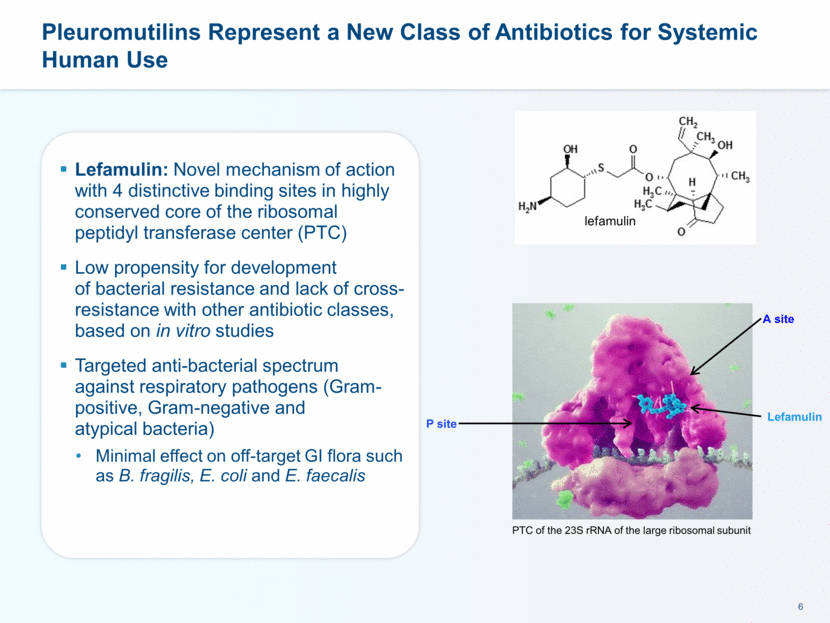
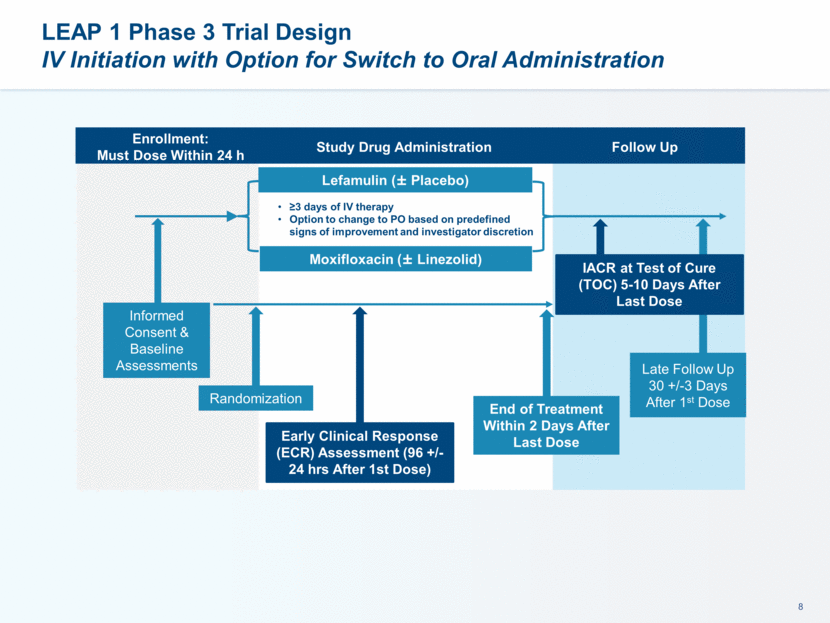
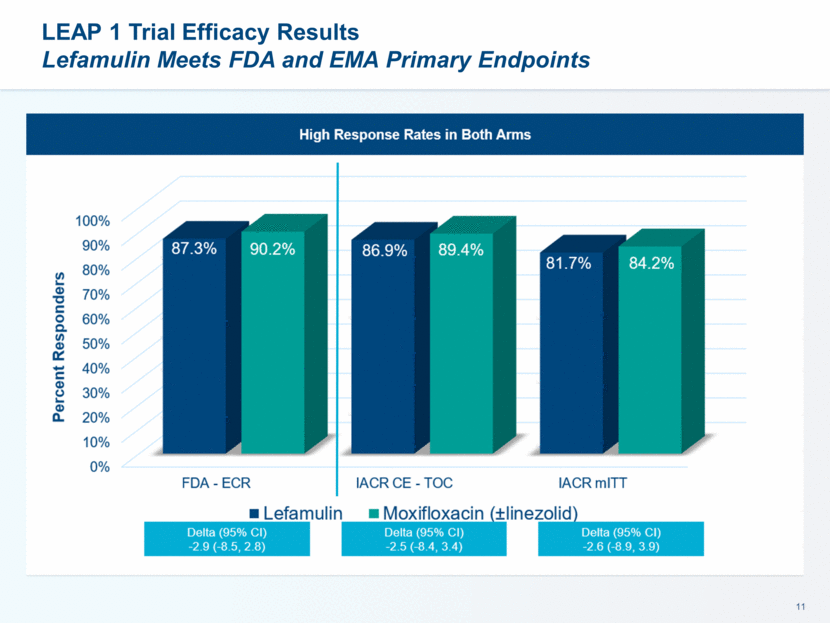
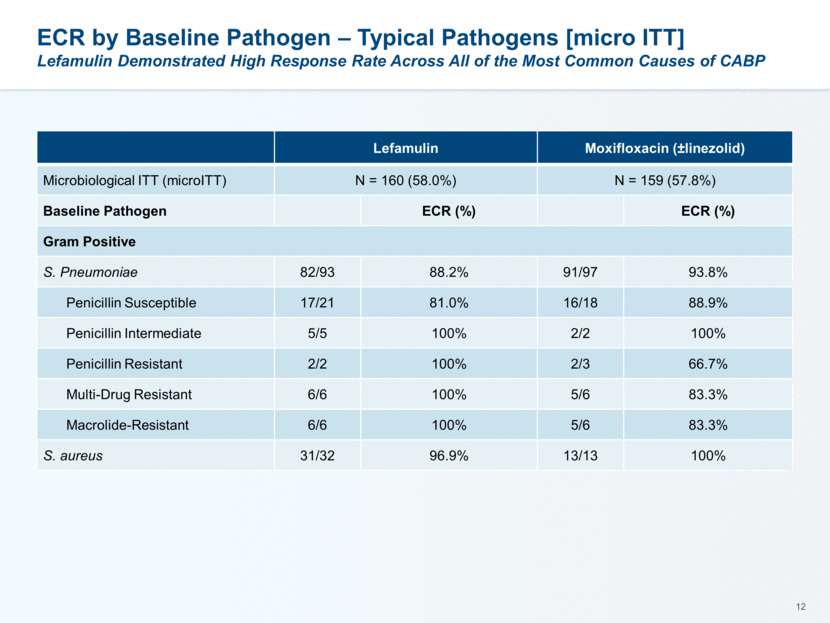
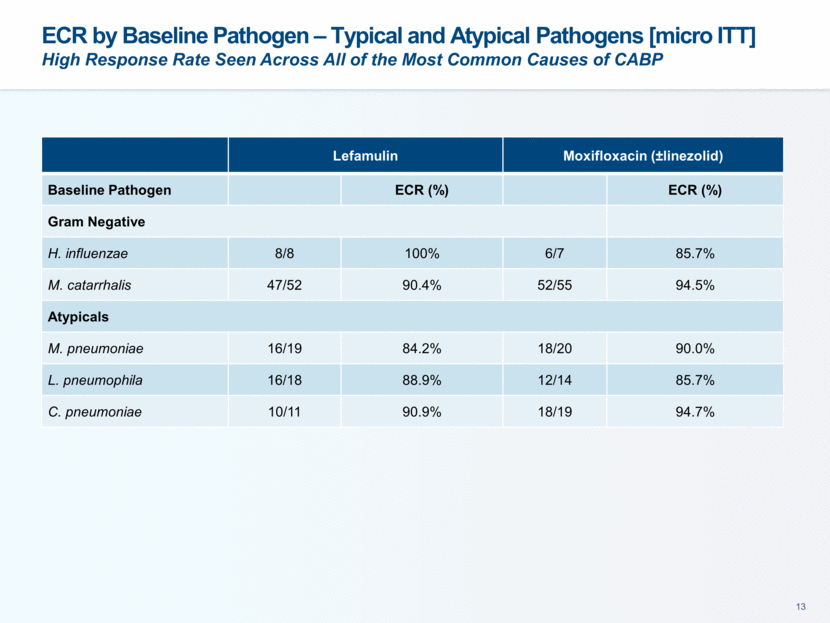
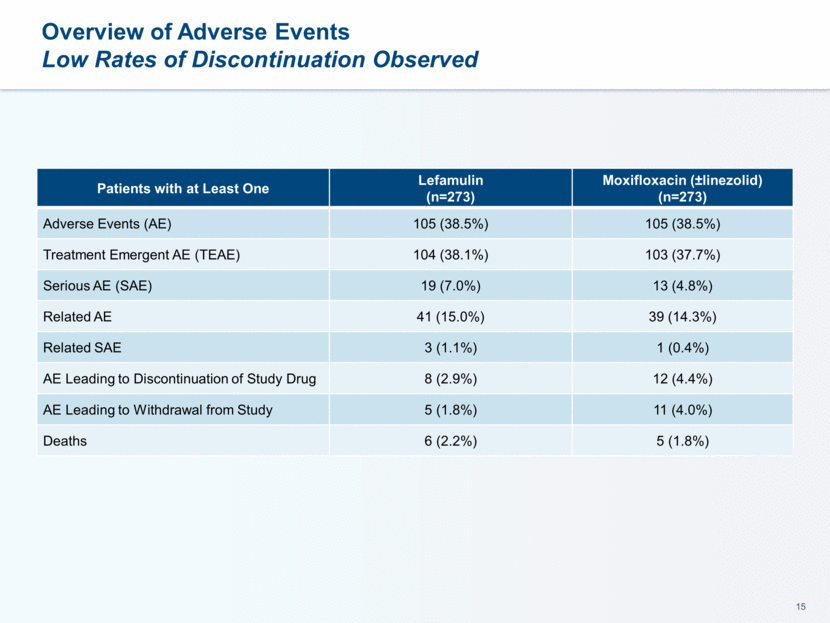
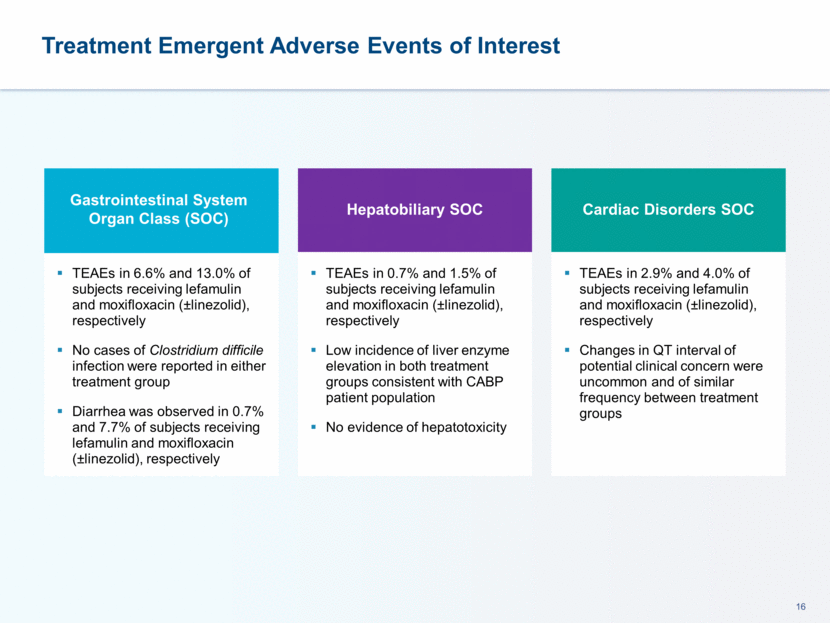
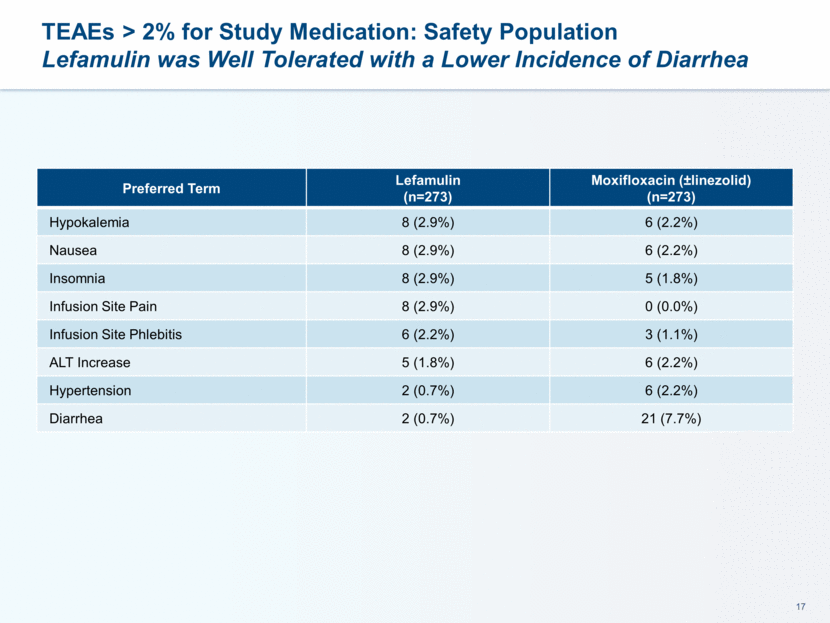
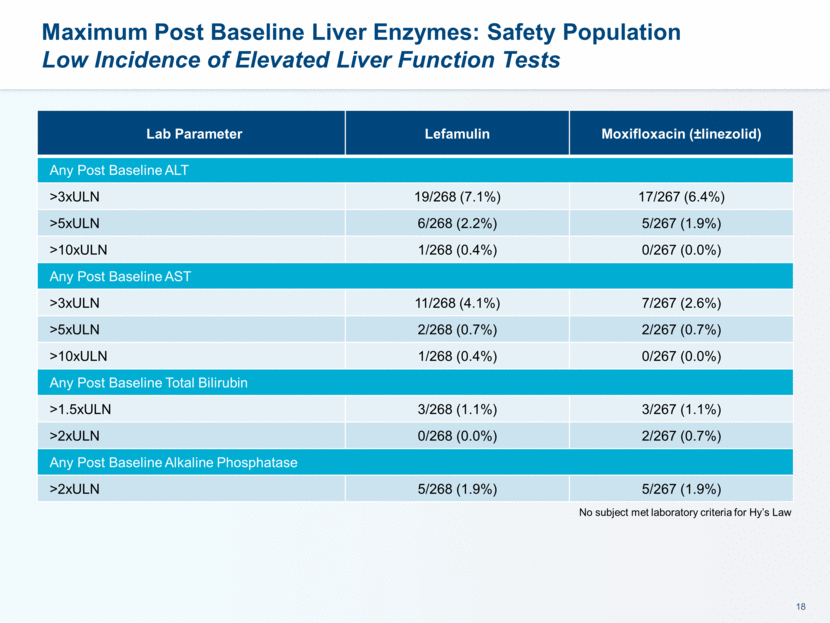
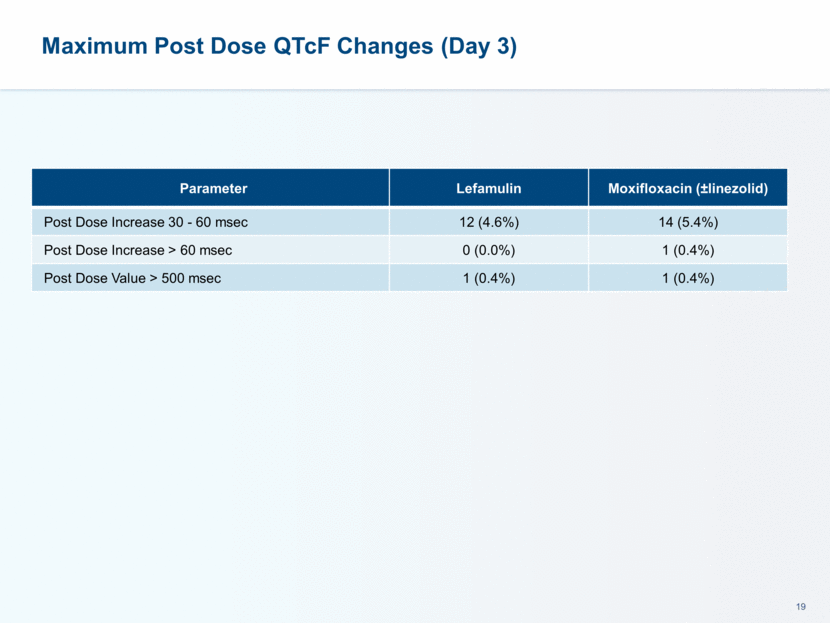
