Exhibit 13.1
Index of Materials:
| 3. | Transcripts of videos found on the Wefunder Campaign Page and Quadrant Investor Page |




The Science Behind The Clarifi Test
What we’ve been able to do over the last 5 or 7 years, working together with a very dedicated team, is develop a test that utilizes saliva and can accurately distinguish whether a child has autism spectrum disorder or does not. This relies on a new type of molecular analysis that wasn't even possible until recently.
We’ve published five different studies to date on the specific use of saliva for accurately diagnosing autism spectrum disorder. We had initial pilot work that was published using smaller samples. It was really a proof of principle. And then we’ve had a number of different publications since then stretching back almost 6 years, where we looked at different classes of molecules individually and reported on their ability to differentiate autism from their peers. Last year we published the culminations. This was the largest study involving about 450 children. It was published in a peer reviewed journal, it was very thoroughly reviewed, and it was also very warmly accepted by our scientific and medical colleagues, who were very surprised to see that saliva could be used to accurately distinguish children with autism from those who did not have autism, and that there was science behind it that backed it up.
Saliva, it turns out, is a treasure trove for molecules that might reflect brain function, and we felt that was a key component for the development of a test that’s going to essentially be evaluating a change in brain function. The reason that saliva is so well characteristic, or reflective, of what’s going on in the brain has a lot to do with the simple fact that the brain stem innervates so much of the oropharynx where saliva is made, where you swallow saliva, where you speak, where you encounter the foods that you’re putting in your mouth all the time.
It became very apparent that the molecule types we were interested in evaluating were actually present in saliva in higher levels than you’d find in blood, in other biofluids and in other tissues. And what was in saliva was indeed highly reflective of brain content or brain contributions to the oraphynx.
The reason it’s not a genetic test is because we’re not assessing or looking for a change in DNA sequence. We are specifically pushing a technology that utilizes epigenetic testing. We believe that the epigenetic level of testing that we are providing represents a final common path, so whether a child may have developed ASD as a result of one particular DNA sequence change or another one or potentially some environmental exposure that pushes them toward autism spectrum disorder, we believe there will be a consvere change at the epigenetic level that we can measure in the saliva.
The term epigenetics refers to any trait that could be passed from one generation of cells or individuals to the next generation that does not rely on a change in DNA sequence for that transmission. The markers that we have chosen to study, we didn’t even know existed 20 years ago, and it turns out that these are master regulators that don’t rely on a change in DNA sequence to exert their effects, and yet they control the expression of most of the genes in the human genome.
All the cells in your body can create proteins. The central dogma of biology has held for over half a century that that process involves going from DNA to RNA to protein. We taught for many years that that’s all you needed to know. We learned about two decades ago that there’s a whole class of molecules that we refer to as non coding RNA that strongly affect this whole process, going from DNA to RNA to protein. These are known as non coding RNAs. They don't themselves code for proteins, but they are essential for this action to take place. There are specific classes of non coding RNAs that are quite important in our test. These specific classes include micro RNAs.
Micro RNAs are made in all cells of the body. They are released in all cells of the body and they travel in extracellular fluids, like hormones, throughout the body and can affect the process of making a protein in cells that are distant from the cells in which they were originally made. In the case of our saliva test, we are looking at miRNAs, some of which have been made in brain cells and yet are found in the saliva. And that’s one of the key reasons this particular saliva test is providing a window into brain function.
The microbiome refers to the conjunction of microorganisms that live in a particular portion of the body or on a particular portion of the body and are quite distinct from the host cell or the host organism. The interactions of those microorganisms with the host collectively forms a microbiome like a mini atmosphere or small world in which lots of interactions take place.
Machine learning that we use in the development of the saliva test allowed us to utilize two features of machine learning to our advantage. One of those is you can consider all possible components equally at the beginning, and when we talk about saliva, we had about 20,000 different features that would have contributed to the final molecular test.
Through a machine learning algorithm, the important ones were narrowed down really by trial and error, a rope process that would take a human thousands of years to go through, the machine literally the computer can go through and test different combinations of features until a test set performs really well. Another critical component of machine learning is the ability to take that test and evaluate it in a replication set, a hold out set, that was not involved in deciding what the important features were at the beginning. And that whole process of going through and selecting molecules you’d think would perform well on the test, evaluating them both in the training set and the independent test set, doing that over and over again is what machine learning is really based on, regardless of the discipline in which you are using it.
What is sensitivity? It’s actually indicating the percent of times that you get an accurate result from the test when you have the actual condition that you’re testing for.
So some of the times when you use a test and you have the condition, you will get a false negative. We see with the autism saliva test very high sensitivity.
There’s another metric that’s used to evaluate diagnostic tests and that’s the specificity. When you have a result that indicates you do not have a condition, is that accurate or not, specificity is really referring to the simple fact of when you do not have a disease or a condition, does the test actually report that result, or does the test sometimes say you do have it?
When evaluating the performance of any molecular diagnostic test, you want to pay very careful attention to overall sensitivity and specificity. One of the statistical measures that combines both of those would be the overall accuracy of the test, or what we would refer to as the area under the curve and a receiver operating characteristic curve analysis.
Early intervention through accurate referrals and accurate diagnoses is what this all centered on achieving. The advantage of the saliva test from a pediatrician's perspective is that it will back up the opinion that they may have formed, or been forming over the previous experience they had with the child, and provide them a sound objective, medically-based, scientifically-tested, read out, that can be used to confirm their suspicion. The saliva test takes a few seconds to obtain a sample and really a few weeks to get a full report that explains the results to the pediatrician. And we feel that that’s something they will want to have when they make the decision to either refer a child to a developmental specialist for a formal, full length behavioral evaluation or not.
When the test is properly applied and interpreted, can be used as an aid to that process and really facilitate the initiation of appropriate therapies and interventions that can make a difference in the child’s lifetime, and in the parents lifetime.
What Is Clarifi?
What we have been able to do over the last five or six years working together with a very dedicated team is develop a test that utilizes saliva and can accurately distinguish whether a child has autism spectrum disorder or does not and this relies on a new type of molecular analysis that really wasn’t even possible until recently.
The Clarifi Story
Richard Uhlig, Founder & CEO of Quadrant Biosciences : We’re seeing a very significant increase in prevalence of autism spectrum disorder in our society. As recently as 2006 1 in 150 children in the United States were diagnosed with autism spectrum disorder. As we sit here right now the statics are now 1 in 59. The causes of that are generally unknown, what we do know is that currently in the United States the average age of diagnosis is between four and five years old. Our goal is to really change that, to have the average age of diagnosis in the second year of life. Access to those types of services at that very early age is nothing short of changing the trajectory of that child's life.
VO: We discovered really pretty early on in our research that children with autism did not want to have needles stuck in their arms and parents did not want to have to hold them down for an autism test and we understood that we could sample some of the same epigenetic molecules in saliva as we could in blood. Clarifi harnesses new RNA molecules that most scientists didn’t even know existed ten years ago. These molecules are critical for brain development and what’s more they’re present in high levels in the saliva. This allows us to do a non-invasive test in an out-patient pediatrics office and identify changes in the levels of molecules which differentiate kids with autism with kids who have typical development.
Frank Middleton, Associate Professor in the Department of Neurosciences and Physiology at Upstate Medical University: All other evaluations and diagnoses of autism are based on an observation of a child’s behavior. The saliva test has the advantage of being objective.
Angela Saturno, Assistant Clinical Director ABA Services at AAPSA: Imagine if you have a child that is very aversive to going into the doctor's office. This test is quick, it is non-invasive, and it’s something that will give you the answer you need. Now that will open the door for them to come to clinics and try to get treatment that is evidence based and effective.
