Filed pursuant to General Instruction II.L. of Form F-10
File No. 333-205596
A copy of this preliminary prospectus supplement has been filed with the securities regulatory authorities in each of the provinces of Canada, but has not yet become final for the purposes of the sale of securities. Information contained in this preliminary prospectus supplement is not complete and may have to be amended.
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. This prospectus supplement, together with the accompanying short form base shelf prospectus dated July 16, 2015 to which it relates, and each document incorporated by reference therein constitutes a public offering of these securities only in those jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such securities in these jurisdictions.
Information has been incorporated by reference in the accompanying prospectus from documents filed with securities commissions or similar authorities in Canada.Copies of the documents incorporated herein by reference may be obtained on request without charge from the Secretary of Concordia Healthcare Corp. at 277 Lakeshore Rd. East, Suite 302, Oakville, Ontario, L6J 1H9, telephone (905) 842-5150, and are also available electronically at www.sedar.com.
SUBJECT TO COMPLETION, DATED SEPTEMBER 21, 2015
PRELIMINARY PROSPECTUS SUPPLEMENT
TO THE SHORT FORM BASE SHELF PROSPECTUS DATED JULY 16, 2015
| | |
| New Issue | | September 21, 2015 |

CONCORDIA HEALTHCARE CORP.
US$[●]
8,000,000 Common Shares
This prospectus supplement (the “Prospectus Supplement”) of Concordia Healthcare Corp. (“Concordia”, or the “Corporation”) qualifies the distribution (the “Offering”) of 8,000,000 common shares of the Corporation (the “Offered Shares”) at a price of US$[●] per Offered Share (the “Offering Price”).
The Offered Shares will be issued pursuant to an underwriting agreement (the “Underwriting Agreement”) dated September [●], 2015, entered into among the Corporation and Goldman, Sachs & Co. and RBC Dominion Securities Inc., as representatives for the Underwriters (as defined below) (together the “Representatives”), Credit Suisse Securities (USA) LLC and Jefferies LLC. Each of the Representatives, Credit Suisse Securities (USA) LLC and Jefferies LLC are acting as joint bookrunners in connection with the Offering. Goldman, Sachs & Co., RBC Dominion Securities Inc., Credit Suisse Securities (USA) LLC and Jefferies LLC are collectively referred to herein as the “Underwriters”. The Offering Price was determined by negotiation between the Corporation and the Representatives on behalf of the Underwriters. See “Plan of Distribution”.
On September 4, 2015, the Corporation entered into an agreement (the “AMCo Purchase Agreement”) to purchase all of the outstanding shares in the capital of Amdipharm Mercury Limited (“AMCo”) from the holders of such shares (the “Vendors”) for aggregate consideration equal to approximately US$3.3 billion (the “Acquisition”). The purchase price for the Acquisition (the “Purchase Price”) will consist of (i) approximately £800 million (approximately US$1.2 billion) in cash (the “Total Cash Consideration”), (ii) 8,490,000 common shares of the Corporation (with a value of approximately US$0.7 billion) (the “Consideration Shares”), and (iii) the repayment of AMCo’s existing senior secured facilities in the respective principal amounts of £581 million and €440 million (approximately US$1.4 billion in the aggregate) plus accrued interest and related cross-currency swaps. In addition, pursuant to the AMCo Purchase Agreement, Concordia will pay the Vendors (i) an amount of £272,801 (approximately US$414,000) accruing daily from June 30, 2015 to the completion of the Acquisition, and (ii) to the extent earned, a cash earn-out in a maximum amount of £144 million (approximately US$220 million) calculated with reference to the future gross profit of the AMCo group over a period of 12 months from October 1, 2015. The US dollar amounts and the value of the Consideration Shares set forth above are based on the GBP/US$ and EUR/US$ foreign exchange rates, and the closing price of the common shares of the Corporation (the “Common Shares”) on the Toronto Stock Exchange (“TSX”), at September 4, 2015. At completion of the Acquisition (the “Acquisition Closing”), Concordia will procure, on behalf of AMCo, the redemption of the capital loan notes held by certain Vendors and the payment of certain other amounts, all of which will be deducted from the Total Cash Consideration. The Acquisition Closing is subject to certain closing conditions. The Corporation intends to use a portion of the net proceeds of the Offering to fund a portion of the Purchase Price. See“Details of the Acquisition”.
| | | | | | | | | | | | |
| | | Price: US$[●] per Offered Share | | | | |
| | | Price to the Public | | | Underwriters’ Fee(1) | | | Net Proceeds to the
Corporation(2) | |
Per Offered Share | | | US$[● | ] | | | US$[● | ] | | | US$[● | ] |
Total(3) | | | US$[● | ] | | | US$[● | ] | | | US$[● | ] |
Notes:
| (1) | In consideration for the services rendered by the Underwriters in connection with the Offering, the Underwriters will be paid a cash commission (the “Underwriters’ Fee”) equal to 3.75% of the Offering Price for each Offered Share purchased on the Closing Date (as defined herein). See “Plan of Distribution”. |
| (2) | After deducting the Underwriters’ Fee, but before deducting expenses of the Offering, including in connection with the preparation and filing of this Prospectus Supplement, which are estimated to be $[●] and which will be paid from the proceeds of the Offering. |
| (3) | The Corporation has granted the Underwriters an option (the “Underwriters’ Option”), exercisable in whole or in part, at any time and from time to time, in the sole discretion of the Underwriters, up to 30 days after and including the Closing Date to purchase up to an additional[●] Common Shares (the “Additional Offered Shares”) at a price of US$[●] per Additional Offered Share, to cover sales by the Underwriters of a greater number of Common Shares than the number set out above, if any. The Additional Offered Shares issuable upon exercise of the Underwriters’ Option are hereby qualified for distribution under this Prospectus Supplement. A person who acquires Additional Offered Shares acquires such Additional Offered Shares under this Prospectus Supplement. If the Underwriters’ Option is exercised in full, the total price to the public, Underwriters’ Fee and net proceeds to the Corporation (before payment of the expenses of the Offering) will be US$[●], US$[●] and US$[●], respectively. Unless the context otherwise requires, references to “Offered Shares” in this Prospectus Supplement include the Additional Offered Shares, if the context requires. See“Plan of Distribution”. |
The following table sets out the number of Additional Offered Shares that may be issued by the Corporation to the Underwriters pursuant to the Underwriters’ Option:
| | | | | | |
Underwriters’ Position | | Number of Securities Available | | Exercise Period | | Exercise Price |
Underwriters’ Option | | [●] Additional Offered Shares | | Up to 30 days after the Closing Date | | US$[●] per Additional Offered Share |
The Common Shares are listed and posted for trading on the TSX under the symbol “CXR”. On [●], 2015, the last trading day prior to the date of this Prospectus Supplement, the closing price of the Common Shares on the TSX was $[●] and on September 18, 2015, the last trading day prior to the announcement of the Offering, the closing price of the Common Shares on the TSX was $96.50. The Common Shares are also listed on the NASDAQ Global Select Market® (“NASDAQ”) under the symbol “CXRX”. On [●], 2015, the last trading day prior to the date of this Prospectus Supplement, the closing price of the Common Shares on NASDAQ was US$[●] and on September 18, 2015, the last trading day prior to the announcement of the Offering, the closing price of the Common Shares on NASDAQ was US$72.23. The Corporation has applied to list the Offered Shares and Additional Offered Shares on the TSX. Listing is subject to the Corporation fulfilling all of the listing requirements of the TSX. The Offered Shares and Additional Offered Shares will be listed on NASDAQ, upon official notice of issuance.
Subject to applicable laws, the Underwriters may, in connection with the Offering, effect transactions intended to stabilize or maintain the market price of the Common Shares at levels other than those which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time. See“Plan of Distribution”.
After the Underwriters have made reasonable efforts to sell the Offered Shares at the Offering Price, the Underwriters may decrease the Offering Price for the Offered Shares and may further change the Offering Price from time to time to amounts no greater than the Offering Price. In the event the Offering Price is reduced, the compensation received by the Underwriters will be decreased by the amount that the aggregate price paid by the purchasers for the Offered Shares is less than the gross proceeds paid by the Underwriters for the Offered Shares. Any such reduction will not affect the proceeds received by the Corporation. See“Plan of Distribution”.
The Offering is being made concurrently in the United States and in all of the provinces of Canada on an underwritten basis for 100% of the Offered Shares offered hereunder. Goldman Sachs Canada Inc., RBC Dominion Securities Inc., and Credit Suisse Securities (Canada), Inc. in Canadian jurisdictions and Goldman, Sachs & Co., RBC Capital Markets, LLC, Credit Suisse Securities (USA) LLC, and Jefferies LLC in the United States, as principals, conditionally offer the Offered Shares, subject to prior sale, if, as and when issued by Concordia and accepted by the Underwriters in accordance with the conditions contained in the Underwriting Agreement referred to under “Plan of Distribution”, and subject to the approval of certain legal matters on Concordia’s behalf by Sullivan & Cromwell LLP with respect to matters of U.S. law and Fasken Martineau DuMoulin LLP with respect to matters of Canadian law, and on behalf of the Underwriters by Cahill, Gordon &
ii
Reindel LLP with respect to matters of U.S. law and by Blake, Cassels & Graydon LLP with respect to matters of Canadian law. Jefferies LLC is not registered to sell securities in any Canadian jurisdiction and, accordingly, will only sell Offered Shares in the United States.
Subscriptions for the Offered Shares will be received subject to rejection or allotment in whole or in part and the right is reserved to close the subscription books at any time without notice. It is expected that the closing of the Offering will occur on or about September 30, 2015, or on such other date (which date shall not be later than 42 days after the date of this Prospectus Supplement) as may be agreed upon by the Corporation and the Representatives, on behalf of the Underwriters (the “Closing Date”).
An investment in the Offered Shares involves significant risks that should be carefully considered by prospective investors before purchasing such securities. The risks outlined in this Prospectus Supplement, the Prospectus (as defined below) and in the documents incorporated by reference therein should be carefully reviewed and considered by prospective investors in connection with an investment in the Offered Shares. See“Cautionary Note Regarding Forward-Looking Information” and“Risk Factors”.
Each of RBC Dominion Securities Inc. and Credit Suisse Securities (Canada), Inc. are respective affiliates of financial institutions that, among other lenders, have provided the Corporation with a portion of the Existing Bank Facilities (as defined herein), and, along with Goldman, Sachs & Co. and Jefferies LLC, have entered into the Commitment Letter (as defined herein) pursuant to which they have agreed to provide, among other things, certain financing in connection with the Acquisition. Consequently, the Corporation may be considered to be a “connected issuer” of Goldman Sachs Canada Inc., RBC Dominion Securities Inc., Credit Suisse Securities (Canada), Inc. and Jefferies LLC under applicable Canadian securities laws. See “Relationship Between the Underwriters and the Corporation”.
The Offering is made by a Canadian issuer that is permitted, under a multijurisdictional disclosure system adopted by the United States and Canada, to prepare this Prospectus Supplement and the accompanying base shelf prospectus dated July 16, 2015 (the “Prospectus”) in accordance with Canadian disclosure requirements. Prospective investors should be aware that such requirements are different from those applicable to issuers in the United States. Certain financial statements incorporated by reference in the Prospectus have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”), and may not be comparable to financial statements of United States companies.
Prospective investors should be aware that the acquisition of the Offered Shares may have tax consequences both in the United States and in Canada. Such tax consequences for investors, including investors who are resident in or citizens of the United States, may not be described fully herein.
The enforcement by investors of civil liabilities under United States federal securities laws may be affected adversely by the fact that the Corporation is incorporated under the laws of the Province of Ontario, Canada, that most of its officers and directors are residents of Canada, that some of the experts named in this Prospectus Supplement are residents of Canada, and that all or a substantial portion of the assets of the Corporation and said persons are located outside the United States.
THE OFFERED SHARES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION (THE “SEC”) NOR HAS THE SEC PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS SUPPLEMENT. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
Canadian investors should be aware that Keiter, Certified Public Accountants, auditors of certain financial statements in respect of Donnatal® contained in the business acquisition report of the Corporation dated June 9, 2014, incorporated by reference in the Prospectus, is incorporated, continued, or otherwise organized under the laws of the United States; Crowe Horwath LLP, auditors of the carve-out financial statements for the Covis Business (as defined herein) contained in the business acquisition report of the Corporation dated July 3, 2015 incorporated by reference in the Prospectus, is incorporated, continued, or otherwise organized under the laws of the United States; and that Mr. Edward Borkowski and Ms. Rochelle Fuhrmann, directors of the Corporation, reside outside of Canada. Each of Mr. Borkowski and Ms. Fuhrmann have appointed the Corporation as his or her agent for service of process. Canadian investors are advised that it may not be possible for investors to enforce judgements obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process in Canada.
iii
US investors should be aware that Collins Barrow Toronto LLP, auditors of certain financial statements of the Corporation incorporated by reference in the Prospectus, is incorporated, continued, or otherwise organized under the laws of Canada; and that each of Mr. Jordan Kupinsky and Mr. Doug Deeth, directors of the Corporation, Mr. Mark Thompson, an officer and director of the Corporation, and Mr. Adrian de Saldanha, the Chief Financial Officer of the Corporation, reside outside the United States. Each of Mr. Thompson, Mr. Kupinsky, Mr. Deeth and Mr. de Saldanha have appointed CT Corporation System, located at 111 Eighth Avenue, New York, NY 10011, as their agent for service of process. United States investors are advised that it may not be possible for investors to enforce judgements obtained in the United States against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of the United States, even if the party has appointed an agent for service of process in the United States.
The registered and head office of the Corporation is located at 277 Lakeshore Rd. East, Suite 302, Oakville, Ontario, L6J 1H9. The Corporation’s records office is located at 333 Bay St., Suite 2400, Toronto, Ontario M56 2T6.
iv
TABLE OF CONTENTS
PROSPECTUS SUPPLEMENT
PROSPECTUS
ABOUT THIS PROSPECTUS SUPPLEMENT AND ACCOMPANYING PROSPECTUS
Explanatory Notes
This document is in two parts. The first part is this Prospectus Supplement, which describes the specific terms of the Offered Shares and also adds to and updates certain information contained in the accompanying Prospectus and the documents incorporated by reference therein. The second part, the Prospectus, gives more general information, some of which may not apply to the Offered Shares being offered under this Prospectus Supplement. In this Prospectus Supplement, all capitalized terms used and not otherwise defined herein have the meanings ascribed thereto in the Prospectus.
In this Prospectus Supplement, the Corporation and its subsidiaries are collectively referred to as the “Corporation” or “Concordia”, unless the context otherwise requires. Readers of this Prospectus Supplement should rely only on information contained in this Prospectus Supplement and contained or incorporated by reference in the Prospectus. The Corporation has not authorized anyone to provide the reader with different information. The Corporation takes no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give readers of this Prospectus Supplement.
Readers should not assume that the information contained in this Prospectus Supplement is accurate as of any date other than the date on the front of this Prospectus Supplement or the respective dates of the documents incorporated by reference in the accompanying Prospectus.
The Corporation is not making an offer of the Offered Shares in any jurisdiction where the offer is not permitted. This Prospectus Supplement and the accompanying Prospectus must not be used by anyone for any purpose other than in connection with the Offering. The Corporation does not undertake to update the information contained in this Prospectus Supplement or contained or incorporated by reference in the Prospectus, except as required by applicable securities laws. Information contained on, or otherwise accessed through, the website of the Corporation, www.concordiarx.com, shall not be deemed to be a part of this Prospectus Supplement or the accompanying Prospectus and such information is not incorporated by reference herein or therein and should not be relied upon by readers for the purpose of determining whether to invest in the Offered Shares.
Trademarks
This Prospectus Supplement, or the accompanying Prospectus or documents incorporated by reference therein, include references to trademarks which are protected under applicable intellectual property laws and are the property of the Corporation, AMCo, or their respective affiliates or licensors. Solely for convenience, the trademarks of the Corporation, AMCo, their respective affiliates and/or their respective licensors referred to in this Prospectus Supplement or the accompanying Prospectus, or documents incorporated by reference therein may appear with or without the® or ™ symbol, but such references or the absence thereof are not intended to indicate, in any way, that the Corporation, AMCo, or their respective affiliates or licensors will not assert, to the fullest extent under applicable law, their respective rights to these trademarks. Any other trademarks used in this Prospectus Supplement, or the accompanying Prospectus or documents incorporated by reference therein, are the property of their respective owners.
Market Data
This Prospectus Supplement, or the accompanying Prospectus or documents incorporated by reference therein, contains certain statistical data, market research and industry forecasts that were obtained, unless otherwise indicated, from independent industry and government publications and reports or based on estimates derived from such publications and reports and management of Concordia’s knowledge of, and experience in, the markets in which the Corporation operates. Industry and government publications and reports generally indicate that they have obtained their information from sources believed to be reliable, but do not guarantee the accuracy and completeness of their information. While the Corporation believes this data to be reliable, market and industry data is subject to variation and cannot be verified due to limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties inherent in any statistical survey. The Corporation has not independently verified the accuracy or completeness of such information contained in this Prospectus Supplement, the Prospectus, or incorporated by reference therein. In addition, projections, assumptions and estimates of the Corporation’s future performance and the future performance of the industry in which the Corporation operates are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described under the heading“Risk Factors” in this Prospectus Supplement and under the heading“Risk Factors” in the AIF (as defined herein).
S-3
Information Pertaining to AMCo
The information contained in this Prospectus Supplement with respect to AMCo and its business has been provided by management of AMCo.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
Certain statements contained in this Prospectus Supplement, the accompanying Prospectus, or in documents incorporated by reference therein, constitute “forward-looking information” within the meaning of applicable Canadian provincial securities laws (“forward-looking statements”), which are based upon the Corporation’s current internal expectations, estimates, projections, assumptions and beliefs. Statements concerning the Corporation’s objectives, goals, strategies, intentions, plans, beliefs, expectations and estimates, and the business, operations, future financial performance and condition of the Corporation and/or AMCo are forward-looking statements. The words “believe”, “expect”, “anticipate”, “estimate”, “intend”, “may”, “will”, “would” and similar expressions, including the negative and grammatical variations of such expressions, are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These statements are not guarantees of future performance and involve known and unknown risks, uncertainties and other factors that may cause actual results or events to differ materially from those anticipated in the forward-looking statements. In addition, this Prospectus Supplement, the accompanying Prospectus, and/or the documents incorporated by reference therein, may contain forward-looking statements attributed to third-party industry sources.
By their nature, forward-looking statements involve numerous assumptions, known and unknown risks and uncertainties, both general and specific, that contribute to the possibility that the predictions, forecasts and projections that constitute forward-looking statements will not occur. Such forward-looking statements in this Prospectus Supplement, in the accompanying Prospectus, or in documents incorporated by reference therein, speak only as of the date of such document. Forward-looking statements in this Prospectus Supplement, in the accompanying Prospectus, or in documents incorporated by reference therein, include, but are not limited to, statements with respect to:
| | • | | the proposed settlement agreement with the FTC (as defined herein); |
| | • | | the use of net proceeds of the Offering; |
| | • | | the Acquisition, including but not limited to: |
| | • | | the completion and timing thereof; |
| | • | | synergies resulting from the Acquisition; |
| | • | | the completion of the related debt financings and the expected terms of those financings; |
| | • | | the entering into of documentation with respect to the related debt financings; and |
| | • | | the impact of the Acquisition on Concordia’s financial performance (including with respect to its revenues, margins, Adjusted EPS, EBITDA and Adjusted EBITDA); |
| | • | | the performance of the Corporation’s and AMCo’s business and operations; |
| | • | | the ability of the Corporation to integrate the business of AMCo with that of the Corporation; |
| | • | | the retention of key personnel of AMCo; |
| | • | | the Corporation’s capital expenditure programs; |
| | • | | the future development of the Corporation, its growth strategy and the timing thereof; |
| | • | | the acquisition strategy of the Corporation; |
| | • | | the completion and timing of the Offering; |
| | • | | the estimated future contractual obligations of the Corporation; |
| | • | | the Corporation’s future liquidity and financial capacity; |
| | • | | the supply and demand for pharmaceutical products and services similar to the Corporation’s and AMCo’s products and services; |
| | • | | cost and reimbursement for the Corporation’s and AMCo’s products; |
| | • | | expectations regarding the Corporation’s ability to raise capital; |
| | • | | the Corporation’s treatment under government regulatory and taxation regimes; |
| | • | | the Corporation’s and AMCo’s net sales of all or any one of their respective products; |
| | • | | sales relating to the specialty healthcare distribution division; and |
| | • | | potential synergies available through the combination with AMCo. |
With respect to the forward-looking statements contained in this Prospectus Supplement, in the accompanying Prospectus, or in documents incorporated by reference therein, the Corporation has made assumptions regarding, among other things:
S-4
| | • | | the ability of the Corporation to comply with its contractual obligations, including, without limitation, its obligations under debt arrangements; |
| | • | | the ability of the Corporation to complete the Acquisition and the related debt financings on certain terms; |
| | • | | the successful licensing of products to third parties to market and distribute such products on terms favorable to the Corporation; |
| | • | | the ability of the Corporation to maintain key strategic alliances, and licensing and partnering arrangements, including in relation to the business of AMCo, now and in the future; |
| | • | | the ability of the Corporation to maintain its and AMCo’s distribution networks and distribute its products effectively despite significant geographical expansion; |
| | • | | the general regulatory environment in which the Corporation and AMCo operate, including the areas of taxation, environmental protection, consumer safety and health regulation; |
| | • | | the timely receipt of any required regulatory approvals; |
| | • | | the general economic, financial, market and political conditions impacting the industry in which the Corporation and AMCo operate; |
| | • | | the tax treatment of the Corporation, AMCo, and their subsidiaries and the materiality of legal proceedings; |
| | • | | the ability of the Corporation to sustain or increase profitability, fund its operations with existing capital, and/or raise additional capital to fund future acquisitions; |
| | • | | the ability of the Corporation to acquire any necessary technology, products or businesses and effectively integrate such acquisitions, including the ability to complete the Acquisition and integrate AMCo; |
| | • | | the development and clinical testing of products under development; |
| | • | | the ability of the Corporation and AMCo to obtain necessary approvals for commercialization of the Corporation’s products from the United States Food and Drug Administration or other regulatory authorities; |
| | • | | future currency exchange and interest rates; |
| | • | | reliance on third party contract manufacturers to manufacture the Corporation’s and AMCo’s products on favourable terms; |
| | • | | the ability of the Corporation to generate sufficient cash flow from operations and to access existing and proposed credit facilities and the capital markets to meet its future obligations on acceptable terms; |
| | • | | the availability of raw materials and finished products necessary for the Corporation’s and AMCo’s products; |
| | • | | the impact of increasing competition; |
| | • | | the ability of the Corporation and AMCo to obtain and retain qualified staff, equipment and services in a timely and efficient manner; |
| | • | | the ability of the Corporation and AMCo to maintain and enforce the protection afforded by any patents or other intellectual property rights; |
| | • | | the ability of the Corporation and AMCo to conduct operations in a safe, efficient and effective manner; |
| | • | | the results of continuing and future safety and efficacy studies by industry and government agencies related to the Corporation’s and AMCo’s products; and |
| | • | | the ability of the Corporation to successfully market its products and services and the products and services of AMCo upon completion of the Acquisition. |
Forward-looking statements contained in this Prospectus Supplement, in the accompanying Prospectus, or in documents incorporated by reference therein, are based on the key assumptions described herein or therein. The reader is cautioned that such assumptions, although considered reasonable by the Corporation, may prove to be incorrect. Actual results achieved during the forecast period will vary from the information provided in this Prospectus Supplement, in the accompanying Prospectus, or in documents incorporated by reference therein, as a result of numerous known and unknown risks and uncertainties and other factors. The Corporation cannot guarantee future results.
Some of the risks and other factors which could cause actual results to differ materially from those expressed in the forward-looking statements contained in this Prospectus Supplement, in the accompanying Prospectus, or in documents incorporated by reference therein, include, but are not limited to, the risk factors included under the heading“Risk Factors” in this Prospectus Supplement and under the heading“Risk Factors” in the AIF.
Forward-looking statements contained in this Prospectus Supplement, in the accompanying Prospectus, or in documents incorporated by reference therein, are based on management’s current plans, expectations, estimates, projections, beliefs and opinions and the assumptions relating to those plans, expectations, estimates, projections, beliefs and opinions may change. Management of the Corporation has included the above summary of assumptions and risks related to forward-looking statements included in this Prospectus Supplement, in the accompanying Prospectus, or in documents incorporated by reference therein, for the purpose of assisting the reader in understanding management’s current views regarding those future outcomes.Readers are cautioned that this information may not be
S-5
appropriate for other purposes. Readers are cautioned that the lists of assumptions and risk factors contained herein are not exhaustive. Neither the Corporation nor any other person assumes responsibility for the accuracy or completeness of the forward-looking statements contained herein.
Such forward-looking statements are made as of the date of this Prospectus Supplement, or in the case of the accompanying Prospectus or any documents incorporated by reference therein, as of the dates of such documents, and the Corporation disclaims any intention or obligation to update publicly any such forward-looking statements, whether as a result of new information, future events or results or otherwise, other than as required by applicable Canadian securities laws.
All of the forward-looking statements made in this Prospectus Supplement, in the accompanying Prospectus, or in documents incorporated by reference therein are expressly qualified by these cautionary statements and other cautionary statements or factors contained or incorporated by reference herein or therein, and there can be no assurances that the actual results or developments will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, the Corporation.
CAUTIONARY NOTE REGARDING FUTURE-ORIENTED FINANCIAL INFORMATION
To the extent any forward-looking statements in this Prospectus Supplement, in the accompanying Prospectus, or in documents incorporated by reference therein, constitute “future-oriented financial information” or “financial outlooks” within the meaning of applicable Canadian securities laws, such information is being provided to demonstrate the potential benefits of the Acquisition and the debt financings described herein and the reader is cautioned that this information may not be appropriate for any other purpose and the reader should not place undue reliance on such future-oriented financial information and financial outlooks. Future-oriented financial information and financial outlooks, as with forward-looking statements generally, are, without limitation, based on the assumptions and subject to the risks set out above under the heading“Cautionary Note Regarding Forward-Looking Information”.
CURRENCY PRESENTATION AND EXCHANGE RATE INFORMATION
All references to “$” in this Prospectus Supplement refer to Canadian dollars, all references to “US$” are to United States dollars, all references to “£” or “GBP” are to United Kingdom pounds sterling, and all references to “€” or “EUR” are to European Union Euro, unless otherwise indicated. The following table reflects the high and low rates of exchange for one United States dollar, expressed in Canadian dollars, during the periods noted, the rates of exchange at the end of such periods, and the average rates of exchange during such periods, based on the Bank of Canada noon spot rate of exchange.
| | | | | | | | | | | | | | | | |
| | | Six months ended June 30 | | | Year ended December 31, | |
| | | 2015 | | | 2014 | | | 2014 | | | 2013 | |
High for the period | | | 1.2803 | | | | 1.1251 | | | | 1.1643 | | | | 1.0697 | |
Low for the period | | | 1.1728 | | | | 1.0614 | | | | 1.0614 | | | | 0.9839 | |
Rate at the end of the period | | | 1.2474 | | | | 1.0676 | | | | 1.1601 | | | | 1.0636 | |
Average noon spot rate for the period | | | 1.2354 | | | | 1.0968 | | | | 1.1045 | | | | 1.0299 | |
On September 18, 2015, the Bank of Canada noon rate of exchange for US$ was US$1.00 = $1.3147.
The following table reflects the high and low rates of exchange for one GBP, expressed in Canadian dollars, during the periods noted, the rates of exchange at the end of such periods, and the average rates of exchange during such periods, based on the Bank of Canada noon spot rate of exchange
| | | | | | | | | | | | | | | | |
| | | Six months ended June 30 | | | Year ended December 31, | |
| | | 2015 | | | 2014 | | | 2014 | | | 2013 | |
High for the period | | | 1.9614 | | | | 1.8594 | | | | 1.8594 | | | | 1.7639 | |
Low for the period | | | 1.7856 | | | | 1.7432 | | | | 1.7432 | | | | 1.5263 | |
Rate at the end of the period | | | 1.9614 | | | | 1.8261 | | | | 1.8071 | | | | 1.7627 | |
Average noon spot rate for the period | | | 1.8816 | | | | 1.8306 | | | | 1.8190 | | | | 1.6113 | |
On September 18, 2015, the Bank of Canada noon rate of exchange for GBP was £1.00 = $2.0471.
S-6
On September 18, 2015, the Bank of Canada noon rate of exchange for EUR was €1.00 = $1.4932.
PRESENTATION OF FINANCIAL INFORMATION
The financial statements of the Corporation incorporated by reference in the Prospectus are reported in United States dollars and have been prepared in accordance with IFRS as issued by the IASB.
The financial statements of AMCo included in this Prospectus Supplement are reported in GBP and have been prepared in accordance with IFRS as issued by the IASB.
The: (i) financial statements and information contained in the business acquisition report dated July 3, 2015 relating to the acquisition of substantially all of the commercial assets of Covis Pharma S.à.r.l (“Covis Pharma”) and Covis Injectables S.à.r.l (collectively, the “Covis Business”) incorporated by reference in the Prospectus; (ii) financial statements and information contained in the business acquisition report dated June 9, 2014 relating to the acquisition of Donnatal® incorporated by reference in the Prospectus; and (iii) financial statements and information contained in the business acquisition report dated December 12, 2014 relating to the acquisition of Zonegran® incorporated by reference in the Prospectus, each are reported in United States dollars and have been prepared in accordance with IFRS or United States generally accepted accounting principles (“U.S. GAAP”), as the case may be, and may not be comparable to the financial statements of the Corporation or any companies prepared in accordance with IFRS.
NON-IFRS MEASURES
This Prospectus Supplement contains references to certain measures that are not recognized under IFRS. These non-IFRS measures are used as indicators of financial performance and may differ from similar computations as reported by other similar entities and, accordingly, may not be comparable. The Corporation believes these measures provide useful supplemental information that may assist investors in assessing an investment in the Offered Shares.
The non-IFRS measures used in relation to the Corporation in this Prospectus Supplement are EBITDA, Adjusted EBITDA and Adjusted EPS of the Corporation and/or AMCo, which have the following meanings:
“EBITDA” is defined as net income adjusted for net interest expense, income tax expense, depreciation and amortization. Management uses EBITDA to assess the Corporation’s operating performance, as applicable.
“Adjusted EBITDA” is defined as EBITDA adjusted for one-time charges, including costs associated with acquisitions, exchange listing expenses, non-recurring gains, non-cash items such as unrealized gains/losses on derivative instruments, share based compensation, changes in fair value of contingent consideration, and realized/unrealized gains/losses related to foreign exchange revaluation.
“Adjusted EPS” is earnings per share presented on a fully diluted basis and is calculated as net income, adding back one-time charges, including costs associated with acquisitions, exchange listing expenses, non-recurring gains, non-cash items such as unrealized gains/losses on derivative instruments, share based compensation, changes in fair value of contingent consideration, and realized/unrealized gains/losses related to foreign exchange revaluation, depreciation, amortization, accretion, and tax effect of all adjustments, divided by the weighted average number of Common Shares outstanding on a fully-diluted basis.
Investors are cautioned that these non-IFRS measures should not be considered an alternative to net earnings for the period (as determined in accordance with IFRS) as an indicator of the Corporation’s performance, or an alternative to cash flows from operating, financing and investing activities as a measure of liquidity. The Corporation’s method of calculating such non-IFRS measures may differ from the methods used by other issuers and, accordingly, non-IFRS measures used in relation to the Corporation may not be comparable to similar measures used by other issuers.
Please refer to“Summary - Selected Pro Forma Consolidated Financial Information” for a reconciliation of the non-IFRS measures used in relation to the Corporation in this Prospectus Supplement.
S-7
SUMMARY
The following is a summary of the principal features of the Offering and the Acquisition and should be read together with the more detailed information and financial data and statements contained elsewhere in this Prospectus Supplement.
CONCORDIA HEALTHCARE CORP.
Concordia is a diverse healthcare company that is primarily focused on sustainable established legacy pharmaceutical products, which are off-patent drugs and have a track record of safety and efficacy, a history of stable prescription demand, and competitive barriers to entry. Some of Concordia’s products are often difficult to manufacture, have specialized regulatory status and/or possess strong brand loyalty that allows them to maintain ongoing sales after patent expiration. Concordia’s business strategy involves seeking to acquire products and maximize their value through:
| | • | | optimizing the sales and pricing strategy; |
| | • | | managing regulatory affairs and supply chain; |
| | • | | optimizing its corporate structure; |
| | • | | identifying authorized generic opportunities; and |
| | • | | exploring targeted promotion or co-promotion. |
The Corporation has utilized this strategy in six acquisitions since 2013 (not including the Acquisition), which has added a portfolio of products, including branded products such as Nilandron®, for the treatment of metastatic prostate cancer; Dibenzyline®, for the treatment of pheochromocytoma; Lanoxin®, for the treatment of mild-to moderate heart failure and atrial fibrillation; Plaquenil®, for the treatment of lupus and rheumatoid arthritis; Donnatal® for the treatment of irritable bowel syndrome; and Zonegran® (zonisamide) for treatment of partial seizures in adults with epilepsy. Donnatal® accounted for 21% of the Corporation’s 2014 total revenue, Lanoxin® accounted for 16% of 2014 total revenue, Plaquenil® accounted for 9% of 2014 total revenue, and each of Zonegran® and Dibenzyline® accounted for 8% of 2014 total revenue.
After giving effect to the Acquisition, the Corporation will have a high level of financial diversification with sales in over 100 countries and no single product representing more than 10% of revenues. Concordia’s global operations after the Acquisition are expected to provide the Corporation with the opportunity to maximize brand value worldwide as well as to identify products that may have specialized value propositions in certain specific markets.
Concordia also markets orphan drugs through an Orphan Drug Division, currently consisting of Photofrin® for the treatment of rare forms of cancer. The Corporation is currently undergoing testing for multiple new indications for Photofrin® in order to enable expanded usage if approved. In addition, Concordia operates a Specialty Healthcare Distribution Division that provides a distribution capability for specialty pharmaceutical products and orphan drugs once acquired and/or developed. Concordia currently operates out of facilities in Oakville, Ontario; Bridgetown, Barbados; Roanoke, Virginia; Kansas City, Missouri; and Chicago, Illinois.
For a description of Concordia’s business and certain recent developments, including the acquisition of the Covis Business completed in April 2015, see the section titled “General Development and Description of the Business” in the AIF and the section titled “Company Overview” in the Interim MD&A. The AIF and the Interim MD&A are incorporated by reference into the Prospectus and this Prospectus Supplement, respectively.
THE ACQUISITION
On September 4, 2015, the Corporation entered into the AMCo Purchase Agreement with the Vendors pursuant to which the Corporation agreed to acquire all of the outstanding shares in the capital of AMCo from the Vendors.
The Purchase Price for the Acquisition is expected to be equal to approximately US$3.3 billion, comprising (i) approximately £800 million (approximately US$1.2 billion) in cash; (ii) 8,490,000 Consideration Shares (with a value of approximately US$0.7 billion); and (iii) the repayment of AMCo’s existing senior secured facilities in the respective
S-8
principal amounts of £581 million and €440 million (approximately US$1.4 billion in the aggregate) plus accrued interest and related cross-currency swaps. In addition, pursuant to the AMCo Purchase Agreement, Concordia will pay the Vendors (i) an amount of £272,801 (approximately US$414,000) accruing daily from June 30, 2015 to the completion of the Acquisition, and (ii) to the extent earned, a cash earn-out in a maximum amount of £144 million (approximately US$220 million) calculated with reference to the future gross profit of the AMCo group over a period of 12 months from October 1, 2015. The US dollar amounts and the value of the Consideration Shares set forth above are based on the GBP/US$ and EUR/US$ foreign exchange rates, and the closing price of the Common Shares on the TSX, at September 4, 2015. On the Acquisition Closing, Concordia will procure, on behalf of AMCo, the redemption of the capital loan notes held by certain Vendors and the payment of certain other amounts, all of which will be deducted from the Total Cash Consideration. In addition, pursuant to the AMCo Purchase Agreement, if the Corporation acquires certain specified pharmaceutical products from a specified third party introduced to the Corporation by certain of the Vendors within 12 months of the Acquisition Closing, the Corporation will pay to the Vendors an additional US$72 million.
Completion of the Acquisition is subject to certain closing conditions. The Acquisition will be financed through the net proceeds of the Offering and certain debt financings described in this Prospectus Supplement. In addition, a portion of the Purchase Price will be satisfied through the issuance of the Consideration Shares. See“The Acquisition” and“Details of the Acquisition”.
DESCRIPTION OF THE AMCO BUSINESS
General Description of the AMCo Business
AMCo is an international specialty pharmaceutical company, owning a diversified portfolio of branded and generic prescription products which are sold to wholesalers, hospitals and pharmacies in over 100 countries. AMCo specializes in the acquisition and development of hard-to-make, niche, off-patent prescription medicines in selected therapeutic areas. AMCo’s medicines are manufactured and sold through an out-sourced production and distribution network and marketed internationally through a combination of direct sales and local partnerships.
As of December 31, 2014, AMCo had a diversified product portfolio consisting of 180 molecules that represent a range of dosage strengths, formulations and geographic markets, covering a range of therapeutic categories, including endocrinology, ophthalmology and urology. As of June 30, 2015, AMCo’s product portfolio had increased to 190 molecules. For the year ended December 31, 2014, the largest molecule by revenue in AMCo’s portfolio accounted for less than 10% of total revenue, and the top ten molecules in AMCo’s portfolio accounted for less than 60% of total revenue.
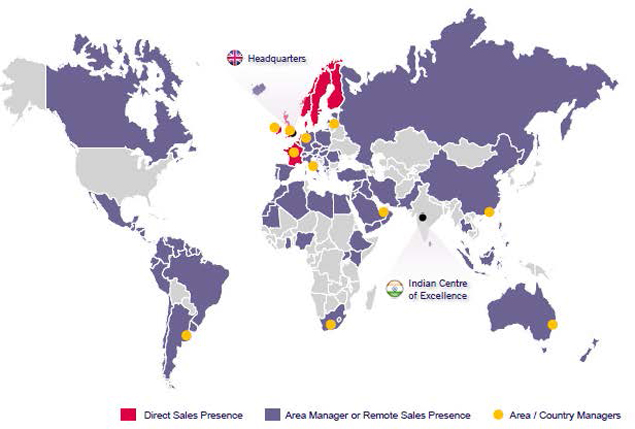
As at the date of this Prospectus Supplement, AMCo has a direct sales presence in the United Kingdom, Ireland, France, the Nordic region, the Benelux region and the Middle East and North African (“MENA”) region and a distribution network worldwide. AMCo utilizes an asset-light business model with a core management team in the United Kingdom and Ireland supported by commercial and regulatory teams in certain international markets and a Centre of Excellence in India that has operational functions ranging from regulatory, supply chain, medical marketing, customer service, information technology and finance. AMCo focuses on the registration and regulatory maintenance of acquired, in-licensed and own-developed products. This model requires no basic research and minimal development spend. New products are acquired and in-licensed as well as developed by contract research organizations that are managed by an AMCo in-house team, mainly to develop new formulations and dosage strengths of existing products. AMCo outsources all product manufacturing services to over 73 well-known, reputable third-party contract manufacturers in the United Kingdom and Europe.
AMCo Business Divisions
AMCo’s operations are divided into two major business divisions, direct markets and distributor markets, which are the basis for its segment reporting in its financial results.
Direct markets
AMCo’s Direct markets division is engaged in direct sales through commercial teams in each geographic region that target public health organizations (for example, Clinical Commissioning Groups (“CCGs”) in the United Kingdom), key opinion leaders, wholesalers, pharmacies, and general and specialist physicians. AMCo’s revenue from the Direct
S-9
markets division increased from £182.5 million in 2013 to £232.3 million in 2014. For the year ended December 31, 2014, the Direct markets division accounted for 79.4% of AMCo’s revenue and included AMCo’s operations in the United Kingdom, Ireland, France, the Nordic region and the Benelux region.
Distributor markets
AMCo’s Distributor markets division is engaged in sales worldwide through distribution partnerships monitored both on the ground by an area or country manager and remotely from AMCo’s United Kingdom headquarters and/or its operations center in India. AMCo’s revenue from the Distributor markets division increased from £58.5 million in 2013 to £60.5 million in 2014. For the year ended December 31, 2014, the Distributor markets division accounted for 20.6% of AMCo’s revenue. The Distributor markets division in 2014 included AMCo’s operations in the Middle-East, the Americas, Asia-Pacific, Southern Europe, Eastern Europe and South Africa.
The following figure shows AMCo’s geographic presence as of December 31, 2014.
Products
AMCo has a diversified product portfolio, with over 190 molecules across several therapeutics areas, sold in over 100 countries. In 2014, no one molecule accounted for more than 10% of AMCo’s total revenue, and AMCo’s top ten molecules accounted for less than 60% of total revenue. AMCo’s top ten molecules in the United Kingdom (its largest market) accounted for less than 44% of total revenue.
AMCo specializes in the acquisition and development of hard-to-make, niche, off-patent prescription medicines in selected therapeutic areas. The following table provides an overview of AMCo’s top ten molecules during the year ended December 31, 2014 and illustrates the level of diversity across AMCo’s product portfolio:
S-10
| | | | | | | | | | | | |
AMCo’s Top 10 Molecules | |
Molecule | | Indication | | Sold in no. of
countries | | International
Rights | | FY 2014 Revenue
(£ million) | | % of FY 2014
Revenue | |
Levothyroxine Sodium | | Hypothyroidism | | 12 | | ü | | 28.7 | | | 9.8 | % |
| | | | | |
Nitrofurantoin | | Urinary tract infections | | 16 | | ü | | 26.9 | | | 9.2 | % |
| | | | | |
Prednisolone | | Inflammatory conditions | | 2 | | ü | | 21.0 | | | 7.2 | % |
| | | | | |
Carbimazole | | Hyperthyroidism | | 27 | | Defined
territories | | 17.4 | | | 5.9 | % |
| | | | | |
Liothyronine
Sodium | | Severe
hypothyroid states | | 9 | | ü | | 16.6 | | | 5.7 | % |
| | | | | |
Fusidic Acid | | Bacterial conjunctivitis | | 70 | | ü | | 15.8 | | | 5.4 | % |
| | | | | |
Erythromycin | | Bacterial infections | | 37 | | Defined
territories | | 13.4 | | | 4.6 | % |
| | | | | |
Codeine
Phosphate + Paracetamol | | Moderate/severe pain | | 3 | | ü | | 12.0 | | | 4.1 | % |
| | | | | |
Biperiden Hydrochloride | | Parkinson’s disease | | 19 | | Defined
territories | | 6.9 | | | 2.3 | % |
| | | | | |
Tranylcypromine Sulphate | | Depression | | 11 | | ü | | 6.6 | | | 2.3 | % |
Launch of New Products
AMCo grows and diversifies its product portfolio through acquisitions and organic growth, pursuant to which AMCo has developed a pipeline of products which consists of approximately 60 product launches over the next three years, mostly in the form of new dosages or formulations of existing drugs. This pipeline consists of reformulations or new dosages of existing products, which are developed in-house, the launch of existing products in new countries and in-licensed products that reach across AMCo’s various markets. In-licensed products include niche branded or un-branded products that are currently sold by other companies. AMCo identifies in-licensing opportunities and seeks to reach an agreement with these companies to allow AMCo to market the product in some or all geographic regions, typically in return for milestone payments and/or profit share agreements.
Production and Supply
AMCo outsources all product manufacturing services to over 73 third-party contract manufacturers (“CMOs”) in the United Kingdom and Europe. AMCo partners with well-known, reputable CMOs. AMCo has purposefully concentrated its supplier base in Europe in order to be close to its manufacturers, which allows AMCo to more readily monitor the manufacturing process and reduce the risk of any supply disruptions.
AMCo’s operations are managed by a core management team in the United Kingdom and Ireland supported by commercial and regulatory teams in certain international markets and a Centre of Excellence in India that has operational functions ranging from regulatory, supply chain, medical marketing, customer service, information technology and finance. AMCo operates a Centre of Excellence in India to support its main operations team in the United Kingdom and Ireland and its other teams globally. In 2014, the cost of AMCo’s Centre of Excellence in India was equivalent to 1.4% of AMCo’s total revenue.
S-11
See “Description of the AMCo Business.”
ACQUISITION RATIONALE
Concordia believes the Acquisition is aligned with its strategy of pursuing accretive acquisitions of legacy pharmaceutical products. The Corporation believes the Acquisition is highly complementary to its existing business and is expected to result in the following significant strategic and financial benefits to Concordia:
| | • | | Strong Financial Returns - The Acquisition is expected to be accretive to both revenue and adjusted earnings per share; |
| | • | | Platform for International Expansion - The Corporation anticipates that AMCo will provide it with an international platform for continued expansion; |
| | • | | Commercial Footprint - AMCo conducts business in over 100 countries, which will significantly expand the Corporation’s geographical commercial reach after the Acquisition; |
| | • | | Diversification - AMCo will diversify the Corporation’s product portfolio by adding more than 190 complementary, niche pharmaceutical products that may present technical barriers to entry as they are difficult to manufacture, and entail complex regulatory approval processes (88% of AMCo’s product portfolio is estimated to have two or fewer competitors), with no AMCo product representing more than 10% of AMCo’s total revenue in 2014; |
| | • | | Meaningful Operating Leverage - AMCo’s Indian operations provide a low-cost operations support centre that includes functions such as regulatory, supply chain, medical marketing, customer service, information technology and finance; |
| | • | | Complementary Business Strategy - AMCo’s flexible and asset-light business strategy, with an operating model based on the Indian operations support centre, third party manufacturers, and a limited marketing and promotion spend, is a strategic fit for Concordia and mirrors Concordia’s philosophy of focusing on niche life-cycle management opportunities for off-patent prescription drugs; |
| | • | | Opportunities for Organic Growth - The Acquisition is expected to present the Corporation with attractive growth opportunities through the continued optimization of AMCo’s existing portfolio and its pipeline consisting of over 60 product launches based on reformulations and new dosages; |
| | • | | Financial Scale for Future Acquisitions - The Acquisition is expected to enable the Corporation to focus its investments and resources on further growing its business by providing the financial scale to pursue future acquisitions and increase Concordia’s ability to compete for global, quality assets; |
| | • | | Improved Access to Capital - The Acquisition is expected to improve Concordia’s access to the capital markets as a result of enhanced size and diversification, as well benefit the Corporation’s credit profile by providing the Corporation with the ability to manage its indebtedness and de-lever over time; and |
| | • | | Experienced Management Team - The Acquisition provides the Corporation with a complementary team of senior executives that have extensive experience in the pharmaceutical industry. |
See“Acquisition Rationale”.
CONCORDIA FOLLOWING THE ACQUISITION
Concordia believes that, subsequent to the completion of the Acquisition, the Corporation will possess a number of attributes and competitive advantages that should enable it to maintain and grow revenues and operating cash flow.
| | • | | Sustainable Products Portfolio -Concordia’s portfolio pro forma for the Acquisition will consist of established, branded, off-patent and authorized generic products. The majority of the Corporation’s products and the products of AMCo have a long prescription |
S-12
| | history, an established prescriber base and a proven safety and efficacy profile. The United Kingdom has historically represented a large market for generic pharmaceutical products in Europe, with a value of approximately £6.5 billion in 2014 and forecasted growth of approximately 4% until 2019. Additionally, some of Concordia’s products benefit from barriers to entry such as complex formulation, manufacturing or regulatory challenges. The Corporation believes products with these characteristics will face a lesser degree of competition. |
| | • | | Diversified Revenue Stream - Giving effect to the Acquisition, the Corporation’s product portfolio will be comprised of a total of over 190 products across several therapeutics areas. As a result, Concordia expects that the Acquisition will significantly increase its size and scale while reducing its reliance on any individual product. No single product will represent more than 10% of pro forma 2014 revenue. After giving effect to the Acquisition, Donnatal® would account for approximately 8% of 2014 pro forma total revenue, Lanoxin®, Eltroxin, and Macrobid would each represent 6% of 2014 pro forma total revenue and Predsol would account for approximately 4% of 2014 pro forma total revenue. The Corporation believes that this enhanced diversification will increase the expected stability of its aggregate revenue base going forward. In addition, AMCo has developed a pipeline of products which consists of approximately 60 product launches over the next three years mostly in the form of new dosages or formulations of existing drugs. |
| | • | | Scalable Business Model with Reliable Outsourcing - Concordia’s and AMCo’s partnerships with service providers, including CMOs, are expected to continue to enable the Corporation to produce and deliver quality, safe and reliable products without being exposed to the headcount and fixed costs of operating a pharmaceutical manufacturing facility. The Corporation believes that its limited fixed costs and capital investments together with its targeted development and promotional spending will continue to enable the Corporation to maintain attractive Adjusted EBITDA margins and generate free cash flow. The acquisition of AMCo adds further capabilities as the Centre of Excellence in India provides operational functions ranging from regulatory, supply chain, medical marketing, customer service, information technology and finance that can be leveraged by Concordia. |
| | • | | Strong Financial Profile with High Margins and Cash Flow Generation - Concordia benefits from a high cash flow conversion profile. The Corporation’s cash flow generation can be attributed to attractive gross margins, controlled operating expenses and modest capital expenditures. The Corporation expects that the Acquisition will further enhance its ability to generate cash flow. For the twelve months ended December 31, 2014, the pro forma Adjusted EBITDA margin for the Corporation was 59%. The Corporation intends to employ its cash flow to manage its indebtedness and de-lever over time while opportunistically pursuing its acquisition-driven growth strategy to continue to enhance its product portfolio. |
| | • | | Track Record of Successfully Growing the Business through Acquisitions - The current Concordia management team has a demonstrated track record of successfully identifying, acquiring and integrating products and businesses in order to drive growth and realize synergies. Since 2013, the Corporation has successfully completed six acquisitions (not including the Acquisition) of varying size and complexity. |
| | • | | Strong Management with Extensive Industry Experience - Certain members of the Corporation’s executive management team and Board have, on average, 15 years of pharmaceutical product acquisition and operational experience. The Acquisition of AMCo and its experienced senior management team is expected to be complementary to and enhance the Corporation’s management team. |
See“Concordia Following the Acquisition”.
SELECTED PRO FORMA CONSOLIDATED FINANCIAL INFORMATION
The following tables set forth Concordia’s selected unaudited pro forma consolidated financial information (i) for the year ended December 31, 2014, (ii) for the year ended December 31, 2014 after giving effect to the acquisition of Donnatal®, the acquisition of Zonegran®, the acquisition of the Covis Business, and the Acquisition, in each case as if such acquisitions had been effective on January 1, 2014, (iii) as at June 30, 2015 and for the six months ended June 30, 2015; and (iv) as at June 30, 2015 and for the six months ended June 30, 2015 after giving effect to the acquisition of the Covis Business and the Acquisition, in each case as if such acquisitions had been effective on January 1, 2014.
The selected unaudited pro forma consolidated financial information set forth below and included elsewhere in this Prospectus Supplement are for illustrative purposes only and not necessarily indicative of results of operations that
S-13
would have occurred as at or for the year ended December 31, 2014 or for the six months ended June 30, 2015 had the acquisitions of Donnatal® and Zonegran®, the Covis Business, and the Acquisition been effective January 1, 2014, or of the results of operations expected in 2015 and future years. The following pro forma financial information has been prepared by management of the Corporation on the basis of the information contained in this Prospectus Supplement, the Prospectus, or in documents incorporated by reference therein. This pro forma financial information is not a forecast or a projection of future results. The actual results of the combined operations for any period, whether before or after the Acquisition Closing, will likely vary from the amounts set forth in the following pro forma financial information and such variation may be material. See “Non-IFRS Measures” and “Risk Factors - Risk Factors Related to the Acquisition and the Debt Financing”.
Year ended December 31, 2014
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Year ended December 31, 2014 (US$ thousands) | | Concordia
Healthcare
Corp. | | | Donnatal®
Jan 1 - May
15, 2014 | | | Zonegran®
Jan 1 - Sep
30, 2014 | | | | | | Amdipharm
Mercury
Limited | | | Adjustments | | | Concordia
Healthcare | |
| | | | | Covis
Portfolio | | | | Pro Forma
Adjustments
(1)(2) | | | Corp. Pro
Forma
Consolidated(2) | |
Consolidated Statement of Earnings | | | | | | | | | | | | | | | | | | | | |
Revenue | | | 122,191 | | | | 17,607 | | | | 17,281 | | | | 151,534 | | | | 482,248 | | | | — | | | | 790,861 | |
Cost of sales | | | 17,989 | | | | 900 | | | | 4,311 | | | | 12,939 | | | | 120,618 | | | | — | | | | 156,757 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 104,202 | | | | 16,707 | | | | 12,970 | | | | 138,595 | | | | 361,630 | | | | — | | | | 634,104 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating expenses | | | | | | | | | | | | | | | | |
General and administrative | | | 20,663 | | | | 3,280 | | | | 1,994 | | | | 32,920 | | | | 77,315 | | | | — | | | | 136,172 | |
Acquisition, restructuring and other | | | 13,521 | | | | — | | | | — | | | | — | | | | 7,627 | | | | (12,163 | ) | | | 8,985 | |
Selling and marketing | | | 10,229 | | | | 1,159 | | | | 566 | | | | — | | | | 37,069 | | | | — | | | | 49,023 | |
Research and development | | | 9,301 | | | | 94 | | | | — | | | | — | | | | 5,076 | | | | — | | | | 14,471 | |
Share-based compensation | | | 4,484 | | | | — | | | | — | | | | 2,690 | | | | — | | | | — | | | | 7,174 | |
Amortization of intangible assets | | | 11,039 | | | | — | | | | — | | | | 36,117 | | | | 73,702 | | | | (36,117 | ) | | | 163,862 | |
| | | | | | | | | | | | | | | 47,822 | | | | | |
| | | | | | | | | | | | | | | 8,696 | | | | | |
| | | | | | | | | | | | | | | 22,603 | | | | | |
Depreciation expense | | | 131 | | | | 114 | | | | — | | | | — | | | | 1,476 | | | | — | | | | 1,721 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 69,368 | | | | 4,647 | | | | 2,560 | | | | 71,727 | | | | 202,265 | | | | 30,841 | | | | 381,408 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating income | | | 34,834 | | | | 12,060 | | | | 10,410 | | | | 66,868 | | | | 159,365 | | | | (30,841 | ) | | | 252,696 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Other income and expenses | | | | | | | | | | | | | | | | | | | | |
Interest and accretion expense | | | 12,194 | | | | — | | | | — | | | | 10,523 | | | | 160,194 | | | | (10,523 | ) | | | 223,601 | |
| | | | | | | | | | | | | | | (6,332 | ) | | | | |
| | | | | | | | | | | | | | | 78,763 | | | | | |
| | | | | | | | | | | | | | | 6,113 | | | | | |
| | | | | | | | | | | | | | | 162,957 | | | | | |
| | | | | | | | | | | | | | | (30,094 | ) | | | | |
| | | | | | | | | | | | | | | (160,194 | ) | | | | |
Finance income | | | — | | | | — | | | | — | | | | — | | | | (1,304 | ) | | | — | | | | (1,304 | ) |
Change in fair value of contingent consideration | | | 2,629 | | | | — | | | | — | | | | — | | | | 1,820 | | | | — | | | | 4,449 | |
Foreign exchanges loss (gain) | | | 696 | | | | — | | | | — | | | | — | | | | (15,055 | ) | | | 15,055 | | | | 696 | |
Other (income) expense | | | 203 | | | | — | | | | 38 | | | | (159 | ) | | | (545 | ) | | | — | | | | (463 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income (loss) before tax | | | 19,112 | | | | 12,060 | | | | 10,372 | | | | 56,504 | | | | 14,255 | | | | (86,586 | ) | | | 25,717 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income taxes | | | 7,522 | | | | — | | | | 3,994 | | | | 3,353 | | | | 4,040 | | | | (24,507 | ) | | | 43,416 | |
Net income (loss) | | | 11,590 | | | | 12,060 | | | | 6,378 | | | | 53,151 | | | | 10,215 | | | | (111,093 | ) | | | (17,699 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
S-14
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Year ended December 31, 2014 (US$ thousands) | | Concordia
Healthcare
Corp. | | | | | | Covis
Portfolio | | | Amdipharm
Mercury
Limited | | | Adjustments | | | Concordia
Healthcare | |
| | | Donnatal®
Jan 1 - May
15, 2014 | | | Zonegran®
Jan 1 - Sep
30, 2014 | | | | | Pro Forma
Adjustments
(1)(2) | | | Corp. Pro
Forma
Consolidated(2) | |
Other financial and operating metrics: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Adjusted EBITDA (3) | | | 64,009 | | | | 12,174 | | | | 10,410 | | | | 123,507 | | | | 255,572 | | | | | | | | 463,065 | |
Adjusted EBITDA margin(4) | | | 52 | % | | | 69 | % | | | 60 | % | | | 82 | % | | | 53 | % | | | | | | | 59 | % |
Pro forma Adjusted EBITDA (3)(5) | | | | | | | | | | | | | | | | | | | | | | | | | | | 500,617 | |
Reconciliation of net income to EBITDA and Adjusted EBITDA: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | 11,590 | | | | 12,060 | | | | 6,378 | | | | 53,151 | | | | 10,215 | | | | (111,093 | ) | | | (17,699 | ) |
Depreciation and amortization (6) | | | 11,170 | | | | 114 | | | | — | | | | 36,280 | | | | 75,177 | | | | 42,842 | | | | 165,583 | |
Interest expense | | | 12,194 | | | | — | | | | — | | | | 10,523 | | | | 160,194 | | | | 40,690 | | | | 223,601 | |
Finance income | | | — | | | | — | | | | — | | | | — | | | | (1,304 | ) | | | — | | | | (1,304 | ) |
Income taxes | | | 7,522 | | | | — | | | | 3,994 | | | | 3,353 | | | | 4,040 | | | | 24,507 | | | | 43,416 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
EBITDA(3) | | | 42,476 | | | | 12,174 | | | | 10,372 | | | | 103,307 | | | | 248,322 | | | | (3,054 | ) | | | 413,597 | |
Change in fair value of contingent consideration | | | 2,629 | | | | — | | | | — | | | | — | | | | 1,820 | | | | | | | | 4,449 | |
Stock-based compensation | | | 4,484 | | | | — | | | | — | | | | 2,690 | | | | — | | | | — | | | | 7,174 | |
Acquisition, restructuring and other (7) | | | 13,521 | | | | — | | | | — | | | | 2,445 | | | | 7,627 | | | | (14,608 | ) | | | 8,985 | |
Foreign exchange loss (gain) | | | 696 | | | | — | | | | — | | | | — | | | | (15,055 | ) | | | 15,055 | | | | 696 | |
Other (income) expense | | | 203 | | | | — | | | | 38 | | | | (159 | ) | | | (545 | ) | | | — | | | | (463 | ) |
Discontinued sales & marketing expenses (8) | | | — | | | | — | | | | — | | | | 15,224 | | | | — | | | | — | | | | 15,224 | |
Other non-recurring costs (9) | | | — | | | | — | | | | — | | | | — | | | | 13,403 | | | | — | | | | 13,403 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Adjusted EBITDA(3) | | | 64,009 | | | | 12,174 | | | | 10,410 | | | | 123,507 | | | | 255,572 | | | | (2,607 | ) | | | 463,065 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Pro forma adjustment for acquisition of pharmaceutical products(10) | | | | | | | | | | | | | | | | | | | | | | | | | | | 26,023 | |
Impact of AMCo’s cost savings programs(11) | | | | | | | | | | | | | | | | | | | | | | | | | | | 11,529 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Pro forma Adjusted
EBITDA (3)(5) | | | | | | | | | | | | | | | | | | | | | | | | | | | 500,617 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Notes:
| (1) | See the pro forma financial statements of the Corporation attached hereto as Appendix “C” for details regarding the pro forma adjustments. |
| (2) | Includes estimated gross proceeds from the Offering of US$577,840, assuming the issuance of 8,000,000 Offered Shares at a price of US$72.23, which was the closing price of the Common Shares on NASDAQ on September 18, 2015. |
| (3) | EBITDA, Adjusted EBITDA and pro forma Adjusted EBITDA each represent non-IFRS measures. See “Non-IFRS Measures” for the definitions of EBITDA and Adjusted EBITDA. Please refer to the above table for a reconciliation of reported net income to EBITDA and Adjusted EBITDA. Pro forma Adjusted EBITDA includes additional pro forma adjustments related to AMCo which were not included in the pro forma financial statements attached hereto as Appendix “C”. |
| (4) | Adjusted EBITDA margin represents Adjusted EBITDA divided by revenue. |
| (5) | Pro forma Adjusted EBITDA includes additional pro forma adjustments related to AMCo which were not included in the pro forma financial statements attached hereto as Appendix “C”. |
| (6) | Includes $36,117 of amortization and $163 of depreciation expense included in the Covis Business’ general and administrative expenses. |
| (7) | Acquisition related costs of $2,445 are included in the Covis Business’ general and administrative expenses. |
| (8) | Represents one-time costs related to a selling and marketing initiative, which was discontinued in October 2014. |
| (9) | Represents non-recurring one-time costs primarily related to dual sourcing and other non-recurring special projects. |
| (10) | Represents EBITDA from acquisitions that are not included in AMCo’s results. |
| (11) | Represents the pro forma impact of cost savings initiatives implemented by AMCo during the year ended December 31, 2014. |
Six months ended June 30, 2015
| | | | | | | | | | | | | | | | | | | | |
6 months ended June 30, 2015 (US$ thousands) | | Concordia
Healthcare
Corp.(1) | | | Covis
Portfolio(2) | | | Amdipharm
Mercury
Limited | | | Adjustments | | | Concordia
Healthcare | |
| | | | | Pro Forma
Adjustments
(3)(4) | | | Corp. Pro
Forma
Consolidated(4) | |
Consolidated Statement of Earnings | | | | | | | | | | | | | | | | | | | | |
Revenue | | | 113,949 | | | | 60,880 | | | | 251,729 | | | | (2,124 | ) | | | 424,434 | |
Cost of sales | | | 11,150 | | | | 5,638 | | | | 69,775 | | | | (106 | ) | | | 86,457 | |
| | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 102,779 | | | | 55,242 | | | | 181,954 | | | | (2,018 | ) | | | 337,977 | |
| | | | | | | | | | | | | | | | | | | | |
Operating Expenses | | | | | | | | | | | | | | | | | | | | |
General and administrative | | | 14,616 | | | | 5,280 | | | | 33,109 | | | | — | | | | 53,005 | |
Acquisition, restructuring and other | | | 12,556 | | | | — | | | | 3,548 | | | | (10,820 | ) | | | 5,284 | |
S-15
| | | | | | | | | | | | | | | | | | | | |
6 months ended June 30, 2015 (US$ thousands) | | Concordia
Healthcare
Corp.(1) | | | Covis
Portfolio(2) | | | Amdipharm
Mercury
Limited | | | Adjustments | | | Concordia
Healthcare | |
| | | | | Pro Forma
Adjustments
(3)(4) | | | Corp. Pro
Forma
Consolidated(4) | |
Selling and marketing | | | 7,329 | | | | — | | | | 18,297 | | | | — | | | | 25,626 | |
Research and development | | | 5,792 | | | | — | | | | 620 | | | | — | | | | 6,412 | |
Share-based compensation | | | 5,017 | | | | 698 | | | | — | | | | — | | | | 5,715 | |
Exchange listing expenses | | | 574 | | | | — | | | | — | | | | — | | | | 574 | |
Amortization of intangible assets | | | 20,260 | | | | 10,522 | | | | 36,434 | | | | (10,522 | ) | | | 80,369 | |
| | | | | | | | | | | | | | | 11,956 | | | | | |
| | | | | | | | | | | | | | | 11,719 | | | | | |
Depreciation expense | | | 128 | | | | — | | | | 905 | | | | — | | | | 1,033 | |
| | | | | | | | | | | | | | | | | | | | |
Total Operating Expenses | | | 66,272 | | | | 16,500 | | | | 92,913 | | | | 2,333 | | | | 178,018 | |
| | | | | | | | | | | | | | | | | | | | |
Operating income | | | 36,527 | | | | 38,742 | | | | 89,041 | | | | (4,351 | ) | | | 159,959 | |
| | | | | | | | | | | | | | | | | | | | |
Other income and expenses | | | | | | | | | | | | | | | | | | | | |
Interest and accretion expense | | | 27,653 | | | | 2,598 | | | | 89,597 | | | | (2,598 | ) | | | 108,180 | |
| | | | | | | | | | | | | | | (8,641 | ) | | | | |
| | | | | | | | | | | | | | | 19,691 | | | | | |
| | | | | | | | | | | | | | | 1,528 | | | | | |
| | | | | | | | | | | | | | | 81,479 | | | | | |
| | | | | | | | | | | | | | | (13,530 | ) | | | | |
| | | | | | | | | | | | | | | (89,597 | ) | | | | |
Finance income | | | — | | | | — | | | | (631 | ) | | | — | | | | (631 | ) |
Impairment loss | | | 668 | | | | — | | | | — | | | | — | | | | 668 | |
Change in fair value of contingent consideration | | | (6,224 | ) | | | — | | | | — | | | | — | | | | (6,224 | ) |
Foreign exchanges loss (gain) | | | (282 | ) | | | — | | | | (52,139 | ) | | | 52,139 | | | | (282 | ) |
Fair value loss on foreign exchange forward contract | | | 5,126 | | | | — | | | | — | | | | — | | | | 5,126 | |
Other (income) expense | | | 400 | | | | (78 | ) | | | (238 | ) | | | — | | | | 84 | |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) before tax | | | 9,186 | | | | 36,222 | | | | 52,452 | | | | (44,822 | ) | | | 53,038 | |
| | | | | | | | | | | | | | | | | | | | |
Income taxes | | | | | | | | | | | | | | | | | | | | |
Current | | | 475 | | | | 3,412 | | | | 16,642 | | | | 7,088 | | | | 27,617 | |
Deferred | | | 3,598 | | | | — | | | | (4,373 | ) | | | (2,344 | ) | | | (3,119 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | 5,113 | | | | 32,810 | | | | 40,183 | | | | (49,566 | ) | | | 28,540 | |
| | | | | | | | | | | | | | | | | | | | |
Consolidated Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | | 140,207 | | | | n/a | | | | 67,910 | | | | (114,132) | | | | 93,985 | |
Working capital balance(5) | | | 168,426 | | | | n/a | | | | 123,736 | | | | (114,132) | | | | 178,030 | |
Total assets | | | 1,938,452 | | | | n/a | | | | 1,750,534 | | | | 2,196,012 | | | | 5,884,998 | |
Total debt(6) | | | 1,295,927 | | | | n/a | | | | 1,472,323 | | | | 867,720 | | | | 3,635,970 | |
Total shareholders’ equity | | | 559,791 | | | | n/a | | | | (7,828 | ) | | | 1,124,194 | | | | 1,676,157 | |
Other Financial and Operating Metrics: | | | | | | | | | | | | | | | | | | | | |
Adjusted EBITDA(7) | | | 75,062 | | | | 49,962 | | | | 133,652 | | | | | | | | 256,658 | |
Adjusted EBITDA margin(8) | | | 66 | % | | | 82 | % | | | 53 | % | | | | | | | 60 | % |
Pro forma Adjusted EBITDA(7)(9) | | | | | | | | | | | | | | | | | | | 264,124 | |
Reconciliation of net income to EBITDA and Adjusted EBITDA: | | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | | 5,113 | | | | 32,810 | | | | 40,183 | | | | (49,566 | ) | | | 28,540 | |
Depreciation and amortization | | | 20,388 | | | | 10,522 | | | | 37,339 | | | | 13,153 | | | | 81,402 | |
Interest expense | | | 27,653 | | | | 2,598 | | | | 89,597 | | | | (11,668 | ) | | | 108,180 | |
Finance income | | | — | | | | — | | | | (631 | ) | | | — | | | | (631 | ) |
Income taxes | | | 4,073 | | | | 3,412 | | | | 12,269 | | | | 4,744 | | | | 24,498 | |
| | | | | | | | | | | | | | | | | | | | |
EBITDA(7) | | | 57,227 | | | | 49,342 | | | | 178,757 | | | | (43,337 | ) | | | 241,989 | |
Change in fair value of contingent consideration | | | (6,224 | ) | | | — | | | | — | | | | — | | | | (6,224 | ) |
Stock-based compensation | | | 5,017 | | | | 698 | | | | — | | | | — | | | | 5,715 | |
Acquisition, restructuring and other | | | 12,556 | | | | — | | | | 3,548 | | | | (10,820 | ) | | | 5,284 | |
impairment loss | | | 668 | | | | — | | | | — | | | | — | | | | 668 | |
Exchange listing expenses | | | 574 | | | | — | | | | — | | | | — | | | | 574 | |
Foreign exchange loss (gain) | | | (282 | ) | | | — | | | | (52,139 | ) | | | 52,139 | | | | (282 | ) |
Loss on foreign exchange forward contract | | | 5,126 | | | | | | | | | | | | | | | | 5,126 | |
Other (income) expense | | | 400 | | | | (78 | ) | | | (238 | ) | | | — | | | | 84 | |
S-16
| | | | | | | | | | | | | | | | | | | | |
6 months ended June 30, 2015 (US$ thousands) | | Concordia
Healthcare
Corp.(1) | | | Covis
Portfolio
(2) | | | Amdipharm
Mercury
Limited | | | Adjustments | | | Concordia
Healthcare | |
| | | | | Pro Forma
Adjustments
(3)(4) | | | Corp. Pro
Forma
Consolidated (4) | |
Other non-recurring costs(10) | | | — | | | | — | | | | 3,724 | | | | — | | | | 3,724 | |
| | | | | | | | | | | | | | | | | | | | |
Adjusted EBITDA(7) | | | 75,062 | | | | 49,962 | | | | 133,652 | | | | (2,018 | ) | | | 256,658 | |
| | | | | | | | | | | | | | | | | | | | |
Pro forma adjustment for acquisition of pharmaceutical products(11) | | | | | | | | | | | | | | | | | | | 7,466 | |
| | | | | | | | | | | | | | | | | | | | |
Pro forma Adjusted EBITDA(7)(9) | | | | | | | | | | | | | | | | | | | 264,124 | |
| | | | | | | | | | | | | | | | | | | | |
Notes:
| (1) | Includes Covis Business results from the period from April 21, 2015, being the date of the Covis Acquisition, to June 30, 2015. |
| (2) | Represents Covis Business results for the period January 1, 2015 to March 31, 2015. |
| (3) | See the pro forma financial statements of the Corporation attached hereto as Appendix “C” for details regarding the pro forma adjustments. |
| (4) | Includes estimated gross proceeds from the Offering of US$577,840, assuming the issuance of 8,000,000 Offered Shares at a price of US$72.23, which was the closing price of the Common Shares on NASDAQ on September 18, 2015. |
| (5) | Working capital is defined as current assets less accounts payable, accrued liabilities, provisions and royalties payable. |
| (6) | Total debt includes short-term and long-term portions of long-term debt, notes payable and purchase consideration payable. |
| (7) | EBITDA, Adjusted EBITDA and pro forma Adjusted EBITDA each represent non-IFRS measures. See “Non-IFRS Measures” for the definitions of EBITDA and Adjusted EBITDA. Please refer to the above table for a reconciliation of reported net income to EBITDA and Adjusted EBITDA. See the pro forma financial statements of the Corporation attached hereto as Appendix “C” for details regarding the pro forma adjustments. |
| (8) | Adjusted EBITDA margin represents Adjusted EBITDA divided by revenue. |
| (9) | Pro forma Adjusted EBITDA includes additional pro forma adjustments related to AMCo which were not included in the pro forma financial statements attached hereto as Appendix “C”. |
| (10) | Represents non-recurring one-time costs primarily related to dual sourcing and other non-recurring special projects. |
| (11) | Represents EBITDA from acquisitions that are not included in AMCo’s results. |
S-17
THE OFFERING
| | |
| |
| Issuer: | | Concordia Healthcare Corp. |
| |
| Offering Price: | | US$[●] per Offered Share |
| |
| Amount: | | US$[●] |
| |
| Closing Date: | | On or about September 30, or on such other date (which date shall not be later than 42 days after the date of this Prospectus Supplement) as may be agreed upon by the Corporation and the Representatives, on behalf of the Underwriters. |
| |
| Issue: | | 8,000,000 Common Shares. See “Description of Common Shares”. |
| |
| Underwriters’ Option: | | The Corporation has granted the Underwriters the Underwriters’ Option, exercisable in whole or in part, at any time and from time to time, in the sole discretion of the Underwriters, up to 30 days after and including the Closing Date, to cover sales by the Underwriters of a greater number of Common Shares than the number set out above, if any. The Additional Offered Shares issuable upon exercise of the Underwriters’ Option are hereby qualified for distribution under this Prospectus Supplement. A person who acquires Additional Offered Shares acquires such Additional Offered Shares under this Prospectus Supplement. See“Plan of Distribution”. |
| |
| Use of Proceeds: | | The net proceeds to the Corporation from this Offering, after deducting the Underwriters’ Fee and expenses of the Offering, are estimated to be approximately US$[●], or approximately US$[●] if the Underwriters exercise the Underwriters’ Option in full. The Corporation intends to use the net proceeds from the Offering to fund, in part: (i) a portion of the Purchase Price; and (ii) the fees and expenses incurred in connection with the Acquisition. In the event the Underwriters’ Option is exercised, any additional net proceeds will be allocated to the partial satisfaction of the Purchase Price and/or general corporate purposes. See“Use of Proceeds”. In the event that the Acquisition Closing does not occur, the net proceeds from the Offering (before deducting expenses of the Offering of US$[●]) will initially be added to the Corporation’s working capital and will subsequently be applied to fund future acquisitions in furtherance of the Corporation’s business plan and for general corporate purposes and, with available cash on hand, to potentially repay certain debt obligations of the Corporation. See“Use of Proceeds”,“Details of the Acquisition” and“Risk Factors”. |
| |
| Listing and Trading: | | The Corporation has applied to list the Offered Shares and the Additional Offered Shares on the TSX. Listing is subject to the Corporation fulfilling all of the listing requirements of the TSX. The Offered Shares and the Additional Offered Shares will be listed on NASDAQ, upon official notice of issuance. See“Plan of Distribution”. |
| |
| Risk Factors: | | An investment in the Offered Shares involves significant risks that should be carefully considered by prospective investors before purchasing such securities. The risks outlined in this Prospectus Supplement, in the accompanying Prospectus, and in the documents incorporated by reference therein should be carefully reviewed and considered by prospective investors in connection with an investment in the Offered Shares. See“Cautionary Note Regarding Forward-Looking Information” and“Risk Factors”. |
S-18
DOCUMENTS INCORPORATED BY REFERENCE
This Prospectus Supplement is deemed, as of the date hereof, to be incorporated by reference into the accompanying Prospectus only for the purposes of the Offering.
