verdeca for use in soybeans. The Group will grant an exclusive, worldwide, sub licensable license to Verdeca for its technologies, including HB4, for use in soybeans. Both partners also will grant Verdeca a right of first offer to obtain a license to the intellectual property rights that are owned, licensed to, or acquired by the Group or Arcadia, as applicable, in the future and that are reasonably necessary or useful with respect to soybean traits.
The first product in Verdeca pipeline is the Group`s HB4 trait, a drought and salinity tolerance trait that is in the advanced development phase. Verdeca`s pipeline also includes Arcadia’s nutrient use efficiency and water use efficiency technologies, which are combined in a two-trait stack that will be further stacked with HB4. Verdeca has successfully negotiated favorable market access in South America and is growing its partnerships in USA, India and China.
The contractual arrangement provides the Group with only the rights to the net assets of the joint arrangement. The rights to the assets and obligation for liabilities of the joint arrangement rest primarily with Verdeca. Under IFRS 11 this joint arrangement is classified as a joint venture and has been included in the consolidated financial statements using the equity method. The shares of Verdeca are not quoted.
F-131
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
There are no significant restrictions on the ability of the joint venture to transfer funds to the Group in the form of cash dividends, or to repay loans or advances made by the Group.
At June 30, 2017, December 31, 2016 and 2015, and until the date of issue of these consolidated financial statements, the contracts had not been formalized, so the partners (Bioceres and Arcadia) will grant Verdeca licenses for use and commercialization of their HB4 Soybean, Nue Wue, Tilling and other technologies. Therefore, at those dates, Verdeca has not recorded any assets, liabilities or results because it does not have control over those technologies.
(iii) SEMYA S.A.
This joint venture was launched in 2012 and formed as a separate vehicle in August 2014 with Rizobacter and Bioceres. Equity interest is equally shared between both participants.
The primary activity of SEMYA is being engaged in the development and deregulation of second generation agricultural biological products, in particular, seed treatments tailored for particular soil environments, which is in line with the Group’s core business.
The joint venture agreement provides for each party to render the services required to advance a mutually agreed work plan and which each party agrees to fund in proportion to its equity interest. Under the services agreements, both the Group and Rizobacter agree to provide to SEMYA the licenses required for product development and commercialization, while results obtained in the provision of the services are owned by SEMYA. SEMYA creates customized seed treatments based on soil conditions on a given agricultural environment and on specific trait-germplasm combinations.
At October 19, 2016 and after the acquisition of 60% of Rizobacter, the Group also acquired a controlling interest in SEMYA, reaching 80% (Bioceres directly owns 50% of SEMYA and indirectly a further 30% (60% of 50%) through Rizobacter). For this reason, and since the business combination date, SEMYA has no longer been classified as a joint venture and is now a subsidiary.
The effects of the business combination are described in Note 1.
Summarized financial information in relation to the joint venture until October 19, 2016 is presented below:
Summary statements of financial position: | |||||||||
Semya S.A. | |||||||||
10/19/2016 | 12/31/2015 | 12/31/2014 | |||||||
CURRENT ASSETS | |||||||||
Cash and cash equivalents | 5,093 | — | — | ||||||
Other receivables | 2,257 | 34 | 175,384 | ||||||
NON-CURRENT ASSETS | |||||||||
Other receivables | 189,690 | 165,610 | 1,773 | ||||||
Income and minimum presumed income taxes recoverable | 21,946 | 646,426 | — | ||||||
Deferred tax assets | 753,306 | — | 449,400 | ||||||
TOTAL ASSETS | 972,292 | 812,070 | 626,557 | ||||||
F-132
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
10/19/2016 | 12/31/2015 | 12/31/2014 | |||||||
CURRENT LIABILITIES | |||||||||
Trade and other payables | 421,854 | 673,652 | 1,430,068 | ||||||
Borrowings | — | 3,318 | — | ||||||
Income and minimum presumed income taxes payable | 13,414 | — | — | ||||||
Other fiscal charges | — | 12,492 | — | ||||||
NON-CURRENT LIABILITIES | |||||||||
Trade and other payables | 3,920 | 4,580 | — | ||||||
Borrowings | 804,433 | — | — | ||||||
TOTAL LIABILITIES | 1,243,621 | 694,042 | 1,430,068 | ||||||
Equity before loss for the period / year | 118,028 | 1,097,413 | 14,599 | ||||||
Loss for the period / year | (389,357 | ) | (979,385 | ) | (818,110 | ) | |||
EQUITY | (271,329 | ) | 118,028 | (803,511 | ) | ||||
TOTAL EQUITY AND LIABILITIES | 972,292 | 812,070 | 626,557 | ||||||
Statement of comprehensive Income or Loss | Semya S.A. | ||||||||
Period / year ended | |||||||||
10/19/2016 | 06/30/2016 | 12/31/2015 | |||||||
(unaudited) | |||||||||
Research and development | (360,968 | ) | (221,688 | ) | (945,638 | ) | |||
Administrative, Selling and distribution Expenses | (10,039 | ) | (6,656 | ) | (8,794 | ) | |||
Finance results | (125,231 | ) | (111,523 | ) | (221,980 | ) | |||
Loss before income tax | (496,238 | ) | (339,867 | ) | (1,176,412 | ) | |||
Income tax benefit | 106,881 | 60,096 | 197,027 | ||||||
Loss before income tax | (389,357 | ) | (279,771 | ) | (979,385 | ) | |||
Other comprehensive income | — | — | — | ||||||
Total comprehensive loss | (389,357 | ) | (279,771 | ) | (979,385 | ) | |||
As part of the business combination and as a result of the valuation at fair value of the acquired assets and assumed liabilities, intangible assets for USD 2,380,203 and deferred tax liabilities for USD 833,071 were identified.
Accounting recognition was given at December 31, 2016, to the result of the acquisition of control over Semya equivalent to USD 4,453,284, and arises from the difference between the fair value measurement of the previously held interests and the equity value prior to the business combination.
As December 31, 2015, the Group recognized in the consolidated financial statements a negative carrying amount of the investment in SEMYA USD 59,015
There are no significant restrictions on the ability of the joint venture to transfer funds to the Group in the form of cash dividends, or to repay loans or advances made by the Group, except for the obligation to establish a legal reserve for 5% of the profit for the year until reaching 20% of the capital.
F-133
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
(iv) S&W Semillas S.A.
This joint venture was formed on Friday, September 18, 2015 with S&W Seed Company, a US-based company that has alfalfa germ-plasm assets, mainly in relation to non-dormant alfalfa varieties), and with Bioceres. Both parties’ equity interest is 50%.
S&W Semillas is a separately structured vehicle created and operating in Argentina.
The main line of business of S&W Semillas is the participation in the development and deregulation of alfalfa events, in line with the main business activity of the Group.
The joint venture agreement sets forth that each of the members must render the necessary services to achieve a mutually agreed work plan and that each party must finance the project in proportion to their equity interest.
Under the service agreement entered into, the Company and S&W Seed Company undertake to grant S&W Semillas the necessary licenses for the development and commercialization of products, and the results obtained from the provision of the services are the property of S&W Semillas.
The agreements set forth that the joint venture shall act as a sales agent for the conventional genetics products of S&W Seed Company in South America, and that S&W Seed Company shall grant considerable financing for these sales of seeds.
The contractual agreement only gives the Company rights over the joint arrangement net assets, and the rights over the assets and obligations related to the liabilities under the joint arrangement will primarily remain with S&W Semillas. Under IFRS 11, this joint arrangement is classified as a joint venture and has been equity-accounted in the separate financial statements.
S&W Semillas shares are not listed for trading in a public offering.
The summarized financial information on the joint venture is disclosed below:
S&W Semillas S.A. | |||||||||
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
CURRENT ASSETS | |||||||||
Other receivables | 8,734 | 8,651 | 346,888 | ||||||
NON-CURRENT ASSETS | |||||||||
Other receivables | 104,853 | 84,311 | 42,347 | ||||||
Deferred tax assets | 173,589 | 186,613 | 141,552 | ||||||
TOTAL ASSETS | 287,176 | 279,575 | 530,787 | ||||||
CURRENT LIABILITIES | |||||||||
Trade and other payables | 95,215 | 87,789 | 50,265 | ||||||
Finance results | 24 | 10 | — | ||||||
TOTAL LIABILITIES | 95,239 | 87,799 | 50,265 | ||||||
Equity before loss for the period / year | 306,062 | 302,591 | 890,867 | ||||||
Loss for the period / year | (114,125 | ) | (110,815 | ) | (410,345 | ) | |||
EQUITY | 191,937 | 191,776 | 480,522 | ||||||
TOTAL EQUITY AND LIABILITIES | 287,176 | 279,575 | 530,787 | ||||||
F-134
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Statement of comprehensive Income or loss | S&W Semillas S.A. | |||||||||||
Period / year ended | ||||||||||||
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
(unaudited) | ||||||||||||
Research and development | (144,682 | ) | (198,187 | ) | (144,020 | ) | (474,088 | ) | ||||
Administrative, Selling and distribution Expenses | (10,682 | ) | — | (4,928 | ) | (2,582 | ) | |||||
Finance results | 10,302 | (21,720 | ) | (5,303 | ) | (25,449 | ) | |||||
Loss before income tax | (145,062 | ) | (219,907 | ) | (154,251 | ) | (502,119 | ) | ||||
Income tax benefit | 30,937 | 51,403 | 43,436 | 91,774 | ||||||||
Loss before income tax | (114,125 | ) | (168,504 | ) | (110,815 | ) | (410,345 | ) | ||||
Other comprehensive income | — | (9,427 | ) | — | — | |||||||
Total comprehensive loss | (114,125 | ) | (177,931 | ) | (110,815 | ) | (410,345 | ) | ||||
The first fiscal year ended December 31, 2015 is an irregular period of 104 days.
As of June 30, 2016, and December 31, 2016 and 2015, the Group recognized in the consolidated financial statements a positive carrying value of the investment in S&W Seeds for USD 95,968, USD 95,968 and USD 240,261, respectively.
There are no significant restrictions on the joint venture’s ability to transfer funds to the Group as cash dividends, or to repay loans or advances made by the Group, except for the obligation to set up a legal reserve for 5% of the profit for the fiscal year until 20% of capital is reached.
(v) AGBM
This joint venture between Porta Hnos. S.A. and Bioceres S.A. was formed in December 2016. The equity interest is 49.999% for both parties and a minority shareholder with a 0.002% non-controlling interest.
AGBM is a separately structured vehicle created and operating in Córdoba, Argentina. AGBM’s main business activity is the industrial production and commercialization of chymosin.
AGBM has a complete portfolio of SPC® technology patents and an industrial plant for the production of chymosin using the molecular farming technology. The production capacity is 2,000,000 liters of chymosin (600 imcu/mL) per year, representing approximately 15% of the global market.
The contractual agreement sets forth that the Group only has rights over the net assets under the joint arrangement. The rights over the assets and obligations for the liabilities assumed under the joint arrangement primarily remain with AGBM. Under IFRS 11, this joint arrangement is classified as a joint venture and has been equity-accounted in the consolidated financial statements. AGBM shares are not listed for trading in a public offering.
F-135
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The summarized financial information relating to the joint venture is disclosed below:
Summary statements of financial position: | ||||||
AGBM S.A. | ||||||
06/30/2017 | 12/31/2016 | |||||
CURRENT ASSETS | ||||||
Cash and cash equivalents | 6,980 | — | ||||
Trade receivables | 21,666 | — | ||||
Other receivables | 30,916 | 9,513 | ||||
Inventories | 1,208,421 | — | ||||
NON-CURRENT ASSETS | ||||||
Other receivables | 440,534 | 35,703 | ||||
Property Plant & Equipment | 1,838,777 | 1,814,083 | ||||
Intangible assets | 1,939,417 | 1,756,191 | ||||
TOTAL ASSETS | 5,486,711 | 3,615,490 | ||||
CURRENT LIABILITIES | ||||||
Trade and other payables | 2,273,955 | — | ||||
Income and minimum presumed income taxes payable | 208 | 35,704 | ||||
TOTAL LIABILITIES | 2,274,163 | 35,704 | ||||
Equity before loss for the period / year | 3,431,187 | 3,579,786 | ||||
Loss for the period / year | (218,639 | ) | — | |||
EQUITY | 3,212,548 | 3,579,786 | ||||
TOTAL EQUITY AND LIABILITIES | 5,486,711 | 3,615,490 | ||||
Statement of comprehensive Income or loss | ||||||
Period / year ended | ||||||
06/30/2017 | 12/31/2016 | |||||
Revenue | 54,186 | |||||
Cost of sales | (37,481 | ) | — | |||
Administrative, Selling and distribution Expenses | (205,581 | ) | — | |||
Finance results | (29,763 | ) | — | |||
Loss before income tax | (218,639 | ) | — | |||
Income tax | — | — | ||||
Loss | (218,639 | ) | — | |||
Other comprehensive loss | — | — | ||||
Total comprehensive loss | (218,639 | ) | — | |||
F-136
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
At December 31, 2016, the Group recognized in the consolidated financial statements a positive carrying value of the investment in AGBM for USD 1,661,080, arising from 49.999% of the net equity from which is deducted the result obtained by the Group upon the transfer of the SPC chymosin molecular farming intangible.
AGBM reported no results in the period ended on December 31, 2016.
As of June 30, 2017, the Group recognized in the consolidated financial statements a positive carrying value of the investment in AGBM for USD 1,483,208.
There are no significant restrictions on the joint venture’s ability to transfer funds to the Group as cash dividends, or to repay loans or advances made by the Group, except for the obligation to set up a legal reserve for 5% of the profit for the fiscal year until 20% of capital is reached.
(vi) Synertech
Synertech has been included in the Bioceres Group after the acquisition of the majority interest in Rizobacter. This joint venture has arisen from the association between Synertech and De Sangosse, which jointly opened a new fertilizer manufacturing plant in Pergamino, Argentina, on June 28, 2017. Both parties’ equity interest is 50%.
Construction and start-up of the new plant required an investment of more than USD 30 million, and was conceived for research, production and commercialization of the Microstar PZ® microgranulated fertilizer, its byproducts and other possible developments in the agricultural fertilization area to supply both the domestic and export markets, such as Brazil, Paraguay, Bolivia, Uruguay, Chile, Central America and North America, among others, accompanying the exponential growth of Rizobacter in the world markets with a strong international insertion.
The contractual agreement sets forth that the Group only has rights over the net assets under the joint arrangement. The rights over the assets and obligations for the liabilities assumed under the joint arrangement primarily remain with Synertech. Under IFRS 11, this joint arrangement is classified as a joint venture and has been equity-accounted in the consolidated financial statements. Synertech shares are not listed for trading in a public offering.
The summarized financial information relating to the joint venture is disclosed below:
Summary statements of financial position: | ||||||
Synertech S.A. | ||||||
06/30/2017 | 12/31/2016 | |||||
Current assets | ||||||
Cash and cash equivalents | 85,654 | 749,952 | ||||
Financial assets at fair value through profit or loss | 10 | 143,686 | ||||
Trade receivables | 1,481,838 | 2,427,430 | ||||
Other receivables | 2,773,524 | 24,817 | ||||
Inventories | 1,623,155 | 116,933 | ||||
Non-current assets | ||||||
Other receivables | 1,449,117 | 771,660 | ||||
Property, plant and equipment(1) | 16,066,496 | 17,918,935 | ||||
Intangible assets(1) | 1,897,589 | 2,010,169 | ||||
Total assets | 25,377,383 | 24,163,582 | ||||
F-137
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Synertech S.A. | ||||||
06/30/2017 | 12/31/2016 | |||||
Current liabilities | ||||||
Trade and other payables | 5,061,342 | 2,468,224 | ||||
Other tax payables | 27,128 | 9,531 | ||||
Borrowings | 1,428,481 | 1,270,472 | ||||
Employee benefits and social security | 70,254 | 22,308 | ||||
Non-current liabilities | ||||||
Trade and other payables | 5,378,861 | 5,854,523 | ||||
Borrowings | 2,898,800 | 3,544,841 | ||||
Deferred tax liabilities(1) | 869,328 | 1,041,195 | ||||
Total liabilities | 15,734,194 | 14,211,094 | ||||
Equity before results | 12,045,743 | 10,273,097 | ||||
Results | (2,402,554 | ) | (320,609 | ) | ||
Total equity | 9,643,189 | 9,952,488 | ||||
Total equity and liabilities | 25,377,383 | 24,163,582 | ||||
| (1) | Included fair value of intangible assets and deferred tax liabilities identified in the purchase price allocation (PPA) of the business combination. |
Statement of comprehensive Income or loss | ||||||
Synertech S.A. Period ended | ||||||
06/30/2017 | 12/31/2016 | |||||
Revenue | 1,386,728 | — | ||||
Cost of sales | (2,055,043 | ) | — | |||
Selling, general and administrative expenses | (545,457 | ) | (288,356 | ) | ||
Other loss | (75,488 | ) | — | |||
Finance result | (1,691,154 | ) | (204,889 | ) | ||
Loss before income tax | (2,980,414 | ) | (493,245 | ) | ||
Income tax benefit | 1,159,476 | 172,636 | ||||
Loss | (1,820,938 | ) | (320,609 | ) | ||
Other comprehensive loss | (581,616 | ) | — | |||
Total comprehensive loss | (2,402,554 | ) | (320,609 | ) | ||
At June 30, 2017 and December 31, 2016, the Group recognized in the consolidated financial statements a positive carrying value of the investment in Synertech for USD 26,844,410 and USD 27,520,779, respectively, arising from 50% of the net equity, with assets and liabilities measured at fair value from the business combination.
There are no significant restrictions on the joint venture’s ability to transfer funds to the Group as cash dividends, or to repay loans or advances made by the Group, except for the obligation to set up a legal reserve for 5% of the profit for the fiscal year until 20% of capital is reached.
F-138
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
(vii) Héritas
At the beginning of 2017, INDEAR subscribed shares at the capital increase decided by the Extraordinary Shareholders’ Meeting of Héritas S.A held in December 2016 and it became a shareholder, with 33% of the capital stock and the possibility of increasing its equity interest up to 40% of Héritas capital stock.
Héritas is the result of an agreement between CIBIC and INDEAR to provide services of precision medical research in the fields of clinical genomics, human microbiome and reproductive genomics.
The summarized financial information relating to the joint venture is disclosed below:
Summary statements of financial position: | |||
Héritas S.A. 06/30/2017 | |||
Current assets | |||
Cash and cash equivalents | 622,354 | ||
Trade receivables | 91,907 | ||
Other receivables | 516,375 | ||
Non-current assets | |||
Income and minimum presumed income taxes recoverable | 72,596 | ||
Total assets | 1,303,232 | ||
Current liabilities | |||
Trade and other payables | 224,483 | ||
Total liabilities | 224,483 | ||
Equity before results | 1,220,832 | ||
Results | (142,083 | ) | |
Total equity | 1,078,749 | ||
Total equity and liabilities | 1,303,232 | ||
Statement of comprehensive Income or loss | |||
Héritas S.A. Period ended | |||
06/30/2017 | |||
Revenue | 121,648 | ||
Cost of sales | (111,511 | ) | |
Research and development expenses | — | ||
Selling, general and administrative expenses | (304,398 | ) | |
Finance result | 75,672 | ||
Loss before income tax | (218,589 | ) | |
Income tax benefit | 76,506 | ||
Loss | (142,083 | ) | |
Other comprehensive loss | — | ||
Total comprehensive loss | (142,083 | ) | |
At June 30, 2017 the Group recognized in the consolidated financial statements a positive carrying value of the investment in Héritas for USD 9,191,102.
F-139
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
There are no significant restrictions on the joint venture’s ability to transfer funds to the Group as cash dividends, or to repay loans or advances made by the Group, except for the obligation to set up a legal reserve for 5% of the profit for the fiscal year until 20% of capital is reached.
13.2. Joint operations
The Group engages in joint arrangements not structured through a separate vehicle with strategic partners with a focus on product development or with governmental entities and internationally recognized research institutions with whom the Group collaborates in pre-competitive technology sourcing and early-stage research.
The collaborative agreements that focus on product development are primarily driven by the Group’s commercial interest in a particular product. Some of the most important collaborations of this nature are:
| - | SenesTech Inc (USA): a service agreement for the development and synthetic production of the triptolíde active ingredient used for the production of ContraPest (a product against rodents) and an agency agreement to deregulate the product ContraPest in Argentina for its future commercialization in that country, through a company to be established between SenesTech and the subsidiary INMET. |
| - | TripCo: TripCo is partnership between SenesTech Inc. and Bioceres Inc. for the synthetic development of the active ingredient used for the production of ContraPest, among other uses. |
| - | YPF (Argentina): A joint research and development project to obtain second generation bioethanol. |
| - | Danziger (Israel): A research and collaboration project to obtain new soybean events with the use and development of Danziger’s technology. |
| - | CO Sistemas S.A. (Argentina): A project to jointly develop technologies and methods for fermentation of starch from Lemna-duckweed as a source of biomass to obtain lactic acid and its biopolymer byproducts. |
| - | IDEN Biotechnology S.L. (Spain): A project for the co-development of technology to obtain wheat varieties resistant to low temperatures. |
| - | INIS Biotech (Argentina): A joint research and development project to obtain transgenic organisms for the cultivation of soybean. |
On the other hand, the collaborations that focus on technology sourcing are primarily driven by the Group`s ability to accelerate in a capital-efficient manner the validation of promising technology discovered by internationally recognized scientific groups or institutions. Some of the most important collaborations of this nature are:
| - | CONICET and National University of the Litoral: for creation of traits, with a focus on transcription factors for the development of drought tolerant transgenic plants. |
| - | INTA (Argentina): an agreement for the multiplication and marketing of wheat bread varieties owned by INTA. |
14. SEGMENT INFORMATION
The Group is organized into four main operating segments:
| - | Seed and integrated products |
The seed and integrated products segment focuses mainly on the development and commercialization of seed technologies and products that increase yield per hectare, with a focus on the provision of seed technologies and integrated crop protection and crop nutrition products designed to control weeds, insects or diseases, to increase their quality characteristics, to improve nutritional value and other benefits. The segment focuses on the commercialization of
F-140
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
integrated products that combine three complementary components biotechnological events, germplasm and seed treatments—in order to increase crop productivity and create value for customers. While each component can increase yield independently, through an integrated technology strategy the segment offers biotechnological events, germplasm and treatments for seeds that complement and integrate with each other to generate higher yields in crops.
Currently the segment generates revenue from ordinary activities through the sale of seeds, integrated packs, royalties and licenses charged to third parties, among others.
| - | Crop protection |
The crop protection segment mainly includes the development, production and marketing of adjuvants, insecticides and fungicides. The adjuvants are used in mixtures to facilitate greater efficiency of the products to be applied (such as fertilizers and agrochemicals). Insecticides and fungicides can significantly reduce disease or insect problems during the germination period.
The segment currently generates revenue from ordinary activities through the sale of adjuvants, insecticides, fungicides and baits, among others.
| - | Crop nutrition |
The crop nutrition segment focuses mainly on the development, production and commercialization of inoculants that allow the biological fixation of nitrogen in the crops, and of fertilizers including biofertilizers and microgranulated fertilizers that optimize the productivity and yield of the crops.
Currently the segment generates income from ordinary activities through the sale of inoculants, bio-inductors, biological fertilizers and microgranulated fertilizers, among others
| - | Emerging solutions |
This segment focuses mainly on providing R&D services, biotechnology capabilities and expertise to facilitate the incorporation of technologies and the Group’s product development efforts and R&D services to third parties.
Currently, this segment generates revenues from the provision R&D services by the Group’s R&D subsidiaries, INDEAR and INMET, and to joint ventures, licensees and third parties. These R&D subsidiaries also enter into collaboration agreements in the early stages of research with external research groups as part of the technology development effort. Some of these partnerships are funded through government grants. INDEAR is dedicated to R&D across a wide range of platforms and develops technologies that are used in the seed technology and related industries businesses, and more recently in the field of precision medicine. INMET was created to develop and commercialize environmentally responsible production of fermentation solutions with high-value compounds by converting low-cost feedstock using genetically improved bacteria.
This segment also includes other sources of income mainly related to agribusiness biotechnology businesses, such as those generated by the production and commercialization of industrial enzymes and fermentation technology to convert sugars and oils into molecules or compounds of high value. Currently, income is generated through the production and sale of chymosin, an enzyme used in the manufacture of cheese, through our joint venture, AGBM, established in 2016 to expand the production of chymosin at an industrial level. Other sources of income include fermentation solutions for environmentally responsible production of high-value compounds by converting low-cost raw materials using genetically improved bacteria, including solutions being developed and implemented to replace petroleum products through the use of biodegradable and sustainable biological alternatives.
The measurement principles for the Group’s segment reporting structure are based on the IFRS principles adopted in the consolidated financial statements. Revenue generated by products and services exchanged between segments and entities within the Group are calculated on the basis of market prices.
F-141
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The following tables present information with respect to the Group’s reporting segments:
Period ended June 30, 2017 | Seed and integrated products | Crop protection | Crop nutrition | Emerging solutions | Consolidated | ||||||||||
Revenues | |||||||||||||||
Sale of goods | 8,911,341 | 31,191,970 | 6,640,228 | — | 46,743,539 | ||||||||||
Rendering of Services | — | — | — | 1,214,901 | 1,214,901 | ||||||||||
Royalties | 102,178 | — | — | — | 102,178 | ||||||||||
Licenses | 7,480 | — | — | — | 7,480 | ||||||||||
Government grants | |||||||||||||||
Grants | — | — | — | 273,023 | 273,023 | ||||||||||
Total revenue | 9,020,999 | 31,191,970 | 6,640,228 | 1,487,924 | 48,341,121 | ||||||||||
Cost of sales | (4,851,444 | ) | (22,641,887 | ) | (2,084,652 | ) | (607,463 | ) | (30,185,446 | ) | |||||
Gross margin per segment | 4,169,555 | 8,550,083 | 4,555,576 | 880,461 | 18,155,675 | ||||||||||
% | 46 | % | 27 | % | 69 | % | 59 | % | 38 | % | |||||
Other Income | — | — | — | — | 121,065 | ||||||||||
Operating costs | — | — | — | — | (20,926,031 | ) | |||||||||
Share of loss of joint ventures | — | — | — | — | (786,805 | ) | |||||||||
Financial results | — | — | — | — | (12,809,230 | ) | |||||||||
Loss before income tax | — | — | — | — | (16,245,326 | ) | |||||||||
Income tax | — | — | — | — | 5,090,723 | ||||||||||
Loss | — | — | — | — | (11,154,603 | ) | |||||||||
Period ended June 30, 2016 (unaudited) | Seed and integrated products | Crop protection | Crop nutrition | Emerging solutions | Consolidated | ||||||||||
Revenues | |||||||||||||||
Sale of goods | 828,251 | — | — | 36,236 | 864,487 | ||||||||||
Rendering of Services | — | — | — | 1,313,192 | 1,313,192 | ||||||||||
Royalties | 82,500 | — | — | — | 82,500 | ||||||||||
Licenses | 8,014 | — | — | — | 8,014 | ||||||||||
Government grants | |||||||||||||||
Grants | — | — | — | 270,206 | 270,206 | ||||||||||
Total revenue | 918,765 | — | — | 1,619,634 | 2,538,399 | ||||||||||
Cost of sales | (790,671 | ) | — | — | (499,585 | ) | (1,290,256 | ) | |||||||
Gross margin per segment | 128,094 | — | — | 1,120,049 | 1,248,143 | ||||||||||
% | 14 | % | 69 | % | 49 | % | |||||||||
Operating costs | — | — | — | — | (4,809,719 | ) | |||||||||
Share of loss of joint ventures | — | — | — | — | (455,182 | ) | |||||||||
Net financing costs | — | — | — | — | (789,136 | ) | |||||||||
Loss before income tax | — | — | — | — | (4,805,894 | ) | |||||||||
Income tax | — | — | — | — | 1,040,923 | ||||||||||
Loss | — | — | — | — | (3,764,971 | ) | |||||||||
F-142
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Year ended December 31, 2016 | Seed and integrated products | Crop protection | Crop nutrition | Emerging solutions | Consolidated | ||||||||||
Revenues | |||||||||||||||
Sale of goods | 13,879,750 | 20,719,445 | 5,227,394 | 37,334 | 39,863,923 | ||||||||||
Rendering of Services | — | 773,974 | — | 2,584,720 | 3,358,694 | ||||||||||
Royalties | 349,760 | — | — | — | 349,760 | ||||||||||
Licenses | 15,457 | — | — | — | 15,457 | ||||||||||
Other | — | — | — | — | — | ||||||||||
Government grants | |||||||||||||||
Grants | — | — | — | 761,429 | 761,429 | ||||||||||
Total revenue | 14,244,967 | 21,493,419 | 5,227,394 | 3,383,483 | 44,349,263 | ||||||||||
Cost of sales | (8,895,386 | ) | (16,825,572 | ) | (4,819,455 | ) | (1,060,585 | ) | (31,600,998 | ) | |||||
Gross margin per segment | 5,349,581 | 4,667,847 | 407,939 | 2,322,898 | 12,748,265 | ||||||||||
% | 38 | % | 22 | % | 8 | % | 69 | % | 29 | % | |||||
Other Income | — | — | — | — | 48,495 | ||||||||||
Operating costs | — | — | — | — | (15,766,792 | ) | |||||||||
Share of loss of joint ventures | — | — | — | — | (936,769 | ) | |||||||||
Profit of acquisition of control over Semya | — | — | — | — | 4,453,284 | ||||||||||
Financial results | — | — | — | — | (9,916,425 | ) | |||||||||
Loss before income tax | — | — | — | — | (9,369,942 | ) | |||||||||
Income tax | — | — | — | — | 4,140,028 | ||||||||||
Loss | — | — | — | — | (5,229,914 | ) | |||||||||
Year ended December 31, 2015 | Seed and integrated products | Crop protection | Crop nutrition | Emerging solutions | Consolidated | ||||||||||
Revenues | |||||||||||||||
Sale of goods | 4,016,898 | — | — | 166,530 | 4,183,428 | ||||||||||
Rendering of Services | — | — | — | 4,303,738 | 4,303,738 | ||||||||||
Royalties | 354,118 | — | — | — | 354,118 | ||||||||||
Licenses | 124,026 | — | — | — | 124,026 | ||||||||||
Government grants | |||||||||||||||
Grants | — | — | — | 1,230,574 | 1,230,574 | ||||||||||
Total revenue | 4,495,042 | — | — | 5,700,842 | 10,195,884 | ||||||||||
Cost of sales | (3,733,701 | ) | — | — | (1,065,644 | ) | (4,799,345 | ) | |||||||
Gross margin per segment | 761,341 | — | — | 4,635,198 | 5,396,539 | ||||||||||
% | 81.3 | % | 53 | % | |||||||||||
Operating costs | — | — | — | — | (6,769,784 | ) | |||||||||
Net financing costs | — | — | — | — | (394,833 | ) | |||||||||
Share of loss of joint ventures | — | — | — | — | (1,553,022 | ) | |||||||||
Loss before income tax | — | — | — | — | (3,321,100 | ) | |||||||||
Income tax | — | — | — | — | (411,342 | ) | |||||||||
Loss | — | — | — | — | (3,732,442 | ) | |||||||||
F-143
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Revenue by geography | ||||||||||||
Period ended | ||||||||||||
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
Revenue from external customers | (unaudited) | |||||||||||
Argentina | 42,507,131 | 1,573,196 | 38,528,676 | 6,438,440 | ||||||||
Austria | 8,400 | — | 336,795 | — | ||||||||
Brazil | 275,545 | — | 909,142 | — | ||||||||
China | 193,911 | 258,585 | 267,312 | 170,673 | ||||||||
Lebanon | 262,428 | — | — | |||||||||
United States of America | 380,310 | — | 484,202 | 811,470 | ||||||||
Paraguay | 1,052,088 | 622,635 | — | |||||||||
United Kingdom | 129,297 | — | 232,775 | — | ||||||||
South Africa | 1,931,908 | — | 8,654 | — | ||||||||
Ukraine | 822,976 | — | 152,521 | — | ||||||||
Uruguay | 447,248 | 436,412 | 1,641,752 | 1,540,909 | ||||||||
Rest of the world | 56,856 | — | 403,370 | 3,818 | ||||||||
Total income (without grants) | 48,068,098 | 2,268,193 | 43,587,834 | 8,965,310 | ||||||||
As of | |||||||||
06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||
Non-current assets | |||||||||
Argentina | 124,250,950 | 128,642,257 | 6,599,876 | ||||||
United States of America | 631,063 | 495,187 | 471,167 | ||||||
Paraguay | 589,418 | 538,831 | — | ||||||
Brazil | 347,649 | 188,627 | — | ||||||
Bolivia | 22,876 | 25,325 | — | ||||||
South Africa | 22,706 | 25,442 | — | ||||||
Total | 125,864,662 | 129,915,669 | 7,071,043 | ||||||
Revenue by products | ||||||||||||
Period ended | ||||||||||||
06/30/2017 | 06/30/2016 | 12/31/2016 | 12/31/2015 | |||||||||
Seed and integrated products | 9,020,999 | 918,765 | 14,244,967 | 4,495,042 | ||||||||
Seeds, royalties & licences | 2,394,755 | 918,765 | 4,226,284 | 4,495,042 | ||||||||
Packs | 6,626,244 | — | 10,018,683 | — | ||||||||
Crop protection | 31,191,970 | — | 21,493,419 | — | ||||||||
Adjuvants | 15,008,963 | — | 12,526,504 | — | ||||||||
Insecticides & fungicides | 9,914,886 | — | 2,059,936 | — | ||||||||
Other | 6,268,121 | — | 6,906,979 | — | ||||||||
Crop nutritions | 6,640,228 | — | 5,227,394 | — | ||||||||
Inoculants | 4,245,712 | — | 2,098,826 | — | ||||||||
Fertilizers | 2,394,516 | — | 3,128,568 | — | ||||||||
Emerging solutions | 1,487,924 | 1,619,634 | 3,383,483 | 5,700,842 | ||||||||
Research & development | 1,487,924 | 1,583,398 | 3,346,149 | 5,534,312 | ||||||||
Agro-industrial biotecnology | — | 36,236 | 37,334 | 166,530 | ||||||||
Total revenue | 48,341,121 | 2,538,399 | 44,349,263 | 10,195,884 | ||||||||
F-144
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
15. FINANCIAL INSTRUMENTS – RISK MANAGEMENT
15.1. Principal financial instruments
The principal financial instruments used by the Group, from which financial instrument risk arise, are as follows:
| - | Cash and cash equivalents |
| - | Financial assets at fair value through profit or loss |
| - | Trade receivables |
| - | Other receivables |
| - | Trade and other payables |
| - | Bank overdrafts |
| - | Other loans |
| - | Financed payment for the acquisition of business |
| - | Puttable instruments |
The Group is exposed to the following financial risks that arise from its activities and from its use of financial instruments:
| - | Credit risk |
| - | Liquidity risk |
| - | Currency risk |
| - | Interest rate risk |
| - | Market risk |
This Note provides information on the Group’s exposure to each of the above risks, the Group’s objectives, policies and processes regarding the measurement and management of each risk.
