FIRST QUARTER ENDED MARCH 31, 2018
MANAGEMENT’S
DISCUSSION AND ANALYSIS
May 15, 2018
|
| | | |
| Table of Contents | | | |
| | 1 | Management's Discussion and Analysis | |
| | 2 | Business Overview and Segments | |
| | 3 | Recent Events | |
| | 4 | Results of Operations | |
| | 5 | Segment Performance | |
| | 6 | Corporate and Other Costs | |
| | 7 | Selected Quarterly Financial Information | |
| | 8 | Balance Sheet Analysis | |
| | 9 | Liquidity and Capital Realignment | |
| | 10 | Lending Arrangements and Debt | |
| | 11 | Contractual Obligations | |
| | 12 | Related Party Transactions | |
| | 13 | Non-IFRS Financial Measures | |
| | 14 | Critical Accounting Estimates | |
| | 15 | Contingencies | |
| | 16 | Outstanding Share Data | |
| | 17 | Control Environment | |
| | 18 | Forward-looking Statements | |
1 Management Discussion and Analysis
The following Management’s Discussion and Analysis ("MD&A") summarizes Concordia International Corp.’s ("Concordia" or the "Company", or "we" or "us" or "our") consolidated operating results and cash flows for the three month period ended March 31, 2018 with a comparative prior period, and the Company’s balance sheet as at March 31, 2018 with a comparative period to December 31, 2017. The MD&A was prepared as of May 15, 2018 and should be read in conjunction with the unaudited condensed interim consolidated financial statements and the notes thereto as at and for the three month period ended March 31, 2018 and the consolidated financial statements and Management's Discussion and Analysis for the year ended December 31, 2017. Financial information in this MD&A is based on financial statements that have been prepared in accordance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB") and amounts are stated in thousands of United States Dollars ("USD"), which is the reporting currency of the Company, unless otherwise noted. The significant exchange rates used in the translation to the reporting currency are:
|
| | |
| | US$ per Great British pound (£) |
| As at, and for the periods ended | Spot | Average |
| January 1, 2016 to March 31, 2016 | 1.4395 | 1.4321 |
| April 1, 2016 to June 30, 2016 | 1.3395 | 1.4354 |
| July 1, 2016 to September 30, 2016 | 1.3008 | 1.3136 |
| October 1, 2016 to December 31, 2016 | 1.2305 | 1.2438 |
| January 1, 2017 to March 31, 2017 | 1.2489 | 1.2387 |
| April 1, 2017 to June 30, 2017 | 1.3004 | 1.2781 |
| July 1, 2017 to September 30, 2017 | 1.3402 | 1.3088 |
| October 1, 2017 to December 31, 2017 | 1.3494 | 1.3276 |
| January 1, 2018 to March 31, 2018 | 1.4037 | 1.3910 |
Certain prior period financial information has been presented to conform to the current period presentation.
Some of the statements contained in this MD&A constitute forward-looking information within the meaning of applicable Canadian securities legislation and forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995 (collectively, "forward-looking statements"), which are based upon the current internal expectations, estimates, projections, assumptions and beliefs of the Company's management ("Management"). Refer to the "Forward-Looking Statements" section of this MD&A for a discussion of certain risks, uncertainties, and assumptions relating to forward-looking statements. Additional information relating to the Company, including the Company’s Annual Report on Form 20-F, is available on SEDAR at www.sedar.com and on EDGAR at www.sec.gov. The results of operations, business prospects and financial condition of Concordia will be affected by, among other things, the "Risk Factors" set out in Concordia’s Annual Report on Form 20-F dated March 8, 2018 and other documents filed with the Canadian Securities Administrators and the United States Securities and Exchange Commission, available on SEDAR at www.sedar.com and EDGAR at www.sec.gov.
Certain measures used in this MD&A do not have any standardized meaning under IFRS. When used, these measures are defined in such terms as to allow the reconciliation to the closest IFRS measure. See "Results of Operations", "Segment Performance", "Selected Quarterly Financial Information", and "Non-IFRS Financial Measures".
|
| |
| Concordia Management's Discussion and Analysis | Page 2 |
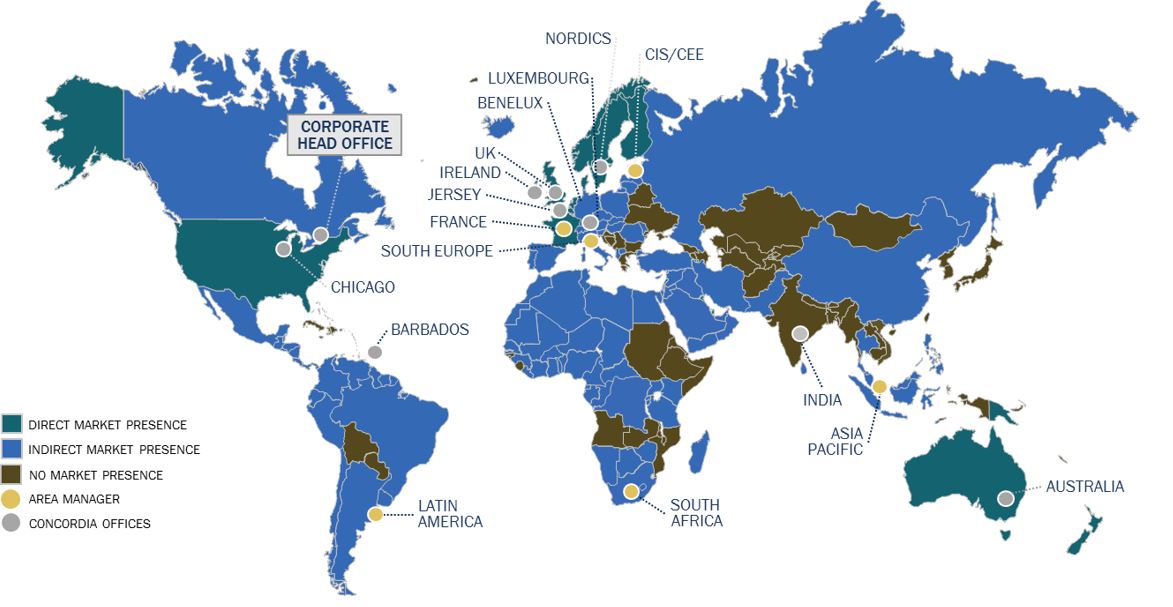
2 Business Overview and Segments
Concordia is an international specialty pharmaceutical company, owning or licensing, through its subsidiaries, a diversified portfolio of branded and generic prescription products. The Company has two reporting segments, which consist of Concordia International and Concordia North America, in addition to its Corporate cost centre.
* In above, “CIS” means the Commonwealth of Independent States and “CEE” means Central and Eastern Europe.
The registered and head office of the Company is located at 277 Lakeshore Rd. East, Suite 302, Oakville, Ontario, L6J 1H9. The Company’s records office is located at 333 Bay St., Suite 2400, Toronto, Ontario, M56 2T6. The Company’s common shares are listed on the Toronto Stock Exchange under the symbol "CXR" and on the NASDAQ under the symbol "CXRX".
Concordia International
The Concordia International segment consists of a diversified portfolio of branded and generic products that are sold to wholesalers, hospitals and pharmacies in over 90 countries. The Concordia International segment specializes in the acquisition, licensing and development of off-patent prescription medicines, which may be niche, hard to make products. The segment’s over 200 products are manufactured and sold through an out-sourced manufacturing network and marketed internationally through a combination of direct sales and local distribution relationships. The Concordia International segment operates primarily outside of the North American marketplace.
Concordia North America
The Concordia North America segment has a diversified product portfolio that focuses primarily on the United States pharmaceutical market. These products include, but are not limited to, Donnatal® for the treatment of irritable bowel syndrome; Zonegran® for the treatment of partial seizures in adults with epilepsy; Nilandron® for the treatment of metastatic prostate cancer; Lanoxin® for the treatment of mild to moderate heart failure and atrial fibrillation; Plaquenil® for the treatment of lupus and rheumatoid arthritis; and Photofrin® for the treatment of certain types of cancer. Concordia North America’s product portfolio consists of branded products and authorized generic contracts. The segment’s products are manufactured through an out-sourced production network and sold primarily through a third party distribution network in the United States.
Corporate
The Corporate cost centre represents certain centralized costs including costs associated with the Company's head office and senior management located in Canada and costs associated with being a public reporting entity.
|
| |
| Concordia Management's Discussion and Analysis | Page 3 |
3 Recent Events
Canada Business Corporations Act (the "CBCA") Proceedings
On October 20, 2017, the Company announced that it and one of its wholly-owned subsidiaries commenced a court proceeding under the CBCA.
On May 2, 2018, the Company announced a proposed transaction to realign its capital structure (the “Recapitalization Transaction”).
The proposed Recapitalization Transaction would raise new equity capital of $586.5 million, and to reduce the Company’s total outstanding debt by approximately $2.4 billion.
In connection with the proposed Recapitalization Transaction, the Company entered into a support agreement (the “Support Agreement”) with certain holders of the Company’s existing secured debt (the “Secured Debt”) and certain holders of the Company’s existing unsecured debt (the “Unsecured Debt”) that are subject to confidentiality agreements with Concordia and which hold in the aggregate approximately $1.6 billion in principal amount, or approximately 72%, of the Company’s Secured Debt and approximately $1.0 billion in principal amount, or approximately 64% of the Company’s Unsecured Debt (the “Initial Consenting Debtholders”). The Initial Consenting Debtholders are comprised of an ad hoc committee of holders of Secured Debt and an ad hoc committee of holders of Unsecured Debt. Pursuant to the Support Agreement, the Initial Consenting Debtholders have, among other things, agreed to support the Recapitalization Transaction and vote in favour of the plan of arrangement (the “CBCA Plan”) in Concordia’s previously announced proceedings (the "CBCA Proceedings") under the CBCA pursuant to which the Recapitalization Transaction is expected to be implemented.
In addition, on May 2, 2018 Concordia obtained an interim order (the “Interim Order”) issued by the Ontario Superior Court of Justice (the "Court") in the CBCA Proceedings authorizing, among other things, the holding of the following meetings (the “Meetings”) scheduled for June 19, 2018: (i) a meeting of holders of the Secured Debt (the “Secured Debtholders”); (ii) a meeting of holders of the Unsecured Debt (the “Unsecured Debtholders”); and (iii) a meeting (the "Shareholders' Meeting") of holders of the Company’s common shares (the “Shareholders”), in each case to consider and vote upon, among other things, the CBCA Plan to implement the Recapitalization Transaction.
Certain Key Recapitalization Transaction Terms
The Recapitalization Transaction contemplates the following key terms and conditions:
Secured Debt
| |
| • | The Company’s Secured Debt in the aggregate principal amount of approximately $2.2 billion, plus accrued and unpaid interest, will be exchanged for (i) cash in an amount equal to any outstanding accrued and unpaid interest (at contractual non-default rates) in respect of the Secured Debt, (ii) cash in the amount of $500 million (the “Secured Creditor Cash Pool”), (iii) any Additional Cash Amount (as defined below) and (iv) new secured debt (the “New Secured Debt”) comprised of new senior secured term loans (“New Senior Secured Term Loans”) and new senior secured notes (“New Senior Secured Notes”). The Company expects the aggregate principal amount of the New Secured Debt to be issued to Secured Debtholders pursuant to the Recapitalization Transaction to be approximately $1.4 billion; |
| |
| • | Each Secured Debtholder will receive its pro rata share of the New Secured Debt, in the form of either New Senior Secured Term Loans or New Senior Secured Notes depending on the type of Secured Debt held by such Secured Debtholder, subject to (i) holders of the Company’s existing secured term loans as of the record date of May 9, 2018 (the “Record Date”) having the right to elect to receive their New Secured Debt in the form of New Senior Secured Notes, provided that any such elections may be subject to certain re-allocations pursuant to the terms of the Recapitalization Transaction, and (ii) Secured Debtholders receiving New Senior Secured Term Loans having the right to elect to receive their New Senior Secured Term Loans denominated in USD or Euros, provided that any such elections may be subject to certain re-allocations pursuant to the terms of the Recapitalization Transaction; |
| |
| • | Secured Debtholders as of the Record Date who vote in favour of the CBCA Plan on or prior to the early consent date of June 6, 2018 (the “Early Consent Date”), as it may be extended by Concordia (the “Early Consenting Secured Debtholders”) will be entitled to receive on implementation of the Recapitalization Transaction pursuant to the CBCA Plan early consent consideration in the form of cash equal to 5% of the principal amount of Secured Debt owing to such Early Consenting Secured Debtholder as of the Record Date and voted in favour of the CBCA Plan (the “Secured Debtholder Early Consent Cash Consideration”) as additional consideration in exchange for their Secured Debt; |
| |
| • | If the aggregate amount of Secured Debtholder Early Consent Cash Consideration that becomes payable pursuant to the Recapitalization Transaction is less than $100 million, then an amount equal to the difference between $100 million and the amount of Secured Debtholder Early Consent Cash Consideration that becomes payable (the “Additional Cash Amount”) will be paid on a pro rata basis to each Secured Debtholder as additional consideration in exchange for their Secured Debt; and |
| |
| • | The final principal amount of New Secured Debt to be issued pursuant to the Recapitalization Transaction shall be in such amount that results in the aggregate consideration payable to Secured Debtholders pursuant to the Recapitalization Transaction by way of the Secured Creditor Cash Pool, the New Secured Debt and the Secured Debtholder Early Consent Cash Consideration (but not including the payment of accrued and unpaid interest or the Additional Cash Amount) being equal to 93.3835% of the principal amount of Secured Debt owing to such Secured Debtholders if such Secured Debtholders are Early Consenting Secured Debtholders, |
|
| |
| Concordia Management's Discussion and Analysis | Page 4 |
and approximately 88.3835% of the principal amount of Secured Debt owing to such Secured Debtholders if such Secured Debtholders are not Early Consenting Secured Debtholders.
