
November 15, 2021 Uniquely Powerful Approaches to Tackling the Toughest Diseases SELECTIVE TARGETS. BROAD IMPACT. Kezar Life Sciences 1 Exhibit 99.1

This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “target,” and similar expressions (as well as other words or expressions referencing future events or circumstances) are intended to identify forward-looking statements. Each of these forward-looking statements involves substantial risks and uncertainties that could cause actual results to differ significantly from those expressed or implied by such forward-looking statements. Forward-looking statements contained in this presentation include, but are not limited to, statements about the company’s financial position and cash runway, statements about the potential use of our product candidates to treat patients, the association of data with treatment outcomes, the design, timing and progress of clinical trials, the expected timing of data disclosures, the likelihood that data, including interim or topline data, will support future development, the likelihood of obtaining regulatory approval for our product candidates, and the regulatory pathway and competitive landscape for our product candidates. Data from the MISSION Phase 2 clinical trial are preliminary and will require confirmation in additional patients as well as longer follow-up to draw any clinical conclusions. Interim top-line data and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data. Forward-looking statements in this presentation reflect Kezar's current beliefs and expectations. Many factors may cause differences between current expectations and actual results, including the availability of additional data, confirmation of data resulting from trial auditing and verification procedures, unexpected safety or efficacy data observed during preclinical or clinical studies, upon study completion, clinical trial enrollment rates that are lower than expected, changes in expected or existing competition, and changes in the regulatory environment. Other factors that may cause our actual results to differ from current expectations are discussed in Kezar’s most recent Form 10-K or Form 10-Q filed with the U.S. Securities and Exchange Commission (SEC), under the caption “Risk Factors” and elsewhere in such reports. Except as required by law, we assume no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available. Forward-Looking Statements and Interim Data Disclaimer 2
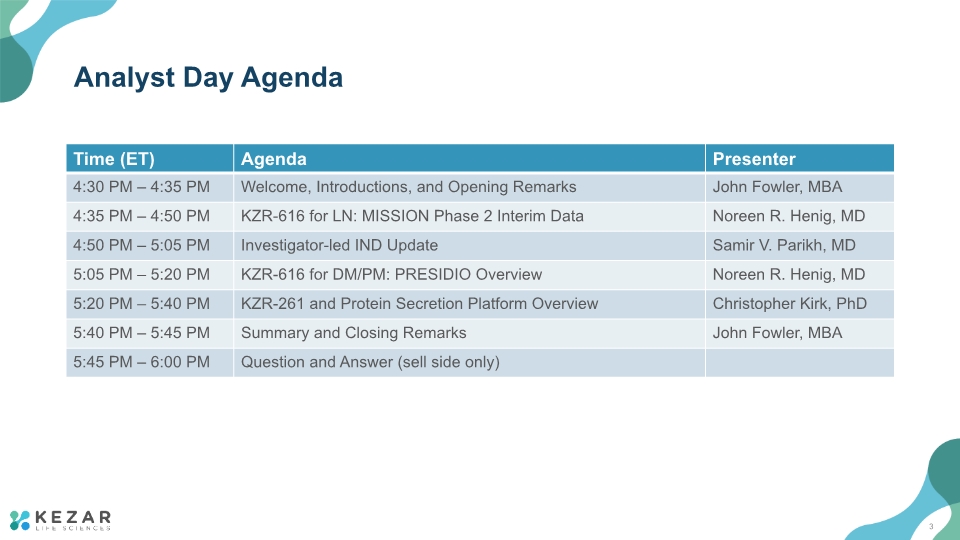
Analyst Day Agenda 3
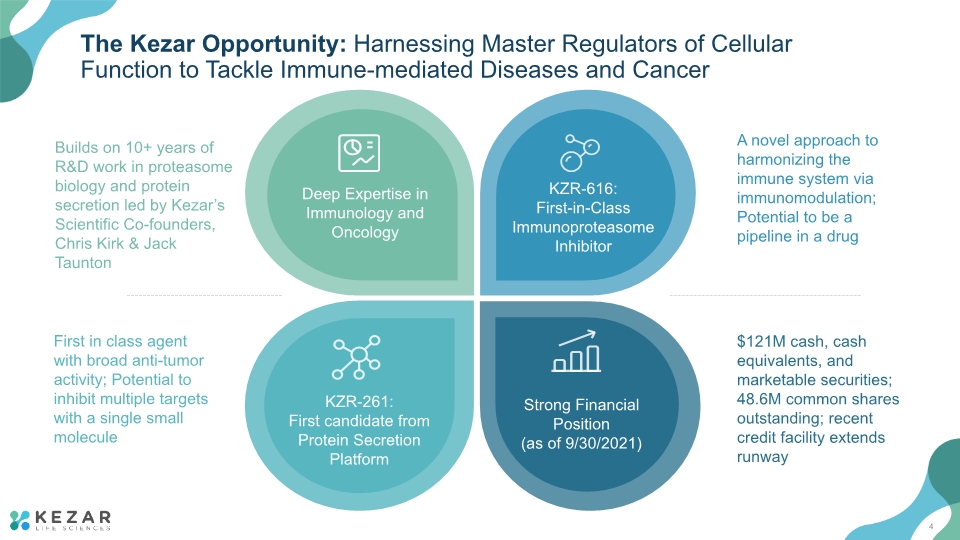
The Kezar Opportunity: Harnessing Master Regulators of Cellular Function to Tackle Immune-mediated Diseases and Cancer A novel approach to harmonizing the immune system via immunomodulation; Potential to be a pipeline in a drug First in class agent with broad anti-tumor activity; Potential to inhibit multiple targets with a single small molecule Builds on 10+ years of R&D work in proteasome biology and protein secretion led by Kezar’s Scientific Co-founders, Chris Kirk & Jack Taunton $121M cash, cash equivalents, and marketable securities; 48.6M common shares outstanding; recent credit facility extends runway 4
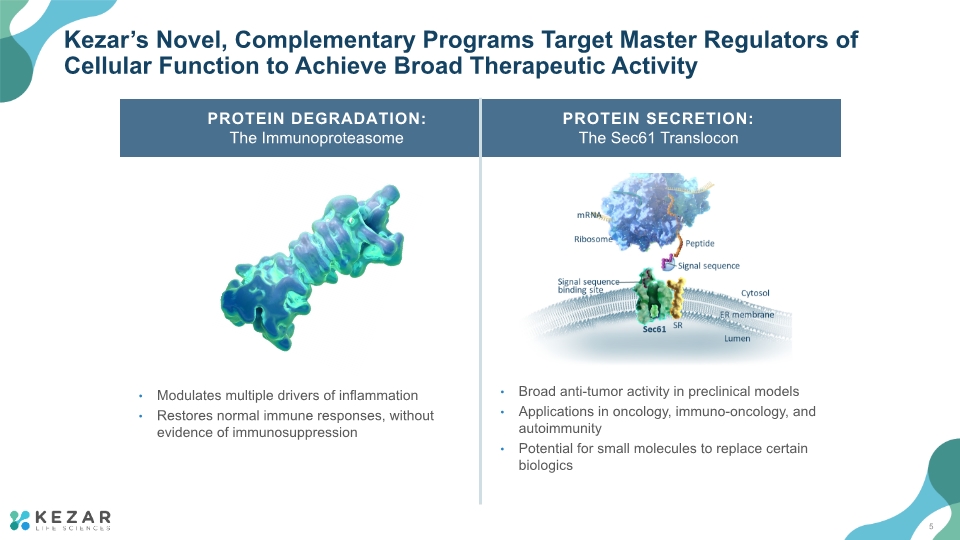
Kezar’s Novel, Complementary Programs Target Master Regulators of Cellular Function to Achieve Broad Therapeutic Activity Modulates multiple drivers of inflammation Restores normal immune responses, without evidence of immunosuppression PROTEIN DEGRADATION: The Immunoproteasome PROTEIN SECRETION: The Sec61 Translocon N Broad anti-tumor activity in preclinical models Applications in oncology, immuno-oncology, and autoimmunity Potential for small molecules to replace certain biologics 5
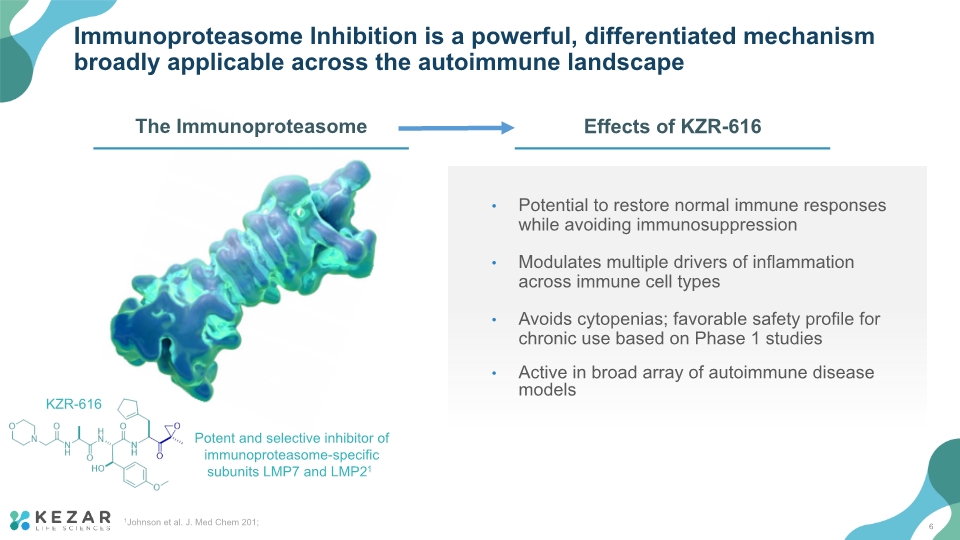
Potential to restore normal immune responses while avoiding immunosuppression Modulates multiple drivers of inflammation across immune cell types Avoids cytopenias; favorable safety profile for chronic use based on Phase 1 studies Active in broad array of autoimmune disease models Immunoproteasome Inhibition is a powerful, differentiated mechanism broadly applicable across the autoimmune landscape 1Johnson et al. J. Med Chem 201; Potent and selective inhibitor of immunoproteasome-specific subunits LMP7 and LMP21 KZR-616 6
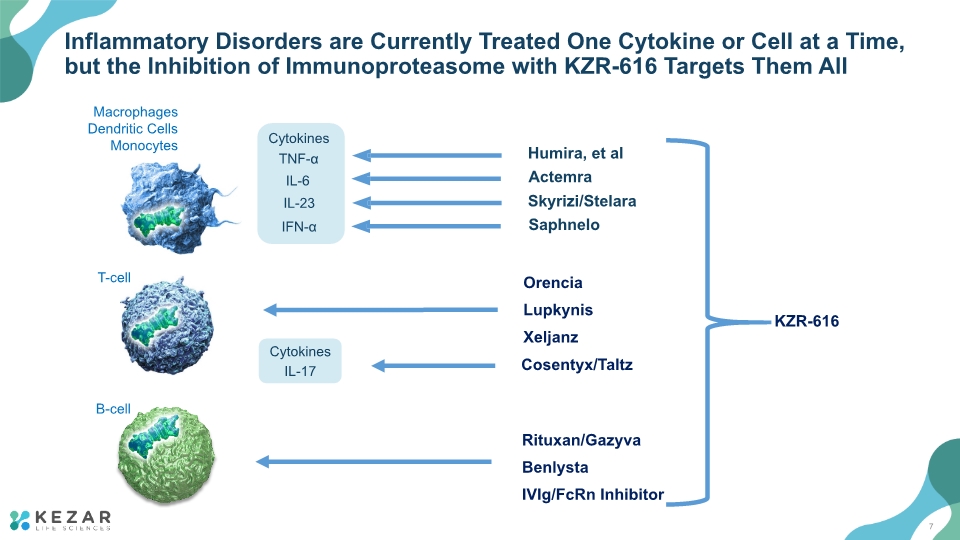
Inflammatory Disorders are Currently Treated One Cytokine or Cell at a Time, but the Inhibition of Immunoproteasome with KZR-616 Targets Them All Macrophages Dendritic Cells Monocytes T-cell B-cell Rituxan/Gazyva Benlysta IVIg/FcRn Inhibitor Orencia Lupkynis Xeljanz Cytokines IL-17 Cosentyx/Taltz KZR-616 7
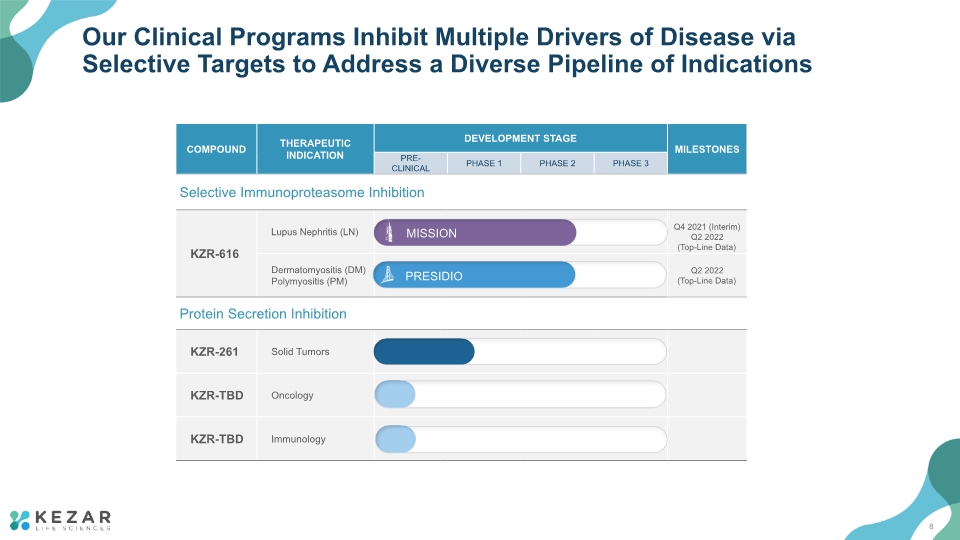
Our Clinical Programs Inhibit Multiple Drivers of Disease via Selective Targets to Address a Diverse Pipeline of Indications 8

Noreen R. Henig, MD Chief Medical Officer MISSION: Phase 1b/2 Study to Evaluate the Safety and Efficacy of KZR-616 in Systemic Lupus Erythematosus/Lupus Nephritis 9 Interim data reported and are subject to audit and verification procedures that could result in material changes in the final data.