The Group does not use derivative financial instruments to hedge any of the above risks.
F-145
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
15.2. Financial instruments by category
The following tables show additional information required under IFRS 7 on the financial assets and liabilities recorded as of June 30, 2017, December 31, 2016 and 2015.
Amortized Cost | Mandatorily measured at fair value through profit or loss | |||||||||||||||||
Financial asset | 06/30/2017 | 12/31/2016 | 12/31/2015 | 06/30/2017 | 12/31/2016 | 12/31/2015 | ||||||||||||
Cash and Cash Equivalents | 2,119,883 | 1,256,696 | 58,425 | — | — | — | ||||||||||||
Other financial assets | 4,260,517 | — | — | 3,559,201 | 7,637,380 | 844,000 | ||||||||||||
Trade Receivables | 45,693,673 | 57,033,051 | 5,498,974 | — | — | — | ||||||||||||
Other Receivables(*) | 10,304,989 | 9,922,740 | 4,695,081 | — | — | — | ||||||||||||
Total | 62,379,062 | 68,212,487 | 10,252,480 | 3,559,201 | 7,637,380 | 844,000 | ||||||||||||
| (*) | Not include advances, deferred expenses and tax balances |
Amortized Cost | Mandatorily measured at fair value through profit or loss | |||||||||||||||||
Financial liability | 06/30/2017 | 12/31/2016 | 12/31/2015 | 06/30/2017 | 12/31/2016 | 12/31/2015 | ||||||||||||
Trade Payables and other payables | 23,748,712 | 37,050,214 | 7,091,742 | — | — | — | ||||||||||||
Borrowings | 92,127,476 | 77,746,010 | 3,030,783 | — | — | — | ||||||||||||
Financed payment - Acquisition of business | 59,880,365 | 62,562,187 | — | — | — | — | ||||||||||||
Puttable Instruments | 2,508,467 | 2,397,397 | 1,612,168 | 2,500,000 | 2,500,000 | — | ||||||||||||
Total | 178,265,020 | 179,755,808 | 11,734,693 | 2,500,000 | 2,500,000 | — | ||||||||||||
15.3. Financial instruments measured at fair value
Fair value by hierarchy
According to the requirements of IFRS 7, the Group classifies each class of financial instrument valued at fair value into three levels, depending on the relevance of the judgment associated to the assumptions used for measuring the fair value.
Level 1 comprises financial assets and liabilities with fair values determined by reference to quoted prices (unadjusted) in active markets for identical assets or liabilities;
Level 2 comprises inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. derived from prices);
Level 3 comprises financial instruments with inputs for estimating fair value that are not based on observable market data.
F-146
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Measurement at fair value at 06/30/2017 | Level 1 | Level 2 | Level 3 | ||||||
Financial assets at fair value | |||||||||
Other financial assets | 266,442 | — | 3,292,757 | ||||||
Financial liabilities valued at fair value | |||||||||
Puttable Instruments | — | 2,500,000 | — | ||||||
Measurement at fair value at 12/31/2016 | Level 1 | Level 2 | Level 3 | ||||||
Financial assets at fair value | |||||||||
Other financial assets | 4,558,463 | — | 3,078,917 | ||||||
Financial liabilities valued at fair value | |||||||||
Puttable Instruments | — | 2,500,000 | — | ||||||
Measurement at fair value at 12/31/2015 | Level 1 | Level 2 | Level 3 | ||||||
Financial assets at fair value | |||||||||
Financial assets at fair value through profit or loss | 844,000 | — | — | ||||||
Estimation of fair value
The fair value of marketable securities is calculated using the market approach using quoted prices in active markets for identical assets (Level 1 of the fair value hierarchy).
The income approach method used for the valuation of the participation of Chemotécnica requires the use of assumptions, including: sales projections, EBITDA, rate marginal of taxes, weighted average cost of capital and discount rate. Management has developed these assumptions on the basis of historical knowledge of the business and the projected financial information of the Company analyzed. These assumptions may vary depending on the occurrence of future events, perceptions of different market participants and other factors outside the control of the Company, and such variations may be significant in comparison to estimated values. The sensitivity analysis for the discounted cash flows was based on a 13.5% reduction in the discount rate or a 10% reduction in future cash flows. Those decreases in isolation would have increased / decreased the amount of the financial asset in USD 2.0 million, USD 0.6 million, respectively
The Group’s financial liabilities, which were not traded in an active market were determined using valuation techniques that maximize the use of available market information, and thus rely as little as possible on specific estimates of the entity. The rates applicable to similar loans in the market (Level 2 of the fair value hierarchy) were compared.
The Group’s policy is to recognize transfers between different categories of the fair value hierarchy at the time they occur or when there are changes in the circumstances that cause the transfer.
There were no transfers between levels of the fair value hierarchy. There were no changes in economic or business circumstances affecting fair value.
F-147
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
15.4. Financial instruments not measured at fair value
The financial instruments not measured at fair value include cash and cash equivalents, trade accounts receivable, other accounts receivable, trade payables and other debts, loans, financed payments and instruments with a put option.
The carrying value of financial instruments not measured at fair value does not differ significantly from their fair value.
Management estimates that the carrying value of the financial instruments measured at amortized cost approximates their fair value.
15.5. General objectives, policies and processes
The Board of Directors has overall responsibility for establishing and monitoring the Group’s risk management objectives and policies and, whilst retaining ultimate responsibility for them, it has delegated the function to design and operate processes that ensure the effective implementation of the objectives and policies to the Group’s finance function that periodically reports to the Board of Directors on the evolution of the risk management activities and results. The overall objective of the Board of Directors is to set policies that seek to reduce risk as much as possible without unduly affecting the Group’s competitiveness and flexibility.
The Group’s risk management policy is established to identify and analyze the risks facing the Group, to set appropriate risk limits and controls and to monitor risks and adherence to limits. The risks and methods for managing the risks are reviewed regularly in order to reflect changes in market conditions and the Group’s activities. The Group, through training and management standards and procedures, aims to develop a disciplined and constructive control environment in which all the employees understand their roles and obligations.
The Group seeks to use suitable means of financing to minimize the Group’s capital costs and to manage and control the Group’s financial risks effectively. There have been no substantive changes in the Group’s exposure to financial instrument risks, its objectives, policies and processes for managing those risks or the methods used to measure them from previous periods unless otherwise stated in this note.
The Group adopted a code of ethics applicable to its principal executive, financial and accounting officers and all persons performing similar functions.
The principal risks and uncertainties facing the business, set out below, do not appear in any particular order of potential materiality or probability of occurrence.
a) Credit risk
Credit risk is the risk of financial loss to the Group if a customer or counterparty fails to meet its contractual obligations, which derives mainly from trade and other receivables, as well as from cash and deposits in financial institutions.
The credit risk to which the Group is exposed is mainly defined in the Group’s accounts receivable followed by cash and cash equivalents, with the logical importance of being able to satisfy the Group’s needs in the short term.
Trade and other receivables
Credit risk is the risk of financial loss to the Group if a customer or counterparty fails to meet its contractual obligations, and derives mainly from trade receivables and other receivables generated by services and product sales, as well as from cash and deposits in financial institutions. The Group is also exposed to political and economic risk events, which may cause nonpayment of local and foreign currency obligations to the Group owed by customers, partners, contractors and suppliers.
F-148
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The Group sells seeds and integrated products, crop protection products, crop nutrition products, and offers R&D and other products and services to a diverse base of customers. Customers include multi-national and local agricultural companies, distributors, research and educational institutions and farmers who purchase the Group’s seed products, integrated products, crop protection products, crop nutrition products and the Group’s R&D and technical services. Type and class of customers may differ depending on the Group’s business segments.
The Group’s finance function determines concentrations of credit risk by periodically monitoring the credit worthiness rating of existing customers and through a monthly review of the trade receivables’ aging analysis. In monitoring the customers’ credit risk, customers are grouped according to their credit characteristics.
The Group’s policy is to manage credit exposure to counterparties through a process of credit rating. The Group performs credit evaluations of existing and new customers, and every new customer is examined thoroughly regarding the quality of its credit before offering the customer transaction terms. The examination made by the Group includes outside credit rating information, if available. Additionally, and even if there is no independent outside rating, the Group assesses the credit quality of the customer taking into account its financial position, past experience, bank references and other factors. A credit limit is prescribed for each customer. These limits are examined annually. Customers that do not meet the Group’s criteria for credit quality may do business with the Group on a prepayment basis or by furnishing collateral satisfactory to the Group. The Group may still seek collateral and guarantees as it may consider appropriate regardless the credit profile of any customer.
In monitoring customer credit risk, the customers are grouped according to a characterization of their credit, based on geographical location, industry, aging of receivables, maturity, and existence of past financial difficulties. Customers defined as “high risk” are classified into the restricted customer list and are supervised by management. In a case of a doubtful debt, the Group records a provision for the amount of the debt less the value of the collateral provided and acts to realize the collateral.
To cover trade receivables related to Rizobacter and its subsidiaries, the Group has taken out credit insurance from Grupo Insur SRL, which periodically analyzes its customer portfolio and currently covers 50% of the portfolio.
The financial statements contain specific provisions for doubtful debts, which properly reflect, in Management’s estimate, the loss embedded in debts, the collection of which is doubtful.
The maximum exposure to credit risk is represented by the carrying amount of each financial asset in the statement of financial position after deducting any impairment allowance.
Cash and deposits in banks
The Group is exposed to counterparty credit risk on cash and cash equivalent balances. The Group holds cash on deposit with a number of financial institutions. The Group manages its credit risk exposure by limiting individual deposits to clearly defined limits. The Group only deposits with high quality banks and financial institutions.
The maximum exposure to credit risk is represented by the carrying amount of cash and cash equivalents in the statement of financial position.
b) Liquidity risk
Liquidity risk is the risk that the Group will encounter difficulty in meeting its financial obligations when they come due.
The Group’s approach to managing its liquidity risk is to manage the profile of debt maturities and funding sources, maintaining sufficient cash, and ensuring the availability of funding from an adequate amount of committed credit
F-149
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
facilities. The Group’s ability to fund its existing and prospective debt requirements is managed by maintaining diversified funding sources with adequate committed funding lines from high quality lenders.
The liquidity risk of each of the Group entities is managed centrally by the Group’s finance function.
The cash flow forecast is determined at both an entity level and consolidated level. The forecasts are reviewed by the Board of Directors in advance, enabling the Group’s cash requirements to be anticipated. The Group examines the forecasts of its liquidity requirements in order to ascertain that there is sufficient cash for the operating needs, including the amounts required in order to settle financial liabilities.
The following table sets out the contractual maturities of financial liabilities:
As of June 30, 2017 | Up to 3 months | 3 to 12 months | Between one and three years | Between three and five years | Subsequent years | ||||||||||
Trade Payables and other payables | 18,674,805 | 5,073,903 | — | — | — | ||||||||||
Borrowings | 15,333,457 | 41,207,517 | 26,955,690 | 18,773,427 | — | ||||||||||
Financed payment - Acquisition of business | 24,882,332 | 2,937,500 | 36,277,500 | — | — | ||||||||||
Contingent consideration - Acquisition of business | — | — | 17,300,000 | — | — | ||||||||||
Puttable Instruments | — | — | 1,176,549 | 5,889,427 | 518,375 | ||||||||||
Total | 58,890,594 | 49,218,920 | 81,709,739 | 24,662,854 | 518,375 | ||||||||||
As of June 30, 2017, December 31, 2016 and 2015, the Group had no exposure to derivative liabilities.
The current actions being carried out by the Group for planning to meet payments and BAF debt extension were explained in Note 1.
c) Currency risk
Foreign currency risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in foreign exchange rate. Currency on foreign exchange risk arises when the Group enters into transactions denominated in a currency other than its functional currency. A significant part of our business activities is conducted in Argentine pesos. However, some of our subsidiaries using the Argentine peso as their functional currency also have significant transactions denominated in U.S. dollars, mainly with respect to sales and financing activities.
Our policy is, where possible, to allow the Group entities to settle liabilities denominated in U.S. dollars with the cash generated from their own operations in U.S. dollars. We have liabilities denominated in U.S. dollars in entities utilizing the Argentine peso as functional currency, which expose us to foreign currency exchange risks. Such risks are partially mitigated by our revenues, which are also partly denominated in U.S. dollars (mainly exports) or Argentine pesos but adjusted to reflect changes in U.S. Dollars.
We do not use foreign exchange derivatives to hedge our foreign exchange rate exposure. We periodically evaluate the use of derivatives and other financial instruments to hedge our foreign exchange rate exposure, but do not have any exchange rate related financial instruments in place.
F-150
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The table below sets forth our net exposure to currency risk as of June 30, 2017 and December 31, 2016 and 2015
Net foreign currency position | 06/30/2017 | 12/31/2016 | 12/31/2015 | ||||||
Amount expressed in USD | (69,106,000 | ) | (47,511,149 | ) | 1,212,452 | ||||
Considering only this net currency exposure at June 30, 2017, if an Argentine peso/US dollar revaluation or depreciation in relation to other foreign currencies with the remaining variables remaining constant, would have a positive or a negative impact on comprehensive income as a result of foreign exchange gains or losses.
We estimate that a devaluation of the Argentine peso against the U.S. dollar of 20% during the transition period ended June 30, 2017 would have resulted in a net pre-tax loss of approximately USD 13.8 million. We estimate that an appreciation of the Argentine peso against the U.S. dollar of 20% during the same period would have resulted in a net pre-tax gain of approximately USD 13.8 million.
d) Interest Rate risk
The Group’s financing costs may be affected by interest rate volatility. Borrowings under the Group’s interest rate management policy may be fixed or floating rate. The Group maintains adequate committed borrowing facilities and holds most of its financial assets primarily in cash or checks collected from customers that are readily convertible into known amounts of cash.
The Group’s interest rate risk arises from long-term borrowings. Borrowings issued at floating rates expose the Group to cash flow interest rate risk. Borrowings issued at fixed rates expose the Group to fair value interest rate risk.
The Group has not entered into derivative contracts to hedge this exposure.
The Group’s debt composition, consisting of the loans and the payment financed for the acquisition of Rizobacter, is set out below.
06/30/2017 | |||
Carrying amount | |||
Fixed-rate instruments | |||
Current financial liabilities | 70,678,974 | ||
Non-current financial liabilities | 73,798,769 | ||
Variable-rate instruments | |||
Current financial liabilities | 6,818,938 | ||
Non-current financial liabilities | 711,157 | ||
During the transition period ended June 30, 2017, an increase (decrease) in 1 percentage point in the rate related to the portion of financial liabilities with variable interest rate and considering all other variables would have resulted in an increase (decrease) in the profit for the year of $ 0.4 million
The Company does not use derivative financial instruments to hedge its interest rate risk exposure.
F-151
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
e) Market risk
The shares of S&W Seed Company held in the portfolio expose the Group to market risk derived from fluctuations in the market price of the shares.
The estimated effect of an appreciation in the value per share of USD 0.5 at June 30, 2017 would have resulted in a pre-tax gain of approximately USD 31,230. The estimated effect of a decrease in the value per share of USD 0.5 at June 30, 2017 would have resulted in a pre-tax loss of approximately USD 31,230.
The Group’s policy to manage market risk derived from the fluctuations in the market price of the S&W Seed Company shares consists of monitoring the fluctuations in the market price of those shares and analyzing whether or not those variations will revert in the short term.
5) Capital risk management
The Group’s objectives when managing capital are to safeguard the Group’s ability to continue as a going concern, in order to provide returns for shareholders and benefits for other stakeholders, and to maintain an optimal capital structure to reduce the cost of capital.
The Group manages its capital structure and makes adjustments to it in the light of changes in economic conditions and the risk characteristics of the underlying assets. In order to maintain or adjust the capital structure, the Group may adjust the amount of any dividends it could pay to shareholders, return capital to shareholders, issue new shares, or sell assets to reduce debt.
The current actions being carried out by the Group for the management of capital were explained in Note 1.
16. LEASES
16.1. Finance lease - lessee
Detail of leased equipment:
Giver | Object of the contract | Number of leasing contracts | Present Value of Minimum Payments | ||||
HP Financial Services | Computer equipment | 8 | 299.486 | ||||
Banco Comafi | Machinery and equipment | 2 | 58.691 | ||||
Banco Galicia | Machinery and equipment | 1 | 189.839 | ||||
Banco Supervielle | Machinery and equipment | 1 | 597.030 | ||||
Banco Galicia | Vehicles | 2 | 647 | ||||
Totals | 1.145.693 | ||||||
Such contracts were classified as finance leases as the rental period amounts to the estimated useful economic life of the assets concerned and the Group has the right to purchase the assets outright at the end of the minimum lease term by paying a low nominal amount. The leases are payable in ARS.
F-152
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Future minimum lease payments due as follows:
Nominal value - Minimum payments of financial leases
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Less than 1 year | 701,025 | 767,105 | 8,317 | ||||||
1 year- 5 years | 961,476 | 1,224,799 | 4,005 | ||||||
Total | 1,662,501 | 1,991,904 | 12,322 | ||||||
Financial charges to be accrued | (516,819 | ) | (709,937 | ) | (1,758 | ) | |||
Debt for financial leases | 1,145,682 | 1,281,967 | 10,564 | ||||||
Present value - Minimum payments of financial leases
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Less than 1 year | 466,689 | 457,613 | 6,559 | ||||||
1 year- 5 years | 678,993 | 824,354 | 4,005 | ||||||
Total | 1,145,682 | 1,281,967 | 10,564 | ||||||
There are no contingent rents related to finance leases and no subleases. There are no material restrictions imposed by lease arrangements.
16.2. Operating lease - lessee
The Group maintains leased land under operating leases.
06/30/2017 | 12/31/2016 | 12/31/2015 | |||||||
Less than 1 year | — | 6,798 | 83,254 | ||||||
1 year- 5 years | — | — | 333,017 | ||||||
Total | — | 6,798 | 416,271 | ||||||
Minimum lease payments are denominated in USD and payable in ARS at the USD/ARS exchange rate applicable as of the payment date.
The total lease payments recognized as expenses during the transition period ended June 30, 2017, the years ended December 31, 2016, 2015 and for the period ended June 30, 2016 are:
06/30/2017 | 06/30/2016 (unaudited) | 12/31/2016 | 12/31/2015 | |||||||||
Minimum lease payments | 40,469 | 27,898 | 83,564 | 90,607 | ||||||||
Total | 40,469 | 27,898 | 83,564 | 90,607 | ||||||||
There are no subleases. There are no material restrictions imposed by lease arrangements.
F-153
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
17. SHAREHOLDERS AND OTHER RELATED PARTIES BALANCES AND TRANSACTIONS
During the transition period ended June 30, 2017, the years ended December 31, 2016, 2015 and the six-month period ended June 30, 2016, the transactions between the Group and related parties, and the related balances owed by and to them, are as follows:
Amount of the transactions | |||||||||||||
Party | Transaction type | 06/30/2017 | 06/30/2016 (unaudited) | 12/31/2016 | 12/31/2015 | ||||||||
Joint ventures | R & D Sales and Services | 546,859 | 741,370 | 1,466,358 | 2,374,985 | ||||||||
Joint ventures | Equity Contributions | 1,450,357 | 82,804 | 1,712,395 | 2,031,409 | ||||||||
Joint ventures | Business combination | — | — | 29,143,156 | — | ||||||||
Joint ventures | Earned interests | 72,445 | 53,638 | 116,379 | 99,326 | ||||||||
Key management personnel | Salaries, social security benefits and benefits | 823,023 | 675,253 | 1,131,510 | 732,655 | ||||||||
Key management personnel | Interest loans | — | (1,867 | ) | (1,867 | ) | (15,666 | ) | |||||
Shareholders and other related parties | Dividends | (4,359 | ) | (13,790 | ) | (13,790 | ) | — | |||||
Shareholders and other related parties | Puttable instruments | — | (814,570 | ) | (602,012 | ) | 204,085 | ||||||
Shareholders and other related parties | Receipt of own shares by subsidiary | — | (270,342 | ) | (270,342 | ) | — | ||||||
Shareholders and other related parties | Receipt of own shares in business combination | — | — | (456,480 | ) | — | |||||||
Shareholders and other related parties | Sales of goods and services | 1,060,954 | 180,551 | 3,312,186 | 2,432,767 | ||||||||
Shareholders and other related parties | Purchases of goods and services | (514,962 | ) | (882,495 | ) | (2,192,714 | ) | (3,906,452 | ) | ||||
Total | 3,434,317 | (249,448 | ) | 33,344,779 | 3,953,109 | ||||||||
Amounts receivable from related parties
Amounts receivable from related parties | ||||||||||
Party | Transaction type | 06/30/2017 | 12/31/2016 | 12/31/2015 | ||||||
Shareholders and other related parties | Trade receivables | 4,217,840 | 1,868,899 | 2,002,887 | ||||||
Shareholders and other related parties | Allowance for impairment | (206,196 | ) | (135,938 | ) | (94,274 | ) | |||
Shareholders and other related parties | Deferred checks | — | 59,090 | 719,977 | ||||||
Shareholders and other related parties | Other accounts receivable | 137,859 | 1,493,838 | 143,574 | ||||||
Shareholders and other related parties | Advances to suppliers | 98,167 | 453,399 | 470,414 | ||||||
Joint ventures | Trade receivables | 592,700 | 593,941 | 1,280,822 | ||||||
Joint ventures | Other accounts receivable | 9,654 | 3,873 | 2,800 | ||||||
Joint ventures | Loans and other receivables | 3,812,469 | 3,601,925 | 1,383,134 | ||||||
Total | 8,662,493 | 7,939,027 | 5,909,334 | |||||||
F-154
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Amounts payable to related parties
Amounts payable to related parties | ||||||||||
Party | Transaction type | 06/30/2017 | 12/31/2016 | 12/31/2015 | ||||||
Key management personnel | Salaries, social security benefits and benefits | 74,893 | 65,774 | 60,847 | ||||||
Shareholders and other related parties | Trade payables | 2,113,149 | 1,570,497 | 2,146,162 | ||||||
Shareholders and other related parties | Puttable instruments | 2,508,467 | 2,397,397 | 1,612,168 | ||||||
Joint ventures | Other accounts receivable | 427,092 | 6,705 | 6,185 | ||||||
Total | 5,123,601 | 4,040,373 | 3,825,362 | |||||||
The Group has collateral for compliance with the obligations arising under the Credit Agreement entered into with Banco Patagonia, in the form of a pledge on 11,000 shares in the Company, owned by certain shareholders, as a guarantee of a maximum credit amount of ARS 8,000,000.
18. KEY MANAGEMENT PERSONNEL COMPENSATION
Key management personnel are those persons having authority and responsibility for planning, directing and controlling the activities of the Group.
The compensation of directors and other members of key management personnel, including social contributions and others benefits, was as follows for the transition period ended June 30, 2017, 2016 and for the years ended December 31, 2016 and 2015:
Period ended | ||||||||||||
06/30/2017 | 06/30/2016 (unaudited) | 12/31/2016 | 12/31/2015 | |||||||||
Salaries, social security and others benefits | 542,230 | 236,784 | 649,410 | 696,517 | ||||||||
Incentives based on stock options | 280,793 | 438,469 | 482,100 | 36,138 | ||||||||
Total | 823,023 | 675,253 | 1,131,510 | 732,655 | ||||||||
The Company entered into indemnification agreements with each of its directors and executive officers. These agreements generally provide that the relevant director or officer will be indemnified by the Company to the fullest extent permitted by law against liability and against all expenses reasonably incurred or paid by him or her in connection with any claim, action, suit or proceeding which he or she becomes involved as a party or otherwise by virtue of his or her being or having been such a director or officer of the Company and against amounts paid or incurred by him or her in the settlement thereof.
The agreements are subject to certain exceptions, including that no indemnification will be provided to any director or officer against any liability to the Group or its shareholder (i) by reason of intentional fraudulent conduct, dishonesty, willful misconduct, or gross negligence on the part of the director or officer; or (ii) by reason of payment made under an insurance policy or any third party that has no recourse against the indemnitee director or officer.
The compensation of key executives is determined by the Board of Directors based on the performance of individuals and market trends.
The Group currently does not pay any compensation to any of its non-employee board members.
F-155
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
19. SHARE-BASED PAYMENTS
In December 2014, the Company’s shareholders approved the issue of options to purchase shares (“Incentive Option Plan”) that can be exercised in relation to up to 632,000 common shares and up to 632,000 shares to be granted as an incentive to the employees (“Share Incentive Plan”). On December 16, 2015 purchase options were granted to certain Group executives with respect to 464,520 ordinary shares in accordance with the Argentine Capital Markets Law. At the date of issue of these consolidated financial statements, no benefits were granted by means of the Share Incentive Plan.
Under the Incentive Option Plan, options on Company shares were granted to certain Group executives and directors (the beneficiaries). The exercise price of such options is USD 15.85. Stock options are vested when the beneficiaries complete a specified period of service, from the grant date (December 16, 2015) to the expiration date (April 1, 2017), on condition that the initial public offering of the Company shares is successful. In order to be able to exercise the purchase option, the beneficiaries must remain with the Group at the date of exercise. Purchase options on shares can be exercised during a 2-year period after expiration (i.e., until April 1, 2019). The shares that can be purchased through exercising the options may not exceed, in the aggregate, 5% of the Company’s ordinary shares then outstanding after the shares subject to the option have been issued.
Beneficiaries may request financing from the Company, up to 100% of the option exercise price, and to that end, the Company will determine the period of the loan, the interest payable with respect thereto, the payment schedule, the method of payment for interest and principal, the causes for early repayment (which will include, among others, the termination of the eligible job position for the beneficiary), and the guarantees (including, but not limited to, the issuance of promissory notes, deferred payment checks and/or the setting up of a senior pledge on the shares under the option).
The Incentive Option Plan will be administered and implemented by the Company’s Compensation Committee. The Incentive Option Plan will expire in December 2024.
The fair value of the purchase options on shares at the grant date is estimated using the Black-Scholes formula, considering the terms and conditions under which the options were granted, and adjusted to consider the possible dilutive effect of the future exercise of the share options granted on their estimated fair value at grant date, as established in paragraph B41 of IFRS 2. Non-market related service and performance conditions are taken into account when determining the number of equity instruments that will vest. There are no cash payment alternatives.
The charge to income/loss for the transition period ended June 30, 2017 and the six-month period ended June 30, 2016 as well as the fiscal years ended December 31, 2016 and 2015 related to share-based payments (corresponding to equity-settled share-based payment transactions) was USD 374,724, USD 585.955, USD 644,261 and USD 48,293, respectively and was recognized as follows:
| - | Under the Administrative, selling and other expenses line, in the statement of comprehensive income: USD 257,765, USD 183,838, USD 512,941 and USD 48,293 for the period ended June 30, 2017 and 2016 and the fiscal years ended December 31, 2016 and 2015, respectively. |
| - | Under the Research expenses line, in the statement of comprehensive income: USD 117,356, USD 183,838 and USD 131,320 for the period ended June 30, 2017 and 2016 and the fiscal year ended December 31, 2016, respectively |
There were no modifications or cancellations of agreements in the period ended June 30, 2017 and 2016 and the fiscal years ended December 31, 2016 and 2015.
F-156
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
The number, weighted average exercise price, and activity in share options during the period ended June 30, 2017 and the years ended December 31, 2016 and 2015 are shown in the table below.
06/30/2017 | 12/31/2016 | 12/31/2015 | ||||||||||||||||
Number of options | Weighted average exercise price | Number of options | Weighted average exercise price | Number of options | Weighted average exercise price | |||||||||||||
At the beginning | 464,520 | 15.85 | 464,520 | 15.85 | — | — | ||||||||||||
Granted during the period / year | — | — | — | — | 464,520 | 15.85 | ||||||||||||
Annulled during the period /year | — | — | — | — | — | — | ||||||||||||
Exercised during the period / year | — | — | — | — | — | — | ||||||||||||
Expired during the period / year | — | — | — | — | — | — | ||||||||||||
Effective at period / year | 464,520 | 15.85 | 464,520 | 15.85 | 464,520 | 15.85 | ||||||||||||
The Incentive Option Plan was calculated considering 50-1 split that was authorized by the shareholders of the Company on December 17, 2014. On April 27, 2017, the shareholders approved a modification to the split such that it is nowa 100-1 split of the Company’s ordinary shares and an increase of 24,000,000 shares of nominal value ARS 1.
For the share purchase options effective as of June 30, 2017, December 31, 2016 and 2015, the exercise price is USD 15.85 and the remaining weighted average contractual life of such options is approximately 1.75, 2.25 years and 3.25 years, respectively.
The fair value of the share options granted and effective during the period ended June 30, 2017 and the years ended December 31, 2016 and 2015 and the factors considered for its calculation are shown in the table below:
Factor | Incentive Option Plan |
Weighted average fair value at measurement date (per option) | USD 4.09 |
Value of the share (per share) | USD 15.85 |
Expected dividend rate | 0% |
Expected volatility | 47.92% |
Risk-free interest rate | 1.05% |
Weighted average expected life of stock options | 2 years |
Weighted average exercise price (per share) | USD 15.85 |
Model used | Black and Scholes (adjusted to consider the possible dilutive effect of the future exercise of options) |
The expected life of the share options is estimated based on the contractual life of the option adjusted according to the expectations about early exercise of the options. To estimate the effect of early exercise of the options, Management considered the factors mentioned in paragraphs B16 to B21 of IFRS 2 as a whole and put forward the hypothesis that the options would be exercised, on the average, at a time somewhat earlier than the halfway point between the expiration date (April 1, 2017) and the cutoff date for exercising the option (April 1, 2019).
F-157
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
Expected volatility was estimated based on the historical volatility in similar public companies. The measure of volatility used is the annualized standard deviation of rates of return on the shares over a period similar to the expected life of the shares, calculated using continuous capitalization.
There are no market-related performance conditions or non-vesting conditions that should be considered for determining the fair value of options.
The Group estimates that 80% of the share options will be exercised, taking into account historical patterns of executives maintaining their jobs and the probability of the exercising the options. This estimate is reviewed at the end of each annual or interim period.
20. CONTINGENCIES, COMMITMENTS AND RESTRICTIONS ON THE DISTRIBUTION OF PROFITS
Funding Agreements for Hahb 4 between Bioceres and private collaborators
Between 2005 and 2007, the Company entered into agreements with various investors in order to obtain funding in the aggregate amount of approximately USD 1,038,000 for research related to early stage technology for the development of drought tolerant crops (¨Hahb 4¨), with some results of this research to be potentially included in the HB4 trait. Such agreements granted the investors the right to receive a percentage of the rights and royalties payable to the Company in the event of the successful commercialization of the resulting technology. As of the date of authorization for issue of these consolidated financial statements, Hahb 4 is incorporated into a leading product being developed by Verdeca solely in respect of drought resistance in soybeans. In consideration of the exclusive license granted to Verdeca to use and commercialize soybean HB4, Bioceres Inc. will receive from Verdeca 15% of the net revenue realized for the commercialization of soybean HB4 which contains HB4 trait and Hahb 4. Thus, upon the successful commercialization of soybean HB4, the Company will be contractually obligated to pay investors up to an aggregate of 54.40% of the royalties received from a portion of the 15% license fee that the Company will receive from Verdeca (i.e. the portion which corresponds to the initial technology or Hahb 4), but not on the total of such income.
Pledged and restricted assets, commitments and restrictions
Pledged and restricted assets at June 30, 2017 are as follows:
Detail | Asset value | Type of restriction | ||
Computer equipment | 87,574 | Leasing | ||
Machinery and equipment | 115,451 | Leasing | ||
Machinery and equipment | 620,905 | Leasing | ||
Vehicles | 11,480 | Leasing | ||
Land and buildings | 197,467 | Mortgage security(a) | ||
Vehicles | 97,142 | Mortgage security(b) | ||
Other financial assets | 4,260,517 | Collateral(c) | ||
Machinery and equipment | 71,753 | Collateral(d) | ||
Machinery and equipment | 44,112 | Collateral(e) | ||
Vehicles | 13,115 | Collateral(e) | ||
Fixtures and fittings | 17,059 | Collateral(e) | ||
Machinery and equipment | 34,593 | Collateral(f) | ||
Totals | 5,571,168 | |||
F-158
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
| a. | On September 29, 2015, Rizobacter assumed, jointly and severally, all of the obligations for partial financing of investments of Synertech. This loan was granted by Banco Provincia de Buenos Aires directly to Synertech for completing the financing stipulated to finalize the construction. The total approved amount is ARS 80,000,000. Also, is guaranteed by the company De Sangosse by 50% through the extension of a stand by Banco Paribas, and a mortgage of the site where the plant of Synertech is currently being built. |
| b. | On June 24, 2016, Rizobacter obtained financing from Compañía Financiera Rombo S.A. for the purchase of five Renault Duster vans to renew the automotive fleet, which is secured by the pledge of such vehicles by Rizobacter. |
| c. | Rizobacter entered into a USD 45 million syndicated loan and signed a guarantee and pledge. On June 13, 2017 Rizobacter granted a pledge of a fixed-term certificate at Banco Galicia for an amount of USD 4,260,517. |
| d. | Corresponds to the guarantee for the CFI loan, granted by the Nuevo Banco de Santa Fe to INDEAR for ARS 750,000. |
| e. | Corresponds to guarantees for the Línea Verde ARS 15 million loan granted by Banco Municipal de Rosario to INDEAR. |
| f. | Guarantee for the FORBIO loan granted to INMET for an amount of ARS 614,000 |
There is a senior pledge set up in favor of BLD Avales S.G.R on 726,250 Class B shares of Bioceres Semillas. This pledge guarantees the obligations undertaken by INDEAR for the Línea Verde loan from Banco Municipal de Rosario before BLD Avales S.G.R. and it will be effective until full compliance with those obligations. If, during the effective term of this agreement the obligations undertaken by INDEAR with BLD Avales S.G.R. are not complied with, BLD Avales S.G.R. shall foreclose on the pledged shares.
Of the total number of shares purchased by RASA Holding, an attachment was levied on 7,600,000 shares representing 19% of Rizobacter capital stock under a provisional remedy ordered by the court in re. “Harnan Miguel, Marcos y Martina vs. Mac Mullen Jorge et al - Provisional Remedies, affecting 44% of Rizobacter’s total capital. Likewise, 30% of dividends that could accrue on the encumbered shares are subject to attachment.
Bioceres Inc. granted BAF a Put Option, giving BAF a right to sell the ordinary shares of Bioceres and/or RASA Holding that BAF acquires in the face of a financing event as defined in the participation rights agreement and/or in the case of a mandatory conversion into common shares of RASA Holding in 5 years’ time, or in the case of a public offering by RASA Holding or Rizobacter. The price of the Put Option is the nominal value of the shares of Bioceres and/or the preferred and common shares of RASA Holding at the time that the pledge was amde and the participation rights agreement was entered into, i.e. on October 14, 2016.
In the context of the loan granted by BAF to Bioceres for USD 12 million, Bioceres set up a pledge in favor of BAF on 800,000 preferred shares, and Bioceres Inc. set up a pledge on 400,000 RASA Holding common shares owned by Bioceres Inc in order to secure the loan.