Unsecured Debt
| |
| • | The Company’s Unsecured Debt in the aggregate principal amount of approximately $1.6 billion, plus accrued and unpaid interest, will be exchanged for (i) new common shares of Concordia representing approximately 8% of the outstanding common shares of Concordia immediately following the implementation of the Recapitalization Transaction (the “Unsecured Debt Exchange Shares”) and (ii) any Reallocated Unsecured Shares (as defined below); |
| |
| • | Unsecured Debtholders as of the Record Date who vote in favour of the CBCA Plan on or prior to the Early Consent Date, as it may be extended by Concordia (the “Early Consenting Unsecured Debtholders”) will be entitled to receive on implementation of the Recapitalization Transaction pursuant to the CBCA Plan early consent consideration in the form of new common shares of Concordia equal to their pro rata share (calculated based on the principal amount of Unsecured Debt held by such Early Consenting Unsecured Debtholders as at the Record Date and voted in favour of the CBCA Plan, divided by the aggregate principal amount of Unsecured Debt outstanding as at the Record Date) of a pool of common shares (the “Unsecured Early Consent Share Pool”) representing approximately 4% of the outstanding common shares of Concordia immediately following implementation of the Recapitalization Transaction pursuant to the CBCA Plan (the “Unsecured Debtholder Early Consent Shares”) as additional consideration in exchange for their Unsecured Debt; and |
| |
| • | If less than 100% of Unsecured Debt is voted in favour of the CBCA Plan by Early Consenting Unsecured Debtholders, any shares remaining in the Unsecured Early Consent Share Pool not issued as Unsecured Debtholder Early Consent Shares (the “Reallocated Unsecured Shares”) will be issued to all holders of Unsecured Debt on a pro rata basis as additional consideration for their Unsecured Debt. |
Private Placement
| |
| • | Approximately $586.5 million (the “Total Offering Size”) in cash will be invested to acquire new common shares of Concordia representing in the aggregate approximately 88% of the outstanding common shares of Concordia immediately following the implementation of the Recapitalization Transaction (the “Private Placement Shares”) by certain parties who executed a subscription agreement with the Company (the “Subscription Agreement”) concurrently with the execution of the Support Agreement (the “Private Placement Parties”) pursuant to a private placement (the “Private Placement”); |
| |
| • | The proceeds of the Private Placement will be used towards paying the Secured Creditor Cash Pool and the Secured Debtholder Early Consent Cash Consideration to be paid as part of the consideration for the exchange of the Secured Debt; |
| |
| • | Each of the Private Placement Parties will be entitled to receive its pro rata share (based on its subscription commitment) of cash consideration in the aggregate amount of $44 million (subject to any corresponding adjustments to the extent the Total Offering Size is reduced pursuant to the terms of the Subscription Agreement) (the “Private Placement Consideration”), which is payable on the terms set out in the Subscription Agreement, including on completion of the Recapitalization Transaction and certain earlier events; and |
| |
| • | Pursuant to the Subscription Agreement, the Private Placement Parties and the Company expect to agree on certain governance terms and registration rights. The governance terms will be described in more detail in the Company’s management information circular to be mailed to Secured Debtholders, Unsecured Debtholders and Shareholders in connection with the various Meetings to be held to approve the CBCA Plan. |
Existing Shares and Equity Claims
| |
| • | Upon completion of the Recapitalization Transaction, existing Shareholders will retain their existing common shares of Concordia, subject to a share consolidation of one common share in exchange for 300 existing common shares to be implemented as part of the Recapitalization Transaction and the dilution resulting from the issuance of common shares pursuant to the Recapitalization Transaction, such that the existing Shareholders will own approximately 0.35% of the outstanding common shares of Concordia immediately following implementation of the Recapitalization Transaction; and |
| |
| • | All other equity interests in Concordia, including all options, warrants, rights or similar instruments, will be cancelled on implementation of the Recapitalization Transaction pursuant to the CBCA Plan, and all equity claims, other than existing equity class action claims against Concordia (the “Existing Equity Class Action Claims”), will be released pursuant to the CBCA Plan, provided that any recovery in respect of any Existing Equity Class Action Claims will be limited to recovery as against any applicable insurance policies maintained by the Company. |
Share Dilution
| |
| • | The existing common shares retained by the Shareholders upon implementation of the Recapitalization Transaction and the common shares to be issued under the CBCA Plan, including the Unsecured Debt Exchange Shares, the Reallocated Unsecured Shares, the Unsecured Debtholder Early Consent Shares and the Private Placement Shares, shall be subject to dilution following the completion of the Recapitalization Transaction pursuant to the issuance of any new common shares under the management equity incentive plan to be adopted pursuant to the Recapitalization Transaction. |
|
| |
| Concordia Management's Discussion and Analysis | Page 5 |
Subject to the satisfaction or waiver of applicable conditions, the Recapitalization Transaction is expected to be completed by July 31, 2018.
In connection with the Recapitalization Transaction, it is anticipated that Concordia will continue from the Business Corporations Act (Ontario) to the CBCA.
Alternative Implementation Process
The Recapitalization Transaction is being implemented pursuant to the CBCA Plan. The Company is also soliciting votes to advance the Recapitalization Transaction pursuant to insolvency proceedings under Chapter 11 of the United States Bankruptcy Code (a “Chapter 11 Process”), contemporaneously with soliciting votes in respect of the CBCA Plan. Concordia currently intends to complete and implement the CBCA Plan pursuant to the CBCA Proceedings. Contemporaneous solicitation of votes in respect of a Chapter 11 Process ensures that the Company has the future ability to also complete the Recapitalization Transaction under such an alternative implementation process if the Company elects to do so in the future, subject to certain conditions and consent requirements as provided for in the Support Agreement. In addition, in accordance with the terms of the Interim Order, a vote cast in favour of the CBCA Plan at the Meetings may also be counted in favour of implementing a plan of arrangement on substantially similar terms in any insolvency proceedings under the Companies’ Creditors Arrangement Act (Canada) that may be commenced by the Company, to the extent such proceedings are consented to by the majority private placement parties and the majority initial consenting debtholders.
Management and Board of Director Changes
On May 2, 2018, the Company announced that it appointed Graeme Duncan as its interim Chief Executive Officer, and also announced the departure of Allan Oberman, the Company's former Chief Executive Officer and Board Member. The Company paid approximately $7.7 million in severance to the former Chief Executive Officer.
On May 2, 2018, the Company also announced that it appointed Guy Clark as the Company's Chief Corporate Development Officer, and also announced the departure of Sarwar Islam, the Company's former Chief Corporate Development Officer.
Relocation of Corporate Head Office
During the second quarter of 2018, the Company's corporate head office is expected to be relocated to 5770 Hurontario Street, Suite 310, Mississauga, Ontario, L5R 3G5. At the Shareholders' Meeting, Shareholders will be asked to approve a resolution to move the corporate head office to Mississauga, Ontario.
Notification of the termination of the Currency Swaps and termination of Revolving Commitments
On October 20, 2017, the counterparty to the Company's August 17, 2016 cross currency swap agreement ("August Swap Agreement") and November 3, 2016 cross currency swap agreement ("November Swap Agreement", and together with the August Swap Agreement, the "Currency Swaps") notified the Company that it would be terminating the Currency Swaps effective October 23, 2017 due to commencement of the CBCA Proceedings. As part of the Recapitalization Transaction, the Company has agreed to the amount of the Currency Swaps liability ($114,431), which amount will be addressed in the same manner as the Secured Debt under the Recapitalization Transaction. In addition, on October 27, 2017, the Company terminated the revolving commitments under the Company's credit agreement dated October 21, 2015, as amended ("Credit Agreement"). No amounts had been drawn or were outstanding in respect of the revolving commitments at such time.
Rating Agency Changes
On May 7, 2018, Standard & Poors Global Ratings ("S&P") lowered its issue-level ratings on Concordia’s Secured Debt to "CC" from "CCC-". S&P noted that its “SD” corporate credit rating and "D" rating on Concordia’s Unsecured Debt remained unchanged. In addition, on May 8, 2018, Moody’s Investors Service ("Moody’s") placed the Company’s “Ca” Corporate Family Rating and Ca-PD/LD Probability of Default Rating under review for upgrade. Moody’s also affirmed its "Caa2" senior secured ratings and "C" senior unsecured ratings. The SGL-4 Speculative Grade Liquidity Rating was also affirmed. To the extent that the Company intends to complete any future transactions following the Recapitalization Transaction, the Company’s ability to complete any such transactions may be effected by credit rating agency decisions.
Business Impact in Relation to Brexit
On June 23, 2016, the United Kingdom held a referendum and voted to withdraw from the European Union ("Brexit"). On March 29, 2017, the United Kingdom delivered notice to the European Council in accordance with Article 50 of the Treaty on European Union of the United Kingdom’s intention to withdraw from the European Union. The Company understands that the timeframe for the negotiated withdrawal of the United Kingdom from the European Union is approximately two (2) years from the date of the withdrawal notification. However, as no member state has formally withdrawn from the European Union in the past, there is no precedent for the operation of Article 50 and, as a result, the timing and outcome of Brexit continues to be uncertain at this time. The Concordia International segment has significant operations within the United Kingdom and other parts of the European Union, and therefore continues to monitor developments related to Brexit, including the impact resulting from currency market movements.
|
| |
| Concordia Management's Discussion and Analysis | Page 6 |
Business Impact in Relation to the UK Health Service Medical Supplies (Costs) Act 2017 (the "Act")
The Act received Royal Assent on April 27, 2017. The Act introduces provisions in connection with controlling the cost of health service medicines and other medical supplies. The Act also introduces provisions in connection with the provision of pricing and other information by manufacturers, distributors and suppliers of those medicines and medical supplies. The Company continues to monitor the implementation of the Act and its impact on its business. Refer to the "Risk Factors" section of the Company's Annual Report on Form 20-F dated March 8, 2018.
|
| |
| Concordia Management's Discussion and Analysis | Page 7 |
4 Results of Operations
|
| | | | |
| | Three months ended |
| (in $000's, except per share data) | Mar 31, 2018 |
| Mar 31, 2017 |
|
| Revenue | 152,264 |
| 160,557 |
|
| Gross profit | 101,106 |
| 115,415 |
|
| Gross profit % | 66 | % | 72 | % |
| Total operating expenses | 112,345 |
| 97,049 |
|
| Operating income (loss) | (11,239 | ) | 18,366 |
|
| | | |
| Income tax expense (recovery) | 4,704 |
| 4,489 |
|
| Net loss | (55,694 | ) | (78,824 | ) |
| | | |
| Loss per share | | |
| Basic | (1.09 | ) | (1.54 | ) |
| Diluted | (1.09 | ) | (1.54 | ) |
| | | |
EBITDA (1) | 94,503 |
| 56,932 |
|
Adjusted EBITDA (1) | 72,024 |
| 84,242 |
|
Adjusted EPS (1) | (0.19 | ) | 0.22 |
|
Notes:
| |
| (1) | Represents a non-IFRS measure. For the relevant definitions and reconciliation to reported results, see "Non-IFRS Financial Measures" section of this MD&A. Management believes non-IFRS measures, including Adjusted EBITDA, provide supplementary information to IFRS measures used in assessing the performance of the business. |
Revenue
Revenue for the first quarter of 2018 decreased by $8,293, or 5%, compared to the corresponding period in 2017. This decrease was due to lower sales from both segments, partially offset by higher foreign exchange rates impacting translated revenues from the Concordia International segment for the first quarter of 2018 compared to the corresponding period in 2017. Revenues were lower primarily due to lower volumes resulting from competition on a number of the Company's products in both segments. The Concordia International segment revenue for the first quarter of 2018 decreased by $5,779, or 5%, due to $20,463 lower revenue primarily as a result of volume and price declines on key products, including Liothyronine Sodium, Trazodone and Predisolone, partially offset by $14,684 higher revenue as a result of favourable foreign exchange rates positively impacting translated results. The Concordia North America segment revenue for the first quarter of 2018 decreased by 6% when compared to the corresponding period in 2017, mainly as a result of lower volumes on key products, including Donnatal® and Kapvay®. Refer to the "Segment Performance" section of this MD&A for a further discussion on segmental and product specific performance.
