As filed with the U.S. Securities and Exchange Commission on June 29, 2017
Registration no. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
CLEMENTIA PHARMACEUTICALS INC.
(Exact name of Registrant as specified in its charter)
|
|
|
|
|
Canada | 2834 | 98-1128564 |
Clementia Pharmaceuticals Inc.
4150 St Catherine Street West, Suite 550
Montreal, Quebec, Canada H3Z 2Y5
(514) 940-3600
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
The Corporation Trust Company
Corporation Trust Center
1209 Orange Street
Wilmington, DE 19801
Telephone: (302) 658-7581
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
|
|
Kevin T. Collins | Patrick O’Brien |
Approximate date of commencement of proposed offering to the public:As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box.o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.o
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth companyR
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.R
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.o
CALCULATION OF REGISTRATION FEE
|
|
|
|
|
| ||||
Title of Each Class of | Proposed | Amount of | ||
| ||||
Common Shares, no par value | US$115,000,000 | US$13,328.50 | ||
| ||||
(1) | Estimated solely for the purpose of determining the amount of registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended. Includes common shares that the underwriters may purchase pursuant to their option to purchase additional common shares, if any. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to such Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the United States Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
|
|
|
PROSPECTUS (Subject to Completion) |
|
|
Shares

Common Shares
Clementia Pharmaceuticals Inc. is offering shares of its common shares. This is our initial public offering and no public market exists for our shares. We anticipate that the initial public offering price per share will be between $ and $ .
We have applied to list our common shares on The Nasdaq Global Market under the symbol “CMTA.”
We are an “emerging growth company” as defined under the federal securities laws. Investing in our common shares involves risks. See “Risk Factors” beginning on page 10 of this prospectus.
PRICE$ A SHARE
|
|
|
|
|
|
| |||||||||||||||
| Price to Public | Underwriting | Proceeds to | ||||||||||||||||||
Per share |
|
| US$ |
|
| US$ |
|
| US$ | ||||||||||||
Total |
|
| US$ |
|
| US$ |
|
| US$ | ||||||||||||
(1) See “Underwriters” for a description of the compensation payable to the underwriters.
Neither the United States Securities and Exchange Commission nor any state securities regulators have not approved or disapproved of these securities, or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
We have granted the underwriters an option to purchase up to an additional common shares to cover over-allotments. The underwriters can exercise this option at any time within 30 days after the date of this prospectus.
The underwriters expect to deliver the common shares on or about , 2017.
|
|
|
MORGAN STANLEY | LEERINK PARTNERS | |
WEDBUSH PACGROW | BTIG |
The date of this prospectus is , 2017.
TABLE OF CONTENTS
|
|
| |||||
|
| 1 | |||||
|
| 10 | |||||
|
| 48 | |||||
|
| 50 | |||||
|
| 51 | |||||
|
| 52 | |||||
|
| 53 | |||||
|
| 55 | |||||
|
| 57 | |||||
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
|
| 59 | ||||
|
| 83 | |||||
|
| 125 | |||||
|
| 131 | |||||
|
| 146 | |||||
|
| 149 | |||||
|
| 151 | |||||
|
| 157 | |||||
|
| 159 | |||||
|
| 166 | |||||
|
| 173 | |||||
|
| 176 | |||||
|
| 181 | |||||
|
| 181 | |||||
|
| 181 | |||||
|
| 181 | |||||
|
| 182 | |||||
|
| 182 | |||||
|
| F-1 | |||||
Neither we nor the underwriters have authorized anyone to provide information different from that contained in this prospectus, any amendment or supplement to this prospectus or in any free writing prospectus prepared by us or on our behalf. Neither we nor the underwriters take any responsibility for, and can provide no assurance as to the reliability of, any information other than the information in this prospectus, any amendment or supplement to this prospectus, and any free writing prospectus prepared by us or on our behalf. Neither the delivery of this prospectus nor the sale of our common shares means that information contained in this prospectus is correct after the date of this prospectus. This prospectus is not an offer to sell or the solicitation of an offer to buy our common shares in any circumstances under which such offer or solicitation is unlawful.
For investors outside the United States, neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
i
ABOUT THIS PROSPECTUS
All references in this prospectus to “the Company,” “Clementia,” “we,” “us,” or “our” refer to Clementia Pharmaceuticals Inc. and the subsidiaries through which it conducts its business unless otherwise indicated.
This prospectus includes statistical data, market data and other industry data and forecasts, which we obtained from various sources, including internal surveys, market research, publicly available information and independent industry publications and reports. Industry surveys, publications, consultant surveys and forecasts generally state that the information contained therein has been obtained from sources believed to be reliable, but that the accuracy and completeness of such information is not guaranteed. We have not independently verified any of the data from third-party sources nor have we ascertained the underlying economic assumptions relied upon therein. Similarly, internal surveys, industry forecasts and market research, which we believe to be reliable based upon our management’s knowledge of the industry, have not been independently verified. The future performance of the industry in which we operate is necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the sections entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” and elsewhere in this prospectus. These and other factors could cause results to differ materially from those expressed in these publications and reports.
“Clementia” and our other registered or common law trademarks, service marks or trade names appearing in this prospectus are the property of Clementia Pharmaceuticals, Inc. Other trademarks and trade names referred to in this prospectus are the property of their respective owners.
Unless otherwise indicated, all references to “dollars” or the use of the symbol “$” are to U.S. dollars, the Company’s functional currency, and all references to “Canadian dollars” or “C$” are to Canadian dollars. Unless otherwise specified, all financial information has been prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB).
ii
This summary highlights information contained in other parts of this prospectus. Because it is only a summary, it does not contain all of the information that you should consider before investing in our common shares and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere in this prospectus. You should read the entire prospectus carefully, especially “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business” and our consolidated financial statements and the related notes, before deciding to buy our common shares.
OUR COMPANY
We are a clinical stage biopharmaceutical company that is developing disease-modifying treatments for patients suffering from debilitating bone and other diseases with high unmet medical need. Our lead product candidate, palovarotene, is an oral small molecule that binds and activates retinoic acid receptor gamma (an RARg agonist), and has shown potent activity in preventing abnormal new bone formation as well as scar tissue formation (or fibrosis) in a variety of tissues in animal models. We are developing palovarotene for the treatment of Fibrodysplasia Ossificans Progressiva (FOP) and Multiple Osteochondroma (MO) and have one Phase 3 trial and one Phase 2/3 trial, for two separate indications, planned to commence in 2017 with data read-outs planned in 2019 and 2020. We believe that if approved in FOP or MO, palovarotene could become the standard of care in either or both of these indications.
FOP is an ultra-rare, chronic and severely disabling disease of abnormal bone formation, known as heterotopic ossification (HO). FOP is characterized by painful, recurrent episodes of soft tissue swelling (flare-ups) that result in bone formation in areas of the body where bone is not normally present, such as muscles, tendons and ligaments. Flare-ups begin early in life and may occur spontaneously or after certain events, including soft tissue trauma, vaccinations or influenza infections. Recurrent flare-ups and new bone formation progressively restrict movement by locking joints, leading to cumulative loss of function, disability and early death due to reduced respiratory function. FOP is caused by a mutation of the bone morphogenetic protein (BMP) Type I receptor or ACVR1 (also known as ALK2) that leads to excess BMP signaling and new bone formation. Virtually all known patients have the same point mutation and have congenital malformations of the big toes at birth. We estimate that the prevalence of FOP is approximately 1.3 individuals per million lives, or approximately 9,000 globally. As of October 2016, there were known to be 800 diagnosed FOP patients worldwide. There are currently no approved medical treatment options to prevent the formation of heterotopic bone in FOP.
A 2011 Nature Medicine paper showed that palovarotene potently inhibited HO in animal models. Palovarotene had been previously tested by Roche Pharmaceuticals in 825 subjects, including healthy volunteers and patients with chronic obstructive pulmonary disease, where it was well-tolerated. Upon evaluation of the RARg agonist landscape, we determined that palovarotene had the most immediate potential in this class. As a result, we exclusively in-licensed palovarotene from Roche to form the basis of Clementia. In July 2014, the FDA granted Orphan Drug Designation for palovarotene as a treatment for FOP and in November 2014, we were granted orphan drug status in the EU. Orphan Drug Designation by the FDA allows for seven years of market exclusivity in the U.S. upon approval of the drug for the indication for which it was designated except in certain limited circumstances. In Europe, marketing authorization for an orphan drug generally leads to a ten-year period of market exclusivity. Also, in November 2014 we received Fast Track Designation from the FDA, which allows for more frequent interactions with the FDA during the drug development and review process. We have also secured IP related to palovarotene and in-licensed additional next generation RARg agonists.
Our Programs
Our programs currently focus on diseases involving tissue transformation via retinoic acid receptors (RARs). RARs are expressed in a variety of tissues and are involved in the growth, shape and maintenance of tissues (morphogenesis). In particular, the RARg receptor sub-type is expressed in cells that produce cartilage and plays a role in biological pathways responsible for endochondral bone
1
formation (the process of new bone formation which occurs via cartilage formation). RARg is also present in multiple other cells and tissues where it mediates the growth and differentiation of specific cell types, including those involved in fibrosis.
We believe that RARg agonists, such as palovarotene, have the potential for therapeutic use in a broad range of conditions, including diseases like FOP and MO that involve pathological bone formation as well as other indications characterized by excessive fibrosis or scarring such as dry eye disease.
The following table summarizes our development programs:
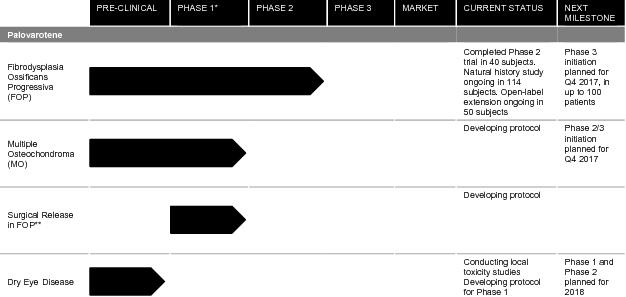
* | Phase 1 trials for palovarotene in FOP provide basis for proceeding directly to Phase 2/3 trials in MO | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
** | To our knowledge, no animal models for surgical release in FOP currently exist | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Palovarotene for FOP
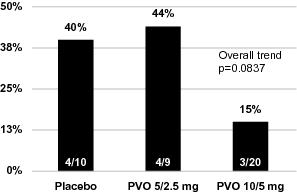
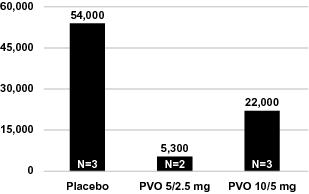
In advance of the commencement of our pivotal palovarotene trial program, we completed the first, randomized, placebo-controlled, adaptive design Phase 2 study in FOP, which enrolled 40 patients. The results of the Phase 2 study along with our open label extension, which reported a total of 67 flare-ups, saw positive trends on certain of our secondary endpoints. Importantly, while our clinical trials have not demonstrated statistically significant results, the data suggest that palovarotene reduced the incidence of new HO by approximately 50% as determined by CT scan at 12 weeks. In those subjects who formed new HO, the mean volumes of new HO were reduced by approximately 70% as compared to the placebo-treated subjects. Palovarotene was well-tolerated in this study and no patient discontinued drug or dose de-escalated.
Our Phase 2 trial and open label extensions as well as additional insights have led us to design our registration trial (considered to be a clinical trial expected to form the basis for regulatory approval) for palovarotene in FOP as well as our clinical trial for palovarotene for surgical excision of HO in FOP. Our Phase 3 trial, MOVE, for the treatment of FOP in adults and children, will enroll up to 100 patients who will be treated chronically with palovarotene and with increased doses during flare-ups. Based on clinical data generated from our Phase 2 study, open label extensions and our natural history study, the FDA has indicated that new HO is a clinically meaningful outcome provided we pre-specify the magnitude of treatment effect and support the HO findings with secondary endpoints and is sufficient as a primary endpoint for a registration trial supporting approval. We expect to initiate our Phase 3 trial in 2017 and report data in 2020 with an interim read-out in 2019.
We are also planning for a trial which aims to determine if palovarotene can inhibit new HO formation after surgical excision of HO. Patients will be treated prophylactically and after surgery at
2
specific previously locked joints, in an effort to prevent the re-growth of abnormal bone typically observed in FOP patients and to attempt to increase range of motion at such joints. We intend to discuss the details and timing of this trial with FDA in the future.
In parallel with our Phase 2 trials, we have also completed enrollment in a first of its kind natural history study with 114 patients worldwide to characterize the progression of FOP across numerous outcomes. This study is tracking new HO formation across the body using whole body CT scans (WBCTs) as well as measuring range of motion across all joints. Cross-sectional data indicates a strong correlation between losses in physical function with age. Also, the total body volume of HO in individual patients as well as the number of joints with heterotopic ossification shows strong correlations with these functional outcomes. The findings of this study have been instrumental in establishing that HO is a clinically meaningful endpoint in FOP. Further, given that patients in this study have not received palovarotene, they could be eligible to enroll in our planned registration trial for FOP and we anticipate that many of them will choose to do so.
Palovarotene for MO
Like FOP, MO, also called multiple hereditary exostoses, is an ultra-rare genetic disease of new bone formation in children, which is mediated by excess BMP signaling. Patients with MO develop multiple benign bone tumors, also known as osteochondromas (OCs) or exostoses, on bones. MO affects approximately 20 individuals per million lives, or approximately 150,000 globally, which is approximately 15 times greater than FOP. Patients suffer from substantial morbidities that worsen over time until they reach skeletal maturity. Since it is believed that the mutations which cause MO also result in excess BMP signaling, we believe palovarotene can also inhibit this pathway in MO.
We have generated pre-clinical data demonstrating that palovarotene inhibits the number of OCs by approximately 80% in an animal model of MO as compared to vehicle-treated animals. Based on our knowledge of the safety and tolerability profile of palovarotene and our pre-clinical animal model data, we are planning to initiate a placebo-controlled Phase 2/3 study of palovarotene in MO this year. We expect to report Phase 2/3 clinical data for this trial in 2020 with a potential interim read-out in 2019.
Palovarotene for Dry Eye Disease
We also believe that RARg agonists have great potential as inhibitors of BMP signaling in other indications. Palovarotene has been shown to exert multiple effects in various tissues including in ocular tissues, where RARg agonists generally demonstrate anti-fibrotic properties. As a result, we have conducted pre-clinical proof-of-concept studies in dry eye disease that show that an eye drop formulation of palovarotene can potently increase tear production and decrease corneal damage. Following the completion of these studies, we plan to initiate toxicity studies of an ophthalmologic formulation in order to satisfy the requirements for an investigational new drug (IND) submission, which is required to begin Phase 1 and 2 clinical trials in dry eye disease in 2018.
Other RARg Agonists
We are also focused on developing our RARg agonist platform beyond palovarotene for larger, related disease markets, such as in ankylosing spondylitis or trauma-induced HO. Ankylosing spondylitis is a type of arthritis associated with excess BMP signaling, which the National Institute of Health estimates affects greater than 500,000 people in the United States and represents a high unmet medical need. Other indications, such as those characterized by excessive fibrosis or scarring, are also potential target indications for RARg agonists. On the basis of our scientific know-how and other clinical and commercial insights, a number of indications have been prioritized for animal model proof-of-concept studies in 2017 and 2018. Should these studies be successful, we plan to initiate the pre-IND activities necessary to initiate clinical trials in these new indications.
3
Our Strategy
We strive to become a leading fully-integrated biopharmaceutical company that provides disease modifying treatments to patients suffering from debilitating bone and other diseases with high unmet medical need. We are rapidly developing our lead product candidate, palovarotene, to treat FOP and MO. To achieve our goals, we are executing the following strategy:
Complete development and obtain regulatory approval for our lead product candidate, palovarotene, in FOP and MO.Following the completion of our Phase 2 double-blind, placebo-controlled clinical trial of palovarotene in FOP, we are planning to commence a global multi-site Phase 3 clinical trial for palovarotene in FOP in 2017. In addition, we are planning a study of palovarotene in subjects with FOP who will undergo surgical excision of HO. Following the completion of our pre-clinical proof-of-concept studies showing that palovarotene potently suppresses the number of OCs expressed in animal models of MO, and based on the safety and tolerability profile of palovarotene observed to date, we are planning to initiate a placebo-controlled Phase 2/3 study of palovarotene in MO in 2017. Based on observations we made in our Phase 2 clinical trials, we believe that success in any one of our planned clinical trials can form the basis of FDA approval of palovarotene for the targeted indication. We expect to file for worldwide regulatory approvals of palovarotene in FOP and MO including in the United States, Europe and Japan after generating the relevant Phase 3 and Phase 2/3 clinical data, which we expect to read out in 2019 and 2020.
Independently commercialize palovarotene and improve patient care in FOP and MO.We intend to establish our own commercial organization and have begun to develop a global commercial plan under the leadership of our chief commercial officer. Our plan includes establishing the sales, marketing and reimbursement functions required to commercialize palovarotene in global markets. We actively collaborate with patient groups through a number of initiatives including participation in local meetings and educational initiatives such that we better understand the burdens and unmet needs that patients face, and so that we can better facilitate their access to palovarotene, should it be approved.
Develop palovarotene for other indications including dry eye disease.Palovarotene as a RARg agonist is an inhibitor of BMP signaling and has been shown to exert multiple effects in various tissues including bone, muscle and ocular tissues where it generally demonstrates anti-fibrotic properties. Following the completion of our pre-clinical proof-of-concept studies showing that an eye drop formulation of palovarotene can potently increase tear production and decrease corneal damage, we are initiating IND-enabling toxicity studies of an ophthalmologic formulation in order to begin Phase 1 and 2 clinical trials in dry eye disease in 2018.
Expand our RARg agonists platform.We believe that RARg agonists beyond palovarotene have great potential as inhibitors of BMP signaling in other indications and in particular in inhibiting HO in larger disease markets, such as ankylosing spondylitis or trauma-induced HO. As a result, we intend to further develop our RARg agonist platform beyond palovarotene. We are currently in the process of characterizing second generation RARg agonists recently licensed from Galderma.
Evaluate opportunities to expand our leadership in our areas of expertise. We may also selectively form collaborative alliances to expand our capabilities and product offerings into new therapeutic areas and potentially accelerate commercialization in select geographic markets. Additionally, we may pursue acquisition or in-licensing of product candidates, particularly in our core focus area of rare bone diseases.
Our Team
We have assembled a team of highly skilled and experienced employees, directors and consultants with broad capabilities in drug discovery, development, regulation and commercialization and particular expertise in orphan diseases. Seventy percent of our employees possess advanced scientific degrees. Our management team has substantial industry experience in the orphan disease space and has an average of 24 years of industry experience, with a successful track record of developing and commercializing drug candidates such as Aldurazyme®, Cerezyme®, Fabrazyme®, Myozyme®, Soliris® and Vyndaqel®. Our board members include the former CEOs of companies that developed Synagis®, FluMist®, Gattex®, Natpara® and Strensiq®, the latter two drugs being for the treatment of rare bone diseases. We
4
continue to leverage this specialized expertise and experience to rapidly pursue the development and commercialization of palovarotene in multiple indications. We are backed by a group of leading institutional life science investors, including OrbiMed, New Enterprise Associates, RA Capital, a fund managed by Janus Capital Management LLC, Rock Springs Capital, EcoR1 Capital, UCB Biopharma SPRL, BDC and Fonds de solidarité des travailleurs du Quebec.
Summary Risk Factors
Investing in our common shares involves a high degree of risk. You should carefully consider the risks described in “Risk Factors” before making a decision to invest in our common shares. If any of these risks actually occur, our business, financial condition and results of operations would likely be materially adversely affected. In such case, the trading price of our common shares would likely decline and you may lose part or all of your investment. Below is a summary of some of the principal risks we face:
• | our ability to generate revenue and become profitable; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the ability to obtain, on satisfactory terms or at all, the financing required to support operations, development, clinical trials, and commercialization of products; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risks related to our heavy reliance on palovarotene, our only current product candidate; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risks associated with the development of palovarotene and any future product candidate, including the demonstration of efficacy and safety; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risks related to clinical trials including the risk of negative results, potential delays, cost overruns and potential adverse events or unacceptable side effects; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risks of reliance on third-parties for the planning, conduct and monitoring of clinical trials and for the manufacture of clinical drug supplies and drug product; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | potential changes in regulatory requirements, and delays or negative outcomes from the regulatory approval process; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our ability to successfully compete in our targeted markets, including the risk that competing therapies could emerge; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risks related to healthcare reimbursement policies and potential healthcare reform; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our heavy dependence on licensed intellectual property, including our ability to source and maintain licenses from third-party owners; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risk of patent or other intellectual property related litigation; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risks of adverse tax consequences for our U.S. shareholders if we are characterized as a passive foreign investment company (PFIC). We expect to qualify as a PFIC for our taxable year ending December 31, 2017 and, very possibly, for subsequent years. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Implications of Being an Emerging Growth Company and a Foreign Private Issuer
As a company with less than $1.07 billion in revenue during our most recently completed fiscal year as of the initial filing date of the registration statement of which this prospectus is a part, we qualify as an “emerging growth company” as defined in Section 2(a) of the Securities Act of 1933, as amended (the Securities Act) as modified by the Jumpstart our Business Startups Act of 2012 (the JOBS Act). As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies that are not emerging growth companies. These provisions include an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting.
We may take advantage of these exemptions for up to five years or such time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have $1.07 billion or more in annual revenues as of the end of our fiscal year, more than $700 million in market value of our stock held by non-affiliates as of the end of our second fiscal quarter, or we issue more than $1.0 billion of non-convertible debt securities over a three-year period. We may choose to take advantage of some but not all of these reduced disclosure obligations. If we do, the information
5
that we provide shareholders may be different than you might get from other public companies in which you hold stock.
The JOBS Act permits an emerging growth company such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies. We prepare our financial statements in accordance with IFRS as issued by the IASB, which make no distinction between public and private companies for purposes of compliance with new or revised accounting standards. As a result, the requirements for our compliance as a private company and as a public company are the same.
Upon consummation of this offering, we will report under the Securities Exchange Act of 1934, as amended (the Exchange Act) as a non-U.S. company with foreign private issuer status. As a foreign private issuer, we may take advantage of certain provisions in the Nasdaq Listing Rules that allow us to follow Canadian law for certain corporate governance matters. Even after we no longer qualify as an emerging growth company, as long as we qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
• | the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the rules under the Exchange Act requiring the filing with the U.S. Securities and Exchange Commission of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the sections of the Exchange Act requiring U.S. GAAP financial statements (rather than financial statements pursuant to IFRS as issued by the IASB used by the Company); and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Regulation Fair Disclosure, or Regulation FD, which regulates selective disclosures of material information by issuers. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Both foreign private issuers and emerging growth companies are also exempt from certain more stringent executive compensation disclosure rules. Thus, even if we no longer qualify as an emerging growth company, but remain a foreign private issuer, we will continue to be exempt from the more stringent compensation disclosures required of companies that are neither an emerging growth company nor a foreign private issuer.
Corporate Information
Clementia Pharmaceuticals Inc. was incorporated under the Canada Business Corporations Act on November 5, 2010. The principal executive offices of Clementia Pharmaceuticals Inc. are currently located at 4150 Sainte-Catherine Street West, Suite 550, Montreal, Quebec, Canada H3Z 2Y5. Our telephone number is (514) 940-3600.
Clementia Pharmaceuticals Inc. has a wholly-owned subsidiary, Clementia Pharmaceuticals USA Inc., which was incorporated in the state of Delaware, with a registered office located at 275 Grove Street, Suite 2-400, Newton, Massachusetts, USA.
6
|
|
|
Common shares offered by us | shares (or shares if the underwriters exercise their option to purchase additional shares in full) | |
Common shares to be outstanding immediately after this offering |
| |
Use of proceeds | We estimate that the net proceeds to us from this offering, after deducting underwriting discounts and estimated offering expenses payable by us, will be approximately $ , or approximately $ million if the underwriters exercise their option to purchase additional shares in full, based on an assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus. | |
| We anticipate that we will use the net proceeds of this offering, together with our existing cash on hand, to fund expenses primarily incurred in conducting a Phase 3 and an additional clinical trial of palovarotene for the treatment of FOP, to fund expenses incurred in conducting a Phase 2/3 trial of palovarotene for the treatment of MO, to fund expenses incurred in conducting Phase 1 and Phase 2 clinical trials of palovarotene for the treatment of dry eye disease, and for working capital and other general corporate purposes. See “Use of Proceeds” for more information. | |
Proposed Nasdaq Global Market | “CMTA” | |
Risk factors | See “Risk Factors” beginning on page 10 of this prospectus for a discussion of factors you should consider before deciding to invest in our common shares. |
The number of common shares to be outstanding after this offering is based on of our common shares outstanding as of , 2017 and excludes:
• | common shares issuable upon the exercise of options outstanding as of , 2017 pursuant to our stock option plans, at a weighted-average exercise price of $ per share; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | common shares available for future issuance under our stock option plans. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Except as otherwise noted or the context otherwise requires, all information in this prospectus:
• | assumes no issuance or exercise of options after , 2017; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the automatic conversion of all outstanding shares of our Class A, Class B and Class C convertible preferred shares into shares of common shares; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | reflects a 1-for- stock split, which was effected on | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | assumes the amendment of our articles of incorporation and the amendment and restatement of our by-laws in connection with the consummation of this offering; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | assumes no exercise by the underwriters of their option to purchase additional common shares. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
7
SUMMARY CONSOLIDATED FINANCIAL DATA
The following tables present our historical summary consolidated financial data for the periods, and as of the dates, indicated. Our consolidated financial statements have been prepared in accordance with IFRS as issued by the IASB.
We derived the consolidated statements of net loss and comprehensive loss data for the years ended December 31, 2016, 2015 and 2014 from our audited consolidated financial statements and the related notes thereto appearing elsewhere in this prospectus. We derived the consolidated statements of net loss and comprehensive loss data for the three-month periods ended March 31, 2017 and 2016 and the consolidated statement of financial position data as of March 31, 2017 from our unaudited interim condensed consolidated financial statements and the related notes thereto appearing elsewhere in this prospectus. In the opinion of management, the unaudited interim condensed consolidated financial statements reflect all adjustments necessary for a fair presentation of the financial statements. Our historical results are not necessarily indicative of the results that should be expected in the future, and the results for the three-month period ended March 31, 2017 are not necessarily indicative of the results that may be expected for the full year or any other period.
You should read this summary consolidated financial data together with our consolidated financial statements and related notes and the information under the sections titled “Selected Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Consolidated Statements of Net Loss and Comprehensive Loss Data | Three-months Ended | Year Ended | |||||||||||||||||||||||||||||||||
2017 | 2016 | 2016 | 2015 | 2014 | |||||||||||||||||||||||||||||||
| (in thousands, except share and per share data) | ||||||||||||||||||||||||||||||||||
Expenses |
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Research and development |
|
| $ |
| 3,408 |
|
| $ |
| 3,669 |
|
| $ |
| 16,852 |
|
| $ |
| 14,396 |
|
| $ |
| 7,797 | ||||||||||
Investment tax credits |
|
| (50 | ) |
|
|
| (47 | ) |
|
|
| (139 | ) |
|
|
| (165 | ) |
|
|
| (214 | ) |
| ||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
|
|
| 3,358 |
|
| 3,622 |
|
| 16,713 |
|
| 14,231 |
|
| 7,583 | ||||||||||||||||||||
General and administrative |
|
| 1,668 |
|
| 1,080 |
|
| 3,406 |
|
| 5,479 |
|
| 2,266 | ||||||||||||||||||||
Interest income |
|
| (81 | ) |
|
|
| (103 | ) |
|
|
| (399 | ) |
|
|
| (109 | ) |
|
|
| (19 | ) |
| ||||||||||
Financial expenses |
|
| 36,347 |
|
| 1,432 |
|
| 37,646 |
|
| 56,140 |
|
| 2,364 | ||||||||||||||||||||
Income tax expense |
|
| 44 |
|
| 33 |
|
| 146 |
|
| 156 |
|
| 95 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Net loss and comprehensive loss |
|
| $ |
| (41,336 | ) |
|
|
| $ |
| (6,064 | ) |
|
|
| $ |
| (57,512 | ) |
|
|
| $ |
| (75,897 | ) |
|
|
| $ |
| (12,289 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Net loss per share applicable to common shareholders—basic and diluted |
|
| $ |
| (209.64 | ) |
|
|
| $ |
| (30.92 | ) |
|
|
| $ |
| (293.27 | ) |
|
|
| $ |
| (396.45 | ) |
|
|
| $ |
| (68.32 | ) |
|
Weighted average number of common shares used in net loss per share applicable to common shareholders—basic and diluted |
|
| 197,181 |
|
| 196,109 |
|
| 196,109 |
|
| 191,443 |
|
| 179,874 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Pro forma net loss per share applicable to common shareholders—basic and diluted (unaudited)(1) |
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Pro forma weighted average number of common shares used in net loss per share applicable to common shareholders—basic and diluted (unaudited)(1) |
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
(1) | See the section titled “Selected Consolidated Financial Data” for a discussion of the pro forma weighted-average common shares and pro forma net loss used to compute pro forma net loss per share | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The following table sets forth summary statement of financial position data as of March 31, 2017:
• | on an actual basis, which retrospectively reflects a 1-for- stock split of our common shares; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | on a pro forma basis to give effect to the conversion of all outstanding Class A, Class B and Class C preferred shares into common shares and the resulting re-measurement of the | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
8
| embedded derivative liability, the reclassifications of the original stated capital of the preferred shares into capital stock, the reclassification of the excess of the total carrying value of the preferred shares over the stated capital of the preferred shares and embedded derivative into contributed surplus, and the elimination of the contributed surplus thus created against deficit, as further described in the section titled “Capitalization”; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | on a pro forma basis as adjusted to give further effect to our issuance and sale of common shares in this offering at an assumed initial public offering price of $ per share, the midpoint of the estimated price range set forth on the cover page of this prospectus, after deducting underwriting discounts and estimated offering expenses payable by us. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
| ||||||||||||||||
Consolidated Balance Sheet Data | As at | March 31, | March 31, | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Cash and short-term investments |
|
| $ |
| 43,722 |
|
| $ |
|
| $ | ||||||||||
Preferred share and embedded derivative liabilities |
|
| 231,916 |
|
|
|
| ||||||||||||||
Total equity |
|
| (189,899 | ) |
|
|
|
|
| ||||||||||||
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) the amount of cash and short-term investments, working capital, total assets and total equity by approximately $ , assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and estimated offering expenses payable by us. Similarly, each increase (decrease) of 1,000,000 shares in the number of common shares offered by us would increase (decrease) the amount of cash and short-term investments, working capital, total assets and total equity by approximately $ , assuming that the assumed initial public offering price remains the same and after deducting underwriting discounts and estimated offering expenses payable by us. The pro forma and pro forma as adjusted information discussed above is illustrative only and will change based on the actual initial public offering price and other terms in this offering determined at pricing.
9
Investing in our common shares involves a high degree of risk. You should carefully consider the following risks and uncertainties, together with all other information in this prospectus, including our financial statements and related notes thereto, before investing in our common shares. Any of the risk factors we describe below could adversely affect our business, financial condition or results of operations. The market price of our common shares could decline if one or more of these risks or uncertainties occur, causing you to lose all or part of the money you paid to buy our common shares. Additional risks that we currently do not know about or that we currently believe to be immaterial may also impair our business. Certain statements below are forward-looking statements. See “Cautionary Statement Regarding Forward-Looking Statements” in this prospectus.
Risks Related To Our Financial Position and Need For Capital
We are a clinical stage biopharmaceutical company with a limited operating history and have not generated any revenue from product sales. We have incurred significant operating losses and negative operating cash flows since our inception and anticipate that we will incur continued losses for the foreseeable future.
We are a clinical stage company with a limited operating history on which to base your investment decision. Biopharmaceutical product development is a highly speculative undertaking and involves a substantial degree of risk. We were incorporated in November 2010. Our operations to date have been limited primarily to organizing and staffing our Company, raising capital and conducting research and development activities for palovarotene and any other product candidate. We have never generated any revenue from product sales. We have not obtained regulatory approvals for palovarotene or any other product candidate.
We have funded our operations to date primarily through proceeds from issuances of redeemable convertible preferred stock. From our inception through March 31, 2017, we received gross proceeds of $102.2 million from the sale of our convertible and redeemable preferred shares. As of March 31, 2017 our cash and short-term investments were $43.7 million. We have incurred net losses in each year since our inception. Our net losses were $41.3 million for the three-month period ended March 31, 2017 and $57.5, $75.9 and $12.3 million for the years ended December 31, 2016, 2015 and 2014, respectively, and our negative operating cash flows were $5.9 million for the three-month period ended March 31, 2017 and $18.8, $17.6 and $9.7 million for the years ended December 31, 2016, 2015 and 2014, respectively. Substantially all of our operating losses have resulted from costs incurred in connection with our research and development programs and from general and administrative costs associated with our operations. We expect to incur increasing levels of operating losses over the next several years and for the foreseeable future. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our stockholders’ deficit and working capital. In addition, if we obtain marketing approval for palovarotene or any other product candidate, we will incur significant sales, marketing and outsourced-manufacturing expenses. Once we are a public company, we will incur additional costs associated with operating as a public company. As a result, we expect to continue to incur significant and increasing operating losses for the foreseeable future. Because of the numerous risks and uncertainties associated with developing pharmaceutical products, we are unable to predict the extent of any future losses or when we will become profitable, if at all. Even if we do become profitable, we may not be able to sustain or increase our profitability on a quarterly or annual basis.
Our ability to become profitable depends upon our ability to generate revenue. To date, we have not generated any revenue from palovarotene or any other product candidate and we do not know when, or if, we will generate any revenue. We do not expect to generate significant revenue unless and until we obtain marketing approval of, and begin to sell, palovarotene. Our ability to generate revenue depends on a number of factors, including, but not limited to, our ability to:
• | initiate and successfully complete clinical trials that meet their clinical endpoints; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | initiate and successfully complete all safety studies required to obtain U.S. and foreign marketing approval for palovarotene or any other product candidates; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
10
• | commercialize palovarotene or any other product candidates, if approved, by developing a sales force or entering into collaborations with third parties; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | achieve market acceptance of palovarotene or any other product candidates in the medical community and with third-party payors. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Absent our entering into a collaboration or partnership agreement, we expect to incur significant sales and marketing costs as we prepare to commercialize palovarotene or any other product candidates. Even if we initiate and successfully complete pivotal clinical trials of palovarotene or any other product candidates, and any such product candidate is approved for commercial sale, and despite expending these costs, our product candidate may not be a commercially successful drug. We may not achieve profitability soon after generating product sales, if ever. If we are unable to generate product revenue, we will not become profitable and may be unable to continue operations without continued funding.
Even if this offering is successful, we expect to need to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
We are currently advancing palovarotene through clinical development. Developing our product candidates is expensive, and we expect our research and development and commercialization expenses to increase substantially in connection with our ongoing activities, particularly as we advance palovarotene in clinical trials. Depending on the status of regulatory approval or, if approved, commercialization of palovarotene or any other product candidates, as well as the progress we make in selling palovarotene or any other product candidates, we will require additional capital to fund operating needs thereafter. We may also need to raise additional funds sooner if we choose to pursue additional indications and/or geographies for palovarotene or any other product candidates or otherwise expand more rapidly than we presently anticipate.
As of March 31, 2017, our cash and short-term investments were $43.7 million. We expect that our existing cash and short-term investments will be sufficient to fund our operating expenses for at least the twelve months following March 31, 2017. We estimate that the net proceeds from this offering will be approximately $ , based on the initial public offering price of $ per share, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements or a combination of these approaches. In any event, we will require additional capital to obtain regulatory approval for, and to commercialize, palovarotene or any other product candidate. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize palovarotene or any other product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any product candidates, or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
11
Risks Related To Product Development, Regulatory Approval and Commercialization
We depend heavily on the success of our only current product candidate, palovarotene, for which as of the date of this prospectus, we have completed a Phase 2 clinical trial and we are planning to initiate one registration trial and one Phase 2/3 clinical trial in 2017. We cannot be certain that we will be able to develop, obtain regulatory approval of, or successfully commercialize palovarotene for FOP, MO or any other indication.
We currently have no drug products for sale and may never be able to successfully develop drug products. We have invested the vast majority of our efforts and resources into the development of our only current product candidate, palovarotene, for the treatment of FOP and MO. Our business thus depends heavily on the successful non-clinical and clinical development, regulatory approval and commercialization of palovarotene, for which we are planning two clinical trials for the treatment of FOP and a clinical trial for the treatment of MO.
Palovarotene will require substantial additional clinical development, testing and regulatory approval before we may be permitted to commence its commercialization. The clinical trials of our product candidate are, and the manufacturing and marketing of our product candidate will be, subject to extensive and rigorous review and regulation by numerous government authorities in the United States and in other countries where we intend to test and, if approved, market this or any other product candidate. Before obtaining regulatory approvals for the commercial sale of any product candidate, we must demonstrate through clinical trials that the applicable product candidate is safe and effective for use for the target indication. Drug development is a long, expensive and uncertain process, and delay or failure can occur at any stage of any of our clinical trials. This process can take many years and may include post-marketing studies and surveillance, which may require the expenditure of substantial resources beyond the proceeds we raise in this offering. Of the large number of drugs in development in the United States at any time, only a small percentage will successfully complete the U.S. Food and Drug Administration (FDA) regulatory approval process and will be commercialized. Accordingly, even if we are able to obtain the requisite financing to continue to fund our development and clinical trials, we cannot assure you that our product candidate will be successfully developed or commercialized.
We are not permitted to market our product candidate in the United States until we receive approval of a new drug application (NDA) from the FDA, or market in any foreign countries until we receive the requisite approval from such countries. We are currently planning to initiate a Phase 3 clinical trial to study safety, tolerability and efficacy of palovarotene in patients with FOP. We expect that the FDA will require us to complete one Phase 3 trial in order to submit an NDA for palovarotene as a treatment for FOP patients. We are also currently planning a Phase 2/3 trial using palovarotene for the treatment of MO. We believe that successful completion of this Phase 2/3 study may be sufficient for us to file an NDA for palovarotene for MO. We also intend to discuss the details and timing of a clinical trial in surgical excision of HO using palovarotene with the FDA. The FDA has indicated that we need additional positive data from our trials in FOP prior to commencing this trial. We cannot be certain that the FDA will not require that we conduct additional pivotal trials before we can submit an NDA for palovarotene. We have only sought general feedback to date from the FDA on what would be required in a Phase 3 clinical trial of palovarotene for the treatment of FOP, and we have not yet met with the FDA to discuss our trial plans for surgical excision of HO in FOP, or for the treatment of MO. We intend to discuss our protocols for our proposed clinical trials of palovarotene for FOP and MO prior to initiating the trials. We cannot be certain that the FDA will agree on all aspects of the design of our clinical trials. The FDA may require that we conduct additional toxicity studies and may also require us to conduct additional non-clinical studies before submitting an NDA for palovarotene.
Obtaining approval of an NDA is a complex, lengthy, expensive and uncertain process, and the FDA may delay, limit or deny approval of our product candidate for many reasons, including, among others:
• | we may not be able to demonstrate that palovarotene or any other product candidate is safe and effective in treating FOP to the satisfaction of the FDA; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
12
• | the results of our clinical trials may not meet the level of statistical or clinical significance required by the FDA for marketing approval; for example, FDA may not agree new HO volume is a clinically meaningful endpoint or that the magnitude of change in HO volume is clinically meaningful without the support of secondary endpoints; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the FDA may disagree with the number, design, size, conduct or implementation of our clinical trials; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the FDA may require that we conduct non-clinical studies and additional clinical trials; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the FDA may not approve the formulation, labeling or specifications of palovarotene or any other product candidate; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the contract research organizations (CROs) that we retain to conduct our non-clinical studies and clinical trials may take actions outside of our control that adversely impact such studies or trials; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the FDA may find the data from non-clinical studies and clinical trials insufficient to demonstrate that palovarotene or any other product candidate’s clinical and other benefits outweigh its safety risks; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the FDA may disagree with our interpretation of data from our non-clinical studies or clinical trials; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the FDA may not accept some or all of the data generated at our non-clinical studies and clinical trial sites; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | if our NDA, if and when submitted, is reviewed by an advisory committee, the FDA may have difficulties scheduling an advisory committee meeting in a timely manner or the advisory committee may recommend against approval of our application or may recommend that the FDA require, as a condition of approval, non-clinical studies or additional clinical trials, limitations on approved labeling or distribution and use restrictions; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the FDA may determine that the manufacturing processes or facilities of third-party manufacturers with which we contract do not conform to applicable requirements, including current Good Manufacturing Practices (cGMPs); or | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the FDA may change its approval policies or adopt new regulations. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Any of these factors and others, many of which are beyond our control, could jeopardize our ability to obtain regulatory approval for and successfully market palovarotene or any other product candidate. Any such setback in our pursuit of regulatory approval would have a material adverse effect on our business and prospects.
The number of patients suffering from FOP and MO is small and has not been established with precision. If the actual number of patients with FOP or MO is smaller than we anticipate, we may encounter difficulties in enrolling patients in our clinical trials, thereby delaying or preventing development of palovarotene or any other product candidate, and if palovarotene or any other product candidate is approved, our revenue and ability to achieve profitability may be materially adversely affected.
There is no precise method of establishing the actual number of patients with FOP or MO in any geography over any time period. We estimate that the number of individuals affected by FOP is approximately 1.3 individuals per million lives globally and the number of individuals affected by MO is approximately 20 individuals per million lives worldwide. If we are not able to identify and recruit a sufficient number of patients, we will have difficulty completing our clinical trials. Moreover, other companies are trying to recruit patients for their trials which may negatively impact our ability to recruit patients for our trials. Because the estimated number of patients is so small, and particularly if the actual number of patients with FOP or MO is lower than we believe, we may experience difficulty in enrolling patients in our clinical trials, thereby delaying development of palovarotene.
Further, if palovarotene or any other product candidate is approved, the markets for FOP or MO could be smaller than we anticipate, which could limit our ability to achieve profitability.
13
If serious adverse events or unacceptable side effects are identified during the development of palovarotene or at any other time, we may need to delay, limit or terminate our clinical development activities, and such adverse events or unacceptable side effects may negatively impact the regulatory approval and commercial profile of palovarotene, and have other significant negative consequences.
Clinical trials by their nature utilize a sample of the potential patient population. Accordingly, any rare and severe side effects of palovarotene may be uncovered only in later stages of our product development activities or only in subsequent trials that we may conduct. Many product candidates that initially showed promise in early stage testing have later been found to cause side effects that prevented their further development. We have observed an increase in specific mucocutaneous side effects when higher doses of palovarotene are administered. These included pruritus (or itchiness), generalized pruritus, excoriation (or skin abrasion), rash and alopecia (or hair loss), none of which were considered severe. These mucocutaneous side-effects required dose de-escalations in approximately 20% of subjects due primarily to dry skin, alopecia and pruritus. We are planning to evaluate in our registration trial chronic dosing for a duration that will support lifetime chronic dosing of palovarotene and as a result the severity of side effects observed to date may increase and new side effects may emerge. Since palovarotene is a retinoid and therefore a teratogen, women who are or expect to become pregnant will not be able to take palovarotene and major fetal abnormalities may occur if it is taken, as any fetus that is exposed can be affected. Moreover, the development of palovarotene or any other product candidate may cause certain undesirable side effects in certain patient populations or have characteristics that are unexpected, and we may need to abandon development or limit development to more narrow uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective, any of which could adversely affect our business, prospects, financial condition and results of operations.
Further, it is possible that rare and severe side effects of palovarotene or any other product candidate may only be uncovered after palovarotene or any other product candidate receives marketing approval. This would result in a number of potentially significant negative consequences, including:
• | regulatory authorities may withdraw or limit their approval of such product candidate; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | regulatory authorities may require the addition of labeling statements, such as a “boxed” warning or a contraindication; for example, palovarotene, like all retinoids, is a teratogen and its labeling will communicate that it must not be taken by pregnant women or women who may become pregnant; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | we may be required to change the way such product candidate is distributed or administered, conduct additional clinical trials or change the labeling of the product candidate; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | we may be subject to regulatory investigations and government enforcement actions; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | we may decide to remove such product candidate from the marketplace; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | we could be sued and held liable for injury caused to individuals exposed to or taking our product candidate; or | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our reputation may suffer. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
We believe that any of such events could prevent us from achieving or maintaining market acceptance of the affected product candidate and could substantially increase the costs of commercializing palovarotene or any other product candidate and significantly impact our ability to successfully commercialize palovarotene or any other product candidate and generate revenues.
Results from early clinical trials of palovarotene or any other product candidate are not necessarily predictive of the results of later non-clinical studies and clinical trials of palovarotene or any other product candidate. If later clinical trials are not successful, we will not be able to successfully develop, obtain regulatory approval for and commercialize palovarotene or any other product candidate.
Any trends we observe and results we obtain from our early clinical trials of palovarotene or any other product candidate may not necessarily be predictive of the results from later clinical trials. For example, as is common with Phase 2 trials, particularly with the first clinical trials to be conducted in a
14
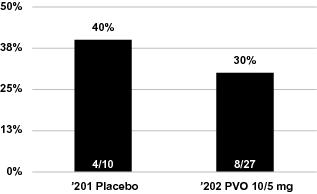
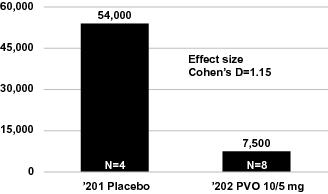
patient population, we explored numerous endpoints and analyzed the data from our Phase 2 clinical trials of palovarotene in a number of ways. Product candidates such as palovarotene in Phase 3 clinical trials may fail to demonstrate sufficient efficacy despite having progressed through initial clinical trials, even if certain analyses of primary or secondary endpoints or cross-study comparisons of pooled results in those early trials showed trends toward efficacy. Much of the data we present on the use of palovarotene for the treatment of FOP is drawn from the results of multiple clinical trials which have been pooled and then analyzed. While we believe this data is useful in informing the design of future clinical trials in palovarotene, cross-study comparisons of pooled results involve the inherent bias of post-hoc manipulation of data and choice of analytical methods, as well as methodological issues surrounding heterogeneity among studies contributing to the analyses; therefore, it is important to view such results in light of the totality of all available information, such as individual study results on pre-specified analyses of endpoints. Prior to obtaining approval for palovarotene, the results of our registration trials will have to demonstrate statistically significant improvement in the pre-specified primary endpoint in the applicable registration trial. To date, our clinical trials of palovarotene have not demonstrated statistically significant results. Further, different results may be achieved depending upon whether the Per Protocol (PP) population is used to report results or the Full Analysis Set or an Intent-to-Treat (ITT) population is used. While we believe the PP analysis may be more applicable for our Phase 2 studies because their primary purpose is in determining the biological effect of palovarotene such as by eliminating data relating to study participants that did not adhere to the trial protocol, we expect that the primary analysis for any registration trial would need to show efficacy on the full analysis set population or an ITT population. Also, our later-stage clinical trials could differ in significant ways from our Phase 2 clinical trials of palovarotene, which may cause the outcome of these later-stage trials to differ from our earlier stage clinical trials. These differences may include changes to inclusion and exclusion criteria, efficacy endpoints and statistical design. For example, we expect our Phase 3 clinical trial in FOP to implement a chronic dosing regimen with increased dosing in the case of flare-ups and a primary endpoint of new HO volume as measured by whole body CT scans. This dosing regimen and primary endpoint of annualized change in new HO volume is different from those of our ‘201 study and Part A of our ‘202 study, and different from the data we present herein on the use of palovarotene for the treatment of FOP; this Phase 3 primary endpoint is a continuous variable rather than a discrete, responder analysis, as was performed in our ‘201 study and Part A of our ‘202 study. These differences may have an effect on the ability of our Phase 2 clinical trials to predict the outcome of our Phase 3 clinical trial. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials after achieving positive results in early-stage development, and we cannot be certain that we will not face similar setbacks. These setbacks have been caused by, among other things, non-clinical findings made while clinical trials were underway or safety or efficacy observations made in non-clinical studies and clinical trials, including previously unreported adverse events. Moreover, non-clinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in non-clinical studies and clinical trials nonetheless failed to obtain FDA approval. If we fail to produce positive results in our clinical trials of palovarotene or any other product candidate, the development timeline and regulatory approval and commercialization prospects for palovarotene or any other product candidate, and, correspondingly, our business and financial prospects, would be materially adversely affected.
Failures or delays in the commencement or completion of our planned clinical trials of palovarotene or any other product candidate could result in increased costs to us and could delay, prevent or limit our ability to generate revenue and continue our business.
We believe we will need to complete at least one additional trial prior to the submission of an NDA for palovarotene. Successful completion of our clinical trials is a prerequisite to submitting an NDA to the FDA and, consequently, the ultimate approval and commercial marketing of palovarotene. We are currently planning to initiate one registration trial and one Phase 2/3 clinical trial in 2017; however, we do not know if these trials or any future clinical trials will begin or be completed on schedule, if at all, as the commencement and completion of clinical trials can be delayed or prevented for a number of reasons, including, among others:
15
• | the FDA may deny permission to proceed with our planned clinical trials or any other clinical trials we may initiate, or may place a clinical trial on hold; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | delays in filing or obtaining permission to proceed under additional investigational new drug applications (INDs) that may be required; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | delays in reaching or failing to reach agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | inadequate quantity or quality of palovarotene or any other product candidate or other materials necessary to conduct clinical trials, for example delays in the manufacturing of sufficient supply of finished drug product; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | difficulties obtaining Institutional Review Board (IRB) approval to conduct a clinical trial at a prospective site or sites; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | challenges in recruiting and enrolling patients to participate in clinical trials, including the small size of the patient population, acute nature of the flare-ups in and chronic nature of the disease FOP, the proximity of patients to trial sites, eligibility criteria for the clinical trial, the nature of the clinical trial protocol and competition from other clinical trial programs for similar indications; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | severe or unexpected drug-related side effects experienced by patients in a clinical trial; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | delays in validating any endpoints utilized in a clinical trial that we may choose to use, particularly any clinical outcome measures, such as the FOP-Physical Function Questionnaire that we have developed; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the FDA may disagree with our clinical trial design and our interpretation of data from clinical trials, or may change the requirements for approval even after it has reviewed and commented on the design for our clinical trials; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | reports from non-clinical or clinical testing of other FOP and MO treatments that raise safety or efficacy concerns; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | difficulties retaining patients who have enrolled in a clinical trial but may be prone to withdraw due to rigors of the clinical trials, lack of efficacy, side effects, personal issues or loss of interest. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinical trials may also be delayed or terminated as a result of ambiguous or negative interim results. In addition, a clinical trial may be suspended or terminated by us, the FDA, the IRBs at the sites where the IRBs are overseeing a clinical trial, a data and safety monitoring board, overseeing the clinical trial at issue or other regulatory authorities due to a number of factors, including, among others:
• | failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | inspection of the clinical trial operations or trial sites by the FDA or other regulatory authorities that reveals deficiencies or violations that require us to undertake corrective action, including the imposition of a clinical hold; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | unforeseen safety issues, adverse side effects or lack of effectiveness; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | changes in government regulations or administrative actions; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | problems with clinical supply materials; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | lack of adequate funding to continue clinical trials. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Product development costs for palovarotene for FOP, MO or for any other future indications we may pursue or for any other product candidates we may develop in the future will increase if we have delays in testing, or if we need to perform more or larger clinical studies than planned. If we experience delays in completion of any of our clinical trials, or if we, the FDA, other regulatory authorities, IRBs or other reviewing entities, or any of our clinical trial sites suspend or terminate any of our clinical trials of palovarotene for any indication, its commercial prospects may be harmed and our ability to generate product revenues will be delayed, if we are able to generate product revenue at all. Any delays in completing our clinical trials will increase our costs, slow down our development and approval process
16
and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, termination or suspension of, or a delay in the commencement or completion of, clinical trials may also ultimately lead to the denial or even withdrawal of regulatory approval of palovarotene for any indication. In addition, if one or more clinical trials are delayed, our competitors may be able to bring products to market before we do, and the commercial viability of palovarotene could be significantly reduced.
We have never completed a Phase 3 clinical trial or registration trial, or submitted an NDA before and may be unable to do so for palovarotene.
The conduct of Phase 3 clinical trials and registration trials, and the submission of a successful NDA is a complicated process. We have never conducted a Phase 3 clinical trial or registration trial before, have limited experience in preparing and submitting regulatory filings, and have not submitted an NDA before. Consequently, we may be unable to successfully and efficiently execute and complete these planned clinical trials in a way that leads to NDA submission and approval of palovarotene. We may require more time and incur greater costs than our competitors and may not succeed in obtaining regulatory approvals of drug candidates that we develop. Failure to commence or complete, or delays in, our planned clinical trials would prevent or delay commercialization of palovarotene.
Potential changes in regulatory requirements, FDA guidance or unanticipated events during clinical trials of palovarotene or any other product candidate may result in changes to clinical trial protocols or additional non-clinical studies and clinical trial requirements, which could result in increased costs to us and could delay our development timeline.
Changes in regulatory requirements, FDA guidance or unanticipated events during our clinical trials may force us to amend clinical trial protocols or cause the FDA to impose new or additional clinical trial requirements. For example, the endpoints in our clinical trials may change. Based on our discussions with the FDA to date, the FDA has indicated that new HO volume is a clinically meaningful outcome for our Phase 3 clinical trial for palovarotene in FOP provided we pre-specify the magnitude of treatment effect and support the HO findings with secondary endpoints. However, there can be no assurance that the FDA may not later determine that mean new HO volume does not qualify as a clinically meaningful endpoint for our Phase 3 clinical trial for palovarotene. Further, we have not discussed with the FDA endpoints related to our Phase 2/3 clinical trial in MO or clinical trial for surgical release in FOP.
Amendments or changes to our clinical trial protocols would require resubmission to the FDA and IRBs for review and approval, which may adversely impact the cost, timing or successful completion of clinical trials. If we experience delays completing, or if we terminate, any of our clinical trials, or if we are required to conduct non-clinical studies or additional clinical trials, the commercial prospects for palovarotene and any other product candidate may be harmed and our ability to generate product revenue will be delayed, if we are able to generate product revenue at all.
We rely, and expect that we will continue to rely, on third parties to conduct clinical trials for palovarotene and any other product candidate. If these third parties do not successfully carry out their contractual duties or fail to meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize palovarotene and any other product candidate and our business could be substantially harmed.
We do not have the ability to independently conduct clinical trials. We rely on universities, contract laboratories and other third parties, such as CROs, to conduct clinical trials on palovarotene and any other product candidate. We enter into agreements with third-party CROs to provide monitors for and to manage data for our ongoing clinical trials. We rely heavily on these parties for execution of clinical trials for palovarotene and any other product candidate and control only certain aspects of their activities. As a result, we have less direct control over the conduct, timing and completion of these clinical trials and the management of data developed through clinical trials than would be the case if we were relying entirely upon our own staff. Communicating with outside parties can also be challenging,
17
potentially leading to mistakes as well as difficulties in coordinating activities. Outside parties may, among other things:
• | have staffing difficulties; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | fail to comply with contractual obligations; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | experience regulatory compliance issues; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | undergo changes in priorities or become financially distressed; or | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | form relationships with other entities, some of which may be our competitors. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
These factors may materially adversely affect the willingness or ability of third parties to conduct our clinical trials and may subject us to unexpected cost increases that are beyond our control. Nevertheless, we are responsible for ensuring that each of our clinical trials is conducted in accordance with the applicable protocol, legal, regulatory and scientific requirements and standards, and our reliance on CROs does not relieve us of our regulatory responsibilities. We and our CROs are required to comply with regulations and guidelines, including current Good Clinical Practices (cGCPs) for conducting, monitoring, recording and reporting the results of clinical trials to ensure that the data and results are scientifically credible and accurate, and that the trial patients are adequately informed of the potential risks of participating in clinical trials. These regulations and standards are enforced by the FDA, the Competent Authorities of the Member States of the European Economic Area and comparable foreign regulatory authorities for any products in clinical development. The FDA enforces cGCP regulations and standards through periodic inspections of clinical trial sponsors, principal investigators and trial sites. If we or our CROs fail to comply with applicable cGCPs, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, the FDA will determine that any of our clinical trials comply with cGCPs. In addition, our clinical trials must be conducted with product candidates produced under cGMPs regulations and will require a large number of test patients. Our failure or the failure of our CROs to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process and could also subject us to enforcement action up to and including civil and criminal penalties.
Although we design our clinical trials for palovarotene and will do so for any future product candidate, CROs conduct all of the clinical trials. As a result, many important aspects of our drug development programs are outside of our direct control. In addition, the CROs may not perform all of their obligations under arrangements with us or in compliance with regulatory requirements, but we remain responsible and are subject to enforcement action that may include civil penalties up to and including criminal prosecution for any violations of FDA laws and regulations during the conduct of our clinical trials. If the CROs do not perform clinical trials in a satisfactory manner, breach their obligations to us or fail to comply with regulatory requirements, the development and commercialization of palovarotene and any other product candidates may be delayed or our development program materially and irreversibly harmed. We cannot control the amount and timing of resources these CROs devote to our program or our clinical products. If we are unable to rely on clinical data collected by our CROs, we could be required to repeat, extend the duration of, or increase the size of our clinical trials and this could significantly delay commercialization and require significantly greater expenditures.
If any of our relationships with these third-party CROs terminates, we may not be able to enter into arrangements with alternative CROs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, any clinical trials such CROs are associated with may be extended, delayed or terminated, and we may not be able to obtain regulatory approval for or successfully commercialize palovarotene and any other product candidate. As a result, we believe that our financial results and the commercial prospects for palovarotene and any other product candidate in the subject indication would be harmed, our costs could increase and our ability to generate revenue could be delayed.
18
Sales of counterfeit palovarotene or unauthorized sales of palovarotene may have a material adverse effect on our revenues, business, results of operations and damage our brand and reputation.
Palovarotene may become subject to competition from counterfeit pharmaceutical products, which are pharmaceutical products sold under the same or very similar brand names and/or having a similar appearance to genuine products, but which are sold without proper licenses or approvals. Such products divert sales from genuine products, often are of lower cost, often are of lower quality (having different ingredients or formulations, for example), and have the potential to damage the reputation for quality and effectiveness of the genuine product.
Obtaining regulatory approval for palovarotene is a complex and lengthy process. If during the period while the regulatory approval is pending illegal sales of counterfeit palovarotene begin, consumers may buy such counterfeit palovarotene, which could have an adverse impact on our revenues, business and results of operations. In addition, if illegal sales of counterfeit palovarotene result in adverse side effects to consumers, we may be associated with any negative publicity resulting from such incidents.
Although pharmaceutical regulation control and enforcement systems throughout the world have been increasingly active in policing counterfeit pharmaceuticals, we may not be able to prevent third parties from manufacturing, selling or purporting to sell counterfeit products competing with our product candidate.
Further, we are aware that third parties are manufacturing unauthorized palovarotene for research use, and it is possible that unauthorized sales of palovarotene may be made through such channels without our knowledge.
The existence and any increase in production or sales of counterfeit palovarotene or unauthorized palovarotene could negatively impact our revenues, brand reputation, business and results of operations.
We rely completely on one third-party supplier to manufacture the active pharmaceutical ingredient (API) for palovarotene and another third party supplier to manufacture final drug product, and we intend to rely on third parties to produce non-clinical, clinical and commercial supplies of any future product candidates.
We do not currently have, nor do we plan to acquire, the infrastructure or capability to internally manufacture our clinical drug supply of palovarotene, or any future product candidates, for use in the conduct of our non-clinical studies and clinical trials, and we lack the internal resources and the capability to manufacture any product candidates on a clinical or commercial scale. We currently have the API for palovarotene manufactured by one supplier and final drug product supplied by another supplier. At this time, we have not identified secondary sources of clinical supplies of palovarotene, and identifying an additional manufacturer would require significant delay and likely significant additional cost.
The facilities used by contract manufacturers to manufacture the active pharmaceutical ingredient and final drug product will generally be required to complete a pre-approval inspection by the FDA and other comparable foreign regulatory agencies to assess compliance with applicable requirements, including cGMPs, after we submit our NDA or relevant foreign regulatory submission to the applicable regulatory agency.
We do not control the manufacturing process of, and are completely dependent on, our contract supplier to comply with cGMPs for manufacture of the active pharmaceutical ingredient for our clinical trials, and upon another supplier for manufacture of final drug product. These manufacturers must successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or applicable foreign regulatory agencies. In addition, although we have no direct control over these manufacturers’ ability to maintain adequate quality control, quality assurance and qualified personnel, we are responsible for ensuring that our materials and product candidates are manufactured in accordance with cGMPs. Furthermore, these manufacturers are engaged with other companies to supply and/or manufacture materials or products for such companies, which exposes our manufacturers to regulatory risks for the production of such materials and products. As a result, failure to satisfy the regulatory requirements for the production of those materials and products may affect the
19
status of their facilities generally. If the FDA or an applicable foreign regulatory agency determines now or in the future that these facilities for the manufacture of our product candidate are noncompliant, any pending NDAs or other applications for marketing authorization may not be approved until the facilities have a compliance status that is acceptable to FDA or an applicable foreign regulatory agency. In such case, we may need to find alternative manufacturing facilities, which would adversely impact our ability to develop, obtain regulatory approval for or market palovarotene and any future product candidate. Our reliance on these manufacturers also exposes us to the possibility that they, or third parties with access to their facilities, will have access to and may appropriate our trade secrets or other proprietary information.
We do not have long-term supply agreements in place with any party, and each batch of palovarotene is individually contracted under a quality and supply agreement. If we engage new contractors, such contractors must complete an inspection by the FDA and other applicable foreign regulatory agencies. We plan to continue to rely upon the same manufacturers and, potentially, collaboration partners to manufacture commercial quantities of palovarotene, if approved.
If we are unable to obtain the supplies we need at a reasonable price or on a timely basis, it could have a material adverse effect on our ability to complete the development of palovarotene or, if we obtain regulatory approval for palovarotene, to properly commercialize it.
Even though we have obtained orphan drug designation for palovarotene as a treatment for FOP, there may be limits to the regulatory exclusivity afforded by such designation.
Even though we obtained orphan drug designation in July 2014 for palovarotene for treatment of FOP by the FDA and the EMA, there are limitations to exclusivity afforded by such designation. In the United States, the company that first obtains FDA approval for a designated orphan drug for the specified rare disease or condition receives orphan drug marketing exclusivity for that drug for a period of seven years. This orphan drug exclusivity prevents the FDA from approving another application, including a full NDA to market the same drug for the same orphan indication, except in very limited circumstances, including when the FDA concludes that the later drug is safer, more effective or makes a major contribution to patient care. For purposes of small molecule drugs such as palovarotene, the FDA defines “same drug” as a drug that contains the same active moiety and is intended for the same use as the drug in question. To obtain orphan drug exclusivity for a drug that shares the same active moiety as an already approved drug, it must be demonstrated to the FDA that the drug is safer or more effective than the approved orphan designated drug, or that it makes a major contribution to patient care. In addition, a designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition or if another drug with the same active moiety is determined to be safer, more effective, or represents a major contribution to patient care.
In Europe, orphan drugs may be able to obtain ten years of marketing exclusivity and up to an additional two years on the basis of qualifying pediatric studies. However, orphan exclusivity may be reduced to six years if the drug no longer satisfies the original designation criteria. Additionally, a marketing authorization holder may lose its orphan exclusivity if it consents to a second orphan drug application or cannot supply enough drug. Orphan drug exclusivity also can be lost when a second applicant demonstrates its drug is “clinically superior” to the original orphan drug.
Even though we have obtained Fast Track Designation from the FDA for palovarotene for the treatment of FOP, it may not lead to a faster development or regulatory review or approval process, and does not increase the likelihood that palovarotene will receive marketing approval.
If a drug is intended for the treatment of a serious or life-threatening condition and the drug demonstrates the potential to address unmet medical needs for this condition, the drug sponsor may apply to the FDA for Fast Track Designation. Even though we have obtained Fast Track Designation for palovarotene for treatment of FOP, we may not experience a faster development process, review or approval compared to conventional FDA procedures. The FDA may withdraw Fast Track Designation
20
if it believes that the designation is no longer supported by data from our clinical development program. Many drugs that have received Fast Track Designation have failed to obtain approval.
Even if we receive marketing approval for palovarotene in the United States, we may never receive regulatory approval to market palovarotene outside of the United States.
We have selected markets outside of the United States, including but not limited to parts of Europe, Asia, South America and Canada, where we intend to seek regulatory approval to market palovarotene. In order to market any product outside of the United States, however, we must establish and comply with the numerous and varying safety, efficacy and other regulatory requirements of any such other country. Approval procedures vary among countries and can involve additional product candidate testing and additional administrative review periods. The time required to obtain approvals in other countries might differ from that required to obtain FDA approval. The marketing approval processes in other countries may implicate all of the risks detailed above regarding FDA approval in the United States as well as other risks. In particular, in many countries outside of the United States, products must receive pricing and reimbursement approval before the product can be commercialized. Obtaining this approval can result in substantial delays in bringing products to market in such countries. Marketing approval in one country does not ensure marketing approval in another, but a failure or delay in obtaining marketing approval in one country may have a negative effect on the regulatory process in others. Failure to obtain marketing approval in other countries or any delay or other setback in obtaining such approval would impair our ability to market palovarotene in such foreign markets. Any such impairment would reduce the size of our potential market, which could have a material adverse impact on our business, results of operations and prospects.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell palovarotene or any other product candidate, we may not be able to generate any revenue.
We do not currently have an infrastructure for the sales, marketing and distribution of pharmaceutical products. In order to market palovarotene or any other product candidate, if approved by the FDA or any other regulatory body, we must build our sales, marketing, managerial and other non-technical capabilities or make arrangements with third parties to perform these services. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, or if we are unable to do so on commercially reasonable terms, our business, results of operations, financial condition and prospects will be materially adversely affected.
Even if we receive marketing approval for palovarotene or any other product candidate, our product candidate may not achieve broad market acceptance, which would limit the revenue that we generate from its sales.
The commercial success of palovarotene or any other product candidate, if approved by the FDA or other applicable regulatory authorities, will depend upon the awareness and acceptance of palovarotene or any other product candidate among the medical community, including physicians, patients and healthcare payors. Market acceptance of palovarotene or any other product candidate, if approved, will depend on a number of factors, including, among others:
• | the efficacy of palovarotene or any other product candidate as demonstrated in clinical trials; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | limitations or warnings contained in the labeling approved for palovarotene or any other product candidate by the FDA or other applicable regulatory authorities, including that palovarotene, like all retinoids, is a teratogen and must not be taken by pregnant women or women who may become pregnant; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the clinical indications for which palovarotene or any other product candidate is approved; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | availability of alternative treatments already approved or expected to be commercially launched in the near future; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the potential and perceived advantages of palovarotene or any other product candidate over current treatment options or alternative treatments, including future alternative treatments; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
21
• | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the strength of marketing and distribution support and timing of market introduction of competitive products; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | publicity concerning our product or any competing products and treatments; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | pricing and cost effectiveness; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the effectiveness of our sales and marketing strategies; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our ability to increase awareness of palovarotene or any other product candidate through marketing efforts; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our ability to obtain sufficient third-party coverage or reimbursement; or | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the willingness of patients to pay out-of-pocket in the absence of third-party coverage. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If palovarotene or any other product candidate is approved but does not achieve an adequate level of acceptance by patients, physicians and payors, we may not generate sufficient revenue from palovarotene or any other product candidate to become or remain profitable. Before granting reimbursement approval, healthcare payors may require us to demonstrate that palovarotene or any other product candidate, in addition to treating its intended target indication, also provides incremental health benefits to patients. Our efforts to educate the medical community and third-party payors about the benefits of palovarotene and any other product candidate may require significant resources and may have limited or no success.
If we obtain approval to commercialize palovarotene or any other product candidate outside of the United States, a variety of risks associated with international operations could materially adversely affect our business.
If palovarotene or any other product candidate is approved for commercialization, we may enter into agreements with third parties to market it outside of the United States. We expect that we will be subject to additional risks related to entering into international business relationships, including:
• | different regulatory requirements for drug approvals in foreign countries; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | reduced protection for intellectual property rights; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | unexpected changes in tariffs, trade barriers and regulatory requirements; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | economic weakness, including inflation, or political instability in particular foreign economies and markets; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | foreign taxes, including withholding of payroll taxes; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, or other obligations incidental to doing business in another country; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | workforce uncertainty in countries where labor unrest is more common than in the United States; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; or | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters, including earthquakes, typhoons, floods and fires. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Even if we receive marketing approval for palovarotene or any other product candidate, we may still face future development and regulatory difficulties.
Even if we receive marketing approval for palovarotene or any other product candidate, regulatory authorities may still impose significant restrictions on palovarotene or any other product candidate, indicated uses or marketing, or impose ongoing requirements for potentially costly post-approval studies. Palovarotene and any other product candidate will also be subject to ongoing FDA
22
requirements governing the labeling, packaging, storage and promotion of the product as well as record keeping, submission of safety and other post-market information, and prohibitions regarding the promotion of off-label uses.
The FDA has significant post-marketing authority, including, for example, the authority to require labeling changes based on new safety information and to require post-marketing studies or clinical trials to evaluate serious safety risks related to the use of a drug. The FDA also has the authority to require, as part of an NDA or post-approval, the submission of a Risk Evaluation and Mitigation Strategy. Any Risk Evaluation and Mitigation Strategy required by the FDA may lead to increased costs to assure compliance with new post-approval regulatory requirements and potential requirements or restrictions on the sale of approved products, all of which could lead to lower sales volume and revenue. Further, the FDA and other regulatory agencies strictly regulate the promotional claims that may be made about prescription products, and we could be subject to significant liability if, for example, physicians prescribe palovarotene to their patients in a manner that is inconsistent with the approved label and we are found to have promoted such off-label uses.
Manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with cGMPs and other regulations. If we or a regulatory agency discover problems with palovarotene or any other product candidate, such as adverse events of unanticipated severity or frequency, or problems with the facility where our product candidate is manufactured, a regulatory agency may impose restrictions or penalties on our product candidate, the manufacturer or us, including requiring withdrawal of our product candidate from the market or suspension of manufacturing.
The occurrence of any regulatory penalty may inhibit or preclude our ability to commercialize palovarotene and any other future product candidates we may develop which in turn would inhibit or preclude our ability to generate revenue.
Competing therapies could emerge, adversely affecting our opportunity to generate revenue from the sale of our product candidate.
The biopharmaceuticals industry is highly competitive. There are many public and private biopharmaceutical companies, universities, governmental agencies and other research organizations actively engaged in the research and development of products that may be similar to palovarotene or any other product candidate or address similar markets. We believe it is probable that the number of companies seeking to develop products and therapies similar to our product will increase. Gene therapy, cell therapy and other approaches may also emerge for the treatment of any of the disease areas in which we focus.
Currently, there are no therapies that have been specifically approved for treatment of FOP. However, products approved for other indications, for example, cortisteroids and non-steroidal anti-inflammatory drugs, are used off-label to manage FOP symptoms during flare-ups, though none of these medications has been shown to prevent HO. Further, we are aware that Regeneron Pharmaceuticals Inc., Blueprint Medicines and La Jolla Pharmaceutical Company are each in early stages of development of therapies for the treatment of FOP. See “Business—Competition” for a more detailed discussion.
Many of our potential competitors, alone or with their strategic partners, could have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of treatments and the commercialization of those treatments. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
23
We may not be able to establish collaborations, and, if we are able to establish collaborations we may not be able to establish them on commercially reasonable terms, which could result in alterations or delays of our development and commercialization plans.
Our drug development program and the potential commercialization of palovarotene and any other product candidate will require substantial additional cash to fund expenses. We may decide to collaborate with pharmaceutical and biotechnology companies for the development and potential commercialization of palovarotene and any other product candidate. We are restricted under existing collaboration agreements, such as pursuant to certain rights granted to Roche under our agreement with it from entering into future agreements on certain terms with potential collaborators.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the product candidate, the costs and complexities of manufacturing and delivering the product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our product candidate. The terms of any collaborations or other arrangements that we may establish may not be favorable to us.
Collaborations are complex and time-consuming to negotiate and document. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
We may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of the product candidate, reduce or delay its development program, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to further develop our product candidate or bring it to market and generate product revenue.
In addition, any future collaborations that we enter into may not be successful. The success of our collaboration arrangements will depend heavily on the efforts and activities of our collaborators. Collaborators generally have significant discretion in determining the efforts and resources that they will apply to these collaborations. Disagreements between parties to a collaboration arrangement regarding clinical development and commercialization matters can lead to delays in the development process or commercializing the applicable product candidate and, in some cases, termination of the collaboration arrangement. These disagreements can be difficult to resolve if neither of the parties has final decision-making authority. Collaborations with pharmaceutical or biotechnology companies and other third parties often are terminated or allowed to expire by the other party. Any such termination or expiration would adversely affect us financially and could harm our business reputation.
We may not be successful in potential future efforts to identify or discover additional product candidates or we may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
The success of our business depends primarily upon our ability to identify, develop and commercialize products. Although palovarotene is in clinical development, our research programs may fail to identify other potential product candidates for clinical development for a number of reasons. Our research methodology may be unsuccessful in identifying potential product candidates or our potential
24
product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval.
Because we have limited financial and management resources, we are currently focused on our FOP and MO programs. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial drugs or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable drugs. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through future collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
If any of these events occur, we may be forced to abandon our development efforts for a program or programs, which would have a material adverse effect on our business and could potentially cause us to cease operations.
We are subject to healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Although we do not currently have any products on the market, once we begin commercializing our product, we may be subject to additional healthcare statutory and regulatory requirements and enforcement by the federal government and the states and foreign governments in which we conduct our business. Healthcare providers, physicians and others will play a primary role in the recommendation and prescription of our product candidate, if approved. Our future arrangements with third-party payors will expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our product candidate, if we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations include the following:
• | The federal anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal healthcare programs such as Medicare and Medicaid. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | The federal False Claims Act imposes criminal and civil penalties, including those from civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | The federal Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | The federal false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | The federal transparency requirements, sometimes referred to as the "Sunshine Act," under the Patient Protection and Affordable Care Act, require manufacturers of drugs, devices, biologics and medical supplies that are reimbursable under Medicare, Medicaid, or the Children’s Health Insurance Program to report on an annual basis to the Department of Health and Human Services information related to transfers of value to physicians and teaching hospitals and physician ownership and investment interests. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Analogous state laws and regulations, such as state anti-kickback and false claims laws and transparency laws, may apply to sales or marketing arrangements and claims involving healthcare | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
25
| items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures and drug pricing. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Ensuring that our future business arrangements with third parties comply with applicable healthcare laws and regulations could be costly. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations, including anticipated activities to be conducted by our sales team, were found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines and exclusion from government funded healthcare programs, such as Medicare and Medicaid, any of which could substantially disrupt our operations. If any of the physicians or other providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Healthcare legislative reform measures may have a material adverse effect on our business and results of operations.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. For example, in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively referred to as the Affordable Care Act, was enacted, which substantially changes the way health care is financed by both governmental and private insurers, and significantly impacts the U.S. pharmaceutical industry. The Affordable Care Act, among other things, imposed reporting requirements on manufacturers related to drug samples and financial relationships with physicians and teaching hospitals, increased the minimum Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program and extended the rebate program to individuals enrolled in Medicaid managed care organizations, established annual fees on manufacturers of certain branded prescription drugs, and established a Medicare Part D coverage gap discount program. The healthcare regulatory environment in the U.S. is still in flux, and judicial challenges and legislative initiatives to modify, limit, or repeal the Affordable Care Act continue, and may increase in light of the change in administration following the 2016 U.S. presidential election. For example, a recent Executive Order signed by the U.S. President directed executive departments and federal agencies to waive, defer, grant exemptions from, or delay the implementation of provisions of the Affordable Care Act that would impose a fiscal or regulatory burden on individuals and certain entities to the maximum extent permitted by law. In addition, the future implementation of the Affordable Care Act is not assured. By way of example, in January 2017, Congress voted to adopt a budget resolution for fiscal year 2017, or the Budget Resolution, that authorizes the implementation of legislation that would repeal portions of the Affordable Care Act. The Budget Resolution is not a law; however, it is widely viewed as the first step toward the passage of legislation that would repeal certain aspects of the Affordable Care Act. Congress has also proposed and may in the future propose legislation to replace elements of the Affordable Care Act that are repealed. We cannot predict the impact on our business of changes to current laws and regulations. However, any changes that lower reimbursements for products for which we may obtain regulatory approval, or that impose administrative and financial burdens on us, could adversely affect our business.
In addition, other legislative changes have been proposed and adopted in the United States since the Affordable Care Act was enacted. These changes include, among others, aggregate reductions of Medicare payments to providers of up to 2% per fiscal year, starting in 2013. We expect that additional state and federal healthcare reform measures will be adopted in the future, which may alter or completely replace the existing healthcare financing structure. Any of these reform measures could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for any such product candidate that we may have developed or additional pricing pressures on our business.
