
BDC-1001 Interim Clinical Data ESMO Immuno-Oncology 2021 December 2021 1 Exhibit 99.2

Today’s Agenda 2

This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements other than statements of historical facts contained in this presentation, including statements regarding Bolt Biotherapeutics, Inc. (the "Company," "we," "us," or "our")’s ability to achieve upcoming milestones for our product candidates and the success and results of our pipeline programs, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potentially” “predict,” “should,” “will” or the negative of these terms or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, among other things: the success, cost and timing of our product development activities and clinical trials; our expectations about the timing of achieving regulatory approval and the cost of our development programs; our ability to obtain funding for our operations, including funding necessary to complete further development and commercialization of our product candidates; our ability to fund our clinical programs and the sufficiency of our cash, cash equivalents, and marketable securities to fund achievement of key milestones; the commercialization of our product candidates, if approved; our plans to research, develop and commercialize our product candidates; our ability to attract collaborators with development, regulatory and commercialization expertise; future agreements with third parties in connection with the commercialization of our product candidates; the success of our current collaborations with third parties, including our collaborations with Bristol-Myers Squibb Company, Innovent Biologics, Inc., Genmab A/S and Toray Industries, Inc.; the achievement of milestone payments or any tiered royalties related to our collaborations; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; the rate and degree of market acceptance of our product candidates; and regulatory developments in the United States and foreign countries. These risks are not exhaustive. For a detailed discussion of the risk factors that could affect our actual results, please refer to the risk factors identified in our SEC reports, including, but not limited to our Annual Report on Form 10-K for the year ended December 31, 2020. New risk factors emerge from time to time and it is not possible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward- looking statements. You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this presentation. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this presentation, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and investors are cautioned not to unduly rely upon these statements. Disclaimer 3
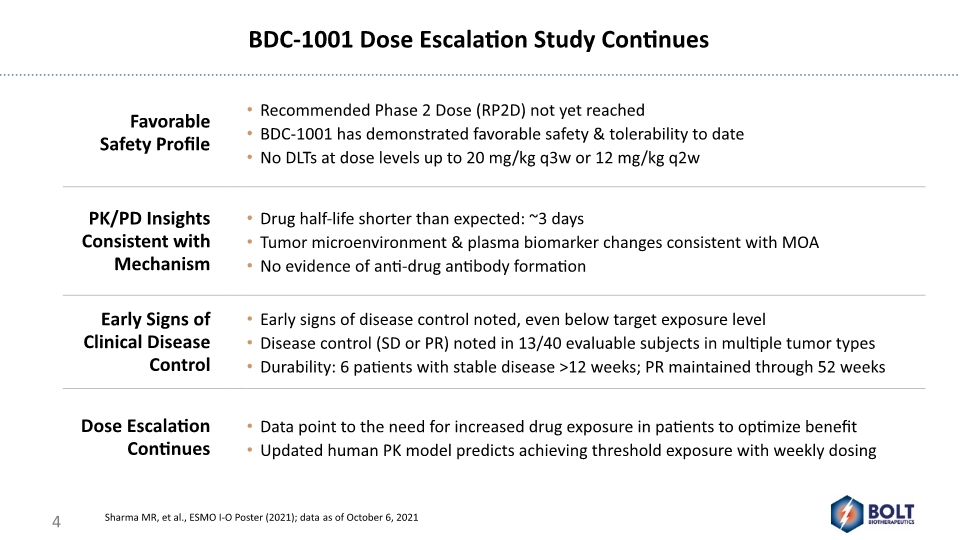
BDC-1001 Dose Escalation Study Continues Sharma MR, et al., ESMO I-O Poster (2021); data as of October 6, 2021 4

Review of BDC-1001 Mechanism & Preclinical Data 5
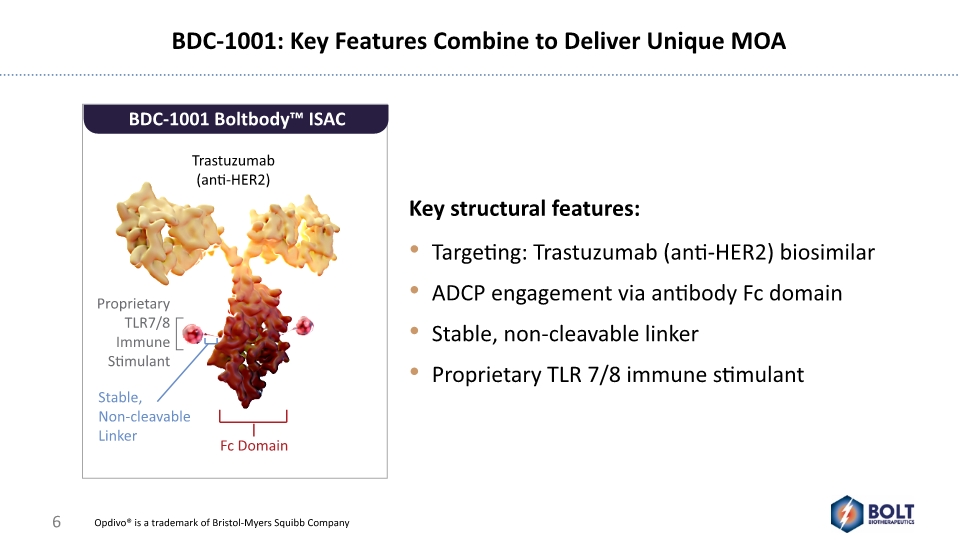
BDC-1001: Key Features Combine to Deliver Unique MOA BDC-1001 Boltbody™ ISAC Trastuzumab (anti-HER2) Proprietary TLR7/8 Immune Stimulant Fc Domain Stable, Non-cleavable Linker Opdivo® is a trademark of Bristol-Myers Squibb Company 6