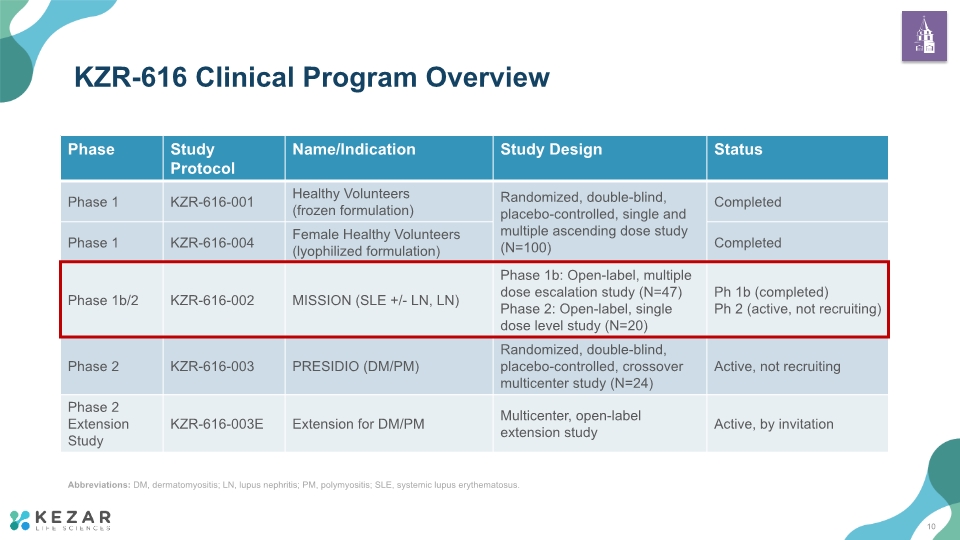
KZR-616 Clinical Program Overview Abbreviations: DM, dermatomyositis; LN, lupus nephritis; PM, polymyositis; SLE, systemic lupus erythematosus. 10
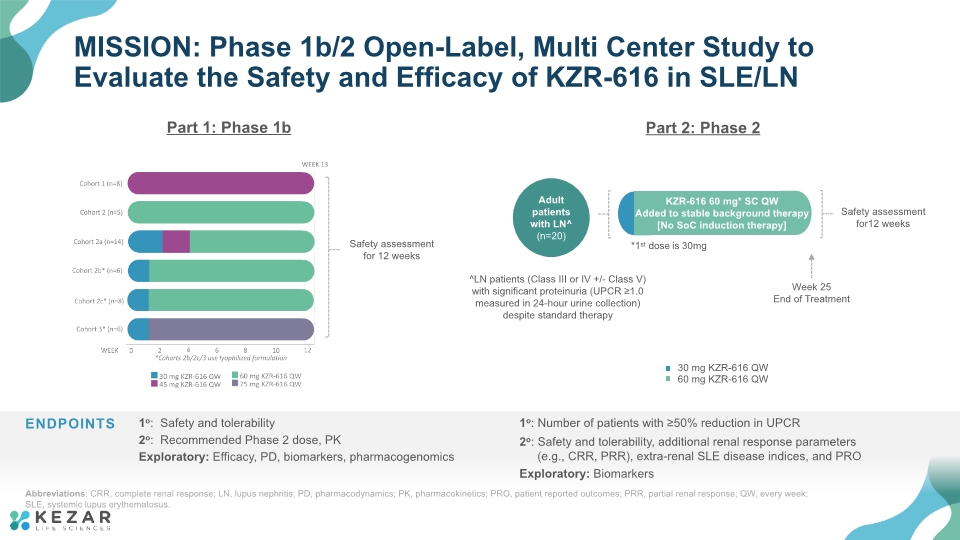
MISSION: Phase 1b/2 Open-Label, Multi Center Study to Evaluate the Safety and Efficacy of KZR-616 in SLE/LN Part 1: Phase 1b Part 2: Phase 2 Abbreviations: CRR, complete renal response; LN, lupus nephritis; PD, pharmacodynamics; PK, pharmacokinetics; PRO, patient reported outcomes; PRR, partial renal response; QW, every week; SLE, systemic lupus erythematosus.
Modulates innate and acquired immune responses without signs of immunosuppression to date Weekly administration results in consistent pharmacokinetics and pharmacodynamics Selective inhibition of the immunoproteasome via targeting of specific subunits LMP2 and LMP7 Weekly dose leads to consistent exposure and clearance (T1/2 <5 hours) Not predicted to result in clinically significant DDI Demonstrates rapid and sustained immunomodulatory gene expression changes Favorable safety and tolerability profile Improved signs and symptoms of SLE and LN as measured with exploratory endpoints in symptoms, serologic markers, reduction in proteinuria, and reduction in markers of specific kidney inflammation KZR-616: Phase 1 Findings to Date Well-Positioned to Be Chronic Therapy for Autoimmune Diseases Abbreviations: DDI, drug-drug interactions; SLE, systemic lupus erythematosus; LN, lupus nephritis. Furie et al, EULAR 2021 and Data on File. 12
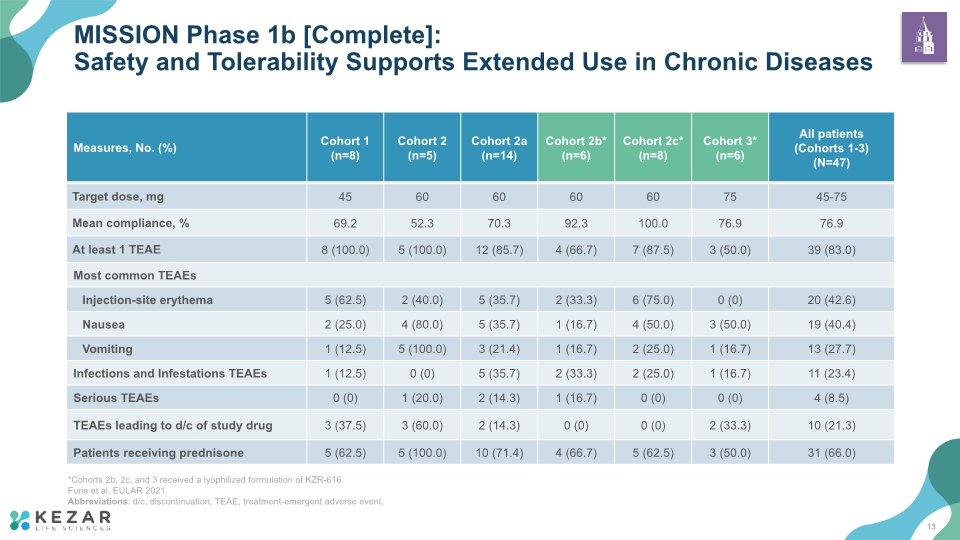
MISSION Phase 1b [Complete]: Safety and Tolerability Supports Extended Use in Chronic Diseases *Cohorts 2b, 2c, and 3 received a lyophilized formulation of KZR-616. Furie et al, EULAR 2021. Abbreviations: d/c, discontinuation; TEAE, treatment-emergent adverse event. 13
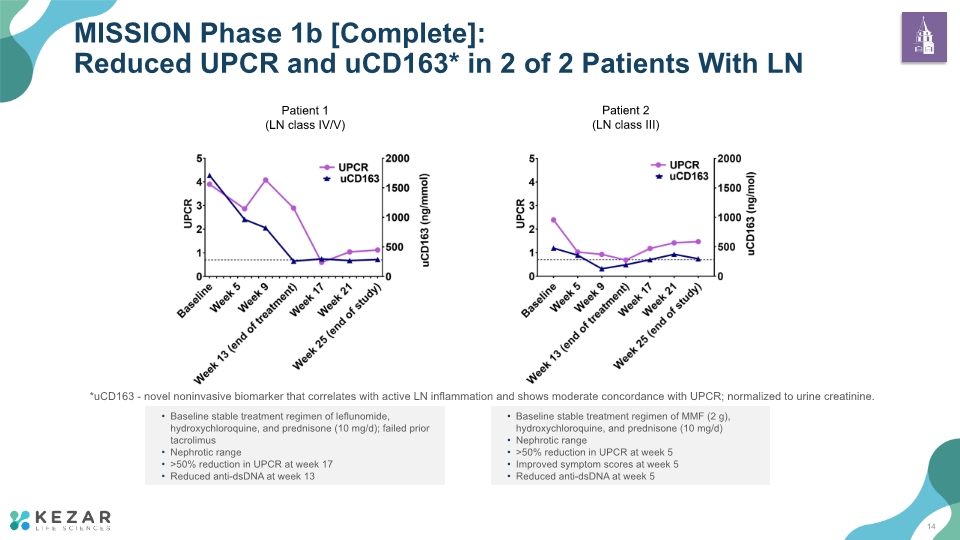
MISSION Phase 1b [Complete]: Reduced UPCR and uCD163* in 2 of 2 Patients With LN Baseline stable treatment regimen of leflunomide, hydroxychloroquine, and prednisone (10 mg/d); failed prior tacrolimus Nephrotic range >50% reduction in UPCR at week 17 Reduced anti-dsDNA at week 13 Baseline stable treatment regimen of MMF (2 g), hydroxychloroquine, and prednisone (10 mg/d) Nephrotic range >50% reduction in UPCR at week 5 Improved symptom scores at week 5 Reduced anti-dsDNA at week 5 *uCD163 - novel noninvasive biomarker that correlates with active LN inflammation and shows moderate concordance with UPCR; normalized to urine creatinine. 14

Interim Data MISSION Phase 2 Data from the MISSION Phase 2 clinical trial are preliminary and will require confirmation in additional patients as well as longer follow-up to draw any clinical conclusion. Interim top-line and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data. 15
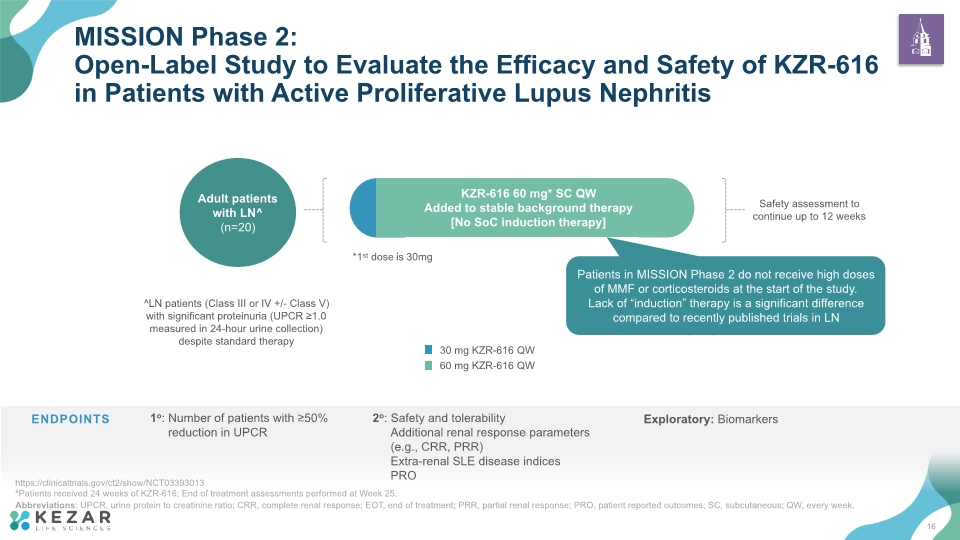
*Patients received 24 weeks of KZR-616; End of treatment assessments performed at Week 25. Abbreviations: UPCR, urine protein to creatinine ratio; CRR, complete renal response; EOT, end of treatment; PRR, partial renal response; PRO, patient reported outcomes; SC, subcutaneous; QW, every week. MISSION Phase 2: Open-Label Study to Evaluate the Efficacy and Safety of KZR-616 in Patients with Active Proliferative Lupus Nephritis https://clinicaltrials.gov/ct2/show/NCT03393013 Week 25 End of Treatment (EOT)* Adult patients with LN^ (n=20) ^LN patients (Class III or IV +/- Class V) with significant proteinuria (UPCR ≥1.0 measured in 24-hour urine collection) despite standard therapy Safety assessment to continue up to 12 weeks *1st dose is 30mg KZR-616 60 mg* SC QW Added to stable background therapy [No SoC induction therapy] Patients in MISSION Phase 2 do not receive high doses of MMF or corticosteroids at the start of the study. Lack of “induction” therapy is a significant difference compared to recently published trials in LN 16
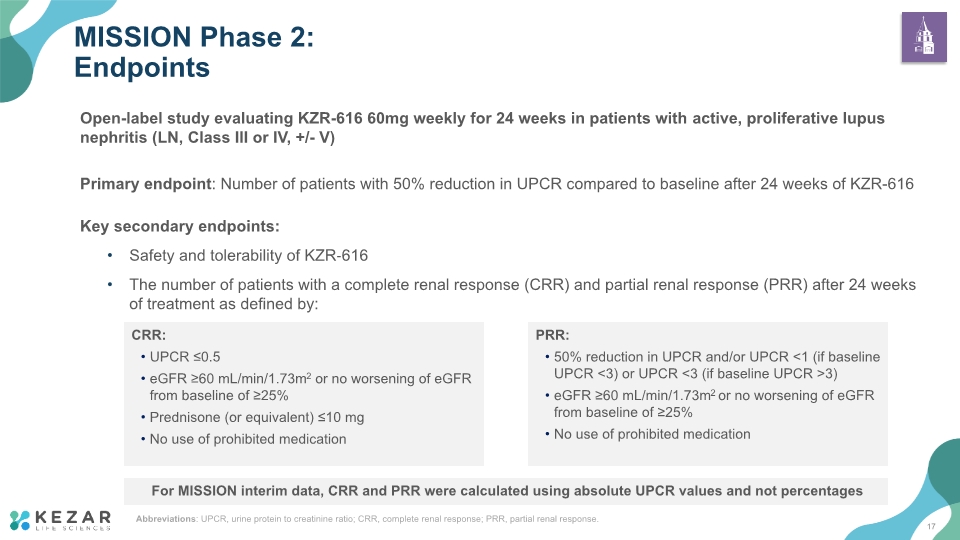
