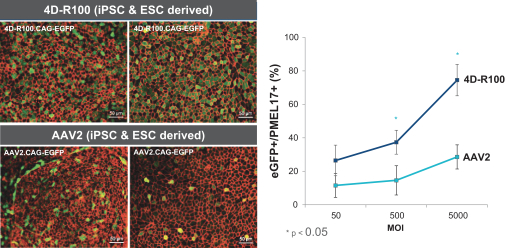
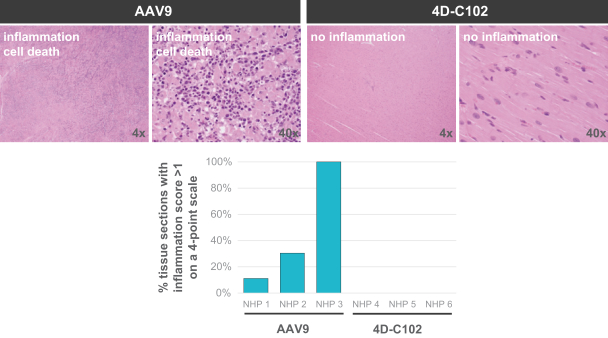
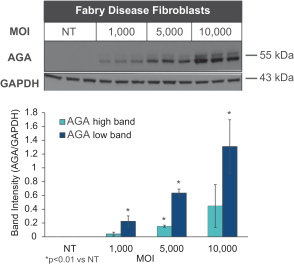
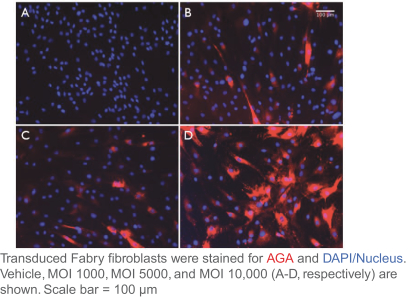
Hatch-Waxman Amendments of 1984. In the European Union member countries, a supplementary protection certificate, if obtained, provides a maximum five years of market exclusivity.
In other jurisdictions (currently, Australia, Bahrain, Brazil, Canada, Chile, China, Colombia, Costa Rica, Egypt, India, Indonesia, Iran, Israel, Japan, Korea, Kuwait, Malaysia, Mexico, New Zealand, Oman, Peru, Philippines, Qatar, Russia, Saudi Arabia, Singapore, Thailand, United Arab Emirates, Ukraine, Vietnam and South Africa), the patent applications relating to our product and lead optimization candidates, including composition of matter and various other patents, including dosage unit form, method-of-treatment and medical use patents, where applicable, are expected to expire between May 12, 2037 and November 26, 2038, if the appropriate maintenance, renewal, annuity, and other government fees are paid. These patents and patent applications (if applicable), depending on the national laws, may benefit from extension of patent term in individual countries if regulatory approval of any of our product or lead optimization candidates is obtained in those countries. For example, in Japan, the term of a patent may be extended by a maximum of five years in certain circumstances.
Our in-licensed patent portfolio includes four granted U.S. patents and six granted foreign patents; each of these patents is expected to expire between June 28, 2024. and June 13, 2029. Our in-licensed patent portfolio also includes three pending U.S. non-provisional patent applications, five pending foreign patent applications and one pending international patent application.
In other jurisdictions (currently, Canada, China, France, Germany, Great Britain, Hong Kong, Japan and Italy), the term and patent applications relating to our product and lead optimization candidates, including composition of matter and various other patents, including dosage unit form, method-of-treatment and medical use patents, where applicable, are expected to expire between June 29, 2024 and June 28, 2038, if the appropriate maintenance, renewal, annuity, and other government fees are paid. These patents and patent applications (if applicable), depending on the national laws, may benefit from extension of patent term in individual countries if regulatory approval of any of our product or lead optimization candidates is obtained in those countries. For example, in Japan, the term of a patent may be extended by a maximum of five years in certain circumstances.
Individual patents extend for varying periods depending on the date of filing of the patent application or the date of patent issuance and the legal term of patents in the countries in which they are obtained. Generally, patents issued for regularly filed applications in the United States are effective for 20 years from the earliest effective non-provisional filing date. In addition, in certain instances, a patent term can be extended to recapture a portion of the U.S. Patent and Trademark Office (USPTO) delay in issuing the patent as well as a portion of the term effectively lost as a result of the FDA regulatory review period. However, as to the FDA component, the restoration period cannot be longer than five years and the total patent term including the restoration period must not exceed 14 years following FDA approval. The duration of foreign patents varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest effective filing date. The actual protection afforded by a patent varies on a product by product basis, from country to country and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent.
We also protect our proprietary technology and processes, in part, by confidentiality and invention assignment agreements with our employees, consultants, scientific advisors and other contractors. These agreements may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our employees, consultants, scientific advisors or other contractors use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
147