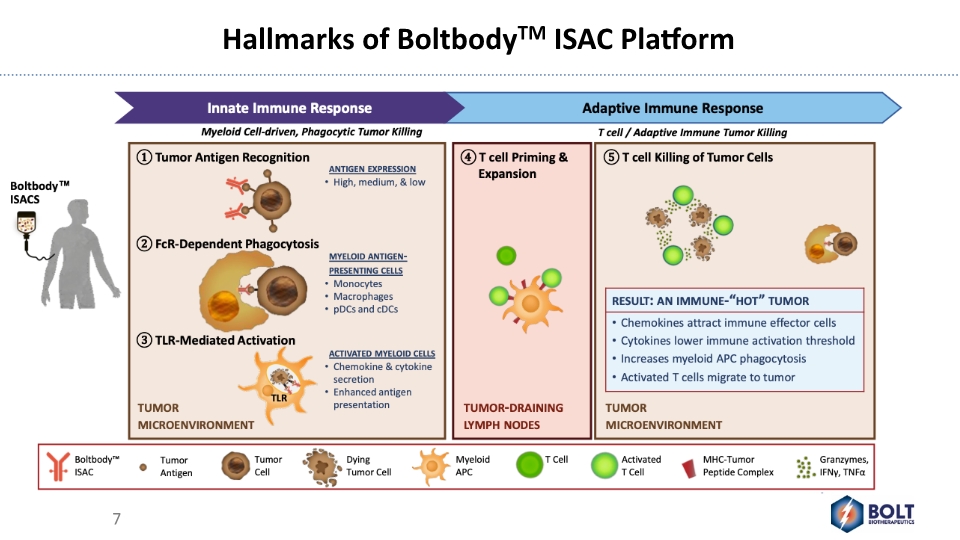
Hallmarks of BoltbodyTM ISAC Platform 7
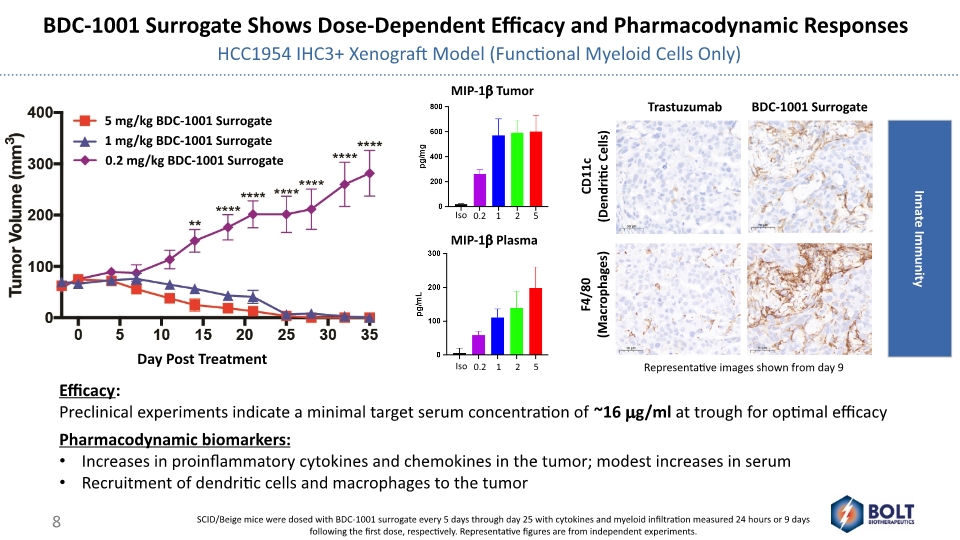
BDC-1001 Surrogate Shows Dose-Dependent Efficacy and Pharmacodynamic Responses HCC1954 IHC3+ Xenograft Model (Functional Myeloid Cells Only) Efficacy: Preclinical experiments indicate a minimal target serum concentration of ~16 mg/ml at trough for optimal efficacy Pharmacodynamic biomarkers: Increases in proinflammatory cytokines and chemokines in the tumor; modest increases in serum Recruitment of dendritic cells and macrophages to the tumor MIP-1b Tumor MIP-1b Plasma SCID/Beige mice were dosed with BDC-1001 surrogate every 5 days through day 25 with cytokines and myeloid infiltration measured 24 hours or 9 days following the first dose, respectively. Representative figures are from independent experiments. Representative images shown from day 9 8
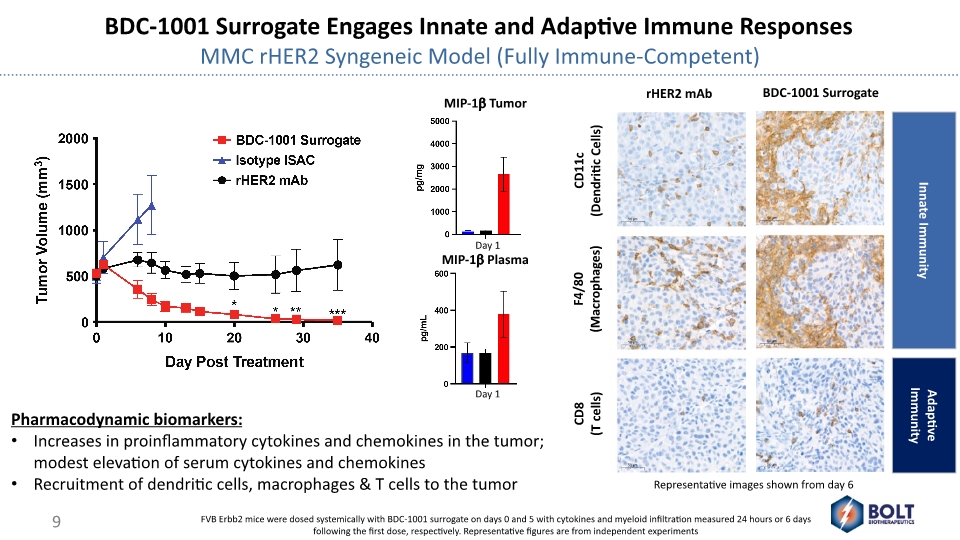
BDC-1001 Surrogate Engages Innate and Adaptive Immune Responses MMC rHER2 Syngeneic Model (Fully Immune-Competent) CD11c (Dendritic Cells) F4/80 (Macrophages) rHER2 mAb BDC-1001 Surrogate CD8 (T cells) Representative images shown from day 6 Adaptive Immunity Innate Immunity MIP-1b Plasma MIP-1b Tumor FVB Erbb2 mice were dosed systemically with BDC-1001 surrogate on days 0 and 5 with cytokines and myeloid infiltration measured 24 hours or 6 days following the first dose, respectively. Representative figures are from