Olivia: If i had the opportunity to do this, I would do it as early as I could. It gives you a head start, it gives you an opportunity to take advantage of new things and new resources, and he’s a different kid because we got the diagnosis and we got the help early on.
Richard Uhlig: To have the opportunity to bring a product like Clarifi to market is frankly, humbling. This is a product that’s going to positively affect the lives of millions of people around the World. We feel very honored to be the company that is bringing this product to market.
Clarifi Parent Perspective
It gives you a headstart, it gives you an opportunity to take advantage of new things and new resources and he’s a different kid because we got the diagnosis and got the help early on.
Investor Relations Video
Hi I’m Rich Uhlig, Founder and CEO of Quadrant Biosciences.
It’s not every day you have the opportunity to make a difference in someone’s life. It’s even more rare to be in a position to positively change the trajectory of millions of lives. I believe our company is on the verge of doing just that through our scientific breakthroughs in the area of epigenetic biomarkers for autism spectrum disorder. And you can be a part of that too.
Quadrant Biosciences has just released Clarifi ASD, the world’s first epigenetic saliva test for autism. And it all started here, at this research lab at SUNY Upstate Medical University in Syracuse New York.
Seven years ago,using state of the art next-generation sequencing, researchers discovered that certain molecules in the saliva called microRNA could accurately differentiate children with autism from their peers,
This groundbreaking discovery led to a series of peer- reviewed research publications [show publications] that culminated in the finding that by combining these miRNAs and specific bacteria in the oral microbiome, and applying sophisticated machined-learning tools, an accurate autism diagnostic test could be developed. That research provided the scientific foundation for the Clarifi ASD test.
But why is this so important?
As you may have heard, the rate of autism in the U.S. has rapidly increased over the past several decades and currently stands at 1 in 59 children, making it themost commonly diagnosed developmental disability in the United States. Unfortunately, existing screening tools are causing pediatricians to over-refer patients for ASD evaluations and geographically, there are a limited number of trained experts diagnosing autism, causing wait times for diagnostic evaluation to exceed 12 months on average and may exceed 18 months in some locations.
What this means is that autism is being diagnosed on average in the fifth year of life when it should be reliably diagnosed earlier for access to intervention services. Indeed, studies have shown that with early intensive behavioral intervention beginning the second year of life, between 45 and 50% of children on the autism spectrum become indistinguishable from their peers by the time they get to first grade. This outcome can dramatically improve the quality of life for ASD children and their families.
Thats where Clariri ASD comes in. Now with a simple saliva swab, we can provide clinicians biological information to support earlier diagnosis and expedite the start of services. Our goal is to help move the average age of diagnosis in the US from 4-5 years of age, to the second year of life when therapy is most effective.
Why consider investing?
I’ll give you five reasons
| 1. | First, as you heard we have just launched the world’s first epigenetic diagnostic aid for autism spectrum disorder. |
| | |
| 2. | We have successfully raised over 30 million from private investors who have recognized the value in what we are doing. |
| | |
| 3. | Our pipeline - Clarifi ASD is just the beginning. Several other products based on the Clarifi epigenetic diagnostic platform are under currently development including saliva tests for concussion and Parkinson’s disease. |
| | |
| 4. | We have assembled a seasoned management team, as well as a world class medical advisory board comprised of recognized leaders in the areas of autism and epigenetic research to confidently guide us into the future. |
| | |
| 5. | Fifth and probably the most important factor in our success, is our people. Our entire team recognizes that we are fundamentally in the service of others, and this drives us every day. |
So if our mission to develop diagnostic solutions to today’s most pressing neurological challenges appeals to you, please consider joining us as an investor. We would love to have join our Quadrant Biosciences team; and work together to help make the world a better place.
Austin Mom
Amber: When a behavior test piggybacks with this wonderful scientific test you’re looking at that child’s biological makeup. In addition to how they behave, how they’re acting, how they’re developing, what they’re doing, milestones they’re meeting, with those two together you can’t go wrong.
Clarifi HCP Informational
Part 1: What’s inside the kit
Inside your test kit will be six items:
| 1. | A collection swab |
| | |
| 2. | Four matching barcode stickers and |
| | |
| 3. | Collection instructions |
| | |
| Behind the panel you will find |
| | |
| 4. | A requisition form to be completed by the clinician |
| | |
| 5. | A patient instruction card to be given to the patient |
| | |
| 6. | And a biospecimen bag for return shipment of the sample |
Part 2: Administering the test
Select an unopened kit and verify the expiration date.
While opening the kit be sure to keep the box intact because it will be used for return shipment.
Verify the child has not had food or drink for 15 minutes prior to collection.
Rinse the child’s mouth before collection.
The child may swallow or spit out the water.
With gloves on, take the collection swab out of the packaging being sure not to touch the end of the swab.
Do not use if the collection swab appears to be contaminated.
When collecting the sample you will want to be sure the swab tip touches three places:
| 1. | The right side of the mouth between the gums and cheek where saliva builds up |
| | |
| 2. | The left side of the mouth between the gums and cheek where saliva builds up and |
| | |
| 3. | Under the tongue |
Your goal is to saturate the swab.
During this step you will want to avoid touching the tongue and the teeth.
After swabbing is complete, hold the collection tube upright and unscrew the cap.
Turn the cap upside down and place it in the stabilizing solution.
Make sure the cap is on tight, then invert the tube and shake vigorously 15 times.
Write the date of birth, name, date and time of collection on the tube in the space provided. If you have to use a separate label, make sure not to cover the barcode label.
Place the sample in the biospecimen bag provided and seal.
Do not remove the absorbent pad.
Pull out the requisition form and instruction card and attach barcode stickers to both forms. Note, there are two additional barcodes for office use.
Complete all fields on the requisition form, including a signature from both the clinician and parent.
Provide the patient instruction card with barcode to the patient’s guardian so they can register and pay for the test.
Part 3: Shipping test kit
Place the biospecimen bag containing the collected sample and the completed requisition form in the box, seal closed.
All items need to be complete and included within the kit otherwise the sample cannot be processed.
Ship the completed test kit within 24 hours of sample collection via UPS.
Part 4: Results
Results will be delivered to the clinician identified on the requisition form in 3 to 6 weeks via the web site.
Review the results with the parent and provide the parent with a supplemental information sheet.
Thank you for choosing Clarifi.
What to Expect
What to expect during your Clarifi test appointment:
When you arrive at the doctor’s office you will need to confirm that your child hasn’t had food or drink 15 min prior to the test. Next, the doctor will gently swab the child’s mouth. First the right and left side next to the gums, and then under the tongue. This should take less than 30 seconds.
Before leaving the office, you and your child’s doctor will complete a requisition form. You will be given a patient instruction card that explains how to pay for and activate the test when you get home. The doctor will apply a barcode sticker to the patient instruction card for this purpose.
Back home, use the code on the patient instruction card to log into ClarifiASD.com. Then, activate the kit by submitting payment and completing the required questionnaire. Clarifi test results will be sent to your child’s doctor in 3 to 6 weeks. Schedule a followup appointment to discuss the results and options moving forward. At that appointment, you will be given a password to access comprehensive resources to help guide your family moving forward.