In the context of the loan granted by BAF to Bioceres Inc. for USD 20 million, Bioceres Inc. pledged 2,000,000 RASA Holding preferred shares in favor of BAF on in order to secure the loan.
21. EVENTS OCCURRING AFTER THE REPORTING PERIOD
On August 10, 2017, the U.S. FDA completed its full review of the safety evaluation for HB4 soybeans, clearing it for use in human food and animal food (pending USDA approval).
On September 11, 2017, the Group sold the 50,600 Bioceres shares that were received by Bioceres Semillas S.A. as payment of the commercial debt that a shareholder had with that subsidiary. The transaction amount of the sale was ARS 7,050,029 (equivalent to USD 408,461).
The Group has extended the maturity of BAF loans until October 2018.
On October 2017, the first due payments of “Financed payment to sellers” have been canceled. For the cancellation of these, the Group obtained a loan with Banco Santander Río for USD 4.5M at an annual rate of 4% and maturing at 120 days.
F-159
BIOCERES S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As of June 30, 2017, December 31, 2016 and 2015, for the six-month transition period ended June 30, 2017,
for the years ended December 31, 2016 and 2015 and for the six-month period ended June 30, 2016
(Amounts in USD, except otherwise indicated)
In addition to those mentioned above, after June 30, 2017, there have been no other situations or circumstances that may require significant adjustments to the consolidated financial statements or require disclosure in the Notes thereto.
The Group analyzed transactions that occurred after June 30, 2017 until the date of approval of these consolidated financial statements.
F-160

Rizobacter Argentina S.A.
Avda. Dr. Arturo Frondizi Nº 1150, Industrial Park
2700 Pergamino (Province of Buenos Aires)
INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
As of September 30, 2017 and June 30, 2017 and for the three-month periods ended on September 30, 2017 and 2016
F-161
Rizobacter Argentina S.A.
Interim Condensed Consolidated Financial Statements
As of September 30, 2017 and June 30, 2017 and for the three-month periods ended on September 30, 2017 and 2016.
Contents
Interim Condensed Consolidated Financial Statements
Interim Condensed Consolidated Statement of Comprehensive Income
Interim Condensed Consolidated Statement of Financial Position
Interim Condensed Consolidated Statement of Changes in Equity
Interim Condensed Consolidated Statement of Cash Flows
Notes to the Interim Condensed Consolidated Financial Statements
F-162
RIZOBACTER ARGENTINA S.A.
INTERIM CONDENSED CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME
For the three-month periods ended September 30, 2017 and 2016 (amounts in US dollars)
Three-month period ended | |||||||||
9/30/2017 | 9/30/2016 | ||||||||
(Unaudited) | |||||||||
Revenue | 3.1 | 32,852,813 | 26,153,338 | ||||||
Cost of sales | 4 | (18,060,896 | ) | (11,608,790 | ) | ||||
Gross income | 14,791,917 | 14,544,548 | |||||||
Administrative expenses | 5 | (2,371,453 | ) | (2,125,642 | ) | ||||
Distribution expenses | 6 | (5,262,815 | ) | (4,668,990 | ) | ||||
Research expenses | 7 | (579,634 | ) | (533,601 | ) | ||||
Other operating expenses, net | 8 | (175,320 | ) | (23,699 | ) | ||||
Operating income | 6,402,695 | 7,192,616 | |||||||
Financial income | 9 | 1,198,552 | 1,000,264 | ||||||
Financial costs | 9 | (5,719,583 | ) | (5,028,406 | ) | ||||
Share of profit / (loss) of joint ventures and associates accounted under the equity method | 10 | 297,939 | (232,404 | ) | |||||
Profit before income tax | 2,179,603 | 2,932,070 | |||||||
Income tax (expense) | (489,761 | ) | (1,052,834 | ) | |||||
PROFIT FOR THE PERIOD | 1,689,842 | 1,879,236 | |||||||
Profit for the period attributable to the equity holders of the parent company | 1,697,872 | 1,854,367 | |||||||
Profit (loss) / attributable to the non-controlling interests | (8,030 | ) | 24,869 | ||||||
1,689,842 | 1,879,236 | ||||||||
Basic and diluted earnings per share attributable to shareholders of the Company (stated in USD per share) | 11 | 0.04 | 0.05 | ||||||
Three-month period ended | ||||||
OTHER CONSOLIDATED COMPREHENSIVE INCOME | 9/30/2017 | 9/30/2016 | ||||
(Unaudited) | ||||||
Profit for the period | 1,689,842 | 1,879,236 | ||||
Other comprehensive income: | ||||||
Items that may be subsequently reclassified to profit and loss | ||||||
Exchange differences on translation of foreign operations | (893,658 | ) | (365,643 | ) | ||
Other comprehensive loss for the period | (893,658 | ) | (365,643 | ) | ||
Total comprehensive income for the period | 796,184 | 1,513,593 | ||||
Comprehensive income / (loss) attributable to: | ||||||
Equity holders of the parent company | 803,696 | 1,487,993 | ||||
Non-controlling interests | (7,512 | ) | 25,600 | |||
796,184 | 1,513,593 | |||||
The accompanying notes are an integral part of these interim condensed consolidated financial statements.
F-163
RIZOBACTER ARGENTINA S.A.
INTERIM CONDENSED CONSOLIDATED STATEMENT OF FINANCIAL POSITION
As of September 30, 2017 and June 30, 2017 (amounts in US dollars)
ASSETS | Notes | 9/30/2017 | 6/30/2017 | ||||||
(Unaudited) | |||||||||
NON-CURRENT ASSETS | |||||||||
Property, plant and equipment | 12 | 36,889,065 | 38,604,264 | ||||||
Intangible assets | 13 | 3,998,382 | 4,208,429 | ||||||
Investment property | 14 | 870 | 906 | ||||||
Investments in joint arrangements | 15 | 4,748,559 | 4,632,304 | ||||||
Other financial assets | 16 | 128,471 | 92,805 | ||||||
Trade and other receivables | 18 | 1,848,596 | 1,810,063 | ||||||
Deferred tax assets | 2,125 | — | |||||||
Total non-current assets | 47,616,068 | 49,348,771 | |||||||
CURRENT ASSETS | |||||||||
Inventories | 17 | 33,981,196 | 27,574,042 | ||||||
Trade and other receivables | 18 | 62,995,027 | 49,354,529 | ||||||
Financial assets at fair value through profit or loss | 19 | 3,990 | 4,275 | ||||||
Income tax credit | 1,209,791 | 1,701,381 | |||||||
Other financial assets | 16 | 4,250,715 | 4,247,669 | ||||||
Cash and cash equivalents | 20 | 1,175,691 | 940,895 | ||||||
Total current assets | 103,616,410 | 83,822,791 | |||||||
Total assets | 151,232,478 | 133,171,562 | |||||||
EQUITY | |||||||||
Share capital and capital adjustments | 7,113,060 | 7,113,060 | |||||||
Reserved profits and other equity components | 16,612,872 | 15,809,176 | |||||||
Non-controlling interests | 41,135 | 48,647 | |||||||
Total equity | 23,767,067 | 22,970,883 | |||||||
LIABILITIES | |||||||||
NON-CURRENT LIABILITIES | |||||||||
Borrowings | 21 | 36,462,304 | 40,450,586 | ||||||
Provisions | 22 | 996,143 | 1,036,998 | ||||||
Deferred tax liabilities | 7,627,799 | 8,234,885 | |||||||
Trade and other payables | 23 | 145 | 151 | ||||||
Employee benefit obligations | 24 | 274,753 | 286,022 | ||||||
Total non-current liabilities | 45,361,144 | 50,008,642 | |||||||
CURRENT LIABILITIES | |||||||||
Borrowings | 21 | 41,280,799 | 33,344,287 | ||||||
Income tax provision | 77,748 | 29,789 | |||||||
Trade and other payables | 23 | 32,898,158 | 20,634,144 | ||||||
Employee benefit obligations | 24 | 5,347,823 | 4,995,477 | ||||||
Customer advances | 2,499,739 | 1,188,340 | |||||||
Total current liabilities | 82,104,267 | 60,192,037 | |||||||
Total liabilities | 127,465,411 | 110,200,679 | |||||||
Total liabilities and equity | 151,232,478 | 133,171,562 | |||||||
The accompanying notes are an integral part of these interim condensed consolidated financial statements.
F-164
RIZOBACTER ARGENTINA S.A.
INTERIM CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
For the three-month periods ended September 30, 2017 and 2016 (amounts in US dollars)
Attributable to the parent company's owners | Non-controlling interests | Total equity | ||||||||||||||||||||||||||||
Item | Share capital | Capital Adjustment | Legal reserve | Statutory reserve | Revaluation Surplus | Foreign currency translation | Retained earnings / (Accumulated losses) | Total | ||||||||||||||||||||||
Balance at June 30, 2016 | 6,541,236 | 571,824 | 1,266,023 | 22,270,432 | 21,676,334 | (23,703,897 | ) | (6,187,416 | ) | 22,434,536 | 46,607 | 22,481,143 | ||||||||||||||||||
Shareholders’ Meeting dated September 23, 2016 | ||||||||||||||||||||||||||||||
Distribution of dividends in cash (1) | — | — | — | (1,436,969 | ) | — | — | — | (1,436,969 | ) | — | (1,436,969 | ) | |||||||||||||||||
Sub-total | — | — | — | (1,436,969 | ) | — | — | — | (1,436,969 | ) | — | (1,436,969 | ) | |||||||||||||||||
Net profit for the period | — | — | — | — | — | — | 1,854,367 | 1,854,367 | 24,869 | 1,879,236 | ||||||||||||||||||||
Other comprehensive income | — | — | — | — | — | (366,374 | ) | — | (366,374 | ) | 731 | (365,643 | ) | |||||||||||||||||
Total comprehensive income | — | — | — | — | — | (366,374 | ) | 1,854,367 | 1,487,993 | 25,600 | 1,513,593 | |||||||||||||||||||
Increase in the participation in subsidiaries | — | — | — | — | — | — | — | — | 363 | 363 | ||||||||||||||||||||
Balance at September 30, 2016 (Unaudited) | 6,541,236 | 571,824 | 1,266,023 | 20,833,463 | 21,676,334 | (24,070,271 | ) | (4,333,049 | ) | 22,485,560 | 72,570 | 22,558,130 | ||||||||||||||||||
Balance at June 30, 2017 | 6,541,236 | 571,824 | 1,266,023 | 20,819,819 | 23,932,888 | (26,788,716 | ) | (3,420,838 | ) | 22,922,236 | 48,647 | 22,970,883 | ||||||||||||||||||
Net profit / (loss) for the period | — | — | — | — | — | — | 1,697,872 | 1,697,872 | (8,030 | ) | 1,689,842 | |||||||||||||||||||
Other comprehensive income | — | — | — | — | — | (894,176 | ) | — | (894,176 | ) | 518 | (893,658 | ) | |||||||||||||||||
Total comprehensive income | (894,176 | ) | 1,697,872 | 803,696 | (7,512 | ) | 796,184 | |||||||||||||||||||||||
Balance at September 30, 2017 (Unaudited) | 6,541,236 | 571,824 | 1,266,023 | 20,819,819 | 23,932,888 | (27,682,892 | ) | (1,722,966 | ) | 23,725,932 | 41,135 | 23,767,067 | ||||||||||||||||||
| (1) | The dividend distributed in cash represents USD 0.07 per share on the share capital at the beginning of the period ended September 30, 2016. |
The accompanying notes are an integral part of these interim condensed consolidated financial statements.
F-165
RIZOBACTER ARGENTINA S.A.
INTERIM CONDENSED CONSOLIDATED STATEMENT OF CASH FLOWS
For the three-month periods ended September 30, 2017 and 2016 (amounts in US dollars)
Notes | 9/30/2017 | 9/30/2016 | |||||||
(Unaudited) | |||||||||
Cash from operating activities | |||||||||
Net profit for the period | 1,689,842 | 1,879,236 | |||||||
Adjustments for: | |||||||||
Income tax | 489,761 | 1,052,834 | |||||||
Property, plant and equipment depreciation | 469,397 | 431,272 | |||||||
Intangible assets amortization | 154,945 | 10,567 | |||||||
Accrued interest | 2,616,112 | 3,041,850 | |||||||
Exchange differences and other financial results, net | (265,806 | ) | 34,588 | ||||||
Bad debts accrual | 382,091 | 720,527 | |||||||
Charges for obsolescence | — | 99,519 | |||||||
Charges for contingencies | 19,923 | 106,738 | |||||||
Share of income / (loss) of joint ventures | (297,939 | ) | 232,404 | ||||||
Gain/loss from the sale of fixed assets | 49,470 | — | |||||||
Changes in operating assets and liabilities: | |||||||||
Increase in inventories | (7,492,930 | ) | (7,649,308 | ) | |||||
Increase in operating receivables | (13,966,157 | ) | (12,653,163 | ) | |||||
Increase in operating liabilities | 14,929,729 | 14,418,621 | |||||||
Interest received | 125,575 | 113,163 | |||||||
Income tax paid | (301,362 | ) | (128,419 | ) | |||||
Net cash flows (used in) / generated from operating activities | (1,397,349 | ) | 1,710,429 | ||||||
Cash from investing activities | |||||||||
Payments of property, plant and equipment | (295,100 | ) | (387,453 | ) | |||||
Loans to joint ventures | (2,054,184 | ) | — | ||||||
Payment of intangible assets | (66,868 | ) | (263,765 | ) | |||||
Proceeds from sale of property, plant and equipment | 129,230 | — | |||||||
Net cash flow used in investing activities | (2.286,922 | ) | (651,218 | ) | |||||
Cash from financing activities | |||||||||
Interest paid | (2,112,860 | ) | (3,240,062 | ) | |||||
Dividends paid | — | (546,154 | ) | ||||||
Proceeds from borrowings | 73,034,668 | 17,031,029 | |||||||
Repayment of borrowings | (67,221,844 | ) | (15,004,731 | ) | |||||
Net cash flows generated from / (used in) financing activities | 3,699,964 | (1,759,918 | ) | ||||||
Net increase / (decrease) in cash and cash equivalents | 15,693 | (700,707 | ) | ||||||
Cash, cash equivalents and overdraft at the beginning of the period | 26 | 940,895 | 98,268 | ||||||
Cash, cash equivalents and overdraft at the end of the period | 26 | 956,588 | (602,439 | ) | |||||
The accompanying notes are an integral part of these interim condensed consolidated financial statements.
F-166
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
Contents of the notes to the interim condensed consolidated financial statements
| 1. | General information |
| 2. | Accounting standards and basis for preparation |
| 3. | Segment reporting |
| 4. | Cost of sales |
| 5. | Administrative expenses |
| 6. | Distribution expenses |
| 7. | Research expenses |
| 8. | Other operating income and expenses, net |
| 9. | Financial income and financial costs |
| 10. | Share of net profit / (loss) of joint ventures accounted for using the equity method |
| 11. | Earnings per share |
| 12. | Property, plant and equipment |
| 13. | Intangible assets |
| 14. | Investment property |
| 15. | Investment in joint arrangements |
| 16. | Other financial assets |
| 17. | Inventories |
| 18. | Trade and other receivables |
| 19. | Financial assets at fair value through profit or loss |
| 20. | Cash and cash equivalents |
| 21. | Borrowings |
| 22. | Provisions |
| 23. | Trade and other payables |
| 24. | Employee benefit obligations |
| 25. | Transactions and balances with related parties |
| 26. | Additional information on the Statement of Cash Flow |
| 27. | Transactions with non-controlling interests |
| 28. | Contingencies, commitments and restrictions on the distribution of profits |
| 29. | Pledged and restricted assets |
| 30. | Subsequent events |
F-167
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| 1. | General information |
Rizobacter Argentina S.A. (hereinafter “Rizobacter” or “the Company”) was created on October 20, 1983 in the city of Pergamino and is engaged in the development of microbiological products applied to the agricultural market. Through the Company and its subsidiaries (“the Group”), Rizobacter performs operations in Argentina, Brazil, Uruguay, Paraguay, Bolivia and United States, and it is in the process of creating subsidiaries in South Africa and India.
Rizobacter is a company whose articles of incorporation were registered with the Superintendence of Commercial Companies of the Province of Buenos Aires on October 20, 1983 under number 15284, registration No. 1/32.501 with a term established until October 19, 2082. The last amendment of its by-laws was approved by the Extraordinary Shareholders’ Meeting No. 49, dated March 31, 2015, and is pending registration with the Public Registry of Commerce.
Below is a description of the capital status of Rizobacter.
Common shares | Subscribed and paid-up shares (ARG pesos) | ||
Class “A” par value 1 Argentine Peso – 5 voting rights | 40,000,000 | ||
The capital status is as follows:
9/30/2017 (ARG pesos) | 9/30/2016 (ARG pesos) | |||||
Share capital at the beginning of the year | 40,000,000 | 40,000,000 | ||||
Share capital at the end of the year | 40,000,000 | 40,000,000 | ||||
On March 31, 2015, the Shareholders’ Meeting of the Company approved the establishment of a global program of Negotiable Obligations (“the Program”) for the issue of one or more series of simple Negotiable Obligations, through public offering, for their future listing on stock exchanges and other markets, up to a revolving outstanding amount of USD 40,000,000 or the equivalent in other currencies, or a lower amount to be determined by the Board of Directors, with a maximum term of five years.
On July 16, 2015, the Argentine National Securities Commission (CNV for its acronym in Spanish) approved the Program and the Company was included in the CNV’s public offering regime of corporate bonds. The details of the program and the series issued by the Company are described in Note 21.
| 2. | Accounting standards and basis for preparation |
The accounting policies in these interim condensed financial statements are consistent with those of the previous annual financial statements and have been applied consistently for all the periods.
| 2.1. | Basis for preparation |
The CNV has established the application of Technical Pronouncement (“RT”) Nos. 26 and 29 issued by the F.A.C.P.C.E. adopting International Financial Reporting Standards (“IFRS”), the standards issued by the International Accounting Standards Board (“IASB”), for those entities included in the public offering regime of Law No. 17811, due either to their share capital or negotiable obligations, or having requested listing authorization to be included in this regime. The application of these standards was mandatory for the Company as from the fiscal year commenced July 1, 2014, and financial statements at June 30, 2015 were the first financial statements presented under these standards.
The Group’s interim condensed financial statements are unaudited and in the opinion of management reflect all adjustments necessary to fairly state the financial position of the Company as of September 30, 2017, and the results of
F-168
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
operations and cash flows for the three-month period ended on September 30, 2017 and 2016. These interim financial statements have been prepared in accordance with International Accounting Standards (“IAS”) 34, 'Interim financial reporting', and they should be read in conjunction with the annual financial statements for the year ended June 30, 2017, which have been prepared in accordance with IFRS as issued by the IASB and the Interpretations of the International Financial Reporting Interpretations Committee (“IFRIC”). All IFRS issued by the IASB, effective at the time of preparing these consolidated interim financial statements, have been applied.
These interim condensed consolidated financial statements were approved by the Company’s Board of Directors (the “Board”) on December 4, 2017.
The financial statements are stated in USD with no cents except the earnings/losses per share.
| 2.2. | Critical accounting estimates |
The preparation of financial statements requires the use of estimates. It also requires Group Management to exercise its judgment in the process of applying the accounting policies. The estimates and judgments are assessed on an ongoing basis and are based on historical experience and other factors, including the expectations of future events considered reasonable under the circumstances.
The Group makes estimates and hypotheses with regard to the future. The resulting accounting estimates may not be equal to the results actually obtained. The Company’s most significant estimates and judgments are explained below.
| (a) | Income tax |
The Group is subject to income tax in the countries in which it operates. In the determination of the income tax provision in each of the jurisdictions in which the Group pays this tax, it applies professional judgment to show the tax consequences of the economic events of each year, based on current tax legislation, making the best estimates based on the information available at the date of the interim condensed consolidated financial statements.
| (b) | Provisions for lawsuits and contingencies |
The evaluation of contingent liabilities is made by the Board and the Group’s legal advisors based on the judgment elements available at the date of these interim condensed consolidated financial statements. In the estimate of the amounts, we have considered, among other issues, the probability of occurrence of such liabilities. If in the evaluation of the contingency there is a probability that a loss may materialize and the amount of such loss may be estimated in a reliable manner, a liability is accounted for in the caption provisions for contingencies. If the potential loss is not probable, or if it is probable but its amount cannot be reliably estimated, the nature of the contingent liability and an estimate of the possibility of occurrence is disclosed in a note to the interim condensed consolidated financial statements.
| (c) | Allowance for obsolescence and slow moving inventories |
Management assesses the recoverability of its inventories by considering their sales prices as if the inventories were damaged or as if they were to totally or partially become obsolete.
Net realizable value is the sale price estimated in the normal course of business, less costs of completion and other sales expenses.
The Group establishes an allowance for obsolescence or slow moving inventories in relation to finished products and in-process products. The allowance for slow moving inventories is recognized for finished products and in-process products based on an analysis of Management on the aging of stock.
F-169
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| (d) | Calculation of useful lives and impairment of property, plant and equipment |
As described in Note 2.5 of the consolidated financial statements, the Group reviews the estimated useful life of property, plant and equipment at the end of each reporting period. During the current period, the estimated useful lives remained unchanged.
| (e) | Allowance for bad debts |
Management makes estimates regarding its inability to collect recorded receivables. Management analyzes trade account receivables in accordance with conventional criteria, adjusting the amount through a charge of an allowance for bad debts upon recognition of the inability of third parties to afford their financial obligations with the Group. Management analyses specifically the accounts receivable, the historical bad debts, solvency of customers, current economic trends and changes to the payment conditions of customers to assess the proper allowance for bad debts.
| (f) | Fair value of property, plant and equipment |
Assets recorded in lines “Land and buildings” are valued through the revaluation model indicated in IAS 16. To obtain reasonable values, Management considers whether an active market exists for goods in their current condition. For those assets for which an active market in their current status exists, the fair values were determined based on market values. For the remaining cases, market values were analyzed for new assets, applying a discount based on the status and wear of each asset.
Management considers the characteristics of each of the revalued assets (for example, improvements, maintenance status, productivity levels, use, etc.).
| (g) | Recoverability of intangible assets internally generated |
Management analyzes the recoverability of intangible assets internally generated by the development of several microbiological products with commercial potential, which are included in the interim condensed consolidated statement of financial position. Projects continue to be developed satisfactorily and the market reaction has reconfirmed the prior estimates of projected income for projects. No events have been detected during the period which may have influenced Management to reconsider their hypotheses as to the future participation in the market and projected margins as to these products. We have made a detailed sensitivity analysis, and Management trusts that the book value of the assets will be fully recovered, even if profits decrease. This situation is closely monitored and adjustments will be made to future periods if the market activity indicates that those adjustments are pertinent.
| 2.3. | Changes in accounting policies |
(a) New standards, amendments and interpretations adopted by the Group:
IFRS 9 “Financial instruments” (2010) prescribes the classification, measurement and recognition of financial assets and liabilities. It replaces the parts of the IAS No. 39 that relate to the classification and measurement of financial instruments. IFRS 9 requires financial assets to be classified into two categories: those measured at fair value and those measured at amortized cost.
The complete version of this standard was issued in July 2014. It replaces the guidelines of IAS 39 and the prior version of IFRS 9, which are related to the classification and measurement of financial instruments. IFRS 9 preserves and simplifies the mixed measurement model and establishes three main categories to measure financial assets: amortized cost, fair value through other comprehensive income and fair value through profit or loss. The basis of this classification depends on the business model of the entity used to manage its financial assets and the characteristics of the financial asset’s contractual cash flows. In its initial recognition, an entity can make an irrevocable election to disclose in other comprehensive income the subsequent changes in the fair value of an investment in an equity instrument not held for
F-170
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
trading. There were no changes in the classification and measurement of financial liabilities, except for the recognition of changes in its own credit risks (own credit rating) in other comprehensive income, for liabilities designated at fair value through profit or loss. Furthermore, IFRS 9 adjusts the requirements for effectiveness of hedge instruments. It requires the existence of an economic relation between the item hedged and the hedge instrument and that the reason for hedging be the same as that used by management for risk management. The requirement of a formal documentation of the hedge relationship at the beginning remains in effect but it is different to that under IAS 39. Also, this standard adds a model of receivable losses expected which substitutes the impairment model of financial assets used in IAS 39. The standard is effective for accounting periods commencing on or after January 1, 2018.
The Group has adopted IFRS 9 (2010) as from July 1, 2013, as well as important changes relating to other IFRS, as this new accounting policy provides more relevant and reliable information for the users to assess figures, timing and uncertainty of future cash flows.
(b) New standards, amendments and interpretation which have come into effect as from the year beginning on July 1, 2017 and which have not generated effects on the interim condensed consolidated financial statements:
Amendments to IAS 12 “Income Tax”: these amendments to the recognition of deferred tax assets for unrealized losses clarify how to account for such assets when they are related to debt instruments measured at fair value. This standard is applicable for the years beginning on or after January 1, 2017. The Company does not estimate that its application will have a significant impact.
(c) New published standards, amendments and interpretations which have not yet come into force for fiscal years beginning on or after July 1, 2017 and have not been early adopted:
IFRS 15 Revenue from contracts with Customers is a standard on revenue recognition agreed between the IASB and FASB which allows for improvement in financial reports over revenue, enabling their comparability at international level. It was published in May 2014 and it is effective for annual periods commencing as from January 1, 2017. The Group is assessing the possible impacts of this standard, but it is not possible at this moment to estimate the potential effects in the Company’s revenue recognition accounting policies.
IFRS 16 “Leases”: In January 2016, the IASB published IFRS 16 “Leases” which establishes the principles for the recognition, measurement, presentation and disclosure of leases. This standard is applicable for the years commencing as from January 1, 2019.
Amendments to IAS 7 “Statement of cash flows” introduce additional disclosures which enable users to assess changes to liabilities for financing activities. These include changes implying “cash flows”, such as withdrawal of funds and loan reimbursements, and changes which do not imply “cash flows”, such as acquisitions, disposals and unrealized exchange differences. This standard is applicable for the years commencing as from January 1, 2017. The Company does not estimate that its application will have a significant impact.
IFRIC 22 “Foreign Currency Transactions and Advance Consideration”: This interpretation addresses how to determine the date of the transaction for the purpose of determining the exchange rate to use on initial recognition of the related asset, expense or income (or part of it) on the derecognition of a non-monetary asset or non-monetary liability arising from the payment or receipt of advance consideration in a foreign currency. This interpretation is applicable for the years commencing as from January 1, 2018. The Company does not estimate that its application will have a significant impact.
There are no other IFRS or IFRIC interpretations that are not yet effective and which are expected to have a significant effect on the Group.
F-171
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| 3. | Segment reporting |
The Board is the maximum authority for the operating decision making. The Board has determined the operating segments based on information that it reviews with the aim of allocating resources and assessing performance.
The Board considers the business from the geographic and product viewpoint. At geographical level, the Board considers the performance in Argentina, Brazil and Paraguay, which were not disclosed as they do not exceed the quantitative parameters required by IFRS 8. From the product perspective, the Board considers separately the activities of the product families.
The Board assesses the performance of operating segments based on the gross results. This valuation basis excludes commercial, administrative, research and financial expenses, as these types of activities are carried out through the central administration, which manages the Group’s consolidated position.
The Group develops its main activities through the following family of products:
| ■ | Inoculants |
| ■ | Packs |
| ■ | Seed therapies |
| ■ | Adjuvants |
| ■ | Fertilizers |
| ■ | Others |
Packs
Packs offer services that comprise more than one Group product and include:
| ■ | Inoculants, which ensure an adequate Biological Nitrogen Fixation. |
| ■ | Seed therapies that control a wide range of germs. |
| ■ | Fertilizers applied to seeds that allow for adequate plant nutrition. |
Other:
The following family of products is included within this segment:
Biofertilizers:
Biofertilizers are biotechnology products that increase crop yields through non-contaminant technologies.
Micronutrients:
Regarding the fertilization of crops, apart from obtaining and using the elements called macronutrients, as nitrogen and phosphorus, and mesonutrients such as sulphur and calcium, there is a wide range of micronutrients which are essential for a good development of metabolic processes that maximize the productivity of crops.
Seed pelleting
Seed pelleting technology provides important advantages and benefits for crops including:
| − | Uniformity and homogenization of forage seeds, which ease sowing tasks. |
F-172
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| − | The addition to seeds, generally of a smaller size, a complex of fungicides, insecticides and even fertilizers to achieve a better nutrition for treated crops, turning the seed into a “technology pack”. |
| − | The addition to leguminous species of specific bacteria of the genus Rhizobium, specific for each one of these species, with the purpose of maximizing the capacity to produce nodules of this family of plants. |
Formulation for third parties
A service provided by the Company which includes:
| − | Formulation of Fungicides and Insecticides as Concentrated Suspensions, which consists in scattering active principles in a water-based suspension, adjusting granulometry, concentration and viscosity, for which a pre-scattering stage is required, as well a humid grinding stage and a stage for adjusting concentration and viscosity. |
| − | Formulation of Fungicides and Insecticides as Concentrated Emulsions, which consists in dissolving active principles in a blend of solvents, which will subsequently form an emulsion when mixed with water. |
| − | Packing of formulated products in the plant, in accordance with customer specifications, including exports. |
| − | Preparation of packs, with formulated products at the plant and materials supplied by the customer. |
| 3.1. | Segment reporting as of September 30, 2017 and 2016 |
Below is a description of the main indicators of each of the segments above described and grouped based on IFRS 8.
Reporting segments as of September 30, 2017 (Unaudited):
Item | Inoculants | Seed therapies | Adjuvants | Packs | Fertilizers | Others | Total | ||||||||||||||
Revenue | 6,306,921 | 2,409,728 | 7,993,798 | 6,515,983 | 3,233,962 | 6,392,421 | 32,852,813 | ||||||||||||||
Cost of sales | (4,436,796 | ) | (1,224,196 | ) | (3,015,946 | ) | (1,554,859 | ) | (2,635,415 | ) | (5,193,684 | ) | (18,060,896 | ) | |||||||
Gross income | 1,870,125 | 1,185,532 | 4,977,852 | 4,961,124 | 598,547 | 1,198,737 | 14,791,917 | ||||||||||||||
Total inventories | 4,074,526 | 6,931,436 | 8,751,214 | 4,287,835 | 3,988,446 | 5,947,739 | 33,981,196 | ||||||||||||||
Total intangible assets (microbiological products) | 2,201,734 | — | 550,434 | — | — | — | 2,752,168 | ||||||||||||||
Reporting segments as of September 30, 2016 (Unaudited):
Item | Inoculants | Seed therapies | Adjuvants | Packs | Fertilizers | Others | Total | ||||||||||||||
Revenue | 4,993,062 | 2,887,265 | 6,764,431 | 5,767,778 | 2,983,251 | 2,757,551 | 26,153,338 | ||||||||||||||
Cost of sales | (1,730,079 | ) | (1,482,593 | ) | (2,863,643 | ) | (1,521,077 | ) | (2,327,058 | ) | (1,684,340 | ) | (11,608,790 | ) | |||||||
Gross income | 3,262,983 | 1,404,672 | 3,900,788 | 4,246,701 | 656,193 | 1,073,211 | 14,544,548 | ||||||||||||||
Total inventories | 3,802,129 | 6,552,520 | 7,582,751 | 3,954,560 | 1,348,959 | 6,963,104 | 30,204,023 | ||||||||||||||
Total intangible assets (microbiological products) | 2,088,628 | — | 806,376 | — | — | — | 2,895,004 | ||||||||||||||
F-173
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| 4. | Cost of sales |
9/30/2017 | 9/30/2016 | |||||
(Unaudited) | (Unaudited) | |||||
Inventory at the beginning of the period (1) | 27,712,433 | 23,278,198 | ||||
Add: charges for the period | ||||||
Purchases for the period | 22,746,696 | 13,110,646 | ||||
Staff | 1,920,490 | 1,427,464 | ||||
Professional fees | — | 11,891 | ||||
Maintenance | 250,709 | 116,341 | ||||
Import expenses | 24,683 | 10,986 | ||||
Freight and transportation | 52,111 | 50,655 | ||||
Fuel and energy | 47,380 | 77,930 | ||||
Insurance | 26,206 | 12,143 | ||||
Vehicle expenses | 3,621 | 1,514 | ||||
Travel expenses | 5,172 | 4,713 | ||||
Taxes | 6,789 | 15,746 | ||||
Office expenses | 9,230 | 18,851 | ||||
Materials and packages | 47,763 | 37,381 | ||||
Depreciation of property, plant and equipment | 278,779 | 237,744 | ||||
Sundry | 23,870 | 35,144 | ||||
Total manufacturing expenses | 2,696,803 | 2,058,503 | ||||
Less: Inventory at the end of the period(1) | (34,049,447 | ) | (26,428,034 | ) | ||
Effect of currency translation | (1,045,589 | ) | (410,523 | ) | ||
Cost of sales | 18,060,896 | 11,608,790 | ||||
| (1) | Neither allowance for inventory obsolescence nor goods in transit are included. |
| 5. | Administrative expenses |
9/30/2017 | 9/30/2016 | |||||
(Unaudited) | (Unaudited) | |||||
Staff | 1,555,496 | 1,311,815 | ||||
Advertising and publicity | 801 | — | ||||
Directors’ and Syndic’s fees | — | 94,478 | ||||
Professional fees | 234,251 | 249,725 | ||||
Maintenance | 46,369 | 31,576 | ||||
Fuel and energy | 38,676 | 22,692 | ||||
Insurance | 28,048 | 14,914 | ||||
Vehicle expenses | 3,006 | 11,179 | ||||
Travel expenses | 30,191 | 30,674 | ||||
Taxes | 24,424 | 10,759 | ||||
Office expenses | 125,081 | 94,417 | ||||
Lawsuits and claims | — | 99,519 | ||||
Depreciation of property, plant and equipment | 79,981 | 124,494 | ||||
Amortization of Intangible assets | 90,798 | 5,975 | ||||
Sundry | 114,331 | 23,425 | ||||
Total | 2,371,453 | 2,125,642 | ||||
F-174
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| 6. | Distribution expenses |
9/30/2017 | 9/30/2016 | |||||
(Unaudited) | (Unaudited) | |||||
Staff | 1,889,094 | 1,393,333 | ||||
Advertising and publicity | 515,232 | 337,805 | ||||
Professional fees | 96,115 | 176,148 | ||||
Maintenance | 97,364 | 204,862 | ||||
Commissions and royalties | 93,384 | 125,157 | ||||
Import and export expenses | 365,374 | 217,484 | ||||
Freight and transportation | 399,790 | 272,115 | ||||
Fuel and energy | 89,801 | 79,487 | ||||
Insurance | 105,043 | 72,284 | ||||
Vehicle expenses | 23,442 | 56,178 | ||||
Travel expenses | 117,712 | 137,020 | ||||
Taxes | 747,446 | 654,401 | ||||
Office expenses | 38,076 | 27,060 | ||||
Depreciation of property, plant and equipment | 83,211 | 63,643 | ||||
Amortization of Intangible assets | 64,147 | 4,592 | ||||
Allowance for bad debts | 382,091 | 720,527 | ||||
Obsolescence | 19,923 | 106,738 | ||||
Sundry | 135,570 | 20,156 | ||||
Total | 5,262,815 | 4,668,990 | ||||
| 7. | Research expenses |
9/30/2017 | 9/30/2016 | |||||
(Unaudited) | (Unaudited) | |||||
Staff | 328,913 | 260,226 | ||||
Professional fees | 34,548 | 27,980 | ||||
Maintenance | 8,381 | 12,623 | ||||
Import and export expenses | 4,228 | — | ||||
Freight and transportation | 181 | 86 | ||||
Fuel and energy | 11,410 | 30,082 | ||||
Insurance | 5,579 | 12,407 | ||||
Vehicle expenses | 7,626 | 10,041 | ||||
Travel expenses | 12,697 | 23,051 | ||||
Office expenses | 14,772 | 25,967 | ||||
Agreements and other expenses | 103,910 | 109,094 | ||||
Depreciation of property, plant and equipment | 27,426 | 5,391 | ||||
Taxes | 1,406 | 4,975 | ||||
Materials and packages | 4,000 | — | ||||
Sundry | 14,557 | 11,678 | ||||
Total | 579,634 | 533,601 | ||||
F-175
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| 8. | Other operating income and expenses, net |
9/30/2017 | 9/30/2016 | |||||
(Unaudited) | (Unaudited) | |||||
Gain/loss from the sale of fixed assets | 49,470 | — | ||||
Other income and expenses | (224,790 | ) | (23,699 | ) | ||
Total | (175,320 | ) | (23,699 | ) | ||
| 9. | Financial income and financial costs |
Financial Income | 9/30/2017 | 9/30/2016 | ||||
(Unaudited) | (Unaudited) | |||||
Interest gain for financial assets | 125,575 | 103,715 | ||||
Net exchange gain generated by assets | 1,072,208 | 792,369 | ||||
Other financial results | 769 | 104,180 | ||||
Total | 1,198,552 | 1,000,264 | ||||
Financial Costs | 9/30/2017 | 9/30/2016 | ||||
(Unaudited) | (Unaudited) | |||||
Interest charge paid for financial liabilities | (2,792,043 | ) | (3,240,062 | ) | ||
Net exchange losses generated by liabilities | (2,449,089 | ) | (1,549,409 | ) | ||
Bank and financial commissions | (450,463 | ) | (271,360 | ) | ||
Other financial costs | (78,344 | ) | (62,072 | ) | ||
Sub-total | (5,769,939 | ) | (5,122,903 | ) | ||
Less: amounts capitalized in qualifying assets | 50,356 | 94,497 | ||||
Total | (5,719,583 | ) | (5,028,406 | ) | ||
| 10. | Share of net profit / (loss) of joint ventures accounted for using the equity method |
9/30/2017 | 9/30/2016 | |||||
(Unaudited) | ||||||
Semya S.A. | (7,043 | ) | (57,230 | ) | ||
Synertech Industrias S.A. | 304,982 | (175,174 | ) | |||
Total | 297,939 | (232,404 | ) | |||
F-176
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| 11. | Earnings per share |
Basic earnings per share are calculated by dividing the result attributable to the shareholders of the Company by the number of outstanding common shares at the end of the period.