Open-label study evaluating KZR-616 60mg weekly for 24 weeks in patients with active, proliferative lupus nephritis (LN, Class III or IV, +/- V) Primary endpoint: Number of patients with 50% reduction in UPCR compared to baseline after 24 weeks of KZR-616 Key secondary endpoints: Safety and tolerability of KZR‑616 The number of patients with a complete renal response (CRR) and partial renal response (PRR) after 24 weeks of treatment as defined by: MISSION Phase 2: Endpoints PRR: 50% reduction in UPCR and/or UPCR <1 (if baseline UPCR <3) or UPCR <3 (if baseline UPCR >3) eGFR ≥60 mL/min/1.73m2 or no worsening of eGFR from baseline of ≥25% No use of prohibited medication CRR: UPCR ≤0.5 eGFR ≥60 mL/min/1.73m2 or no worsening of eGFR from baseline of ≥25% Prednisone (or equivalent) ≤10 mg No use of prohibited medication Abbreviations: UPCR, urine protein to creatinine ratio; CRR, complete renal response; PRR, partial renal response. For MISSION interim data, CRR and PRR were calculated using absolute UPCR values and not percentages 17

Target enrollment of 20 patients met for MISSION Phase 2 study Interim analysis is based on laboratory and safety analysis; a full data analysis will occur at the completion of the study 10 patients who completed at least 13 weeks of treatment with KZR-616 SC QW were included in the analysis 5 of the 10 patients reached EOT (W25) at the time of the interim analysis Patients did not receive induction therapy, and there was no mandated taper of glucocorticoids or other agents. Patients included in the interim analysis participated from 4 countries [US, Australia, Russia and Ukraine] Abbreviation: EOT, end of treatment; SC, subcutaneous; QW, once weekly. Patients received 24 weeks of KZR-616; end of treatment assessments performed at Week 25 Data from the MISSION Phase 2 clinical trial are preliminary and will require confirmation in additional patients as well as longer follow-up to draw any clinical conclusion. Interim top-line and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data. MISSION Phase 2: KZR-616 for the Treatment of LN Interim Data Overview 18
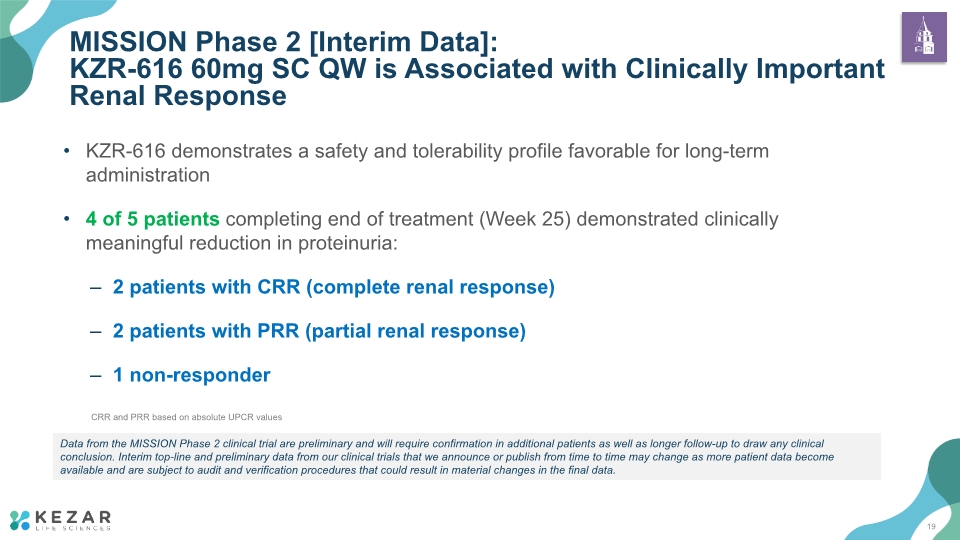
KZR-616 demonstrates a safety and tolerability profile favorable for long-term administration 4 of 5 patients completing end of treatment (Week 25) demonstrated clinically meaningful reduction in proteinuria: 2 patients with CRR (complete renal response) 2 patients with PRR (partial renal response) 1 non-responder MISSION Phase 2 [Interim Data]: KZR-616 60mg SC QW is Associated with Clinically Important Renal Response 19 Data from the MISSION Phase 2 clinical trial are preliminary and will require confirmation in additional patients as well as longer follow-up to draw any clinical conclusion. Interim top-line and preliminary data from our clinical trials that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data. CRR and PRR based on absolute UPCR values
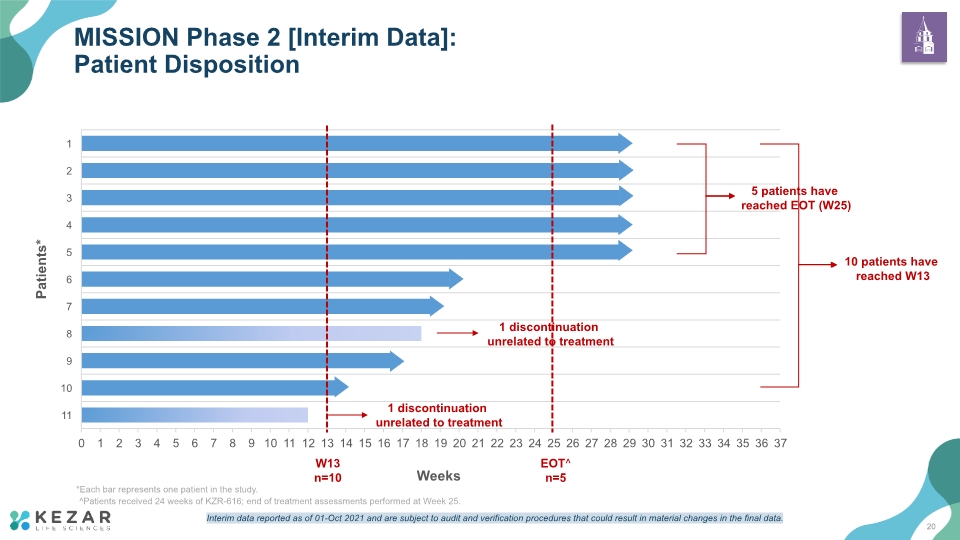
MISSION Phase 2 [Interim Data]: Patient Disposition Weeks EOT^ n=5 W13 n=10 Patients* *Each bar represents one patient in the study. ^Patients received 24 weeks of KZR-616; end of treatment assessments performed at Week 25. 20 Interim data reported as of 01-Oct 2021 and are subject to audit and verification procedures that could result in material changes in the final data.
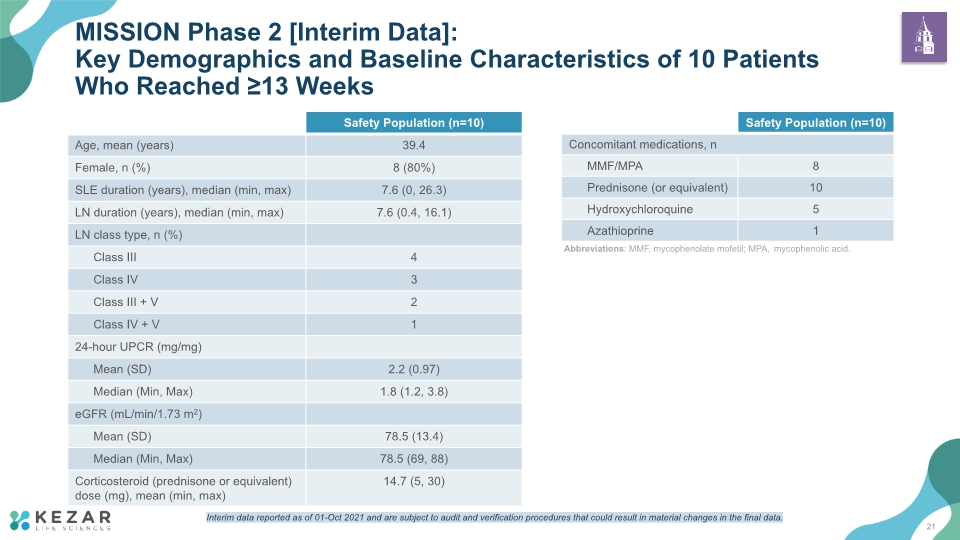
MISSION Phase 2 [Interim Data]: Key Demographics and Baseline Characteristics of 10 Patients Who Reached ≥13 Weeks Abbreviations: MMF, mycophenolate mofetil; MPA, mycophenolic acid. 21 Interim data reported as of 01-Oct 2021 and are subject to audit and verification procedures that could result in material changes in the final data.
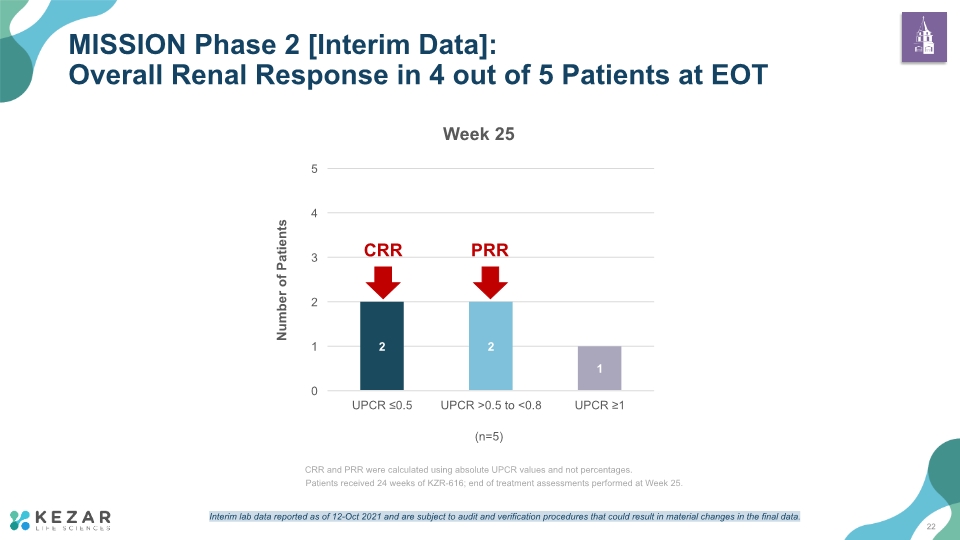
MISSION Phase 2 [Interim Data]: Overall Renal Response in 4 out of 5 Patients at EOT (n=5) 22 Interim lab data reported as of 12-Oct 2021 and are subject to audit and verification procedures that could result in material changes in the final data. Patients received 24 weeks of KZR-616; end of treatment assessments performed at Week 25.