uadrant biosciences Quadrant Biosciences Inc. Richard Uhlig,Founder and CEO February 6,2020 coMPANY wHo DATE
Professional Biographies Contact Us News Leadership and governance Intellectual property Product pipeline Clari Business model About the Company

About the Company

Section A-About the Company quadrantbiosciences.com Quadrant Biosciences is an epigenetic diagnostics company with an initial focus on the early detection of autism spectrum disorder, Parkinson's Disease, concussion injuries, and other large-scale health issues. Al

We are strategically located in the Institute for Human Performance on the SUNY Upstate Medical University campus to rapidly translate cutting edge scientic advancements into commercial products. Our business is co-located with the University Hospital’s: ●Neuroscience Department ●Molecular Analysis Core Facility ●Concussion Management Clinic ●Concussion Research and Motion Laboratory We co-developed innovative epigenetic technologies with two major research university hospitals. Quadrant Biosciences Inc. was founded in 2015

Quadrant awarded 2019 Technology Business of the Year We are honored to be recognized as the 2019 Technology Business of the Year by the boldest, brightest, and best small companies in New York State. Development Center. The award is given annually to the New York State Small Business

$330,000 $2.0 M $225,000 $201,000 Quadrant has received grants in excess of $2.7 million
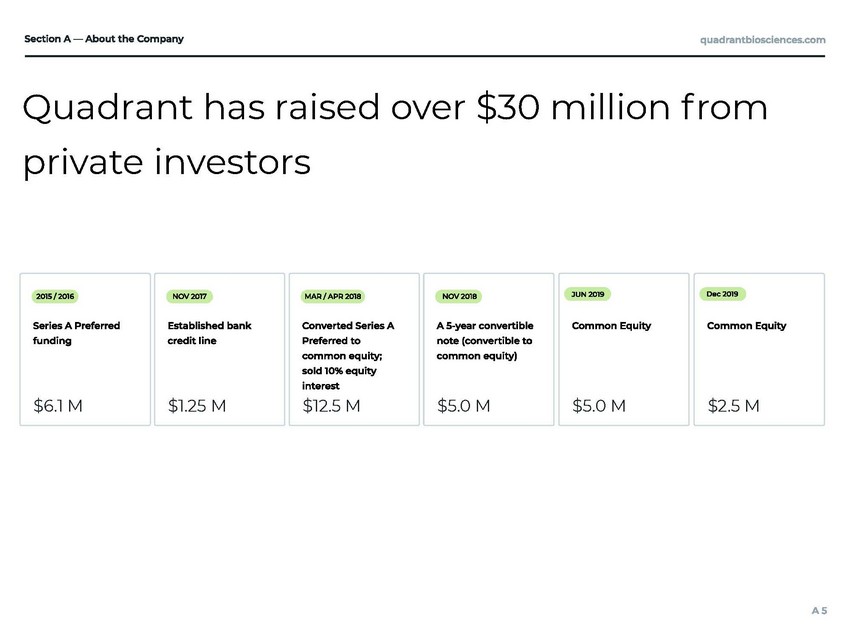
$6.1 M $1.25 M $12.5 M $5.0 M $5.0 M $2.5 M Quadrant has raised over $30 million f rom private investors

Business Model
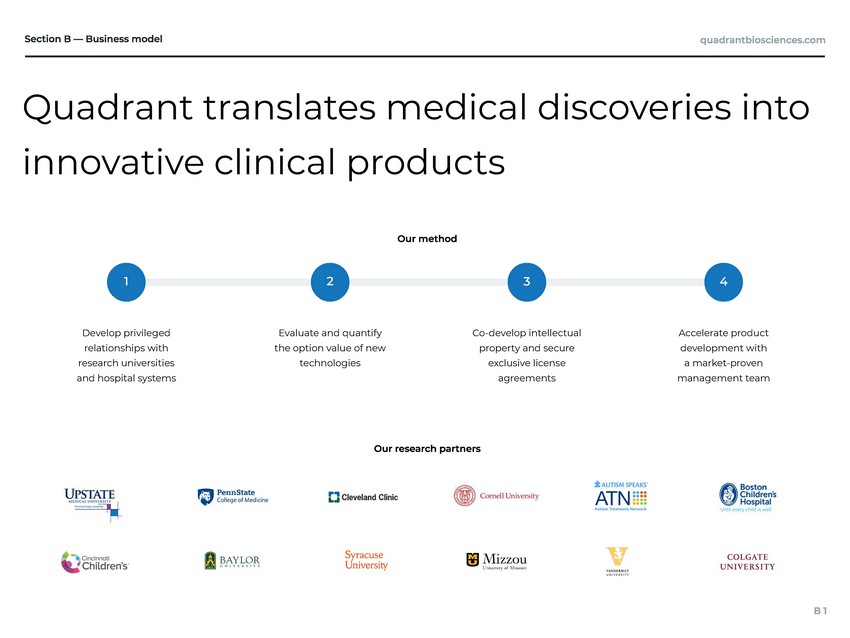
Quadrant translates medical discoveries into innovative clinical products Evaluate and quantify the option value of new technologies Co-develop intellectual property and secure exclusive license agreements Accelerate product development with a market-proven management team

We are global leaders in the development of epigenetic diagnostics Learn about our process

Quadrant has developed a world-class data management and analytics system A growing portfolio of analytic techniques that leverage big data Big data — managed in AWS Configure parameters for an experiment — then go Protects the privacy of our customers and security of our proprietary data Computational resources only exist while being used Traceable, versionable, and safe Technology stack used for research, development and production — infrastructure as code (IaC) methodology Systems available in multiple regions with established disaster recovery plans
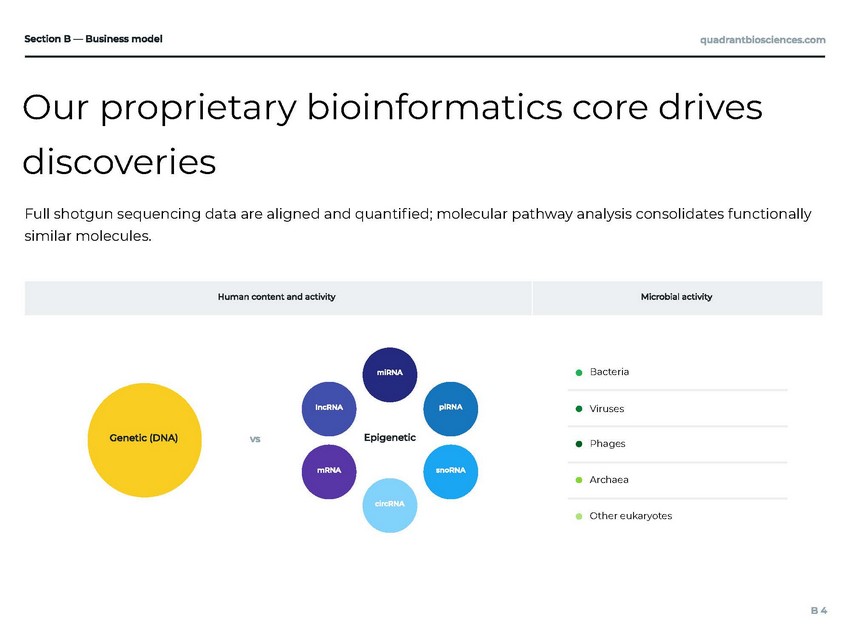
Our proprietary bioinformatics core drives discoveries Bacteria Viruses Phages Archaea Other eukaryotes

Quadrant’s modular system architecture accelerates new product development AWS Cloud Orchestration Inventory Provenance Questionnaires Records Ticketing
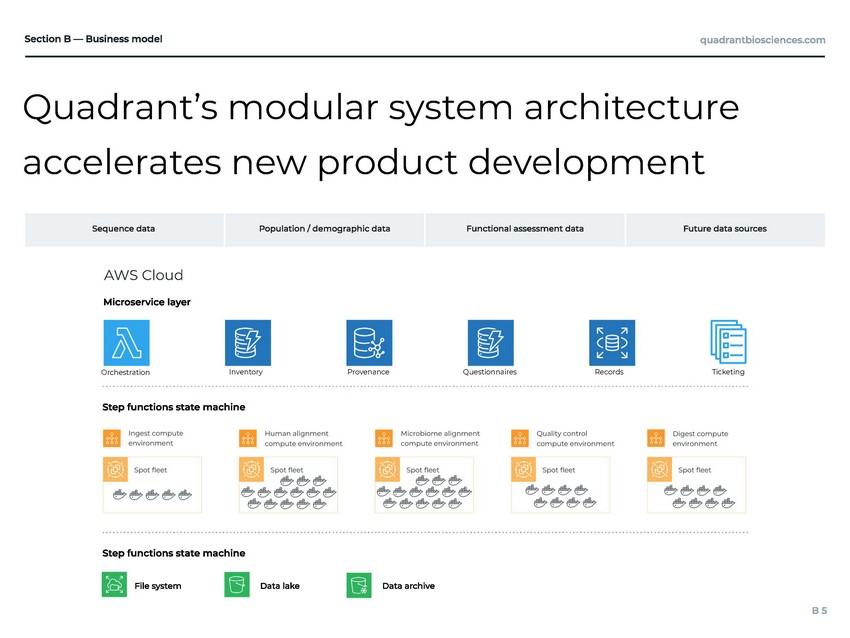
Quadrant’s modular system architecture accelerates new product development AWS Cloud Orchestration Inventory Provenance Questionnaires Records Ticketing

The first product from our epigenetic research pipeline is Clarifi ASD Clarifi ASD is an easy to administer, non-invasive, molecular test that accurately identifies children likely to have Autism Spectrum Disorder. This test provides objective support for earlier diagnosis, when treatment is most effective. Clarifi ASD was launched nationally (X-NY) in December 2019.