26
Similar initiatives and legislations may come into force in other jurisdictions as well, such as in the different countries of the European Union, where price ceilings and rebate systems are essential parts of the reimbursement of medicinal products. Some of these systems are being adapted on a regular or irregular basis to further reduce drug prices.
Even if approved, reimbursement policies could limit our ability to sell palovarotene and any other product candidate.
Market acceptance and sales of palovarotene and any other product candidate will depend on reimbursement policies and may be affected by healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels for those medications. Cost containment is a primary concern in the U.S. healthcare industry and elsewhere. Government authorities and these third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. We cannot be sure what the level of reimbursement will be for palovarotene and any future product candidate and, if any reimbursement is available, the level of such reimbursement. Reimbursement may impact the demand for, or the price of, palovarotene and any other product candidate. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize palovarotene and any other product candidate. In some foreign countries, particularly in Canada and European countries, the pricing of prescription pharmaceuticals is subject to strict governmental control. In these countries, pricing negotiations with governmental authorities can take six to twelve months or longer after the receipt of regulatory approval and product launch. To obtain favorable reimbursement for the indications sought or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product candidate with other available therapies. If reimbursement for our product candidate is unavailable in any country in which we seek reimbursement, limited in scope or amount, conditioned upon our completion of additional clinical trials, or set at unsatisfactory levels, our operating results could be materially adversely affected.
Risks Related To Our Intellectual Property Rights
We are heavily dependent on licensed intellectual property. If we were to lose our rights to licensed intellectual property, we would not be able to continue developing or commercializing palovarotene or any other product candidate, if approved. If we breach any of the agreements under which we license the use, development and commercialization rights to palovarotene or any other product candidate or technology from third parties or if certain insolvency events were to occur, we could lose license rights that are important to our business.
We are a party to a number of license agreements under which we are granted rights to intellectual property that are important to our business and we may need to enter into additional license agreements in the future. Our existing license agreements impose, and we expect that any future license agreements will impose on us, various development, regulatory and/or commercial diligence obligations, payment of milestones and/or royalties and other obligations. If we fail to comply with our obligations under these agreements, or we are subject to a bankruptcy, the licensor may have the right to terminate the license, in which event we would not be able to market products covered by the license. Our business could suffer, for example, if any current or future licenses terminate, if the licensors fail to abide by the terms of the license, if the licensed patents or other rights are found to be invalid or unenforceable, or if we are unable to enter into necessary licenses on acceptable terms. See “Business—License Agreements” for a description of our existing license agreements, which includes a description of the termination provisions of each such agreement.
As we have done previously, we may need to obtain licenses from third parties to advance our research or allow commercialization of palovarotene or any other product candidates, and we cannot provide any assurances that third-party patents do not exist that might be enforced against palovarotene or any other product candidates or future products in the absence of such a license. We may fail to obtain any of these licenses on commercially reasonable terms, if at all. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies
27
licensed to us. In that event, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected product candidates, which could materially harm our business and the third parties owning such intellectual property rights could seek either an injunction prohibiting our sales, or, with respect to our sales, an obligation on our part to pay royalties and/or other forms of compensation.
Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues. Disputes may arise between us and our licensors regarding intellectual property subject to a license agreement, including:
• | the scope of rights granted under the license agreement and other interpretation-related issues; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | whether and the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our right to sublicense patent and other rights to third parties under collaborative development relationships; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization of our product candidates, and what activities satisfy those diligence obligations; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates.
We may enter into additional licenses to third-party intellectual property that are necessary or useful to our business. Our current licenses and any future licenses that we may enter into impose various royalty payments, milestones, diligence and other obligations on us. If we fail to comply with any of our obligations under a current or future license agreement, such licensor may allege that we have breached our license agreement and may accordingly seek to terminate our license with them. In addition, future licensors may decide to terminate any such license at will. Termination of any of our current or future licenses could result in our loss of the right to use the licensed intellectual property, which could materially adversely affect our ability to develop and commercialize palovarotene or any other product candidate, if approved, as well as harm our competitive business position and our business prospects. Moreover, our current or future licenses may provide for a reversion to the licensor of our rights in regulatory filings or other intellectual property or data that we regard as our own in the event the license terminates under certain circumstances, such as due to breach.
In addition, if our current or future licensors fail to abide by the terms of the applicable license, if the licensors fail to prevent infringement by third parties, if the licensed patents or other rights are found to be invalid or unenforceable, or if we are unable to enter into necessary licenses on acceptable terms, if at all, our business could suffer.
Moreover, our licensors under current licenses retain and our licensors under future licenses may retain certain rights and obligations. For example, the licensor may retain control over patent prosecution and maintenance under a license agreement, in which case we may not be able to adequately influence patent prosecution or prevent inadvertent lapses of coverage due to failure to pay maintenance fees. Similarly, the licensor may retain the sole right or a first right to enforce the licensed patents against infringement, in which case we may not be able to adequately influence or may be delayed in enforcing certain licensed patents.
Some intellectual property that we have licensed may have been discovered through government funded programs and thus may be subject to federal regulations such as “march-in” rights, certain reporting requirements, and a preference for U.S. industry. Compliance with such regulations may limit our exclusive rights, subject us to expenditure of resources with respect to reporting requirements, and limit our ability to contract with non-U.S. manufacturers.
28
Some of the intellectual property rights we have licensed may have been generated through the use of U.S. government funding and may therefore be subject to certain federal regulations. As a result, the U.S. government may have certain rights to intellectual property embodied in our current or future product candidates pursuant to the Bayh-Dole Act of 1980 (Bayh-Dole Act). Moreover, intellectual property that we may own or license in the future may be subject to the applicable provisions of the Bayh-Dole Act. These U.S. government rights in certain inventions developed under a government-funded program include a non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the U.S. government has the right to require us to grant exclusive, partially exclusive, or non-exclusive licenses to any of these inventions to a third party if it determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; or (iii) government action is necessary to meet requirements for public use under federal regulations (also referred to as “march-in rights”). The U.S. government also has the right to take title to these inventions if we fail, or the applicable licensor fails, to disclose the invention to the government and fail to file an application to register the intellectual property within specified time limits. In addition, the U.S. government may acquire title to these inventions in any country in which a patent application is not filed within specified time limits. Intellectual property generated under a government funded program is also subject to certain reporting requirements, compliance with which may require us, or the applicable licensor, to expend substantial resources. In addition, the U.S. government requires that any products embodying the subject invention or produced through the use of the subject invention be manufactured substantially in the United States. The manufacturing preference requirement can be waived if the owner of the intellectual property can show that reasonable but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would be likely to manufacture substantially in the United States or that under the circumstances domestic manufacture is not commercially feasible. This preference for U.S. manufacturers may limit our ability to contract with non-U.S. product manufacturers for products covered by such intellectual property.
We currently do not plan to apply for U.S. government funding, but if we do, and we discover compounds or drug candidates as a result of such funding, intellectual property rights to such discoveries may be subject to the applicable provisions of the Bayh-Dole Act.
If we are unable to adequately protect our proprietary technologies, or obtain and maintain issued in-licensed patents that are sufficient to protect palovarotene or any future product candidates, others could compete against us more directly, which would have a material adverse impact on our business, results of operations, financial condition and prospects.
Our commercial success will depend in part on our success in obtaining and maintaining issued owned and in-licensed patents and other intellectual property rights in the United States and elsewhere and protecting our proprietary technologies. We will seek to protect our proprietary position by filing patent applications in the United States and abroad related to our business plans. We also rely on trade secrets that protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. If we do not adequately protect our intellectual property and proprietary technologies, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability.
We cannot provide any assurances that any of our in-licensed patents have, or that any of our pending owned or in-licensed patent applications will mature into issued patents that include claims with a scope sufficient to protect palovarotene or any other product candidate, any additional features we develop for palovarotene or any other product candidate, or any new products or otherwise provide any competitive advantage. Other parties may have developed technologies that may be related or competitive to our approach, and may have filed or may file patent applications and may have received or may receive patents that may overlap or conflict with our owned or in-licensed patent applications, either by claiming the same methods or formulations or by claiming subject matter that could dominate our patent position. Such third-party patent positions may limit or even eliminate our ability to obtain patent protection for certain inventions.
The patent positions of biotechnology and pharmaceutical companies, including our patent position, involve complex legal and factual questions, and, therefore, the issuance, scope, validity and
29
enforceability of any patent claims that we have or may obtain cannot be predicted with certainty. Patents may be challenged, deemed unenforceable, invalidated, or circumvented. U.S. patents and patent applications may also be subject to interference proceedings,ex partereexamination, orinter partesreview proceedings, supplemental examination and challenges in district court. Patents may be subjected to opposition, post-grant review, or comparable proceedings lodged in various foreign, both national and regional, patent offices. These proceedings could result in either loss of the patent or denial of the patent application or loss or reduction in the scope of one or more of the claims of the patent or patent application. In addition, such proceedings may be costly. Thus, any patents that we may own or exclusively license may not provide any protection against competitors. Furthermore, an adverse decision in an interference proceeding can result in a third party receiving the patent right sought by us, which in turn could affect our ability to develop, market or otherwise commercialize palovarotene or any other product candidate.
Furthermore, though an issued patent is presumed valid and enforceable, its issuance is not conclusive as to its validity or its enforceability and it may be interpreted in a manner adverse to our interests, or not provide us with adequate proprietary protection or competitive advantages against competitors with similar products. Even if an issued patent is held to be valid and enforceable, it may be interpreted in a manner adverse to our interests, or competitors may be able to design around any patents that we may own or in-license, such as through the use of pre-existing or newly developed technology. Other parties may develop and obtain patent protection for more effective technologies, designs or methods. Moreover, the life of a patent and the term of any protection it may provide is limited. Given the period of time it takes to bring a drug to market, it is possible that some of our patent rights will expire or be near the end of their term before we obtain approval to sell palovarotene or any other product candidate, if such approval is obtained. For example, a U.S. patent licensed from Roche claiming palovarotene as a composition of matter has a statutory expiration date in 2021, without extensions or adjustments.
We may not be able to prevent the unauthorized disclosure or use of our technical knowledge or trade secrets by consultants, vendors, former employees and current employees. The laws of some foreign countries do not protect our proprietary rights to the same extent as the laws of the United States, and we may encounter significant problems in protecting our proprietary rights in these countries, such as China. If these developments were to occur, they could have a material adverse effect on our sales.
Our ability to enforce our patent rights depends on our ability to detect infringement. It is difficult to detect infringers who do not advertise the components that are used in their products. Moreover, it may be difficult or impossible to obtain evidence of infringement in a competitor’s or potential competitor’s product. Any litigation to enforce or defend our in-licensed patent rights, even if we were to prevail, could be costly and time-consuming and would divert the attention of our management and key personnel from our business operations. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded if we were to prevail may not be commercially meaningful.
In addition, proceedings to enforce or defend our owned or in-licensed patents, if and when issued, could put our patents at risk of being invalidated, held unenforceable, or interpreted narrowly. Such proceedings could also provoke third parties to assert claims against us, including that some or all of the claims in one or more of our patents are invalid or otherwise unenforceable. If any of our owned or in-licensed patents, if and when issued, that cover or which we believe cover palovarotene or any other product candidate (including any method of use) are invalidated or found unenforceable or interpreted in a manner adverse to our interests, such as to not cover the relevant product candidate, our financial position and results of operations would be materially and adversely impacted. In addition, if a court found that valid, enforceable patents held by third parties covered palovarotene or any other product candidate, our financial position and results of operations would also be materially and adversely impacted.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
• | any of our in-licensed patents or pending owned or in-licensed patent applications, if issued, will include claims having a scope sufficient to protect palovarotene or any other product candidates; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | any of our pending owned or in-licensed patent applications will issue as patents at all; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
30
• | we will be able to successfully commercialize palovarotene or any other product candidate, if approved, before our relevant in-licensed patents expire; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our licensors were the first to make the inventions covered by each of our in-licensed patents, and we or our licensors were the first to make the inventions covered by each of our pending owned or in-licensed patent applications; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | we or our licensors were the first to file patent applications for these inventions; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | others will not develop similar or alternative technologies that do not infringe our in-licensed patents, or our owned or in-licensed patent applications, if issued; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | others will not use pre-existing technology to effectively compete against us; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | any of our in-licensed patents, if issued, will be found to ultimately be valid and enforceable; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | any patents issued to us or our licensors will provide a basis for an exclusive market for our commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | we will develop additional proprietary technologies or product candidates that are separately patentable; or | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | that our commercial activities or products will not infringe upon the patents or proprietary rights of others. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
We rely upon unpatented trade secrets, unpatented know-how and continuing technological innovation to develop and maintain our competitive position, which we seek to protect, in part, by confidentiality agreements with our employees and our collaborators and consultants. It is possible that technology relevant to our business will be independently developed by a person that is not a party to such an agreement. Furthermore, if the employees and consultants who are parties to these agreements breach or violate the terms of these agreements, we may not have adequate remedies for any such breach or violation, and we could lose our trade secrets through such breaches or violations. Further, our trade secrets could otherwise become known or be independently discovered by our competitors.
We may infringe the intellectual property rights of others, which may prevent or delay our product development efforts and stop us from commercializing or increase the costs of commercializing palovarotene or any other product candidates, if approved.
Our success will depend in part on our ability to operate without infringing the intellectual property and proprietary rights of third parties. We cannot assure you that our business, products and methods do not or will not infringe the patents or other intellectual property rights of third parties.
The pharmaceutical industry is characterized by extensive litigation regarding patents and other intellectual property rights. Other parties may allege that palovarotene or any other product candidate or the use of our technologies infringes patent claims or other intellectual property rights held by them or that we are employing their proprietary technology without authorization. As we continue to develop and, if approved, commercialize palovarotene or any future product candidates, competitors may claim that our technology infringes their intellectual property rights as part of business strategies designed to impede our successful commercialization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of palovarotene or any other product candidate. Because patent applications can take many years to issue, third parties may have currently pending patent applications which may later result in issued patents that palovarotene or any other product candidate may infringe, or which such third parties claim are infringed by our technologies. Moreover, we may fail to identify relevant third party patents or patent applications, or we may incorrectly conclude that the claims of an issued patent were not relevant to our activities.
The outcome of intellectual property litigation is subject to uncertainties that cannot be adequately quantified in advance. The coverage of patents is subject to interpretation by the courts, and the interpretation is not always uniform. If we are sued for patent infringement, we would need to demonstrate that our product candidate, products or methods either do not infringe the patent claims of the relevant patent or that the patent claims are invalid, and we may not be able to do this. Even if we
31
are successful in these proceedings, we may incur substantial costs and the time and attention of our management and scientific personnel could be diverted in pursuing these proceedings, which could have a material adverse effect on us. In addition, we may not have sufficient resources to bring these actions to a successful conclusion.
Patent and other types of intellectual property litigation can involve complex factual and legal questions, and their outcome is uncertain. Any claim relating to intellectual property infringement that is successfully asserted against us may require us to pay substantial damages, including treble damages and attorney’s fees if we are found to be willfully infringing another party’s patents, for past use of the asserted intellectual property and royalties and other consideration going forward if we are forced to take a license. In addition, if any such claim were successfully asserted against us and we could not obtain such a license, we may be forced to stop or delay developing, manufacturing, selling or otherwise commercializing palovarotene or any other product candidate.
Even if we are successful in these proceedings, we may incur substantial costs and divert management time and attention in pursuing these proceedings, which could have a material adverse effect on us. If we are unable to avoid infringing the patent rights of others, we may be required or may choose to seek a license, defend an infringement action or challenge the validity of the patents in court, or redesign our product. Patent litigation is costly and time-consuming. We may not have sufficient resources to bring these actions to a successful conclusion. In addition, intellectual property litigation or claims could force us to do one or more of the following:
• | cease developing, selling or otherwise commercializing palovarotene or any other product candidate; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | pay substantial damages for past use of the asserted intellectual property; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | obtain a license from the holder of the asserted intellectual property, which license may not be available on reasonable terms, if at all; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | in the case of trademark claims, redesign or rename palovarotene or any other product candidate to avoid infringing the intellectual property rights of third parties, which may not be possible and, even if possible, could be costly and time-consuming. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Any of these risks coming to fruition could have a material adverse effect on our business, results of operations, financial condition and prospects.
We may be subject to claims challenging the inventorship or ownership of our owned or in-licensed patents and other intellectual property.
We generally enter into confidentiality and intellectual property assignment agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors. However, these agreements may not be honored and may not effectively assign intellectual property rights to us. For example, inventorship disputes may arise from conflicting obligations of consultants or others who are involved in developing palovarotene or any other product candidates.
Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. The owners of intellectual property in-licensed to us could also face such claims. If we or our licensors fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we or our licensors are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Obtaining and maintaining our patent protection, including patents licensed from third parties, depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The U.S. Patent and Trademark Office (U.S. PTO), and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in
32
abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case.
If we or our licensors fail to maintain the patents and patent applications covering or otherwise protecting our product candidates, it could materially harm our business. In addition, to the extent that we have responsibility for taking any action related to the prosecution or maintenance of patents or patent applications in-licensed from a third party, any failure on our part to maintain the in-licensed intellectual property could jeopardize our rights under the relevant license and may expose us to liability.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Even if the patent applications we own or license are issued, competitors may infringe these patents. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid, is unenforceable and/or is not infringed, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put our patents or our licensors’ patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
Interference proceedings provoked by third parties or brought by us may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common shares.
Issued patents covering palovarotene or any other product candidate could be found invalid or unenforceable if challenged in court.
If we or one of our licensing partners initiated legal proceedings against a third party to enforce a patent covering palovarotene or any other product candidate, the defendant could counterclaim that the patent covering palovarotene or any other product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge include alleged failures to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement. Grounds for unenforceability assertions include allegations that someone connected with prosecution of the patent withheld relevant information from the U.S. PTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review and equivalent proceedings in foreign jurisdictions, e.g. opposition proceedings. Such proceedings could result in revocation or amendment of our patents or our licensors’ patents in such a way that they no longer cover palovarotene or any other product candidate or competitive products. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to validity, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were
33
unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on palovarotene or any other product candidate. Such a loss of patent protection would have a material adverse impact on our business.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on product candidates in all countries and jurisdictions throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States could be less extensive than those offered in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions.
Competitors may use our technologies in jurisdictions where we do not have, or where we do not pursue and obtain, patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our product and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing. Even if we pursue and obtain issued patents in particular jurisdictions, our patent claims or other intellectual property rights may not be effective or sufficient to prevent third parties from so competing.
Further, the laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of some countries, particularly developing countries, do not favor the enforcement of patents and other intellectual property protection, especially those relating to biotechnology. This could make it difficult for us to stop the infringement of our patents, if obtained, or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. Patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.
Moreover, proceedings to enforce our patent rights, or those of our licensors or partners, in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our in-licensed patents, or any patents that we may own in the future, at risk of being invalidated or interpreted narrowly, could put our owned or in-licensed patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
If we do not obtain additional protection under the Hatch-Waxman Amendments and similar foreign legislation by extending the patent term and obtaining data exclusivity for our product candidates, our business may be materially harmed.
Depending upon the timing, duration and specifics of FDA marketing approval of palovarotene or any other product candidate, one or more of the U.S. patents we may own or license may be eligible for limited patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a restoration of patent term for one patent of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, we may not be granted an extension because of, for example, failing to apply within applicable deadlines, failing to apply prior to expiration of relevant patents or otherwise failing to satisfy applicable requirements. Moreover, the
34
applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain patent term extension or restoration or the term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our ability to generate revenues could be materially adversely affected.
Changes in either U.S. or foreign patent law or interpretation of such laws could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biotechnology companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biotechnology industry involve both technological and legal complexity, and it therefore is costly, time-consuming and inherently uncertain. In addition, on September 16, 2011, the Leahy-Smith America Invents Act (AIA), was signed into law. The AIA includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation.
An important change introduced by the AIA is that, as of March 16, 2013, the United States transitioned to a “first-to-file” system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that files a patent application in the U.S. PTO after that date but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by the third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application.
Among some of the other changes introduced by the AIA are changes that limit where a patentee may file a patent infringement suit and providing opportunities for third parties to challenge any issued patent in the U.S. PTO. This applies to all of our U.S. patents, even those issued before March 16, 2013. Because of a lower evidentiary standard necessary to invalidate a patent claim in U.S. PTO proceedings compared to the evidentiary standard in United States federal court, a third party could potentially provide evidence in a U.S. PTO proceeding sufficient for the U.S. PTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the U.S. PTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action.
Depending on decisions by the United States Congress, the federal courts, the U.S. PTO, or similar authorities in foreign jurisdictions, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing in-licensed patents and patents that we might obtain in the future.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Some of our employees have been previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. We also engage advisors and consultants who are concurrently employed at universities or who perform services for other entities.
Although we are not aware of any claims currently pending against us, we may be subject to claims that we or our employees, advisors or consultants have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third party. We may be subject to claims that an employee, advisor or consultant performed work for us that conflicts with that person’s obligations to a third party, such as an employer, and thus, that the third party has an ownership interest in the intellectual property arising out of work performed for us. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. If we fail in defending such claims, in addition to paying money claims, we may lose valuable intellectual property rights or personnel. A loss of key personnel or their work product could hamper or prevent our ability to commercialize our current and future product candidates, which would materially adversely affect our commercial development efforts.
35
General Company-Related Risks
We will need to develop and expand the size and scope of our Company, and we may encounter difficulties in managing this development and expansion, which could disrupt our operations.
As of March 31, 2017, we had 24 full-time employees and in connection with becoming a public company and advancing our growth plans, we expect to increase our number of employees and the scope of our operations. To manage our anticipated development and expansion, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Also, our management may need to divert a disproportionate amount of its attention away from its day-to-day activities and devote a substantial amount of time to managing these development activities. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. This may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. The physical expansion of our operations may lead to significant costs and may divert financial resources from other projects, such as the development of palovarotene and any other product candidates. If our management is unable to effectively manage our expected development and expansion, our expenses may increase more than expected, our ability to generate or increase our revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize our product candidates, if approved, and compete effectively will depend, in part, on our ability to effectively manage the future development and expansion of our Company.
We have limited experience operating internationally, are subject to a number of risks associated with our international activities and operations, and may not be successful in our efforts to expand internationally.
We have collaboration, clinical trial and other relationships outside the United States, but we currently have very limited operations outside of the United States and Canada. In order to meet our long-term goals, we would need to grow our international operations significantly. Consequently, we are and will continue to be subject to additional risks related to operating in foreign countries, including:
• | the fact that we have limited experience operating our business internationally; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | local, economic and political conditions, including inflation, geopolitical events, such as war and terrorism, foreign currency fluctuations and exchange risks, which could result in increased or unpredictable operating expenses and reduced revenues and other obligations incident to doing business in, or with a company located in, another country; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our customers’ ability to obtain reimbursement for palovarotene or any other product candidate in foreign markets, and unexpected changes in reimbursement and pricing requirements, tariffs, trade barriers and regulatory requirements; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | different medical practices and customs in foreign countries affecting acceptance in the marketplace; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | longer lead times for shipping and longer accounts receivable collection times; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the interpretation of contractual provisions governed by foreign laws in the event of a contract dispute; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | reduced protection of intellectual property rights in some foreign countries or the existence of additional potentially relevant third party intellectual property rights; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | compliance with foreign or U.S. laws, rules and regulations, including data privacy requirements, labor relations laws, tax laws, accounting requirements, anti-competition regulations, import, export and trade restrictions, anti-bribery/anti-corruption laws, regulations or rules, which could lead to actions by us or our licensees, distributors, manufacturers, other third parties who act on our behalf or with whom we do business in foreign countries or our employees who are working abroad that could subject us to investigation or prosecution under such foreign or U.S. laws. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
36
We face potential product liability exposure, and, if claims are brought against us, we may incur substantial liability.
The use of our product candidates in clinical trials and the sale of our product candidates, if approved, exposes us to the risk of product liability claims. Product liability claims might be brought against us by patients, healthcare providers or others selling or otherwise coming into contact with our product candidates. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, including as a result of interactions with alcohol or other drugs, negligence, strict liability and a breach of warranties. Claims could also be asserted under state consumer protection acts. If we become subject to product liability claims and cannot successfully defend ourselves against them, we could incur substantial liabilities. In addition, regardless of merit or eventual outcome, product liability claims may result in, among other things:
• | withdrawal of patients from our clinical trials; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | substantial monetary awards to patients or other claimants; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | decreased demand for our product candidates or any future product candidates following marketing approval, if obtained; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | damage to our reputation and exposure to adverse publicity; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | increased FDA warnings on product labels; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | litigation costs; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | distraction of management’s attention from our primary business; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | loss of revenue; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the inability to successfully commercialize our product candidates or any future product candidates, if approved. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
We maintain product liability and clinical trial insurance coverage in the U.S., United Kingdom (UK), France, Italy, Argentina, Spain and Australia for our clinical trials with annual aggregate coverage limits varying from $2.7 million to $15.0 million. Nevertheless, our insurance coverage may be insufficient to reimburse us for any expenses or losses we may suffer. Moreover, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses, including if insurance coverage becomes increasingly expensive. If and when we obtain marketing approval for our product candidates, we intend to expand our insurance coverage to include the sale of commercial products; however, we may not be able to obtain this product liability insurance on commercially reasonable terms. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. The cost of any product liability litigation or other proceedings, even if resolved in our favor, could be substantial, particularly in light of the size of our business and financial resources. A product liability claim or series of claims brought against us could cause our stock price to decline and, if we are unsuccessful in defending such a claim or claims and the resulting judgments exceed our insurance coverage, our financial condition, business and prospects could be materially adversely affected.
Our future success depends on our ability to retain key executives and to attract, retain and motivate qualified personnel, including qualified accounting and financial personnel with appropriate public company experience.
We are highly dependent on the principal members of our executive team listed under “Management” located elsewhere in this prospectus, the loss of whose services may adversely impact the achievement of our objectives. Any of our executive officers could leave our employment at any time, as all of our employees are “at will” employees. Recruiting and retaining other qualified employees for our business, including scientific and technical personnel, will also be critical to our success. There is currently a shortage of skilled executives in our industry, which is likely to continue. As a result, competition for skilled personnel is intense and the turnover rate can be high. We may not be able to attract and retain personnel on acceptable terms given the competition among numerous
37
pharmaceutical companies for individuals with similar skill sets. In addition, failure to succeed in clinical trials may make it more challenging to recruit and retain qualified personnel. The inability to recruit key executives or the loss of the services of any executive or key employee might impede the progress of our development and commercialization objectives.
Further, as a newly public company, we may need to hire additional accounting and financial personnel with appropriate public company experience and technical accounting knowledge, and it may be difficult to recruit and maintain such personnel. Even if we are able to hire appropriate personnel, our existing operating expenses and operations will be impacted by the direct costs of their employment and the indirect consequences related to the diversion of management resources from product development efforts.
We will incur increased costs as a result of operating as a public company, and our management team will be required to devote substantial time to new compliance initiatives.
As a public company, and particularly after we are no longer an “emerging growth company,” we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act of 2002 and rules subsequently implemented by the U.S. Securities and Exchange Commission (SEC) and The Nasdaq Global Market have imposed various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
After we are no longer an emerging growth company, pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 (Section 404), we will be required to furnish a report by our management on our internal control over financial reporting, including, after we are no longer an emerging growth company, an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting pursuant to the Committee of Sponsoring Organizations of the Treadway Commission 2013, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting.
Changes in our effective income tax rate could adversely affect our results of operations.
We are subject to income taxes in the Unites States and Canada and may be subject to income taxes in various foreign jurisdictions in the future, and our domestic and foreign tax liabilities are largely dependent upon the distribution of pre-tax earnings among these different jurisdictions. Various factors may have favorable or unfavorable effects on our effective income tax rate. These factors include, but are not limited to, interpretations of existing tax laws, changes in tax laws and rates, the accounting for stock options and other share-based compensation, changes in accounting standards, future levels of research and development spending, changes in the mix and level of pre-tax earnings by taxing jurisdiction, the outcome of examinations by the U.S. Internal Revenue Service, Canadian Revenue Agency and related provincial tax authorities, and other jurisdictions, the accuracy of our estimates for unrecognized tax benefits, the realization of deferred tax assets, or by changes to our ownership or capital structure. The impact on our income tax provision resulting from the above-mentioned factors and others may be significant and could adversely affect our results of operations.
Our ability to use our net operating loss carryforwards to offset future taxable income may be subject to certain limitations.
38
In general, under Section 382 of the U.S. Internal Revenue Code of 1986, as amended (the Code) a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its pre-change net operating loss carryforwards (NOLs) to offset future taxable income. Similarly, where control of a corporation has been acquired by a person or group of persons, subsection 111(5) of the Canadian Income Tax Act (Canadian Tax Act), and equivalent provincial income tax legislation restrict the corporation’s ability to carry forward non-capital losses from preceding taxation years. We have not performed a detailed analysis to determine whether an ownership change under Section 382 of the Internal Revenue Code or an acquisition of control for the purposes of subsection 111(5) of the Canadian Tax Act has occurred after each of our previous issuances of common shares, preferred shares and convertible debt. In addition, if we undergo an ownership change or acquisition of control after this public offering, our ability to utilize NOLs and non-capital losses could be limited by Section 382 of the Internal Revenue Code and subsection 111(5) of the Canadian Tax Act. As of December 31, 2016, we had Canadian federal and provincial net operating loss carry forwards of $49.2 million and $48.3 million, respectively, and unused federal tax credits of $0.7 million, all of which are set to expire over time to 2036. We also had Canadian federal and provincial research and development expenditure pools of $4.1 million and $4.9 million, respectively, without time limitations. Future changes in our share ownership, some of which are outside of our control, could result in an ownership change under Section 382 of the Internal Revenue Code or an acquisition of control for the purposes of subsection 111(5) of the Canadian Tax Act. Furthermore, our ability to utilize NOLs and non-capital losses of companies that we may acquire in the future may be subject to limitations.
We may become a “controlled foreign corporation,” which may have adverse U.S. federal income tax consequences for a U.S. investor that owns substantial amounts of our common shares.
Although we believe we qualified as a controlled foreign corporation (CFC) in prior years, based on our current ownership structure, we do not believe we are currently a CFC for U.S. federal income tax purposes for the taxable year ending December 31, 2017. Given our ownership concentration, however, it is possible we could become a CFC if U.S. persons, each of whom own, directly or indirectly, including through non-U.S. persons (or is considered to own under applicable constructive ownership rules of the Code), 10% or more of the total combined voting power of all our classes of stock entitled to vote (U.S. Shareholders), together own more than 50% of the total vote or value of our stock. In such case, U.S. Shareholders may be subject to current U.S. federal income tax on certain types of income (generally passive income) regardless of whether corresponding cash distributions are made by us. In addition, gains recognized by U.S. Shareholders on the sale of our common shares may be reclassified, in whole or in part, as a dividend. The CFC rules are complex, and investors are urged to consult their own tax advisors regarding the possible application of the CFC rules in their particular circumstances. See “Material U.S. Federal Income Tax Considerations—Controlled Foreign Corporation Considerations.”
Transfer pricing rules may adversely affect our income tax expense.
Many of the jurisdictions in which we will conduct business have detailed transfer pricing rules which require that all transactions with non-resident related parties be priced using arm’s length pricing principles. Contemporaneous documentation must exist to support this pricing. The taxation authorities in these jurisdictions could challenge our arm’s length related party transfer pricing policies. International transfer pricing is an area of taxation that depends heavily on the underlying facts and circumstances and generally involves a significant degree of judgment. If any of these taxation authorities are successful in challenging our transfer pricing policies, our income tax expense may be adversely affected and we could also be subjected to interest and penalty charges. Any increase in our income tax expense and related interest and penalties could have a significant impact on our future earnings and future cash flows.
Our deductions and credits in respect of scientific research and experimental development expenditures may be challenged by the Canadian tax authorities.
The Canadian taxation authorities may not necessarily agree with our determinations of the expenses and tax credits claimed by us, including research and development expenses and related tax credits. If the Canadian taxation authorities successfully challenge such expenses or the correctness of
39
such income tax credits claimed, our operating results could be materially adversely affected. Furthermore, if the Canadian taxation authorities reduce the tax credit either by reducing the rate of the credit or the eligibility of some research and development expenses in the future, our operating results will be materially adversely affected.
We are likely a “passive foreign investment company,” which may have adverse U.S. federal income tax consequences for U.S. investors.
U.S. investors should be aware that we believe that we qualified as a passive foreign investment company (PFIC) for our taxable year ended December 31, 2016 and that we expect to qualify as a PFIC for our taxable year ending December 31, 2017 and very possibly, for subsequent years. If we are a PFIC for any taxable year during a U.S. investor’s holding period of our common shares, a U.S. investor generally will be required to treat any gain realized upon a disposition of our common shares, or any so-called “excess distribution” received on our common shares, as ordinary income (not capital gain) earned over the U.S. investor’s holding period of our common shares, and to pay applicable taxes on such ordinary income as well as an interest charge on the gain or distribution as if the resulting taxes had been earned (and resulting tax due) ratably over the holding period. A U.S. investor may make certain elections (such as a “mark-to-market” election or a “qualified electing fund” election) to mitigate certain of the adverse U.S. federal income tax consequences of owning PFIC stock, but such elections have their own set of consequences and may not be available in certain circumstances. The PFIC rules are complex and they and any available elections may have a significant effect on U.S. investors. U.S. investors are urged to consult their own tax advisors regarding all aspects of the PFIC rules that may be applicable. See “Material U.S. Federal Income Tax Considerations—Passive Foreign Investment Company Considerations.”
We may acquire businesses or products, or form strategic alliances, in the future, and we may not realize the benefits of such acquisitions.
We may acquire additional businesses or products, form strategic alliances or create joint ventures with third parties that we believe will complement or augment our existing business. If we acquire businesses with promising products or technologies, we may not be able to realize the benefit of acquiring such businesses if we are unable to successfully integrate them with our existing operations and company culture. We may encounter numerous difficulties in developing, manufacturing and marketing any new products resulting from a strategic alliance or acquisition that delay or prevent us from realizing their expected benefits or enhancing our business. We cannot assure you that, following any such acquisition, we will achieve the expected synergies to justify the transaction.
Risks Related To Our Common Shares and This Offering
Market volatility may affect our stock price and the value of your investment.
Following this offering, the market price for our common shares is likely to be volatile, in part because our common shares have not been previously traded publicly. In addition, the market price of our common shares may fluctuate significantly in response to a number of factors, most of which we cannot control, including, among others:
• | plans for, progress of or results from non-clinical studies and clinical trials of palovarotene and any other product candidates; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the FDA’s approval of or failure to approve palovarotene or any other product candidate; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | announcements of new products, technologies, commercial relationships, acquisitions or other events by us or our competitors; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the success or failure of other FOP or MO therapies; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | regulatory or legal developments in the United States and other countries; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | failure of palovarotene or any other product candidates, if approved, to achieve commercial success; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
40
• | fluctuations in stock market prices and trading volumes of similar companies; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | general market conditions and overall fluctuations in U.S. equity markets; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | variations in our operating results; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | changes in our financial guidance or securities analysts’ estimates of our financial performance; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | changes in accounting principles; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our ability to raise additional capital and the terms on which we can raise it; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | sales of large blocks of our common shares, including sales by our executive officers, directors and significant stockholders; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | additions or departures of key personnel; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | discussion of us or our stock price by the press and by online investor communities; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | other risks and uncertainties described in these risk factors. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
An active trading market for our common shares may not develop, and you may not be able to resell your shares at or above the initial public offering price.
Prior to this offering, there has been no public market for our common shares. Although we have applied to list our common shares on The Nasdaq Global Market, an active trading market for our shares may never develop or be sustained following this offering. The initial public offering price of our common shares will be determined through negotiations between us and the underwriters. The initial public offering price may not be indicative of the market price of our common shares after this offering. In the absence of an active trading market for our common shares, investors may not be able to sell their common shares at or above the initial public offering price or at the time that they would like to sell.
We are an “emerging growth company,” and as a result of the reduced disclosure and governance requirements applicable to emerging growth companies, our common shares may be less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and we may choose to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. If we choose not to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, our auditors will not be required to attest to the effectiveness of our internal control over financial reporting. As a result, investors may become less comfortable with the effectiveness of our internal controls and the risk that material weaknesses or other deficiencies in our internal controls go undetected may increase. If we choose to provide reduced disclosures in our periodic reports and proxy statements while we are an emerging growth company, investors would have access to less information and analysis about our executive compensation, which may make it difficult for investors to evaluate our executive compensation practices. We cannot predict if investors will find our common shares less attractive because we may rely on these exemptions. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and our stock price may be more volatile.
As a foreign private issuer, we are not subject to certain U.S. securities law disclosure requirements that apply to domestic U.S. issuers, which may limit the information publicly available to our shareholders, and we may lose such status in the future.
As a foreign private issuer, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Securities and Exchange Act of 1934 (Exchange Act) and therefore there may be less publicly available information about us than if we were a U.S. domestic
41
issuer. For example, we are not subject to the proxy rules in the United States and disclosure with respect to our annual meetings will be governed by Canadian requirements. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and the rules thereunder. Therefore, our shareholders may not know on a timely basis when our officers, directors and principal shareholders purchase or sell our shares. Furthermore, as a foreign private issuer, we may take advantage of certain provisions in the Nasdaq listing rules that allow us to follow Canadian law for certain governance matters.
We may also lose our status as a foreign private issuer. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter, and, accordingly, the next determination will be made with respect to us on June 30, 2017. We would lose our foreign private issuer status if, for example, more than 50% of our common shares are directly or indirectly held by residents of the United States on June 30, 2017 and we fail to meet additional requirements necessary to maintain our foreign private issuer status. More than 50% of our common shares are currently held by U.S. shareholders. We nonetheless meet the definition of a foreign private issuer as we have determined that a majority of our executive officers and directors are not, for purposes of this test, U.S. citizens or U.S. residents, a majority of our assets are not located in the U.S. and our business is administered principally in Canada. If we lose our foreign private issuer status on this date, we will be required to file with the SEC periodic reports and registration statements on U.S. domestic issuer forms beginning on January 1, 2018, which are more detailed and extensive than the forms available to a foreign private issuer. We will also have to mandatorily comply with U.S. federal proxy requirements, and our officers, directors and principal shareholders will become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, we will lose our ability to rely upon exemptions from certain corporate governance requirements under the listing rules of The Nasdaq Global Market. As a U.S. listed public company that is not a foreign private issuer, we will incur significant additional legal, accounting and other expenses that we will not incur as a foreign private issuer, and accounting, reporting and other expenses in order to maintain a listing on a U.S. securities exchange. These expenses will relate to, among other things, the obligation to prepare our financial information in accordance with U.S. generally accepted accounting principles in the future.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common shares.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition and when required, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act, or the subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retrospective changes to our consolidated financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common shares.
The two material weaknesses in internal controls over financial reporting identified as of December 31, 2016 were: (i) the valuation of the embedded derivative and related preferred shares liability accounting, and (ii) lack of segregation of duties due to super-user access and insufficient journal entry review throughout the entire fiscal year. The preferred shares will be converted to common shares at the time of the closing of this initial public offering. Management introduced a new control in the fourth quarter of 2016 related to journal entry review, and in April 2017 management implemented another new control related to super-user access. We expect that these new controls combined will remediate the material weakness related to segregation of duties. Despite our efforts to remediate existing material weaknesses or due to the existence of other material weaknesses, there is a risk that neither we nor our independent registered public accounting firm will be able to conclude
42
within the prescribed timeframe that our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
Our charter documents and certain Canadian legislation could delay or deter a change of control, limit attempts by our shareholders to replace or remove our current management and limit the market price of our common shares.
Our authorized preferred shares are available for issuance from time to time at the discretion of our board of directors, without shareholder approval. Our articles grant our board of directors the authority, subject to the CBCA, to determine the special rights and restrictions granted to or imposed on any unissued series of preferred shares, and those rights may be superior to those of our common shares.
Any of the foregoing could prevent or delay a change of control and may deprive or limit strategic opportunities to our shareholders to sell their shares.
In addition, provisions in the CBCA and in our articles of incorporation and by-laws, as amended and/or restated in connection with this offering, may have the effect of delaying or preventing changes in our management, including provisions that:
• | require that any action to be taken by our shareholders be effected at a duly called annual or special meeting and not by written consent; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | establish an advance notice procedure for shareholder proposals to be brought before an annual meeting, including proposed nominations of persons for election to our board of directors; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | prohibit cumulative voting in the election of directors; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | require the approval of our board of directors or the holders of a supermajority of our outstanding share capital to amend our by-laws and certain provisions of our articles of incorporation. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
These provisions may frustrate or prevent any attempts by our shareholders to replace or remove our current management by making it more difficult for shareholders to replace members of our board of directors, which is responsible for appointing the members of our management.
Our executive officers, directors, principal stockholders and their affiliates will continue to exercise significant control over our Company after this offering, which will limit your ability to influence corporate matters and could delay or prevent a change in corporate control.
Immediately following the completion of this offering, and disregarding any common shares that they purchase in this offering, the existing holdings of our executive officers, directors, principal stockholders and their affiliates, including OrbiMed Private Investments IV, LP, BDC, and New Enterprise Associates, Inc. and its affiliates will represent beneficial ownership, in the aggregate, of approximately % of our outstanding common shares, assuming no exercise of the underwriters’ option to acquire additional common shares in this offering and assuming we issue the number of common shares as set forth on the cover page of this prospectus. As a result, these stockholders, if they act together, will have significant influence over our management and affairs and control the outcome of matters submitted to our stockholders for approval, including the election of directors and any sale, merger, consolidation, or sale of all or substantially all of our assets. These stockholders acquired their common shares for substantially less than the price of the common shares being acquired in this offering, and these stockholders may have interests, with respect to their common shares, that are different from those of investors in this offering and the concentration of voting power among these stockholders may have an adverse effect on the price of our common shares. In addition, this concentration of ownership might adversely affect the market price of our common shares by:
• | delaying, deferring or preventing a change of control of us; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | impeding a merger, consolidation, takeover or other business combination involving us; or | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
43
See “Principal Shareholders” in this prospectus for more information regarding the ownership of our outstanding common shares by our executive officers, directors, principal stockholders and their affiliates.
Limitations on the ability to acquire and hold our common shares may be imposed under the Hart-Scott Rodino Act, the Competition Act (Canada) and other applicable antitrust legislation.
Limitations on the ability to acquire and hold our common shares may be imposed under the Hart-Scott Rodino Act, the Competition Act (Canada) and other applicable antitrust legislation. Such legislation generally permits the relevant governmental authority review any acquisition of control over or of significant interest in us, and grants the authority to challenge or prevent an acquisition on the basis that it would, or would be likely to, result in a substantial prevention or lessening of competition. In addition, the Investment Canada Act subjects an “acquisition of control” of a “Canadian business” (as those terms are defined therein) by a non-Canadian to governmental review if the book value of the Canadian business’ assets as calculated pursuant to the legislation exceeds a threshold amount. A reviewable acquisition may not proceed unless the relevant minister is satisfied that the investment is likely to be of net benefit to Canada. Any of the foregoing could prevent or delay a change of control and may deprive our shareholders of the opportunity to sell their common shares at a control premium.
Sales of a substantial number of our common shares in the public market could cause our stock price to fall.
If our existing stockholders sell, or indicate an intention to sell, substantial amounts of our common shares in the public market after the lock-up and other legal restrictions on resale discussed in this prospectus lapse, the market price of our common shares could decline. Based upon the number of common shares, on an as-converted basis, outstanding as of , upon the completion of this offering, we will have outstanding a total of common shares, assuming no exercise of the underwriters’ option to purchase additional shares. Of these shares, as of the date of this prospectus, approximately of our common shares, plus any shares sold upon exercise of the underwriters’ option to purchase additional shares, will be freely tradable, without restriction, in the public market immediately following this offering, assuming that current stockholders do not purchase shares in this offering. The representatives of the underwriters, however, may, in their sole discretion, permit our officers, directors and other stockholders who are subject to these lock-up agreements to sell shares prior to the expiration of the lock-up agreements.
The lock-up agreements pertaining to this offering will expire 180 days from the date of this prospectus. After the lock-up agreements expire, based upon the number of common shares, on an as-converted basis, outstanding as of , up to an additional common shares will be eligible for sale in the public market, % of which shares are held by directors, executive officers and other affiliates and will therefore be subject to certain limitations of Rule 144 under the Securities Act of 1933, as amended (the Securities Act).
Upon completion of this offering, common shares that are either subject to outstanding options or reserved for future issuance under our equity incentive plans will become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules, the lock-up agreements and Rule 144 and Rule 701 under the Securities Act. If these additional common shares are sold, or if it is perceived that they will be sold, in the public market, the market price of our common shares could decline.
After this offering, the holders of approximately shares of our common share will be entitled to rights with respect to the registration of their shares under the Securities Act, subject to the lock-up agreements described above. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates. Any sales of securities by these stockholders could have a material adverse effect on the market our common shares.
44
If you purchase common shares in this offering, you will incur immediate and substantial dilution in the book value of your shares.
You will suffer immediate and substantial dilution in the net tangible book value of the common shares you purchase in this offering. Based on the initial public offering price of $ per share, purchasers of common shares in this offering will experience immediate dilution of $ per share in net tangible book value of the common shares. In addition, investors purchasing common shares in this offering will contribute % of the total amount invested by stockholders since inception but will only own % of our common shares outstanding. In the past, we issued options to acquire common shares at prices significantly below the initial public offering price. To the extent these outstanding securities are ultimately exercised, investors purchasing common shares in this offering will sustain further dilution. See “Dilution” for a more detailed description of the dilution to new investors in the offering.
Future sales and issuances of our common shares or rights to purchase common shares, including pursuant to additional financings or our equity incentive plans could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. To the extent we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. We may sell common shares, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common shares, convertible securities or other equity securities in more than one transaction, investors may be materially diluted by subsequent sales. These sales may also result in material dilution to our existing stockholders, and new investors could gain rights superior to our existing stockholders.
The terms of any financing may adversely affect the holdings or the rights of our stockholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our shares to decline. The incurrence of indebtedness would result in increased fixed payment obligations and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or at an earlier stage than otherwise would be desirable and we may be required to relinquish rights to some of our technologies or palovarotene or any other product candidate or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects.
Moreover, pursuant to the stock option plan, our board of directors are authorized to grant stock options and other equity-based awards to our employees, directors and consultants. The number of shares available for future grant under the 2017 Omnibus Plan will automatically increase by an annual amount to be added the first day of each fiscal year, subject to the ability of our board of directors to take action to reduce the size of the increase in any given year. Currently, we plan to register the increased number of shares available for issuance under the stock option plan each year. If our board of directors elects to increase the number of shares available for future grant by the maximum amount each year, our stockholders may experience additional dilution, which could cause our stock price to fall.
After the completion of this offering, we may be at an increased risk of securities class action litigation.
Historically, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and pharmaceutical companies have experienced significant stock price volatility in recent years. If we were to be sued, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
45
We have broad discretion in how we use the proceeds of this offering and may not use these proceeds effectively, which could affect our results of operations and cause our stock price to decline.
Our management will have considerable discretion in the application of the net proceeds of this offering, including for any purpose described in the section of this prospectus entitled “Use of Proceeds.” However, our needs may change as our business and industry evolve and, as a result, the proceeds we receive from this offering may be used in a manner substantially different from our current expectations. We may use the net proceeds for purposes that do not yield a significant return or any return at all for our stockholders. In addition, pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value. You will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately and, as a result, you will be relying on our management’s judgment.
We are governed by the corporate laws of Canada which in some cases have a different effect on shareholders than the corporate laws of Delaware.
We are governed by the CBCA, and other relevant laws, which may affect the rights of shareholders differently than those of a company governed by the laws of a U.S. jurisdiction, and may, together with our charter documents, have the effect of delaying, deferring or discouraging another party from acquiring control of our Company by means of a tender offer, a proxy contest or otherwise, or may affect the price an acquiring party would be willing to offer in such an instance. The material differences between the CBCA and Delaware General Corporation Law (DGCL), that may have the greatest such effect include but are not limited to the following: (i) for material corporate transactions (such as mergers and amalgamations, other extraordinary corporate transactions or amendments to our articles) the CBCA generally requires a two-thirds majority vote by shareholders, whereas DGCL generally only requires a majority vote; and (ii) under the CBCA a holder of 5% or more of our common shares can requisition a special meeting of shareholders, whereas such right does not exist under the DGCL. Refer to the heading titled “Material Differences between the Canada Business Corporations Act and Delaware General Corporation Law” for more information.
U.S. civil liabilities may not be enforceable against us, our directors, our officers or certain experts named in this prospectus.
We are governed by the CBCA and our principal place of business is in Canada. Many of our directors and officers, as well as certain experts named herein, reside outside of the U.S., and all or a substantial portion of their assets as well as all or a substantial portion of our assets are located outside the U.S. As a result, it may be difficult for investors to effect service of process within the U.S. upon us and such directors, officers and experts or to enforce judgments obtained against us or such persons, in U.S. courts, in any action, including actions predicated upon the civil liability provisions of U.S. federal securities laws or any other laws of the U.S. Additionally, rights predicated solely upon civil liability provisions of U.S. federal securities laws or any other laws of the U.S. may not be enforceable in original actions, or actions to enforce judgments obtained in U.S. courts, brought in Canadian courts, including courts in the Province of Quebec.
We do not intend to pay dividends on our common shares and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common shares.
We have never declared or paid any cash dividend on our common shares and do not currently intend to do so in the foreseeable future. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends in the foreseeable future. Therefore, the success of an investment in our common shares will depend upon any future appreciation in their value. There is no guarantee that our common shares will appreciate in value or even maintain the price at which you purchased them.
46
If securities or industry analysts do not publish or cease publishing research or reports or publish misleading, inaccurate or unfavorable research about us, our business or our market, our stock price and trading volume could decline.
The trading market for our common share will be influenced by the research and reports that securities or industry analysts publish about us, our business, our market or our competitors. We do not currently have and may never obtain research coverage by securities and industry analysts. If no or few securities or industry analysts cover our Company, the trading price and volume of our stock would likely be negatively impacted. If we obtain securities or industry analyst coverage and if one or more of the analysts who covers us downgrades our stock or publishes inaccurate or unfavorable research about our business, or provides more favorable relative recommendations about our competitors, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, demand for our stock could decrease, which could cause our stock price or trading volume to decline.
47
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements within the meaning of applicable securities laws. All statements contained herein that are not clearly historical in nature are forward-looking, and the words “anticipate,” “believe,” “expect,” “estimate,” “may,” “will,” “could,” “leading,” “intend,” “contemplate,” “shall” and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements in this prospectus include, but are not limited to, statements with respect to:
• | our ability to achieve profitability in the future; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our projected financial position and estimated cash burn rate; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our expectations about the timing of achieving milestones and the cost of our development programs; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our observations and expectations regarding the efficacy of palovarotene and the potential benefits to patients; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our requirements for, and the ability to obtain, future funding on favorable terms or at all; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our projections regarding the timely and successful completion of studies and trials and availability of results from such studies and trials; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our expectations about palovarotene’s safety and efficacy; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our expectations regarding our ability to arrange for the manufacturing of our products and technologies; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our expectations regarding the progress, and the successful and timely completion, of the various stages of the regulatory approval process; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our plans to market, sell and distribute our products and technologies; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our expectations regarding the acceptance of our products and technologies by the market; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our ability to retain and access appropriate staff, management, and expert advisers; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our expectations with respect to existing and future corporate alliances and licensing transactions with third parties, and the receipt and timing of any payments to be made by us or to us in respect of such arrangements; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our strategy with respect to the protection of our intellectual property. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
All forward-looking statements reflect our beliefs and assumptions based on information available at the time the assumption was made. These forward-looking statements are not based on historical facts but rather on management’s expectations regarding future activities, results of operations, performance, future capital and other expenditures (including the amount, nature and sources of funding thereof), competitive advantages, business prospects and opportunities. By its nature, forward-looking information involves numerous assumptions, inherent risks and uncertainties, both general and specific, known and unknown, that contribute to the possibility that the predictions, forecasts, projections or other forward-looking statements will not occur. Factors which could cause future outcomes to differ materially from those set forth in the forward-looking statements include, but are not limited to:
• | our ability to generate revenue and become profitable; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the ability to obtain, on satisfactory terms or at all, the financing required to support operations, development, clinical trials, and commercialization of products; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risks related to our heavy reliance on palovarotene, our only current product candidate; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risks of delays and inability to complete clinical trials due to difficulties enrolling patients; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risks associated with the development of palovarotene and any future product candidate, including the demonstration of efficacy and safety; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risks related to clinical trials including the risk of negative results, potential delays, cost overruns and potential adverse events or unacceptable side effects; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
48
• | the risks of reliance on third-parties for the planning, conduct and monitoring of clinical trials and for the manufacture of clinical drug supplies and drug product; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | potential changes in regulatory requirements, and delays or negative outcomes from the regulatory approval process; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our ability to successfully compete in our targeted markets, including the risk that competing therapies could emerge; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risks related to healthcare reimbursement policies and potential healthcare reform; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our heavy dependence on licensed intellectual property, including our ability to source and maintain licenses from third-party owners; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our ability to adequately protect proprietary information, trade secrets, and technology from competitors; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the risk of patent or other intellectual property related litigation; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | risks related to changes in patent laws and their interpretations; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | risks relating to our ability to manage the expansion of the size and scope of our Company, including risks associated with international operations; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the potential for product liability claims; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our ability to attract, retain and motivate key personnel. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The above are further and more fully described under the section of this prospectus entitled “Risk Factors.”
Although the forward-looking statements contained in this prospectus are based upon what our management believes to be reasonable assumptions, we cannot assure readers that actual results will be consistent with these forward-looking statements.
Any forward-looking statements represent our estimates only as of the date of this prospectus and should not be relied upon as representing our estimates as of any subsequent date. We undertake no obligation to update any forward-looking statement or statements to reflect events or circumstances after the date on which such statement is made or to reflect the occurrence of unanticipated events, except as may be required by applicable securities legislation.
49
The following table sets forth, for the periods indicated, the high, low, average and end of period noon rates of exchange for one U.S. dollar, expressed in Canadian dollars, published by the Bank of Canada during the respective periods.
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
| Three-Month | Year Ended December 31, | ||||||||||||||||||||||||||
2017 | 2016 | 2015 | 2014 | |||||||||||||||||||||||||
Highest noon rate during the period |
|
| 1.3513 |
|
| 1.4589 |
|
| 1.3990 |
|
| 1.1643 | ||||||||||||||||
Lowest noon rate during the period |
|
| 1.3016 |
|
| 1.2544 |
|
| 1.1728 |
|
| 1.0614 | ||||||||||||||||
Average noon spot rate for the period |
|
| 1.3238 |
|
| 1.3248 |
|
| 1.2787 |
|
| 1.1045 | ||||||||||||||||
Noon rate at the end of the period |
|
| 1.3310 |
|
| 1.3427 |
|
| 1.3840 |
|
| 1.1601 | ||||||||||||||||
On June 28, 2017, the Bank of Canada indicative rate of exchange was $1.00 = C$1.3060.
50
We estimate that we will receive net proceeds from this offering of approximately $ million based upon an assumed initial public offering price of $ per common share, the midpoint of the estimated price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters’ over-allotment option to purchase additional shares in this offering is exercised in full, we estimate that our net proceeds will be approximately $ million, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the estimated price range set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us from this offering by approximately $ , assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of one million shares in the number of common shares offered by us would increase (decrease) the net proceeds to us from this offering by approximately $ , assuming the assumed initial public offering price remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
The principal reasons for this offering are to increase our capitalization and financial flexibility, create a public market for our common shares, and facilitate our access to the public equity markets. We anticipate that we will use the net proceeds of this offering, together with our existing cash on hand, as follows:
• | Approximately $ to fund expenses incurred in pursuing the registration of palovarotene in FOP, including conducting the Phase 3 MOVE clinical trial and an additional clinical trial of palovarotene for the treatment of FOP; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Approximately $ to fund expenses incurred in conducting the Phase 2/3 clinical trial of palovarotene for the treatment of MO; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Approximately $ to fund expenses incurred in conducting the Phase 1 and Phase 2 clinical trials of palovarotene for the treatment of dry eye disease; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | The remainder for working capital, general and administrative expenses, research and development expenses, and other general corporate purposes. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Our expected use of net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering or the amounts that we will actually spend on the uses set forth above. We may also use a portion of the net proceeds to in-license, acquire or invest in complementary technologies, products or assets. The amounts and timing of our actual use of net proceeds will vary depending on numerous factors, including the status of and results from clinical trials, the timing of regulatory submissions, and the progress of our development and commercialization efforts. As a result, our management will have broad discretion over the use of the net proceeds from this offering.
Pending its use, we intend to invest the net proceeds to us from the offering in accordance with the terms of our investment policy, as approved by our board of directors, or hold them as cash.
51
We have never declared or paid cash dividends on our common shares. We currently intend to retain any future earnings to fund the development and growth of our business and we do not currently anticipate paying dividends on our common shares. Any determination to pay dividends to holders of our common shares in the future will be at the discretion of our board of directors and will depend on many factors, including our financial condition, earnings, legal requirements and other factors as our board of directors deems relevant.
52
The following table sets forth our cash and short-term investments and capitalization as of March 31, 2017:
(1) | on an actual basis, which retrospectively reflects a 1-for- stock split of our common shares; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) | on a pro forma basis to give effect to (1) the automatic conversion of all of our outstanding preferred shares into common shares upon the closing of this offering; (2) the recording of a non-recurring, non-cash charge of approximately $ million to the consolidated statement of net loss and comprehensive loss (reflected as a pro forma increase in preferred share embedded derivative liability and deficit in connection with the mark-to-market of the preferred shares and embedded derivative liabilities to the fair value of our common shares prior to conversion of our preferred shares into common shares), assuming an initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus (see footnote (1) to the table set forth in the section titled “Selected Consolidated Financial Data” for more information); (3) the inclusion in share capital of the original stated capital of the preferred shares (in accordance with subsection 39(4) of the CBCA); (4) the inclusion in contributed surplus of the excess of the total carrying value of the preferred shares, including the preferred shares and embedded derivative liabilities, over the stated capital of the preferred shares, and a corresponding increase in deficit (as resolved by the Company’s board of directors); and (5) the elimination of the contributed surplus created by the conversion of the preferred shares into common shares, and a corresponding reduction in deficit (as resolved by the Company’s board of directors); and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) | on a pro forma as adjusted basis to give effect to the sale by us of common shares in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and estimated offering expenses payable by us. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
You should read the information in this table together with our financial statements and accompanying notes thereto, “Selected Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” appearing elsewhere in this prospectus.
|
|
|
|
|
|
| |||||||||||||||
| As of March 31, 2017 | ||||||||||||||||||||
Actual | Pro Forma | Pro Forma | |||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Cash and short-term investments |
|
| $ |
| 43,722 |
|
|
|
|
|
| ||||||||||
|
|
|
|
|
|
| |||||||||||||||
Preferred shares liability(1) |
|
| $ |
| 76,059 |
|
|
|
| ||||||||||||
Preferred shares embedded derivative |
|
| $ |
| 155,857 |
|
|
|
| ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
Total long-term liabilities |
|
| $ |
| 231,916 |
|
|
|
| ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
Equity |
|
|
|
|
|
| |||||||||||||||
Common shares(2) |
|
| $ |
| 314 |
|
|
|
| ||||||||||||
Contributed surplus(3) |
|
| $ |
| 566 |
|
|
|
| ||||||||||||
Deficit |
|
| $ |
| (190,779 | ) |
|
|
|
|
| ||||||||||
|
|
|
|
|
|
| |||||||||||||||
Total equity |
|
| $ |
| (189,899 | ) |
|
|
|
|
| ||||||||||
|
|
|
|
|
|
| |||||||||||||||
Consolidated capitalization |
|
| $ |
| 42,017 |
|
|
|
| ||||||||||||
(1) | Actual: no par value, unlimited shares authorized, 1,118,415 Class A, 485,823 Class B and 70,176 Class C convertible and redeemable preferred shares issued and outstanding. Pro forma and pro forma as adjusted: no shares authorized, issued and outstanding. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) | Actual: no par value, unlimited shares authorized, 202,693 shares issued and outstanding. Pro forma: unlimited shares authorized; shares issued and outstanding. Pro forma as adjusted: unlimited shares authorized, shares issued and outstanding. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) | At March 31, 2017, there were 212,602 stock options outstanding at a weighted average exercise price of $12.87 per share. Share-based compensation expense recognized as stock options vest is | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
53
| recorded in contributed surplus. 39,100 stock options were granted subsequent to March 31, 2017 with a weighted average exercise price of $120.36 per share. The grants subsequent to March 31, 2017 are not reflected in the above table as they will only be recorded as vesting occurs. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) | A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the estimated price range set forth on the cover page of this prospectus, would increase (decrease) pro forma as adjusted cash, short-term investments and total equity by $ , assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of one million shares in the number of common shares offered by us would increase (decrease) pro forma as adjusted cash, short-term investments and total equity by approximately $ , assuming the assumed initial public offering price remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
54
If you invest in our common shares, your interest will be diluted to the extent of the difference between the initial public offering price per share of our common shares and the pro forma as adjusted net tangible book value per share of our common shares immediately after this offering.
Our historical net tangible book value as of , 2017 was $ or $ per share. The historical net tangible book value per share represents the amount of our total tangible assets less our total liabilities, divided by the number of common shares outstanding as of , 2017. The negative amount is attributable to our preferred shares being classified as a liability and the related embedded derivative liability, which have been included in this calculation based on the amounts in the consolidated financial statements as of , 2017.
Our pro forma net tangible book value as of , 2017 was $ , or $ per share. Pro forma net tangible book value per share represents the amount of our total tangible assets less our total liabilities, divided by the number of common shares as of after giving effect to the automatic conversion of our preferred shares into common shares and the 1-for- stock split of our common shares.
After giving further effect to the sale by us of common shares in this offering at an assumed initial public offering price of $ per share, the midpoint of the estimated price range set forth on the cover page of this prospectus, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of would have been $ , or $ per share. This represents an immediate increase in pro forma as adjusted net tangible book value of $ per share to our existing shareholders and an immediate dilution in pro forma as adjusted net tangible book value of $ per share to new investors in this offering. The following table illustrates this dilution on a per share basis:
|
|
|
|
| ||||||||||
Assumed initial public offering price per share |
|
|
|
|
| $ |
|
| ||||||
Historical net tangible book value per share as of , 2017 |
|
| $ |
|
| |||||||||
Increase in pro forma net tangible book value per share attributed to automatic conversion of preferred shares into common shares |
|
| $ |
|
| |||||||||
|
|
|
|
| ||||||||||
Pro forma net tangible book value per share as of , 2017 |
|
| $ |
|
| |||||||||
Increase in pro forma as adjusted net tangible book value per share attributed to new investors purchasing shares in this offering |
|
| $ |
|
| |||||||||
|
|
|
|
| ||||||||||
Pro forma as adjusted net tangible book value per share after giving effect to this offering |
|
|
|
| $ | |||||||||
|
|
|
|
| ||||||||||
Dilution in pro forma as adjusted net tangible book value per share to new investors in this offering |
|
|
|
| $ | |||||||||
|
|
|
|
| ||||||||||
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) the pro forma as adjusted net tangible book value by $ per share and the dilution to new investors by $ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and estimated offering expenses payable by us. Similarly, each increase (decrease) of 1,000,000 shares in the number of common shares offered by us would increase (decrease) the pro forma as adjusted net tangible book value by approximately $ per share and the dilution to new investors by $ per share, assuming the assumed initial public offering price remains the same and after deducting underwriting discounts and estimated offering expenses payable by us. If the underwriters exercise their option to purchase additional shares in full, the pro forma as adjusted net tangible book value per share would be $ per share, and the dilution in pro forma as adjusted net tangible book value per share to investors in this offering would be $ per share.
If the underwriters’ option to purchase additional common shares from us is exercised in full, the pro forma as adjusted net tangible book value per share of our common shares immediately after the completion of this offering would be approximately $ per share, and the dilution in pro forma net tangible book value per share to investors purchasing shares of our common shares in this offering would be approximately $ per share, assuming no change in the assumed initial public offering price and after deducting underwriting discounts and estimated offering expenses payable by us.
55
The table below summarizes as of , 2017 on a pro forma as adjusted basis described above, the number of our common shares, the total consideration and the average price per share (i) paid to us by our existing shareholders and (ii) to be paid by new investors purchasing our common shares in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, before deducting underwriting discounts and estimated offering expenses payable by us.
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
| Shares Purchased | Total Consideration | Average | ||||||||||||||||||||||||||||||||||
Number | Percent | Amount | Percent | ||||||||||||||||||||||||||||||||||
| (In thousands) |
|
| ||||||||||||||||||||||||||||||||||
Existing shareholders |
|
|
|
|
| % |
|
|
|
|
| % |
|
|
|
|
| ||||||||||||||||||||
New investors |
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||
Total |
|
|
|
| 100.0 | % |
|
|
|
|
|
| 100.0 | % |
|
|
|
|
|
| |||||||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
The above discussion and tables are based on of our common shares outstanding as of , 2017 after giving effect to a 1-for stock split of our common shares and the automatic conversion of all of our outstanding preferred shares into common shares, and exclude:
• | common shares issuable upon the exercise of options outstanding as of , 2017 pursuant to our Second Amended and Restated Stock Option Plan (Existing Stock Option Plan) and 2017 Omnibus Plan, at a weighted-average exercise price of $ per share; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | common shares available for future issuance under our 2017 Omnibus Plan. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
To the extent that any outstanding options are exercised, new options are issued under our 2017 Omnibus Plan or we issue additional common shares in the future, there will be further dilution to investors participating in this offering. If all outstanding options under our Existing Stock Option Plan and 2017 Omnibus Plan as of were exercised, then our existing shareholders, including the holders of these options, would own %, and our new investors would own % of the total number of our common shares outstanding upon the closing of this offering. In such event, the total consideration paid by our existing shareholders, including the holders of these options, would be approximately $ , or % the total consideration paid by our new investors would be $ million, or % the average price per share paid by our existing shareholders would be $ and the average price per share paid by our new investors would be $ .
56
SELECTED CONSOLIDATED FINANCIAL DATA
The following table sets forth our selected consolidated financial data for the periods, and as of the dates, indicated. The information set forth below does not take into account the 1-for- stock split of our common shares or the automatic conversion of our preferred shares into common shares, which will occur prior to the consummation of this offering. The following selected consolidated financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes thereto, which are included elsewhere in this prospectus.
We have derived the selected consolidated statements of net loss and comprehensive loss data for the years ended December 31, 2016, 2015 and 2014 and the selected consolidated statement of financial position data as of December 31, 2016, 2015 and 2014 from our audited consolidated financial statements included elsewhere in this prospectus. We derived the consolidated statements of net loss and comprehensive loss data for the three-month periods ended March 31, 2017 and 2016 and the consolidated statement of financial position data as of March 31, 2017 from our unaudited interim condensed consolidated financial statements and the related notes thereto appearing elsewhere in this prospectus. Our consolidated financial statements have been prepared in accordance with IFRS, as issued by the IASB. In the opinion of management, the unaudited interim condensed consolidated financial statements reflect all adjustments necessary for a fair presentation of the financial statements. Our historical results are not necessarily indicative of the results that should be expected in any future period, and the results for the three-month period ended March 31, 2017 are not necessarily indicative of the results that may be expected for the full year or any other period.
All of our operations are continuing operations and we have not paid any dividends since inception.
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Consolidated Statements of Net Loss and Comprehensive Loss Data | Three-months Ended | Year Ended | |||||||||||||||||||||||||||||||||
2017 | 2016 | 2016 | 2015 | 2014 | |||||||||||||||||||||||||||||||
| (in thousands, except share and per share data) | ||||||||||||||||||||||||||||||||||
Expenses |
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Research and development |
|
| $ |
| 3,408 |
|
| $ |
| 3,669 |
|
| $ |
| 16,852 |
|
| $ |
| 14,396 |
|
| $ |
| 7,797 | ||||||||||
Investment tax credits |
|
| (50 | ) |
|
|
| (47 | ) |
|
|
| (139 | ) |
|
|
| (165 | ) |
|
|
| (214 | ) |
| ||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
|
|
| 3,358 |
|
| 3,622 |
|
| 16,713 |
|
| 14,231 |
|
| 7,583 | ||||||||||||||||||||
General and administrative |
|
| 1,668 |
|
| 1,080 |
|
| 3,406 |
|
| 5,479 |
|
| 2,266 | ||||||||||||||||||||
Interest income |
|
| (81 | ) |
|
|
| (103 | ) |
|
|
| (399 | ) |
|
|
| (109 | ) |
|
|
| (19 | ) |
| ||||||||||
Financial expenses |
|
| 36,347 |
|
| 1,432 |
|
| 37,646 |
|
| 56,140 |
|
| 2,364 | ||||||||||||||||||||
Income tax expense |
|
| 44 |
|
| 33 |
|
| 146 |
|
| 156 |
|
| 95 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Net loss and comprehensive loss |
|
| $ |
| (41,336 | ) |
|
|
| $ |
| (6,064 | ) |
|
|
| $ |
| (57,512 | ) |
|
|
| $ |
| (75,897 | ) |
|
|
| $ |
| (12,289 | ) |
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Net loss per share applicable to common shareholders—basic and diluted |
|
| $ |
| (209.64 | ) |
|
|
| $ |
| (30.92 | ) |
|
|
| $ |
| (293.27 | ) |
|
|
| $ |
| (396.45 | ) |
|
|
| $ |
| (68.32 | ) |
|
Weighted average number of common shares used in net loss per share applicable to common shareholders—basic and diluted |
|
| 197,181 |
|
| 196,109 |
|
| 196,109 |
|
| 191,443 |
|
| 179,874 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Pro forma net loss per share applicable to common shareholders—basic and diluted (unaudited)(1) |
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Pro forma weighted average number of common shares used in net loss per share applicable to common shareholders—basic and diluted (unaudited)(1) |
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
57
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
Consolidated Statements of Financial Position Data | As of | As of December 31, | ||||||||||||||||||||||||||
2017 | 2016 | 2015 | 2014 | |||||||||||||||||||||||||
| (in thousands) | |||||||||||||||||||||||||||
Cash and short-term investments |
|
| $ |
| 43,722 |
|
| $ |
| 39,434 |
|
| $ |
| 58,107 |
|
| $ |
| 5,504 | ||||||||
Working capital(2) |
|
| 40,118 |
|
| 36,101 |
|
| 55,569 |
|
| 4,885 | ||||||||||||||||
Total assets |
|
| 46,670 |
|
| 41,557 |
|
| 60,657 |
|
| 8,000 | ||||||||||||||||
Preferred share and embedded derivative liabilities |
|
| 231,916 |
|
| 185,706 |
|
| 147,981 |
|
| 21,598 | ||||||||||||||||
Common shares |
|
| 314 |
|
| 272 |
|
| 272 |
|
| 195 | ||||||||||||||||
Total equity |
|
| (189,899 | ) |
|
|
| (148,672 | ) |
|
|
| (91,335 | ) |
|
|
| (15,652 | ) |
| ||||||||
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
(1) | Pro-forma net loss per share has been computed to give retrospective effect to the conversion of all of the outstanding preferred shares into common shares on a one-for-one basis using the if-converted method for the three-month period ended March 31, 2017 and the year-ended December 31, 2016 as if the conversion had occurred on January 1, 2016 or the original issue date of the preferred shares if later and after reflecting a 1-for- stock split of our common shares. A non-recurring, non-cash charge will be recorded in the statement of net loss at the time of the conversion of the preferred shares immediately prior to the initial public offering. This charge is not reflected in the pro forma net loss per share. In addition, the following charges are not reflected in the pro forma net loss per share: charges related to incurred or upcoming listing and related expenses, and share-based compensation expense related to 39,100 stock options granted subsequent to March 31, 2017. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) | Working capital equals current assets less current liabilities. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
58
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with the section entitled “Selected Consolidated Financial Data,” our audited annual consolidated financial statements and related notes thereto, and our unaudited interim condensed consolidated financial statements and notes thereto included elsewhere in this prospectus. This discussion and analysis and other parts of this prospectus contain forward-looking statements that involve risks and uncertainties, such as statements regarding our plans, objectives, expectations, intentions, and projections. Our actual results could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the “Risk Factors” section of this prospectus.
Overview
We are a clinical stage biopharmaceutical company that is developing disease-modifying treatments for patients suffering from debilitating bone and other diseases with high unmet medical need. Our lead product candidate, palovarotene, is an oral small molecule that has shown potent activity in preventing abnormal new bone formation as well as fibrosis in a variety of tissues. We are developing palovarotene for the treatment of Fibrodysplasia Ossificans Progressiva (FOP) and Multiple Osteochondroma (MO).
Our most advanced program is palovarotene for the treatment of FOP. In July 2014, the FDA granted Orphan Drug Designation for palovarotene as a treatment for FOP and in November 2014, we received Fast Track Designation. We expect to initiate a registration trial of palovarotene in FOP in 2017 and we are also developing a protocol for another clinical trial in FOP. The registration trial is the Phase 3 MOVE trial for the treatment of FOP in adults and children, which will enroll up to 100 patients who will be treated chronically with palovarotene and with increased doses during flare-ups. The second is a trial which aims to determine if palovarotene can inhibit new HO formation after surgical excision of HO. We expect to report data from the MOVE trial in 2020 with an interim read-out in 2019.
We are also developing palovarotene for the treatment of MO. Following the completion of our pre-clinical proof-of-concept studies showing that palovarotene inhibits the number of osteochondromas expressed in animal models of MO, and based on our knowledge of the safety and tolerability profile of palovarotene and our pre-clinical animal model data, we are planning to initiate a placebo-controlled Phase 2/3 study of palovarotene in MO this year. We expect to report data for this trial in 2020 with a potential interim read-out in 2019.
We also believe that RARg agonists have great potential as inhibitors of bone morphogenetic protein (BMP) signaling in other indications. There are several other potential large indications for the prevention of HO, such as ankylosing spondylitis, a type of arthritis associated with BMP signaling, which represents a high unmet medical need. Other indications, such as those characterized by excessive fibrosis or scarring, are also potential target indications for RARg agonists. As a result, we have conducted pre-clinical proof-of-concept studies in dry eye disease that show an eye drop formulation of palovarotene can potently increase tear production and decrease corneal damage. Following the completion of these studies, we plan to initiate IND-enabling toxicity studies of an ophthalmologic formulation in order to begin Phase 1 and 2 clinical trials in dry eye disease in 2018. We are also focused on developing our RARg agonist platform beyond palovarotene for larger, related disease markets, such as in ankylosing spondylitis or trauma-induced HO. As part of this development process, we recently licensed a number of second generation RARg agonists from Galderma. On the basis of our scientific know-how and other clinical and commercial insights, a number of indications have been prioritized for animal model proof-of-concept studies in 2017 and 2018. Should these studies be successful, we plan to initiate the pre-IND activities necessary to initiate clinical trials in these new indications.
Since our inception in November 2010, we have devoted substantially all of our resources to organizing and staffing our Company, business planning, raising capital, developing our product candidates, preparing and conducting clinical studies of our product candidates, providing general and administrative support for these operations and protecting our intellectual property. We have funded
59
our operations primarily through issuances of redeemable convertible preferred shares and to a lesser extent, the issuance of convertible notes and common shares. From our inception through March 31, 2017, we have received gross proceeds of $102.8 million from such transactions, of which $102.2 million was in the form of gross proceeds from the sale of preferred shares, $0.5 million was in the form of gross proceeds from the sale of convertible notes and $0.1 million was in the form of gross proceeds from the sale of common shares. As at March 31, 2017 we had cash and short-term investments of $43.7 million.
We are a development stage company and have not generated any revenue. We have incurred net losses since our inception and as of March 31, 2017, we had an accumulated deficit of $190.8 million. Substantially all of our net losses have resulted from non-cash charges incurred in connection with the accounting of our preferred shares and embedded derivatives, as well as research and development activities and general and administrative costs associated with our operations.
We expect to incur significant expenses and increasing operating losses for the foreseeable future. We expect our expenses will increase substantially in connection with our ongoing activities, particularly as we advance clinical development of palovarotene, our lead product candidate for the treatment of FOP, including conducting two clinical trials; advance development of palovarotene in the treatment of MO; continue research and development efforts to support clinical development of additional RARg agonist candidates; continue to engage contract manufacturing organizations (CMOs) to manufacture our clinical study materials and to develop large-scale manufacturing capabilities; seek regulatory approval for our product candidates; add personnel to support our product development and future commercialization; add operational, financial and management information systems; maintain, leverage and expand our intellectual property portfolio; and operate as a public company.
We do not expect to generate revenue from product sales unless and until we successfully complete development and obtain regulatory approval for palovarotene or any other product candidate, which we expect will take a number of years and is subject to significant uncertainty. If we obtain regulatory approval for any of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing, and distribution. As a result, we will need additional financing to support our continuing operations. Until such time that we can generate significant revenue from product sales, if ever, we expect to finance our operations through a combination of public or private equity or debt financings or other sources, which may include collaborations with third parties. Arrangements with collaborators or others may require us to relinquish rights to certain of our technologies or product candidates, obtain adequate patent protection for our technology, obtain necessary regulatory approval for our product candidates or achieve commercial viability for any approved product candidates. Adequate additional financing may not be available to us on acceptable terms, or at all. Our inability to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy. We will need to generate significant revenue to achieve profitability, and we may never do so.