9/30/2017 | 9/30/2016 | |||||
(Unaudited) | ||||||
Profit for the period attributable to the shareholders | 1,689,842 | 1,879,236 | ||||
Outstanding common shares | 40,000,000 | 40,000,000 | ||||
Earnings per basic and diluted share (USD per share)(1) | 0.04 | 0.05 | ||||
| (1) | At September 30, 2017 and September 30, 2016, the Company has not issued financial instruments or other contracts that grant to their holder rights on the capital represented by Company common shares which might modify their current shareholding; therefore, earnings per basic and diluted share agree. |
| 12. | Property, plant and equipment |
| (a) | Three-month period ended September 30, 2017 (Unaudited) |
Item | Original value | ||||||||||||||
At beginning of year | Additions/Transfers(1) | Disposals | Effect of currency translation | At end of the period | |||||||||||
Land and Buildings | 36,932,600 | 333,085 | — | (1,435,549 | ) | 35,830,136 | |||||||||
Machinery | 5,612,678 | 52,150 | (25,990 | ) | (216,692 | ) | 5,422,146 | ||||||||
Vehicles | 1,810,436 | 107,828 | (40,783 | ) | (42,500 | ) | 1,834,981 | ||||||||
Furniture and fixtures | 570,281 | 4,122 | (4,799 | ) | (19,146 | ) | 550,458 | ||||||||
Computer equipment | 788,304 | 12,600 | — | (27,292 | ) | 773,612 | |||||||||
Assets under construction | 870,469 | (208,281 | ) | (46,113 | ) | (28,060 | ) | 588,015 | |||||||
Total | 46,584,768 | 301,504 | (117,685 | ) | (1,769,239 | ) | 44,999,348 | ||||||||
Item | Accumulated depreciation | Book value as of 9/30/2017 | ||||||||||||||||
At beginning of year | Disposals | For the period | Effect of currency translation | At end of the period | ||||||||||||||
Land and Buildings | 4,148,710 | — | 231,699 | (162,424 | ) | 4,217,985 | 31,612,151 | |||||||||||
Machinery | 2,223,906 | — | 138,427 | (86,424 | ) | 2,275,909 | 3,146,237 | |||||||||||
Vehicles | 774,990 | (37,881 | ) | 57,514 | (23,391 | ) | 771,232 | 1,063,749 | ||||||||||
Furniture and fixtures | 352,355 | — | 7,931 | (13,450 | ) | 346,836 | 203,622 | |||||||||||
Computer equipment | 480,543 | — | 33,826 | (16,048 | ) | 498,321 | 275,291 | |||||||||||
Assets under construction | — | — | — | — | — | 588,015 | ||||||||||||
Total | 7,980,504 | (37,881 | ) | 469,397 | (301,737 | ) | 8,110,283 | 36,889,065 | ||||||||||
| (1) | Additions include the capitalization of financial costs in qualifying assets amounting to USD 6,404. |
F-177
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| (b) | Three-month period ended September 30, 2016 (Unaudited) |
Item | Original value | ||||||||||||||
At beginning of year | Additions/Transfers(1) | Disposals | Effect of currency translation | At end of the period | |||||||||||
Land and Buildings | 37,475,102 | — | — | (651,794 | ) | 36,823,308 | |||||||||
Machinery | 6,025,470 | 22,130 | — | (103,340 | ) | 5,944,260 | |||||||||
Vehicles | 1,448,031 | 37,713 | — | (9,067 | ) | 1,476,677 | |||||||||
Furniture and fixtures | 587,272 | 4,711 | — | (9,352 | ) | 582,631 | |||||||||
Computer equipment | 854,378 | 3,161 | (3,056 | ) | (14,487 | ) | 839,996 | ||||||||
Assets under construction | 169,994 | 319,738 | — | (7,532 | ) | 482,200 | |||||||||
Total | 46,560,247 | 387,453 | (3,056 | ) | (795,572 | ) | 46,149,072 | ||||||||
Item | Accumulated depreciation | Book value as of 9/30/2016 | ||||||||||||||||
At beginning of year | Disposals | For the period | Effect of currency translation | At end of the period | ||||||||||||||
Land and Buildings | 3,159,774 | — | 219,231 | (58,644 | ) | 3,320,361 | 33,502,947 | |||||||||||
Machinery | 1,891,852 | — | 118,205 | (33,149 | ) | 1,976,908 | 3,967,352 | |||||||||||
Vehicles | 597,713 | — | 49,732 | (9,292 | ) | 638,153 | 838,524 | |||||||||||
Furniture and fixtures | 354,436 | — | 934 | (5.277 | ) | 350,093 | 232,538 | |||||||||||
Computer equipment | 358,947 | (3,056 | ) | 43,170 | (6,611 | ) | 392,450 | 447,546 | ||||||||||
Assets under construction | — | — | — | — | — | 482,200 | ||||||||||||
Total | 6,362,722 | (3,056 | ) | 431,272 | (112,973 | ) | 6,677,965 | 39,471,107 | ||||||||||
| 13. | Intangible assets |
9/30/2017 | 9/30/2016 | |||||
(Unaudited) | (Unaudited) | |||||
Microbiological products(1) | 2,752,168 | 2,895,004 | ||||
Software(2) | 1,246,214 | 439,426 | ||||
Total | 3,998,382 | 3,334,430 | ||||
| (1) | It corresponds to the capitalization of expenses from the development of different microbiological products. |
| (2) | It corresponds to software development expenses. |
Cost | Microbiological products | Software | Total | ||||||
Balance at 06/30/2016 | 2,853,618 | 272,818 | 3,126,436 | ||||||
Additions(1) | 153,794 | 204,468 | 358,262 | ||||||
Effect of currency translation | (52,712 | ) | (7,983 | ) | (60,695 | ) | |||
Balance at 09/30/2016 (Unaudited) | 2,954,700 | 469,303 | 3,424,003 | ||||||
Balance at 06/30/2017 | 3,032,299 | 1,773,906 | 4,806,205 | ||||||
Additions(1) | 110,820 | — | 110,820 | ||||||
Effect of currency translation | (119,161 | ) | (69,887 | ) | (189,048 | ) | |||
Balance at 09/30/2017 (Unaudited) | 3,023,958 | 1,704,019 | 4,727,977 | ||||||
F-178
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
Accumulated amortization | Microbiological products | Software | Total | ||||||
Balance at 06/30/2016 | (56,095 | ) | (24,330 | ) | (80,425 | ) | |||
Amortization expense(2) | (4,592 | ) | (5,975 | ) | (10,567 | ) | |||
Effect of currency translation | 991 | 428 | 1,419 | ||||||
Balance at 09/30/2016 (Unaudited) | (59,696 | ) | (29,877 | ) | (89,573 | ) | |||
Carrying value at 09/30/2016 | 2,895,004 | 439,426 | 3,334,430 | ||||||
Balance at 06/30/2017 | (215,976 | ) | (381,800 | ) | (597,776 | ) | |||
Amortization expense(2) | (64,147 | ) | (90,798 | ) | (154,945 | ) | |||
Effect of currency translation | 8,333 | 14,793 | 23,126 | ||||||
Balance at 09/30/2017 (Unaudited) | (271,790 | ) | (457,805 | ) | (729,595 | ) | |||
Carrying value at 09/30/2017 | 2,752,168 | 1,246,214 | 3,998,382 | ||||||
| (1) | Additions include the capitalization of costs for interest in qualifying assets. The amounts capitalized were USD 43,952 and USD 94,497 at September 30, 2017 and September 30, 2016, respectively. |
| (2) | Amortization of Microbiological Products is allocated to distribution expenses. |
| 14. | Investment property |
Non-Current | 9/30/2017 | 6/30/2017 | ||||
(Unaudited) | ||||||
Land | 870 | 906 | ||||
Total | 870 | 906 | ||||
| 15. | Investment in joint arrangements |
Entity | Percentage of equity interest | 9/30/2017 | 6/30/2017 | ||||||
(Unaudited) | |||||||||
Semya S.A | 50.00 | % | 151,819 | 165,397 | |||||
Synertech Industrias S.A. | 50.00 | % | 4,596,740 | 4,466,907 | |||||
Total | 4,748,559 | 4,632,304 | |||||||
Below is a detail of the financial information selected at September 30, 2017 and June 30, 2017 in USD of the joint arrangements:
| (a) | At September 30, 2017 (Unaudited) |
Information on the issuer | |||||||||||||||||||||
Entity | Date | Current assets | Non-current assets | Current liabilities | Non-current liabilities | Revenue | Net profit/ (loss) for the period | ||||||||||||||
Semya S.A. | 9/30/2017 | 253,947 | 687,565 | 651,636 | — | — | (15,379 | ) | |||||||||||||
Synertech Industrias S.A. | 9/30/2017 | 10,567,434 | 16,481,436 | 12,653,890 | 6,070,932 | 4,309,731 | 683,451 | ||||||||||||||
F-179
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| (b) | At June 30, 2017 |
Information on the issuer | |||||||||||||||||||||
Entity | Date | Current assets | Non-current assets | Current liabilities | Non-current liabilities | Revenue | Net loss for the year | ||||||||||||||
Semya S.A. | 6/30/2017 | 76 | 992,143 | 657,805 | 3,619 | — | (198,726 | ) | |||||||||||||
Synertech Industrias S.A. | 6/30/2017 | 6,069,809 | 16,823,936 | 6,587,205 | 8,277,660 | 1,315,901 | (2,544,244 | ) | |||||||||||||
| 16. | Other financial assets |
Non-Current
Entity | 9/30/2017 | 6/30/2017 | ||||
Unaudited | ||||||
Bioceres S.A. | 3,453 | 3,474 | ||||
Coop. Eléctrica Pergamino Ltda. | 3,082 | 3,208 | ||||
FAID 2015 Fondo Agrícola de inversión directa (Direct Investment Agricultural Fund) | 579 | 602 | ||||
Other investments | 121,357 | 85,521 | ||||
Total | 128,471 | 92,805 | ||||
Current
9/30/2017 | 6/30/2017 | |||||
Unaudited | ||||||
Restricted short-term deposit | 4,250,715 | 4,247,669 | ||||
Total | 4,250,715 | 4,247,669 | ||||
| 17. | Inventories |
9/30/2017 | 6/30/2017 | |||||
Unaudited | ||||||
Raw materials | 4,384,806 | 4,773,742 | ||||
Packaging | 2,766,436 | 2,637,222 | ||||
Finished goods | 11,437,490 | 8,931,124 | ||||
Resale goods | 15,460,715 | 11,370,345 | ||||
Goods in transit | 321,262 | 482,186 | ||||
Inventory obsolescence allowance (Note 23) | (389,513 | ) | (620,577 | ) | ||
Total | 33,981,196 | 27,574,042 | ||||
The Company has delivered goods on consignment for third parties for its resale for USD 5,475,549 and USD 3,919,192, at September 30, 2017 and June 30, 2017, respectively, which are included in lines “finish goods” and “resale goods”.
F-180
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| 18. | Trade and other receivables |
9/30/2017 | 6/30/2017 | |||||
(Unaudited) | ||||||
Non-Current | ||||||
Tax credits | 130,978 | 124,735 | ||||
Reimbursements over exports | 414,424 | 472,275 | ||||
Related parties (Note 25) | 1,298,086 | 1,213,053 | ||||
Sundry | 5,108 | — | ||||
Total | 1,848,596 | 1,810,063 | ||||
9/30/2017 | 6/30/2017 | |||||
(Unaudited) | ||||||
Current | ||||||
Trade receivables in local currency | 684,959 | 215,497 | ||||
Trade receivables in foreign currency | 32,743,672 | 28,701,893 | ||||
Deferred checks | 297,467 | 398,916 | ||||
Checks discounted with resource not yet due | 13,943,143 | 10,359,675 | ||||
Notes receivable in local currency | 1,093,136 | 842,956 | ||||
Receivables in litigation | 3,484,348 | 2,877,099 | ||||
Reimbursements over exports | 165,416 | 122,584 | ||||
Advances to suppliers in local currency | 1,343,035 | 685,562 | ||||
Advances to suppliers in foreign currency | 570,848 | 412,878 | ||||
Tax credits | 2,784,021 | 1,878,251 | ||||
Related parties (Note 25) | 8,900,361 | 6,360,368 | ||||
Sundry | 850,873 | 671,072 | ||||
Provision of credit notes to be issued | (391,130 | ) | (1,393,059 | ) | ||
Allowance for bad debts (Note 22) | (3,475,122 | ) | (2,779,163 | ) | ||
Total | 62,995,027 | 49,354,529 | ||||
Trade and other receivables per currency | 9/30/2017 | 6/30/2017 | ||||
(Unaudited) | ||||||
In Argentine pesos | 25,518,119 | 17,124,551 | ||||
In United States dollars | 38,359,478 | 33,323,430 | ||||
In Euros | 23,073 | 10,821 | ||||
In other currencies | 942,953 | 705,790 | ||||
Total | 64,843,623 | 51,164,592 | ||||
During the period ended September 30, 2017 and the period ended June 30, 2017, Rizobacter Argentina assigned accounts receivable for USD 13,943,143 and USD 10,359,675, respectively, to financial institutions in exchange for cash. The transaction was recorded as a secured loan (Note 21). In case of default by the entities under the loan agreement, the bank has the right to receive the cash flows of the accounts receivable transferred. If there is no default, the entities will collect accounts receivable.
F-181
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
The increase in the allowance for bad debts is included in line “distribution expenses” in the income statement (Note 6). The amounts charged to the allowance for bad debts are usually deleted from accounting when there are no expectations of receiving additional cash. The activity for the period of the allowance for bad debts is disclosed in Note 22.
The rest of the accounts included in trade receivables and other accounts receivable do not contain assets that have been impaired.
The maximum credit risk exposure at the date of these financial statements is the carrying amount of each type of account receivable mentioned above.
The fair values of current trade and other receivables approximate their respective carrying amounts due to their short-term nature. The fair values of non-current other receivables approximate their carrying amount, as the impact of discounting is not significant.
| 19. | Financial assets at fair value through profit or loss |
9/30/2017 | 6/30/2017 | |||||
(Unaudited) | ||||||
Marketable shares | 3,990 | 4,275 | ||||
Total | 3,990 | 4,275 | ||||
Changes in the fair value of financial assets at fair value through profit or loss is disclosed in line “Other operating income and expenses, net” of the Income Statement (Note 8). The fair value of all equity securities is based on the current price offered in an active market.
| 20. | Cash and cash equivalents |
9/30/2017 | 6/30/2017 | |||||
(Unaudited) | ||||||
Cash and banks | 1,175,691 | 940,895 | ||||
Total (Excluding overdraft) | 1,175,691 | 940,895 | ||||
Cash and cash equivalents per currency | 9/30/2017 | 6/30/2017 | ||||
(Unaudited) | ||||||
In Argentine pesos | 256,949 | 229,408 | ||||
In United States dollars | 221,506 | 643,174 | ||||
In Euros | 2,761 | 3,255 | ||||
In other currencies | 694,475 | 65,058 | ||||
Total (Excluding overdraft) | 1,175,691 | 940,895 | ||||
F-182
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| 21. | Borrowings |
9/30/2017 | 6/30/2017 | |||||
(Unaudited) | ||||||
Non-current | ||||||
Bank loans | 35,874,865 | 35,881,720 | ||||
Financial leases | 587,439 | 678,993 | ||||
Corporate bonds | — | 3,889,873 | ||||
Total | 36,462,304 | 40,450,586 | ||||
Current | ||||||
Bank loans | 21,871,981 | 18,594,823 | ||||
Financial leases | 327,912 | 466,055 | ||||
Corporate bonds | 6,136,687 | 4,644,621 | ||||
Discounted checks | 12,725,116 | 9,638,788 | ||||
Bank overdraft | 219,103 | — | ||||
Total | 41,280,799 | 33,344,287 | ||||
Borrowings by type of rate | 9/30/2017 | 6/30/2017 | ||||
(Unaudited) | ||||||
Fixed rate | 71,315,042 | 66,340,807 | ||||
Variable rate | 6,428,061 | 7,454,066 | ||||
Total | 77,743,103 | 73,794,873 | ||||
Borrowings by type of currency | 9/30/2017 | 6/30/2017 | ||||
(Unaudited) | ||||||
In Argentine pesos | 21,071,042 | 17,950,005 | ||||
In United States dollars | 56,672,061 | 55,844,868 | ||||
Total | 77,743,103 | 73,794,873 | ||||
Bank debts have an average effective rate of 23.50% (June 2017: 24.07% annual rate). The fair value of current loans is equivalent to their book value, since the impact of applying the adjustment is not significant.
| 22. | Provisions |
| (a) | Three-months period ended September 30, 2017 (Unaudited) |
Description | Balances at the beginning of period | Increases | Effect of currency translation | Reversals / Uses | Balances at period end | ||||||||||
Provision for contingencies | 1,036,998 | — | (40,855 | ) | — | 996,143 | |||||||||
Inventory obsolescence allowance | 620,577 | 19,923 | (38,355 | ) | (212,632 | ) | 389,513 | ||||||||
Allowance for bad debts | 2,779,163 | 382,091 | 313,868 | — | 3,475,122 | ||||||||||
F-183
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| (b) | Three-months period ended September 30, 2016 (Unaudited) |
Description | Balances at the beginning of period | Increases | Effect of currency translation | Reversals / Uses | Balances at period end | ||||||||||
Provision for contingencies | 1,286,524 | 99,519 | (23,815 | ) | — | 1,362,228 | |||||||||
Inventory obsolescence allowance | 322,506 | 106,738 | (7,344 | ) | — | 421,900 | |||||||||
Allowance for bad debts | 2,149,802 | 720,527 | (285,473 | ) | (123,423 | ) | 2,461,433 | ||||||||
| 23. | Trade and other payables |
9/30/2017 | 6/30/2017 | |||||
(Unaudited) | ||||||
Non-Current | ||||||
Related parties (Note 25) | 145 | 151 | ||||
Total | 145 | 151 | ||||
9/30/2017 | 6/30/2017 | |||||
(Unaudited) | ||||||
Current | ||||||
Trade payables in local currency | 9,239,652 | 7,664,088 | ||||
Trade payables in foreign currency | 17,587,119 | 10,358,420 | ||||
Taxes | 580,238 | 363,127 | ||||
Syndic’ fees payable | 5,840 | 6,080 | ||||
Related parties (Note 25) | 5,337,637 | 2,101,975 | ||||
Sundry debts | 147,672 | 140,454 | ||||
Total | 32,898,158 | 20,634,144 | ||||
Trade and other payable by type of currency | 9/30/2017 | 6/30/2017 | ||||
(Unaudited) | ||||||
In Argentine pesos | 9,039,148 | 8,209,643 | ||||
In United States dollars | 22,283,383 | 11,081,847 | ||||
In other currencies | 1,720 | 8,135 | ||||
In Euros | 1,574,052 | 1,334,670 | ||||
Total | 32,898,303 | 20,634,295 | ||||
The carrying of trade and other accounts payable are considered to be the same as their fair values, due to their short-term maturity.
F-184
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| 24. | Employee benefit obligations |
9/30/2017 | 6/30/2017 | |||||
(Unaudited) | ||||||
Non-Current | ||||||
Payroll and social security charges | 274,753 | 286,022 | ||||
Total | 274,753 | 286,022 | ||||
Current | ||||||
Payroll and social security charges | 5,347,823 | 4,995,477 | ||||
Total | 5,347,823 | 4,995,477 | ||||
| 25. | Transactions and balances with related parties |
For the purposes of these financial statements, related parties are those individuals or legal entities related to the Group, under the terms of IAS 24.
| (a) | Balances with related parties |
Balances with related parties at September 30, 2017 (unaudited) are the following:
Related Party | Current trade and other receivables | Non-Current trade and other receivables | Current trade and other payables | Other non- current accounts payable | ||||||||
Parent | ||||||||||||
RASA Holding LLC | 22,848 | — | — | — | ||||||||
Under joint control | ||||||||||||
Semya S.A. | 317,647 | — | — | — | ||||||||
Synertech Industrias S.A. | 5,257,468 | 1,298,086 | 4,959,148 | — | ||||||||
Other related parties | ||||||||||||
Bioceres Semilla S.A. | 3,302,398 | — | 224,911 | — | ||||||||
Management staff and others | — | — | 30,764 | 145 | ||||||||
Shareholders | — | — | 122,814 | — | ||||||||
TOTAL | 8,900,361 | 1,298,086 | 5,337,637 | 145 | ||||||||
F-185
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
Balances with related parties at June 30, 2017 (unaudited) are the following:
Related Party | Current trade and other receivables | Non-Current trade and other receivables | Current trade and other payables | Other non- current accounts payable | ||||||||
Under joint control | ||||||||||||
Synertech Industrias S.A. | 2,981,258 | 1,213,053 | 1,649,367 | — | ||||||||
Semya S.A. | 321,761 | — | — | — | ||||||||
Other related parties | ||||||||||||
Bioceres Semillas S.A. | 3,057,349 | — | 152,549 | — | ||||||||
Management staff and others | — | — | 53,304 | 151 | ||||||||
Shareholders | — | — | 246,755 | — | ||||||||
TOTAL | 6,360,368 | 1,213,053 | 2,101,975 | 151 | ||||||||
| (b) | Operations with related parties |
Operations with related parties for the three-month period ended September 30, 2017 and 2016 are the following:
9/30/2017 | 9/30/2016 | |||||
(Unaudited) | (Unaudited) | |||||
Sale of goods and services | ||||||
Other related parties | ||||||
Bioceres Semillas S.A. | 2,493 | — | ||||
Total | 2,493 | — | ||||
F-186
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
9/30/2017 | 9/30/2016 | |||||
(Unaudited) | (Unaudited) | |||||
Other operations | ||||||
Entities under joint control | ||||||
Semya S.A. | ||||||
Expenses refund | — | 121,602 | ||||
Capital contributions | — | 79,207 | ||||
Other | 907 | — | ||||
Synertech Industrias S.A. | ||||||
Expenses refund | 91,447 | 177,312 | ||||
Purchases | 4,200,135 | — | ||||
Loans | 2,054,184 | 616,092 | ||||
Other related parties | ||||||
Bioceres Semillas S.A. | ||||||
Purchases | 20,389 | — | ||||
Interest | 49,016 | — | ||||
Shareholders | ||||||
Cash dividends | — | 1,459,613 | ||||
Key management personal | ||||||
Fees | — | 144,954 | ||||
Payroll | 843,197 | 803,801 | ||||
Sale of property, plant and equipment | 6,303 | — | ||||
The upper management includes directors (both executive and non-executive) and the senior management. Compensations paid or payable to the upper management for labor services rendered are shown in the previous table.
| 26. | Additional information on the Statement of Cash Flow |
For the purposes of the Statement of Cash Flow, cash and cash equivalents include:
9/30/2017 | 9/30/2016 | |||||
(Unaudited) | (Unaudited) | |||||
Cash and cash equivalents (Note 20) | 1,175,691 | 859,157 | ||||
Bank overdrafts (Note 21) | (219,103 | ) | (1,461,596 | ) | ||
Cash and cash equivalents net of bank overdrafts | 956,588 | (602,439 | ) | |||
Significant investment or funding transactions not entailing outlays of cash and cash equivalents are shown below:
9/30/2017 | 9/30/2016 | |||||
(Unaudited) | (Unaudited) | |||||
Unpaid distributed dividends | — | 898,930 | ||||
F-187
RIZOBACTER ARGENTINA S.A.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in US dollars, except otherwise indicated)
| 27. | Transactions with non-controlling interests |
There are no significant transactions with non-controlling interests to report.
| 28. | Contingencies, commitments and restrictions on the distribution of profits |
| (a) | Contingencies |
There are several administrative, judicial and out-of-court proceedings in the ordinary course of business to which the subsidiaries and/or related companies of Rizobacter are a party. The Group believes that none of these cases, individually or cumulatively, will produce a significant adverse effect on the financial position of the Group, nor on the future results of its operations, bearing in mind the opinion of the legal counsel and professional advisors and the provisions for contingencies recorded at year end.
| (b) | Restrictions to the distribution of profits |
Pursuant to Section 70 of the General Companies Law No. 19,550 of Argentina, companies must allocate 5% of the net profit of each year to a statutory reserve until reaching 20% of their adjusted capital. In addition, distribution of profits will be subject to retained earnings and statutory reserves in the statutory financial statements presented in ARG pesos. Retained earnings and statutory reserves as of September 30, 2017 amounts to ARS 55,331,419. Covenants restrictions to the distribution of profits are disclosed in Note 21 of the annual consolidated financial statements.
| 29. | Pledged and restricted assets |
Pledged and restricted assets at September 30, 2017 are as follows:
Item | Asset value | Type of debt | Amount of debt | Type of restriction | ||||
Computer equipment | 64,665 | Commercial | 75,811 | Leasing | ||||
Machinery and equipment | 103,453 | Bank | 38,628 | Leasing | ||||
Machinery and equipment | 576,076 | Bank | 546,991 | Leasing | ||||
Land and Buildings | 189,687 | Bank | 1,937,254 | Mortgage security(a) | ||||
Vehicles | 59,080 | Commercial | 9,223 | Collateral(b) | ||||
Short-term deposit | 4,250,715 | Bank | 45,130,359 | Collateral(c) | ||||
Total | 5,243,676 | 47,738,266 | ||||||
| a. | On June 29, 2015, the Company acquired real property in the Pergamino Industrial Park, where it installed a Phytosanitary Plant, using a mortgage loan on the property acquired from Banco de la Provincia de Buenos Aires. In addition, the shareholders Jorge Mac Mullen and Enrique Ripoll provided security deposits. |
| b. | On June 24, 2016, the Company entered into five pledge loans with Rombo Compañía Financiera. Each of those loans amounted to Argentine peso 285,000 (equivalent to USD 18,949), payable in 24 monthly consecutive principal and interest installments, the first of which was due on August 5, 2016. Interest is accrued on the balance at an annual rate of 18.9%. As collateral for those loans, the Company has pledged five Renault Dusters 2.0, model year 2016 in favor of Rombo Cía. Financiera. |
| c. | Rizobacter took a syndicated loan for USD 45,000,000 and signed a guarantee and pledge. On June 13, 2017, Rizobacter granted a pledge of a fixed-term certificate at Banco Galicia for an amount of USD 4,250,715. |
| 30. | Subsequent events |
On October 30, 2016, the Company’s shareholders approved the creation of a ARS 22,046,536 statutory reserve (equivalent to USD 1,277,320) and also approved the Board’s fees accrued during the fiscal year ended June 30, 2017.
No other events, situations or circumstances have taken place affecting or which may significantly affect the equity, economic or financial position of the Group.
F-188
 | |
Rizobacter Argentina S.A. | |
Avda. Dr. Arturo Frondizi Nº 1150, Industrial Park 2700 Pergamino (Province of Buenos Aires) | |
CONSOLIDATED FINANCIAL STATEMENTS | |
As of and for the for the years ended June 30, 2017, 2016 and 2015 |
F-189
Rizobacter Argentina S.A.
Consolidated Financial Statements
As of and for the years ended June 30, 2017, 2016 and 2015
Contents
Report of Independent Auditors
Consolidated Financial Statements
Consolidated Statement of Comprehensive Income
Consolidated Statement of Financial Position
Consolidated Statement of Changes in Equity
Consolidated Statement of Cash Flows
Notes to the Consolidated Financial Statements
F-190
Report of Independent Auditors
To the Board of Directors and Shareholders of Rizobacter Argentina S.A.
We have audited the accompanying consolidated financial statements of Rizobacter Argentina and its subsidiaries, which comprise the consolidated statement of financial position as of June 30, 2017, 2016 and 2015, and the related consolidated statements of comprehensive income, of changes in equity and of cash flows for the years then ended.
Management's Responsibility for the Consolidated Financial Statements
Management is responsible for the preparation and fair presentation of the consolidated financial statements in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board; this includes the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
Auditors’ Responsibility
Our responsibility is to express an opinion on the consolidated financial statements based on our audits. We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the consolidated financial statements. The procedures selected depend on our judgment, including the assessment of the risks of material misstatement of the consolidated financial statements, whether due to fraud or error. In making those risk assessments, we consider internal control relevant to the Company's preparation and fair presentation of the consolidated financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control. Accordingly, we express no such opinion. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Rizobacter Argentina S.A and its subsidiaries as of June 2017, 2016 and 2015, and the results of their operations and their cash flows for the years then ended in accordance with the International Financial Reporting Standards as issued by the International Accounting Standards Board.
Price Waterhouse & Co. S.R.L. | |
/s/ GABRIEL MARCELO PERRONE | |
Gabriel Marcelo Perrone | |
Partner | |
Pergamino, Argentina | |
October 30, 2017 |
F-191
RIZOBACTER ARGENTINA S.A.
CONSOLIDATED STATEMENT OF COMPREHENSIVE INCOME
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars)
Year ended | ||||||||||
6/30/2017 | 6/30/2016 | 6/30/2015 | ||||||||
Revenue | 3.1 | 112,296,212 | 93,405,678 | 99,163,146 | ||||||
Cost of sales | 4 | (58,838,471 | ) | (49,073,466 | ) | (47,357,049 | ) | |||
Gross income | 53,457,741 | 44,332,212 | 51,806,097 | |||||||
Administrative expenses | 5 | (9,765,385 | ) | (8,363,830 | ) | (7,677,468 | ) | |||
Distribution expenses | 6 | (19,502,749 | ) | (18,824,875 | ) | (20,102,818 | ) | |||
Research expenses | 7 | (2,423,428 | ) | (2,254,885 | ) | (655,941 | ) | |||
Other operating income, net | 8 | 49,654 | 446,739 | 158,731 | ||||||
Operating income | 21,815,833 | 15,335,361 | 23,528,601 | |||||||
Financial income | 9 | 2,080,709 | 4,633,611 | 2,032,019 | ||||||
Financial costs | 9 | (19,450,815 | ) | (27,025,501 | ) | (15,944,826 | ) | |||
Share of net losses of joint ventures accounted under the equity method | 10 | (1,109,131 | ) | (848,948 | ) | (584,728 | ) | |||
Net profit / (loss) before income tax | 3,336,596 | (7,905,477 | ) | 9,031,066 | ||||||
Income tax (expense) / benefit | 26 | (1,789,654 | ) | 2,443,866 | (3,198,198 | ) | ||||
NET PROFIT / (LOSS) | 1,546,942 | (5,461,611 | ) | 5,832,868 | ||||||
Net profit / (loss) attributable to the equity holders of the parent company | 1,545,891 | (5,469,358 | ) | 5,834,608 | ||||||
Net profit / (loss) attributable to the non-controlling interests | 1,051 | 7,747 | (1,740 | ) | ||||||
1,546,942 | (5,461,611 | ) | 5,832,868 | |||||||
Basic and diluted earnings / (loss) per share attributable to shareholders of the Company | 11 | 0.04 | (0.14 | ) | 0.15 | |||||
Year ended | |||||||||
OTHER CONSOLIDATED COMPREHENSIVE INCOME | 6/30/2017 | 6/30/2016 | 6/30/2015 | ||||||
Net profit / (loss) | 1,546,942 | (5,461,611 | ) | 5,832,868 | |||||
Other comprehensive income: | |||||||||
Items that may be subsequently reclassified to profit and loss | |||||||||
Exchange differences on translation of foreign operations | (1,865,399 | ) | (12,641,968 | ) | (2,790,627 | ) | |||
Items that will not be subsequently reclassified to profit and loss | |||||||||
Revaluation of property, plant and equipment, net of tax | 2,256,554 | 10,908,784 | 10,767,550 | ||||||
Other comprehensive income / (loss) | 391,155 | (1,733,184 | ) | 7,976,923 | |||||
Total comprehensive income / (loss) | 1,938,097 | (7,194,795 | ) | 13,809,791 | |||||
Comprehensive income (loss) attributable to: | |||||||||
Equity holders of the parent company | 1,938,313 | (7,187,957 | ) | 13,803,650 | |||||
Non-controlling interests | (216 | ) | (6,838 | ) | 6,141 | ||||
1,938,097 | (7,194,795 | ) | 13,809,791 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-192
RIZOBACTER ARGENTINA S.A.