Gross Profit and Gross Profit %
Gross profit for the first quarter of 2018 decreased by $14,309, or 12%, compared to the corresponding period in 2017 primarily due to the revenue decreases described above. The decrease in gross profit percentage of 6% for the first quarter of 2018 compared to the corresponding period in 2017, is primarily due to a change in the mix of product sales within both segments. Refer to the "Segment Performance" section of this MD&A for a further discussion on segmental and product specific performance.
Operating Expenses
Operating expenses for the first quarter of 2018 increased by $15,296, or 16%, compared to the corresponding period in 2017. Operating expenses were higher during the first quarter of 2018 primarily due to $10,278 higher restructuring costs arising from the Company's initiative to realign its capital structure and $8,890 higher amortization charges on intangible assets, partially offset by $1,685 lower share based compensation expense and $1,570 lower general and administrative costs. For a further detailed description of operating expenses, refer to the "Corporate and Other Costs" section of this MD&A. For a further detailed description of certain segment operating expenses, refer to "Segment Performance" section of this MD&A.
Operating income (loss) for the first quarter of 2018 decreased by $29,605 compared to the corresponding period in 2017 due to the decrease in gross profit and higher operating expenses as described above.
|
| |
| Concordia Management's Discussion and Analysis | Page 8 |
The current income tax expense recorded for the first quarter of 2018 decreased by $1,296 compared to the corresponding period in 2017. Income taxes were lower primarily due to lower taxable income compared to the corresponding period in 2017, partially offset by the impact of foreign exchange translation of the income tax expense from the Concordia International segment. The deferred income tax expense recorded for the first quarter of 2018 increased by $1,511 and is mainly the result of movements in the foreign exchange rates, partially offset by the reversal of certain temporary differences.
The net loss for the first quarter of 2018 was $55,694 and EPS loss was $1.09 per share. Significant components comprising the net loss for the first quarter of 2018 are interest and accretion expenses of $80,122 and amortization of intangible assets of $65,607 offset by gross profit of $101,106. Refer to the "Corporate and Other Costs" section of this MD&A for further information related to expenses impacting net loss.
EBITDA and Adjusted EBITDA
EBITDA is higher than the net loss as it excludes: interest and accretion expense; interest income; income taxes; depreciation; and amortization of intangible assets. Refer to the "Non-IFRS Financial Measures" section of this MD&A for a full reconciliation. EBITDA for the first quarter of 2018 increased by $37,571 compared to the corresponding period in 2017. The increase in EBITDA was primarily due to $27,314 lower fair value loss on derivative financial instruments and $31,341 higher unrealized foreign exchange gain, partially offset by $14,309 lower gross profit and $10,278 higher acquisition related, restructuring and other costs.
Adjusted EBITDA also includes adjustments for: impairments; fair value adjustments to acquired inventory; acquisition related, restructuring and other costs; share-based compensation; fair value (gain) loss including purchase consideration and derivative financial instruments; foreign exchange (gain) loss; unrealized foreign exchange (gain) loss; and legal settlements and related legal costs (refer to the "Non-IFRS Financial Measures" section of this MD&A for a full reconciliation and description of these expenses). Adjusted EBITDA for the first quarter of 2018 decreased by $12,218, or 15%, compared to the corresponding period in 2017. The decline is primarily due to lower sales and gross margins from both segments, partially offset by higher foreign exchange rates impacting translated results during the first quarter of 2018. Adjusted EBITDA by segment for the three month period ended March 31, 2018 was $51,748 from Concordia International and $24,273 from Concordia North America. Refer to the "Segment Performance" section of this MD&A for a further discussion on segment performance. In addition, during the first quarter of 2018 the Company incurred $3,997 of Corporate costs related to the Corporate Head Office. Corporate expenses decreased by $1,641 compared to the corresponding period in 2017, primarily due to lower general and administrative expenses, including professional fees incurred during the first quarter of 2018.
|
| |
| Concordia Management's Discussion and Analysis | Page 9 |
5 Segment Performance
Concordia International |
| | | | |
| | Three months ended |
| (in $000's) | Mar 31, 2018 |
| Mar 31, 2017 |
|
| Revenue | 112,950 |
| 118,729 |
|
| Cost of sales | 41,673 |
| 37,501 |
|
| Gross profit | 71,277 |
| 81,228 |
|
| Gross profit % | 63 | % | 68 | % |
Adjusted Gross Profit (1) | 71,277 |
| 81,539 |
|
Adjusted Gross Profit %(1) | 63 | % | 69 | % |
| General and Administrative, Selling and Marketing and Research and Development Expenses | 19,529 |
| 18,298 |
|
Adjusted EBITDA(1) | 51,748 |
| 63,241 |
|
Notes:
| |
| (1) | Represents a non-IFRS measure. For the relevant definitions see "Non-IFRS Financial Measures" section of this MD&A. |
Revenue for the first quarter of 2018 decreased by $5,779 or 5%, compared to the corresponding period in 2017. A $20,463 decrease in revenue was partially offset by a $14,684 increase in revenue as a result of the Great British Pound ("GBP") strengthening against the USD, given a significant portion of the segment revenues are earned in GBP. Declines to revenue attributable to key products during the quarter, excluding the impact of foreign currency translation, were: (i) a $9,488 decrease from Liothyronine Sodium; (ii) a $2,384 decrease from Trazodone; (iii) a $2,064 decrease from Prednisolone; (vi) a $1,643 decrease from Biperiden Hydrochloride; and (v) a $1,244 decrease from Prochlorperazine. These lower product volumes and revenues are primarily due to ongoing competitive market pressures resulting in market share erosion. These revenue decreases were partially offset by $2,751 increased revenue from Nitrofurantoin. The remaining decrease was primarily due to general competitive market pressures across the segment's product portfolio.
Cost of sales for the first quarter of 2018 increased by $4,172 or 11%, compared to the corresponding period in 2017. The increase in cost of sales during the first quarter of 2018 is primarily due to the impact of foreign exchange, partially offset by volume declines from products described above.
Gross profit for the first quarter of 2018 decreased by $9,951 primarily due to the factors described above.
Gross profit as a percentage of revenue for the first quarter of 2018 decreased by 5% compared to the corresponding period in 2017. The decrease was primarily due to a shift in product mix, with certain higher margin products experiencing additional market competition, when compared to the first quarter of 2017.
General and administrative, selling and marketing and research and development costs increased by $1,231 primarily due to the impact of foreign exchange. Excluding the $2,249 unfavorable impact of foreign exchange, these costs decreased by $1,018 as a result of lower general and administrative expenses.
|
| |
| Concordia Management's Discussion and Analysis | Page 10 |
Concordia North America |
| | | | |
| | Three months ended |
| (in $000's) | Mar 31, 2018 |
| Mar 31, 2017 |
|
| Revenue | 39,314 |
| 41,828 |
|
| Cost of sales | 9,485 |
| 7,641 |
|
| Gross profit | 29,829 |
| 34,187 |
|
| Gross profit % | 76 | % | 82 | % |
| General and Administrative, Selling and Marketing and Research and Development Expenses | 5,556 |
| 7,548 |
|
Adjusted EBITDA(1) | 24,273 |
| 26,639 |
|
Notes:
| |
| (1) | Represents a non-IFRS measure. For the relevant definitions, see "Non-IFRS Financial Measures" section of this MD&A. |
Revenue for the first quarter of 2018 decreased by $2,514 or 6%, compared to the corresponding period in 2017. The decrease was primarily due to: (i) a $3,533 decrease from Donnatal®, as a result of additional competitive pressures that have resulted in a loss of market share; and (ii) a $2,456 decrease from Kapvay®. In the first quarter of 2018, Donnatal® continued to face pressure from a non-FDA approved product being distributed by a competitor and an additional competitive product launched in the second quarter of 2017. These decreases were partially offset by a $1,516 increase in revenue from Plaquenil® authorized generic. The remaining decrease was primarily due to general competitive market pressures across the segment's product portfolio.
Cost of sales for the first quarter of 2018 increased by $1,844, or 24%, compared to the corresponding period in 2017. The increase in cost of sales when compared with the decrease in revenue is due to a shift in product mix to lower margin products.
Gross profit for the first quarter of 2018 decreased by $4,358, or 13%, primarily due to lower revenue as described above.
Gross profit as a percentage of revenue for the first quarter of 2018 decreased by 6%, compared to the corresponding period in 2017. The decrease was primarily due to a shift in product mix.
General and administrative, selling and marketing and research and development costs decreased by $1,992 primarily due to lower bad debt expenses and a refund of regulatory fees, partially offset by higher selling and marketing costs associated with the co-promotion agreement for sales of Donnatal®.
|
| |
| Concordia Management's Discussion and Analysis | Page 11 |
6 Corporate and Other Costs
The following table details expenses from the Company's Corporate cost centre and other operating expenses from the business segments: |
| | | | |
| | Three months ended |
| (in $000's) | Mar 31, 2018 |
| Mar 31, 2017 |
|
| General and administrative | 12,178 |
| 13,748 |
|
| Selling and marketing | 9,798 |
| 9,752 |
|
| Research and development | 7,106 |
| 7,984 |
|
| Acquisition related, restructuring and other | 15,494 |
| 5,216 |
|
| Share-based compensation | 1,267 |
| 2,952 |
|
| Amortization of intangible assets | 65,607 |
| 56,717 |
|
| Depreciation expense | 470 |
| 488 |
|
| Fair value (gain) loss | 425 |
| 192 |
|
| Interest and accretion expense | 80,122 |
| 92,541 |
|
| Interest income | (706 | ) | (18,479 | ) |
| Fair value (gain) loss on derivative financial instruments | — |
| 27,314 |
|
| Foreign exchange (gain) loss | 1,341 |
| 990 |
|
| Unrealized foreign exchange (gain) loss | (41,006 | ) | (9,665 | ) |
| Total | 152,096 |
| 189,750 |
|
General and Administrative Expenses
General and administrative expenses reflect costs related to salaries and benefits, professional and consulting fees, public company costs, travel, facility leases and other administrative expenditures. General and administrative expenses for the first quarter of 2018 decreased by 11%, compared to the corresponding period in 2017. This decrease is a result of the Company's objective to reduce operating costs across the business.
Selling and Marketing Expenses
Selling and marketing expenses reflect costs incurred by the Company for the marketing, promotion and sale of the Company’s broad portfolio of products across the Company's segments. Selling and marketing costs for the first quarter of 2018 increased by $46 compared to the corresponding period in 2017 primarily as a result of unfavorable foreign exchange rate movements impacting translation.
Research and Development Expenses
Research and development expenses reflect costs for clinical trial activities, product development, professional and consulting fees and services associated with the activities of the medical, clinical and scientific affairs, quality assurance costs, regulatory compliance and drug safety costs (Pharmacovigilence) of the Company. Research and development costs for the first quarter of 2018 decreased by $878, or 11%, compared to the corresponding period in 2017. This decrease is primarily due to a refund of regulatory fees.
Acquisition Related, Restructuring and Other Costs
Acquisition related, restructuring and other costs during the first quarter of 2018 were $15,494. Acquisition related, restructuring and other costs for the first quarter of 2018 increased by 197% primarily due to costs associated with consultants involved in the Company's capital realignment initiative.
Significant costs incurred during the first quarter of 2018 include $8,261 of costs associated with the Company's realignment of its capital structure which include costs of the Company's advisors and advisors of the lending syndicate, $2,326 of management retention costs, $1,931 of costs related to severance, and $2,487 related to ongoing regulatory matters relating to the UK Competition and Markets Authority ("CMA") investigations (refer to the "Litigation and Arbitration" section of this MD&A for further details). The remaining costs relate primarily to costs associated with the ongoing class action lawsuits involving the Company (refer to the "Litigation and Arbitration" section of this MD&A for further details).
|
| |
| Concordia Management's Discussion and Analysis | Page 12 |
Share-Based Compensation
The share based compensation expense relates to the fair value of share-based option, restricted share unit ("RSU") and deferred share unit ("DSU") awards to employees, management and directors of the Company. Share based compensation during the first quarter of 2018 was $1,267. The decrease in the expense of $1,685 for the period is primarily due to no RSUs or DSUs being issued since the second quarter of 2017, and the impact of the staged vesting of the outstanding share-based options and RSUs.