The Corporation’s filings through SEDAR are not incorporated by reference in the Prospectus except as specifically set out herein or therein.
As at the date hereof, the following documents filed by the Corporation with the securities commissions or similar authorities in each of the Provinces of Canada are specifically incorporated by reference into the Prospectus for the purposes of the Offering, and form an integral part of the Prospectus, as supplemented by this Prospectus Supplement:
| | (a) | annual information form of the Corporation for the year ended December 31, 2014 dated March 19, 2015 (“AIF”); |
| | (b) | audited consolidated financial statements of the Corporation for the year ended December 31, 2014, together with the notes thereto and the auditor’s report thereon (the “Annual Financial Statements”); |
| | (c) | management’s discussion and analysis of financial condition and results of operations of the Corporation for the year ended December 31, 2014; |
| | (d) | unaudited condensed interim consolidated financial statements of the Corporation for the three and six months ended June 30, 2015, together with the notes thereto (the “Interim Financial Statements”); |
| | (e) | management’s discussion and analysis of financial condition and results of operations of the Corporation for the three and six months ended June 30, 2015 (the “Interim MD&A”); |
| | (f) | business acquisition report dated June 9, 2014 relating to the acquisition of Donnatal®; |
| | (g) | business acquisition report dated December 12, 2014 relating to the acquisition of Zonegran®; |
| | (h) | business acquisition report dated July 3, 2015 relating to the acquisition of the Covis Business (the “Covis Acquisition”); |
| | (i) | the management information circular of the Corporation dated May 25, 2015 relating to the Corporation’s annual general meeting of shareholders held on June 25, 2015; |
| | (j) | the material change report dated March 16, 2015 relating to the announcement of the Covis Acquisition; |
| | (k) | the material change report dated March 17, 2015 relating to the announcement of an underwritten offering of 3,764,720 subscription receipts at a price of $85.00 per subscription receipt for aggregate gross proceeds to Concordia of $320,001,200 in connection with the Covis Acquisition; |
| | (l) | the material change report dated April 14, 2015 relating to among other things, the pricing of a private placement of US$735,000,000 principal amount of 7.00% notes of the Corporation, undertaken in connection with the Covis Acquisition; |
| | (m) | the material change report dated May 1, 2015 relating to the closing of the Covis Acquisition and the related offering of notes, the conversion of outstanding subscription receipts into Common Shares, and the entering into of certain credit facilities in connection with the Covis Acquisition; and |
| | (n) | the material change report dated September 14, 2015 relating to the announcement of the Acquisition. |
Any documents of the type required by National Instrument 44-101 -Short Form Prospectus Distributions (“NI 44-101”) to be incorporated by reference in a short form prospectus, including those types of documents referred to above
S-19
and press releases issued by Concordia referencing incorporation by reference into the Prospectus, if filed by Concordia with the provincial securities commissions or similar authorities in Canada after the date of this Prospectus Supplement and prior to the completion or termination of the Offering, shall be deemed to be incorporated by reference into the Prospectus, as supplemented by this Prospectus Supplement, for the purposes of the Offering.
Upon a new annual information form and annual consolidated financial statements being filed by the Corporation with the applicable Canadian securities commissions or similar regulatory authorities in Canada during the period that this Prospectus Supplement is effective, the previous annual information form, the previous annual consolidated financial statements and all interim consolidated financial statements, and in each case the accompanying management’s discussion and analysis of financial condition and results of operations, and material change reports, filed prior to the commencement of the financial year of the Corporation in which the new annual information form is filed shall be deemed to no longer be incorporated into the Prospectus for purposes of offers and sales of Offered Shares under this Prospectus Supplement. Upon interim consolidated financial statements and the accompanying management’s discussion and analysis of financial condition and results of operations being filed by the Corporation with the applicable Canadian securities commissions or similar regulatory authorities during the period that this Prospectus Supplement is effective, all interim consolidated financial statements and the accompanying management’s discussion and analysis of financial condition and results of operations filed prior to such new interim consolidated financial statements and management’s discussion and analysis of financial condition and results of operations shall be deemed to no longer be incorporated into the Prospectus for purposes of offers and sales of Offered Shares under this Prospectus Supplement. In addition, upon a new management information circular for an annual meeting of shareholders being filed by the Corporation with the applicable Canadian securities commissions or similar regulatory authorities during the period that this Prospectus Supplement is effective, the previous management information circular filed in respect of the prior annual meeting of shareholders shall no longer be deemed to be incorporated into the Prospectus for offers and sales of Offered Shares under this Prospectus Supplement.
Documents referenced in any of the documents incorporated by reference in the Prospectus, as supplemented by this Prospectus Supplement, but not expressly incorporated by reference therein, and not otherwise required to be incorporated by reference therein, are not incorporated by reference in the Prospectus.
Any statement contained in this Prospectus Supplement, in the accompanying Prospectus, or in a document incorporated or deemed to be incorporated by reference into the Prospectus shall be deemed to be modified or superseded to the extent that a statement contained herein or therein, or in any subsequently filed document that also is, or is deemed to be, incorporated by reference into the Prospectus modifies, replaces or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this Prospectus Supplement or the Prospectus. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document or statement that it modifies or supersedes.
The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made.
MARKETING MATERIALS
Any “template version” of “marketing materials” (as such terms are defined in NI 44-101) will be incorporated by reference in this Prospectus Supplement. However, such “template version” of “marketing materials” will not form part of this Prospectus Supplement or the Prospectus to the extent that the contents of the “template version” of “marketing materials” are modified or superseded by a statement contained in this Prospectus Supplement or any amendment thereto. Any “template version” of “marketing materials” filed on SEDAR after the date of this Prospectus Supplement and before the termination of the distribution of Offered Shares under the Offering will be deemed to be incorporated by reference into this Prospectus Supplement and the accompanying Prospectus.
ELIGIBILITY FOR INVESTMENT
In the opinion of Fasken Martineau DuMoulin LLP, Canadian counsel to the Corporation, and Blake, Cassels & Graydon LLP, Canadian counsel to the Underwriters, based on the current provisions of theIncome Tax Act (Canada) (the “Tax Act”) and the regulations thereunder (the “Regulations”) in force on the date hereof, provided that the
S-20
Common Shares are listed on the date hereof on a “designated stock exchange” as defined in the Tax Act (which currently includes the TSX), the Offered Shares, if issued on the date hereof, would be qualified investments under the Tax Act on the date hereof for a trust governed by a registered retirement savings plan (“RRSP”), a registered retirement income fund (“RRIF”), a deferred profit sharing plan, a registered education savings plan, a tax-free savings account (“TFSA”), or a registered disability savings plan (collectively, “Registered Plans”).
The Offered Shares will not be “prohibited investments” for a trust governed by a TFSA, RRSP or RRIF provided the holder of the TFSA or the annuitant under the RRSP or RRIF, as applicable: (i) deals at arm’s length with the Corporation for purposes of the Tax Act; and (ii) does not have a “significant interest” (within the meaning of subsection 207.01(4) of the Tax Act) in the Corporation. In addition, the Offered Shares will not be a “prohibited investment” for such a trust if such Offered Shares are “excluded property” for such trust as defined in subsection 207.01(1) of the Tax Act. If the Offered Shares are a “prohibited investment”, the holder or annuitant, as the case may be, will be subject to penalty taxes as set out in the Tax Act. Holders of trusts governed by TFSAs and annuitants under trusts governed by RRSPs or RRIFs should consult their own tax advisors to ensure that the Offered Shares would not be a prohibited investment in their particular circumstances.
S-21
CONCORDIA HEALTHCARE CORP.
General Description of the Business
Concordia is a diverse healthcare company that is primarily focused on sustainable established legacy pharmaceutical products, which are off-patent drugs and have a track record of safety and efficacy, a history of stable prescription demand, and competitive barriers to entry. Some of Concordia’s products are often difficult to manufacture, have specialized regulatory status and/or possess strong brand loyalty that allows them to maintain ongoing sales after patent expiration. Concordia’s business strategy involves seeking to acquire products and maximize their value through:
| | • | | optimizing the sales and pricing strategy; |
| | • | | managing regulatory affairs and supply chain; |
| | • | | optimizing its corporate structure; |
| | • | | identifying authorized generic opportunities; and |
| | • | | exploring targeted promotion or co-promotion. |
The Corporation has utilized this strategy in six acquisitions since 2013 (not including the Acquisition), which has added a portfolio of products, including branded products such as Nilandron®, for the treatment of metastatic prostate cancer; Dibenzyline®, for the treatment of pheochromocytoma; Lanoxin®, for the treatment of mild-to moderate heart failure and atrial fibrillation; Plaquenil®, for the treatment of lupus and rheumatoid arthritis; Donnatal® for the treatment of irritable bowel syndrome; and Zonegran® (zonisamide) for treatment of partial seizures in adults with epilepsy. Donnatal® accounted for 21% of the Corporation’s 2014 total revenue, Lanoxin® accounted for 16% of 2014 total revenue, Plaquenil® accounted for 9% of 2014 total revenue, and each of Zonegran® and Dibenzyline® accounted for 8% of 2014 total revenue.
After giving effect to the Acquisition, the Corporation will have a high level of financial diversification with sales in over 100 countries and no single product representing more than 10% of revenues. Concordia’s global operations after the Acquisition are expected to provide the Corporation with the opportunity to maximize brand value worldwide as well as to identify products that may have specialized value propositions in certain specific markets.
Concordia also markets orphan drugs through an Orphan Drug Division, currently consisting of Photofrin® for the treatment of rare forms of cancer. The Corporation is currently undergoing testing for new indications for Photofrin® in order to enable expanded usage if approved. In addition, Concordia operates a Specialty Healthcare Distribution Division that provides a distribution capability for specialty pharmaceutical products and orphan drugs once acquired and/or developed.
Concordia operates out of facilities in Oakville, Ontario; Bridgetown, Barbados; Roanoke, Virginia and its Specialty Healthcare Distribution Division that operates out of Kansas City, Missouri. Pinnacle Biologics, Inc. is located in Chicago, Illinois. The Corporation intends to continue its Barbadian subsidiaries, Concordia Pharmaceuticals Inc. and Concordia Laboratories Inc., under the laws of the Grand Duchy of Luxembourg at or about the end of September 2015.
For a description of Concordia’s business and certain recent developments, including the Acquisition of the Covis Business completed in April 2015, see the section titled “General Development and Description of the Business” in the AIF and the section titled “Company Overview” in the Interim MD&A. The AIF and the Interim MD&A are incorporated by reference into the Prospectus and this Prospectus Supplement, respectively.
THE ACQUISITION
On September 4, 2015, the Corporation entered into the AMCo Purchase Agreement with the Vendors pursuant to which the Corporation has agreed to acquire all of the outstanding shares in the capital of AMCo from the Vendors. The Purchase Price for the Acquisition is expected to be equal to approximately US$3.3 billion, comprising (i) approximately £800 million (approximately US$1.2 billion) in cash; (ii) 8,490,000 Consideration Shares (with a value of approximately US$0.7 billion); and (iii) the repayment of AMCo’s existing senior secured facilities in the respective principal amounts of £581 million and €440 million (approximately US$1.4 billion in the aggregate) plus accrued interest and related cross-currency swaps. In addition, pursuant to the AMCo Purchase Agreement, Concordia will pay
S-22
the Vendors (i) an amount of £272,801 (approximately US$414,000) accruing daily from June 30, 2015 to the completion of the Acquisition, and (ii) to the extent earned, a cash earn-out in a maximum amount of £144 million (approximately US$220 million) calculated with reference to the future gross profit of the AMCo group over a period of 12 months from October 1, 2015. The US dollar amounts and the value of the Consideration Shares set forth above are based on the GBP/US$ and EUR/US$ foreign exchange rates, and the closing price of the Common Shares on the TSX, at September 4, 2015. On the Acquisition Closing, Concordia will procure, on behalf of AMCo, the redemption of the capital loan notes held by certain Vendors and the payment of certain other amounts, all of which will be deducted from the Total Cash Consideration. In addition, pursuant to the AMCo Purchase Agreement, if the Corporation acquires certain specified pharmaceutical products from a specified third party introduced to the Corporation by certain of the Vendors within 12 months of the Acquisition Closing, the Corporation will pay to the Vendors an additional US$72 million.
Completion of the Acquisition is subject to certain closing conditions. The Acquisition will be financed through the net proceeds of the Offering and certain debt financings described in this Prospectus Supplement. In addition, a portion of the Purchase Price will be satisfied through the issuance of the Consideration Shares.
DESCRIPTION OF THE AMCO BUSINESS
General Description of the AMCo Business
AMCo is an international specialty pharmaceutical company, owning a broad portfolio of branded and generic prescription products which are sold to wholesalers, hospitals and pharmacies in over 100 countries. AMCo specializes in the acquisition and development of hard-to-make, niche, off-patent prescription medicines in selected therapeutic areas. AMCo’s medicines are manufactured and sold through an out-sourced production and distribution network and marketed internationally through a combination of direct sales and local partnerships.
As of December 31, 2014, AMCo had a diversified product portfolio consisting of 180 molecules that represent a range of dosage strengths, formulations and geographic markets, covering a range of therapeutic categories, including endocrinology, ophthalmology and urology. As of June 30, 2015, AMCo’s product portfolio had increased to 190 molecules. For the year ended December 31, 2014, the largest molecule by revenue in AMCo’s portfolio accounted for less than 10% of total revenue, and the top ten molecules in AMCo’s portfolio accounted for less than 60% of total revenue.
AMCo focuses on the end of a typical pharmaceutical product lifecycle where the market has stabilized in terms of competitive landscape, pricing and volume, and prescribers remain loyal to the treatment regime. In many cases, AMCo’s products have prescription histories dating back decades, which reduces the risk of any new undetected side-effect profiles that could materially alter prescribing habits. AMCo’s top products benefit from stable demand and often have limited competition because the products are complex to manufacture and register with applicable drug product regulatory authorities, and face a low risk of innovation due to the off-patent stage of their life cycle. The figure below illustrates the various stages in the typical lifecycle of a pharmaceutical product and where AMCo’s products fit within this lifecycle.
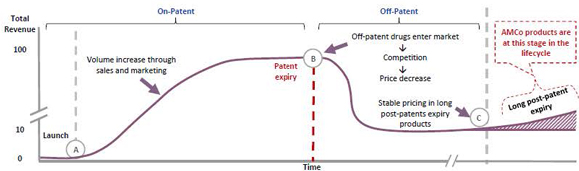
As at the date of this Prospectus Supplement, AMCo has a direct sales presence in the United Kingdom, Ireland, France, the Nordic region, the Benelux region and the MENA region, and a distribution network worldwide.
AMCo utilizes an asset-light business model focusing on the registration and regulatory maintenance of acquired, in-licensed and own-developed products. This model requires no basic research and minimal development spend. New products are acquired and in-licensed as well as developed by contract research organizations that are managed by an
S-23
AMCo in-house team, mainly to develop new formulations and dosage strengths of existing products. AMCo’s medicines are predominantly manufactured in Western Europe. AMCo’s operations are managed by a core management team in the United Kingdom and Ireland supported by commercial and regulatory teams in certain international markets and a Centre of Excellence in India that has operational functions ranging from regulatory, supply chain, medical marketing, customer service, information technology and finance.
History and Development
AMCo was created through the merger of Mercury Pharma Group Limited (“Mercury Pharma”) and Amdipharm International Holdings Limited (“Amdipharm”). Mercury Pharma was founded in the United Kingdom in 1989 and was listed on the London Stock Exchange from 1998 to 2009. In December 2009, Mercury Pharma was taken private by HgCapital LLP, a private equity investor, and subsequently acquired by Cinven in August 2012. Amdipharm was founded in the United Kingdom in 2002 as a part of Waymade Group, a privately-owned healthcare company, and subsequently acquired by CCM Pharma Limited, the holding company of Mercury Pharma, in October 2012. The merger between Mercury Pharma and Amdipharm became effective in December 2012 and the AMCo brand was subsequently launched in March 2013.
Throughout its history, AMCo has aimed to acquire other speciality pharmaceutical companies and products and cross-border rights to niche medicines in an effort to expand its portfolio and reach across various markets. AMCo has made a number of acquisitions during the period from 2013 through to 2015 as further described below.
In March 2013, AMCo acquired the worldwide rights to Fucithalmic, an ophthalmic gel containing fusidic acid for the topical treatment of bacterial conjunctivitis sold in over 90 countries, from LEO Pharma (“LEO Pharma”). As a part of the transaction, LEO Pharma will continue to manufacture the product in Denmark and Ireland for at least ten years. Over time, LEO Pharma will transfer over marketing authorizations to AMCo and AMCo’s third-party distributors.
In December 2013, AMCo acquired Abcur AB (“Abcur”), a specialty pharmaceutical company operating in the Nordic region that had previously acted as a distributor for a number of AMCo’s products including Diamox, a treatment for glaucoma.
On October 1, 2014, AMCo acquired Focus Pharmaceuticals (“Focus”), a specialty pharmaceutical company operating in the United Kingdom, thereby acquiring the rights to over 60 different molecules ranging across different types of formulations and therapeutic areas. Since the acquisition, Focus’s products have been sold globally by AMCo through its worldwide distribution network.
On June 2, 2015, AMCo acquired Primegen Limited (“Primegen”), a specialty pharmaceutical company based in the United Kingdom and its portfolio of five marketed products and pipeline of near-term products. Primegen sells a number of niche medicines such as Ciprofibrate and Dicycloverine, predominantly in the United Kingdom.
On July 31, 2015, AMCo acquired Boucher and Muir Pty Ltd (“BNM”), an Australian company founded in 1957 which sells specialist medical products in Australia, New Zealand and the Pacific Islands. BNM’s products range from vaccines to anesthetics, as well as more general products such as solid dose tablets and capsules. Previously, BNM had been one of AMCo’s main distributors in the region.
AMCo Business Divisions
AMCo’s operations are divided into two major business divisions, Direct markets and Distributor markets, which are the basis for its segment reporting in its financial results.
Direct markets
AMCo’s Direct markets division is engaged in direct sales through commercial teams in each geographic region that target public health organizations (for example, CCGs in the United Kingdom), key opinion leaders, wholesalers, pharmacies, and general and specialist physicians. For example, AMCo markets nitrofurantonin, an antibiotic used to treat urinary tract infections, directly to microbiologists.
S-24
The Direct markets division expanded in 2014 to include France, Ireland and the Benelux region, increasing the regions where AMCo has full control over its sales and marketing efforts. AMCo’s revenue from the Direct markets division increased from £182.5 million in 2013 to £232.3 million in 2014. For the year ended December 31, 2014, the Direct markets division accounted for 79.4% of AMCo’s revenue and included AMCo’s operations in the United Kingdom, Ireland, France, the Nordic region and the Benelux region.
As a result of organic growth and the acquisition of BNM, AMCo’s operations in the MENA region and Australia/New Zealand will be included in the Direct markets division in 2015.
Distributor markets
AMCo’s Distributor markets division is engaged in sales worldwide through distribution partnerships monitored both on the ground by an area or country manager and remotely from AMCo’s United Kingdom headquarters and/or its operations center in India. AMCo’s revenue from the Distributor markets division increased from £58.5 million in 2013 to £60.5 million in 2014. For the year ended December 31, 2014, the Distributor markets division accounted for 20.6% of AMCo’s revenue. The Distributor markets division in 2014 included AMCo’s operations in the Middle-East, the Americas, Asia-Pacific, Southern Europe, Eastern Europe and South Africa.
The following figure shows AMCo’s geographic presence as of December 31, 2014.
Key Markets and Marketing Strategy
The Corporation expects the global pharmaceutical market to grow over the next five years due to an aging population in developed countries and continued economic growth in developing countries. Additionally, the Corporation believes the off-patent market will grow as governments carry out cost cutting initiatives to curb high-priced patent protected products and shift volumes to off-patent products, which are typically lower-priced.
All of AMCo’s pharmaceutical products are off-patent medicines with well-established prescribing histories that are at the end of their product lifecycle. AMCo typically does not participate in areas where other pharmaceutical companies continue to invest significantly to find more effective treatments that will disrupt the market for existing medications when they are released, such as products relating to cancer or diabetes. Also, AMCo’s off-patent products generally do not face a “revenue cliff” from expiration of product patent or marketing exclusivity and are sold across developed and emerging market countries. AMCo’s diverse product portfolio consists of off-patent branded prescription products and niche, un-branded products in various oral, injectable and topical forms.
S-25
In 2014, sales to customers in the United Kingdom accounted for 64.0% of AMCo’s revenue while sales in over 100 other countries including France, Ireland, the Netherlands and Nordic countries accounted for the remaining 36.0%. AMCo’s revenue in the United Kingdom is derived mainly from un-branded drugs while the majority of its international revenue is from branded drugs, which enables AMCo to take advantage of the different market dynamics in various markets.
United Kingdom
The United Kingdom pharmaceutical industry is one of the largest pharmaceutical industries in Europe. The Corporation believes that there are favorable market drivers in the United Kingdom including population growth, an increase in the number of patients with chronic diseases, favorable demographics and increased life expectancy and government commitment. The off-patent pharmaceutical market in the United Kingdom has different regulatory schemes for branded and un-branded medicines. For un-branded products, the government has set a free pricing, self-regulating market designed to increase competition and drive costs down. For branded products, the Pharmaceutical Price Regulation Scheme (“PPRS”) established in 1952 controls the profits earned by pharmaceutical companies on their overall portfolio of branded products. The proportion of un-branded drugs sold in the United Kingdom pharmaceutical market is significantly higher than other markets. This is due to:
| | • | | the existence of national bodies, such as the National Institute of Health and Clinical Experience, that limit the availability of new patented drugs; |
| | • | | the high propensity of doctors to prescribe drugs on an International Nonproprietary Name basis driven by training and promotion by CCGs; and |
| | • | | the ability of pharmacists to substitute un-branded equivalent products from different manufacturers. |
Due to the legacy and history of AMCo, the United Kingdom remains its largest market. Most of AMCo’s products sold in the United Kingdom are niche un-branded products, in various oral, injectable and topical forms, that are not subject to PPRS but rather priced through market dynamics. AMCo has targeted strategies for three different categories of the United Kingdom’s pharmaceutical market: Specialty/Hard to make, Generics and Medicines optimisation.
Specialty/Hard to make
Due to the relatively old age of certain medicines and the corresponding manufacturing processes, AMCo considers such medicines to be hard to make and maintain. In this market, AMCo identifies, develops and markets niche, essential medicines and acts as the exclusive or semi-exclusive supplier of these products. These medicines can come in the form of unique formulations, dosages, delivery mechanisms and molecules. They are, however, often essential medicines that patients depend upon. AMCo sells products such as liothyronine, nitrofurantoin and levothyroxine within this category of the pharmaceutical market.
Generics
The United Kingdom’s generics market is one of the largest generics markets in the world. In this market, AMCo is able to leverage its “pure play” generic capabilities through the acquisitions of Focus and Primegen. AMCo’s generic franchise in the United Kingdom consists of products such as carbimazole, prochlorperazine and colchicine.
Medicines optimisation
Medicines optimisation is an official government policy designed to promote medicines through understanding the patient experience, utilizing an evidence-based model for the choice of medicines, ensuring medicine use is as safe as possible and making medicines optimisation part of routine practice. In this category, pharmaceutical companies such as AMCo can approach the Department of Health directly, or local procurement bodies individually, with certain products that fit this model. These products are typically affordable branded products, with direct patient benefits that are supported by the Department of Health as part of this medicines optimisation agenda. AMCo’s products in this market include, among others, Eltroxin, Pevanti, Zapain, Detrunorm and Macrobid. AMCo has also promoted products such as Morphgesic through this program.
S-26
International Markets
AMCo’s international sales are conducted across more than 100 developing as well as emerging market countries and have increased in revenue from £91.3 million in 2013 to £105.4 million in 2014. The international pharmaceutical market has historically directed marketing efforts to prescribers of its products, such as physicians and other healthcare professionals, instead of patients. As governments have relaxed regulation over pharmaceutical advertising, patients have increasingly become aware of new treatments and, as a result, patient demand for new prescription medicines has increased. Governments have also focused on reducing overall healthcare spending by cutting down the prices of pharmaceutical products, mainly with respect to on-patent drugs. Additionally, AMCo believes the number of prescriptions for un-branded pharmaceutical products from physicians is relatively lower in international markets as compared to the United Kingdom. The originator brand (the brand name that was used by the pharmaceutical company when the drug was on patent) is often perceived to be of higher quality due to the large amount spent on development, testing and marketing by the originator company while the drug was patent protected. As a result, there is loyalty and recognition attached to the branded pharmaceutical products. AMCo tactically promotes certain of its off-patent, branded products, directly or indirectly through distributors, in the international markets and has developed focused marketing teams in select international markets. The Corporation believes that AMCo will continue to benefit from these trends and build brand equity in the international market.
Products
AMCo retains a strong competitive position for its key products in its main markets. AMCo adopts a tailored growth strategy depending on the competitive and market dynamics for each of its key products. In 2014, no one molecule accounted for more than 10% of AMCo’s total revenue, and AMCo’s top ten molecules accounted for less than 60% of total revenue. AMCo’s top ten molecules in the United Kingdom (its largest market) accounted for less than 44% of total revenue. The following table and subsequent molecule descriptions provides an overview of AMCo’s top ten molecules during the year ended December 31, 2014 and illustrates the level of diversity across AMCo’s product portfolio:
| | | | | | | | | | | | |
AMCo’s Top 10 Molecules | |
Molecule | | Indication | | Sold in no. of
countries | | International
Rights | | FY 2014 Revenue
(£ million) | | % of FY 2014
Revenue | |
Levothyroxine Sodium | | Hypothyroidism | | 12 | | ü | | 28.7 | | | 9.8 | % |
| | | | | |
Nitrofurantoin | | Urinary tract infections | | 16 | | ü | | 26.9 | | | 9.2 | % |
| | | | | |
Prednisolone | | Inflammatory conditions | | 2 | | ü | | 21.0 | | | 7.2 | % |
| | | | | |
Carbimazole | | Hyperthyroidism | | 27 | | Defined
territories | | 17.4 | | | 5.9 | % |
| | | | | |
Liothyronine Sodium | | Severe hypothyroid states | | 9 | | ü | | 16.6 | | | 5.7 | % |
| | | | | |
Fusidic Acid | | Bacterial conjunctivitis | | 70 | | ü | | 15.8 | | | 5.4 | % |
| | | | | |
Erythromycin | | Bacterial infections | | 37 | | Defined
territories | | 13.4 | | | 4.6 | % |
| | | | | |
Codeine Phosphate and Paracetamol | | Moderate/severe pain | | 3 | | ü | | 12.0 | | | 4.1 | % |
| | | | | |
Biperiden Hydrochloride | | Parkinson’s disease | | 19 | | Defined
territories | | 6.9 | | | 2.3 | % |
| | | | | |
Tranylcypromine Sulphate | | Depression | | 11 | | ü | | 6.6 | | | 2.3 | % |
S-27
Levothyroxine Sodium
Levothyroxine Sodium treats patients with an underactive thyroid gland, which leads to a deficiency of the hormone thyroxine (a condition known as hypothyroidism). Hypothyroidism is treated by this daily treatment, which is continued and monitored for life.
Nitrofurantoin
Nitrofurantoin is used to treat bladder infections, such as cystitis and urinary tract infections. These are common infections where many antibiotics are used and, as a result, bacteria are becoming resistant to some of them. At this time, there is less resistance to nitrofurantoin. The product is used across all ages and can also be used to prevent bladder infections in vulnerable patients.
Prednisolone
Prednisolone is a powerful anti-inflammatory drug that is used to treat several inflammatory conditions. Inflammation can cause many long-term and acute illnesses, the most common of which are acute attacks of asthma or bronchitis. Other common conditions include inflammation of the joints and rheumatoid arthritis.
Carbimazole
The thyroid gland can become overactive and produce excess thryroxine, which leads to symptoms such as weight loss, increased heart rate and sweating. Carbimazole works to lower thyroid hormone production to normal levels. It is a treatment that is often taken for life and has to be monitored regularly.
Liothyronine Sodium
The deficiency of thyroxine is sometimes particularly severe and causes extreme symptoms (including coma) that requires a drug’s immediate onset. Liothyronine is therefore often a life saving and essential drug as it works quickly and is sometimes used in combination with levothyroxine.
Fusidic acid
Fusidic acid takes the form of a topical gel treatment for patients of all ages suffering with a bacterial infection of the surface lining of the front of the eye (a condition known as conjunctivitis). Conjunctivitis causes the eye to be red, painful and sticky, resulting in a considerable disability for patients and significant inconvenience for the carers of babies, children or the elderly suffering with this condition.
Erythromycin
Erythromycin is a commonly used antibiotic used to treat many types of bacterial infections, such as those of the throat, chest and skin. It is particularly used in patients known or suspected to be allergic to penicillin. Depending on the severity of the infection, erythromycin can be taken as tablets, capsules or given as an injection.
Codeine Phosphate and Paracetamol
Both codeine phosphate and paracetamol are well known and popular painkillers that work differently to relieve pain. Combining the two gives stronger pain relief and can be used for conditions of severe pain such as back pain, toothache, severe sprains and strains and pain after operations. Depending on the severity of the pain, there are a few possible doses that vary in the content of codeine.
Tranylcypromine sulphate
Tranylcypromine sulphate is used to treat forms of depression that are often long-term, severe and have not responded to other treatments. This drug can be essential as it is often the only one that works for certain patients.
S-28
Biperiden Hydrochloride
Biperiden hydrochloride is used alone or in combination with other drugs to treat Parkinson’s disease – a condition of shaky, involuntary movements and stiffness. The same symptoms can also occur as side effects to commonly used drugs and Biperiden Hydrochloride is often prescribed to manage these patients as well.
Launch of New Products
AMCo grows and diversifies its product portfolio through acquisitions and organic growth, pursuant to which AMCo has developed a pipeline of products, which consists of approximately 60 product launches over the next three years, mostly in the form of new dosages or formulations of existing drugs. This pipeline consists of reformulations or new dosages of existing products, which are developed in-house, the launch of existing products in new countries and in-licensed products that reach across AMCo’s various markets. In-licensed products include niche branded or un-branded products that are currently sold by other companies. AMCo identifies in-licensing opportunities and seeks to reach an agreement with these companies to allow AMCo to market the product in some or all geographic regions, typically in return for milestone payments and/or profit share agreements.
Reformulations and new dosages of existing products, as well as the launch of existing products in new countries, have a lower risk compared to typical pharmaceutical products due to the proven medical efficacy, lack of new product development risk and AMCo’s existing experience with the molecules. Strong market insight helps AMCo identify unmet customer needs in terms of dosage, packaging, formulations and ease of use. For example, in 2009, AMCo identified a market opportunity for a reformulation of levothyroxine, a tablet manufactured and marketed in various doses. AMCo launched an oral solution formulation of levothyroxine in 2012 in the United Kingdom and Ireland and recorded an increase in revenue from the new solution from £3.2 million in 2013 to £4.9 million in 2014.
Production and Supply
AMCo outsources all product manufacturing services to over 73 third-party CMOs in the United Kingdom and Europe. AMCo partners with well-known, reputable CMOs, such as Haupt Pharma, Cenexi, B Braun Medical UK and Aesica, among others. AMCo has maintained long-term relationships with its key suppliers, managed through its Irish hub. AMCo has purposefully concentrated its supplier base in Europe in order to be close to its manufacturers, which allows AMCo to more readily monitor the manufacturing process and reduce the risk of any supply disruptions.
Manufacturing AMCo’s products is typically challenging due to factors such as low microgram formulations or difficult biostability profiles. Technologies used to make the products often date back decades to the time regulatory approvals were obtained under previous regulatory regimes. Additionally, a number of AMCo’s products are non-solid oral dosage forms (such as creams, oral liquids and injectables) that require specialized manufacturing operations that are less prevalent.
Through a program of operational optimization initiatives, AMCo has improved its supplier network by hiring a senior supply chain manager, increasing the size of the operations management team and implementing business interruption insurance, a safety stock policy and a dual sourcing program for key products, which establishes secondary sources of finished dosage form and active pharmaceutical ingredients for key products. AMCo utilizes long-term supplier contracts and its products are distributed to customers via third-party distributors.
Centre of Excellence
AMCo operates a Centre of Excellence in India to support its main operations team in the United Kingdom and Ireland and its other teams globally. In 2014, the cost of the Centre of Excellence was equivalent to 1.4% of AMCo’s total revenue.
AMCo utilizes a balanced mix of employees in India with varied academic backgrounds to provide a full range of business services and support. Such services and support include:
| | • | | Commercial activities such as order processing, business intelligence, market analytics and marketing campaign development; |
| | • | | Regulatory and medical activities such as license management and document management; |
S-29
| | • | | Global operations activities such as planning, order processing and vendor management; |
| | • | | Strategic development activities such as regulatory filings and dossier reviews; |
| | • | | Finance activities such as financial reporting and consolidation; and |
| | • | | Human resources, information technology and other support functions. |
The key business functions of sales and marketing, supply chain and regulatory and medical affairs operate through a model whereby the team in India supports the core team based in the United Kingdom. Finance, human resources, information technology and facilities all operate from India with a support team based in the United Kingdom. Additionally, the Centre of Excellence operates an international sales and marketing team.
Sales and Distribution
AMCo’s products are sold and distributed through two models: the direct model and the indirect model. AMCo utilizes the direct model for sales within its Direct market division and utilizes the indirect model for sales within its Distributor market division. When AMCo sees fit based on strategic considerations, it will shift a region from the indirect model to the direct model.
Direct Model
AMCo has a direct sales presence and commercial teams in the United Kingdom, Ireland, France, the Nordic region, the Benelux region and, for 2015, the MENA region. Through this model, AMCo has full control over its sales and marketing efforts with the use of an in-house, on-the-ground commercial team that targets potential customers in specific segments of the local market. AMCo may shift to a direct sales model from an indirect sales model in additional international markets in the future to further drive growth in its existing product portfolio by utilizing a targeted sales force.
United Kingdom
AMCo’s products are delivered by CMOs to a pre-wholesaler who then further distributes the products to the wholesaler. AMCo utilizes short-line wholesalers as well as exclusive arrangements with wholesalers and distributors.
The United Kingdom market is managed by the management team based at AMCo’s office in London, England. Sales in the United Kingdom are handled directly through AMCo’s in-house sales team. AMCo’s product portfolio in the United Kingdom is its largest and is predominantly comprised of products such as Levothyroxine, Liothyronine and Nitrofurantoin. For the year ended December 31, 2014, AMCo’s revenue generated in the United Kingdom was £187.4 million.
Ireland
The AMCo country manager for Ireland is based in Dublin and manages a team of account managers that promotes certain medicines in the primary care field, such as Detrunorm, Fucithalmic, Macrobid and Codipar. AMCo also maintains a portfolio of non-promoted products that are passively sold in response to naturally occurring market demand. Physical distribution in Ireland is managed by third-party distributors.
France
The AMCo country manager for France is based in Paris and manages a team of account managers, each covering a major metropolitan area. The France team also includes one marketing executive and a contracted team of telesales personnel that markets to pharmacies. Marketing activity is particularly targeted for Esberiven, a product which is used to improve blood flow and appearance in “heavy legs”.
The product portfolio in France is comprised of Esberiven, sold both by prescription and over-the-counter in pharmacies, Neomercazole, sold to thyroid specialists, Fucithalmic, sold to primary care physicians, and a range of legacy products that are passively sold in response to naturally occurring market demand. Physical distribution in France is managed by a distributor who also manages the relationship with the French regulatory authority.
S-30
Nordic Region
The Nordic region is managed by the regional office in Helsingborg, Sweden, which makes sales through direct representatives in Sweden and Finland and through third-party agents in Norway and Denmark. The portfolio is predominantly comprised of the products obtained through a previous acquisition by AMCo, such as phenylephrine, dexamethasone, morphine, noradrenaline, sodium chloride and thiamine as well as Fucithalmic and the legacy Amdipharm portfolio.
Benelux Region
The area manager for the Benelux region is based in Amsterdam and manages a team of key account managers in the Netherlands and a third-party distributor in Belgium and Luxembourg. The product portfolio is comprised of Furabid, Parnate and Fucithalmic. Physical distribution is managed by a distributor that has depots in both the Netherlands and Belgium and maintain AMCo’s consignment stock.
MENA (Middle East and North African) Region
The regional business for the MENA region is managed from AMCo’s Dubai office. Importation and physical distribution is managed by local agents and AMCo employs direct sales teams in an expanding number of countries in the MENA region. AMCo divides the region into five areas: the Gulf, Saudi Arabia, Egypt/Sudan, Maghreb (Algeria/Morocco/Tunisia) and the Levant (Jordan/Lebanon/Iraq).
The Dubai office is comprised of the area general manager, the regulatory affairs manager, one office manager and houses the Gulf first line manager and hot desks for the United Arab Emirates team.
Indirect Model
In markets where AMCo uses an indirect model for sales and distribution, AMCo utilizes on-the-ground area/country managers. The area/country managers manage distributors, train and monitor third-party sales forces and identify portfolio optimisation opportunities. In certain markets, such as Turkey and Canada, AMCo manages distributors remotely and uses its marketing and/or distribution partner to drive sales.
Europe
The remaining markets in Europe that are not managed under the direct model are classified as “European Distributor Markets” and are broken down into the following regions.
Southern Europe
The Southern Europe region is managed by an area manager who is based in Rome and works closely with AMCo’s third-party commercial and distribution partners across Italy, Spain and Portugal. A key product in the area is Transact, which is a non-steroidal anti-inflammatory patch and actively marketed across Italy and Portugal by one of AMCo’s strategic partners. Currently, the majority of AMCo’s product portfolio in the area is comprised of brands with a stable user base.
Eastern Europe (Central and Eastern Europe/Commonwealth of Independent States/Russia)
The Eastern Europe region, which includes, CEE and CIS countries as well as Russia, is managed by an area manager based in Riga, Latvia who works closely with AMCo’s network of partners, which range from logistical organizations to pharmaceutical companies. The largest market for AMCo in the region is Turkey and AMCo’s product portfolio is distributed by a number of local companies. AMCo’s key product in Turkey is Fucithalmic. Following AMCo’s acquisition of the rights to Fucithalmic, AMCo was able to market its first product in Russia. In Poland, AMCo has a distribution agreement for the sale of Hygroton, an antihypertensive drug.
DACH (Germany, Austria and Switzerland)
The DACH region is currently managed by the Head of European Distributors. Germany is the largest market in the region and AMCo’s portfolio consists of a range of well-established, branded pharmaceutics. In Germany and Austria, products are distributed via a third-party logistical provider and promotion is not required given the well-established
S-31
nature of the products. In Switzerland, the product portfolio is licensed to two different partners who also manage local distribution and are responsible for the relationship with regulators as well as undertake limited promotion for a few select products.
Australia/New Zealand
The Australia/New Zealand region is managed by a local area general manager based in Sydney. The acquisition of BNM provided AMCo with the infrastructure to build upon its pre-existing capabilities in the region. AMCo partners with two distributors in New Zealand for Fucithalmic.
The product portfolio in Australia and New Zealand is comprised of both of the original Mercury and Amdipharm portfolios as well as the BNM portfolio. The BNM business has a portfolio supplying Australia, New Zealand and the South Pacific Islands.
Americas
The Americas region includes Canada as well as Latin America. The region is managed by an area manager based in Buenos Aires. The Latin American pharmaceutical patients and prescribers are brand loyal and AMCo is able to utilize its branded portfolio in this region. AMCo’s products are mainly distributed and marketed to the private sector through AMCo’s strategic alliances. The primary product sold by AMCo in Canada is Fucithalmic.
The largest product in AMCo’s Brazilian and Mexican portfolio is Akineton, which is used to treat Parkinson’s disease as well as tremors associated with the use of certain psychotics. Sales of Akineton have continued to grow in the region with minimal promotional efforts, which AMCo believes is due to the brand loyal nature of prescribers and patients in Brazil and Mexico.
Asia-Pacific
The Asia-Pacific region is managed by an area manager based in Hong Kong. AMCo’s product portfolio is distributed and marketed through a network of distributors that have been selected based on their product specific capabilities. A variety of branded pharmaceutical products are sold in the region, all of which stem from historical acquisitions from pharmaceutical companies. Similar to the Americas, prescribers and patients in the Asia-Pacific region are typically brand loyal with a preference to imported originator brands over local generics. Following AMCo’s acquisition of the rights to Fucithalmic, AMCo was able to market its first product in China. Fucithalmic is the only twice daily product in China for bacterial conjunctivitis.
South Africa
The South African portfolio consists of well-established brands which are imported, distributed and, in some cases, marketed by three strategic partners. The AMCo country manager is based in Johannesburg and works closely with all of AMCo’s partners. One of the products AMCo distributes in South Africa is Ecotrin, which is a low dose aspirin used for cardiac protection. Ecotrin is actively promoted to general practitioners and pharmacists as well as through certain direct-to-consumer activities by AMCo’s South African distributors.
Intellectual Property Matters
AMCo controls the intellectual property in the vast majority of its products, giving AMCo the option to launch its products in various geographies, to develop new formulations and to select its contract manufacturer(s) of choice. AMCo’s intellectual property rights include its brand names, trademarks worldwide, marketing authorizations, regulatory dossiers and manufacturing know-how. AMCo’s intellectual property portfolio has been built from product and company acquisitions over the past 25 years, including product acquisitions from large originator pharmaceutical companies. AMCo has successfully developed a number of these acquired products into new and more patient-friendly formulations. In addition, AMCo has a number of exclusive in-licensing arrangements with third party dossier development boutiques under which AMCo licenses territorial rights to strategic medicines which have, or are shortly to, come off patent. This in-licensing business has enabled AMCo to extend the breadth of its portfolio and make ‘Day 1’ generic launches on the first day after patent expiry.
S-32
Employees
AMCo’s senior management team has extensive experience in the pharmaceutical industry. As at December 31, 2014, AMCo had 118 full time employees at its United Kingdom headquarters who are engaged in commercial, operations, regulatory and medical, strategic development and other support units. As at December 31, 2014, there were 180 India-based employees at AMCo’s India Centre of Excellence, which constituted 54% of the total employee base at that time. In addition to the teams in the United Kingdom and India, as at December 31, 2014, AMCo employed 34 individuals in the rest of the world.
Regulatory Matters
AMCo’s regulatory matters are generally managed through established: (i) internal regulatory capabilities; and (ii) external third party providers. The internal team registers and maintains products with regulatory authorities across AMCo’s key markets. In some international markets, AMCo’s teams are assisted by local regulatory partners. Being primarily based in the United Kingdom, AMCo is subject to inspections by the UK regulatory authority, the Medicines and Healthcare products Regulatory Agency. As a marketing authorization holder, AMCo is required to comply with (among other things) the following EU regulations and guidelines:
| | • | | Good Manufacturing Practice, which ensures that medicinal products are consistently produced and controlled to the appropriate quality standards for their intended use and as required by the marketing authorization or product specification; |
| | • | | Good Distribution Practice, which ensures that products are consistently stored, transported and handled under suitable conditions as required by the marketing authorization or product specification; and |
| | • | | Good Pharmacovigilance Practice, which regulates the processes for monitoring the safety of medicines. |
Legal Proceedings
Focus, acquired by AMCo in October 2014, is involved in patent litigation with Novartis AG concerning Focus Voleze® Rivastigmine Transdermal Patches. Novartis AG commenced legal proceedings against Focus in August 2013, alleging that the Voleze® Rivastigmine Transdermal Patches infringed a Novartis AG patent. After a trial in London in March 2015, the High Court ruled that Novartis AG’s patent was invalid on two counts, including that the invention was obvious over an earlier published Novartis AG patent. Novartis AG is currently appealing this decision, which appeal AMCo expects to be heard in 15-18 months. AMCo estimates its financial exposure from an unfavourable decision to be in the range of £2-3 million, assuming AMCo is able to recover certain amounts from the vendors of Focus pursuant to indemnities granted in the purchase agreement. See “Risk Factors”.
ACQUISITION RATIONALE
Concordia believes the Acquisition is aligned with its strategy of pursuing accretive acquisitions of legacy pharmaceutical products. The Corporation believes the Acquisition is highly complementary to its existing business and is expected to result in the following significant strategic and financial benefits to Concordia:
| | • | | Strong Financial Returns - The Acquisition is expected to be accretive to both revenue and adjusted earnings per share; |
| | • | | Platform for International Expansion - The Corporation anticipates that AMCo will provide it with an international platform for continued expansion; |
| | • | | Commercial Footprint - AMCo conducts business in over 100 countries, which will significantly expand the Corporation’s geographical commercial reach after the Acquisition; |
| | • | | Diversification - AMCo will diversify the Corporation’s product portfolio by adding more than 190 complementary, niche pharmaceutical products that may present technical barriers to entry as they are difficult to manufacture and entail complex regulatory approval processes (88% of AMCo’s product portfolio is estimated to have two or fewer competitors), with no AMCo product representing more than 10% of AMCo’s total revenue in 2014; |
S-33
| | • | | Meaningful Operating Leverage - AMCo’s Indian operations provide a low-cost operations support centre that includes functions such as regulatory, supply chain, medical marketing, customer service, information technology and finance; |
| | • | | Complementary Business Strategy - AMCo’s flexible and asset-light business strategy, with an operating model based on the Indian operations support centre, third party manufacturers, and a limited marketing and promotion spend, is a strategic fit for Concordia and mirrors Concordia’s philosophy of focusing on niche life-cycle management opportunities for off-patent prescription drugs; |
| | • | | Opportunities for Organic Growth - The Acquisition is expected to present the Corporation with attractive growth opportunities through the continued optimization of AMCo’s existing portfolio and its pipeline consisting of over 60 product launches based on reformulations and new dosages; |
| | • | | Financial Scale for Future Acquisitions - The Acquisition is expected to enable the Corporation to focus its investments and resources on further growing its business by providing the financial scale to pursue future acquisitions and increase Concordia’s ability to compete for global, quality assets; |
| | • | | Improved Access to Capital - The Acquisition is expected to improve Concordia’s access to the capital markets as a result of enhanced size and diversification, as well benefit the Corporation’s credit profile by providing the Corporation with the ability to manage its indebtedness and de-lever over time; and |
| | • | | Experienced Management Team - The Acquisition provides the Corporation with a complementary team of senior executives that have extensive experience in the pharmaceutical industry. |
CONCORDIA FOLLOWING THE ACQUISITION
Competitive Strengths Following the Acquisition
Concordia believes that, subsequent to the completion of the Acquisition, the Corporation will possess a number of attributes and competitive advantages that should enable it to maintain and grow revenues and operating cash flow.
Sustainable Products Portfolio
Concordia’s portfolio pro forma for the Acquisition will consist of established, branded, off-patent and authorized generic products. The majority of the Corporation’s products and the products of AMCo have a long prescription history, an established prescriber base and a proven safety and efficacy profile. The United Kingdom has historically represented a large market for generic pharmaceutical products in Europe, with a value of approximately £6.5 billion in 2014 and forecasted growth of approximately 4% until 2019. Additionally, some of Concordia’s products benefit from barriers to entry such as complex formulation, manufacturing or regulatory challenges. The Corporation believes products with these characteristics will face a lesser degree of competition. Concordia believes that its products portfolio, when combined with the products of AMCo, will continue to provide a stable revenue base that may be supplemented through the acquisition of additional established brands and products.
Diversified Revenue Stream
Giving effect to the Acquisition, the Corporation’s product portfolio will be comprised of a total of over 190 products across several therapeutics areas. As a result, Concordia expects that the Acquisition will significantly increase its size and scale while reducing its reliance on any individual product. No single product will represent more than 10% of pro forma 2014 revenue. After giving effect to the Acquisition, Donnatal® would account for approximately 8% of 2014 pro forma total revenue, Lanoxin®, Eltroxin, and Macrobid would each represent 6% of 2014 pro forma total revenue and Predsol would account for approximately 4% of 2014 pro forma total revenue. The Corporation believes that this enhanced diversification will increase the expected stability of its aggregate revenue base going forward. In addition, AMCo has developed a pipeline of products which consists of approximately 60 product launches over the next three years mostly in the form of new dosages or formulations of existing drugs.
Scalable Business Model and Reliable Outsourcing
Concordia’s and AMCo’s partnerships with service providers, including CMOs, are expected to continue to enable the Corporation to produce and deliver quality, safe and reliable products without being exposed to the headcount and fixed costs of operating a pharmaceutical manufacturing facility. The Corporation believes that its limited fixed costs and capital investments together with its targeted development and promotional spending will continue to enable the Corporation to
S-34
maintain attractive Adjusted EBITDA margins and generate free cash flow. The acquisition of AMCo adds further capabilities as the Centre of Excellence in India provides operational functions ranging from regulatory, supply chain, medical marketing, customer service, information technology and finance that can be leveraged by Concordia.
Strong Financial Profile with High Margins and Cash Flow Generation
Concordia benefits from a high cash flow conversion profile. The Corporation’s cash flow generation can be attributed to attractive gross margins, controlled operating expenses and modest capital expenditures. The Corporation expects that the Acquisition will further enhance its ability to generate cash flow. For the twelve months ended December 31, 2014, the pro forma Adjusted EBITDA margin for the Corporation was 59%. The Corporation intends to employ its cash flow to manage its indebtedness and de-lever over time while opportunistically pursuing its acquisition-driven growth strategy to continue to enhance its product portfolio.
Track Record of Successfully Growing the Business through Acquisitions
The current Concordia management team has a demonstrated track record of successfully identifying, acquiring and integrating products and businesses in order to drive growth and realize synergies. Since 2013, the Corporation has successfully completed six acquisitions (not including the Acquisition) of varying size and complexity. Examples of these acquisitions include the additions of Zonegran® in September 2014 and Donnatal® in May 2014. These acquisitions contributed to Concordia’s growth in revenue of approximately 202% in 2014, from reported revenue of US$40 million in 2013 to US$122.2 million in 2014. The Acquisition is expected to increase the Corporation’s scale, earnings and cash flow which the Corporation believes will improve access to capital markets and the ability to manage its indebtedness and de-lever over time while opportunistically pursuing the Corporation’s acquisition-driven growth strategy.
Strong Management with Extensive Industry Experience
Certain members of the Corporation’s executive management team and Board have, on average, 15 years of pharmaceutical product acquisition and operational experience. The Corporation’s Chief Executive Officer, Mark Thompson, has spent his career in pharmaceutical mergers and acquisitions, having completed more than US$3.5 billion of product transactions (not including the Acquisition). As a result, Mr. Thompson, as well as other senior executives of Concordia, have a broad network within the industry. Certain members of the Corporation’s management team are also experienced in various aspects of pharmaceutical operations, including product development, clinical research, technology transfer, manufacturing, regulatory affairs, sales and marketing. The Acquisition of AMCo and its experienced senior management team is expected to be complementary to and enhance the Corporation’s management team.
DETAILS OF THE ACQUISITION
On September 4, 2015, the Corporation entered into the AMCo Purchase Agreement with the Vendors to acquire all of the outstanding shares in the capital of AMCo. The following is a summary of the material terms of the AMCo Purchase Agreement. This summary does not purport to be complete and is subject to, and is qualified in its entirety by reference to, the provisions of the AMCo Purchase Agreement, a copy of which has been filed on SEDAR at www.sedar.com.
The AMCo Purchase Agreement
On September 4, 2015, the Corporation entered into the AMCo Purchase Agreement with the Vendors pursuant to which the Corporation has agreed to acquire all of the outstanding shares in the capital of AMCo from Cinven (which may mean, depending on the context, any of or collectively, Cinven Group Limited, Cinven Partners LLP, Cinven (LuxCo1) S.A., Cinven Capital Management (V) General Partner Limited and each of their respective associates and/or funds managed or advised by the group) (collectively referred to herein as “Cinven”), members of AMCo management and other individual sellers. The Purchase Price for the Acquisition is expected to be equal to approximately US$3.3 billion, comprising (i) approximately £800 million (approximately US$1.2 billion) in cash; (ii) 8,490,000 Consideration Shares (with a value of approximately US$0.7 billion) (the amounts referred to in (i) and (ii) are collectively the “Implied Fixed Consideration”); and (iii) the repayment of AMCo’s existing senior secured facilities in the respective principal amounts of £581 million and €440 million (approximately US$1.4 billion in the aggregate) plus accrued interest and related cross-currency swaps. In addition, pursuant to the AMCo Purchase Agreement, Concordia will pay the Vendors (i) an amount of £272,801 (approximately US$414,000) accruing daily from June 30, 2015 to the
S-35
completion of the Acquisition, and (ii) to the extent earned, a cash earn-out in a maximum amount of £144 million (approximately US$220 million) calculated with reference to the future gross profit of the AMCo group over a period of 12 months from October 1, 2015. The US dollar amounts and the value of the Consideration Shares set forth above are based on the GBP/US$ and EUR/US$ foreign exchange rates, and the closing price of the Common Shares on the TSX, at September 4, 2015. On the Acquisition Closing, Concordia will procure, on behalf of AMCo, the redemption of the capital loan notes held by certain Vendors and the payment of certain other amounts, all which will be deducted from the Total Cash Consideration. In addition, pursuant to the AMCo Purchase Agreement, if the Corporation acquires certain specified pharmaceutical products from a specified third party introduced to the Corporation by certain of the Vendors within 12 months of the Acquisition Closing, the Corporation will pay to the Vendors an additional US$72 million. Pursuant to the AMCo Purchase Agreement, Concordia may use a special purpose vehicle for the purpose of implementing the Acquisition.
The Implied Fixed Consideration has been fixed under a “locked box” mechanism by reference to the consolidated financial condition of AMCo as of June 30, 2015. If, during the period from June 30, 2015 to the Acquisition Closing, any member of the AMCo group makes certain transfers of value to the Vendors, their affiliates or portfolio companies, including, among other things, by (i) paying or declaring dividends or other distributions, (ii) returning any of its share capital, (iii) transferring material assets, rights or other benefits, other than on arm’s length commercial terms, (iv) amending the terms of borrowing or indebtedness owed by it to a Vendor or any of its affiliates or portfolio companies, (v) creating an encumbrance over any of its assets in favour of or for the benefit of a Vendor or any of its affiliates or portfolio companies, (vi) making certain payments or (vii) waiving, deferring or forgiving amounts owed to it by a Vendor or any its affiliates or portfolio companies, such Vendors will be required to pay Concordia a cash amount equal to the amount of leakage so received plus interest. The Corporation has agreed to certain permitted leakages pursuant to the AMCo Purchase Agreement.
Warranties and Covenants
Certain AMCo management Vendors have given to Concordia customary warranties on the AMCo business and other matters, subject to customary exceptions and limitations, under a separate English law-governed warranty deed. Consequently, the Corporation will have no recourse against Cinven for breach of any warranties with respect to the AMCo business. The aggregate liability of the AMCo management vendors is subject to a maximum amount of £17.5 million, on a damages, rather than an indemnity basis. See “Risk Factors”. In addition, under the AMCo Purchase Agreement, Cinven and certain AMCo management Vendors have agreed to pre-completion covenants, including, among other things, to procure that the affairs of the AMCo group are conducted in the ordinary course of business and that all members of the AMCo group refrain from taking specifically enumerated actions. These commitments are subject to exceptions as set forth in the AMCo Purchase Agreement or Concordia’s approval of the taking of any such actions.
Under the AMCo Purchase Agreement, Concordia has agreed to carry out the business of AMCo in a commercially reasonable manner until the end of the earn-out period on September 30, 2016.
Conditions of Closing
The Acquisition Closing is subject to the TSX’s conditional approval of the listing of the Consideration Shares, which conditional approval has been received, the absence of any orders, judgments, decrees or injunctions preventing completion or making the Acquisition unlawful, and completion of the Acquisition not resulting in certain material violations of law or regulation. If the Acquisition Closing has not occurred on or before March 4, 2016, and if such outside closing date has not been extended by the parties, the AMCo Purchase Agreement will automatically terminate. On the Acquisition Closing, the Corporation will enter into the Governance Agreement described below, and a customary registration rights agreement with Cinven.
The Governance Agreement
In connection with the Acquisition, Cinven will receive, among other consideration, Consideration Shares representing approximately 19.9% of the outstanding Common Shares on a non-diluted basis immediately after the Acquisition (and prior to giving effect to the Offering). As a result, Concordia has agreed with Cinven that, concurrently with the completion of the Acquisition, it will enter into a governance agreement (the “Governance Agreement”) with Cinven. No Vendors other than Cinven will be a party to the Governance Agreement. The following is a summary of certain terms of the Governance Agreement, does not purport to be complete and is subject to, and qualified in its entirety by, reference to the terms of the Governance Agreement, a copy of which will be filed on SEDAR at www.sedar.com in connection with the Acquisition Closing.
S-36
Term
The Governance Agreement will terminate on the earlier of (i) the date upon which Cinven and its controlled affiliates no longer beneficially own at least 1% of the then outstanding Common Shares, and (ii) the date upon which any person completes any business combination or reorganization transaction where the shareholders of Concordia immediately prior to the completion of such transaction hold less than 50% of the equity securities in the capital of the entity resulting upon the completion of such transaction.