Financial Operations Overview
Revenue
We have not generated any revenues from product sales since our inception and do not expect to generate any revenues from the sale of products in the near future. If our development efforts result in clinical success and regulatory approval or collaboration agreements with third parties for our product candidates, we may generate revenue from those product candidates.
Research and development expenses
Research and development expenses consist primarily of costs associated with our product research and efforts, and predominantly include:
• | personnel costs, including salaries, benefits, bonuses, stock-based compensation expense and related travel for employees engaged in scientific research and development functions; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | expenses incurred under agreements with contract research organizations, or CROs and investigative sites that conduct our non-clinical studies and clinical trials; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
60
• | expenses associated with manufacturing clinical study materials and developing external manufacturing capabilities; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | costs of outside consultants, including their fees and related travel expenses; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | other expenses related to our non-clinical studies and expenses related to our regulatory activities; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | payments made under our third-party licensing agreements. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Research and development costs are generally expensed as incurred unless they meet specific criteria for recognition as internally-generated intangible assets as per IFRS. We have not recognized any internally-generated intangible asset to date.
Costs for certain development activities are recognized based on an evaluation of the progress to completion of specific tasks using information and data provided to us by our vendors and our clinical sites.
We have been focused on developing palovarotene, our product candidate for the treatment of patients with FOP and MO. Our research and development expenses consist principally of external costs, such as start-up fees paid to investigators, consultants, central laboratories and CROs in connection with our clinical and non-clinical studies, and costs related to acquiring and manufacturing clinical study materials. We do not allocate personnel-related costs, depreciation or other indirect costs to specific programs, as they are deployed across various projects under development and, as such, are separately classified as personnel and other expenses.
The following table summarizes our research and development expenses by program:
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
| Three-Months | Years Ended | |||||||||||||||||||||||||||||||||
2017 | 2016 | 2016 | 2015 | 2014 | |||||||||||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||||||||||||
Palovarotene for FOP research and development expenses |
|
| $ |
| 2,073 |
|
| $ |
| 2,666 |
|
| $ |
| 13,014 |
|
| $ |
| 11,318 |
|
| $ |
| 5,700 | ||||||||||
Palovarotene for MO research and development expenses |
|
| 35 |
|
| 50 |
|
| 369 |
|
| — |
|
| — | ||||||||||||||||||||
Palovarotene for ocular research and development expenses |
|
| 2 |
|
| 61 |
|
| 229 |
|
| 75 |
|
| — | ||||||||||||||||||||
Other research and development expenses |
|
| 176 |
|
| 141 |
|
| 149 |
|
| 501 |
|
| — | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Total direct research and development expenses |
|
| 2,286 |
|
| 2,918 |
|
| 13,761 |
|
| 11,894 |
|
| 5,700 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Personnel-related expenses |
|
| 781 |
|
| 628 |
|
| 2,353 |
|
| 1,971 |
|
| 1,692 | ||||||||||||||||||||
Facility and other expenses |
|
| 341 |
|
| 123 |
|
| 738 |
|
| 531 |
|
| 405 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Total personnel, facility and other expenses |
|
| 1,122 |
|
| 751 |
|
| 3,091 |
|
| 2,502 |
|
| 2,097 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Total research and development expenses |
|
| $ |
| 3,408 |
|
| $ |
| 3,669 |
|
| $ |
| 16,852 |
|
| $ |
| 14,396 |
|
| $ |
| 7,797 | ||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Research and development activities are central to our business. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. From inception through March 31, 2017, we incurred $44.5 million in research and development expenses. We expect that our research and development expenses will continue to increase in the foreseeable future as we pursue later stages of clinical development of our product candidates.
We cannot determine with certainty the duration and completion costs of the current or future clinical studies of our product candidates or if, when, or to what extent we will generate revenues from the commercialization and sale of any of our product candidates that obtain regulatory approval. We may never succeed in achieving regulatory approval for any of our product candidates. The duration,
61
costs, and timing of clinical studies and development of our product candidates will depend on a variety of factors, including:
• | the scope, rate of progress, and expense of our ongoing as well as any additional clinical studies and other research and development activities; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | future clinical study results; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | uncertainties in clinical study enrollment rate or design; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | significant and changing government regulation; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the timing and receipt of any regulatory approvals. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A change in the outcome of any of these variables with respect to the development of a product candidate could mean a significant change in the costs and timing associated with the development of that product candidate. For example, if the FDA, or another regulatory authority were to require us to conduct clinical studies beyond those that we currently anticipate will be required for the completion of clinical development of a product candidate or if we experience significant delays in enrollment in any of our clinical studies, we could be required to expend significant additional financial resources and time on the completion of clinical development.
General and administrative expenses
General and administrative expenses consist primarily of personnel costs, including salaries, related benefits, bonuses, stock-based compensation and travel expenses for our executive, finance, business and corporate development and other administrative functions. General and administrative expenses also include facilities and other expenses, including rent, depreciation, maintenance of facilities, insurance and supplies; and professional fees for accounting, tax and legal services, including legal expenses to pursue patent protection of our intellectual property.
We anticipate that our general and administrative expenses will increase in the future as we increase our headcount to support our expected growth in our continued research and development activities and the potential commercialization of our product candidates. We also anticipate increased expenses related to audit, legal, regulatory, and tax-related services associated with maintaining compliance with our exchange listing on The Nasdaq Global Market and SEC requirements, director and officer insurance premiums, and investor relations costs. Additionally, if and when we believe a regulatory approval of palovarotene or any other product candidate appears likely, we anticipate an increase in payroll and related expenses as a result of our preparation for commercial operations, especially as it relates to the sales and marketing of palovarotene or any other product candidate.
Interest income and financial expenses
Interest income consists of interest earned on our cash and short-term investments. Our interest income has not been significant due to low interest rates earned on invested funds. We anticipate that our interest income will increase in the future primarily due to the anticipated net proceeds from this offering.
Financial expenses consist mainly of losses on the re-measurement of embedded derivatives at fair value at each reporting date, accretion expense and foreign exchange gains and losses. Accretion expense consists of accreted interest expense on our outstanding Class A, Class B and Class C redeemable convertible preferred stock to bring the debt components of our preferred stock back to their face value over time. The consummation of this offering will result in the conversion of all classes of our preferred stock into common shares and as such, losses on the re-measurement of embedded derivatives at fair value and accretion expense will be eliminated following this offering. Foreign exchange gains and losses consist of the realized and unrealized net gains and losses from holding cash and short-term investments in foreign currency and foreign currency-denominated other current assets and accounts payable.
62
Results of Operations
Comparison of the three-months ended March 31, 2017 and 2016
The following table summarizes our results of operations for the three-months ended March 31, 2017 and 2016:
|
|
|
|
|
|
| |||||||||||||||
| Three-months ended | Increase | |||||||||||||||||||
2017 | 2016 | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Expenses: |
|
|
|
|
|
| |||||||||||||||
Research and development |
|
| $ |
| 3,408 |
|
| $ |
| 3,669 |
|
| $ |
| (261 | ) |
| ||||
Investment tax credits |
|
| (50 | ) |
|
|
| (47 | ) |
|
|
| (3 | ) |
| ||||||
|
|
|
|
|
|
| |||||||||||||||
|
|
| 3,358 |
|
| 3,622 |
|
| (264 | ) |
| ||||||||||
|
|
|
|
|
|
| |||||||||||||||
General and administrative expenses |
|
| 1,668 |
|
| 1,080 |
|
| 588 | ||||||||||||
Interest income |
|
| (81 | ) |
|
|
| (103 | ) |
|
|
| 22 | ||||||||
Financial expenses |
|
| 36,347 |
|
| 1,432 |
|
| 34,915 | ||||||||||||
Income tax expense |
|
| 45 |
|
| 33 |
|
| 12 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
Net loss and comprehensive loss |
|
| $ |
| (41,337 | ) |
|
|
| $ |
| (6,064 | ) |
|
|
| $ |
| (35,273 | ) |
|
|
|
|
|
|
|
| |||||||||||||||
Research and development expenses
Research and development expenses were $3.4 million for the three-months ended March 31, 2017, compared to $3.7 million for the three-months ended March 31, 2016. The $0.3 million decrease was primarily due to reduced activities on completion of the Phase 2 clinical trial in FOP and completing enrollment into the Phase 2 open-label extension study in 2016.
General and administrative expenses
General and administrative expenses were $1.7 million for the three-months ended March 31, 2017, compared to $1.1 million for the three-months ended March 31, 2016. The increase of $0.6 million in expenses is primarily due to a $0.4 million increase in financing costs associated with the initial public offering and a $0.2 million increase in pre-commercial marketing activities and other general and administrative support activities.
Interest income
Interest income remained stable at $0.1 million for the three-months ended March 31, 2017, compared to $0.1 million for the three-months ended March 31, 2016.
Financial expenses
Financial expenses were $36.3 million for the three-months ended March 31, 2017, compared to $1.4 million for the three-months ended March 31, 2016. The $34.9 million increase in financial expenses is primarily due to an increase in losses on the re-measurement at fair value of the embedded derivative in our preferred stock as a result of a greater step-up in value of the conversion option in the first quarter of 2017 as compared to the first quarter of 2016.
63
Comparison of the years ended December 31, 2016 and 2015
The following table summarizes our results of operations for the years ended December 31, 2016 and 2015:
|
|
|
|
|
|
| |||||||||||||||
| Years ended | Increase | |||||||||||||||||||
2016 | 2015 | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Expenses: |
|
|
|
|
|
| |||||||||||||||
Research and development |
|
| $ |
| 16,852 |
|
| $ |
| 14,396 |
|
| $ |
| 2,456 | ||||||
Investment tax credits |
|
| (139 | ) |
|
|
| (165 | ) |
|
|
| 26 | ||||||||
|
|
|
|
|
|
| |||||||||||||||
|
|
| 16,713 |
|
| 14,231 |
|
| 2,482 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
General and administrative expenses |
|
| 3,406 |
|
| 5,479 |
|
| (2,073 | ) |
| ||||||||||
Interest income |
|
| (399 | ) |
|
|
| (109 | ) |
|
|
| (290 | ) |
| ||||||
Financial expenses |
|
| 37,646 |
|
| 56,140 |
|
| (18,494 | ) |
| ||||||||||
Income tax expense |
|
| 146 |
|
| 156 |
|
| (10 | ) |
| ||||||||||
|
|
|
|
|
|
| |||||||||||||||
Net loss and comprehensive loss |
|
| $ |
| (57,512 | ) |
|
|
| $ |
| (75,897 | ) |
|
|
| $ |
| 18,385 | ||
|
|
|
|
|
|
| |||||||||||||||
Research and development expenses
Research and development expenses were $16.9 million for the year ended December 31, 2016, compared to $14.4 million for the year ended December 31, 2015. The $2.5 million increase was primarily due to:
• | $1.7 million increase in clinical studies and CRO related activities as a result of three FOP clinical studies initiated in 2014 and 2015; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | $0.5 million for initiation of pre-clinical research activities for new potential product candidates in MO and ocular indications; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | $0.4 million decrease in pre-clinical research activities for other potential indications; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | $0.4 million increase in personnel related expenses in support of increased development activities; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | $0.2 million increase in facility and other expenses in support of increased development activities. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
General and administrative expenses
General and administrative expenses were $3.4 million for the year ended December 31, 2016, compared to $5.5 million for the year ended December 31, 2015. The decrease of $2.1 million in expenses is primarily due to a $1.3 million reduction in pre-commercial marketing activities as certain projects undertaken in 2015 were completed in 2016, a $0.5 million reduction in professional fees and a $0.3 million reduction in other general and administrative supporting expenditures.
Interest income
Interest income was $0.4 million for the year ended December 31, 2016, compared to $0.1 million for the year ended December 31, 2015. The increase of $0.3 million in interest income is primarily due to higher invested capital throughout 2016 at higher rates of return.
Financial expenses
Financial expenses were $37.6 million for the year ended December 31, 2016, compared to $56.1 million for the year ended December 31, 2015. The $18.5 million decrease in financial expenses is primarily due to a reduction in losses on the re-measurement at fair value of the embedded derivative as a result of a smaller step-up in value of the conversion option in 2016 than in 2015.
64
Comparison of the years ended December 31, 2015 and 2014
The following table summarizes our results of operations for the years ended December 31, 2015 and 2014:
|
|
|
|
|
|
| |||||||||||||||
| Years ended | Increase | |||||||||||||||||||
2015 | 2014 | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Expenses: |
|
|
|
|
|
| |||||||||||||||
Research and development |
|
| $ |
| 14,396 |
|
| $ |
| 7,797 |
|
| $ |
| 6,599 | ||||||
Investment tax credits |
|
| (165 | ) |
|
|
| (214 | ) |
|
|
| 49 | ||||||||
|
|
|
|
|
|
| |||||||||||||||
|
|
| 14,231 |
|
| 7,583 |
|
| 6,648 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
General and administrative expenses |
|
| 5,479 |
|
| 2,266 |
|
| 3,213 | ||||||||||||
Interest income |
|
| (109 | ) |
|
|
| (19 | ) |
|
|
| (90 | ) |
| ||||||
Financial expenses |
|
| 56,140 |
|
| 2,364 |
|
| 53,776 | ||||||||||||
Income tax expense |
|
| 156 |
|
| 95 |
|
| 61 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
Net loss and comprehensive loss |
|
| $ |
| (75,897 | ) |
|
|
| $ |
| (12,289 | ) |
|
|
| $ |
| (63,608 | ) |
|
|
|
|
|
|
|
| |||||||||||||||
Research and development expenses
Research and development expenses were $14.4 million for the year ended December 31, 2015, compared to $7.8 million for the year ended December 31, 2014. The $6.6 million increase was primarily due to:
• | $5.6 million increase in clinical studies and CRO related activities as a result of three FOP clinical studies initiated in 2014 and 2015; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | $0.6 million increase in pre-clinical research activities for other potential indications; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | $0.3 million increase in personnel related expenses in support of increased development activities; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | $0.1 million increase in facility and other expenses in support of increased development activities. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
General and administrative expenses
General and administrative expenses were $5.5 million for the year ended December 31, 2015, compared to $2.3 million for the year ended December 31, 2014. The increase of $3.2 million in expenses is primarily due to a $1.4 million increase in pre-commercial marketing activities, a $0.7 million increase in personnel related expenses in support of increased development activities, a $0.6 million increase in professional fees and a $0.5 million increase in other general and administrative supporting expenditures.
Interest income
Interest income was $0.1 million for the year ended December 31, 2015, compared to $19,000 for the year ended December 31, 2014. The increase of $0.1 million in interest income is primarily due to an increase in short-term investments throughout 2015 at higher rates of return.
Financial expenses
Financial expenses were $56.1 million for the year ended December 31, 2015, compared to $2.3 million for the year ended December 31, 2014. The $53.8 million increase in financial expenses is primarily due to increased losses on the re-measurement at fair value of the embedded derivative as a result of a higher step-up in value of the conversion option in 2015 than in 2014.
65
Liquidity and Capital Resources
Sources of liquidity
We have funded our operations principally from the issuance of common shares, preferred stock and convertible notes to purchase common shares. In addition, we have recorded investment tax credits of $0.6 million since inception. As of March 31, 2017, we had cash and short-term investment of $43.7 million. Cash in excess of immediate working capital requirements is invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Currently, our short-term investments are held in guaranteed investment certificates with a Canadian chartered bank.
The Company is not subject to any externally imposed restrictions, covenants or capital requirements and has no arranged sources of financing.
Cash flows
Comparison of the three-month periods ended March 31, 2017 and 2016
The following table sets forth the primary sources and uses of cash for each of the periods set forth below:
|
|
|
|
|
|
| |||||||||||||||
| Three-months ended | Increase | |||||||||||||||||||
2017 | 2016 | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Net cash provided by (used in): |
|
|
|
|
|
| |||||||||||||||
Operating activities |
|
| $ |
| (5,938 | ) |
|
|
| $ |
| (5,645 | ) |
|
|
| $ |
| (293 | ) |
|
Investing activities |
|
| 10,324 |
|
| (40,006 | ) |
|
|
| 50,330 | ||||||||||
Financing activities |
|
| 9,896 |
|
| — |
|
| 9,896 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
Net (decrease) increase in cash |
|
| $ |
| 14,282 |
|
| $ |
| (45,651 | ) |
|
|
| $ |
| 59,933 | ||||
|
|
|
|
|
|
| |||||||||||||||
Operating activities
The increase in cash used in operating activities for the three-months ended March 31, 2017, compared to the three-months ended March 31, 2016 is primarily due to an increase in general and administrative expenses related to the initial public offering. The use of cash in all periods resulted primarily from our net losses adjusted for non-cash charges and changes in working capital.
During the three-months ended March 31, 2017, operating activities used $5.9 million in cash, primarily resulting from our research and development and general and administrative expenses, as well as cash used for changes in working capital. The significant items accounting for the change in working capital include a decrease in accounts payable and accrued liabilities, an increase in deferred financing costs and a decrease in other current assets.
During the three-months ended March 31, 2016, operating activities used $5.6 million in cash, primarily resulting from research and development and general and administrative expenses, as well as cash used for changes in working capital. The significant items accounting for the change in working capital include decreases in accounts payable and accrued liabilities as well as prepaid expenses.
Investing activities
Net cash used in investing activities primarily consists of acquisition and maturity of short-term investments, in-licensing of intellectual property and purchases of fixed assets.
During the three-months ended March 31, 2017, investing activities provided $10.3 million in cash primarily resulting from the maturity of guaranteed investment certificates.
During the three-months ended March 31, 2016, investing activities used $40.0 million in cash primarily for investments in guaranteed investment certificates with a Canadian chartered bank.
66
Financing activities
During the three-months ended March 31, 2017, net cash provided by financing activities was $9.9 million resulting from net proceeds received from the issuance of 70,176 shares of our Class C redeemable convertible preferred stock.
During the three-months ended March 31, 2016, there were no financing activities.
Comparison of the years ended December 31, 2016 and 2015
The following table sets forth the primary sources and uses of cash for each of the periods set forth below:
|
|
|
|
|
|
| |||||||||||||||
| Years ended | Increase | |||||||||||||||||||
2016 | 2015 | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Net cash provided by (used in): |
|
|
|
|
|
| |||||||||||||||
Operating activities |
|
| $ |
| (18,828 | ) |
|
|
| $ |
| (17,623 | ) |
|
|
| $ |
| (1,205 | ) |
|
Investing activities |
|
| (29,932 | ) |
|
|
| (60 | ) |
|
|
| (29,872 | ) |
| ||||||
Financing activities |
|
| — |
|
| 70,671 |
|
| (70,671 | ) |
| ||||||||||
|
|
|
|
|
|
| |||||||||||||||
Net (decrease) increase in cash |
|
| $ |
| (48,760 | ) |
|
|
| $ |
| 52,988 |
|
| $ |
| (101,748 | ) |
| ||
|
|
|
|
|
|
| |||||||||||||||
Operating activities
The increase in cash used in operating activities for the year ended December 31, 2016, compared to December 31, 2015 is primarily due to a $2.5 million increase in research and development expenses as we continue the development of FOP, which includes an increase in personnel related costs as well as in pre-clinical and clinical study costs. In addition, general and administrative expenses decreased by $2.1 million due to a reduction in pre-commercial marketing related activities. The use of cash in all periods resulted primarily from our net losses adjusted for non-cash charges and changes in components of working capital.
During the year ended December 31, 2016, operating activities used $18.8 million in cash, primarily resulting from our research and development and general and administrative expenses, partially offset by cash provided from changes in working capital. The significant items accounting for the change in working capital include an increase in accounts payable and accrued liabilities, a decrease in prepaid expenses and other current assets.
During the year ended December 31, 2015, operating activities used $17.6 million in cash, primarily resulting from research and development and general and administrative expenses, partially offset by cash provided from changes in working capital. The significant item accounting for the change in working capital is the increase in accounts payable and accrued liabilities.
Investing activities
Net cash used in investing activities primarily consists of acquisition and maturity of short-term investments, in-licensing of intellectual property and purchases of fixed assets.
During the year ended December 31, 2016, investing activities used $29.9 million in cash primarily for investments in guaranteed investment certificates with a Canadian chartered bank.
During the year ended December 31, 2015, investing activities used $0.1 million in cash primarily for in-licensing of intellectual property.
Financing activities
During the year ended December 31, 2016, we had no net cash provided by financing activities.
67
During the year ended December 31, 2015, net cash provided by financing activities was $70.7 million resulting from net proceeds received from the issuance of 450,798 and 485,823 shares of our Class A and Class B redeemable convertible preferred stock, respectively.
Comparison of the years ended December 31, 2015 and 2014
The following table sets forth the primary sources and uses of cash for each of the periods set forth below:
|
|
|
|
|
|
| |||||||||||||||
| Years ended | Increase | |||||||||||||||||||
2015 | 2014 | ||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Net cash provided by (used in): |
|
|
|
|
|
| |||||||||||||||
Operating activities |
|
| $ |
| (17,623 | ) |
|
|
| $ |
| (9,658 | ) |
|
|
| $ |
| (7,965 | ) |
|
Investing activities |
|
| (60 | ) |
|
|
| (120 | ) |
|
|
| 60 | ||||||||
Financing activities |
|
| 70,671 |
|
| 11,987 |
|
| 58,684 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
Net (decrease) increase in cash |
|
| $ |
| 52,988 |
|
| $ |
| 2,209 |
|
| $ |
| 50,779 | ||||||
|
|
|
|
|
|
| |||||||||||||||
Operating activities
The increase in cash used in operating activities for the year ended December 31, 2015, compared to December 31, 2014 is primarily due to a $6.6 million increase in research and development expenses for the continued development of FOP, which includes an increase in personnel related costs as well as in pre-clinical and clinical study costs. In addition, general and administrative expenses increased by $3.2 million due to increases in pre-commercial marketing related activities, personnel related costs, professional fees and other corporate supporting expenditures. The use of cash in all periods resulted primarily from our net losses adjusted for non-cash charges and changes in working capital.
During the year ended December 31, 2015, operating activities used $17.6 million in cash, primarily resulting from our research and development and general and administrative operating expenses, partially offset by cash provided from changes in working capital. The significant item accounting for the change in working capital was an increase in accounts payable and accrued liabilities.
During the year ended December 31, 2014, operating activities used $9.7 million in cash, primarily resulting from research and development and general and administrative operating expenses.
Investing activities
Net cash used in investing activities primarily consisted of acquisitions and maturities of short-term investments, in-licensing of intellectual property and purchases of fixed assets.
During the year ended December 31, 2015, investing activities used $0.1 million in cash primarily for in-licensing of intellectual property.
During the year ended December 31, 2014, investing activities used $0.1 million in cash also primarily for in-licensing of intellectual property.
Financing activities
During the year ended December 31, 2015, net cash provided by financing activities was $70.7 million resulting from net proceeds received from the issuance of 450,798 and 485,823 shares of our Class A and Class B redeemable convertible preferred stock, respectively.
During the year ended December 31, 2014, net cash provided by financing activities was $12.0 million resulting from net proceeds received from the issuance of 410,326 shares of our Class A redeemable convertible preferred stock.
Funding Requirements and Planned Operations
To date, we have not generated any revenue from product sales and we do not know when, or if, we will generate any revenue from product sales in the future. We do not expect to generate significant
68
revenue from product sales unless and until we obtain regulatory approval to commercialize our current product candidate or future product candidates. We anticipate that we will continue to generate losses for the foreseeable future, and we expect these losses to increase as we continue the development of, and seek regulatory approvals for, our product candidates, and begin to commercialize any approved products. We are subject to all of the risks incident in the development of new therapeutic products, and we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. Upon the closing of this offering, we expect to incur additional costs associated with operating as a public company. We anticipate that we will need additional funding in connection with our continuing operations.
We believe that our existing cash and short-term investments as of March 31, 2017 will be sufficient to fund our anticipated operating expenses for at least the next twelve months from March 31, 2017. However, we will eventually require additional capital for the further development of our existing product candidate and may also need to raise additional funds sooner to pursue other development activities related to additional product candidates.
Until we can generate a sufficient amount of revenue from our products, if ever, we expect to finance future cash needs through public or private equity or debt offerings. Additional capital may not be available on reasonable terms, if at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our product candidates. If we raise additional funds through the issuance of additional debt or equity securities, it could result in dilution to our existing stockholders, increased fixed payment obligations and these securities may have rights senior to those of our common shares. If we incur indebtedness, we could become subject to covenants that would restrict our operations and potentially impair our competitiveness, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. Any of these events could significantly harm our business, financial condition and prospects.
Contractual Obligations and Commitments
The following table summarizes our contractual obligations at March 31, 2017.
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
| Less than | 1 to 3 | 3 to 5 | More than | Total | ||||||||||||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||||||||||||
Operating lease obligations |
|
| 559 |
|
| 777 |
|
| 39 |
|
| — |
|
| $ |
| 1,375 | ||||||||||||||||||
On July 2, 2015, we entered into a non-cancelable operating lease that expires on June 30, 2020 for office space at 4150 Sainte-Catherine Street West, Suite 550 in Montreal, Quebec, Canada. We also lease office space at 275 Grove Street, Suite 2-400 in Newton, Massachusetts under a non-cancelable operating lease that expires on April 30, 2019.
We also have obligations to make future payments to third parties that become due and payable on the achievement of certain development, regulatory and commercial milestones (such as initiation of a clinical trial, filing of an NDA, approval by the FDA or product launch). We have not included these commitments on our statement of financial position or in the table above because the achievement and timing of these milestones is not fixed or determinable. These commitments include:
• | In accordance with an exclusive licensing agreement with Hoffman-La Roche, we are committed to pay Roche (i) a total of $1,000,000 in milestone payments upon the achievement of certain clinical milestones, (ii) up to a total of $11,000,000 in milestone payments upon the achievement of certain regulatory milestones in connection with the three clinical trial programs currently underway with an additional $1 million in milestone payments upon the achievement of certain regulatory milestones in connection with each subsequent indication, if any, and (iii) up to a total of $37,500,000 in milestone payments upon the achievement of certain sales milestones. Future royalty payments in the low teens based on net sales are also stipulated in the licensing agreement. The likelihood and timing of these payments is not known at this time. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
69
• | In accordance with an exclusive licensing agreement with Thomas Jefferson University, we are committed to make a total of $100,000 in milestone payments upon the achievement of certain clinical milestones and a total of $250,000 in milestone payments upon the achievement of certain regulatory milestones, in each case in connection with the first licensed product or licensed process that meets the relevant milestones. Future low single digit royalty payments based on net sales are also stipulated in the licensing agreement. Annual license maintenance royalty payments are also required as per the terms of the licensing agreement. Such maintenance royalty payments are non-refundable, but can be applied to royalties owing on sales per calendar year. The likelihood and timing of these payments is not known at this time. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | In accordance with an exclusive licensing agreement with Yamaguchi University, we are committed to make a total of $75,000 in milestone payments upon the achievement of certain clinical milestones and a total of $150,000 in milestone payments upon the achievement of certain regulatory milestones, in each case in connection with the first licensed product that meets the relevant milestones. Future low single digit royalty payments based on net sales are also stipulated in the licensing agreement. We also have a royalty buy-out option pursuant to which we can terminate at any time in our sole discretion upon payment of a certain amount in exchange for our obligation to pay royalties to Yamaguchi University under the license agreement. The likelihood and timing of these payments is not known at this time. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | In March 2017, we entered into an exclusive licensing agreement with Galderma to obtain access to RARg agonists and were granted exclusive rights to use these RARg agonists in nondermatological indications. In accordance with this agreement with Galderma, we are committed to pay Galderma a total of $2,000,000 in milestone payments upon the achievement of certain clinical milestones and up to a total of $25,500,000 in milestone payments upon the achievement of certain regulatory milestones, in each case in connection with the first product that meets the relevant milestones. Future single digit royalty payments based on net sales are also stipulated in the licensing agreement. The likelihood and timing of these payments is unknown at this time. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
We enter into contracts in the normal course of business with CROs for pre-clinical research and clinical studies, research supplies and other services and products for operating purposes. These contracts generally provide for termination on notice, and therefore are cancelable contracts and not included in the table of contractual obligations and commitments.
Our preferred shares are also not considered a contractual obligation and commitment as the preferred shares will automatically convert to common shares upon the completion of this offering.
Quantitative and Qualitative Disclosures about Market Risks
We are exposed to certain financial risks, including the financial risk related to fluctuations of foreign exchange rates. We incur a portion of our expenditures in Canadian dollars and in Euros. A change in the currency exchange rates between the U.S. dollar relative to the Canadian dollar or the Euro could have a significant effect on our results of operations, financial position or cash flows. We do not have in place any tools to manage our foreign exchange risk, other than keeping expected foreign currency cash requirements in the foreign currency to form a natural hedge. We are exposed to currency risk through our cash, sales tax and other receivables and accounts payable and accrued liabilities denominated in Canadian dollars and Euros. Based on our net exposures as at March 31, 2017, and assuming all other variables remain constant, a 10% depreciation or appreciation of the U.S. dollar against the Canadian dollar and the Euro would result in an increase/decrease of less than $0.1 million on the Company’s results of operations.
Critical accounting judgments and key sources of estimation uncertainty
The management’s discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with IFRS as issued by the IASB. The preparation of these financial statements in conformity with IFRS requires us to make
70
judgments, estimates and assumptions that affect the application of accounting policies and the reported amounts of revenues, expenses, assets and liabilities. Actual results could differ from those estimates.
On an ongoing basis, we evaluate our estimates and judgments. We base our estimates on the most probable set of economic conditions and planned course of action, historical experience, known trends and events, and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in the notes to our financial statements appearing elsewhere in this prospectus, we believe the following accounting policies to be most critical to the judgments and estimates used in the preparation of our financial statements.
Estimation of accrued expenses
As part of the process of preparing our financial statements, we are required to estimate our accrued expenses. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. The majority of our service providers invoice us in arrears for services performed, on a pre-determined schedule or when contractual milestones are met; however, some require advanced payments. We make estimates of our accrued expenses as of each statement of financial position date in our financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and make adjustments if necessary. Examples of estimated accrued research and development expenses include fees paid to:
• | CROs in connection with performing research and development services on our behalf; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | investigative sites or other providers in connection with clinical trials; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | vendors in connection with non-clinical development activities; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | vendors related to product manufacturing, development and distribution of clinical supplies; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | various external consultants. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
We base our expenses related to clinical trials on our estimates of the services received and efforts expended pursuant to contracts with multiple CROs that conduct and manage non-clinical studies and clinical trials on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the clinical expense. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing service fees, we estimate the time period over which services will be performed, enrollment of patients, number of sites activated and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual or prepaid accordingly. Although we do not expect our estimates to be materially different from amounts actually incurred, if our estimates of the status and timing of services performed differs from the actual status and timing of services performed we may report amounts that are too high or too low in any particular period. To date, we have not made any material adjustment to our prior estimates of accrued research and development expenses.
Valuation of embedded derivative conversion options
As part of assessing whether an instrument is a hybrid financial instrument and contains an embedded derivative, significant judgment is required in evaluating whether the host contract is more akin to debt or equity and whether the embedded derivative is clearly and closely related to the underlying host contract. We concluded that the host instrument of the preferred shares was a debt host due in part to the holder’s right to redeem the instrument at a point in time in the future based only
71
based on passage of time. We determined that the conversion option was not closely related to the debt host, and that the conversion option was required to be separated from the host instrument and accounted for as an embedded derivative due to its down-round protection feature. In applying our judgment, we rely primarily on the economic characteristics and risks of the instrument as well as the substance of the contractual arrangement.
The initial fair values of the embedded derivative conversion options and subsequent re-measurement at fair value at each reporting date up to and including December 31, 2016 were determined by using the Monte Carlo simulation model. The Monte Carlo simulation model better reflects non-static inputs, such as the anti-dilution (down-round protection) features of the preferred shares. A Monte Carlo simulation model is a valuation model that relies on random sampling and is often used when modeling systems with a large number of inputs and where there is a significant uncertainty in the future value of inputs and where the movement in inputs can be independent of each other.
Moreover, the use of this valuation model requires highly subjective assumptions. These assumptions are determined as of the measurement date and include the risk-free interest rate, the expected dividend yield, the expected volatility, the timing and amounts of subsequent rounds of financing, the expected timing and probability of exit events, and the underlying value of the company. Assumptions with regards to volatility, subsequent rounds of financing, time to exit and underlying value of the company are particularly important and sensitive, requiring significant judgment by management. Accordingly, any changes in the assumptions used in this model could significantly impact the values recognized as embedded derivative conversion options at inception and on subsequent re-measurement at each reporting date.
The risk-free rate is the rate of return on the U.S. Department of Treasury daily treasury yield curve rates over a period equal to the expected timing of an exit event. The expected dividend yield is nil as we do not expect to pay dividends in the near future. The expected volatility reflects the assumption that the volatility used in estimating the value of the embedded derivative in indicative of future trends, which may not necessarily be the actual outcome. Due to the last of a public market for the trading of our shares and a lack of company specific historical and implied volatility data, we have based our estimate of expected volatility on the historical volatility of a group of similar companies that are publicly traded. For these analyses, we selected companies with comparable characteristics to us, including risk profiles, orphan drugs within their portfolios, positions within the industry and with historical share price information sufficient to meet the expected timing of an exit event.
The expected timing and amounts of subsequent rounds of financing reflect our best estimate of subsequent rounds of financing based on contracted commitments for subsequent rounds of financing, our financial condition, including cash on hand, and our historical and forecasted performance and cash burn.
The expected timing of exit events are based on our best estimate of possible exit events and their likelihood, considering the progress of our research and development programs, including the status of non-clinical studies and clinical trials of our product candidates, our stage of development and our commercialization and business strategy, our financial condition, including cash on hand, our historical and forecasted performance and operating results, the likelihood of achieving a liquidity event, such as the sale of the company or an initial public offering given prevailing market conditions, external market conditions affecting the biopharmaceutical industry and trends within the biopharmaceutical industry.
In the absence of a public trading market, the underlying value of our Company was performed using methodologies, approaches and assumptions consistent with theAmerican Institute of Certified Public Accounts Audit and Accounting Practice Aid Series, with the assistance of a third-party specialist, and is subject to estimated based on the valuation techniques selected and an evaluation of the inputs used in creating the valuation. Valuation techniques used include the probability-weighted expected return method and the option-pricing method or a hybrid of both methods. In addition, various objective and subjective factors were also considered, including the prices at which we sold preferred shares and the superior rights and preferences of the preferred shares relative to our common shares, the progress of our research and development programs, our stage of development and our commercialization and business strategy, our financial condition, including cash on hand, our historical
72
and forecasted performance and operating results, the lack of a public market for our common shares and preferred shares, the likelihood of achieving a liquidity event, such as a sale of the company or an initial public offering given prevailing market conditions, the analysis of initial public offerings and the market performance of similar companies in the biopharmaceutical industry, external market conditions affecting the biopharmaceutical industry and trends within the biopharmaceutical industry. There are significant judgments and estimates inherent in these valuations. These judgments and estimates include assumptions regarding our future operating performance, the timing and likelihood of potential liquidity events and the determination of the appropriate valuation methodology at each valuation date. If different assumptions had been made, the fair value of our embedded derivatives and the resulting preferred share liability and accretion expense, as well as the re-measurement of the embedded derivatives could have been materially different.
The fair value of the embedded derivative conversion options at March 31, 2017, and at inception for the Class C convertible redeemable preferred shares, were estimated using a hybrid of the probability-weighted expected return method (PWERM), weighted at 75%, and a Monte Carlo simulation model, weighted at 25%. We integrated a PWERM model into our valuation methodology as, during the first quarter of 2017, we had undertaken tangible steps toward a qualifying IPO and we believe this model to be a more accurate estimation method of the conversion option as a result of the IPO commitments taken.
Under the PWERM methodology, the fair value was estimated based upon the future implied equity values using a range of low, medium and high exit multiples. Exit multiples were derived from comparable public company transactions that compared the invested capital (being the aggregate of debt and shares) to the pre-IPO equity values. The estimated implied equity value was discounted back from the estimated time to exit to the valuation date.
Share-based Payments
We issue share-based payments to employees and non-employee directors, generally in the form of stock options. We account for our share-based payment in accordance with IFRS 2,Share-Based Payments.IFRS 2 requires all equity-settled share-based payments to employees and others providing similar services to be measured at the fair value of the equity instruments at the grant date. The fair value determined at the grant date of the equity-settled share-based payments is expensed over the vesting period, based on our estimate of equity instruments that will eventually vest, or net of estimated forfeitures. On an ongoing basis, we revise our estimate of the number of instruments expected to vest. The impact of revisions, if any, is recognized under share-based compensation such that the cumulative expense reflects the revised estimate. We recognize the compensation cost of share-based payments to employees and directors using a graded vesting method where each installment that vests is treated as its own award and each installment is measured and recognized separately. Described below is the methodology we have utilized in measuring share-based compensation expense. Following the consummation of this offering, stock option values will be determined based on the quoted market price of our common shares.
We estimate the fair value of our stock-based awards to employees and directors using the Black-Scholes option pricing model that was developed to estimate the fair value of freely tradable, fully transferable stock options without vesting restrictions. The terms of the stock options that we have awarded differ significantly from actual options for which the Black-Scholes model was designed to evaluate. The Black-Scholes model requires the input of highly subjective assumptions, including (a) the expected volatility of our stock, (b) the expected term of the award, (c) the risk-free interest rate, and (d) expected dividends. The expected volatility reflects the assumption that the volatility used in estimating the fair value of the share-based compensation is indicative of future trends, which may not necessarily be the actual outcome. Due to the lack of a public market for the trading of our common shares and a lack of company specific historical and implied volatility data, we have based our estimate of expected volatility on the historical volatility of a group of similar companies that are publicly traded. For these analyses, we have selected companies with comparable characteristics to ours including risk profiles, pre-commercial initial public offerings, orphan drugs within their portfolios, position within the industry, and with historical share price information sufficient to meet the expected life of the stock-
73
based awards. We will continue to apply this process until a sufficient amount of historical information regarding the volatility of our own stock price becomes available and weigh the result with our own limited stock price history that is available. The expected life of the options reflects the assumption that the expected life of the options used in estimating the fair value of share-based compensation is indicative of future exercise patterns that may occur which many not necessarily be the actual outcome. Due to our limited operating history, we have estimated the expected life of our employee stock options using the “simplified” method, whereby, the expected life equals the average of the vesting term and the original contractual term of the option. The risk-free interest rates for periods within the expected life of the option are based on the U.S. Department of Treasury daily treasury yield curve rates in effect at grant date for time periods approximately equal to the expected life of the option. The expected dividend yield has been estimated at nil as we have never paid cash dividends and we do not expect to pay any cash dividends in the foreseeable future.
The assumptions we used to determine the fair value of stock options granted to employees and directors are as follows, presented on a weighted average basis.
|
|
|
|
|
|
|
| |||||||||||||||||||||
| Three-months | Years ended | ||||||||||||||||||||||||||
2017 | 2016 | 2015 | 2014 | |||||||||||||||||||||||||
Share price |
|
| $ |
| 116.29 |
|
| n/a |
|
| $ |
| 11.08 |
|
| $ |
| 3.50 | ||||||||||
Expected volatility |
|
| 81.56 | % |
|
|
| n/a |
|
| 74.8 | % |
|
|
| 82.1 | % |
| ||||||||||
Expected term (in years) |
|
| 6.0 |
|
| n/a |
|
| 6.0 |
|
| 6.0 | ||||||||||||||||
Risk-free interest rate |
|
| 2.04 | % |
|
|
| n/a |
|
| 1.6 | % |
|
|
| 1.9 | % |
| ||||||||||
Expected dividend yield |
|
| 0.0 | % |
|
|
| n/a |
|
| 0.0 | % |
|
|
| 0.0 | % |
| ||||||||||
These assumptions represented our best estimates, but the estimates involved inherent uncertainties and the application of our judgment. We recognize compensation expense for only the portion of awards that are expected to vest. In developing a forfeiture rate estimate for pre-vesting forfeitures, we have considered our historical experience of actual forfeitures and expected forfeiture behaviors.
Award grants
The following table presents the grant dates, number of underlying shares, along with the corresponding exercise price for each option grant and the common shares fair value per share on the date of grant:
|
|
|
|
|
|
| |||||||||||||||
Date of grant | Number | Exercise | Common | ||||||||||||||||||
2/20/2014 |
|
| 26,801 |
|
| $ |
| 3.50 |
|
| $ |
| 3.50 | ||||||||
5/28/2014 |
|
| 1,334 |
|
| $ |
| 3.50 |
|
| $ |
| 3.50 | ||||||||
8/27/2014 |
|
| 94,626 |
|
| $ |
| 3.50 |
|
| $ |
| 3.50 | ||||||||
11/14/2014 |
|
| 3,160 |
|
| $ |
| 3.50 |
|
| $ |
| 3.50 | ||||||||
4/22/2015 |
|
| 23,418 |
|
| $ |
| 8.33 |
|
| $ |
| 4.72 | ||||||||
12/14/2015 |
|
| 4,502 |
|
| $ |
| 57.68 |
|
| $ |
| 44.15 | ||||||||
02/28/2017 |
|
| 14,550 |
|
| $ |
| 116.29 |
|
| $ |
| 116.29 | ||||||||
04/30/2017 |
|
| 39,100 |
|
| $ |
| 120.36 |
|
| $ |
| 120.36 | ||||||||
During the three-month period ended March 31, 2017, we recognized stock-based compensation expense of $83,741, of which $58,486 was recorded as research and development expense and $25,255 was recorded as general and administrative expense in our statement of operations. During the three-month period ended March 31, 2016, we recognized stock-based compensation expense of $52,739, of which $31,326 was recorded as research and development expense and $21,413 was recorded as general and administrative expense in our statement of operations.
During the year ended December 31, 2016, we recognized stock-based compensation expense of $174,419, of which $109,421 was recorded as research and development expense and $64,998 was recorded as general and administrative expense in our statement of operations. During the year ended December 31, 2015, we recognized stock-based compensation expense of $164,456, of which $63,941 was
74
recorded as research and development expense and $100,515 was recorded as general and administrative expense in our statement of operations. During the year ended December 31, 2014, we recognized stock-based compensation of $142,811 of which $54,282 was recorded as research and development expense and $88,529 was recorded as general and administrative expense in our statement of operations.
As of March 31, 2017, we had $1.2 million of total unrecognized compensation expense, net of related forfeiture estimates, which is expected to be recognized over a weighted-average remaining vesting period of approximately 1.5 years. While compensation expense recognized to date has not been significant, we expect the impact of our stock-based compensation expense for stock option grants to increase in future periods from potential increases in the value of our common shares and anticipated increases in headcount.
Determination of fair value at grant date
We have historically granted stock options at exercise prices not less than the fair value of our common shares. We are a privately held company with no active public market for our common shares. Therefore, our board of directors has estimated the fair value of our common shares at various dates, with input from management, considering our most recently available third-party valuations of common shares and its assessment of additional objective and subjective factors that it believed were relevant and which may have changed from the date of the most recent valuation through the date of the grant. Once a public trading market for our common shares has been established in connection with the completion of this offering, it will no longer be necessary for our board of directors to estimate the fair value of our common shares in connection with our accounting for granted stock options as the fair value of our common shares will be its trading price on The Nasdaq Global Market.
In the absence of a public trading market for our common shares, our determination of the fair value of our common shares was performed using methodologies, approaches and assumptions consistent with theAmerican Institute of Certified Public Accounts Audit in their Audit and Accounting Practice Aid Series: Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or the Practice Aid. We performed these contemporaneous valuations, at times with the assistance of a third-party specialist as of May 31, 2013, February 28, 2015, June 30, 2015, February 28, 2017 and April 30, 2017 which resulted in valuations of our common shares of $3.50, $4.72, $44.15, $116.29 and $120.36 per share, respectively, as of those dates. In addition, our board of directors considered various objective and subjective factors, along with input from management, to determine its best estimate of the fair value of our common shares as of each grant date, including the following:
• | the prices at which we sold shares of preferred stock and the superior rights and preferences of the preferred stock relative to our common shares; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the progress of our research and development programs, including the status of non-clinical studies and clinical trials for our product candidates; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our stage of development and our commercialization and business strategy; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our financial condition, including cash on hand; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our historical and forecasted performance and operating results; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the composition of, and changes to, our management team and board of directors; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the lack of an active public market for our common shares and our preferred stock; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the likelihood of achieving a liquidity event, such as an initial public offering, or IPO, given prevailing market conditions; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the analysis of IPOs and the market performance of similar companies in the biopharmaceutical industry; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | external market conditions affecting the biopharmaceutical industry; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | trends within the biopharmaceutical industry. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
There are significant judgments and estimates inherent in these valuations. These judgments and estimates include assumptions regarding our future operating performance, the stage of development of
75
our product candidates, the timing and likelihood of a potential IPO or other liquidity event, and the determination of the appropriate valuation methodology at each valuation date. If reasonable alternative assumptions had been used, our stock-based compensation expense, net loss attributable to common shareholders, and net loss per share attributable to common shareholders would not have been materially different.
Valuation methodologies
Our common share valuations were prepared using a hybrid of the probability-weighted expected return method, or PWERM, and the option-pricing method, or OPM.
OPM
The OPM treats common shares and convertible preferred stock as call options on the total equity value of a company, with exercise prices based on the value thresholds at which the allocation among various holders of a company’s securities changes. Under this method, the common shares has a value only if the funds available for distribution to stockholders exceed the value of the liquidation preferences at the time of a liquidity event, such as a strategic sale or merger. The common shares are modeled as a call option on the underlying equity value at a predetermined exercise price. In this mode, the exercise price is based on a comparison with the total equity value rather than, as in the case of a regular call option, a comparison with a per share stock price. Thus, common shares are considered to be a call option with a claim on the enterprise at an exercise price equal to the remaining value immediately after the convertible stock liquidation preference is paid.
The OPM uses the Black-Scholes option-pricing model to price the call options. This model defines the securities’ fair values as functions of the current fair value of a company and uses assumptions, such as the anticipated timing of a potential liquidity event and the estimated volatility of the equity securities. The aggregate value of the common shares derived from the OPM is then divided by the number of shares of common shares outstanding to arrive at the per share value.
We used the OPM back-solve approach to estimate enterprise value under the OPM. The OPM back-solve approach uses the OPM to derive an implied equity value for one type of a company’s equity securities from a contemporaneous sale transaction involving another type of the company’s equity securities. For the OPM, we based our assumed volatility factor on the historical trading volatility of our publicly traded peer companies. At each valuation date, we determined the appropriate volatility to be used, considering such factors as our expected time to a liquidity event and our stage of development.
To derive the fair value of our common shares using the OPM, we calculated the proceeds to our common shareholders based on the preferences and priorities of our convertible preferred stock and common shares. We then applied a discount for lack of marketability to the common shares to account for the lack of access to an active public market.
PWERM
Under the PWERM methodology, the fair value of a company’s common shares is estimated based upon an analysis of future values for the company, assuming various outcomes. The common share value is based on the probability-weighted present value of expected future investment returns considering each of the possible outcomes available as well as the rights of each class of stock. The future value of the common shares under each outcome is discounted back to the valuation date at an appropriate risk-adjusted discount rate and probability weighted to arrive at an indication of value for the common shares.
Hybrid Method
The hybrid method is a PWERM where the equity value in one of the scenarios is calculated using an OPM. In the hybrid method used by us, we considered two to four types of future-event scenarios: an IPO, a merger or acquisition transaction, an unspecified liquidity event and a liquidation scenario.
76
The enterprise value for the IPO scenario was determined using the guideline public company method (GPC) and/or the guideline transactions method (GTC) under the market approach. The enterprise value for the unspecified liquidity event scenario was determined using the OPM back-solve approach. The liquidity scenario assumed the liquidation preference of the convertible preferred stock rendered the common share value to nil. The relative probability of each type of future-event scenario was determined based on an analysis of market conditions at the time, including then-current IPO valuations of similarly situated companies, and our expectations as to the timing and likely prospects of the future-event scenarios.
In our application of the GPC method, we considered publicly traded companies in the biopharmaceutical industry that recently completed IPOs as indicators of our estimated future value in an IPO. We then discounted that future value back to the valuation date at an appropriate risk-adjusted discount rate. We applied a discount for lack of marketability to the common shares to account for the lack of access to an active public market. In our application of the GTC method, we considered comparable companies in the biopharmaceutical industry that recently completed merger and acquisition transactions as indicators of our estimated future value. We then discounted the future value back to the valuation date at an appropriate risk-adjusted discount rate. We applied a discount for lack of marketability to the common shares to account for the lack of access to an active public market.
Our contemporaneous common share valuation as of May 31, 2013 was prepared using the PWERM method.
Our contemporaneous common shares valuations as of February 28, 2015, June 22, 2015, February 28, 2017 and April 30, 2017 were prepared using the hybrid method.
May 31, 2013 valuation
The following table summarizes the significant assumptions used to determine the fair value of our common shares of $3.50 as of May 31, 2013:
|
|
|
|
|
|
| |||||||||||||||
May 31, 2013 valuation | Liquidation | IPO | M&A | ||||||||||||||||||
Key assumptions |
|
|
|
|
|
| |||||||||||||||
Probability weighting |
|
| 60 | % |
|
|
| 20 | % |
|
|
| 20 | % |
| ||||||
Liquidity date |
|
| n/a |
|
| 5/31/2017 |
|
| 5/31/2017 | ||||||||||||
Weighted average cost of capital range |
|
| n/a |
|
| 30%-35 | % |
|
|
| 30%-35 | % |
| ||||||||
Discount for lack of marketability (DLOM) |
|
| n/a |
|
| 51 | % |
|
|
| 51 | % |
| ||||||||
Estimated per share present value range of marketable common shares (before DLOM and probability weighting) |
|
| $ |
| 0.00 |
|
| $ |
| 10.90-$15.97 |
|
| $ |
| 23.82-$27.70 | ||||||
February 28, 2015 valuation
The following table summarizes the significant assumptions used to determine the fair value of our common shares of $4.72 as of February 28, 2015:
|
|
|
|
| ||||||||||
February 28, 2015 valuation | Liquidation | OPM(1) | ||||||||||||
Key assumptions |
|
|
|
| ||||||||||
Probability weighting |
|
| 35 | % |
|
|
| 65 | % |
| ||||
Liquidity date |
|
| n/a |
|
| 2/28/2017 | ||||||||
Annual volatility |
|
| n/a |
|
| 77 | % |
| ||||||
Risk-free interest rate |
|
| n/a |
|
| 0.70 | % |
| ||||||
Discount for lack of marketability (DLOM) |
|
| n/a |
|
| 35 | % |
| ||||||
Estimated per share present value of marketable common shares (before DLOM and probability weighting) |
|
| $ |
| 0.00 |
|
| $11.18 | ||||||
(1) | We used the back-solve method of the OPM to solve for the equity value and corresponding value of common shares based on the $29.245 per share price of the Class A preferred stock extension agreed to in October 2014 and closed in March 2015. Given the proximity to the Class A preferred stock | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
77
| extension, we believe the per share issuance price of the Class A preferred stock extension provides an indication of the fair value of our equity as of February 28, 2015. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The estimated per share fair value of our common shares calculated in our valuation as of February 28, 2015 of $4.72 per share increased from previous valuations of $3.50 per share primarily due to the following factors:
• | our improved financial position resulting from the $10 million Class A extension in October 2014; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | initiation of our Phase 2 study of palovarotene in FOP patients and completion of enrollment of the first cohort of patients; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | initiation of our natural history study in patients with FOP; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | receipt of orphan drug designation from the FDA and the EMA; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | receipt of fast track designation from the FDA. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
June 30, 2015 valuation
The following table summarizes the significant assumptions used in the hybrid method to determine the fair value of our common shares of $44.15 as of June 30, 2015:
|
|
|
|
|
| ||||||||||||||||
June 30, 2015 valuation | Liquidation | IPO | OPM(1) | ||||||||||||||||||
Key assumptions |
|
|
|
|
|
| |||||||||||||||
Probability weighting |
|
| 25 | % |
|
|
| 25 | % |
|
|
| 50 | % |
| ||||||
Liquidity date |
|
| n/a |
|
| 6/30/2016 |
|
| 6/30/2017 | ||||||||||||
Weighted average cost of capital |
|
| n/a |
|
| 25 | % |
|
|
| n/a | ||||||||||
Annual volatility |
|
| n/a |
|
| n/a |
|
| 73 | % |
| ||||||||||
Risk-free interest rate |
|
| n/a |
|
| n/a |
|
| 0.64 | % |
| ||||||||||
Discount for lack of marketability (DLOM) |
|
| n/a |
|
| 21 | % |
|
|
| 28 | % |
| ||||||||
Estimated per share present value of marketable common shares (before DLOM and probability weighting) |
|
| $ |
| — |
|
| $158.93 |
|
| $35.44 | ||||||||||
(1) | We used the back-solve method of the OPM to solve for the equity value and corresponding value of common shares based on the $123.50 per share price of the Class B preferred stock issuance in June 2015. Given the proximity to the Class B preferred stock issuance, we believe the per share issuance price of the Class B preferred stock extension provides an indication of the fair value of our equity as of June 30, 2015. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The estimated per share fair value of our common shares calculated in our valuation as of June 30, 2015 of $44.15 per share increased from the February 28, 2015 valuation of $4.72 per share primarily due to the following factors:
• | enhanced Class B valuation; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | addition of tier 1 investors in our Class B financing; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | enhanced financial position on completion of our $60 million Class B financing; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | increased probability of advancing towards an IPO scenario; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | increased valuation thresholds at IPO pursuant to Class A and Class B financings; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | data monitoring committee recommendation to continue the Phase 2 adaptive-design, dose-ranging study as designed, based on their review of initial efficacy and safety data obtained from the first cohort of patients; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | initiation of enrollment in the second cohort of the Phase 2 trial; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | completion of the juvenile toxicity study allowing for the planned expansion of the Phase 2 trial to include children with FOP. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
78
February 28, 2017 valuation
The following table summarizes the significant assumptions used in the hybrid method to determine the fair value of our common shares of $116.29 as of February 28, 2017:
|
|
|
|
|
| ||||||||||||||||
February 28, 2017 valuation | Liquidation | IPO | OPM(1) | ||||||||||||||||||
Key assumptions |
|
|
|
|
|
| |||||||||||||||
Probability weighting |
|
| 5 | % |
|
|
| 75 | % |
|
|
| 20 | % |
| ||||||
Liquidity date |
|
| NA |
|
| 9/30/2017 |
|
| 2/28/2019 | ||||||||||||
Weighted average cost of capital |
|
| NA |
|
| 25 | % |
|
|
| NA | ||||||||||
Annual volatility |
|
| NA |
|
| NA |
|
| 78 | % |
| ||||||||||
Risk-free interest rate |
|
| NA |
|
| NA |
|
| 1.2 | % |
| ||||||||||
Discount for lack of marketability (DLOM) |
|
| NA |
|
| 11 | % |
|
|
| 27 | % |
| ||||||||
Estimated per share present value of marketable common stock (before DLOM and probability weighting) |
|
| $ |
| — |
|
| $164.77 |
|
| $43.23 | ||||||||||
(1) | We used the back-solve method of the OPM to solve for the equity value and corresponding value of common shares based on the $142.50 per share price of the Class C preferred stock issuance on March 16, 2017. Given the proximity to the Class C preferred stock issuance, we believe the per share issuance price of the Class C preferred stock extension provides an indication of the fair value of our equity as of February 28, 2017. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The estimated per share fair value of our common shares calculated in our valuation as of February 28, 2017 of $116.29 per share increased from the June 30, 2015 valuation of $44.15 per share primarily due to the following factors:
• | enhanced Class C valuation; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | enhanced financial position on completion of our $10 million Class C financing; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | increased probability of advancing towards an IPO scenario; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | positive results in the 40 subject, placebo controlled Phase 2 ’201 clinical trial, though none reached statistical significance; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | preliminary data from the Phase 2 open label extension ’202 clinical trial supporting the results obtained in the Phase 2 ’201 clinical trial. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
April 30, 2017 valuation
The following table summarizes the significant assumptions used in the hybrid method to determine the fair value of our common shares of $120.36 as of April 30, 2017:
|
|
|
|
|
| ||||||||||||||||
April 30, 2017 valuation | Liquidation | IPO | OPM(1) | ||||||||||||||||||
Key assumptions |
|
|
|
|
|
| |||||||||||||||
Probability weighting |
|
| 5 | % |
|
|
| 75 | % |
|
|
| 20 | % |
| ||||||
Liquidity date |
|
| n/a |
|
| 9/30/2017 |
|
| 4/30/2019 | ||||||||||||
Weighted average cost of capital |
|
| n/a |
|
| 25 | % |
|
|
| n/a | ||||||||||
Annual volatility |
|
| n/a |
|
| n/a |
|
| 80 | % |
| ||||||||||
Risk-free interest rate |
|
| n/a |
|
| n/a |
|
| 1.3 | % |
| ||||||||||
Discount for lack of marketability (DLOM) |
|
| n/a |
|
| 11 | % |
|
|
| 27 | % |
| ||||||||
Estimated per share present value of marketable common stock (before DLOM and probability weighting) |
|
| $ |
| — |
|
| $170.61 |
|
| $44.35 | ||||||||||
(1) | We used the back-solve method of the OPM to solve for the equity value and corresponding value of common shares based on the $142.50 per share price of the Class C preferred stock issuance on March 16, 2017. Given the proximity to the Class C preferred stock issuance with no material changes to our business, we believe the per share issuance price of the Class C preferred stock extension provides an indication of the fair value of our equity as of April 30, 2017. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
79
The estimated per share fair value of our common shares calculated in our valuation as of April 30, 2017 of $120.36 per share remained materially unchanged as compared to the February 27, 2017 valuation of $116.29 per share primarily due to no material changes in the Company’s operations and no material changes in the estimated time to exit due to passage of time, as well as updated volatility, risk-free interest rate and DLOM inputs.
Initial public offering price
In consultation with the underwriters for this offering, we determined the estimated price range for this offering, as set forth on the cover page of this prospectus. The midpoint of the price range is $ per share. In comparison, our estimate of the fair value of our common stock was $ per share as of . We note that, as is typical in IPOs, the estimated price range for this offering was not derived using a formal determination of fair value, but was determined by negotiation between us and the underwriters. Among the factors that were considered in setting this range were the following:
• | an analysis of the typical valuation ranges seen in recent IPOs for companies in our industry; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the general condition of the securities markets and the recent market prices of, and the demand for, publicly traded common stock of generally comparable companies; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | an assumption that there would be a receptive public trading market for pre-commercial biotechnology companies such as us; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | an assumption that there would be sufficient demand for our common stock to support an offering of the size contemplated by this prospectus. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The midpoint of the estimated price range for this offering reflects an increase over the estimated valuation as of of $ per share. Investors should be aware of this difference and recognize that the price range for this offering is in excess of our prior valuations. Further, investors are cautioned not to place undue reliance on the valuation methodologies discussed above as an indicator of future stock prices. We believe the difference may be due to the following factors:
• | The initial offering price range necessarily assumes that this offering has occurred, a public market for our common stock has been created and that our preferred stock has converted into common stock in connection with this offering and, therefore, excludes the marketability or illiquidity discounts associated with the timing or likelihood of an initial public offering, the superior rights and preferences of our preferred stock and the alternative scenarios considered in the contemporaneous valuations over the past two years. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | In the public markets we believe there are investors who may apply more qualitative valuation criteria to certain of our clinical assets than the valuation methods applied in our valuations. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | The price that investors are willing to pay in this offering, for which the price range is intended to serve as an estimate, may take into account other things that have not been expressly considered in our prior valuations, are not objectively determinable and that valuation models are not able to quantify. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Investors should be cautioned that the midpoint of the price range set forth on the cover of this prospectus does not necessarily represent the fair value of our common stock, but rather reflects an estimate of the offer price determined in consultation with the underwriters.
There are significant judgments and estimates inherent in the determination of these valuations. These judgments and estimates include assumptions regarding our future performance, including the successful enrollment and completion of our clinical studies as well as the determination of the appropriate valuation methods. If we had made different assumptions, our stock-based compensation expense could have been different. The foregoing valuation methodologies are not the only methodologies available and they will not be used to value our common stock once this offering is complete. We cannot make assurances as to any particular valuation for our common stock. Accordingly, investors are cautioned not to place undue reliance on the foregoing valuation methodologies as an indicator of future stock prices.
80
Off-balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
JOBS Act Transition Period
In April 2012, the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. Thus, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements under the JOBS Act. Subject to certain conditions, as an emerging growth company, we may rely on certain of these exemptions, including without limitation, providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act. We would cease to be an emerging growth company upon the earliest of: (1) the last day of the fiscal year in which we have more than $1.07 billion in annual revenue; (2) the date we qualify as a “large accelerated filer,” with at least $700.0 million of equity securities held by non-affiliates; (3) the issuance, in any three-year period, by our Company of more than $1.0 billion in non-convertible debt securities held by non-affiliates; or (4) the last day of the fiscal year ending after the fifth anniversary of our initial public offering.
Future Accounting Standards
The IASB has issued several new standards and amendments to standards and interpretations that are not yet effective for the year ended December 31, 2017, and although early adoption is permitted, they have not been applied in preparing our consolidated financial statements. We are currently evaluating the effect, if any, the following new standards and amendments will have on our financial results.
(i) | Financial Instruments(IFRS 9), effective for annual periods beginning on or after January 1, 2018, replaces the requirements of International Accounting Standard (IAS) 39,Financial Instruments, Recognition and Measurementfor classification and measurement of financial assets and liabilities. IFRS 9 introduces a single classification and measurement approach for financial instruments, which is driven by cash flow characteristics and the business model in which an assets is held. This single, principle-based approach replaces existing rule-based requirements and results in a single impairment model being applied to all financial instruments. IFRS 9 also modifies the hedge accounting model to incorporate the risk management practices of an entity. Additional disclosures will also be required under the new standard. Early adoption of IFRS 9 is permitted. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(ii) | Leases(IFRS 16), effective for annual periods beginning on or after January 1, 2019, provides a comprehensive model for the identification of lease arrangements and their treatment in the financial statements of both lessees and lessors. It supersedes IAS 17,Leasesand its associated interpretive guidance. Significant changes were made to lessee accounting with the distinction between operating and finance leases removed and assets and liabilities recognized in respect of all leases (subject to limited exceptions for short-term leases and leases of low value assets). Earlier application of IFRS 16 is permitted for companies that have also adopted IFRS 15,Revenues from Contracts with Customers. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Internal Controls and Procedures
A company’s internal control over financial reporting, or ICFR, is a process designed by, or under the supervision of, a company’s principal executive and principal financial officers, or persons
81
performing similar functions, and effected by a company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with IFRS. A material weakness is defined as a deficiency, or a combination of deficiencies, in ICFR, such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis.
The two material weaknesses in ICFR identified as of December 31, 2016 were: (i) the valuation of the embedded derivative and related preferred shares liability accounting, and (ii) lack of segregation of duties due to super-user access and insufficient journal entry review throughout the entire fiscal year. The preferred shares will be converted to common shares at the time of the closing of this initial public offering. Management introduced a new control in the fourth quarter of 2016 related to journal entry review, and in April 2017 management introduced another new control related to super-user access. We expect that these new controls combined will remediate the material weakness related to segregation of duties. Despite our efforts to remediate existing material weaknesses or due to the existence of other material weaknesses, there is a risk that neither we nor our independent registered public accounting firm will be able to conclude within the prescribed timeframe that our ICFR is effective as required by Section 404.
82
Overview
We are a clinical stage biopharmaceutical company that is developing disease-modifying treatments for patients suffering from debilitating bone and other diseases with high unmet medical need. Our lead product candidate, palovarotene, is an oral small molecule that has shown potent activity in preventing abnormal new bone formation as well as fibrosis in a variety of tissues. We are developing palovarotene for the treatment of Fibrodysplasia Ossificans Progressiva (FOP) and Multiple Osteochondroma (MO) and have one registration trial and one Phase 2/3 clinical trial for two separate indications planned to commence in 2017 with data read-outs planned in 2019 and 2020. We believe that if approved in FOP or MO, palovarotene could become the standard of care in either or both of these indications.
FOP is an ultra-rare, chronic and severely disabling disease of abnormal bone formation, known as heterotopic ossification (HO). FOP is characterized by painful, recurrent episodes of soft tissue swelling (flare-ups) that result in bone formation in areas of the body where bone is not normally present, such as muscles, tendons and ligaments. Flare-ups begin early in life and may occur spontaneously or after certain events, including soft tissue trauma, vaccinations or influenza infections. Recurrent flare-ups and new bone formation progressively restrict movement by locking joints, leading to cumulative loss of function, disability and early death often due to reduced respiratory function. FOP is caused by a mutation of the bone morphogenetic protein (BMP) Type I receptor or ACVR1 (also known as ALK2) that leads to excess BMP signaling and new bone formation. Virtually all known patients have the same point mutation and have congenital malformations of the big toes at birth. We estimate that the prevalence of FOP is approximately 1.3 individuals per million lives, or approximately 9,000 globally. As of October 2016, there were known to be 800 diagnosed FOP patients worldwide. There are currently no approved medical treatment options to prevent the formation of heterotopic bone in FOP.
A 2011 Nature Medicine paper showed that palovarotene potently inhibited HO in animal models. Palovarotene had been previously tested by Roche Pharmaceuticals in 825 subjects, including healthy volunteers and patients with chronic obstructive pulmonary disease, where it was well-tolerated. Upon evaluation of the RARg agonist landscape, we determined that palovarotene had the most immediate potential in this class. As a result, we exclusively in-licensed palovarotene from Roche to form the basis of Clementia. In July 2014, the FDA granted Orphan Drug Designation for palovarotene as a treatment for FOP and in November 2014, we received Fast Track Designation. In November 2014, we were granted orphan drug status in the EU. We have also secured IP related to palovarotene and in-licensed additional next generation RARg agonists.
In advance of the commencement of our pivotal palovarotene trial program, we completed the first, randomized, placebo-controlled, adaptive design Phase 2 study in FOP, which enrolled 40 patients. This study showed encouraging safety and efficacy results, as well as unique insights regarding the disease in general and how to better dose patients and measure disease impact. The results of the Phase 2 study along with our open label extension, which reported a total of 67 flare-ups, saw positive trends on certain of our secondary endpoints. Importantly, while our clinical trials have not demonstrated statistically significant results, the data suggest that palovarotene reduced the incidence of new HO by approximately 50% as determined by CT scan at 12 weeks. In those subjects who formed new HO, the mean volumes of new HO were reduced by approximately 70% as compared to the placebo-treated subjects. Palovarotene was well-tolerated in this study and no patient discontinued drug or dose de-escalated.
Our Phase 2 trial and open label extensions as well as additional insights have led us to design our registration trial and an additional clinical trial for palovarotene in FOP. Our Phase 3 trial, MOVE, for the treatment of FOP in adults and children, will enroll up to 100 patients who will be treated chronically with palovarotene and with increased doses during flare-ups. Based on clinical data generated from our Phase 2 study, open label extensions and our natural history study, the FDA has indicated that new HO is a clinically meaningful outcome provided we pre-specify the magnitude of treatment effect and support the HO findings with secondary endpoints, and is sufficient as a primary endpoint for a registration trial supporting approval. We expect to initiate the MOVE trial in 2017 and report data in 2020 with an interim read-out in 2019.
83
We are also planning for a trial which aims to determine if palovarotene can inhibit new HO formation after surgical excision of HO. Patients will be treated prophylactically and after surgery at specific previously locked joints, in an effort to prevent the re-growth of abnormal bone typically observed in FOP patients and to attempt to increase range of motion at such joints. We intend to discuss the details and timing of this trial with FDA in the future.
In parallel with our Phase 2 trials, we have also completed enrollment in a first of its kind natural history study with 114 patients worldwide to characterize the progression of FOP across numerous outcomes. This study is tracking new HO formation across the body using whole body CT scans (WBCTs) as well as measuring range of motion across all joints. Cross-sectional data indicates a strong correlation between losses in physical function with age. Also, the total body volume of HO in individual patients as well as the number of joints with heterotopic ossification shows strong correlations with these functional outcomes. The findings of this study have been instrumental in establishing that HO is a clinically meaningful endpoint in FOP. Further, given that patients in this study have not received palovarotene, they could be eligible to enroll in our planned registration trial for FOP and we anticipate that many of them will choose to do so.
Like FOP, MO, also called multiple hereditary exostoses, is an ultra-rare genetic disease of new bone formation in children, which is mediated by excess BMP signaling. Patients with MO develop multiple benign bone tumors, also known as osteochondromas (OCs) or exostoses, on bones. MO affects approximately 20 individuals per million lives, or approximately 150,000 globally, which is approximately 15 times greater than that of FOP. Patients suffer from substantial morbidities that worsen over time until they reach skeletal maturity. Since it is believed that the mutations which cause MO also result in excess BMP signaling, we believe palovarotene can also inhibit this pathway in MO.
We have generated pre-clinical data demonstrating that palovarotene inhibits the number of OCs by approximately 80% in an animal model of MO as compared to vehicle-treated animals. Based on our knowledge of the safety and tolerability profile of palovarotene and our pre-clinical animal model data, we are planning to initiate a placebo-controlled Phase 2/3 study of palovarotene in MO this year. We expect to report Phase 2/3 clinical data for this trial in 2020 with a potential interim read-out in 2019.
We also believe that RARg agonists have great potential as inhibitors of BMP signaling in other indications. Palovarotene has been shown to exert multiple effects in various tissues including in ocular tissues, where RARg agonists generally demonstrate anti-fibrotic properties. As a result, we have conducted pre-clinical proof-of-concept studies in dry eye disease that show that an eye drop formulation of palovarotene can potently increase tear production and decrease corneal damage. Following the completion of these studies, we plan to initiate IND-enabling toxicity studies of an ophthalmologic formulation in order to begin Phase 1 and 2 clinical trials in dry eye disease in 2018. We are also focused on developing our RARg agonist platform beyond palovarotene for larger, related disease markets, such as in ankylosing spondylitis or trauma-induced HO. As part of this development process, we are currently in the process of characterizing second generation RARg agonists that we recently licensed from Galderma.
Our Team
We have assembled a team of highly skilled and experienced employees, directors and consultants with broad capabilities in drug discovery, development, regulation and commercialization and particular expertise in orphan diseases. Seventy percent of our employees possess advanced scientific degrees. Our management team has substantial industry experience in the orphan disease space and has an average of 24 years of industry experience, with a successful track record of developing and commercializing drug candidates such as Aldurazyme®, Cerezyme®, Fabrazyme®, Myozyme®, Soliris® and Vyndaqel®. Our board members include the former CEOs of companies that developed Synagis®, FluMist®, Gattex®, Natpara® and Strensiq®, the latter two drugs being for the treatment of rare bone diseases. We continue to leverage this specialized expertise and experience to rapidly pursue the development and commercialization of palovarotene in multiple indications. We are backed by a group of leading institutional life science investors, including OrbiMed, New Enterprise Associates, RA Capital, a fund managed by Janus Capital Management LLC, Rock Springs Capital, EcoR1 Capital, UCB Biopharma SPRL, BDC and Fonds de solidarité des travailleurs du Québec.
84
Our Pipeline
We believe that RARg agonists, such as palovarotene, have the potential for therapeutic use in a broad range of conditions, including diseases like FOP and MO that involve pathological bone formation as well as other indications characterized by excessive fibrosis or scarring such as dry eye disease. Our product pipeline consists of:

* | Phase 1 trials for palovarotene in FOP provide basis for proceeding directly to Phase 2/3 trials in MO | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
** | To our knowledge, no animal models for surgical release in FOP currently exist | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Our Strategy
We strive to become a leading fully-integrated biopharmaceutical company that provides disease-modifying treatments to patients suffering from debilitating bone and other diseases with high unmet medical need. We are rapidly developing our lead product candidate, palovarotene, to treat FOP and MO. To achieve our goals, we are executing the following strategy:
Complete development and obtain regulatory approval for our lead product candidate, palovarotene, in FOP and MO. Based on observations we made in our Phase 2 clinical trials, we believe that success in any one of our planned clinical trials can form the basis of FDA approval of palovarotene for the targeted indication. We expect to file for worldwide regulatory approvals of palovarotene in FOP and MO including in the United States, Europe and Japan after generating the relevant clinical data, which we expect to read out in 2019 and 2020.
Following the completion of our Phase 2 double-blind, placebo-controlled clinical trial of palovarotene in FOP, we are planning to commence a Phase 3 clinical trial for palovarotene in FOP in 2017, which will include multiple clinical sites around the world, including the U.S., Europe, Japan and South America. We anticipate enrolling up to 100 patients in this Phase 3 clinical trial. We expect to report clinical data from this trial in 2019.
85
Following the completion of our pre-clinical proof-of-concept studies showing that palovarotene potently suppresses the number of OCs expressed in animal models of MO, and based on the safety and tolerability profile of palovarotene observed to date, we are planning to initiate a placebo-controlled Phase 2/3 study of palovarotene in MO this year. We expect to report clinical data from this trial in 2020 with a potential interim read-out in 2019.
In addition, we are planning a study of palovarotene in subjects with FOP who will undergo surgical excision of HO. The primary objective of this study is to determine whether treatment with palovarotene immediately prior to and following surgical excision of HO at an ankylosed joint in subjects with FOP can improve range of motion. We intend to discuss the details and timing of this trial with FDA in the future.
Independently commercialize palovarotene and improve patient care in FOP and MO. We intend to establish our own commercial organization and have begun to develop a global commercial plan under the leadership of our chief commercial officer. Our plan includes establishing the sales, marketing and reimbursement functions required to commercialize palovarotene in global markets. Advocacy groups, patients, caregivers and thought leaders are extremely active and vocal in the FOP and MO communities. We actively collaborate with these patient groups through a number of initiatives including participation in local meetings and educational initiatives such that we better understand the burdens and unmet needs that patients face, and so that we can better facilitate their access to palovarotene, should it be approved.
Develop palovarotene for other indications including Dry Eye Disease.Palovarotene as a RARg agonist is an inhibitor of BMP signaling and has been shown to exert multiple effects in various tissues including bone, muscle and ocular tissues where it generally demonstrates anti-fibrotic properties. Following the completion of our pre-clinical proof-of-concept studies showing that an eye drop formulation of palovarotene can potently increase tear production and decrease corneal damage, we are initiating IND-enabling toxicity studies of an ophthalmologic formulation in order to begin Phase 1 and 2 clinical trials in dry eye disease in 2018.
Expand our RARg agonists platform.We believe that RARg agonists other than palovarotene have great potential as inhibitors of BMP signaling in other indications and in particular in inhibiting HO in larger disease markets, such as ankylosing spondylitis or trauma-induced HO. As a result, we intend to further develop our RARg agonist platform beyond palovarotene. We are currently in the process of characterizing second generation RARg agonists recently licensed from Galderma. From these compounds, derived from four different structural families, we expect to select leads to be developed internally as well as through out-licensing to external partners.
Evaluate opportunities to expand our leadership in our areas of expertise.We may also selectively form collaborative alliances to expand our capabilities and product offerings into new therapeutic areas and potentially accelerate commercialization in select geographic markets. Additionally, we may pursue acquisition or in-licensing of product candidates, particularly in our core focus area of rare bone diseases.
Our History
A 2011 paper published in Nature Medicine by the team of Dr. Maurizio Pacifici showing that palovarotene, a RARg agonist and drug previously developed by Roche, potently inhibited new bone formation in animal models of FOP, was the basis for the creation of Clementia. Shortly after this publication, we evaluated the RARg agonist landscape and determined that palovarotene had the most immediate potential of the agonists in this class. The scientific breakthrough of Dr. Pacifici and his team represented a key opportunity for several reasons. First, palovarotene had an established safety profile, reflected in the fact that it was well-tolerated in 825 subjects previously tested by Roche, some of whom were followed for up to two years. Also, palovarotene is a small molecule and is taken as a once a day pill, an ideal route of administration. Furthermore, there were no clinical trials being conducted in FOP, a debilitating disease which is life-shortening and for which there is a significant unmet medical need. Consistent with our belief that RARg agonists as a class have great potential, we also secured IP and options to in-license additional second generation RARg agonists, which we have exercised.
86
Our Programs
Our programs currently focus on diseases involving tissue transformation via retinoic acid receptors (RARs). RARs are expressed in a variety of tissues and are involved in the growth, shape and maintenance of tissues (morphogenesis). In particular, the RARg receptor sub-type is expressed in cells that produce cartilage and plays a role in biological pathways responsible for endochondral bone formation (the process of new bone formation which occurs via cartilage formation). RARg is also present in multiple other cells and tissues where it mediates the growth and differentiation of specific cell types, including those involved in fibrosis.
