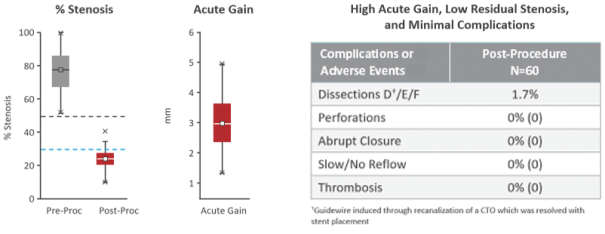
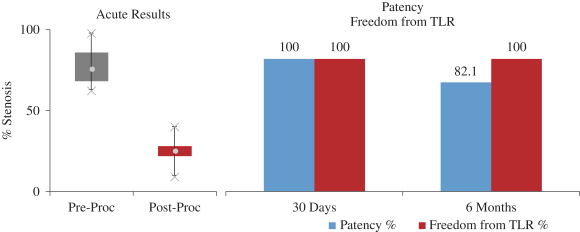
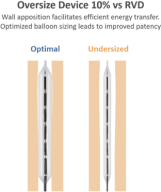
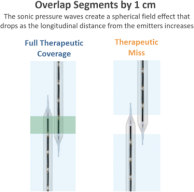
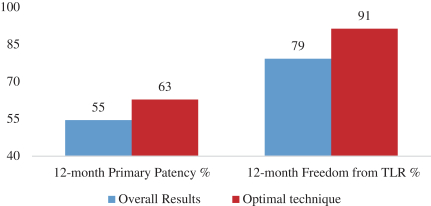
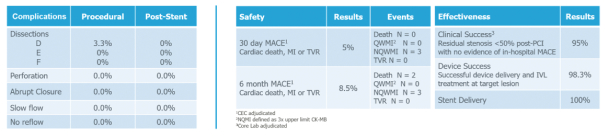
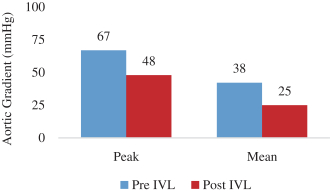
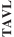and expect to make additional modifications in the future that we believe do not or will not require additional clearances or approvals. If the FDA disagrees and requires new clearances or approvals for these modifications, we may be required to recall and to stop selling or marketing such products as modified, which could harm our operating results and require us to redesign such products. In these circumstances, we may be subject to significant enforcement actions.
Our employees, independent contractors, consultants, commercial partners, distributors and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk that our employees, independent contractors, consultants, commercial partners, distributors and vendors may engage in fraudulent or illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates: (i) the laws of the FDA and other similar foreign regulatory bodies, including those laws requiring the reporting of true, complete and accurate information to such regulators; (ii) manufacturing standards; (iii) healthcare fraud and abuse laws in the United States and similar foreign fraudulent misconduct laws; or (iv) laws that require the true, complete and accurate reporting of financial information or data. These laws may impact, among other things, future sales, marketing and education programs. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, structuring and commissions, certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials.
We have adopted a code of business conduct and ethics, but it is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent these activities may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant fines or other sanctions, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, disgorgement, individual imprisonment, additional integrity reporting and oversight obligations, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings and curtailment of operations, any of which could adversely affect our ability to operate our business and our results of operations. Whether or not we are successful in defending against any such actions or investigations, we could incur substantial costs, including legal fees, and divert the attention of management in defending ourselves against any of these claims or investigations, which could have a material adverse effect on our business, financial condition and results of operations.
Environmental and health safety laws may result in liabilities, expenses and restrictions on our operations. Failure to comply with environmental laws and regulations could subject us to significant liability.
Federal, state, local and foreign laws regarding environmental protection, hazardous substances and human health and safety may adversely affect our business. Our research and development and manufacturing operations involve the use of hazardous substances and are subject to a variety of federal, state, local and foreign environmental laws and regulations relating to the storage, use, discharge, disposal and remediation of, as well as human exposure to, hazardous substances and the sale, labeling, collection, recycling, treatment and disposal of products containing hazardous substances. These operations are permitted by regulatory authorities, and the resultant waste materials are disposed of in material compliance with environmental laws and regulations. Using hazardous substances in our operations exposes us to the risk of accidental injury, contamination or other liability from the use, storage, importation, handling or disposal of hazardous materials. If our or our suppliers’ operations
49