Alcyone Therapeutics, Inc., Amicus Therapeutics, Inc., Apic Bio, Inc., Applied Genetic Technologies Corporation, Asklepios BioPharmaceutical, Inc., or AskBio (acquired by Bayer), Audentes Therapeutics, Inc. (acquired by Astellas Pharma Inc.), Biogen, Inc., or Biogen, Brain Neurotherapy Bio, Inc. (merged with AskBio), Encoded Therapeutics, Inc., GenSight Biologics SA, Homology Medicines, Inc., LEXEO Therapeutics, Inc., LogicBio Therapeutics, Inc., Lysogene SA, MeiraGTx Ltd., or MeiraGTx, Neurogene, Inc., Novartis Gene Therapies, Inc. (formerly AveXis, Inc.), Passage Bio, Inc., Pfizer, Inc., Prevail Therapeutics, Inc. (acquired by Eli Lilly), PTC Therapeutics, Inc., REGENXBio Inc., Sarepta Therapeutics, Inc., Sio Gene Therapies, Inc., Solid Biosciences, Inc., Spark Therapeutics, Inc. (acquired by Roche), StrideBio, Inc., Taysha Gene Therapies, Inc. and uniQure, as well as several companies addressing other methods for modifying genes and regulating gene expression. Any advances in gene therapy technology made by a competitor may be used to develop therapies that could compete against any of our product candidates.
We expect that VY-AADC (NBIb-1817) will potentially compete with a variety of therapies currently marketed and in development for Parkinson’s disease, including DBS marketed by Medtronic plc, Abbott Laboratories (acquired from St. Jude Medical in 2017), and other medical device companies, DUOPA/Duodopa marketed by AbbVie, as well as other novel, non-oral forms of levodopa, including Mitsubishi Tanabe Pharma’s ND0612 (acquired from NeuroDerm in 2017), Acorda Therapeutics’ inhaled levodopa, INBRIJA, and Sunovion Pharmaceuticals’, or Sunovion’s, sublingual apomorphine, KYNMOBI. Gene therapy competition for Parkinson’s disease includes AAV2-GDNF being developed by Brain Neurotherapy Bio, Inc. and AAV-GAD being developed by MeiraGTx. Sio Gene Therapies, Inc. is developing a second generation LentiVector gene therapy, AXO-Lenti-PD (previously OXB-102, licensed from Oxford Biomedica in 2018).
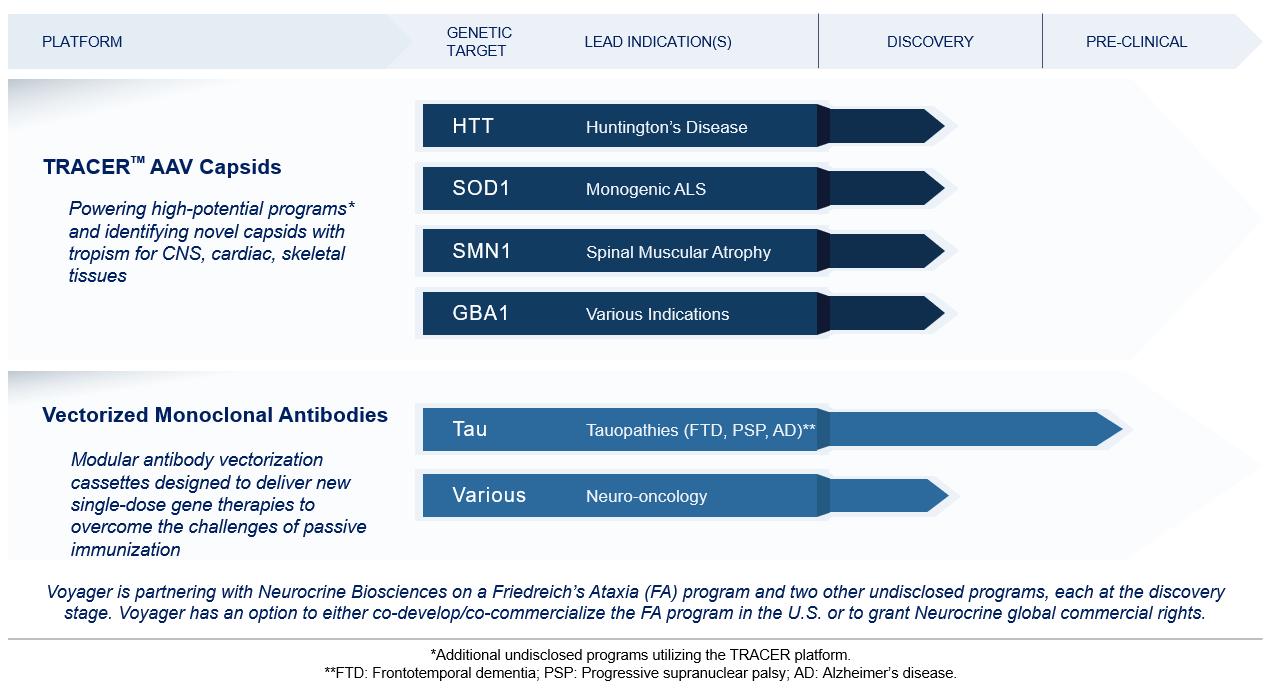
We expect that our preclinical programs will compete with a variety of therapies in development, including:
| ● | Our program for Huntington’s disease will potentially compete with TAK-686 being developed by Sangamo Therapeutics, Inc. in collaboration with Takeda, and AMT-130, an AAV gene therapy being developed by uniQure and a gene therapy being developed by Spark; |
| ● | Our program for a monogenic form of ALS will potentially compete with BIIB067 (IONIS-SOD1Rx) being developed by Biogen, in collaboration with Ionis, and gene therapies being developed by Novartis Gene Therapies, Inc. and Apic Bio, Inc.; |
| ● | Our program for Friedreich’s ataxia will potentially compete with AAV gene therapies being developed by Pfizer, Inc., PTC Therapeutics, Inc., StrideBio, Inc. in collaboration with Takeda, AAVANTIBio, Inc., Novartis Gene Therapies, and LEXEO Therapeutics, Inc.; |
| ● | Our program for tauopathies including Alzheimer’s disease, progressive supranuclear palsy, and frontotemporal dementia will potentially compete with tau antibodies being developed by Roche Genentech Inc. in collaboration with AC Immune SA, Eli Lilly & Co., AbbVie, Biogen, and several other companies, as well as an antisense oligonucleotide program being developed by Ionis in collaboration with Biogen; |
| ● | Our program for Spinal muscular atrophy, or SMA, will potentially compete with Zolgensma being marketed by Novartis Gene Therapies, Spinraza being marked by Biogen, and Evrysdi being marked by Roche; and |
| ● | Our program for diseases linked to GBA1 mutations will potentially compete with AAV gene therapies being developed by Prevail Therapeutics Inc., Freeline Therapeutics Holdings plc, Pfizer, Inc., Biogen, Lysogene SA, and Coave Therapeutics; |
Many of our potential competitors, alone or with their strategic partners, have substantially greater financial, technical and other resources, such as larger research and development, clinical, marketing and manufacturing organizations. Mergers and acquisitions in the biotechnology and pharmaceutical industries, including recent transactions involving a number of gene therapy companies, may result in even more resources being concentrated among a smaller number of competitors. Smaller and other early stage companies may also prove to be significant competitors,