
Jounce Therapeutics A Next Gen Immunotherapy Company

Legal Disclaimer Various statements concerning Jounce’s future expectations, plans and prospects, including without limitation, Jounce’s expectations regarding the timing, progress and results of discovery programs, preclinical studies and clinical trials for Jounce’s product candidates and any future product candidates, the potential benefits of any of these product candidates and the timing or likelihood of regulatory filings may constitute forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws and are subject to substantial risks, uncertainties and assumptions. You should not place reliance on these forward looking statements, which often include words such as “anticipate,” “can,” “estimate,” “expect,” “explore,” “goal,” “initiate,” “intend,” “in development,” “may,” “on track,” “plan,” “position,” “potential,” “predict,” “predictive,” “target,” “tracking,” “up to,” or similar terms, variations of such terms or the negative of those terms. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, the Company cannot guarantee such outcomes. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including, without limitation, Jounce’s ability to successfully demonstrate the efficacy and safety of its product candidates and future product candidates, the preclinical and clinical results for its product candidates, which may not support further development and marketing approval, the potential advantages of Jounce’s product candidates, the development plans of its product candidates, actions of regulatory agencies, which may affect the initiation, timing and progress of preclinical studies and clinical trials of its product candidates, Jounce’s anticipated milestones, Jounce’s ability to obtain, maintain and protect its intellectual property, Jounce’s ability to enforce its patents against infringers and defend its patent portfolio against challenges from third parties, the timing, cost or other aspects of a potential commercial launch of Jounce’s product candidates and potential future sales of our current product candidates or any other potential products if any are approved for marketing, competition from others developing products for similar uses, Jounce’s ability to manage operating expenses, Jounce’s ability to establish or maintain future collaborations, Jounce’s dependence on third parties for development, manufacture, marketing, sales and distribution of product candidates and unexpected expenditures, as well as those risks more fully discussed in the section entitled “Risk Factors” in Jounce’s most recent Annual Report on Form 10-K or Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission as well as discussions of potential risks, uncertainties, and other important factors in Jounce’s subsequent filings with the Securities and Exchange Commission. All such statements speak only as of the date made, and the Company undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise. Keytruda® is a registered trademark of Merck, Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. 2 | Jounce Therapeutics, Inc. © January 2020

Jounce: Our Approach Novel Discovery Development Moving Beyond Today’s The right immunotherapies Immunotherapies for the right patients 3 | Jounce Therapeutics, Inc. © January 2020

The Jounce Difference Clinical Stage IO Pipeline • Vopratelimab: ICOS Agonist in Phase 2 – Two development paths, guided by clinical data and biomarkers – EMERGE preliminary efficacy data in 2H20 • JTX-4014: Phase 2 ready PD-1 Inhibitor (PD-1i) – First use in combo with vopra in SELECT Translational Science Platform & Pipeline • Highly productive platform, from discovery through development – Rapid advancement of four programs to development stage – Pipeline targeting multiple immune cell types – Proven value generation Foundation For Success • Experienced founders and management ( ) – Coley, Lasker and Nobel awards • Strong balance sheet 4 | Jounce Therapeutics, Inc. © January 2020

The Core of Our Approach: Translational Science Platform Translational Drug Discovery Reverse Translational Analysis • Sustainable engine for 1st in class • Patients with clinical outcomes • Focused on multiple immune cell • PD* and predictive biomarkers types • Patients more likely to respond *PD= Pharmacodynamic 5 | Jounce Therapeutics, Inc. © January 2020

Jounce Immunotherapy Pipeline Preclinical Clinical Program Target Biology Discovery IND Enabling Phase 1 Phase 2 Phase 3 Development Programs CD4 T cell Vopratelimab ICOS focused CD8 T cell JTX-4014 PD-1 focused Suppressive JTX-8064 LILRB2 macrophage Not JTX-1811 T-regulatory Disclosed Discovery Programs Not Disclosed T-regulatory Not Disclosed Stromal Not Disclosed Macrophage Jounce Wholly Owned Out-licensed; Potential Milestones and Royalties 6 | Jounce Therapeutics, Inc. © January 2020