independent experiments Pharmacodynamic biomarkers: Increases in proinflammatory cytokines and chemokines in the tumor; modest elevation of serum cytokines and chemokines Recruitment of dendritic cells, macrophages & T cells to the tumor Day 1 Day 1 9

BDC-1001 Interim Results Presented at ESMO Immuno-Oncology 2021 December 2021 10
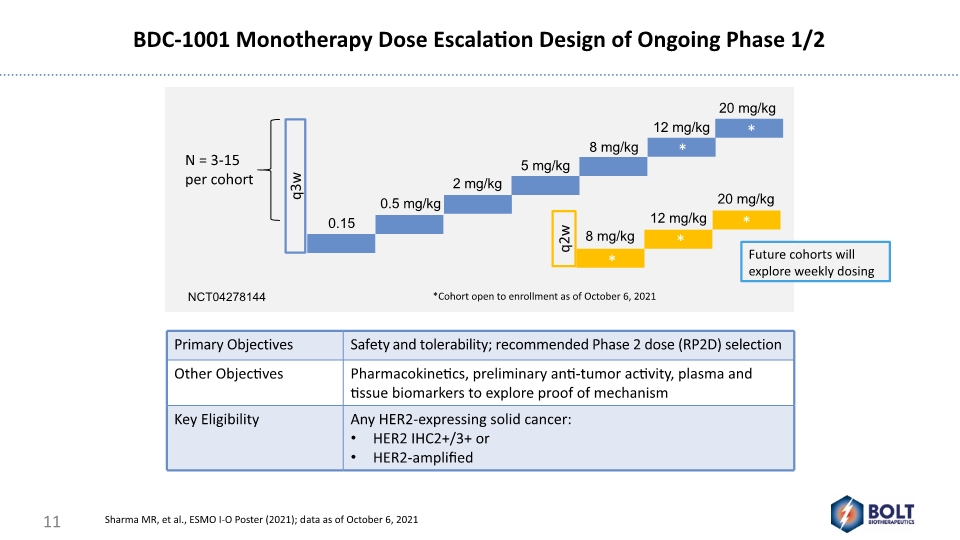
BDC-1001 Monotherapy Dose Escalation Design of Ongoing Phase 1/2 11 Future cohorts will explore weekly dosing Sharma MR, et al., ESMO I-O Poster (2021); data as of October 6, 2021
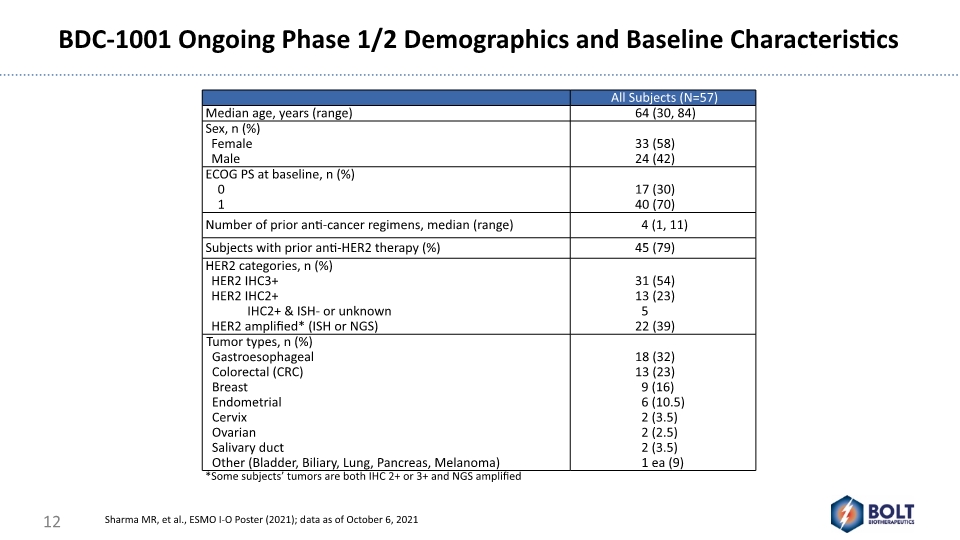
*Some subjects’ tumors are both IHC 2+ or 3+ and NGS amplified BDC-1001 Ongoing Phase 1/2 Demographics and Baseline Characteristics 12 Sharma MR, et al., ESMO I-O Poster (2021); data as of October 6, 2021
BDC-1001 Overall Safety Summary Consistent with BDC-1001’s design, this agent has demonstrated good safety and tolerability at doses tested to date No DLTs observed to date; MTD has not been reached at up to 20 mg/kg q3w or 12mg/kg q2w dose levels Two treatment-related SAEs, both of which led to treatment discontinuation Asymptomatic Grade 3 ejection fraction decrease (>20%) after 4 cycles of therapy in an anti-HER2 therapy naïve subject with history of hypertension Grade 4 bronchopulmonary hemorrhage in a subject who had a lung biopsy 5d prior to treatment Grade 1/2 infusion-related reactions (IRRs) occurred in 11 subjects starting at the 5mg/kg q3w cohort; none related to treatment discontinuation Non-steroid pre-medication introduced at the 8 mg/kg dose level No AEs consistent with cytokine release syndrome (CRS) reported 13 Sharma MR, et al., ESMO I-O Poster (2021); data as of October 6, 2021