Transfer Restrictions - Restricted Period
Until the later of (i) January 31, 2016 and (ii) 90 days after the date of the Governance Agreement (the “Restricted Period”), Cinven may not directly or indirectly sell, offer to sell, pledge, or otherwise transfer any Common Shares, or enter into any instrument or arrangement that transfers the economic risk of ownership of any Common Shares, including short selling Common Shares or securities convertible into or exercisable or exchangeable for Common Shares. Despite these restrictions, Cinven may undertake the following limited transfers of Common Shares during the Restricted Period (“Permitted Transfers”):
| | (i) | transfers to Cinven entities that hold Common Shares and any controlled affiliate of Cinven as long as such transferee agrees to be bound by the Governance Agreement; |
| | (ii) | a transfer pursuant to a business combination or other similar transaction that has been approved and publicly recommended for acceptance by shareholders of Concordia by the Board; |
| | (iii) | a transfer pursuant to a take-over bid that meets certain criteria prescribed by the Governance Agreement; |
| | (iv) | a transfer to Concordia or a subsidiary of Concordia; or |
| | (v) | a transfer of Consideration Shares pursuant to certain registration rights granted by Concordia to Cinven. |
Transfer Restrictions - Post Restricted Period Transfers
After the Restricted Period, as long as Cinven holds at least 10% of the issued and outstanding Common Shares, Cinven collectively may not transfer more than 2% of the then issued and outstanding Common Shares in any calendar month, unless Cinven engages Concordia, in good faith, to seek to implement a marketed transfer of such Common Shares (whether by private placement or public offering). However, in any event, Cinven may transfer such Common Shares through a non-publicized pre-arranged off-exchange block trade using a securities dealer acceptable to Cinven and Concordia.
Transfer Restrictions - Block Transfers
Cinven may only transfer Common Shares representing 10% or more of the then issued and outstanding Common Shares in the aggregate to any one person and its affiliates with Concordia’s prior written consent (not to be unreasonably withheld) (i) after the Restricted Period and to persons on a list of certain pre-determined permitted transferees, or (ii) pursuant to a Permitted Transfer.
Transfer Restrictions - Prohibited Transfers
Cinven will not transfer any Common Shares to any Concordia competitor or to any activist investor as set forth on certain lists (as such lists may be adjusted from time to time).
Voting
As long as Cinven has the right to nominate a director to the Board, Cinven must use reasonable best efforts to cause all Common Shares and any other securities of Concordia held by it and its controlled affiliates not to be voted against, or
S-37
withheld from voting for, as applicable (i) persons nominated and publicly recommended to serve as directors of Concordia by management or the Board, and (ii) with respect to any other matter to be voted on by shareholders of Concordia, the public recommendation of the Board.
Despite the preceding paragraph, Cinven and its respective controlled affiliates may vote at their discretion in respect of (i) the issuance of equity securities in connection with any arrangement, business combination or other similar transaction, and (ii) any arrangement, business combination, or similar transaction of Concordia. However, Cinven may not publicly announce the manner in which they will vote without Concordia’s prior written consent. If such proposal has not been approved, or has been publicly rejected or not recommended for acceptance by Concordia shareholders by the Board, and while Cinven has the right to nominate a director to the Board, each of Cinven and their controlled affiliates are required to use reasonable best efforts to vote against such proposal.
Standstill
Until the first business day that Cinven and their respective controlled affiliates collectively beneficially own less than 5% of the then issued and outstanding Common Shares (on a non-diluted basis) (except in the case of (a) below, which shall be effective until none of Cinven and their respective controlled affiliates beneficially own any Common Shares), Cinven and their controlled affiliates will not, directly or indirectly, without the prior written consent or waiver of Concordia:
| | (i) | acquire, offer, seek to, agree to, or make a proposal to, acquire, any securities or rights to acquire securities of Concordia or any subsidiary, although this restriction does not apply if, immediately following such acquisition, the aggregate beneficial ownership of Common Shares of Cinven and their respective controlled affiliates, as a group, would not exceed the greater of (a) the initial percentage of Common Shares held by Cinven upon the issuance of the Consideration Shares, as such percentage may be lowered from time to time pursuant to any transfer (other than to Cinven or a controlled affiliate) and (b) 4.99% of the number of outstanding Common Shares on such date (on a non-diluted basis); and |
| | (ii) | undertake certain additional actions that may affect control of or which are hostile to Concordia (including, among others, offering to acquire assets of the Corporation; conducting any take-over bid, merger, business combination or other similar transaction; soliciting proxies; calling a meeting of shareholders or initiating a shareholder proposal). |
These standstill prohibitions will not apply to the activities of Cinven or any of its respective affiliates in connection with:
| | (i) | acquisitions made as a result of a share dividend, capital reorganization, or other like changes approved and/or publicly recommended by the Board; |
| | (ii) | acquisitions where Cinven (or its affiliates) acquire a previously unaffiliated business entity that beneficially owns securities of the Corporation, as long as those securities are divested within a prescribed period of time and, prior to such divestment, continue to be voted in accordance with the requirements of the Governance Agreement; |
| | (iii) | Permitted Transfers; or |
| | (iv) | hedging activities involving index-linked instruments, provided that securities of Concordia represent not more than 5% of the underlying index. |
Board Nomination Right
As long as Cinven holds at least an aggregate of 10% or more of the then issued and outstanding Common Shares (on a non-diluted basis), Concordia agrees to use commercially reasonable efforts to (i) nominate to the Board one nominee selected by Cinven, who shall be acceptable to the Board and legally eligible to act as a director (the “Cinven Nominee”), (ii) recommend that Concordia’s shareholders vote in favour of or consent to the election of (or against the removal of, as the case may be) the Cinven Nominee as a director, and (iii) cause all properly completed proxies regarding the election or removal of directors whereby the proxyholder is a management appointee of Concordia to be voted at the relevant time in the manner specified in such proxies.
S-38
Concordia has agreed to discuss the transfer and assignment of Cinven’s right to nominate a person for election to the Board to bona fide proposed purchasers of Common Shares held by Cinven, provided that the proposed purchaser acquires at least 10% of the issued and outstanding Common Shares, and provided further that such proposed purchase is not made by an activist investor or a competitor of Concordia as set forth on certain lists (as such lists may be adjusted from time to time).
Financing the Acquisition
The Acquisition will be financed through the net proceeds of the Offering and debt financing. In addition, a portion of the Purchase Price will be satisfied through the issuance of the Consideration Shares.
To complete the debt financing, the Corporation has entered into a commitment letter (the “Commitment Letter”) with Goldman Sachs Lending Partners LLC, Goldman Sachs Bank USA, Credit Suisse Securities (USA) LLC, Credit Suisse AG, Cayman Islands Branch, Jefferies Finance LLC and a Canadian chartered bank affiliate of RBC Dominion Securities Inc. (collectively, the “Commitment Parties”) pursuant to which the Commitment Parties and other lenders will (i) make secured term loans in the aggregate amounts of up to US$1.1 billion in one tranche and £500 million in a separate tranche, in each case available to the Corporation on the Acquisition Closing Date (collectively, the “Term Loans”) and (ii) make secured revolving loans in the aggregate outstanding principal amount of up to US$200 million available to the Corporation on or after the Acquisition Closing Date (the “Revolver” and, collectively with the Term Loans, the “Bank Loans”). The Term Loans will be used by the Corporation to fund payment of a portion of the Purchase Price, repay existing indebtedness owing by the Corporation and AMCo and pay transaction costs and the Revolver will be used by the Corporation for working capital and other general corporate purposes. All obligations of the Corporation under the Bank Loans, subject to certain customary exceptions, will be guaranteed by all material subsidiaries of the Corporation and secured by first priority (subject to permitted liens) perfected security interests in the assets of the Corporation and the assets of and equity interests in its material subsidiaries.
In addition to the Bank Loans, the Commitment Parties have agreed to provide the Corporation on the Acquisition Closing Date (i) a senior unsecured bond bridge facility (the “Bond Bridge Facility”) in an aggregate principal amount of up to US$790 million less the aggregate gross proceeds provided by any senior unsecured notes and gross proceeds in excess of US$700 million provided by any new equity issued by the Corporation, including the Offered Shares to be issued under the Offering, on or before the Acquisition Closing Date, (ii) a senior unsecured equity bridge facility (the “Equity Bridge Facility”) in an aggregate principal amount of up to US$700 million less the aggregate amount of any new equity issued by the Corporation, including the Offered Shares to be issued under the Offering, on or before the Acquisition Closing Date and (iii) a senior unsecured backstop bridge facility in an aggregate principal amount of up to US$881 million (the “Backstop Bridge Facility”). The proceeds of the Bond Bridge Facility and Equity Bridge Facility, if any, will be used to fund payment of a portion of the Purchase Price, repay existing indebtedness owing by the Corporation and pay transaction costs. The proceeds of the Backstop Bridge Facility will be used to fund the purchase of the Corporation’s existing senior notes if such senior notes are required to be purchased by the Corporation on the Acquisition Closing Date. All obligations of the Corporation under the Bond Bridge Facility, the Equity Bridge Facility and the Backstop Bridge Facility will be guaranteed by all material subsidiaries of the Corporation.
The availability of the Bank Loans, the Bond Bridge Facility, the Equity Bridge Facility and the Backstop Bridge Facility is subject to the completion of definitive documentation which will contain customary representations and warranties and restrictive covenants for facilities of this nature.
Subject to market conditions, the Corporation intends to raise up to US$890 million by way of a private placement of debt securities, in which case no loans will be drawn under the Bond Bridge Facility at the Acquisition Closing. There can be no assurances that the Corporation will be able to issue and sell such debt securities in the manner contemplated or that such private placement or the Offering will be completed.
Prior Valuations
Neither the Corporation nor AMCo has obtained, within the last 12 months, a valuation opinion required by securities legislation or a Canadian exchange or market to support the consideration to be paid pursuant to the Acquisition.
Parties to the Acquisition
The Acquisition does not involve an informed person, associate or affiliate of the Corporation.
S-39
CONSOLIDATED CAPITALIZATION
The following table sets forth the Corporation’s cash and cash equivalents and capitalization at June 30, 2015, on an (i) actual basis, (ii) as adjusted basis to give effect to the Offering, and (iii) as adjusted basis to give effect to the Offering, the Acquisition, a private placement of debt securities of US$890 million and the Bank Loans. The Bank Loans will be used by the Corporation to fund a portion of the cash portion of the Purchase Price. The following table should be read in conjunction with the Interim Financial Statements and Annual Financial Statements that are incorporated by reference into this Prospectus Supplement.
| | | | | | | | |
Designation | | Authorized | | As at June 30, 2015 | | As at June 30, 2015, after giving effect to the Offering(1) | | As at June 30, 2015, after giving
effect to the Offering(2), the
Acquisition, a private placement
and the Bank Loans(3) |
Cash and Cash Equivalents | | N/A | | US$140,207,000 | | US$[●] | | US$[●] (1) |
Long Term Debt | | N/A | | US$1,264,387,000(4) | | US$[●] (4) | | US$[●] (2)(4) |
Purchase Consideration Payable | | N/A | | US$31,540,000 | | US$[●] | | US$[●] (5) |
Share Capital | | Unlimited Common Shares | | US$538,310,000 (33,876,115 Common Shares) | | US$[●] ([●]Common Shares) | | US$[●] (2)(5) ([●] Common Shares) |
Notes:
| (1) | There is a risk that the Corporation may not complete the Acquisition. See “Risk Factors”. |
| (2) | After giving effect to the Acquisition (including the issuance of 8,490,000 Consideration Shares) but without giving effect to the exercise of the Underwriters’ Option and based on the issuance of an aggregate of [●] Offered Shares pursuant to the Offering for gross proceeds of US$[●] less the Underwriters’ Fee of US$[●] and the deduction of the expenses of the Offering, estimated to be approximately [●]. If the Underwriters’ Option was exercised in full, after giving effect to the Acquisition (including the issuance of 8,490,000 Consideration Shares) and such exercise of the Underwriters’ Option, based on the issuance of an aggregate of [●] Offered Shares for gross proceeds of US$[●] less the Underwriters’ Fees of US$[●] and the deduction of the expenses of the Offering, estimated to be approximately $[●], shareholders’ equity would be US$[●] ([●] Common Shares). |
| (3) | Assuming the Corporation raises US$890 million by way of a private placement of debt securities, draws down US$[●] under the Bank Loans and incurs debt issuance costs of US$[●]. Long-term debt is presented net of debt issuance costs. See“Details of the Acquisition - Financing the Acquisition”. |
| (4) | The Corporation’s long term debt transactions were completed in US$. |
| (5) | Based on exchange rates of US$1.00 = £0.6454 and US$1.00 = €0.8880, being the forward contract rates obtained by the Corporation, using an estimated Acquisition Closing Date of October 30, 2015. While the forward contract rate does not apply to any proceeds raised pursuant to any exercise of the Underwriters’ Option, such exchange rates have, for illustrative purposes, been used for converting any amounts into United States dollars described in the table above. See “Use of Proceeds”. |
There have been no material changes to the Corporation’s share and loan capitalization on a consolidated basis since June 30, 2015, except as set forth under the sections entitled“Prior Sales” and“Details of the Acquisition - Financing the Acquisition”.
PLAN OF DISTRIBUTION
Pursuant to the Underwriting Agreement, the Underwriters have severally agreed to purchase, as principals, and the Corporation has agreed to sell, subject to compliance with all necessary legal requirements and pursuant to the terms and conditions of the Underwriting Agreement, on the Closing Date, not less than all of the Offered Shares at the Offering Price, payable in cash to the Corporation against delivery of the Offered Shares.
The obligations of the Underwriters under the Underwriting Agreement are several and may be terminated at their discretion upon the occurrence of certain stated events, including in the event that: (a) there shall be any material change in the affairs of the Corporation, which, in the reasonable opinion of the Underwriters (or any of them), has or would be expected to have a significant adverse effect on the market price or value of the Common Shares; (b) (i) any inquiry, action, suit, investigation or other proceeding (whether formal or informal) is commenced, announced or threatened or any order made by any federal, provincial, state, municipal or other governmental department, commission, board, bureau, agency or instrumentality including, without limitation, the TSX or any securities regulatory authority or any law or regulation is enacted or changed which in the sole opinion of the Underwriters (or any of them), acting reasonably, could operate to prevent or materially restrict the trading of the Common Shares or materially and adversely affects or will materially and adversely affect the market price or value of the Common Shares; or (ii) if there should develop, occur or come into effect or existence any event, action, state, condition or major financial occurrence of national or international consequence or any law or regulation which in the sole opinion of the Underwriters seriously adversely affects, or involves, or will seriously adversely affect, or involve, the financial markets or the business, operations or affairs of the Corporation and certain of its subsidiaries taken as a whole; (c) the Corporation is in breach
S-40
of any material term, condition or covenant of the Underwriting Agreement or any material representation or warranty given by the Corporation in the Underwriting Agreement is or becomes false; and (d) any Underwriter and the Corporation agree in writing to terminate the Underwriting Agreement. In the event that one or more, but not all of the Underwriters exercises the right of termination herein, the other Underwriter(s) have the right, but are not obligated, to purchase all of the Offered Shares which would otherwise have been purchased by the Underwriter(s) which so terminated. The Underwriters are, however, obligated to take up and pay for all of the Offered Shares if any of the Offered Shares are purchased under the Underwriting Agreement.
In consideration of the agreement of the Underwriters to purchase the Offered Shares and for the services rendered by the Underwriters in connection with the Offering, the Underwriters will be paid an aggregate cash commission representing 3.75% of the gross proceeds of the Offering (including gross proceeds realized on the sale of Additional Offered Shares issuable upon the exercise of the Underwriters’ Option). The Offering Price was determined by negotiation between the Corporation and the Representatives, on behalf of the Underwriters.
The Corporation has granted the Underwriters the Underwriters’ Option, exercisable in whole or in part, at any time and from time to time, in the sole discretion of the Underwriters, up to 30 days after and including the Closing Date, to purchase up to [●] Additional Offered Shares at a price of US$[●] per Additional Offered Share, to cover sales by the Underwriters of a greater number of Common Shares than the number set out above, if any. The Additional Offered Shares issuable upon exercise of the Underwriters’ Option are hereby qualified for distribution under this Prospectus Supplement. A person who acquires Additional Offered Shares acquires such Additional Offered Shares under this Prospectus Supplement. If the Underwriters’ Option is exercised in full, the total price to the public, Underwriters’ Fee and net proceeds to the Corporation (before payment of the expenses of the Offering) will be US$[●], US$[●] and US$[●], respectively.
Subscriptions for the Offered Shares will be received subject to rejection or allotment in whole or in part and the right is reserved to close the subscription books at any time without notice.
The Underwriters are entitled under the Underwriting Agreement to customary indemnification by the Corporation against certain liabilities (including liabilities under the U.S. Securities Act of 1933), and expenses.
Pursuant to the Underwriting Agreement, the Corporation has agreed that it will not, directly or indirectly, issue, announce an intention to issue, sell, or agree or offer to sell, any Common Shares or any securities convertible into or exchangeable for or exercisable to acquire Common Shares from the date of the Underwriting Agreement until 90 days after the Closing Date without the prior written consent of Goldman, Sachs & Co., on behalf of the Underwriters (which consent shall not be unreasonably withheld, delayed or conditioned), except in connection with: (i) the issuance and sale of the Additional Offered Shares (if the Underwriters’ Option is exercised); (ii) the grant or exercise of outstanding options or incentives and other similar issuances pursuant to the Corporation’s stock option plan, long term incentive plan and any other share compensation arrangements outstanding as of the date of the Underwriting Agreement, including, for greater certainty, the sale of any Common Shares issued thereunder and the issuance of Common Shares pursuant to outstanding compensation options and restricted share units granted to current and former directors and officers of the Corporation; (iii) the issuance of up to 5% of the outstanding Common Shares of the Corporation in connection with property, share and/or asset acquisitions, (iv) the issuance of Common Shares in connection with the Corporation’s prior purchase of its Specialty Healthcare Distribution Division; and (v) the issuance of an aggregate of 8,490,000 Consideration Shares pursuant to the Acquisition.
The Corporation has agreed to use its commercially reasonable efforts to cause certain directors and officers (as specified by Goldman, Sachs & Co.) of the Corporation and their respective associates to execute agreements, in favour of the Underwriters, agreeing not to, for a period ending on the date that is 90 days following the Closing Date, directly or indirectly: (i) offer, sell, contract to sell, transfer, assign, secure, pledge, lend or otherwise dispose of or deal with, whether through the facilities of a stock exchange, by private placement or otherwise, any Common Shares or other securities of the Corporation owned, directly or indirectly, by such officers or directors or their associates; or (ii) make any short sale, engage in any hedging transaction, or enter into any swap or other arrangement or agreement that transfers any of the economic consequences of ownership of any Common Shares or securities of the Corporation; or (iii) otherwise publicly announce any intention to do any of the restricted activities, whether through the facilities of a
S-41
stock exchange, by private placement or otherwise, unless (a) the prior written consent of Goldman, Sachs & Co. on behalf of the Underwriters (such consent not to be unreasonably withheld, delayed or conditioned) has been obtained, or (b) there is a take-over bid or similar transaction, or plan of arrangement or other business combination, involving a change of control of the Corporation generally made to all shareholders of the Corporation to which such persons will tender their Common Shares, and subject to such other customary exceptions as may be agreed by the Corporation and Goldman, Sachs & Co.
Until the distribution of the Offered Shares is complete, SEC rules may limit the Underwriters from bidding for and purchasing Common Shares. However, the Underwriters may engage in transactions that stabilize, maintain or otherwise affect the market price of the Common Shares, such as bids or purchases to peg, fix or maintain that price in accordance with Regulation M under the U.S. Exchange Act of 1934.
Pursuant to policy statements of certain Canadian provincial securities regulators, the Underwriters may not, throughout the period of distribution under this Prospectus Supplement, bid for or purchase Common Shares for their own account or for accounts over which they exercise control or direction. The foregoing restriction is subject to exceptions, provided the bid or purchase is not engaged in for the purposes of creating actual or apparent trading in, or raising the price of, the Common Shares. These exceptions include bid or purchases permitted by the by-laws and rules of applicable regulatory authorities and stock exchanges, including the Universal Market Integrity Rules for Canadian Marketplaces administered by the Investment Industry Regulatory Organization of Canada, relating to passive market making activities, and a bid or purchase made for and on behalf of a customer where the order was not solicited during the period of distribution. Subject to applicable laws, the Underwriters may, pursuant to the first mentioned exception, in connection with the Offering, over-allot or effect transactions intended to maintain the market price of the Common Shares at levels other than those which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time.
If the Underwriters create a short position in the Common Shares in connection with the Offering, i.e., if they sell more Offered Shares than are listed on the cover of this Prospectus Supplement, the Underwriters may reduce that short position by purchasing Common Shares in the open market. The Underwriters may also elect to reduce any short position by exercising the Underwriters’ Option to purchase Additional Offered Shares described above. Purchases of Common Shares to stabilize the price or to reduce a short position may cause the price of the Common Shares to be higher than it might otherwise be in the absence of such purchases. No representation is made as to the magnitude or effect of any such stabilization or other activities. The Underwriters are not required to engage in these activities.
After the Underwriters have made reasonable efforts to sell the Offered Shares at the Offering Price, the Underwriters may decrease the Offering Price for the Offered Shares and may further change the Offering Price from time to time to amounts no greater than the Offering Price. In the event the Offering Price of the Offered Shares is reduced, the compensation received by the Underwriters will be decreased by the amount that the aggregate price paid by the purchasers for the Offered Shares is less than the gross proceeds paid by the Underwriters for the Offered Shares. Any such reduction will not affect the proceeds received by the Corporation.
The Corporation has applied to list the Offered Shares on the TSX. Listing is subject to the Corporation fulfilling all of the listing requirements of the TSX. The Offered Shares will be listed on NASDAQ, upon official notice of issuance.
The Offering is being made concurrently in the United States and in all of the provinces of Canada on an underwritten basis for 100% of the Offered Shares offered hereunder. Goldman Sachs Canada Inc., RBC Dominion Securities Inc., and Credit Suisse Securities (Canada), Inc. in Canadian jurisdictions, and Goldman, Sachs & Co., RBC Capital Markets, LLC, Credit Suisse Securities (USA) LLC, and Jefferies LLC in the United States, as principals, conditionally offer the Offered Shares, subject to prior sale, if, as and when issued by Concordia and accepted by the Underwriters in accordance with the conditions contained in the Underwriting Agreement. Jefferies LLC is not registered to sell securities in any Canadian jurisdiction and, accordingly, will only sell Offered Shares in the United States.
The Offered Shares will be offered in each of the provinces of Canada through those Underwriters or their affiliates who are registered to offer the Offered Shares for sale in such provinces and such other registered dealers as may be designated by the Underwriters.
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”), an offer of shares to the public may not be made in that Relevant Member State, except that an offer of shares to the public may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
| | (i) | to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
S-42
| | (ii) | to fewer than 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive) subject to obtaining the prior consent of the relevant underwriter or underwriters nominated by the Corporation for any such offer; or |
| | (iii) | in any other circumstances falling within Article 3(2) of the Prospectus Directive, |
provided that no such offer of shares shall result in a requirement for the publication of a prospectus pursuant to Article 3 of the Prospectus Directive or any measure implementing the Prospectus Directive in a Relevant Member State and each person who initially acquires any shares or to whom an offer is made will be deemed to have represented, warranted and agreed to and with the Underwriters that it is a qualified investor within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive.
For the purposes of this provision, the expression an “offer of shares to the public” in relation to any shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares to be offered so as to enable an investor to decide to purchase, or subscribe for, the shares, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State, the expression Prospectus Directive means Directive 2003/71/EC (as amended, including by Directive 2010/73/EU) and includes any relevant implementing measure in each Relevant Member State.
In the case of any shares being offered to a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, such financial intermediary will also be deemed to have represented, acknowledged and agreed that the shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of shares to the public other than their offer or resale in a Relevant Member State to qualified investors as so defined or in circumstances in which the prior consent of the underwriters has been obtained to each such proposed offer or resale.
In the United Kingdom, this Prospectus Supplement is only addressed to and directed as qualified investors who are (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 (the “Order”); or (ii) high net worth entities and other persons to whom it may lawfully be communicated, falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). Any investment or investment activity to which this Prospectus Supplement relates is available only to relevant persons and will only be engaged with relevant persons. Any person who is not a relevant person should not act or relay on this Prospectus Supplement or any of its contents.
The shares may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32 of the Laws of Hong Kong) (“Companies (Winding Up and Miscellaneous Provisions) Ordinance”) or which do not constitute an invitation to the public within the meaning of the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (“Securities and Futures Ordinance”), or (ii) to “professional investors” as defined in the Securities and Futures Ordinance and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance, and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” in Hong Kong as defined in the Securities and Futures Ordinance and any rules made thereunder.
This Prospectus Supplement has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this Prospectus Supplement and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the Offered Shares may not be circulated or distributed, nor may the Offered Shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor (as defined under Section 4A of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”)) under Section 274 of the SFA, (ii) to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to conditions set forth in the SFA.
S-43
Where the Offered Shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor, the securities (as defined in Section 239(1) of the SFA) of that corporation shall not be transferable for 6 months after that corporation has acquired the shares under Section 275 of the SFA except: (i) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (ii) where such transfer arises from an offer in that corporation’s securities pursuant to Section 275(1A) of the SFA, (iii) where no consideration is or will be given for the transfer, (iv) where the transfer is by operation of law, (v) as specified in Section 276(7) of the SFA, or (vi) as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore (“Regulation 32”)
Where the Offered Shares are subscribed for or purchased under Section 275 of the SFA by a relevant person which is a trust (where the trustee is not an accredited investor (as defined in Section 4A of the SFA)) whose sole purpose is to hold investments and each beneficiary of the trust is an accredited investor, the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferable for 6 months after that trust has acquired the shares under Section 275 of the SFA except: (i) to an institutional investor under Section 274 of the SFA or to a relevant person (as defined in Section 275(2) of the SFA), (ii) where such transfer arises from an offer that is made on terms that such rights or interest are acquired at a consideration of not less than S$200,000 (or its equivalent in a foreign currency) for each transaction (whether such amount is to be paid for in cash or by exchange of securities or other assets), (iii) where no consideration is or will be given for the transfer, (iv) where the transfer is by operation of law, (v) as specified in Section 276(7) of the SFA, or (vi) as specified in Regulation 32.
The Offered Shares have not been and will not be registered under the Financial Instruments and Exchange Act of Japan (Act No. 25 of 1948, as amended), (“FIEA”). The Offered Shares may not be offered or sold, directly or indirectly, in Japan or to or for the benefit of any resident of Japan (including any person resident in Japan or any corporation or other entity organized under the laws of Japan) or to others for reoffering or resale, directly or indirectly, in Japan or to or for the benefit of any resident of Japan, except pursuant to an exemption from the registration requirements of the FIEA and otherwise in compliance with any relevant laws and regulations of Japan.
In the ordinary course of their various business activities, the Underwriters and their respective affiliates, officers, directors and employees may purchase, sell or hold a broad array of investments and actively trade securities, derivatives, loans, commodities, currencies, credit default swaps and other financial instruments for their own account and for the accounts of their customers, and such investment and trading activities may involve or relate to assets, securities and/or instruments of the Corporation (directly, as collateral securing other obligations or otherwise) and/or persons and entities with relationships with the Corporation. The Underwriters and their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such assets, securities or instruments and may at any time hold, or recommend to clients that they should acquire, long and/or short positions in such assets, securities and instruments.
USE OF PROCEEDS
Proceeds
The net proceeds to the Corporation from this Offering, after deducting the Underwriters’ Fee and expenses of the Offering, are estimated to be approximately US$[●], or approximately US$[●] if the Underwriters exercise the Underwriters’ Option in full. See“Plan of Distribution”.
Principal Purposes assuming Completion of the Acquisition
The Corporation intends to use the proceeds from the Offering to fund, in part: (i) the Purchase Price; and (ii) the fees and expenses incurred in connection with the Acquisition.
S-44
The following table illustrates the estimated uses of the proceeds of the Offering.
| | | | |
Use of Proceeds | | Amount(1) | |
Partial satisfaction of the Purchase Price(2) | | | US$[● | ]3) |
Partial satisfaction of fees and expenses incurred in connection with the Acquisition | | | US$[● | ](3) |
| | | | |
Net proceeds to the Corporation | | | US$[● | ] |
Notes:
| (1) | Without giving effect to the exercise of the Underwriters’ Option. |
| (2) | The Purchase Price will be financed through the net proceeds of the Offering and the debt financings described herein. In addition, a portion of the Purchase Price will be satisfied through the issuance of the Consideration Shares. The partial satisfaction of the Purchase Price attributable to the proceeds of the Offering in GBP is [●], based on an exchange rate of US$1.00 = £0.6454, being the forward contract rate obtained by the Corporation, using an estimated Acquisition Closing Date of October 30, 2015. See“Details of the Acquisition - Financing the Acquisition”. |
| (3) | Based on an exchange rate of US$1.00 = £0.6454 and US$1.00 = €0.8880, being the forward contract rates obtained by the Corporation, using an estimated Acquisition Closing Date of October 30, 2015. |
In the event the Underwriters’ Option is exercised, any additional net proceeds will be allocated to the partial satisfaction of the Purchase Price and/or general corporate purposes.
The Offering is denominated in US dollars. The Purchase Price is payable in GBP and EUR, and certain debt being assumed pursuant to the Acquisition is denominated in GBP and EUR. To manage this foreign exchange risk the Corporation will be borrowing GBP denominated loans under the Bank Loans. In addition, the Corporation has entered into unsecured contingent forward foreign exchange contracts to hedge its US$ exposure to exchange rate fluctuations relative to GBP and EUR between now and the Acquisition Closing Date. The Corporation intends to use the US dollars received from the Offering and from US dollar denominated loans under the Bank Loans to satisfy its US dollar denominated obligations under these contingent forward foreign exchange contracts, and receive GBP and EUR amounts determined at agreed fixed exchange rates in order to pay these foreign currency denominated obligations payable on completion of the Acquisition Closing. As a result, the Corporation should not be exposed to material foreign exchange risk relative to its obligations under the AMCo Purchase Agreement between now and Acquisition Closing. See“Risk Factors”.
Principal Purposes if the Acquisition is not Completed
In the event that the Acquisition Closing does not occur, the net proceeds from the Offering of US$[•] (before deducting expenses of the Offering) will initially be added to the Corporation’s working capital and will subsequently be applied to fund future acquisitions in furtherance of the Corporation’s business plan and for general corporate purposes and, with available cash on hand, to potentially repay certain debt obligations of the Corporation. Other than the Acquisition, the Corporation does not currently have an intention to use the net proceeds of the Offering for any specific acquisition. See “Cautionary Note Regarding Forward-Looking Information”, “Plan of Distribution” and “Risk Factors”. From time to time, the Corporation evaluates various potential acquisition opportunities, some of which could, if consummated, have a material impact on the Corporation. There can be no assurance that the Corporation will be able to identify acquisition opportunities that meet its strategic objectives, or to the extent such opportunities are identified, that it will be able to negotiate acquisition terms that are acceptable to it or complete any transaction. No commitments have been made with respect to any transactions. However, the Corporation cannot preclude the possibility that agreement on one or more acquisition transactions will be reached in the weeks or months following the Closing Date. As noted above, if the Acquisition Closing does not occur, all or a portion of the net proceeds of the Offering received by the Corporation may be allocated to effect acquisitions.
In the event the Underwriters’ Option is exercised and the Acquisition is not completed, any additional net proceeds will be used for working capital and general corporate purposes.
The Corporation intends to use the funds available to it as stated in this Prospectus Supplement; however, there may be circumstances where, for sound business reasons, a reallocation of funds may be deemed prudent or necessary. See“Risk Factors - Risks Relating to the Offering”.
DESCRIPTION OF COMMON SHARES
The authorized capital of the Corporation consists of an unlimited number of Common Shares. As of September18, 2015,34,346,876Common Shares were issued and outstanding.
There are no special rights or restrictions attached to the Common Shares. The Common Shares rank equally as to all benefits which might accrue to the holders thereof, including the right to receive dividends out of monies of the Corporation properly applicable to the payment of dividends if and when declared by the Board and to participate rateably in the remaining assets of the Corporation in any distribution on a dissolution or winding-up. There are no provisions restricting the issuance of Common Shares or any other material restrictions.
S-45
All shareholders are entitled to receive a notice of all meetings of shareholders to be convened by the Corporation. At any general meeting, on a show of hands every shareholder who is present in person or by proxy and entitled to vote has one vote, and on a poll, every shareholder who is entitled to vote has one vote for each Common Share held and may exercise such vote either in person or by proxy.
Book-Based System
Delivery and Form
Offered Shares will be issued in the form of fully-registered global Offered Shares (the “Global Offered Share Certificates”) held by, or on behalf of, The Depository Trust Company (“DTC”) as custodian for its participants (“DTC Participants”), or recorded electronically through the facilities of DTC.
Except in limited circumstances, all Offered Shares will be represented in the form of Global Offered Share Certificates registered in the name of DTC or its nominee or will be delivered electronically. Purchasers of Offered Shares represented by a Global Offered Shares Certificate or recorded electronically will not receive definitive Offered Shares in fully-registered form (“Definitive Offered Shares”). Rather, the Offered Shares will be represented only in “book-entry only” form (unless the Corporation, in its sole discretion, elects to prepare and deliver Definitive Offered Shares). Beneficial interests in the Offered Shares, constituting ownership of the Offered Shares, will be represented through book-entry accounts of institutions (including the Underwriters) acting on behalf of beneficial owners, as direct and indirect DTC Participants. Each purchaser of an Offered Share represented by a Global Offered Share Certificate or recorded electronically will receive a customer confirmation of purchase from the Underwriter or registered dealer from whom the Offered Share is purchased in accordance with the practices and procedures of the selling Underwriter or registered dealer. The practices of registered dealers may vary, but generally customer confirmations are issued promptly after execution of a customer order. DTC will be responsible for establishing and maintaining book-entry accounts for its DTC Participants having interests in Offered Shares.
If DTC notifies the Corporation that it is unwilling or unable to continue as depository in connection with the Offered Shares, or if at any time DTC ceases to be a clearing agency or otherwise ceases to be eligible to be a depository and the Corporation is unable to locate a qualified successor, or if the Corporation elects, in its sole discretion, to terminate the book-entry system, beneficial owners of Offered Shares represented by Global Offered Share Certificates or recorded electronically at such time will receive Definitive Offered Shares.
Transfers of ownership of Offered Shares must be effected through a DTC Participant, directly or indirectly, which includes CDS Clearing and Depository Services Inc., securities brokers and dealers, banks and trust companies. All rights of shareholders who hold Common Shares in DTC must be exercised through, and all payments or other property to which such shareholders are entitled, will be made or delivered by DTC or the DTC Participant through which the shareholder holds such Common Shares. A holder of a Common Share recorded electronically will not be entitled to a certificate or other instrument from the Corporation or the Corporation’s transfer agent evidencing that person’s interest in or ownership of Common Shares, nor, to the extent applicable, will such holder be shown on the records maintained by DTC, except through an agent who is a DTC Participant, directly or indirectly.
Transfer and Exchange of Offered Shares
Transfers of beneficial ownership in Offered Shares represented by Global Offered Share Certificates or recorded electronically will be effected through records maintained by DTC for such Offered Shares or its nominees (with respect to interests of DTC Participants) and on the records of DTC Participants (with respect to interests of persons other than DTC Participants). Unless the Corporation elects, in its sole discretion, to prepare and deliver Definitive Offered Shares, beneficial owners who are not DTC Participants in the depository’s book-entry system, but who desire to purchase, sell or otherwise transfer ownership of or other interests in Global Offered Shares Certificates or recorded electronically, may do so only through DTC Participants in the depository’s book-entry system.
The ability of a beneficial owner of an interest in a Offered Share represented by a Global Offered Share Certificate to pledge such Offered Share or otherwise take action with respect to such owner’s interest in a Offered Share may be limited due to the lack of a physical certificate.
S-46
Neither the Corporation nor the Underwriters will assume any liability for: (i) any aspect of the records relating to the beneficial ownership of the Offered Shares held by DTC or its nominee or the payments relating thereto; (ii) maintaining, supervising or reviewing any records relating to the Offered Shares; or (iii) any advice or representation made by or with respect to DTC and contained in this Prospectus Supplement and relating to the rules governing DTC or any action to be taken by DTC or at the direction of a DTC Participant. The rules governing DTC provide that it acts as the agent and depositary for the DTC Participants. As a result, DTC Participants must look solely to DTC and beneficial owners of the Offered Shares must look solely to DTC Participants for any payments on the Offered Shares paid by, or on behalf of, the Corporation to DTC.
DIVIDEND POLICY
There are no restrictions in the Corporation’s articles preventing the Corporation from paying dividends. Any dividend to be approved by the Board may require third-party consents under the Corporation’s loan facilities. All of the Common Shares are entitled to an equal share in any dividends declared and paid. The Board will determine if, and when, dividends will be declared and paid in the future from funds properly applicable to the payment of dividends based on the Corporation’s financial position at the relevant time. See“Risk Factors” in this Prospectus Supplement, the accompanying Prospectus, and in the AIF.
Commencing during the six month period ended June 30, 2014, the Corporation has paid a quarterly dividend of US$0.075 on each Common Share. Future dividend decisions will consider the Corporation’s then-current business results, cash requirements and financial condition.
The Board has approved a dividend of US$0.075 per Common Share for the quarter ended September 30, 2015, with a record date of October 15, 2015. Payment will be made in US dollars, and the distribution of proceeds is expected on October 30, 2015. If the Closing and settlement of the Offering occurs prior to the record date, purchasers of Offered Shares who continue to hold such Common Shares on the record date of October 15, 2015 will be entitled to receive the dividend.
PRIOR SALES
Common Shares
The following table summarizes details of the Common Shares issued by the Corporation during the 12-month period prior to the date of this Prospectus Supplement:
| | | | |
Date of Issuance | | Price per Security | | Number of Securities |
February 20, 2015 | | US$3.00 | | 12,500(1) |
April 2, 2015 | | $11.50 | | 7,500(1) |
April 21, 2015 | | $85.00 | | 4,329,428(2) |
April 24, 2015 | | $14.95 | | 12,500(1) |
April 29, 2015 | | $34.30 | | 3,500(1) |
May 6, 2015 | | $86.68 | | 93(3) |
May 19, 2015 | | US$3.00 | | 100,000(1) |
May 19, 2015 | | $6.25 | | 87,500(1) |
May 19, 2015 | | $14.95 | | 85,000(1) |
May 19, 2015 | | $34.30 | | 10,000(1) |
May 26, 2015 | | US$3.00 | | 150,000(1) |
June 1, 2015 | | $82.67 | | 14,575(3) |
June 2, 2015 | | $11.50 | | 25,000(1) |
June 18, 2015 | | US$3.00 | | 100,000(1) |
June 26, 2015 | | $6.25 | | 62,500(1) |
July 2, 2015 | | US$3.00 | | 100,000(1) |
July 2, 2015 | | $91.77 | | 6,628(3) |
July 7, 2015 | | $90.89 | | 383(3) |
July 17, 2015 | | $11.50 | | 50,000(1) |
July 17, 2015 | | $34.30 | | 2,500(1) |
August 11, 2015 | | US$3.00 | | 100,000(1) |
August 17, 2015 | | $58.38 | | 6,250(1) |
August 18, 2015 | | $32.50 | | 2,500(1) |
August 18, 2015 | | $34.30 | | 2,500(1) |
August 19, 2015 | | $34.30 | | 10,000(1) |
August 19, 2015 | | $58.38 | | 1,250(1) |
August 25, 2015 | | $6.25 | | 125,000(1) |
September 2, 2015 | | $58.38 | | 1,250(1) |
S-47
Notes:
| (1) | Issued upon exercise of options by certain current or former, as applicable, directors and/or executives and/or employees of the Corporation and/or its subsidiaries. |
| (2) | Issued upon the automatic conversion of 4,329,428 subscription receipts issued pursuant to a short-form prospectus offering undertaken in connection with funding a portion of the purchase price of the Covis Acquisition. |
| (3) | Issued upon vesting of RSUs that were satisfied by delivery of Common Shares to certain current or former, as applicable, directors and/or executives and/or employees of the Corporation and/or its subsidiaries. |
Stock Options
The following table summarizes details of the stock options to purchase common shares issued or to be issued by the Corporation during the 12-month period prior to the date of this Prospectus Supplement:
| | | | |
Date of Issuance | | Price per Security | | Number of Securities |
February 11, 2015 | | $58.38 | | 50,000(1) |
May 25, 2015 | | $83.61 | | 200,000(1) |
May 26, 2015 | | $81.88 | | 89,735(1) |
August 14, 2015 | | $101.89 | | 90,000(1) |
August 17, 2015 | | $102.58 | | 20,000(1) |
Note:
| (1) | Issued to certain employees of the Corporation and/or its subsidiaries pursuant to the Corporation’s stock option plan. |
Restricted Share Units
The following table summarizes details of the RSUs issued or to be issued by the Corporation during the 12-month period prior to the date of this Prospectus Supplement:
| | | | |
Date of Issuance | | Price per Security | | Number of Securities |
February 13, 2015 | | N/A | | 1,000(1) |
April 22, 2015 | | N/A | | 1,009(2) |
May 15, 2015 | | N/A | | 50,965(2) |
May 26, 2015 | | N/A | | 201,441(2) |
June 23, 2015 | | N/A | | 6,179(2) |
July 27, 2015 | | N/A | | 2,936(2) |
July 31, 2015 | | N/A | | 244(2) |
Notes:
| (1) | Issued to a former non-executive director of the Corporation. These RSUs vested on February 27, 2015 and were paid out by the Corporation in cash in the amount of US$55,326.86. |
| (2) | Issued to certain directors and/or executives and/or employees of the Corporation and/or its subsidiaries pursuant to the Corporation’s long term incentive plan. |
Deferred Share Units
The Corporation did not issue any deferred share units under the Corporation’s long term incentive plan during the 12-month period prior to the date of this Prospectus Supplement.
TRADING PRICE AND VOLUME
The Common Shares are currently listed on the TSX under the trading symbol “CXR” and on NASDAQ under the trading symbol “CXRX”. On [●], 2015, the last trading day prior to the date of this Prospectus Supplement, the closing price of the Common Shares on the TSX was $[●] and on NASDAQ was US$[●], and on September 18, 2015, the last trading day prior to the announcement of the Offering, the closing price of the Common Shares on the TSX was $96.50 and on NASDAQ was US$72.23.
The following table sets forth the reported intraday high and low prices and the trading volume for the Common Shares on the TSX for the 12-month period prior to the date of this Prospectus Supplement.
| | | | | | | | | | | | |
| | | TSX(1) | |
Month | | High | | | Low | | | Volume | |
| | | $ | | | $ | | | | |
2014 | | | | | | | | | | | | |
September | | | 37.22 | | | | 32.81 | | | | 4,950,997 | |
October | | | 43.01 | | | | 33.42 | | | | 4,697,327 | |
November | | | 49.00 | | | | 40.20 | | | | 4,063,579 | |
December | | | 48.31 | | | | 41.34 | | | | 4,068,435 | |
2015 | | | | | | | | | | | | |
January | | | 55.01 | | | | 45.09 | | | | 4,559,993 | |
February | | | 66.11 | | | | 54.65 | | | | 4,524,810 | |
March | | | 91.16 | | | | 62.80 | | | | 9,483,728 | |
April | | | 104.74 | | | | 82.00 | | | | 6,639,113 | |
May | | | 91.05 | | | | 78.62 | | | | 5,551,987 | |
June | | | 97.26 | | | | 82.44 | | | | 6,799,897 | |
July | | | 107.15 | | | | 88.00 | | | | 4,400,652 | |
August | | | 109.77 | | | | 82.50 | | | | 5,251,716 | |
September 1-18 | | | 117.75 | | | | 91.25 | | | | 7,307,027 | |
Note:
| (1) | High and low price based on intraday high and low share prices. Source for data in the above table is TMX Group. Past performance should not be seen as an indicator of future performance. |
S-48
The following table sets forth the reported intraday high and low prices and the trading volume for the Common Shares on NASDAQ for the 12-month period prior to the date of this Prospectus Supplement.
| | | | | | | | | | | | |
| | | NASDAQ(1) | |
Month | | High | | | Low | | | Volume | |
| | | US$ | | | US$ | | | | |
2015 | | | | | | | | | | | | |
June 29-30 | | | 73.23 | | | | 70.72 | | | | 27,339 | |
July | | | 83.24 | | | | 68.54 | | | | 134,024 | |
August | | | 83.94 | | | | 51.25 | | | | 288,339 | |
September 1-18 | | | 89.10 | | | | 69.05 | | | | 152,684 | |
Note:
| (1) | High and low price based on intraday high and low share prices. Source for data in the above table is Bloomberg. Past performance should not be seen as an indicator of future performance. The Corporation commenced trading on NASDAQ on June 29, 2015. |
RELATIONSHIP BETWEEN THE UNDERWRITERS AND THE CORPORATION
On April 21, 2015, a group of lenders provided senior secured credit facilities to the Corporation in an aggregate principal amount of US$700 million comprising: (i) a senior secured revolving credit facility in an aggregate principal amount of US$125 million; and (ii) a senior secured term loan facility in an aggregate principal amount of US$575 million (collectively, the “Existing Bank Facilities”). Affiliates of RBC Dominion Securities Inc. and Credit Suisse Securities Canada Inc. are lenders under the Existing Bank Facilities and an affiliate of RBC Dominion Securities Inc. acted as administrative agent and collateral agent in connection therewith.
In connection with the Acquisition, the Corporation will refinance the Existing Bank Facilities and repay AMCo’s existing senior secured credit facilities. Currently, affiliates of RBC Dominion Securities Inc. and Credit Suisse Securities (Canada), Inc. are lenders to the Corporation pursuant to the Existing Bank Facilities. It is anticipated that affiliates of each of the Underwriters will be lenders under the Bank Loans contemplated to be provided to the Corporation pursuant to the Commitment Letter. It is anticipated that affiliates of the Underwriters will be lenders or initial purchasers under the Bond Bridge Facility and/or the Backstop Bridge Facility (or the debt securities issued in lieu thereof) contemplated to be provided to the Corporation pursuant to the Commitment Letter. Consequently, the Corporation may be considered to be a “connected issuer” of each of Goldman Sachs Canada Inc., RBC Dominion Securities Inc., and Credit Suisse Securities (Canada), Inc. under applicable Canadian securities laws. The Corporation has not yet been provided any financing under the Bank Loans contemplated in the Commitment Letter. It is anticipated that the Bank Loans will be issued on terms substantially similar to the Existing Bank Facilities. All obligations of the Corporation under the Existing Bank Facilities are secured, subject to certain customary limitations, by first priority perfected security interests in the assets of the Corporation and the assets of and equity interests in its material subsidiaries. As at September 18, 2015, the Corporation was indebted to a syndicate of lenders under the Existing Bank Facilities in the amount of US$554,928,569 (the carrying value under IFRS) or US$573,562,500 (the face amount less principal repayments). There has been no material adverse change in the financial position of the Corporation, and the value of the assets pledged as security for the Existing Bank Facilities have not materially changed since the indebtedness was incurred. The Corporation is not in default of any of its obligations to such lenders under the Existing Bank Facilities. Since the execution of the agreements relating to the Existing Bank Facilities, the lenders thereunder have not waived a breach on the Corporation’s part or on the part of the Corporation’s subsidiaries. In addition, affiliates of Jefferies LLC and Credit Suisse Securities (USA) LLC are lenders to AMCo under AMCo’s existing senior secured facilities.
The Underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include sales and trading, commercial and investment banking, advisory, investment management, investment research, principal investment, hedging, market making, brokerage and other financial and non-financial activities and services. Certain of the Underwriters and their respective affiliates have provided, and may in the future provide, a variety of these services to the Corporation and to persons and entities with relationships with the Corporation, for
S-49
which they received or will receive customary fees and expenses. Jefferies International Ltd., an affiliate of Jefferies LLC, is a sell side advisor for AMCo. Goldman, Sachs & Co. or certain of its affiliates has been retained by the Corporation as a financial advisor in connection with the Acquisition and, as such, will receive customary fees and expenses. Affiliates of the Underwriters have provided commitments to the Corporation in respect of the Bank Loans, the Bond Bridge Facility, the Equity Bridge Facility and the Backstop Bridge Facility and will receive customary fees and expenses in connection therewith. In addition, affiliates of each of the Underwriters will act as arrangers and bookrunners in respect of the Bank Loans, the Bond Bridge Facility, the Equity Bridge Facility and the Backstop Bridge Facility and in connection with any debt securities issued in lieu thereof. An affiliate of Goldman, Sachs & Co. will act as administrative agent under the Bank Loans.
The decision to issue the Offered Shares and the determination of the terms of the distribution of the Offered Shares were made through negotiation between the Corporation and the Representatives, on behalf of the Underwriters. The Canadian financial institutions of which Goldman Sachs Canada Inc., RBC Dominion Securities Inc. and Credit Suisse Securities (Canada), Inc. are an affiliate do not have any involvement in such decision or determination. As a consequence of the Offering, each of the Underwriters will receive its proportionate share of the Underwriters’ Fee and the affiliates of the Underwriters that are lenders under the Existing Bank Facilities and/or AMCo’s existing senior secured facilities may receive a portion of the proceeds from the financing contemplated by the Commitment Letter as a repayment of any indebtedness outstanding to them.
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
In the opinion of Fasken Martineau DuMoulin LLP, counsel to the Corporation, and Blake, Cassels & Graydon LLP, counsel to the Underwriters, the following summary describes the principal Canadian federal income tax considerations that generally apply to a purchaser who acquires Offered Shares as beneficial owner pursuant to the Offering and who, at all relevant times, for purposes of the Tax Act: (i) deals at arm’s length with the Corporation and the Underwriters; (ii) is not affiliated with the Corporation or the Underwriters; and (iii) holds the Offered Shares as capital property (a “Holder”). Generally, the Offered Shares will be capital property to a Holder provided the Holder does not acquire or hold them in the course of carrying on a business or as part of an adventure or concern in the nature of trade.
This summary is based on the current provisions of the Tax Act and the Regulations, and counsel’s understanding of the current administrative policies and assessing practices of the Canada Revenue Agency made publicly available prior to the date hereof. This summary takes into account all specific proposals to amend the Tax Act and the Regulations publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Proposed Amendments”) and assumes that all Proposed Amendments will be enacted in the form proposed. However, no assurances can be given that the Proposed Amendments will be enacted as proposed, or at all. This summary does not otherwise take into account or anticipate any changes in law or administrative policy or assessing practice whether by legislative, administrative or judicial action nor does it take into account tax legislation or considerations of any province, territory or foreign jurisdiction, which may differ from those discussed herein.
In general, amounts relevant to the computation of income under the Tax Act are reported in Canadian dollars. Accordingly, adjusted cost base, dividends and proceeds of disposition in respect of the Offered Shares must be converted into Canadian dollars generally based on the exchange rate quoted by the Bank of Canada at noon on the date each such amount arises.
This summary is of a general nature only and is not, and is not intended to be, legal or tax advice to any particular holder. This summary is not exhaustive of all Canadian federal income tax considerations. Accordingly, prospective purchasers of Offered Shares should consult their own tax advisors having regard to their own particular circumstances.
Holders Resident in Canada
This portion of the summary only applies to a Holder who, at all relevant times, for purposes of the Tax Act, is, or is deemed to be, resident in Canada (a “Resident Holder”). Certain Resident Holders who may not otherwise be considered to hold their Offered Shares as capital property may be entitled to make the irrevocable election permitted by subsection 39(4) of the Tax Act to have their Offered Shares (and all other “Canadian securities”, as defined in the Tax Act) owned by such Resident Holder in the taxation year in which the election is made and in all subsequent taxation years deemed to be capital property. Resident Holders whose Offered Shares might not otherwise be considered to be capital property should consult their own tax advisors. This portion of the summary does not apply to: (i) a purchaser that is a “specified financial institution”; (ii) a purchaser an interest in which would be a “tax shelter investment”; (iii) a purchaser that is, for purposes of certain rules (referred to as the mark-to-market rules) applicable to securities held by
��
S-50
financial institutions, a “financial institution”; (iv) a purchaser that reports its “Canadian tax results” in a currency other than Canadian currency; (v) a purchaser that is, or becomes as part of a transaction or event or series of transactions or events that includes the acquisition of Offered Shares, controlled by a non-resident corporation for the purposes of the foreign affiliate dumping rules in section 212.3 of the Tax Act, or (vi) a purchaser that has entered into or will enter into with respect to the purchaser’s Offered Shares, a “derivative forward agreement”, as defined in the Tax Act. Such purchasers should consult their own tax advisors.
Dispositions of Offered Shares
On the disposition or deemed disposition of an Offered Share (other than to the Corporation, unless purchased by the Corporation in the open market in the manner in which shares are normally purchased by any member of the public in the open market, in which case other considerations may arise), a Resident Holder will generally realize a capital gain (or a capital loss) equal to the amount by which the proceeds of disposition exceed (or are exceeded by) the aggregate of the Resident Holder’s adjusted cost base of the Offered Share and any reasonable costs of disposition. See “Tax Treatment of Capital Gains and Losses” below. The cost to a Resident Holder of an Offered Share will generally be the amount paid to acquire the Offered Share. The adjusted cost base to a Resident Holder of Offered Shares acquired pursuant to this Offering will be determined by averaging the cost of such Offered Shares with the adjusted cost base of all other Common Shares (if any) held by the Resident Holder immediately before the time of acquisition.
Dividends on Offered Shares
A Resident Holder will be required to include in computing its income for a taxation year any dividends received (or deemed to be received) on the Offered Shares. In the case of a Resident Holder that is an individual (other than certain trusts), such dividends will be subject to the gross-up and dividend tax credit rules that apply to taxable dividends received from taxable Canadian corporations, including the enhanced gross-up and dividend tax credit applicable to any dividends designated by the Corporation as “eligible dividends” in accordance with the provisions of the Tax Act. There may be limitations on the Corporation’s ability to designate its dividends on the Offered Shares as “eligible dividends”.
In the case of a Resident Holder that is a corporation, such dividends received or deemed to be received on Offered Shares held by the Resident Holder generally will be deductible in computing its taxable income. In certain circumstances, subsection 55(2) of the Tax Act (as proposed to be amended by Proposed Amendments released on July 31, 2015) will treat a taxable dividend received by a Resident Holder that is a corporation as proceeds of disposition or a capital gain.
A Resident Holder that is a “private corporation”, for the purposes of Part IV of the Tax Act, or any other corporation controlled, whether because of a beneficial interest in one or more trusts or otherwise, by or for the benefit of an individual (other than a trust) or a related group of individuals (other than trusts), will generally be liable to pay a refundable tax of 33 1⁄3% under Part IV of the Tax Act on dividends received (or deemed to be received) on the Offered Shares to the extent such dividends are deductible in computing the Resident Holder’s taxable income for the taxation year.
A dividend received by a Resident Holder who is an individual (other than certain trusts) on the Offered Shares may give rise to alternative minimum tax under the Tax Act, depending on the individual’s circumstances.
Tax Treatment of Capital Gains and Losses
Generally, a Resident Holder is required to include in computing its income for a taxation year one-half of the amount of any capital gain (a “taxable capital gain”) realized in the year. Subject to and in accordance with the provisions of the Tax Act, a Resident Holder is required to deduct one-half of the amount of any capital loss (an “allowable capital loss”) realized in a taxation year from taxable capital gains realized by the Resident Holder in the year. Allowable capital losses in excess of taxable capital gains realized in a taxation year may be carried back and deducted in any of the three preceding taxation years or carried forward and deducted in any subsequent taxation year against net taxable capital gains realized in such years subject to the detailed rules in the Tax Act.
The amount of any capital loss realized by a Resident Holder that is a corporation on the disposition of an Offered Share may be reduced by the amount of any dividends received (or deemed to be received) by the Resident Holder on such Offered Share or a share for which the Offered Share is substituted or exchanged to the extent and under the circumstances described by the Tax Act. Similar rules may apply where an Offered Share is owned by a partnership or trust of which a corporation, trust or partnership is a member or beneficiary. Such Resident Holders should consult their own advisors.
S-51
Capital gains realized by a Resident Holder who is an individual (other than certain trusts) may give rise to a liability for alternative minimum tax under the Tax Act.
If the Resident Holder is throughout the relevant taxation year a “Canadian-controlled private corporation” (as defined in the Tax Act), the Resident Holder may also be liable to pay a refundable tax on its “aggregate investment income”, which is defined in the Tax Act to include taxable capital gains.
Holders Not Resident in Canada
This portion of the summary only applies to a Holder who, at all relevant times, for purposes of the Tax Act, is not, and is not deemed to be, resident in Canada and does not use or hold and is not deemed to use or hold the Offered Shares in a business carried on in Canada (a “Non-Resident Holder”). Special rules, which are not discussed in this summary, may apply to a non-Canadian Holder that is an insurer that carries on an insurance business in Canada and elsewhere.
Dividends on Offered Shares
Dividends paid or credited on the Offered Shares or deemed to be paid or credited on the Offered Shares to a Non-Resident Holder will be subject to Canadian withholding tax at the rate of 25%, subject to any reduction in the rate of withholding to which the Non-Resident Holder is entitled under any applicable income tax convention. For example, under the Canada-U.S. Income Tax Convention (1980) (the “Convention”), as amended, where dividends on the Offered Shares are considered to be paid to or derived by a Non-Resident Holder that is the beneficial owner of the dividends and is a United States resident for the purposes of, and is entitled to full benefits in accordance with, the provisions of the Convention, the applicable rate of Canadian withholding tax is generally reduced to 15%.
Dispositions of Offered Shares
A Non-Resident Holder will not be subject to tax under the Tax Act on any capital gain realized on a disposition or deemed disposition of Offered Shares (other than to the Corporation, unless purchased by the Corporation in the open market in the manner in which shares are normally purchased by any member of the public in the open market, in which case other considerations may arise), unless the Offered Shares are “taxable Canadian property” of the Non-Resident Holder for purposes of the Tax Act and the Non-Resident Holder is not entitled to relief under an applicable income tax convention between Canada and the country in which the Non-Resident Holder is resident.
Generally, the Offered Shares will not constitute “taxable Canadian property” of a Non-Resident Holder at a particular time provided that the Offered Shares are listed at that time on a “designated stock exchange” for purposes of the Tax Act (which currently includes the TSX), unless at any particular time during the 60-month period that ends at that time: both: (i) (A) the Non-Resident Holder, (B) persons with whom the Non-Resident Holder does not deal with at arm’s length, (C) partnerships in which the Non-Resident Holder or a person described in (B) holds an interest directly or indirectly through one or more partnerships or (D) any combination of (A) to (C) owned 25% or more of the issued shares of any class or series of the capital stock of the Corporation; and (ii) more than 50% of the fair market value of the Offered Shares was derived directly or indirectly from one or any combination of: (a) real or immovable properties situated in Canada, (b) “Canadian resource properties” (as defined in the Tax Act), (c) “timber resource properties” (as defined in the Tax Act), and (d) options in respect of, or interests in, or for civil law rights in, property in any of the foregoing whether or not the property exists. Notwithstanding the foregoing, in certain circumstances set out in the Tax Act, Offered Shares may be deemed to be taxable Canadian property. Non-Resident Holders whose Offered Shares may constitute taxable Canadian property should consult their own tax advisors.
CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
This section describes the material United States federal income tax consequences of owning and disposing of Offered Shares. It applies to you only if you acquire your Offered Shares in the Offering and you hold your Offered Shares as capital assets for tax purposes. This section does not apply to you if you are a member of a special class of holders subject to special rules, including:
| | • | | a dealer in securities; |
S-52
| | • | | a trader in securities that elects to use a mark-to-market method of accounting for securities holdings; |
| | • | | a tax-exempt organization; |
| | • | | a life insurance company; |
| | • | | a person liable for alternative minimum tax; |
| | • | | a person that actually or constructively owns 10% or more of the Corporation’s voting stock; |
| | • | | a person that holds offered shares as part of a straddle or a hedging or conversion transaction; |
| | • | | a person that purchases or sells Offered Shares as part of a wash sale for tax purposes; or |
| | • | | a U.S. holder (as defined below) whose functional currency is not the U.S. dollar. |
This discussion addresses only United States federal income taxation. This section is based on the Internal Revenue Code of 1986, as amended, its legislative history, existing and proposed regulations, published rulings and court decisions, all as currently in effect, as well as on the Convention. These laws are subject to change, possibly on a retroactive basis.
If an arrangement treated as a partnership for United States federal income tax purposes holds Offered Shares, the United States federal income tax treatment of a partner will generally depend on the status of the partner and the tax treatment of the partnership. A partner in a partnership holding Offered Shares should consult its tax advisor with regard to the United States federal income tax treatment of an investment in the Offered Shares.
You are a U.S. holder if you are a beneficial owner of Offered Shares and you are for United States federal income tax purposes:
| | • | | a citizen or resident of the United States, |
| | • | | a corporation created or organized in or under the laws of the United States, any state thereof or the District of Columbia, |
| | • | | an estate whose income is subject to United States federal income tax regardless of its source, or |
| | • | | a trust if a United States court can exercise primary supervision over the trust’s administration and one or more United States persons are authorized to control all substantial decisions of the trust. |
A “non-U.S. holder” is a beneficial owner of Offered Shares that is neither a U.S. holder nor a partnership or an entity treated as a partnership for United States federal income tax purposes.
You should consult your own tax advisor regarding the United States federal, state and local and other tax consequences of owning and disposing of Offered Shares in your particular circumstances.
U.S. Holders
Dividends
Subject to the passive foreign investment company, or PFIC, rules discussed below, if we pay dividends in U.S. dollars, the gross amount of any dividend we pay out of our current or accumulated earnings and profits (as determined for United States federal income tax purposes) is subject to United States federal income taxation. If you are a noncorporate U.S. holder, dividends that constitute qualified dividend income will be taxable to you at the preferential rates applicable to long-term capital gains provided that you hold the Offered Shares for more than 60 days during the 121-day period beginning 60 days before the ex-dividend date and meet other holding period requirements. Dividends the Corporation pays with respect to the Offered Shares generally will be qualified dividend income.
You must include any Canadian tax withheld from the dividend payment in this gross amount even though you do not in fact receive it. The dividend is taxable to you when you receive the dividend, actually or constructively. The dividend
S-53
will not be eligible for the dividends-received deduction generally allowed to United States corporations in respect of dividends received from other United States corporations. Distributions in excess of current and accumulated earnings and profits, as determined for United States federal income tax purposes, will be treated as a non-taxable return of capital to the extent of your basis in the Offered Shares and thereafter as capital gain. However, we do not expect to calculate earnings and profits in accordance with United States federal income tax principles. Accordingly, you should expect to generally treat distributions we make as dividends.
Dividends will be income from sources outside the United States and will, depending on your circumstances, be either “passive” or “general” income for purposes of computing the foreign tax credit allowable to you. Subject to certain limitations, the Canadian tax withheld from dividend payments to you will be creditable or deductible against your United States federal income tax liability. The rules governing the calculation and timing of foreign tax credits and the deduction of foreign taxes are complex and depend upon your particular circumstances. U.S. holders should consult their tax advisors regarding the availability of the foreign tax credit in their particular circumstances.
Distributions paid in a currency other than U.S. dollars will be included in your gross income in a U.S. dollar amount based on the spot exchange rate in effect on the date of actual or constructive receipt of the payment, regardless of whether the payment is converted into U.S. dollars at that time. You will have a tax basis in that currency equal to such U.S. dollar amount. Any gain or loss recognized upon a subsequent sale or conversion of the foreign currency for a different U.S. dollar amount will be U.S. source ordinary income or loss. If the distribution is converted into U.S. dollars on the date of receipt, you should generally not recognize foreign currency gain or loss with respect to the distribution.
Capital Gains
Subject to the PFIC rules discussed below, if you sell or otherwise dispose of your Offered Shares, you will recognize capital gain or loss for United States federal income tax purposes in an amount equal to the difference between the U.S. dollar value of the amount that you realize on the disposition and your tax basis in the disposed Offered Shares, determined in U.S. dollars. Capital gain of a noncorporate U.S. holder is generally taxed at preferential rates where the property is held for more than one year. The gain or loss will generally be income or loss from sources within the United States for foreign tax credit limitation purposes.
PFIC Status
Certain adverse tax consequences could apply to a U.S. holder if the Corporation is treated as a PFIC for any taxable year during which the U.S. holder owns the Corporation’s shares. A non-U.S. corporation, such as Concordia, will be classified as a PFIC for any taxable year in which (i) 75% or more of the corporation’s gross income for such year consists of certain types of passive income or (ii) 50% or more of the value of the corporation’s assets during such year produce or are held for the production of passive income. Passive income for these purposes generally includes dividends, interest, royalties, rents, annuities, net gains from the sale or exchange of property producing such income, and net foreign currency gains.
If the Corporation were to be treated as a PFIC, gain realized on the sale or other disposition of your Offered Shares would in general not be treated as capital gain. Instead, unless you elect to be taxed annually on a mark-to-market basis with respect to your Offered Shares, you would be treated as if you had realized such gain and certain “excess distributions” ratably over your holding period for the Offered Shares. Any such gain or excess distribution would generally be taxed at the highest tax rate in effect for each such year to which the gain or distribution, as applicable, is allocated, and would be subject to an interest charge in respect of the tax attributable to each such year. With certain exceptions, your Offered Shares would be treated as stock in a PFIC if we were a PFIC at any time during your holding period in your Offered Shares. In addition, dividends that you receive from us would not be eligible for the special tax rates applicable to qualified dividend income if we are a PFIC (or are treated as a PFIC with respect to you) either in the taxable year of the distribution or the preceding taxable year, but instead would be taxable at rates applicable to ordinary income.
The Corporation believes that it is not currently a PFIC and is not likely to become a PFIC in the foreseeable future. The determination of whether it is a PFIC, however, is made annually as of the close of each taxable year. Because the determination whether a foreign corporation is a PFIC is primarily factual and there is little administrative or judicial authority on which to rely to make a determination, the Internal Revenue Service (“IRS”) might not agree that the Corporation is not a PFIC. Moreover, no assurance can be given that the Corporation would not become a PFIC for any future taxable year if there were to be changes in Concordia’s assets, income or operations.