Development Programs Vopratelimab JTX-4014 JTX-1811

Vopratelimab Harnesses a Different Element of the Immune Response Mechanism of Action on CD4 T Cells, from Clinical Data Activation of ICOS lo to Vopra Stimulates ICOS hi but Potential for Clinical Benefit ICOS hi CD4 T Cells not ICOS lo CD4 T Cells ICOS ICOS Vopratelimab Vopratelimab Cancer Antigen Priming + Resting ICOS lo ICOS hi primed Differentiated Immune Approach: CD4 T cell CD4 T effector cell • Engages CD4, not CD8 cells • ICOS hi CD4 cells orchestrate a more complete immune response • May lead to amplified and more sustained clinical benefit Harvey, C., AACR 2019, 4053 Hanson, A., SITC 2018, P52 8 | Jounce Therapeutics, Inc. © January 2020

Vopratelimab Value Proposition BiologicalBiological activity activity beyond distinct CD8 from focused CD8 focused PD-1 inhibitors PD(L)-1i* Biomarker driven development paths based on clinical data Key Learnings from ICONIC** Lead to Changes in New Ph 2 Studies • Peripheral ICOS hi CD4 T cells as a PD biomarker • ICOS hi CD4 T cells cornerstone of vopra development – Emerge due to vopra (not PD(L)-1i), correlate with clinical benefit • Phase 2 studies: – Expand and persist throughout durable responses (some >2 years) • Tumor RNA signature predicts ICOS hi CD4 T cells & potential – EMERGE: Induction of ICOS hi CD4 T cells by ipi, then expand clinical benefit and sustain with vopra • Safe and well tolerated – SELECT: Predictive