Our lead product candidate, palovarotene, is a RARg selective agonist that has shown potent activity in preventing chondrogenesis (cartilage formation) as well as fibrosis in a variety of tissues. We are currently developing palovarotene for the treatment of several diseases of abnormal new bone formation or fibrosis. We expect to initiate a Phase 3 clinical trial of palovarotene in FOP in 2017, MOVE trial, for the treatment of FOP in adults and children. We are also planning a clinical trial, which similarly aims to inhibit abnormal bone growth caused by FOP in patients after surgical release of locked joints. We are also conducting a natural history study of FOP, which tracks the progression of this disease prospectively over time. We believe that a substantial majority of the participants in the natural history study will enroll in our Phase 3 trial in FOP. We are also developing palovarotene for the treatment of MO and plan to initiate a Phase 2/3 clinical trial for palovarotene in MO in 2017. We believe that success in any one of our planned clinical trials can form the basis of FDA approval of palovarotene for the targeted indication. Finally, we believe that the anti-fibrotic properties of palovarotene may have benefit in the treatment of dry eye disease and we are planning to initiate a Phase 1 trial in this indication in 2018.
In addition to palovarotene, we have pre-clinical and discovery programs evaluating next-generation RARg agonists.
Palovarotene
Palovarotene is an oral, once-daily pill that was initially developed by Roche which we exclusively licensed in January 2013. Palovarotene has been evaluated in a number of Phase 1 clinical trials as well as several Phase 2 clinical trials in COPD and emphysema. These trials enrolled a total of 825 individuals including 450 patients who were on 5 mg palovarotene for up to two years. While palovarotene did not demonstrate efficacy sufficient to warrant further development in these indications, its safety and toxicity profile was consistent with other retinoids and we deemed it acceptable to treat conditions such as FOP and MO.
RARg agonists, such as palovarotene exert their action on bone formation through regulation of the BMP pathway. RARg receptors are expressed in chondrogenic cells and chondrocytes and repress certain genes even in the absence of ligand. BMPs are part of the transforming growth factorb (TGF-b) family of extracellular signaling proteins. They regulate various cellular activities including differentiation and proliferation and are particularly involved in fibrosis. BMP signaling induces the complete pathway of endochondral bone formation during embryonic development and skeletal formation in children.
87
Figure 1. Palovarotene Mechanism of Action: Suppressing Mediators of BMP Signaling
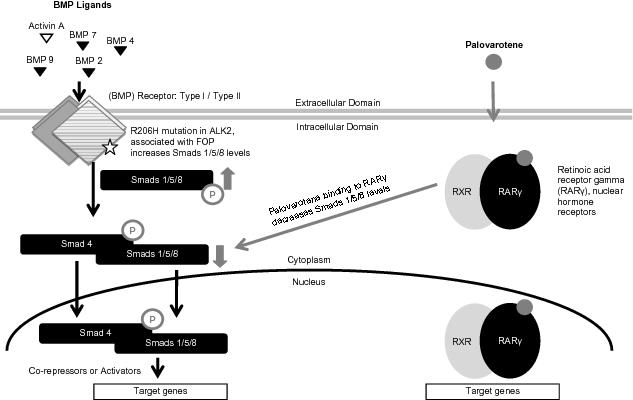
As illustrated in Figure 1, the BMP pathway begins with the binding of any of several extracellular BMP ligands such as BMP 2, 4, 7 and 9 to the BMP receptor which is membrane-bound. The receptor is comprised of two BMP Type II subunits and two BMP Type I subunits (also known as ACVR1 or ALK2), which when activated by ligands, recruit, bind and phosphorylate cytoplasmic signal transduction proteins called Smads 1/5/8. When Smads 1/5/8 become phosphorylated, they are able to bind to Smad 4, which together travel to the nucleus where they regulate the transcription of many genes including all those necessary for new bone formation.
Our lead indication, FOP, is caused by excess BMP signaling and is an example of the inappropriate activation of this pathway. Approximately 97% of patients with FOP have the R206H mutation in the ALK2 receptor which is believed to make the receptor overactive both on its own and in the presence of ligands. The mutated, overactive receptor leads to excess phosphorylation of Smads 1/5/8 and enhanced signals to the nucleus to form heterotopic bone. The result is that the abnormal gene sends signals to the body’s muscles and soft tissues instructing the muscles to repair themselves by forming bone rather than muscle or scar tissue. RARg agonists reduce the level of phosphorylated Smads 1/5/8 and the overall protein levels of Smads 1/5/8 and thereby repress excess BMP signaling.
Our second indication, MO, is also a disease believed to be mediated by excess BMP signaling. In this case, mutations in the Ext1 and Ext2 genes are believed to cause decreases in the heparan sulfate chains on proteoglycans and indirectly cause local increases in BMP at the surface of certain cells. Cell surface heparan sulfate is believed to act as a kind of reservoir for growth factors including BMP and their absence has been shown to result in local increases in BMP signaling and intracellular phosphorylated Smad levels. Since palovarotene has been shown to act directly on this pathway in FOP, we hypothesize that palovarotene could also be a potential treatment in MO.
Palovarotene’s unique mechanism of action confers upon it the ability to repress bone formation and fibrosis and is potentially suited to a variety of indications beyond those currently in development.
In July 2014, the FDA granted Orphan Drug Designation for palovarotene as a treatment for FOP and in November 2014, we received Fast Track Designation. In November 2014, we were granted
88
orphan drug status in the EU. In Japan, we briefed the PMDA on our palovarotene FOP program in January 2017.
Palovarotene for Fibrodysplasia Ossificans Progressiva (FOP)
Background
FOP is an ultra-rare, chronic and severely disabling disease of abnormal bone formation, known as HO. FOP is characterized by painful, recurrent episodes of soft tissue swelling (flare-ups) that result in bone formation in areas of the body where bone is not normally present, such as muscles, tendons and ligaments. Flare-ups begin early in life and may occur spontaneously or after soft tissue trauma, vaccinations or influenza infections. Recurrent flare-ups and new bone formation progressively restrict movement by locking joints, leading to cumulative loss of function, disability and early death often due to reduced respiratory function. FOP is caused by a mutation of ALK2 that leads to excess BMP signaling and new bone formation via cartilage formation.
At birth, subjects with FOP appear healthy but virtually all have a hallmark toe malformation in which both big toes are shortened and bent inwards. We estimate that the prevalence of FOP is approximately 1.3 individuals per million lives, or approximately 9,000 patients globally. As of October 2016, there were known to be 800 diagnosed FOP patients worldwide.
Patients with FOP typically experience episodic flare-ups, large painful swellings, which are usually red and warm to the touch. These flare-ups eventually resolve within weeks to months, often leaving behind new lesions of heterotopic bone. HO proceeds in two phases: a catabolic phase involving inflammation and muscle destruction followed by an anabolic phase leading to the formation of mature heterotopic bone.
The course of HO formation is episodic and cumulative throughout life and results in development of segments, sheets, and ribbons of extra bone throughout the body and across joints, thereby progressively restricting movement (Figure 2).
Figure 2. Heterotopic Ossification in a Patient with FOP
The result is cumulative immobility, with most FOP patients confined to a wheelchair by their mid-20s, if not much sooner, and requiring caregiver assistance to perform daily living activities. Life-threatening complications include severe weight loss due to locked jaws, and thoracic insufficiency syndrome due to constriction of the rib cage or severe deformity of the spine. The thoracic insufficiency
89
syndrome commonly causes complications such as pneumonia and right-sided heart failure, leading to markedly shortened survival (median age at death of 40 years).
Current Therapies – None
Currently there are no approved medical treatment options to prevent the formation of heterotopic bone in FOP. We believe Clementia’s palovarotene program includes the first well-controlled trial for the treatment of this disease. Palliation is administered for symptomatic management of the disease. Removal of heterotopic bone is contraindicated, because surgical trauma to tissues can induce additional vigorous new bone formation. Glucocorticoids are used to manage symptoms of flare-ups affecting major joints. Non-steroidal anti-inflammatory agents, cyclooxygenase-2 inhibitors, mast cell stabilizers, and leukotriene inhibitors are reported by patients to manage chronic symptoms but have not been shown to inhibit new bone formation.
Our Approach
The goal of our development program is to obtain regulatory approval for palovarotene as a treatment for FOP in adults and children around the world. In order to do so, we developed an extensive clinical program which involved three overarching objectives. The first was to conduct Phase 2 clinical trials which could reproduce the effect of palovarotene seen in animal models of FOP. The second was to conduct a natural history study, which would enable us to describe disease progression in the absence of treatment and correlate the total amount of bone a patient has throughout their body with functional outcomes such as mobility or a patient’s report of physical function. Finally, if these first two objectives were successfully achieved, we would confirm these findings in a global Phase 3 trial.
As outlined below, our Phase 2 clinical studies examining the episodic treatment of flare-ups at varying doses of palovarotene were successful in answering many of our questions about the disease as well as showing trends toward the efficacy of palovarotene in FOP. As a result of our learnings from these clinical trials, we modified our dosing regimen from episodic treatment to chronic treatment in our open-label extension ‘202 Part B study. We are also conducting the first natural history study ever in FOP, which successfully enrolled more than 14% of the known worldwide population of FOP subjects and demonstrated strong correlations between the total body volume of HO and functional outcomes. To comply with Japanese regulatory requirements and eventually achieve marketing approval in Japan, we conducted a study in ethnic Japanese healthy volunteers, which enables us to enroll Japanese subjects into our Phase 3 clinical trial.
Based on clinical data generated from our Phase 2 randomized controlled trial, Part A of our Phase 2 open label extension study, and our natural history study, the FDA has indicated that new HO is a clinically meaningful outcome provided we pre-specify the magnitude of treatment effect and support the HO findings with secondary endpoints, and is sufficient as a primary endpoint for a registration trial supporting approval of palovarotene in FOP.
Phase 2 Studies to Support Pivotal Trials for Palovarotene
Our Phase 2 studies were designed to evaluate whether palovarotene could prevent new HO formation after a flare-up and address a series of fundamental questions about FOP. Our initial randomized Phase 2 clinical trial was designed as an episodic treatment trial in which palovarotene was administered at the time of a flare-up. The reasons for conducting an episodic treatment trial as opposed to a chronic treatment trial were that (i) this strategy more closely mimicked the animal studies where HO was induced by muscle injury (flare-up like) and treated with several doses of palovarotene, (ii) flare-up occurrence is unpredictable, thus enrolling patients at the time of a flare-up eliminated this variable, (iii) new HO formation had been previously detected by 6 weeks post flare-up, thus providing a relatively quick read-out of results, (iv) it was believed that HO formed approximately 80% of the time following a flare-up, thus potentially providing a high baseline with which to measure efficacy of palovarotene, and (v) we believed at that time that episodic treatment was less likely to affect the growth plate of children.
90
Clementia is the trial sponsor of an IND submission for PVO-1A-201, which was filed on March 28, 2014. The purpose of the IND submission was to begin a Phase 2 randomized, double-blind, placebo-controlled efficacy and safety study of palovarotene for the treatment of subjects with FOP.
As can be seen in Figure 3 below, palovarotene was tested initially at three different doses (placebo, 10/5 mg and 5/2.5 mg) for 6 weeks followed by an observational period of an additional 6 weeks with no treatment in 40 subjects (‘201 study). This observational period was to ensure that no rebound in flare-ups occurred after withdrawal of drug. Also, the two-dose step-down regimen was based on animal model work which optimized efficacy while minimizing side effects. After this double-blind period, all subjects elected to enroll in the open-label extension trial (‘202 Part A), where they received the 10/5 mg dose for any subsequent flare-ups they experienced.
When we pooled the results of these Phase 2 trials, which reported a total of 67 flare-ups, and made cross-study comparisons, we saw positive trends on certain of our secondary endpoints. Importantly, the pooled data suggest that palovarotene reduced the incidence of new HO by approximately 50% as determined by CT scan at 12 weeks. In those subjects who formed new HO, the mean volumes of new HO were reduced by approximately 70% as compared to placebo-treated subjects. Aside from these positive trends in bone reduction following treatment with palovarotene, several other important lessons were learned from these Phase 2 trials. We learned that (i) CT scans are more sensitive than x-ray in detecting and measuring HO volume, (ii) in some subjects, it took longer than 6 weeks to form new HO, (iii) the process initiating HO formation was possibly occurring before patients could detect flare-up symptoms, and (iv) certain flare-ups could last longer than 6 weeks.
As a result of what was learned in our Phase 2 trials as well as critical new information from animal models showing the benefits of chronic palovarotene treatment, we decided to test a new chronic dosing regimen of palovarotene in the subjects already enrolled in our open-label extension study and termed this ‘202 Part B (Figure 3). We also opened this clinical trial to enroll additional subjects. This regimen includes a 5 mg chronic daily dose of palovarotene (in adults) with increased dosing at the time of a flare-up (20 mg for 4 weeks followed by 10 mg for 8 weeks). In children, only the weight-adjusted 20/10 mg regimen for flare-ups is being administered. Our decision was also informed by the fact that in several other diseases, such as multiple sclerosis and hemophilia, chronic treatment regimens have been found to be superior to episodic treatments alone.
91
Figure 3. Palovarotene Clinical Trials: Overview
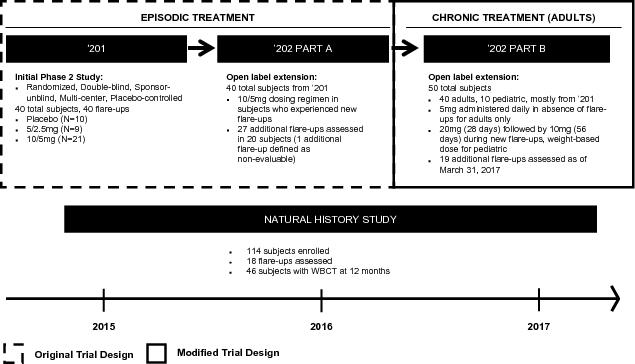
PVO-1A-201
Study PVO-1A-201, which we believe to be the first-ever placebo-controlled clinical trial in FOP, was a Phase 2, multi-center, randomized, double-blind trial (3:1 randomization) which was adaptive for dose, duration of treatment and timing of assessments. Within 1 week of a confirmed flare-up, subjects were randomized to receive either 10 mg palovarotene for 2 weeks followed by 5 mg for 4 weeks (10/5 mg, n=21), 5 mg palovarotene for 2 weeks followed by 2.5 mg for 4 weeks (5/2.5 mg, n=9), or placebo for 6 weeks (n=10); which in all cases was followed by a 6-week period without treatment. The study enrolled subjects as young as 7 years of age.
The study included imaging endpoints that assessed for new HO formation by x-ray and CT, and for the presence of soft tissue edema by magnetic resonance imaging (MRI) or ultrasound. Imaging was interpreted by a central laboratory using two blinded procedures: primary reads performed by two musculoskeletal radiologists who interpreted images relative to baseline within a single imaging modality and measured HO volume; and global reads performed by one of the central laboratory musculoskeletal radiologists, independent musculoskeletal and ultrasound radiologists, and the investigators who interpreted all images, across all imaging modalities and time points but without volume measurements.
The pre-specified primary endpoint of the study was the percent of responders (defined as subjects with no or minimal new HO at the flare-up site as assessed by x-ray) at Week 6 compared to placebo as assessed by primary reads. The statistical assumptions were that 80% of placebo-treated subjects would form new HO versus 20% of palovarotene-treated subjects in the 6-week period following the onset of a flare. These theoretical results would have had an 80% likelihood of demonstrating a statistically significant difference between the groups, which is usually defined as a p-value of less than 0.05 and generally understood to be an observed effect that has not occured by chance. However, as it is more challenging to find statistically significant results in small cohorts, there is heightened difficulty achieving statistical significance for ultra-orphan diseases, like FOP and MO.
In reviewing the data, we made a number of observations. On the primary endpoint of percent of responders at Week 6 as assessed by x-ray, the study did not show a difference between the groups. The placebo group had one out of ten subjects with new HO, the 5/2.5 mg group had one out of nine
92
subjects with new HO and the 10/5 mg group had no subjects with new HO. We believe this result was due to the fact that x-ray was not sensitive enough to detect new HO in all cases and that some subjects demonstrated new HO only at the Week 12 time point. The results of certain of our secondary endpoints presented below reflect the global read analyses using the more sensitive imaging modality (CT scan) at Week 12 in the per protocol population, which excludes one subject in the 10/5 mg group who received less than 80% of the required dose, except where noted.
• | HO Formation.A lower percentage of flare-ups in subjects on 10/5 mg (15%) had new HO as assessed by CT (or x-ray if CT was not available) at Week 12 than those on placebo (40%) and 5/2.5 mg (44.4%). This finding, which represents a 62% decline in the rate of new HO (p=0.0837), did not meet the 0.05 significance level with a sample size of 21. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | HO Volume.In addition, the volume of new HO at Week 12 (primary read via CT) was lower in the 10/5 mg (21,841 mm3) and 5/2.5 mg (5,332 mm3) groups than in the placebo group (53,938 mm3). While the dataset is small and the differences did not meet the 0.05 significance level (p=0.4871), the results directionally support a treatment effect on volume of new HO. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Figure 4. Palovarotene inhibits new bone formation after flare-ups:
Study ’201: New HO by CT scan at Week 12
|
|
|
Percent of Flare-ups with New HO | Mean Volume (mm3) of New HO by CT | |
|
|
• | Patient reported flare-up symptoms. Flare-up pain and swelling symptoms were measured at baseline and study days 14, 28, 42, 63, and 84 using a Numeric Rating Scale (NRS) (0 to 10). Time to flare-up resolution was determined based on each patient’s reporting of flare-up status contained in a daily diary. There was no statistically significant difference in pain, swelling, or time to flare-up resolution in those subjects treated with palovarotene compared with placebo. However, there was a numerical trend toward a reduction in days to flare-up resolution in the 10/5 mg (34 days) and 5/2.5 mg (46 days) groups versus placebo (64 days). There was also a numerical trend toward a greater reduction in NRS of pain in the 10/5 mg group (-3.3) versus placebo (-2.1) at day 84. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Tolerability.No subjects discontinued treatment due to an AE. All subjects had at least one treatment-emergent adverse event (TEAE); most of which were mild in severity (62.5%), the majority were dry skin. There were dose-related increases in the number and severity of retinoid-associated TEAEs (60.0% in the placebo group, 88.9% in the 5/2.5 mg group, and 95.2% in the 10/5 mg group). The most common AE was dry skin (30.0%, 55.6%, and 81.0%, respectively). No other safety signals were observed. We believe that palovarotene showed an acceptable safety profile and was well-tolerated in this study. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | SAEs.Four subjects had serious AEs: asthmatic crisis (placebo), hemorrhagic ovarian cyst (5/2.5 mg); myoclonus (10/5 mg) in a subject known to have this condition; and FOP flare-up (10/5 mg). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
93
PVO-1A-202 Part A Open Label Extension
Upon completion of each subject’s participation in Study PVO-1A-201, all subjects enrolled into open-label extension study, Part A (PVO-1A-202) in which the 10/5 mg palovarotene dosing regimen was evaluated in any subjects who experienced new flare-ups. Of these, 20 subjects experienced 28 new flare-ups, which were assessed in the same manner as flare-ups in the ‘201 study.
• | HO Formation.30% of flare-ups (8 out of 27 evaluable) in subjects receiving palovarotene (10/5 mg) had new HO as assessed by CT at Week 12. However, 4 of these flare-ups (in 3 subjects) were determined to be continuations of their original flare-ups including from a placebo subject. If these flare-ups are removed from the analysis as not representing a distinct new flare-up, then the percentage of new HO in this study is 18%. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | HO Volume.In addition, the volume of new HO at Week 12 (primary read) was measured in 27 evaluable flare-ups, 8 of which showed new HO and including those from ongoing flare-ups from the ‘201 study. Mean bone volume in these subjects (10/5 mg) was 7,506 mm3 (compared with 53,938 mm3 in placebo) indicating a large effect size (Cohen’s d=1.15). Effect sizes, unlike p values, are independent of sample size. Cohen’s d is an effect size index that measures the standardized difference between group means divided by the standard deviation and classifies effect sizes as small, medium, large and very large (Cohen’s d≥0.2, 0.5, 0.8 and 1.3, respectively). | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Figure 5. Palovarotene inhibits new bone formation after flare-ups:
Study ‘202 Open Label Extension: New HO by CT scan at Week 12
|
|
|
Percent of Flare-ups with New HO | Mean Volume (mm3) of New HO by CT | |
|
|
In summary, in the pooled results from episodic treatment of 67 flare-ups in the ‘201 and ‘202 Part A clinical trials, we observed that palovarotene, relative to the placebo group in the ‘201 study:
• | reduced the percentage of subjects who developed HO after a flare-up by approximately 50%; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | decreased the volume of HO in those who formed HO by approximately 70%; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | diminished subject-reported time to flare-up resolution. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This evidence indicates that palovarotene may yield substantial improvement in clinically meaningful endpoints in FOP, a disease for which there are no approved drugs. The results also warrant moving forward with the design of a Phase 3 program in FOP with assessment of new HO volume as a primary endpoint.
Natural History Study
In December 2014, in parallel with our Phase 2 clinical trials, we initiated, and have since completed enrollment of 114 subjects in our study, “A Natural History Study of Fibrodysplasia Ossificans Progressiva (FOP).” Based on the International Fibrodysplasia Ossificans Progressiva Association’s finding that as of October 2016, there were 800 confirmed FOP patients worldwide, the patients enrolled in our natural history study represent more than 14% of the patients in the world confirmed to have the disease. The natural history study is being conducted to characterize FOP
94
progression of disease across numerous outcomes and to understand the relationship between new bone formation in FOP and functional outcomes. Imaging outcomes include whole body CT scans (WBCTs) measured at yearly timepoints, as well as CT imaging at the location of flare-ups. Functional endpoints include range of motion, Cumulative Analogue Joint Involvement Score (CAJIS Score), and physical function measured by a patient-reported outcome questionnaire (FOP-PFQ) we specifically adapted to FOP and global health scales.
Cross-sectional data available for 114 of these patients indicates a strong correlation between losses in range of motion, physical function and age. Also, the total body volume of HO in patients is strongly correlated with functional outcomes. The measurement of HO volume is objective and quantifiable. Furthermore, HO is the foremost feature of FOP.
Our natural history study is also collecting data on flare-ups occurring in untreated subjects in the same manner as was collected in our Phase 2 clinical trials. These results indicate that untreated subjects formed new HO 44% of the time following a flare-up and the mean volume of new HO in those who did form bone was 34,625 mm3. These results are largely in line with what was observed in placebo treated subjects in the ‘201 trial (40% incidence of new bone and mean bone volume of new HO of 53,938 mm3), lending further support to the efficacy signal of palovarotene in FOP.
Thus far, we have collected information on the progression of HO at 12 months in 46 of our natural history study subjects. The location and volume of all new HO across the body was measured. 41% of the individuals with 12 month WBCTs showed evidence of new HO. The mean bone volume of new HO lesions was 41,662 mm3, a number consistent with the amount of bone formed in untreated subjects after an individual flare-up. We believe these results are consistent with our observations in placebo-treated patients in the ‘201 trial.
We anticipate that by the end of the year, we will have 12 month WBCT data on a majority of our natural history study patients as well as 24 month WBCT data on a subset of subjects. Given that patients in our natural history study have not received palovarotene, they could be eligible to enroll in our planned Phase 3 trial, and we anticipate that many of them will choose to do so.
PVO-1A-202 Part B Open Label Extension
During the course of our ‘202 Part A open-label study and based on specific outcomes we were seeing in individual cases in our studies, we believed it was appropriate to amend this study and add a Part B to evaluate chronic dosing and escalation of dosing during flare-ups. Part B of this open-label study is now evaluating chronic daily treatment of 5 mg in adults as well as higher and longer dosing during flare-ups, for adults and weight-based equivalent doses for children.
Despite the fact that our episodic dosing regimen provided an efficacy signal, we felt that this new chronic dosing regimen could potentially improve efficacy. For one, animal model studies with palovarotene indicated dose-dependent reduction in HO; therefore, we believed that as long as palovarotene was well-tolerated, higher doses would further reduce new HO. Also, we learned that for at least one subject who enrolled with nascent HO at baseline the process of HO formation may be difficult to suppress once it has begun, therefore administering palovarotene prior to detection of any symptoms could be advantageous. We also noted that of the subjects who formed new HO in our ‘202 Part A study, most of them had either formed HO in the ‘201 study or had ongoing symptoms in the 6 week observational period in which no palovarotene was received. This indicated to us that chronic treatment without interruptions in drug dosing might be preferable to episodic treatment alone. Importantly, new data published in the R206H mouse model (the mouse model which most closely mimics human FOP), indicated that chronic doses of palovarotene (5 mg HED) were not detrimental to growing bones but may be beneficial in that palovarotene treatment restored the appearance of growth plates and preserved bone growth as compared to the R206H FOP animal model. Although this animal model data suggested a potential benefit from chronic palovarotene treatment in children with FOP, the tolerability profile of our new chronic dosing regimen with higher dose treatment for flare-ups had not yet been established. Therefore, only adults are currently being treated with chronic daily dosing. Now that preliminary data has been gathered, we intend to amend the ‘202 Part B protocol to administer chronic daily dosing in children.
95
We currently have 50 subjects enrolled in our ‘202 Part B open-label extension study: 40 adults and 10 pediatric subjects. All subjects who are receiving chronic treatment underwent baseline WBCTs at study entry and will be assessed with WBCT at 12 months. Adults are being administered 5 mg of palovarotene daily in the absence of a flare-up. Children who have not achieved skeletal maturity receive treatment for flare-ups but not chronic daily treatment. During a flare-up, 20 mg for 28 days followed by 10 mg for 56 days is administered to all subjects, with weight-based dosing for children.
Based on preliminary flare-up data obtained for this new dosing regimen in our ‘202 Part B, we believe that we are seeing improved efficacy over our previous episodic treatment regimen. Overall, as of March 31, 2017, 19 flare-ups have been evaluated in ‘202 Part B for whom we have 12 week CT data, 9 flare-ups in pediatric subjects who received only episodic treatment and 10 flare-ups in adults who received prior chronic treatment. One subject had a fracture of heterotopic bone in the hip area that appeared to heal normally while on palovarotene. Although these data are sparse and this study is ongoing, subjects mean bone volume of new HO in the chronically treated group has thus far been much lower (approximately 90%, see Figure 6) than that observed in our ‘201 and ‘202 episodic treated groups.
Since palovarotene’s mechanism of action may also include anti-inflammatory activity, we examined the possibility that subjects on chronic daily dosing might also be experiencing less flare-ups. Preliminary analysis suggests an approximate 19% decrease in the flare-up rate of subjects on chronic daily dosing as compared to the flare-up rate of subjects on episodic dosing in our ‘201 and ‘202 clinical trials (as measured by the number of flare-ups per patient month exposure). Taken together, we believe these data suggest improved efficacy over our previous episodic dosing regimens.
Since flare-ups from all our studies were assessed in the same manner, we compared the volume of new HO formation following a flare-up in each of three groups: (i) untreated patients (from our ‘201 placebo group and the natural history study), (ii) patients who received only either the 10/5 mg or 20/10 mg doses episodically during flare-ups (from the ‘201 and ‘202 Part A and B studies) and (iii) patients receiving chronic daily dosing (from ‘202 Part B). In each group we included flare-ups that did not form bone (0 mm3). See Figure 6.
96
Figure 6. All flare-up combined cross-study results comparing episodic treatment to chronic treatment: New HO by CT scan at Week 12
|
Mean Volume (mm3) of New HO—All Flare-ups |
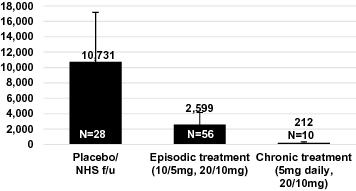
The endpoint we are proposing in our Phase 3 clinical trial is one that measures the annualized change in new HO volume by WBCT. This is the precise endpoint we have measured and are continuing to measure in our natural history subjects. In general, the mean HO volumes observed at 12 months in the natural history study are similar to what we observed in our placebo subjects from the ’201 flare-up study both in terms of the proportion who formed new HO after a flare-up as well as the mean bone volume of HO in those who did form bone. Although we have not collected data on this endpoint in our previous clinical trials, we believe that the flare-up data is suggestive of what will be seen across the body. In addition, we will have preliminary data on this endpoint from subjects in our ‘202 Part B by early 2018 and anticipate reporting these results at that time.
We have observed an increase in certain specific mucocutaneous side-effects as compared to our ‘201 and ‘202 Part A episodic dosing regimen, although the overall level of side-effects was similar. These included pruritus (or itchiness), excoriation (or skin abrasion), rash and alopecia (or hair loss), of which none were considered severe. In contrast to our previous studies, four out of twenty subjects have required dose de-escalation during flare-up dosing due to intolerable mucocutaneous side-effects attributed to dry skin, alopecia and pruritus. In general, symptoms improve at about 3 days after dose de-escalation. Overall, we believe that the predicted benefit of chronic daily dosing and 20/10 mg for flare-ups greatly outweigh the manageable tolerability issues observed and we intend to carry this regimen forward in our Phase 3 clinical trial.
Planned Clinical Trials
We plan to initiate a global Phase 3 clinical trial, the MOVE trial, which we believe, if successful, could lead to regulatory approval for palovarotene for the treatment of FOP in adults and children in the United States, as well as other major markets worldwide. The treatment regimen to be administered will be identical to that currently being tested in our ‘202 Part B study, except that children will also receive chronic dosing in addition to higher dose treatment for flare-ups.
We are also planning a trial to determine whether treatment with palovarotene immediately prior to and following surgical excision of HO in an ankylosed joint in subjects with FOP can improve range of motion at 3 months. If this trial is successful, it could allow patients who have certain locked joints to regain mobility.
MOVE Trial for FOP
We anticipate that the Phase 3 MOVE trial will be a global multi-site study which will evaluate the efficacy and safety of palovarotene in preventing new HO in subjects with FOP. We anticipate that adults and children as young as 4 years of age will receive oral palovarotene 5 mg once daily or weight-based equivalent for children. During flare-ups, all subjects will receive 20 mg for 28 days followed by
97
10 mg for 56 days or weight-based equivalent and will then return to the 5 mg daily dose. Patients will not be required to come to the clinic during flare-ups unless requested by principal investigators.
We anticipate enrolling up to a maximum of 100 subjects at approximately 15-20 clinical sites worldwide. WBCTs will be assessed at baseline and months 6, 12, 18 and 24. We anticipate our primary endpoint will be annualized change in new HO volume as measured by WBCT. Pre-specified interim analyses will be performed to evaluate for early stopping based on efficacy or futility. Since at any given time point, individual patients will have differing follow-up durations depending on when they enrolled into the clinical trial, this endpoint has the advantage of accounting for all of the data available at the time of analysis. Imaging reads will be conducted by a central imaging lab with musculosketelal radiologists blinded to patient treatment group. HO is outlined at baseline using image analysis software and any new HO measured at subsequent time points is also outlined and reported as new HO in mm3. Recent regulatory interactions support this endpoint as a clinically meaningful outcome measure and sufficient as the primary endpoint in Phase 3 to support approval, provided we pre-specify the magnitude of the treatment effect and support the HO findings with secondary endpoints. Secondary and exploratory endpoints will include the percent of subjects with new HO at 6, 12, 18 and 24 months, change in the number of body regions with any HO, CAJIS measure of physical mobility, our FOP-PFQ and PROMIS Global Health Scale which will be assessed every 6 months. We anticipate that our clinical trial will commence in 2017 with planned clinical read-outs in 2019 and 2020.
Detailed safety evaluations will include AE and serious AE reporting, electrocardiograms, vital signs, laboratory parameters and concomitant medication reporting. Also, as required with all retinoids, suicide ideation will be assessed with the C-SSRS questionnaire. For subjects with open epiphyses (active growth plate), knee and hand or wrist radiographs will be taken as well as knee height measurements for the assessment of linear growth. We believe, but cannot be certain, based on previous juvenile toxicity data, that the growth plate of children will be either minimally affected or unaffected.
We anticipate that many patients currently enrolled in our natural history study will elect to enroll in our Phase 3 MOVE trial. Our natural history study represents the largest collection of data on untreated FOP patients in the world and is unlikely to be reproduced. Since our natural history study measured and is continuing to measure WBCT annually, we are exploring ways to maximally utilize these data in the Phase 3 study.
The fact that our primary endpoint in Phase 3 will be annualized change in new HO volume by WBCT, meaning the change in HO from baseline to final scan divided by the number of years between baseline and final scan, has many advantages. First, using an annualized change enables us to integrate all the CT scan volume information we will have at our pre-specified analysis time points, including from subjects who may have assessments that go beyond the 12 month new HO volume data. Also, by using new HO volume as our endpoint, we are capturing much more information than in our percent responder analysis in the ’201 study, since both the presence and absence of HO (bone volume of 0 mm3) as well as the volume of new HO across the body are included in the same endpoint. This provides greater sensitivity to capture all of palovarotene’s potential effects, including reductions in the proportion of subjects who form new HO, reductions in new HO bone volume and potential reductions in the flare-up rate in treated subjects.
We believe that the efficacy seen in our Phase 2 studies after a single flare-up may translate to similar efficacy when evaluating new HO across the body since the biological action and the bioavailability of palovarotene is likely similar at different locations in the body. We may also see additional improvement in the reduction of HO volume from potential reductions in the flare-up rate.
In parallel with our Phase 3 trial, we will be conducting other supportive clinical and non-clinical studies including, among others, drug-drug interaction and food affect studies.
Surgical Release Trial for FOP
We are also planning a clinical trial which will be an efficacy and safety study of palovarotene in subjects with FOP undergoing surgical excision of HO. The objective is to determine whether treatment with palovarotene immediately prior to and following surgical excision of HO in an ankylosed joint in subjects with FOP can improve range of motion. We intend to discuss the timing and details of the protocol in the future.
98
Currently, there are no approved therapeutic treatments which can reduce or eliminate heterotopic bone which locks a joint into place. In non-FOP subjects, surgical removal of heterotopic bone is possible and can result in increased range of motion. Patients with FOP are currently advised not to undergo surgeries since these have been reported to lead to new HO formation. Certain patients with FOP also have compromised respiratory function due to restrictions in the ability of the chest wall to expand, and other issues related to lack of mobility in the neck and other body regions. Nonetheless, patients have undergone surgeries, of the jaw, for example, to change the position of the locked jaw and create a space for eating and speaking.
Since palovarotene has shown an efficacy signal in reducing HO after flare-ups, we intend to cautiously determine whether it can also prevent regrowth of HO after surgery.
Our proposed primary efficacy endpoint will be change in range of motion at the joint where surgical excision occured at day 84 as compared to pre-operative baseline. Proposed secondary endpoints will include change in range of motion at the joint where surgical excision occured at day 42, change in physical function using the upper extremity items from the FOP-PFQ as compared to pre-operative baseline, change from pre-operative baseline in HO volume by CT scan at day 84 as well as change from post-operative HO volume by CT scan at discharge (day 5 or 6) compared to day 84.
We intend to discuss the surgical release clinical trial with FDA prior to initiation.
Earlier Development Work
Extensive pre-clinical animal model work has consistently demonstrated dose-dependent declines in new HO formation with palovarotene treatment. In the ALK2 (Q207D) mouse model of HO, animals were injected with cardiotoxin to initiate the inflammatory triggering event leading to HO. Treatment started on the day of muscle injury, and continued with various concentrations of palovarotene or vehicle for 14 days. Soft tissue x-ray andmCT images after 15 days revealed the presence of HO in vehicle controls, and a dose-dependent decrease in the presence and volume of HO in palovarotene-treated mice, including near abrogation of HO formation (Figure 7).
Figure 7. Palovarotene Dose Response in Q207D Mouse Model
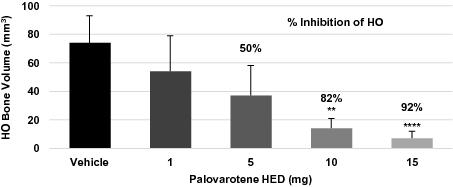
**p<0.01; ****p<0.0001
A full non-clinical toxicology program has been conducted, including juvenile toxicology, which we believe supports chronic dosing in both adults and children with FOP. From these studies, we predicted that at the doses being tested (10/5 mg weight-based equivalent), the 6 week treatment of pediatric patients with palovarotene would have minimal to no impact on the growth plate. In fact, using radiographic measures of growth plate function and linear growth velocity, we observed no evident impact on the growth plate in children in our studies. In the preliminary data we have collected in pediatric subjects tested with 20/10 mg, there has also been no evident impact on the growth plate.
The effectiveness of palovarotene was also tested in the ALK2 R206H knock-in mouse model of FOP. In this highly physiological animal model of FOP (which has abnormal posterior toe formation), growth plate abnormalities were discovered as was the observation that long bone elongation was
99
impaired in the absence of treatment. This was not previously known. Palovarotene administered on alternate days at an average human equivalent dose of approximately 5 mg surprisingly preserved long bone growth and near-normalized growth plate organization and cartilage matrix deposition. Furthermore, palovarotene treatment significantly reduced the formation of spontaneous HO in these mice. These data, along with the efficacy signal emerging from our Phase 2 studies at the time, provided one of the key elements in our decision to modify our regimen to one which included a chronic treatment component in addition to flare-up treatment.
Palovarotene in Multiple Osteochondroma (MO)
Background
Palovarotene has been previously shown to inhibit the downstream signaling of BMP receptors via Smads 1/5/8 in FOP. Since it is believed that the mutations which cause MO also result in excess BMP signaling through Smads 1/5/8, we believe palovarotene may also inhibit this pathway in MO. In this manner, palovarotene treatment could potentially reduce morbidity and deformity and preserve function in patients with MO.
MO, also called multiple hereditary exostoses, is an ultra-rare genetic musculoskeletal condition in which multiple benign bone tumors, also known as OCs or exostoses, develop on bones. MO affects approximately 20 individuals per million lives or approximately 150,000 globally, which is approximately 15 times greater than FOP. MO is typically diagnosed in early childhood with a median age at diagnosis of 3 years due to symptomatic OCs. These are comprised of growth plate-like cartilage cap overlying a bony base. They originate as an outgrowth of growth plates but frequently detach from the growth plate as a child grows. OCs form at the end of most long bones and on flat bones, such as the hip, shoulder blade or ribs. MO is phenotypically variable and associated with skeletal abnormalities including short stature, joint deformity including dislocation of the hand or valgus deformity of the knee, bowed bones and limb length discrepancies, and early onset osteoarthritis. Functional problems and morbidity occur due to pain, reduced mobility and range of motion, entrapment of blood vessels, nerves, tendons and spinal cord compression. Of patients with MO, 70% often undergo surgeries, sometimes in excess of 20, to remove OCs or address deformities. In 2-5% of patients with MO, OCs become neoplastic during adulthood.
Once bone growth is complete in late adolescence and early adulthood, it is believed that new OCs do not form; however, existing OCs can still grow and cause morbidity. Disease severity has been classified according to the number of OCs detected at a certain age as well as the number of sites with deformities or functional limitations. Class I represent less severe subjects with exostoses but without functional limitations or deformities. Class II and III represent those with multiple OCs and with functional limitations. From a registry of 529 patients, 62% were deemed to be moderate to severe, or Class II and III, while the remainder presented with milder disease. Figure 8 illustrates the appearance of OCs at multiple locations around the knees and ankles. These can be discerned visually and by clinical exam. The inward bent of the knees (valgus of the knee) is also apparent. (Images reproduced from Bovée. J.V. (2008) Orphanet Journal of Rare Diseases, 3, 3)
100
Figure 8. Appearance of OCs
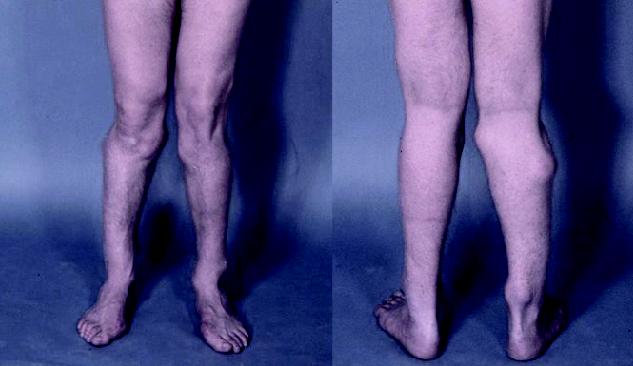
Like FOP, MO is an ultra-rare genetic disease of new bone formation in children, which is mediated by excess BMP signaling. Loss-of-function mutations in Exostosin1 (Ext1) and Exostosin2 (Ext2) genes are thought to be causal in 90% of patients with MO. Ext1 and Ext2 genes encode glycosyl transferases responsible for the elongation of heparin sulfate chains present on proteoglycans at the surface of cells. It has been proposed that reductions in cell surface heparin sulfate resulting from these mutations lead to local increases in BMP and Smad signaling. These local increases in BMP likely mediate OC formation.
Palovarotene acts by inhibiting Smad-mediated BMP signaling and is therefore being investigated as a potential treatment for the prevention of new bone formation in FOP and MO.
Current Therapies
There are currently no approved therapies for MO nor are we aware of any drug therapies in development for this disease. Surgical excision is the only treatment available for symptomatic OCs and can be associated with serious complications. No Orphan Drug Designations have been granted to any company for MO.
Our Approach
The goal of our clinical development program in MO is to obtain regulatory approval for the use of palovarotene for the treatment of MO worldwide. We intend to use all of our learnings from the development program in FOP, to design the most efficient path to approval for MO. Since we have obtained access to an extensive registry of MO patients via our collaboration with the Rizzoli Institute, we do not believe that it will be necessary to conduct a natural history study. Also, because of our extensive studies, including juvenile toxicity studies, and experience with oral palovarotene in the clinic, we do not believe we will need to conduct any additional pre-clinical or clinical studies prior to initiating our clinical trial in MO. Because of the rarity, severity and unmet medical need in MO, we believe it may be possible to conduct a single Phase 2/3 trial, which, if successful, could support our application for regulatory approval.
101
Earlier Development Work
Dr. Yu Yamaguchi (a professor at Sandford Burnham Prebys Medical Discovery Institute) developed an animal model of MO, the Ext1 gene knockout mouse model (Ext1-CKO). A study with this model was completed and BMP signaling was shown to be enhanced in the outermost cells of the growth plate, or perichondrium. In collaboration with Dr. Yamaguchi, we have generated pre-clinical data showing that a 2.6 mg human equivalent daily dose of palovarotene inhibits the number of OCs by 80% as compared to vehicle-treated Ext1-CKO animals.
Figure 9. Palovarotene Treatment Significantly Reduces Osteochondroma Formation in a Animal Model of Multiple Osteochondroma
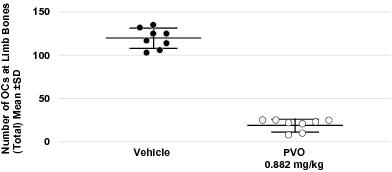
Briefly, conditional knock-out of Ext1 in mice leads to the formation of phenotypes characteristic of MO. Bony tuberosities with a cartilage cap are first detectable at 2 weeks and by one month, all animals develop multiple OCs with histological features consistent with human MO.
The Ext1-CKO mice were treated with palovarotene (0.882 mg/kg) or vehicle by daily oral gavage for 4 weeks. The effect of palovarotene on the formation of OCs in the mouse model was evaluated using whole-mount skeletal preparations stained with alcian blue. OCs were identified and counted in multiple limb bones (humerus, radius, ulna, femur and tibia) and each of the 24 rib bones from each mouse.
The sum of these counts (total number of OCs in limb bones) versus vehicle treated bones is presented in Figure 9 above. In vehicle-treated Ext1-CKO mice (n=8), 100% of the animals showed presence of OCs at all bones after 4 weeks of treatment. The mean total number of OCs at the limb bones (119±11.2) was significantly greater in vehicle treated Ext1-CKO mice compared to palovarotene treated Ext1-CKO mice (n=8;19.9±6.9) representing an 83% decrease (p<0.0001). Furthermore, results in rib bones also showed a similar 80% decline in the number of new OCs in the palovarotene treated group (35.4±9.5) vs. vehicle-treated animals (178.1±27.1) p<0.0001.
Palovarotene treatment had no effect on crown-rump length after 4 weeks of daily oral treatment in Ext1-CKO mice compared to vehicle controls. There was a small effect on long bone length in palovarotene treated animals, which may be attributable to the young age at which these animals were treated. This effect was not seen in a subsequent experiment where dosing began in animals that were one week older.
We believe that the consistency of these results on OC numbers, the magnitude of the effect and the relatively low dose of palovarotene used in these animal model studies, could potentially translate into the clinic. We note that other examples of animal models of bone disease such as osteoporosis and hypophosphatasia have previously translated in vivo findings into the clinic.
Planned Phase 2/3 Trial
Following these proof-of-concept animal studies, and as a result of our extensive clinical experience with palovarotene, we are planning to initiate a Phase 2/3 clinical trial in MO. The objective of the study will be to evaluate the efficacy and safety of palovarotene in delaying time to disease progression in MO in subjects with confirmed Ext1 or Ext2 mutations. A protocol for a multi-center, randomized
102
placebo-controlled study is being finalized. We submitted a request for a Type B Pre-IND meeting to the FDA on May 19, 2017.
Patients will be treated with one of 2 doses of oral palovarotene or with placebo using 1:1:1 randomization. The doses being considered are weight-based equivalents of 5 mg or 2.5 mg daily oral. The primary endpoint is expected to be time to MO progression across treatment groups (progression being defined as a multidomain endpoint including new OC, new joint deformity or new functional impairment and surgery. Secondary endpoints will include the number, size and location of OCs as assessed by whole body MRI, pain and quality of life measures. Enrollment will likely be limited to children as young as 4 years old.
As with our FOP clinical studies, detailed safety evaluations will include AE and serious AE reporting, electrocardiograms, vital signs, laboratory parameters and concomitant medication reporting. Also, as required with all retinoids, suicide ideation will be assessed with the C-SSRS questionnaire. For subjects with open epiphyses (active growth plates), knee and hand or wrist radiographs will be taken as well as knee height measurements for the assessment of linear growth. We believe but cannot be certain that due to the relatively low doses being tested, and based on our previous juvenile toxicity data that the growth plate of children will be either minimally affected or unaffected.
In order to better understand the epidemiology and natural history of MO, we have concluded an agreement with Dr. Luca Sangiorgi, M.D. Ph.D., the head of the Medical Genetics and Rare Orthopaedic Diseases department of Istituto Ortopedico Rizzoli di Bologna (the Rizzoli Institute), who has built a registry of over 600 subjects, 200 of which are pediatric subjects. We anticipate using the rate of progression of this disease to estimate the time required to measure a significantly different number of events in palovarotene treated subjects as compared to placebo. We anticipate that this will commence in 2017 and report data in 2020 with a potential interim read-out in 2019 with planned data read-outs in 2019 and 2020. The terms of this agreement are more fully described under “Sponsored Research Agreements” below.
We are planning a pre-IND meeting to discuss our Phase 2/3 MO program with FDA later this year. We intend to apply for Orphan Drug Designation for MO in the U.S. and in Europe in 2017 and later in Japan. We also anticipate applying for Fast Track Designation.
Palovarotene for Dry Eye Disease
Background
Dry eye disease is a multifactorial ocular condition in which the eye does not adequately produce tears. Dry eye disease is one of the most common ocular morbidities, affecting about 7% of the U.S. population. General estimates for the prevalence of dry eye disease are 14.5%. The disorder is most prevalent in elderly patients and women, in particular menopausal or post-menopausal women. There are both primary and secondary causes for dry eye disease, which result in disruptions to the precorneal tear film. The primary causes include systemic disorders, while the secondary causes can be from hormonal imbalances, environmental conditions (extreme temperatures, low humidity) and inflammatory disease. Other risk factors for dry eye disease include extensive use of display screens, refractive surgery, contact lens wear and certain medications. Hyper-osmolarity of the tears stimulates inflammatory mediators. Consequently, dry eye disease is characterized by ocular discomfort (including redness, gritty or burning eyes, and foreign body sensation), mucous discharge, disturbed vision, and tear film instability. Advanced dry eye disease can lead to pain, ulcers, or scars on the cornea, and some loss of vision.
Histopathologic changes with dry eye disease involve gradual pathologic transition of nonkeratinized stratified epithelium, including nonsecretory-cornea and/or secretory-conjunctiva, to a nonsecretory, keratinized epithelium. This process involves an increase of cellular stratification, a loss of conjunctival goblet cells, an abnormal enlargement of non-goblet epithelial cells and keratinization, making this a keratoconjunctive disorder.
The precursor to retinoids, Vitamin A, is required for ocular health. Specifically, Vitamin A is required for the maintenance of the cornea and conjunctiva as well as the retina. Vitamin A deficiency causes xeropthalmia, a severe form of dry eye disease that can lead to keratomalacia and blindness
103
when untreated. Conditions resulting from Vitamin A deficiency include squamous metaplasia of the cornea and conjunctiva, corneal ulcerations, night blindness and retinopathy. Meanwhile, the literature indicates that replacement with Vitamin A or all-trans retinoic acid (ATRA) can restore corneal health.
Receptors for RARa,b andg are widely distributed in ocular tissues. In vitro data from Dr. Kazuhiro Kimura, affiliated with Yamaguchi University, suggest that the previously observed beneficial effects of ATRA on ocular health may be mediated via RARg receptors since ligands such as palovarotene, which are selective for RARg receptors were able to completely reproduce the effects of ATRA in an ex vivo model of corneal fibrosis whereas ligands which preferentially bind RARa or RARb receptors had minimal or no effect. Specifically, palovarotene inhibits IL-1b induced fibrosis and specific matrix metalloproteases. In vivo data from Dr. Kimura supports a role for palovarotene in preventing fibrosis after corneal ulcerations and laser-induced CNV.
There is an extensive literature on the beneficial effects of retinoids in dry eye disease. Numerous studies using Vitamin A, ATRA or retinyl palmitate demonstrate statistically significant effects on signs and symptoms of dry eye disease in the clinic. For example, in a 2009 study by Kim et al., retinyl palmitate demonstrated statistically significant improvements in tear production, impression cytology grade, corneal staining, change in goblet cell number and changed in blurred vision. Furthermore, retinyl palmitate’s effects, as compared to cyclosporine, the active ingredient in Restasis®, were better in terms of tear production, impression cytology grade and blurred vision. These clinical findings are consistent with known in vitro actions of retinoids on ocular tissues including the transcriptional regulation of genes which promote ocular surface hydration and corneal epithelial healing. Other genomic effects of retinoids include the reduction of keratinization, protection of cornea from dissolution and the suppression of oncogenic proliferation of neoplasia.
There are contradictory reports in the literature with respect to the effect of retinoids on Meibomian gland function. Most if not all studies that report a detrimental effect of retinoids on this gland come from studies using 13-cis-retinoic acid or Accutane. Accutane has been found to induce dry eye disease in a subset of patients and is likely detrimental to Meibomian glands. However, this was not observed in Roche’s long term studies with palovarotene and we have found no detrimental effects to Meibomian gland histology in our animal model studies. We believe this is likely a drug specific effect which does not apply to other retinoids.
Despite the compelling evidence of the beneficial effects of Vitamin A and other retinoids on dry eye disease and other keratoconjunctive disorders, these have not been developed as commercial drugs except for a few small suppliers. We believe that one reason for this is that retinoids are believed to cause dry eye disease and be detrimental to Meibomian glands due to the experience with Accutane. We also believe that the relative instability of most retinoids in solution has impeded their commercial development as eye drops. Palovarotene is stable in solution and our eye drop formulation has been found to be stable at room temperature for at least 3 months. Another potential reason for lack of development of retinoids for ocular disorders is the fact that most known retinoids lack intellectual property protection.
The scientific insights of Dr. Kimura on the relative importance of RARg receptors for mediating anti-fibrotic effects in the eye, our own in vivo studies with palovarotene as well as the historical evidence supporting the clinical benefits of retinoids in dry eye disease, we believe provide a strong rationale for the development of palovarotene eye drop formulation for the treatment of dry eye disease.
Current Therapies
The treatment options available for dry eye disease do not treat the underlying disease process. Available treatment options are encompassed in the following categories: avoidance of exacerbating factors, eyelid hygiene, tear supplementation, tear retention, tear stimulation, and anti-inflammatory agents. These symptomatic treatments include artificial tears, lubricants, anti-inflammatory drugs, including topical steroids, topical cyclosporine (generic), Restasis® (cyclosporine emulsion), Lifitegrast®, tear retention devices (plug lacrimal puncta preventing tear drainage), topical antihistamines and mast cell stabilizers. Allergan’s Restasis® as well as Shire’s Lifitegrast® are currently the standard of care.
104
Our Approach
Our goal for the dry eye disease development program is to develop our ophthalmic formulation of palovarotene until early proof-of-concept clinical studies in man and consider potential licensing partners at that time. The path to approval in dry eye disease has been well established and the endpoints are well defined. Furthermore, the costs of early development up to proof-of-concept clinical studies in man are relatively small as compared to other programs. We intend to perform the local toxicology and pharmacokinetic studies necessary to initiate a Phase 1 and Phase 2 program in 2018.
Pre-clinical Work
In order to develop an eye drop formulation for palovarotene, several aqueous additives were tested as part of a formulation screen and the best combination selected for pre-clinical proof-of-concept studies. This formulation has been found to be stable at room temperature for at least 3 months.
Our palovarotene eye drop formulation was initially tested in an ocular irritation study in rabbits. The study was designed to evaluate tolerability of palovarotene formulation dosed for 5 consecutive days, 3 times a day. No treatment related immediate signs of ocular irritation or toxicity were observed and no abnormal clinical observations were recorded at two different doses of palovarotene including in daily ophthalmic examinations (anterior and posterior segments, body weight or weight change, ocular scoring scale, or changes in skin, fur, eyes, mucous membranes or other organ systems).
The palovarotene ocular formulation was also tested in an animal model of dry eye disease, the botulinum toxin B (BTX-B) model, which involves injection of BTX-B into the lacrimal gland and has been well characterized as a model of dry eye disease syndrome in humans. Eye drop formulations at 3 doses of palovarotene (low, mid, high) compared to current standard of care, Restasis®, were used in this model. Endpoints included ophthalmic examinations, tear production measurement, corneal fluorescein staining and histopathology. These endpoints (except histopathology) are the same as those measured in human clinical trials. As shown in Figures 10 and 11, the high and mid doses of palovarotene were more effective than Restasis® in restoring tear production and reducing corneal fluorescein in this animal model.
Figure 10. Palovarotene dose-dependently and significantly increases tear production in an animal model of dry eye disease
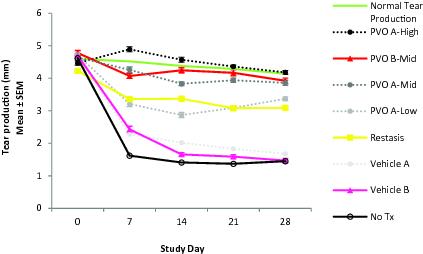
These results suggest a dose response effect with the high and mid doses of palovarotene leading to levels of tear production that are equivalent to normal tear production and low dose providing a smaller effect. Corneal fluorescein staining provides a measure of the effect of treatment on corneal scarring or fibrosis associated with dry eye disease. Figure 11 shows the results we obtained for this end point in the BTX-B model.
105
Figure 11. Palovarotene dose-dependently and significantly decreases corneal fluorescein staining in an animal model of dry eye disease
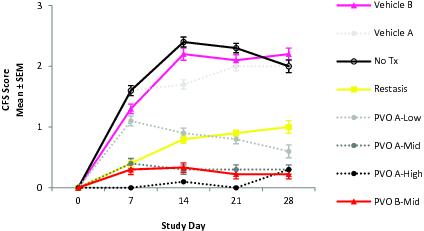
These data indicate that the palovarotene eye drops dose-dependently reduce corneal damage in this animal model. The effect of high and mid doses of palovarotene is greater than the effect of Restasis®. Together, these in vivo proof-of-concept studies in an animal model of dry eye disease support the idea that palovarotene eye drops could provide an effective treatment for dry eye disease.
Non-clinical Safety Evaluation
We are currently planning the non-clinical safety studies necessary to initiate our Phase 1 and 2 clinical trials. For previously used products intended for administration by an alternate route, generally, only local (i.e., eye) toxicity studies are required. Repeat dose toxicity studies are being planned in two distinct species. Palovarotene ocular tissue distribution will be measured in several ocular tissues and systemic levels of palovarotene will also be measured. We anticipate that due to the relatively small doses of palovarotene being administered to the eye, systemic exposure will be well below what has been previously administered in the chronic toxicology studies that support oral dosing in humans.
Planned Phase 1 and Phase 2 Trials
We are currently developing protocols for Phase 1 and Phase 2 studies. We intend to initiate a Phase 1 trial and a Phase 2 trial for palovarotene in dry eye disease in 2018. The Phase 1 clinical trial will likely be a single-center, masked, vehicle-controlled dose-ranging study with the objective to evaluate pharmacokinetics, safety and tolerability of topical eye drops containing palovarotene versus vehicle in healthy volunteers. The Phase 2 study will likely be a multi-center, masked, vehicle-controlled dose-ranging study with the objective to evaluate safety and tolerability of two doses of palovarotene versus vehicle in subjects with moderate to severe dry eye disease.
Our Pipeline of Other RARg Agonist Candidates
Our work in FOP, MO, and ocular disorders, as well as in non-clinical studies designed to elucidate RARg agonist biology in a variety of cell systems has provided us with unique insights into the biological effects of systemically administered RARg agonists and their potential therapeutic applications. We believe that RARg selective agonists have substantial untapped therapeutic potential because of their potential anti-fibrotic and tissue regeneration and repair activities and because of their predicted safety profile (if palovarotene is representative of the group). Because of this, we undertook a systematic search for second generation RARg agonists which we could deploy in different indications. This search led us to our license agreement with Galderma for several novel RARg agonists in their portfolio. These agonists, derived from four structural families, possess numerous advantageous properties including high selectivity to the RARg receptor. Some of these next-generation RARg agonists have associated data packages, such as oral formulation and absorption, distribution,
106
metabolism and excretion studies. These data are also licensed to Clementia through our agreement with Galderma.
An initial focus for the development of novel RARg agonists will be for therapeutic use in diseases, like FOP or MO, which involve pathological bone formation. There are several other potential indications for the prevention of HO with a large potential patient population, such as ankylosing spondylitis, a type of arthritis associated with excess BMP signaling, which the National Institute of Health estimates affects greater than 500,000 people in the United States and represents a high unmet medical need. Other indications, such as those characterized by excessive fibrosis or scarring, are also potential target indications for RARg agonists. On the basis of our scientific know-how and other clinical and commercial insights, a number of indications have been prioritized for animal model proof-of-concept studies in 2017 and 2018. Should these studies be successful, we plan to initiate the pre-IND activities necessary to initiate clinical trials in these new indications.
We believe that RARg agonists represent a new class of compounds with broad therapeutic potential comparable to other hormones such as corticosteroids. Our plans are to fully exploit this potential internally and in partnership with others. We intend to focus initially on our core competencies in HO and orphan drug development and access additional expertise through collaborations over time as we aim to become a fully-integrated pharmaceutical company.
Commercialization Strategy
Clementia currently intends to build the commercial infrastructure to support global commercialization of palovarotene for the debilitating bone disorders of FOP and MO, if approved. This will be accomplished by a targeted infrastructure because FOP and MO patients are managed by the same sub-specialist physicians focused on metabolic and skeletal dysplasia. A specialty sales force calling on this limited and focused group of physicians would be supported by sales management, medical liaison, internal sales support, an internal marketing group and distribution support. Also, patients, caregivers and advocacy groups are active, well organized, and networked through rare disease advocacy groups such as Rare Bone Disease Alliance, National Organization for Rare Diseases, Global Genes and EURORDIS.
Clementia already actively collaborates with the FOP and MO constituents through a number of initiatives including participation in patient meetings and educational initiatives, such that we better understand the burdens and unmet needs patients face, and so that we can better facilitate their access to palovarotene, should it be approved.
We estimate that the prevalence of FOP is approximately 1.3 individuals per million lives, or approximately 9,000 globally. As of October 2016, there were known to be 800 diagnosed patients worldwide. MO prevalence is estimated at 20 individuals per million lives, or approximately 150,000 globally. We will drive disease diagnosis and subsequent treatment of these identified patients by providing information, increasing physician awareness and creating more efficient referral pathways.
Outside of the United States and Europe, where appropriate, we may elect in the future to utilize strategic partners, distributors, or contract sales forces to assist in the commercialization of our products.
As our product candidates advance through our pipeline, our commercial plans may change. In particular, some of our research programs target potentially larger indications. Data, the size of the development programs, the size of the target market, the size of a commercial infrastructure, and manufacturing needs may all influence our strategies in the United States, Europe, and the rest of the world.
Manufacturing
Palovarotene drug substance synthesis was originally developed and optimized by Roche. Clementia further optimized this synthesis route due to the availability of novel chemistries. Our drug substance is currently manufactured by one manufacturer. It has demonstrated greater than two years stability under ambient conditions protected from light. Our drug product consists of a hard shell capsule, which can be opened by patients and sprinkled on food for those with locked jaws and pediatric subjects. Multiple dosage strengths are available to account for all potential doses
107
administered in the clinic, including for weight-based dosing in children. We monitor stability of our drug substance and drug product according to ICH compliant stability programs.
Competition
Currently, there are no therapies that have been specifically approved for the treatment of FOP. To our knowledge, palovarotene is the only orally available RARg agonist that has been clinically tested in FOP. However, products approved for other indications, for example, cortisteroids and non-steroidal anti-inflammatory drugs, are used off-label to manage FOP symptoms during flare-ups. None of these medications has been shown to prevent HO.
Regeneron Pharmaceuticals Inc. has completed a Phase 1 trial in Belgium with an antibody against Activin A. As an antibody, it would be administered either via intravenous infusion or subcutaneous injection. Regeneron has stated that they will start a Phase 2 clinical trial in FOP in 2017.
Blueprint Medicines concluded a partnership with Alexion for the development of ALK2 specific serine-threonine kinase inhibitors. To our knowledge, these kinase inhibitors being developed for FOP are at the pre-clinical stage. A number of academic groups are also pursuing ALK2 kinase inhibitors and to our knowledge, these are also at the pre-clinical stage in FOP.
Regeneron and La Jolla Pharmaceutical Company have been granted orphan drug designation for their respective approaches in FOP. To our knowledge, the compounds being developed by La Jolla Pharmaceutical Company are pre-clinical small-molecule kinase inhibitors designed to bind to ALK2.
GRI Bio is developing an RARg selective agonist, GRI-0621, in a Phase 2 clinical trial in chronic liver disease in South Africa.
License Agreements
We have entered into exclusive license agreements to help support our development efforts.
Roche Agreement
In January 2013, we entered into a license agreement with Roche for the composition of matter of palovarotene, for which we have the worldwide rights to develop and commercialize. We are obligated to pay Roche certain clinical/regulatory/sales milestones and royalties on products developed from this technology. Termination of our license agreement with Roche would have a material adverse impact on our ability to develop and commercialize palovarotene.
Pursuant to the terms of the agreement, we were granted the exclusive worldwide right to research, develop, make, have made, use, sell, have sold, offer for sale and import palovarotene, any other compounds covered by certain Roche patents, and any pharmaceutical or therapeutic products containing either palovarotene or such other licensed compounds, for all human pharmaceutical uses and indications. Addtionally, Roche transferred to us certain regulatory information on the licensed compounds, including palovarotene, pursuant to the license agreement. The Roche license also grants us a right, subject to Roche’s exclusive negotiation rights described below, to sublicense our licensed rights to third parties, provided each sublicensee enters into a written agreement with us with terms consistent with our agreement with Roche.
Roche has the first right, but not the obligation, to prepare, file, prosecute and maintain certain patent rights at Roche’s sole expense.
The license agreement requires us to use commercially reasonable efforts to develop, obtain regulatory approval of, and commercialize a product containing palovarotene. The license agreement includes commitments to pay Roche (i) a total of $1,000,000 in milestone payments upon the achievement of certain clinical milestones, (ii) up to a total of $11,000,000 in milestone payments upon the achievement of certain regulatory milestones in connection with the three clinical trial programs currently underway with an additional $1 million in milestone payments upon the achievement of certain regulatory milestones in connection with each subsequent indication, if any, and (iii) up to a total of $37,500,000 in milestone payments upon the achievement of certain sales milestones. The agreement also requires us to pay Roche a royalty rate in the low teens based on net sales of products containing palovarotene or the other licensed compounds during the royalty term. The Roche agreement provides that the royalty rate
108
will be adjusted significantly downward on a country-by-country basis if generic versions of the licensed products are introduced and sold in the relevant country and the sale of such generic versions have a certain impact on our net sales. Under the agreement, we are also required to pay any consideration owed to third parties related to such third parties’ intellectual property rights covering the licensed technology, though such payments may be at least partially offset by a reduction in royalties payable to Roche.
The Roche Agreement will expire on a licensed product-by-product and country-by-country basis upon the later of (i) the date of expiration of the last to expire patent having a valid claim relating to such licensed product in a particular country, or (ii) 10 years after the first commercial sale of a licensed product in such country. Either party may terminate the agreement for the material breach or insolvency of the other party. In the event the agreement is terminated by Roche for our material breach or insolvency, the rights and licenses granted to us under the agreement would terminate. In addition, upon termination under certain circumstances, rights in regulatory filings and certain other intellectual property may revert to Roche. Termination of our rights under the Roche license would have a material adverse effect on us.
Thomas Jefferson University (TJU) Agreement
In February 2014, we entered into a license agreement with TJU to use palovarotene for treating muscle tissue damage. We are obligated to pay TJU certain clinical/regulatory/sales milestones and royalties on the sales of certain licensed products and processes. Termination of our license agreement with TJU would have a material adverse impact on our ability to develop and commercialize palovarotene in its current formulation.
Pursuant to the terms of the agreement, we were granted the exclusive worldwide right to make and have made, to use and have used, to sell and have sold, to offer for sale, to import, to export, to research, develop and improve upon methods for muscle repair and regeneration comprising administering therapeutically effective amounts of RAR agonists (such as palovarotene) for treating muscle tissues damage. The TJU license also grants us a right to sublicense our licensed rights to third parties, provided that our license from TJU is exclusive at the time of the granting of the sublicense and that each sublicensee enters into a written agreement with us with terms consistent with our agreement with TJU.
The license agreement requires us to use commercially reasonable diligent efforts to effect introduction of a product containing palovarotene into the commercial market as soon as practicable. At any time after three (3) years from the effective date of the TJU license, TJU may terminate the agreement or render the license non-exclusive if, in TJU’s reasonable judgment, the progress reports provided by us substantially demonstrate our failure to satisfy certain diligence obligations.
The agreement requires us to make a total of $100,000 in milestone payments upon the achievement of certain clinical milestones and a total of $250,000 in milestone payments upon the achievement of certain regulatory milestones, in each case in connection with the first licensed product or licensed process that meets the relevant milestones. The agreement also requires us to pay TJU a low single digit royalty rate on the amount of total net sales of licensed products or licensed processes during the term of the agreement. We must also pay TJU a low double digit royalty rate on the amount of non-royalty sub-license income we receive from a sub-licensee if we sub-license our license under the TJU agreement and we receive a revenue stream under the sub-license. Under the agreement, non-royalty sublicense income shall include the amount paid to us by a sublicensee pursuant to the sublicense, including but not limited to license fees, milestone payments and the fair market value in cash of any non-cash consideration of any kind for such sublicense.
The term of the license agreement remains in effect until the last patent or patent application containing a valid claim in the patent rights under the license have expired or been abandoned. Either party may terminate the agreement for the material breach or insolvency of the other party. In the event the agreement is terminated by TJU for our material breach or insolvency or on account of their determination that our progress reports substantially demonstrate that we neither used commercially reasonable efforts to put the licensed subject matter into commercial use and/or are not keeping it reasonably available to the public, nor engaged in research, development, manufacturing, marketing or
109
sublicensing activity to achieve the above, the rights and licenses granted to us under the agreement would terminate. Termination of our rights under the TJU license would have a material adverse effect on us.
Upon the expiration of the TJU patent portfolio, our license agreement with and our license payment obligations to TJU will terminate and we will have a fully-paid, royalty-free, sublicensable license.
Yamaguchi University Agreement
In April 2015, we entered into a license agreement with Yamaguchi University for a patent family titled “therapeutic agent for keratoconjunctive disorders” and a patent family titled “an inhibitor for Retinochoroidal disorders.” We are obligated to pay Yamaguchi University certain clinical/regulatory milestones and royalties on sales of certain licensed products and processes. Termination of our license agreement with Yamaguchi University would have a material adverse impact on our ability to develop and commercialize palovarotene for the treatment of certain ocular disorders.
Pursuant to the terms of the agreement, we were granted the exclusive worldwide right to make and have made, to use and have used, to sell and have sold, to offer for sale, to import, to export, to research, develop and improve upon therapeutic agents for keratoconjunctive disorders and related patents. The Yamaguchi University license also grants us a right to sublicense our licensed rights to third parties, provided each sublicensee enters into a written agreement with us with terms consistent with our agreement with Yamaguchi University.
The agreement requires us to use commercially reasonable efforts to effect introduction of the licensed products into the commercial market as soon as practicable. The agreement requires us to make a total of $75,000 in milestone payments upon the achievement of certain clinical milestones and a total of $150,000 in milestone payments upon the achievement of certain regulatory milestones, in each case in connection with the first licensed product that meets the relevant milestones. The agreement also requires us to pay Yamaguchi University a low single digit royalty rate on the amount of total net sales of licensed products during the term of the agreement. We also have a royalty buy-out option pursuant to which we can terminate at any time in our sole discretion our obligation to pay royalties and make milestone payments, as well as our reporting obligations, in exchange for a one time payment to Yamaguchi University.
The Yamaguchi University agreement will expire on a licensed product-by-product and country-by-country basis upon the later of (i) the date of expiration of the last to expire patent having a valid claim relating to such licensed product in a particular country, or (ii) 10 years after the first commercial sale of a licensed product in such country. Yamaguchi University can terminate the agreement for our material breach or insolvency. In the event the agreement is terminated by Yamaguchi University for our material breach or insolvency, the rights and licenses granted to us under the agreement would terminate. We can terminate the license agreement upon 90 days’ notice.
Galderma Agreement
In March 2017, we exercised our option to enter into an exclusive license agreement with Galderma for certain retinoic acid receptor gamma agonists compounds. We are committed to make certain future payments based on the successful achievement of specific development and commercialization milestones related to the licensed Galderma compounds.
Pursuant to the terms of the agreement, we have been granted an exclusive worldwide right in certain fields and subject to certain exceptions, to (i) develop, make and use the retinoic acid receptor gamma agonists compounds in order to research, develop, manufacture and commercialize products that incorporate such compounds and (ii) research, develop, manufacture and commercialize such products. The Galderma license also grants us a right to sublicense our licensed rights to third parties, provided that, in case of (i), each sublicensee shall enter into a written agreement with terms consistent with our agreement with Galderma, and, in case of (ii), we will not be relieved from our obligations under the agreement with Galderma and will secure all covenants, obligations, and rights of the sublicensee.
110
The license agreement requires us to use commercially reasonable efforts to develop at least one of the compounds as well as to commercialize the products incorporating the compounds. The agreement requires us to pay Galderma a total of $2,000,000 in milestone payments upon the achievement of certain clinical milestones and up to a total of $25,500,000 in milestone payments upon the achievement of certain regulatory milestones, in each case in connection with the first product that meets the relevant milestones. The agreement also requires us to pay Galderma a single digit royalty rate that varies based on the amount of total worldwide net sales of licensed products during the term of the agreement (including royalty on the net sales by the sublicensees).
The license agreement will remain in effect as long as we develop or commercialize the licensed product. Either party has a right to terminate the agreement for the material breach or insolvency of the other party. Termination by Galderma for our material breach or insolvency causes termination of the rights and licenses that are granted to us under the agreement. We have the right to terminate the agreement upon 90 days prior written notice to Galderma both without cause and because of our decision to discontinue development or commercialization.
Sponsored Research Agreements
Dr. Luca Sangiorgi
On September 18, 2015, we entered into an agreement (the Sangiorgi Agreement) with Dr. Luca Sangiorgi, M.D., Ph.D., the head of the Medical Genetics and Rare Orthopaedic Diseases at the Rizzoli Institute. Under the Sangiorgi Agreement, Dr. Sangiorgi performs clinical consulting services in support of our palovarotene development program and the potential role of palovarotene as a treatment for MO (contract field). The agreement prohibits Dr. Sangiorgi from providing any services in the contract field for any other party during its term without our prior written consent. The Sangiorgi Agreement is effective until September 18, 2018. Either party may terminate the agreement in the event of material uncured breach of the other party or for convenience subject to a prior written notification of the other party. No material payments have been made to date pursuant to this agreement.
Instituto Ortopedico Rizzoli
On April 27, 2017, we entered into an agreement (the Rizzoli Agreement) with the Rizzoli Institute itself. Under the terms of the Rizzoli Agreement, the Rizzoli Institute will perform research activities (consisting of elaborations and analyses on data extracted from Rizzoli Institute’s registries and databases) and provide us with a scientific report for use in our MO development program. Dr. Sangiorgi is the chief scientific investigator responsible for the analysis. The Rizzoli Agreement contains bilateral confidentiality provisions. Confidential information shared by the parties under the agreement is for the purpose of the research project only, with no further disclosure permitted. We are not allowed to publish or distribute the report; however, we can use the results for regulatory purposes. Rizzoli Institute is free to use and supply to third parties the analyses and registry data in its sole discretion. We will own intellectual property rights in the analyses and the final scientific report.
The Rizzoli Agreement is effective until December 15, 2018. Either party may terminate the agreement in the event of material uncured breach of the other party or at will subject to a prior written notification of the other party. No material payments have been made to date pursuant to this agreement.
Sanford Burnham Prebys Medical Discovery Institute (SBP)
We entered into a Commercial Sponsored Research Agreement with SBP on June 21, 2016. This agreement relates to services provided by SBP to us as part of a research program led by Dr. Yamaguchi, a professor of human genetics at SBP. This agreement provides us with an exclusive option to license any joint-IP developed under the agreement. We have a 3 month period following disclosure to exercise the option. The agreement provides SBP with an exclusive license to use the IP generated under the project (Clementia IP or Joint-IP) for non-commercial, internal research and educational purposes only. SBP has the right to publish any results of the research program after
111
disclosure to us. After disclosure of a draft manuscript by SBP, we have up to 90 days to protect any IP related to the disclosed content.
The research program provided for in the agreement is to end within 12 to 18 months after the effective date of the agreement. Either party can terminate the agreement in the event of material uncured breach of the other party. We can also terminate the agreement with or without cause upon 60 days prior written notice. SBP can also terminate the agreement upon our bankruptcy or certain similar events. No material payments have been made to date pursuant to this agreement.
Intellectual Property
We strive to protect the proprietary technology that we believe is important to our business, including seeking and maintaining patents intended to cover our product candidates and compositions, their methods of use and processes for their manufacture, and any other aspects of inventions that are commercially important to the development of our business. We also rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection.
We plan to continue to expand our intellectual property estate by filing patent applications directed to compositions, formulations and methods of treatment created or identified from our ongoing development of our product candidates. Our success will depend on our ability to obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know-how related to our business, defend and enforce our patents, preserve the confidentiality of our trade secrets and operate without infringing the valid and enforceable patents and proprietary rights of third parties. We also rely on know-how, continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position. We seek to obtain domestic and international patent protection, and endeavor to promptly file patent applications for new commercially valuable inventions.
The patent positions of biopharmaceutical companies like us are generally uncertain and involve complex legal, scientific and factual questions. In addition, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and patent scope can be reinterpreted by the courts after issuance. Moreover, many jurisdictions permit third parties to challenge issued patents in administrative proceedings, which may result in further narrowing or even cancellation of patent claims. We cannot predict whether the patent applications we or our strategic partners are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient protection from competitors.
Because patent applications in the United States and certain other jurisdictions are maintained in secrecy for 18 months or potentially even longer, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain of the priority of inventions covered by pending patent applications. Moreover, we may have to participate in interference proceedings or derivation proceedings declared by the U.S. PTO, to determine priority of invention.
Clementia Licensed Intellectual Property
We have exclusively licensed patents claiming palovarotene as a composition of matter from Roche (the Roche patent portfolio), which has a statutory expiration date in 2021. We have an exclusive worldwide license to patents claiming the use of palovarotene for treating muscle tissue damage from TJU (the TJU patent portfolio), which has a statutory expiration date in 2031. We have an exclusive worldwide license to patents claiming the use of certain RARg agonists, including palovarotene, for treating keratoconjunctive disorders and claiming the use of palovarotene for treating retinochoroidal disorders from Yamaguchi University (the Yamaguchi University patent portfolio), which have a statutory expiration date in 2033 or 2034.
The licensed Galderma RARgagonists include lead compounds from four distinct structural families. Two families are claimed in recently filed patent applications; one with an expected statutory patent expiry date of December 2034 and a second with an expected statutory patent expiry date of December 2035. The third family includes lead compounds that have not been publicly disclosed, nor
112
has any patent specifically claiming these compounds been filed. The forth structural family includes lead compounds that are disclosed in expired Galderma patents.