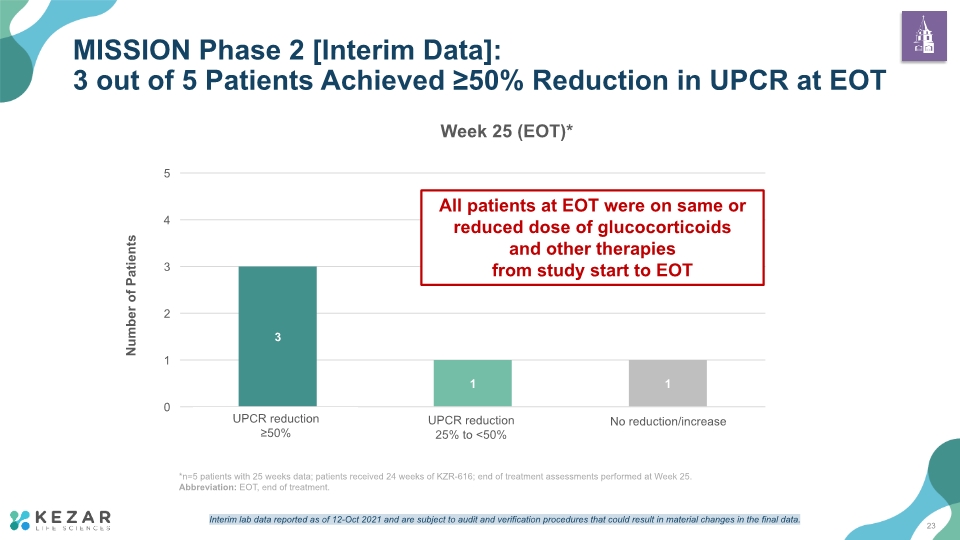
MISSION Phase 2 [Interim Data]: 3 out of 5 Patients Achieved ≥50% Reduction in UPCR at EOT 23 * UPCR reduction 25% to <50% UPCR reduction ≥50% *n=5 patients with 25 weeks data; patients received 24 weeks of KZR-616; end of treatment assessments performed at Week 25. Abbreviation: EOT, end of treatment. All patients at EOT were on same or reduced dose of glucocorticoids and other therapies from study start to EOT Interim lab data reported as of 12-Oct 2021 and are subject to audit and verification procedures that could result in material changes in the final data.
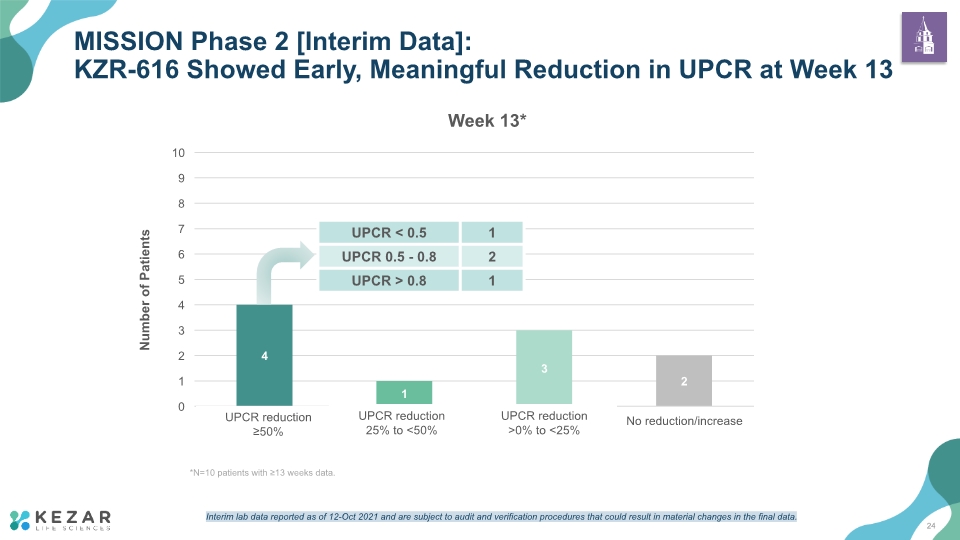
MISSION Phase 2 [Interim Data]: KZR-616 Showed Early, Meaningful Reduction in UPCR at Week 13 * UPCR reduction 25% to <50% UPCR reduction ≥50% UPCR reduction >0% to <25% *N=10 patients with ≥13 weeks data. 24 Interim lab data reported as of 12-Oct 2021 and are subject to audit and verification procedures that could result in material changes in the final data.
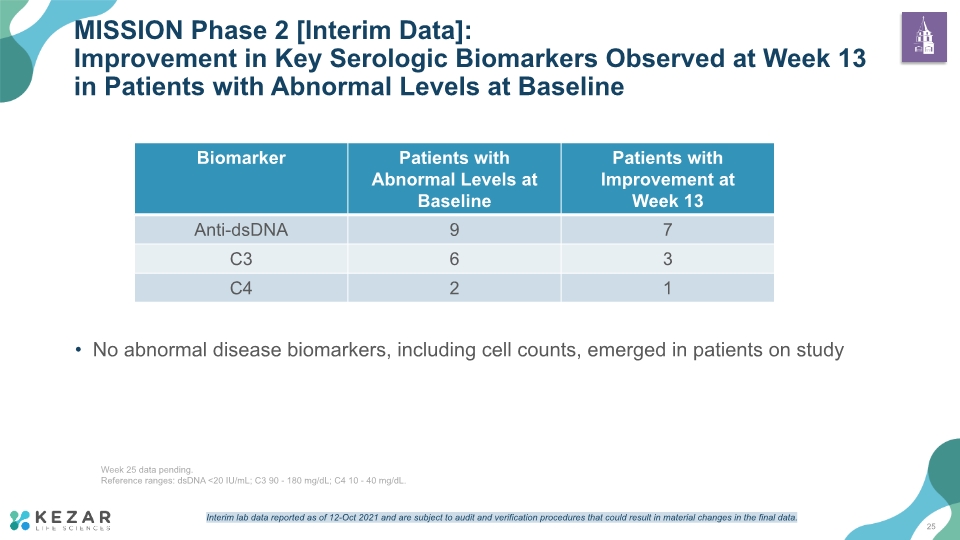
No abnormal disease biomarkers, including cell counts, emerged in patients on study MISSION Phase 2 [Interim Data]: Improvement in Key Serologic Biomarkers Observed at Week 13 in Patients with Abnormal Levels at Baseline 25 Week 25 data pending. Reference ranges: dsDNA <20 IU/mL; C3 90 - 180 mg/dL; C4 10 - 40 mg/dL. Interim lab data reported as of 12-Oct 2021 and are subject to audit and verification procedures that could result in material changes in the final data.
All reported AEs were mild to moderate (≤Grade 2) except 1 related SAE of Grade 3 migraine occurred, which led to temporary interruption of study drug. Patient fully recovered and resumed study treatment at the same 60mg dose level. 1 unrelated SAE of worsening PAH and AKI; led to discontinuation No study discontinuations due to related AEs Injection site reactions continue to be the most commonly reported AE MISSION Phase 2 shows nausea and vomiting is episodic and may occur at any point throughout 24 weeks of the study 10 reports of nausea and 6 reports of vomiting occurred in 4 patients, with nausea and vomiting often occurring together Any nausea and/or vomiting lasts ≤1 day in duration No patients reported nausea or vomiting with first dose of KZR-616 Some incidents of N/V were reported as attributed to the SAE, as in the case of the patient with migraine headache 204 doses of KZR-616 received in total at the time of the interim analysis --> 3-5% rate of N/V which is same or less than commonly used therapies for the treatment of SLE/LN No opportunistic infections reported to date MISSION Phase 2 [Interim Data]: KZR-616 Continues to Show Favorable Safety and Tolerability Profile Abbreviations: SAE, serious adverse event; PAH, pulmonary arterial hypertension; AKI, acute kidney injury; N/V, nausea and vomiting. 26 Interim data reported as of 01-Oct 2021 and are subject to audit and verification procedures that could result in material changes in the final data.
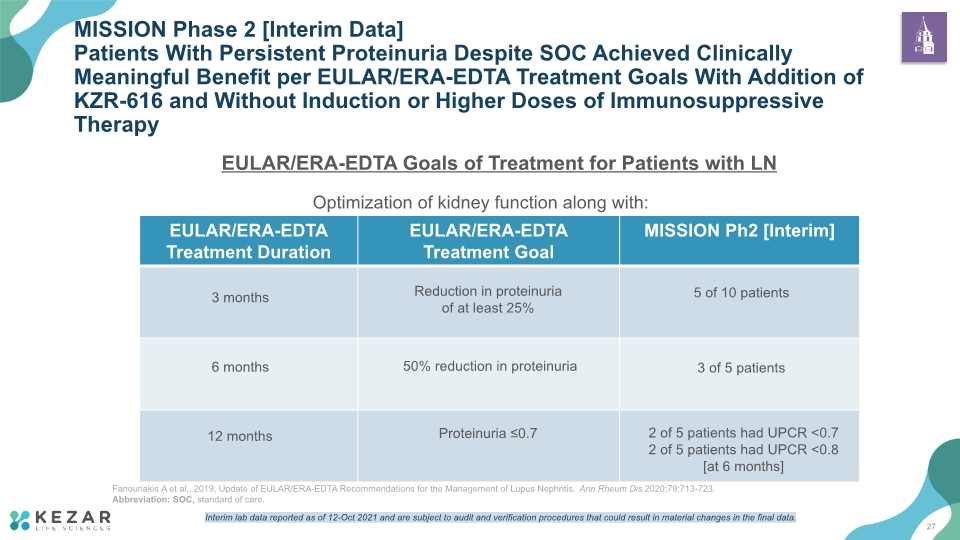
EULAR/ERA-EDTA Goals of Treatment for Patients with LN MISSION Phase 2 [Interim Data] Patients With Persistent Proteinuria Despite SOC Achieved Clinically Meaningful Benefit per EULAR/ERA-EDTA Treatment Goals With Addition of KZR-616 and Without Induction or Higher Doses of Immunosuppressive Therapy Fanouriakis A et al., 2019, Update of EULAR/ERA-EDTA Recommendations for the Management of Lupus Nephritis, Ann Rheum Dis 2020;79:713-723. Abbreviation: SOC, standard of care. Optimization of kidney function along with: Reduction in proteinuria of at least 25% 50% reduction in proteinuria Proteinuria ≤0.7 5 of 10 patients 3 of 5 patients 2 of 5 patients had UPCR <0.7 2 of 5 patients had UPCR <0.8 [at 6 months] 3 months 6 months 12 months 27 Interim lab data reported as of 12-Oct 2021 and are subject to audit and verification procedures that could result in material changes in the final data.
KZR-616 MISSION Phase 2 interim data learnings KZR-616 60mg SC QW dosing (starting with “step-up” dose) demonstrates a favorable safety and tolerability profile KZR-616 60mg SC QW demonstrated meaningful reductions in proteinuria in patients with LN, including those who are refractory or hard to treat KZR-616 continues to appear to be immunomodulatory rather than immunosuppressive The top-line data is anticipated for late second quarter 2022 Given the strength of the interim analysis, Kezar is planning next studies of KZR-616 for the treatment of LN MISSION Phase 2 [Interim Data]: KZR-616 Interim Analysis Suggests Clinically Significant Renal Activity and Kezar is Planning for Later Phase Studies 28 Interim data reported are subject to audit and verification procedures that could result in material changes in the final data.