CONSOLIDATED STATEMENT OF FINANCIAL POSITION
At June 30, 2017, 2016 and 2015 (amounts in US dollars)
ASSETS | Notes | 6/30/2017 | 6/30/2016 | 6/30/2015 | ||||||||
NON-CURRENT ASSETS | ||||||||||||
Property, plant and equipment | 12 | 38,604,264 | 40,197,525 | 35,955,482 | ||||||||
Intangible assets | 13 | 4,208,429 | 3,046,011 | 3,278,335 | ||||||||
Investment property | 14 | 906 | 999 | 1,653 | ||||||||
Investments in joint arrangements | 15 | 4,632,304 | 4,558,512 | 7,553,916 | ||||||||
Other financial assets | 16 | 92,805 | 14,669 | 25,637 | ||||||||
Trade and other receivables | 18 | 1,810,063 | 1,491,829 | 464,301 | ||||||||
Deferred tax assets | 26 | — | 8,813 | — | ||||||||
Total non-current assets | 49,348,771 | 49,318,358 | 47,279,324 | |||||||||
CURRENT ASSETS | ||||||||||||
Inventories | 17 | 27,574,042 | 23,187,382 | 31,608,911 | ||||||||
Trade and other receivables | 18 | 49,354,529 | 26,247,601 | 34,070,055 | ||||||||
Financial assets at fair value through profit or loss | 19 | 4,275 | 4,712 | 9,909 | ||||||||
Income tax credit | 1,701,381 | 1,800,997 | 676,727 | |||||||||
Other financial assets | 16 | 4,247,669 | — | — | ||||||||
Cash and cash equivalents | 20 | 940,895 | 1,030,067 | 3,671,219 | ||||||||
Total current assets | 83,822,791 | 52,270,759 | 70,036,821 | |||||||||
Total assets | 133,171,562 | 101,589,117 | 117,316,145 | |||||||||
EQUITY | ||||||||||||
Share capital and capital adjustments | 7,113,060 | 7,113,060 | 6,044,570 | |||||||||
Reserved profits and other equity components | 15,809,176 | 15,321,476 | 25,714,903 | |||||||||
Non-controlling interests | 48,647 | 46,607 | 43,480 | |||||||||
Total equity | 22,970,883 | 22,481,143 | 31,802,953 | |||||||||
LIABILITIES | ||||||||||||
NON-CURRENT LIABILITIES | ||||||||||||
Borrowings | 21 | 40,450,586 | 13,225,810 | 12,377,904 | ||||||||
Provisions | 23 | 1,036,998 | 1,286,524 | 1,705,990 | ||||||||
Deferred tax liabilities | 26 | 8,234,885 | 6,925,457 | 5,130,246 | ||||||||
Trade and other payables | 24 | 286,173 | 80,824 | 56,362 | ||||||||
Total non-current liabilities | 50,008,642 | 21,518,615 | 19,270,502 | |||||||||
CURRENT LIABILITIES | ||||||||||||
Borrowings | 21 | 33,344,287 | 39,011,807 | 41,482,011 | ||||||||
Income tax provision | 29,789 | — | — | |||||||||
Trade and other payables | 24 | 26,817,961 | 18,577,552 | 24,760,679 | ||||||||
Total current liabilities | 60,192,037 | 57,589,359 | 66,242,690 | |||||||||
Total liabilities | 110,200,679 | 79,107,974 | 85,513,192 | |||||||||
Total liabilities and equity | 133,171,562 | 101,589,117 | 117,316,145 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-193
RIZOBACTER ARGENTINA S.A.
CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars)
Attributable to the parent company's owners | Non- controlling interests | Total equity | ||||||||||||||||||||||||||||
Item | Share capital | Capital Adjustment | Legal reserve | Statutory reserve | Revaluation Surplus | Foreign currency translation | Retained earnings / (Accumulated losses) | Total | ||||||||||||||||||||||
Balance at July 1, 2016 | 6,541,236 | 571,824 | 1,266,023 | 22,270,432 | 21,676,334 | (24,924,584 | ) | (4,966,729 | ) | 22,434,536 | 46,607 | 22,481,143 | ||||||||||||||||||
Shareholders’ Meeting dated September 23, 2016 | ||||||||||||||||||||||||||||||
-Distribution of dividends in shares | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||
-Distribution of dividends in cash(1) | — | — | — | (1,450,613 | ) | — | — | — | (1,450,613 | ) | — | (1,450,613 | ) | |||||||||||||||||
-Setting-up of legal reserve | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||
-Setting-up of statutory reserve | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||
Sub-total | — | — | — | (1,450,613 | ) | — | — | — | (1,450,613 | ) | — | (1,450,613 | ) | |||||||||||||||||
Net profit for the year | — | — | — | — | — | — | 1,545,891 | 1,545,891 | 1,051 | 1,546,942 | ||||||||||||||||||||
Other comprehensive income | — | — | — | — | 2,256,554 | (1,864,132 | ) | — | 392,422 | (1,267 | ) | 391,155 | ||||||||||||||||||
Total comprehensive income | — | — | — | — | 2,256,554 | (1,864,132 | ) | 1,545,891 | 1,938,313 | (216 | ) | 1,938,097 | ||||||||||||||||||
Increase in the participation in subsidiaries | — | — | — | — | — | — | — | — | 2,256 | 2,256 | ||||||||||||||||||||
Balance at June 30, 2017 | 6,541,236 | 571,824 | 1,266,023 | 20,819,819 | 23,932,888 | (26,788,716 | ) | (3,420,838 | ) | 22,922,236 | 48,647 | 22,970,883 | ||||||||||||||||||
Attributable to the parent company's owners | Non- controlling interests | Total equity | ||||||||||||||||||||||||||||
Item | Share capital | Capital Adjustment | Legal reserve | Statutory reserve | Revaluation Surplus | Foreign currency translation | Retained earnings / (Accumulated losses) | Total | ||||||||||||||||||||||
Balance at July 1, 2015 | 5,472,746 | 571,824 | 1,100,407 | 20,793,962 | 10,767,550 | (12,297,201 | ) | 5,350,185 | 31,759,473 | 43,480 | 31,802,953 | |||||||||||||||||||
Shareholders’ Meeting dated September 14, 2015 | ||||||||||||||||||||||||||||||
-Distribution of dividends in shares | 1,068,490 | — | — | — | — | — | (1,068,490 | ) | — | — | — | |||||||||||||||||||
-Distribution of dividends in cash(1) | — | — | — | — | — | — | (2,136,980 | ) | (2,136,980 | ) | — | (2,136,980 | ) | |||||||||||||||||
-Setting-up of legal reserve | — | — | 165,616 | — | — | — | (165,616 | ) | — | — | — | |||||||||||||||||||
-Setting-up of statutory reserve | — | — | — | 1,476,470 | — | — | (1,476,470 | ) | — | — | — | |||||||||||||||||||
Sub-total | 1,068,490 | — | 165,616 | 1,476,470 | — | — | (4,847,556 | ) | (2,136,980 | ) | — | (2,136,980 | ) | |||||||||||||||||
Net loss/profit for the year | — | — | — | — | — | — | (5,469,358 | ) | (5,469,358 | ) | 7,747 | (5,461,611 | ) | |||||||||||||||||
Other comprehensive income | — | — | — | — | 10,908,784 | (12,627,383 | ) | — | (1,718,599 | ) | (14,585 | ) | (1,733,184 | ) | ||||||||||||||||
Total comprehensive income | — | — | — | — | 10,908,784 | (12,627,383 | ) | (5,469,358 | ) | (7,187,957 | ) | (6,838 | ) | (7,194,795 | ) | |||||||||||||||
Increase in the participation in subsidiaries | — | — | — | — | — | — | — | — | 9,965 | 9,965 | ||||||||||||||||||||
Balance at June 30, 2016 | 6,541,236 | 571,824 | 1,266,023 | 22,270,432 | 21,676,334 | (24,924,584 | ) | (4,966,729 | ) | 22,434,536 | 46,607 | 22,481,143 | ||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-194
RIZOBACTER ARGENTINA S.A.
CONSOLIDATED STATEMENT OF CHANGES IN EQUITY
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars)
Attributable to the parent company's owners | Non- controlling interests | Total equity | ||||||||||||||||||||||||||||
Item | Share capital | Capital Adjustment | Legal reserve | Statutory reserve | Revaluation Surplus | Foreign currency translation | Retained earnings / (Accumulated losses) | Total | ||||||||||||||||||||||
Balance at July 1, 2014 | 4,763,107 | 571,824 | 905,256 | 16,500,469 | — | (10,419,719 | ) | 7,586,394 | 19,907,331 | 17,110 | 19,924,441 | |||||||||||||||||||
Shareholders’ Meeting dated October 6, 2014 | ||||||||||||||||||||||||||||||
-Distribution of dividends in shares | 709,639 | — | — | — | — | — | (709,639 | ) | — | — | — | |||||||||||||||||||
-Distribution of dividends in cash(1) | — | — | — | — | — | — | (1,951,508 | ) | (1,951,508 | ) | — | (1,951,508 | ) | |||||||||||||||||
-Setting-up of statutory reserve | — | — | — | 4,293,493 | — | — | (4,293,493 | ) | — | — | — | |||||||||||||||||||
-Setting-up of legal reserve | - | — | 195,151 | — | — | — | (195,151 | ) | — | — | — | |||||||||||||||||||
Sub-total | 709,639 | — | 195,151 | 4,293,493 | — | — | (7,149,791 | ) | (1,951,508 | ) | — | (1,951,508 | ) | |||||||||||||||||
Net profit/loss for the year | — | — | — | — | — | — | 5,834,608 | 5,834,608 | (1,740 | ) | 5,832,868 | |||||||||||||||||||
Other comprehensive income | - | — | — | — | 10,767,550 | (1,877,482 | ) | (921,026 | ) | 7,969,042 | 7,881 | 7,976,923 | ||||||||||||||||||
Total comprehensive income | — | — | — | — | 10,767,550 | (1,877,482 | ) | 4,913,582 | 13,803,650 | 6,141 | 13,809,791 | |||||||||||||||||||
Increase in the participation in subsidiaries | — | — | — | — | — | — | — | — | 20,229 | 20,229 | ||||||||||||||||||||
Balance at June 30, 2015 | 5,472,746 | 571,824 | 1,100,407 | 20,793,962 | 10,767,550 | (12,297,201 | ) | 5,350,185 | 31,759,473 | 43,480 | 31,802,953 | |||||||||||||||||||
| (1) | The dividend distributed in cash represents USD 0.04, 0.07 and 0.08 per share on the share capital at the beginning of the years ended June 30, 2017, June 30, 2016 and June 30, 2015, respectively. |
The accompanying notes are an integral part of these consolidated financial statements.
F-195
RIZOBACTER ARGENTINA S.A.
CONSOLIDATED STATEMENT OF CASH FLOWS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars)
Notes | 6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||||
Cash from operating activities | ||||||||||||
Net profit / (loss) | 1,546,942 | (5,461,611 | ) | 5,832,868 | ||||||||
Adjustments for: | ||||||||||||
Income tax | 1,789,654 | (2,443,866 | ) | 3,198,198 | ||||||||
Property, plant and equipment depreciation | 2,038,380 | 1,761,478 | 920,271 | |||||||||
Intangible assets amortization | 556,051 | 55,991 | 32,681 | |||||||||
Accrued interest | 12,805,446 | 16,519,760 | 11,061,544 | |||||||||
Exchange differences and other financial results, net | 3,798,465 | 1,373,368 | 46,774 | |||||||||
Bad debts charge | 809,487 | 1,331,189 | 606,872 | |||||||||
Charge for obsolescence | 481,692 | 319,229 | — | |||||||||
(Recovery) / Charge for contingencies | (2,205 | ) | 268,761 | 556,474 | ||||||||
Net losses on investments in joint ventures | 1,109,131 | 848,948 | 584,728 | |||||||||
Gain from the sale of fixed assets | (47,680 | ) | (26,079 | ) | (184,606 | ) | ||||||
Changes in operating assets and liabilities: | ||||||||||||
Increase in inventories | (4,762,057 | ) | (5,584,908 | ) | (4,613,400 | ) | ||||||
Increase in operating receivables | (24,418,775 | ) | (10,040,532 | ) | (6,686,155 | ) | ||||||
Increase in operating liabilities | 11,037,864 | 4,763,441 | 1,924,419 | |||||||||
Interest received | 332,147 | 174,368 | 1,048,953 | |||||||||
Income tax paid | (1,278,038 | ) | (2,724,561 | ) | (5,533,106 | ) | ||||||
Net cash flows generated from operating activities | 5,796,504 | 1,134,976 | 8,796,515 | |||||||||
Cash from investing activities | ||||||||||||
Payments of property, plant and equipment | (1,292,629 | ) | (3,385,056 | ) | (7,608,243 | ) | ||||||
Payment of intangible assets | (1,452,377 | ) | (636,609 | ) | (1,468,781 | ) | ||||||
Proceeds from sale of property, plant and equipment | 130,011 | 67,621 | 410,471 | |||||||||
Shor-term investments | (4,247,669 | ) | — | — | ||||||||
Capital increases in joint ventures | (1,101,081 | ) | (1,257,553 | ) | (8,430,014 | ) | ||||||
Net cash flow used in investing activities | (7,963,745 | ) | (5,211,597 | ) | (17,096,567 | ) | ||||||
Cash from financing activities | ||||||||||||
Interest paid | (12,453,083 | ) | (17,323,176 | ) | (13,305,763 | ) | ||||||
Dividends paid | (1,203,858 | ) | (1,541,964 | ) | (1,862,629 | ) | ||||||
Proceeds from borrowings | 79.784.507 | 66,359,460 | 58,329,284 | |||||||||
Repayment of borrowings | (63,119,098 | ) | (46,993,776 | ) | (31,816,238 | ) | ||||||
Increase in non-controlling interest | 1,400 | 3,126 | 26,369 | |||||||||
Net cash flows generated from financing activities | 3,009,868 | 503,670 | 11,371,023 | |||||||||
Net increase / (decrease) in cash and cash equivalents | 842,627 | (3,572,951 | ) | 3,070,971 | ||||||||
Cash, cash equivalents and overdraft at the beginning of the year | 27 | 98,268 | 3,671,219 | 600,248 | ||||||||
Cash, cash equivalents and overdraft at the end of the year | 27 | 940,895 | 98,268 | 3,671,219 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-196
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Contents of the notes to the consolidated financial statements
| 1. | General information |
| 2. | Accounting standards and basis for preparation |
| 3. | Segment reporting |
| 4. | Cost of sales |
| 5. | Administrative expenses |
| 6. | Distribution expenses |
| 7. | Research expenses |
| 8. | Other operating income and expenses, net |
| 9. | Financial income and financial costs |
| 10. | Share of net losses of joint ventures accounted for using the equity method |
| 11. | Earnings per share |
| 12. | Property, plant and equipment |
| 13. | Intangible assets |
| 14. | Investment property |
| 15. | Investment in joint arrangements |
| 16. | Other investments |
| 17. | Inventories |
| 18. | Trade and other receivables |
| 19. | Financial assets at fair value through profit or loss |
| 20. | Cash and cash equivalents |
| 21. | Borrowings |
| 22. | Finance leases |
| 23. | Provisions |
| 24. | Trade and other payables |
| 25. | Transactions and balances with related parties |
| 26. | Income tax |
| 27. | Additional information on the Statement of Cash Flow |
| 28. | Transactions with non-controlling interests |
| 29. | Contingencies, commitments and restrictions on the distribution of profits |
| 30. | Pledged and restricted assets |
| 31. | Financial Risk Management |
| 32. | Subsequent events |
F-197
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
1. General information
Rizobacter Argentina S.A. (hereinafter “Rizobacter” or “the Company”) was created on October 20, 1983 in the city of Pergamino and is engaged in the development of microbiological products applied to the agricultural market. Through the Company and its subsidiaries (“the Group”), Rizobacter operates in Argentina, Brazil, Uruguay, Paraguay, Bolivia, France, United States, South Africa and it is in the process of creating subsidiaries in Colombia and India.
Rizobacter is a company whose articles of incorporation were registered with the Superintendence of Commercial Companies of the Province of Buenos Aires on October 20, 1983 under number 15284, registration No. 1/32.501 with a term established until October 19, 2082. The last amendment of its by-laws was approved by the Extraordinary Shareholders’ Meeting No. 49, dated March 31, 2015 and is pending registration with the Public Registry of Commerce.
On October 19, 2016 RASA Holding LLC acquired the 50.01% of Rizobacter Argentina S.A. shares. The sole shareholder of RASA Holding LLC is Bioceres Inc., which is controlled by Bioceres S.A., a company incorporated in Argentina. Additionally on that date, Marcelo Antonio Carrique took place in the position of board president and Ricardo Luis Yapur in the position of board vice president. These consolidated financial statements do not reflect a stepped-up basis of accounting related to the change in control.
Below is a description of the capital status of Rizobacter.
Common shares | Subscribed and paid-up shares (ARG pesos) | ||
Class “A” par value 1 Argentine Peso – 5 voting rights | 40,000,000 | ||
The capital status is as follows:
6/30/2017 (ARG pesos) | 6/30/2016 (ARG pesos) | 6/30/2015 (ARG pesos) | |||||||
Share capital at the beginning of the year | 40,000,000 | 30,000,000 | 24,000,000 | ||||||
Share capital at the end of the year | 40,000,000 | 40,000,000 | 30,000,000 | ||||||
On March 31, 2015, the Shareholders’ Meeting of the Company approved the establishment of a global program of Negotiable Obligations (“the Program”) for the issue of one or more series of simple Negotiable Obligations, through public offering, for their future listing on stock exchanges and other markets, up to a revolving outstanding amount of USD 40,000,000 or the equivalent in other currencies, or a lower amount to be determined by the Board of Directors, with a maximum term of five years.
On July 16, 2015, the Argentine National Securities Commission (CNV for its acronym in Spanish) approved the Program and the Company was included in the CNV’s public offering regime of corporate bonds. The details of the program and the series issues by the Company are described in Note 21.
2. Accounting standards and basis for preparation
The main accounting standards used in the preparation of these consolidated financial statements are summarized below. These accounting standards have been applied consistently for all the periods presented.
2.1. Basis for preparation
The CNV has established the application of Technical Pronouncement (RT) Nos. 26 and 29 issued by the F.A.C.P.C.E. adopting International Financial Reporting Standards (IFRS), the standards issued by the International Accounting Standards Board (IASB), for those entities included in the public offering regime of Law No. 17811, due either to their
F-198
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
share capital or negotiable obligations, or having requested listing authorization to be included in this regime. The application of these standards was mandatory for the Company as from the fiscal year commenced July 1, 2014, and financial statements at June 30, 2015 were the first financial statements presented under these standards.
The Company’s consolidated financial statements have been prepared in accordance with the IFRS issued by IASB. Also, the accounting policies applied are based on the IFRS issued by the IASB and interpretations issued by the IFRIC that are applicable at the date of the separate financial statements.
The preparation of these financial statements in accordance with IFRS requires the use of certain critical accounting estimates. It also requires that management exercises its judgment to apply the accounting policies of the Group. The areas involving a higher degree of judgment or complexity, or areas where assumptions and estimates are significant to the consolidated financial statements are disclosed in Note 2.20.
These consolidated financial statements were approved by the Company's Board of Directors on October 30, 2017.
The financial statements are stated in USD with no cents except the earnings/losses per share and otherwise indicated.
2.2. Consolidation
(a) Subsidiaries
Subsidiaries are all entities (including special purpose entities) in which the Group is exposed or has the right to variable returns from its participation in the subsidiary and has the ability to affect those returns through its power over the subsidiary, which is generally accompanied by an interest exceeding 50% of available voting rights. The existence and effect of potential voting rights that are currently exercisable or convertible are considered when assessing whether the Group controls another entity.
The Group also assesses the existence of control when it has not more than 50% of the voting rights but may direct the operating and financial policies in view of the “the facto control”. The “the facto control” may arise in circumstances where the relative size of voting rights of the Group in relation with the number and dispersion of other shareholders provides the Group with power to direct the operating and financial policies, etc. The subsidiaries are consolidated as from the date in which the control is transferred to the Group and excluded from the date that control ceases.
(b) Joint ventures and operations
They are entities jointly controlled over which the Group has a joint control. Interest in jointly controlled entities are classified into i) joint operations and ii) joint ventures, according to IFRS 11. Joint ventures are equity accounted. Joint operations are accounted for by proportionate consolidation, i.e., the share of their individual income and expenses, assets, liabilities, and cash flow is recognized on a line-by-line basis in the Group's financial statements. The Group recognizes the portion of gains and losses on the sale of assets by the Group to the joint venture or operation that is attributable to the other venturers. When the Group purchases assets from a joint venture or operation, it recognizes its portion of the gain or loss when the assets are re-sold to a third party. However, the loss on that sale is recognized immediately if it represents a reduction of the recoverable value of the asset or an impairment of the asset.
F-199
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
The Company has investments in the following joint ventures:
Interest and votes at | ||||||||||||||||||||
Name | Country | Re. | 6/30/2017 | 6/30/2016 | 6/30/2015 | Line of business | ||||||||||||||
- Synertech Industrias S.A. | Argentina | (1 | ) | 50.00 | % | 50.00 | % | 50.00 | % | Production and selling of fertilizers | ||||||||||
- Semya S.A. | Argentina | (1 | ) | 50.00 | % | 50.00 | % | 50.00 | % | Development of new biological products | ||||||||||
| (1) | Represents the direct holding percentage held by Rizobacter Argentina S.A. |
(c) Consolidation Structure
i) Companies consolidated at 100% are as follows:
Interest and votes at | ||||||||||||
Name | Country | Re. | 6/30/2017 | 6/30/2016 | 6/30/2015 | Line of business | ||||||
- Rizobacter do Brasil Ltda. | Brazil | (1) | 99.90 | % | 99.90 | % | 99.90 | % | Selling of agricultural inputs | |||
- Rizobacter del Paraguay S.A. | Paraguay | (1) | 95.00 | % | 95.00 | % | 95.00 | % | Selling of agricultural inputs | |||
- Rizobacter Uruguay S.A | Uruguay | (1) | 99.00 | % | 99.00 | % | 99.00 | % | Selling of agricultural inputs | |||
- Rizobacter South Africa | South Africa | (1) | 95.00 | % | 95.00 | % | 95.00 | % | Selling of agricultural inputs | |||
- Comer. Agrop. Rizobacter de Bolivia S.A. | Bolivia | (1) | 95.00 | % | 95.00 | % | 95.00 | % | Selling of agricultural inputs | |||
- Rizobacter USA, LLC | United States of America | (1) | 95.00 | % | 95.00 | % | 95.00 | % | Selling of agricultural inputs | |||
- Indrasa Biotecnología S.A. | Argentina | (1) | 52.50 | % | 52.50 | % | 52.50 | % | Development of new biological products | |||
- Synertech SAS | France | (1) and (2) | 100.00 | % | — | — | Development of new biological products | |||||
Reference
| (1) | Represents the direct holding percentage held by Rizobacter Argentina S.A. |
| (2) | Company not yet incorporated |
2.3. Segment reporting
The operating segments are presented consistently with the internal information provided to the person in authority in charge of the Group's operating decision-making. The Board of Directors making strategic decisions has been identified as the CODM which is responsible for allocating resources and assessing the performance of operating segments. Segment reporting is detailed in Note 3 below.
2.4. Foreign currency translation
(a) Functional and presentation currency
The financial statement figures of each of the Group’s entities were measured using the currency of the primary economic environment in which the entity operates (the ‘functional currency’), which is the Argentine Peso for Rizobacter Argentina S.A. The group consolidated financial statements are presented in US Dollars (USD), which is the presentation currency. For the case of investments, functional currency has been defined as the currency of the primary economic environment in which those entities operate.
F-200
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
IAS 29 “Financial Reporting in hyperinflationary economies” requires that the financial statements of an entity that reports in the functional currency of a hyperinflationary economy should be stated in terms of the measuring unit current at the closing of the reporting period, irrespective of whether they are based on the historical cost or current cost methods. To this end, in general terms, non-monetary items include inflation from the acquisition date of the item or the restatement date, as applicable. To determine whether there is a hyperinflationary economy, the standard provides a series of factors to be taken into account, among others, a cumulative inflation rate over three years that is approaching, or exceeds, 100%.
At June 30, 2017, it is not possible to calculate the cumulative inflation rate for the three-year period then ended based on official data from the National Institute of Statistics and Census (INDEC), given that in October 2015 this agency discontinued the calculation of the Domestic Wholesale Price Index, only resuming its calculation from January 2016 onwards.
At the end of the reporting period, the Management has evaluated that the Argentine peso does not meet the characteristics to be classified as the currency of a hyperinflationary economy, according to the guidelines of IAS 29 and the Government’s expectations towards a lower inflation level; therefore, these separate financial statements have not been restated in constant currency.
Nevertheless, in the last few years, certain macroeconomic variables that affect the Group’s business, such as labour costs and the prices of inputs, have recorded yearly variations of some importance. This circumstance must be taken into account in the assessment and interpretation of the financial position and the results disclosed by the Group in these consolidated financial statements.
Foreign currency balances and transactions
Foreign currency transactions are translated into the functional currency using the exchange rates prevailing at the dates of the transactions (or of valuation, if transactions that are to be re-measured are involved). Foreign exchange gains and losses resulting from the settlement of such transactions and from the measurement at period end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognized in the statement of income, except for cash flow or net investment hedges that qualify for deferral as other comprehensive income. Exchange differences related to loans and cash and cash equivalents are disclosed in the income statement within “financial income or cost”.
(b) Translation of financial statements of foreign entities
The results and financial position of all Group entities (none of which has the currency of a hyperinflationary economy) that have a functional currency other than the presentation currency of the Group are converted into the presentation currency as follows:
| ■ | Assets and liabilities at year end are translated at the exchange rate prevailing at that date; |
| ■ | Income and expenses are translated at the average exchange rate (unless this average does not represent a reasonable approximation of the cumulative effect of the exchange rates prevailing at the date of each transaction, in which case those income and expenses are translated at the exchange rates prevailing at the date of each transaction); and |
| ■ | The resulting exchange differences are presented in other comprehensive income. |
Goodwill and adjustments at fair value resulting from the acquisition of foreign entities are treated as assets and liabilities of the foreign entity and translated at the year-end exchange rate. The resulting exchange differences are recorded in other comprehensive income.
When an investment is sold or disposed of in whole or in part, the exchange differences are recognized in the statement of comprehensive income as part of the gain or loss on that sale/disposal.
F-201
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
2.5. Property, plant and equipment
Property, plant and equipment are valued at acquisition cost, which includes additional costs produced until the goods are prepared for operation, less accumulated depreciation and losses for cumulative impairment, if any. Subsequent costs are included in the asset's carrying amounts only if future economic benefits are expected to arise from their disposals and their cost can be measured reliably. The value of replacement parts is written off. The other repair and maintenance expenses are charged to earnings in the year when incurred. Financial costs were capitalized in accordance with Note 2.14.
The amount of depreciation is recorded in the profit/loss for the year following a lineal method as from the useful life of the different types of assets. The Group checks the net carrying value, useful life and depreciation method for Property, plant and equipment at year end. Changes of criteria initially established are recognized, as the case may be, as a change of estimate. Land is not depreciated. Depreciation of assets is calculated using the straight-line method over their estimated useful life, as follows:
Years | |||
Buildings | 50 | ||
Facilities | 10 | ||
Machinery and equipment | 10 | ||
Furniture and fixtures | 10 | ||
Vehicles | 5 | ||
Computer hardware | 3 | ||
The amount of property, plant and equipment elements is written down to its recoverable amount if the asset’s book value is greater than its estimated recoverable amount. Gains and losses on sales of assets are measured by comparing the income received with their carrying value and are disclosed within "Other operating income, net", in the statement of income.
Further, assets under items Buildings and Land, are accounted for at fair value arising from the last revaluation performed, applying the revaluation model indicated by IAS 16. This policy was adopted by the entity as from the year ended June 30, 2015.
Revaluations are performed on a regular basis, when there are signs that the book value differs significantly from that to be determined using the fair value at the end of the reporting year.
To obtain fair values, the existence of an active market is considered for the assets in their current status. For those assets for which an active market in their current status exists, the fair values were determined based on their market values. For the remaining cases, the market values of new assets have been analysed, applying a discount based on the status and wear of each asset and considering the characteristics of each of the revalued assets (for example, improvements made, maintenance status, level of productivity, use, etc.)
2.6. Intangible assets
Intangible assets are non-monetary assets, without physical substance, that are identifiable separately or which result from legal or contractual rights. They are recorded when they can be measured reliably and are expected to produce benefits for the Group.
2.6.1 Intangible assets acquired separately
Intangible assets with defined useful lives acquired separately are accounted for at cost less accumulated amortization and less accumulated losses for impairment. Amortization is recognized based on the straight-line method on their
F-202
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
estimated useful lives. The estimated useful life and the amortization method are reviewed at the end of each reporting period, with the effect of any changes in those estimated recorded on a prospective basis. Intangible assets with indefinite useful life acquired separately are recorded at cost less accumulated losses for impairment.
2.6.2 Intangible assets internally generated - disbursements for research and development
Disbursements generated by research activities are recognized as an expense in the period they are incurred.
An intangible asset internally generated as a result of development activities (or the development phase of an internal project) is recognized if all conditions mentioned below have been met:
| ■ | Technical feasibility to complete the intangible asset in a way it may be available for use or sale; |
| ■ | Intention to complete the intangible asset at issue, to use it or sell it; |
| ■ | Ability to use or sell the intangible asset; |
| ■ | The way in which the intangible asset is to generate probable future economic benefits; |
| ■ | Availability of certain technical, financial or other resources to complete the development and for using or selling the intangible asset; and |
| ■ | The ability to measure, reliably, the disbursement attributable to the intangible asset during its development. |
The amount initially recognized for an intangible asset internally generated is the sum of the disbursement incurred as from the first time in which the element complies with the conditions for its recognition, listed above.
When an internally generated intangible asset cannot be recognized, the disbursements are allocated to profits and loss in the period they are incurred. The Group started the recognition of intangible assets as from the year 2014. As from that year, the Group generated accounting information to identify separately development expenses from research expenses.
Subsequent to initial recognition, an intangible asset internally generated is reported by its cost less accumulated amortization and the cumulative amount of losses due to impairment, on the same basis of the intangible assets that are acquired separately. Financial costs were capitalized in accordance with Note 2.14.
2.7. Financial assets
In accordance with IFRS 9, financial assets, subsequent to their initial recognition are measured at amortized cost or fair value, on the basis of:
| (a) | Group’s business model for managing the financial assets, and |
| (b) | Contractual cash flow characteristics of the financial asset. |
Financial assets at amortized cost
Financial assets are measured at amortized cost if both the following conditions are met:
| (a) | Asset is held within a business model aimed at maintaining the assets to obtain contractual cash flows; and |
| (b) | Contractual conditions of the financial asset give rise on specified dates to cash flows that are only payments of the principal and interest on the outstanding principal amount. |
F-203
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Financial assets at fair value through profit or loss
Within this category are those financial assets held for trading. A financial asset is classified in this category if it is mainly acquired to be sold in the short-term. Derivative instruments are included in this category unless they have been designated as hedge instruments. Assets in this category are classified as current if expected to be settled within 12 months, otherwise they are classified as non-current.
Financial assets at fair value through profit or loss are recorded at fair value, recognizing any profit or loss from the re-measurement in the income statements. Net profits or losses recognized in the income statements include all dividends or interest earned on the financial asset and is included under “Other income and expenses” in the consolidated statement of comprehensive income.
Recognition and measurement
Regular purchases and sales of financial assets are recognized on the trade-date (the date on which the Company commits to purchase or sell the asset). Investments are initially recognized at fair value plus transaction costs for all financial assets at amortized cost. Financial assets valued at fair value through profit or loss are initially recognized at fair value, and the transaction costs are recognized in the statement of income. Financial assets are derecognised when the rights to receive cash flows from investments have expired or have been transferred.
Offsetting of financial assets against financial liabilities
Financial assets and financial liabilities are offset, and presented net on the statement of financial position, when there is a legally enforceable right to offset the recognized amounts, and it is the Group's intention to settle the net amount, or to simultaneously realize the asset and settle the liability.
Impairment of financial assets
At each period closing, the Group assesses whether there is objective evidence that a financial asset or a group of financial assets is impaired. Impairment losses of financial assets are recognized when there is objective evidence of the impairment as a result of one or more events occurred after the initial recognition of the financial asset and that event has impact on the estimated cash flows for that financial asset or group of financial assets, which may be reliably estimated.
Evidence of the impairment loss may include indicators where the debtors or Group of debtors are undergoing significant financial trouble, non-payments or delays in the payment of interest and principal, the probability of a bankruptcy proceedings or any other situation involving a financial reorganization, and as regards observable data
They indicate a decrease that may be measured, such as changes in the payment methods or in the economic conditions correlated with non-payments.
If in a subsequent period, the amount of the impairment loss decreases, and the decrease may be objectively attributed to an event occurred after recognition of the impairment (for example, an improvement in the debtor's credit standing), the reversal of the previously recognized impairment loss will be recognized in the income statement.
2.8. Inventories
Inventories are valued at the lower of cost or net realizable value. Cost is determined applying the weighted average price method. The cost of finished products and products in process includes the costs of design, raw material, direct labour, other direct costs and overhead manufacturing expenses (based on normal operating capacity) but it excludes loan costs. Net realizable value is the sale price estimated in the normal course of business, less applicable variable costs to sell. Inventory costs includes transfers of equity of the profits/loss for hedge cash flow transactions related to purchases of raw material.
F-204
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
2.9. Trade and other receivables
The following assets are mainly included in this caption:
Trade receivables: They are amounts owed by customers for goods sold or services provided in the normal course of business. If the collection is expected within a year or less (or over a year, in the normal course of business), they are classified as non-current assets. Otherwise, they are disclosed as non-current assets. Trade receivables are initially recognized at fair value and subsequently measured at amortized cost in accordance with the effective interest rate method, less the allowance for bad debts.
Tax credit balances: it corresponds to amounts paid for national, provincial or municipal taxes which may be applied to the cancellation of future taxes. These assets are recognized as long as their use against future taxes of the same nature is feasible, or if applicable, they are returned by the pertinent tax authorities.
Advances to suppliers: They relate to sums advanced to suppliers for services pending reception, as well as expenses paid pending accrual. They are recognized by the amount delivered, net of the value of services already received and expenses accrued.
Other credits receivable: they are financial assets which represent balances receivable initially recognized at fair value and subsequently measured at amortized cost applying the effective interest rate method.
The allowance for bad debts is recorded when there is objective evidence that the Group may not recover all pending amounts through its collection or future use. The allowance has been determined based on estimates as to the probability of recovery of the assets, taking into account the reports from legal advisors, securities received and the pertinent debtors’ equity and financial condition.
The assets' carrying amount is written down through an allowance account and the amount of the loss is recognized in the consolidated statement of income and disclosed in the item “Selling expenses”. The recovery of the amounts previously recognized as impairment losses are recognized as credits in the line “Other operating income and expenses” of the consolidated income statement.