Amortization of Intangible Assets
Amortization of intangible assets was $8,890 higher for the first quarter of 2018 compared to the corresponding period in 2017. The higher expense is due to the Company's change in accounting estimate with respect to amortizing intangible assets in both segments. The expense for the first quarter of 2018 of $65,607 is comprised of the following amounts:
| |
| • | Amortization related to acquired product rights and manufacturing processes for the first quarter of 2018 was $57,255; |
| |
| • | Amortization related to distribution and supplier contracts for the first quarter of 2018 was $7,751. Distribution and supplier contracts are amortized on a straight-line basis over 5 years; and |
| |
| • | Amortization related to other intangibles for the first quarter of 2018 was $601. |
Interest and Accretion
Interest and accretion expenses for the first quarter of 2018 were $80,122 representing a decrease of $12,419 compared to the corresponding period in 2017. The decrease is primarily due to:
| |
| • | The termination of the Currency Swaps during the fourth quarter of 2017 which resulted in $18,303 lower interest expense during the first quarter of 2018 (as well as lower interest income as described below); and |
| |
| • | The acceleration of all deferred financing fees during the fourth quarter of 2017 which resulted in $7,461 lower costs compared to the corresponding period in 2017. |
Offset primarily by:
| |
| • | A $12,230 higher interest expense on the Company's debt agreements as a result of: (i) events of default on certain of the Company's debt agreements; (ii) higher LIBOR rates; and (iii) a change in foreign exchange rates resulting in higher interest expense on the Company's GBP denominated term loan. |
Interest Income
Interest income for the first quarter of 2018 was $706, representing a decrease of $17,773 due to the termination of the Currency Swaps during the fourth quarter of 2017 as a result of the CBCA Proceedings.
Fair value loss on Derivative Contracts
The fair value loss on derivative contracts was $nil for the first quarter of 2018 compared with $27,314 in the corresponding period in 2017 as a result of the termination of the Currency Swaps. On October 20, 2017, the Company was notified by the counterparty to the Currency Swaps that one or more events of default had occurred under the Currency Swaps as a result of the Company obtaining a preliminary interim order from the Court pursuant to the arrangement provisions of the CBCA. As a result of the foregoing, the counterparty designated October 23, 2017 as the early termination date with respect to all transactions under the Currency Swaps. As part of the Recapitalization Transaction, the Company has agreed to the amount of the Currency Swaps liability ($114,431), which amount will be addressed in the same manner as the Secured Debt under the Recapitalization Transaction.
Foreign Exchange (Gain) Loss and Unrealized Foreign Exchange (Gain) Loss
Foreign exchange (gain) loss for the first quarter of 2018 was a loss of $1,341.
Unrealized foreign exchange gain for the first quarter of 2018 was $41,006. The primary component of the unrealized foreign exchange gain is a result of IFRS requiring that inter-company trading balances denominated in a currency other than the functional currency of an entity being retranslated with the exchange differences flowing through the consolidated statement of loss with the off-set within other comprehensive income (loss).
The foreign exchange translation impact of the Concordia International segment is recorded within other comprehensive loss. During the first quarter of 2018, there was a total of $10,569 foreign exchange gains, net of tax, associated with the translation of entities with a different functional currency, primarily within the Concordia International segment, offset by $22,690 of foreign exchange losses associated with the translation of the Company's GBP denominated term loan.
|
| |
| Concordia Management's Discussion and Analysis | Page 13 |
7 Selected Quarterly Financial Information
|
| | | | | | | | | | | | | | | | |
| For the three months ended (in $000’s, except per share amounts) | Q1-2018 |
| Q4-2017 |
| Q3-2017 |
| Q2-2017 |
| Q1-2017 |
| Q4-2016 |
| Q3-2016 |
| Q2-2016 |
|
| Revenue | 152,264 |
| 150,205 |
| 154,622 |
| 160,785 |
| 160,557 |
| 170,408 |
| 185,504 |
| 231,712 |
|
| Gross profit | 101,106 |
| 100,200 |
| 108,610 |
| 111,312 |
| 115,415 |
| 120,464 |
| 137,034 |
| 177,607 |
|
Adjusted Gross profit (1) | 101,106 |
| 100,200 |
| 108,610 |
| 111,312 |
| 115,726 |
| 120,858 |
| 138,540 |
| 178,476 |
|
| Operating income (loss) | (11,239 | ) | (211,648 | ) | 9,589 |
| (981,255 | ) | 18,366 |
| (524,962 | ) | 42,636 |
| (514,931 | ) |
| Net income (loss), continuing operations | (55,694 | ) | (431,773 | ) | (69,485 | ) | (1,010,653 | ) | (78,824 | ) | (663,761 | ) | (75,147 | ) | (570,384 | ) |
| | | | | | |
|
| | |
| Cash | 343,834 |
| 327,030 |
| 341,303 |
| 301,782 |
| 336,156 |
| 397,917 |
| 162,616 |
| 145,341 |
|
| Total assets | 2,324,626 |
| 2,322,335 |
| 2,651,844 |
| 2,611,489 |
| 3,619,665 |
| 3,731,574 |
| 4,229,695 |
| 4,349,554 |
|
| Total liabilities | 4,301,682 |
| 4,232,848 |
| 4,128,960 |
| 4,022,218 |
| 4,058,725 |
| 4,109,147 |
| 3,928,646 |
| 3,982,125 |
|
| | | | | | |
|
| | |
EBITDA (1) | 94,503 |
| (170,126 | ) | 63,144 |
| (903,563 | ) | 56,932 |
| (569,997 | ) | 30,213 |
| (454,285 | ) |
Adjusted EBITDA (1) | 72,024 |
| 70,778 |
| 78,582 |
| 81,808 |
| 84,242 |
| 80,508 |
| 104,444 |
| 142,344 |
|
| | | | | | |
|
| | |
| Earnings (Loss) per share | | | | | |
|
| | |
| Basic | (1.09 | ) | (8.42 | ) | (1.36 | ) | (19.78 | ) | (1.54 | ) | (13.00 | ) | (1.47 | ) | (11.18 | ) |
| Diluted | (1.09 | ) | (8.42 | ) | (1.36 | ) | (19.78 | ) | (1.54 | ) | (13.00 | ) | (1.47 | ) | (11.18 | ) |
Adjusted (1) | (0.19 | ) | (0.28 | ) | 0.06 |
| 0.19 |
| 0.22 |
| 0.13 |
| 0.69 |
| 1.38 |
|
Amounts shown above are results from continuing operations, excluding discontinued operations, except for total assets and liabilities amounts.
Notes:
(1) Represents a non-IFRS measure. For the relevant definitions see the "Non-IFRS Financial Measures" section of this MD&A. For the relevant reconciliation to reported results, see the "Non-IFRS Financial Measures" section of this MD&A for the first quarter of 2018 and corresponding period in 2017, and for other periods presented, refer to previous publicly filed MD&As.
During the quarterly periods presented above, the Company has experienced a declining trend in operating results. Subsequent to the second quarter of 2016, the business experienced greater than expected market competition on certain products and industry specific environmental changes, which together have resulted in the Company recording a significant amount of impairment charges with respect to acquired intangible assets from its acquisitions, including intellectual property rights and goodwill. Management has focused the discussion and analysis below on comparing to the most recent quarters presented above in order to describe the most current business trends that have occurred in the first quarter of 2018.
Revenues in the first quarter of 2018 were $152,264 which consisted of $112,950 from the Concordia International segment, and $39,314 from the Concordia North America segment. Revenues during the fourth quarter of 2017 were $150,205 which consisted of $113,663 from the Concordia International segment, and $36,542 from the Concordia North America segment. Revenue from the Concordia International segment decreased by $713, or 1%, primarily due to the impact of: (i) $1,992 lower revenue from Fusidic Acid; (ii) $1,355 lower revenue from Carbimazole; and (iii) $1,188 lower revenue from Hydralazine Hcl, partially offset by: (i) a $5,492 increase in revenue as a result of the GBP strengthening against the USD; (ii) $1,505 higher revenue from Nitrofurantoin; and (iii) $1,442 higher revenue from Agripressin. The remaining decrease was primarily due to general competitive market pressures across the Concordia International segment's product portfolio. The Concordia North America segment revenue increase of $2,772 is primarily due to $3,737 higher revenue from the Plaquenil® authorized generic product. Donnatal® continues to experience competitive pressure from a non-FDA approved product being distributed by a third party and most recently the launch of an additional competitive product in the second quarter of 2017. Refer to the "Litigation and Arbitration" section of this MD&A.
Gross profit and adjusted gross profit in the first quarter of 2018 increased by $906 compared to the fourth quarter of 2017. Gross profit as a percentage of revenue in the first quarter of 2018 was 66% compared with the fourth quarter of 2017 of 67%. The decrease in gross profit as a percentage of revenue is primarily due to the shift in product mix within the Concordia North America segment as gross profit as a percentage of revenue within the Concordia International segment was flat.
Net loss from continuing operations during the first quarter of 2018 compared to the fourth quarter of 2017, decreased by $376,079. The decrease in net loss is primarily due to the fourth quarter of 2017 including impairment charges totaling $207,662, and $143,969 higher accretion expenses as a result of accelerating the accretion of deferred financing costs.
|
| |
| Concordia Management's Discussion and Analysis | Page 14 |
Adjusted EBITDA in the first quarter of 2018 of $72,024 consisted of $51,748 related to Concordia International, $24,273 related to Concordia North America, offset by $3,997 related to Corporate expenses. This is compared with fourth quarter 2017 Adjusted EBITDA of $70,778, which consisted of $56,116 related to Concordia International, $19,880 related to North America, offset by $5,218 related to Corporate expenses. Adjusted EBITDA for the first quarter of 2018 was $1,246 higher than the fourth quarter of 2017. The net increase of total adjusted EBITDA of $1,246 is primarily due to favourable foreign exchange rate movements of $2,680 and $1,221 lower Corporate expenses, partially offset by net product declines as described above.
|
| |
| Concordia Management's Discussion and Analysis | Page 15 |
8 Balance Sheet Analysis
|
| | | | | | | | |
| As at (in $000's) | Mar 31, 2018 |
| Dec 31, 2017 |
| Change |
| $ | % |
| Working capital | 243,277 |
| 281,288 |
| (38,011 | ) | (14 | )% |
| Long-lived assets | 1,740,063 |
| 1,752,261 |
| (12,198 | ) | (1 | )% |
| Other current liabilities | 3,824,774 |
| 3,804,684 |
| 20,090 |
| 1 | % |
| Long-term liabilities | 138,597 |
| 141,844 |
| (3,247 | ) | (2 | )% |
| Shareholder's deficit | (1,977,056 | ) | (1,910,513 | ) | (66,543 | ) | 3 | % |
Working capital
Concordia defines working capital as total current assets less accounts payable and accrued liabilities, income taxes payable and provisions. The $38,011 decrease in working capital from December 31, 2017 to March 31, 2018 is primarily due to the following factors:
| |
| • | Inventory decreased by $6,236 primarily due to $2,390 and $3,846 lower inventory on hand within the Concordia International and Concordia North America segments, respectively. These decreases are primarily due to product sales during the first quarter of 2018; |
| |
| • | Accounts payable and accrued liabilities increased by $53,993. The increase in accounts payable and accrued liabilities is primarily due to an increase in interest payable on the Company's debt of $52,563. Interest payments on Unsecured Debt have not been paid as a result of the CBCA Proceedings resulting in a stay of interest and principal payments on these facilities; and |
| |
| • | Provisions decreased by $1,875. The decrease is primarily due to lower expected returns within the Concordia North America segment. |
Offset primarily by:
| |
| • | Cash and cash equivalents increased by $16,804 primarily due to cash from operating activities of $50,558, primarily offset by debt amortization and interest payments of $38,859, as further discussed in the "Liquidity and Capital Realignment" section of this MD&A; and |
| |
| • | Accounts receivable increased by $2,054. Concordia International segment accounts receivable increased by $3,173 primarily as a result of foreign exchange rate movements. Concordia North America accounts receivable decreased by $1,119 primarily due to the timing of receipts from customers. |
Long-lived assets
Long-lived assets consist of fixed assets, intangible assets and goodwill. The $12,198 decrease in long-lived assets from December 31, 2017 to March 31, 2018 is primarily due to intangible asset amortization recorded during the first quarter of 2018 of $65,607, partially offset by a $52,015 increase due to foreign exchange translation. This increase from foreign exchange translation is a result of the movement in the GBP/USD period end exchange rates from 1.3494 as at December 31, 2017 to 1.4037 as at March 31, 2018.
Other current liabilities
Other current liabilities consist of the current portion of long-term debt, purchase consideration payable and cross currency swap liability. The $20,090 increase from December 31, 2017 to March 31, 2018 is primarily due to the increase in the current portion of long-term debt of $14,939 as a result of $26,221 foreign exchange rate movement from December 31, 2017 to March 31, 2018, partially offset by $11,282 of principal repayments.