Medicare Tax
A U.S. holder who is an individual, or that is an estate or a trust that does not fall into a special class of trusts that is exempt from such tax, is subject to a 3.8% tax on the lesser of (1) the U.S. holder’s “net investment income” (or “undistributed net investment income” in the case of an estate or trust) for the relevant taxable year and (2) the excess of the U.S. holder’s modified adjusted gross income for the taxable year over a certain threshold (which in the case of an individual is between $125,000 and $250,000, depending on the individual’s circumstances). A U.S. holder’s net
S-54
investment income generally includes its dividend income and its net gains from the disposition of Offered Shares, unless such dividend income or net gains are derived in the ordinary course of the conduct of a trade or business (other than a trade or business that consists of certain passive or trading activities). If you are a U.S. holder who is an individual, or that is an estate or trust, you should consult your tax advisors regarding the applicability of the Medicare tax to your income and gains in respect of your investment in the Offered Shares.
Non-U.S. Holders
Dividends
If you are a non-U.S. holder, dividends paid to you in respect of Offered Shares will not be subject to United States federal income tax unless (i) the dividends are “effectively connected” with your conduct of a trade or business within the United States, and (ii) the dividends are attributable to a permanent establishment that you maintain in the United States, if that is required by an applicable income tax treaty as a condition for subjecting you to United States taxation on a net income basis. In such cases, you generally will be taxed in the same manner as a U.S. holder. If you are a corporate non-U.S. holder, “effectively connected” dividends may, under certain circumstances, be subject to an additional “branch profits tax” at a 30% rate or at a lower rate if you are eligible for the benefits of an income tax treaty that provides for a lower rate.
Capital Gains
If you are a non-U.S. holder, you will not be subject to United States federal income tax on gain recognized on the sale or other disposition of your Offered Shares unless:
| | • | | (i) the gain is “effectively connected” with your conduct of a trade or business in the United States, and (ii) the gain is attributable to a permanent establishment that you maintain in the United States, if that is required by an applicable income tax treaty as a condition for subjecting you to United States taxation on a net income basis, or |
| | • | | you are an individual, you are present in the United States for 183 or more days in the taxable year of the sale and certain other conditions exist. |
If you are a corporate non-U.S. holder, “effectively connected” gains that you recognize may also, under certain circumstances, be subject to an additional “branch profits tax” at a 30% rate or at a lower rate if you are eligible for the benefits of an income tax treaty that provides for a lower rate.
Information with respect to Foreign Financial Assets
Owners of “specified foreign financial assets” with an aggregate value in excess of $50,000 (and in some circumstances, a higher threshold) may be required to file an information report with respect to such assets with their tax returns. “Specified foreign financial assets” may include financial accounts maintained by foreign financial institutions, as well as the following, but only if they are held for investment and not held in accounts maintained by financial institutions: (i) stocks and securities issued by non-United States persons, (ii) financial instruments and contracts that have non-United States issuers or counterparties, and (iii) interests in foreign entities. Holders are urged to consult their tax advisors regarding the application of this reporting requirement to their ownership of Offered Shares.
Backup Withholding and Information Reporting
If you are a noncorporate U.S. holder, information reporting requirements, on IRS Form 1099, generally will apply to dividend payments or other taxable distributions made to you within the United States, and the payment of proceeds to you from the sale of Offered Shares effected by a United States broker or a foreign broker with certain connections to the United States. Additionally, backup withholding may apply to such payments if you fail to comply with applicable certification requirements or are notified by the IRS that you have failed to report all interest and dividends required to be shown on your federal income tax returns.
If you are a non-U.S. holder, you will generally be exempt from backup withholding and information reporting requirements with respect to dividend payments made to you outside the United States by us or another non-United States payor. You will also generally be exempt from backup withholding and information reporting requirements in respect of dividend payments made within the United States and the payment of the proceeds from the sale of Offered
S-55
Shares effected at a United States office of a broker, as long as either (i) the payor or broker does not have actual knowledge or reason to know that you are a United States person and you have furnished a valid IRS Form W-8 or other documentation upon which the payor or broker may rely to treat the payments as made to a non-United States person, or (ii) you otherwise establish an exemption.
Payment of the proceeds from the sale of Offered Shares effected at a foreign office of a broker that has no connections to the United States generally will not be subject to information reporting or backup withholding. However, a sale effected at a foreign office of a broker could be subject to information reporting in the same manner as a sale within the United States (and in certain cases may be subject to backup withholding as well) if (i) the broker has certain connections to the United States, (ii) the proceeds or confirmation are sent to the United States or (iii) the sale has certain other specified connections with the United States.
Backup withholding is not an additional tax. You generally may obtain a refund of any amounts withheld under the backup withholding rules that exceed your income tax liability by timely filing a refund claim with the IRS.
RISK FACTORS
Before making an investment decision, prospective purchasers of Offered Shares should carefully consider the information described in this Prospectus Supplement, the accompanying Prospectus, and in the documents incorporated by reference therein. There are certain risks inherent in an investment in the Offered Shares, including the following factors, the factors described under the heading “Risk Factors - Risk Factors Related to the Business” in the AIF (pages 46 through 64) and “Risk Factors - Risk Factors Related to the Common Shares” in the AIF (on pages 64 through 68), and any other factors described in a document incorporated by reference in the accompanying Prospectus, which investors should carefully consider before investing. The risk factors pertaining to the Corporation’s business, financial condition and results of operations would also apply to AMCo and its business that is being acquired by the Corporation pursuant to the Acquisition. Some of the following factors and the factors described in the documents incorporated by reference in the accompanying Prospectus are interrelated and, consequently, investors should treat such risk factors as a whole. The following information is a summary only of certain risk factors and is qualified in its entirety by reference to, and must be read in conjunction with, the detailed information appearing elsewhere in this Prospectus Supplement, the accompanying Prospectus, and in the documents incorporated by reference therein. The risks described in this Prospectus Supplement, the accompanying Prospectus and in the documents incorporated by reference therein describe certain currently known material factors, any of which could have a material adverse effect on the Corporation’s business, financial condition and results of operations. If any of the following or other risks occur, it could have a material adverse effect on the business, financial condition and results of operations of the Corporation and on the trading price of the Common Shares, which could materially decline, and investors may lose all or part of their investment. Additional risks and uncertainties of which the Corporation is currently unaware or that are unknown or that it currently deems to be immaterial could also have a material adverse effect on the Corporation’s business, financial condition and results of operations. The Corporation cannot assure you that it will successfully address any or all of these risks. There is no assurance that any risk management steps taken will avoid future loss due to the occurrence of any of the risks described in this Prospectus Supplement, the accompanying Prospectus, and in the documents incorporated by reference therein, or other unforeseen risks.
Risk Factors Related to the Business
The Corporation is subject to risks associated with the enforcement of anti-trust and competition laws.
Pharmaceutical companies in the United States and other countries have faced lawsuits and investigations pertaining to violations of antitrust and competition laws from time to time. AMCo’s drug products acquired pursuant to the Acquisition could be subject to antitrust or competition law challenge that, if successful, could affect the Corporation’s ability to set prices for its drug products or enter into agreements with respect thereto. A successful antitrust or competition law challenge against the Corporation could result in the imposition of significant fines by one or more authorities, and/or in decisions preventing the Corporation from further expanding its business, and/or third parties (such as competitors and customers) initiating civil litigation claiming damages caused by anticompetitive practices. A violation of any such law could result in civil penalties, mitigation, significant capital expenditures or require changes in the Corporation’s business practices, which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
On February 9, 2015, Concordia and CPI, received a civil investigative demand (“CID”) from the United States Federal Trade Commission (“FTC”) regarding its attention deficit hyperactivity disorder product Kapvay®. The CID is a request for documents and information relating to CPI’s agreements with Par Pharmaceutical, Inc. (“Par”) with respect to Kapvay® (the “Par Agreements”).
S-56
While Concordia maintains that the Par Agreements are lawful, it expects to enter into a settlement agreement with the FTC to settle the FTC investigation in September 2015. The agreement with the FTC does not constitute an admission by Concordia that it has violated the law. Under the terms of the settlement agreement, Concordia will be prohibited from enforcing the provision of the Par Agreements which entitle Concordia to a royalty payment corresponding to Par’s sales of generic Kapvay® and from entering any future agreement with a generic company that would prevent the marketing of an authorized generic version of a branded drug after all patents have expired on the drug.
On December 5, 2014, Covis Pharma received a subpoena from the U.S. Department of Justice, Antitrust Division, requesting certain documents relating to its product Lanoxin®. Pursuant to the purchase agreement entered into in connection with the Covis Acquisition, the Corporation did not assume liabilities associated with any potential action arising in connection therewith and has a right to indemnification, under certain circumstances, for losses directly arising from any such action. However, to the extent market or regulatory conditions require the Corporation to alter its pricing of Lanoxin®, it could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Corporation may become subject to litigation.
Defense and settlement costs of legal claims can be substantial, even with respect to claims that have no merit. At any time, the Corporation is subject to the threat of litigation and may be involved in disputes with other parties in the future which may result in litigation or other proceedings. The results of litigation or any other proceedings cannot be predicted with certainty. If the Corporation is unable to resolve these disputes favourably, it could have a material adverse effect on the Corporation and its business, financial position, and results of operations. In particular, the pharmaceutical industry has been characterized by frequent litigation regarding patent and other intellectual property rights. There may be certain issued patents and patent applications claiming subject matter that the Corporation’s licensors or the Corporation may be required to license and the Corporation cannot be certain whether such patents and patent applications would be available to license on commercially reasonable terms, or at all. Any claims of patent infringement asserted by third parties would be time-consuming and may result in costly litigation, divert the time and attention of the Corporation’s technical personnel and management, require the Corporation to cease or modify its use of the product, and/or require the Corporation to enter into royalty or licensing agreements.
Focus, acquired by AMCo in October 2014, is currently involved in patent litigation with Novartis AG concerning Focus Voleze® Rivastigmine Transdermal Patches. Novartis AG commenced legal proceedings against Focus in August 2013, alleging that the Voleze® Rivastigmine Transdermal Patches infringed a Novartis AG patent. After a trial in London in March 2015, the High Court ruled that Novartis AG’s patent was invalid on two counts, including that the invention was obvious over an earlier published Novartis AG patent. Novartis AG is currently appealing this decision, which AMCo expects to be heard in 15-18 months. There is no guarantee the appeal will be held in AMCo’s favour and the results of this or any other litigation involving AMCo or the Corporation from time to time could have an adverse effect on the Corporation’s business, reputation, results of operations, and financial position.
There can be no assurance that the Corporation may not be treated as a “passive foreign investment company” under the U.S. Internal Revenue Code. If the Corporation were treated as a PFIC, such treatment could result in adverse tax consequences for investors in the United States.
There can be no assurance that the Corporation may not be treated as a PFIC under the U.S. Internal Revenue Code. If the Corporation were treated as a PFIC, such treatment could result in adverse tax consequences for investors in the United States.
Certain adverse tax consequences could apply to a U.S. holder if the Corporation were treated as a PFIC for any taxable year during which the U.S. holder owns the Corporation’s shares. The Corporation believes that it is not currently a PFIC and is not likely to become a PFIC in the foreseeable future. The determination of whether it is a PFIC, however, is made annually as of the close of each taxable year. Because the determination whether a foreign corporation is a PFIC is primarily factual and there is little administrative or judicial authority on which to rely to make a determination, the IRS might not agree that the Corporation is not a PFIC. Moreover, no assurance can be given that the Corporation would not become a PFIC for any future taxable year if there were to be changes in Concordia’s assets, income or operations. See “Certain United States Federal Income Tax Considerations”.
Risk Factors Related to the Acquisition and Debt Financing
The Corporation will have a new significant shareholder upon completion of the Acquisition.
Upon completion of the Offering and the Acquisition, it is expected that Cinven will hold approximately [●]% of the Common Shares, which will limit other shareholders’ ability to influence certain corporate matters and the direction of the Corporation. Cinven will, for the foreseeable future, have significant influence over corporate management and affairs, and, although restricted in the manner in which it may vote on certain matters pursuant to the Governance Agreement, Cinven will still be able to significantly affect the outcome of important matters affecting Concordia that require shareholder approval, including the issuance of securities in business combination transactions, and business combination or other transactions that have been recommended for acceptance by Concordia shareholders by the Board. Cinven will also be able to appoint one nominee to stand for election to the Board. As a result of its significant shareholdings, Cinven may have significant influence over the management and the strategic direction of the Corporation. It is possible that the interests of Cinven may in some circumstances conflict with the Corporation’s interests and the interests of other Concordia shareholders. In addition, Cinven is in the business of making or advising on investments in other companies and may hold securities of, and may from time to time in the future acquire interests in or provide advice to, businesses that directly or indirectly compete with all or a portion of the Corporation’s business
S-57
or the businesses of its suppliers. Cinven has not entered into any non-competition agreements with the Corporation, or provided any covenants not to compete with the Corporation under the AMCo Purchase Agreement. The specific knowledge of AMCo’s business and the Corporation’s industry that Cinven possesses and the absence of restrictions on its ability to invest in and operate businesses in direct or indirect competition with the Corporation may pose a competitive threat to the Corporation that could lead to a material adverse effect on the Corporation’s business, financial condition, and results of operations.
The Corporation could fail to complete the Acquisition or complete the Acquisition on different terms.
The completion of the Acquisition is subject to the satisfaction of certain conditions and may not occur. These conditions include the absence of any orders, judgments, decrees or injunctions preventing completion or making the Acquisition unlawful, and completion of the Acquisition not resulting in certain material violations of law or regulation. In addition, if the Acquisition Closing has not occurred on or before March 4, 2016, and such outside closing date has not been extended by the parties, the AMCo Purchase Agreement will automatically terminate. If these conditions are not met or the Acquisition is not completed by March 4, 2016, either the Vendors or the Corporation may choose not to proceed with the Acquisition. In that case, while holders of Common Shares will have had their holdings diluted by the Offering, the Corporation would not realize any anticipated benefits from the Acquisition, which could have a material adverse effect on the Corporation’s business, financial condition, and results of operations, and the market price of the Common Shares. See also “Risk Factors-Management will have discretion with respect to the use of proceeds from the Offering if the Acquisition Closing does not Occur.”
Even if the Acquisition is completed, there is no assurance that it will be completed on the same or similar terms to those described in this Prospectus Supplement. In addition, the ongoing business of the Corporation may be adversely affected as a result of the costs (including opportunity costs) incurred in respect of pursuing the Acquisition, and the Corporation could experience negative reactions from the financial markets, which could cause a decrease in the market price of the Corporation’s securities. The Corporation may also experience negative reactions from its or AMCo’s customers and employees and there could be negative impact on the Corporation’s ability to attract future acquisition or in-license opportunities. Failure to complete the Acquisition or a change in the terms of the Acquisition could each have a material adverse effect on the Corporation’s business, financial condition and results of operations and the market price of the Common Shares.
Purchasers of Offered Shares may be subject to immediate and substantial dilution.
Purchasers of the Offered Shares offered pursuant to this Prospectus Supplement may pay more for the Offered Shares than the amounts paid by existing shareholders or securityholders of the Corporation for Common Shares. As a result, purchasers may incur immediate and substantial dilution. Convertible securities have been issued and may be issued in the future by the Corporation at a lower price than the current market value of the Common Shares and, consequently, purchasers who purchase Offered Shares under this Prospectus Supplement may also incur substantial dilution in the near future.
S-58
AMCo’s current business is operated in numerous foreign countries that may be subject to a higher degree of political, social and economic risk than jurisdictions in which it currently operates.
AMCo’s business is conducted in approximately 100 countries, whereas the business of the Corporation historically has been conducted predominantly in the United States. A number of risks are inherent in international operations, including risks associated with: (i) foreign currency fluctuations and devaluations; (ii) political, social, security and economic instability in foreign countries; (iii) changes in and compliance with local laws and regulations or uncertainty regarding the interpretation and/or application of applicable laws, including export and import control laws, sanctions regulations, tax laws, labour laws, employee benefits, currency restrictions and other requirements; (iv) differences in tax regimes and potentially adverse tax consequences of operating in foreign countries or unfavourable or arbitrary tax enforcement; (v) customizing products for foreign countries; (vi) legal uncertainties regarding liability, export and import restrictions, tariffs and other trade barriers; (vii) changes in governmental regulations regarding currency or price controls, profit repatriation, labour, or health and safety matters; (viii) hiring qualified foreign employees; and (viii) difficulty in accounts receivable collection and longer collection periods. Accordingly, upon completion of the Acquisition, Concordia’s exposure to risks involved with operating in foreign countries will be increased, which could have a material adverse effect on the Corporation’s business, financial conditions and results of operations.
The Corporation could fail to successfully integrate the AMCo business into the business of the Corporation.
If the Acquisition is completed, the success of the Acquisition will depend, in part, on the ability of the Corporation to realize the anticipated benefits, accretion and synergies from integrating AMCo’s business into the businesses of the Corporation. If the Corporation is not able to achieve these objectives in whole or in part, or is not able to achieve these objectives on a timely basis, the anticipated benefits of the Acquisition may not be realized fully or at all. In addition, the actual integration of AMCo may result in additional and unforeseen expenses, which could reduce the anticipated benefits, accretion and synergies of the Acquisition, and may also result in additional and unforeseen matters that require the attention of management, which could divert management’s focus and the Corporation’s resources from other strategic opportunities and operational matters. Failure to successfully integrate AMCo’s business for any of these reasons could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Corporation may have difficulties maintaining or growing AMCo’s business.
AMCo’s business may involve the selling of products that the Corporation has limited experience operating or managing. The Corporation may experience unanticipated challenges or difficulties maintaining AMCo’s business at its current level or growing AMCo’s business. Factors that may impair the Corporation’s ability to maintain or grow AMCo’s business, its customers and personnel may include, but are not limited to:
| | • | | challenges in integrating AMCo’s business with the Corporation’s business; |
| | • | | loss of customers of, and/or suppliers to or distributors of, AMCo’s business, including as a result of the exercise of change of control provisions in connection with the Acquisition or otherwise; |
| | • | | in the case of certain service agreements, the ability of a counterparty to terminate such agreement for any breach of the terms of the agreement; |
| | • | | loss of key members of the AMCo management team; |
| | • | | risk relating to infringement of third party intellectual property rights by AMCo’s business; |
| | • | | non-compatible business cultures; |
| | • | | difficulties in gaining necessary approvals in international markets to maintain and/or expand AMCo’s business; |
| | • | | additional demands on resources, systems, procedures and controls; and |
| | • | | dealing with unfamiliar laws, customs and practices in foreign jurisdictions. |
S-59
In addition, the Corporation may not have identified all risks or have fully assessed risks identified with the Acquisition. There is also a risk that the expected benefits of the Acquisition may not be achieved in the expected timeframe or to the extent expected. The individual or combined effect of these risks could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Corporation could sell, spin off or otherwise dispose of products of AMCo instead of integrating them into the business of the Corporation.
The Corporation may decide to sell, spin off or otherwise dispose of certain products of AMCo in connection with or shortly after the Acquisition Closing. If it does, it may not properly value such products and dispose of products that are more valuable than the Corporation expected, or it may underestimate liabilities that would be retained by the Corporation following the disposition of such products. To the extent the Corporation does not properly determine the materiality, value and liabilities associated with any such assets that it may choose to dispose of, the Corporation could fail to realize the benefits it expects from the Acquisition, which in turn could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Acquisition could impact the operations of the Corporation.
In connection with the Acquisition, some customers of the Corporation or AMCo may delay or defer purchasing decisions, which could negatively impact the revenues, earnings, cash flows and expenses of the Corporation and AMCo, regardless of whether the Acquisition is completed. The Corporation may also be subject to successor liability claims, including matters related to taxes, product liability, and government proceedings in connection with AMCo. Any contractual claims the Corporation may have under the Acquisition documentation may be of limited benefit because of time limits, enforceability, deductibles and caps on claim amounts, creditworthiness, and other factors. The Corporation will have no recourse against Cinven for breach of any warranties with respect to AMCo. The only Vendors who have provided the Corporation with warranties with respect to AMCo and its business are senior members of management, and such warranties have been provided on a damages basis, as opposed to a dollar-for-dollar indemnity basis, to a maximum of £17.5 million. Any such successor liability claims could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Corporation may be unable to complete the Acquisition or, in order to do so, the Corporation and AMCo may be required to comply with material restrictions or satisfy material conditions.
The completion of the Acquisition is subject to conditions, including the absence of any orders, judgments, decrees or injunctions preventing completion or making the Acquisition unlawful, and completion of the Acquisition not resulting in certain material violations of law or regulation. The Corporation can provide no assurance that any required regulatory clearances will be obtained in order to complete the Acquisition. There can be no assurance as to the cost, scope or impact of the actions that may be required to obtain any required regulatory approval.
We may be unable to realize anticipated cost synergies or may incur additional and/or unexpected costs in order to realize them.
There can be no assurance that the Corporation will be able to realize synergies from the proposed transaction in any anticipated amounts or within anticipated timeframes, or at all. The Corporation may implement cost saving initiatives at the combined company in the expectation that it will result in annual cost synergies. The Corporation may incur costs to achieve such synergies. These or any other costs or synergies that the Corporation realizes may differ materially from the Corporation’s estimates and expectations. Concordia cannot provide assurances that anticipated cost or other synergies will be achieved or that its programs and improvements will be completed as anticipated or at all. In addition, any cost synergies that the Corporation realizes may be offset, in whole or in part, by reductions in revenues or through increases in other expenses.
Neither Concordia’s independent auditors nor any other independent auditors, have examined, compiled or performed any procedures with respect to synergies, nor have they expressed any opinion, or any other form of assurance on such information or their achievability. Assumptions relating to cost or other synergies involve subjective decisions and judgments. Although management of the Corporation believes these estimates and assumptions to be reasonable, any of the assumptions could be inaccurate and, therefore, there can be no assurance that the objectives and plans with respect to synergies will be achieved. The internal financial projections used to calculate anticipated cost synergies also do not take into account any circumstances or events occurring after the date on which they were prepared. These internal financial projections reflect assumptions as to certain business decisions that are subject to change. As a result, actual
S-60
results may differ materially from those contained in these internal financial projections. Accordingly, there can be no assurance that the internal financial projections will be realized or that actual results will not be significantly higher or lower than projected. Concordia undertakes no obligation to update or otherwise revise or reconcile these internal financial projections whether as a result of new information, future events or otherwise.
The Corporation will incur substantial transaction-related costs in connection with the Acquisition such that the Corporation may not be able to achieve the benefits and cost savings expected to be realized as a result of the Acquisition.
The Corporation expects to incur a number of non-recurring transaction-related costs associated with assessing and completing the Acquisition, integrating AMCo and its business with the business of the Corporation and achieving desired synergies. These fees and costs will be substantial. Non-recurring transaction costs include, but are not limited to, fees paid to legal, financial and accounting advisors, filing fees and printing costs. Additional unanticipated costs may be incurred in the integration of the operations and businesses of the Corporation with AMCo. Such costs may include increases in other expenses unrelated to the Acquisition, which may offset the cost savings and other synergies from the Acquisition.
The estimates of benefits and cost savings associated with the Acquisition reflect estimates and assumptions made by the Corporation, and it is possible that these estimates and assumptions may not ultimately reflect actual results. In addition, these estimated cost savings may not actually be achieved in the timeframe anticipated or at all. If the Corporation fails to realize anticipated benefits, cost savings, synergies or revenue enhancements, it could have a material adverse effect on the Corporation’s business, financial condition and results of operations. There can be no assurance that the elimination of certain duplicative costs, as well as the realization of other efficiencies related to the integration of AMCo into the Corporation’s business, will offset the incremental transaction-related costs over time. Thus, any net benefit may not be achieved in the near term, the long term or at all.
In addition, the Corporation may be required to pay up to £144 million (US$220 million) to satisfy the earn-out that may be payable in connection with the Acquisition, as well as an additional US$72 million to the Vendors if the Corporation acquires certain specified pharmaceutical products from a specified third party within 12 months of the Acquisition Closing, which payments could offset certain expected financial benefits of the Acquisition and negatively impact the Corporations’ financial condition and results of operations.
The Corporation is expanding and diversifying its business in connection with the Acquisition, which expansion and diversification may not be successful.
The Corporation may encounter financial and operational difficulties in integrating AMCo’s business with the business of the Corporation or in managing AMCo successfully. The Corporation cannot be certain of the degree and scope of operational and integration problems that may arise. In addition, with the Acquisition, the Corporation is significantly diversifying its involvement in the pharmaceutical industry. The Corporation may not be successful in developing and integrating AMCo’s products and this could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
The Corporation will acquire liabilities, some of which may be contingent or latent, through the Acquisition that could adversely affect the Corporation.
The Corporation will acquire liabilities in connection with the Acquisition, some of which may be contingent or latent. Although the Corporation’s management has estimated the risks associated with these liabilities and the likelihood that contingent or latent liabilities will materialize, their estimates could differ materially from the liabilities actually incurred.
In addition, as the acquirer of AMCo, the Corporation may acquire contingent liabilities in addition to liabilities assumed pursuant to the AMCo Purchase Agreement, such as statutory liabilities imposed on the acquirer of a business pursuant to applicable laws, such as creditor protection legislation, legislation relating to the protection of personal information, and anti-bribery legislation. Any of the contingent liabilities referred to above may be material and could materially adversely affect the Corporation’s business, financial condition and results of operations.
S-61
Following the Acquisition, the Corporation’s actual financial position and results of operations may differ materially from the unaudited pro forma consolidated financial information included in this Prospectus Supplement.
The unaudited pro forma consolidated financial information contained in this Prospectus Supplement is presented for illustrative purposes only and may not be an indication of what the Corporation’s financial position or results of operations would have been had the Acquisition been completed on the dates indicated. The unaudited pro forma consolidated financial information has been derived from the audited and unaudited financial statements of the Corporation, AMCo, the Covis Business, and other businesses acquired by the Corporation since the beginning of the fiscal year in respect of which the Annual Financial Statements have been filed, and certain adjustments and assumptions have been made regarding the Corporation after giving effect to such acquisitions. The assets and liabilities of the Corporation have been measured at fair value based on various preliminary estimates using assumptions that management believes are reasonable based on information currently available. The process for estimating the fair value of AMCo or other acquired businesses and assumed liabilities requires the use of judgment in determining the appropriate assumptions and estimates. These estimates and assumptions may be revised as additional information becomes available and as additional analyses are performed, but there is no guarantee these estimates and assumptions can be correct. Management may also omit certain periods from pro forma information to accord with financial reporting periods. While management believes such omissions do not have a material effect on the presentation of the pro forma financial information, there is no guarantee such belief may be correct.
Differences between preliminary estimates in the unaudited pro forma consolidated financial information and the final acquisition accounting will occur and could have a material impact on the pro forma consolidated financial information and the Corporation’s financial position and future results of operations. In addition, the assumptions used in preparing the pro forma consolidated financial information may not prove to be accurate, and other factors may affect the Corporation’s financial condition or results of operations following the Acquisition. Any potential decline in the Corporation’s financial condition or results of operations may cause significant variations in the trading price of the Common Shares following the Acquisition.
Forward-looking statements, future-oriented financial information, and financial outlooks may prove inaccurate.
Investors are cautioned not to place undue reliance on forward-looking statements, future-oriented financial information and financial outlooks. By their nature, forward-looking statements, future-oriented financial information and financial outlooks involve numerous assumptions, known and unknown risks and uncertainties, of both a general and specific nature, that could cause actual results to differ materially from those suggested by the forward-looking statements or contribute to the possibility that predictions, forecasts or projections will prove to be materially inaccurate. Future-oriented financial information and financial outlooks presented in this Prospectus Supplement are based upon the completion of the Offering and the Acquisition and if these transactions are not completed or not completed on the terms or timelines contemplated, this will impact the future-oriented financial information and financial outlooks provided herein and such impact may be material. Additional information on the risks, assumptions and uncertainties are found in this Prospectus Supplement under the headings“Cautionary Note Regarding Forward-Looking Information” and“Cautionary Note Regarding Future-Oriented Financial Information”.
The Corporation’s consolidated financial statements may be impacted in future periods based on the accuracy of the Corporation’s valuations of AMCo.
Accounting for business combinations and other agreements may involve complex and subjective valuations of the assets and liabilities recorded as a result of the business combination or other agreement, and in some instances contingent consideration, which is recorded in the Corporation’s consolidated financial statements pursuant to the standards applicable for business combinations in accordance with generally accepted accounting principles. Differences between the inputs and assumptions used in the valuations and actual results could have a material effect on the Corporation’s consolidated financial statements in future periods.
The Corporation does not currently control AMCo and no assurance of AMCo’s future performance can be given.
The Corporation will not control AMCo until after the completion of the Acquisition. Although the AMCo Purchase Agreement contains covenants on the part of the Vendors regarding the operation of AMCo’s business prior to the Acquisition Closing, the Corporation will not assume control of AMCo until completion of the Acquisition and there may be a material adverse effect on AMCo’s business, financial condition and results of operations based on events that are outside of the Corporation’s control during the intervening period. Prior to completion of the Acquisition, the Corporation cannot assure you that AMCo will operate its business in the same way its business would be operated
S-62
under the control of the Corporation. Furthermore, AMCo’s historical and current performance may not be indicative of success in future periods. There is no provision of the AMCo Purchase Agreement that allows the Corporation to terminate the agreement as a result of a material adverse effect on, or a material adverse change with respect to, AMCo or its business. Therefore, the Corporation is assuming the risk with respect to such material adverse effects or changes as they may occur with respect to AMCo prior to the Acquisition Closing.
Because the Corporation does not yet control AMCo, the information contained in this Prospectus Supplement relating to AMCo has been derived from information provided to the Corporation by AMCo and the Vendors. Moreover, as AMCo is a privately held company, its internal controls and reporting procedures may not be comparable to those of a public company. The Corporation has relied on its diligence and information supplied to it by AMCo and the Vendors in the preparation of this Prospectus Supplement.
The AMCo Purchase Agreement obligates the Corporation to cooperate and use its reasonable best efforts to complete the related financings for the Acquisition. If the Corporation is unable to obtain financing, it could adversely affect the Corporation’s business prospects.
Under the terms of the AMCo Purchase Agreement, the Corporation has agreed to use reasonable best efforts to take, or cause to be taken, all actions, and do, or cause to be done, all things necessary, proper or advisable to obtain the proceeds of the debt financing on the terms and conditions described in the AMCo Purchase Agreement and in the Commitment Letter. If the Corporation is unable to obtain the related debt financing, it may be subject to claims for breach of contract, in addition to being subject to higher interest rates or other adverse effects as a result of having to draw on alternative or additional sources of financing. See“Details of the Acquisition - AMCo Purchase Agreement”.
The Acquisition is not conditional on obtaining financing.
There exists no condition for financing under the AMCo Purchase Agreement on which the Corporation can rely to terminate the AMCo Purchase Agreement. The Corporation has obtained a commitment from the Commitment Parties to provide the Bank Loans which, together with the net proceeds of the Offering and contemplated private placement of debt securities, is expected to provide funds sufficient to pay the Purchase Price and pay related transaction expenses. Such financing arrangements are subject to specific terms and conditions for funding and closing to take place. If the Bank Loans are not available to the Corporation, the Corporation would have to find alternative forms of financing to pay a portion of the Purchase Price, which may not be available on acceptable terms, or at all. Any variation in the terms of, or inability to complete, the contemplated debt financings could result in, among other things, higher interest rates, more onerous security packages, or additional restrictive covenants on the Corporation’s business, each of which could materially adversely affect the Corporation’s business, financial condition and results of operations.
The Corporation may encounter limitations against the Vendors with respect to any claims it may have in connection with the Acquisition.
The warranties provided by certain management Vendors pursuant to the AMCo Purchase Agreement are customary for a transaction of this nature. Cinven has not provided any warranties with respect to AMCo or its business. While the Corporation has limited recourse for damages as a result of breach of warranties against certain management Vendors under the warranty deed entered into in connection with the AMCo Purchase Agreement, in the event that the Corporation suffers such damages, the management Vendors’ aggregate liability is capped at £17.5 million, which is substantially less than the estimated net proceeds of the Offering and the Purchase Price, respectively. There can be no assurance of adequate recovery by the Corporation from the Vendors for any breach of warranties and covenants under the AMCo Purchase Agreement, or that the length and amounts of the warranties provided will be sufficient to satisfy such obligations, or that the Vendors will have the financial ability to satisfy such obligations. Similarly, there can be no assurance of recovery from the Vendors under the AMCo Purchase Agreement.
Prospective investors are cautioned that: (i) neither AMCo nor the Vendors have signed the certificate page to this Prospectus Supplement, certifying that the disclosure in this Prospectus Supplement represents full, true and plain disclosure of all material facts relating to AMCo or its business and does not contain a misrepresentation relating to AMCo or its business; and (ii) the Vendors will have no liability to investors participating in the Offering in the event that the disclosure contained in this Prospectus Supplement relating to AMCo or its business contains a misrepresentation.
S-63
The Corporation expects to incur substantial indebtedness to finance the Acquisition and to refinance certain of its prior indebtedness, which total indebtedness imposes operating and financial restrictions on the Corporation that, together with the resulting debt service obligations from such total indebtedness, may significantly limit the Corporation’s ability to execute its business plans.
In connection with the Acquisition, the Corporation entered into the Commitment Letter pursuant to which the Commitment Parties and certain other lenders have agreed to provide the Bank Loans.
The funding of such debt financing is contingent on the Acquisition Closing and certain other conditions set forth in the Commitment Letter, however, the funding of such debt financing is not a condition to the obligations of the Corporation under the terms of the AMCo Purchase Agreement. The Corporation expects to finance the Acquisition by issuing the Offered Shares offered hereby and through the issuance of senior unsecured notes and borrowings under the Bank Loans such that the Bond Bridge Facility and Equity Bridge Facility will not be drawn upon to finance the Acquisition. However, it is possible that, after this Offering is complete, it will still be necessary to draw on the Bond Bridge Facility and the Equity Bridge Facility to complete the Acquisition, which could require the Corporation to pay higher interest payments than expected. In addition, if the Corporation is required to purchase its existing senior notes on the Acquisition Closing Date it may be required to draw on the Backstop Bridge Facility, and/or if the Corporation is unable to complete its contemplated private placement of notes to finance a portion of the Purchase Price on favourable terms or at all, it may be required to draw on the Bond Bridge Facility, which, in each case, will also lead to higher interest payments than expected. Any of the occurrences described above could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
Moreover, if the terms of the Bank Loans or the anticipated private placement of debt securities differ from those currently contemplated by the Corporation, it could have a material adverse impact on the Corporation’s anticipated benefits of the Acquisition, which could, in turn, have a material adverse effect on the Corporation’s business, financial condition and results of operations.
After giving effect to the Offering and the debt incurred to finance the Acquisition and to refinance the Corporation’s existing indebtedness, the Corporation will have a substantial amount of indebtedness. As of June 30, 2015, on an adjusted basis after giving effect to the Offering and the debt incurred to finance the Acquisition and to refinance a portion of the Corporation’s existing indebtedness, the Corporation would have had approximately US$[•] of total indebtedness.
The Corporation’s indebtedness could have important consequences to holders of the Common Shares. For example, the Corporation anticipates that its indebtedness will:
| | • | | require the Corporation to dedicate a substantial portion of its cash flow from operations to payments on its indebtedness, reducing the availability of cash flow to fund working capital, capital expenditures, development activity, acquisitions and other general corporate purposes; |
| | • | | increase the Corporation’s vulnerability to adverse general economic or industry conditions; |
| | • | | limit the Corporation’s flexibility in planning for, or reacting to, changes in its business or the industries in which it operates; |
| | • | | limit the Corporation’s ability to obtain additional financing in the future for working capital or other purposes; |
| | • | | place the Corporation at a competitive disadvantage compared to competitors that have less indebtedness; |
| | • | | result in increased sensitivity of overall cash flow and profitability to changes in interest rates; and |
| | • | | place certain limits or constraints on the Corporation’s ability to pay dividends on its Common Shares. |
If the Corporation is deemed to be in violation of applicable sanctions laws and regulations, its reputation, business, results of operations and financial condition could be adversely affected.
Various governments and supranational organizations, including Canada and the United States, maintain economic sanctions targeting various countries, persons and entities. The various sanctions regimes vary. In some cases, the sanctions may amount to a near-absolute prohibition on trade and investment with or involving the sanctions target for persons required to comply with the sanctions laws. In other cases, only specific goods, services or other dealings may be prohibited. AMCo operates in and has sales into various jurisdictions in which the Corporation does not currently operate or have sales. After the Acquisition Closing, the Corporation may have to cease AMCo’s operations or the sale of any of AMCo’s products if and to the extent that such operations or the export of such products would be in violation of the sanctions laws and regulations of Canada or the United States with which the Corporation is required to comply. If AMCo or the Corporation is deemed to be in violation of applicable sanctions laws and regulations, whether due to
S-64
ongoing business or business conducted in the past, or to have engaged in any conduct that is sanctionable, the Corporation could be subject to sanctions or other governmental actions that could lead to civil or criminal penalties, including fines. In addition, such violations or engaging in such conduct could damage the Corporation’s reputation. All of the foregoing could have a material adverse effect on Concordia’s business, results of operations and financial condition.
The Corporation is exposed to fluctuations in exchange rates.
AMCo operates in more than 100 countries, and after the Acquisition, the Corporation will be subject to making and receiving payments in a number of foreign currencies. The Corporation reports its financial results in US dollars, but significant portions of the Corporation’s costs and revenue streams may be denominated in currencies other than the U.S. dollar. To the extent that there are fluctuations in the US dollar relative to other currencies, the Corporation’s revenue and operating results may be negatively impacted.
The Acquisition may be consummated on terms different from those described in this Prospectus Supplement, and as a result, the benefits of the Acquisition may not be fully realized.
The Corporation may, in its sole discretion, waive certain closing conditions in its favour in the AMCo Purchase Agreement or agree to amend the AMCo Purchase Agreement and consummate the Acquisition on terms that may be different from those described in this Prospectus Supplement, subject to the consent on behalf of the Underwriters in certain circumstances. As a result, the expected benefits of the Acquisition may not be fully realized.
Risk Factors Related to the Offering and the Common Shares
There can be no assurance that the Offering will be completed.
The completion of the Offering is subject to the completion of definitive binding documentation and satisfaction of a number of conditions, including approval of the Offering and the conditional approval of the listing of the Offered Shares by the TSX. There can be no certainty that the Offering will be completed.
Management will have discretion with respect to the use of proceeds from the Offering if the Acquisition Closing does not occur.
If the Acquisition Closing does not occur, the Corporation’s management will have broad discretion in the application of the net proceeds of the Offering. Accordingly, a purchaser of Offered Shares will have to rely upon the judgment of the Corporation’s management with respect to the use of the proceeds in that event, with only limited information concerning management’s specific intentions. The Corporation’s management may spend a portion or all of the net proceeds from the Offering in ways that the Corporation’s shareholders may not desire, that may not yield a favourable return and that may not increase the value of the Common Shares. The failure by the Corporation’s management to apply such funds effectively could harm the Corporation’s business. Pending their use, the Corporation may invest the net proceeds from the Offering in a manner that does not produce income or that loses value. If the Acquisition Closing does not occur, the results and effectiveness of the application of the net proceeds are uncertain. If the proceeds of the Offering are not applied effectively, the Corporation’s results of operations may suffer.
Enforcement of judgments against foreign persons may not be possible.
Canadian investors should be aware that Mr. Edward Borkowski and Ms. Rochelle Fuhrmann, each a director of the Corporation, as well as certain of the experts named in this Prospectus Supplement, are located outside of Canada and, as a result, it may not be possible for Canadian purchasers of Offered Shares to effect service of process within Canada upon these persons. All or a substantial portion of the assets of these persons are likely to be located outside of Canada and, as a result, it may not be possible to satisfy a judgment against such persons in Canada or to enforce a judgment obtained in Canadian courts against such persons outside of Canada.
US investors should be aware that each of Mr. Jordan Kupinsky and Mr. Doug Deeth, directors of the Corporation, Mr. Mark Thompson, an officer and a director of the Corporation, and Mr. Adrian de Saldanha, the Chief Financial Officer of the Corporation, as well as certain of the experts named in this Prospectus Supplement, are located outside the United States and, as a result, it may not be possible for purchasers of Offered Shares to effect service of process within the United States upon these persons. All or a substantial portion of the assets of these persons are likely to be located outside of the United States and, as a result, it may not be possible to satisfy a judgment against such persons in the United States or to enforce a judgment obtained in US courts against such persons outside of the United States.
S-65
The Corporation is subject to risks related to additional regulatory burden and controls over financial reporting.
The Corporation is subject to the continuous and timely disclosure requirements of Canadian securities laws and the rules, regulations and policies of the TSX and NASDAQ. These rules, regulations and policies relate to, among other things, corporate governance, corporate controls, internal audit, disclosure controls and procedures and financial reporting and accounting systems. The Corporation has made, and will continue to make, changes in these and other areas, including the Corporation’s internal controls over financial reporting. However, there is no assurance that these and other measures that it may take will be sufficient to allow the Corporation to satisfy its obligations as a public company on a timely basis. In addition, compliance with reporting and other requirements applicable to public companies create additional costs for the Corporation and require the time and attention of management of the Corporation. The Corporation cannot predict the amount of the additional costs that the Corporation may incur, the timing of such costs or the impact that management’s attention to these matters will have on the Corporation’s business.
In addition, Concordia’s inability to maintain effective internal controls over financial reporting could increase the risk of an error in its financial statements. Concordia’s management, with the participation of the Corporation’s Chief Executive Officer and Chief Financial Officer, is responsible for establishing and maintaining adequate internal control over financial reporting. The Corporation’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS. Internal control over financial reporting cannot provide absolute assurance of achieving financial reporting objectives due to its inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is therefore subject to error, improper override or improper application of the internal controls. Because of such limitations, there is a risk that material misstatements may not be prevented or detected on a timely basis, and although it is possible to incorporate into the financial reporting process safeguards to reduce this risk, they cannot be guaranteed to entirely eliminate it. If the Corporation fails to maintain effective internal control over financial reporting, then there is an increased risk of an error in the Corporation’s financial statements that could result in the Corporation being required to restate previously issued financial statements at a later date.
INTEREST OF EXPERTS
Legal Matters
Certain Canadian legal matters in connection with the Offering will be passed upon by Fasken Martineau DuMoulin LLP, on behalf of the Corporation, and by Blake Cassels & Graydon LLP, on behalf of the Underwriters.
As of the date hereof, the partners and associates of Fasken Martineau DuMoulin LLP, as a group, own, directly or indirectly, in the aggregate, less than 1% of the Common Shares.
As of the date hereof, the partners and associates of Blake Cassels & Graydon LLP, as a group, own, directly or indirectly, in the aggregate, less than 1% of the Common Shares.
Financial Matters
Collins Barrow Toronto LLP, was the auditor in respect of the consolidated financial statements of the Corporation as at December 31, 2014 and the financial statements in respect of Zonegran® included in the business acquisition report of the Corporation dated December 12, 2014, each incorporated by reference in the Prospectus, as set forth in its reports thereon. Collins Barrow Toronto LLP has advised the Corporation that it is independent of the Corporation in accordance with the Rules of Professional Conduct of the Chartered Professional Accountants of Ontario.
Keiter, Certified Public Accountants, was the auditor in respect of the financial statements in respect of Donnatal® included in the business acquisition report dated June 9, 2014, incorporated by reference in the Prospectus, as set forth in its report thereon. Keiter, Certified Public Accountants, has advised the Corporation that it is independent of the Corporation under the American Institute of Certified Public Accountants rules on auditor independence.
Crowe Horwath LLP was the auditor in respect of the carve-out financial statements for the Covis Business for the years ended December 31, 2014 and December 31, 2013 included in the business acquisition report dated July 3, 2015, incorporated by reference in the Prospectus, as set forth in its report thereon. Crowe Horwath LLP has advised the Corporation that it is independent of the Corporation under the American Institute of Certified Public Accountants rules on auditor independence.
S-66
KPMG LLP is the auditor of the consolidated financial statements of AMCo for the years ended December 31, 2014 and December 31, 2013 included in this Prospectus Supplement, as set forth in its report thereon. KPMG LLP has advised the Corporation that it is independent of AMCo under the Ethical Standards issued by the UK Auditing Practices Board rules on auditor independence.
No director, officer or employee of any of the aforementioned companies, is or is expected to be elected, appointed or employed as a director, officer or employee of the Corporation or any associate or affiliate of the Corporation.
PROMOTER
Mark Thompson, the CEO, President, founder and a director of the Corporation, may be considered a promoter of the Corporation. As at September 18, 2015, Mr. Thompson beneficially owns, controls or directs, directly or indirectly on a non-diluted basis and prior to taking into account any Common Shares issuable under the Offering 2,101,491 Common Shares, representing 6.12% of the issued and outstanding Common Shares as at that date.
S-67
APPENDIX A - AUDITED CONSOLIDATED FINANCIAL STATEMENTS OF AMDIPHARM MERCURY LIMITED FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
Amdipharm Mercury Limited
Consolidated financial statements
For the year ended 31 December 2014
11-15 Seaton Place,
ST Helier,
Jersey
JE4 0QH
A-1
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Company registration number: 111126
Registered office:
11-15 Seaton Place,
ST Helier,
Jersey
JE4 0QH
Board of Directors:
Simon Radford
Mark Wanless
Aztec Directors Limited
Supraj Rajagopalan
Alexander Leslie
Jeremy Caplan
Auditor:
KPMG LLP
Botanic House,
100 Hills Road,
Cambridge,
CB2 1AR,
United Kingdom.
A-2
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Index
A-3
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Directors’ Report
The directors present their report and the consolidated financial statements of Amdipharm Mercury Limited (“the Group”) for the year ended 31 December 2014.
Principal activity
The principal activity of Amdipharm Mercury Limited (the “Company”) is that of a holding company of the operating and group services businesses within the Group. The Group is engaged in the marketing and distribution of pharmaceutical products. The trading results for the year and the Group’s financial position at the end of the year are shown in the attached financial statements. It is intended that the company will continue in its present capacity in the coming year.
Affiliated undertaking
The Company is owned by funds managed by Fifth Cinven Fund (No. 1) Limited Partnership; Fifth Cinven Fund (No. 2) Limited Partnership; Fifth Cinven Fund (No. 3) Limited Partnership; Fifth Cinven Fund (No. 4) Limited Partnership; Fifth Cinven Fund (No. 5) Limited Partnership; Fifth Cinven Fund (No. 6) Limited Partnership; Fifth Cinven Fund FCP-SIF; Fifth Cinven FundCo-Investment Partnership, Verdot Limited and the management of Mercury Pharma Group Limited.
Acquisition of subsidiary and product rights
On 1 October 2014 a fellow group company, Mercury Pharma Group Limited acquired the entire ordinary share capital of Focus Pharmaceuticals Limited, a speciality pharma business in the UK, for a total consideration of£48.00 million (including cash acquired) and a contingent consideration to be paid over a period of 3 years amounting to£20.93 million (present value£17.16 million). Focus sales are all currently in the UK, although it has developed a number of products that can be registered and sold throughout Europe. The company’s range currently comprises of over 60 different molecules, ranging across almost all different types of formulation and therapeutic areas. The acquisition of Focus is in line with the Group’s strategy of identifying niche medicines and opportunities to expand its sales base throughout the Group’s growing international platform.
Financial position and funding
As at 31 December 2014, the Consolidated Statement of Financial Position shows net current assets of£40.22 million (2013:£89.34 million) and total assets of£1,042.58 million (2013:£1,029.69 million). On 28 November 2014 the group successfully completed a refinancing process, which involved raising new debt and paying off the Mezzanine debt.
Results and dividends
Revenue for the year was£292.80 million (2013:£240.96 million) and the profit for the financial year was£6.20 million (2013: loss of£18.29 million). The company did not make any dividend payments during the year.
A-4
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Directors’ Report (continued)
Future prospects for the business
The Group’s pharmaceuticals product portfolio has performed well during the year benefiting from a combination of favourable product mix and operational efficiencies. The Group intends to use its resources to invest in new product introductions via development, in-licensing and acquisitions. Long term sustainable growth is an area that is at the forefront of the Group’s objectives, so that it can maximise value for its stakeholders.
Other matters
The Group has incurred costs of£3,082,000 (2013:£1,082,000) in relation to research and development activities during the year.
The Group has not been involved in the purchase of its own shares during the current year or previous year.
Directors
The directors who served the Company during the year and subsequent to the year end were as follows:
Simon Radford
Mark Wanless
Aztec Directors Limited
Supraj Rajagopalan
Alexander Leslie
Jeremy Caplan
Vijay Patel (resigned on 30 July 2014)
Principal risks and uncertainties
The management of the business and the nature of the Group’s strategy are subject to certain risks:
Capacity constraints of suppliers of key products
The planned sales growth of some of our key products may be impacted by the capacity constraints of some of our suppliers. Senior management are working on initiatives to mitigate these risks in order to deliver the forecasted sales growth.
Foreign exchange risk
Foreign exchange rates have undergone a period of volatility due to economic uncertainty and relative economic performance in different parts of the world. While the Group hedging policy attempts to mitigate this risk, a net exposure will remain to currencies which may depreciate against the GBP in future. The Group operates a centralised treasury model to mitigate foreign exchange risk.
High proportion of fixed overheads and variable revenues
A large proportion of the Group’s overheads are fixed. Senior management closely monitor fixed overheads against budget on a monthly basis and costs saving exercises are implemented in case there is an anticipated decline in revenues.
A-5
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Directors’ Report (continued)
Principal risks and uncertainties (continued)
Competition
A small segment of the business operates in a competitive environment which puts pressure on margins and the ability to meet customers’ expectations. Policies of constant price monitoring and ongoing market research are in place to mitigate such risks.
Interest rate risk
The Group adopts a policy of ensuring that an appropriate proportion of its exposure to changes in interest rates on borrowings is covered by effective conversion to a fixed rate. Interest rate swaps are entered into to achieve an appropriate mix of fixed and floating exposure that is consistent with the Group’s policy.
Essential contracts
The Group’s performance is largely reliant on sourcing high quality products at the lowest possible prices. The Group has benefited from a number of contracts with suppliers which have proved essential in enabling the Group to deliver quality products at the lowest possible price.
Statement of directors’ responsibilities
The directors are responsible for preparing the financial statements in accordance with applicable law and International Financial Reporting Standards.
Company law requires the directors to prepare financial statements for each financial year which give a true and fair view of the state of affairs of the Group and of the profit or loss of the Group for that year. In preparing those financial statements, the directors are required to:
| | • | | select suitable accounting policies and then apply them consistently; |
| | • | | make judgements and estimates that are reasonable and prudent; |
| | • | | state whether applicable accounting standards have been followed, subject to any material departures disclosed and explained in the financial statements; and |
| | • | | prepare the financial statements on the going concern basis (unless it is inappropriate to presume that the Group will continue in business). |
The directors are responsible for keeping proper accounting records that disclose with reasonable accuracy at any time the financial position of the Group and to enable them to ensure that the financial statements comply with the Companies (Jersey) Law 1991. They are also responsible for safeguarding the assets of the Group and hence for taking reasonable steps for the prevention and detection of fraud and other irregularities.
Independent auditor
KPMG LLP was appointed as auditor during the year.
Approval of accounts
The Directors’ Report, Consolidated Statement of Financial Position, Consolidated Statement of Comprehensive Income, Consolidated Statement of Changes in Equity, Consolidated Cash Flow Statement and Notes to the Consolidated Financial Statements are approved by the Directors.
A-6
Independent Auditor’s Report to the Members of Amdipharm Mercury Limited
We have audited the accompanying consolidated financial statements of Amdipharm Mercury Limited (“the Company”), which comprise the consolidated statement of financial position as at 31 December 2014 and 31 December 2013, the consolidated statement of comprehensive income, consolidated statement of changes in equity and consolidated cash flow statement for the years then ended, and notes to the consolidated financial statements, comprising a summary of significant accounting policies and other explanatory information. The consolidated financial statements have been prepared by management based on the requirements of International Financial Reporting Standards as issued by the IASB.
Directors’ Responsibility for the Consolidated Financial Statements
The directors are responsible for the preparation of the consolidated financial statements which give a true and fair view, and for such internal control as the directors determine is necessary to enable the preparation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
Auditors’ Responsibility
Our responsibility is to express an opinion on these consolidated financial statements based on our audit. We conducted our audit in accordance with International Standards on Auditing. Those standards require that we comply with ethical requirements and plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the consolidated financial statements. The procedures selected depend on our judgment, including the assessment of the risks of material misstatement of the consolidated financial statements, whether due to fraud or error. In making those risk assessments, we consider internal control relevant to the entity’s preparation of the consolidated financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the consolidated financial statements of the Company for the year ended 31 December 2014 and year ended 31 December 2013:
| | • | | give a true and fair view of the state of the group’s affairs as at 31 December 2014 and 31 December 2013 and of its profit for the year ended 31 December 2014 and its loss for the year ended 31 December 2013; |
| | • | | have been properly prepared in accordance with International Financial Reporting Standards; and |
| | • | | have been properly prepared in accordance with the Companies (Jersey) Law 1991. |
|
 |
| Stephen Muncey |
|
| for and on behalf of KPMG LLP |
|
| Chartered Accountants |
|
| Botanic House, |
| 100 Hills Road, |
| Cambridge, |
| CB2 1AR, |
| United Kingdom. |
| 11 September 2015 |
A-7
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Consolidated Statement of Comprehensive Income
| | | | | | | | | | |
| | | Notes | | Year ended
31 December
2014
£‘000 | | | Year ended
31 December
2013
£‘000 | |
| | | |
Revenue | | 2 | | | 292,804 | | | | 240,963 | |
Cost of sales | | | | | (73,235 | ) | | | (57,735 | ) |
| | | | | | | | | | |
Gross profit | | | | | 219,569 | | | | 183,228 | |
| | | |
Other income | | | | | 331 | | | | 459 | |
Distribution costs | | | | | (15,262 | ) | | | (13,261 | ) |
| | | |
Non recurring items | | 21 | | | (13,874 | ) | | | (8,123 | ) |
Other administrative expenses | | | | | (98,415 | ) | | | (92,389 | ) |
| | | | | | | | | | |
Administrative expenses | | | | | (112,289 | ) | | | (100,512 | ) |
| | | |
Operating profit | | | | | 92,349 | | | | 69,914 | |
Finance costs | | 5 | | | (97,264 | ) | | | (91,329 | ) |
Finance income | | 5 | | | 13,571 | | | | 2,592 | |
| | | | | | | | | | |
Profit/(loss) before tax | | | | | 8,656 | | | | (18,823 | ) |
| | | |
Income tax (charge)/credit | | 6 | | | (2,453 | ) | | | 531 | |
| | | | | | | | | | |
Profit/(loss) for the financial year | | | | | 6,203 | | | | (18,292 | ) |
| | | |
Other comprehensive income/(expense) | | | | | | | | | | |
Items that may be reclassified subsequently to profit or loss: | | | | | | | | | | |
Currency translation differences | | | | | 1,132 | | | | (703 | ) |
| | | | | | | | | | |
Other comprehensive income/(expense) for the year, net of tax | | | | | 1,132 | | | | (703 | ) |
| | | | | | | | | | |
Total comprehensive profit/(loss) for the year | | | | | 7,335 | | | | (18,995 | ) |
| | | | | | | | | | |
The accompanying accounting policies and notes form an integral part of these consolidated financial statements.
A-8
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Consolidated Statement of Financial Position
| | | | | | | | | | |
| | | Notes | | As at
31 December
2014 £‘000 | | | As at
31 December
2013 £‘000 | |
Assets | | | | | | | | | | |
Non-current | | | | | | | | | | |
Intangible assets | | 7 | | | 903,213 | | | | 878,135 | |
Property, plant and equipment | | 8 | | | 2,386 | | | | 2,334 | |
Deferred tax assets | | 13 | | | 191 | | | | 345 | |
| | | | | | | | | | |
| | | | | 905,790 | | | | 880,814 | |
Current | | | | | | | | | | |
Inventories | | 9 | | | 35,028 | | | | 27,115 | |
Trade and other receivables | | 10 | | | 72,916 | | | | 55,790 | |
Current tax asset | | | | | 1,012 | | | | 1,695 | |
Other financial assets | | 17 | | | 61 | | | | 1,490 | |
Cash and cash equivalents | | 11 | | | 27,780 | | | | 62,790 | |
| | | | | | | | | | |
| | | | | 136,797 | | | | 148,880 | |
| | | | | | | | | | |
Total assets | | | | | 1,042,587 | | | | 1,029,694 | |
| | | | | | | | | | |
| | | |
Equity | | | | | | | | | | |
Share capital | | 12 | | | 94 | | | | 94 | |
Share premium | | | | | 16,480 | | | | 16,480 | |
Profit and loss account | | | | | (44,177 | ) | | | (50,380 | ) |
Currency translation reserve | | | | | 353 | | | | (779 | ) |
| | | | | | | | | | |
Total equity | | | | | (27,250 | ) | | | (34,585 | ) |
| | | | | | | | | | |
| | | |
Liabilities | | | | | | | | | | |
Non-current | | | | | | | | | | |
Long term borrowings | | 19 | | | 913,282 | | | | 946,501 | |
Deferred tax liabilities | | 13 | | | 59,979 | | | | 58,238 | |
| | | | | | | | | | |
| | | | | 973,261 | | | | 1,004,739 | |
Current | | | | | | | | | | |
Short term borrowings | | 19 | | | 22,120 | | | | 4,831 | |
Trade and other payables | | 14 | | | 44,318 | | | | 41,045 | |
Other financial liabilities | | 17 | | | 3,191 | | | | 158 | |
Other liabilities | | 15 | | | 26,947 | | | | 13,506 | |
| | | | | | | | | | |
| | | | | 96,576 | | | | 59,540 | |
| | | | | | | | | | |
Total liabilities | | | | | 1,069,837 | | | | 1,064,279 | |
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
Total equity and liabilities | | | | | 1,042,587 | | | | 1,029,694 | |
| | | | | | | | | | |
The consolidated financial statements were approved by the Board of Directors on 11 September 2015 and signed on their behalf by:
| | | | |
 | | | |  |
| Mark Wanless, Director | | | | Simon Radford, Director |
The accompanying accounting policies and notes form an integral part of these consolidated financial statements.
A-9
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Consolidated Statement of Changes in Equity
| | | | | | | | | | | | | | | | | | | | |
| | | Share
capital | | | Share
premium | | | Currency
translation
reserve | | | Profit and
loss account | | | Total
equity | |
| | | £‘000 | | | £‘000 | | | £‘000 | | | £‘000 | | | £‘000 | |
Balance at 1 January 2013 | | | 93 | | | | 16,380 | | | | (76 | ) | | | (32,088 | ) | | | (15,691 | ) |
Comprehensive expense | | | | | | | | | | | | | | | | | | | | |
Loss for the financial year | | | — | | | | — | | | | — | | | | (18,292 | ) | | | (18,292 | ) |
| | | | | |
Other comprehensive expense | | | | | | | | | | | | | | | | | | | | |
Currency translation differences | | | — | | | | — | | | | (703 | ) | | | — | | | | (703 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total comprehensive income | | | — | | | | — | | | | (703 | ) | | | (18,292 | ) | | | (18,995 | ) |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Transactions with owners | | | | | | | | | | | | | | | | | | | | |
Issue of shares | | | 1 | | | | 100 | | | | — | | | | — | | | | 101 | |
| | | | | | | | | | | | | | | | | | | | |
Total transactions with owners | | | 1 | | | | 100 | | | | — | | | | — | | | | 101 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Balance at 31 December 2013 | | | 94 | | | | 16,480 | | | | (779 | ) | | | (50,380 | ) | | | (34,585 | ) |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | Share
capital | | | Share
premium | | | Currency
translation
reserve | | | Profit and
loss account | | | Total
equity | |
| | | £‘000 | | | £‘000 | | | £‘000 | | | £‘000 | | | £‘000 | |
Balance at 1 January 2014 | | | 94 | | | | 16,480 | | | | (779 | ) | | | (50,380 | ) | | | (34,585 | ) |
Comprehensive income | | | | | | | | | | | | | | | | | | | | |
Profit for the financial year | | | — | | | | — | | | | — | | | | 6,203 | | | | 6,203 | |
| | | | | |
Other comprehensive income | | | | | | | | | | | | | | | | | | | | |
Currency translation differences | | | — | | | | — | | | | 1,132 | | | | — | | | | 1,132 | |
| | | | | | | | | | | | | | | | | | | | |
Total comprehensive income | | | — | | | | — | | | | 1,132 | | | | 6,203 | | | | 7,335 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Balance at 31 December 2014 | | | 94 | | | | 16,480 | | | | 353 | | | | (44,177 | ) | | | (27,250 | ) |
| | | | | | | | | | | | | | | | | | | | |
Currency translation differences recognised in other comprehensive income may be reclassified subsequently to the Statement of Comprehensive Income in future periods.
The accompanying accounting policies and notes form an integral part of these consolidated financial statements.
A-10
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Consolidated Cash Flow Statement
| | | | | | | | | | |
| | | Notes | | Year ended
31 December
2014 £‘000 | | | Year ended
31 December
2013
£‘000 | |
| | | |
Cash flows from operating activities | | | | | | | | | | |
Result for the period before tax | | | | | 8,656 | | | | (18,823 | ) |
Depreciation | | 8 | | | 896 | | | | 2,385 | |
Amortisation of intangible assets | | 7 | | | 44,749 | | | | 40,941 | |
Foreign exchange (gain)/loss on borrowings | | 5 | | | (12,779 | ) | | | 3,285 | |
Profit/(loss) on sale of assets | | | | | (3 | ) | | | 261 | |
Loss on sale of investments | | | | | — | | | | 7 | |
Finance costs | | 5 | | | 97,264 | | | | 88,044 | |
Finance income | | 5 | | | (792 | ) | | | (2,592 | ) |
| | | | | | | | | | |
| | | | | 137,991 | | | | 113,508 | |
| | | |
(Increase)/Decrease in inventories | | 9 | | | (3,979 | ) | | | 2,463 | |
(Increase) in trade and other receivables | | 10 | | | (12,225 | ) | | | (4,038 | ) |
(Decrease)/Increase in provisions, trade payables and other liabilities | | | | | (8,690 | ) | | | 6,677 | |
Taxes paid | | | | | (6,430 | ) | | | (10,892 | ) |
| | | | | | | | | | |
Net cash inflow from operating activities | | | | | 106,667 | | | | 107,718 | |
| | | |
Cash flows from investing activities | | | | | | | | | | |
Additions of: | | | | | | | | | | |
- intangible assets | | 7 | | | (2,978 | ) | | | (74,189 | ) |
- property, plant and equipment | | 8 | | | (781 | ) | | | (2,068 | ) |
Proceeds on sale of assets | | | | | 9 | | | | 892 | |
Proceeds on sale of investment | | | | | — | | | | 26 | |
Acquisition of subsidiaries (net of cash acquired) | | 20 | | | (45,166 | ) | | | (11,118 | ) |
Interest received | | 5 | | | 792 | | | | 1,260 | |
| | | | | | | | | | |
Net cash outflow from investing activities | | | | | (48,124 | ) | | | (85,197 | ) |
| | | |
Cash flows from financing activities | | | | | | | | | | |
Bank drawdowns | | | | | 935,019 | | | | 65,000 | |
Bank repayments | | | | | (482,966 | ) | | | (4,000 | ) |
Mezzanine debt paid | | | | | (128,422 | ) | | | — | |
Arrangement fees paid | | | | | (11,427 | ) | | | (6,559 | ) |
Interest paid | | | | | (38,906 | ) | | | (37,401 | ) |
Fixed rate unsecured loan notes 2052 repayments | | | | | (289,318 | ) | | | (112,949 | ) |
Interest paid on fixed rate unsecured loan notes 2052 | | | | | (79,902 | ) | | | (5,102 | ) |
Repayment of advances | | | | | — | | | | (600 | ) |
| | | | | | | | | | |
Net cash inflow from financing activities | | | | | (95,922 | ) | | | (101,611 | ) |
| | | |
Net decrease in cash and cash equivalents | | | | | (37,379 | ) | | | (79,090 | ) |
Exchange gain/(loss) on cash and cash equivalents | | | | | 2,369 | | | | (1,003 | ) |
Cash and cash equivalents at beginning of the year | | | | | 62,790 | | | | 142,883 | |
| | | | | | | | | | |
Cash and cash equivalents at the end of the year | | 11 | | | 27,780 | | | | 62,790 | |
| | | | | | | | | | |
The accompanying accounting policies and notes form an integral part of these consolidated financial statements.
A-11
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements
1. PRINCIPAL ACCOUNTING POLICIES
Corporate information
The consolidated financial statements of Amdipharm Mercury Limited (“the Company”) for the year ended 31 December 2014 were authorised for issue by the Board of Directors on 11 September 2015 and the Consolidated Statement of Financial Position was signed on the Board’s behalf by Mark Wanless and Simon Radford. Amdipharm Mercury Limited, a private limited company was incorporated in Jersey on 22 November 2012 as an ultimate holding company.
The principal activities of the Group are described in the Directors’ Report that accompanies these consolidated financial statements.
Basis of preparation
The consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (IFRSs) as issued by the International Accounting Standards Board (IASB).
The accounting policies set out below have, unless otherwise stated, been applied consistently to all periods presented in these financial statements and in preparing an opening IFRS balance sheet at 31 December 2013 for the purposes of the transition to Adopted IFRSs. A summary of the accounting policies applied in the preparation of these consolidated financial statements is given below. In addition, note 17 to the financial statements includes the Group’s objectives, policies and processes for managing its capital, its financial risk management risk objectives and details of its financial instruments.
The Group recognised a profit of£6.20 million (2013: loss of£18.29 million) for the year ended 31 December 2014. Consistent with this fact the financial statements have been prepared on a going concern basis which the Board of Directors believe is appropriate for the following reasons.