Clarifi
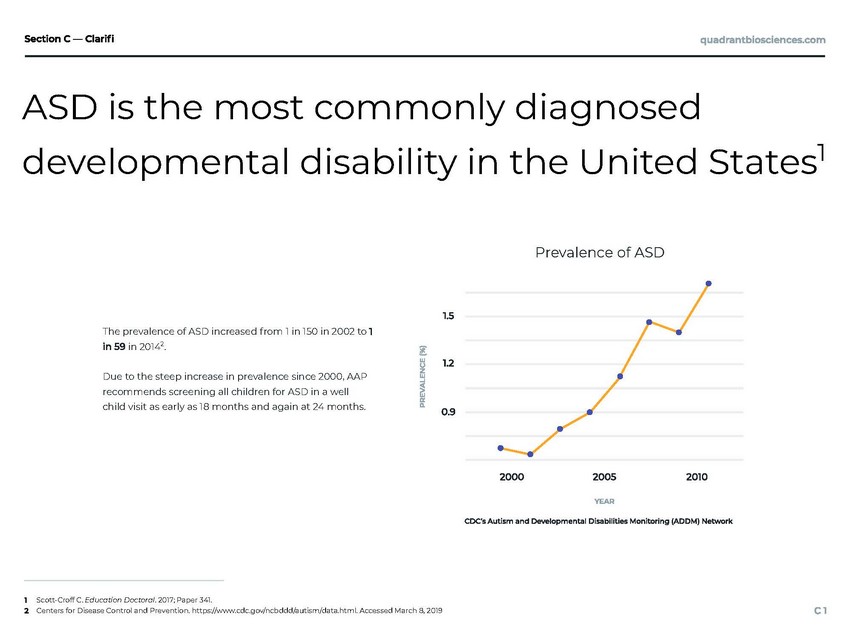
ASD is the most commonly diagnosed developmental disability in the United States1 Scott-Croff C. Education Doctoral. 2017; Paper 341. Centers for Disease Control and Prevention. https://www.cdc.gov/ncbddd/autism/data.html. Accessed March 8, 2019 Prevalence of ASD
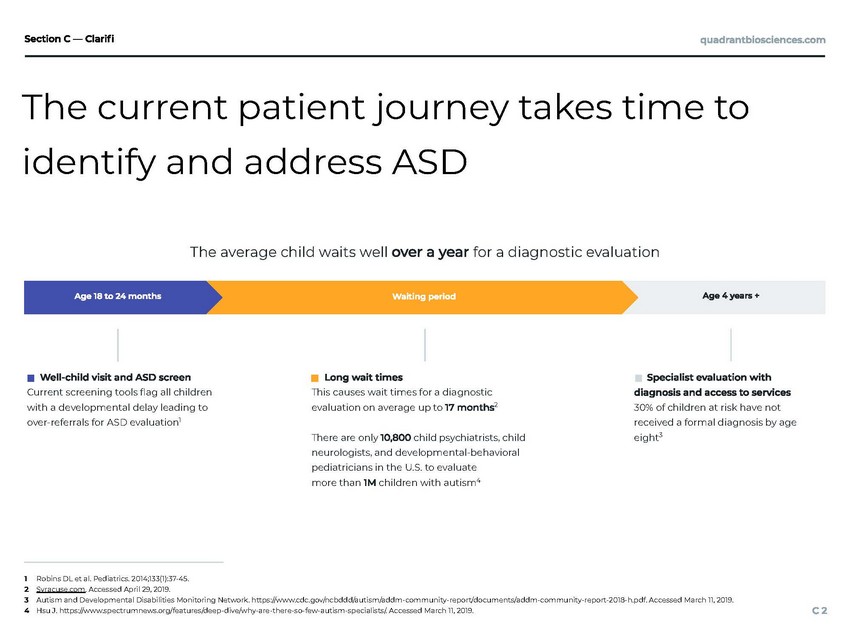
The current patient journey takes time to identify and address ASD Robins DL et al. Pediatrics. 2014;133(1):37-45. Syracuse.com. Accessed April 29, 2019. Autism and Developmental Disabilities Monitoring Network. https://www.cdc.gov/ncbddd/autism/addm-community-report/documents/addm-community-report-2018-h.pdf. Accessed March 11, 2019. Hsu J. https://www.spectrumnews.org/features/deep-dive/why-are-there-so-few-autism-specialists/. Accessed March 11, 2019.

The current patient journey takes time to identify and address ASD Robins DL et al. Pediatrics. 2014;133(1):37-45. Syracuse.com. Accessed April 29, 2019. Autism and Developmental Disabilities Monitoring Network. https://www.cdc.gov/ncbddd/autism/addm-community-report/documents/addm-community-report-2018-h.pdf. Accessed March 11, 2019. Hsu J. https://www.spectrumnews.org/features/deep-dive/why-are-there-so-few-autism-specialists/. Accessed March 11, 2019.