2.10. Cash and cash equivalents
In the consolidated statement of cash flows, cash and cash equivalents include cash on hand, time deposits with financial institutions and other short-term highly liquid investments with original maturities of three months or less as well as bank overdrafts. In the statement of financial position, bank overdrafts, if any, are classified as loans in the current liabilities.
2.11. Share capital
The corporate capital is made up of 40,000,000 Class "A" common shares, of 1 Argentine peso par value each and entitled to five votes per share and has been subscribed and fully paid up. The capital status is described in Note 1.
2.12. Trade and other payables
The following liabilities are mainly included in this caption:
Trade payables represent payment obligations for goods and services purchased from suppliers in the normal course of business. They are disclosed under current liabilities if their payment is enforceable within one year or less (or over a year, in the normal course of business).
Accounts payable are initially recognized at fair value and subsequently measured at amortized cost using the effective interest rate method.
F-205
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Tax debts: they comprise balances to be settled for taxes, rates and contributions. Their measurement is made at nominal value of the amounts payable, except for the case in which the financial effects are significant, in which case, the measurement at each closing is made on the basis of the current value of the amounts to be disbursed, discounted using a rate that reflects the evaluations of the market as to time value of money as well as specific risks of the obligation to be settled.
Advances from customers and services billed in advance: they comprise balances collected in advance for products pending delivery. They are initially recognized at fair value and subsequently measured at amortized cost using the effective interest rate method.
Other accounts payable: they are financial liabilities which represent balances payable initially recognized at fair value and subsequently measured at amortized cost applying the effective interest rate method.
2.13. Borrowings
Loans are initially recognized at fair value, net of the transaction costs incurred. They are subsequently valued at amortized cost and any difference between the proceeds (net of transaction costs) and the reimbursement value is recognized in the statement of income over the period of the loan using the effective interest method.
Fees paid in the establishment for lines of credit are recognized as transaction costs of the loan, as long as it is probable that some or all of them are made available. In this case, the fee is deferred until the loan is available. Provided that there is no evidence that it is probable that some or all of the credit lines be used, the fee will be capitalized as an advance payment for liquidity services and is amortized during the period to which the credit lines refers.
The effective interest method is a method for calculating the amortized cost of a debt instrument and for allocating the financial income throughout the corresponding period. The effective interest rate is the discount rate that exactly equals the estimated future cash flows receivable (including commission, basic points of interest paid or earned, cost of transaction and other premiums or discounts included in the calculation of the effective interest rate) throughout the expected life of the debt instrument or, when applicable, over a shorter period, with the net book amount based on its initial recognition.
2.14. Borrowing costs
Borrowing costs, either generic or specific, attributable to the acquisition, construction or production of assets that necessarily take a substantial period of time to get ready for their intended use or sale (qualifying assets) are included in the cost of the assets until the moment in which they are substantially ready for use or sale.
Income earned on the temporary investments of funds generated in specific borrowings still pending use in the qualifying assets, are deducted from the total of financing costs potentially eligible for capitalization.
During the year ended June 30, 2017 the Company has capitalized financial costs in qualifying assets for USD 338,600 (2016: 743,837, 2015: USD 1,195,266) in qualifying assets. Costs for interest were capitalized at the average weighted rate of their general borrowings of 29.50%.
All the other borrowing costs are recognized in the period when incurred.
F-206
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
2.15. Leases
The Group classifies leases to which it is a party, either in financial or operating, based on the following:
(a) Financial leases
The Group leases some items of property, plant and equipment. Leases of property, plant and equipment, where the Group has all the risks and rewards of the ownership, are classified as financial leases. Finance leases are capitalized at the lease’s inception at the lower of the fair value of the leased property and the present value of the minimum lease payments.
Each lease payment is allocated between the liability and finance charges. The corresponding lease obligations, net of finance charges, are included in long-term payables. The interest element of the finance cost is charged to income over the lease period so as to produce a constant periodic rate of interest on the outstanding debt in each period. Assets acquired through finance leases are depreciated during the shortest period between the useful life of the asset or the leasing period.
(b) Operating leases
Leases where the lessor retains a significant portion of the risks and rewards of ownership are classified as operating leases. Payments made under operating leases (net of any incentives received from the lessor) are charged to income on a straight-line basis over the period of the lease.
2.16. Income tax and minimum notional income tax
(a) Income tax
The income tax charge comprises current and deferred taxes. The tax is recognized in the consolidated statement of income, except for the items that must be recognized directly in other comprehensive income. In this case, the income tax related to these items is also recognized in the statement of comprehensive income.
The current income tax charge is calculated on the basis of the tax laws in force at the statement of financial position date, in the countries in which the Company, its subsidiaries and associates operate and generate taxable income.
Deferred income tax is computed in its entirety according to the liability method, on the basis of the temporary differences arising between the tax bases of assets and liabilities and their respective carrying amounts shown in the consolidated financial statements. However, the deferred tax generated by the initial recognition of an asset or a liability in a transaction not corresponding to a business combination and that at the time of the transaction affects neither accounting profit or loss nor taxable profit, is not recorded. Deferred tax is computed using the tax rates in effect at the date of the consolidated statement of financial position and which are expected to apply when the deferred tax asset is realized or the deferred tax liability is settled.
Deferred tax assets are recognized only to the extent that tax benefits are likely to be obtained in the future to be able to offset the temporary differences.
The Group records a deferred tax liability on taxable temporary differences related to investments in subsidiaries and associates, unless both the following conditions are met:
| i) | the Group controls the timing of reversal of the temporary differences; and |
| ii) | it is probable that the temporary difference will not reverse in the foreseeable future. |
F-207
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
The balances of deferred income tax assets and liabilities are offset when there is a legally enforceable right to offset current tax assets against current tax liabilities and when they relate to the same taxation authority for the Company or the different subsidiaries where there is an intention and possibility to settle the tax balances on a net basis.
(b) Minimum notional income tax
The Company and its subsidiaries in Argentina compute the minimum notional income tax by applying the current 1% rate on computable assets at the end of the period. This tax complements income tax. The Company’s tax obligation will be the higher of the two taxes.
However, if in a fiscal year minimum notional income tax obligation exceeds income tax liability, the surplus will be computable as a payment on account of income tax through the next ten years.
2.17. Provisions and allowances
Provisions are recognized in the financial statements when:
| (a) | the Group has a present legal or constructive obligation as a result of past events; |
| (b) | it is more likely than not that an outflow of resources will be required to settle the obligation; and |
| (c) | and the amount has been reliably estimated. |
Provisions are measured at the present value of the expenditure required to settle the obligation considering the best information available at the financial statements date and are re-estimated at the end of each reporting period. The discount rate used to determine the present value is a rate before taxes that reflects market assessments, at the balance sheet date, of the time value of money and the risks specific to the liability. Higher provisions as a result of the passage of time are recognized as an interest expense.
2.18. Revenue recognition
Revenue is recognized at the fair value of the consideration received or receivable, and represents the amounts receivable for sales of goods and/or services, net of discounts, returns and value added tax. Revenue is recognized by the Group when the amounts can be reliably measured; when it is probable that future economic benefits will flow to the entity; and when specific criteria are met for each of the Group's business operations, as described below.
(a) Sales of goods
The Group manufactures and sells a wide range of microbiological products applied to the agricultural market. Income from ordinary activities from the sale of goods are recognized when all and each of the following conditions are met:
| ■ | The Group has transferred to the purchaser all significant risks and rewards of ownership of goods. |
| ■ | The Group neither keeps for itself any implication in the current management of goods sold, in the degree usually associated to the ownership, nor retains effective control on the goods sold; |
| ■ | The amount of the income from ordinary activities can be reliably measured. |
| ■ | It is probable that the economic benefits associated with the transaction will flow to the Group; and |
| ■ | The costs incurred or to be incurred in respect of the transaction may be measured reliably. |
b) Provision of services
The Group provides services for the application of microbiological products. For the sale of services, income is recognized in the period when they are provided, with reference to the finalization stage of the specific transaction and assessed on the basis of the service currently provided as a proportion of the total of services that will be provided.
F-208
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
2.19. Distribution of dividends
Dividends are recognized as a liability in the Group’s financial statements in the period when they are approved by the Group shareholders.
2.20. Critical accounting estimates
The preparation of financial statements requires the use of estimates. It also requires Group Management to exercise its judgment in the process of applying the accounting policies. The estimates and judgments are assessed on an ongoing basis, and are based on historical experience and other factors, including the expectations of future events considered reasonable under the circumstances.
The Group makes estimates and hypothesis with regard to the future. The resulting accounting estimates may not be equal to the results actually obtained. The Company’s most significant estimates and judgments are explained below.
(a) Income tax
The Group is subject to income tax in the countries in which it operates. In the determination of the income tax provision in each of the jurisdictions in which the Group pays this tax, it applies professional judgment to show the tax consequences of the economic events of each year, based on current tax legislation, making the best estimates based on the information available at the date of the consolidated financial statements.
(b) Provisions for lawsuits and contingencies
The evaluation of contingent liabilities is made by the Board and the Group’s legal advisors based on the judgment elements available at the date of these consolidated financial statements. In the estimate of the amounts we have considered, among other issues, the probability of occurrence. If in the evaluation of the contingency there is a probability that a loss is materialized and the amount may be estimated in a reliable manner, a liability is accounted for in the caption provisions for contingencies. If the potential loss is not probable, or if it is probable but its amount cannot be reliably estimated, the nature of the contingent liability and an estimate of the possibility of occurrence is disclosed in a note to the consolidated financial statements.
(c) Allowance for obsolescence and slow moving inventories
Management assesses the recoverability of its inventories considering their sales prices if the inventories are damaged or if they have total or partially become obsolete.
Net realizable value is the sale price estimated in the normal course of business, less costs of completion and other sales expenses.
The Group establishes an allowance for obsolescence or slow moving inventories in relation to finished products and in-process products. The allowance for slow moving inventories is recognized for finished products and in-process products based on an analysis of Management on the aging of stock.
(d) Calculation of useful lives and impairment of property, plant and equipment
As described in Note 2.5, the Group reviews the estimated useful life of property, plant and equipment at the end of each reporting period. During the current period, the estimated useful lives remained unchanged.
(e) Allowance for bad debts
Management makes estimates as regards the uncollectibility of its recorded receivables. Management analyses trade account receivables in accordance with conventional criteria, adjusting the amount through a charge of an allowance for
F-209
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
bad debts upon recognition of the inability of third parties to afford their financial obligations with the Group. Management analyses specifically the accounts receivable, the historical bad debts, solvency of customers, current economic trends and changes to the payment conditions of customers to assess the proper allowance for bad debts.
(f) Fair value of property, plant and equipment:
Assets recorded in lines “Land and buildings” are valued through the revaluation model indicated in IAS 16. To obtain reasonable values, we considered the existence or not of an active market for goods in their current condition. For those assets for which an active market in their current status exists, the fair values were determined based on their market values. For the remaining cases, market values were analysed for new assets, applying a discount based on the status and wear of each asset considering the characteristics of each of the revalued assets (for example, improvements, maintenance status, productivity levels, use, etc.).
(g) Recoverability of intangible assets internally generated
Directors analyse the recoverability of intangible assets internally generated arisen from the development of several microbiological products with commercial potential, which are included in the consolidated statement of financial position at the end of the year.
Projects continue to be developed satisfactorily and the market reaction has reconfirmed the prior estimates of projected income for projects. No events have been detected during the period which may have had influence in the Directors to reconsider their hypotheses as to the future participation in the market and projected margins as to these products. We have made a detailed sensitivity analysis and the Directors trust that the book value of the assets will be fully recovered, even if profits decrease. This situation is closely monitored and adjustments will be made to future periods if the market activity indicates that those adjustments are pertinent.
2.21. Changes in accounting policies
| (a) | New standards, amendments and interpretations adopted by the Group: |
IFRS 9 "Financial instruments" (2010): prescribes the classification, measurement and recognition of financial assets and liabilities. It replaces the parts of the International Accounting Standard No. 39 (IAS 39) that relate to the classification and measurement of financial instruments. IFRS 9 requires financial assets to be classified into two categories: those measured at fair value and those measured at amortized cost.
The complete version of this standard was issued in July 2014. It replaces the guidelines of IAS 39 and the prior version of IFRS 9, which are related to the classification and measurement of financial instruments. IFRS 9 keeps but simplifies the mixed measurement model and establishes three main categories to measure financial assets: amortized cost, fair value through other comprehensive income and fair value through profit or loss. The basis of this classification depends on the business model of the entity to manage its financial assets and the characteristics of the financial asset's contractual cash flows. In their initial recognition, an entity can make an irrevocable election to disclose in other comprehensive income the subsequent changes in the fair value of an investment in an equity instrument not held for trading. There were no changes in the classification and measurement of financial liabilities, except for the recognition of changes in its own credit risks (own credit rating) in other comprehensive income, for liabilities designated at fair value through profit or loss. Furthermore, IFRS 9 adjusts the requirements for effectiveness of hedge instruments. It requires the existence of an economic relation between the item hedged and the hedge instrument and that the reason for hedging be the same as that used by management for risk management. The requirement of a formal documentation of the hedge relationship at the beginning remains in effect but it is different to that under IAS 39. Also, this standard adds a model of receivable losses expected which substitutes the impairment model of financial assets used in IAS 39. The standard is effective for accounting periods commencing on or after January 1, 2018.
F-210
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
The Group has adopted IFRS 9 (2010) as from July 1, 2013, as well as important changes relating to other IFRS as this new accounting policy provides more relevant and reliable information for the users to assess figures, timing and uncertainty of future cash flows.
| (b) | New standards, amendments and interpretation which have come into effect as from the year beginning on July 1, 2016 and which have not generated effects on the consolidated financial statements: |
Amendments to IAS 16 “Property, plant and equipment” and IAS 41 “Agriculture” by “Bearer Plants”: amendments to the registration model of “bearer plants” have been introduced which must be accounted for in a similar manner as elements of property, plant and equipment, as their productive operation schemes are comparable under the scope of IAS 16, keeping the agricultural products (that are) developed therein within the scope of IAS 41. This regulatory changes are effective for annual periods beginning on or after January 1, 2016 and allows for early adoption. The application of these amendments has not had any significant impact on these consolidated financial statements.
| (c) | New published standards, amendments and interpretations which have not yet come into force for fiscal years beginning on or after July 1, 2015 and have not been early adopted: |
IFRS 15 Revenue from contracts with Customers: is a standard on revenue recognition agreed between the IASB and FASB which allows for improvement in financial reports over revenue, enabling their comparability at international level. It was published in May 2014 and it is effective for annual periods commencing as from January 1, 2017. The Group is assessing the possible impacts of this standard, but it is not possible at this moment to estimate the potential effects in the Company’s revenue recognition accounting policies.
IFRS 16 “Leases”: In January 2016, the IASB published IFRS 16 “Leases” which establishes the principles for the recognition, measurement, presentation and disclosure of leases. This standard is applicable for the years commencing as from January 1, 2019.
Amendments to IAS 12 “Income Tax”: these amendments to the recognition of deferred tax assets for unrealized losses clarify how to account for such assets when they are related to debt instruments measured at fair value. This standard is applicable for the years beginning on or after January 1, 2017. The Company does not estimate that its application will have a significant impact.
Amendments to IAS 7, “Statement of cash flows”: These amendments to the IAS 17 introduce additional disclosures which enable users to assess changes to liabilities for financing activities. This includes changes implying "cash flows", such as withdrawal of funds and loan reimbursements; and changes which do not imply "cash flows", such as acquisitions, disposals and unrealized exchange differences. This standard is applicable for the years commencing as from January 1, 2017. The Company does not estimate that its application will have a significant impact.
There are no other IFRS or IFRIC interpretations that are not yet effective and which are expected to have a significant effect on the Group.
3. Segment reporting
The Board of directors is the maximum authority for the operating decision making. The Board of directors has determined the operating segments based on information that it reviews with the aim of allocating resources and assessing performance.
The Board of directors considers the business from the geographic and product viewpoint. At geographical level, the Board considers the performance in Argentina, Brazil and Paraguay, which were not disclosed as they do not exceed the quantitative parameters required by IFRS 8. From the product perspective, the Board of directors considers separately the activities of the product families.
F-211
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
The Board assesses the performance of operating segments based on the gross results. This valuation basis excludes commercial, administrative, research and financial expenses, as these type of activities are carried out through the central administration, which manages the Group’s consolidated position.
The Group develops its main activities through the following family of products.
| ■ | Inoculants |
| ■ | Packs |
| ■ | Seed therapics |
| ■ | Adjuvants |
| ■ | Others |
Packs
Packs offer services that comprise more than one Group product and include:
| ■ | Inoculants, which ensure an adequate Biological Nitrogen Fixation. |
| ■ | Seed therapics that controls a wide range of germs. |
| ■ | Fertilizers applied to seeds that allow for an adequate plant nutrition. |
Other:
Within this segment we include the following family of products:
Biofertilizers:
Biofertilizers are biotechnology products that allow, to increase the yields of crops, through non-contaminant technologies.
Micronutrients:
Regarding the fertilization of crops, apart from obtaining and using the elements called macronutrients, as nitrogen and phosphorus, and mesonutrients such as sulphur and calcium, there is a wide range of micronutrients which are essential for a good development of the metabolic processes that maximize the productivity of crops.
Seed pelleting
Seed pelleting technology provides important advantages and benefits for crops among which we can mention:
| ■ | Uniformity and homogenization of forage seeds, which eases the sowing tasks. |
| ■ | Adding to seeds - generally of a smaller size, a complex of fungicides, insecticides and even fertilizers to achieve a better nutrition for treated crops, turning the seed into a “technology pack”. |
| ■ | The addition to leguminous species of specific bacteria of the genus Rhizobium, specific for each one of these species, with the purpose of maximizing the capacity to produce nodules of this family of plants. |
F-212
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Formulation for third parties
A service provided by the Company which includes:
| ■ | Formulation of Fungicides and Insecticides as Concentrated Suspensions, which consist in scattering the active principles in a water-based suspension, adjusting granulometry, concentration and viscosity, for which a pre-scattering stage is required, as well a humid grinding stage and one for adjusting concentration and viscosity. |
| ■ | Formulation of Fungicides and Insecticides as Concentrated Emulsions, which consists in dissolving the active principles in a blend of solvents, which will subsequently form an emulsion when mixed with water for its use. |
| ■ | Packing of formulated products in the plant, in accordance with customer specifications, including exports. |
| ■ | Preparation of packs, with formulated products at the plant and materials supplied by the customer. |
3.1. Segment reporting
Below is a description of the main indicators of each of the segments above described and grouped based on IFRS 8.
June 30, 2017:
Item | Inoculants | Seed therapics | Adjuvants | Packs | Others | Total | ||||||||||||
Revenue | 12,482,786 | 21,450,510 | 35,653,018 | 23,111,291 | 19,598,607 | 112,296,212 | ||||||||||||
Cost of sales | (3,915,337 | ) | (14,834,044 | ) | (15,218,434 | ) | (6,789,019 | ) | (18,081,636 | ) | (58,838,471 | ) | ||||||
Gross income | 8,567,449 | 6,616,466 | 20,434,584 | 16,322,271 | 1,516,971 | 53,457,741 | ||||||||||||
Total inventories | 4,419,177 | 3,412,839 | 10,540,364 | 8,419,193 | 782,469 | 27,574,042 | ||||||||||||
Total intangible assets (microbiological products) | 1,971,426 | — | 844,897 | — | — | 2,816,323 | ||||||||||||
June 30, 2016:
Item | Inoculants | Seed therapics | Adjuvants | Packs | Others | Total | ||||||||||||
Revenue | 12,759,410 | 13,023,454 | 29,869,904 | 20,843,223 | 16,909,687 | 93,405,678 | ||||||||||||
Cost of sales | (7,936,177 | ) | (8,949,443 | ) | (10,678,992 | ) | (6,836,804 | ) | (14,672,050 | ) | (49,073,466 | ) | ||||||
Gross income | 4,823,233 | 4,074,011 | 19,190,912 | 14,006,419 | 2,237,637 | 44,332,212 | ||||||||||||
Total inventories | 4,887,767 | 6,322,241 | 5,756,493 | 1,008,105 | 5,212,776 | 23,187,382 | ||||||||||||
Total intangible assets (microbiological products) | 2,005,319 | — | 792,204 | — | — | 2,797,523 | ||||||||||||
F-213
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
June 30, 2015:
Item | Inoculants | Seed therapics | Adjuvants | Packs | Others | Total | ||||||||||||
Revenue | 16,663,499 | 10,701,871 | 34,593,977 | 24,497,906 | 12,705,893 | 99,163,146 | ||||||||||||
Cost of sales | (9,580,869 | ) | (7,326,369 | ) | (13,593,551 | ) | (6,621,439 | ) | (10,234,821 | ) | (47,357,049 | ) | ||||||
Gross income | 7,082,630 | 3,375,502 | 21,000,426 | 17,876,467 | 2,471,072 | 51,806,097 | ||||||||||||
Total inventories | 7,773,781 | 7,150,661 | 9,344,004 | 2,017,703 | 5,322,762 | 31,608,911 | ||||||||||||
Total intangible assets (microbiological products) | 2,111,653 | — | 1,045,893 | — | — | 3,157,546 | ||||||||||||
4. Cost of sales
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Inventory at the beginning of the year(1) | 23,278,198 | 26,038,949 | 22,681,207 | ||||||
Add: charges for the period | |||||||||
Purchases for the year | 55,197,121 | 47,849,063 | 41,076,912 | ||||||
Staff | 7,658,263 | 6,666,118 | 7,996,177 | ||||||
Professional fees | 41,806 | 91,779 | 193,715 | ||||||
Maintenance | 636,934 | 926,880 | 706,228 | ||||||
Import expenses | 116,950 | 280,007 | 229,323 | ||||||
Freight and transportation | 202,305 | 172,078 | 560,830 | ||||||
Fuel and energy | 381,805 | 360,531 | 427,157 | ||||||
Insurance | 75,391 | 169,375 | 44,215 | ||||||
Vehicle expenses | 12,698 | 9,984 | 36,132 | ||||||
Travel expenses | 19,771 | 30,212 | 50,311 | ||||||
Taxes | 55,913 | 80,623 | 114,806 | ||||||
Office expenses | 29,275 | 167,303 | 153,404 | ||||||
Materials and packages | 177,124 | 425,379 | 633,252 | ||||||
Depreciation of property, plant and equipment | 942,207 | 994,158 | 530,707 | ||||||
Sundry | 424,224 | 508,949 | 645,356 | ||||||
Total manufacturing expenses | 10,774,666 | 10,883,376 | 12,321,613 | ||||||
Less: Inventory at the end of the year(1) | (27,712,433 | ) | (23,278,198 | ) | (26,038,949 | ) | |||
Effect of currency translation | (2,699,081 | ) | (12,419,724 | ) | (2,683,734 | ) | |||
Cost of sales | 58,838,471 | 49,073,466 | 47,357,049 | ||||||
| (1) | It does neither include allowance for inventory obsolescence nor goods in transit. |
F-214
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
5. Administrative expenses
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Staff | 5,227,359 | 4,147,403 | 3,861,691 | ||||||
Directors’ and Syndic’s fees | 117,419 | 865,444 | 802,690 | ||||||
Professional fees | 1,570,620 | 1,481,254 | 1,071,039 | ||||||
Maintenance | 233,763 | 139,147 | 105,468 | ||||||
Fuel and energy | 126,940 | 89,633 | 2,708 | ||||||
Insurance | 109,800 | 30,476 | 28,641 | ||||||
Vehicle expenses | 52,790 | 26,903 | 46,423 | ||||||
Travel expenses | 282,381 | 281,225 | 229,785 | ||||||
Taxes | 190,127 | 89,456 | 116,117 | ||||||
Office expenses | 324,595 | 261,663 | 379,159 | ||||||
Lawsuits and claims | 238,123 | 268,761 | 699,534 | ||||||
Depreciation of property, plant and equipment | 571,171 | 463,717 | 105,001 | ||||||
Amortization of Intangible assets | 380,475 | 31,249 | — | ||||||
Sundry | 339,822 | 187,499 | 229,212 | ||||||
Total | 9,765,385 | 8,363,830 | 7,677,468 | ||||||
6. Distribution expenses
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Staff | 7,443,159 | 5,867,205 | 4,846,448 | ||||||
Advertising and publicity | 1,700,694 | 1,704,282 | 2,992,857 | ||||||
Directors’ and Syndic’s fees | 16,029 | — | — | ||||||
Professional fees | 324,252 | 821,651 | 973,321 | ||||||
Maintenance | 400,982 | 300,227 | 146,506 | ||||||
Commissions and royalties | 394,769 | 335,122 | 398,317 | ||||||
Import and export expenses | 206,606 | 1,260,955 | 2,141,923 | ||||||
Freight and transportation | 1,971,512 | 1,645,710 | 1,885,169 | ||||||
Fuel and energy | 396,651 | 337,381 | 350,051 | ||||||
Insurance | 345,777 | 395,612 | 390,896 | ||||||
Vehicle expenses | 251,474 | 200,057 | 179,096 | ||||||
Travel expenses | 574,683 | 523,222 | 765,810 | ||||||
Taxes | 3,359,472 | 3,461,061 | 3,441,964 | ||||||
Office expenses | 179,837 | 165,612 | 411,303 | ||||||
Depreciation of property, plant and equipment | 417,577 | 256,992 | 213,073 | ||||||
Allowance for bad debts | 937,257 | 1,331,189 | 606,872 | ||||||
Amortization of Intangible assets | 175,576 | 24,742 | 32,681 | ||||||
Sundry | 406,442 | 193,855 | 326,531 | ||||||
Total | 19,502,749 | 18,824,875 | 20,102,818 | ||||||
F-215
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
7. Research expenses
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Staff | 1,154,511 | 1,024,165 | 185,682 | ||||||
Directors’ and Syndic’s fees | — | 37,844 | — | ||||||
Professional fees | 145,422 | 126,763 | 70,636 | ||||||
Maintenance | 53,608 | 58,087 | 34,768 | ||||||
Import and export expenses | 15,956 | 12,155 | 2,562 | ||||||
Freight and transportation | 7 | 1,530 | 1,956 | ||||||
Fuel and energy | 107,760 | 97,570 | 13,544 | ||||||
Insurance | 39,240 | 26,128 | 1,898 | ||||||
Vehicle expenses | 51,956 | 42,432 | 7,989 | ||||||
Travel expenses | 75,692 | 86,595 | 29,711 | ||||||
Office expenses | 131,744 | 188,477 | 16,930 | ||||||
Agreements and other expenses | 528,906 | 398,587 | 188,751 | ||||||
Depreciation of property, plant and equipment | 107,425 | 46,611 | 71,490 | ||||||
Sundry | 11,201 | 107,941 | 30,024 | ||||||
Total | 2,423,428 | 2,254,885 | 655,941 | ||||||
8. Other operating income, net
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Expenses recovery | 66,521 | 971,315 | — | ||||||
Reversal of provision for contingeciones | 240,328 | — | — | ||||||
Gain/loss from the sale of fixed assets | 47,680 | 26,079 | 184,606 | ||||||
Other income and expenses | (304,875 | ) | (550,655 | ) | (25,875 | ) | |||
Total | 49,654 | 446,739 | 158,731 | ||||||
9. Financial income and financial costs
Financial income | 6/30/2017 | 6/30/2016 | 6/30/2015 | ||||||
Interest gain | 356,199 | 174,368 | 1,048,953 | ||||||
Net exchange gain generated by assets | 1,695,722 | 3,887,370 | 983,066 | ||||||
Other financial results | 28,788 | 571,873 | — | ||||||
Total | 2,080,709 | 4,633,611 | 2,032,019 | ||||||
F-216
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Financial costs | 6/30/2017 | 6/30/2016 | 6/30/2015 | ||||||
Interest loss | (13,500,245 | ) | (17,437,965 | ) | (13,305,763 | ) | |||
Net exchange losses generated by liabilities | (4,405,346 | ) | (8,703,659 | ) | (1,480,863 | ) | |||
Bank and financial commissions | (1,834,999 | ) | (1,619,079 | ) | (1,727,762 | ) | |||
Other financial costs | (48,825 | ) | (8,635 | ) | (625,704 | ) | |||
Financial costs | (19,789,415 | ) | (27,769,338 | ) | (17,140,092 | ) | |||
Less: amounts capitalized in qualifying assets | 338,600 | 743,837 | 1,195,266 | ||||||
Total | (19,450,815 | ) | (27,025,501 | ) | (15,944,826 | ) | |||
10. Share of net losses of joint ventures accounted for under the equity method
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Semya S.A. | (78,036 | ) | (418,745 | ) | (581,740 | ) | |||
Synertech Industrias S.A. | (1,031,095 | ) | (430,203 | ) | (2,988 | ) | |||
Total | (1,109,131 | ) | (848,948 | ) | (584,728 | ) | |||
11. Earnings per share
Basic earnings per share are calculated by dividing the result attributable to the shareholders of the Company by the number of outstanding common shares at the end of the year.
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Profit / (loss) for the year attributable to the shareholders | 1,545,891 | (5,469,358 | ) | 5,834,608 | |||||
Adjusted outstanding common shares | 40,000,000 | 40,000,000 | 40,000,000 | ||||||
Earnings / (loss) per basic and diluted share (USD per share)(1) | 0.04 | (0.14 | ) | 0.15 | |||||
| (1) | At June 30, 2017, 2016 and 2015, the Company has not issued financial instruments and other contracts that grant to their holder rights on the capital represented by Company common shares which might modify their current shareholding; therefore, earnings per basic and diluted share agree. |
12. Property, plant and equipment
(a) Year ended June 30, 2017
Original value | ||||||||||||||||||
Item | At beginning of year | Additions/ Transfers(1) | Disposals | Effect of currency translation | Revaluation Adjustment | At end of the period | ||||||||||||
Land and Buildings | 33,479,394 | 234,763 | (208,551 | ) | (3,089,506 | ) | 3,077,833 | 33,493,933 | ||||||||||
Facilities | 3,680,459 | 105,358 | — | (347,150 | ) | — | 3,438,667 | |||||||||||
Machinery | 6,104,577 | 80,742 | — | (572,641 | ) | — | 5,612,678 | |||||||||||
Vehicles | 1,448,031 | 646,423 | (162,985 | ) | (121,033 | ) | — | 1,810,436 | ||||||||||
Furniture and fixtures | 588,243 | 36,815 | — | (54,777 | ) | — | 570,281 | |||||||||||
Computer equipment | 854,378 | 50,491 | (36,713 | ) | (79,852 | ) | — | 788,304 | ||||||||||
Assets under construction | 408,130 | 537,303 | — | (74,964 | ) | — | 870,469 | |||||||||||
Total | 46,563,212 | 1,691,895 | (408,249 | ) | (4,339,923 | ) | 3,077,833 | 46,584,768 | ||||||||||
| (1) | Additions include the capitalization of financial costs in qualifying assets amounted to USD 79,729. See detail in Note 2.5 to the financial statements. |
F-217
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Accumulated depreciation | Book value as of 6/30/2017 | ||||||||||||||||||||
Item | At beginning of year | Disposals | For the year | Effect of currency translation | Revaluation Adjustment | At end of the year | |||||||||||||||
Land and Buildings | 2,704,773 | — | 584,230 | (289,557 | ) | 391,371 | 3,390,817 | 30,103,116 | |||||||||||||
Facilities | 455,001 | — | 368,651 | (65,759 | ) | — | 757,893 | 2,680,774 | |||||||||||||
Machinery | 1,893,847 | — | 544,000 | (212,507 | ) | — | 2,225,340 | 3,387,338 | |||||||||||||
Vehicles | 597,713 | (73,841 | ) | 310,658 | (59,540 | ) | — | 774,990 | 1,035,446 | ||||||||||||
Furniture and fixtures | 355,407 | — | 30,462 | (34,948 | ) | — | 350,921 | 219,360 | |||||||||||||
Computer equipment | 358,947 | (35,096 | ) | 200,379 | (43,687 | ) | — | 480,543 | 307,761 | ||||||||||||
Assets under construction | — | — | — | — | — | — | 870,469 | ||||||||||||||
Total | 6,365,688 | (108,937 | ) | 2,038,380 | (705,998 | ) | 391,371 | 7,980,504 | 38,604,264 | ||||||||||||
(b) Year ended June 30, 2016
Original value | ||||||||||||||||||
Item | At beginning of year | Additions/ Transfers(1) | Disposals | Effect of currency translation | Revaluation adjustment | At end of year | ||||||||||||
Land and Buildings | 22,933,021 | 3,217,524 | — | (9,775,707 | ) | 17,553,150 | 33,927,988 | |||||||||||
Facilities | 4,115,311 | 1,719,020 | — | (2,287,217 | ) | — | 3,547,114 | |||||||||||
Machinery | 8,742,289 | 1,084,451 | (7,766 | ) | (3,793,504 | ) | — | 6,025,470 | ||||||||||
Vehicles | 1,535,628 | 529,853 | (55,832 | ) | (561,618 | ) | — | 1,448,031 | ||||||||||
Furniture and fixtures | 883,770 | 64,702 | — | (361,200 | ) | — | 587,272 | |||||||||||
Computer equipment | 852,870 | 439,655 | (7,164 | ) | (430,983 | ) | — | 854,378 | ||||||||||
Assets under construction | 3,823,868 | (3,133,774 | ) | (12,155 | ) | (507,945 | ) | — | 169,994 | |||||||||
Total | 42,886,757 | 3,921,431 | (82,917 | ) | (17,718,174 | ) | 17,553,150 | 46,560,247 | ||||||||||
| (2) | Additions include the capitalization of financial costs in qualifying assets amounted to USD 45,723. See detail in Note 2.5 to the financial statements. |
Accumulated depreciation | Book value as of 6/30/2016 | ||||||||||||||||||||
Item | At beginning of year | Disposals | For the year | Effect of currency translation | Revaluation adjustment | At end of year | |||||||||||||||
Land and Buildings | 2,597,207 | — | 462,781 | (1,168,980 | ) | 813,765 | 2,704,773 | 31,223,215 | |||||||||||||
Facilities | 171,508 | — | 423,327 | (139,834 | ) | — | 455,001 | 3,092,113 | |||||||||||||
Machinery | 2,399,848 | — | 563,222 | (1,071,218 | ) | — | 1,891,852 | 4,133,618 | |||||||||||||
Vehicles | 765,809 | (34,439 | ) | 176,891 | (310,548 | ) | — | 597,713 | 850,318 | ||||||||||||
Furniture and fixtures | 549,663 | — | 30,012 | (225,239 | ) | — | 354,436 | 232,836 | |||||||||||||
Computer equipment | 447,240 | (6,936 | ) | 105,245 | (186,602 | ) | — | 358,947 | 495,431 | ||||||||||||
Assets under construction | — | — | — | — | — | — | 169,994 | ||||||||||||||
Total | 6,931,275 | (41,375 | ) | 1,761,478 | (3,102,421 | ) | 813,765 | 6,362,722 | 40,197,525 | ||||||||||||
F-218
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
(c) Year ended June 30, 2015
Original Value | ||||||||||||||||||
Item | At beginning of year | Additions/ Transfers(1) | Disposals | Effect of currency translation | Revaluation adjustment | At end of year | ||||||||||||
Land and Buildings | 5,938,322 | 819,456 | (81,375 | ) | (633,976 | ) | 16,890,594 | 22,933,021 | ||||||||||
Facilities | 732,894 | 2,208,335 | — | (124,300 | ) | 1,298,382 | 4,115,311 | |||||||||||
Machinery | 5,128,966 | 4,363,592 | (110,321 | ) | (639,948 | ) | — | 8,742,289 | ||||||||||
Vehicles | 1,207,740 | 563,122 | (86,132 | ) | (149,102 | ) | — | 1,535,628 | ||||||||||
Furniture and fixtures | 872,289 | 105,096 | — | (93,615 | ) | — | 883,770 | |||||||||||
Computer equipment | 799,663 | 145,989 | — | (92,782 | ) | — | 852,870 | |||||||||||
Assets under construction | 3,237,154 | 1,095,631 | — | (508,917 | ) | — | 3,823,868 | |||||||||||
Total | 17,917,028 | 9,301,221 | (277,828 | ) | (2,242,640 | ) | 18,188,976 | 42,886,757 | ||||||||||
| (1) | Additions include the capitalization of financial costs in qualifying assets amounted to USD 621,538.See detail in Note 2.5 to the financial statements. |
Accumulated depreciation | Book value as of 6/30/2015 | ||||||||||||||||||||
Item | At beginning of year | Disposals | For the year | Effect of currency translation | Revaluation adjustment | At end of year | |||||||||||||||
Land and Buildings | 1,057,815 | — | 93,977 | (129,911 | ) | 1,575,326 | 2,597,207 | 20,335,814 | |||||||||||||
Facilities | 66,962 | — | 71,532 | (15,174 | ) | 48,188 | 171,508 | 3,943,803 | |||||||||||||
Machinery | 2,318,424 | (49,931 | ) | 392,844 | (261,489 | ) | — | 2,399,848 | 6,342,441 | ||||||||||||
Vehicles | 708,789 | (2,032 | ) | 221,363 | (162,311 | ) | — | 765,809 | 769,819 | ||||||||||||
Furniture and fixtures | 535,190 | — | 69,649 | (55,176 | ) | — | 549,663 | 334,107 | |||||||||||||
Computer equipment | 427,403 | — | 70,906 | (51,069 | ) | — | 447,240 | 405,630 | |||||||||||||
Assets under construction | — | — | — | — | — | — | 3,823,868 | ||||||||||||||
Total | 5,114,583 | (51,963 | ) | 920,271 | (675,130 | ) | 1,623,514 | 6,931,275 | 35,955,482 | ||||||||||||
(d) Revaluation made in land and buildings
Assets recorded under “Land and Buildings” are valued through the revaluation model indicated in IAS 16.