Long term liabilities
Long-term obligations consist of purchase consideration payable, other liabilities and deferred income tax liabilities. The $3,247 decrease in long term liabilities from December 31, 2017 to March 31, 2018 is primarily due to the decrease in deferred tax liabilities of $1,491 due to the reversal of certain deferred tax liabilities in respect of assets recorded as a result of purchase price accounting and changes to the carrying value of certain assets due to their impairment and/or changes in the applicable foreign exchange rate.
|
| |
| Concordia Management's Discussion and Analysis | Page 16 |
Shareholders’ deficit
Shareholders’ deficit increased by $66,543 from December 31, 2017 to March 31, 2018. The increase is primarily related to:
| |
| • | A net loss for the first quarter of 2018 of $55,694; and |
| |
| • | A net foreign exchange impact of $12,121 from the translation of the Concordia International segment, the Currency Swaps and the GBP denominated term loan. |
Offset primarily by:
| |
| • | A $1,272 net change in equity for share based compensation expense, issuance of options, vesting of RSUs and related reversal of deferred income tax assets. |
|
| |
| Concordia Management's Discussion and Analysis | Page 17 |
9 Liquidity and Capital Realignment
Realignment of Capital Structure and Going Concern
During the 2017 fiscal year, the Company announced as part of its long-term strategy an objective to realign its capital structure, which includes an intention to significantly reduce the Company’s existing secured and unsecured debt obligations. On October 20, 2017, as part of the Company’s efforts to realign its capital structure, the Company and one of its wholly-owned direct subsidiaries commenced a court proceeding under the CBCA. The CBCA is a Canadian corporate statute that includes provisions that allow Canadian corporations to restructure certain debt obligations, and is not a bankruptcy or insolvency statute. The preliminary interim order issued by the Court provides a stay of proceedings, which continues to be effective, against any third party that is party to, or a beneficiary of, any loan, note, commitment, contract or other agreement with the Company or any of its subsidiaries, including the Company's debtholders, from exercising any rights or remedy or any proceeding, including, without limitation, terminating, demanding, accelerating, setting-off, amending, declaring in default or taking any other action under or in connection with any loan, note, commitment, contract, or other agreement of the Company and its subsidiaries on the terms set out in the Court order.
In connection with the Company's efforts to realign its capital structure and as contemplated by the CBCA Proceedings, the Company has elected to not make scheduled payments on the following debt obligations: payments under its 7% unsecured senior notes; payments under its 9.5% unsecured senior notes; and payments under its unsecured extended equity bridge facility. During the CBCA Proceedings, the Company has been, and intends to, continue to make scheduled ordinary course interest and principal payments under its secured debt facilities, as applicable. The commencement of the CBCA Proceedings and non-payment of the scheduled payments noted above resulted in events of default under the Company's credit facilities and the Company's Currency Swaps, and therefore the outstanding debt and cross currency swap liabilities have been presented as current liabilities. Refer to the "Lending Arrangements and Debt" section of this MD&A for additional details associated with events of default applicable under certain of the Company's credit facilities and Currency Swaps.
Proposed Recapitalization Transaction
On May 2, 2018, the Company announced a proposed Recapitalization Transaction to realign its capital structure. The details of the Recapitalization Transaction are described within the "Recent Events" section of this MD&A, and in the Company's term sheet dated as of May 1, 2018, the support agreement between the Company, certain subsidiaries of the Company and certain holders of the Company’s secured debt and unsecured debt dated as of May 1, 2018, and the subscription agreement between the Company, certain subsidiaries of the Company, and certain holders of the Company’s secured debt and unsecured debt dated as of May 1, 2018. A management information circular outlining all key terms of the Recapitalization Transaction is expected to be mailed to relevant stakeholders prior to the scheduled June 19, 2018 Meetings where secured and unsecured debtholders and shareholders will vote on the approval of, among other things, the Company's CBCA Plan. The Company believes the Recapitalization Transaction, once completed, will realign the Company's capital structure, and significantly reduce the Company's outstanding debt.
Upon completion of the Recapitalization Transaction, the Company expects to have New Secured Debt of approximately $1.4 billion and cash on hand in excess of $200 million, after payment of related advisor fees and recapitalization transaction costs.
Going Concern
Future liquidity and operations of the Company are dependent on the ability of the Company to restructure its debt obligations and to generate sufficient operating cash flows to fund its on-going operations. If the Company does not complete the realignment of its capital structure through the CBCA Plan described in the "Recent Events" section of this MD&A, it will be necessary to pursue other restructuring strategies, which may include, among other alternatives, proceedings under the Companies Creditors Arrangement Act and / or a filing under the United States Bankruptcy Code. The Company may not be able to restructure and reduce its debt obligations and this results in a material uncertainty that may cast significant doubt upon the Company’s ability to continue as a going concern.
The Company had approximately $344 million of cash on hand as of March 31, 2018 (December 31, 2017 - $327 million) and believes it has sufficient liquidity in the near term to operate its business and meet its ordinary course financial commitments while it works toward implementing the Recapitalization Transaction.
|
| |
| Concordia Management's Discussion and Analysis | Page 18 |
Sources and Uses of Cash |
| | | | |
| | Three months ended |
| (in $000’s) | Mar 31, 2018 |
| Mar 31, 2017 |
|
| Cash flow from Operating Activities | 50,558 |
| 86,204 |
|
| Cash flow used in Investing Activities | (1,583 | ) | (317 | ) |
| Cash flow used in Financing Activities | (38,859 | ) | (151,515 | ) |
| Total | 10,116 |
| (65,628 | ) |
The Company's business continues to generate cash flow from operating activities. Cash flows from operations represent net income adjusted for changes in working capital, non-cash items and excludes interest paid as this is recorded within cash used in financing activities.
Cash flow from operating activities for the first quarter of 2018 decreased by $35,646 compared to the corresponding period in 2017. The decrease is primarily due to $14,309 lower gross profit from both segments, $10,278 higher acquisition related, restructuring and other costs primarily as a result of the Company's capital realignment initiative and $13,546 lower favourable working capital movements.
Cash flow used in financing activities during the three months ended March 31, 2018 is comprised of: $27,577 of contractual interest payments; and $11,282 of scheduled long-term debt principal repayments.
Cash and Capital Management
The purpose of cash and capital management is to ensure that there is sufficient cash to meet all the financial commitments and obligations of the Company as they come due. Since inception, the Company has financed its cash requirements primarily through the issuances of securities, short-term borrowings, long-term debt as well as cash flows generated from operations.
Liquidity risk is the risk that the Company may encounter difficulty meeting obligations associated with financial liabilities. The Company manages liquidity risk through the management of its capital structure.
As described above and in the "Recent Events" section of this MD&A, the Company's capital management plan was to enter into the CBCA Proceedings to realign its capital structure. During this period, the Company has been, and intends to, continue to make scheduled ordinary course interest and principal payments under its Secured Debt facilities, as applicable. The Company has not made scheduled payments under its Unsecured Debt facilities As a result of certain events associated with the CBCA Proceedings, the Company may not have the ability to transfer certain funds, which could have an impact on the Company's liquidity.
In managing the Company’s capital, Management estimates future cash requirements by preparing annual financial forecasts for review and approval by the Board. The financial forecasts are reviewed and updated periodically and establish approved activities for the year and estimates the costs associated with those activities. Forecast to actual variances are prepared and reviewed by Management and are presented regularly to the Board.
|
| |
| Concordia Management's Discussion and Analysis | Page 19 |
10 Lending Arrangements and Debt
|
| | | | |
| As at (in $000’s) | Mar 31, 2018 |
| Dec 31, 2017 |
|
| Term Loan | | |
| - USD term loan | 1,054,625 |
| 1,061,500 |
|
| - GBP term loan | 672,900 |
| 651,086 |
|
| Extended Bridge Facility | 100,832 |
| 100,832 |
|
| 9.5% Senior Notes | 790,000 |
| 790,000 |
|
| 7% Senior Notes | 735,000 |
| 735,000 |
|
| 9% Secured Notes | 350,000 |
| 350,000 |
|
| Total Debt | 3,703,357 |
| 3,688,418 |
|
| Less: Current Portion | 3,703,357 |
| 3,688,418 |
|
| Long-Term Debt | — |
| — |
|
As at March 31, 2018, approximately 82% of total long term debt was denominated in USD (December 31, 2017 - 82%) and 18% denominated in GBP (December 31, 2017 - 18%).
The commencement of the CBCA Proceedings on October 20, 2017 resulted in an event of default under the Credit Agreement, indentures governing the Company's 9% senior secured notes and 9.5% unsecured notes and the Currency Swaps, which defaults are subject to the stay of proceedings granted by the Court. As a result of the foregoing events of default, a cross default was triggered under the indenture governing the 7% unsecured senior notes and the unsecured extended equity bridge facility, however any demand for payment of this debt has been stayed by the preliminary interim order granted by the Court in the CBCA Proceedings. As a result of these events all debt arrangements are presented as current liabilities. On October 20, 2017, the Company was notified by the counterparty to the Currency Swaps that one or more events of default occurred under the swap agreements as a result of the Company obtaining a preliminary interim order from the Court pursuant to the arrangement provisions of the CBCA. As a result of the foregoing, the counterparty to the Currency Swaps designated October 23, 2017 as the early termination date with respect to all transactions under the Currency Swaps. As part of the Recapitalization Transaction, the Company has agreed to the amount of the Currency Swaps liability ($114,431), which amount will be addressed in the same manner as the Secured Debt under the Recapitalization Transaction.
During the three months ended March 31, 2018, the Company made $11,282 of principal repayments and paid $27,577 of cash interest expense on its secured debt facilities, including the Currency Swaps, as applicable.
Details of the Company's lending arrangements are further disclosed in the notes to the consolidated financial statements for the first quarter of 2018.
The following table presents repayments of long-term debt principal, interest payments on long-term debt, payments on cross currency swap liability and purchase consideration on an undiscounted basis:
|
| | | | | | | | | | | | | | |
| (in $000's) | < 3 months |
| 3 to 6 months |
| 6 months to 1 year |
| 1 to 2 years |
| 2 to 5 years |
| Thereafter |
| Total |
|
| | | | | | | | |
Long-term debt (1) | 3,703,357 |
| — |
| — |
| — |
| — |
| — |
| 3,703,357 |
|
Interest on long-term debt(2) | 159,131 |
| — |
| — |
| — |
| — |
| — |
| 159,131 |
|
| Cross currency swap liability | 114,431 |
| — |
| — |
| — |
| — |
| — |
| 114,431 |
|
Purchase consideration(3) | 6,986 |
| — |
| — |
| — |
| 3,770 |
| — |
| 10,756 |
|
| Total | 3,983,905 |
| — |
| — |
| — |
| 3,770 |
| — |
| 3,987,675 |
|
| |
| (1) | All long-term debt as at March 31, 2018 has been presented as a current liability. Refer to the discussion above on long-term debt classification and the CBCA Proceedings. |
| |
| (2) | The contractual interest amount as at March 31, 2018 reflects the accrued interest payable on long-term debt. |
| |
| (3) | Refer to the "Contractual Obligations" section of this MD&A for further information. |
|
| |
| Concordia Management's Discussion and Analysis | Page 20 |
11 Contractual Obligations
Contractual Obligations
The Company enters into contractual obligations in the normal course of business. There have been no significant changes to the specified contractual obligations during the three months ended March 31, 2018. Details of the contractual obligations are further disclosed in the notes to the consolidated financial statements for the three months ended March 31, 2018.
During the three months ended March 31, 2018, the Company did not engage in any off-balance sheet financing transactions.
|
| |
| Concordia Management's Discussion and Analysis | Page 21 |
12 Related Party Transactions
Compensation for directors and key management, consisting of salaries, performance and retention bonuses, other benefits, severance and director fees for the first quarter of 2018 amounted to $1,946 (2017 - $1,315).
Share based compensation expense recorded for key management and directors, for the first quarter of 2018 amounted to $494 (2017 - $1,883).