MISSION 1b enrolled two patients with active LN, which were reported as Patient #1 and Patient #2 Following completion of the MISSION 1b study, the site investigator treating Patient #1 approached Kezar about re-starting KZR-616 as the patient reported improvements in SLE symptoms and showed reduced proteinuria while on the open-label Phase 1b study The site investigator took necessary steps to open an Investigator-led IND application and agreed to share safety and efficacy data with Kezar This case report is distinct from the MISSION 1b and MISSION 2 study Samir V. Parikh, MD will be presenting this patient’s experience today Introduction to Investigator-led IND Study Restarting KZR-616 in MISSION Phase 1b: Patient #1 29

Investigator-led IND Study: KZR-616 for the Treatment of Refractory LN Samir V. Parikh, MD, FASN Associate Professor, Division of Nephrology The Ohio State University Wexner Medical Center November 15, 2021 30
Case Report: Past Medical History 29-year-old Asian female diagnosed with SLE and LN in 2015-Jan LN Biopsy (2019-May): Class IV/V LN Treatment: Initially treated with high dose steroids and MMF. Developed leukopenia on MMF so discontinued Failed prior therapy with tacrolimus and no improvement on leflunomide. Remained steroid dependent on prednisone 10mg daily At baseline on stable regimen of hydroxychloroquine, leflunomide, prednisone (10mg/d) but still active disease Nephrotic range proteinuria at baseline (UPCR – 3.9) 31
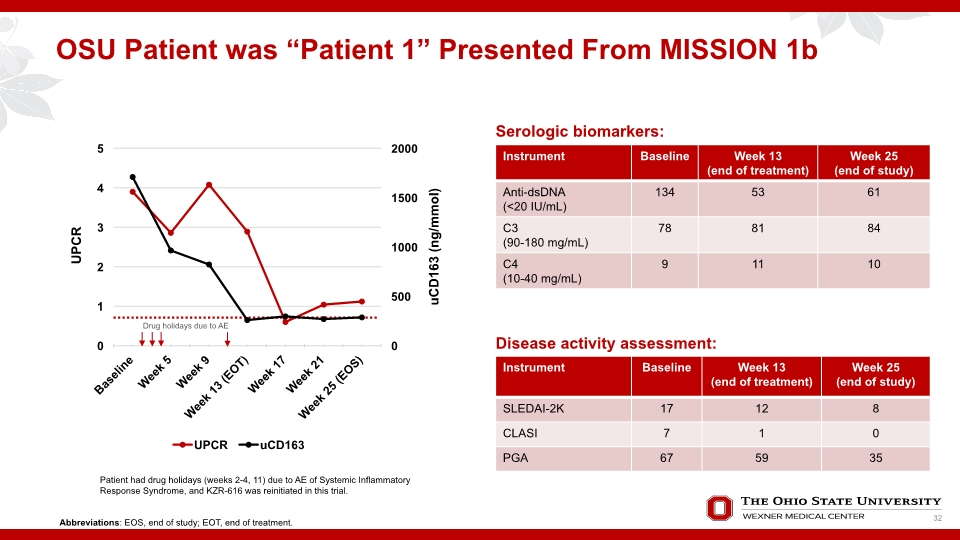
OSU Patient was “Patient 1” Presented From MISSION 1b Serologic biomarkers: Disease activity assessment: Patient had drug holidays (weeks 2-4, 11) due to AE of Systemic Inflammatory Response Syndrome, and KZR-616 was reinitiated in this trial. Abbreviations: EOS, end of study; EOT, end of treatment. Drug holidays due to AE 32

Patient 1 Experienced Worsening Course of Disease Following Completion of MISSION Phase 1b Study No significant changes to medications UPCR: 0.56 (3 months following completion of MISSION Ph1b) 5.5 (9 months following completion of MISSION Ph1b) Symptoms at 9 months following completion of MISSION Ph1b: Alopecia, arthralgia, higher BP (140s systolic), low C3/C4 (69/11) Started on high dose prednisone 40mg/d for 30 days and then tapered by 10mg every 2 weeks to 20mg daily and then to 15mg daily until she started Inv-led IND study Following prednisone therapy, proteinuria improved from 5.5 to 2.4 and remained there until resuming KZR-616 33
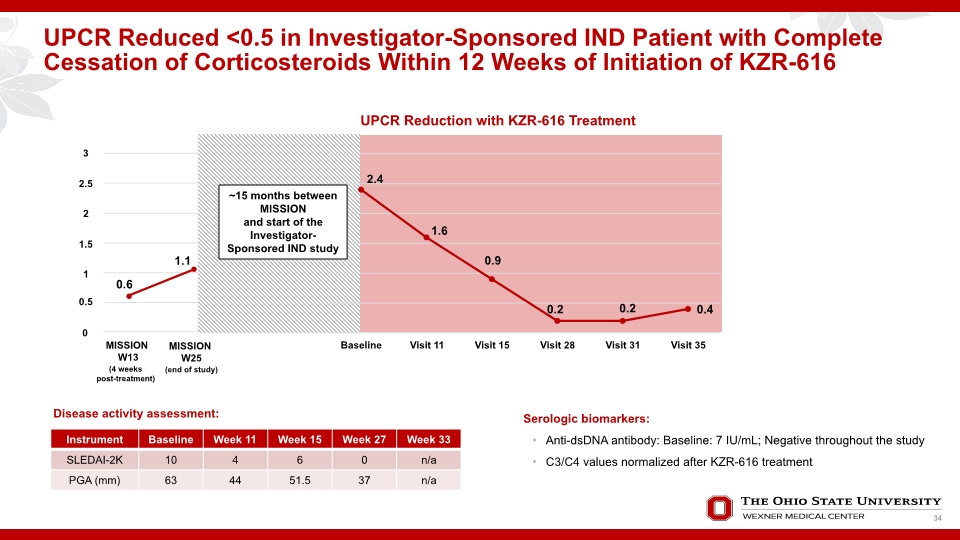
UPCR Reduced <0.5 in Investigator-Sponsored IND Patient with Complete Cessation of Corticosteroids Within 12 Weeks of Initiation of KZR-616 Serologic biomarkers: Anti-dsDNA antibody: Baseline: 7 IU/mL; Negative throughout the study C3/C4 values normalized after KZR-616 treatment Disease activity assessment: 34

SUMMARY: Patient Improves on KZR-616 Treatment Rapid improvement in proteinuria and clinical disease activity seen in the MISSION Phase 1b Patient experienced worsening of disease with a return to nephrotic range proteinuria 9 months following completion of MISSION Phase 1b Improvements seen early on and continued once initiated back on KZR-616 in Investigator-led IND Study >50% reduction in proteinuria observed at Week 14 Discontinuation of corticosteroid therapy within 12 weeks UPCR = 0.4 at Week 34 Patient reports feeling well and has no extra-renal symptoms 35

KZR-616 Potential in the LN Treatment Landscape Preclinical and early clinical data suggest that KZR-616 has broad immunomodulatory potential, with no major safety signals to date Possible role as: Potent anti-inflammatory Anti-autoimmunity therapy Steroid-sparing Consolidation therapy to limit number of concomitant therapies required for LN treatment 36

Noreen R. Henig, MD Chief Medical Officer PRESIDIO: Phase 2 Trial to Evaluate the Safety and Efficacy of KZR-616 in Dermatomyositis and Polymyositis 37
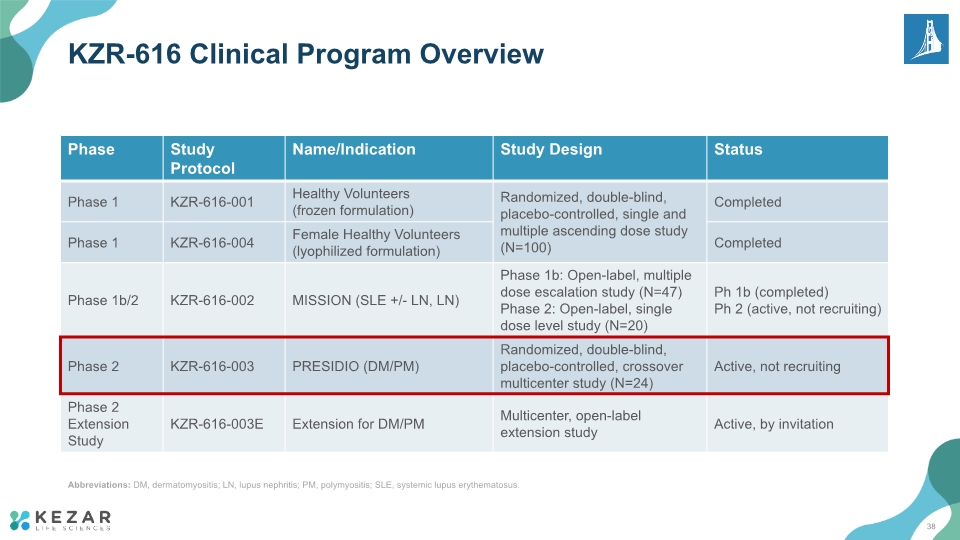