The Group is funded by a combination of ordinary share capital, cash holdings, bank term loans and loan notes. As highlighted in note 19, the bank term loans and revolving credit facilities are for a period of between 4 and 5 years. Repayments are ongoing and primarily on a half yearly basis. As with other groups with similar financing structures, the banking facilities are subject to periodic covenant tests.
The Directors have prepared projections for the next fifteen months from the date of these financial statements, including sensitivity analysis on key assumptions made, and consider the forecasts to be reasonable and realistic.
On the basis of these projections, and based on the current level of trading early in 2015, the Directors consider the Group will continue to operate within its facilities, remain compliant with its banking covenants and continue in operational existence for the foreseeable future. Hence the going concern basis is appropriate.
Standards, amendments and interpretations effective in 2014 adopted by the company
The following new standards and amendments to standards are mandatory for the first time for the financial year beginning 1st January 2014:
| • | | Offsetting financial assets and financial liabilities – amendments to IAS 32. |
| • | | Recoverable amount disclosures for non-financial assets – amendments to IAS 36. |
Standards, amendments and interpretations effective in 2014 but not relevant to group’s operation:
| • | | Investment entities (amendments to IFRS 10, IFRS 12 and IAS 27). |
| • | | Continuing hedge accounting after derivative novations – amendments to IAS 39. |
Standards, amendments and interpretations that are not yet effective and have not been early adopted:
| • | | Annual improvements to IFRS’s – 2010-2012 cycle. |
| • | | Annual improvements to IFRS’s – 2011-2013 cycle. |
| • | | Clarification of acceptable methods of depreciation and amortisation – amendments to IAS 36 and IAS 38. |
| • | | Annual improvements to IFRS’s – 2012-2014 cycle. |
| • | | IFRS 15 – Revenue from contracts with customers. |
| • | | IFRS 9 – Financial instruments. |
A-12
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
1. PRINCIPAL ACCOUNTING POLICIES (continued)
Standards, amendments and interpretations that are not yet effective and not currently relevant to the Group:
| • | | Defined benefit plans – employee contributions – amendments to IAS 19. |
| • | | IFRS 14 – Regulatory deferral accounts. |
| • | | Accounting for acquisitions of interests in joint operations – amendments to IFRS 11. |
| • | | Agriculture: bearer plants – amendments to IAS 16 and IAS 41. |
| • | | Equity method in separate financial statements – amendments to IAS 27. |
| • | | Sale or contribution of assets between an investor and its associate or joint venture – amendments to IFRS 10 and IAS 28. |
Basis of consolidation
The consolidated financial statements of the Group incorporate the results of the Company and its subsidiaries drawn up to 31 December 2014.
Control of the Companies subsidiaries was obtained in the year 2012 by transfer of the investments in the Mercury Pharma and Amdipharm groups to Amdipharm Mercury Debtco Limited, an intermediate holding company, registered in Jersey, under the common control of the Company. As these transfers occurred under common control, they are outside the scope of IFRS 3 ‘Business Combinations’.
The Consolidated Statement of Financial Position consolidates the assets and liabilities of those entities controlled by the Company as at 31 December 2014. The Consolidated Statement of Comprehensive Income, the Consolidated Statement of Changes in Equity and the Consolidated Cash Flow Statement consolidate the income, expenses, gains, losses and cash flows of entities controlled by the Company from the point at which control was obtained by the ultimate controlling party, Amdipharm Mercury Limited, to 31 December 2014.
The original acquisitions by the group headed by the Company of the Mercury Pharma and Amdipharm groups from unrelated third parties are business combinations that fall within the scope of IFRS 3. The acquisition method of accounting has therefore been applied to these transactions.
The cost of an acquisition is measured as the fair value of the assets given, equity instruments issued and liabilities incurred or assumed at the date of exchange. Identifiable assets acquired and liabilities and contingent liabilities assumed in a business combination are measured initially at their fair values on the date of acquisition, irrespective of the extent of any non-controlling interest. The excess of the cost of the acquisition over the fair value of the Group’s share of identifiable net assets, including intangible assets acquired, is recorded as goodwill. Acquisition costs are charged to the Consolidated Statement of Comprehensive Income as incurred.
As the transfers took place under the control of the Company they are considered to be transactions with these entities in their capacity as equity holders and the resulting change in equity has been recognised outside of total comprehensive income.
On consolidation, all intra group transactions, balances, income and expenditure are eliminated. An Employee Benefit Trust that is controlled by its sponsoring entity, which is a member of the Group, is consolidated into the financial statements.
A-13
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
1. PRINCIPAL ACCOUNTING POLICIES (continued)
Revenue
Revenue mainly comprises the fair value of the consideration received or receivable for the sale of goods.
Revenue is recognised when the significant risks and rewards of ownership have been transferred to a third party and generally this is upon delivery of the products to the wholesaler or distributor.
Revenue is shown net of value added tax, estimated returns and discounts. The methodology and assumptions used to estimate provision for discounts and returns are monitored and adjusted in the light of contractual and historical information.
Royalty income is recognised on an accruals basis in accordance with the substance of the relevant agreements.
Goodwill
Goodwill represents the excess of the cost of an acquisition over the fair value of the Group’s share of the net identifiable assets of the acquired subsidiary at the date of acquisition. Goodwill on acquisitions of subsidiaries is included in ‘intangible assets’. Goodwill is tested annually for impairment and carried at cost less accumulated impairment losses. Impairment losses on goodwill are not reversed. Gains and losses on the disposal of an entity include the carrying amount of goodwill relating to the entity sold.
Goodwill is allocated to cash-generating units for the purpose of impairment testing. The allocation is made to those cash-generating units or groups of cash-generating units that are expected to benefit from the business combination in which the goodwill arose identified according to operating segment.
Trademarks, licenses and product rights
Separately acquired trademarks, licences and product rights are shown at historical cost. Trademarks, licences and product rights acquired in a business combination are recognised at fair value at the acquisition date. Trademarks, licences and product rights have a finite useful life and are carried at cost less accumulated amortisation. Amortisation is calculated using the straight-line method to allocate the cost of trademarks, licences and product rights over their estimated useful lives of 5 to 20 years.
Technology under development
Costs incurred on development projects (relating to own development branded products and line extensions) are recognised as intangible assets when it is probable that the project will be a success considering its commercial and technological feasibility and costs can be measured reliably. Other development expenditures are recognised as an expense as incurred. Development costs previously recognised as an expense are not recognised as an asset in a subsequent year.
Technology under development acquired in a business combination is recognised separately as an intangible asset if and only if it meets the definition of an intangible asset in accordance with IAS 38 and its fair value can be measured reliably.
All development costs with a finite useful life that have been capitalised are amortised from the commencement of the commercial production of the product on a straight-line basis over the period of its expected benefit. Prior to commercial production of the product the asset is tested annually for impairment. Provision is made for any impairment.
Customer contracts
Customer contracts acquired in a business combination are recognised at fair value at the acquisition date. Customer contracts have a finite useful life and are carried at cost less accumulated amortisation. Amortisation is calculated using the straight-line method to allocate the cost of contract over their estimated useful lives of 5 years.
Distributor relationships
Distributor relationships acquired in a business combination are recognised at fair value at the acquisition date. The distributor relationships have a finite useful life and are carried at cost less accumulated amortisation. Amortisation is calculated using the straight-line method over the expected life of the distributor relationships. The estimated useful life of the existing distributor relationships is 5 to 10 years.
A-14
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
1. PRINCIPAL ACCOUNTING POLICIES (continued)
Software
Acquired software licences are capitalised on the basis of the costs incurred to acquire and bring into use the specific software. These costs are amortised over their estimated useful lives of between 3 to 5 years. The amortisation cost has been included within administrative expenses in the Statement of Comprehensive Income.
Property, plant and equipment
Property, plant and equipment is stated at cost less accumulated depreciation. Capitalised costs include borrowing costs where applicable in accordance with IAS 23. Depreciation is charged on a straight line basis over the estimated useful lives on the cost of the assets less their residual value. Land is not depreciated.
The estimated useful lives are as follows:
| | | | |
| Freehold buildings and leasehold improvements | | – | | 25 years or over the period of lease |
| Office equipment | | – | | 3 to 5 years |
| Plant and equipment | | – | | 6 to 7 years |
| Motor vehicles | | – | | 5 years |
Residual values are re-assessed annually.
Assets which have been purchased but are not ready for their intended use and directly attributable costs for construction of assets are shown under assets under the course of construction. These assets will be transferred to the relevant asset class on completion of construction of the asset or when the asset is ready for their intended use. Assets under the course of construction are carried at cost, less any recognised impairment loss. Depreciation commences when the assets are ready for their intended use.
Impairment of non-financial assets
Assets that have an indefinite useful life, excluding land, for example goodwill, are not subject to amortisation and are tested annually for impairment. Assets that are subject to amortisation are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. An impairment loss is recognised for the amount by which the asset’s carrying amount exceeds its recoverable amount. For the purposes of assessing impairment, assets are grouped at the lowest levels for which there are separately identifiable cash flows (cash generating units). An impairment loss is recognised as the amount by which the asset’s or cash generating unit’s carrying amount exceeds its recoverable amount. The recoverable amount is based on the higher of the fair value less costs to sell and value in use.
If at the balance sheet date there is any indication that an impairment loss recognised in prior periods for an asset other than goodwill no longer exists, the recoverable amount is reassessed and the asset is reflected at the recoverable amount.
Inventories
Inventories are stated at the lower of cost and net realisable value. The cost is determined using the weighted average price method. The cost of finished goods comprises product cost, its packaging and applicable duties and taxes. Net realisable value is the estimated selling price in the ordinary course of business, less applicable variable selling expenses.
Accounting for income taxes
Current income tax assets and/or liabilities comprise those obligations to, or claims from, fiscal authorities relating to the current or prior reporting period, that are unpaid at the balance sheet date. They are calculated according to the substantively enacted tax rates and tax laws applicable to the fiscal periods to which they relate, based on the taxable profit for the period.
Deferred tax is the tax expected to be payable or recoverable on differences between the carrying amounts of assets and liabilities in the financial statements and the corresponding tax bases used in the computation of taxable profit and is accounted for using the balance sheet liability method. This involves comparison of the carrying amount of assets and liabilities in the consolidated financial statements with their respective tax bases. However, deferred tax is not provided on the initial recognition of goodwill, nor on the initial recognition of an asset or liability unless the related transaction is a business combination or affects tax or accounting profit. Deferred tax liabilities are always provided for in full. Deferred tax assets and liabilities are calculated, without discounting, at tax rates that are expected to apply to the period when the asset is realised or the liability is settled, based on tax rates (tax laws) that have been enacted or substantively enacted by the balance sheet date. All changes in deferred tax assets or liabilities are recognised as a
A-15
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
1. PRINCIPAL ACCOUNTING POLICIES (continued)
Accounting for income taxes (continued)
component of tax expense in the Statement of Comprehensive Income, except where they relate to items that are charged or credited directly to equity (such as currency translation differences) in which case the related deferred tax is also charged or credited directly to equity.
Tax losses available to be carried forward as well as other income tax credits to the Group are assessed for recognition as deferred tax assets. Deferred tax assets are only recognised to the extent that it is probable that future taxable profits will be available against which the asset can be recognised and are reduced to the extent that it is no longer probable that the related tax benefit will be realised.
Deferred income tax is provided on temporary differences arising on investments in subsidiaries except where the timing of the reversal of the temporary differences is controlled by the Group and it is probable that the temporary difference will not reverse in the foreseeable future.
Cash and cash equivalents
Cash and cash equivalents comprise cash balances and call deposits with an original maturity of three months or less. Bank overdrafts that are repayable on demand and form an integral part of the Group’s cash management are included as a component of cash.
Employee Benefit Trust
The assets and liabilities of the Employee Benefit Trust (EBT) have been included in the consolidated financial statements. Any assets held by the EBT cease to be recognised in the Statement of Financial Position when the assets vest unconditionally in identified beneficiaries.
Employee benefits
The Group operates a defined contribution pension scheme whereby contributions are made to individual employee pension plans of certain employees. These costs are charged against profits in respect of the accounting period in which they are paid.
Leased assets
All leased assets are identified as operating leases if they do not transfer substantially all the risks and rewards to the lessee.
Payments made under operating leases are charged to the Statement of Comprehensive Income on a straight line basis over the period of the lease.
Foreign currencies
The reporting currency for these financial statements is UK sterling (£) which is the Company’s functional currency.
Transactions in foreign currencies are translated at the exchange rate ruling at the date of the transaction into the relevant company’s functional currency. Monetary assets and liabilities in foreign currencies are translated at the rates of exchange ruling at the balance sheet date. Foreign exchange differences arising on translation are recognised in profit or loss. Non monetary assets and liabilities that are measured in terms of historical cost in a foreign entity are translated using the exchange rate at the date of the transaction.
All assets and liabilities in the financial statements of foreign subsidiaries are translated at the closing rate at the balance sheet date. The results of foreign operations have been converted into the Group’s reporting currency at the average rates over the reporting period and the exchange differences arising have been taken to a currency translation reserve, a component of equity. The exchange differences arising from re-translation of the net investments in subsidiaries are directly taken to the currency translation reserve.
Dividends
Dividends proposed or declared after the balance sheet date is not recognised as a liability. However the amounts of such dividends are disclosed in the financial statements.
A-16
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
1. PRINCIPAL ACCOUNTING POLICIES (continued)
Segmental reporting
During the year, the results were reported separately under direct and distributor markets to the Board of Directors and constituted the distinct operating segments of the Group.
Financial instruments
Financial assets and financial liabilities are recognised in the Statement of Financial Position when the Group becomes a party to the contractual terms of the instrument.
Loans and receivables
Trade receivables are recognised initially at fair value and subsequently measured at amortised cost using the effective interest method, less provision for impairment. A provision for impairment of trade receivables is established when there is objective evidence that the Group will not be able to collect all amounts due according to the original terms of the receivables. Significant financial difficulties of the debtor, probability that the debtor will enter bankruptcy or financial reorganisation, and default or delinquency in payments (more than GO days overdue) are considered indicators that the trade receivable is impaired. The amount of the provision is the difference between the asset’s carrying amount and the present value of estimated future cash flows, discounted at the original effective interest rate. The carrying amount of the asset is reduced through the use of an allowance account, and the amount of the loss is recognised in the Statement of Comprehensive Income within ‘other administrative expenses’. When a trade receivable is uncollectible, it is written off against the allowance account for trade receivables. Subsequent recoveries of amounts previously written off are credited against ‘other administrative expenses’ in the Statement of Comprehensive Income.
Bank borrowings and loan notes
Borrowings are recognised initially at fair value, net of transaction costs incurred. Borrowings are subsequently carried at amortised cost; any difference between the proceeds (net of transaction costs) and the redemption value is recognised in profit and loss over the period of the borrowings using the effective interest method.
Fees paid on the establishment of loan facilities are recognised as transaction costs of the loan and are charged to profit and loss over the period of the loan.
Trade payables
Trade payables are obligations to pay for goods or services that have been acquired in the ordinary course of business from suppliers. Accounts payable are classified as current liabilities if payment is due within one year or less (or in the normal operating cycle of the business if longer). If not, they are presented as non-current liabilities.
Trade payables are recognised initially at fair value and subsequently measured at amortised cost using the effective interest method.
Equity instruments
Equity instruments issued by the Group are recorded at the proceeds received, net of direct issue costs.
| | | | | | |
| - | | Share capital | | - | | represents the nominal value of equity shares |
| - | | Share premium | | - | | represents amounts paid for equity shares in excess of their nominal value |
| - | | Retained earnings | | - | | represents the accumulated retained profits |
| - | | Currency translation reserve | | - | | represents gains or losses on foreign currency differences arising on consolidation of the net investment in subsidiaries. |
A-17
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
1. PRINCIPAL ACCOUNTING POLICIES (continued)
Critical accounting estimates and judgements
The preparation of financial statements requires management to make judgements, estimates and assumptions that affect the amounts reported for assets and liabilities as at the balance sheet date and the amounts reported for revenues and expenses during the year. However, the nature of estimation means that actual outcomes could differ from those estimates.
Estimates and judgements are continually evaluated and are based on historical experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances.
The Group makes estimates and assumptions concerning the future. The resulting accounting estimates will, by definition, seldom equal the related actual results. The estimates and assumptions that have a significant risk of causing material adjustment to the carrying amounts of assets and liabilities within the next financial year are discussed below.
Impairment of goodwill and other intangibles
The Group tests goodwill annually for impairment, and other intangible assets where indicators exist, in accordance with the accounting policy set out above. The recoverable amounts of cash generating units have been determined using value in use calculations. These calculations require the use of estimates.
Income taxes
The Group is subject to taxes in various jurisdictions. Significant judgment is required in determining the Group provision for income taxes. There are many transactions and calculations for which the ultimate tax determination is uncertain during the ordinary course of business. The Group recognises liabilities for anticipated tax audit issues based on estimates as to whether additional taxes will be due. Where the final tax outcome of these matters is different from the amounts that were initially recorded, such differences will impact the income tax and deferred tax provision in the year in which such determination is made.
Measurement of fair value
The Group is subject to payment of contingent consideration on various acquisitions over different periods. The Group has an established control framework with respect to the measurement of fair values and has an internal reporting and monitoring process for the contingent consideration. The measurement at fair value of contingent consideration on acquisition is performed by independent third party experts and relies on management’s estimates of achievement of contracted earn out milestones.
Capitalisation of development costs
The Group capitalises costs related to in-licensing and development projects related to its product pipeline, however there could be uncertainty around the timing of launch of the pipeline products and the commercial potential when these products are launched. The Group has a on-going process of monitoring these projects and assumptions around the commercial forecasts and launch dates of these products, which is a area of judgement.
A-18
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
2. SEGMENTAL REPORTING
Segment information is presented in the consolidated financial statements in respect of the Group’s business segments. The business segment-reporting format reflects the Group’s management and internal reporting structure. All operating segments’ operating results are reviewed regularly by the Board of Directors and the Group CEO to make decisions about resources to be allocated to the segment and assess its performance.
Business segments
The Group is currently organised into two major divisions: Direct markets and Distributor markets. These divisions form the basis for the Group’s reporting of segment information. Direct markets comprise of UK, Ireland, France, Nordics and Benelux regions.
Geographical segments
In presenting information based on the geographical segments, segment revenue is based on the geographical location of the customer.
Assets are identified to the segment on the basis of their place of use.
Segment results
Segment results include items directly attributable to a segment as well as those that can be allocated on a reasonable basis. The segment results are pre-tax.
All inter-segment revenues are priced and carried out at arm’s length; however there are no inter-segment revenues during the year.
Segment assets and liabilities
Segment assets include all operating assets used by a segment and consist principally of operating cash, trade and other receivables and property, plant and equipment, net of allowances and provisions which are reported as direct offsets in the Consolidated Statement of Financial Position. Segment liabilities include all operating Liabilities and consist principally of trade and other payable and other liabilities.
Unallocated corporate assets include mark to market receivable of interest rate and cross currency swaps.
Unallocated corporate liabilities include long term and short term borrowings, mark-to market liability of interest rate and cross currency swaps and accrued interest on borrowings.
A-19
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
2. SEGMENTAL REPORTING (continued)
Business segments
| | | | | | | | | | | | |
| Year ended 31 December 2014 | | Direct Markets | | | Distributor Markets | | | Total | |
| | | £‘000 | | | £‘000 | | | £‘000 | |
| | | |
Revenue | | | | | | | | | | | | |
External sales | | | 232,347 | | | | 60,457 | | | | 292,804 | |
Segment result | | | 93,458 | | | | 12,765 | | | | 106,223 | |
Non recurring items | | | | | | | | | | | (13,874 | ) |
| | | | | | | | | | | | |
Operating profit | | | | | | | | | | | 92,349 | |
Finance costs | | | | | | | | | | | (97,264 | ) |
Finance income | | | | | | | | | | | 13,571 | |
| | | | | | | | | | | | |
Profit before tax | | | | | | | | | | | 8,656 | |
Income tax expense | | | | | | | | | | | (2,453 | ) |
| | | | | | | | | | | | |
Profit for the financial year | | | | | | | | | | | 6,203 | |
| | | | | | | | | | | | |
| | | |
Other information | | | | | | | | | | | | |
Segment assets | | | 588,161 | | | | 454,365 | | | | 1,042,526 | |
Unallocated corporate assets | | | | | | | | | | | 61 | |
| | | | | | | | | | | | |
Consolidated total assets | | | | | | | | | | | 1,042,587 | |
| | | | | | | | | | | | |
| | | |
Segment Liabilities | | | 83,369 | | | | 47,875 | | | | 131,244 | |
Unallocated corporate liabilities | | | | | | | | | | | 938,593 | |
| | | | | | | | | | | | |
Consolidated total liabilities | | | | | | | | | | | 1,069,837 | |
| | | | | | | | | | | | |
| | | |
Capital expenditure (tangible and intangible assets) | | | 3,759 | | | | — | | | | 3,759 | |
Depreciation and amortization | | | 26,126 | | | | 19,519 | | | | 45,645 | |
Geographical segments
| | | | | | | | | | | | |
| | | Direct Markets £‘000 | | | Distributor Markets £‘000 | | | Total £‘000 | |
Revenue by destination of customer | | | | | | | | | | | | |
Year ended 31 December 2014 | | | | | | | | | | | | |
| | | |
United Kingdom | | | 187,398 | | | | — | | | | 187,398 | |
Rest of World | | | 44,949 | | | | 60,457 | | | | 105,406 | |
| | | | | | | | | | | | |
Total | | | 232,347 | | | | 60,457 | | | | 292,804 | |
| | | | | | | | | | | | |
| | | |
| | | Direct Markets
£‘000 | | | Distributor Markets
£‘000 | | | Total £‘000 | |
Assets by place of use | | | | | | | | | | | | |
At 31 December 2014 | | | | | | | | | | | | |
| | | |
United Kingdom | | | 588,161 | | | | — | | | | 588,161 | |
Rest of World | | | — | | | | 454,365 | | | | 454,365 | |
| | | | | | | | | | | | |
Total | | | 588,161 | | | | 454,365 | | | | 1,042,526 | |
| | | | | | | | | | | | |
A-20
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
2. SEGMENTAL REPORTING (continued)
| | | | | | | | | | | | |
| | | Direct
Markets
£‘000 | | | Distributor
Markets
£‘000 | | | Total £‘000 | |
Capital expenditure (tangible and intangible assets) | | | | | | | | | | | | |
Year ended 31 December 2014 | | | | | | | | | | | | |
| | | |
United Kingdom | | | 1,748 | | | | — | | | | 1,748 | |
Rest of World | | | — | | | | 2,011 | | | | 2,011 | |
| | | | | | | | | | | | |
Total | | | 1,748 | | | | 2,011 | | | | 3,759 | |
| | | | | | | | | | | | |
Business segments
| | | | | | | | | | | | |
| | | Direct | | | Distributor | | | | |
| Year ended 31 December 2013 | | Markets | | | Markets | | | Total | |
| | | £‘000 | | | £‘000 | | | £‘000 | |
| | | |
Revenue | | | | | | | | | | | | |
External sales | | | 182,473 | | | | 58,490 | | | | 240,963 | |
Segment result | | | 62,285 | | | | 15,752 | | | | 78,037 | |
Non recurring items | | | | | | | | | | | (8,123 | ) |
| | | | | | | | | | | | |
Operating profit | | | | | | | | | | | 69,914 | |
Finance costs | | | | | | | | | | | (91,329 | ) |
Finance income | | | | | | | | | | | 2,592 | |
| | | | | | | | | | | | |
Loss before tax | | | | | | | | | | | (18,823 | ) |
Income tax expense | | | | | | | | | | | 531 | |
| | | | | | | | | | | | |
Loss for the financial year | | | | | | | | | | | (18,292 | ) |
| | | | | | | | | | | | |
| | | |
Other information | | | | | | | | | | | | |
Segment assets | | | 538,595 | | | | 489,609 | | | | 1,028,204 | |
Unallocated corporate assets | | | | | | | | | | | 1,490 | |
| | | | | | | | | | | | |
Consolidated total assets | | | | | | | | | | | 1,029,694 | |
| | | | | | | | | | | | |
| | | |
Segment liabilities | | | 67,992 | | | | 44,797 | | | | 112,789 | |
Unallocated corporate liabilities | | | | | | | | | | | 951,490 | |
| | | | | | | | | | | | |
Consolidated total liabilities | | | | | | | | | | | 1,064,279 | |
| | | | | | | | | | | | |
| | | |
Capital expenditure (tangible and intangible assets) | | | 31,057 | | | | 45,200 | | | | 76,257 | |
Depreciation and amortization | | | 24,663 | | | | 18,663 | | | | 43,326 | |
Geographical segments
| | | | | | | | | | | | |
| | | Direct
Markets
£‘000 | | | Distributor
Markets
£‘000 | | | Total
£‘000 | |
Revenue by destination of customer | | | | | | | | | | | | |
Year ended 31 December 2013 | | | | | | | | | | | | |
| | | |
United Kingdom | | | 149,676 | | | | — | | | | 149,676 | |
Rest of World | | | 32,797 | | | | 58,490 | | | | 91,287 | |
| | | | | | | | | | | | |
Total | | | 182,473 | | | | 58,490 | | | | 240,963 | |
| | | | | | | | | | | | |
A-21
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
2. SEGMENTAL REPORTING (continued)
| | | | | | | | | | | | |
| | | Direct
Markets
£‘000 | | | Distributor
Markets
£‘000 | | | Total £‘000 | |
Assets by place of use | | | | | | | | | | | | |
United Kingdom | | | 538,595 | | | | — | | | | 538,595 | |
Rest of World | | | — | | | | 489,609 | | | | 489,609 | |
| | | | | | | | | | | | |
Total | | | 538,595 | | | | 489,609 | | | | 1,028,204 | |
| | | | | | | | | | | | |
| | | |
| | | Direct
Markets
£‘000 | | | Distributor
Markets
£‘000 | | | Total £‘000 | |
Capital expenditure (tangible and intangible assets) | | | | | | | | | | | | |
United Kingdom | | | 2,365 | | | | — | | | | 2,365 | |
Rest of World | | | — | | | | 73,892 | | | | 73,892 | |
| | | | | | | | | | | | |
Total | | | 2,365 | | | | 73,892 | | | | 76,257 | |
| | | | | | | | | | | | |
3. OPERATING PROFIT
The operating profit is stated after charging/(crediting):
| | | | | | | | |
| | | Year ended
31 December
2014 £‘000 | | | Year ended
31 December
2013 £‘000 | |
Depreciation and amortisation: | | | | | | | | |
- Intangible assets | | | 44,749 | | | | 40,941 | |
- Property, plant and equipment | | | 896 | | | | 2,385 | |
Hire of plant and machinery | | | 159 | | | | 61 | |
Other operating lease rentals | | | 1,429 | | | | 1,039 | |
Foreign exchange (gain)/losses: | | | | | | | | |
- Realised losses | | | 1,295 | | | | 772 | |
- Unrealised losses | | | 2,343 | | | | 1,500 | |
Research and development expenditure | | | 3,082 | | | | 1,082 | |
Auditors’ remuneration for audit and non audit services in jurisdictions in which the Group has a presence are analysed below:
| | | | | | | | |
| | | Year ended
31 December
2014 £‘000 | | | Year ended
31 December
2013 £‘000 | |
Group audit | | | | | | | | |
Audit fees to Company’s auditor for audit of the Group’s and subsidiaries annual accounts | | | 180 | | | | 190 | |
Other services | | | 6 | | | | 38 | |
The 2013 auditors’ remuneration for statutory audit services and non-audit services relate solely to amounts paid to KPMG Audit Plc. The 2014 amounts relate solely to amounts paid to KPMG LLP.
A-22
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
4. DIRECTORS AND EMPLOYEES
Employees
Staff costs during the year were as follows:
| | | | | | | | |
| | | Year ended
31 December
2014 £‘000 | | | Year ended
31 December
2013 £‘000 | |
Wages and salaries | | | 15,733 | | | | 14,489 | |
Social security costs | | | 2,034 | | | | 1,260 | |
Other pension costs | | | 960 | | | | 817 | |
| | | | | | | | |
| | | 18,727 | | | | 16,566 | |
| | | | | | | | |
The average number of employees is analysed below:
| | | | | | | | |
| | | Year ended
31 December
2014
Number | | | Year ended
31 December
2013
Number | |
Administration | | | 257 | | | | 190 | |
Marketing and Selling | | | 35 | | | | 24 | |
Management | | | 33 | | | | 28 | |
| | | | | | | | |
| | | 325 | | | | 242 | |
| | | | | | | | |
The Group contributes to employee money purchase pension schemes at a percentage of pay (depending on grade).
Key management remuneration
The key management personnel have been identified as the Directors of the Mercury Pharma Group and Aztec Directors Limited.
| | | | | | | | |
| | | Year ended
31 December
2014 £‘000 | | | Year ended
31 December
2013 £‘000 | |
Emoluments | | | 1,303 | | | | 1,169 | |
Pension contributions to money purchase pension schemes | | | 47 | | | | 43 | |
| | | | | | | | |
| | | 1,350 | | | | 1,212 | |
| | | | | | | | |
During the year, two directors (2013: two directors) participated in money purchase pension schemes.
A-23
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
5. FINANCE COSTS AND FINANCE INCOME
Finance costs and finance income includes all interest related income and expenses. The following amounts have been included in the Consolidated Statement of Comprehensive Income for the reporting periods presented:
| | | | | | | | |
| | | Year ended
31 December
2014 £‘000 | | | Year ended
31 December
2013 £‘000 | |
Interest expense resulting from | | | | | | | | |
-bank loans and overdrafts | | | 41,766 | | | | 42,199 | |
-amortisation of loan cost | | | 8,576 | | | | 5,136 | |
-unrealised forex loss on borrowings | | | — | | | | 3,285 | |
-currency and interest rate swaps | | | 4,462 | | | | — | |
-fixed unsecured loan notes 2052 | | | 42,177 | | | | 40,589 | |
-others | | | 283 | | | | 120 | |
| | | | | | | | |
Finance costs | | | 97,264 | | | | 91,329 | |
| | | | | | | | |
| | |
Interest income resulting from | | | | | | | | |
-unrealised forex gain on borrowings | | | 12,779 | | | | — | |
-short term bank deposits | | | 626 | | | | 550 | |
-corporation tax | | | 81 | | | | 3 | |
-interest rate swaps | | | — | | | | 1,332 | |
-others | | | 85 | | | | 707 | |
| | | | | | | | |
Finance income | | | 13,571 | | | | 2,592 | |
| | | | | | | | |
6. INCOME TAX (CHARGE) / CREDIT
| | | | | | | | |
| | | Year ended
31 December
2014 £‘000 | | | Year ended
31 December
2013 £‘000 | |
Current tax (charge)/credit: | | | | | | | | |
| | |
Current tax: | | | | | | | | |
Foreign taxation | | | (12,076 | ) | | | (14,707 | ) |
Adjustment to tax charges in respect of prior periods | | | 4,509 | | | | 2,772 | |
| | | | | | | | |
| | | (7,567 | ) | | | (11,935 | ) |
| | | | | | | | |
| | |
Deferred tax (note 13): | | | | | | | | |
| | | | | | | | |
- origination and reversal of temporary differences | | | 5,114 | | | | 12,466 | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Total income tax (charge)/credit (see below) | | | (2,453 | ) | | | 531 | |
| | | | | | | | |
Factors affecting current tax charge:
The tax assessed on profit/(loss) on ordinary activities for the year differs from the standard rate of corporation tax in Jersey which is 0%. As per IAS -12, the blended tax rate @ 23.81% (2013: -37.13%) is taken into consideration.
A-24
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
6. INCOME TAX (CHARGE) / CREDIT (continued)
The effective tax rate (ETR) of 23.81% has been arrived at by calculating the weighted average standard rate of tax, taking into consideration the tax charge as per the standard rate in the respective jurisdiction over the profit before tax of such jurisdictions.
The differences are explained below:
Reconciliation of effective tax rate:
| | | | | | | | |
| | | Year ended
31 December
2014 £‘000 | | | Year ended
31 December
2013 £‘000 | |
Profit/(loss) for the financial year before tax | | | 8,656 | | | | (18,823 | ) |
| | |
Weighted average tax rate | | | 23.81 | % | | | (37.13 | %) |
| | | | | | | | |
Expected tax (charge) | | | (2,061 | ) | | | (6,989 | ) |
Adjustment for: | | | | | | | | |
Non-deductible expenses | | | (10,122 | ) | | | (8,417 | ) |
Deferred tax movement | | | 5,114 | | | | 12,466 | |
Adjustment to tax charges in respect of prior periods | | | 4,509 | | | | 2,771 | |
Prior year loss adjusted | | | 107 | | | | 700 | |
| | | | | | | | |
Actual tax (charge)/credit, net (see above) | | | (2,453 | ) | | | 531 | |
| | | | | | | | |
The effective tax rate is impacted by the finance cost of the fixed unsecured loan notes 2052 of£42.18 million (2013:£40.59 million) which is a non-deductible expense as it attracts a tax rate of 0% in Jersey.
A reduction in the UK corporation tax rate from 24% to 23% (effective 1 April 2013) was substantively enacted on 3 July 2012. Further reductions to 21% (effective from 1 April 2014) and 20% (effective from 1 April 2015) were substantively enacted on 2 July 2013. In the Budget on 8 July 2015, the Chancellor announced additional planned reductions to 18% by 2020. This will reduce the company’s future current tax charge accordingly. The deferred tax liability at 31 December 2014 has been calculated based on the rate of 20% substantively enacted at the balance sheet date.
A-25
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
7. INTANGIBLE ASSETS
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Product rights, licensing agreement, product brands
£‘000 | | | Development costs £‘000 | | | Distributor relationships
£‘000 | | | Goodwill
£‘000 | | | Software
£‘000 | | | Total
£‘000 | |
Cost | | | | | | | | | | | | | | | | | | | | | | | | |
At 1 January 2013 | | | 526,645 | | | | 93 | | | | 13,300 | | | | 298,041 | | | | 755 | | | | 838,834 | |
Additions through business combination (note 20) | | | 9,915 | | | | — | | | | — | | | | 4,176 | | | | — | | | | 14,091 | |
Additions | | | 73,557 | | | | 404 | | | | — | | | | — | | | | 228 | | | | 74,189 | |
Exchange differences | | | — | | | | (12 | ) | | | — | | | | — | | | | (62 | ) | | | (74 | ) |
Disposals | | | — | | | | — | | | | — | | | | — | | | | (85 | ) | | | (85 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
At 31 December 2013 | | | 610,117 | | | | 485 | | | | 13,300 | | | | 302,217 | | | | 836 | | | | 926,955 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
At 1 January 2014 | | | 610,117 | | | | 485 | | | | 13,300 | | | | 302,217 | | | | 836 | | | | 926,955 | |
Additions through business combination (note 20) | | | 33,504 | | | | — | | | | 1,523 | | | | 33,330 | | | | — | | | | 68,357 | |
Additions | | | 417 | | | | 2,243 | | | | — | | | | — | | | | 318 | | | | 2,978 | |
Transfers | | | (508 | ) | | | 238 | | | | — | | | | — | | | | 270 | | | | —�� | |
Exchange differences | | | (1,179 | ) | | | 14 | | | | — | | | | (476 | ) | | | 10 | | | | (1,631 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
At 31 December 2014 | | | 642,351 | | | | 2,980 | | | | 14,823 | | | | 335,071 | | | | 1,434 | | | | 996,659 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
Amortisation | | | | | | | | | | | | | | | | | | | | | | | | |
At 1 January 2013 | | | 7,378 | | | | — | | | | 444 | | | | — | | | | 129 | | | | 7,951 | |
Charge for the year | | | 38,300 | | | | — | | | | 2,150 | | | | — | | | | 491 | | | | 40,941 | |
Disposals | | | — | | | | 32 | | | | — | | | | — | | | | — | | | | 32 | |
Exchange differences | | | — | | | | (4 | ) | | | — | | | | — | | | | (100 | ) | | | (104 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
At 31 December 2013 | | | 45,678 | | | | 28 | | | | 2,594 | | | | — | | | | 520 | | | | 48,820 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
At 1 January 2014 | | | 45,678 | | | | 28 | | | | 2,594 | | | | — | | | | 520 | | | | 48,820 | |
Charge for the year | | | 42,199 | | | | — | | | | 2,227 | | | | — | | | | 323 | | | | 44,749 | |
Exchange differences | | | (135 | ) | | | 1 | | | | — | | | | — | | | | 11 | | | | (123 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
At 31 December 2014 | | | 87,742 | | | | 29 | | | | 4,821 | | | | — | | | | 854 | | | | 93,446 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
Carrying amount | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
At 31 December 2013 | | | 564,439 | | | | 457 | | | | 10,706 | | | | 302,217 | | | | 316 | | | | 878,135 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
At 31 December 2014 | | | 554,609 | | | | 2,951 | | | | 10,002 | | | | 335,071 | | | | 580 | | | | 903,213 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
During the previous year, the Group acquired Fucithalmic®, a global ophthalmic product, from LEO Pharma for a consideration of£72 million which has been included under Product rights, licensing agreement, product brands.
A-26
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
7. INTANGIBLE ASSETS (continued)
The carrying amount of goodwill is allocated to the following cash generating units:
| | | | | | | | |
| | | Year ended
31 December
2014 £‘000 | | | Year ended
31 December
2013 £‘000 | |
Mercury Pharma Group | | | 232,774 | | | | 199,920 | |
Amdipharm Group | | | 102,297 | | | | 102,297 | |
| | | | | | | | |
| | | 335,071 | | | | 302,217 | |
| | | | | | | | |
The recoverable amounts of the cash generating units are determined from value in use calculations which use discounted pre tax cash flows based on the future forecasts for the estimated useful life between the ranges of 2-20 years.
Goodwill is not amortized but tested annually for impairment or more frequently if there are indications that it may be impaired. Goodwill has been allocated to two cash generating units in the current year for the purpose of impairment testing. The recoverable amount of the cash generating units is determined from value in use calculations. The value in use calculations are based on discounted cash flow forecasts over five years and then grown at 3% in perpetuity. The discount rate applied is 14%. The Directors believe that any reasonably possible change in the key assumptions on which the recoverable amounts are based would not cause the carrying amount of goodwill to exceed its recoverable amount.
A-27
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
8. PROPERTY, PLANT AND EQUIPMENT
| | | | | | | | | | | | | | | | | | | | |
| | | Freehold
land and
building
£‘000 | | | Assets under
course of
construction
£‘000 | | | Office equipment
£‘000 | | | Plant and
equipment
£‘000 | | | Total
£‘000 | |
Cost | | | | | | | | | | | | | | | | | | | | |
At 1 January 2013 | | | 891 | | | | — | | | | 802 | | | | 2,026 | | | | 3,719 | |
Addition through business combination (note 20) | | | — | | | | — | | | | — | | | | 19 | | | | 19 | |
Additions | | | 1,199 | | | | — | | | | 677 | | | | 192 | | | | 2,068 | |
Disposals | | | (739 | ) | | | — | | | | — | | | | (1,854 | ) | | | (2,593 | ) |
Exchange differences | | | (115 | ) | | | — | | | | (102 | ) | | | (1 | ) | | | (218 | ) |
| | | | | | | | | | | | | | | | | | | | |
At 31 December 2013 | | | 1,236 | | | | — | | | | 1,377 | | | | 382 | | | | 2,995 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
At 1 January 2014 | | | 1,236 | | | | — | | | | 1,377 | | | | 382 | | | | 2,995 | |
Addition through business combination (note 20) | | | 118 | | | | — | | | | 48 | | | | — | | | | 166 | |
Additions | | | 260 | | | | 150 | | | | 285 | | | | 86 | | | | 781 | |
Disposals | | | — | | | | — | | | | (207 | ) | | | — | | | | (207 | ) |
Exchange differences | | | 2 | | | | — | | | | 29 | | | | (2 | ) | | | 29 | |
| | | | | | | | | | | | | | | | | | | | |
At 31 December 2014 | | | 1,616 | | | | 150 | | | | 1,532 | | | | 466 | | | | 3,764 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Depreciation | | | | | | | | | | | | | | | | | | | | |
At 1 January 2013 | | | 10 | | | | — | | | | 126 | | | | 93 | | | | 229 | |
Charge for the year | | | 29 | | | | — | | | | 556 | | | | 1,800 | | | | 2,385 | |
Disposals | | | (6 | ) | | | — | | | | (60 | ) | | | (1,761 | ) | | | (1,827 | ) |
Exchange differences | | | (5 | ) | | | — | | | | (120 | ) | | | (1 | ) | | | (126 | ) |
| | | | | | | | | | | | | | | | | | | | |
At 31 December 2013 | | | 28 | | | | — | | | | 502 | | | | 131 | | | | 661 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
At 1 January 2014 | | | 28 | | | | — | | | | 502 | | | | 131 | | | | 661 | |
Charge for the year | | | 284 | | | | — | | | | 480 | | | | 132 | | | | 896 | |
Disposals | | | — | | | | — | | | | (201 | ) | | | — | | | | (201 | ) |
Exchange differences | | | 1 | | | | — | | | | 21 | | | | — | | | | 22 | |
| | | | | | | | | | | | | | | | | | | | |
At 31 December 2014 | | | 313 | | | | — | | | | 802 | | | | 263 | | | | 1,378 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Carrying amount | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
At 31 December 2013 | | | 1,208 | | | | — | | | | 875 | | | | 251 | | | | 2,334 | |
| | | | | | | | | | | | | | | | | | | | |
At 31 December 2014 | | | 1,303 | | | | 150 | | | | 730 | | | | 203 | | | | 2,386 | |
| | | | | | | | | | | | | | | | | | | | |
A-28
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
9. INVENTORIES
| | | | | | | | |
| | | 2014
£‘000 | | | 2013
£‘000 | |
Finished goods and goods for resale | | | 42,874 | | | | 34,454 | |
Write down on inventories | | | (7,846 | ) | | | (7,339 | ) |
| | | | | | | | |
| | | 35,028 | | | | 27,115 | |
| | | | | | | | |
In the year ended 31 December 2014,£73,235,000 (2013:£57,735,000) of inventories is included in the Consolidated Statement of Comprehensive Income in cost of sales. An amount of£1,954,000 (2013:£5,995,000) for write down of inventories has been included within other administrative expenses in the Consolidated Statement of Comprehensive Income for the year ended 31 December 2014.
10. TRADE AND OTHER RECEIVABLES
| | | | | | | | |
| | | 2014
£‘000 | | | 2013
£‘000 | |
Trade receivables | | | 70,013 | | | | 55,241 | |
Provision for impairment of trade receivables | | | (645 | ) | | | (1,060 | ) |
| | | | | | | | |
Trade receivables, net | | | 69,368 | | | | 54,181 | |
| | |
Prepayments and accrued income | | | 3,548 | | | | 1,577 | |
Salary advance to director | | | — | | | | 32 | |
| | | | | | | | |
| | | 72,916 | | | | 55,790 | |
| | | | | | | | |
Trade receivables that are less than 60 days past due are not considered impaired. As of 31 December 2014, trade receivables of£15,857,000 (2013:£9,334,000) were past due but not impaired. These relate to a number of independent customers for whom there is no recent history of default. The ageing analysis of these trade receivables is as follows:
| | | | | | | | |
| | | 2014
£‘000 | | | 2013
£‘000 | |
Up to two months | | | 11,203 | | | | 5,259 | |
Two to six months | | | 4,654 | | | | 4,075 | |
| | | | | | | | |
| | | 15,857 | | | | 9,334 | |
| | | | | | | | |
As at 31 December 2014, trade receivables of£645,000 (2013:£1,060,000) were considered to be impaired and provided for. The individually impaired receivables mainly relate to wholesalers/distributors, which are in unexpectedly difficult economic situations. The ageing of these receivables is as follows:
| | | | | | | | |
| | | 2014
£‘000 | | | 2013
£‘000 | |
Two to six months | | | 62 | | | | 22 | |
Over six months | | | 583 | | | | 1,038 | |
| | | | | | | | |
| | | 645 | | | | 1,060 | |
| | | | | | | | |
A-29
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
10. TRADE AND OTHER RECEIVABLES (continued)
The carrying amounts of the Group’s trade receivables are denominated in the following currencies:
| | | | | | | | |
| Currency | | 2014
£‘000 | | | 2013
£‘000 | |
Sterling | | | 38,292 | | | | 29,726 | |
Euro | | | 12,717 | | | | 11,196 | |
Dollar | | | 5,224 | | | | 4,591 | |
Other currencies | | | 13,135 | | | | 8,668 | |
| | | | | | | | |
| | | 69,368 | | | | 54,181 | |
| | | | | | | | |
Movements on the Group’s provision for impairment of trade receivables are as follows:
| | | | | | | | |
| | | 2014
£‘000 | | | 2013
£‘000 | |
At 1 January | | | 1,060 | | | | 1,516 | |
Provision for receivables impairment | | | 475 | | | | 11 | |
Receivables written off during the year as uncollectible | | | (890 | ) | | | (363 | ) |
Unused amounts reversed | | | — | | | | (104 | ) |
| | | | | | | | |
At 31 December | | | 645 | | | | 1,060 | |
| | | | | | | | |
The creation and release of provision for impaired receivables have been included in ‘other administrative expenses’ in the Consolidated Statement of Comprehensive Income. Amounts charged to the allowance account are generally written off, when there is no expectation of recovering additional cash.
The other classes within trade and other receivables do not contain impaired assets.
Trade receivables are usually due within 80 days and do not bear any effective interest rate. All trade receivables except the factored portion of the Retail Generics segment are subject to credit risk exposure. However the Group does not identify specific concentration of credit risk with regards to trade receivables, as the amount recognised resemble a large number of receivables from various customers.
The fair value of these short term financial assets is not individually determined as the carrying amount is a reasonable approximation of fair value.
11. CASH AND CASH EQUIVALENTS
| | | | | | | | |
| | | 2014
£‘000 | | | 2013
£‘000 | |
Cash at bank and in hand | | | 21,164 | | | | 57,384 | |
Short-term bank deposits | | | 6,616 | | | | 5,406 | |
| | | | | | | | |
| | | 27,780 | | | | 62,790 | |
| | | | | | | | |
The effective interest rate on short-term bank deposits was 8.4% (2013: 8.5%). These deposits have an average maturity of 112 days (2013: 120 days).
A-30
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
12. SHARE CAPITAL
| | | | | | | | |
| | | 2014
£‘000 | | | 2013
£‘000 | |
Allotted, called up and fully paid: | | | | | | | | |
14,058,617 A1 ordinary shares of 0.5 pence each (2013: 14,058,617 A1 ordinary shares of 0.5 pence each) | | | 70 | | | | 70 | |
405,939 A2 ordinary shares of 0.5 pence each (2013: 405,939 A2 ordinary shares of 0.5 pence each) | | | 2 | | | | 2 | |
1,180,877 A3 ordinary shares of 0.5 pence each (2013: 1,180,877 A3 ordinary shares of 0.5 pence each) | | | 6 | | | | 6 | |
900,000 B ordinary shares of 0.5 pence each (2013: 900,000 B ordinary shares of 0.5 pence each) | | | 5 | | | | 5 | |
900,000 C ordinary shares of 0.01 pence each (2013: 900,000 C ordinary shares of 0.01 pence each) | | | — | | | | — | |
1,568,000 E ordinary shares of 0.7 pence each (2013: 1,568,000 E ordinary shares of 0.7 pence each) | | | 11 | | | | 11 | |
| | | | | | | | |
| | | 94 | | | | 94 | |
| | | | | | | | |
During the prior year, the Company issued 98,820 C ordinary shares with a par value of 0.01 pence each for consideration of£101,000, which resulted in a credit to share capital of£1,000 and to share premium of£100,000.
13. DEFERRED TAX ASSETS AND LIABILITIES
Deferred tax assets
| | | | | | | | | | | | | | | | | | | | |
| | | Intangible
assets
£‘000 | | | Property, plant
and equipment
£‘000 | | | Other assets
£‘000 | | | Unused tax
losses
£‘000 | | | Total
£‘000 | |
At 1 January 2013 | | | 341 | | | | 50 | | | | 8 | | | | 554 | | | | 953 | |
Recognised in income | | | (154 | ) | | | (14 | ) | | | (8 | ) | | | (435 | ) | | | (611 | ) |
Reclassification | | | — | | | | 3 | | | | — | | | | — | | | | 3 | |
| | | | | | | | | | | | | | | | | | | | |
At 31 December 2013 | | | 187 | | | | 39 | | | | — | | | | 119 | | | | 345 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
| | | Intangible
assets
£‘000 | | | Property, plant
and equipment
£‘000 | | | Other assets
£‘000 | | | Unused tax
losses
£‘000 | | | Total
£‘000 | |
At 1 January 2014 | | | 187 | | | | 39 | | | | — | | | | 119 | | | | 345 | |
Recognised in income | | | (55 | ) | | | 21 | | | | — | | | | (119 | ) | | | (153 | ) |
Exchange differences | | | — | | | | (1 | ) | | | — | | | | — | | | | (1 | ) |
| | | | | | | | | | | | | | | | | | | | |
At 31 December 2014 | | | 132 | | | | 59 | | | | — | | | | — | | | | 191 | |
| | | | | | | | | | | | | | | | | | | | |
A-31
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
13. DEFERRED TAX ASSETS AND LIABILITIES (continued)
Deferred tax liabilities
| | | | | | | | | | | | | | | | | | | | |
| | | Intangible
assets
£‘000 | | | Property, plant
and equipment
£‘000 | | | Other
liabilities
£‘000 | | | Unused tax
losses
£‘000 | | | Total
£‘000 | |
At 1st January 2013 | | | 68,885 | | | | 42 | | | | — | | | | — | | | | 68,927 | |
Recognised on acquisition | | | | | | | | | | | | | | | | | | | | |
Abcur (note 20) | | | 2,181 | | | | — | | | | — | | | | 205 | | | | 2,386 | |
Recognised in income | | | (13,092 | ) | | | 16 | | | | — | | | | — | | | | (13,076 | ) |
Reclassification | | | — | | | | 3 | | | | — | | | | — | | | | 3 | |
Exchange differences | | | — | | | | (2 | ) | | | — | | | | — | | | | (2 | ) |
| | | | | | | | | | | | | | | | | | | | |
At 31 December 2013 | | | 57,974 | | | | 59 | | | | — | | | | 205 | | | | 58,238 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
| | | Intangible
assets
£‘000 | | | Property, plant
and equipment
£‘000 | | | Other
liabilities
£‘000 | | | Unused tax
losses
£‘000 | | | Total
£‘000 | |
At 1st January 2014 | | | 57,974 | | | | 59 | | | | — | | | | 205 | | | | 58,238 | |
Recognised on acquisition | | | | | | | | | | | | | | | | | | | | |
Focus (note 20) | | | 7,005 | | | | — | | | | — | | | | — | | | | 7,005 | |
Recognised in income | | | (5,572 | ) | | | 87 | | | | — | | | | 218 | | | | (5,267 | ) |
Exchange differences | | | — | | | | 3 | | | | — | | | | — | | | | 3 | |
| | | | | | | | | | | | | | | | | | | | |
At 31 December 2014 | | | 59,407 | | | | 149 | | | | — | | | | 423 | | | | 59,979 | |
| | | | | | | | | | | | | | | | | | | | |
Unrecognised deferred taxes
| | | | | | | | |
| | | 2014
£‘000 | | | 2013
£‘000 | |
India | | | 1,005 | | | | 901 | |
UK | | | 1,047 | | | | 1,262 | |
| | | | | | | | |
The unrecognised deferred tax is related to tax losses for Indian subsidiaries which will expire in year 2018. For UK entities the losses pertain to December 2012. Deferred tax assets on the tax losses have not been recognised as the realisation of the related tax benefit through the future taxable profits cannot be forecast with sufficient certainty.
14. TRADE AND OTHER PAYABLES
| | | | | | | | |
| | | 2014
£‘000 | | | 2013
£‘000 | |
Trade payables | | | 14,788 | | | | 10,647 | |
Accruals | | | 29,530 | | | | 30,398 | |
| | | | | | | | |
| | | 44,318 | | | | 41,045 | |
| | | | | | | | |
Trade payables are non-interest bearing and are settled on an average 43 days (2013: 49 days). Accruals have an average term of 90 to 120 days until they are settled.
A-32
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
15. OTHER LIABILITIES
| | | | | | | | |
| | | 2014
£‘000 | | | 2013
£‘000 | |
Social security and other taxes | | | 5,415 | | | | 4,721 | |
Other creditors | | | 21,532 | | | | 8,785 | |
| | | | | | | | |
| | | 26,947 | | | | 13,506 | |
| | | | | | | | |
16. OPERATING LEASES
The Group’s minimum operating lease payments are as follows:
| | | | | | | | |
| | | 2014
Land and
buildings
£‘000 | | | 2013
Land and
buildings
£‘000 | |
Within one year | | | 775 | | | | 1,084 | |
Between two and five years | | | 1,611 | | | | 2,727 | |
More than 5 years | | | 77 | | | | — | |
| | | | | | | | |
| | | 2,463 | | | | 3,811 | |
| | | | | | | | |
Lease costs recognised as an expense during the year amount to£1,429,000 (2013:£1,039,000). No sublease income is expected as all assets held under lease agreements are used exclusively by the Group.
No operating lease agreements contain any contingent rent clauses.
The Group did not have any finance leases at 31 December 2014 (2013: Nil)
17. FINANCIAL INSTRUMENTS
Monitoring of financial risk is part of the Board’s ongoing risk assessment process.
The Group uses financial instruments, comprising cash, short term and long terms borrowings, trade receivables and trade payables, which arise directly from its operations. The main purpose of these financial instruments is to raise finance for the Group’s operations.
Financial assets are measured at amortised cost or fair value and comprise trade receivables, accrued income, interest rate swaps and cash and cash equivalents. Financial liabilities are measured at amortised cost or fair value and comprise trade payables, accruals, other creditors, interest rate swaps and long and short term borrowings.
Credit risk
The Group’s principal financial assets are bank balances, cash and trade receivables, which represent the Group’s maximum exposure to credit risk in relation to financial assets.
The Group’s credit risk is primarily attributable to its trade receivables. Credit risk is managed by monitoring the aggregate amount and duration of exposure to any one customer depending upon their credit rating. Where export customers are granted credit terms, credit insurance of some, or all of the balances is generally sought. The amounts presented in the Statement of Financial Position are net of allowances for doubtful debts, estimated by management based on prior experience and their assessment of the current economic environment.
The Group has no significant concentration of credit risk with exposure spread over a large number of counterparties and customers.
A-33
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
17. FINANCIAL INSTRUMENTS (continued)
Cash flow and fair value interest rate risk
The Group’s interest rate risk arises from long-term borrowings. Borrowings issued at variable rates expose the Group to cash flow interest rate risk which is partially offset by cash held at variable rates. Borrowings issued at fixed rates expose the Group to fair value interest rate risk. During 2014, the Group’s borrowings at variable rate were denominated in UK sterling (£) and Euro (€).
The Group does not have significant interest-bearing assets and therefore the Group’s income and operating cash flows are substantially independent of changes in market interest rates.
The Group finances its operations through a mixture of retained profits and bank facilities. Bank borrowings are made using variable interest rates and loan notes have a fixed 12% interest rate.
An interest rate sensitivity analysis assumes an instantaneous 100 basis point change in interest rates in all currencies from their levels at 31 December 2014, with all other variables held constant. Based on the composition of the Group’s debt portfolio as at 31 December 2014, a 1% increase/decrease in interest rates would result in an additional£8,796,648 (2013:£9,499,698) in interest expense/income for the year.
| | | | | | | | |
| | | 2014 | |
| | | Fixed rate
£‘000 | | | Floating rate
£‘000 | |
Financial liabilities | | | | | | | | |
Interest bearing loans and borrowings | | | — | | | | 958,186 | |
| | |
Financial assets | | | | | | | | |
Cash and cash equivalents | | | — | | | | 27,780 | |
A-34
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
17. FINANCIAL INSTRUMENTS (continued)
| | | | | | | | |
| | | 2013 | |
| | | Fixed rate
£‘000 | | | Floating rate
£‘000 | |
Financial liabilities | | | | | | | | |
Interest bearing loans and borrowings | | | — | | | | 974,407 | |
| | |
Financial assets | | | | | | | | |
Cash and cash equivalents | | | — | | | | 62,790 | |
Liquidity risk
Cash flow forecasting is performed in the operating entities of the Group and aggregated by Group finance. Group finance monitors rolling forecasts of the Group’s liquidity requirements to ensure it has sufficient cash to meet operational needs while maintaining sufficient headroom on its undrawn committed borrowing facilities (note 19) at all times so that the Group does not breach borrowing limits or covenants (where applicable) on any of its borrowing facilities. Such forecasting takes into consideration the Group’s debt financing plans, covenant compliance and compliance with internal balance sheet ratio targets.
Currency risk
The Group is exposed to translation and transaction foreign exchange risk. In relation to translation risk the proportion of assets held in the foreign currency are matched to an appropriate level of borrowings in the same currency. Transaction exposures are hedged when known, mainly using the forward exchange hedge market.
Capital management
The Group’s objectives when managing capital (retained profits and borrowings) are to safeguard the Group’s ability to continue as a going concern, support the growth of the business and to maintain an optimal capital structure to reduce the cost of borrowing. The Board of Directors review the Group’s funding requirements annually. The financing of the Group consists of borrowings (see note 19, ‘Long term and short term borrowings’), cash and cash equivalents and shareholders’ equity (see ‘Consolidated Statement of Changes in Equity’).
The Group seeks to hedge its exposures using a variety of financial instruments, with the objective of minimising the impact of fluctuations in exchange rates on future transactions and cash flows.
£105.41 million (2013:£91.29 million) of the sales of the Group’s business is to customers in continental Europe/foreign markets. The majority of these sales are invoiced in the currencies of the customers involved. The Group policy is to minimise all currency exposures on any balance not expected to mature within 60 days of its arising through the use of forward currency contracts, however there were none in place at the year end. All other sales of UK business are denominated in sterling.
A-35
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
17. FINANCIAL INSTRUMENTS (continued)
The tables below show the extent to which Group companies have monetary assets and liabilities in currencies other than the presentation currency of the Group.
Functional currency of operation
| | | | | | | | | | | | | | | | | | | | |
| | | Net foreign currency monetary assets/ (liabilities) | |
| | | US Dollars
£000 | | | Euro £000 | | | Indian Rupees £000 | | | Other currencies £000 | | | Total
£000 | |
2014 | | | | | | | | | | | | | | | | | | | | |
Sterling | | | 4,843 | | | | 8,905 | | | | — | | | | 10,803 | | | | 24,551 | |
Indian Rupees | | | — | | | | — | | | | 6,760 | | | | — | | | | 6,760 | |
Euro | | | — | | | | 27 | | | | — | | | | — | | | | 27 | |
Swedish Krona | | | 634 | | | | 161 | | | | — | | | | 2,396 | | | | 3,191 | |
United Arab Emirates Dirham | | | — | | | | — | | | | — | | | | 7 | | | | 7 | |
Hong Kong Dollar | | | — | | | | — | | | | — | | | | 16 | | | | 16 | |
| | | | | | | | | | | | | | | | | | | | |
| | | 5,477 | | | | 9,093 | | | | 6,760 | | | | 13,222 | | | | 34,552 | |
| | | | | | | | | | | | | | | | | | | | |
| |
| | | Net foreign currency monetary assets/ (liabilities) | |
| | | US Dollars
£000 | | | Euro £000 | | | Indian Rupees £000 | | | Other currencies £000 | | | Total
£000 | |
2013 | | | | | | | | | | | | | | | | | | | | |
Sterling | | | 6,623 | | | | 16,664 | | | | (4 | ) | | | 14,505 | | | | 37,788 | |
Indian Rupees | | | — | | | | — | | | | 7,227 | | | | — | | | | 7,227 | |
Swedish Krona | | | — | | | | — | | | | — | | | | 1,547 | | | | 1,547 | |
| | | | | | | | | | | | | | | | | | | | |
| | | 6,623 | | | | 16,664 | | | | 7,223 | | | | 16,052 | | | | 46,562 | |
| | | | | | | | | | | | | | | | | | | | |
Fair value of financial assets and liabilities
The fair value of a financial asset or liability is the amount at which the instrument could be exchanged in a current transaction between willing parties, other than in a forced or liquidation sale. For the financial assets and liabilities of the Group, the fair values have been estimated as described below:
| | | | |
| Cash and cash equivalents | | - | | approximates to the carrying amount; |
| Short-term borrowings | | - | | approximates to the carrying amount because of the short maturity of these instruments; |
| Long-term borrowings | | - | | approximates to the carrying amount in the case of floating rate bank loans and other loans; |
| Receivables and payables | | - | | approximates to the carrying amount. |
A-36
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
17. FINANCIAL INSTRUMENTS (continued)
FINANCIAL INSTRUMENTS- FAIR VALUES AND RISK MANAGEMENT
| | A) | Accounting classifications and fair values |
The following table shows the carrying amounts and fair values of financial assets and financial liabilities, including their levels in the fair value hierarchy. It does not include fair value information for financial assets and financial liabilities not measured at fair value if the carrying amount is a reasonable approximation of fair value
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 31 December 2014 | | | | | Carrying amount | | | Fair value | |
| £000’s | | Note | | | Designated at fair value | | | Fair value
hedging
instruments | | | Loans and
receivables | | | Other financial liabilities | | | Total | | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
| | | | | | | | | |
Financial assets measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest rate swaps | | | | | | | — | | | | 61 | | | | — | | | | — | | | | 61 | | | | — | | | | 61 | | | | — | | | | 61 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | — | | | | 61 | | | | — | | | | — | | | | 61 | | | | — | | | | 61 | | | | — | | | | 61 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | |
Financial assets not measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Trade and other receivables | | | 10 | | | | — | | | | — | | | | 72,916 | | | | — | | | | 72,916 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | |
Cash and cash equivalents | | | 11 | | | | — | | | | — | | | | 27,780 | | | | — | | | | 27,780 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | — | | | | — | | | | 100,696 | | | | — | | | | 100,696 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
Financial liabilities measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest rate swaps | | | | | | | — | | | | (3,191 | ) | | | — | | | | — | | | | (3,191 | ) | | | — | | | | (3,191 | ) | | | — | | | | (3,191 | ) |
| | | | | | | | | | |
Focus contingent consideration (2014) | | | 20 | | | | (17,155 | ) | | | — | | | | — | | | | — | | | | (17,155 | ) | | | — | | | | — | | | | (17,155 | ) | | | (17,155 | ) |
| | | | | | | | | | |
Abcur contingent consideration (2013) | | | 20 | | | | (3,318 | ) | | | — | | | | — | | | | — | | | | (3,318 | ) | | | — | | | | — | | | | (3,318 | ) | | | (3,318 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | (20,473 | ) | | | (3,191 | ) | | | — | | | | — | | | | (23,664 | ) | | | — | | | | (3,191 | ) | | | (20,473 | ) | | | (23,664 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | |
Financial liabilities not measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Secured bank loans | | | 19 | | | | — | | | | — | | | | — | | | | (905,494 | ) | | | (905,494 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | |
Fixed unsecured loan notes 2052 | | | 19 | | | | — | | | | — | | | | — | | | | (40,913 | ) | | | (40,913 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | |
Trade payables | | | 14 | | | | — | | | | — | | | | — | | | | (44,318 | ) | | | (44,318 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | — | | | | — | | | | — | | | | (990,725 | ) | | | (990,725 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | |
| 31 December 2013 | | | | | Carrying amount | | | Fair value | |
| £000’s | | Note | | | Designated at
fair value | | | Fair value
hedging
instruments | | | Loans and
receivables | | | Other financial liabilities | | | Total | | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
| | | | | | | | | |
Financial assets measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
Interest rate swaps | | | | | | | — | | | | 1,490 | | | | — | | | | — | | | | 1,490 | | | | — | | | | 1,490 | | | | — | | | | 1,490 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | — | | | | 1,490 | | | | — | | | | — | | | | 1,490 | | | | — | | | | 1,490 | | | | — | | | | 1,490 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | |
Financial assets not measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
Trade and other receivables | | | 10 | | | | — | | | | — | | | | 55,790 | | | | — | | | | 55,790 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | |
Cash and cash equivalents | | | 11 | | | | — | | | | — | | | | 62,790 | | | | — | | | | 62,790 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | — | | | | — | | | | 118,580 | | | | — | | | | 118,560 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | |
Financial liabilities measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
Interest rate swaps | | | | | | | — | | | | (158 | ) | | | — | | | | — | | | | (158 | ) | | | — | | | | (158 | ) | | | — | | | | (158 | ) |
| | | | | | | | | | |
Abcur contingent consideration (2013) | | | 20 | | | | (2,213 | ) | | | — | | | | — | | | | — | | | | (2,213 | ) | | | — | | | | — | | | | (2,213 | ) | | | (2,213 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | (2,213 | ) | | | (158 | ) | | | — | | | | — | | | | (2,371 | ) | | | — | | | | (158 | ) | | | (2,213 | ) | | | (2,371 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | |
Financial liabilities not measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Secured bank loans | | | 19 | | | | — | | | | — | | | | — | | | | (466,250 | ) | | | (466,250 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | |
Fixed unsecured loan notes 2052 | | | 19 | | | | — | | | | — | | | | — | | | | (330,231 | ) | | | (330,231 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | |
Trade payables | | | 14 | | | | — | | | | — | | | | — | | | | (41,045 | ) | | | (41,045 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | — | | | | — | | | | — | | | | (837,526 | ) | | | (837,526 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
A-37
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
17. FINANCIAL INSTRUMENTS (continued)
| | B) | Measurement of fair values |
i) Valuation techniques and significant unobservable inputs
The following tables show the valuation techniques used in measuring Level 2 and Level 3 fair values, as well as the significant unobservable inputs used.