The Roche patent portfolio includes granted patents in over 45 countries, including the United States, Canada and the primary countries of the European Union (26 countries). The Roche patent portfolio includes a United States patent that is scheduled to expire in 2021, without taking into account any potential patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act. The Roche patent portfolio includes a European patent, validated in numerous European jurisdictions, that has a statutory expiration date in 2021, absent any adjustments or extensions.
The TJU patent portfolio includes granted patents issued in the United States, Australia, Japan, New Zealand and South Africa, and pending patent applications in Brazil, Canada, China, Europe, the United States, and other countries. The TJU patent portfolio includes a United States patent that has a statutory expiration date in 2031, without taking into account any potential patent term restoration under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act. The TJU patent portfolio includes a European patent application that, if granted, would have a statutory expiration date in 2031, absent any adjustments, extensions or supplementary protections.
The Yamaguchi University patent portfolio includes a granted U.S. patent and patent applications pending in Canada, China, Europe, Japan, the United States, and other countries.
Clementia Owned Intellectual Property
Three patent applications have been filed by and assigned to Clementia:
A pending U.S. utility patent application (filed December 2016) with claims directed to antisense oligonucleotides and their use for treating FOP;
Patent Cooperation Treaty (PCT) application (filed June 2017) with claims directed to therapeutic treatment regimens for treating heterotopic ossification in FOP using palovarotene; and
A U.S. provisional patent application (filed November 2016) with claims directed to the use of RARg agonists, including palovarotene, in treating MO.
With respect to our U.S. provisional patent application, we will have to decide whether to pursue patent protection by filing one or more non-provisional patent applications before the statutory deadline that expires one year from the filing date of a provisional patent application. With respect to our pending PCT application, we will need to decide whether and in which jurisdictions to pursue protection before expiration of certain statutory deadlines, and we will only have the opportunity to obtain protection in such pursued jurisdictions.
The base term of a U.S. patent is 20 years from the filing date of the earliest-filed non-provisional patent application from which the patent claims priority. The term of a U.S. patent can be lengthened by patent term adjustment, which compensates the owner of the patent for administrative delays at the U.S. PTO. In some cases, the term of a U.S. patent is shortened by terminal disclaimer that reduces its term to that of an earlier-expiring patent.
The term of a U.S. patent may be eligible for patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act, to account for at least some of the time the drug is under development and regulatory review after the patent is granted. With regard to a drug for which FDA approval is the first permitted marketing of the active ingredient, the Hatch-Waxman Act allows for extension of the term of one U.S. patent that includes at least one claim covering the composition of matter of an FDA-approved drug, an FDA-approved method of treatment using the drug, and/or a method of manufacturing the FDA-approved drug. The extended patent term cannot exceed the shorter of five years beyond the non-extended expiration of the patent or 14 years from the date of the FDA approval of the drug. Some foreign jurisdictions, including Europe and Japan, have analogous patent term extension provisions, which allow for extension of the term of a patent that covers a drug approved by the applicable foreign regulatory agency. In the future, if and when our pharmaceutical products receive FDA approval, we expect to apply for patent term extension where available on patents covering those products, their methods of use, and/or methods of manufacture.
113
Trade Secrets
In addition to patents, we rely on trade secrets and know-how to develop and maintain our competitive position. We typically rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. We protect trade secrets and know-how by establishing confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and partners. These agreements generally provide that all confidential information developed or made known during the course of an individual or entity’s relationship with us must be kept confidential during and after the relationship. These agreements also generally provide that all inventions resulting from work performed for us or relating to our business and conceived or completed during the period of employment or assignment, as applicable, shall be our exclusive property. In addition, we take other appropriate precautions, such as physical and technological security measures, to guard against misappropriation of our proprietary information by third parties.
Government Regulation
FDA Approval Process
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug, and Cosmetic Act (the FDC Act) and other federal and state statutes and regulations, govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of pharmaceutical products. Failure to comply with applicable U.S. requirements may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending NDAs, warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties, and criminal prosecution.
Pharmaceutical product development for a new product or certain changes to an approved product in the U.S. typically involves pre-clinical laboratory and animal tests, the submission to the FDA of an IND, which must become effective before clinical testing may commence, and adequate and well-controlled clinical trials to establish the safety and effectiveness of the drug for each indication for which FDA approval is sought. Satisfaction of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the type, complexity, and novelty of the product or disease.
Pre-clinical tests include laboratory evaluation of product chemistry, formulation, and toxicity, as well as animal trials to assess the characteristics and potential safety and efficacy of the product. The conduct of the pre-clinical tests must comply with federal regulations and requirements, including good laboratory practices. The results of pre-clinical testing are submitted to the FDA as part of an IND along with other information, including information about product chemistry, manufacturing and controls, and a proposed clinical trial protocol. Long term pre-clinical tests, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has neither commented on nor questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or patients under the supervision of a qualified investigator. Clinical trials must be conducted: (i) in compliance with federal regulations; (ii) in compliance with good clinical practice (GCP), an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators, and monitors; as well as (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND.
The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance
114
with FDA requirements or presents an unacceptable risk to the clinical trial patients. The study protocol and informed consent information for patients in clinical trials must also be submitted to an IRB for approval. An IRB may also require the clinical trial at the site to be halted, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions.
Clinical trials to support NDAs for marketing approval are typically conducted in three sequential phases, but the phases may overlap. In Phase 1, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess metabolism, pharmacokinetics, pharmacological actions, side effects associated with increasing doses, and, if possible, early evidence on effectiveness. Phase 2 usually involves trials in a limited patient population to determine the effectiveness of the drug for a particular indication, dosage tolerance, and optimum dosage, and to identify common adverse effects and safety risks. If a compound demonstrates evidence of effectiveness and an acceptable safety profile in Phase 2 evaluations, Phase 3 trials are undertaken to obtain the additional information about clinical efficacy and safety in a larger number of patients, typically at geographically dispersed clinical trial sites, to permit FDA to evaluate the overall benefit-risk relationship of the drug and to provide adequate information for the labeling of the drug. In most cases FDA requires two adequate and well-controlled Phase 3 clinical trials to demonstrate the efficacy of the drug. A single Phase 3 trial with other confirmatory evidence may be sufficient in rare instances where the study is a large multi-center trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible.
Pursuant to the 21st Century Cures Act, which was enacted on December 13, 2016, the manufacturer of an investigational drug for a serious or life-threatening disease is required to make available, such as by posting on its website, its policy on evaluating and responding to requests for expanded access. This requirement applies on the later of 60 days after the date of enactment or the first initiation of a Phase 2 or Phase 3 trial of the investigational drug.
After completion of the required clinical testing, an NDA is prepared and submitted to the FDA. FDA approval of the NDA is required before marketing of the product may begin in the U.S. The NDA must include the results of all pre-clinical, clinical, and other testing and a compilation of data relating to the product’s pharmacology, chemistry, manufacture, and controls. The cost of preparing and submitting an NDA is substantial. The submission of most NDAs is additionally subject to a substantial application user fee, currently exceeding $2,038,000 for Fiscal Year 2017, and the manufacturer and/or sponsor under an approved new drug application are also subject to annual product and establishment user fees, currently exceeding $97,000 per product and $512,000 per establishment for Fiscal Year 2017. While these fees are typically increased annually, they decreased from Fiscal Year 2016 to Fiscal Year 2017.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of new drug applications. Most such applications for standard review drug products are reviewed within ten to twelve months; most applications for priority review drugs are reviewed in six to eight months. Priority review can be applied to drugs that the FDA determines offer major advances in treatment, or provide a treatment where no adequate therapy exists. For biologics, priority review is further limited only for drugs intended to treat a serious or life-threatening disease relative to the currently approved products. The review process for both standard and priority review may be extended by FDA for three additional months to consider certain late-submitted information, or information intended to clarify information already provided in the submission.
The FDA may also refer applications for novel drug products, or drug products that present difficult questions of safety or efficacy, to an advisory committee—typically a panel that includes clinicians and other experts—for review, evaluation, and a recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. Additionally, the FDA will
115
inspect the facility or the facilities at which the drug is manufactured. FDA will not approve the product unless compliance with cGMP is satisfactory and the NDA contains data that provide substantial evidence that the drug is safe and effective in the indication studied.
After FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing, or information, in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. FDA has committed to reviewing such resubmissions in two or six months depending on the type of information included.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require a risk evaluation and mitigation strategy (REMS) to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use (ETASU). ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. The requirement for a REMS can materially affect the potential market and profitability of the drug. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
The Hatch-Waxman Act
Orange Book Listing
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent whose claims cover the applicant’s product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an abbreviated new drug application (ANDA). An ANDA provides for marketing of a drug product that has the same active ingredients in the same strengths and dosage form as the listed drug and has been shown to be bioequivalent to the listed drug. Other than the requirement for bioequivalence testing, ANDA applicants are not required to conduct, or submit results of, pre-clinical or clinical tests to prove the safety or effectiveness of their drug product. Drugs approved in this way are commonly referred to as “generic equivalents” to the listed drug, and can often be substituted by pharmacists under prescriptions written for the original listed drug.
The ANDA applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Orange Book. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The ANDA applicant may also elect to submit a section viii statement certifying that its proposed ANDA label does not contain (or carves out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent. If the applicant does not challenge the listed patents, the ANDA application will not be approved until all the listed patents claiming the referenced product have expired.
A certification that the new product will not infringe the already approved product’s listed patents, or that such patents are invalid or unenforceable, is called a Paragraph IV certification. If the ANDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of
116
the Paragraph IV certification to the NDA and patent holders once the ANDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA until the earlier of 30 months, expiration of the patent, settlement of the lawsuit, or a decision in the infringement case that is favorable to the ANDA applicant.
The ANDA application also will not be approved until any applicable non-patent exclusivity listed in the Orange Book for the referenced product has expired.
Exclusivity
Upon NDA approval of a new chemical entity (NCE), which is a drug that contains no active moiety that has been approved by FDA in any other NDA, that drug receives five years of marketing exclusivity during which FDA cannot receive any ANDA seeking approval of a generic version of that drug. Certain changes to a drug, such as the addition of a new indication to the package insert, are associated with a three-year period of exclusivity during which FDA cannot approve an ANDA for a generic drug that includes the change.
An ANDA may be submitted one year before NCE exclusivity expires if a Paragraph IV certification is filed. If there is no listed patent in the Orange Book, there may not be a Paragraph IV certification, and, thus, no ANDA may be filed before the expiration of the exclusivity period.
Patent Term Extension
After NDA approval, owners of relevant drug patents may apply for up to a five year patent extension for one patent. The allowable patent term extension is calculated as half of the drug’s testing phase—the time between IND application and NDA submission—and all of the review phase—the time between NDA submission and approval up to a maximum of five years. The time can be shortened if FDA determines that the applicant did not pursue approval with due diligence. The total patent term after the extension may not exceed 14 years from approval.
For patents that might expire during the application phase, the patent owner may request an interim patent extension. An interim patent extension increases the patent term by one year and may be renewed up to four times. For each interim patent extension granted, the post-approval patent extension is reduced by one year. The director of the United States Patent and Trademark Office must determine that approval of the drug covered by the patent for which a patent extension is being sought is likely. Interim patent extensions are not available for a drug for which an NDA has not been submitted.
Post-Approval Requirements
Once an NDA is approved, a product will be subject to certain post-approval requirements. For instance, FDA closely regulates the post-approval marketing and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities involving the internet. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved labeling.
Adverse event reporting and submission of periodic reports is required following FDA approval of an NDA. The FDA also may require post-marketing testing, known as Phase 4 testing, REMs, and surveillance to monitor the effects of an approved product, or the FDA may place conditions on an approval that could restrict the distribution or use of the product. In addition, quality-control, drug manufacture, packaging, and labeling procedures must continue to conform to cGMPs after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with FDA and certain state agencies. Registration with the FDA subjects entities to periodic unannounced inspections by the FDA, during which the agency inspects manufacturing facilities to assess compliance with cGMPs. Accordingly, manufacturers must continue to expend time, money, and effort in the areas of production and quality-control to maintain compliance with cGMPs. Regulatory
117
authorities may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards, if it encounters problems following initial marketing, or if previously unrecognized problems are subsequently discovered.
Pediatric Information
Under the Pediatric Research Equity Act (PREA), NDAs or supplements to NDAs must contain data to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the drug is safe and effective. The FDA may grant full or partial waivers, or deferrals, for submission of data. Unless otherwise required by regulation, PREA does not apply to any drug for an indication for which orphan designation has been granted.
The Best Pharmaceuticals for Children Act (BPCA), provides NDA holders a six-month extension of any exclusivity—patent or non-patent—for a drug if certain conditions are met. Conditions for exclusivity include the FDA’s determination that information relating to the use of a new drug in the pediatric population may produce health benefits in that population, FDA making a written request for pediatric studies, and the applicant agreeing to perform, and reporting on, the requested studies within the statutory timeframe. Applications under the BPCA are treated as priority applications, with all of the benefits that designation confers.
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition—generally a disease or condition that affects fewer than 200,000 individuals in the U.S. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process. The first NDA applicant to receive FDA approval for a particular active ingredient to treat a particular disease with FDA orphan drug designation is entitled to a seven-year exclusive marketing period in the U.S. for that product, for that indication. During the seven-year exclusivity period, the FDA may not approve any other applications to market the same drug for the same disease, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA application user fee.
Fast Track Designation and Accelerated Approval
FDA is required to facilitate the development, and expedite the review, of drugs that are intended for the treatment of a serious or life-threatening disease or condition for which there is no effective treatment and which demonstrate the potential to address unmet medical needs for the condition. Under the fast track program, the sponsor of a new drug candidate may request that FDA designate the drug candidate for a specific indication as a fast track drug concurrent with, or after, the filing of the IND for the drug candidate. FDA must determine if the drug candidate qualifies for fast track designation within 60 days of receipt of the sponsor’s request.
Under the fast track program and FDA’s accelerated approval regulations, FDA may approve a drug for a serious or life-threatening illness that provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.
In clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions, or survives.
118
Surrogate endpoints can often be measured more easily or more rapidly than clinical endpoints. A drug candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, will allow FDA to withdraw the drug from the market on an expedited basis. All promotional materials for drug candidates approved under accelerated regulations are subject to prior review by FDA.
In addition to other benefits such as the ability to use surrogate endpoints and engage in more frequent interactions with FDA, FDA may initiate review of sections of a fast track drug’s NDA before the application is complete. This rolling review is available if the applicant provides, and FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, FDA’s time period goal for reviewing an application does not begin until the last section of the NDA is submitted. Additionally, the fast track designation may be withdrawn by FDA if FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Breakthrough Therapy Designation
FDA is also required to expedite the development and review of the application for approval of drugs that are intended to treat a serious or life-threatening disease or condition where preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. Under the breakthrough therapy program, the sponsor of a new drug candidate may request that FDA designate the drug candidate for a specific indication as a breakthrough therapy concurrent with, or after, the filing of the IND for the drug candidate. FDA must determine if the drug candidate qualifies for breakthrough therapy designation within 60 days of receipt of the sponsor’s request.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of FDA regulated products, including drugs, are required to register and disclose certain clinical trial information. Information related to the product, patient population, phase of investigation, study sites and investigators, and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to discuss the results of their clinical trials after completion. Disclosure of the results of these trials can be delayed in certain circumstances for up to two years after the date of completion of the trial. Competitors may use this publicly available information to gain knowledge regarding the progress of development programs.
Anti-Kickback, False Claims Laws
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the pharmaceutical industry in recent years. These laws include anti-kickback statutes, false claims statutes, and other statutes pertaining to health care fraud and abuse. The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce, or in return for, purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid, or other federally financed healthcare programs. The Patient Protection and Affordable Care Act (ACA) amended the intent element of the federal statute so that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers, and formulary managers on the other. Violations of the anti-kickback statute are punishable by imprisonment, criminal fines, civil monetary penalties, and exclusion from participation in federal healthcare programs. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution or other regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases, or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor.
119
Federal false claims laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to have a false claim paid. This includes claims made to programs where the federal government reimburses, such as Medicaid, as well as programs where the federal government is a direct purchaser, such as when it purchases off the Federal Supply Schedule. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly inflating drug prices they report to pricing services, which in turn were used by the government to set Medicare and Medicaid reimbursement rates, and for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. In addition, certain marketing practices, including off-label promotion, may also violate false claims laws. Additionally, the ACA amended the federal false claims law such that a violation of the federal healthcare program anti-kickback statute can serve as a basis for liability under the federal false claims law. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
Other federal statutes pertaining to healthcare fraud and abuse include the civil monetary penalties statute, which prohibits the offer or payment of remuneration to a Medicaid or Medicare beneficiary that the offerer/payor knows or should know is likely to influence the beneficiary to order a receive a reimbursable item or service from a particular supplier, and the healthcare fraud statute, which prohibits knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare benefit program or obtain by means of false or fraudulent pretenses, representations, or promises any money or property owned by or under the control of any healthcare benefit program in connection with the delivery of or payment for healthcare benefits, items, or services.
Other Federal and State Regulatory Requirements
Pursuant to the ACA, the Centers for Medicare & Medicaid Services (CMS) has issued a final rule that requires manufacturers of prescription drugs to collect and report information on payments or transfers of value to physicians and teaching hospitals, as well as investment interests held by physicians and their immediate family members. The reports are due on an annual basis, and the reported data is posted in searchable form on a public website. Failure to submit required information may result in civil monetary penalties.
In addition, several states now require prescription drug companies to report expenses relating to the marketing and promotion of drug products and to report gifts and payments to individual physicians in these states. Other states prohibit various other marketing-related activities. Still other states require the posting of information relating to clinical studies and their outcomes. In addition, California, Connecticut, Nevada, and Massachusetts require pharmaceutical companies to implement compliance programs and/or marketing codes. Several additional states are considering similar proposals. Compliance with these laws is difficult and time consuming, and companies that do not comply with these state laws face civil penalties.
European Union Drug Development
In the European Union, our future products may also be subject to extensive regulatory requirements. As in the United States, medicinal products can only be marketed if a marketing authorization from the competent regulatory agencies has been obtained.
Similar to the United States, the various phases of non-clinical and clinical research in the European Union are subject to significant regulatory controls. Although the EU Clinical Trials Directive 2001/20/EC has sought to harmonize the EU clinical trials regulatory framework, setting out common rules for the control and authorization of clinical trials in the EU, the EU Member States have transposed and applied the provisions of the Directive differently. This has led to significant variations in the member state regimes. Under the current regime, before a clinical trial can be initiated it must be approved in each of the EU countries where the trial is to be conducted by two distinct bodies: the National Competent Authority (NCA), and one or more Ethics Committees (ECs). Under the current regime all suspected unexpected serious adverse reactions to the investigated drug that occur during the clinical trial have to be reported to the NCA and ECs of the Member State where they occurred.
120
The EU clinical trials legislation is currently undergoing a revision process mainly aimed at harmonizing and streamlining the clinical trials authorization process, simplifying adverse event reporting procedures, improving the supervision of clinical trials, and increasing their transparency.
European Union Drug Review and Approval
In the European Economic Area, (EEA), (which is comprised of the 27 Member States of the European Union (excluding Croatia) plus Norway, Iceland and Liechtenstein), medicinal products can only be commercialized after obtaining a Marketing Authorization (MA). There are two types of marketing authorizations:
The Community MA is issued by the European Commission through the Centralized Procedure, based on the opinion of the Committee for Medicinal Products for Human Use (CHMP), of the European Medicines Agency (EMA), and is valid throughout the entire territory of the EEA. The Centralized Procedure is mandatory for certain types of products, such as biotechnology medicinal products, orphan medicinal products, and medicinal products containing a new active substance indicated for the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and viral diseases. The Centralized Procedure is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant therapeutic, scientific or technical innovation or which are in the interest of public health in the EU.
National MAs, which are issued by the competent authorities of the Member States of the EEA and only cover their respective territory, are available for products not falling within the mandatory scope of the Centralized Procedure. Where a product has already been authorized for marketing in a Member State of the EEA, this National MA can be recognized in another Member States through the Mutual Recognition Procedure. If the product has not received a National MA in any Member State at the time of application, it can be approved simultaneously in various Member States through the Decentralized Procedure. Under the Decentralized Procedure an identical dossier is submitted to the competent authorities of each of the Member States in which the MA is sought, one of which is selected by the applicant as the Reference Member State. The competent authority of the Reference Member State prepares a draft assessment report, a draft summary of the product characteristics (SPC) and a draft of the labeling and package leaflet, which are sent to the other Member States (referred to as the Member States Concerned) for their approval. If the Member States Concerned raise no objections, based on a potential serious risk to public health, to the assessment, SPC, labeling, or packaging proposed by the Reference Member State, the product is subsequently granted a national MA in all the Member States (i.e., in the Reference Member State and the Member States Concerned).
Under the above described procedures, before granting the MA, the EMA or the competent authorities of the Member States of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
European Union New Chemical Entity Exclusivity
In the European Union, new chemical entities, sometimes referred to as new active substances, qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. This data exclusivity, if granted, prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a generic application for eight years, after which generic marketing authorization can be submitted, and the innovator’s data may be referenced, but not approved for two years. The overall ten-year period will be extended to a maximum of 11 years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies.
European Union Orphan Designation and Exclusivity
In the European Union, the EMA’s Committee for Orphan Medicinal Products grants orphan drug designation to promote the development of products that are intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions affecting not more than 5 in 10,000 persons in the European Union Community and for which no satisfactory method of diagnosis,
121
prevention, or treatment has been authorized (or the product would be a significant benefit to those affected). Additionally, designation is granted for products intended for the diagnosis, prevention, or treatment of a life-threatening, seriously debilitating or serious and chronic condition and when, without incentives, it is unlikely that sales of the drug in the European Union would be sufficient to justify the necessary investment in developing the medicinal product.
In the European Union, orphan drug designation entitles a party to financial incentives such as reduction of fees or fee waivers and ten years of market exclusivity is granted following medicinal product approval. This period may be reduced to six years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity. Orphan drug designation must be requested before submitting an application for marketing approval. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
Rest of the World Regulation
For other countries outside of the European Union and the United States, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases the clinical trials must be conducted in accordance with cGCP requirements and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Healthcare Payor Reimbursement
Sales of our products will depend, in part, on the extent to which our products will be covered by third-party payors, such as government health programs, commercial insurance and managed healthcare organizations. In the United States no uniform policy of coverage and reimbursement for drug products exists. Accordingly, decisions regarding the extent of coverage and amount of reimbursement to be provided for any of our products will be made on a payor by payor basis. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our product candidates to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained.
Third-party payors are increasingly reducing reimbursements for medical products and services. Additionally, the containment of healthcare costs has become a priority of federal and state governments, and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Decreases in third-party reimbursement for our product candidate or a decision by a third-party payor to not cover our product candidate could reduce physician usage of the product candidate and have a material adverse effect on our sales, results of operations and financial condition.
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA), established the Medicare Part D program to provide a voluntary prescription drug benefit to Medicare beneficiaries. Under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities that provide coverage of outpatient prescription drugs. Unlike Medicare Part A and B, Part D coverage is not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. However, Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, though not necessarily all the drugs in each category or class. Any formulary used by a Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee. Government payment for some of the costs of prescription drugs may increase demand for products for which we receive marketing approval. However, any negotiated prices for our products covered by a Part D prescription drug plan
122
will likely be lower than the prices we might otherwise obtain. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own payment rates. Any reduction in payment that results from the MMA may result in a similar reduction in payments from non-governmental payors.
The American Recovery and Reinvestment Act of 2009 provides funding for the federal government to compare the effectiveness of different treatments for the same illness. The plan for the research was published in 2012 by the Department of Health and Human Services, the Agency for Healthcare Research and Quality and the National Institutes for Health, and periodic reports on the status of the research and related expenditures will be made to Congress. Although the results of the comparative effectiveness studies are not intended to mandate coverage policies for public or private payors, it is not clear what effect, if any, the research will have on the sales of our product candidate, if any such product or the condition that it is intended to treat is the subject of a trial. It is also possible that comparative effectiveness research demonstrating benefits in a competitor’s product could adversely affect the sales of our product candidate. If third-party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
The ACA is expected to have a significant impact on the health care industry. The ACA is expected to expand coverage for the uninsured while at the same time containing overall healthcare costs. With regard to pharmaceutical products, among other things, the ACA is expected to expand and increase industry rebates for drugs covered under Medicaid programs and make changes to the coverage requirements under the Medicare Part D program. We cannot predict the full impact of the ACA on our business as many of the ACA reforms require the promulgation of detailed regulations implementing the statutory provisions that has not yet occurred. In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted. On August 2, 2011, the Budget Control Act of 2011 among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, started in April 2013. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012 (TRA), which delayed for another two months the budget cuts mandated by these sequestration provisions of the Budget Control Act of 2011. The TRA, among other things, also reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. However, President Trump has made statements that suggest he supports repeal of all or portions of the ACA, and Congress has introduced and may introduce in the future new legislation to repeal and replace portions of the ACA. There is uncertainty with respect to the impact these changes, if any, may have, and any changes likely will take time to unfold. Any additional federal healthcare reform measures adopted in the future could limit the amounts that federal and state governments will pay for healthcare products and services, and in turn could significantly reduce the projected value of certain development projects and reduce our profitability.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the United States and generally prices tend to be significantly lower.
123
Employees
As of March 31, 2017, we employed 24 full-time employees, including 16 in research and development and 8 in general and administrative. Seven of our employees hold M.D., Sc.D., or Ph.D. degrees and an additional 10 hold M.Sc. degrees. As of March 31, 2017, 16 of our employees were located in Montreal and 8 were located in Newton, Massachusetts. We have never had a work stoppage, and none of our employees is represented by a labor organization or under any collective-bargaining arrangements. We consider our employee relations to be good.
Facilities
We lease our office space, which consists of 6,200 square feet, in Montreal, Canada. We also lease office space, which consists of approximately 2,850 square feet, in Newton, Massachusetts.
Corporate Structure
We were incorporated under the Canada Business Corporation Act on November 5, 2010. We have one wholly-owned subsidiary, Clementia Pharmaceuticals USA Inc., which is incorporated in the state of Delaware.
Legal Proceedings
We are not a party to or engaged in any material legal proceedings. From time to time we may be subject to legal proceedings and claims arising in the ordinary course of business.
124
Executive Officers and Directors
The business address for our directors and senior management is Clementia Pharmaceuticals Inc., 4150 Sainte-Catherine Street West, Suite 550, Montreal, Quebec, Canada H3Z 2Y5.
Executive Officers
The following table sets forth the name, age (as of March 31, 2017) and position of individuals who currently serve as the executive officers of Clementia Pharmaceuticals, Inc. The following also includes certain information regarding our officers’ individual experience, qualifications, attributes and skills.
|
|
|
|
| |||||
Name | Age | Positions | |||||||
Clarissa Desjardins |
|
| 50 | President, Chief Executive Officer, Director | |||||
Michael Singer |
|
| 52 | Chief Financial Officer, Corporate Secretary | |||||
Donna Roy Grogan |
|
| 60 | Chief Medical Officer | |||||
Jeffrey Packman |
|
| 50 | Chief Development Officer | |||||
Eric Grinstead |
|
| 59 | Chief Commercial Officer | |||||
Dr. Clarissa Desjardinsco-founded Clementia in 2010 and has served as our President since inception and as Chief Executive Officer since June 2012. Prior to founding Clementia, Dr. Desjardins served as Chief Executive Officer at the Centre d’excellence en médecine personnalisée from 2009 until 2012. Prior to that, Dr. Desjardins founded Advanced Bioconcept, which was sold to NEN Life Sciences (Perkin Elmer) in 1998, and cofounded Caprion Pharmaceuticals Inc. in 1998, a biotechnology company focused on proteomic biomarker discovery and drug development, where she was executive vice-president of corporate development from 1998 until 2007. Dr. Desjardins has taken part in many aspects of company creation, from conception to financing to the marketplace. Dr. Desjardins has received the BRIO award for outstanding contributions to the biotechnology industry from the Quebec Biotechnology Association and was nominated for Ernst & Young’s Entrepreneur of the Year award. Dr. Desjardins earned a PhD in Neurology and Neurosurgery from McGill’s Faculty of Medicine and was a Medical Research Council postdoctoral fellow at the Douglas Hospital Research Centre. We believe Dr. Desjardins provides significant leadership and operational experience to our board of directors as our Chief Executive Officer.
Michael Singer has served as our Chief Financial Officer and as Corporate Secretary since May 2015. Mr. Singer has been a President of 8115966 Canada Inc. since February 2012 and a director of Aurora Cannabis Inc., a TSX-V listed company, since May 2015. Prior to Clementia, Mr. Singer served as Chief Financial Officer Consultant of Bedrocan Cannabis Corporation from May 2014 until June 2015. Prior to Bedrocan, from 2000 until August 2013, Mr. Singer served as Chief Financial Officer and Corporate Secretary of Thallion Pharmaceuticals Inc. and its predecessor Caprion Pharmaceuticals Inc., clinical-stage biotechnology companies. From August 2011 to July 2014, Mr. Singer served as Chairman of the board of Warnex Inc. until successful completion of the company’s amalgamation with Diagnos Inc., at which time he continued on the board of Diagnos Inc. until May 2016. Mr. Singer received his Bachelor of Commerce in Accounting/Finance from Concordia University, and received his Graduate Diploma in Public Accounting from McGill University. He is a Certified Professional Accountant—Certified General Accountant with the Ordre des CPA du Québec.
Dr. Donna Roy Groganhas served as our Chief Medical Officer since September 2013, when she joined Clementia. As Chief Medical Officer, Dr. Grogan is responsible for implementing the Company’s clinical strategy and the design of Clementia’s clinical trials for its lead product candidate. Since June 2014 Dr. Grogan has also served as a President of Clementia Pharmaceuticals USA Inc. Dr. Grogan is a board certified Internal Medicine physician with over 15 years of experience in clinical medicine and the pharmaceutical industry. She has been responsible for numerous INDs, the design and execution of clinical development programs and new drug applications across multiple therapeutic areas, including respiratory, cardiovascular and orphan drug development. Prior to joining Clementia, from March 2012 to August 2013, Dr. Grogan was a Medical Officer at Health Care Ventures Focused Companies. From August 2011 to March 2012, Dr. Grogan was an independent consultant, and from
125
February 2007 to August 2011, she was Chief Medical Officer at FoldRx Pharmaceuticals. Dr. Grogan received her BA in Psychology from College of the Holy Cross, and her MD from the University of Illinois College of Medicine.
Jeffrey Packmanhas served as our Chief Development Officer since November 2013, when he joined Clementia. Mr. Packman has more than two decades of international experience in regulatory affairs, manufacturing and clinical operations management and has led drug development efforts at several biotechnology companies. Since 1996, Mr. Packman has been a director of Recycline, Inc. From January 2012 to October 2013, Mr. Packman served as Chief Development Officer of Apofore Corporation, a start-up company focused on the development of novel diabetes therapeutics. From October 2010 to June 2011, he was the Tafamidis Development Team Leader at Pfizer. Prior to Pfizer, Mr. Packman held positions in drug development operations at FoldRx Pharmaceuticals and Oscient Pharmaceuticals. Mr. Packman earned his BA in biology from Colby College and a MBA with highest honors from the F. W. Olin Graduate School of Business Administration at Babson College.
Eric Grinsteadhas served as our Chief Commercial Officer since January 2014, when he joined Clementia. Mr. Grinstead is responsible for our commercial operations, including the pricing and reimbursement strategies and the global commercial organization. From 2013 to 2014, Mr. Grinstead held the position of Consultant at Medical Marketing Economics, a premier value and pricing firm in the pharmaceutical industry. From 2009 to 2012, Mr. Grinstead served as Senior Vice President of Global Commercial Operations at Synageva BioPharma Corp. Prior to Synageva, Mr. Grinstead had worked for over 20 years in the biopharmaceutical industry, with responsibilities spanning operations, market access, sales, marketing, reimbursement and government affairs at Alexion, Genzyme, Amgen and GSK. Mr. Grinstead received his BA in Religious Studies from Westmont College.
Directors
The following table sets forth the name, age (as of March 31, 2017) and position of individuals who currently serve as the directors of Clementia Pharmaceuticals, Inc. The following also includes certain information regarding our directors’ individual experience, qualifications, attributes and skills, and brief statements of those aspects of our directors’ backgrounds that led us to conclude that they should serve as directors.
|
|
|
|
| |||||
Name | Age | Positions | |||||||
David P. Bonita |
|
| 41 | Chairman of the Board of Directors | |||||
Robert Heft |
|
| 62 | Director | |||||
Allan Mandelzys |
|
| 52 | Director | |||||
David Mott |
|
| 51 | Director | |||||
Francois Nader |
|
| 60 | Director | |||||
Jean-François Pariseau |
|
| 47 | Director | |||||
Clarissa Desjardins |
|
| 50 | President, Chief Executive Officer, Director | |||||
Dr. David P. Bonitahas served as Chairman of our Board since June 2013 and served as a director since April 2013. Since June 2013, Dr. Bonita has held the position of Private Equity Partner at OrbiMed Advisors LLC, an affiliate of one of our principal shareholders. From June 2004 to June 2013, Dr. Bonita held other positions at OrbiMed. Within the past five years, Dr. Bonita held directorships with the following companies: Acutus Medical, Ambit Biosciences Corporation, CardiAQ Valve Technologies, Cryterion Medical Inc., Enobia Pharma Inc., Keystone Heart Ltd., Kyn Therapeutics Inc., Loxo Oncology Inc., Prelude Therapeutics Inc., Si-Bone Inc., Tricida Inc. and ViewRay Inc. Dr. Bonita has also worked as a corporate finance analyst in the healthcare investment banking groups of Morgan Stanley and UBS. He has published scientific articles in peer-reviewed journals based on signal transduction research performed at the Harvard Medical School. Dr. Bonita received his BA in Biological Sciences from Harvard University and his joint MD/MBA from Columbia University. We believe Dr. Bonita provides significant scientific and industry knowledge to our board of directors, as well as valuable experience gained from prior board service.
Dr. Robert Hefthas served as a director since June 2013. Dr. Heft is Chief Executive Officer and a director of Zingenix Ltd. He also holds directorships with the following companies: ELOXX
126
Pharmaceuticals Ltd. (where he is chairman of the board), Ra Pharma, VisionGate Inc., and Lumos Pharma Inc. Dr. Heft is a member of advisory board and an executive in residence in Sectoral Asset Management Inc. Prior to Clementia, Dr. Heft served as President, Chief Executive Officer, and a member of the board of directors of Enobia Pharma Inc. from 2005 until February 2012, when Enobia was sold to Alexion Pharmaceuticals. From 2001 until 2004, Dr. Heft held several senior positions with Biomarin Pharmaceuticals. Prior to that, Dr. Heft founded IBEX Pharmaceuticals in 1986, where he served as its President, Chief Scientist, and director until 2001. Dr. Heft received his PhD in Genetic Engineering/Radiological Sciences from the Massachusetts Institute of Technology and was a Neurology Fellow at the Massachusetts General Hospital. He has also received a Masters degree in Nuclear Engineering from Cornell University and a Bachelor of Mechanical Engineering from McGill University. We believe Dr. Heft provides significant executive and business skills, and scientific and industry knowledge to our board of directors, as well as valuable experience gained from prior and current board service.
Dr. Allan Mandelzyshas served as a director since June 2013. He also serves as a director of Matrizyme Pharma Corp. and is President and a director of 8569975 Canada Inc. and 9639439 Canada Inc., companies involved in corporate and business development consulting for the pharmaceutical and biotechnology industries. From August 2014 to September 2015, Dr. Mandelzys served as a director of Bedrocan Cannabis Corporation and acted as chairman of the Special Committee of the Board leading to its acquisition by Tweed Marijuana Inc. Dr. Mandelzys was the Chief Executive Officer and a director of Thallion Pharmaceuticals Inc. from January 2010 to July 2013, when Thallion was sold to Bellus Health Inc. Prior to that, Dr. Mandelzys served as Executive Vice President of Licensing & Corporate Development at Thallion and from 2000-2006, Vice President of Business Development at Labopharm Inc., where he helped to transform the drug delivery company into a product development company with commercial sales via multiple distribution partners. He earned his PhD in physiology and MBA from McGill University. Dr. Mandelzys also holds a BSc from the University of Toronto and received his post-doctoral training at the Roche Institute of Molecular Biology. We believe Dr. Mandelzys provides significant executive and business skills, and scientific and industry knowledge to our board of directors, as well as valuable experience gained from prior and current board service.
David M. Motthas served on our board of directors since August 2015. Mr. Mott has served as a general partner of New Enterprise Associates, an investment firm focused on venture capital and growth equity investments, since September 2008, where he leads the healthcare investing practice. From 1992 until 2008, Mr. Mott worked at MedImmune Limited, a biotechnology company and subsidiary of AstraZeneca Plc, and served in numerous roles during his tenure including from October 2000 to July 2008 as president and chief executive officer, and previously as chief financial officer, and as president and chief operating officer. During that time, Mr. Mott also served as executive vice president of AstraZeneca Plc from June 2007 to July 2008 following AstraZeneca Plc’s acquisition of MedImmune Limited in June 2007. Prior to joining MedImmune Limited, Mr. Mott was a vice president in the healthcare investment banking group at Smith Barney, Harris Upham & Co. Inc. Mr. Mott received a Bachelor of Arts degree from Dartmouth College. Mr. Mott serves as the chairman of the board of directors for Adaptimmune, Ardelyx, Epizyme, and TESARO. He also serves on the boards of directors of several privately held life sciences companies, namely: 3-V Biosciences, Cydan, Imara, Mersana, NightstaRx, Vtesse, and Xtuit. Mr. Mott has previously been a director of Prosensa Holding NV and Omthera Pharmaceuticals Inc. We believe Mr. Mott provides significant executive and business skills and industry knowledge to our board of directors, as well as valuable experience gained from prior and current board service.
Dr. Francois Naderhas served as a director since February 2014. Since February 2015, Dr. Nader has been the Chief Executive Officer of Jesra Advisors LLC. Prior to that Dr. Nader was President, Chief Executive Officer and a member of the board of directors of NPS Pharmaceuticals from 2008 until 2015, when NPS was acquired by Shire. He joined NPS in 2006 as Chief Medical and Commercial Officer, and was promoted to Chief Operating Officer in 2007. Before NPS, from 2004 to 2006, he was a CMO and venture partner at Care Capital. Prior to that, he served on the North America Leadership Team of Aventis and its predecessor companies holding a number of executive positions. Dr. Nader was recognized as the Ernst and Young National Life Science Entrepreneur of the Year in 2013. Dr. Nader is the Chairman of the Board of BioNJ, a trade association representing the biotechnology industry in
127
New Jersey. Dr. Nader currently serves as chairman of the board of directors of Acceleron Pharma Inc. and as a director of Advanced Accelerator Applications and ArRett Neuroscience. From 2015 to 2016, he served as a director at Baxalta Inc. and from 2014 to 2015, as director of Trevena. Dr. Nader is a board member of the Biotechnology Industry Organization and the New Jersey Chamber of Commerce. Dr. Nader earned his French Doctorate in Medicine from St. Joseph University in Lebanon and his Physician Executive MBA from the University of Tennessee. We believe Dr. Nader provides significant executive and business skills, and scientific and industry knowledge to our board of directors, as well as valuable experience gained from prior and current board service.
Jean-François Pariseauhas served as a director since June 2012. Since July 2001 he has been a partner in the Healthcare Venture Fund at BDC Capital Inc., a wholly owned subsidiary of the Business Development of Canada and one of our principal shareholders. From 2001 until March 2011, he served as director of BDC Venture Capital. Mr. Pariseau has served as Advisor for Hacking Health since April 2013. He currently serves on the board of directors of Imagia Cybenetics Inc., Profound Medical Inc. and Angiochem. Mr. Pariseau also served as director of Clearwater Clinical Inc. and Milestone Pharmaceuticals Inc. Prior to joining BDC, Mr. Pariseau was an investment manager with CDP Capital Technology Ventures, a global fund investing in healthcare, information technology and advanced technologies where he was responsible for healthcare investments in Canada and the U.S. Mr. Pariseau holds a BS in Biotechnology from Université de Sherbrooke, a MS in Biomedical Sciences from Université de Montréal, and an MBA from HEC Montréal. We believe Mr. Pariseau provides significant executive and business skills and industry knowledge to our board of directors, as well as valuable experience gained from prior and current board service.
Board of Directors
Our board of directors currently consists of 7 members. The articles and by-laws provide that the number of directors shall be a maximum of 10 members and will be fixed from time to time by resolution of the board of directors. Our board of directors are elected at each annual meeting of our shareholders and serve until their successors are elected or appointed, unless their office is vacated earlier. The term of office for each of the directors will expire at the time of our next annual shareholders meeting. Under the CBCA, one quarter of our directors must be resident Canadians, as defined in the CBCA.
Family Relationships and Other Arrangements
There are no family relationships among our directors and senior management.
Dr. Bonita was designated as a director by OrbiMed in connection with a shareholders agreement, which will terminate upon the closing of this offering.
Mr. Pariseau was designated as a director by BDC Venture Capital in connection with a shareholders agreement, which will terminate upon the closing of this offering.
Mr. Mott was designated as a director by New Enterprise Associates in connection with a shareholders agreement, which will terminate upon the closing of this offering.
Director Independence
As a foreign private issuer, under the listing requirements and rules of The Nasdaq Global Market, we are not required to have independent directors on our board of directors, except to the extent that our audit committee is required to consist of independent directors, subject to certain phase-in schedules. Nevertheless, our board of directors has undertaken a review of the independence of the directors and considered whether any director has a material relationship with us that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. Our board of directors determined that 6 of our 7 directors are “independent directors” as defined under current rules and regulations of the SEC and the listing standards of The Nasdaq Global Market. In making these determinations, our board of directors considered the current and prior relationships that each non-employee director has with our Company and all other facts and circumstances that our board of directors deemed relevant in determining their independence.
128
Board Committees
The standing committees of our board of directors consist of an audit committee, compensation committee and, upon the completion of this offering, a nominating and corporate governance committee.
Audit Committee
Our audit committee is comprised of Dr. Allan Mandelzys (Chair), Dr. David Bonita and David Mott.
Our board of directors has determined that each of the members of our audit committee is financially literate and has sufficient financial expertise, and is independent within the meaning of such term in the rules of The Nasdaq Global Market and the SEC. Our board of directors has determined that Dr. Allan Mandelzys is a financial expert in accordance with the applicable rules and regulations of the SEC.
The purpose of our audit committee is to assist our board of directors in overseeing:
• | the integrity of our financial statements and our accounting and financial reporting processes and financial statement audits; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | our compliance with legal and regulatory requirements; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the performance, qualifications and independence of our registered public accounting firm (independent auditor); | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the results of our audits; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the design, implementation and ongoing effectiveness of our systems of disclosure controls and procedures, risk management systems, internal control over financial reporting and compliance with ethical standards adopted by us. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Prior to the completion of this offering, our Board of Directors will adopt a new written charter for the Audit Committee that will satisfy the applicable requirements of the SEC and The Nasdaq Global Market and will be available on our website.
Compensation Committee
Our compensation committee is comprised of Jean-François Pariseau (Chair), Dr. David Bonita and Dr. Robert Heft, all of whom are “independent” in accordance with the provisions of Rule 10C-1(b)(1) under the Exchange Act and Nasdaq Rule 5605(a)(2).
Our board of directors has determined that each member of our compensation committee is independent within the meaning of such term in the rules and regulations of the SEC and The Nasdaq Global Market. In affirmatively determining the independence of any member of our compensation committee, our board of directors must consider all factors specifically relevant to determining whether a director has a relationship to the Company that is material to that director’s ability to be independent from management in connection with the duties of a compensation committee member, including, but not limited to: (i) the source of compensation of such director, including any consulting, advisory or other compensatory fee paid by the Company to such director; and (ii) whether such director is affiliated with the Company, a subsidiary of the Company or an affiliate of a subsidiary of the Company.
The purpose of our compensation committee is to ensure that the compensation programs and values transferred to management through cash pay, share and share-based awards, whether immediate, deferred, or contingent are fair and appropriate to attract, retain and motivate management and are reasonable in view of company economics and of the relevant practices of other similar companies. Our compensation committee also recommends to our board of directors’ compensation arrangements for board members.
Prior to the completion of this offering, our Board of Directors will adopt a new written charter for the Compensation Committee that will satisfy the applicable requirements of the SEC and The Nasdaq Global Market and will be available on our website.
129
Nominating and Corporate Governance Committee
Upon the completion of this offering the nominating and corporate governance committee will be comprised of Dr. Francois Nader (Chair), Dr. David Bonita and Dr. Allan Mandelzys, all of whom will be “independent directors” within the meaning of Nasdaq Rule 5605(a)(2). In affirmatively determining the independence of any member of our corporate governance and nominating committee, our board must consider all factors specifically relevant to determining whether a director has a relationship to us that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
The purpose of our corporate governance and nominating committee is to:
• | assist our board of directors in identifying prospective director nominees and recommend to our board of directors the director nominees for each annual meeting of shareholders; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | recommend members for each board committee; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | ensure that our board of directors is properly constituted to meet its fiduciary obligations to the Company and its shareholders and that the Company follows appropriate governance standards; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | develop and recommend to our board of directors governance principles applicable to the Company; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | review and recommend to the board of directors the functions, duties and compositions of the committees of the board of directors; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | oversee the succession planning for senior management; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | oversee the evaluation of our board of directors and management. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Prior to the completion of this offering, our Board of Directors will adopt a written charter for the nominating and corporate governance committee that will satisfy the applicable requirements of the SEC and The Nasdaq Global Market and will be available on our website.
Code of Business Conduct and Ethics
Prior to the completion of this offering, we intend to adopt a Code of Business Conduct and Ethics (the Code of Conduct), applicable to all of our employees, executive officers and directors. The Code of Conduct will be available on our website. The nominating and corporate governance committee of our board of directors will be responsible for overseeing the Code of Conduct and must approve any waivers of the Code of Conduct for employees, executive officers and directors. We expect that any amendments to the Code of Conduct, or any waivers of its requirements, will be disclosed on our website.
130
EXECUTIVE AND DIRECTOR COMPENSATION
Determining Compensation
In the first half of 2017, Radford, part of Aon Hewitt (a business unit of Aon plc), was retained by the compensation committee to conduct a competitive review and assessment of Clementia’s director and executive compensation program and recommend go-forward strategies. The compensation committee was involved in and approved the adoption of the following procedures during Radford’s assessment:
(1) | Establishing the public company peer group used in the director and executive compensation assessment; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) | Reviewing the detailed assessment of Clementia’s director compensation program versus the market, approving and implementing certain recommendations; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) | Reviewing the detailed assessment of Clementia’s executive compensation program versus the market, approving and implementing certain recommendations; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) | Reviewing Clementia’s equity incentive program, approving and implementing certain recommendations; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) | Reviewing equity ownership levels, and approving and implementing certain recommendations. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The compensation committee has and will continue to utilize these strategies when contemplating director and executive compensation matters.
Executive Compensation
For the year ended December 31, 2016, our named executive officers (NEOs) received compensation for services, as follows:
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
Name and Principal Position | Salary earned(1) | Non-equity | Other | Total | ||||||||||||||||||||||||
Clarissa Desjardins, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
President, Chief Executive Officer, Director(4) |
|
| $ |
| 265,335 |
|
| $ |
| 69,732 |
|
| — |
|
| $ |
| 335,067 | ||||||||||
Michael Singer, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Chief Financial Officer, Corporate Secretary(5) |
|
| $ |
| 191,989 |
|
| $ |
| 40,845 |
|
| — |
|
| $ |
| 232,834 | ||||||||||
Donna Roy Grogan, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Chief Medical Officer(6) |
|
| $ |
| 356,566 |
|
| $ |
| 72,739 |
|
| — |
|
| $ |
| 429,305 | ||||||||||
Jeffrey Packman, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Chief Development Officer(7) |
|
| $ |
| 262,181 |
|
| $ |
| 63,513 |
|
| — |
|
| $ |
| 325,694 | ||||||||||
Eric Grinstead, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Chief Commercial Officer(8) |
|
| $ |
| 349,574 |
|
| $ |
| 80,227 |
|
| — |
|
| $ |
| 429,801 | ||||||||||
(1) | Compensation amounts paid in Canadian dollars have been converted to U.S. dollars for purposes of the table. For 2016, the U.S. dollar per Canadian dollar exchange rate used for such conversion was $0.7581 for salaries earned, which was the Company’s average exchange rate for the year ended December 31, 2016, along with $0.7441 for non-equity inventive plan compensation, which was the Company’s closing exchange rate at December 31, 2016, both based on Bloomberg Markets CADUSD:CUR spot exchange rates. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) | Non-equity incentive plan compensation reflects annual bonuses earned with respect to fiscal 2016, based on the achievement of corporate goals and objectives, as well as personal performance assessments. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) | No options were granted in the year ended December 31, 2016. Furthermore, compensation amounts do not include taxable benefits for group health, dental, life insurance and disability insurance premiums paid by the Company on behalf of the named executive officers as these benefits are offered to all employees of the Company. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
131
(4) | On January 1, 2017, Clarissa Desjardins’ annual base salary was increased to C$490,000. On the effective date of the offering, the non-equity incentive plan compensation will be increased from 35% to 50% of Ms. Desjardins’ base salary. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) | On January 1, 2017, Michael Singer’s annual base salary was increased to C$290,000. On the effective date of the offering, the annual base salary will be increased to C$331,000. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) | On January 1, 2017, Donna Roy Grogan’s annual base salary was increased to $361,201. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(7) | On January 1, 2017, Jeffrey Packman’s annual base salary was increased to $265,589. On the effective date of the offering, the annual base salary will be increased to $290,000. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(8) | On January 1, 2017, Eric Grinstead’s annual base salary was increased to $354,118. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Employment Agreements
On March 31, 2017, the board of directors approved certain amendments to the existing employment agreements of our NEOs. As such and pursuant to the consummation of this offering, we intend to enter into new employment agreements (the Employment Agreements) with our NEOs which include the following terms.
Term of Employment
All of our NEOs are employed for an indefinite term, subject to termination in accordance with the terms of the Employment Agreements.
Base Salaries and Bonus Opportunities
The Employment Agreements will establish a base salary for each of our NEOs, subject to annual review and possible increase by our board of directors. A decrease in the base salary of our NEOs by the board of directors will only be allowed if implemented throughout the Company and is proportional to the decrease in base salaries of all the employees of the Company.
The annual base salaries for Ms. Desjardins, Ms. Grogan, Mr. Grinstead, Mr. Singer and Mr. Packman are established at C$490,000, $361,201, $354,118, C$290,000 and $265,589, respectively. The annual base salary of Mr. Singer and Mr. Packman will be increased to C$331,000 and $290,000, respectively, upon the completion of the offering.
Our NEOs are also eligible for an annual bonus based on achievement of performance goals established for the employees and the Company at the beginning of each year and/or in accordance with the terms of the applicable bonus plan in effect from time to time. The maximum annual bonus that may be earned, expressed as a percentage of the NEO’s annual base salary, are 50% for Ms. Desjardins and 30% for each of Ms. Grogan, Mr. Grinstead, Mr. Singer and Mr. Packman.
The Employment Agreements will also provide that our NEOs will be eligible to participate in any benefit plans made generally available to the Company’s similarly-situated employees.
Severance
Each of the Employment Agreements will provide that if the applicable NEO is terminated by the Company for cause or if his or her employment is terminated at such NEO’s initiative, the NEO will be entitled to the following standard payments: (i) base salary earned by her or him up to the date of termination; (ii) unused vacation pay calculated as of the date of termination and (iii) reimbursement of expenses up to and including the date of termination.
Each of the Employment Agreements will also provide that if the applicable NEO is terminated by the Company other than for cause, in addition to the standard payments, the NEO will also be entitled to the following additional severance payments, subject to the execution of a general release and waiver: (i) base salary and (ii) health continuation coverage reimbursements, in each case during the severance period, which is equal to 12 months from the date of termination for Ms. Desjardins and 9 months from the date of termination for each of Ms. Grogan, Mr. Grinstead, Mr. Singer and Mr. Packman.
132
In the event any NEO is terminated by the Company for any reason other than cause within 12 months after a change of control, in addition to the standard payments and the additional severance payments, the Employment Agreements will provide that the NEO will also be entitled to the following change of control benefits, subject to the execution of a general release and waiver: (i) target bonus for the year in which date of termination occurs and (ii) vesting of all outstanding equity grants including any outstanding stock options as of the date of termination.
The severance payments related to terminations other than for cause and the change of control benefits of each of Ms. Grogan, Mr. Grinstead and Mr. Packman pursuant to the Employment Agreements will be conditioned upon such NEO’s compliance with the confidentiality, non-compete and non-solicitation obligations provided in their Employment Agreements.
Each of the Employment Agreements with Ms. Grogan, Mr. Grinstead and Mr. Packman will also provide that if any payments or benefits payable to them under the Employment Agreements (such as parachute payments) would be subject to an excise tax pursuant to the Section 4999 of the Internal Revenue Code, the respective NEO will receive the greater on an after-tax basis of (i) all parachute payments or (ii) parachute payments as reduced to avoid the exercise tax.
Restrictive Covenants
Each of the Employment Agreements will contain standard confidentiality, non-solicitation and non-competition restrictions.
Confidentiality obligations will continue during and indefinitely after the term of employment for each of Ms. Grogan, Mr. Grinstead and Mr. Packman. The confidentiality obligations of Ms. Desjardins and Mr. Singer will be effective during the term of their employment and for 36 months following the date of termination (subject to limited exceptions pursuant to which their confidentiality obligations continue indefinitely).
Non-solicitation and non-competition restrictions pursuant to the Employment Agreements will be effective during the term of each of our NEOs’ employment and remain in place during the severance period applicable to the respective NEO.
Potential Payments upon Termination or Change in Control
Our severance benefits plan (the Severance Plan), provides severance benefits to our NEOs, and other employees designated by our board of directors or an authorized committee thereof, if their employment is terminated by us “without cause” or, only in connection with a “change in control” of our Company, they terminate employment with us for “good reason” (as each of those terms is defined in the Severance Plan).
Under the Severance Plan, if we terminate an NEO’s employment without cause absent a change in control, the NEO is entitled to (a) 12 months’ base salary (in the case of our Chief Executive Officer) and 9 months’ base salary for all remaining NEOs following the date of termination, which we refer to as the Severance Period, and (b) company contributions to the cost of health care continuation for the Severance Period.
The Severance Plan also provides that, if, following the closing of a change in control of our Company, we terminate an NEO’s employment without cause or such NEO terminates his or her employment with us for good reason, each of which events we refer to as a Change in Control Termination, the NEO is entitled to (a) 12 months’ base salary (in the case of our Chief Executive Officer) and 9 months’ base salary for all remaining NEO following the date of termination, which we refer to as the Change in Control Severance Period, (b) company contributions to the cost of health care continuation during the Change in Control Severance Period, and (c) the amount of any unpaid annual bonus determined by our board of directors to be payable to the NEO for any completed bonus period which ended prior to the date of such NEO termination. In addition, in the event of a Change in Control Termination, all of the NEO outstanding unvested equity awards will immediately vest in full on the date of such termination.
133
All payments and benefits provided under the Severance Plan are contingent upon the execution and effectiveness of a release of claims by the NEO in our favor and continued compliance by the NEO with any proprietary information and inventions, nondisclosure, non-competition, and non-solicitation (or similar) agreement to which we and the executive are party.
The table below reflects amounts payable by the Company to the NEOs, assuming that their employment was terminated as of the effective date of this offering, either without cause or upon change of control of the Company.
|
|
|
|
|
|
|
|
|
|
|
|
|
Name | Severance | Continuation | Total | Bonus(1) | Accelerated | Total upon | ||||||
Clarissa Desjardins | C$490,000 | C$7,600 | C$497,600 | C$ | C$ | C$ | ||||||
Michael Singer | C$248,250 | C$5,050 | C$253,300 | C$ | C$ | C$ | ||||||
Donna Grogan | $270,901 | $25,551 | $296,452 | $ | $ | $ | ||||||
Jeffrey Packman | $217,500 | $25,551 | $243,051 | $ | $ | $ | ||||||
Eric Grinstead | $265,589 | $18,329 | $283,918 | $ | $ | $ |
(1) | In the event of a Change in Control, NEOs will also receive the amount of any unpaid annual bonus determined by our board of directors to be payable to the NEO for any completed bonus period which ended prior to the date of such NEO termination. Amounts included in the table above assume 100% of the target annual bonus percentage of each NEO, prorated for the period ended prior to the effective date of this offering. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) | In the event of a Change in Control, all of the NEO outstanding unvested equity awards will immediately vest in full on the date of such termination. Amounts included in the table above reflect the in-the-money value each NEO’s unvested options at the effective date of this offering, based upon the difference between an assumed initial public offering price of $ per share, the midpoint of the estimated price range set forth on the cover page of this prospectus, and the exercise price of the options. The actual value of the awards realized by each NEO, if any, will depend on the price of our common shares at the time of exercise. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Director Compensation
For the year ended December 31, 2016, our directors received compensation for services, as follows:
|
|
|
|
|
|
| |||||||||||||||
Name and Principal Position | Director | Other | Total | ||||||||||||||||||
David P. Bonita, |
|
|
|
|
|
| |||||||||||||||
Chairman of the Board, Director |
|
| — |
|
| — |
|
| — | ||||||||||||
Jean-Francois Pariseau, |
|
|
|
|
|
| |||||||||||||||
Chairman of the Compensation Committee, Director |
|
| — |
|
| — |
|
| — | ||||||||||||
David Mott, |
|
|
|
|
|
| |||||||||||||||
Director |
|
| — |
|
| — |
|
| — | ||||||||||||
Robert Heft, |
|
|
|
|
|
| |||||||||||||||
Director |
|
| $ |
| 15,162 |
|
| — |
|
| $ |
| 15,162 | ||||||||
Allan Mandelzys, |
|
|
|
|
|
| |||||||||||||||
Director, Chairman of the Audit Committee |
|
| $ |
| 15,162 |
|
| $ |
| 4,833 |
|
| $ |
| 19,995 | ||||||
Francois Nader, |
|
|
|
|
|
| |||||||||||||||
Director |
|
| $ |
| 15,162 |
|
| — |
|
| $ |
| 15,162 | ||||||||
Clarissa Desjardins, |
|
|
|
|
|
| |||||||||||||||
President, Chief Executive Officer, Director |
|
| — |
|
| — |
|
| — | ||||||||||||
(1) | Compensation amounts paid in Canadian dollars have been converted to U.S. dollars for purposes of the table. For 2016, the U.S. dollar per Canadian dollar exchange rate used for such conversion was | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
134
| $0.7581, which was the Company’s average exchange rate for the year ended December 31, 2016, based on Bloomberg Markets CADUSD:CUR spot exchange rates. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) | For the year ended December 31, 2016, we compensated each director with quarterly cash retainers of C$5,000 per quarter, or C$20,000 per annum. Directors appointed by investors to the Company’s board of directors received no compensation. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) | Other compensation represents amounts paid to a company controlled by Dr. Mandelzys for consulting services provided to the Company. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) | No options were granted in the year ended December 31, 2016. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
On March 31, 2017, our board of directors approved a director compensation program to be effective on the effective date of this offering. Under this director compensation program, we will pay our non-employee directors a cash retainer for service on the board of directors and for service on each committee on which the director is a member. The chairman of the board and of each committee will receive higher retainers for such service. These fees are payable in arrears in four equal quarterly installments on the last day of each quarter, provided that the amount of such payment will be prorated for any portion of such quarter that the director is not serving on our board of directors and no fee shall be payable in respect of any period prior to the effective date of the offering. The fees paid to non-employee directors for service on the board of directors and for service on each committee of the board of directors on which the director is a member are as follows:
|
|
|
|
| ||||||||||
| Member | Chairman | ||||||||||||
Board of Directors |
|
| $ |
| 35,000 |
|
| $ |
| 60,000 | ||||
Audit Committee |
|
| $ |
| 7,500 |
|
| $ |
| 15,000 | ||||
Compensation Committee |
|
| $ |
| 5,000 |
|
| $ |
| 10,000 | ||||
Nominating and Corporate Governance Committee |
|
| $ |
| 3,750 |
|
| $ |
| 7,500 | ||||
The annual retainer fee, including fees for chairing the Board and committees, will be paid in cash unless a director instead elects to receive the fair value equivalent in stock options.
We also will continue to reimburse our non-employee directors for reasonable travel and other expenses incurred in connection with attending our board of directors and committee meetings.
On April 30, 2017, the board of directors approved a new stock option grant to each non-employee director, except for Jean-Francois Pariseau. Pursuant to this grant, each non-employee director, except for Jean-Francois Pariseau, received under the Existing Stock Option Plan, an option to purchase 2,000 common shares at an exercise price of $120.36 per share, subject to a 1-for- stock split. Each of these options will vest as to one-third of the shares of our common shares underlying such option on each anniversary of the date of this offering until the third anniversary of the date of this offering, subject to the non-employee director’s continued service as a director. Further, each non-employee director, except for Jean-Francois Pariseau, also received under the Existing Stock Option Plan, an option to purchase 1,000 common shares at an exercise price of $120.36 per share, subject to a 1-for- stock split. Each of these options will vest in full on the one-year anniversary of the date of this offering, subject to the non-employee director’s continued service as a director.
In addition, under our director compensation program to be effective on the effective date of this offering, each non-employee director will receive under the 2017 Omnibus Plan, upon his or her initial election to our board of directors, an option to purchase 2,000 common shares, subject to a 1-for- stock split. Each of these options will vest as to one-third of the shares of our common shares underlying such option on each anniversary of the grant date until the third anniversary of the grant date, subject to the non-employee director’s continued service as a director. Further, on the date of the first board meeting held after each annual meeting of stockholders, each non-employee director will receive, under the 2017 Omnibus Plan, an option to purchase 1,000 common shares, subject to a 1-for- stock split. Each of these options will vest in full on the one-year anniversary of the grant date unless otherwise provided at the time of grant, subject to the non-employee director’s continued service as a director. All options issued to our non-employee directors under our director compensation program will be issued at exercise prices equal to the fair market value of our common
135
shares on the date of grant and will become exercisable in full upon a change in control of our Company.
We do not pay any compensation to our Chief Executive Officer in connection with her service on our board of directors. The compensation that we pay to our Chief Executive Officer is discussed previously in the “Executive Compensation” section.
Existing Stock Option Plan
Our Existing Stock Option Plan provides for the granting of stock options to officers, directors, employees and consultants of ours and our affiliates. The purpose of the Existing Stock Option Plan is to develop the interest and incentive of our eligible employees, directors, officers and consultants in our growth and development by giving eligible participants an opportunity to purchase common shares of our stock, providing incentives thereby advancing our interests, enhancing the value of the common shares for the benefit of all the shareholders and increasing our ability to attract, motivate and retain skilled and motivated individuals.
The following is a summary only, and is qualified in its entirety by the terms and conditions of the Existing Stock Option Plan, which is filed as an exhibit to the registration statement of which this prospectus forms a part.
Administration by the Board of Directors
The Existing Stock Option Plan is administered by our board of directors, which has sole and complete authority and discretion, subject to the express provisions of the Existing Stock Option Plan, to interpret the Existing Stock Option Plan, to prescribe, amend and rescind rules and regulations relating to it and to make all other determinations deemed necessary or advisable for the administration of the Existing Stock Option Plan. This includes the discretion of our board of directors to decide who will participate in the Existing Stock Option Plan, including directors, officers, employees or consultants (each a Participant). Our board of directors also has authority to delegate its duties to a committee of the board.
Expiry
Options granted under the Existing Stock Option Plan (Options), are non-transferable. Unless an earlier termination date is specified by the board and/or stock option agreement, each Option expires on the earlier of the following: (i) on the tenth anniversary of its date of grant and (ii) the time immediately following the closing of a merger or consolidation or other transaction in which greater than or equal to 50% of the voting power of shareholders of Clementia is transferred to a third party or a sale, lease, license, transfer or other disposition of all or substantially all of the assets or intellectual property rights of Clementia (except to an affiliate) (a liquidity event).
Exercise Price
Subject to the rules of any stock exchange upon which the Common Shares may become listed, the exercise price of common shares under any option will be determined by the Board.
Insider Participation Limit
The aggregate number of common shares issuable (or reserved for issuance) to Participants under the Existing Stock Option Plan is 315,837, subject to adjustment as described in the plan. If our common shares become listed and posted for trading on a major stock exchange in the United States, Canada, Europe or Asia, unless otherwise approved by holders of a majority of the voting shares, the aggregate number of common shares that may be reserved for issuance under the Plan will not exceed the maximum permitted by the rules and policies of such stock exchange.
136
Amendment Provisions
Subject to the provisions of our shareholders agreement, which will be terminated in connection with this offering, our board of directors has the discretion to amend, suspend, or terminate the Existing Stock Option Plan, although such changes may not alter or impair the rights or obligations arising from any Option previously granted.
Termination, Resignation, Death, etc.
Options granted under the Existing Stock Option Plan are evidenced by a stock option agreement entered between us and the Participant. All vested and unvested options granted under the plan terminate immediately if a Participant is dismissed with cause. If, at any time, a Participant ceases to be an employee as a result of the Participant’s death, disability, or termination for any reason other than for cause, the Options granted to the Participant and vested as of the Termination Date (as defined therein) remain exercisable for 90 days after the Termination Date, unless such vested Options expire sooner in accordance with their terms. All unvested Options granted to the Participant will expire. Where, in the case of a consultant, the Participant’s consulting agreement terminates by reason of the Participant’s death, disability, termination for any reason other than for breach of the consulting agreement, or voluntary termination by the Participant, then any Options granted to the Participant and vested as of the Termination Date, or as of the date of the death or disability of the participant, as the case may be, remain exercisable for 30 days following the Termination Date, or the date of the death or disability of the Participant, as the case may be, unless such vested Options expire earlier in accordance with their terms. All unvested Options granted to the Participant will expire. Where, in the case of a consultant, the Participant’s consulting agreement is terminated for breach or for cause, then all vested and unvested Options granted to the Participant will expire. If a Participant ceases to be a director or officer, the Options granted to the Participant and vested as of the Termination Date may be exercised by the Participant pursuant to the same terms and conditions described above, as if the director or officer were a consultant unless such director or officer continues as a consultant or full time employee of the company.
Change of Control
The Existing Stock Option Plan provides that in the event that the Company or its shareholders accept an offer in respect of a transaction that will result in a Liquidity Event, the board of directors, without the consent of any Participant, shall take such steps as are necessary or desirable to cause the conversion or exchange of any outstanding Options into or for, rights or other securities of substantially equivalent value (or greater value), as determined by the board of directors in its discretion, in any entity participating in or resulting from a Liquidity Event (an Alternative Award). If no Alternative Awards are available, the board of directors shall, without the consent of any Participant, provide that all unvested options vest immediately prior to the consummation of the transaction constituting the Liquidity Event. Unless otherwise determined by the board of directors at or after the time of grant, any Participant whose service is terminated by his or her employer for any reason other than for Cause within 12 months following the consummation of a Liquidity Event, then with respect to all Options granted to the Participant prior to the date of the consummation of the Liquidity Event which were still outstanding at the time of such termination of service, all such unvested Options shall vest and to the extent exercisable shall remain exercisable until the earlier of the one-year anniversary of such termination of service or until the Option’s normal expiration date.
Maximum Limit
The aggregate number of Common Shares that may be issued pursuant to the exercise of Options shall not exceed 315,837 Common Shares, subject to adjustments related to a reorganization of our capital, amalgamation, combination, merger, or similar events.
137
Option Grant Summary
The following table sets forth the outstanding option-based awards outstanding for each of our directors and officers as at March 31, 2017, subject to a 1-for- stock split:
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
Name and Office Held | Number of | Option | Option | Value of | ||||||||||||||||||||||||
David P. Bonita, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Chairman of the Board, Director(2) |
|
| — |
|
| — |
|
| — |
|
| — | ||||||||||||||||
Jean-Francois Pariseau, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Chairman of the Compensation Committee, Director(2) |
|
| — |
|
| — |
|
| — |
|
| — | ||||||||||||||||
David Mott, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Director(2) |
|
| — |
|
| — |
|
| — |
|
| — | ||||||||||||||||
Robert Heft, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Director(2) |
|
| 3,336 |
|
| $ |
| 3.50 |
|
| June 17, 2023 |
|
| |||||||||||||||
|
| 1,228 |
|
| $ |
| 3.50 |
|
| August 27, 2024 |
|
| ||||||||||||||||
Allan Mandelzys, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Chairman of the Audit Committee, Director(2) |
|
| 6,672 |
|
| $ |
| 3.50 |
|
| June 17, 2023 |
|
| |||||||||||||||
|
|
| 1,228 |
|
| $ |
| 3.50 |
|
| August 27, 2024 |
|
| |||||||||||||||
Francois Nader, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Director(2) |
|
| 3,336 |
|
| $ |
| 3.50 |
|
| February 20, 2024 |
|
| |||||||||||||||
|
| 1,228 |
|
| $ |
| 3.50 |
|
| August 27, 2024 |
|
| ||||||||||||||||
Clarissa Desjardins, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
President, Chief Executive Officer, Director(3) |
|
| 33,364 |
|
| $ |
| 3.50 |
|
| June 17, 2023 |
|
| |||||||||||||||
|
|
| 27,479 |
|
| $ |
| 3.50 |
|
| August 27, 2024 |
|
| |||||||||||||||
Michael Singer, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Chief Financial Officer, Corporate Secretary(4) |
|
| 15,250 |
|
| $ |
| 8.33 |
|
| April 20, 2025 |
|
| |||||||||||||||
Donna Roy Grogan, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Chief Medical Officer |
|
| 6,488 |
|
| $ |
| 3.50 |
|
| September 23, 2023 |
|
| |||||||||||||||
|
| 18,107 |
|
| $ |
| 3.50 |
|
| August 27, 2024 |
|
| ||||||||||||||||
Jeffrey Packman, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Chief Development Officer |
|
| 1,911 |
|
| $ |
| 3.50 |
|
| February 20, 2024 |
|
| |||||||||||||||
|
|
| 12,174 |
|
| $ |
| 3.50 |
|
| August 27, 2024 |
|
| |||||||||||||||
Eric Grinstead, |
|
|
|
|
|
|
|
| ||||||||||||||||||||
Chief Commercial Officer |
|
| 11,122 |
|
| $ |
| 3.50 |
|
| February 20, 2024 |
|
| |||||||||||||||
|
| 19,300 |
|
| $ |
| 3.50 |
|
| August 27, 2024 |
|
| ||||||||||||||||
(1) | This column represents the in-the-money value of all unexercised options based upon the difference between an assumed initial public offering price of $ per share, the midpoint of the estimate price range set forth on the cover page of this prospectus, and the exercise price of the options. An option is in-the-money if the market price of common shares is greater than the exercise price. The actual value of the awards realized by each of the directors and officers, if any, will depend on the price of our common shares at the time of exercise. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) | On April 30, 2017, the board of directors approved a new stock option grant to each non-employee director, except for Jean-Francois Pariseau. Pursuant to this grant, each non-employee director, except for Jean-Francois Pariseau, received under the Existing Stock Option Plan, an option to purchase 2,000 common shares at an exercise price equal of $120.36 per share, subject to a 1-for- stock split. Each of these options will vest as to one-third of the shares of our common shares underlying such option on each anniversary of the date of this offering until the third anniversary of the date of this offering, subject to the non-employee director’s continued service as a director. Further, each non-employee director, except for Jean-Francois Pariseau, also received | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
138
| under the Existing Stock Option Plan, an option to purchase 1,000 common shares at an exercise price equal of $120.36 per share, subject to a 1-for- stock split. Each of these options will vest in full on the one-year anniversary of the date of this offering, subject to the non-employee director’s continued service as a director. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) | On April 30, 2017, Clarissa Desjardins received an equity based award of 5,000 stock options at an exercise price of $120.36 per share, subject to a 1-for- stock split under the Existing Stock Option Plan. These stock options will expire 10 years after their initial grant date and commence vesting upon closing of the initial public offering over a period of 4 years. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) | On April 30, 2017, Michael Singer received an equity based award of 5,800 stock options at an exercise price of $120.36 per share, subject to a 1-for- stock split under the Existing Stock Option Plan. These stock options will expire 10 years after their initial grant date and commence vesting upon closing of the initial public offering over a period of 4 years. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Following the adoption of our 2017 Omnibus Plan by our board prior to the completion of this offering, all equity-based awards will be granted under the 2017 Omnibus Plan described below.
2017 Omnibus Plan
Prior to the completion of this offering, our board of directors will adopt and our shareholders will approve the 2017 Omnibus Plan and, following its adoption, all equity-based awards are granted under the 2017 Omnibus Plan. The following summary describes the terms of the 2017 Omnibus Plan. This summary of the 2017 Omnibus Plan is not a complete description of all provisions of the 2017 Omnibus Plan and is qualified in its entirety by reference to the 2017 Omnibus Plan, which is filed as an exhibit to the registration statement of which this prospectus is a part.
Administration
The 2017 Omnibus Plan will be administered by our compensation committee. Our compensation committee will have the authority to, among other things, determine eligibility for awards to be granted, determine, modify or waive the type or types of, form of settlement (whether in cash, our common shares or other property) of, and terms and conditions of awards, to accelerate the vesting or exercisability of awards, to adopt rules, guidelines and practices governing the operation of the 2017 Omnibus Plan as the compensation committee deems advisable, to interpret the terms and provisions of the 2017 Omnibus Plan and any award agreement, and to otherwise do all things necessary or appropriate to carry out the purposes of the 2017 Omnibus Plan. The compensation committee’s decisions with respect to the 2017 Omnibus Plan and any award under the 2017 Omnibus Plan will be binding upon all persons.
Eligibility
Our key employees and non-employee directors on our board of directors who, in the opinion of the compensation committee, have the capacity to contribute to our and our affiliates’ success are eligible to participate in the 2017 Omnibus Plan.
Authorized Shares
Subject to adjustment, as described below, the maximum number of our common shares that will be available for issuance under the 2017 Omnibus Plan is shares, equal to 6% of the number of shares of our common shares outstanding on the effective date of the offering, in addition to 46,623 remaining options from our Existing Stock Option Plan, all of which may be delivered in satisfaction of incentive stock options (ISOs), awarded under the 2017 Omnibus Plan. Common shares issued under the 2017 Omnibus Plan may be shares held in treasury or authorized but unissued shares of the Company not reserved for any other purpose. The number of shares reserved for issuance under the 2017 Omnibus Plan will automatically increase by an annual amount to be added the first day of each fiscal year, beginning January 1, 2018 and continuing until, and including, the fiscal year ending December 31, 2027, equal to the lower of 4% of the number of shares of our common shares
139
outstanding as of December 31 of the prior calendar year and an amount determined by our board of directors. The increase in the number of shares available for issuance will be registered each year.
Common shares subject to an award that for any reason expires without having been exercised, is cancelled, forfeited or terminated or otherwise is settled without the issuance of shares will again be available for grant under the 2017 Omnibus Plan. The grant of a tandem award of a stock option and a stock appreciation right (SAR) will reduce the number of our common shares available for awards under the 2017 Omnibus Plan by the number of shares subject to the related stock option (and not as to both awards). To the extent consistent with applicable legal requirements (including applicable stock exchange requirements), common shares issued under awards of an acquired company that are converted, replaced or adjusted in connection with the acquisition will not reduce the number of shares available for awards under the 2017 Omnibus Plan.
Types of Awards
The 2017 Omnibus Plan will provide for awards of stock options, SARs, restricted stock, unrestricted stock, stock units (including RSUs), performance awards, deferred share units, elective deferred share units and other awards convertible into or otherwise based on our common shares. Eligibility for stock options intended to be ISOs will be limited to our employees. Dividend equivalents may also be provided in connection with an award under the 2017 Omnibus Plan.