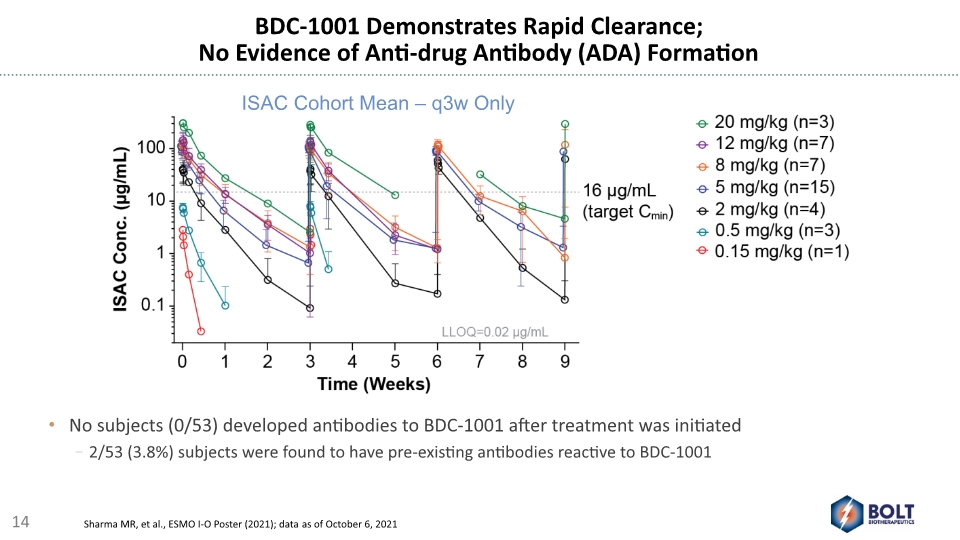
BDC-1001 Demonstrates Rapid Clearance; No Evidence of Anti-drug Antibody (ADA) Formation No subjects (0/53) developed antibodies to BDC-1001 after treatment was initiated 2/53 (3.8%) subjects were found to have pre-existing antibodies reactive to BDC-1001 14 Sharma MR, et al., ESMO I-O Poster (2021); data as of October 6, 2021 ISAC Cohort Mean – q3w Only
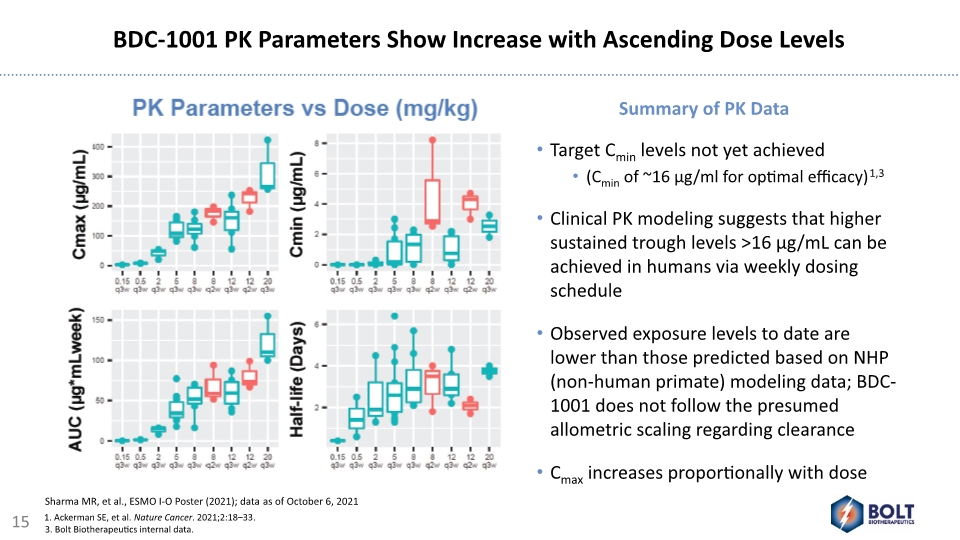
Target Cmin levels not yet achieved (Cmin of ~16 µg/ml for optimal efficacy)1,3 Clinical PK modeling suggests that higher sustained trough levels >16 µg/mL can be achieved in humans via weekly dosing schedule Observed exposure levels to date are lower than those predicted based on NHP (non-human primate) modeling data; BDC-1001 does not follow the presumed allometric scaling regarding clearance Cmax increases proportionally with dose Summary of PK Data BDC-1001 PK Parameters Show Increase with Ascending Dose Levels 15 1. Ackerman SE, et al. Nature Cancer. 2021;2:18–33. 3. Bolt Biotherapeutics internal data. Sharma MR, et al., ESMO I-O Poster (2021); data as of October 6, 2021
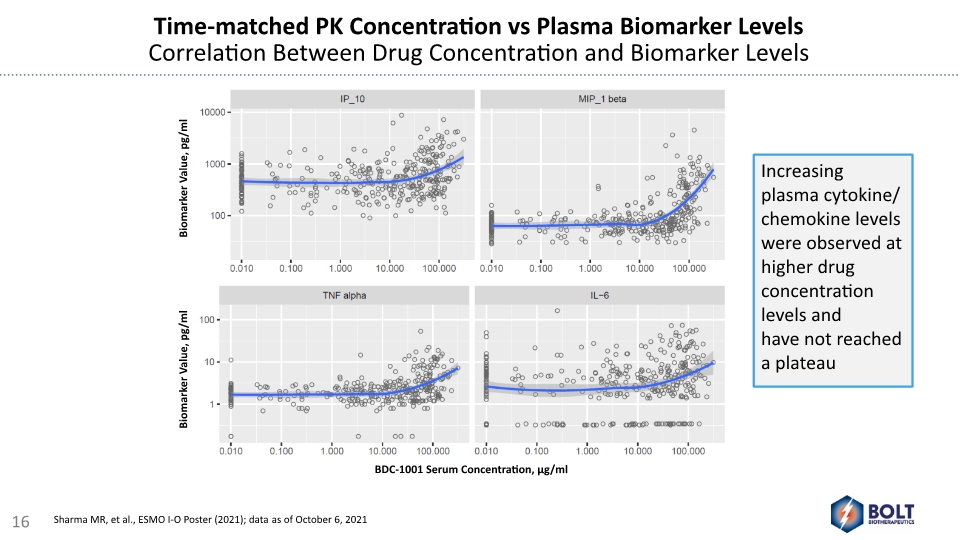
Time-matched PK Concentration vs Plasma Biomarker Levels Correlation Between Drug Concentration and Biomarker Levels Increasing plasma cytokine/ chemokine levels were observed at higher drug concentration levels and have not reached a plateau BDC-1001 Serum Concentration, µg/ml Biomarker Value, pg/ml Biomarker Value, pg/ml 16 Sharma MR, et al., ESMO I-O Poster (2021); data as of October 6, 2021