KZR-616 Clinical Program Overview Abbreviations: DM, dermatomyositis; LN, lupus nephritis; PM, polymyositis; SLE, systemic lupus erythematosus. 38
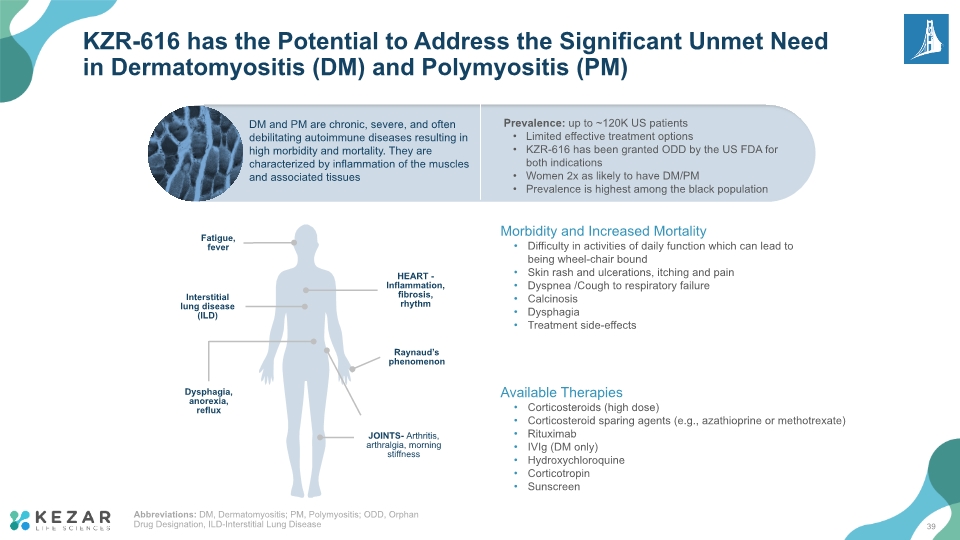
KZR-616 has the Potential to Address the Significant Unmet Need in Dermatomyositis (DM) and Polymyositis (PM) Prevalence: up to ~120K US patients Limited effective treatment options KZR-616 has been granted ODD by the US FDA for both indications Women 2x as likely to have DM/PM Prevalence is highest among the black population DM and PM are chronic, severe, and often debilitating autoimmune diseases resulting in high morbidity and mortality. They are characterized by inflammation of the muscles and associated tissues Fatigue, fever Interstitial lung disease (ILD) Dysphagia, anorexia, reflux HEART - Inflammation, fibrosis, rhythm JOINTS- Arthritis, arthralgia, morning stiffness Raynaud’s phenomenon Morbidity and Increased Mortality Difficulty in activities of daily function which can lead to being wheel-chair bound Skin rash and ulcerations, itching and pain Dyspnea /Cough to respiratory failure Calcinosis Dysphagia Treatment side-effects Available Therapies Corticosteroids (high dose) Corticosteroid sparing agents (e.g., azathioprine or methotrexate) Rituximab IVIg (DM only) Hydroxychloroquine Corticotropin Sunscreen Abbreviations: DM, Dermatomyositis; PM, Polymyositis; ODD, Orphan Drug Designation, ILD-Interstitial Lung Disease 39

Despite Currently Available Therapies, Most of Which Have Been Available for Decades, There Remains a Large Unmet Medical Need and Increased Health Care Utilization for Patients with DM and PM ACR 2021 Convergence [08-Nov 2021] KZR-616 is the only agent in the US granted Orphan Drug Designation (ODD) for DM and PM 40
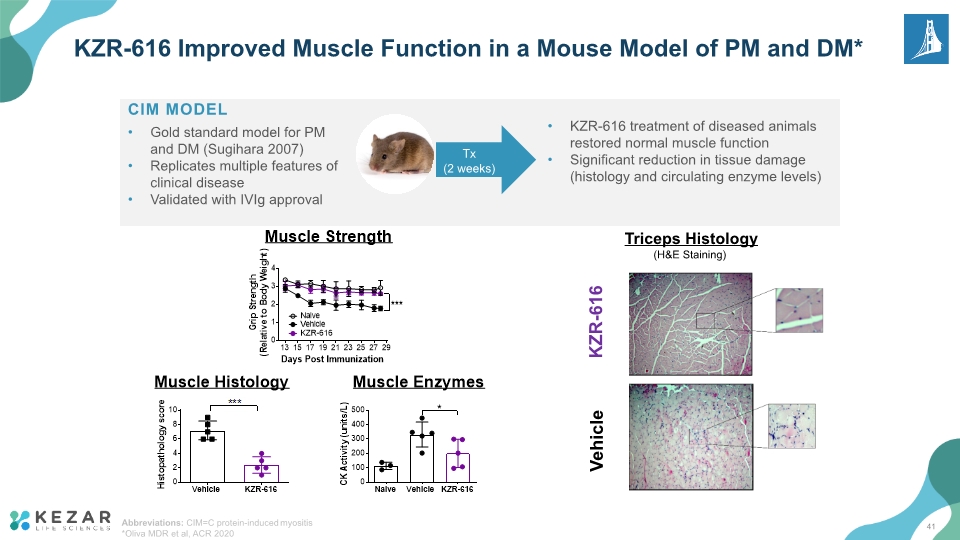
KZR-616 Improved Muscle Function in a Mouse Model of PM and DM* KZR-616 Vehicle Triceps Histology (H&E Staining) CIM MODEL Gold standard model for PM and DM (Sugihara 2007) Replicates multiple features of clinical disease Validated with IVIg approval Tx (2 weeks) KZR-616 treatment of diseased animals restored normal muscle function Significant reduction in tissue damage (histology and circulating enzyme levels) Abbreviations: CIM=C protein-induced myositis *Oliva MDR et al, ACR 2020 41
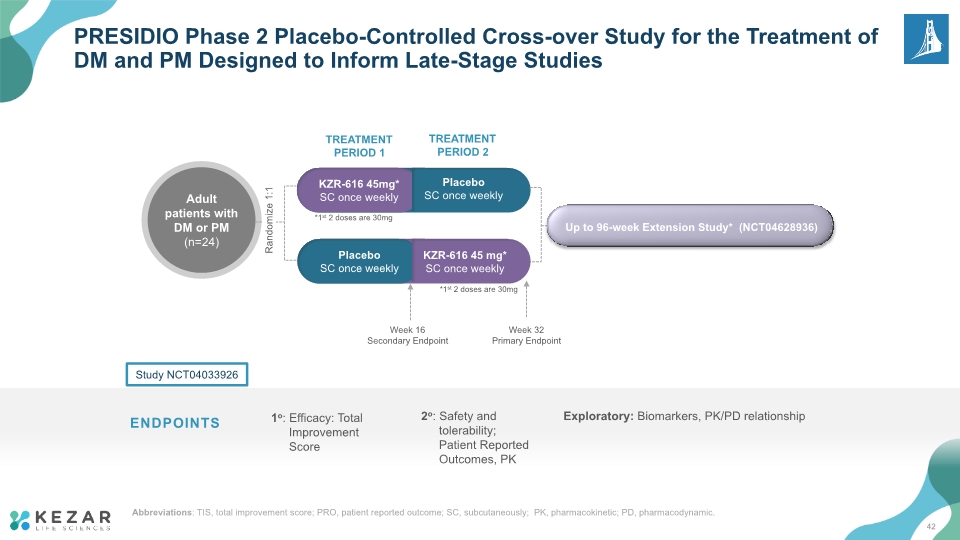
PRESIDIO Phase 2 Placebo-Controlled Cross-over Study for the Treatment of DM and PM Designed to Inform Late-Stage Studies TREATMENT PERIOD 1 TREATMENT PERIOD 2 Week 16 Secondary Endpoint Week 32 Primary Endpoint *1st 2 doses are 30mg ENDPOINTS Study NCT04033926 1o: Efficacy: Total Improvement Score 2o: Safety and tolerability; Patient Reported Outcomes, PK Exploratory: Biomarkers, PK/PD relationship Placebo SC once weekly KZR-616 45 mg* SC once weekly *1st 2 doses are 30mg Randomize 1:1 Up to 96-week Extension Study* (NCT04628936) 42 Abbreviations: TIS, total improvement score; PRO, patient reported outcome; SC, subcutaneously; PK, pharmacokinetic; PD, pharmacodynamic.
TIS is the same endpoint used in label expansion study for Octagam 10% [immune globulin intravenous (human)] for the treatment of DM. Also used in the Phase 3 trial of lenabasum for the treatment of DM TIS is a composite score which includes measures of muscle strength, serum enzymes which correspond to muscle injury, patient and physician directed symptom scores, and extra-muscular symptoms Kezar included patients with both DM and PM in the PRESIDIO study PM patients have not been included in recent interventional studies We will be looking to see if one or both patient groups show an efficacy signal KZR-616 for the Treatment of DM/PM Top-Line Results to Be Available Q2 2022 43 Rohit A et al. Ann Rheum Dis. 2017;76(5):792-801. Abbreviations: TIS, total improvement score.

