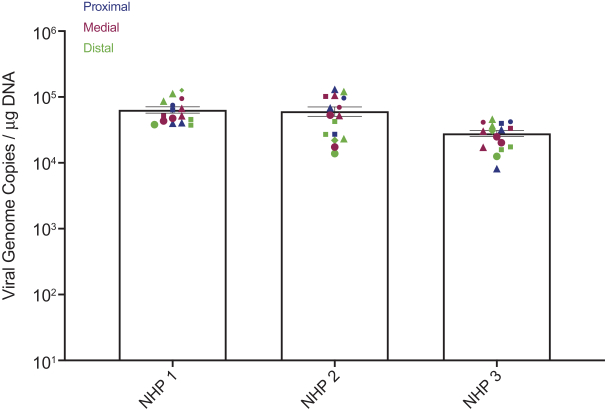
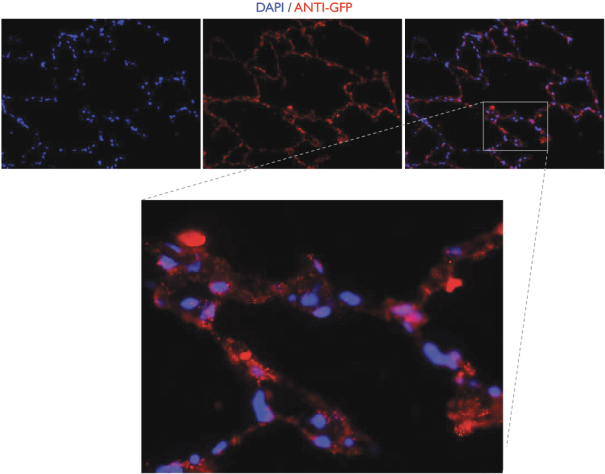
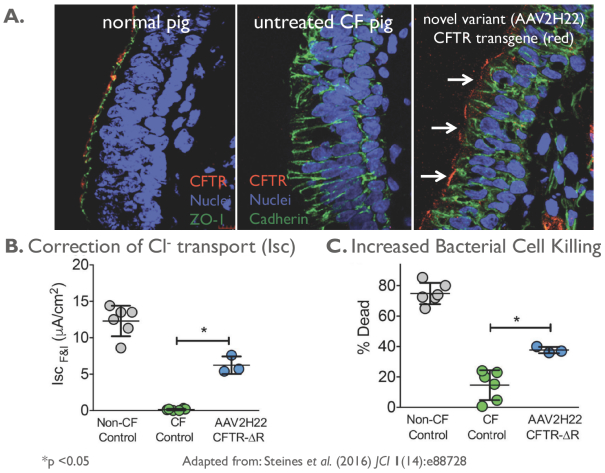
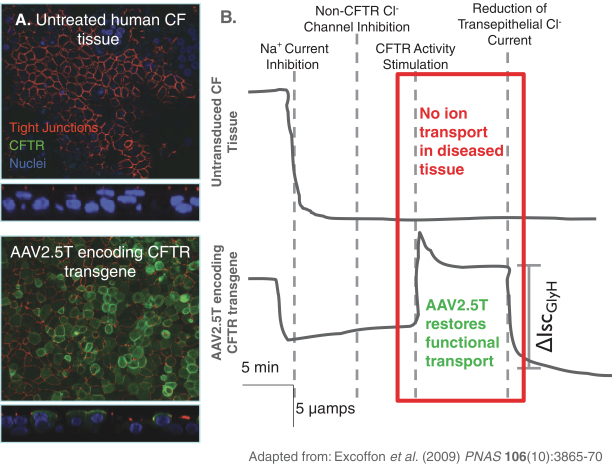
Team
Our team of approximately 20 highly-trained individuals is led by our Chief Technical Officer, Dr. Kamal. Collectively, they have significant experience in viral vector manufacturing, chemistry-manufacturing-controls (CMC), regulatory affairs, analytical and process development, and quality assurance and controls. Our team also has experience with manufacturing multiple AAV vectors from preclinical studies through to multiple Phase 3 trials. For example, Dr. Kamal helped to write and compile the AAV gene therapy BLA for AVXS-101 or Zolgensma (Novartis).
Intellectual Property
Our commercial success depends in part on our ability to obtain and maintain proprietary protection for our product candidates, manufacturing and process discoveries, and other know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development and implementation of our business. We also rely on trade secrets, know-how, continuing technological innovation and potential in-licensing opportunities to develop and maintain our proprietary position.
Our product and lead optimization candidates were discovered by us utilizing our proprietary technology. We have filed several non-provisional and provisional patent applications, all owned by us, relating to our product and lead optimization candidates in the United States, certain foreign countries, and the World Intellectual Property Organization that are directed to compositions-of-matter, dosage unit forms, methods-of-treatment and medical use. We have also licensed several non-provisional patent applications, granted patents and international patent applications relating to our product and lead optimization candidates from U.C. Berkeley.
Our solely owned patent portfolio includes one pending U.S. non-provisional application, thirty-one pending foreign applications, one of which has been allowed, one granted foreign patent and two pending international applications. We expect that United States and European patents and the patent applications in this portfolio, if issued, would expire between May 12, 2037 and November 26, 2038, excluding any additional term from patent term adjustment or patent term extension if appropriate maintenance and other governmental fees are paid. Additional patent term for the presently-issued or later issued U.S. patents may be awarded as a result of the patent term extension provision of the Hatch-Waxman Amendments of 1984. In the European Union member countries, a supplementary protection certificate, if obtained, provides a maximum five years of market exclusivity.
In other jurisdictions (currently, Australia, Bahrain, Brazil, Canada, Chile, China, Colombia, Costa Rica, Egypt, India, Indonesia, Iran, Israel, Japan, Korea, Kuwait, Malaysia, Mexico, New Zealand, Oman, Peru, Philippines, Qatar, Russia, Saudi Arabia, Singapore, Thailand, United Arab Emirates, Ukraine, Vietnam and South Africa), the patent applications relating to our product and lead optimization candidates, including composition of matter and various other patents, including dosage unit form, method-of-treatment and medical use patents, where applicable, are expected to expire between May 12, 2037 and November 26, 2038, if the appropriate maintenance, renewal, annuity, and other government fees are paid. These patents and patent applications (if applicable), depending on the national laws, may benefit from extension of patent term in individual countries if regulatory approval of any of our product or lead optimization candidates is obtained in those countries. For example, in Japan, the term of a patent may be extended by a maximum of five years in certain circumstances.
Our in-licensed patent portfolio includes four granted U.S. patents and six granted foreign patents; each of these patents is expected to expire between June 28, 2024. and June 13, 2029. Our
153