Clinicians need better tools to diagnose ASD Shortfalls of existing screening tools are causing pediatricians to over-refer patients for ASD evaluations. The availability of developmental specialists are limited (both by number and geography); this causes wait times for evaluation to exceed 12 months on average and may exceed 17 months in some locations. Autism is being diagnosed on average in the fifth year of life and should be reliably diagnosed earlier for access to intervention services. An objective test to aid in diagnosis and expedite access to intervention services by differentiating ASD from other developmental delays and reducing the number of referrals. A tool that can be used earlier in development (18 months to 6 years) than the current average age of diagnosis (five years). Clinically acceptable statistics similar to other rapid tests today (e.g., rapid strep and influenza test).
 | test for ASD Clari ASD is an easy to administer, non-invasive, molecular test that accurately identies children likely to have ASD. This test provides objective support for earlier diagnosis, when treatment is most efcacious. Clari ASD is intended to be utilized by pediatricians and family physicians with patients (18 months through 6 years of age) with a positive screening test or a clinical suspicion of ASD. Clari ASD accelerates the autism diagnostic process with results available in 3 to 6 weeks, ultimately providing access to important services earlier. Introducing Clari ASD, the rst biological |
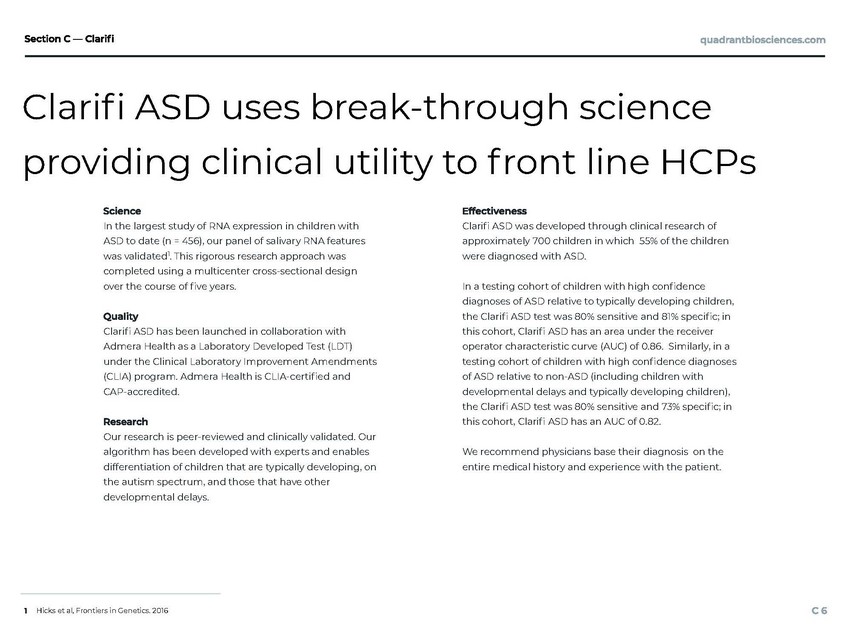 | In the largest study of RNA expression in children with ASD to date (n = 456), our panel of salivary RNA features was validated1. This rigorous research approach was Clari ASD was developed through clinical research of approximately 700 children in which 55% of the children were diagnosed with ASD. completed using a multicenter cross-sectional design over the course of ve years. In a testing cohort of children with high condence diagnoses of ASD relative to typically developing children, the Clari ASD test was 80% sensitive and 81% specic; in Clari ASD has been launched in collaboration with Admera Health as a Laboratory Developed Test (LDT) under the Clinical Laboratory Improvement Amendments (CLIA) program. Admera Health is CLIA-certied and CAP-accredited. this cohort, Clari ASD has an area under the receiver operator characteristic curve (AUC) of 0.86. Similarly, in a testing cohort of children with high condence diagnoses of ASD relative to non-ASD (including children with developmental delays and typically developing children), the Clari ASD test was 80% sensitive and 73% specic; in this cohort, Clari ASD has an AUC of 0.82. Our research is peer-reviewed and clinically validated. Our algorithm has been developed with experts and enables differentiation of children that are typically developing, on the autism spectrum, and those that have other developmental delays. We recommend physicians base their diagnosis on the entire medical history and experience with the patient. Hicks et al, Frontiers in Genetics. 2016 Clari ASD uses break-through science providing clinical utility to f ront line HCPs |
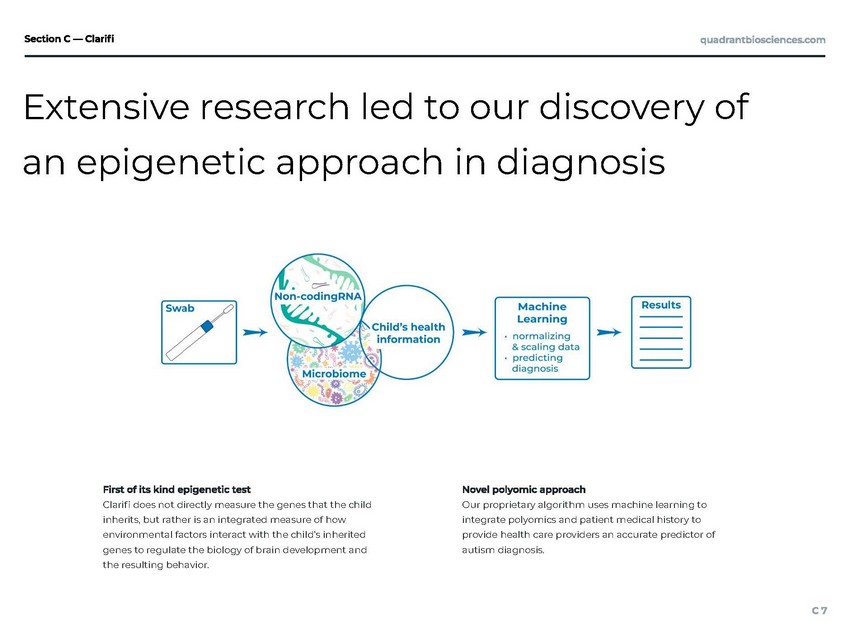 | an epigenetic approach in diagnosis Our proprietary algorithm uses machine learning to integrate polyomics and patient medical history to provide health care providers an accurate predictor of autism diagnosis. Clari does not directly measure the genes that the child inherits, but rather is an integrated measure of how environmental factors interact with the child's inherited genes to regulate the biology of brain development and the resulting behavior. Extensive research led to our discovery of |
 | product f rom existing tools Autism DiagnosticSemi-structured 75% agreement with Approx. $2,000Used in diagnostic assessment Takes an hour to Observation Schedule, play-based DSM-5 diagnosisto examine autism symptoms;administer and requires second edition (ADOS-2)observational (multiple-study Viewed as the gold standard extensive training assessment estimated)assessment tool utilized by clinicians (not utilized as sole indicator) Chromosomal Microarray Saliva / blood2 to 4% of people withRange f rom $2,000 to Identies known genetic A genetic test designed Analysis (CMA) (E.g., autism have rare $4,000syndromes associated withto detect small regions of Lineagen-FirstStep Dx genetic disorders that autism, but does not diagnose abnormalities called copy PLUS, Affymetrix CytoScan are thought to be the autism number variants (CNV) DxAssay)cause of the conditionsuch as deletions and duplications on chromosomes Our novel approach differentiates our |
 | Clari ASD and an ASD diagnosis Genetic testing can help provide parents answers by indicating the presence of genetic conditions associated with ASD. Many want to understand the recurrence risk for other family members, what other comorbid medical conditions their child may experience, and the expectation around prognosis. Genetic tests are primarily not to diagnose, but rather to better understand a condition and create more targeted treatment plans. Genetic testing can be useful following |
 | We have added information and resources to facilitate discussions and support both parents and providers in the diagnostic process. ●Digital M-CHAT-R score ●Autism Speaks 100 Day Kit ●Several other comprehensive resources are provided to inform parents ●Information about risk factors identied in research to date is provided based on questionnaire responses We believe it is important for parents and HCPs to be working in harmony |
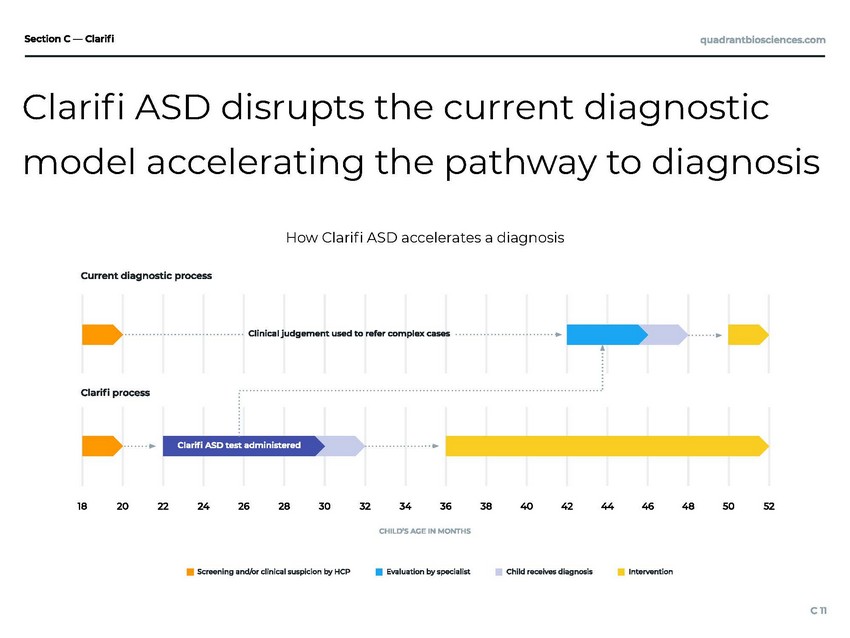 | model accelerating the pathway to diagnosis How Clari ASD accelerates a diagnosis Clari ASD disrupts the current diagnostic |
 | care providers accelerates market adoption We believe parents will be the primary drivers of interest in Clari ASD. Our aggressive digital marketing efforts have specically targeted parents of children 18 months through 6 years of age. The primary clinical audience is comprised of general pediatricians, family medicine physicians and integrative medicine physicians. We also are targeting a secondary audience that includes developmental pediatricians, pediatric neurologists, and clinical therapists. The Clari ASD sales and marketing approach is bifurcated, targeting both parents and health care providers Demand driven by both parents and health |