Next Steps for the Development of KZR-616 in DM and PM PRESIDIO study achieved target enrollment in August 2021 Top-line results anticipated in the second quarter of 2022 Kezar is currently planning registrational studies of KZR-616 in inflammatory myositis based on High unmet need Strong preclinical data Demonstration of immunoproteasome activity in inflamed muscle Data from the MISSION trial demonstrating that KZR-616 is an active agent 44
Kezar Will Consider Development in Other Inflammatory Myopathies if PRESIDIO Generates Positive Results Dermatomyositis and polymyositis are two of four subtypes of the idiopathic inflammatory myositis Dermatomyositis has a bimodal age distribution with one peak in adults and the other in pediatric patients aged 5-15 years. Juvenile PM is described but considered rare. Kezar is evaluating clinical trials for juvenile DM and PM Necrotizing myopathy and inclusion body myositis are two additional subtypes of idiopathic inflammatory myositis and will be considered as potential opportunities in the future 45

Christopher Kirk, PhD President and Chief Scientific Officer, Co-Founder KZR-261 & Protein Secretion Platform 46
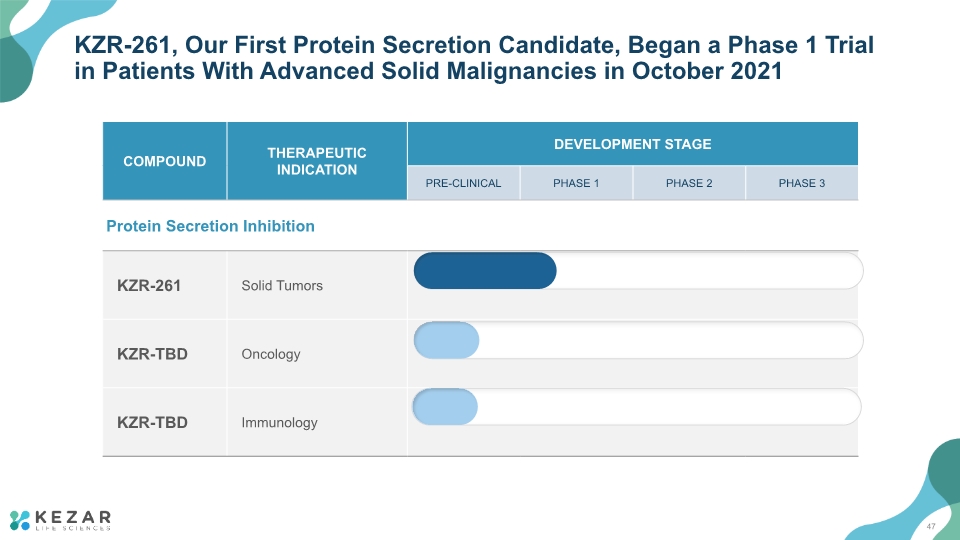
KZR-261, Our First Protein Secretion Candidate, Began a Phase 1 Trial in Patients With Advanced Solid Malignancies in October 2021 47
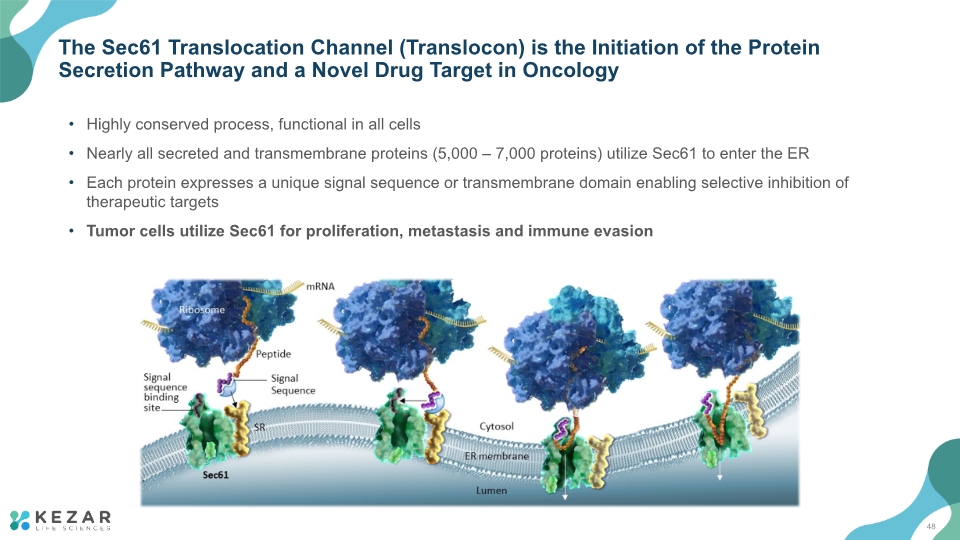
The Sec61 Translocation Channel (Translocon) is the Initiation of the Protein Secretion Pathway and a Novel Drug Target in Oncology Highly conserved process, functional in all cells Nearly all secreted and transmembrane proteins (5,000 – 7,000 proteins) utilize Sec61 to enter the ER Each protein expresses a unique signal sequence or transmembrane domain enabling selective inhibition of therapeutic targets Tumor cells utilize Sec61 for proliferation, metastasis and immune evasion 48
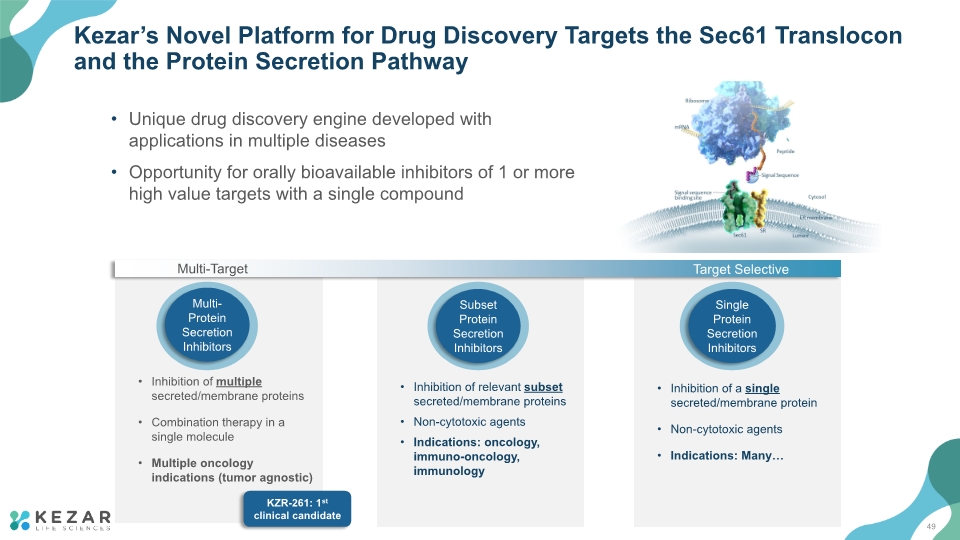
Inhibition of a single secreted/membrane protein Non-cytotoxic agents Indications: Many… Inhibition of multiple secreted/membrane proteins Combination therapy in a single molecule Multiple oncology indications (tumor agnostic) Inhibition of relevant subset secreted/membrane proteins Non-cytotoxic agents Indications: oncology, immuno-oncology, immunology Kezar’s Novel Platform for Drug Discovery Targets the Sec61 Translocon and the Protein Secretion Pathway Unique drug discovery engine developed with applications in multiple diseases Opportunity for orally bioavailable inhibitors of 1 or more high value targets with a single compound Multi-Target Target Selective KZR-261: 1st clinical candidate 49
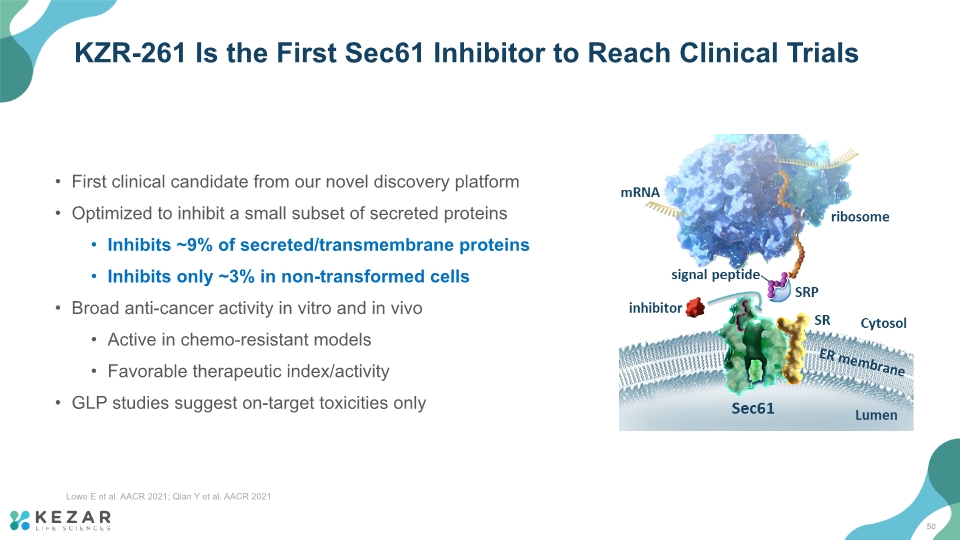
KZR-261 Is the First Sec61 Inhibitor to Reach Clinical Trials First clinical candidate from our novel discovery platform Optimized to inhibit a small subset of secreted proteins Inhibits ~9% of secreted/transmembrane proteins Inhibits only ~3% in non-transformed cells Broad anti-cancer activity in vitro and in vivo Active in chemo-resistant models Favorable therapeutic index/activity GLP studies suggest on-target toxicities only 50 Lowe E et al. AACR 2021; Qian Y et al. AACR 2021
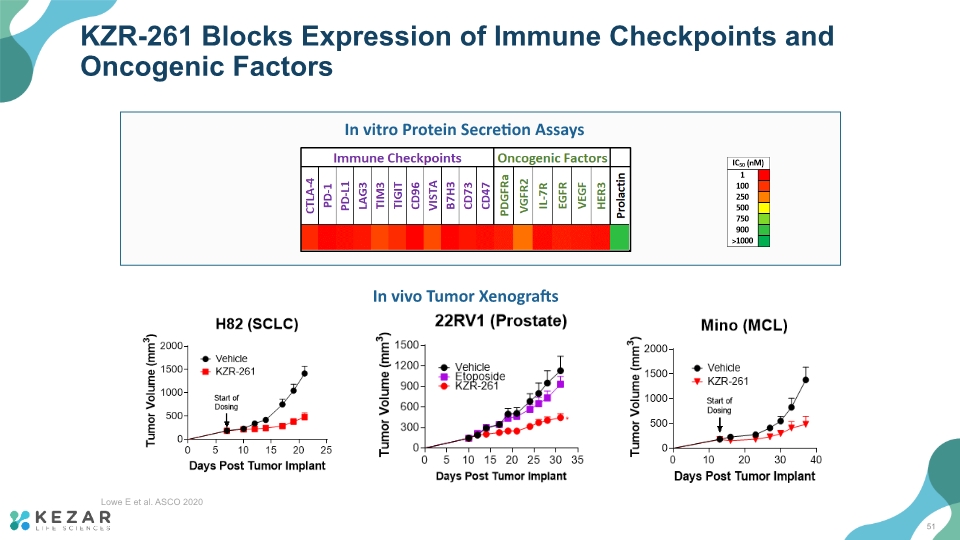
KZR-261 Blocks Expression of Immune Checkpoints and Oncogenic Factors In vitro Protein Secretion Assays In vivo Tumor Xenografts Lowe E et al. ASCO 2020 51
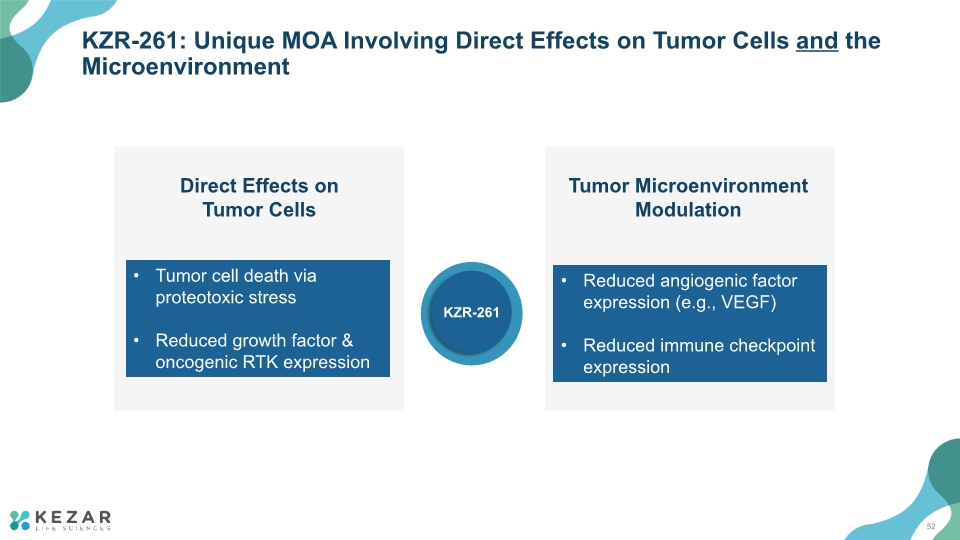
KZR-261: Unique MOA Involving Direct Effects on Tumor Cells and the Microenvironment KZR-261 52
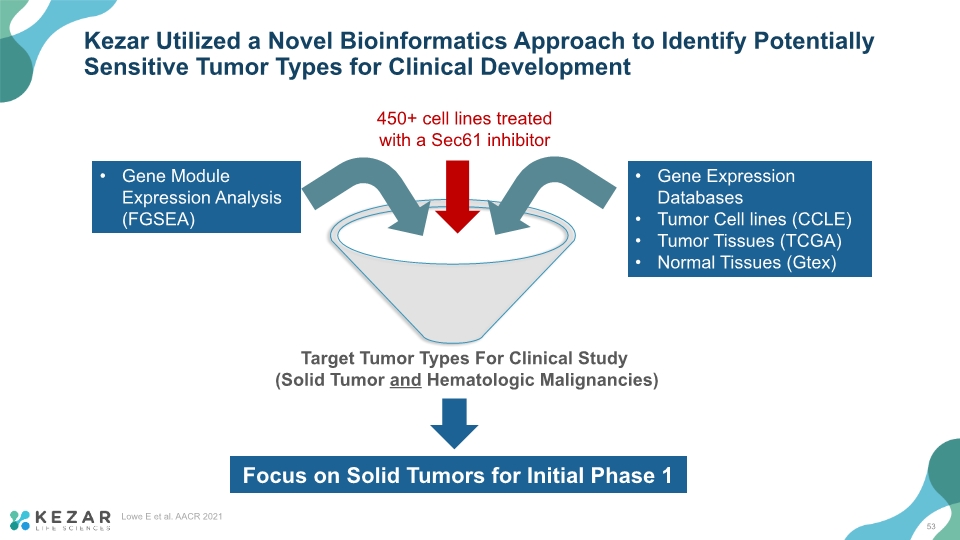
Kezar Utilized a Novel Bioinformatics Approach to Identify Potentially Sensitive Tumor Types for Clinical Development Lowe E et al. AACR 2021 450+ cell lines treated with a Sec61 inhibitor Gene Module Expression Analysis (FGSEA) Gene Expression Databases Tumor Cell lines (CCLE) Tumor Tissues (TCGA) Normal Tissues (Gtex) Target Tumor Types For Clinical Study (Solid Tumor and Hematologic Malignancies) Focus on Solid Tumors for Initial Phase 1 53
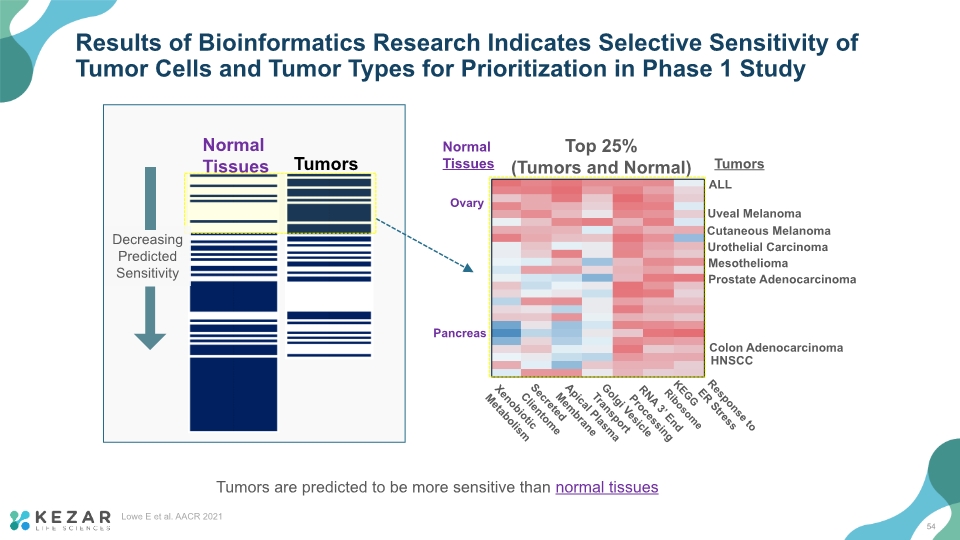