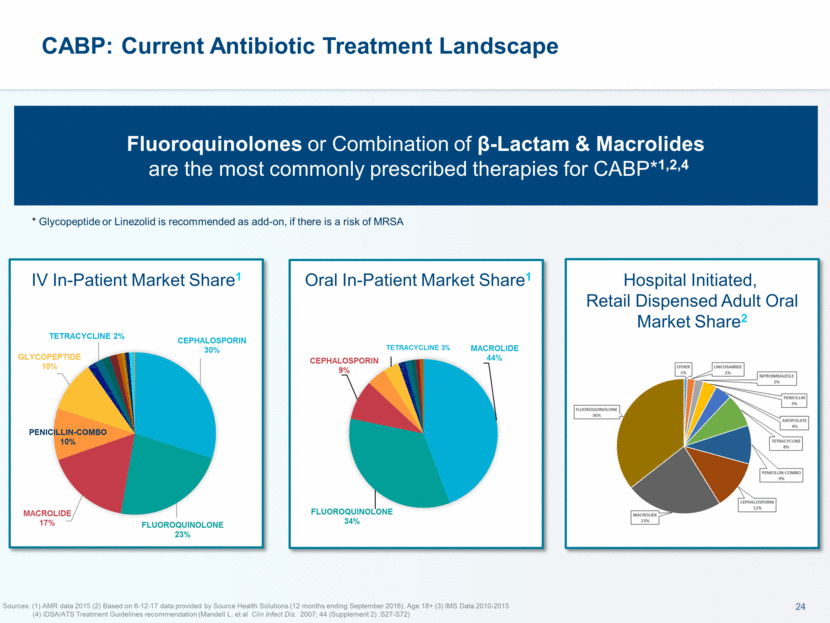
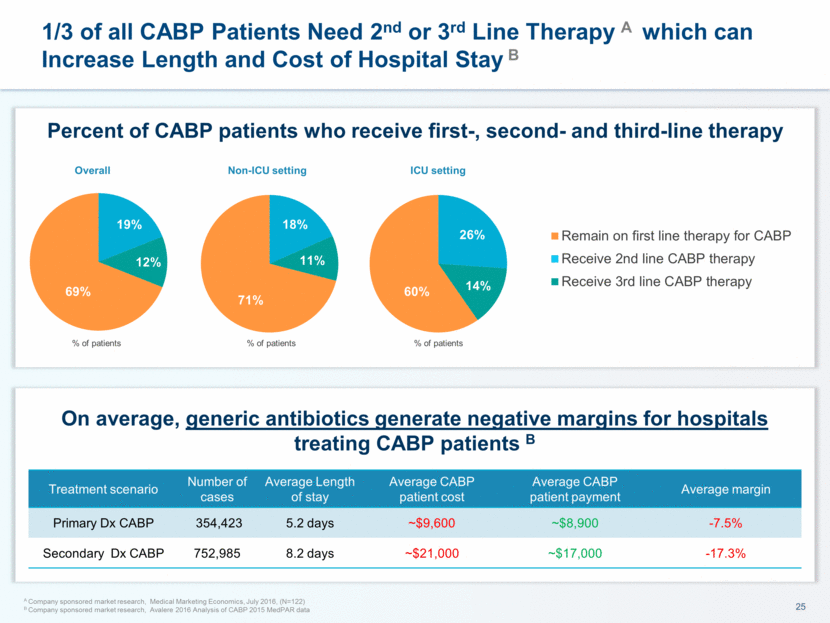
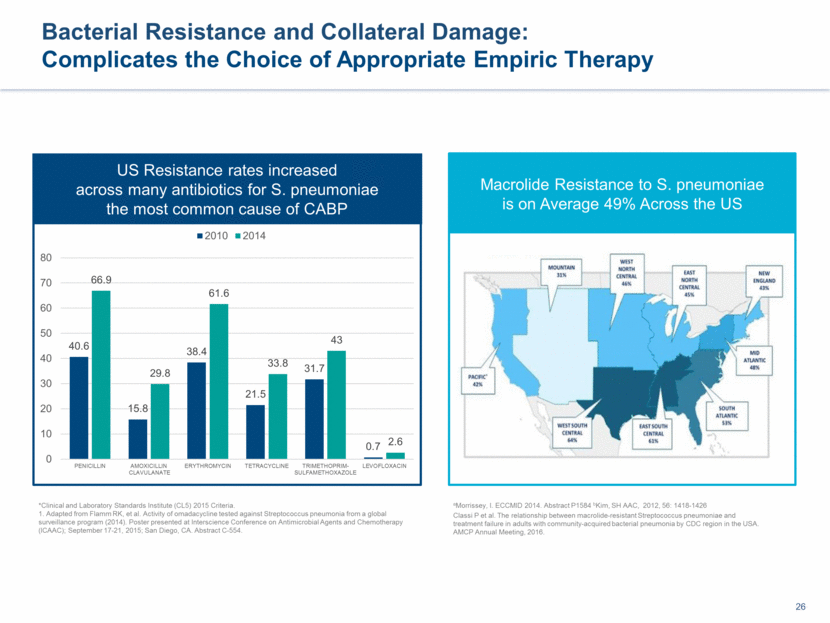
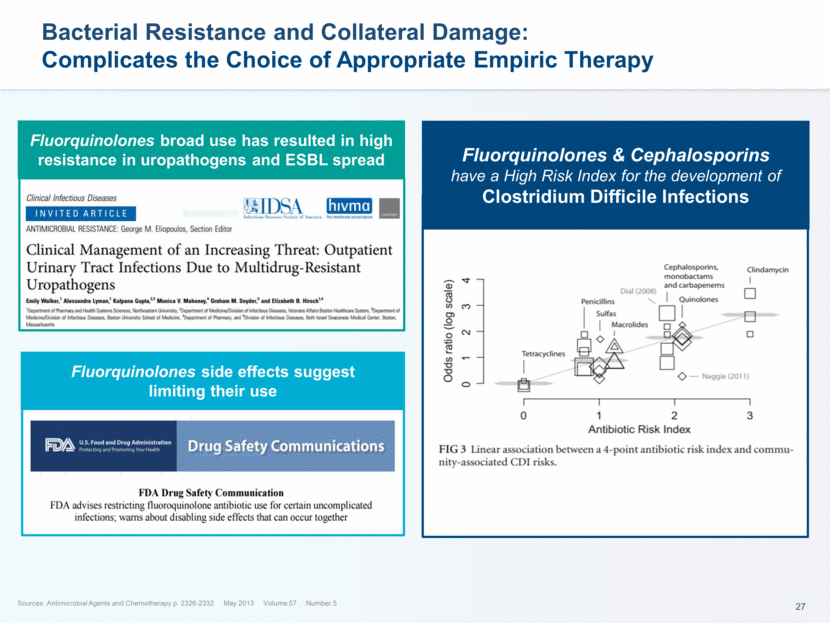
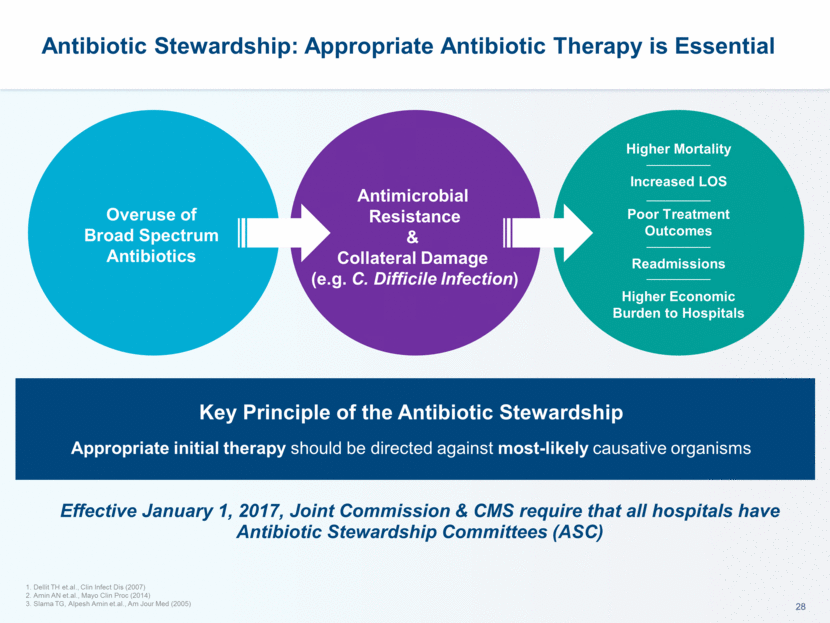
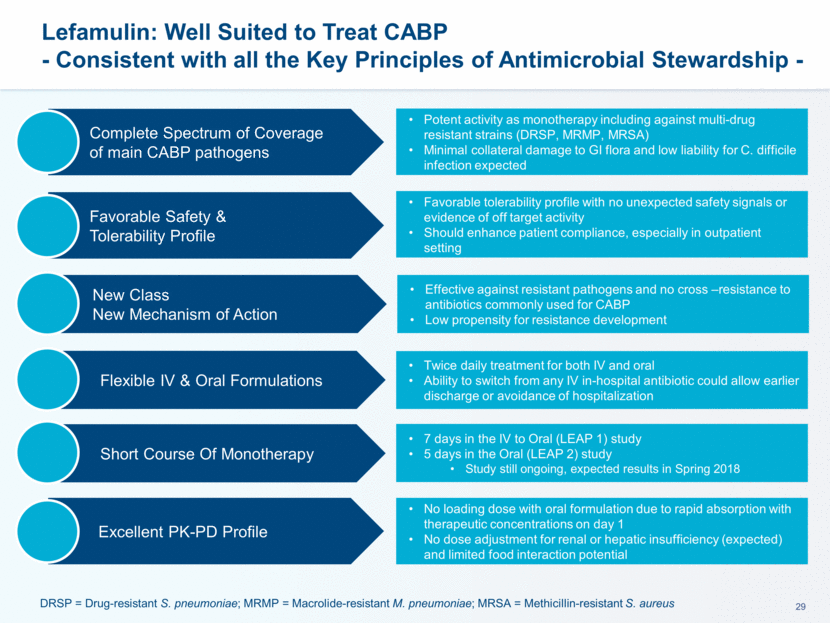
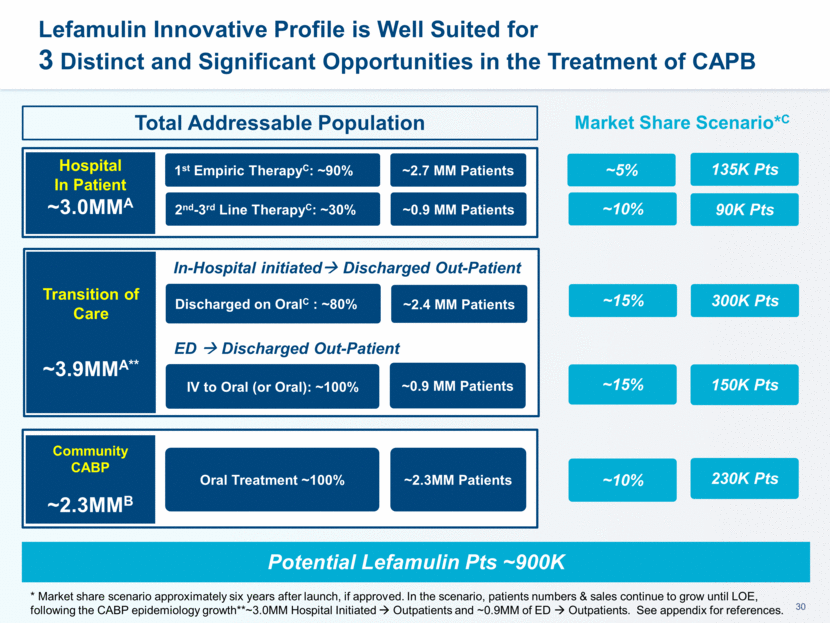
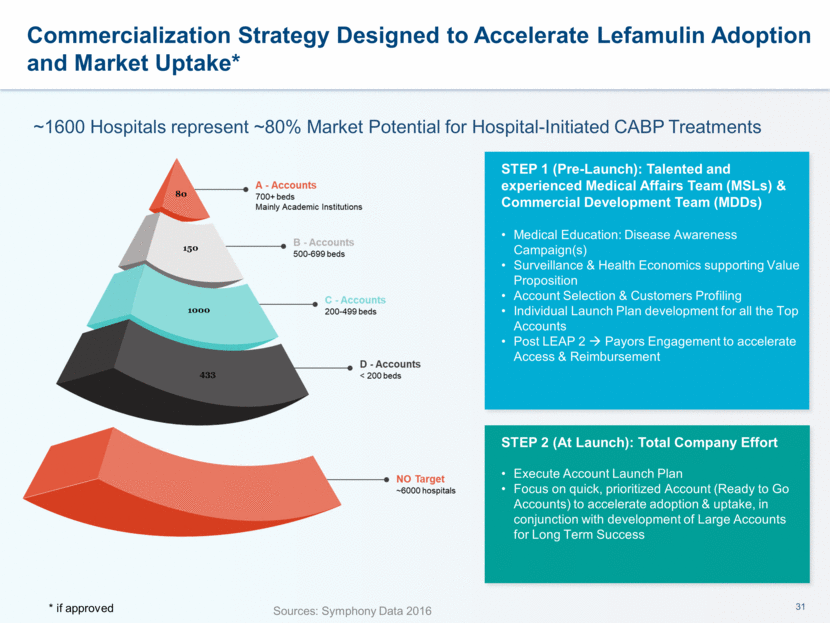
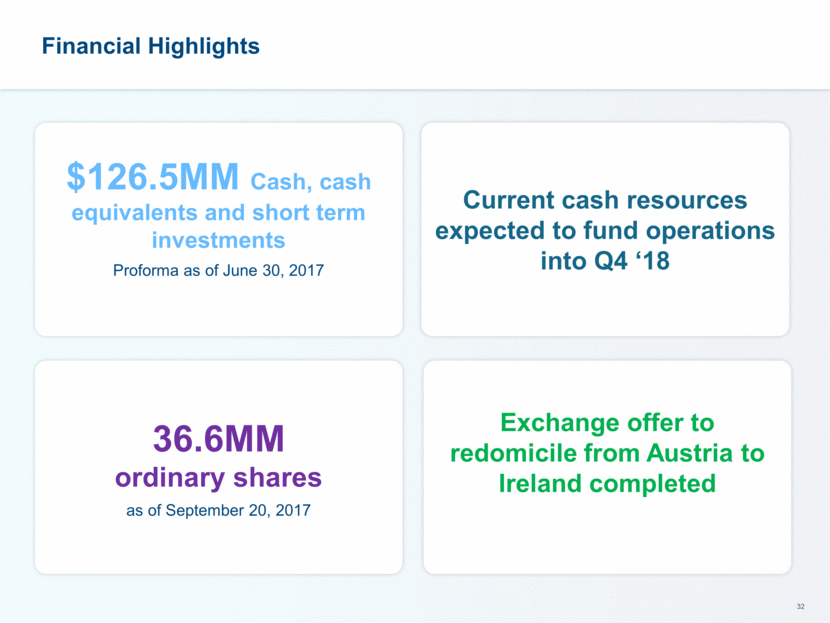
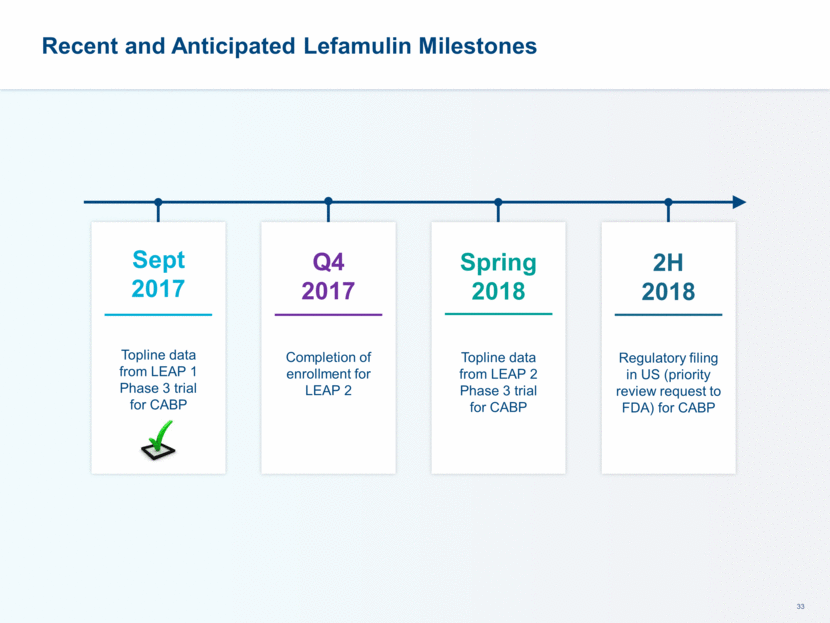