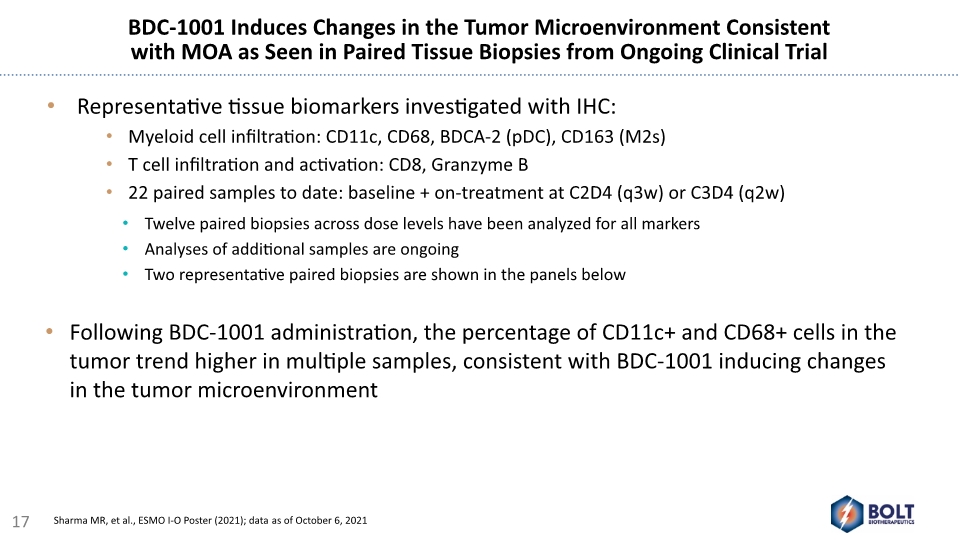
BDC-1001 Induces Changes in the Tumor Microenvironment Consistent with MOA as Seen in Paired Tissue Biopsies from Ongoing Clinical Trial Representative tissue biomarkers investigated with IHC: Myeloid cell infiltration: CD11c, CD68, BDCA-2 (pDC), CD163 (M2s) T cell infiltration and activation: CD8, Granzyme B 22 paired samples to date: baseline + on-treatment at C2D4 (q3w) or C3D4 (q2w) Twelve paired biopsies across dose levels have been analyzed for all markers Analyses of additional samples are ongoing Two representative paired biopsies are shown in the panels below Following BDC-1001 administration, the percentage of CD11c+ and CD68+ cells in the tumor trend higher in multiple samples, consistent with BDC-1001 inducing changes in the tumor microenvironment 17 Sharma MR, et al., ESMO I-O Poster (2021); data as of October 6, 2021
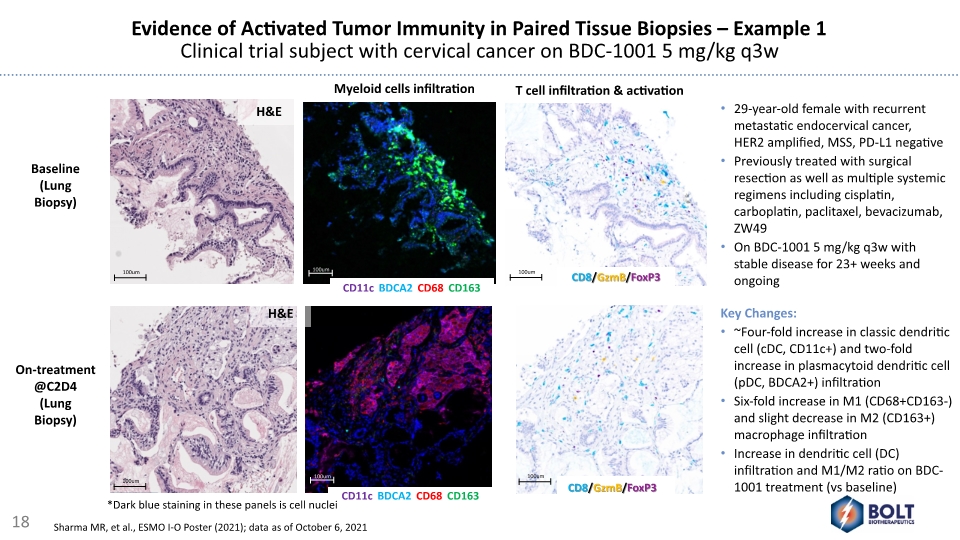
Evidence of Activated Tumor Immunity in Paired Tissue Biopsies – Example 1 Clinical trial subject with cervical cancer on BDC-1001 5 mg/kg q3w H&E H&E CD11c/BDCA2/CD68/CD163 CD11c/BDCA2/CD68/CD163 Myeloid cells infiltration T cell infiltration & activation CD8/GzmB/FoxP3 CD8/GzmB/FoxP3 Baseline (Lung Biopsy) On-treatment @C2D4 (Lung Biopsy) *Dark blue staining in these panels is cell nuclei 29-year-old female with recurrent