Results of Bioinformatics Research Indicates Selective Sensitivity of Tumor Cells and Tumor Types for Prioritization in Phase 1 Study Tumors are predicted to be more sensitive than normal tissues Prostate Adenocarcinoma Uveal Melanoma Cutaneous Melanoma Mesothelioma HNSCC Colon Adenocarcinoma Urothelial Carcinoma ALL Top 25% (Tumors and Normal) Ovary Pancreas Xenobiotic Metabolism Secreted Clientome Apical Plasma Membrane Golgi Vesicle Transport RNA 3’ End Processing KEGG Ribosome Response to ER Stress Normal Tissues Tumors Normal Tissues Tumors Decreasing Predicted Sensitivity 54 Lowe E et al. AACR 2021
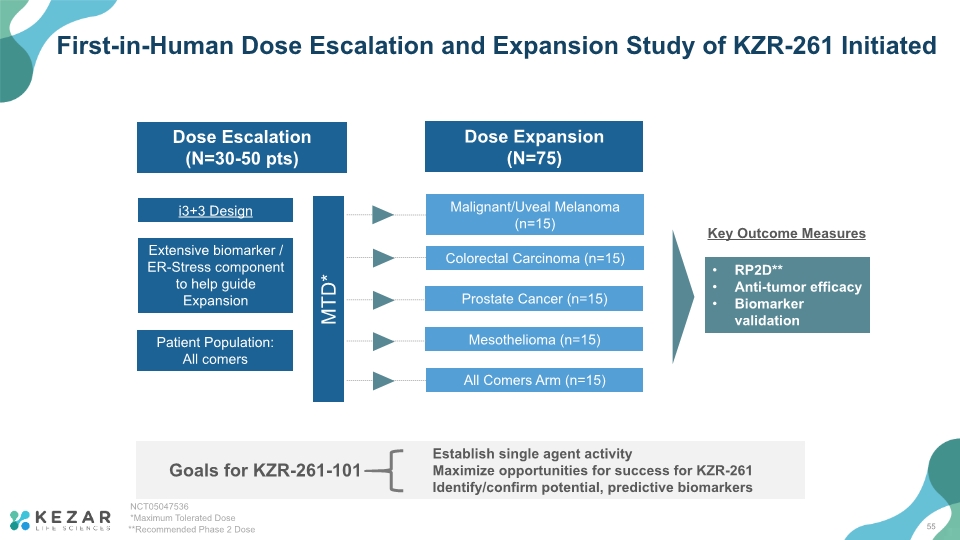
First-in-Human Dose Escalation and Expansion Study of KZR-261 Initiated RP2D** Anti-tumor efficacy Biomarker validation Key Outcome Measures 55 Goals for KZR-261-101 Establish single agent activity Maximize opportunities for success for KZR-261 Identify/confirm potential, predictive biomarkers

Inhibition of a single secreted/membrane protein Non-cytotoxic agents Indications: Many… Inhibition of multiple secreted/membrane proteins Combination therapy in a single molecule Multiple oncology indications (tumor agnostic) Inhibition of relevant subset secreted/membrane proteins Non-cytotoxic agents Indications: oncology, immuno-oncology, autoimmunity Kezar’s Novel Platform for Drug Discovery Targets the Sec61 Translocon and the Protein Secretion Pathway Unique drug discovery engine developed with applications in multiple diseases Opportunity for orally bioavailable inhibitors of 1 or more high value targets with a single compound Multi-Target Target Selective KZR-261: 1st clinical candidate 56
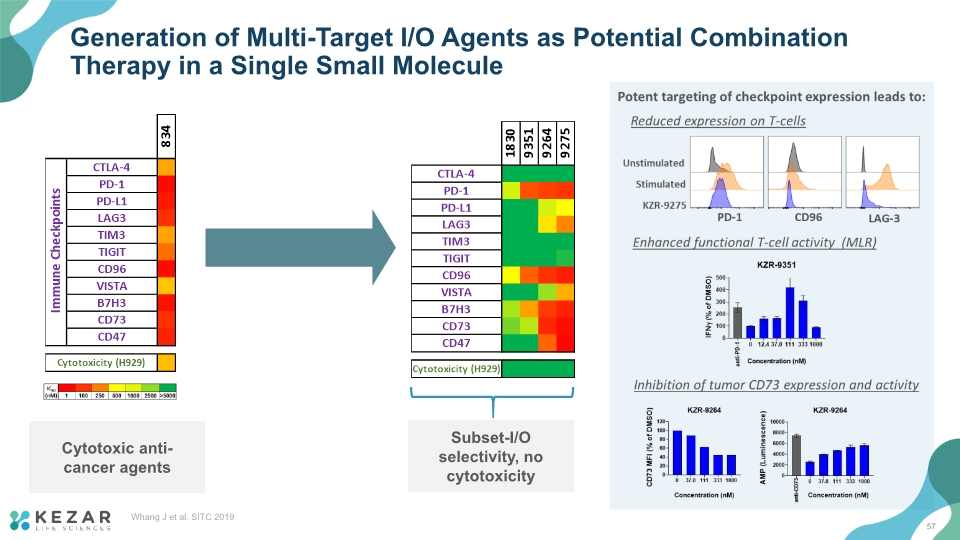
Generation of Multi-Target I/O Agents as Potential Combination Therapy in a Single Small Molecule Cytotoxic anti-cancer agents 57 Whang J et al. SITC 2019
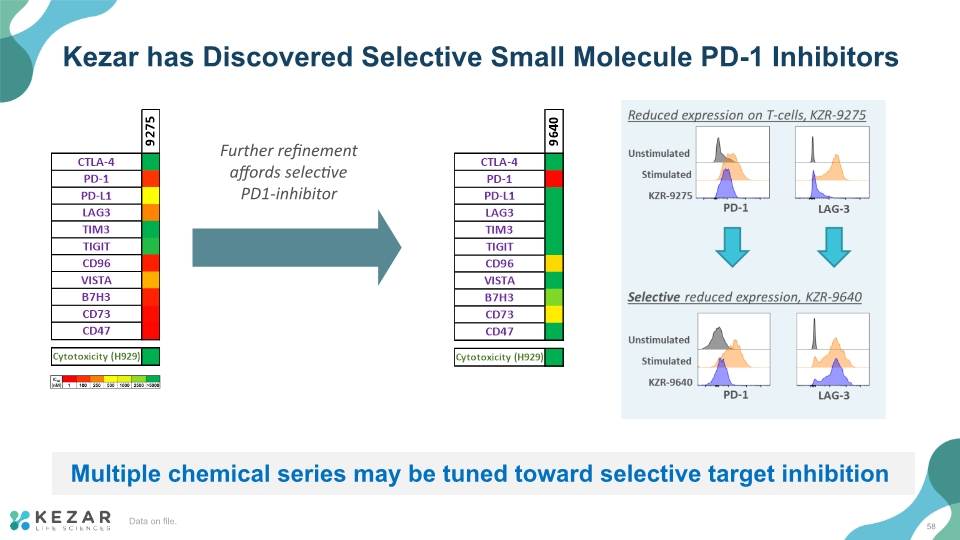
Kezar has Discovered Selective Small Molecule PD-1 Inhibitors Multiple chemical series may be tuned toward selective target inhibition Further refinement affords selective PD1-inhibitor 58 Data on file.
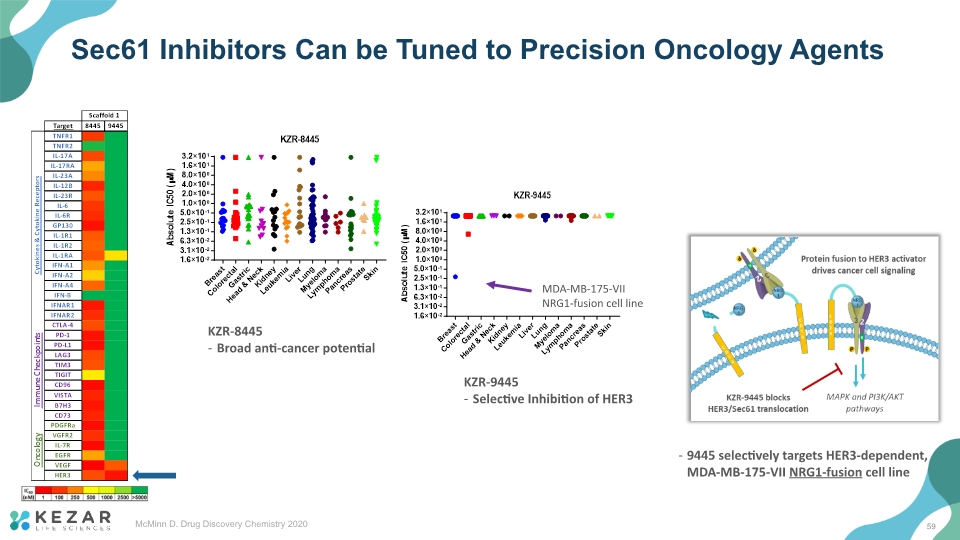
Sec61 Inhibitors Can be Tuned to Precision Oncology Agents KZR-8445 Broad anti-cancer potential KZR-9445 Selective Inhibition of HER3 59 McMinn D. Drug Discovery Chemistry 2020
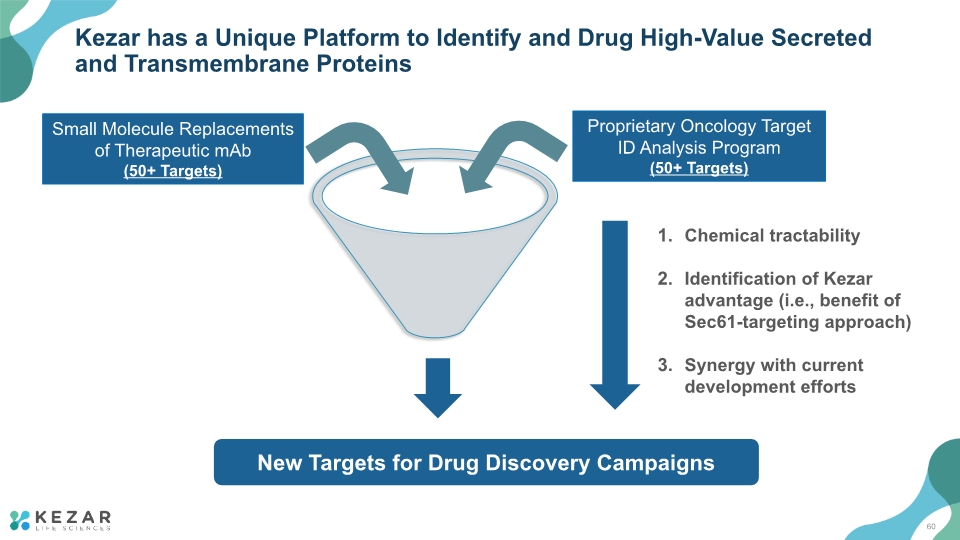
Kezar has a Unique Platform to Identify and Drug High-Value Secreted and Transmembrane Proteins Small Molecule Replacements of Therapeutic mAb (50+ Targets) Proprietary Oncology Target ID Analysis Program (50+ Targets) Chemical tractability Identification of Kezar advantage (i.e., benefit of Sec61-targeting approach) Synergy with current development efforts New Targets for Drug Discovery Campaigns 60
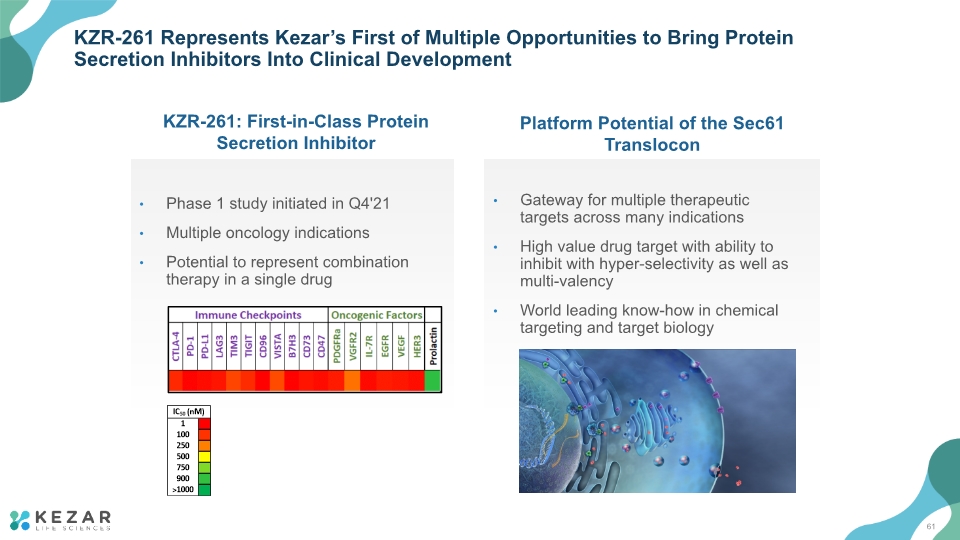
KZR-261 Represents Kezar’s First of Multiple Opportunities to Bring Protein Secretion Inhibitors Into Clinical Development KZR-261: First-in-Class Protein Secretion Inhibitor Platform Potential of the Sec61 Translocon Phase 1 study initiated in Q4'21 Multiple oncology indications Potential to represent combination therapy in a single drug Gateway for multiple therapeutic targets across many indications High value drug target with ability to inhibit with hyper-selectivity as well as multi-valency World leading know-how in chemical targeting and target biology 61
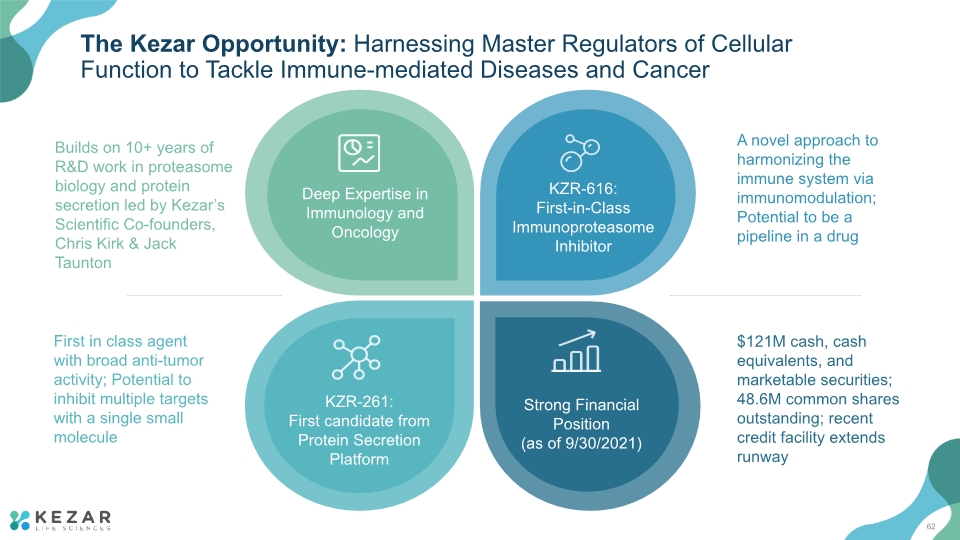
The Kezar Opportunity: Harnessing Master Regulators of Cellular Function to Tackle Immune-mediated Diseases and Cancer A novel approach to harmonizing the immune system via immunomodulation; Potential to be a pipeline in a drug First in class agent with broad anti-tumor activity; Potential to inhibit multiple targets with a single small molecule Builds on 10+ years of R&D work in proteasome biology and protein secretion led by Kezar’s Scientific Co-founders, Chris Kirk & Jack Taunton $121M cash, cash equivalents, and marketable securities; 48.6M common shares outstanding; recent credit facility extends runway 62





























