 | The focus of our aggressive digital marketing campaign has been on building awareness of Clari ASD among parents and clinicians. We have achieved this by saturating the target audiences with social media, native and programmatic ads, and relevant content such as Clari ASD related videos, press releases, and blog posts. Aggressive brand awareness campaign has resulted in signicant public awareness |
 | Several key metrics strongly indicate that our pre-launch focus on brand awareness and audience building will translate into ordering test kits. This includes thousands of parent and clinician subscribers who have expressed interest in obtaining the test as soon as it becomes available on the market, as well as over 13,000 parents that have downloaded a Clari ASD informational sheet to bring to their child’s clinician. In addition to our subscribers, we have captured who have actively engaged in some way with our content such as sharing or liking a social media post, visiting our website, or watching a video. Once we launch, this considerable audience will be retargeted with Clari ASD ads and content. Millions of parents and clinicians in our target market know about Clari ASD |
 | CLARIFI SUBSCRIBER MAP Our pre-launch focus has been on a) identifying Key Opinion Leaders, b) meeting with the largest ASD Diagnosing Centers within 38 target market territories, c) participating in local and national pediatric conferences, and d) arranging presentations to pediatric societies and pediatric residency programs across the country. While our marketing efforts are national in scope, we have identied 38 metropolitan areas in U.S. where our initial sales efforts will be focused. These locations were selected based on the following criteria: ●Highest per capita income ●Population less than ve years of age ●Physician population We have identied over 40 independent sales representatives to support our aggressive digital marketing strategy in these 38 top metro areas. Our sales management team is based strategically in Texas where the greatest level of interest and concentration of early adopters of Clari ASD are located Clari sales inf rastructure is well prepared for product launch |
 | Peer-reviewed research validates the science behind Clari |
 | Our clinical advisory board is comprised of leading autism scientists and clinicians |
 | Product pipeline |
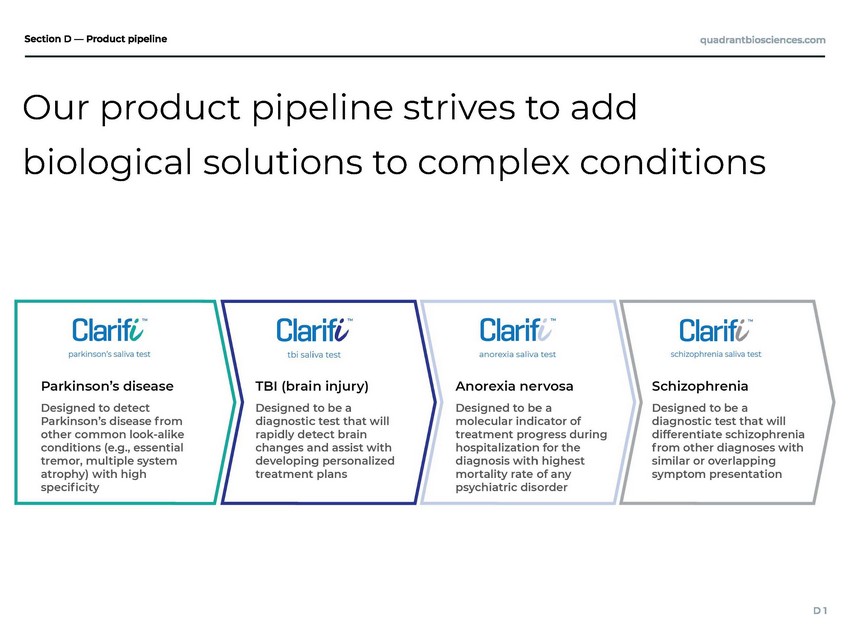 | Our product pipeline strives to add biological solutions to complex conditions |
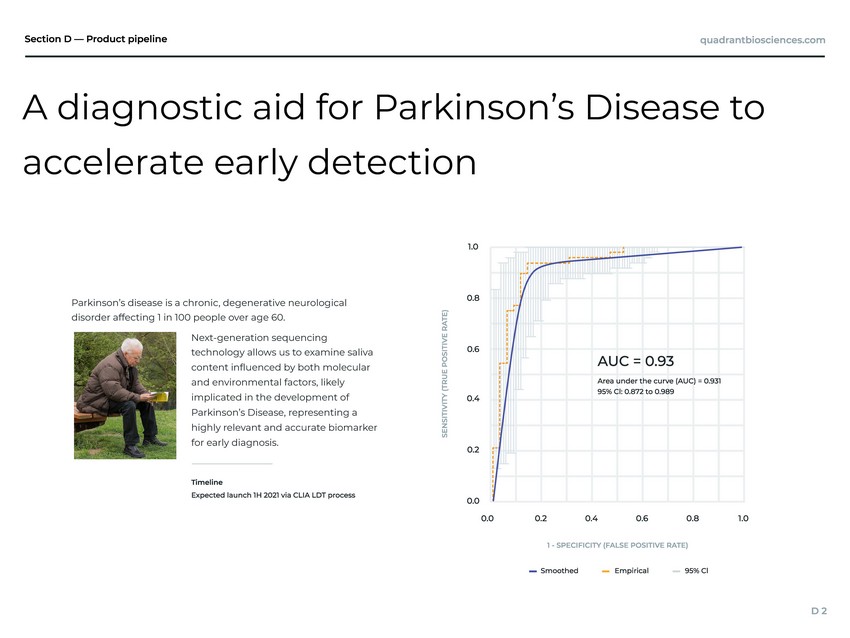 | chronic, degenerative neurological 0 people over age 60. er for early diagnosis. Parkinson’s disease is a disorder affecting 1 in 10 Next-generation sequencing technology allows us to examine saliva content inuenced by both molecular and environmental factors, likely implicated in the development of Parkinson’s Disease, representing a highly relevant and accurate biomark A diagnostic aid for Parkinson’s Disease to accelerate early detection |
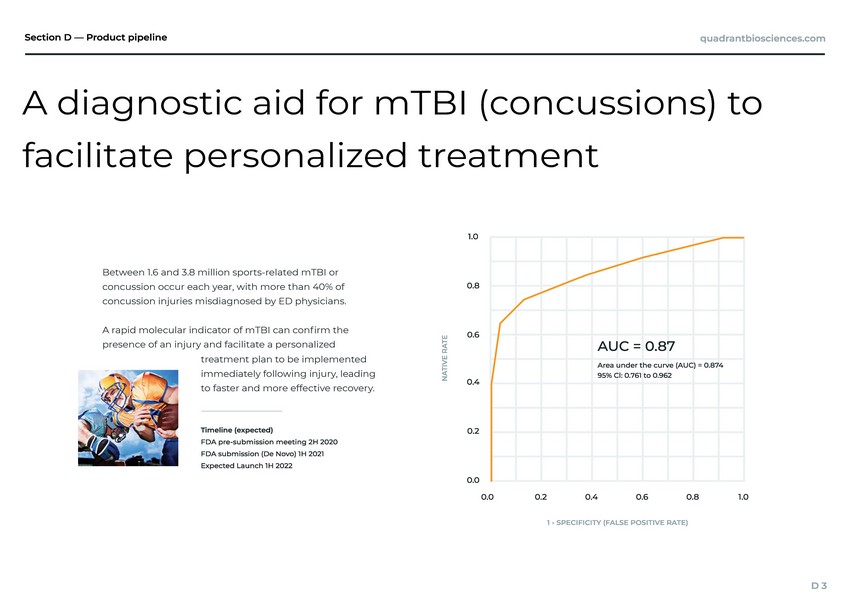 | 8 million sports-related mTBI or ach year, with more than 40% of misdiagnosed by ED physicians. indicator of mTBI can conrm the injury and facilitate a personalized immediately following injury, leading to faster and more effective recovery. Between 1.6 and 3. concussion occur e concussion injuries A rapid molecular presence of an treatment plan to be implemented A diagnostic aid for mTBI (concussions) to facilitate personalized treatment |
 | Our product pipeline was built f rom years of research with leading academic institutions |
 | Intellectual property |
 | “We believe our portfolio of IP will differentiate our competitive position in the market and establish and catalyze a positive feedback loop. Additional discoveries will build upon our aggregate portfolio and create signicant value for the company and its shareholders over time.” Quadrant is developing a unique and compelling intellectual property portfolio |
 | Quadrant has actively pursued the registration of the Clari mark, ling 34 applications in the U.S. and numerous countries. ● ●United States, Brazil, Hong Kong, Taiwan, and Malaysia. Vietnam Arabia, Singapore, Switzerland, Thailand, and Korea, Norway, New Zealand, Philippines, Saudi Union, Great Britain, Indonesia, India, Japan, Australia, Canada, China, European The company has led 29 patent applications for biomarker panels and diagnostic methods ●Autism Spectrum Disorder: 9 applications; 1 US and 8 international ●mild Traumatic Brain Injury (mTBI): 7 applications; 1 US and 6 international ●Parkinson’s Disease: 1 PCT application* ●Anorexia nervosa: 1 PCT application* ●Machine learning methods: 2 PCT applications* ●Normalization of biomarkers for expected physiological variation: 8 applications; 2 US and 6 international ●microRNA signature of runner’s high: 1 US provisional application Leveraging our expertise and product pipeline to develop signicant IP portfolio |
 | Leadership and governance |
 | Meet the leadership team |
 | Meet the leadership team |
 | Meet the leadership team |
 | Board of directors |
 | News |
 | Board of directors |
 | Quadrant research has been featured numerous times in the media |
 | Contact us |
 | investors@quadrantbiosciences.com |
 | Professional biographies |