metastatic endocervical cancer, HER2 amplified, MSS, PD-L1 negative Previously treated with surgical resection as well as multiple systemic regimens including cisplatin, carboplatin, paclitaxel, bevacizumab, ZW49 On BDC-1001 5 mg/kg q3w with stable disease for 23+ weeks and ongoing Key Changes: ~Four-fold increase in classic dendritic cell (cDC, CD11c+) and two-fold increase in plasmacytoid dendritic cell (pDC, BDCA2+) infiltration Six-fold increase in M1 (CD68+CD163-) and slight decrease in M2 (CD163+) macrophage infiltration Increase in dendritic cell (DC) infiltration and M1/M2 ratio on BDC-1001 treatment (vs baseline) 18 Sharma MR, et al., ESMO I-O Poster (2021); data as of October 6, 2021
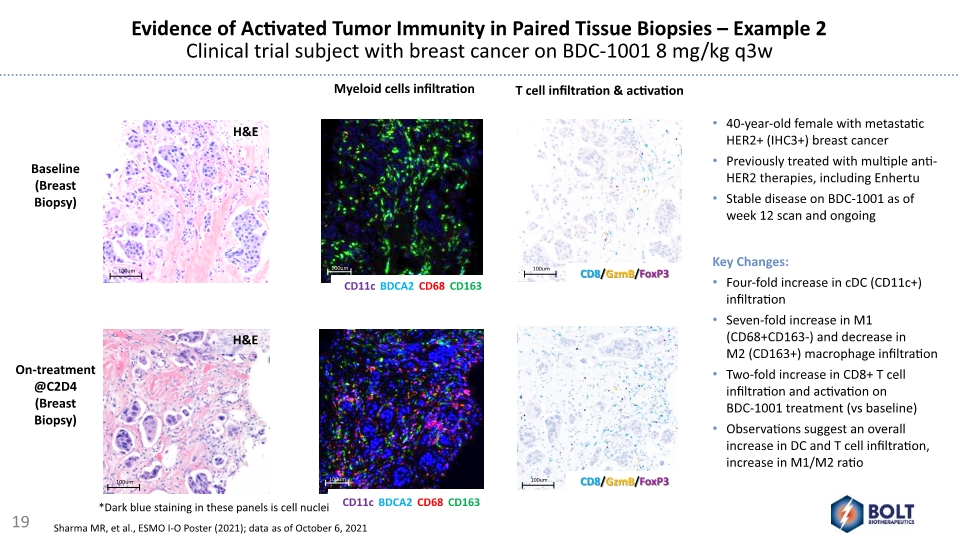
Evidence of Activated Tumor Immunity in Paired Tissue Biopsies – Example 2 Clinical trial subject with breast cancer on BDC-1001 8 mg/kg q3w H&E H&E 40-year-old female with metastatic HER2+ (IHC3+) breast cancer Previously treated with multiple anti-HER2 therapies, including Enhertu Stable disease on BDC-1001 as of week 12 scan and ongoing Myeloid cells infiltration T cell infiltration & activation Baseline (Breast Biopsy) On-treatment @C2D4 (Breast Biopsy) *Dark blue staining in these panels is cell nuclei Key Changes: Four-fold increase in cDC (CD11c+) infiltration Seven-fold increase in M1 (CD68+CD163-) and decrease in M2 (CD163+) macrophage infiltration Two-fold increase in CD8+ T cell infiltration and activation on BDC-1001 treatment (vs baseline) Observations suggest an overall increase in DC and T cell infiltration, increase in M1/M2 ratio 19 Sharma MR, et al., ESMO I-O Poster (2021); data as of October 6, 2021