RNA signature to select patients • Dose: continuous target saturation may not be ideal • New dose and schedule may be more suitable for vopra *PD(L)-1i = PD-1 inhibitor or PD-L1 inhibitor **Yap, T, ASCO 2018, 3000; Harvey, C., AACR 2019, 4053; Hanson, A., SITC 2018, P52 9 | Jounce Therapeutics, Inc. © January 2020
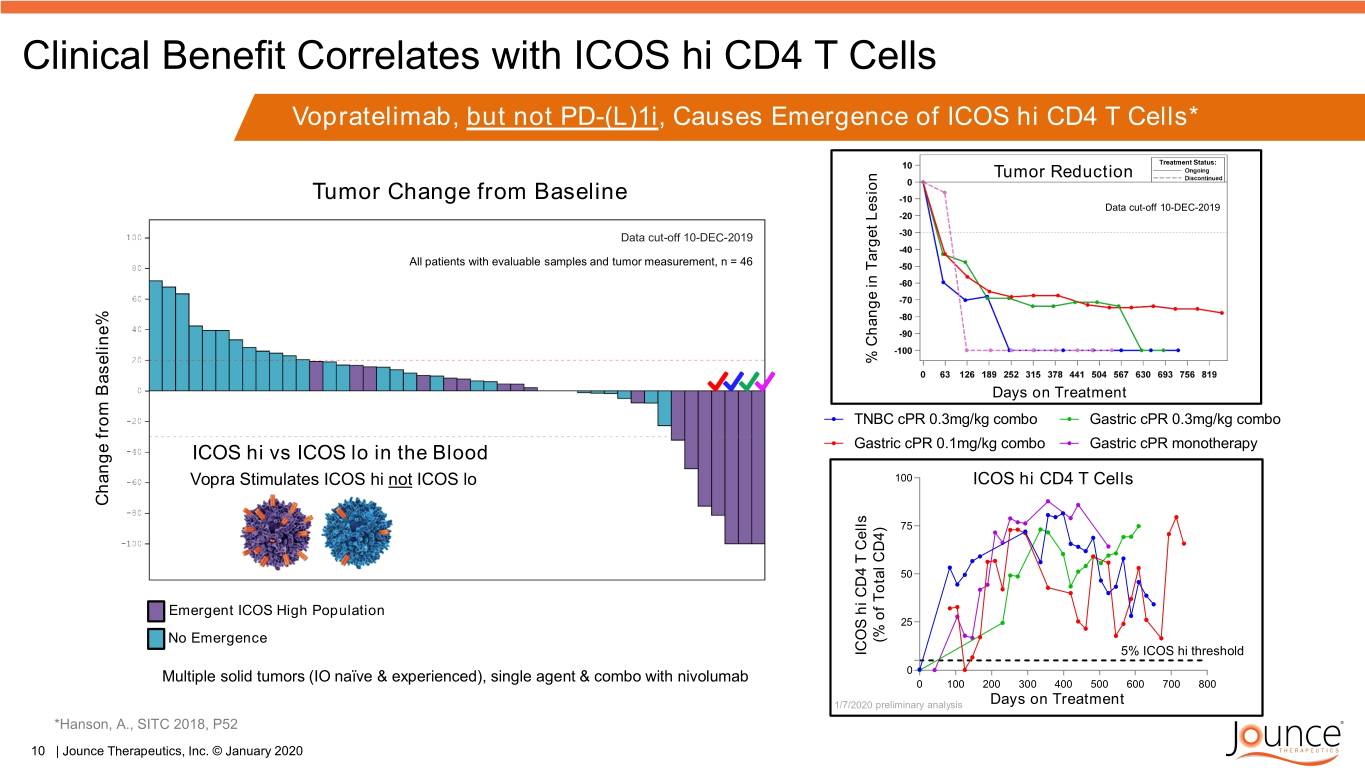
Clinical Benefit Correlates with ICOS hi CD4 T Cells Vopratelimab, but not PD-(L)1i, Causes Emergence of ICOS hi CD4 T Cells* Tumor Reduction Tumor Change from Baseline 100 Data cut-off 10-DEC-2019 75 Data cut-off 10-DEC-2019 All patients with evaluable samples and tumor measurement, n = 46 50 25 % Change in Target Lesion Target in Change% 0 0 100 200 300 400 500 600 700 800 Days on Treatment TNBC cPR 0.3mg/kg combo Gastric cPR 0.3mg/kg combo ICOS hi vs ICOS lo in the Blood Gastric cPR 0.1mg/kg combo Gastric cPR monotherapy Vopra Stimulates ICOS hi not ICOS lo 100 ICOS hi CD4 T Cells Change Change Baseline% from 75 50 Emergent ICOS High Population 25 No Emergence CD4) Total of (% ICOS hi CD4 T Cells Cells T CD4 hi ICOS 5% ICOS hi threshold 0 Multiple solid tumors (IO naïve & experienced), single agent & combo with nivolumab 0 100 200 300 400 500 600 700 800 1/7/2020 preliminary analysis Days on Treatment *Hanson, A., SITC 2018, P52 TNBC cPR 0.3mg/kg combo Gastric cPR 0.3mg/kg combo 10 | Jounce Therapeutics, Inc. © January 2020 Gastric cPR 0.1mg/kg combo Gastric cPR monotherapy