Certain current employees of the Concordia International segment had an equity interest in the Concordia International segment at the time of its sale to the Company. As a result, pursuant to the share purchase agreement entered into by the Company in connection with the acquisition of the Concordia International segment, these employees received a portion of the consideration paid by the Company to the vendors of the Concordia International segment (including the earn-out consideration paid in December 2016 and February 2017, respectively).
|
| |
| Concordia Management's Discussion and Analysis | Page 22 |
13 Non-IFRS Financial Measures
This MD&A makes reference to certain non-IFRS measures. These non-IFRS measures are not recognized measures under IFRS and do not have a standardized meaning prescribed by IFRS, and are therefore unlikely to be comparable to similar measures presented by other companies. When used, these measures are defined in such terms as to allow the reconciliation to the closest IFRS measure. These measures are provided as additional information to complement those IFRS measures by providing further understanding of the Company’s results of operations from Management’s perspective. Accordingly, they should not be considered in isolation nor as a substitute to the Company’s financial information reported under IFRS. Management uses non-IFRS measures such as EBITDA, Adjusted EBITDA, Adjusted Gross Profit, Adjusted Net Income and Adjusted EPS to provide investors with supplemental information of the Company’s operating performance and thus highlight trends in the Company’s core business that may not otherwise be apparent when relying solely on IFRS financial measures. Securities analysts, investors and other interested parties frequently use non-IFRS measures in the evaluation of issuers. Management also uses non-IFRS measures in order to facilitate operating performance comparisons from period to period, prepare annual operating budgets, and to assess its ability to meet future debt service requirements, in making capital expenditures, and to consider the business's working capital requirements.
The definition and reconciliation of Adjusted Gross Profit, EBITDA, Adjusted EBITDA, Adjusted Net Income and Adjusted EPS used and presented by the Company to the most directly comparable IFRS measures follows below.
Adjusted Gross Profit
Adjusted Gross Profit is defined as gross profit adjusted for non-cash fair value increases to the cost of acquired inventory from a business combination. Under IFRS, acquired inventory is required to be written-up to fair value at the date of acquisition. As this inventory is sold the fair value adjustment represents a non-cash cost of sale amount that has been excluded in adjusted gross profit in order to normalize gross profit for this non-cash component.
|
| | | | |
| | Three months ended |
| (in $000’s) | Mar 31, 2018 |
| Mar 31, 2017 |
|
| Gross profit per financial statements | 101,106 |
| 115,415 |
|
| Add back: Fair value adjustment to acquired inventory | — |
| 311 |
|
| Adjusted Gross profit | 101,106 |
| 115,726 |
|
EBITDA
EBITDA is defined as net income / loss adjusted for interest and accretion expense, interest income, income taxes, depreciation and amortization. Management uses EBITDA to assess the Company’s operating performance.
Adjusted EBITDA
Adjusted EBITDA is defined as EBITDA adjusted for certain charges including costs associated with acquisitions, restructuring initiatives, and other costs (which includes onerous contract costs and direct costs associated with contractual terminations), management retention costs, non-operating gains / losses, integration costs, legal settlements (net of insurance recoveries) and related legal costs, non-cash items such as unrealized gains / losses on derivative instruments, share based compensation, fair value changes including purchase consideration and derivative financial instruments, asset impairments, fair value increases to inventory arising from purchased inventory from a business combination, gains / losses from the sale of assets and unrealized gains / losses related to foreign exchange. Management uses Adjusted EBITDA, among other Non-IFRS financial measures, as the key metric in assessing business performance when comparing actual results to budgets and forecasts. Management believes Adjusted EBITDA is an important measure of operating performance and cash flow, and provides useful information to investors because it highlights trends in the underlying business that may not otherwise be apparent when relying solely on IFRS measures.
|
| |
| Concordia Management's Discussion and Analysis | Page 23 |
|
| | | | |
| | Three months ended |
| (in $000’s) | Mar 31, 2018 |
| Mar 31, 2017 |
|
| Net loss | (55,694 | ) | (78,824 | ) |
| | | |
| Interest and accretion expense | 80,122 |
| 92,541 |
|
| Interest income | (706 | ) | (18,479 | ) |
| Income taxes | 4,704 |
| 4,489 |
|
| Depreciation | 470 |
| 488 |
|
| Amortization of intangible assets | 65,607 |
| 56,717 |
|
| EBITDA | 94,503 |
| 56,932 |
|
| Fair value adjustment to acquired inventory | — |
| 311 |
|
| Acquisition related, restructuring and other | 15,494 |
| 5,216 |
|
| Share-based compensation | 1,267 |
| 2,952 |
|
| Fair value (gain) loss on purchase consideration and derivatives | 425 |
| 27,506 |
|
| Foreign exchange (gain) loss | 1,341 |
| 990 |
|
| Unrealized foreign exchange (gain) loss | (41,006 | ) | (9,665 | ) |
| Adjusted EBITDA | 72,024 |
| 84,242 |
|
|
| |
| Concordia Management's Discussion and Analysis | Page 24 |
Adjusted Net Income and Adjusted EPS
Adjusted EPS is defined as adjusted net income divided by the weighted average number of fully diluted shares outstanding. Adjusted net income is defined as net income (loss) adjusted for certain charges including costs associated with acquisitions, restructuring initiatives, and other costs (which includes onerous contract costs and direct costs associated with contractual terminations), management retention costs, non-operating gains / losses, integration costs, legal settlements (net of insurance recoveries) and related legal costs, non-cash items such as unrealized gains / losses on derivative instruments, share based compensation, fair value changes including purchase consideration and derivative financial instruments, asset impairments, fair value increases to inventory arising from purchased inventory from a business combination, gains / losses from the sale of assets and unrealized gains / losses related to foreign exchange, non-cash accretion expense and the tax impact of the above items. Management believes Adjusted EPS is an important measure of operating performance and cash flow, and provides useful information to investors. |
| | | | | | | | | | | | | | | | |
| In $000’s, except per share amounts | Q1-2018 |
| Q4-2017 |
| Q3-2017 |
| Q2-2017 |
| Q1-2017 |
| Q4-2016 |
| Q3-2016 |
| Q2-2016 |
|
| |
| | |
|
| | |
| Weighted average number of fully diluted shares | 53,685,667 |
| 53,747,659 |
| 52,481,324 |
| 53,732,989 |
| 52,690,190 |
| 51,623,190 |
| 51,862,590 |
| 52,081,161 |
|
| Net income (loss) | (55,694 | ) | (431,773 | ) | (69,485 | ) | (1,010,653 | ) | (78,824 | ) | (663,761 | ) | (75,147 | ) | (570,384 | ) |
| Adjustments: | |
|
| |
|
|
|
|
|
|
|
|
|
|
| Fair value adjustment to acquired inventory | — |
| — |
| — |
| — |
| 311 |
| 394 |
| 1,506 |
| 869 |
|
| Share-based compensation | 1,267 |
| 285 |
| 2,999 |
| 2,475 |
| 2,952 |
| 3,438 |
| 10,069 |
| 8,889 |
|
| Acquisition related, restructuring and other | 15,494 |
| 21,129 |
| 14,266 |
| 6,167 |
| 5,216 |
| 20,309 |
| 4,251 |
| 7,860 |
|
| Depreciation | 470 |
| 475 |
| 499 |
| 500 |
| 488 |
| 512 |
| 528 |
| 469 |
|
| Amortization of intangible assets | 65,607 |
| 51,162 |
| 51,076 |
| 67,470 |
| 56,717 |
| 41,148 |
| 42,715 |
| 52,361 |
|
| Impairments | — |
| 207,662 |
| — |
| 987,103 |
| — |
| 562,105 |
| 3,062 |
| 567,076 |
|
| Foreign exchange (gain) loss | (39,665 | ) | (8,967 | ) | (23,184 | ) | (30,514 | ) | (8,675 | ) | 84,075 |
| 55,666 |
| (7,816 | ) |
| Fair value (gain) loss on purchase consideration and derivatives | 425 |
| 41,983 |
| 21,357 |
| 20,140 |
| 27,506 |
| (20,599 | ) | (323 | ) | 6,288 |
|
| Gain on debt settlement | — |
| (21,188 | ) | — |
| — |
| — |
| — |
| — |
| — |
|
| Interest accretion | — |
| 140,254 |
| 7,995 |
| 8,381 |
| 7,461 |
| 7,453 |
| 7,348 |
| 7,692 |
|
| Legal settlement and related legal cost | — |
| — |
| — |
| — |
| — |
| 783 |
| — |
| 13,463 |
|
Tax adjustments (1) | 2,146 |
| (15,925 | ) | (2,621 | ) | (40,930 | ) | (1,484 | ) | (29,125 | ) | (14,047 | ) | (15,052 | ) |
| Adjusted net income | (9,950 | ) | (14,903 | ) | 2,902 |
| 10,139 |
| 11,668 |
| 6,732 |
| 35,628 |
| 71,715 |
|
| Adjusted EPS diluted, continuing operations | (0.19 | ) | (0.28 | ) | 0.06 |
| 0.19 |
| 0.22 |
| 0.13 |
| 0.69 |
| 1.38 |
|
Notes:
(1) The Company has included in tax adjustments the current and deferred income taxes presented in the consolidated statements of income (loss) to the extent that these relate to adjustments made to net income (loss) from continuing operations. The income taxes presented in the consolidated statements of income (loss), after including the tax adjustments, represents the Company’s estimate of the income taxes in respect of adjusted net income ("Tax on Adjusted Net Income"). Tax on Adjusted Net Income does not represent the Company’s expectation of its current cash income tax obligations as such obligations are further impacted by: (i) the tax impact of certain adjustments made to net income (loss) from continuing operations but which do impact current cash income tax obligations, e.g., the tax impact of adjustments for stock based compensation, depreciation and amortization; and (ii) when such income tax obligations are required to be paid, which is a function of the laws applicable in the jurisdiction to which the payment is due.
|
| |
| Concordia Management's Discussion and Analysis | Page 25 |
14 Critical Accounting Estimates
The preparation of financial statements in accordance with IFRS requires management to make estimates, judgments and assumptions that affect reported assets, liabilities, revenues and expenses, gains and losses, and disclosures of contingencies. These estimates and assumptions are subject to change based on experience and new information. Critical accounting estimates are those that require management to make assumptions about matters that are highly uncertain at the time the estimate is made. Critical accounting estimates are also those estimates which, where a different estimate could have been used or where changes in the estimate that are reasonably likely to occur, would have a material impact on the company’s financial condition, changes in financial condition or financial performance. Critical accounting estimates and judgments are reviewed annually by the Audit Committee of the Board of Directors.
A detailed description of the Company’s critical accounting estimates is provided in Note 4 of the consolidated financial statements for the year ended December 31, 2017 and in the "Critical Accounting Estimates" section of the Company's MD&A for the year ended December 31, 2017, dated March 8, 2018 (the "2017 Annual MD&A").
Change in estimate
During the first quarter of 2018, the Company assessed the use of the straight line amortization method for certain intangible assets in both reportable segments and determined that, based on recent developments and historical patterns of commercial benefit, certain assets should be amortized based on a declining balance model to align with corresponding expected future cash flows. Specifically, the Company determined that this method of amortization better reflects the pattern in which acquired product rights and manufacturing processes future economic benefits are expected to be realized by the Company.
This change in estimate resulted in an increase in amortization expense of approximately $18 million in the three month period ended March 31, 2018. The impact for the remainder of the year is estimated to be approximately $55 million.
Current and Future Accounting Pronouncements
Note 3 of the consolidated financial statements as at and for the three month period ended March 31, 2018 describes information relating to current and future significant accounting policies applicable to the Company. The Company adopted IFRS 15, "Revenue from Contracts with Customers" and IFRS 9, “Financial Instruments” on January 1, 2018.
|
| |
| Concordia Management's Discussion and Analysis | Page 26 |
15 Contingencies
Royalties
The Company has a commitment to pay royalties on certain products acquired from Shionogi Inc. in May 2013 and certain products acquired from Covis Pharma S.à R.L. on April 21, 2015, at certain prescribed rates. These royalties are payable on a quarterly basis. During the three month period ended March 31, 2018 the royalty expense was $101 (2017 - $408).
Litigation and Arbitration
From time to time, the Company becomes involved in various legal and administrative proceedings, which include product liability, intellectual property, commercial, antitrust, government and regulatory investigations, related private litigation and ordinary course employment-related issues. From time to time, the Company also initiates actions or files counterclaims. The Company could be subject to counterclaims or other suits in response to actions it may initiate. The Company believes that the prosecution of these actions and counterclaims is important to preserve and protect the Company, its reputation and its assets. Certain of these proceedings and actions are described below.
Unless otherwise indicated the Company cannot reasonably predict the outcome of these legal proceedings, nor can it currently estimate the amount of loss, or range of loss, if any, that may result from these proceedings. An adverse outcome in certain of these proceedings could have a material adverse effect on the Company's business, financial condition and results of operations, and could cause the market value of its common shares and/or debt securities to decline.
The Company and certain of its former executive officers are the subject of various class action complaints relating to the Company’s August 12, 2016 press release, whereby the Company revised its 2016 guidance. The complaints allege that the Company issued false and misleading statements to investors and/or failed to disclose that: the Company was experiencing a substantial increase in market competition against its drug Donnatal®, and other products; as a result, Concordia’s financial results would suffer, and Concordia would be forced to suspend its dividend; and as a result Concordia’s statements about its business, operations and prospects were materially false and misleading and/or lacked a reasonable basis at all relevant times. The class action lawsuits have been consolidated into a single case and a motion to dismiss this action was filed by the Company on February 20, 2017. On March 21, 2017, the plaintiffs in this action filed a response to the motion to dismiss, and on April 5, 2017 the Company filed a reply to plaintiffs' response. On July 28, 2017, the United States District Court, Southern District of New York denied the motion to dismiss in part and granted it in part. On February 7, 2018, the plaintiffs filed a motion for class certification. The Company filed an opposition to the plaintiffs' motion for class certification on March 12, 2018, and the plaintiffs filed a reply in support of class certification on March 28, 2018.