• | Stock options and SARs.The exercise price of a stock option, and the base price against which a SAR is to be measured, may not be less than the fair market value (or, in the case of an ISO granted to a 10% shareholder, 110% of the fair market value) of our common shares on the date of grant. Our compensation committee will determine the time or times at which stock options or SARs become exercisable and the terms on which such awards remain exercisable. The maximum term of stock options and SARs is ten years, except that if a participant still holding an outstanding non-statutory stock option or SAR ten years from the date of grant (or in the case of such an award with a maximum term of less than ten years, such maximum term) is prohibited by applicable law or written policy of the Company applicable to similarly situated employees from engaging in any open-market sales of our common shares, and if at such time the stock is publicly traded, the maximum term of the award will instead by deemed to expire on the 30th day following the date the participant is no longer prohibited from engaging in such open market sales. A SAR that is granted in tandem with a stock option will become vested and exercisable on the same date or dates as the related stock option and may only be exercised upon the surrender of the right to exercise the related option for an equivalent number of our common shares. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Restricted and unrestricted stock.A restricted stock award is an award of our common shares subject to forfeiture restrictions, while an unrestricted stock award is not subject to such restrictions. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Restricted stock units.An RSU award is an award denominated in our common shares that entitles the participant to receive our common shares or cash measured by the value of our common shares in the future. The delivery of our common shares or cash under an RSU award may be subject to the satisfaction of performance conditions or other vesting conditions. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Performance awards.A performance award is an award the vesting, settlement or exercisability of which is subject to specified performance criteria. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Deferred share units.A deferred share unit is an award of a notional investment in our common shares reflected on an unfunded, book-entry account maintained by the Company. Deferred share units may be subject to performance conditions or other vesting conditions. Each deferred share unit may be settled, at the compensation committee’s discretion, in a share of our common shares, cash measured by the value of a share of our common shares or a combination thereof. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Elective deferred share unit.An elective deferred share unit is an award of a notional investment in a share of our common shares reflected on an unfunded, book-entry account maintained by the Company, where the number of elective deferred share units awarded to a participant is determined by the amount of a participant’s elective deferral of his or her eligible compensation and/or bonus divided by the fair market value of a share of our common shares on the date of | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
140
| the payment of such amounts to be deferred, in accordance with and subject to the conditions of the 2017 Omnibus Plan. Elective deferred share units are at all times fully vested and may be settled, at the compensation committee’s discretion, in our common shares, cash measured by the value of our common shares or a combination thereof. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Other awards.Other awards are awards that are convertible into or otherwise based on our common shares. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Performance Awards
The 2017 Omnibus Plan will provide for the grant of performance awards that will be made based upon, and subject to achieving, performance objectives. Performance objectives with respect to those awards that are intended to qualify as “performance-based compensation” for purposes of Section 162(m) of the U.S. Internal Revenue Code of 1986, as amended (the Code), to the extent applicable, will be limited to an objectively determinable measure or measures of performance relating to any, or any combination, of the following (measured either in absolute terms or relative to the performance of one or more similarly situated companies or a published index covering the performance of a number of companies and determined either on a consolidated basis or, as the context permits, with respect to one or more business units, divisions, subsidiaries, products, projects or geographic locations, or on combinations thereof): stockholder return; sales; assets; expenses; earnings before or after deduction for all or any portion of interest, taxes, depreciation, amortization or equity expense, whether or not on a continuing operation or an aggregate or per share basis; return on equity, investment, capital, capital employed or assets; operating earnings; one or more operating ratios; operating income or profit, including on an after-tax basis; net earnings; net income; income; earnings per share; revenues; stock price; economic value added; cash flow; expenses; capital expenditures; working capital levels; borrowing levels, leverage ratios or credit rating; gross profit; market share; workplace safety goals; workforce satisfaction and diversity goals; employee retention; completion of key projects; implementation and achievement of synergy targets; joint ventures and strategic alliances, licenses or collaborations; sales of particular products or services; customer acquisition or retention; acquisitions and divestitures (in whole or in part); spin-offs, split-ups and the like; reorganizations; recapitalizations, restructurings, financings (issuance of debt or equity); or refinancings. When establishing performance objectives, the compensation committee may exclude any or all unusual items as determined by the board of directors, including, without limitation, the charges or costs associated with closures and restructurings of the Company or an affiliate, discontinued operations, extraordinary items, capital gains and losses, dividends, share repurchase, other unusual or non-recurring items, and the cumulative effects of accounting changes.
To the extent consistent with Section 162(m), if applicable, the compensation committee may provide that one or more performance objectives applicable to awards intended to qualify for the performance-based compensation exception under Section 162(m) will be adjusted in an objectively determinable manner to reflect events occurring during the performance period that affect the applicable performance objective.
Individual Limits
The maximum aggregate number of our common shares subject to all awards under the 2017 Omnibus Plan (including stock options, SARs, restricted stock, unrestricted stock, RSUs, performance awards, deferred share units, elective deferred share units and any other award under the 2017 Omnibus Plan) that may be granted to any participant in the 2017 Omnibus Plan in any calendar year will be 100,000 shares, subject to a 1-for- stock split.
A participant who is a non-employee director on our board of directors, in any calendar year, may not receive initial awards with respect to the greater of an aggregate of 3,000 common shares subject to a 1-for- stock split, or $0.3 million and annual awards with respect to the greater of an aggregate of 2,000 common shares, subject to a 1-for- stock split, or $0.2 million in aggregate grant date fair value. In addition, the aggregate number of our common shares issuable under outstanding awards to non-employee directors, at any time, may not exceed 1% of our common shares that are issued and
141
outstanding. These limitations, however, will not apply to any award or common shares granted pursuant to a non-employee director’s election to receive our common shares in lieu of cash fees.
Vesting; Termination of Employment or Service
Our compensation committee has the authority to determine the vesting schedule applicable to each award, and to accelerate the vesting or exercisability of any award. Our compensation committee will determine the effect of termination of employment or service on an award. Unless otherwise provided by our compensation committee, upon a termination of a participant’s employment or service under the following circumstances the following treatment will apply:
• | Death.All time-based awards will immediately vest and performance awards will vest at the target level of performance. Awards subject to exercise will remain exercisable until the earlier of the one-year anniversary of the participant’s death or the award’s normal expiration date. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Disability.All time-based awards will immediately vest and performance awards will remain eligible to vest to the extent the applicable performance goals are achieved. Awards subject to exercise will remain exercisable until the earlier of the one-year anniversary of the participant’s termination of employment or service due to disability or the award’s normal expiration date. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Retirement.All time-based awards will be deemed vested with respect to a portion of the award determined by multiplying the number of shares underlying the award by a fraction, the numerator of which is the number of days from the grant date to the date of retirement and the denominator of which is the number of days from the grant date to the final vesting date of the award. Performance awards will remain eligible to vest, to the extent the applicable performance goals are achieved, with respect to a portion of the award determined by multiplying the number of shares underlying the award by a fraction, the numerator of which is the number of days from the beginning of the applicable performance period through the date of retirement and the denominator of which is the number of days in the performance period. Awards subject to exercise will remain exercisable until the earlier of the one-year anniversary of the Participant’s “retirement” or the award’s normal expiration date. Unless otherwise defined in a participant’s employment or award agreement, under the 2017 Omnibus Plan, “retirement” means a termination of the participant’s employment or service at or after the time the participant reaches age 65. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Resignation by Participant.All unvested awards will be forfeited. Awards subject to exercise will remain exercisable until the earlier of the 90 day anniversary of the participant’s termination of employment or the award’s normal expiration date. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Termination by the Company for Cause.All stock options and SARs, whether vested or unvested, will be forfeited and all other awards, to the extent unvested, will be forfeited. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Termination by the Company other than for Cause.Awards other than RSUs, restricted stock awards and performance awards will be forfeited to the extent then unvested. Awards subject to exercise, to the extent vested, will remain exercisable until the earlier of the 90 day anniversary of the participant’s termination of service or the award’s normal expiration date. RSUs, restricted stock awards and performance awards will be deemed vested with respect to a portion of the award determined by multiplying the number of shares underlying the award by a fraction, the numerator of which is the number of days from the grant date to the six-month anniversary of the date of the participant’s termination of employment or service and the denominator of which is the number of days from the grant date to third anniversary of the grant date, with the vesting of such performance awards to be subject to performance assessed as of the date of such termination of employment or service. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Termination by the Company other than for Cause within 12 months following a Change in Control.To the extent granted prior to the time of the change in control and then outstanding at the time of termination, all time-based awards will vest and performance awards will vest at the target level of performance. Awards subject to exercise will remain exercisable until the earlier of the one-year anniversary of the participant’s termination of employment or service due to disability or the award’s normal expiration date. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
142
Non-Transferability of Awards
Awards under the 2017 Omnibus Plan may not be sold, assigned, transferred, pledged or otherwise encumbered other than by the laws of succession or descent and distribution or, in the case of awards other than ISOs, to a permitted assign (within the meaning of the National Instrument 45-106 Prospectus and Registration Exemptions of the Canadian Securities Administrators).
Recovery of Compensation
Our compensation committee may cancel, rescind, withhold or otherwise limit or restrict any award at any time under the 2017 Omnibus Plan if the participant is not in compliance with the provisions of the 2017 Omnibus Plan or any award thereunder or if the participant breaches any agreement with our Company with respect to non-competition, non-solicitation or confidentiality. Our compensation committee also may recover any award or payments or gain in respect of any award under the 2017 Omnibus Plan in accordance with any applicable Company recoupment policy or as otherwise required by applicable law or applicable stock exchange listing standards.
Change in Control
In the event of certain consolidations, mergers or similar transactions, certain sales or other disposition of our common shares, certain liquidations of the Company or sales or dispositions of all or substantially all of the Company’s assets, or members of our board of directors cease to constitute a majority of our board of directors or the board of directors of a successor entity, our compensation committee may provide for the conversion or exchange of outstanding awards for new awards, or, if no equivalent awards are available, for the accelerated vesting or delivery of shares under awards, or for a cash-out of outstanding awards.
Certain Adjustments
In the event of an extraordinary dividend, stock dividend, stock split or share combination (including a reverse stock split) or any recapitalization, business combination, merger, consolidation, spin-off, exchange of shares, liquidation or dissolution of the Company or other similar transaction affecting our common shares our board of directors will make adjustments as it determines in its sole discretion to the number and kind of shares available for issuance under the 2017 Omnibus Plan, the maximum number of shares that may be issued upon the exercise of ISOs, the annual per-participant share limits, the number, class, exercise price (base value), performance objectives applicable to outstanding awards and any other terms of outstanding awards affected by such transaction. Our compensation committee may also make adjustments of the type described in the preceding sentence to take into account distributions and events other than those listed above if it determines that adjustments are appropriate to avoid distortion in the operation of the 2017 Omnibus Plan.
Term
The 2017 Omnibus Plan became effective on the date it was adopted by our board and will terminate on the tenth anniversary of such date, unless terminated earlier by our board of directors.
Amendment; Termination
Our compensation committee may amend the 2017 Omnibus Plan or outstanding awards, or terminate the 2017 Omnibus Plan as to future grants of awards, except that our compensation committee will not be able to alter the terms of an award if it would affect materially and adversely a participant’s rights under the award without the participant’s consent (unless expressly provided in the 2017 Omnibus Plan or the right to alter the terms of an award was expressly reserved by our compensation committee at the time the award was granted). Shareholder approval will be required for any amendment to the 2017 Omnibus Plan that increases the number of our common shares available for issuance under the 2017 Omnibus Plan or the individual award limitations specified in the 2017 Omnibus Plan (except with respect to certain adjustments described above), modifies the class of
143
persons eligible for participation in the 2017 Omnibus Plan, allows options to be issued with an exercise price below fair market value on the date of grant, extends the term of any award granted under the 2017 Omnibus Plan beyond its original expiration date, permits an award to be exercisable beyond 10 years from its grant date (except where an expiration date would have fallen within a blackout period of the Company), permits awards to be transferred other than for normal estate settlement purposes, or deletes or reduces the range of amendments which require approval of the holders of voting shares of the Company, or to the extent required by law.
Employee Share Purchase Plan
Prior to the completion of this offering, we will adopt an employee share purchase plan (ESPP) pursuant to which eligible employees will be able to acquire our common shares at a discount from the average market price on the purchase date in a convenient and systematic manner through payroll deductions. The ESPP is intended to qualify as an “employee stock purchase plan” within the meaning of Section 423 of the Code for employees who are United States taxpayers. The following discussion is qualified in its entirety by the full text of the ESPP.
Unless otherwise determined by our board of directors, participation in the ESPP will be open to our employees in Canada and the United States who are customarily employed for at least 20 hours per week. Participation in the ESPP will be voluntary. Eligible employees will be able to contribute up to 15% of their gross base earnings for purchases under the ESPP through regular payroll deductions. The maximum number of common shares issued to insiders within any six month period, or issuable to insiders at any time, under the ESPP and all private placements must not exceed 10% of the number of common shares issued and outstanding at that time. No employee will be eligible for the grant of any purchase rights under the ESPP if immediately after such rights are granted, such employee has voting power over 5% or more of our outstanding common shares measured by vote or value under Section 424(d) of the Code. In addition, no employee may purchase shares under the ESPP at a rate in excess of US$25,000 worth of our common shares (determined on the grant date of the purchase right) for each year such purchase right is outstanding.
The ESPP will be implemented through a series of offerings under which eligible employees are granted rights to purchase our common shares at the end of specified purchase periods. We currently expect to hold offerings consisting of a single six-month purchase period commencing on January 1 and July 1 of each calendar year, with a single purchase date at the end of the purchase period on June 30 and December 31 of each calendar year. However, our compensation committee may establish different offerings and purchase periods from time to time, which may have a duration of between three months to twenty-four months. No offering may commence prior to July 1, 2017, unless otherwise determined by our compensation committee. Common shares purchased under the ESPP will be issued from treasury at a purchase price equal to 85% of the average market price of the common shares on such date, all in accordance with applicable laws and the terms and conditions of the ESPP. For the purposes of the ESPP, the average market price of the common shares as at a given date shall be the weighted average trading price on the trading day immediately preceding such date.
The number of common shares reserved for issuance under the ESPP will not exceed common shares, plus the number of common shares that are automatically added on January 1st of each year, commencing on (and including) January 1, 2018 and ending on (and including) January 1, 2027, in an amount equal to the lesser of (i) 1% of the total number of common shares issued and outstanding on December 31st of the preceding calendar year, and (ii) common shares. No rights to purchase common shares may be issued under the plan from and after the tenth anniversary of the date the plan becomes effective, unless otherwise approved by our shareholders.
The ESPP will be administered by the compensation committee of our board of directors. The compensation committee will have the authority, in the event the common shares are subdivided or consolidated, or in the event the common shares will be exchanged for shares of another issuer in the context of a reorganization, split-up, liquidation, recapitalization or similar transaction, to determine appropriate equitable adjustments, if any, to be made under the ESPP, including adjustments to the number of common shares which have been authorized for issuance under the ESPP.
144
In the event of certain significant corporate transactions such as an acquisition, merger or sale of all or substantially all of our assets, then either (i) a participant’s then-outstanding purchase right shall be continued or substituted for by the surviving or acquiring entity, or (ii) such purchase right shall be terminated in exchange for a cash payment equal to the fair market value of a number of our common shares on the date of such transaction that the participant’s accumulated payroll deductions as of the date of the transaction could purchase, determined with reference only to the first business day of the applicable purchase period, less the result of multiplying such number of shares by such purchase price.
Our board of directors will have the right to amend or terminate the ESPP, in whole or in part, at any time, subject to applicable laws and requirements of any stock exchange or governmental or regulatory body (including any requirement for shareholder approval). Subject to certain exceptions, our board of directors will be entitled to make amendments to the ESPP without shareholder approval.
Rule 10b5-1 Sales Plans
Our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, in which they will contract with a broker to buy or sell shares of our common shares on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or officer when entering into the plan, without further direction from the director or officer. The director or officer may amend or terminate the plan in some circumstances. Our directors and executive officers may buy or sell additional shares outside of a Rule 10b5-1 plan when they are not in possession of material, nonpublic information.
145
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following is a description of the material terms of those transactions with related parties to which we are party and which we are required to disclose pursuant to the disclosure rules of the SEC.
Private Placement
Class A Preferred Shares Financing
Between April 10, 2013 and March 18, 2015, we issued an aggregate of 1,118,415 Class A preferred shares for an aggregate purchase price of approximately $32,708,059. The table below sets forth the number of these Class A preferred shares. Upon completion of this offering, each Class A preferred share will convert into one common share, subject to adjustment to account for the 1-for- stock split.
|
|
|
|
| ||||||||||
NAME | NUMBER OF SHARES OF | AGGREGATE PURCHASE | ||||||||||||
BDC Capital, Inc.(1) |
|
| 366,096 |
|
| $ |
| 10,706,490 | ||||||
OrbiMed Private Investments IV, LP(2) |
|
| 746,042 |
|
| $ |
| 21,817,998 | ||||||
Francois Nader(3) |
|
| 1,883 |
|
| $ |
| 55,068 | ||||||
Allan Mandelzys(4) |
|
| 1,500 |
|
| $ |
| 43,868 | ||||||
Clarissa Desjardins(5) |
|
| 2,394 |
|
| $ |
| 70,013 | ||||||
Michael Singer(6) |
|
| 500 |
|
| $ |
| 14,622 | ||||||
(1) | BDC Capital, Inc. is a 5% shareholder. In addition, one of our directors, Jean-François Pariseau is a partner in the Healthcare Fund at BDC Venture Capital. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) | OrbiMed Private Investments IV, LP is a 5% shareholder. In addition, one of our directors, Dr. David P. Bonita, is a private equity partner at OrbiMed Advisors LLC. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) | Dr. Nader is one of our directors. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) | Dr. Mandelzys is one of our directors. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) | Dr. Desjardins is one of our NEOs and directors. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) | Mr. Singer is one of our NEOs. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Class B Preferred Shares Financing
In June 2015, we issued an aggregate of 485,823 Class B preferred shares for an aggregate purchase price of approximately $59,999,141. The table below sets forth the number of these Class B preferred shares. Upon completion of this offering, each Class B preferred share will convert into one common share, subject to adjustment to account for the 1-for- stock split.
146
|
|
|
|
| ||||||||||
NAME | NUMBER OF SHARES OF | AGGREGATE PURCHASE | ||||||||||||
OrbiMed Private Investments IV, LP(1) |
|
| 109,523 |
|
| $ |
| 13,526,091 | ||||||
BDC Capital, Inc.(2) |
|
| 52,420 |
|
| $ |
| 6,473,870 | ||||||
Entities Affiliated with New Enterprise Associates 15, L.P.(3) |
|
| 161,943 |
|
| $ |
| 19,999,961 | ||||||
Francois Nader(4) |
|
| 1,983 |
|
| $ |
| 244,900 | ||||||
Janus Global Life Sciences Fund |
|
| 44,534 |
|
| $ |
| 5,499,949 | ||||||
Entities Affiliated with RA Capital Healthcare Fund, L.P. |
|
| 40,484 |
|
| $ |
| 4,999,774 | ||||||
UCB BioPharma |
|
| 24,291 |
|
| $ |
| 2,999,939 | ||||||
Rock Springs Capital Master Fund LP |
|
| 24,291 |
|
| $ |
| 2,999,939 | ||||||
Entities Affiliated with EcoR1 Capital Fund L.P. |
|
| 16,194 |
|
| $ |
| 1,999,959 | ||||||
Franklin Berger |
|
| 1,983 |
|
| $ |
| 244,900 | ||||||
Entities Affiliated with Leerink Holdings LLC |
|
| 8,097 |
|
| $ |
| 999,979 | ||||||
Sara Nayeem |
|
| 80 |
|
| $ |
| 9,880 | ||||||
(1) | BDC Capital, Inc. is a 5% shareholder. In addition, one of our directors, Jean-François Pariseau is a partner in the Healthcare Fund at BDC Venture Capital. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) | OrbiMed Private Investments IV, LP is a 5% shareholder. In addition, one of our directors, David P. Bonita, is a private equity partner at OrbiMed Advisors LLC. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) | New Enterprise Associates 15, L.P. is a 5% shareholder. In addition, one of our directors, David Mott, is a General Partner at New Enterprise Associates 15, L.P. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) | Dr. Nader is one of our directors. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Class C Preferred Shares Financing
In March 2017, we issued an aggregate of 70,176 Class C preferred shares for an aggregate purchase price of approximately $10,000,080. The Class C preferred shares are held by a single investor, Fonds de Solidarité des Travailleurs du Québec, which is not a 5% shareholder or an otherwise related party. Upon completion of this offering, each Class C preferred share will convert into one common share, subject to adjustment to account for the 1-for- stock split.
Registration Rights
We are a party to a second amended and restated registration rights agreement (the Registration Rights Agreement) dated as of March 16, 2017, with certain holders of our preferred shares, including our 5% stockholders and our director Francois Nader. The Registration Rights Agreement provides certain registration rights and other rights in respect of the preferred shares, as well as the common shares issuable upon conversion of the preferred shares filing. See “Description of Share Capital—Registration Rights” for additional information regarding these registration rights.
Indemnity Agreements
We have entered into indemnity agreements with each of our current directors and officers undertaking to indemnify each of them to the fullest extent permitted by law from and against all liabilities, costs, charges and expenses incurred as a result of actions in the exercise of their duties as a director or officer. See “Description of Share Capital—Limitations on Liability and Indemnification of Directors and Officers.”
Employment Agreements
We intend to enter into new employment agreements with our executive officers as described in “Executive and Director Compensation—Employment Agreements.”
147
Equity Awards
Since our inception, we have granted equity awards to all of our officers. We describe our equity plans under “Executive and Director Compensation—Stock Option Plan.”
Related Person Transaction Policy
Following the completion of this offering, our audit committee will have the primary responsibility for reviewing and approving or disapproving “related party transactions,” which are transactions between us and related persons in which the aggregate amount involved exceeds or may be expected to exceed $120,000 and in which a related person has or will have a direct or indirect material interest. Upon the completion of this offering, our policy regarding transactions between us and related persons will provide that a related person is defined as a director, executive officer, nominee for director or greater than 5% beneficial owner of our common shares, in each case since the beginning of the most recently completed year, and any of their immediate family members.
148
The following table and accompanying footnotes sets forth, as of March 31, 2017, information regarding beneficial ownership of our common shares by:
• | each person, or group of affiliated persons, known by us to beneficially own more than 5% of our common shares; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | each of our executive officers; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | each of our directors; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | all of our executive officers and directors as a group. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Beneficial ownership is determined in accordance with the rules of the SEC, which generally attribute beneficial ownership of securities to persons who possess sole or shared voting or investment power with respect to those securities, including options and warrants that are currently exercisable or exercisable within 60 days of March 31, 2017. Common shares subject to options and warrants currently exercisable or exercisable within 60 days of March 31, 2017 are deemed to be outstanding for computing the percentage ownership of the person holding these options and/or warrants and the percentage ownership of any group of which the holder is a member, but are not deemed outstanding for computing the percentage of any other person.
Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons named in the table below have sole voting and investment power with respect to all common shares shown that they beneficially own, subject to community property laws where applicable. The table is based upon information supplied to us by officers, directors and principal shareholders.
Our calculation of the percentage of beneficial ownership prior to this offering is based on 2,004,205 shares, which represents the aggregate of all common shares issued and outstanding as at March 31, 2017, all preferred shares issued and outstanding as of March 31, 2017 and shares issuable pursuant to outstanding stock options that are exercisable within 60 days at March 31, 2017. Beneficial ownership representing less than 1% is denoted with an asterisk (*).
Unless otherwise indicated, the business address of each beneficial owner listed in the table below is c/o Clementia Pharmaceuticals Inc., 4150 Sainte-Catherine Street West, Suite 550, Montreal, Quebec, Canada H3Z 2Y5.
|
|
|
|
|
|
|
|
| |||||||||||||||
Name of Beneficial Owner | Number of | Number of | Percentage of shares | ||||||||||||||||||||
Before offering | After offering | ||||||||||||||||||||||
5% or greater shareholders |
|
|
|
|
|
|
|
| |||||||||||||||
OrbiMed Private Investments IV, LP(1) |
|
| 855,565 |
|
|
|
| 42.7 | % |
|
|
| % |
| |||||||||
BDC Capital Inc.(2) |
|
| 458,516 |
|
|
|
| 22.9 | % |
|
|
| % |
| |||||||||
Entities Affiliated with New Enterprise Associates 15, L.P.(3) |
|
| 161,943 |
|
|
|
| 8.1 | % |
|
|
| % |
| |||||||||
Executive officers and directors |
|
|
|
|
|
|
|
| |||||||||||||||
Clarissa Desjardins(4) |
|
| 114,053 |
|
|
|
| 5.7 | % |
|
|
|
| % |
| ||||||||
Eric Grinstead(5) |
|
| 16,552 |
|
|
|
| * |
|
| % |
| |||||||||||
Donna Roy Grogan(6) |
|
| 17,711 | * |
|
|
|
|
| * |
|
| % |
| |||||||||
Robert Heft(7) |
|
| 9,568 | * |
|
|
|
|
| * |
|
| % |
| |||||||||
Allan Mandelzys(8) |
|
| 12,405 | * |
|
|
|
|
| * |
|
| % |
| |||||||||
Francois Nader(9) |
|
| 10,318 | * |
|
|
|
|
| * |
|
| % |
| |||||||||
Jeffrey Packman(10) |
|
| 12,414 | * |
|
|
|
|
| * |
|
| % |
| |||||||||
Michael Singer(11) |
|
| 10,142 | * |
|
|
|
|
| * |
|
| % |
| |||||||||
All executive officers and directors as a group (8 persons) |
|
| 208,445 |
|
|
|
| 10.4 | % |
|
|
| % |
| |||||||||
149
Notes:
(1) | Consists of 746,042 Class A preferred shares and 109,523 Class B preferred shares, all of which will be converted into common shares in connection with this offering. The mailing address of OrbiMed Private Investments IV, LP is 601 Lexington Anenue, 54th Floor, New York, NY 10022. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(2) | Consists of 40,000 common shares, 366,096 Class A preferred shares and 52,420 Class B preferred shares, all of which will be converted into common shares in connection with this offering. The mailing address of BDC Capital Inc. is 5 Place Ville-Marie, Suite 400 Montreal, Québec H3B 5E7. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(3) | Consists of 161,822 Class B preferred shares held by New Enterprise Associates 15 IV, L.P., and 121 Class B preferred shares held by its affiliate, New Enterprise Associates Ventures 2015, L.P., all of which will be converted into common shares in connection with this offering. The mailing address of the entities affiliated with New Enterprise Associates 15, L.P. is 1954 Greenspring Drive, Suite 600 Timonium, Maryland 21093. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(4) | Consists of 65,000 common shares, 2,394 Class A preferred shares, which will be converted into common shares in connection with this offering, and 46,659 shares issuable pursuant to outstanding stock options that are exercisable within 60 days of March 31, 2017. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(5) | Consists of 16,552 shares issuable pursuant to outstanding stock options that are exercisable within 60 days of March 31, 2017. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(6) | Consists of 5,827 common shares, and 11,884 shares issuable pursuant to outstanding stock options that are exercisable within 60 days of March 31, 2017. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(7) | Consists of 5,329 common shares held by Gren Trust, for which Dr. Heft serves as sole trustee, and 4,239 shares issuable pursuant to outstanding stock options that are exercisable within 60 days of March 31, 2017. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(8) | Consists of 3,400 common shares, 1,500 Class A preferred shares, which will be converted into common shares in connection with this offering, and 7,505 shares issuable pursuant to outstanding stock options that are exercisable within 60 days of March 31, 2017. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(9) | Consists of 4,752 common shares, 1,883 Class A preferred shares, which will be converted into common shares in connection with this offering, 1,983 Class B preferred shares, which will be converted into common shares in connection with this offering, and 3,683 shares issuable pursuant to outstanding stock options that are exercisable within 60 days of March 31, 2017. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(10) | Consists of 8,731 common shares and 3,683 shares issuable pursuant to outstanding stock options that are exercisable within 60 days of March 31, 2017. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(11) | Consists of 1,700 common shares, 500 Class A preferred shares, which will be converted into common shares in connection with this offering, and 7,943 shares issuable pursuant to outstanding stock options that are exercisable within 60 days of March 31, 2017. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(12) | Common share numbers included in footnotes (1) to (11) have been adjusted to account for the 1-for- stock split and preferred share numbers included in footnotes (1) to (11) have been adjusted to account for the 1-for- stock split. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
150
General
The following is a description of the material terms of our common and preferred shares as set forth in our articles of incorporation, as amended, and as further amended in connection with this offering, and certain related sections of the CBCA. For more detailed information, please see our articles of incorporation and amendments thereto, which are filed as exhibits to the registration statement of which this prospectus is a part.
As of March 31, 2017, we had 202,693 common shares outstanding, which were held by 12 shareholders of record which are subject to a 1-for- stock split. As of March 31, 2017, we had 1,118,415 Class A preferred shares outstanding, which were held by six shareholders of record, 485,823 Class B preferred shares outstanding, which were held by approximately 16 shareholders of record and 70,176 Class C preferred shares outstanding, which were held by one shareholder of record. On or immediately prior to the completion of this offering, each of our outstanding preferred shares will be converted into common shares as adjusted to account for the 1-for- stock split. Both our common and preferred shares have no par value.
In the last three years, we have amended and restated our articles of incorporation twice to create new classes of shares (in June 2015 to create our Class B preferred shares, and in March 2017 to create our Class C preferred shares). Since January 1, 2014, we have issued an aggregate of 168,391 stock options common shares under our Existing Employee Stock Option Plan; issued and sold 26,216 common shares to our directors, officers and employees under our equity compensation plans; issued 861,124 Class A preferred shares in connection with our Class A financing; issued 485,823 Class B preferred shares in connection with our Class B financing; and issued 70,176 Class C preferred shares in connection with our Class C financing.
Upon closing of this offering, our authorized share capital will consist of an unlimited number of common shares, no par value per share, of which will be issued and outstanding or which will be issued and outstanding if the overallotment option is exercised and an unlimited number of preferred shares, issuable in series, no par value per share, none of which will be issued and outstanding.
Common Shares
Under the articles of incorporation to be in effect upon the closing of this offering, the holders of our common shares will be entitled to one vote for each share held at any meeting of the shareholders. Subject to the prior rights of any holders of our preferred shares, the holders of our common shares will be entitled to receive dividends as and when declared by our board of directors. See “Dividend Policy.” Subject to the prior payment to any holders of our preferred shares, in the event of our liquidation, dissolution or winding-up or other distribution of our assets among our shareholders, the holders of our common shares will be entitled to share pro rata in the distribution of the balance of our assets. Holders of common shares will have no preemptive, redemption, conversion rights or other rights. The rights, preferences and privileges of the holders of common shares will be subject to and may be adversely affected by, the rights of the holders of any series of preferred shares that we may designate in the future.
Preferred Shares
Upon or immediately prior to the closing of this offering, our articles of incorporation will be amended to delete all references to our Class A preferred shares, Class B preferred shares and Class C preferred shares. Under our amended articles, we will be authorized to issue, without shareholder approval, an unlimited number of preferred shares, issuable in one or more series, and, subject to the provisions of the CBCA, having such designations, rights, privileges, restrictions and conditions, including dividend and voting rights, as our board of directors may determine, and such rights and privileges, including dividend and voting rights, may be superior to those of the common shares. The issuance of preferred shares, while providing flexibility in connection with possible acquisitions and
151
other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of our Company and might adversely affect the market price of our common shares and the voting and other rights of the holders of common shares. We have no current plans to issue any preferred shares.
Options
As of March 31, 2017, we have granted to employees, officers, directors and consultants 230,892 options to purchase our common shares under our stock option plans under our Existing Stock Option Plan, of which 212,602 are outstanding, all subject to a 1-for- stock split. We currently have 46,623 remaining options from our Existing Stock Option Plan which will be available for issuance under our 2017 Omnibus Plan. See “Executive and Director Compensation—Stock Option Plan.”
On April 30, 2017, we granted 39,100 options under our Existing Stock Option Plan.
Limitations on Liability and Indemnification of Directors and Officers
Under the CBCA, we may indemnify our current or former directors or officers or another individual who acts or acted at our request as a director or officer, or an individual acting in a similar capacity, of another entity, against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by the individual in respect of any civil, criminal, administrative, investigative or other proceeding in which the individual is involved because of his or her association with us or another entity. The CBCA also provides that we may also advance moneys to a director, officer or other individual for costs, charges and expenses reasonably incurred in connection with such a proceeding; provided that such individual shall repay the moneys if the individual does not fulfill the conditions described below.
However, indemnification is prohibited under the CBCA unless the individual:
• | acted honestly and in good faith with a view to our best interests, or the best interests of the other entity for which the individual acted as director or officer or in a similar capacity at our request; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | in the case of a criminal or administrative action or proceeding that is enforced by a monetary penalty, the individual had reasonable grounds for believing that his or her conduct was lawful; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | was not judged by the court or other competent authority to have committed any fault or omitted to do anything that the individual ought to have done. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The by-laws to be in effect upon the closing of this offering require us to indemnify each of our current or former directors or officers and each individual who acts or acted at our request as a director or officer, or in a similar capacity, of another entity, and well as well as their respective heirs and legal representatives to the full extent permitted by the CBCA.
The by-laws to be in effect upon the closing of this offering authorize us to purchase and maintain insurance for the benefit of each of our current or former directors or officers and each person who acts or acted at our request as a director or officer, or an individual acting in a similar capacity, of another entity.
We have entered into indemnity agreements with our directors and our executive officers which provide, among other things, that we will indemnify him or her to the fullest extent permitted by law from and against all liabilities, claims, damages, costs, charges or expenses reasonably incurred in any proceeding in which the Indemnified Party is involved in or made a party to, or that arises because the Indemnified Party is or was a director or officer of the Company; provided that, we shall not indemnify such individual if, among other things, he or she did not act honestly and in good faith with a view to our best interests and, in the case of a criminal or penal action, the individual did not have reasonable grounds for believing that his or her conduct was lawful.
At present, we are not aware of any pending or threatened litigation or proceeding involving any of our directors, officers, employees or agents in which indemnification would be required or permitted.
152
Other Important Provisions of Our Articles of Incorporation, By-Laws and the CBCA
The following is a summary of certain other important provisions of the articles of incorporation and by-laws to be in effect upon the closing of this offering, as well as certain related sections of the CBCA. Please note that this is only a summary and is not intended to be exhaustive. For further information please refer to the full versions of the articles of incorporation and by-laws to be in effect upon closing of the offering, filed as exhibits to the registration statement of which this prospectus is a part.
Stated Objects or Purposes
Our articles of incorporation will not contain stated objectives or purposes and do not place any limitations on the business that we may carry on.
Directors
Residency and Independence. Under the articles of incorporation that will be in effect upon the closing of this offering, at least 25% of our directors must be resident Canadians. Furthermore, under the CBCA, no business may be transacted at a meeting of our board of directors unless 25% of the directors present are resident Canadians. The minimum number of directors we may have is one and the maximum number we may have is ten. The CBCA provides that any amendment to our articles to increase or decrease the minimum or maximum number of our directors requires the approval of our shareholders by a special resolution.
Power to vote on matters in which a director is materially interested.The CBCA states that a director must disclose to us, in accordance with the provisions of the CBCA, the nature and extent of an interest that the director has in a material contract or material transaction, whether made or proposed, with us, if the director is a party to the contract or transaction, is a director or an officer or an individual acting in a similar capacity of a party to the contract or transaction, or has a material interest in a party to the contract or transaction.
A director who holds an interest in respect of any material contract or transaction into which we have entered or propose to enter is not entitled to vote on any directors’ resolution to approve that contract or transaction, unless the contract or transaction:
• | relates primarily to the director’s remuneration as a director, officer, employee or agent of us or an affiliate; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | is for indemnity or insurance otherwise permitted under the CBCA; or | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | is with an affiliate. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Directors’ power to determine the remuneration of directors.The CBCA provides that the remuneration of our directors, if any, may be determined by our directors subject to our articles of incorporation and by-laws. That remuneration may be in addition to any salary or other remuneration paid to any of our employees who are also directors.
Retirement or non-retirement of directors under an age limit requirement.Neither the articles of incorporation to be in effect upon the closing of this offering nor the CBCA imposes any mandatory age-related retirement or non-retirement requirement for our directors.
Number of shares required to be owned by a director.Neither our articles of incorporation to be in effect upon the closing of this offering nor the CBCA provides that a director is required to hold any of our shares as a qualification for holding his or her office. Our board of directors has discretion to prescribe minimum share ownership requirements for directors.
Action Necessary to Change the Rights of Holders of Our Shares
Our shareholders can authorize the alteration of our articles of incorporation to create or vary the special rights or restrictions attached to any of our shares by passing a special resolution. However, a right or special right attached to any class or series of shares may not be prejudiced or interfered with unless the shareholders holding shares of that class or series to which the right or special right is
153
attached consent by a separate special resolution. A special resolution means a resolution passed by: (a) a majority of not less than two-thirds of the votes cast by the applicable class or series of shareholders who vote in person or by proxy at a meeting, or (b) a resolution consented to in writing by all of the shareholders entitled to vote holding the applicable class or series of shares.
Shareholder Meetings
Under the articles of incorporation to be in effect upon the closing of this offering, we must hold an annual general meeting of our shareholders at least once every year at a time and place determined by our board of directors, provided that the meeting must not be held later than 15 months after the preceding annual general meeting. A meeting of our shareholders may be held anywhere in Canada, or provided that shareholders agree, anywhere outside Canada.
A notice to convene a meeting, specifying the date, time and location of the meeting, and, where a meeting is to consider special business, the general nature of the special business, must be sent to shareholders, to each director and the auditor not less than 48 hours prior to the meeting, although, as a result of applicable securities laws, the time for notice is effectively longer. Under the CBCA, shareholders entitled to notice of a meeting may waive or reduce the period of notice for that meeting, provided applicable securities laws are met. The accidental omission to send notice of any meeting of shareholders to, or the non-receipt of any notice by, any person entitled to notice does not invalidate any proceedings at that meeting.
A quorum for meetings will be one or more persons present in person or by means of a telephonic, electronic or other communication facility that permits all participants to communicate adequately with each other during the meeting and each entitled to vote at the meeting and holding or representing by proxy not less than 20% of the votes entitled to be cast at the meeting.
Holders of our common shares will be entitled to attend meetings of our shareholders. Except as otherwise provided with respect to any particular series of preferred shares, and except as otherwise required by law, the holders of our preferred shares are not entitled as a class to receive notice of, or to attend or vote at any meetings of our shareholders. Our directors, our secretary (if any), our auditor and any other persons invited by our chairman or directors or with the consent of those at the meeting will be entitled to attend at any meeting of our shareholders but will not be counted in the quorum or be entitled to vote at the meeting unless he or she is a shareholder or proxyholder entitled to vote at the meeting.
Change of Control
The articles of incorporation to be in effect upon the closing of this offering do not contain any change of control limitations with respect to a merger, acquisition or corporate restructuring that involves us.
Shareholder Ownership Disclosure
Although applicable securities laws regarding shareholder ownership by certain persons require disclosure, the articles of incorporation to be in effect upon the closing of this offering do not provide for any ownership threshold above which shareholder ownership must be disclosed.
Ownership and Exchange Controls
Limitations on the ability to acquire and hold our common shares may be imposed by theCompetition Act(Canada). This legislation permits the Commissioner of Competition (the Commissioner) to review any acquisition of control over or of a significant interest in us. This legislation grants the Commissioner jurisdiction, for up to one year, to challenge this type of acquisition before the Canadian Competition Tribunal on the basis that it would, or would be likely to, substantially prevent or lessen competition in any market in Canada.
This legislation also requires any person who intends to acquire our common shares to file a notification with the Canadian Competition Bureau if certain financial thresholds are exceeded and if
154
that person (and their affiliates) would hold more than 20% of our common shares. If a person already owns more than 20% of our common shares, a notification must be filed when the acquisition of additional shares would bring that person’s holdings to over 50%. Where a notification is required, the legislation prohibits completion of the acquisition until the expiration of a statutory waiting period, unless the Commissioner provides written notice that she does not intend to challenge the acquisition.
There is no limitation imposed by Canadian law or our articles of incorporation on the right of non-residents to hold or vote our common shares, other than those imposed by theInvestment Canada Act.
The Investment Canada Act requires any person that is not a “Canadian” (as defined in the Investment Canada Act) who acquires “control” (as defined in the Investment Canada Act) of an existing Canadian business to file either a pre-closing application for review or a post-closing notification with Industry Canada. Effective April 24, 2017, the threshold for review of a direct acquisition of control of a non-cultural Canadian business by a World Trade Organization member country investor (other than a state-owned enterprise) is an enterprise value of the assets of the Canadian business that equals or exceeds C$800 million. Amendments to the Investment Canada Act have been proposed which would increase the review threshold to C$1 billion. For purposes of a publicly traded company, the “enterprise value” of the assets of the Canadian business is equal to the market capitalization of the entity, plus its liabilities (excluding its operating liabilities), minus its cash and cash equivalents (all terms being described in the Investment Canada regulations).
Where the acquisition of control is a reviewable transaction, theInvestment Canada Actgenerally requires pre-closing approval of the reviewable transaction by the relevant minister on the basis that he is satisfied that the acquisition is likely to be of net benefit to Canada.
There is no law, governmental decree or regulation in Canada that restricts the export or import of capital or which would affect the remittance of dividends or other payments by us to non-Canadian holders of our common shares or preferred shares, other than withholding tax requirements.
This summary is not a comprehensive description of relevant or applicable considerations regarding such requirements and, accordingly, is not intended to be, and should not be interpreted as, legal advice to any prospective purchaser and no representation with respect to such requirements to any prospective purchaser is made. Prospective investors should consult their own Canadian legal advisors with respect to any questions regarding securities law in the provinces and territories of Canada.
Registration Rights
After the completion of this offering, certain holders of our common shares will be entitled to rights with respect to the registration of their shares. The Registration Rights Agreement provides for the registration of the shares held by such shareholders under the securities laws of the United States and/or the qualification for distribution of the shares held by such shareholders under the securities laws of the provinces and territories of Canada. These shareholders will collectively hold % of our common shares upon the completion of this offering, assuming no exercise of the underwriters’ over-allotment option. Pursuant to the Registration Rights Agreement, any one or more of the shareholders holding at least 51% of the then-outstanding registrable securities may require us to file and take such other steps as may be necessary under securities laws to facilitate a public offering with respect to all or part of such shareholders’ registrable securities. The registration rights set out above may be postponed in certain circumstances. These demand registration rights do not apply until six months following the closing of this offering.
In addition to the demand registration rights described above, these shareholders also have “piggy-back” registration rights relating to the inclusion of their common shares on certain registration statements or prospectuses filed by us. Pursuant to the piggy-back registration rights, we have certain obligations to include the common shares of such shareholders in such registration statements or prospectuses, subject to a qualified right to reduce the number of such common shares we are obligated to include if the underwriters participating in such transaction determine in writing that, in their opinion, the number of securities to be included in the offering relating to such piggy-back registration exceeds the number of securities that can be sold in such offering and that the number of securities
155
proposed to be included in such offering would adversely affect the price per security to be sold in such offering. All such piggyback rights related to this offering have been waived by the shareholders party to the Registration Rights Agreement.
We have agreed to pay all costs and expenses in connection with each registration and prospectus qualification described above, except underwriting fees, discounts and commissions, fees and disbursements of the selling shareholders’ counsel (other than for one counsel for all shareholders), and any transfer taxes applicable to the securities sold by the shareholders. We have agreed to indemnify the shareholders against certain liabilities, and the shareholders have agreed to indemnify us for certain liabilities, including liabilities under the Securities Act and Canadian provincial securities laws.
Listing
We have applied to list our common shares on The Nasdaq Global Market under the symbol “CMTA.”
Transfer Agent, Registrar and Auditor
Computershare Investor Services Inc., with its principal office in Toronto, Ontario, is the transfer agent for our common shares in Canada. Computershare Trust Company, N.A., with its principal office in Canton, Massachusetts is the transfer agent and registrar for our common shares in the United States.
KPMG LLP is our independent registered public accounting firm and is located in Montreal, Canada.
156
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there was no public market for our common shares. Future sales of our common shares in the public market, or the availability for sale of substantial amounts of our common shares in the public market, could adversely affect prevailing market prices and could impair our ability to raise equity capital in the future.
Upon completion of this offering, a total of of our common shares will be outstanding, assuming no exercise of the underwriters’ over-allotment option. All of the common shares sold in this offering will be freely tradable without restriction or further registration under the Securities Act, unless held by our “affiliates,” as that term is defined in Rule 144 under the Securities Act.
As of , 2017 common shares had been issued under our equity incentive plans, common shares were issuable upon exercise of vested and unvested stock options outstanding under our equity incentive plans and an additional common shares were reserved for issuance under our equity incentive plans. Subject to the lock-up agreements described below and limitations imposed by U.S. and Canadian securities laws on resales by our affiliates, common shares already issued pursuant to these plans or issuable upon exercises of these stock options will be freely tradeable in the public markets.
Rule 144
In general, under Rule 144 under the Securities Act as currently in effect, a person who is not deemed to have been one of our “affiliates” for purposes of the Securities Act at any time during 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than our “affiliates,” is entitled to sell those shares without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our “affiliates,” then such person is entitled to sell such shares without complying with any of the requirements of Rule 144. In general, under Rule 144, as currently in effect, our “affiliates” or persons selling shares on behalf of our “affiliates” are entitled to sell upon expiration of the lock-up agreements described above, within any three-month period beginning 90 days after the date of this prospectus, a number of shares that does not exceed the greater of:
• | 1% of the then outstanding common shares, which will equal approximately common shares after this offering; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the average weekly trading volume of our common shares during the four calendar weeks preceding the filing of a notice on Form 144 with respect to that sale. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Such sales under Rule 144 are also subject to provisions relating to notice, manner of sale, volume limitations and the availability of current public information about us.
In general, under Rule 144 of the Securities Act as currently in effect, beginning 90 days after the date of this prospectus, an “affiliate” who has beneficially owned our shares for a period of at least six months is entitled to sell within any three-month period a number of shares that does not exceed the greater of either 1% of the then outstanding shares or the average weekly trading volume of our shares on The Nasdaq Global Market during the four calendar weeks preceding the filing with the SEC of a notice on Form 144 with respect to such sale. Such sales under Rule 144 of the Securities Act are also subject to prescribed requirements relating to the manner of sale, notice and availability of current public information about us.
Under Rule 144, a person who is not deemed to have been an affiliate of ours at any time during the 90 days preceding a sale, and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior holder other than an affiliate, is entitled to sell such shares without restriction, provided we have been in compliance with our reporting requirements under the Exchange Act for 90 days preceding such sale. To the extent that our affiliates sell their shares, other than pursuant to Rule 144 or a registration statement, the purchaser’s holding period for the purpose of effecting a sale under Rule 144 commences on the date of transfer from the affiliate.
157
Registration Rights
Under the terms of the Registration Rights Agreement, the shares held by shareholders party to the agreement may be registered under the securities laws of the United States and/or qualified for distribution under the securities laws of the provinces and territories of Canada. The rights to make registration demands under the Registration Rights Agreement are limited in a number of respects, including the number of demands that can be made. The registration rights set out above may be postponed in certain circumstances. The demand registration rights provided in the Registration Rights Agreement do not apply until six months after the closing of this offering. The piggyback rights related to this offering have been waived by the shareholders party to the Registration Rights Agreement. See “Description of Share Capital—Registration Rights” for more information.
Lock-up Agreements
All of our shareholders, optionholders, executive officers and directors have entered into lock-up agreements. For a description of the lock-up agreements, see “Underwriters.”
10b5-1 Plans
Following this offering, certain of our employees, including our executive officers and/or our directors, may enter into written trading plans that are intended to comply with Rule 10b5-1 under the Exchange Act. Sales under these trading plans will not be permitted until the expiration of the lock-up agreements related to this offering that are described above.
158
Our corporate affairs are governed by our articles and by-laws and the provisions of the CBCA. The CBCA differs from the various state laws applicable to U.S. corporations and their shareholders. The following table provides a summary of the material differences between the provisions of the CBCA and the DGCL, taking into account certain specific provisions in our amended articles and our by-laws that will be in effect upon the closing of this offering.
|
|
|
|
|
| CBCA | DGCL | ||
Authorized | Upon closing, under our amended articles, as permitted by the CBCA, the authorized share capital will consist of (i) an unlimited number of common shares without par value and (ii) an unlimited number of new preferred shares without par value, issuable in series. | Under the DGCL, a corporation’s certificate of incorporation must specify the number of shares of each class of stock and their par value, or include a statement that such shares are without par value. The certificate of incorporation must also set forth the designations, powers, preferences, rights, qualifications, limitations and restrictions of each class of shares, if any. Under the DGCL, a corporation’s certificate of incorporation may give the board of directors the authority to issue preferred stock in one or more series, with such designations and special rights and restrictions as determined by the board of directors. | ||
Dividends | Under the CBCA and our amended articles, dividends may be declared on the common shares at the discretion of our board of directors. Any dividends declared shall be subject to the rights, if any, of shareholders holding shares with special rights as to dividends. Our directors shall not declare dividends if there are reasonable grounds for believing that we are, or would after the payment be, unable to pay our liabilities as they become due or the realizable value of our assets would thereby be less than the aggregate of our liabilities and stated capital of all classes. | The DGCL generally provides that, subject to certain restrictions, the directors of a corporation may declare and pay dividends upon the shares of its capital stock either out of the corporation’s surplus or, if there is no such surplus, out of its net profits for the fiscal year in which the dividend is declared and/or the preceding fiscal year. Further, the holders of preferred or special stock of any class or series may be entitled to receive dividends at such rates, on such conditions and at such times as stated in the certificate of incorporation. | ||
Vote Required for Certain Transactions | Under the CBCA, certain extraordinary corporate actions, including, without limitation, continuances, certain amalgamations and sales, leases or exchanges of all, or substantially all, of the property of a corporation (other than in the ordinary course of business), and liquidations, dissolutions and certain arrangements, are required to be approved by special resolution of our shareholders. | Under the DGCL, certain mergers, consolidation, sale, lease, exchange or other disposition of all, or substantially all, the property and assets of a corporation or dissolution of the corporation requires the approval of a majority of the outstanding voting stock of the corporation entitled to vote thereon. | ||
|
|
|
|
159
|
|
|
|
|
| CBCA | DGCL | ||
| A special resolution is a resolution passed by a majority of not less than two-thirds of the votes cast by the shareholders who voted in respect of the resolution or signed by all shareholders entitled to vote on that resolution. |
|
| |
Amendment of Organizing | Under the CBCA, an amendment to our articles generally requires approval by special resolution of holders of our voting shares. Specified amendments may also require the approval of other classes of our shares. In the event that an amendment to our articles would prejudice or interfere with a right or special right attached to our issued shares of a class or series of our shares, such amendment must be approved separately by the holders of the class or series of shares being affected. | The DGCL provides that a corporation may amend its certificate of incorporation if its board of directors has adopted such amendment, followed by the affirmative vote of a majority of the outstanding voting stock and a majority of the outstanding shares of each class entitled to vote on the amendment as a class. In the event the amendment would alter the aggregate number of authorized shares of a class of stock, their par value, or the powers, preferences or special rights of the shares of a class so as to affect them adversely, the holders of the outstanding shares of the class are entitled to vote as a class upon a proposed amendment, whether or not entitled to vote thereon by the certificate of incorporation. | ||
Amendment of | Under the CBCA, our board of directors may, by resolution, make, amend or repeal any by-law that regulates our business or affairs. Where our board of directors makes, amends or repeals a by-law, they are required under the CBCA to submit that action to our shareholders at the next meeting of shareholders and our shareholders may confirm, reject or amend that action by ordinary resolution. If the action is rejected by our shareholders, or our board of directors does not submit the action to our shareholders at the next meeting of shareholders, the action will cease to be effective and no subsequent resolution of our directors to make, amend or repeal a by-law having substantially the same purpose or effect will be effective until it is confirmed. | The DGCL provides that the stockholders entitled to vote have the power to adopt, amend or repeal bylaws. A corporation may also confer, in its certificate of incorporation, that power upon the board of directors. | ||
|
|
|
|
160
|
|
|
|
|
| CBCA | DGCL | ||
Quorum of | As permitted under the CBCA, our by-laws provide that quorum for meetings of shareholders is one person present or representing by proxy, shareholders holding no less than % of the issued shares entitled to be voted at the meeting. | Under the DGCL, unless otherwise provided in the certificate of incorporation, with respect to any matter, a quorum for a meeting of stockholders requires the holders of a majority of the shares entitled to vote are represented at the meeting in person or by proxy. | ||
Annual Meetings of Shareholders | Under the CBCA, we must hold an annual general meeting of our shareholders at least once every year at a time and place determined by our board of directors, provided that the meeting must not be held later than 15 months after the preceding annual general meeting but no later than six months after the end of our preceding financial year. The CBCA requires that a meeting of our shareholders may be held anywhere in Canada as our board of directors may determine. Under the CBCA, and our by-laws, we must provide notice of an annual general meeting to each shareholder entitled to vote thereat, to each director, and to our auditor at least 21 days in advance of the meeting. | Under the DGCL, a corporation must hold an annual meeting of stockholders in a place designated by the certificate of incorporation or bylaws, whether inside or outside of Delaware, or, if not so designated, as determined by the board of directors and on a date and at a time designated in the bylaws, except as otherwise provided by law. Written notice of every meeting of stockholders must be given to each stockholder of record not less than ten and not more than 60 days before the date of the meeting. | ||
Special Meetings of Shareholders | Under the CBCA and our by-laws, our board of directors has the power at any time to call a special meeting of shareholders. Under the CBCA, the holders of not less than 5% of our issued shares that carry the right to vote at a meeting sought to be held can also requisition our directors to call a meeting of shareholders for the purposes stated in the requisition. | Under the DGCL, special meetings of stockholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or the bylaws. | ||
|
|
|
|
161
|
|
|
|
|
| CBCA | DGCL | ||
Anti-takeover Provisions and Interested Shareholder Transactions | As permitted by the CBCA, our amended articles provide that our board of directors may fix the number of preferred shares in, and determine the designation of the shares of, each series and create, define and attach rights and restrictions to the preferred shares without shareholder approval. | Under the DGCL, a certificate of incorporation may provide the board of directors with the ability to designate the terms of and issue a new class or series of preferred stock, and to issue a stockholder rights plan. Delaware corporations are subject to Delaware’s “business combination” statute. In general, such statute prohibits a corporation from engaging in any business combination transactions with an interested stockholder for a period of three years after the time that the stockholder became an interested stockholder, unless approved by the board of directors beforehand or upon satisfaction of other criteria. | ||
Interested Director Transactions | Under the CBCA, a director who holds a disclosable interest in a material contract or transaction into which we have entered or propose to enter may generally not vote on any directors’ resolution to approve the contract or transaction. A director or officer has a disclosable interest in a material contract or transaction if the director or officer is: | Under the DGCL, a transaction in which a director of the corporation has a conflict of interest is not void or voidable solely because of the director’s conflict, solely because the director is present at or participates in the meeting of the board of directors or committee which authorizes the transaction or solely because any such director’s vote is counted for such purpose, if (a) the material facts of the conflict of interest are known to or disclosed to the board of directors or the committee and the board of directors or committee in good faith authorizes the transaction by a majority of the votes of the disinterested directors, (b) the material facts of the conflict of interest are known or disclosed to the stockholders of the corporation and the transaction is approved in good faith by the stockholders, or (c) the board of directors can demonstrate that the transaction is fair as to the corporation as of the time it is approved by the board of directors, committee or stockholders. | ||
|
|
|
|
162
|
|
|
|
|
| CBCA | DGCL | ||
Directors’ and | As permitted under the CBCA, our by-laws, subject to certain limitations, require us to indemnify our directors and officers and our former directors and officers and any persons acting, at our request, as a director or officer, or in a similar capacity, of a body corporate. | Under the DGCL, a corporation has the power to indemnify any person who was, is or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, or any person who was, is or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor, in each case by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, against expenses, judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interest of the corporation, and subject to certain other limitations. | ||
Dissent or | Under the CBCA dissenters’ rights are generally only available in connection with: | Under the DGCL, dissenters’ rights are generally only available in connection with cash mergers or mergers where the target stockholders hold stock other than stock of a widely held corporation. | ||
| * an amendment to our articles to add, change or remove any restriction upon the business or businesses that we may carry on; |
|
| |
| * a continuance under the laws of another jurisdiction; |
|
| |
| * a sale, lease or exchange of all, or substantially all, of our property other than in the ordinary course of business; |
|
| |
| * the carrying out of a going-private or a squeeze-out transaction; |
|
| |
|
|
|
|
163
|
|
|
|
|
| CBCA | DGCL | ||
| * a court order permitting a shareholder to dissent in connection with an application to the court for an order approving an arrangement proposed by us; and |
|
| |
| * certain amendments to our articles which require a separate class or series vote by a holder of shares of any class or series. |
|
| |
Oppression Remedy | The CBCA provides an oppression remedy that enables a court to make any order, whether interim or final, to rectify matters that are oppressive or unfairly prejudicial to or that unfairly disregard the interests of any of our securityholders, creditors, directors or officers if an application is made to a court by a “complainant.” | The DGCL does not expressly provide for a similar remedy. | ||
| A “complainant” with respect to a corporation means any of the following: |
|
| |
| * a present or former registered holder or beneficial owner of securities of the corporation or any of its affiliates; |
|
| |
| * a present or former officer or director of the corporation or any of its affiliates; |
|
| |
| * the director responsible for the application of the CBCA; and |
|
| |
| * any other person who in the discretion of the court is a proper person to make the application. |
|
| |
| The oppression remedy provides the court with broad and flexible powers to intervene in corporate affairs to protect our shareholders and other complainants. |
|
| |
Shareholder Derivative Actions | Under the CBCA, a complainant may also apply to a Canadian court for leave to bring an action in the name of, and on behalf of us, or to intervene in an existing action to which we are a party, for the purpose of prosecuting, defending or discontinuing an action on our behalf. Under the CBCA, no action may be brought and no intervention in an action may be made unless a court is satisfied that: | Under the DGCL, stockholders may bring derivative actions on behalf of, and for the benefit of, the corporation. The plaintiff in a derivative action on behalf of the corporation either must be or have been a stockholder of the corporation at the time of the transaction or must be a stockholder who became a stockholder by operation of law. | ||
|
|
|
|
164
|
|
|
|
|
| CBCA | DGCL | ||
| * the complainant has given the required notice to our board of directors of the shareholder’s intention to apply to the court if our board of directors does not bring, diligently prosecute or defend or discontinue the action; |
|
| |
| * the complainant is acting in good faith; and |
|
| |
| * it appears to be in our interests or the interest of the relevant subsidiary that the action be brought, prosecuted, defended or discontinued. |
|
| |
| Under the CBCA, the court in a derivative action may make any order it thinks fit. |
|
| |
Director Qualification | Generally, at least 25% of the directors of a CBCA corporation must be resident Canadians. Furthermore, under the CBCA, no business may be transacted at a meeting of our board of directors unless 25% of the directors present, or able to provide approval of the business transacted at the meeting in writing, by telephone or other means of communication, are resident Canadians. | The DGCL does not have director residency requirements although a corporation may prescribe qualifications for directors under its certificate of incorporation or bylaws. |
165
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following discussion is a general summary of the material U.S. federal income tax considerations for U.S. Holders (defined below) relating to the acquisition, ownership, and disposition, as a capital asset, of common shares issued by our Company (Common Stock). This discussion is based upon the Code, U.S. Treasury regulations (the Treasury Regulations) promulgated thereunder, published rulings, court decisions and other applicable authorities, all as in effect on the date hereof and all of which are subject to change or differing interpretations (possibly with retroactive effect).
This summary does not address, except as explicitly set forth below, the U.S. federal income tax considerations that may be applicable to any particular taxpayer nor to taxpayers that may be subject to special tax rules (e.g., financial institutions, insurance companies, real estate investment trusts, regulated investment companies, broker-dealers, tax-exempt organizations, holders that are, or that own their Common Stock through, partnerships or other pass-through entities, traders that elect mark-to-market treatment, persons that do not acquire their Common Stock upon original issuance, holders for whom Common Stock is not a “capital asset,” holders that are subject to the alternative minimum tax, holders that are not U.S. Holders, holders that have a functional currency other than the U.S. dollar, expatriates and former long-term residents of the United States, persons holding Common Stock as part of a “straddle,” “hedge,” “constructive sale” or “conversion” or other integrated transaction for U.S. federal income tax purposes, persons that acquire Common Stock pursuant to any employee share option or otherwise as compensation, or holders that own (directly, indirectly or constructively) 10% or more of our total combined voting power). This summary does not address the U.S. estate and gift, alternative minimum, state, local or non-U.S. tax consequences to U.S. Holders (defined below) of the acquisition, ownership, and disposition of the Common Stock. Each U.S. Holder should consult its own tax advisor regarding the U.S. estate and gift, alternative minimum, state, local and non-U.S. tax consequences arising from and relating to the acquisition, ownership, and disposition of the Common Stock.
IN VIEW OF THE FOREGOING, EACH PROSPECTIVE INVESTOR SHOULD CONSULT ITS OWN TAX ADVISOR REGARDING ALL U.S. FEDERAL, STATE, AND LOCAL AND NON-U.S. INCOME AND OTHER TAX CONSEQUENCES OF AN INVESTMENT IN COMMON STOCK, WITH SPECIFIC REFERENCE TO SUCH INVESTOR’S OWN PARTICULAR TAX SITUATION AND RECENT OR PROPOSED CHANGES IN APPLICABLE LAW.
For purposes of this discussion, a “U.S. Person” is:
(i) | a citizen or resident of the United States, | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(ii) | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created in or organized under the law of the United States or any political subdivision thereof, | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(iii) | an estate the income of which is subject to U.S. federal income taxation regardless of its source, or | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(iv) | a trust which (A) is subject to the primary supervision of a United States court and one or more U.S. Persons have the authority to control all substantial decisions of the trust or (B) has otherwise validly elected to be treated as a U.S. Person under the Code. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A “U.S. Holder” is a U.S. Person that is a beneficial owner of Common Stock for U.S. federal income tax purposes.
If a partnership (or other entity treated as a partnership for U.S. federal income tax purposes) is a beneficial owner of Common Stock, the tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of the partnership. If a U.S. Holder is a partner of a partnership holding Common Stock, such U.S. Holder is urged to consult its tax advisor regarding an investment in Common Stock.
Controlled Foreign Corporation Considerations
Our Company is a corporation organized under the laws of Canada. Generally, a non-U.S. corporation, such as us, will be classified as a CFC if more than 50% (by vote or value) of the shares of the corporation are held, directly, indirectly or constructively, by “U.S. Shareholders.” For purposes of this test, a U.S. Shareholder is generally any U.S. Person that possesses, directly, indirectly or
166
constructively, at least 10% of the combined voting power of all classes of shares of a non-U.S. corporation. If a non-U.S. corporation is a CFC for an uninterrupted period of 30 days or more during any taxable year, the U.S. Shareholders of the CFC will generally be subject to current U.S. federal income tax on certain types of income of the CFC (generally passive forms of income; including dividends, interest, certain rents and royalties, gain from the sale of property producing such income, gain from commodities transactions, foreign currency gain, income from notional principal contracts, and certain income from certain sales and services) and, in certain circumstances, treated as having received a taxable dividend with respect to earnings of the CFC that are invested in U.S. property (including loans to or investments in certain obligations of a U.S. Person). Such income is currently taxable to U.S. Shareholders regardless of whether corresponding cash distributions are made by the CFC. Gain recognized on the sale of a CFC’s stock may be classified, in whole or in part, as a dividend, if, at any time during the five-year period ending on the sale date, the seller was a U.S. Shareholder with respect to such non-U.S. corporation while it was a CFC. Gain taxable as a dividend pursuant to the CFC rules may be eligible for reduced rates of taxation to non-corporate taxpayers in certain circumstances. See “Distributions on Common Stock,” below.
Although we believe we qualified as a CFC in prior taxable years, based on our current ownership structure, we do not believe that we are currently a CFC for U.S. Federal income tax year ending December 31, 2017. However, our ownership includes U.S. Holders that are U.S. Shareholders for U.S. federal income tax purposes, and we expect to have U.S. Holders who qualify as U.S. Shareholders following this offering. Therefore, while we do not believe we are a CFC at the time of this offering, it is possible that, following this offering, a shareholder treated as a U.S. Person could acquire, directly or indirectly, enough shares to be treated as a U.S. Shareholder after application of the constructive ownership rules and, together with any other U.S. Shareholders of our Company, cause our Company to be treated as a CFC for U.S. federal income tax purposes. If we are classified as both a CFC and a PFIC (as discussed below), U.S. Holders that meet the definition of a U.S. Shareholder during the period in which we are a CFC generally will not also be subject to the PFIC rules during such period. A U.S. Holder who is a shareholder may be required to file Form 5471 (Information Return of U.S. Persons with Respect to Certain Foreign Corporations) with the IRS for one or more taxable years. This information return requires certain disclosures concerning the filing U.S. Shareholder, other U.S. Shareholders and us. The CFC rules are complex and may have a significant effect on U.S. Holders that are U.S. Shareholders. EACH PROSPECTIVE U.S. HOLDER SHOULD CONSULT ITS TAX ADVISOR REGARDING ALL ASPECTS OF THE CFC RULES.
Passive Foreign Investment Company Considerations
A non-U.S. corporation, such as us, will be classified as a PFIC for U.S. federal income tax purposes in any taxable year in which, after applying certain look-through rules with respect to the income and assets of certain subsidiaries, either: (i) at least 75% of its gross income for the taxable year is “passive income”; or (ii) at least 50% of the average quarterly value of its total gross assets (which may be determined in part by the market value of outstanding shares of Common Stock, if our Company is a publicly traded corporation) is attributable to assets that produce passive income or are held for the production of passive income. For this purpose, passive income generally includes most types of income subject to current taxation under the CFC rules described above (other than certain sales and service income) and if a non-U.S. corporation owns at least 25% by value of the stock of another corporation, the non-U.S. corporation is treated as owning its proportionate share of the assets of the other corporation and as receiving directly its proportionate share of such other corporation’s income.
There are no minimum stock ownership requirements exempting U.S. investors from the PFIC rules. If we are a PFIC for any tax year during which a U.S. Holder owns Common Stock, we will generally continue to be treated as a PFIC with respect to such U.S. Holder in all succeeding years during which such U.S. Holder owns Common Stock, regardless of whether we continue to qualify as a PFIC under the tests described above.
Based on certain estimates of our gross income and gross assets, our receipt and intended use of proceeds of this offering, and the nature of our business, we believe that we qualified as a PFIC for our
167
taxable year ended December 31, 2016 and we expect that we will qualify as a PFIC for our current taxable year ending December 31, 2017 and very possibly, for subsequent years.
General PFIC Rules
If we are a PFIC, and unless a U.S. Holder makes one of the elections described below, a special tax regime will apply to both (i) any “excess distribution” received by such holder from us (generally, such holder’s ratable portion of any distributions in a year which exceeds 125% of the average annual distribution received by such holder during the shorter of the three preceding tax years or such holder’s holding period for Common Stock) and (ii) any gain realized by such holder on the sale or other disposition of Common Stock. Under the PFIC rules, any such excess distribution and realized gain will generally be treated as though realized ratably over such U.S. Holder’s holding period of Common Stock and subject to tax rates applicable to ordinary income. Such income is generally allocated to each previous year when we were a PFIC, taxed at the highest tax rate then in effect applicable to such U.S. Holder for that year and, in addition, is subject to an interest charge. The interest charge is based on the tax deemed deferred from prior years based on the allocation of the income and the resulting tax liability across the U.S. Holder’s holding period. If we are determined to be a PFIC, a U.S. Holder will generally be treated as owning a proportionate amount (by value) of shares owned by us in any direct or indirect subsidiaries that are also PFICs (lower-tier PFIC), and will be subject to similar adverse rules with respect to any distributions we receive from, or dispositions we make of, the shares of such subsidiaries. The mark-to-market election (as described below) is not permitted for the shares of any of our subsidiaries that are also classified as PFICs. U.S. holders are urged to consult their tax advisors about the application of the PFIC rules to any of our subsidiaries.
Mark-to-Market Election
If we are a PFIC and our Common Stock is treated as “marketable stock” (as described below), a U.S. Holder may make an election to “mark to market” its Common Stock. If a U.S. Holder makes the mark-to-market election, then, in lieu of being subject to the PFIC tax and interest charge rules discussed above, the U.S. Holder generally will recognize as ordinary income any excess of the fair market value of the Common Stock at the end of each taxable year over such U.S. Holder’s adjusted tax basis, and will recognize an ordinary loss in respect of any excess of the adjusted tax basis of the ordinary shares over its fair market value at the end of the taxable year (but in the case of losses, only to the extent of the net amount of income previously included as a result of the mark-to-market election). If a U.S. Holder makes the election, the U.S. Holder’s tax basis in the Common Stock will be adjusted up or down to reflect these income or loss amounts taken into income annually. Any gain recognized on the sale or other disposition of Common Stock in a year when we are a PFIC will continue to be treated as ordinary income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included as a result of the mark-to-market election) but no interest charge is imposed. If a U.S. Holder makes a mark-to market election, it will be effective for the taxable year for which the election is made and for all subsequent taxable years unless Common Stock ceases to be marketable stock or the U.S. Internal Revenue Service (IRS) consents to the revocation of the election.
We expect that our Common Stock will be regularly traded in The Nasdaq Global Market after this offering and therefore will be considered marketable stock for purposes of the mark-to-market election. However, there can be no assurance that our Common Stock will continue to be treated as marketable stock.
QEF Election
Alternatively, a U.S. Holder may make an election to treat us (and each lower-tier PFIC, if any) as a “qualified electing fund” (QEF Election). If a U.S. Holder makes a QEF Election, in lieu of the treatment described in “General PFIC Rules” above, such U.S. Holder would be required to include in income each year a portion of the “ordinary earnings” and “net capital gains” of our Company or such lower-tier PFIC, even if not distributed to such U.S. Holder. Such U.S. Holder will not be permitted to recognize our current capital losses or ordinary losses. For this purpose, “net capital gain,” which is
168
taxed as long-term capital gain, is generally the excess of (a) net long-term capital gain over (b) net short-term capital loss, and “ordinary earnings,” which are taxed as ordinary income, are the excess of (a) ”earnings and profits” over (b) net capital gain.
A U.S. Holder that makes a QEF Election with respect to us (or a lower-tier PFIC, if any) generally may receive a tax-free distribution from us to the extent that such distribution represents our “earnings and profits” that were previously included in income by the U.S. Holder because of the QEF Election. The tax basis in such U.S. Holder’s Common Stock will be adjusted to reflect the amount included in income or allowed as a tax-free distribution because of the QEF Election. In addition, a U.S. Holder that makes a QEF Election generally will recognize capital gain or loss on the sale or other taxable disposition of its Common Stock.
If a U.S. Holder does not make a QEF Election for the first year of the U.S. Holder’s holding period for its Common Stock in which we were a PFIC with respect to such U.S. Holder, the U.S. Holder may still be able to make a retroactive QEF Election in a subsequent year if such U.S. Holder meets certain requirements and makes a “purging” election to recognize gain (which will be taxed under the general PFIC rules discussed above) as if its Common Stock were sold for its fair market value on the day when the QEF Election becomes effective. If a U.S. Holder is permitted to make a retroactive QEF Election but does not make a “purging” election to recognize gain as discussed in the preceding sentence, then such U.S. Holder shall continue to be subject to tax under the general PFIC rules discussed above (see, “General PFIC Rules,” above.)
A QEF Election will apply to the tax year for which such QEF Election is timely made and to all subsequent tax years, unless such QEF Election is invalidated or terminated or the IRS consents to revocation of such QEF Election. A U.S. Holder will not be currently taxed on the ordinary income and net capital gain of a PFIC with respect to which a QEF Election was made for any taxable year of the non-U.S. corporation during which such corporation does not qualify as a PFIC.
We will make due inquiry with our U.S. tax advisors at least annually regarding our status as a PFIC. For each year in which we determine our Company to be a PFIC, we will endeavor to provide to a U.S. Holder, upon its written request, all information necessary for such U.S. Holder to make or maintain a valid QEF Election, with respect to us (and any of our subsidiaries which are lower-tier PFICs (as discussed below). However, there is no assurance that we will have timely knowledge of the status of any such lower-tier PFIC. In addition, we may not hold a controlling interest in any such lower-tier PFIC and thus there can be no assurance we will be able to cause the lower-tier PFIC to provide the required information. U.S. Holders are urged to consult their own tax advisors regarding the tax issues raised by lower-tier PFICs and the procedure for and adjustability of making a QEF Election.
Other PFIC Rules
Pursuant to proposed Treasury Regulations, subject to certain exceptions, a U.S. Holder that has not made a timely QEF Election may be required to recognize gain (but not loss) upon certain transfers of its Common Stock that would otherwise be tax-deferred (e.g., gifts or exchanges pursuant to corporate reorganization provisions). However, the specific U.S. federal income tax consequences to a U.S. Holder may vary based on the manner in which such stock is transferred.
Certain additional adverse rules may apply with respect to a U.S. Holder if we are a PFIC, regardless of whether such U.S. Holder makes a QEF Election. For example, a U.S. Holder that uses its Common Stock as securities for a loan will, except as may be provided in Treasury Regulations, be treated as having made a taxable disposition of such stock. Furthermore, non-corporate U.S. Holders will not qualify for the preferential rates of taxation applicable to qualified dividend income as discussed in “Distributions on Common Stock” below.
A tax-exempt U.S. Holder will be subject to the PFIC rules discussed above only if a dividend from a PFIC would be treated as unrelated business taxable income to such tax-exempt U.S. Holder.
If a U.S. holder owns Common Stock during any taxaable year in which we are a PFIC, the U.S. Holder generally will be required to file an IRS Form 8621—(Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund) with respect to the company,
169
generally with the U.S. Holder’s federal income tax return for that year. If our company were a PFIC for a given taxable year, each U.S. Holder should consult its tax advisor concerning any applicable filing requirements. The PFIC rules are complex and may have a significant effect on U.S. Holders. Each prospective U.S. Holder should consult its tax advisor regarding all other aspects of the PFIC rules.
Distributions on Common Stock
We do not currently make distributions on our common shares and we currently intend to retain all available funds and any future earnings for use in the operation of our business. See “Dividend Policy.” Subject to the CFC and PFIC rules discussed above, a U.S. Holder that receives a distribution with respect to its Common Stock (without reduction for any Canadian tax withheld from such distribution) will be required to include the amount of such distribution in gross income as a dividend to the extent of our current and accumulated “earnings and profits,” as determined for U.S. federal income tax purposes. To the extent that a distribution exceeds our current and accumulated “earnings and profits,” such distribution will be treated first as a tax-free return of capital to the extent of a U.S. Holder’s tax basis in its Common Stock and thereafter as gain from the sale or exchange of such stock. (See “Sale or Other Disposition of Common Stock,” below.) There can be no assurance that we will maintain the calculations of our earnings and profits in accordance with U.S. federal income tax principles, and each U.S. Holder may have to assume that any distribution by us with respect to its Common Stock will constitute ordinary dividend income.
Dividends received by corporate U.S. Holders on their Common Stock generally will not be eligible for the “dividends received deduction.” If we are not a PFIC in the tax year of distribution or in the preceding tax year, dividends received by non-corporate U.S. Holders with respect to their Common Stock may constitute “qualified dividend income” and thus, may be eligible for the preferential tax rates applicable to long-term capital gains, provided that (1) we are eligible for the benefits of the United States-Canada income tax treaty as determined in accordance with the provisions of such treaty and certain U.S. federal income tax rules or such Common stock is readily tradable on an established securities market in the United States and (2) certain holding period requirements are satisfied. We expect to be eligible for the benefits of the United States-Canada income tax treaty. In addition, The Nasdaq Global Market should be treated as an established securities market for this purpose, although there can be no assurance that Common Stock will be readily tradable.
Dividends will constitute foreign source income for foreign tax credit limitation purposes. If the dividends are qualified dividend income, the amount of the dividend taken into account for purposes of calculating the U.S. foreign tax credit limitation will be limited to the gross amount of the dividend, multiplied by the reduced rate divided by the highest rate of tax normally applicable to dividends. The limitation of foreign taxes eligible for credit is calculated separately with respect to specific classes of income. Dividends distributed by us with respect to Common Stock will generally constitute “passive category income” but could, in the case of certain U.S. Holders, constitute “general category income.” Special rules may also apply to the amount of foreign tax credit that U.S. Holder may claim on a distribution from a PFIC. The rules relating to foreign tax credits are complex and the availability of a foreign tax credit depends on numerous factors. EACH U.S. HOLDER SHOULD CONSULT ITS OWN TAX ADVISOR CONCERNING THE APPLICATION OF THE UNITED STATES FOREIGN TAX CREDIT RULES TO ITS PARTICULAR SITUATION.
If dividends are paid in foreign currency, the amount includible in gross income will be the U.S. dollar value of such dividends, calculated based on the exchange rate applicable on the date of receipt, regardless of whether such foreign currency is converted into U.S. dollars at that time. Any U.S. Holder who converts or otherwise disposes of the foreign currency after the date of receipt may have a foreign currency exchange gain or loss that would be treated as ordinary income or loss, and generally will be U.S. source income or loss for foreign tax credit purposes. Each U.S. Holder should consult its own U.S. tax advisors regarding foreign currency gain or loss for U.S. federal income tax purposes if any portion of dividends in foreign currency is not converted into U.S. dollars on the date of receipt.
170
Sale or Other Disposition of Common Stock
Subject to the PFIC and CFC rules discussed above, a U.S. Holder will generally recognize capital gain or loss upon the sale or other disposition of its Common Stock in an amount equal to the difference between the amount realized upon the disposition and the U.S. Holder’s adjusted tax basis in such stock. If Canadian tax is imposed on the sale or other disposition of Common Stock, a U.S. Holder’s amount realized will include the gross amount of the proceeds before deduction of such Canadian tax. See “Material Canadian Federal Income Tax Considerations—Holders Not Resident in Canada—Dispositions.” Subject to the PFIC and CFC rules discussed above, for non-corporate U.S. Holders, capital gains from the sale or other disposition of Common Stock held for more than one year are generally eligible for preferential rates applicable to long-term capital gains. The deductibility of a capital loss is subject to certain limitations. Any gain or loss recognized on the sale of stock of a non-U.S. corporation by a U.S. Holder will generally be treated as U.S. source income or loss for foreign tax credit limitation purposes, except as otherwise provided in an applicable income tax treaty, and if an election is properly made under the Code. Each prospective U.S. Holder should consult its own tax advisor regarding the tax consequences if a Canadian withholding tax is imposed on a disposition of its Common Stock, including the availability of the foreign tax credit under their particular circumstances.