At the end of the year, the Company’s Board reviewed the valuation of the assets described above, to determine the variations between fair values and book values, in conformity with current regulations for those using fair values as primary measurement criteria, For this purpose, we have obtained and approved valuations made by external independent appraisers. Fair values thus obtained implied an increase in the book value of revalued assets which was recorded in the Statement of Changes in Equity, net of the effects in income tax.
The book value for the types of property, plant and equipment revalued that were reported at June 30, 2017, 2016 and 2015 if the revaluation model were not applied, would be as follows:
June 30, 2017 | June 30, 2016 | June 30, 2015 | |||||||
Land and Buildings | 5,538,935 | 7,533,700 | 7,714,155 | ||||||
F-219
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
All revalued assets form a single category under IFRS 13 for the purpose of determining their fair values. For this type of assets, there are no relevant observable data (level 3) and their valuation was based on the economic value of the assets for the Company when they are used, given the non-existence of an active, dynamic and representative market for these assets in their current status.
Reports from independent appraisers are used which apply valuation techniques based on the location, existent buildings, status and remaining useful lives of the buildings, the possibility of access, potential benefits of improvements, among other factors.
13. Intangible assets
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Microbiological products(1) | 2,816,323 | 2,797,523 | 3,157,546 | ||||||
Software(2) | 1,392,106 | 248,488 | 120,789 | ||||||
Total | 4,208,429 | 3,046,011 | 3,278,335 | ||||||
| (1) | It corresponds to the capitalization of expenses from the development of different microbiological products. |
| (2) | It corresponds to software development expenses. |
Cost | Microbiological products | Software | Total | ||||||
Balance at 30.06.2014 | 1,569,200 | — | 1,569,200 | ||||||
Additions(1) | 1,919,852 | 122,657 | 2,042,509 | ||||||
Effect of currency translation | (269,617 | ) | (1,868 | ) | (271,485 | ) | |||
Balance at 30.06.2015 | 3,219,435 | 120,789 | 3,340,224 | ||||||
Additions(1) | 1,123,457 | 211,266 | 1,334,723 | ||||||
Effect of currency translation | (1,489,274 | ) | (59,237 | ) | (1,548,511 | ) | |||
Balance at 30.06.2016 | 2,853,618 | 272,818 | 3,126,436 | ||||||
Additions(1) | 477,960 | 1,628,630 | 2,106,590 | ||||||
Effect of currency translation | (299,279 | ) | (127,542 | ) | (426,821 | ) | |||
Balance at 30.06.2017 | 3,032,299 | 1,773,906 | 4,806,205 | ||||||
Accumulated amortization | Microbiological products | Software | Total | ||||||
Balance at 30.06.2014 | (34,578 | ) | — | (34,578 | ) | ||||
Amortization expense(2) | (32,681 | ) | — | (32,681 | ) | ||||
Effect of currency translation | 5,370 | — | 5,370 | ||||||
Balance at 30.06.2015 | (61,889 | ) | — | (61,889 | ) | ||||
Amortization expense(2) | (24,742 | ) | (31,249 | ) | (55,991 | ) | |||
Effect of currency translation | 30,536 | 6,919 | 37,455 | ||||||
Balance at 30.06.2016 | (56,095 | ) | (24,330 | ) | (80,425 | ) | |||
Amortization expense(2) | (175,576 | ) | (380,475 | ) | (556,051 | ) | |||
Effect of currency translation | 15,695 | 23,005 | 38,700 | ||||||
Balance at 30.06.2017 | (215,976 | ) | (381,800 | ) | (597,776 | ) | |||
Carrying value at 30.06.2017 | 2,816,323 | 1,392,106 | 4,208,429 | ||||||
| (1) | Additions include the capitalization of costs for interest in qualifying assets. The amounts capitalized were USD 258,871 USD 698,114 and USD 573,728 at June 30, 2017, 2016 and June 30, 2015. See detail in Note 2.6 to the financial statements. |
| (2) | Amortization of Microbiological Products is allocated to distribution expenses. |
F-220
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
14. Investment property
Non-Current | 6/30/2017 | 6/30/2016 | 6/30/2015 | ||||||
Land | 906 | 999 | 1,653 | ||||||
Total | 906 | 999 | 1,653 | ||||||
15. Investment in joint arrangements
Entity | Percentage of equity interest(1) | 6/30/2017 | 6/30/2016 | 6/30/2015 | ||||||||
Semya S.A. | 50.00 | % | 165,397 | — | 180,086 | |||||||
Synertech Industrias S.A. | 50.00 | % | 4,466,907 | 4,558,512 | 7,373,830 | |||||||
Total | 4,632,304 | 4,558,512 | 7,553,916 | |||||||||
| (1) | For the purpose of applying the equity method, the Group has used the financial statements of the joint arrangements at June 30, 2017, 2016 and 2015. |
Below is a detail of the caption at June 30, 2017, 2016 and 2015:
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Opening balance | 4,558,512 | 7,553,916 | 6,148 | ||||||
Share of loss | (1,109,131 | ) | (848,948 | ) | (584,728 | ) | |||
Revaluation surplus in JV | 506,136 | — | — | ||||||
Reclassification of other debts | (73,165 | ) | (210,208 | ) | — | ||||
Capital contributions | 1,101,081 | 1,257,553 | 8,430,014 | ||||||
Effect of currency translation | (351,129 | ) | (3,193,801 | ) | (297,518 | ) | |||
Closing balance | 4,632,304 | 4,558,512 | 7,553,916 | ||||||
Below is a detail of the financial information selected at June 30, 2017, 2016 and 2015 in USD of the main joint arrangements:
(a) At June 30, 2017
Information on the issuer | |||||||||||||||||||||
Entity | Date | Current assets | Non- current assets | Current liabilities | Non- current liabilities | Revenue | Net profit/loss for the year | ||||||||||||||
Semya S.A. | 6/30/2017 | 76 | 992,143 | 657,805 | 3,619 | — | (198,726 | ) | |||||||||||||
Synertech Industrias S.A. | 6/30/2017 | 6,069,809 | 16,823,936 | 6,587,205 | 8,277,660 | 1,315,901 | (2,544,244 | ) | |||||||||||||
F-221
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
(b) At June 30, 2016
Information on the issuer | |||||||||||||||||||||
Entity | Date | Current assets | Non- current assets | Current liabilities | Non- current liabilities | Revenue | Net profit/loss for the year | ||||||||||||||
Semya S.A. | 6/30/2016 | 17,793 | 875,438 | 1,050,555 | 3,989 | — | (334,481 | ) | |||||||||||||
Synertech Industrias S.A. | 6/30/2016 | 4,477,298 | 15,171,361 | 6,373,446 | 4,158,189 | — | (973,189 | ) | |||||||||||||
(c) At June 30, 2015
Information on the issuer | |||||||||||||||||||||
Entity | Date | Current assets | Non- current assets | Current liabilities | Non- current liabilities | Revenue | Net profit/loss for the year | ||||||||||||||
Semya S.A. | 6/30/2015 | 19,102 | 758,253 | 417,183 | — | — | (1,209,113 | ) | |||||||||||||
Synertech Industrias S.A. | 6/30/2015 | 8,750,913 | 6,887,110 | (890,363 | ) | — | — | (8,508 | ) | ||||||||||||
16. Other investments
Non-Current
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Bioceres S.A. | 3,474 | 10,468 | 15,578 | ||||||
Coop, Eléctrica Pergamino Ltda, | 3,208 | 3,537 | 4,063 | ||||||
FAID 2015 Fondo Agrícola de inversión directa (Direct Investment Agricultural Fund) | 602 | 664 | 1,099 | ||||||
Other investments | 85,521 | — | 4,897 | ||||||
Total | 92,805 | 14,669 | 25,637 | ||||||
Current
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Restricted short-term deposit | 4,247,669 | — | — | ||||||
Total | 4,247,669 | — | — | ||||||
17. Inventories
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Raw materials | 4,773,742 | 3,725,687 | 3,087,436 | ||||||
Packaging | 2,637,222 | 2,006,744 | 3,373,952 | ||||||
Finish goods | 8,931,124 | 10,261,354 | 15,474,822 | ||||||
Resale goods | 11,370,345 | 7,284,413 | 4,102,739 | ||||||
Goods in transit | 482,186 | 231,690 | 5,569,962 | ||||||
Inventory obsolescence allowance (Note 23) | (620,577 | ) | (322,506 | ) | — | ||||
Total | 27,574,042 | 23,187,382 | 31,608,911 | ||||||
F-222
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
The Company has delivered goods on consignment for third parties for its resale for USD 3,919,192, USD 2,818,458 and USD 1,641,531 at June 30, 2017, 2016 and 2015, respectively, which are disclosed in lines "finish goods" and "resale goods".
18. Trade and other receivables
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Non-Current | |||||||||
Tax credits | 124,735 | 388,062 | 207,055 | ||||||
Reimbursements over exports | 472,275 | 425,413 | 257,246 | ||||||
Minimum notional income tax credit | — | 678,354 | — | ||||||
Related parties (Note 25) | 1,213,053 | — | — | ||||||
Total | 1,810,063 | 1,491,829 | 464,301 | ||||||
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Current | |||||||||
Trade receivables in local currency | 215,497 | 61,143 | 82,249 | ||||||
Trade receivables in foreign currency | 28,701,893 | 14,236,653 | 23,373,603 | ||||||
Deferred checks | 398,916 | 215,913 | 848,243 | ||||||
Checks discounted with resource not yet due | 10,359,675 | 7,970,949 | 6,965,577 | ||||||
Notes receivable in local currency | 842,956 | 452,089 | 506,203 | ||||||
Notes receivable in foreign currency | — | 147,431 | 344,977 | ||||||
Receivables in litigation | 2,877,099 | 1,893,970 | 1,719,373 | ||||||
Reimbursements over exports | 122,584 | 178,145 | 544,902 | ||||||
Advances to suppliers in local currency | 685,562 | 666,145 | 733,553 | ||||||
Advances to suppliers in foreign currency | 412,878 | 7,146 | 5,483 | ||||||
Tax credits | 1,878,251 | 1,084,715 | 1,202,122 | ||||||
Related parties (Note 25) | 6,360,368 | 1,393,866 | 215,911 | ||||||
Sundry | 671,072 | 400,194 | 502,866 | ||||||
Provision of credit notes to be issued | (1,393,059 | ) | (310,956 | ) | (1,319,503 | ) | |||
Allowance for bad debts (Note 23) | (2,779,163 | ) | (2,149,802 | ) | (1,655,504 | ) | |||
Total | 49,354,529 | 26,247,601 | 34,070,055 | ||||||
The aging of the trade receivables is shown in the following table:
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
To be due | 41,448,728 | 16,397,397 | 25,560,091 | ||||||
Past due up to three months | 5,255,239 | 4,451,984 | 5,376,074 | ||||||
Past due from three to six months | 3,637,384 | 1,950,495 | 530,065 | ||||||
Past due for more than six months | 823,241 | 4,939,554 | 3,068,126 | ||||||
Total | 51,164,592 | 27,739,430 | 34,534,356 | ||||||
F-223
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Trade and other receivables per currency | 6/30/2017 | 6/30/2016 | 6/30/2015 | ||||||
In Argentine pesos | 17,124,551 | 11,550,705 | 12,248,532 | ||||||
In United States dollars | 33,323,430 | 14,679,952 | 19,365,112 | ||||||
In Euros | 10,821 | — | — | ||||||
In other currencies | 705,790 | 1,508,773 | 2,920,712 | ||||||
Total | 51,164,592 | 27,739,430 | 34,534,356 | ||||||
During the year ended June 30, 2017, 2016 and 2015, Rizobacter Argentina assigned accounts receivable for USD 10,359,675, USD 7,970,949 and USD 6,965,577, respectively, to financial institutions in exchange for cash. The transaction was recorded as a secured loan (Note 21). In case of default by the entities under the loan agreement, the bank has the right to receive the cash flows of the accounts receivable transferred. If there is no default, the entities will collect accounts receivable.
The increase in the allowance for bad debts is included in line “distribution expenses” in the income statement (Note 6). The amounts charged to the allowance for bad debts are usually deleted from accounting when there are no expectations of receiving additional cash. The activity for the year of the allowance for bad debts is disclosed in Note 23.
The rest of the accounts included in trade receivables and other accounts receivable do not contain assets that have been impaired.
The accounts receivable past due for more than six months not provided for amount to USD 823,242 at June 30, 2017, USD 4,939,553 at June 30, 2016 and USD 3,068,126 at June 30, 2015.
The maximum credit risk exposure at the date of these financial statements is the carrying amount of each type of account receivable mentioned above.
19. Financial assets at fair value through profit or loss
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Marketable shares | 4,275 | 4,712 | 9,909 | ||||||
Total | 4,275 | 4,712 | 9,909 | ||||||
Changes in the fair value of financial assets at fair value through profit or loss is disclosed in line “Other operating income, net” of the Income Statement (Note 8). The fair value of all equity securities is based on the current price offered in an active market.
20. Cash and cash equivalents
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Cash and banks | 940,895 | 1,030,067 | 3,671,219 | ||||||
Total (Excluding overdraft) | 940,895 | 1,030,067 | 3,671,219 | ||||||
F-224
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Cash and cash equivalents per currency | 6/30/2017 | 6/30/2016 | 6/30/2015 | ||||||
In Argentine pesos | 229,408 | 157,987 | 3,365,735 | ||||||
In United States dollars | 643,174 | 228,175 | 156,685 | ||||||
In Euros | 3,255 | 3,172 | 5,179 | ||||||
In other currencies | 65,058 | 640,733 | 143,620 | ||||||
Total (Excluding overdraft) | 940,895 | 1,030,067 | 3,671,219 | ||||||
21. Borrowings
6/30/2016 | 6/30/2016 | 6/30/2015 | |||||||
Non-current | |||||||||
Bank loans | 35,881,720 | 3,582,606 | 10,840,263 | ||||||
Financial leases (Note 22) | 678,993 | 887,218 | 1,207,535 | ||||||
Corporate bonds | 3,889,873 | 8,755,986 | 330,106 | ||||||
Total | 40,450,586 | 13,225,810 | 12,377,904 | ||||||
Current | |||||||||
Bank loans | 18,594,823 | 25,004,704 | 33,802,394 | ||||||
Financial leases (Note 22) | 466,055 | 437,597 | 776,326 | ||||||
Corporate bonds | 4,644,621 | 5,088,481 | 354,720 | ||||||
Discounted checks | 9,638,788 | 7,549,226 | 6,548,571 | ||||||
Bank overdraft | — | 931,799 | — | ||||||
Total | 33,344,287 | 39,011,807 | 41,482,011 | ||||||
Borrowings by type of rate | 6/30/2017 | 6/30/2016 | 6/30/2015 | ||||||
Fixed rate | 66,340,807 | 20,105,270 | 36,731,034 | ||||||
Variable rate | 7,454,066 | 32,132,347 | 17,128,881 | ||||||
Total | 73,794,873 | 52,237,617 | 53,859,915 | ||||||
Borrowings by type of currency | 6/30/2017 | 6/30/2016 | 6/30/2015 | ||||||
In Argentine pesos | 17,950,005 | 28,997,018 | 39,926,777 | ||||||
In United States dollars | 55,844,868 | 23,001,813 | 13,598,498 | ||||||
In other currencies | — | 238,786 | 334,640 | ||||||
Total | 73,794,873 | 52,237,617 | 53,859,915 | ||||||
Bank debts have an average effective rate of 24.07% (2016: 29.50% annual rate). The fair value of current loans is equivalent to their book value, since the impact of applying the adjustment is not significant.
F-225
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Debt Public Offering
a) Debt public offering under the system for SMEs
On June 9, 2011, the Extraordinary Shareholders’ Meeting of the Company approved the decision to go public and listing under the special system for Small and Medium sized Enterprise (SME), The issue of Corporate Bonds (CB) was approved for a principal amount up to Argentine Peso 15,000,000 (equivalent to USD 997,340), to be placed in one or more series.
On September 26, 2011 the Executive Board of the Rosario Stock Exchange issued Resolution N° 38/2012 authorizing the Company to trade Small and Medium sized Enterprise CB for a principal amount of Argentine Peso 15,000,000 (equivalent to USD 997,340), with half-yearly amortizations as from the twelfth month following the date of subscription (November 21, 2011). Each of the first eight instalments is equivalent to 10% of the principal amount, and the ninth and last instalment is equal to 20% of the principal amount.
The bonds accrue a variable annual nominal interest rate equivalent to the reference rate (the simple arithmetic average of private banks interest rates on 30 to 35 day deposits of more than one million pesos: “Private BADLAR”) plus a differential 3,9% rate, payable half-yearly as from the date of subscription.
According to the Prospectus for the Issue filed before the Rosario Stock Exchange, if the Company fails to duly adhere to or comply with any of the terms or commitments established in the conditions for the issue of the CB – provided any such noncompliance significantly affects the capacity to pay the Services on the issue of CB - and such noncompliance continues without being corrected for thirty (30) days after any investor has filed written notice to the Company concerning the matter, this will constitute grounds for noncompliance.
Additionally, pursuant to the conditions for the issue of SME bonds, the Company undertakes not to carry out certain acts save with the express authorization of a majority of the holders of CB issued and outstanding, in the extent that the payment of any service relating to the CB to be placed remains pending. The acts referred to include:
| a) | Disposal of assets of any type, or establishment in relation to those assets of any right pertaining to tangible property or any fiduciary encumbrance in favour of third parties in the case of assets that individually or as a whole are worth more than Argentine Peso 5,000,000 (equivalent to USD 332,447) per their book value or market value, whichever is lowest; |
| b) | Voluntary capital reduction or redemption of its shares; |
| c) | Spin-off or merger; |
| d) | Discontinue its regular activities; |
| e) | Issue other negotiable obligations. |
The Board of Directors of the Company considers that the issue of CB Series I described in paragraph b) below does not constitute Grounds for Noncompliance according to the conditions for the issue of SME CB, since it does not significantly affect the capacity to pay Services on the SME CB.
The Company fulfilled the proposed allocation of funds according to the prospectus. The destinations of the funds are included in those envisaged in article 36 of Law 23576.
b) Issue of corporate bonds (Principal Market)
With the aim of consolidating its financial situation and obtaining funds for investments and working capital, on March 31, 2015 the Shareholders' Meeting of the Company approved the establishment of a global program of CB in the
F-226
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Argentine principal market (“the Program”) for the issue of one or more series of simple CB through public offering, for their future listing on stock exchanges and other markets, up to a revolving outstanding amount of USD 40,000,000 or the equivalent in other currencies, or a lower amount to be determined by the Board of Directors, with a maximum term of five years.
Series I: On August 13, 2015 the Argentine National Securities Commission (CNV for its acronym in Spanish) approved the Program and the issue of series I of the Negotiable Obligations for USD 10,000,000 (increasable to a maximum of USD 17,000,000 or the equivalent in pesos), through Resolution N° 17737, according to the main terms and conditions summarized in the Prospectus Supplement dated August 13, 2015, published in the Daily Gazette of the Buenos Aires Stock Exchange and the Rosario Stock Exchange on the same date.
On August 26, 2015 the notice of the result of the placement was issued, informing the results of the placement once the auction period had ended.
Series I (USD)
Amount of the Issue: USD 7,786,327
Date of Issue and Subscription: August 31, 2015
Applicable rate: 6% annual nominal rate
Maturity: August 31, 2018
Initial Exchange rate: 9.28 Argentine peso / USD
Amortization: The principal will be amortized in 5 half-yearly instalments as from the twelfth month following the Date of Issue. The first four instalments will be identical, each representing 17.50% of the principal amount issued, and the last will be equal to 30% of the principal amount issued.
Date of Payment of Services: Interest will be paid on a quarterly basis at a fixed annual nominal rate (Fixed Rate applicable to CB Series I Class USD). If any service payment date is not a working day, the services will be paid on the immediately subsequent working day without any interest accruing on this payment in respect of the days that elapse from the date of payment of the services to the actual payment date.
At June 30, 2016, the principal due under Series I (Class USD) reached USD 7,786,326.
Series I (Class I – Pesos)
Amount of the Issue: Argentine Peso 84,115,789 (equivalent to USD 5,592,805)
Date of Issue and Subscription: August 31, 2015
Applicable rate (initial): 26.50% annual nominal rate
Maturity: August 31, 2018
Amortization: The principal will be amortized in 5 half-yearly instalments as from the twelfth month following the Date of Issue, The first four instalments will be identical, each representing 17.50% of the principal amount issued, and the last will be equivalent to 30% of the principal amount issued,
Date of Payment of Services: Interest will be paid on a quarterly basis at a mixed annual nominal rate as follows: (a) from the Date of Issue up to an including the expiry of the ninth (9) month, interest will accrue at the Fixed Rate for NOs Series I Class I Pesos, and (b) from the start of the tenth (10) month up to the Maturity Date, interest will accrue at a Variable Rate equal to the Reference Rate plus a Differential Rate of 550 (five hundred and fifty) points. The Fixed Rate for NOs Series I Class I Pesos cannot exceed 35%. The Variable Rate for CB Series I Class I Pesos cannot be lower than
F-227
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
an annual nominal 16% rate or exceed a 32% annual nominal rate. On June 15, 2015 the National Insurance Superintendency (“SSN”) issued Communication No, 4568 establishing that CB Series I Class I Pesos constitute productive investments under the framework of paragraph k) of item 35.8.1 of the General Regulation Governing Insurance Activities (Resolution SSN No, 21,523/1992).
Series I (Class II – Pesos)
Amount of the Issue: Argentine Peso 1,377,882 (equivalent to USD 91,614)
Date of Issue and Subscription: August 31, 2015
Applicable rate (initial): 27% annual nominal rate
Maturity: August 31, 2018
Amortization: The principal will be amortized in 5 half-yearly instalments as from the twelfth month following the Date of Issue, The first four instalments will be identical, each representing 17.50% of the principal amount issued, and the last will be equivalent to 30% of the principal amount issued.
Date of Payment of Services: Interest will be paid on a quarterly basis at a mixed annual nominal rate as follows: (a) from the Date of Issue up to an including the expiry of the ninth (9) month, interest will accrue at the Fixed Rate for CB Series I Class II Pesos, and (b) from the start of the tenth (10) month up to the Maturity Date, interest will accrue at a Variable Rate equal to the Reference Rate plus a Differential Rate of 550 (five hundred and fifty) points.
In compliance with the law governing Negotiable Obligations, the net funds obtained from the issue of each Series and/or Class of negotiable Obligations will be used by the Company for one or more of the following purposes: (i) as working capital (this principal amount must be subscribed in the country), (ii) to refinance liabilities, (iii) investments in tangible assets located in the country, (iv) subscription of capital contributions in controlled or related companies of the Issuer (the proceeds from the subscription will be applied exclusively to the destinations specified in article 36 of the Law governing Negotiable Obligations).
On August 26, 2016 the first payment of services on the principal, and the fourth interest payment were made corresponding to the Negotiable Obligations from Series I Class I Pesos, Series I Class II Pesos, and Series I Class USD.
On March 1, 2017 the second payment of services on the principal, and the sixth interest payment were made corresponding to the Negotiable Obligations from Series I Class I Pesos, Series I Class II Pesos, and Series I Class USD.
c) Syndicated loan
With the objectives of financing working capital and improving its capital structure, Rizobacter consummated a USD 45 million syndicated loan, with Banco de Galicia y Buenos Aires S,A, as administrator, together with Banco Santander Río S,A,, Banco BBVA Francés S,A,, Banco Ciudad de Buenos Aires, Banco Provincia de Córdoba S,A,,, Banco Hipotecario S,A, and Banco Mariva S,A, as lenders, This loan was funded in two disbursements, the first one was made on March 15, 2017 for an amount of USD 22,000,000, and the second was made on April 25, 2017 for an amount of USD 23,000,000.
Amount: USD 45,000,000
Amortization: The principal will be amortized in 13 quarterly instalments as from the twelfth month following the date of Issue,
Applicable rate: 6.50%
Collaterals: Cash and short-term bank deposits collaterals and Bioceres guarantees.
F-228
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Covenants: Under the terms of the borrowing facility, Rizobacter is required to comply with the following financial covenants:
Restrictions on assets dispositions
Restrictions on the payment of dividends
Restriction on loans to related parties, including Joint Ventures (USD 5 million per entity)
Keep the following ratios:
| ■ | Net Debt to EBITDA ratio must be less than 3x. |
| ■ | EBITDA to interest ratio must be more than (i) 1.2x for 2017. (ii) 1.5x for 2018 and (iii) 2x for the years 2019 and 2020, and |
| ■ | Liabilities to assets ratio less than (i) 0.85x for 2017, (ii) 0.825 for 2018 and (iii) 0.8 for 2019 and 2020 |
Rizobacter complied with these covenants as of June 2017.
22. Finance leases
The financial lease agreements in force at June 30, 2017, 2016 and 2015, along with the present value of the minimum payments grouped by lessor, are described below.
(a) At June 30, 2017
Lessor | Subject-matter of the agreement | Number of leasing agreements | Present value of the minimum payments | ||||
HP Financial Services | Computer hardware | 8 | 299,486 | ||||
Banco Comafi | Machinery and equipment | 2 | 58,691 | ||||
Banco Supervielle | Machinery and equipment | 1 | 597,030 | ||||
Banco Galicia | Machinery and equipment | 1 | 189,841 | ||||
Total | 1,145,048 | ||||||
(b) At June 30, 2016
Lessor | Subject-matter of the agreement | Number of leasing agreements | Present value of the minimum payments | ||||
HP Financial Services | Computer hardware | 11 | 405,745 | ||||
Banco Comafi | Machinery and equipment | 9 | 79,474 | ||||
CGM Leasing Argentina | Vehicles | 3 | 5,638 | ||||
CGM Leasing Argentina | Machinery and equipment | 1 | 74,282 | ||||
Banco Supervielle | Machinery and equipment | 4 | 759,676 | ||||
Total | 1,324,815 | ||||||
F-229
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
(c) At June 30, 2015
Lessor | Subject-matter of the agreement | Number of leasing agreements | Present value of the minimum payments | ||||
HP Financial Services | Computer hardware | 7 | 322,427 | ||||
Banco Comafi | Vehicles | 2 | 26,029 | ||||
Banco Comafi | Machinery and equipment | 12 | 337,392 | ||||
Banco Comafi | Computer hardware | 3 | 20,878 | ||||
CGM Leasing | Vehicles | 1 | 339,581 | ||||
CGM Leasing | Machinery and equipment | 4 | 937,554 | ||||
Total | 1,983,861 | ||||||
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Nominal value - Minimum payment of financial leases | |||||||||
Up to a year | 700,369 | 736,230 | 1,117,877 | ||||||
From one to five years | 961,477 | 1,306,957 | 1,906,123 | ||||||
Total | 1,661,846 | 2,043,187 | 3,024,000 | ||||||
Financial charges to be accrued | (516,798 | ) | (718,372 | ) | (1,040,139 | ) | |||
Liabilities from financial leases | 1,145,048 | 1,324,815 | 1,983,861 | ||||||
The present value of the obligations for finance leases is as follows:
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Present value - Minimum payment of financial leases | |||||||||
Up to a year | 466,055 | 437,597 | 776,326 | ||||||
From one to five years | 678,993 | 887,218 | 1,207,535 | ||||||
Total | 1,145,048 | 1,324,815 | 1,983,861 | ||||||
23. Provisions
(a) Year ended June 30, 2017
Description | Balances at the beginning of year | Increases | Effect of currency translation | Uses / Reversals | Balances at year-end | ||||||||||
Provision for contingencies | 1,211,324 | 238,123 | (172,121 | ) | (240,328 | ) | 1,036,998 | ||||||||
Inventory obsolescence allowance | 322,506 | 481,692 | (133,529 | ) | (50,092 | ) | 620,577 | ||||||||
Allowance for bad debts | 2,149,802 | 937,257 | (180,126 | ) | (127,770 | ) | 2,779,163 | ||||||||
F-230
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
(b) Year ended June 30, 2016
Description | Balances at the beginning of year | Increases | Effect of currency translation | Uses / Reversals | Balances at year-end | ||||||||||
Provision for contingencies | 1,705,990 | 268,761 | (688,227 | ) | — | 1,286,524 | |||||||||
Inventory obsolescence allowance | — | 319,229 | 3,277 | — | 322,506 | ||||||||||
Allowance for bad debts | 1,655,504 | 1,331,189 | (836,891 | ) | — | 2,149,802 | |||||||||
(c) Year ended June 30, 2015
Description | Balances at the beginning of year | Increases | Effect of currency translation | Uses / Reversals | Balances at year-end | ||||||||||
Provision for contingencies | 1,278,740 | 699,534 | (143,060 | ) | (129,224 | ) | 1,705,990 | ||||||||
Allowance for bad debts | 1,365,087 | 606,872 | (260,949 | ) | (55,506 | ) | 1,655,504 | ||||||||
24. Trade and other payables
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Non-Current | |||||||||
Payroll and social security charges | 286,022 | — | — | ||||||
Related parties (Note 25) | 151 | 166 | 275 | ||||||
Sundry debts | — | 80,658 | 56,087 | ||||||
Total | 286,173 | 80,824 | 56,362 | ||||||
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Current | |||||||||
Trade payables in local currency | 7,664,088 | 3,734,878 | 7,180,317 | ||||||
Trade payables in foreign currency | 10,358,420 | 9,772,474 | 9,593,983 | ||||||
Payroll and social security charges | 4,995,477 | 2,145,663 | 3,640,511 | ||||||
Taxes | 363,127 | 306,350 | 381,825 | ||||||
Minimum notional income tax | — | 678,354 | — | ||||||
Customer advances | 1,188,340 | 1,543,593 | 1,578,182 | ||||||
Syndic’ fees payable | 6,080 | 9,574 | 12,324 | ||||||
Directors’ fees payable | — | 91,729 | — | ||||||
Related parties (Note 25) | 2,101,975 | 258,058 | 648,778 | ||||||
Sundry debts | 140,454 | 36,879 | 1,724,759 | ||||||
Total | 26,817,961 | 18,577,552 | 24,760,679 | ||||||
F-231
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Trade and other payable by type of currency | 6/30/2017 | 6/30/2016 | 6/30/2015 | ||||||
In Argentine pesos | 14,679,482 | 6,292,013 | 15,813,374 | ||||||
In United States dollars | 11,081,847 | 11,854,152 | 7,309,781 | ||||||
In other currencies | 1,334,670 | 309,958 | 58,462 | ||||||
In Euros | 8,135 | 202,253 | 1,635,424 | ||||||
Total | 27,104,134 | 18,658,376 | 24,817,041 | ||||||
25. Transactions and balances with related parties
For the purposes of these financial statements, related parties are those individuals or legal entities related to Rizobacter Group, under the terms of IAS 24.