 | Richard has been our Chairman and CEO since 2015. He has more than 30 years of business experience focused on the design and development of innovative products across various industries, Richard’s management capabilities range f rom ownership of regional retail businesses to the start-up and management of major corporate divisions with domestic and international product sourcing and sales experience. Prior to serving as Chairman and CEO of Quadrant Biosciences, he was the Sole Member of Motion Intelligence LLC, a biotechnology company he founded in 2012 and which was merged into Quadrant in 2015. Previously, he was the Chairman and Chief Executive Ofcer of Morgan Stanley Bank, the principal banking subsidiary of Morgan Stanley, and was the Chief Investment Ofcer at Merrill Lynch Bank. He held other signicant posts in the nancial industry and served as an Executive in Residence at Cornell University’s Johnson Graduate School of Management. Richard is a graduate of Cornell University. Chief Executive Ofcer Benjamin’s career originates f rom a technical background, where he spent over ten years in the software development industry. During that time, he both led and contributed to several successful projects in the public, private, and academic sectors. Benjamin’s expertise revolves around a wide breadth of disciplines including cloud computing architecture, blockchain technology, and agile project management, where his experience spans all facets of the product development lifecycle. He strives to build both human and technical systems that are accessible to all, and scale rapidly with demand. Ben has been with Quadrant Biosciences since April 2016. Until his recent promotion to President of the company in November 2019, he served as the Chief Technology Ofcer for both Quadrant and our subsidiary Motion Intelligence LLC. Prior to 2018, he was the Vice President of Technology at both organizations. Prior to Quadrant Biosciences, Benjamin led projects for the federal statistical system and public opinion domain. More specically, he was a software developer for the Cornell Institute for Social and Economic Research f rom June 2013 – April 2016, where his metadata management software was installed within the US Census Bureau as part of an NSF grant. In addition, Benjamin ran a consulting business that provided data management expertise, and software development services. He received his master’s degree in Information Science f rom Cornell University. President Jim has been our General Counsel since January 2018. He is currently a principal at the law ofcers of James Croke, LLC where he has been since April 2014. Jim was previously a structured nance/banking partner at Chapman & Cutler, Orrick Herrington, Cadwalader Wickersham & Taft, and Hunton & Williams. Throughout his career, he served as counsel to underwriters and issuers in U.S. and global public offerings and private placements. Jim has been a member of the board of directors of the American Securitization Forum and a faculty member of the Practicing Law Institute. He has written and lectured on a variety of topics regarding legal and regulatory issues and annually served as a guest lecturer regarding U.S. corporate and nance law at The Universidad Panamericana in Guadalajara, Mexico. Jim practiced U.S. law in London f rom 1999 - 2004, as the head of Cadwalader, Wickersham & Taft LLP's London capital markets department. Jim earned his undergraduate degree in Mathematics f rom the University of Kentucky (in three years) and his J.D. degree f rom the University of Notre Dame Law School. General Counsel & Director Richard has over 30 years of nance experience while working at many major Wall Street rms. Richard has been our Chief Financial Ofcer since May 2018. He most recently was a Managing Director at BNP Paribas (April 2006 through April 2018), one of Europe's largest banks, and has also worked at such rms as Lehman Brothers, Credit Suisse, Merrill Lynch and Bank of America. Richard’s experience spans several different disciplines in structured nance including structuring, trading and sales at the institutional level, where he helped to usher in several cutting-edge nancial investment products such as Collateralized Mortgage Obligations and Commercial Mortgage Backed Securities and Collateralized Loan Obligations. Richard began his career at Coopers & Lybrand (Pricewaterhouse Coopers) where he received his CPA. He holds degrees in both Computer Information Systems and Accounting f rom Kings College. Chief Financial Ofcer & Director David has more than 25 years of business, research and legal experience. He recently produced two award-winning feature and documentary lms and founded an MMA website and clothing company. David was the co-owner of a mixed-martial arts promotion company and co-owner of a medical-device sales distribution company, MacLean Surgical Instruments, which he managed for over 20 years prior to joining us as our chief marketing ofcer in August 2015. Previously, he was a litigator at the rms of LeBoeuf, Lamb, Lieby & MacRae, and Nixon Hargrave Devans & Doyle. Earlier, he was a research biologist for the Cornell University Lab of Ornithology. David earned a BS f rom Cornell University and a JD f rom the University of Buffalo Law School, where he was a member of the Law Review. Chief Marketing Ofcer Bryan brings more than 15 years of experience in medical device operations, manufacturing, validation and new-product introduction at both large multinational and start-up corporations. He has been in charge of our operations since October 2015. He has a proven track record of successfully introducing Class I, II and III products at Life Technologies (Thermo Fisher Scientic), Pall Corporation and ImClone System (Eli Lilly). Most recently, Bryan was the manufacturing and operations leader during establishment and implementation of an FDA 21CFR820 compliant system at Rheonix, a medical device start-up (January 2013 – July 2015) and production and operations quality manager (July 2015 –October 2015) at Unilife Corporation. He received a BS in Chemical Engineering f rom Clarkson University. Chief Operating Ofcer Jeremy has developed software solutions in the nancial sector, the GIS industry and in support of large-scale social science research. He has held leadership roles in a number of successful projects, both academically and commercially. He was responsible for designing a citizen science project funded by the NEH and used in secondary schools and colleges across the country. He has also led the development of restricted access Census Bureau metadata repository software funded by the NSF. His experience spans complex systems integration, mapping software used by state and municipal governments to manage geo-spatial data, and applications for research data analysis and dissemination. During Jeremy’s career, he has developed expertise in a variety of technologies, with a focus on information system architecture. Until his recent promotion to Chief Technology Ofcer of the company in November 2019, he served as a Vice President of Technology starting in April 2016. Prior to Quadrant Biosciences, Jeremy was a software developer for the Cornell Institute for Social and Economic Research f rom September 2007 – April 2016. He graduated with a master’s degree in Information Science f rom Cornell University. Chief Technology Ofcer Leadership team |