Subject to the discussion below under “Backup Withholding,” a non-U.S. Holder will not be subject to U.S. federal income or withholding tax on any gain realized on the sale or other disposition of its Common Stock unless (i) such gain is effectively connected with its conduct of a trade or business in the United States (or, if required by an applicable income tax treaty, the gain is attributable to a permanent establishment or fixed base that such holder maintains in the United States); (ii) such non-U.S. Holder is an individual and has been present in the United States for 183 days or more in the taxable year of such sale or disposition or as determined under a special “lookback” formula, and certain other conditions are met, or (iii) such non-U.S. Holder is subject to rules applicable to certain expatriates or former long-term residents of the United States.
Medicare Tax
Certain U.S. Holders that are individuals, estates or trusts (other than trusts that are exempt from tax) will be subject to an additional 3.8% tax on all or a portion of their “net investment income,” which includes, among others, dividends on their Common Stock (including excess distributions treated as dividends), and net gains from the disposition of their Common Stock. U.S. Holders making a mark-to-market election or a QEF Election may be subject to special rules for purposes of this additional tax. Prospective U.S. Holders that are individuals, estates or trusts should consult their tax advisors regarding the applicability of this additional tax to their income and gains in respect of their investments in Common Stock.
Certain Reporting Requirements
U.S. Persons (and in certain cases, certain non-resident aliens) that are treated as holding certain specified foreign financial assets in excess of certain thresholds may be subject to various U.S. return disclosure obligations. For these purposes, specified foreign financial assets include not only financial accounts maintained with foreign financial institutions, but also, unless held in accounts maintained by a financial institution, any stock or security issued by a foreign person or entity. U.S. Holders may be subject to these reporting requirements unless their shares of Common Stock are held in an account at certain financial institutions. Penalties for failure to file certain of these information returns are substantial. EACH PROSPECTIVE U.S. HOLDER SHOULD CONSULT ITS TAX ADVISOR WITH REGARD TO THE U.S. FEDERAL, STATE, LOCAL AND NON-U.S. TAX REPORTING REQUIREMENTS ASSOCIATED WITH AN INVESTMENT IN COMMON STOCK.
Backup Withholding
Dividend payments with respect to Common Stock and proceeds from the sale, exchange or redemption of Common Stock may be subject to certain information reporting to the IRS and possible United States backup withholding currently at a rate of 28%. Backup withholding will not apply,
171
however, to a U.S. Holder who furnishes a correct taxpayer identification number and makes other required certifications, or who is otherwise exempt from backup withholding. U.S. Holders that are required to establish their exempt status generally must provide such certification on IRS Form W-9.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against a U.S. Holder’s United States federal income tax liability, and a U.S. Holder generally may obtain a refund of any excess amounts withheld under the backup withholding rules by timely filing the appropriate claim for refund with the IRS and furnishing any required information. U.S. Holders should consult their tax advisors regarding the application of the United States backup withholding rules.
172
MATERIAL CANADIAN FEDERAL INCOME TAX CONSIDERATIONS
The following is a general summary, as of the date hereof, of certain material Canadian federal income tax considerations under Canada’sIncome Tax Act(Canadian Tax Act) generally applicable to a holder who acquires, as beneficial owner, Common Stock pursuant to this offering. This summary only applies to such a holder who, for the purposes of the Canadian Tax Act and at all relevant times: (i) deals at arm’s length and is not affiliated with us, (ii) acquires and holds the Common Stock as capital property, and (iii) has not entered into, and will not enter into, with respect to the Common Stock, a “derivative forward agreement” as that term is defined in the Canadian Tax Act (a Holder). The Common Stock will generally be considered to be capital property to a Holder unless they are held in the course of carrying on a business or were acquired in one or more transactions considered to be an adventure or concern in the nature of trade.
This summary is based upon: (i) the current provisions of the Canadian Tax Act in force as of the date hereof; (ii) all specific proposals (the Tax Proposals) to amend the Canadian Tax Act that have been publicly announced by, or on behalf of, the Minister of Finance (Canada) prior to the date hereof; and (iii) counsels’ understanding of the current published administrative policies and assessing practices of the Canada Revenue Agency made publicly available prior to the date hereof. This summary assumes that all such Tax Proposals will be enacted in the form currently proposed but no assurance can be given that they will be enacted in the form proposed or at all. This summary does not otherwise take into account or anticipate any changes in law, administrative policy or assessing practice, whether by legislative, regulatory, administrative, governmental or judicial interpretation, decision or action, nor does it take into account the tax laws of any province or territory of Canada or of any jurisdiction outside of Canada, which may differ from the Canadian federal income tax considerations described herein.
Subject to certain exceptions that are not discussed in this summary, for the purposes of the Canadian Tax Act, all amounts relating to the acquisition, holding or disposition of Common Stock must be determined in Canadian dollars based on exchange rates as determined in accordance with the Canadian Tax Act. The amount of any dividends required to be included in the income of, and capital gains or capital losses realized by, a Holder may be affected by fluctuations in the relevant exchange rate.
This summary is not exhaustive of all possible Canadian federal income tax considerations of purchasing, holding or disposing of the Common Stock. Moreover, this summary is of a general nature only and is not intended to be, nor should it be construed to be, legal or tax advice to any particular Holder and no representation with respect to the income tax consequences to any particular Holder is made. Accordingly, Holders are urged to consult their own tax advisors about the specific tax consequences to them of acquiring, holding and disposing of Common Stock in their particular circumstances.
Holder Resident in Canada
This portion of the summary is generally applicable to a Holder who, for the purposes of the Canadian Tax Act and at all relevant times, is or is deemed to be, a resident of Canada (a Resident Holder). A Resident Holder whose Common Stock might not otherwise qualify as capital property may, in certain circumstances, be entitled to make the irrevocable election provided by subsection 39(4) of the Canadian Tax Act to have its Common Stock and every other “Canadian security” (as defined in the Canadian Tax Act) owned by such Resident Holder in the taxation year of the election and in all subsequent taxation years deemed to be capital property. Such Resident Holders should consult their own tax advisors as to whether an election under subsection 39(4) of the Canadian Tax Act is available and/or advisable in their particular circumstances.
This portion of the summary is not applicable to a Resident Holder: (i) that is a “financial institution” within the meaning of the Canadian Tax Act (for the purposes of the mark-to-market rules); (ii) that is a “specified financial institution” within the meaning of the Canadian Tax Act; (iii) that reports its “Canadian tax results” within the meaning of the Canadian Tax Act in a currency other than Canadian currency; or (iv) an interest in which is a “tax shelter investment” within the meaning of the Canadian Tax Act. This summary does not address the possible application of the
173
“foreign affiliate dumping” rules that may be applicable to a Resident Holder that is a corporation that is or becomes, or does not deal at arm’s length for purposes of the Canadian Tax Act with a corporation resident in Canada that is or becomes, as part of a transaction or event or series of transactions or events that include the acquisition of the Common Stock, controlled by a non-resident corporation for the purposes of the rules in section 212.3 of the Canadian Tax Act. Any such Resident Holder to which this portion of the summary does not apply should consult its own tax advisor with respect to the tax consequences of this offering.
Dividends on Common Stock
A Resident Holder will be required to include in computing its income for a taxation year any dividend received or deemed to be received on the Common Stock. In the case of a Resident Holder that is an individual (other than certain trusts), such dividend will be subject to the gross-up and dividend tax credit rules normally applicable under the Canadian Tax Act to taxable dividends received from taxable Canadian corporations. Taxable dividends that are designated by us as “eligible dividends” will be subject to an enhanced gross-up and tax credit regime in accordance with the rules in the Canadian Tax Act. There may be limitations on our ability to designate dividends as eligible dividends. In the case of a Resident Holder that is a corporation, the amount of any such dividend that is included in its income for a taxation year will generally be deductible in computing its taxable income for that taxation year. In certain circumstances, however, subsection 55(2) of the Canadian Tax Act will treat a dividend received or deemed to be received by a Resident Holder that is a corporation as proceeds of disposition or a capital gain. Resident Holders that are corporations should consult their own tax advisors having regard to their own circumstances.
Dispositions of Common Stock
A Resident Holder who disposes of or is deemed for the purposes of the Canadian Tax Act to have disposed of Common Stock (other than to the company unless purchased by the company in the open market in the manner in which shares are normally purchased by any member of the public in the open market) will generally realize a capital gain (or capital loss) in the taxation year of the disposition equal to the amount by which the proceeds of disposition are greater (or are less) than the total of: (i) the adjusted cost base as defined in the Canadian Tax Act to the Resident Holder of the Common Stock immediately before the disposition or deemed disposition, and (ii) any reasonable costs of disposition.
A Resident Holder will generally be required to include in computing its income for the taxation year of disposition, one half of the amount of any capital gain (a “taxable capital gain”) realized in such year. Subject to and in accordance with the provisions of the Canadian Tax Act, a Resident Holder will generally be required to deduct one-half of the amount of any capital loss (an “allowable capital loss”) realized in the taxation year of disposition against taxable capital gains realized in the same taxation year. Allowable capital losses in excess of taxable capital gains for the taxation year of disposition generally may be carried back and deducted in any of the three preceding taxation years or carried forward and deducted in any subsequent taxation year against net taxable capital gains realized in such taxation years, to the extent and under the circumstances specified in the Canadian Tax Act.
If a Resident Holder is a corporation, any capital loss realized on a disposition or deemed disposition of Common Stock (or a share for which the Common Stock has been substituted) may, in certain circumstances prescribed by the Canadian Tax Act, be reduced by the amount of any dividends which have been received or which are deemed to have been received on such Common Stock. Similar rules may apply where a corporation is a member of a partnership or a beneficiary of a trust that owns Common Stock directly or indirectly through a partnership or a trust. Resident Holders to whom these rules may be relevant should consult their own tax advisors.
Other Taxes
A Resident Holder that is a “private corporation” or a “subject corporation,” as defined in the Canadian Tax Act, will generally be liable to pay a refundable tax of 38 1/3% under Part IV of the
174
Canadian Tax Act on dividends received or deemed to be received on the Common Stock to the extent such dividends are deductible in computing the Resident Holder’s taxable income for the year.
A Resident Holder that is throughout the relevant taxation year a “Canadian-controlled private corporation” (as defined in the Canadian Tax Act) may be liable to pay an additional refundable tax of 10 2/3% on its “aggregate investment income” (as defined in the Canadian Tax Act) for the year, including taxable capital gains realized on the disposition of Common Stock.
Capital gains and taxable dividends received or deemed to be received by a Resident Holder who is an individual (other than certain trusts) may result in such Resident Holder being liable for alternative minimum tax under the Canadian Tax Act. Such Resident Holders should consult their own tax advisors in this regard.
Holders Not Resident in Canada
This portion of the summary is generally applicable to a Holder who, at all relevant times, for the purposes of the application of the Canadian Tax Act, (i) is not, and is not deemed to be, resident in Canada for purposes of the Canadian Tax Act and (ii) does not use or hold, and is not deemed to use or hold, Common Stock in a business carried on in Canada (a Non-Canadian Holder). Special rules, which are not discussed in this summary, may apply to a Non-Canadian Holder that is an insurer carrying on an insurance business in Canada and elsewhere.
Dividends
Dividends paid or credited on the Common Stock or deemed to be paid or credited on the Common Stock to a Non-Canadian Holder will be subject to Canadian withholding tax at the rate of 25%, subject to any reduction in the rate of withholding to which the Non-Canadian Holder is entitled under any applicable income tax convention between Canada and the country in which the Non-Canadian Holder is resident. For example, under the Canada-United States Tax Convention (1980), as amended, where dividends on the Common Stock are considered to be paid to or derived by a Non-Canadian Holder that is a beneficial owner of the dividends and is a U.S. resident for the purposes of, and is entitled to benefits of, such treaty, the applicable rate of Canadian withholding tax is generally reduced to 15% or, if such beneficial owner is a corporation that owns at least 10% of our voting shares, to 5%.
Dispositions
A Non-Canadian Holder will not be subject to tax under the Canadian Tax Act on any capital gain realized on a disposition or deemed disposition of Common Stock, unless the Common Stock are “taxable Canadian property” to the Non-Canadian Holder for purposes of the Canadian Tax Act and the Non-Canadian Holder is not entitled to relief under an applicable income tax convention between Canada and the country in which the Non-Canadian Holder is resident.
Generally, the Common Stock will not constitute “taxable Canadian property” to a Non-Canadian Holder at a particular time provided that the Common Stock are listed at that time on a “designated stock exchange” (as defined in the Canadian Tax Act), which includes The Nasdaq Global Market, unless at any particular time during the 60-month period that ends at that time (i) one or any combination of (a) the Non-Canadian Holder, (b) persons with whom the Non-Canadian Holder does not deal at arm’s length, and (c) partnerships in which the Non-Canadian Holder or a person described in (b) holds a membership interest directly or indirectly through one or more partnerships, has owned 25% or more of the issued shares of any class or series of our capital stock, and (ii) more than 50% of the fair market value of the Common Stock was derived, directly or indirectly, from one or any combination of: (i) real or immoveable property situated in Canada, (ii) “Canadian resource properties” (as defined in the Canadian Tax Act), (iii) “timber resource properties” (as defined in the Canadian Tax Act) and (iv) options in respect of, or interests in, or for civil law rights in, property in any of the foregoing whether or not the property exists. Notwithstanding the foregoing, in certain circumstances set out in the Canadian Tax Act, Common Stock could be deemed to be “taxable Canadian property.” Non-Canadian Holders whose Common Stock may constitute “taxable Canadian property” should consult their own tax advisors.
175
Under the terms and subject to the conditions in an underwriting agreement dated the date of this prospectus, the underwriters named below, for whom Morgan Stanley & Co. LLC and Leerink Partners LLC are acting as representative, have severally agreed to purchase, and we have agreed to sell to them, severally, the number of shares indicated below:
|
|
|
Name | Number of | |
Morgan Stanley & Co. LLC |
|
|
Leerink Partners LLC |
|
|
Wedbush Securities Inc. |
|
|
BTIG, LLC |
|
|
Total: |
|
|
The underwriters and the representative are collectively referred to as the “underwriters” and the “representative,” respectively. The underwriters are offering the common shares subject to their acceptance of the shares from us and subject to prior sale. The underwriting agreement provides that the obligations of the several underwriters to pay for and accept delivery of the common shares offered by this prospectus are subject to the approval of certain legal matters by their counsel and to certain other conditions. The underwriters are obligated to take and pay for all of the common shares offered by this prospectus if any such shares are taken. However, the underwriters are not required to take or pay for the shares covered by the underwriters’ over-allotment option described below.
The underwriters initially propose to offer part of the common shares directly to the public at the offering price listed on the cover page of this prospectus and part to certain dealers at a price that represents a concession not in excess of $ per share under the public offering price. After the initial offering of the common shares, the offering price and other selling terms may from time to time be varied by the representative.
We have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase up to additional common shares at the public offering price listed on the cover page of this prospectus, less underwriting discounts and commissions. The underwriters may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with the offering of the common shares offered by this prospectus. To the extent the option is exercised, each underwriter will become obligated, subject to certain conditions, to purchase approximately the same percentage of the additional common shares as the number listed next to the underwriter’s name in the preceding table bears to the total number of common shares listed next to the names of all underwriters in the preceding table.
The following table shows the per share and total public offering price, underwriting discounts and commissions, and proceeds before expenses to us. These amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase up to an additional common shares.
|
|
|
|
|
|
| |||||||||||||||
| Per Share | Total | |||||||||||||||||||
No Exercise | Full Exercise | ||||||||||||||||||||
Public offering price |
|
| $ |
|
|
|
| $ |
|
|
|
| $ |
|
| ||||||
Underwriting discounts and commissions to be paid by us |
|
| $ |
|
| $ |
|
| $ | ||||||||||||
Proceeds, before expenses, to us |
|
| $ |
|
| $ |
|
| $ | ||||||||||||
The estimated offering expenses payable by us, exclusive of the underwriting discounts and commissions, are approximately $ . We have agreed to reimburse the underwriters for expense relating to clearance of this offering with the Financial Industry Regulatory Authority up to $ .
The underwriters have informed us that they do not intend sales to discretionary accounts to exceed 5% of the total number of common shares offered by them.
We have applied to quote our common shares on The Nasdaq Global Market under the trading symbol “CMTA.” In order to meet the requirements for listing on that exchange, the underwriters have
176
undertaken to sell a minimum number of shares to a minimum number of beneficial owners as required by that exchange.
We and all directors and officers and the holders of all of our outstanding stock and stock options have agreed that, without the prior written consent of Morgan Stanley & Co. LLC on behalf of the underwriters, we and they will not, during the period ending 180 days after the date of this prospectus (the “restricted period”):
• | offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any common shares or any securities convertible into or exercisable or exchangeable for common shares or publicly disclose the intention to do so; or | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common shares; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
whether any such transaction described above is to be settled by delivery of common shares or such other securities, in cash or otherwise. In addition, we and each such person agrees that, without the prior written consent of Morgan Stanley & Co. LLC and Leerink Partners LLC on behalf of the underwriters, we or such other person will not, during the restricted period, make any demand for, or exercise any right with respect to, the registration of any common shares or any security convertible into or exercisable or exchangeable for common shares.
The restrictions described in the immediately preceding paragraph to do not apply, among other things, to:
• | transactions relating to our common shares or other securities acquired in this offering or in open market transactions after the completion of this offering, provided that no filing or public announcement under Section 16(a) of the Exchange Act, under any of the securities laws (including rules, regulations, policy statements or other such instruments or rulings) of each of the provinces and territories of Canada (collectively, the Canadian Securities Laws) or the equivalent thereof in other non-U.S. jurisdictions shall be required or shall be voluntarily made during the restricted period in connection with any such subsequent sales of common shares or other securities acquired in such open market transactions; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the exercise of stock options or other similar awards granted pursuant to the company’s equity incentive plans as described in this prospectus, provided that any of the signatory’s common shares or any security convertible into or exchangeable for common shares issued or received upon such exercise shall be subject to the restrictions described herein; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | transfers of common shares or any security convertible or exercisable or exchangeable into common shares as a bona fide gift, including as a result of estate or intestate succession, or pursuant to a will or other testamentary document, provided that (i) each donee, distributee or transferee shall concurrently with such transfer or distribution sign and deliver a lock up letter substantially in the form of the restrictions described herein and (ii) no filing or public announcement under Section 16(a) of the Exchange Act, under Canadian Securities Laws or the equivalent thereof in other non-U.S. jurisdictions, shall be required or shall be voluntarily made during the restricted period; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | transfers of common shares or any security convertible or exercisable or exchangeable into common shares: if the signatory is a natural person, to a member of the immediate family of the signatory or to any trust or other like entity for the direct or indirect benefit of the signatory or the immediate family of the signatory; if the signatory is a corporation, partnership, limited liability company or other business entity, to any affiliate, wholly-owned subsidiary, limited partner or member of the signatory or to any investment fund or other entity controlled by the signatory, provided in all cases that (i) such transfer shall not involve a disposition of value, (ii) each donee, distributee or transferee shall concurrently with such transfer or distribution sign and deliver a lock up letter substantially in the form of the restrictions described herein and (iii) no filing or public announcement under Section 16(a) of the Exchange Act, under Canadian Securities Laws or the equivalent thereof in other non-U.S. jurisdictions, shall be required or shall be voluntarily made during the restricted period; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
177
• | distributions of common shares or any security convertible into common shares to limited partners or stockholders of the signatory, provided that (i) each donee, distributee or transferee shall concurrently with such transfer or distribution sign and deliver a lock up letter substantially in the form of the restrictions described herein and (ii) no filing or public announcement under Section 16(a) of the Exchange Act, under Canadian Securities Laws or the equivalent thereof in other non-U.S. jurisdictions, shall be required or shall be voluntarily made during the restricted period; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the establishment of a trading plan pursuant to Rule 10b5-1 under the Exchange Act or similar plan under Canadian Securities Laws or the equivalent thereof in other non-U.S. jurisdictions for the transfer of common shares, provided that (i) such plan does not provide for the transfer of common shares during the restricted period and (ii) to the extent a public announcement or filing under the Exchange Act, under Canadian Securities Laws or the equivalent thereof in other non-U.S. jurisdictions, if any, is required or voluntarily made by or on behalf of the signatory or the company regarding the establishment of such plan, such announcement or filing shall include a statement to the effect that no transfer of common shares may be made under such plan during the restricted period; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the transfer of common shares or any security convertible into or exercisable or exchangeable for common shares to the company, pursuant to agreements or rights in existence on the date hereof which have been disclosed to the Representative and under which the company has the option to repurchase such shares or a right of first refusal with respect to transfers of such shares, in each case, solely in connection with the termination of the signatory’s employment or other service relationship with the company, provided that any public filing or public announcement under Section 16(a) of the Exchange Act, Canadian Securities Laws or the equivalent thereof in other non-U.S. jurisdictions, required or voluntarily made during the restricted period shall clearly indicate in the footnotes thereto or comments section thereof that such transfer was made solely to the company pursuant to the circumstances described herein; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the transfer of common shares or any securities convertible into or exercisable or exchangeable for common shares from the signatory to the company (or the purchase and cancellation of same by the company) upon a vesting event of the company’s securities or upon the exercise of options to purchase common shares by the signatory, in each case on a “cashless” or “net exercise” basis solely to cover tax withholding obligations of the signatory in connection with such vesting or exercise, provided that no filing or public announcement under Section 16(a) of the Exchange Act, under Canadian Securities Laws or the equivalent thereof in other non-U.S. jurisdictions, shall be required or shall be voluntarily made during the restricted period; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | the transfer of common shares or any security convertible into or exercisable or exchangeable for common shares pursuant to a bona fide third party tender offer, merger, amalgamation, consolidation or other similar transaction occurring after this offering, in each case, made to all holders of the common shares involving a change of control of the company; provided, that in the event that the tender offer, merger, amalgamation, consolidation or other such similar transaction is not completed, the common shares owned by the signatory shall remain subject to the restrictions contained in the restrictions described herein and provided, further, that no such transfer of common shares shall be permitted if such bona fide third-party tender offer, merger, amalgamation, consolidation or other similar transaction is not approved by the board of directors of the company, unless either (A) such transfer is required pursuant to mandatory take-over or squeeze-out provisions under applicable law or (B) the failure to so transfer such signatory’s common shares would result in such signatory’s common shares being extinguished without value being received by the signatory; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | any transfer of common shares that occurs by operation of law pursuant to a qualified domestic order in connection with a divorce settlement or other court order, provided that any public filing or public announcement under Section 16(a) of the Exchange Act, Canadian Securities Laws or the equivalent thereof in other non-U.S. jurisdictions, required or voluntarily made during the restricted period shall clearly indicate in the footnotes thereto or comments section thereof that such transfer was made pursuant to the circumstances described herein; or | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
178
• | the transfer of common shares or any securities convertible into or exercisable or exchangeable for common shares that is required to effect the reorganization of the company as described in this prospectus. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The representatives, in their sole discretion, may release the common shares and other securities subject to the lock-up agreements described above in whole or in part at any time.
In order to facilitate the offering of the common shares, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of the common shares. Specifically, the underwriters may sell more shares than they are obligated to purchase under the underwriting agreement, creating a short position. A short sale is covered if the short position is no greater than the number of shares available for purchase by the underwriters under the over-allotment option. The underwriters can close out a covered short sale by exercising the over-allotment option or purchasing shares in the open market. In determining the source of shares to close out a covered short sale, the underwriters will consider, among other things, the open market price of shares compared to the price available under the over-allotment option. The underwriters may also sell shares in excess of the over-allotment option, creating a naked short position. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common shares in the open market after pricing that could adversely affect investors who purchase in this offering. As an additional means of facilitating this offering, the underwriters may bid for, and purchase, common shares in the open market to stabilize the price of the common shares. These activities may raise or maintain the market price of the common shares above independent market levels or prevent or retard a decline in the market price of the common shares. Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of the effect, if any, that any one or more of these activities may have on the price of our common shares, were they to occur at all. The underwriters are not required to engage in these activities and may end any of these activities at any time.
We and the underwriters have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
A prospectus in electronic format may be made available on websites maintained by one or more underwriters, or selling group members, if any, participating in this offering. The representative may agree to allocate a number of common shares to underwriters for sale to their online brokerage account holders. Internet distributions will be allocated by the representative to underwriters that may make Internet distributions on the same basis as other allocations.
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The address of Morgan Stanley & Co. LLC is 1585 Broadway, New York, New York. The address of Leerink Partners LLC is 299 Park Avenue, New York, New York. Certain of the underwriters and their respective affiliates have, from time to time, performed, and may in the future perform, various financial advisory and investment banking services for us, for which they received or will receive customary fees and expenses.
In addition, in the ordinary course of their various business activities, the underwriters and their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers and may at any time hold long and short positions in such securities and instruments. Such investment and securities activities may involve our securities and instruments. On June 22, 2015, Clementia issued an aggregate of 485,823 Class B preferred shares for $123.50 per share, of which 8,195 were purchased and remain held by certain affiliates of Leerink Partners LLC, in connection with its Class B financing for an aggregate purchase price of approximately $59,999,141. The shares were issued in reliance on the exemption from registration under Section 4(a)(2) of the Securities Act on the basis that the transaction did not involve a public offering. Leerink Partners LLC served as placement agent for the transaction, and received a sales commission of $2,145,300. The 8,097 Class B preferred shares held by certain affiliates of Leerink Partners LLC will,
179
upon the completion of this offering, convert into common shares. The underwriters and their respective affiliates may also make investment recommendations or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long or short positions in such securities and instruments.
Pricing of the Offering
Prior to this offering, there has been no public market for our common shares. The initial public offering price will be determined by negotiations between us and the representative. Among the factors considered in determining the initial public offering price will be our future prospects and those of our industry in general, our results of operations and certain other financial and operating information in recent periods, the price-earnings ratios, price-sales ratios, market prices of securities, and certain financial and operating information of companies engaged in activities similar to ours.
Selling Restrictions
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”) an offer to the public of any of our common shares may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of any of our common may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
(a) | to any legal entity which is a qualified investor as defined in the Prospectus Directive; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) | to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representatives for any such offer; or | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(c) | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of our common shall result in a requirement for the publication by us or any underwriter of a prospectus pursuant to Article 3 of the Prospectus Directive. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
For the purposes of this provision, the expression an “offer to the public” in relation to any of our common shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any of our common shares to be offered so as to enable an investor to decide to purchase any of our common shares, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive” means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the Relevant Member State, and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
United Kingdom
Each underwriter has represented and agreed that:
(a) | it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 (FSMA) received by it in connection with the issue or sale of our common shares in circumstances in which Section 21(1) of the FSMA does not apply to us; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(b) | it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to our common shares in, from or otherwise involving the United Kingdom. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
180
EXPENSES RELATED TO THIS OFFERING
The following table sets forth the costs and expenses, other than underwriting discounts and commissions, payable by us in connection with the offer and sale of common shares in this offering. All amounts listed below are estimates except the SEC registration fee, The Nasdaq Global Market listing fee and FINRA filing fee.
|
|
| |||||
Itemized expense | Amount | ||||||
SEC registration fee |
|
| $ |
|
| ||
Nasdaq listing fee |
|
| |||||
FINRA filing fee |
|
| |||||
Printing and engraving expenses |
|
| |||||
Transfer agent and registrar fees |
|
| |||||
Legal fees and expenses |
|
| |||||
Accounting fees and expenses |
|
| |||||
Public Relations fees |
|
| |||||
|
|
| |||||
Total |
|
| |||||
|
|
| |||||
ENFORCEABILITY OF CIVIL LIABILITIES
We are incorporated under the laws of Canada. Substantially all of our assets are located outside the United States. In addition, several of our directors and officers are nationals and/or residents of countries other than the United States, and all or a substantial portion of such persons’ assets may be located outside the United States. As a result, it may be difficult for investors to effect service of process within the United States upon us or such persons or to enforce against them or against us, judgments obtained in United States courts, including judgments predicated upon the civil liability provisions of the securities laws of the United States or any state thereof. In addition, investors should not assume that the courts of Canada (i) would enforce judgments of U.S. courts obtained in actions against us, our officers or directors, or other said persons, predicated upon the civil liability provisions of the U.S. federal securities laws or other laws of the United States or (ii) would enforce, in original actions, liabilities against us or such directors, officers or experts predicated upon the United States federal securities laws or any securities or other laws of any state or jurisdiction of the United States.
In addition, there is doubt as to the applicability of the civil liability provisions of U.S. federal securities law to original actions instituted in Canada. It may be difficult for an investor, or any other person or entity, to assert U.S. securities laws claims in original actions instituted in Canada.
The Corporation Trust Company is our agent to receive service of process with respect to any action brought against us in the United States. The Corporation Trust Company is located at 1209 Orange Street, Wilmington, Delaware 19801.
Our consolidated financial statements as at and for the years ended December 31, 2016, 2015 and 2014 have been included herein and in the registration statement in reliance upon the report of KPMG LLP, independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
On June 8, 2016, with the approval of its shareholders, the Company dismissed Deloitte LLP as its outside auditor and appointed KPMG LLP as its new independent audit firm. Deloitte LLP’s report on our 2015 consolidated financial statements pursuant to IFRS not included herein was audited pursuant to Canadian generally accepted auditing standards and contained no adverse opinion or disclaimer of opinion and was not qualified or modified as to uncertainty, audit scope, or accounting principles. Deloitte LLP’s report issued under Canadian generally accepted auditing standards is not included in this filing. In addition, during the period from 2013 to March 1, 2016, there were no disagreements with Deloitte LLP on any matter of accounting principles or practices, financial statement disclosure, or
181
auditing scope or procedure, which disagreements, if not resolved to the satisfaction of Deloitte LLP, would have caused it to make a reference to the subject matter of the disagreements in connection with its report for such year and there were no reportable events, noting that Deloitte LLP’s audit was performed under Canadian generally accepted auditing standards. We did not consult KPMG LLP on any financial or accounting reporting matters in the period before their appointment. We provided Deloitte LLP with a copy of the disclosure in this paragraph and requested them to furnish a letter to us stating whether they agree with the statements made in this paragraph and if not, stating the respect in which they do not agree, which is included as an exhibit to the registration statement of which this prospectus forms a part.
Certain legal matters in connection with this offering relating to U.S. law will be passed upon for us by Jenner & Block LLP, New York, New York, United States. The validity of the common shares being offered by this prospectus will be passed upon for us by Dentons Canada LLP, Montreal, Quebec, Canada. Certain legal matters in connection with this offering will be passed upon for the underwriters by Ropes & Gray LLP, Boston, Massachusetts, with respect to U.S. law, and by Osler, Hoskin & Harcourt LLP, Toronto, Canada, with respect to Canadian law.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form F-1 under the Securities Act with respect to the common shares offered by this prospectus. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement, some items of which are contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and the common shares offered hereby, we refer you to the registration statement, including the exhibits filed as a part of the registration statement. Statements contained in this prospectus concerning the contents of any contract or document referred to are not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, please see the copy of the contract or document that has been filed. Each statement is this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit.
You may read and copy the registration statement, including the exhibits and schedules thereto, at the Public Reference Room of the SEC, 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy statements and other information about issuers, like us, that file electronically with the SEC. The address of that website is http://www.sec.gov.
After this offering, we will be subject to the reporting requirements of the Exchange Act applicable to foreign private issuers. As a foreign private issuer, we are exempt from, among other things, certain rules prescribing the furnishing and content of proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act.
We also maintain a corporate website at http://www.clementiapharma.com. Information contained on, or that can be accessed through, our website does not constitute a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference.
182
INDEX TO THE CONSOLIDATED FINANCIAL STATEMENTS
|
|
| |||||
Unaudited Interim Condensed Consolidated Financial Statements |
|
| |||||
|
| F-2 | |||||
|
| F-3 | |||||
|
| F-4 | |||||
|
| F-5 | |||||
Notes to Interim Condensed Consolidated Financial Statements |
|
| F-6 | ||||
Audited Consolidated Financial Statements |
|
| |||||
|
| F-13 | |||||
Consolidated Statements of Financial Position as of December 31, 2016, 2015 and 2014 |
|
| F-14 | ||||
Consolidated Statements of Changes in Equity for the years ended December 31, 2016, 2015 and 2014 |
|
| F-15 | ||||
|
| F-16 | |||||
Consolidated Statements of Cash Flows for the years ended December 31, 2016, 2015 and 2014 |
|
| F-17 | ||||
|
| F-18 | |||||
F-1
CLEMENTIA PHARMACEUTICALS INC.
INTERIM CONDENSED CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
(unaudited)
|
|
|
|
|
|
|
|
| ||||||||||
As at | Note | March 31, | December 31, |
|
| |||||||||||||
|
|
| (in US dollars) |
|
| |||||||||||||
ASSETS |
|
|
|
|
|
|
|
| ||||||||||
Current Assets |
|
|
|
|
|
|
|
| ||||||||||
Cash |
|
|
|
| $ |
| 23,722,034 |
|
| $ |
| 9,434,495 |
|
| ||||
Short-term investments |
|
|
|
| 20,000,000 |
|
| 30,000,000 |
|
| ||||||||
Interest receivable |
|
|
|
| 57,533 |
|
| 307,579 |
|
| ||||||||
Sales tax and other receivables |
|
|
|
| 52,431 |
|
| 90,966 |
|
| ||||||||
Investment tax credits receivable |
|
|
|
| 188,849 |
|
| 139,223 |
|
| ||||||||
Deferred financing costs |
|
|
|
| 93,544 |
|
| — |
|
| ||||||||
Prepaid expenses |
|
|
|
| 656,619 |
|
| 652,158 |
|
| ||||||||
|
|
|
|
|
|
| ||||||||||||
Total current assets |
|
|
|
| 44,771,010 |
|
| 40,624,421 |
|
| ||||||||
Non-current assets |
|
|
|
|
|
|
|
| ||||||||||
Property and equipment |
|
|
|
| 38,212 |
|
| 38,163 |
|
| ||||||||
Intangible assets | 9 |
|
| 1,860,766 |
|
| 894,584 |
|
| |||||||||
|
|
|
|
|
|
| ||||||||||||
Total non-current assets |
|
|
|
| 1,898,978 |
|
| 932,747 |
|
| ||||||||
|
|
|
|
|
|
| ||||||||||||
Total assets |
|
|
|
| $ |
| 46,669,988 |
|
| $ |
| 41,557,168 |
|
| ||||
|
|
|
|
|
|
| ||||||||||||
LIABILITIES |
|
|
|
|
|
|
|
| ||||||||||
Current Liabilities |
|
|
|
|
|
|
|
| ||||||||||
Accounts payable and accrued liabilities |
|
|
|
| $ |
| 4,652,868 |
|
| $ |
| 4,523,713 |
|
| ||||
Non-current liabilities |
|
|
|
|
|
|
|
| ||||||||||
Preferred shares | 4 |
|
| 76,058,914 |
|
| 67,880,952 |
|
| |||||||||
Embedded derivatives | 4 |
|
| 155,857,471 |
|
| 117,824,611 |
|
| |||||||||
|
|
|
|
|
|
| ||||||||||||
Total non-current liabilities |
|
|
|
| 231,916,385 |
|
| 185,705,563 |
|
| ||||||||
|
|
|
|
|
|
| ||||||||||||
Total liabilities |
|
|
|
| 236,569,253 |
|
| 190,229,276 |
|
| ||||||||
|
|
|
|
|
|
| ||||||||||||
EQUITY |
|
|
|
|
|
|
|
| ||||||||||
Common shares |
|
|
|
| 314,136 |
|
| 272,391 |
|
| ||||||||
Contributed surplus |
|
|
|
| 566,196 |
|
| 498,471 |
|
| ||||||||
Deficit |
|
|
|
| (190,779,597 | ) |
|
|
| (149,442,970 | ) |
|
|
| ||||
|
|
|
|
|
|
| ||||||||||||
Total equity |
|
|
|
| (189,899,265 | ) |
|
|
| (148,672,108 | ) |
|
|
| ||||
|
|
|
|
|
|
| ||||||||||||
Total equity and liabilities |
|
|
|
| $ |
| 46,669,988 |
|
| $ |
| 41,557,168 |
|
| ||||
|
|
|
|
|
|
| ||||||||||||
The accompanying notes are an integral part of these interim condensed consolidated financial statements.
F-2
CLEMENTIA PHARMACEUTICALS INC.
INTERIM CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
(unaudited)
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Common shares | Contributed | Deficit | Equity | ||||||||||||||||||||||||||||||||
Shares | $ | ||||||||||||||||||||||||||||||||||
| (in US dollars) | ||||||||||||||||||||||||||||||||||
December 31, 2016 |
|
| 196,109 |
|
| 272,391 |
|
| 498,471 |
|
| (149,442,970 | ) |
|
|
| (148,672,108 | ) |
| ||||||||||||||||
Exercise of stock options |
|
| 6,584 |
|
| 41,745 |
|
| (16,016 | ) |
|
|
| — |
|
| 25,729 | ||||||||||||||||||
Share-based compensation |
|
| — |
|
| — |
|
| 83,741 |
|
| — |
|
| 83,741 | ||||||||||||||||||||
Net loss and comprehensive loss |
|
| — |
|
| — |
|
| — |
|
| (41,336,627 | ) |
|
|
| (41,336,627 | ) |
| ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
March 31, 2017 |
|
| 202,693 |
|
| 314,136 |
|
| 566,196 |
|
| (190,779,597 | ) |
|
|
| (189,899,265 | ) |
| ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
December 31, 2015 |
|
| 196,109 |
|
| 272,391 |
|
| 324,052 |
|
| (91,930,991 | ) |
|
|
| (91,334,548 | ) |
| ||||||||||||||||
Share-based compensation |
|
| — |
|
| — |
|
| 52,739 |
|
| — |
|
| 52,739 | ||||||||||||||||||||
Net loss and comprehensive loss |
|
| — |
|
| — |
|
| — |
|
| (6,063,778 | ) |
|
|
| (6,063,778 | ) |
| ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
March 31, 2016 |
|
| 196,109 |
|
| 272,391 |
|
| 376,791 |
|
| (97,994,769 | ) |
|
|
| (97,345,587 | ) |
| ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
The accompanying notes are an integral part of these interim condensed consolidated financial statements.
F-3
CLEMENTIA PHARMACEUTICALS INC.
INTERIM CONDENSED CONSOLIDATED STATEMENTS OF NET LOSS AND
COMPREHENSIVE LOSS
(unaudited)
|
|
|
|
|
|
| ||||||||||
| Note | Three-month | Three-month | |||||||||||||
|
|
| (in US dollars) | |||||||||||||
Expenses |
|
|
|
|
|
| ||||||||||
Research and development expenses |
|
|
|
| $ |
| 3,407,511 |
|
| $ |
| 3,669,342 | ||||
Investment tax credits |
|
|
|
| (49,626 | ) |
|
|
| (46,922 | ) |
| ||||
|
|
|
|
|
|
| ||||||||||
|
|
|
|
| 3,357,885 |
|
| 3,622,420 | ||||||||
General and administrative expenses |
|
|
|
| 1,668,292 |
|
| 1,080,424 | ||||||||
Interest income |
|
|
|
| (80,997 | ) |
|
|
| (103,144 | ) |
| ||||
Financial expenses | 7 |
|
| 36,347,084 |
|
| 1,431,620 | |||||||||
|
|
|
|
|
|
| ||||||||||
Net loss before income taxes |
|
|
|
| 41,292,264 |
|
| 6,031,320 | ||||||||
Income tax expense |
|
|
|
| 44,363 |
|
| 32,458 | ||||||||
|
|
|
|
|
|
| ||||||||||
Net loss and comprehensive loss |
|
|
|
| $ |
| (41,336,627 | ) |
|
|
| $ |
| (6,063,778 | ) |
|
|
|
|
|
|
|
| ||||||||||
Basic and diluted loss per share |
|
|
|
| $ |
| (209.64 | ) |
|
|
| $ |
| (30.92 | ) |
|
Weighted average number of outstanding basic and diluted shares |
|
|
|
| 197,181 |
|
| 196,109 | ||||||||
|
|
|
|
|
|
| ||||||||||
The accompanying notes are an integral part of these interim condensed consolidated financial statements.
F-4
CLEMENTIA PHARMACEUTICALS INC.
INTERIM CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(unaudited)
|
|
|
|
|
|
| ||||||||||
| Note | Three-month | Three-month | |||||||||||||
|
|
| (in US dollars) | |||||||||||||
OPERATING ACTIVITIES |
|
|
|
|
|
| ||||||||||
Net loss |
|
|
|
| $ |
| (41,336,627 | ) |
|
|
| $ |
| (6,063,778 | ) |
|
Adjusting items |
|
|
|
|
|
| ||||||||||
Interest income recognized in net loss |
|
|
|
| (80,997 | ) |
|
|
| (103,144 | ) |
| ||||
Depreciation of property and equipment | 6 |
|
| 7,478 |
|
| 8,946 | |||||||||
Amortization of intangible assets | 6 |
|
| 33,818 |
|
| 34,193 | |||||||||
Transaction costs recognized in net loss | 7 |
|
| 35,175 |
|
| — | |||||||||
Embedded derivative loss recognized in net loss | 4 |
|
| 35,317,049 |
|
| 638,760 | |||||||||
Accretion of preferred shares | 4 |
|
| 988,038 |
|
| 915,371 | |||||||||
Share-based compensation |
|
|
|
| 83,741 |
|
| 52,739 | ||||||||
Net foreign exchange gain |
|
|
|
| (7,039 | ) |
|
|
| (128,821 | ) |
| ||||
Income tax expense recognized in net loss |
|
|
|
| 44,363 |
|
| 32,458 | ||||||||
Income taxes paid |
|
|
|
| (45,589 | ) |
|
|
| — | ||||||
Net changes in working capital |
|
|
|
|
|
| ||||||||||
Sales tax and other receivables |
|
|
|
| 39,292 |
|
| 20,999 | ||||||||
Investment tax credits receivable |
|
|
|
| (49,626 | ) |
|
|
| (46,922 | ) |
| ||||
Deferred financing costs |
|
|
|
| (93,544 | ) |
|
|
| — | ||||||
Prepaid expenses |
|
|
|
| (4,461 | ) |
|
|
| (551,121 | ) |
| ||||
Accounts payable and accrued liabilities |
|
|
|
| (868,716 | ) |
|
|
| (454,926 | ) |
| ||||
|
|
|
|
|
|
| ||||||||||
Net operating cash flows |
|
|
|
| (5,937,645 | ) |
|
|
| (5,645,246 | ) |
| ||||
INVESTING ACTIVITIES |
|
|
|
|
|
| ||||||||||
Interest income received |
|
|
|
| 331,043 |
|
| 13,397 | ||||||||
Acquisition of short-term investments |
|
|
|
| (20,000,000 | ) |
|
|
| (40,000,000 | ) |
| ||||
Maturity of short-term investment |
|
|
|
| 30,000,000 |
|
| — | ||||||||
Acquisition of property and equipment |
|
|
|
| (7,527 | ) |
|
|
| (19,475 | ) |
| ||||
|
|
|
|
|
|
| ||||||||||
Net investing cash flows |
|
|
|
| 10,323,516 |
|
| (40,006,078 | ) |
| ||||||
FINANCING ACTIVITIES |
|
|
|
|
|
| ||||||||||
Issuance of common shares |
|
|
|
| 25,729 |
|
| — | ||||||||
Issuance of preferred shares |
|
|
|
| 10,000,080 |
|
| — | ||||||||
Issues costs of preferred shares |
|
|
|
| (129,520 | ) |
|
|
| — | ||||||
|
|
|
|
|
| �� | ||||||||||
Net financing cash flows |
|
|
|
| 9,896,289 |
|
| — | ||||||||
|
|
|
|
|
|
| ||||||||||
Net increase (decrease) in cash |
|
|
|
| 14,282,160 |
|
| (45,651,324 | ) |
| ||||||
Cash at beginning of period |
|
|
|
| 9,434,495 |
|
| 58,106,885 | ||||||||
Effect of exchange rate fluctuations on cash held |
|
|
|
| 5,379 |
|
| 134,159 | ||||||||
|
|
|
|
|
|
| ||||||||||
CASH AT END OF PERIOD |
|
|
|
| $ |
| 23,722,034 |
|
| $ |
| 12,589,720 | ||||
|
|
|
|
|
|
| ||||||||||
The accompanying notes are an integral part of these interim condensed consolidated financial statements.
F-5
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL
STATEMENTS (unaudited)
Three-month periods ended March 31, 2017 and 2016 (in US dollars)
1. General information
Clementia Pharmaceuticals Inc. (the Company or Clementia) is a clinical stage biopharmaceutical company that is developing disease-modifying treatments for patients suffering from debilitating bone and other diseases with high unmet medical need. The Company’s lead product candidate, palovarotene, is an oral small molecule, first-in class, retinoic acid receptor gamma (RARg) agonist that has shown potent activity in preventing abnormal new bone formation as well as fibrosis in a variety of tissues. The Company is developing palovarotene for the treatment of Fibrodysplasia Ossificans Progressiva (FOP) and Multiple Osteochondroma (MO).
Clementia has not generated any revenue and has incurred net losses in each year since its inception and has an accumulated deficit of $190,779,597 as at March 31, 2017. Net losses were $41,336,627 for the three-month period ended March 31, 2017 and $57,511,979 for the year ended December 31, 2016 resulting primarily from financing activities as well as costs incurred in connection with research and development activities and general and administrative costs associated with operations. Operating activities used $5,937,645 in cash for the three-month period ended March 31, 2017 and $18,828,083 for the year ended December 31, 2016. The Company expects that its existing cash and short-term investments as of March 31, 2017 will enable it to fund its planned operating expenses for at least the next twelve months from March 31, 2017.
We expect to incur significant expenses and increasing operating losses for the foreseeable future. We expect our expenses will increase substantially in connection with our ongoing activities, particularly as we advance clinical development of palovarotene, our lead product candidate for the treatment of FOP, including conducting two clinical trials; advance development of palovarotene in the treatment of MO; continue research and development efforts to support clinical development of additional RARg agonist candidates; continue to engage contract manufacturing organizations (CMOs) to manufacture our clinical study materials and to develop large-scale manufacturing capabilities; seek regulatory approval for our product candidates; add personnel to support our product development and future commercialization; add operational, financial and management information systems; maintain, leverage and expand our intellectual property portfolio; and operate as a public company.
We do not expect to generate revenue from product sales unless and until we successfully complete development and obtain regulatory approval for palovarotene or any other product candidate, which we expect will take a number of years and is subject to significant uncertainty. If we obtain regulatory approval for any of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing, and distribution. As a result, we will need additional financing to support our continuing operations. Until such time that we can generate significant revenue from product sales, if ever, we expect to finance our operations through a combination of public or private equity or debt financings or other sources, which may include collaborations with third parties. Arrangements with collaborators or others may require us to relinquish rights to certain of our technologies or product candidates, obtain adequate patent protection for our technology, obtain necessary regulatory approval for our product candidates or achieve commercial viability for any approved product candidates. Adequate additional financing may not be available to us on acceptable terms, or at all. Our inability to raise capital as and when needed would have a negative impact on our financial condition and our ability to pursue our business strategy. We will need to generate significant revenue to achieve profitability, and we may never do so.
Clementia is incorporated under the laws of Canada. The address of the Company’s registered office is 4150 Sainte-Catherine Street West, Suite 550, Montréal, Québec, Canada, H3Z 2Y5.
F-6
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL
STATEMENTS (unaudited)—(Continued)
Three-month periods ended March 31, 2017 and 2016 (in US dollars)
2. Significant accounting policies
Statement of compliance and basis of preparation
These interim condensed consolidated financial statements have been prepared in accordance with International Accounting Standard (IAS) 34,Interim Financial Reporting, as issued by the International Accounting Standards Board (IASB), and were approved by the board of directors and authorized for issue on May 12, 2017, except as it relates to subequent event disclosures in Note 11, which were authorized on June 29, 2017.
These interim condensed consolidated financial statements were prepared using the same accounting policies as set forth in notes 2 and 3 in the audited consolidated financial statements of the Company for the year ended December 31, 2016, except as discussed in note 4. These interim condensed consolidated financial statements do not include all the notes required in annual financial statements. Therefore, these interim condensed consolidated financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto of the Company for the year ended December 31, 2016.
The preparation of the Company’s interim condensed consolidated financial statements in conformity with IFRS requires management to make judgements, estimates and assumptions that affect the application of accounting policies and the reported amounts of revenues, expenditures, assets and liabilities. Actual results could differ from those estimates. On an ongoing basis, estimates and judgements are evaluated. The Company bases its estimates on the most probable set of economic conditions and planned course of action, historical experience, known trends and events, and various other factors believed to be reasonable under the circumstances, the results of which form the basis for making judgements about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions and conditions. Uncertainty about these assumptions and estimates could result in outcomes that require material adjustments to the carrying amount of the asset or liability in future periods. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which these estimates are revised and in any future periods affected. Balances and transactions that are subject to a high degree of estimation are the estimation of accrued expenses and the valuation of the embedded derivatives of the preferred shares. The critical accounting judgements and key sources of estimate uncertainty are consistent with those in the audited consolidated financial statements and notes thereto of the Company for the year ended December 31, 2016.
Deferred financing costs
Financing costs consist of legal and other advisory costs related to the Company’s proposed initial public offering (IPO) of its common shares. Financing costs allocated to the listing of the Company’s existing common shares in the amount of $371,752 are expensed as incurred under general and administrative expenses. Financing costs allocated to estimated new common shares issued as part of the IPO are deferred until completion of the IPO. At March 31, 2017, the Company had deferred financing costs of $93,544. Upon completion of the IPO, deferred financing costs will be transferred to share capital as a reduction of the IPO proceeds. Deferred financing costs will be expensed under general and administrative expenses should the proposed IPO not be completed.
3. Future changes in accounting policies
The IASB has issued several new standards and amendments to standards and interpretations that are not effective for the year ended December 31, 2016, and although early adoption is permitted, they have not been applied in preparing these consolidated financial statements. The Company is currently
F-7
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL
STATEMENTS (unaudited)—(Continued)
Three-month periods ended March 31, 2017 and 2016 (in US dollars)
evaluating the effect, if any, the following new standards and amendments will have on its financial results.
(i) | Financial Instruments(IFRS 9), effective for annual periods beginning on or after January 1, 2018, replaces the requirements in IAS 39,Financial Instruments, Recognition and Measurementfor classification and measurement of financial assets and liabilities. IFRS 9 introduces a single classification and measurement approach for financial instruments, which is driven by cash flow characteristics and the business model in which an asset is held. This single, principle-based approach replaces existing rule-based requirements and results in a single impairment model being applied to all financial instruments. IFRS 9 also modified the hedge accounting model to incorporate the risk management practices of an entity. Additional disclosures will also be required under the new standard. Early adoption of IFRS 9 is permitted. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
(ii) | Leases(IFRS 16), effective for annual periods beginning on or after January 1, 2019, provides a comprehensive model for the identification of lease arrangements and their treatment in the financial statements of both lessees and lessors. It supersedes IAS 17 Leases and its associated interpretive guidance. Significant changes were made to lessee accounting with the distinction between operating and finance leases removed and assets and liabilities recognized in respect of all leases (subject to limited exceptions for short-term leases and leases of low value assets). Earlier application of IFRS 16 is permitted for companies that have also adopted IFRS 15,Revenue from Contracts with Customers. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
4. Preferred shares
On March 16, 2017, the Company completed a $10,000,080 Class C financing with a new investor. Under the agreed terms, the Company issued 70,176 Class C redeemable convertible preferred shares at $142.50 per share for a total consideration of $10,000,080, less $129,520 in share issuance costs. The terms of the Class C redeemable convertible preferred shares are substantially similar as those of the Class A and B redeemable convertible preferred shares.
As at March 31, 2017, there were 1,118,415 Class A redeemable convertible preferred shares issued and outstanding at a price of $29.245 per share (1,118,415 as at December 31, 2016), 485,823 Class B redeemable convertible preferred shares issued and outstanding at a price of $123.50 per share (485,823 as at December 31, 2016) and 70,176 Class C redeemable convertible preferred shares issued and outstanding at a price of $142.50 per shares (nil as at December 31, 2016). As at March 31, 2017 and pursuant to certain terms and conditions, the Class A, B and C redeemable convertible preferred shares are convertible on a one-for-one basis into 1,674,414 common shares.
Changes in preferred shares and embedded derivatives for the three-months ended March 31, 2017 and 2016 were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||
| Preferred shares | Embedded derivative | ||||||||||||||||||||||||||||||||||||||||
Class A | Class B | Class C | Class A | Class B | Class C | |||||||||||||||||||||||||||||||||||||
Balance, December 31, 2016 |
|
| $ |
| 24,993,486 |
|
| $ |
| 42,887,466 |
|
| $ |
| — |
|
| $ |
| 83,355,470 |
|
| $ |
| 34,469,141 |
|
| $ |
| — | ||||||||||||
Issuance of preferred shares |
|
| — |
|
| — |
|
| 7,284,269 |
|
| — |
|
| — |
|
| 2,715,811 | ||||||||||||||||||||||||
Transaction costs |
|
| — |
|
| — |
|
| (94,345 | ) |
|
|
| — |
|
| — |
|
| — | ||||||||||||||||||||||
Accretion during the period |
|
| 307,595 |
|
| 662,239 |
|
| 18,204 |
|
| — |
|
| — |
|
| — | ||||||||||||||||||||||||
Loss (gain) on re-measurement at fair value |
|
| — |
|
| — |
|
| — |
|
| 44,814,889 |
|
| (9,497,840 | ) |
|
|
| — | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||
Balance, March 31, 2017 |
|
| $ |
| 25,301,081 |
|
| $ |
| 43,549,705 |
|
| $ |
| 7,208,128 |
|
| $ |
| 128,170,359 |
|
| $ |
| 24,971,301 |
|
| $ |
| 2,715,811 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||
F-8
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL
STATEMENTS (unaudited)—(Continued)
Three-month periods ended March 31, 2017 and 2016 (in US dollars)
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||
| Preferred shares | Embedded derivative | ||||||||||||||||||||||||||||||||||||||||
Class A | Class B | Class C | Class A | Class B | Class C | |||||||||||||||||||||||||||||||||||||
Balance, December 31, 2015 |
|
| $ |
| 23,801,078 |
|
| $ |
| 40,337,696 |
|
| $ |
| — |
|
| $ |
| 61,893,086 |
|
| $ |
| 21,949,483 |
|
| $ |
| — | ||||||||||||
Issuance of preferred shares |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — | ||||||||||||||||||||||||
Transaction costs |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — | ||||||||||||||||||||||||
Accretion during the period |
|
| 292,504 |
|
| 622,867 |
|
| — |
|
| — |
|
| — |
|
| — | ||||||||||||||||||||||||
Loss (gain) on re-measurement at fair value |
|
| — |
|
| — |
|
| — |
|
| 424,997 |
|
| 213,763 |
|
| — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||
Balance, March 31, 2016 |
|
| $ |
| 24,093,582 |
|
| $ |
| 40,960,563 |
|
| $ |
| — |
|
| $ |
| 62,318,083 |
|
| $ |
| 22,163,246 |
|
| $ |
| — | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||
The fair value of the embedded derivative conversion options prior to March 16, 2017 were estimated using a Monte Carlo simulation model.
The fair value of the embedded derivative conversion options at March 31, 2017, and at inception for the Class C preferred shares, were estimated using a hybrid of the probability-weighted expected return method (PWERM), weighted at 75%, and a Monte Carlo simulation model, weighted at 25%. The Company integrated a PWERM model into its valuation methodology as, during the first quarter of 2017, it has undertaken tangible steps towards a qualifying IPO and it believes this model to be a more accurate estimation method of the conversion option.
Under the PWERM methodology, the fair value was estimated based upon the future implied equity values using a range of low, medium and high exit multiples. Exit multiples were derived from comparable public company transactions that compared the invested capital (being the aggregate of debt and shares) to the pre-IPO equity values. The estimated implied equity value was discounted back from the estimated time to exit to the March 31, 2017 valuation date.
The fair value of the embedded derivative conversion options were estimated at inception and on a recurring basis using the following key assumptions, including a nil dividend yield.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Mar 31, 2017 | Inception | Dec 31, 2016 | March 31, 2016 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Class A | Class B | Class C | Class C | Class A | Class B | Class C | Class A | Class B | Class C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fair value of embedded derivative per share |
|
| $ |
| 114.60 |
|
| $ |
| 51.40 |
|
| $ |
| 38.70 |
|
| $ |
| 38.70 |
|
| $ |
| 74.53 |
|
| $ |
| 70.95 |
|
| — |
|
| $ |
| 55.72 |
|
| $ |
| 45.62 |
|
| — | ||||||||||||||||||||||||
PWERM assumptions: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
Range of exit multiples |
|
| 3.4-4.1 |
|
| 3.4-4.1 |
|
| 3.4-4.1 |
|
| 3.4-4.1 |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — | ||||||||||||||||||||||||||||||||||||||||
Time to exit (in years) |
|
| 0.50 |
|
| 0.50 |
|
| 0.50 |
|
| 0.50 |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — |
|
| — | ||||||||||||||||||||||||||||||||||||||||
Monte Carlo assumptions: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||
Starting equity value (in millions of $) |
|
| $ |
| 298.1 |
|
| $ |
| 298.1 |
|
| $ |
| 298.1 |
|
| $ |
| 298.1 |
|
| $ |
| 249.6 |
|
| $ |
| 249.6 |
|
| — |
|
| $ |
| 170.9 |
|
| $ |
| 170.9 |
|
| — | ||||||||||||||||||||||||
Volatility |
|
| 74 | % |
|
|
| 74 | % |
|
|
| 74 | % |
|
|
| 74 | % |
|
|
| 68 | % |
|
|
| 68 | % |
|
|
| — |
|
| 89 | % |
|
|
| 89 | % |
|
|
| — | ||||||||||||||||||||||||
Weighted average time to exit (in years) |
|
| 0.75 |
|
| 0.75 |
|
| 0.75 |
|
| 0.75 |
|
| 0.85 |
|
| 0.85 |
|
| — |
|
| 2.00 |
|
| 2.00 |
|
| — | ||||||||||||||||||||||||||||||||||||||||
These derivative liabilities are classified as a Level 3 in the fair value hierarchy. A reasonably possible movement in the estimated starting equity value, expected volatility or expected time to exit could significantly impact the fair value of the embedded derivative.
As at March 31, 2017, and assuming that all other variables remain constant, a 10% increase and decrease in the following assumptions would result in the following approximate increases and
F-9
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL
STATEMENTS (unaudited)—(Continued)
Three-month periods ended March 31, 2017 and 2016 (in US dollars)
decreases in the per-share fair value of the embedded derivative, respectively, for Class A, B and C preferred shares.
|
|
|
|
|
|
| |||||||||||||||
| Embedded derivative per-share fair value increase/decrease | ||||||||||||||||||||
Class A | Class B | Class C | |||||||||||||||||||
10% increase in low-medium-high exit multiples |
|
| $ |
| 11.14 |
|
| $ |
| 11.12 |
|
| $ |
| 11.11 | ||||||
10% decrease in low-medium-high exit multiples |
|
| $ |
| (11.08 | ) |
|
|
| $ |
| (11.09 | ) |
|
|
| $ |
| (11.11 | ) |
|
10% increase in volatility |
|
| $ |
| 0.20 |
|
| $ |
| 0.21 |
|
| $ |
| 0.21 | ||||||
10% decrease in volatility |
|
| $ |
| (0.19 | ) |
|
|
| $ |
| (0.19 | ) |
|
|
| $ |
| (0.20 | ) |
|
10% increase in estimated starting equity value |
|
| $ |
| 3.30 |
|
| $ |
| 3.30 |
|
| $ |
| 3.29 | ||||||
10% decrease in estimated starting equity value |
|
| $ |
| (3.26 | ) |
|
|
| $ |
| (3.25 | ) |
|
|
| $ |
| (3.26 | ) |
|
5. Share-based payments
Under the Company’s Employee Stock Option Plan (ESOP), the Company can grant to its directors, management and employees non-transferrable stock options for the purchase of common shares. The maximum number of common shares available for issuance under the ESOP is limited to 315,837 as at March 31, 2017 (315,837 as at December 31, 2016).
Changes in the number of stock options outstanding are as follows:
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
| Three-months ended | Three-months ended | ||||||||||||||||||||||||||
Options | Weighted | Options | Weighted | |||||||||||||||||||||||||
Balance at beginning of year |
|
| 204,636 |
|
| $ |
| 5.23 |
|
| 205,414 |
|
| $ |
| 5.24 | ||||||||||||
Issued during the period |
|
| 14,550 |
|
| $ |
| 116.29 |
|
| — |
|
| — | ||||||||||||||
Exercised during the period |
|
| (6,584 | ) |
|
|
| $ |
| 3.91 |
|
| — |
|
| — | ||||||||||||
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
Balance at end of period |
|
| 212,602 |
|
| $ |
| 12.87 |
|
| 205,414 |
|
| $ |
| 5.24 | ||||||||||||
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
The following table summarizes the information related to outstanding stock options as at March 31, 2017.
|
|
|
|
|
|
|
|
|
|
|
Exercise price | Outstanding stock options | Exercisable stock options | ||||||||
Number of | Weighted average | Weighted | Number of | Weighted | ||||||
$3.50 | 171,466 | 7.0 |
|
| 106,427 |
|
| |||
$8.33 | 22,084 | 8.1 |
|
| 10,658 |
|
| |||
$57.68 | 4,502 | 8.7 |
|
| 1,650 |
|
| |||
$116.29 | 14,550 | 9.9 |
|
| — |
|
| |||
|
|
|
|
|
|
|
|
|
|
|
| 212,602 | 7.3 | $12.87 | 118,735 | $4.69 | |||||
|
|
|
|
|
|
|
|
|
|
|
During the three-month period ended March 31, 2017, the Company recorded a stock-based compensation expense of $83,741 ($52,739 during the three-month period ended March 31, 2016) of which $25,255 ($21,413 in 2016) was recorded in general and administrative expenses and $58,486 ($31,326 in 2016) in research and development expenses.
As of March 31, 2017, the Company had $1.2 million of total unrecognized stock-based compensation expense, net of related forfeiture estimates, which is expected to be recognized over a weighted-average remaining vesting period of approximately 1.5 years.
F-10
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL
STATEMENTS (unaudited)—(Continued)
Three-month periods ended March 31, 2017 and 2016 (in US dollars)
The fair value of the stock options granted on February 28, 2017 was estimated at the date of grant using the Black-Scholes option pricing model with the following weighted average assumptions:
|
|
|
|
| ||||||||||
| 2017 | 2016 | ||||||||||||
Grant (number of stock options) |
|
| 14,550 |
|
| — | ||||||||
Weighted average fair value of stock options |
|
| $ |
| 81.56 |
|
| — | ||||||
Weighted average exercise price |
|
| $ |
| 116.29 |
|
| — | ||||||
Weighted average assumptions: |
|
|
|
| ||||||||||
Share price |
|
| $ |
| 116.29 |
|
| — | ||||||
Risk-free interest rate |
|
| 2.04 | % |
|
|
| — | ||||||
Expected dividend yield |
|
| Nil |
|
| — | ||||||||
Volatility factor |
|
| 81.56 | % |
|
|
| — | ||||||
Expected life (in years) |
|
| 6.00 |
|
| — | ||||||||
The Black-Scholes model requires subjective assumptions, which affect the calculated values. The assumptions used represent the Company’s best estimates at the time of grant.
6. Additional information on the consolidated statements of loss and comprehensive loss
|
|
|
|
| ||||||||||
| 2017 | 2016 | ||||||||||||
Included in research and development expenses: |
|
|
|
| ||||||||||
Employee benefits expense |
|
| $ |
| 780,871 |
|
| $ |
| 627,726 | ||||
Depreciation of property and equipment |
|
| $ |
| 4,673 |
|
| $ |
| 5,515 | ||||
Expenses related to minimum operating lease payments |
|
| $ |
| 101,623 |
|
| $ |
| 71,036 | ||||
Included in general and administrative expenses: |
|
|
|
| ||||||||||
Employee benefits expense |
|
| $ |
| 516,806 |
|
| $ |
| 637,707 | ||||
Depreciation of property and equipment |
|
| $ |
| 2,805 |
|
| $ |
| 3,431 | ||||
Amortization of intangible assets |
|
| $ |
| 33,818 |
|
| $ |
| 34,193 | ||||
Expenses related to minimum operating lease payments |
|
| $ |
| 29,888 |
|
| $ |
| 39,426 | ||||
7. Financial expenses
|
|
|
|
| ||||||||||
| 2017 | 2016 | ||||||||||||
Financial expenses |
|
|
|
| ||||||||||
Transaction costs—embedded derivatives |
|
| $ |
| 35,175 |
|
| $ |
| — | ||||
Accretion—preferred shares |
|
| 988,038 |
|
| 915,371 | ||||||||
Loss on re-measurement at fair value |
|
| 35,317,049 |
|
| 638,760 | ||||||||
Bank charges and other interest |
|
| 2,176 |
|
| 3,934 | ||||||||
Foreign exchange losses (gains) |
|
| 4,646 |
|
| (126,445 | ) |
| ||||||
|
|
|
|
| ||||||||||
Total financial expenses |
|
| $ |
| 36,347,084 |
|
| $ |
| 1,431,620 | ||||
|
|
|
|
| ||||||||||
8. Financial instruments
The Company has determined that the carrying amounts of its short-term financial assets and liabilities, including cash, short-term investments and accounts payable and accrued liabilities approximate their fair value due to the relatively short periods to maturity for these instruments.
9. Commitments
On March 29, 2017, the Company entered into an exclusive licensing agreement with Galderma to obtain access to retinoic acid receptor gamma agonist compounds and was granted exclusive rights to
F-11
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE INTERIM CONDENSED CONSOLIDATED FINANCIAL
STATEMENTS (unaudited)—(Continued)
Three-month periods ended March 31, 2017 and 2016 (in US dollars)
use these in non-dermatological indications. In accordance with this agreement, and subsequent to March 31, 2017, the Company has paid a one-time license fee, which was recorded as an intangible asset, and is committed to making certain future payments based on the successful achievement of specific development and commercialization milestones related to the licensed Galderma compounds. Future royalty payments based on net sales are also stipulated in the licensing agreement. The likelihood and timing of these payments is unknown at this time.
10. Segmented information
The Company manages its operations as a single segment for the purposes of assessing performance and making operating decisions, being the biopharmaceutical segment. The Company’s focus is on advancing treatments for people living with rare diseases, including FOP and MO.
All of the Company’s intangible assets are held in Canada. As at March 31, 2017, the Company’s property and equipment are held as follows: 83% held in Canada and 17% held in the United States.
11. Subsequent events
On April 30, 2017, the Company granted 39,100 stock options at an exercise price of $120.36.
F-12
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors of Clementia Pharmaceuticals Inc.
We have audited the accompanying consolidated statements of financial position of Clementia Pharmaceuticals Inc. as of December 31, 2016, December 31, 2015 and December 31, 2014 and the related consolidated statements of net loss and comprehensive loss, changes in equity and cash flows for the years then ended. These consolidated financial statements are the responsibility of Clementia Pharmaceuticals Inc.’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with Canadian generally accepted auditing standards and the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of Clementia Pharmaceuticals Inc. as of December 31, 2016, December 31, 2015 and December 31, 2014 and its consolidated financial performance and its consolidated cash flows for the years then ended in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
/s/ KPMG LLP*
February 28, 2017, except as it relates to Note 17, which is dated as of June 29, 2017
Montréal, Canada
*CPA auditor, CA, public accounting permit No. A125211.
KPMG LLP is a Canadian limited liability partnership and a member firm of the KPMG
network of independent member firms affiliated with KPMG International Cooperative
("KPMG International"), a Swiss entity.
KPMG Canada provides services to KPMG LLP.
F-13
CLEMENTIA PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
|
|
|
|
|
|
|
|
| |||||||||||||||
As at | Note | December 31, | December 31, | December 31, | |||||||||||||||||||
|
|
| (in US dollars) | ||||||||||||||||||||
ASSETS |
|
|
|
|
|
|
|
| |||||||||||||||
Current Assets |
|
|
|
|
|
|
|
| |||||||||||||||
Cash |
|
|
|
| $ |
| 9,434,495 |
|
| $ |
| 58,106,885 |
|
| $ |
| 5,503,938 | ||||||
Short-term investments |
|
|
|
| 30,000,000 |
|
| — |
|
| — | ||||||||||||
Interest receivable |
|
|
|
| 307,579 |
|
| 4,546 |
|
| — | ||||||||||||
Sales tax and other receivables |
|
|
|
| 90,966 |
|
| 41,860 |
|
| 74,647 | ||||||||||||
Investment tax credits receivable |
|
|
|
| 139,223 |
|
| 377,913 |
|
| 292,553 | ||||||||||||
Prepaid expenses |
|
|
|
| 652,158 |
|
| 1,002,287 |
|
| 1,068,044 | ||||||||||||
Prepaid income taxes |
|
|
|
| — |
|
| 46,060 |
|
| — | ||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Total current assets |
|
|
|
| 40,624,421 |
|
| 59,579,551 |
|
| 6,939,182 | ||||||||||||
Non-current assets |
|
|
|
|
|
|
|
| |||||||||||||||
Property and equipment |
|
|
|
| 38,163 |
|
| 45,300 |
|
| 10,951 | ||||||||||||
Intangible assets | 5 |
|
| 894,584 |
|
| 1,032,110 |
|
| 1,050,092 | |||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Total non-current assets |
|
|
|
| 932,747 |
|
| 1,077,410 |
|
| 1,061,043 | ||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Total assets |
|
|
|
| $ |
| 41,557,168 |
|
| $ |
| 60,656,961 |
|
| $ |
| 8,000,225 | ||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
LIABILITIES |
|
|
|
|
|
|
|
| |||||||||||||||
Current Liabilities |
|
|
|
|
|
|
|
| |||||||||||||||
Accounts payable and accrued liabilities |
|
|
|
| $ |
| 4,521,537 |
|
| $ |
| 4,010,166 |
|
| $ |
| 1,960,083 | ||||||
Income taxes payable |
|
|
|
| 2,176 |
|
| — |
|
| 94,350 | ||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Total current liabilities |
|
|
|
| 4,523,713 |
|
| 4,010,166 |
|
| 2,054,433 | ||||||||||||
Non-current liabilities |
|
|
|
|
|
|
|
| |||||||||||||||
Preferred shares | 6 |
|
| 67,880,952 |
|
| 64,138,774 |
|
| 14,107,447 | |||||||||||||
Embedded derivatives | 6 |
|
| 117,824,611 |
|
| 83,842,569 |
|
| 7,490,662 | |||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Total non-current liabilities |
|
|
|
| 185,705,563 |
|
| 147,981,343 |
|
| 21,598,109 | ||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Total liabilities |
|
|
|
| 190,229,276 |
|
| 151,991,509 |
|
| 23,652,542 | ||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
EQUITY |
|
|
|
|
|
|
|
| |||||||||||||||
Common shares | 7 |
|
| 272,391 |
|
| 272,391 |
|
| 194,651 | |||||||||||||
Contributed surplus |
|
|
| �� | 498,471 |
|
| 324,052 |
|
| 187,080 | ||||||||||||
Deficit |
|
|
|
| (149,442,970 | ) |
|
|
| (91,930,991 | ) |
|
|
| (16,034,048 | ) |
| ||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Total equity |
|
|
|
| (148,672,108 | ) |
|
|
| (91,334,548 | ) |
|
|
| (15,652,317 | ) |
| ||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Commitments and contingencies | 13 |
|
|
|
|
|
| ||||||||||||||||
Subsequent events | 17 |
|
|
|
|
|
| ||||||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Total equity and liabilities |
|
|
|
| $ |
| 41,557,168 |
|
| $ |
| 60,656,961 |
|
| $ |
| 8,000,225 | ||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-14
CLEMENTIA PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
Note | Common shares | Contributed | Deficit | Equity | |||||||||||||||||||||||||||||||||
Shares | $ | ||||||||||||||||||||||||||||||||||||
|
|
| (in US dollars) | ||||||||||||||||||||||||||||||||||
January 1, 2014 |
|
|
|
| 176,477 |
|
| 176,192 |
|
| 44,269 |
|
| (3,745,439 | ) |
|
|
| (3,524,978 | ) |
| ||||||||||||||||
Issuance of common shares | 7 |
|
| 5,273 |
|
| 18,459 |
|
| — |
|
| — |
|
| 18,459 | |||||||||||||||||||||
Share-based compensation | 8 |
|
| — |
|
| — |
|
| 142,811 |
|
| — |
|
| 142,811 | |||||||||||||||||||||
Net loss and comprehensive loss |
|
|
|
| — |
|
| — |
|
| — |
|
| (12,288,609 | ) |
|
|
| (12,288,609 | ) |
| ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
December 31, 2014 |
|
|
|
| 181,750 |
|
| 194,651 |
|
| 187,080 |
|
| (16,034,048 | ) |
|
|
| (15,652,317 | ) |
| ||||||||||||||||
Issuance of common shares | 7 |
|
| 3,431 |
|
| 12,008 |
|
| — |
|
| — |
|
| 12,008 | |||||||||||||||||||||
Exercise of stock options |
|
|
|
| 10,928 |
|
| 65,732 |
|
| (27,484 | ) |
|
|
| — |
|
| 38,248 | ||||||||||||||||||
Share-based compensation | 8 |
|
| — |
|
| — |
|
| 164,456 |
|
| — |
|
| 164,456 | |||||||||||||||||||||
Net loss and comprehensive loss |
|
|
|
| — |
|
| — |
|
| — |
|
| (75,896,943 | ) |
|
|
| (75,896,943 | ) |
| ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
December 31, 2015 |
|
|
|
| 196,109 |
|
| 272,391 |
|
| 324,052 |
|
| (91,930,991 | ) |
|
|
| (91,334,548 | ) |
| ||||||||||||||||
Share-based compensation | 8 |
|
| — |
|
| — |
|
| 174,419 |
|
| — |
|
| 174,419 | |||||||||||||||||||||
Net loss and comprehensive loss |
|
|
|
| — |
|
| — |
|
| — |
|
| (57,511,979 | ) |
|
|
| (57,511,979 | ) |
| ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
December 31, 2016 |
|
|
|
| 196,109 |
|
| 272,391 |
|
| 498,471 |
|
| (149,442,970 | ) |
|
|
| (148,672,108 | ) |
| ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-15
CLEMENTIA PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF NET LOSS AND COMPREHENSIVE LOSS
|
|
|
|
|
|
|
|
| |||||||||||||||
| Note | Year ended | Year ended | Year ended | |||||||||||||||||||
|
|
| (In US dollars) | ||||||||||||||||||||
Expenses |
|
|
|
|
|
|
|
| |||||||||||||||
Research and development expenses |
|
|
|
| $ |
| 16,851,974 |
|
| $ |
| 14,396,563 |
|
| $ |
| 7,797,081 | ||||||
Investment tax credits |
|
|
|
| (139,212 | ) |
|
|
| (165,124 | ) |
|
|
| (213,917 | ) |
| ||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
|
|
|
|
| 16,712,762 |
|
| 14,231,439 |
|
| 7,583,164 | ||||||||||||
General and administrative expenses |
|
|
|
| 3,405,615 |
|
| 5,478,833 |
|
| 2,266,021 | ||||||||||||
Interest income |
|
|
|
| (398,559 | ) |
|
|
| (109,670 | ) |
|
|
| (18,852 | ) |
| ||||||
Financial expenses | 12 |
|
| 37,645,707 |
|
| 56,140,121 |
|
| 2,363,410 | |||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Net loss before income taxes |
|
|
|
| 57,365,525 |
|
| 75,740,723 |
|
| 12,913,743 | ||||||||||||
Income tax expense | 10 |
|
| 146,454 |
|
| 156,220 |
|
| 94,866 | |||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Net loss and comprehensive loss |
|
|
|
| $ |
| (57,511,979 | ) |
|
|
| $ |
| (75,896,943 | ) |
|
|
| $ |
| (12,288,609 | ) |
|
|
|
|
|
|
|
|
|
| |||||||||||||||
Additional information | 11 |
|
|
|
|
|
| ||||||||||||||||
Basic and diluted loss per share | 9 |
|
| $ |
| (293.27 | ) |
|
|
| $ |
| (396.45 | ) |
|
|
| $ |
| (68.32 | ) |
| |
Weighted average number of outstanding basic and diluted shares |
|
|
|
| 196,109 |
|
| 191,443 |
|
| 179,874 | ||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-16
CLEMENTIA PHARMACEUTICALS INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
|
|
|
|
|
|
| |||||||||||||||
| Note | Year ended | Year ended | Year ended | |||||||||||||||||||
|
|
| (In US dollars) | ||||||||||||||||||||
OPERATING ACTIVITIES |
|
|
|
|
|
|
|
| |||||||||||||||
Net loss |
|
|
|
| $ |
| (57,511,979 | ) |
|
|
| $ |
| (75,896,943 | ) |
|
|
| $ |
| (12,288,609 | ) |
|
Adjusting items |
|
|
|
|
|
|
|
| |||||||||||||||
Interest income recognized in net loss |
|
|
|
| (398,559 | ) |
|
|
| (109,670 | ) |
|
|
| (18,852 | ) |
| ||||||
Depreciation of property and equipment |
|
|
|
| 35,055 |
|
| 14,506 |
|
| 5,719 | ||||||||||||
Amortization of intangible assets | 5 |
|
| 137,526 |
|
| 134,258 |
|
| 130,129 | |||||||||||||
Transaction costs recognized in net loss |
|
|
|
| — |
|
| 819,271 |
|
| 10,374 | ||||||||||||
Embedded derivative loss recognized in net loss | 6 |
|
| 33,982,042 |
|
| 52,563,759 |
|
| 1,608,871 | |||||||||||||
Accretion of preferred shares | 6 |
|
| 3,742,178 |
|
| 2,378,992 |
|
| 606,153 | |||||||||||||
Share-based compensation | 8 |
|
| 174,419 |
|
| 164,456 |
|
| 142,811 | |||||||||||||
Net foreign exchange (gain) loss |
|
|
|
| (82,589 | ) |
|
|
| 382,982 |
|
| 129,245 | ||||||||||
Income tax expense recognized in net loss | 10 |
|
| 146,454 |
|
| 156,220 |
|
| 94,866 | |||||||||||||
Income taxes paid |
|
|
|
| (98,218 | ) |
|
|
| (296,630 | ) |
|
|
| (516 | ) |
| ||||||
Net changes in working capital |
|
|
|
|
|
|
|
| |||||||||||||||
Sales tax and other receivable |
|
|
|
| (48,207 | ) |
|
|
| 24,435 |
|
| (60,511 | ) |
| ||||||||
Investment tax credits receivable |
|
|
|
| 238,690 |
|
| (85,360 | ) |
|
|
| (213,917 | ) |
| ||||||||
Prepaid expenses |
|
|
|
| 350,129 |
|
| 65,757 |
|
| (820,665 | ) |
| ||||||||||
Accounts payable and accrued liabilities |
|
|
|
| 504,976 |
|
| 2,060,688 |
|
| 1,017,276 | ||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Net operating cash flows |
|
|
|
| (18,828,083 | ) |
|
|
| (17,623,279 | ) |
|
|
| (9,657,626 | ) |
| ||||||
INVESTING ACTIVITIES |
|
|
|
|
|
|
|
| |||||||||||||||
Interest income received |
|
|
|
| 95,526 |
|
| 105,124 |
|
| 18,852 | ||||||||||||
Acquisition of short-term investments |
|
|
|
| (40,000,000 | ) |
|
|
| (40,000,000 | ) |
|
|
| — | ||||||||
Maturity of short-term investments |
|
|
|
| 10,000,000 |
|
| 40,000,000 |
|
| — | ||||||||||||
Acquisition of property and equipment |
|
|
|
| (27,918 | ) |
|
|
| (48,855 | ) |
|
|
| (9,081 | ) |
| ||||||
Acquisition of intangible assets | 5 |
|
| — |
|
| (116,276 | ) |
|
|
| (130,661 | ) |
| |||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Net investing cash flows |
|
|
|
| (29,932,392 | ) |
|
|
| (60,007 | ) |
|
|
| (120,890 | ) |
| ||||||
FINANCING ACTIVITIES |
|
|
|
|
|
|
|
| |||||||||||||||
Issuance of common shares | 7 |
|
| — |
|
| 50,256 |
|
| 18,459 | |||||||||||||
Issuance of preferred shares | 6 |
|
| — |
|
| 73,182,730 |
|
| 11,999,983 | |||||||||||||
Issuance costs of preferred shares | 6 |
|
| — |
|
| (2,561,518 | ) |
|
|
| (31,128 | ) |
| |||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Net financing cash flows |
|
|
|
| — |
|
| 70,671,468 |
|
| 11,987,314 | ||||||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
Net (decrease) increase in cash |
|
|
|
| (48,760,475 | ) |
|
|
| 52,988,181 |
|
| 2,208,798 | ||||||||||
Cash, beginning of year |
|
|
|
| 58,106,885 |
|
| 5,503,938 |
|
| 3,414,749 | ||||||||||||
Effect of exchange rate fluctuations on cash held |
|
|
|
| 88,085 |
|
| (385,235 | ) |
|
|
| (119,609 | ) |
| ||||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
CASH, END OF YEAR |
|
|
|
| $ |
| 9,434,495 |
|
| $ |
| 58,106,885 |
|
| $ |
| 5,503,938 | ||||||
|
|
|
|
|
|
|
|
| |||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-17
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
1. General information
Clementia Pharmaceuticals Inc. (the Company or Clementia) is a privately-held, clinical stage biopharmaceutical company dedicated to the research and development of treatments for people living with rare diseases by exploiting the use of retinoic acid reception gamma agonists (RARg) to address diseases of heterotopic ossification, including Fibrodysplasia Ossificans Progressiva (FOP). FOP is a rare, severely disabling and life-shortening disease characterized by spontaneous and recurrent episodes of heterotopic bone formation. Clementia is advancing its lead product candidate, palovarotene and has completed Phase 2 clinical trials.