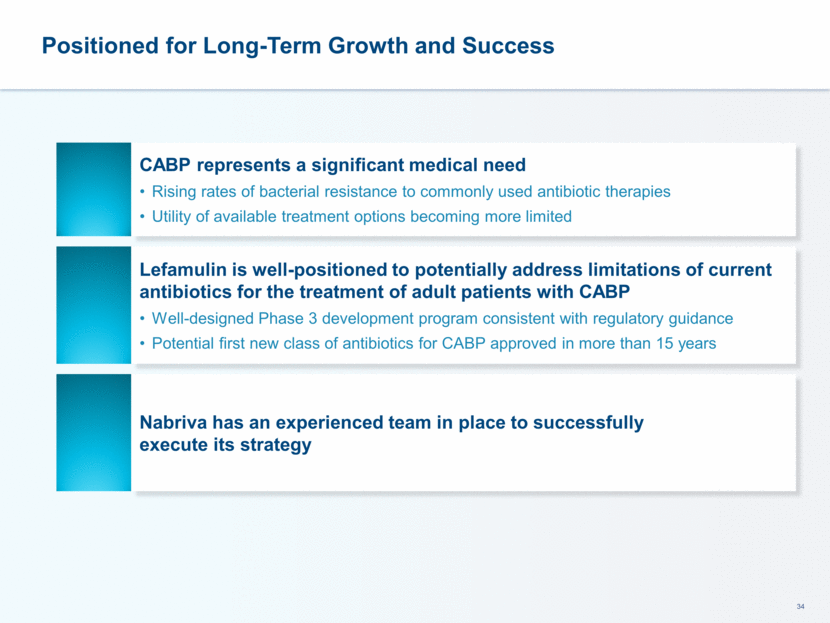
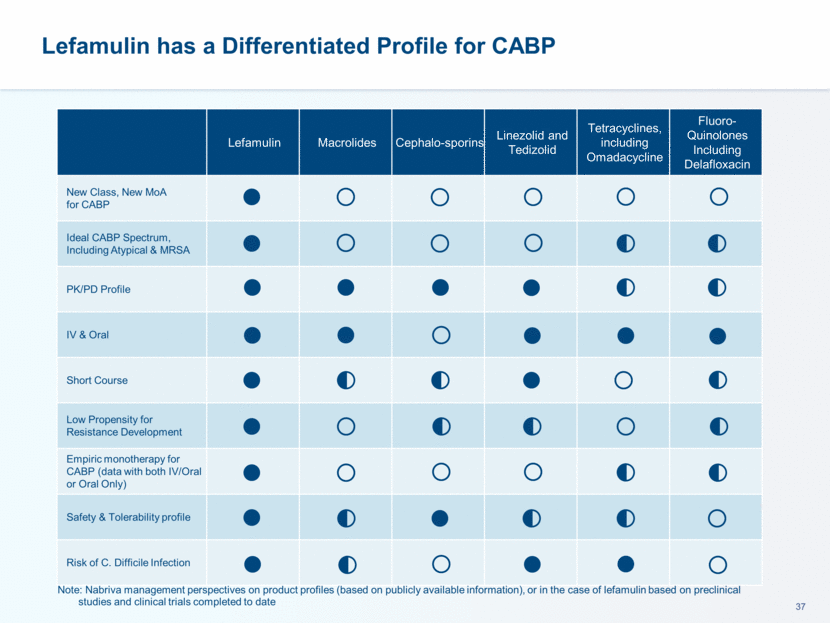
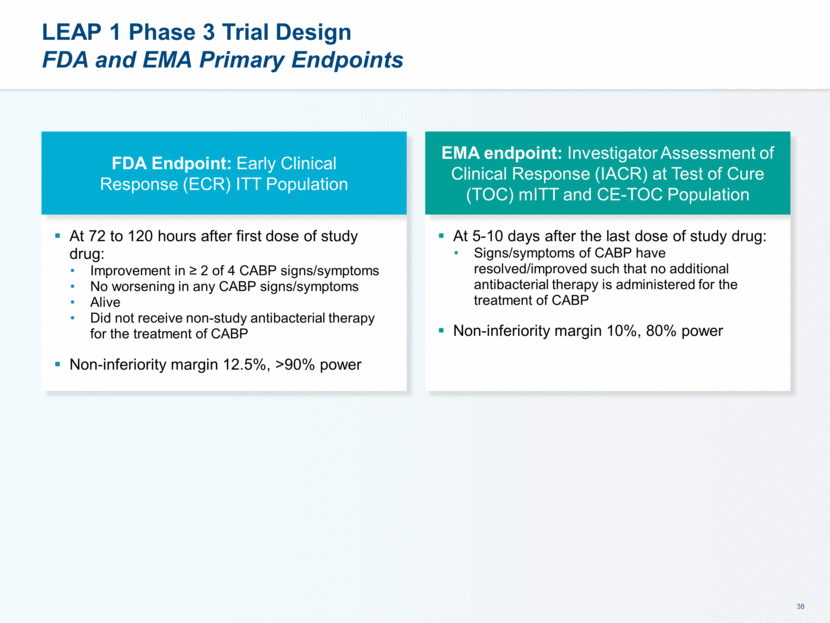
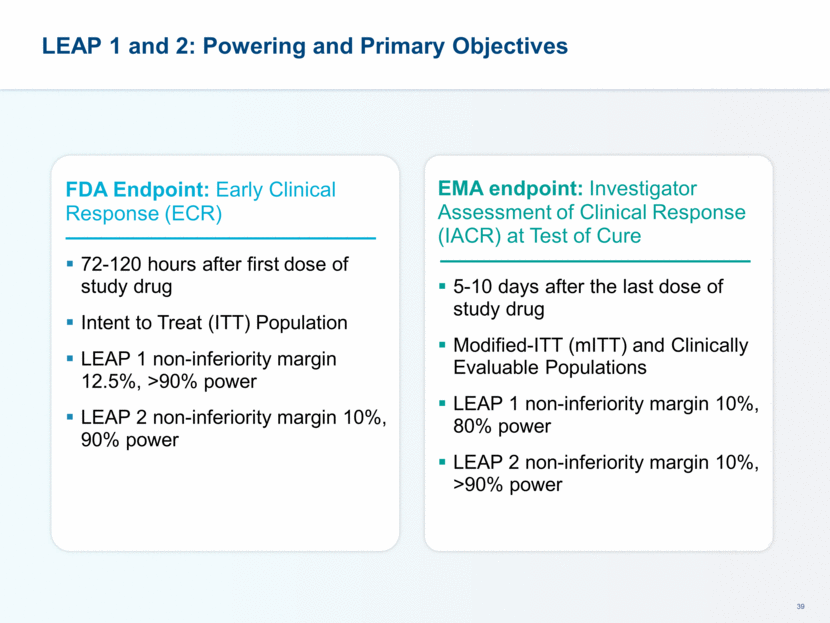
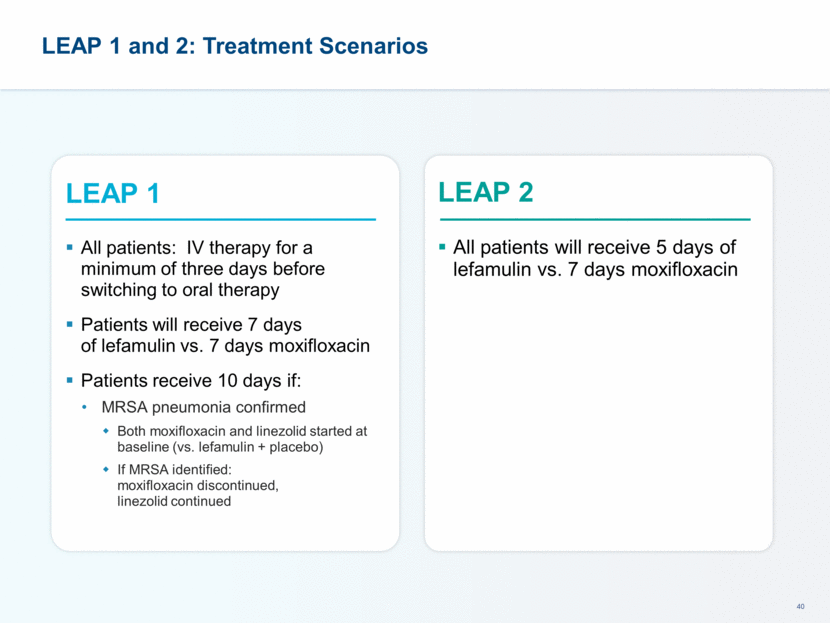
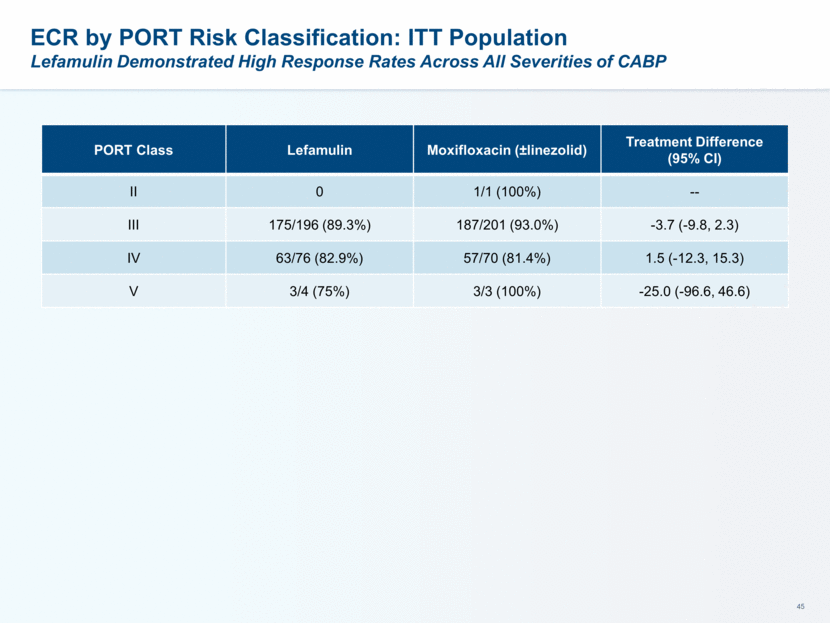
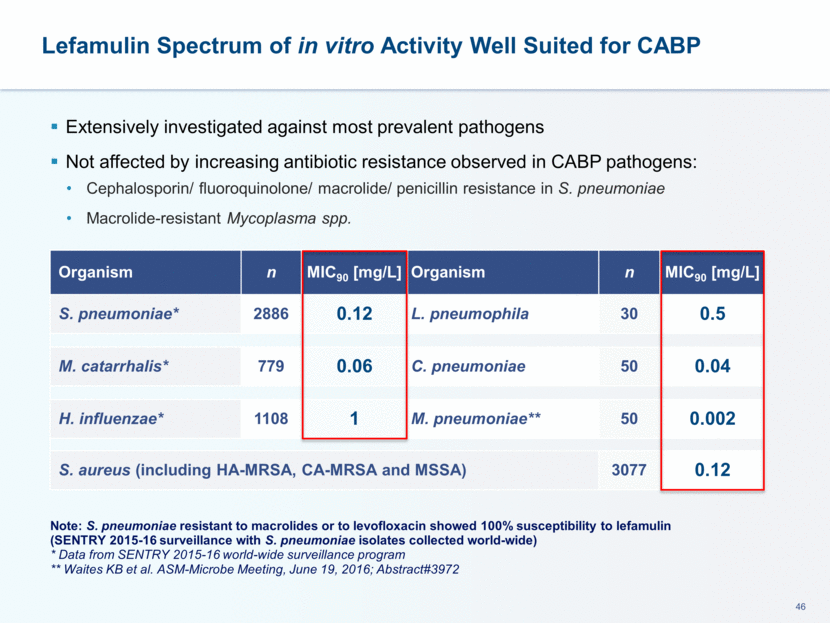
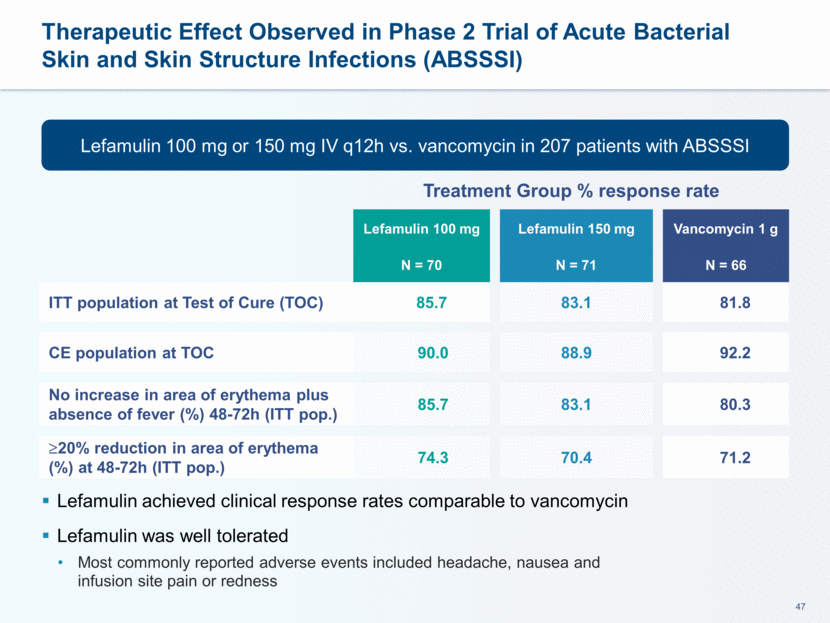
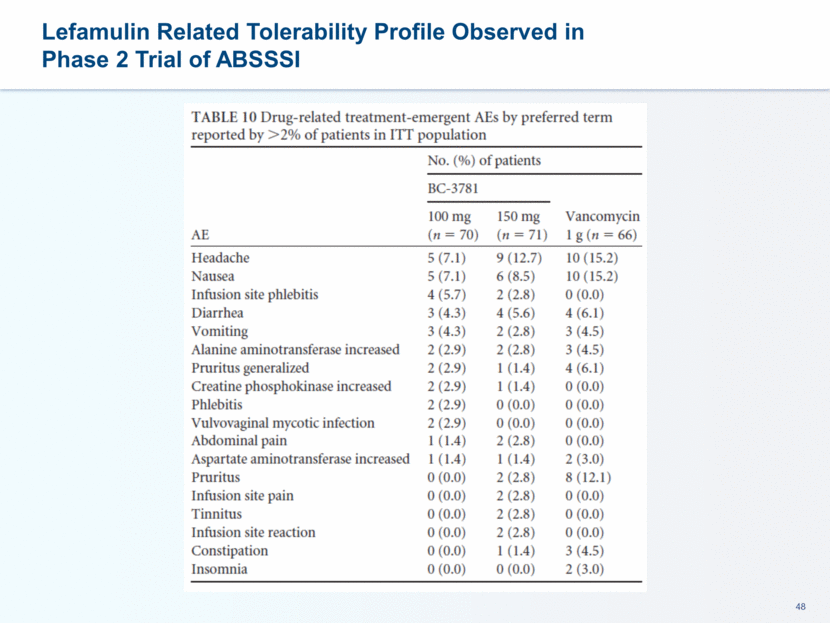
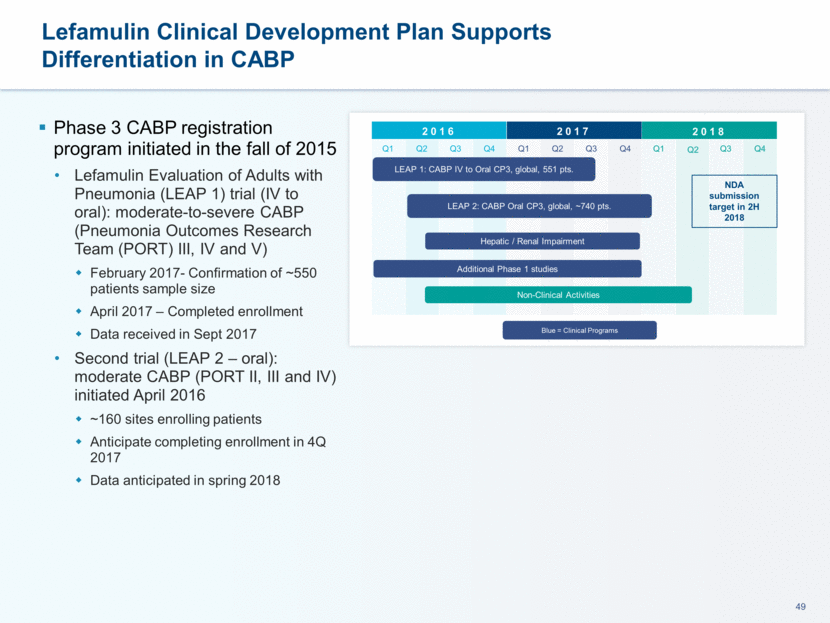