Clinical Benefit Correlates with ICOS hi CD4 T Cells ICONIC Clinical Benefit: ICOS hi vs. ICOS lo CD4 T Cells* ICOS hi (n=18) ICOS lo (n=32) RECIST 1.1 ORR 4 (22.2%) 0 Median PFS 6.2 months 2 months Median OS 20.7 months 9 months Progression Free Survival Overall Survival Data cut-off 10-DEC-2019, central radiology review Data cut-off 10-DEC-2019 ICOS hi ICOS hi ICOS lo ICOS lo Durable = PR ≥ 6 months *Subset of 50 patients with evaluable blood samples and tumor measurements, All Patients n=201; central radiology review; data cut December 10, 2019 11 | Jounce Therapeutics, Inc. © January 2020

100 t n e 80 m saturation of receptor ICONIC Data Leadse to Unique Dosing Strategy g a 60 g n E saturation of signaling t 40 Target Engagement from ICONIC Patients e g r a 0.03 mg/kg vopra + ipi T 20 100100 t % t 0.1 mg/kg vopra + ipi n n Dose used in ICONIC: e e 0 80 80 0.3 mg/kg vopra + ipi m m 0 1s0atusraattiu2o0rna toiof nre o3cf0e pretocrep4to0r 50 e e g g Days on Study a a 60 60 g g n n E E satusraattiuorna otiof nsi gonf asliignngaling t t 40 40 e e g Doses for New Studies : g r r a a 0.03 mg/kg vopra + ipi T 20 0.03 mg/kg vopra + ipi T 20 % % 0.1 m0g./1k gm vgo/pkgra v +o pipria + ipi 0 0 0.3 mg/kg vopra + ipi 0 0 10 10 20 20 30 30 40 40 50 50 0.3 mg/kg vopra + ipi DayDs aoyns S otund Sytudy New Strategy: Optimize Dosing for Pulsed Periods of Target Engagement 12 | Jounce Therapeutics, Inc. © January 2020