The Company and certain of its former executive officers were also subject to a class action complaint alleging that the Company made false and/or misleading statements, as well as, failed to disclose material adverse facts about the Company's business operations and prospects, in the Company's Registration Statement, Prospectus and Supplemental Prospectus issued in connection with the Company's secondary offering completed on September 30, 2015. Specifically, the claim alleged that the statements were false and/or misleading and/or failed to disclose that: (i) the Company was experiencing a substantial increase in market competition against Donnatal®, and other products; (ii) consequently the Company's financial results would suffer and the Company would be forced to suspend its dividends; and (iii) as a result of the foregoing, the defendant's statements about the Company's business operations and prospects were false and misleading and/or lacked a reasonable basis. On June 27, 2017, the plaintiff in this action voluntarily dismissed the complaint on a without prejudice basis.
The Company and certain of its former executive officers and a former director are subject to a securities class action filed in Quebec, Canada. The amended statement of claim alleges that the Company failed to disclose adverse material facts relating to, and misrepresented, among other things, the Company's business model, growth platforms, proforma revenues and dividend payments in certain disclosures from March 23, 2016 to August 11, 2016. This class action has not yet been certified nor has leave to bring a statutory claim under securities legislation yet been granted. On June 15, 2017, the plaintiff in the action discontinued their claim against the Company's Board of Directors (other than the one former director) and certain of its former executive officers.
On October 19, 2017, a statement of claim was filed in Ontario, Canada against the Company and certain of its former executive officers on behalf of all persons and entities, other than persons resident in Quebec, Canada, which alleges substantially the same claims as the Quebec action described above. This class action has not yet been certified nor has leave to bring a statutory claim under securities legislation yet been granted.
On October 25, 2016, the Company announced that the UK Competition and Markets Authority (CMA) commenced an investigation into various issues in relation to the UK pharmaceutical sector, and that the Concordia International segment was part of the inquiry. The CMA’s investigation includes matters that pre-date Concordia’s ownership of the Concordia International segment and relates to the Company’s pricing of three products. On May 31, 2017, the Company announced that the CMA notified the Company that it was continuing its investigation after an initial stop/go decision. On November 21, 2017, the Company announced that the CMA issued a statement of objections to the Company, and the former owners of the Concordia International segment, Hg Capital and Cinven, in relation to the pricing of one of the three
|
| |
| Concordia Management's Discussion and Analysis | Page 27 |
products, liothyronine, in the United Kingdom between November 2007 and at least July 2017. A statement of objections is a formal statement by the CMA that it considers that a competition infringement may have occurred. On February 15, 2018, the Company announced that the CMA notified the Company that it was closing its investigation related to Fusidic Acid, also one of the three products under investigation. On April 20, 2018, the Company responded in detail to the statement of objections.
On March 3, 2017, the Company announced that the CMA issued a statement of objections to a third party and the Company in relation to the supply of 10mg hydrocortisone tablets in the UK between 2013 and 2016. On May 26, 2017, the Company responded in detail to the statement of objections and on July 20, 2017 the Company attended an oral hearing to present the key points of its response to the CMA decision panel. This investigation includes matters that pre-date the Company’s ownership of the Concordia International segment.
On October 11, 2017, the Company announced that the CMA commenced additional investigations in relation to the UK pharmaceutical sector, and that the Concordia International segment and certain of its products are part of the inquiry. These investigations are at an early information gathering stage and the CMA has confirmed that, at this time, it has not reached a conclusion on whether competition law has been infringed. These investigations include matters that predate the Company's ownership of the Concordia International segment, and involve the following products: Carbimazole, Nitrofurantoin, Prochlorperazine, Dicycloverine, Trazodone and Nefopam.
The Company was subject to a class action proceeding in relation to one of its third party distributors purportedly faxing unsolicited advertisements to market Ulesfia® in violation of the Telephone Consumer Protection Act. On April 9, 2017, the court in this action dismissed the Company's motion to dismiss and on June 8, 2017 the court denied the Company's motion for reconsideration. On November 6, 2017, the court issued an order re-evaluating its previous finding of personal jurisdiction, which order required the plaintiffs in this action to make a new submission rebutting the evidence submitted by defendants showing that there is no personal jurisdiction. On December 1, 2017, the court dismissed this claim against the Company for lack of personal jurisdiction.
During the first quarter of 2016, the Company became aware that a third party had notified wholesalers, through listing services, of its intent to distribute and sell in certain US regions a non-FDA approved copy of Donnatal®. On January 6, 2016, the Company commenced a lawsuit against the third party and its principal owner claiming damages from such conduct, and on April 29, 2016 and May 3, 2016 commenced proceedings against two listing services for the continued listing of the products in their database. In May 2016, the Company became aware that this non-FDA approved product was introduced into certain US regions. On October 4, 2016 and November 16, 2016, the Company dismissed its claims against the listing services on a without prejudice basis, respectively. On March 15, 2017, the court ruled on the third party's motion to dismiss the Company's claim, denying such motion in part and granting it in part. On March 29, 2017, the third party filed its answer and counter claim in response to the Company's claim. On August 16, 2017, this third party filed a motion to amend its counterclaim to add factual allegations detailing the scope of the Company's campaign to disparage its products and interfere with its contractual and business relationships. On November 8, 2017, the court granted the Company's motion for leave to file its second amended complaint, permitting the Company to include its direct false advertising claim. The Company continues to pursue this lawsuit vigorously. In a similar lawsuit commenced against Method Pharmaceuticals, LLC ("Method") and its principal owner, the Company received a favorable jury verdict on April 21, 2016 and was awarded damages in the amount of approximately $733. On March 2, 2017, the United States District Court - Western District of Virginia, Charlottesville Division, granted the Company's motion for enhanced damages in part, to amend the judgment against Method and its principal owner to reflect an award of damages in the total amount of approximately $2.2 million. On March 30, 2017, Method filed a motion to reconsider the order on enhanced damages. On April 13, 2017, the Company filed an opposition to Method's motion to reconsider. On July 19, 2017, the court denied Method's motion to reconsider and further awarded the Company an additional $15 in costs. On August 30, 2017, Method filed a notice of appearance with the United States Court of Appeals for the Fourth Circuit to appeal the enhanced damages award. On February 1, 2018, Method and its principal owner and the Company settled the enhanced damages award.
During the second quarter of 2017, the Company became aware that an additional third party had launched a competitor product to Donnatal®. The Company continues to assess its legal rights against such third party.
On September 16, 2016, the Company announced the introduction of a bill into the U.K. House of Commons to amend and extend existing provisions of the National Health Service Act 2006 to enable the Secretary of State to help manage the cost of health service medicines. On April 27 2017, the U.K. government accorded Royal Assent to the Act. The Act introduces provisions in connection with controlling the cost of health service medicines and other medical supplies. The Act also introduces provisions in connection with the provision of pricing and other information by manufacturers, distributors and suppliers of those medicines and medical supplies. The Company continues to monitor the implementation of the Act. While the effects of the Act are unknown at this time, the Act could impose certain risks and uncertainties on the Company's operations and cash flows.
|
| |
| Concordia Management's Discussion and Analysis | Page 28 |
16 Outstanding Share Data
The authorized capital of the Company consists of an unlimited number of common shares. As at March 31, 2018 and May 15, 2018 the Company had, respectively, 51,283,574 and 51,283,800 common shares issued and outstanding. As at March 31, 2018 and May 15, 2018, there were, respectively, 1,377,500 and 1,336,667 stock options outstanding that entitle the holders thereof to purchase one common share of the Company per stock option held.
As at March 31, 2018 and May 15, 2018, the Company had, respectively, 2,358,571 and 288,791 unvested RSUs outstanding. Each RSU can be settled either in cash or common shares issued from treasury or a combination of cash and common shares issued from treasury at the sole discretion of the Company.
As at March 31, 2018 and May 15, 2018, the Company had 30,033 unvested DSUs outstanding. Each DSU can be settled either in cash or common shares issued from treasury or a combination of cash and common shares issued from treasury at the sole discretion of the Company.
|
| |
| Concordia Management's Discussion and Analysis | Page 29 |
17 Control Environment
Management is responsible for establishing and maintaining adequate Internal Control over Financial Reporting and disclosure controls and procedures as defined in the 2017 Annual MD&A.
Based on their evaluation as at March 31, 2018, Management concluded that the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the United States Securities Exchange Act of 1934, as amended (the "Exchange Act")), are effective to ensure that information required to be disclosed by the Company in reports that are filed or submitted to Canadian and U.S. securities authorities is recorded, processed, summarized and reported within the time periods specified in Canadian and U.S. securities laws. In addition, as at March 31, 2018, there were no changes in the internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) that occurred during the three month period ended March 31, 2018 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting. As a result, Management's conclusion on the effectiveness of the Company's internal control over financial reporting and its disclosure controls and procedures that were operating effectively as at December 31, 2017 has not changed.
Management will continue to periodically evaluate the Company’s disclosure controls and procedures and internal control over financial reporting, and will make any modifications from time to time as deemed necessary. Based on their inherent limitations, disclosure controls and procedures and internal control over financial reporting may not prevent or detect misstatements, and even those controls determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
|
| |
| Concordia Management's Discussion and Analysis | Page 30 |
18 Forward-looking Statements
Certain statements contained in this MD&A constitute "forward looking statements" within the meaning of the United States Private Securities Litigation Reform Act of 1995 and "forward-looking information" within the meaning of applicable Canadian securities laws (collectively, "forward-looking statements"), which are based upon the current internal expectations, estimates, projections, assumptions and beliefs of Management. Statements concerning the Company’s objectives, goals, strategies, intentions, plans, beliefs, assumptions, projections, predictions, expectations and estimates, and the business, operations, future financial performance and condition of the Company are forward-looking statements. This MD&A uses words such as "believe", "expect", "anticipate", "estimate", "intend", "may", "will", "would", "could", "plan", "create", "designed", "predict", "project", "seek", "ongoing", "increase", "upside" and similar expressions and the negative and grammatical variations of such expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Such forward-looking statements reflect the current beliefs of Management based on information currently available to them, and are based on assumptions and subject to risks and uncertainties. These statements are not guarantees of future performance and involve known and unknown risks, uncertainties and other factors that may cause actual results or events to differ materially from those anticipated in the forward-looking statements. In addition, this MD&A may contain forward-looking statements attributed to third-party industry sources.