(a) Balances with related parties
Balances with related parties at June 30, 2017, are the following:
Related Party | Current trade and other receivables | Other current accounts receivable | Other non- current accounts receivable | Current trade and other payables | Other non- current accounts payable | ||||||||||
Under joint control | |||||||||||||||
Semya S.A. | — | 2,981,258 | 1,213,053 | 1,649,367 | — | ||||||||||
Synertech Industrias S.A. | 217,963 | 103,798 | — | — | — | ||||||||||
Other related parties | |||||||||||||||
Bioceres Semillas S.A. | — | 3,057,349 | — | 152,549 | — | ||||||||||
Management staff and others | — | — | — | 53,304 | 151 | ||||||||||
Shareholders | — | — | — | 246,755 | — | ||||||||||
TOTAL | 217,963 | 6,142,405 | 1,213,053 | 2,101,975 | 151 | ||||||||||
Balances with related parties at June 30, 2016, are the following:
Related Party | Current trade and other receivables | Current trade and other payables | Non-current trade and other payables | ||||||
Under joint control | |||||||||
Semya S.A. | 612,002 | — | — | ||||||
Synertech Industrias S.A. | 771,981 | — | — | ||||||
Other related parties | |||||||||
Management staff and others | 9,883 | 145,859 | 166 | ||||||
Shareholders | — | 112,199 | — | ||||||
TOTAL | 1,393,866 | 258,058 | 166 | ||||||
F-232
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Balances with related parties at June 30, 2015, are the following:
Related Party | Current trade and other receivables | Current trade and other payables | Non-current trade and other payables | ||||||
Under joint control | |||||||||
Semya S.A. | 209,835 | 385,123 | — | ||||||
Other related parties | |||||||||
Management staff and others | 6,076 | 174,776 | 275 | ||||||
Shareholders | — | 88,879 | — | ||||||
TOTAL | 215,911 | 648,778 | 275 | ||||||
(b) Operations with related parties
Operations with related parties for the years ended June 30, 2017, 2016 and 2015, are the following:
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Sale of goods and services | |||||||||
Other related parties | |||||||||
Key management personal | 125,409 | 408,848 | 1,225,161 | ||||||
Total | 125,409 | 408,848 | 1,225,161 | ||||||
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Other operations | |||||||||
Entities under joint control | |||||||||
Semya S.A. | |||||||||
Expenses refund | 165,390 | 685,772 | 502,761 | ||||||
Capital contributions | (324,828 | ) | (494,011 | ) | (757,913 | ) | |||
Synertech Industrias S.A. | |||||||||
Expenses refund | 236,514 | 816,158 | — | ||||||
Capital contributions | (776,253 | ) | (763,542 | ) | (7,672,101 | ) | |||
Loans | (4,209,465 | ) | — | — | |||||
Parent | |||||||||
RASA Holding LLC | |||||||||
Expenses refund | 25,120 | — | — | ||||||
Bioceres S.A. | |||||||||
Expenses refund | 2,866 | — | — | ||||||
Other related parties | |||||||||
Bioceres Semillas S.A. | |||||||||
Expenses refund | 111,142 | — | — | ||||||
Loans | (2,998,351 | ) | — | — | |||||
Shareholders | |||||||||
F-233
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Cash dividends | (1,450,613 | ) | (2,136,980 | ) | (1,951,508 | ) | |||
Key management personal | |||||||||
Fees | (146,198 | ) | (192,466 | ) | (164,460 | ) | |||
Payroll | (2,279,633 | ) | (5,310,145 | ) | (5,708,025 | ) | |||
Total | (11,644,309 | ) | (7,395,214 | ) | (15,751,246 | ) | |||
The upper management includes directors (both executive and non-executive) and the senior management, Compensations paid or payable to the upper management for labour services rendered are shown in the previous table.
26. Income tax
The income tax charge for the year is made up of:
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Current income tax | (797,050 | ) | (106,607 | ) | (3,217,584 | ) | |||
Deferred income tax | (992,604 | ) | 2,550,473 | 19,386 | |||||
Total | (1,789,654 | ) | 2,443,866 | (3,198,198 | ) | ||||
The income tax charge for the year differs from the result obtained by applying the income tax rate in effect Argentina where the Company operate to the income before taxes, due to the following:
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Net income / (loss) before income tax | 3,336,596 | (7,905,477 | ) | 9,031,066 | |||||
Income tax at the tax rate of 35% | (1,167,809 | ) | 2,766,917 | (3,160,873 | ) | ||||
Non-deductible fees to directors | (135,998 | ) | (307,309 | ) | (29,424 | ) | |||
Losses in joint agreements | (388,196 | ) | (297,132 | ) | (204,655 | ) | |||
Non-recognized tax losses | (59,587 | ) | (433,479 | ) | — | ||||
Other non-deductible expenses | — | (103,184 | ) | (10,568 | ) | ||||
Difference in income tax return of the previous year | — | (97,097 | ) | 54,192 | |||||
Other, net | (56,740 | ) | (11,651 | ) | 48,320 | ||||
Effect of currency translation | 18,676 | 926,801 | 148,298 | ||||||
Income tax (expense) / benefit | (1,789,654 | ) | 2,443,866 | (3,198,198 | ) | ||||
F-234
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Deferred income tax
All charges for deferred income tax are fully calculated on the basis of temporary differences according to the liability method, applying the tax rates in force in each country,
Deferred tax account activity is as follows:
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
At beginning of year | (6,916,644 | ) | (5,130,246 | ) | 725,537 | ||||
Deferred tax credit | (992,604 | ) | 2,550,473 | 19,386 | |||||
Expense charged to other comprehensive income | (936,029 | ) | (5,830,601 | ) | (5,797,912 | ) | |||
Effect of currency translation | 610,392 | 1,493,730 | (77,257 | ) | |||||
At year end | (8,234,885 | ) | (6,916,644 | ) | (5,130,246 | ) | |||
Changes in deferred tax assets and liabilities occurred in the fiscal year before the offsetting of balances, are the following:
(a) Year ended June 30, 2017
Deferred tax assets:
At beginning of year | Deferred tax credit (charge) | Charged to other comprehensive income | Effect of currency translation | At year end | |||||||||||
Trade receivables | 800,624 | 99,619 | — | (71,148 | ) | 829,095 | |||||||||
Inventories | 1,342,332 | (16,669 | ) | — | (125,218 | ) | 1,200,445 | ||||||||
Other receivables and liabilities | 95,542 | 32,619 | — | (7,821 | ) | 120,340 | |||||||||
Allowances | 401,822 | 69,918 | — | (35,065 | ) | 436,675 | |||||||||
Tax loss carry forwards | 1,402,402 | (1,232,355 | ) | — | (170,047 | ) | — | ||||||||
Total deferred tax credit | 4,042,722 | (1,046,868 | ) | — | (409,299 | ) | 2,586,555 | ||||||||
Deferred tax liabilities:
At beginning of year | Deferred tax credit (charge) | Charged to other comprehensive income | Effect of currency translation | At year end | |||||||||||
Intangible Assets | (554,020 | ) | 26,781 | — | 52,323 | (474,916 | ) | ||||||||
Property, plant and equipment | (10,344,457 | ) | 27,451 | (936,029 | ) | 961,710 | (10,291,325 | ) | |||||||
Financial debts | (43,277 | ) | — | — | 4,020 | (39,257 | ) | ||||||||
Other | (17,612 | ) | 32 | — | 1,638 | (15,942 | ) | ||||||||
Total deferred tax liabilities | (10,959,366 | ) | 54,264 | (936,029 | ) | 1,019,691 | (10,821,440 | ) | |||||||
F-235
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
(b) Year ended June 30, 2016
Deferred tax assets:
At beginning of year | Deferred tax credit (charge) | Charged to other comprehensive income | Effect of currency translation | At year end | |||||||||||
Trade receivables | 865,331 | 351,737 | — | (416,444 | ) | 800,624 | |||||||||
Inventories | 714,737 | 1,153,002 | — | (525,407 | ) | 1,342,332 | |||||||||
Other receivables and liabilities | 358,557 | (153,385 | ) | — | (109,630 | ) | 95,542 | ||||||||
Allowances | 539,732 | 95,850 | — | (233,760 | ) | 401,822 | |||||||||
Tax loss carry forwards | — | 1,776,018 | — | (373,616 | ) | 1,402,402 | |||||||||
Total deferred tax credit | 2,478,357 | 3,223,222 | — | (1,658,857 | ) | 4,042,722 | |||||||||
Deferred tax liabilities:
At beginning of year | Deferred tax credit (charge) | Charged to other comprehensive income | Effect of currency translation | At year end | |||||||||||
Intangible Assets | (660,802 | ) | (195,948 | ) | — | 302,730 | (554,020 | ) | |||||||
Property, plant and equipment | (6,813,623 | ) | (502,370 | ) | (5,830,601 | ) | 2,802,137 | (10,344,457 | ) | ||||||
Financial debts | — | (54,806 | ) | — | 11,529 | (43,277 | ) | ||||||||
Other | (134,178 | ) | 80,375 | — | 36,191 | (17,612 | ) | ||||||||
Total deferred tax liabilities | (7,608,603 | ) | (672,749 | ) | (5,830,601 | ) | 3,152,587 | (10,959,366 | ) | ||||||
(c) Year ended June 30, 2015
Deferred tax assets:
At beginning of year | Deferred tax credit (charge) | Effect of currency translation | At year end | |||||||||
Trade receivables | 641,185 | 307,640 | (83,494 | ) | 865,331 | |||||||
Inventories | 462,129 | 317,819 | (65,211 | ) | 714,737 | |||||||
Other liabilities | 242,175 | 149,671 | (33,289 | ) | 358,557 | |||||||
Allowances | 393,776 | 197,691 | (51,735 | ) | 539,732 | |||||||
Total deferred tax credit | 1,739,265 | 972,821 | (233,729 | ) | 2,478,357 | |||||||
Deferred tax liabilities:
At beginning of year | Deferred tax credit (charge) | Charged to other comprehensive income | Effect of currency translation | At year end | |||||||||||
Property, plant and equipment | (425,529 | ) | (669,996 | ) | (5,797,912 | ) | 79,814 | (6,813,623 | ) | ||||||
Intangible Assets | (537,117 | ) | (190,084 | ) | — | 66,399 | (660,802 | ) | |||||||
Other | (51,082 | ) | (93,355 | ) | — | 10,259 | (134,178 | ) | |||||||
Total deferred tax liabilities | (1,013,728 | ) | (953,435 | ) | (5,797,912 | ) | 156,472 | (7,608,603 | ) | ||||||
F-236
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
The deferred tax assets and liabilities are offset when a) the Company and its subsidiaries have a legally recognized right to offset before the tax authorities the amounts recognized for those items; and b) the deferred tax assets and liabilities derived from the pertinent income tax payable to those tax authorities.
The amounts disclosed in the Statement of Financial Position as of June 30, 2017, 2016 and 2015 after the offsetting are the following:
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Net deferred tax assets to be recovered / reversed before 12 months | — | 20,086 | — | ||||||
Net deferred tax assets to be recovered / reversed after 12 months | — | (11,273 | ) | — | |||||
Deferred tax assets, net | — | 8,813 | — | ||||||
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Net deferred tax liabilities to be recovered / reversed before 12 months | 1,264,540 | 3,559,925 | 1,804,447 | ||||||
Net deferred tax liabilities to be recovered / reversed after 12 months | (9,499,425 | ) | (10,485,382 | ) | (6,934,693 | ) | |||
Deferred tax liabilities, net | (8,234,885 | ) | (6,925,457 | ) | (5,130,246 | ) | |||
27. Additional information on the Statement of Cash Flow
For the purposes of the Statement of Cash Flow, Cash and cash equivalents include:
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Cash and cash equivalents (Note 20) | 940,895 | 1,030,067 | 3,671,265 | ||||||
Bank overdrafts (Note 21) | — | (931,799 | ) | — | |||||
Cash and cash equivalents, net of bank overdrafts | 940,895 | 98,268 | 3,671,265 | ||||||
Significant investment or funding transactions not entailing outlays of cash and cash equivalents are shown below:
6/30/2017 | 6/30/2016 | 6/30/2015 | |||||||
Acquisition of Property, plant and equipment by leasing agreement | 223,866 | 490,652 | 1,071,440 | ||||||
Unpaid distributed dividends | 246,755 | 595,016 | 88,879 | ||||||
28. Transactions with non-controlling interests
There are no transactions with non-controlling interests to report.
29. Contingencies, commitments and restrictions on the distribution of profits
(a) Contingencies
There are several administrative, judicial and out-of-court proceedings in the ordinary course of business to which the subsidiaries and/or related companies of Rizobacter are a party. The Group believes that these cases, or the cumulative effect of all of them taken as a whole, will not produce a significant adverse effect on the financial position of the Group, or on the future results of its operations, bearing in mind the opinion of the legal counsel and professional advisors and the provisions for contingencies recorded at year end.
F-237
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
(b) Restrictions to the distribution of profits
Pursuant to Section 70 of the General Companies Law No, 19,550 of Argentina, companies must allocate 5% of the net profit of each year to a statutory reserve until reaching 20% of their adjusted capital, In addition, distribution of profits will be subject to retained earnings and statutory reserves in the statutory financial statements presented in ARG pesos. Retained earnings and statutory reserves as of June 30, 2017 amounts to ARS 25,945,786. Covenants restrictions to the distribution of profits are disclosed in note 21.
30. Pledged and restricted assets
Pledged and restricted assets at June 30, 2017 are as follows:
Item | Asset value | Type of debt | Amount of debt | Type of restriction | ||||
IT equipment | 87,574 | Commercial | 286,376 | Leasing | ||||
Machinery and equipment | 115,451 | Bank | 56,072 | Leasing | ||||
Machinery and equipment | 620,904 | Bank | 597,030 | Leasing | ||||
Land and buildings | 197,467 | Bank | 2,055,131 | Mortgage security(a) | ||||
Vehicles | 97,142 | Bank | 50,524 | Collateral(b) | ||||
Other financial assets | 4,260,517 | Bank | 41,876,960 | Collateral(c) | ||||
Total | 5,379,055 | 44,922,093 | ||||||
| (a) | On September 29, 2015, Rizobacter assumes jointly and severally all of the obligations for partial financing of investments of Synertech. This loan is granted by Banco Provincia de Buenos Aires directly to Synertech for completing the financing stipulated to finalize the construction. The total approved amount is ARS 80,000,000. Also, is guaranteed by the company De Sangosse by 50% through the extension of a stand by Banco Paribas, and a mortgage of the site where the plant of Synertech is currently being built. |
| (b) | On June 24, 2016, Rizobacter obtained financing from Compañía Financiera Rombo S.A. for the purchase of five vans model Duster, of Renault make, to renew the automotive fleet, setting up a pledge on the vehicles. |
| (c) | Rizobacter took a syndicated loan for USD 45,000,000 and signed a guarantee and pledge. On June 13, 2017 Rizobacter granted a pledge of a fixed-term certificate at Banco Galicia for an amount of USD 4,260,517. |
31. Financial Risk Management
(a) Financial risk factors
In performing its business activities, the Group faces various financial risks, namely: market risk (including exchange risk, fair value and cash flow interest rate risk, and price risk), credit risk and liquidity risk.
The Administration and Finance management is responsible for managing risk under policies approved by the Board of Directors, Management identifies, evaluates and covers financial risks in collaboration with the Group's operating units. The financial risk management program focuses on the unpredictability of financial markets and seeks to reduce potential adverse effects on financial performance.
(i) Market risk
Foreign exchange risks
The Group faces foreign exchange risks derived from various currency exposures, mainly in respect of the US dollar. This risk derives from commercial and financial transactions and, therefore, exchange rate variations may have a negative or positive effect on the Company's financial and economic position.
F-238
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
The following table shows the currency exposure of financial instruments denominated in foreign currencies.
6/30/2017 | ||||||||||||
(Amount in USD) | Net assets (liabilities) currency exposure | |||||||||||
USD | Other currencies | Euro | Total | |||||||||
Assets | 38,214,273 | 770,850 | 14,076 | 38,999,199 | ||||||||
Liabilities | (66,926,715 | ) | (1,160,714 | ) | (8,135 | ) | (68,095,564 | ) | ||||
Total | (28,712,442 | ) | (389,864 | ) | 5,941 | (29,096,365 | ) | |||||
6/30/2016 | ||||||||||||
(Amount in USD) | Net assets (liabilities) currency exposure | |||||||||||
USD | Other currencies | Euro | Total | |||||||||
Assets | 14,908,126 | 2,011,640 | 141,037 | 17,060,803 | ||||||||
Liabilities | (34,855,965 | ) | (548,743 | ) | (202,253 | ) | (35,606,961 | ) | ||||
Total | (19,947,839 | ) | 1,462,897 | (61,216 | ) | (18,546,158 | ) | |||||
6/30/2015 | ||||||||||||
(Amount in USD) | Net assets (liabilities) currency exposure | |||||||||||
USD | Other currencies | Euro | Total | |||||||||
Assets | 19,521,797 | 3,064,331 | 5,179 | 22,591,307 | ||||||||
Liabilities | (20,908,279 | ) | (393,104 | ) | (1,635,424 | ) | (22,936,807 | ) | ||||
Total | (1,386,482 | ) | 2,671,227 | (1,630,245 | ) | (345,500 | ) | |||||
Considering only this net currency exposure at June 30, 2017, if an Argentine peso revaluation or depreciation in relation to other foreign currencies with the remaining variables remaining constant, would have a positive or a negative impact on comprehensive income as a result of foreign exchange gains or losses, and these variations would be mainly caused by trade receivables in foreign currency and bank and financial debts in foreign currency. The Group estimates that the impact net of the effect of foreign currency derivatives of a 20% and 25% favourable/unfavourable variation in the main exchange rates (while the remaining variables remain constant) would be as follows:
Exchange rate variation | Probable | Possible | ||
(-) 20% | (+) 20% | (-) 25% | (+) 25% | |
Impact on income | 5,298,891 | (5,298,891) | 6,623,614 | (6,623,614) |
The Group has recorded Trade receivables in foreign currency from sales on the Argentina domestic market for USD 28,701,893, USD 14,236,653 and USD 23,373,603 at June 30, 2017, 2016 and 2015, respectively, for which there is a currency exposure risk that could have a negative/positive impact on the Company's financial and economic position.
Cash flow and fair value interest rate risks
The Group manages its interest rate volatility exposure through financial mechanisms. Variable-rate loans expose the Company to the risk of an increase in interest expense in the event of an increase in interest rates, while fixed-rate loans expose the Company to variations in fair value. The Group's general policy consists in maintaining a proper balance between instruments subject to fixed rate and variable rate exposure -excluding documents payable and overdrafts- which may be modified considering long-term market conditions.
F-239
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
These liabilities comprise short and long-term, fixed and variable-rate loans from leading financial institutions, in addition to variable-rate negotiable obligations.
The Group has obtained financing through short-term and long-term loans, and repayment has been made when due. Most of them are single name bank loans, pledge loans, leasing, exports prefinancing, import financing and factoring.
The Group's debts presented in USD at June 30, 2017 are broken down as follows:
Type of debt | Interest rate | Denominated in Argentine pesos | Denominated in USD | Total | Percentage | |||||||
Short-term | Fixed | 12,813,391 | 13,753,112 | 26,566,503 | 80% | |||||||
Variable | 1,973,547 | 4,804,237 | 6,777,784 | 20% | ||||||||
Total short-term | 14,786,938 | 18,557,349 | 33,344,287 | 45% | ||||||||
Long-term | Fixed | 2,544,985 | 37,229,319 | 39,774,304 | 98% | |||||||
Variable | 618,082 | 58,200 | 676,282 | 2% | ||||||||
Total long-term | 3,163,067 | 37,287,519 | 40,450,586 | 55% | ||||||||
A decrease or increase in interest rates would imply a positive or negative impact on income as a result of lower or higher losses derived from accrual of interest, mainly on variable-rate bank and financial debts.
(ii) Credit risk
The Group's credit risk mainly relates to cash and cash equivalents and trade receivables from customers.
To mitigate the risk generated by cash and cash equivalents, the Group operates with high credit rating financial institutions and with low-risk financial instruments.
The Group's customer base is diversified among distributors, seedbed businesses, leading multinationals in the agricultural sector and producers.
All of its customers are required to meet the Group's credit policies, procedures and controls, Credit limits are set based on internal ratings, which consider the analysis of customers' economic and financial position, their past performance and their overall qualifications,
The Group regularly controls the use of credit limits, The Group has taken out credit insurance from Insur SRL, which regularly analyses our customer base and currently covers approximately 50% of our portfolio.
(iii) Liquidity risk
Management holds sufficient cash and credit facilities to finance normal business activities, and monitors the Group's reserves liquidity forecasts based on expected cash flows. The Administration and Finance Management prepares a projection of the Group's cash flows, and monitors the Group's liquidity needs to ensure that it has sufficient resources to meet its operating needs. These projections take into account the Group's debt financing plans, compliance with internal objectives and, where applicable, the external or internal regulatory and legal requirements.
F-240
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
The following table summarizes bank and financial debts according to their remaining maturities at the date of the Statement of Financial Position, with respect to the maturities established by contract, Amounts shown in the table are undiscounted contractual cash flows:
At June 30, 2017 | Less than 3 months | From 3 months to 1 year | More than 1 year | ||||||
Financial debt (excluding liabilities from financial leases) | 14,581,318 | 21,543,906 | 44,587,264 | ||||||
Financial leases | 186,818 | 513,551 | 961,477 | ||||||
Trade and other payables(1) | 16,411,513 | 10,406,448 | 286,173 | ||||||
Total at June 30, 2017 | 31,179,649 | 32,463,905 | 45,834,914 | ||||||
At June 30, 2016 | Less than 3 months | From 3 months to 1 year | More than 1 year | ||||||
Financial debt (excluding liabilities from financial leases) | 24,355,458 | 19,295,232 | 17,406,621 | ||||||
Financial leases | 437,597 | 298,633 | 1,306,957 | ||||||
Trade and other payables(1) | 12,463,453 | 6,194,757 | 166 | ||||||
Total at June 30, 2016 | 37,256,508 | 25,788,622 | 18,713,744 | ||||||
At June 30, 2015 | Less than 3 months | From 3 months to 1 year | More than 1 year | ||||||
Financial debt (excluding liabilities from financial leases) | 14,813,756 | 33,502,435 | 16,277,049 | ||||||
Financial leases | 391,776 | 384,550 | 1,906,123 | ||||||
Trade and other payables(1) | 14,856,407 | 9,904,272 | 56,362 | ||||||
Total at June 30, 2015 | 30,061,939 | 43,791,257 | 18,239,534 | ||||||
| (1) | The analysis of maturities is only applicable to financial instruments and, therefore, it excludes non-financial liabilities. |
At June 30, 2017, the Group has set up the necessary provisions to meet its operating expenses, The Group has unused credit lines from public and private banks that, if necessary, would allow it to meet its debt obligations.
(b) Capital management
The Group's capital management objectives consist in safeguarding its capacity and maintain an optimal structure to reduce financial costs and, thus, contribute to increasing return on invested capital. The Group is not subject to external capital requirements except for the covenants described in the borrowings note.
F-241
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
(c) Financial instruments by category and fair value hierarchy
The following tables show additional information required by IFRS 7 and a breakdown of profit and loss per category of financial instrument according to the categories established by IFRS 9 with respect to financial assets and liabilities recorded at June 30, 2017, 2016 and 2015.
At June 30, 2017 | Amortized cost | Fair value through profit or loss | Fair value through other comprehensive profit or loss | ||||||
(1) Assets as per Statement of Financial Position | |||||||||
Trade and other receivables | 46,179,041 | — | — | ||||||
Financial assets | — | 4,275 | — | ||||||
Other financial assets | 4,247,669 | — | — | ||||||
Cash and cash equivalents | 940,895 | — | — | ||||||
Total | 51,367,605 | 4,275 | — | ||||||
| (1) | Only includes financial assets covered by IFRS 7. |
At June 30, 2016 | Amortized cost | Fair value through profit or loss | Fair value through other comprehensive profit or loss | ||||||
(1) Assets as per Statement of Financial Position | |||||||||
Trade and other receivables | 24,504,931 | — | — | ||||||
Financial assets | — | 4,712 | — | ||||||
Cash and cash equivalents | 1,030,067 | — | — | ||||||
Total | 25,534,998 | 4,712 | — | ||||||
| (1) | Only includes financial assets covered by IFRS 7. |
At June 30, 2015 | Amortized cost | Fair value through profit or loss | Fair value through other comprehensive profit or loss | ||||||
(1) Assets as per Statement of Financial Position | |||||||||
Trade and other receivables | 31,619,952 | — | — | ||||||
Financial assets | — | 9,909 | — | ||||||
Cash and cash equivalents | 3,671,219 | — | — | ||||||
Total | 35,291,171 | 9,909 | — | ||||||
| (1) | Only includes financial assets covered by IFRS 7. |
At June 30, 2017 | Amortized cost | Fair value through profit or loss | ||||
(1) Liabilities as per Statement of Financial Position | ||||||
Trade and other payables | 12,252,749 | — | ||||
Borrowings | 73,794,873 | — | ||||
Total | 86,047,622 | — | ||||
| (1) | Only includes financial liabilities covered by IFRS 7. |
F-242
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
At June 30, 2016 | Amortized cost | Fair value through profit or loss | ||||
(1) Liabilities as per Statement of Financial Position | ||||||
Trade and other payables | 13,507,352 | — | ||||
Borrowings | 52,237,617 | — | ||||
Total | 65,744,969 | — | ||||
| (1) | Only includes financial liabilities covered by IFRS 7. |
At June 30, 2015 | Amortized cost | Fair value through profit or loss | ||||
(1) Liabilities as per Statement of Financial Position | ||||||
Trade and other payables | 16,774,300 | — | ||||
Borrowings | 53,859,915 | — | ||||
Total | 70,634,215 | — | ||||
| (2) | Only includes financial liabilities covered by IFRS 7. |
Fair value by hierarchy
According to the requirements of IFRS 7, the Group classifies each class of financial instrument valued at fair value in the Statement of Financial Position into three levels, depending on the relevance of the judgment associated to the assumptions used for measuring the fair value.
Level 1 comprises financial assets and liabilities with fair values determined by reference to quoted prices (unadjusted) in active markets for identical assets or liabilities;
Level 2 comprises inputs other than quoted prices included within Level 1 that are observable for the asset or liability, either directly (i.e. as prices) or indirectly (i.e. derived from prices) (Level 2);
Level 3 comprises financial instruments with inputs for estimating fair value that are not based on observable market data.
Measurement at fair value at June 30, 2017 | |||||||||
Description | Level 1 | Level 2 | Level 3 | ||||||
Financial assets at fair value through profit or loss | |||||||||
Financial assets at fair value through profit or loss | 4,275 | — | — | ||||||
Total | 4,275 | — | — | ||||||
Measurement at fair value at June 30, 2016 | |||||||||
Description | Level 1 | Level 2 | Level 3 | ||||||
Financial assets at fair value through profit or loss | |||||||||
Financial assets at fair value through profit or loss | 4,712 | — | — | ||||||
Total | 4,712 | — | — | ||||||
F-243
RIZOBACTER ARGENTINA S.A.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
For the years ended June 30, 2017, 2016 and 2015 (amounts in US dollars, except otherwise indicated)
Measurement at fair value at June 30, 2015 | |||||||||
Description | Level 1 | Level 2 | Level 3 | ||||||
Financial assets at fair value through profit or loss | |||||||||
Financial assets at fair value through profit or loss | 9,909 | — | — | ||||||
Total | 9,909 | — | — | ||||||
Fair value estimation
The fair value of financial instruments traded in active markets is based on market quoted prices at the balance sheet date, A market is regarded as active if quoted prices are readily and regularly available from a stock exchange, broker, industry group, pricing service or regulatory agency, and those prices represent actual and regularly occurring market transactions on an arm’s length basis. The quoted price used for financial assets held by the Group is the current price offered, These instruments are included in Level 1, Instruments included in Level 1 comprise investments in stocks in the Merval index.
No transfers between levels were made during the year.
32. Subsequent events
After June 30, 2017, no other events, situations or circumstances have taken place affecting or which may significantly affect the equity, economic or financial position of the Group.
F-244
American Depositary Shares

BIOCERES S.A.
Representing ordinary shares
PROSPECTUS
Sole Global Coordinator
Jefferies
Joint book-running managers
Jefferies | Piper Jaffray |
, 2017
Through and including , 2017 (the 25th day after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 6. Indemnification of Directors and Officers
The indemnity agreements that the registrant maintains with its directors and other officers provide that, subject to Argentine Corporate Law, every person who is or was at any time a director or other officer (excluding an auditor) of the registrant may be indemnified out of the assets of the registrant against all costs, charges, expenses, losses or liabilities incurred by him in performing his duties or the exercise of his powers or otherwise in relation to or in connection with his duties, powers or office.
The registrant plans to maintain insurance for its directors regarding negligence or fault under the terms and to the extent allowed by Argentine Corporate Law.
Subject to the exceptions and limitations set forth below and mandatory provisions of law, every person who is, or has been, a director or officer of Bioceres will be indemnified by Bioceres to the fullest extent permitted by law against liability and against all expenses reasonably incurred or paid by him or her in connection with any claim, action, suit or proceeding which he or she becomes involved as a party or otherwise by virtue of his or her being or having been such a director or officer of Bioceres and against amounts paid or incurred by him or her in the settlement thereof. The words “claim,” “action,” “suit” or “proceeding” refer to all claims, actions, suits or proceedings (civil, criminal or otherwise including appeals) actual or threatened and the words “liability” and “expenses” include without limitation attorneys’ fees, costs, judgments, amounts paid in settlement and other liabilities.
No indemnification, however, will be provided to any director or officer against any liability to Bioceres or its shareholders: (i) by reason of intentional fraudulent conduct, dishonesty, willful misconduct, or gross negligence on the part of such indemnitee director or officer; or (ii) by reason of payment made under the insurance policy or any third party that has no recourse against the indemnitee director or officer.
The rights of indemnification described above continue as to a person who has ceased to be such director or officer for a period of no longer than two years. Nothing contained in Bioceres S.A.’s bylaws affect any rights to indemnification to which corporate personnel, including directors and officers, may be entitled by contract or otherwise under law
Expenses in connection with the preparation and representation of a defense of any claim, action, suit or proceeding of the character described above will be advanced by prior to final disposition thereof upon receipt of any undertaking by or on behalf of the officer or director, who must repay such amount if it is ultimately determined that he is not entitled to indemnification.
Bioceres maintains an insurance policy that protects its directors and officers from liabilities incurred as a result of actions taken in their official capacity.
In the underwriting agreement, the international underwriters will agree to indemnify, under certain conditions, the registrant, members of the registrant’s board of directors, members of the executive management board and persons who control the registrant within the meaning of the Securities Act, against certain liabilities. See “Item 9. Undertakings” for a description of the Commission’s position regarding such indemnification provisions.
Item 7. Recent Sales of Unregistered Securities
In the two years and six months ended June 30, 2017, no shares have been sold by Bioceres.
II-1
Item 8. Exhibits and Financial Statement Schedules
(a) EXHIBIT INDEX
Exhibit Number | Exhibit |
1.1* | Form of Underwriting Agreement |
3.1** | Bylaws of Bioceres S.A. |
4.1* | Specimen American Depositary Receipt (included in Exhibit 4.2) |
4.2* | Form of Deposit Agreement among the Registrant, the depositary and holder of the American Depositary Receipts |
5.1* | Opinion of Marval O’Farrell Mairal as to the validity of the ordinary shares of Bioceres S.A. |
8.1* | Opinion of Marval O’Farrell Mairal as to Argentine tax matters |
10.1**† | Specific Agreement dated 2003 between Bioceres S.A., CONICET and National University of Litoral |
10.2**† | Amendment to the Specific Agreement dated May 5, 2010 between Bioceres S.A., CONICET and National University of Litoral |
10.3**† | Agreement dated February 28, 2012, between Bioceres S.A., CONICET and National University of Litoral |
10.4**† | Framework Agreement dated February 26, 2013 between Bioceres, Inc. and FD Admiral SAS |
10.5**† | Letter Agreement dated February 24, 2012 between Bioceres, Inc. and Arcadia Biosciences, Inc. |
10.6** | Lease Agreement dated November 30, 2004 between INDEAR and CONICET |
10.7** | Form of Indemnity Agreement by and between Bioceres S.A. and each of its directors and executive officers |
10.8**† | Credit Facility Agreement dated October 14, 2016 among Bioceres S.A., as borrower, Bioceres Inc. and Rizobacter Argentina S.A., as guarantors, and BAF Latam Trade Finance Fund B.V. |
10.9**† | Credit Facility Agreement dated October 14, 2016 among Bioceres Inc., as borrower, Bioceres S.A. and Rizobacter Argentina S.A., as guarantors, and BAF Latam Trade Finance Fund B.V. |
10.10*† | Amendment to the Credit Facility Agreement dated October 26, 2017 among Bioceres S.A., as borrower, Bioceres Inc. and Rizobacter Argentina S.A., as guarantors, and BAF Latam Trade Finance Fund B.V. |
10.11*† | Amendment to the Credit Facility Agreement dated October 26, 2017 among Bioceres Inc., as borrower, Bioceres S.A. and Rizobacter Argentina S.A., as guarantors, and BAF Latam Trade Finance Fund B.V. |
10.12**† | Share Purchase Agreement dated October 18, 2016 among, Bioceres Inc., International Property Services Corp and RASA Holding LLC |
10.13**† | Shareholders’ Agreement dated October 19, 2016 among International Property Services Corp, Maria Marta Mac Mullen, Pedro Enrique Mac Mullen and RASA Holding LLC |
10.14** | Stock Option Incentive Plan |
10.15** | Stock Grant Incentive Plan |
10.16**† | Syndicated Loan Facility dated March 15, 2017 among Rizobacter Argentina S.A., Banco de Galicia y Buenos Aires S.A., Banco Santander Río S.A., Banco Hipotecario S.A. and Banco Mariva S.A. |
10.17† | Call Option Agreement dated October 19, 2016 among International Property Services Corp, Maria Marta Mac Mullen, Pedro Enrique Mac Mullen, Bioceres S.A., Bioceres Inc. and Indear S.A. |
21.1** | List of Subsidiaries |
23.1* | Consent of Price Waterhouse & Co. S.R.L., independent registered public accounting firm, with respect to the Bioceres Audited Financial Statements |
23.2* | Consent of Price Waterhouse & Co. S.R.L., independent registered public accounting firm, with respect to the Rizobacter Audited Financial Statements |
23.3* | Consent of Marval O’Farrell Mairal (included in Exhibit 5.1) |
23.4* | Consent of Marval O’Farrell Mairal (included in Exhibit 8.1) |
24.1* | Powers of Attorney (included on signature page) |
| * | To be filed by amendment |
| ** | Filed previously |
| † | Confidential treatment has been or will be requested with respect to certain portions of this exhibit. The omitted portions have been filed separately with the Securities and Exchange Commission. |
II-2
| (b) | Financial Statement Schedules. |
All schedules have been omitted because they are not required, are not applicable or the information is otherwise set forth in the Consolidated Financial Statements and related notes thereto.
Item 9. Undertakings
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions described in Item 6 hereof, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes:
| (1) | To provide the international underwriters specified in the Underwriting Agreement, at the closing, certificates in such denominations and registered in such names as required by the international underwriters to permit prompt delivery to each purchaser. |
| (2) | That for purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4), or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (3) | That for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and this offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-3
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in on this day of .
Bioceres S.A. | ||
Name: | ||
Title: | ||
POWER OF ATTORNEY
We, the undersigned directors and officers of Bioceres S.A., hereby severally constitute and appoint , and each of them singly, his/her true and lawful attorneys-in-fact and agents, with full power of substitution and re-substitution for him/her and in his/her name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and any subsequent registration statements pursuant to Rule 462 of the United States Securities Act of 1933, as amended, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that each of said attorney-in-fact or his/her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
Signatures | Title | Date | |
Chief Executive Officer (Principal Executive Officer) and Director | |||
Federico Trucco | |||
Chief Financial Officer (Principal Financial Officer & Principal Accounting Officer) | |||
Enrique López Lecoube | |||
Chairman of the Board | |||
Marcelo Adolfo Carrique | |||
Director | |||
Cintia Guillermina Castagnino | |||
Director | |||
Matías Hugo Kugler | |||
Director | |||
Santiago Sacerdote | |||
Director | |||
Manuel Alberto Sobrado | |||
II-4
Signatures | Title | Date | |
Director | |||
Ignacio Lartirigoyen | |||
Vice-Chairman | |||
Aimar Dimo | |||
Director | |||
Carlos Popik | |||
By: | Authorized Representative in the United States | ||
Name: | |||
Title: | |||
II-5