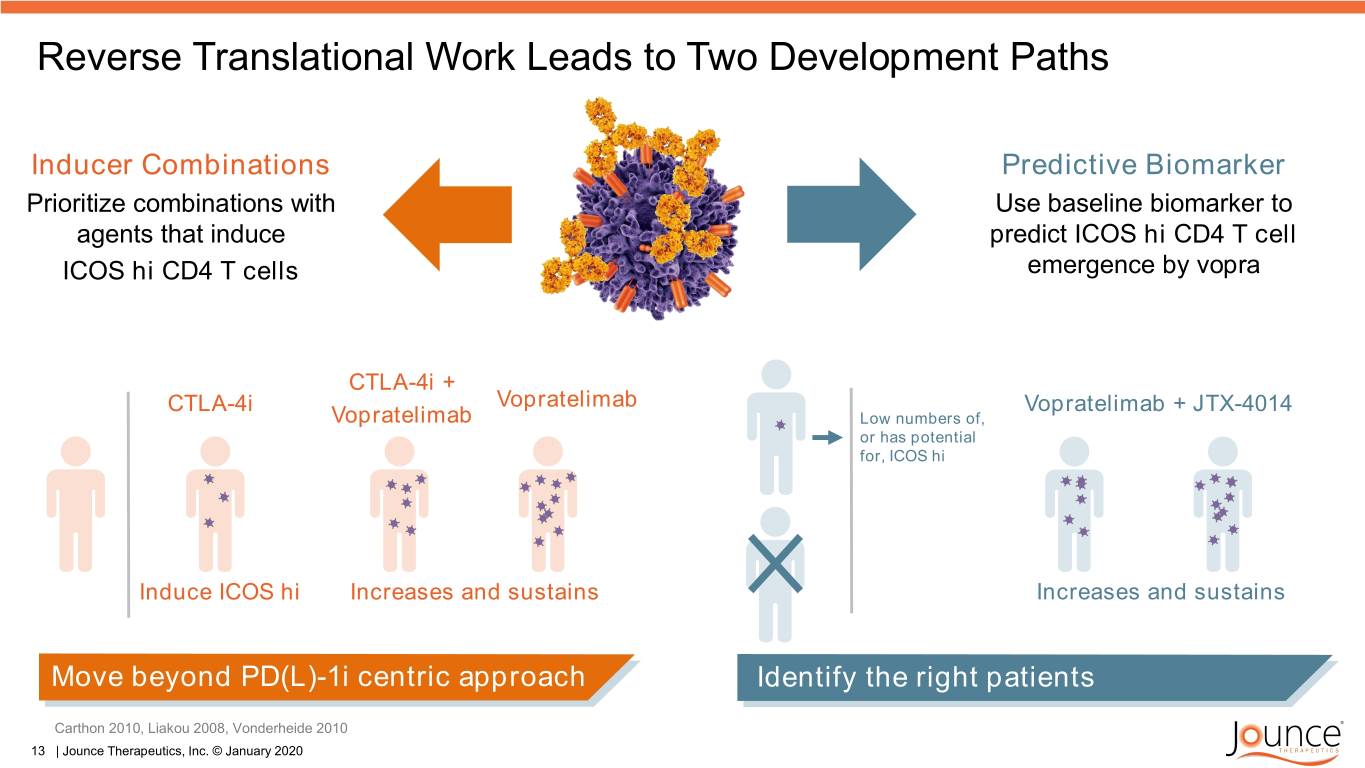
Reverse Translational Work Leads to Two Development Paths Inducer Combinations Predictive Biomarker Prioritize combinations with Use baseline biomarker to agents that induce predict ICOS hi CD4 T cell ICOS hi CD4 T cells emergence by vopra CTLA-4i + CTLA-4i Vopratelimab Vopratelimab + JTX-4014 Vopratelimab Low numbers of, or has potential for, ICOS hi Induce ICOS hi Increases and sustains Increases and sustains Move beyond PD(L)-1i centric approach Identify the right patients Carthon 2010, Liakou 2008, Vonderheide 2010 13 | Jounce Therapeutics, Inc. © January 2020
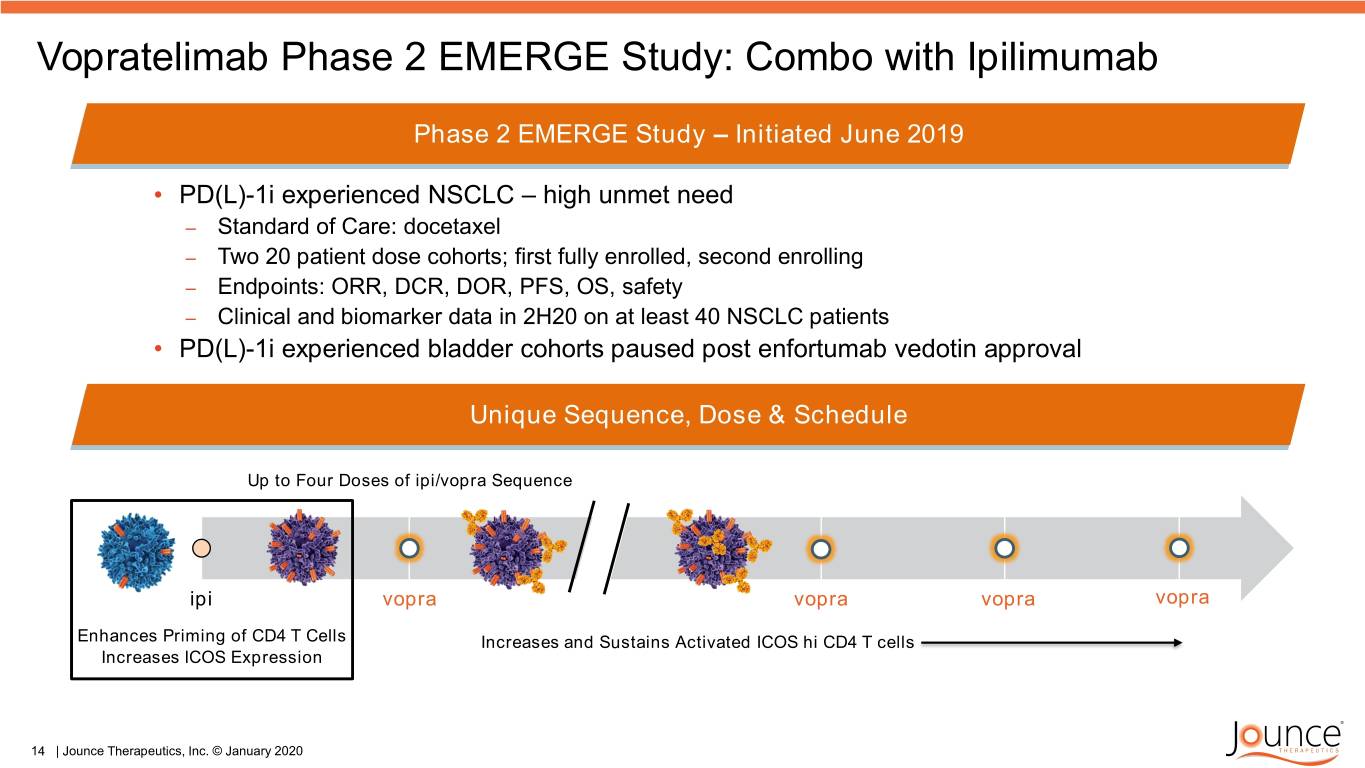
Vopratelimab Phase 2 EMERGE Study: Combo with Ipilimumab Phase 2 EMERGE Study – Initiated June 2019 • PD(L)-1i experienced NSCLC – high unmet need – Standard of Care: docetaxel – Two 20 patient dose cohorts; first fully enrolled, second enrolling – Endpoints: ORR, DCR, DOR, PFS, OS, safety – Clinical and biomarker data in 2H20 on at least 40 NSCLC patients • PD(L)-1i experienced bladder cohorts paused post enfortumab vedotin approval Unique Sequence, Dose & Schedule Up to Four Doses of ipi/vopra Sequence ipi vopra vopra vopra vopra Enhances Priming of CD4 T Cells Increases and Sustains Activated ICOS hi CD4 T cells Increases ICOS Expression 14 | Jounce Therapeutics, Inc. © January 2020

