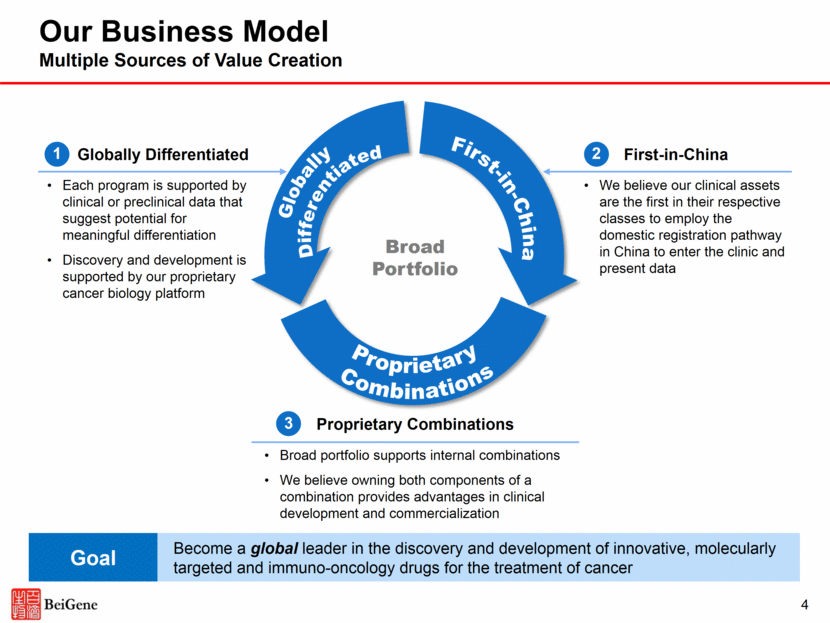
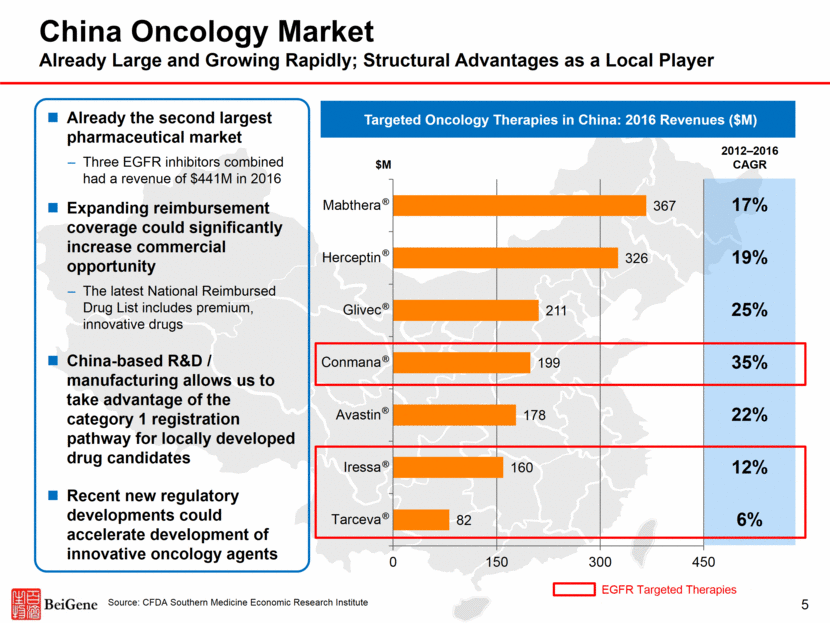
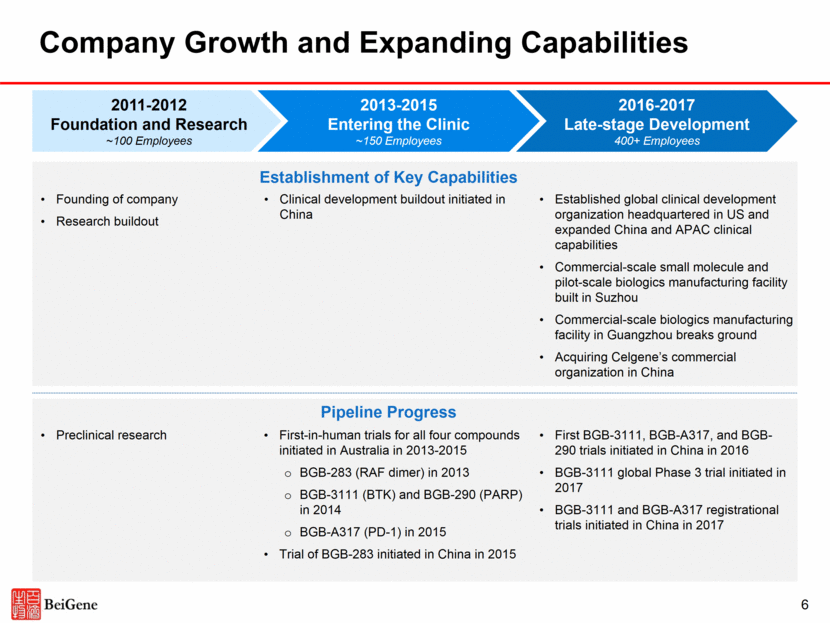
2 Disclosures Certain statements contained in this presentation and in the accompanying oral presentation, other than statements of fact that are independently verifiable at the date hereof, may constitute forward-looking statements. Examples of such forward-looking statements include those regarding investigational drug candidates and clinical trials and the status and related results thereto, as well those regarding continuing and further development efforts and transactions with third parties. Such statements, based as they are on the current analysis and expectations of management, inherently involve numerous risks and uncertainties, known and unknown, many of which are beyond BeiGene’s control. Such risks include but are not limited to: the impact of general economic conditions, general conditions in the pharmaceutical industries, changes in the global and regional regulatory environments in the jurisdictions in which BeiGene does business, market volatility, fluctuations in costs and changes to the competitive environment. Consequently, actual future results may differ materially from the anticipated results expressed in the forward-looking statements. In the case of forward-looking statements regarding investigational drug candidates and continuing further development efforts, specific risks which could cause actual results to differ materially from BeiGene’s current analysis and expectations include: failure to demonstrate the safety, tolerability and efficacy of our drug candidates, final and quality controlled verification of data and the related analyses, the expense and uncertainty of obtaining regulatory approval, including from the FDA, CFDA and EMA, and the possibility of having to conduct additional clinical trials. Further, even if regulatory approval is obtained, pharmaceutical products are generally subject to stringent on-going governmental regulation, challenges in gaining market acceptance and competition. These statements are also subject to a number of material risks and uncertainties that are described in BeiGene’s filings with the Securities and Exchange Commission (SEC). The reader should not place undue reliance on any forward-looking statements included in this presentation or in the accompanying oral presentation. These statements speak only as of the date made, and BeiGene is under no obligation and disavows any obligation to update or revise such statements as a result of any event, circumstances or otherwise, unless required by applicable legislation or regulation. Clinical data in this presentation relating to BeiGene’s investigational drug candidates is from early phase, single-arm trials. When such data are presented in relation to other investigational or marketed drug products, the presentation and discussion are not based on head-to-head trials between BeiGene’s investigational drug candidates and other products. BeiGene is still conducting clinical trials and, as additional patients are enrolled and evaluated, data on BeiGene’s investigational drug candidates may change. This presentation and the accompanying oral presentation contains data and information obtained from third-party studies and internal company analysis of such data and information. BeiGene has not independently verified the data and information obtained from these sources. Forward-looking information obtained from these sources is subject to the same qualifications noted above.