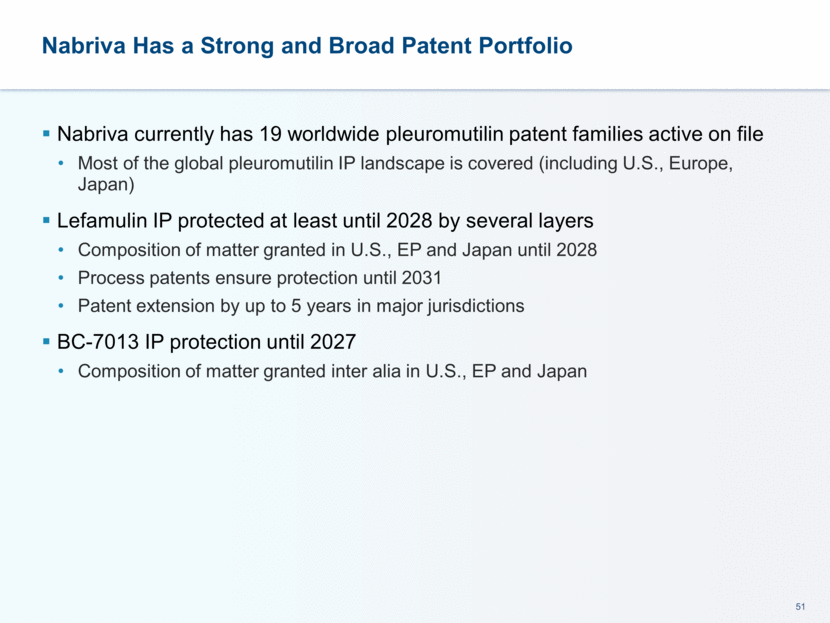
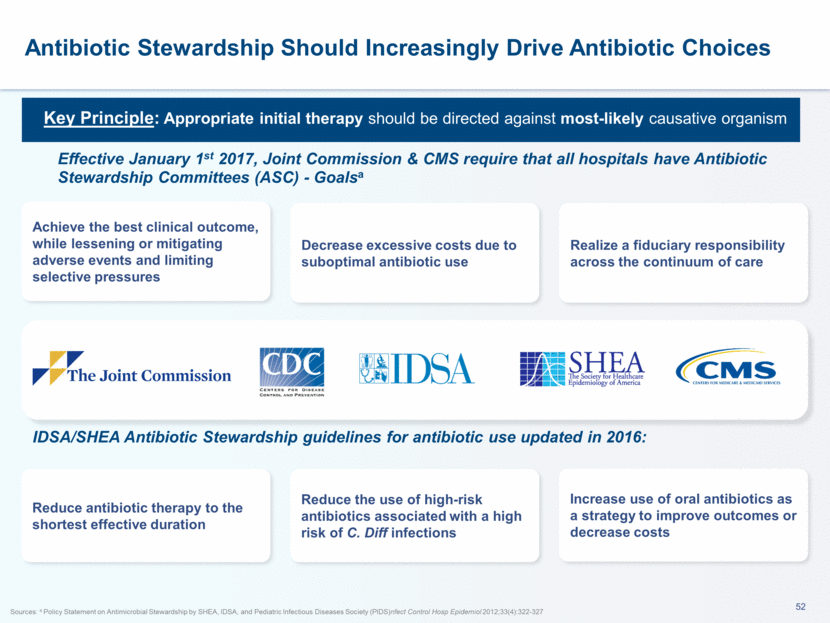
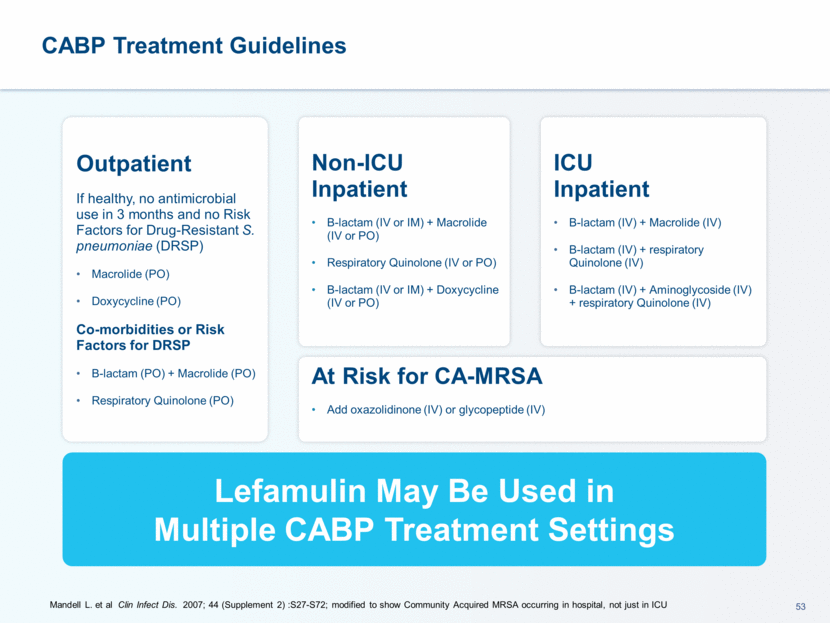
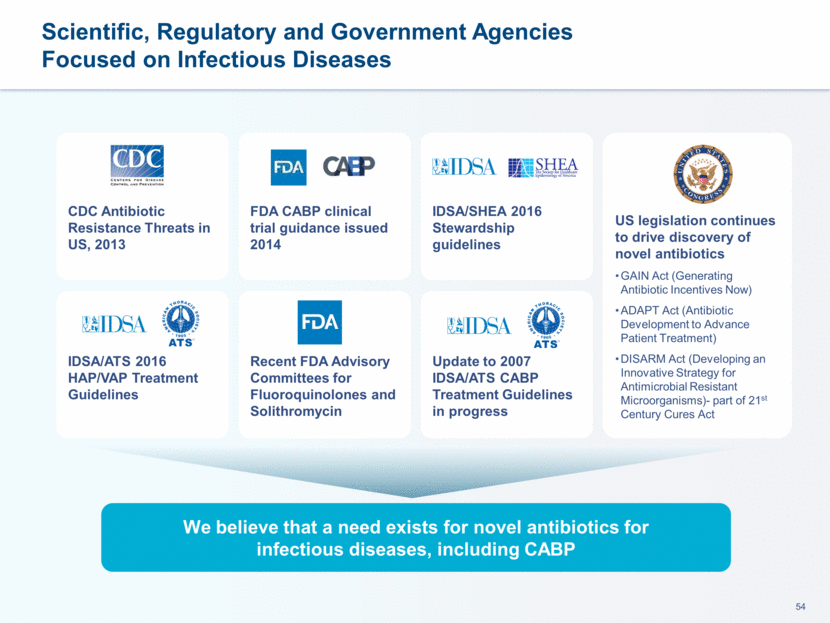
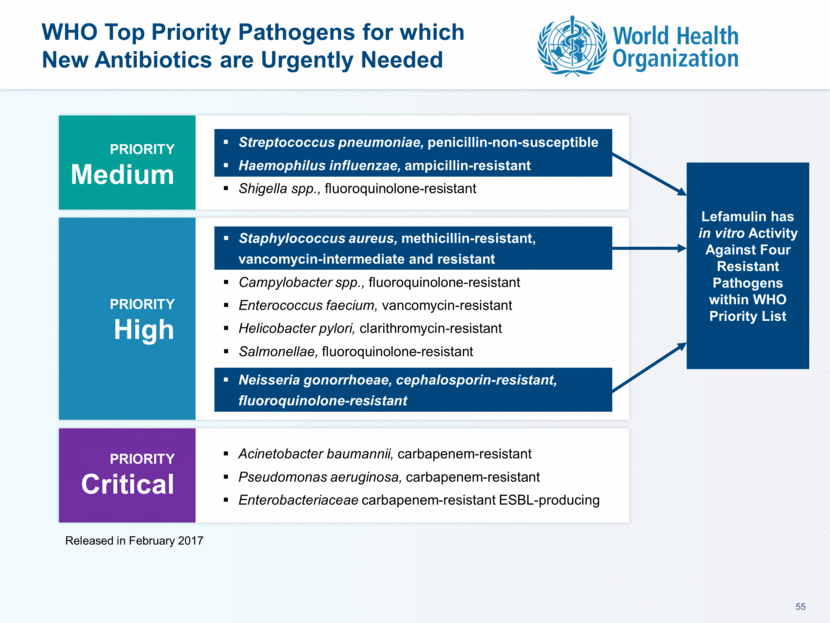
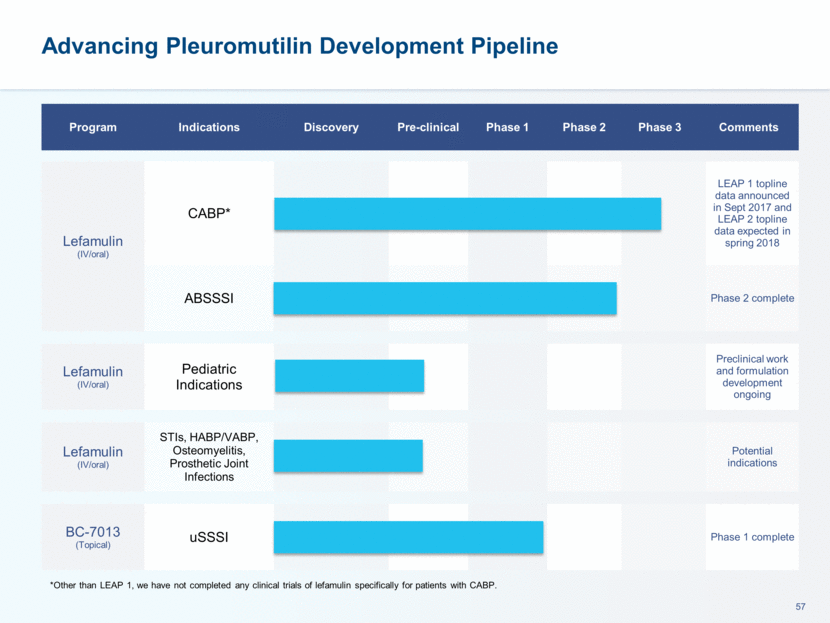
This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements about our future expectations, plans and prospects, including but not limited to statements about, our redomicile from Austria to Ireland, the development of our product candidates, such as plans for the design, conduct and timelines of Phase 3 clinical trials of lefamulin for CABP, the clinical utility of lefamulin for CABP and our plans for filing of regulatory approvals and efforts to bring lefamulin to market, the development of lefamulin for additional indications, the development of additional formulations of lefamulin, plans to pursue research and development of other product candidates, and other statements containing the words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: the uncertainties inherent in the initiation and conduct of clinical trials, availability and timing of data from clinical trials, whether results of early clinical trials or trials in different disease indications will be indicative of the results of ongoing or future trials, uncertainties associated with regulatory review of clinical trials and applications for marketing approvals, the availability or commercial potential of product candidates, the sufficiency of cash resources, whether research programs will result in product candidates that are advanced into future clinical trials, and need for additional financing and such other important factors as are set forth under the caption "Risk Factors" in the annual and quarterly reports we file with the United States Securities and Exchange Commission. In addition, the forward-looking statements included in this presentation represent our views as of the date of this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. Safe Harbor and Disclaimer