Baseline RNA Signature Predicts ICOS hi CD4 T Cells and Clinical Benefit ICOS hi CD4 T Cells: RNA Signature (RS) Predicts Emergence RS Predicts Overall Survival Vopra PD Biomarker (Treatment Emergent) of ICOS hi CD4 T Cells Overall Survival: ICONIC Patients with Longitudinal T Cells Overall Survival: ICONIC Patients with Longitudinal T Cells Available Available and Baseline Tumor RNA ICONIC Patients with Baseline Tumor RNA PPV = 78% NPV = 83% Above RS Cut-Off Median OS = 16.9 mo ICOS hi Median OS = 20.7 mo ICOS lo Median OS = 9 mo Below RS Cut-Off Median OS = 6.2 mo Peripheral ICOS hi CD4 RS (n = 32) AUC= 0.79 Above Biomarker Cut-Off No ICOS hi CD4 PD-L1 IHC (TPS) (n = 27) AUC= 0.52 Below Biomarker Cut-Off Data cut-off 10-DEC-2019, n = 50 Data cut-off 10-DEC-2019 Data cut-off 10-DEC-2019, n = 89 PD-L1 IHC did not predict ICOS hi CD4 T cells PPV = Positive Predictive Value; NPV = Negative Predictive Value; AUC = Area Under the ROC Curve 15 | Jounce Therapeutics, Inc. © January 2020
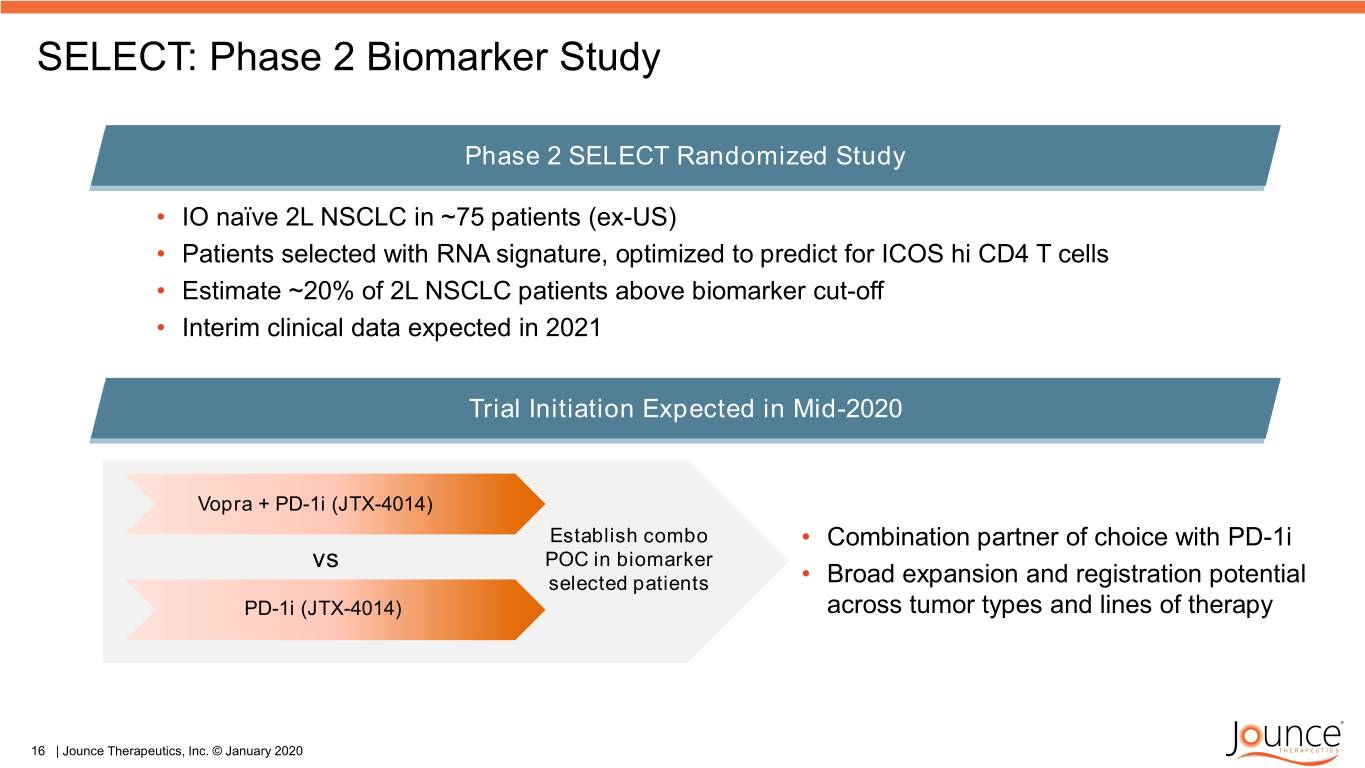
SELECT: Phase 2 Biomarker Study Phase 2 SELECT Randomized Study • IO naïve 2L NSCLC in ~75 patients (ex-US) • Patients selected with RNA signature, optimized to predict for ICOS hi CD4 T cells • Estimate ~20% of 2L NSCLC patients above biomarker cut-off • Interim clinical data expected in 2021 Trial Initiation Expected in Mid-2020 Vopra + PD-1i (JTX-4014) Establish combo • Combination partner of choice with PD-1i vs POC in biomarker selected patients • Broad expansion and registration potential PD-1i (JTX-4014) across tumor types and lines of therapy 16 | Jounce Therapeutics, Inc. © January 2020