Clementia is a development stage company and has not generated any revenue. It has incurred net losses in each year since its inception and has an accumulated deficit of $149,442,970 as at December 31, 2016. Net losses were $57,511,979, $75,896,943 and $12,288,609 for the years ended December 31, 2016, 2015 and 2014, respectively. Net losses have resulted primarily from costs incurred in connection with research and development activities, general and administrative costs associated with operations as well as financing activities. Negative cash flows from operations were $18,828,083, $17,623,279 and $9,657,626 for the years ended December 31, 2016, 2015 and 2014, respectively.
The Company expects to incur significant expenses and increase its operating losses for the foreseeable future. Expenses are expected to increase substantially in connection with ongoing activities as the Company advances the clinical development of its lead product candidate for the treatment of FOP, advances development in potential follow-on programs, continues research and development efforts for the discovery and development of additional product candidates, continues to engage contract manufacturing organizations to manufacture its clinical study materials and to develop large-scale manufacturing capabilities, seeks regulatory approval for its product candidates, adds personnel to support its product development and future commercialization, adds operational, financial and management information systems, as well as maintains, leverages and expands its intellectual property portfolio.
The Company does not expect to generate revenue from product sales unless and until it successfully completes development and obtains regulatory approval for one or more of its product candidates, which it expects will take a number of years and is subject to significant uncertainty. If the Company obtains regulatory approval for any of its product candidates, the Company expects to incur significant commercialization expenses related to product sales, marketing, manufacturing, and distribution. As a result, the Company will need additional financing to support its continuing operations. Until such time as it can generate significant revenue from product sales, if ever, the Company expects to finance its operations through a combination of public or private equity or debt financings or other sources, which may include collaborations with third parties. Arrangements with collaborators or others may require the Company to relinquish rights to certain of its technologies or product candidates, obtain adequate patent protection for its technology, obtain necessary regulatory approval for its product candidates or achieve commercial viability for any approved product candidate. Adequate additional financing may not be available to the Company on acceptable terms or at all. The Company’s inability to raise capital as and when needed would have a negative impact on its financial condition and its ability to pursue its business strategy. The Company will need to generate significant revenue to achieve profitability, and it may never do so.
The Company expects that its existing cash and short-term investments as of December 31, 2016 will enable it to fund its operating expenses for at least the next twelve months from December 31, 2016.
Clementia is incorporated under the laws of Canada. The address of the Company’s registered office is 4150 Sainte-Catherine Street West, Suite 550, Montréal, Québec, H3Z 2Y5, Canada.
The consolidated financial statements of the Company as at and for the years ended December 31, 2016, 2015 and 2014 were approved by the board of directors of the Company and authorized for issue
F-18
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
on February 28, 2017, except as it relates to subsequent event disclosures in Note 17, which were authorized on June 29, 2017.
2. Significant accounting policies
a. Statement of compliance and basis of preparation
These consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB).
These consolidated financial statements have been prepared on the historical cost basis except for the valuation of the preferred shares, embedded derivatives, as well as the share-based payment transactions as explained in the accounting policies below. Historical cost is generally based on the fair value of the consideration given in exchange for goods and services at the time of acquisition.
Fair value is the price that would be received in selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date, regardless of whether that price is directly observable or estimated using another valuation technique. In estimating the fair value of an asset or a liability, the Company takes into account the characteristics of the asset or liability if market participants would take those characteristics into account when pricing the asset or liability at the measurement date.
In addition, for financial reporting purposes, fair value measurements are categorized into Level 1, 2, or 3 based on the degree to which the inputs to the fair value measurements are observable and the significance of the inputs to the fair value measurement in its entirety, which are described as follows:
• | Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities that the entity can access at the measurement date; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Level 2 inputs are inputs, other than quoted prices included within Level 1, that are observable for the asset or liability, either directly or indirectly; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | Level 3 inputs are unobservable inputs for the asset or liability. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The principal accounting policies are set out below.
b. Basis of consolidation
These consolidated financial statements include the accounts of the Company and those of its wholly-owned subsidiary, Clementia Pharmaceuticals USA Inc., together, the “Group”. Clementia Pharmaceuticals USA Inc. is an entity incorporated in the state of Delaware, with a registered office in Newton, Massachusetts, in the United States.
Subsidiaries are fully consolidated from the date of acquisition, being the date on which the Company obtains control, and continue to be consolidated until the date that such control ceases. Control is achieved when the Company has the power over the investee; is exposed, or has the rights to variable returns from its involvement with the investee; and has the ability to use its power to affect its return. The Company reassesses whether or not it controls an investee if facts and circumstances indicate that there are changes to one or more of the three elements of control listed beforehand.
All intragroup assets and liabilities, equity, income, expenses and cash flows relating to transaction between members of the Group are eliminated in full on consolidation.
c. Functional and presentation currency
Items included in the consolidated financial statements of the Company are measured using the currency of the primary economic environment in which the Company operates (the functional
F-19
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
currency). These consolidated financial statements are presented in United States dollars (USD), which is the Company’s functional and presentation currency.
d. Foreign currency transactions
In preparing these consolidated financial statements, transactions in currencies other that the Company’s functional currency are recognized at rates of exchange prevailing at the dates of the transactions. At the end of each reporting period, monetary items denominated in foreign currencies are retranslated at the rates prevailing at that date. Non-monetary items that are measured in terms of historical cost in a foreign currency are not retranslated. Exchange differences on monetary items are recognized in profit or loss in the period in which they arise.
e. Cash
Cash is limited to current balances with banks and similar institutions that have an insignificant risk of change in value.
f. Short-term investments
Short-term investments are highly liquid investments with an original maturity greater than three months but less than one year. The Company’s investment portfolio consists of guaranteed investment certificates.
g. Property and equipment
Equipment is stated at historical cost less accumulated depreciation and accumulated impairment losses, if any. Historical cost includes expenditures that are directly attributable to the acquisition of such items. Subsequent costs are included in the asset’s carrying amount or recognized as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Company and the cost of the item can be measured reliably. All other repairs and maintenance are charged to profit and loss in the period in which they are incurred.
Depreciation is recognized so as to write off the cost or valuation of assets less their residual values over their estimated useful lives, using the straight-line method as follows.
|
|
|
Computer hardware | Straight-line over 2 years | |
Office equipment | Straight-line over 2 years | |
Furniture | Straight-line over 5 years | |
Leasehold improvements | Straight-line over the term of the lease |
The estimated useful lives, residual values and depreciation methods are reviewed at the end of each reporting period, with the effect of any changes in estimate accounted for on a prospective basis.
An item of property and equipment is derecognized upon disposal or when no future economic benefits are expected to arise from the continued use of the asset. Any gain or loss arising on the disposal or retirement of an item of property and equipment is determined as the difference between the sales proceeds and the carrying amount of the asset and is recognized in profit or loss.
h. Intangible assets
Intangible assets with finite useful lives that are acquired separately are carried at cost less accumulated amortization and accumulated impairment losses, if any. Amortization is recognized on a straight-line basis over their estimated useful lives ranging from 9 to 20 years. The estimated useful life and amortization method are reviewed at the end of each reporting period, with the effect of any
F-20
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
changes in estimate being accounted for on a prospective basis. All intangible assets have been purchased from third parties and have not been internally generated.
An intangible asset is derecognized on disposal, or when no future economic benefits are expected from use or disposal. Gains or losses from derecognition of an intangible asset, measured as the difference between the net disposal proceeds and the carrying amount of the asset, are recognized in profit or loss when the asset is derecognized.
i. Impairment of tangible and intangible assets
At the end of each reporting period, the Company reviews the carrying amounts of its tangible and intangible assets to determine whether there is any indication that those assets have suffered an impairment loss. If any such indication exists, the recoverable amount of the asset is estimated in order to determine the extent of the impairment loss, if any. When it is not possible to estimate the recoverable amount of an individual asset, the Company estimates the recoverable amount of the cash-generating unit to which the asset belongs. When a reasonable and consistent basis of allocation can be identified, corporate assets are also allocated to individual cash-generating units, or otherwise they are allocated to the smallest group of cash-generating units for which a reasonable and consistent allocation basis can be identified.
Recoverable amount of a cash-generating unit is the higher of fair value less costs of disposal and value in use. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset for which the estimates of future cash flows have not been adjusted. If the recoverable amount of an asset or cash-generating unit is estimated to be less than its carrying amount, the carrying amount of the asset or cash-generating unit is reduced to its recoverable amount. An impairment loss is recognized immediately in profit or loss.
When an impairment loss subsequently reverses, the carrying amount of the asset or cash-generating unit is increased to the revised estimate of its recoverable amount, but so that the increased carrying amount does not exceed the carrying amount that would have been determined had no impairment loss been recognized for the asset or cash-generating unit in prior periods. A reversal of an impairment loss is recognized immediately in profit or loss.
j. Government assistance
Government assistance, consisting of investment tax credits, is recorded as a reduction of the related expense or against the cost of the asset acquired. Government assistance is recognized when there is reasonable assurance that the Company has met the requirements of the approved research program, when there is reasonable assurance that benefits will be received and all attached conditions have been complied with.
Investment tax credits consist of direct and indirect expenditures, including a reasonable allocation of overhead expenses, associated with the Company’s research and development programs. Research and development costs are expensed as incurred. Overhead expenses comprise general and administrative support provided to the research and development program and involve costs associated with support activities such as office services, information technology and human resources.
Investment tax credits are recorded based on management estimates of amounts for which there is reasonable assurance that they will be received and are subject to audit by the taxation authorities and, accordingly, these amounts may vary materially which could result in adjustments to profits or losses.
F-21
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
k. Share-based payments
Equity-settled share-based payments to employees and directors providing similar services are measured at the fair value of the equity instruments at the grant date.
The Company recognizes the compensation cost of share-based payments using a graded vesting method where each installment that vests is treated as its own award and each installment is measured and recognized separately. The fair value determined at the grant date for each tranche of the equity-settled share-based payments is expensed over the vesting period on a straight-line basis, based on the Company’s estimate of equity instruments that will eventually vest, with a corresponding increase in contributed surplus.
At the end of each reporting period, the Company revises its estimate of the number of equity instruments expected to vest. The impact of the revision of the original estimates, if any, is recognized in profit or loss such that the cumulative expense reflects the revised estimate, with a corresponding adjustment to contributed surplus.
l. Share capital
Common shares are classified as equity. Incremental costs directly attributable to the issuance of shares and stock options are recognized as a deduction from share capital, net of any tax effects.
m. Income taxes
Income tax expense represents the sum of the tax currently payable and deferred tax.
Current tax
The tax currently payable is based on taxable profit for the year. Taxable profits differ from profits or losses as reported in the consolidated statements of net loss and comprehensive loss because items of income or expense that are taxable or deductible in other years and items that are never taxable or deductible. The Group’s current tax is calculated using tax rates that have been enacted or substantively enacted at the end of the reporting period.
Deferred tax
Deferred tax is recognized on temporary differences between the carrying amounts of assets and liabilities in the consolidated financial statements and the corresponding tax bases used in the computation of taxable profit.
Deferred tax assets and liabilities are measured at the tax rates that are expected to apply in the period in which the liability is settled or the asset realized, based on tax rates and tax laws that have been enacted or substantively enacted by the end of the reporting period. The measurement of deferred tax liabilities and assets reflect the tax consequences that would flow from the manner in which the Company expects, at the end of the reporting period, to recover or settle the carrying amount of its assets and liabilities. The effect of a change in tax rates is recognized in the period during which the tax rate change occurs.
Deferred tax assets and liabilities are offset if there is a legally enforceable right to offset deferred tax liabilities and assets, and they relate to income taxes levied by the same tax authority on the same taxable entity, or on different tax entities, but they intend to settle deferred tax liabilities and assets on a net basis or their tax assets and liabilities will be realized simultaneously.
A deferred tax asset is recognized for unused tax losses and deductible temporary differences, to the extent that it is probable that future taxable profits will be available against which they can be utilized. In the Company’s case, recurring operating losses expected to be incurred throughout the
F-22
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
development years create tax assets that may reduce future taxable earnings, if any. The carrying amount of deferred tax assets is reviewed at each reporting period and reduced to the extent that it is no longer probable that sufficient taxable profits will be available to allow all or a part of the asset to be recovered.
In assessing whether deferred tax assets may be realized, management considers the likelihood that some portion or all of the tax assets will be realized. The ultimate use of net deferred tax assets is dependent upon the generation of future taxable income or available tax planning strategies in making this assessment. Since the Company is a development stage company, the generation of future taxable income is dependent on the successful commercialization of its products and technologies. Management has determined that it is not “more likely than not” that the benefits of the deferred tax assets will be recovered and have thus not been recorded.
n. Loss per share
Loss per share is determined using the weighted average number of common shares outstanding during the period.
Diluted loss per share is determined by adjusting the loss and the weighted average number of common shares for the effects of all dilutive potential common shares, which comprise the preferred shares and employee stock options.
o. Employee benefits
Short-term employee benefit obligations are measured at the undiscounted amount of the benefits expected to be paid in exchange for the related service and are expensed as the related service is provided.
A liability is recognized for the amount expected to be paid under short-term cash bonus plans if the Company has a present, legal or constructive obligation to pay this amount as a result of past service provided by the employee, and the obligation can be estimated reliably.
p. Financial instruments
Financial assets and financial liabilities are recognized when the Company becomes party to the contractual obligations of the instruments.
Financial assets and financial liabilities are initially measured at fair value. Transaction costs that are directly attributable to the acquisition or issue of financial assets or financial liabilities other than financial assets and financial liabilities at fair value through profit or loss are added to or deducted from the fair value of the financial assets or financial liabilities, as appropriate, on initial recognition.
Transaction costs directly attributable to the acquisition of financial assets or financial liabilities at fair value through profit or loss are recognized immediately in profit or loss.
Financial instruments are classified into the following specified categories: ‘at fair value through profit or loss’ (FVTPL), ‘held to maturity’ investments, ‘available for sale’ (AFS), ‘loans and receivables’ and ‘other financial liabilities’. The classification depends on the nature and purpose of the
F-23
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
financial instrument and is determined at the time of initial recognition. The Company’s financial instruments have been classified as follows:
|
|
|
|
| ||||||||||
Financial instrument | Classification | Measurement | ||||||||||||
Cash |
|
| Loans and receivables |
|
| Amortized cost | ||||||||
Short-term investments |
|
| Loans and receivables |
|
| Amortized cost | ||||||||
Interest and other receivables |
|
| Loans and receivables |
|
| Amortized cost | ||||||||
Accounts payable and accrued liabilities |
|
| Other financial liabilities |
|
| Amortized cost | ||||||||
Preferred shares |
|
| Other financial liabilities |
|
| Amortized cost | ||||||||
Embedded derivatives |
|
| FVTPL |
|
| Fair value | ||||||||
Loans and receivables are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market. Loans and receivables are recorded at fair value plus any directly attributable transaction costs and are subsequently measured at amortized cost using the effective interest method, less any impairment. Interest income is recognized by applying the effective interest rate, except for short-term receivables when the effect of discounting is immaterial.
Financial assets, other than those at FVTPL, are assessed for indicators of impairment at the end of each reporting period. Financial assets are considered to be impaired when there is objective evidence that, as a result of one or more events that occurred after the initial recognition of the financial asset, the estimated future cash flows of the investment have been affected. At the end of each reporting period, the Company assesses whether there is objective evidence that a financial asset is impaired as a result of one or more events that occurred after the initial recognition of the financial asset and which have an impact on the estimated future cash flows of the asset. The losses arising from impairment, if any, are recognized in profit and loss in the period in which they were incurred.
Other financial liabilities are initially recognized at fair value, net of directly attributable transaction costs, and subsequently measured at amortized cost using the effective interest method. A financial liability is derecognized when the obligation underlying the liability is discharged or cancelled or expires.
Financial liabilities at FVTPL are recognized and subsequently measured at fair value at each reporting date with changes in fair value recorded in profit and loss in the period in which they arise. Transaction costs are allocated to financial liabilities at FVTPL and are expensed in profit and loss at inception.
Classification as debt or equity
Debt and equity instruments issued by the Company are classified as either financial liabilities or as equity in accordance with the substance of the contractual arrangements and the definitions of a financial liability and an equity instrument.
An equity instrument is any contract that evidences a residual interest in the assets of the Company after deducting all of its liabilities. Equity instruments issued by the Company are recognized at the proceeds received, net of direct issue costs.
Financial liabilities are classified as either financial liabilities at FVTPL or other financial liabilities. A financial liability is derecognized when its contractual obligations are discharged, cancelled or expire.
Embedded derivatives
An embedded derivative is a feature within a contract, such that the cash flows associated with that feature behave in a similar fashion to a stand-alone derivative. Embedded derivatives that are separated from the host contract are accounted for at FVTPL.
The terms of the preferred shares include an embedded conversion feature which provides for the conversion of the preferred shares into common shares at a variable rate due to a price protection
F-24
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
feature (down-round protection). The Company determined that the conversion feature was an embedded derivative liability that required separation from the financial liability host contract. The embedded derivative liability is recognized and measured at fair value at each reporting date with changes in fair value recorded in financial expenses in profit and loss in the period in which they arise. The liability component is determined at inception by deducting the fair value of the embedded derivative from the fair value of the instrument as a whole.
Transaction costs that relate to the issue of the preferred shares are allocated to the liability and embedded derivative components in proportion to the allocation of the gross proceeds. Transaction costs relating to the embedded derivative liability component are expensed directly in profit and loss and transaction costs relating to the financial liability component are included in the carrying amount of the liability component and are amortized over the expected lives of the convertible instrument using the effective interest method.
Effective interest method
The effective interest method is a method of calculating the amortized cost of a debt instrument and of allocating interest over the relevant period. The effective interest rate is the rate that discounts estimated future cash receipts (including transaction costs and other premiums or discounts) through the expected term of the debt instrument, or, where appropriate, a shorter period, to the net carrying amount on initial recognition.
q. Interest income
The Company earns interest on its cash in current bank accounts and on its short-term investments. Interest income from a financial asset is recognized when it is probable that the economic benefits will flow to the Company and the amount of interest can be measured reliably. Interest income is accrued on a time basis, by reference to the principal amount outstanding and at the effective interest rate applicable, which is the rate that exactly discounts estimated future cash receipts through the expected life of the financial asset to that asset’s net carrying amount on initial recognition.
r. Operating leases
The Company’s leases are operating leases. The leased assets are not recognized in the Company’s consolidated statements of financial position as the Company does not substantially assume all risks and rewards of ownership of the leased assets. Operating lease payments are recognized as an expense on a straight-line basis over the lease term.
In the event that lease incentives are received to enter into operating leases, such incentives are recognised as a liability. The aggregate benefit of incentives is recognized as a reduction of rental expense on a straight-line basis.
3. Critical accounting judgements and key sources of estimation uncertainty
The preparation of the Company’s consolidated financial statements in conformity with IFRS requires management to make judgements, estimates and assumptions that affect the application of accounting policies and the reported amounts of revenues, expenditures, assets and liabilities. Actual results could differ from those estimates.
On an ongoing basis, estimates and judgements are evaluated. The Company bases its estimates on the most probable set of economic conditions and planned course of action, historical experience, known trends and events, and various other factors believed to be reasonable under the circumstances, the results of which form the basis for making judgements about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these
F-25
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
estimates under different assumptions or conditions. Uncertainty about these assumptions and estimates could result in outcomes that require material adjustments to the carrying amount of the asset or liability affected in future periods. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which these estimates are revised and in any future periods affected.
Balances and transactions that are subject to a high degree of estimation are the estimation of accrued expenses and the valuation of the embedded derivatives of the preferred shares.
a. Estimation of accrued expenses
As part of the process of preparing its financial statements, the Company is required to estimate its accrued expenses. This process involves reviewing open contracts and purchase orders, communicating with personnel to identify services that have been performed on the Company’s behalf and estimating the level of service performed and the associated cost incurred for the service when the Company has not yet been invoiced or otherwise notified of the actual cost.
The majority of service providers invoice the Company in arrears for services performed, on a pre-determined schedule or when contractual milestones are met; however, some require advanced payments. The Company estimates its accrued expenses as of each statement of financial position date in its financial statements based on facts and circumstances known at that time. The Company periodically confirms the accuracy of its estimates with service providers and makes adjustments if necessary.
Examples of accrued research and development expenses include fees paid to: i) contract research organizations in connection with performing research and development services on the Company’s behalf; ii) investigative sites or other providers in connection with clinical trials; iii) vendors in connection with non-clinical development activities; iv) vendors related to product manufacturing, development and distribution of clinical supplies; and v) various external consultants.
The Company bases its expenses related to clinical trials on its estimate of the services received and efforts expended pursuant to contracts with multiple contract research organizations that conduct and manage non-clinical studies and clinical trials on the Company’s behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments to the Company’s vendors will exceed the level of services provided and result in a prepayment of the clinical expense. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones.
In accruing service fees, the Company estimates the time period over which services will be performed, enrollment of patients, number of sites activated and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of efforts varies from its estimate, the Company adjusts the accrual or prepaid expense accordingly. Although the Company does not expect its estimates to be materially different from amounts actually incurred, if its estimates of the status and timing of services performed differs from the actual status and timing of services performed, the Company may report amounts that are too high or too low in any particular period.
b. Valuation of the embedded derivative of the preferred shares
As part of assessing whether an instrument is a hybrid financial instrument and contains an embedded derivative, significant judgement is required in evaluating whether the host contract is more akin to debt or equity and whether the embedded derivative is clearly and closely related to the underlying host contract. In applying its judgement, the Company relies primarily on the economic characteristics and risks of the instrument as well as the substance of the contractual arrangement.
F-26
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
The initial fair values of the embedded derivative conversion options and subsequent re-measurements at fair value at each reporting date were determined by using the Monte Carlo simulation model. The Monte Carlo simulation model better reflects non-static inputs, such as the anti-dilution (down-round protection) features of the preferred shares. A Monte Carlo simulation model is a valuation model that relies on random sampling and is often used when modeling systems with a large number of inputs and where there is a significant uncertainty in the future value of inputs and where the movement in inputs can be independent of each other.
Moreover, the use of this valuation model requires highly subjective assumptions. These assumptions are determined as of the measurement date and include the risk-free interest rate, the expected dividend yield, the expected volatility, the timing and amounts of subsequent rounds of financing, the expected timing and probability of exit events, and the underlying value of the Company. Assumptions with regards to volatility, subsequent rounds of financing, time to exit and underlying value of the Company are particularly important and sensitive, requiring significant judgement by management. Accordingly, any changes in the assumptions used in this model could significantly impact the values recognized as embedded derivative conversion options at inception and on subsequent re-measurement at each reporting date.
The risk-free rate is the rate of return on the US Department of Treasury daily treasury yield curve rates over a period equal to the expected timing of an exit event. The expected dividend yield is nil as the Company does not expect to pay dividends in the near future. The expected volatility reflects the assumption that the volatility used in estimating the value of the embedded derivative is indicative of future trends, which may not necessarily be the actual outcome. Due to the lack of a public market for the trading of the Company’s shares and a lack of company specific historical and implied volatility data, the Company has based its estimate of expected volatility on the historical volatility of a group of similar companies that are publicly traded. For these analyses, the Company has selected companies with comparable characteristics to it including risk profiles, orphan drugs within their portfolios, positions within the industry and with historical share price information sufficient to meet the expected timing of an exit event.
The expected timing and amounts of subsequent rounds of financing reflect management’s best estimate of subsequent rounds of financing based on contracted commitments for subsequent rounds of financing; the Company’s financial condition, including cash on hand; and the Company’s historical and forecasted performance and budgeted cash burn.
The expected timing of exit events are based on management’s best estimate of possible exit events and their likelihood, considering the progress of the Company’s research and development programs, including the status of non-clinical studies and clinical trials for the Company’s product candidates; the Company’s stage of development and its commercialization and business strategy; the Company’s financial condition, including cash on hand; the Company’s historical and forecasted performance and operating results; the likelihood of achieving a liquidity event, such as a sale of the Company or an IPO given prevailing market conditions; external market conditions affecting the biopharmaceutical industry; and trends within the biopharmaceutical industry.
In the absence of a public trading market, the underlying value of the Company was performed using methodologies, approaches and assumptions consistent with theAmerican Institute of Certified Public Accounts Audit and Accounting Practice Aid Series, with the assistance of a third-party specialist, and is subject to estimation based on the valuation techniques selected and an evaluation of the inputs used in creating the valuation. Valuation techniques used include the probability-weighted expected return method and the option-pricing method or a hybrid of both methods. In addition, various objective and subjective factors were also considered, including the prices at which the Company sold preferred shares and the superior rights and preferences of the preferred shares relative to the Company’s common shares; the progress of the Company’s research and development programs; the Company’s stage of development and its commercialization and business strategy; the Company’s
F-27
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
financial condition, including cash on hand; the Company’s historical and forecasted performance and operating results; the lack of an active public market for the Company’s common shares and preferred shares; the likelihood of achieving a liquidity event, such as a sale of the Company or an initial public offering (IPO) given prevailing market conditions; the analysis of IPOs and the market performance of similar companies in the biopharmaceutical industry; external market conditions affecting the biopharmaceutical industry; and trends within the biopharmaceutical industry. There are significant judgements and estimates inherent in these valuations. These judgements and estimates include assumptions regarding the Company’s future operating performance, the timing and likelihood of potential liquidity events and the determination of the appropriate valuation methodology at each valuation date. If different assumptions had been made, the Company’s fair value of embedded derivatives and the resulting preferred share liability and accretion expense, as well as the re-measurement of the embedded derivatives could have been materially different.
4. Future changes in accounting policies
The IASB has issued several new standards and amendments to standards and interpretations that are not effective for the year ended December 31, 2016, and although early adoption is permitted, they have not been applied in preparing these consolidated financial statements. The Company is currently evaluating the effect, if any, the following new standards and amendments will have on its financial results.
i) | Financial Instruments(IFRS 9), effective for annual periods beginning on or after January 1, 2018, replaces the requirements in IAS 39,Financial Instruments, Recognition and Measurementfor classification and measurement of financial assets and liabilities. IFRS 9 introduces a single classification and measurement approach for financial instruments, which is driven by cash flow characteristics and the business model in which an asset is held. This single, principle-based approach replaces existing rule-based requirements and results in a single impairment model being applied to all financial instruments. IFRS 9 also modified the hedge accounting model to incorporate the risk management practices of an entity. Additional disclosures will also be required under the new standard. Early adoption of IFRS 9 is permitted. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Leases(IFRS 16), effective for annual periods beginning on or after January 1, 2019, provides a comprehensive model for the identification of lease arrangements and their treatment in the financial statements of both lessees and lessors. It supersedes IAS 17Leasesand its associated interpretive guidance. Significant changes were made to lessee accounting with the distinction between operating and finance leases removed and assets and liabilities recognized in respect of all leases (subject to limited exceptions for short-term leases and leases of low value assets). Earlier application of IFRS 16 is permitted for companies that have also adopted IFRS 15,Revenue from Contracts with Customers. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
5. Intangible assets
|
|
|
|
|
|
| |||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||
Cost: |
|
|
|
|
|
| |||||||||||||||
Opening balance as at January 1, |
|
| 1,388,399 |
|
| 1,272,123 |
|
| 1,141,462 | ||||||||||||
Additions |
|
| — |
|
| 116,276 |
|
| 130,661 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
Closing balance as at December 31, |
|
| 1,388,399 |
|
| 1,388,399 |
|
| 1,272,123 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
Accumulated amortization: |
|
|
|
|
|
| |||||||||||||||
Opening balance as at January 1, |
|
| 356,289 |
|
| 222,031 |
|
| 91,902 | ||||||||||||
Amortization |
|
| 137,526 |
|
| 134,258 |
|
| 130,129 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
Closing balance as at December 31, |
|
| 493,815 |
|
| 356,289 |
|
| 222,031 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
Net book value as at December 31, |
|
| 894,584 |
|
| 1,032,110 |
|
| 1,050,092 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
F-28
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
All intangible assets are related to licensing agreements and have a finite life.
During the year ended December 31, 2014, the Company entered into an exclusive worldwide license agreement with Thomas Jefferson University (TJU) to exploit TJU’s patent rights related to a method of muscle repair and regeneration elicited by a retinoid agonist. During the year ended December 31, 2014, the Company paid TJU a license fee of $100,000 plus accumulated legal costs and is responsible for additional payments upon achieving certain clinical and regulatory milestones and royalties on related product sales (see note 13).
During the year ended December 31, 2015, the Company entered into an exclusive worldwide license with Yamaguchi University (Yamaguchi) in Japan to exploit Yamaguchi’s patent rights related to retinoid agonists as an anti-fibrotic agent in corneal and retinal repair. During the year ended December 31, 2015, the Company paid Yamaguchi a license fee of $100,000 plus accumulated legal costs and is responsible for additional payments upon achieving certain clinical and regulatory milestones and royalties on related product sales (see note 13).
6. Preferred shares
The Company has an unlimited number of voting and participating Preferred Shares authorized for issuance in series and without par value.
On April 10, 2013, the Company closed a $22,500,000 Class A financing led by OrbiMed Advisors LLC (OrbiMed), with a participation from an existing investor, BDC Capital Inc. On October 7, 2014, the Company secured an additional $10,200,000 from its current investors to support the development of its lead compound palovarotene for the treatment of FOP thus bringing the total amount raised in the Class A financing to $32,700,000.
The Class A financing was disbursed over four tranches. The first tranche, amounting to $3,547,197 was issued on April 10, 2013, upon closing the Class A financing, less $195,631 in share issuance costs. The additional first tranche, amounting to $477,278 was issued on May 30, 2013, less $6,619 in share issuance costs. The second tranche, amounting to $3,500,012, was issued on November 4, 2013 upon completion of the technology transfer from Roche, the Company’s licensor, and access to pre-clinical animal models, less $9,685 in share issuance costs. The timing of the third and fourth tranches was amended in February 2014. The revised third tranche, amounting to $11,999,983, less $31,128 in share issuance costs, was received on April 24, 2014, upon the filing of a pre-investigational new drug (pre-IND) application with the FDA (Food and Drug Administration of the United States). On March 18, 2015, the Company received the fourth and final tranche, amounting to $13,183,589 upon the achievement of certain clinical milestones.
On June 22, 2015, the Company entered into a Class B share subscription agreement with new and existing investors and completed a $59,999,141 round of financing to further support the ongoing development of its lead compound palovarotene for the potential treatment of FOP, less $2,561,518 in share issuance costs.
As at December 31, 2016, there were 1,118,415 Class A redeemable and convertible preferred stock issued and outstanding at a price of $29.245 per share (1,118,415 as at December 31, 2015 and 667,617 as at December 31, 2014) and 485,823 Class B redeemable and convertible preferred stock issued and outstanding at a price of $123.50 per share (485,823 as at December 31, 2015 and nil as at December 31, 2014). As at December 31, 2016, the Class A and B redeemable and convertible preferred shares are convertible into 1,604,238 common shares.
The Class A and B redeemable and convertible preferred shares have substantially the same terms. The preferred shares are convertible into common shares any time at the option of the holder, on a one-to-one basis subject to normal and customary anti-dilutive provisions, but are mandatorily convertible upon closing of a qualified IPO, defined as an IPO with a minimum multiple of the original issue price of the preferred shares and with a minimum gross proceeds threshold. Also, the preferred
F-29
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
shares are redeemable at face value at the option of the holder at any time commencing seven years after the Class B issuance. The preferred shares also have a liquidation preference whereby upon dissolution or sale of the Company, the preferred shares are redeemable prior to the distribution of the remaining property, profits and surplus assets of the Company to preferred and common shareholders.
The preferred shares carry an 8% fixed, non-cumulative, preferential dividend, as and when declared by the Company’s Board of Directors. In order to maximize the full value of the Company’s assets and its ongoing development efforts, the Company does not expect to pay out dividends.
The preferred shares also contain provisions that protect holders from a decline in the issue price of the Company’s shares (or down-round provisions). These down-round provisions reduce the conversion price of the Class A and Class B preferred shares if the Company issues a subsequent financing round at a price that is lower than the conversion price of the Class A and Class B preferred shares. As a result, the Company accounted for the conversion options of the Class A and Class B preferred shares as embedded derivative liabilities, measured at FVTPL. The difference between the fair values allocated to the embedded derivative liabilities at inception and the proceeds received were accounted for as financial liabilities due to their redemption feature. The financial liabilities are being accreted back to their face values through a charge to financial expenses over its expected term using the effective interest rate method.
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
| Preferred shares | Embedded derivative | ||||||||||||||||||||||||||
Class A | Class B | Class A | Class B | |||||||||||||||||||||||||
Balance—December 31, 2013 |
|
| $ |
| 5,268,342 |
|
| $ |
| — |
|
| $ |
| 2,135,514 |
|
| $ |
| — | ||||||||
Issuance of preferred shares |
|
| 8,253,706 |
|
| — |
|
| 3,746,277 |
|
| — | ||||||||||||||||
Transaction costs |
|
| (20,754 | ) |
|
|
| — |
|
| — |
|
| — | ||||||||||||||
Accretion during the year |
|
| 606,153 |
|
| — |
|
| — |
|
| — | ||||||||||||||||
Loss on re-measurement at fair value |
|
| — |
|
| — |
|
| 1,608,871 |
|
| — | ||||||||||||||||
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
Balance—December 31, 2014 |
|
| 14,107,447 |
|
| — |
|
| 7,490,662 |
|
| — | ||||||||||||||||
Issuance of preferred shares |
|
| 8,585,449 |
|
| 40,809,133 |
|
| 4,598,140 |
|
| 19,190,008 | ||||||||||||||||
Transaction costs |
|
| — |
|
| (1,742,247 | ) |
|
|
| — |
|
| — | ||||||||||||||
Accretion during the year |
|
| 1,108,182 |
|
| 1,270,810 |
|
| — |
|
| — | ||||||||||||||||
Loss on re-measurement at fair value |
|
| — |
|
| — |
|
| 49,804,284 |
|
| 2,759,475 | ||||||||||||||||
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
Balance—December 31, 2015 |
|
| 23,801,078 |
|
| 40,337,696 |
|
| 61,893,086 |
|
| 21,949,483 | ||||||||||||||||
Accretion during the year |
|
| 1,192,408 |
|
| 2,549,770 |
|
| — |
|
| — | ||||||||||||||||
Loss on re-measurement at fair value |
|
| — |
|
| — |
|
| 21,462,384 |
|
| 12,519,658 | ||||||||||||||||
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
Balance—December 31, 2016 |
|
| $ |
| 24,993,486 |
|
| $ |
| 42,887,466 |
|
| $ |
| 83,355,470 |
|
| $ |
| 34,469,141 | ||||||||
|
|
|
|
|
|
|
|
| ||||||||||||||||||||
The fair value of the embedded derivative conversion options were estimated at inception and on a recurring basis using the Monte Carlo simulation model with the following key assumptions, including a nil dividend yield:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||
| Dec. 31, 2016 | Dec. 31, 2015 | 2015 (inception) | Dec. 31, 2014 | 2014 (inception) | |||||||||||||||||||||||||||||||||||||||||||||||||||
Class A | Class B | Class A | Class B | Class A | Class B | Class A | Class A | |||||||||||||||||||||||||||||||||||||||||||||||||
Fair value of embedded derivative per share |
|
| $ |
| 74.53 |
|
| $ |
| 70.95 |
|
| $ |
| 55.34 |
|
| $ |
| 45.18 |
|
| $ |
| 10.20 |
|
| $ |
| 39.50 |
|
| $ |
| 11.22 |
|
| $ |
| 9.13 | ||||||||||||||||
Assumptions: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||||||||||||
Starting equity value (in millions of $) |
|
| $ |
| 249.6 |
|
| $ |
| 249.6 |
|
| $ |
| 170.9 |
|
| $ |
| 170.9 |
|
| $ |
| 36.2 |
|
| $ |
| 170.9 |
|
| $ |
| 22.9 |
|
| $ |
| 22.1 | ||||||||||||||||
Volatility |
|
| 68 | % |
|
|
| 68 | % |
|
|
| 81 | % |
|
|
| 81 | % |
|
|
| 78 | % |
|
|
| 72 | % |
|
|
| 88 | % |
|
|
| 73 | % |
| ||||||||||||||||
Weighted average time to exit (in years) |
|
| 0.85 |
|
| 0.85 |
|
| 2.25 |
|
| 2.25 |
|
| 1.54 |
|
| 1.38 |
|
| 1.74 |
|
| 1.77 | ||||||||||||||||||||||||||||||||
F-30
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
These derivative liabilities are classified as a Level 3 in the fair value hierarchy. A reasonably possible movement in the estimated starting equity value, expected volatility or expected time to exit could significantly impact the fair value of the embedded derivative.
As at December 31, 2016, and assuming that all other variables remain constant, a 10% increase in volatility would result in an increase of $1.91 and $0.34 in the per-share fair value of the embedded derivative, respectively for Class A and Class B. Inversely, a decrease of 10% in volatility would result in an decrease of $1.58 and $0.10 in the per-share fair value of the embedded derivative, respectively for Class A and Class B.
As at December 31, 2016, and assuming that all other variables remain constant, a 10% increase in the estimated starting equity value would result in an increase of $11.09 and $10.58 in the per-share fair value of the embedded derivative, respectively for Class A and Class B. Inversely, a decrease of 10% in the estimate starting equity value would result in a decrease of $11.26 and $10.55 in the per-share fair value of the embedded derivative, respectively for Class A and Class B.
7. Share capital
The Company has an unlimited number of voting and participating common shares authorized for issuance without par value. As at December 31, 2016, there were 196,109 common shares issued and outstanding (196,109 as at December 31, 2015 and 181,750 as at December 31, 2014).
Changes in the number of common shares were as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||
| 2016 | 2015 | 2014 | |||||||||||||||||||||||||||||||||||||||
Shares | $ | Shares | $ | Shares | $ | |||||||||||||||||||||||||||||||||||||
Balance at beginning of year |
|
| 196,109 |
|
| 272,391 |
|
| 181,750 |
|
| 194,651 |
|
| 176,477 |
|
| 176,192 | ||||||||||||||||||||||||
Issued during the year |
|
| — |
|
| — |
|
| 3,431 |
|
| 12,008 |
|
| 5,273 |
|
| 18,459 | ||||||||||||||||||||||||
Exercise of stock options |
|
| — |
|
| — |
|
| 10,928 |
|
| 38,248 |
|
| — |
|
| — | ||||||||||||||||||||||||
Reclassification of share-based compensation pursuant to the exercise of stock options |
|
| — |
|
| — |
|
| — |
|
| 27,484 |
|
| — |
|
| — | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||
Balance at end of year |
|
| 196,109 |
|
| 272,391 |
|
| 196,109 |
|
| 272,391 |
|
| 181,750 |
|
| 194,651 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||
8. Share-based payments
Under the Company’s Employee Stock Option Plan (ESOP), the Company can grant to its directors, management and employees non-transferrable stock options for the purchase of common shares. The maximum number of common shares available for issuance under the ESOP is limited to 315,837 as at December 31, 2016. Stock options expire 10 years after their initial grant date. Time-based stock options vest over a period of 4 years: 25% cliff vesting after one year and equal monthly installments thereafter. Performance-based stock options vest 25% upon achievement of a milestone event with monthly vesting over the subsequent 36 months. Milestone events are specific to corporate goals and are currently assessed as probable of achievement.
The exercise price for stock option grants is determined at the date of grant by the Company’s Board of Directors and cannot be less than the fair value of the Company’s common shares as established by various valuation analyses performed.
F-31
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
Changes in the number of stock options outstanding are as follows:
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||
| 2016 | 2015 | 2014 | |||||||||||||||||||||||||||||||||||||||
Options | Weighted | Options | Weighted | Options | Weighted | |||||||||||||||||||||||||||||||||||||
Balance at beginning of year |
|
| 205,414 |
|
| $ |
| 5.24 |
|
| 188,422 |
|
| $ |
| 3.50 |
|
| 62,501 |
|
| $ |
| 3.50 | ||||||||||||||||||
Issued during the year |
|
| — |
|
| — |
|
| 27,920 |
|
| $ |
| 16.29 |
|
| 125,921 |
|
| $ |
| 3.50 | ||||||||||||||||||||
Exercised during the year |
|
| — |
|
| — |
|
| (10,928 | ) |
|
|
| $ |
| 3.50 |
|
| — |
|
| — | ||||||||||||||||||||
Cancelled during the year |
|
| (778 | ) |
|
|
| $ |
| 8.33 |
|
|
|
|
|
| — |
|
| — | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||
Balance at end of year |
|
| 204,636 |
|
| $ |
| 5.23 |
|
| 205,414 |
|
| $ |
| 5.24 |
|
| 188,422 |
|
| $ |
| 3.50 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||||||||||||||||||||||||||
The following table summarizes the information on outstanding stock options as at December 31, 2016.
|
|
|
|
|
|
|
|
|
|
|
Exercise price | Outstanding stock options | Exercisable stock options | ||||||||
Number of | Weighted average | Weighted | Number of | Weighted | ||||||
$3.50 | 177,494 | 7.2 |
|
| 89,982 |
|
| |||
$8.33 | 22,640 | 8.3 |
|
| 9,834 |
|
| |||
$57.68 | 4,502 | 9.0 |
|
| 1,369 |
|
| |||
|
|
|
|
|
|
|
|
|
|
|
| 204,636 | 7.4 | $5.23 | 101,185 | $4.70 | |||||
|
|
|
|
|
|
|
|
|
|
|
During the year ended December 31, 2016, the Company recorded a stock-based compensation expense of $174,419 ($164,456 in 2015 and $142,811 in 2014) of which $64,998 ($100,515 in 2015 and $88,529 in 2014) was recorded in general and administrative expenses and $109,421 ($63,941 in 2015 and $54,282 in 2014) in research and development expenses.
The fair value of the stock options granted was estimated at the date of grant using the Black-Scholes option pricing model with the following weighted average assumptions:
|
|
|
|
|
|
| |||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||
Grant (number of stock options) |
|
| — |
|
| 27,920 |
|
| 125,921 | ||||||||||||
Weighted average fair value of stock options |
|
| — |
|
| $ |
| 6.53 |
|
| $ |
| 2.46 | ||||||||
Weighted average exercise price |
|
| — |
|
| $ |
| 16.29 |
|
| $ |
| 3.50 | ||||||||
Weighted average assumptions: |
|
|
|
|
|
| |||||||||||||||
Share price |
|
| — |
|
| $ |
| 11.08 |
|
| $ |
| 3.50 | ||||||||
Risk-free interest rate |
|
| — |
|
| 1.62 | % |
|
|
| 1.86 | % |
| ||||||||
Expected dividend yield |
|
| — |
|
| Nil |
|
| Nil | ||||||||||||
Volatility factor |
|
| — |
|
| 74.76 | % |
|
|
| 82.13 | % |
| ||||||||
Expected life (in years) |
|
| — |
|
| 6.00 |
|
| 6.00 | ||||||||||||
The Black-Scholes model requires subjective assumptions, which affect the calculated values. The assumptions used represent the Company’s best estimates at the time of grant.
9. Loss per share
For the years ended December 31, 2016, 2015 and 2014, diluted loss per share is the same as basic loss per share as the effect of stock options, the Class A preferred shares and the Class B preferred shares would have been anti-dilutive.
F-32
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
10. Income taxes
The effective tax rate on the Company’s net loss differs from the expected amount that would arise using the statutory income tax rates. A reconciliation of the difference is as follows:
|
|
|
|
|
|
| |||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||
Loss before income taxes |
|
| $ |
| (57,365,525 | ) |
|
|
| $ |
| (75,740,723 | ) |
|
|
| $ |
| (12,193,743 | ) |
|
Basic income tax rate |
|
| 26.90 | % |
|
|
| 26.90 | % |
|
|
| $ |
| 26.90 | % |
| ||||
|
|
|
|
|
|
| |||||||||||||||
Computed income tax recovery |
|
| (15,431,326 | ) |
|
|
| (20,374,255 | ) |
|
|
| (3,280,117 | ) |
| ||||||
Adjustment in income taxes resulting from: |
|
|
|
|
|
| |||||||||||||||
Unrecorded potential tax benefits |
|
| 5,165,985 |
|
| 5,654,937 |
|
| 2,665,853 | ||||||||||||
Increase in deferred tax assets resulting from a reduction in tax rate |
|
| 217,675 |
|
| — |
|
| — | ||||||||||||
Income taxable at a different foreign tax rate |
|
| 43,494 |
|
| 40,602 |
|
| 91,319 | ||||||||||||
Accounting charges not deducted for tax |
|
| 10,150,626 |
|
| 14,834,936 |
|
| 617,811 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
Income tax expense |
|
| 146,454 |
|
| 156,220 |
|
| 94,866 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
The Company’s applicable statutory tax rate is the Canadian combined rate applicable in the jurisdictions in which the Company operates.
The income tax effect of temporary differences that give rise to net deferred tax assets and deferred tax liabilities is presented below.
|
|
|
|
|
|
| |||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||
Deferred tax assets |
|
|
|
|
|
| |||||||||||||||
Net operating loss carryforwards |
|
| $ |
| 12,938,264 |
|
| $ |
| 8,020,146 |
|
| $ |
| 2,163,766 | ||||||
Unused research and development expenditures |
|
| 1,178,910 |
|
| 958,240 |
|
| 507,982 | ||||||||||||
Financing costs |
|
| 17,201 |
|
| 108,847 |
|
| — | ||||||||||||
Costs relating to patents and others |
|
| 118,564 |
|
| 96,524 |
|
| 42,637 | ||||||||||||
Property and equipment |
|
| 174,725 |
|
| 64,272 |
|
| 17,417 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
|
| 14,427,664 |
|
| 9,248,029 |
|
| 2,731,802 | |||||||||||||
Deferred tax liabilities |
|
|
|
|
|
| |||||||||||||||
Property and equipment |
|
| (3,209 | ) |
|
|
| (1,857 | ) |
|
|
| — | ||||||||
Net deferred income tax assets |
|
| 14,424,455 |
|
| 9,246,172 |
|
| 2,731,802 | ||||||||||||
Unrecorded deferred income tax assets |
|
| (14,424,455 | ) |
|
|
| (9,246,172 | ) |
|
|
| (2,731,802 | ) |
| ||||||
|
|
|
|
|
|
| |||||||||||||||
|
|
| — |
|
| — |
|
| — | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
As at December 31, 2016, the Company had the following loss carryforwards and unclaimed deductions and credits available for carryforward. Investment tax credits, loss carryforwards and scientific research and experimental development expenditure amounts are based on management
F-33
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
estimates and are subject to verification by taxation authorities. Accordingly, these amounts may vary significantly.
|
|
|
|
| ||||||||||
| Provincial | Federal | ||||||||||||
Research and development expenditures, without time limitation |
|
| 4,938,487 |
|
| 4,073,224 | ||||||||
|
|
|
|
| ||||||||||
Losses carried forward, expiring |
|
|
|
| ||||||||||
2029 |
|
| 39,492 |
|
| 39,508 | ||||||||
2030 |
|
| 153,102 |
|
| 153,353 | ||||||||
2031 |
|
| 88,677 |
|
| 89,381 | ||||||||
2032 |
|
| 77,368 |
|
| 92,095 | ||||||||
2033 |
|
| 2,631,911 |
|
| 2,722,029 | ||||||||
2034 |
|
| 7,902,544 |
|
| 8,103,485 | ||||||||
2035 |
|
| 18,593,985 |
|
| 18,868,654 | ||||||||
2036 |
|
| 18,814,505 |
|
| 19,149,511 | ||||||||
|
|
|
|
| ||||||||||
|
| 48,301,584 |
|
| 49,218,016 | |||||||||
|
|
|
|
| ||||||||||
Unused tax credits, expiring |
|
|
|
| ||||||||||
2033 |
|
|
|
| 76,818 | |||||||||
2034 |
|
|
|
| 260,594 | |||||||||
2035 |
|
|
|
| 203,856 | |||||||||
2036 |
|
|
|
| 167,778 | |||||||||
|
|
|
|
| ||||||||||
|
|
|
| 709,046 | ||||||||||
|
|
|
|
| ||||||||||
11. Additional information on the consolidated statements of net loss and comprehensive loss
|
|
|
|
|
|
| |||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||
Included in research and development expenses: |
|
|
|
|
|
| |||||||||||||||
Employee benefits expense |
|
| $ |
| 2,352,591 |
|
| $ |
| 1,971,266 |
|
| $ |
| 1,691,801 | ||||||
Depreciation of property and equipment |
|
| $ |
| 20,544 |
|
| $ |
| 6,450 |
|
| $ |
| 2,892 | ||||||
Expenses related to minimum operating lease payments |
|
| $ |
| 360,565 |
|
| $ |
| 219,611 |
|
| $ |
| 148,259 | ||||||
Included in general and administrative expenses: |
|
|
|
|
|
| |||||||||||||||
Employee benefits expense |
|
| $ |
| 1,677,808 |
|
| $ |
| 1,814,930 |
|
| $ |
| 1,097,900 | ||||||
Depreciation of property and equipment |
|
| $ |
| 14,511 |
|
| $ |
| 8,056 |
|
| $ |
| 2,827 | ||||||
Amortization of intangible assets |
|
| $ |
| 137,526 |
|
| $ |
| 134,258 |
|
| $ |
| 130,129 | ||||||
Expenses related to minimum operating lease payments |
|
| $ |
| 132,110 |
|
| $ |
| 117,703 |
|
| $ |
| 59,303 | ||||||
12. Financial expenses
|
|
|
|
|
|
| |||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||
Financial expenses |
|
|
|
|
|
| |||||||||||||||
Transaction costs—embedded derivatives |
|
| $ |
| — |
|
| $ |
| 819,271 |
|
| $ |
| 10,374 | ||||||
Accretion—preferred shares |
|
| 3,742,178 |
|
| 2,378,992 |
|
| 606,153 | ||||||||||||
Loss on re-measurement at fair value |
|
| 33,982,042 |
|
| 52,563,759 |
|
| 1,608,871 | ||||||||||||
Bank charges and other interest |
|
| 11,487 |
|
| 11,334 |
|
| 6,597 | ||||||||||||
Foreign exchange (gains) losses |
|
| (90,000 | ) |
|
|
| 366,765 |
|
| 131,415 | ||||||||||
|
|
|
|
|
|
| |||||||||||||||
Total financial expenses |
|
| $ |
| 37,645,707 |
|
| $ |
| 56,140,121 |
|
| $ |
| 2,363,410 | ||||||
|
|
|
|
|
|
| |||||||||||||||
F-34
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
13. Commitments and contingencies
As at December 31, 2016, the Company has commitments under various leased premises in Montreal, Quebec, Canada and Boston, Massachusetts, USA.
Minimum annual payments under all operating leases are expected to be as follows:
|
|
| |||||
| Operating lease | ||||||
2017 |
|
| 547,718 | ||||
2018 |
|
| 576,588 | ||||
2019 |
|
| 298,706 | ||||
2020 |
|
| 77,897 | ||||
2021 |
|
| — | ||||
|
|
| |||||
|
|
| 1,500,909 | ||||
|
|
| |||||
In accordance with a licensing agreement with Roche, the Company may incur future payments with a potential aggregate value of $12 million based on the successful achievement of specific development, regulatory and commercial milestones relating to the current palovarotene development program, as well as additional future payments for additional indications. Future royalty payments, in the low teens, based on net sales are also stipulated in the licensing agreement along with one-time sales-based event payments with a potential aggregate value of $37.5 million. The likelihood and timing of these payments is unknown at this time.
In accordance with a licensing agreement with TJU, the Company may incur future payments with a potential aggregate value of $0.35 million based on the successful achievement of specific milestones relating to the current palovarotene development program. Future low single digit royalty payments based on net sales are also stipulated in the licensing agreement. Annual license maintenance royalties are also required as per the terms of the TJU licensing agreement. Such maintenance royalties are non-refundable, but can be applied to royalties owing on sales per calendar year. The likelihood and timing of these payments is unknown at this time.
In accordance with a licensing agreement with Yamaguchi, the Company may incur future payments with a potential aggregate value of $0.23 million based on the successful achievement of specific milestones relating to the palovarotene development program in corneal and retinal repair. Future low single digit royalty payments based on net sales are also stipulated in the licensing agreement. The likelihood and timing of these payments is unknown at this time.
In accordance with an exclusive option agreement signed with Galderma Research & Development SNC (Galderma) on April 1, 2016, the Company is currently evaluating certain Galderma retinoic acid receptor gamma agonist compounds in exchange for technology access payments with a potential aggregate amount of $0.7 million during a two-year option period. The technology access fees are being expensed over the period to which they relate. Should the Company decide to enter into a pre-negotiated and exclusive licensing agreement, the aforementioned technology access fees would immediately terminate.
On March 29, 2017, the Company entered into an exclusive licensing agreement with Galderma to obtain access to retinoic acid receptor gamma agonist compounds and was granted exclusive rights to use these in non-dermatological indications. In accordance with this agreement, the Company has paid a one-time licensing fee of $1 million and is committed to making certain future payments with a potential aggregate value of $27.5 million for up to three indications based on the successful achievement of specific development and commercialization milestones related to the licensed Galderma compounds. Future single digit royalty payments based on net sales are also stipulated in the licensing agreement. The likelihood and timing of these payments is unknown at this time.
The Company enters into contracts in the normal course of business with contract research organizations for preclinical research and clinical studies, research supplies and other services and
F-35
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
products for operating purposes. These contracts generally provide for termination on notice, and therefore are cancelable contracts.
The preferred shares are also not considered a contractual obligations and commitments as the preferred shares will automatically convert to common shares upon a qualifying event and are only redeemable after seven years.
14. Related party transactions
The Company has not been party to any material related party transactions which might reasonably be expected to influence decisions made by the users of these consolidated financial statements, other than the following.
During the year ended December 31, 2016, one of the Company’s executive officers provided limited services to a company controlled by an investor. The salary and related expenses incurred, such as travel costs, totaled $59,022 for the year ended December 31, 2016 (nil in 2015 and 2014), and were invoiced to the company controlled by an investor, all of which is receivable at December 31, 2016. The Company recorded this expense reimbursement as a reduction of general and administrative expenses.
The transactions referred to above are measured at the exchange amount, being the consideration established and agreed to by the related parties.
Accounts payable and accrued liabilities, including accumulated payroll, vacation and bonus accruals for key management personnel, as well as business expense reimbursements for key management personnel amounted to $355,327 as at December 31, 2016 ($379,462 at December 31, 2015 and $387,921 at December 31, 2014).
Compensation of key management personnel
The Executive Committee and the members of the Board of Directors are considered to be key management personnel. The aggregate compensation for the years ended December 31, 2016 and 2015 to key management personnel of the Company is set out below:
|
|
|
|
|
|
| |||||||||||||||
| 2016 | 2015 | 2014 | ||||||||||||||||||
Short-term |
|
| $ |
| 1,742,021 |
|
| $ |
| 1,672,327 |
|
| $ |
| 1,623,096 | ||||||
Director fees |
|
| 45,540 |
|
| 46,920 |
|
| 52,530 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
|
| 1,787,561 |
|
| 1,719,247 |
|
| 1,675,626 | |||||||||||||
Stock-based compensation |
|
| 83,270 |
|
| 126,889 |
|
| 120,991 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
|
| $ |
| 1,870,831 |
|
| $ |
| 1,846,136 |
|
| $ |
| 1,796,617 | |||||||
|
|
|
|
|
|
| |||||||||||||||
15. Financial instruments
a. Capital management
The Company’s primary objective when managing capital is to ensure its ability to continue as a going concern in order to pursue the development of its product candidates.
The capital structure consists of preferred shares and equity offset by cash and short-term investments.
Cash in excess of immediate working capital requirements is invested in accordance with the Company’s investment policy, primarily with a view to liquidity and capital preservation. At December 31, 2016, short-term investments are held in guaranteed investment certificates.
The Company monitors its cash requirements and market conditions to anticipate the timing of requiring additional capital to finance the development of its product candidates.
F-36
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
The Company has incurred losses and cumulative negative operating cash flows since its inception. The Company anticipates that it will continue to incur losses for at least the next several years in order to fund its operations. The Company expects its research and development and general and administrative expenses will continue to increase and, as a result, the Company will need additional capital to fund its operations, which it may raise through any one or a combination of equity offerings, debt financings, other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliance and licensing arrangements.
To date, the Company has financed its cash requirements primarily from the issuance of common shares, preferred shares and convertible notes. Although the Company’s cash position as at December 31, 2016 exceeds the Company’s anticipated level of gross expenditures for at least the next twelve months, the Company believes that its current liquidities will not be sufficient to carry its current development program through to commercialization. If the Company is unable to secure additional financing, it will be required to curtail some or all of its operations. There can be no assurance that such financing will materialize on a timely basis or be obtained on acceptable terms.
In order to maximize the full value of the Company’s assets and its ongoing development efforts, the Company does not pay out dividends.
The Company is not subject to any externally imposed restrictions, covenants or capital requirements.
b. Fair value of financial instruments
The Company has determined that the carrying amount of its cash, short-term investments, interest receivable, other receivables and accounts payable and accrued liabilities approximates their fair value because of the relatively short periods to maturity of these instruments.
c. Financial risk management objectives
The Company is exposed to certain financial risks, including liquidity risk, foreign currency risk and credit risk. Management is responsible for setting acceptable levels of risk and reviewing risk management activities as necessary.
d. Liquidity risk management
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they fall due. The Company manages liquidity risk through the management of its capital structure and financial leverage as outlined in note 15 a) above.
The balance of accounts payable and accrued liabilities is due within one year. For information on the maturity of the Class A and Class B preferred shares and commitment and contingencies, see note 6 and Note 13 respectively.
e. Foreign currency risk management
The Company is exposed to the financial risk related to the fluctuation of foreign exchange rates. The Company incurs a portion of its expenses in Canadian dollars and in Euros as well as other currencies to a lesser extent. A change in the currency exchange rates between the US dollar relative to the Canadian dollar or the Euro could have a significant effect on the Company’s results of operations, financial position or cash flows. The Company does not have in place any tools to manage its foreign exchange risk, other than keeping expected foreign currency cash requirements in the foreign currency to form a natural hedge.
F-37
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
The Company is exposed to currency risk through its cash, interest, sales tax and other receivables and accounts payable and accrued liabilities denominated in Canadian dollars and Euros as follows.
|
|
|
|
|
|
| |||||||||||||||
| December 31, | December 31, | December 31, | ||||||||||||||||||
Cash |
|
| 931,082 |
|
| 4,328,341 |
|
| 2,808,696 | ||||||||||||
Interest, sales tax and other receivables |
|
| 42,930 |
|
| 60,681 |
|
| 86,598 | ||||||||||||
Accounts payable and accrued liabilities |
|
| (569,958 | ) |
|
|
| (881,747 | ) |
|
|
| (544,333 | ) |
| ||||||
|
|
|
|
|
|
| |||||||||||||||
Net exposure |
|
| 404,054 |
|
| 3,507,275 |
|
| 2,350,961 | ||||||||||||
|
|
|
|
|
|
| |||||||||||||||
|
|
|
|
|
|
| |||||||||||||||
| December 31, | December 31, | December 31, | ||||||||||||||||||
Cash |
|
| 63,033 |
|
| 98,060 |
|
| — | ||||||||||||
Accounts payable and accrued liabilities |
|
| (421,451 | ) |
|
|
| (362,978 | ) |
|
|
| (23,875 | ) |
| ||||||
|
|
|
|
|
|
| |||||||||||||||
Net exposure |
|
| (358,418 | ) |
|
|
| (264,918 | ) |
|
|
| (23,875 | ) |
| ||||||
|
|
|
|
|
|
| |||||||||||||||
Based on the above net exposures as at December 31, 2016, and assuming that all other variables remain constant, a 10% depreciation or appreciation of the US dollar against the Canadian dollar would result in an increase/decrease of $30,065 on the Company’s net loss.
Based on the above net exposures as at December 31, 2016, and assuming that all other variables remain constant, a 10% depreciation or appreciation of the US dollar against the Euro would result in an increase/decrease of $37,695 on the Company’s net loss.
The following exchange rates applied during the year ended December 31, 2016:
|
|
|
|
| ||||||||||
| Average rate | Reporting date rate | ||||||||||||
CA$—US$ |
|
| 0.7581 |
|
| 0.7441 | ||||||||
EURO—US$ |
|
| 1.1355 |
|
| 1.0517 | ||||||||
f. Interest rate risk management
Interest rate risk is the risk that the fair value or future cash flows of a financial instrument will fluctuate because of changes in market interest rates.
The Company has no exposure to interest rate risk on its borrowing activities as all outstanding debt as at December 31, 2016 (preferred shares) is interest-free. The Company is exposed to interest rate risk on its short-term investments.
g. Credit risk management
Credit risk arises from cash, short-term investments, interest, and other receivables.
The Company holds its cash and short-term investments at Canadian and US chartered banks. The credit risk of cash, short-term investments and interest receivable from short-term investments is limited because the counter-parties are chartered banks with high credit ratings assigned by international credit rating agencies.
16. Operating segments
The Company manages its operations as a single segment for the purposes of assessing performance and making operating decisions, being the biopharmaceutical segment. The Company’s singular focus is on advancing treatments for people living with rare diseases, including FOP.
F-38
CLEMENTIA PHARMACEUTICALS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
Years ended December 31, 2016, 2015 and 2014 (in US dollars)
All of the Company’s intangible assets are held in Canada. The Company’s property and equipment are held as follows: 80% held in Canada and 20% in the United States.
17. Subsequent events
a) Class C financing
On March 16, 2017, the Company completed a $10,000,080 Class C financing with the Fonds de solidarité des travailleurs du Québec (FTQ). Under the agreed terms, the Company issued 70,176 Class C redeemable convertible preferred shares at $142.50 per share for a total consideration of $10,000,080. The terms of the Class C redeemable convertible preferred shares are substantially the same as those of Class A and Class B preferred shares.
b) Granting of stock options
On February 28, 2017, the Company granted 14,550 stock options at an exercise price of $116.29.
On April 30, 2017, the Company granted 39,100 stock options at an exercise price of $120.36.
F-39
Through and including , 2017 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
Shares

Common Shares
PROSPECTUS
|
|
|
MORGAN STANLEY | LEERINK PARTNERS | |
WEDBUSH PACGROW | BTIG |
, 2017
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors and Officers
Under the Canada Business Corporations Act (CBCA), we may indemnify our current or former directors or officers or another individual who acts or acted at our request as a director or officer, or an individual acting in a similar capacity, of another entity, against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by the individual in respect of any civil, criminal, administrative, investigative or other proceeding in which the individual is involved because of his or her association with us or another entity. The CBCA also provides that we may advance moneys to a director, officer or other individual for costs, charges and expenses reasonably incurred in connection with such a proceeding; provided that such individual shall repay the moneys if the individual does not fulfill the conditions described below.
However, indemnification is prohibited under the CBCA unless the individual:
• | acted honestly and in good faith with a view to our best interests, or the best interests of the other entity for which the individual acted as director or officer or in a similar capacity at our request; and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
• | in the case of a criminal or administrative action or proceeding that is enforced by a monetary penalty, the individual had reasonable grounds for believing that his or her conduct was lawful | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Our by-laws require us to indemnify each of our current or former directors or officers and each individual who acts or acted at our request as a director or officer, or an individual acting in a similar capacity, of another entity, against all costs, charges and expenses, including, without limitation, an amount paid to settle an action or satisfy a judgment, reasonably incurred by the individual in respect of any civil, criminal, administrative, investigative or other proceeding in which the individual is involved because of his or her association with us or another entity.
Our by-laws authorize us to purchase and maintain insurance for the benefit of each of our current or former directors or officers and each person who acts or acted at our request as a director or officer, or an individual acting in a similar capacity, of another entity.
We have entered into indemnity agreements with our directors and certain officers which provide, among other things, that we will indemnify him or her to the fullest extent permitted by law from and against all liabilities, costs, charges and expenses incurred as a result of his or her actions in the exercise of his or her duties as a director or officer.
At present, we are not aware of any pending or threatened litigation or proceeding involving any of our directors, officers, employees or agents in which indemnification would be required or permitted.
The Underwriting Agreement filed as Exhibit 1.1 to this Registration Statement provides for indemnification of our officers and directors by the underwriters against certain liabilities.
Item 7. Recent Sales of Unregistered Securities
Set forth below is information regarding all securities issued by Clementia without registration under the Securities Act since January 1, 2014. The following sales have not been adjusted to give effect to the 1-for- split of our common shares.
Common Share and Option Issuances
Since January 1, 2014, Clementia has granted to its directors, officers, employees, consultants and advisors options to purchase an aggregate of 207,491 common shares under its Existing Employee Stock Option Plan at a weighted average exercise price of $35.15 per share.
Since January 1, 2014, Clementia has issued and sold to its directors, officers, employees, consultants and advisors an aggregate of 19,186 common shares in connection with the exercise of
II-1
options granted under its equity compensation plans, at a weighted average exercise price of $3.64 per share.
Preferred Share Issuances
Between January 1, 2014 and March 18, 2015, Clementia issued an aggregate of 861,124 Class A preferred shares for $29.245 per share in connection with its Class A financing for an aggregate gross purchase price of approximately $25,183,571. The shares were issued in reliance on the exemption from registration under Section 4(a)(2) of the Securities Act on the basis that the transaction did not involve a public offering.
On June 22, 2015, Clementia issued an aggregate of 485,823 Class B shares for $123.50 per share in connection with its Class B financing for an aggregate gross purchase price of approximately $59,999,141. The shares were issued in reliance on the exemption from registration under Section 4(a)(2) of the Securities Act on the basis that the transaction did not involve a public offering. Leerink Partners LLC served as placement agent for the transaction, and received a sales commission of $2,145,300.
On March 16, 2017, Clementia issued an aggregate of 70,176 Class C preferred shares for $142.50 per share in connection with its Class C financing for an aggregate gross purchase price of approximately $10,000,080. The shares were issued to a single non-U.S. investor.
Except as noted above, the issuance of securities described above did not involve underwriters. All recipients had adequate access, through their relationships with us, to information about us. The recipients of securities in each such transaction represented their intention to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the share certificates and other instruments issued in such transactions. The sales of these securities were made without general solicitation or advertising.
Item 8. Exhibits and Financial Statement Schedules.
The exhibits listed in the exhibits index, appearing elsewhere in this Registration Statement, have been filed as part of this Registration Statement.
All financial statement schedules have been omitted because either they are not required, are not applicable or the information required therein is otherwise set forth in the registrant’s financial statements and related notes thereto.
Item 9. Undertakings.
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreements, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the undersigned registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
1. For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A
II-2
and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
2. For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-3
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Montreal, Province of Quebec on this 29th day of June, 2017.
CLEMENTIA PHARMACEUTICALS INC.
By: | /S/ CLARISSA DESJARDINS
Name: Clarissa Desjardins |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTED,that each person whose signature appears below hereby constitutes and appoints Clarissa Desjardins and Michael Singer as the undersigned’s true and lawful attorney-in-fact and agents, with full power of substitution and resubstitution for such person and in such person’s name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments, exhibits thereto, and other documents in connection therewith to this registration statement and any related registration statements necessary to register additional securities) and to file the same with exhibits thereto and other documents in connection therewith with the SEC, granting unto said attorney-in-fact and agent full power and authority to do and perform each and every act and thing requisite and necessary to be done, as fully to all intents and purposes as such person might or could do in person, hereby ratifying and confirming all that each of said attorney-in-fact and agent, or their substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities indicated on this 29th day of June, 2017.
Signature | Title | |||
| ||||
/S/ CLARISSA DESJARDINS
Clarissa Desjardins | President and Chief Executive | |||
/S/ MICHAEL SINGER
Michael Singer | Chief Financial Officer (principal | |||
/S/ DAVID BONITA
David Bonita | Chairman of the Board of Directors | |||
/S/ ROBERT HEFT
Robert Heft | Director | |||
/S/ ALLAN MANDELZYS
Allan Mandelzys | Director | |||
/S/ DAVID MOTT
David Mott | Director | |||
/S/ FRANCOIS NADER
Francois Nader | Director | |||
/S/ JEAN-FRANÇOIS PARISEAU
Jean-François Pariseau | Director | |||
II-4
AUTHORIZED REPRESENTATIVE
Pursuant to the requirements of Section 6(a) of the Securities Act of 1933, the undersigned has signed this Registration Statement, solely in the capacity of the duly authorized representative of Clementia Pharmaceuticals Inc. in the United States, on this 29th day of June 2017.
CLEMENTIA PHARMACEUTICALS INC.
By: | /S/ CLARISSA DESJARDINS
Name: Clarissa Desjardins |
II-5
|
|
| |||||
Exhibit No. | Description | ||||||
1.1** | Underwriting Agreement. | ||||||
| 3.1** | Articles of Amendment of the Registrant, to be in effect upon closing of the offering. | |||||
3.2** | Form of Amended and Restated By-Laws of the Registrant, to be in effect upon closing of the offering. | ||||||
| 4.1** | Form of Common Share Certificate. | |||||
4.2* | Second Amended and Restated Registration Rights Agreement, dated March 16, 2017. | ||||||
| 5.1** | Opinion of Dentons Canada LLP. | |||||
10.1*† | License Agreement, by and among F. Hoffman-La Roche Ltd, Hoffman-La Roche Inc. and Clementia Pharmaceuticals Inc., dated January 4, 2013. | ||||||
| 10.2*† | Exclusive License Agreement by and between Thomas Jefferson University and Clementia Pharmaceuticals Inc., dated February 10, 2014. | |||||
10.3*† | Exclusive License Agreement by and among Yamaguchi University, Yamaguchi Technology Licensing Organization and Clementia Pharmaceuticals Inc., dated April 8, 2015. | ||||||
| 10.4*† | Exclusive License Agreement by and between Galderma Research & Development SNC and Clementia Pharmaceuticals Inc., dated March 29, 2017. | |||||
10.5*† | Palovarotene Program Consultancy Agreement between Clementia Pharmaceuticals Inc. and Luca Sangiorgi dated September 18, 2015. | ||||||
| 10.6*† | Agreement for fee-per-service between Clementia Pharmaceuticals Inc. and Instituto Ortopedico Rizzoli dated April 27, 2017. | |||||
10.7*† | Commercial Sponsored Research Agreement by Stanford Burnham Prebys Medical Discovery Institute and Clementia Pharmaceuticals Inc. dated June 21, 2016. | ||||||
| 10.8** | Third Amended and Restated Stock Option Plan. | |||||
10.9** | 2017 Omnibus Plan. | ||||||
| 10.10** | Employee Share Purchase Plan. | |||||
10.11** | Form of Indemnification Agreement for Directors and Officers. | ||||||
| 10.12** | Employment Agreement with Clarissa Desjardins, dated , 2017, to be in effect upon closing of the offering. | |||||
10.13** | Employment Agreement with Donna Roy Grogan, dated , 2017, to be in effect upon closing of the offering. | ||||||
| 10.14** | Employment Agreement with Eric Grinstead, dated , 2017, to be in effect upon closing of the offering. | |||||
10.15** | Employment Agreement with Jeffrey Packman, dated , 2017, to be in effect upon closing of the offering. | ||||||
| 10.16** | Employment Agreement with Michael Singer, dated , 2017, to be in effect upon closing of the offering. | |||||
16.1* | Letter from former auditor, Deloitte LLP. | ||||||
| 21.1* | List of Subsidiaries of the Registrant. | |||||
23.1* | Consent of KPMG LLP. | ||||||
| 23.2** | Consent of Jenner & Block LLP. | |||||
23.3** | Consent of Dentons Canada LLP (included in Exhibit 5.1). | ||||||
| 24.1* | Power of Attorney (included on signature page). | |||||
* | Included herewith. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
** | To be provided by amendment. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
† | Confidential treatment has been requested for portions of this exhibit. These portions will be omitted from the registration statement and have been filed separately with the United States Securities and Exchange Commission. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||