By their nature, forward-looking statements involve numerous assumptions, known and unknown risks and uncertainties, both general and specific, that contribute to the possibility that the predictions, forecasts, projections or other characterizations of future events or circumstances that constitute forward-looking statements will not occur. Such forward-looking statements in this MD&A speak only as of the date of this MD&A. Forward-looking statements in this MD&A include, but are not limited to, statements with respect to:
| |
| • | the ability of the Company to compete against companies that are larger and have greater financial, technical and human resources than that of the Company, as well as other competitive factors, such as technological advances achieved, patents obtained and new products introduced by competitors; |
| |
| • | the performance of the Company’s business and operations; |
| |
| • | the Company’s capital expenditure programs; |
| |
| • | the future development of the Company, its growth strategy (including its DELIVER strategy) and the timing thereof; |
| |
| • | the acquisition strategy of the Company; |
| |
| • | the Company’s ability to achieve all of its estimated synergies as a result of cost reductions and/or integration initiatives; |
| |
| • | the estimated future contractual obligations of the Company; |
| |
| • | the Company’s future liquidity and financial capacity; |
| |
| • | the Company's ability to satisfy its financial obligations in future periods; |
| |
| • | the supply and market changes in demand for pharmaceutical products within the Company’s portfolio of pharmaceutical products; |
| |
| • | cost and reimbursement of the Company’s products; |
| |
| • | expectations regarding the Company’s ability to raise capital and/or restructure its capital structure; |
| |
| • | the availability and extent to which the Company’s products are reimbursed by government authorities and other third party payors, as well as the impact of obtaining or maintaining such reimbursement on the price of the Company’s products; |
| |
| • | the Company's business priorities, long-term growth strategy and/or stabilization programs or initiatives; |
| |
| • | changes in regulatory rules or practices in the U.S., United Kingdom or in other jurisdictions in which the Company sells products; |
| |
| • | changes in prescription recommendations or behaviours by clinical commissioning groups or other healthcare groups in the U.S., United Kingdom, or in any other jurisdictions in which the Company sells its products; |
| |
| • | the inclusion of the Company’s products on formularies or clinical commissioning groups providing guidance to prescribe the Company's products or the Company’s ability to achieve favourable formulary or clinical commissioning group status, as well as the impact on the price of the Company’s products in connection therewith; |
| |
| • | the acquisition, in-licensing and/or launch of new products including, but not limited to, the acceptance and demand for new pharmaceutical products, and the impact of competitive products and prices; |
| |
| • | the market size for the Company's products including, without limitation, its pipeline products; |
| |
| • | the Company's intention to reduce its debt; |
| |
| • | the Company's intention to realign its capital structure and the timing thereof; |
| |
| • | the Company's filing with the Court and the preliminary interim order and Interim Order under the CBCA; |
| |
| • | the CBCA process staying certain defaults under the Company's agreements, including its debt agreements; |
| |
| • | the ability of the Company to operate its business and satisfy its obligations to service providers, employees, suppliers and contractors in the ordinary course during the CBCA Proceedings; |
| |
| • | the Company's ability to complete the Recapitalization Transaction; and |
| |
| • | the going concern nature of the Company. |
|
| |
| Concordia Management's Discussion and Analysis | Page 31 |
With respect to the forward-looking statements contained in this MD&A, such statements are subject to certain risks, including those risks set forth below and in the Company's Annual Report on Form 20-F dated March 8, 2018, and the Company has made assumptions regarding, among other factors:
| |
| • | the ability of the Company to significantly reduce its debt and the terms of any such reduction; |
| |
| • | the ability of the Company to realign its capital structure and the timing thereof; |
| |
| • | the completion of the Recapitalization Transaction; |
| |
| • | third parties respecting the court order under the CBCA process or not taking steps to violate such order; |
| |
| • | the ability of the Company to maintain its listings on the NASDAQ and/or TSX, given the initial notification letter received from the NASDAQ, the current trading price of the Company's common shares and the Company's CBCA filing; |
| |
| • | alternatives available to the Company to strengthen the Company’s capital structure; |
| |
| • | the ability of the Company to create a financial foundation for the Company that will be able to support its long-term growth; |
| |
| • | the ability of the Company to achieve the Company’s financial goals including with respect to the nature of any agreement with its lenders; |
| |
| • | the ability of the Company to reduce the Company’s debt and interest payments; |
| |
| • | the ability of the Company to operate in the ordinary course during the CBCA process, including with respect to satisfying obligations to service providers, suppliers, contractors and employees; |
| |
| • | the CBCA process enabling the Company to stay defaults under its and its subsidiaries agreements, including debt agreements; |
| |
| • | the ability of the Company to continue as a going concern, and its ability to continue to realize its assets and discharge its liabilities and commitments; |
| |
| • | the ability of the Company to comply with its contractual obligations, including, without limitation, its obligations under debt arrangements; |
| |
| • | the Company's future liquidity position, and access to capital, to fund ongoing operations and obligations (including debt obligations); |
| |
| • | the ability of the Company to stabilize its business; |
| |
| • | the ability of the Company to implement and successfully achieve its business priorities in order to stabilize the Company's business and financial condition; |
| |
| • | the impact of the downgrade of the Company's corporate and debt ratings; |
| |
| • | the ability of the Company to execute its long-term growth strategy and/or not being delayed in executing such strategy; |
| |
| • | the successful licensing of products to third parties or to the Company, as applicable, to market and distribute such products on terms favourable to the Company; |
| |
| • | the ability of the Company to maintain key partnerships, and licensing and partnering arrangements, now and in the future; |
| |
| • | the ability of the Company to maintain its distribution networks and distribute its products effectively despite significant geographical expansion; |
| |
| • | the general regulatory environment in which the Company operates, including the areas of taxation, environmental protection, consumer safety and health regulation; |
| |
| • | the tax treatment of the Company and its subsidiaries and the materiality of legal and regulatory proceedings; |
| |
| • | the timely receipt of any required regulatory approvals, including in respect of the Company's restructuring process; |
| |
| • | the general economic, financial, market and political conditions impacting the industry and countries in which the Company operates; |
| |
| • | the ability of the Company to sustain or increase profitability, fund its operations with existing capital, and/or raise additional capital to fund its operations or future acquisitions; |
| |
| • | the ability of the Company to meet its financial forecasts and projections over the next twelve months and beyond; |
| |
| • | the ability of the Company to acquire or in-license any necessary technology, products or businesses and effectively integrate such acquisitions or such in-licensed technology or products; |
| |
| • | the development and clinical testing of products under development; |
| |
| • | the ability of the Company to obtain necessary approvals for commercialization of the Company’s products from the U.S. Food and Drug Administration ("FDA"), the U.K. Medicines and Healthcare products Regulatory Agency, the EMA or other regulatory authorities; |
| |
| • | future currency exchange and interest rates; |
| |
| • | reliance on third party contract manufacturers to manufacture the Company’s products on favourable terms; |
| |
| • | reliance on third party distributors to distribute the Company's products on favourable terms; |
| |
| • | reliance on development partners to develop the Company's products; |
| |
| • | the ability of the Company to generate sufficient cash flow from operations and to access existing and proposed credit facilities and the capital markets to meet its future obligations on acceptable terms; |
| |
| • | potential competition to the Company’s pharmaceutical products, including competition created by pharmaceutical parallel trade; |
| |
| • | the availability of raw materials and finished products necessary for the Company’s products; |
| |
| • | the impact of increasing competition; |
| |
| • | the impact of the entry of competitive products, including the timing of the entry of such products in the market place; |
| |
| • | the ability of the Company to obtain and retain qualified staff, equipment and services in a timely and efficient manner (particularly in light of the Company's efforts to restructure its debt obligations); |
|
| |
| Concordia Management's Discussion and Analysis | Page 32 |
| |
| • | the ability of the Company to maintain and enforce the protection afforded by any patents or other intellectual property rights; |
| |
| • | the ability of the Company to conduct operations in a safe, efficient and effective manner; |
| |
| • | the results of continuing and future safety and efficacy studies by industry and government agencies related to the Company’s products; |
| |
| • | the ability of the Company to retain members of the senior management team, including but not limited to, the officers of the Company; |
| |
| • | the ability of the Company to successfully market its products and services; |
| |
| • | clinical commissioning groups and/or other healthcare groups in the markets in which the Company sells its products, including the United Kingdom and United States, not making adverse prescribing recommendations against the Company's products; |
| |
| • | the impact of the United Kingdom's referendum through which voters supported a withdrawal from the European Union. A significant portion of the Company’s business is in the United Kingdom pharmaceutical industry and a significant portion of the Company's contract manufacturers are in mainland Europe. The United Kingdom’s exit from the European Union could result in a number of developments, including, without limitation, regulatory changes in the pharmaceutical industry, cross-border tariff and cost structure changes or loss of access to European Union global trade markets. Therefore, the United Kingdom’s exit from the European Union could have a material adverse effect on the Company’s business, financial condition and results of operations. In addition, the United Kingdom's exit from the European Union may result in a period of uncertainty while the terms of such exit are being negotiated; |
| |
| • | a significant number of the Company’s products are vulnerable to price competition driven by pharmaceutical parallel trade ("PPT"). PPT refers to pharmaceutical products that are put on the market in one country by the owner of the intellectual property rights to such products, or with the consent of the owner, that are subsequently imported into another country by a third party for secondary sale without the consent or authorization of the intellectual property right owner. Many of the Company’s products are distributed in the European Union, where PPT is common and, as a result, some of the Company’s products may be subject to price competition caused by PPT, which could have a material adverse effect on the Company’s business, financial condition and results of operations. In addition, PPT may restrict the Company’s ability to ensure that patients receive products designed for their local preferences and needs and possibly to the satisfaction of applicable governmental regulations in the jurisdiction of import. Moreover, as a result of PPT, packaging, manuals and instructions may be provided in a foreign language and may lack domestic telephone numbers and other important contact information for patient support, which may result in a diminished experience for the patient and diminished product reputation, which could have a material adverse effect on the Company’s business, financial condition and results of operations; |
| |
| • | the impact of the recently enacted UK Health Service Medical Supplies (Costs) Act on the Company's business, including, without limitation, on the pricing of the Company's products in the United Kingdom; and |
| |
| • | the Company’s operating results, financial condition and financial forecasts may fluctuate from period to period for a number of reasons, including as a result of events or occurrences disclosed in the Company’s public filings (including, without limitation, under the heading "Risk Factors" in the Company's Annual Report on Form 20-F dated March 8, 2018). As a result, the Company believes that quarter-to-quarter comparisons of results from operations or financial forecasts, or any other similar period-to-period comparisons, should not be construed as reliable indicators of the Company’s future performance. The events or occurrences described in the Company’s public filings, including, without limitation, under the heading "Risk Factors" in the Company's Annual Report on Form 20-F dated March 8, 2018, may cause the Company’s operating results and/or financial forecasts to fluctuate and such events or occurrences could have a material adverse effect on the Company’s business, financial condition and results of operations. In any period, the Company’s results may be below the expectations of market analysts and investors, which could cause the trading price of the Company’s securities to decline. |
Forward-looking statements contained in this MD&A are based on the key assumptions described herein. Readers are cautioned that such assumptions, although considered reasonable by the Company, may prove to be incorrect. Actual results achieved during the forecast period will vary from the information provided in this MD&A as a result of numerous known and unknown risks and uncertainties and other factors. The Company cannot guarantee future results.
Risks related to forward-looking statements include those risks referenced herein and in the Company’s other filings with the Canadian Securities Administrators and the SEC. Some of the risks and other factors which could cause actual results to differ materially from those expressed in the forward-looking statements contained in this MD&A include, but are not limited to, the risk factors described herein and included under the heading "Risk Factors" in the Company’s Annual Report on Form 20-F dated March 8, 2018, which is available on SEDAR, online at www.sedar.com and on EDGAR, online at www.sec.gov, as applicable.
Forward-looking statements contained in this MD&A are based on Management’s current plans, expectations, estimates, projections, beliefs and opinions and the assumptions relating to those plans, expectations, estimates, projections, beliefs and opinions may change. Management has included the summary of assumptions and risks related to forward-looking statements included in this MD&A for the purpose of assisting the reader in understanding Management’s current views regarding those future outcomes. Readers are cautioned that this information may not be appropriate for other purposes. Readers are cautioned that the lists of assumptions and risk factors contained herein are not exhaustive.
|
| |
| Concordia Management's Discussion and Analysis | Page 33 |
Neither the Company nor any other person assumes responsibility for the accuracy or completeness of the forward-looking statements contained herein.
Such forward-looking statements are made as of the date of this MD&A and the Company disclaims any intention or obligation to update publicly any such forward-looking statements, whether as a result of new information, future events or results or otherwise, other than as required by applicable securities laws.
All of the forward-looking statements made in this MD&A are expressly qualified by these cautionary statements and other cautionary statements or factors contained herein, and there can be no assurance that the actual results or developments will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, the Company.
Actual results, performance or achievements could differ materially from those expressed in, or implied by, any forward-looking statement in this MD&A, and, accordingly, investors should not place undue reliance on any such forward-looking statement. New factors emerge from time to time and the importance of current factors may change from time to time and it is not possible for Management to predict all of such factors, or changes in such factors, or to assess in advance the impact of each such factors on the business of Concordia or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statement contained in this MD&A.
Refer to the "Liquidity and Capital Realignment" and "Lending Arrangements and Debt" sections of this MD&A for a further discussion on the Company's financial position, liquidity and future outlook.
Trademarks
This MD&A includes trademarks that are protected under applicable intellectual property laws and are the property of Concordia or its affiliates or its licensors. Solely for convenience, the trademarks of Concordia, its affiliates and/or its licensors referred to in this MD&A may appear with or without the ® or TM symbol, but such references or the absence thereof are not intended to indicate, in any way, that the Company or its affiliates or licensors will not assert, to the fullest extent under applicable law, their respective rights to these trademarks. Any other trademarks used in this MD&A are the property of their respective owners.
Market and Industry Data
The market industry data contained in this MD&A is based upon information from independent industry and other publications and Concordia's Management's knowledge of, and experience in, the industry in which Concordia operates. None of the sources of market and industry data have provided any form of consultation, advice or counsel regarding any aspects of, or is in anyway whatsoever associated with, the matters described herein (including any related transactions). Market and industry data is subject to variations and cannot be verified with complete certainty due to limits on the availability and reliability of raw data at any particular point in time, the voluntary nature of the data gathering process or the limitations and uncertainties inherent in any statistical survey. Accordingly, the accuracy and completeness of this data are not guaranteed. Concordia has not in dependently verified any of the data from third party sources referred to in this MD&A and has not ascertained the underlying assumptions relied upon by such sources.
|
| |
| Concordia Management's Discussion and Analysis | Page 34 |