Development Programs Vopratelimab JTX-4014 JTX-1811
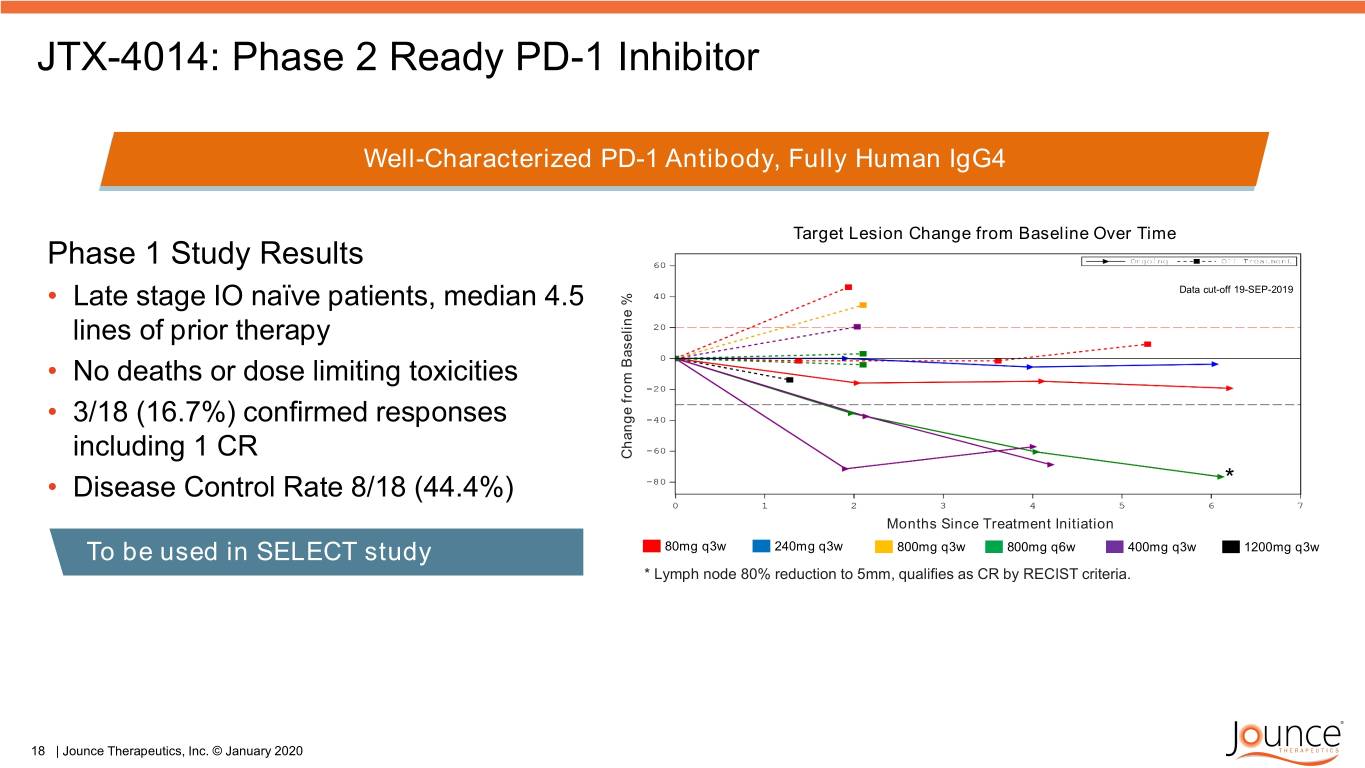
JTX-4014: Phase 2 Ready PD-1 Inhibitor Well-Characterized PD-1 Antibody, Fully Human IgG4 Target Lesion Change from Baseline Over Time Phase 1 Study Results • Late stage IO naïve patients, median 4.5 Data cut-off 19-SEP-2019 lines of prior therapy • No deaths or dose limiting toxicities • 3/18 (16.7%) confirmed responses including 1 CR Change% from Baseline • Disease Control Rate 8/18 (44.4%) * Months Since Treatment Initiation To be used in SELECT study 80mg q3w 240mg q3w 800mg q3w 800mg q6w 400mg q3w 1200mg q3w * Lymph node 80% reduction to 5mm, qualifies as CR by RECIST criteria. 18 | Jounce Therapeutics, Inc. © January 2020