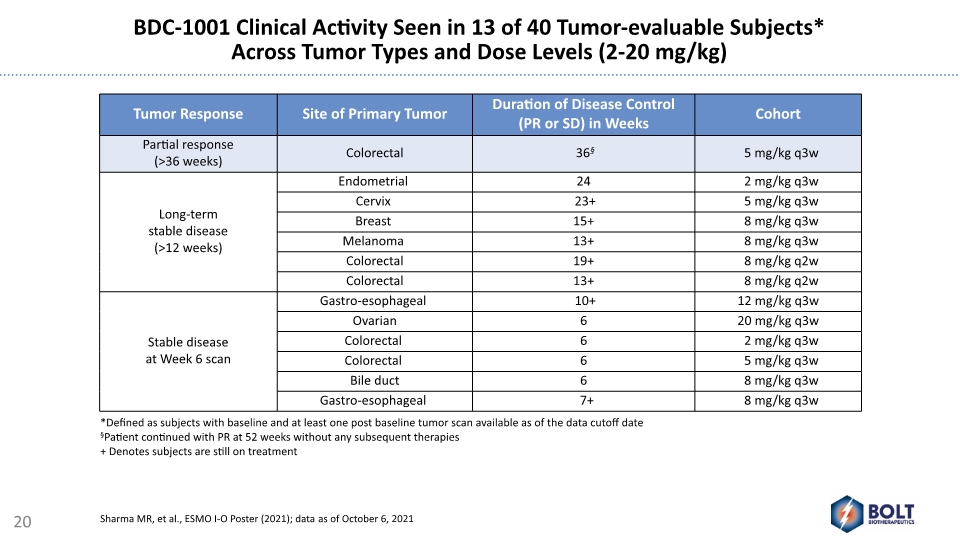
BDC-1001 Clinical Activity Seen in 13 of 40 Tumor-evaluable Subjects* Across Tumor Types and Dose Levels (2-20 mg/kg) *Defined as subjects with baseline and at least one post baseline tumor scan available as of the data cutoff date §Patient continued with PR at 52 weeks without any subsequent therapies + Denotes subjects are still on treatment 20 Sharma MR, et al., ESMO I-O Poster (2021); data as of October 6, 2021
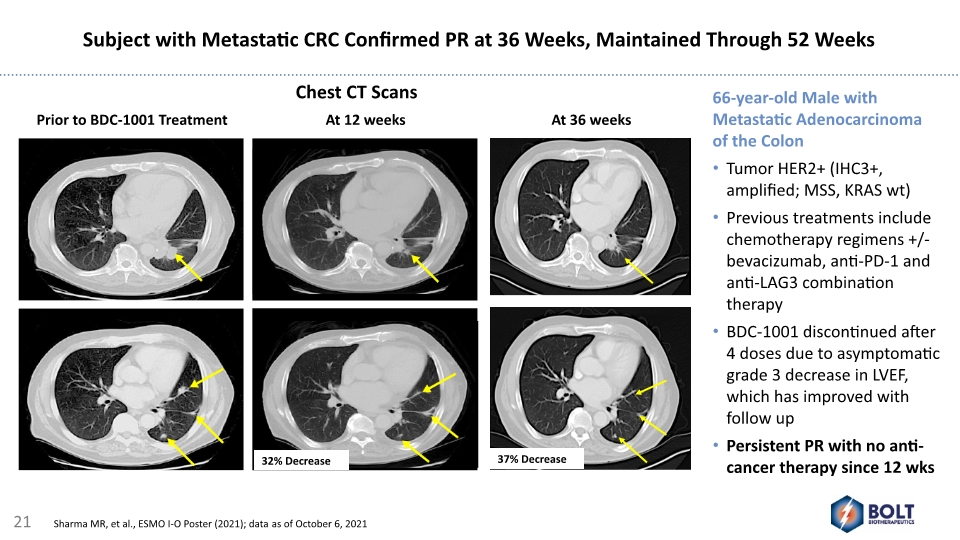
2014 Prior to BDC-1001 Treatment At 12 weeks 66-year-old Male with Metastatic Adenocarcinoma of the Colon Tumor HER2+ (IHC3+, amplified; MSS, KRAS wt) Previous treatments include chemotherapy regimens +/- bevacizumab, anti-PD-1 and anti-LAG3 combination therapy BDC-1001 discontinued after 4 doses due to asymptomatic grade 3 decrease in LVEF, which has improved with follow up Persistent PR with no anti-cancer therapy since 12 wks At 36 weeks Subject with Metastatic CRC Confirmed PR at 36 Weeks, Maintained Through 52 Weeks Chest CT Scans 21 Sharma MR, et al., ESMO I-O Poster (2021); data as of October 6, 2021

Summary 22
Progress in Our Pioneering Journey 1 Proforma Cash, cash equivalents, and marketable securities as of September 30, 2021: $290.5 million balance plus $5 million Innovent receivable Opdivo® is a trademark of Bristol-Myers Squibb Company 23

Thank You 24