Financial instruments measured at fair values
| | | | | | |
| Type | | Valuation technique | | Significant unobservable inputs | | Inter-relationship between significant
unobservable inputs and fair value
measurements |
| Interest rate swaps | | Market comparison technique: The fair values are based on bank quotes. Similar contracts are traded in an active market and the quotes reflect the actual transactions in similar instruments | | Not applicable | | Not applicable |
| | | | |
| Focus contingent consideration (2014) | | Discounted cash flows: The valuation model considers the present value of expected payment, discounted using a risk - adjusted discount rate. The expected payment is determined by considering the possible scenarios of gross profit threshold, receiving market authorisations and ensuring continuity of supply of the products, the amount to be paid under each scenario and the probability of each scenario. | | Gross profit thresholds for 12 months ending December 2015 and 2016, subject to a cap of£7 million and£4 million respectively. Contingent consideration of£934k paid in January 2015 contributes to the estimate of fair value. | | The estimated fair value would decrease if: - the annual gross profit growth rates were lower. |
| | | | |
| Abcur contingent consideration (2013) | | Discounted cash flows: The valuation model considers the present value of expected payment, discounted using a risk - adjusted discount rate. The expected payment is determined by considering the possible scenarios of forecast revenue and EBITDA , the amount to be paid under each scenario and the probability of each scenario | | Certain revenue and EBITDA thresholds for 12 months ending November 2014 and 2015 and also SEK 500k is payable for every 1% outperformance (subject to cap of 20%) of EBITDA and revenue thresholds. Contingent consideration of£1.3 million paid in January 2015 contributes to the estimate of fair value. | | The estimated fair value would increase/(decrease) if: - the annual revenue growth rate were lower,-the EBITDA margin were lower or;- the risk adjusted discount rate were lower/ (higher). Generally, a change in the annual revenue growth rate is accompanied by a directionally similar change in EBITDA. |
A-38
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
17. FINANCIAL INSTRUMENTS (continued)
| B) | Measurement of fair values (continued) |
ii) Level 3 fair values
Reconciliation of Level 3 fair values
The following table shows reconciliation from the opening balances to the closing balances for Level 3 fair values.
| | | | |
| | | Contingent consideration | |
Balance as at 1 January 2013 | | | (851 | ) |
Paid during the year | | | 851 | |
Assumed in business combination (note 20) | | | (2,213 | ) |
| | | | |
Balance as at 31 December 2013 | | | (2,213 | ) |
| | | | |
Balance as at 1 January 2014 | | | (2,213 | ) |
Recognised in profit and loss account (Abcur) | | | (1,105 | ) |
Assumed in business combination (note 20) | | | (17,155 | ) |
| | | | |
| |
Balance as at 31 December 2014 | | | (20,473 | ) |
| | | | |
Sensitivity analysis
Focus contingent consideration:
Management have assumed that the earn out targets will be reached in period one and period two and have therefore recognised the maximum fair value payable. In order for the contingent consideration to be affected, it would require a fall in forecast gross profit of 19% in earn out period one and 57% in earn out period two. The second element of the contingent consideration is in respect of securing approval for particular Market Authorisations. The current fair value assumes these Market Authorisations will be approved, if this is not achieved then this element of the contingent consideration would reduce to£nil.
Abcur contingent consideration:
The fair value of the contingent consideration is based upon two earn out period calculations with target revenue and EBITDA figures for November 2014 and November 2015. In January 2015 a payment was made in respect of the first earn out period and this has been deemed to be fair value and further sensitivity analysis is not considered necessary. Management have assumed the maximum earn out targets will be reached in period two and have therefore recognised the maximum fair value amount. In order for this element of the contingent consideration to be affected it would require a fall in forecast revenue of 14 % and a fall in forecast EBITDA of 17%.
18. CAPITAL COMMITMENTS
At 31 December 2014, the Group had entered into a contract to purchase property, plant and equipment of£566,000 (2013:£nil). These commitments are expected to be settled in the following financial year.
A-39
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
19. LONG TERM AND SHORT TERM BORROWINGS
On 28 November 2014 the group successfully completed a refinancing process, which involved raising new debt and paying off the Mezzanine debt. The proceeds were used to make a more efficient use of borrowings by paying off the Mezzanine debt and to make repayments to the fixed unsecured loan notes 2052.
| | | | | | | | |
| | | 2014
£‘000 | | | 2013
£‘000 | |
Short-term borrowings: | | | | | | | | |
Senior Debt A | | | 25,957 | | | | — | |
Senior Debt A1 | | | — | | | | 6,000 | |
Senior Debt A2 | | | — | | | | 4,000 | |
Interest accrued | | | 3,391 | | | | 248 | |
Arrangement fees capitalised | | | (7,228 | ) | | | (5,417 | ) |
| | | | | | | | |
| | | 22,120 | | | | 4,831 | |
| | | | | | | | |
| | |
Long-term borrowings: | | | | | | | | |
Senior Debt A | | | 133,493 | | | | — | |
Senior Debt A1 | | | — | | | | 51,600 | |
Senior Debt A2 | | | — | | | | 34,400 | |
Senior Debt B1 | | | 352,332 | | | | 155,000 | |
Senior Debt B2 | | | 343,949 | | | | 165,250 | |
Acquisition facility | | | 49,763 | | | | 50,000 | |
Mezzanine | | | — | | | | 128,422 | |
Arrangement fees capitalised | | | (18,947 | ) | | | (17,906 | ) |
Fixed unsecured loan notes 2052 | | | 52,692 | | | | 379,735 | |
| | | | | | | | |
| | | 913,282 | | | | 946,501 | |
| | | | | | | | |
The borrowings are denominated in UK Sterling (£) currency except Senior Debt B2 being denominated in Euro (€).
| | | | | | | | | | | | | | | | | | | | | | | | |
| Analysis by maturity | | 2014 | |
| | | Bank loan
£‘000 | | | Loan notes | | | Interest
accrued
on loan
notes | | | Interest
accrued on
bank loan
£‘000 | | | Arrangement fees £‘000 | | | Total
£‘000 | |
Within one year | | | 25,957 | | | | — | | | | — | | | | 3,391 | | | | (7,228 | ) | | | 22,120 | |
In the second year | | | 35,227 | | | | — | | | | — | | | | — | | | | (6,397 | ) | | | 28,830 | |
In third year | | | 65,671 | | | | — | | | | — | | | | — | | | | (5,256 | ) | | | 60,415 | |
In the fourth year | | | 82,358 | | | | — | | | | — | | | | — | | | | (4,062 | ) | | | 78,296 | |
In the fifth year | | | 696,281 | | | | — | | | | — | | | | — | | | | (3,232 | ) | | | 693,049 | |
Thereafter | | | — | | | | 40,913 | | | | 11,779 | | | | — | | | | — | | | | 52,692 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total | | | 905,494 | | | | 40,913 | | | | 11,779 | | | | 3,391 | | | | (26,175 | ) | | | 935,402 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
A-40
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
19. LONG TERM AND SHORT TERM BORROWINGS (continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Analysis by maturity | | 2013 | |
| | | Bank loan
£‘000 | | | Loan notes
£‘000 | | | Interest
accrued
on loan
notes | | | Interest
accrued
on bank
loan
£‘000 | | | Mezzanine
debt £‘000 | | | Arrangement
fees £‘000 | | | Total
£‘000 | |
Within one year | | | 10,000 | | | | — | | | | — | | | | 248 | | | | — | | | | (5,417 | ) | | | 4,831 | |
In the second year | | | 14,000 | | | | — | | | | — | | | | — | | | | — | | | | (5,136 | ) | | | 8,864 | |
In third year | | | 19,000 | | | | — | | | | — | | | | — | | | | — | | | | (4,769 | ) | | | 14,231 | |
In the fourth year | | | 47,000 | | | | — | | | | — | | | | — | | | | — | | | | (4,258 | ) | | | 42,742 | |
In the fifth year | | | 47,000 | | | | — | | | | — | | | | — | | | | — | | | | (3,742 | ) | | | 43,258 | |
Thereafter | | | 329,250 | | | | 330,231 | | | | 49,503 | | | | — | | | | 128,422 | | | | — | | | | 837,406 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 466,250 | | | | 330,231 | | | | 49,503 | | | | 248 | | | | 128,422 | | | | (23,322 | ) | | | 951,332 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The Group borrowings consist of term loans, namely Senior Debt A and Senior Debt B1 and B2. Senior Debt A is repayable in installments with the final payment falling due on 31 December 2018. The Senior Debt B is repayable on 31 December 2019.
Interest is charged at 4% above 3 month LIBOR for Senior Debt A, 5.25% above 3 month LIBOR for Senior Debt B1 and 4.75% above 3 month EURIBOR for Senior Debt B2.
The initial costs of establishing the loan facility are being amortised over term of the loan.
The bank term loan facility is secured by a cross debenture and guarantee between Amdipharm Mercury Debtco Limited, Amdipharm Mercury Newco Limited, Amdipharm Mercury Holdco UK Ltd, Amdipharm Mercury Midco UK Limited, Amdipharm Mercury UK Limited, Mercury Pharma Group Limited, Mercury Pharmaceuticals Limited, Mercury Pharma (Generics) Limited, Mercury Pharma Overseas Limited, Mercury Pharma International Limited, Amdipharm AG, Amdipharm International Ltd, Amdipharm Sales & Marketing Ltd, Amdipharm Marketing Ltd, Amdipharm Ltd and by a debenture granting fixed and floating security over all assets of the above mentioned companies with the company’s lending banks.
The Group has an undrawn revolving loan facility of£24.25 million (2013:£24.65 million) and acquisition facility commitments of£nil (2013:£25 million) as at 31 December 2014. The revolving loan facility is subject to commitment fees of 1.6% per annum and is valid for the remaining life of the term loans.
The Company issued a first tranche of A1 fixed rate unsecured loan notes 2052 worth£244.32 million on 31 August 2012 and a second tranche of£153.64 million on 31 October 2012.£11.41 million of A2 fixed rate unsecured loan notes 2052 and£33.82 million of A3 fixed rate unsecured loan notes 2052 were also issued by the Group on 31 October 2012. The Series A1 loan notes carry the same rights and rank pari passu among themselves, and both the A1, A2 and A3 loan notes mature in 2052.
An amount of£369.20 million including interest has been paid during 2014.
The Company had originally issued the A1 loan notes to the Cinven funds and certain private shareholders, A2 loan notes to Cinven funds and the management team of Amdipharm Mercury Group Limited (formerly known as Mercury Pharma Group Limited) and A3 Loan notes to Verdot Limited (the management team of the Amdipharm group). The loan notes confer upon the holders the right to receive interest at a rate of 12 per cent per annum, compounding annually.
A-41
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
20. ACQUISITIONS
On 1 October 2014, Mercury Pharma Group limited purchased 100% of the ordinary shares of Focus Pharmaceuticals Limited, a company incorporated in the United Kingdom.
Focus specialise in the licensing and marketing of generic products in the UK. The company’s range currently comprises of over 60 different molecules, ranging across almost all different types of formulation and therapeutic areas.
Since Mercury Pharma Group Limited is under the control of Amdipharm Mercury Limited, the results of the acquired business are included from the date of acquisition in the statement of comprehensive income.
The acquisition was for net cash of£45.17 million (including cash acquired) and a total consideration of£65.16 million. Focus Pharmaceuticals limited had a turnover of£38.37 million and a profit before tax of£4.13 million for the year, of which£9.50 million of turnover and£0.89 million of profit before tax related to the period since acquisition are included in Group accounts.
The following table summarises the consideration paid for Focus Pharmaceuticals Limited and the amounts of the assets acquired and liabilities assumed recognised at the acquisition date, as well as the fair value at the acquisition date. The valuations in the table are provisional fair values and may change in the future.
| | | | | | | | | | | | |
| | | Book
value
£‘000 | | | Fair value
adjustment
£‘000 | | | Fair
value
£‘000 | |
Intangible assets | | | — | | | | 35,027 | | | | 35,027 | |
Machinery and equipment | | | 166 | | | | — | | | | 166 | |
Inventories | | | 3,934 | | | | — | | | | 3,934 | |
Trade and other receivables | | | 4,901 | | | | — | | | | 4,901 | |
Cash and cash equivalents | | | 2,835 | | | | — | | | | 2,835 | |
Trade and other payables | | | (7,841 | ) | | | — | | | | (7,841 | ) |
Corporation tax | | | 217 | | | | — | | | | 217 | |
Other liabilities | | | (408 | ) | | | — | | | | (408 | ) |
Deferred tax liability | | | — | | | | (7,005 | ) | | | (7,005 | ) |
Net identifiable assets and liabilities | | | 3,804 | | | | 28,022 | | | | 31,826 | |
| | | |
Goodwill on acquisition (note 7) | | | | | | | | | | | 33,330 | |
| | | | | | | | | | | | |
Total purchase consideration | | | | | | | | | | | 65,156 | |
| | | | | | | | | | | | |
| | | |
Less: Cash acquired | | | | | | | | | | | (2,835 | ) |
Less: contingent consideration | | | | | | | | | | | (17,155 | ) |
| | | | | | | | | | | | |
Cash outflow on acquisition | | | | | | | | | | | 45,166 | |
| | | | | | | | | | | | |
The goodwill is mainly attributable to the synergies expected to be achieved from integrating the company into the Group’s existing business and also on expansion of Focus product portfolio into the Group’s international sales network. None of this goodwill is expected to be tax deductible.
The Group has agreed to pay the selling shareholders over a period of 3 years additional consideration of£20.93 million (present value£17.16 million) based on the company meeting a gross profit threshold, receiving Market Authorisation approval for certain molecules and continuity of supply of the products relating to relevant contracts, for the period ending December 2015 and 2016. In January 2015, an amount of£0.93 million has been paid to the selling shareholders as part payment towards the contingent consideration.
Acquisition-related costs amounting to£1,241,000 has been shown within non-recurring items in the Statement of Comprehensive Income (see note 21) for the period ended 31 December 2014.
A-42
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
20. ACQUISITIONS (continued)
On 5 December 2013, Mercury Pharma Group Limited purchased 100% of the ordinary shares of Abcur AB, a company incorporated in Sweden from Vimixor AB. It operates within the Nordic Markets of Sweden, Finland, Norway, Denmark and Iceland and has a number of therapeutic areas, including the treatment of addiction, intensive care, pain and anaesthesia.
Since Mercury Pharma Group Limited is under the control of Amdipharm Mercury Limited, the results of the acquired business are included from the date of acquisition in the Statement of Comprehensive Income.
The acquisition was for net cash of£11.12 million (including cash acquired) and a total consideration of£12.70 million. Abcur Ab had a turnover of£7.00 million and a profit of£1.40 million for the year, of which£0.80 million of turnover and£0.20 million of profit after tax related to the period since acquisition are included in Group accounts for the year, ended 31 December 2013.
The following table summarises the consideration paid for Abcur AB and the amounts of the assets acquired and liabilities assumed recognised at the acquisition dare, as well as the fair value at the acquisition date. The valuations in the table are provisional fair values and may change in the future.
| | | | | | | | | | | | |
| | | Book
value
£‘000 | | | Fair value
adjustment
£‘000 | | | Fair
value
£‘000 | |
Intangible assets | | | — | | | | 9,915 | | | | 9,915 | |
Machinery and equipment | | | 19 | | | | — | | | | 19 | |
Inventories | | | 989 | | | | — | | | | 989 | |
Trade and other receivables | | | 1,630 | | | | — | | | | 1,630 | |
Cash and cash equivalents | | | 90 | | | | — | | | | 90 | |
Trade and other payables | | | (688 | ) | | | — | | | | (688 | ) |
Corporation tax | | | (71 | ) | | | — | | | | (71 | ) |
Other liabilities | | | (253 | ) | | | — | | | | (253 | ) |
Liability to group company | | | (717 | ) | | | — | | | | (717 | ) |
Deferred tax liability | | | (205 | ) | | | (2,181 | ) | | | (2,386 | ) |
Net identifiable assets and liabilities | | | 794 | | | | 7,734 | | | | 8,528 | |
| | | |
Goodwill on acquisition (note 7) | | | | | | | | | | | 4,176 | |
| | | | | | | | | | | | |
Total purchase consideration | | | | | | | | | | | 12,704 | |
| | | | | | | | | | | | |
| | | |
Less: Cash acquired | | | | | | | | | | | (90 | ) |
Less: Contingent consideration | | | | | | | | | | | (2,213 | ) |
Add: Liability to Group company | | | | | | | | | | | 717 | |
| | | | | | | | | | | | |
Cash outflow on acquisition | | | | | | | | | | | 11,118 | |
| | | | | | | | | | | | |
The Group has agreed to pay the selling shareholders in 2 years’ time additional consideration of£2.20 million based on the company meeting certain revenue and EBITDA thresholds for the 12 months ending November 2014 and November 2015. More specifically, contingent consideration of£0.05 million is payable for every 1% outperformance (subject to cap of 20%) of EBITDA and revenue thresholds. In January 2015, an amount of£1.30 million has been paid to selling shareholders as part payment towards the contingent consideration.
Acquisition-related costs amounting to£450,000 have been shown within non-recurring items in the Statement of Comprehensive Income (see note 21) for the year ended 31 December 2013.
Goodwill is attributable to portfolio optimisation opportunities between Group and Abcur along with the future product pipeline from the Abcur business. None of this goodwill is expected to be tax deductible.
A-43
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
21. ACQUISITION, DEAL TRANSACTION COSTS AND NON RECURRING ITEMS
| | | | | | | | |
| | | 2014
£‘000 | | | 2013
£‘000 | |
Dual sourcing and other operations projects | | | 6,118 | | | | 250 | |
Integration costs | | | 3,958 | | | | 7,217 | |
Project P3 | | | 1,835 | | | | — | |
Acquisition and deal transaction costs | | | 1,778 | | | | 484 | |
Others | | | 185 | | | | 172 | |
| | | | | | | | |
| | | 13,874 | | | | 8,123 | |
| | | | | | | | |
Dual sourcing and other operational projects include one-off costs incurred through the diversification of the company’s sources of finished dosage and active product ingredients for the top products. The expenditure is primarily related to the transfer of technologies, validation of sample batches and stability studies.
Integration costs consist of legal and professional fees incurred in relation to employee related settlement payments, project management, infrastructure costs and similarly identifiable expenditure.
Project P3 costs consist of one-off expenditure incurred by the company in updating the summary of product characteristics for the legacy Amdipharm portfolio across all territories in line with regulatory requirements.
Acquisition and deal costs consists primarily of advisor fees incurred for the due diligence work for acquisitions projects, along with related stamp duty and legal costs for post acquisition restructuring.
22. SUBSIDIARY UNDERTAKINGS
At 31 December 2014 the Company held more than 20% of the allotted share capital of the following significant undertakings. All subsidiary undertakings are wholly owned.
| | | | |
| Name | | Country of registration or incorporation | | Class of capital held |
| Intermediate holding Companies | | | | |
| Amdipharm Mercury Holdco Limited | | Jersey | | Ordinary shares |
| Amdipharm Mercury Midco Limited | | Jersey | | Ordinary shares |
| Amdipharm Mercury Debtco Limited | | Jersey | | Ordinary shares |
| Amdipharm Mercury Newco Limited | | Jersey | | 1 pence ordinary shares |
| Amdipharm Mercury Holdco UK Limited | | England and Wales | | £1 ordinary shares |
| Amdipharm Mercury Midco UK Limited | | England and Wales | | £1 ordinary shares |
| Amdipharm Mercury UK Limited | | England and Wales | | 1 pence ordinary shares |
| Midas Bidco Limited | | England and Wales | | 1 pence ordinary shares |
| Mercury Pharma Group Limited | | England and Wales | | 5 pence ordinary shares |
| Mercury Pharmaceuticals (Ireland) Limited | | Ireland | | Ordinary shares |
| Amdipharm Holdings SàRL | | Luxemburg | | Ordinary shares |
| Amdipharm Coöperatief UA | | Netherlands | | Capital Account |
| Focus Pharma Holdings Limited | | England and Wales | | Ordinary shares |
| Holding companies for intellectual property rights | | | | |
| Amdipharm AG | | Switzerland | | Ordinary shares |
| Amdipharm Mercury International Limited | | Jersey | | Ordinary shares |
| Exploitation of intellectual property | | | | |
| Amdipharm BV | | Netherlands | | Ordinary shares |
| Amdipharm Limited | | Ireland | | Ordinary shares |
| Amdipharm Sales and Marketing Limited | | Jersey | | Ordinary shares |
| Amdipharm UK Limited | | England and Wales | | Ordinary shares |
A-44
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
22. SUBSIDIARY UNDERTAKINGS (continued)
| | | | |
| Marketing and distribution of pharmaceutical products | | | | |
| Mercury Pharmaceuticals Limited | | England and Wales | | £1 ordinary shares |
| Mercury Pharma (Generics) Limited | | England and Wales | | £1 ordinary shares |
| Mercury Pharma International Limited | | Ireland | | Ordinary shares |
| Mercury Pharma Overseas limited | | Ireland | | Ordinary shares |
| MercuryPharm Limited | | Ireland | | Ordinary shares |
| Goldshield Healthcare Private Limited | | India | | Ordinary shares |
| Abcur AB | | Sweden | | Ordinary shares |
| Focus Pharmaceuticals Limited | | England and Wales | | £1 ordinary shares |
| Exploitation of pharmaceutical licences | | | | |
| Amdipharm Marketing Limited | | England and Wales | | Ordinary shares |
| Amdipharm BV International | | Netherlands | | Ordinary shares |
| Management services | | | | |
| Amdipharm Mercury Company Limited | | England and Wales | | £1 ordinary shares |
| Amdipharm Mercury Middle East FZ-LLC | | United Arab Emirates | | Ordinary shares |
| Amdipharm Mercury (Hong Kong) Limited | | Hong Kong | | Ordinary shares |
| Amdipharm Mercury Services Private Limited | | India | | Ordinary shares |
| AMCo GmBH | | Germany | | Ordinary shares |
| AMCo S.r.l. Milan | | Italy | | Ordinary shares |
| Amdipharm Mercury Pharma Pty Ltd. | | Australia | | Ordinary shares |
| AMCo France Sarl Paris | | France | | Ordinary shares |
| Mercury RSA Pty Limited | | South Africa | | Ordinary shares |
| Development of Wellbeing villages and resorts | | | | |
| Goldshield Real Estate Private Limited | | India | | Ordinary shares |
| Finance company | | | | |
| WIHL Finance PLC | | England and Wales | | Ordinary shares |
23. CONTINGENT LIABILITIES
Indemnities and guarantees
The Group has given indemnities in respect of advance payments, contingent purchase consideration and import duty guarantees issued on its behalf in the normal course of business. The indemnities given at 31 December 2014 were£490,000 (2013:£337,000).
24. RELATED PARTIES
Fixed rate unsecured loan notes 2052 totalling£43.17 million (2013:£311.15 million), as disclosed in note 19, are in issue to the following identified related parties: Fifth Cinven Fund (No. 1) Limited Partnership; Fifth Cinven Fund (No. 2) Limited Partnership; Fifth Cinven Fund (No. 3) Limited Partnership; Fifth Cinven Fund (No. 4) Limited Partnership; Fifth Cinven Fund (No. 5) Limited Partnership; Fifth Cinven Fund (No. 6) Limited Partnership; Fifth Cinven Fund FCP-SIF; Fifth Cinven Fund Co-Investment Partnership. In addition, the key management personnel of Amdipharm Mercury Limited have fixed unsecured loan notes 2052 totalling£0.81 million (2013:£5.86 million). The total interest expensed to Comprehensive Income during the year was£35.39 million (2013:£33.79 million).
During the year, a monitoring fee of£250,000 (2013:£250,000) was charged by Cinven Partners LLP, a member of the Cinven group of companies and an administration fee of£173,000 (2013:£174,000) was charged by Aztec Financial Services (Jersey) Limited, a member of Aztec Group.
A-45
Amdipharm Mercury Limited
Consolidated financial statements for the year ended 31 December 2014
Notes to the Consolidated Financial Statements (continued)
25. POST BALANCE SHEET EVENTS
On 2 June 2015 Mercury Pharma Group Limited purchased 100% of the ordinary shares of Primegen Limited (“Primegen”), a specialty pharma business based in the UK for an initial consideration of£45 million and contingent consideration of£22.50 million. Primegen sells a number of niche medicines, predominantly in the UK, and has an attractive pipeline of UK and international registrations.
On 31 July 2015 the Group acquired an Australian company Boucher and Muir Pty Ltd (BNM) for an initial consideration of£6.59 million and contingent consideration of£2.95 million. BNM sells specialist medical products ranging from vaccines to anaesthetics, as well as more general products such as solid dose tablets and capsules.
Since BNM is under the control of Amdipharm Mercury Limited, the results of the acquired business will be included from the date of acquisition in the statement of comprehensive income. Full disclosure of this acquisition under IFRS 3 will be included within the consolidated financial statements for the year ended 31 December 2015.
On 8 September 2015, the Group’s ultimate controlling party, Cinven, announced it had entered into a definitive agreement to sell the Group to Concordia Healthcare Corp. (“Concordia”) for US$3.5 billion to be paid through a combination of cash, common shares of Concordia, and performance-based earn-out payable in cash. The acquisition has been approved by the Board of Directors of both Concordia and Cinven, and is strongly supported by the Amdipharm Mercury Limited management team.
A-46
APPENDIX B - UNAUDITED INTERIM CONDENSED CONSOLIDATED FINANCIAL STATEMENTS OF AMDIPHARM MERCURY LIMITED FOR THE SIX MONTHS ENDED JUNE 30, 2015
Amdipharm Mercury Limited
Condensed financial statements
For the period ended 30 June 2015
11-15 Seaton Place,
ST Heller,
Jersey
JE4 0QH
B-1
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Company registration number: 111126
Registered office:
11-15 Seaton Place,
ST Helier,
Jersey
JE4 0QH
Board of Directors:
Simon Radford
Mark Wanless
Aztec Directors Limited
Supraj Rajagopalan
Alexander Leslie
Jeremy Caplan
Auditor:
KPMG LLP
Botanic House,
100 Hills Road,
Cambridge,
CB2 1AR,
United Kingdom.
B-2
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Index
B-3
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Condensed Consolidated Statement of Comprehensive Income
| | | | | | | | | | | | | | |
| | | | | Six months
ended
30 June
2015 £‘000 | | | Six months ended 30 June 2014 £‘000 | | | Twelve months ended 31 December 2014 £‘000 | |
| | | Notes | | (unaudited) | | | (unaudited) | | | (audited) | |
| | | | |
Revenue | | 4 | | | 165,220 | | | | 142,584 | | | | 292,804 | |
Cost of sales | | | | | (45,796 | ) | | | (33,056 | ) | | | (73,235 | ) |
| | | | | | | | | | | | | | |
Gross profit | | | | | 119,424 | | | | 109,528 | | | | 219,569 | |
| | | | |
Other income | | | | | 156 | | | | 118 | | | | 331 | |
Distribution costs | | | | | (6,262 | ) | | | (7,482 | ) | | | (15,262 | ) |
| | | | |
Non recurring items | | 11 | | | (4,774 | ) | | | (4,705 | ) | | | (13,874 | ) |
Other administrative expenses | | | | | (49,391 | ) | | | (45,726 | ) | | | (98,415 | ) |
| | | | | | | | | | | | | | |
Administrative expenses | | | | | (54,165 | ) | | | (50,431 | ) | | | (112,289 | ) |
| | | | |
Operating profit | | 5 | | | 59,153 | | | | 51,733 | | | | 92,349 | |
Finance costs | | | | | (58,806 | ) | | | (45,069 | ) | | | (97,264 | ) |
Finance income | | | | | 34,079 | | | | 7,014 | | | | 13,571 | |
| | | | | | | | | | | | | | |
Profit before tax | | | | | 34,426 | | | | 13,678 | | | | 8,656 | |
| | | | |
Income tax charge | | 7 | | | (8,053 | ) | | | (5,688 | ) | | | (2,453 | ) |
| | | | | | | | | | | | | | |
Profit for the financial period | | | | | 26,373 | | | | 7,990 | | | | 6,203 | |
| | | | |
Other comprehensive income/(expense) | | | | | | | | | | | | | | |
| | | | |
Items that may be reclassified subsequently to profit or loss: | | | | | | | | | | | | | | |
Currency translation differences | | | | | (4,113 | ) | | | (1,066 | ) | | | 1,132 | |
| | | | | | | | | | | | | | |
| | | | |
Other comprehensive (expense)/income for the period, net of tax | | | | | (4,113 | ) | | | (1,066 | ) | | | 1,132 | |
| | | | | | | | | | | | | | |
Total comprehensive profit for the period | | | | | 22,260 | | | | 6,924 | | | | 7,335 | |
| | | | | | | | | | | | | | |
The accompanying accounting policies and notes form an integral part of these condensed consolidated financial statements.
B-4
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Condensed Consolidated Statement of Financial Position
| | | | | | | | | | | | | | |
| | | | | As at 30 June 2015 £‘000 | | | As at 30 June 2014 £‘000 | | | As at 31
December 2014 £‘000 | |
| | | Notes | | (unaudited) | | | (unaudited) | | | (audited) | |
Assets | | | | | | | | | | | | | | |
Non-current | | | | | | | | | | | | | | |
Intangible assets | | | | | 951,733 | | | | 856,984 | | | | 903,213 | |
Property, plant and equipment | | | | | 2,638 | | | | 2,337 | | | | 2,386 | |
Deferred tax assets | | | | | 141 | | | | 304 | | | | 191 | |
| | | | | | | | | | | | | | |
| | | | | 954,512 | | | | 859,625 | | | | 905,790 | |
| | | | |
Current | | | | | | | | | | | | | | |
Inventories | | | | | 36,959 | | | | 27,165 | | | | 35,028 | |
Trade and other receivables | | | | | 81,358 | | | | 61,736 | | | | 72,916 | |
Current tax asset | | | | | — | | | | — | | | | 1,012 | |
Other financial assets | | 8 | | | — | | | | 1,490 | | | | 61 | |
Cash and cash equivalents | | | | | 43,300 | | | | 59,547 | | | | 27,780 | |
| | | | | | | | | | | | | | |
| | | | | 161,617 | | | | 149,938 | | | | 136,797 | |
| | | | | | | | | | | | | | |
Total assets | | | | | 1,116,129 | | | | 1,009,563 | | | | 1,042,587 | |
| | | | | | | | | | | | | | |
| | | | |
Equity | | | | | | | | | | | | | | |
Share capital | | | | | 94 | | | | 94 | | | | 94 | |
Share premium | | | | | 16,480 | | | | 16,480 | | | | 16,480 | |
Profit and loss account | | | | | (17,804 | ) | | | (42,390 | ) | | | (44,177 | ) |
Currency translation reserve | | | | | (3,760 | ) | | | (1,845 | ) | | | 353 | |
| | | | | | | | | | | | | | |
Total equity | | | | | (4,990 | ) | | | (27,661 | ) | | | (27,250 | ) |
| | | | | | | | | | | | | | |
| | | | |
Liabilities | | | | | | | | | | | | | | |
Non-current | | | | | | | | | | | | | | |
Long-term borrowings | | 9 | | | 908,980 | | | | 924,083 | | | | 913,282 | |
Deferred tax liabilities | | | | | 65,340 | | | | 55,408 | | | | 59,979 | |
| | | | | | | | | | | | | | |
| | | | | 974,320 | | | | 979,491 | | | | 973,261 | |
| | | | |
Current | | | | | | | | | | | | | | |
Short-term borrowings | | 9 | | | 29,762 | | | | 12,207 | | | | 22,120 | |
Trade and other payables | | | | | 38,644 | | | | 35,425 | | | | 44,318 | |
Other financial liabilities | | 8 | | | 29,267 | | | | 158 | | | | 3,191 | |
Other liabilities | | | | | 44,079 | | | | 6,597 | | | | 26,947 | |
Current tax liabilities | | | | | 5,047 | | | | 3,346 | | | | — | |
| | | | | | | | | | | | | | |
| | | | | 146,799 | | | | 57,733 | | | | 96,576 | |
| | | | | | | | | | | | | | |
Total liabilities | | | | | 1,121,119 | | | | 1,037,224 | | | | 1,069,837 | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Total equity and liabilities | | | | | 1,116,129 | | | | 1,009,563 | | | | 1,042,587 | |
| | | | | | | | | | | | | | |
The condensed consolidated financial statements are approved by the Board of Directors on 11 September 2015 and signed on their behalf by:
| | | | |
 | | | |  |
| Mark Wanless, Director | | | | Simon Radford, Director |
The accompanying accounting policies and notes form an integral part of these condensed consolidated financial statements.
B-5
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Condensed Consolidated Statement of Changes in Equity
| | | | | | | | | | | | | | | | | | | | |
| | | Share capital £‘000 | | | Share premium £‘000 | | | Currency translation reserve £‘000 | | | Profit and
Loss
account
£‘000 | | | Total equity
£‘000 | |
| | | | | |
Balance at 1 January 2014 | | | 94 | | | | 16,480 | | | | (779 | ) | | | (50,380 | ) | | | (34,585 | ) |
| | | | | |
Comprehensive income | | | | | | | | | | | | | | | | | | | | |
Profit for the financial period | | | — | | | | — | | | | — | | | | 7,990 | | | | 7,990 | |
| | | | | |
Other comprehensive expense | | | | | | | | | | | | | | | | | | | | |
Currency translation differences | | | — | | | | — | | | | (1,066 | ) | | | — | | | | (1,066 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total comprehensive profit | | | — | | | | — | | | | (1,066 | ) | | | 7,990 | | | | 6,924 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Balance at 30 June 2014 | | | 94 | | | | 16,480 | | | | (1,845 | ) | | | (42,390 | ) | | | (27,661 | ) |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Balance at 1 January 2014 | | | 94 | | | | 16,480 | | | | (779 | ) | | | (50,380 | ) | | | (34,585 | ) |
| | | | | |
Comprehensive income | | | | | | | | | | | | | | | | | | | | |
Profit for the financial period | | | — | | | | — | | | | — | | | | 6,203 | | | | 6,203 | |
| | | | | |
Other comprehensive income | | | | | | | | | | | | | | | | | | | | |
Currency translation differences | | | — | | | | — | | | | 1,132 | | | | — | | | | 1,132 | |
| | | | | | | | | | | | | | | | | | | | |
Total comprehensive profit | | | — | | | | — | | | | 1,132 | | | | 6,203 | | | | 7,335 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Balance at 31 December 2014 | | | 94 | | | | 16,480 | | | | 353 | | | | (44,177 | ) | | | (27,250 | ) |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Balance at 1 January 2015 | | | 94 | | | | 16,480 | | | | 353 | | | | (44,177 | ) | | | (27,250 | ) |
| | | | | |
Comprehensive income | | | | | | | | | | | | | | | | | | | | |
Profit for the financial period | | | — | | | | — | | | | — | | | | 26,373 | | | | 26,373 | |
| | | | | |
Other comprehensive expense | | | | | | | | | | | | | | | | | | | | |
Currency translation differences | | | — | | | | — | | | | (4,113 | ) | | | — | | | | (4,113 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total comprehensive profit | | | — | | | | — | | | | (4,113 | ) | | | 26,373 | | | | 22,260 | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Balance at 30 June 2015 | | | 94 | | | | 16,480 | | | | (3,760 | ) | | | (17,804 | ) | | | (4,990 | ) |
| | | | | | | | | | | | | | | | | | | | |
Currency translation differences recognised in other comprehensive income may be reclassified subsequently to the Statement of Comprehensive Income in future periods.
The accompanying accounting policies and notes form an integral part of these condensed consolidated financial statements.
B-6
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Condensed Consolidated Statement of Cash Flows
| | | | | | | | | | | | |
| | | Six months
ended 30 June 2015 £‘000 | | | Six months
ended 30 June 2014 £‘000 | | | Twelve months ended 31 December 2014 £‘000 | |
| | | (unaudited) | | | (unaudited) | | | (audited) | |
Cash flows from operating activities | | | | | | | | | | | | |
Result for the period before tax | | | 34,426 | | | | 13,678 | | | | 8,656 | |
Depreciation | | | 594 | | | | 430 | | | | 896 | |
Amortisation of intangible assets | | | 23,913 | | | | 21,145 | | | | 44,749 | |
Foreign exchange (gain)/loss on borrowings | | | (33,665 | ) | | | (6,640 | ) | | | (12,779 | ) |
Profit/(loss) on sale of assets | | | — | | | | (1 | ) | | | (3 | ) |
Finance costs | | | 58,806 | | | | 45,069 | | | | 97,264 | |
Finance income | | | (414 | ) | | | (374 | ) | | | (792 | ) |
| | | | | | | | | | | | |
| | | 83,660 | | | | 73,307 | | | | 137,991 | |
(Increase) in inventories | | | (1,444 | ) | | | (50 | ) | | | (3,979 | ) |
(Increase) in trade and other receivables | | | (7,994 | ) | | | (5,945 | ) | | | (12,225 | ) |
(Decrease) in provisions, trade payables and other liabilities | | | (7,587 | ) | | | (12,530 | ) | | | (8,690 | ) |
Taxes paid | | | (4,851 | ) | | | (3,241 | ) | | | (6,430 | ) |
| | | | | | | | | | | | |
Net cash inflow from operating activities | | | 61,784 | | | | 51,541 | | | | 106,667 | |
| | | |
Cash flows from investing activities | | | | | | | | | | | | |
Additions of: | | | | | | | | | | | | |
- intangible assets | | | (1,370 | ) | | | (1,050 | ) | | | (2,978 | ) |
- property, plant and equipment | | | (862 | ) | | | (431 | ) | | | (781 | ) |
Proceeds on sale of assets | | | — | | | | 3 | | | | 9 | |
Acquisition of subsidiaries (net of cash acquired) | | | (45,400 | ) | | | — | | | | (45,166 | ) |
Interest received | | | 414 | | | | 374 | | | | 792 | |
| | | | | | | | | | | | |
Net cash outflow from investing activities | | | (47,218 | ) | | | (1,104 | ) | | | (48,124 | ) |
| | | |
Cash flows from financing activities | | | | | | | | | | | | |
Bank drawdowns | | | 45,500 | | | | — | | | | 935,019 | |
Bank repayments | | | (11,124 | ) | | | (36,220 | ) | | | (482,966 | ) |
Mezzanine debt paid | | | — | | | | — | | | | (128,422 | ) |
Arrangement fees paid | | | (894 | ) | | | — | | | | (11,427 | ) |
Interest paid | | | (29,146 | ) | | | (17,251 | ) | | | (38,906 | ) |
Fixed rate unsecured loan notes 2052 repayments | | | — | | | | — | | | | (289,318 | ) |
Interest paid on fixed rate unsecured loan notes 2052 | | | — | | | | — | | | | (79,902 | ) |
| | | | | | | | | | | | |
Net cash inflow/(outflow) from financing activities | | | 4,336 | | | | (53,471 | ) | | | (95,922 | ) |
| | | |
Net increase/(decrease) in cash and cash equivalents | | | 18,902 | | | | (3,034 | ) | | | (37,379 | ) |
Exchange gain/(loss) on cash and cash equivalents | | | (3,382 | ) | | | (209 | ) | | | 2,369 | |
Cash and cash equivalents at beginning of the year | | | 27,780 | | | | 62,790 | | | | 62,790 | |
| | | | | | | | | | | | |
Cash and cash equivalents at the end of the year | | | 43,300 | | | | 59,547 | | | | 27,780 | |
| | | | | | | | | | | | |
The accompanying accounting policies and notes form an integral part of these condensed consolidated financial statements.
B-7
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Notes to the Interim Financial Information for the six months ended 30 June 2015
1. General information
The condensed financial statements of Amdipharm Mercury Limited have been prepared for the six months ended 30 June 2015. These financial statements have been reviewed and not audited and were approved for issue by the Directors on 11 September 2015. Annual accounts for the year ended 31 December 2014 were approved by the Board of Directors on 11 September 2015. The report of the auditors on those financial statements was unqualified, and did not contain an emphasis of matter paragraph.
The principal activity of the Company is that of a holding company of the operating and group services businesses within the Group. The Group is engaged in the marketing and distribution of pharmaceutical products. It is intended that the company will continue in its present capacity in the coming year.
2. Basis of preparation
This half yearly financial report for the six months ended 30 June 2015 has been prepared in accordance with IAS 34, ‘Interim financial reporting’. The half yearly financial report should be read in conjunction with the annual report and financial statements for the year ended 31 December 2014, which have been prepared in accordance with International Financial Reporting Standards (IFRSs).
The Group recognised a profit of£26.37 million for the six months ended 30 June 2015 (six months ended 30 June 2014:£7.99 million). Consistent with this fact the financial statements have been prepared on a going concern basis which the Board of Directors believe is appropriate for the following reasons.
The Group is funded by a combination of ordinary share capital, cash holdings, bank term loans and loan notes. The bank term loans and revolving credit facilities are for a period of between 4 and 5 years. Repayments are on-going and primarily on a half yearly basis. As with other groups with similar financing structures, the banking facilities are subject to periodic covenant tests.
The Directors have prepared projections for the next fifteen months from the date of these financial statements, including sensitivity analysis on key assumptions made, and consider the forecasts to be reasonable and realistic.
On the basis of these projections, and based on the actual trading first 6 months of the year 2015, the Directors consider the Group will continue to operate within its facilities, remain compliant with its banking covenants and continue in operational existence for the foreseeable future. Hence the going concern basis is appropriate.
3. Accounting Policies
The accounting policies applied are consistent with those of the annual report and financial statements for the year ended 31 December 2014, as described in the Annual Report and Accounts.
4. Segmental Reporting
Segment information is presented in the condensed financial statements in respect of the Group’s business segments. The business segment-reporting format reflects the Group’s management and internal reporting structure. All operating segments’ operating results are reviewed regularly by the Board of Directors and the Group CEO to make decisions about resources to be allocated to the segment and assess its performance.
Business segments
The Group is currently organised into two major divisions: Direct markets and Distributor markets. These divisions form the basis for the Group’s reporting of segment information. Direct markets comprise of UK, Ireland, France, Nordics and Benelux regions.
Geographical segments
In presenting information based on the geographical segments, segment revenue is based on the geographical location of the customer.
Segment results
Segment results include items directly attributable to a segment as well as those that can be allocated on a reasonable basis. The segment results are pre-tax.
All inter-segment revenues are priced and carried out at arm’s length; however there are no inter-segment revenues during the year.
B-8
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Notes to the Interim Financial Information for the six months ended 30 June 2015 (continued)
4. Segmental Reporting (continued)
Business segments
| | | | | | | | | | | | |
| 6 months ended 30 June 2015 | | Direct
Markets
£‘000 | | | Distributor
Markets
£‘000 | | | Total
£‘000 | |
Revenue | | | | | | | | | | | | |
External sales | | | 133,082 | | | | 32,138 | | | | 165,220 | |
Segment result | | | 56,533 | | | | 7,394 | | | | 63,927 | |
Non recurring items | | | | | | | | | | | (4,774 | ) |
| | | | | | | | | | | | |
Operating profit | | | | | | | | | | | 59,153 | |
Finance costs | | | | | | | | | | | (58,806 | ) |
Finance income | | | | | | | | | | | 34,079 | |
| | | | | | | | | | | | |
Profit before tax | | | | | | | | | | | 34,426 | |
Income tax expense | | | | | | | | | | | (8,053 | ) |
| | | | | | | | | | | | |
Profit for the financial period | | | | | | | | | | | 26,373 | |
| | | | | | | | | | | | |
Geographical segments
Revenue by destination of customer
| | | | | | | | | | | | |
| 6 months ended 30 June 2015 | | Direct
Markets
£‘000 | | | Distributor
Markets
£‘000 | | | Total
£‘000 | |
United Kingdom | | | 109,290 | | | | — | | | | 109,290 | |
Rest of World | | | 23,792 | | | | 32,138 | | | | 55,930 | |
| | | | | | | | | | | | |
Total | | | 133,082 | | | | 32,138 | | | | 165,220 | |
| | | | | | | | | | | | |
Business segments
| | | | | | | | | | | | |
| 6 months ended 30 June 2014 | | Direct
Markets
£‘000 | | | Distributor
Markets
£‘000 | | | Total
£‘000 | |
Revenue | | | | | | | | | | | | |
External sales | | | 112,557 | | | | 30,027 | | | | 142,584 | |
Segment result | | | 48,250 | | | | 8,188 | | | | 56,438 | |
Non recurring items | | | | | | | | | | | (4,705 | ) |
| | | | | | | | | | | | |
Operating profit | | | | | | | | | | | 51,733 | |
Finance costs | | | | | | | | | | | (45,069 | ) |
Finance income | | | | | | | | | | | 7,014 | |
| | | | | | | | | | | | |
Profit before tax | | | | | | | | | | | 13,678 | |
Income tax expense | | | | | | | | | | | (5,688 | ) |
| | | | | | | | | | | | |
Profit for the financial period | | | | | | | | | | | 7,990 | |
| | | | | | | | | | | | |
Geographical segments
Revenue by destination of customer
| | | | | | | | | | | | |
| 6 months ended 30 June 2014 | | Direct
Markets
£‘000 | | | Distributor
Markets
£‘000 | | | Total
£‘000 | |
United Kingdom | | | 90,193 | | | | — | | | | 90,193 | |
Rest of World | | | 22,364 | | | | 30,027 | | | | 52,391 | |
| | | | | | | | | | | | |
Total | | | 112,557 | | | | 30,027 | | | | 142,584 | |
| | | | | | | | | | | | |
B-9
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Notes to the Interim Financial Information for the six months ended 30 June 2015 (continued)
4. Segmental Reporting (continued)
Business segments
| | | | | | | | | | | | |
| 12 months ended 31 December 2014 | | Direct
Markets
£‘000 | | | Distributor
Markets
£‘000 | | | Total
£‘000 | |
Revenue | | | | | | | | | | | | |
External sales | | | 232,347 | | | | 60,457 | | | | 292,804 | |
Segment result | | | 93,458 | | | | 12,765 | | | | 106,223 | |
Non recurring items | | | | | | | | | | | (13,874 | ) |
| | | | | | | | | | | | |
Operating profit | | | | | | | | | | | 92,349 | |
Finance costs | | | | | | | | | | | (97,264 | ) |
Finance income | | | | | | | | | | | 13,571 | |
| | | | | | | | | | | | |
Profit before tax | | | | | | | | | | | 8,656 | |
Income tax expense | | | | | | | | | | | (2,453 | ) |
| | | | | | | | | | | | |
Profit for the financial period | | | | | | | | | | | 6,203 | |
| | | | | | | | | | | | |
Geographical segments
Revenue by destination of customer
| | | | | | | | | | | | |
| 12 months ended 31 December 2014 | | Direct
Markets
£‘000 | | | Distributor
Markets
£‘000 | | | Total
£‘000 | |
United Kingdom | | | 187,398 | | | | — | | | | 187,398 | |
Rest of World | | | 44,949 | | | | 60,457 | | | | 105,406 | |
| | | | | | | | | | | | |
Total | | | 232,347 | | | | 60,457 | | | | 292,804 | |
| | | | | | | | | | | | |
5. Operating Profit
The operating profit is stated after charging/(crediting):
| | | | | | | | | | | | |
| | | Six months ended 30 June 2015 £‘000 | | | Six months ended 30 June 2014 £‘000 | | | Twelve months ended 31 December 2014 £‘000 | |
Depreciation and amortisation: | | | | | | | | | | | | |
- Intangible assets | | | 23,913 | | | | 21,145 | | | | 44,749 | |
- Property, plant and equipment | | | 594 | | | | 430 | | | | 896 | |
Foreign exchange (gain)/losses: | | | | | | | | | | | | |
- Realised losses | | | 685 | | | | 476 | | | | 1,295 | |
- Unrealised (gain) / losses | | | (1,241 | ) | | | 979 | | | | 2,343 | |
Research and development expenditure | | | 407 | | | | 1,102 | | | | 3,082 | |
| | | | | | | | | | | | |
6. Seasonality
The Group operates in markets where no significant seasonal or cyclical variation in sales are experienced during the period.
B-10
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Notes to the Interim Financial Information for the six months ended 30 June 2015 (continued)
7. Income tax charge
| | | | | | | | | | | | |
| | | Six months ended 30 June 2015 | | | Six months ended 30 June 2014 | | | Twelve months ended 31 December 2014 | |
| | | £‘000 | | | £‘000 | | | £‘000 | |
Current tax charge: | | | | | | | | | | | | |
| | | |
Current tax: | | | | | | | | | | | | |
Foreign taxation | | | (10,228 | ) | | | (8,281 | ) | | | (12,076 | ) |
Adjustment to tax charges in respect of prior periods | | | (695 | ) | | | (10 | ) | | | 4,509 | |
| | | | | | | | | | | | |
| | | (10,923 | ) | | | (8,291 | ) | | | (7,567 | ) |
| | | | | | | | | | | | |
| | | |
Deferred tax: | | | | | | | | | | | | |
- origination and reversal of temporary differences | | | 2,870 | | | | 2,603 | | | | 5,114 | |
| | | | | | | | | | | | |
Total income tax (charge) (see below) | | | (8,053 | ) | | | (5,688 | ) | | | (2,453 | ) |
| | | | | | | | | | | | |
Factors affecting current tax charge:
The tax assessed on profit on ordinary activities for the period differs from the standard rate of corporation tax in Jersey which is 0%. As per IAS -12, the blended tax rate @ 11.83% (2014: 12.97%) is taken into consideration.
The effective tax rate (ETR) of 11.83% has been arrived at by calculating the weighted average standard rate of tax, taking into consideration the tax charge as per the standard rate in the respective jurisdiction over the profit before tax of such jurisdictions.
The differences are explained below:
Reconciliation of effective tax rate:
| | | | | | | | | | | | |
| | | Six months ended 30 June 2015 | | | Six months ended 30 June 2014 | | | Twelve months ended 31 December 2014 | |
| | | £‘000 | | | £‘000 | | | £‘000 | |
| | | |
Profit for the financial period before tax | | | 34,426 | | | | 13,678 | | | | 8,656 | |
Weighted average tax rate | | | 11.83 | % | | | 12.97 | % | | | 23.81 | % |
| | | | | | | | | | | | |
Expected tax charge | | | (4,073 | ) | | | (1,774 | ) | | | (2,061 | ) |
Adjustment for: | | | | | | | | | | | | |
Non-deductible expenses | | | (3,398 | ) | | | (6,507 | ) | | | (10,122 | ) |
Origination and reversal of temporary differences | | | 2,871 | | | | 2,603 | | | | 5,114 | |
Adjustment to tax charges in respect of prior periods | | | (695 | ) | | | (10 | ) | | | 4,509 | |
Capital gains tax | | | (2,758 | ) | | | — | | | | 107 | |
| | | | | | | | | | | | |
Actual tax charge, net (see above) | | | (8,053 | ) | | | (5,688 | ) | | | (2,453 | ) |
| | | | | | | | | | | | |
A reduction in the UK corporation tax rate from 24% to 23% (effective 1 April 2013) was substantively enacted on 3 July 2012, Further reductions to 21% (effective from 1 April 2014) and 20% (effective from 1 April 2015) were substantively enacted on 2 July 2013. In the Budget on 8 July 2015, the Chancellor announced additional planned reductions to 18% by 2020. This will reduce the company’s future current tax charge accordingly. The deferred tax liability at 30 June 2015 has been calculated based on the rate of 20% substantively enacted at the balance sheet date.
B-11
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Notes to the Interim Financial Information for the six months ended 30 June 2015 (continued)
8. Financial Instruments
Fair value of financial assets and liabilities
The fair value of a financial asset or liability is the amount at which the instrument could be exchanged in a current transaction between willing parties, other than in a forced or liquidation sale. For the financial assets and liabilities of the Group, the fair values have been estimated as described below:
| | | | |
| Cash and cash equivalents | | - | | approximates to the carrying amount; |
| Short-term borrowings | | - | | approximates to the carrying amount because of the short maturity of these instruments; |
| Long-term borrowings | | - | | approximates to the carrying amount in the case of floating rate bank loans and other loans; |
| Receivables and payables | | - | | approximates to the carrying amount. |
Fixed rate unsecured loan note 2052 | | - | | approximates to the carrying amount. |
FINANCIAL INSTRUMENTS- FAIR VALUES AND RISK MANAGEMENT
| | A) | Accounting classifications and fair values |
The following table shows the carrying amounts and fair values of financial assets and financial liabilities, including their levels in the fair value hierarchy. It does not include fair value information for financial assets and financial liabilities not measured at fair value if the carrying amount is a reasonable approximation of fair value
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 30 June 2015 | | | | | Carrying amount | | | Fair value | |
| £000’s | | Note | | | Designated
at fair
value | | | Fair value
hedging
instruments | | | Loans and
receivables | | | Other financial liabilities | | | Total | | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
| | | | | | | | | | |
Financial assets not measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Trade and other receivables | | | | | | | — | | | | — | | | | 81,358 | | | | — | | | | 81,358 | | | | — | | | | — | | | | — | | | | — | |
Cash and cash equivalents | | | | | | | — | | | | — | | | | 43,300 | | | | — | | | | 43,300 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | — | | | | — | | | | 124,658 | | | | — | | | | 124,658 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
Financial liabilities measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest rate swaps | | | | | | | — | | | | (29,267 | ) | | | — | | | | — | | | | (29,267 | ) | | | — | | | | (29,267 | ) | | | — | | | | (29,267 | ) |
Primegen contingent consideration (2015) | | | | | | | (19,044 | ) | | | — | | | | — | | | | — | | | | (19,044 | ) | | | — | | | | — | | | | (19,044 | ) | | | (19,044 | ) |
Focus contingent consideration (2014) | | | | | | | (16,221 | ) | | | — | | | | — | | | | — | | | | (16,221 | ) | | | — | | | | — | | | | (16,221 | ) | | | (16,221 | ) |
Abcur contingent consideration (2013) | | | | | | | (1,979 | ) | | | — | | | | — | | | | — | | | | (1,979 | ) | | | — | | | | — | | | | (1,979 | ) | | | (1,979 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | (37,244 | ) | | | (29,267 | ) | | | — | | | | — | | | | (66,511 | ) | | | — | | | | (29,267 | ) | | | (37,244 | ) | | | (66,511 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
Financial liabilities not measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Secured bank loans | | | 9 | | | | — | | | | — | | | | — | | | | (906,205 | ) | | | (906,205 | ) | | | — | | | | — | | | | — | | | | — | |
Fixed unsecured loan notes 2052 | | | 9 | | | | — | | | | — | | | | — | | | | (40,913 | ) | | | (40,913 | ) | | | — | | | | — | | | | — | | | | — | |
Trade payables | | | | | | | — | | | | — | | | | — | | | | (38,644 | ) | | | (38,644 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | — | | | | — | | | | — | | | | (985,762 | ) | | | (985,762 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
B-12
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Notes to the Interim Financial Information for the six months ended 30 June 2015 (continued)
8. Financial Instruments (continued)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 30 June 2014 | | | | | Carrying amount | | | Fair value | |
| £000’s | | Note | | | Designated
at fair
value | | | Fair value hedging instruments | | | Loans and
receivables | | | Other financial liabilities | | | Total | | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
| | | | | | | | | | |
Financial assets measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest rate swaps | | | | | | | — | | | | 1,490 | | | | — | | | | — | | | | 1,490 | | | | — | | | | 1,490 | | | | — | | | | 1,490 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | — | | | | 1,490 | | | | — | | | | — | | | | 1,490 | | | | — | | | | 1,490 | | | | — | | | | 1,490 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
Financial assets not measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Trade and other receivables | | | | | | | — | | | | — | | | | 61,736 | | | | — | | | | 61,736 | | | | — | | | | — | | | | — | | | | — | |
Cash and cash equivalents | | | | | | | — | | | | — | | | | 59,547 | | | | — | | | | 59347 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | — | | | | — | | | | 121,283 | | | | — | | | | 121,283 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
Financial liabilities measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest rate swaps | | | | | | | — | | | | (158 | ) | | | — | | | | — | | | | (158 | ) | | | — | | | | (158 | ) | | | — | | | | (158 | ) |
Abcur contingent consideration (2013) | | | | | | | (2,213 | ) | | | — | | | | — | | | | — | | | | (2,213 | ) | | | — | | | | — | | | | (2,213 | ) | | | (2,213 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | (2,213 | ) | | | (158 | ) | | | — | | | | — | | | | (2,371 | ) | | | — | | | | (158 | ) | | | (2,213 | ) | | | (2,371 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
Financial liabilities not measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Secured bank loans | | | 9 | | | | — | | | | — | | | | — | | | | (554,359 | ) | | | (554,359 | ) | | | — | | | | — | | | | — | | | | — | |
Fixed unsecured loan notes 2052 | | | 9 | | | | — | | | | — | | | | — | | | | (410,133 | ) | | | (410,133 | ) | | | — | | | | — | | | | — | | | | — | |
Trade payables | | | | | | | — | | | | — | | | | — | | | | (35,425 | ) | | | (35,425 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | — | | | | — | | | | — | | | | (999,917 | ) | | | (999,917 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | |
| 31 December 2014 | | | | | Carrying amount | | | Fair value | |
| £000’s | | Note | | | Designated
at fair
value | | | Fair value
hedging
instruments | | | Loans and
receivables | | | Other
financial
liabilities | | | Total | | | Level 1 | | | Level 2 | | | Level 3 | | | Total | |
| | | | | | | | | | |
Financial assets measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest rate swaps | | | | | | | — | | | | 61 | | | | — | | | | — | | | | 61 | | | | — | | | | 61 | | | | — | | | | 61 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | — | | | | 61 | | | | — | | | | — | | | | 61 | | | | — | | | | 61 | | | | — | | | | 61 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
Financial assets not measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Trade and other receivables | | | | | | | — | | | | — | | | | 72,916 | | | | — | | | | 72,916 | | | | — | | | | — | | | | — | | | | — | |
Cash and cash equivalents | | | | | | | — | | | | — | | | | 27,780 | | | | — | | | | 27,780 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | — | | | | — | | | | 100,696 | | | | — | | | | 100,696 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
Financial liabilities measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest rate swaps | | | | | | | — | | | | (3,191 | ) | | | — | | | | — | | | | (3,191 | ) | | | — | | | | (3,191 | ) | | | — | | | | (3,191 | ) |
Focus contingent consideration (2014) | | | | | | | (17,155 | ) | | | — | | | | — | | | | — | | | | (17,155 | ) | | | — | | | | — | | | | (17,155 | ) | | | (17,155 | ) |
Abcur contingent consideration (2013) | | | | | | | (3,318 | ) | | | — | | | | — | | | | — | | | | (3,318 | ) | | | — | | | | — | | | | (3,318 | ) | | | (3,318 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | (20,473 | ) | | | (3,191 | ) | | | — | | | | — | | | | (23,664 | ) | | | — | | | | (3,191 | ) | | | (20,473 | ) | | | (23,664 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | |
Financial liabilities not measured at fair value | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Secured bank loans | | | 9 | | | | — | | | | — | | | | — | | | | (905,494 | ) | | | (905,494 | ) | | | — | | | | — | | | | — | | | | — | |
Fixed unsecured loan notes 2052 | | | 9 | | | | — | | | | — | | | | — | | | | (40,913 | ) | | | (40,913 | ) | | | — | | | | — | | | | — | | | | — | |
Trade payables | | | | | | | — | | | | — | | | | — | | | | (44,318 | ) | | | (44,318 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | — | | | | — | | | | — | | | | (990,725 | ) | | | (990,725 | ) | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
B-13
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Notes to the Interim Financial Information for the six months ended 30 June 2015 (continued)
8. Financial Instruments (continued)
| | B) | Measurement of fair values |
i) Valuation techniques and significant unobservable inputs
The following tables show the valuation techniques used in measuring Level 2 and Level 3 fair values, as well as the significant unobservable inputs used.
Financial instruments measured at fair values
| | | | | | |
| Type | | Valuation technique | | Significant unobservable inputs | | Inter-relationship between significant
unobservable inputs and fair value
measurements |
| Interest rate swaps | | Market comparison technique: The fair values are based on bank quotes. Similar contracts are traded in an active market and the quotes reflect the actual transactions in similar instruments. | | Not applicable | | Not applicable |
| | | | |
Primegen contingent consideration (2015) | | Discounted cash flows: The valuation model considers the present value of expected payment, discounted using a risk-adjusted discount rate. The expected payment is determined by considering the possible scenarios of receiving market authorisations and ensuring continuity of supply of the products, the amount to be paid under each scenario and the probability of each scenario. | | Certain revenue thresholds for 12 months ending June 2016 subject to a cap of£10 million and marketing authorisations being granted. | | The estimated fair value would decrease if: - the annual revenue growth rates were lower and marketing authorisations are not granted. |
| | | | |
Focus contingent consideration (2014) | | Discounted cash flows: The valuation model considers the present value of expected payment, discounted using a risk-adjusted discount rate. The expected payment is determined by considering the possible scenarios of gross profit threshold, receiving market authorisations and ensuring continuity of supply of the products, the amount to be paid under each scenario and the probability of each scenario. | | Gross profit thresholds for 12 months ending December 2015 and 2016, subject to a cap of£7 million and£4 million respectively. Contingent consideration of£934k paid in January 2015 contributes to the estimate of fair value. | | The estimated fair value would decrease if: - the annual gross profit growth rates were lower. |
| | | | |
Abcur contingent consideration (2013) | | Discounted cash flows: The valuation model considers the present value of expected payment, discounted using a risk-adjusted discount rate. The expected payment is determined by considering the possible scenarios of forecast revenue and EBITDA, the amount to be paid under each scenario and the probability of each scenario. | | Certain revenue and EBITDA thresholds for 12 months ending November 2014 and 2015 and also SEK 500k is payable for every 1% outperformance (subject to cap of 20%) of EBITDA and revenue thresholds. contingent consideration of£1.3 million paid in January 2015 contributes to the estimate of fair value. | | The estimated fair value would increase/(decrease) if: - the annual revenue growth rate were lower; -the EBITDA margin were lower or;- the risk adjusted discount rate were lower/ (higher). Generally, a change in the annual revenue growth rate is accompanied by a directionally similar change in EBITDA. |
B-14
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Notes to the Interim Financial Information for the six months ended 30 June 2015 (continued)
8. Financial Instruments (continued)
| B) | Measurement of fair values (continued) |
ii) Level 3 fair values
Reconciliation of Level 3 fair values
The following table shows reconciliation from the opening balances to the closing balances for Level 3 fair values.
| | | | |
| | | Contingent consideration | |
Balance as at 1 January 2014 | | | (2,213 | ) |
| | | | |
Balance as at 30 June 2014 | | | (2,213 | ) |
| | | | |
Balance as at 1 July 2014 | | | (2,213 | ) |
Recognised in profit and loss account (Abcur) | | | (1,105 | ) |
Assumed in business combination (Focus) | | | (17,155 | ) |
| | | | |
Balance as at 31 December 2014 | | | (20,473 | ) |
| | | | |
Balance as at 1 January 2015 | | | (20,473 | ) |
Paid during the period | | | 2,273 | |
Assumed in business combination (Primegen) | | | (19,044 | ) |
| | | | |
| | | (37,244 | ) |
| | | | |
Sensitivity analysis
Primegen contingent consideration:
Management have assumed that the cam out targets will be reached in period one and period two and have therefore recognised the maximum fair value payable. In order for the contingent consideration to be affected, it would require a fall in forecast revenue by 28%. The second element of the contingent consideration is in respect of securing approval for particular Market Authorisations. The current fair value assumes these Market Authorisations will be approved, if this is not achieved then this element of the contingent consideration would reduce to£nil.
Focus contingent consideration:
Management have assumed that the earn out targets will be reached in period one and period two and have therefore recognised the maximum fair value payable. In order for the contingent consideration to be affected, it would require a fall in forecast gross profit of 7% in earn out period one and 57% in earn out period two. The second element of the contingent consideration is in respect of securing approval for particular Market Authorisations. The current fair value assumes these Market Authorisations will be approved, if this is not achieved then this element of the contingent consideration would reduce to£nil.
Abcur contingent consideration:
The fair value of the contingent consideration is based upon two earn out period calculations with target revenue and EBITDA figures for November 2014 and November 2015. In January 2015 a payment was made in respect of the first earn out period and this has been deemed to be fair value and further sensitivity analysis is not considered necessary. Management have assumed the maximum earn out targets will be reached in period two and have therefore recognised the maximum fair value amount. In order for this element of the contingent consideration to be affected it would require a fall in forecast revenue of 13 % and a fall in forecast EBITDA of 20 %.
B-15
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Notes to the Interim Financial Information for the six months ended 30 June 2015 (continued)
9. Long term and short-term borrowings
| | | | | | | | | | | | |
| | | As at 30 June
2015 | | | As at 30 June
2014 | | | As at 31 December 2014 | |
| | | £‘000 | | | £‘000 | | | £‘000 | |
| | | |
Short-term borrowings: | | | | | | | | | | | | |
Senior Debt A | | | 29,665 | | | | 12,000 | | | | 25,957 | |
Interest accrued | | | 97 | | | | 207 | | | | 3,391 | |
Arrangement fees capitalised | | | — | | | | — | | | | (7,228 | ) |
| | | | | | | | | | | | |
| | | 29,762 | | | | 12,207 | | | | 22,120 | |
| | | | | | | | | | | | |
| | | |
Long-term borrowings: | | | | | | | | | | | | |
Senior Debt A | | | 118,660 | | | | 54,897 | | | | 133,493 | |
Senior Debt B1 | | | 352,332 | | | | 153,343 | | | | 352,332 | |
Senior Debt B2 | | | 310,284 | | | | 156,651 | | | | 343,949 | |
Acquisition facility | | | 95,264 | | | | 46,498 | | | | 49,763 | |
Mezzanine | | | — | | | | 130,970 | | | | — | |
Arrangement fees capitalised | | | (23,387 | ) | | | (20,607 | ) | | | (18,947 | ) |
Fixed unsecured loan notes 2052 | | | 55,827 | | | | 402,331 | | | | 52,692 | |
| | | | | | | | | | | | |
| | | 908,980 | | | | 924,083 | | | | 913,282 | |
| | | | | | | | | | | | |
The Group bank borrowings consist of term loans, namely Senior Debt A and Senior Debt B1 and B2. Senior Debt A is repayable in installments with the final payment falling due on 31 December 2018. The Senior Debt B is repayable on 31 December 2019.
Interest is charged at 4% above 3 month LIBOR for Senior Debt A, 5.25% above 3 month LIBOR for Senior Debt B1 and 4.75% above 3 month EURIBOR for Senior Debt B2.