Development Programs Vopratelimab JTX-4014 JTX-1811

JTX-1811: Designed to Deplete Immunosuppressive T regulatory Cells • T regulatory cells (T regs) • JTX-1811 is a monoclonal – Diminish productive immune responses antibody designed to deplete – Immune response to tumors may require T regs in the tumor reducing T regulatory effect microenvironment JTX-1811 CD4 Suboptimal Immune Response CD4 Remove Immunosuppression T regs Diminish a Productive Immune JTX-1811 Depletes Response T regs CD8 Suboptimal Immune Response CD8 Remove Immunosuppression 20 | Jounce Therapeutics, Inc. © January 2020

JTX-1811: Target Specificity to T regs in the Tumor JTX-1811 Selectively Binds to T regs from TILs* Peripheral Blood** Lung Tumor** (Healthy) Similar binding to T regs from multiple Tregs Tregs tumor types (n=25) including: Tconv Tconv Iso Iso • NSCLC • Breast • Colon • Head & Neck Cell Count Cell • Ovarian Target Expression Level Opportunity to Specifically Deplete T regs in the Tumor *Tumor infiltrating lymphocytes **Flow cytometry 21 | Jounce Therapeutics, Inc. © January 2020

Single Agent Anti-Tumor Activity in PD-1 Inhibitor Resistant Tumors Also Restores PD-1i Activity PD-1 Inhibitor Resistant Mouse Tumor Models Selective Depletion of Tumor T regs Colon-26 Tumor MBT-2 Tumor Tumor Resident Immunosuppressive T regs 5/10 complete responder 5/9 complete JTX-1811 responder + Isotype anti-PD-1 Surrogate JTX-1811 mIgG2a Surrogate JTX-1811 mIgG2a + anti-PD-1 Loss of Tumor T regs and Function 22 | Jounce Therapeutics, Inc. © January 2020

The Jounce Approach: Sustainable Discovery for 1st in Class Immunotherapies Translational Science Platform Innovative & Sustainable Discovery Pipeline Immunosuppressive T regulatory cells Inhibit immunosuppressive activity Stromal cells Approach for cold tumors that lack T cells New target ID from 1000s of human tumors Immunosuppressive Prioritized targets from different Macrophages immune cell types in tumors Approach for macrophage dominated TME 23 | Jounce Therapeutics, Inc. © January 2020

Financial Strength and Flexibility Financial Overview Funding History NASDAQ: JNCE Event Date Gross $ Comment Series A 2013 $47M Third Rock Strong balance sheet: Ventures • $185.1M cash and investments, Series B 2015 $56M Fidelity, as of September 30, 2019 Wellington, others Updated Financial guidance: CELG 2016 $225M Non-refundable • 2019 gross cash burn $75M - $85M Collaboration upfront upfront payment o Actual ~$79M $36M equity • 2020 gross cash burn $80M - $95M Initial Public 2017 $117M Upsized deal Common stock outstanding: Offering • 33M shares as of November 1, 2019 Exclusively 2019 $50M Potential Out-licensed upfront milestones $50M ATM put in place Dec 2019 JTX-8064 & royalties 24 | Jounce Therapeutics, Inc. © January 2020

Milestones 2019 2020 ▪ Three immunotherapies in the clinic ▪ Vopratelimab: Preliminary efficacy and related ✓ Two wholly owned immunotherapies in the biomarker data from EMERGE in 2H20 clinic ▪ Vopratelimab + JTX-4014: Initiation of predictive ✓ Exclusively out-licensed JTX-8064 biomarker SELECT study mid-year ✓ Vopratelimab: Data update including PFS and ▪ JTX-1811: OS from ICONIC at AACR ▪ Presentation at a scientific meeting in 1H20 ✓ Vopratelimab: Initiate new Phase 2 studies ▪ Continue IND enabling activities for a 1H21 IND filing ✓ JTX-4014: Establish safety and RP2D ▪ Continue to advance multiple new targets ✓ Advance next development candidate into IND through discovery pipeline enabling studies (JTX-1811) 25 | Jounce Therapeutics, Inc. © January 2020

Jounce Therapeutics A Next Gen Immunotherapy Company

