 | Wade brings over 39 years of entrepreneurial business experience in leadership, sales, marketing and business development. He has been our executive vice president of sales since January 2019. Prior to that and for over thirty years, he has been the founder and President of General Orthopedics, orthopedic and neurosurgery focused medical sales and distribution company based in San Antonio, Texas. Wade began his career with Minolta Reprographic Systems, eventually becoming a national account executive. He then transitioned into the orthopedic market, taking a sales position with Biomet where he was recognized as the top sales representative for four consecutive years. In 1992, Wade became the rst Biomet Spine distributor in the United States, at which time he founded General Orthopedics. A driven entrepreneur, Wade has provided consulting services to a number of startups and growing companies in the orthopedic and medical device industry. He is actively involved in several charitable organizations including Wish For Our Heroes. Executive Vice President - Sales & Director Naved joined Quadrant in August 2019 and has over 25 years’ experience working in a variety of nancial roles. He started his career in investment banking at Smith Barney, advising institutions ranging f rom credit card issuers, state housing authorities to the Resolution Trust Corporation. A majority of his career was involved with creating, marketing and trading innovative nancial products for investors of every type both domestically and internationally. He also has experience as an investor as a xed income portfolio manager at Dillon Read and most recently f rom August 2014 through March 2017 as founder and portfolio manager of Tevere Capital a hedge fund catering to insurance companies. Naved has lead trading desks at Bank of America, Lehman Brothers and Morgan Stanley. He holds a Bachelor’s Degree in Economics f rom The University of Pennsylvania. Executive Vice President - Corporate Strategy Chris has more than 20 years of experience as corporate counsel on matters including transactions, business development, intellectual property, and regulatory compliance. Chris joined Quadrant in January 2018. Previously, he served as Deputy General Counsel and Compliance Ofcer for Welch Allyn, Inc. for more than 10 years, and in that capacity managed the attorneys in the legal department who worked in the areas of transactions, intellectual property, and compliance, and also served on the executive team that managed the Quality and Regulatory departments. Chris is an adjunct professor at the Syracuse College of Law and teaches a section of the class on technology commercialization. He received a BS in Medicine f rom the University of Nebraska, and a JD, with distinction, f rom the University of Nebraska College of Law. Deputy General Counsel Kayla has spent her career dedicated to working with children and adolescents with neurodevelopmental disorders in both clinical and research settings. In her clinical work she provided diagnostic and counseling services to children and adolescents with psychiatric disorders. Her research spans many areas of child psychopathology, including autism spectrum disorder, ADHD and 22q11.2 Deletion Syndrome. More specically, she has investigated the comorbidity between autism and ADHD, genetic and familial relationships within RDOC constructs, and factors related to poor social outcomes (e.g., relationship development/maintenance with f riends, family, romantic partners) in children, adolescents, and adults with neurodevelopmental disorders. Kayla joined Quadrant in May 2017; previously, she was a Therapist at Syracuse University (January 2017 to May 2017) and a Research Coordinator at SUNY Upstate Medical University (August 2014 to August 2016). Kayla earned a BS in neuroscience and psychology and an MS in Clinical Psychology at Syracuse University. Vice President - Product Management Dr. Carpenter joined Quadrant in January 2016 and has over 30 years’ experience in primary care, academic medicine, basic science and clinical research, and pharmaceutical drug development. His experience in the pharmaceutical and biotechnology industries include Co-Founder, President and CEO of Seaside Therapeutics (September 2005 – December 2013), President and CEO of Sention, VP of Clinical Research & Development and Regulatory Affairs at Adolor Corporation and member of Astra Pain Control’s Global Therapeutic Area Team. While in industry, Dr. Carpenter led translational medicine teams responsible for 8 successful IND submissions and dozens of GCP-compliant clinical trials. Prior to joining industry, he held academic faculty appointments at Virginia Mason Medical Centre, the University of Washington and Wake Forest University where he specialized in anaesthesiology, pain management and translational medicine. Executive Vice President - Clinical Research Alexander has a varied career in biomedical sciences, f rom basic and translational research, life science and computational biology education and outreach, emergency medicine, and biotech startups. He began his research career investigating autism diagnostics, and has managed, led, and consulted on research projects in vision, somatosensation, proprioception, and motor systems involving biomechanics, neuroanatomy, electrophysiology, and stimulation. His funding sources have included the NSF, NIH, and DARPA. His startup work has been in sales and marketing and in research and development. Prior to joining Quadrant in October 2017, Alexander was the Technical Sales Lead at Ripple LLC (June 2016 to February 2017) and a PhD candidate (September 2009 to May 2016). Alexander earned a BS in biopsychology f rom the University of California, Davis and a PhD in computational neuroscience f rom the University of Chicago. Executive Vice President - Articial Intelligence Leadership team |































