The initial costs of establishing the loan facility are being amortised over term of the loan.
The bank term loan facility is secured by a cross debenture and guarantee between Amdipharm Mercury Debtco Limited, Amdipharm Mercury Newco Limited, Amdipharm Mercury Holdco UK Ltd, Amdipharm Mercury Midco UK Limited, Amdipharm Mercury UK Limited, Mercury Pharma Group Limited, Mercury Pharmaceuticals Limited, Mercury Pharma (Generics) Limited, Mercury Pharma Overseas Limited, Mercury Pharma International Limited, Amdipharm AG, Amdipharm International Ltd, Amdipharm Sales & Marketing Ltd, Amdipharm Marketing Ltd, Amdipharm Ltd and by a debenture granting fixed and floating security over all assets of the above mentioned companies with the company’s lending banks.
The Group has an undrawn revolving loan facility of£24.25 million (2014:£24.65 million) and acquisition facility commitments of£nil (2014:£25 million). The revolving loan facility is subject to commitment fees of 1.6% per annum and is valid for the remaining life of the term loans.
The Company issued a first tranche of A1 fixed rate unsecured loan notes 2052 worth£244.32 million on 31 August 2012 and a second tranche of£153.64 million on 31 October 2012.£11.41 million of A2 fixed rate unsecured loan notes 2052 and£33.82 million of A3 fixed rate unsecured loan notes 2052 were also issued by the Group on 31 October 2012. The Series A1 loan notes carry the same rights and rank pari passu among themselves, and both the A1, A2 and A3 loan notes mature in 2052.
The Company had originally issued the A1 loan notes to the Cinven funds and certain private shareholders, A2 loan notes to Cinven funds and the management team of Amdipharm Mercury Group Limited (formerly known as Mercury Pharma Group Limited) and A3 Loan notes to Verdot Limited (the management team of the Amdipharm group). The loan notes confer upon the holders the right to receive interest at a rate of 12 per cent per annum, compounding annually.
B-16
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Notes to the Interim Financial Information for the six months ended 30 June 2015 (continued)
10. ACQUISITIONS
On 2 June 2015 Mercury Pharma Group Limited purchased 100% of the ordinary shares of Primegen Limited (“Primegen”), a specialty pharma business based in the United Kingdom. Primegen sells a number of niche medicines, predominantly in the UK, and has an attractive pipeline of UK and international registrations.
Since Primegen Limited is under the control of Amdipharm Mercury Limited, the results of the acquired business are included from the date of acquisition in the Statement of Comprehensive Income.
The acquisition was for net cash of£45.40 million (including cash acquired) and a total consideration of£64.65 million. Primegen limited had a turnover of£2.83 million and a profit before tax from operations of£1.70 million for the period, of which£1.25 million of turnover and£1.09 million of profit before tax related to the period since acquisition are included in Group accounts.
The following table summarises the consideration paid for Primegen Limited and the amounts of the assets acquired and liabilities assumed recognised at the acquisition date, as well as the fair value at the acquisition date. The valuations in the table are provisional fair values and may change in the future.
| | | | | | | | | | | | |
| | | Book
value | | | Fair value
adjustment | | | Fair
value | |
| | | £‘000 | | | £‘000 | | | £‘000 | |
| | | |
Intangible assets | | | — | | | | 41,630 | | | | 41,630 | |
Inventories | | | 486 | | | | — | | | | 486 | |
Trade and other receivables | | | 449 | | | | — | | | | 449 | |
Cash and cash equivalents | | | 205 | | | | — | | | �� | 205 | |
Deferred tax liability | | | — | | | | (8,326 | ) | | | (8,326 | ) |
| | | | | | | | | | | | |
Net identifiable assets and liabilities | | | 1,140 | | | | 33,304 | | | | 34,444 | |
| | | | | | | | | | | | |
| | | |
Goodwill on acquisition | | | | | | | | | | | 30,205 | |
| | | | | | | | | | | | |
Total purchase consideration | | | | | | | | | | | 64,649 | |
| | | | | | | | | | | | |
| | | |
Less: Cash acquired | | | | | | | | | | | (205 | ) |
Less: Contingent consideration | | | | | | | | | | | (19,044 | ) |
| | | | | | | | | | | | |
Cash outflow on acquisition | | | | | | | | | | | 45,400 | |
| | | | | | | | | | | | |
The goodwill is mainly attributable to the product pipeline additional revenues to be generated from expansion of Primegen’s product portfolio into the Group’s international sales network. None of this goodwill is expected to be tax deductible.
The Group has agreed to pay the selling shareholders over a period of 2 years additional consideration of£22.5 million (present value of£19.04 million) based on the company meeting existing product sales target and receiving Market Authorisation approval for certain molecules, for the year ending December 2016 and 2017.
Acquisition-related costs amounting to£748,000 have been shown within non-recurring items in the Statement of Comprehensive Income for the period ended 30 June 2015.
B-17
Amdipharm Mercury Limited
Condensed financial statements for the period ended 30 June 2015
Notes to the Interim Financial Information for the six months ended 30 June 2015 (continued)
11. Acquisition, deal transaction costs and non recurring items
| | | | | | | | | | | | |
| | | As at
30 Jun-15 | | | As at 30 Jun-14 | | | As at 31 December
2014 | |
| | | £‘000 | | | £‘000 | | | £‘000 | |
| | | |
Dual sourcing and other operations projects | | | 1,788 | | | | 1,682 | | | | 6,118 | |
Acquisition and deal transaction costs | | | 1,703 | | | | 573 | | | | 1,778 | |
Integration costs | | | 626 | | | | 1,783 | | | | 3,958 | |
Others | | | 383 | | | | 80 | | | | 185 | |
Project P3 | | | 274 | | | | 587 | | | | 1,835 | |
| | | | | | | | | | | | |
| | | 4,774 | | | | 4,705 | | | | 13,874 | |
| | | | | | | | | | | | |
Dual sourcing and other operational projects include one-off costs incurred through the diversification of the company’s sources of finished dosage and active product ingredients for the top products. The expenditure is primarily related to the transfer of technologies, validation of sample batches and stability studies.
Integration costs consist of legal and professional fees incurred in relation to employee related settlement payments, project management, infrastructure costs and similarly identifiable expenditure.
Project P3 costs consist of one-off expenditure incurred by the company in updating the summary of product characteristics for the legacy Amdipharm portfolio across all territories in line with regulatory requirements.
Acquisition and deal costs consists primarily of advisor fees incurred for the due diligence work for acquisitions projects, along with related stamp duty and legal costs for post acquisition restructuring.
12. Subsequent events
On 31 July 2015 the Group acquired an Australian company Boucher and Muir Pty Ltd (BNM) for an initial consideration of£6.59 million and contingent consideration of£2.95 million. BNM sells specialist medical products ranging from vaccines to anaesthetics, as well as more general products such as solid dose tablets and capsules.
Since BNM is under the control of Amdipharm Mercury Limited, the results of the acquired business will be included from the date of acquisition in the statement of comprehensive income. Full disclosure of this acquisition under IFRS 3 will be included within the consolidated financial statements for the year ended 31 December 2015.
On 8 September 2015, the Group’s ultimate controlling party, Cinven, announced it had entered into a definitive agreement to sell the Group to Concordia Healthcare Corp. (“Concordia”) for US$3.5 billion to be paid through a combination of cash, common shares of Concordia, and performance-based earn-out payable in cash. The acquisition has been approved by the Board of Directors of both Concordia and Cinven, and is strongly supported by the Amdipharm Mercury Limited management team.
B-18
Independent Auditors’ Report on Review of Interim Financial Information
To the Board of Directors
Amdipharm Mercury Limited
Introduction
We have reviewed the accompanying condensed consolidated statement of financial position of Amdipharm Mercury Limited as at 30 June 2015, the condensed consolidated statement of comprehensive income, changes in equity and cash flows for the six month period then ended, and notes to the interim financial information (“the condensed consolidated interim financial information”). Management is responsible for the preparation and presentation of this condensed consolidated interim financial information in accordance with IAS 34, ‘Interim Financial Reporting’. Our responsibility is to express a conclusion on this condensed consolidated interim financial information based on our review.
Scope of Review
We conducted our review in accordance with the International Standard on Review Engagements 2410, ‘‘Review of Interim Financial Information Performed by the Independent Auditor of the Entity”. A review of interim financial information consists of making inquiries, primarily of persons responsible for financial and accounting matters, and applying analytical and other review procedures. A review is substantially less in scope than an audit conducted in accordance with International Standards on Auditing and consequently does not enable us to obtain assurance that we would become aware of all significant matters that might be identified in an audit. Accordingly, we do not express an audit opinion.
Conclusion
Based on our review, nothing has come to our attention that causes us to believe that the accompanying condensed consolidated interim financial information as at 30 June 2015 is not prepared, in all material respects, in accordance with IAS 34, ‘Interim Financial Reporting’.
|
 |
| KPMG LLP |
|
| 11 September 2015 |
Botanic House
100 Hills Road
Cambridge
CB2 1AR
B-19
APPENDIX C - PRO FORMA FINANCIAL INFORMATION FOR THE YEAR ENDED DECEMBER 31, 2014 AND THE SIX MONTHS ENDED JUNE 30, 2015
CONCORDIA HEALTHCARE CORP.
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
C-1
Concordia Healthcare Corp
Unaudited Pro Forma Condensed Consolidated Balance Sheet
As at June 30, 2015
US $000’s
| | | | | | | | | | | | | | | | | | | | |
| | | Concordia
Healthcare
Corp. | | | Amdipharm
Mercury
Limited | | | Pro Forma
Adjustments | | | Reference | | | Concordia
Healthcare
Corp. Pro
Forma
Consolidated | |
Assets | | | | | | | | | | | | | | | | | | | | |
Current | | | | | | | | | | | | | | | | | | | | |
Cash | | $ | 140,207 | | | $ | 67,910 | | | $ | (3,456,972 | ) | | | 6(a) | | | $ | 93,985 | |
| | | | | | | | | | $ | 3,342,840 | | | | 6(a) | | | | | |
Accounts receivable | | | 68,320 | | | | 127,602 | | | | — | | | | | | | | 195,922 | |
Inventory | | | 14,932 | | | | 57,966 | | | | — | | | | | | | | 72,898 | |
Current income taxes recoverable | | | 4,769 | | | | — | | | | — | | | | | | | | 4,769 | |
Prepaid expenses and other current assets | | | 14,262 | | | | — | | | | — | | | | | | | | 14,262 | |
| | | | | | | | | | | | | | | | | | | | |
| | | 242,490 | | | | 253,478 | | | | (114,132 | ) | | | | | | | 381,836 | |
Fixed assets | | | 1,622 | | | | 4,137 | | | | — | | | | | | | | 5,759 | |
Intangible assets | | | 1,645,528 | | | | 1,157,627 | | | | 1,250,000 | | | | 4 | | | | 4,053,155 | |
Goodwill | | | 43,944 | | | | 335,071 | | | | 1,060,144 | | | | 4 | | | | 1,439,159 | |
Deferred income taxes | | | 4,868 | | | | 221 | | | | — | | | | | | | | 5,089 | |
Deferred financing costs, net | | | — | | | | — | | | | — | | | | | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total Assets | | $ | 1,938,452 | | | $ | 1,750,534 | | | $ | 2,196,012 | | | | | | | $ | 5,884,998 | |
| | | | | | | | | | | | | | | | | | | | |
Liabilities | | | | | | | | | | | | | | | | | | | | |
Current | | | | | | | | | | | | | | | | | | | | |
Accounts payable | | $ | 17,585 | | | $ | 60,608 | | | $ | — | | | | | | | $ | 78,193 | |
Accrued liabilities | | | 20,055 | | | | 69,134 | | | | — | | | | | | | | 89,189 | |
Provisions | | | 36,401 | | | | — | | | | — | | | | | | | | 36,401 | |
Royalties payable | | | 23 | | | | — | | | | — | | | | | | | | 23 | |
Dividend payable | | | 2,553 | | | | — | | | | — | | | | | | | | 2,553 | |
Taxes payable | | | 165 | | | | 7,916 | | | | — | | | | | | | | 8,081 | |
Financial liabilities | | | — | | | | 45,902 | | | | (45,902 | ) | | | 6(e) | | | | — | |
Current portion of notes payable | | | — | | | | — | | | | — | | | | | | | | — | |
Current portion of long-term debt | | | 5,750 | | | | 46,679 | | | | (46,679 | ) | | | 6(e) | | | | 5,750 | |
Current portion of purchase consideration payable | | | 5,965 | | | | — | | | | — | | | | | | | | 5,965 | |
| | | | | | | | | | | | | | | | | | | | |
| | | 88,497 | | | | 230,239 | | | | (92,581 | ) | | | | | | | 226,155 | |
Long-term debt | | | 1,258,637 | | | | 1,425,644 | | | | (1,358,089 | ) | | | 6(e) | | | | 3,390,082 | |
| | | | | | | | | | | (575,000 | ) | | | 6(d) | | | | | |
| | | | | | | | | | | 2,687,355 | | | | 6(c) | | | | | |
| | | | | | | | | | | 19,090 | | | | 6(d) | | | | | |
| | | | | | | | | | | (86,532 | ) | | | 5(c) | | | | | |
| | | | | | | | | | | 18,977 | | | | 5(d) | | | | | |
Purchase consideration payable | | | 25,575 | | | | — | | | | 208,598 | | | | 4 | | | | 234,173 | |
Deferred income taxes | | | 5,589 | | | | 102,479 | | | | 250,000 | | | | 4 | | | | 358,068 | |
Other liabilities | | | 363 | | | | — | | | | — | | | | | | | | 363 | |
| | | | | | | | | | | | | | | | | | | | |
Total Liabilities | | | 1,378,661 | | | | 1,758,362 | | | | 1,071,818 | | | | | | | | 4,208,841 | |
| | | | | | | | | | | | | | | | | | | | |
Shareholders’ Equity | | | | | | | | | | | | | | | | | | | | |
Share capital | | | 538,310 | | | | — | | | | 556,171 | | | | 6(b) | | | | 1,714,166 | |
| | | | | | | | | | | 619,685 | | | | 4 | | | | | |
Net equity | | | — | | | | (7,828 | ) | | | 7,828 | | | | 5(e) | | | | — | |
Reserve for share based compensation | | | 15,877 | | | | — | | | | — | | | | | | | | 15,877 | |
Accumulated other comprehensive income | | | (551 | ) | | | — | | | | — | | | | | | | | (551 | ) |
Retained earnings (deficit) | | | 6,155 | | | | — | | | | (19,090 | ) | | | 6(d) | | | | (53,335 | ) |
| | | | | | | | | | | (40,400 | ) | | | 6(f) | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Total Shareholders’ Equity | | | 559,791 | | | | (7,828 | ) | | | 1,124,194 | | | | | | | | 1,676,157 | |
| | | | | | | | | | | | | | | | | | | | |
Total Liabilities and Shareholders’ Equity | | $ | 1,938,452 | | | $ | 1,750,534 | | | $ | 2,196,012 | | | | | | | $ | 5,884,998 | |
| | | | | | | | | | | | | | | | | | | | |
C-2
Concordia Healthcare Corp
Unaudited Pro Forma Condensed Consolidated Statement of Income (Loss)
Six Months Ended June 30, 2015
US $000’s
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Concordia
Healthcare
Corp. | | | Covis
Portfolio | | | Amdipharm
Mercury
Limited | | | Pro Forma
Adjustments | | | Reference | | | Concordia
Healthcare
Corp. Pro
Forma
Consolidated | |
Revenue | | $ | 113,949 | | | $ | 60,880 | | | $ | 251,729 | | | | (2,124 | ) | | | | | | $ | 424,434 | |
Cost of sales | | | 11,150 | | | | 5,638 | | | | 69,775 | | | | (106 | ) | | | | | | | 86,457 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 102,799 | | | | 55,242 | | | | 181,954 | | | | (2,018 | ) | | | 7(i) | | | | 337,977 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Operating expenses | | | | | | | | | | | | | | | | | | | | | | | | |
General and administrative | | | 14,616 | | | | 5,280 | | | | 33,109 | | | | — | | | | | | | | 53,005 | |
Acquisition, restructuring and other | | | 12,556 | | | | — | | | | 3,548 | | | | (10,820 | ) | | | 7(h) | | | | 5,284 | |
Selling and marketing | | | 7,329 | | | | — | | | | 18,297 | | | | — | | | | | | | | 25,626 | |
Research and development | | | 5,792 | | | | — | | | | 620 | | | | — | | | | | | | | 6,412 | |
Share-based compensation | | | 5,017 | | | | 698 | | | | — | | | | | | | | | | | | 5,715 | |
Exchange listing expenses | | | 574 | | | | — | | | | — | | | | — | | | | | | | | 574 | |
Amortization of intangible assets | | | 20,260 | | | | 10,522 | | | | 36,434 | | | | (10,522 | ) | | | 7(f) | | | | 80,369 | |
| | | | | | | | | | | | | | | 11,956 | | | | 7(f) | | | | | |
| | | | | | | | | | | | | | | 11,719 | | | | 5(a) | | | | | |
Depreciation expense | | | 128 | | | | — | | | | 905 | | | | — | | | | | | | | 1,033 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 66,272 | | | | 16,500 | | | | 92,913 | | | | 2,333 | | | | | | | | 178,018 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Operating income | | | 36,527 | | | | 38,742 | | | | 89,041 | | | | (4,351 | ) | | | | | | | 159,959 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Other income and expense | | | | | | | | | | | | | | | | | | | | | | | | |
Interest and accretion expense | | | 27,653 | | | | 2,598 | | | | 89,597 | | | | (2,598 | ) | | | 7(d) | | | | 108,180 | |
| | | | | | | | | | | | | | | (8,641 | ) | | | 7(e) | | | | | |
| | | | | | | | | | | | | | | 19,691 | | | | 7(c) | | | | | |
| | | | | | | | | | | | | | | 1,528 | | | | 7(c) | | | | | |
| | | | | | | | | | | | | | | 81,479 | | | | 6(c) | | | | | |
| | | | | | | | | | | | | | | (13,530 | ) | | | 6(d) | | | | | |
| | | | | | | | | | | | | | | (89,597 | ) | | | 6(e) | | | | | |
Finance income | | | — | | | | — | | | | (631 | ) | | | — | | | | | | | | (631 | ) |
Impairment loss | | | 668 | | | | — | | | | — | | | | — | | | | | | | | 668 | |
Change in fair value of contingent consideration | | | (6,224 | ) | | | — | | | | — | | | | — | | | | | | | | (6,224 | ) |
Foreign exchange (gain) loss | | | (282 | ) | | | — | | | | (52,139 | ) | | | 52,139 | | | | 6(e) | | | | (282 | ) |
Fair value loss on foreign exchange forward contract | | | 5,126 | | | | — | | | | — | | | | — | | | | | | | | 5,126 | |
Other (income) expense | | | 400 | | | | (78 | ) | | | (238 | ) | | | — | | | | | | | | 84 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Income (loss) before tax | | | 9,186 | | | | 36,222 | | | | 52,452 | | | | (44,822 | ) | | | | | | | 53,038 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Income taxes | | | | | | | | | | | | | | | | | | | | | | | | |
Current | | | 475 | | | | 3,412 | | | | 16,642 | | | | 7,088 | | | | 5(f) | | | | 27,617 | |
Deferred | | | 3,598 | | | | — | | | | (4,373 | ) | | | (2,344 | ) | | | 5(f) | | | | (3,119 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Net Income (loss) | | | 5,113 | | | | 32,810 | | | | 40,183 | | | | (49,566 | ) | | | | | | | 28,540 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
C-3
Concordia Healthcare Corp
Unaudited Pro Forma Condensed Consolidated Statement of Income (Loss)
Year ended December 31, 2014
US $000’s
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Concordia
Healthcare
Corp. | | | Donnatal®
Jan 1 -
May 15,
2014 | | | Zonegran®
Jan 1 -
Sep 30,
2014 | | | Covis
Portfolio | | | Amdipharm
Mercury
Limited | | | Pro Forma
Adjustments | | | Reference | | | Concordia
Healthcare
Corp. Pro
Forma
Consolidated | |
Revenue | | $ | 122,191 | | | $ | 17,607 | | | $ | 17,281 | | | $ | 151,534 | | | $ | 482,248 | | | | — | | | | | | | | 790,861 | |
Cost of sales | | | 17,989 | | | | 900 | | | | 4,311 | | | | 12,939 | | | | 120,618 | | | | — | | | | | | | | 156,757 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Gross profit | | | 104,202 | | | | 16,707 | | | | 12,970 | | | | 138,595 | | | | 361,630 | | | | — | | | | | | | | 634,104 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating expenses | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
General and administrative | | | 20,663 | | | | 3,280 | | | | 1,994 | | | | 32,920 | | | | 77,315 | | | | — | | | | | | | | 136,172 | |
Acquisition, restructuring and other | | | 13,521 | | | | — | | | | — | | | | — | | | | 7,627 | | | | (12,163 | ) | | | 7(h) | | | | 8,985 | |
Selling and marketing | | | 10,229 | | | | 1,159 | | | | 566 | | | | — | | | | 37,069 | | | | — | | | | | | | | 49,023 | |
Research and development | | | 9,301 | | | | 94 | | | | — | | | | — | | | | 5,076 | | | | — | | | | | | | | 14,471 | |
Share-based compensation | | | 4,484 | | | | — | | | | — | | | | 2,690 | | | | — | | | | — | | | | | | | | 7,174 | |
Amortization of intangible assets | | | 11,039 | | | | — | | | | — | | | | 36,117 | | | | 73,702 | | | | (36,117 | ) | | | 7(f) | | | | 163,862 | |
| | | | | | | | | | | | | | | | | | | | | | | 47,822 | | | | 7(f) | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | 8,696 | | | | 7(g) | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | 22,603 | | | | 5(a) | | | | | |
Depreciation expense | | | 131 | | | | 114 | | | | — | | | | — | | | | 1,476 | | | | — | | | | | | | | 1,721 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 69,368 | | | | 4,647 | | | | 2,560 | | | | 71,727 | | | | 202,265 | | | | 30,841 | | | | | | | | 381,408 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Operating income | | | 34,834 | | | | 12,060 | | | | 10,410 | | | | 66,868 | | | | 159,365 | | | | (30,841 | ) | | | | | | | 252,696 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Other income and expense | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Interest and accretion expense | | | 12,194 | | | | — | | | | — | | | | 10,523 | | | | 160,194 | | | | (10,523 | ) | | | 7(a) | | | | 223,601 | |
| | | | | | | | | | | | | | | | | | | | | | | (6,332 | ) | | | 7(b) | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | 78,763 | | | | 7(c) | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | 6,113 | | | | 7(c) | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | 162,957 | | | | 6(c) | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | (30,094 | ) | | | 6(d) | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | (160,194 | ) | | | 6(e) | | | | | |
Finance income | | | — | | | | — | | | | — | | | | — | | | | (1,304 | ) | | | — | | | | | | | | (1,304 | ) |
Change in fair value of contingent consideration | | | 2,629 | | | | — | | | | — | | | | — | | | | 1,820 | | | | — | | | | | | | | 4,449 | |
Foreign exchange loss (gain) | | | 696 | | | | — | | | | — | | | | — | | | | (15,055 | ) | | | 15,055 | | | | 6(e) | | | | 696 | |
Other (income) expense | | | 203 | | | | — | | | | 38 | | | | (159 | ) | | | (545 | ) | | | — | | | | | | | | (463 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income (loss) before tax | | | 19,112 | | | | 12,060 | | | | 10,372 | | | | 56,504 | | | | 14,255 | | | | (86,586 | ) | | | | | | | 25,717 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Income taxes | | | 7,522 | | | | — | | | | 3,994 | | | | 3,353 | | | | 4,040 | | | | 24,507 | | | | 5(g) | | | | 43,416 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net Income (loss) | | | 11,590 | | | | 12,060 | | | | 6,378 | | | | 53,151 | | | | 10,215 | | | | (111,093 | ) | | | | | | | (17,699 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
C-4
NOTES TO THE UNAUDITED PRO FORMA CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
(all dollar amounts expressed in thousands of U.S. dollars. All Pounds Sterling amounts
expressed in thousands of Pounds Sterling)
| 1. | DESCRIPTION OF THE TRANSACTION |
Concordia Healthcare Corp. (the “Company” or “Concordia”) is an integrated healthcare company that targets three areas: (a) legacy pharmaceutical products; (b) specialized healthcare distribution that services the growing diabetic market; and (c) the acquisition and/or development of orphan drugs. These three business units are run as separate divisions but are inter-related.
On September 4, 2015, the Company entered into an agreement (“AMCo Purchase Agreement”) to purchase all of the outstanding shares in the capital of Amdipharm Mercury Limited (“AMCo”) from the holders of such shares for aggregate consideration equal to approximately $3.3 billion (the “Acquisition”). The purchase price for the Acquisition (the “Purchase Price”) will consist of (i) approximately £800 million (approximately US$1.2 billion) in cash, (ii) 8,490,000 common shares of the Company, and (iii) the repayment of AMCo’s existing senior secured facilities in the respective principal amounts of £581 million and €440 million (approximately US$1.4 billion in the aggregate) plus accrued interest and related cross-currency swaps. In addition, pursuant to the AMCo Purchase Agreement, Concordia will pay (i) an amount of £272,801 (approximately US$414,000) accruing daily from June 30, 2015 to the completion of the Acquisition, and (ii) to the extent earned, a cash earn-out in a maximum amount of £144 million (approximately US$220 million) calculated with reference to the future gross profit of the AMCo group over a period of 12 months from October 1, 2015. At completion of the Acquisition, Concordia will procure, on behalf of AMCo, the redemption of the capital loan notes and the payment of certain other amounts, all of which will be deducted from the cash consideration described above.
The accompanying unaudited pro forma condensed consolidated financial statements of the Company also reflect adjustments to the historical financial statements to give effect to the Company’s acquisitions of a portfolio of pharmaceutical products (the “Covis Portfolio”) from Covis Pharma S.à.r.l and Covis Injectables S.à.r.l (collectively, “Covis”) on April 21, 2015, and the acquisitions of Donnatal® and Zonegran® completed on May 15, 2014 and September 30, 2014, respectively (collectively, “Pharmaceutical Assets”).
The unauditedpro formacondensed consolidated financial statements have been prepared based upon currently available information and assumptions deemed appropriate by management. The unauditedpro formacondensed consolidated financial statements are provided for information purposes only and may not be indicative of the results that would have occurred if the above transactions had been effected on the dates indicated. The accounting for certain of the above transactions will require the determination of fair value estimates at the date of the transactions. Thepro formaadjustments are preliminary and have been made solely for the purpose of providing unauditedpro formacondensed consolidated financial information. Differences between these preliminary estimates and the final accounting for these transactions may occur and these differences could have a material impact on the accompanying unauditedpro formacondensed consolidated financial statements.
The Company’s unauditedpro forma condensed consolidated statement of financial position as at June 30, 2015 has been prepared assuming the Acquisition had occurred as of June 30, 2015 and the unauditedpro formacondensed consolidated statements of income (loss) for the six months ended June 30, 2015 and unauditedpro formacondensed consolidated statements of income (loss) for the year ended December 31, 2014 have been prepared assuming all the transactions described above had each occurred as of January 1, 2014. The unauditedpro forma adjustments are described herein and are based upon currently available information and certain assumptions made.
C-5
The Company’s unauditedpro forma condensed consolidated financial statements have been prepared using the following information:
| i. | Audited consolidated financial statements of the Company as at and for the year ended December 31, 2014, which include the results of Donnatal® and Zonegran® post their acquisition dates of May 15, 2014 and September 30, 2014; |
| ii. | Unaudited consolidated financial statements of the Company as of and for the six months ended June 30, 2015, which include the results of the Covis Portfolio post the acquisition date of April 21, 2015; |
| iii. | Unaudited carve out financial information of Donnatal® for the period January 1, 2014 to May 15, 2014; |
| iv. | Audited carve out financial statements of Zonegran® for the year ended March 31, 2014 and unaudited carve out financial statements of Zonegran® for the six months ended September 30, 2014; |
| v. | Audited carve out financial statements of the Covis Portfolio for the year ended December 31, 2014; |
| vi. | Unaudited carve out financial statements of Covis Pharma S.à.r.l and Covis Injectables S.à.r.l as of and for the three months ended March 31, 2015; |
| vii. | Audited consolidated financial statements of AMCo as at and for the year ended December 31, 2014, for which the statement of income was translated using the average GBP:USD rate of 1.647 for the year ended December 31, 2014. |
| viii. | Unaudited consolidated financial statements of AMCo as at and for the six months ended June 30, 2015 denominated in UK pounds. For inclusion in thepro forma condensed consolidated financial statements, the unaudited balance sheet was translated at the spot rate of 1.5684 as of June 30, 2015 and the statement of income was translated using the average GBP:USD rate of 1.5236 for the six months ended June 30, 2015. |
All financial data in these unauditedpro formacondensed consolidated financial statements is presented in US dollars and has been prepared using the accounting policies used to prepare the Company’s financial statements which are in accordance with International Financial Reporting Standards as issued by the IASB (“IFRS”). Management has determined, based on their initial assessment, that no accounting adjustments are necessary to conform the relevant items of the financial statements of the Covis Portfolio, Donnatal®, Zonegran® and AMCo to the Company’s accounting policies. Certain reclassification were required to present AMCo’s income statement in accordance with the presentation of the Company’s income statement – please refer to note 8.
The unauditedpro forma condensed consolidated financial statements have been prepared for informational purposes only and should be read in conjunction with the financial statements described above and related disclosures used to prepare these statements. The preparation of these unauditedpro forma condensed consolidated financial statements requires management to make estimates and assumptions deemed appropriate. The unauditedpro forma condensed consolidated financial statements are not intended to present or be indicative of the actual financial position and results of operations that would have occurred if the transactions described above had been effected on the dates indicated.
C-6
The Company’s unauditedpro forma condensed consolidated financial statements adjust the Company’s historic financial statements to give effect to the following transactions:
| | • | | Those that are directly attributable to the Acquisition; |
| | • | | Those that are directly attributable to the Company’s prior acquisitions of the Pharmaceutical Assets as described in note 1; |
| | • | | Those with respect to the unauditedpro forma condensed consolidated statements of income (loss) that are directly attributable to the Acquisition and the prior acquisitions of the Pharmaceutical Assets that are expected to have a continuing impact on the consolidated results of the Company; |
| | • | | Those with respect to the unauditedpro formacondensed consolidated statements of income (loss) that are directly attributable to the Acquisition and the prior acquisitions of the Pharmaceuticals Assets that are expected to have both a continuing and non-recurring impact on the consolidated results of the Company; |
| | • | | Debt and equity financing transactions directly attributable to the Acquisition and the prior acquisitions of the Pharmaceutical Assets; and |
| | • | | Debt and equity financing transactions directly attributable to this equity offering. |
The unauditedpro forma condensed consolidated financial statements do not reflect the impact of potential cost savings and other synergies.
| 4. | PURCHASE PRICE ALLOCATION - AMCO |
The Acquisition is considered to be a business combination under IFRS 3 with Concordia as the acquirer and AMCo as the acquiree. The unauditedpro forma condensed consolidated financial statements have been prepared using the acquisition method of accounting in accordance with IFRS 3. Accordingly, the purchase price calculations and purchase price allocations are dependent upon fair value estimates and assumptions as at the Acquisition date. In certain instances adequate information is not available at the time of the preparation of these unauditedpro forma condensed consolidated financial statements to perform a final estimate of fair value. The Company will finalize all amounts as it obtains the information necessary to complete the measurement process, which, pursuant to IFRS 3, will be no later than one year from the Acquisition date.
Certain pro forma adjustments contained herein are preliminary and have been made solely for the purpose of providing unauditedpro forma condensed consolidated financial statements. Differences between preliminary estimates and final amounts may occur and these differences could be material to the accompanying unauditedpro forma condensed consolidated financial statements of the Company and the Company’s future performance and financial position.
C-7
The preliminary purchase price allocation to the following identifiable assets and liabilities is based on their estimated fair values:
| | | | |
Net Assets Acquired | | | | |
Working capital | | $ | 69,918 | |
Fixed assets | | | 4,137 | |
Intangible assets | | | 2,407,627 | |
Goodwill | | | 1,395,215 | |
Deferred income tax assets | | | 221 | |
| |
Current portion of long-term debt | | | (46,679 | ) |
Long-term debt | | | (1,358,089 | ) |
Deferred income taxes | | | (352,479 | ) |
| | | | |
Total | | $ | 2,119,871 | |
| | | | |
| |
Consideration Comprised of: | | | | |
Cash | | | 1,291,588 | |
Contingent consideration payable | | | 208,598 | |
Equity | | | 619,685 | |
| | | | |
Total Consideration | | $ | 2,119,871 | |
| | | | |
The purchase consideration for the Acquisition of $2,119,871 includes estimated contigent consideration of $208,598. As agreed upon with the sellers of AMCo, the Company has agreed to pay an additional amount equal to three times the amount by which the total actual gross contribution of certain specified products exceeds $375,720 (£242,400) for the period of October 1, 2015 to September 30, 2016, provided that such amount shall not in any event exceed $220,000 (£144,000). The Company estimates that the targeted gross contribution will be achieved, and accordingly, has accrued $208,598 representing the present value of the expected future cash outflow.
In addition, if the Company acquires certain specified pharmaceutical products from a specified third party introduced to the Company by certain of the sellers of AMCo within 12 months of the date of the Acquisition, the Company will pay to the sellers of AMCo an additional $72,000.
The equity consideration of 8,490,000 shares included in the purchase consideration has been determined using a share price of $72.99 as listed on the NASDAQ as of September 15, 2015. In the event the share price increases or decreases by 10%, the equity consideration will range from $557,717 to $681,654.
| 5. | PRO FORMA ADJUSTMENTS RELATING TO THE ACQUISITION OF AMCO |
| | a. | The unaudited pro forma consolidated income statements for the six months ended June 30, 2015 and for the year ended December 31, 2014 include adjustments of $11,719 and $22,603 respectively, to increase amortization expense of intangible assets acquired on acquisition of AMCo. Amortization of intangible assets acquired has been determined using an estimated life of 25 years, which is subject to change as the Company obtains further information regarding the estimated future cash flows and valuation of these assets. |
| | b. | Goodwill of $1,395,215 was recognized in the preliminary purchase price allocation of the Acquisition, which includes $250,000 relating to deferred tax liabilities. |
| | c. | AMCo had approximately $86,532 of shareholder loans outstanding as at June 30, 2015, which will not be acquired by the Company. |
C-8
| | d. | An adjustment of $18,977 was recognized in the preliminary purchase price allocation of the Acquisition to re-measure long-term debt to its fair value, which is the amount expected to be repaid on the Acquisition date. |
| | e. | The previous net equity of AMCo of $7,828 is eliminated upon acquisition. |
| | f. | The unaudited pro forma consolidated income statement for the six months ended June 30, 2015 include adjustments to increase current tax expenses by $7,088 and decrease deferred tax expenses by $2,344 to reflect the tax impact of non-tax related pro forma adjustments related to the Acquisition. |
| | g. | The unaudited pro forma consolidated income statement for the year ended December 31, 2014 include an adjustment to increase income tax expenses by $24,507 to reflect the tax impact of non-tax related pro forma adjustments related to the Acquisition. |
| 6. | PRO FORMA ADJUSTMENTS RELATED TO EQUITY AND DEBT FINANCING OF THE ACQUISITION |
| | a. | The Acquisition is expected to be financed through the combination of an equity issuance, debt financing and the Company’s cash on-hand. The following table details the cash sources and uses of funds: |
| | | | |
Sources of funds | | | | |
Issuance of equity | | $ | 577,840 | |
Term loan | | | 1,875,000 | |
Senior notes | | | 890,000 | |
Cash on-hand | | | 114,132 | |
| | | | |
Total | | $ | 3,456,972 | |
| | | | |
| |
Uses of funds | | | | |
Cash consideration for acquisition of AMCo | | $ | 1,239,876 | |
Additional AMCo consideration re: lockbox interest | | $ | 51,712 | |
Refinance of the Company’s term loan | | | 575,000 | |
Repayment of AMCo’s debt facilities | | | 1,404,768 | |
Settlement of AMCo financial instruments | | | 45,902 | |
Equity issuance costs | | | 21,669 | |
Debt financing costs | | | 77,645 | |
Acquisition costs | | | 40,400 | |
| | | | |
| | $ | 3,456,972 | |
| | | | |
C-9
| | b. | To finance the Acquisition, the Company expects to raise funds through an equity issuance, which will result in adjusting the unaudited pro forma balance sheet as follows: |
| | | | |
Equity net of issuance costs | | | | |
Issuance of equity | | $ | 577,840 | |
Less: equity issuance costs | | | (21,669 | ) |
| | | | |
| | $ | 556,171 | |
| | | | |
Gross proceeds from the issuance of equity of $577,840 assumes the issuance of 8,000,000 shares at a price of $72.23, which was the closing price of the Company’s stock on NASDAQ on September 18, 2015.
| | c. | To finance the Acquisition, the Company will enter into agreements relating to term loans and senior unsecured debt totaling $2,765,000, as follows: |
| | | | | | |
| | | Principal | | | Term |
Debt financing facilities | | | | | | |
US denominated term loan | | $ | 1,100,000 | | | 7 years |
GBP denominated term loan | | £ | 500,000 | | | 7 years |
Senior unsecured debt | | $ | 890,000 | | | 8 years |
The Company estimates that the weighted average interest rate of the debt financings will be approximately 5.51%.
The unaudited pro forma consolidated balance sheet has been adjusted by $2,687,355 to reflect the above-noted debt facilities of $2,765,000 less related financing costs of $77,645.
As a result of issuing the term loans, the unaudited pro forma consolidated income statements for the six months ended June 30, 2015 and for the year ended December 31, 2014 include adjustments to increase interest and accretion expense by $81,479 and $162,957, respectively.
If the interest rate for each of the debt financing facilities were to have increased 1/8 of a percent during the periods presented, the combined interest and accretion expense would have been $83,207 for the six months ended June 30, 2015 and $166,414 for the year ended December 31, 2014.
| | d. | The term loans described in note 6(c) will replace the Company’s previous term loan outstanding of $575,000. The Company will treat the refinancing as an extinguishment in accordance with IAS 39Financial Instruments – Recognition and Measurement and will expense the remaining unamortized financing costs of $19,090. |
Consequently, the unaudited pro forma consolidated income statements for the six months ended June 30, 2015 and for the year ended December 31, 2014 include adjustments of $13,530 and $30,094, respectively, to reduce interest and accretion expense for the extinguished term loan.
| | e. | Upon acquisition of AMCo, the Company is required to settle approximately $1,404,768 of AMCo’s outstanding debt facilities and $45,902 of financial liabilities acquired. As a result, the unaudited pro forma consolidated income statements for the six months ended June 30, 2015 and for the year ended December 31, 2014 include adjustments of $89,597 and $160,194, respectively, to reduce interest and accretion expense for the repayment of these liabilities. |
Additionally, the unaudited pro forma consolidated income statements for the six months ended June 30, 2015 and for the year ended December 31, 2014 include adjustments to eliminate the foreign exchange gains related to the translation of AMCo’s debt facility denominated in Euros of $52,139 and $15,055, respectively.
| | f. | The Company’s retained earnings will be decreased by $40,400 for transaction costs expected to be incurred related to the Acquisition. |
C-10
| 7. | ACQUISITION OF PHARMACEUTICAL ASSETS AND RELATED PRO FORMA ADJUSTMENTS |
During the year ended December 31, 2014 and during the first quarter of 2015, the Company acquired the Pharmaceutical Assets. For purposes of presenting pro forma consolidated financial statements for the year ended December 31, 2014 and six months ended June 30, 2015, the Company has recorded the following pro forma adjustments related to the acquisition of the Pharmaceutical Assets:
| | a. | The unaudited pro forma consolidated income statement for the year ended December 31, 2014 includes an adjustment to decrease interest and accretion expense by $10,523 incurred by Covis on debt that was not assumed by the Company as part of the acquisition of the Covis Portfolio. |
| | b. | The unaudited pro forma consolidated income statement for the year ended December 31, 2014 includes an adjustment to decrease interest and accretion expense incurred by the Company related to its credit facilities with GE Capital Canada Finance Inc. of $6,332. These credit facilities were retired as part of the financing related to the acquisition of the Covis Portfolio. |
| | c. | The unaudited pro forma consolidated income statements for the six months ended June 30, 2015 and for the year ended December 31, 2014 include adjustments to increase interest and accretion expense to reflect the impact of the term loans and senior notes that were incurred to fund the acquisition of the Pharmaceutical Assets. For the purposes of the unaudited pro forma consolidated income statements it is assumed that the term loans and senior notes were issued on January 1, 2014. The following table details the calculations of the adjustments related to interest expense: |
| | | | | | | | | | | | | | | | |
| | | Principal | | | Interest
Rate | | | 2014 Interest
Expense | | | Q1-15 Interest
Expense | |
Debt financing sources | | | | | | | | | | | | | | | | |
Term loan facility | | | 575,000 | | | | 4.75 | % | | | 27,313 | | | | 6,828 | |
Senior notes | | | 735,000 | | | | 7.00 | % | | | 51,450 | | | | 12,863 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 1,310,000 | | | | | | | $ | 78,763 | | | $ | 19,691 | |
| | | | | | | | | | | | | | | | |
Debt issuance costs of $46,122 are included as a reduction to the carrying amount of the liability and will be amortized through interest and accretion expense using the effective interest rate method. For the purposes of the unaudited pro forma consolidated income statements a straight-line amortization over the maturity period of the term loans and senior notes has been assumed. The following table details the calculation of the adjustment related to accretion interest:
| | | | | | | | | | | | | | | | |
| | | Costs | | | Years | | | 2014
Accretion | | | Q1-2015
Accretion | |
Amortization of Debt Issuance Costs | | | | | | | | | | | | | | | | |
Term loan facility | | | 19,472 | | | | 7 | | | | 2,782 | | | | 695 | |
Senior notes | | | 26,650 | | | | 8 | | | | 3,331 | | | | 833 | |
| | | | | | | | | | | | | | | | |
Total | | $ | 46,122 | | | | | | | $ | 6,113 | | | $ | 1,528 | |
| | | | | | | | | | | | | | | | |
| | d. | The unaudited pro forma consolidated income statement for the six months ended June 30, 2015 includes an adjustment to decrease interest and accretion expense by $2,598 incurred by Covis on debt that was not assumed by the Company as part of the acquisition of the Covis Portfolio. |
| | e. | The unaudited pro forma consolidated income statement for the six months ended June 30, 2015 includes an adjustment to decrease interest and accretion expense incurred by Concordia related to its credit facilities with GE Capital Canada Finance Inc. of $8,641. These credit facilities were retired as part of the financing related to the acquisition of the Covis Portfolio. |
C-11
| | f. | The unaudited pro forma consolidated income statement for the six months ended June 30, 2015 and for the year ended December 31, 2014 include adjustments to decrease amortization expense by $10,522 and $36,117, respectively, representing the amortization of intangible assets recorded by Covis. The statements also include adjustments to increase amortization expense by $11,956 and $47,822, respectively. These adjustments represent the amortization that Concordia would have recognized in each period assuming that the acquired product rights of Covis of $1,195,560 were amortized over a period of 25 years. As noted in the audited financial statements of Concordia, the Company amortizes intangible assets over a period of 15 – 30 years. The Company is continuing to assess and review the fair value of net assets acquired pursuant to the acquisition of the Covis Portfolio and finalize the associated purchase price accounting including the valuation of intangible assets acquired. As part of the valuation the Company will also determine the useful life of each of the products acquired. As a result, the actual amortization expense recognized by the Company could vary significantly from the amounts used in the unaudited pro forma consolidated financial statements. |
| | g. | The unaudited pro forma consolidated income statement for the year ended December 31, 2014 includes an adjustment to increase amortization expense by $8,696. This adjustment represents amortization of the acquired product rights from the acquisitions of Donnatal® and Zonegran® that Concordia would have recognized in 2014 during the period prior to Concordia’s acquisition of these assets. The following table details the calculation of the adjustment to amortization expense: |
| | | | | | | | | | | | | | | | | | |
| | | Acquired
Product
Rights | | | Date of
Acquisition | | Pre-
Acquisition
Period
(months) | | | Useful Life
(years) | | | Pro Forma
Amortization
Adjustment | |
Donnatal® | | | 327,523 | | | 2014 - 05 -15 | | | 4.5 | | | | 30 | | | | 4,094 | |
Zonegran® | | | 92,040 | | | 2014 - 09 - 30 | | | 9.0 | | | | 15 | | | | 4,602 | |
| | | | | | | | | | | | | | | | | | |
Total | | $ | 419,563 | | | | | | | | | | | | | $ | 8,696 | |
| | | | | | | | | | | | | | | | | | |
C-12
| | h. | The unaudited pro forma consolidated income statement for the six months ended June 30, 2015 and the year ended December 31, 2014 has been adjusted by $10,820 and $12,163, respectively, to eliminate acquisition costs incurred related to the acquisition of the Pharmaceutical Assets. |
C-13
| | i. | The pro forma consolidated financial statements for the six months ended June 30, 2015 include an adjustment to reduce Covis gross profit by $2,018. This adjustment quantifies the net impact to the pro forma financial statements of aligning the Covis distribution policies with the Company in the transition of the business to the Company in the second quarter of 2015. |
| 8. | PRESENTATION AND TRANSLATION OF AMCO INCOME STATEMENTS |
Certain reclassifications were recorded to adjust the presentation of AMCo’s income statements to align with the manner in which the Company presents its consolidated income statement. Net income was not impacted by the reclassifications posted.
C-14
The following table presents a reconciliation of the AMCo’s income statement for the six months ended June 30, 2015 and translates AMCo’s income statement from British pounds to US dollars at an average exchange rate of 1.5236:
| | | | | | | | | | | | | | | | |
| | | Amdipharm
Mercury
Limited
as presented | | | Amdipharm
Mercury
Limited
re-allocations | | | Amdipharm
Mercury
Limited
Reclassified | | | Amdipharm
Mercury
Limited
Translated to
US$ | |
Revenue | | £ | 165,220 | | | | — | | | £ | 165,220 | | | $ | 251,729 | |
Cost of sales | | | 45,796 | | | | — | | | | 45,796 | | | | 69,775 | |
| | | | | | | | | | | | | | | | |
Gross profit | | | 119,424 | | | | — | | | | 119,424 | | | | 181,954 | |
| | | | | | | | | | | | | | | | |
Operating expenses | | | | | | | | | | | | | | | | |
General and administrative | | | 54,165 | | | | (32,434 | ) | | | 21,731 | | | | 33,109 | |
Business acquisition related costs | | | — | | | | 2,329 | | | | 2,329 | | | | 3,548 | |
Selling and marketing | | | 6,262 | | | | 5,747 | | | | 12,009 | | | | 18,297 | |
Research and development | | | — | | | | 407 | | | | 407 | | | | 620 | |
Share-based compensation | | | — | | | | — | | | | — | | | | — | |
Amortization of intangible assets | | | — | | | | 23,913 | | | | 23,913 | | | | 36,434 | |
Depreciation expense | | | — | | | | 594 | | | | 594 | | | | 905 | |
| | | | | | | | | | | | | | | | |
Total operating expenses | | | 60,427 | | | | 556 | | | | 60,983 | | | | 92,913 | |
| | | | | | | | | | | | | | | | |
Operating income | | | 58,997 | | | | (556 | ) | | | 58,441 | | | | 89,041 | |
| | | | | | | | | | | | | | | | |
Other income and expense | | | | | | | | | | | | | | | | |
Interest and accretion expense | | | 58,806 | | | | — | | | | 58,806 | | | | 89,597 | |
Finance income | | | (34,079 | ) | | | 33,665 | | | | (414 | ) | | | (631 | ) |
Change in fair value of contingent consideration | | | — | | | | — | | | | — | | | | — | |
Foreign exchange gain | | | — | | | | (34,221 | ) | | | (34,221 | ) | | | (52,139 | ) |
Other income | | | (156 | ) | | | — | | | | (156 | ) | | | (238 | ) |
| | | | | | | | | | | | | | | | |
Income before tax | | | 34,426 | | | | — | | | | 34,426 | | | | 52,452 | |
| | | | | | | | | | | | | | | | |
Income taxes | | | | | | | | | | | | | | | | |
Current | | | 10,923 | | | | — | | | | 10,923 | | | | 16,642 | |
Deferred | | | (2,870 | ) | | | — | | | | (2,870 | ) | | | (4,373 | ) |
| | | | | | | | | | | | | | | | |
Net Income | | £ | 26,373 | | | | — | | | £ | 26,373 | | | $ | 40,183 | |
| | | | | | | | | | | | | | | | |
C-15
The following table presents a reconciliation of the AMCo’s income statement for the year ended December 31, 2014 and translates AMCo’s income statement from British pounds to US dollars at an average exchange rate of 1.647:
| | | | | | | | | | | | | | | | |
| | | Amdipharm
Mercury
Limited
as presented | | | Amdipharm
Mercury
Limited
re-allocations | | | Amdipharm
Mercury
Limited
Reclassified | | | Amdipharm
Mercury
Limited
Translated to
US$ | |
Revenue | | £ | 292,804 | | | | — | | | £ | 292,804 | | | $ | 482,248 | |
Cost of sales | | | 73,235 | | | | — | | | | 73,235 | | | | 120,618 | |
| | | | | | | | | | | | | | | | |
Gross profit | | | 219,569 | | | | — | | | | 219,569 | | | | 361,630 | |
| | | | | | | | | | | | | | | | |
Operating expenses | | | | | | | | | | | | | | | | |
General and administrative | | | 112,289 | | | | (65,346 | ) | | | 46,943 | | | | 77,315 | |
Business acquisition related costs | | | — | | | | 4,631 | | | | 4,631 | | | | 7,627 | |
Selling and marketing | | | 15,262 | | | | 7,245 | | | | 22,507 | | | | 37,069 | |
Research and development | | | — | | | | 3,082 | | | | 3,082 | | | | 5,076 | |
Share-based compensation | | | — | | | | — | | | | — | | | | — | |
Amortization of intangible assets | | | — | | | | 44,749 | | | | 44,749 | | | | 73,702 | |
Depreciation expense | | | — | | | | 896 | | | | 896 | | | | 1,476 | |
| | | | | | | | | | | | | | | | |
Total operating expenses | | | 127,551 | | | | (4,743 | ) | | | 122,808 | | | | 202,265 | |
| | | | | | | | | | | | | | | | |
Operating income | | | 92,018 | | | | 4,743 | | | | 96,761 | | | | 159,365 | |
| | | | | | | | | | | | | | | | |
Other income and expense | | | | | | | | | | | | | | | | |
Interest and accretion expense | | | 97,264 | | | | — | | | | 97,264 | | | | 160,194 | |
Finance income | | | (13,571 | ) | | | 12,779 | | | | (792 | ) | | | (1,304 | ) |
Change in fair value of contingent consideration | | | — | | | | 1,105 | | | | 1,105 | | | | 1,820 | |
Foreign exchange gain | | | — | | | | (9,141 | ) | | | (9,141 | ) | | | (15,055 | ) |
Other income | | | (331 | ) | | | — | | | | (331 | ) | | | (545 | ) |
| | | | | | | | | | | | | | | | |
Income before tax | | | 8,656 | | | | — | | | | 8,656 | | | | 14,255 | |
| | | | | | | | | | | | | | | | |
Income taxes | | | 2,453 | | | | — | | | | 2,453 | | | | 4,040 | |
| | | | | | | | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
Net Income | | £ | 6,203 | | | | — | | | £ | 6,203 | | | $ | 10,215 | |
| | | | | | | | | | | | | | | | |
C-16
| 9. | TRANSLATION OF AMCO BALANCE SHEET |
The following table presents the translated amounts of AMCo’s balance sheet from British pounds to US dollars at an average exchange rate of 1.5684:
| | | | | | | | |
| | | Amdipharm
Mercury
Limited
in GBP | | | Amdipharm
Mercury
Limited
Translatedto
US$ | |
Assets | | | | | | | | |
Current | | | | | | | | |
Cash | | £ | 43,300 | | | $ | 67,910 | |
Accounts receivable | | | 81,358 | | | | 127,602 | |
Inventory | | | 36,959 | | | | 57,966 | |
Current income taxes recoverable | | | — | | | | — | |
Prepaid expenses and other current assets | | | — | | | | — | |
| | | | | | | | |
| | | 161,617 | | | | 253,478 | |
Fixed assets | | | 2,638 | | | | 4,137 | |
Intangible assets | | | 738,094 | | | | 1,157,627 | |
Deferred income taxes | | | 141 | | | | 221 | |
Deferred financing costs, net | | | — | | | | — | |
Goodwill | | | 213,639 | | | | 335,071 | |
| | | | | | | | |
Total Assets | | £ | 1,116,129 | | | $ | 1,750,534 | |
| | | | | | | | |
Liabilities | | | | | | | | |
Current | | | | | | | | |
Accounts payable | | £ | 38,644 | | | $ | 60,608 | |
Accrued liabilities | | | 44,079 | | | | 69,134 | |
Provisions | | | — | | | | — | |
Financial liabilities | | | 29,267 | | | | 45,902 | |
Royalties payable | | | — | | | | — | |
Dividend payable | | | — | | | | — | |
Taxes payable | | | 5,047 | | | | 7,916 | |
Current portion of notes payable | | | — | | | | — | |
Current portion of long-term debt | | | 29,762 | | | | 46,679 | |
Current portion of purchase consideration payable | | | — | | | | — | |
| | | | | | | | |
| | | 146,799 | | | | 230,239 | |
Long-term debt | | | 908,980 | | | | 1,425,644 | |
Term loans and senior notes - (net of debt issuance costs) | | | — | | | | — | |
Notes payable | | | — | | | | — | |
Purchase consideration payable | | | — | | | | — | |
Deferred income taxes | | | 65,340 | | | | 102,479 | |
Other liabilities | | | — | | | | — | |
| | | | | | | | |
Total Liabilities | | | 1,121,119 | | | | 1,758,362 | |
| | | | | | | | |
Shareholders’ Equity | | | | | | | | |
Share capital | | | 16,574 | | | | 25,995 | |
Net equity | | | — | | | | — | |
Reserve for share based compensation | | | — | | | | — | |
Accumulated other comprehensive income | | | (3,760 | ) | | | (5,898 | ) |
Retained earnings (deficit) | | | (17,804 | ) | | | (27,925 | ) |
| | | | | | | | |
Total Shareholders’ Equity | | | (4,990 | ) | | | (7,828 | ) |
| | | | | | | | |
Total Liabilities and Shareholders’ Equity | | £ | 1,116,129 | | | $ | 1,750,534 | |
| | | | | | | | |
C-17
This short form base shelf prospectus has been filed under legislation in each of the provinces of Canada that permits certain information about these securities to be determined after this prospectus has become final and that permits the omission from this prospectus of that information. The legislation requires the delivery to purchasers of a prospectus supplement containing the omitted information within a specified period of time after agreeing to purchase any of these securities.
No securities regulatory authority has expressed an opinion about these securities and it is an offence to claim otherwise. This short form base shelf prospectus constitutes a public offering of these securities only in those jurisdictions where they may be lawfully offered for sale and therein only by persons permitted to sell such securities in these jurisdictions.
Information has been incorporated by reference in this short form base shelf prospectus from documents filed with securities commissions or similar authorities in Canada.Copies of the documents incorporated herein by reference may be obtained on request without charge from the Secretary of Concordia Healthcare Corp. at 277 Lakeshore Rd. East, Suite 302, Oakville, Ontario, L6J 1H9, telephone (905) 842-5150, and are also available electronically at www.sedar.com.
SHORT FORM BASE SHELF PROSPECTUS

CONCORDIA HEALTHCARE CORP.
$3,000,000,000
Common Shares
Preferred Shares
Warrants
Subscription Receipts
Debt Securities
Units
Concordia Healthcare Corp. (the “Corporation” or “Concordia”) may offer and sell from time to time its common shares (“Common Shares”), preferred shares (“Preferred Shares”), warrants (“Warrants”) to purchase any of the other securities that are described in this short form base shelf prospectus (the “Prospectus”), subscription receipts (“Subscription Receipts”), debt securities (“Debt Securities”), units (“Units”) comprised of one or more of any of the other securities that are described in this Prospectus, or any combination of such securities (all of the foregoing collectively, the “Securities” and individually, a “Security”) for up to an aggregate offering price of $3,000,000,000, in one or more transactions during the 25-month period that this Prospectus, including any amendments hereto, remains effective.
The Corporation will provide the specific terms of any offering of Securities, including the specific terms of the Securities with respect to a particular offering and the terms of such offering, in one or more prospectus supplements (each a “Prospectus Supplement”) to this Prospectus. The Securities may be offered separately or together or in any combination, and as separate series. An investor should read this Prospectus and the applicable Prospectus Supplement carefully before investing in any Securities.
All dollar amounts in this Prospectus refer to Canadian dollars, unless otherwise indicated. See “Currency Presentation and Exchange Rate Information”.
If required by applicable law, when Securities are offered in currencies other than Canadian dollars, appropriate disclosure of foreign exchange rates applicable to the Securities will be included in the Prospectus Supplement describing the Securities. A Prospectus Supplement may include specific variable terms pertaining to the Securities that are not within the alternatives and parameters described in this Prospectus.
All information permitted under applicable securities laws to be omitted from this Prospectus will be contained in one or more Prospectus Supplements that will be delivered to purchasers together with this Prospectus. For the purposes of applicable securities laws, each Prospectus Supplement will be incorporated by reference into this Prospectus as of the date of the Prospectus Supplement and only for the purposes of the distribution of the Securities to which that Prospectus Supplement pertains.
The Corporation may sell Securities directly to investors, or through agents, underwriters or dealers it selects. If the Corporation uses agents, underwriters or dealers to sell the Securities, it will name them and describe their compensation in a Prospectus Supplement. The net proceeds that the Corporation expects to receive from an offering of Securities will be described in the Prospectus Supplement relating to that offering.
The Common Shares are listed and posted for trading on the Toronto Stock Exchange (the “TSX”) under the symbol “CXR”. On July 15, 2015, the last trading day prior to the date of this Prospectus, the closing price of the Common Shares on the TSX was $103.00. In addition, the Common Shares are listed on the NASDAQ Global Select Market® (“NASDAQ”) under the symbol “CXRX”. On July 15, 2015, the last trading day prior to the date of this Prospectus, the closing price of the Common Shares on the NASDAQ was US$79.50. The applicable Prospectus Supplement will contain information, where applicable, with respect to any listing on the TSX or any other securities exchange of the Securities distributed under that Prospectus Supplement.Unless otherwise specified in the applicable Prospectus Supplement, Securities other than Common Shares will not be listed on any securities exchange. There is currently no market through which such Securities may be sold and purchasers may not be able to resell any such Securities purchased under this Prospectus and the Prospectus Supplement relating to such Securities. This may affect the pricing of such Securities in the secondary market, the transparency and availability of trading prices, the liquidity of such Securities and the extent of issuer regulation. See “Risk Factors”.
THESE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION (THE “SEC”) NOR HAS THE SEC PASSED UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
This offering is made by a foreign issuer that is permitted, under a multi-jurisdictional disclosure system (“MJDS”) adopted by the United States of America, to prepare this Prospectus in accordance with Canadian disclosure requirements. Prospective investors should be aware that such requirements are different from those of the United States. The Corporation prepares its financial statements in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board (“IFRS”). Thus, the Corporation’s financial statements may not be comparable to the financial statements of U.S. companies.
Purchasers of Securities should be aware that the acquisition of Securities may have tax consequences both in the United States and in Canada. Such consequences for purchasers who are resident in the United States may not be described fully herein. Purchasers of Securities should read the tax discussion contained in the applicable Prospectus Supplement with respect to a particular offering of Securities.
The enforcement by investors of civil liabilities under United States federal securities laws may be affected adversely by the fact that the Corporation is incorporated under the laws of the Province of Ontario, Canada, that most of its officers and directors are residents of Canada, that some of the experts named in this Prospectus are residents of Canada, and that all or a substantial portion of the assets of the Corporation and said persons are located outside the United States.
An investment in Securities involves significant risks that should be carefully considered by prospective investors before purchasing Securities. The risks outlined in this Prospectus and in the documents incorporated by reference herein, including the applicable Prospectus Supplement, should be carefully reviewed and considered by prospective investors in connection with any investment in Securities. See“Cautionary Note Regarding Forward-Looking Information” and“Risk Factors”.
No underwriter has been involved in the preparation of this Prospectus nor has any underwriter performed any review of the contents of this Prospectus.
Keiter, Certified Public Accountants, auditor of certain financial statements in respect of Donnatal® contained in the business acquisition report of the Corporation dated June 9, 2014, incorporated by reference herein, is incorporated, continued, or otherwise organized under the laws of a foreign jurisdiction. Crowe Horwath LLP, auditor of certain financial statements in respect of the Covis Portfolio (as hereinafter defined) contained in the business acquisition report of the Corporation dated July 3, 2015, incorporated by reference herein, is incorporated, continued, or otherwise organized under the laws of a foreign jurisdiction. Edward Borkowski and Rochelle Fuhrmann reside outside of Canada. Each of Mr. Borkowski and Ms. Fuhrmann has appointed the Corporation as his or her agent for service of process in Canada. Purchasers are advised that it may not be possible for investors to enforce judgements obtained in Canada against any person or company that is incorporated, continued or otherwise organized under the laws of a foreign jurisdiction or resides outside of Canada, even if the party has appointed an agent for service of process in Canada.
The registered and head office of the Corporation is located at 277 Lakeshore Rd. East, Suite 302, Oakville, Ontario, L6J 1H9. The Corporation’s records office is located at 333 Bay St., Suite 2400, Toronto, Ontario M56 2T6.
ii
TABLE OF CONTENTS
ABOUT THIS SHORT FORM PROSPECTUS
Explanatory Notes
In this Prospectus, the Corporation and its subsidiaries are collectively referred to as the “Corporation” or “Concordia”, unless the context otherwise requires. The Corporation has not authorized anyone to provide readers with information different from that contained in this Prospectus or in any free writing prospectus prepared by the Corporation. The Corporation takes no responsibility for, and can provide no assurance as to the reliability of any other information that others may give readers of this Prospectus. The Corporation is not making an offer of Securities in any jurisdiction where the offer is not permitted.
Readers should not assume that the information contained or incorporated by reference in this Prospectus is accurate as of any date other than the date of this Prospectus or the respective dates of the documents incorporated by reference herein, unless otherwise noted herein or as required by law. It should be assumed that the information appearing in this Prospectus, any Prospectus Supplement and the documents incorporated by reference herein and therein are accurate only as of their respective dates. The business, financial condition, results of operations and prospects of the Corporation may have changed since those dates.
This Prospectus shall not be used by anyone for any purpose other than in connection with an offering of Securities as described in one or more Prospectus Supplements. The Corporation does not undertake to update the information contained or incorporated by reference herein, including any Prospectus Supplement, except as required by applicable securities laws. Information contained on, or otherwise accessed through, the website of the Corporation, www.concordiarx.com, shall not be deemed to be a part of this Prospectus and such information is not incorporated by reference herein.
Trademarks
This Prospectus, or documents incorporated by reference herein, include trademarks which are protected under applicable intellectual property laws and are the property of the Corporation or its affiliates or its licensors. Solely for convenience, the trademarks of the Corporation, its affiliates and/or its licensors referred to in this Prospectus, or documents incorporated by reference herein, may appear with or without the® or ™ symbol, but such references or the absence thereof are not intended to indicate, in any way, that the Corporation, its affiliates or its licensors will not assert, to the fullest extent under applicable law, their respective rights or the right of the applicable licensor to these trademarks. Any other trademarks used in this Prospectus, or documents incorporated by reference herein, are the property of their respective owners.
Market Data
This Prospectus, or documents incorporated by reference herein, contains statistical data, market research and industry forecasts that were obtained, unless otherwise indicated, from independent industry and government publications and reports or based on estimates derived from such publications and reports and management’s knowledge of, and experience in, the markets in which the Corporation operates. Industry and government publications and reports generally indicate that they have obtained their information from sources believed to be reliable, but do not guarantee the accuracy and completeness of their information. While the Corporation believes this data to be reliable, market and industry data is subject to variation and cannot be verified due to limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties inherent in any statistical survey. The Corporation has not independently verified the accuracy or completeness of any such information contained or incorporated by reference herein. In addition, projections, assumptions and estimates of the Corporation’s future performance and the future performance of the industry in which the Corporation operates are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described under the heading“Risk Factors” in this Prospectus and under the heading“Risk Factors” in the AIF (as hereinafter defined).
1
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
Certain statements contained in this Prospectus, or in documents incorporated by reference herein, constitute “forward-looking information” within the meaning of applicable Canadian provincial securities laws (“forward-looking statements”), which are based upon the Corporation’s current internal expectations, estimates, projections, assumptions, opinions and beliefs. Statements concerning the Corporation’s objectives, goals, strategies, intentions, plans, beliefs, expectations and estimates, and the business, operations, future financial performance and condition of the Corporation are forward-looking statements. The words “believe”, “expect”, “anticipate”, “estimate”, “intend”, “may”, “will”, “would” and similar expressions and the negative and grammatical variations of such expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These statements are not guarantees of future performance and involve known and unknown risks, uncertainties and other factors that may cause actual results or events to differ materially from those anticipated in the forward-looking statements. In addition, this Prospectus, or the documents incorporated by reference herein, may contain forward-looking statements attributed to third-party industry sources.
