reference from our most recent Annual Report on Form10-K and most recent Quarterly Report on Form10-Q, as well as any amendments thereto, filed with the SEC. You should not rely upon forward-looking statements as predictions of future events.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Except as required by law, we are under no duty to update or revise any of the forward-looking statements in this Exhibit 99.1, whether as a result of new information, future events or otherwise.
Company Overview
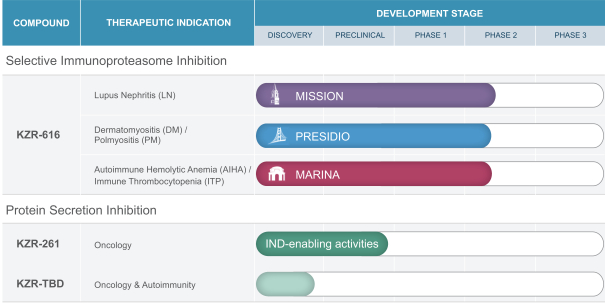
We are a clinical-stage biotechnology company, discovering and developing novel small molecule therapeutics to treat unmet needs in autoimmunity and cancer. Our lead product candidate, KZR-616, afirst-in-class selective immunoproteasome inhibitor, has completed testing in healthy volunteers, and we are now leveraging its broad therapeutic potential in three Phase 2 clinical trials across five separate autoimmune indications: lupus nephritis (the MISSION trial), autoimmune hemolytic anemia, or AIHA, immune thrombocytopenia, or ITP (the MARINA trial), dermatomyositis, or DM, and polymyositis, or PM (the PRESIDIO trial). We are also continuing to enroll patients in the Phase 1b portion of the MISSION trial, a Phase 1b/2 clinical trial in systemic lupus erythematosus, also known as lupus or SLE, and lupus nephritis.
We believe that the immunoproteasome is a validated target for the treatment of a wide variety of autoimmune diseases based on its ability to target cells in both the adaptive and innate immune system as bolstered by compelling published activity seenwith non-selective proteasome inhibitors administered to patients with severe autoimmune diseases. Based on results from our Phase 1a studies in healthy volunteers and the preliminary results from the Phase 1b portion of the MISSION trial,KZR-616 has largely avoided adverse effects associated with currentlymarketed non-selective proteasome inhibitors, as exhibited in clinical studies conducted by third parties, including side effects which we believe could prevent them from being utilized as a chronic treatment in autoimmune disorders. We intend todevelop KZR-616 to address chronic, severe and underserved autoimmune diseases.
Additionally, we are advancing our novel research platform targeting the Sec61 translocon and the protein secretion pathway to discover and develop small molecule therapeutics targeting oncology indications. Our first clinical candidate in thisprogram, KZR-261, has demonstrated broad anti-tumor activity in preclinical models of both solid and hematologicmalignancies. KZR-261 is undergoing laboratory studies and manufacturing activities in support of an investigational new drug, or IND, application, which we anticipate submitting to the FDA in the first quarter of 2021 for a Phase 1 clinical trial in solid tumors. We believe this discovery platform has the potential to yield oral small molecule candidates to act as cytotoxic anti-cancer agents or to block the secretion of novel targets of interest in immuno-oncology or inflammation and that, if successfully developed and approved, could serve as alternatives to currently marketed biologic therapeutics.
KZR-616: Selective Immunoproteasome Inhibitor
We believethat KZR-616 has potential to be developed for the treatment of multiple autoimmune disease indications. In the last decade, research directed by our Chief Scientific Officer, along with work performed in multiple academic laboratories, has led to over 15 peer-reviewed publications showing that selective immunoproteasome inhibition resulted in a broad anti-inflammatory response, reducing autoimmune disease in animal models of lupus, lupus nephritis, rheumatoid arthritis, inflammatory bowel disease, multiple sclerosis, Type 1 diabetes and other indications. This immunomodulatory response was broadly seen across many cell types of the immune system, including both T cells and B cells, and was demonstrated in a safe