By their nature, forward-looking statements involve numerous assumptions, known and unknown risks and uncertainties, both general and specific, that contribute to the possibility that the predictions, forecasts and projections that constitute forward-looking statements will not occur. Such forward-looking statements in this Prospectus, or in documents incorporated by reference herein, speak only as of the date of such document. Forward-looking statements in this Prospectus, or in documents incorporated by reference herein, include, but are not limited to, statements with respect to:
| | • | | the proposed changes to management of the Corporation; |
| | • | | the proposed settlement agreement with the FTC (as hereinafter defined); |
| | • | | the use of net proceeds of any offering of Securities; |
| | • | | the performance of the Corporation’s business and operations; |
| | • | | the Corporation’s capital expenditure programs; |
| | • | | the future development of the Corporation, its growth strategy and the timing thereof; |
| | • | | the acquisition strategy of the Corporation; |
| | • | | the completion and timing of any offering of Securities and the entering into of documentation in respect thereof; |
| | • | | the future contractual obligations of the Corporation; |
| | • | | the Corporation’s future liquidity and financial capacity; |
| | • | | the supply and demand for pharmaceutical products and services similar to the Corporation’s products and services; |
| | • | | cost and reimbursement for the Corporation’s products; |
| | • | | the Corporation’s ability to raise capital; |
| | • | | the Corporation’s treatment under government regulatory and taxation regimes; |
| | • | | the Corporation’s net sales of all or any one of the Corporation’s legacy pharmaceutical products, orphan drugs and other products; and |
| | • | | sales relating to the specialty healthcare distribution (SHD) division. |
With respect to the forward-looking statements contained in this Prospectus, or in documents incorporated by reference herein, the Corporation has made assumptions regarding, among other things:
| | • | | the ability of the Corporation to comply with its contractual obligations, including, without limitation, its obligations under debt arrangements; |
| | • | | the successful licensing of products to third parties to market and distribute such products on terms favorable to the Corporation; |
2
| | • | | the ability of the Corporation to maintain key strategic alliances, out licensing and partnering arrangements, now and in the future; |
| | • | | the general regulatory environment in which the Corporation operates, including the areas of taxation, environmental protection, consumer safety and health regulation; |
| | • | | the timely receipt of any required regulatory approvals; |
| | • | | the general economic, financial, market and political conditions impacting the industry in which the Corporation operates; |
| | • | | the tax treatment of the Corporation and its subsidiaries; |
| | • | | the materiality of legal proceedings; |
| | • | | the ability of the Corporation to sustain or increase profitability, fund its operations and/or raise additional capital to fund future acquisitions; |
| | • | | the ability of the Corporation to acquire any necessary technology, products or businesses and effectively integrate such acquisitions; |
| | • | | the development and clinical testing of products under development; |
| | • | | the ability of the Corporation to obtain necessary approvals for commercialization of the Corporation’s products from the United States Food and Drug Administration (“FDA”) or other regulatory authorities; |
| | • | | currency exchange and interest rates; |
| | • | | reliance on third party contract manufacturers to manufacture the Corporation’s products on favourable terms; |
| | • | | the ability of the Corporation to generate sufficient cash flow from operations and to access existing and proposed credit facilities and the capital markets to meet its future obligations on acceptable terms; |
| | • | | the availability of raw materials and finished products necessary for the Corporation’s products; |
| | • | | the impact of increasing competition; |
| | • | | the ability of the Corporation to obtain and retain qualified staff, equipment and services in a timely and efficient manner; |
| | • | | the ability of the Corporation to maintain and enforce the protection afforded by any patents or other intellectual property rights; |
| | • | | the ability of the Corporation to conduct operations in a safe, efficient and effective manner; |
| | • | | the results of continuing and future safety and efficacy studies by industry and government agencies related to the Corporation’s products; and |
| | • | | the ability of the Corporation to successfully market its products and services. |
Forward-looking statements contained in this Prospectus, or in documents incorporated by reference herein, are based on the key assumptions described herein or therein. The reader is cautioned that such assumptions, although considered reasonable by the Corporation, may prove to be incorrect. Actual results achieved during the forecast period will vary from the information provided in this Prospectus, or in documents incorporated by reference herein, as a result of numerous known and unknown risks and uncertainties and other factors. The Corporation cannot guarantee future results. Moreover, neither the Corporation nor any other person assumes responsibility for the accuracy or completeness of the forward-looking statements contained herein.
Some of the risks and other factors which could cause actual results to differ materially from those expressed in the forward-looking statements contained in this Prospectus, or in documents incorporated by reference herein, include, but are not limited to, the risk factors included under the heading“Risk Factors” in this Prospectus and under the heading“Risk Factors” in the AIF.
3
Forward-looking statements contained in this Prospectus, or in documents incorporated by reference herein, are based on management’s current plans, expectations, estimates, projections, beliefs and opinions and the assumptions relating to those plans, expectations, estimates, projections, beliefs and opinions may change. Management of the Corporation has included the above summary of assumptions and risks related to forward-looking statements included in this Prospectus, or in documents incorporated by reference herein, for the purpose of assisting the reader in understanding management’s current views regarding those future outcomes.Readers are cautioned that this information may not be appropriate for other purposes. Readers are cautioned that the lists of assumptions and risk factors contained herein are not exhaustive.Some of these and other risk factors are discussed in greater detail under the heading“Risk Factors” in the AIF. Additional information regarding these and other risk factors included in the Corporation’s public filings with the securities commissions or similar authorities in each of the provinces of Canada can be found through the System for Electronic Document Analysis and Retrieval (“SEDAR”) on the Corporation’s profile at www.sedar.com.
Such forward-looking statements are made as of the date of this Prospectus, or in the case of any documents incorporated by reference herein, as of the dates of such documents. The forward-looking statements contained in the Prospectus represent the Corporation’s views only as of such date. The Corporation disclaims any intention or obligation to update publicly any such forward-looking statements, whether as a result of new information, future events or results or otherwise, other than as required by applicable Canadian securities laws.
All of the forward-looking statements made in this Prospectus are expressly qualified by these cautionary statements and other cautionary statements or factors contained or incorporated by reference herein, and there can be no assurances that the actual results or developments will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, the Corporation.
AVAILABLE INFORMATION
Concordia is subject to the informational reporting requirements of the United States Securities Exchange Act of 1934 (the “Exchange Act”) as the Common Shares are registered under Section 12(b) of the Exchange Act. Accordingly, the Corporation is required to publicly file reports and other information with the SEC. Under the MJDS, the Corporation is permitted to prepare such reports and other information in accordance with Canadian disclosure requirements, which are different from United States disclosure requirements. As a foreign private issuer, the Corporation is exempt from the rules under the Exchange Act prescribing the furnishing and content of proxy statements, and the Corporation’s officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
Reports and other information concerning the Corporation can be inspected and copied at the public reference facilities maintained by the SEC at: 100 F. Street, N.E., Washington, D.C. 20549. Copies of these materials can be obtained from the Public Reference section of the SEC at 100 F Street, N.E., Washington, D.C. at prescribed rates. Please call the SEC at 1-800-SEC-0330 for further information on the public reference room. Certain of the Corporation’s filings are also electronically available from the SEC’s Electronic Document Gathering and Retrieval System (“EDGAR”), and which may be accessed atwww.sec.gov.
The Corporation has filed with the SEC a registration statement onForm F-10 under the Securities Act of 1933 (as amended, the “U.S. Securities Act”) with respect to the Securities. This Prospectus, including the documents incorporated by reference in this Prospectus, which forms a part of the registration statement, does not contain all of the information set forth in the registration statement, certain parts of which are contained in the exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to the Corporation and the Securities, reference is made to the registration statement and the exhibits thereto. The registration statement can be found on EDGAR atwww.sec.gov.
4
CURRENCY PRESENTATION AND EXCHANGE RATE INFORMATION
All references to “$” in this Prospectus refer to Canadian dollars and all references to “US$” are to United States dollars, unless otherwise indicated. The following table reflects the low and high rates of exchange during the periods noted, the rates of exchange at the end of such periods and the average noon spot rates of exchange during such periods, for one United States dollar expressed in Canadian dollars, based on the Bank of Canada noon spot rate of exchange.
| | | | | | | | | | | | |
| | | Three months ended
March 31,
2015 | | | Year ended
December 31, | |
| | | | 2014 | | | 2013 | |
High for the period | | | 1.2803 | | | | 1.1643 | | | | 1.0697 | |
Low for the period | | | 1.1728 | | | | 1.0614 | | | | 0.9839 | |
Rate at the end of the period | | | 1.2683 | | | | 1.1601 | | | | 1.0636 | |
Average noon spot rate for the period | | | 1.2412 | | | | 1.1047 | | | | 1.0301 | |
On July 15, 2015, the Bank of Canada noon rate of exchange was US$1.00 = $1.2937.
NON-IFRS FINANCIAL MEASURES
The Q1 MD&A and the 2014 MD&A (each as defined below and incorporated by reference herein) each contain references to certain financial measures that are not recognized under IFRS. Management uses non-IFRS financial measures such as EBITDA, Adjusted EBITDA and Adjusted EPS to provide investors with a supplemental measure of the Corporation’s operating performance and thus highlight trends in the Corporation’s core business that may not otherwise be apparent when relying solely on IFRS financial measures. Management also believes that securities analysts, investors and other interested parties frequently use non-IFRS financial measures in the evaluation of issuers. Management also uses non-IFRS financial measures in order to facilitate operating performance comparisons from period to period, prepare annual operating budgets, and to assess its ability to meet future debt service, capital expenditure, and working capital requirements. Non-IFRS financial measures do not have standardized meanings and are unlikely to be comparable to any similar measures presented by other companies.
Definitions of non-IFRS financial measures and a reconciliation of non-IFRS to IFRS financial measures related to the Corporation can be found under the heading“Non IFRS Financial Measures” in each of the Q1 MD&A and the 2014 MD&A.
DOCUMENTS INCORPORATED BY REFERENCE
Information has been incorporated by reference in this Prospectus from documents filed with the securities commissions or similar authorities in each of the provinces of Canada and with the SEC. Copies of the documents incorporated by reference herein may be obtained on request without charge from the Secretary of the Corporation at 277 Lakeshore Rd. East, Suite 302, Oakville, Ontario, L6J 1H9, telephone (905) 842-5150, and are also available electronically at www.sedar.com. The filings of the Corporation through SEDAR are not incorporated by reference in this Prospectus except as specifically set out herein.
The information incorporated by reference is considered part of this Prospectus, and information filed with the securities commission or similar authorities in each of the provinces of Canada subsequent to this Prospectus and prior to the termination of a particular offering of Securities referred to in any Prospectus Supplement will be deemed to update and, if applicable, supersede this information. Except as may be set forth in a Prospectus Supplement, the following documents, filed by the Corporation with the securities commissions or similar authorities in each of the provinces of Canada, are specifically incorporated by reference into, and form an integral part of, this Prospectus:
| | (a) | annual information form of the Corporation for the year ended December 31, 2014 dated March 19, 2015 (“AIF”); |
| | (b) | audited consolidated financial statements of the Corporation as at and for the year ended December 31, 2014, together with the notes thereto and the auditor’s report thereon; |
5
| | (c) | management’s discussion and analysis of financial condition and result of operations of the Corporation for the year ended December 31, 2014 (the “2014 MD&A”); |
| | (d) | unaudited condensed interim consolidated financial statements of the Corporation as at and for the three-month period ended March 31, 2015, together with the notes thereto; |
| | (e) | management’s discussion and analysis of financial condition and result of operations of the Corporation for the three-month period ended March 31, 2015 (the “Q1 MD&A”); |
| | (f) | business acquisition report of the Corporation dated June 9, 2014 relating to the acquisition of Donnatal®; |
| | (g) | business acquisition report of the Corporation dated December 12, 2014 relating to the acquisition of Zonegran®; |
| | (h) | business acquisition report of the Corporation dated July 3, 2015 relating to the Covis Acquisition (as hereinafter defined); |
| | (i) | management information circular of the Corporation dated May 25, 2015 relating to the Meeting (as hereinafter defined); |
| | (j) | material change report of the Corporation dated March 16, 2015 relating to the announcement of the Covis Acquisition; |
| | (k) | material change report of the Corporation dated March 17, 2015 relating to the announcement of the Equity Offering (as hereinafter defined); |
| | (l) | material change report of the Corporation dated April 14, 2015 relating to the announcement and pricing of the Note Offering (as hereinafter defined); and |
| | (m) | material change report of the Corporation dated May 1, 2015 relating to the closing of the Covis Acquisition and the Note Offering, the conversion of the Equity Offering Subscription Receipts (as hereinafter defined) into Common Shares and the entering into of the Bank Facilities (as hereinafter defined). |
Any document of the type referred to in section 11.1 of Form 44-101F1 of National Instrument 44-101 –Prospectus Distributions (excluding confidential material change reports), if filed by the Corporation with a securities commission or similar regulatory authority in Canada after the date of this Prospectus and all Prospectus Supplements (only in respect of the offering of Securities to which that particular Prospectus Supplement relates) disclosing additional or updated information including the documents incorporated by reference therein, filed pursuant to the requirements of applicable securities legislation in Canada and during the period that this Prospectus is effective, shall be deemed to be incorporated by reference in this Prospectus. In addition, all documents filed onForm 6-K orForm 40-F by the Corporation with the SEC on or after the date of this Prospectus shall be deemed to be incorporated by reference into this Prospectus and the registration statement onForm F-10 of which this Prospectus forms a part, if and to the extent expressly provided in such document. The documents incorporated or deemed to be incorporated herein by reference contain meaningful and material information relating to the Corporation and the readers should review all information contained in this Prospectus, the applicable Prospectus Supplement and the documents incorporated or deemed to be incorporated by reference herein and therein.
Upon a new annual information form and annual consolidated financial statements being filed by the Corporation with the applicable Canadian securities commissions or similar regulatory authorities in Canada during the period that this Prospectus is effective, the previous annual information form, the previous annual consolidated financial statements and all interim consolidated financial statements and in each case the accompanying management’s discussion and analysis of financial condition and results of operations, and material change reports, filed prior to the commencement of the financial year of the Corporation in which the new annual information form is filed shall be deemed to no longer be incorporated into this Prospectus for purpose of future offers and sales of Securities under this Prospectus. Upon interim consolidated financial statements and the accompanying management’s discussion and analysis of financial condition and results of operations being filed by the Corporation with the applicable Canadian securities commissions or similar regulatory authorities during the period that this Prospectus is effective, all interim consolidated financial statements and the accompanying management’s discussion and analysis of financial condition and results of operations filed prior to such new interim consolidated financial statements and management’s discussion and analysis of financial condition and results of operations shall be deemed to no longer be incorporated into this Prospectus for purposes of future offers and sales of Securities under this Prospectus. In addition, upon a new management information circular for an annual meeting of shareholders being filed by the Corporation with the applicable Canadian
6
securities commissions or similar regulatory authorities during the period that this Prospectus is effective, the previous management information circular filed in respect of the prior annual meeting of shareholders shall no longer be deemed to be incorporated into this Prospectus for purposes of future offers and sales of Securities under this Prospectus.
A Prospectus Supplement containing the specific terms of an offering of Securities and other information relating to the Securities will be delivered to prospective purchasers of such Securities, together with this Prospectus, and will be deemed to be incorporated into this Prospectus as of the date of such Prospectus Supplement but only for the purpose of the offering of the Securities covered by that Prospectus Supplement.
Any statement contained in this Prospectus or in a document incorporated or deemed to be incorporated by reference herein shall be deemed to be modified or superseded, for purposes of this Prospectus, to the extent that a statement contained herein or in any other subsequently filed document that also is, or is deemed to be, incorporated by reference herein modifies, replaces or supersedes such statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this Prospectus. The modifying or superseding statement need not state that it has modified or superseded a prior statement or include any other information set forth in the document or statement that it modifies or supersedes.
The making of a modifying or superseding statement shall not be deemed an admission for any purposes that the modified or superseded statement, when made, constituted a misrepresentation, an untrue statement of a material fact or an omission to state a material fact that is required to be stated or that is necessary to make a statement not misleading in light of the circumstances in which it was made.
7
CONCORDIA HEALTHCARE CORP.
Name, Address and Incorporation
Concordia Healthcare Corp. was incorporated pursuant to the provisions of theBusiness Corporations Act(Ontario) on January 20, 2010, under the name “Mercari Acquisition Corp.”. The Corporation completed its initial public offering on May 6, 2010, and was listed on the TSX Venture Exchange (“TSX-V”) as a capital pool company and subsequently on the NEX board of the TSX-V. On December 18, 2013, and prior to the completion of its qualifying transaction, the Corporation changed its name to “Concordia Healthcare Corp.” and completed a consolidation of its share capital on a basis of one post-consolidation Common Share for every 48.08 common shares existing immediately before the consolidation. The Corporation completed its qualifying transaction pursuant to the policies of the TSX-V by way of a reverse takeover of the Corporation by the shareholders of Concordia Healthcare Inc. on December 20, 2013. The Common Shares were delisted from the NEX and relisted for trading on the TSX under the symbol “CXR” on December 24, 2013. On June 29, 2015, the Common Shares were listed on the NASDAQ, under the trading symbol “CXRX”.
Concordia is a reporting issuer or the equivalent in all provinces of Canada and a foreign private issuer as defined in Rule 3b-4 under the Exchange Act in the United States, eligible to file disclosure documents pursuant to the MJDS adopted and implemented by securities regulatory authorities in the United States and Canada.
The registered and head office of the Corporation is located at 277 Lakeshore Rd. East, Suite 302, Oakville, Ontario, L6J 1H9. The Corporation’s records office is located at 333 Bay St., Suite 2400, Toronto, Ontario M56 2T6.
Intercorporate Relationships
The Corporation’s business is carried on through its various subsidiaries. The following chart illustrates, as at July 15, 2015, the Corporation’s subsidiaries, including their respective jurisdiction of incorporation and percentage of voting securities in each that are held by the Corporation either directly or indirectly:
Note:
| (1) | On April 27, 2015, Concordia Healthcare Corp. transferred all shares held by it in Concordia Pharmaceuticals Inc. to Concordia Healthcare Inc. |
| (2) | Pinnacle Biologics, Inc. has a dormant subsidiary existing under the laws of India, Pinnacle Biopharma India Private Limited. |
8
General Description of the Business
Concordia is a diverse healthcare company focused on legacy pharmaceutical products and orphan drugs. Concordia’s legacy pharmaceutical division, Concordia Pharmaceuticals Inc. (“CPI”), consists of a portfolio of branded products and authorized generic contracts, including branded products such as Nilandron®, for the treatment of metastatic prostate cancer; Dibenzyline®, for the treatment of pheochromocytoma; Lanoxin®, for the treatment of mild-to-moderate heart failure and atrial fibrillation; Plaquenil®, for the treatment of lupus and rheumatoid arthritis; Donnatal® for the treatment of irritable bowel syndrome; and Zonegran® (zonisamide) for treatment of partial seizures in adults with epilepsy. Concordia’s orphan drugs division, Concordia Laboratories Inc., owns Photofrin®. Photofrin® is marketed by Pinnacle Biologics, Inc. in the United States.
Concordia operates out of facilities in Oakville, Ontario; Bridgetown, Barbados; Roanoke, Virginia and has a specialty healthcare distribution division that operates out of Kansas City, Missouri. Pinnacle Biologics, Inc. is located in Chicago, Illinois.
Concordia focuses on the acquisition and sale of legacy pharmaceutical products through its Legacy Pharmaceutical Division and the acquisition and/or development of orphan drugs through its Orphan Drug Division. The cash flow generated from the sale of legacy pharmaceutical products is used to fund operations and is also intended to fund the expansion of indications for orphan drugs. In addition, through its registered pharmacy operation, the Corporation’s Specialty Healthcare Distribution Division provides a distribution capability for specialty pharmaceutical products and orphan drugs once acquired and/or developed. These three divisions are identified as reportable segments under IFRS for the purposes of disclosure in the Corporation’s financial statements and the Corporation’s accounting records are kept in accordance with IFRS. The Corporation’s fiscal year end is December 31.
Recent Developments
Covis Acquisition
On April 21, 2015, Concordia completed its acquisition of 18 products (the “Covis Portfolio”), being comprised of 12 branded products and five authorized generic contracts and a product distributed by a third party in Australia pursuant to the terms of a distribution agreement, from Covis Pharma S.à.r.l and Covis Injectables, S.à.r.l (collectively, “Covis”) for US$1.2 billion in cash (the “Covis Acquisition”).
The Covis Portfolio includes branded pharmaceuticals, injectables and authorized generics that address life threatening and other serious medical conditions in various therapeutic areas including cardiovascular, central nervous system, oncology and acute care markets. Key products acquired from Covis include: Nilandron®, for the treatment of metastatic prostate cancer; Dibenzyline®, for the treatment of pheochromocytoma; Lanoxin®, for the treatment of mild-to-moderate heart failure and atrial fibrillation; and, Plaquenil® for the treatment of lupus and rheumatoid arthritis. Almost all of Covis’ net sales for the year ended December 31, 2014 were generated in the United States.
The Corporation paid for the Covis Acquisition through a mix of equity, senior notes and bank facilities as described below.
Equity Offering
On April 8, 2015, Concordia closed a short form prospectus offering (the “Equity Offering”), on a “bought deal” basis, of 4,329,428 subscription receipts (the “Equity Offering Subscription Receipts”) of the Corporation, which included the exercise by the underwriters of an over-allotment option of 15%, for aggregate gross proceeds to the Corporation of $368,001,380. The Equity Offering was completed at a price per Equity Offering Subscription Receipt of $85.00 by a syndicate of underwriters led by RBC Capital Markets, as sole bookrunner and co-lead manager, and including GMP Securities L.P., as co-lead manager, and TD Securities Inc. Upon closing of the Covis Acquisition, each holder of Equity Offering Subscription Receipts automatically received, without payment of additional consideration or further action, one Common Share in exchange for each Equity Offering Subscription Receipt held.
The net proceeds of the Equity Offering were used to partially fund: (i) the purchase price for the Covis Acquisition; and (ii) the fees and expenses incurred in connection with the Covis Acquisition.
9
Senior Notes due 2023
In connection with the Covis Acquisition, on April 21, 2015 Concordia closed a private offering (the “Note Offering”) of US$735,000,000 of 7.00% Senior Notes due 2023 (the “Notes”) issued pursuant to an indenture (the “Note Indenture”). The Notes were priced at an issue price of 100.00% of their face amount to yield 7.00%. The Notes were offered and sold to qualified institutional buyers pursuant to Rule 144A under the U.S. Securities Act, and to certain non-U.S. persons, including persons resident in Canada, in accordance with Regulation S under the U.S. Securities Act and other applicable securities laws.
The net proceeds of the offering of the Notes were used to partially fund: (i) the purchase price for the Covis Acquisition; and (ii) the fees and expenses incurred in connection with the Covis Acquisition.
Bank Facilities
Concurrent with the closing of the Covis Acquisition, Concordia entered into a credit agreement (“Credit Agreement”) dated April 21, 2015, by and among the Corporation, certain of the Corporation’s subsidiaries, the Royal Bank of Canada, Morgan Stanley Senior Funding, Inc., TD Securities (USA) LLC, GE Capital Markets, Inc., Fifth Third Bank and certain lenders party thereto (collectively, the “Lenders”). Pursuant to the terms of the Credit Agreement, the Lenders agreed to provide senior secured credit facilities to the Corporation in an aggregate principal amount of up to US$700 million comprising: (i) a senior secured revolving credit facility (the “Revolving Facility”) in an aggregate principal amount of up to US$125 million; and (ii) a senior secured term loan facility (the “Term Facility”) in an aggregate principal amount of US$575 million (together, the “Bank Facilities”). All obligations of the Corporation under the Bank Facilities are guaranteed by all material subsidiaries of the Corporation and are secured by first priority (subject to permitted liens) perfected security interests in the assets of the Corporation and the assets of and equity interests in its material subsidiaries. The Term Facility will mature on the seventh anniversary of the Covis Acquisition date (unless extended by the lenders under the Term Facility) and the Revolving Facility will mature on the fifth anniversary of the Covis Acquisition date (unless extended by the lenders under the Revolving Facility).
The funds made available to the Corporation under the Term Facility were used to partially fund: (i) the purchase price for the Covis Acquisition; (ii) the fees and expenses incurred in connection with the Covis Acquisition; and (iii) the repayment and retirement of the Corporation’s outstanding debt issued pursuant to the terms and provisions of the amended and restated senior secured credit facility with GE Capital Canada Finance Inc. and a syndicate of lenders dated September 30, 2014. All security interests in the assets of the Corporation and guarantees given in connection therewith by the subsidiaries of the Corporation in favour of GE Capital Canada Finance Inc. were discharged and released.
Update on PDT with Photofrin®
On May 7, 2015, Concordia provided a clinical update on its orphan drug development program with Photodynamic Therapy (PDT) with Photofrin® (porfimer sodium), a drug-device combination therapy.
Due to its ability to target cancer cells, PDT with Photofrin® is being studied in a range of hard-to-treat, hard-to-reach cancers including adult and pediatric brain cancer, mesothelioma and cholangiocarcinoma (bile duct cancer).
Researchers at the Medical College of Wisconsin (MCW) are currently initiating a Phase 2 study of PDT with Photofrin® in adults with recurrent high-grade gliomas, a diverse group of tumors of the brain and spinal cord that grow quickly and have the ability to spread through the brain aggressively making them very difficult to treat.
Changes to the Board of Directors and Management Team
On May 25, 2015, Concordia announced several key changes to the Corporation’s board of directors (the “Board”) and management team.
Board of Directors
Both Mr. Ronald Schmeichel and Mr. John Huss did not stand for re-election at the Corporation’s annual general meeting of shareholders that took place on June 25, 2015 (the “Meeting”). In their place, Mr. Edward Borkowski and Ms. Rochelle Fuhrmann have been elected as directors of the Corporation, effective June 25, 2015.
10
Mr. Borkowski has over 15 years of experience in executive financial positions in the pharmaceutical sector, and is currently the Chief Financial Officer of Amerigen Pharmaceuticals. Prior to joining Amerigen Pharmaceuticals, Mr. Borkowski held several executive level finance positions with various entities, notably as the Chief Financial Officer and Executive Vice President of Mylan N.V. Mr. Borkowski has an MBA in accounting from Rutgers University and a degree in Economics and Political Science from Allegheny College.
Ms. Fuhrmann has over 20 years of broad financial experience and is currently the Chief Financial Officer, Life Sciences of Becton, Dickinson and Company was previously the Chief Financial Officer of Amneal Pharmaceuticals LLC. Prior to joining Amneal, Ms. Fuhrmann was Senior Vice President, Finance at Warner Chilcott plc, a specialty pharmaceuticals company, prior to its acquisition by Actavis, Inc. in 2013. She spent the early part of her career in telecommunications and public accounting having held various positions at AT&T and Coopers and Lybrand LLC (now PricewaterhouseCoopers LLP). Ms. Fuhrmann earned her certified public accountant designation (inactive) in 1996 and has a B.Sc. degree in accounting from the University of Rhode Island.
Effective upon conclusion of the Meeting, Mr. Thompson replaced Mr. Schmeichel as the Chairman of the Board and Jordan Kupinsky became Lead Independent Director of the Board.
Management Team
Effective May 25, 2015, Mr. Adrian de Saldanha was appointed the Chief Financial Officer of the Corporation. In his role as Chief Financial Officer, Mr. de Saldanha has succeeded Mr. Leith Tessy.
Mr. de Saldanha is a Chartered Professional Accountant (CPA, CA) with extensive experience as a senior finance executive in roles covering a variety of industries. Most recently Mr. de Saldanha was Chief Financial Officer at Syncordia Technologies and Healthcare Solutions, Inc., previous to which he was a Managing Vice President at OMERS Strategic Investments where his responsibilities included management of investments in entities involved in aviation, engineering and industrial services. Mr. de Saldanha also spent over seven years at Biovail Corporation (now Valeant Pharmaceuticals) in a number of senior finance roles including Interim Chief Financial Officer, Vice President Finance & Treasurer, Vice President Manufacturing Finance as well as Vice President, Controller at Biovail Pharmaceuticals Canada. Before joining Biovail, Mr. de Saldanha spent eight years at Molson Inc. in a number of progressively senior finance positions.
In addition, effective upon conclusion of the Meeting, Mr. Thompson relinquished his role as President of the Corporation and Mr. Wayne Kreppner was promoted to the role of President, in addition to his role as Chief Operating Officer of the Corporation.
Mr. Arijit Mookerjee is expected to join CPI, the Corporation’s Barbados subsidiary, upon receipt of a work permit to work in Barbados. Mr. Mookerjee was a consultant for SMEC International Pty Limited, an Audit Director with PricewaterhouseCoopers LLP, and acted as the Group Financial Controller with Cordlife Limited a healthcare and stem-cell biotechnology firm.
Change of Auditor
Collins Barrow Toronto LLP, Chartered Accountants were not proposed for re-appointment as auditor of the Corporation at the Meeting. At the Meeting, Concordia’s shareholders approved the appointment of PricewaterhouseCoopers LLP, as auditor of the Corporation. Effective upon conclusion of the Meeting, the Corporation’s auditor is PricewaterhouseCoopers LLP.
FTC Consent Decree
Pharmaceutical companies in the United States have faced lawsuits and investigations pertaining to violations of antitrust and competition laws. The Corporation’s drug products could be subject to antitrust or competition law challenge that, if successful, could affect the Corporation’s ability to set prices for its drug products or enter into agreements with respect thereto. A successful antitrust or competition law challenge against the Corporation could result in the imposition of significant fines by one or more authorities, and/or in decisions preventing the Corporation from further expanding its business, and/or third parties (such as competitors and customers) initiating civil litigation claiming damages caused by anticompetitive practices. A violation of any such law could result in civil penalties, the implementation of mitigation measures, significant capital expenditures or require changes in the Corporation’s
11
business practices, which could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
On February 9, 2015, Concordia and its subsidiary, CPI, received a civil investigative demand (“CID”) from the United States Federal Trade Commission (“FTC”) regarding its attention deficit hyperactivity disorder product Kapvay®. The CID is a request for documents and information relating to CPI’s agreements with Par Pharmaceutical, Inc. (“Par”) with respect to Kapvay® (the “Par Agreements”).
While Concordia maintains that the Par Agreements are lawful, it has negotiated the terms of a proposed settlement agreement with the FTC and expects to settle the FTC investigation. The proposed agreement with the FTC does not constitute an admission by Concordia that it has violated the law. Under the terms of the proposed settlement agreement, Concordia will be prohibited from enforcing the provision of the Par Agreements which entitle Concordia to a royalty payment corresponding to Par’s sales of generic Kapvay® and from entering any future agreement with a generic company that would prevent the marketing of an authorized generic version of a branded drug after all patents have expired on the drug.
Concordia will also be subject to standard antitrust compliance and reporting requirements. The proposed settlement agreement is subject to initial acceptance by the FTC, publication of the settlement’s terms and a 30-day public comment period, and final acceptance by the FTC.
On December 5, 2014, Covis received a subpoena from the U.S. Department of Justice, Antitrust Division, requesting certain documents relating to its product Lanoxin®. Pursuant to the purchase agreement for the Covis Portfolio, the Corporation did not assume liabilities associated with any potential action arising in connection therewith and has a right to indemnification, under certain circumstances and subject to certain limits as set out in the asset purchase agreement with respect to the Covis Acquisition, for losses directly arising from any such action. However, to the extent market or regulatory conditions require the Corporation to alter its pricing of Lanoxin®, it could have a material adverse effect on the Corporation’s business, financial condition and results of operations.
U.S. Listing
On June 1, 2015, Concordia filed an application to list the Common Shares on the NASDAQ, and filed the requisite registration documentation with the SEC. On June 18, 2015, NASDAQ approved the listing of the Common Shares, and trading of the Common Shares commenced on June 29, 2015.
CONSOLIDATED CAPITALIZATION
The applicable Prospectus Supplement will describe any material change, and the effect of such material change, on the share and loan capitalization of the Corporation that will result from the issuance of Securities pursuant to such Prospectus Supplement.
There have been no material changes to the Corporation’s share and loan capitalization on a consolidated basis since March 31, 2015, except as set forth under the sections entitled “Concordia Healthcare Corp. – Recent Developments – Equity Offering”, “Concordia Healthcare Corp. – Recent Developments – Senior Notes due 2023” and “Concordia Healthcare Corp. – Recent Developments – Bank Facilities”.
PLAN OF DISTRIBUTION
The Corporation may offer and sell Securities directly to one or more purchasers, through agents, or through underwriters or dealers designated by the Corporation from time to time. The Corporation may distribute the Securities from time to time in one or more transactions at a fixed price or prices (which may be changed from time to time), at market prices prevailing at the times of sale, at prices related to prevailing market prices or at negotiated prices. A description of such pricing will be disclosed in the applicable Prospectus Supplement. The Corporation may offer Securities in the same offering, or it may offer Securities in separate offerings. A Prospectus Supplement will describe the terms of each specific offering of Securities, including:
| | • | | the terms of the Securities to which the Prospectus Supplement relates, including the type of Security being offered; |
12
| | • | | the name or names of any agents, underwriters or dealers involved in such offering of Securities; |
| | • | | the purchase price of the Securities offered thereby and the proceeds to, and the portion of expenses borne by, the Corporation from the sale of such Securities; |
| | • | | any agents’ commission, underwriting discounts and other items constituting compensation payable to agents, underwriters or dealers; and |
| | • | | any discounts or concessions allowed or re-allowed or paid to agents, underwriters or dealers. |
If underwriters are used in an offering, the Securities offered thereby will be acquired by the underwriters for their own account and may be resold from time to time in one or more transactions at a fixed public offering price or at varying prices determined at the time of sale. Securities may be either offered to the public through underwriting syndicates represented by managing underwriters or by underwriters without a syndicate. Only underwriters named in the Prospectus Supplement are deemed to be underwriters in connection with the Securities offered thereby. The obligations of the underwriters to purchase Securities will be subject to the conditions precedent agreed upon by the parties and outlined in the applicable Prospectus Supplement and the underwriters will be obligated to purchase all Securities under that offering if any are purchased. Any public offering price and any discounts or concessions allowed or re-allowed or paid to agents, underwriters or dealers may be changed from time to time.
The Securities may also be sold: (i) directly by the Corporation at such prices and upon such terms as agreed to by the Corporation and the purchaser of such Securities; or (ii) through agents designated by the Corporation from time to time. Any agent involved in the offering and sale of the Securities in respect of which this Prospectus is delivered will be named, and any commissions payable by the Corporation to such agent will be set forth, in the Prospectus Supplement. Unless otherwise indicated in the Prospectus Supplement, any agent is acting on a “best efforts” basis for the period of its appointment.
The Corporation may agree to pay the underwriters a commission for various services relating to the issue and sale of any Securities offered under any Prospectus Supplement. Agents, underwriters or dealers who participate in the distribution of the Securities may be entitled under agreements to be entered into with the Corporation to indemnification by the Corporation against certain liabilities, including liabilities under securities legislation, or to contribution with respect to payments which such underwriters, dealers or agents may be required to make in respect thereof.
Agents, underwriters or dealers may make sales of Securities in privately negotiated transactions and/or any other method permitted by law, including sales deemed to be an “at-the-market” offering as defined in and subject to limitations imposed by and the terms of any regulatory approvals required and obtained under, applicable Canadian securities laws which includes sales made directly on an existing trading market for the Common Shares, or sales made to or through a market maker other than on an exchange. In connection with any offering of Securities, except with respect to “at-the-market” offerings, underwriters may over-allot or effect transactions which stabilize or maintain the market price of the offered Securities at a level above that which might otherwise prevail in the open market. Such transactions may be commenced, interrupted or discontinued at any time. No underwriter or dealer involved in an “at-the-market” offering, as defined under applicable Canadian securities laws, no affiliate of such an underwriter or dealer and no person or company acting jointly or in concert with such an underwriter or dealer will over-allot Securities in connection with such distribution or effect any other transactions that are intended to stabilize or maintain the market price of the Securities.
The Corporation may authorize agents or underwriters to solicit offers by eligible institutions to purchase Securities from the Corporation at the public offering price set forth in the applicable Prospectus Supplement under delayed delivery contracts providing for payment and delivery on a specified date in the future. The conditions to these contracts and the commissions payable for solicitation of these contracts will be set forth in the applicable Prospectus Supplement.
Each class or series of Securities, other than the Common Shares, will be a new issue of Securities with no established trading market. Subject to applicable laws, any underwriter may make a market in such Securities, but will not be obligated to do so and may discontinue any market making at any time without notice. There may be limited liquidity in the trading market for any such Securities.
13
USE OF PROCEEDS
The net proceeds to the Corporation from any offering of Securities, the proposed use of those proceeds and the specific business objectives which the Corporation expects to accomplish with such proceeds will be set forth in the applicable Prospectus Supplement relating to that offering of Securities.
There may be circumstances where, on the basis of results obtained or for other sound business reasons, a re-allocation of funds may be necessary or prudent. Accordingly, management of the Corporation will have broad discretion in the application of the proceeds of an offering of Securities. The actual amount that the Corporation spends in connection with each intended use of proceeds may vary significantly from the amounts specified in the applicable Prospectus Supplement and will depend on a number of factors, including those referred to under “Risk Factors” and any other factors set forth in the applicable Prospectus Supplement.
DESCRIPTION OF COMMON SHARES
The authorized capital of the Corporation consists of an unlimited number of Common Shares. As of July 15, 2015, 34,045,243 Common Shares were issued and outstanding. The Corporation may issue Common Shares separately or together, with Preferred Shares, Warrants, Subscription Receipts, Debt Securities or Units or any combination thereof, as the case may be.
There are no special rights or restrictions attached to the Common Shares. The Common Shares rank equally as to all benefits which might accrue to the holders thereof, including the right to receive dividends out of monies of the Corporation properly applicable to the payment of dividends if and when declared by the Board and to participate rateably in the remaining assets of the Corporation in any distribution on a dissolution or winding-up. There are no provisions restricting the issuance of Common Shares or any other material restrictions.
All shareholders are entitled to receive a notice of all meetings of shareholders to be convened by the Corporation. At any general meeting, on a show of hands every shareholder who is present in person or by proxy and entitled to vote has one vote, and on a poll, every shareholder who is entitled to vote has one vote for each Common Share held and may exercise such vote either in person or by proxy.
DESCRIPTION OF PREFERRED SHARES
As of the date of this Prospectus, the Corporation is not authorized to issue Preferred Shares in the capital of the Corporation and, as a result, there are no Preferred Shares of the Corporation issued and outstanding. Any issuance of Preferred Shares by the Corporation would require an amendment to the Corporation’s then-current constating documents, which amendment would require shareholder approval. In the event that such shareholder approval was obtained, and the constating documents were appropriately amended, Preferred Shares may then be issued by the Corporation, separately or together, with Common Shares, Warrants, Subscription Receipts, Debt Securities or Units or any combination thereof, as the case may be. The following describes the general terms that will apply to any Preferred Shares that may be offered by the Corporation pursuant to this Prospectus. The terms and provisions of any Preferred Shares offered under a Prospectus Supplement may differ from the terms described below, and may not be subject to or contain any or all of the terms described below.
The specific terms and provisions of the Preferred Shares, and the extent to which the general terms of the Preferred Shares described in this Prospectus apply to those Preferred Shares, will be set forth in the applicable Prospectus Supplement. This description will include, where applicable:
| | • | | the number of Preferred Shares offered; |
| | • | | the price or prices, if any, at which the Preferred Shares will be issued; |
| | • | | the manner of determining the offering price(s) (in the event that the offering is not a fixed price distribution); |
| | • | | the currency at which the Preferred Shares will be offered; |
| | • | | the title and designation of number of shares of the series of Preferred Shares; |
14
| | • | | the dividend rate or method of calculation, the payment dates for dividends and the place or places where the dividends will be paid, whether dividends will be cumulative or noncumulative, and, if cumulative, the dates from which dividends will begin to accumulate; |
| | • | | any conversion or exchange features or rights; |
| | • | | whether the Preferred Shares will be subject to redemption or retraction and the redemption or retraction price and other terms and conditions relative to the redemption or retraction rights; |
| | • | | any liquidation rights; |
| | • | | any sinking fund provisions; |
| | • | | whether the Preferred Shares will be listed on any securities exchange; |
| | • | | whether the Preferred Shares will be issued with any other securities and, if so, the amount and terms of these securities; |
| | • | | any minimum or maximum subscription amount; |
| | • | | whether the Preferred Shares are to be issued in registered form, “book-entry only” form, non-certificated inventory system form, bearer form or in the form of temporary or permanent global securities and the basis of exchange, transfer and ownership thereof; |
| | • | | any material risk factors relating to such Preferred Shares; |
| | • | | material Canadian federal income tax consequences and U.S. federal income tax consequences of owning the Preferred Shares; |
| | • | | any other rights, privileges, restrictions and conditions attaching to the Preferred Shares; and |
| | • | | any other material terms or conditions of the Preferred Shares. |
DESCRIPTION OF WARRANTS
As of the date of this Prospectus, the Corporation has no Warrants outstanding. The Corporation may issue Warrants, separately or together, with Common Shares, Preferred Shares, Subscription Receipts, Debt Securities or Units or any combination thereof, as the case may be. The Warrants will be issued under a separate Warrant agreement or indenture. A copy of the Warrant agreement or indenture relating to an offering of Warrants will be filed by the Corporation with securities regulatory authorities in Canada after it has been entered into by the Corporation. The following describes the general terms that will apply to any Warrants that may be offered by the Corporation pursuant to this Prospectus. The terms and provisions of any Warrants offered under a Prospectus Supplement may differ from the terms described below, and may not be subject to or contain any or all of the terms described below.
The specific terms and provisions of the Warrants, and the extent to which the general terms of the Warrants described in this Prospectus apply to those Warrants, will be set forth in the applicable Prospectus Supplement. This description will include, where applicable:
| | • | | the number of Warrants offered; |
| | • | | the price or prices, if any, at which the Warrants will be issued; |
| | • | | the manner of determining the offering price(s) (in the event that the offering is not a fixed price distribution); |
| | • | | the currency at which the Warrants will be offered and in which the exercise price under the Warrants may be payable; |
| | • | | the securities for which the Warrants are exercisable; |
| | • | | conditions to the exercise of Warrants into securities, and the consequences of such conditions not being satisfied; |
| | • | | the number of securities that may be issued upon the exercise of each Warrant and the price per security or the aggregate principal amount, denominations and terms of the series of debt securities that may be issued |
15
| | upon exercise of the Warrant, and the events or conditions under which the amount of securities may be subject to adjustment; |
| | • | | the date on which the right to exercise such Warrants shall commence and the date on which such right shall expire; |
| | • | | the circumstances, if any, which will cause the Warrants to be deemed to be automatically exercised; |
| | • | | if applicable, the identity of the Warrant agent; |
| | • | | whether the Warrants will be listed on any securities exchange; |
| | • | | whether the Warrants will be issued with any other securities and, if so, the amount and terms of these securities; |
| | • | | any minimum or maximum subscription amount; |
| | • | | whether the Warrants are to be issued in registered form, “book-entry only” form, non-certificated inventory system form, bearer form or in the form of temporary or permanent global securities and the basis of exchange, transfer and ownership thereof; |
| | • | | any material risk factors relating to such Warrants and the securities to be issued upon exercise of the Warrants; |
| | • | | material Canadian federal income tax consequences and U.S. federal income tax consequences of owning the Warrants and the securities to be issued upon exercise of the Warrants; |
| | • | | any other rights, privileges, restrictions and conditions attaching to the Warrants and the securities to be issued upon exercise of the Warrants; and |
| | • | | any other material terms or conditions of the Warrants and the securities to be issued upon exercise of the Warrants. |
Prior to the exercise of any Warrants, holders of such Warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including the right to receive payments of dividends or the right to vote such underlying securities.
DESCRIPTION OF SUBSCRIPTION RECEIPTS
As of the date of this Prospectus, the Corporation has no Subscription Receipts outstanding. The Corporation may issue Subscription Receipts, separately or together, with Common Shares, Preferred Shares, Warrants, Debt Securities or Units or any combination thereof, as the case may be. The Subscription Receipts will be issued under an agreement or indenture. A copy of the Subscription Receipts agreement or indenture relating to an offering of Subscription Receipts will be filed by the Corporation with securities regulatory authorities in Canada after it has been entered into by the Corporation. The following describes the general terms that will apply to any Subscription Receipts that may be offered by the Corporation pursuant to this Prospectus. The terms and provisions of any Subscription Receipts offered under a Prospectus Supplement may differ from the terms described below, and may not be subject to or contain any or all of the terms described below.
The specific terms and provisions of the Subscription Receipts, and the extent to which the general terms of the Subscription Receipts described in this Prospectus apply to those Subscription Receipts, will be set forth in the applicable Prospectus Supplement. This description will include, where applicable:
| | • | | the number of Subscription Receipts offered; |
| | • | | the price or prices, if any, at which the Subscription Receipts will be issued; |
| | • | | the manner of determining the offering price(s) (in the event that the offering is not a fixed price distribution); |
| | • | | the currency at which the Subscription Receipts will be offered and whether the price is payable in installments; |
| | • | | the securities into which the Subscription Receipts may be exchanged; |
16
| | • | | conditions to the exchange of Subscription Receipts into securities and the consequences of such conditions not being satisfied; |
| | • | | the number of securities that may be issued upon the exchange of each Subscription Receipt and the price per security or the aggregate principal amount, denominations and terms of the series of debt securities that may be issued upon exchange of the Subscription Receipts, and the events or conditions under which the amount of securities may be subject to adjustment; |
| | • | | the dates or periods during which the Subscription Receipts may be exchanged; |
| | • | | the circumstances, if any, which will cause the Subscription Receipts to be deemed to be automatically exchanged; |
| | • | | provisions applicable to any escrow of the gross or net proceeds from the sale of the Subscription Receipts plus any interest or income earned thereon, and for the release of such proceeds from such escrow; |
| | • | | if applicable, the identity of the Subscription Receipt agent; |
| | • | | whether the Subscription Receipts will be listed on any securities exchange; |
| | • | | whether the Subscription Receipts will be issued with any other securities and, if so, the amount and terms of these securities; |
| | • | | any minimum or maximum subscription amount; |
| | • | | whether the Subscription Receipts are to be issued in registered form, “book-entry only” form, non-certificated inventory system form, bearer form or in the form of temporary or permanent global securities and the basis of exchange, transfer and ownership thereof; |
| | • | | any material risk factors relating to such Subscription Receipts and the securities to be issued upon exchange of the Subscription Receipts; |
| | • | | material Canadian federal income tax consequences and U.S. federal income tax consequences of owning the Subscription Receipts and the securities to be issued upon exchange of the Subscription Receipts; |
| | • | | any other rights, privileges, restrictions and conditions attaching to the Subscription Receipts and the securities to be issued upon exchange of the Subscription Receipts; and |
| | • | | any other material terms or conditions of the Subscription Receipts and the securities to be issued upon exchange of the Subscription Receipts. |
Prior to the exchange of any Subscription Receipts, holders of such Subscription Receipts will not have any of the rights of holders of the securities for which the Subscription Receipts may be exchanged, including the right to receive payments of dividends or the right to vote such underlying securities.
DESCRIPTION OF DEBT SECURITIES
The Corporation may issue Debt Securities, separately or together, with Common Shares, Preferred Shares, Warrants, Subscription Receipts or Units or any combination thereof, as the case may be. The Debt Securities will be issued under an indenture with a trustee to be named in a Prospectus Supplement. A form of indenture to be used in connection with offerings of Debt Securities will be filed by the Corporation with the SEC as an exhibit to the registration statement on Form F-10. The following describes the general terms that will apply to any Debt Securities that may be offered by the Corporation pursuant to this Prospectus. The terms and provisions of any Debt Securities offered under a Prospectus Supplement may differ from the terms described below, and may not be subject to or contain any or all of the terms described below.
The specific terms and provisions of the Debt Securities, and the extent to which the general terms of the Debt Securities described in this Prospectus apply to those Debt Securities, will be set forth in the applicable Prospectus Supplement. This description will include, where applicable:
| | • | | the designation, aggregate principal amount and authorized denominations of such Debt Securities; |
| | • | | the manner of determining the offering price(s) (in the event that the offering is not a fixed price distribution); |
17
| | • | | the currency or currency units for which the Debt Securities may be purchased and the currency or currency unit in which the principal and any interest is payable; |
| | • | | the percentage of the principal amount at which such Debt Securities will be issued; |
| | • | | the date or dates on which such Debt Securities will mature; |
| | • | | any mandatory or optional redemption provisions applicable to the Debt Securities; |
| | • | | any sinking fund or analogous redemption provisions applicable to the Debt Securities; |
| | • | | the rate or rates per annum at which such Debt Securities will bear interest (if any), or the method of determination of such rates (if any); |
| | • | | the dates on which any such interest will be payable and the record dates for such payments; |
| | • | | the form of consideration for payment of any interest and/or principal payments (whether by cash, Common Shares or other securities, or a combination thereof); |
| | • | | the trustee under the indenture pursuant to which the Debt Securities are to be issued; |
| | • | | the designation and terms of any Debt Securities which will be offered, if any, and the number of Debt Securities that will be offered; |
| | • | | any redemption term or terms under which such Debt Securities may be defeased; |
| | • | | any exchange or conversion terms; |
| | • | | any provisions relating to any security provided for the Debt Securities; |
| | • | | event of default provisions contained in the indenture pursuant to which the Debt Securities are to be issued; |
| | • | | whether the Debt Securities will be senior or subordinated to other liabilities of the Corporation; |
| | • | | if applicable, the identity of the Debt Security agent; |
| | • | | whether the Debt Securities will be listed on any securities exchange; |
| | • | | whether the Debt Securities will be issued with any other securities and, if so, the amount and terms of these securities; |
| | • | | any minimum or maximum subscription amount; |
| | • | | whether the Debt Securities are to be issued in registered form, “book-entry only” form, non-certificated inventory system form, bearer form or in the form of temporary or permanent global securities and the basis of exchange, transfer and ownership thereof; |
| | • | | any material risk factors relating to such Debt Securities; |
| | • | | material Canadian federal income tax consequences and U.S. federal income tax consequences of owning the Debt Securities; |
| | • | | any other rights, privileges, restrictions and conditions attaching to the Debt Securities; and |
| | • | | any other material terms or conditions of the Debt Securities. |
If the Corporation denominates the purchase price of any of the Debt Securities in a foreign currency or currencies or a foreign currency unit or units, or if the principal of and any premium and interest on any Debt Securities is payable in a foreign currency or currencies or a foreign currency unit or units, the Corporation will provide investors with information on the restrictions, elections, general tax considerations, specific terms and other information with respect to that issue of Debt Securities and such foreign currency or currencies or foreign currency unit or units in the applicable Prospectus Supplement.
Each series of Debt Securities may be issued at various times with different maturity dates, may bear interest at different rates and may otherwise vary.
To the extent any Debt Securities are convertible into other securities, prior to such conversion the holders of such Debt Securities will not have any of the rights of holders of the securities into which the Debt Securities are convertible, including the right to receive payments of dividends or the right to vote such underlying securities.
18
DESCRIPTION OF UNITS
As of the date of this Prospectus, the Corporation has no Units outstanding. The Corporation may issue Units, separately or together, with Common Shares, Preferred Shares, Warrants, Subscription Receipts, or Debt Securities or any combination thereof, as the case may be. Each Unit will be issued so that the holder of the Unit is also the holder of each Security comprising the Unit. Thus, the holder of a Unit will have the rights and obligations of a holder of each Security. The following describes the general terms that will apply to any Units that may be offered by the Corporation pursuant to this Prospectus. The terms and provisions of any Units offered under a Prospectus Supplement may differ from the terms described below, and may not be subject to or contain any or all of the terms described below.
The specific terms and provisions of the Units, and the extent to which the general terms of the Units described in this Prospectus apply to those Units, will be set forth in the applicable Prospectus Supplement. This description will include, where applicable:
| | • | | the number of Units offered; |
| | • | | the price or prices, if any, at which the Units will be issued; |
| | • | | the manner of determining the offering price(s) (in the event that the offering is not a fixed price distribution); |
| | • | | the currency at which the Units will be offered; |
| | • | | the securities comprising the Units; |
| | • | | whether the Units will be issued with any other securities and, if so, the amount and terms of these securities; |
| | • | | any minimum or maximum subscription amount; |
| | • | | whether the Units and the Securities comprising the Units are to be issued in registered form, “book-entry only” form, non-certificated inventory system form, bearer form or in the form of temporary or permanent global securities and the basis of exchange, transfer and ownership thereof; |
| | • | | any material risk factors relating to such Units or the Securities comprising the Units; |
| | • | | material Canadian federal income tax consequences and U.S. federal income tax consequences of owning the Securities comprising the Units; |
| | • | | any other rights, privileges, restrictions and conditions attaching to the Units or the Securities comprising the Units; and |
| | • | | any other material terms or conditions of the Units or the Securities comprising the Units, including whether and under what circumstances the Securities comprising the Units may be held or transferred separately. |
DIVIDEND POLICY
There are no restrictions in the Corporation’s articles preventing the Corporation from paying dividends. Any dividend to be approved by the Board may require third-party consents under the Corporation’s loan facilities and Note Indenture. All of the Common Shares are entitled to an equal share in any dividends declared and paid. The Board will determine if, and when, dividends will be declared and paid in the future from funds properly applicable to the payment of dividends based on the Corporation’s financial position at the relevant time. See“Risk Factors” in this Prospectus and in the AIF.
Commencing during the six month period ended June 30, 2014, the Corporation has paid a quarterly dividend of US$0.075 on each Common Share. Future dividend decisions made by the Board will be based on the Corporation’s then-current business results, cash requirements and financial condition.
19
BOOK-ENTRY ONLY SECURITIES
Securities issued in “book-entry only” form must be purchased, transferred or redeemed through participants (“CDS Participants”) in the depository service of CDS Clearing and Depository Services Inc. or a successor (collectively, “CDS”). Each of the agents, underwriters or dealers, as the case may be, named in a Prospectus Supplement will be a CDS Participant or will have arrangements with a CDS Participant. On the closing of a book-entry only offering of Securities, the Corporation may cause a global certificate or certificates representing the aggregate number of Securities subscribed for under such offering to be delivered to, and registered in the name of, CDS or its nominee. Except as described below, no purchaser of Securities issued in book-entry-only form or non-certificated inventory system form will be entitled to a certificate or other instrument from the Corporation or CDS evidencing that purchaser’s ownership thereof, and no purchaser will be shown on the records maintained by CDS except through a book-entry account of a CDS Participant acting on behalf of such purchaser. Each purchaser of Securities will receive a customer confirmation of purchase from the registered dealer from which the Securities are purchased in accordance with the practices and procedures of that registered dealer. The practices of registered dealers may vary, but generally customer confirmations are issued promptly after execution of a customer order. CDS will be responsible for establishing and maintaining book-entry accounts for its CDS Participants having interests in the Securities. Reference in this Prospectus to a holder of Securities means, unless the context otherwise requires, the owner of the beneficial interest in the Securities.
If the Corporation determines, or CDS notifies the Corporation in writing, that CDS is no longer willing or able to discharge properly its responsibilities as depository with respect to the Securities and the Corporation is unable to locate a qualified successor, or if the Corporation at its option elects, or is required by law, to terminate the book-entry system, then the Securities will be issued in fully registered form to holders or their nominees.
Transfer, Conversion or Redemption of Securities
Transfer of ownership, conversion or redemption of Securities will be effected through records maintained by CDS or its nominee for such Securities with respect to interests of CDS Participants, and on the records of CDS Participants with respect to interests of persons other than CDS Participants. Holders who desire to purchase, sell or otherwise transfer ownership of or other interests in the Securities may do so only through CDS Participants.
The ability of a holder to pledge a Security or otherwise take action with respect to such holder’s interest in a Security (other than through a CDS Participant) may be limited due to the lack of a physical certificate.
Payments and Notices
Any payments on a Security will be made by the Corporation to CDS or its nominee, as the case may be, as the registered holder of the Security and the Corporation understands that such payments will be credited by CDS or its nominee in the appropriate amounts to the relevant CDS Participants. Payments to holders of Securities of amounts so credited will be the responsibility of the CDS Participants.
As long as CDS or its nominee is the registered holder of the Securities, CDS or its nominee, as the case may be, will be considered the sole owner of the Securities for the purposes of receiving notices or payments on the Securities. In such circumstances, the responsibility and liability of the Corporation in respect of notices or payments on the Securities is limited to giving of notice or making of the payment on the Securities to CDS or its nominee.
Each holder must rely on the procedures of CDS and, if such holder is not a CDS Participant, on the procedures of the CDS Participant through which such holder owns its interest, to exercise any rights with respect to the Securities. The Corporation understands that under existing policies of CDS and industry practices, if the Corporation requests any action of holders or if a holder desires to give any notice or take any action which a registered holder is entitled to give or take with respect to the Securities, CDS would authorize the CDS Participant acting on behalf of the holder to give such notice or to take such action, in accordance with the procedures established by CDS or agreed to from time to time by the Corporation, any trustee and CDS. Any holder that is not a CDS Participant must rely on the contractual arrangement it has directly, or indirectly through its financial intermediary, with its CDS Participant to give such notice or take such action.
The Corporation, agents, underwriters or dealers and any trustee identified in a Prospectus Supplement, as applicable, will not have any liability or responsibility for: (i) records maintained by CDS relating to beneficial ownership interest
20
in the Securities held by CDS or the book-entry accounts maintained by CDS; (ii) maintaining, supervising or reviewing any records relating to any such beneficial ownership interest; or (iii) any advice or representation made by or with respect to CDS and contained herein or in any other document with respect to the rules and regulations of CDS or based on the directions of the CDS Participants.
EARNINGS COVERAGE RATIO
The applicable Prospectus Supplement will provide, as required, the earnings coverage ratios with respect to the issuance of Securities pursuant to such Prospectus Supplement.
PRIOR SALES
The applicable Prospectus Supplement will describe prior sales of Concordia as required in a Prospectus Supplement with respect to the issuance of Securities pursuant to such Prospectus Supplement.
TRADING PRICE AND VOLUME
The Common Shares are currently listed on the TSX under the trading symbol “CXR” and the NASDAQ under the trading symbol “CXRX”. On July 15, 2015, the last trading day prior to the date of this Prospectus, the closing price of the Common Shares on the TSX and the NASDAQ was $103.00 and US$79.50, respectively.
The following table sets forth the reported intraday high and low prices and the trading volume for the Common Shares on the TSX for the 12-month period prior to the date of this Prospectus.
| | | | | | | | | | | | |
| | | TSX(1) | |
Month | | High | | | Low | | | Volume | |
| | | $ | | | $ | | | | |
2014 | | | | | | | | | | | | |
July | | | 38.75 | | | | 33.74 | | | | 2,944,954 | |
August | | | 36.32 | | | | 32.58 | | | | 3,098,506 | |
September | | | 37.22 | | | | 32.81 | | | | 4,950,997 | |
October | | | 43.01 | | | | 33.42 | | | | 4,697,327 | |
November | | | 49.00 | | | | 40.20 | | | | 4,063,579 | |
December | | | 48.31 | | | | 41.34 | | | | 4,068,435 | |
2015 | | | | | | | | | | | | |
January | | | 55.01 | | | | 45.09 | | | | 4,559,993 | |
February | | | 66.11 | | | | 54.65 | | | | 4,524,810 | |
March | | | 91.16 | | | | 62.80 | | | | 9,483,728 | |
April | | | 104.74 | | | | 82.00 | | | | 6,639,113 | |
May | | | 91.05 | | | | 78.62 | | | | 5,551,987 | |
June | | | 97.26 | | | | 82.44 | | | | 6,799,897 | |
July 1 – 15 | | | 104.94 | | | | 88.00 | | | | 2,305,637 | |
Note:
| (1) | High and low price based on intraday high and low share prices. Source for data in the above table is TMX Group. Past performance should not be seen as an indicator of future performance. |
The following table sets forth the reported intraday high and low prices and the trading volume for the Common Shares on the NASDAQ for the period from the Corporation’s listing date to the date prior to the date of this Prospectus.
| | | | | | | | | | | | |
| | | NASDAQ | |
| | | High | | | Low | | | Volume | |
| | | US$ | | | US$ | | | | |
2015 | | | | | | | | | | | | |
June 29 – 30 | | | 73.23 | | | | 70.72 | | | | 27,339 | |
July 1 – 15 | | | 83.24 | | | | 68.54 | | | | 43,757 | |
21
CERTAIN CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
The applicable Prospectus Supplement may describe certain Canadian federal income tax consequences to an investor acquiring any Securities offered thereunder.
CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The applicable Prospectus Supplement may describe certain United States federal income tax consequences to an investor acquiring any Securities offered thereunder.
RISK FACTORS
Before making an investment decision, prospective purchasers of Securities should carefully consider the information described in this Prospectus and the documents incorporated by reference herein, including the applicable Prospectus Supplement. There are certain risks inherent in an investment in the Securities, including the factors described under the heading “Risk Factors – Risk Factors Related to the Business” in the AIF (pages 46 through 64) and “Risk Factors – Risk Factors Related to the Common Shares” in the AIF (on pages 64 through 68), and any other risk factors described herein or in a document incorporated by reference herein, which investors should carefully consider before investing. Additional risk factors relating to a specific offering of Securities will be described in the applicable Prospectus Supplement. Some of the factors described herein, in the documents incorporated by reference herein, and/or the applicable Prospectus Supplement are interrelated and, consequently, investors should treat such risk factors as a whole. If any of the risk factors described herein, in the AIF, in another document incorporated by reference herein or in the applicable Prospectus Supplement occur, it could have a material adverse effect on the business, financial condition and results of operations of the Corporation. Additional risks and uncertainties of which the Corporation currently is unaware or that are unknown or that it currently deems to be immaterial could have a material adverse effect on the Corporation’s business, financial condition and results of operation. The Corporation cannot assure you that it will successfully address any or all of these risks. There is no assurance that any risk management steps taken will avoid future loss due to the occurrence of the risks described herein, in the AIF, in the other documents incorporated by reference herein or in the applicable Prospectus Supplement or other unforeseen risks.
LEGAL MATTERS
Unless otherwise specified in a Prospectus Supplement, certain Canadian legal matters in connection with the Securities offered hereby will be passed upon by Fasken Martineau DuMoulin LLP, on behalf of the Corporation.
As of the date hereof, the partners and associates of Fasken Martineau DuMoulin LLP, as a group, own, directly or indirectly, in the aggregate, less than 1% of the Common Shares.
EXPERTS
The (i) consolidated annual financial statements of the Corporation for the years ended December 31, 2014 and 2013 and (ii) the financial statements in respect of Zonegran® included in the business acquisition report of the Corporation dated December 12, 2014, incorporated by reference in this Prospectus have been so incorporated in reliance on the audit reports, which are also incorporated by reference in this Prospectus, of Collins Barrow Toronto LLP, on the authority of such firm as experts in auditing and accounting. Collins Barrow Toronto LLP has advised the Corporation that it is independent of the Corporation in accordance with the Rules of Professional Conduct of the Chartered Professional Accountants of Ontario.
The financial statements in respect of Donnatal® included in the business acquisition report of the Corporation dated June 9, 2014, incorporated by reference in this Prospectus have been so incorporated in reliance on the audit report, which is also incorporated by reference in this Prospectus, of Keiter, Certified Public Accountants, on the authority of such firm as experts in auditing and accounting. Keiter, Certified Public Accountants has advised the Corporation that it is independent of the Corporation under the American Institute of Certified Public Accountants’ rules on auditor independence.
22
The financial statements in respect of the Covis Portfolio included in the business acquisition report of the Corporation dated July 3, 2015, incorporated by reference in this Prospectus have been so incorporated in reliance on the audit report included therein of Crowe Horwath LLP, on the authority of such firm as experts in auditing and accounting. Crowe Horwath LLP has advised the Corporation that it is independent of the Corporation under the American Institute of Certified Public Accountants’ rules on auditor independence.
AUDITORS, TRANSFER AGENT AND REGISTRAR
The auditor of the Corporation is PricewaterhouseCoopers LLP, 354 Davis Road, Suite 600, Oakville, Ontario, Canada L6J 0C5.
The transfer agent and registrar for the Common Shares is Equity Financial Trust Company, 200 University Avenue, Suite 300, Toronto, Ontario, M5H 4H1. Continental Stock Transfer & Trust Company, 17 Battery Place, 8th Floor, New York, New York, USA 10004, is the transfer agent and registrar for the Common Shares in the United States.
PURCHASERS’ STATUTORY AND CONTRACTUAL RIGHTS OF WITHDRAWAL AND RESCISSION
Securities legislation in certain of the provinces of Canada provides purchasers with the right to withdraw from an agreement to purchase securities. This right may be exercised within two business days after receipt or deemed receipt of a prospectus and any amendment. In several of the provinces of Canada, the securities legislation further provides a purchaser with remedies for rescission or, in some jurisdictions, revisions of the price or damages, if the prospectus and any amendment contains a misrepresentation or is not delivered to the purchaser, provided that the remedies for rescission, revisions of the price or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of these rights or consult with a legal adviser.
In addition, original purchasers of Subscription Receipts, Warrants or convertible or exchangeable Debt Securities (or Units comprised of any such Securities) will have a contractual right of rescission against the Corporation in respect of the conversion, exchange or exercise of a Subscription Receipt, Warrant or a convertible or exchangeable Debt Security. The contractual right of rescission will entitle such original purchasers to receive the amount paid upon conversion, exchange or exercise, upon surrender of the underlying securities acquired thereby, in the event that this Prospectus (as supplemented or amended) contains a misrepresentation, provided that: (i) the conversion, exchange or exercise takes place within 180 days of the date of the purchase of the convertible, exchangeable or exercisable Security under this Prospectus; and (ii) the right of rescission is exercised within 180 days of the date of the purchase of the convertible, exchangeable or exercisable Security under this Prospectus.
In an offering of Subscription Receipts, Warrants or convertible or exchangeable Debt Securities (or Units comprised of any such Securities), investors are cautioned that the statutory right of action for damages for a misrepresentation contained in the prospectus is limited, in certain provincial securities legislation, to the price at which Subscription Receipts, Warrants or convertible or exchangeable Debt Securities (or Units comprised of any such Securities) are offered to the public under the prospectus offering. This means that, under the securities legislation of certain provinces, if the purchaser pays additional amounts upon the conversion, exchange or exercise of the security, those amounts may not be recoverable under the statutory right of action for damages that applies in those provinces. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province for the particulars of this right of action for damages or consult with a legal adviser.
23











